Abstract
Introduction:
Implementation science frameworks with a focus on health equity have emerged to help guide the introduction of new interventions into healthcare and community settings while limiting health disparities. The purpose of this research was to explore the applicability of such frameworks to guide the equitable implementation of population genetic screening programs.
Methods:
We searched PubMed and reference lists for relevant frameworks and examples of their use in health settings. We then assessed if and how selected frameworks provide guidance for different stages of population genetic screening: recruitment, sample collection, result return, follow-up care and long-term management, and cascade screening. Findings were synthesized into a list of health equity considerations specific to each stage.
Results:
We identified 5 implementation frameworks that focus on health equity. Guidance varied by framework type: determinant (explaining what affects implementation outcomes), process (translating research into practice), or evaluation (assessing implementation). Common characteristics included focusing implementation efforts on populations who have historically experienced health inequities and adapting interventions to fit local contexts. Process models also highlighted the importance of community partnerships.
Discussion:
Overall, frameworks offered broad recommendations applicable to population genetic screening program implementation. However, gaps still exist in guidance provided for later stages of population genetic screening. To improve the equitable implementation of genetic screening, future programs may benefit from utilizing one or more of these frameworks or by incorporating the health equity considerations and outcomes compiled in this analysis.
Introduction
Population genetic screening, or genetic screening of people regardless of personal or family history of disease, has been proposed to increase the reach of genetic services and identify more people at risk for preventable conditions (1–3). However, population screening is complex for many reasons including the need to appropriately inform large numbers of people about the benefits and harms of genetic screening, collecting DNA samples, and following people over time to ensure they receive appropriate care based on their results. If not implemented with care and the needs of underserved people in mind, population genetic screening may perpetuate or further exacerbate already existing health disparities (4, 5).
To limit harmful consequences, health equity must be a central consideration in the design and implementation of population genetic screening programs. Health equity is defined as everyone having a fair and just opportunity to be as healthy as possible (6). Striving for health equity requires focusing on the needs of those who are at greatest risk of poor health due to social circumstances (7). It involves the elimination of health differences that are linked to social determinants historically connected to exclusion, such as race, ethnicity, socioeconomic status, gender, age, religion, disability, sexual orientation, gender identity, and geographic location (7).
Implementation science frameworks with a focus on health equity have emerged to guide the introduction of new interventions into healthcare and community settings, and their principles could improve the incorporation of genomic discoveries into healthcare. How well such frameworks may guide the implementation of population genomic screening programs is not well understood, as both efforts to incorporate implementation science in genomic settings and the integration of health equity with implementation science have occurred relatively recently (5, 8, 9).
To aid researchers with incorporating both implementation science and health equity concepts in population genetic screening programs, we conducted a systematic literature search to identify and describe published equity-focused implementation science frameworks. We then assessed the applicability of these frameworks to population genetic screening programs and additionally compiled a list of health equity considerations for the different stages of population genetic screening, including questions and outcomes to consider. Results from this work can simplify framework selection and utilization when implementing population genetic screening programs and promote health equity during all phases of implementation.
Materials and methods
Population genetic screening stages
We conceptualized population genetic screening in 5 major stages (Figure 1) based on the design of existing pilot screening programs (4, 10–12). Our descriptive model's stages include recruitment, sample collection, return of results, follow-up care and long-term management, and cascade screening. Importantly, follow-up care and long-term management includes not just initial conversations about genetic results with a provider, but also the adherence to appropriate screening or other medical intervention over time, maintaining screening results in medical records over time, and re-contact if variants are re-classified. Cascade screening moves beyond the initial person screened and involves notifying biological relatives about genetic risk. This model formed the basis for our analysis of framework applicability to population screening.
Figure 1
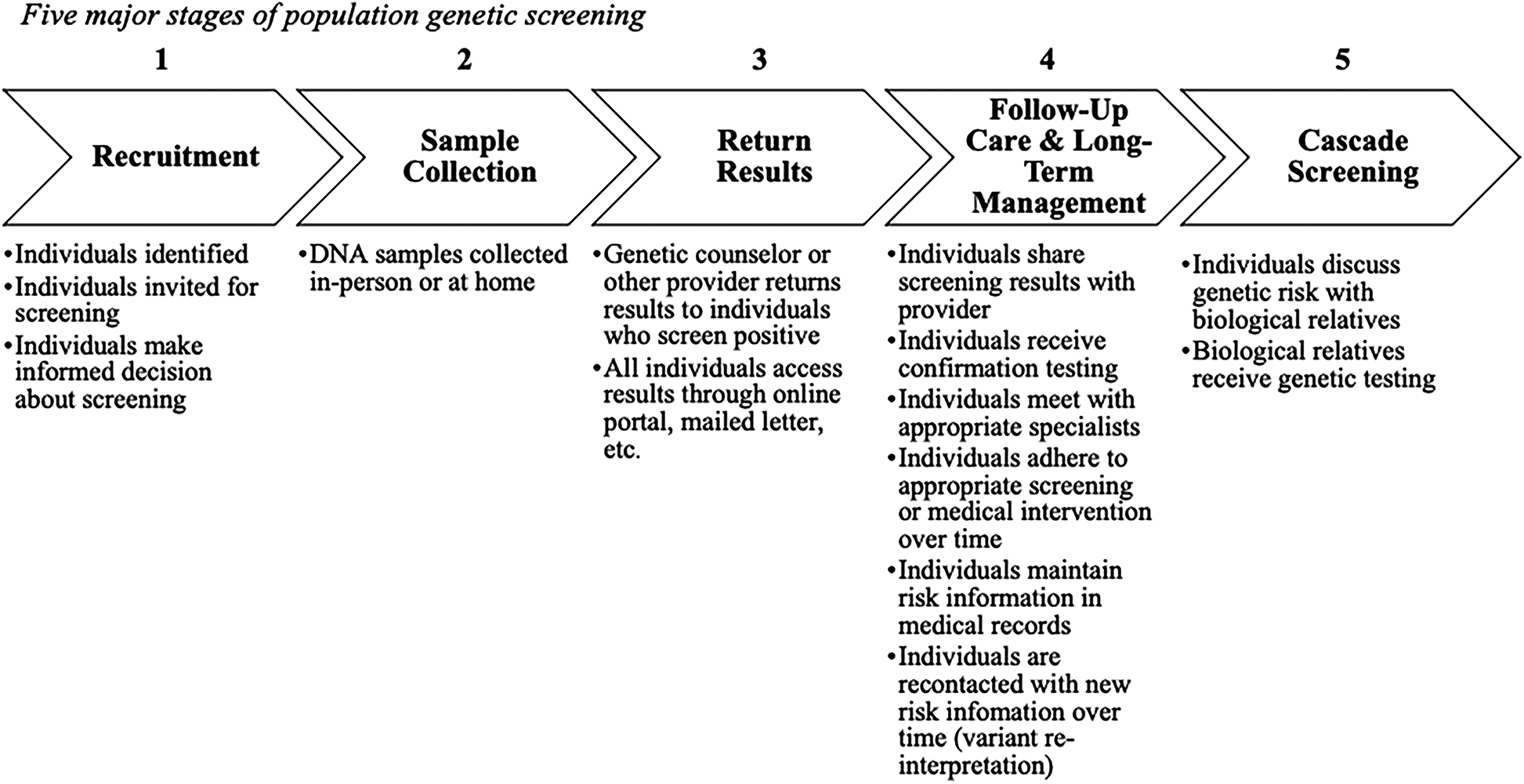
Five major stages of population genetic screening.
Framework identification
We searched PubMed for frameworks designed to promote health equity during the implementation of health interventions using the following keywords: (“health equity” or “health disparities” or “health inequalities”) and (“implementation” or “translation”) and (“framework” or “model” or “theory”). One author, NDR, screened the resulting titles and abstracts. When articles cited potentially relevant frameworks or included a review of frameworks, NDR examined the reference lists for pertinent publications. Other frameworks previously known to the authors were also considered. Article review was restricted to work published between January 2010 and December 2021, as the focus on health equity in implementation science has become more prominent relatively recently (13).
Criteria for inclusion were frameworks focused on health equity and implementation of health services, and that were developed for high-resource settings, as these are the most relevant to population genetic screening. Frameworks were excluded if they were specific to a certain health condition or intervention, provided little guidance for implementation or if the article was not available in English. Discussion papers, or those that only described a need for considering health equity during program implementation or provided no explicit framework or model, were also excluded.
Data extraction and evaluation
For each of the selected frameworks, the following data was extracted: name, author, year of publication, type, audience, development, and description. Framework type was determined according to Nilsen's categorizations of implementation science theories, models, and frameworks: determinant frameworks, process models, or evaluation frameworks (14). Determinant frameworks are designed to assist with understanding barriers or facilitators that influence implementation outcomes; process models to guide the process of translating research into practice; and evaluation frameworks to specify implementation outcomes (14).
Data synthesis
We assessed the applicability of each framework to population genetic screening by evaluating if and how the framework provided guidance for the 5 major stages of population genetic screening we identified. To further our understanding of potential framework application, we looked for examples of how each framework may have been used in other settings by examining articles that cited our selected frameworks. We also searched for evidence of framework validation beyond additional development, including evidence of feedback from experts or verification of definitions of framework concepts.
We then compared the selected frameworks and discussed their strengths and weaknesses with respect to guiding the implementation of population genetic screening. Using findings from our applicability assessment, we also compiled a list of health equity considerations and outcomes specific to each stage of population genetic screening. The goal of these considerations is to provide a starting point of questions and measures to account for when designing and implementing a population genetic screening program.
Results
The initial PubMed search yielded 1,013 results. An additional 37 articles were identified through reference lists or because they were previously known to authors. Records were screened by title and abstract followed by full text review (Figure 2). We identified five frameworks designed to reduce or prevent health disparities during the implementation of health interventions (Table 1). One framework was described in two of the selected articles.
Figure 2
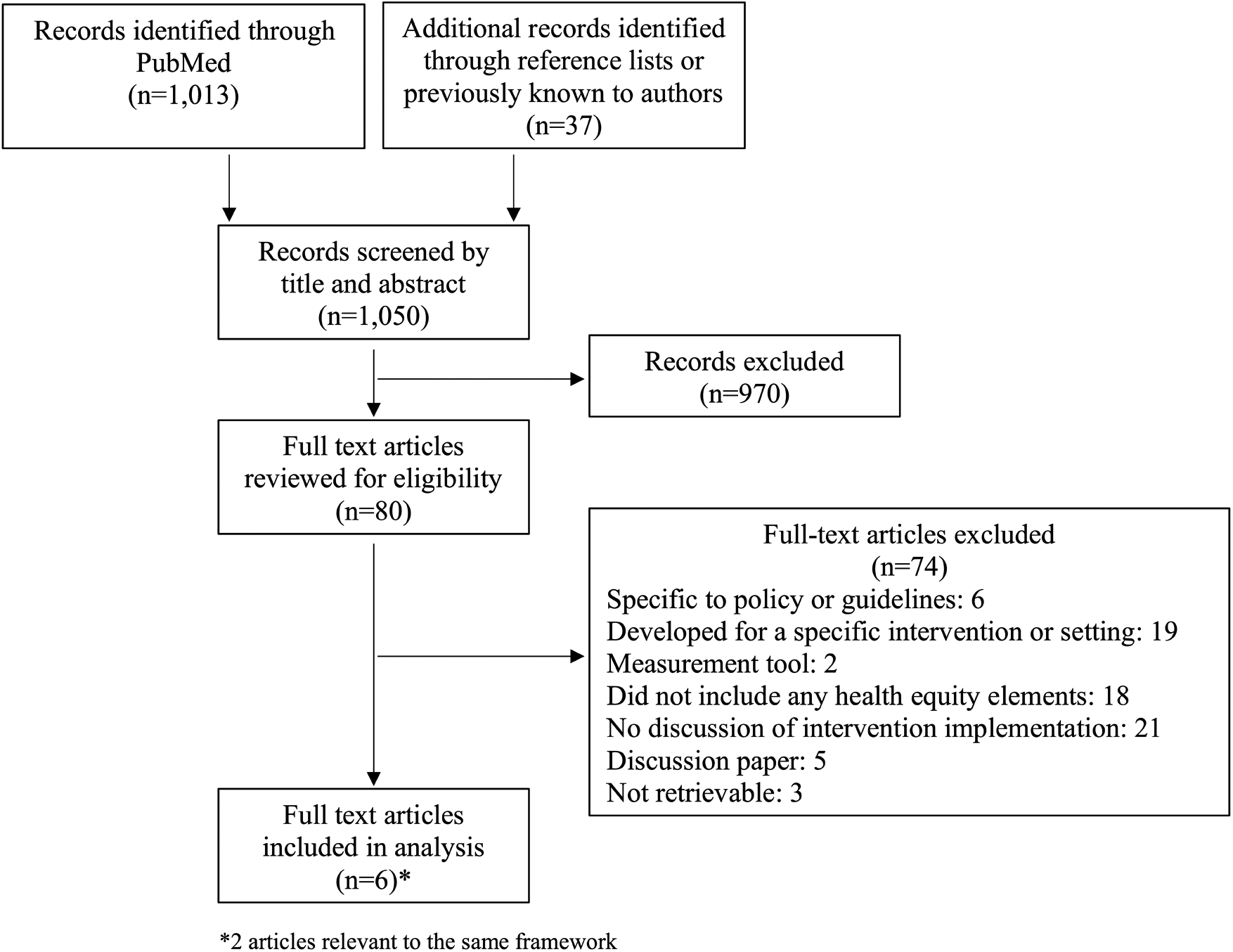
Diagram of article search and selection process.
Table 1
| Framework | Type | Audience | Development | Description |
|---|---|---|---|---|
| Health Equity Implementation Framework (HEIF) (15) | Determinant | Researchers | Integration of the implementation science framework, i-PARIHS (16), and the Health Care Disparities Framework (17). | Framework to assist studying and modifying multilevel implementation and healthcare disparity factors. |
| Reframing implementation science to address inequities in healthcare delivery (Proctor reframed) (18) | Process | Researchers | Reframes Proctor et al.'s conceptual model of implementation research (19) to study healthcare inequities. | Framework seeking to address inequities in healthcare by proactively tailoring interventions and implementation strategies to address social determinants of health and explicitly meet the needs of vulnerable communities/settings. |
| Transcreation: an implementation science framework for community-engaged behavioral interventions to reduce health disparities (20) | Process | Community partners and researchers | Prior methodological frameworks, training resources, authors’ experience. | Framework for designing and implementing behavioral interventions specifically for communities experiencing health disparities. |
| Conceptual framework of equity-focused implementation research for health programs (EquIR) (21) | Process | Decision makers and researchers | Literature review, stakeholder analysis. | Conceptual framework designed to reduce or prevent the increase of existing inequalities during the implementation of programs, policies or health. |
| An extension of RE-AIM to enhance sustainability: addressing dynamic context and promoting health equity over time (22) | Evaluation | Not stated | Builds upon the previously developed RE-AIM framework (23, 24). | Evaluates public health interventions across reach, efficacy, adoption, implementation, and maintenance domains. Focused on sustainability, with the goal of increasing health impact and health equity over time. |
Characteristics of included frameworks.
We categorized the frameworks as one determinant framework [HEIF, (15)], three process frameworks [Proctor reframed, (18); Transcreation, (20); EquIR, (21)], and one evaluation framework [RE-AIM extension, (22)]. Researchers were the primary intended audience for each framework. Frameworks were largely conceptually developed by the authors, though one, EquIR (21), was developed using stakeholder engagement (Table 2). Our search for evidence of framework validation yielded no information about if and how any of the five frameworks had been validated.
Table 2
| Framework | Components/Steps |
|---|---|
| HEIF (15) | Factors to understand healthcare disparity determinants:
|
| Proctor reframed (18) | Steps to design intervention and implementation strategies to address healthcare inequities:
|
| Transcreation (20) | Steps involved in designing, delivering, and evaluating interventions to reduce health disparities:
|
| EquIR (21) | Cyclical steps to prevent the increase of inequalities during intervention implementation:
|
| RE-AIM extension (22) | Health equity considerations for evaluation domains:
|
Components of included frameworks.
The health equity implementation framework (HEIF)
Description
Woodward and colleagues developed HEIF by integrating the i-PARIHS implementation science framework (16) and the Health Care Disparities Framework (17). HEIF is designed to help researchers determine factors related to innovation uptake and disparities in healthcare to improve outcomes for marginalized populations (15). Health equity domains include culturally relevant factors, the clinical encounter, and societal context (Table 2). Culturally relevant factors are specific to intervention recipients based on their lived experience and can include characteristics such as socioeconomic status, implicit bias, health literacy, trust in providers, language, race and ethnicity. The clinical encounter encompasses interactions between providers and patients, which influence if an intervention is offered by a provider or accepted by a patient. These encounters are influenced by inner context at the local (e.g., clinic) and organizational (e.g., hospital) levels, and outer context (e.g., the healthcare system). Finally, the societal context includes economies, physical structures (how environments are built or arranged), and sociopolitical forces (social norms or political forces). These impact health disparities by influencing the inner and outer context, the clinical encounter, and culturally relevant factors. The HEIF has previously been applied to design an interview guide and direct content analysis to identify implementation factors and best practices for social needs screening in primary care settings (25).
Application to population genetic screening
The HEIF is well suited to provide guidance for anticipating possible barriers or facilitators to implementation across all stages of population genetic screening (Table 3). For example, during recruitment, attention to cultural factors can help researchers anticipate how language and cultural beliefs influence informed consent and enrollment. HEIF's physical structures domain can inform how in-person sample collection and return of results may facilitate or impede screening depending on access to reliable transportation and the location of facilities. Similarly, during follow-up care and long-term management, understanding potentially inequitable physical spaces can inform implementation. In the cascade screening stage, reflecting on sociopolitical forces, such genetic privacy laws, may illuminate barriers to information sharing among relatives.
Table 3
| Framework/Population Screening Stages | Recruitment | Sample Collection | Return Results | Follow-Up Care & Long-Term Management | Cascade Screening |
|---|---|---|---|---|---|
| HEIF (15) | Anticipate and identify barriers and facilitators using health equity domains: culturally relevant factors, the clinical encounter and societal context | ||||
| Proctor reframed (18) | Include populations experiencing inequities. Conduct programs in non-traditional settings. | ||||
| Collaborate with stakeholders and community members | |||||
| Assess acceptability and adapt interventions | |||||
| Transcreation (20) | Focus on populations experiencing inequities. Adopt recruitment strategies that have worked in similar settings. | ||||
| Stakeholder and community participation | |||||
| Involve and train community health workers | |||||
| EquIR (21) | Consider how programs and procedures may exclude disadvantaged communities | ||||
| Quantify potential inequities | |||||
| Develop recommendations to address inequities | |||||
| Relevant outcomes: acceptability, appropriateness, coverage | Relevant outcomes: acceptability, appropriateness, coverage, fidelity | Relevant outcomes: acceptability, appropriateness, coverage, fidelity | Relevant outcomes: coverage, fidelity | Relevant outcomes: acceptability, coverage, fidelity | |
| RE-AIM extension (22) | Proportion of eligible people offered screening, proportion who enroll | Proportion who provide samples | Proportion who have results available, proportion who receive results, proportion who experience psychosocial harms from results | Proportion who engage in preventive interventions who desire it, proportion who experience psychosocial harms because of difficulties accessing care, proportion who are re-contacted about new risk information (measure over time) | Proportion who communicate about risk with relatives, proportion of biological relatives who receive testing (measure over time) |
Applicability of frameworks to population genetic screening programs.
Proctor reframed
Description
Baumann and Cabassa (18) reframed the Proctor implementation science framework to provide an example of how to apply an existing framework to address healthcare inequities. The original Proctor framework posits that interventions differ from their implementation strategies and requires the involvement of various stakeholders at multiple levels (19). The original Proctor also proposes outcomes in three interrelated but distinct domains: implementation (e.g., feasibility, fidelity, acceptance), service (e.g., efficiency, safety, effectiveness), and client (e.g., satisfaction, function). The reframed Proctor framework emphasizes collaborating with stakeholders and community members throughout intervention planning, design, and implementation in order to understand and meet the needs of historically underserved communities (Table 2) (18). It proposes continually adapting programs based on the needs of populations with the goal of reducing inequities through systematic changes to intervention and implementation strategies. Finally, Proctor reframed suggests conducting descriptive and explanatory studies to identify factors that contribute to inequities in implementation outcomes. Proctor reframed has been used to assist with the summarization of determinants and strategies concerning the effective implementation of HIV-related health interventions (26).
Application to population genetic screening
Proctor-reframed specifies guidance most relevant to the recruitment stage, including ensuring that populations that have previously experienced inequities in genetic services are included in population genetic screening programs (Table 3). Suggested strategies for enhancing inclusion are conducting programs in non-traditional settings such as in faith communities or community centers. This framework also discusses how face-to-face presentations with community members and person-to-person recruitment can assist with enrolling people who would otherwise not participate.
Transcreation
Description
Transcreation is defined as the process of planning and delivering interventions to reduce health disparities that resonate with the intended community (20). Nápoles and colleagues created this framework to address the differences that occur between original intervention implementation settings (often among mainstream populations or in academic settings) and when interventions are adopted among a population facing health disparities.
Collaboration is a central principle of Transcreation, which proposes stakeholder and community involvement through the entire process of intervention design, implementation, and adaptation (Table 2) (20). This framework assumes the presence of an established partnership between researchers and community members and a shared understanding of the disparity to be addressed. As part of the framework's proposed collaboration, Transcreation suggests involving community workers in implementation by training them in intervention delivery. Fitting interventions to context and population needs is also prominent.
Transcreation has previously been applied in other health settings; for example, it has been used to adapt a stress management intervention for Latina breast cancer survivors living in rural settings (27).
Application to population genetic screening
Transcreation provides guidance most relevant for the initial recruitment stage of population genetic screening by suggesting focusing attention on populations who experience disparities in access to and utilization of genetic services (Table 3). Through the incorporation of scientific evidence, programs can also adopt recruitment strategies that have been proven to work in similar settings.
Unique to Transcreation is training community members in intervention delivery. This idea is relevant for all population screening stages, as members can be trained to provide cultural, informational and logistical support to specific communities within a general population. During recruitment, this can promote informed decision-making. In the follow-up and long-term management stage, community members acting as patient navigators can assist individuals with information about insurance or recommended medical interventions.
Equity-Based framework for implementation research (EquIR)
Description
Eslava-Schmalbach and colleagues developed EquIR for researchers and decision makers to reduce or prevent health inequities during the implementation of health programs or policies (21). This conceptual framework is cyclic, with social determinants of health considered throughout. The cycle begins with identifying disadvantaged populations and quantifying current health inequalities (Table 2). It then suggests developing and implementing recommendations to meet the needs of disadvantaged populations with key players such as health professionals, patients, community members, and stakeholders. It finishes by recommending the monitoring of implementation outcomes (Table 2) and identifying how the intervention has impacted the health status of populations receiving the intervention. From here the cycle continues and the new population health status becomes the starting point of the intervention. The EquIR has been used to investigate adaptations to improve emergency preparedness made by outreach programs for underserved and uninsured Mexican immigrants during the COVID-19 pandemic (28).
Application to population genetic screening
Of the guidance proposed by EquIR, the described outcomes are most readily applied to population genetic screening and can be used to understand how programs impact disadvantaged populations at each stage. For example, measures of acceptability and appropriateness can be applied during recruitment, sample collection, and return of results stages to understand stakeholder perceptions of fit and usefulness of program procedures (Table 3). Measures of fidelity and coverage may be useful during follow-up care and cascade screening stages for understanding how often people receiving positive screening results are able to act on these results and how often risk information is shared with biological relatives. In addition, the cyclical nature of EquIR promotes ongoing program adjustments informed by these outcome measures.
Reach, effectiveness, adoption, implementation, maintenance (RE-AIM) extension
Description
The extension to the RE-AIM framework authored by Shelton and colleagues is designed to promote sustainability and health equity. The original RE-AIM framework focuses on evaluation and includes both individual and staff/setting level domains: Reach and effectiveness (individual), adoption and implementation (staff/setting), and maintenance (individual and staff/setting) (23, 24). While the extension to RE-AIM discusses these same domains and previously described indicators, Shelton et al. provide additional guidance to consider health equity during the measurement of these indicators (Table 2). This guidance focuses on assessing indicators over time across different populations of focus (defined by age, race, ethnicity, disability, insurance status, literacy level or other social determinants of health), to identify and address health inequities (22). The extension to RE-AIM also considers the link between health equity and costs or resources and suggests incorporating cost estimates and resource requirements into planning discussions with stakeholders. This framework has previously been used to evaluate the implementation of a COVID-19 vaccine program seeking to facilitate equitable vaccine access and uptake among Latinx community members (29).
Application to population genetic screening
The outcome indicators and health equity considerations listed by RE-AIM extension give measures that can be monitored at each stage of population genetic screening (Table 3). During recruitment, relevant indicators include the proportion of people who are offered screening among those who are eligible and the proportion of people who agree to screening. Taking into account social determinants of health when interpreting these indicators can determine if all populations are offered and enroll in screening similarly and reveal which populations are not reached. Reach can also be ascertained for sample collection, return of results, and cascade screening to find inequities that may be emerging during these stages.
Measures of effectiveness across social determinants of health are also relevant for the return of results, follow-up care, and cascade screening stages. For return of results, indicators include the proportion of people experiencing psychosocial harms upon learning results. For follow-up care and long-term management, relevant indicators are the proportion of people who are able to engage in preventive interventions who desire it, the proportion of people who experience psychosocial harms because of difficulties accessing care, and the proportion of people who are re-contacted about new risk information as it become available over time. During cascade screening, indicators include the proportion of biological relatives who receive testing.
Comparing frameworks
A number of characteristics were shared across the analyzed frameworks. The first was to consider populations who have historically experienced health inequities early in the implementation process. This is a crucial consideration as placing specific emphasis on underserved populations at the beginning of implementation planning can reorient design and procedures to better prioritize the needs of such communities. Another common element across frameworks was to adapt interventions to fit local context and meet the needs of marginalized communities. Doing so can limit the implementation gap, which occurs when the context where interventions are designed and developed does not align with realities of implementation settings. Constraining this gap can increase the appropriateness of an intervention (18).
The identified frameworks were conceptually focused, rather than validated theories, and the guidance provided varied by framework type, as expected. The process models, Proctor reframed (18), Transcreation (20), and EquIR (21), for example, tended to be high-level, and provided overarching considerations and recommendations for program design, implementation, and evaluation rather than specific guidance that lends itself to individual stages of an intervention like population genetic screening. Regarding screening, Proctor reframed (18) and Transcreation (20) recommendations applied most directly to the recruitment stage. While all three process models described evaluating implementation outcomes keeping social determinants and differences in outcomes across populations in mind, they varied in the specificity with which they described and defined these outcomes.
In contrast, the determinant framework, HEIF (15), provides an explicit means to identify barriers to program implementation throughout all stages of population genetic screening. Similarly, the evaluation framework, RE-AIM extension (22), detailed indicators and health equity considerations for monitoring program outcomes relevant to all program stages.
Among the process models, Proctor reframed (18), Transcreation (20), and EquIR (21), another main concept was the importance of involving community partners and other stakeholders throughout implementation. Such collaboration allows researchers to learn more about local customs and build trust with community members (30, 31). Interventions can better be tailored to a specific population and integrate relevant perspectives, norms, and social and cultural values. As a result, this may improve intervention acceptability and effectiveness and prevent health disparities from emerging.
Discussion
In this analysis we outline relevant equity considerations for population genetic screening program implementation guided by five selected frameworks: HEIF (15), Proctor reframed (18), Transcreation (20), EquIR (21), and RE-AIM extension (22). The HEIF (15), RE-AIM extension (22), and outcome measures provided in EquIR (21) were applicable to all stages of population screening. Remaining guidance from EquIR (21) and ideas proposed by Proctor reframed (18) and Transcreation (20) tended to be broad so less clearly applicable to each individual screening stage.
Results of our analysis may offer insights for researchers designing new population genetic screening programs and assist with identification and selection of relevant frameworks to direct implementation. To make the best use of the variety of recommendations brought up by the different frameworks, these frameworks may best be used in tandem. Depending on the implementation effort (e.g., planning vs. evaluating), different frameworks may provide more or less guidance (32). For instance, determinant domains can be used when process model steps suggest identifying implementation barriers and specific indicators can be drawn from evaluation frameworks when steps call for assessing implementation outcomes. In addition, some frameworks may be better suited for guiding implementation at different levels (e.g., provider, organization, system) or circumstances, such as engaging with community members or partners. By drawing upon multiple frameworks, each for a specific purpose, researchers may better be able to address their needs (32).
We found that guidance for later stages of population genetic screening programs, such as follow-up care and cascade screening, was limited beyond central framework characteristics. For true public health impact, individuals receiving positive screening results must have access to services to delay or prevent disease onset. Health benefits may also be seen if genetic risk information is communicated to relatives. However, frameworks lacked specific guidance about how to ensure equitable referrals to follow-up care or promote adherence to recommended medical interventions across populations over time, issues that are applicable beyond population genetic screening or even genomics. Additionally, population genetic screening programs focused on health equity must continually incorporate new genetic information, ensure that providers are up to date on genetic recommendations, and assist with risk communication among relatives. These are continual processes that need to be sustained and maintained.
While the cyclical nature of EquIR (21) and ongoing evaluation measures provided by RE-AIM extension (22) can be used to some extent to promote program maintenance and adherence to care over time, the other frameworks we identified are limited when it comes to guiding longer-term sustainability. While there are additional implementation science tools and frameworks that emphasize sustainability (33, 34), they lack attention to health equity. However, integrating concepts from these sustainability focused tools with the health equity implementation science frameworks identified here may assist with maintaining programs in an equitable manner.
We found that some aspects specific to population genetic screening were particularly difficult to apply our identified frameworks to. This included discussion about sharing health insights and how to engage biological relatives who may be impacted by an individual's risk results, data storage of genetic results so that information moves with an individual regardless of changes in insurance status or health system, and incorporating new genetic risk information into programs over time. Part of the challenge of these specific population genetic screening components is that they require ongoing efforts and, as mentioned previously, sustainability is not emphasized across our identified frameworks. An additional challenge of these later components is that they build on initial implementation efforts but may require a new set of stakeholders and their own unique adaptations. While the frameworks we identified may be well-suited to the implementation of a health intervention with fewer stages, they provide little guidance for interventions that have multiple stages that build upon each other (e.g., population genetic screening). Finally, as the frameworks we identified were not designed to be genetics specific, gaps in guidance are expected.
Population genetic screening health equity considerations
Synthesizing findings from the included frameworks, we have compiled a list of relevant health equity questions and outcomes that warrant consideration during the implementation of population genetic screening programs in order to limit health disparities (Table 4). Though not exhaustive, questions may be useful throughout the design and implementation of future screening programs and spur further discussion related to pursing health equity. Broadly, considerations include the accessibility and cultural sensitivity of different population screening processes. Outcomes focus on understanding the distribution of benefits and harms from genetic screening, and the acceptability of program procedures across various demographic and equity-relevant subgroups.
Table 4
| Stage | Health equity-focused questions | Outcomes assessed across equity-relevant subgroups |
|---|---|---|
| Recruitment |
|
|
| Sample collection |
|
|
| Return of results |
|
|
| Follow-up care & long-term management |
|
|
| Cascade screening |
|
|
| Overall considerations |
|
|
Health equity considerations for population genetic screening programs.
Consider by type of screening result (e.g., positive or uninformative).
One of the overall considerations for pursuing health equity is involving community partners (Table 4). As members of the community are likely more in-tune with local settings compared to researchers, they may be better equipped to understand and identify drivers behind complex inequities (35). Through community engagement, researchers and public health professionals can ascertain what communities identify as problems to be addressed and what community health priorities are. This can inform if population genetic screening is a suitable intervention in a particular setting and truly meeting community needs. Investment by communities in population genetic screening programs can also promote sustainability of such programs.
Even with these health equity considerations identified, challenges may emerge when incorporating these ideas into practice. For example, answers to these questions may vary by communities included in a single screening program. Resource constraints may also prevent the adoption of more equitable practices. Additionally, outcome measures may be difficult to ascertain if they involve time-intensive data collection and the continued engagement of people who have taken part in genetic screening. As such, researchers and health professionals looking to implement screening programs may benefit from using these considerations to appropriately plan and allocate resources.
Limitations
Our study may have been limited by the frameworks considered for analysis. It is possible that our specific search terms and strategy did not identify all relevant frameworks. In addition, though we give a broad overview of implementation science frameworks that center health equity, we did not assess the quality of these frameworks by rigorously evaluating usability, applicability, and testability (36). However, our analysis of how frameworks have been used in other settings provides an indirect measure of utility and quality. Our assessment of framework applicability to population genetic screening programs may also have been limited. Relevant stages, such as sample lab testing, were not included and some of our analysis may be applicable to multiple stages though not detailed here. Despite these limitations, our study is a first step in describing the current state of implementation science frameworks that explicitly focus on health equity and how they can be applied to improve the equitable implementation of population genetic screening programs.
Conclusion
Current implementation science frameworks that emphasize health equity offer broad recommendations applicable to the implementation of population genetic screening programs. However, gaps still exist in guidance provided for stages of screening that are ongoing, such as follow-up care and cascade screening. Through our application of frameworks to population genetic screening, we have created a list of considerations and outcomes that may assist with more equitable implementation. Researchers planning to implement screening programs may benefit from consulting these considerations or following guidance from analyzed frameworks.
Statements
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Author contributions
NR: Conceptualization, Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. SF: Writing – review & editing. BS: Writing – review & editing. AC: Writing – review & editing. NH: Conceptualization, Data curation, Formal Analysis, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. AC, BS, NR, and SF received funding from the Brotman Baty Institute for Precision Medicine for this project. NBH received funding from the National Institutes of Health R01HG010144 (Henrikson).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
King M-C Levy-Lahad E Lahad A . Population-based screening for BRCA1 and BRCA2: 2014 lasker award. JAMA. (2014) 312(11):1091–92. 10.1001/jama.2014.12483
2.
Grzymski JJ Elhanan G Morales Rosado JA Smith E Schlauch KA Read R et al Population genetic screening efficiently identifies carriers of autosomal dominant diseases. Nat Med. (2020) 26(8):1235–39. 10.1038/s41591-020-0982-5
3.
Limburg PJ Harmsen WS Chen HH Gallinger S Haile RW Baron JA et al Prevalence of alterations in DNA mismatch repair genes in patients with young-onset colorectal cancer. Clin Gastroenterol Hepatol. (2011) 9 (6):497–502. 10.1016/j.cgh.2010.10.021
4.
Grzymski JJ Coppes MJ Metcalf J Galanopoulos C Rowan C Henderson M et al The healthy Nevada project: rapid recruitment for population health study. bioRxiv. (2018). 10.1101/250274
5.
Allen CG Olstad DL Kahkoska AR Guan Y Ramos PS Steinberg J et al Extending an antiracism lens to the implementation of precision public health interventions. Am J Public Health. (2023) 13(11):1210–18. 10.2105/AJPH.2023.307386
6.
Centers for Disease Control and Prevention. What Is Health Equity? (n.d.). Available online at:https://www.cdc.gov/nchhstp/healthequity/index.html(accessed July 15, 2022).
7.
Braveman P . What are health disparities and health equity? We need to be clear. Public Health Rep. (2014) 129(1_Suppl 2):5–8. 10.1177/00333549141291S203
8.
Chambers DA Gregory Feero W Khoury MJ . Convergence of implementation science, precision medicine, and the learning health care system: a new model for biomedical research. JAMA. (2016) 315(18):1941–42. 10.1001/jama.2016.3867
9.
Roberts MC Kennedy AE Chambers DA Khoury MJ . The current state of implementation science in genomic medicine: opportunities for improvement. Genet Med. (2017) 19(8):858–63. 10.1038/gim.2016.210
10.
David SP Dunnenberger HM Ali R Matsil A Lemke AA Singh L et al Implementing primary care mediated population genetic screening within an integrated health system. J Am Board Fam Med. (2021) 34(4):861–65. 10.3122/jabfm.2021.04.200381
11.
East KM Kelley WV Cannon A Cochran ME Moss IP May T et al A state-based approach to genomics for rare disease and population screening. Genet Med. (2021) 23(4):777–81. 10.1038/s41436-020-01034-4
12.
Rao ND Kaganovsky J Malouf EA Coe S Huey J Tsinajinne D et al Diagnostic yield of genetic screening in a diverse, community-ascertained cohort. Genome Med. (2023) 15(1):26. 10.1186/s13073-023-01174-7
13.
Odeny B . Closing the health equity gap: a role for implementation science?PLoS Med. (2021) 18(9):e1003762. 10.1371/journal.pmed.1003762
14.
Nilsen P . Making sense of implementation theories, models and frameworks. Implement Sci. (2015) 10(1):53. 10.1186/s13012-015-0242-0
15.
Woodward EN Matthieu MM Uchendu US Rogal S Kirchner JE . The health equity implementation framework: proposal and preliminary study of hepatitis C virus treatment. Implement Sci. (2019) 14(1):26. 10.1186/s13012-019-0861-y
16.
Harvey G Kitson A . PARIHS revisited: from heuristic to integrated framework for the successful implementation of knowledge into practice. Implement Sci. (2016) 11(1):33. 10.1186/s13012-016-0398-2
17.
Kilbourne AM Switzer G Hyman K Crowley-Matoka M Fine MJ . Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health. (2006) 96(12):2113–21. 10.2105/AJPH.2005.077628
18.
Baumann AA Cabassa LJ . Reframing implementation science to address inequities in healthcare delivery. BMC Health Serv Res. (2020) 20(1):190. 10.1186/s12913-020-4975-3
19.
Proctor EK Landsverk J Aarons G Chambers D Glisson C Mittman B . Implementation research in mental health services: an emerging science with conceptual, methodological, and training challenges. Adm Policy Ment Health Ment Health Serv Res. (2009) 36(1):24–34. 10.1007/s10488-008-0197-4
20.
Nápoles AM Stewart AL . Transcreation: an implementation science framework for community-engaged behavioral interventions to reduce health disparities. BMC Health Serv Res. (2018) 18(1):710. 10.1186/s12913-018-3521-z
21.
Eslava-Schmalbach J Garzón-Orjuela N Elias V Reveiz L Tran N Langlois EV . Conceptual framework of equity-focused implementation research for health programs (EquIR). Int J Equity Health. (2019) 18(1):80. 10.1186/s12939-019-0984-4
22.
Shelton RC Chambers DA Glasgow RE . An extension of RE-AIM to enhance sustainability: addressing dynamic context and promoting health equity over time. Front Public Health. (2020) 8. 10.3389/fpubh.2020.00134
23.
Glasgow RE Harden SM Gaglio B Rabin B Smith ML Porter GC et al RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. Front Public Health. (2019) 7. 10.3389/fpubh.2019.00064
24.
Glasgow RE Vogt TM Boles SM . Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. (1999) 89(9):1322–27. 10.2105/AJPH.89.9.1322
25.
Drake C Batchelder H Lian T Cannady M Weinberger M Eisenson H et al Implementation of social needs screening in primary care: a qualitative study using the health equity implementation framework. BMC Health Serv Res. (2021) 21(1):975. 10.1186/s12913-021-06991-3
26.
Mustanski B Queiroz A Merle JL Zamantakis A Zapata JP Li DH et al A systematic review of implementation research on determinants and strategies of effective HIV interventions for men who have sex with men in the United States. Annu Rev Psychol. (2024) 75:55–85. 10.1146/annurev-psych-032620-035725
27.
Santoyo-Olsson J Stewart AL Samayoa C Palomino H Urias A Gonzalez N et al Translating a stress management intervention for rural Latina breast cancer survivors: the nuevo amanecer-II. PLOS ONE. (2019) 14(10):e0224068. 10.1371/journal.pone.0224068
28.
Gaitán-Rossi P Vilar-Compte M Bustamante AV . Adaptation of a community health outreach model during the COVID-19 pandemic: the case of the Mexican consulates in the United States of America. Int J Equity Health. (2023) 22(1):138. 10.1186/s12939-023-01911-9
29.
Marquez C Kerkhoff AD Naso J Contreras MG Diaz EC Rojas S et al A multi-component, community-based strategy to facilitate COVID-19 vaccine uptake among latinx populations: from theory to practice. PLOS ONE. (2021) 16(9):e0257111. 10.1371/journal.pone.0257111
30.
Cabassa LJ Baumann AA . A two-way street: bridging implementation science and cultural adaptations of mental health treatments. Implement Sci. (2013) 8(1):90. 10.1186/1748-5908-8-90
31.
Wallerstein N Duran B . Community-based participatory research contributions to intervention research: the intersection of science and practice to improve health equity. Am J Public Health. (2010) 100(S1):S40–46. 10.2105/AJPH.2009.184036
32.
Moullin JC Dickson KS Stadnick NA Albers B Nilsen P Broder-Fingert S et al Ten recommendations for using implementation frameworks in research and practice. Implement Sci Commun. (2020) 1:42. 10.1186/s43058-020-00023-7
33.
Shelton RC Cooper BR Stirman SW . The sustainability of evidence-based interventions and practices in public health and health care. Annu Rev Public Health. (2018) 39:55–76. 10.1146/annurev-publhealth-040617-014731
34.
Luke DA Calhoun A Robichaux CB Elliott MB Moreland-Russell S . The program sustainability assessment tool: a new instrument for public health programs. Prev Chronic Dis. (2014) 11:130184. 10.5888/pcd11.130184
35.
Urban Institute. Leveraging Community Expertise to Advance Health Equity (2021). Available online at:https://www.urban.org/research/publication/leveraging-community-expertise-advance-health-equity(accessed July 1, 2021).
36.
Wang Y Wong EL-Y Nilsen P Chung VC-h Tian Y Yeoh E-K . A scoping review of implementation science theories, models, and frameworks—an appraisal of purpose, characteristics, usability, applicability, and testability. Implement Sci. (2023) 18:43. 10.1186/s13012-023-01296-x
Summary
Keywords
health equity, population genetic screening, implementation science, hereditary cancer, genetic testing
Citation
Rao ND, Fullerton SM, Shirts BH, Chen AT and Henrikson NB (2024) Applying health equity implementation science frameworks to population genetic screening. Front. Health Serv. 4:1455365. doi: 10.3389/frhs.2024.1455365
Received
26 June 2024
Accepted
31 October 2024
Published
21 November 2024
Volume
4 - 2024
Edited by
Laura V. Milko, University of North Carolina at Chapel Hill, United States
Reviewed by
Katherine W. Saylor, University of Pennsylvania, United States
Yue Guan, Emory University, United States
Updates
Copyright
© 2024 Rao, Fullerton, Shirts, Chen and Henrikson.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Nandana D. Rao ndrao@uw.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.