Abstract
Introduction:
Hospital-at-Home interventions have been shown to be clinically and cost-effective, and many healthcare systems internationally are investing in scaling-up such interventions. However, most existing studies focus on how effective the intervention is, rather than how to successfully scale it up. We report a study protocol for a theory-driven investigation of a Hospital-at-Home intervention. We propose a novel combination of two established implementation science frameworks—the EPIS framework and the Scale-Up framework—and apply it to a planned scale-up of a Hospital-at-Home intervention in Singapore.
Methods:
and analysis: This will be an observational cohort study across 23 months (May 2022 to April 2024) to evaluate the association of outer and inner contextual factors on key implementation outcomes—the volume of patients admitted, operational efficiency and levels of adoption. Statistical process control graphs will be used to examine variation in the implementation outcomes over time. Linear regression will be applied to assess associations of outcomes with contextual factors that are continuous variables; logistic regression will be applied to assess the associations of outcomes with binary/descriptive contextual factors. To supplement these, qualitative methods will be applied using a content analysis of monthly meeting minutes and focus group discussions with the implementation team to understand and explain the outcomes of the observational cohort study.
Ethics and dissemination:
This protocol has been reviewed and approved by the National Health Group Domain Specific Review Board: Reference Number: 2023/00245. Apart from the end-of-study focus group discussions, waiver of informed consent was sought as the data sources were a review of routinely collected retrospective data. The results of this study will be disseminated to peer-reviewed journals, presented at conferences and shared with policy-level stakeholders.
Introduction
In many health systems, the demand for hospital beds driven by ageing populations is increasing faster than the available supply. As a result, sustainable and effective alternatives to hospitalization are becoming increasingly important. One such strategy is the Hospital-at-Home (HaH) service, which substitutes traditional inpatient care with treatment delivered in the patient's home (1). HaH is a complex intervention providing hospital-level services at home that involves a daily “ward round” via virtual or home visits, administration of intravenous therapy, and simple investigations at home, with 24/7 access to the care team. These services can be technology-enabled, utilizing remote monitoring and telecommunication systems. Across multiple randomized controlled trials conducted in Australia, Europe, and the USA, HaH interventions have demonstrated similar or improved clinical outcomes and positive patient experiences, often at a lower cost to the healthcare system (2–5).
In recent years, many healthcare systems have invested heavily in the development of HaH programs, including Singapore (Mobile Inpatient Care @ Home) (6), NHS England (Hospital-at-Home/Virtual Wards) (7, 8), and the Center for Medicaid and Medicare Services (Hospital-at-Home) in the United States (9). Although a number of studies have addressed implementation (10, 11), most ongoing evaluations remain focused on safety, efficacy, and cost-effectiveness (2, 3). Despite generally positive results, many pilot programs struggle to scale from a census of 3–5 patients per day to 50–100 patients. Apart from limitations in operational models, we believe that a limited understanding of implementation factors and the lack of systematic application of implementation science concepts (as opposed to intuitive ‘trial and error’) account for at least part of the lack of why scalable HaH models remain elusive globally.
In this protocol, we report a retrospective study of the planned scale-up of a HaH program in Singapore, from its pilot phase to full scale implementation. Our aim is to gain insights into barriers and facilitators influencing the scale-up process of HaH services. The primary aim is to apply well-established implementation research concepts to a HaH scale-up process to understand the barriers and drivers of the process and the associations with implementation success (or failure). Our overarching question is: How do we scale up HaH successfully?
Methods and analysis
The intervention, NUHS@Home
NUHS@Home is the HaH service of the National University Health System (NUHS), an academic health system in Western Singapore (12). The NUHS comprises two emergency departments, one urgent care center, three acute hospitals, three national specialist centers (cancer, cardiology and transplant), and a network of primary care clinics (including government-funded polyclinics and private general practitioner clinics as part of a primary care network).
With preliminary studies showing demand and interest from patients and providers (13, 14), NUHS@Home started was launched in September 2020 as a small-scale pilot to test the feasibility of the care model. In the pilot, multi-disciplinary care teams delivered inpatient-level care to patients at home, aligned with international HaH programs. NUHS@Home adopts an inpatient acute bed substitution approach, led by a consultant specializing in general/acute medicine. Key features of care include: (1) daily virtual ward rounds by doctors followed by home visits if physical reviews are indicated; (2) home visits by nurses and allied health professionals for procedures (e.g., intravenous medication or venipuncture) or therapy; (3) remote vital signs monitoring, and (4) a round-the-clock hotline to the care team.
Evaluation of this one-year pilot, operating with a virtual capacity of 3 beds and caring for 108 patient episodes, demonstrated that acute care at home is feasible, safe, and effective (15). From September 2021, due to the COVID-19 surge, NUHS@Home rapidly scaled to over 100 virtual beds, delivering protocolized COVID-19 care to over 2,000 patient episodes (16). Although COVID-19 service demands and patient acuity are lower and not representative of typical patients admitted to HaH programs, insights gained during this period were useful for plans for further growth.
The success of the pilot helped drive financial, regulatory, and policy shifts to support HaH scale-up nationally. The Ministry of Health launched a 2-year “sandbox” from 2022 to 2024 to support and finance these efforts. With this sandbox, patients admitted to HaH programs are eligible for subsidies similar to traditional inpatient care, with an aim to eventually transition to mainstream healthcare delivery (6). As such, NUHS@Home has planned for major expansion from 3 to 50–100 virtual beds within 3–5 years.
This protocol focuses on the evaluation of the scale-up of NUHS@Home, aiming to identify barriers and facilitators to the successful expansion of the HaH services.
Implementation frameworks
This evaluation will combine two established implementation frameworks—the Exploration, Preparation, Implementation, Sustainment (EPIS) Framework (17, 18) and the Scale-Up Framework (19).
EPIS provides a useful macro-framework for analyzing implementation processes. It outlines four key phases—Exploration, Preparation, Implementation, and Sustainment—and identifies contextual influences across each phase. Contextual influences, defined as factors external to the intervention itself (20), may be further classified into outer context (i.e., healthcare system) and inner context (i.e., local organizational aspects). Examples of these influences include elements of leadership (within and outside the NUHS@Home team and NUHS), characteristics of the local patient population, fidelity of implementation (i.e., implementing NUHS@Home as originally designed), workforce characteristics, and more. In the field of implementation science, contextual factors have consistently been identified as key to the success or failure of implementing evidence-based interventions (21), even more than the quality and nature of supporting evidence or the complexity of the implementation process. These contextual influences are therefore the primary focus for our research and evaluation. Furthermore, EPIS offers a (chrono)logical lens through which the process of implementation can be studied as it takes place and evolves. This is useful and applicable as we study the scale-up process in situ, i.e., as it happens within the NUHS setting.
The macro approach of EPIS is complemented by an action-orientated framework to define and operationalize the specific elements of the scale-up process. This is a critically important aspect of the research, as it will lead to the ultimate ‘manualization’ of the scale-up process to create generalizable findings. The Scale-Up framework offers precisely this, in an ideal complement to EPIS. The Scale-Up framework breaks down scaling-up into 4 phases: (1) set-up, (2) develop the scalable unit, (3) test of scale-up, and (4) go to full-scale (Figure 1). Table 1 maps out the growth of NUHS@Home following these phases. The focus of our research is to study the process of developing a scalable unit (phase 2) and test of scale-up (phase 3).
Figure 1
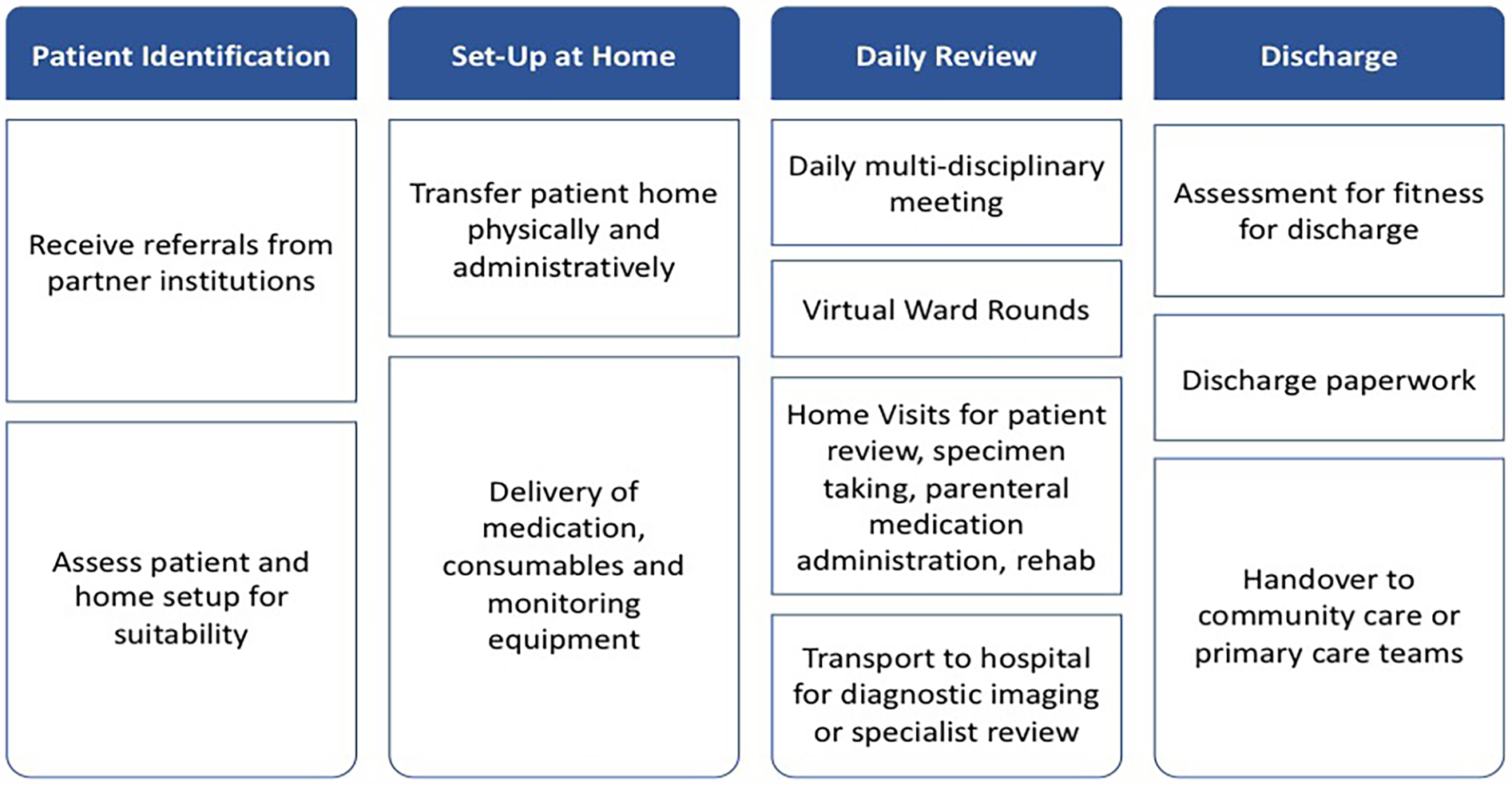
NUHS@home patient journey.
Table 1
| Phases | Set-up | Develop the scalable unit | Test of scale-up | Go to full-scale |
|---|---|---|---|---|
| Timeline | 2020–2022 | 2022–2024 | 2024–2025 | 2025–2030 |
| Bed capacity | 3 beds | 25 beds | 50–100 beds | 300 beds |
The scale-up framework as applied to NUHS@home.
Study design
This is a retrospective observational cohort study to evaluate implementation outcomes using the EPIS framework during the planned scale-up of NUHS@Home over 23 months. The primary methodology will be quantitative in approach, supported by an explanatory qualitative study with the implementation team to understand which factors are associated with the assessed implementation outcomes and how. The period of data collection will begin with the transition to phase 2 (‘develop the scalable unit’), between May 2022 to April 2024.
Sample selection
This study will evaluate NUHS@Home, the HaH service at NUHS. NUHS@Home functions as an inpatient substitute service, replicating elements of ward-based care in the home environment. Referrals are accepted from all partner institutions in the health system, including wards, emergency departments, and primary and community care facilities. Patients are assessed for suitability based on the following criteria: (1) clinical stability, (2) ability to receive the required clinical interventions at home, (3) ability to manage toileting independently or with an available caregiver, and (4) living within the geographical catchment (Western Singapore).
The service delivery process can be summarized in four steps (Figure 1)—patient identification, set-up, daily review at home, and discharge.
Key members of the implementation team include leads from physicians, nurses, pharmacy, allied health, and operations. During the initial pilot phase, the implementation team identified 4 key priorities for scale-up: (1) development of new clinical pathways to generate demand for virtual beds, (2) recruitment and training of staff to provide inpatient-level care at home, (3) optimization of internal workflows and processes to ensure both patient safety and provider efficiency, and (4) advocacy for sustainable healthcare financing and billing. The relevant activities and EPIS contextual factors are detailed in Table 2.
Table 2
| Priorities for scale up | Activities | Relevant contextual factors (EPIS) |
|---|---|---|
| Overall | Building an implementation team to plan and execute the following activities | Organizational characteristics (IC) Leadership (IC) |
| Development of new clinical pathways to generate demand for virtual beds | Working with multiple clinical stakeholders to expand clinical pathways. | Institutional leadership (OC) Patient/client characteristics (OC) Patient/client advocacy (OC) |
| Recruitment and training of staff to be able to provide inpatient level care in the home | Development of accreditation frameworks, careers tracks and training programs for new clinical staff | Organizational staffing (IC) Individual characteristics (IC) |
| Optimizing internal workflows and processes to achieve both patient safety and provider efficiency | Engaging clinical governance, medicolegal and medical informatics to develop efficient and effective work processes | Quality and fidelity of monitoring/support (IC) Infrastructure (IC) Patient/client advocacy (IC) |
| Advocating for sustainable healthcare financing and billing | Advocacy for healthcare policy shifts | Funding (OC) External networks (OC) |
Priorities and activities for scale-up.
OC, outer context; IC, inner context.
Measurements and outcomes
The primary implementation outcomes—defined as ‘the effects of deliberate and purposive actions to implement new treatments […] and are distinct from service and client (patient) outcomes’ (
22)—of the scale-up process are as follows:
- 1.
Volume: number of patient admissions to NUHS@Home and associated bed days per month
- 2.
Operational efficiency: bed occupancy rate of NUHS@Home, defined as the number of bed days occupied/virtual bed capacity * number of days that month
- 3.
Adoption: proportion of patients admitted to NUHS@Home/total patients admitted to affiliated hospital wards for established clinical pathways. Within the NUHS setting, these currently include: cellulitis, urinary tract infections, gastroenteritis, dengue fever, and exertional rhabdomyolysis, but may increase with the development of new clinical pathways.
Relevant clinical outcomes will include the rate of unplanned returns to hospital, 30-day readmission rate, HaH mortality rate, and rate of patient safety incidents. At the initial set-up of the NUHS@Home service, our team developed a list of patient safety indicators by reviewing inpatient hospital reporting guidelines and adapting them for NUHS@Home. These indicators include: (1) diagnosis, treatment and procedure-related complications, (2) laboratory medicine and sample-related complications, (3) peripheral venous complication-related complications, (4) medication-related complications, (5) patient falls, (6) pressure injuries, (7) sharps injury and body fluid splash related and (8) staff and visitor incidents. These indicators will be tracked throughout the study period.
In addition, basic patient demographic data (e.g., age, gender) will also be collected to provide descriptive context for the implementation analysis.
A key tenet of implementation theory is that the scale-up process and the associated outcomes of that process (summarized above) will potentially be impacted by, or at least associated with, the context of the implementation. To better understand the impact of context, we applied the EPIS framework to define contextual influences on the outcomes described above and developed a corresponding measurement plan, summarized in Table 3.
Table 3
| Measurement (per month) | Definition |
|---|---|
| Implementation outcomes (IO) | |
| Volume |
|
| Operational efficiency |
|
| Adoption |
|
| Clinical outcomes (CO) | |
| Clinical outcomes |
|
| Outer context (OC) | |
| Service environment |
|
| Funding |
|
| Institutional leadership |
|
| External networks |
|
| Patient/client characteristics |
|
| Patient/client advocacy |
|
| Inner context (IC) | |
| Organizational characteristics |
|
| Leadership |
|
| Quality and fidelity of monitoring/support |
|
| Organizational staffing processes |
|
| Individual characteristics |
|
| Infrastructure |
|
| Patient/Client Advocacy |
|
Measurements of contextual influences.
Data collection and data sources
Data sources for this study will include chart review from electronic medical records, human resources data, operational data, and standardized questionnaires disseminated to the NUHS@Home Implementation Team. Collected data will be consolidated into data collection forms (DCFs) on a monthly basis (i.e., 23 data points in total) by a study team member (SL). The DCFs are available in the Supplementary Material, and the data collection strategy is summarized in Table 4.
Table 4
| DCF | Data | Participants | Data sources |
|---|---|---|---|
| DCF 1 (operational data) |
|
Operations team | Chart review Clinical operations databases Patient satisfaction team |
| DCF 2 (service structure and organization) |
|
Program lead | Operations data |
| DCF 3 (clinical operations) |
|
Clinical service lead | Operations data Chart review |
| DCF 4 (service development activities) |
|
Operations team to draft a response but all leads to review | Operations data |
| DCF 5 (staffing) |
|
Clinical, nursing, pharmacy, allied health and operations lead to fill in separately | Operations data Human resources data |
Data collection strategy.
DCF, data collection form.
Color coding: implementation outcome, clinical outcome, outer context, inner context.
Qualitative methods
We will embed qualitative methods in this study through two key components. First, a document analysis of monthly implementation team meeting minutes will be conducted retrospectively. A content analysis will be carried out by one of the study team members (SL) to identify key problems and implementation activities (administrative, management, operations, or clinical) each month. Following each meeting, the lead author (SK), who also leads the implementation team, will prospectively record any important matters not captured in the minutes or the quantitative DCFs, using rapid auto-ethnography approach (23). Our team has previously applied this method in acute medical settings (24), enabling the timely capture of contextual information and real-time adjustments to the implementation process. This ensures that research remains closely aligned with the evolving NUHS@Home program.
Second, we will conduct end-of-study focus group discussions with the core implementation team after quantitative data collection concludes. These discussions will be led by trained researchers, external to both the implementation and study teams, with expertise in qualitative methods and implementation research. They will be supervised by a senior implementation scientist (NS)—a non-clinician with over 20 years of experience in health services research in hospital settings. The focus groups will explore the following thematic areas: (1) what facilitated the scale-up process and why, (2) what did not work or hindered and why, (3) explanations of the implementation outcomes elicited in the first component of the study, and (4) contextual factors that were critical in success or failure of implementation and how precisely they affected the process. If needed, two additional implementation researchers from the NUS Centre for Behavioural and Implementation Science Interventions will be trained by NS to support the data collection. Each session is expected to last approximately 90 min and include 6–8 participants. If logistical challenges prevent group discussions, semi-structured interviews will be conducted instead.
Sample size
Based on our pilot study, which averaged 8–10 patient episodes per month with a virtual capacity of 3 beds, we anticipate approximately 1,012 patients admitted to NUHS@Home over the 23-month study period. As this is a descriptive study with no comparison group, we have not set a minimum sample size target. If a sample size becomes necessary, we will submit an amendment to the ethics borad to extend the study period.
We expect 15–25 members of the implementation team will provide data (as outlined in Table 4) and in the focus group discussions. This will include service leads from clinical, nursing, pharmacy, allied health, and operations teams within NUHS@Home.
Data analysis
Both implementation and clinical outcomes will be described descriptively over time to contextualize the scale-up process. We will also use statistical process control (SPC) graphs to examine variation in the three defined implementation outcomes over time. To determine the association of different contextual factors with these outcomes, each contextual factor will first be categorized as either continuous (e.g., bed occupancy rates of referring hospitals) or binary/descriptive (e.g., change in funding source). For continuous variables, unadjusted and adjusted linear regression will be used to statistically assess associations between the number of contextual factors and their values and each implementation outcome to determine whether certain contextual factors are more influential than others. For binary/descriptive variables, contextual events will be annotated on the SPC graphs to assess if a clinically significant relationship exists. These analyses will be supplemented with multivariable regression to adjust for potential confounders. This approach has been previously applied in a similar ‘naturalistic’ implementation evaluation of a hospital-based scaled intervention in the UK (25).
Qualitative data from focus group discussions and meeting minutes will be analyzed using Atlas.ti software, guided by Braun and Clarke's thematic analysis approach (26). Chat logs, transcripts, and observations will be coded inductively, i.e., without any theoretical preconceptions, and themes reflecting implementation aspects will be identified and summarized. In the first stage, data will be deconstructed systematically into first-order concepts. In the second construction stage, the concepts previously developed will be reassembled into new patterns and a constant comparison technique will be carried out to compare the incidents applicable to each emerging theme. The third and final stage of analysis will include the confirmation of the conceptualization of the new phenomenon and a descriptive narrative summary will be created using second-order concepts. The analysis will seek to establish whether the emerging theme represents a barrier or a driver. Results from the different methods will be triangulated for the final data analysis.
Interim analyses will be conducted every three months, with feedback provided to the implementation team as needed.
We anticipate that some data points may be missing due to the retrospective nature of the data collection from operational and administrative datasets. If the proportion of missing data is low (<10%) and missing at random, a complete case analysis will be conducted. If missingness is substantial or not random, we will apply appropriate methods such as multiple imputation or sensitivity analyses to assess its impact on the findings.
Discussion
This study aims to deepen our understanding of how to effectively scale up HaH interventions, which hold significant promise in addressing critical challenges in healthcare systems. Large-scale HaH programs may reduce the demand for expanding or constructing new hospitals, curb rising healthcare costs, and shift care towards more patient-centered, home-based care models.
A key emphasis of this study is the adoption of a systematic theory-driven approach to evaluating the scale-up process, with a focus on identifying both barriers and facilitators to full-scale implementation. In doing so, the study contributes to the growing body of HaH literature, aligning with current discourse that moves beyond assessing efficacy alone and toward understanding large-scale adoption strategies.
To our knowledge, this is the first study to integrate two established implementation science frameworks—the EPIS framework and the Scale-Up framework—to analyze the scaling process of HaH interventions. This integrated approach allows for a comprehensive analysis of both the process and the contextual drivers of success or failure associated with scaling. Moreover, the study design was developed in collaboration with key implementation stakeholders from the outset, ensuring practical relevance and robust assessment of real-world conditions.
Nonetheless, there are limitations. This is an observational study treating the scale-up process as a natural experiment. As such, the evaluation is based on the service as implemented, without a control group, which restricts the ability to draw causal inferences. Consequently, the findings are limited to identifying associations rather than establishing definitive causal relationships.
Although a formal cost analysis is not included in this study, we recognize that economic viability is central to the successful scale-up and long-term sustainability of HaH services. A future follow-up economic evaluation study can be conducted to assess the cost implications and potential savings of HaH compared to conventional inpatient care, to inform healthcare financing strategies and policy decisions.
Finally, while this study protocol focuses on the evaluation of implementation and understanding contextual factors from the perspective of the implementation team, we acknowledge the importance of understanding the perspectives of referring providers. A separate qualitative study is currently underway to explore providers’ perceptions of HaH, their referral decision-making processes, and perceived barriers or facilitators to referral. Findings from that work will complement the present study and help contextualize adoption outcomes.
Ethics and dissemination
This protocol has been approved by the National Health Group Domain Specific Review Board: Reference Number: 2023/00245. A waiver of informed consent was sought for the cohort study and document analysis of meeting minutes as individual patient data will not be collected and it involves a review of routinely collected retrospective data. For the end-of-study focus group, informed consent will be taken. The reporting of the study will follow the STROBE guidelines (27) for reporting observational studies. The results of this study will be disseminated to peer-reviewed journals, presented at conferences, and shared with policy-level stakeholders.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
SK: Conceptualization, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing. SL: Writing – review & editing. NS: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work is supported by the National Medical Research Council Population Health Research Grant (PHRGOC23Jan-0002). NS's research is funded directly by the Centre for Behavioural and Implementation Science Interventions (BISI) at the NUS [grant number: N/A]. The funders did not influence the study design, writing or decision to publish this protocol.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frhs.2025.1571090/full#supplementary-material
References
1.
Leff B . Defining and disseminating the hospital-at-home model. Can Med Assoc J. (2009) 180(2):156–7. 10.1503/cmaj.081891
2.
Shepperd S Iliffe S Doll HA Clarke MJ Kalra L Wilson AD et al Admission avoidance hospital at home. Cochrane Database Syst Rev. (2016) (9):CD007491. 10.1002/14651858.CD007491.pub2
3.
Gonçalves-Bradley DC Iliffe S Doll HA Broad J Gladman J Langhorne P et al Early discharge hospital at home. Cochrane Database Syst Rev. (2017) (6):CD000356. 10.1002/14651858.CD000356.pub4
4.
Caplan GA . A meta-analysis of “hospital in the home”. Med J Aust. (2013) 198(4):195–6. 10.5694/mja12.11841
5.
Leong MQ Lim CW Lai YF . Comparison of hospital-at-home models: a systematic review of reviews. BMJ Open. (2021) 11(1):e043285. 10.1136/bmjopen-2020-043285
6.
Chong C . Some Patients can now be Hospitalised at Home and be Cared for via Teleconsultations, Home Visits. The Straits Times (2022). Available at:https://www.straitstimes.com/singapore/some-patients-can-now-be-hospitalised-at-home-and-be-cared-for-via-teleconsultations-home-visits (Accessed December 28, 2022).
7.
NHS UK. Expansion of NHS 111 to Transform Patient Access. National Health Service England (2023). Available at:https://www.england.nhs.uk/2023/01/expansion-of-nhs-111-to-transform-patient-access/ (Accessed June 3, 2023).
8.
Digital Health. Government Plans 500% Expansion of Virtual Wards. Digital Health (2023). Available at:https://www.digitalhealth.net/2023/01/government-plans-500-expansion-of-virtual-wards/ (Accessed March 6, 2023).
9.
Gorbenko K Baim-Lance A Franzosa E Wurtz H Schiller G Masse S et al A national qualitative study of hospital-at-home implementation under the CMS acute hospital care at home waiver. J Am Geriatr Soc. (2023) 71(1):245–58. 10.1111/jgs.18071
10.
Brody AA Arbaje AI DeCherrie LV Federman AD Leff B Siu AL . Starting up a hospital at home program: facilitators and barriers to implementation. J Am Geriatr Soc. (2019) 67(3):588–95. 10.1111/jgs.15782
11.
Siu AL Zimbroff RM Federman AD DeCherrie LV Garrido M Morano B et al The effect of adapting hospital at home to facilitate implementation and sustainment on program drift or voltage drop. BMC Health Serv Res. (2019) 19(1):264. 10.1186/s12913-019-4063-8
12.
National University Health System. National University Health System: Patient Care Institutions. National University Health System (n.d.). Available at:https://www.nuhs.edu.sg/For-Patients-Visitors/Pages/our-institutions (Accessed January 8, 2023).
13.
Lai YF Lim YW Kuan WS Goh J Soong JTY Shorey S et al Asian Attitudes and perceptions toward hospital-at-home: a cross-sectional study. Front Public Health. (2021) 9:704465. 10.3389/fpubh.2021.704465
14.
Chua CMS Ko SQ Lai YF Lim YW Shorey S . Perceptions of stakeholders toward “hospital at home” program in Singapore: a descriptive qualitative study. J Patient Saf. (2022) 18(3):e606–12. 10.1097/PTS.0000000000000890
15.
Ko SQ Goh J Tay YK Nashi N Hooi BMY Luo N et al Treating acutely ill patients at home: data from Singapore. Ann Acad Med Singapore. (2022) 51(7):392–9. 10.47102/annals-acadmedsg.2021465
16.
Ko SQ Kumar SK Jacob J Hooi BMY Soo M Nashi N et al Technology-enabled virtual ward for COVID management of the elderly and immunocompromised in Singapore: a descriptive cohort. BMC Infect Dis. (2023) 23(1):102. 10.1186/s12879-023-08040-2
17.
Aarons GA Hurlburt M Horwitz SM . Advancing a conceptual model of evidence-based practice implementation in public service sectors. Adm Policy Ment Health. (2011) 38(1):4–23. 10.1007/s10488-010-0327-7
18.
Moullin JC Dickson KS Stadnick NA Rabin B Aarons GA . Systematic review of the exploration, preparation, implementation, sustainment (EPIS) framework. Implement Sci. (2019) 14(1):1–16. 10.1186/s13012-018-0842-6
19.
Barker PM Reid A Schall MW . A framework for scaling up health interventions: lessons from large-scale improvement initiatives in Africa. Implement Sci iS. (2016) 11:12. 10.1186/s13012-016-0374-x
20.
Moore GF Audrey S Barker M Bond L Bonell C Hardeman W et al Process evaluation of complex interventions: medical research council guidance. Br Med J. (2015) 350(mar19 6):h1258–h1258. 10.1136/bmj.h1258
21.
Pfadenhauer LM Gerhardus A Mozygemba K Lysdahl KB Booth A Hofmann B et al Making sense of complexity in context and implementation: the context and implementation of Complex interventions (CICI) framework. Implement Sci. (2017) 12(1):21. 10.1186/s13012-017-0552-5
22.
Proctor E Silmere H Raghavan R Hovmand P Aarons G Bunger A et al Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health Ment Health Serv Res. (2011) 38(2):65–76. 10.1007/s10488-010-0319-7
23.
Vindrola-Padros C . Rapid Ethnographies: A Practical Guide. 1st edn. Cambridge: Cambridge University Press (2020).
24.
Pannick S Archer S Johnston MJ Beveridge I Long SJ Athanasiou T et al Translating concerns into action: a detailed qualitative evaluation of an interdisciplinary intervention on medical wards. BMJ Open. (2017) 7(4):e014401. 10.1136/bmjopen-2016-014401
25.
IMPHS group, WilliamsJFairbairnEMcGrathRClarkAHealeyAet alDevelopment and rapid evaluation of services to support the physical health of people using psychiatric inpatient units during the COVID-19 pandemic: study protocol. Implement Sci Commun. (2021) 2(1):12. 10.1186/s43058-021-00113-0
26.
Braun V Clarke V . Using thematic analysis in psychology. Qual Res Psychol. (2006) 3(2):77–101. 10.1191/1478088706qp063oa
27.
von Elm E Altman DG Egger M Pocock SJ Gotzsche PC Vandenbroucke JP et al Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Br Med J. (2007) 335(7624):806–8. 10.1136/bmj.39335.541782.AD
Summary
Keywords
Hospital-at-Home, Singapore, barriers and facilitators, EPIS framework, scale-up framework, contextual influences
Citation
Ko SQ, Low SY and Sevdalis N (2025) Barriers and facilitators to scale-up of hospital-at-home: an observational cohort study protocol. Front. Health Serv. 5:1571090. doi: 10.3389/frhs.2025.1571090
Received
04 February 2025
Accepted
19 May 2025
Published
06 June 2025
Volume
5 - 2025
Edited by
Linda DeCherrie, Medically Home Group, United States
Reviewed by
Michael Maniaci, Mayo Clinic Florida, United States
David W. Walsh, Augusta University, United States
Stephanie Murphy, Medically Home Group, United States
Updates
Copyright
© 2025 Ko, Low and Sevdalis.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Stephanie Q. Ko stephanie_qianwen_ko@nuhs.edu.sg
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.