Abstract
Introduction:
Spinal muscular atrophy (SMA) is a rare disease characterized by challenges in its management both for patients and parents/caregivers. In Greece, these challenges are exacerbated by the lack of an integrated SMA patient pathway. This survey mapped the experiences of parents of SMA pediatric patients with the Greek healthcare system, highlighting the actual pathway, its impact on quality of life, and associated financial burden.
Methods:
Parents of children living with SMA, who were members of MDA Hellas, a non-profit organization for people with neuromuscular diseases in Greece, were invited to complete a novel online questionnaire, prepared in collaboration with the Health Policy Institute, a non-profit research organization specializing in health policy evaluation and design. The survey ran from May to September 2023. The results were analyzed using SPSS V26.0.
Results:
Forty-four parents participated in the survey, with a mean age of 43.2 years. In the absence of universal prenatal or newborn screening, parents were the first to recognize any symptoms in the majority (73.5%) of cases. A total of 38.6% of parents had to travel from their place of residence to receive a diagnosis for their child. More than half had to pay out-of-pocket for diagnosis-related services. Parents shouldered additional treatment services and assistive aids' costs in excess of 300 euros a month. The majority reported feelings of agony and fear (90.9%) and guilt (25%) upon receiving the diagnosis. The average PedsQol score was 67 (SD = 15.8), whereas Zarit scores confirmed a higher burden for caregivers of children with graver SMA types.
Conclusion:
This survey details the concerns and needs and quantifies SMA burden on parents/caregivers of children with SMA in Greece to inform health policy advocacy. Such advocacy should be translated into a national action plan with a focus on integrating care organization and delivery options.
1 Introduction
Spinal muscular atrophy (SMA) is a severe neurodegenerative condition caused by recessive mutations in the survival motor neuron (SMN) 1 gene, resulting in insufficient SMN proteins. It is considered a rare disease, with a prevalence of 1–2 cases per 100,000 persons and an incidence of 1 in 10,000 live births (1). SMA leads to progressive muscle weakness, atrophy, and multisystem physical dysfunction, which severely impacts patients' quality of life (2). Recent, rapid advances in diagnosis and treatment have significantly changed the clinical picture of the disease (3), yet timely access is of paramount importance for patients to reap any benefits.
The disease's management pathway across the public health continuum should include the following areas: (a) prevention and early diagnosis, including preconception, prenatal and newborn screening; (b) in the absence of universal screening, symptoms' recognition and referral to diagnostic services; (c) diagnosis and linkage to care; (d) care delivery, including a wide array of health and welfare services and education; and (e) transition to adult care. Across these areas, patients and their caregivers journey through (i) services, which include access to health, welfare, educational, and other supportive services; (ii) feelings and needs, and what would have probably sustained or helped them; and (iii) costs, which they might have to incur to ensure access to and delivery of services.
This journey is further aggravated by the nature of SMA as a rare disease. In rare diseases, where the population is small and geographically dispersed, harmonizing management processes and practices is difficult, as the health system lacks the critical demand mass to both prioritize and streamline its function to address patient needs. This compounds the challenges faced by patients with SMA and their parents and caregivers, adding to their burden.
To ameliorate some of these challenges and provide a universally agreed minimum set of processes and outcomes that should be targeted during each area of the disease management continuum, standards of care were agreed upon among the global scientific community (4–6). These are supplemented by emerging literature (7) guiding ongoing management of SMA, as various therapies and processes become available, and the disease paradigm evolves to incorporate them.
Nonetheless, in Greece, such standards are hard to uphold. Healthcare services struggle with integration (8), with the geography of the country further undermining equity in access to care (9), and staff shortages, whereas patients are faced with high out-of-pocket payments (10), fueled by years of healthcare system underfunding (11). The situation was further exacerbated by the economic crisis and the COVID-19 pandemic, which have both, in sequence, tested the sustainability of the system. For the 150 people, children and adults, living with SMA in Greece and registered with MDA Hellas, and the additional 1–6 live births (8 per 100,000 live births) per annum (12), the struggle with overcoming these barriers may be even harder. This is due to the fact that, despite the full reimbursement of innovative treatments, including gene therapies, by the third-party social insurance payer, i.e., the National Organization for Health Services Provision (EOPYY), there persists a lack of definition of an optimal patient pathway that enhances patient experience with the system and, ultimately, improves care outcomes.
This survey shares the journeys of parents of children with SMA through care in Greece. It details the challenges faced in each stage of this journey, by detailing what parents had to do, what they had to pay, and how they felt. It also used validated scales (PedsQol, Barthel index, and the Zarit scale) to correlate areas of quality of life of the child and ability to perform activities with the level of strain placed on parents, as well as record defined values in each index that would assist benchmarking across diseases, primarily rare, within Greece and beyond. Finally, the study provides a platform to support the definition of actionable priorities for health policy advocacy at the healthcare system level on the basis of actual evidence from parents of children with SMA.
2 Materials and methods
The study recruited adult parents of children with an SMA diagnosis who, at the time of study, were members of MDA Hellas. Dissemination was the sole responsibility and decision of MDA Hellas. Participants were sent an invitation directly by MDA to participate in the survey by completing an online, novel questionnaire through the Jotform platform. All responses were collected by MDA Hellas. Only one of the two parents of each child could participate in the survey. This approach was deemed appropriate to ensure data could be gathered concurrently by all participants in a structured manner.
2.1 Survey tool
The questionnaire was developed and customized to the survey audience, i.e., to parents and/or unpaid caregivers of pediatric patients. The questionnaire was conditioned by the specifics of the Greek healthcare system, which is plagued by a lack of integration of healthcare services, suboptimal care networks, and informal, out-of-pocket payments, over and above the formal reimbursement of care services by EOPYY, which are reported as persistently higher than the EU average (13). Prior to finalizing the questionnaire, we held two unstructured, lengthy interviews with two parents of children with SMA, members of MDA, to ensure the relevance of all questionnaire sections to their major challenges and concerns. Section 1 of the questionnaire recorded basic sociodemographic characteristics and disease history. Section 2 mapped the full patient journey across a continuum, per disease phase, i.e., (a) before and during diagnosis and (b) from treatment start to disease management to date. In addition, the participants were requested to assess their child's status regarding functional autonomy, using the validated Barthel index scale (14). Moreover, they assessed the overall quality of life of the child using the Pediatric Quality of life (PedsQol) parent/carer proxy reports (15, 16) and their own burden, using the Zarit scale (17). Finally, the questionnaire recorded disease-related personal or family financial burden. The latter included financial burden due to travel from the place of residence to access services, the cost of disease-related healthcare services, and aids that the parents had to pay out of their pocket, if not reimbursed, either in part or in full, by EOPYY. For all expenditures, the mean value was computed by summing the corresponding cost across parents who had reported this cost and dividing it by the number of parents who reported it. Cronbach's alpha reliability index was 0.83 for the Zarit scale and 0.90 for the Barthel index scale. For PedsQol, reliability indexes ranged from 0.71 to 0.85, indicating acceptable reliability of all scales.
2.2 Data management processes
The Health Policy Institute prepared the online survey questionnaire, which was reviewed and approved by MDA Hellas. The questionnaire was fully anonymized, and responses could not be traced back to participants by those performing the analysis. Great attention was paid to promoting the use of ranges to responses to all questions for which this was deemed appropriate for this type of survey, to ensure any risk of re-identifying is minimized. The Health Policy Institute, which was scientifically responsible for the analysis of responses, never collected, acquired access to, stored, or processed non-anonymized respondent data that were not provided by MDA Hellas.
2.3 Pilot survey
Questionnaires were sent by MDA Hellas to four parents of pediatric patients to pilot completion and test content validity and completeness. As there were no suggestions for further improvements of the questionnaire, pilot survey responses were accounted for in the total survey sample.
2.4 Statistical analysis
To describe the data, quantitative variables were expressed as mean values (standard deviation) and as median (interquartile range), while categorical variables were expressed as absolute and relative frequencies. To investigate the correlation between areas of quality of life of the child and functional capacity with parental burden, we used Pearson’s correlation coefficient (r). In addition, to investigate the differences in children's QoL, functional capacity, and parental burden among types of SMA, we used analysis of variance (ANOVA). To investigate the association of parental needs with their educational level and their annual income, we used Pearson's chi-square tests or Fisher's exact tests. All reported p-values are two-tailed. Statistical significance was set at p < 0.05, and analyses were conducted using Statistical Package for the Social Sciences (SPSS) software (version 26.0).
3 Results
3.1 Sample sociodemographic characteristics, disease background, and treatment patterns
Forty-four parents, each representing one family, participated in the survey, out of a total of 56 parents who were members of MDA Hellas at the time of the study. The mean age of parents was 43.2 years (SD = 7.1 years). Their characteristics, along with those of their children, are presented in Table 1. Most participants were women (70.5%), university alumni (45.5%), and married (95.5%), had an annual family income of 10,000–30,000 euros (59.1%), lived with the child (97.7%), and had to accompany the child to all medical/disease management appointments (97.7%). The majority (88.5%) reported taking time off work to attend to the child’s needs. Specifically, 34.8% had to take time off work once every 3 months, while 26.1% did so once a month. The children had a mean age of 8.5 years (SD = 4.6 years), with a mean time from diagnosis of 7.1 years (SD = 4.2 years). Half of the children (50%) had been diagnosed with type II disease, 90.9% were currently under treatment, and 54.5% (N = 24) received their treatment once every 4 months. Furthermore, 27.3% of children had experienced a treatment-related adverse event, and 25.0% of them were hospitalized for this event.
Table 1
| n = 44 | n | % |
|---|---|---|
| Parents' characteristics | ||
| Gender | ||
| Men | 13 | 29.5 |
| Women | 31 | 70.5 |
| Educational level | ||
| Middle school | 2 | 4.5 |
| High school | 12 | 27.3 |
| University | 20 | 45.5 |
| MSc/PhD | 10 | 22.7 |
| Employment status | ||
| State employee | 10 | 22.7 |
| Private employee (full-time) | 10 | 22.7 |
| Private employee (part-time) | 2 | 4.5 |
| Freelance | 3 | 6.8 |
| Household | 6 | 13.6 |
| Unemployed | 12 | 27.3 |
| Other | 1 | 2.3 |
| Family status | ||
| Married | 42 | 95.5 |
| Divorced | 2 | 4.5 |
| Annual family income (euros) | ||
| <10,000 | 13 | 29.5 |
| 10,000–30,000 | 26 | 59.1 |
| 30,000–60,000 | 5 | 11.4 |
| Private insurancec | 14 | 31.8 |
| What is the distance from the hospital (e.g., pediatric neurology clinic) that attends the patient? (km) | ||
| <10 | 9 | 20.5 |
| 10–50 | 19 | 43.2 |
| 50–100 | 6 | 13.6 |
| >100 | 10 | 22.7 |
| Has your family situation changed since your child was diagnosed with spinal muscular atrophy (i.e., separated, divorced, and married)?a | 10 | 22.7 |
| Has there been a change in your employment since your child was diagnosed with spinal muscular atrophy?b | ||
| I have stopped working | 1 | 3.8 |
| I have reduced my working hours | 6 | 23.1 |
| No change | 17 | 65.4 |
| I have increased my working hours | 2 | 7.7 |
| Do you live with the child?a | 43 | 97.7 |
| Do you accompany the child to all medical/disease management appointments?a | 43 | 97.7 |
| Do you take time off work to be present when the child needs anything?a,b | 23 | 88.5 |
| On average, how long is the absence from work each time?b | ||
| One day | 12 | 52.2 |
| Some days | 10 | 43.5 |
| One week | 1 | 4.3 |
| Missing | 3 | |
| Children's characteristics | Age, mean (SD) | |
| Years from diagnosis, mean (SD) | 7.1 (4.2) | 8.5 (4.6) |
| Type | ||
| Ι | 15 | 34.1 |
| ΙΙ | 22 | 50.0 |
| ΙΙΙ | 7 | 15.9 |
| Currently under treatmenta | 40 | 90.9 |
| Frequency of treatment | ||
| Daily | 12 | 27.3 |
| Once every 4 months | 24 | 54.5 |
| Other | 8 | 18.2 |
| To date, what types of treatment has the child received?c | ||
| Gene therapy | 16 | 36.4 |
| Disease modifiers | 20 | 45.5 |
| Management of symptoms only | 12 | 27.3 |
| Other | 9 | 20.5 |
| Did the child experience any side effects from the treatment? | 12 | 27.3 |
| Management of side effects required hospitalization?d | 3 | 25.0 |
Parents' and children's characteristics.
SD, standard deviation.
Possible answers were yes/no. Numbers provided refer to “yes.”
Refers to parents who work (n = 26).
Participants could select more than one answer.
Refers to cases with reported treatment side effects (n = 12).
3.2 Journey phase I: diagnosis
SMA was diagnosed through newborn screening in only 22.7% of the total sample (N = 10), while no prenatal screening cases were reported by participants. In the remaining cases, the first symptom was primarily identified by the parent (73.5%). Post symptom identification, 73.5% of respondents contacted a pediatric neurologist, and 70.6% underwent diagnostic tests, of which 95.8% confirmed the child's diagnosis. In the majority of cases (52.9%), the diagnosis was confirmed within 3 months of first seeking care.
More than one in three (38.6%, N = 17) were required to travel from their place of residence to receive a diagnosis, and all (N = 17) covered this cost out of their pocket. Among those required to travel to receive a diagnosis, 70.6% had to travel for more than 100 km. Of the 26 parents in the sample who were employed at the time of the survey, 19 (73.1%) had to take leave of absence from their work to receive a diagnosis, and 42.1% of these had to take more than 3 days off work.
In addition, more than half (65.9%) had to pay out-of-pocket for diagnosis-related services (Table 2), primarily diagnostic tests (82.8%) and medical visits (69.0%). The majority of children (86.4%) started their treatment more than 1 month after confirming the diagnosis. The most frequent reason (76.9%) for this delay was getting treatment approval by EOPYY.
Table 2
| Variables | n | % |
|---|---|---|
| Did you have to pay out-of-pocket for any health services related to the diagnosis?a | 29 | 65.9 |
| If yes, for which one?b,c | 15 | 34.1 |
| Medical visit | 20 | 69.0 |
| Medical visit (co-payment only) | 3 | 10.3 |
| Diagnostic tests | 24 | 82.8 |
| Diagnostic tests (co-payment only) | 6 | 20.7 |
| Pharmaceutical treatments | 3 | 10.3 |
| Pharmaceutical treatments (co-payment only) | 0 | 0.0 |
| Other | 1 | 3.4 |
| In how many days after the diagnosis did the child start treatment? | ||
| 1 week | 1 | 2.3 |
| 15 days | 2 | 4.5 |
| 1 month | 2 | 4.5 |
| >1 month | 38 | 86.4 |
| Other | 1 | 2.3 |
| Did you experience problems/delays with starting the (drug) treatment?a | 26 | 59.1 |
| If yes, definec,d | ||
| Treatment approval (SEP/EOPYY) | 20 | 76.9 |
| Availability of treatment (e.g., delayed importation from abroad) | 9 | 34.6 |
| Availability of hospital/clinic (appointment) for the administration of the treatment | 9 | 34.6 |
| Other | 4 | 15.4 |
Diagnosis-related services paid out-of-pocket and barriers in receiving treatment.
EOPYY, national organization for health services provision; SEP, system for electronic preauthorization.
Possible answers were yes/no. Numbers provided refer to “yes.”
Refers to parents who paid out-of-pocket for services related to the diagnosis (n = 29).
Participants could select more than one answer.
Refers to cases that experienced a delay in treatment start (n = 26).
During the period from first symptom recognition to treatment start, parents reported a heavy emotional burden and underlined a need for accurate, comprehensive, and timely information provision. Figure 1 depicts participants’ self-reported needs and requests for support during this phase of the journey. A total of 65.9% of participants reported that detailed information on the disease, treatment options, and prognosis could have helped them manage the fear and anxiety that came with the diagnosis. Another 47.7% of parents stressed the importance of having easy access to detailed information on special services and assistive equipment/aids, social insurance benefits, and patient rights, whereas one in three (34.1%) would welcome centralized organization of the various diagnosis-related appointments and tests. Finally, an additional 34.1% stressed the need for psychological support for parents and carers, to assist them in maintaining their optimism. The need for information about the disease, treatment options, and prognosis was similar across parental education (64.3% for middle/high school graduates and 66.7% for university alumni/MSc/PhD holders; p > 0.999) and income levels (53.8% for those with less than 10,000 euros annual income and 71.0% for those with more than 10,000 euros annual income; p = 0.313) (Table 3). Furthermore, need for information on special services, aids, benefits, and rights was significantly greater in university alumni/MSc/PhD holders (21.4% for middle/high school graduates and 60.0% for university alumni/MSc/PhD holders; p = 0.017), yet similar across income levels (38.5% for those with less than 10,000 euros annual income and 51.6% for those with more than 10,000 euros annual income; p = 0.426). In addition, the need for referral information, before diagnosis, so that they can be prepared for the appointment with the specialist was similar across parental education (21.4% for middle/high school graduates and 23.3% for university alumni/MSc/PhD holders; p > 0.999) and income levels (23.1% for those with less than 10,000 euros annual income and 22.6% for those with more than 10,000 euros annual income; p > 0.999). Figure 2 presents the feelings reported by participants upon receiving the diagnosis. The majority of participants (90.9%) felt agony and fear for the future, and one in four (25%) felt guilt, as they considered themselves responsible for their child's illness.
Figure 1
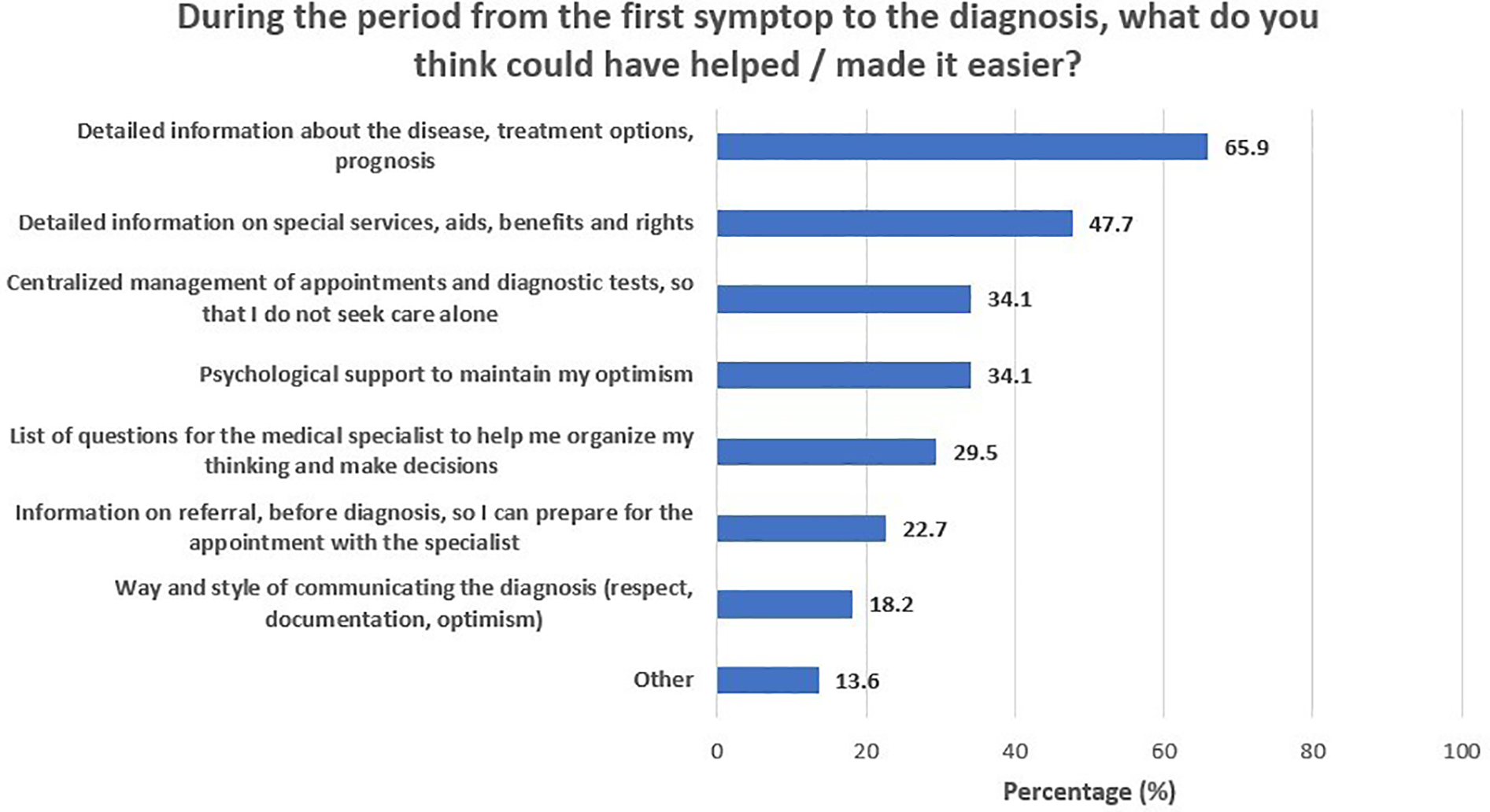
During the period from the first symptom to the diagnosis, what do you think could have helped/made it easier?
Table 3
| Variables | Need for information about the disease, treatment options, and prognosis | Need for information on special services, aids, benefits, and rights | Need for information on referral, before diagnosis, so that parents can be prepared for the appointment with the specialist | |||
|---|---|---|---|---|---|---|
| n (%) | p | n (%) | p | n (%) | p | |
| Educational level | ||||||
| Middle/high school graduates | 9 (64.3) | >0.999b | 3 (21.4) | 0.017a | 3 (21.4) | >0.999b |
| University alumni/ MSc/PhD holders | 20 (66.7) | 18 (60) | 7 (23.3) | |||
| Family's annual income (euros) | ||||||
| <10,000 | 7 (53.8) | 0.313b | 5 (38.5) | 0.426b | 3 (23.1) | >0.999b |
| ≥10,000 | 22 (71) | 16 (51.6) | 7 (22.6) | |||
Association of parental needs with their educational level and their annual income.
Pearson's chi-square test.
Fisher's exact test.
Figure 2
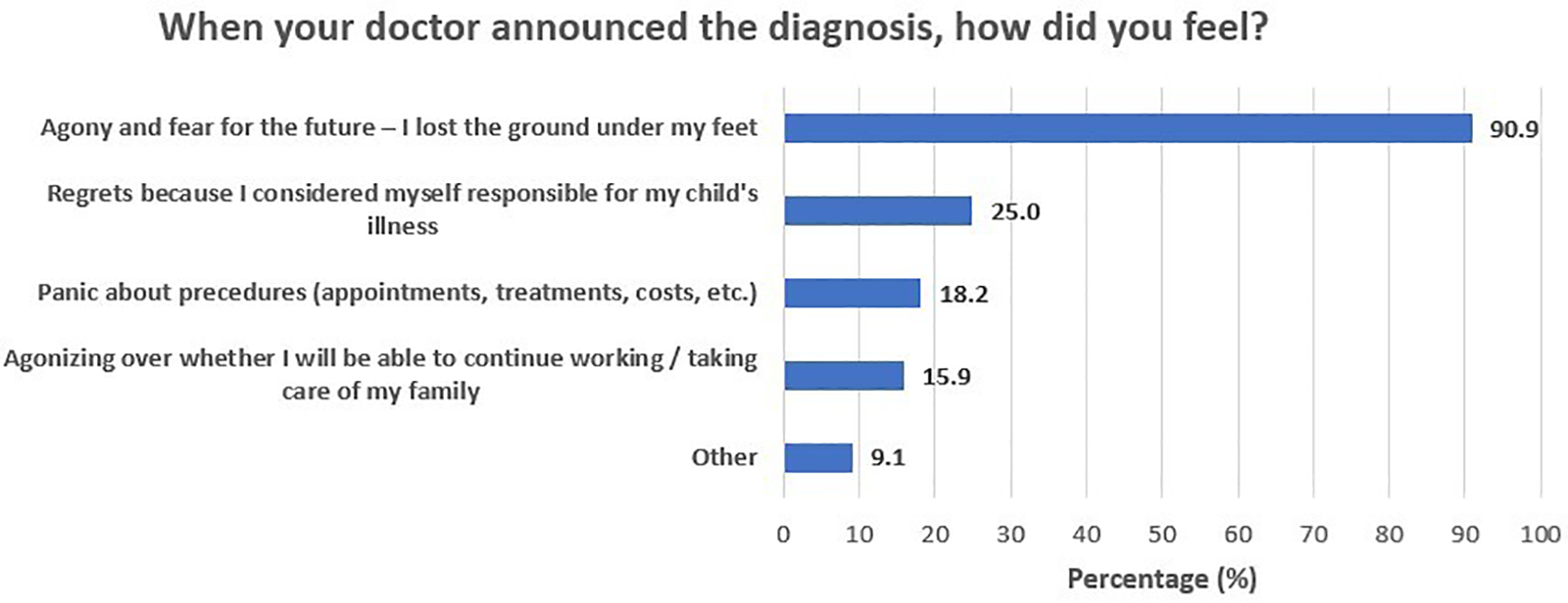
When your doctor announced the diagnosis, how did you feel?
3.3 Journey phase 2: linkage to care and treatment
Physiotherapy/occupational therapy was used by 86.4% of the children and orthopedic monitoring/tests [e.g., dual-energy x-ray absorptiometry (DEXA)] by 43.2% (Table 4). Speech therapy and orthopedic monitoring/tests were the two types of expenses most frequently reported as reimbursed by EOPYY, in 50% and 47.4% of the sample, respectively. In those cases, parents had to shoulder a monthly expense of on average 319.5 euros (SD = 154.1 euros) for physiotherapy/occupational therapy and 185.7 euros (SD = 146.8 euros) for speech therapy. Two out of three children (66.7%) used orthotic aids and 79.4% splints, none of which were fully reimbursed by EOPYY (Table 5). Moreover, 75% of the children used a wheelchair, which was fully reimbursed in only 9.5% of the cases. In total, the overall cost that parents had to shoulder, as it was not reimbursed by EOPYY, ranged from 80 to 166,238€, with a mean of 8,371.11€ (SD = 28,329.09€) and a median of 1,450€ (IQR: 350–4,680€).
Table 4
| Type of expenseb | Children’s usea | Expense reimbursed by EOPYYa | Monthly out-of-pocket amount spent (€), if not reimbursed by EOPYY | |||
|---|---|---|---|---|---|---|
| n | % | n | % | Mean (SD) | Median (IQR) | |
| Food supplements/vitamins | 19 | 43.2 | 6 | 31.6 | 71.4 (66.8) | 30 (20–100) |
| Physiotherapy/occupational therapy | 38 | 86.4 | 16 | 42.1 | 319.5 (154.1) | 285 (200–450) |
| Speech therapy | 14 | 31.8 | 7 | 50.0 | 185.7 (146.8) | 120 (100–240) |
| Nutritional monitoring/assessment/intervention | 10 | 22.7 | 2 | 20.0 | 86.3 (64.1) | 60 (35–135) |
| Orthopedic monitoring/tests (e.g., DEXA) | 19 | 43.2 | 9 | 47.4 | 53 (27.5) | 50 (30–60) |
| Dental/orthodontic follow-up/intervention (e.g., due to jaw problems) | 12 | 27.3 | 2 | 16.7 | 50 (44.7) | 45 (20–50) |
| Psychological support (for me or my partner) | 6 | 13.6 | 1 | 16.7 | 84 (70.2) | 50 (40–100) |
| Physiotherapy (for me or my partner) | 14 | 31.8 | 6 | 42.9 | 178.8 (183.3) | 150 (45–200) |
| Other | 2 | 4.5 | 0 | 0.0 | 55 (63.6) | 55 (10–100) |
Parents' out-of-pocket expenses.
DEXA, dual-energy x-ray absorptiometry; EOPYY, national organization for health services provision; IQR, interquartile range; SD, standard deviation.
Possible answers were yes/no. Numbers provided refer to “yes.”
Participants could select more than one answer.
Table 5
| Type of aidd | Have it at homea | If yes, was the cost of buying it reimbursed by EOPYY?b | Out-of-pocket amount spent (€)c | ||
|---|---|---|---|---|---|
| No | Partly | In full | |||
| n (%) | n (%) | n (%) | n (%) | Mean (SD) | |
| Simple continuous positive airway pressure (CPAP) device | 5 (20) | 0 (0) | 1 (33.3) | 2 (66.7) | 4,500 (—) |
| Missing | 19 | 2 | |||
| Simple biphasic positive airway pressure (BiPAP S/T) device | 15 (48.4) | 1 (9.1) | 6 (54.5) | 4 (36.4) | 1,736.7 (2,586) |
| Missing | 13 | 4 | 1 | ||
| Volume/pressure ventilator | 11 (37.9) | 0 (0) | 5 (71.4) | 2 (28.6) | 125 (35.4) |
| Missing | 15 | 4 | 3 | ||
| Orthotic means | 20 (66.7) | 2 (11.1) | 13 (72.2) | 3 (16.7) | 1,261.8 (468.3) |
| Missing | 14 | 2 | 4 | ||
| Knee guards | 12 (42.9) | 5 (45.5) | 5 (45.5) | 1 (9.1) | 564.3 (857.7) |
| Missing | 16 | 1 | 3 | ||
| Shoulder guards | 5 (20) | 2 (40) | 3 (60) | 0 (0) | 1,633.3 (2,064.8) |
| Missing | 19 | 2 | |||
| Splints | 27 (79.4) | 3 (13) | 20 (87) | 0 (0) | 800 (1,027.6) |
| Missing | 10 | 4 | 5 | ||
| Wheelchair | 27 (75) | 3 (14.3) | 16 (76.2) | 2 (9.5) | 2,516.7 (1,977.8) |
| Missing | 8 | 6 | 4 | ||
| Special van/vehicle that allows the transportation of a wheelchair | 4 (14.8) | 4 (100) | 0 (0) | 0 (0) | 95,000 (91,923.9) |
| Missing | 17 | 2 | |||
Aids used by children and their reimbursement.
BiPAP, biphasic positive airway pressure; CPAP, continuous positive airway pressure; EOPYY, national organization for health services provision; SD, standard deviation; S/T, spontaneous/timed.
Possible answers were yes/no. Numbers provided refer to “yes.”
Answered only by participants who had aid at home.
Answered only by participants who stated that the cost was not reimbursed by EOPYY either in full or in part.
Participants could select more than one answer.
3.4 Correlation of areas of quality of life of the child and functional capacity with parental burden
PedsQol scales' descriptives and their correlation with the Barthel index and Zarit scale are presented in Table 6. Children's quality of life was not significantly correlated with their functional capacity (as measured by the Barthel index). On the contrary, a higher score in the Zarit scale, i.e., greater burden, was significantly associated with lower physical functioning, lower social functioning, and lower QoL overall. Mean functional capacity was 37.7 (SD = 26.7), and mean burden was 14.1 (SD = 7.5). Higher functional capacity in children was significantly associated with lower parental burden (r = −0.52; p < 0.001).
Table 6
| Variables | Mean (SD) | Barthel index | Zarit scale | ||
|---|---|---|---|---|---|
| r | p | r | p | ||
| Physical functioning | 68.9 (28) | 0.14 | 0.379 | −0.31 | 0.041 |
| Emotional functioning | 69.9 (17.7) | 0.11 | 0.482 | −0.29 | 0.056 |
| Social functioning | 58.1 (22.6) | 0.04 | 0.778 | −0.37 | 0.013 |
| School functioning | 71 (24.1) | −0.03 | 0.848 | −0.04 | 0.815 |
| Total PedsQol score | 67 (15.8) | 0.09 | 0.540 | −0.36 | 0.015 |
| Barthel index | 37.7 (26.7) | −0.52 | <0.001 | ||
PedsQol scales' descriptives and their correlation with the Barthel index and Zarit scale.
PedsQol, pediatric quality of life; SD, standard deviation.
3.5 Differences in children’s QoL, functional capacity, and parental burden among types of SMA
To examine if children's QoL, functional capacity, and parental burden differed between SMA types, we compared all scale scores across the three SMA types (Table 7). The mean functional capacity score was 15.3 (SD = 14.3) in participants with SMA type I, 38.6 (SD = 14.4) in SMA type II, and 82.9 (SD = 16.0) in SMA type III. Functional capacity was significantly improved in type III compared with types I and II, and significantly greater in type II compared with type I (p < 0.001 for all comparisons). The mean score on the burden scale was 19.9 (SD = 7.1) for participants with SMA type I, 12.2 (SD = 5.1) for participants with SMA type II, and 7.4 (SD = 6.9) for participants with SMA type III. Parental burden was significantly lower in children with SMA type III as compared with types I (p < 0.001) and II (p = 0.002). QoL was not significantly different among the different SMA types (p > 0.05).
Table 7
| Variables | SMA type | p ANOVA | p a | ||||
|---|---|---|---|---|---|---|---|
| Ι | ΙΙ | ΙΙΙ | |||||
| Mean (SD) | Mean (SD) | Mean (SD) | I vs. II | I vs. III | II vs. III | ||
| Barthel index | 15.3 (14.3) | 38.6 (14.4) | 82.9 (16.0) | <0.001 | <0.001 | <0.001 | <0.001 |
| Zarit scale | 19.9 (7.1) | 12.2 (5.1) | 7.4 (6.9) | <0.001 | 0.002 | <0.001 | ns |
| Physical functioning | 59.2 (35.3) | 72.9 (25) | 77.2 (12.7) | 0.243 | ns | ns | ns |
| Emotional functioning | 70.3 (16.7) | 70.7 (18) | 66.4 (21.2) | 0.858 | ns | ns | ns |
| Social functioning | 54.3 (27.8) | 61.1 (18.6) | 56.4 (23.8) | 0.663 | ns | ns | ns |
| School functioning | 71.4 (28.8) | 70.5 (21.9) | 71.9 (23.4) | 0.988 | ns | ns | ns |
| Total PedsQol score | 63.8 (19.1) | 68.8 (13.3) | 68 (16.9) | 0.645 | ns | ns | ns |
PedsQol scales, Barthel index, and Zarit scale according to SMA type.
p-value from pairwise comparisons (after Bonferroni correction).
4 Discussion
Our results confirm that, in the majority of cases and given the lack of organized prenatal or newborn screening, parents are the first to recognize any symptoms and seek care. Over one-third of parents have to travel from their place of residence to receive a diagnosis for their child, and more than half have to pay out-of-pocket for diagnosis-related services. In addition, parents shoulder treatment services and assistive aids' costs in excess of 300 euros a month. The majority report feelings of agony, fear, and guilt upon receiving the diagnosis. Caregivers of children with severe SMA types report a higher burden.
Our findings confirm results from a previous study, in which among types I, II, and III patients, 63%, 75%, and 85% of cases were first recognized by parents, respectively (18). In the same study, the mean time between symptom onset and genetic diagnosis was 1.94 months (SD ± 1.84; range: 0–10.3 months) in type I patients, 5.28 months (SD ± 4.68; range: 0–35 months) in type II, and 16.8 months (SD ± 18.72; range: 0–102 months) in type III (18), which is longer than the average of 3 months reported in our study. Lack of specialized knowledge to recognize symptoms or even assert a behavior as abnormal by parents may be related to a delayed diagnosis (19), which inevitably impacts the prospects of successful early intervention. A recent systematic review (20) revealed that many parents described an early feeling that something was wrong with their child—nonetheless, most of them received the diagnosis with a delay.
The majority of our sample reported feelings of shock, loss, and guilt upon receiving the formal diagnosis. Such sentiments are reported as related to the confrontation with the possible premature death of the child and lost expectations for a normal life (20). This “anticipatory loss’s” main themes include (1) enduring the helplessness and pressure of care, (2) suffering due to the child's rare and unknown condition, (3) loss of hope and reinforcement of the parent–child attachment, and (4) avoiding the pressure of death and enriching the child's life (21).
In this light, parents' most pressing need, mentioned consistently in the literature and confirmed by our survey, is information (22–27), particularly about treatment options. The need for information about the child's options, prospects, and the institutions that can benefit the child repeatedly comes to the fore and, in the literature, appears to be unrelated to the educational level of the mother or father and related to family income, with the need for information relatively decreasing as income increases (22). Likewise, our survey highlights that parental need for information appears unrelated to educational level, except for specialized information on special services, aids, benefits, and rights, which was significantly greater in university alumni/MSc/PhD holders. This may be explained by the fact that parents with a higher education may be better positioned to fully understand and advocate for their child's rights. On the contrary, our survey revealed that parental need for information is wholly unrelated to family income. This may be explained by the fact that the disease is rare and all parents, irrespective of income, are faced first and foremost with the same need for information. Another recent review (20) revealed that parents express a consistent need for coordinated and integrated care, including not only medical treatment but also psychological and practical care support, from very early on, from the point of diagnosis. These findings are fully reflected in our study. Parents often report feeling left alone after the diagnosis, struggling to find the right solutions for their child, because of complex and time-consuming navigation through the healthcare and support system (20). The lack of clearly defined and integrated support pathways in the current structure and operation of the Greek National Health System (NHS) further confounds this situation and is confirmed by participants in this study as of critical importance both at the stage of diagnosis and during the ongoing management of pediatric SMA.
Out-of-pocket expenditure is also a major worry for parents of pediatric SMA patients. Shouldering a cost that may exceed 300 euros a month for a series of additional health services or assistive aids and equipment places a substantial burden not only on the financial but also on the psychological capacity of parents. With challenges related to maintaining employment despite the need for constant leave of absence and the increasing requirements for support services as the child grows, the fear of being unable to make ends meet is prevalent in most survey respondents. Particularly for some expenses, such as wheelchairs and vans, for which the respective out-of-pocket expenditure could be considered as catastrophic (28). Being unable to shoulder such costs may, in addition, impact actual disease outcomes. Our study confirmed that children, whose parents had shouldered out-of-pocket expenses as early as the diagnosis phase, had significantly improved school functioning scores. In a study of Thai children with SMA (29), whose health-related quality of life (HRQoL) was compared with those of healthy Thai children, household income less than 18,500 Thai baht/month was statistically significantly associated with lower HRQoL, as costs of assistive devices that allow these patients to live and function more independently were not covered by Thailand's universal healthcare coverage insurance scheme. Although not confirmed by our data due to the sample size, this aspect warrants careful consideration in settings where access to assistive devices is limited or problematic, as reported in this survey for Greece.
Indirect costs, associated with income loss, are also highlighted in our study, across the disease continuum, for those parents who can maintain their employment post-diagnosis. This is in line with a recent review of the economic impact of SMA across Europe (30), which highlighted that direct non-healthcare costs ranged between a whopping 79%–86% of total SMA costs, with informal care costs being the main component. This confirms older results from Spain (31) that attributed 67.8% of total SMA costs to direct non-healthcare costs. Of these, informal caregiving constituted the major cost component.
Among our sample, the average PedsQol score was 67 (SD = 15.8), which is higher than scores recently reported in Thailand (54.3 ± 14.8) (29) and Chile (51.92 ± 17) (32). Zarit scores per SMA type were higher for type I SMA vs. types II and III, revealing a higher burden for caregivers in the graver SMA types. These results are substantially different from those recently reported in Aranda-Reneo et al. (33) [25.1 (23.2) for type I, 34.8 (13.3) for type II, and 29.6 (17.1) for type III]. These differences, especially when considering Zarit scores by SMA type, may be attributed to the low participant numbers in such surveys, which may lead to biases when extrapolating to the entire SMA population.
5 Limitations
All data in this study are self-reported. Some refer to past events and may, thus, be affected by recall bias, as some of the costs (e.g., aids and supportive equipment) incurred out of pocket or self-reported quality of life may be affected by participants' incomplete memories. This recall bias may impact the true relationship between variables.
The survey was performed in Greece through MDA Hellas to ensure patient concerns and perspectives are input into formal health policy advocacy. As such, results may be conditioned by personal patient experiences with the Greek healthcare system’s specifics regarding funding and access to care. Nonetheless, the survey is replicable in other countries, through a patient association or an advocacy organization, and as such can provide insights into similarities and differences in perceptions and challenges faced by parents of SMA children in different healthcare systems.
Participants, all parents of children diagnosed with SMA, were members of MDA Hellas in Greece. For this study, only one of the two parents was included to accurately reflect the number of children represented in the study. Our sample size of 44 parents may, therefore, be considered representative for a study among parents of underage children with such a rare disease as SMA in Greece.
6 Conclusions
Given the nature of SMA as a rare genetic disorder, pediatric patients and their caregivers are faced with additional challenges as they navigate their journey, related primarily to the rarity and severity of the condition. This paper details their concerns and needs at each phase of their patient journey to ensure these can be measurably addressed through targeted health policy advocacy. Such advocacy should be translated into a national action plan, which adequately addresses these specific patient and caregivers' needs, as highlighted in this study. In addition, future studies are required to follow patients over time to understand long-term effects on caregivers and the impact of different SMA treatment modalities or care organization and delivery options.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the corresponding author, upon request.
Ethics statement
This study was approved by the Ethics Committee of the Health Policy Institute and the Board of Directors of MDA Hellas. The study was conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
KS: Writing – review & editing, Supervision, Writing – original draft, Conceptualization, Methodology. CG: Writing – original draft, Writing – review & editing, Investigation, Methodology, Project administration, Conceptualization. CT: Data curation, Writing – original draft, Formal analysis, Writing – review & editing. CV: Investigation, Writing – review & editing, Project administration. PG: Writing – review & editing, Investigation. AN: Investigation, Data curation, Writing – review & editing. KB: Investigation, Writing – review & editing. AK: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by MDA Hellas (Grant Number 01/23.02.2022).
Acknowledgments
The authors would like to thank the SMA patients and their parents and carers in Greece for their time, commitment, and energy in participating in this survey and in sharing their journey with us.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence, and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Verhaart IE Robertson A Wilson IJ Aartsma-Rus A Cameron S Jones CC et al Prevalence, incidence and carrier frequency of 5q–linked spinal muscular atrophy—a literature review. Orphanet J Rare Dis. (2017) 12(1):124. 10.1186/s13023-017-0671-8
2.
Zhang W Feng Y Yan Y Yao M Gao F Lin W et al Health information literacy among children with spinal muscular atrophy and their caregivers. Ital J Pediatr. (2024) 50(1):157. 10.1186/s13052-024-01723-9
3.
Bieniaszewska A Sobieska M Gajewska E . Functional and structural changes in patients with spinal muscular atrophy treated in Poland during 12-month follow-up: a prospective cohort study. J Clin Med. (2024) 13(14):4232. 10.3390/jcm13144232
4.
TREAT-NMD. Standards of Care for Spinal Muscular Atrophy (SMA) (2017). Available online at:https://www.treat-nmd.org/resources-and-support/care-guides/sma-care/ (Accessed September 28, 2025).
5.
Mercuri E Finkel RS Muntoni F Wirth B Montes J Main M et al Diagnosis and management of spinal muscular atrophy: part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. (2018) 28(2):103–15. 10.1016/j.nmd.2017.11.005
6.
Finkel RS Mercuri E Meyer OH Simonds AK Schroth MK Graham RJ et al Diagnosis and management of spinal muscular atrophy: part 2: pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul Disord. (2018) 28(3):197–207. 10.1016/j.nmd.2017.11.004
7.
Ropars J Peudenier S Genot A Barnerias C Espil C . Multidisciplinary approach and psychosocial management of spinal muscular atrophy (SMA). Arch Pédiatr. (2020) 27(7, Suppl):7S45–9. 10.1016/S0929-693X(20)30277-3
8.
Souliotis K Lionis C . Creating an integrated health care system in Greece: a primary care perspective. J Med Syst. (2005) 29(2):187–96. 10.1007/s10916-005-3006-6
9.
Athanasakis K Souliotis K Kyriopoulos E-J Loukidou E Kritikou P Kyriopoulos J . Inequalities in access to cancer treatment: an analysis of cross-regional patient mobility in Greece. Support Care Cancer. (2012) 20(3):455–60. 10.1007/s00520-011-1093-0
10.
Giannouchos TV Ukert B Vozikis A Steletou E Souliotis K . Informal out-of-pocket payments experience and individuals’ willingness-to-pay for healthcare services in Greece. Health Policy. (2021) 125(6):693–700. 10.1016/j.healthpol.2021.04.001
11.
Kanavos P Souliotis K . “9 Reforming health care in Greece: balancing fiscal adjustment with health care needs1”. In: MeghirCPissaridesCAVayanosDVettasN, editors. Beyond Austerity: Reforming the Greek Economy. London: The MIT Press (2017). p. 359–402.
12.
Kekou K Svingou M Sofocleous C Mourtzi N Nitsa E Konstantinidis G et al Evaluation of genotypes and epidemiology of spinal muscular atrophy in Greece: a nationwide study spanning 24 years. J Neuromuscul Dis. (2020) 7(3):247–56. 10.3233/JND-190466
13.
OECD. “European observatory on health systems and policies”. In: Greece: Country Health Profile 2023, State of Health in the EU. Paris: OECD Publishing (2023). p. 1–24.
14.
Ferfeli S Galanos A Dontas IA Triantafyllou A Triantafyllopoulos IK Chronopoulos E . Reliability and validity of the Greek adaptation of the modified Barthel index in neurorehabilitation patients. Eur J Phys Rehabil Med. (2024) 60(1):44–54. 10.23736/S1973-9087.23.08056-5
15.
Varni JW Burwinkle TM Seid M Skarr D . The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. (2003) 3(6):329–41. 10.1367/1539-4409(2003)003%3C0329:TPAAPP%3E2.0.CO;2
16.
Gkoltsiou K Dimitrakaki C Tzavara C Papaevangelou V Varni JW Tountas Y . Measuring health-related quality of life in Greek children: psychometric properties of the Greek version of the Pediatric Quality of Life InventoryTM 4.0 generic core scales. Qual Life Res. (2008) 17(2):299–305. 10.1007/s11136-007-9294-1
17.
Parpa E Katsantonis N Tsilika E Galanos A Papazoglou I Mystakidou K . Validity and reliability of the Greek version of the ZBI in informal carers of adults with intellectual disabilities. Int J Mental Health Psychiatr. (2017) 3(03):1–7. 10.4172/2471-4372.1000145
18.
Pera MC Coratti G Berti B D'Amico A Sframeli M Albamonte E et al Diagnostic journey in spinal muscular atrophy: is it still an odyssey? PLoS One. (2020) 15(3):e0230677. 10.1371/journal.pone.0230677
19.
Qian Y McGraw S Henne J Jarecki J Hobby K Yeh W-S . Understanding the experiences and needs of individuals with spinal muscular atrophy and their parents: a qualitative study. BMC Neurol. (2015) 15(1):217. 10.1186/s12883-015-0473-3
20.
Brandt M Johannsen L Inhestern L Bergelt C . Parents as informal caregivers of children and adolescents with spinal muscular atrophy: a systematic review of quantitative and qualitative data on the psychosocial situation, caregiver burden, and family needs. Orphanet J Rare Dis. (2022) 17(1):274. 10.1186/s13023-022-02407-5
21.
Yang B-H Mu P-F Wang W-S . The experiences of families living with the anticipatory loss of a school-age child with spinal muscular atrophy—the parents’ perspectives. J Clin Nurs. (2016) 25(17–18):2648–57. 10.1111/jocn.13312
22.
Evkaya Acar A Karadağ Saygı E İmamoğlu S Öztürk G Ünver O Ergenekon P et al The burden of primary caregivers of spinal muscular atrophy patients and their needs. Turk Arch Pediatr. (2021) 56(4):366–73. 10.5152/TurkArchPediatr.2021.20117
23.
Farrar MA Carey KA Paguinto S-G Kasparian NA De Abreu Lourenço R . “The whole game is changing and you’ve got hope”: Australian perspectives on treatment decision making in spinal muscular atrophy. Patient. (2020) 13:389–400. 10.1007/s40271-020-00415-w
24.
Kariyawasam DST D’Silva AM Vetsch J Wakefield CE Wiley V Farrar MA . “We needed this”: perspectives of parents and healthcare professionals involved in a pilot newborn screening program for spinal muscular atrophy. eClinicalMedicine. (2021) 33:100742. 10.1016/j.eclinm.2021.100742
25.
Lawton S Hickerton C Archibald AD McClaren BJ Metcalfe SA . A mixed methods exploration of families’ experiences of the diagnosis of childhood spinal muscular atrophy. Eur J Hum Genet. (2015) 23(5):575–80. 10.1038/ejhg.2014.147
26.
Oude Lansink ILB van Stam PCC Schafrat ECWM Mocking M Prins SD Beelen A et al This battle, between your gut feeling and your mind. Try to find the right balance’: parental experiences of children with spinal muscular atrophy during COVID-19 pandemic. Child Care Health Dev. (2022) 48(6):1062–70. 10.1111/cch.13014
27.
van Kruijsbergen M Schröder CD Ketelaar M van der Pol WL Cuppen I van der Geest A et al Parents’ perspectives on nusinersen treatment for children with spinal muscular atrophy. Dev Med Child Neurol. (2021) 63(7):816–23. 10.1111/dmcn.14825
28.
World Health Organization. Households with Out-of-Pocket Payments Greater than 40% of Capacity to Pay for Health Care (Food, Housing and Utilities Approach—Developed by WHO/Europe) (2023). Available online at:https://www.who.int/data/gho/indicator-metadata-registry/imr-details/4989 (Accessed September 28, 2025).
29.
Aksaralikitsunti M Sanmaneechai O . Health-related quality of life in Thai children with spinal muscular atrophy. Pediatr Neonatol. (2022) 63(3):291–7. 10.1016/j.pedneo.2022.01.002
30.
Peña-Longobardo LM Aranda-Reneo I Oliva-Moreno J Litzkendorf S Durand-Zaleski I Tizzano E et al The economic impact and health-related quality of life of spinal muscular atrophy. An analysis across Europe. Int J Environ Res Public Health. (2020) 17(16):5640. 10.3390/ijerph17165640
31.
López-Bastida J Peña-Longobardo LM Aranda-Reneo I Tizzano E Sefton M Oliva-Moreno J . Social/economic costs and health-related quality of life in patients with spinal muscular atrophy (SMA) in Spain. Orphanet J Rare Dis. (2017) 12(1):141. 10.1186/s13023-017-0695-0
32.
Vega P Glisser C Castiglioni C Amézquita MV Quirola M Barja S . Quality of life in children and adolescents with spinal muscular atrophy. Rev Chil Pediatr. (2020) 91(4):512–20. 10.32641/rchped.v91i4.1443
33.
Aranda-Reneo I Peña-Longobardo LM Oliva-Moreno J Litzkendorf S Durand-Zaleski I Tizzano EF et al The burden of spinal muscular atrophy on informal caregivers. Int J Environ Res Public Health. (2020) 17(23):8989. 10.3390/ijerph17238989
Summary
Keywords
patient journey, spinal muscular atrophy, disease burden, quality of life, health policy, patient advocacy
Citation
Souliotis K, Golna C, Tzavara C, Vasileiadi C, Golnas P, Ntokou A, Binou K and Karras A (2025) Caring for children with spinal muscular atrophy in Greece: parents' and caregivers' experience with the healthcare system. Front. Health Serv. 5:1612270. doi: 10.3389/frhs.2025.1612270
Received
15 April 2025
Accepted
29 September 2025
Published
23 October 2025
Volume
5 - 2025
Edited by
Daniel Schwarzkopf, University Hospital Jena, Germany
Reviewed by
Raman Kaur, Reed Elsevier, United States
Juan Garcia, National University of Colombia, Colombia
Timothy Rotarius, University of Central Florida, United States
Antje Freytag, University Hospital Jena, Germany
Updates
Copyright
© 2025 Souliotis, Golna, Tzavara, Vasileiadi, Golnas, Ntokou, Binou and Karras.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Kyriakos Souliotis ksouliotis@uop.gr
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.