- 1Department of Public and Occupational Health, Amsterdam Public Health Research Institute, Amsterdam University Medical Center, Amsterdam, Netherlands
- 2Department of Rehabilitation Medicine, University Medical Center Groningen, University of Groningen, Groningen, Netherlands
- 3School of Sports Studies, Hanze University of Applied Sciences, Groningen, Netherlands
- 4Department Strategy and Policy, University Medical Center Groningen, University of Groningen, Groningen, Netherlands
- 5Department of Orthopedics, University Medical Center Groningen, University of Groningen, Groningen, Netherlands
- 6Department of Orthopedics, Orthopaedic Surgery, University Medical Center Groningen, University of Groningen, Groningen, Netherlands
- 7Center for Human Movement Sciences, University Medical Center Groningen, University of Groningen, Groningen, Netherlands
- 8Knowledge Centre for Sport and Physical Activity, Utrecht, Netherlands
- 9Sports Valley, Department of Sports Medicine, Gelderse Vallei Hospital, Ede, Netherlands
Introduction: Several barriers, such as lack of time, knowledge and support, hinder clinicians from providing an individually tailored physical activity (PA) prescription and referral to their patients. As a result, “exercise is medicine” (E = M) is not systematically implemented in clinical care today. Many studies have identified facilitators and barriers to implementation, yet linking these factors to tailored implementation strategies is still an under-researched area. Therefore, this study aimed to apply Implementation Mapping to develop an implementation protocol to support the individually tailored PA prescription in hospital care.
Methods: We used strong stakeholder participation and, we applied the five tasks of the systematic Implementation Mapping approach to match implementation strategies to implementation barriers and facilitators identified through interviews with clinicians working at two university hospitals in the Netherlands.
Results: We identified clinicians as primary actors. Secondary actors were managers of the departments and stakeholders in the broader context. For each actor group, performance objectives were defined. We matched previously identified facilitators and barriers to theory and evidence-informed implementation strategies from the Effective Practice and Organisation of Care taxonomy using the CFIR Strategy Matching Tool. Next, we translated these implementation strategies (e.g., active learning, audit, and feedback, technical assistance, peer education) into practical activities to support the implementation of the E = M tool, such as training for clinicians, creating overviews of possible local exercise referral options, and appointing role models for clinicians. Lastly, these activities were bundled into an implementation protocol. The implementation protocol consisted of a set of implementation activities to support and guide clinicians during the adoption, implementation, and sustainability process of the prescription of E = M. All activities were supported by implementation tools, practical applications, and materials while allowing tailoring to the specific clinical context.
Discussion/conclusion: This study illustrates the application of Implementation Mapping to design an implementation protocol to support and guide the prescription of E = M by clinicians in the hospital environment, using strong stakeholder participation in the development process. The stepwise development of the implementation protocol can serve as an example for researchers or practitioners preparing for E = M implementation.
Introduction
Today, we face a global pandemic of physical inactivity (1, 2). Being physically inactive implies not meeting the current World Health Organization physical activity (PA) guidelines of 150 min of moderate to vigorous intensity PA per week and muscle-strengthening activities two times per week (3). Physical inactivity is associated with chronic non-communicable diseases, such as cardiovascular disease, diabetes, osteoarthritis (4, 5), obesity, cancer (2, 6), and mental health disorder, such as depression (7). It is an important risk factor for impaired daily functioning and participation, especially in patient populations (8). In addition, patient populations show lower PA levels than healthy adults (9). Evidence has shown that improving PA can reverse disease pathogenesis and reduce associated symptoms (10). Promotion of PA among the inactive population is therefore regarded as a top priority, especially as it is known that the biggest health gain can be achieved by increasing PA levels of inactive people to being a little active (11, 12). As a result, many PA interventions and initiatives have emerged.
One of those initiatives is the global concept of “exercise is medicine” (E = M), also known as exercise on prescription by clinicians (13). E = M encourages clinicians and other health care providers to refer patients to evidence-based physical activity programs and qualified exercise professionals when designing treatment plans (14). Although some initiatives exist (15, 16), routine implementation of E = M is not yet achieved in many countries (17). Several barriers hinder clinicians from prescribing exercise or more general forms of PA to their patients, such as lack of time, insufficient knowledge about referral options, and lack of support (18–21). As a result, despite all its potential benefits, E = M is not systematically implemented in clinical care in the Netherlands.
To address the issue of limited time for clinicians, we created an online tool (The E = M tool) integrated within the electronic medical records system in two Dutch Academic hospitals to support the individually tailored prescription of PA in clinical care (22). However, implementing this tool involves a complex process. Therefore, an implementation protocol, including implementation strategies, should be designed and tailored to meet the specific contextual needs for successfully introducing and using this tool. Implementation strategies can be defined as methods or techniques used to enhance the adoption, implementation, and sustainability of a clinical program or practice (23, 24), i.e., they describe the “how to” of implementation. Powell et al. describe 73 implementation strategies in their taxonomy (23). Implementation strategies can consist of a single component strategy. However, most often, several strategies are combined to form a multifaceted strategy, such as combined training, consultation, audit, and feedback.
To develop an implementation protocol, the Implementation Mapping approach was created (25). This approach encompasses five iterative tasks that enable circling back throughout the development process to ensure all actors, outcomes, determinants, and objectives are addressed. Therefore, this study aimed to apply Implementation Mapping to develop an implementation protocol, existing of a tailored set of strategies to support the individually tailored PA prescription in hospital care.
Materials and methods
This study is part of the Physicians Implement Exercise is Medicine (PIE = M) project, in which two Dutch university medical centers [University Medical Center Groningen (UMCG) and Amsterdam University Medical Center (Amsterdam UMC)] worked towards implementing E = M into routine clinical care; i.e., Rehabilitation Medicine and Medical Oncology of the Amsterdam UMC and the departments of Rehabilitation Medicine, Orthopedics, and Sports Medicine of UMCG. In general, these clinical departments focus on PA, which makes them suitable for pilot-testing the feasibility of individually tailored prescription of PA for patients.
The medical ethical committees of the UMCG and Amsterdam UMC approved the study design (METc UMCG 2017/517 and Amsterdam UMC 2018/219). All participants gave informed consent to participate in the study before taking part. The study design and protocol were published elsewhere (26).
The tool
The E = M tool exists of an algorithm, including patient characteristics assessed with a digital questionnaire [i.e., age, gender, PA behavior, Body Mass Index (BMI), medical diagnosis, motivation to change PA and preference to discuss PA with their clinician], set against norm values. The tool and its algorithm are described in more detail elsewhere (22). The online E = M tool provides an individual E = M-prescription for patients and referral options to local PA interventions in- and outside the hospital. An E = M decision guide was developed to support clinicians in the referral of patients to tailored PA interventions (22).
Applying implementation mapping
We applied the Implementation Mapping approach, using strong stakeholder engagement in a co-creation process, to develop an implementation protocol to support and guide clinicians during the adoption, implementation and sustainability process of prescription of E = M in a clinical setting. Implementation Mapping is a systematic, multi-step approach for developing implementation strategies that incorporates theory, evidence, and stakeholder perspectives (25) and has been applied previously in our field (27–29).
We operationalized the 5 tasks as follows:
In task 1 – Conduct needs assessment and identifying key actors - we conducted a mixed methods study among clinicians and their managers working at the two participating university hospitals to identify determinants (i.e., barriers and facilitators) for implementing E = M in clinical practice. The needs assessment results are described in more detail elsewhere (18, 22). Therefore, we will only briefly describe these results and how they were used to inform the development process of the implementation protocol. Building on the needs assessment and discussions with the working group of each department and the consortium, we selected key actors to implement E = M in clinical care.
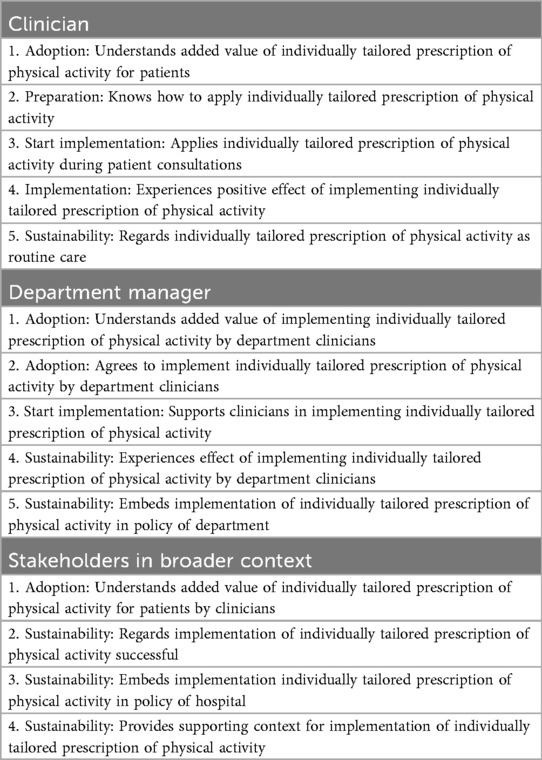
During task 2 - State implementation outcomes - we defined performance objectives for adopting, implementing and maintaining the prescription of E = M in clinical care for each actor. Performance objectives make clear “who has to do what”.
Next in task 3 – Select implementation strategies - we were guided by the Effective Practice and Organisation of Care taxonomy from Powell et al. (23). We used a structured approach by matching our identified barriers and facilitators from Task 1 to the Consolidated Framework for Implementation Research (CFIR) framework classification (30). Waltz et al. have conducted an expert based ranking procedure to identify which implementation strategies would best address specific implementation barriers, resulting in a practical tool for matching implementation strategies (31). This Strategy Matching Tool helps users to choose strategies based on CFIR barriers (30), and complements Fernandez and colleagues' Implementation Mapping (25). Therefore, we linked each identified barrier to a CFIR construct and then selected the top 3 highest ranked proposed strategies by the matching tool.
In task 4 - Produce protocol for implementation and materials - we (FN, JN, AB) matched the implementation strategies (result of task 3) to the suggested practical solutions gathered during the interviews and during discussions with the working group of each department and the stakeholders in our consortium. To operationalize the strategies, we held design sessions with working groups of each department to ask input for the materials. We produced final materials tailored to each department's specific needs and context-specific factors. The tailored implementation strategies were operationalized in a way that delineates what they entail and how they will be delivered and described according to Proctor's reporting guidelines (24).
Lastly in Task 5 – Evaluate implementation outcomes - we developed a mixed methods process evaluation plan for both the processes and outcomes of the set of implementation strategies using the RE-AIM framework (32). The process evaluation was conducted during the pilot implementation in the four departments of two university hospitals (Amsterdam UMC, UMCG), where interviews were initially conducted (33).
Stakeholder engagement
During each task of the Implementation Mapping approach, we strongly engaged with stakeholders within our consortium, working groups at the participating departments and patient representatives. The PIE = M project is supported by a large consortium of clinicians working in the participating clinical departments, researchers, information technology professionals, implementation experts, sports organizations, municipalities, lifestyle professionals and patients.
In each department, we installed a working group led by researchers consisting of the manager, clinician, and administrative support. In addition, during six meetings (May, Jul, Sept, Dec 2018, and May, Dec 2019) with the consortium, several aspects of the PIE = M project were discussed and active participation, brainstorms over and reflections on the Implementation Mapping results were gathered.
Although the primary focus of the PIE = M project is on individually tailored prescription of PA by hospital clinicians, a panel discussion with patient representatives was organized to reflect on the developed strategies for implementing E = M from a patient perspective.
Results
Task 1: Needs assessment and identify key actors
The needs assessment comprised of a questionnaire (n = 45 clinicians) and a semi-structured interview (n = 19 clinicians). Our mixed methods needs assessment resulted in 52 identified facilitators and barriers for implementation. A full description of the assessment and its results are described in detail elsewhere (18). Briefly, we identified four main themes: (1) beliefs toward the implementation of E = M (e.g., clinicians knowledge and skills, or social support), (2) factors related to the patient perspective (e.g., patient priorities or motivation), (3) factors related to the E = M referral options (e.g., knowledge of and trust in local referral options) and (4) practical considerations when implementing E = M (e.g., time constraints).
Discussing these determinants with our consortium stakeholders enriched the content and explanation of factors influencing implementation of E = M, but did not lead to new determinants. Yet, the stakeholders emphasized that some determinants were beyond this project's scope, such as knowledge among the general population about the PA guidelines. Consequently, those determinants were not included in the next tasks of the Implementation Mapping approach.
In addition, the stakeholders endorsed that not all determinants will have the same impact at each department. Therefore, tailoring the strategies to the specific departments was mentioned to be essential. We have considered this in Task 4 when designing the implementation materials.
Key actors
Building on the interviews and input from consortium stakeholders, we identified the following three key actor groups for the adoption, implementation and sustainability of the E = M in clinical care:
Primary actors are clinicians. Clinicians see the patient during their consultation and then (1) decide with which patients they will discuss their current PA levels; (2) identify a possible need for more daily PA; (3) discuss patients' potential benefits; (4) discuss guidelines for PA; and (5) have the option to refer the patient to tailored PA programs in- or outside the hospital. So, implementation strategies had to target clinicians to support their implementation behavior.
Secondary actors are managers of the departments. From the interviews it became clear that embedding the E = M in routine care needed endorsement of the department managers.
Tertiary actors are stakeholders in the broader context, such as hospital board, health insurance companies and providers of exercise programs in and around the hospital. They are not involved in the primary process of applying E = M in practice, but they play a key role in providing an enabling (financial) context in which the clinicians can implement the E = M.
Task 2: State implementation outcomes
To realize adoption, implementation and sustainability of the E = M in routine clinical care, together with involved stakeholders, we designed objectives for clinicians, department managers and stakeholders in the broader context, by specifying who and what will change due to the implementation strategies. Table 1 provides an overview of implementation outcomes for each actor group to facilitate successful implementation of E = M in routine clinical care.
Task 3: Select implementation strategies and justification
In Task 3 evidence-based and theoretically sound implementation strategies were selected. Table 2 shows the six themes of determinants (result of Task 1), their equivalent CFIR construct(s), selected implementation strategies based on the Strategy Matching Tool, and timing.
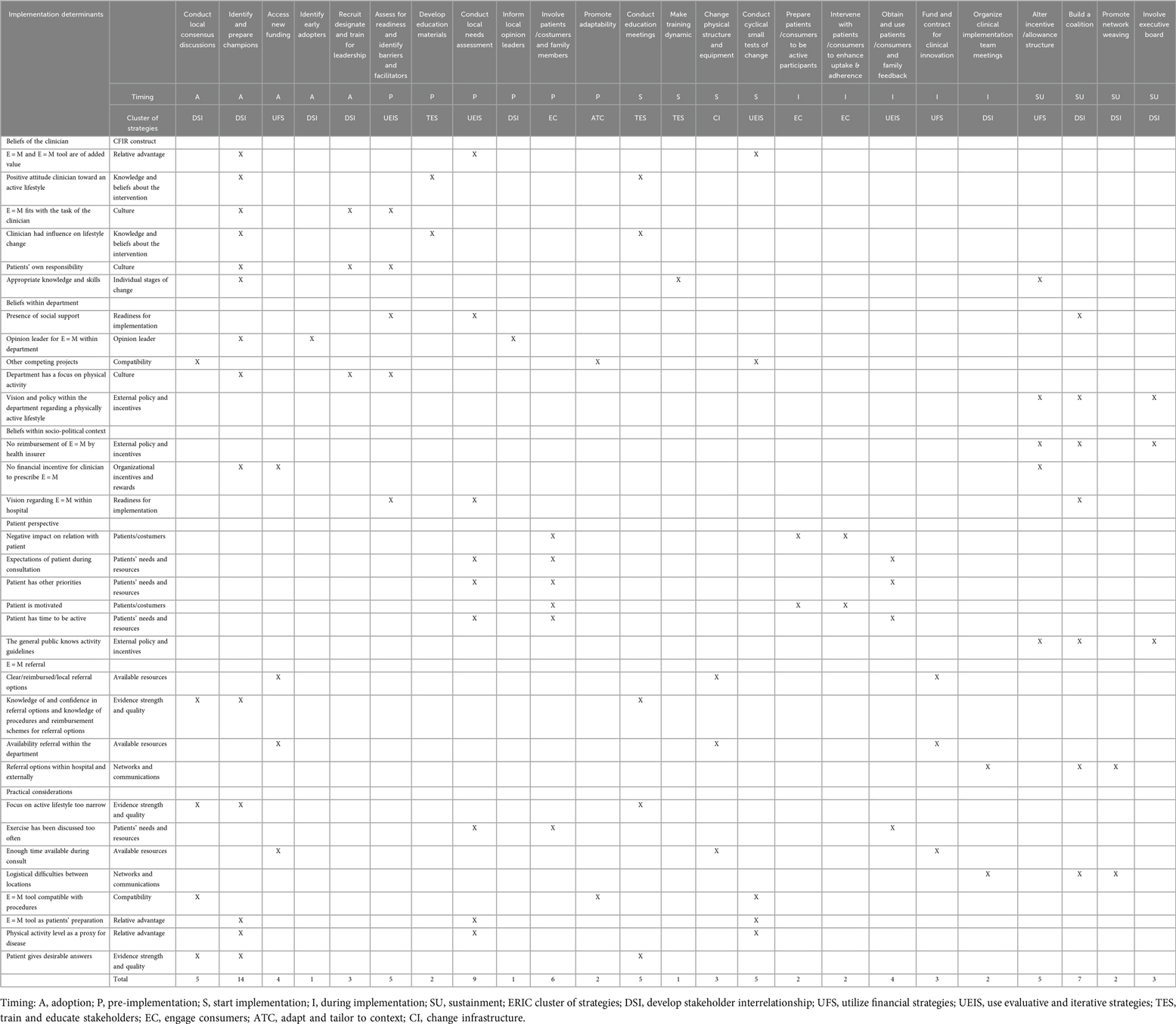
Table 2. Identified determinants matched with implementation strategies according to the timing in the implementation process (justification).
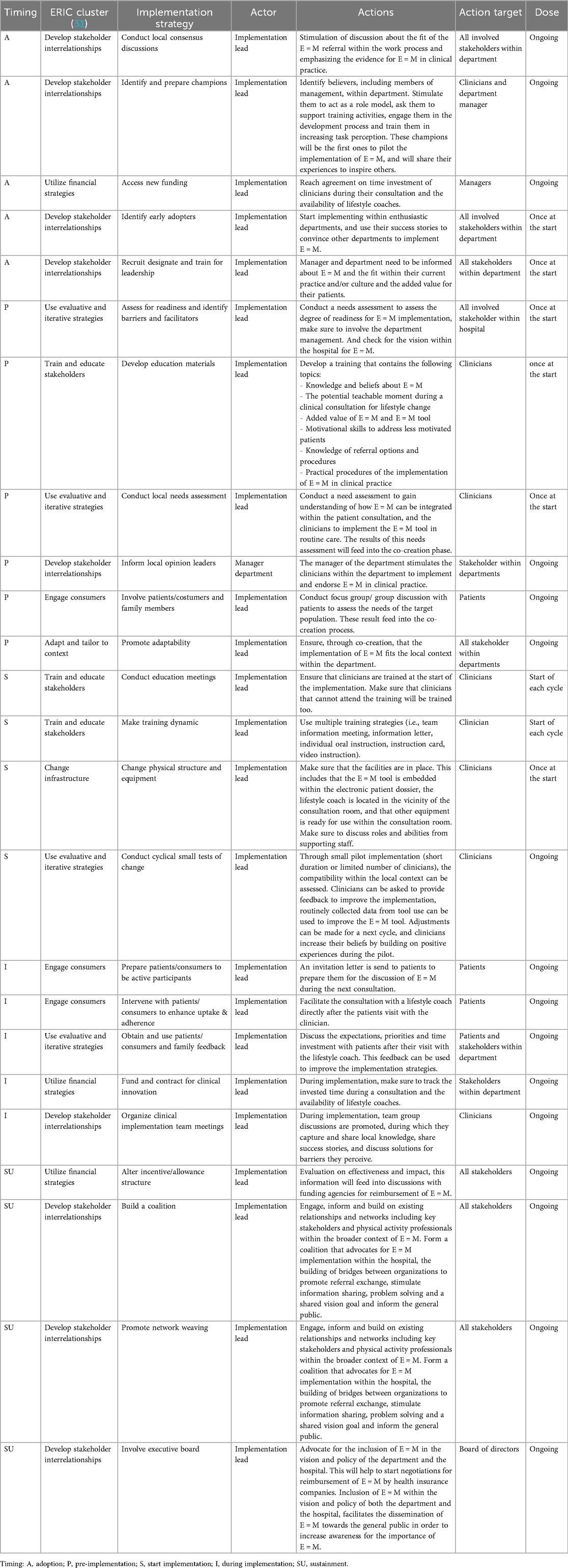
In total, 24 strategies were matched to 32 determinants. We identified 5 strategies for the adoption phase, 6 for the pre-implementation phase, 4 during the start of implementation, 5 for during implementation and 4 strategies aimed at the sustainability phase.
Most often the strategy Identify and prepare champions was matched to address determinants (n = 14, 44%), followed by Conduct local needs assessment (n = 9, 28%). All clusters of strategies were represented, except for the Support & provide assistance clusters.
Task 4: Produce protocol for implementation and materials
Guided by Table 2 of Task 3, interview data of Task 1 and by input from our consortium, we translated the theoretical strategies into practical applications included in the implementation protocol (see Table 3).
We operationalized actions needed in the adoption phase to facilitate momentum for the implementation of E = M, such as stimulating discussion, identifying champions, and discussing the added value. During the pre-implementation phase, readiness and needs among clinicians and patients were assessed to tailor the tool for individually tailored prescription of PA for patients to the local context. Furthermore, a training was developed to support clinicians in using the E = M tool, as well as the other strategies. At the start of the implementation process, the training was delivered, all facilities were set to start, and a plan for evaluation was developed.
During implementation, strategies to engage patients were implemented, as well as clinical implementation team meetings to discuss the successes and to address potential barriers.
Lastly, strategies were operationalized to support implementation on the long term (i.e., sustainability) by network weaving, and advocacy for reimbursement by the health insurance company.
Although no strategies of the Support & provide assistance clusters were matched to determinants, we included these in our implementation protocol in the form of appointing a local implementation lead (i.e., formally appoint internal implementation leader responsible for the coordination of the implementation process), as the need for such support was often mentioned during stakeholders interviews and by members of our consortium.
Actors for the designed implementation strategies were mainly the implementation lead, and the managers of implementing departments targeting their actions towards clinicians, managers, patients, other stakeholders involved in the broader context and the board of directors.
Furthermore, our needs assessment highlighted the necessity for lifestyle coaches appointed at the implementing departments and/or hospitals. Lifestyle coaches are health care professionals working in the outpatient clinic, closely collaborating with clinicians. They qualify for lifestyle counselling and give eligible patients one-off consultations aimed at knowledge transfer, improve patients' motivation, and regional referral options.
Task 5: Evaluation plan
According to the RE-AIM framework (32), we conducted short cyclical tests to evaluate the feasibility (i.e., reach, effectiveness, adoption, implementation and sustainability) of the implementation protocol in 5 pilots in UMCG between October 2019 and October 2020. A full description of the results is described in detail elsewhere (33). The same departments were also participating departments in the Implementation Mapping tasks, therefore, many adoption strategies were not evaluated during the pilot phase, as they were already applied during the co-creation phase. After the co-creation process, 5 strategies were piloted in the Departments of Orthopedics and Rehabilitation Medicine of UMCG, the Netherlands. The implementation lead was a researcher (AB), who was responsible for (1) educating clinicians on E = M, (2) incorporating the E = M tool in the Electronic Medical Records, (3) appointing of lifestyle coaches, (4) generating an overview of local E = M referral options, and (5) providing support to implementing teams. Through short cyclical evaluation, each implementation informed adaptations that were put into practice in the next implementation cycle. In total, 12 clinicians of 2 departments participated in 5 pilots. During the pilots, 210 patients were invited for participation and clinicians reported that they discussed the tailored advice with their patients in 47% (Orthopedics) to 62% (Rehabilitation Medicine) of their consultations.
Interview data of the process evaluation showed that clinicians particularly valued three of the five main implementation strategies: namely education on E = M, the lifestyle coach appointed within the department, and the presence of an implementation lead. While the E = M tool in the Electronic Medical Records was appreciated, clinicians identified several barriers that hindered its effective use in practice. Additionally, the overview of local E = M providers was rarely used by clinicians. Based on the evaluation, deployment of a lifestyle coach within a department, and implementation lead deemed essential. Clinicians expressed a need to structurally embed lifestyle coaches in the hospital, with especially knowledge about local E = M providers.
Discussion
The aim of this study was to apply Implementation Mapping to develop an implementation protocol, existing of a tailored set of strategies to support the individually tailored PA prescription in hospital care. In total, 24 strategies were matched to 32 determinants, and operationalized into an implementation protocol evaluated during a pilot study.
Selecting the most appropriate implementation strategies is a complex and challenging task. The literature highlights several issues in selecting these strategies, including the limited use of theory to guide this process (34, 35). As a result, implementation efforts often face difficulties in achieving desired outcomes. Therefore, our matching process was guided by expert-based knowledge incorporated in the CFIR matching tool (31). This resulted in a multifaceted strategy implementation protocol, in which the top 3 ranked strategies addressed one determinant. Still, at the same time, one strategy addressed multiple determinants, such as the strategy Identify and prepare champions that matched to 14 determinants. Others have used other approaches to use the matching tool, such as combining all determinants together and then select the top 10 strategies (36). We combined this matching result in the operationalization with solutions provided by our stakeholders; solutions they perceived to be successful in previous implementation efforts. Although this was a step-by-step approach combining multiple inputs, our evaluation showed that not all strategies were perceived as useful. It might well be that the CFIR matching tool relies too much on experts' opinions and not on empirical evidence, or our stakeholders did not provide us with the best solutions to address the determinants.
This is related to the fact that there is currently an insufficient understanding of the determinants and mechanisms that drive successful implementation (35). Although efforts are made to describe mechanisms of strategies, this is hindered by the fact that many implementation strategies are implemented simultaneously, resulting in a complex system of intertwined mechanisms. More need for empirical research into strategy mechanisms is needed to further unravel these underlying pathways, as such, insights from the system science field are introduced in the implementation science field to further unravel these pathways to implementation success (37, 38).
One other pitfall observed in previous studies is the tendency of implementers to rely on a narrow set of strategies—such as educational workshops—without critically assessing their appropriateness for the specific context. This pragmatic “one-size-fits-all” approach can lead to ineffective outcomes. For example, in a review of 20 studies, Bosch and colleagues (39) found that attempts to match implementation strategies to identified barriers often resulted in a theoretical mismatch, such as applying clinician-focused strategies to address organizational-level barriers. In our study, we used the expert derived CFIR-matching tool, complemented with stakeholders input to match strategies to determinants, in which we did not solely focus on one strategy. Still, we developed a multifaced strategy for the different implementation phases to overcome this problem. By incorporating opinions of several stakeholders with our strategies (i.e., clinicians, managers, broader stakeholders and patient representatives), we aimed to support implementation on different levels. However, during our evaluation phase, we could only conduct a small pilot in two departments of one hospital, resulting in implementing strategies mainly focused on the clinicians. Therefore, we do not yet know if the other strategies targeting other levels are successful.
Lastly, to effectively implement strategies, it is crucial to operationalize them in a way tailored to the specific context. Tailoring, which involves an iterative process of selecting and adapting strategies, has proven to be an effective method for addressing local determinants (40). The key mechanisms linking tailoring to improved implementation outcomes include raising awareness of relevant issues, building stakeholder consensus, obtaining buy-in or acceptance, and ensuring greater alignment between the context and the chosen implementation strategies (41). Decisions made during the prioritization of determinants are often based on stakeholder perceptions of the modifiability and importance of these determinants, while strategy selection is typically guided by the perceived feasibility and impact of the strategies (41, 42). In our pilot study, feasibility was a main driver for decision making in the tailoring process. This implied that not all strategies were implemented during the pilot phase. As the pilot departments were already part of the co-creation process, the researcher was the implementation lead, and strategies were mainly focused on the clinicians, and not on the other stakeholders, such as management and broader context. Building on the pilot experiences, we have developed a website in which all the strategies and tools are presented (43). This allows other “new” hospitals considering implementing individually tailored prescription of PA in clinical care to follow the protocol to design their own E = M process, whilst building on the experiences and knowledge of our departments that have gone through this development process.
Strengths and limitations
This study has several strengths. First, the study follows a systematic approach to develop an implementation protocol, ensuring that the selected strategies are grounded in theory and practice. A second strength is the use of a matching tool, which has been central to the study's methodology. Given the current stage of the field, this tool remains the best available method for matching strategies to determinants. Lastly, multiple data sources were used, including interviews, input from different departments, and insights gained during consortium meetings, providing a rich and diverse perspective to inform the implementation protocol.
Several limitations need to be considered. First, the generalizability of the findings to other departments or hospitals is limited, as the study primarily focused on the Dutch academic clinical setting, and departments that already focused on PA. Yet, the already matched list of potential strategies described in our manuscript in Table 2 can serve as a starting point for selection of strategies, that match local barriers. Other hospitals can then adapt these to their local contexts, and use our RE-AIM evaluation protocol to evaluate implementation processes.
Another limitation lies in the process of matching our barriers and facilitators to CFIR constructs. Many of the terms used by our stakeholders (e.g., during interviews or consortium meetings) are framed in practical, everyday language, which we may have misclassified to CFIR constructs. However, during the operationalization, we aligned all tasks with our consortium members and patient representatives to avoid a mismatch of our strategies.
Conclusions
This study illustrates the application of Implementation Mapping to design an implementation protocol to support and guide prescription of E = M by clinicians, using strong stakeholder participation in the development process. Implementation Mapping proved to help develop the protocol with our stakeholders. As such our stepwise development of the implementation protocol can serve as an example for researchers or practitioners preparing for E = M implementation.
Data availability statement
The datasets presented in this article are not readily available because this would likely compromise participants' anonymity. Some descriptive data may be available from the corresponding author on reasonable request. Requests to access the datasets should be directed toZi52YW5uYXNzYXVAYW1zdGVyZGFtdW1jLm5s.
Ethics statement
This study involved human participants. The medical ethical committees of the UMCG and Amsterdam UMC approved the study design (METc UMCG 2017/517 and Amsterdam UMC 2018/219). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FN: Conceptualization, Investigation, Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. JN: Conceptualization, Investigation, Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. AB: Conceptualization, Investigation, Data curation, Formal analysis, Writing – review & editing. LK: Supervision, Project administration, Conceptualization, Writing – review & editing. IA-S: Writing – review & editing, Supervision. RDi: Writing – review & editing. JJ: Writing – review & editing. HL: Writing – review & editing. MS: Writing – review & editing. ST: Conceptualization, Writing – review & editing. WM: Funding acquisition, Writing – review & editing, Supervision. JZ: Supervision, Writing – review & editing, Funding acquisition. EV: Funding acquisition, Writing – review & editing, Supervision. LW: Funding acquisition, Writing – review & editing, Supervision. HK: Writing – review & editing, Supervision. HP: Conceptualization, Writing – review & editing, Supervision. RDe: Supervision, Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by a grant from ZonMw (project number 546001002).
Acknowledgments
We would like to thank the following interns for their support during the various phases of the PIE = M project: Yvon Douma, Anouk Driessen, Äaron Spapens, Nanick van der Wal, and Kim Wolffenbuttel. And we would like to extend our gratitude toward all participating clinicians, department managers, lifestyle coaches, and patients from the departments of orthopedics and rehabilitation medicine at University Medical Center Groningen and Rehabilitation Medicine at Amsterdam University Medical Center.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kohl HW 3rd, Craig CL, Lambert EV, Inoue S, Alkandari JR, Leetongin G, et al. The pandemic of physical inactivity: global action for public health. Lancet. (2012) 380(9838):294–305. doi: 10.1016/S0140-6736(12)60898-8
2. Andersen LB, Mota J, Di Pietro L. Update on the global pandemic of physical inactivity. Lancet. (2016) 388(10051):1255–6. doi: 10.1016/S0140-6736(16)30960-6
3. Andersen LB. WHO Guidelines approved by the guidelines review committee. In: WHO Steering Group, editor. WHO Guidelines on Physical Activity and Sedentary Behaviour. Geneva: World Health Organization (2020). p. 32.
4. Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. (2011) 377(9783):2115–26. doi: 10.1016/S0140-6736(11)60243-2
5. Hawker GA. Osteoarthritis is a serious disease. Clin Exp Rheumatol. (2019) 37 Suppl 120(5):3–6.31621562
6. Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. (2012) 380(9838):219–29. doi: 10.1016/S0140-6736(12)61031-9
7. Noetel M, Sanders T, Gallardo-Gómez D, Taylor P, Del Pozo Cruz B, van den Hoek D, et al. Effect of exercise for depression: systematic review and network meta-analysis of randomised controlled trials. BMJ. (2024) 384:e075847. doi: 10.1136/bmj-2023-075847
8. Ekelund U, Steene-Johannessen J, Brown WJ, Fagerland MW, Owen N, Powell KE, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet. (2016) 388(10051):1302–10. doi: 10.1016/S0140-6736(16)30370-1
9. Marks-Vieveen JM, Uijtdewilligen L, Motazedi E, Stijnman DPM, van den Akker-Scheek I, Bouma AJ, et al. Physical activity levels, correlates, and all-cause mortality risk in people living with different health conditions. J Phys Act Health. (2024) 21(4):394–404. doi: 10.1123/jpah.2023-0387
10. Pedersen BK, Saltin B. Exercise as medicine—evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. (2015) 25 Suppl 3:1–72. doi: 10.1111/sms.12581
11. Saqib ZA, Dai J, Menhas R, Mahmood S, Karim M, Sang X, et al. Physical activity is a medicine for non-communicable diseases: a survey study regarding the perception of physical activity impact on health wellbeing. Risk Manag Healthc Policy. (2020) 13:2949–62. doi: 10.2147/RMHP.S280339
12. Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. (2017) 32(5):541–56. doi: 10.1097/HCO.0000000000000437
13. Cowan RE. Exercise is medicine initiative: physical activity as a vital sign and prescription in adult rehabilitation practice. Arch Phys Med Rehabil. (2016) 97(9 Suppl):S232–7. doi: 10.1016/j.apmr.2016.01.040
14. Exercise is Medicine (2025). Available online at: https://www.exerciseismedicine.org/ (Accessed September 14, 2025).
15. Roempler J, Petzold MB, Bendau A, Plag J, Ströhle A. Tracking changes in physical activity during inpatient treatment in a psychiatric clinic in Germany by asking two simple questions. Eur Arch Psychiatry Clin Neurosci. (2023) 273(4):983–94. doi: 10.1007/s00406-023-01565-2
16. Moving Medicine UK (2025). Available online at: https://movingmedicine.ac.uk/ (Accessed September 14, 2025).
17. Bowen PG, Mankowski RT, Harper SA, Buford TW. Exercise is medicine as a vital sign: challenges and opportunities. Transl J Am Coll Sports Med. (2019) 4(1):1–7.30828640
18. Nauta J, van Nassau F, Bouma AJ, Krops LA, van der Ploeg HP, Verhagen E, et al. Facilitators and barriers for the implementation of exercise are medicine in routine clinical care in Dutch university medical centres: a mixed methodology study on clinicians’ perceptions. BMJ Open. (2022) 12(3):e052920. doi: 10.1136/bmjopen-2021-052920
19. Bouma S, Stevens M, van der Woude L, Diercks R, van den Akker-Scheek I. Barriers and facilitators for implementing lifestyle-related treatment modalities in osteoarthritis: a cross-sectional study among primary and secondary healthcare professionals. Health Policy. (2023) 136:104898. doi: 10.1016/j.healthpol.2023.104898
20. Hoekstra F, Hettinga FJ, den Breejen M, Duijf M, van der Woude LHV, Dekker R, et al. Professionals’ perceptions of factors affecting implementation and continuation of a physical activity promotion programme in rehabilitation: a qualitative study. J Rehabil Med. (2017) 49(5):385–94. doi: 10.2340/16501977-2220
21. Te Loo LM, Holla JFM, Vrijsen J, Driessen A, van Dijk ML, Linders L, et al. Implementation barriers and facilitators for referral from the hospital to community-based lifestyle interventions from the perspective of lifestyle professionals: a qualitative study. PLoS One. (2024) 19(6):e0304053. doi: 10.1371/journal.pone.0304053
22. Bouma A, van Nassau F, Nauta J, Krops L, van der Ploeg H, Verhagen E, et al. Implementing exercise=medicine in routine clinical care; needs for an online tool and key decisions for implementation of exercise=medicine within two Dutch academic hospitals. BMC Med Inform Decis Mak. (2022) 22(1):250. doi: 10.1186/s12911-022-01993-5
23. Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, et al. A refined compilation of implementation strategies: results from the expert recommendations for implementing change (ERIC) project. Implement Sci. (2015) 10:21. doi: 10.1186/s13012-015-0209-1
24. Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci. (2013) 8:139. doi: 10.1186/1748-5908-8-139
25. Fernandez ME, Ten Hoor GA, van Lieshout S, Rodriguez SA, Beidas RS, Parcel G, et al. Implementation mapping: using intervention mapping to develop implementation strategies. Front Public Health. (2019) 7:158. doi: 10.3389/fpubh.2019.00158
26. Krops LA, Bouma AJ, Van Nassau F, Nauta J, van den Akker-Scheek I, Bossers WJ, et al. Implementing individually tailored prescription of physical activity in routine clinical care: protocol of the physicians implement exercise=medicine (PIE=M) development and implementation project. JMIR Res Protoc. (2020) 9(11):e19397. doi: 10.2196/19397
27. Peels DA, Boekhout JM, van Nassau F, Lechner L, Bolman CAW, Berendsen BAJ. Promoting the implementation of a computer-tailored physical activity intervention: development and feasibility testing of an implementation intervention. Implement Sci Commun. (2024) 5(1):90. doi: 10.1186/s43058-024-00622-8
28. Jenniskens K, Rasing S, Popma A, Creemers D, Ghalit C, van Vuuren L, et al. Development of an implementation plan for a school-based multimodal approach for depression and suicide prevention in adolescents. Front Public Health. (2024) 12:1386031. doi: 10.3389/fpubh.2024.1386031
29. Kennedy MA, Bayes S, Newton RU, Zissiadis Y, Spry NA, Taaffe DR, et al. We have the program, what now? Development of an implementation plan to bridge the research-practice gap prevalent in exercise oncology. Int J Behav Nutr Phys Act. (2020) 17(1):128. doi: 10.1186/s12966-020-01032-4
30. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. (2009) 4:50. doi: 10.1186/1748-5908-4-50
31. Waltz TJ, Powell BJ, Fernández ME, Abadie B, Damschroder LJ. Choosing implementation strategies to address contextual barriers: diversity in recommendations and future directions. Implement Sci. (2019) 14(1):42. doi: 10.1186/s13012-019-0892-4
32. Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. (1999) 89(9):1322–7. doi: 10.2105/AJPH.89.9.1322
33. Bouma AJ, Nauta J, van Nassau F, Krops LA, van den Akker-Scheek I, Diercks RL, et al. Implementing individually tailored prescription of physical activity in routine clinical care: a process evaluation of the physicians implement exercise=medicine project. J Phys Act Health. (2024) 21(9):916–27. doi: 10.1123/jpah.2023-0625
34. Powell BJ, Beidas RS, Lewis CC, Aarons GA, McMillen JC, Proctor EK, et al. Methods to improve the selection and tailoring of implementation strategies. J Behav Health Serv Res. (2017) 44(2):177–94. doi: 10.1007/s11414-015-9475-6
35. Lewis CC, Klasnja P, Powell BJ, Lyon AR, Tuzzio L, Jones S, et al. From classification to causality: advancing understanding of mechanisms of change in implementation science. Front Public Health. (2018) 6:136. doi: 10.3389/fpubh.2018.00136
36. Bouma S, van den Akker-Scheek I, Schiphof D, van der Woude L, Diercks R, Stevens M. Implementing lifestyle-related treatment modalities in osteoarthritis care: identification of implementation strategies using the consolidated framework for implementation research-expert recommendations for implementing change matching tool. Musculoskeletal Care. (2023) 21(4):1125–34. doi: 10.1002/msc.1791
37. Northridge ME, Metcalf SS. Enhancing implementation science by applying best principles of systems science. Health Res Policy Syst. (2016) 14(1):74. doi: 10.1186/s12961-016-0146-8
38. Whelan J, Fraser P, Bolton KA, Love P, Strugnell C, Boelsen-Robinson T, et al. Combining systems thinking approaches and implementation science constructs within community-based prevention: a systematic review. Health Res Policy Syst. (2023) 21(1):85. doi: 10.1186/s12961-023-01023-4
39. Bosch M, van der Weijden T, Wensing M, Grol R. Tailoring quality improvement interventions to identified barriers: a multiple case analysis. J Eval Clin Pract. (2007) 13(2):161–8. doi: 10.1111/j.1365-2753.2006.00660.x
40. Baker R, Camosso-Stefinovic J, Gillies C, Shaw EJ, Cheater F, Flottorp S, et al. Tailored interventions to address determinants of practice. Cochrane Database Syst Rev. (2015) 2015(4):Cd005470. doi: 10.1002/14651858.CD005470.pub3
41. McHugh SM, Riordan F, Curran GM, Lewis CC, Wolfenden L, Presseau J, et al. Conceptual tensions and practical trade-offs in tailoring implementation interventions. Front Health Serv. (2022) 2:974095. doi: 10.3389/frhs.2022.974095
42. Proctor EK, Landsverk J, Aarons G, Chambers D, Glisson C, Mittman B. Implementation research in mental health services: an emerging science with conceptual, methodological, and training challenges. Adm Policy Ment Health. (2009) 36(1):24–34. doi: 10.1007/s10488-008-0197-4
43. Website with tools for E = M (2025). Available online at: https://www.specialheroes.nl/zorg/beweegstappenplan/ (Accessed September 14, 2025).
Keywords: implementation, strategies, exercise is medicine, exercise, physical activity, clinical practice, patients, prevention
Citation: van Nassau F, Nauta J, Bouma AJ, Krops LA, van den Akker- Scheek I, Diercks RL, de Jong J, Leutscher H, Stevens M, van Twillert S, van Mechelen W, Zwerver J, Verhagen EALM, van der Woude LHV, van Keeken H, van der Ploeg HP and Dekker R (2025) Stepwise development of an implementation protocol to support the prescription of Exercise = Medicine by clinicians using the Implementation Mapping approach. Front. Health Serv. 5:1645456. doi: 10.3389/frhs.2025.1645456
Received: 11 June 2025; Accepted: 24 September 2025;
Published: 27 October 2025.
Edited by:
Obasanjo Bolarinwa, York St John University, United KingdomReviewed by:
Oleksandr Nazarchuk, National Pirogov Memorial Medical University, UkraineMoritz Bruno Petzold, Medical School Berlin, Germany
Copyright: © 2025 van Nassau, Nauta, Bouma, Krops, van den Akker- Scheek, Diercks, de Jong, Leutscher, Stevens, van Twillert, van Mechelen, Zwerver, Verhagen, van der Woude, van Keeken, van der Ploeg and Dekker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: F. van Nassau, Zi52YW5uYXNzYXVAYW1zdGVyZGFtdW1jLm5s
 F. van Nassau
F. van Nassau J. Nauta
J. Nauta A. J. Bouma2,3,4
A. J. Bouma2,3,4 J. de Jong
J. de Jong E. A. L. M. Verhagen
E. A. L. M. Verhagen L. H. V. van der Woude
L. H. V. van der Woude H. van Keeken
H. van Keeken R. Dekker
R. Dekker