- 1Department of Horticulture Science, Faculty of Agriculture, University of Zanjan, Zanjan, Iran
- 2Department of Plant Tissue Culture, Fartak Oxin Baft Knowledge Based Company, Karaj, Iran
- 3Department of Horticultural Science and Landscape Architecture, Faculty of Agriculture, Ferdowsi University of Mashhad, Mashhad, Iran
- 4Department of Genetic Engineering and Biosafety, Agricultural Biotechnology Research Institute of Iran (ABRII), Agricultural Research, Education and Extension Organization (AREEO), Karaj, Iran
- 5Ornamental Plants Research Center, Horticulture Research Institute, Agricultural Research, Education and Extension Organization (AREEO), Mahallat, Iran
Gladiolus is an important and economically valued ornamental plant grown worldwide. One of the major challenges in its micropropagation is maintaining genetic stability during indirect regeneration and long-term callus maintenance. The objective of this study was to develop an optimized indirect shoot regeneration protocol for three commercial Gladiolus cultivars with consistent genetic traits. Callus initiated from the basal part of extended mother corm sprout (EMCS) explants in MS medium supplemented with 2 mgL-1 2,4-D, 2 mgL-1 NAA and 1 mgL-1 BAP. The synthesis of phenolic compounds was effectively controlled by the addition of 150 mgL-1 ascorbic acid, 100 mgL-1 citric acid, and 500 mgL-1 activated charcoal. This medium led to an 80% decrease in the accumulation of phenolic compounds across all cultivars in comparison to the control. For shoot regeneration, calli which were maintained over the long term were transferred to MS medium supplemented with 2 mgL-1 BAP, 2 mgL-1 Kin and 0.25 mgL-1 NAA. This significantly enhanced shoot regeneration percentage (95.55%) and number (39.44 shoots per explant). Additionally, cormel formation was significantly enhanced (16.66 cormels per explant) at the base of regenerated plantlets using MS medium containing 9% sucrose and 2 mgL-1 indole-3-acetic acid, without any cormel formation in the control. Cormels were effectively acclimatized in the greenhouse with 100% survival rate. To demonstrate genetic stability, regenerated plantlets were evaluated by flow cytometry and Inter Simple Sequence Repeat (ISSR) markers, verifying their genetic identification with the mother plants. This study provides a reliable and scalable protocol for the commercial micropropagation of gladiolus, with promising applications in breeding programs that aim at transferring desirable traits such as disease resistance or specific floral features.
1 Introduction
The gladiolus (Gladiolus grandiflorus L.), a well-known ornamental plant in the Iridaceae family, is prized for its attractive flower spikes and remarkable vase life. Gladiolus is a cross-pollinated, diploid species (2n = 2x = 30) (Azimi, 2020), with significant economic value evidenced by Flora Holland auctions, which generate 9 million euros annually (Sharma and Tripathi, 2008; Ramos-Garci et al., 2009; Azadi et al., 2016 The plant is known as the “queen of bulbous flowers” because of its tall, colorful flower stems, which come in a range of colors, including pink, red, purple, white, cream, and orange, as well as different flower shapes (Singh et al., 2018).
In their natural environment, gladiolus plants primarily reproduce through corms and cormels. Breeders often use seeds after hybridization to create new and improved cultivars (Swaroop et al., 2018). Despite their effectiveness, both approaches come with challenges, such as slow propagation rates, vulnerability to diseases, and limited genetic variation. Tissue culture has emerged as an effective method for the mass propagation and large-scale commercial production of ornamental plants, offering a promising solution to overcome existing limitations (Jaryal et al, 2025; Ntui et al., 2010; Kumar and Nandi, 2015; Nalousi et al., 2019);. Both direct and indirect organogenesis have been investigated for effective micropropagation (Sinha and Roy, 2002; Wu et al., 2015; Kumar et al., 2024a). Indirect regeneration yet, has demonstrated advantages for both propagation and as a foundation for genetic engineering (Wu et al., 2015). A significant problem associated with indirect regeneration is the risk of somaclonal variation, which may result in genetic instability in the regenerated plants (Endemann et al., 2001; Ferreira et al., 2023; Krishna et al., 2016; Long et al., 2022).
Several factors contribute to this instability, including explant source (Dey et al., 2015), wounding during the culture process (Debnath and Ghosh, 2022), concentration of plant growth regulators (PGR) (Rakoczy-Trojanowska, 2002; Rai, 2021), in vitro environmental conditions (Polanco and Ruiz, 2002; Ferreira et al., 2023; Joshi et al., 2024), prolonged culture durations (Sun et al., 2013), and oxidative stress caused by free radicals (Krishna et al., 2016; Smulders and de Klerk, 2011). To mitigate these issues, reliable genetic stability evaluation methods are critical. Flow cytometry (FCM) is a rapid and efficient technique for evaluating ploidy levels in plants (Ochatt et al., 2011; Zafar et al., 2019). In addition, molecular markers such as Random Amplified Polymorphic DNA (RAPD), Simple Sequence Repeats (SSR), and Inter Simple Sequence Repeats (ISSR) are commonly used to confirm genetic stability in regenerated plants (Dey et al., 2015; Nalousi et al., 2019; Singh et al., 2017). Of these, ISSR markers are distinguished for their high reproducibility and the ability to detect genetic variation without requiring species-specific primers (Faisal et al., 2014).
While significant progress has been made, comprehensive studies addressing the combination of long-term callus maintenance, high regeneration efficiency, and robust genetic stability assessment in gladiolus are insufficient. This study aims to fill this gap by developing an optimized protocol for indirect organogenesis using elongated mother corm sprout (EMCS) explants from three popular Gladiolus cultivars ‘Rose Supreme’, ‘Amsterdam’, and ‘Advance Red’ known for their vibrant colors (pink, white, and red). For the first time, the genetic stability of regenerated plants was evaluated using both FCM and ISSR markers, ensuring the reliability of the proposed protocol.
The optimized protocol offers numerous advantages, including the ability to maintain callus cultures for extended periods, achieve high shoot regeneration rates, and efficiently produce cormels. These attributes make the method suitable for both commercial micropropagation and genetic engineering applications aimed at enhancing disease resistance and other desirable traits in gladiolus.
Gladiolus (Gladiolus grandiflorus) is a cross-pollinating and diploid (2n = 2x = 30) plant (Azimi, 2020).
2 Materials and methods
2.1 Plant materials and surface sterilization procedures
Gladiolus corms of three commercial cultivars including ‘Rose Supreme’ (RS), ‘Amsterdam’ (A), and ‘Advance Red’ (AR) were obtained from the research greenhouse of the Iranian Floriculture and Ornamental Plants Research Institute (Latitude: 33° 53’ N, Longitude: 50° 29’ E; altitude: 1,732 m; average temperature: 23.4°C; relative humidity: 57.1%). Uniform mother corm sprouts (3–3.5 cm diameter) were selected as explant sources. For sterilization, the corms’ surface scales were removed, and sprouts were divided into 1 cm² explants (Figure 1a). The explants were first washed under running tap water for 30 min with a mild detergent, followed by a 45-min heat treatment at 45°C in a water bath to eliminate internal contaminants. Surface disinfection was then performed by immersion in 70% (v/v) ethanol (1 min) and 1.5% (w/v) sodium hypochlorite (10 min). Finally, the explants were rinsed four times with sterile distilled water in a laminar airflow cabinet and cultured on MS medium supplemented with 0.5 mg L1 BAP for establishment.

Figure 1. Flow diagram of indirect regeneration of Gladiolus grandifloras ‘Advance red’ from Elongated Mother Corm Sprout (EMCS) explants; (a) Slices of corm sprouts explant, (b) Establishment of EMCS explants, (c) Callus induction in bottom of the EMCS explants (in callus induction medium), (d) Long-term maintenance of callus, (e) Early regeneration steps (in shoot induction medium), (f) Shoot regeneration (in shoot induction medium), (g) Development of shoot (in shoot induction medium), (h) Multiple regenerated shoots (in shoot Proliferation medium) , (i) Rooted plantlets (in root induction medium), (j) Cormel production (in cormel induction medium). Bar: 10 mm.
2.2 Callus induction
Two weeks after the sprouts growth, EMCS explants (with an average length 50 mm) were transferred to the Murashige and Skoog (1962) (MS) medium supplemented with various combinations of 2,4-D (0, 1, 2 and 3 mgL-1), NAA (0, 1, 2 and 3 mgL-1) and BAP (0, 1 and 2 mgL-1) (Table 1).
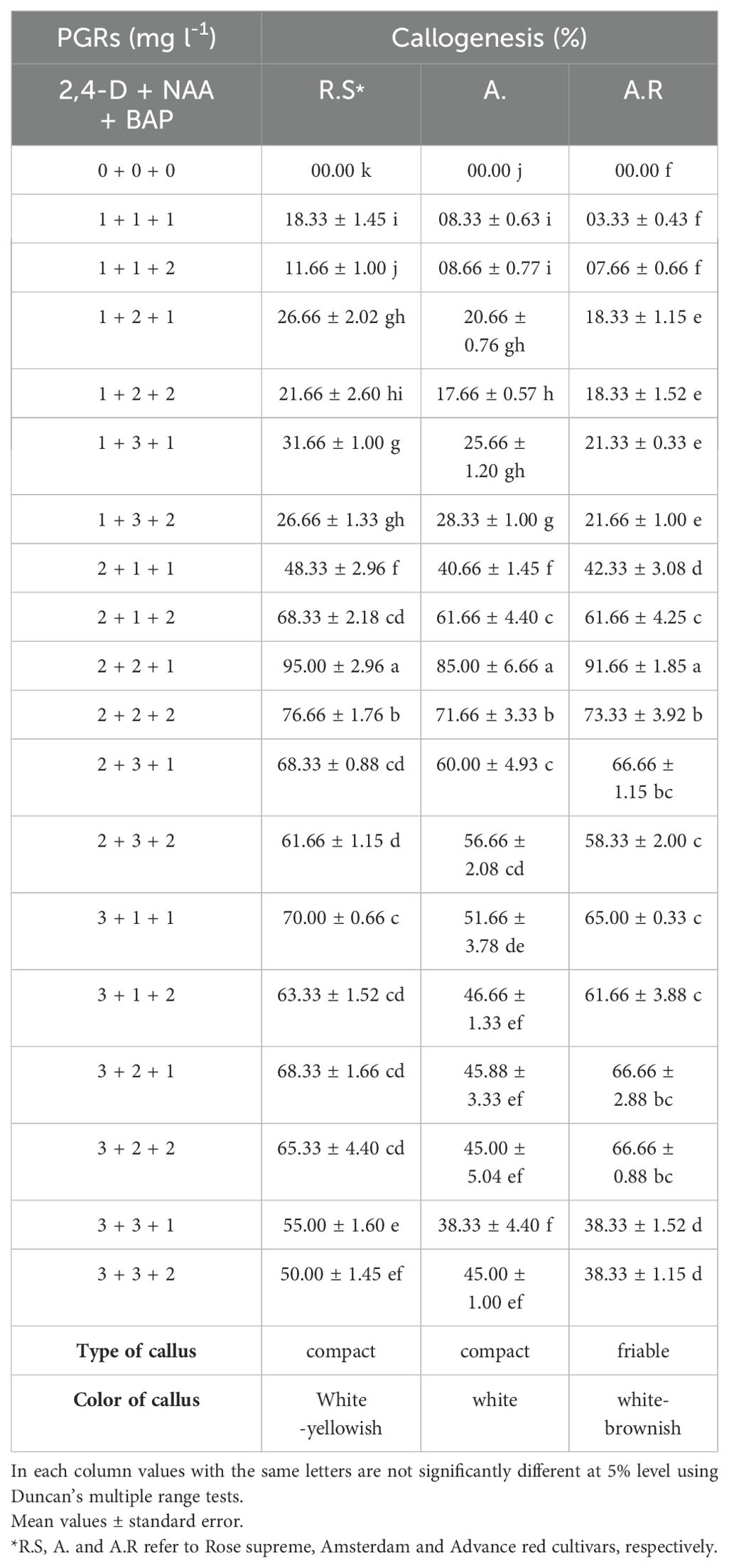
Table 1. Effects of PGR combinations on callogenesis of EMCS explants in three Gladiolus grandiflorus cultivars after three weeks.
2.3 Long-term maintenance of callus
The callus tissue was excised from the base of EMCS explants and subsequently transferred to MS medium supplemented with 0.5 mg L-1 of 2,4-D. This medium also contained various concentrations of ascorbic acid (0, 100, and 150 mg L-1), citric acid (0, 50, and 100 mg L-1), and activated charcoal (0, 500, and 1000 mg L-1) to reduce the production of phenolic compounds and facilitate long-term callus culture (Table 2).
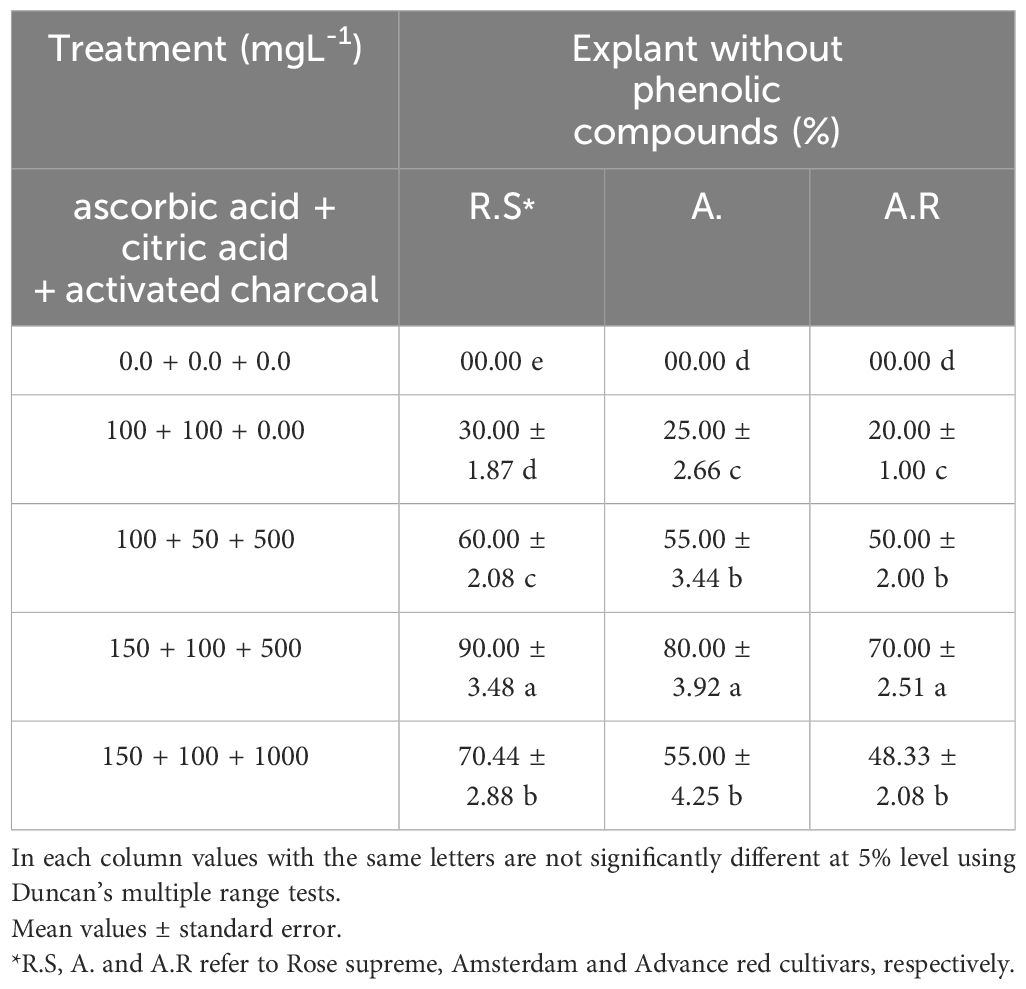
Table 2. Effects of various inhibitor combinations on phenolic compound reduction in three Gladiolus grandiflorus cultivars after 16 weeks.
2.4 Shoot regeneration of callus explants
In order to investigation the shoot induction potential from the long-term callus cultures (5 months), the callus was divided into equal pieces (2 cm2) and cultures on MS medium supplemented with various combinations of BAP (0, 1, 2 and 4 mgL-1), Kin (0, 1, 2 and 4 mgL-1) and NAA (0, 0.25 and 0.50 mgL-1) (Table 3).
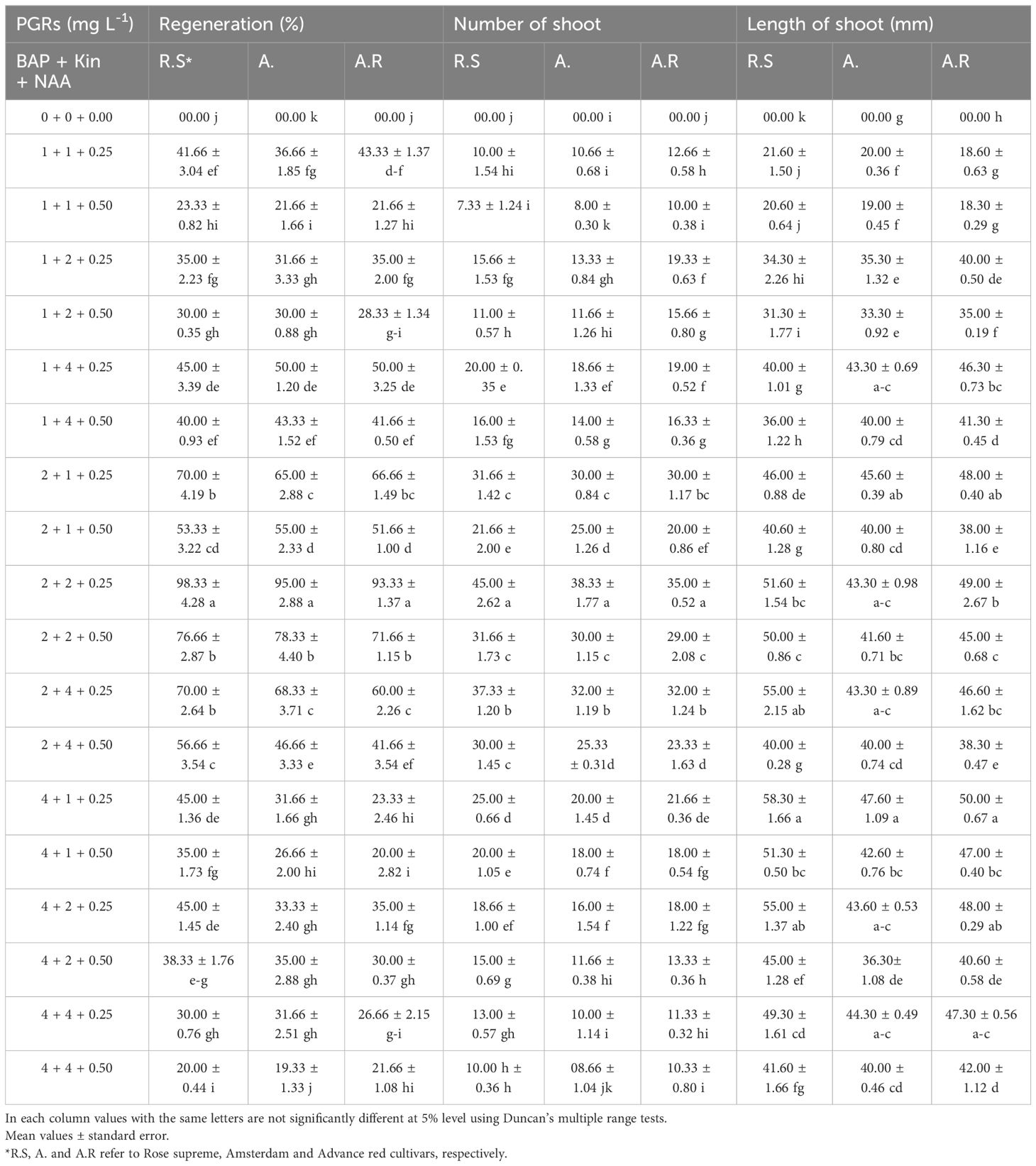
Table 3. The effects of PGRs combinations on plant regeneration from long term callus cultures in three Gladiolus grandiflorus cultivars after six weeks.
2.5 Formation of cormels at shoot base
Regenerated shoots were isolated and sub cultured on MS medium supplemented with 2 mgL-1 BAP and 0.25 mgL-1 NAA for shoot proliferation for six weeks. Proliferated shoots, averaging 60 mm in height were excised from the basal part and cultured on MS medium supplemented with various concentrations of sucrose (0, 3, 6 and 9%) and filter-sterilized IAA (0, 2 and 4 mgL-1) along with 1000 mgL-1 activated charcoal (Table 4) for 10 weeks.
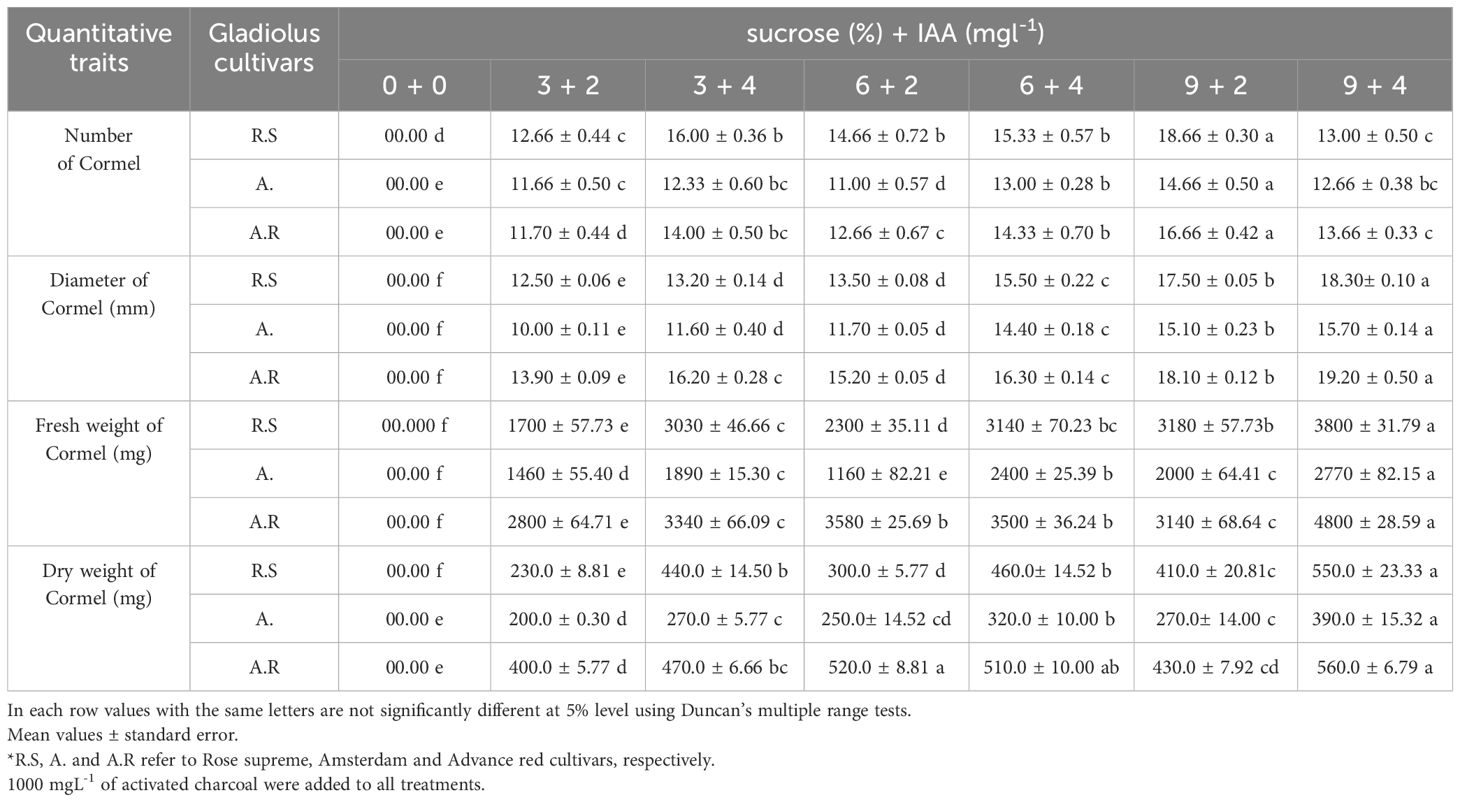
Table 4. Effects of sucrose and IAA concentrations on cormel formation in three Gladiolus grandiflorus cultivars after 10 weeks.
2.6 Culture medium and conditions
In all experiments (except cormel production), the MS medium supplemented with 3% (w/v) sucrose and 7 gL-1 agar. The pH of the medium was adjusted to 5.8 prior to autoclaving at 121°C and 1.5 kg cm-1 pressure for 15 min. The explants were sub cultured every two weeks and were kept under 16-h light photoperiod at 25 ± 2°C. All chemicals used in this study were purchased from Duchefa Biochemie (Haarlem, The Netherlands).
2.7 Germination of cormels
In order to break the dormancy of regenerated cormels, they were stored at a low temperature (4 ± 1°C) in complete darkness for two months. Matured cormels were then planted in a sterilized substrate consisting of 50% cocopeat and 50% perlite. They were maintained under plastic bags in controlled conditions with the bags removed after two weeks. The germinated cormels were subsequently transferred to larger pots containing a sterile medium of coco peat and perlite mixed with sand (1:1). These pots were kept in a shaded greenhouse at 28 ± 2°C and 60 ± 5% relative humidity) for two weeks.
2.8 Evaluation of genetic stability
2.8.1 Flow cytometry analysis
The Ploidy levels of leaves derived from mother plants and regenerated plants obtained through indirect shoot regeneration were assessed using ice-cold nuclei isolation buffer in flow cytometry (FCM) (Ebrahimzadeh et al., 2018). The mother plants served as control plants, and their nuclear DNA content were used as the reference comparison.
2.8.2 ISSR marker
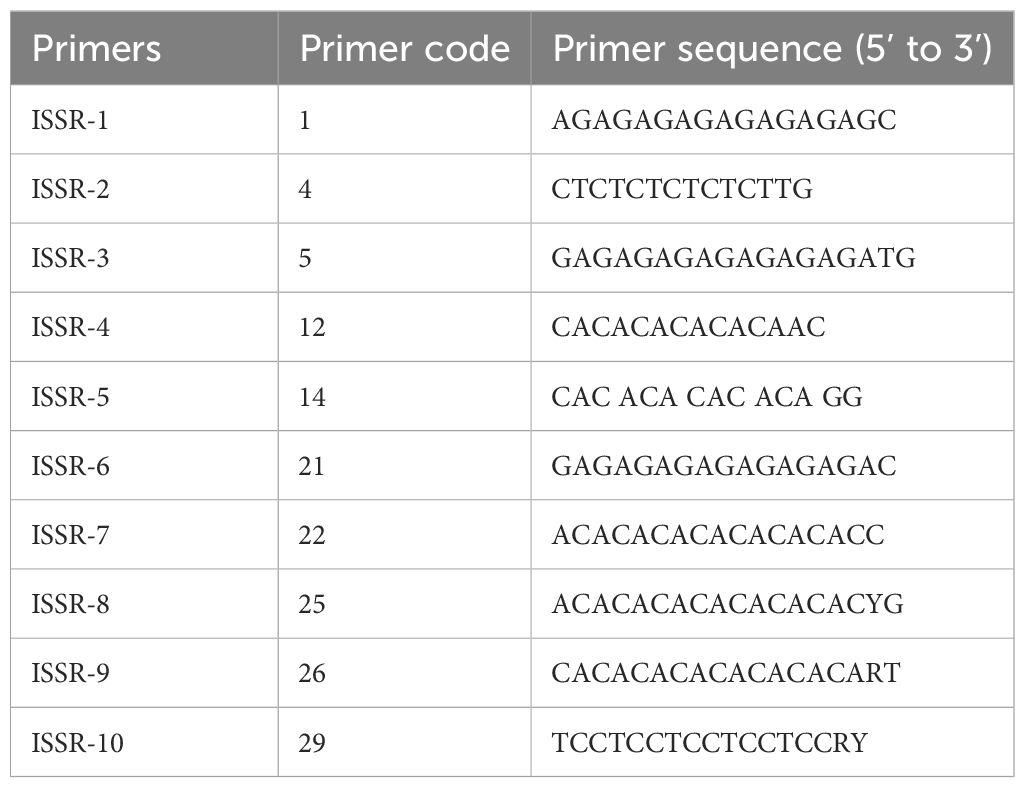
100 mg of young leaves from both regenerated cormels (3 plantlets of each cultivar) and mother corms were used for genomic DNA isolation. The modified CTAB method (Murray and Thompson, 1980) was applied as described by Azadi et al., 2010. Ten Inter Simple Sequence Repeat (ISSR) markers were used to evaluate the genetic stability of the regenerated comels (Table 5). PCR amplifications were performed using a master mix (Master Mix RED, Ampliqon, Denmark) in 25 μl reaction mixture. The reaction was initiated with DNA denaturation at 94°C for 5 minutes, followed by 35 cycles of amplification (94°C for 30 s, 55°C for 30 s, 72°C for 30 s), and a final extension at 72°C for 10 minutes. After amplification, the PCR products were loaded onto a 1% agarose gel and electrophoresis at 85 V for 30 min.
2.9 Statistical analysis
The explants in the experiments were arranged in a completely randomized design (CRD) with three replications, each containing five explants. Data was analyzed using Duncan’s multiple range test with SAS 9.1 software. Differences were significant at P values <0.05 or 0.01, compared to control values.
3 Results and discussion
3.1 Effect of PGRs on callus induction
Three weeks after the EMCS explants were cultured on the callus induction medium, callus was initiated to form at the basal region of the explants (Figures 1b, c). Callus cultured on the media with various concentrations of PGRS displayed friable and proliferative characteristics. In contrast, the medium lacking PGRS did not induce any callus which indicates the essential role of PGRs in callus induction. The MS medium supplemented with 2 mgL-1 2,4-D+ 2 mgL-1 NAA+ 1 mgL-1 BAP exhibited the highest callogenesis across all three cultivars with average induction rate of 95.00, 85.00, 91.66% for R.S., A. and A.R. cultivars, respectively. Additionally the quality of the callus varied among the tested cultivars, as displayed in Table 1. Our findings indicate that the combination of two auxins (2 mgL-1 2,4-D + 2 mgL-1 NAA) with BAP (1 mgL-1) significantly enhanced callus formation (Table 1). Previous studies on gladiolus have highlighted the key factors influencing the callogenesis including the composition of the culture medium and PGRs, appropriate explant and genotype (Kamo, 1994; Kumar et al., 2024a, b; Mujib et al., 2016; Tripathi et al., 2017). In this study, all three cultivars exhibited optimal callogenesis with the same PGR composition using EMCS explants, suggesting that genotype did not significantly influence callus formation.
3.2 Effect of different treatments on long-term maintenance of callus
The calli lost their regenerative capacity two weeks after excision from the EMCS explant bases, primarily due to the accumulation of phenolic compounds, which are known to damage callus tissue. It has been shown that the addition of phenolic inhibitors, along with darkness and low temperature, enhances callus viability in various plant species (Ko et al., 2009; Mingliang et al., 2011; Mohamed et al., 2018; Su et al., 2023).
For maintain the calli in a regenerable state, they were transferred to MS medium supplemented with phenolic inhibitors. The results demonstrated that the inclusion of 150 mg/L ascorbic acid, 100 mg/L citric acid, and 500 mg/L activated charcoal in the callus proliferation medium (containing 0.5 mg/L 2,4-D) significantly improved callus quality (Figure 1d) and effectively controlled phenolic compound production. This treatment resulted in a marked increase in the percentage of viable calli with no phenolic compound production (with averages of 90.00%, 80.00%, and 70.00% for R.S, A., and A.R cultivars, respectively) (Table 2). Furthermore, the calli retained their regenerative potential for up to one year. This long-term callus culture enables consistent corm production throughout the year which is a critical factor for commercial applications. Additionally, it provides a reliable system for in vitro breeding programs (De Klerk, 2012).
3.3 Effect of PGRs on shoot induction
In the present study, shoot regeneration was successfully induced after six weeks of sub culturing calli (five months old) onto the shoot induction medium (Figures 1e–g). MS medium supplemented with 2 mg/L BAP, 2 mg/L Kin, and 0.25 mg/L NAA resulted in the highest regeneration rates (average of 98.33%, 95.00%, and 93.33% for R.S, A., and A.R cultivars, respectively). This medium also yielded the greatest shoot number per explant, with averages of 45.00, 38.33, and 35.00 shoots for the R.S, A., and A.R cultivars, respectively. However, the longest shoot with averages of 58.30, 47.60 and 50.00 mm for R.S, A. and A.R respectively, obtained in the medium supplemented with high concentrations of BAP (4 mgL-1 BAP + 1 mgL-1 Kin + 0.25 mgL-1 NAA). Regeneration through callogenesis is primarily influenced by the quality of callus, the composition, as well as the concentration of cytokines, and its efficiency tend to decreases over time (Memon, 2012). Both direct and indirect regeneration methods have been reported for gladiolus tissue culture (Xu et al., 2009; Bera et al., 2015; Kumar et al., 2024a, b). Indirect organogenesis not only enhances the rate of reproduction but also significantly reduces production costs and time (Azadi et al., 2017; Kumar et al., 2018; Lalthafamkimi et al., 2022).
3.4 Effect of sucrose and IAA on cormel formation
Plantlet clusters were cultured on cormel production media (Figure 1h). In all treatments, root formation occurred first (Figure 1i), followed by cormel initiation after three weeks. By the 10th week, cormels had formed in all plantlets (Figure 1j).
The maximum number of cormels per plantlet (with an average of 18.66, 14.66, 16.66 cormels for R.S, A. and A.R cultivars, respectively) was obtained in MS medium supplemented with 9% Sucrose + 2 mgL-1 IAA + 1000 mgL-1 activated charcoal. However, the maximum diameter (with an average of 18.30, 15.70, 19.20 mm for R.S, A. and A.R cultivars, respectively), fresh weight (with an average of 3800, 2770, 4800 mg for R.S, A. and A.R cultivars, respectively) and dry weight (with average of 550, 390, 560 mg for R.S, A. and A.R cultivars, respectively) of cormels were obtained in MS medium supplemented with 9% sucrose + 4 mgL-1 IAA + 1000 mgL-1 activated charcoal (Table 4).
The type of PGRs, concentration of salts, sucrose, and activated charcoal are effective in rooting and cormel formation in gladiolus (De Bruyn and Ferreira, 1992; Nagaraju et al., 2002; Sinha and Roy, 2002; Kumar et al., 2011). Different auxins such as NAA, IBA and IAA have the significant impact on rooting and cormel formation (Dantu and Bhojwani, 1995; Tripathi et al., 2017; Naik et al., 2024);. Increasing the concentration of sucrose (4 - 10%) improved the formation of storage organs (Kumar et al., 1999; Sinha and Roy, 2002; Ascough et al., 2009; Kumar et al., 2011). Activated charcoal can also promote growth, adsorption of ions and PGRs, darkening of culture media, and creation of similar conditions as the soil does in nature (Nhut et al., 2001; Thomas, 2008; Martins et al., 2024).
Under natural conditions, one mother corm typically produces approximately 1–2 corms and 5–50 cormels depending on the genotypes (Pragya et al., 2012). Cormels produced under such condition require 3–4 growth seasons to reach the standard size necessary for producing flowering spike. Therefore, they need several years to produce a commercial colony (Bajaj et al., 1983). However, regenerated plantlets in vitro require 16 to 24 weeks for cormel formation and development (Steinitz and Lilien-kipnis, 1989). In the present study, we observed a high yield of cormels in approximately ten weeks, resulting in a complete protocol cycle of around 20 weeks. This represents a substantial reduction in time compared to conventional methods, highlighting the efficiency of the proposed approach.
3.5 Germination and acclimatization of cormel
Acclimatization and transfer of in vitro regenerated plantlets to an ex-vitro environment with minimal cost, and high survival rates are critical steps for successful propagation (Chandra et al., 2010). In geophytes, the production of storage organs provides an effective strategy for ensuring high adaptation rates. However, these storage organs often exhibit dormancy, which can be overcome using various strategies, such as exposure to low temperature, darkness, gibberellic acid (GA3), or specific medium compositions (Kumar and Raju, 2007; Padhi et al., 2018; Kumari et al., 2024). In this study, the produced cormels were removed from culture media, washed thoroughly and dried at room temperature for two weeks. In order to break the dormancy of the cormels, they were placed at low temperature (4 °C) in darkness for two months. The cormels were then cultured in disinfected substrate consisting of 50% coco peat + 50% perlite. All cormels successfully germinated (100%) and survived upon transfer to greenhouse condition.
3.6 Assessment of genetic stability
One of the key features of an effective plant regeneration protocol is its ability to produce healthy, true-to-type plantlets in short time (Mujib et al., 2016). In this study, we evaluated the genetic stability of regenerated gladiolus plantlets using two techniques: flow cytometry (FCM) and inter-simple sequence repeat (ISSR) markers. FCM analysis showed that the DNA ratios of regenerated plants (Figures 2b, d, f) were stable and matched those of the mother plants (Figures 2a, c, e). For the ISSR analysis, we screened the regenerated plantlets using ten primers (Table 5). Three of these primers produced consistent, monomorphic banding patterns across all plants (Figures 3a–c, Supplementary Figure 1), confirming that the regenerated gladiolus plantlets were genetically stable (Table 6).
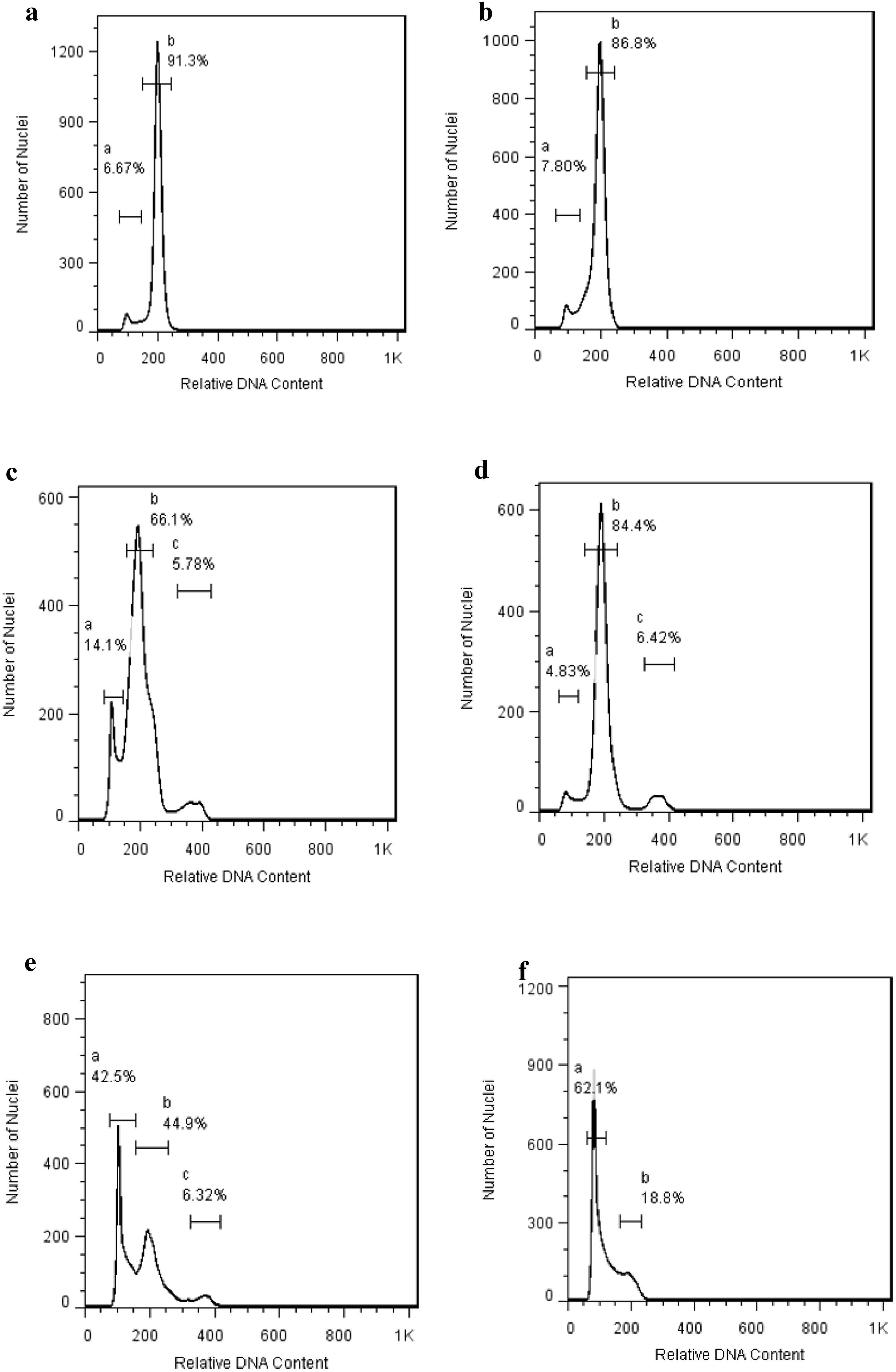
Figure 2. Flow cytometry histogram of Gladiolus grandiflorus; (a) G. grandifloras ‘Rose Supreme’ (mother plant). (b) G. grandifloras ‘Rose Supreme’ (in vitro regenerated plant). (c) G. grandifloras ‘Amsterdam’ (mother plant) (d) G. grandifloras ‘Amsterdam’ (in vitro regenerated plant) (e) G. grandifloras ‘Advance red’ (mother plant) (f) G. grandifloras ‘Advance red’ (in vitro regenerated plant) R1 R2 R3 MP C_ L.
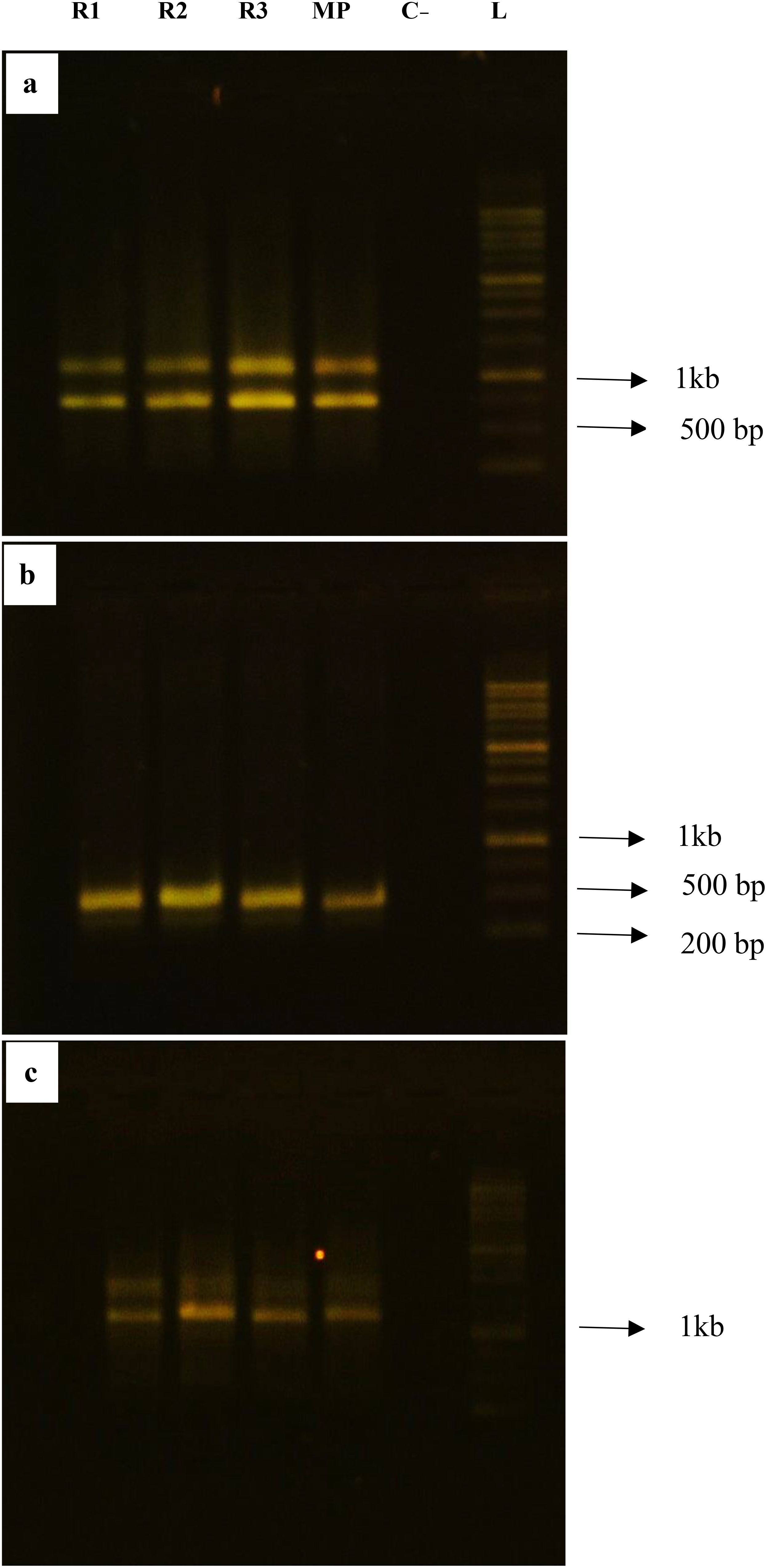
Figure 3. Genetic stability assessment of Gladiolus grandiflorus L. with ISSR markers; (a–c) represent profiles of ISSR markers 2, 5 and 10, respectively. R1–3 randomly selected regenerated plants, C- Negative control, MP Mother plant, L Ladder. (Unprocessed electrophoresis gel images of Figure are provided in Supplementary Figure 1).
Genetic stability is a crucial for successful plant regeneration, as somaclonal variation can lead to undesirable changes in plant characteristics (Mujib et al., 2016. FCM analysis revealed complete nuclear DNA content stability between mother plants and their in vitro-regenerated counterparts (Figure 2). The 2C DNA content values were determined to be 14.32 ± 0.18 pg for ‘Rose Supreme’, 14.15 ± 0.22 pg for ‘Amsterdam’, and 14.41 ± 0.15 pg for ‘Advance Red’, with no significant differences (p > 0.05) between source plants and regenerants. These measurements correspond to the G0/G1 peaks shown in Figure 2 panels (a-f), confirming the diploid nature (2n = 2x = 60) of all analyzed plants. The consistent DNA ploidy levels across all cultivars demonstrate the genomic stability of our regeneration protocol.
To further validate genetic stability at the molecular level, ISSR markers were employed. These markers are highly reproducible, cost-effective, and capable of detecting genetic variations without requiring species-specific primers (Abouseada et al., 2023; Bhatia et al., 2011). The monomorphic banding patterns we observed (Figure 3a–c; Supplementary Figure 1) are consistent with findings from other species, such as apple (Bisht et al, 2024), Muehlenbeckia platyclade (Badhepuri et al., 2024), Rhododendron formosum (Marwein et al, 2024) and Polianthes tuberosa (Nalousi et al, 2019). In a recent study, alternative molecular marker such as Sequence-Related Amplified Polymorphism (SRAP) has also been employed to assess the genetic diversity and stability among Gladiolus hybridus L. cultivars with high accuracy (Jadhav et al,2025).
3.7 Conclusion
Plant tissue culture has become an incredibly useful method for rapid production of genetically identical plants. However, it faces challenges, particularly the phenolic compounds accumulation and the risk of somaclonal variation. In this study, we successfully achieved notable improvements over traditional methods, which often take more than a year to complete, by efficiently controlling the production of phenolic compounds, creating long-term callus cultures, and cutting the production time to just 20 weeks. To verify the genetic stability of the regenerated plantlets, we utilized ISSR marker which is a reliable PCR-based method along with FCM for the detection of genetic variations. Our analysis confirmed that the regenerated plants grown in greenhouse conditions were genetically stable, demonstrating the effectiveness of the protocol. This protocol has significant potential for commercial gladiolus production, as it enables the rapid and reliable propagation of genetically identical plants. Moreover, it prepares a robust foundation for genetic transformation studies that aimed at improving desirable traits. Further research based on this protocol is already continuing, focusing on the genetic engineering of gladiolus for enhancing its ornamental and commercial value.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SM: Project administration, Writing – original draft, Data curation. SNM: Methodology, Writing – review & editing. LS: Formal Analysis, Validation, Writing – review & editing. PA: Conceptualization, Data curation, Methodology, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Ornamental Plants Research Center, Mahallat, Iran.
Acknowledgments
The authors wish to thank Ornamental Plants Research Center for supporting this research.
Conflict of interest
The author SM is CEO of Fartak Oxin Baft Knowledge Based Company.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. This manuscript was edited with limited use of Chat GPT (GPT-4-) solely to refine sentence structure. The authors meticulously reviewed and verified the content to ensure accuracy and scientific integrity.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fhort.2025.1571042/full#supplementary-material
Supplementary Figure 1 | Raw data of electrophoresis. Pictures show unprocessed electrophoresis gel images of Figure 3.
Abbreviations
2,4-D, 2,4-Dichlorophenoxyacetic acid; BAP, 6-benzylaminopurine; EMCS, Elongated mother corm sprout; IAA, Indole-3-acetic acid; ISSR, Inter Simple Sequence Repeats; Kin, Kinetin; MS, Murashige and Skoog; NAA, Naphthalene acetic acid.
References
Abouseada H. H., Mohamed A. S. H., Teleb S. S., Badr A., Tantawy M. E., Ibrahim S. D., et al. (2023). Genetic diversity analysis in wheat cultivars using SCoT and ISSR markers, chloroplast DNA barcoding and grain SEM. BMC Plant Biol. 23. doi: 10.1186/s12870-023-04196-w
Ascough G. D., Erwin J. E., and Staden J. V. (2009). Micropropagation of iridaceae-a review. Plant Cell Tissue Organ Cult. 97, 1–19. doi: 10.1007/s11240-009-9499-9
Azadi P., Bagheri H., Gholami M., Mirmasoumi M., Moradi A., and Sharafi A. (2017). Thin cell layer, a suitable explant for in vitro regeneration of saffron (Crocus sativus L.). J. Agric. Sci. Technol. 19, 1429–1435.
Azadi P., Bagheri H., Nalousi M. A., Nazari F., and Chandel S. F. (2016). Current status and biotechnological advances in genetic engineering of ornamental plants. Biotechnol. Adv. 34, 1073–1090. doi: 10.1016/j.bioteChadv.2016.06.006
Azadi P., Chin D. P., Kuroda K., Khan R. S., and Mii M. (2010). Macro elements in inoculation and co-cultivation medium strongly affect the efficiency of Agrobacterium-mediated transformation in lilium. Plant Cell Tissue Organ Cult. 101, 201–209. doi: 10.1007/s11240-010-9677-9
Azimi M. H. (2020). Assessment and ranking of new gladiolus hybrids in Iran. J. Hortic. Postharvest Res. 3, 235–244. doi: 10.22077/jhpr.2020.2972.1112
Badhepuri M. K., Beeravelli P. R., Arolla R. G., Jogam P., Rohela G. K., and Singisala N. R. (2024). Micropropagation and genetic fidelity analysis using SCoT and ISSR markers in Muehlenbeckia platyclada (F. Muell.) meisn. Plant Cell Tissue Organ Cult. 157 . doi: 10.1007/s11240-024-02763-z
Bajaj Y. P. S., Sidhu M. M. S., and Gill A. P. S. (1983). Some factors affecting the in vitro propagation of Gladiolus. Sci. Horti. 18, 269–275. doi: 10.1016/0304-4238(83)90031-6
Bera A. K., Maity T. R., Samanta A., Dolai A., Saha A. B., and Datta S. (2015). Enhancement of in vitro corm production in gladiolus by periodically replacement of liquid media using coir matrix. J. Appl. Horti. 17, 222–224. doi: 10.37855/jah.2015.v17i03.42
Bhatia R., Singh K. P., Sharma T. R., and Jhang T. (2011). Evalaution of the genetic fidelity of in vitro propagated gerbera (Gerbera jamesonii) using DNA- based markers. Plant Cell Tissue Organ Cult. 104, 131–135. doi: 10.1007/s11240-010-9806-5
Bisht V., Rawat J. M., Gaira K. S., Purohit S., Anand J., Sinha S., et al. (2024). Assessment of genetic homogeneity of in-vitro propagated apple root stock MM 104 using ISSR and SCoT primers. BMC Plant Biol. 24 , 1–14. doi: 10.1186/s12870-024-04939-3
Chandra S., Bandopadhyay R., Kumar V., and Chandra R. (2010). Acclimatization of tissue cultured plantlets: From laboratory to land. Biotechnol. Lett. 32, 1199–1205. doi: 10.1007/s10529-010-0290-0
Dantu P. K. and Bhojwani S. S. (1995). In vitro corm formation and field evaluation of corm-derived plants of gladiolus. Sci. Horti. 61, 115–129. doi: 10.1016/0304-4238(94)00722-R
Debnath S. C. and Ghosh A. (2022). Phenotypic variation and epigenetic insight into tissue culture of berry crops. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1042726
De Bruyn M. H. and Ferreira D. I. (1992). In-vitro corm production of Gladiolus dalenii and G. tristis. Plant Cell Tissue Organ Cult. 31, 123–128. doi: 10.1007/BF00037696
De Klerk G. (2012). Micropropagation of bulbous crops: technology and present state. Floriculture Ornamental Biotech. 6 , 1–8.
Dey T., Saha S., and Ghosh P. D. (2015). Somaclonal variation among somatic embryo derived plants- evaluation of agronomically important somaclones and detection of genetic changes by RAPD in Cymbopogon winterianus. Safr J. Bot. 96, 112–121. doi: 10.1016/j.sajb.2014.10.010
Ebrahimzadeh H., Shariatpanahi M. E., Ahmadi B., Soltanloo H., Lotfi M., and Zarifi E. (2018). Efficient parthenogenesis induction and in vitro haploid plant regeneration in cucumber (Cucumis sativus L.) using putrescine, spermidine, and cycocel. Plant Growth Regul. 37, 1127–1134. doi: 10.1007/s00344-018-9803-1
Endemann M., Hristoforoglu K., Stauber T., and Wilhelm E. (2001). Assessment of age-related polyploidy in Quercus robur L. somatic embryos and regenerated plants using DNA flow cytometry. Biol. Plant 44, 339–345. doi: 10.1023/A:1012426306493
Faisal M., Alatar A., Hegazy A. K., Alharbia S. A., El-Sheikh M., and Okla M. K. (2014). Thidiazuron induced in vitro multiplication of Mentha arvensis and evaluation of genetic stability by flow cytometry and molecular markers. Ind. Crop Prod. 62, 100–106. doi: 10.1016/j.indcrop.2014.08.019
Ferreira M., d. S., d. J. A., d. S. F., d. S. W. D., d. S. J. M., et al. (2023). The role of somaclonal variation in plant genetic improvement: a systematic review. Agronomy 13, 730. doi: 10.3390/agronomy13030730
Jadhav P. R., Jagtap A. Y., Gadge A. D., Solanke A. U., Pagariya M. C., Kadam G. B., et al. (2025). Agro-morphological characterization and SRAP-based genetic diversity analysis of Gladiolus hybridus L. cultivars. Genet. Resour. Crop Evol., 1–23. doi: 10.1007/s10722-024-02309-4
Jaryal P., Pathak P., and Warghat A. R. (2025). An efficient clonal propagation of a medicinally important and endangered Himalayan herb, Dactylorhiza hatagirea D. Don Soo using shoot meristem culture and genetic fidelity analysis. Plant Cell Tissue Organ Cult. 160 , 1–10. doi: 10.1007/s11240-024-02958-4
Joshi A., Tripathi A., Sharma N., and Tailor A. (2024). Somaclonal Variation for the Improvement of Tree Species. In: Thomas T D., Razdan M. K., and Kumar A. (eds) Biotechnological Approaches for Sustaining Forest Trees and Their Products. (Singapore: Springer). doi: 10.1007/978-981-97-4363-6_4
Kamo K. (1994). Effect of phytohormones on plant regeneration from callus of gladiolus cultivar “Jenny Lee”. In Vitro Cell. Dev. Biol. Plant 30, 26–31. doi: 10.1007/BF02632116
Ko W. H., Su C. C., Chen C. L., and Chao C. P. (2009). Control of lethal browning of tissue culture plantlets of Cavendish banana cv. Formosana ascorbic acid. Plant Cell Tissue Organ Cult. 96, 137–141. doi: 10.1007/s11240-008-9469
Krishna H., Alizadeh M., Singh D., Singh U., Chauhan N., Eftekhari M., et al. (2016). Somaclonal variations and their applications in horticultural crops improvement. Biotech. 6, 1–18. doi: 10.1007/s13205-016-0389-7
Kumar M., Chaudhary V., Sirohi U., Singh J., Yadav M. K., Prakash S., et al. (2024a). In vitro propagation journey of ornamental gladiolus (Gladiolus species): a systematic review analysis based on more than 50 years research. Horticulturae 10 , 148. doi: 10.3390/horticulturae10020148
Kumar A., Kumar A., Sharma V., Mishra A., Singh S., and Kumar P. (2018). In vitro regeneration of gladiolus (Gladiolus hybrida L.): optimization of growth media and assessment of genetic fidelity. Int. J. Curr. Microbiol. App. Sci. 7, 2900–2909. doi: 10.20546/ijcmas.2018.710.337
Kumar A., Palni L. M. S., and Sood A. (1999). In vitro propagation of Gladiolus hybridus hort.: synergistic effect of heat shock and sucrose on morphogenesis. Plant Cell Tissue Organ Cult. 57, 105–112. doi: 10.1023/A:1006373314814
Kumar A., Palni L. M. S., and Sood A. (2011). Factors affecting in vitro formation of cormlets in Gladiolus hybridus Hort. their Field performance. Acta Physiol. Plant 33, 509–515. doi: 10.1007/s11738-010-0574-y
Kumar P. N. and Raju D. V. C. (2007). Dormancy in gladiolus: the cause and remedy - a review. Agri Rev. 28, 309–312.
Kumar M., Sirohi U., Yadav M. K., and Chaudhary V. (2024b). In vitro culture technology and advanced biotechnology tools toward improvement in gladiolus (Gladiolus species): present scenario and future prospects. Mol. Biotechnol. 66, 1806–1835. doi: 10.1007/s12033-023-00818-8
Kumar M. S. and Nandi S. C. (2015). High frequency plant regeneration with histological analysis of organogenic callus from internode explants of Asteracantha longifolia Nees. Genet. Eng. Biotechnol. J. 13 (1), 31–37. doi: 10.1016/j.jgeb.2014.12.002
Kumari N., Kumari S., Jose-Santhi J., Kalia D., Sheikh F. R., and Singh R. K. (2024). Emerging into the world: regulation and control of dormancy and sprouting in geophytes. J. Exp. Bot. 75, 6125–6141. doi: 10.1093/jxb/erae216
Lalthafamkimi L., Bhau B. S., Kumar S., and Mukhia S. (2022). Indirect organogenesis-mediated high frequency conversion of non-embryonic synthetic seeds, essential oil profiling and antibacterial activity in genetically stable plants of Patchouli. Biotech. 12, 349. doi: 10.1007/s13205-022-03302-3
Long Y., Yang Y., Pan G., and Shen Y. (2022). New insights into tissue culture plant-regeneration mechanisms. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.926752
Martins J. P. R., Wawrzyniak M. K., Kalemba E. M., Ley-López J. M., Lira J. M. S., and Chmielarz P. (2024). In vitro rooting of Quercus robur, activated charcoal vs. exogenous auxin: a morphophysiological approach. Plant Cell Tissue Organ Cult. 156, 24. doi: 10.1007/s11240-023-02656-7
Marwein D., Prasad G., Vijayan D., and Mao A. A. (2024). Assessment of genetic stability of micropropagated Rhododendron formosum Wall. an endemic plant to Eastern Himalaya. In Vitro Cell. Dev. Biol.-Plant. 60, 681–692. doi: 10.1007/s11627-024-10466-5
Memon N. (2012). In-vitro propagation of gladiolus plantlets and cormels. J. Hortic. Sci. Ornam. Plants. 3, 280–291. doi: 10.5829/idosi.jhsop.2012.4.3.258
Mingliang C., Yufang J., Shu C., Zhongshan G., Hefei W., Lulu L., et al. (2011). Callus induction, plant regeneration, and long-term maintenance of embryogenic cultures in Zoysia matrella [L.] Merr. Plant Cell Tissue Organ Cult. 104, 187–192. doi: 10.1007/s11240-010-9817-2
Mohamed S. A., Hattem M. E., Amira S., and Mai A. S. (2018). Optimization of germination, callus induction, and cell suspension culture of African locust beans Parkia biglobosa (Jacq.). Benth. Genet. Eng. Biotechnol. J. 16, 191–201. doi: 10.1016/j.jgeb.2017.10.012
Mujib A., Ali M., Tonk D., Isah T., and Zafar N. (2016). Embryogenesis in Ornamental Monocots: Plant Growth Regulators as Signalling. In Mujib A. (ed), Somatic embryogenesis in ornamentals and its applications (New Delhi, India: Springer), 187–201. doi: 10.1007/978-81-322-2683-3_12
Murashige T. and Skoog F. A. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant 15, 473–492. doi: 10.1111/j.1399-3054.1962.tb08052.x
Murray M. G. and Thompson W. F. (1980). Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 8, 4321–4325. doi: 10.1093/nar/8.19.4321
Nagaraju V., Howmik G., and Parthasarathy V. A. (2002). Effect of paclobutrazol and sucrose on in vitro cormel formation in gladiolus. Acta Bot. Croat. 61, :27–:33.
Naik M., Nazki I. T., and Husaini A. M. (2024). A high-efficiency 3-Step in vitro protocol for commercial cormel micropropagation of Gladiolus hybridus Hort. cv ‘Red Majesty’. Vegetos 38, 157–168. doi: 10.1007/s42535-024-01134-5
Nalousi M. A., Hatamzadeh A., Azadi P., Mohsenpour M., and Samizadeh Lahiji H. (2019). A procedure for indirect shoot organogenesis of Polianthes tuberosa L. and analysis of genetic stability using ISSR markers in regenerated plants. Sci. Hortic. 244, 315–321. doi: 10.1016/j.scienta.2018.09.066
Nhut D. T., Le B. V., Fukai S., Tanaka M., and Van K. T. (2001). Effects of activated charcoal, explant size, explant position and sucrose concentration on plant and shoot regeneration of Lilium longiflorum via young stem culture. Plant Growth Regul. 33, 59–65. doi: 10.1023/A:1010701024963
Ntui V., Azadi P., Supaporn H., and Mii M. (2010). Plant regeneration from stem segment-derived friable callus of “Fonio” (Digitaria exilis (L.) Stapf.). Sci. Hortic. 125, 494–499. doi: 10.1016/j.scienta.2010.04.017
Ochatt S. J., Patat-Ochatt E. M., and Moessner A. (2011). Ploidy level determination within the context of in vitro breeding. Plant Cell Tissue Organ Cult. 104, 329–341. doi: 10.1007/s11240-011-9918-6
Padhi M., Sisodia A., Pal S., Kapri M., Singh, and Sing A. K. (2018). Growing media, GA3 and Thiourea stimulates growth and rooting in gladiolus cormels cv. Tiger Flame. J. Pharmacogn Phytochem. 7, 1919–1922.
Polanco C. and Ruiz M. L. (2002). AFLP analysis of somaclonal variation in Arabidopsis thaliana regenerated plants. Plant Sci. J. 162 , 817–824. doi: 10.1016/S0168-9452(02)00029-8
Pragya S., Sing K., Misra R. L., and Ranjan J. K. (2012). In-vitro shoot regeneration from cormel derived callus of gladiolus and bio-hardening of plantlets. Indian J. Biotech. 11, 99–104.
Rai M. K. (2021). “Somaclonal variation in improvement of agricultural crops: recent progress,” in Agricultural Biotechnology: latest research and trends. Eds. Kumar D. Srivastava, Kumar Thakur A., and Kumar P. (Springer, Singapore). doi: 10.1007/978-981-16-2339-4_6
Rakoczy-Trojanowska M. (2002). The effects of growth regulators on somaclonal variation in rye (Secale cereale L.) and selection of somaclonal variants with increased agronomic traits. Cell. Mol. Biol. Lett. 7, 111–120. doi: 10.2478/s11658-002-0009-4
Ramos-Garci M., Ortega-Centeno S., Herna A., Lauzardo I., Tejacal A., Bosquez-Molina E., et al. (2009). Response of gladiolus (Gladiolus spp) plants after exposure corms to chitosan and hot water treatments. Sci. Hortic. 121, 480–484. doi: 10.1016/j.scienta.2009.03.002
Sharma N. and Tripathi A. (2008). Integrated management of postharvest fusarium rot of gladiolus corms using hot water, UV-C and Hyptis suaveolens (L.) Poit. essential oil. Postharvest Biol. Technol. 47, 246–254. doi: 10.1016/j.postharvbio.2007.07.001
Singh N., Pal A. K., Roy R. K., Tamta S., and Rana T. S. (2017). Development of cpSSR markers for analysis of genetic diversity in gladiolus cultivars. Plant Gene 10, 31–36. doi: 10.1016/j.plgene.2017.05.003
Singh D., Singh. V. K., Fayaz K., Verty P., and Bhuj B. D. (2018). Review article impact of plant growth regulators in gladiolus. Int. J. Curr. Microbiol. Appli. Sci. 7, 139–148.
Sinha P. and Roy S. K. (2002). Plant regeneration through in-vitro cormel formation from callus culture of Gladiolus primulinus Baker. Plant Tissue Cult 2, 139–145.
Smulders M. and de Klerk G. (2011). Epigenetics in plant tissue culture. Plant Growth Regul. 63, 137–146. doi: 10.1007/s10725-010-9531-4
Steinitz B. and Lilien-Kipnis H. (1989). Control of precocious gladiolus corm and cormel formation in tissue culture. J. Plant Physiol. 135, 495–500. doi: 10.1016/S0176-1617(89)80110-5
Su Y., Wei M., Guo Q., Hunang J., Zhao K., and Huang J. (2023). Investigating the relationships between callus browning in Isatis indigotica Fortune, total phenol content, and PPO and POD activities. Plant Cell Tissue Organ Cult. 155, 175–182. doi: 10.1007/s11240-023-02567-7
Sun S. L., Zhong J. Q., Li S. H., and Wang X. J. (2013). Tissue culture-induced somaclonal variation of decreased pollen viability in torenia (Torenia fournieri Lind.). Bot. Stud. 23, 36–54. doi: 10.1186/1999-3110-54-36
Swaroop K., Singh K. P., Kumar P., and Sindhu S. S. (2018). Improvement and performance of gladiolus hybrids for flower traits novel colour and higher corm multiplication. Int. J. Agric. Innov. Res. 6, 4–9. doi: 10.56093/ijas.v86i12.65617
Thomas T. D. (2008). The role of activated charcoal in plant tissue culture. Biotechnol. Adv. 26, 618–631. doi: 10.1016/j.bioteChadv.2008.08.003
Tripathi M. K., Malviya R. K., Vidhyashankar M., and Patel R. P. (2017). Effect of plant growth regulators on in vitro morphogenesis in Gladiolus (Gladiolus hybridus Hort.) from cultured corm slice. Int. J. Agric. Technol. 13, 583–599.
Wu J., Liu C., Seng S., Khan M. A., Sui J., Gong B., et al. (2015). Somatic embryogenesis and Agrobacterium-mediated transformation of Gladiolus hybridus cv. ‘Advance Red’. Plant Cell Tissue Organ Cult. 120, 717–728. doi: 10.5555/20153079632
Xu Z., Hao J., He X., and Yi M. (2009). Callus induction and plant regeneration of Gladiolus hybridus Hort. Plant Physiol. Commun. 45, 473–478. doi: 10.5555/20093336709
Zafar M., Mujib A., Muzamil A., Tonk D., Basit G., Malik M., et al. (2019). Genome size analysis of field grown and tissue culture regenerated Rauvolfia serpentina L. by flow cytometry: Histology and scanning electron microscopic study for in vitro morphogenesis. Ind. Crop Prod. 128, 545–555. doi: 10.5555/20193253391
Keywords: gladiolus micropropagation, genetic stability, flow cytometry, ISSR markers, cormel formation
Citation: Mousavimatin S, Mortazavi SN, Samiei L and Azadi P (2025) Indirect regeneration of long-term callus cultures in gladiolus: a protocol for stable genetic fidelity. Front. Hortic. 4:1571042. doi: 10.3389/fhort.2025.1571042
Received: 04 February 2025; Accepted: 21 April 2025;
Published: 21 May 2025.
Edited by:
Abdul Mujib, Jamia Hamdard University, IndiaReviewed by:
Yelda Özden Çiftçi, Gebze Technical University, TürkiyeAnelia Iantcheva, Anelia Veneva Iantcheva, Bulgaria
Copyright © 2025 Mousavimatin, Mortazavi, Samiei and Azadi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pejman Azadi, YXphZGlwMjJAZ21haWwuY29t
 Seyedehraziyeh Mousavimatin
Seyedehraziyeh Mousavimatin Seyed Najmmaddin Mortazavi1
Seyed Najmmaddin Mortazavi1 Leila Samiei
Leila Samiei Pejman Azadi
Pejman Azadi