- 1Key Laboratory for Critical Care Medicine of the Ministry of Health, Tianjin First Central Hospital, Tianjin, China
- 2State Key Laboratory of Medicinal Chemical Biology, Nankai University, Tianjin, China
- 3School of Life Sciences, Tianjin University, Tianjin, China
Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are primary risk factors for a wide spectrum of liver diseases that severely affect human health. The liver is an immunological organ that has an abundance of immune cells. Thus, various innate or adaptive immune cells are involved in the progression of HBV or HCV infection. Among those cells, a unique kind of immune cell, the γδ T cell, contributes to promoting or inhibiting the progression of liver diseases. To reveal the diverse roles of γδ T cells in HBV or HCV infection, the properties and functions of these cells in human and mouse models are analyzed. Here, we briefly describe the characteristics and functions of γδ T cells subsets in liver diseases. Then, we fully discuss the diverse roles of γδ T cells in the progression of HBV or HCV infection, including stages of acute infection, chronic infection, liver cirrhosis, and hepatocellular carcinoma. Finally, the functions and existing problems of γδ T cells in HBV or HCV infection are summarized. A better understanding of the function of γδ T cells during the progression of HBV and HCV infection will be helpful for the treatment of virus infection.
Introduction
Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are major risk factors for a wide spectrum of liver diseases. Although most adults recover from HBV infection, about 5% of patients are unable to clear HBV and thus develop chronic HBV infection (1) and experience virus flares and long-term morbidity. Similarly, acute HCV infection can easily convert into chronic HCV infection (2). The persistent inflammatory environment in chronic HBV (CHB) or chronic HCV (CHC) infection patients is associated with the elevated expression of α-smooth muscle actin and collagen fibers in hepatic stellate cells (HSCs), which then develop into liver cirrhosis (2–4). Hepatocellular carcinoma (HCC) is a common cancer and is mainly caused by HBV or HCV infection. HCV patients show a higher probability of developing HCC than HBV patients (5).
The liver is known as an immune tolerance organ. Aside from hepatocytes and stellate cells, there are various hepatic residential immune cells, including Küpffer cells (hepatic macrophages), T cells, natural killer (NK) cells, and dendritic cells (6). These cells play crucial roles in the pathogenesis of HBV or HCV infection. During acute HBV or HCV infection, innate immune cells such as NK cells are activated and further induce antiviral function of adaptive immune cells (7). In chronic HBV and HCV infections, the liver is infiltrated with impaired antiviral T cells and activated inflammatory cells such as IL-17-producing CD4+ T cells that further exacerbate liver inflammation (8, 9). Moreover, other hepatic immune cells, including regulatory T cells and myeloid-derived suppressor cells (MDSC), prompt the pathogenesis of chronic HBV or HCV infection, liver cirrhosis, or even liver cancer (10). The proportion of hepatic γδ T cells in hepatic T cells in humans and mice is found to be 15%–25% and 4.5%, respectively (6, 11), indicating the crucial role of these cells in liver diseases. However, the current understanding of the function of γδ T cells compared with other immune cells in HBV or HCV infection is limited.
γδ T cells, as the bridge of innate and adaptive immunity, play critical roles in various diseases, including liver diseases, infections, and cancer. γδ T cells can be divided into different subsets through γ and δ TCR chains. Based on δ TCR chains, human γδ T cells can mainly be separated into Vδ1 (in peripheral blood or organs), Vδ2 (peripheral blood dominant γδ T cells, usually combined with Vγ9), and Vδ3 (in intestine and lamina propria) T cell subsets. Based on γ TCR chains, mouse γδ T cells can be divided into Vγ1, Vγ4, Vγ5, Vγ6, and Vγ7 T cell subsets (12). In liver diseases, hepatic γδ T cells usually include Vγ1, Vγ4, and Vγ6 in mice and Vδ1, Vδ2, and Vδ3 in humans (13–15). These cells can produce cytokines such as IFN-γ, TNF-α, IL-17, and IL-22, as well as express cytotoxic and regulatory molecules such as Granzyme B (GrB), perforin, NK receptor, and Toll-like receptors (16). γδ T cells play different roles in the pathogenesis of HBV and HCV infections. In acute HBV infection, human γδ T cells are activated and exhibit antiviral functions by secreting IFN-γ and TNF-α. During other stages of HBV and HCV infections (chronic infection, liver cirrhosis, and HCC), these cells can inhibit or promote progression of the diseases. Surprisingly, different subsets of γδ T cells play contradictory roles in the same stage of liver infection. For example, in chronic HBV infection, human Vδ2 T cell subsets inhibit HBV infection progression by inhibiting Th17-induced liver damage (17). However, human CD4-CD8- γδ T cell (18) and mouse IL-17-producing Vγ4 T cell (19) subsets are found to inhibit the function of T cells and promote HBV infection in CHB patients and an HBV mouse model. Similar contradictory functions are also observed in other stages. In HCC, human Vδ2 T cells, which can be activated and proliferate in vitro (20), are used in the clinic to prolong the survival time of HCC patients (21).
To determine the precise role of these cells, we summarize the functions of different human and mouse γδ T cells subsets in the different stages of HBV and HCV infections. Moreover, we indicate the opportunities and challenges in clinical application of γδ T cells.
Role of γδ T Cells in Acute and Chronic HBV Infection
During human acute HBV infection, about 5% of adult patients progress to chronic hepatitis B infection, whereas the rest go through a self-limited process that results in recovery (1). Accumulating data have demonstrated that different outcomes of HBV infection are associated with the intensity of antiviral immune responses (22). As shown in our previous study, the numbers of γδ T cells increase in liver tissue, but decrease in the peripheral blood of acute hepatitis B (AHB) patients (3). These peripheral γδ T cells are highly activated and terminally differentiated into memory phenotype, which has increased cytotoxic capacity and enhanced antiviral activity. Interestingly, in asymptomatic HBV infection patients, the frequencies of peripheral Vδ1 and Vδ2 T cells are higher, and the level of peripheral IFN-γ+Vδ2 T cells is also significantly elevated compared to healthy controls (23). Furthermore, in an AHB infection mouse model, the number of hepatic γδ T cells significantly increases with the upregulation of HBV markers and exhibits elevated expression of the activation marker CD69, IFN-γ production, and IFN-β mRNA abundance in liver tissues (24). The above studies indicate that the antiviral function of γδ T cells in AHB patients can inhibit the progression of AHB infection.
γδ T cells display contradictory roles in CHB infection. Several studies have shown that these cells are impaired and exhibit liver protective functions to inhibit the progression of CHB infection (17). Our study and others show that the frequency of human peripheral and hepatic Vδ2 T cells is significantly lower in severe CHB patients with impaired chemotaxis (17) or degranulation (25). Although they display an active effector-memory phenotype (17), the IFN-γ or TNF-α-induced cytotoxicity of Vδ2 T cells is impaired (26) and can be reversed by IFN-α treatment in vitro and in vivo (27). In addition, in vitro proliferated human Vδ2 T cells can inhibit inflammatory cytokines production in pathogenic Th17 cells (17), which contributes to significant liver damage and pathology. However, a recent study indicates that the frequency of human γδ T cells and their subsets barely change and antiviral function of Vδ2 T cells is enhanced in CHB patients (28). This opposite result maybe because of the different applied standard for patient enrollment, including age, gender, and race, which would interfere the characteristics of γδ T cells (29).
However, other studies report that γδ T cells promote the progression of chronic HBV infection. By suppressing the secretion of HBV core peptide-stimulated IFN-γ and TNF-α by CD8+ T cells, human CD4-CD8- γδ T cells limit T cell responses to HBV partially through NKG2A and may impede HBeAg seroconversion during antiviral therapy of CHB patients (18). Moreover, in HBV-associated acute-on-chronic liver failure (CHB-ACLF) patients, more human peripheral γδ T cells exhibit upregulation of TNF-α or IL-17 and GrB or CD107, demonstrating the participation of γδ T cells in liver injury which in turn promote the progression of liver diseases (30). Meanwhile, in an immune tolerance chronic HBV infection mouse model, IL-17-producing Vγ4 T cells recruit MDSCs into the liver and induce CD8+ T cell exhaustion (19).
In conclusion, IFN-γ- or TNF-α-producing γδ T cells can inhibit AHB and CHB infection, while human CD4-CD8- γδ T cells and mouse IL-17-producing Vγ4 T cell subsets promote the progression of chronic HBV infection. The opposite roles of these cells can be attributed to the different subsets of γδ T cells and their variable cytokine production (IFN-γ, TNF-α, or IL-17).
Role of γδ T Cells in Chronic HCV Infection
Numerous researchers have focused on the function of γδ T cells in chronic HCV (CHC) infection. The number of hepatic γδ T cells is higher in CHC patients, and Vδ1 T cells are the predominant subset of hepatic γδ T cells (31, 32). However, the number of peripheral Vγ9Vδ2 and Vδ1 T cells decrease in CHC patients compared with healthy control and asymptomatic HCV carriers (33). Moreover, in mice, the level of hepatic γδ T cells is significantly higher in HCV transgenic mice compared with wild-type mice (34). It is assumed that peripheral γδ T cells are recruited into the liver and contribute to the pathogenesis of HCV infection.
γδ T cells play different roles in the pathogenesis of CHC infection. In some studies, γδ T cells manifest their antiviral role and inhibit the progression of CHC infection. In CHC patients, the cytotoxicity of hepatic γδ T cells is higher than that of hepatic αβ T cells. This is attributable to their elevated secretion of IFN-γ, TNF-α, and IL-8 (31) and their expression of activation marker (human leukocyte antigen-DR) and memory/effector (CD62L-CD45RO+ CD95+) marker (32). In particular, the frequency of human hepatic IFN-γ+Vδ1 T cells is positively correlated with the degree of liver necroinflammation, indicating their involvement in liver pathogenesis and liver damage (32). Furthermore, the expression of CD56 and CD16 (markers of natural killer cells) increase in peripheral Vγ9Vδ2 T cells and is further enhanced in hepatic Vγ9Vδ2 T cells of CHC patients (35). In humans, after stimulation by non-peptide antigen-isopentenyl diphosphate (IPP), activated peripheral Vγ9Vδ2 T cells are associated with a dramatic reduction in HCV RNA levels. Neutralizing experiments have further revealed the function of IFN-γ in HCV clearance (36). Moreover, in a mouse model, the number of hepatic γδ T cells increases and activated CD69+ γδ T cells produce more IFN-γ and TNF-α during MHV (mouse hepatitis virus) infection than controls. Interestingly, those activated hepatic γδ T cells can kill MHV-infected hepatocytes in vitro by secreting IFN-γ and TNF-α (37).
However, several studies have indicated that human peripheral γδ T cells exhibit impaired function in CHC patients even after antiviral treatment. Human peripheral Vγ9Vδ2 T cells are activated and differentiate into effector cells with upregulated GrB and perforin expression, but have a markedly impaired capacity to produce IFN-γ in CHC patients (38). Furthermore, IFN-α treatments result in the upregulation of cytotoxic markers such as GrB, perforin, and CD107a, but not the IFN-γ production capacity of peripheral Vγ9Vδ2 T cells in CHC patients (35, 38). The above results suggest a functional dichotomy of Vγ9Vδ2 T cells in chronic HCV infections that contribute to both liver inflammation and HCV persistence. Moreover, dysfunction of γδ T cells in CHC patients has also been observed in antiviral therapy. Direct-active antiviral agents (DAAs) are widely used in the treatment of chronic HCV infection. In clinical trials, DAAs have induced minor changes in γδ T cells both in terms of numbers and in alterations of TRG and TRD repertoires 1 year after treatment (39). Although human peripheral Vγ9Vδ2 T cells display an elevated effector phenotype in sustained virologic-response HCV patients, recent DAA treatment research demonstrates that these cells show poor cytokine response and proliferative responses to antigens (40).
In summary, human and mouse hepatic γδ T cells as well as in vitro stimulated human peripheral Vγ9Vδ2 T cells can inhibit HCV pathogenesis. However, impaired cytokine response of peripheral Vγ9Vδ2 T cells in CHC patients contributes to HCV infection progression, even after DAA treatment. Further studies on recovery from the cytokine response impairment of Vγ9Vδ2 T cells is very important for CHC treatment.
Role of γδ T Cells in Liver Cirrhosis and HCC
Persistent inflammation of HBV or HCV can lead to liver fibrosis and liver cirrhosis. HSCs are critical cells in the pathogenesis of liver cirrhosis. Activation of these cells promote the progression of liver cirrhosis (41). A liver cirrhosis mouse model shows different relationships between HSCs and hepatic γδ T cells. IL-17-producing CCR6+ γδ T cells induce apoptosis of HSCs in a FasL-dependent manner to inhibit the progression of liver cirrhosis (42). Moreover, IFN-γ-producing γδ T cells can directly kill activated HSCs and increase NK cell-mediated cytotoxicity against activated HSCs partially through a 4-1BB dependent manner (43). However, hepatocyte-secreted exosomes can activate HSCs via Toll-like receptor 3. These HSCs further enhance the activity of IL-17-producing γδ T cells, which exacerbates liver fibrosis and promotes the progression of liver cirrhosis (4). In view of the contradictory roles of IL-17-producing γδ T cells in the same mouse model, further studies involving patients and a virus-induced liver cirrhosis mouse model should be performed to elucidate the exact role of γδ T cells.
A recent study has shown that the increased peritumor ratio in human γδ T cells contributes to the progression and recurrence of HCC, indicating the important role of γδ T cells in HCC (44). Interestingly, γδ T cells play different roles in the pathogenesis of HCC. In several studies, γδ T cells display cytotoxicity and inhibit proliferation of tumor cells in vivo and in vitro. In HCC patients, the number of human peritumoral γδ T cells is positively related to better prognosis of HCC curative resection (45). A recent biostatistics study has shown that the increase of human tumor-infiltrated γδ T cells, which is driven by the accumulation of chemokines such as CCL4 and CCL5, is significantly positively correlated with the survival rate and negatively correlated with HCC recurrence. γδ T cells play protective roles by regulating the infiltration and differentiation of CD8+ T cells in HCC procession (46). Furthermore, human γδ T cells can induce the death of HCC cell lines and reverse the immune escape of HCC in vitro (47). Moreover, the anti-HCC function of peripheral γδ T cells, especially Vγ9Vδ2 T cells, can be further enhanced by activating agents, including histone deacetylase inhibitors (48), pyrophosphate (49), zoledronate (20), CD226 (50), and even the Chinese herb artesunate (51).
However, other studies reveal that impaired human γδ T cells or mouse γδ T cells can also contribute to the progression of HCC. In an immunosuppressed tumor microenvironment, γδ T cells show impaired IFN-γ production and degranulation (perforin and CD107a) capacity, which is attributed to the secretion of TGF-β and IL-10 by tumor-infiltrating Tregs (52). In addition, a decrease in the number and cytotoxicity of peripheral Vδ2 T cells is observed in HCC patients and possibly associated with the lack of IL-2 and IL-21 (53). The total number of γδ T cells and effector γδ T cells is significantly lower in tumors than in peritumoral tissues and non-tumor livers (52, 54). In addition, in an HCC mouse model, IL-17-producing Vγ4 T cells recruit MDSCs in a CXCL5/CXCR2-dependent manner and further suppress the anti-tumor function of CD8+ T cells (55).
Human peripheral Vδ2 T cells can proliferate in vitro and kill HCC and thus have been used in clinical immunotherapy of HCC patients. Zoledronate induces the proliferation of γδ T cells in HCC patients who exhibit upregulated expression of IFN-γ, TNF-α, GrB, perforin, and lysosome-associated membrane protein 1 (47). A clinical trial has shown that the combined use of γδ T cells, NK cells, and cytokine-induced killer (CIK) therapy significantly inhibits virus replication and prolongs the survival rate of HCV-positive HCC patients (21).
In conclusion, γδ T cells and their subsets play opposite roles in liver cancer, and their underlying mechanisms require further investigation.
Conclusions and Perspectives
Different subsets of γδ T cells play various roles in pathogenesis of HBV or HCV infection. Most of the mouse and human studies are summarized in Figure 1.
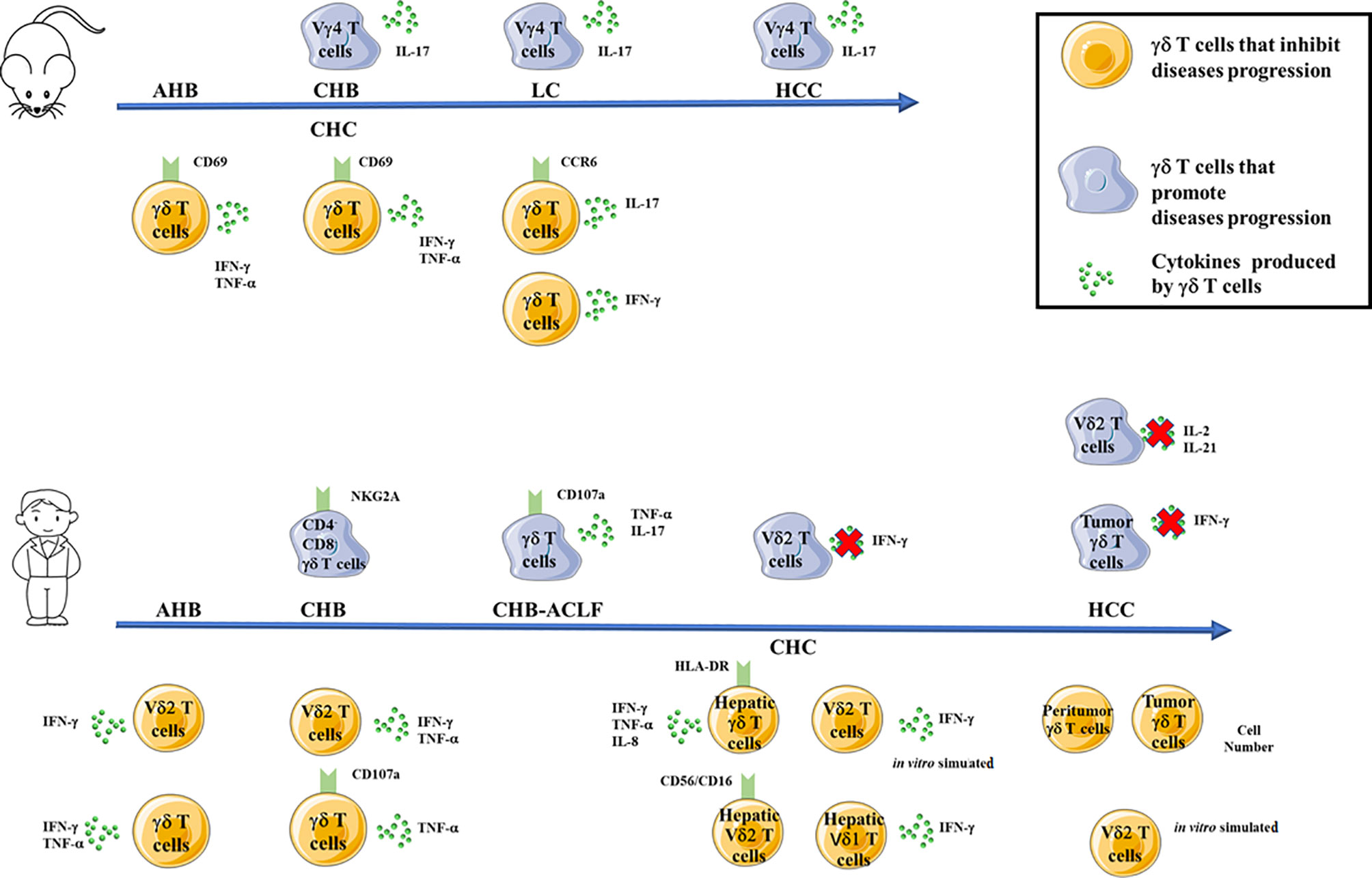
Figure 1 Functions of mouse (top) or human (bottom) γδ T cells and their subsets in inhibiting (yellow cells) or promoting (blue cells) the pathogenesis of HBV or HCV infection. The arrows indicate the progression of HBV or HCV infections, the red fox indicate impaired secretion of cytokines by γδ T cells and their subsets. AHB, acute hepatitis B infection; CHB, chronic hepatitis B infection; CHB-ACLF, HBV-associated acute-on-chronic liver failure; CHC, chronic hepatitis C infection; LC, liver cirrhosis; HCC, hepatocellular carcinoma.
In mouse model, IL-17-producing Vγ4 T cells subsets promote the progression of CHB, LC and HCC. However, in other studies, IFN-γ and TNF-α-producing CD69+ mouse γδ T cells can inhibit the progression of AHB and CHC. Furthermore, IL-17-producing CCR6+ mouse γδ T cells or IFN-γ producing mouse γδ T cells inhibit the progression of LC. (Figure 1, top).
In human studies (Figure 1, bottom), CD4- CD8- γδ T cells subsets and IL-17/TNF-a+ γδ T cells promote the progression of CHB and CHB-ACLF patients, respectively. Impairment secretion of IFN-γ by peripheral Vδ2 T cells contributes to the progression of CHC. Moreover, impairment secretions of IL-2 and IL-21 by peripheral Vδ2 T cells and IFN-γ by tumor-infiltrating γδ T cells contribute to the progression of HCC. Contradictorily, in AHB patients, IFN-γ-producing peripheral Vδ2 T cells and IFN-γ and TNF-α-producing peripheral γδ T cells can inhibit AHB infection. In addition, IFN-γ and TNF-α-producing peripheral Vδ2 T cells and TNF-α-producing CD107a+ peripheral γδ T cells inhibit the progression of CHB infection. Furthermore, hepatic γδ T cells as well as in vitro activated peripheral Vδ2 T cells inhibit the progression of CHC infection. Furthermore, increased number of peritumor and tumor γδ T cells as well as in vitro activated peripheral Vδ2 T cells inhibit the progression of HCC (Figure 1, bottom).
Although functions of γδ T cells are summarized above, some of their roles in virus infection remain obscure. For instance, IL-17-producing Vγ4 T cells display diverse roles to influence the development of liver cirrhosis in the same mouse model. Furthermore, the role of human peripheral γδ T cells but not hepatic γδ T cells has been extensively studied. Thus, the impact of cytokine production and the functions of hepatic γδ T cell subsets in the pathogenesis of HBV and HCV infections require further investigation. The frequency and function of γδ T cells can be distinguished based on human race, age, and gender, thus these factors have to be considered in related researches (28, 29). Asian Americans display two- to three-fold higher number of peripheral Vδ2 T cells compared to non-Asian Americans (28), which in turn may partially contribute to the immune responses and outcome of virus infection. Moreover, the fate of transferred γδ T cells in the human body as well as the indication and race of liver cancer patients should be assessed to achieve better therapeutic outcomes during treatment. Last but not least, in view of their antiviral function, IFN-γ-producing γδ T cell-based therapies should be developed for patients in stages of virus infection other than HCC. Understanding the roles of γδ T cells in relation to the pathogenesis of HBV and HCV infections may facilitate in the development of γδ T cell-based therapy or γδ T cell-based targets for the treatment of virus infections.
Author Contributions
WH wrote the main part of the review. XW wrote the Introduction and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Natural Science Foundation of Tianjin City (18JCZDJC34500), the State Key Laboratory of Medicinal Chemical Biology, Nankai University (2019003), and Tianjin First Central Hospital Spring Funding (CM201813).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Rajoriya N, Fergusson JR, Leithead JA, Klenerman P. Gamma Delta T-lymphocytes in Hepatitis C and Chronic Liver Disease. Front Immunol (2014) 5:400. doi: 10.3389/fimmu.2014.00400
2. Lee HM, Banini BA. Updates on Chronic HBV: Current Challenges and Future Goals. Curr Treat Options Gastroenterol (2019) 17:271–91. doi: 10.1007/s11938-019-00236-3
3. Jia ZH, Li YY, Wang JY, Zhang JY, Huang A, Guo XD, et al. Activated gammadelta T cells exhibit cytotoxicity and the capacity for viral clearance in patients with acute hepatitis B. Clin Immunol (2019) 202:40–8. doi: 10.1016/j.clim.2019.03.005
4. Seo W, Eun HS, Kim SY, Yi HS, Lee YS, Park SH, et al. Exosome-mediated activation of toll-like receptor 3 in stellate cells stimulates interleukin-17 production by gammadelta T cells in liver fibrosis. Hepatology (2016) 64:616–31. doi: 10.1002/hep.28644
5. Lafaro KJ, Demirjian AN, Pawlik TM. Epidemiology of hepatocellular carcinoma. Surg Oncol Clinics North America (2015) 24(1):1–17. doi: 10.1016/j.soc.2014.09.001
6. Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology (2006) 43:S54–62. doi: 10.1002/hep.21060
7. Shin EC, Sung PS, Park SH. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat Rev Immunol (2016) 16:509–23. doi: 10.1038/nri.2016.69
8. Saeidi A, Zandi K, Cheok YY, Saeidi H, Wong WF, Lee CYQ, et al. T-Cell Exhaustion in Chronic Infections: Reversing the State of Exhaustion and Reinvigorating Optimal Protective Immune Responses. Front Immunol (2018) 9:2569. doi: 10.3389/fimmu.2018.02569
9. Zhang JY, Zhang Z, Lin F, Zou ZS, Xu RN, Jin L, et al. Interleukin-17-producing CD4(+) T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology (2010) 51:81–91. doi: 10.1002/hep.23273
10. Li TY, Yang Y, Zhou G, Tu ZK. Immune suppression in chronic hepatitis B infection associated liver disease: A review. World J Gastroenterol (2019) 25:3527–37. doi: 10.3748/wjg.v25.i27.3527
11. Kohlgruber AC, Gal-Oz ST, LaMarche NM, Shimazaki M, Duquette D, Koay HF, et al. γδ T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat Immunol (2018) 19:464–74. doi: 10.1038/s41590-018-0094-2
12. Pellicci DG, Koay HF, Berzins SP. Thymic development of unconventional T cells: how NKT cells, MAIT cells and gammadelta T cells emerge. Nat Rev Immunol (2020) 20:756–70. doi: 10.1038/s41577-020-0345-y
13. Wang X, Tian Z. gammadelta T cells in liver diseases. Front Med (2018) 12:262–8. doi: 10.1007/s11684-017-0584-x
14. Papadopoulou M, Sanchez SG, Vermijlen D. Innate and adaptive γδ T cells: How, when, and why. Immunol Rev (2020) 00:1–18. doi: 10.1111/imr.12926
15. Hunter S, Willcox CR, Davey MS, Kasatskaya SA, Jeffery HC, Chudakov DM, et al. Human liver infiltrating γδ T cells are composed of clonally expanded circulating and tissue-resident populations. J Hepatol (2018) 69(3):654–65. doi: 10.1016/j.jhep.2018.05.007
16. Benova K, Hanckova M, Koci K, Kudelova M, Betakova T. T cells and their function in the immune response to viruses. Acta Virol (2020) 64:131–43. doi: 10.4149/av_2020_203
17. Wu X, Zhang JY, Huang A, Li YY, Zhang S, Wei J, et al. Decreased Vdelta2 gammadelta T cells associated with liver damage by regulation of Th17 response in patients with chronic hepatitis B. J Infect Dis (2013) 208:1294–304. doi: 10.1093/infdis/jit312
18. Lai Q, Ma S, Ge J, Huang Z, Huang X, Jiang X, et al. TCR gammadelta(+)CD4(-)CD8(-) T cells suppress the CD8(+) T-cell response to hepatitis B virus peptides, and are associated with viral control in chronic hepatitis B. PloS One (2014) 9:e88475. doi: 10.1371/journal.pone.0088475
19. Kong X, Sun R, Chen Y, Wei H, Tian Z. gammadeltaT cells drive myeloid-derived suppressor cell-mediated CD8+ T cell exhaustion in hepatitis B virus-induced immunotolerance. J Immunol (2014) 193:1645–53. doi: 10.4049/jimmunol.1303432
20. Sugai S, Yoshikawa T, Iwama T, Tsuchiya N, Ueda N, Fujinami N, et al. Hepatocellular carcinoma cell sensitivity to Vgamma9Vdelta2 T lymphocyte-mediated killing is increased by zoledronate. Int J Oncol (2016) 48:1794–804. doi: 10.3892/ijo.2016.3403
21. Qian L, Wang N, Tian H, Jin H, Zhao H, Niu C, et al. Dual Effects of Cellular Immunotherapy in Inhibition of Virus Replication and Prolongation of Survival in HCV-Positive Hepatocellular Carcinoma Patients. J Immunol Res (2016) 2016:6837241. doi: 10.1155/2016/6837241
22. Ferrari C. HBV and the immune response. Liver Int (2015) 35(Suppl 1):121–8. doi: 10.1111/liv.12749
23. Conroy MJ, Mac Nicholas R, Taylor M, O’dea S, Mulcahy F, Norris S, et al. Increased Frequencies of Circulating IFN-gamma-Producing Vdelta1(+) and Vdelta2(+) gammadelta T Cells in Patients with Asymptomatic Persistent Hepatitis B Virus Infection. Viral Immunol (2015) 28:201–8. doi: 10.1089/vim.2014.0133
24. Chang L, Wang L, Ling N, Peng H, Chen M. Increase in liver gammadelta T cells with concurrent augmentation of IFN-beta production during the early stages of a mouse model of acute experimental hepatitis B virus infection. Exp Ther Med (2020) 19:67–78. doi: 10.3892/etm.2019.8197
25. Cannizzo ES, Tincati C, Binda F, Ronzi P, Cazzaniga FA, Antinori S, et al. Unconventional T cells in chronic hepatitis B patients on long-term suppressive therapy with tenofovir followed by a Peg-IFN add-on strategy: A randomized study. J Viral Hepat (2018) 25:381–90. doi: 10.1111/jvh.12820
26. Chen M, Zhang D, Zhen W, Shi Q, Liu Y, Ling N, et al. Characteristics of circulating T cell receptor gamma-delta T cells from individuals chronically infected with hepatitis B virus (HBV): an association between V(delta)2 subtype and chronic HBV infection. J Infect Dis (2008) 198:1643–50. doi: 10.1086/593065
27. Chen M, Hu P, Ling N, Peng H, Lei Y, Hu H, et al. Enhanced functions of peripheral gammadelta T cells in chronic hepatitis B infection during interferon alpha treatment in vivo and in vitro. PLoS One (2015) 10:e0120086. doi: 10.1371/journal.pone.0120086
28. Chang KM, Traum D, Park JJ, Ho S, Ojiro K, Wong DK, et al. Distinct phenotype and function of circulating Vdelta1+ and Vdelta2+ gammadeltaT-cells in acute and chronic hepatitis B. PLoS Pathog (2019) 15:e1007715. doi: 10.1371/journal.ppat.1007715
29. Cairo C, Armstrong CL, Cummings JS, Deetz CO, Tan M, Lu C, et al. Impact of age, gender, and race on circulating γδ T cells. Hum Immunol (2010) 71(10):968–75. doi: 10.1016/j.humimm.2010.06.014
30. Chen M, Hu P, Peng H, Zeng W, Shi X, Lei Y, et al. Enhanced peripheral gammadeltaT cells cytotoxicity potential in patients with HBV-associated acute-on-chronic liver failure might contribute to the disease progression. J Clin Immunol (2012) 32:877–85. doi: 10.1007/s10875-012-9678-z
31. Tseng CT, Miskovsky E, Houghton M, Klimpel GR. Characterization of liver T-cell receptor gammadelta T cells obtained from individuals chronically infected with hepatitis C virus (HCV): evidence for these T cells playing a role in the liver pathology associated with HCV infections. Hepatology (2001) 33:1312–20. doi: 10.1053/jhep.2001.24269
32. Agrati C, D’offizi G, Narciso P, Abrignani S, Ippolito G, Colizzi V, et al. Vdelta1 T lymphocytes expressing a Th1 phenotype are the major gammadelta T cell subset infiltrating the liver of HCV-infected persons. Mol Med (2001) 7:11–9. doi: 10.1007/BF03401834
33. Par G, Rukavina D, Podack ER, Horanyi M, Szekeres-Bartho J, Hegedus G, et al. Decrease in CD3-negative-CD8dim(+) and Vdelta2/Vgamma9 TcR+ peripheral blood lymphocyte counts, low perforin expression and the impairment of natural killer cell activity is associated with chronic hepatitis C virus infection. J Hepatol (2002) 37:514–22. doi: 10.1016/S0168-8278(02)00218-0
34. Alonzi T, Agrati C, Costabile B, Cicchini C, Amicone L, Cavallari C, et al. Steatosis and intrahepatic lymphocyte recruitment in hepatitis C virus transgenic mice. J Gen Virol (2004) 85:1509–20. doi: 10.1099/vir.0.19724-0
35. Yin W, Tong S, Zhang Q, Shao J, Liu Q, Peng H, et al. Functional dichotomy of Vdelta2 gammadelta T cells in chronic hepatitis C virus infections: role in cytotoxicity but not for IFN-gamma production. Sci Rep (2016) 6:26296. doi: 10.1038/srep26296
36. Agrati C, Alonzi T, De Santis R, Castilletti C, Abbate I, Capobianchi MR, et al. Activation of Vgamma9Vdelta2 T cells by non-peptidic antigens induces the inhibition of subgenomic HCV replication. Int Immunol (2006) 18:11–8. doi: 10.1093/intimm/dxh337
37. Wu D, Yan WM, Wang HW, Huang D, Luo XP, Ning Q. gammadelta T Cells Contribute to the Outcome of Murine Fulminant Viral Hepatitis via Effector Cytokines TNF-alpha and IFN-gamma. Curr Med Sci (2018) 38:648–55. doi: 10.1007/s11596-018-1926-x
38. Lee JW, Kim W, Kwon EK, Kim Y, Shin HM, Kim DH, et al. Immunological dynamics associated with rapid virological response during the early phase of type I interferon therapy in patients with chronic hepatitis C. PLoS One (2017) 12:e0179094. doi: 10.1371/journal.pone.0179094
39. Ravens S, Hengst J, Schlapphoff V, Deterding K, Dhingra A, Schultze-Florey C, et al. Human gammadelta T Cell Receptor Repertoires in Peripheral Blood Remain Stable Despite Clearance of Persistent Hepatitis C Virus Infection by Direct-Acting Antiviral Drug Therapy. Front Immunol (2018) 9:510. doi: 10.3389/fimmu.2018.00510
40. Ghosh A, Mondal RK, Romani S, Bagchi S, Cairo C, Pauza CD, et al. Persistent gamma delta T-cell dysfunction in chronic HCV infection despite direct-acting antiviral therapy induced cure. J Viral Hepat (2019) 26:1105–16. doi: 10.1111/jvh.13121
41. Dewidar B, Meyer C, Dooley S, Meindl-Beinker AN. TGF-beta in Hepatic Stellate Cell Activation and Liver Fibrogenesis-Updated 2019. Cells (2019) 8(11):1419. doi: 10.3390/cells8111419
42. Hammerich L, Bangen JM, Govaere O, Zimmermann HW, Gassler N, Huss S, et al. Chemokine receptor CCR6-dependent accumulation of gammadelta T cells in injured liver restricts hepatic inflammation and fibrosis. Hepatology (2014) 59:630–42. doi: 10.1002/hep.26697
43. Liu M, Hu Y, Yuan Y, Tian Z, Zhang C. gammadeltaT Cells Suppress Liver Fibrosis via Strong Cytolysis and Enhanced NK Cell-Mediated Cytotoxicity Against Hepatic Stellate Cells. Front Immunol (2019) 10:477. doi: 10.3389/fimmu.2019.00477
44. Zhou BY, Gong JH, Cai XY, Wang JX, Luo F, Jiang N, et al. An imbalance between stellate cells and gammadeltaT cells contributes to hepatocellular carcinoma aggressiveness and recurrence. Hepatol Int (2019) 13:631–40. doi: 10.1007/s12072-019-09969-w
45. Cai XY, Wang JX, Yi Y, He HW, Ni XC, Zhou J, et al. Low counts of gammadelta T cells in peritumoral liver tissue are related to more frequent recurrence in patients with hepatocellular carcinoma after curative resection. Asian Pac J Cancer Prev (2014) 15:775–80. doi: 10.7314/APJCP.2014.15.2.775
46. Zhao N, Dang H, Ma L, Martin SP, Forgues M, Ylaya K, et al. Intratumoral gammadelta T-cell infiltrates, CCL4/5 protein expression and survival in patients with hepatocellular carcinoma. Hepatology (2020). doi: 10.1002/hep.31412
47. Tian W, Ma J, Shi R, Ren C, He J, Zhao H. gammadelta T cell-mediated individualized immunotherapy for hepatocellular carcinoma considering clinicopathological characteristics and immunosuppressive factors. Oncol Lett (2018) 15:5433–42. doi: 10.3892/ol.2018.8026
48. Hoh A, Dewerth A, Vogt F, Wenz J, Baeuerle PA, Warmann SW, et al. The activity of gammadelta T cells against paediatric liver tumour cells and spheroids in cell culture. Liver Int (2013) 33:127–36. doi: 10.1111/liv.12011
49. Tanaka Y, Kobayashi H, Terasaki T, Toma H, Aruga A, Uchiyama T, et al. Synthesis of pyrophosphate-containing compounds that stimulate Vgamma2Vdelta2 T cells: application to cancer immunotherapy. Med Chem (2007) 3:85–99. doi: 10.2174/157340607779317544
50. Toutirais O, Cabillic F, Le Friec G, Salot S, Loyer P, Le Gallo M, et al. DNAX accessory molecule-1 (CD226) promotes human hepatocellular carcinoma cell lysis by Vgamma9Vdelta2 T cells. Eur J Immunol (2009) 39:1361–8. doi: 10.1002/eji.200838409
51. Qian P, Zhang YW, Zhou ZH, Liu JQ, Yue SY, Guo XL, et al. Artesunate enhances gammadelta T-cell-mediated antitumor activity through augmenting gammadelta T-cell function and reversing immune escape of HepG2 cells. Immunopharmacol Immunotoxicol (2018) 40:107–16. doi: 10.1080/08923973.2017.1386212
52. Yi Y, He HW, Wang JX, Cai XY, Li YW, Zhou J, et al. The functional impairment of HCC-infiltrating gammadelta T cells, partially mediated by regulatory T cells in a TGFbeta- and IL-10-dependent manner. J Hepatol (2013) 58:977–83. doi: 10.1016/j.jhep.2012.12.015
53. Jiang H, Yang Z, Song Z, Green M, Song H, Shao Q. gammadelta T cells in hepatocellular carcinoma patients present cytotoxic activity but are reduced in potency due to IL-2 and IL-21 pathways. Int Immunopharmacol (2019) 70:167–73. doi: 10.1016/j.intimp.2019.02.019
54. Di Blasi D, Boldanova T, Mori L, Terracciano L, Heim MH, De Libero G. Unique T-Cell Populations Define Immune-Inflamed Hepatocellular Carcinoma. Cell Mol Gastroenterol Hepatol (2020) 9:195–218. doi: 10.1016/j.jcmgh.2019.08.004
Keywords: γδ T cells, hepatitis B virus, hepatitis C virus, progression, cytokines
Citation: Hou W and Wu X (2021) Diverse Functions of γδ T Cells in the Progression of Hepatitis B Virus and Hepatitis C Virus Infection. Front. Immunol. 11:619872. doi: 10.3389/fimmu.2020.619872
Received: 21 October 2020; Accepted: 17 December 2020;
Published: 01 February 2021.
Edited by:
Jianlei Hao, Jinan University, ChinaReviewed by:
David Vermijlen, Université libre de Bruxelles, BelgiumSarina Ravens, Hannover Medical School, Germany
Carolina Cristina Jancic, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina
Copyright © 2021 Hou and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoli Wu, wuxiaoli@tju.edu.cn
 Wen Hou
Wen Hou Xiaoli Wu
Xiaoli Wu