- Department of Plant Biology and Plant Biotechnology, Presidency College (Autonomous), Chennai, Tamil Nadu, India
Cyanobacteria are widely distributed across numerous ecosystems that include aquatic, terrestrial as well as extreme habitats such as the polar regions, hypersaline waters, hot springs, and deserts. They are known to play a key role in bringing oxygen on Earth. The present investigation reports the potential of Desertifilum sp. PSL17, a freshwater blue-green algae, to produce indole 3-acetic acid (IAA) and ammonia and also trigger the efficient germination of rice and promote the growth of green gram (Vigna radiata). Desertifilum sp. PSL17 was found to produce significant amounts of IAA (87.3 µg/mL) under optimal growth conditions. Tryptophan (trp) induction, promoting a 2.3-fold increase in IAA production, signifies trp-mediated IAA production in Desertifilum sp. PSL17. Rice seeds exhibited remarkable germination ability in the presence of the alga in comparison to untreated seeds. When co-cultivated with green gram (Vigna radiata), it exhibited a profound impact on plant growth promotion, resulting in increased root length, shoot length, and biomass production compared to the control. The substantial production of IAA and ammonia by Desertifilum sp. PSL17 plays a pivotal role in plant growth promotion. Our findings suggest that this freshwater Desertifilum sp. PSL17 has enormous prospective as a biofertilizer for promoting sustainable agriculture practices, particularly for green gram cultivation.
1 Introduction
Cyanobacteria, also called blue-green algae, are the oldest forms of life. They take part in biological processes such as the biogeochemical cycling of oxygen, nitrogen, and carbon and are known as one of the most important primary producers (Sharma et al., 2011). Cyanobacteria have long been recognized for their capacity to produce a wide array of bioactive compounds, including pigments, polysaccharides, and fatty acids. These secondary metabolites are of significant interest due to their antioxidant, antimicrobial, and anti-inflammatory properties, among other health benefits (Bouyahya et al., 2024). Desertifilum, a relatively recent addition to the genus of filamentous cyanobacteria, represents a fascinating subject of study due to its remarkable adaptations to extreme environments such as desert soil crusts and saline–alkaline lakes. This genus, first described in 2012 (Dadheech et al., 2012), is known for its unique ability to survive and thrive under harsh conditions, including high temperatures, intense UV radiation, and desiccation (Cellamare et al., 2018). The growing interest in Desertifilum is driven not only by its ecological significance but also by its potential applications in biotechnology and medicine, given its rich phytochemical composition and diverse biological activities (Shawer et al., 2022). Desertifilum, with its unique habitat and physiological traits, presents a promising source of such valuable compounds. However, comprehensive studies focusing on its phytochemical profile, detailed characterization, and the potential biological activities of its metabolites remain sparse.
Cyanobacteria have been reported to produce important growth regulators such as gibberellins, auxins, ethylene, cytokinin, abscisic acid, and jasmonic acid (Wang and Irving, 2011). One of the most physiologically active members of the auxin family of phytohormones is IAA. Root initiation and elongation and a number of other processes concerned with the differentiation and proliferation of plant tissues are carried out by IAA (Aloni, 2013). Sergeeva et al. (2002) have demonstrated the ability of cyanobacteria to produce IAA. It is a universal fact that trp serves as a precursor for the biosynthesis of auxin in plants and microorganisms, and the addition of trp always has a stimulating effect (Spaepen et al., 2007). Microorganisms utilize trp-dependent pathways in the production of IAA. The growth of rice treated with the culture of Phormidium sp. exhibited varied growth parameters based on the type of fractions used, the culture concentrations, and the form of culture used for the extraction process (Jaiswal et al., 2018). Microorganisms, in addition to balancing mineral nutrition and naturally fertilizing the soils, synthesize growth-promoting substances which improve plant health (Jacob and Paranthaman, 2023; Lelapalli et al., 2021). Cyanobacteria, in addition to nitrogen fixation, carry out oxygenic photosynthesis and release an ample number of substances, both organic and inorganic, in the culture medium, which are considered as significant factors toward plant growth promotion (Singh, 2014; Toribio et al., 2020). Researchers have reported promising results on the utilization of cyanobacteria in a hydroponic system by exhibiting plant growth promotion (Kholssi et al., 2021; Bharti et al., 2019).
A unique algal isolate from freshwater of Permabalum Lake, Tamil Nadu, India, caught our attention, sparking curiosity and prompting further investigation into its characteristics, which led to the identification of this isolate as Desertifilum. Significant phytochemicals and compounds were present in this algal extract which exhibited antibacterial properties against various human pathogens (Paheshwari et al., 2024). Understanding the chemical makeup of Desertifilum is essential for the exploration of its biotechnological potential. Considering the demand for sustainable agricultural practices and the need for eco-friendly alternatives, this study is aimed to investigate the potential of a freshwater Desertifilum sp. PSL17 to produce IAA and ammonia and promote the growth of green gram and induce seed germination in rice. By undertaking this study, we hoped to contribute to the development of a novel, sustainable, and environment-friendly approach to agricultural practices and to provide a scientific basis for the potential use of Desertifilum sp. PSL17 as a biofertilizer in rice and green gram cultivation.
2 Materials and methods
2.1 Production and quantification of IAA in Desertifilum sp. PSL17 culture
Screening for IAA production was evaluated by Salkowski’s colorimetric method (Babu et al., 2013). A 10-day-old culture grown in BG11 medium without trp supplement was taken to determine the IAA production. Various concentrations of the culture supernatant were taken, to which distilled water was added to make the volume up to 2 mL, followed by the addition of 2 mL of Salkowski’s reagent. The reaction mixture was kept in the dark for 30 min, followed by reading the absorbance at 535 nm in a UV spectrophotometer. For the standard solution, 1.0 mg of IAA (Hi-media) was dissolved in 1 mL of methanolic water and used as a stock.
2.2 Evaluation of IAA production in tryptophan-supplemented culture
The Desertifilum sp. PSL17 strain was inoculated in 10 test tubes containing 4 mL of BG11 medium with various concentrations of trp ranging from 10 to 100 μg/mL. Two test tubes were maintained as controls, one with BG11 containing a known concentration of IAA and the other contained culture in BG11 without trp. The contents of the test tubes were adjusted to a final volume of 5 mL each. All of the test tubes were incubated at 28°C–30°C for 3 days. The experiment was carried out in triplicates thrice. IAA estimation was conducted after 10, 20, and 30 days of incubation as per the standard protocol (Patten and Glick, 2002).
2.3 Methanol extraction of tryptophan-supplemented culture
To detect for the presence of IAA, methanol extract of Desertifilum sp. PSL17 grown in BG11 supplemented with trp was subjected to TLC (Ahmed et al., 2010). The 15-day-old culture was centrifuged and the supernatant was transferred to a fresh tube. Furthermore, 5 mL of the culture supernatant was taken in a tube and was added with twice the volume of methanol. The resultant mixture was poured in a petri plate and left undisturbed at room temperature until it completely evaporates. The IAA extract dissolved in methanol was spotted and developed on silica gel TLC plates using isopropanol/ammonia/water [10/1/1 (v/v/v)] as the mobile phase. The plates were then sprayed with the reagent (1% H2SO4 in 20 mL of methanol containing 10 mg of FeCl3) and heated in an oven at 80°C until color development.
2.4 Screening for ammonia production
Test tubes with 2 mL of peptone water were taken. To the sample tube, 200 µL of Desertifilum sp. PSL17 culture was added, and the total volume was made up to 3 mL using peptone water. One test tube was maintained as control where 3 mL of peptone water without the culture was taken. All of the tubes were incubated at 28°C for 4 days, followed by the addition of 1 mL of Nessler’s reagent to each tube, and the color change was observed and recorded (Afify and Ashour, 2018). The experiment was performed thrice in triplicates.
2.5 Effect of Desertifilum sp. PSL17 on rice germination on agar plates
Cell-free culture supernatant was evaluated for the ability to promote or inhibit seed germination and seedling growth (Jaiswal et al., 2018). Then, 5 mL each of 14- and 21-day-old culture supernatant (14-DOCS and 21-DOCS) were used for the bio-assay on rice seeds. The rice seeds were surface-sterilized with 0.1% HgCl2 for 3 min prior to the bio-assay, and three different variables of germinations in sterilized petri plates were taken in triplicates for the study. The first set of control plates was poured with 20 mL BG11 medium and agar, the second set of control plates was poured with 20 mL sterile water and agar, and the third set was the test plates where 5 mL of culture supernatant and agar were poured. An equal number of surface-sterilized seeds was placed on the agar, and all of the plates were incubated at 28°C in the dark to promote germination. After 15 days, the percentage of germination and the length (cm) of the shoots and roots were measured and recorded.
2.6 Evaluation of the growth promotion effect of Desertifilum sp. PSL17 on Vigna radiata
The plant-growth-promoting effect of Desertifilum sp. PSL17 was evaluated on green grams (Vigna radiata). Among the two variables employed to test the growth promotion effect of the alga, one was by soaking the seeds for 30 min in 15-day-old Desertifilum sp. PSL17 culture (algal seed priming) and the other was by directly mixing the surface-sterilized seeds with 15-day-old culture biomass (algal seed coating) before sowing in the cups. For the control, seeds without any treatment were used. Appropriate conditions were maintained for both the treated and control seeds, and growth parameters such as height of the seedling, shoot length, leaf length, leaf breadth, number of flowers, number of pods, and number of seeds were observed and recorded at 24-h interval for 30 days. After 30 days, the plants from one cup from both the treatment and control were uprooted, and their fresh weight, dry weight, root length, and shoot length were recorded and tabulated.
Data analysis was performed using OriginLab software trial version. The results were considered to be significant at P < 0.05. Correlation coefficient was calculated for the data wherever necessary.
3 Results
3.1 Production of IAA and ammonia by Desertifilum sp. PSL17
Desertifilum sp. PSL17 exhibited the ability to produce IAA and ammonia. The total amount of IAA synthesized was quantified by using the Salkowski colorimetric method, and it was compared with an authentic IAA curve. Using the standard as reference, it was found that the IAA concentration present in the 10-day-old culture extract was 87.3 µg/mL. No significant increase in the IAA concentration was observed with an increase in the days of incubation. Desertifilum sp. PSL17 showed remarkable results for IAA production when grown in the presence of varied concentrations of trp. The IAA estimation carried out after 10, 20, and 30 days showed an increase in the production of IAA as the days of incubation increased. The quantity of IAA produced at the 10th, 20th, and 30th day was 169.7, 209.2, and 247.3 µg/mL, respectively, in 50 µg/mL of trp-supplemented culture, which is a 2.3-fold increase in concentration when compared to the IAA produced in culture without trp supplement (Figure 1a). Ammonia production was evaluated by a color change observed in peptone water. Post-incubation, upon adding Nessler’s reagent, there was no color change in the peptone water without the culture (Figure 1b-A), whereas in the peptone broth with the culture, brown color formation was observed after the addition of Nessler’s reagent, which indicated the production of ammonia by Desertifilum sp. PSL17 (Figure 1b-B).
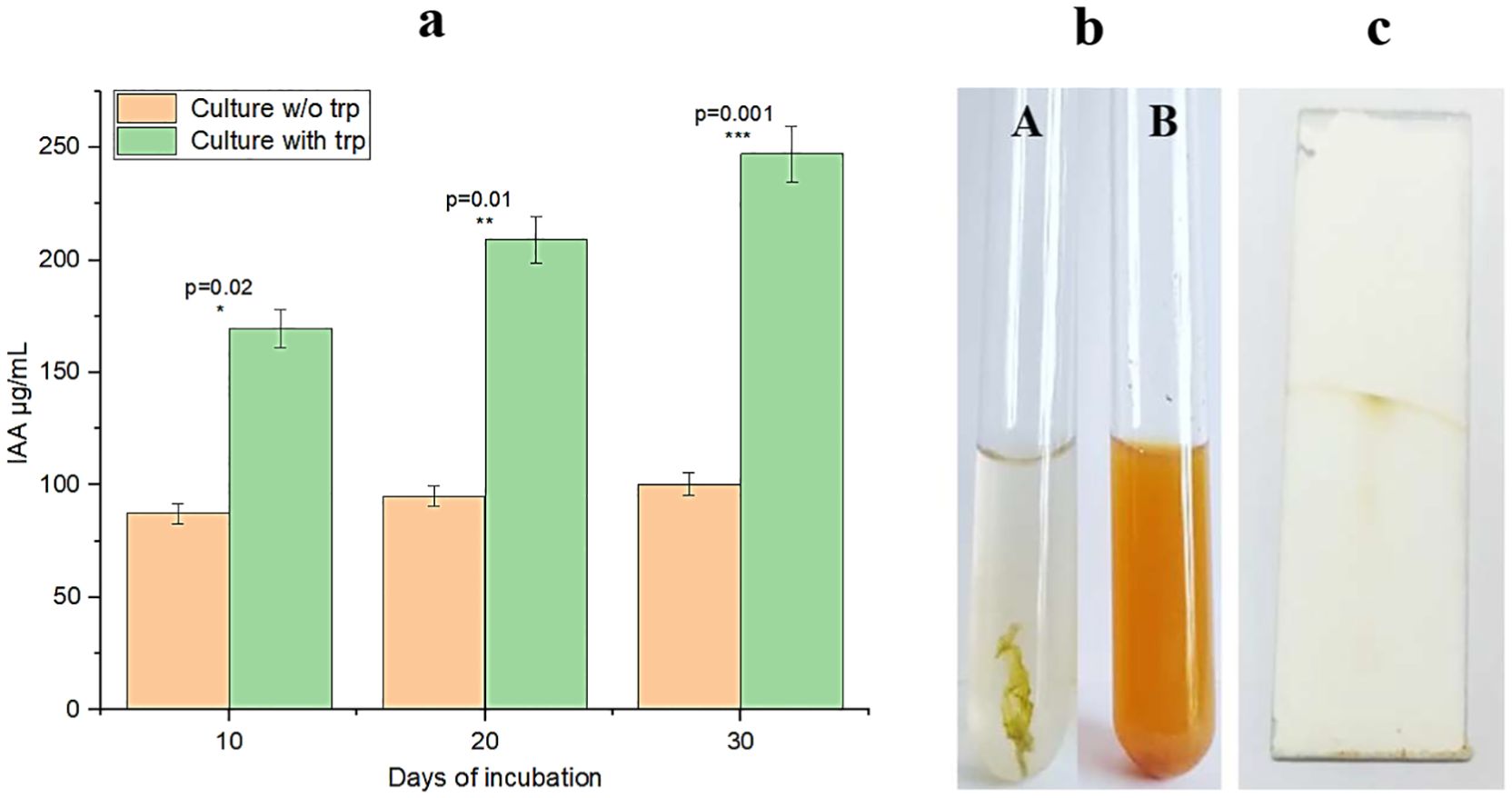
Figure 1. Production of IAA and ammonia by Desertifilum sp. PSL17. (a) Effect of tryptophan supplementation on IAA production in Desertifilum sp. PSL17 culture over time. IAA production was significantly higher in the trp-supplemented (50 µg/mL) culture compared to the culture without trp with a 2.3-fold increase observed (p < 0.001). At day 10, IAA produced without trp was only 87.3 µg/mL, whereas in the trp-supplemented culture, IAA was 169.7 µg/mL. An increase in IAA production was observed with an increase in incubation time reaching a maximum of 247.3 µg/mL on day 30 in the trp-supplemented culture. In contrast, no significant increase in IAA production was observed in the culture without trp supplementation beyond day 30. The experiment was conducted in triplicates. OriginLab software was used to perform the graphical and statistical analysis. (b) Ammonia production in Desertifilum sp. PSL17 culture. Ammonia production by Desertifilum sp. PSL17 (A) peptone water without culture and (B) peptone water with culture after the addition of Nessler’s reagent, indicating the presence of ammonia. (c) TLC analysis of IAA in Desertifilum sp. PSL17 methanol extract. Thin-layer chromatography analysis of the methanol extract of Desertifilum sp. PSL17, showing the presence of IAA as indicated by the red color on the TLC plate. *-significant; **-very significant; ***-highly significant.
3.2 IAA detection in methanol extract using TLC
Detection of IAA in the methanolic extract on TLC revealed a red color on the TLC plate after spraying the chromatoplate with a reagent containing H2SO4, methanol, and FeCl3, indicating the production of IAA by Desertifilum sp. PSL17 (Figure 1c).
3.3 Evaluation of the plant growth promotion ability of Desertifilum sp. PSL17
3.3.1 Effect of Desertifilum sp. PSL17 on rice germination
Desertifilum sp. PSL17 cultures showed a positive effect on the seed germination of rice. After 15 days of incubation, the germination frequency was higher in the presence of cell-free supernatant as compared to control 2 (Figure 2a). In control 1, no germination was observed (result not shown). The germination percentage of the control was only 30% (Figures 2a-A, C), whereas the treated seeds were exhibiting a remarkable percentage of germination. The cell-free supernatant of 14-DOCS-treated rice grains resulted in 100% germination (Figure 2a-B), and the 21-DOCS-treated ones exhibited 93% germination (Figure 2a-D). The average root and shoot length of the control seedling was 1.6 ± 0.2 and 4.5 ± 0.5 cm, respectively, and the 14-DOCS-treated plants exhibited an average root and shoot length of 2.8 ± 0.3 and 5.6 ± 0.6 cm, respectively. In a similar manner, the average root and shoot length of the control seedling was 3.2 ± 0.4 and 3.9 ± 0.3 cm, respectively, and the 21-DOCS-treated plants exhibited an average root and shoot length of 5 ± 0.5 and 5.8 ± 0.4, cm respectively (Figure 2b). Increased shoot length and root length, respectively, was observed in the treated seedlings when compared to the control seedlings.
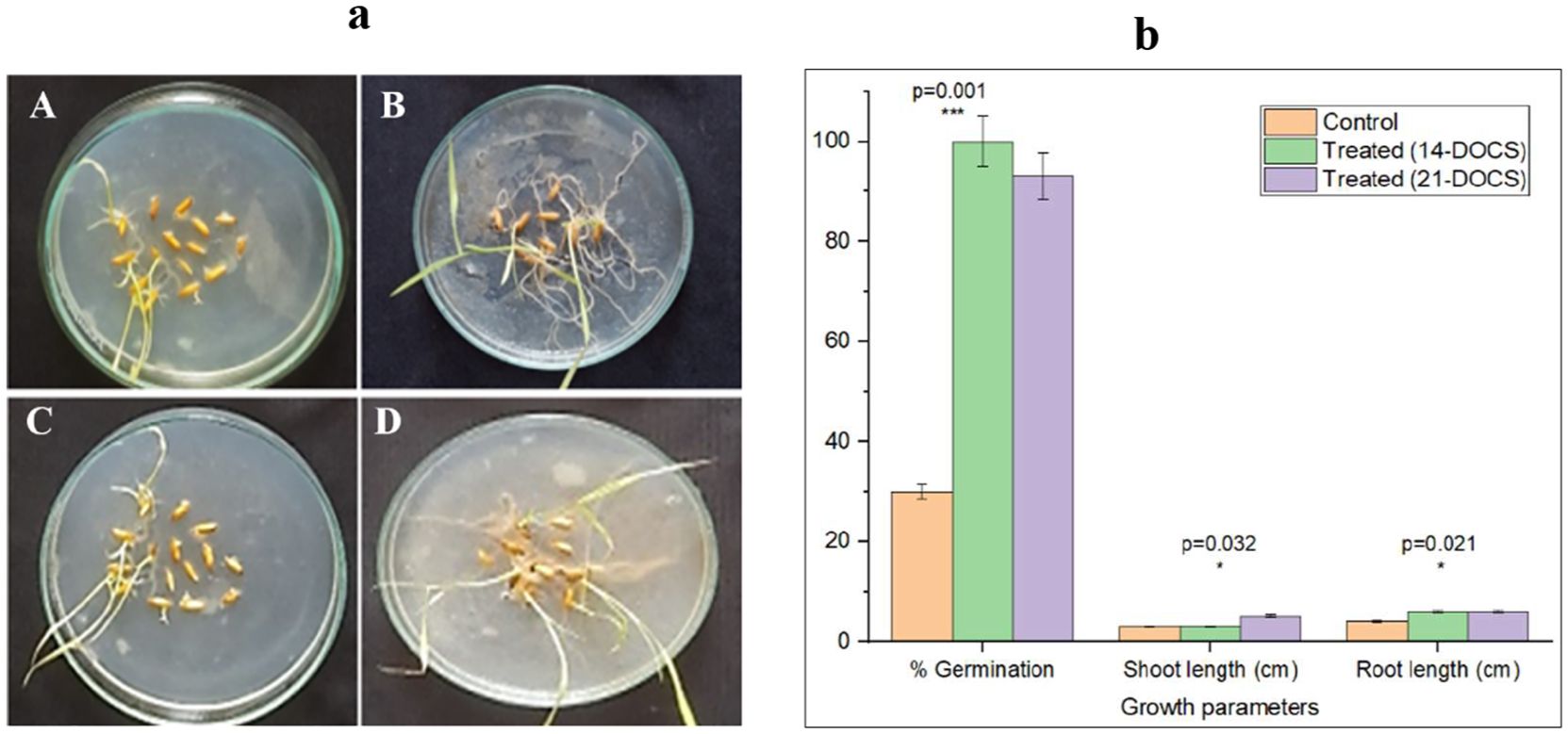
Figure 2. Effect of Desertifilum sp. PSL17 culture supernatant on rice seed germination. (a) Rice seed germination after 15 days of treatment with Desertifilum sp. PSL17 culture supernatant. (A, C) Control 2 (sterile water + agar) showed 30% germination, while no germination was observed in control 1 (BG11 medium + agar), result not shown. In contrast, seeds treated with (B) 14-DOCS and (D) 21-DOCS of Desertifilum sp. PSL17 exhibited 100% and 93% germination, respectively. The experiment was conducted in triplicates. *DOCS-days old culture supernatant. (b) Effect of Desertifilum sp. PSL17 culture supernatant on rice seed germination and seedling growth. Rice seeds treated with Desertifilum sp. PSL17 culture supernatant showed a significant improvement in germination and growth parameters after 15 days. No germination was observed in control 1 (BG11 + agar medium). In contrast, control 2 (sterile water + agar) showed 30% germination, while the seeds treated with 14-DOCS + agar and 21-DOCS + agar exhibited significantly higher germination rates (100% and 93%, respectively; p < 0.001). Moreover, the treated seedlings showed enhanced shoot and root lengths compared to the control seedlings (p < 0.05). The experiment was conducted in triplicates. OriginLab software was used to perform the graphical and statistical analysis. *-significant; ***-highly significant.
3.3.2 Evaluation of growth promotion of Vigna radiata (green gram)
The evaluation of Desertifilum sp. PSL17 for its growth-promoting ability was assessed in green gram (Vigna radiata). Right from sowing until harvesting, the seedlings/plants were maintained in sterile soil in paper cups. The plant-growth-promoting ability of Desertifilum sp. PSL17, when tested on green gram, revealed significant growth promotion that was observed in terms of various parameters such as fresh and dry weight of the seedlings, length of root, shoot and pods, length and breadth of leaf, and number of flowers, fruits, and seeds in each pod. One control and two treatment variables in triplicates were maintained, and the parameter in terms of plant growth was assessed. The overall growth was inferior in the control when compared to the treated plants (Figure 3a). Among the two treatments, treatment 2 (algal seed coating) was found to be superior when compared to the plants in treatment 1 (algal seed priming). The PSL17-treated seeds gave rise to seedlings that were superior in all of the parameters. The root length of treatment 1 and treatment 2 was 14.2 ± 1.5 and 17.1 ± 1.8 cm, respectively, compared to control, which had a root length of 12.0 ± 1.2 cm. Similarly, the shoot length of treatment 1 and treatment 2 was 41.2 ± 2.5 and 58.4 ± 2.2 cm, respectively, whereas the control had a shoot length of 36.1 ± 2.5 cm. Additionally, the treated plants exhibited larger leaf sizes compared to the control plants, which had smaller leaves (Figure 3b). The control plants produced flowers on the 65th day of germination, whereas the treated plants produced flowers on the 42nd day of germination itself. In treatment 2, the total number of flowers produced was six by the end of the 65th day, of which one withered, whereas the control plant produced only two flowers, of which 50% withered. The pod size was 2.5 ± 0.1 and 2.7± 0.1 cm in the treatment 1 and treatment 2 plants, respectively, and the pod size of the control plant was 1.9 ± 0.1 cm only. In contrast, the number of rootlets was greater in the control when compared to the treated plants (Figure 3c). The fresh weight and dry weight of the leaflets of the treated plants were twice as that of the control leaflet. The most interesting part was the number of seeds and the fresh and dry weight of the seeds, which exhibited remarkable differences. The treatment 1 and treatment 2 plants produced three and five seeds per pod, whereas the control plant produced pods with only one seed. The control leaves exhibited significantly lower fresh and dry weights compared to treatment 1 and treatment 2 leaves, which were larger and heavier. The fresh weight of the seeds per pod of the treatment 1 and treatment 2 plants was 2.3 ± 0.2 and 2.8 ± 0.3 g, respectively, whereas the seeds per pod of the control plants weighed only 1.2 ± 0.1 g, which is half the weight of the seeds of the treated plants. The dry weight of the seeds of the treatment 1 and treatment 2 plant pods was 1.4 ± 0.1 and 1.7 ± 0.2 g, respectively, whereas that of the control plant pods was 0.5 ± 0.05 g, which was one-third of the treated ones (Figure 3d). Overall height, appearance, and yield were superior in the case of the treated plants when compared to the control plants.
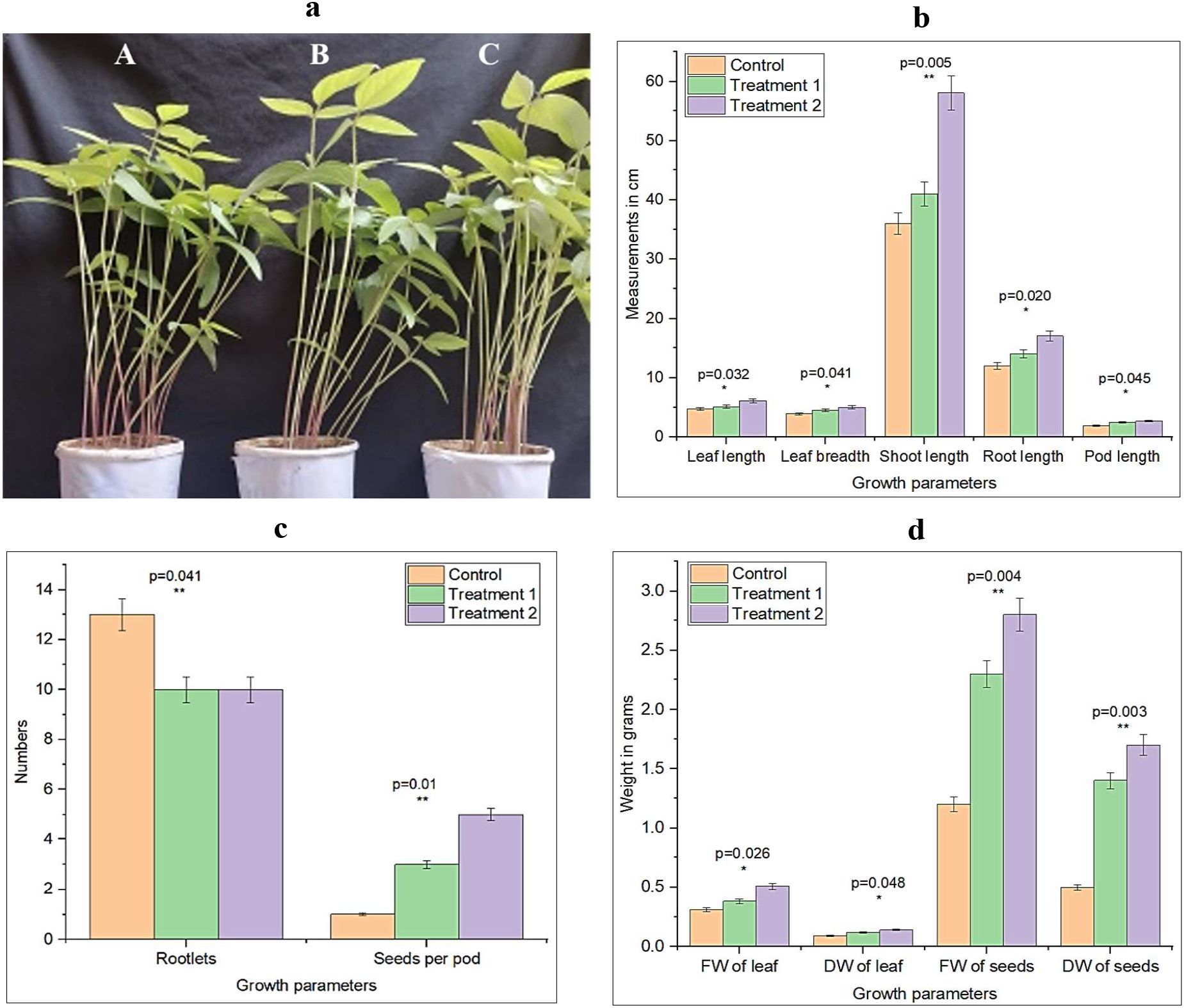
Figure 3. Growth promotion ability of Desertifilum sp. PSL17 on Vigna radiata (green gram). (a) Effect of Desertifilum sp. PSL17 on the overall growth of Vigna radiata. Desertifilum sp. PSL17 treatment significantly enhanced the overall growth of Vigna radiata seedlings. (A) Control, (B) treatment 1 (algal seed priming), and (C) treatment 2 (algal seed coating) showed varying degrees of growth promotion. (b) Effect of Desertifilum sp. PSL17 on the growth parameters of Vigna radiata. Treatment with Desertifilum sp. PSL17 significantly improved the growth parameters, including shoot length, root length, leaf length, and leaf breadth, compared to the control (p < 0.05). Both treatment 1 (algal seed priming) and treatment 2 (algal seed coating) exhibited enhanced growth attributes. The experiment was conducted in triplicates. OriginLab software was used to perform the graphical and statistical analysis. (c) Effect of Desertifilum sp. PSL17 on yield-related parameters. Treatment 2 (algal seed coating) significantly increased the number of seeds per pod compared to treatment 1 and control (p < 0.01). However, the control plants had more rootlets than the treated plants (p < 0.05). (d) Effect of Desertifilum sp. PSL17 on biomass and yield. Desertifilum sp. PSL17 treatment significantly increased the fresh and dry weights of leaves and seed weight per pod, resulting in enhanced crop yield (p < 0.001). Both treatment 1 and treatment 2 showed improved biomass and seed weight compared to the control. FW, fresh weight; DW, dry weight. *-significant; **-very significant.
4 Discussion
4.1 Production of IAA and ammonia by Desertifilum sp. PSL17
An essential biological element of the soil microbiota is cyanobacteria. Since they may produce physiologically active compounds that stimulate root development through nitrogen fixation, nutrient mobilization, and carbon sequestration in higher plants, several of them are regarded as plant-growth-boosting agents (Babu et al., 2015). Studies have indicated that the presence of auxin-like growth-promoting substances by cyanobacteria including Nostoc (Hashtroudi et al., 2013; Sergeeva et al., 2002) and Leptolyngbya sp. from freshwater and Geitlerinema sp. from an estuarine had the ability to produce IAA (Babu et al., 2013). Desertifilum sp. PSL17 exhibited the production of IAA at a concentration of 87.3 µg/mL after 10 days of incubation. The production of IAA by Desertifilum sp. PSL17 indicates that this species might play a role in plant growth promotion. One of our unpublished findings has shown the presence of gibberellic acid both in the aqueous and methanol extracts of Desertifilum sp. PSL17. The release of IAA by several Anabaena strains and IAA production with trp induction by free-living and associated cyanobacteria was well documented (Prasanna et al., 2010). The evaluation of trp-induced IAA production in Desertifilum sp. PSL17 exhibited an increase in IAA production with the increase in trp concentration. When IAA production was compared in the presence and absence of trp, IAA production was more prominent in the culture extract with trp. IAA at concentrations of 169.7, 209.2, and 247.3 µg/mL at the 10th, 20th, and 30th day, respectively, was produced in the presence of 50 µg/mL of trp. The remarkable increase in the IAA concentration was observed as the incubation days increased from 10 to 30 days. The 2.3-fold increase in IAA production in the culture supplemented with trp when compared to the IAA observed in the culture without trp supplement indicates that this alga could possess a trp-dependent pathway for the production of IAA. Studies on the role of IAA production in cyanobacteria are scarce, and so far, there are no reports to prove the trp-independent IAA production via the indole-3- pyruvic acid pathway. Sergeeva et al. (2002) reported that the secretion of IAA from the cyanobacterium Nostoc increased with incubation time, and Arthrospira platensis MMG-9 also accumulated IAA intracellularly along with release into the medium. Researchers have indicated that a number of bacteria produce IAA without trp induction (Lelapalli et al., 2021). Plants continuously interact with microorganisms, and some of them are capable of imparting plant-growth-promoting and biocontrol potential. Cyanobacteria are one among the microbial flora which contribute protection to plants from phytopathogens due to ammonia, hydrogen cyanide, and lytic enzyme production (Bagul et al., 2022; Prasanna et al., 2015; Kremer and Souissi, 2001). Desertifilum sp. PSL17 was positive for ammonia production, indicating the biocontrol activity and plant growth promotion potential of this cyanobacteria. Cyanobacterial exopolysaccharides (EPS) play a crucial role in plant growth promotion by improving the soil structure and water-holding capacity and stimulating plant root growth and development. Anabaena and Synechococcus produce siderophores, which are known to induce plant growth by solubilizing iron present in the soil, making it available to plants and promoting plant growth (Mohan et al., 2020). Studies have shown that exopolysaccharides and siderophore produced by a certain blue-green algae can induce defense mechanisms in plants through the production of enzymes, thereby enhancing resistance against pathogens (Drira et al., 2021; Righini et al., 2022).
4.2 Plant-growth-promoting ability of Desertifilum sp. PSL17
4.2.1 Desertifilum sp. PSL17 influences rice germination
Cyanobacteria can form a symbiotic relationship with plants and induce growth by improving soil fertility and crop productivity. In the current investigation, Desertifilum sp. PSL17 extracts have shown a significant effect on germination and an increase in the growth of rice seedlings. The effect of Desertifilum sp. PSL17 culture filtrates ranging from log phase and late log phase has been evaluated on rice seed germination and growth. The evaluation of extracellular exudates of Desertifilum sp. PSL17 cultures exhibited 100% positive effect in germination assay as well as growth promotion of the rice seedlings. Varying degrees of effect on the growth of roots and shoot length of seedlings were also observed. In terms of length of shoot and root recorded after 15 days, the seedlings with 21-DOCS treatment showed significantly higher values when compared to 14-DOCS treatment and the control. The average increase in root and shoot lengths was 1.7- and 1.2-fold, respectively, more than the control in the case of 14-DOCS and 1.5- and 1.4-fold more root and shoot length than the control was observed in 21-DOCS. A similar pattern was observed in the stimulation of both root and shoot of rice seedlings by the cell supernatant of Desertifilum sp. PSL17. The potential of cyanobacteria in inducing the germination and growth of rice seedlings by Nostoc sp. is well recorded (Yadav et al., 2022; Abedi Firoozjaei et al., 2021). The biologically active metabolites produced during the growth and proliferation of cyanobacteria in soil have often been interpreted with the potential of the organism to induce the growth promotion of crops. The release of miscellaneous metabolites by the growing cyanobacterial colonies in the soil may also assist in enhancing plant growth and yields in unfertile soils. However, the synthesis and production of phytohormones like auxin, gibberellin, and cytokinin by genera like Anabaenopsis, Anabaena, and Calothrix not only increase the acquisition of nutrients by plants but also actively promote their germination, growth, and development (Singh, 2014; Nawaz et al., 2024). Anabaena variabilis has been reported to enhance the growth and yield of rice and wheat seedlings (Bao et al., 2021). It is necessary to propose their role as PGPR because cyanobacteria naturally colonize the root surface of rice in saline soil. It is hypothesized that they promote plant growth in the soil having stress conditions. It can change the physical and chemical properties of soils together with nitrogen fixation and exopolysaccharide that are secreted from cyanobacteria, which may increase the organic carbon and nitrogen content and improve the water-holding capacity of infertile soils (Malam Issa et al., 2007).
4.2.2 Desertifilum sp. PSL17 augments the growth of Vigna radiata (green gram)
The plant-growth-promoting potential of the extracellular products of cyanobacteria is underexplored. Few investigations on the ability of cyanobacteria to induce the growth promotion of green gram have been recorded (Dineshkumar et al., 2020; Dey et al., 2017). Alallaf et al. (2023) have reported for the first time the ability of Desertifilum to induce the growth promotion of tomato as well as biocontrol of wilt disease caused by Fusarium oxysporum. Enhanced crop productivity was observed when Desertifilum sp. was co-cultivated with Pseudomonas (Khemka and Saraf, 2015). The present investigation is the third study on this alga toward plant growth promotion and the first study to evaluate the ability to induce the growth of green gram. In the current research, remarkable results were obtained in plant growth promotion when the green gram (Vigna radiata) seeds were treated with Desertifilum sp. PSL17 culture at 30°C for 30 min. A continuous observation made until day 90 gave a comprehensive finding on the growth promotion ability of this cyanobacteria on green grams. The treated seeds resulted in plants with increased shoot length and root length and leaves that were also bigger than those in the controls plants. Among the two treatments used in the test, the seeds treated with the biomass (treatment 2) exhibited promising results in terms of growth of root, shoot, leaf color, size, etc., when compared to the seeds soaked in culture supernatant, i.e., treatment 1. The results indicate that both treatments had a positive effect on pod size, with treatment 2 showing the most substantial increase. In contrast, the number of rootlets was greater in the control when compared to the treatment, indicating that growth promotion induced by the alga might be through hormonal or nutrient-related mechanisms accompanied with the reduction in rootlet formation, whereas the control plants are thriving hard to absorb nutrients and water by developing more rootlets. Bioformulations prepared with cyanobacteria are reported to cause growth promotion ability (Chabili et al., 2024; Prasanna et al., 2016). Recent studies have shown that inoculation with cyanobacteria can improve plant growth and soil health and reduce the need for chemical fertilizers (Win et al., 2018; Chittora et al., 2020). The present study demonstrated the plant-growth-promoting ability of Desertifilum sp. PSL17 and indicated that this strain is a promising blue-green alga which could be used in the formulation of biofertilizers for an eco-friendly and sustainable agriculture toward a green technology approach. Consequently, cyanobacteria play a significant role in reducing environmental pollution and promoting soil biodiversity and sustainable agricultural practices by generating high-value biomass and reducing carbon dioxide levels.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
PP: Methodology, Conceptualization, Investigation, Writing – original draft, Formal analysis. US: Data curation, Writing – original draft, Formal analysis. ZZ: Methodology, Data curation, Writing – original draft. SP: Supervision, Formal analysis, Conceptualization, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to thank the institution for providing the infrastructure.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abedi Firoozjaei M. H., Hassani S. B., Nazifi E., and Keypour S. (2021). Study the effect of the terrestrial cyanobacterium Nostoc commune aqueous extract on seed germination and seedling growth of rice. Plant Algae Environ. 5, 642–653. doi: 10.48308/jpr.2021.223334.1008
Afify A. and Ashour A. (2018). Use of cyanobacteria for controlling flax seedling blight. J. Agric. Chem. Biotechnol. 9, 259–261. doi: 10.21608/jacb.2018.36313
Ahmed M., Stal L. J., and Hasnain S. (2010). Production of indole-3-acetic acid by the cyanobacterium Arthrospira platensis strain MMG-9. J. Microbiol. Biotechnol. 20, 1259–1265. doi: 10.4014/jmb.1004.04033
Alallaf A. L., Kottb M. R., El-Sayed A. K., and Shafik H. M. (2023). Desertifilum tharense methanol extract inhibits vascular wilt caused by Fusarium oxysporum f. sp. lycopersici and promotes tomato growth. Egyptian J. Bot. 63, 505–523. doi: 10.21608/ejbo.2023.151351.2050
Aloni R. (2013). Role of hormones in controlling vascular differentiation and the mechanism of lateral root initiation. Planta 238, 819–830. doi: 10.1007/s00425-013-1927-8
Babu S. V., Ashokkumar B., Sivakumar N., Sudhakarsamy P., and Varalakshmi P. (2013). Indole-3-acetic acid from filamentous cyanobacteria: screening, strain identification and production. J. Sci. Ind. Res. 72, 581–584. Available at: http://nopr.niscpr.res.in/handle/123456789/20951.
Babu S., Bidyarani N., Chopra P., Monga D., Kumar R., Prasanna R., et al. (2015). Evaluating microbe-plant interactions and varietal differences for enhancing biocontrol efficacy in root rot disease challenged cotton crop. Eur. J. Plant Pathol. 142, 345–362. doi: 10.1007/s10658-015-0619-6
Bagul S. Y., Vishwakarma R., Chakdar H., Pandiyan K., and Bandeppa S. (2022). Plant growth-promoting and biocontrol potential of selected cyanobacteria from stress environment. Int. J. Plant Soil Sci. 23, 485–494. doi: 10.9734/IJPSS/2022/v34i2231400
Bao J., Zhuo C., Zhang D., Li Y., Hu F., Li H., et al. (2021). Potential applicability of a cyanobacterium as a biofertilizer and biopesticide in rice fields. Plant Soil 463, 97–112. doi: 10.1007/s11104-021-04899-9
Bharti A., Prasanna R., Kumar G., Kumar A., and Nain L. (2019). Co-cultivation of cyanobacteria for raising nursery of chrysanthemum using a hydroponic system. J. Appl. Phycol 31, 3625–3635. doi: 10.1007/s10811-019-01830-9
Bouyahya A., Bakrim S., Chamkhi I., Taha D., El Omari N., El Mneyiy N., et al. (2024). Bioactive substances of cyanobacteria and microalgae: sources, metabolism, and anticancer mechanism insights. Biomed Pharmacother 170, 115989. doi: 10.1016/j.biopha.2023.115989
Cellamare M., Duval C., Drelin Y., Djediat C., Touibi N., Agogué H., et al. (2018). Characterization of phototrophic microorganisms and description of new cyanobacteria isolated from the saline-alkaline crater-lake Dziani Dzaha (Mayotte, Indian Ocean). FEMS Microbiol. Ecol. 94, 108. doi: 10.1093/femsec/fiy108
Chabili A., Minaoui F., Hakkoum Z., Douma M., Meddich A., and Loudiki M. (2024). A comprehensive review of microalgae and cyanobacteria-based biostimulants for agriculture uses. Plants 13, 159. doi: 10.3390/plants13020159
Chittora D., Meena M., Barupal T., Swapnil P., and Sharma K. (2020). Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochem. Biophys Rep. 22, 100737. doi: 10.1016/j.bbrep.2020.100737
Dadheech P. K., Mahmoud H., Kotut K., and Krienitz L. (2012). Haloleptolyngbya alcalis gen. et sp. nov., a new filamentous cyanobacterium from the soda lake Nakuru, Kenya. Hydrobiologia 691, 269–283. doi: 10.1007/s10750-012-1080-6
Dey S. K., Chakrabarti B., Prasanna R., Singh S. D., Purakayastha T. J., Datta A., et al. (2017). Productivity of mungbean (Vigna radiata) with elevated carbon dioxide at various phosphorus levels and cyanobacteria inoculation. Legume Res An Int. J. 40, 497–505. doi: 10.18805/lr.v0iOF.3765
Dineshkumar R., Duraimurugan M., Sharmiladevi N., Lakshmi L. P., Rasheeq A. A., Arumugam A., et al. (2020). Microalgal liquid biofertilizer and biostimulant effect on green gram (Vigna radiata L) an experimental cultivation. Biomass Convers Biorefin 12, 3007–3027. doi: 10.1007/s13399-020-00857-0
Drira M., Elleuch J., Ben Hlima H., Hentati F., Gardarin C., Rihouey C., et al. (2021). Optimization of exopolysaccharides production by Porphyridium sordidum and their potential to induce defense responses in Arabidopsis thaliana against Fusarium oxysporum. Biomolecules 11, 282. doi: 10.3390/biom11020282
Hashtroudi M. S., Ghassempour A., Riahi H., Shariatmadari Z., and Khanjir M. (2013). Endogenous auxins in plant growth-promoting Cyanobacteria—Anabaena vaginicola and Nostoc calcicola. J. Appl. Phycol 25, 379–386. doi: 10.1007/s10811-012-9872-7
Jacob S. M. and Paranthaman S. (2023). Biofertilizers: An advent for eco-friendly and sustainable agriculture development. Vegetos 36, 1141–1153. doi: 10.1007/s42535-022-00550-9
Jaiswal A., Das K., Koli D. K., and Pabbi S. (2018). Characterization of cyanobacteria for IAA and siderophore production and their effect on rice seed germination. Int. J. Curr. Microbiol. Appl. Sci. 5, 212–222. doi: 10.22034/gjesm.2019.02.03
Khemka A. and Saraf M. (2015). Emerging role of co-inoculation of Pseudomonas with cyanobacteria for increasing crop productivity. J. Indian Botanical Soc. 94, 111–117.
Kholssi R., Marks E. A., Miñón J., Maté A. P., Sacristán G., Montero O., et al. (2021). A consortium of cyanobacteria and plant growth promoting rhizobacteria for wheat growth improvement in a hydroponic system. South Afr. J. Bot. 142, 247–258. doi: 10.1016/j.sajb.2021.06.035
Kremer R. J. and Souissi T. (2001). Cyanide production by rhizobacteria and potential for suppression of weed seedling growth. Curr. Microbiol. 43, 182–186. doi: 10.1007/s002840010284
Lelapalli S., Baskar S., Jacob S. M., and Paranthaman S. (2021). Characterization of phosphate solubilizing plant growth promoting rhizobacterium Lysinibacillus Pakistanensis strain PCPSMR15 isolated from Oryza sativa. Curr. Res. Microb Sci. 2, 100080. doi: 10.1016/j.crmicr.2021.100080
Malam Issa O., Défarge C., Le Bissonnais Y., Marin B., Duval O., Bruand A., et al. (2007). Effects of the inoculation of cyanobacteria on the microstructure and the structural stability of a tropical soil. Plant Soil 290, 209–219. doi: 10.1007/s11104-006-9153-9
Mohan A., Kumar M., and Kumar B. (2020). Siderophore production by some soil cyanobacteria. J. Pharm. Biol. Sci. 15, 34–44. doi: 10.9790/3008-1505033444
Nawaz T., Saud S., Gu L., Khan I., Fahad S., and Zhou R. (2024). Cyanobacteria: harnessing the power of microorganisms for plant growth promotion, stress alleviation, and phytoremediation in the Era of sustainable agriculture. Plant Stress 11, 1–16. doi: 10.1016/j.stress.2024.100399
Paheshwari P., Zingran Zimik, Selvi P. V., and Sripriya P. (2024). Phytochemical screening, GC-MS analysis and anti-bacterial activity of Desertifilum sp. PSL17 extract. Indian Hydrobiol 23, 123–130.
Patten C. L. and Glick B. R. (2002). Role of Pseudomonas putida indole 3-acetic acid in development of the host plant root system. Appl. Environ. Microbiol. 68, 3795–3801. doi: 10.1128/aem.68.8.3795-3801.2002
Prasanna R., Babu S., Bidyarani N., Kumar A., Triveni S., Monga D., et al. (2015). Prospecting cyanobacteria-fortified composts as plant growth promoting and biocontrol agents in cotton. Exp. Agric. 51, 42–65. doi: 10.1017/S0014479714000143
Prasanna R., Joshi M., Rana A., and Nain L. (2010). Modulation of IAA production in cyanobacteria by tryptophan and light. Polish J. Microbiol. 59, 99. doi: 10.33073/pjm-2010-015
Prasanna R., Kanchan A., Ramakrishnan B., Ranjan K., Venkatachalam S., Hossain F., et al. (2016). Cyanobacteria-based bioinoculants influence growth and yields by modulating the microbial communities favorably in the rhizospheres of maize hybrids. Eur. J. Soil Biol. 75, 15–23. doi: 10.1016/j.ejsobi.2016.04.001
Righini H., Francioso O., Martel Quintana A., and Roberti R. (2022). Cyanobacteria: a natural source for controlling agricultural plant diseases caused by fungi and oomycetes and improving plant growth. Horticulturae 8, 58. doi: 10.3390/horticulturae8010058
Sergeeva E., Liaimer A., and Bergman B. (2002). Evidence for production of the phytohormone indole-3-acetic acid by cyanobacteria. Planta 215, 229–238. doi: 10.1007/s00425-002-0749-x
Sharma N. K., Tiwari S. P., Tripathi K., and Rai A. K. (2011). Sustainability and cyanobacteria (blue-green algae): facts and challenges. J. Appl. Phycol 23, 1059–1081. doi: 10.1007/s10811-010-9626-3
Shawer E. E., El-Gamal A. D., Sabae S. Z., and Elsaied H. E. (2022). Identifying of the bioactive compounds from two aquatic cyanobacteria, Leptolyngbya sp. and Desertifilum sp., with antioxidant and antimicrobial activities. Egyptian J. Phycol 23, 57–88. doi: 10.21608/egyjs.2022.143340.1013
Singh S. (2014). A review on possible elicitor molecules of cyanobacteria: their role in improving plant growth and providing tolerance against biotic or abiotic stress. J. Appl. Microbiol. 117, 1221–1244. doi: 10.1111/jam.12612
Spaepen S., Vanderleyden J., and Remans R. (2007). Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 31, 425–448. doi: 10.1111/j.1574-6976.2007.00072.x
Toribio A. J., Suárez-Estrella F., Jurado M. M., López M. J., López-González J. A., and Moreno J. (2020). Prospection of cyanobacteria producing bioactive substances and their application as potential phytostimulating agents. Biotechnol. Rep. 26, e00449. doi: 10.1016/j.btre.2020.e00449
Wang Y. H. and Irving H. R. (2011). Developing a model of plant hormone interactions. Plant Signaling Behav. 6, 494–500. doi: 10.4161/psb.6.4.14558
Win T. T., Barone G. D., Secundo F., and Fu P. (2018). Algal biofertilizers and plant growth stimulants for sustainable agriculture. Ind. Biotechnol. 14, 203–211. doi: 10.1089/ind.2018.0010
Keywords: Desertifilum sp. PSL17, indole 3-acetic acid, ammonia, plant growth promotion, Vigna radiata, biofertilizer, sustainable agriculture
Citation: Palraj P, Subramani U, Zimik Z and Paranthaman S (2025) Indole 3-acetic acid production and growth-promoting effect of Desertifilum sp. PSL17 on Vigna radiata. Front. Ind. Microbiol. 3:1594031. doi: 10.3389/finmi.2025.1594031
Received: 15 March 2025; Accepted: 02 May 2025;
Published: 02 June 2025.
Edited by:
Marika Pellegrini, University of L’Aquila, ItalyReviewed by:
Srijana Mukhia, Donald Danforth Plant Science Center, United StatesNaima Mahreen, National Institute for Biotechnology and Genetic Engineering, Pakistan
Copyright © 2025 Palraj, Subramani, Zimik and Paranthaman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sripriya Paranthaman, c3JpcHJpeWEuaWl0bUBnbWFpbC5jb20=
†ORCID: Sripriya Paranthaman, orcid.org/0000-0002-1631-7809
 Paheshwari Palraj
Paheshwari Palraj Sripriya Paranthaman
Sripriya Paranthaman