- 1Department of Electronic, Mechatronic, and Biomedical Engineering, Universidad del Valle de Guatemala, Guatemala City, Guatemala
- 2Department of Molecular Biosciences, The Wenner-Gren Institutet, Stockholm University, Stockholm, Sweden
Animal models have long supported biomedical research, particularly in the development of drugs and preclinical testing. Yet, persistent discrepancies between animal data and human clinical outcomes have prompted a critical reassessment of their translational value. Challenges, including biological variability, inadequate methodological reporting, and limited regulatory oversight, particularly in low- and middle-income countries, undermine the reliability of animal research in guiding clinical practice. Organ-on-a-chip (OoC) technology offers a compelling alternative, especially relevant for resource-limited contexts. These microengineered systems enable more accurate modeling of human physiology and better predictions of drug safety and effectiveness, yielding direct benefits for underserved populations. By incorporating patient-derived cells, OoC platforms allow the study of region-specific diseases while fostering international research collaboration. Moreover, such approaches reduce reliance on costly animal research infrastructure, addressing critical barriers in countries like Guatemala, where the legal framework and funding remain limited. We argue that broader adoption of OoC technology is essential to improving research equity, quality, and accessibility worldwide. This perspective reflects the realities and aspirations of the Guatemalan scientific community, where advancing alternatives to animal models is not only a scientific priority but also a pathway to greater participation in global biomedical research.
Introduction
Animal models have long supported biomedical research, providing critical insights into human physiology, disease mechanisms, and the development of therapeutic interventions (Chang and Grieder, 2024). Their principal value lies in their extensive use for drug discovery and preclinical assessment of efficacy, toxicity, and safety (Sántha, 2020; Loewa et al., 2023). Despite their contributions, mounting evidence highlights fundamental limitations in the ability of animal studies to predict human clinical outcomes. Discrepancies between animal data and human responses have led to false negatives, false positives, and unexpected safety risks in clinical trials, underscoring the challenges of translating animal findings to humans (Loewa et al., 2023). Key factors include species-specific biological differences and the artificial induction of disease states that do not adequately capture the complexity of human pathologies (Van Norman, 2019).
Compounding these issues is a broader lack of transparency and methodological rigor in biomedical research, which undermines reproducibility and trust in published (Goodman et al., 2016). Insufficient detail in published studies hampers reproducibility and undermines confidence in the translational validity of preclinical findings (Freedman et al., 2017; Begley and Ioannidis, 2015). This recognition led to the introduction of the PREPARE and ARRIVE guidelines, which established standards to enhance the quality, transparency, and reproducibility of animal research (Percie du Sert et al., 2020a; Percie du Sert et al., 2020b; Smith et al., 2018).
In high-income countries, strong regulatory oversight, ethical review systems, and access to specialized infrastructure, such as validated protocols, qualified personnel, and compliance with Good Laboratory Practice (GLP), help ensure the quality of animal-based research (Srinivasan et al., 2021). In contrast, Low- or Middle-Income Countries (LMICs) often face significant barriers, including limited infrastructure and shortages of trained staff, which impede their ability to generate reliable and internationally comparable data (Vandresen et al., 2025; Crowther et al., 2005; Del Valle, 2025). Consequently, differences in animal care practices and regulatory standards across countries can compromise consistency in research quality, contributing to data variability and translational failure across institutions (Parlasca et al., 2023).
Against this backdrop, the adoption of the new organ-on-a-chip (OoC) technology presents significant opportunities for resource-limited settings. By using patient-derived cells, these microphysiological systems can replicate key aspects of human physiology and disease, enabling more accurate modeling of population-specific conditions and enhancing the predictive power of preclinical testing (Momoli et al., 2025; Huang et al., 2024). OoC platforms are being integrated into drug development pipelines to assess efficacy, pharmacokinetics, and toxicity with greater translational relevance (Ma et al., 2021). Their standardized and modular design further supports reproducibility and fosters international collaboration, positioning OoC systems as scalable and accessible alternatives to animal research. In this Perspective, we focus on the Guatemalan context, exploring how OoC technology could be leveraged to build biomedical research capacity, tackle locally relevant health challenges, and advance the inclusion of resource-limited settings within the global scientific community.
Inadequate regulatory and ethical frameworks limit animal research in resource-constrained settings
The Guatemalan Animal Protection and Welfare Law (Decree 5–2017), enacted in 2017, includes provisions for animals used in research and teaching. Article 43 mandates that institutions conducting animal studies establish Institutional Ethics Committees for Animal Use and Care (CICUAL), in accordance with international standards ratified by Guatemala, and register them through the Animal Welfare Unit of MAGA (Congress of Guatemala, 2017). These committees should include a veterinarian, a researcher, a community representative, and other relevant professionals. As of this writing, only Universidad de San Carlos de Guatemala (USAC) and Universidad del Valle de Guatemala (UVG), among the 15 higher education institutions existing in the country, have registered ethics committees with MAGA.
Despite existing legislation, Guatemala lacks a unified national framework or technical oversight for animal research, resulting in inconsistent ethical review and limited alignment with international protocols. Guatemala is not listed in the directory maintained by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC), the primary body for external accreditation of animal care and use programs, which includes over 1,140 accredited institutions worldwide (National Research Council Institute for Laboratory Animal, 2004). The country has also not formally adopted global standards such as those from the Federation of European Laboratory Animal Science Associations (FELASA) or Good Laboratory Practice (GLP). The practical effect is a fragmented regulatory environment with inconsistent enforcement. Nonetheless, the existence of a legal infrastructure provides a foundation for developing more robust and transparent oversight that aligns with international best practices.
The lack of a unified regulatory and legislative framework not only limits access to animal research but also significantly compromises the rigor, reproducibility, and international credibility of findings from smaller research groups. Without urgent reforms to establish consistent oversight aligned with global standards, Guatemala and other LMICs in the same situation risk marginalizing their biomedical research communities and diminishing their contributions to the broader scientific community.
OoC as a cost-effective solution amid animal facility infrastructure and resource limitations
International best practices require significant ongoing investment in laboratory animal infrastructure. According to the US National Academies, “animal facilities are among the most expensive facilities to construct and operate” due to continuous operational requirements, sophisticated engineered systems, specialized equipment, and high personnel costs, which can constitute over 55% of operating expenses (Cork et al., 1997; National Research Council, 2011). Construction costs are estimated at US$400–700 per square foot, with additional requirements for ongoing maintenance by external specialists. Notably, less than half of the total investment in such facilities is reflected in visible infrastructure and equipment (Lang, 2009).
Additionally, animal use in preclinical studies entails substantial costs, encompassing not only the infrastructure but also the purchase, care, and compliance with societal and regulatory requirements. Licenses and approvals from relevant committees or agencies carry associated fees, after which animals are procured from authorized breeders at prices that vary by species, age, and genetic background (Colby et al., 2017; Jaric et al., 2024). On arrival, animals must be housed in accredited facilities that meet enrichment and welfare standards in line with the principle of “Refinement” (Smith et al., 2018). Daily husbandry is provided by trained personnel, with costs typically borne by research institutions (National Research Council, 2011).
Experimental design further dictates the need for specialized materials, sterile surgical equipment, in vivo imaging techniques, anesthetics, analgesics, and other drugs, with expenses increasing in proportion to the complexity of the procedure (Barrow, 2019). Collectively, these requirements represent a significant financial investment for research institutions. These costs are commonly funded by non-profits or government agencies in developed countries, with a rational budget for animal research (da Silva and Blasimme, 2023). Ongoing efforts at both academic and industrial levels seek to minimize animal use, improve data accuracy, reduce waste of resources, and address ethical and societal concerns, underscoring the importance of developing viable alternatives to animal experimentation (Kola and Landis, 2004).
In 2022, the U.S. Food and Drug Administration (FDA) signaled a shift in policy by supporting the FDA Modernization Act 2.0, which removes the legal mandate for animal testing in drug development, allowing alternatives such as OoC and computational models (Nuwer, 2022). The agency now actively supports New Approach Methodologies (NAMs), including OoC and other in vitro systems. These technologies replicate organ-level physiology by integrating patient-derived or engineered human tissues into miniaturized, microfluidic devices, which enable real-time analysis while reducing dependence on animal models (Leung et al., 2022).
OoC platforms require substantially less physical infrastructure, are more scalable and amenable to automation, and do not necessarily demand highly specialized biomedical personnel (Ewart et al., 2022). With OoC systems evolving from a theoretical concept to an actual alternative in drug discovery and development, decision makers are challenged to determine their commercial viability. A recent study, based on experts’ input, estimated a reduction of 10%–26% in research and development (R&D) costs per new drug, resulting in a positive cost impact (Franzen et al., 2019).
The FDA’s removal of mandatory animal testing requirements for drug approval also presents an encouraging opportunity for countries like Guatemala and other LMICs to embark on microfluidics-based research, fostering local innovation and adoption of advanced OoC technologies (Nuwer, 2022). For example, in 2023, the budget of Guatemala’s Ministry of Agriculture, Livestock, and Food (MAGA) allocated only 0.7% (Q10.8 million) to Program 12: Support for Animal Protection and Welfare, which primarily focuses on companion, working, abused, or abandoned animals. None of these allocations is directed explicitly toward scientific research infrastructure (Ministerio de Agricultura, 2022). Consequently, the funding, legal, infrastructural, and institutional limitations underscore that Guatemala is currently ill-equipped to support and sustain large-scale animal research programs (Del Valle, 2023).
Guatemala’s investment in R&D is markedly low, averaging only 0.04% of its gross domestic product (GDP) between 2005 and 2021 and reaching 0.06% in 2021. This level is substantially below the global average of 1.25% reported across 78 countries (Tariq Mahmood et al., 2021; Cardozo, 2021; Bonilla et al., 2021). The country is served by two veterinary schools—USAC and Universidad Mariano Gálvez (UMG)—neither of which offers specialized training or academic programs in laboratory animal care or animal facility design (Tadich et al., 2010). The Faculty of Chemical Sciences and Pharmacy at USAC operates a small-scale animal facility, primarily used for teaching and research purposes. Staffed by a multidisciplinary team, including a veterinary doctor, pharmaceutical scientists, and laboratory technicians, and accredited by the International Council for Laboratory Animal Science (ICLAS), this is the only facility of its kind in Guatemala and is limited by its capacity, staffing, and resources, thus precluding its use for standardized or large-scale biomedical research (Granados, 2022). Annually, USAC graduates approximately 32 veterinarians and 11 zootechnicians, the majority of whom lack specialized training in laboratory animal science or biomedical research (Granados, 2022; Lepe-López et al., 2018). Collectively, these factors render Guatemala unprepared to perform compliant, high-quality animal experiments necessary for advancing biomedical research.
To counteract the cost of animal research and regulatory gaps, while also implementing and advancing biomedical research in LMICs, recent examples have shown that OoC technologies are feasible. In Brazil, researchers created a modular microfluidic platform that combines liver organoids made from primary human hepatocytes with cardiac organoids built from induced pluripotent stem cell-derived cardiomyocytes (Goyes-Balladares et al., 2025). This system allowed continuous monitoring for up to 5 days and supported large-scale drug screening, demonstrating both its scalability and translational potential in resource-limited environments. In Mexico, tumor-on-a-chip research (miniaturized cancer models for personalized medicine) is highlighted by institutes such as Tecnológico de Monterrey, indicating movement toward application-driven studies for regional health challenges (Sánchez-Salazar et al., 2021). PhD careers focused on biotechnology have also been introduced at the Tecnológico de Monterrey, demonstrating the advancement of technology implementation in the area (Sosa-Hernández et al., 2018). Latin American teams have increasingly focused on disease modeling, drug toxicity, and personalized medicine, often leveraging strengths in mammalian cell culture and material sciences (Rodríguez-Salvador et al., 2019). Nevertheless, a recent publication by researchers from Mexico has also utilized microfluidic technology for chloroplast isolation to address the limitations of conventional methods, which typically require complex protocols, specialized equipment, and trained personnel (Chavez-Pineda et al., 2025). Consequently, in countries with limited animal facilities infrastructure but stronger technical training capacities, OoC technologies represent an opportunity for innovation of feasible and scalable alternatives to conventional research (Leung et al., 2022).
Disease-specific modelling capabilities of OoC
Besides its cost-effective benefits of OoC, it has increasingly been validated against clinical benchmarks, demonstrating predictive accuracy for human pathophysiology. A large-scale study involving 870 human Liver-Chips reported that the system correctly identified 87% of drugs known to cause drug-induced liver injury (DILI) in patients, while achieving 100% specificity by not falsely labeling safe drugs as toxic (Ewart et al., 2022). Beyond hepatotoxicity, OoC models have been shown to recapitulate complex human clinical responses to drugs, toxins, pathogens, and even host–microbiome interactions, offering a level of physiological relevance not achievable with conventional animal models (Ingber, 2022). Recent advances in lung-on-chip technology now enable modeling of pulmonary edema, inflammation, and infection with high fidelity to human clinical responses—highlighted by models that mimic epithelial-endothelial barrier injury, immune cell extravasation, and pathogen-induced damage (Bai and Ingber, 2022). Moreover, recent analyses have emphasized the potential of chip-based platforms to enhance the prediction of oral bioavailability and intrinsic clearance, two crucial pharmacokinetic parameters that often do not translate from animal studies to humans (Keuper-Navis et al., 2023).
In LMICs, the need for disease modeling of endemic vector-borne diseases, including malaria, dengue, and Chagas disease, is a priority in healthcare since they cause substantial morbidity and mortality in tropical regions and are highly sensitive to demographic, environmental, and climate drivers (Sutherst, 2004; Forum on Microbial Threats, 2016). These diseases are understudied owing to low investment and because rodent models are often unsuitable (Zompi and Harris, 2012). OoC systems can use cells from local human donors, enabling scientists to explore region-specific health burdens. For example, OoC technologies have been leveraged to model a broad spectrum of diseases, including asthma, pulmonary edema (Shah et al., 2024), inflammatory bowel disease, and viral infections such as SARS-CoV-2 (Sheng et al., 2025), by using patient-derived cells and recapitulating tissue interfaces. These models can facilitate the study of diseases in genetically diverse or underrepresented populations, providing a platform to investigate how genetic and environmental factors influence disease progression and therapeutic response. Allowing, at the same time, scientific advances in the understanding of the disease and potential pharmacological treatments.
Research on OoC technology in Latin America is progressing, with Brazil recognized as the leading regional hub for both market growth and research activity, followed by Mexico and Argentina (Rodríguez-Salvador et al., 2019). Brazil’s implementation of OoC is supported by public institutions and universities promoting biomedical innovation. The government has initiated discussions to align its regulatory frameworks with global standards, enabling the use of OoC data in pharmaceutical approval processes. At CNPEM (National Center for Research in Energy and Materials), researchers have established a liver-on-a-chip platform to test hepatotoxicity, one of the first regionally developed systems with translational potential (Indolfo et al., 2023).
Ethical advancements and reduction of animal use
The “3Rs” (Replacement, Reduction, and Refinement) form the ethical framework for responsible animal research established by Russell and Burch over 50 years ago (Hubrecht and Carter, 2019; Lauwereyns et al., 2024). OoC systems exemplify these principles: they enable replacement of animals for studies of disease mechanisms, drug responses, and toxicity; they facilitate reduction by minimizing or eliminating animal use; and they inherently refine experimental procedures through more human-relevant models that better recapitulate physiological responses. Consequently, OoC technologies have the potential to advance both scientific discovery and animal welfare by providing more accurate, scalable, and ethical research tools. For instance, Brazil’s CNPEM has developed human-on-a-chip systems, including liver, skin, and gut tissue modules, for systemic toxicity testing and to reduce the use of animal models, especially for cosmetic testing (Indolfo et al., 2023). In the European Union and other jurisdictions, regulations prohibit the use of animal testing for cosmetic products, limiting the ability to assess cosmetic toxicity using traditional in vivo approaches (Abbott, 2009).
Barriers to adoption and strategies for implementation
While OoC technologies show considerable promise, their adoption faces significant barriers (Danku et al., 2022). A key obstacle is the lack of local microfabrication infrastructure, as producing microfluidic devices requires specialized cleanroom facilities, advanced materials, and technical expertise that are rarely available outside high-income regions (Skardal, 2024; Zhao et al., 2024). Furthermore, the limited availability of personnel with interdisciplinary expertise spanning microengineering, stem cell biology, and bioinformatics poses a critical barrier to implementation in resource-constrained settings. Equally limiting is the lack of standardized protocols and context-specific regulatory frameworks, which generates uncertainty in validation, delays approval processes, and impedes clinical translation (Rogal et al., 2022). Collectively, these challenges restrict the effective integration of OoC platforms into global biomedical research and healthcare systems. Several strategies have been advanced to address these barriers and foster equitable access to OoC technologies. Establishing regional manufacturing hubs could lower costs and reduce dependence on imported devices, while international training partnerships would build local expertise and support sustainable knowledge transfer. Progress in modular, user-friendly device design can further decrease technical barriers, facilitating adoption in decentralized laboratory settings. Equally critical are efforts to align with international regulatory standards, providing a transparent pathway for validation and approval while ensuring consistency with existing preclinical testing guidelines (Alver et al., 2024). By addressing infrastructural, human capital, and regulatory limitations in parallel, these initiatives can enhance the feasibility of OoC deployment globally.
Discussion
The role of animal models in biomedical research is under increasing scrutiny, particularly given their persistent limitations in predicting human clinical outcomes. Historically, animal studies have underpinned drug discovery and preclinical testing; yet, mounting evidence demonstrates their frequent failure to translate effectively to human biology, underscoring the need for a paradigm shift in preclinical research.
A primary concern lies in the considerable biological variability inherent in animal models. Species-specific differences in physiology, genetics, and environmental exposures confound the extrapolation of findings to humans and often obscure the underlying mechanisms of human disease. These challenges are exacerbated by inconsistent reporting practices, which hinder reproducibility and risk misleading conclusions. The PREPARE and ARRIVE guidelines were established to enhance transparency and methodological rigor in animal research; however, their implementation remains uneven, particularly in low- and middle-income countries that lack robust research infrastructure.
In resource-limited settings such as Guatemala, the shortcomings of traditional animal research are further amplified by constrained funding, shortages of trained personnel, and underdeveloped regulatory frameworks. Such barriers jeopardize research quality and impede the generation of data that meets international standards, perpetuating global disparities in biomedical advances. Reliance on animal models in these contexts also restricts the investigation of endemic diseases that disproportionately affect underserved populations.
OoC technology offers a compelling alternative that addresses many of these challenges. By recreating key aspects of human physiology in microengineered systems, OoC platforms enable more precise modeling of disease and improved prediction of drug efficacy and safety. The use of patient-derived cells in OoC systems enhances the relevance and personalization of research findings, facilitating the study of mechanisms and therapeutic responses that reflect the characteristics of specific populations (Figure 1). OoC platforms enable automated, high-throughput culture setups that reduce variability and improve reproducibility (Papamichail et al., 2025). Moreover, they provide highly reproducible results and allow the simultaneous use of multiple cell types, supporting their scalability in complex biological studies. In contrast, conventional 2D cell cultures and spheroid models often lack the structural and microenvironmental complexity needed for accurate drug response prediction, and animal models are limited by interspecies differences that compromise translational accuracy (Ma et al., 2021). Compared to these approaches, OoC systems offer superior scalability for drug screening, improved data reproducibility, and long-term cost-effectiveness, while more closely aligning with human clinical outcomes.
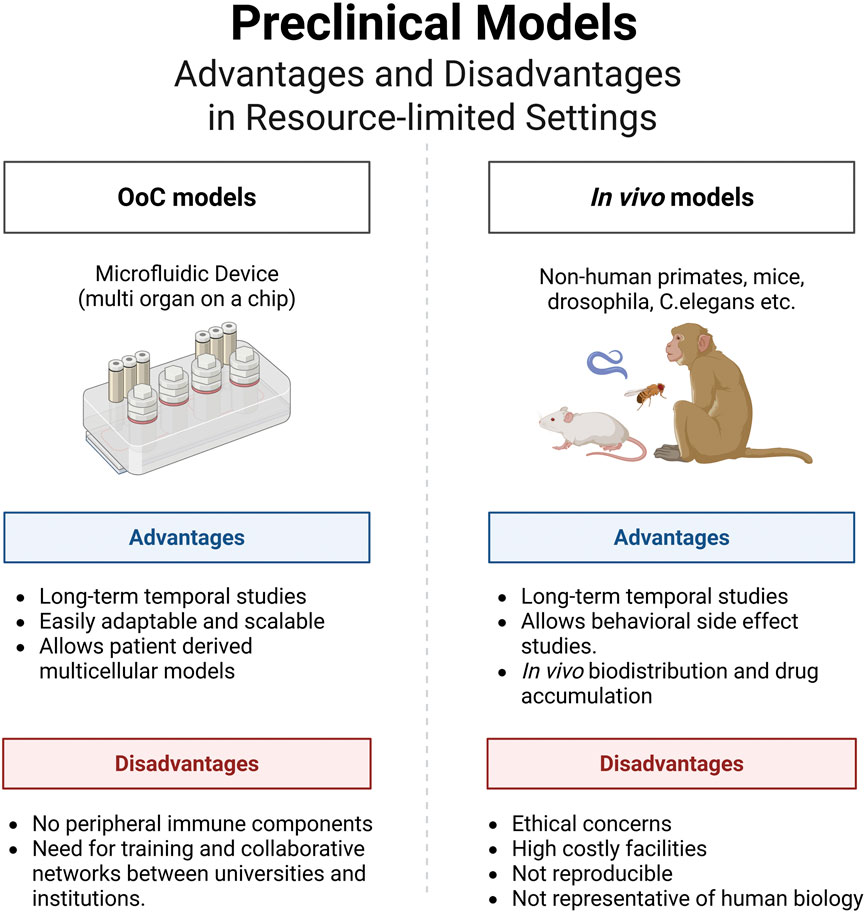
Figure 1. Organs-on-a-Chip (OoC) technology offers a transformative solution for biomedical research in resource-limited settings, such as Guatemala, where traditional animal models face significant challenges due to limited funding, personnel, and regulatory infrastructure. By replicating human physiology using patient-derived cells, OoC platforms enable more accurate, ethical, and population-relevant disease modeling and drug testing, helping to bridge global disparities in healthcare research.
Furthermore, OoC technology is particularly well-suited to the needs of resource-constrained environments. These platforms require less infrastructure than animal research facilities and can be implemented more feasibly where resources are limited. Their adoption also opens new avenues for international collaboration, fostering knowledge exchange and capacity building between high- and low-income countries (Alver et al., 2024).
OoC technologies have gained traction in North America, Europe, and Asia; some Latin American institutions are already pioneering their integration. Although no institutions in Guatemala currently operate dedicated OoC research programs, several universities offer academic programs in disciplines closely related to OoC development, which are essential for the interdisciplinary nature of OoC technology (Alver et al., 2024). For instance, the Universidad del Valle de Guatemala (UVG) provides degrees in biomedical engineering, electronics, mechatronics, and biotechnology; Universidad Galileo offers programs in electronics and mechatronics; and UMG includes biomedical engineering and biology-related degrees with biotechnology components. UVG has been leading the creation of chip technology and opening OoC and bioreactors courses for the undergraduate students, who have started to engineer and validate their prototypes in-house (UVG, 2025). We believe that these educational opportunities establish a robust foundation for future OoC initiatives in Guatemala, which could be realized through international collaborations or access to regional funding.
To address the growing demand for decentralized and point-of-care testing (POCT) applications, we propose a strategic roadmap for the phased deployment of OoC platforms in resource-limited settings (Figure 2). The framework prioritizes technology simplification and modularization to minimize operational complexity, followed by targeted capacity-building and training initiatives to equip local operators with the necessary skills. To mitigate infrastructural constraints, we envision the establishment of regional resource-sharing hubs, complemented by pilot demonstration projects within LMIC healthcare systems to assess feasibility and clinical utility. Finally, alignment with regulatory and reimbursement pathways is emphasized to secure long-term sustainability and broad adoption. Collectively, this roadmap offers a pragmatic path for translating OoC innovations into accessible and scalable decentralized healthcare solutions.
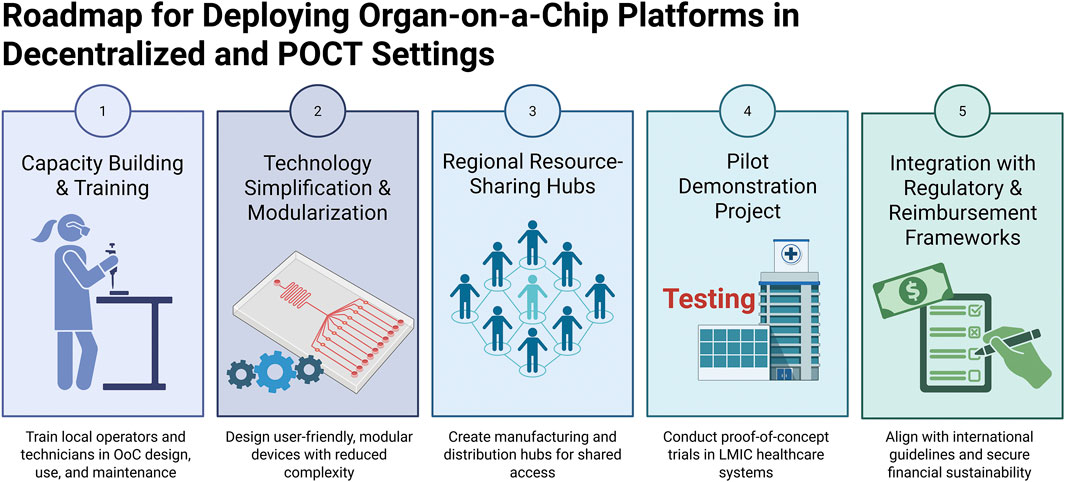
Figure 2. Roadmap for deploying OoC platforms in decentralized and point-of-care testing (POCT) settings.
We propose the development of national training programs that integrate biology, electronics, and fluidics, supported by open-source hardware platforms and standardized protocols. Such initiatives have the potential to broaden access to OoC systems and address persistent gaps in biomedical research in Guatemala. Leveraging existing laboratory infrastructure within engineering and biomedical faculties would further facilitate pilot projects targeting locally relevant health challenges, thereby fostering the adoption of cost-effective, scalable alternatives to conventional experimental models. We acknowledge that although animal models have been essential in biomedical science, their well-known limitations require the use of alternative methods in resource-limited environments. OoC technology has the potential to transform preclinical research in low-income countries, enhance fidelity, and enable inclusivity, ultimately promoting the development of safer and more effective medical interventions for diverse global populations. The continued evolution of research methodologies is essential for bridging the gap between scientific advancements and clinical relevance.
Conclusion
In summary, the adoption of OoC technology offers a transformative advancement for biomedical research, particularly in resource-limited settings such as Guatemala. By overcoming key limitations of traditional animal models, including restricted translational relevance, biological variability, and lack of methodological standardization. OoC platforms substantially improve the predictive accuracy and reproducibility of preclinical studies. The integration of patient-derived cells further enables the development of disease models that reflect the genetic and environmental diversity of local populations, thereby advancing research with greater global relevance. Broad implementation of OoC technology is therefore essential to accelerate biomedical innovation, strengthen clinical translation, and promote a more equitable and inclusive research ecosystem worldwide.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MC: Investigation, Writing – original draft. AdV: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank Qiaolin Deng and the Deng Lab at Stockholm University for the insightful discussions and guidance in organoid methodology. Figures were generated using Biorender.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbott, A. (2009). Fresh hope for German stem-cell patent case. Nature 462, 265. doi:10.1038/462265a
Alver, C. G., Drabbe, E., Ishahak, M., and Agarwal, A. (2024). Roadblocks confronting widespread dissemination and deployment of organs on chips. Nat. Commun. 15, 5118. doi:10.1038/s41467-024-48864-3
Bai, H., and Ingber, D. E. (2022). What can an Organ-on-a-Chip teach us about human lung pathophysiology? Physiology 37, 242–252. doi:10.1152/physiol.00012.2022
Barrow, J. C. (2019). A medicinal chemist's perspective on transitioning from industry to academic drug discovery. ACS Med. Chem. Lett. 10, 687–689. doi:10.1021/acsmedchemlett.9b00107
Begley, C. G., and Ioannidis, J. P. (2015). Reproducibility in science: improving the standard for basic and preclinical research. Circ. Res. 116, 116–126. doi:10.1161/CIRCRESAHA.114.303819
Bonilla, K., and Serafim, M. (2021). “Relevance of science, technology and innovation diplomacy for STI capacity building in central America: the cases of Guatemala, El Salvador and Honduras,” in Science, technology, and higher education: Governance approaches on social inclusion and sustainability in Latin America. Editors L. A. Orozco, G. Ordóñez-Matamoros, J. H. Sierra-González, J. García-Estévez, and I. Bortagaray (Cham: Springer International Publishing), 21–53.
Cardozo, M. L. (2021). Characterization of gross domestic expenditure on r&d in Latin American countries during 2008-2017. TURCOMAT 12, 684–688.
Chang, M. C. J., and Grieder, F. B. (2024). The continued importance of animals in biomedical research. Lab. Anim. 53, 295–297. doi:10.1038/s41684-024-01458-4
Chavez-Pineda, O. G., Guevara-Pantoja, P. E., Marín-Lizarraga, V., Caballero-Robledo, G. A., Patiño-Lopez, L. D., May-Arrioja, D. A., et al. (2025). Parallel DLD microfluidics for chloroplast isolation and sorting. Lab a Chip 25, 4609–4619. doi:10.1039/d5lc00348b
Colby, L. A., Quenee, L. E., and Zitzow, L. A. (2017). Considerations for infectious disease research studies using animals. Comp. Med. 67, 222–231.
Congress of Guatemala (2017). Decreto número 5-2017: ley de protección y bienestar animal. Ministerio de Agricultura, Ganadería y Alimentación (MAGA). Available online at: https://www.maga.gob.gt/wpfb-file/reglamento-p-pdf-3/.
Cork, L. C., Clarkson, T. B., Jacoby, R. O., Gaertner, D. J., Leary, S. L., Linn, J. M., et al. (1997). The costs of animal research: origins and options. Science 276, 758–759. doi:10.1126/science.276.5313.758
Crowther, J. R., and Jeggo, M. H. (2005). “Challenges and opportunities for controlling and preventing animal diseases in developing countries through gene-based technologies,” in Applications of gene-based technologies for improving animal production and health in developing countries. Editors H. P. S. Makkar, and G. J. Viljoen (Dordrecht: Springer Netherlands), 53–71.
da Silva, R. G. L., and Blasimme, A. (2023). Organ chip research in Europe: players, initiatives, and policies. Front. Bioeng. Biotechnol. 2023, 11. doi:10.3389/fbioe.2023.1237561
Danku, A. E., Dulf, E.-H., Braicu, C., Jurj, A., and Berindan-Neagoe, I. (2022). Organ-On-A-Chip: a survey of technical results and problems. Front. Bioeng. Biotechnol. 10, 840674. doi:10.3389/fbioe.2022.840674
Del Valle, A. C. (2023). Latino scientists need professional incentives to return home. Nature 613, 437. doi:10.1038/d41586-023-00098-x
Del Valle, A. C. (2025). Organs on chips could make biomedical research more equitable. Nature 644, 338. doi:10.1038/d41586-025-02569-9
Ewart, L., Apostolou, A., Briggs, S. A., Carman, C. V., Chaff, J. T., Heng, A. R., et al. (2022). Performance assessment and economic analysis of a human liver-chip for predictive toxicology. Commun. Med. 2, 154. doi:10.1038/s43856-022-00209-1
Forum on Microbial Threats (2016). “Workshop overview,” in Global health impacts of vector-borne diseases: workshop summary (Washington, DC: National Academies Press).
Franzen, N., van Harten, W. H., Retèl, V. P., Loskill, P., van den Eijnden-van Raaij, J., and Ijzerman, M. (2019). Impact of organ-on-a-chip technology on pharmaceutical R&D costs. Drug Discov. Today 24, 1720–1724. doi:10.1016/j.drudis.2019.06.003
Freedman, L. P., Venugopalan, G., and Wisman, R. (2017). Reproducibility2020: progress and priorities. F1000Res 6, 604. doi:10.12688/f1000research.11334.1
Goodman, S. N., Fanelli, D., and Ioannidis, J. P. (2016). What does research reproducibility mean? Sci. Transl. Med. 8, 341ps12. doi:10.1126/scitranslmed.aaf5027
Goyes-Balladares, A., Moya-Jiménez, R., Molina-Dueñas, V., Chaca-Espinoza, W., and Magal-Royo, T. (2025). What inspires biomimicry in construction? Patterns, trends, and applications. Biomimetics 10, 259. doi:10.3390/biomimetics10050259
Granados, I. V. V. (2022). Jorge David El uso de animales en investigación y docencia en Guatemala: explorando la percepción del bienestar animal en la comunidad académica veterinaria. Rev. UVG, 107–118.
Huang, Y., Liu, T., Huang, Q., and Wang, Y. (2024). From Organ-on-a-Chip to Human-on-a-Chip: a review of research progress and latest applications. ACS Sens. 9, 3466–3488. doi:10.1021/acssensors.4c00004
Hubrecht, R. C., and Carter, E. (2019). The 3Rs and humane experimental technique: implementing change. Anim. (Basel) 9, 754. doi:10.3390/ani9100754
Indolfo, N. d. C., Ganzerla, M. D., Doratioto, T. R., Avelino, T. M., Tofani, L. B., Peroni, L. A., et al. (2023). Combining a microphysiological system of three organ equivalents and transcriptomics to assess toxicological endpoints for cosmetic ingredients. Lab. Chip 23, 5092–5106. doi:10.1039/d3lc00546a
Ingber, D. E. (2022). Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 23, 467–491. doi:10.1038/s41576-022-00466-9
Jaric, I., Voelkl, B., Amrein, I., Wolfer, D. P., Novak, J., Detotto, C., et al. (2024). Using mice from different breeding sites fails to improve replicability of results from single-laboratory studies. Lab. Anim. 53, 18–22. doi:10.1038/s41684-023-01307-w
Keuper-Navis, M., Walles, M., Poller, B., Myszczyszyn, A., van der Made, T. K., Donkers, J., et al. (2023). The application of organ-on-chip models for the prediction of human pharmacokinetic profiles during drug development. Pharmacol. Res. 195, 106853. doi:10.1016/j.phrs.2023.106853
Kola, I., and Landis, J. (2004). Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 3, 711–716. doi:10.1038/nrd1470
Lauwereyns, J., Bajramovic, J., Bert, B., Camenzind, S., De Kock, J., Elezović, A., et al. (2024). Toward a common interpretation of the 3Rs principles in animal research. Lab. Anim. 53, 347–350. doi:10.1038/s41684-024-01476-2
Lepe-López, M. A., Gabriela, F., Patricia, L., Flor, G., Amilcar, D. H., Luis, V. R., et al. (2018). Incremento del número de estudiantes del género femenino egresados de la carrera de medicina veterinaria en Guatemala. Ciencias Sociales Humanidades 5, 31–38.
Leung, C. M., de Haan, P., Ronaldson-Bouchard, K., Kim, G. A., Ko, J., Rho, H. S., et al. (2022). A guide to the organ-on-a-chip. Nat. Rev. Methods Prim. 2, 33. doi:10.1038/s43586-022-00118-6
Loewa, A., Feng, J. J., and Hedtrich, S. (2023). Human disease models in drug development. Nat. Rev. Bioeng. 1, 545–559. doi:10.1038/s44222-023-00063-3
Ma, C., Peng, Y., Li, H., and Chen, W. (2021). Organ-on-a-Chip: a new paradigm for drug development. Trends Pharmacol. Sci. 42, 119–133. doi:10.1016/j.tips.2020.11.009
Ministerio de Agricultura (2022). Presupuesto General de Egresos del Estado 2023 – programa 14 Apoyo a la Protección y Bienestar Animal.
Momoli, C., Costa, B., Lenti, L., Tubertini, M., Parenti, M. D., Martella, E., et al. (2025). Correction: momoli et al. The Evolution of Anticancer 3D in vitro Models: the Potential Role of Machine Learning and AI in the Next Generation of Animal-Free Experiments. Cancers 2025, 17, 700. Cancers 17, 1106. doi:10.3390/cancers17071106
National Research Council (2011). Guide for the care and use of laboratory animals. Washington (DC): National Academies Press.
National Research Council Institute for Laboratory Animal (2004). The development of science-Based guidelines for laboratory animal care: proceedings of the November 2003 international workshop. Washington (DC): National Academies Press.
Nuwer, R. (2022). US agency seeks to phase out animal testing. Nature. doi:10.1038/d41586-022-03569-9
Papamichail, L., Koch, L. S., Veerman, D., Broersen, K., and van der Meer, A. D. (2025). Organoids-on-a-chip: microfluidic technology enables culture of organoids with enhanced tissue function and potential for disease modeling. Front. Bioeng. Biotechnol. 13, 1515340. doi:10.3389/fbioe.2025.1515340
Parlasca, M., Knößlsdorfer, I., Alemayehu, G., and Doyle, R. (2023). How and why animal welfare concerns evolve in developing countries. Anim. Front. 13, 26–33. doi:10.1093/af/vfac082
Percie du Sert, N., Hurst, V., Ahluwalia, A., Alam, S., Avey, M. T., Baker, M., et al. (2020a). The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. BMC Vet. Res. 16, 242. doi:10.1186/s12917-020-02451-y
Percie du Sert, N., Hurst, V., Ahluwalia, A., Alam, S., Avey, M. T., Baker, M., et al. (2020b). The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 18, e3000410. doi:10.1371/journal.pbio.3000410
Rodríguez-Salvador, M., Villarreal-Garza, D., Álvarez, M. M., and Santiago, G. T. (2019). Analysis of the knowledge landscape of three-dimensional bioprinting in Latin America. Int. J. Bioprinting 5, 240. doi:10.18063/ijb.v5i2.3.240
Rogal, J., Schlünder, K., and Loskill, P. (2022). Developer’s guide to an organ-on-chip model. ACS Biomater. Sci. Eng. 8, 4643–4647. doi:10.1021/acsbiomaterials.1c01536
Sánchez-Salazar, M. G., Álvarez, M. M., and Trujillo-de Santiago, G. (2021). Advances in 3D bioprinting for the biofabrication of tumor models. Bioprinting 21, e00120. doi:10.1016/j.bprint.2020.e00120
Sántha, M. (2020). Biologia futura: animal testing in drug Development—The past, the present and the future. Biol. Futura 71, 443–452. doi:10.1007/s42977-020-00050-4
Shah, D., Dave, B., Chorawala, M. R., Prajapati, B. G., Singh, S., M. Elossaily, G., et al. (2024). An insight on microfluidic Organ-on-a-Chip models for PM2.5-Induced pulmonary complications. ACS Omega 9, 13534–13555. doi:10.1021/acsomega.3c10271
Sheng, H., Liang, Y., Lauschke, V. M., and Wang, Y. (2025). Organoids and organ-on-chip models for COVID-19 research and its application in modernization of traditional Chinese medicine: opportunities and challenges. Engineering. doi:10.1016/j.eng.2025.01.019
Skardal, A. (2024). Grand challenges in organoid and organ-on-a-chip technologies. Front. Bioeng. Biotechnol. 12, 1366280. doi:10.3389/fbioe.2024.1366280
Smith, A. J., Clutton, R. E., Lilley, E., Hansen, K. E. A., and Brattelid, T. (2018). PREPARE: guidelines for planning animal research and testing. Lab. Anim. 52, 135–141. doi:10.1177/0023677217724823
Sosa-Hernández, J. E., Villalba-Rodríguez, A. M., Romero-Castillo, K. D., Aguilar-Aguila-Isaías, M. A., García-Reyes, I. E., Hernández-Antonio, A., et al. (2018). Organs-on-a-Chip module: a review from the development and applications perspective. Micromachines (Basel) 9, 536. doi:10.3390/mi9100536
Srinivasan, K., Tikoo, K., and Jena, G. B. (2021). “Good laboratory practice (GLP) requirements for preclinical animal studies,” in Essentials of laboratory animal science: principles and practices. Editors P. Nagarajan, R. Gudde, and R. Srinivasan (Singapore: Springer Singapore), 655–677.
Sutherst, R. W. (2004). Global change and human vulnerability to vector-borne diseases. Clin. Microbiol. Rev. 17, 136–173. doi:10.1128/cmr.17.1.136-173.2004
Tadich, N. A., Molento, C. F. M., and Gallo, C. B. (2010). Teaching animal welfare in some veterinary schools in Latin America. J. Veterinary Med. Educ. 37, 69–73. doi:10.3138/jvme.37.1.69
Tariq Mahmood, A., Adiqa Kausar, K., Tariq, B., and Talah Numan, K. R. & D. (2021). Expenditure as an accelerator of economic growth with special reference to developing countries. J. Bus. Soc. Rev. Emerg. Econ. 7. doi:10.26710/jbsee.v7i3.1842
UVG (2025). UVG in El primer nanochip diseñado en Guatemala. Available online at: https://www.uvg.edu.gt/nanochip/.
Van Norman, G. A. (2019). Limitations of animal studies for predicting toxicity in clinical trials: is it time to rethink our current approach? JACC Basic Transl. Sci. 4, 845–854. doi:10.1016/j.jacbts.2019.10.008
Vandresen, B., Nogues, E., and von Keyserlingk, M. A. G. (2025). Navigating challenges in applied animal behaviour and welfare research: a focus group study. Appl. Animal Behav. Sci. 287, 106637. doi:10.1016/j.applanim.2025.106637
Zhao, Y., Landau, S., Okhovatian, S., Liu, C., Lu, R. X. Z., Lai, B. F. L., et al. (2024). Integrating organoids and organ-on-a-chip devices. Nat. Rev. Bioeng. 2, 588–608. doi:10.1038/s44222-024-00207-z
Keywords: organs on a chip, developing and developed countries, animal experiments, clinical translation, biomedical research, Latin America and caribbean
Citation: Cabrera MD and del Valle AC (2025) Organs-on-a-chip for global equity: a perspective from Guatemala on advancing biomedical research in resource-limited settings. Front. Lab Chip Technol. 4:1669220. doi: 10.3389/frlct.2025.1669220
Received: 19 July 2025; Accepted: 17 September 2025;
Published: 01 October 2025.
Edited by:
Angeliki Tserepi, National Centre of Scientific Research Demokritos, GreeceReviewed by:
Arpana Parihar, Advanced Materials and Processes Research Institute (CSIR), IndiaCopyright © 2025 Cabrera and del Valle. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea C. del Valle, YW5kcmVhLmRlbHZhbGxlQHN1LnNl
 Maria D. Cabrera
Maria D. Cabrera Andrea C. del Valle
Andrea C. del Valle