- 1Department of Biology, Pennsylvania State University, University Park, PA, USA
- 2Division of Math, Science and Technology, Nova Southeastern University, Fort Lauderdale, FL, USA
- 3Center for Marine Environmental Studies, University of the Virgin Islands, St. Thomas, USVI, USA
- 4Khaled Bin Sultan Living Oceans Foundation, Landover, MD, USA
Coral populations at distributional margins frequently experience suboptimal and variable conditions. Recurrent El Niño-Southern Oscillation (ENSO) warming events have caused extensive mortality of reef-building corals in the Eastern Pacific, and particularly impacted branching pocilloporid corals in the Galápagos Islands. Pocillopora spp. were previously more common and formed incipient reefs at several locations in the archipelago but now occur as scattered colonies. Here, we report an unusually concentrated aggregation of colonies and evaluate their current genetic diversity. In particular we focus on a large population of 1614 live Pocillopora colonies found in a volcanic lagoon along the southern shore of Isabela Island. Forty seven colonies were sampled, primarily using a spatially explicit sampling design, and all colonies belonged to Pocillopora mitochondrial open reading frame lineage type 3a. Typing of additional Pocillopora samples (n = 40) from three other islands indicated that this stand is the only known representative of type 3a in the Galápagos Islands. The Isabela Pocillopora type 3a colonies harbored Symbiodinium ITS-2 clade C1d. Multilocus genotyping (n = 6 microsatellites) capable of resolving individual clones indicated that this stand is monogenotypic and thus the high density of colonies is a result of asexual reproduction, likely via fragmentation. Colony size distribution, while an imperfect measure, suggested the stand regrew from remnant colonies that survived the 1997/98 ENSO event but may postdate the 1982/83 ENSO. The community of Pocillopora colonies at Isabela is of particular ecological value due to its high density and support of associated organisms such as fish and benthic invertebrates. The Galapagos Pocillopora corals will continue to provide insights into the genetic structure and population dynamics of marginal coral populations.
Introduction
Many reef building corals occur over large geographic ranges and experience suboptimal and variable conditions especially at their distribution margins. Hence, marginal populations can provide unique insights into how corals might respond to climate change (Guinotte et al., 2003; Lirman and Manzello, 2009; Hennige et al., 2010; Goodkin et al., 2011). For example, coral communities in the Tropical Eastern Pacific (TEP) already experience seasonal cold upwelling, El Nino Southern Oscillation warm events and reduced aragonite saturation states (Glynn and Colgan, 1992; Fong and Glynn, 2000).
The Galápagos Islands harbor some of the most vibrant coral communities in the remote Tropical Eastern Pacific. The center of the archipelago is located 1000 km offshore from the equatorial South American coastline and 1200 km away from the more diverse Central Pacific coral communities. Recent analyses show that the offshore islands are well connected with coral populations along the Central American coast (Pinzón and Lajeunesse, 2011; Baums et al., 2012). Coral communities in the Galápagos Islands have experienced large scale bleaching events killing 97–100% of colonies during the 1982/83 El Niño-Southern Oscillation (ENSO) event (Glynn, 1988). Recent (primarily 1982/83 and 1997/98) ENSO events left a legacy of depressed coral populations (Glynn, 2003). Whereas Porites mostly recovered at the northern-most reefs at Darwin Island, Pocillopora density is still lower than prior to the ENSO events (Glynn et al., 2009). Even more limited recovery of Pocillopora has occurred in the central and southern Archipelago (Feingold and Glynn, 2014).
Branching corals in the genus Pocillopora form ecologically important reef structures throughout the TEP. Pocillopora is the primary constructor of modern reefs in the Eastern Pacific (Toth et al., 2012) and provides habitat for associated reef species in this low-diversity coral system (Glynn, 2004). In the Galápagos Islands, pocilloporid reef structures were known within the shallow basin of the nearly submerged volcanic cone, Devil's Crown, Floreana (Glynn and Wellington, 1983). Also, aggregations of colonies that formed incipient reefs were observed within semi-enclosed lava pools at Punta Espinosa, Fernandina Island, and well-developed communities occurred on the islands of San Cristobal, Española and Darwin (Glynn, 1994, 2003; Glynn et al., 2009). However, these structures were lost due to impacts associated with the 1982–83 ENSO event and subsequent bio-erosion. In all previously studied research sites in the archipelago, Pocillopora now occurs only as isolated, scattered colonies. One such recovering population of scattered Pocillopora is now present at the former reef site in Devil's Crown (Feingold and Glynn, 2014), but no live colonies have been noted in the lava rock pools of Punta Espinosa (Glynn, 2003). Recently, high densities of Pocillopora colonies were observed in the Concha y Perla Lagoon on the southern coast of Isabela Island (M Schmale, personal communication). Here, we set out to characterize the genetic diversity of the corals and their associated Symbiodinium dinoflagellates in this isolated yet highly dense population of Pocillopora and compare it to other Pocillopora collections from throughout the Galápagos Islands.
Pocillopora species designations were traditionally based on morphological characteristics and 8 or 9 (Hickman, 2008) separate species were identified within the Galápagos Islands. However, within the genus Pocillopora there is little correlation between morphology and species designation in the TEP. Only three evolutionary divergent lineages were found based on mitochondrial sequencing phylogenies and Bayesian clustering analysis (Flot et al., 2008; Pinzón and Lajeunesse, 2011). The mismatch between genetic data and traditional species designations based on morphology calls into question previously published species distributions and occurrences of Pocillopora in the TEP and elsewhere (Combosch and Vollmer, 2011; Pinzón et al., 2013; Schmidt-Roach et al., 2013). A re-evaluation of Pocillopora species distribution in the TEP is thus necessary especially in light of recent large-scale disturbances during ENSO events that can cause local extirpations (Glynn and Deweerdt, 1991; Toth et al., 2012). Here, we employ genetic markers to determine species and clonal diversity of Pocillopora and their dinoflagellate symbionts at Isabela Island and throughout the Galápagos Archipelago.
Size frequency distributions of colonies can provide insights into the recovery process from large scale disturbance events such as ENSO. However, correlating age and size is complicated in fragmenting corals such as Pocillopora damicornis. In addition to asexual reproduction via fragmentation, P. damicornis can produce asexual (ameiotic) (Yeoh and Dai, 2010) as well as sexual planula larvae leading to populations of mixed asexual and sexual origin, e.g., in the Western Australia, Panama, Hawaii and the Ryukyu Islands (Stoddart, 1984; Richmond, 1987; Adjeroud and Tsuchiya, 1999; Whitaker, 2006). In contrast, on the Great Barrier Reef and Lord Howe Island reef, sexual reproduction dominates (Benzie et al., 1995; Ayre et al., 1997; Ayre and Miller, 2004; Miller and Ayre, 2004). Sexual reproduction in Eastern Pacific pocilloporids occurs via spawning of female and male gametes into the water column where fertilization occurs (Glynn et al., 1991). Larvae can spend considerable time in the plankton and are already inoculated with Symbiodinium, their dinoflagellate symbionts (Richmond, 1987). Pocillopora colonies thus may achieve high population densities via either sexual or asexual reproduction. Fingerprinting with high-resolution genetic markers allows for identification of asexually produced colonies (Coffroth and Lasker, 1998; Baums et al., 2006), and in combination with size frequency distributions of colonies can provide insights into population growth and recovery processes.
While asexual reproduction allows for population expansion, it does not allow genetic recombination and, thus, only preserves existing genotypic variation rather than increasing it. Considerable variability in genotypic evenness and richness on small spatial scales is common in corals, ranging from minimal clonal replication to reefs dominated by just one genet (Hunter, 1993; Ayre and Hughes, 2000; Miller and Ayre, 2004; Baums et al., 2006; Sherman et al., 2006). Often asexual reproduction is common at the edges of a species range where sexual partners may be absent (Baums, 2008; Silvertown, 2008). Asexual reproduction allows genets to persist potentially indefinitely in the absence of a sexual partner. Locally well adapted coral clones may thus extend the range of a species (Boulay et al., 2014). Little is known about the contribution of asexual vs. sexual reproduction to population maintenance in Pocillopora corals in the Galápagos. Surveys of Pocillopora clonal structure in the SW Gulf of California, Mexico revealed that a site with little physical disturbance were dominated by a large clone whereas more disturbed sites had a higher occurrence of sexual recruits (Pinzón et al., 2012).
Here, we extend previous efforts (Combosch and Vollmer, 2011; Pinzón and Lajeunesse, 2011; Cunning et al., 2013; Pinzón et al., 2013) to evaluate the genetic diversity and population structure of Pocillopora in the Eastern Pacific at the geographic margins of this genus' range. By applying multilocus genotyping methods we discovered that the high density stand of Pocillopora corals at Isabela Islands was monogenotypic and aimed to determine whether this clone was a recent colonizer or a survivor of the large-scale ENSO events in 1982/83 and 1997/98. The community of Pocillopora colonies at Isabela is of particular ecological value due to its unique presence in the archipelago and support of associated organisms such as fish and benthic invertebrates. Its proximity to the population center of Puerto Villamil gives this ecological oasis high touristic appeal and consequently high economic value.
Materials and Methods
Sample Collection and DNA Extraction
Species diversity survey
Pocillopora corals were collected during the Global Reef Expedition onboard the M/V Golden Shadow to the Galápagos Islands in 2012. Forty colonies (Table 1) were sampled from across the Galápagos Islands, 6 from Darwin (01.67603°N, 091.99481°W), 24 from Marchena (00.30779°N, 090.40228°W), and 10 from Wolf (01.3856°N, 091.8146°W). Further, three neighboring aggregations of Pocillopora colonies were sampled on Isabela Island during the same cruise in 2012 (Table 2). They were located in 2–3 m depth just east of the tourist area of Concha y Perla lagoon at 00.96294°S, 090.95600°W. The colonies were found in a volcanic lagoon separated by a basalt sill into a small and large basin. A small sample was clipped from the tips of colonies using bone cutters and the colonies were photographed. Samples were preserved in ethanol and extracted using the DNeasy tissue kit (Qiagen) according to the manufacturer's instruction; however, extraction time in the lysis buffer was extended to 12 h.
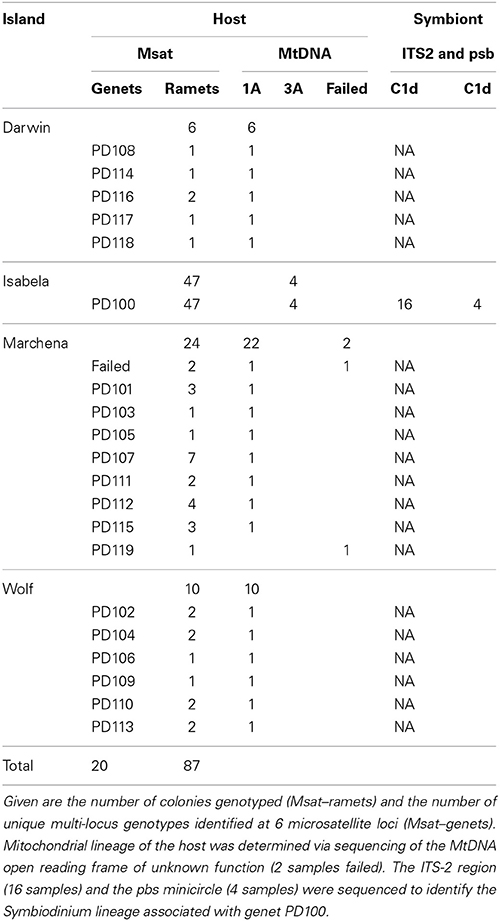
Table 1. Pocillopora colonies collected at Darwin, Isabela, Marchena and Wolf Islands, Galápagos Islands.
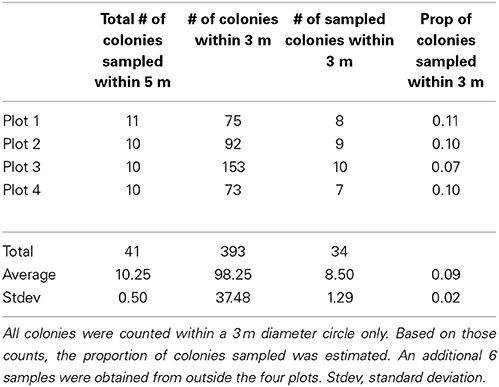
Table 2. Pocillopora colonies in the Concha y Perla lagoon on Isabela Island, Galápagos Islands were sampled (n = 41) in four plots of 5 m diameter.
Clonal structure in the Concha y Perla lagoon
The three Pocillopora aggregations in the Isabela volcanic lagoon were sampled for clonal structure following the sampling design of Baums et al. (2006). Briefly, coral branch tips (n = 41) were collected haphazardly in 5 m radius circular plots for a total of 4 plots within the volcanic pools on Isabela Island (Figure 1). Plots 3 and 4 were located in the same aggregation. Coordinates had a precision of 5° of arc and of 0.5 m along strike. Using a compass and a measuring tape secured to the center point of the circle, colonies were located by a team of SCUBA divers and mapped. The center of the plot was diver selected to maximize colony density and therefore sampling feasibility. An additional 6 colonies were sampled from areas outside of the four plots. A total of 47 branch tips from individual colonies were collected and preserved in 95% non-denatured ethanol. Samples were extracted for Genomic DNA using the DNeasy tissue kit (Qiagen) as above.
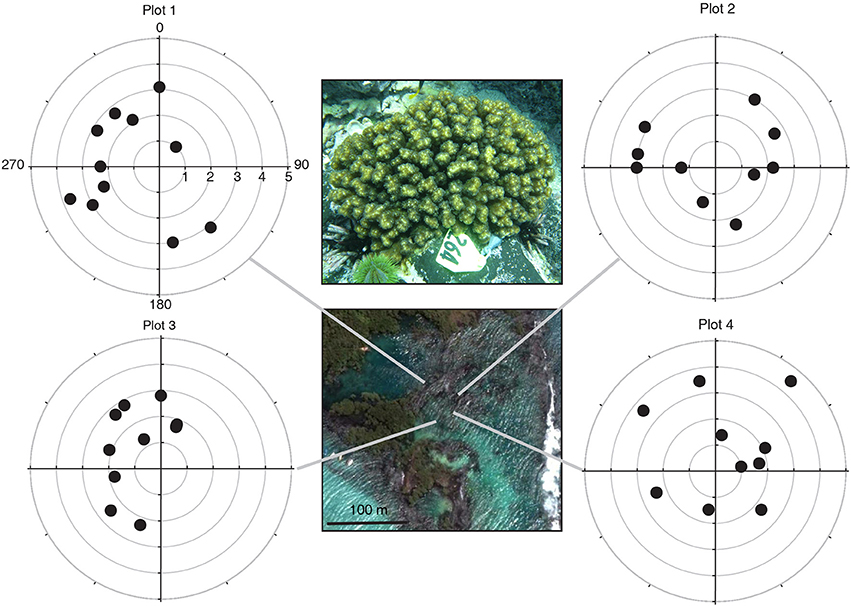
Figure 1. Pocillopora colonies were sampled in four polar plots within the volcanic pools at Concha Y Perla, Isabela Island, Galápagos. All colonies shared the same host multilocus genotype (indicated by the symbol shape) and harbored Symbiodinium ITS-2 clade C1d (indicated by fill color of the symbol). The host genet assigned to the Pocillopora mtDNA-ORF of unknown function lineage 3a. Polar plots: radial axis in m, angular axis in degrees. Satellite image from Google Earth.
Colony Size Measurements and Percent Mortality
The extent of each of the three Pocillopora aggregations was outlined using a handheld GPS while snorkeling around the perimeter of each. A series of photographic images were obtained over the complete area of the coral aggregations in the Concha y Perla Lagoon. A Nikon D5100 with a Nikon 10–24 mm lens and Ikelite waterproof housing and a housed Canon G12 camera were used without flash units. These images were taken as perpendicular as possible to the substrate, rather than strictly vertically, and care was taken to not overlap or repeat sections of the aggregation. A 1-m stick with graduated millimeter increments was used for scale and included in each image. Images were obtained only in areas with live colonies.
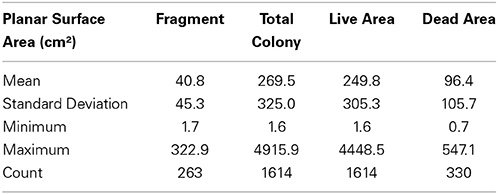
Coral Point Count with Excel extensions (CPCe) was used to measure the circumference of the colonies contained within each image (Kohler and Gill, 2006). The 2-D projection of each colony was outlined around the perimeter to calculate planar surface area. These data do not provide measurements of the actual 3-dimensional tissue area, only the planar (2-D) surface area. Measurements were made of individual colonies and fragments. For colonies with partial mortality two measurements were made, the total area and the portion that had died. Adjacent colonies were discriminated from each other by growth pattern, tissue color, and other distinctive patterns. These boundaries would be clear in some cases, but in others close consideration of which way the coral was growing or how they were connected helped determine boundaries. Fragments were distinguished in a similar fashion. A fragment would normally be clearly unattached from the aggregation and typically much smaller in size and laying on the benthic substrata. Some fragments showed partial mortality, but this was not discriminated. Instead a single measurement of the total planar surface area of each fragment was made. Dead areas were determined mostly by pigment differences from live tissue and the presence of turf algae on the skeleton.
Colony Age Estimation
Area estimates from colony sizes were used with published data on Pocillopora spp. growth rates to estimate age ranges of the colonies in the pool and to assess if any of the colonies were older than the 1982–83 and 1997–98 El Niño disturbances. The area of each colony was converted to colony radii assuming a circular colony shape with the formula
Age was estimated as the radius divided by the linear extension rate (cm year−1). Linear extension rates were estimated at 2.24 cm year−1 and were derived from measurements for pocilloporids (P. damicornis and P. elegans) from the Galápagos Islands based on Glynn et al. (1979). These estimates are lower than the mean linear extension rates from all studies conducted on pocilloporids in the Eastern Pacific (mean = 3.31 cm yr−1 ± 0.24 s.e.m., n = 11 studies, colony range 2.13–7.56; see Table 2 in Manzello, 2010). Estimation of ages from colony sizes is made difficult by processes that allow colony fission or fusion (Hughes, 1984). Assuming that fission (fragmentation) is the more important process, then linear extension likely overestimates colony growth rates from a group of colonies because it is usually measured as pristine growth (i.e., damaged colonies were excluded, Glynn et al., 1979) and thus, underestimates age. Therefore, these age estimates are likely conservative.
Polymerase Chain Reaction (PCR) Amplification of the Mitochondrial Open Reading Frame of Unknown Function
The mitochondrial open reading frame of unknown function (ORF) was amplified with the FATP6.1 and the RORF primers (Flot and Tillier, 2007; Flot et al., 2008). This was done for a subset of samples; 4 from inside the volcanic pools and all 40 from the islands of Darwin, Wolf, and Marchena. Amplified products were sequenced on the ABI Hitachi 3730XL genetic analyzer. DNA sequence chromatograms were reviewed and edited using CodonCode Aligner (CodonCode Corporation, Centerville, MA). Sequences (GenBank Accession #s: KM610241-KM610280, Supplementary Table 1) were aligned using ClustalW (Thompson et al., 1994) and neighbor-joining phylogenetic trees were constructed for the mitochondrial ORF using MEGA (Kumar et al., 2001). Trees (Figure 2) were generated using the Bootstrap method with 500 replications and the p-distance model. A representative of each previously-described Pocillopora mitochondrial lineage type sensu Pinzón and Lajeunesse (2011) was included in the phylogenetic analysis: four unique haplotypes (GenBank Accession #s: HQ378758–HQ378761) from the Eastern Pacific and 16 from the Indo-Pacific (GenBank Accession #s: JX994072–JX994088) were included for the phylogenetic tree.
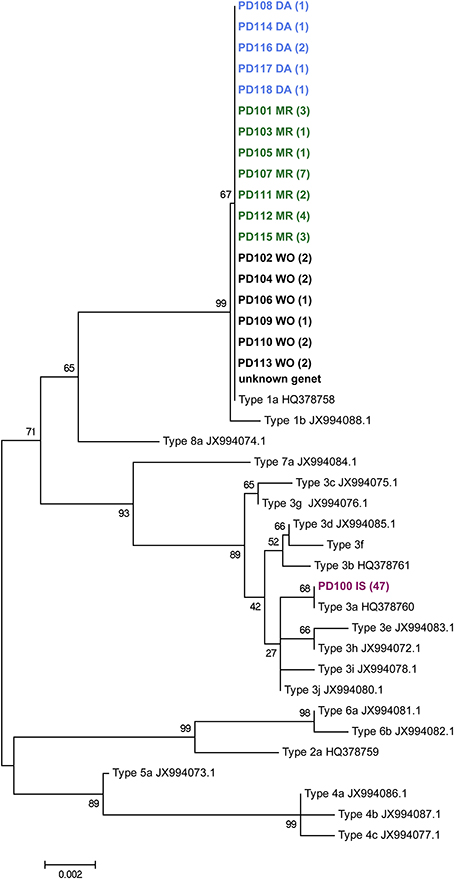
Figure 2. Neighbor-joining phylogenetic tree of the Pocillopora mtDNA open reading frame of unknown function. Each genet (names begin with letters PD) was included once in this dataset. Each genet name includes its geographic location as the last two letters, with “DA” = Darwin, “MR” = Marchena, “WO” = Wolf, “IS” = Isabela. The number of times a genet was observed is indicated in parentheses. Genet PD 119 failed to amplify for this marker. The topology of the tree matches the one published by Pinzón et al. (2013), however Type 4 clusters with Type 5 here rather than with Types 3 and 7. Pinzon et al. reported clustering of Type 4 with Type 5 in their Structure analysis. Gene Bank accession numbers: KM610241-KM610280.
Host Microsatellite Genotyping
Pocillopora colonies were genotyped using six published microsatellite loci: Pd3-002, Pd3-005, Pd2- 006, Pd2-007, Pd3-008, and Pd3-009 (Starger et al., 2008, Supplement 1). Single-plex reactions consisted of: 1X Taq polymerase buffer, 2.5 mM magnesium chloride, 0.5 mg/mL Bovine Serum Albumin (BSA), 0.2 mM of dNTPs, 0.15 μM forward primers, 0.15 μM reverse primers, 0.5U/μL Taq polymerase and 1 μL of DNA (concentrations ranged from 37 ng/μL to 240 ng/μL). PCR products were visualized using an ABI3730 (Applied Biosystems) automated DNA sequencer with an internal size standard (Gene Scan 500-Liz, Applied Biosystems) for accurate sizing. Electropherograms were analyzed using GeneMapper Software 5.0 (Applied Biosystems). These 6 markers should have enough power to accurately distinguish between closely related genotypes and those produced by asexual reproduction (probability of identity = 4.2 × 10−6; Waits et al., 2001).
Denaturing-Gradient Gel Electrophoresis (DGGE) and Minicircle Analysis
A denaturing-gradient gel electrophoresis (DGGE) was used to analyze the Internal Transcribed Spacer 2 (ITS2) of nuclear ribosomal RNA genes (Lajeunesse, 2001) for a total of 16 samples, 4 from each plot in the volcanic pools. The PCR was conducted using the forward primer, “ITSintfor2” (Lajeunesse and Trench, 2000), which anneals to a “Symbiodinium-conserved” region in the middle of the 5.8 s ribosomal gene and an ITS-reverse universal primer modified with a 39-bp GC clamp (Lajeunesse and Trench, 2000). Samples and a ladder containing a mix of C1, D1a, and B1 were loaded onto an 8% polyacrylamide denaturing gradient gel (45–80% urea-formamide gradient; 100% consists of 7 mol L21 urea and 40% deionized formamide) and separated by electrophoresis for 15 h at 115 V at a constant temperature of 60°C (Lajeunesse, 2002). The gel was stained with Sybr Green (Molecular Probes) for 25 min according to the manufacturer's specifications and photographed (Figure 3). Comparison of the samples with the ladder indicated that all samples contained ITS-2 Clade C1. To determine the ITS2-subclade, the non-coding region of the psbA minicircle, an element in the chloroplast genome that allows high resolution comparisons among Symbiodinium clades, was sequenced on the Applied Biosystems 3730XL using the primers miniC-F and miniC-Rev and protocol as specified by Moore et al. (2003).

Figure 3. Internal transcribed spacer 2-DGGE analysis of 16 samples belonging to genet PD100 from the volcanic pools at Isabela identified Symbiodinium ITS-2 sublcade C1d as the major symbiont in all samples. Second to last lane from the right is the size standard (mixture of clades D1, B1, and C1).
Results
Microsatellite Analysis Reveals Only one Genet in Isabela's Lava Pools
Using 6 microsatellite markers, multi-locus genotypes were determined for 47 colonies from within the lava pools on Isabela and 40 samples haphazardly collected from Darwin, Marchena and Wolf Islands (Table 1). All 47 colonies sampled from within the volcanic pools of Isabela Island were of the same multi locus genotype (Table 1), that is they were all clonemates of the same genet (PD100, Figure 1). Within each of the four plots, about 10% of colonies were genotyped (Table 2). In contrast, the maximum number of clonemates per genet was seven (genet PD107) for any of the samples collected from Darwin, Marchena and Wolf (Table 1). However, note that sampling of colonies outside of Isabela occurred over a larger area within each site than sampling within the lava pools. Greater spatial dispersion of sampled colonies could lead to less genetic similarity.
Typing of the Host's Mitochondrial Open Reading Frame
Four colonies belonging to genet PD100 from within the lava pools at Isabela Island were typed for the ORF of unknown function of the host's mitochondria and found to be of lineage 3a (Figure 2). In addition to the lava pool samples, 40 of the 42 samples randomly collected throughout the Galápagos Islands including Marchena, Wolf and Darwin Islands, successfully amplified for the mitochondrial lineage and were found to be of type 1a.
DGGE Reveals Genet PD100 Harbors Symbiodinium ITS2-Clade C1d
Internal transcribed spacer 2-DGGE analysis of 16 samples belonging to genet PD100 from the volcanic pools at Isabela identified Symbiodinium ITS-2 clade C1 as the major symbiont in all samples (Table 1, Figure 3). No other ITS-2 clades appeared to be present at detectable levels. Sequencing of the non-coding psbA region of the minicircle of two of the samples from within the volcanic pools further resolved the identified Symbiodinium ITS-2 type as sublcade C1d (Table 1).
Colony Size Measurements and Percent Mortality
The three aggregations of Pocillopora colonies in the Concha y Perla lava pools occupied areas of 53 m2, 104 m2, and 291 m2. These aggregations contained a total of 1614 colonies at a density of 3.6 colonies m−2 (Table 3). There was a total of 43.5 m2 of overall colony area (planar view of live tissue and dead skeleton), of which 40.3 m2 was live coral tissue. The average live tissue area of each colony was 249.8 cm2. Of the total colony surface area, 92.7% was live tissue. In addition, 263 fragments were observed, indicating that asexual reproduction was occurring.
Age Estimates
An estimate of colony ages based on southern Galápagos Pocillopora spp. growth rate averages of Glynn et al.[1979, 2.24 cm year−1] gave a mean colony age of 3.59 years ± 2.05 SD. The range was 1.68–3.59 years when using the average growth rate of all 11 ETP studies. The three largest colonies found within the three aggregations had estimated ages of 14, 15, and 18 years using the Glynn et al. growth rates. When assuming minimum ages based on the fastest Eastern Pacific growth rate from the Gulf of Papagayo, Costa Rica (4.78 cm year−1; Manzello, 2010) the three largest colonies were 7, 7, and 8 years old.
Discussion
The Galápagos Islands harbor some of the most vibrant coral communities in the Tropical Eastern Pacific. Here, we showed that the densest known Pocillopora population in the entire Galápagos Archipelago was the result of asexual reproduction. We cannot say for certain whether this clone is a survivor of the 1982/83 ENSO or a later arrival but preliminary age estimates from colony sizes indicate that the birth of the clone may predate the 1997/98 ENSO event. The three largest colonies found within the three aggregations had estimated ages of 14, 15, and 18 years, suggesting a conservative estimated recruitment date of at or just before the 1997–98 El Niño, whereas the remaining 1611 colonies were estimated to be younger than the 1997–98 El Niño. If only three colonies survived 1997–98, they were probably remnants from a larger population. This bottleneck makes it impossible to determine if the clone survived through the 1982–83 El Niño in the volcanic pool or recruited afterwards from more distant locations.
Mitochondrial Markers Define Two Distinct Lineages in the Galápagos Archipelago
Pocillopora damicornis is a small branching coral (Figure 1) that forms dense stands in shallow reefs throughout the Pacific (Goreau, 1959). Morphological identification is a challenge (Combosch et al., 2008; Souter, 2010) but sequencing of the mitochondrial ORF allows for designation of distinct lineages (Flot et al., 2008; Souter et al., 2009; Pinzón and Lajeunesse, 2011; Pinzón et al., 2013). Three types (Type 1–3) can be distinguished genetically that appear to be broadcast spawners (Toonen unpubl. data, Pinzón and Lajeunesse, 2011). An additional four types (4–7) appear to be brooders (Pinzón, 2011). Type 3 and 5 are prevalent throughout the Pacific. Co-occurrence of types might reconcile observations of broadcast spawning and brooding in colonies identified as Pocillopora damicornis from the same reef (Ward, 1992). Both brooding and broadcasting types are hermaphroditic (Sier and Olive, 1994; Kruger and Schleyer, 1998).
From inside the volcanic pools at Isabela Island, all samples typed for the mt-ORF were found to be of lineage 3a (Figure 2) making the Isabela Island genet the only known representative of this lineage in the Galápagos Archipelago albeit sampling has not been exhaustive thus far. In Panama, type 3a is commonly found on reefs in Taboga and Uraba. Pinzón and Lajeunesse (2011) also found three Pocillopora colonies of type 3b in the Galápagos; 1 on Marchena Island and 2 on Darwin Island. The remainder of the Pocillopora colonies analyzed by Pinzón and Lajeunesse (n = 19, 2011) and here (n = 38, Table 1) from throughout the Galápagos Island were of type 1a. Lineages 3a and 3b are only separated by 2 nucleotide changes whereas types 3 and 1 are separated by 14 nucleotide differences (Pinzón and Lajeunesse, 2011). It is not known if mitochondrial lineage types 3a and 3b are sexually compatible (i.e., if they represent different species), however type 3b appears to be rare in the Eastern Pacific (Cunning et al., 2013; Pinzón et al., 2013). Therefore, it is possible that the Isabela colonies represent a founder or remnant genet.
Population Dynamics of Marginal Coral Populations
Populations at the edges of a species' range may only receive sporadic immigrants from more central populations. The “abundant center” model makes specific predictions about the demographic properties and genetic diversity of marginal populations (Antonovics, 1976; Brussard, 1984; Lawton, 1993; Hoffmann and Blows, 1994; Lesica and Allendorf, 1995; Vucetich and Waite, 2003) such as those in the Tropical Eastern Pacific, Japan and the Red Sea. Evidence for the model has been equivocal in terrestrial and marine systems (reviewed in Sagarin and Gaines, 2002; Eckert et al., 2008) and we do not directly test its validity here. However, according to the hypothesis, physical isolation is expected to increase and population size is expected to decrease with increasing distance from the geographic center of a species' range (reviewed in Sagarin and Gaines, 2002; Eckert et al., 2008). If gene flow is correlated with distance, differentiation will be higher among peripheral populations than central populations, and so enhance the probability of inbreeding and the loss of allelic diversity in marginal populations. Because corals can reproduce locally by asexual means, reduced gene flow into marginal populations can result in increased clonality (i.e., decreased genotypic diversity).
Because successful fertilization of gametes is dependent on the distance among adults in broadcast spawning organisms (Levitan, 1992), marginal populations frequently experience Allee effects (Eckert, 2002; Baums et al., 2006). In species capable of asexual reproduction and/or self-fertilization, a rare migrant to a novel environment can successfully establish high local population densities via fragmentation and local recruitment of selfed larvae even in the absence of other sexual partners (Eckert, 2002). Such genetically depauperate populations can persist for extended periods of time until additional migrants arrive. In the Eastern Pacific, ENSO events change current patterns sometimes bringing migrants to locations where these species are not normally found (Glynn and Ault, 2000). Often the species fail to establish due to a lack of mates and other stochastic factors. Because of the lack of genetic diversity, such populations are vulnerable to disease outbreaks, and they carry an extinction debt (Honnay and Bossuyt, 2005).
Conversely, marginal conditions combined with reduced gene flow can lead to evolution of locally adapted genotypes in edge populations (Bell and Gonzalez, 2011). Asymmetrical gene flow from the center to the margins (driven by the higher densities in the center) can offset the loss of genetic diversity on the edges (Kirkpatrick and Barton, 1997) and improve fitness (Sexton et al., 2011) but also swamp locally adapted genotypes (Haldane, 1956; Case and Taper, 2000). Given this complexity, it remains unknown whether marginal coral populations retain enough functional genetic diversity to adapt to changing conditions and if those adaptations are shared among populations.
Dispersal of type 3a larvae from other TEP locations to Isabela may occur in the future. This assessment is supported by limited data on gene flow and connectivity in corals across the TEP. Of the Pocillopora types, Type 1a is the only one with sufficient sample sizes across the region to allow for population-level analysis. Structure results, utilizing seven microsatellite markers, suggested limited partitioning, however Fst and Rst calcuations were not significant, indicating panmixia within this region which includes the Mexican mainland, Revillagigedo Island, Clipperton Atoll, the Galápagos and Panama (Pinzón and Lajeunesse, 2011). Porites lobata was similarly well connected throughout the TEP (Baums et al., 2012). A more comprehensive assessment of coral gene flow patterns within the TEP across a range of species is needed to determine routes of successful larval dispersal within the region (Lessios and Baums, in preparation).
The Densest Known Community of Pocillopora in the Galápagos Archipelago Formed Asexually
Initial establishment of the Pocillopora community in Concha y Perla lagoon could have been via sexually or asexually produced (ameiotic) planula larvae that settled on available basalt substrata. Once established at the study site, the high density of the Isabela Pocillopora aggregations resulted from asexual reproduction, either via fragmentation or ameiotic larvae (Table 1, Figure 1). While we cannot say for certain, the data indicate that fragmentation is the dominant reproductive process generating the high population density. Accordingly, a high number of fragments were observed within the lava pools (Table 3). Large fragments have a higher chance of survival (Lirman, 2000) so dispersal is limited but over time genets can extend over 10 s of meters (Lasker, 1990; Baums et al., 2006; Foster et al., 2007; Pinzón et al., 2012).
Asexually produced propagules of Pocillopora are not always the result of fragmentation. Pocillopora and other coral species release ameiotic planulae as evidenced by having multilocus genotypes identical to their mothers' (Stoddart, 1983; Stoddart et al., 1988; Brazeau et al., 1998; Sherman et al., 2006; Yeoh and Dai, 2010). Ameiotic planulae have, theoretically, the same dispersal potential as their sexually produced counterparts and thus could be transported further than fragments (Stoddart, 1983). Several clones of the coral P. damicornis were found distributed over 8 reefs in Hawaii (Stoddart, 1983) and over 800 km in Australia (Whitaker, 2006). However, we did not find evidence of genet PD100 outside of the larva pools despite searching habitat around Isabela that previously had been settled by Pocillopora. Had we found PD100 elsewhere, this would have indicated that the clone produced ameiotic planulae with dispersal potential. The pools are flushed daily—the tidal flow is quite strong so that larvae should have been able to disperse outside the pool. However, larvae may not find suitable habitat easily in the southern Galápagos due to low temperatures and unfavorable alkalinity (Manzello, 2010). Nevertheless, there is a chance that further searches may yet reveal evidence of PD100 outside the pools.
Symbiodinium
The three mt-DNA lineages of Pocillopora in the Tropical Eastern Pacific identified by Pinzón and Lajeunesse (2011) associate primarily with one or two Symbiodinium ITS-2 clade types. Pocillopora mt-DNA Lineage 1a was found to harbor both Symbiodinium C1b-c and S. glynni (clade D) whereas Pocillopora mt-DNA Lineage type 3 contained only Symbiodinium C1d (Lajeunesse et al., 2008; Pinzón and Lajeunesse, 2011). Analysis of a larger dataset from the Eastern Pacific subsequently also discovered Symbiodinium clade D in Pocillopora lineage 3 (Cunning et al., 2013). Nevertheless, all 16 tested Pocillopora mt-DNA Lineage type 3a samples from within the volcanic pools at Isabela harbored only Symbiodinium ITS-2 clade C1d.
The uniformity of the host genet-Symbiodinium association in the lava pools at the subclade level is not surprising (Thornhill et al., 2014). Analysis of Symbiodinium ITS-2 clade C1d from within the Isabela pools with multiple microsatellite markers may reveal additional subcladal genetic and thereby, perhaps, functional diversity (Howells et al., 2012). However, in other coral species with extensive asexual reproduction, colonies usually associate with just one clonal strain of Symbiodinium (Andras et al., 2011, 2013; Baums et al., 2014) and clonemates of the same host genet often harbor the same clonal strain of Symbiodinium (Baums et al., 2014).
Conservation Implications
The clone of Pocillopora mtORF type 3a in the lava pools of Concha y Perla is the only known representative of its type in the Galápagos. While local density is quite high, the low genotypic diversity may limit the evolutionary potential to selfing and somatic mutations (Van Oppen et al., 2011). No evidence of selfing was found within the pools as that would have generated distinct albeit similar genotypes rather than identical ones. We are quite confident in the conclusion that all sampled colonies were the result of asexual reproduction due to the high number of microsatellite markers used which results in high power to distinguish between closely related and identical genotypes. We cannot exclude the possibility that additional sampling may have detected other Pocillopora genotypes, however the chances seem remote. Moreover, all tested colonies only harbored one ITS-2 clade type, Symbiodinium ITS-2 clade C1d. This apparent absence of genetic diversity makes the Isabela population vulnerable to infectious disease outbreaks and environmental perturbations. While other coral species are rare in the pool, the pool is heavily visited by snorkelers who generally have traveled to other areas of the Archipelago and may serve as disease vectors. Physical contact via fins is one way to spread infectious coral diseases (Williams and Miller, 2005). Rinsing of snorkel gear in a mild bleach solution can reduce the risk of introducing an infectious disease. The Pocillopora population should be monitored for arrival of new, genetically diverse recruits.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The data presented here represent one component of a larger assessment of coral reefs undertaken by the Khaled bin Sultan Living Oceans Foundation and their partners during the Global Reef Expedition. Samples were collected and exported with appropriate permissions from the Galápagos National Park (Permiso de investigacion cientifoca pc-07-12, No. 0059922, issued 28/05/2012), and logistical support was provided by the Charles Darwin Research Station. Special thanks to Peter W Glynn for his leadership during this expedition. Thanks also to the other expedition members and Francesca Fourney who helped process coral population data. Thanks to the LaJeunesse lab for help with DGGE analysis. Funding was provided by NSF grant OCE 0928764 to Iliana B. Baums and an Undergraduate Discovery grant from the PSU Eberly College of Science to Beatrice A. A. Laing and Iliana B. Baums.
Supplementary Material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fmars.2014.00059/abstract
References
Adjeroud, M., and Tsuchiya, M. (1999). Genetic variation and clonal structure in the scleractinian coral Pocillopora damicornis in the Ryukyu Archipelago, southern Japan. Marine Biology 134, 753–760. doi: 10.1007/s002270050592
Andras, J. P., Kirk, N. L., and Drew Harvell, C. (2011). Range-wide population genetic structure of Symbiodinium associated with the Caribbean Sea fan coral, Gorgonia ventalina. Mol. Ecol. 20, 2525–2542. doi: 10.1111/j.1365-294X.2011.05115.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Andras, J. P., Rypien, K. L., and Harvell, C. D. (2013). Range-wide population genetic structure of the Caribbean sea fan coral, Gorgonia ventalina. Mol. Ecol. 22, 56–73. doi: 10.1111/mec.12104
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Antonovics, J. (1976). Nature of limits to natuarl selection. Ann. Mo. Bot. Gard. 63, 224–247. doi: 10.2307/2395303
Ayre, D. J., and Hughes, T. P. (2000). Genotypic diversity and gene flow in brooding and spawning corals along the Great Barrier Reef, Australia. Evolution 54, 1590–1605. doi: 10.1111/j.0014-3820.2000.tb00704.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ayre, D. J., Hughes, T. P., and Standish, R. S. (1997). Genetic differentiation, reproductive mode, and gene flow in the brooding coral Pocillopora damicornis along the Great Barrier Reef, Australia. Marine Ecology-Progress Series 159, 175–187. doi: 10.3354/meps159175
Ayre, D. J., and Miller, K. J. (2004). Where do clonal coral larvae go? Adult genotypic diversity conflicts with reproductive effort in the brooding coral Pocillopora damicornis. Marine Ecology-Progress Series 277, 95–105. doi: 10.3354/meps277095
Baums, I. B. (2008). A restoration genetics guide for coral reef conservation. Mol. Ecol. 17, 2796–2811. doi: 10.1111/j.1365-294X.2008.03787.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Baums, I. B., Boulay, J., Polato, N. R., and Hellberg, M. E. (2012). No gene flow across the Eastern Pacific Barrier in the reef-building coral Porites lobata. Mol. Ecol. 21, 5418–5433. doi: 10.1111/j.1365-294X.2012.05733.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Baums, I. B., Devlin-Durante, M. K., and Lajeunesse, T. C. (2014). New insights into the dynamics between reef corals and their associated dinoflagellate endosymbionts from population genetic studies. Mol. Ecol. 23, 4203–4215. doi: 10.1111/mec.12788
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Baums, I. B., Miller, M. W., and Hellberg, M. E. (2006). Geographic variation in clonal structure in a reef building Caribbean coral, Acropora palmata. Ecol. Monogr. 76, 503–519. doi: 10.1890/0012-9615(2006)076[0503:GVICSI]2.0.CO;2
Bell, G., and Gonzalez, A. (2011). Adaptation and evolutionary rescue in metapopulations experiencing environmental deterioration. Science 332, 1327–1330. doi: 10.1126/science.1203105
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Benzie, J. A. H., Haskell, A., and Lehman, H. (1995). Variation in the genetic composition of coral (Pocillopora damicornis and Acropora palifera) populations from different reef habitats. Marine Biology 121, 731–739. doi: 10.1007/BF00349309
Boulay, J. N., Hellberg, M. E., Cortés, J., and Baums, I. B. (2014). Unrecognized coral species diversity masks differences in functional ecology. Proc. Biol. Sci. 281:20131580. doi: 10.1098/rspb.2013.1580
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Brazeau, D. A., Gleason, D. F., and Morgan, M. E. (1998). Self-fertilization in brooding hermaphroditic Caribbean corals: evidence from molecular markers. J. Exp. Mar. Biol. Ecol. 231, 225–238. doi: 10.1016/S0022-0981(98)00097-5
Brussard, P. F. (1984). Geographical patterns and environmental gradients - the central-marginal model in Drosophila revisited. Annu. Rev. Ecol. Syst. 15, 25–64. doi: 10.1146/annurev.es.15.110184.000325
Case, T. J., and Taper, M. L. (2000). Interspecific competition, environmental gradients, gene flow, and the coevolution of species' borders. Am. Nat. 155, 583–605. doi: 10.1086/303351
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Coffroth, M. A., and Lasker, H. R. (1998). Population structure of a clonal gorgonian coral: the interplay between clonal reproduction and disturbance. Evolution 52, 379–393. doi: 10.2307/2411075
Combosch, D. J., Guzman, H. M., Schuhmacher, H., and Vollmer, S. V. (2008). Interspecific hybridization and restricted trans-Pacific gene flow in the Tropical Eastern Pacific Pocillopora. Mol. Ecol. 17, 1304–1312. doi: 10.1111/j.1365-294X.2007.03672.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Combosch, D. J., and Vollmer, S. V. (2011). Population genetics of an ecosystem-defining reef coral Pocillopora damicornis in the Tropical Eastern Pacific. PLoS ONE 6:e21200. doi: 10.1371/journal.pone.0021200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cunning, R., Glynn, P. W., and Baker, A. C. (2013). Flexible associations between Pocillopora corals and Symbiodinium limit utility of symbiosis ecology in defining species. Coral Reefs 32, 795–801. doi: 10.1007/s00338-013-1036-y
Eckert, C. G. (2002). The loss of sex in clonal plants. Evol. Ecol. 15, 501–520. doi: 10.1023/A:1016005519651
Eckert, C. G., Samis, K. E., and Lougheed, S. C. (2008). Genetic variation across species' geographical ranges: the central-marginal hypothesis and beyond. Mol. Ecol. 17, 1170–1188. doi: 10.1111/j.1365-294X.2007.03659.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Feingold, J. S., and Glynn, P. W. (2014). “Coral Research in the Galápagos Islands, Ecuador,” in The Galapagos Marine Reserve. A Dynamic Social-Ecological System, eds J. Denkinger and L. Vinueza (Heidelberg; New York, NY; Dordrecht; London: Springer), 3–22. doi: 10.1007/978-3-319-02769-2_1
Flot, J. F., Magalon, H., Cruaud, C., Couloux, A., and Tillier, S. (2008). Patterns of genetic structure among Hawaiian corals of the genus Pocillopora yield clusters of individuals that are compatible with morphology. C. R. Biol. 331, 239–247. doi: 10.1016/j.crvi.2007.12.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Flot, J. F., and Tillier, S. (2007). The mitochondrial genome of Pocillopora (Cnidaria: Scleractinia) contains two variable regions: the putative D-loop and a novel ORF of unknown function. Gene 401, 80–87. doi: 10.1016/j.gene.2007.07.006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Fong, P., and Glynn, P. W. (2000). A regional model to predict coral population dynamics in response to El Nino-Southern Oscillation. Ecol. Appl. 10, 842–854. doi: 10.2307/2641049
Foster, N. L., Baums, I. B., and Mumby, P. J. (2007). Sexual vs. asexual reproduction in an ecosystem engineer: the massive coral Montastraea annularis. J. Anim. Ecol. 76, 384–391. doi: 10.1111/j.1365-2656.2006.01207.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Glynn, P. W. (1988). El-Niño Southern Oscillation 1982-1983 - Nearshore population, community, and ecoystem responses. Annu. Rev. Ecol. Syst. 19, U309.
Glynn, P. W. (1994). State of coral-reefs in the Galapagos-Island - Natural vs anthropogenic Impacts. Mar. Pollut. Bull. 29, 131–140. doi: 10.1016/0025-326X(94)90437-5
Glynn, P. W. (2003). “Coral communities and coral reefs of ecuador,” in Latin America Coral Reefs, ed J. Cortes (Amsterdam: Elsevier), 449–472.
Glynn, P. W. (2004). High complexity food webs in low-diversity Eastern Pacific reef-coral communities. Ecosystems 7, 358–367. doi: 10.1007/s10021-004-0184-x
Glynn, P. W., and Ault, J. S. (2000). A biogeographic analysis and review of the far Eastern Pacific coral reef region. Coral Reefs 19, 1–23. doi: 10.1007/s003380050220
Glynn, P. W., and Colgan, M. W. (1992). Sporadic disturbances in fluctuating coral-reef environments - El Nino and coral-reef development in the Eastern Pacific. Am. Zool. 32, 707–718.
Glynn, P. W., and Deweerdt, W. H. (1991). Elimination of 2 reef-building hydrocorals following the 1982-83 el-nino warming event. Science 253, 69–71. doi: 10.1126/science.253.5015.69
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Glynn, P. W., Gassman, N. J., Eakin, C. M., Cortes, J., Smith, D. B., and Guzman, H. M. (1991). Reef coral reproduction in the Eastern Pacific - Costa-Rica, Panama, and Galapagos-Islands (Ecuador).1. Pocilloporidae. Mar. Biol. 109, 355–368. doi: 10.1007/BF01313501
Glynn, P. W., Riegl, B., Correa, A. M. S., and Baums, I. B. (2009). Rapid recovery of a coral reef at Darwin Island, Galapagos Islands. Galapagos Res. 66, 6–13. Available online at: http://www.darwinfoundation.org/datazone/galapagos-research/
Glynn, P. W., and Wellington, G. M. (1983). Corals and Coral Reefs of the Galápagos Islands. Berkeley, CA: University of California Press.
Glynn, P. W., Wellington, G. M., and Birkeland, C. (1979). Coral reef growth in galapagos - limitation by sea-urchins. Science 203, 47–49. doi: 10.1126/science.203.4375.47
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Goodkin, N. F., Switzer, A. D., McCorry, D., Devantier, L., True, J. D., Hughen, K. A., et al. (2011). Coral communities of Hong Kong: long-lived corals in a marginal reef environment. Mar. Ecol. Prog. Ser. 426, 185–196. doi: 10.3354/meps09019
Goreau, T. F. (1959). The ecology of Jamaican coral reefs: species composition and zonation. Ecology 40, 67–90. doi: 10.2307/1929924
Guinotte, J. M., Buddemeier, R. W., and Kleypas, J. A. (2003). Future coral reef habitat marginality: temporal and spatial effects of climate change in the Pacific basin. Coral Reefs 22, 551–558. doi: 10.1007/s00338-003-0331-4
Haldane, J. B. S. (1956). The relation between density regulation and natural selection. Proc. R. Soc. Lond. B. Biol. Sci. 145, 306–308. doi: 10.1098/rspb.1956.0039
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hennige, S. J., Smith, D. J., Walsh, S. J., McGinley, M. P., Warner, M. E., and Suggett, D. J. (2010). Acclimation and adaptation of scleractinian coral communities along environmental gradients within an Indonesian reef system. J. Exp. Mar. Biol. Ecol. 391, 143–152. doi: 10.1016/j.jembe.2010.06.019
Hickman, C. P. (2008). A Field Guide to Corals and Other Radiates of Galápagos. Lexington, VA: Sugar Spring Press.
Hoffmann, A. A., and Blows, M. W. (1994). Species borders - ecological and evolutionary perspectives. Trends Ecol. Evol. 9, 223–227. doi: 10.1016/0169-5347(94)90248-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Honnay, O., and Bossuyt, B. (2005). Prolonged clonal growth: escape route or route to extinction? Oikos 108, 427–432. doi: 10.1111/j.0030-1299.2005.13569.x
Howells, E. J., Beltran, V. H., Larsen, N. W., Bay, L. K., Willis, B. L., and Van Oppen, M. J. H. (2012). Coral thermal tolerance shaped by local adaptation of photosymbionts. Nat. Clim. Change 2, 116–120. doi: 10.1038/nclimate1330
Hughes, T. P. (1984). Population dynamics based on individual size rather than age: a general model with a reef coral example. Am. Nat. 123, 778–795. doi: 10.1086/284239
Hunter, C. L. (1993). Genotypic variation and clonal structure in coral populations with different disturbance histories. Evolution 47, 1213–1228. doi: 10.2307/2409987
Kirkpatrick, M., and Barton, N. H. (1997). Evolution of a species' range. Am. Nat. 150, 1–23. doi: 10.1086/286054
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kohler, K. E., and Gill, S. M. (2006). Coral point count with excel extensions (CPCe): a visual basic program for the determination of coral and substrate coverage using random point count methodology. Comput. Geosci. 32, 1259–1269. doi: 10.1016/j.cageo.2005.11.009
Kruger, A., and Schleyer, M. H. (1998). Sexual reproduction in the coral Pocillopora verrucosa (Cnidaria: Scleractinia) in KwaZulu-Natal, South Africa. Mar. Biol. 132, 703–710. doi: 10.1007/s002270050434
Kumar, S., Tamura, K., Jakobsen, I. B., and Nei, M. (2001). MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17, 1244–1245. doi: 10.1093/bioinformatics/17.12.1244
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lajeunesse, T. C. (2001). Investigating the biodiversity, ecology, and phylogeny of endosymbiotic dinoflagellates in the genus Symbiodinium using the its region: In search of a “species” level marker. J. Phycol. 37, 866–880. doi: 10.1046/j.1529-8817.2001.01031.x
Lajeunesse, T. C. (2002). Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar. Biol. 141, 387–400. doi: 10.1007/s00227-002-0829-2
Lajeunesse, T. C., Bonilla, H. R., Warner, M. E., Wills, M., Schmidt, G. W., and Fitt, W. K. (2008). Specificity and stability in high latitude Eastern Pacific coral-algal symbioses. Limnol. Oceanogr. 53, 719–727. doi: 10.4319/lo.2008.53.2.0719
Lajeunesse, T. C., and Trench, R. K. (2000). Biogeography of two species of Symbiodinium (Freudenthal) inhabiting the intertidal sea anemone Anthopleura elegantissima (Brandt). Biol. Bull. 199, 126–134. doi: 10.2307/1542872
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lasker, H. R. (1990). Clonal propagation and population-dynamics of a gorgonian coral. Ecology 71, 1578–1589. doi: 10.2307/1938293
Lawton, J. H. (1993). Range, population abundance and conservation. Trends Ecol. Evol. 8, 409–413. doi: 10.1016/0169-5347(93)90043-O
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lesica, P., and Allendorf, F. W. (1995). When are peripheral populations valuable for conservation? Conserv. Biol. 9, 753–760. doi: 10.1046/j.1523-1739.1995.09040753.x
Levitan, D. R. (1992). Community structure in times past - influence of human fishing pressure on algal urchin interactions. Ecology 73, 1597–1605. doi: 10.2307/1940013
Lirman, D. (2000). Lesion regeneration in the branching coral Acropora palmata: effects of colonization, colony size, lesion size, and lesion shape. Mar. Ecol. Prog. Ser. 197, 209–215. doi: 10.3354/meps197209
Lirman, D., and Manzello, D. (2009). Patterns of resistance and resilience of the stress-tolerant coral Siderastrea radians (Pallas) to sub-optimal salinity and sediment burial. J. Exp. Mar. Biol. Ecol. 369, 72–77. doi: 10.1016/j.jembe.2008.10.024
Manzello, D. P. (2010). Coral growth with thermal stress and ocean acidification: lessons from the eastern tropical Pacific. Coral Reefs 29, 749–758. doi: 10.1007/s00338-010-0623-4
Miller, K. J., and Ayre, D. J. (2004). The role of sexual and asexual reproduction in structuring high latitude populations of the reef coral Pocillopora damicornis. Heredity 92, 557–568. doi: 10.1038/sj.hdy.6800459
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Moore, R. B., Ferguson, K. M., Loh, W. K. W., Hoegh-Guldberg, C., and Carter, D. A. (2003). Highly organized structure in the non-coding region of the psbA minicircle from clade C Symbiodinium. Int. J. Syst. Evol. Microbiol. 53, 1725–1734. doi: 10.1099/ijs.0.02594-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pinzón, J. (2011). The Evolution of Animal-Microbe Mutualisms. Ph.D. The Pennsylvania State University.
Pinzón, J. H., and Lajeunesse, T. C. (2011). Species delimitation of common reef corals in the genus Pocillopora using nucleotide sequence phylogenies, population genetics and symbiosis ecology. Mol. Ecol. 20, 311–325. doi: 10.1111/j.1365-294X.2010.04939.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pinzón, J. H., Sampayo, E., Cox, E., Chauka, L. J., Chen, C. A., Voolstra, C. R., et al. (2013). Blind to morphology: genetics identifies several widespread ecologically common species and few endemics among Indo-Pacific cauliflower corals (Pocillopora, Scleractinia). J. Biogeogr. 40, 1595–1608. doi: 10.1111/jbi.12110
Pinzón, J., Reyes-Bonilla, H., Baums, I., and Lajeunesse, T. (2012). Contrasting clonal structure among Pocillopora (Scleractinia) communities at two environmentally distinct sites in the Gulf of California. Coral Reefs 3, 765–777. doi: 10.1007/s00338-012-0887-y
Richmond, R. H. (1987). Energetics, competence, and long-distance dispersal of planula larvae of the coral Pocillopora damicornis. Mar. Biol. 93, 527–533. doi: 10.1007/BF00392790
Sagarin, R. D., and Gaines, S. D. (2002). The ‘abundant centre’ distribution: to what extent is it a biogeographical rule? Ecol. Lett. 5, 137–147. doi: 10.1046/j.1461-0248.2002.00297.x
Schmidt-Roach, S., Lundgren, P., Miller, K. J., Gerlach, G., Noreen, A. M. E., and Andreakis, N. (2013). Assessing hidden species diversity in the coral Pocillopora damicornis from Eastern Australia. Coral Reefs 32, 161–172. doi: 10.1007/s00338-012-0959-z
Sexton, J. P., Strauss, S. Y., and Rice, K. J. (2011). Gene flow increases fitness at the warm edge of a species' range. Proc. Natl. Acad. Sci. U.S.A. 108, 11704–11709. doi: 10.1073/pnas.1100404108
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sherman, C. D. H., Ayre, D. J., and Miller, K. J. (2006). Asexual reproduction does not produce clonal populations of the brooding coral Pocillopora damicornis on the Great Barrier Reef, Australia. Coral Reefs 25, 7–18. doi: 10.1007/s00338-005-0053-x
Sier, C. J. S., and Olive, P. J. W. (1994). Reproduction and reproductive variability in the coral Pocillopora verrucosa from the Republic of Maldives. Mar. Biol. 118, 713–722. doi: 10.1007/BF00347520
Silvertown, J. (2008). The evolutionary maintenance of sexual reproduction: evidence from the ecological distribution of asexual reproduction in clonal plants. Int. J. Plant Sci. 169, 157–168. doi: 10.1086/523357
Souter, P. (2010). Hidden genetic diversity in a key model species of coral. Mar. Biol. 157, 875–885. doi: 10.1007/s00227-009-1370-3
Souter, P., Henriksson, O., Olsson, N., and Grahn, M. (2009). Patterns of genetic structuring in the coral Pocillopora damicornis on reefs in East Africa. BMC Ecol. 9:19. doi: 10.1186/1472-6785-9-19
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Starger, C. J., Yeoh, S. S. R., Dai, C.-F., Baker, A. C., and Desalle, R. O. B. (2008). Ten polymorphic STR loci in the cosmopolitan reef coral, Pocillopora damicornis. Mol. Ecol. Resour. 8, 619–621. doi: 10.1111/j.1471-8286.2007.02017.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Stoddart, J. A. (1983). Asexual production of planulae in the coral Pocillopora damicornis. Mar. Biol. 76, 279–284. doi: 10.1007/BF00393029
Stoddart, J. A. (1984). Genetical structure within populations of the coral Pocillopora damicornis. Marine Biology 81, 19–30. doi: 10.1007/BF00397621
Stoddart, J. A., Babcock, R. C., and Heyward, A. J. (1988). Self-fertilization and maternal enzymes in the planulae of the coral Goniastrea favulus. Mar. Biol. 99, 489–494. doi: 10.1007/BF00392556
Thompson, J. D., Higgins, D. G., and Gibson, T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680. doi: 10.1093/nar/22.22.4673
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Thornhill, D. J., Lewis, A. M., Wham, D. C., and Lajeunesse, T. C. (2014). Host-specialist lineages dominate the adaptive radiation of reef coral endosymbionts. Evolution 68, 352–367. doi: 10.1111/evo.12270
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Toth, L. T., Aronson, R. B., Vollmer, S. V., Hobbs, J. W., Urrego, D. H., Cheng, H., et al. (2012). ENSO drove 2500-year collapse of Eastern Pacific coral reefs. Science 337, 81–84. doi: 10.1126/science.1221168
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Van Oppen, M. J. H., Souter, P., Howells, E. J., Heyward, A., and Berkelmans, R. (2011). Novel genetic diversity through somatic mutations: fuel for adaptation of reef corals? Diversity 3, 405–423. doi: 10.3390/d3030405
Vucetich, J. A., and Waite, T. A. (2003). Spatial patterns of demography and genetic processes across the species' range: null hypotheses for landscape conservation genetics. Conserv. Genet. 4, 639–645. doi: 10.1023/A:1025671831349
Waits, L. P., Luikart, G., and Taberlet, P. (2001). Estimating the probability of identity among genotypes in natural populations: cautions and guidelines. Mol. Ecol. 10, 249–256. doi: 10.1046/j.1365-294X.2001.01185.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ward, S. (1992). Evidence for broadcast spawning as well as brooding in the scleractinian coral Pocillopora damicornis. Mar. Biol. 112, 641–646. doi: 10.1007/BF00346182
Whitaker, K. (2006). Genetic evidence for mixed modes of reproduction in the coral Pocillopora damicornis and its effect on population structure. Mar. Ecol. Prog. Ser. 306, 115–124. doi: 10.3354/meps306115
Williams, D. E., and Miller, M. W. (2005). Coral disease outbreak: pattern, prevalence and transmission in Acropora cervicornis. Mar. Ecol. Prog. Ser. 301, 119–128. doi: 10.3354/meps301119
Keywords: coral, asexual reproduction, clones, ENSO, El Niño-Southern Oscillation, Symbiodinium, Galápagos Islands, fragmentation
Citation: Baums IB, Devlin-Durante M, Laing BAA, Feingold J, Smith T, Bruckner A and Monteiro J (2014) Marginal coral populations: the densest known aggregation of Pocillopora in the Galápagos Archipelago is of asexual origin. Front. Mar. Sci. 1:59. doi: 10.3389/fmars.2014.00059
Received: 16 August 2014; Accepted: 26 October 2014;
Published online: 12 November 2014.
Edited by:
Sandie M. Degnan, The University of Queensland, AustraliaReviewed by:
Mikhail V. Matz, The University of Texas at Austin, USAShane Lavery, University of Auckland, New Zealand
Copyright © 2014 Baums, Devlin-Durante, Laing, Feingold, Smith, Bruckner and Monteiro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Iliana B. Baums, Biology Department, Pennsylvania State University, 208 Mueller Lab, University Park, PA 16802, USA e-mail:YmF1bXNAcHN1LmVkdQ==
 Iliana B. Baums
Iliana B. Baums Meghann Devlin-Durante1
Meghann Devlin-Durante1 Joshua Feingold
Joshua Feingold