Abstract
The Indus River dolphin (IRD; Platanista gangetica minor) is an endangered and blind freshwater cetacean, endemic to the Indus River system of Pakistan and India. This review article provides detailed information about the major challenges IRDs are facing, and their possible consequences on the population dynamics of the IRD. Furthermore, we have suggested future conservation strategies for the IRD based on the lesson learned from the conservation of the Yangtze finless porpoise (YFP; Neophocaena asiaeorientalis), a Critically Endangered freshwater cetacean. The major challenges for IRDs are habitat degradation, habitat fragmentation, and several types of industrial and agricultural pollutants. Worsening climatic changes, illegal fishing, and overfishing are additional threats. The construction of several barrages has fragmented the population into several short segments, some of which are too small for the IRDs to survive. In some segments, the population status of the IRD is unknown. In the remaining populations, genetic inbreeding, water shortage, canal entrapment, and altered ecological environment are potent negative factors for the survival of the IRD. Conservation strategies including fishing bans, translocation, and future research (tagging, periodic health assessments, necropsy and virtopsy, understanding the reproductive biology, and genomics) are possible recommendations. Very serious conservation efforts are needed to save the IRD from decline keeping in view the water shortage, pollution, lack of health assessment studies, and habitat degradation and fragmentation.
Introduction
The primary drivers of the loss of biodiversity worldwide are habitat loss and degradation (Sala et al., 2000; Newbold et al., 2014; Arroyo-Rodríguez et al., 2017; Fink et al., 2017; Nabi et al., 2017c). In fact, there are only a few places on earth that remain free of human influence (Kareiva et al., 2007). It is believed that biodiversity is more at-risk in freshwater systems than in other systems (Allan and Flecker, 1993; Master et al., 1998; Ricciardi and Rasmussen, 1999; Aloo, 2003; Schelle, 2010).
The Indus River dolphin (IRD; Platanista gangetica minor) is a freshwater cetacean, endemic to the Indus River of Pakistan and India (Braulik et al., 2015a). Due to highly fragmented populations and an 80% reduction in its distributional range, IRDs are listed in the International Union for Conservation of Nature (IUCN) Red List as an endangered species (Braulik et al., 2015a). In 2006, the meta-population estimates of IRDs were in the range of 1,550–1,750 (Braulik et al., 2012a). In 2011, the population declined to 1,452 (Noureen, 2013), but surprisingly increased to approximately, 1,816 in 2017 (WWF, 2017). Although, a recent survey showed an increase in the IRD population (WWF, 2017). However, still, they are exposed to several overwhelming potential threats including worsening water pollution, stranding in irrigation canals, dams and barrages construction, accidental capture in fishing nets, loss of genetic diversity, drying rivers and lakes, altered Indus River ecological conditions, conflict with India about the Indus River discharge, climatic changes, and illegal and overfishing (Braulik et al., 2012a,b; Mirza and Mirza, 2014; Nabi et al., 2019a). Although, being a top predator, information about the IRD basic biology is limited (Braulik et al., 2015b). Furthermore, there are few comprehensive review articles that have highlighted several major challenges that the IRDs are facing (Waqas et al., 2012; Braulik et al., 2015b). However, based on the lesson learned from the conservation practices of the critically endangered freshwater cetacean, the Yangtze finless porpoise (YFP; Neophocaena asiaeorientalis), we have listed several threats, their consequences, and strategies for the conservation of IRD population (Wang, 2009, 2015). Both the IRDs and the YFPs are exposed to various anthropogenic stressors (Wang, 2009; Afzal et al., 2012; Braulik et al., 2014, 2015b). Similarly, dolphins and porpoises are warm-blooded, air-breathing, give birth, nurse their young with milk, consume some of the same common foods, and use echolocation for navigation, communication, escape from predators and finding food (Berta, 2015). Both belong to the same order “Cetacea” however, porpoises belong to family “Phocoenidae” and dolphins to family “Delphinidae” and have some morphological and anatomical differences (Berta, 2015). The average declining rate of YFPs was first estimated 6.06% per year (Mei et al., 2012). However, later, the average declining rate per annum was increased and estimated doubled (13.73%) to the previous study (Mei et al., 2014). To conserve the YFPs, scientists at the Institute of Hydrobiology (IHB), University of the Chinese Academy of Sciences, Wuhan, China has used several conservation practices and have fruitful results (Wang et al., 2005; Wang, 2015). Therefore, based on the lesson learned from the conservation practices of YFPs, the aims of the present article were to highlight the key threats that the IRDs are experiencing and suggest strategies and research direction for the IRD conservation.
Materials and Methods
We reviewed published peer-reviewed books, journal articles, theses, as well as magazines, and newspapers in English and Chinese related to the IRD and YFP through the Thomson Reuters “Web of Science” and “Google Scholar” databases. We refined our review article by using the following keywords in different combinations: threats, anthropogenic activities, habitat quality, pollution, population status, conservation practices, conservation physiology, reproductive biology, and molecular biology. The review article is organized as follows: (1) background information of the IRD and YFP (2) threats and challenges to the IRD and their consequences, and (3) future conservation strategies and research direction for the IRD.
Major Challenges
The IRDs face a lot of threats and challenges. These challenges are discussed individually below and summarized in Figure 1.
FIGURE 1
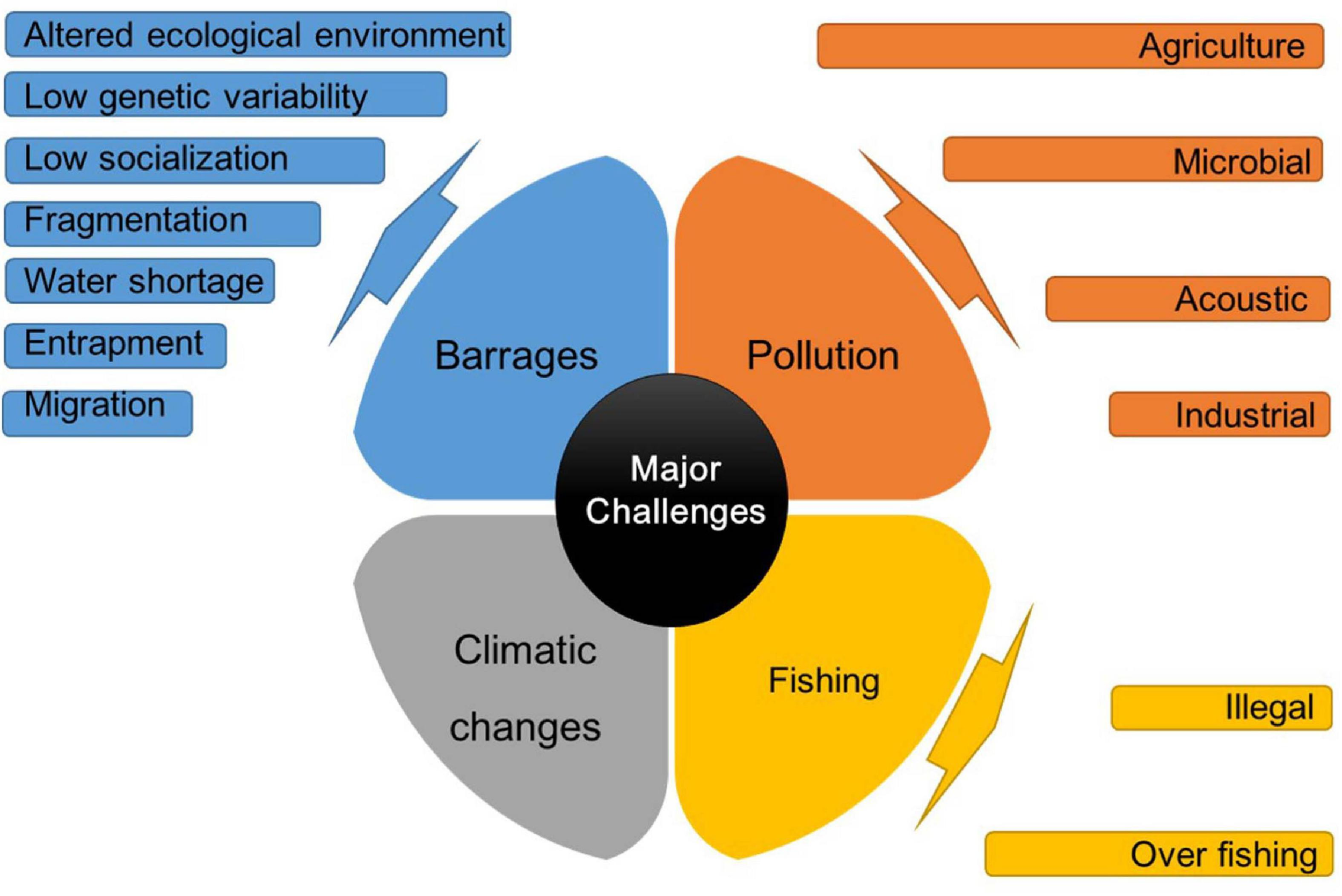
Summary of the major challenges to Indus River Dolphins.
Barrages
The Indus basin irrigation is the largest irrigation system in the world, consisting of 19 irrigation barrages and a series of gates (Braulik et al., 2015b). The purpose of the system is not to store water, but to divert water into lateral canals (Braulik et al., 2015b). These barrages have fragmented the dolphin population into 17 small sections. In some sections, their status is currently unknown (Braulik et al., 2014). In the Indus River, historically, dolphins were found throughout in the approximately, 3,400 km main channel and tributaries (Braulik, 2006). To date, they are only found in approximately, 1,000 km stretch of the downstream reach of the main channel, with 99% of the population confined to a 690 km linear segment (Braulik, 2006). The IRD is now divided into five subpopulations which are bounded by the Kotri, Sukkur, Guddu, Panjnad, Taunsa, Chashma, and Jinnah barrages while one population is found in Beas River India (Figure 2; Braulik et al., 2015b). In fact, approximately, 70% of the dolphin population is found in a short 190 km section of the Indus River between the Sukkur and Guddu barrages (Braulik et al., 2015b). These barrages isolate different dolphin populations which are detrimental in several ways such as:
FIGURE 2
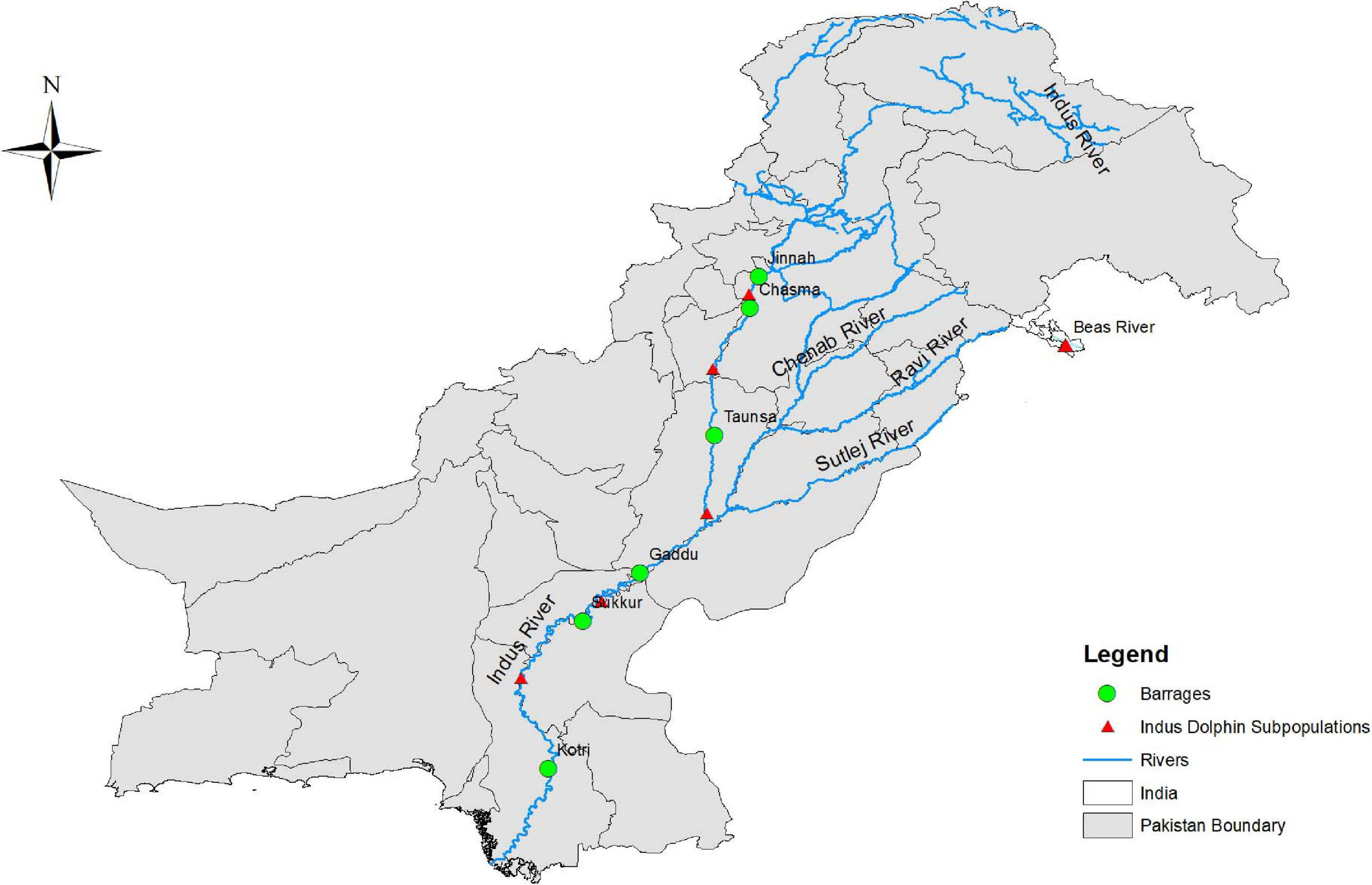
The Indus River Dolphin subpopulations.
Habitat Fragmentation
The construction of dams and barrages can cause habitat isolation, habitat loss, habitat fragmentation, and an alteration in the downstream flow (Wu et al., 2004). These detrimental effects can ultimately lead to species decline and extinction (Wang, 2009). Approximately, 54% of the world’s freshwater megafaunal species are already listed as threatened (vulnerable, endangered or critically endangered) (IUCN, 2016). Various animal species such as isolated subpopulations of the Ganges River Dolphin (Platanista gangetica gangetica;Reeves et al., 2000), IRD (Paudel et al., 2015), Gharial (Gavialis gangeticus;Choudhury et al., 2007), Chinese sturgeon (Acipenser sinensis;Wu et al., 2015), Russian sturgeon (Acipenser gueldenstaedtii;Gesner et al., 2010), YFP (Mei et al., 2014), Chinese paddlefish (Psephurus gladius;Zhang et al., 2016), Yangtze sturgeon (Acipenser dabryanus;Wu et al., 2014), Adriatic sturgeon (Acipenser naccarii;Bronzi et al., 2013), and Atlantic sturgeon (Acipenser sturio;Williot et al., 2009) have either become extinct or have experienced critical population reductions as a result of the barrier effects of dams and barrages. In 2007, the Chinese river dolphin (Lipotes vexillifer) was reported to be extinct partly due to the construction of barriers and pollution (Turvey et al., 2007). This is the first extinction of a cetacean caused by anthropogenic factors (Turvey et al., 2007). In the Yangtze River, approximately, 40 fish species will be detrimentally affected by dams and other barriers (Fu et al., 2003). As the IRD population is highly fragmented, it is therefore, exposed to various detrimental effects like other freshwater cetaceans (Wang, 2009; Braulik et al., 2015b).
Loss of Genetic Variability
The species reproductive biology is also affected by habitat fragmentation (Yates and Ladd, 2005). The most immediate effect of habitat fragmentation/isolation is inbreeding in a population (Barrett and Kohn, 1991). The inbreeding further reduces the frequency of heterozygotes and increases the frequency of recessive, deleterious traits (Charlesworth and Charlesworth, 1987). Inbreeding often results from a population bottleneck (Charlesworth and Charlesworth, 1987; Barrett and Kohn, 1991). As a result, the risk of population extinction increases. Therefore, for long-term population survival, a continuous gene flow is crucial through functional connectivity between habitat patches (Melissa et al., 2003). It has also been known that species with low reproductive rates require more habitat area, and thus are more prone to extinction, than species with high reproductive rates (Melissa et al., 2003). Unfortunately, the majority of IRDs are confined to a small 190 km section of the Indus River (Braulik et al., 2015b). In past, the population has been declined (Braulik, 2006; Noureen, 2013), which could in part be due to lower reproductive output. Furthermore, the lack of knowledge of reproductive biology and the minimum habitat requirement for the IRD further complicate the conservation efforts. With only two dolphins living between Jinnah–Chashma Barrages and 18 dolphins living between Sukkur–Kotri Barrages (Braulik, 2006), loss of genetic diversity due to inbreeding can occur as observed in the populations of YFPs (Chen et al., 2014).
Block Migration
Dams and other barriers disrupt the river’s continuity and retard the free movement of aquatic organisms to their breeding as well as feeding grounds (Lucas and Baras, 2001). Curtailing the long-term upstream and downstream movements of the river can negatively affect the long-term population viability by reducing the effective breeding of the population which can affect population abundance (Morita et al., 2009). In the Indus River, within the barrages gate, turbulence and water velocity is very high, which allows only downstream rather than upstream movements. This leads to downstream migratory attrition of upstream subpopulations (Reeves, 1991; Reeves et al., 1991). Based on a single individual with radio-tagged, dolphin either moved up and down or trapped downstream (Toosy et al., 2009; Braulik et al., 2012a). However, the design and operation of each barrage is different (Braulik et al., 2015b) and there is no detail study to prove a regular upstream and downstream movement across the barrages. If the movement of individuals is primarily in one direction, this would be another important factor to consider in the IRD population dynamics.
Alteration in Ecological Environment
Barriers cause an alteration in water velocity, discharge, siltation and suspended solids, riverbed movement, dissolved oxygen, temperature (Crisp, 1993), stream habitat, and biogeochemistry (Larinier, 2001). These factors further compromise different biotic compositions, de-synchronize aquatic species life history categories, and break longitudinal and lateral hydraulic connectivity (Bunn and Arthington, 2002). Additionally, the decline in water flow during a dry season can affect the dolphins by increasing water temperatures and reduce the average water depths, the water velocity, and the physical space available to them (Nilsson et al., 2005; Xenopoulos and Lodge, 2006). Indirectly, flow regulation could affect dolphins by increasing the success of invasive species, the dominance of generalist fish species, and the decline in fish diversity (Nilsson et al., 2005; Xenopoulos and Lodge, 2006). As the Indus River runs through semi-desert land, the river is naturally highly turbid, broad, shallow and braided, sand-bedded, and is constantly eroding its bed and banks (Braulik et al., 2015b). Furthermore, little vegetation and an extreme rise in temperature (Braulik et al., 2015b) can create a worse scenario for the IRD survival. Therefore, based on the historical pattern of decline, IRDs are most likely to disappear in the future from locations with a low river discharge (Braulik et al., 2014).
Low Socialization/Group Cohesion
Cetaceans are social animals (Peter and Raga, 2001). Social living has many advantages such as communal care, especially of calves, defense from predators, co-operative feeding, the formation of breeding coalitions and a variety of forms of social organization, and adapted to different environmental conditions and lifestyles (Peter and Raga, 2001). This social cohesion is not only crucial for an individual’s own survival, but also for the survival of the entire group. By remaining connected, the knowledge collectively held by the group, especially by more mature members, can be used to enhance survival (Whitehead and Rendell, 2015).
Entrapment
Occasionally, IRDs close to the irrigation barrages enter irrigation canals and remain either close to the river or travel hundreds of kilometers (Bhaagat, 2002; Khan, 2005). Each year, all canals are drained for over several week periods (Waqas et al., 2012), which can cause mortality and morbidity for entrapped stressed dolphins (Waqas et al., 2012). A total of 34 recorded entrapped dolphins died between 1992 and 2014 (Waqas et al., 2012). Furthermore, the lack of monitoring the number of dolphins entering the canals and how and when they traverse the gates (Braulik et al., 2015b) further complicate their survival.
Water Shortage
The Indus Basin Irrigation System (IBIS) has reduced the annual downstream water flow from greater than 150 billion m3 to less than 45 billion m3 (Inam et al., 2007) due to its extensive use for agriculture (95%), industry, sanitation, and drinking (Kamal, 2008). Especially during the dry season, the IBIS severely affects the water flow to such a level that the river virtually ceases to flow (Dudgeon, 2005), causing habitat degradation, reducing physical space, and a decline in the range of the IRD (Braulik et al., 2014, 2015b). Therefore, to increase the chances of foraging opportunities and decrease the risk of being isolated in a small pool, the dolphins will move and congregate in a relatively deep area (Braulik et al., 2012b). However, the congregation can increase the chances of negative interactions with humans (Braulik et al., 2012b). Furthermore, with an expanding agriculture and economic sector, there is an increased demand for more cultivable land. A conflict with India about the Indus River discharge and the newly planned and under constructed hydropower stations, river linking projects, barrages, and dams are additional potent threats to IRDs (Asian Development Bank, 2010).
Pollutions
Industrial Pollution
Due to rapid industrialization and urbanization, water pollution has significantly increased in Pakistan (Qadir et al., 2008). More than 90% of the municipal and industrial effluent from paper factories, sugar mills, tanneries, wood mills, jute mills, textile mills, and distilleries enter the lakes and rivers, causing chemical pollution (World Bank, 2005; Directorate of Land Reclamation Punjab, 2007). Streams of sewage just upstream of the Sukkur Barrage and the dumping of the waste into the Indus River (Gachal and Slater, 2003) further amplify the water pollution problem. Various industrial pollutants such as cadmium, lead, mercury, copper, and arsenic were recorded in higher concentrations in the Indus River as well as in the tissue samples of fish (Gachal et al., 2006a,b; Humerah et al., 2011; Afzal et al., 2012). Since fish tend to accumulate heavy metals in their body (Jia et al., 2017). Dolphins are at the top of the food chain and can, therefore, accumulate high metal loads from their prey (Aguilar et al., 1999). The large quantity of prey consumed, and the long lifespan of dolphins further enhances their capacity to accumulate metals (Aguilar et al., 1999; Bowles, 1999). Like other cetaceans, several toxic pollutants have been reported in the various tissues of YFPs (Xiong et al., 2019). Though not specifically studied in detailed in the IRD, the bioaccumulation of metals could cause higher mortality, morbidity as well as could negatively compromise reproduction, putting the population health and growth at-risk (Aguilar et al., 1999; Murphy et al., 2015). Chemical pollutants can affect the cetacean immune system in several ways including alterations in the immunoglobulin production, cytokine gene expression, hematology/circulating immune cell populations, and cytotoxicity (Desforges et al., 2016; Nabi et al., 2020b). Chronic exposure to immunotoxic pollutants may have population-level consequences and can cause infectious disease outbreaks which typically occur near heavily urbanized and polluted areas (Duignan et al., 2014).
Agricultural Pollution
The plain areas of Pakistan are intensively cultivated with rice, wheat, sugarcane, and cotton (World Bank, 2005; Ali et al., 2016). To combat pests, about 25,000 tons of pesticides and chemical compounds are used with an annual 6% increase (Memon, 2004; World Bank, 2005; Humerah et al., 2011). Commonly used pesticides include Endosulfan, Cypermethrin, Chlordanes, Heptachlor, Hexachlorobenzene, Hexachlorocyclohexane, DDT, and several others (Sultana et al., 2014; Ali et al., 2016). These pesticides are sprayed on crops using irrigation water. The water containing the chemicals leaches through the soil and enters into various water sources (Ali et al., 2016). Some of these chemicals have also been found in the tissue of three dead IRDs (WWF-Pakistan, 2011). Analysis of water samples indicates heavy organic pollutants and tissue samples of different fish species indicate a heavy fertilizer load (Gachal et al., 2006a,b). Some fishermen use poisonous pesticides in the Indus River to maximize their fish catch (Waqas et al., 2012). All these pesticides both directly and indirectly, through prey consumption, affect the IRD. Similarly, YFPs exposed to heavy pesticides load showed elevated levels of liver enzymes in the Tian-E-Zhou Oxbow (Nabi et al., 2017a). Furthermore, the slow degradation of pesticides and accumulation in different biotic and abiotic matrices (Yang et al., 2013) can increase the pesticide load, and possibly can lead to carcinogenesis, immunological disorders, reproductive disorders, and finally death of the animals (Sanpera et al., 2003).
Microbial Pollution
Analysis of the Indus River water showed a heavy load of total coliform and thermo-tolerant coliforms bacterial pathogens (Humerah et al., 2011). These bacterial pathogens are not limited to fecal sources but also are commonly found in sugar beet processing wastewater, cotton mill effluents, textile processing-plant effluents, and pulp and paper mill effluents (Dufour, 1976) which are discharged into the Indus River. However, a detailed study of the micro biota of the Indus River is lacking. There is a great possibility that there are a number of bacterial, fungal, and viral pathogens as well as parasites in the Indus River which pose a serious health risk to the IRD.
Acoustic Pollution
The Indus River is not immensely used for traffic because of several barrages, but still, vessels are present in the form of motorized or oar-powered ferries and fishing boats (Braulik et al., 2015b). Dolphins use sound for prey localization, escape, navigation, and communication, because their vision is limited underwater (Nabi et al., 2018c). Aquatic animals are also exposed to natural noises such as wave noise and rainfall and lightning strikes on water, but over the evolutionary time, they genetically adapted (Rabin and Greene, 2002). However, noises from anthropogenic sources are recent additions which pose a serious survival risk to animals (Rabin and Greene, 2002; Mei et al., 2021). Acoustic pollution can cause death (Claridge, 2006), and a variety of health issues such as physiological stress, behavioral alteration, cardiovascular collapse and hemorrhages in the subarachnoid, cochlear duct, lungs, kidney, and intracranial areas (Balcomb and Claridge, 2001; Freitas, 2004; Cox et al., 2006; Miller et al., 2008; Nabi et al., 2018a). Noise can affect reproduction in all marine animals regardless of their age (Wright et al., 2007) by directly suppressing the hypothalamic-pituitary-gonadal (HPG) axis (Rengarajan and Balasubramanian, 2008) or through hypothalamic-pituitary-adrenal (HPA) axis activation (Rolland et al., 2012). The indirect effect of noise is hearing loss (Cox et al., 2006).
Dolphin Hunting and Fishing
Dolphin Hunting
For a long time, IRDs were hunted by different indigenous groups (McNair, 1908) using well-equipped boats for their capture (Pilleri, 1972). The meat was used for food by the highly marginalized communities. The oil was used as fuel in lamps and for medical purposes (Braulik et al., 2015b). The hunters then moved to the Punjab province of Pakistan after the enforcement of a ban on dolphin hunting in Sindh province (Reeves et al., 1991). Currently, there is no evidence that IRD hunting has continued anywhere in Pakistan since then (Braulik et al., 2015b), however, still, overfishing and using illegal fishing gears are potential threats for the IRD population (WWF-Pakistan, 2011).
Illegal Fishing
The irrigation canals are heavily fished, and, therefore, net entanglement of dolphins is very common (Khan, 1947) which poses risks to the IRD survival. In 2011, within the protected area, six dolphins were killed by insecticides dumped into the river to increase fish catch (WWF-Pakistan, 2011). Furthermore, with the increased use of the Indus River by unskilled fishermen, an increase in dolphin mortality has been observed (Waqas et al., 2012). There is also a possibility of using others harmful, illegal, and non-selective methods for fishing such as gill nets, rolling hooks, and electrofishing which was one of the factors in the extinction of the Lipotes vexillifer (Turvey et al., 2007).
Overfishing
More than 180 fish species are found in the Indus River (Mirza and Mirza, 2014). However, they are exposed to various threats such as the westward shifting of the river’s course, global warming, extensive deforestation, illegal hunting of fish, pollution, and most importantly overfishing (Mirza and Mirza, 2014). At the Taunsa Barrage, fish diversity is poor as compared to other rivers in Asia such as the Yangtze River, the Yellow River, the Mekong River, the Salween River, the Brahmaputra River, and the Ganges River (Muhammad et al., 2016). Much stress has been placed on commercial fish species due to overfishing (Muhammad et al., 2016). Commercial exploitation of fishes, poverty, and illegal fishing not only leads to a reduction in prey for the IRD, but also a scarcity of food (WWF-Pakistan, 2011; Waqas et al., 2012). Poor nutrition in cetaceans can affect immunity and reproduction (Beineke et al., 2010; Murphy et al., 2015). Furthermore, maternal body condition can alter the sex ratio of the offspring (Wiley and Clapham, 1993). if the uneven birth sex ratios in an endangered population persist for a long time, it can hamper the conservation efforts (Wiley and Clapham, 1993; Saragusty et al., 2009).
Climatic Changes
Climate changes can cause a dramatic alteration in ecosystems and can negatively impact freshwater cetaceans (Smith et al., 2010). Alteration in the thickness of glaciers (Hewitt, 2007), seasonal snow melting, rainfall (Archer et al., 2010), an increase in temperature (Hijioka et al., 2014), and the lack of water conservation practices results in the deterioration of the ecosystem where the IRD dwell in. The IRDs, through the passage of time, have evolved the ability to adapt to the rising temperature (Braulik et al., 2015b). However, an increasing global temperature and other climatic changes which affect biodiversity, and the ecosystem can negatively affect cetaceans (MacLeod, 2009). Although cetaceans in response to adverse climatic changes can change their geographical range (MacLeod, 2009), but unfortunately, the IRDs are restricted to a 190 km stretch of the Indus River (Braulik et al., 2015b). Therefore, exposure to adverse climatic conditions can potentially have life-threatening consequences.
Future Conservation Strategies
After the extinction of the Yangtze River dolphin in 2006, YFPs are the only fresh-water cetacean, endemic in the Yangtze River, Poyang, and Dongting Lakes of China (Turvey et al., 2007; Mei et al., 2014). Like IRDs, YFPs are also exposed to a variety of anthropogenic factors, and declining continuously (IUCN, 1996; Wang et al., 1998, 2000; Wang, 2009). Due to a tremendous decline in population, YFPs are further reclassified as critically endangered cetacean (Wang et al., 2013). Various conservative approaches are used for YFPs conservation, some of which have fruitful results. For example, the first freshwater cetacean birth in captivity and the higher fecundity and a net increase of 108% in the population of Tian-E-Zhou Oxbow (Wang et al., 2005; Wang, 2015). Based on our experience with the YFPs conservation, we have suggested the following strategies for the IRD conservation, summarized in Figure 3.
FIGURE 3
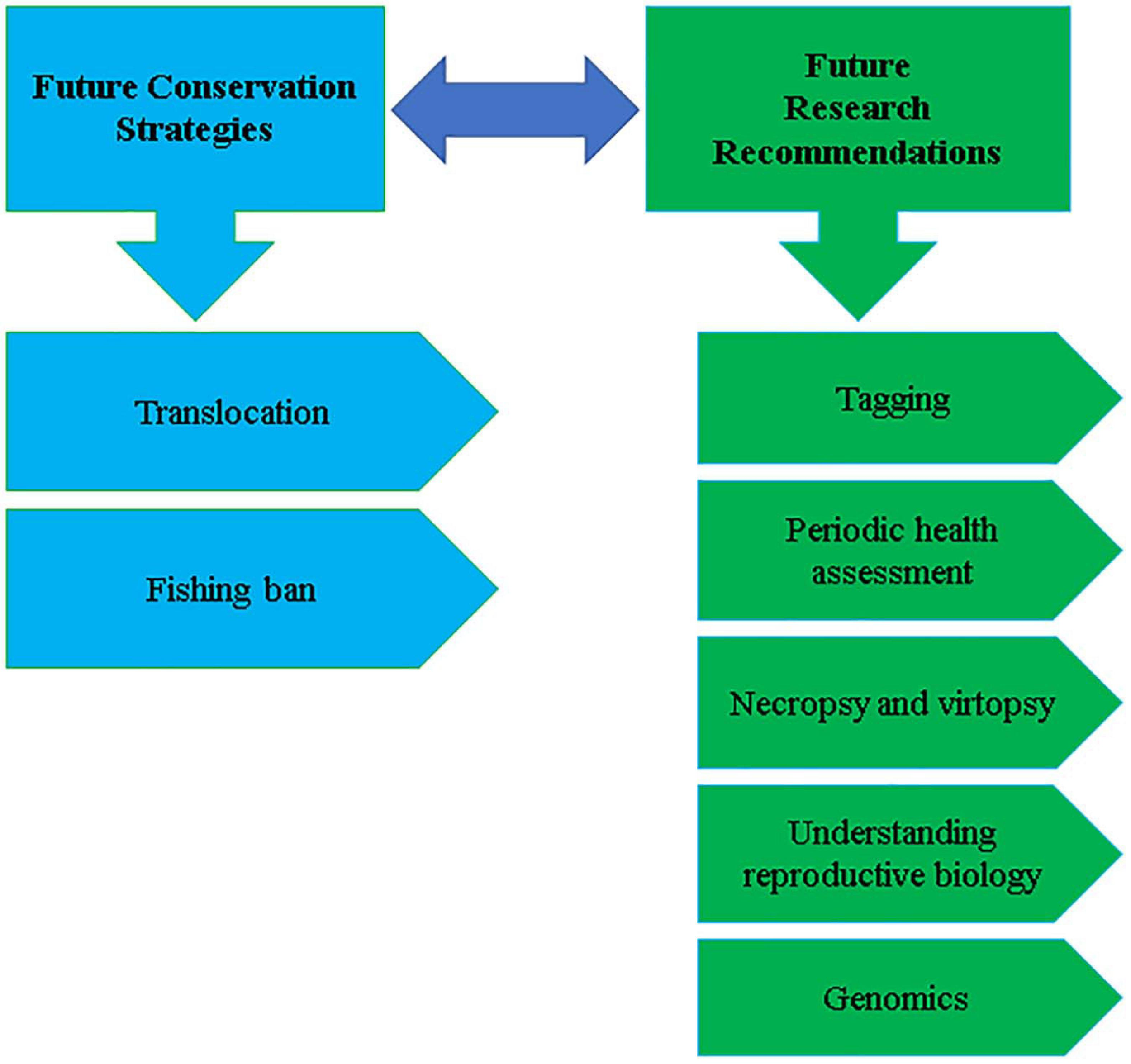
Summary of the strategies for future conservation of Indus River Dolphins.
Translocation
There are various goals which can be achieved by using a translocation program such as speeding the recovery of a species after habitat restoration, establishing satellite populations, and bolstering genetic heterogeneity (Griffith et al., 1989). The subpopulation of the IRD between Sukkur and Guddu is relatively large (n = 1289) (Braulik et al., 2012a). This is compared to the few dolphins found at Jinnah-Chashma, the 96 animals living at Chashma-Taunsa (Noureen, 2013), and the 10 animals living in the Beas River (Behera et al., 2008). Furthermore, these animals, due to downstream migration are declining continuously and are concentrating between the Sukkur and Guddu barrage (Braulik et al., 2015b). To prevent the extirpation of these small subpopulations, translocation of animals, especially those trapped in irrigation canals, may provide a better solution. The translocation of the IRDs from an area of low population to an area of high population through well-trained staff could help the conservation of specie in several ways. For the conservation of YFPs, porpoises have been translocated either to new habitats or other reserves to boost the genetic diversity of existing population, and ultimately, save them from extinction (WWF, 2015).
Fishing Ban
The conversion of a fishing system in Sindh province from contract to license has resulted in an exploitation of fish resources and an abrupt increase in the use of illegal fishing practices, such as fishing without a legal permit, pesticide use, and overnight netting (WWF-Pakistan, 2011; Waqas et al., 2012). Furthermore, many local and native peoples depend on the Indus River as a source of subsistence. All these activities have adversely affected the fish resources of the Indus River (Waqas et al., 2012). As a result, the IRDs travel into water channels and lakes due to high fish availability. Unfortunately, in these environments, they are exposed to many negative anthropogenic activities (Waqas et al., 2012). There is an intense need to enforce ban on the illegal fishing and using harmful fishing gears in the Indus River and its adjoining lakes. The Pakistani government needs to provide jobs or facilities for fish farming, cattle farming, agriculture, and other alternative facilities for the local people who depend on the Indus River. Furthermore, there is also a need to investigate fisheries resources in the deteriorating habitats of the Indus River. To save the YFPs and other biodiversity in the Yangtze River from extinction, Chinese government first initiated an annual 3 months fishing ban in 2002, then 4 months in 2016, and in January 2020, China instituted a 10-year ban on fishing in the Yangtze River and its adjoining lakes (Mei et al., 2020). This new ban is estimated to affect more than 110,000 fishing boats and approximately, 280,000 fishermen in ten provincial regions along the Yangtze River. However, the government has promised to provide vocational training, financial support, and social security services for fishermen who have to find new ways of living (Xinhua, 2020).
Future Research Recommendations
Tagging
Tagging is an essential research tool that permits tracking species survival, growth, migration, and reproduction (Nielsen, 1992). Instead of group tagging, nowadays individual tagging in the form of passive integrated transponder (PIT) is effectively used in different species, (Baras et al., 2000) including YFPs. The PIT tag has not only a vast number of individual code combinations but also operates for an unlimited period of time (Baras et al., 2000). The use of PIT tagging in IRDs will help in identifying each individual. In addition, PIT tagging can be used to monitor canal entrapment, survival, growth, disease, behavior and downstream migration of the dolphins.
Periodic Health Assessment
For the assessment of animal health, several different assessments such as physiological, disease, blood, and biochemical are very crucial for making management and treatment decisions for the animals (Moore et al., 2007). Furthermore, physiological indices such as fats reserves, body growth, hematological, and biochemical parameters can be used to rank habitat quality indirectly (Nabi et al., 2017a). Several studies regarding different marine mammals including YFPs have been performed to investigate population health for effective management and conservation and have focused research on understanding the implications of stressors such as infectious diseases, marine toxins, and pollutants on the health of marine mammal populations (Wells et al., 2004; Fair et al., 2006; Goldstein et al., 2006; Nabi et al., 2017b,c, 2018b, 2019b, 2020a, 2021). In the light of the above studies, an annual periodic health assessment is needed for the IRDs in order to understand their growth rate, fertility, and the diseases which cause morbidity and mortality.
Necropsy and Virtopsy
Most of the literature suggests that the worsening ecological environment is the causative factor for the IRDs decline (Braulik, 2006; Noureen, 2013; Braulik et al., 2015b). However, it is unknown how these various environmental factors can cause morbidity or mortality. Therefore, it is very crucial from a conservation point of view to identify the exact cause of illness or death using necropsy, virtopsy, pathology, histopathology, microbiology, and biochemistry techniques. All this information will guide the conservationist in designing conservation strategies.
Understanding Reproductive Biology of the IRDs
Escalating anthropogenic activities are the major drivers for animal extinction, and therefore, to conserve and manage wild species, basic and applied knowledge about their reproductive biology is essential (Comizzoli and Holt, 2019). Unfortunately, for most of the wild endangered species including the IRDs, very little is known about their reproductive biology (Frankham, 2008; Comizzoli and Holt, 2019). Unlike YFPs, IRDs do not have a specific breeding season and can breed at any time of the year (Dolphins-World, 2017). However, the low reproductive rate of the IRD warrants further investigations (Khan, 2016). Although cetacean chasing, catching, and handling is difficult and there are several logistics, ethical, and physiological issues (Nabi et al., 2018a). However, the IRDs in the irrigation canals and during the rescue operations could provide an opportunity to study them for a while (for example mother and calf interaction, lactation, parental care, and sexual behavior). These opportunities could also help us to record the sex ratio and anthropometric measurements. Blood, milk, and blowhole samples collected can be used to assay various reproductive parameters. Furthermore, studying the fresh carcasses could also provide a unique opportunity to understand the basic reproductive biology of the IRDs (Xiao et al., 2018; Ji et al., 2019). In 2005, the first YFP was born in captivity at the Baiji Dolphinarium, located in Wuhan, China (Wang et al., 2005). The long-term objective of this natural captive breeding program is to establish a colony of animals in which some can be released into the wild (Wang et al., 2005). The birth in captivity of this first ever freshwater cetacean not only indicates the success of the natural breeding program, but it also provides hope for other freshwater cetaceans which could potentially be bred naturally in captivity. Furthermore, reproductive biology of the YFP is studied both in the captive and free-ranging populations (Zeng et al., 2017, 2018, 2019; Hao et al., 2019). The IRD, unlike, YFP is not critically endangered and due to several other reasons, it is very early to start a captive breeding program like started for several other animal species (Suresh et al., 2010; Sandler, 2012). However, understanding the basic reproductive biology will help the natural or artificial breeding programs whenever it is applicable. Furthermore, attention from national and international organizations for funds and collaboration with international institutions for technical aid is needed to understand the reproductive biology of IRD.
Genomics
Advancement in molecular technologies including genetic engineering and advanced genomics can help conservation researchers and provide important scientific information to policy makers and managers (Supple and Shapiro, 2018). These techniques can help in identification and enhance the expression of the genes responsible for adaptation and increase our understanding about micro-evolution. Tools can be developed which are essential for the advanced monitoring of endangered biodiversity (Khan et al., 2016). Genomics studies provide information about speciation time, recombination rates, origin, relationships, estimation of the current and the ancestral effective population size (Wilding et al., 2001; Locke et al., 2011), the genetic architecture of inbreeding depression, and genetic mechanisms including epistasis, over-dominance, and gene-environment interactions (Steiner et al., 2013). Genome-wide association studies (GWAS) (Charlier et al., 2008), gene expression profiles (Paige, 2010), and genomic sequencing (Charlesworth and Willis, 2009) are broadly used to identify inbreeding depression related loci. Advanced genomics can more effectively detect relevant susceptible genes and can provide a better comprehension into protective and pathogenic mechanisms and can determine new molecular targets for therapeutic and prophylactic interventions (Hill, 1999).
Conclusion
The conservation status of the IRD is not as serious as the status of the YFPs. However, still, it is one of the world most endangered mammals. The Indus River is highly polluted and fragmented by several barrages. In addition, fisheries resources are exploited, and climatic changes have had a negative impact. Furthermore, the shortage of water and the decreased level of the Indus River due to agriculture, installation of new hydropower projects, and a conflict with India have complicated survival of the IRD in the long term. Despite such major challenges, the current conservation progress and practices are not enough, and it seems that its conservation status will be drastically compromised in the future. We have suggested translocation and fishing ban practices for the conservation of IRD. These practices have significantly contributed to the conservation of YFPs. Furthermore, we have suggested several future research directions for IRDs conservation including genomics, tagging, necropsy and virtopsy, periodic health assessment, and to understand basic reproductive biology of the IRD. These research studies have played an important role in the conservation of YFPs.
Statements
Author contributions
GN conceived and drafted the manuscript. ShA, RM, YH, NA, and MK critically reviewed and edited the manuscript. DL, SK, SaA, and WU polished the manuscript. DL funded the study. All authors contributed to the article and approved the submitted version.
Funding
This study was conducted with the support of the National Natural Science Foundation of China (NSFC, 31971413), the biodiversity investigation, observation, and assessment program (2019–2023) of the Ministry of Ecology and Environment of China, and the Natural Science Foundation of Hebei Province (NSFHB, C2020205038) to DL.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Afzal M. F. Shaukat S. S. Zafar M. U. Abbas Q. (2012). Variation pattern of heavy metal concentrations during pre- and post-monsoon seasons in the surface water of River Indus (Sindh Province).World Appl. Sci. J.19582–587. 10.5829/idosi.wasj.2012.19.04.64233
2
Aguilar A. Borrell A. Pastor T. (1999). Biological factors affecting the variability of persistent pollutant concentrations in cetaceans.J. Cetacean Res. Manag. Spec. Issue183–116. 10.47536/jcrm.v1i1.264
3
Ali U. Bajwa A. Iqbal Chaudhry M. J. Mahmood A. Syed J. H. Li J. et al (2016). Significance of black carbon in the sediment-water partitioning of organochlorine pesticides (OCPs) in the Indus River, Pakistan.Ecotoxicol. Environ. Saf.126177–185. 10.1016/j.ecoenv.2015.12.024
4
Allan J. D. Flecker A. S. (1993). Biodiversity conservation in running waters: identifying the major factors that threaten destruction of riverine species and ecosystems.BioScience4332–43. 10.2307/1312104
5
Aloo P. (2003). Biological diversity of the Yala Swamp lakes, with special emphasis on fish species composition, in relation to changes in the Lake Victoria Basin (Kenya): threats and conservation measures.Biodivers. Conserv.12905–920. 10.1023/A:1022869624524
6
Archer D. R. Forsythe N. Fowler H. J. Shah S. M. (2010). Sustainability of water resources management in the Indus Basin under changing climatic and socio-economic conditions.Hydrol. Earth Syst. Sci.141669–1680. 10.5194/hess-14-1669-2010
7
Arroyo-Rodríguez V. Melo F. P. L. Martinez-Ramos M. Bongers F. Chazdon R. L. Meave J. A. et al (2017). Multiple successional pathways in human-modified tropical landscapes: new insights from forest succession, forest fragmentation and landscape ecology research.Biol. Rev. Camb. Philos. Soc.92326–340. 10.1111/brv.12231
8
Asian Development Bank (2010). Asian Development Outlook 2010: The Future of Growth in Asia.Mandaluyong: Asian Development Bank.
9
Balcomb K. C. Claridge D. E. (2001). Mass whale mortality: US Navy exercises cause strandings.Bahamas. J. Sci.81–12.
10
Baras E. Malbrouck C. Houbart M. Kestemont P. Mélard C. (2000). The effect of PIT tags on growth and physiology of age-0 cultured Eurasian perch Perca fluviatilis of variable size.Aquaculture185159–173. 10.1016/S0044-8486(99)00346-4
11
Barrett S. C. H. Kohn J. R. (1991). “Genetic and evolutionary consequences of small population size in plants: implications for conservation,” in Genetics and Conservation of Rare Plants, edsFalkD. A.HolsingerK. E. (New York, NY: Oxford University Press), 3–30.
12
Behera S. K. Nawab A. Rajkumar B. (2008). Preliminary investigations confirming the occurrence of Indus River dolphin Platanista gangeticaminor in River Beas, Punjab, India.J. Bombay Nat. Hist. Soc.10590–126.
13
Beineke A. Siebert U. Wohlsein P. Baumgärtner W. (2010). Immunology of whales and dolphins.Vet. Immunol. Immunop.13381–94. 10.1016/j.vetimm.2009.06.019
14
Berta A. (2015). Whales, Dolphins and Porpoises: A Natural History and Species Guide.Chicago, IL: University of Chicago press.
15
Bhaagat H. B. (2002). Status, population abundance, strandings and rescues of Indus blind dolphin (Platanista minor) in River Indus (Pakistan).Tiger Paper299–12.
16
Bowles D. (1999). An overview of the concentrations and effects of metals in cetacean species.J. Cetacean Res. Manag. Spec. Issue1125–148. 10.47536/jcrm.v1i1.267
17
Braulik G. T. (2006). Status assessment of the Indus River dolphin, Platanista gangetica minor, March-April 2001.Biol. Conserv.129579–590. 10.1016/j.biocon.2005.11.026
18
Braulik G. T. Arshad M. Noureen U. Northridge S. P. (2014). Habitat fragmentation and species extirpation in freshwater ecosystems; causes of range decline of the Indus River dolphin (Platanista gangetica minor).PLoS One9:e101657. 10.1371/journal.pone.0101657
19
Braulik G. T. Barnett R. Odon V. Islas-Villanueva V. Hoelzel A. R. Graves J. A. (2015a). One species or two? Vicariance, lineage divergence and low mtDNA Diversity in geographically isolated populations of South Asian river dolphin.J. Mamm. Evol.22111–120. 10.1007/s10914-014-9265-6
20
Braulik G. T. Bhatti Z. I. Ehsan T. Hussain B. Khan A. R. Khan A. et al (2012a). Robust abundance estimate for endangered river dolphin subspecies in South Asia.Endanger. Species Res.17201–215. 10.3354/esr00425
21
Braulik G. T. Reichert A. P. Ehsan T. Khan S. Northridge S. P. Alexander J. S. et al (2012b). Habitat use by a freshwater dolphin in the low-water season.Aquat. Conserv. Mar. Freshwat. Ecosyst.22533–546. 10.1002/aqc.2246
22
Braulik G. T. Uzma N. Masood A. Randall R. R. (2015b). Review of status, threats, and conservation management options for the endangered Indus River blind dolphin.Biol. Conserv.19230–41. 10.1016/j.biocon.2015.09.008
23
Bronzi P. Congiu L. Rossi R. Zerunian S. Arlati G. (2013). Acipenser naccarii. The IUCN Red List of Threatened Species 2013: e.T224A13037056. Available online at: http://www.iucnredlist.org/details/224/0(accessed May 4, 2017).
24
Bunn S. E. Arthington A. H. (2002). Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity.Environ. Manage.30492–507. 10.1007/s00267-002-2737-0
25
Charlesworth D. Charlesworth B. (1987). Inbreeding depression and its evolutionary consequences.Annu. Rev. Ecol. Syst.18237–268. 10.1146/annurev.es.18.110187.001321
26
Charlesworth D. Willis J. H. (2009). The genetics of inbreeding depression.Nat. Rev. Genet.10783–796. 10.1038/nrg2664
27
Charlier C. Coppieters W. Rollin F. Desmecht D. Agerholm J. S. Cambisano N. et al (2008). Highly effective SNP-based association mapping and management of recessive defects in livestock.Nat. Genet.40449–454. 10.1038/ng.96
28
Chen M. Zheng J. Wu M. Ruan R. Zhao Q. Wang D. (2014). Genetic diversity and population structure of the critically endangered Yangtze finless porpoise (Neophocaena asiaeorientalis asiaeorientalis) as revealed by mitochondrial and microsatellite DNA.Int. J. Mol. Sci.511307–11323. 10.3390/ijms150711307
29
Choudhury B. C. Singh L. A. K. Rao R. J. Basu D. Sharma R. K. Hussain S. A. et al (2007). Gavialis Gangeticus. The IUCN Red List of Threatened Species 2007: e. T8966A12939997.Gland: IUCN.
30
Claridge D. E. (2006). Fine-Scale Distribution and Habitat Selection of Beaked Whales. Dissertation. Scotland: Department of Zoology, University of Aberdeen.
31
Comizzoli P. Holt W. V. (2019). Breakthroughs and new horizons in reproductive biology of rare and endangered animal species.Biol. Reprod.101514–525. 10.1093/biolre/ioz031
32
Cox T. M. Ragen T. J. Read A. J. Vos E. Baird R. W. Balcomb K. et al (2006). Understanding the impacts of anthropogenic sound on beaked whales.J. Cetacean Res. Manage.7177–187.
33
Crisp D. (1993). The environmental requirements of salmon and trout in fresh water.Freshw. Forum3176–202.
34
Desforges J. P. Sonne C. Levin M. Siebert U. De Guise S. Dietz R. (2016). Immunotoxic effects of environmental pollutants in marine mammals.Environ. Int.86126–139. 10.1016/j.envint.2015.10.007
35
Directorate of Land Reclamation Punjab (2007). Surface Water Quality Monitoring Plan.Lahore: Irrigation and Power Department, Government of Punjab.
36
Dolphins-World (2017). Indus River dolphin (Platanista gangetica minor). Available online at: http://www.dolphins-world.com/indus-river-dolphin/(accessed May 2, 2017).
37
Dudgeon D. (2005). River rehabilitation for conservation of fish biodiversity in monsoonal Asia.Ecol. Soc.10:15. 10.1127/1868-5749/2010/019-0015
38
Dufour A. P. (1976). “Escherichia coli: the fecal coliform, in bacterial indicators/health hazards associated with water,” in ASTM STP 635: American Society for Testing and Materials, edsHoadleyA. A.DutkaB. J. (West Conshohocken: ASTM), 48–58.
39
Duignan P. J. Van Bressem M. F. Baker J. D. Barbieri M. Colegrove K. M. De Guise S. et al (2014). Phocine distemper virus: current knowledge and future directions.Viruses65093–5134. 10.3390/v6125093
40
Fair P. Hulsey T. Varela R. Goldstein J. D. Adams J. Zolman E. S. et al (2006). Hematology, serum chemistry, and cytology findings from apparently healthy Atlantic bottlenose dolphins (Tursiops truncatus) inhabiting the estuarine waters of Charleston, South Carolina.Aquat. Mamm.32182–195. 10.1578/AM.32.2.2006.182
41
Fink S. Lanz T. Stecher R. Scheidegger C. (2017). Colonization potential of an endangered riparian shrub species.Biodivers. Conserv.262099–2214. 10.1007/s10531-017-1347-3
42
Frankham R. (2008). Genetic adaptation to captivity.Mol. Ecol.17325–333. 10.1111/j.1365-294X.2007.03399.x
43
Freitas L. (2004). The stranding of three Cuvier’s beaked whales Ziphius caviostris in Madeira archipelago – May 2000.ECS Newsletter4228–32.
44
Fu C. Wu J. Chen J. Wu Q. Lei G. (2003). Freshwater fish biodiversity in the Yangtze River basin of China: patterns, threats and conservation.Biodivers. Conserv.121649–1685. 10.1023/A:1023697714517
45
Gachal G. S. Slater F. M. (2003). Historical and current status of the Indus River dolphin (Platanista minor) Owen 1853: its conservation and future.Sind. Univ. Res. J.3551–62.
46
Gachal G. S. Slater F. M. Nisa Z. Qadri A. H. (2006a). Ecological effect to the status of the Indus dolphin.Pak. J. Biol. Sci.92117–2121. 10.3923/pjbs.2006.2117.2121
47
Gachal G. S. Slater F. M. Qadri A. H. Nisa Z. Zuhra S. (2006b). Environmental impact to the conservation of the Indus River dolphin (Platanista minor).Pak. J. Biol. Sci.91496–1503. 10.3923/pjbs.2006.1497.1503
48
Gesner J. Freyhof J. Kottelat M. (2010). Acipenser gueldenstaedtii. The IUCN Red List of Threatened Species 2010: e.T232A13042340. Available online at: http://www.iucnredlist.org/details/biblio/232/0(Accessed 4 May 2017).
49
Goldstein J. D. Reese E. Reif J. S. Varela R. A. McCulloch S. D. Defran R. H. et al (2006). Hematologic, biochemical, and cytologic findings from apparently healthy Atlantic bottlenose dolphins (Tursiops truncatus) inhabiting the Indian River Lagoon, Florida, USA.J. Wildl. Dis.42447–454. 10.7589/0090-3558-42.2.447
50
Griffith B. Scott J. M. Carpenter J. W. Reed C. (1989). Translocation as a species conservation tool: status and strategy.Science245477–480. 10.1126/science.245.4917.477
51
Hao Y. Nabi G. Deng X. J. Wang D. (2019). Non-invasive fecal steroid measurements for monitoring the reproductive status of a critically endangered yangtze finless porpoises (Neophocaena asiaeorientalis asiaeorientalis).Front. Endocrinol10:606. 10.3389/fendo.2019.00606
52
Hewitt K. (2007). Tributary glacier surges: An exceptional concentration at Panmah Glacier, Karakoram Himalaya.J. Glaciol.53181–188. 10.3189/172756507782202829
53
Hijioka Y. Lin E. Pereira J. J. Corlett R. T. Cui X. Insarov G. E. et al (2014). “Climate change 2014: impacts, adaptation, and vulnerability. part b: regional aspects,” in Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, edsBarrosV. R.FieldC. B.DokkenD. J.MastrandreaM. D.MachK. J.BilirT. E. (Cambridge, MA: Cambridge University Press), 1327–1370.
54
Hill A. V. S. (1999). Genetics and genomics of infectious disease susceptibility.Br. Med. Bull.55401–413. 10.1258/0007142991902457
55
Humerah B. S. Munazza A. Sheikh A. R. (2011). Bacterial and toxic pollutants in lakes of River Indus.Pak. J. Bot.431765–1772.
56
Inam A. Clift P. D. Giosan L. Tabrez A. R. Tahir M. Rabbani M. M. et al (2007). “The geographic, geological and oceanographic setting of the Indus River,” in Large Rivers: Geomorphology and Management, ed.GuptaA. (Hoboken, NJ: John Wiley & Sons, Ltd), 333–346. 10.1002/9780470723722.ch16
57
IUCN (1996). IUCN Red List of Threatened Animals.Gland: IUCN.
58
IUCN (2016). The IUCN Red List of Threatened Species, Version 2016–1. Available online at: http://www.iucnredlist.org(accessed August 2, 2016).
59
Ji J. Nabi G. Zeng X. Hao Y. Wang D. (2019). Histological variation of blubber morphology in the endangered east Asian finless porpoise (Neophocaena asiaeorientalis sunameri) with ontogeny and reproductive states.Zool. Stud.58:42. 10.6620/ZS.2019.58-42
60
Jia Y. Wang L. Qu Z. Wang C. Yang Z. (2017). Effects on heavy metal accumulation in freshwater fishes: species, tissues, and sizes.Environ. Sci. Pollut. Res. Int.249379–9386. 10.1007/s11356-017-8606-4
61
Kamal A. (2008). “Environmental flows Indus River system in Pakistan,” in Proceedings of the 3rd International Conference on Water Resources and Arid Environments and the 1st Arab Water Forum, Lahore.
62
Kareiva P. Watts S. McDonald R. Boucher T. (2007). Domesticated nature: shaping landscapes and ecosystems for human welfare.Science3161866–1869. 10.1126/science.1140170
63
Khan H. (1947). A fishery survey of River Indus.J. Bombay Nat. Hist. Soc.46529–535.
64
Khan M. S. (2016). Factors affecting the survival of Indus River dolphin and species tolerance towards anthropogenic pressures.Mar. Freshw. Res.681245–1250. 10.1071/MF16001
65
Khan S. Nabi G. Ullah M. W. Yousaf M. Manan S. Siddique R. et al (2016). Overview on the role of advance genomics in conservation biology of endangered species.Int. J. Genomics2016:3460416. 10.1155/2016/3460416
66
Khan U. (2005). Cetacean Conservation: A Case Study of the Indus Dolphin Rescue Project: A Collaboration for Rescuing Stranded River Dolphin from Sindh to Punjab.Lahore: WWF Pakistan.
67
Larinier M. (2001). “Environmental issues, dams and fish migration,” in FAO Fisheries Technical Paper 419. Dams, Fish and Fisheries. Opportunities, challenges and conflict resolution, ed.MarmullaG. (Rome: FAO), 45–89.
68
Locke D. P. Hillier L. W. Warren W. C. Worley K. C. Nazareth L. V. Muzny D. M. et al (2011). Comparative and demographic analysis of orangutan genomes.Nature469529–533. 10.1038/nature09687
69
Lucas M. C. Baras E. (2001). Migration of Freshwater Fishes.Oxford: Black Science.
70
MacLeod C. D. (2009). Global climate change, range changes and potential implications for the conservation of marine cetaceans: a review and synthesis.Endang. Spec. Res.7125–136. 10.3354/esr00197
71
Master L. L. Flack S. R. Stein B. A. (1998). Rivers of Life: Critical Watersheds for Protecting Freshwater Biodiversity.Arlington: The Nature Conservancy.
72
McNair J. F. A. (1908). Oral Tradition from the Indus.Brighton: Gosden R.
73
Mei Z. Cheng P. Wang K. Wei Q. Barlow J. Wang D. (2020). A first step for the Yangtze.Science3671314–1314. 10.1126/science.abb5537
74
Mei Z. Han Y. Turvey S. T. Liu J. Wang Z. Nabi G. et al (2021). Mitigating the effect of shipping on freshwater cetaceans: The case study of the Yangtze finless porpoise.Biol. Conserv.257:109132. 10.1016/j.biocon.2021.109132
75
Mei Z. Huang S. L. Hao Y. Turvey S. T. Gong W. Wang D. (2012). Accelerating population decline of Yangtze finless porpoise (Neophocaena asiaeorientalis asiaeorientalis).Biol. Conserv.153192–200. 10.1016/j.biocon.2012.04.029
76
Mei Z. Zhang X. Huang S. L. Zhao X. Hao Y. Zhang L. et al (2014). The Yangtze finless porpoise: on an accelerating path to extinction?Biol. Conserv.172117–123. 10.1016/j.biocon.2014.02.033
77
Melissa D. V. Lenore F. Curtis H. F. (2003). Effect of reproductive rate on minimum habitat requirements of forest-breeding birds.Ecology842643–2653. 10.1890/02-0159
78
Memon A. A. (2004). Evaluation of impacts on the lower Indus River Basin due to upstream water storage and diversion. Critical transitions in water and environmental resources management.World Water Congress2004:10.
79
Miller L. J. Solangi M. Kuczaj S. A. (2008). Immediate response of Atlantic bottlenose dolphins to high-speed personal watercraft in the Mississippi Sound.J. Mar. Biol. Assoc. U.K.881139–1143. 10.1017/S0025315408000908
80
Mirza M. R. Mirza Z. S. (2014). Longitudinal zonation in the fish fauna of the Indus River in Pakistan.Biologia60149–152. 10.17582/journal.pjz/2017.49.1.149.154
81
Moore M. Early G. Touhey K. Barco S. Gulland F. Wells R. (2007). Rehabilitation and release of marine mammals in the United States: risks and benefits.Mar. Mamm. Sci.23731–750. 10.1111/j.1748-7692.2007.00146.x
82
Morita K. Morita S. Yamamoto S. (2009). Effects of habitat fragmentation by damming on salmonid fishes: lessons from white-spotted charr in Japan.Ecol. Res.24711–722. 10.1007/s11284-008-0579-9
83
Muhammad H. Iqbal Z. Saleemi S. (2016). Diversity and distribution of fish fauna of Indus River at Taunsa Barrage in Punjab, Pakistan.Pakistan J. Zool.49149–154. 10.17582/journal.pjz/2017.49.1.149.154
84
Murphy S. Barber J. L. Learmonth J. A. Read F. L. Deaville R. Perkins M. W. et al (2015). Reproductive failure in UK harbour porpoises Phocoena phocoena: legacy of pollutant exposure?PLoS One10:e0131085. 10.1371/journal.pone.0131085
85
Nabi G. Ali M. Khan S. Kumar S. (2019a). The crisis of water shortage and pollution in Pakistan: risk to public health, biodiversity, and ecosystem.Environ. Sci. Pollut. Res.26:10443. 10.1007/s11356-019-04483-w
86
Nabi G. Hao Y. McLaughlin R. W. Wang D. (2018a). The possible effects of high vessel traffic on the physiological parameters of the critically endangered Yangtze Finless Porpoise (Neophocaena asiaeorientalis ssp. asiaeorientalis).Front. Physiol.9:1665. 10.3389/fphys.2018.01665
87
Nabi G. Hao Y. Robeck T. R. Jinsong Z. Wang D. (2018b). Physiological consequences of biologic state and habitat dynamics on the critically endangered Yangtze finless porpoises (Neophocaena asiaeorientalis ssp. asiaeorientalis) dwelling in the wild and semi-natural environment.Conserv. Physiol.6:coy072. 10.1093/conphys/coy072
88
Nabi G. Hao Y. Zeng X. Jinsong Z. McLaughlin R. W. Wang D. (2017a). Hematologic and biochemical differences between two free ranging Yangtze finless porpoise populations: the implications of habitat.PLoS One12:e0188570. 10.1371/journal.pone.0188570
89
Nabi G. Hao Y. Zeng X. Wang D. (2017b). Assessment of yangtze finless porpoises (Neophocaena asiaorientalis) through biochemical and hematological parameters.Zool. Stud.56:31. 10.6620/ZS.2017.56-31
90
Nabi G. Khan S. Ahmad S. Khan A. Siddique R. (2017c). China–Pakistan Economic Corridor (CPEC): an alarming threat to the biodiversity of Northern Pakistan.Biodivers. Conserv.263003–3004. 10.1007/s10531-017-1402-0
91
Nabi G. Li Y. McLaughlin R. W. Mei Z. Wang K. Hao Y. et al (2020a). Immune responses of the critically endangered yangtze finless porpoises (Neophocaena asiaeorientalis ssp. asiaeorientalis) to escalating anthropogenic stressors in the wild and seminatural environments.Front. Physiol.10:1594. 10.3389/fphys.2019.01594
92
Nabi G. McLaughlin R. W. Hao Y. Wang K. Zeng X. Khan S. et al (2018c). The possible effects of anthropogenic acoustic pollution on marine mammals’ reproduction: an emerging threat to animal extinction.Environ. Sci. Pollut. Res. Int.2519338–19345. 10.1007/s11356-018-2208-7
93
Nabi G. McLaughlin R. W. Khan S. Hao Y. Chang M. X. (2020b). Pneumonia in endangered aquatic mammals and the need for developing low-coverage vaccination for their management and conservation.Anim. Health Res. Rev.21122–130. 10.1017/S1466252320000158
94
Nabi G. Robeck T. R. Hao Y. Wang D. (2019b). Hematologic and biochemical reference interval development and the effect of age, sex, season, and location on hematologic analyte concentrations in critically endangered yangtze finless porpoise (Neophocaena asiaeorientalis ssp. asiaeorientalis).Front. Physiol.10:792. 10.3389/fphys.2019.00792
95
Nabi G. Robeck T. R. Yujiang H. Tang B. Zheng J. Wang K. et al (2021). Circulating concentrations of thyroid hormones and cortisol in wild and semi-natural Yangtze finless porpoise (Neophocaena asiaeorientalis).Conserv. Physiol.9:coab034. 10.1093/conphys/coab034
96
Newbold T. Hudson L. N. Phillips H. R. P. Hill S. L. L. Contu S. Lysenko I. et al (2014). A global model of the response of tropical and sub-tropical forest biodiversity to anthropogenic pressures.Proc. Biol. Sci.281:20141371. 10.1098/rspb.2014.1371
97
Nielsen L. A. (1992). Methods of marking fish and shellfish.Am. Fish. Soc. Special Publ.23:208.
98
Nilsson C. Reidy C. A. Dynesius M. Revenga C. (2005). Fragmentation and flow regulation of the world’s large river systems.Science308405–408. 10.1126/science.1107887
99
Noureen U. (2013). Indus River Dolphin (Platanista gangetica minor) Abundance Estimations Between Chashma and Sukkur Barrages, in the Indus River, Pakistan.Islamabad: Quaid-e-Azam University.
100
Paige K. N. (2010). The functional genomics of inbreeding depression: a new approach to an old problem.BioScience60267–277. 10.1525/bio.2010.60.4.5
101
Paudel S. Timilsina Y. P. Lewis J. Ingersoli T. Jnawali S. R. (2015). Population status and habitat occupancy of endangered river dolphins in the Karnali River system of Nepal during low water season.Mar. Mamm. Sci.31707–719. 10.1111/mms.12192
102
Peter G. H. E. Raga J. A. (2001). Marine Mammals: Biology and Conservation (Eds 1).New York, NY: Springer, 181–182.
103
Pilleri G. (1972). Field observations carried out on the Indus dolphin Platanista indi in the winter of 1972.Investig. Cetacea423–29.
104
Qadir A. Malik R. N. Husain S. Z. (2008). Spatio-temporal variations in water quality of nullah Aik-tributary of the river Chenab, Pakistan.Environ. Monit. Assess14043–59. 10.1007/s10661-007-9846-4
105
Rabin L. A. Greene C. M. (2002). Changes in acoustic communication systems in human-altered environments.J. Comp. Psychol.116137–141. 10.1037//0735-7036.116.2.137
106
Reeves R. R. (1991). Conservation of the bhulan (blind river dolphin) in the Punjab.Natura53–22.
107
Reeves R. R. Chaudhry A. A. Khalid U. (1991). Competing for water on the Indus Plain: is there a future for Pakistan’s river dolphins?Environ. Conserv.18341–349. 10.1017/S0376892900022591
108
Reeves R. R. Smith B. D. Kasuya T. (2000). Biology and Conservation of Freshwater Cetaceans in Asia.Gland: IUCN.
109
Rengarajan S. Balasubramanian K. (2008). Corticosterone induces steroidogenic lesion in cultured adult rat Leydig cells by reducing the expression of star protein and steroidogenic enzymes.J. Cell. Biochem.1031472–1487. 10.1002/jcb.21533
110
Ricciardi A. Rasmussen J. B. (1999). Extinction rates in north American freshwater fauna.Conserv. Biol.131220–1222. 10.1046/j.1523-1739.1999.98380.x
111
Rolland R. M. Parks S. E. Hunt K. E. Castellote M. Corkeron P. J. Nowacek D. P. et al (2012). Evidence that ship noise increases stress in right whales.Proc. Biol. Sci.2792363–2368. 10.1098/rspb.2011.2429
112
Sala O. E. Chapin I. I. I. F. S. Armesto J. J. Berlow E. Bloomfield J. Dirzo R. et al (2000). Global biodiversity scenarios for the year 2100.Science2871770–1774. 10.1126/science.287.5459.1770
113
Sandler R. L. (2012). The Ethics of Species. An Introduction.Cambridge, MA: Cambridge University Press.
114
Sanpera C. Ruiz X. Jover L. Llorente G. Jabeen R. Muhammad A. et al (2003). Persistent organic pollutants in little egret eggs from selected wetlands in Pakistan.Arch. Environ. Contam. Toxicol.44360–368. 10.1007/s00244-002-2044-z
115
Saragusty J. Hermes R. Göritz F. Schmitt D. L. Hildebrandt T. B. (2009). Skewed birth sex ratio and premature mortality in elephants.Anim. Reprod. Sci.115247–254. 10.1016/j.anireprosci.2008.10.019
116
Schelle P. (2010). River Dolphins & People: Shared Rivers, Shared Future.Gland: WWF International.
117
Smith B. D. Reeves R. R. Sonderegger C. (2010). Comprehensive Assessment Needed on the Implications of Climate Change for Freshwater-Dependent Cetaceans. International Whaling Commission Report SC\N10\CC4. Cambridge, MA: International Whaling Commission.
118
Steiner C. C. Putnam A. S. Hoeck P. E. A. Ryder O. A. (2013). Conservation genomics of threatened animal species.Annu. Rev. Anim. Biosci.1261–281. 10.1146/annurev-animal-031412-103636
119
Sultana J. Syed J. H. Mahmood A. Ali U. Rehman M. Malik R. N. et al (2014). Investigation of organochlorine pesticides from the Indus Basin, Pakistan: sources, air-soil exchange fluxes and risk assessment.Sci. Total. Environ.497-498113–122. 10.1016/j.scitotenv.2014.07.066
120
Supple M. A. Shapiro B. (2018). Conservation of biodiversity in the genomics era.Genome. Biol.19:131. 10.1186/s13059-018-1520-3
121
Suresh A. Seema R. Disha D. Shubhakara G. (2010). Captive Breeding: A Potential Method for Conservation of Species. Wetlands, Biodiversity and Climate Change. Available online at: http://www.ces.iisc.ernet.in/energy/lake2010/theme5/ashitha_suresh.pdf(accessed July 17, 2017).
122
Toosy A. H. Khan U. Mahmood R. Bhagat H. B. (2009). First tagging with a radiotransmitter of a rescued Indus River dolphin near Sukkur barrage, Pakistan.Wildl. Middle East.3:6.
123
Turvey S. T. Pitman R. L. Taylor B. L. Barlow J. Akamatsu T. Barrett L. A. (2007). First human-caused extinction of a cetacean species?Biol. Lett.3537–540. 10.1098/rsbl.2007.0292
124
Wang D. (2009). Population status, threats and conservation of the Yangtze finless porpoise.Chin. Sci. Bull.543473–3484. 10.1007/s11434-009-0522-7
125
Wang D. (2015). Natural Ex situ Conservation of Yangtze Finless Porpoise Saw Progress.Guangzhou: Chinese Academy of Sciences.
126
Wang D. Liu R. Zhang X. Yang J. Wei Z. Zhao Q. et al (2000). Status and conservation of the Yangtze finless porpoise.Biol. Conserv. Fresh. Cetaceans Asia481–85.
127
Wang D. Turvey S. T. Zhao X. Mei Z. (2013). Neophocaena asiaeorientalis ssp. Asiaeorientalis. The IUCN Red List of Threatened Species 2013: e.T43205774A45893487.Gland: IUCN.
128
Wang D. Yujiang H. Kexiong W. Qingzhong Z. Daoquang C. Zhuo W. et al (2005). The first Yangtze finless porpoise successfully born in captivity.Environ. Sci. Pollut. Res.5247–250. 10.1065/espr2005.08.284
129
Wang D. Zhang X. Liu R. (1998). Conservation Status and The Future of Baiji and Finless Porpoise in the Yangtze River of China. Ecology and Environment Protection in the Large Water Conservancy Projects of the Yangtze River. Environmental.Beijing: Science Press, 218–226.
130
Waqas U. Muhammad I. M. Liaquat A. K. (2012). Conservation of Indus River Dolphin (Platanista gangetica minor) in the Indus River system, Pakistan: an overview.Rec. Zool. Surv. Pakistan2182–85.
131
Wells R. S. Rhinehart H. L. Hansen L. J. Hansen L. J. Sweeney J. C. Townsend F. I. et al (2004). Bottlenose dolphins as marine ecosystem sentinels: developing a health monitoring system.Ecol. Health1246–254. 10.1007/s10393-004-0094-6
132
Whitehead H. Rendell L. (2015). The Cultural Lives of Whales and Dolphins.Chicago, IL: The University of Chicago Press, 259–260.
133
Wilding C. S. Butlin R. K. Grahame J. (2001). Differential gene exchange between parapatric morphs of Littorina saxatilis detected using AFLP markers.J. Evol. Biol.14611–619. 10.1046/j.1420-9101.2001.00304.x
134
Wiley D. N. Clapham P. J. (1993). Does maternal condition affect the sex ratio of offspring in humpback whales?Anim. Behav.46321–324. 10.1006/anbe.1993.1193
135
Williot P. Rochard E. Rouault T. Kirschbaum F. (2009). “Acipenser sturio recovery research actions in France,” in Biology, Conservation and Sustainable Development of Sturgeons, edsCarmonaC. M.CarmonaR.DomezainA.GallegoM. G.HernandoJ. A.RodríguezF.et al (Amsterdam: Springer), 247–263. 10.1007/978-1-4020-8437-9_15
136
World Bank (2005). Pakistan Country Water Resources Assistance Strategy. Agriculture and Rural Development Unit, South Asia Region.Washington, DC: World Bank.
137
Wright A. J. Soto N. A. Baldwin A. L. Bateson M. Beale C. M. Clark C. et al (2007). Do marine mammals experience stress related to anthropogenic noise?Int. J. Comp. Psychol.20274–316.
138
Wu J. Huang J. Han X. Gao X. He F. Jiang M. et al (2004). The Three Gorges Dam: an ecological perspective.Front. Ecol. Environ.2241–248.
139
Wu J. M. Wang C. Y. Zhang H. Du H. Liu Z. G. Shen L. et al (2015). Drastic decline in spawning activity of Chinese sturgeon Acipenser sinensis Gray the remaining spawning ground of the Yangtze River since the construction of hydrodams.J. Appl. Ichthyol.31839–842. 10.1111/jai.12882
140
Wu J. M. Wei Q. W. Du H. Wang C. Y. Zhang H. (2014). Initial evaluation of the release programme for Dabry’s sturgeon (Acipenser dabryanus Dumeril, 1868) in the upper Yangtze River.J. Appl. Ichthyol.301423–1427. 10.1111/jai.12597
141
WWF (2015). Milestone in Race to Save Yangtze Finless Porpoise. Available: https://wwf.panda.org/wwf_news/?242311/Milestone-in-race-to-save-Yangtze-finless-porpoise%E2%80%9(accessed on 23rd August 2020).
142
WWF (2017). Indus River Dolphin Numbers on the Rise with the Help of Local Communities.Washington DC: World Wildlife Fund.
143
WWF-Pakistan (2011). Report on Indus River Dolphin Mortality: Analysis of Dead Dolphin Samples for Pesticides.Lahore: WWF-Pakistan.
144
Xenopoulos M. A. Lodge D. M. (2006). Going with the flow: using species-discharge relationships to forecast losses in fish biodiversity.Ecology871907–1914. 10.1890/0012-9658(2006)87[1907:gwtfus]2.0.co;2
145
Xiao Y. Nabi G. Yang J. Hao Y. Wang D. (2018). Hormonal regulation of testicular development in the finless Porpoise Neophocaena asiaeorientalis sunameri: preliminary evidence from testicular histology and immunohistochemistry.Zool. Stud.57:41. 10.6620/ZS.2018.57-41
146
Xinhua (2020). China Starts 10-Year Fishing Ban on Yangtze River. Available online at: http://www.xinhuanet.com/english/2020-01/02/c_138672069.htm#:~:text=BEIJING%2C%20Jan.,of%20Agriculture%20and%20Rural%20Affairs(accessed August 23, 2020).
147
Xiong X. Qian Z. Mei Z. Wu J. Hao Y. Wang K. et al (2019). Trace elements accumulation in the Yangtze finless porpoise (Neophocaena asiaeorientalis asiaeorientalis) - A threat to the endangered freshwater cetacean.Sci. Total. Environ.10797–804. 10.1016/j.scitotenv.2019.06.031
148
Yang D. Qi S. Zhang J. Wu C. Xing X. (2013). Organochlorine pesticides in soil, water and sediment along the Jinjiang River mainstream to Quanzhou Bay, southeast China.Ecotoxicol. Environ. Saf.8959–65. 10.1016/j.ecoenv.2012.11.014
149
Yates C. J. Ladd P. G. (2005). Relative importance of reproductive biology and establishment ecology for persistence of a rare shrub in a fragmented landscape.Conserv. Biol.19239–249. 10.1111/j.1523-1739.2005.00286.x
150
Zeng X. Chen M. Liu Z. Yu D. Huang S. L. Yang J. et al (2019). Characterization of milk protein composition of the Yangtze finless porpoise.Mar. Mam. Sci35252–260. 10.1111/mms.12508
151
Zeng X. Huang S. L. Hao Y. Wang D. Ji J. Deng X. et al (2018). Ultrasonography of mammary glands in finless porpoises (Neophocaena asiaeorientalis) at different reproductive stages.Mar. Mam. Sci.34529–540. 10.1111/mms.12453
152
Zeng X. Huang S. L. Qian Z. Hao Y. Wang D. Ji J. et al (2017). Characterization of milk composition in narrow-ridged finless porpoises (Neophocaena asiaeorientalis) at different lactation stages.Mar. Mam. Sci.33803–816. 10.1111/mms.12398
153
Zhang H. Balk H. Wang C. Wu J. Du H. Shen L. et al (2016). Search for Chinese paddlefish (Psephurus gladius) in the upper Yangtze River during 2009–2013 including reevaluation of data from 2006 to 2008.Aquat. Living Resour.29101–109. 10.1051/alr/2016008
Summary
Keywords
conservation, endangered, extinction, Indus River dolphin, potential threats, translocation
Citation
Nabi G, Ahmad S, McLaughlin RW, Hao Y, Khan S, Ahmad N, Ahmad S, Kiani MS, Wu Y and Li D (2021) Deteriorating Habitats and Conservation Strategies to Repopulate the Endangered Indus River Dolphin (Platanista gangetica minor); a Lesson Learned From the Conservation Practices of the Yangtze Finless Porpoise (Neophocaena asiaeorientalis). Front. Mar. Sci. 8:561905. doi: 10.3389/fmars.2021.561905
Received
14 May 2020
Accepted
07 June 2021
Published
05 July 2021
Volume
8 - 2021
Edited by
Diego Horacio Rodriguez, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina
Reviewed by
Farooq Rashid, Southern Medical University, China; Haroon Haroon, Northwest University, China
Updates
Copyright
© 2021 Nabi, Ahmad, McLaughlin, Hao, Khan, Ahmad, Ahmad, Kiani, Wu and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ghulam Nabi, ghulamnabiqau@gmail.comDongming Li, lidongming@hebtu.edu.cn
This article was submitted to Marine Megafauna, a section of the journal Frontiers in Marine Science
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.