Abstract
Under certain conditions, dispersed crude oil in the sea combines with organisms, organic matter, and minerals to form marine oil snow (MOS), thereby contributing to the sinking of oil to the seafloor. Marine microbes are the main players in MOS formation, particularly via the production of extracellular polymeric substances. Distinct groups of microbes also consume the majority of the hydrocarbons during descent, leading to enrichment of the less bioavailable hydrocarbons and asphaltenes in the residue. Here we discuss the dynamics of microbial communities in MOS together with their impacts on MOS evolution. We explore the effects of dispersant application on MOS formation, and consider ways in which laboratory experiments investigating MOS formation can be more representative of the situation in the marine environment, which in turn will improve our understanding of the contribution of MOS to the fate of spilled oil.
Introduction and Scope
In order to respond effectively to crude oil released to the marine environment it is imperative to understand the interplay between the physical, chemical, and biological factors that determine petroleum fate, transport, and impact on the biota and surrounding environment. Marine snow (MS) came to prominence as a vehicle for transporting oil to the benthos during the 2010 Macondo spill (hereafter referred to as the MC-252 spill, because the Macondo Prospect is located in Mississippi Canyon Block 252 of the Gulf of Mexico), and has stimulated a lot of research to determine its quantitative importance, resulting in several reviews on the topic (e.g., Daly et al., 2016; Passow and Ziervogel, 2016; Quigg et al., 2016, 2020; Decho and Gutierrez, 2017; Brakstad et al., 2018b; Burd et al., 2020; Kujawinski et al., 2020; Passow and Overton, 2021). Here we consider the microbial communities associated with marine oil snow (MOS), including producers and degraders of extracellular polymeric substances (EPS). We discuss the evidence for biodegradation of different hydrocarbon components during the descent of MOS, the impacts of MOS on the benthos, and the potential influence of oil on the formation of MOS and on its associated microbiome. We discuss the efficacy of the methods used to investigate aspects of MOS, and conclude by suggesting ways to advance research into the formation and fate of MOS.
Marine Snow (MS) Formation
The ocean contains ~600 gigatonnes of carbon in the form of dissolved organic matter (DOM; Hansell et al., 2009), the vast majority in the nanometre size range (Benner and Amon, 2015). Around 10% of this DOM can self-assemble to form marine microgels, three-dimensional polymer networks transiently suspended in seawater, which can aggregate into particulate organic matter (POM; Chin et al., 1998; Verdugo, 2012; Orellana and Leck, 2015; Figure 1). This transition from DOM to POM plays a critical role in marine snow (MS) formation (Passow et al., 2012). MS is defined as aggregates ≥500 μm in diameter that descend from the euphotic layer of the epipelagic zone (0–200 m depth) into the mesopelagic zone and beyond, ultimately reaching the sea floor (Turner, 2015; Figure 1). MS is made up of various organic and inorganic particles, eukaryotic microalgae, and Bacteria, along with Archaea, microzooplankton, fungi, viruses, fecal material, detritus, and feeding structures, all bound together by biopolymers (Shanks and Trent, 1980; Alldredge and Silver, 1988; Alldredge et al., 1993; Simon et al., 2002; Volkman and Tanoue, 2002; Bochdansky et al., 2017). Bacteria are abundant in MS (Mestre et al., 2018), where their large surface-area to volume ratio makes them particularly effective in transporting and transforming organic matter and cycling nutrients (Azam and Long, 2001). Analysis of large, slowly sinking MS particles in the bathypelagic zone showed that, while bacteria were extremely numerically dominant, two distinct eukaryotic groups, the fungi and the labyrinthulomycetes, together contributed about twice the bacterial biomass on the MS (Bochdansky et al., 2017). Marine snow transports microbes from the surface (Mestre et al., 2018), provides an important food source for organisms living in the deep water column, and drives the organic-carbon pump that exports carbon and energy to the aphotic zone, sequestering atmospheric CO2 into the deep ocean and sediment (DeVries et al., 2012). MS sinking velocities range from 1 to 368 m/day (median 95 m/day) (Alldredge and Silver, 1988), dependent on particle size, density, and porosity (De La Rocha and Passow, 2007; Brakstad et al., 2018b), and can change while sinking.
Figure 1
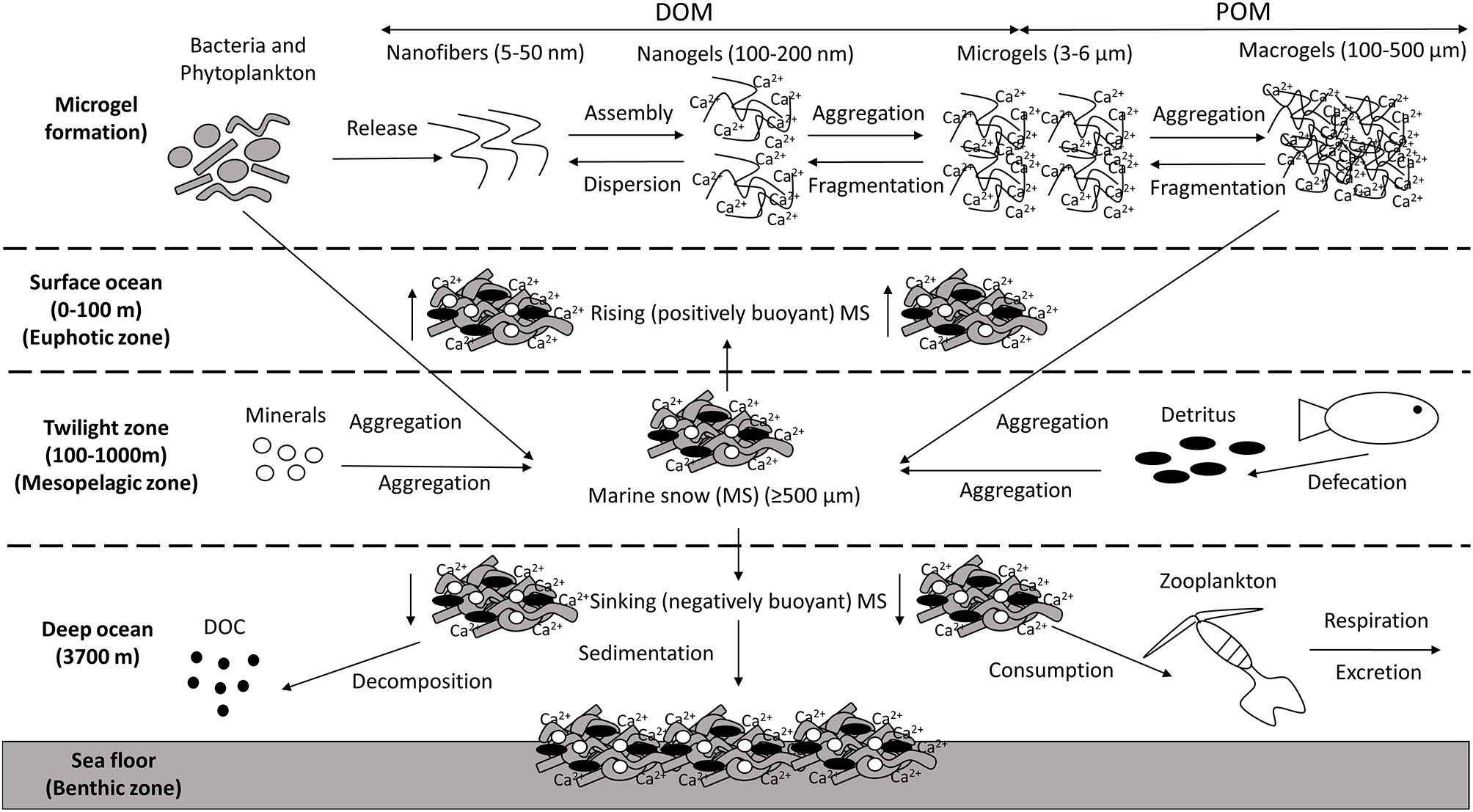
Marine snow (MS) formation and sedimentation. Macromolecules/nanofibres are released by microbes (e.g., bacteria and phytoplankton), which assemble, forming nanogels stabilized by entanglements and Ca2+ bonds. There is a transition in the size continuum of marine gels from dissolved organic matter (DOM) to particulate organic matter (POM) as these nanogels interpenetrate each other forming microgels. Neighboring microgels then aggregate forming macrogels [e.g., Transparent Exopolymer Particles (TEP)]. This process occurs in the euphotic zone, mesopelagic zone and the deep ocean. MS forms from aggregation of microbes and other particles, which are held together by a combination of Extracellular Polymeric Substances (EPS) and the macrogels generated. Other material can be incorporated into these aggregates, such as minerals and detritus. Positively buoyant MS particles rise and can float on the ocean surface. Negatively buoyant MS particles sink, where they can be altered by the activities of heterotrophic microbes and zooplankton prior to sedimentation on the seafloor.
Extracellular polymeric substances (EPS) are a major component of the total DOM pool in the ocean. They consist primarily of polysaccharides (up to 90%), but also proteins, glycoproteins, nucleic acids, and lipids, and are secreted or released by phytoplankton and heterotrophic microbes (Mühlenbruch et al., 2018). Polysaccharide-rich EPS are transparent, but can be visualized with Alcian Blue (Alldredge et al., 1993), and hereafter, this form of EPS will be referred to by its widely used descriptor of “transparent exopolymer particles” (TEP; Alldredge et al., 1993; Passow and Alldredge, 1995). Protein-rich EPS (which are also transparent in nature but not in name), can be distinguished by staining with Coomassie Blue (Long and Azam, 1996). TEP are “sticky,” largely due to acidic sugar units, and so they are often considered to be most significant in the formation of MS (Daly et al., 2016).
Thus, EPS can enhance the aggregation of particles, enabling the formation of MS (Quigg et al., 2016; Decho and Gutierrez, 2017), although not all EPS assembles to form these discrete particles (Passow, 2002a). Firstly, EPS nanofibers (5–50 nm) assemble, forming nanogels (100–200 nm) that are stabilized by entanglement and, due to their polyanionic nature, cross-linking with divalent Ca2+ (Verdugo, 2012; Wakeham and Lee, 2019). These DOM polymer networks diffuse into the bulk seawater and collide with neighboring nanogels, forming microgels (~3–6 μm) in a reversible process (Verdugo, 2012). Microgels are ubiquitous throughout the oceans, generally decreasing in abundance below the photic zone (Engel et al., 2020), and continue to collide and aggregate, eventually forming stable macrogels of several hundred micrometers, operationally defined as particles retained on 0.4 μm polycarbonate filters (though some researchers use 0.2 μm filters).
Aggregate formation depends on the number of particle collisions, and is a function of the abundance of particles, including cells, present in the medium, and their cohesiveness, which enhances the probability that such particles will stay attached after collision (Jackson, 1990; Burd and Jackson, 2009; Cruz and Neuer, 2019). TEP aggregate with other particles and act as a biological glue to form sinking MS (Engel et al., 2004; Mari et al., 2017). Several studies have established a link between increases in TEP concentration and the enhanced aggregation and sinking of MS (Passow et al., 1994, 2001; Logan et al., 1995; Gärdes et al., 2011). By contrast, Coomassie-blue stainable particles are not considered to be as important as TEP in MS formation (Prieto et al., 2002; Cisternas-Novoa et al., 2015). However, protein-rich EPS, which are as common as TEP in the ocean (Busch et al., 2017), consist of both hydrophilic and hydrophobic domains, which result in surface processes that are less predictable and more dependent on the context (e.g., types of associated minerals, presence of hydrocarbons), and may be important for MS formation (Santschi et al., 2020). Actually, the world of hydrated gels is less binary than portrayed here, with the existence of hybrid and intermediate forms, and understanding the varied nature of these gels and their roles in, inter alia, the microbial loop and marine snow formation remains work in progress (Thornton, 2018).
In addition to these biochemical processes, hydrodynamics influence the formation and transport of MS and marine oil snow (MOS), indirectly (e.g., by transport of limiting nutrients to microbiota), and directly (e.g., by turbulence-enhanced aggregation of particles due to increased collision rate or, when excessive, disaggregation of MS) (Burd and Jackson, 2009; Daly et al., 2016). Currents, eddies, subduction, upwelling, river discharge, benthic resuspension, and other physical processes can all alter the formation and distribution of MS/MOS (see Daly et al., 2016, 2020). Also, density gradients in the water column can decrease the settling rate of MS/MOS particles (Prairie et al., 2015).
Marine Oil Snow (MOS) Formation and Its Contribution to Transporting Weathered Crude Oil to Deep-Sea Sediments
Composition of MOS and Potential Impact on the Benthos and Associated Biota
Crude oil spilled on the sea is rapidly weathered by processes such as spreading, evaporation, natural dispersion, emulsification, and photooxidation. The most soluble hydrocarbons, such as benzene and toluene dissolve into the underlying water, and hydrocarbons with up to 15 carbon atoms evaporate in the first day or so. Floating oil undergoes photooxidation and emulsification, and any wave action will disperse some of the oil into the water column. Oil-spill dispersants lower the interfacial tension between oil and water, and thereby allow dispersion with much less wave energy, and the generation of much smaller droplets. Crude oil spilled from a deep-sea well blowout will rise to the surface, losing most of its soluble components during the ascent (Ryerson et al., 2012), and at least partially dispersing as small droplets. Again, this process can be stimulated with dispersants (Gros et al., 2017).
Dispersed oil droplets are subject to rapid biodegradation, and can also interact with minerals and organic matter. In coastal areas oil-mineral aggregates are common (Owens, 1999; Loh and Yim, 2016; Li et al., 2020) and result in sinking or suspension in the water column depending on the nature of the aggregate (Quigg et al., 2020). Suspended sediment particles from the Mississippi River outflow generated oil mineral aggregates during the MC-252 spill (Figure 2), although their quantitative influence is not clear (Daly et al., 2016). A substantial settling of MS during the early days of the MC-252 spill led to the suggestion that MS could also transport oil to the sediment (Passow et al., 2012; Passow, 2016; Passow and Ziervogel, 2016; Larson et al., 2018). There are many examples from mesocosm studies of the formation of oil-algal-bacterial complexes (e.g., Lee et al., 1985; Macnaughton et al., 2003; Coulon et al., 2012). In the open sea the resultant oil-containing flocs have been called marine oil snow (MOS) or oil-related marine snow (Brakstad et al., 2018b) among other names (Quigg et al., 2020), shown schematically in Figure 3. From analysis of MOS captured in sediment traps (Stout and German, 2018) it has been estimated that the extensively biodegraded residue of 217,700 to 229,900 barrels of oil was deposited in the form of MOS over an area of at least 7,600 km2 following the MC-252 spill, representing around 7% of the estimated 3.19 million barrels of spilled oil (Barbier, 2015). MOS particles consist of a mixture of organic matter (e.g., phytoplankton, heterotrophic microbes, fecal material, dead, or decaying material), sediment particles and weathered crude oil within a matrix of EPS and especially TEP (Daly et al., 2016; Passow and Ziervogel, 2016; Brakstad et al., 2018b). Thus, TEP can be considered as the glue, while the cells and other particles act as ballast, providing the density to sink the less dense oil and TEP. In addition, the presence of oil may lead to a tighter packaging of microbial cells causing MOS particles to sink faster than MS (Passow et al., 2019). However, as with MS, MOS particles are hot-spots of microbial activity (Ziervogel et al., 2012), and constantly changing as colonization, consumption, clumping, and fragmentation alter the rate of their descent. The sinking of these MOS particles and their accumulation on the seafloor of the Gulf of Mexico was referred to as a marine oil snow sedimentation and flocculent accumulation event (MOSSFA; Valentine et al., 2014; Brooks et al., 2015; Chanton et al., 2015; Romero et al., 2015, 2017; Yan et al., 2016; Schwing et al., 2017). Oil is also transported to deep-water sediments by other mechanisms, such as incorporation into invertebrate fecal pellets (Lee et al., 2012; Almeda et al., 2016), direct sinking of very degraded oil (Murray et al., 2020), sinking of degraded oil attached to mineral particles (Li et al., 2020), settling of oil-mud complexes formed from the injection of drilling fluids (Stout and Payne, 2017), and sinking of heavy residue from burning oil (Stout and German, 2018). For example, in-situ burning removed 220,000–310,000 barrels of surfaced oil following the MC-252 spill, generating some 33,800–54,700 barrels of heavy viscous residue that sank to the seafloor (Stout and Payne, 2016b). Also, 4% of the burned oil was emitted as soot particles (Middlebrook et al., 2012), which may have contributed to MOS formation due to aerosol deposition and adsorption of DOM on the surfaces of soot particles, thereby stimulating aggregation (Mari et al., 2014; Figure 2).
Figure 2
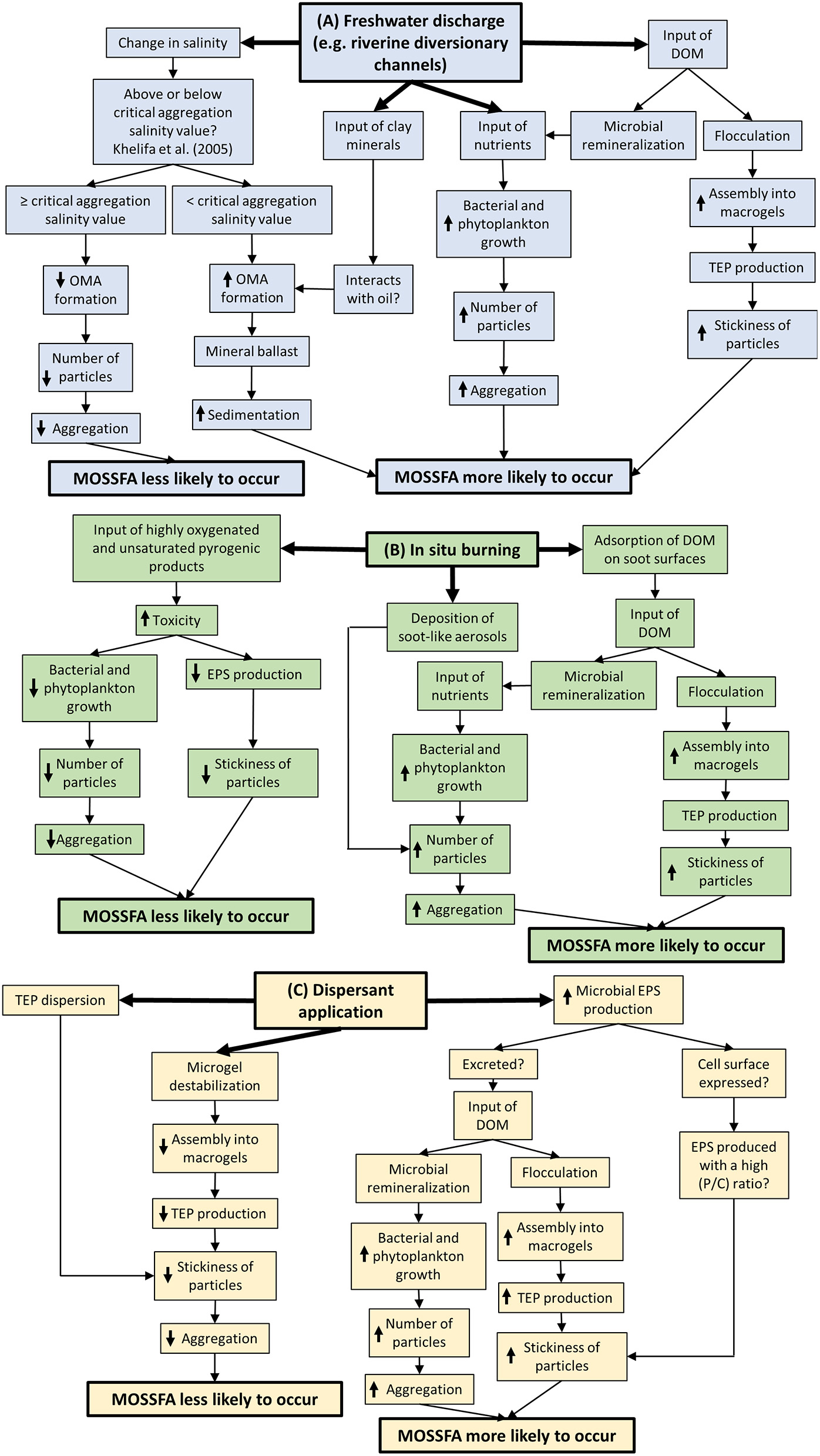
A mind-map showing the factors that may influence the likelihood of a marine oil snow sedimentation and flocculent accumulation (MOSSFA) event, (A) in the event of freshwater influence from rivers, and (B) and (C) after common oil-spill-response strategies, such as (B) in-situ burning, and (C) dispersant application. Abbreviations include: dissolved organic matter (DOM), extracellular polymeric substances (EPS), oil-mineral aggregates (OMA), protein/carbohydrate ratio (P/C ratio) and transparent exopolymer particles (TEP). Up-arrows denote an increase of the process and down-arrows denote a decrease.
Figure 3
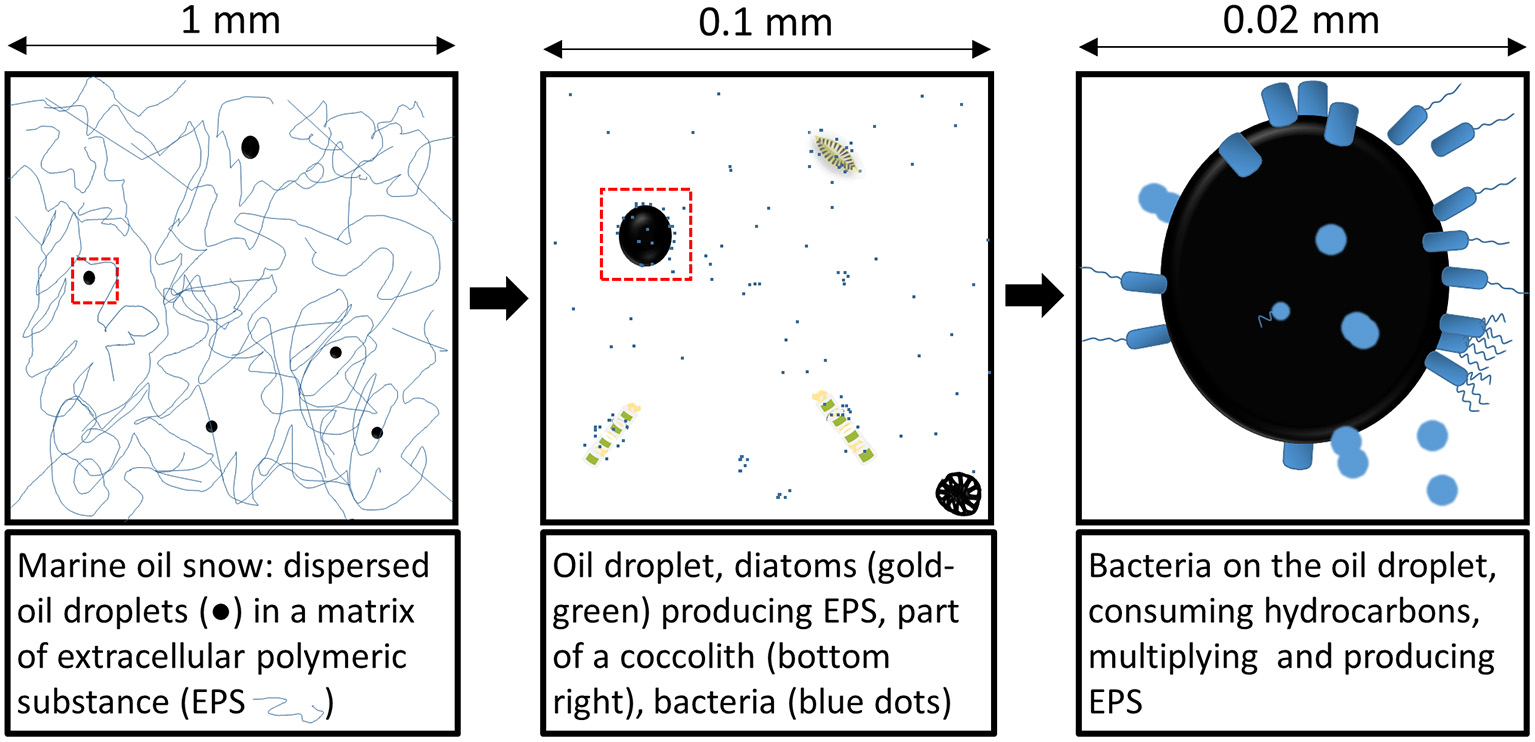
Schematic representation of microbes and dispersed oil droplets in marine snow at different scales.
Oil and sediment deposition following the MC-252 spill impacted the benthic macrofauna and meiofauna (including foraminifera whose carbonate shells provide a temporal sedimentary record), reducing their abundance and biodiversity (Montagna et al., 2013; Baguley et al., 2015; Schwing et al., 2015, 2017, 2018; Washburn et al., 2017). The main drivers of these changes have been assigned to the introduction of toxic oil compounds (van Eenennaam et al., 2018, 2019), an increase in anoxic conditions due to aerobic consumption of the added organic carbon (Hastings et al., 2016, 2020), or a combination of both (Schwing et al., 2015). However, the composition of the oil assumed in these predictions did not take into account the biodegradation that occurs during descent through the water column prior to deposition on the seafloor (Stout and Payne, 2016a; Bagby et al., 2017; Supplementary Table 1). For example, van Eenennaam et al. (2018, 2019) applied 3 or 10 g per m2 of fresh Macondo surrogate oil in their mesocosm experiments. The use of fresh oil is unrepresentative of what was actually deposited on the seafloor, which was a wax-rich, severely biodegraded residue lacking the more bioavailable and toxic components of the oil (Stout and Payne, 2016a). Any future toxicity studies should use weathered oil in order to fully assess the impact of MOS on the benthos.
MOS may have formed after other major spills. For example, MOS is thought to have formed during the 1979 Ixtoc I blowout in the Bay of Campeche based on the presence of river-derived POM (Vonk et al., 2015). Two lines of evidence support a significant depositional pulse of oil following the spill: (1) petroleum biomarkers and molecular compound classes indicative of the Ixtoc I oil were detected in seafloor sediments in the Bay of Campeche from deep (>500 m) waters (Lincoln et al., 2020); and (2) similar laminations and visible redox boundaries in sediment cores to those seen following the MC-252 spill (Brooks et al., 2015; Schwing et al., 2020). However, it cannot be determined what proportion of oil was deposited due to MOS sedimentation compared to the alternative mechanisms discussed above.
Mechanisms of MOS Formation
To our knowledge, controlled field studies to investigate MOS formation and fate soon after an oil spill have yet to be performed as it would require researchers to be present (and divert resources) during an emergency incident. MOS formation and fate are also difficult to investigate experimentally at sea due to stringent regulations on controlled oil releases. In addition, monitoring MOS formation would be technically challenging as this needs an in-situ camera system that follows the aggregation process (Iversen and Lampitt, 2020). As an alternative, MOS experiments have been performed in microcosms with the aim of replicating in-situ conditions, often comparing MS/MOS formation in uncontaminated and crude-oil-amended seawater. Most groups use closed bottles on their sides on roller tables, where the changing direction of gravity keeps any flocs in suspension. If the bottles are full there is minimal turbulence, and if there is an air space the rotation simulates surface turbulence under mild sea conditions. The aggregates formed are generally morphologically and chemically similar to MS particles collected in situ (Unanue et al., 1998; Ziervogel et al., 2012), and rotating bottle experiments have been widely adopted to investigate MOS formation (e.g., Passow et al., 2012; Ziervogel et al., 2012). However, aggregates formed in full bottles may grow larger than those that descend through marine water layers where there is varying turbulence.
MOS may develop as: (1) pre-formed MS interacting with oil, or (2) oil acting as a nucleus for microbial biofilm growth and floc formation. The latter is referred to as a bacteria-oil aggregate (BOA; Passow and Overton, 2021). These mechanisms are not mutually exclusive, and given the temporal and spatial extent of the MC-252 spill, both mechanisms are highly likely to have been responsible for MOS formation. Mechanism 1 was probably enhanced by river outflows (e.g., diversionary channels of the Mississippi River) providing nutrients for microbial growth, as well as directly supplying microbes, clay and other particulate matter to the water column (Figure 2; Vonk et al., 2015; Daly et al., 2016; van Eenennaam et al., 2016). In addition, the transient acute toxicity of dispersed oil immediately upon dispersion may have negatively impacted grazing zooplankton, diminishing their effect on phytoplankton populations (Almeda et al., 2013a,b). Thus, the stimulated phytoplankton blooms (Hu et al., 2011; Bianchi et al., 2014), with their high levels of EPS/TEP, coupled with greater abundance of particles, increased the probability of collisions and aggregation that lead to MOS formation (Jackson, 1990, 2005; Burd and Jackson, 2009).
Furthermore, the unusual features of the MC-252 spill, i.e., its occurrence in deep water and extensive subsurface dispersant treatment leading to a deep-water plume of highly dispersed oil, make it highly likely that some MOS formed below the photic zone. For example, it is known that hydrocarbon-degrading enrichments in the dark (Baelum et al., 2012) as well as pure cultures of hydrocarbon-degrading bacteria (Alcanivorax borkumensis, Omarova et al., 2019; Marinobacter hydrocarbonoclasticus, Vaysse et al., 2011; Halomonas sp., Gutierrez et al., 2013b; and Alteromonas sp., Gutierrez et al., 2018), can form EPS-rich films around oil droplets, sometimes creating flocs (Gutierrez et al., 2013a,b, 2018), even causing gelation (Radwan et al., 2017), and microbial coatings on oil droplets will alter their surface properties and may result in reduced coalescence (Omarova et al., 2019). Unfortunately, the oil concentrations used in many of these studies were very high (up to 5,000 ppm), and nutrient levels were often many orders of magnitude above those available in the open sea (Bejarano et al., 2014; Wade et al., 2016), so it is not clear how well they mimic what happens in the ocean. However, enrichments using seawater without added nutrients and applying light-dark cycles demonstrated bacterial colonization of oil droplets and the formation of flocs (Doyle et al., 2018).
The microfluidic system developed in the laboratory of Jian Sheng (White et al., 2019) shows the colonization of an oil droplet by Pseudomonas sp. ATCC 27259 (strain P62) in real time. This coating of EPS dramatically increased drag, and would thus reduce the rising velocity of oil droplets in the water column. With further studies, applying natural communities and lower nutrient concentrations, this experimental set-up should help to explain and model various aspects of rising plumes of sub-millimeter oil droplets, as was seen upon well-head dispersant addition to the high-pressure MC-252 blowout, e.g., (1) rising velocity; (2) potential for biodegradation; and (3) potential to form MOS (White et al., 2020a,b).
During the MC-252 spill it is possible that biofilms on highly dispersed oil droplets created small flocs in the water column that were captured by downward flux of larger flocs of MS or MOS from the surface (Passow and Stout, 2020), but, it is also feasible that they reached the sediment of their own accord after extensive biodegradation (Murray et al., 2020), or by a combination of both means (Suja et al., 2017; Quigg et al., 2020).
It is noteworthy that droplets of dispersed oil persisted in the environment at low concentrations long after the MC-252 well was capped. Passow and Stout (2020) analyzed MOS captured in a sediment trap 6 weeks to 13 months later, and found low levels of very degraded oil in MOS which they believe originated in the subsurface “plume” and had never reached the surface. Four pulses of MOS were captured, demonstrating that periodic “blooms” of MS, with varying compositions of diatoms, fecal pellets, etc., occur regardless of major oil intrusion. Oil was highly degraded, and concentrations were low (the residue of 1.45 mg fresh oil/m2/d, median percentage degradation of 85%).
Changes in Hydrocarbon Composition During Sinking of MOS
In assessing the impact of MOS on the benthos it has sometimes been assumed, erroneously, that the concentration and composition of crude-oil entrained in marine snow near the surface will be the same when it reaches the seafloor. During the downward transport of MOS, weathering of the oil by evaporation and photooxidation stops, and the relative contribution of biodegradation, as well as dissolution, increases. It is important to understand how rapidly hydrocarbon abundance and composition change during transit and sedimentation, as well as the environmental factors influencing biodegradation, and thus the likelihood of harm to the benthic biota from MOS. Hazen et al. (2010) reported a half-life of alkanes in dispersed oil following the MC-252 spill of a few days, and several papers have confirmed this result under laboratory conditions that mimic environmentally-relevant concentrations (Prince et al., 2013, 2017; Brakstad et al., 2015a; Wang et al., 2016). A key to determining these rates is the use of a conserved internal marker within the oil; hopanes are used most commonly (Prince et al., 1994), although even this pentacyclic saturated hydrocarbon is known to be consumed under some conditions (Frontera-Suau et al., 2002). Therefore, estimates based on hopane's stability may underestimate the extent of biodegradation. Alternative biomarkers can be used, such as C28 20S-triaromatic steroid (Douglas et al., 2012), or even long chain n-alkanes (Bagby et al., 2017), although it must be borne in mind that especially the latter may be biogenic (Dehmer, 1993). Oil residues in deep-sea sediments were found to be very biodegraded, with most of the resolvable alkanes and PAHs degraded >90–95%. The residues did contain measurable amounts of n-alkanes longer than about C30 (Stout and Payne, 2016a; Bagby et al., 2017; Stout and German, 2018). These studies agree that the extent of biodegradation increased with distance from the well, suggesting that the biodegradation occurred while the oil was in transit. Nevertheless, some biodegradation may also have occurred once the oil was in the sediment. The concentrations of oil were sufficiently low that there was likely enough bioavailable N and P so that biodegradation was not severely nutrient limited. The foregoing evidence highlights that biodegradation severely alters the oil composition of residues deposited on the seafloor, and so, to fully understand MOS' impact on the benthos, we must consider the influence of oil degradation within MOS prior to deposition.
Microbial Production and Degradation of Transparent Exopolymer Particles (TEP) and Their Impacts on MOS
It is well-known that diverse eukaryotic phytoplankton produce copious amounts of EPS that aggregate to form TEP, with the quantity produced dependent on the species and environmental conditions (Passow, 2002a,b; Passow et al., 2019). Diatoms, dinoflagellates, and prymnesiophytes are perhaps the most relevant taxa in the context of MOS, as they tend to be the most abundant blooming microalgae in coastal areas where spills are most likely to occur. Many diatoms produce silica and some prymnesiophytes (e.g., coccolithophores) produce calcium carbonate, which cause cells to aggregate and ballast marine snow, causing it to sink (Tyrrell and Young, 2009; Lombard et al., 2013; Quigg et al., 2016). Cyanobacteria also contribute TEP, with naturally forming flocs of Trichodesmium trapping degrading oil in their mucus (Passow et al., 2012). The abundant Prochlorococcus produces TEP (Iuculano et al., 2017). Some associated heterotrophic bacteria can enhance cellular aggregation (Cruz and Neuer, 2019), but other phytoplankton blooms are accompanied by bacteria that consume TEP, such as Flavobacteria, Verrucomicrobia, and Rhodobacterales species, as well as those feeding on other algal products, especially Gammaproteobacteria, such as Alteromonadaceae (Buchan et al., 2014; Taylor et al., 2014; Arnosti et al., 2016; Needham and Fuhrman, 2016; Bohórquez et al., 2017; Decho and Gutierrez, 2017).
Heterotrophs produce their own EPS, including polysaccharides that are often rich in uronic acids and protein-rich EPS, and the protein-to-carbohydrate ratio of bacterial EPS varies from 0.04 to 20 (see Santschi et al., 2020). Moreover, heterotrophs can influence the EPS quantity/type synthesized by proximal phytoplankton (see Quigg et al., 2020). The EPS produced by phototrophs can be used as a source of carbon and energy by heterotrophs (Abed, 2019), which release and return nutrients (e.g., N, P, and trace metals) from the EPS to the phototroph in a somewhat mutualistic system (Christie-Oleza et al., 2017). Importantly, both MS and MOS formation appears to be enhanced by these bacterial-phytoplankton interactions (Fu et al., 2014; van Eenennaam et al., 2016; Cruz and Neuer, 2019). For example, van Eenennaam et al. (2016) showed that more EPS was released by bacteria associated with phytoplankton (Dunaliella tertiolecta and Phaeodactylum tricornutum) compared to bacteria-free phytoplankton (which did not produce any EPS) or the phytoplankton-associated bacteria grown on their own (up to 2-fold less). In addition to a greater quantity of EPS being produced due to these interactions, the chemical composition was also different. The EPS produced by the bacteria associated with phytoplankton had a higher protein-to-carbohydrate ratio, which may alter how EPS aggregates and interacts with oil (Bacosa et al., 2018; Santschi et al., 2020), e.g., by influencing: (1) hydrophobicity (Xu et al., 2011); (2) surface tension (Schwehr et al., 2018); (3) light-induced chemical crosslinking (Sun et al., 2017, 2018, 2019); and, overall, affecting MS/MOS sinking rates (Xu et al., 2018a,b).
Therefore, when we talk about TEP derived from a phytoplankton bloom, the TEP will actually come from an array of microbial species—phototrophs (e.g., phytoplankton) and heterotrophs (e.g., bacteria)—plus bacteria from the gut contents of grazing microzooplankton (Turner, 2015), some of which may include ingested oil (Almeda et al., 2016). Moreover, the rapid turnover of phytoplankton and associated heterotrophic Bacteria and Archaea during blooms (Needham and Fuhrman, 2016) will add to the structural variety of TEP. Different EPS/TEP-degrading microbes will colonize sinking MS/MOS flocs (Busch et al., 2017; Duret et al., 2019), which are additionally grazed by animals (Giering et al., 2014; Figure 1), leading to structural, chemical, and biological succession during descent, and potentially altering sinking velocity (Turner, 2015; Dang and Lovell, 2016). Clearly, the presence of weathered crude oil as a substrate in the MS adds an extra microbial dimension.
Which Hydrocarbon-Degrading Microorganisms Are Associated With MOS?
General Considerations of Microbial Community Analysis of MOS
The analytical work discussed above has demonstrated that the oil residues from the MC-252 well blowout in the sediments of the Gulf of Mexico are highly degraded, and at least some arrived in association with marine snow. Here we discuss the microbial communities associated with MOS, followed by an appraisal of the main taxa found, and their potential roles in MOS formation and oil degradation. The capacity to degrade hydrocarbons is found in more than 300 genera of Bacteria (Prince et al., 2018) as well as in numerous fungal taxa (Prince, 2018) and in some Archaea (Oren, 2017; Prince et al., 2018). However, most investigations into MOS have focused on Bacteria, as, after viruses, they are the most abundant microbes in the surface ocean, are often associated with phytoplankton and typically dominate hydrocarbon degradation in the marine environment (McGenity et al., 2012; Gutierrez, 2019; Yakimov et al., 2019). We note that most of the experiments focusing on MOS have used concentrations of dispersed oil and dispersant that are substantially (sometimes >100-fold) higher than found in the marine water column after a spill (Bejarano et al., 2014; Wade et al., 2016), which may have resulted in toxic responses from some organisms as well as unnaturally high activity from others.
Some studies assessing hydrocarbon degradation have assigned this function to particular taxa by testing isolates, applying DNA-stable isotope probing (DNA-SIP) with 13C-labeled hydrocarbons (Gutierrez et al., 2013b), or drawing inferences from metatranscriptomics, metagenome-assembled genomes and single-cell amplified genomes (Mason et al., 2012), but the majority of these studies have been on bulk water samples rather than specifically focussing on MOS. In many cases the identification of a taxon's capacity for hydrocarbon degradation is based on an increase in relative abundance of their 16S rRNA gene sequences in oiled samples vs. non-oiled controls, as well as an understanding of taxon-trait relationships. Therefore, we briefly assess the degree to which phylogenetic data can be used to infer the function of particular microbial taxa. Some genera contain species that can degrade hydrocarbons and others that cannot (e.g., Pseudomonas and Marinobacter; Duran, 2010; Palleroni et al., 2010), and so an increase in abundance in oil samples may be a direct or indirect effect of the oil, requiring complementary techniques to understand their role. In this respect, Netzer et al. (2018) provide a relevant example in the microbial community succession in MOS formed in cold (4–5°C) seawater microcosms additionally containing the obligately psychrophilic diatom Fragilariopsis cylindrus and 30 ppm of chemically dispersed oil (30 ppm oil and 0.3 ppm Corexit 9500). Degradation of hydrocarbons was similar with and without diatoms, but Nonlabens, a genus within the Bacteroidetes, was dominant in the diatom-containing aggregates, and thus proposed to be a hydrocarbon degrader. However, Nonlabens, clearly co-habiting with the diatom culture, was also dominant on aggregates formed in diatom-containing microcosms without hydrocarbons, and so its role needs further investigation (Netzer et al., 2018). These bacterial communities differed significantly from those in diatom-free oiled samples that were dominated by Oleispira, a psychrophilic alkane degrader (Yakimov et al., 2003), and members of the family Sphingomonadaceae (Netzer et al., 2018). Whilst there is no evidence for hydrocarbon utilization by most of the seawater-derived strains of Sphingomonadaceae, marine sediments have yielded isolates from this family that degrade polycyclic aromatic hydrocarbons (PAHs) (Gilewicz et al., 1997; Johnson and Hill, 2003; Kertesz et al., 2019). Thus, in this example, other approaches (e.g., cultivation or DNA-SIP) are needed to confirm hydrocarbon degradation by the identified Sphingomonadaceae and Nonlabens. By contrast, we can have much more confidence that Oleispira was degrading alkanes as they belong to a group of marine bacteria that use hydrocarbons almost exclusively as a source of carbon and energy, known as obligate hydrocarbonoclastic bacteria (OHCB). These microbes are present in non-polluted marine environments in low numbers, but often bloom and dominate the microbial community following a spill (McKew et al., 2007a,b; Yakimov et al., 2007; Teramoto et al., 2009; Vila et al., 2010). Although some species designated as OHCB will use small organic acids as a source of carbon and energy, the word “obligate” is understood in the context that OHCB cannot compete with generalists in an environment lacking hydrocarbons. OHCB include genera with species that degrade aliphatic (Alcanivorax, Oleibacter, Oleiphilus, Oleispira, Thalassolituus) and aromatic (Cycloclasticus, Neptunomonas) hydrocarbons (McGenity et al., 2012; Gutierrez, 2017). There are also obligate (as well as some facultative) methane-oxidizing bacteria that are termed methanotrophs (Kalyuzhnaya et al., 2019).
Baelum et al. (2012) found that cells aggregated to form bacterial-oil aggregates during incubation of enrichment cultures consisting of uncontaminated seawater from the Gulf of Mexico, 100 ppm of MC-252 oil and 60 ppm of the dispersant Corexit 9500. The aggregates were dominated by members of the family Colwelliaceae, which is discussed in detail later. Methylococcaceae also increased in abundance from <1% at 10 days to 16% at 40 days. Methylococcaceae are methanotrophs, meaning they can metabolize only methane or methanol as carbon and energy sources. An increase in the abundance of Methylococcaceae following the MC-252 spill was not surprising given the unprecedented amount of methane that entered the water column (~250,000 tons) (Rogener et al., 2018). However, the increase of Methylococcaceae in experimentally formed aggregates (Baelum et al., 2012) was surprising, as there was no obvious source of methane or methanol. The presence of these methanotrophs may suggest the existence of anoxic microenvironments in the aggregates that could allow growth of methanogenic Archaea, and although Baelum et al. (2012) adapted bacterial primers to improve archaeal detection, it is not clear whether they would have amplified methanogens. Suboxic and anoxic conditions develop as a consequence of oxygen consumption during aerobic degradation of MS organic matter and insufficient oxygen diffusion into flocs (Ploug et al., 1997; Rath et al., 1998; Vojvoda et al., 2014). Reduced levels of dissolved oxygen enhance the use of alternative electron acceptors for anaerobic respiration by microorganisms (Torres-Beltrán et al., 2016) and can provide a favorable environment for methanogens (Shanks and Reeder, 1993; Vojvoda et al., 2014). The methane produced would diffuse to oxic zones and serve as a source of carbon and energy for methanotrophs.
Thus, there are numerous microbes implicated in MOS formation, oil emulsification/degradation and other activities induced indirectly by their existence on an oil-containing floc. Below, we discuss the main genera of heterotrophic bacteria involved in the formation and fate of MOS.
Role of the Generalist Genera Alteromonas, Pseudoalteromonas and Halomonas in the Formation and Fate of MOS
These genera are cosmopolitan marine aerobic generalists capable of consuming a wide range of organic compounds, including, in several species, hydrocarbons. Their potential involvement in hydrocarbon degradation tends to be overlooked in sequencing surveys, as their increased abundance relative to controls is not enough to suggest a direct role in degrading oil-derived hydrocarbons in that environment (Gutierrez et al., 2013a,b, 2018; Gutierrez, 2017; Gutierrez and Kleindienst, 2020).
Nevertheless, Alteromonas, Pseudoalteromonas, and Halomonas constitute a significant proportion of hydrocarbon-degrading communities in oil-contaminated marine environments. For example, Alteromonas and Pseudoalteromonas were enriched from surface waters and water-column samples collected following the MC-252 spill (Hazen et al., 2010; Valentine et al., 2010; Yang et al., 2016a). Halomonas was regularly detected in sequencing surveys of surface oil samples collected 1 month after the MC-252 spill (Gutierrez et al., 2013b) and water-column samples collected after 6 months (Yang et al., 2016a). The ability of strains within these genera to use hydrocarbons as growth substrates was confirmed with isolations of Alteromonas strain TK-46(2), Pseudoalteromonas TK-105 and three Halomonas strains (GOS-2, GOS-3a, and TGOS-10) from samples collected in the Gulf of Mexico (Gutierrez et al., 2013b, 2018; Bacosa et al., 2018). In addition, the similarity and co-location of these strains to those enriched in surface oil slicks suggests that they made a major contribution to the degradation of Macondo oil (Hazen et al., 2010; Valentine et al., 2010; Gutierrez et al., 2013b, 2018). There are also many other species within these genera that degrade hydrocarbons. For example, Alteromonas napthalenivorans was responsible for the in-situ biodegradation of multiple PAHs in oil-contaminated tidal-flat sediment from the Yellow Sea (Jin et al., 2012; Math et al., 2012). Pseudoalteromonas was enriched in ship-board mesocosms containing North Sea water and crude oil, and many isolates were able to grow on PAHs, and branched and straight-chain alkanes (Chronopoulou et al., 2015). Halomonas species degrade a variety of hydrocarbons including monoaromatics (Garcia et al., 2004; Abdelkafi et al., 2006), polycyclic aromatics (Melcher et al., 2002; Yang et al., 2010) and aliphatics (Pepi et al., 2005; Mnif et al., 2009).
Sequencing surveys demonstrated an increase in the relative abundance of Alteromonas, Pseudoalteromonas, and Halomonas in MOS particles compared to the surrounding seawater (Arnosti et al., 2016; Suja et al., 2017). Moreover, an Alteromonas strain isolated from a MOS particle that formed in roller-bottle incubations with sea surface water collected during the active phase of the MC-252 spill, matched the aforementioned hydrocarbon-degrading strain Alteromonas TK-46(2) with 100% 16S rRNA sequence identity (Gutierrez et al., 2018). Fluorescence in-situ hybridization assays also detected Halomonas in bacterial-oil aggregates generated in the laboratory in alkane enrichments from deep plume water (McKay et al., 2016).
Roller-bottle experiments with Alteromonas strain TK-46(2) (Gutierrez et al., 2018) and Halomonas strain TGOS-10 (Gutierrez et al., 2013a) formed MOS even when cells had been inactivated by sodium azide, suggesting that the cell surface and/or their EPS, play a role in MOS formation/colonization. Epifluorescence microscopy revealed the attachment of Alteromonas and Halomonas cells to the MOS particles. The EPS produced by these bacteria enhance aggregation of particles to form MS or MOS (Passow et al., 2012; Ziervogel et al., 2012; Gutierrez et al., 2013a), and can exhibit amphiphilic properties, leading to emulsification or dispersion of hydrophobic oil constituents, making them more amenable to biodegradation (Gutierrez et al., 2007, 2008, 2018).
Colwelliaceae
Numerous amplicon and metagenomic sequencing studies from the MC-252 spill (and others) have shown an increased relative abundance of Colwelliaceae in the presence of crude oil (Brakstad et al., 2015b; Kleindienst et al., 2015b; Campeão et al., 2017; Hu et al., 2017; Bacosa et al., 2018; Lofthus et al., 2018; Ribicic et al., 2018). Following the initial enrichment of Oceanospirillales (May 2010) there was a shift in dominance to Colwellia (until August 2010) (Redmond and Valentine, 2012). Furthermore, Mason et al. (2014) suggested that the high abundance of Colwellia in the sediments below the oil plume was due to its transport by MOS. Many species of Colwellia are psychrophilic and/or piezophilic (Bowman, 2014; Liu et al., 2020), and one strain, RC25 was able to degrade 75% of MC-252 oil at 5°C (Baelum et al., 2012). While some of this loss can be attributed to evaporation, Colwellia species are probably important hydrocarbon degraders in deep-sea MOS (e.g., MC-252 at 4 to 6°C and 15 MPa). As an aside, phylogenetic analysis of strain RC25 (Supplementary Figure 1) reveals that it clusters within Colwelliaceae Clade 1, which according to Liu et al. (2020) should be renamed as Cognaticolwellia.
Mesocosm experiments with deep-sea (1,100–1,240 m depth) Gulf of Mexico bacterial communities have indicated that Corexit-9500 components provide additional carbon sources for bacteria, leading to an increase in the relative abundance of Colwelliaceae (e.g., Kleindienst et al., 2015b; Techtmann et al., 2017). Colwellia strain RC25, for example, degraded dioctyl sodium sulfosuccinate, a surfactant component of Corexit 9500 (Chakraborty et al., 2012), which in part explains its high abundance on MOS in the study by Baelum et al. (2012). In other studies, Colwelliaceae made up <1% of the community in MOS particles formed at subarctic conditions (Suja et al., 2017) and was detected at low abundances (max. 6%) in oil-related aggregates formed in cold seawater (Netzer et al., 2018). Whilst it is difficult to compare across experiments in which more than one variable has changed, this difference might be a function of differing methodologies, which include: (1) different initial microbial communities present between the Gulf of Mexico, Faroe-Shetland Channel and Norwegian Sea; (2) direct addition of oil/dispersant vs. the use of water accommodated fractions; (3) different dispersant concentrations (Baelum et al. (2012) used 60 ppm, Suja et al. (2017) used 17.6 ppm, and Netzer et al. (2018) used 0.3 ppm), probably more so than the type of dispersant used (Corexit 9500 and Superdispersant-25); and (4) incubations remaining static or continuously mixed (Supplementary Table 1). Again, it must also be borne in mind that the concentrations of oil and dispersants used in most of these experiments were much higher than those found in the environment (Bejarano et al., 2014; Wade et al., 2016); lower concentrations might have much smaller effects.
Obligate Hydrocarbonoclastic Bacteria (OHCB)
Alcanivorax spp. are undetectable or at low abundances in pristine marine environments, but they typically bloom to make up a significant proportion of the bacterial community after an oil spill (Coulon et al., 2012; Teramoto et al., 2013), and so play an important role in the natural bioremediation of oil spills worldwide (McKew et al., 2007a,b; Yakimov et al., 2007).
Suja et al. (2017) prepared MOS from surface waters of the Faroe-Shetland Channel and 35,000 ppm dispersed oil, enriching for Alcanivorax, Alteromonas, and Pseudoalteromonas, which collectively represented 80% of the sequence reads at 2.5 weeks incubation. In a subsequent roller-bottle study using surface water from the same environment, and similar high concentrations of oil, a comparison was made between the bacterial communities associated with MOS aggregates and marine dispersant snow, a product of adding the dispersant Superdispersant-25 to seawater in the absence of oil (Suja et al., 2019). Both marine dispersant snow and MOS aggregates had similar bacterial communities dominated by Alcanivorax with a consistent but minor contribution from Oleispira, as well as a range of other genera (Suja et al., 2019). Whilst the exact formulation of commercial dispersants, such as Superdispersant-25 used by Suja et al. (2017, 2019), are proprietary, they typically consist of a mixture of solvents and surfactants. The solvent fraction is usually a paraffinic or synthetic hydrocarbon, while the surfactant fraction of at least some includes dioctyl sodium sulfosuccinate, Tween 80, Tween 85, and Span 80 (Place et al., 2010, 2016; Brakstad et al., 2018b). Some species of Alcanivorax, as many other heterotrophs, are able to grow on components of the surfactant fraction, such as Tween (Liu and Shao, 2005) and likely the solvent hydrocarbon, so the Alcanivorax on marine dispersant snow aggregates may have been metabolizing the solvent or surfactant fraction of the dispersant.
There are numerous biochemical and physiological reasons why Alcanivorax has an ecological advantage over other hydrocarbon degraders, which may explain their frequent dominance in MOS particles, including: (1) cellular hydrophobicity that allows attachment to oil (Godfrin et al., 2018); (2) production of biosurfactants to facilitate their access to hydrocarbons (Abraham et al., 1998; Yakimov et al., 1998; Qiao and Shao, 2010); (3) biosynthesis of exopolysaccharides (including alginate) and type IV pili which mediate the formation of biofilms (Schneiker et al., 2006; Sabirova et al., 2011), including associations with marine plankton (Coulon et al., 2012; Yakimov et al., 2019); (4) expression of permeases and iron-scavenging siderophores that concentrate nutrients in oligotrophic marine environments (Schneiker et al., 2006; Sabirova et al., 2011; Denaro et al., 2014; Kem et al., 2014); (5) the ability to use both branched and linear alkanes as growth substrates (Yakimov et al., 2007; Gregson et al., 2019). Nevertheless, Alcanivorax is not universally detected in studies which generated MOS, e.g., with surface (Arnosti et al., 2016) and deep-sea (Baelum et al., 2012) water from the Gulf of Mexico. Some Alcanivorax species are piezosensitive, so higher pressure in the deep-sea may have inhibited their growth (Scoma et al., 2016a,b); however other species of Alcanivorax are piezotolerant (Liu et al., 2019).
Members of the genus Oleispira are known to flourish in cold, oil-contaminated seawater (Yakimov et al., 2003, 2007; Coulon et al., 2007; Tremblay et al., 2019). Oleispira were dominant in deep-water samples following the MC-252 spill (Hazen et al., 2010; Mason et al., 2012), and on MOS formed in mesocosms using seawater from the deep-sea of the Gulf of Mexico (4.8°C; Baelum et al., 2012), the Faroe-Shetland Channel (8.7°C; Suja et al., 2017, 2019) and the Trondheim Fjord (6–8°C; Netzer et al., 2018). The type strain, Oleispira antarctica RB-8, is a psychrophilic alkane degrader with a broad growth optimum of 1–15°C (Yakimov et al., 2003). It uses multiple mechanisms to compete at low temperatures, such as changes in the flagella structure/output to overcome increased viscosity, switching of flagella rotation to accumulate cells in an environment where it can outcompete other bacteria, and proline metabolism to counteract oxidative stress (Gregson et al., 2020). These psychrophilic adaptations of Oleispira most probably explain its absence from MOS generated from 25°C surface water collected following the MC-252 spill (Arnosti et al., 2016). This single trait of temperature requirement for growth highlights the importance of understanding the succession of microbial communities, and hydrocarbon-degradation potential, in MOS that sinks from warm surface waters to cold hadal depths.
Biodegradation of PAHs in oil-contaminated marine environments is usually associated with Cycloclasticus spp. (Dyksterhouse et al., 1995; McKew et al., 2007a; Teira et al., 2007). Arnosti et al. (2016) demonstrated, using 1% floating oil, that bacterial communities on MOS particles were distinct from those in surrounding seawater and dominated by a Cycloclasticus phylotype that was nearly identical in 16S rRNA sequence to PAH-degrading Cycloclasticus strain TK8 (Gutierrez et al., 2013b) and C. pugetti (Dyksterhouse et al., 1995). This highly enriched Cycloclasticus phylotype in the MOS was distinct from Cycloclasticus found in the experimental inoculum and the water column following the MC-252 spill (Valentine et al., 2010; Redmond and Valentine, 2012; Yang et al., 2016a) [for phylogenetic analysis see Yang et al. (2016b) and Redmond and Valentine (2019)].
In the aforementioned study by Suja et al. (2017), the relative abundance of Cycloclasticus in the community after 4 weeks was 3.5-fold higher in MOS particles than in surrounding seawater, although it contributed only 1.6% of total sequence reads. Doyle et al. (2018) demonstrated that Cycloclasticus was sensitive to chemically dispersed oil at high concentrations (i.e., chemically enhanced water-accommodated fraction) but thrived in lower concentrations of physically dispersed oil. Also, given that most Cycloclasticus spp. from the identified clade are able to metabolize only aromatic hydrocarbons, in some cases they are outcompeted by aliphatic hydrocarbon degraders (e.g., Alcanivorax) for nutrients, and do not reach high levels until after the aliphatic fraction of the oil is depleted (Röling et al., 2002). There is now evidence that some Cycloclasticus phylotypes do not degrade PAHs but are capable of short-chain alkane degradation (e.g., mussel endosymbionts; Rubin-Blum et al., 2017), and that others may degrade both types of hydrocarbon (see Gutierrez et al., 2018; Redmond and Valentine, 2019), and so interpretation of the function of Cycloclasticus from 16S rRNA sequences alone requires caution.
Does the Use of Dispersant Enhance or Inhibit MOS Formation?
Dispersants lower the interfacial tension between water and oil, and so reduce the energy required to convert a slick (or plume) into small oil droplets in the water column where they undergo rapid biodegradation (Prince, 2015b; Brandvik et al., 2019). However, there is currently a debate over whether dispersants have an effect on the biodegradation of crude-oil constituents (Kleindienst et al., 2015b, 2016; Prince et al., 2016b), with conflicting reports in the literature showing it can be enhanced (Baelum et al., 2012; Techtmann et al., 2017; Tremblay et al., 2017), inhibited (Hamdan and Fulmer, 2011; Kleindienst et al., 2015a,b), or essentially unaffected (Prince et al., 2013; McFarlin et al., 2014; Louvado et al., 2019). In part this reflects a misunderstanding of the scale at which dispersants are designed to operate—they are formulated to encourage the dispersion of oil at sea that would otherwise remain as a floating slick, thereby dramatically increasing the surface area available for microbial colonization. Laboratory experiments cannot mimic this behavior at environmentally relevant concentrations unless oil is prevented from dispersing by corralling it in an appropriately scaled boom (Prince and Butler, 2014). Most of the studies listed above were conducted under conditions where the added oil dispersed regardless of the presence of dispersant, and the small stimulations and inhibitions reported are of no environmental significance. The substantial stimulation of biodegradation by dispersants (many-fold) occurs after oil goes from a floating slick to small dispersed droplets (Prince and Butler, 2014; Prince et al., 2017). Once dispersed, the different surfactants of the dispersant leave the droplets at different rates (Riehm and McCormick, 2014), and are biodegraded quite rapidly (Brakstad et al., 2018a,c; McFarlin et al., 2018).
Equally, the effect of oil on MOS formation, and any additional effects of dispersant application are a major point of contention. Some workers have found that dispersant alone generates MS (Baelum et al., 2012; van Eenennaam et al., 2016), and that adding dispersant with oil stimulates flocculation (van Eenennaam et al., 2016; Doyle et al., 2018; Bretherton et al., 2019), albeit sometimes only when extra nutrients were added (Kleindienst et al., 2015b). Wirth et al. (2018) saw larger, more stable MOS in the presence of Corexit, but other work suggests that dispersants inhibit MOS (Passow, 2016; Passow et al., 2017), while others claim they were essential for their formation (Suja et al., 2017, 2019).
A major problem seems to be that many of these studies are far-removed from the conditions found at sea. Perhaps the first thing to consider is how much dispersant and oil was available during the MC-252 oil spill response to cause an effect. Dispersants are typically applied at a nominal rate of 5 gallons (US) per acre; 47 liters per hectare or 4.7 ml/m2 (a teaspoon/m2), which when diluted into the top meter of the sea becomes a concentration of 4.7 ppm [The critical micelle concentration—the concentration above which the dispersant forms micelles—for Corexit 9527 is about 400 ppm (Singer et al., 1995)]. Such dilution is generally accepted to occur within hours, with continued dilution as time progresses (Lunel, 1995; Bejarano et al., 2013, 2014; Lee et al., 2013). Claims that “the average Corexit concentration in the water after the Deepwater Horizon spill was estimated at 0.5%” (Passow, 2016) cannot be correct.
The amount of oil in dispersed oil “plumes” is similarly dilute—immediately after dispersion the concentration will be several hundred to thousands of ppm (Bejarano et al., 2013, 2014), but this mixes and dilutes to less than a ppm within a day. The vast majority of samples collected in the vicinity of MC-252 during the spill contained only background levels of total petroleum hydrocarbons, despite the 3.19 million barrels spilled (Barbier, 2015); only 5% of the 1,372 samples had more than 250 μg of total petroleum hydrocarbons per liter (250 ppb), and only 24 had above 3 ppm (Wade et al., 2016). These amounts are in line with the concentrations of oil residue found at the bottom of the Gulf of Mexico; Stout et al. (2017) estimated that the heavily biodegraded residue of ~233,000 bbl of fresh oil was deposited on ~1,470 km2 of Gulf deep-sea sediment. This is the residue of an initial 25 ml fresh oil/m2. If 80% of the oil is GC-detectable, and the residue is 80% degraded (Stout et al., 2017), this is 6.25 g of extensively biodegraded oil residue in the top 1–3 cm per m2 in the sediment. By extension, a total of 18.75 g of hydrocarbon had been consumed in the 1 m2 × 1,500 m water column, or in the sediment after deposition. Data from sediment traps is also consistent: Stout and German (2018) estimated that the biodegraded residue of 10 barrels/km2 fell through the water column some 58 km northeast of the MC-252 site during the blowout—this is equivalent to the residue of 1.6 ml fresh oil per square meter of sediment, ~0.3 ml/m2 of weathered oil.
As pointed out by Brakstad et al. (2018b), the majority of papers claiming to provide insight into the effects of dispersants on oil degradation and on MOS used concentrations that are substantially higher, typically by several orders of magnitude. This becomes especially important when discussing whether marine snow is generated from oil and/or dispersants, or whether the surfactants in dispersants might have disruptive effects on MOS.
Another confusing aspect is the use of Water Accommodated Fractions (WAF) instead of oil-in-water dispersions. WAF were developed by toxicologists in an attempt to distinguish true chemical toxicity from physical smothering (Widdows et al., 1982; Singer et al., 2000). Essentially the oil is stirred into seawater for a defined time, allowed to “settle” (i.e., for oil droplets to coalesce and float), and then WAF is drawn off from below the slick (see Prince et al., 2016a). If dispersant is also present, the liquid is known as Chemically Enhanced Water Accommodated Fraction (CEWAF), and other abbreviations abound to describe dilutions, higher energies of mixing, etc. While such methods do provide a way of reproducibly preparing samples for toxicity testing, say for comparing different species, their composition is specific to the precise conditions used; WAF and CEWAF are indeed “accommodated” fractions, including both truly soluble compounds and tiny droplets. The concentration of the latter depends on the mixing energy and the “settling time,” which in turn defines how much of a reservoir the drops provide for resupplying soluble compounds should they be consumed from the aqueous phase by biodegradation or evaporation. WAF with nominally similar concentrations have significantly different concentrations of soluble compounds if they are made directly at the nominal concentration, or by dilution from a more concentrated “stock” (Prince et al., 2016a), and one cannot readily predict the chemical composition of either. In contrast, while oil-in-water dispersions may have variable droplet sizes depending on the energy used to generate the dispersion, the chemical composition of what is in the vessel is reproducible anywhere, and is readily calculated. Another major confusion of such work is that CEWAF have much more hydrocarbon per volume than WAF; without dilution to equivalent oil concentrations it is impossible to disentangle any effects of the presence of dispersants from the presence of more oil. Nevertheless, few papers address this issue, and assume any effects are due to the dispersants rather than the extra oil.
The widespread use of WAF/CEWAF in MOS experiments may be due to the fact that crude oil adheres to the walls and lids of the roller bottles. To overcome this issue several modifications can be made to the experimental setup, including pre-treatment of glassware (Brakstad et al., 2015a), use of hydrophobic seals/lids (Prince, 2015a), and slower rotation (Brakstad et al., 2015a; Netzer et al., 2018; Henry et al., 2020, 2021).
Clearly there needs to be a concerted effort to make sense of the disparate conclusions described above. All of the papers claim to be aimed at understanding what happens to spilled oil after a major oil spill, but few are done at environmentally relevant conditions. While there is much to be learned from careful studies with well-characterized and appropriate microbial isolates, the diversity already revealed (e.g., Passow et al., 2019) indicates that extrapolating to the real world remains premature.
Understanding MOS Formation and Behavior Beyond the Gulf of Mexico
Investigations into MOS formation/deposition and the microbial communities associated with these aggregates have focused on the Gulf of Mexico with only a few studies in other areas (Suja et al., 2017, 2019; Netzer et al., 2018). To be of value to oil spill response in future spills, studies need to include other locations with environmental features that may influence MOS processes, such as areas with both deep-sea oil exploration/production and periods with high biological productivity and/or inorganic matter abundance. Overlaid maps of these features or their proxies (oil platforms, chlorophyll, and suspended mineral concentration) will help to predict locations where marine oil snow sedimentation and flocculent accumulation may occur (Murk et al., 2020), even as we are still unclear whether we can limit or stimulate MOS formation. Many oil refineries operate on estuaries, and gradients for MOS formation are expected with distance from river systems as they supply nutrients/clay to promote aggregation (Figure 2; Daly et al., 2016; Quigg et al., 2020). The Arctic region has been predicted to be extremely sensitive to MOS formation (Murk et al., 2020), and the environmental conditions, especially temperature, light and the presence of ice, will be very different from the Gulf of Mexico. Dispersants may be the only possible oil-spill response option in many cases (Lewis and Prince, 2018). Psychrophilic hydrocarbon degraders are well-characterized (Brakstad et al., 2015b; Gregson et al., 2020), and although overall biodegradation of oil components may be a little slower in incubations with polar compared with temperate seawater (McFarlin et al., 2014; Garneau et al., 2016; Brakstad et al., 2018a,c), this may reflect the particularly pristine waters that were used in these studies, and the lower natural abundance of oil-degrading microbes. In support of this, Coulon et al. (2007) showed that the oil-degradation rate in North Sea microcosms at 4°C was only half that at 20°C. Slower degradation would lead to increased exposure of the (micro)biota to oil, but dilution of dispersed oil would still dominate physical processes, and Arctic species are no more sensitive to oil toxicity than temperate ones (Bejarano et al., 2017).
Further Experimental Considerations
We have highlighted the overarching need to employ environmentally relevant oil and dispersant concentrations/ratios for experiments to be useful in modeling the fate of oil in the sea. Oil concentrations of 0.3 to 3 ppm should be sufficient for MOS formation, while also limiting depletion of autochthonous nutrients and oxygen. Oil in sealed roller bottles should be at least partially weathered to allow benzene and other small hydrocarbons to evaporate, as they do in the field. Also, where model microbes are used in MOS studies they must be relevant marine species, and not as occasionally seen, surrogates from terrestrial or freshwater environments. After all, no-one interested in understanding apex predators on the savannah of southern Africa would think that the best approach would be to study Polar Bears, yet several papers use terrestrial and freshwater microbes as surrogates for marine species. Here we outline a number of other important factors that must be born in mind when studying MOS.
Light
Light plays a central role in MS formation by stimulating the growth of phototrophs and thus their exudates, but it also has a photochemical role by cross-linking EPS (Sun et al., 2017, 2018, 2019), and photooxidizing hydrocarbons (Prince et al., 2003; Bacosa et al., 2015a; Aeppli et al., 2018). Passow (2016) revealed that photooxidation of oil over a period of 3 weeks promoted MS formation, but the particles were smaller and more compact than MOS that accumulated at the sea surface after the MC-252 spill (diameters of 2.5 vs. 7 mm, respectively). Presumably oil's interaction with marine snow depends on how the photooxidation changes specific oil compounds. Insoluble compounds (e.g., high molecular weight n-alkanes and PAHs) are incorporated into MOS within entire oil droplets, whereas more soluble compounds (e.g., low molecular weight n-alkanes (<C7) and simple aromatics) might be sorbed by cells in the MOS (Wirth et al., 2018).
Light intensity, spectrum, and photoperiod all have effects on photosynthesis, phytoplankton communities, and the metabolites produced (Schuback and Tortell, 2019), which lead to changes in the associated heterotrophic communities (Bacosa et al., 2015b). For example, incubations with sunlight (compared with dark incubations) greatly reduced the abundance of cyanobacteria, such as Synechocococcus and Prochlorococcus, in seawater samples containing oil (200 ppm) or dispersant (10 ppm; Bacosa et al., 2015b). However, there was no analysis of eukaryotic phototrophs in this study, and so we do not know how the overall phytoplankton community responded. In the presence of crude oil (+/– dispersant) and sunlight, Alteromonas and Marinobacter were among the most enriched genera, and had much higher relative abundance than in the equivalent dark bottles (Bacosa et al., 2015b), suggesting an indirect effect of light, perhaps as a consequence of the death of cyanobacteria providing organic matter for the metabolically versatile copiotroph, Alteromonas (Pedler et al., 2014) Alternatively (or additionally) the growth of eukaryotic phytoplankton that potentially thrived in the absence of cyanobacteria may have changed the composition of released organic matter in favor of this genus. The microbial communities formed by different treatments (light vs. dark) may have contributed to the dissimilar size and structure of MOS particles in Passow (2016), but since community composition of the MOS particles was not determined, this cannot be addressed.
Thus, future MOS studies need to mimic natural light conditions, including light intensity, spectral composition, and light-dark cycles, in order to assess the aforementioned direct and indirect influences on MOS formation and fate. Due consideration must be shown to the light intensity used—it is very difficult to mimic noon sunlight in the laboratory, and different glass bottles absorb different fractions of the UV component of sunlight, so it may only be possible to mimic some depth into the natural photic zone in the laboratory (Ryther, 1956).
Aeration
The natural concentrations of MOS and dispersed oil in the water column are so low that aerobic seawater will remain oxygenated during bottle experiments at environmentally relevant levels. Surface seawater typically contains 200 μM dissolved oxygen, and although there is usually an oxygen minimum of about 120 μM oxygen around 500 m depth, most ocean waters are aerobic throughout (e.g., Camilli et al., 2010, but see Paulmier and Ruiz-Pino, 2009 for a discussion of truly low-oxygen water). This is enough oxygen for the complete oxidation of several ppm of oil, so sealed bottles with only a few ppm of oil will remain aerobic even in the dark. Incubation in the light would allow phototrophs to generate even more oxygen, for it is well-known that the surface layers of the ocean are supersaturated with oxygen during the day (Craig and Hayward, 1987). It is noteworthy that the initial discovery of the subsea plume of dispersed oil following the MC-252 spill included the detection of local consumption (10%) of the indigenous oxygen as microbes degraded the hydrocarbons (Hazen et al., 2010), but the plume was far from anoxic.
Ocean sediments are typically anoxic within a centimeter or two of the sediment surface even though the water itself contains typically >100 μM oxygen (Reimers et al., 1986). [The oligotrophic deep-sea sediments are an exception (Fischer et al., 2009) but are also unlikely to be impacted by oil.] Experiments aiming to study MOS and its effects once it lands on the sediment should ensure that such conditions exist in the mesocosm before adding MOS.
Temperature and Pressure
The impacts of temperature on hydrocarbon bioavailability, degradation rates and microbial communities are well-established (e.g., Coulon et al., 2007), and so in-situ temperatures are usually replicated in MOS experiments. However, there are few studies that apply a decreasing temperature regime to mimic the sinking of particles from warm surface waters to colder hadal depths. Increases in pressure can reduce the rate of hydrocarbon biodegradation (Prince et al., 2016c) and also alter microbial communities, e.g., selecting against piezo-sensitive microbes, such as some Alcanivorax (Scoma et al., 2016a,b). Although high pressure can be simulated in the laboratory, it is less straightforward to effect a gradual pressure change that would allow piezotolerant microbes time to acclimate. Moreover, in the marine environment MOS sinks into new waters, where particle association is the normal mode of existence for microbes (Zhao et al., 2020) and where they are adapted to low temperature and high pressure. Thus, new microbes would attach to the MOS during its decent, a phenomenon that would be challenging to replicate experimentally.
Nutrients
Numerous papers have shown that the natural levels of nutrients in seawater are enough for the rapid biodegradation of ppm levels of oil (Prince et al., 2013, 2017; McFarlin et al., 2014; Brakstad et al., 2015a, 2018a; Wang et al., 2016). Furthermore, regulators are unlikely to allow the addition of nutrients to the open ocean for fear of eutrophication (Altieri and Diaz, 2019). Depending on the environment under investigation, experiments intending to inform oil-spill response measures should either not add nutrients or should supply them continuously at in-situ concentrations in order to mimic replenishment in the natural environment.
Oil and Dispersant Type
The majority of studies investigating MOS have focused on environmental effects (e.g., the presence of POM, the proximity of river outflow providing clay minerals and phytoplankton biomass, and the presence of EPS-producing biota), while the influence of oil and dispersant type has not received much attention.
Most MOS studies have used either oil collected directly from MC-252 (Macondo oil) or closely related surrogates that are similarly light and high in volatile hydrocarbons (Hemmer et al., 2011; Wade et al., 2017), but other spilled oils can have significantly different compositions and densities. For example, the Bunker B fuel oil spilt from the Tsesis tanker in the brackish (6%0) Baltic was a heavy (though lighter than water), viscous, refined fluid, containing low levels of volatile hydrocarbons (Boehm et al., 1982). We have no knowledge of whether such a spill might lead to MOS. Passow et al. (2019) compared Macondo surrogate oil with a heavy California oil, and found that the latter led to a greater reduction in diatom abundance, which they predicted would negatively impact MOS formation, but the mechanisms by which this would occur are obscure. Oils are very complex mixtures, and some characterization of the oil, focusing on the progress of its biodegradation, should be part of all experiments.
By far the most studied dispersant is Corexit 9500 which was used on the surface and injected at the well-head during the MC-252 blowout (Atlas and Hazen, 2011). Other approved dispersants stockpiled in large quantities around the world include Finasol OSR 52 (Total Fluids, France) and SlickGone NS (Dasic International Ltd, UK) (Carter-Groves, 2014). Korea used HiClean II in the response to the Hebei Spirit spill (Jung et al., 2009), and Superdispersant-25 is licensed in the UK (Scarlett et al., 2005). The chemical composition of Corexit 9500 is known (Riehm and McCormick, 2014; Place et al., 2016), whilst limited information is available on the material safety data sheets for the others. It would certainly be interesting to see whether the different dispersants have different effects, with and without different oils, on microbial communities at environmentally-relevant concentrations. For example, several studies have shown an increase in the abundance of Colwellia in experimental incubations containing only Corexit 9500 (Kleindienst et al., 2015b; Techtmann et al., 2017; McFarlin et al., 2018), likely because they are degrading the Tween surfactant (Bowman, 2014) and the hydrocarbon solvent.
Concluding Remarks and Recommendations
MOS research abounds with controversies, mostly because of extrapolations to the marine environment from experiments employing unrealistic conditions, particularly oil/dispersant concentrations/ratios. Despite the difficulties in both capturing and investigating patchily distributed MOS in the deep sea and mimicking in-situ conditions experimentally, our understanding of MOS formation and fate has advanced over the last decade in terms of the microbes present and their potential roles in the formation of MOS and the fate of hydrocarbons and dispersants. Nevertheless, an overarching concern for practitioners is whether particular oil-spill responses, such as dispersant application, will enhance or inhibit MOS formation (Figure 2). Dispersants can reduce the overall stickiness of particles through either destabilization of TEP precursors (e.g., microgels; Chiu et al., 2017) or direct dispersal of TEP (Passow et al., 2017). This means that particles may collide but not stick together. However, dispersants also cause microbes to alter both EPS abundance and composition (e.g., protein-carbohydrate ratio; Xu et al., 2018a,b, 2019). Protein-rich/hydrophobic EPS shows more resistance to dispersion and can facilitate MOS formation due to their high stickiness (Chiu et al., 2019; Santschi et al., 2020; Shiu et al., 2020). Therefore, MOS formation appears to be dependent on the relative strength of opposing mechanisms (e.g., microgel destabilization and TEP dispersion vs. changes in EPS release; Figure 2). However, there is currently insufficient evidence that results from these laboratory incubations will be replicated in the field.
Oil spill responders would benefit from models of the fate of hydrocarbons, from the formation of MOS to sediment deposition (Passow et al., 2019; Daly et al., 2020; Quigg et al., 2020), accounting for factors that influence MOS sinking rate, such as the degree of hydrocarbon degradation and loss. Due consideration should be given to how oil degradation will change while sinking, given that: (1) less structurally complex hydrocarbons will be degraded rapidly first (rapid degradation earlier); (2) colonization, growth and surfactant production by hydrocarbon-degrading microbes will take time (rapid degradation later); (3) temperatures are higher at the surface (rapid degradation earlier); (4) nutrient concentrations are generally higher at depth (rapid degradation later). More unpredictable features include: (1) the effects of photic processes on the alteration of hydrocarbons that could enhance their loss or diminish their biodegradability; (2) the poorly studied impact of pressure on hydrocarbon biodegradation; (3) changes in the buoyancy of MOS (e.g., due to change in hydrocarbon composition; de-novo EPS production as well as its consumption); (4) feeding on, and disaggregation of, MOS.
A more fundamental understanding of the interacting forces that influence MOS sinking rates and hydrocarbon biodegradation will come from applying novel techniques. For example, Fourier transform ion cyclotron resonance mass spectrometry (FTICR-MS; Wozniak et al., 2019) and solid-state 13C nuclear magnetic resonance (NMR; Hatcher et al., 2018) allow detailed analysis of polar species, hydrocarbons and their oxidation products. Synchrotron radiation-based Fourier transform infrared (SR-FTIR) spectromicroscopy is particularly valuable in illustrating the spatial organization in MOS of microorganisms, biopolymers, oil, and its degradation products (Baelum et al., 2012). Although many studies have described the bulk microbial composition of MOS, more analysis of the active microbial component is needed (e.g., via metatranscriptomics and metaproteomics). Also, new methods are being developed for studying MS that could be applied to MOS, which involve capturing (Duret et al., 2019), preserving, embedding and imaging particles to investigate the spatial distribution of microbes and polymers (Flintrop et al., 2018; Rogge et al., 2018), and even allowing single-cell functional analysis by nanoscale secondary ion mass spectrometry (nano-SIMS; Rogge et al., 2018). However, such analyses must be coupled with realistic experiments and/or in-situ investigations if they are to aid oil-spill response.
It remains to be seen how important MOS is in the overall fate of spilled oil in light of the roles of adhesion to mineral and biological particles, and the simple loss of buoyancy as oil droplets degrade. It is clear that dispersed oil degrades promptly at sea, while undispersed and weathered oil can reach the shoreline (for example as a black tide or as tar balls), where the ecological impacts can be prolonged and the clean-up effort is often expensive and time consuming. This alternate fate must be borne in mind when addressing the environmental effects of sunken dilute degraded oil.
Statements
Author contributions
TM, BM, and RH conceived the review. BG wrote the first draft and produced the figures. TM, TN, and RP wrote sections. All authors contributed to the writing and structure of the review.
Acknowledgments
We would like to thank the ESRC for funding this research through the University of Essex Impact Acceleration Account (grant number ES/M500537/1) and the Natural Environment Research Council (NE/R016569/1). The research was supported by the Eastern Academic Research Consortium (Eastern ARC).
Conflict of interest
RH is employed by the company Oil Spill Response Limited. TN is employed by ExxonMobil Upstream Research Company. RP is retired from ExxonMobil, and received no payments for this work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.619484/full#supplementary-material
Supplementary Figure 1Phylogenetic tree based on 16S rRNA sequences for different species of the family Colwelliaceae. The 16S rRNA sequence of Colwellia RC25 which was isolated from marine oil snow (MOS) particles in Baelum et al. (2012) is highlighted in bold. The evolutionary history was inferred using the Neighbor-Joining method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Maximum Composite Likelihood method and are in the units of the number of base substitutions per site. Evolutionary analyses were conducted in MEGA X.
Supplementary Table 1Details of conditions used in experiments on Marine Oil Snow (MOS) and Marine Dispersant Snow (MDS) formation.
- CEWAF
Chemically Enhanced Water Accommodated Fraction
- DOM
Dissolved Organic Matter
- EPS
Extracellular Polymeric Substances
- MC-252
Mississippi Canyon Block 252
- MOS
Marine Oil Snow
- MS
Marine Snow
- OHCB
Obligate Hydrocarbonoclastic Bacteria
- POM
Particulate Organic Matter
- PAH
Polycyclic Aromatic Hydrocarbons
- TEP
Transparent Exopolymer Particles
- WAF
Water Accommodated Fraction.
Abbreviations
References
1
Abdelkafi S. Sayadi S. Ali Gam Z. Ben Casalot L. Labat M. (2006). Bioconversion of ferulic acid to vanillic acid by Halomonas elongata isolated from table-olive fermentation. FEMS Microbiol. Lett. 262, 115–120. 10.1111/j.1574-6968.2006.00381.x
2
Abed R. M. M. (2019). Phototroph-heterotroph oil-degrading partnerships, in Microbial Communities Utilizing Hydrocarbons and Lipids: Members, Metagenomics and Ecophysiology, ed T. J. McGenity (Cham: Springer), 1–14. 10.1007/978-3-319-60063-5_15-1
3
Abraham W. R. Meyer H. Yakimov M. (1998). Novel glycine containing glucolipids from the alkane using bacterium Alcanivorax borkumensis. Biochim. Biophys. Acta1393, 57–62. 10.1016/s0005-2760(98)00058-7
4
Aeppli C. Swarthout R. F. O'Neil G. W. Katz S. D. Nabi D. Ward C. P. et al . (2018). How persistent and bioavailable are oxygenated Deepwater Horizon oil transformation products?Environ. Sci. Technol. 52, 7250–7258. 10.1021/acs.est.8b01001
5
Alldredge A. L. Passow U. Logan B. E. (1993). The abundance and significance of a class of large, transparent organic particles in the ocean. Deep Sea Res. Part I Oceanogr. Res. Pap. 40, 1131–1140. 10.1016/0967-0637(93)90129-Q
6
Alldredge A. L. Silver M. W. (1988). Characteristics, dynamics and significance of marine snow. Prog. Oceanogr. 20, 41–82. 10.1016/0079-6611(88)90053-5
7
Almeda R. Connelly T. L. Buskey E. J. (2016). How much crude oil can zooplankton ingest? Estimating the quantity of dispersed crude oil defecated by planktonic copepods. Environ. Pollut. 208, 645–654. 10.1016/J.ENVPOL.2015.10.041
8
Almeda R. Wambaugh Z. Chai C. Wang Z. Liu Z. Buskey E. J. (2013a). Effects of crude oil exposure on bioaccumulation of polycyclic aromatic hydrocarbons and survival of adult and larval stages of gelatinous zooplankton. PLoS ONE8:e74476. 10.1371/journal.pone.0074476
9
Almeda R. Wambaugh Z. Wang Z. Hyatt C. Liu Z. Buskey E. J. (2013b). Interactions between zooplankton and crude oil: toxic effects and bioaccumulation of polycyclic aromatic hydrocarbons. PLoS ONE8:e67212. 10.1371/journal.pone.0067212
10
Altieri A. Diaz D. J. (2019). Dead zones: oxygen depletion in coastal ecosystems, in World Seas: An Environmental Evaluation Volume III: Ecological Issues and Environmental Impacts. eds SheppardC. (Academic Press), 453–473. 10.1016/B978-0-12-805052-1.00021-8
11
Arnosti C. Ziervogel K. Yang T. Teske A. (2016). Oil-derived marine aggregates – hot spots of polysaccharide degradation by specialized bacterial communities. Deep Sea Res. Part II Top. Stud. Oceanogr. 129, 179–186. 10.1016/J.DSR2.2014.12.008
12
Atlas R. M. Hazen T. C. (2011). Oil biodegradation and bioremediation: a tale of the two worst spills in U.S. history. Environ. Sci. Technol. 45, 6709–6715. 10.1021/es2013227
13
Azam F. Long R. A. (2001). Sea snow microcosms. Nature414, 495–498. 10.1038/35107174
14
Bacosa H. P. Erdner D. L. Liu Z. (2015a). Differentiating the roles of photooxidation and biodegradation in the weathering of Light Louisiana Sweet crude oil in surface water from the Deepwater Horizon site. Mar. Pollut. Bull. 95, 265–272. 10.1016/J.MARPOLBUL.2015.04.005
15
Bacosa H. P. Erdner D. L. Rosenheim B. E. Shetty P. Seitz K. W. Baker B. J. et al . (2018). Hydrocarbon degradation and response of seafloor sediment bacterial community in the northern Gulf of Mexico to light Louisiana sweet crude oil. ISME J. 12, 2532–2543. 10.1038/s41396-018-0190-1
16
Bacosa H. P. Liu Z. Erdner D. L. (2015b). Natural sunlight shapes crude oil-degrading bacterial communities in Northern Gulf of Mexico surface waters. Front. Microbiol. 6:1325. 10.3389/fmicb.2015.01325
17
Baelum J. Borglin S. Chakraborty R. Fortney J. L. Lamendella R. Mason O. U. et al . (2012). Deep-sea bacteria enriched by oil and dispersant from the Deepwater Horizon spill. Environ. Microbiol. 14, 2405–2416. 10.1111/j.1462-2920.2012.02780.x
18
Bagby S. C. Reddy C. M. Aeppli C. Fisher G. B. Valentine D. L. (2017). Persistence and biodegradation of oil at the ocean floor following Deepwater Horizon. Proc. Natl. Acad. Sci. U.S.A.114, E9–E18. 10.1073/pnas.1610110114
19
Baguley J. Montagna P. Cooksey C. Hyland J. Bang H. Morrison C. et al . (2015). Community response of deep-sea soft-sediment metazoan meiofauna to the Deepwater Horizon blowout and oil spill. Mar. Ecol. Prog. Ser. 528, 127–140. 10.3354/meps11290
20
Barbier C. J. (2015). MDL - 2179 Oil Spill by the Oil Rig “Deepwater Horizon”. Available online at: http://www.laed.uscourts.gov/sites/default/files/OilSpill/Orders/1152015FindingsPhaseTwo.pdf
21
Bejarano A. C. Clark J. R. Coelho G. M. (2014). Issues and challenges with oil toxicity data and implications for their use in decision making: a quantitative review. Environ. Toxicol. Chem. 33, 732–742. 10.1002/etc.2501
22
Bejarano A. C. Gardiner W. W. Barron M. G. Word J. Q. (2017). Relative sensitivity of Arctic species to physically and chemically dispersed oil determined from three hydrocarbon measures of aquatic toxicity. Mar. Pollut. Bull. 122, 316–322. 10.1016/J.MARPOLBUL.2017.06.064
23
Bejarano A. C. Levine E. Mearns A. J. (2013). Effectiveness and potential ecological effects of offshore surface dispersant use during the Deepwater Horizon oil spill: a retrospective analysis of monitoring data. Environ. Monit. Assess. 185, 10281–10295. 10.1007/s10661-013-3332-y
24
Benner R. Amon R. M. W. (2015). The size-reactivity continuum of major bioelements in the ocean. Ann. Rev. Mar. Sci. 7, 185–205. 10.1146/annurev-marine-010213-135126
25
Bianchi T. S. Osburn C. Shields M. R. Yvon-Lewis S. Young J. Guo L. et al . (2014). Deepwater Horizon oil in Gulf of Mexico waters after 2 years: transformation into the dissolved organic matter pool. Environ. Sci. Technol. 48, 9288–9297. 10.1021/es501547b
26
Bochdansky A. B. Clouse M. A. Herndl G. J. (2017). Eukaryotic microbes, principally fungi and labyrinthulomycetes, dominate biomass on bathypelagic marine snow. ISME J. 11, 362–373. 10.1038/ismej.2016.113
27
Boehm P. D. Barak J. E. Fiest D. L. Elskus A. A. (1982). A chemical investigation of the transport and fate of petroleum hydrocarbons in littoral and benthic environments: the Tsesis oil spill. Mar. Environ. Res. 6, 157–188. 10.1016/0141-1136(82)90052-6
28
Bohórquez J. McGenity T. J. Papaspyrou S. García-Robledo E. Corzo A. Underwood G. J. C. (2017). Different types of diatom-derived extracellular polymeric substances drive changes in heterotrophic bacterial communities from intertidal sediments. Front. Microbiol. 8:245. 10.3389/fmicb.2017.00245
29
Bowman J. P. (2014). The family Colwelliaceae, in The Prokaryotes: Gammaproteobacteria, eds RosenbergE.DeLongE. F.LoryS.StackebrandtE.ThompsonF. (Berlin; Heidelberg: Springer), 179–195. 10.1007/978-3-642-38922-1_230
30
Brakstad O. G. Davies E. J. Ribicic D. Winkler A. Brönner U. Netzer R. (2018a). Biodegradation of dispersed oil in natural seawaters from Western Greenland and a Norwegian fjord. Polar Biol. 41, 2435–2450. 10.1007/s00300-018-2380-8
31
Brakstad O. G. Lewis A. Beegle-Krause C. J. (2018b). A critical review of marine snow in the context of oil spills and oil spill dispersant treatment with focus on the Deepwater Horizon oil spill. Mar. Pollut. Bull. 135, 346–356. 10.1016/J.MARPOLBUL.2018.07.028
32
Brakstad O. G. Nordtug T. Throne-Holst M. (2015a). Biodegradation of dispersed Macondo oil in seawater at low temperature and different oil droplet sizes. Mar. Pollut. Bull. 93, 144–152. 10.1016/J.MARPOLBUL.2015.02.006
33
Brakstad O. G. Størseth T. R. Brunsvik A. Bonaunet K. Faksness L.-G. (2018c). Biodegradation of oil spill dispersant surfactants in cold seawater. Chemosphere204, 290–293. 10.1016/J.CHEMOSPHERE.2018.04.051
34
Brakstad O. G. Throne-Holst M. Netzer R. Stoeckel D. M. Atlas R. M. (2015b). Microbial communities related to biodegradation of dispersed Macondo oil at low seawater temperature with Norwegian coastal seawater. Microb. Biotechnol. 8, 989–998. 10.1111/1751-7915.12303
35
Brandvik P. J. Daling P. S. Leirvik F. Krause D. F. (2019). Interfacial tension between oil and seawater as a function of dispersant dosage. Mar. Pollut. Bull. 143, 109–114. 10.1016/J.MARPOLBUL.2019.04.019
36
Bretherton L. Kamalanathan M. Genzer J. Hillhouse J. Setta S. Liang Y. et al . (2019). Response of natural phytoplankton communities exposed to crude oil and chemical dispersants during a mesocosm experiment. Aquat. Toxicol. 206, 43–53. 10.1016/J.AQUATOX.2018.11.004
37
Brooks G. R. Larson R. A. Schwing P. T. Romero I. Moore C. Reichart G.-J. et al . (2015). Sedimentation pulse in the NE Gulf of Mexico following the 2010 DWH Blowout. PLoS ONE10:e0132341. 10.1371/journal.pone.0132341
38
Buchan A. LeCleir G. R. Gulvik C. A. González J. M. (2014). Master recyclers: features and functions of bacteria associated with phytoplankton blooms. Nat. Rev. Microbiol. 12, 686–698. 10.1038/nrmicro3326
39
Burd A. B. Chanton J. P. Daly K. L. Gilbert S. Passow U. Quigg A. (2020). The science behind marine-oil snow and MOSSFA: past, present, and future. Prog. Oceanogr. 187:102398. 10.1016/J.POCEAN.2020.102398
40
Burd A. B. Jackson G. A. (2009). Particle aggregation. Annu. Rev. Mar. Sci. 1, 65–90. 10.1146/annurev.marine.010908.163904
41
Busch K. Endres S. Iversen M. H. Michels J. Nöthig E.-M. Engel A. (2017). Bacterial colonization and vertical distribution of marine gel particles (TEP and CSP) in the Arctic Fram Strait. Front. Mar. Sci. 4:166. 10.3389/fmars.2017.00166
42
Camilli R. Reddy C. M. Yoerger D. R. Van Mooy B. A. S. Jakuba M. V. Kinsey J. C. et al . (2010). Tracking hydrocarbon plume transport and biodegradation at Deepwater Horizon. Science330, 201–204. 10.1126/science.1195223
43
Campeão M. E. Reis L. Leomil L. de Oliveira L. Otsuki K. Gardinali P. et al . (2017). The deep-sea nicrobial community from the Amazonian Basin associated with oil degradation. Front. Microbiol. 8:1019. 10.3389/fmicb.2017.01019
44
Carter-Groves M. (2014). Global dispersant stockpile: part of the industry solution to worst case scenario readiness. Int. Oil Spill Conf. Proc. 2014, 504–515. 10.7901/2169-3358-2014.1.504
45
Chakraborty R. Borglin S. E. Dubinsky E. A. Andersen G. L. Hazen T. C. (2012). Microbial response to the MC-252 oil and Corexit 9500 in the Gulf of Mexico. Front. Microbiol. 3:357. 10.3389/fmicb.2012.00357
46
Chanton J. Zhao T. Rosenheim B. E. Joye S. Bosman S. Brunner C. et al . (2015). Using natural abundance radiocarbon to trace the flux of petrocarbon to the seafloor following the Deepwater Horizon oil spill. Environ. Sci. Technol. 49, 847–854. 10.1021/es5046524
47
Chin W.-C. Orellana M. V. Verdugo P. (1998). Spontaneous assembly of marine dissolved organic matter into polymer gels. Nature391, 568–572. 10.1038/35345
48
Chiu M.-H. Garcia S. G. Hwang B. Claiche D. Sanchez G. Aldayafleh R. et al . (2017). Corexit, oil and marine microgels. Mar. Pollut. Bull. 122, 376–378. 10.1016/j.marpolbul.2017.06.077
49
Chiu M.-H. Vazquez C. I. Shiu R.-F. Le C. Sanchez N. R. Kagiri A. et al . (2019). Impact of exposure of crude oil and dispersant (Corexit) on aggregation of extracellular polymeric substances. Sci. Total Environ. 657, 1535–1542. 10.1016/j.scitotenv.2018.12.147
50
Christie-Oleza J. A. Sousoni D. Lloyd M. Armengaud J. Scanlan D. J. (2017). Nutrient recycling facilitates long-term stability of marine microbial phototroph–heterotroph interactions. Nat. Microbiol. 2:17100. 10.1038/nmicrobiol.2017.100
51
Chronopoulou P.-M. Sanni G. O. Silas-Olu D. I. van der Meer J. R. Timmis K. N. Brussaard C. P. D. et al . (2015). Generalist hydrocarbon-degrading bacterial communities in the oil-polluted water column of the North Sea. Microb. Biotechnol. 8, 434–447. 10.1111/1751-7915.12176
52
Cisternas-Novoa C. Lee C. Engel A. (2015). Transparent exopolymer particles (TEP) and Coomassie stainable particles (CSP): differences between their origin and vertical distributions in the ocean. Mar. Chem. 175, 56–71. 10.1016/J.MARCHEM.2015.03.009
53
Coulon F. Chronopoulou P.-M. Fahy A. Païssé S. Goñi-Urriza M. Peperzak L. et al . (2012). Central role of dynamic tidal biofilms dominated by aerobic hydrocarbonoclastic bacteria and diatoms in the biodegradation of hydrocarbons in coastal mudflats. Appl. Environ. Microbiol. 78, 3638–3648. 10.1128/AEM.00072-12
54
Coulon F. McKew B. A. Osborn A. M. McGenity T. J. Timmis K. N. (2007). Effects of temperature and biostimulation on oil-degrading microbial communities in temperate estuarine waters. Environ. Microbiol. 9, 177–186. 10.1111/j.1462-2920.2006.01126.x
55
Craig H. Hayward T. (1987). Oxygen supersaturation in the ocean: biological versus physical contributions. Science235, 199–202. 10.1126/science.235.4785.199
56
Cruz B. N. Neuer S. (2019). Heterotrophic bacteria enhance the aggregation of the marine picocyanobacteria Prochlorococcus and Synechococcus. Front. Microbiol. 10:1864. 10.3389/fmicb.2019.01864
57
Daly K. L. Passow U. Chanton J. Hollander D. (2016). Assessing the impacts of oil-associated marine snow formation and sedimentation during and after the Deepwater Horizon oil spill. Anthropocene13, 18–33. 10.1016/J.ANCENE.2016.01.006
58
Daly K. L. Vaz A. Paris C. B. (2020). Physical processes influencing the sedimentation and lateral transport of MOSSFA in the NE Gulf of Mexico, in Scenarios and Reponses to Future Deep Oil Spills, eds MurawskiS.HollanderD.AimsworthC.ParisC. B.SchluterM.WetzerD. (New York, NY: Springer), 300–314. 10.1007/978-3-030-12963-7-18
59
Dang H. Lovell C. R. (2016). Microbial surface colonization and biofilm development in marine environments. Microbiol. Mol. Biol. Rev. 80, 91–138. 10.1128/MMBR.00037-15
60
De La Rocha C. L. Passow U. (2007). Factors influencing the sinking of POC and the efficiency of the biological carbon pump. Deep Sea Res. Part II Top. Stud. Oceanogr. 54, 639–658. 10.1016/J.DSR2.2007.01.004
61
Decho A. W. Gutierrez T. (2017). Microbial extracellular polymeric substances (EPSs) in ocean systems. Front. Microbiol. 8:922. 10.3389/fmicb.2017.00922
62
Dehmer J. (1993). Petrology and organic geochemistry of peat samples from a raised bog in Kalimantan (Borneo). Org. Geochem. 20, 349–362. 10.1016/0146-6380(93)90125-U
63
Denaro R. Crisafi F. Russo D. Genovese M. Messina E. Genovese L. et al . (2014). Alcanivorax borkumensis produces an extracellular siderophore in iron-limitation condition maintaining the hydrocarbon-degradation efficiency. Mar. Genomics17, 43–52. 10.1016/j.margen.2014.07.004
64
DeVries T. Primeau F. Deutsch C. (2012). The sequestration efficiency of the biological pump. Geophys. Res. Lett. 39:L13601. 10.1029/2012GL051963
65
Douglas G. S. Hardenstine J. H. Liu B. Uhler A. D. (2012). Laboratory and field verification of a method to estimate the extent of petroleum biodegradation in soil. Environ. Sci. Technol. 46, 8279–8287. 10.1021/es203976a
66
Doyle S. M. Whitaker E. A. De Pascuale V. Wade T. L. Knap A. H. Santschi P. H. et al . (2018). Rapid formation of microbe-oil aggregates and changes in community composition in coastal surface water following exposure to oil and the dispersant Corexit. Front. Microbiol. 9:689. 10.3389/fmicb.2018.00689
67
Duran R. (2010). Marinobacter, in Handbook of Hydrocarbon and Lipid Microbiology, eds TimmisK. N.McGenityT. J.van der MeerJ. R.de LorenzoV. (Berlin; Heidelberg: Springer), 1725–1735. 10.1007/978-3-540-77587-4_122
68
Duret M. T. Lampitt R. S. Lam P. (2019). Prokaryotic niche partitioning between suspended and sinking marine particles. Environ. Microbiol. Rep. 11, 386–400. 10.1111/1758-2229.12692
69
Dyksterhouse S. E. Gray J. P. Herwig R. P. Lara J. C. Staley J. T. (1995). Cycloclasticus pugetii gen. nov., sp. nov., an aromatic hydrocarbon-degrading bacterium from marine sediments. Int. J. Syst. Bacteriol. 45, 116–123. 10.1099/00207713-45-1-116
70
Engel A. Delille B. Jacquet S. Riebesell U. Rochelle-Newall E. Terbrüggen A. et al . (2004). Transparent exopolymer particles and dissolved organic carbon production by Emiliania huxleyi exposed to different CO2 concentrations: a mesocosm experiment. Aquat. Microb. Ecol. 34, 93–104. 10.3354/ame034093
71
Engel A. Endres S. Galgani L. Schartau M. (2020). Marvelous marine microgels: on the distribution and impact of gel-like particles in the oceanic water-column. Front. Mar. Sci. 7:405. 10.3389/fmars.2020.00405
72
Fischer J. P. Ferdelman T. G. D'Hondt S. Røy H. Wenzhöfer F. (2009). Oxygen penetration deep into the sediment of the South Pacific gyre. Biogeosciences6, 1467–1478. 10.5194/bg-6-1467-2009
73
Flintrop C. M. Rogge A. Miksch S. Thiele S. Waite A. M. Iversen M. H. (2018). Embedding and slicing of intact in situ collected marine snow. Limnol. Oceanogr. Methods16, 339–355. 10.1002/lom3.10251
74
Frontera-Suau R. Bost F. D. McDonald T. J. Morris P. J. (2002). Aerobic biodegradation of hopanes and other biomarkers by crude oil-degrading enrichment cultures. Environ. Sci. Technol. 36, 4585–4592. 10.1021/es025894x
75
Fu J. Gong Y. Zhao X. O'Reilly S. E. Zhao D. (2014). Effects of oil and dispersant on formation of marine oil snow and transport of oil hydrocarbons. Environ. Sci. Technol. 48, 14392–14399. 10.1021/es5042157
76
Garcia M. T. Mellado E. Ostos J. C. Ventosa A. (2004). Halomonas organivorans sp. nov., a moderate halophile able to degrade aromatic compounds. Int. J. Syst. Evol. Microbiol. 54, 1723–1728. 10.1099/ijs.0.63114-0
77
Gärdes A. Iversen M. H. Grossart H.-P. Passow U. Ullrich M. S. (2011). Diatom-associated bacteria are required for aggregation of Thalassiosira weissflogii. ISME J. 5, 436–445. 10.1038/ismej.2010.145
78
Garneau M.-È. Michel C. Meisterhans G. Fortin N. King T. L. Greer C. W. et al . (2016). Hydrocarbon biodegradation by Arctic sea-ice and sub-ice microbial communities during microcosm experiments, Northwest Passage (Nunavut, Canada). FEMS Microbiol. Ecol.92:fiw130. 10.1093/femsec/fiw130
79
Giering S. L. C. Sanders R. Lampitt R. S. Anderson T. R. Tamburini C. Boutrif M. et al . (2014). Reconciliation of the carbon budget in the ocean's twilight zone. Nature507, 480–483. 10.1038/nature13123
80
Gilewicz M. Ni'matuzahroh Nadalig T. Budzinski H. Doumenq P. Michotey V. et al . (1997). Isolation and characterization of a marine bacterium capable of utilizing 2-methylphenanthrene. Appl. Microbiol. Biotechnol. 48, 528–533. 10.1007/s002530051091
81
Godfrin M. P. Sihlabela M. Bose A. Tripathi A. (2018). Behavior of marine bacteria in clean environment and oil spill conditions. Langmuir34, 9047–9053. 10.1021/acs.langmuir.8b01319
82
Gregson B. H. Metodieva G. Metodiev M. V. Golyshin P. N. McKew B. A. (2020). Protein expression in the obligate hydrocarbon-degrading psychrophile Oleispira antarctica RB-8 during alkane degradation and cold tolerance. Environ. Microbiol. 22, 1870–1883. 10.1111/1462-2920.14956
83
Gregson B. H. Metodieva G. Metodiev M. V. McKew B. A. (2019). Differential protein expression during growth on linear versus branched alkanes in the obligate marine hydrocarbon-degrading bacterium Alcanivorax borkumensis SK2T. Environ. Microbiol. 21, 2347–2359. 10.1111/1462-2920.14620
84
Gros J. Socolofsky S. A. Dissanayake A. L. Jun I. Zhao L. Boufadel M. C. et al . (2017). Petroleum dynamics in the sea and influence of subsea dispersant injection during Deepwater Horizon. Proc. Natl. Acad. Sci. U.S.A.114, 10065–10070. 10.1073/pnas.1612518114
85
Gutierrez T. (2017). Marine, aerobic hydrocarbon-degrading gammaproteobacteria: overview, in Taxonomy, Genomics and Ecophysiology of Hydrocarbon-Degrading Microbes, ed T. J. McGenity (Cham: Springer), 1–10. 10.1007/978-3-319-60053-6_22-1
86
Gutierrez T. (2019). Aerobic hydrocarbon-degrading Gammaproteobacteria: Porticoccus, in Taxonomy, Genomics and Ecophysiology of Hydrocarbon-Degrading Microbes, ed T. J. McGenity (Cham: Springer), 181–189. 10.1007/978-3-030-14796-9_32
87
Gutierrez T. Berry D. Yang T. Mishamandani S. McKay L. Teske A. et al . (2013a). Role of bacterial exopolysaccharides (EPS) in the fate of the oil released during the Deepwater Horizon oil spill. PLoS ONE8:e67717. 10.1371/journal.pone.0067717
88
Gutierrez T. Kleindienst S. (2020). Uncovering microbial hydrocarbon degradation processes: the promise of stable isotope probing, in Marine Hydrocarbon Seeps, eds TeskeA.CarvalhoV. (Berlin: Springer), 183–199.
89
Gutierrez T. Morris G. Ellis D. Bowler B. Jones M. Salek K. et al . (2018). Hydrocarbon-degradation and MOS-formation capabilities of the dominant bacteria enriched in sea surface oil slicks during the Deepwater Horizon oil spill. Mar. Pollut. Bull. 135, 205–215. 10.1016/j.marpolbul.2018.07.027
90
Gutiérrez T. Mulloy B. Black K. Green D. H. (2007). Glycoprotein emulsifiers from two marine Halomonas species: chemical and physical characterization. J. Appl. Microbiol. 103, 1716–1727. 10.1111/j.1365-2672.2007.03407.x
91
Gutierrez T. Shimmield T. Haidon C. Black K. Green D. H. (2008). Emulsifying and metal ion binding activity of a glycoprotein exopolymer produced by Pseudoalteromonas sp. strain TG12. Appl. Environ. Microbiol.74, 4867–4876. 10.1128/AEM.00316-08
92
Gutierrez T. Singleton D. R. Berry D. Yang T. Aitken M. D. Teske A. (2013b). Hydrocarbon-degrading bacteria enriched by the Deepwater Horizon oil spill identified by cultivation and DNA-SIP. ISME J. 7, 2091–2104. 10.1038/ismej.2013.98
93
Hamdan L. Fulmer P. (2011). Effects of COREXIT® EC9500A on bacteria from a beach oiled by the Deepwater Horizon spill. Aquat. Microb. Ecol. 63, 101–109. 10.3354/ame01482
94
Hansell D. Carlson C. Repeta D. Schlitzer R. (2009). Dissolved organic matter in the ocean: a controversy stimulates new insights. Oceanography22, 202–211. 10.5670/oceanog.2009.109
95
Hastings D. W. Bartlett T. Brooks G. R. Larson R. A. Quinn K. A. Razionale D. et al . (2020). Changes in redox conditions of surface sediments following the Deepwater Horizon and Ixtoc 1 events.” in Deep Oil Spills. Facts, Fates and Effects, eds MurawskiS.AinsworthC.GilbertS.HollanderD.ParisC.SchlüterM.WetzelD. (Cham: Springer International Publishing), 269–284. 10.1007/978-3-030-11605-7_16
96
Hastings D. W. Schwing P. T. Brooks G. R. Larson R. A. Morford J. L. Roeder T. et al . (2016). Changes in sediment redox conditions following the BP DWH blowout event. Deep Sea Res. Part II Top. Stud. Oceanogr. 129, 167–178. 10.1016/J.DSR2.2014.12.009
97
Hatcher P. G. Obeid W. Wozniak A. S. Xu C. Zhang S. Santschi P. H. et al . (2018). Identifying oil/marine snow associations in mesocosm simulations of the Deepwater Horizon oil spill event using solid-state 13C NMR spectroscopy. Mar. Pollut. Bull. 126, 159–165. 10.1016/J.MARPOLBUL.2017.11.004
98
Hazen T. C. Dubinsky E. A. DeSantis T. Z. Andersen G. L. Piceno Y. M. Singh N. et al . (2010). Deep-sea oil plume enriches indigenous oil-degrading bacteria. Science330, 204–208. 10.1126/science.1195979
99
Hemmer M. J. Barron M. G. Greene R. M. (2011). Comparative toxicity of eight oil dispersants, Louisiana sweet crude oil (LSC), and chemically dispersed LSC to two aquatic test species. Environ. Toxicol. Chem. 30, 2244–2252. 10.1002/etc.619
100
Henry I. A. Netzer R. Davies E. Brakstad O. G. (2021). The influences of phytoplankton species, mineral particles and concentrations of dispersed oil on the formation and fate of marine oil-related aggregates. Sci. Total Environ. 752:141786. 10.1016/J.SCITOTENV.2020.141786
101
Henry I. A. Netzer R. Davies E. J. Brakstad O. G. (2020). Formation and fate of oil-related aggregates (ORAs) in seawater at different temperatures. Mar. Pollut. Bull. 159:111483. 10.1016/J.MARPOLBUL.2020.111483
102
Hu C. Weisberg R. H. Liu Y. Zheng L. Daly K. L. English D. C. et al . (2011). Did the northeastern Gulf of Mexico become greener after the Deepwater Horizon oil spill?Geophys. Res. Lett.38:L09601. 10.1029/2011GL047184
103
Hu P. Dubinsky E. A. Probst A. J. Wang J. Sieber C. M. K. Tom L. M. et al . (2017). Simulation of Deepwater Horizon oil plume reveals substrate specialization within a complex community of hydrocarbon degraders. Proc. Natl. Acad. Sci. U.S.A. 114, 7432–7437. 10.1073/pnas.1703424114
104
Iuculano F. Mazuecos I. P. Reche I. Agustí S. (2017). Prochlorococcus as a possible source for transparent exopolymer particles (TEP). Front. Microbiol.8:709. 10.3389/fmicb.2017.00709
105
Iversen M. H. Lampitt R. S. (2020). Size does not matter after all: no evidence for a size-sinking relationship for marine snow. Prog. Oceanogr. 189:102445. 10.1016/J.POCEAN.2020.102445
106
Jackson G. A. (1990). A model of the formation of marine algal flocs by physical coagulation processes. Deep Sea Res. Part A. Oceanogr. Res. Pap. 37, 1197–1211. 10.1016/0198-0149(90)90038-W
107
Jackson G. A. (2005). Coagulation theory and models of oceanic plankton aggregation, in Flocculation in Natural and Engineered Environments, eds DroppoI.LeppardG.LissS.MilliganT. (Boca Raton, FL: CRC Press), 271–292.
108
Jin H. M. Kim J. M. Lee H. J. Madsen E. L. Jeon C. O. (2012). Alteromonas as a key agent of polycyclic aromatic hydrocarbon biodegradation in crude oil-contaminated coastal sediment. Environ. Sci. Technol. 46, 7731–7740. 10.1021/es3018545
109
Johnson J. E. Hill R. T. (2003). Sediment microbes of deep-sea bioherms on the Northwest Shelf of Australia. Microb. Ecol. 46, 55–61. 10.1007/s00248-002-2031-y
110
Jung J. H. Yim U. H. Han G. M. Shim W. J. (2009). Biochemical changes in rockfish, Sebastes schlegeli, exposed to dispersed crude oil. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 150, 218–223. 10.1016/J.CBPC.2009.04.009
111
Kalyuzhnaya M. G. Gomez O. A. Murrell J. C. (2019). The methane-oxidizing bacteria (methanotrophs), in Taxonomy, Genomics and Ecophysiology of Hydrocarbon-Degrading Microbes, ed T. J. McGenity (Cham: Springer), 245–278. 10.1007/978-3-319-60053-6_10-1
112
Kem M. P. Zane H. K. Springer S. D. Gauglitz J. M. Butler A. (2014). Amphiphilic siderophore production by oil-associating microbes. Metallomics6, 1150–1155. 10.1039/c4mt00047a
113
Kertesz M. A. Kawasaki A. Stolz A. (2019). Aerobic hydrocarbon-degrading Alphaproteobacteria: Sphingomonadales, in Taxonomy, Genomics and Ecophysiology of Hydrocarbon-Degrading Microbes, ed T. J. McGenity (Cham: Springer), 105–124. 10.1007/978-3-030-14796-9_9
114
Khelifa A. Stoffyn-Egli P. Hill P. S. Lee K. (2005). Effects of salinity and clay type on oil-mineral aggregation. Mar. Environ. Res. 59, 235–254. 10.1016/j.marenvres.2004.05.003
115
Kleindienst S. Paul J. H. Joye S. B. (2015a). Using dispersants after oil spills: impacts on the composition and activity of microbial communities. Nat. Rev. Microbiol. 13, 388–396. 10.1038/nrmicro3452
116
Kleindienst S. Seidel M. Ziervogel K. Grim S. Loftis K. Harrison S. et al . (2015b). Chemical dispersants can suppress the activity of natural oil-degrading microorganisms. Proc. Natl. Acad. Sci. U.S.A.112, 14900–14905. 10.1073/pnas.1507380112
117
Kleindienst S. Seidel M. Ziervogel K. Grim S. Loftis K. Harrison S. et al . (2016). Reply to Prince et al.: ability of chemical dispersants to reduce oil spill impacts remains unclear. Proc. Natl. Acad. Sci. U.S.A.113, E1422–E1423. 10.1073/pnas.1600498113
118
Kujawinski E. B. Reddy C. M. Rodgers R. P. Thrash J. C. Valentine D. L. White H. K. (2020). The first decade of scientific insights from the Deepwater Horizon oil release. Nat. Rev. Earth Environ. 1, 237–250. 10.1038/s43017-020-0046-x
119
Larson R. A. Brooks G. R. Schwing P. T. Holmes C. W. Carter S. R. Hollander D. J. (2018). High-resolution investigation of event driven sedimentation: Northeastern Gulf of Mexico. Anthropocene24, 40–50. 10.1016/J.ANCENE.2018.11.002
120
Lee K. Nedwed T. Prince R. C. Palandro D. (2013). Lab tests on the biodegradation of chemically dispersed oil should consider the rapid dilution that occurs at sea. Mar. Pollut. Bull. 73, 314–318. 10.1016/J.MARPOLBUL.2013.06.005
121
Lee K. Wong C. S. Cretney W. J. Whitney F. A. Parsons T. R. Lalli C. M. et al . (1985). Microbial response to crude oil and Corexit 9527: SEAFLUXES enclosure study. Microb. Ecol. 11, 337–351. 10.1007/BF02016816
122
Lee R. F. Koster M. Paffenhofer G.-A. (2012). Ingestion and defecation of dispersed oil droplets by pelagic tunicates. J. Plankton Res. 34, 1058–1063. 10.1093/plankt/fbs065
123
Lewis A. Prince R. C. (2018). Integrating dispersants in oil spill response in Arctic and other icy environments. Environ. Sci. Technol. 52, 6098–6112. 10.1021/acs.est.7b06463
124
Li H. Bao M. Li Y. Zhao L. King T. Xie Y. (2020). Effects of suspended particulate matter, surface oil layer thickness and surfactants on the formation and transport of oil-sediment aggregates (OSA). Int. Biodeterior. Biodegradation149:104925. 10.1016/J.IBIOD.2020.104925
125
Lincoln S. A. Radović J. R. Gracia A. Jaggi A. Oldenburg T. B. P. Larter S. R. et al . (2020). Molecular legacy of the 1979 Ixtoc 1 oil spill in deep-sea sediments of the Southern Gulf of Mexico, in Deep Oil Spills. Facts, Fates and Effects, eds MurawskiS.AinsworthC.GilbertS.HollanderD.ParisC.SchlüterM.WetzelD. (Cham: Springer International Publishing), 312–327. 10.1007/978-3-030-11605-7_19
126
Liu A. Zhang Y.-J. Cheng P. Peng Y.-J. Blom J. Xue Q.-J. (2020). Whole genome analysis calls for a taxonomic rearrangement of the genus Colwellia. Antonie Van Leeuwenhoek113, 919–931. 10.1007/s10482-020-01405-6
127
Liu C. Shao Z. (2005). Alcanivorax dieselolei sp. nov., a novel alkane-degrading bacterium isolated from sea water and deep-sea sediment. Int. J. Syst. Evol. Microbiol. 55, 1181–1186. 10.1099/ijs.0.63443-0
128
Liu J. Zheng Y. Lin H. Wang X. Li M. Liu Y. et al . (2019). Proliferation of hydrocarbon-degrading microbes at the bottom of the Mariana Trench. Microbiome7:47. 10.1186/s40168-019-0652-3
129
Lofthus S. Netzer R. Lewin A. S. Heggeset T. M. B. Haugen T. Brakstad O. G. (2018). Biodegradation of n-alkanes on oil?seawater interfaces at different temperatures and microbial communities associated with the degradation. Biodegradation29, 141–157. 10.1007/s10532-018-9819-z
130
Logan B. E. Passow U. Alldredge A. L. Grossartt H.-P. Simont M. (1995). Rapid formation and sedimentation of large aggregates is predictable from coagulation rates (half-lives) of transparent exopolymer particles (TEP). Deep Sea Res. Part II Top. Stud. Oceanogr. 42, 203–214. 10.1016/0967-0645(95)00012-F
131
Loh A. Yim U. H. (2016). A review of the effects of particle types on oil-suspended particulate matter aggregate formation. Ocean Sci. J. 51, 535–548. 10.1007/s12601-016-0050-8
132
Lombard F. Guidi L. Kiørboe T. (2013). Effect of type and concentration of ballasting particles on sinking rate of marine snow produced by the Appendicularian Oikopleura dioica. PLoS ONE8:e75676. 10.1371/journal.pone.0075676
133
Long R. A. Azam F. (1996). Abundant protein-containing particles in the sea. Aquat. Microb. Ecol.10, 213–221. 10.3354/ame010213
134
Louvado A. Coelho F. J. R. C. Oliveira V. Gomes H. Cleary D. F. R. Simões M. M. Q. et al . (2019). Microcosm evaluation of the impact of oil contamination and chemical dispersant addition on bacterial communities and sediment remediation of an estuarine port environment. J. Appl. Microbiol. 127, 134–149. 10.1111/jam.14261
135
Lunel T. (1995). Dispersant effectiveness at sea, in in International Oil Spill Conference Proceedings (Washington, DC: American Petroleum Institute), 147–155.
136
Macnaughton S. J. Swannell R. Daniel F. Bristow L. (2003). Biodegradation of dispersed Forties crude and Alaskan North slope oils in microcosms under simulated marine conditions. Spill Sci. Technol. Bull. 8, 179–186. 10.1016/S1353-2561(03)00020-3
137
Mari X. Lefèvre J. Torréton J.-P. Bettarel Y. Pringault O. Rochelle-Newall E. et al . (2014). Effects of soot deposition on particle dynamics and microbial processes in marine surface waters. Global Biogeochem. Cycles28, 662–678. 10.1002/2014GB004878
138
Mari X. Passow U. Migon C. Burd A. B. Legendre L. (2017). Transparent exopolymer particles: effects on carbon cycling in the ocean. Prog. Oceanogr. 151, 13–37. 10.1016/J.POCEAN.2016.11.002
139
Mason O. U. Hazen T. C. Borglin S. Chain P. S. G. Dubinsky E. A. Fortney J. L. et al . (2012). Metagenome, metatranscriptome and single-cell sequencing reveal microbial response to Deepwater Horizon oil spill. ISME J. 6, 1715–1727. 10.1038/ismej.2012.59
140
Mason O. U. Scott N. M. Gonzalez A. Robbins-Pianka A. Baelum J. Kimbrel J. et al . (2014). Metagenomics reveals sediment microbial community response to Deepwater Horizon oil spill. ISME J. 8, 1464–1475. 10.1038/ismej.2013.254
141
Math R. K. Jin H. M. Kim J. M. Hahn Y. Park W. Madsen E. L. et al . (2012). Comparative genomics reveals adaptation by Alteromonas sp. SN2 to marine tidal-flat conditions: cold tolerance and aromatic hydrocarbon metabolism. PLoS ONE7:e35784. 10.1371/journal.pone.0035784
142
McFarlin K. M. Perkins M. J. Field J. A. Leigh M. B. (2018). Biodegradation of crude oil and Corexit 9500 in Arctic seawater. Front. Microbiol. 9:1788. 10.3389/fmicb.2018.01788
143
McFarlin K. M. Prince R. C. Perkins R. Leigh M. B. (2014). Biodegradation of dispersed oil in Arctic seawater at−1°C. PLoS ONE9:e84297. 10.1371/JOURNAL.PONE.0084297
144
McGenity T. J. Folwell B. D. McKew B. A. Sanni G. O. (2012). Marine crude-oil biodegradation: a central role for interspecies interactions. Aquat. Biosyst. 8:10. 10.1186/2046-9063-8-10
145
McKay L. J. Gutierrez T. Teske A. P. (2016). Development of a group-specific 16S rRNA-targeted probe set for the identification of Marinobacter by fluorescence in situ hybridization. Deep Sea Res. Part II Top. Stud. Oceanogr. 129, 360–367. 10.1016/J.DSR2.2013.10.009
146
McKew B. A. Coulon F. Osborn A. M. Timmis K. N. McGenity T. J. (2007a). Determining the identity and roles of oil-metabolizing marine bacteria from the Thames estuary, UK. Environ. Microbiol. 9, 165–176. 10.1111/j.1462-2920.2006.01125.x
147
McKew B. A. Coulon F. Yakimov M. M. Denaro R. Genovese M. Smith C. J. et al . (2007b). Efficacy of intervention strategies for bioremediation of crude oil in marine systems and effects on indigenous hydrocarbonoclastic bacteria. Environ. Microbiol. 9, 1562–1571. 10.1111/j.1462-2920.2007.01277.x
148
Melcher R. J. Apitz S. E. Hemmingsen B. B. (2002). Impact of irradiation and polycyclic aromatic hydrocarbon spiking on microbial populations in marine sediment for future aging and biodegradability studies. Appl. Environ. Microbiol. 68, 2858–2868. 10.1128/aem.68.6.2858-2868.2002
149
Mestre M. Ruiz-González C. Logares R. Duarte C. M. Gasol J. M. Sala M. M. (2018). Sinking particles promote vertical connectivity in the ocean microbiome. Proc. Natl. Acad. Sci. U.S.A.115, E6799–E6807. 10.1073/PNAS.1802470115
150
Middlebrook A. M. Murphy D. M. Ahmadov R. Atlas E. L. Bahreini R. Blake D. R. et al . (2012). Air quality implications of the Deepwater Horizon oil spill. Proc. Natl. Acad. Sci.U.S.A.109, 20280–20285. 10.1073/PNAS.1110052108
151
Mnif S. Chamkha M. Sayadi S. (2009). Isolation and characterization of Halomonas sp. strain C2SS100, a hydrocarbon-degrading bacterium under hypersaline conditions. J. Appl. Microbiol. 107, 785–794. 10.1111/j.1365-2672.2009.04251.x
152
Montagna P. A. Baguley J. G. Cooksey C. Hartwell I. Hyde L. J. Hyland J. L. et al . (2013). Deep-sea benthic footprint of the Deepwater Horizon blowout. PLoS ONE8:e70540. 10.1371/journal.pone.0070540
153
Mühlenbruch M. Grossart H.-P. Eigemann F. Voss M. (2018). Mini-review: phytoplankton-derived polysaccharides in the marine environment and their interactions with heterotrophic bacteria. Environ. Microbiol. 20, 2671–2685. 10.1111/1462-2920.14302
154
Murk A. J. Hollander D. J. Chen S. Hu C. Liu Y. Vonk S. M. et al . (2020). A predictive strategy for mapping locations where future MOSSFA events are expected, in Scenarios and Responses to Future Deep Oil Spills Facts, Fate, and Effects, eds MurawskiS.AinsworthC.GilbertS.HollanderD.ParisC.SchlüterM.WetzelD. (Cham: Springer International Publishing), 355–368. 10.1007/978-3-030-12963-7_21
155
Murray K. J. Boehm P. D. Prince R. C. (2020). The importance of understanding transport and degradation of oil and gasses from deep-sea blowouts, in Deep Oil Spills Facts, Fate, and Effects, eds MurawskiS.AinsworthC.GilbertS.HollanderD.ParisC.SchlüterM.WetzelD. (Cham: Springer International Publishing), 86–106. 10.1007/978-3-030-11605-7_6
156
Needham D. M. Fuhrman J. A. (2016). Pronounced daily succession of phytoplankton, archaea and bacteria following a spring bloom. Nat. Microbiol. 1:16005. 10.1038/nmicrobiol.2016.5
157
Netzer R. Henry I. A. Ribicic D. Wibberg D. Brönner U. Brakstad O. G. (2018). Petroleum hydrocarbon and microbial community structure successions in marine oil-related aggregates associated with diatoms relevant for Arctic conditions. Mar. Pollut. Bull. 135, 759–768. 10.1016/J.MARPOLBUL.2018.07.074
158
Omarova M. Swientoniewski L. T. Mkam Tsengam I. K. Blake D. A. John V. McCormick A. et al . (2019). Biofilm formation by hydrocarbon-degrading marine bacteria and its effects on oil dispersion. ACS Sustain. Chem. Eng. 7, 14490–14499. 10.1021/acssuschemeng.9b01923
159
Orellana M. V. Leck C. (2015). Marine microgels, in Biogeochemistry of Marine Dissolved Organic Matter, 2nd Edn, eds HansellD. A.CarlsonC. A. (London: Academic Press), 451–480. 10.1016/B978-0-12-405940-5.00009-1
160
Oren A. (2017). Aerobic hydrocarbon-degrading Archaea, in Taxonomy, Genomics and Ecophysiology of Hydrocarbon-Degrading Microbes, ed T. J. McGenity (Cham: Springer), 1–12. 10.1007/978-3-319-60053-6_5-1
161
Owens E. H. (1999). The interaction of fine particles with stranded oil. Pure Appl. Chem. 71, 83–93. 10.1351/pac199971010083
162
Palleroni N. J. Pieper D. H. Moore E. R. B. (2010). Microbiology of hydrocarbon-degrading Pseudomonas, in Handbook of Hydrocarbon and Lipid Microbiology, eds TimmisK. N.McGenityT. J.van der MeerJ. R.De LorenzoV. (Berlin; Heidelberg: Springer), 1787–1798. 10.1007/978-3-540-77587-4_129
163
Passow U. (2002a). Production of transparent exopolymer particles (TEP) by phyto- and bacterioplankton. Mar. Ecol. Prog. Ser. 236, 1–12. 10.3354/meps236001
164
Passow U. (2002b). Transparent exopolymer particles (TEP) in aquatic environments. Prog. Oceanogr. 55, 287–333. 10.1016/S0079-6611(02)00138-6
165
Passow U. (2016). Formation of rapidly-sinking, oil-associated marine snow. Deep Sea Res. Part II Top. Stud. Oceanogr. 129, 232–240. 10.1016/J.DSR2.2014.10.001
166
Passow U. Alldredge A. L. (1995). Aggregation of a diatom bloom in a mesocosm: the role of transparent exopolymer particles (TEP). Deep Sea Res. Part II Top. Stud. Oceanogr. 42, 99–109. 10.1016/0967-0645(95)00006-C
167
Passow U. Alldredge A. L. Logan B. E. (1994). The role of particulate carbohydrate exudates in the flocculation of diatom blooms. Deep Sea Res. Part I Oceanogr. Res. Pap. 41, 335–357. 10.1016/0967-0637(94)90007-8
168
Passow U. Overton E. B. (2021). The complexity of spills: the fate of the Deepwater Horizon oil. Ann. Rev. Mar. Sci. 13, 109–136. 10.1146/annurev-marine-032320-095153
169
Passow U. Shipe R. Murray A. Pak D. Brzezinski M. Alldredge A. (2001). The origin of transparent exopolymer particles (TEP) and their role in the sedimentation of particulate matter. Cont. Shelf Res. 21, 327–346. 10.1016/S0278-4343(00)00101-1
170
Passow U. Stout S. A. (2020). Character and sedimentation of “lingering” Macondo oil to the deep-sea after the Deepwater Horizon oil spill. Mar. Chem. 218, 103733. 10.1016/J.MARCHEM.2019.103733
171
Passow U. Sweet J. Francis S. Xu C. Dissanayake A. Lin Y. et al . (2019). Incorporation of oil into diatom aggregates. Mar. Ecol. Prog. Ser. 612, 65–86. 10.3354/meps12881
172
Passow U. Sweet J. Quigg A. (2017). How the dispersant Corexit impacts the formation of sinking marine oil snow. Mar. Pollut. Bull. 125, 139–145. 10.1016/J.MARPOLBUL.2017.08.015
173
Passow U. Ziervogel K. (2016). Marine snow sedimented oil released during the Deepwater Horizon spill. Oceanography29, 118–125. 10.5670/oceanog.2016.76
174
Passow U. Ziervogel K. Asper V. Diercks A. (2012). Marine snow formation in the aftermath of the Deepwater Horizon oil spill in the Gulf of Mexico. Environ. Res. Lett. 7:035301. 10.1088/1748-9326/7/3/035301
175
Paulmier A. Ruiz-Pino D. (2009). Oxygen minimum zones (OMZs) in the modern ocean. Prog. Oceanogr. 80, 113–128. 10.1016/J.POCEAN.2008.08.001
176
Pedler B. E. Aluwihare L. I. Azam F. (2014). Single bacterial strain capable of significant contribution to carbon cycling in the surface ocean. Proc. Natl. Acad. Sci.U.S.A.111, 7202–7207. 10.1073/PNAS.1401887111
177
Pepi M. Cesàro A. Liut G. Baldi F. (2005). An antarctic psychrotrophic bacterium Halomonas sp. ANT-3b, growing on n-hexadecane, produces a new emulsyfying glycolipid. FEMS Microbiol. Ecol. 53, 157–166. 10.1016/j.femsec.2004.09.013
178
Place B. Anderson B. Mekebri A. Furlong E. T. Gray J. L. Tjeerdema R. et al . (2010). A role for analytical chemistry in advancing our understanding of the occurrence, fate, and effects of Corexit oil dispersants. Environ. Sci. Technol. 44, 6016–6018. 10.1021/es102319w
179
Place B. J. Perkins M. J. Sinclair E. Barsamian A. L. Blakemore P. R. Field J. A. (2016). Trace analysis of surfactants in Corexit oil dispersant formulations and seawater. Deep Sea Res. Part 2. Top. Stud. Oceanogr.129, 273–281. 10.1016/j.dsr2.2014.01.015
180
Ploug H. Kühl M. Buchholz-Cleven B. Jørgensen B. (1997). Anoxic aggregates - an ephemeral phenomenon in the pelagic environment?Aquat. Microb. Ecol. 13, 285–294. 10.3354/ame013285
181
Prairie J. C. Ziervogel K. Camassa R. McLaughlin R. M. White B. L. Dewald C. et al . (2015). Delayed settling of marine snow: effects of density gradient and particle properties and implications for carbon cycling. Mar. Chem. 175, 28–38. 10.1016/j.marchem.2015.04.006
182
Prieto L. Ruiz J. Echevarria F. Garcia C. M. Bartual A. Gálvez J. A. et al . (2002). Scales and processes in the aggregation of diatom blooms: high time resolution and wide size range records in a mesocosm study. Deep Sea Res. Part I Oceanogr. Res. Pap. 49, 1233–1253. 10.1016/S0967-0637(02)00024-9
183
Prince R. C. (2015a). Introduction: mesocosms and microcosms, in Hydrocarbon and Lipid Microbiology Protocols: Meso- and Microcosms, eds McGenityT. J.TimmisK. N.NogalesB. (Berlin: Springer), 1–13. 10.1007/8623_2015_173
184
Prince R. C. (2015b). Oil spill dispersants: boon or bane?Environ. Sci. Technol. 49, 6376–6384. 10.1021/acs.est.5b00961
185
Prince R. C. (2018). Eukaryotic hydrocarbon degraders, in Taxonomy, Genomics and Ecophysiology of Hydrocarbon-Degrading Microbes, ed T. J. McGenity (Cham: Springer), 1–20. 10.1007/978-3-319-60053-6_16-1
186
Prince R. C. Amande T. J. McGenity T. J. (2018). Prokaryotic hydrocarbon degraders, in Taxonomy, Genomics and Ecophysiology of Hydrocarbon-Degrading Microbes, ed T. J. McGenity (Cham: Springer), 1–41. 10.1007/978-3-319-60053-6_15-1
187
Prince R. C. Butler J. D. (2014). A protocol for assessing the effectiveness of oil spill dispersants in stimulating the biodegradation of oil. Environ. Sci. Pollut. Res. 21, 9506–9510. 10.1007/s11356-013-2053-7
188
Prince R. C. Butler J. D. Bragin G. E. Parkerton T. F. Redman A. D. Kelley B. A. et al . (2016a). Preparing the hydrocarbon/crude oil, in Hydrocarbon and Lipid Microbiology Protocols: Meso- and Microcosms, eds McGenityT. J.TimmisK. N.NogalesB. (Berlin; Heidelberg: Springer), 15–32. 10.1007/8623_2016_220
189
Prince R. C. Butler J. D. Redman A. D. (2017). The rate of crude oil biodegradation in the sea. Environ. Sci. Technol. 51, 1278–1284. 10.1021/acs.est.6b03207
190
Prince R. C. Coolbaugh T. S. Parkerton T. F. (2016b). Oil dispersants do facilitate biodegradation of spilled oil. Proc. Natl. Acad. Sci. U.S.A. 113, E1421. 10.1073/pnas.1525333113
191
Prince R. C. Elmendorf D. L. Lute J. R. Hsu C. S. Haith C. E. Senius J. D. et al . (1994). 17.alpha.(H)-21.beta.(H)-hopane as a conserved internal marker for estimating the biodegradation of crude oil. Environ. Sci. Technol. 28, 142–145. 10.1021/es00050a019
192
Prince R. C. Garrett R. M. Bare R. E. Grossman M. J. Townsend T. Suflita J. M. et al . (2003). The roles of photooxidation and biodegradation in long-term weathering of crude and heavy fuel oils. Spill Sci. Technol. Bull. 8, 145–156. 10.1016/S1353-2561(03)00017-3
193
Prince R. C. McFarlin K. M. Butler J. D. Febbo E. J. Wang F. C. Y. Nedwed T. J. (2013). The primary biodegradation of dispersed crude oil in the sea. Chemosphere90, 521–526. 10.1016/J.CHEMOSPHERE.2012.08.020
194
Prince R. C. Nash G. W. Hill S. J. (2016c). The biodegradation of crude oil in the deep ocean. Mar. Pollut. Bull.111, 354–357. 10.1016/j.marpolbul.2016.06.087
195
Qiao N. Shao Z. (2010). Isolation and characterization of a novel biosurfactant produced by hydrocarbon-degrading bacterium Alcanivorax dieselolei B-5. J. Appl. Microbiol. 108, 1207–1216. 10.1111/j.1365-2672.2009.04513.x
196
Quigg A. Passow U. Chin W.-C. Xu C. Doyle S. Bretherton L. et al . (2016). The role of microbial exopolymers in determining the fate of oil and chemical dispersants in the ocean. Limnol. Oceanogr. Lett. 1, 3–26. 10.1002/lol2.10030
197
Quigg A. Passow U. Daly K. L. Burd A. Hollander D. J. Schwing P. T. et al . (2020). Marine oil snow sedimentation and flocculent accumulation (MOSSFA) events: learning from the past to predict the future, in Deep Oil Spills. Facts, Fate, and Effects, eds MurawskiS.AinsworthC.GilbertS.HollanderD.ParisC.SchlüterM.WetzelD. (Cham: Springer International Publishing), 196–220. 10.1007/978-3-030-11605-7_12
198
Radwan S. S. A. Al-Mailem D. M. Kansour M. K. (2017). Gelatinizing oil in water and its removal via bacteria inhabiting the gels. Sci. Rep. 7:13975. 10.1038/s41598-017-14296-x
199
Rath J. Wu K. Herndl G. DeLong E. (1998). High phylogenetic diversity in a marine-snow-associated bacterial assemblage. Aquat. Microb. Ecol. 14, 261–269. 10.3354/ame014261
200
Redmond M. C. Valentine D. L. (2012). Natural gas and temperature structured a microbial community response to the Deepwater Horizon oil spill. Proc. Natl. Acad. Sci. U.S.A. 109, 20292–20297. 10.1073/pnas.1108756108
201
Redmond M. C. Valentine D. L. (2019). Microbial communities responding to deep-sea hydrocarbon spills, in Microbial Communities Utilizing Hydrocarbons and Lipids: Members, Metagenomics and Ecophysiology, ed T. J. McGenity (Cham: Springer). 10.1007/978-3-319-60063-5_12-1
202
Reimers C. E. Fischer K. M. Merewether R. Smith K. L. Jahnke R. A. (1986). Oxygen microprofiles measured in situ in deep ocean sediments. Nature320, 741–744. 10.1038/320741a0
203
Ribicic D. Netzer R. Hazen T. C. Techtmann S. M. Drabløs F. Brakstad O. G. (2018). Microbial communities in seawater from an Arctic and a temperate Norwegian fjord and their potentials for biodegradation of chemically dispersed oil at low seawater temperatures. Mar. Pollut. Bull. 129, 308–327. 10.1016/J.MARPOLBUL.2018.02.024
204
Riehm D. A. McCormick A. V. (2014). The role of dispersants' dynamic interfacial tension in effective crude oil spill dispersion. Mar. Pollut. Bull. 84, 155–163. 10.1016/J.MARPOLBUL.2014.05.018
205
Rogener M. K. Bracco A. Hunter K. S. Saxton M. A. Joye S. B. (2018). Long-term impact of the Deepwater Horizon oil well blowout on methane oxidation dynamics in the northern Gulf of Mexico. Elem. Sci. Anth.6:73. 10.1525/elementa.332
206
Rogge A. Flintrop C. M. Iversen M. H. Salter I. Fong A. A. Vogts A. et al . (2018). Hard and soft plastic resin embedding for single-cell element uptake investigations of marine-snow-associated microorganisms using nano-scale secondary ion mass spectrometry. Limnol. Oceanogr. Methods16, 484–503. 10.1002/lom3.10261
207
Röling W. F. M. Milner M. G. Jones D. M. Lee K. Daniel F. Swannell R. J. P. et al . (2002). Robust hydrocarbon degradation and dynamics of bacterial communities during nutrient-enhanced oil spill bioremediation. Appl. Environ. Microbiol. 68, 5537–5548. 10.1128/aem.68.11.5537-5548.2002
208
Romero I. C. Schwing P. T. Brooks G. R. Larson R. A. Hastings D. W. Ellis G. et al . (2015). Hydrocarbons in deep-sea dediments following the 2010 Deepwater Horizon blowout in the Northeast Gulf of Mexico. PLoS ONE10:e0128371. 10.1371/journal.pone.0128371
209
Romero I. C. Toro-Farmer G. Diercks A.-R. Schwing P. Muller-Karger F. Murawski S. et al . (2017). Large-scale deposition of weathered oil in the Gulf of Mexico following a deep-water oil spill. Environ. Pollut. 228, 179–189. 10.1016/j.envpol.2017.05.019
210
Rubin-Blum M. Antony C. P. Borowski C. Sayavedra L. Pape T. Sahling H. et al . (2017). Short-chain alkanes fuel mussel and sponge Cycloclasticus symbionts from deep-sea gas and oil seeps. Nat. Microbiol. 2:17093. 10.1038/nmicrobiol.2017.93
211
Ryerson T. B. Camilli R. Kessler J. D. Kujawinski E. B. Reddy C. M. Valentine D. L. et al . (2012). Chemical data quantify Deepwater Horizon hydrocarbon flow rate and environmental distribution. Proc. Natl. Acad. Sci. U.S.A. 109, 20246–20253. 10.1073/pnas.1110564109
212
Ryther J. H. (1956). Photosynthesis in the ocean as a function of light intensity. Limnol. Oceanogr. 1, 61–70. 10.4319/lo.1956.1.1.0061
213
Sabirova J. S. Becker A. Lünsdorf H. Nicaud J.-M. Timmis K. N. Golyshin P. N. (2011). Transcriptional profiling of the marine oil-degrading bacterium Alcanivorax borkumensis during growth on n-alkanes. FEMS Microbiol. Lett. 319, 160–168. 10.1111/j.1574-6968.2011.02279.x
214
Santschi P. H. Xu C. Schwehr K. A. Lin P. Sun L. Chin W.-C. et al . (2020). Can the protein/carbohydrate (P/C) ratio of exopolymeric substances (EPS) be used as a proxy for their ‘stickiness’ and aggregation propensity?Mar. Chem. 218:103734. 10.1016/J.MARCHEM.2019.103734
215
Scarlett A. Galloway T. S. Canty M. Smith E. L. Nilsson J. Rowland S. J. (2005). Comparitive toxicity of two oil dispersantsm Superdispersant-25 and Corexit 9527, to a range of coastal species. Environ. Toxicol. Chem. 24:1219. 10.1897/04-334R.1
216
Schneiker S. Martins dos Santos V. A. P. Bartels D. Bekel T. Brecht M. Buhrmester J. et al . (2006). Genome sequence of the ubiquitous hydrocarbon-degrading marine bacterium Alcanivorax borkumensis. Nat. Biotechnol. 24, 997–1004. 10.1038/nbt1232
217
Schuback N. Tortell P. D. (2019). Diurnal regulation of photosynthetic light absorption, electron transport and carbon fixation in two contrasting oceanic environments. Biogeosciences16, 1381–1399. 10.5194/bg-16-1381-2019
218
Schwehr K. A. Xu C. Chiu M.-H. Zhang S. Sun L. Lin P. et al . (2018). Protein: Polysaccharide ratio in exopolymeric substances controlling the surface tension of seawater in the presence or absence of surrogate Macondo oil with and without Corexit. Mar. Chem.206, 84–92. 10.1016/J.MARCHEM.2018.09.003
219
Schwing P. T. Brooks G. R. Larson R. A. Holmes C. W. O'Malley B. J. Hollander D. J. (2017). Constraining the spatial extent of marine oil snow dedimentation and flocculent accumulation following the Deepwater Horizon event using an excess 210Pb flux approach. Environ. Sci. Technol. 51, 5962–5968. 10.1021/acs.est.7b00450
220
Schwing P. T. Chanton J. P. Romero I. C. Hollander D. J. Goddard E. A. Brooks G. R. et al . (2018). Tracing the incorporation of carbon into benthic foraminiferal calcite following the Deepwater Horizon event. Environ. Pollut. 237, 424–429. 10.1016/J.ENVPOL.2018.02.066
221
Schwing P. T. Hollander D. J. Brooks G. R. Larson R. A. Hastings D. W. Chanton J. P. et al . (2020). The sedimentary record of MOSSFA events in the Gulf of Mexico: a comparison of the Deepwater Horizon (2010) and Ixtoc 1 (1979) Oil Spills, in Deep Oil Spills Facts, Fate, and Effects, eds MurawskiS.AinsworthC.GilbertS.HollanderD.ParisC.SchlüterM.WetzelD. (Cham: Springer International Publishing), 221–234. 10.1007/978-3-030-11605-7_13
222
Schwing P. T. Romero I. C. Brooks G. R. Hastings D. W. Larson R. A. Hollander D. J. (2015). A decline in benthic foraminifera following the Deepwater Horizon event in the Northeastern Gulf of Mexico. PLoS ONE10:e0120565. 10.1371/journal.pone.0120565
223
Scoma A. Barbato M. Borin S. Daffonchio D. Boon N. (2016a). An impaired metabolic response to hydrostatic pressure explains Alcanivorax borkumensis recorded distribution in the deep marine water column. Sci. Rep. 6:31316. 10.1038/srep31316
224
Scoma A. Barbato M. Hernandez-Sanabria E. Mapelli F. Daffonchio D. Borin S. et al . (2016b). Microbial oil-degradation under mild hydrostatic pressure (10 MPa): which pathways are impacted in piezosensitive hydrocarbonoclastic bacteria?Sci. Rep. 6:23526. 10.1038/srep23526
225
Shanks A. Reeder M. (1993). Reducing microzones and sulfide production in marine snow. Mar. Ecol. Prog. Ser. 96, 43–47. 10.3354/meps096043
226
Shanks A. L. Trent J. D. (1980). Marine snow: sinking rates and potential role in vertical flux. Deep Sea Res. Part A. Oceanogr. Res. Pap. 27, 137–143. 10.1016/0198-0149(80)90092-8
227
Shiu R.-F. Chiu M.-H. Vazquez C. I. Tsai Y.-Y. Le A. Kagiri A. et al . (2020). Protein to carbohydrate (P/C) ratio changes in microbial extracellular polymeric substances induced by oil and Corexit. Mar. Chem. 223:103789. 10.1016/J.MARCHEM.2020.103789
228
Simon M. Grossart H. Schweitzer B. Ploug H. (2002). Microbial ecology of organic aggregates in aquatic ecosystems. Aquat. Microb. Ecol. 28, 175–211. 10.3354/ame028175
229
Singer M. Aurand D. Bragin G. Clark J. Coelho G. Sowby M. et al . (2000). Standardization of the preparation and quantitation of water-accommodated fractions of petroleum for toxicity testing. Mar. Pollut. Bull. 40, 1007–1016. 10.1016/S0025-326X(00)00045-X
230
Singer M. M. George S. Tjeerdema R. S. (1995). Relationship of some physical properties of oil dispersants and their toxicity to marine organisms. Arch. Environ. Contam. Toxicol. 29, 33–38. 10.1007/BF00213084
231
Stout S. A. German C. R. (2018). Characterization and flux of marine oil snow settling toward the seafloor in the northern Gulf of Mexico during the Deepwater Horizon incident: Evidence for input from surface oil and impact on shallow shelf sediments. Mar. Pollut. Bull. 129, 695–713. 10.1016/j.marpolbul.2017.10.059
232
Stout S. A. Payne J. R. (2016a). Chemical composition of floating and sunken in-situ burn residues from the Deepwater Horizon oil spill. Mar. Pollut. Bull. 108, 186–202. 10.1016/J.MARPOLBUL.2016.04.031
233
Stout S. A. Payne J. R. (2016b). Macondo oil in deep-sea sediments: part 1 – sub-sea weathering of oil deposited on the seafloor. Mar. Pollut. Bull. 111, 365–380. 10.1016/J.MARPOLBUL.2016.07.036
234
Stout S. A. Payne J. R. (2017). Footprint, weathering, and persistence of synthetic-base drilling mud olefins in deep-sea sediments following the Deepwater Horizon disaster. Mar. Pollut. Bull. 118, 328–340. 10.1016/j.marpolbul.2017.03.013
235
Stout S. A. Rouhani S. Liu B. Oehrig J. Ricker R. W. Baker G. et al . (2017). Assessing the footprint and volume of oil deposited in deep-sea sediments following the Deepwater Horizon oil spill. Mar. Pollut. Bull. 114, 327–342. 10.1016/J.MARPOLBUL.2016.09.046
236
Suja L. D. Chen X. Summers S. Paterson D. M. Gutierrez T. (2019). Chemical dispersant enhances microbial exopolymer (EPS) production and formation of marine oil/dispersant snow in surface waters of the Subarctic Northeast Atlantic. Front. Microbiol. 10:553. 10.3389/fmicb.2019.00553
237
Suja L. D. Summers S. Gutierrez T. (2017). Role of EPS, dispersant and nutrients on the microbial response and MOS formation in the Subarctic Northeast Atlantic. Front. Microbiol. 8:676. 10.3389/fmicb.2017.00676
238
Sun L. Chin W.-C. Chiu M.-H. Xu C. Lin P. Schwehr K. A. et al . (2019). Sunlight induced aggregation of dissolved organic matter: role of proteins in linking organic carbon and nitrogen cycling in seawater. Sci. Total Environ. 654, 872–877. 10.1016/J.SCITOTENV.2018.11.140
239
Sun L. Chiu M.-H. Xu C. Lin P. Schwehr K. A. Bacosa H. et al . (2018). The effects of sunlight on the composition of exopolymeric substances and subsequent aggregate formation during oil spills. Mar. Chem. 203, 49–54. 10.1016/J.MARCHEM.2018.04.006
240
Sun L. Xu C. Zhang S. Lin P. Schwehr K. A. Quigg A. et al . (2017). Light-induced aggregation of microbial exopolymeric substances. Chemosphere181, 675–681. 10.1016/j.chemosphere.2017.04.099
241
Taylor J. D. Cottingham S. D. Billinge J. Cunliffe M. (2014). Seasonal microbial community dynamics correlate with phytoplankton-derived polysaccharides in surface coastal waters. ISME J. 8, 245–248. 10.1038/ismej.2013.178
242
Techtmann S. M. Zhuang M. Campo P. Holder E. Elk M. Hazen T. C. et al . (2017). Corexit 9500 enhances oil biodegradation and changes active bacterial community structure of oil-enriched microcosms. Appl. Environ. Microbiol. 83, e03462–e03416. 10.1128/AEM.03462-16
243
Teira E. Lekunberri I. Gasol J. M. Nieto-Cid M. Alvarez-Salgado X. A. Figueiras F. G. (2007). Dynamics of the hydrocarbon-degrading Cycloclasticus bacteria during mesocosm-simulated oil spills. Environ. Microbiol. 9, 2551–2562. 10.1111/j.1462-2920.2007.01373.x
244
Teramoto M. Queck S. Y. Ohnishi K. (2013). Specialized hydrocarbonoclastic bacteria prevailing in seawater around a port in the Strait of Malacca. PLoS ONE8:e66594. 10.1371/journal.pone.0066594
245
Teramoto M. Suzuki M. Okazaki F. Hatmanti A. Harayama S. (2009). Oceanobacter-related bacteria are important for the degradation of petroleum aliphatic hydrocarbons in the tropical marine environment. Microbiology155, 3362–3370. 10.1099/mic.0.030411-0
246
Thornton D. C. O. (2018). Coomassie stainable particles (CSP): protein containing exopolymer particles in the Ocean. Front. Mar. Sci. 5:206. 10.3389/fmars.2018.00206
247
Torres-Beltrán M. Hawley A. K. Capelle D. W. Bhatia M. P. Evan Durno W. Tortell P. D. et al . (2016). Methanotrophic community dynamics in a seasonally anoxic fjord: Saanich Inlet, British Columbia. Front. Mar. Sci. 3:268. 10.3389/fmars.2016.00268
248
Tremblay J. Fortin N. Elias M. Wasserscheid J. King T. L. Lee K. et al . (2019). Metagenomic and metatranscriptomic responses of natural oil degrading bacteria in the presence of dispersants. Environ. Microbiol. 21, 2307–2319. 10.1111/1462-2920.14609
249
Tremblay J. Yergeau E. Fortin N. Cobanli S. Elias M. King T. L. et al . (2017). Chemical dispersants enhance the activity of oil- and gas condensate-degrading marine bacteria. ISME J. 11, 2793–2808. 10.1038/ismej.2017.129
250
Turner J. T. (2015). Zooplankton fecal pellets, marine snow, phytodetritus and the ocean's biological pump. Prog. Oceanogr. 130, 205–248. 10.1016/J.POCEAN.2014.08.005
251
Tyrrell T. Young J. R. (2009). Coccolithophores, in Encyclopedia of Ocean Sciences, 2nd Edn, eds SteeleJ.TurekianK.ThorpeS. (San Diego, CA: Academic Press), 3568–3576.
252
Unanue M. A. AzÃoa I. Arrieta J. M. Herndl G. J. Iriberri J. (1998). Laboratory-made particles as a useful approach to analyse microbial processes in marine macroaggregates. FEMS Microbiol. Ecol. 26, 325–334. 10.1111/j.1574-6941.1998.tb00517.x
253
Valentine D. L. Fisher G. B. Bagby S. C. Nelson R. K. Reddy C. M. Sylva S. P. et al . (2014). Fallout plume of submerged oil from Deepwater Horizon. Proc. Natl. Acad. Sci. U.S.A. 111, 15906–15911. 10.1073/pnas.1414873111
254
Valentine D. L. Kessler J. D. Redmond M. C. Mendes S. D. Heintz M. B. Farwell C. et al . (2010). Propane respiration jump-starts microbial response to a deep oil spill. Science. 330, 208–211. 10.1126/science.1196830
255
van Eenennaam J. S. Rahsepar S. Radovi,ć J. R. Oldenburg T. B. P. Wonink J. Langenhoff A. A. M. et al . (2018). Marine snow increases the adverse effects of oil on benthic invertebrates. Mar. Pollut. Bull. 126, 339–348. 10.1016/j.marpolbul.2017.11.028
256
van Eenennaam J. S. Rohal M. Montagna P. A. Radović J. R. Oldenburg T. B. P. Romero I. C. et al . (2019). Ecotoxicological benthic impacts of experimental oil-contaminated marine snow deposition. Mar. Pollut. Bull. 141, 164–175. 10.1016/j.marpolbul.2019.02.025
257
van Eenennaam J. S. Wei Y. Grolle K. C. F. Foekema E. M. Murk A. J. (2016). Oil spill dispersants induce formation of marine snow by phytoplankton-associated bacteria. MPB104, 294–302. 10.1016/j.marpolbul.2016.01.005
258
Vaysse P.-J. Sivadon P. Goulas P. Grimaud R. (2011). Cells dispersed from Marinobacter hydrocarbonoclasticus SP17 biofilm exhibit a specific protein profile associated with a higher ability to reinitiate biofilm development at the hexadecane-water interface. Environ. Microbiol. 13, 737–746. 10.1111/j.1462-2920.2010.02377.x
259
Verdugo P. (2012). Marine microgels. Ann. Rev. Mar. Sci. 4, 375–400. 10.1146/annurev-marine-120709-142759
260
Vila J. María Nieto J. Mertens J. Springael D. Grifoll M. (2010). Microbial community structure of a heavy fuel oil-degrading marine consortium: linking microbial dynamics with polycyclic aromatic hydrocarbon utilization. FEMS Microbiol. Ecol. 73, 349–362. 10.1111/j.1574-6941.2010.00902.x
261
Vojvoda J. Lamy D. Sintes E. Garcia J. Turk V. Herndl G. (2014). Seasonal variation in marine-snow-associated and ambient-water prokaryotic communities in the northern Adriatic Sea. Aquat. Microb. Ecol. 73, 211–224. 10.3354/ame01718
262
Volkman J. K. Tanoue E. (2002). Chemical and biological studies of particulate organic matter in the Ocean. J. Oceanogr. 58, 265–279. 10.1023/A:1015809708632
263
Vonk S. M. Hollander D. J. Murk A. J. (2015). Was the extreme and wide-spread marine oil-snow sedimentation and flocculent accumulation (MOSSFA) event during the Deepwater Horizon blow-out unique?Mar. Pollut. Bull. 100, 5–12. 10.1016/j.marpolbul.2015.08.023
264
Wade T. L. Morales-McDevitt M. Bera G. Shi D. Sweet S. Wang B. et al . (2017). A method for the production of large volumes of WAF and CEWAF for dosing mesocosms to understand marine oil snow formation. Heliyon3:e00419. 10.1016/J.HELIYON.2017.E00419
265
Wade T. L. Sericano J. L. Sweet S. T. Knap A. H. Guinasso N. L. (2016). Spatial and temporal distribution of water column total polycyclic aromatic hydrocarbons (PAH) and total petroleum hydrocarbons (TPH) from the Deepwater Horizon (Macondo) incident. Mar. Pollut. Bull. 103, 286–293. 10.1016/J.MARPOLBUL.2015.12.002
266
Wakeham S. G. Lee C. (2019). Limits of our knowledge, part 2: Selected frontiers in marine organic biogeochemistry. Mar. Chem. 212, 16–46. 10.1016/J.MARCHEM.2019.02.005
267
Wang J. Sandoval K. Ding Y. Stoeckel D. Minard-Smith A. Andersen G. et al . (2016). Biodegradation of dispersed Macondo crude oil by indigenous Gulf of Mexico microbial communities. Sci. Total Environ. 557–558, 453–468. 10.1016/J.SCITOTENV.2016.03.015
268
Washburn T. W. Reuscher M. G. Montagna P. A. Cooksey C. Hyland J. L. (2017). Macrobenthic community structure in the deep Gulf of Mexico one year after the Deepwater Horizon blowout. Deep Sea Res. Part I Oceanogr. Res. Pap. 127, 21–30. 10.1016/J.DSR.2017.06.001
269
White A. R. Jalali M. Boufadel M. C. Sheng J. (2020a). Bacteria forming drag-increasing streamers on a drop implicates complementary fates of rising deep-sea oil droplets. Sci. Rep. 10, 1–14. 10.1038/s41598-020-61214-9
270
White A. R. Jalali M. Sheng J. (2019). A new ecology-on-a-chip microfluidic platform to study interactions of microbes with a rising oil droplet. Sci. Rep. 9:13737. 10.1038/s41598-019-50153-9
271
White A. R. Jalali M. Sheng J. (2020b). Hydrodynamics of a rising oil droplet with bacterial extracellular polymeric substance (EPS) streamers using a microfluidic microcosm. Front. Mar. Sci. 7:294. 10.3389/fmars.2020.00294
272
Widdows J. Bakke T. Bayne B. L. Donkin P. Livingstone D. R. Lowe D. M. et al . (1982). Responses of Mytilus edulis on exposure to the water-accommodated fraction of North Sea oil. Mar. Biol. 67, 15–31. 10.1007/BF00397090
273
Wirth M. A. Passow U. Jeschek J. Hand I. Schulz-Bull D. E. (2018). Partitioning of oil compounds into marine oil snow: insights into prevailing mechanisms and dispersant effects. Mar. Chem. 206, 62–73. 10.1016/J.MARCHEM.2018.09.007
274
Wozniak A. S. Prem P. M. Obeid W. Waggoner D. C. Quigg A. Xu C. et al . (2019). Rapid degradation of oil in mesocosm simulations of marine oil snow events. Environ. Sci. Technol. 53, 3441–3450. 10.1021/acs.est.8b06532
275
Xu C. Lin P. Zhang S. Sun L. Xing W. Schwehr K. A. et al . (2019). The interplay of extracellular polymeric substances and oil/Corexit to affect the petroleum incorporation into sinking marine oil snow in four mesocosms. Sci. Total Environ. 693:133626. 10.1016/j.scitotenv.2019.133626
276
Xu C. Zhang S. Beaver M. Lin P. Sun L. Doyle S. M. et al . (2018a). The role of microbially-mediated exopolymeric substances (EPS) in regulating Macondo oil transport in a mesocosm experiment. Mar. Chem. 206, 52–61. 10.1016/J.MARCHEM.2018.09.005
277
Xu C. Zhang S. Beaver M. Wozniak A. Obeid W. Lin Y. et al . (2018b). Decreased sedimentation efficiency of petro- and non-petro-carbon caused by a dispersant for Macondo surrogate oil in a mesocosm simulating a coastal microbial community. Mar. Chem. 206, 34–43. 10.1016/J.MARCHEM.2018.09.002
278
Xu C. Zhang S. Chuang C. Miller E. J. Schwehr K. A. Santschi P. H. (2011). Chemical composition and relative hydrophobicity of microbial exopolymeric substances (EPS) isolated by anion exchange chromatography and their actinide-binding affinities. Mar. Chem. 126, 27–36. 10.1016/J.MARCHEM.2011.03.004
279
Yakimov M. M. Giuliano L. Gentile G. Crisafi E. Chernikova T. N. Abraham W.-R. et al . (2003). Oleispira antarctica gen. nov., sp. nov., a novel hydrocarbonoclastic marine bacterium isolated from Antarctic coastal sea water. Int. J. Syst. Evol. Microbiol. 53, 779–785. 10.1099/ijs.0.02366-0
280
Yakimov M. M. Golyshin P. N. Crisafi F. Denaro R. Giuliano L. (2019). Marine, aerobic hydrocarbon-degrading Gammaproteobacteria: the family Alcanivoracaceae, in Taxonomy, Genomics and Ecophysiology of Hydrocarbon-Degrading Microbes, ed T. J McGenity (Cham: Springer), 167–179. 10.1007/978-3-030-14796-9_24
281
Yakimov M. M. Golyshin P. N. Lang S. Moore E. R. Abraham W. R. Lünsdorf H. et al . (1998). Alcanivorax borkumensis gen. nov., sp. nov., a new, hydrocarbon-degrading and surfactant-producing marine bacterium. Int. J. Syst. Bacteriol. 48, 339–348. 10.1099/00207713-48-2-339
282
Yakimov M. M. Timmis K. N. Golyshin P. N. (2007). Obligate oil-degrading marine bacteria. Curr. Opin. Biotechnol. 18, 257–266. 10.1016/j.copbio.2007.04.006
283
Yan B. Passow U. Chanton J. P. Nöthig E.-M. Asper V. Sweet J. et al . (2016). Sustained deposition of contaminants from the Deepwater Horizon spill. Proc. Natl. Acad. Sci. U.S.A. 113, E3332–E3340. 10.1073/pnas.1513156113
284
Yang C. Wang Z. Li Y. Niu Y. Du M. He X. et al . (2010). Metabolic versatility of halotolerant and alkaliphilic strains of Halomonas isolated from alkaline black liquor. Bioresour. Technol. 101, 6778–6784. 10.1016/j.biortech.2010.03.108
285
Yang T. Nigro L. M. Gutierrez T. D'Ambrosio L. Joye S. B. Highsmith R. et al . (2016a). Pulsed blooms and persistent oil-degrading bacterial populations in the water column during and after the Deepwater Horizon blowout. Deep Sea Res. Part II Top. Stud. Oceanogr. 129, 282–291. 10.1016/J.DSR2.2014.01.014
286
Yang T. Speare K. McKay L. MacGregor B. J. Joye S. B. Teske A. (2016b). Distinct bacterial communities in surficial seafloor sediments following the 2010 Deepwater Horizon blowout. Front. Microbiol. 7:1384. 10.3389/fmicb.2016.01384
287
Zhao Z. Baltar F. Herndl G. J. (2020). Linking extracellular enzymes to phylogeny indicates a predominantly particle-associated lifestyle of deep-sea prokaryotes. Sci. Adv.6:eaaz4354. 10.1126/sciadv.aaz435
288
Ziervogel K. McKay L. Rhodes B. Osburn C. L. Dickson-Brown J. Arnosti C. et al . (2012). Microbial activities and dissolved organic matter dynamics in oil-contaminated surface seawater from the Deepwater Horizon oil spill site. PLoS ONE7:e34816. 10.1371/journal.pone.0034816
Summary
Keywords
marine oil snow, marine snow, hydrocarbon biodegradation, hydrocarbonoclastic bacteria, extracellular polymeric substances, oil-spill response
Citation
Gregson BH, McKew BA, Holland RD, Nedwed TJ, Prince RC and McGenity TJ (2021) Marine Oil Snow, a Microbial Perspective. Front. Mar. Sci. 8:619484. doi: 10.3389/fmars.2021.619484
Received
20 October 2020
Accepted
06 January 2021
Published
28 January 2021
Volume
8 - 2021
Edited by
Xiaoshou Liu, Ocean University of China, China
Reviewed by
Wenzhe Xu, Tianjin University of Science and Technology, China; Hans Uwe Dahms, Kaohsiung Medical University, Taiwan
Updates
Copyright
© 2021 Gregson, McKew, Holland, Nedwed, Prince and McGenity.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Terry J. McGenity tjmcgen@essex.ac.uk
This article was submitted to Marine Pollution, a section of the journal Frontiers in Marine Science
†ORCID: Benjamin H. Gregson orcid.org/0000-0001-8337-227X
Boyd A. McKew orcid.org/0000-0001-5607-6619
Roger C. Prince orcid.org/0000-0002-5174-4216
Terry J. McGenity orcid.org/0000-0002-1497-8822
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.