Abstract
Biomass is defined as organic matter from living organisms represented in all kingdoms. It is recognized to be an excellent source of proteins, polysaccharides and lipids and, as such, embodies a tailored feedstock for new products and processes to apply in green industries. The industrial processes focused on the valorization of terrestrial biomass are well established, but marine sources still represent an untapped resource. Oceans and seas occupy over 70% of the Earth’s surface and are used intensively in worldwide economies through the fishery industry, as logistical routes, for mining ores and exploitation of fossil fuels, among others. All these activities produce waste. The other source of unused biomass derives from the beach wrack or washed-ashore organic material, especially in highly eutrophicated marine ecosystems. The development of high-added-value products from these side streams has been given priority in recent years due to the detection of a broad range of biopolymers, multiple nutrients and functional compounds that could find applications for human consumption or use in livestock/pet food, pharmaceutical and other industries. This review comprises a broad thematic approach in marine waste valorization, addressing the main achievements in marine biotechnology for advancing the circular economy, ranging from bioremediation applications for pollution treatment to energy and valorization for biomedical applications. It also includes a broad overview of the valorization of side streams in three selected case study areas: Norway, Scotland, and the Baltic Sea.
Introduction
Marine biomass represents a biotechnological resource with great diversity in composition and functional properties due to various bioactive compounds, from polyphenols and peptides to polysaccharides. Considering the shortage of terrestrial resources, the constantly increasing and aging population, there is an urgent need to propose alternative sources of food and novel medicine. There is a growing consumer awareness regarding the relationship between diet and health, resulting in elevated demand for new fish products with enhanced nutritional and functional properties (Al Khawli et al., 2019). Moreover, according to the World Health Organization (2021), musculoskeletal conditions are the main contributors that cause disability, thus personalized solutions, methods and materials for tissue regeneration are widely studied. Although often overlooked, marine waste can represent a practical alternative resource to address multiple societal challenges (Chubarenko et al., 2021). This review discusses mainly the state-of-the-art of valorization potential of two sources of marine wastes, (i) marine industries biowaste (focusing on fishery and aquaculture industries), as well as (ii) beach wrack. Indeed, the exploitation of marine biomass and valorization of seafood by-products either directly or by the extraction of biopolymers seems to be a promising alternative, leading to more environmentally sustainable uses of marine resources and higher economic benefits, in line with the circular economy and blue bioeconomy concepts. The realization of such developments is hampered by the lack of appropriate regulatory frames to enable the use of waste and by-products and to ensure the safety, quality, and acceptability of the product.
Beach Wrack
Beach-cast sea wrack or simply beach wrack is an organic material consisting of seagrass or seaweed biomass (Macreadie et al., 2017), various marine invertebrates, as well as human-made litter, mostly plastics (Oliveira et al., 2020), which accumulates on beaches due to the action of waves, tides, and aperiodical water level fluctuations (Suursaar et al., 2014). Despite the natural origin of most of this material and its significant ecological role (Dugan et al., 2003; Orr et al., 2005; Defeo et al., 2009; Nordstrom et al., 2011), beach wrack often becomes a social amenity (Kirkman and Kendrick, 1997; Macreadie et al., 2017) and/or presents environmental issues, if accumulated in excessive amounts (McGwynne et al., 1988; Macreadie et al., 2017). Anthropogenic pressure, such as shoreline reconfiguration (Macreadie et al., 2017), eutrophication (Risén et al., 2017) and climate change (Macreadie et al., 2011), stimulate the accumulation of wrack onshore and multiply the negative impacts on the environment as mentioned above. Likewise, marine eutrophication and climate change do not only affect the accumulation of sea wrack and its degradation, but this may be exacerbated in return by the products of aerobic decomposition as well. It is estimated that the annual global carbon flux from seagrass wrack to the atmosphere is between 1.31 and 19.04 Tg C/yr (Liu et al., 2019). Coordinated collection and valorization of beach wrack could be an intervention to mitigate the eutrophication processes by lowering the nutrient concentrations from the sea as well as lowering the nitrogen to phosphorus ratio. The collection and processing of beach wrack is in line with the European Union (EU) waste law (Regulation, 2008/98/EC), where its recycling is a priority. Importantly, the collection and removal of near-shore beach wrack is associated with estimated costs between 6 and 120 € per ton of disposed wrack or 38 € per meter of beach and an additional 85 € per ton for material drying (Mossbauer et al., 2012; Barbot et al., 2016). Hence, new value chains and business models should be developed to change the perspective of beach wrack from a cost-intensive to a profitable activity. Traditionally, this biomass was either composted (also with fresh-terrestrial green waste, thus contributing to the blue-green innovations) and utilized as a biofertilizer or for biomethane production (Barbot et al., 2016; Weinberger et al., 2020; Borchert et al., 2021). However, there are still unexploited opportunities to maximize its valorization potential in other industries, thus maintaining the circularity of financial and biological resources.
By-Products From Fishery and Aquaculture
According to the Food and Agriculture Organization (FAO), in 2018, about 88% of 179 million tons of total fish production was utilized for direct human consumption, while the remaining 12% was used for non-food purposes (FAO, 2020). Until now, all by-products of fish processing, estimated at up to 75% of the raw material (Rustad et al., 2011), were discarded or used directly as feed, in silage or fertilizers. Such fishery waste includes fish or by-catch species, having low or no commercial value, undersized or damaged commercial species, species of commercial value caught in insufficient amount to warrant a sale, as well as unused fish tissues, including fins, heads, skin and viscera (WRAP, 2012; Caruso, 2016). Nowadays, there is an increasing trend of using these by-products as materials to produce fishmeal and fish oil (currently estimated at 25–35%) (FAO, 2020). Moreover, other aquatic organisms, such as shellfish, seaweeds and aquatic plants, are being increasingly used in experimental projects for the production of food, feed, pharmaceutical and cosmetic products, as well as to produce biomaterials, biofuels and to improve the efficiency of water treatment (Barbot et al., 2016; Nisticò, 2017; Poblete-Castro et al., 2020). Nevertheless, the world’s fisheries discards are still high, exceeding 10%, equivalent to 9.1 million tons of the total production of marine fishery catch (as per 2014 data) and around 5.2 million tons/yr in the European Union (Pérez Roda et al., 2019). In fisheries and aquaculture combined, it is estimated that 35% of the global harvest is either lost or wasted every year (FAO, 2020). Therefore, additional valorization approaches are needed to minimize the amounts of discards and maintain the circularity of resources.
Since January 2019, discards at sea have become an illegal practice in the waters of the EU, increasing the need for a complete valorization of all landed fishery waste, both for large-scale and small fisheries operators. This creates a sociological and environmental imperative for the reduction of these discards and utilization of fishery waste as a potential resource (Rustad et al., 2011; Caruso, 2016). Regulatory frameworks, capacity building, services, infrastructure, as well as physical access to markets are therefore needed to reduce fish loss and waste. The above-mentioned measures can also contribute to improving resource sustainability and food security (FAO, 2020). Furthermore, the need for responsible fisheries and aquaculture practices to help preserve aquatic biodiversity has driven the search for alternative valorization routes for fish waste streams, such as heads, guts, skins, scales, and bones.
General Processing Aspects
When processing beach wrack or fishery by-products, it is crucial to start their processing as early as possible to minimize physical, chemical, and microbial degradation. To preserve the raw materials for as long as possible, chilling, freezing or acidification using organic acids is performed (Rustad et al., 2011). In general, by-products from side streams or waste can be valorized as they are, using appropriate extraction, purification and downstream processing methods (Figure 1). Alternative methods, such as hydrolysis, ensilaging, fermentation and gelation (surimi production from the fish protein fraction) with food-grade enzymes and/or microorganisms have been optimized for extraction and production of concentrated marine oils, functional protein food ingredients and products, as well as pharmaceutical-grade biopolymers and textiles (Kim, 2013; Lopez-Caballero et al., 2014). These methods are easily used to a wide range of industrial applications in the food, nutraceutical or biomedical sectors. This valorization approach favors the circular economy concept, providing an immediate solution for the reuse or recycling of materials. Biobased production of biopolymers can then be coupled with either sourcing the producer organism and its growth in bioreactors or microbial production in heterologous systems to guarantee a sustainable supply of the biopolymers. For example, hyaluronic acid production was demonstrated in Bacillus subtilis, Lactococcus lactis, and Pichia pastoris (de Oliveira et al., 2016; Badri et al., 2019). However, there are still challenges to successfully produce biopolymers using microorganisms, namely the complex regulatory mechanisms and in vivo polymerization. Nevertheless, fine-tuning of the expression of endogenous or heterologous genes has now advanced using molecular techniques, applying inducible and/or controllable genetic switches, such as CRISPR-Cas tools.
FIGURE 1
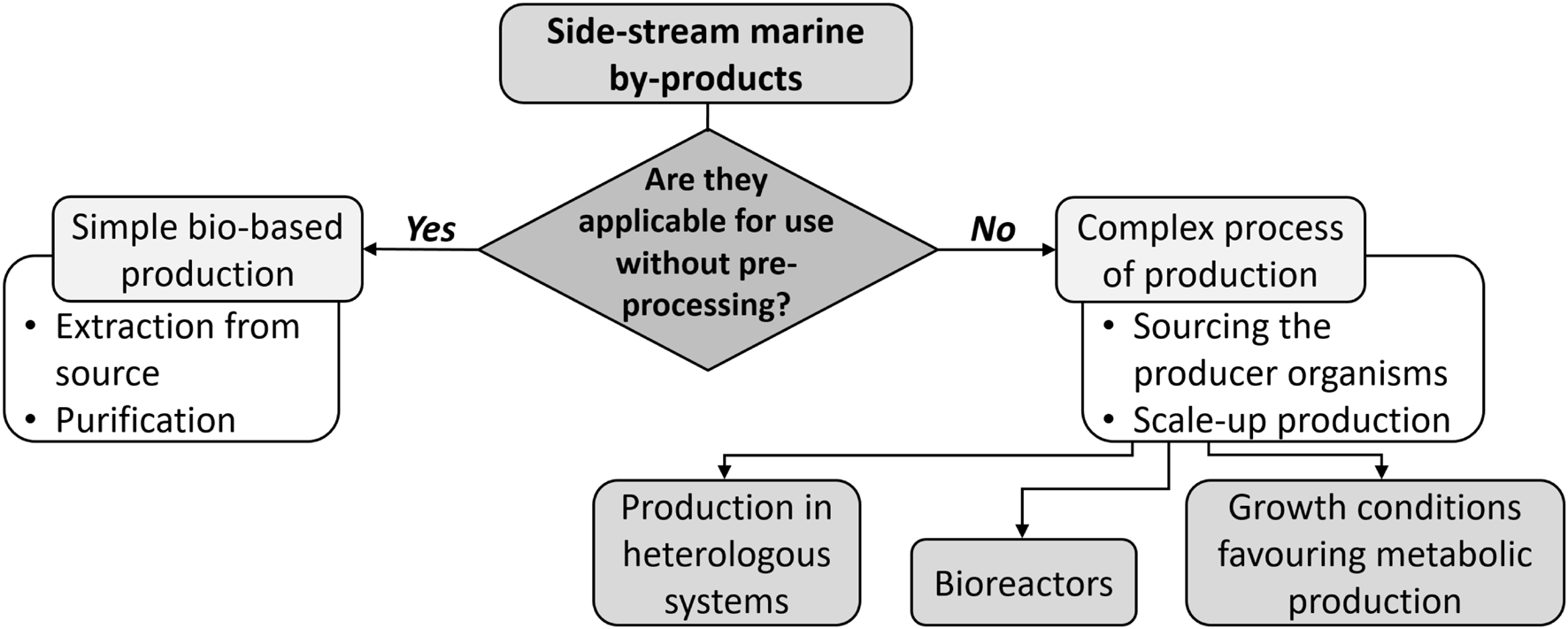
The options for utilization of side-stream marine by-products: simple production or combination of bio-based production and growth of producer species or production in heterologous systems, guaranteeing a sustainable sourcing (in case of bio-based production) or a sustainable supply (in case of the complex process of production) of marine biopolymers.
The remainder of this article is structured as follows. Section “Direct valorization of marine waste biomass” describes the alternatives for direct utilization of marine waste biomass. Section “Valorization of marine biopolymers” presents the potential applications of biopolymers from waste biomass, while the overarching European strategies are depicted in Section “Valorization of marine biomass as a European strategy.” Finally, Section “Case studies” describes selected case studies in Norway, Scotland and the Baltic Sea. These Northern European solutions could serve to provide future transfer of knowledge action plans toward Southern Europe and beyond.
Direct Valorization of Marine Waste Biomass
Biosorbents
The basic definition of biosorption is the removal of various contaminants using materials of biological origin (biomass). It is a process typically independent of energy, employing dead or waste biomass of low cost. Most biological waste materials can be efficiently and directly used as readily available biosorbents for removal of organic and inorganic pollutants and radionuclides from e.g., residual waters (de Freitas et al., 2019; Silva et al., 2019; Beni and Esmaeili, 2020; Fawzy and Gomaa, 2020; Magesh et al., 2020; Ubando et al., 2021; Table 1). Biosorption of pollutants on biosorbents usually includes several mechanisms based on the presence of appropriate functional groups (e.g., hydroxyl, carboxyl, amino, phosphate, sulfate, amide, imidazole, thiol, acetamide, etc.), which can interact with target pollutants. The adsorption efficiency can be increased through appropriate physical or chemical treatments (Bulgariu and Bulgariu, 2016; Safarik et al., 2018). The exhausted biosorbents have to be appropriately treated, including regeneration and reuse of biosorbents in multiple biosorption cycles. The totally exhausted biosorbents can then be used as fertilizers for soil remediation, or to form biochar through pyrolysis (Bãdescu et al., 2018). On the contrary, bioaccumulation employs living (micro-) organisms, and appropriate nutrients must be added continuously. Most pollutants are accumulated inside the cell, and thus the possibility of regeneration and repeated use is very limited (Beni and Esmaeili, 2020).
TABLE 1
| Type of marine biomass | Taxonomy | Target pollutant | References |
| Heavy metals | |||
| Algae | Enteromorpha prolifera (magnetically modified) Cladophora sericea | Cobalt, Nickel Antimony | Zhong et al., 2020 Ungureanu et al., 2016 Ungureanu et al., 2016 |
| Chlorella vulgaris | Cadmium | Cheng et al., 2017 | |
| Sargassum sp. | Lead, Copper, Cadmium, Nickel, Zinc, Cobalt, Mercury | Davis et al., 2003; Sheng et al., 2007; He and Chen, 2014; Saldarriaga-Hernandez et al., 2020 | |
| Sargassum cinereum | Lead | Kishore Kumar et al., 2018 | |
| Sargassum filipendula | Silver, Cadmium, Chromium, Copper, Nickel, Zinc | Cardoso et al., 2017 | |
| Padina pavonia | Uranium, Lead | Aytas et al., 2014; He and Chen, 2014 | |
| Pelvetia canaliculata | Nickel | Bhatnagar et al., 2012 | |
| Ulva fasciata | Chromium, Copper | He and Chen, 2014; Shobier et al., 2020 | |
| Ulva intestinalis | Mercury | Fabre et al., 2020 | |
| Ulva lactuca | Mercury, Cadmium | He and Chen, 2014; Fabre et al., 2020 | |
| Ulva rigida | Antimony | Ungureanu et al., 2016 | |
| Ulva rigida | Arsenic, Selenium | Filote et al., 2017 | |
| Crab shell | Clistocoeloma sinensis | Lead, Zinc | Zhou et al., 2016 |
| Cancer pagurus | Mercury | Rae et al., 2009 | |
| Shrimp shell | Farfantepenaeus aztecus | Nickel | Hernández-Estévez and Cristiani-Urbina, 2014 |
| Industrial dyes | |||
| Algae | Bifurcaria bifurcata | Reactive Blue 19, Methylene Blue | Bouzikri et al., 2020 |
| Caulerpa lentillifera | Astrazon® Blue® FGRL, Astrazon Red GTLN | Marungrueng and Pavasant, 2007 | |
| Cymopolia barbata (magnetically modified) Cystoseira barbata (magnetically modified) Euchema spinosum | Safranin O Methylene Blue Methylene Blue | Mullerova et al., 2019 Ozudogru et al., 2016 Mokhtar et al., 2017 | |
| Kappaphycus alverezii | Methylene Blue | Vijayaraghavan et al., 2015 | |
| Nizamuddina zanardini | Acid Black 1 | Esmaeli et al., 2013 | |
| Sargassum sp. | Anionic dyes, Methylene Blue, Malachite Green, Acid Black | Saldarriaga-Hernandez et al., 2020 | |
| Sargassum horneri (magnetically modified) Sargassum swartzii | Acridine orange, Crystal violet, Malachite Green, Methylene Blue, Safranin O Malachite Green | Angelova et al., 2016 Jerold and Sivasubramanian, 2016 | |
| Sargassum wightii | Brilliant Green | Vigneshpriya et al., 2017 | |
| Seagrass Crab shell | Posidonia oceanica (magnetically modified) Penaeus indicus | Acridine Orange, Bismarck Brown Y, Brilliant Green, Crystal Violet, Methylene Blue, Nile Blue A, Safranin O Acid Blue 25 | Safarik et al., 2016a Daneshvar et al., 2014 |
| Inorganic nutrients | |||
| Algae | Gracilaria birdiae | Nitrates, phosphates | Marinho-Soriano et al., 2009 |
| Kappaphycus alverezii | Phosphates | Rathod et al., 2014 | |
| Organic compounds | |||
| Algae | Chlorella vulgaris | Tetracycline | de Godos et al., 2012 |
| Chlorella pyrenoidosa | Progesterone, Norgestrel, Triclosan | Wang et al., 2013; Peng et al., 2014 | |
| Lessonia nigrescens | Sulfamethoxazole | Navarro et al., 2014 | |
| Macrocystis integrifolia | Sulfacetamide | Navarro et al., 2014 | |
| Synechocystis sp. | Paracetamol | Escapa et al., 2017 | |
Potential of marine biomass to act as biosorbents of organic and inorganic pollutants.
Seagrasses, macroalgae, as well as microalgae, have been extensively studied as biosorbents for the removal of various aquatic pollutants (e.g., metal ions, dyes, etc.) (Table 1). The algal cell wall is composed of polysaccharides (e.g., alginate), which have multiple functional groups that act as binding sites for metals and other pollutants. Brown algae, in particular, are very efficient biosorbents for the removal of different metal ions from water due to their unique features, such as high alginate content, higher uptake capacities, and their unlimited availability in the oceans (Davis et al., 2003). The biosorption capacity of algae for specific metals depends on several factors, such as the number of binding sites in the algae, the sites’ accessibility, the availability of the site and affinity between the binding site and the metal (Davis et al., 2003). For example, Saldarriaga-Hernandez et al. (2020) reviewed the bioremediation potential of Sargassum sp. in a circular economy approach. Brown marine macroalgae as natural cation exchangers for the removal of toxic metals were also reviewed (Figueira et al., 2000; Davis et al., 2003; Bilal et al., 2018; Mazur et al., 2018). He and Chen (2014) published a comprehensive review of heavy metal biosorption by algal biomass and discussed materials, performances, chemistry and modeling simulation tools. Biomass from marine macroalgae and seagrasses can be obtained in large quantities at a low price, provided that their harvesting is sustainable and does not affect the ecosystem balance in coastal areas. Different species of marine algae have been used to remove various metal ions, colorants (dyes) and other pollutants from water (Sheng et al., 2007; Bhatnagar et al., 2012; Aytas et al., 2014; Navarro et al., 2014; Rathod et al., 2014; Vijayaraghavan et al., 2015; Jerold and Sivasubramanian, 2016; Ungureanu et al., 2016; Mokhtar et al., 2017; Vigneshpriya et al., 2017; Arumugam et al., 2018; Kishore Kumar et al., 2018; Silva et al., 2019; Bouzikri et al., 2020; Coração et al., 2020; Fabre et al., 2020; Safarik et al., 2020a,b; Shobier et al., 2020; Table 1). Also, waste macroalgae biomass obtained after selected industrial processes can be employed for the same purposes (Safarik et al., 2018; Santos et al., 2018). Several commercially available biosorbents, such as Alga SORBTM, Bio-recovery Systems Inc., and B.V. Sorbex use marine algae. Despite the ease of the algal biosorption process, the technology is not yet recognized, and it requires further research and development efforts at a larger scale using industrial effluents.
Some studies attempted to modify algae to enhance their biosorption (Volesky, 2003), but this has not been considered favorable since it may increase the cost, both for the treatment onside and of the polluted biosorbent afterward, without always improving the sorption capacity. Moreover, active sorption sites are sometimes destroyed due to chemical treatment, resulting in lower biosorption than the untreated algal biosorbent. Despite these discouraging drawbacks, research is still progressing to modify specific properties of biosorbents to increase the biosorption capacities or simplify their recovery. An interesting strategy employs magnetic modification to obtain smart biomaterials, exhibiting several types of responses to an external magnetic field. Such materials can be easily and selectively separated from desired environments using permanent magnets, electromagnets or appropriate magnetic separators (Kanjilal and Bhattacharjee, 2018; Safarik et al., 2018; Hassan et al., 2020). Thus, magnetically responsive marine-derived biosorbents can find significant applications, especially for removing various inorganic and organic pollutants from wastewater. Several procedures can be employed for magnetic modification of marine-based biomass. Usually, magnetic iron oxide nano- and microparticles (Laurent et al., 2008) are used as magnetic labels for marine biomass modification (Safarik et al., 2016b, 2018, 2020a,b). Magnetically modified Sargassum horneri biomass was employed to remove several organic dyes (Angelova et al., 2016), while Sargassum swartzii modified with nanoscale zero-valence iron particles was used for crystal violet biosorption (Jerold et al., 2017). The brown alga Cystoseira barbata coated with magnetite particles was used for the removal of methylene blue from aqueous solution (Ozudogru et al., 2016), while the tropical marine green calcareous alga Cymopolia barbata, magnetically modified with microwave-synthesized iron oxide nano- and microparticles, was used for the removal of safranin O (Mullerova et al., 2019). The marine seagrass Posidonia oceanica was magnetically modified using three procedures and subsequently used to remove seven water-soluble organic dyes (Safarik et al., 2016a; Table 1).
Biochar is an intensively studied carbon-based material produced by pyrolysis of biomass in the absence of oxygen. The study of Macreadie et al. (2017) provided clear evidence that the conversion of beach wrack to biochar could be a viable environmental solution for dealing with unwanted wrack, offsetting carbon emissions and providing a commercially valuable product for agriculture and wastewater and its sludge treatment. The use of macroalgae biomass for biochar (charcoal) production, with energy co-generation potential, provides a value-driven model to sequester carbon and recycle nutrients (Bird et al., 2011). In specific cases, macroalgal biomass can be used as biochar and magnetic biochar precursor, as shown in several cases using brown or green macroalgae (Jung et al., 2016; Son et al., 2018a, b; Foroutan et al., 2019; Jung et al., 2019; Yang et al., 2019). The prepared magnetic biochars were employed as efficient adsorbents of acetylsalicylic acid, water-soluble organic dyes and copper ions (Jung et al., 2019).
Biofuels
Almost 80% of the world’s energy supply is provided by fossil fuels (Balachandar et al., 2013). Energy demands are increasing worldwide due to industrialization, population growth and modernization, leading to the over exploitation of limited available natural fossil fuel reserves (Kumar and Thakur, 2018; Kumar et al., 2020). These fuels represent a significant threat to the environment due to their greenhouse gases (GHG) emissions, which are the main cause of global warming. This stimulates the research on bioenergy production from biomass (Kumar and Thakur, 2018; Karkal and Kudre, 2020). Biofuel and related technologies are considered renewable alternatives to fossil-based fuels due to their sustainable features to overcome the global energy demand (Klavins et al., 2018). As biomass production can be quite expensive to meet the energy needs alone, energy production from waste biomass with the biorefinery approach is alternatively used. Waste biorefineries are attracting significant interest worldwide as sustainable waste management solutions (Khoo et al., 2019). In this case, both required energy needs are met, and a solution to the waste management problem is found in the circular economy context (Tuck et al., 2012; Ahrens et al., 2017).
Production of the first-generation of biofuels is mainly based on the biomass of terrestrial plants, such as corn, soybean, sugar cane, palm oil, among others (Chen et al., 2017; Yu and Tsang, 2017; Shuba and Kifle, 2018). However, their utilization also creates ecosystem damage, water shortage and food vs. fuel debate. Considering the problems related to the first-generation of biofuels, the second- and the third- generations have become alternative options, which are respectively produced from waste materials (plant and agricultural waste, municipal sludge) and microorganisms, without disrupting the environment and natural resources (Kumar et al., 2016, 2018; Shuba and Kifle, 2018).
The incorporation of wastewater treatment with microalgae for biofuel production has both environmental and economic benefits. In this process, microalgae are used as biosorbents before biofuel production. Different conversion technologies are used for the production of biofuels (Figure 2), such as biochemical – anaerobic digestion (biogas) and fermentation (bioethanol), and chemical conversion – extraction and transesterification (biodiesel) (Chen et al., 2015; Sikarwar et al., 2017; Kumar et al., 2020). In addition, several non-fermentation options for the production of energy from macroalgae are available, including direct combustion (heat energy), gasification (syngas for heat and power generation, liquefaction and production of hydrogen) and pyrolysis (production of liquid bio-oil, syngas and charcoal) (Bruhn et al., 2011; Luo and Zhou, 2012; Rowbotham et al., 2012).
FIGURE 2
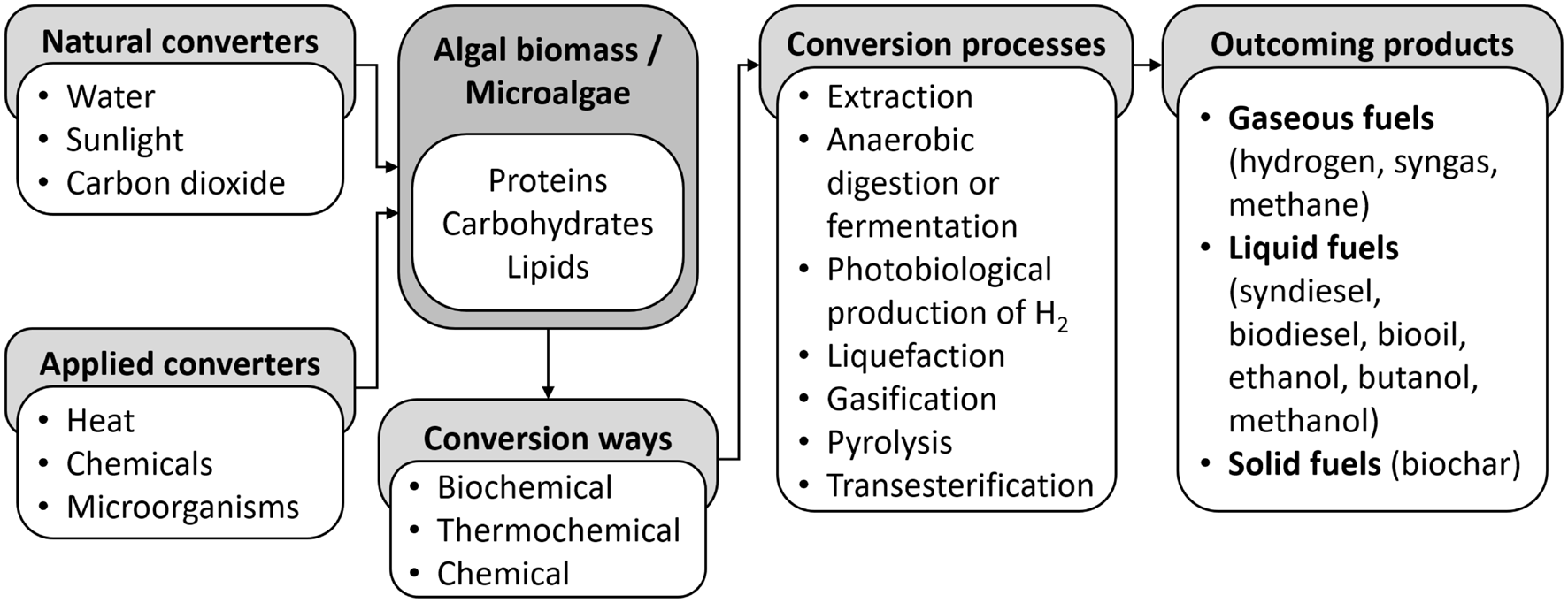
Schematic overview of conversion of algal biomass into economically significant products – biofuels, indicating the major converters, conversion ways and processes (Sikarwar et al., 2017; Kumar et al., 2020).
Torrefaction, also known as destructive drying and slow pyrolysis, is a mild pyrolytic process that recently received wide attention from the scientific community, as both a method of pre-treatment and upgrade of low-quality fuels (Chew and Doshi, 2011; Chen et al., 2015), as well as for the production of biochar. This process may be organized at scales ranging from extensive industrial facilities down to the individual farms (Lehmann and Joseph, 2009) and even at the domestic level (Whitman and Lehmann, 2009), making it applicable to various socioeconomic situations.
Table 2 summarizes the most important algal biofuels, their production mechanisms and applications. Sustainability is the most important issue for biofuel production. Hence, currently, algae, especially microalgae are the most promising source for biofuels due to their availability and continuous supply. In addition, different biofuel production techniques can be applied depending on the type of algae and biofuel. Thus, the use of algae can still be regarded as a viable option for the next generation of biofuels.
TABLE 2
| Type of biofuel | Content | Process | Extraction protocol | Potential algae | Application | References |
| Biodiesel | Mixture of fatty acid alkyl esters. All the algal lipid contents are extracted from the biomass | Transesterification | These extracted lipid contents are majorly composed of triglycerides, which react with alcohol to produce fatty acid methyl esters (FAMEs). | Scenedesmus dimorphus, Nannochloropsis sp., Chlorella vulgaris | Recently FAME is mixed in petro-diesel in a specific proportion (max 20%) and is directly used in the diesel engines without any engine modifications | Chisti, 2007; Adeniyi et al., 2018 |
| Bioethanol | Algae store high amounts of carbohydrates mainly glucose, galactose, and mannitol | Hydrolysis followed by fermentation of sugars | Liquefaction is carried out to increase the efficiency of the produced ethanol. | Eucheuma spinosum | The production of bioethanol for fuel from algae waste could be quite efficient as the industrial waste of Eucheuma spinosum can be converted into bioethanol with an efficiency of 75%. | Chen et al., 2015; Khan et al., 2018; Alfonsín et al., 2019 |
| Biogas | Algal biomass | Methane and carbon dioxide | Anaerobically digested to convert its organic contents into bio-gas. | Ulva lactuca, Chlorella minutissima | Domestic as well as industrial purposes | Cirne et al., 2007; Koçer and Özçimen, 2018 |
| Bio-oil | Algal biomass | Thermal cracking Pyrolysis | Thermal cracking is carried out to extract the lipid contents from the biomass at a very low calorific power range. Pyrolysis is the conversion of algal biomass into bio-oil in the absence of oxygen. | Desmodesmus sp., Gelidium sesquipedale | Conversion into liquid fuel (bio-oil) | Chaiwong et al., 2013; Gang et al., 2017 |
The main algal biofuels and their use.
Fertilizers and Soil Improvers
Beach wrack can be utilized as a biofertilizer for the cultivation and growth of plants. Nowadays, biofertilizers are preferred over chemical fertilizers due to their environmentally friendly and cost-effective nature. Biofertilizers contain microorganisms that can fix nitrogen, solubilize phosphate and promote plant growth. The shells of many bivalves, e.g., blue mussels and oysters, are rich in CaCO3, a mineral currently mined from limestone, representing a widely exploited resource for many industrial applications in agriculture, as a biofilter medium for wastewater treatment, or even cement production (Oso et al., 2011; Yao et al., 2014; Morris et al., 2019; Scialla et al., 2020). In Galicia (Spain), the second global largest aquaculture producer of blue mussels (Mytilus galloprovincialis), their shells are commonly used in agriculture for liming the acidified soil (Morris et al., 2019) or for the absorption of heavy metals (e.g., arsenic) to reduce soil pollution (Osorio-López et al., 2014). Marine organic waste, such as seagrasses washed ashore, can also be considered as an alternative and sustainable fertilizer source because of its content of essential macro- and microelements (Bãdescu et al., 2017; Emadodin et al., 2020). Algae contain regulatory macro- and micronutrients, plant hormones such as cytokines, auxins, gibberellins and betaines that can increase plant growth, as well as vitamins, amino acids and metabolites with antibacterial and antifungal activity, which improve productivity. However, the low concentration of phosphorus, the presence of litter or toxic materials in the biomass, especially if the sampling area is subjected to high levels of anthropogenic pressure, can be of concern for using beach wrack as fertilizer (Villares et al., 2016). The salinity of seaweed leachates can be another obstacle; thus, applying it at appropriate rates and leaching of salts before the application can be crucial to obtain an optimal beneficial effect on plant root development. Although already applied in practice, the use of marine algae as biofertilizers is an ongoing field of research. One of the remaining research questions is to understand what role fertilizers from seaweed play in marginal coastal conditions by stimulating the growth of terrestrial plants or for the provision of specific nutrient elements. As an example, beer barley, grown in Scotland, is traditionally fertilized with seaweed and is known for the ability to cope with marginal, high pH soils without inorganic fertilizer addition (Brown et al., 2020).
Biochar has demonstrated applications as a soil enhancer, capable of improving water holding capacity, nutrient status and microbial ecology of many soils (Lehmann et al., 2006; Lehmann and Joseph, 2009; Thies and Rillig, 2012). Bird et al. (2011) showed that macroalgal biochar has properties that provide direct nutrient benefits to soils and stimulate crop productivity and are especially useful for application on acidic soils. In contrast to bioenergy, in which all CO2 that is fixed in the biomass by photosynthesis is returned to the atmosphere quickly as fossil carbon emissions are offset, biochar has the potential for more significant impact on climate through its enhancement of the productivity of infertile soils and its effects on soil GHG fluxes (Woolf et al., 2010).
Feed
For decades, fishery waste has largely been used in fishmeal production (due to its high protein and lipid contents), but this application is no longer considered the only option. Vázquez et al. (2019a, b) described alternative processes to valorize fish discards and produce fish mince, gelatins, oils and fish protein hydrolysates to be used as aquaculture feed ingredients. Wastes generated from the industrial processing of various fish species can also be turned into peptones (water-soluble products of partial hydrolysis of proteins to be used as a liquid medium for growing bacteria) (Vázquez et al., 2020). Mussel meat is rich in protein, lipids, carbohydrates, minerals and carotenoid pigments (Grienke et al., 2014) with potential application as food/feed supplements, preservation agents and enzymes. Seaweeds were traditionally used for animal feeding, either as aquaculture or cattle feed (Araújo et al., 2021). The interest in their use as feed was increased after the 1960s when Norway started producing seaweed meal from kelp (Makkar et al., 2016). Nowadays, they are still used as additional feed for free-range ruminants grazing on beach cast seaweeds in the coastal areas (Bay-Larsen et al., 2016). Brown seaweeds are more often used as feed because of their large size and ease of harvesting (Makkar et al., 2016). Seaweeds can supply the rumen with high amounts of rumen-degradable protein or can be used as a source of digestible bypass protein (Tayyab et al., 2016; Molina-Alcaide et al., 2017). In remote regions, like the Arctic, seaweeds are considered as local protein sources for sustainable sheep farming to replace imported soya (Bay-Larsen et al., 2018). However, the latest research highlights the challenges when applying seaweed proteins in animal feed (Novoa-Garrido et al., 2017; Özkan Gülzari et al., 2019; Emblemsvåg et al., 2020; Koesling et al., 2021; Krogdahl et al., 2021). Some seaweed species, for example, Asparagopsis taxiformis, have antimethanogenic activity on fermentation and can inhibit methanogenesis in the rumen at very low inclusion levels (Machado et al., 2016). Hence, the most appropriate method for processing such seaweeds and feeding to livestock in systems with variable feed quality and content has not been determined yet (Kinley et al., 2016).
Seaweeds tend to accumulate heavy metals (e.g., arsenic) or iodine. Consequently, using polluted beach wrack for feeding could negatively affect animal health. The decomposition and pollution, together with variable and undefined composition, may make beach wrack unsuitable for feed, and considerable sorting and cleaning may be required. Moreover, to have a continuous supply for feeding purposes, industrial cultivation of algae might be considered.
Additional Direct Valorization of Side Streams
Shells can be further exploited as a useful source for the production of biocomposites (Gigante et al., 2020), bio-based insulation material in environmentally responsive building solutions (Martínez-García et al., 2020) or even as a substitute for concrete components to reduce the dependency on conventional natural materials and to decrease the emission of GHG (El Biriane and Barbachi, 2020). Furthermore, seashells can be used as a calcium source to produce bioactive materials in tissue engineering, such as hydroxyapatite, which is the main inorganic phase of the bone (Hart, 2020; Hembrick-Holloman et al., 2020). Finally, direct human patch applications (fish skin grafts) of high ω-3 rich fish skins, like cod or tilapia, are also used for tissue regeneration in chronic or trauma wounds (Lima-Junior et al., 2019).
Valorization of Marine Biopolymers
The valorization of side stream biopolymers into useful compounds can have positive environmental, economic, social and technical added values, contributing to the circular economy development. Marine polysaccharides that are present in various marine organisms have the broadest valorization potential. The molecular structure of marine polysaccharides is characterized by long molecular chains of repeating monosaccharide units linked together by glycosidic bonds (Nitta and Numata, 2013). Serving mostly as energy storage and with structural functions, they are derived from various marine resources, including crustaceans and marine algae (Raveendran et al., 2013). Marine polysaccharides are characterized by outstanding chemical and structural diversity, and due to their biocompatibility and biodegradability, they have been used as a material of choice in numerous biomedical applications (Table 3). Exhibiting a wide range of bioactivities (such as anticoagulant, antioxidant, antimicrobial, anticancer, immunomodulatory, or antiviral), they are ideal candidates as low-cost, renewable, non-toxic and abundant biomaterials for the development of novel biosystems, such as 3D scaffolds, nanofibers, membranes, hydrogels, and bioinks for tissue engineering, drug delivery and wound dressing applications (Manivasagan et al., 2017).
TABLE 3
| Biopolymer | Structure | Function | Extraction protocol | Application | References |
| Cellulose | Linear homo-polysaccharide of D-glucose units connected by (1,4)-β-glycosidic linkages | The main structural component of the cell walls in higher plants and many seaweeds | Sequential extraction based on three steps: (1) initial Soxhlet extraction to remove lipidic compounds and pigments using organic solvents, (2) mild acid treatment (usually known as bleaching) to remove lignin and some hemicelluloses and (3) basic treatment to eliminate hemicelluloses | Paper industry, food packaging, and pharmaceutical applications | Abdollahi et al., 2013a; Ramamoorthy et al., 2015; Trache et al., 2016; Khalil et al., 2017 |
| Agar | Mixture of the linear polysaccharide agarose and agaropectin | Structural components of the cell walls in several red seaweeds, such as Gelidium and Gracilaria spp. | Application of alkaline pre-treatments, followed by high-temperature and high-pressure extraction treatments, high-temperature filtration and several freeze-thawing cycles. Alternative extraction protocols, based on simplified heating treatments or using ultrasound-assisted methods, have been recently explored for the production of less purified agar-based extracts. These less purified agar-based extracts show similar rheological behavior to the pure agar, but they form softer gels | One of the phycocolloids more widely used in the microbiology field, as well as in the food industry as gelling agent and stabilizing agent for food products | Chemat et al., 2017; Flórez-Fernández et al., 2017; Martínez-Sanz et al., 2019, 2020b |
| Carrageenan | Backbone of disaccharide repeating units of alternating α-1,3-linked D-galactopyranosyl and β-1,4-linked D-galactopyranosyl groups with 3,6-anhydrogalactose residues | Present in the cell walls and the intercellular matrix of some red seaweeds of the class Rhodophyceae | Before extraction, the seaweeds are washed for removal of impurities and in some cases are subjected to pre-treatment with organic solvents for de-colorization. The extraction process involves the application of physical separation methods, followed by alkaline treatments at high temperatures, removal of insoluble residues through filtration and filtrate concentration | Used in various biomedical applications owing to their anticancer, immunomodulatory, anticoagulant and antihyperlipidemic properties. Additionally, they find applications in the pharmaceutical and cosmetic industries. They are also widely used in the food industry due to their good texturizing properties (thickening and/or gelling capacity) | Fan et al., 2008; Manivasagan et al., 2017; Rahmati et al., 2019 |
| Alginate | Linear anionic polysaccharides, forming a block copolymer of β-1,4-D-mannuronic acid (M-blocks) and α-L-guluronic acid (G-blocks) in which the uronic blocks are organized in a heteropolymeric (alternating M and G residues) or homopolymeric (consecutive M or G residues) arrangement | Found in the cell walls from some brown algae (such as Ascophyllum nodosum, Laminaria spp., Lessonia and Macrocystis, among others) and brown seaweeds of the class Phaeophyceae | Pre-treatment step is usually employed to remove pigments and lipids using various solvents and solvent mixtures. The pre-treated seaweed may be further exposed to vacuum or freeze-drying methods. Seaweeds are then treated with hot aqueous, acidic or salt solutions at increased temperatures and for extended time periods, followed by precipitation | Wound healing and other biomedical applications | Goh et al., 2012; Abdollahi et al., 2013b; Sirviö et al., 2014; Varaprasad et al., 2020 |
| Fucoidans | α-1,3-fucopyranose residues or alternating α-1,3- and linked α-1,4- fucopyranosyls | Structural components in the cell walls, especially those from the families Fucaceae and Laminariaceae | Under acidic conditions, alginate is removed as water-insoluble alginic acid, leaving a rather pure fucoidan extract. Subsequently, the extracted fucoidan is precipitated by the addition of high volumes of alcohol or cationic surfactants, such as hexadecyltrimethylammonium bromide (Cetavlon®) at low temperatures (4°C) | Due to their non-toxic nature, in conjunction with their antioxidant, antiinflammatory, anticoagulant, antithrombotic and antitumor properties, fucoidans have been used in drug delivery and other biomedical applications | Mautner, 1954; Karunanithi et al., 2016; Dobrinčić et al., 2020; Jönsson et al., 2020 |
| Ulvans | Charged anionic sulphated polysaccharides composed mainly of L-rhamnose, D-xylose, D-glucose, and D-glucuronic and iduronic acids | 8–35% of the dry weight in green seaweeds from the class Ulvales | Extraction with hot water and subsequent precipitation with ethanol | Texture modifiers in the food industry, antioxidant, immunomodulation, antiviral, antihyperlipidemic and anticancer pharmaceutical applications | Lahaye and Robic, 2007; Toskas et al., 2012; Kikionis et al., 2015; Tziveleka et al., 2020 |
| Chitin, chitosan | Chitin: linear polysaccharide consisting of β-1,4- linked 2-amino-2-deoxy-β-D-glucopyranose. Chitosan: polycationic linear polysaccharide consisting of β-1,4-linked N-acetyl-2 amino-2-deoxy-D-glucose (N-acetylglucosamine) and 2-amino-2-deoxy-D-glucose (glucosamine) residues | Crustacean exoskeletons, such as crabs and shrimps, also found in squid, corals, sponges, fungi and yeasts | Chitin is commercially extracted chemically or enzymatically from crab and shrimp shells. Chitosan is commercially obtained from chitin involving chemical methods, using corrosive acidic and basic solutions, which have adverse environmental effects | Applications in the biomedical field due to antimicrobial, anticoagulant and wound-healing properties, food packaging | Kumar, 2000; Fan et al., 2008; Ifuku et al., 2009; Danti et al., 2019; Yadav et al., 2019 |
| Laminarin | Low molecular weight β-glucan | Main storage polysaccharide in some brown species (especially those from the Laminariaceae family | Hot water extraction | Natural health product, due to the antitumor, anti-inflammatory, anticoagulant, and antioxidant activity | Guiry and Blunden, 1991; Kadam et al., 2015 |
| Proteins – collagen and gelatin | Collagen: three helical α-chains are tightly packed together, forming a final superhelix with a hydrophobic core. Gelatin is a partially hydrolyzed form of collagen | Structural protein derived from fish skin and bones and marine invertebrates | Bioconversion of fish skin may include extraction by acid-alkaline hydrolysis, enzymatic hydrolysis and fermentation, using microorganisms or ultrasonic treatment without changing the molecule and enzymatic action | Pharmaceutical/cosmeceutical, nutraceutical and food packaging industries due to their biocompatibility and easy biodegradability. Antihypertensive, antioxidant, antimicrobial, neuroprotective, antihyperglycaemic, and antiaging properties | Liu et al., 2015; Huang et al., 2016; León-López et al., 2019 |
The main marine biopolymers and their use.
The major source of marine polysaccharides are algae, with carrageenans mainly present in red algae, alginates and fucoidans in brown algae and ulvans in green algae, ranging from 4 to 76% dry weight (Kraan, 2012). ι-Carrageenan, a sulfated polysaccharide from red seaweed, has been approved by FDA, as Carragelose®, for the treatment of viral and respiratory diseases (Lu et al., 2021). Moreover, many crustacean shells (e.g., those of shrimps) are composed of chitin, a nitrogen-containing linear polysaccharide with wide industrial use, for example, in drug delivery, cosmetics and food. Chitin is regarded as the second most abundant polysaccharide in nature, after cellulose, used for the commercial production of chitosan, a water-soluble derivative obtained by demineralization, deproteinization, and decolourization of chitin (Jiménez-Gómez and Cecilia, 2020).
Novel Materials for Biomedical Applications
The extraordinary biocompatibility, non-antigenicity, chelating ability and bioavailability of marine biopolymers make them suitable materials for biomedical applications. Due to the broad spectrum of reported bioactivities exhibited by marine polysaccharides, they are ideal candidates for novel biomedical systems and have been utilized in various formulations for drug delivery, wound healing and tissue engineering applications. The interest of the biomedical sector in marine polysaccharides is steadily increasing not only because of their natural origin and their unique biological and physicochemical properties, but also due to their stability, safety and high availability at a relatively low cost (Venkatesan et al., 2017; Choi and Ben-Nissan, 2019; Rahmati et al., 2019; Bilal and Iqbal, 2020).
In the pharmaceutical sector, marine polysaccharides have been used as binders, stabilizers, thickeners, matrix materials, emulsifiers, and suspending agents (Figure 3). Over the years, they have been utilized in various formulations, such as gels and hydrogels, micro/nanoparticles (MPs/NPs), films and membranes, nanofibers, as well as 3D porous structures, serving as drug release modifiers, bioadhesives, coatings, wound dressing materials and tissue engineering scaffolds for various biomedical applications (Ruocco et al., 2016; Vanparijs et al., 2017; Joshi et al., 2019).
FIGURE 3
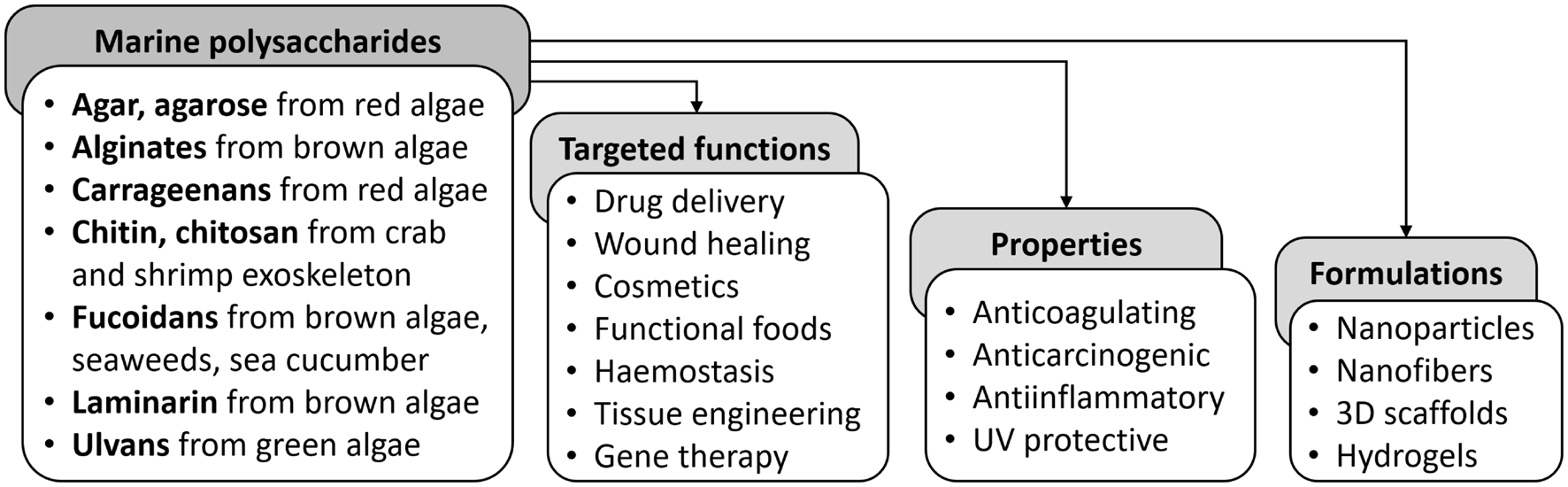
Examples of marine polysaccharides and their applicability for biomedical purposes (Ruocco et al., 2016; Joshi et al., 2019).
Mussel byssus contains high levels of collagen, another widely used raw material (Rodríguez et al., 2017). Collagen extracted from fishery resources is seen as a very promising direction of biotechnological valorization as it is available to a great extent, lacks toxicity and the sociocultural barriers are absent. Mussels easily attach to wet substrates or rocks in wave-battered seashores thanks to adhesive proteins and amino acids (e.g., 3,4-dihydroxyphenylalanine, DOPA). This fact has fueled research on mussel-inspired multifunctional coatings and bioadhesives for use on various surfaces (Lee et al., 2007; Shin et al., 2020). Shell extract of scallop (Pecten maximus) has been shown to stimulate the biosynthesis of extracellular matrix and both type I and type II collagen biosynthesis in primary cells, pointing out their potential in dermatology and cosmetic sectors (Latire et al., 2014).
Gels and Hydrogels
In the biomedical field, gels and hydrogels are recognized as promising biomaterials for drug delivery, tissue engineering, biosensors, self-healing and hemostasis systems due to their highly porous structure, tunable biodegradability and biocompatibility (Venkatesan et al., 2015; Chai et al., 2017). Hydrogels are gels that consist of hydrophilic polymer chains arranged in a 3D cross-linked network. This polymeric network can be controlled and easily manipulated for inclusion and, subsequently, the modified diffusion of various active ingredients (Domalik-Pyzik et al., 2019). Hydrogel scaffolds possess the ability to swell without dissolving in biological fluids; however, due to the fragility of their gel matrix, the need for novel and more stable hydrogel systems is still high (Hoare and Kohane, 2008).
In this respect, carrageenans possess enormous water retaining capacity and gelling properties and have been widely exploited to develop bio-hydrogels (Oun and Rhim, 2017). Alginate hydrogels have been widely used for wound dressing applications and other biomedical applications (Galli et al., 2018; Liao et al., 2018). When cross-linked with natural or synthetic components, they form soft or stiff gels with different physicochemical properties depending on the alginate mannuronic acid:guluronic acid ratio, the material composition and the degree of cross-linking (Gharazi et al., 2018). Chitosan-based hydrogels have been modified with catechol, hydrocaffeic acid, and poly(ethylene glycol) to enhance the bioadhesive, mechanical and antibacterial properties. The developed hydrogel patches and injectable gels can be used as soft tissue engineering materials (Du et al., 2020; Kim et al., 2020; Zheng et al., 2020).
Polymeric Micro- and Nanoparticles
In recent years, various delivery systems have attracted significant attention in the drug delivery sector. Owing to the benefits provided by their small sizes, the application of natural origin MPs/NPs has emerged as a very promising approach for targeted drug delivery. Polymeric MPs/NPs can be fabricated through different methods, such as polyelectrolyte complexation, emulsification and ionic gelation, exhibiting many advantages, such as improved drug solubility, distribution and bioavailability (Chifiriuc and Grumezescu, 2016). Due to their adjustable size and surface characteristics, they can be used as novel carriers for the controlled delivery of active pharmaceutical ingredients with improved pharmacokinetics and pharmacodynamics (Nikam et al., 2014; Manivasagan and Oh, 2016). Several studies have shown the collagen applications as a carrier in different drug delivery systems (Gu et al., 2019), having remarkable abilities and being the focus of extensive research efforts. In particular, collagens from a variety of marine sources have been used to produce micro (diameters between 0.1 and 100 μm) and nano (1–100 nm) bio-based drug delivery systems and are attractive and promising for applications in biomedical and pharmaceutical industries. Marine polysaccharides have been explored to design polymeric MPs/NPs, mainly because of their ionic nature. Oppositely charged polysaccharides can interact with ions, resulting in complex polyelectrolyte structures that can encapsulate various active compounds. The release of the embedded compounds from the complex can be controlled and achieved through various mechanisms, such as charge interactions, ion exchange mechanisms, polymer degradation or dissolution of the polyelectrolyte matrix (Venkatesan et al., 2016).
Numerous studies on the preparation of marine polymer-based nanoparticles have been reported over the years for targeted drug delivery (Bilal and Iqbal, 2020). In a recent report, hybrid alginate/chitosan nanoparticles were investigated for the in vitro release of lovastatin as promising new drug carriers (Thai et al., 2020). Ulvan/lysozyme nanoparticles have exhibited enhanced antibacterial activity against Staphylococcus aureus, while at the same time highlighting the potential of ulvan for the preparation of peptide/protein delivery systems (Tziveleka et al., 2018). The use of carrageenan in MPs/NPs-based drug delivery systems for various biomedical applications has also been actively explored. Insulin-loaded lectin-functionalized carboxymethylated κ-carrageenan microparticles were produced by ionic gelation technique, and their potential use as an improved carrier for the oral delivery of insulin was evaluated (Leong et al., 2011). Oral administration of the lectin-functionalized insulin-carrageenan microparticles (diameter 1273 ± 201 μm) in diabetic rats resulted in a sustained release of the insulin in the intestinal region and a prolonged duration of the hypoglycaemic effect, confirming their therapeutic efficacy. κ-Carrageenan extracted from the red algae Eucheuma cottonii was utilized to encapsulate the poorly soluble coenzyme Q10 (CoQ10) using the spray drying technique. The CoQ10-κ-carrageenan microparticles were shown to represent an efficient model to increase the water solubility of coenzyme Q10, creating a new water-based product for the food industry to be used either as a main ingredient or as an enriched additive (Chan et al., 2016). Microparticles synthesized using carrageenan with a different number of sulfate groups κ, ι, and λ, were prepared by microemulsion polymerization/crosslinking and were shown to include a wide range of particle sizes (0.5–100 μm). The particles and their modified forms were found to have broad biomedical applicability due to their drug delivery capability, antimicrobial activity, anticancer, high blood clotting effect, good biocompatibility, and cell viability (Sahiner et al., 2017).
Despite being one of the most widespread natural polysaccharides, chitin was for a long time considered as an intractable polymer due to its lack of solubility in common solvents, which limits its processing and practical use (Rinaudo, 2006). Recent studies have mainly focused on chitin NPs (Mincea et al., 2012; Zeng et al., 2012) and their applications in different fields. In nature, chitin occurs as micro/nanofibrils that form a composite together with proteins, pigments and calcium carbonate and has a structural role in the exoskeleton of crustaceans and insects (Chen et al., 2008). The unique properties of chitin NPs, such as their renewable and biodegradable characteristics, small size, low density, chemical stability, biological activity, biocompatibility and no cytotoxicity, make them excellent candidates for use in an extensive range of medical applications, nanocomposite fields, water treatment, cosmetics, electronics devices, etc. (Zeng et al., 2012). Several applications of chitin NPs have been developed during the last years in different fields; however, the application in the fields of materials science and health are the most predominant. The addition of nanochitin as a filler in the production of biocomposites enhances the physicochemical properties of the material in addition to its antifungal properties (Salaberria et al., 2014, 2015a, b; Herrera et al., 2017). Its biological activity and non-cytotoxicity have promoted the use of nanochitin for health care and medical applications, such as scaffold and tissue regeneration (Zubillaga et al., 2018; Danti et al., 2019; Smirnova et al., 2019; Zubillaga et al., 2020), drug and cosmetics (Mellou et al., 2019; Coltelli et al., 2020).
Chitosan, obtained from chitin available in the exoskeleton of crustaceans, is a cationic polymer described as an excellent material to design drug delivery systems due to its biocompatibility, biodegradability and non-toxicity. Chitosan NPs have a wide array of applications with excellent oral bioavailability for different biomolecules, such as hydrophobic drugs, nucleic acids, proteins and polysaccharides, retaining their bioactivity, improving stability and enhancing the therapeutic effect (Lang et al., 2020). Moreover, chitosan has mucoadhesive properties and a broad spectrum of bioactivities, namely antioxidant, anti-inflammatory and antimicrobial (Chan et al., 2016; Hafsa et al., 2016), which increase its potential interest for oral drug delivery applications. Chitosan NPs can be produced using different methods, although the most widely described ones are ionotropic gelation and polyelectrolyte complexation. These methods are simple, do not include organic solvents and provide an excellent opportunity to deliver large amounts of nanomaterial into desired products (Divya and Jisha, 2018). Other marine-derived polysaccharides, such as fucoidan, alginate, ulvan, carrageenan, and laminarin, commonly isolated from seaweeds, also have specific and interesting individual properties explored for potential application in drug delivery systems (Venkatesan et al., 2016). These natural anionic polymers can be used to produce NPs of different size, charge and shape for drug delivery applications using methods as emulsion, ionic gelation and polyelectrolyte complexing (Cardoso et al., 2016; Venkatesan et al., 2016). NPs made of marine polysaccharides have been exploited for oral delivery of active pharmaceutical drugs due to their increased stability and resistance to degradation under acidic gastrointestinal conditions leading to improved intestinal drug absorption. Insulin-loaded chitosan-alginate-pentasodium tripolyphosphate (TPP) NPs were produced by ionic gelation. The delivery by nasal administration in rabbits of this hybrid formulation showed enhanced systemic absorption demonstrating its potential in increasing nasal insulin absorption (Goycoolea et al., 2009). Fucoidan-chitosan NPs have been widely described as promising for application as carriers in oral drug delivery systems (Barbosa et al., 2019b). NPs resulting from the encapsulation of curcumin by O-carboxymethyl chitosan-fucoidan were shown to have lower toxicity in mouse fibroblasts when compared with the free form and to be efficiently internalized by Caco-2 cells, demonstrating its potential application for oral drug delivery (Huang et al., 2016). Chitosan-fucoidan NPs containing berberine were developed and shown by in vitro testing in Caco-2 cells/RAW264.7 cells co-culture to restore the barrier function in inflammatory and injured intestinal epithelial (Wu et al., 2014). Also, quercetin loaded fucoidan-chitosan NPs developed for application as a functional food were shown to be stable with controlled drug release under simulated gastrointestinal environment, while maintaining intense antioxidant activity (Barbosa et al., 2019a). Based on the known anticoagulation activity of fucoidan, NPs of chitosan-fucoidan were prepared to encapsulate red ginseng extract and improve its antithrombotic activity and physicochemical properties. Nanoencapsulation improved the ginsenoside solubility and decreased the effect of platelet aggregation in vitro. In vitro studies in the rat model also demonstrated that the NPs caused a significant reduction in thrombus formation when compared with the free red ginseng extract (Kim et al., 2016).
Production of chitosan-fucoidan NPs for pulmonary delivery of the antibiotic chitogentamicin has been described, and results indicate the improvement of antimicrobial efficacy and elimination of systemic toxicity when compared with the intravenous antibiotic administration, with great potential for pneumonia treatment (Huang et al., 2016). Marine-derived drug delivery systems based on chitosan-fucoidan NPs have been recently developed for drug delivery in cancer treatment. Gemcitabine-loaded NPs showed increased toxicity for human breast cancer cells without increasing toxic effects on endothelial cells when compared with free gemcitabine (Oliveira et al., 2018). Piperlongumine is a new class of pro-oxidant drugs with the potential for cancer-specific therapy. Encapsulation of this hydrophobic drug into chitosan-fucoidan NPs increased its solubility and bioavailability, enhancing its anticancer efficacy (Choi et al., 2019).
Studies on the production and application of marine collagen as drug delivery systems for biomedical or as supplements for the food industry are also available in the literature. A MPs protein delivery system was developed using an emulsification-gelation-solvent extraction method and a polymeric matrix of marine collagen extracted from the jellyfish Catostylus tagi. This collagen MPs system (median particle size 9.5 μm) showed promising and versatile results for the controlled release of therapeutic proteins with retained biological activity (Calejo et al., 2012). Collagen from a marine sponge (Porifera, Dictyoceratida) was used to produce a bio-based dressing for topical drug delivery able to absorb the excess wound exudate and at the same time release the drug regulating the healing process (Langasco et al., 2017). Also, collagen extracted from the marine sponge Chondrosia reniformis was used to develop collagen microspheres for dermal delivery of all-trans-retinol (Swatschek et al., 2002). Although the retinol loaded MPs showed a broad size distribution (ranging from 126 ± 2.9 nm to 2179 ± 42 nm), the dermal penetration of retinol-hydrogel-collagen MPs formulations was two-fold higher than when compared to retinol formulations without the MPs. NPs, produced with C. reniformis collagen (size 123 ± 5.5 nm), loaded with 17ß-estradiol-hemihydrate, for application in hormone replacement therapy, were shown to be a promising transdermal drug carrier system of estradiol with enhanced bioavailability, prolonged drug release and increased estradiol absorption compared to a commercial gel (Nicklas et al., 2009).
Marine collagen peptides obtained from Synodontidae fish scales were used to develop alginate NPs, loaded with collagen peptide chelated calcium (diameters approximately 400 nm). This in vivo study demonstrated that the core-shell NPs were able to improve calcium absorption and prevent calcium deficiency in rats treated with this novel biphasic material that could represent an improved calcium supplement for the food industry (Guo et al., 2015).
Polymeric Nanofibers
During the past years, polymeric nanofibers have gained considerable interest due to their unique properties, such as high surface-to-volume area, high and controlled porosity and mechanical flexibility (Kenry and Lim, 2017; Al-Enizi et al., 2018; Cheng et al., 2018). Marine biopolymers, due to their biocompatibility and biodegradability, are considered ideal candidates for the development of multifunctional non-wovens. Exhibiting high encapsulation efficacy and architectural analogy to the natural extracellular matrix, they can be easily produced through the electrospinning technique, which is the most widely used method for the production of polymeric nanofibers with tailor-made properties (Teo and Ramakrishna, 2006; Greiner and Wendorff, 2007; Bhardwaj and Kundu, 2010). Various natural and synthetic polymers can be utilized in nanofibrous matrices, incorporating numerous active agents for different biomedical applications.
In most cases, marine polysaccharides, often lacking chain entanglement, have been utilized in combination with other synthetic or natural biopolymers into hybrid polymeric nanofibrous systems that offer the advantageous properties of the combined ingredients (Zhao et al., 2016). Numerous synthetic polymers, such as polycaprolactone, polyethylene oxide, polyvinyl alcohol (Zia et al., 2017), polylactic acid and cellulose acetate, have been used in the development of such hybrid marine polymer-based electrospun patches. Various nanofibrous scaffolds of alginate, fucoidans, ulvan, chitosan and chitin and other biopolymers (e.g., gelatin, cellulose, hyaluronic acid, collagen and their derivatives) have been developed, exhibiting great potential in tissue regeneration, wound healing and controlled drug delivery (Kikionis et al., 2015; Mendes et al., 2017; Augustine et al., 2020). As a recent example, metformin-loaded polycaprolactone/chitosan nanofibrous patches were reported as potential guided bone regeneration membranes (Zhu et al., 2020), while in another work, electrospun alginate nanofibrous dressings loaded with the aqueous extract of Pinus halepensis bark displayed significant in vivo anti-inflammatory activity in mice (Kotroni et al., 2019).
Membranes and Films
While various membranes and films have been developed from marine biopolymers as wound healing or tissue engineering systems, it is alginate that has been most widely used in wound dressings, either alone or combined with other biomaterials. Due to its gelling and fluid-absorption ability, alginate can promote the skin recovery process, maintaining a physiological moist wound environment. It can be easily cross-linked via electrostatic, ionic interactions, covalent-like bonding, redox reactions and coordination with various metals and oppositely charged polysaccharides. The cation interaction of its guluronate blocks with calcium electrolyte into an egg-box-like structure (Goh et al., 2012) has been employed for various wound healing applications. Furthermore, its combination with other positively charged biopolymers, like chitosan in polyelectrolyte forms, has been shown to increase the mechanical stability of the wound dressing materials. In a similar approach, aiming to develop scaffolds for cell cultivation, anionic ulvan and cationic chitosan have been combined to form novel supramolecular structures of stabilized membranes through electrostatic interactions, showing excellent attachment and proliferation of 7F2 osteoblasts (Toskas et al., 2012). As mentioned before, chitosan has been used in many wound dressing and tissue engineering applications, dressing films and membranes (Khan et al., 2020).
3D Structures
In recent years, 3D bioprinting has risen as a versatile tool in regenerative medicine. Therefore, the demand for suitable bioink materials with good printability, biocompatibility and mechanical integrity is apparent. Marine biopolymers, due to their chemical structures and biological functionalities, satisfy most requirements of 3D bioprinting. 3D bioprinting regenerative medicine techniques for human tissue and organ engineering and biofabrication currently apply to 4% chitosan, 10% gelatin, and 26% collagen from a marine origin in bioink formulations for cellular laying/encapsulation (Zhang et al., 2019). Marine polysaccharide hydrogels are naturally derived bioinks, demonstrating low immune response, sufficient biological cues and excellent biocompatibility for tissue engineering applications. Among various marine-origin macromolecules, alginate, carrageenan and chitosan have been widely used as hydrogels in 3D bioprinting for regenerative medicine, such as tissue repair and regeneration during recent years (Zhang et al., 2019). For example, the excellent biocompatibility and the thermogelation properties of κ-carrageenan and alginate have been used to fabricate the cell-laden scaffolds on alginate/carrageenan hydrogels in 3D bioprinting (Kim et al., 2019). In another approach, cells encapsulated within chitosan-based hydrogels demonstrated the printability and applicability of chitosan as a bioink for 3D bioprinting in bone tissue engineering (Demirtaş et al., 2017).
The freeze-drying technique has also been applied for the fabrication of 3D porous scaffolds. Freeze-dried scaffolds can be produced by removing the frozen solvent of a polymeric solution under vacuum, leaving empty spaces (pores) in the formed polymeric scaffold. The architectural characteristics of the produced scaffolds may be tuned by changing the freezing conditions, the polymer solution concentration and the polymer and solvent type (Reys et al., 2017). In this respect, chitosan freeze-dried sponge-like structures were obtained, exhibiting blood absorbing capacity suitable for haemostasis (Kavitha Sankar et al., 2017). Recently, the preparation of ulvan/gelatin hybrid sponge-like scaffolds was reported, exhibiting efficient mesenchymal stem cell adhesion and proliferation for bone tissue engineering applications (Tziveleka et al., 2020).
Novel Materials for Bio-Based Food Packaging
More than 380 million metric tons of plastics are produced worldwide (Ritchie and Roser, 2018). In Europe, 40% of produced plastic is used in packaging. Despite the tremendous benefits of using plastics for packaging, their single-use feature results in an enormous stream of waste with a significantly negative impact on the environment. Synthetic plastics are petroleum-based, hence consuming large amounts of fossil fuels for their production. Moreover, they are not biodegradable and, thus, after disposal, they can accumulate in natural ecosystems for up to several thousands of years. Consequently, more than 5 trillion plastic particles weighing over 250,000 tons are estimated to be floating in Earth’s oceans (Eriksen et al., 2014), posing a major threat to the trophic chain. Only 14% of plastic packaging is currently recycled (Ellen MacArthur Foundation, 2016), and there is a clear consensus that the industry needs to shift to biodegradable plastics from renewable resources (i.e., biopolymers) for a long-term solution to the current situation (Oliveira et al., 2020). Biopolyesters, such as polylactic acid (PLA), polyhydroxyalkanoates (PHAs) and thermoplastic starch, can be used for food packaging due to their relatively good processability using industrial techniques such as extrusion or thermoforming. However, their properties are still far from synthetic polymers (especially in terms of thermal resistance, barrier and mechanical performance), and their production costs are too high to compete on the market. Moreover, the raw materials typically used to produce biopolymers originate from land crops, whose primary use is the food sector. In this context, the packaging industry is looking for alternative biopolymers with enhanced properties that can be extracted from cheaper, sustainable resources. Given its abundance and interesting composition, marine biomass has received great intererest is being focused on marine biomass as one of the most promising alternative sources for the extraction of biopolymers for food packaging applications.
The structural polysaccharides found in the cell walls from seaweeds, such as cellulose and phycocolloids (Table 3), have excellent properties, which make them promising candidates for the development of bio-based and sustainable food packaging. Cellulose from land biomass has been widely used to produce food packaging materials and applied as a filler to improve the properties of other biopolymers (Ramamoorthy et al., 2015; Trache et al., 2016). In parallel, several recent studies have reported on the outstanding properties of cellulose extracted from marine biomass (Bettaieb et al., 2015; Khalil et al., 2017; Benito-González et al., 2018; Martínez-Sanz et al., 2020a). One particularly interesting approach, given the circular economy policies that are being promoted by the governing bodies, is the valorization of marine waste biomass. For instance, the residues generated after the accumulation of leaves from the seagrass Posidonia oceanica, found in the Mediterranean shores and the industrial waste produced after extraction of agar from red seaweeds have been used to extract cellulosic fractions with different degrees of purity (Benito-González et al., 2018; Benito-González et al., 2019a; Martínez-Sanz et al., 2020a). These cellulosic fractions can be used to produce films, employing a green method based on the production of aqueous suspensions. Even though the properties of the films may vary depending on the biomass source, commonly, the presence of other non-cellulosic components in the less purified fractions may improve the performance of the films (Benito-González et al., 2019a; Martínez-Sanz et al., 2020a). This is particularly interesting since high-performance cellulose-based packaging films could be produced utilizing simplified and more sustainable methods, thus reducing the production costs and the environmental impact. These cellulosic fractions can also be used as fillers to improve the properties of other biopolymers. One recent work has reported the capacity of cellulosic fractions from P. oceanica to produce biopolymeric films with improved mechanical and barrier performance, as well as with better stability upon storage when incorporated into thermoplastic starch by melt mixing (Benito-González et al., 2019b).
Cellulose from marine biomass can also be used in a particular type of structure known as aerogels. Aerogels are lightweight and highly porous structures, which present excellent sorption capacity and can be used in food packaging as absorbent pads (for fresh products such as meat and fish) and as components for the incorporation and sustained release of bioactive compounds in smart packaging, amongst others. Their high specific surface makes them also ideal materials for catalysis and other advanced applications. Many studies have reported the production of cellulose-based aerogels (Nguyen et al., 2014; Feng et al., 2015; Buchtová et al., 2019); however, the preparation methods are often quite complex, involving several steps (disruption of cellulose crystalline structure, gelation, cellulose regeneration, solvent exchange and a final drying step through supercritical CO2 or freeze-drying). Moreover, due to the highly hydrophilic characteristic of cellulose, hydrophobization treatments are usually required to improve the water-resistance of the aerogels. A simple freeze-drying method has been recently reported to yield high-performance aerogels from cellulosic fractions derived from aquatic biomass, and a very simple strategy has been developed for hydrophobization of cellulosic aerogels, making them stable in aqueous solutions (Fontes-Candia et al., 2019; Benito-González et al., 2020; Martínez-Sanz et al., 2020a,b). These materials display a highly porous structure, especially when using less purified cellulosic fractions, conferring a great sorption capacity when soaked in hydrophilic and/or hydrophobic liquids. This has been exploited to develop bioactive aerogels, incorporating an antioxidant extract that could be released upon contact with meat, thus reducing the oxidation processes taking place upon storage (Fontes-Candia et al., 2019).
Although cellulose is, without a doubt, the most widely exploited marine biomass biopolymer for the development of food packaging, other structural polysaccharides such as phycocolloids are currently being studied. In particular, agar (Sousa and Gonçalves, 2015; Malagurski et al., 2017; Martínez-Sanz et al., 2019), carrageenans (Choi et al., 2005; Vu and Won, 2014; Farhan and Hani, 2017) and alginate (Abdollahi et al., 2013a, b; Sirviö et al., 2014; Senturk Parreidt et al., 2018) have excellent potential for the production of food packaging films with interesting properties. The main disadvantages of phycocolloid films are their excessive rigidity (which is counteracted with the addition of plasticizing agents) and their low resistance to high relative humidity conditions (which can be improved by incorporating more hydrophobic fillers). Interestingly, less purified agar-based extracts have been shown to overcome these issues due to the positive effect of other compounds remaining from the native seaweeds, such as proteins and minerals (Martínez-Sanz et al., 2019). These phycocolloids can be used to produce porous aerogels (Quignard et al., 2008; Gonçalves et al., 2016; Manzocco et al., 2017), but similarly to cellulose, complex preparation methods are often required. Further research needs to be carried out to look for alternative manufacturing processes and to identify strategies to adjust the properties of the obtained aerogels to the requirements of different food packaging applications.
Even though there are no studies reported on marine collagen as an alternative food packaging material, there are some studies on bovine/porcine collagen that has already been tested to produce edible films for protection and extension of food products, such as sausages casings (Suurs and Barbut, 2020). Moreover, the addition of chitosan to gelatin films of cuttlefish skin improves the thermal stability of the polymer network and increases the antioxidant and antimicrobial activity against some Gram-positive and Gram-negative bacteria, which is a useful property in packaging production (Hajji et al., 2021). However, there are some restrains in a broad application of collagen as packaging material, as it is sensitive to moisture. Gelatin based packaging coatings have also been explored, but various additives (lysozyme, chitosan, chitin, essential oils, among others) need to be applied to achieve the desired properties (antimicrobial, antioxidative) (Chawla et al., 2021).
Use in the Leather Industry
Among the solutions for reducing fish waste, one potential option is utilizing fish skin to produce exotic leathers for accessories such as bags, gloves, or shoes. The valorization of any animal skin into leather materials involves tanning, a process that alters the skin protein structure, transforming the biodegradable skin into durable and flexible leather. Saranya et al. (2020) explored the potential of fish waste to produce fish oil, which could be used as a fat-liquoring agent in leather processing, and the results were better compared to those of a traditional commercial fat-liquoring agent. Thus, the fish oil fat-liquor produced from fish waste can be regarded as an eco-friendly alternative to lubricate leather as it allows to substantially reduce the sludge disposal issues in the tannery industry and reduce waste in the fisheries sector. To satisfy the trends for greener production, bio-tanning processes are being sought that replace chromium with plant-based tannins or the re-use of tanning floats, which can reduce the water consumption in the process up to 90%. An example of successful valorization of fish skin into leather is the Moroccan company SeaSkin, using a plant-based coloring system and a dry tanning process, thus reducing 95% of water in the process. Another valorization example is the use of salmon skin in leather straps for watches by the Norwegian company Berg Watches, in collaboration with the Icelandic Nordic Fishleather.
Use in Food/Feed Industries
Proteins are a highly valuable resource, present in several raw materials from plant and animal origin, available in different amounts and with the primary dietary function to provide essential amino acids and tissue building material to the human and animal body. Proteins of marine origin demonstrated higher quality due to high amounts of all essential amino acids (Abdul-Hamid et al., 2002; Prihanto et al., 2019). Several studies have also proven that the quality, digestibility and bioavailability of the essential amino acids in marine protein concentrates increase after food-grade hydrolysis processing (Faber et al., 2010; Kim, 2013). Marine protein hydrolysates can also contribute the necessary amounts of bioactive peptides with antioxidative, antihypertensive, metal-chelating, antimicrobial and anti-inflammatory/immunomodulatory properties to the human diet (Kim, 2013; Abdelhedi et al., 2018; Ediriweera et al., 2019).
Marine polysaccharides (e.g., alginates, carrageenans, agar), as well as gelatin, are used in food and feed industries as gelling agents, stabilizers and/or emulsifiers. Their addition can change the product’s viscosity, which impacts the transport of volatile components and affects flavor release (Liu et al., 2015). As an excellent source of proteins, lipids, vitamins and minerals, by-products as fish skin, viscera and blood, as well as crustacean and bivalve shells, are used in the pharmaceutical and agriculture industry and innovative food processing technologies (Beaulieu et al., 2013).
Use in Bioremediation
Several marine polysaccharides have also found useful applications in bioremediation, either as adsorbents of organic and inorganic pollutants or as suitable biopolymers for the immobilization of microbial species with relevant catabolic properties. For instance, chitosan and alginate-based membranes have shown to be highly efficient in the retention of industrial dyes and heavy metals, such as mercury, lead or nickel (Ngah et al., 2010), as well as in the removal of priority organic pollutants, including pesticides (Moraes et al., 2013), pharmaceutical drugs (Vassalini et al., 2020), phenolic compounds and other industry-borne pollutants (Vidal and Moraes, 2019; Vassalini et al., 2020). In fact, several membrane composites, containing these biopolymers, are now being regarded as suitable replacements of current adsorption technologies (e.g., activated carbon) for implementation in treatment plants, depuring industrial effluents (Vidal and Moraes, 2019). On the other hand, several marine biopolymers, including alginate, cellulose and chitosan, have also been widely used for the encapsulation of living cells for many purposes in biotechnology, including bioremediation (Wang et al., 2019). Cellular immobilization is of additional significance in bioremediation strategies that rely on the application of degrading microorganisms to the affected sites (i.e., bioaugmentation), as it maximizes cellular viability and stabilizes the metabolic performance, allowing to achieve productive biodegradation in biomes that often showcase inhospitable conditions for microbial development (e.g., extreme pH levels, low nutrient availability). For such ends, alginate beads, cellulose nanofibers or chitosan NPs have served as suitable microbial interfaces to facilitate the bioremediation of various organic pollutants and to mitigate the nutrient load of wastewaters (Eroglu et al., 2012; Sathishkumar et al., 2014; Khanpour-Alikelayeh et al., 2021), while also bearing minimal environmental impacts due to their biocompatibility and biodegradability.
Valorization of Marine Biomass as a European Strategy
The circular economy and bioeconomy are high on the EU policy making agenda. On the one hand, the Circular Economy Action Plan aims at reducing raw materials and associated environmental pressures, while on the other hand, the Bioeconomy Strategy promotes the exploitation of biomaterials in a sustainable manner. However, the development of novel products demands investment into the optimization of technical procedures, a clear cost-benefit and supply sustainability. That was also recognized by the European Commission that started to support this with new funding opportunities for research and innovation in the field of circular economy within marine biotechnology (Table 4). The first international EU-funded projects focused on exploiting marine organisms. They were financed within Framework Programme 7 - FP7 (2007-2013) and were mainly dedicated to innovative bioprospecting of marine microorganisms and the discovery of high value-added bioactive compounds. Some of these projects investigated the extraction and application potential of marine-derived biopolymers (polysaccharides, proteins, enzymes, among others) in the pharmaceutical, cosmeceutical and medical industries. Two projects within the EU FP7 SME (N-CHITOPACK and SEABIOPLAST) were financed for bioplastic production and food packaging from fish waste and seaweed biomass. At the end of FP7, an ERA-NET was launched for marine biotechnology (ERA-MBT) that funded 21 projects, from which eight were focused on marine biopolymers from a broad range of marine (micro)organisms (bacteria, cyanobacteria, macroalgae, shellfish, crustacean, fish). Polysaccharides, but mostly chitin, chitosan, alginate and laminarin, were the most investigated polymers within ERA-MBT funded projects. H2020 Framework Programme (2014–2020) proceeded with funding projects for marine biomass exploitation for new products. Two projects (PULMO and GoJelly) were focused on jellyfish biomass utilization (usually by-catch) for different applications, from which protein extraction (mainly collagen) was used in cosmeceutical, nutraceutical, medical and agricultural applications. A Public-Private Partnership between the EU and the Bio-based Industries Consortium was established under H2020 called Bio-Based Industries Joint Undertaking (BBI JU). The aim is to reduce Europe’s dependency on fossil-based products and to meet EU climate change goals that would result in greener and eco-friendlier growth. BBI JU funded two projects that foster a cost-effective marine biomass supply (micro- and macro- algae, aquaculture and fisheries side streams) and scale-up the production process of ingredients for further application. On the regional level, INTERREG Programmes also identified marine biomass (mostly seaweed, such as the Interreg Germany-Denmark project FucoSan on fucoidan) as a valuable feedstock for new value chains and biopolymer extraction.
TABLE 4
| Project | Focused on |
Targeted industry | Website | |
| Biopolymers | Organisms | |||
| BLUEandGREEN (EU H2020, Twinning) | Broad | Broad | Boosting scientific excellence and innovation capacity | http://blueandgreen.ciimar.up.pt/ |
| ALGAE4A-B (EU H2020-MSCA-RISE) | Polysaccharides, proteins, enzymes, antioxidants | Microalgae | Cosmetic and Aquaculture industry | http://www.algae4ab.eu/ |
| SEABIOTECH (EU FP7 KBBE) | Polymers | Bacteria, microalgae, cyanobacteria | Pharmaceutical, cosmetic, functional food and industrial chemistry | http://spider.science.strath.ac.uk/seabiotech/ |
| MACRO CASCADE (EU H2020) | Alginate, protein, laminarin, fucoidan, mannitol | Macroalgae | Feed, food, pharmaceuticals, cosmetics | https://www.macrocascade.eu/ |
| GOJELLY (EU H2020) | Collagen, proteins | Jellyfish | Cosmetics, food, feed, fertilizer, nutraceuticals | https://gojelly.eu/ |
| PULMO (EU H2020 MSCA) | Peptides, collagen and gelatin, oligosaccharides, enzymes | Jellyfish | Nutraceutical and pharmaceutical | https://cordis.europa.eu/project/id/708698 |
| BIOSEA (EU H2020 BBI JU) | Lipids, protein, carbohydrates | Microalgae and macroalgae | Food, feed, and cosmetic | http://biosea-project.eu/ |
| AQUABIOPRO-FIT (EU H2020 BBI JU) | Proteins and bioactive compounds | Aquaculture, fisheries, and agricultural side streams | Feed and nutritional supplement food products | https://www.aquabioprofit.eu/index.cfm |
| DISCARDLESS (EU H2020) | Fish pulp production, protein hydrolysate production and fish meal/oil production | Fish discard/by-catch | Food, feed, agriculture, energy, textile industry | http://www.discardless.eu/ |
| OCEAN MEDICINES (EU MSCA RISE) | Bioactive compounds | Microorganisms | Anticancer or antiinfective | https://cordis.europa.eu/project/id/690944 |
| N-CHITOPACK (EU FP7 SME) | Chitin | Fish waste industry | Biodegradable material for food packaging | https://cordis.europa.eu/project/id/315233 |
| SEABIOPLAST (EU FP7 SME) | Polysaccharides | Seaweed | Bioplastic production | https://cordis.europa.eu/project/id/606032 |
| MARPLAST (EU ERA NET ERA-MBT) | Polyhydroxyalkanoates | Bacteria | Biodegradable bioplastic | http://www.marinebiotech.eu/sites/marinebiotech.eu/files/public/marplast_ProjectdescriptionERA-MBTCall2.pdf |
| NOVOFEED (EU ERA NET ERA-MBT) | Peptides | Fish | Novel feed ingredients | http://www.marinebiotech.eu/sites/marinebiotech.eu/files/public/Novofeed_ProjectdescriptionERA-MBTCall2.pdf |
| BLUETEETH (EU ERA NET ERA-MBT) | Biopolymers | Crustacean by-products and waste fraction | Periodontal disease | http://www.marinebiotech.eu/sites/marinebiotech.eu/files/public/blueteeth_ProjectdescriptionERA-MBTCall2.pdf |
| CYANOBESITY (EU ERA NET ERA-MBT) | Broad | Cyanobacteria | Nutraceutical, obesity | https://cyanobesity.ciimar.up.pt/ |
| SEAREFINARY (EU ERA NET ERA-MBT) | Phlorotannins, fucoidan, and laminarin | Macroalgae | Nutraceutical, food industry | http://www.marinebiotech.eu/sites/marinebiotech.eu/files/public/SeaRefinaryProjectdescriptionERA-MBTCall1.pdf |
| MAR3BIO (EU ERA NET ERA-MBT) | Alginate and chitosan | Macroalgae and crustaceans | Materials, cosmeceuticals, nutraceuticals, pharmaceuticals | http://www.marinebiotech.eu/sites/marinebiotech.eu/files/public/Mar3BioProjectdescriptionERA-MBTCall1.pdf |
| BLUESHELL (EU ERA NET ERA-MBT) | Proteins/peptides, unusual fatty acids, pigments, and chitin | Shellfish by-products | Functional foods development, food safety applications and plant health applications | http://www.marinebiotech.eu/sites/marinebiotech.eu/files/public/BlueShell_ProjectdescriptionERA-MBTCall2.pdf |
| CHITOWOUND (EU ERA NET ERA-MBT) | Chitin/chitosan | Crustacean seafood processing industry | Wound healing applications | http://www.marinebiotech.eu/sites/marinebiotech.eu/files/public/CHITOWOUND_ProjectdescriptionCOFASP%26ERA-MBTCall.pdf |
| PRE-SW-GROW (INTERREG NPA) | Broad | Seaweed | Feed, fertilizer; promote innovation | https://keep.eu/projects/20058/ |
| BIOTECMAR (INTERREG Atlantic Area) | Protein, chitin, chitosan, oil, pigments, | Seaweed, crustacean, mussels | Broad | https://keep.eu/projects/5925/ |
| SUBMARINER (INTERREG Baltic Sea) | Broad | Broad | Broad | https://www.submariner-network.eu/ |
| FUCOSAN (INTERREG Germany Denmark) | Fucoidan | Brown algae | Medicine, cosmetics | https://www.fucosan.eu/en/project |
| GRASS (INTERREG Baltic Sea Region) | Broad | Macroalgae | Food industry, packaging, fertilizer | https://www.submariner-network.eu/grass |
| BIOCARB-4-FOOD | Phycocolloids, cellulose | Seaweed, phycocolloids industry waste | Food industry, packaging | https://biooekonomie.uni-hohenheim.de/en/biocarb4food |
Overview of the EU funded international projects on marine biotechnology.
Case Studies
Valorization of side streams (marine and terrestrial ones) could help transition to a more circular economy, where waste is minimized, and resources are used more efficiently by creating new value chains. This strategy is also stated in the EU Action Plan for the Circular Economy (COM/2015/0614), and there have been significant financial contributions to develop new, sustainable value chains, covering marine, and terrestrial sources. This section presents some of these value chains, categorized by geopolitical location: Norway, Scotland, and the Baltic Sea. Norway and Scotland are presented due to their traditional link with fisheries and aquaculture. Both countries have been introducing seaweed aquaculture in the past years, and the valorization potential of this side stream is also presented. Finally, we present the Baltic Sea, which has enormous potential for the valorization of beach wrack (Figure 4).
FIGURE 4
Monthly beach wrack growth production potential in the Baltic Sea by assessing macroalgal biomass amounts.
Status and Utilization of Marine Residual Raw Material in Norway
In Europe, Norway is the major producer of seafood and generates large quantities of residual raw material. Thus, ensuring sustainable harvesting and utilization of the marine resources is at the Norwegian governance strategic forefront (Ween et al., 2018; Ministry of Trade, Industry and Fisheries, 2019). Therefore, advances in the utilization of residual raw materials have been surveyed over several years under the Norwegian Seafood Research Fund (FHF) and earlier through the RUBIN Foundation (Recycling and Utilization of Organic By-Products in Norway). The surveys have given the industry players a better overview of the flow of goods and the possibilities for increased growth and value creation in the seafood sector.
The residual raw materials are defined as the non-primary products obtained from marine raw materials, which are fish and shellfish (crustaceans and molluscs), and seaweeds farmed and caught under Norwegian quotas in Norwegian waters. Opportunities and challenges of three specific sectors of marine waste (fish, shellfish, and seaweed waste) are presented in Table 5, as well as discussed in subparagraphs below.
TABLE 5
| Source | Opportunities | Challenges |
| Fish waste | 85% of the available fish waste was utilized in 2020; the feed market is the most important application in terms of volume. The potential for increased utilization of fish waste is related to the whitefish fishery (cod, haddock, and pollock). Several ongoing research projects have particular focus on utilization for higher added-value products/ingredients like nutraceuticals and pharmaceutical products; bio functional peptides, enzymes, collagen, etc. (www.legasea.no). | Large seasonal fluctuations in fisheries and consequently an unstable access to residual raw material, lack of technological solutions for storage and processing onboard the fishing vessels, economic incentives, infrastructure for new processing methods, documentation, regulations and market authorizations for new marine ingredients or products. |
| Shellfish and crustacean waste | Main consumed shellfish/crustaceans include shrimp, krill, and crabs. The main composition of this type of waste is, as in most of the other countries, of 70% heads and 30% shells. A high amount of nutrients and particularly protein are lost with the heads. The shells are mostly processed or sold by Norwegian fisheries and food processing companies to international partners for chitin/chitosan extraction. Different projects under development are treating the optimization of sustainable processing methods with full utilization of the resources (example: KrillSOFT project, funded by the Norwegian Research Council in 2019) developing a circular bioeconomic approach for full utilization of krill. | Large seasonal fluctuations in fisheries that give variation in access to residual raw material, lack of technological solutions for storage and processing onboard the fishing vessels, economic incentives, infrastructure for new processing methods, documentation, regulations and market authorizations for new marine ingredients or products. An additional bottleneck is the existing waste regulation framework. |
| Seaweed waste | Wild resource origin: The total catch of Ascophyllum nodosum and Laminaria hyperborea combined in 2019 was 149,876 tons, with a first- hand value of 4.06 million EURO (40.59 million NOK). Minor amounts of other seaweed species e.g., Ulva sp., Himanthalia elongata, Vertebrata lanosa, Palmaria palmata are harvested by hand and sold as whole locally to restaurants or directly to the consumer. The exploitation of the last-mentioned species as food results in almost no waste and when so (washing water) in small volume. Alginates can constitute up to 40% of the dry weight of L. hyperborea (Gunn.) Foslie and are extracted in over hundred different qualities for a broad range of applications. During the processing of the A. nodosum biomass few wastes are produced: gravel and sand are removed from the seaweed and up to 82% of moisture is removed. After processing of A. nodosum in meal, 50% of the meal is further processed into extracts, together with fresh seaweed. After extraction, the rest is used as fertilizer by local farmers (∼750 tons/year). Aquaculture origin: Current commercial seaweed farming in Norway is limited to the kelp species Saccharina latissima (sugar kelp) and Alaria esculenta (winged kelp) due to their ability to reach high biomass yields and a favorable content in nutritional and bioactive compounds with multiple industrial applications | Industrial exploitation of A. nodosum and L. hyperborea generate waste. During the harvesting of A. nodosum by-catches composed mostly of Fucus species are occasional and are separated manually from the Ascophyllum and process further mostly as seaweed extract for agricultural use. The extraction of alginate from L. hyperborea results in the production of a cellulosic suspended phase, which today is considered as ‘waste,’ but can represent a valuable resource due to their organic matter content. Aquaculture origin: As the aquaculture industry is still immature, very little investigation has been made to estimate the waste types (biomass or processing liquid). The lost biomass during harvesting is estimated to be around 30% and after the freezing and thawing up to 40% of wet weight. To optimize the productions and have a minimum impact on the environment, the wastes generated during this novel type of production would need to be identified further to both define the volume and develop innovative uses of these wastes. |
Summary of marine waste utilization in Norway.
Norwegian Fish and Shellfish Production Waste for Added Value Products
In Norway, most of the residual raw material from fish and shellfish is currently used, making an important contribution to value creation in the fisheries and aquaculture industry (Johansen et al., 2019). In 2020, about 861,000 tons (85%) of the available residual raw material was utilized to produce various products (Myhre et al., 2021). Large volumes are utilized to varying extents between the different sectors, from 62% in the shellfish industry to almost 100% in the pelagic sector. Norway is currently the largest producer of Atlantic salmon, yielding 93% of total Norwegian aquaculture production (FAO, 2016). In the aquaculture sector, there are strict rules for processing and handling waste in production, and in principle, all biological material is processed. The only fraction that is not being economically exploited is the blood from the slaughter process, for which there is still no usable technology. While in the other marine production sectors (e.g., fisheries), there are large seasonal fluctuations that give variation in access to residual raw material, the residues from the aquaculture industry arise as a relatively steady stream throughout the year. The growth in salmon aquaculture production and the increased use of recirculating aquaculture system (RAS) technology has led to an increased focus on the exploitation of solid waste (feed spill and faeces) from Norwegian aquaculture (Brod et al., 2017; Estevez et al., 2019; Meriac, 2019). The sludge from most land-based aquaculture and smolt production is commonly used for biogas production, and the remaining fraction after biogas production is used as a soil enhancer (Aas and Åsgård, 2017). The production of bioenergy from silage of dead fish from the Norwegian aquaculture industry has increased significantly in recent years. However, in open sea cages, no technology for sludge collection currently exists, and unless resource-efficient solutions like integrated multi-trophic aquaculture (IMTA) are applied, valuable resources are lost. Recently there has also been an increasing focus on producing organic fertilizers from fish waste from captured fish, promoting the recycling of nutrients from the sea and back to terrestrial environments (Ahuja et al., 2020).
Products based on marine residues are mainly used for the feed market (69%), direct and indirect human consumption (13%) and energy/biogas (19%) (SINTEF Ocean and Kontali Analyse, 2020; Myhre et al., 2021). Most marine residues from the production of pelagic fish and aquaculture are used as silage, fishmeal, fish oil and fish protein concentrate. The feed market is the most important application when it comes to volume. Consumer products also include flavorings in foods (extracts) and ingredients for functional food. Other products, for example, cosmetics, nutraceuticals and pharmaceutical products, are produced to a very modest extent from Norwegian based raw material (Pleym et al., 2019).
Norwegian Seaweed Production Waste for Added Value Products
In 2017, over 32.6 million tons of brown, red and green seaweed were produced from capture and aquaculture worldwide, with an annual increase of around 7% in the last 10 years and an average value of 400 USD/ton DW (dry weight) (Buschmann et al., 2017), mainly from the aquaculture sector. On the contrary, the European seaweed industry is mainly based on harvesting of natural resources, as the aquaculture of seaweed is still on the experimental and pilot-scale levels. The European seaweed capture has been stable with around 270,000 tons since 1960, in which Norway contributed with an average of 59.6% (FAO, 2021). Norwegian products from wild-harvested seaweed vary from meal or extracts to highly technological pharmaceutical products. The Norwegian seaweed industry has a long history and relies mostly on the wild harvest of two species: Laminaria hyperborea for alginates production and Ascophyllum nodosum for meal and extracts used for agricultural/horticultural and food or feed supplement purposes. The total harvest of A. nodosum and L. hyperborea combined in 2019 was 149,876 tons, with a first-hand value of 4.06 million EUR. Minor amounts of other seaweed species such as Ulva sp., Himanthalia elongata, Vertebrata lanosa, Palmaria palmata are harvested by hand and sold as a whole, locally to restaurants or directly to the consumer and result in almost no waste.
During the harvesting of A. nodosum, by-catches, mostly composed of Fucus species, are occasional and separated manually and processed further, mostly as seaweed extracts for agricultural use. During the A. nodosum biomass processing, low quantities of waste are produced. For example, gravel and sand are removed from the seaweed using high-pressure air and up to 82% of the moisture is removed by drying the wet biomass directly in a drum. After processing of A. nodosum into meal, 50% of the meal is further processed into extracts, together with fresh seaweed. The remaining material after extraction is used as fertilizer by local farmers (∼750 tons/yr). On the contrary, the industrial exploitation of L. hyperborea generates more waste. Alginates can constitute up to 40% of the dry weight of L. hyperborea (Gunn.) and are extracted in over a hundred different qualities for a broad range of applications. The factory DuPont is licensed for the production of 6,000 tons of alginate and has permission to release the liquid product containing stone dust and kelp dry matter into the seawater, while alginate dust is released to the air. Filtration of the production liquid to collect the rest of the material for soil improvement has been tested but not proven to be cost-effective.
As the aquaculture seaweed industry is still immature (Araújo et al., 2021), minimal investigation has been made to estimate the waste types (biomass or processing liquid). Current commercial seaweed farming in Norway is limited to the kelp species Saccharina latissima (sugar kelp) and Alaria esculenta (winged kelp) due to their ability to reach high biomass yields and a favorable content in nutritional and bioactive compounds with multiple industrial applications (Stévant et al., 2017; Broch et al., 2019). The loss during harvesting is estimated to be around 30% and measured losses of up to 40% when seaweed biomass is frozen and thawed (e.g., Emblemsvåg et al., 2020). To optimize the productions and have a minimum impact on the environment, various wastes generated during this novel type of production would need to be identified further to define the volume and develop innovative uses of these wastes.
Marine Waste in Scotland: Opportunities and Challenges
The Scottish aquaculture industry is dominated by farmed Atlantic salmon. However, rainbow trout, mussels and, more recently, seaweed are gaining attraction as well. Scottish government recognizes the importance of marine resources and has set a target to double the economic contribution of aquaculture from 1.8 billion GBP in 2016 to 3.6 billion GBP by 2030 and also to double the number of jobs to 18,000 by 2030 (Scotland Food and Drink, 2016).
However, aquaculture and fisheries also generate leftover residues and waste such as fish trimmings (guts, heads, tails, frames, and skin), by-catch, aquaculture mortalities, shells and various leftover biomass. This residual biomass can be divided into three categories: (a) co-products which contribute to the profit of the business; (b) by-products that do not generate substantial income but are cash-neutral when accounted for disposal costs; and (c) waste which costs business money to dispose of (Zero Waste Scotland, 2015). As with any market, the costs associated with the above categories fluctuate (e.g., cost of landfill) and are subject to policies and reforms.
In 2015, Zero Waste Scotland (a not-for-profit environmental organization funded by the Scottish Government and European Regional Development Fund) conducted a study, ‘Sector Study on Beer, Whisky, and Fish,’ to evaluate waste practices in Scotland. Their report identified several opportunities in the marine waste sector based on extracting value from leftover residue. However, the authors also highlighted a need for (a) coordinated and staged development of biorefinery strategy; (b) locally adapted innovative low-tech solutions, suitable for small scale and rural areas; (c) cross-sector awareness raising; (d) bioresources mapping; and (e) efficient data recording and sharing. Opportunities and challenges of three specific sectors of marine waste (fish, shellfish and seaweed waste) are presented in Table 6, as well as discussed in subparagraphs below.
TABLE 6
| Source | Opportunities | Challenges |
| Fish waste | Fish waste can be minced for variety of animal feeds. Other uses include composting and fertilizers, anaerobic digestion and biodiesel production, protein hydrolysis, extraction of collagen, guanine, enzymes, carotenoids and hydroxyapatite and other specialty markets (see Zero Waste Scotland, 2015) Number of companies focusing on recycling is increasing (there are 12 in Scotland) | Lack of incentive to investigate other applications for waste due to cheap disposal cost It is cost-prohibitive to sort and/or pretreat the waste Small, disperse and rural fishing processing facilities cannot profit at small scale Seasonality and perishability of the waste Complicated waste regulation |
| Shellfish and crustacean waste | Shellfish and crustacean waste can be used in feed and food flavoring, anaerobic digestion, fertilizers (and pesticides), to extract enzymes, carotenoids, as calcium source in variety of industries, and as structural material (see Zero Waste Scotland, 2015) Shells are a source of chitin which has a well-established market. In addition, new and innovative technologies are emerging (e.g., antimicrobial chitosan film from CuanTec Ltd) | Most shellfish are shipped away whole, so the waste stream is very small and cheap disposal is currently preferred by the industry For some applications, shells must be meat-free for processing which can be costly Complicated waste regulation |
| Seaweed waste and/or beach wrack | Seaweed waste and/or beach wrack can be used for biogas production, soil improvement products and fertilizers, landfill material for sustainable projects Environmental benefits of seaweed growth (nutrient and contaminant absorption, CO2 sequestration, positive climate impact etc.) Seaweed cultivation and/or wild harvesting is a growing industry with new socio-economic opportunities and emerging research and commercial farms (e.g., Hebridean Seaweed Company Ltd and Scottish Association for Marine Science) | Potential for facilitation of disease, alteration of population genetics and negative environmental alteration for birds and insects Knowledge gaps associated with beach wrack collection and separation (see Chubarenko et al., 2021) and large-scale seaweed farming (see Campbell et al., 2019) Need for supportive legal regulations |
Summary of marine waste utilization in Scotland.
Fish Waste
Some fish are immediately frozen upon arrival and exported as a whole, so the processing occurs elsewhere. Nevertheless, most fish are processed locally, which generates a significant amount of waste. During processing, some trimmings (guts, heads, tails, frames, and skin) are removed. Scotland-wide fish processing waste was estimated at 160,000 tons (Pitcairn et al., 2017). While there are well-established international markets for various fish parts in West Africa and East Asia, it is currently not cost-effective to transport fish waste long distances. However, some Scottish fish waste is exported shorter distances and processed into higher-value products (e.g., fish by-products processed in Norway) (Zero Waste Scotland, 2015).
Fish Waste Opportunities
Fish waste can be minced and sold as fish meal, used in animal feed. In Scotland, most fish waste (75%) that is being re-used is sold for blending into aquaculture feeds. Other lower value markets include pig and poultry feeds and pet food (Zero Waste Scotland, 2015). Aquaculture feed in Scotland is custom formulated to address the nutritional needs of specific fish species and their growth stages. Almost all feeds are a blend of materials. The main requirement is to feed with a high content of protein and ω-3 fatty acids. Physical properties are also considered, and losses during feeding are reduced by increasing pellet digestibility and decreasing the speed of sinking in the water. Intra-species recycling (e.g., salmon waste being recycled back into salmon feed) is not permitted under the EU Animal By-Product Regulation (ABPR); therefore, the dominant source of protein and oils in salmon feed is from other wild fish. Other cheaper feed materials (soy, corn, wheat, plant-based oils) are being blended to reduce the overall cost of fish feed, so the ratio of vegetable meal to fish meal increases. Currently, about 15% of fish feed is recycled into fish meal, and fish feed in Scotland remains more expensive (up to 40% in 2014) than in other countries such as Norway (Zero Waste Scotland, 2015).
Various innovative fish waste applications were identified in the ‘Sector Study on Beer, Whisky, and Fish’ report (Zero Waste Scotland, 2015). These include composting, land spreading and organic fertilizers (taking advantage of high nitrogen and phosphorus content), anaerobic digestion and biodiesel production (suitable for fish mortalities that cannot enter any other value chain), protein hydrolysis (protein and peptone refining techniques), a specialist market of manufacturing fish glue, extraction of collagen, guanine, enzymes, carotenoids, and hydroxyapatite. The number of these innovative applications suggests that there is an economic and environmental opportunity for utilizing fish waste in Scotland. However, these innovative uses have been tested only on a small scale in Scotland and generally have a low technology readiness level.
Currently, there are about 12 companies in Scotland focusing on recycling and use of fish waste. These include the production of fish meal, biofuel, fish leather, new foods, oils, and fertilizers. There is a growing demand for bio-based products and renewable energy. Naturally, the demand is driven by price and quality. In 2015, the price range for fish waste was 130–160 GBP/ton of pelagic waste (Zero Waste Scotland, 2015).
Fish Waste Challenges
There are many challenges associated with fish waste processing. Fish industries are often focused on their core and profitable business and lack the incentive to investigate other applications for their waste, especially if disposable is cheap and easy. Any new technology is often profitable only at a large scale, which is not suitable for small, disperse and rural fishing operations in Scotland. There is also an issue of seasonality and perishability (some products and wastes are available only in certain months), which lowers the steady input and consistency of the feedstock. Similarly, biological waste often consists of multiple residues and potential contamination, while it is cost-prohibitive to pretreat the waste before processing. For example, cod liver can be utilized to extract valuable oils, but is often damaged during processing. Similarly, fish skin used for gelatin extraction must be meat-free, which is not a priority during processing. Complicated waste regulation also presents another obstacle because businesses are reluctant to study different regulatory approaches, which is having a negative impact on their decision-making regarding fish waste.
Shellfish Waste
Shellfish production in Scotland is dominated by mussel (Mytilus spp.) and pacific oyster (Crassostrea gigas), with 6,699 tons and 4,610 tons produced, respectively. Scallop (Pecten maximus), queen scallop (Aequipecten opercularis) and native oyster (Ostrea edulis) are also produced, but at a smaller scale (not more than 150 tons combined). Cultivation of common periwinkle (Littorina littorea) was also recorded. In 2019, the total value for all species combined was approximately 7.9 million GBP (Munro, 2019).
Shellfish are shipped away as a whole, which practically eliminates any significant waste stream. Shellfish farmers have, therefore, a small incentive to even investigate any uses for shellfish waste and prefer local disposal.
Waste from crustacean (crabs, lobster, shrimp, etc.) is more dominant than the others. Shells and/or carapaces with no signs of diseases are often applied as organic land fertilizers. In 2008, Scotland produced 3,400–7,000 tons of crab waste and 6,500–13,000 tons of nephrops (Nephrops norvegicus) (Archer and Russel, 2008). The exact information is difficult to access; hence waste generations are largely broad estimates.
Innovative applications from shellfish waste include composting (Lanno et al., 2020), anaerobic digestion, processing into agricultural lime fertilizer, using shells as a calcium source for animal feed, enzyme extraction from viscera and using shells as aggregate for building applications and track surfacing. All the shells must be flesh-free, which is often difficult to achieve cost-effectively. Crustacean waste can be used to produce chitin (which is a well-established global industry), in fish feed, as an ingredient in pet food, to extract carotenoids, to produce stock for flavoring for human consumption, and use as fertilizer and a pesticide (e.g., reduce nematode presence) (Zero Waste Scotland, 2015).
Seaweed Waste
In Scotland, seaweeds are wild-harvested and have been used locally in small quantities for feed, food and fertilizers for centuries; however, large-scale seaweed cultivation is only recently being developed (Pitcairn et al., 2017). Several commercial and research farms (Hebridean Seaweed Company Ltd and Scottish Association for Marine Science) are actively pioneering the industry. Biorefinery report (Pitcairn et al., 2017) has estimated 8-10 million tons/yr of wild, easily and sustainably harvestable seaweed. Seaweed farming is also becoming more popular.
Overall, the most targeted species are S. latissima, A. esculenta, P. palmata, L. hyperborea, and A. nodosum. The seaweed industry creates significant opportunities in Scotland from seaweed applications in food, feed, fertilizers, anaerobic digestions, nutraceutical and pharmaceutical industry, while macroalgae benefit the environment by sequestering CO2 and absorbing nutrients from the water during growth. Similarly, microalgae have also been recognized for their potential in all application fields listed above and wastewater bioremediation (Scottish Enterprise, 2019). The benefits of IMTA, which includes seaweed, are also being investigated. The socio-economic opportunities and potential of the budding seaweed industry in Scotland are considerable and generally recognized. Scottish Enterprise (2019) estimated that high-value products from seaweed (specifically L. hyperborea) could contribute up to 300 million GBP/yr by 2030.
A recent review by Campbell et al. (2019) highlighted the environmental risks and knowledge gaps associated with large-scale seaweed farming in Europe. The authors identified several areas of concern, including facilitation of disease, alteration of population genetics and broader alterations to the local physiochemical environment (e.g., increased noise, altered nutrient fluxes and flow, impact on benthic species, etc.) (Campbell et al., 2019). Current small-scale farms in Scotland were identified as low risk; however, a transition to large-scale cultivation requires more research and monitoring efforts to fully understand the environmental implications and evaluate the balance between environmental risks and benefits of large-scale seaweed cultivation.
Side Stream Valorization in the Baltic Sea
Beach wrack has ecological functions such as providing food and habitat for sandy beach fauna, nutrients for dune vegetation, and protection for coastal dunes. In the framework of the INTERREG project GRASS (Table 7), existing environmental data and expert opinions were gathered to model beach wrack production potential in the Baltic Sea region. A higher amount of beach wrack is expected in the late autumn months and the early winter, along with the end of production season and the onset of heavier storms. High beach wrack production is predicted at shores that have a narrow photic zone (i.e., distance to the 10 m isobath less than 1 km) and are exposed to favorable wave direction. Moreover, higher solar radiance and water salinity are associated with elevated beach wrack.
TABLE 7
| Project | Keywords | Location | Collection | Processing | Economy, other profit | Legislation | Main focuses |
| WRACK4SOIL | Fertilizers, soil improvement products | Bad-Doberan, Germany | Yes | Yes | Yes | No | Improving existed technology of collection and processing, economic feasibility |
| BIO-CHAR | Biochar, products | Island Rügen, Germany | Yes | Yes | Yes | Yes | Improving existed technology of collection and processing, economic feasibility, legislative issues |
| WRA-COVER | Compost material, landfill biocover | Køge Municipality, Denmark | Yes | Yes | Yes | Yes | Improving existed technology of collection and processing, economic feasibility, legislative issues |
| WRACK4COAST | Dune restoration | Curonian and Vistula spits, Russia | Yes | Yes | Yes | Yes | Analysis of possible technology of collection and processing, economic feasibility, legislative issues |
| ALREA | Gasification | Kalmar, Sweden | No | No | No | No | Experimental setup and testing of feasibility – innovation |
| WAIT | Removal of nutrients and pollution | Puck Bay, Poland | Yes | No | Yes | No | Assessment of profit of removal of beach wrack to the Baltic Sea water quality |
| FERTI-WRACK | WWTP, fertilizers | Swarzewo, Puck Bay, Poland | Yes | Yes | Yes | Yes | Improving existed technology of collection and processing, economic feasibility |
| ESMIC | Removal of plastic pollutants | Melnragë, Palanga, Lithuania | Yes | No | Yes | No | Assessment of the feasibility of removal of beach wrack to reduce plastic pollutants in the Baltic Sea |
| GRASS | Macroalgae cultivation and harvesting | Baltic Sea Region | Yes | Yes | Yes | Yes | Raise awareness and develop capacity building in macroalgae cultivation, harvesting and use |
| CONTRA | Sustainable beach wrack management | Baltic Sea Region | Yes | Yes | Yes | Yes | Assess the environmental, social and economic impact of wrack removal and provide its reuse options |
Case studies on beach wrack in the Baltic Sea basin.
Clear hotspots of beach wrack production and harvest emerged throughout the whole Baltic Sea area (including Kattegat, Figure 4). The highest production values (up to 4,000 g per m2 per month) were observed on the west and east coasts of Sweden, all along the southern coast of Finland, west coast of Estonia and in Gdansk Bay. However, some production hotspots were sporadically found even on the east coast of Finland, reaching northernmost parts of the Bothnian Bay as well as on the shores of St. Petersburg. The remaining parts of the Baltic Sea were characterized by lower beach wrack production potential (approximately 0–1,000 g per m2 per month).
Along most of the coast, the beach wrack that is deposited on the beaches does not unduly affect the people who live close by. However, in certain areas, a proportion of the wrack moving onshore is permanently trapped. It creates problems not only for inhabitants of those areas, local authorities responsible for maintaining the beaches or beach visitors (Kataržytė et al., 2019), but also for the local beach ecosystem. Seagrass and algae wrack, during decomposition, release several constituents, which alter the coastal biogeochemical cycles and influence organisms living there. These include nutrients and dissolved organic carbon, which affect flora and microbial activity, and heavy metals (in polluted systems), creating a risk for biota (Rudovica and Bartkevics, 2015). Also, the emission of volatile components from decaying plant material might represent a risk for human health (H2S, Hg0, Cs–137), as well as for climate change (by CH4 emission). Hence, beach wrack is the subject of several research projects (Table 7).
Pollution Prevention
Anthropogenic mercury release remains a problem in the aquatic environment and, based on the sedimentary records in the Baltic Sea, it exceeds Hg coming from natural sources (i.e., hydrothermal processes and rock weathering) by a factor of 5 on average. Recently, the emission of this metal to the environment has substantially decreased (Helsinki Commission, 2009, 2018; Kwasigroch et al., 2021). This has resulted in a noticeable decrease of mercury concentration in macrophytes in the Polish coastal zone of the southern Baltic (Bełdowska et al., 2015, 2016). In parallel, the intense growth of some macrophytobenthos on the sea bottom has been observed in many areas (Carmen et al., 2019; Sokołowski et al., 2021). This is stimulated by an improvement of environmental conditions and lengthening of the growing season. It leads to the rapid inclusion of mercury from the water column (which is introduced from natural and anthropogenic terrestrial sources) and from sediments (which was deposited in the past and can be considered retarded anthropogenic emission) (Bełdowska et al., 2015).
In many areas of the Baltic Sea, due to the pattern of currents and shape of the coastline, large quantities of macrophytobenthos gather in the coastal zone or end up as beach wrack. During the summer season in the Gulf of Gdansk, on 1 km of the beach, the amount of beached seagrass and algae wrack ranges from several dozens to 800 tons (Filipkowska et al., 2008; Weinberger et al., 2020). Considering median total Hg concentration (7.6 ng/g dry weight), it has been calculated that a beach segment that is 1 km long may receive 6 g of mercury per season. Analyses of coastal erosion in the Southern Baltic show that about 39% of the Polish coast is accumulative (Dubrawski et al., 2008). It means that about 200 km of coastline favors phytobenthic accumulation. During the summer season, benthic plants on Polish beaches alone may contain 0.05–1.2 kg of Hg (Bełdowska et al., 2015).
A recent study performed within the CONTRA project in the Puck Bay (sheltered part of Gulf of Gdańsk), Poland, indicate that the concentrations of Hg in the managed beach (P1), where live algae occur, were lower than those collected in the unmanaged site, where decomposing wrack was collected (sampling sites R1 and R2, Figure 5). However, in the unmanaged station, the concentrations of Hg in live algae (sampling site R3) were similar to those at the managed site. This indicates that although biological material from the bay accumulates Hg at the same rate and is characterized by the same mercury concentration in both sites, accumulation does not stop on landing. Decomposing beach wrack in the unmanaged site is rich in organic matter and continuously builds up Hg concentration. This is probably caused by the excellent sorption capabilities of decaying plants and algae material that may capture Hg from coastal water, acting as a filter for surface waters. Another explanation is the Hg capture from the atmosphere, where it originates in low emission from local sources (Bełdowska et al., 2014). This means that unmanaged beaches may not only transfer Hg from beach wrack via accumulation into live algae and subsequent release, but also enhance Hg flux to the beach from local sources.
FIGURE 5
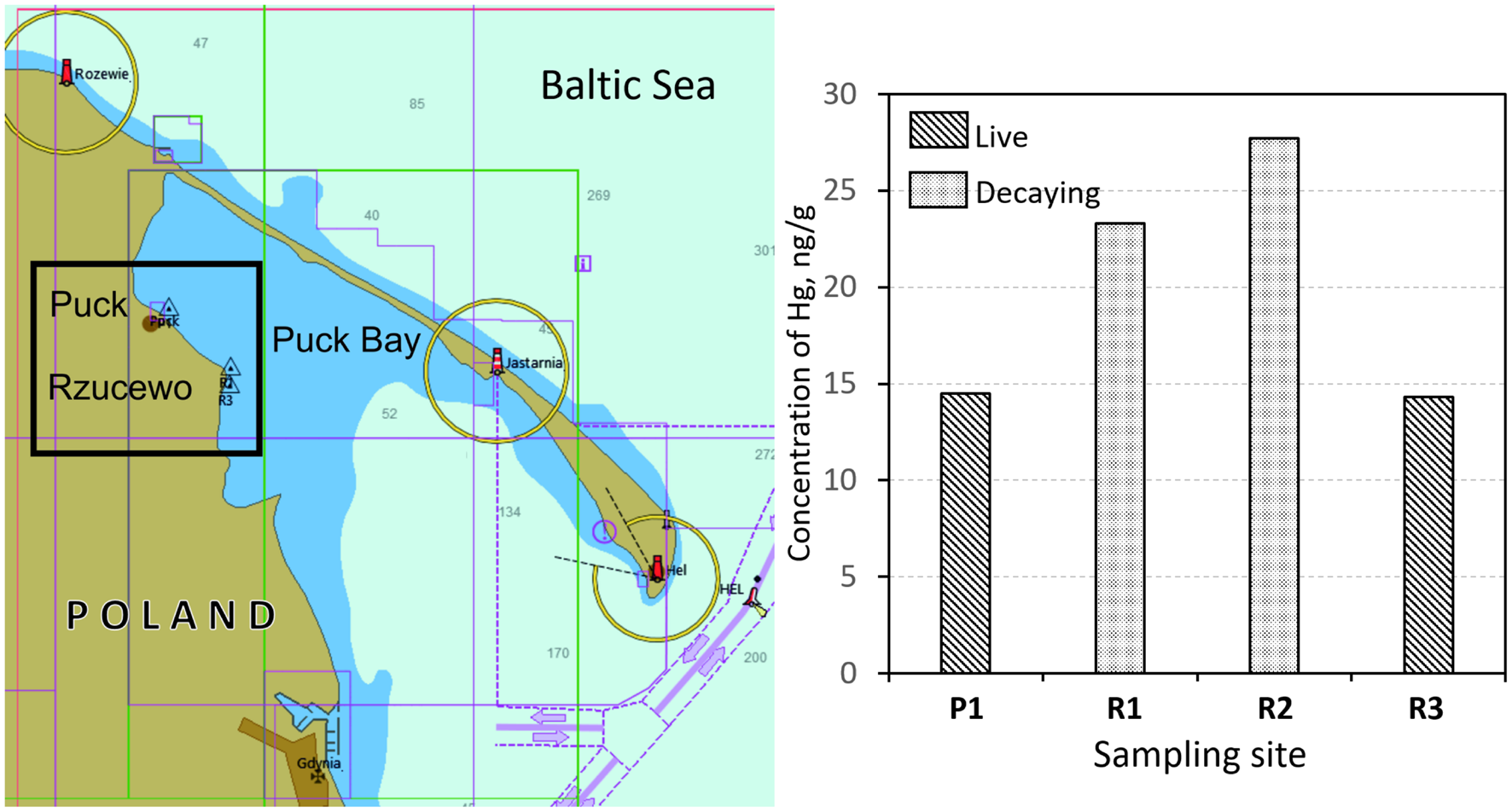
The study area (left) and total mercury concentration (right) in live algae at the managed site (P1) and unmanaged site (R3), decaying beach wrack at the unmanaged site (R1, R2).
Besides pollutants, nutrients are also removed from water by algae and marine plants, which are later released from decomposing beach wrack. Decomposition of organic matter at the bottom causes a higher concentration of phosphate and ammonia in porewaters than nearbottom waters (Graca et al., 2006). Porewaters, collected from areas beneath decaying beach wrack, had similar phosphate and ammonia concentrations (phosphate, p = 0.86; ammonia, p = 0.46) as porewaters in the coastal zone sediments, but higher than those in nearbottom waters (phosphate, p = 0.003; ammonia, p < 0.01) (Figures 6A,B).
FIGURE 6
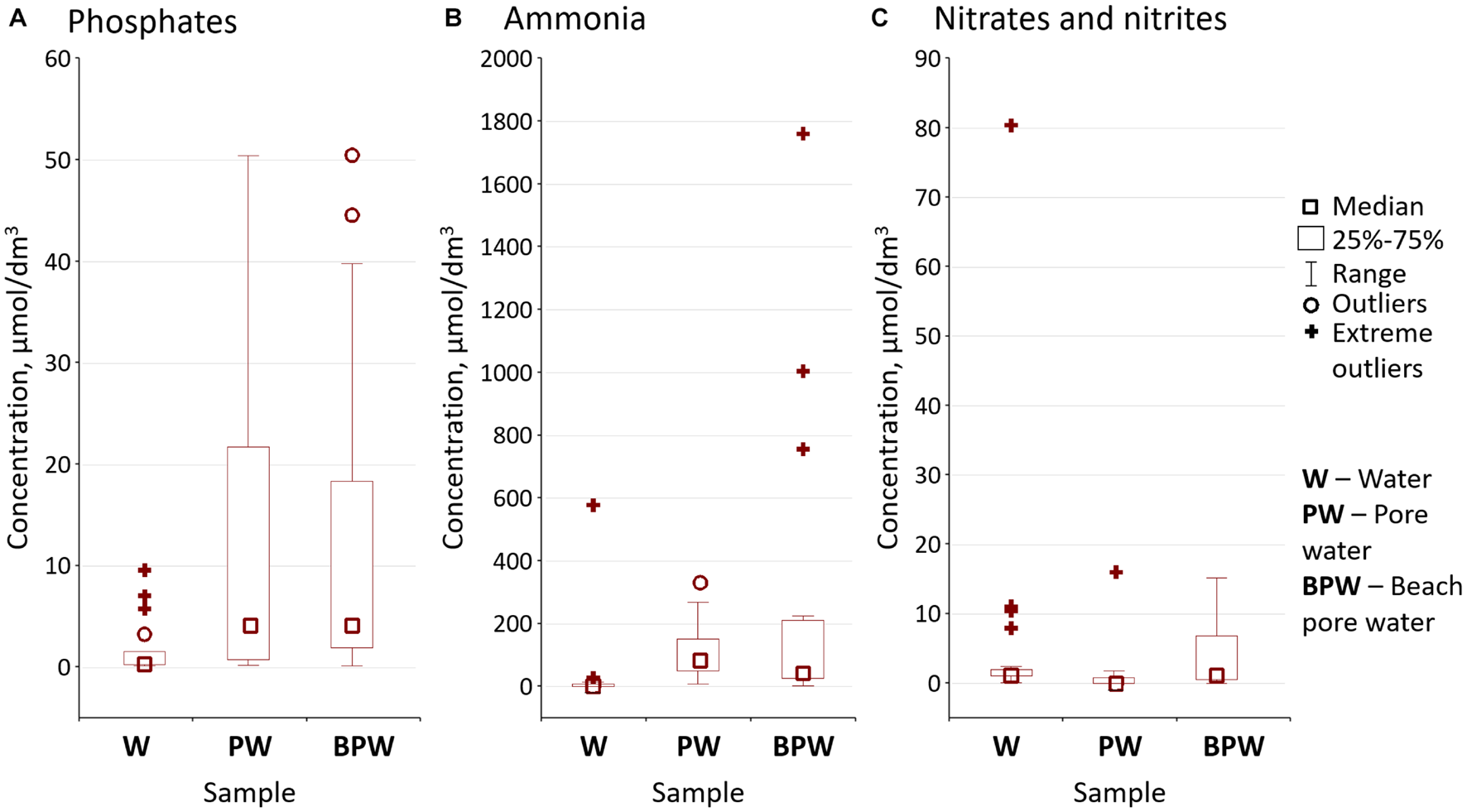
Concentration of (A) phosphates, (B) ammonia, and (C) nitrates + nitrites in water, porewater and beach porewater (sampled from under the detritus) from April (2019) to November (2019) at three sampling sites (P1, R1, R3) in the Bay of Puck (Poland).
Moreover, in porewaters collected beneath beach wrack in unmanaged beaches (sampling sites R1, R3), the median concentration of phosphate and ammonia equaled to 14.5 and 140.9 μmol/dm3, respectively. These concentrations were over three times higher than those from the managed beach in the same area (P1). This suggests that phosphates and ammonia released from decaying beach wrack are partially retained in the beach porewaters. However, in the longer perspective, they return to coastal waters, where they fuel primary production. One ton of dry beach detritus weight contains from 0.5 to slightly above 3 kg of phosphorus and from 5 to 32 kg of nitrogen. Such a load, delivered to sea water, is potentially responsible for the production of 0.5–3 tons of phytoplankton biomass.
Nitrates are also being produced in porewaters. They reach the highest concentration close to the surface, in oxygenated layers. In deeper layers, they are consumed by denitrification (Behrendt et al., 2013). In the study area, nitrate concentrations in porewaters collected beneath decaying beach wrack were similar to those observed in the water column (p = 0.811) and significantly higher than those in porewaters of coastal sediments (Figure 6C). The conditions below decaying beach wrack favor nitrification, which unfortunately reduces the nitrogen removal (in its gaseous form) from the water.
These results indicate that beach wrack removal from the beach can prevent both – pollutants and nutrients scavenged from plants and algae during their lifetime in the sea from re-entry to the coastal waters. Therefore, using beach wrack as a resource could contribute to the clean-up of the marine environment.
Gasification/Torrefaction
As a prospective solution for beach wrack from the Baltic Sea coast, processing, gasification and transformation to biochar has been demonstrated based on an experimental study and pilot-scale tests. Gasification is a chemical process that converts carbonaceous material, such as biomass and coal, into gaseous fuel or chemical feedstock (Basu, 2010), differing substantially from other thermal processes, such as incineration or pyrolysis (Porshnov et al., 2018). This gaseous fuel is known as producer gas or synthesis gas (syngas) containing CO2, H2, CO, H2O, CH4, and N2. Surplus char, formed from the pyrolysis process, is heated by supplying a limited amount of air in the gasifier. Beach wrack sampled from several sites along the Baltic Sea coastline contains relatively high amounts of plastic residues (up to 5%) and inorganic material (ash up to 30%). However, thermogravimetric and proximate/ultimate analysis on beach wrack demonstrates relatively high carbon amounts (up to 30%) with low organic chlorine and sulfur concentration. The highest heating value ranges 8–15 MJ/kg, thus proving the potential to use it for energy production purposes. Also, trace elements and heavy metal concentrations are low in beach wrack (Burlakovs et al., 2019).
The choice of thermochemical conversion technology was driven by the specific nature of the beach wrack. Beach wrack gasification tests were performed on an innovative gasification plant for pyrolysis of various wastes and the thermal cracking of pyrolysis gas products. The apparatus consists of an extruder-type pyrolizer/gasifier, a pyrolysis product separation chamber, a thermal cracker for gaseous pyrolysis products and a gas burning torch (Figure 7). The gasification process does not use air or oxygen as a gasification agent. The process is allothermal in nature, using an external heat source, and the system is used in continuous operation mode. In the extruder-type pyrolyzer (3), the fuel is compacted. The operating temperature of the extruder is set and automatically adjusted to 300–600°C. The primary reforming of the fuel into pyrolysis gas and coal is carried out in the extruder. In the pyrolysis product separation chamber (4), the pyrolysis gas is separated from the coal. The carbon is stored in an airtight container. After cooling, the coal is unloaded from the container and sent to a laboratory for analysis. The pyrolysis gas is fed to a secondary high-temperature reformer (6), where the pyrolysis gas is heated to 800–1200°C. At elevated temperatures, a high turbulent tar thermal cracking occurs, and heavy organic gaseous substances are reformed into the synthesis gas components, such as H2, CO, CO2. At the output of the secondary reformer, the gas is cooled, and the heat consumed in the process is recovered. The resulting synthesis gas has a high concentration of CH4 (up to 60%), but the obtained waste char can be used as biochar or as fuel to replace fossil analogs.
FIGURE 7
Construction of experimental gasification plant (Bisters et al., 2021): (1) feedstock bunker; (2) hydraulic press feeder; (3) extruder-type pyrolizer; (4) separation chamber – gas and char accumulation tank; (5) secondary gas cracking; (6) external inductive heater – temperature reformer; (7) gas cooler; (8) inductive heater resonator; (9) inductive heater power box; (10) flare; (11) control cabinet; (12) hydro-station; (13) hydro-cylinder; (14) prior hydro-presser box; (15) nitrogen balloon.
Anaerobic Digestion
To test the biogas potential obtained from different Baltic Sea coastal beach wrack, three experimental studies were conducted using anaerobic digestion methods. The first two tests were undertaken for three types of selected beach wrack algae from the Riga Gulf coast in Latvia and a mixed sample from the Kalmar coast in Sweden. Anaerobic fermentation (Figure 8) was applied for the samples without specific pre-treatment. In the first study, 16 bioreactors operated in batch mode at 38°C were used to ferment the three common algal types available as beach wrack in the Gulf of Riga. Testing samples were taken from the beach wrack piles in Jaunkemeri, Bigaunciems, and Ragaciems coastal areas in Latvia.
FIGURE 8
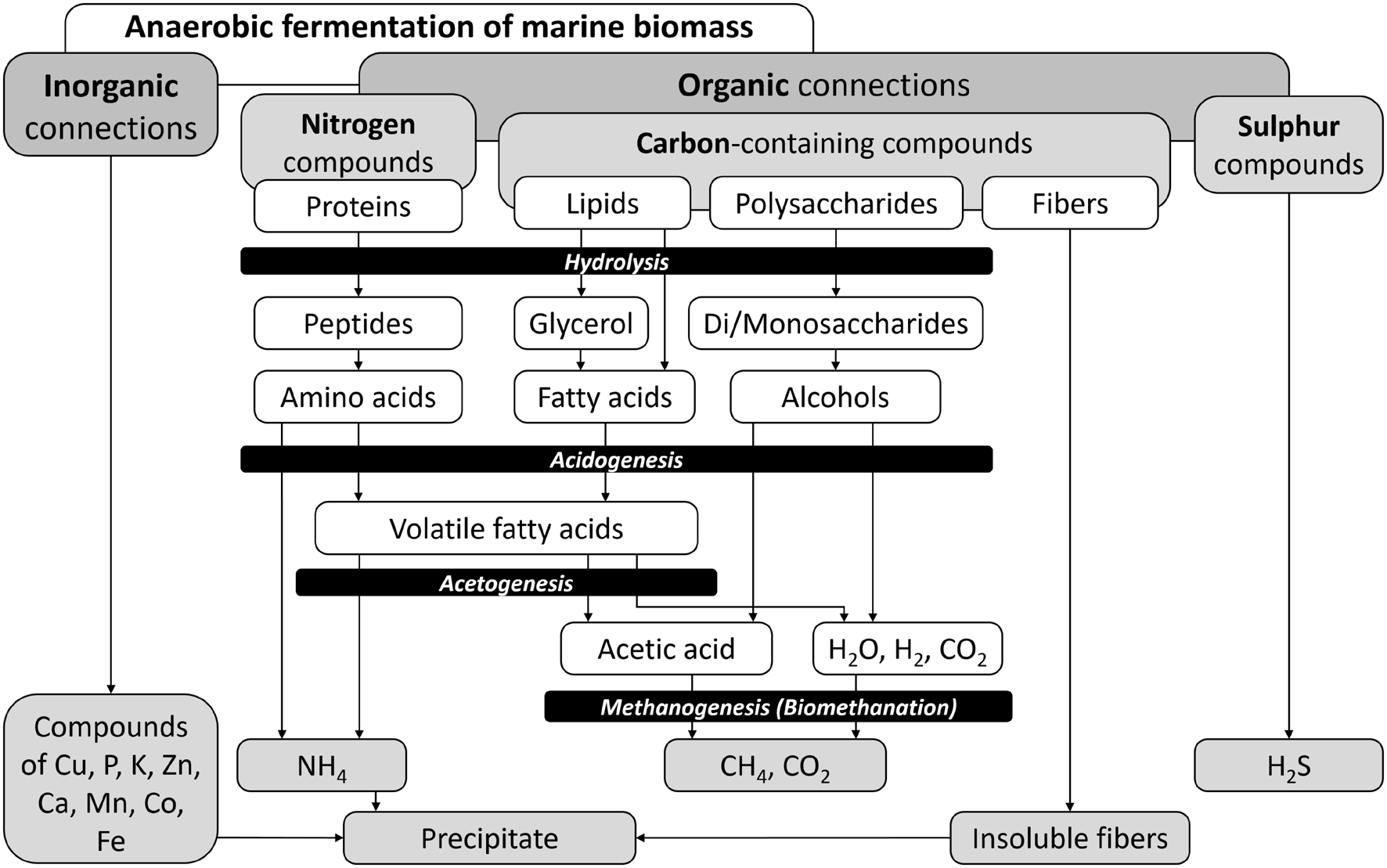
Schematic overview of the anaerobic fermentation of marine biomass, indicating organic and inorganic connections, the main chemical transformation of compounds and resulting intermediate and final products.
From fermentation of 32 days, 0.276 L/g DrOM (dry organic matter) biogas (0.046 L/g DrOM methane) from brown algae and 0.248 L/g DrOM biogas (0.027 L/g DrOM methane) from red algae were obtained. From green algae, 0.425 L/g DrOM biogas (0.071 L/g DrOM methane) were obtained on average. The study showed that from the coast-washed beach wrack, a small amount of methane can be generated per DrOM if there is no pre-treatment and conditioning of the samples. From the mixed sample, due to a higher presence of debris and lignocellulose feedstock, the biogas yield with applied anaerobic fermentation method also shows limited biogas potential.
Further study for measuring brown algae biogas production was tested with three pre-treatment methods: algae being kept for 24 h in tap water, washed for 1 h in a stream of running fresh water and dried. The resulting methane quantities were compared with those obtained from the raw brown algae. From algae that were kept in tap water for 24 h, 0.560 L/g DrOM biogas (0.198 L/g DrOM methane) was obtained, and from the ones washed 1 h in running water, 0.569 L/g DrOM biogas (0.211 L/g DrOM methane) was obtained, but from dried mass, only 0.164 L/g DrOM biogas (0.065 L/g DrOM methane) was gained. The study confirms that washing brown algae as a pre-treatment for anaerobic fermentation avoids salts inhibition and can perform better in biomethane production.
Biogas (methane), generated from brown algae in the study without special pre-treatment, is on average 0.276 L/g DrOM (0.046 L/g DrOM), which is a very low yield, and from red-brown algae in the study without special pre-treatment is on average 0.248 L/g DrOM (0.027 L/g DrOM). Red-green algae in the study without special pre-treatment acquired an average of 0.425 L/g DrOM (0.071 L/g DrOM), which is slightly better but still represents a relatively low result. Biogas (methane) generated from mixed algae with coastal reed mixture in the study without special pre-treatment on average shows 0.267 L/g DrOM (0.32 L/g DrOM), which is a comparably low yield. The retention of brown algae samples in water for 24 h resulted in about 63.6% more methane in the DrOM compared with unwashed brown algae. In the second brown algae study, sample pre-treatment in running water washed for 1 h gained about 74.4% more methane out of DrOM than units from unwashed brown algae. The test results have shown that rinsing seaweed before feeding into anaerobic digestion is preferable to achieve better yields. In cases where it is not possible to apply the described methodology, biogas/methane yields will be negligible, and recovery of the waste into biogas will not be economically feasible. With the pre-treatment of brown algae in running water, the feedstock is well suited for optimal volume biogas generation for energy recovery and use in other bio-SNG (synthetic natural gas) applications. With or without pre-treatment, seaweed biomass can be used in the co-fermentation of other waste streams like sewage sludge or manure. Such co-fermentation will optimize the C:N ratio and will neutralize the inhibiting effect. For clarification of the optimal process parameters, further studies and tests are recommended.
Conclusion
To effectively valorize marine waste, promising perspectives must be considered, namely: (a) the development of mechanical technology for the harvesting of the biomass; (b) the development of chemical and biological pipelines to conserve and/or process marine biomass; (c) the market search to maximize the potential use in the various industries; (d) the employment of communication strategies to raise awareness, increase consumers’ acceptance on the potential greener technologies or public health improvement through enhanced waste-originated food or feed ingredients as well as biomedicals; (e) the promotion of public and private funding toward innovation and technology development, while addressing the potential legislative bottlenecks; and (f) the application of eco-friendly principles to production systems. Even though significant progress has been made in marine-derived biomass research and innovation, there is still the unmet need for technology scale-up and the establishment of business opportunities to promote sustainability and circular economy. Hence, the valorization of waste into useful products entails a tight collaboration between industry and research sectors, as well as governance bodies. It is reflected in the governance promotion of resource efficiency in Europe by several European policy initiatives, the most recent one being the European Green Deal, which is Europe’s roadmap for a sustainable economy. The use of bioactive compounds, valorized from side streams and waste, will significantly contribute to global environmental sustainability as waste valorization companies contribute to a green, blue and circular economy. These companies should include a socially responsible connotation and provide an alternative source of income for communities that are heavily dependent on fisheries and aquaculture. Therefore, future research should, on the one hand, target the optimization of side streams processing into useful products, and guarantee a sustainable supply and product quality on the other hand, regardless of the season or geographical location.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Author contributions
ViR, AR, and JuB designed the manuscript concept and drafted the manuscript. All authors contributed with their specific field of expertise, read, edited and approved the final version of the manuscript.
Funding
This publication is based upon work from COST Action CA18238 (Ocean4Biotech), supported by COST (European Cooperation in Science and Technology). AR and KK: this research was funded by the Slovenian Research Agency (research core funding P1-0245 and P1-0237). AR: this publication has been produced with financial assistance of the Interreg MED Programme, co-financed by the European Regional Development Fund (Project No. 8MED20_4.1_SP_001, internal ref. 8MED20_4.1_SP_001) – B-Blue project. SG, CT, and JO: this work is financed by national funds from FCT – Fundação para a Ciência e a Tecnologia, I.P., in the scope of the project UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences - UCIBIO and the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy – i4HB. JaB and WH: the preparation of the manuscript was supported by the Project CONTRA (Conversion of a Nuisance to a Resource and Asset #R090, 2018–2021) of the INTERREG Baltic Sea Region Program, and Polish Ministry of Science and Higher Education from the 2019–2021 science funding allocated for the implementation of international co-financed project W24/INTERREG BSR/2019. Research of Maris Klavins, VB, and LA was supported by ERDF project 1.1.1.1/16/A/050 “Variable fuel gasification for municipal solid waste recovery.” MC acknowledges the funding from CEEC program supported by FCT/MCTES (CEECIND/02968/2017) and Strategic Funding UIDB/04423/2020 and UIDP/04423/2020 supported by national funds provided by FCT and ERDF. AD acknowledges financial support provided by European Union’s Horizon 2020 research and innovation program under the grant agreement No 857287 and Latvian Council of Science research project No. lzp-2020/1-0054. MKa: the Interreg LAT_LIT Programme, co-financed by the European Regional Development Fund (LLI-525 ESMIC). LB acknowledges the funding from Erasmus + Project No. ECOBIAS 609967-EPP-1-2019-1-RS-EPPKA2-CBHE-JP; GA.2019-1991/001-001. Development of master curricula in ecological monitoring and aquatic bioassessment for Western Balkans HEIs/ECOBIAS. IS and KP acknowledge financial support provided by the projects CZ.02.1.01/0.0/0.0/17_048/0007323 and CZ.02.1.01/0.0/0.0/16_019/0000754 (Ministry of Education, Youth and Sports of the Czech Republic). ZV-G acknowledges support within the project No.1.1.1.2/VIAA/1/16/029 (Formula of peat-free soil conditioner with controlled-release fertilizing effect applicable for soil remediation and quality improvement of agricultural production). IZ: the projects SLTKT20427, KIK 17431 and SARASWATI 2.0. JuB: the project No.1.1.1.2/VIAA/3/19/531 (Innovative technologies for stabilization of landfills – diminishing of environmental impact and resources potential in frames of circular economy). The work conducted by CR, LA-H, and MA was fully financed by Møreforsking AS.
Acknowledgments
Special thanks are addressed to Amanda Ingram (Zero Waste Scotland), Tracey Begg (Scottish Natural Heritage), Iona Campbell (SAMS) and Marine Scotland for the help with compiling the information regarding Scotland, as well as Maris Klavins for help with the gasification chapter.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Aas T. S. Åsgård T. E. (2017). Estimated Content of Nutrients and Energy in Feed Spill and Faeces in Norwegian Salmon Culture.[Nofima Report 19/2017]. Tromsø: Nofima.
2
Abdelhedi O. Nasri R. Mora L. Jridi M. Toldrá F. Nasri M. (2018). In silico analysis and molecular docking study of angiotensin I-converting enzyme inhibitory peptides from smooth-hound viscera protein hydrolysates fractionated by ultrafiltration.Food Chem.239453–463. 10.1016/j.foodchem.2017.06.112
3
Abdollahi M. Alboofetileh M. Behrooz R. Rezaei M. Miraki R. (2013a). Reducing water sensitivity of alginate bio-nanocomposite film using cellulose nanoparticles.Int. J. Biol. Macromol.54166–173. 10.1016/j.ijbiomac.2012.12.016
4
Abdollahi M. Alboofetileh M. Rezaei M. Behrooz R. (2013b). Comparing physico-mechanical and thermal properties of alginate nanocomposite films reinforced with organic and/or inorganic nanofillers.Food Hydrocolloids32416–424. 10.1016/j.foodhyd.2013.02.006
5
Abdul-Hamid A. Bakar J. Bee G. H. (2002). Nutritional quality of spray dried protein hydrolysate from black tilapia (Oreochromis mossambicus).Food Chem.7869–74. 10.1016/S0308-8146(01)00380-6
6
Adeniyi O. M. Azimov U. Burluka A. (2018). Algae biofuel: current status and future applications.Renew. Sustain. Energy Rev.90316–335. 10.1016/j.rser.2018.03.067
7
Ahrens T. Drescher-Hartung S. Anne O. (2017). “Sustainability of the biowaste utilization for energy production,” in Biomass Volume Estimation and Valorization for Energy, ed.TumuluruJ. (London: IntechOpen), 165–187.
8
Ahuja I. Dauksas E. Remme J. F. Richardsen R. Løes A. K. (2020). Fish and fish waste-based fertilizers in organic farming – with status in norway: a review.Waste Manag.11595–112. 10.1016/j.wasman.2020.07.025
9
Al Khawli F. Pateiro M. Domínguez R. Lorenzo J. M. Gullón P. Kousoulaki K. et al (2019). Innovative green technologies of intensification for valorization of seafood and their by-products.Mar. Drugs17:689. 10.3390/md17120689
10
Al-Enizi A. M. Zagho M. M. Elzatahry A. A. (2018). Polymer-based electrospun nanofibers for biomedical applications.Nanomaterials (Basel)8:259. 10.3390/nano8040259
11
Alfonsín V. Maceiras R. Gutiérrez C. (2019). Bioethanol production from industrial algae waste.Waste Manag.87791–797. 10.1016/j.wasman.2019.03.019
12
Angelova R. Baldikova E. Pospiskova K. Maderova Z. Safarikova M. Safarik I. (2016). Magnetically modified Sargassum horneri biomass as an adsorbent for organic dye removal.J. Cleaner Prod.137189–194. 10.1016/j.jclepro.2016.07.068
13
Araújo R. Vázquez Calderón F. Sánchez López J. Azevedo I. C. Bruhn A. Fluch S. et al (2021). Current status of the algae production industry in Europe: an emerging sector of the blue bioeconomy.Front. Mar. Sci.7:626389. 10.3389/fmars.2020.626389
14
Archer M. Russel D. (2008). Crustacea Processing Waste Management.[Seafish Research Report SR593]. Edinburgh: Seafish.
15
Arumugam N. Chelliapan S. Kamyab H. Thirugnana S. Othman N. Nasri N. S. (2018). Treatment of wastewater using seaweed: a review.Int. J. Environ. Res. Public Health15:2851. 10.3390/ijerph15122851
16
Augustine R. Rehman S. R. U. Ahmed R. Zahid A. A. Sharifi M. Falahati M. et al (2020). Electrospun chitosan membranes containing bioactive and therapeutic agents for enhanced wound healing.Int. J. Biol. Macromol.156153–170. 10.1016/j.ijbiomac.2020.03.207
17
Aytas S. Gunduz E. Gok C. (2014). Biosorption of uranium ions by marine macroalga Padina pavonia.Clean Soil Air Water42498–506. 10.1002/clen.201300019
18
Bãdescu I. S. Bulgariu D. Ahmad I. Bulgariu L. (2018). Valorisation possibilities of exhausted biosorbents loaded with metal ions – a review.J. Environ. Manag.224288–297. 10.1016/j.jenvman.2018.07.066
19
Bãdescu I. S. Bulgariu D. Bulgariu L. (2017). Alternative utilization of algal biomass (Ulva sp.) loaded with Zn(II) ions for improving of soil quality.J. Appl. Phycol.291069–1079. 10.1007/s10811-016-0997-y
20
Badri A. Raman K. Jayaraman G. (2019). Uncovering novel pathways for enhancing hyaluronan synthesis in recombinant Lactococcus lactis: genome-scale metabolic modeling and experimental validation.Processes7:43. 10.3390/pr7060343
21
Balachandar G. Khanna N. Das D. (2013). “Biohydrogen production from organic wastes by dark fermentation,” in Biohydrogen, edsPandeyA.ChangJ. S.HallenbeckaP. C.LarrocheC. (Amsterdam: Elsevier), 103–144.
22
Barbosa A. I. Costa Lima S. A. Reis S. (2019a). Application of pH-responsive fucoidan/chitosan nanoparticles to improve oral quercetin delivery.Molecules24:346. 10.3390/molecules24020346
23
Barbosa A. I. Coutinho A. J. Costa Lima S. A. Reis S. (2019b). Marine polysaccharides in pharmaceutical applications: fucoidan and chitosan as key players in the drug delivery match field.Mar. Drugs17:654. 10.3390/md17120654
24
Barbot Y. N. Al-Ghaili H. Benz R. (2016). A review on the valorization of macroalgal wastes for biomethane production.Mar. Drugs14120. 10.3390/md14060120
25
Basu P. (2010). Biomass Gasification and Pyrolysis Practical Design and Theory.Cambridge: Academic Press.
26
Bay-Larsen I. Risvoll C. Vestrum I. Bjørkhaug H. (2018). Local protein sources in animal feed – Perceptions among arctic sheep farmers.J. Rural Stud.5998–110. 10.1016/j.jrurstud.2018.02.004
27
Bay-Larsen I. Vestrum I. K. Lind V. Risvoll C. Novoa-Garrido M. Roleda M. Y. (2016). “Traditional to commercial use of seaweeds: Cross-disciplinary perspectives in using local protein sources in Arctic sheep husbandry,” in Proceedings of the 26th General Meeting of the European Grassland Federation, Trondheim, 693–695.
28
Beaulieu L. Thibodeau J. Bonnet C. Bryl P. Carbonneau M. E. (2013). Evidence of anti-proliferative activities in blue mussel (Mytilus edulis) by-products.Mar. Drugs11975–990. 10.3390/md11040975
29
Behrendt A. de Beer D. Stief P. (2013). Vertical activity distribution of dissimilatory nitrate reduction in coastal marine sediments.Biogeosciences107509–7523. 10.5194/bg-10-7509-2013
30
Bełdowska M. Jędruch A. Słupkowska J. Saniewska D. Saniewski M. (2015). Macrophyta as a vector of contemporary and historical mercury from the marine environment to the trophic web.Environ. Sci. Pollut. Res.225228–5240. 10.1007/s11356-014-4003-4
31
Bełdowska M. Jędruch A. Zgrundo A. Ziółkowska M. Graca B. Gębka K. (2016). The influence of cold season warming on the mercury pool in coastal benthic organisms.Estuar. Coast. Shelf Sci.17199–105. 10.1016/j.ecss.2016.01.033
32
Bełdowska M. Saniewska D. Falkowska L. (2014). Factors influencing variability of mercury input to the southern Baltic Sea.Mar. Pollut. Bull.86283–290. 10.1016/j.marpolbul.2014.07.004
33
Beni A. A. Esmaeili A. (2020). Biosorption, an efficient method for removing heavy metals from industrial effluents: a review.Environ. Technol. Innov.17:100503. 10.1016/j.eti.2019.100503
34
Benito-González I. López-Rubio A. Martínez-Sanz M. (2018). Potential of lignocellulosic fractions from Posidonia oceanica to improve barrier and mechanical properties of bio-based packaging materials.Int. J. Biol. Macromol.118542–551. 10.1016/j.ijbiomac.2018.06.052
35
Benito-González I. López-Rubio A. Gavara R. Martínez-Sanz M. (2019a). Cellulose nanocrystal-based films produced by more sustainable extraction protocols from Posidonia oceanica waste biomass.Cellulose268007–8024. 10.1007/s10570-019-02641-4
36
Benito-González I. López-Rubio A. Martínez-Sanz M. (2019b). High-performance starch biocomposites with celullose from waste biomass: film properties and retrogradation behaviour.Carbohydr. Polym.216180–188. 10.1016/j.carbpol.2019.04.030
37
Benito-González I. López-Rubio A. Gómez-Mascaraque L. G. Martínez-Sanz M. (2020). PLA coating improves the performance of renewable adsorbent pads based on cellulosic aerogels from aquatic waste biomass.Chem. Eng. J.390:124607. 10.1016/j.cej.2020.124607
38
Bettaieb F. Khiari R. Hassan M. L. Belgacem M. N. Bras J. Dufresne A. et al (2015). Preparation and characterization of new cellulose nanocrystals from marine biomass Posidonia oceanica.Ind. Crops Prod.72175–182. 10.1016/j.indcrop.2014.12.038
39
Bhardwaj N. Kundu S. C. (2010). Electrospinning: a fascinating fiber fabrication technique.Biotechnol. Adv.28325–347. 10.1016/j.biotechadv.2010.01.004
40
Bhatnagar A. Vilar V. J. P. Santos J. C. Botelho C. M. S. Boaventura R. A. R. (2012). Valorisation of marine Pelvetia canaliculata Ochrophyta for separation and recovery of nickel from water: equilibrium and kinetics modeling on Na-loaded algae.Chem. Eng. J.200–202365–372. 10.1016/j.cej.2012.06.071
41
Bilal M. Iqbal H. M. N. (2020). Biologically active macromolecules: extraction strategies, therapeutic potential and biomedical perspective.Int. J. Biol. Macromol.1511–18. 10.1016/j.ijbiomac.2020.02.037
42
Bilal M. Rasheed T. Sosa-Hernández J. E. Raza A. Nabeel F. Iqbal H. M. N. (2018). Biosorption: an interplay between marine algae and potentially toxic elements – a review.Mar. Drugs16:65. 10.3390/md16020065
43
Bird M. I. Wurster C. M. de Paula Silva P. H. Bass A. M. de Nys R. (2011). Algal biochar – production and properties.Bioresour. Technol.1021886–1891. 10.1016/j.biortech.2010.07.106
44
Bisters V. Kalviss J. Burlakovs J. Klavins M. (2021). Algae processing for energy production: development of waste pyrolysis technology.Agron. Res.19. 10.15159/AR.21.010
45
Borchert F. Emadodin I. Kluß C. Rotter A. Reinsch T. (2021). Grass growth and N2O emissions from soil after application of jellyfish in coastal areas.Front. Mar. Sci.8:711601. 10.3389/fmars.2021.711601
46
Bouzikri S. Ouasfi N. Benzidia N. Salhi A. Bakkas S. Khamliche L. (2020). Marine alga Bifurcaria bifurcata: biosorption of reactive Blue 19 and methylene blue from aqueous solutions.Environ. Sci. Pollut. Res.2733636–33648. 10.1007/s11356-020-07846-w
47
Broch O. J. Alver M. O. Bekkby T. Gundersen H. Forbord S. Handå A. et al (2019). The kelp cultivation potential in coastal and offshore regions of Norway.Front. Mar. Sci.5:529. 10.3389/fmars.2018.00529
48
Brod E. Oppen J. Kristoffersen A. Ø Haraldsen T. K. Krogstad T. (2017). Drying or anaerobic digestion of fish sludge: nitrogen fertilisation effects and logistics.Ambio46852–864. 10.1007/s13280-017-0927-5
49
Brown L. K. Blanz M. Wishart J. Dieterich B. Schmidt S. B. Russell J. et al (2020). Is Bere barley specifically adapted to fertilisation with seaweed as a nutrient source?Nutr. Cycling Agroecosyst.118149–163. 10.1007/s10705-020-10090-w
50
Bruhn A. Dahl J. Nielsen H. B. Nikolaisen L. Rasmussen M. B. Markager S. et al (2011). Bioenergy potential of Ulva lactuca: biomass yield, methane production and combustion.Bioresour. Technol.1022595–2604. 10.1016/j.biortech.2010.10.010
51
Buchtová N. Pradille C. Bouvard J.-L. Budtova T. (2019). Mechanical properties of cellulose aerogels and cryogels.Soft Matter.157901–7908. 10.1039/C9SM01028A
52
Bulgariu D. Bulgariu L. (2016). Potential use of alkaline treated algae waste biomass as sustainable biosorbent for clean recovery of cadmium(II) from aqueous media: batch and column studies.J. Cleaner Prod.1124525–4533. 10.1016/j.jclepro.2015.05.124
53
Burlakovs J. Kriipsalu M. Porshnov D. Jani Y. Ozols V. Pehme K. M. et al (2019). Gateway of landfilled plastic waste towards circular economy in Europe.Separations6:6020025. 10.3390/separations6020025
54
Buschmann A. H. Camus C. Infante J. Neori A. Israel Á Hernández-González M. C. et al (2017). Seaweed production: overview of the global state of exploitation, farming and emerging research activity.Eur. J. Phycol.52391–406. 10.1080/09670262.2017.1365175
55
Calejo M. T. Almeida A. J. Fernandes A. I. (2012). Exploring a new jellyfish collagen in the production of microparticles for protein delivery.J. Microencapsul.29520–531. 10.3109/02652048.2012.665089
56
Campbell I. Macleod A. Sahlmann C. Neves L. Funderud J. Øverland M. et al (2019). The environmental risks associated with the development of seaweed farming in Europe – Prioritizing key knowledge gaps.Front. Mar. Sci.6:107. 10.3389/fmars.2019.00107
57
Cardoso M. J. Costa R. R. Mano J. F. (2016). Marine origin polysaccharides in drug delivery systems.Mar. Drugs14:34. 10.3390/md14020034
58
Cardoso S. L. Costa C. S. D. Nishikawa E. da Silva M. G. C. Vieira M. G. A. (2017). Biosorption of toxic metals using the alginate extraction residue from the brown algae Sargassum filipendula as a natural ion-exchanger.J. Cleaner Prod.165491–499. 10.1016/j.jclepro.2017.07.114
59
Carmen B. Krause-Jensen D. Alcoverro T. Marbà N. Duarte C. M. Van Katwijk M. M. et al (2019). Recent trend reversal for declining European seagrass meadows.Nat. Commun.101–8. 10.1038/s41467-019-11340-4
60
Caruso G. (2016). Fishery wastes and by-products: a resource to be valorised.J. Fish. Sci.1012–15.
61
Chai Q. Jiao Y. Yu X. (2017). Hydrogels for biomedical applications: their characteristics and the mechanisms behind them.Gels3:6. 10.3390/gels3010006
62
Chaiwong K. Kiatsiriroat T. Vorayos N. Thararax C. (2013). Study of bio-oil and bio-char production from algae by slow pyrolysis.Biomass Bioenergy56600–606. 10.1016/j.biombioe.2013.05.035
63
Chan S. W. Mirhosseini H. Taip F. S. Ling T. C. Nehdi I. A. Tan C. P. (2016). Emulsion formulation optimization and characterization of spray-dried κ-carrageenan microparticles for the encapsulation of CoQ10.Food Sci. Biotechnol.2553–62. 10.1007/s10068-016-0098-3
64
Chawla R. Sivakumar S. Kaur H. (2021). Antimicrobial edible films in food packaging: Current scenario and recent nanotechnological advancements- a review.Carbohydr. Polym. Technol. Appl.2:100024. 10.1016/j.carpta.2020.100024
65
Chemat F. Rombaut N. Sicaire A. G. Meullemiestre A. Fabiano-Tixier A. S. Abert-Vian M. (2017). Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review.Ultrason. Sonochem.34540–560. 10.1016/j.ultsonch.2016.06.035
66
Chen H. Zhou D. Luo G. Zhang S. Chen J. (2015). Macroalgae for biofuels production: progress and perspectives.Renew. Sustain. Energy Rev.47427–437. 10.1016/j.rser.2015.03.086
67
Chen P. Y. Lin A. Y. M. McKittrick J. Meyers M. A. (2008). Structure and mechanical properties of crab exoskeletons.Acta Biomater.4587–596. 10.1016/j.actbio.2007.12.010
68
Chen S. S. Maneerung T. Tsang D. C. W. Ok Y. S. Wang C. H. (2017). Valorization of biomass to hydroxymethylfurfural, levulinic acid, and fatty acid methyl ester by heterogeneous catalysts.Chem. Eng. J.328246–273. 10.1016/j.cej.2017.07.020
69
Cheng H. Yang X. Che X. Yang M. Zhai G. (2018). Biomedical application and controlled drug release of electrospun fibrous materials.Mater. Sci. Eng. C90750–763. 10.1016/j.msec.2018.05.007
70
Cheng J. Yin W. Chang Z. Lundholm N. Jiang Z. (2017). Biosorption capacity and kinetics of cadmium(II) on live and dead Chlorella vulgaris.J. Appl. Phycol.29211–221. 10.1007/s10811-016-0916-2
71
Chew J. J. Doshi V. (2011). Recent advances in biomass pretreatment – Torrefaction fundamentals and technology.Renew. Sustain. Energy Rev.154212–4222. 10.1016/j.rser.2011.09.017
72
Chifiriuc M. C. Grumezescu A. M. (2016). “Iron oxide nanomaterials for functional imaging,” in Nanobiomaterials in Medical Imaging, Applications of Nanobiomaterials, Vol. 8ed.GrumezescuA. M. (Norwich, NY: William Andrew Publishing), 10.1016/B978-0-323-41736-5.00009-1
73
Chisti Y. (2007). Biodiesel from microalgae.Biotechnol. Adv.25294–306.
74
Choi A. H. Ben-Nissan B. (eds) (2019). Marine-Derived Biomaterials for Tissue Engineering Applications, Springer Series in Biomaterials Science and Engineering 14.Singapore: Springer Singapore, 10.1007/978-981-13-8855-2
75
Choi D. G. Venkatesan J. Shim M. S. (2019). Selective anticancer therapy using pro-oxidant drug-loaded chitosan–fucoidan nanoparticles.Int. J. Mol. Sci.20:3220. 10.3390/ijms20133220
76
Choi J. H. Choi W. Y. Cha D. S. Chinnan M. J. Park H. J. Lee D. S. et al (2005). Diffusivity of potassium sorbate in κ-carrageenan based antimicrobial film.LWT Food Sci. Technol.38417–423. 10.1016/j.lwt.2004.07.004
77
Chubarenko B. Woelfel J. Hofmann J. Aldag S. Beldowski J. Burlakovs J. et al (2021). Converting beach wrack into a resource as a challenge for the Baltic Sea (an overview).Ocean Coast. Manag.200:105413. 10.1016/j.ocecoaman.2020.105413
78
Cirne D. G. Lehtomäki A. Björnsson L. Blackall L. L. (2007). Hydrolysis and microbial community analyses in two-stage anaerobic digestion of energy crops.J. Appl. Microbiol.103516–527. 10.1111/j.1365-2672.2006.03270.x
79
Coltelli M.-B. Panariello L. Morganti P. Danti S. Baroni A. Lazzeri A. et al (2020). Skin-compatible biobased beauty masks prepared by extrusion.J. Funct. Biomater.11:23. 10.3390/jfb11020023
80
Coração A. C. de S. dos Santos F. S. Duarte J. A. D. Lopes-Filho E. A. P. De-Paula J. C. et al (2020). What do we know about the utilization of the Sargassum species as biosorbents of trace metals in Brazil?J. Environ. Chem. Eng.8:103941. 10.1016/j.jece.2020.103941
81
Daneshvar E. Sohrabi M. S. Kousha M. Bhatnagar A. Aliakbarian B. Converti A. et al (2014). Shrimp shell as an efficient bioadsorbent for Acid Blue 25 dye removal from aqueous solution.J. Taiwan Inst. Chem. Eng.452926–2934. 10.1016/j.jtice.2014.09.019
82
Danti S. Trombi L. Fusco A. Azimi B. Lazzeri A. Morganti P. et al (2019). Chitin nanofibrils and nanolignin as functional agents in skin regeneration.Int. J. Mol. Sci.20:2669. 10.3390/ijms20112669
83
Davis T. A. Volesky B. Mucci A. (2003). A review of the biochemistry of heavy metal biosorption by brown algae.Water Res.374311–4330. 10.1016/S0043-1354(03)00293-8
84
de Freitas G. R. da Silva M. G. C. Vieira M. G. A. (2019). Biosorption technology for removal of toxic metals: a review of commercial biosorbents and patents.Environ. Sci. Pollut. Res. Int.2619097–19118. 10.1007/s11356-019-05330-8
85
de Godos I. Muñoz R. Guieysse B. (2012). Tetracycline removal during wastewater treatment in high-rate algal ponds.J. Hazard. Mater.229446–449. 10.1016/j.jhazmat.2012.05.106
86
de Oliveira J. D. Carvalho L. S. Gomes A. M. Queiroz L. R. Magalhães B. S. Parachin N. S. (2016). Genetic basis for hyper production of hyaluronic acid in natural and engineered microorganisms.Microb. Cell Fact.15:119. 10.1186/s12934-016-0517-4
87
Defeo O. McLachlan A. Schoeman D. S. Schlacher T. A. Dugan J. Jones A. et al (2009). Threats to sandy beach ecosystems: a review.Estuar. Coast. Shelf Sci.811–12. 10.1016/j.ecss.2008.09.022
88
Demirtaş T. T. Irmak G. Gümüşderelioğlu M. (2017). A bioprintable form of chitosan hydrogel for bone tissue engineering.Biofabrication9:35003. 10.1088/1758-5090/aa7b1d
89
Divya K. Jisha M. S. (2018). Chitosan nanoparticles preparation and applications.Environ. Chem. Lett.16101–112. 10.1007/s10311-017-0670-y
90
Dobrinčić A. Balbino S. Zorić Z. Pedisić S. Kovačević D. B. Garofulić I. E. et al (2020). Advanced technologies for the extraction of marine brown algal polysaccharides.Mar. Drugs18:168. 10.3390/md18030168
91
Domalik-Pyzik P. Chłopek J. Pielichowska K. (2019). “Chitosan-based hydrogels: preparation, properties, and applications,” in Cellulose-Based Superabsorbent Hydrogels. Polymers and Polymeric Composites: A Reference Series, ed.MondalI. H. (Cham: Springer), 1665–1693. 10.1007/978-3-319-77830-3_55
92
Du X. Liu Y. Yan H. Rafique M. Li S. Shan X. et al (2020). Anti-infective and pro-coagulant chitosan-based hydrogel tissue adhesive for sutureless wound closure.Biomacromolecules211243–1253. 10.1021/acs.biomac.9b01707
93
Dubrawski R. Zawadzka E. Gawlik W. Bistram K. (2008). Stan morskiej strefy brzegowej na podstawie wybranych wyników monitoringu brzegów morskich z lat 2004-2006 / The state of the coastal zone on the basis of selected results of the monitoring of sea coasts from 2004 to 2006.Inżynieria Morska I Geotechnika115–27.
94
Dugan J. E. Hubbard D. M. McCrary M. D. Pierson M. O. (2003). The response of macrofauna communities and shorebirds to macrophyte wrack subsidies on exposed sandy beaches of southern California.Estuar. Coast. Shelf Sci.58(Suppl.)25–40. 10.1016/S0272-7714(03)00045-3
95
Ediriweera T. K. Aruppala A. L. H. Abeyrathne E. D. N. S. (2019). Analysis of bioactive properties of fish protein hydrolysates from Scomber japonicus Fin wastes.J. Technol. Value Addition131–45.
96
El Biriane M. Barbachi M. (2020). State-of-the-art review on recycled mussel shell waste in concrete and mortar.Innov. Infrastruct. Solut.6:29. 10.1007/s41062-020-00394-9
97
Ellen MacArthur Foundation (2016). The New Plastics Economy: Rethinking the Future of Plastics.Cowes: Ellen MacArthur Foundation.
98
Emadodin I. Reinsch T. Rotter A. Orlando-Bonaca M. Taube F. Javidpour J. (2020). A perspective on the potential of using marine organic fertilizers for the sustainable management of coastal ecosystem services.Environ. Sustain.3105–115. 10.1007/s42398-020-00097-y
99
Emblemsvåg J. Kvadsheim N. P. Halfdanarson J. Koesling M. Nystrand B. T. Sunde J. et al (2020). Strategic considerations for establishing a large-scale seaweed industry based on fish feed application: a Norwegian case study.J. Appl. Physiol.324159–4169. 10.1007/s10811-020-02234-w
100
Eriksen M. Lebreton L. C. M. Carson H. S. Thiel M. Moore C. J. Borerro J. C. et al (2014). Plastic pollution in the world’s oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea.PLoS One9:e111913. 10.1371/journal.pone.0111913
101
Eroglu E. Agarwal V. Bradshaw M. Chem X. Smith S. M. Raston C. L. et al (2012). Nitrate removal from liquid effluents using microalgae immobilized on chitosan nanofiber mats.Green Chem.142682–2685. 10.1039/C2GC35970G
102
Escapa C. Coimbra R. N. Nuevo C. Vega S. Paniagua S. García A. I. et al (2017). Valorization of microalgae biomass by its use for the removal of paracetamol from contaminated water.Water9:312. 10.3390/w9050312
103
Esmaeli A. Jokar M. Kousha M. Daneshvar E. Zilouei H. Karimi K. (2013). Acidic dye wastewater treatment onto a marine macroalga, Nizamuddina zanardini (Phylum: Ochrophyta).Chem. Eng. J.217329–336. 10.1016/j.cej.2012.11.038
104
Estevez M. M. Tomczak-Wandzel R. Akervold K. Tornes O. (2019). Co-digestion of waste from the salmon aquaculture sector with regional sewage sludge: effects on methane yield and digestate nutrient content.Eco-Energetics229–34. 10.24426/eco-energetics.v2i2.107
105
Faber T. A. Bechtel P. J. Hernot D. C. Parsons C. M. Swanson K. S. Smiley S. et al (2010). Protein digestibility evaluations of meat and fish substrates using laboratory, avian, and ileally cannulated dog assays.J. Anim. Sci.881421–1432. 10.2527/jas.2009-2140
106
Fabre E. Dias M. Costa M. Henriques B. Vale C. Lopes C. B. et al (2020). Negligible effect of potentially toxic elements and rare earth elements on mercury removal from contaminated waters by green, brown and red living marine macroalgae.Sci. Total Environ.724:138133. 10.1016/j.scitotenv.2020.138133
107
Fan Y. Saito T. Isogai A. (2008). Preparation of chitin nanofibers from squid pen β-chitin by simple mechanical treatment under acid conditions.Biomacromolecules91919–1923. 10.1021/bm800178b
108
FAO (2016). The State of the World Fisheries and Aquaculture. Contributing to Food Security and Nutrition For All.Rome: FAO.
109
FAO (2020). The State of World Fisheries and Aquaculture 2020. Sustainability in Action.Rome: FAO.
110
FAO (2021). Fishery Statistical Collections. Global Aquaculture Production. Available online at: http://www.fao.org/fishery/statistics/global-aquaculture-production/en[Accessed January 22, 2021].
111
Farhan A. Hani N. M. (2017). Characterization of edible packaging films based on semi-refined kappa-carrageenan plasticized with glycerol and sorbitol.Food Hydrocoll.6448–58. 10.1016/j.foodhyd.2016.10.034
112
Fawzy M. A. Gomaa M. (2020). Use of algal biorefinery waste and waste office paper in the development of xerogels: a low cost and eco-friendly biosorbent for the effective removal of Congo red and Fe (II) from aqueous solutions.J. Environ. Manag.262:110380. 10.1016/j.jenvman.2020.110380
113
Feng J. Nguyen S. T. Fan Z. Duong H. M. (2015). Advanced fabrication and oil absorption properties of super-hydrophobic recycled cellulose aerogels.Chem. Eng. J.270168–175. 10.1016/j.cej.2015.02.034
114
Figueira M. M. Volesky B. Ciminelli V. S. T. Roddick F. A. (2000). Biosorption of metals in brown seaweed biomass.Water Res.34196–204. 10.1016/S0043-1354(99)00120-7
115
Filipkowska A. Lubecki L. Szymczak-Zyła M. Kowalewska G. Zbikowski R. Szefer P. (2008). Utilisation of macroalgae from the Sopot beach (Baltic Sea).Oceanologia50255–273.
116
Filote C. Ungureanu G. Boaventura R. Santos S. Volf I. Botelho C. (2017). Green macroalgae from the Romanian coast of Black Sea: physico-chemical characterization and future perspectives on their use as metal anions biosorbents.Process Saf. Environ. Prot.10834–43. 10.1016/j.psep.2016.06.002
117
Flórez-Fernández N. López-García M. González-Muñoz M. J. Vilariño J. M. L. Domínguez H. (2017). Ultrasound-assisted extraction of fucoidan from Sargassum muticum.J. Appl. Phycol.291553–1561. 10.1007/s10811-016-1043-9
118
Fontes-Candia C. Erboz E. Martínez-Abad A. López-Rubio A. Martínez-Sanz M. (2019). Superabsorbent food packaging bioactive cellulose-based aerogels from Arundo donax waste biomass.Food Hydrocoll.96151–160. 10.1016/j.foodhyd.2019.05.011
119
Foroutan R. Mohammadi R. Razeghi J. Ramavandi B. (2019). Performance of algal activated carbon/Fe3O4 magnetic composite for cationic dyes removal from aqueous solutions.Algal Res.40:101509. 10.1016/j.algal.2019.101509
120
Galli R. Sitoci-Ficici K. H. Uckermann O. Later R. Marečková M. Koch M. et al (2018). Label-free multiphoton microscopy reveals relevant tissue changes induced by alginate hydrogel implantation in rat spinal cord injury.Sci. Rep.8:10841. 10.1038/s41598-018-29140-z
121
Gang L. Shunan X. Fang J. Yuguang Z. Zhigang H. (2017). Thermal cracking products and bio-oil production from microalgae Desmodesmus sp.Int. J. Agric. Biol. Eng.10198–206. 10.25165/j.ijabe.20171004.3348
122
Gharazi S. Zarket B. C. DeMella K. C. Raghavan S. R. (2018). Nature-inspired hydrogels with soft and stiff zones that exhibit a 100-fold difference in elastic modulus.ACS Appl. Mater. Interf.1034664–34673. 10.1021/acsami.8b14126
123
Gigante V. Cinelli P. Righetti M. C. Sandroni M. Tognotti L. Seggiani M. et al (2020). Evaluation of mussel shells powder as reinforcement for PLA-based biocomposites.Int. J. Mol. Sci.21:5364. 10.3390/ijms21155364
124
Goh C. H. Heng P. W. S. Chan L. W. (2012). Alginates as a useful natural polymer for microencapsulation and therapeutic applications.Carbohydr. Polym.881–12. 10.1016/j.carbpol.2011.11.012
125
Gonçalves V. S. S. Gurikov P. Poejo J. Matias A. A. Heinrich S. Duarte C. M. M. et al (2016). Alginate-based hybrid aerogel microparticles for mucosal drug delivery.Eur. J. Pharm. Biopharm.107160–170. 10.1016/j.ejpb.2016.07.003
126
Goycoolea F. M. Lollo G. Remuñán-López C. Quaglia F. Alonso M. J. (2009). Chitosan-alginate blended nanoparticles as carriers for the transmucosal delivery of macromolecules.Biomacromolecules101736–1743. 10.1021/bm9001377
127
Graca B. Witek Z. Burska D. Białkowska I. Łukawska-Matuszewska K. Bolałek J. (2006). Pore water phosphate and ammonia below the permanent halocline in the south-eastern Baltic Sea and their benthic fluxes under anoxic conditions.J. Mar. Syst.63141–154. 10.1016/j.jmarsys.2006.06.003
128
Greiner A. Wendorff J. H. (2007). Electrospinning: a fascinating method for the preparation of ultrathin fibers.Angew. Chem.465670–5703. 10.1002/anie.200604646
129
Grienke U. Silke J. Tasdemir D. (2014). Bioactive compounds from marine mussels and their effects on human health.Food Chem.14248–60. 10.1016/j.foodchem.2013.07.027
130
Gu L. Shan T. Ma Y.-X. Tay F. R. Niu L. (2019). Novel biomedical applications of crosslinked collagen.Trends Biotechnol.37464–491. 10.1016/j.tibtech.2018.10.007
131
Guiry M. D. Blunden G., eds (1991). Seaweed Resources in Europe: Uses and Potential.Chichester: John Wiley and Sons.
132
Guo H. Hong Z. Yi R. (2015). Core-shell collagen peptide chelated calcium/calcium alginate nanoparticles from fish scales for calcium supplementation.J. Food Sci.801595–1601. 10.1111/1750-3841.12912
133
Hafsa J. Smach M. A. Charfeddine B. Limem K. Majdoub H. Rouatbi S. (2016). Antioxidant and antimicrobial proprieties of chitin and chitosan extracted from Parapenaeus longirostris shrimp shell waste.Ann. Pharm. Fr.7427–33. 10.1016/j.pharma.2015.07.005
134
Hajji S. Kchaou H. Bkhairia I. Salem R. B. S. B. Boufi S. Debeaufort F. et al (2021). Conception of active food packaging films based on crab chitosan and gelatin enriched with crustacean protein hydrolysates with improved functional and biological properties.Food Hydrocoll.116:106639. 10.1016/j.foodhyd.2021.106639
135
Hart A. (2020). Mini-review of waste shell-derived materials’ applications.Waste Manag. Res.38514–527. 10.1177/0734242X19897812
136
Hassan M. Naidu R. Du J. Liu Y. Qi F. (2020). Critical review of magnetic biosorbents: their preparation, application, and regeneration for wastewater treatment.Sci. Total Environ.702:134893. 10.1016/j.scitotenv.2019.134893
137
He J. Chen J. P. (2014). A comprehensive review on biosorption of heavy metals by algal biomass: Materials, performances, chemistry, and modeling simulation tools.Bioresour. Technol.16067–78. 10.1016/j.biortech.2014.01.068
138
Helsinki Commission (2009). Waterborne Inputs of Heavy Metals to the Baltic Sea. HELCOM Indicator Fact Sheets. Available online at: http://archive.iwlearn.net/helcom.fi/environment2/ifs/ifs2009/en_GB/waterborne_hm/index.html[Accessed January 22, 2021].
139
Helsinki Commission (2018). Metals (Lead, Cadmium And Mercury).HELCOM core indicator report. Available online at: https://www.helcom.fi/wp-content/uploads/2019/08/Metals-HELCOM-core-indicator-2018.pdf[Accessed May 31, 2021].
140
Hembrick-Holloman V. Samuel T. Mohammed Z. Jeelani S. Rangari V. K. (2020). Ecofriendly production of bioactive tissue engineering scaffolds derived from egg- and sea-shells.J. Mater. Res. Technol.913729–13739. 10.1016/j.jmrt.2020.09.093
141
Hernández-Estévez A. Cristiani-Urbina E. (2014). Nickel (II) biosorption from aqueous solutions by shrimp head biomass.Environ. Monit. Assess.1867987–7998. 10.1007/s10661-014-3981-5
142
Herrera N. Singh A. A. Salaberria A. M. Labidi J. Mathew A. P. Oksman K. (2017). Triethyl citrate (TEC) as a dispersing aid in polylactic acid/chitin nanocomposites prepared via liquid-assisted extrusion.Polymers (Basel)9:406. 10.3390/polym9090406
143
Hoare T. R. Kohane D. S. (2008). Hydrogels in drug delivery: progress and challenges.Polymer491993–2007. 10.1016/j.polymer.2008.01.027
144
Huang Y. C. Li R. Y. Chen J. Y. Chen J. K. (2016). Biphasic release of gentamicin from chitosan/fucoidan nanoparticles for pulmonary delivery.Carbohydr. Polym.138114–122. 10.1016/j.carbpol.2015.11.072
145
Ifuku S. Nogi M. Abe K. Yoshioka M. Morimoto M. Saimoto H. et al (2009). Preparation of chitin nanofibers with a uniform width as α-chitin from crab shells.Biomacromolecules101584–1588. 10.1021/bm900163d
146
Jerold M. Sivasubramanian V. (2016). Biosorption of malachite green from aqueous solution using brown marine macro algae Sargassum swartzii.Desalination Water Treat.5725288–25300. 10.1080/19443994.2016.1156582
147
Jerold M. Vasantharaj K. Joseph D. Sivasubramanian V. (2017). Fabrication of hybrid biosorbent nanoscale zero-valent iron-Sargassum swartzii biocomposite for the removal of crystal violet from aqueous solution.Int. J. Phytoremed.19214–224. 10.1080/15226514.2016.120760
148
Jiménez-Gómez C. P. Cecilia J. A. (2020). Chitosan: a natural biopolymer with a wide and varied range of applications.Molecules25:3981. 10.3390/molecules25173981
149
Johansen U. Bull-Berg H. Vik L. H. Stokka A. M. Richardsen R. Winther U. (2019). The Norwegian seafood industry – importance for the national economy.Mar. Policy110:103561. 10.1016/j.marpol.2019.103561
150
Jönsson M. Allahgholi L. Sardari R. R. R. Hreggviosson G. O. Karlsson E. N. (2020). Extraction and modification of macroalgal polysaccharides for current and next-generation applications.Molecules25:930. 10.3390/molecules25040930
151
Joshi S. Eshwar S. Jain V. (2019). “Marine polysaccharides: biomedical and tissue engineering applications,” in Marine-Derived Biomaterials for Tissue Engineering Applications, Springer Series in Biomaterials Science and Engineering, Vol. 14edsChoiA.Ben-NissanB. (Singapore: Springer), 443–487. 10.1007/978-981-13-8855-2_19
152
Jung K. W. Choi B. H. Jeong T. U. Ahn K. H. (2016). Facile synthesis of magnetic biochar/Fe3O4 nanocomposites using electro-magnetization technique and its application on the removal of acid orange 7 from aqueous media.Bioresour. Technol.220672–676. 10.1016/j.biortech.2016.09.035
153
Jung K. W. Choi B. H. Song K. G. Choi J. W. (2019). Statistical optimization of preparing marine macroalgae derived activated carbon/iron oxide magnetic composites for sequestering acetylsalicylic acid from aqueous media using response surface methodologys.Chemosphere215432–443. 10.1016/j.chemosphere.2018.10.069
154
Kadam S. U. Tiwari B. K. O’Donnell C. P. (2015). Extraction, structure and biofunctional activities of laminarin from brown algae.Int. J. Food Sci. Technol.5024–31. 10.1111/ijfs.12692
155
Kanjilal T. Bhattacharjee C. (2018). “Green applications of magnetic sorbents for environmental remediation,” in Organic Pollutants in Wastewater I. Methods of Analysis, Removal and Trearment, edsAsiriI. A. M.MohammadA. (Millersville: Materials Research Forum LLC), 1–41. 10.21741/9781945291630
156
Karkal S. S. Kudre T. G. (2020). Valorization of fish discards for the sustainable production of renewable fuels.J. Cleaner Prod.275:122985. 10.1016/j.jclepro.2020.122985
157
Karunanithi P. Murali M. R. Samuel S. Raghavendran H. R. B. Abbas A. A. Kamarul T. (2016). Three dimensional alginate-fucoidan composite hydrogel augments the chondrogenic differentiation of mesenchymal stromal cells.Carbohydr. Polym.147294–303. 10.1016/j.carbpol.2016.03.102
158
Kataržytė M. Vaičiūtė D. Nasvytis P. (2019). Excellent bathing waters in coastal areas: is microbial pollution the only important parameter?Ocean Coast. Manag.182:104922. 10.1016/j.ocecoaman.2019.104922
159
Kavitha Sankar P. C. Rajmohan G. Rosemary M. J. (2017). Physico-chemical characterisation and biological evaluation of freeze dried chitosan sponge for wound care.Mater. Lett.208130–132. 10.1016/j.matlet.2017.05.010
160
Kenry Lim C. T. (2017). Nanofiber technology: current status and emerging developments.Prog. Polym. Sci.701–17. 10.1016/j.progpolymsci.2017.03.002
161
Khalil A. H. Tye Y. Y. Saurabh C. K. Leh C. Lai T. K. Chong E. et al (2017). Biodegradable polymer films from seaweed polysaccharides: a review on cellulose as a reinforcement material.Exp. Polym. Lett.11244–265. 10.3144/expresspolymlett.2017.26
162
Khan A. Z. Jamil S. Akhtar A. Bashir M. M. Yar M. (2020). Chitosan based hybrid materials used for wound healing applications – a short review.Int. J. Polym. Mater. Polym. Biomater.69419–436. 10.1080/00914037.2019.1575828
163
Khan M. I. Shin J. H. Kim J. D. (2018). The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products.Microb. Cell Fact.17:36. 10.1186/s12934-018-0879-x
164
Khanpour-Alikelayeh E. Partovinia A. Talebi A. Kermanian H. (2021). Enhanced biodegradation of light crude oil by immobilized Bacillus licheniformis in fabricated alginate beads through electrospray technique.Environ. Monit. Assess.193:328. 10.1007/s10661-021-09104-z
165
Khoo C. G. Dasan Y. K. Lam M. K. Lee K. T. (2019). Algae biorefinery: Review on a broad spectrum of downstream processes and products.Bioresour. Technol.292:121964. 10.1016/j.biortech.2019.121964
166
Kikionis S. Ioannou E. Toskas G. Roussis V. (2015). Electrospun biocomposite nanofibers of ulvan/PCL and ulvan/PEO.J. Appl. Polym. Sci.132:42153. 10.1002/app.42153
167
Kim E. S. Lee J.-S. Lee H. G. (2016). Nanoencapsulation of red ginseng extracts using chitosan with polyglutamic acid or fucoidan for improving antithrombotic activities.J. Agric. Food Chem.644765–4771. 10.1021/acs.jafc.6b00911
168
Kim M. H. Lee Y. W. Jung W. K. Oh J. Nam S. Y. (2019). Enhanced rheological behaviors of alginate hydrogels with carrageenan for extrusion-based bioprinting.J. Mech. Behav. Biomed. Mater.98187–194. 10.1016/j.jmbbm.2019.06.014
169
Kim M. Ahn Y. Lee K. Jung W. Cha C. (2020). In situ facile-forming chitosan hydrogels with tunable physicomechanical and tissue adhesive properties by polymer graft architecture.Carbohydr. Polym.229:115538. 10.1016/j.carbpol.2019.115538
170
Kim S.-K., ed (2013). Marine Proteins and Peptides: Biological Activities and Applications.Chichester: John Wiley and Sons, 10.1002/9781118375082
171
Kinley R. D. de Nys R. Vucko M. J. Machado L. Tomkins N. W. (2016). The red macroalgae Asparagopsis taxiformis is a potent natural antimethanogenic that reduces methane production during in vitro fermentation with rumen fluid.Anim. Prod. Sci.56282–289. 10.1071/AN15576
172
Kirkman H. Kendrick G. A. (1997). Ecological significance and commercial harvesting of drifting and beach-cast macro-algae and seagrasses in Australia: a review.J. Appl. Phycol.9311–326. 10.1023/A:1007965506873
173
Kishore Kumar K. Prasad M. K. Baburao G. Sudhakar M. Sivajyothi J. Sathish T. et al (2018). A fortunate marine algae biomass, sargassum cinereum for removal of pb(Ii): Studies on thermodynamics, kinetics and characterization.Desalination Water Treat.116179–186. 10.5004/dwt.2018.22544
174
Klavins M. Bisters V. Burlakovs J. (2018). Small scale gasification application and perspectives in circular economy.Environ. Clim. Technol.2242–54. 10.2478/rtuect-2018-0003
175
Koçer A. T. Özçimen D. (2018). Investigation of the biogas production potential from algal wastes.Waste Manag. Res.361100–1105.
176
Koesling M. Kvadsheim N. P. Halfdanarson J. Emblemsvåg J. Rebours C. (2021). Environmental impacts of protein-production from farmed seaweed: comparison of possible scenarios in Norway.J. Clean. Prod.307:127301. 10.1016/j.jclepro.2021.127301
177
Kotroni E. Simirioti E. Kikionis S. Sfiniadakis I. Siamidi A. Karalis V. et al (2019). In vivo evaluation of the anti-inflammatory activity of electrospun micro/nanofibrous patches loaded with Pinus halepensis bark extract on hairless mice skin.Materials12:2596. 10.3390/ma12162596
178
Kraan S. (2012). “Algal polysaccharides, novel applications and outlook,” in Carbohydrates. Comprehensive Studies on Glycobiology and Glycotechnology, ed.ChangC.-F. (London: IntechOpen), 489–532.
179
Krogdahl Å Jaramillo-Torres A. Ahlstrøm Ø Chikwati E. Aasen I. M. Kortner T. M. (2021). Protein value and health aspects of the seaweeds Saccharina latissima and Palmaria palmata evaluated with mink as model for monogastric animals.Anim. Feed Sci. Tech.276:114902. 10.1016/j.anifeedsci.2021.114902
180
Kumar M. N. R. V. (2000). A review of chitin and chitosan applications.React. Funct. Polym.461–27. 10.1016/S1381-5148(00)00038-9
181
Kumar M. Thakur I. S. (2018). Municipal secondary sludge as carbon source for production and characterization of biodiesel from oleaginous bacteria.Bioresour. Technol. Rep.4106–113. 10.1016/j.biteb.2018.09.011
182
Kumar M. Ghosh P. Khosla K. Thakur I. S. (2016). Biodiesel production from municipal secondary sludge.Bioresour. Technol.216165–171. 10.1016/J.BIORTECH.2016.05.078
183
Kumar M. Sun Y. Rathour R. Pandey A. Thakur I. S. Tsang D. C. W. (2020). Algae as potential feedstock for the production of biofuels and value-added products: opportunities and challenges.Sci. Total Environ.716:137116. 10.1016/j.scitotenv.2020.137116
184
Kumar M. Sundaram S. Gnansounou E. Larroche C. Thakur I. S. (2018). Carbon dioxide capture, storage and production of biofuel and biomaterials by bacteria: a review.Bioresour. Technol.2471059–1068. 10.1016/j.biortech.2017.09.050
185
Kwasigroch U. Bełdowska M. Jędruch A. Łukawska-Matuszewska K. (2021). Distribution and bioavailability of mercury in the surface sediments of the Baltic Sea.Environ. Sci. Pollut. Res.2835690–35708. 10.1007/s11356-021-13023-4
186
Lahaye M. Robic A. (2007). Structure and functional properties of ulvan, a polysaccharide from green seaweeds.Biomacromolecules81765–1774. 10.1021/bm061185q
187
Lang X. Wang T. Sun M. Chen X. Liu Y. (2020). Advances and applications of chitosan-based nanomaterials as oral delivery carriers: a review.Int. J. Biol. Macromol.154433–445. 10.1016/j.ijbiomac.2020.03.148
188
Langasco R. Cadeddu B. Formato M. Lepedda A. J. Cossu M. Giunchedi P. et al (2017). Natural collagenic skeleton of marine sponges in pharmaceutics: innovative biomaterial for topical drug delivery.Mater. Sci. Eng.70710–720. 10.1016/j.msec.2016.09.041
189
Lanno M. Silm M. Shanskiy M. Kisand A. Orupõld K. Kriipsalu M. (2020). Open windrow composting of fish waste in Estonia.Agron. Res.182465–2477. 10.15159/AR.20.194
190
Latire T. Legendre F. Bigot N. Carduner L. Kellouche S. Bouyoucef M. et al (2014). Shell extracts from the marine bivalve Pecten maximus regulate the synthesis of extracellular matrix in primary cultured human skin fibroblasts.PLoS One9:e99931. 10.1371/journal.pone.0099931
191
Laurent S. Forge D. Port M. Roch A. Robic C. Vander Elst L. et al (2008). Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications.Chem. Rev.1082064–2110. 10.1021/cr068445e
192
Lee H. Dellatore S. M. Miller W. M. Messersmith P. B. (2007). Mussel-inspired surface chemistry for multifunctional coatings.Science318426–430. 10.1126/science.1147241
193
Lehmann J. Joseph S. (2009). Biochar for Environmental Management. Science, Technology and Implementation.London: Routlege.
194
Lehmann J. Gaunt J. Rondon M. (2006). Bio-char sequestration in terrestrial ecosystems – a review.Mitig. Adapt. Strateg. Glob. Change11403–427. 10.1007/s11027-005-9006-5
195
Leong K. H. Chung L. Y. Noordin M. I. Onuki Y. Morishita M. Takayama K. (2011). Lectin-functionalized carboxymethylated kappa-carrageenan microparticles for oral insulin delivery.Carbohydr. Polym.86555–565. 10.1016/j.carbpol.2011.04.070
196
León-López L. Bañuelos-Piña A. M. Reyes-Moreno C. Milán-Carrillo J. Contreras-Andrade I. Sánchez-Magaña L. M. et al (2019). Optimisation of temperature and time for the dark germination bioprocess of Moringa oleifera seeds to boost nutritional value, total phenolic content and antioxidant activity.Int. Food Res. J.26831–839.
197
Liao J. Jia Y. Wang B. Shi K. Qian Z. (2018). Injectable hybrid poly(ε-caprolactone)-b-poly(ethylene glycol)-b-poly(ε-caprolactone) porous microspheres/alginate hydrogel cross-linked by calcium gluconate crystals deposited in the pores of microspheres improved skin wound healing.ACS Biomater. Sci. Eng.41029–1036. 10.1021/acsbiomaterials.7b00860
198
Lima-Junior E. M. de Moraes Filho M. O. Costa B. A. Fechine F. V. de Moraes M. E. A. Silva-Junior F. R. et al (2019). Innovative treatment using tilapia skin as a xenograft for partial thickness burns after a gunpowder explosion.J. Surg. Case Rep.2019:rjz181. 10.1093/jscr/rjz181
199
Liu G. Zhou Y. Liu D. Wang Q. Ruan Z. He Q. et al (2015). Global transcriptome analysis of the tentacle of the jellyfish Cyanea capillata using deep sequencing and expressed sequence tags: Insight into the toxin-and degenerative disease-related transcripts.PLoS One10:e0142680. 10.1371/journal.pone.0142680
200
Liu S. Trevathan-Tackett S. M. Ewers Lewis C. J. Ollivier Q. R. Jiang Z. Huang X. et al (2019). Beach-cast seagrass wrack contributes substantially to global greenhouse gas emissions.J. Environ. Manag.231329–335. 10.1016/j.jenvman.2018.10.047
201
Lopez-Caballero M. E. Gimenez B. Gomez-Guillen M. C. Montero P. (2014). “Valorization and integral use and of seafood by-products,” in Engineering Aspects of Food Biotechnology, edsTexeiraJ.VicenteA. (Boca Raton, FL: CRC Press), 367–413.
202
Lu W. Y. Li H. J. Li Q. Y. Wu Y. C. (2021). Application of marine natural products in drug research.Bioorgan. Med. Chem.35:116058. 10.1016/j.bmc.2021.116058
203
Luo Z. Zhou J. (2012). “Thermal conversion of biomass,” in Handbook of Climate Change Mitigation, edsChenW.-Y.SeinerJ.SuzukiT.LacknerM. (New York, NY: Springer), 1001–1042. 10.1007/978-1-4419-7991-9_27
204
Machado L. Magnusson M. Paul N. A. Kinley R. de Nys R. Tomkins N. (2016). Identification of bioactives from the red seaweed Asparagopsis taxiformis that promote antimethanogenic activity in vitro.J. Appl. Phycol.283117–3126. 10.1007/s10811-016-0830-7
205
Macreadie P. I. Bishop M. J. Booth D. J. (2011). Implications of climate change for macrophytic rafts and their hitchhikers.Mar. Ecol. Prog. Ser.443285–292. 10.3354/meps09529
206
Macreadie P. I. Trevathan-Tackett S. M. Baldock J. A. Kelleway J. J. (2017). Converting beach-cast seagrass wrack into biochar: a climate-friendly solution to a coastal problem.Sci. Total Environ.57490–94. 10.1016/j.scitotenv.2016.09.021
207
Magesh N. Annam Renita A. Senthil Kumar P. (2020). Practice on treating pharmaceutical compounds (antibiotics) present in wastewater using biosorption techniques with different biowaste compounds. A review.Environ. Prog. Sustain. Energy39:e13429. 10.1002/ep.13429
208
Makkar H. P. S. Tran G. Heuzé V. Giger-Reverdin S. Lessire M. Lebas F. et al (2016). Seaweeds for livestock diets: a review.Anim. Feed Sci. Technol.2121–17. 10.1016/j.anifeedsci.2015.09.018
209
Malagurski I. Levic S. Nesic A. Mitric M. Pavlovic V. Dimitrijevic-Brankovic S. (2017). Mineralized agar-based nanocomposite films: Potential food packaging materials with antimicrobial properties.Carbohydr. Polym.17555–62. 10.1016/j.carbpol.2017.07.064
210
Manivasagan P. Oh J. (2016). Marine polysaccharide-based nanomaterials as a novel source of nanobiotechnological applications.Int. J. Biol. Macromol.82315–327. 10.1016/j.ijbiomac.2015.10.081
211
Manivasagan P. Bharathiraja S. Moorthy M. S. Oh Y.-O. Seo H. Oh J. (2017). Marine biopolymer-based nanomaterials as a novel platform for theranostic applications.Polym. Rev.57631–667. 10.1080/15583724.2017.1311914
212
Manzocco L. Valoppi F. Calligaris S. Andreatta F. Spilimbergo S. Nicoli M. C. (2017). Exploitation of κ-carrageenan aerogels as template for edible oleogel preparation.Food Hydrocoll.7168–75. 10.1016/j.foodhyd.2017.04.021
213
Marinho-Soriano E. Nunes S. O. Carneiro M. A. A. Pereira D. C. (2009). Nutrients’ removal from aquaculture wastewater using the macroalgae Gracilaria birdiae.Biomass Bioenergy33327–331. 10.1016/j.biombioe.2008.07.002
214
Martínez-García C. González-Fonteboa B. Carro-López D. Pérez-Ordóñez J. L. (2020). Mussel shells: A canning industry by-product converted into a bio-based insulation material.J. Cleaner Prod.269:122343. 10.1016/j.jclepro.2020.122343
215
Martínez-Sanz M. Cebrián-Lloret V. Mazarro-Ruiz J. López-Rubio A. (2020a). Improved performance of less purified cellulosic films obtained from agar waste biomass.Carbohydr. Polym.233:115887. 10.1016/j.carbpol.2020.115887
216
Martínez-Sanz M. Gómez-Mascaraque L. G. Ballester A. Martínez-Abad A. Brodkorb A. López-Rubio A. (2019). Production of unpurified agar-based extracts from red seaweed Gelidium sesquipedale by means of simplified extraction protocols.Algal Res.38:101420. 10.1016/j.algal.2019.101420
217
Martínez-Sanz M. Ström A. Lopez-Sanchez P. Knutsen S. H. Ballance S. Zobel H. K. et al (2020b). Advanced structural characterisation of agar-based hydrogels: Rheological and small angle scattering studies.Carbohydr. Polym.236:115655. 10.1016/j.carbpol.2019.115655
218
Marungrueng K. Pavasant P. (2007). High performance biosorbent (Caulerpa lentillifera) for basic dye removal.Bioresour. Technol.981567–1572. 10.1016/j.biortech.2006.06.010
219
Mautner H. G. (1954). The chemistry of brown algae.Econ. Bot.8174–192. 10.1007/BF02984737
220
Mazur L. P. Cechinel M. A. P. de Souza S. M. A. G. U. Boaventura R. A. R. Vilar V. J. P. (2018). Brown marine macroalgae as natural cation exchangers for toxic metal removal from industrial wastewaters: a review.J. Environ. Manag.223215–253. 10.1016/j.jenvman.2018.05.086
221
McGwynne L. E. McLachlan A. Furstenberg J. P. (1988). Wrack breakdown on sandy beaches – Its impact on interstitial meiofauna.Mar. Environ. Res.25213–232. 10.1016/0141-1136(88)90004-9
222
Mellou F. Varvaresou A. Papageorgiou S. (2019). Renewable sources: applications in personal care formulations.Int. J. Cosmet. Sci.41517–525. 10.1111/ics.12564
223
Mendes A. C. Stephansen K. Chronakis I. S. (2017). Electrospinning of food proteins and polysaccharides.Food Hydrocoll.6853–68. 10.1016/j.foodhyd.2016.10.022
224
Meriac A. (2019). Smolt Production and the Potential for Solid Waste Collection in Norway.[Nofima Report 25/2019]. Tromsø: Nofima.
225
Mincea M. Negrulescu A. Ostafe V. (2012). Preparation, modification, and applications of chitin nanowhiskers: a review.Rev. Adv. Mater. Sci.30225–242.
226
Ministry of Trade, Industry and Fisheries (2019). Regjeringa sin strategi for auka verdiskaping frå marint restråstoff / The Government’s Strategy for Increasing Value Creation from Marine Residual Raw Materials.[Strategy W-0029 N]. Oslo: Ministry of Trade, Industry and Fisheries.
227
Mokhtar N. Aziz E. A. Aris A. Ishak W. F. W. Ali N. S. M. (2017). Biosorption of azo-dye using marine macro-alga of Euchema spinosum.J. Environ. Chem. Eng.55721–5731. 10.1016/j.jece.2017.10.043
228
Molina-Alcaide E. Carro M. D. Roleda M. Y. Weisbjerg M. R. Lind V. Novoa-Garrido M. (2017). In vitro ruminal fermentation and methane production of different seaweed species.Anim. Feed Sci. Technol.2281–12. 10.1016/j.anifeedsci.2017.03.012
229
Moraes M. A. Cocenza D. S. Vasconcellos F. C. Fraceto L. F. Beppu M. M. (2013). Chitosan and alginate biopolymer membranes for remediation of contaminated water with herbicides.J. Environ. Manag.131222–227. 10.1016/j.jenvman.2013.09.028
230
Morris J. P. Backeljau T. Chapelle G. (2019). Shells from aquaculture: a valuable biomaterial, not a nuisance waste product.Rev. Aquac.1142–57. 10.1111/raq.12225
231
Mossbauer M. Haller I. Dahlke S. Schernewski G. (2012). Management of stranded eelgrass and macroalgae along the German Baltic coastline.Ocean Coast. Manag.571–9. 10.1016/j.ocecoaman.2011.10.012
232
Mullerova S. Baldikova E. Prochazkova J. Pospiskova K. Safarik I. (2019). Magnetically modified macroalgae Cymopolia barbata biomass as an adsorbent for safranin O removal.Mater. Chem. Phys.225174–180. 10.1016/j.matchemphys.2018.12.074
233
Munro L. (2019). Scottish Fish Farm Production Survey 2018.[Marine Scotland Science Report]. Edinburgh: Scottish Government.
234
Myhre M. Richardsen R. Nystøyl R. Strandheim G. (2021). Analyse marint restråstoff 2020-2022. Tilgjengelighet og anvendelse av marint restråstoff i fra norsk fiskeri- og havbruksnćring.Report number: 2021:00633. Trondheim: SINTEF Ocean Rapporte.
235
Navarro A. E. Lim H. Chang E. Lee Y. Manrique A. S. (2014). Uptake of sulfa drugs from aqueous solutions by marine algae.Sep. Sci. Technol.492175–2181. 10.1080/01496395.2014.926930
236
Ngah W. S. Teong L. C. Hanafiah M. A. K. M. (2010). Adsorption of dyes and heavy metal ions by chitosan composites: a review.Carbohydr. Polym.831446–1456. 10.1016/j.carbpol.2010.11.004
237
Nguyen S. T. Feng J. Ng S. K. Wong J. P. W. Tan V. B. C. Duong H. M. (2014). Advanced thermal insulation and absorption properties of recycled cellulose aerogels.Coll. Surf. A445128–134. 10.1016/j.colsurfa.2014.01.015
238
Nicklas M. Schatton W. Heinemann S. Hanke T. Kreuter J. (2009). Preparation and characterization of marine sponge collagen nanoparticles and employment for the transdermal delivery of 17β-estradiol-hemihydrate.Drug Dev. Ind. Pharm.351035–1042. 10.1080/03639040902755213
239
Nikam A. P. Mukesh P. R. Haudhary S. P. (2014). Nanoparticles – an overview.Int. J. Res. Dev. Pharm. Life Sci.31121–1127.
240
Nisticò R. (2017). Aquatic-derived biomaterials for a sustainable future: a European opportunity.Resources6:65. 10.3390/resources6040065
241
Nitta S. K. Numata K. (2013). Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering.Int. J. Mol. Sci.141629–1654. 10.3390/ijms14011629
242
Nordstrom K. F. Jackson N. L. Korotky K. H. Puleo J. A. (2011). Aeolian transport rates across raked and unraked beaches on a developed coast.Earth Surf. Processes Landf.36779–789. 10.1002/esp.2105
243
Novoa-Garrido M. Rebours C. Aanensen L. Torp T. Lind V. Steinshamn H. (2017). Effect of seaweed on gastrointestinal microbiota isolated from Norwegian White sheep.Acta Agric. Scand. A Anim. Sci.66152–160. 10.1080/09064702.2017.1310287
244
Oliveira C. Neves N. M. Reis R. L. Martins A. Silva T. H. (2018). Gemcitabine delivered by fucoidan/chitosan nanoparticles presents increased toxicity over human breast cancer cells.Nanomedicine (London, England)132037–2050. 10.2217/nnm-2018-0004
245
Oliveira J. Belchior A. da Silva V. D. Rotter A. Petrovski Ž. Almeida P. L. et al (2020). Marine environmental plastic pollution: mitigation by microorganism degradation and recycling valorization.Front. Mar. Sci.7:567126. 10.3389/fmars.2020.567126
246
Orr M. Zimmer M. Jelinski D. E. Mews M. (2005). Wrack deposition on different beach types: Spatial and temporal variation in the pattern of subsidy.Ecology861496–1507. 10.1890/04-1486
247
Oso A. O. Idowu A. A. Niameh O. T. (2011). Growth response, nutrient and mineral retention, bone mineralisation and walking ability of broiler chickens fed with dietary inclusion of various unconventional mineral sources.J. Anim. Physiol. Anim. Nutr.95461–467. 10.1111/j.1439
248
Osorio-López C. Seco-Reigosa N. Garrido-Rodríguez B. Cutillas-Barreiro L. Arias-Estévez M. Fernández-Sanjurjo M. J. et al (2014). As(V) adsorption on forest and vineyard soils and pyritic material with or without mussel shell: kinetics and fractionation.J. Taiwan Inst. Chem. Eng.451007–1014. 10.1016/j.jtice.2013.10.001
249
Oun A. A. Rhim J. W. (2017). Carrageenan-based hydrogels and films: Effect of ZnO and CuO nanoparticles on the physical, mechanical, and antimicrobial properties.Food Hydrocoll.6745–53. 10.1016/j.foodhyd.2016.12.040
250
Özkan Gülzari Ş Lind V. Aasen I. M. Steinshamn H. (2019). Effect of supplementing sheep diets with macroalgae species on in vivo nutrient digestibility, rumen fermentation and blood amino acid profile.Animal132792–2801. 10.1017/S1751731119001502
251
Ozudogru Y. Merdivan M. Göksan T. (2016). Biosorption of methylene blue from an aqueous solution by iron oxide-coated Cystoseira barbata.J. Turkish Chem. Soc. Sec. A3551–564. 10.18596/jotcsa.40601
252
Peng F. Q. Ying G. G. Yang B. Liu S. Lai H. J. Liu Y. S. et al (2014). Biotransformation of progesterone and norgestrel by two freshwater microalgae (Scenedesmus obliquus and Chlorella pyrenoidosa): transformation kinetics and products identification.Chemosphere95581–588. 10.1016/j.chemosphere.2013.10.013
253
Pérez Roda A. Gilman E. Huntington T. Kennelly S. J. Suuronen P. Chaloupka M. et al (2019). A Third Assessment of Global Marine Fisheries Discards.[FAO Fisheries and Aquaculture Technical Paper No. 633]. Rome: FAO.
254
Pitcairn J. Warmington J. Gandy S. Deswarte F. Bell J. (2017). Biorefining potential for Scotland: mapping bioresource arisings across Scotland.Ind. Biotechnol.13301–305. 10.1089/ind.2017.29111.jpi
255
Pleym I. E. Svorken M. Birthe V. (2019). Verdifulle rester. Muligheter for norsk marint restråstoff / Valuable Waste. Opportunities for Norwegian Marine Raw Material.[Nofima Report 9/2019]. Tromsø: Nofima.
256
Poblete-Castro I. Hoffmann S. L. Becker J. Wittmann C. (2020). Cascaded valorization of seaweed using microbial cell factories.Curr. Opin. Biotechnol.65102–113. 10.1016/j.copbio.2020.02.008
257
Porshnov D. Ozols V. Ansone-Bertina L. Burlakovs J. Klavins M. (2018). Thermal decomposition study of major refuse derived fuel components.Energy Proc.14748–53. 10.1016/j.egypro.2018.07.032
258
Prihanto A. A. Nurdiani R. Bagus A. D. (2019). Production and characteristics of fish protein hydrolysate from parrotfish (Chlorurus sordidus) head.PeerJ7:e8297. 10.7717/peerj.8297
259
Quignard F. Valentin R. Di Renzo F. (2008). Aerogel materials from marine polysaccharides.N. J. Chem.321300–1310. 10.1039/B808218A
260
Rae I. B. Gibb S. W. Lu S. (2009). Biosorption of Hg from aqueous solutions by crab carapace.J. Hazard. Mater.1641601–1604. 10.1016/j.jhazmat.2008.09.052
261
Rahmati M. Alipanahi Z. Mozafari M. (2019). Emerging biomedical applications of algal polysaccharides.Curr. Pharm. Design251335–1344. 10.2174/1381612825666190423160357
262
Ramamoorthy S. K. Skrifvars M. Persson A. (2015). A review of natural fibers used in biocomposites: Plant, animal and regenerated cellulose fibers.Polym. Rev.55107–162. 10.1080/15583724.2014.971124
263
Rathod M. Mody K. Basha S. (2014). Efficient removal of phosphate from aqueous solutions by red seaweed Kappaphycus alverezii.J. Cleaner Prod.84484–493. 10.1016/j.jclepro.2014.03.064
264
Raveendran S. Yoshida Y. Maekawa T. Kumar D. S. (2013). Pharmaceutically versatile sulfated polysaccharide based bionano platforms.Nanomedicine9605–626. 10.1016/j.nano.2012.12.006
265
Reys L. L. Silva S. S. Pirraco R. P. Marques A. P. Mano J. F. Silva T. H. et al (2017). Influence of freezing temperature and deacetylation degree on the performance of freeze-dried chitosan scaffolds towards cartilage tissue engineering.Eur. Polym. J.95232–240. 10.1016/j.eurpolymj.2017.08.017
266
Rinaudo M. (2006). Chitin and chitosan: properties and applications.Progress Polym. Sci.31603–632. 10.1016/j.progpolymsci.2006.06.001
267
Risén E. Nordström J. Malmström M. E. Gröndahl F. (2017). Non-market values of algae beach-cast management – study site Trelleborg, Sweden.Ocean Coast. Manag.14059–67. 10.1016/j.ocecoaman.2017.02.009
268
Ritchie H. Roser M. (2018). “Plastic Pollution”. Published online at OurWorldInData.org. Available online at: ‘https://ourworldindata.org/plastic-pollution’ (Accessed May 3, 2021).
269
Rodríguez F. Morán L. González G. Troncoso E. Zúñiga R. N. (2017). Collagen extraction from mussel byssus: a new marine collagen source with physicochemical properties of industrial interest.J. Food Sci. Technol.541228–1238.
270
Rowbotham J. S. Dyer P. W. Greenwell H. C. Theodorou M. K. (2012). Thermochemical processing of macroalgae: a late bloomer in the development of third-generation biofuels?Biofuels3441–461. 10.4155/bfs.12.29
271
Rudovica V. Bartkevics V. (2015). Chemical elements in the muscle tissues of European eel (Anguilla anguilla) from selected lakes in Latvia.Environ. Monit. Assess.187:608. 10.1007/s10661-015-4832-8
272
Ruocco N. Costantini S. Guariniello S. Costantini M. (2016). Polysaccharides from the marine environment with pharmacological, cosmeceutical and nutraceutical potential.Molecules21:551. 10.3390/molecules21050551
273
Rustad T. Storrø I. Slizyte R. (2011). Possibilities for the utilisation of marine by-products.Int. J. Food Sci. Technol.462001–2014. 10.1111/j.1365-2621.2011.02736.x
274
Safarik I. Ashoura N. Maderova Z. Pospiskova K. Baldikova E. Safarikova M. (2016a). Magnetically modified Posidonia oceanica biomass as an adsorbent for organic dyes removal.Mediterr. Mar. Sci.17351–358. 10.12681/mms.1549
275
Safarik I. Baldikova E. Pospiskova K. Safarikova M. (2016b). Magnetic modification of diamagnetic agglomerate forming powder materials.Particuology29169–171. 10.1016/j.partic.2016.05.002
276
Safarik I. Baldikova E. Prochazkova J. Pospiskova K. (2020a). Magnetic particles in algae biotechnology: recent updates.J. Appl. Phycol.321743–1753. 10.1007/s10811-020-02109-0
277
Safarik I. Baldikova E. Prochazkova J. Safarikova M. Pospiskova K. (2018). Magnetically modified agricultural and food waste: preparation and application.J. Agric. Food Chem.662538–2552. 10.1021/acs.jafc.7b06105
278
Safarik I. Prochazkova J. Baldikova E. Pospiskova K. (2020b). “Magnetically responsive algae and seagrass derivatives for pollutant removal,” in Wastes: Solutions, Treatments and Opportunities III, edsVilarinhoC.CastroF.GoncalvesM.FernandoA. L. (Boca Raton, FL: CRC Press), 131–136.
279
Sahiner N. Sagbas S. Yılmaz S. (2017). Microgels derived from different forms of carrageenans, kappa, iota, and lambda for biomedical applications.MRS Adv.22521–2527. 10.1557/adv.2017.415
280
Salaberria A. M. Fernandes S. C. M. Diaz R. H. Labidi J. (2015a). Processing of α-chitin nanofibers by dynamic high pressure homogenization: characterization and antifungal activity against A. niger.Carbohydr. Polym.116286–291. 10.1016/j.carbpol.2014.04.047
281
Salaberria A. M. Labidi J. Fernandes S. C. M. (2014). Chitin nanocrystals and nanofibers as nano-sized fillers into thermoplastic starch-based biocomposites processed by melt-mixing.Chem. Eng. J.256356–364. 10.1016/j.cej.2014.07.009
282
Salaberria A. M. Labidi J. Fernandes S. C. M. (2015b). Different routes to turn chitin into stunning nano-objects.Eur. Polym. J.68503–515. 10.1016/j.eurpolymj.2015.03.005
283
Saldarriaga-Hernandez S. Hernandez-Vargas G. Iqbal H. M. N. Barceló D. Parra-Saldívar R. (2020). Bioremediation potential of Sargassum sp. biomass to tackle pollution in coastal ecosystems: circular economy approach.Sci. Total Environ.715:136978. 10.1016/j.scitotenv.2020.136978
284
Santos S. C. R. Ungureanu G. Volf I. Boaventura R. A. R. Botelho C. M. S. (2018). “Macroalgae biomass as sorbent for metal ions,” in Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value, edsPopaV.VolfI. (Amsterdam: Elsevier), 69–112. 10.1016/B978-0-444-63774-1.00003-X
285
Saranya R. Tamil Selvi A. Jayapriya J. Aravindhan R. (2020). Synthesis of fat liquor through fish waste valorization, characterization and applications in tannery industry.Waste Biomass Valorization116637–6647. 10.1007/s12649-020-00944-3
286
Sathishkumar P. Kamala-Kannan S. Cho M. Kim J. S. Hadibarata T. Salim M. R. et al (2014). Laccase immobilization on cellulose nanofiber: the catalytic efficiency and recyclic application for simulated dye effluent treatment.J. Mol. Catal. B100111–120. 10.1016/j.molcatb.2013.12.008
287
Scialla S. Carella F. Dapporto M. Sprio S. Piancastelli A. Palazzo B. et al (2020). Mussel shell-derived macroporous 3D scaffold: characterization and optimization study of a bioceramic from the circular economy.Mar. Drugs18:309. 10.3390/md18060309
288
Scotland Food and Drink (2016). Aquaculture Growth to 2030. A Strategic Plan for Farming Scotland’s Seas.Edinburgh: Scotland Food and Drink.
289
Scottish Enterprise (2019). Biorefinery roadmap for scotland: building a sustainable future.Ind. Biotechnol.15284–289. 10.1089/ind.2019.29188.sen
290
Senturk Parreidt T. Müller K. Schmid M. (2018). Alginate-based edible films and coatings for food packaging applications.Foods (Basel)7:170. 10.3390/foods7100170
291
Sheng P. X. Ting Y.-P. Chen J. P. (2007). Biosorption of heavy metal ions (Pb, Cu, and Cd) from aqueous solutions by the marine alga Sargassum sp. in single- and multiple-metal systems.Ind. Eng. Chem. Res.462438–2444. 10.1021/ie0615786
292
Shin M. Shin J. Y. Kim K. Yang B. Han J. W. Kim N.-K. et al (2020). The position of lysine controls the catechol-mediated surface adhesion and cohesion in underwater mussel adhesion.J. Coll. Interface Sci.563168–176. 10.1016/j.jcis.2019.12.082
293
Shobier A. H. El-Sadaawy M. M. El-Said G. F. (2020). Removal of hexavalent chromium by ecofriendly raw marine green alga Ulva fasciata: kinetic, thermodynamic and isotherm studies.Egypt. J. Aquat. Res.46325–331. 10.1016/j.ejar.2020.09.003
294
Shuba E. S. Kifle D. (2018). Microalgae to biofuels: ‘Promising’ alternative and renewable energy, review.Renew. Sustain. Energy Rev.81(Part 1)743–755. 10.1016/j.rser.2017.08.042
295
Sikarwar V. S. Zhao M. Fennell P. S. Shah N. Anthony E. J. (2017). Progress in biofuel production from gasification.Progress Energy Combust. Sci.61189–248. 10.1016/j.pecs.2017.04.001
296
Silva A. Delerue-Matos C. Figueiredo S. A. Freitas O. M. (2019). The use of algae and fungi for removal of pharmaceuticals by bioremediation and biosorption processes: a review.Water11:1555. 10.3390/w11081555
297
SINTEF Ocean and Kontali Analyse (2020). Marint Restråstoff – Historisk Database. Available online at: https://www.marintrestrastoff.no/[Accessed July 16, 2021].
298
Sirviö J. A. Kolehmainen A. Liimatainen H. Niinimäki J. Hormi O. E. O. (2014). Biocomposite cellulose-alginate films: promising packaging materials.Food Chem.151343–351. 10.1016/j.foodchem.2013.11.037
299
Smirnova N. V. Kolbe K. A. Dresvyanina E. N. Grebennikov S. F. Dobrovolskaya I. P. Yudin V. E. et al (2019). Effect of chitin nanofibrils on biocompatibility and bioactivity of the chitosan-based composite.Materials (Basel)12:1874. 10.3390/ma12111874
300
Sokołowski A. Jankowska E. Bałazy P. Jędruch A. (2021). Distribution and extent of benthic habitats in Puck Bay (Gulf of Gdañsk, southern Baltic Sea).Oceanologia63301–320. 10.1016/j.oceano.2021.03.001
301
Son E. B. Poo K. M. Chang J. S. Chae K. J. (2018a). Heavy metal removal from aqueous solutions using engineered magnetic biochars derived from waste marine macro-algal biomass.Sci. Total Environ.615161–168. 10.1016/j.scitotenv.2017.09.171
302
Son E. B. Poo K. M. Mohamed H. O. Choi Y. J. Cho W. C. Chae K. J. (2018b). A novel approach to developing a reusable marine macro-algae adsorbent with chitosan and ferric oxide for simultaneous efficient heavy metal removal and easy magnetic separation.Bioresour. Technol.259381–387. 10.1016/j.biortech.2018.03.077
303
Sousa A. M. M. Gonçalves M. P. (2015). Strategies to improve the mechanical strength and water resistance of agar films for food packaging applications.Carbohydr. Polym.132196–204. 10.1016/j.carbpol.2015.06.022
304
Stévant P. Rebours C. Chapman A. (2017). Seaweed aquaculture in Norway: recent industrial developments and future perspectives. Aquac. Int.25, 1373–1390. 10.1007/s10499-017-0120-7
305
Suurs P. Barbut S. (2020). Collagen use for co-extruded sausage casings–a review.Trends Food Sci. Technol.10291–101. 10.1016/j.tifs.2020.06.011
306
Suursaar Ü Torn K. Martin G. Herkül K. Tiit K. (2014). Formation and species composition of stormcast beach wrack in the Gulf of Riga, Baltic Sea.Oceanologia56673–695. 10.5697/oc.56-4.673
307
Swatschek D. Schatton W. Müller W. Kreuter J. (2002). Microparticles derived from marine sponge collagen (SCMPs): preparation, characterization and suitability for dermal delivery of all-trans retinol.Eur. J. Pharm. Biopharm.54125–133. 10.1016/s0939-6411(02)00046-2
308
Tayyab U. Novoa-Garrido M. Roleda M. Y. Lind V. Weisbjerg M. R. (2016). Ruminal and intestinal protein degradability of various seaweed species measured in situ in dairy cows.Anim. Feed Sci. Technol.21344–54. 10.1016/j.anifeedsci.2016.01.003
309
Teo W. E. Ramakrishna S. (2006). A review on electrospinning design and nanofibre assemblies.Nanotechnology17R89–R106. 10.1088/0957-4484/17/14/R01
310
Thai H. Nguyen C. T. Thach L. T. Tran M. T. Mai H. D. Nguyen T. T. T. et al (2020). Characterization of chitosan/alginate/lovastatin nanoparticles and investigation of their toxic effects in vitro and in vivo.Sci. Rep.10:909. 10.1038/s41598-020-57666-8
311
Thies J. E. Rillig M. C. (2012). “Characteristics of biochar: biological properties,” in Biochar for Environmental Management, edsLehmannJ.JosephS. (London: Routledge), 119–138.
312
Toskas G. Heinemann S. Heinemann C. Cherif C. Hund R. D. Roussis V. et al (2012). Ulvan and ulvan/chitosan polyelectrolyte nanofibrous membranes as a potential substrate material for the cultivation of osteoblasts.Carbohydr. Polym.89997–1002. 10.1016/j.carbpol.2012.04.045
313
Trache D. Hussin M. H. Hui Chuin C. T. Sabar S. Fazita M. R. N. Taiwo O. F. A. et al (2016). Microcrystalline cellulose: isolation, characterization and bio-composites application – a review.Int. J. Biol. Macromol.93789–804. 10.1016/j.ijbiomac.2016.09.056
314
Tuck C. O. Pérez E. Horváth I. T. Sheldon R. A. Poliakoff M. (2012). Valorization of biomass: Deriving more value from waste.Science (New York)337695–699. 10.1126/science.1218930
315
Tziveleka L. A. Pippa N. Georgantea P. Ioannou E. Demetzos C. Roussis V. (2018). Marine sulfated polysaccharides as versatile polyelectrolytes for the development of drug delivery nanoplatforms: Complexation of ulvan with lysozyme.Int. J. Biol. Macromol.11869–75. 10.1016/j.ijbiomac.2018.06.050
316
Tziveleka L. A. Sapalidis A. Kikionis S. Aggelidou E. Demiri E. Kritis A. et al (2020). Hybrid sponge-like scaffolds based on ulvan and gelatin: design, characterization and evaluation of their potential use in bone tissue engineering.Materials (Basel)13:1763. 10.3390/ma13071763
317
Ubando A. T. Africa A. D. M. Maniquiz-Redillas M. C. Culaba A. B. Chen W.-H. Chang J.-S. (2021). Microalgal biosorption of heavy metals: a comprehensive bibliometric review.J. Hazard. Mater.402:123431. 10.1016/j.jhazmat.2020.123431
318
Ungureanu G. Filote C. Santos S. C. R. Boaventura R. A. R. Volf I. Botelho C. M. S. (2016). Antimony oxyanions uptake by green marine macroalgae.J. Environ. Chem. Eng.43441–3450. 10.1016/j.jece.2016.07.023
319
Vanparijs N. Nuhn L. De Geest B. G. (2017). Transiently thermoresponsive polymers and their applications in biomedicine.Chem. Soc. Rev.461193–1239. 10.1039/C6CS00748A
320
Varaprasad K. Jayaramudu T. Kanikireddy V. Toro C. Sadiku E. R. (2020). Alginate-based composite materials for wound dressing application: a mini review.Carbohydr. Polym.236:116025. 10.1016/j.carbpol.2020.116025
321
Vassalini I. Gjipalaj J. Crespi S. Gianocelli A. Mella M. Ferroni M. et al (2020). Alginate-derived active blend enhances adsorption and photocatalytic removal of organic pollutants in water.Adv. Sustain. Syst.4:1900112. 10.1002/adsu.201900112
322
Vázquez J. A. Durán A. I. Menduíña A. Nogueira M. (2020). Biotechnological valorization of food marine wastes: microbial productions on peptones obtained from aquaculture by-products.Biomolecules10:1184. 10.3390/biom10081184
323
Vázquez J. A. Fernández-Compás A. Blanco M. Rodríguez-Amado I. Moreno H. Borderías J. et al (2019a). Development of bioprocesses for the integral valorisation of fish discards.Biochem. Eng. J.144198–208. 10.1016/j.bej.2019.02.004
324
Vázquez J. A. Sotelo C. G. Sanz N. Pérez-Martín R. I. Rodríguez-Amado I. Valcarcel J. (2019b). Valorization of aquaculture by-products of salmonids to produce enzymatic hydrolysates: process optimization, chemical characterization and evaluation of bioactives.Mar. Drugs17:676. 10.3390/md17120676
325
Venkatesan J. Anil S. Kim S.-K. (2017). Seaweed Polysaccharides. Isolation, Biological and Biomedical Applications.Amsterdam: Elsevier.
326
Venkatesan J. Anil S. Kim S. K. Shim M. S. (2016). Seaweed polysaccharide-based nanoparticles: preparation and applications for drug delivery.Polymers (Basel)8:30. 10.3390/polym8020030
327
Venkatesan J. Lowe B. Anil S. Manivasagan P. Kheraif A. A. A. Kang K. H. et al (2015). Seaweed polysaccharides and their potential biomedical applications.Starch67381–390. 10.1002/star.201400127
328
Vidal R. R. L. Moraes J. S. (2019). Removal of organic pollutants from wastewater using chitosan: a literature review.Int. J. Environ. Sci. Technol.161741–1754. 10.1007/s13762-018-2061-8
329
Vigneshpriya D. Krishnaveni N. Renganathan S. (2017). Marine brown macroalga Sargassum wightii as a novel biosorbent for removal of brilliant green dye from aqueous solution: Kinetics, equilibrium isotherm modeling and phytotoxicity of treated and untreated dye.Desalination Water Treat.78300–312. 10.5004/dwt.2017.20884
330
Vijayaraghavan J. Bhagavathi Pushpa T. Sardhar Basha S. J. Vijayaraghavan K. Jegan J. (2015). Evaluation of red marine alga Kappaphycus alvarezii as biosorbent for methylene blue: isotherm, kinetic, and mechanism studies.Sep. Sci. Technol.501120–1126. 10.1080/01496395.2014.965260
331
Villares R. Fernández-Lema E. López-Mosquera M. E. (2016). Evaluation of beach wrack for use as an organic fertilizer: temporal survey in different areas.Thalassas3219–36. 10.1007/s41208-015-0003-5
332
Volesky B. (2003). Sorption and Biosorption.Quebec: BV Sorbex.
333
Vu C. H. T. Won K. (2014). Leaching-resistant carrageenan-based colorimetric oxygen indicator films for intelligent food packaging.J.Agric. Food Chem.627263–7267. 10.1021/jf5014764
334
Wang B. Wan Y. Zheng Y. Lee X. Liu T. Yu Z. et al (2019). Alginate-based composites for environmental applications: a critical review.Crit. Rev. Environ. Sci. Technol.49318–356. 10.1080/10643389.2018.1547621
335
Wang S. Wang X. Poon K. Wang Y. Li S. Liu H. et al (2013). Removal and reductive dechlorination of triclosan by Chlorella pyrenoidosa.Chemosphere921498–1505. 10.1016/j.chemosphere.2013.03.067
336
Ween O. Kjerstad M. Stangeland J. (2018). “Strategies for shifting marine proteins up the value chain to develop added-value ingredients,” in Blue Growth: Aquaculture, Fisheries, Market and Health Perspectives, edsThuB. J.Akslen-HoelL. K. (Oslo: Orkana Akademisk).
337
Weinberger F. Paalme T. Wikström S. A. (2020). Seaweed resources of the Baltic Sea, Kattegat and German and Danish North Sea coasts.Bot. Mar.6361–72. 10.1515/bot-2019-0019
338
Whitman T. Lehmann J. (2009). Biochar – one way forward for soil carbon in offset mechanisms in Africa?Environ. Sci. Policy121024–1027. 10.1016/j.envsci.2009.07.013
339
Woolf D. Amonette J. E. Street-Perrott F. A. Lehmann J. Joseph S. (2010). Sustainable biochar to mitigate global climate change.Nat. Commun.1:56. 10.1038/ncomms1053
340
World Health Organization (2021). Musculoskeletal Conditions. Available online at: https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions[Accessed May 7, 2021].
341
WRAP (2012). Sector Guidance Note: Preventing Waste in the Fish Processing Supply Chain.Banbury: Waste and Resources Action Programme (WRAP).
342
Wu S. J. Don T. M. Lin C. W. Mi F. L. (2014). Delivery of berberine using chitosan/fucoidan-taurine conjugate nanoparticles for treatment of defective intestinal epithelial tight junction barrier.Mar. Drugs125677–5697. 10.3390/md12115677
343
Yadav M. Goswami P. Paritosh K. Kumar M. Pareek N. Vivekanand V. (2019). Seafood waste: a source for preparation of commercially employable chitin/chitosan materials.Bioresour. Bioprocess.6:8. 10.1186/s40643-019-0243-y
344
Yang L. Wang Y. Liu A. Zhang Y. (2019). CoOx/MoOy-anchored multi-wrinkled biomass carbon as a promising material for rapidly selective methyl blue removal.J. Mater. Sci.5411024–11036. 10.1007/s10853-019-03612-7
345
Yao Z. Xia M. Li H. Chen T. Ye Y. Zheng H. (2014). Bivalve shell: not an abundant useless waste but a functional and versatile biomaterial.Crit. Rev. Environ. Sci. Technol.442502–2530. 10.1080/10643389.2013.829763
346
Yu I. K. Tsang D. C. W. (2017). Conversion of biomass to hydroxymethylfurfural: a review of catalytic systems and underlying mechanisms.Bioresour. Technol.238716–732. 10.1016/j.biortech.2017.04.026
347
Zeng J.-B. He Y.-S. Li S.-L. Wang Y.-Z. (2012). Chitin whiskers: an overview.Biomacromolecules131–11. 10.1021/bm201564a
348
Zero Waste Scotland (2015). Sector Study on Beer, Whisky and Fish. [Final Report].Harwell: Ricardo-AEA Ltd.
349
Zhang M. Zhao Y. Qin X. Jia W. Chai L. Huang M. et al (2019). Microplastics from mulching film is a distinct habitat for bacteria in farmland soil.Sci. Total Environ.688470–478. 10.1016/j.scitotenv.2019.06.108
350
Zhao Y. Qiu Y. Wang H. Chen Y. Jin S. Chen S. (2016). Preparation of nanofibers with renewable polymers and their application in wound dressing.Int. J. Polym. Sci.2016:4672839. 10.1155/2016/4672839
351
Zheng Z. Bian S. Li Z. Zhang Z. Liu Y. Zhai X. et al (2020). Catechol modified quaternized chitosan enhanced wet adhesive and antibacterial properties of injectable thermo-sensitive hydrogel for wound healing.Carbohydr. Polym.249:116826. 10.1016/j.carbpol.2020.116826
352
Zhong Q.-Q. Zhao Y. Q. Shen L. Hao B. Xu X. Gao B.-Y. et al (2020). Single and binary competitive adsorption of cobalt and nickel onto novel magnetic composites derived from green macroalgae.Environ. Eng. Sci.371–13. 10.1089/ees.2019.0305
353
Zhou C. Gong X. Han J. Guo R. (2016). Removal of Pb (II) and Zn (II) from aqueous solutions by raw crab shell: a comparative study.Water Environ. Res.88374–383. 10.2175/106143016X14504669768174
354
Zhu J. Ye H. Deng D. Li J. Wu Y. (2020). Electrospun metformin-loaded polycaprolactone/chitosan nanofibrous membranes as promoting guided bone regeneration membranes: Preparation and characterization of fibers, drug release, and osteogenic activity in vitro.J. Biomater. Appl.341282–1293. 10.1177/0885328220901807
355
Zia F. Anjum M. N. Saif M. J. Jamil T. Malik K. Anjum S. et al (2017). “Alginate-poly(ethylene) glycol and poly(ethylene) oxide blend materials,” in Algae Based Polymers, Blends, and Composites, edsZiaK. M.ZuberM.AliM. (Amsterdam: Elsevier), 581–601. 10.1016/B978-0-12-812360-7.00016-1
356
Zubillaga V. Alonso-Varona A. Fernandes S. C. M. Salaberria A. M. Palomares T. (2020). Adipose-derived mesenchymal stem cell chondrospheroids cultured in hypoxia and a 3D porous chitosan/chitin nanocrystal scaffold as a platform for cartilage tissue engineering.Int. J. Mol. Sci.21:1004. 10.3390/ijms21031004
357
Zubillaga V. Salaberria A. M. Palomares T. Alonso-Varona A. Kootala S. Labidi J. et al (2018). Chitin nanoforms provide mechanical and topological cues to support growth of human adipose stem cells in chitosan matrices.Biomacromolecules193000–3012. 10.1021/acs.biomac.8b00570
Summary
Keywords
marine waste, marine industrial by-products, marine biopolymers, marine biomass, waste valorization, circular economy, blue biotechnology, beach wrack
Citation
Rudovica V, Rotter A, Gaudêncio SP, Novoveská L, Akgül F, Akslen-Hoel LK, Alexandrino DAM, Anne O, Arbidans L, Atanassova M, Bełdowska M, Bełdowski J, Bhatnagar A, Bikovens O, Bisters V, Carvalho MF, Catalá TS, Dubnika A, Erdoğan A, Ferrans L, Haznedaroglu BZ, Setyobudi RH, Graca B, Grinfelde I, Hogland W, Ioannou E, Jani Y, Kataržytė M, Kikionis S, Klun K, Kotta J, Kriipsalu M, Labidi J, Lukić Bilela L, Martínez-Sanz M, Oliveira J, Ozola-Davidane R, Pilecka-Ulcugaceva J, Pospiskova K, Rebours C, Roussis V, López-Rubio A, Safarik I, Schmieder F, Stankevica K, Tamm T, Tasdemir D, Torres C, Varese GC, Vincevica-Gaile Z, Zekker I and Burlakovs J (2021) Valorization of Marine Waste: Use of Industrial By-Products and Beach Wrack Towards the Production of High Added-Value Products. Front. Mar. Sci. 8:723333. doi: 10.3389/fmars.2021.723333
Received
10 June 2021
Accepted
30 August 2021
Published
20 October 2021
Volume
8 - 2021
Edited by
Sachin Kumar, Sardar Swaran Singh National Institute of Renewable Energy, India
Reviewed by
Bulgariu Laura, Gheorghe Asachi Technical University of Iaşi, Romania; Daniela Giordano, Institute of Bioscience and Bioresources, Italian National Research Council, Italy
Updates
Copyright
© 2021 Rudovica, Rotter, Gaudêncio, Novoveská, Akgül, Akslen-Hoel, Alexandrino, Anne, Arbidans, Atanassova, Bełdowska, Bełdowski, Bhatnagar, Bikovens, Bisters, Carvalho, Catalá, Dubnika, Erdoğan, Ferrans, Haznedaroglu, Setyobudi, Graca, Grinfelde, Hogland, Ioannou, Jani, Kataržytė, Kikionis, Klun, Kotta, Kriipsalu, Labidi, Lukić Bilela, Martínez-Sanz, Oliveira, Ozola-Davidane, Pilecka-Ulcugaceva, Pospiskova, Rebours, Roussis, López-Rubio, Safarik, Schmieder, Stankevica, Tamm, Tasdemir, Torres, Varese, Vincevica-Gaile, Zekker and Burlakovs.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ana Rotter, ana.rotter@nib.si
†These authors share first authorship
This article was submitted to Marine Biotechnology, a section of the journal Frontiers in Marine Science
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.