Abstract
In the current paper, we present the description of five new species of pseudotanaids sampled off the Bass Strait during two campaigns (SLOPE), which took place in 1986/8 and 1994 from the upper continental margin (slope) at depths 200–1550 m, hopefully starting to fill a gap in the knowledge of this major habitat. From five species, two occurred off eastern coast between Gippsland and Jervis Point and three others on the southern coast between Great Otway (Otway Point) and Kangaroo Island. These five species bring the total number of described pseudotanaid species 94 and to six in Australian waters.
Introduction
Pseudotanaidae Sieg, 1976 are small tanaidaceans from the superfamily Paratanaoidea Lang (1949), characterized by their compact (short) body, enlarged chelipeds and a brood pouch composed of one pair of oostegites (Lang, 1949; Sieg, 1977). Currently, the Pseudotanaidae is the third most species-rich family of Paratanaoidea after Leptocheliidae Lang, 1973 and Typhlotanaidae Sieg (1976) (with 132 and 116 nominal species, respectively)1. They are probably epifaunal or shallow sediment burrowers (infauna), and some are unselective predators and hosts for nematode parasites (Błażewicz et al., 2020).
Pseudotanaids are often numerous and a frequent element in macrobenthic communities, an example being the 36% contribution to the tanaid abundance (7% of macrofauna) on the bathyal Chatham Rise, SW Pacific (Bird and Holdich, 1984; Pabis et al., 2014, 2015; Kaiser et al., 2018). They are present in a variety of marine habitats (Bird and Holdich, 1989b; Bird, 1999; Bamber et al., 2009; Błażewicz-Paszkowycz and Bamber, 2011; Jakiel et al., 2015, 2019; Stȩpień et al., 2018) and are recorded over a wide bathymetric range. The shallowest record of the family belongs to Akanthinotanais pedecerritulus Tzeng and Hsueh, 2021 present in the intertidal of Taiwan, while the deepest record was recorded for Pseudotanais longisetosusSieg (1977) and P. nordenskioldiSieg (1977), which were recorded at 6050 m (Kudinova-Pasternak, 1993). Although a few large publications have focused specifically on the diversity of Pseudotanaidae (Sieg, 1977; Bird and Holdich, 1989b; Jakiel et al., 2018, 2019, 2020), knowledge about their diversity, community structure and spatial distribution is still severely limited.
Peracarid pseudotanaids, as with other brooders, are assumed to have limited dispersal ability and narrow zoographical ranges. This was tentatively confirmed with employment of morphometric and molecular methods (Jakiel et al., 2018, 2019, 2020) for investigation of their distribution in the deep North Atlantic and the abyssal of Central and NW Pacific (Bird and Holdich, 1989b; Jakiel et al., 2019, 2020). For this reason, pseudotanaids are possibly good indicators for effective environmental impact assessment, habitat resilience and its potential for reconstruction (Bird and Holdich, 1989b; O’Hara et al., 2020; Francesca et al., 2021).
In the Australian context, 162 tanaid species belonging to 66 genera have been described (e.g., Edgar, 1997, 2008, 2012; Bamber, 2005, 2008; Błażewicz-Paszkowycz and Bamber, 2007, 2009, 2012; Jóźwiak and Błażewicz, 2021). Most of the studies focused on the shelf tanaids and only nine species are formally described from below the shelf break: three from SE Australia (Bathytanais fragilisLarsen and Heard, 2001, Pseudobathytanais gibberosusLarsen and Heard, 2001, and Acinoproskelos vermesBamber and Błażewicz-Paszkowycz, 2013) and six from W Australia (Bunburia primaJóźwiak and Jakiel, 2012, Abrotanais geniculumGellert and Błażewicz, 2018, Macilenta ewaeGellert and Błażewicz, 2018, M. acetabulaGellert and Błażewicz, 2018, M. tworGellert and Błażewicz, 2018, Waki australiensisGellert and Błażewicz, 2018) (Larsen and Heard, 2001; Jóźwiak and Jakiel, 2012; Gellert and Błażewicz, 2018). Only one pseudotanaid, Akanthinotanais scrappiBamber, 2005, has been published, from a sandy bottom with rhodoliths in Esperance Bay at 38.4 m (Bamber, 2005). Two potentially new pseudotanaid species were recorded from two locations of the Great Barrier Reef (Stȩpień et al., 2018), although they stay undescribed.
The continental margins (continental slope) are a narrow oceanic zone covering 11% of the surface (Menot et al., 2010) and the huge extent of Australia’s slopes are relatively understudied. Complicated geomorphology, chemistry and hydrodynamic processes augmented by the steep gradient of temperature, hydrostatic pressure, and oxygen levels make them the most complex and heterogenic zone of the oceanic floor. The steep slope, and often hard and unstable sediments are logistically demanding for sampling and hamper benthic faunal investigations. Analyzing the zoogeographical ranges, natural biodiversity, and factors determining their character makes a baseline for understanding the evolutionary processes and distribution patterns critical for management regimes and conservation reserves (Zardus et al., 2006; Jennings et al., 2013; Poore et al., 2015). In the current paper, we present the description of five new species of pseudotanaids sampled off the Bass Strait during two campaigns (SLOPE), which took place in 1986/8 and 1994 from the upper continental margin (slope) at depths 200–1550 m, hopefully starting to fill a gap in the knowledge of this major habitat.
Materials and Methods
Stations and Collection
Pseudotanaids were recovered from a series of the samples collected at depths greater than 200 m, perpendicular to the East and South coasts of Australia during three campaigns of the O.R.V. Franklin 1986–1988 and 1994, respectively. Altogether, 213 samples were collected with different devices, e.g., Woods Hole Oceanographic Institute epibenthic sled, Reineck box-corer, Beam trawl (Poore et al., 1994; unpublished data). Pseudotanaids were recovered only at six stations (Table 1).
TABLE 1
| Station | Locality | Lat/Long | Date | Gear | Depth (m) |
| SLOPE 40 | Victoria, S of Point Hicks | 38°17.42′S, 149°11.18′E | 24 Jul 1986 | WHOI EBS | 400 |
| SLOPE 53 | New South Wales, 54 km ESE of Nowra | 34°52.43′S–34°54.18′S, 151°15.02′E–151°19.30′E | 22 Oct 1988 | WHOI EBS | 996 |
| SLOPE 67 | Victoria, 67 km S of Point Hicks | 38°23.57′S–38° 23.47′S, 149°17.01′E–149°15.14′E | 25 Oct 1988 | WHOI EBS | 1277 |
| SLOPE 118 | Victoria, Off Portland | 38°48.02′S–38° 48.07 S, 141° 47.14′E–141°47.14′E | 12 May 1994 | WHOI EBS | 209 |
| SLOPE 134 | Victoria, Off Portland | 38°51.02′S, 141°44.47 E | 13 May 1994 | Box Corer | 1021 |
| SLOPE 170 | South Australia, Off Murray River Mouth Encounter Bay | 37°05.53′S, 137°42.32′E | 21 May 1994 | Smith-McIntyre grab | 1548 |
Stations where pseudotanaids were recovered off SE Australian collection made on O.R.V. Franklin 1986–1988 and 1994, respectively, Poore et al. (1994); unpublished data.
Morphological Analyses and Taxonomical Identification
Specimens were dissected with chemically sharpened tungsten needles and the dissected appendages mounted on slides with glycerine as a medium and sealed with paraffin-wax (Błażewicz et al., 2021). Drawings were prepared using a light microscope (Nikon Eclipse 50i) equipped with a camera lucida. Digital drawings were inked and arranged with Photoshop.
Morphological terminology is largely as in Jakiel et al. (2019, 2020);
-
-
the unique blade-like spine, if present, located at the ventrodistal part of the pereopod carpus is characteristic of most pseudotanaids. It is categorized as “long” when is at least 0.6x propodus, “intermediate” when it is 0.5x propodus, and “short” when it is at most 0.3x the propodus;
-
-
setal types are recognized as: (1) simple setae (= without ornamentation), (2) serrate – with serration or denticulation, (3) plumose – with any type of plumose or delicate setulae distributed along the main axis, (4) penicillate – with a tuft of setules located distally and with a small knob on which a seta is fixed to the tegument and (5) rod setae – slightly inflated distally and with a pore; and
-
-
the dorsodistal seta occurring on the carpus of pereopods 4–6 has a chemosensory function – (“rod seta” Jakiel et al., 2019); it is categorized as “long” when it is at least 0.8x propodus, “intermediate” when it is 0.5x propodus, and “short” when it is at most 0.25x propodus.
The classification of the Pseudotanais into morpho-groups (“affinis + longisetosus,” “denticulatus + abathagastor” and “forcipatus”) follows Bird and Holdich (1989b) and Jakiel et al. (2019).
The type material was lodged at the Museums Victoria, Melbourne Museum (Australia).
Classification
In our study and analyses we have applied the system splitting Pseudotanais species into established four morphogroups, e.g., “affinis + longisetosus,” “denticulatus + abathagastor,” “forcipatus,” and “spicatus” (Bird and Holdich, 1989b; Jakiel et al., 2019). The six species (P. borceai Bãcescu, 1960; P. lilljeborgi Sars, 1882; P. falciferBłażewicz-Paszkowycz and Bamber, 2011; P. sigrunisJakiel et al., 2018; P. colonusBird and Holdich, 1989a; P. baresnautiBird, 1999) were gathered into working-group “colonus” characterized by robust chela, acuminate mandible, and relatively long pereonite-1. Three species: P. intortus, P. oculatus and P. shirazi sp. nov. are not classified to any group.
The zoogeographical classification of the marine zoogeographical regions of the oceans followed (Spalding et al., 2007; Watling et al., 2013).
Measurements, Developmental and Stage Identification
Total body length (BL) was measured along the main axis of symmetry from the rostrum to the end of the telson. Body width (BW) was measured at the widest point along the main axis of symmetry. The length was measured along the axis of symmetry, and the width perpendicular to the axis of symmetry at the widest spot. To simplify species descriptions, the expression ‘‘Nx’’ replaces ‘‘N times longer than/as long as’’ and ‘‘N L:W’’ replaces ‘‘N times longer than wide.’’ The measurements were made with a camera connected to the microscope (Nikon Eclipse Ci-L) and NIS-Elements View software.2 The body width and the length of the cephalothorax, pereonites, pleonites, and pleotelson were measured on whole specimens.
All individuals, developmental stages were identified. We refer to the following stages:
-
-
two stages of manca, i.e., “manca-2” and “manca-3” which refer to specimens without or with buds of pereopod-6, respectively;
-
-
preparatory female characterized by undeveloped oostegites (‘buds’) (Bird and Holdich, 1989b) and brooding female (with fully developed oostegites) were not recovered in the studied material;
-
-
neuter – a stage that is morphologically like the juvenile female, but lacking oostegites buds; and
-
-
‘juvenile male’ that shows incompletely developed sexual dimorphic characters, i.e., resembling the neuter but has thicker antennules (equivalent to ‘preparatory male’ sensuBird and Holdich, 1989b).
In our collection sexually mature males (“swimming” male) and brooding females were not recovered.
Results
Nine individuals belonging to Pseudotanaidae were examined in the current paper. All of them were classified to the genus Pseudotanais: two of them represented “affinis + longisetosus” morphogroup (Pseudotanais chardonnayi n. sp. and P. caberneti n. sp.) and two “denticulatus + abathagastor” group: (P. barossai n. sp. and P. coonawarrai n. sp.). The fifth of described species P. shirazi is not assigned to any of the Pseudotanais groups.
Systematics
Order Tanaidacea Dana, 1849
Suborder Tanaidomorpha Sieg, 1980
Superfamily Paratanaoidea Lang, 1949
Family Pseudotanaidae Sieg, 1976
“affinis + longisetosus” group
Diagnosis. After Jakiel et al. (2019).
Species included. Pseudotanais affinis Hansen, 1887; P. chanelaeJakiel et al., 2020; P. curieaeJakiel et al., 2020; P. gaiaeJakiel et al., 2019; P. geraltiJakiel et al., 2019; P. julietaeJakiel et al., 2019; P. longisetosusSieg, 1977; P. longispinusBird and Holdich, 1989b; P. macrocheles Sars, 1882; P. monroeaeJakiel et al., 2020; P. nipponicusMcLelland, 2007; P. nordenskioldiSieg, 1977; P. rapunzelaeBłażewicz et al., 2021; P. romeoJakiel et al., 2019; P. spatulaBird and Holdich, 1989b; P. scalpellumBird and Holdich, 1989b; P. shackletoniBłażewicz et al., 2021; P. svavarssoniJakiel et al., 2018; P. szymborskaeJakiel et al., 2020; P. uranosJakiel et al., 2019; P. vitjazi Kudinova-Pasternak, 1966; P. yenneferaeJakiel et al., 2019; Pseudotanais sp. O (sensuMcLelland, 2008); Pseudotanais sp. P (sensuMcLelland, 2008); P. chardonnayi sp. nov.; P. caberneti sp. nov.
Pseudotanais chardonnayi sp. nov.
-
This species is registered in ZooBank number: LSIDurn:lsid:zoobank.org:act:420075F1-3622-4177-9BBC-4552CE1B3E07.
Diagnosis. Mandible molar subcoronal with distal spines. Pereopod-1 merus with seta. Pereopod-3 carpal blade-like spine long (0.7x propodus). Pereopods 4–6 merus with spine and seta; carpus with long rod seta; propodus with short and long ventral setae. Uropod exopod 0.7x endopod.
Material examined. Holotype, juvenile male 1.3 mm, SLOPE 40 (J61547). Paratypes: neuter 1.1 mm (J61515), dissected in slides, SLOPE 40.
Etymology. The name is after a wine variety grown in the Gippsland area, close to the type locality, as genitive.
Description of neuter. BL = 1.4 mm. Body robust (Figures 1A,B) 3.8 L:W. Cephalothorax 0.7 L:W, 1.0x pereonites 1–3, 0.2 BL. Pereonites 0.5 BL, Pereonite-1 0.5x pereonite-2, pereonites-1–6: 0.1, 0.3, 0.3, 0.5 0.5 and 0.4 L:W, respectively. Pleon short, 0.4 BL. Pleonites 0.9 L:W, pleonites 2–5 with dorsolateral setae on each side of midline. Pleotelson 4.4x pleonite-5, with paired laterodistal setae.
FIGURE 1
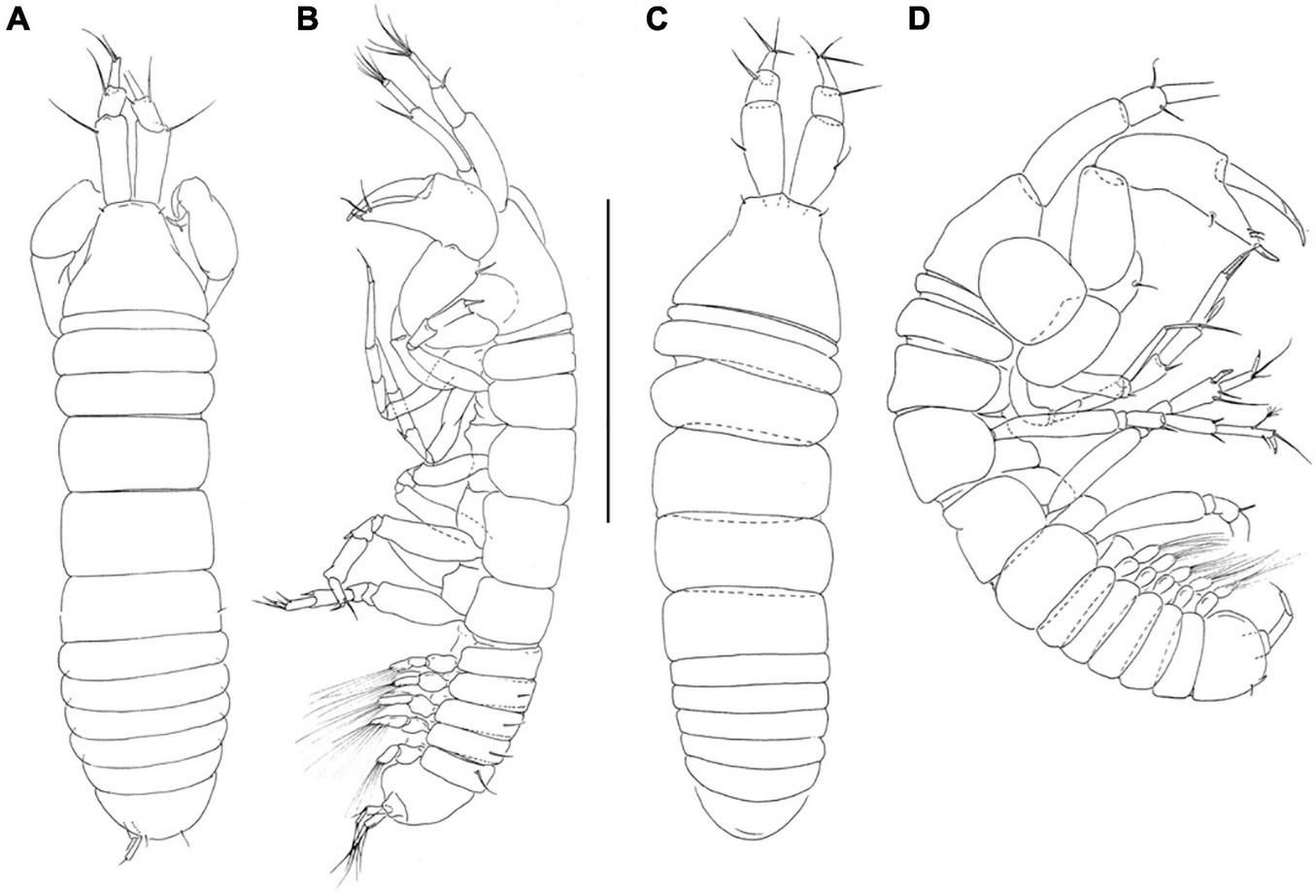
Pseudotanais chardonnayi sp. nov; neuter (J61547), (A), dorsal; (B), lateral; juvenile male (J61515), (C), dorsal, (D), lateral. Scale line = 1 mm.
Antennule (Figure 2A) article-1 6.0 L:W, 2.9x article-2, with long seta at mid-length, and one simple and three penicillate distal setae; article-2 2.9 L:W, 0.7x article-3, with two simple and one penicillate subdistal setae; article-3 5.8 L:W, with one simple, three bifurcated, one penicillate distal setae and one aesthetasc.
FIGURE 2
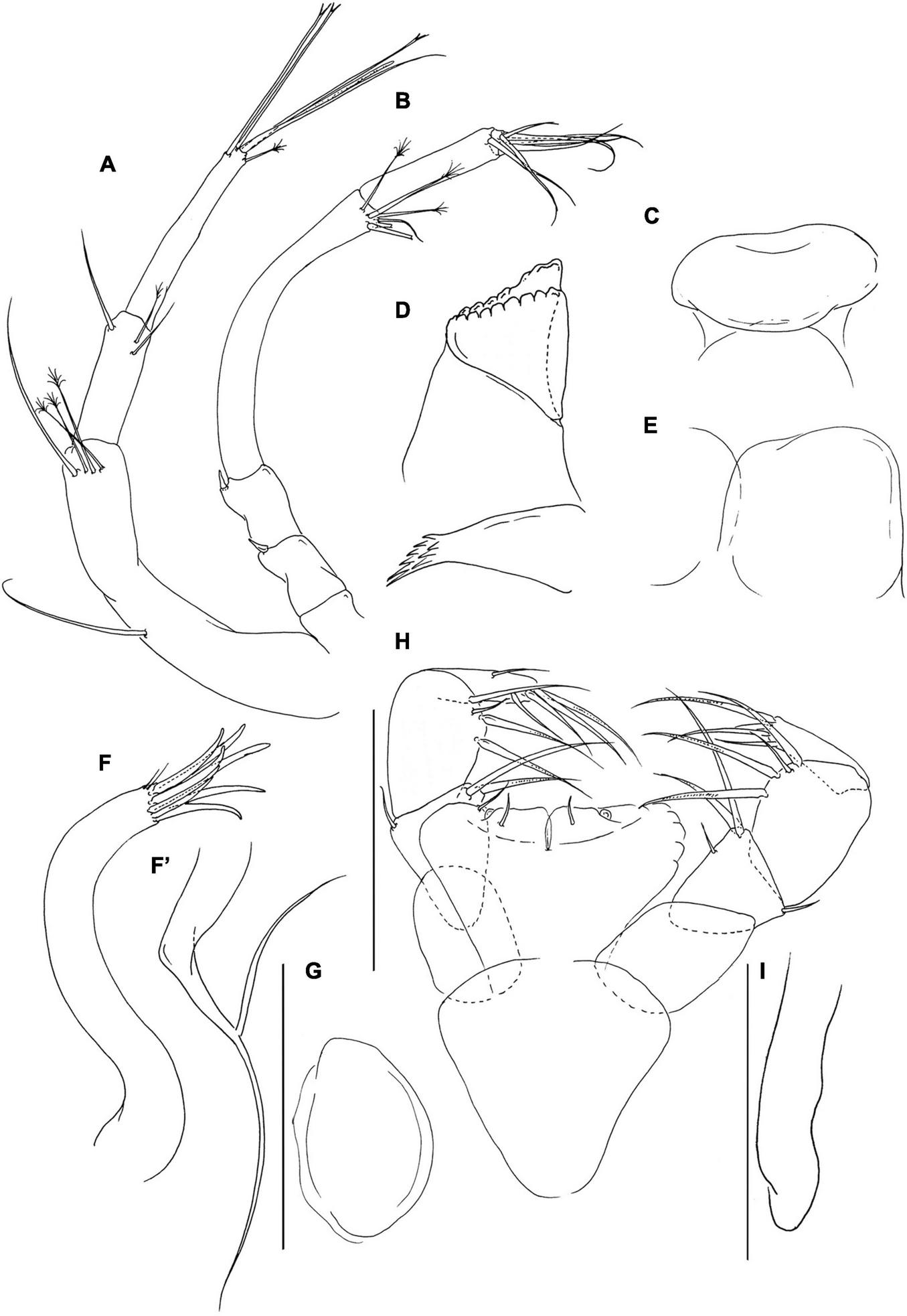
Pseudotanais chardonnayi sp. nov; neuter (J61515), (A), antennule; (B), antenna; (C), labrum; (D), left mandible; (E), labium; (F), maxillule; (F’) maxillule endite; (G), maxilla; (H), maxilliped; (I), epignath. Scale lines = 0.1 mm.
Antenna (Figure 2B) article-2 1.3 L:W; 1.0x article-3, with spine (0.3x article-2); article-3 1.3 L:W, 0.2x article-4, with spine (3.5x article-3); article-4 9.3 L:W, 2.2x article-5, with three simple (two broken) and three penicillate; article-5 5.0 L:W, 10.0x article-6, with simple distal seta; article-6 0.5 L:W, with six distal setae.
Labrum (Figure 2C) rounded, naked. Left mandible (Figure 2D) lacinia mobilis well developed, distally serrate, incisor distal margin beveled, serrate, molar subcoronal/acuminate with distal spines. Right mandible lost. Labium (Figure 2E) simple, rounded, glabrous. Maxillule (Figure 2F) endite with eight distal spines and outer two subdistal setae, palp (Figure 2F’) palp with two distal setae. Maxilla (Figure 2G) almost circular, naked. Maxilliped (Figure 2H) basis heart-shape, naked; palp article-1 1.8 L:W naked; article-2 1.1 L:W with one fine outer and three inner setae (two long and one short); article-3 1.4 L:W with one short and three long inner setae, article-4 3.3 L:W with six distal and subdistal setae; endites mostly fused but with central cleft (1/4 of endite total length), each with inner-distal gustatory cusp and short seta. Epignath (Figure 2I) linguiform, simple, naked.
Cheliped (Figure 3A) basis 1.8 L:W, dorsal seta not seen; merus with ventral seta; carpus 1.5 L:W, 0.8x palm, with two midventral setae and one dorsodistal simple seta; chela non-forcipate, palm 1.7 L:W with seta near dactylus insertion; fixed finger 3.0 L:W, cutting edge simple, poorly calcified, 0.8x palm with ventral seta, and with three setae on cutting edge; dactylus 5.9 L:W, cutting edge smooth, with dorsoproximal seta.
FIGURE 3
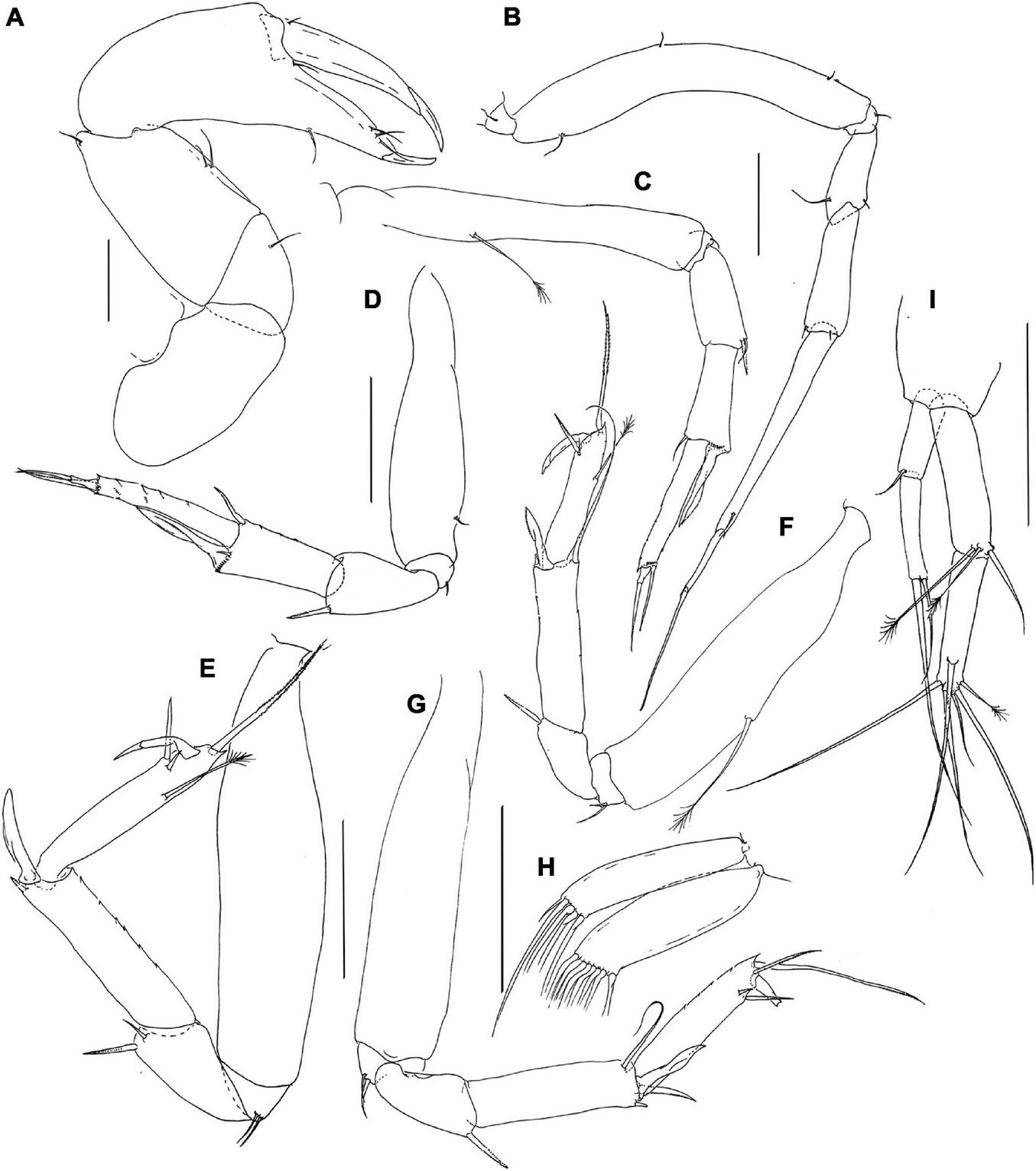
Pseudotanais chardonnayi sp. nov.; neuter (J61515), (A), cheliped; (B), pereopod-1; (C), pereopod-2, (D), pereopod-3; (E), pereopod-4; (F), pereopod-5; (G), pereopod-6; (H), pleopod; (I), uropod. Scale lines = 0.1 mm.
Pereopod-1 (Figure 3B) overall 14.9 L:W; coxa with small seta; basis 6.7 L:W, 4.2x merus, with one dorsoproximal and two ventral setae; ischium with ventral seta; merus 2.0 L:W and 0.7x carpus, with minute ventrodistal and one dorsodistal seta; carpus 3.0 L:W, 0.6x propodus, with two minute distal setae; propodus 8.4 L:W, 0.9x dactylus and unguis combined length, with short ventrodistal seta, dactylus 0.5x unguis.
Pereopod-2 (Figure 3C) overall 12.6 L:W; coxa not dissected; basis 6.5 L:W, 3.6x merus, with mid-dorsal penicillate seta; ischium with ventral seta; merus 2.3 L:W, 1.0x carpus, with seta and spine ventrodistally; carpus 2.0 L:W, 0.7x propodus, with dorsodistal seta, and long ventrodistal blade-like spine (0.6x propodus); propodus 6.3 L:W, 1.5x dactylus and unguis combined length, with long distal seta (0.7x dactylus and unguis combined length); dactylus 0.2x unguis.
Pereopod-3 (Figure 3D) overall 17.4 L:W; basis 3.7 L:W, 2.9x merus, with mid-ventral simple seta; ischium with ventral seta; merus 1.6 L:W, 0.9x carpus, with ventrodistal spine (seta not seen); carpus 2.0 L:W, 0.7x propodus, with dorsodistal robust seta, inner-distal minute seta, and long ventrodistal blade-like spine (0.7x propodus); propodus 5.4 L:W, 1.7x dactylus and unguis combined length, with distal seta (0.6x dactylus and unguis combined length); dactylus 0.5x unguis.
Pereopod-4 (Figure 3E) overall 9.2 L:W; basis 4.2 L:W, 4.2x merus, naked; ischium with two ventral setae; merus 1.4 L:W, 0.5x carpus, with one short seta and one spine; carpus 3.9 L:W, 0.9x propodus, with one short spine and one intermediate blade-like spine (0.5x propodus); dorsal seta not seen; propodus 5.7 L:W, 2.4x dactylus and unguis combined length, with one subdorsal penicillate seta, two spines (short and long) ventrodistally, and long serrate dorsodistal seta (1.7x dactylus and unguis combined length); dactylus 1.8x unguis.
Pereopod-5 (Figure 3F) overall 10.0 L:W; basis 5.0 L:W, 5.9x merus, with long penicillate midlength seta; ischium with two ventral setae; merus 1.2 L:W, 0.4x carpus, with ventral spine (seta not seen); carpus 3.6 L:W, 1.2x propodus, with dorsodistal seta (1.3x propodus), one minute spine and one intermediate blade-like spine (0.5x propodus); propodus 5.0 L:W, 2.2x dactylus and unguis combined length, with one sub-dorsal penicillate seta, one serrate seta, one spine ventrally and one serrate dorsal seta (2.1 x dactylus and unguis combined length); dactylus 2.3x unguis.
Pereopod-6 (Figure 3G) basis 4.8 L:W, 4.5x merus, naked; ischium with two ventral setae; merus 1.5 L:W, 0.5x carpus, with spine (seta not seen); carpus 3.4 L:W, 1.1x propodus, with long dorsodistal seta (0.9x propodus), two spines (short and long) and one intermediate blade-like spine (0.5x propodus) ventrodistally; propodus 4.1 L:W, with two serrate ventral setae and two serrate dorsal setae; dactylus broken.
Pleopods (Figure 3H) rami narrow and elongate; exopod with five, endopod with seven distal setae.
Uropod (Figure 3I) peduncle 0.9 L:W; exopod with two articles; 6.0 L:W; article-1 2.7 L:W, with short distal seta; article-2 5.0 L:W, with two distal setae; endopod 7.9 L:W; article-1 4.3 L:W, with one simple and two penicillate distal setae; article-2 4.2 L:W, with one subdistal, four distal simple setae and one penicillate seta. Exopod 0.7x endopod.
Description of juvenile male. Similar to female, but antennule thicker (Figures 1C,D).
Distribution. The species is known only from the type locality: SE Australia (off Gippsland), at the depth 400 m.
Remarks. Pseudotanais chardonnayi sp. nov. has a dorsodistal spine on antenna articles 2–3, a relatively long propodal distal seta on pereopods 2–3, and a long dorsodistal seta on carpus of pereopods 5–6, that allow classification of the species to the “affinis + longisetosus” morpho-group (Bird and Holdich, 1989b; Jakiel et al., 2019), although the relatively short dorsodistal seta on the pereopod merus (rather long in “affinis + longisetosus” group) is anomalous we have decided to deposit P. chardonnayi in this group as this seta is still longer than in members of other groups where it is minute or absent.
The short dorsodistal seta on the pereopod-4 carpus distinguishes P. chardonnayi from P. chanelae, P. curieae and P. longisetosus, where this seta is long. The combination of a spine and seta on the pereopods 4–6 merus and carpal long rod seta of P. chardonnayi is similar to P. romeo, but it can be separated by the uropod exopod that is 0.7x endopod in P. chardonnayi and 0.9x in P. romeo. Additionally, the blade-like spine on the carpus of pereopod-3 is 0.7x propodus in P. chardonnayi, while P. romeo it is slightly longer (0.8x propodus). Finally, both species can be distinguished by the setation of ischium of pereopods 4–6, with two setae in P. chardonnayi and naked in P. romeo.
Pseudotanais caberneti sp. nov.
-
This species is registered in ZooBank number: LSIDurn:lsid:zoobank.org:act:FE1106E8-DBE6-4CB5-AD67-F631E08F9A1C.
Diagnosis. Mandible molar subcoronal with distal spines. Pereopod-1 merus with seta. Pereopod-3 carpal blade-like spine long (0.7x propodus). Pereopods 4–6 merus with spine and seta; carpus with short dorsodistal seta; propodus with two ventral setae. Uropod exopod 0.7x endopod.
Material examined. Holotype, ovigerous female 1.8 mm, partly dissected (J62735) SLOPE 118.
Etymology. The species name is after one of most widely distributed and best-known wine grape varieties grown in SE Australia, as genitive.
Description of female. BL = 1.7 mm. Body robust (Figures 4A,B) 3.2 L:W. Cephalothorax 0.7 L:W, 1.4x pereonites 1–3 0.2x BL. Pereonites 0.6x BL; pereonite-1 0.4x pereonite-2, pereonites-1–6: 0.1, 0.2, 0.2, 0.5, 0.5, and 0.5 L:W, respectively; pereonites 1, 3–4 with small anterolateral setae. Pleon short, 0.4 BL. Pleonites 0.7 L:W, pleonites 1 and 4 with dorsolateral setae on each side of midline, and pleonite 5 with lateral seta. Pleotelson 5.4x pleonite-5, with pair of mid-distal setae.
FIGURE 4
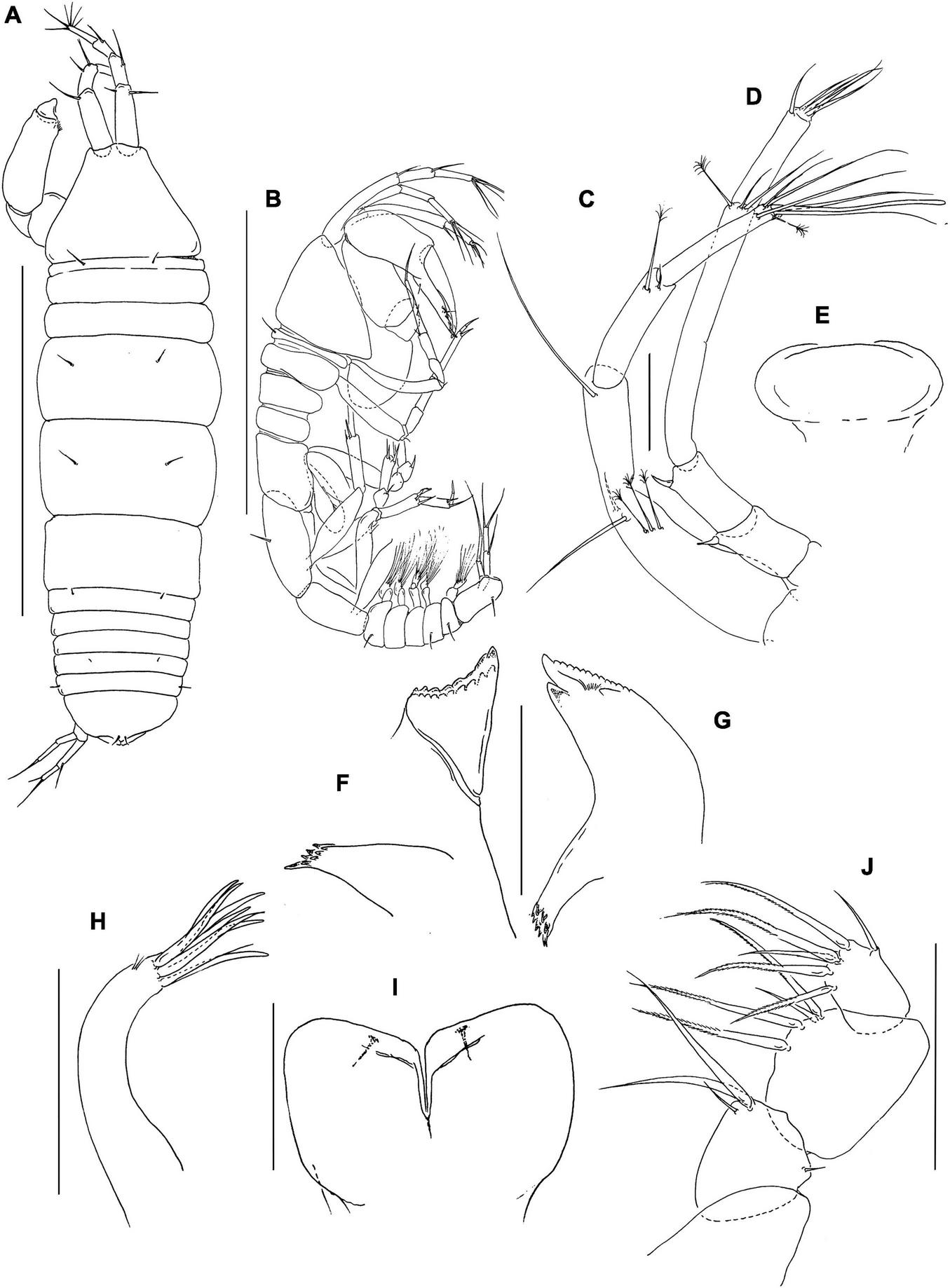
Pseudotanais caberneti sp. nov; female, (J62735) (A), dorsal; (B), lateral; (C), antennule; (D), antenna; (E), labrum; (F), left mandible; (G), right mandible; (H), maxillule; (I), labium; (J), maxilliped palp. Scale lines (A,B) = 1 mm, (C–J) = 0.1 mm.
Antennule (Figure 4C) article-1 3.9 L:W, 2.6x article-2, with two simple and three penicillate midlength setae, and one distal seta; article-2 3.9 L:W, 0.9x article-3, with one simple and one penicillate distal setae; article-3 7.5 L:W, with one subdistal seta and five simple setae and one aesthetasc distally.
Antenna (Figure 4D) article-2 1.6 L:W; 0.8x article-3, with spine (0.3x article-2); article-3 2.2 L:W, 0.4x article-4, with spine (0.2x article-3); article-4 10.0 L:W, 2.6x article-5, with two simple and one penicillate distal setae; article-5 3.8 L:W, 11.5x article-6, with distal seta; article-6 0.4 L:W, with four distal setae.
Labrum (Figure 4E) rounded, naked. Left mandible (Figure 4F) lacinia mobilis well developed, distally serrate, incisor distal margin beveled and serrate, molar subcoronal with distal spines. Right mandible (Figure 4G) incisor unequally bifid, distal margin serrate; molar as in left mandible. Labium simple, semi-rectangular (Figure 4I). Maxillule (Figure 4H) endite with seven distal spines and outer subdistal setae. Maxilla not observed.
Maxilliped (Figure 4J) palp article-1 naked, article-2 1.3 L:W, with fine outer and three inner setae (two long and one short); article-3 1.3 L:W with one shorter and three longer inner setae; article-4 1.4 L:W with six distal and subdistal setae. Maxilliped endite, basis not dissected.
Cheliped (Figure 5A) basis broken; merus ventral seta not seen; carpus 1.9 L:W, 1.2x palm, with two midventral setae and dorsodistal simple seta; chela non-forcipate, palm 1.4 L:W with comb of small setae on inner side, and one seta near dactylus insertion; fixed finger 4.0 L:W, 1.1x palm, with ventral seta, cutting edge poorly calcified, almost simple, and with three setae; dactylus 5.6 L:W, cutting edge smooth, with dorsoproximal seta.
FIGURE 5
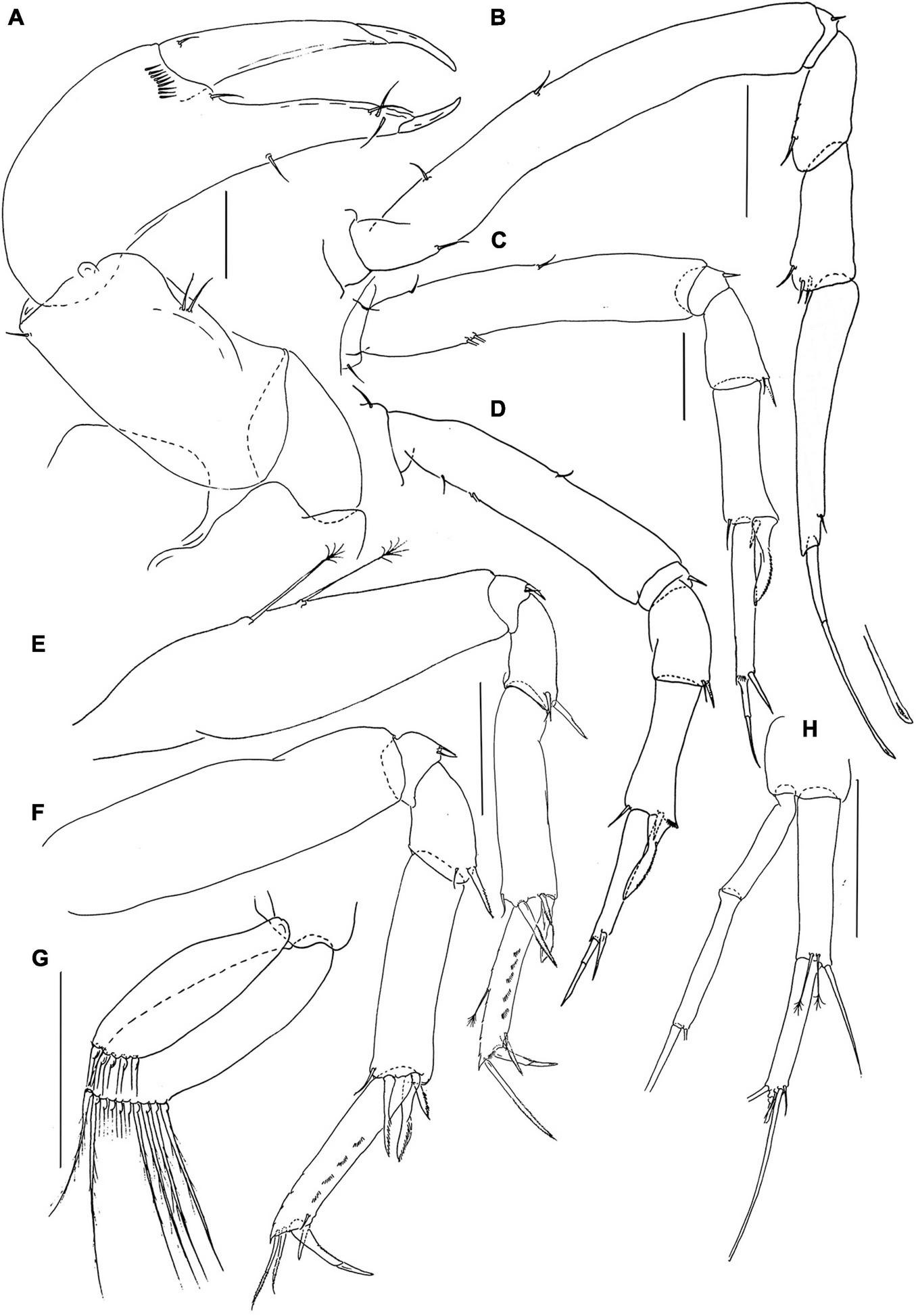
Pseudotanais caberneti sp. nov.; female (J62735), (A), cheliped; (B), pereopod-1; (C), pereopod-2, (D), pereopod-3; (E), pereopod-4; (F), pereopod-6; (G), pleopod; (H), uropod. Scale lines = 0.1 mm.
Pereopod-1 (Figure 5B) overall 16.8 L:W; basis 8.3 L:W, 4.6x merus, with one dorsoproximal and two ventral setae; ischium with ventral seta; merus 2.0 L:W and 0.8x carpus, with dorsodistal seta; carpus 2.2 L:W, 0.5x propodus, with three dorsodistal setae; propodus 6.4 L:W, 0.9x dactylus and unguis combined length, with one subdistal seta, dactylus 0.6x unguis.
Pereopod-2 (Figure 5C) overall 11.8 L:W; coxa with seta; basis 5.4 L:W, 3.6x merus, with two dorsoproximal (broken) and two ventral setae; ischium with ventral seta; merus 1.5 L:W, 0.7x carpus, with seta and spine ventrodistally; carpus 2.6 L:W, 0.9x propodus, with dorsodistal setae, simple ventrodistal spine and intermediate ventrodistal blade-like spine (0.5x propodus); propodus 6.5 L:W, 1.7x dactylus and unguis combined length, with ventrodistal robust seta (0.5x dactylus and unguis combined length); dactylus 0.7x unguis.
Pereopod-3 (Figure 5D) overall 22.8 L:W; coxa with seta; basis 4.8 L:W, 2.7x merus, with two dorsoproximal (one broken) and one ventral setae; ischium with ventral seta; merus 2.0 L:W, 0.8x carpus, with ventrodistal seta and spine; carpus 2.9 L:W, 1.1x propodus, with dorsodistal seta, simple ventrodistal spine and long ventrodistal blade-like spine (0.7x propodus); propodus 5.3 L:W, 1.6x dactylus and unguis combined length, with one distal seta (0.5x dactylus and unguis combined length); dactylus 0.6x unguis.
Pereopod-4 (Figure 5E) overall 8.1 L:W; basis 3.9 L:W, 5.0x merus, with two long penicillate ventral setae; ischium with two ventral setae; merus 1.6 L:W, 0.4x carpus, with one spine and one seta; carpus 4.3 L:W, 4.5x propodus, with small; dorsodistal seta, two distal spines and one short blade-like spine (0.4x propodus); propodus 6.8 L:W, 2.5x dactylus and unguis combined length, with two serrate ventral setae (short and long) and one serrate dorsal seta (1.5x dactylus and unguis combined length); dactylus 1.2x unguis.
Pereopod-5 similar to pereopod-4.
Pereopod-6 (Figure 5F) overall 8.1 L:W; basis 3.3 L:W, 3.3x merus, naked; ischium with two ventral setae; merus 1.6 L:W, 0.5x carpus, with one short seta and one robust spine; carpus 3.5 L:W, 1.1x propodus, with dorsodistal seta, two distal spines, and short blade-like spine (0.4x propodus); propodus 5.5 L:W, 2.2x dactylus and unguis combine length, with two serrate ventral setae (short and long) and two serrate dorsal setae (longer setae 0.9x dactylus and unguis combined length); dactylus 2.0x unguis.
Pleopods (Figure 5G) rami elongate, narrow; exopod with five, endopod with ten distal setae.
Uropod (Figure 5H) peduncle 1.0 L:W; exopod with two articles, 15.3 L:W; article-1 7.3 L:W, naked, article-2 8.0 L:W, with two setae; endopod 8.3 L:W; article-1 4.7 L:W, with one simple seta and two penicillate setae; article-2 7.3 L:W, with five distal setae. Exopod 0.9x endopod.
Distribution: Species known from SE Australia, off Cape Otway, from the depth 209 m.
Remarks. Pseudotanais caberneti sp. nov. with a relatively long seta on merus and carpus of pereopod-1 can be classified in the “affinis + longisetosus” morphogroup, although the short dorsodistal seta on the carpus of pereopods 5–6 differentiates it from fourteen species: Pseudotanais chardonnayi, P. chanelae, P. curieae, P. gaiae, P. julietae, P. longisetosus, P. longispinus, P. monroeae, P. nipponicus, P. rapunzelae, P. romeo, P. spatula, P. uranos, and Pseudotanais sp. O (sensu McLelland), which have a long seta. The combination of a spine and seta on the merus of pereopods 4–6 merus differentiates P. caberneti from P. affinis, P. scalpellum, P. shackletoni, P. svavarssoni and Pseudotanais sp. P. (sensu McLelland), which have either a spine or seta only (the latter P. svavarssoni). Furthermore, two setae on the ischium of pereopods 4–6 of P. caberneti is similar to P. geratli and P. macrocheles, although the uropodal exopod that is only slightly shorter than the endopod (0.9x) separates P. caberneti from both species, where this proportion is at most 0.6x.
Key for identification of Pseudotanais females of the “affinis + longisetosus” morpho-group.
-
1.
Pereopods 5–6 carpus with:
short seta…………………………………………………………………………2
long seta………………………………………………………………………….3
-
2.
Pereopod–4 ischium with:
one seta……………………………………………………………………………4
two setae………………………………………………………………………….5
-
3.
Pereopod–4 carpus rod seta:
short………………………………………………………………………………13
long……………………………………………………………………………….14
-
4.
Uropod exopod to endopod ratio:
< 0.8x……………………………………………………………………………06
> 0.9x………………………………………………………………….P. vitjazi
-
5.
Uropod exopod to endopod ratio:
0.6x………………………………………………………………………………….7
> 0.8x……………………………………………………………………………..8
-
6.
Pereopod–2 carpus blade-like spine to propodus ratio
≤ 0.6x……………………………………………………………………………11
0.8x……………………………………………………………….P. scalpellum
-
7.
Cephalothorax to pereonites 1–3 ratio; pereopod–5 dactylus to unguis ratio:
1.3x;1.6x……………………………………………………. P. macrocheles
0.9x; 2.0x……………………………………………………………..P. geralti
-
8.
Cephalothorax to pereonites 1–3 ratio:
< 1.2x……………………………………………………………………………..9
>1.3x………………………………………………….P. caberneti sp. nov.
-
9.
Pereopods 4–6 merus spine, seta [0-absent, 1-present]:
1.0……………………………………………………………………………….. 10
0.1……………………………………………………………….P. svavarssoni
-
10.
Pereopod-5 dactylus to unguis ratio:
1.0x………………………………………………………Pseudotanais sp. P.
2.3x………………………………………………………………P. shackletoni
3.0x…………………………………………………………………….. P. affinis
-
11.
Cephalothorax to pereonites 1–3 ratio:
< 1.2x……………………………………………………………………………12
> 1.7x…………………………………………………………..P. yenneferae
-
12.
Pereonite-1 to pereonite-2 ratio; pereopods 4–6 merus spine, seta [0-absent, 1-present]:
0.7x; 1,1………………………………………………….. P. nordenskioldi
0.5x; 1,0………………………………………………………………P. spatula
-
13.
Pereopod-3 carpus blade-like spine to propodus ratio
0.5x……………………………………………………………………………… 16
≥ 0.6x…………………………………………………………………………. 17
-
14.
Uropod exopod to endopod ratio:
0.7x……………………………………………………………………………… 15
0.9x…………………………………………………………………… P. curieae
-
15.
Cephalothorax to pereonites 1–3 ratio; pereonite-1 to pereonite-2 ratio:
1.2x; 1,0x……………………………………………………..P. longisetosus
0.9x; 0.5x………………………………………………………… P. chanelae
-
16.
Pereopods 4–6 merus spine, seta [0-absent, 1-present]:
0,2…………………………………………………………………………………18 1,0……………………………………………………………………….. P. gaiae
-
17.
Pereopod-2 carpus blade-like spine to propodus ratio
≥ 0.6x…………………………………………………………………………..19
0.5x……………………………………………………………………. P. romeo
-
18.
Pereonite-1 to pereonite-2 ratio; pereopod-5 dactylus to unguis ratio:
0.3x; 2.0x…………………………………………………………….P. uranos
0.6x; 1.4x………………………………………………………..P. monroeae
-
19.
Pereopods 4–6 ischium with:
One seta………………………………………………………………………..20
Two setae………………………………………………………………………21
-
20.
Cephalothorax to pereonites 1–3 ratio; pereopod-5 dactylus to unguis ratio:
1.0x; 2.0x…………………………………………………….. P. longispinus
1.3x; 2.5x……………………………………………………………P. julietae
-
21.
Pereopods 4–6 merus spine, seta [0-absent, 1-present]:
1,0……………………………………………………………………………….. 22
0,2………………………………………………………………. P. nipponicus
1,1……………………………………………….. P. chardonnayi sp. nov.
-
22.
Pereonite-1 to pereonite-2 length ratio; pereopod-2 blade-like spine to propodus ratio; pereopod-5 dactylus to unguis ratio:
0.6x; 0.8x; 2.3x…………………………………………….. P. rapunzelae
0.4x; 0.6x; 2.4x………………………………………….. P. szymborskae
0.4x; 0.7x; 1.5x…………………………………….. Pseudotanais sp. O
“denticulatus + abathagastor” group
Diagnosis. After Błażewicz et al. (2021).
Species included. Pseudotanais abathagastor Bamber and Błażewicz-Paszkowycz, 2013; P. amundseni Błażewicz et al., 2021; P. barnesi Błażewicz et al., 2021; P. biopearli Błażewicz et al., 2021; P. chaplini Jakiel et al., 2019; P. chopini Jakiel et al., 2019; P. corollatusBird and Holdich, 1989b; P. denticulatusBird and Holdich, 1989b; P. elephas Błażewicz et al., 2021; P. georgesandae Jakiel et al., 2019; P. kitsoni Błażewicz et al., 2021; P. mariae Jakiel et al., 2019; P. livingstoni Błażewicz et al., 2021; P. locueloae Jakiel et al., 2019; P. oloughlini Jakiel et al., 2019; P. palmeri Błażewicz et al., 2021; P. barossai sp. nov.; P. coonawarrai sp. nov.
Pseudotanais barossai sp. nov.
-
This species is registered in ZooBank number: LSIDurn:lsid:zoobank.org:act:F4C1D408-3731-449E-9F00-D18CBEF81DB5.
Diagnosis. Antenna articles 2–3 with slender spine. Mandible molar coronal. Pereopod-2 carpus blade-like spine short (0.4x propodus). Pereopods 2–6 merus with single spine. Uropod exopod 0.8x endopod.
Material examined. Holotype, neuter 1.3 mm, partly dissected (J61545), SLOPE 170.
Etymology. From the Barossa Valley in South Australia, a premium wine-growing region, as genitive.
Description of female. BL = 1.6 mm. Body robust (Figure 6A) 3.0 L:W. Cephalothorax 1.0 L:W, 1.2x pereonites 1–3, 1.3x BL with pair of ocular setae. Pereonites 0.6x BL, pereonite-1 0.6x pereonite-2, pereonites-1–6: 0.2, 0.2, 0.4, 0.5, 0.6 and 0.4 L:W, respectively; pereonites 1, 4–6 with anterolateral setae. Pleon short, 0.5x BL. Pleonites 1.2 L:W. Pleotelson 1.9x pleonite-5, with laterodistal setae.
FIGURE 6
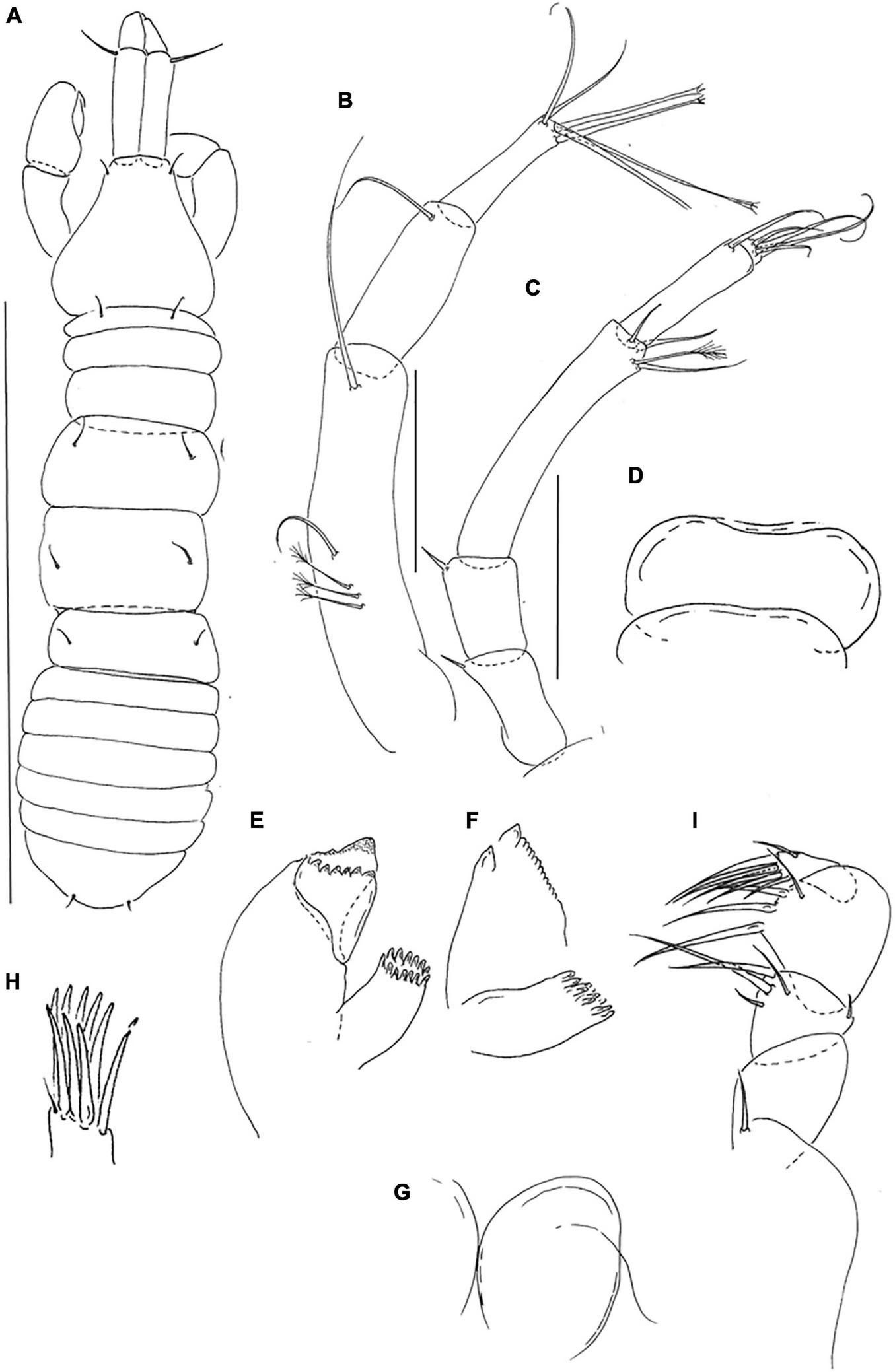
Pseudotanais barossai sp. nov; neuter (J61545), (A), dorsal; (B), antennule; (C), antenna; (D), labrum; (E), left mandible; (F), right mandible; (G), labium; (H), maxillule; endite; (I), maxilliped palp. Scale lines (A) = 1 mm, (B–I) = 0.1 mm.
Antennule (Figure 6B) article-1 4.5 L:W, 2.2x article-2, with one simple and three penicillate midlength setae, and one distal seta; article-2 2.6 L:W, 1.3x article-3, with distal seta; article-3 4.0 L:W, with three simple and three bifurcated setae (aesthetasc not seen).
Antenna (Figure 6C) article-2 1.7 L:W; 1.1x article-3, with slender spine (0.3x article-2); article-3 1.6 L:W, 0.4x article-4, with slender spine (0.3x article-3); article-4 5.5 L:W, 1.7x article-5, with three simple and one penicillate distal setae; article-5 4.4 L:W, 11.7x article-6, with distal seta; article-6 0.4 L:W, with four distal setae.
Labrum (Figure 6D) rounded, naked. Left mandible (Figure 6E) lacinia mobilis well developed, distally serrate, incisor distal margin beveled slightly serrate, molar coronal. Right mandible (Figure 6F) incisor unequally bifid, distal margin serrate molar as left mandible. Labium (Figure 6G) simple, rounded, glabrous. Maxillule (Figure 6H) endite with nine distal spines and outer subdistal seta. Maxilla not recovered.
Maxilliped (Figure 6I) palp article-1 1.5 L:W, naked, article-2 1.0 L:W with fine outer seta and three inner setae; article-3 1.1 L:W with four inner setae, article-4 3.0 L:W with one sub-distal and five distal setae. Epignath not recovered.
Cheliped (Figure 7A) basis 1.9 L:W, with small seta near sclerite articulation; merus with ventral seta; carpus 1.8 L:W, 1.2x palm, with two midventral setae and mid-dorsal and dorsodistal simple setae; chela non-forcipate, palm 1.7 L:W; fixed finger 3.1 L:W, 0.9x palm with one ventral seta, and with three setae on cutting edge; dactylus 6.3 L:W, cutting edge smooth, proximal seta not seen.
FIGURE 7
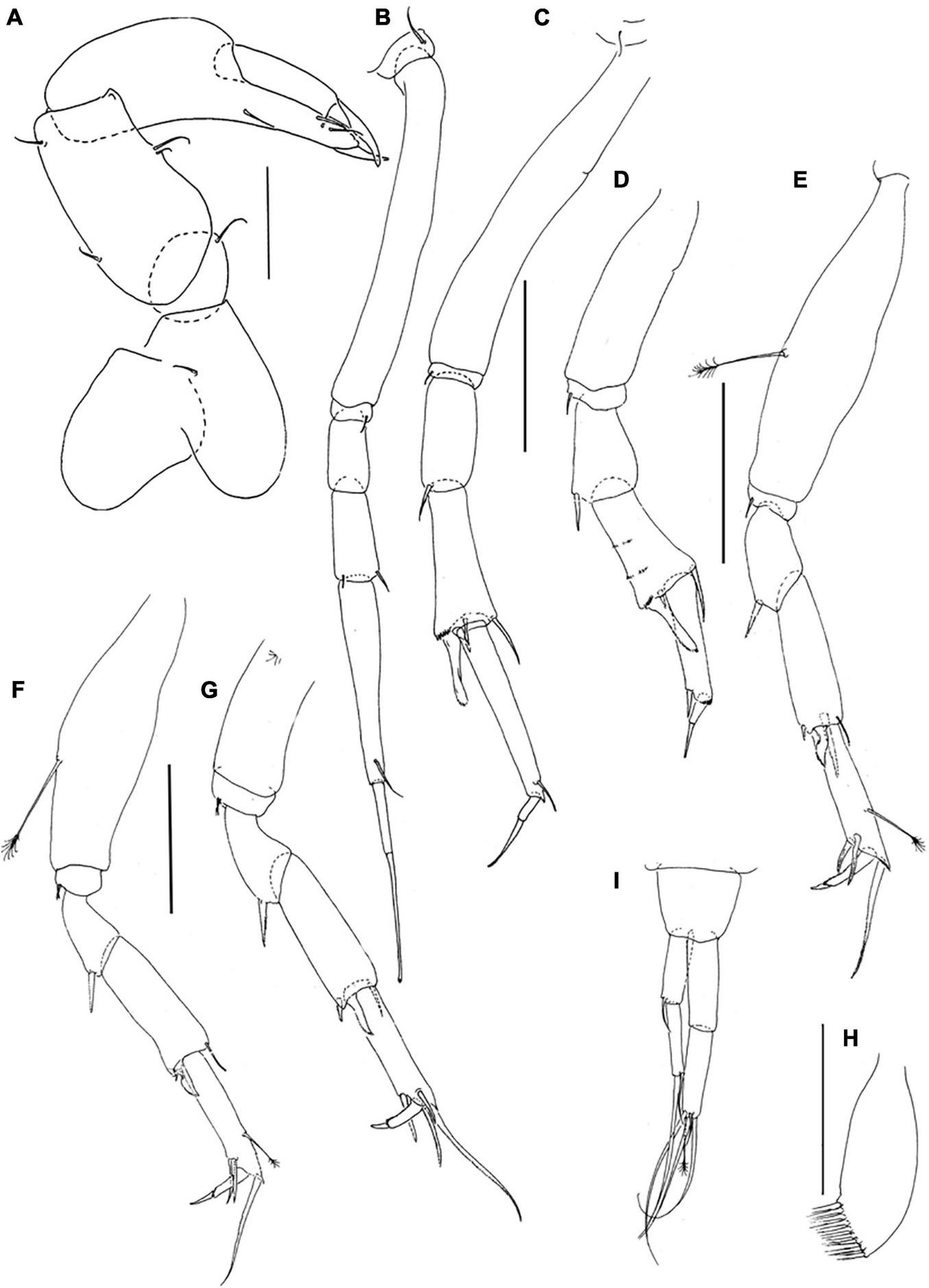
Pseudotanais barossai sp. nov.; neuter (J61545), (A), cheliped; (B), pereopod-1; (C), pereopod-2, (D), pereopod-3; (E), pereopod-4; (F), pereopod-5; (G), pereopod-6; (H), pleopod; (I), uropod. Scale lines = 0.1 mm.
Pereopod-1 (Figure 7B) overall 15.7 L:W; coxa with seta; basis 7.0 L:W, 3.7x merus, naked; ischium with ventral seta; merus 2.1 L:W and 0.8x carpus, naked; carpus 2.4 L:W, 0.5x propodus, with two short distal setae; propodus 5.6 L:W, 0.8x dactylus and unguis combined length, with ventrodistal seta; dactylus 0.6 x unguis.
Pereopod-2 (Figure 7C) overall 13.5 L:W; coxa with seta; basis 6.2 L:W, 3.2x merus, naked; ischium with ventral seta; merus 2.3 L:W, 0.8x carpus, with ventrodistal spine; carpus 2.5 L:W, 0.9x propodus, with dorsodistal spine, inner-distal seta, simple ventrodistal spine and short ventrodistal blade-like spine (0.4x propodus); propodus 6.3 L:W, 2.0x dactylus and unguis combined length, with one distal seta (0.4x dactylus and unguis combined length); dactylus 0.5x unguis.
Pereopod-3 (Figure 7D) basis distally broken, naked; ischium with ventral seta; merus 4.6 L:W, 0.9x carpus, with ventrodistal spine; carpus 1.7 L:W, 1.0x propodus, with dorsodistal seta, simple ventrodistal small spine, and long ventrodistal blade-like spine (0.6x propodus); propodus 3.9 L:W, 3.9x dactylus and unguis combined length, with one ventrodistal robust seta (0.4x dactylus and unguis combined length); dactylus 0.9x unguis.
Pereopod-4 (Figure 7E) overall 8.4 L:W; basis 3.9 L:W, 3.5x merus, with penicillate ventral seta; ischium with ventral seta (second seta not seen); merus 1.8 L:W, 0.6x carpus, with spine; carpus 8.5 L:W, 1.1x propodus, with dorsodistal seta, and two spines (short and long) and one short blade-like spine (0.3x propodus) distally; propodus 4.3 L:W, 1.9x dactylus and unguis combined length, with two serrate ventral spines and one serrate dorsodistal seta (1.7x dactylus and unguis combined length), and one penicillate middorsal seta; dactylus 3.0x unguis.
Pereopod-5 (Figure 7F) overall 7.3 L:W; basis 3.3 L:W, 3.9x merus, with penicillate ventral seta; ischium with two ventral setae; merus 1.5 L:W, 0.6x carpus, with spine; carpus 2.6 L:W, 1.0x propodus, with dorsodistal seta, small spine and short blade-like spine (0.3x propodus) distally; propodus 5.0 L:W, 3.3x dactylus and unguis combined length, with dorsal penicillate seta, two serrate ventrodistal spine, one serrate dorsodistal seta (1.7 dactylus and unguis combined length); dactylus 1.5x unguis.
Pereopod-6 (Figure 7G) basis broken; ischium with two ventral setae; merus 1.7 L:W, 0.6x carpus, with spine; carpus 3.1 L:W, 1.1x propodus, with dorsodistal seta, small spine and short blade-like spine (0.3x propodus) distally; propodus 5.0 L:W, 2.7x dactylus and unguis combine length, with two serrate ventrodistal setae and two serrate dorsodistal setae (longer setae 2.9x dactylus and unguis combined length); dactylus 2.7x unguis.
Pleopods (Figure 7H) poor condition, exopod not observed; endopod with nine setae.
Uropod (Figure 7I) peduncle 0.9 L:W; exopod with two articles; 6.6 L:W; article-1 3.2 L:W, with simple seta; article-2 5.7 L:W, with two distal setae (short and long); endopod 6.0 L:W; article-1 3.1 L:W, naked; article-2 4.0 L:W, with four simple setae and one penicillate distal seta. Exopod 0.8x endopod.
Distribution. Species known only from type locality, off Kangaroo Island (SE Australia) at depth 1548 m.
Remarks. Pseudotanais barossai sp. nov. has a thin spine on antenna article-2 and with this it can be separated from P. abathagastor and P. mariae which have a weaker seta at this position, and P. barnesi that lacks any seta. Furthermore, a thin spine on antennal article-3 also distinguishes P. barossai from P. amundseni, which has a weaker seta. In addition, a single spine on the pereopods 2–6 merus differentiates P. barossai from all other congeners that have a combination of spine and seta, two setae or being naked at this position.
Pseudotanais coonawarrai sp. nov.
-
This species is registered in ZooBank number: LSIDurn:lsid:zoobank.org:act:9C6979E0-B0A2-47FE-8C4B-54B5CA2CAC39.
Diagnosis. Antenna articles 2–3 with spine. Mandible molar coronal with two distal spines. Pereopod-2 carpus blade-like spine short (0.4x propodus). Pereopods 2–6 merus with spine and seta. Uropod exopod 0.8x endopod.
Material examined. Holotype, neuter 2.9 mm, partly dissected (J62734), SLOPE 134.
Etymology. In the Bindjali Aboriginal language, coonawarra is honeysuckle and a wine region from southern Australia, as genitive.
Description of female. BL = 2.9 mm. Body robust (Figure 8A) 4.1 L:W. Cephalothorax 0.9 L:W, 1.1x pereonites 1–3, 0.2x BL. Pereonites 0.6x BL, pereonite-1 0.5x pereonite-2, pereonites 1–6: 0.2, 0.2, 0.4, 0.6, 0.7, and 0.6 L:W, respectively; pereonites 1, 4–6 with small anterolateral setae. Pleon short, 0.4x BL. Pleonites 0.9 L:W, pleonites 1–2 and 4–5 with dorsolateral pair of setae. Pleotelson 5.3x pleonite-5, with pair of laterodistal setae.
FIGURE 8
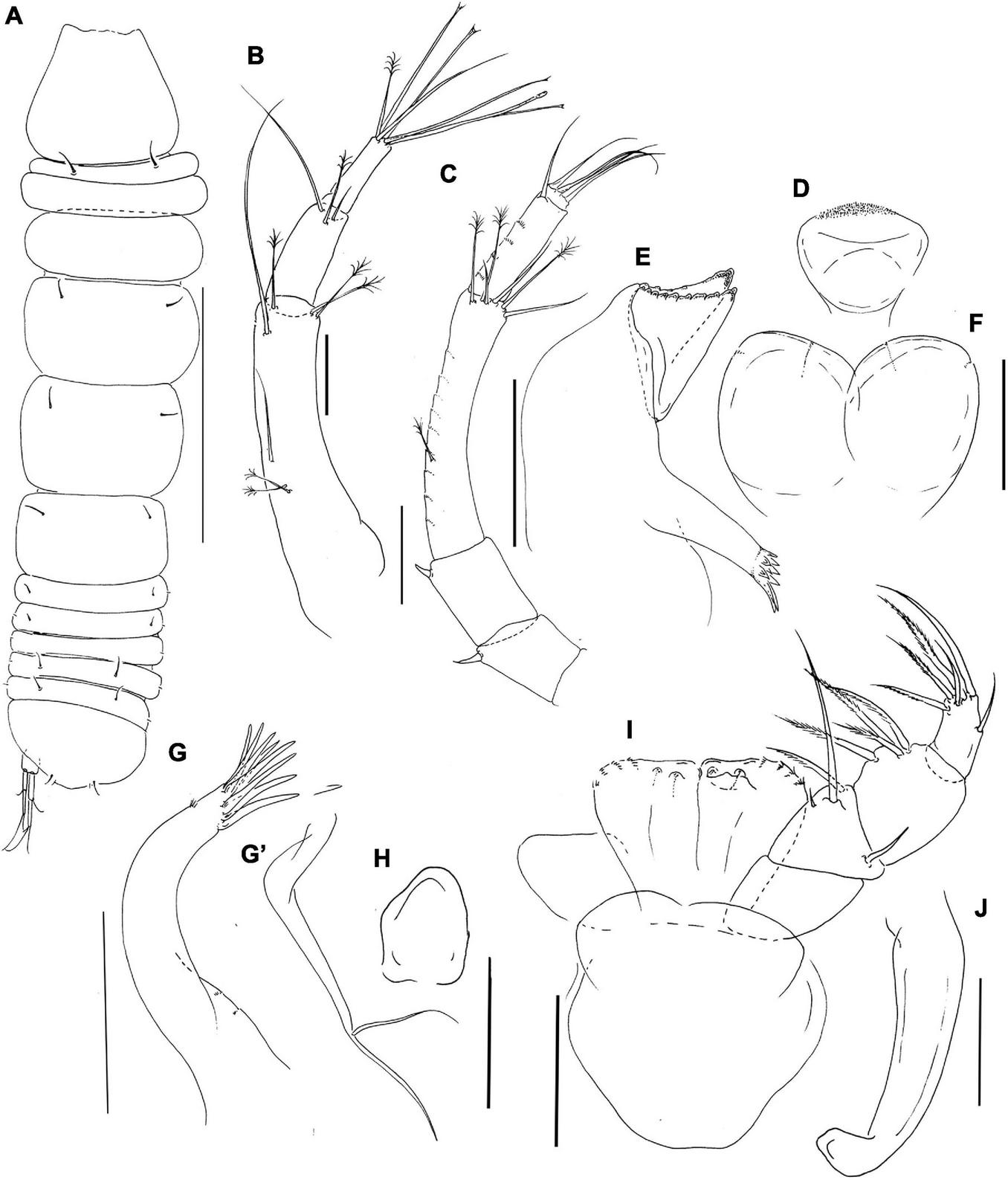
Pseudotanais coonawarrai sp. nov; neuter (J62734), (A), dorsal; (B), antennule; (C), antenna; (D), labrum; (E), left mandible; (F), labium; (G), maxillule; (G’) maxillule endite; (H), maxilla; (I), maxilliped; (J), epignath. Scale lines (A) = 1 mm, (B–I) = 0.1 mm.
Antennule (Figure 8B) article-1 3.9 L:W, 2.8x article-2, with one simple and two penicillate midlength setae, and one simple and three penicillate distal setae; article-2 2.5 L:W, 1.2x article-3, with two simple setae and one distal penicillate seta; article-3 3.7 L:W, with one simple, one penicillate, four bifurcated setae and one aesthetasc.
Antenna (Figure 8C) article-2 1.1 L:W; 0.9x article-3, with spine (0.3x article-2); article-3 1.4 L:W, 0.3x article-4, with spine (0.2x article-3); article-4 6.2 L:W, 2.7x article-5, with middorsal penicillate seta, three simple and three penicillate distal setae; article-5 3.3 L:W, 7.7x article-6, with distal seta; article-6 0.5 L:W, with four distal setae.
Labrum (Figure 8D) rounded, finely setulate. Left mandible (Figure 8E) lacinia mobilis well developed, distally serrate, incisor distal margin beveled and serrate, molar coronal with two longer distal spines. Right mandible not recovered. Labium (Figure 8F) simple, rounded, glabrous. Maxillule (Figure 8G) endite with eight distal spines and outer subdistal setae; palp (Figure 8G’) with two setae. Maxilla (Figure 8H) almost circular, naked.
Maxilliped (Figure 8I) palp article-1 1.2 L:W, naked, article-2 1.0 L:W, with fine outer and three inner setae (one minute, one very long); article-3 0.9 L:W, with one shorter and three longer inner setae, article-4 1.6 L:W, with one sub-distal and five distal setae. Maxilliped endites mostly fused but with distinct central cleft, each with two round gustatory cusps. Epignath (Figure 8J) linguiform, simple, naked.
Cheliped (Figure 9A) basis 1.5 L:W, naked; merus with ventral seta; carpus 1.6 L:W, 1.0x palm, with two midventral setae and middorsal seta; chela non-forcipate, palm 1.6 L:W with seta near the dactylus insertion; fixed finger 3.0 L:W, 0.7x palm, with three setae on cutting edge (ventral seta not seen); dactylus 4.7 L:W, cutting edge smooth, with dorsoproximal seta.
FIGURE 9
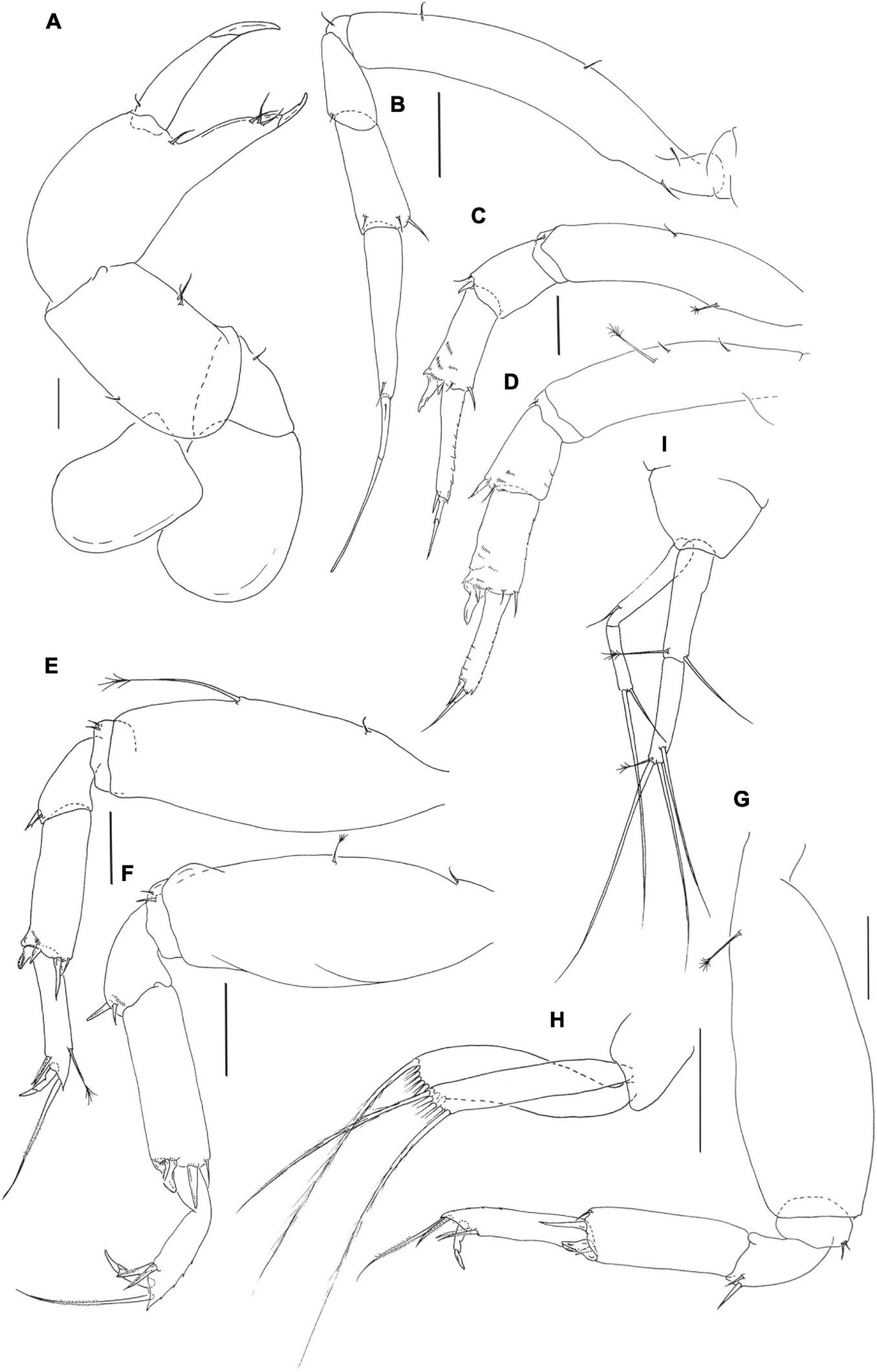
Pseudotanais coonawarrai sp. nov.; neuter (J62734), (A), cheliped; (B), pereopod-1; (C), pereopod-2, (D), pereopod-3; (E), pereopod-4; (F), pereopod-5; (G), pereopod-6; (H), pleopod; (I), uropod. Scale lines = 0.1 mm.
Pereopod-1 (Figure 9B) overall 14.3 L:W; basis 7.2 L:W, 4.0x merus, with one dorsoproximal and two (or three) ventral setae; ischium with ventral seta; merus 2.3 L:W and 0.8x carpus, with minute ventrodistal seta; carpus 2.4 L:W, 0.7x propodus, with one ventrodistal and two dorsodistal setae; propodus 4.4 L:W, 0.6x dactylus and unguis combined length, with one subdistal seta, dactylus 0.5x unguis, with proximal seta.
Pereopod-2 (Figure 9C) overall 9.6 L:W; basis 4.7 L:W, 4.3x merus, with mid-dorsal penicillate seta and mid-ventral simple seta; ischium with ventral seta; merus 1.1 L:W, 0.6x carpus, with seta and spine ventrodistally; carpus 2.3 L:W, 0.9x propodus, with dorsodistal seta, small distal seta, and short spine and blade-like spine (0.4x propodus) ventrodistally, several comb-like scales distally; propodus 5.0 L:W, 1.7x dactylus and unguis combined length, with one distal seta (0.3x dactylus and unguis combined length), with comb-like scales along dorsal margin; dactylus 0.5x unguis.
Pereopod-3 (Figure 9D) overall 15.9 L:W; basis 3.7 L:W, 2.9x merus, with two simple and one penicillate midventral setae; ischium with ventral seta; merus 1.4 L:W, 0.8x carpus, with seta and spine ventrodistally; carpus 2.0 L:W, 1.0x propodus, with dorsodistal seta, small seta, small spine and short ventrodistal blade-like spine (0.4x propodus) distally; propodus 4.5 L:W, 1.5x dactylus and unguis combined length, with one distal seta (0.4x dactylus and unguis combined length); dactylus 0.3x unguis.
Pereopod-4 (Figure 9E) overall 6.2 L:W; basis 2.5 L:W, 3.8x merus, with small simple ventroproximal seta and long penicillate midventral seta; ischium with two ventral setae; merus 1.3 L:W, 0.5x carpus, with seta and spine; carpus 3.3 L:W, 1.2x propodus, with one seta, two spines (short and longer) and short blade-like spine (0.2x propodus); propodus 4.7 L:W, 2.8x dactylus and unguis combined length, with two serrate ventrodistal setae and one serrate dorsal seta (2.3x dactylus and unguis combine length) and penicillate seta dorsally; dactylus 1.5x unguis.
Pereopod-5 (Figure 9F) overall 5.5 L:W; basis 2.7 L:W, 5.0x merus, with simple ventroproximal seta and short penicillate midventral seta; ischium with two ventral setae; merus 1.3 L:W, 0.4x carpus, with seta and spine; carpus 3.0 L:W, 1.2x propodus, with dorsodistal seta, two distal spines and short blade-like spine (0.2x propodus); propodus 4.6 L:W, 2.9x dactylus and unguis combined length, with two serrate ventrodistal setae and serrate dorsodistal seta (2.9x dactylus and unguis combined length); dactylus 1.7x unguis.
Pereopod-6 (Figure 9G) overall 5.4 L:W; basis 2.7 L:W, 4.6x merus, with proximal penicillate seta; ischium with two ventral setae; merus 1.6 L:W, 0.5x carpus, with seta and spine; carpus 2.7 L:W, 1.2x propodus, with one seta, two spines and short blade-like spine (0.3x propodus); propodus 4.6 L:W, 2.9x dactylus and unguis combined length, with two serrate ventral setae and two serrate dorsal setae (longer seta 2.5x dactylus and unguis combined length); dactylus 1.7x unguis.
Pleopods (Figure 9H) rami long and slender, exopod with five, endopod with nine setae.
Uropod (Figure 9I) peduncle 1.2 L:W; exopod with two articles; 9.5 L:W; article-1 6.0 L:W, with subdistal seta; article-2 4.7 L:W, with two setae distally; endopod 7.7 L:W; article-1 3.5 L:W, with one simple and one penicillate setae; article-2 4.5 L:W, with one subdistal and two simple setae, and one penicillate distal seta. Exopod 0.8x endopod.
Distribution. Species known only from the type locality off Cape Otway (SE Australia) at depth 1021 m.
Remarks. The combination of antenna articles 2–3 with spines, coronal mandible molar, short ventrodistal setae on the pereopod-1 merus and carpus, and slender uropods places P. coonawarrai sp. nov. in the “denticulatus + abathagastor” group. A spine on the antenna article-2 distinguishes the new species from P. abathagastor and P. mariae, which have a seta on this article, and from P. barnesi, which has this article naked. The uropod exopod, shorter than the endopod (0.8x), separates P. coonawarrai from P. chaplini and P. oloughlini, where exopod is 1.1x endopod, and from P. palmeri where the exopod and endopod are equal. The presence of a spine and seta on the merus of pereopods 4–6 separates P. coonawarrai from P. biopearli, P. barossai, P. corollatus, P. georgesandae and P. locueloae, which have a spine or two setae in this position. Finely, the absence of wide-based spines on pereopods 2–3 in P. coonawarrai is similar to P. denticulatus and P. kitsoni although it can be distinguished by a short pereopod-2 with an overall proportion of 9.6 L:W compared to > 13 L:W in P. denticulatus and P. kitsoni.
Key for the identification of Pseudotanais females of the “denticulatus + abathagastor” morpho-group.
-
1.
Antenna article-3:
with spine or seta……………………………………………………………..2
naked…………………………………………………………………P. barnesi
-
2.
Antenna article-3 with
seta………………………………………………………………………………… 3
spine……………………………………………………………………………… 4
-
3.
Antenna article-2 with; pereopods 4–6 merus with:
thin spine; spine and seta……………………………..P. amundseni
spine; two spines……………………………………………….. P. elephas
seta; spine…………………………………………………………..P. mariae
-
4.
Pereopods 4–6 ischium with:
one seta…………………………………………………………………………..5
two setae…………………………………………………………………………6
-
5.
Antenna article-4 L:W ratio:
< 7.0 L:W……………………………………………………………………….7
> 9.2 L:W………………………………………………………P. locueloae
-
6.
Antenna article-4 L:W ratio
< 8.2 L:W……………………………………………………………………….9
> 8.3 L:W……………………………………………………………………..10
-
7.
Antenna article-2 with:
spine……………………………………………………………………………….8
seta………………………………………………………….. P. abathagastor
-
8.
Uropod exopod to endopod ratio:
0.8x……………………………………………………………… P. livingstoni
1.1x. ………………………………………………………………P. oloughlini
-
9.
Antenna article-2 with:
spine. ……………………………………………………………………………12
seta…………………………………………………………………… P. chopini
-
10.
Uropod exopod to endopod ratio:
≤ 0.9x0…………………………………………………………………………11
≥ 1.1x. …………………………………………………………….P. chaplini
-
11.
Uropod endopod L:W ratio; pereopod-3 carpus blade-like spine to propodus ratio; pereopod-6 carpus blade-like to propodus ratio:
9.0 L:W; 0.4x; 0.4x………………………………………….. P. biopearli
9.6 L:W; 0.5x; 0.3x…………………………………….. P. denticulatus
6.6 L:W; 0.3x; 0.2x…………………………………… P. georgesandae
-
12.
Pereonite-1 to pereonite-2 length ratio:
≤ 0.6x…………………………………………………………………………..13
0.9x………………………………………………………………..P. corollatus
-
13.
Uropod exopod L:W ratio:
≥ 8.2x…………………………………………………………………………..14
6.6x………………………………………………………P. barossai sp. nov.
-
14.
Uropod endopod L:W ratio:
≤ 8.4 L:W……………………………………………………………………..15
10.0 L:W……………………………………………………………. P. kitsoni
-
15.
Antenna article-4 L:W; uropod exopod to endopod ratio:
6.7 L:W; 1.0x……………………………………………………..P. palmeri
6.2 L:W; 0.8x………………………………….P. coonawarrai sp. nov.
Pseudotanais shirazi sp. nov.
-
This species is registered in ZooBank number: LSIDurn:lsid:zoobank.org:act:A52BE5EE-0195-4981-BBB2-7D953966095F.
Diagnosis. Antenna article 2–3 with seta. Mandible molar acuminate, with distal spines. Maxilliped endites fused with distinct central cleft, with one simple seta and two inner-distal tubercles. Chela non-forcipate, smooth on dorsal margin. Pereopods 4–6 unguis simple (not bifurcated).
Material examined. Holotype, neuter 1.8 mm (J59677), SLOPE 67. Paratype, female dissected 2.1mm (J61517), neuter 1.9 mm (J74952), SLOPE 40; neuter, broken (J62733), SLOPE 53.
Etymology. Shiraz is a grape varied, mostly used in Australia and South Africa, as genitive.
Description of female. BL = 2.1 mm. Body robust (Figures 10A,B) 3.3 L:W. Cephalothorax 0.8 L:W, 1.3x pereonites 1–3, 0.2x BL, with subocular pair of setae on. Pereonites 0.8x BL, pereonite-1 0.4x pereonit-2, pereonites-1–6: 0.1, 0.2, 0.3, 0.5, 0.6, and 0.3 L:W, respectively; pereonite 1 with midlateral seta; pereonites 3–5 each with a pair of small lateral setae. Pleon short, 0.2x BL. Pleonites 0.7 L:W, pleonite-4 with pair of dorsal setae, pereonite-5 with two pairs of setae on each side of midline. Pleotelson 1.2x pleonite-5, with pair of simple and penicillate laterodistal setae.
FIGURE 10
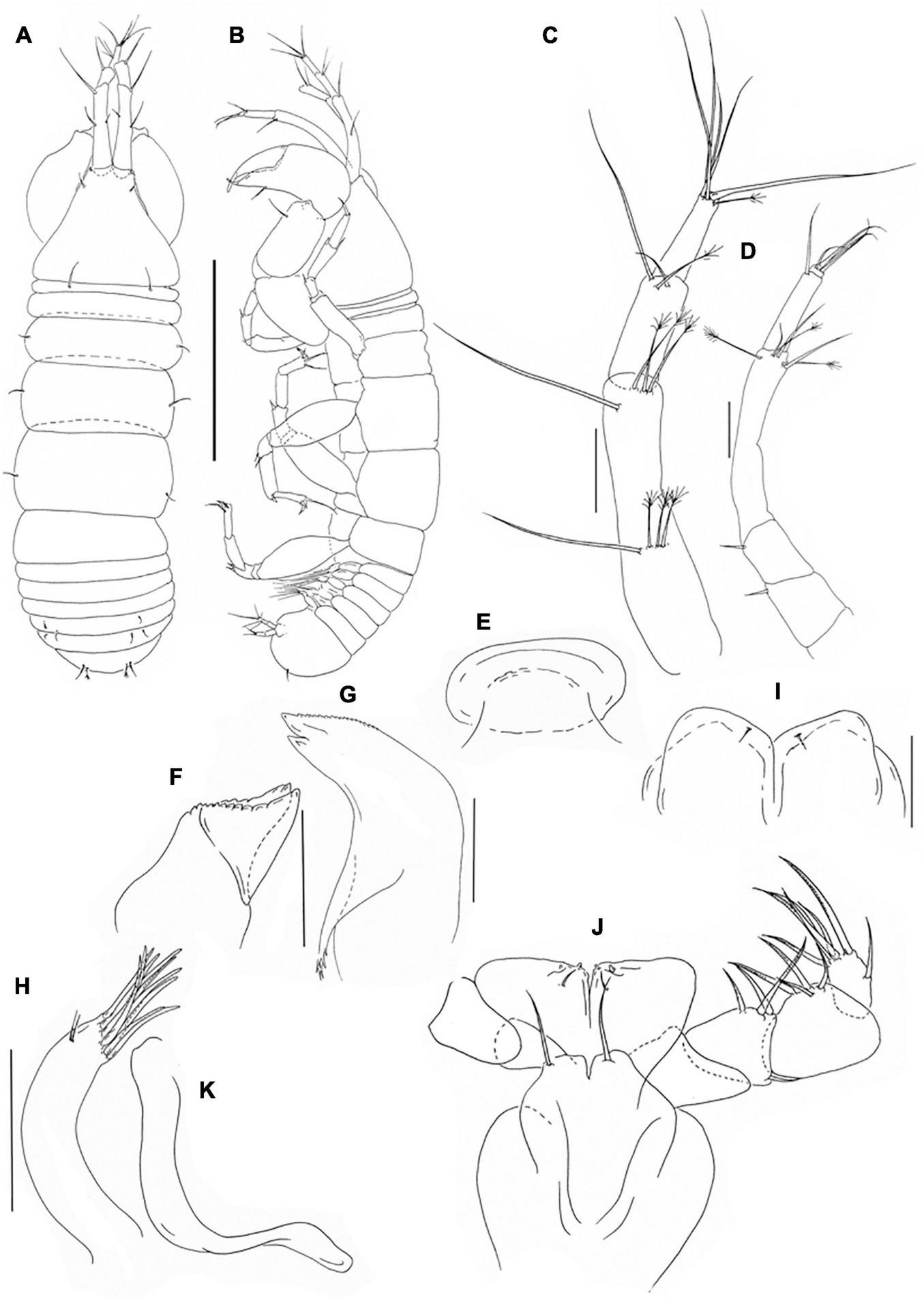
Pseudotanais shirazi sp. nov; neuter (J59677), (A), dorsal; (B), lateral; (C), antennule; (D), antenna; (E), labrum; (F), left mandible; (G), right mandible; (H), maxillule; endite; (I), labium; (J), maxilliped; (K), epignath. Scale lines (A,B) = 1 mm, (C–J) = 0.1 mm.
Antennule (Figure 10C) article-1 3.9 L:W, 2.5x article-2, with one simple and three penicillate midlength setae, and one simple subdistal and three penicillate distal setae; article-2 2.5 L:W, 1.3x article-3, with two simple and one penicillate distal setae; article-3 2.5 L:W, with five simple setae and one penicillate seta, aesthetasc not seen.
Antenna (Figure 10D) article-1 destroyed during dissection; article-2 1.3 L:W; 1.3x article-3, with seta (0.3x article-2); article-3 1.3 L:W, 0.3x article-4, with seta (0.5x article-3); article-4 6.3 L:W, 2.0x article-5, with three simple and three penicillate subdistal or distal setae; article-5 3.2 L:W, 9.5x article-6, with simple seta; article-6 0.5 L:W, with four simple setae.
Labrum (Figure 10E) rounded, naked. Left mandible (Figure 10F) lacinia mobilis well developed, distally serrate, incisor distal margin beveled, serrate. Right mandible (Figure 10G) incisor unequally bifid, distal margin serrate, molar acuminate with distal spines. Labium (Figure 10I) simple, slightly rectangular, glabrous. Maxillule (Figure 10H) endite with ten distal spines and several outer subdistal setae. Maxilla not recovered. Maxilliped (Figure 10J) basis with seta, little shorter than endites; palp article-1 naked, article-2 1.2 L:W with fine outer and three inner setae; article-3 1.4 L:W with one shorter and three longer inner setae; article-4 1.9 L:W with one subdistal and five distal setae.
Maxilliped endites mostly fused but with distinct central cleft, each with small middle seta and two gustatory cusps. Epignath (Figure 10K) linguiform, simple distally rounded.
Cheliped (Figure 11A) basis distally broken; merus with ventral seta; carpus 1.3 L:W, 1.0x palm, with two midventral setae, and one mid-dorsal and one dorsodistal small setae; chela non-forcipate, palm 1.3 L:W; fixed finger 3.0 L:W, 0.8x palm with one ventral seta, three setae on cutting edge, and one simple seta near dactylus insertion; dactylus 3.8 L:W, cutting edge smooth, without proximal seta.
FIGURE 11
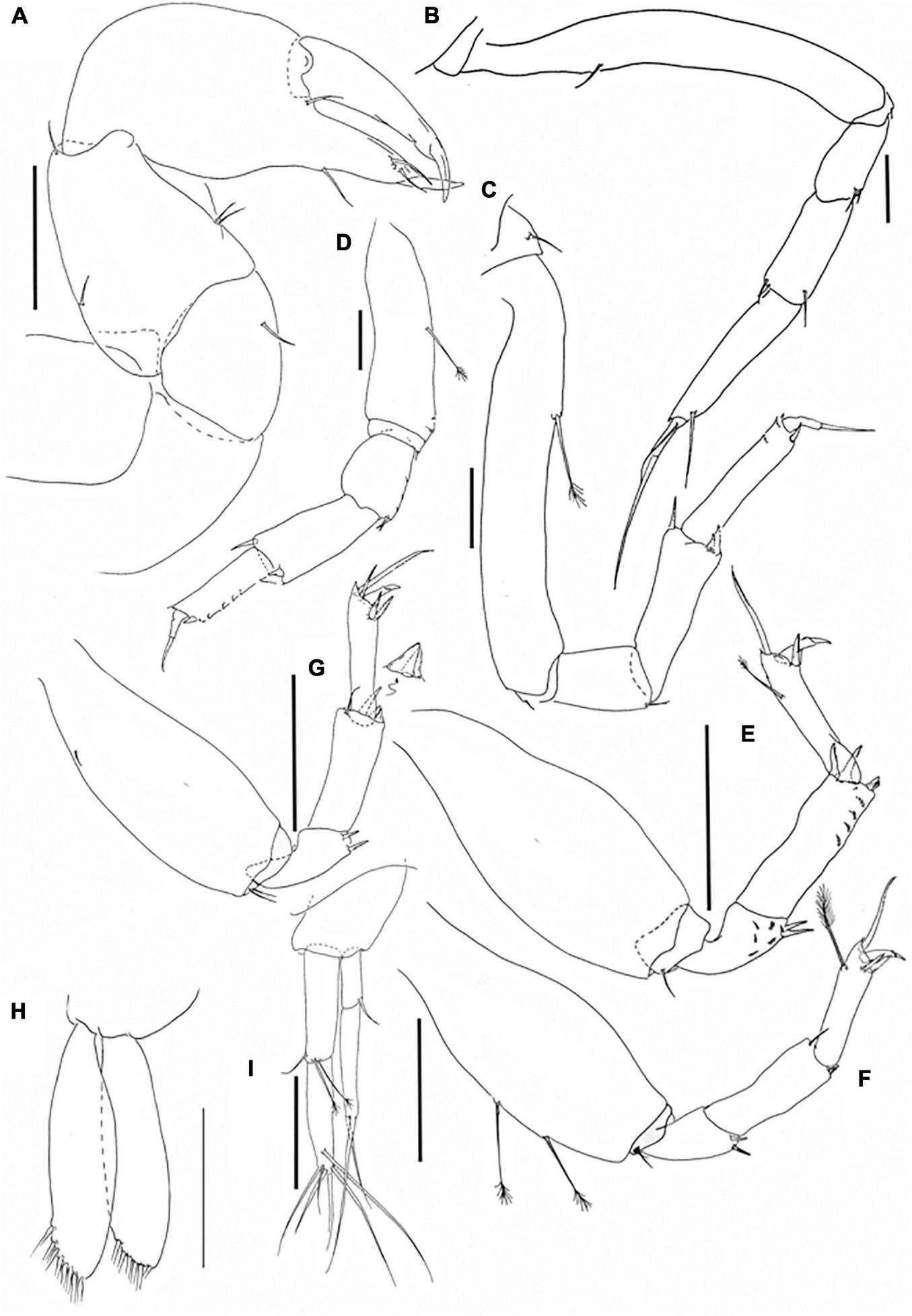
Pseudotanais shirazi sp. nov.; neuter (J74952), (A), cheliped; (B), pereopod-1; (C), pereopod-2, (D), pereopod-3; (E), pereopod-4; (F), pereopod-5; (G), pereopod-6; (H), pleopod; (I), uropod. Scale lines = 0.1 mm.
Pereopod-1 (Figure 11B) overall 14.3 L:W; basis 7.5 L:W, 4.2x merus, with dorsoproximal seta; ischium with ventral seta; merus 2.0 L:W and 0.9x carpus, with two ventrodistal setae (short and long); carpus 2.5 L:W, 0.7x propodus, with two dorsodistal and one ventrodistal setae; propodus 4.3 L:W, 0.9x dactylus and unguis combined length, with one long ventrodistal seta; dactylus 0.4x unguis with proximal seta.
Pereopod-2 (Figure 11C) overall 13.4 L:W; coxa with seta; basis 7.0 L:W, 4.5x merus, with middorsal penicillate seta; ischium with ventral seta; merus 1.5 L:W, 0.6x carpus, with ventrodistal seta; carpus 2.5 L:W, 1.0x propodus, with dorsodistal spine, short ventrodistal spine and short blade-like spine (0.2x propodus); propodus 5.2 L:W, 1.5x dactylus and unguis combined length, with ventroproximal seta (0.2x dactylus and unguis combined length); dactylus 0.7x unguis.
Pereopod-3 (Figure 11D) overall 19.3 L:W; basis 2.6 L:W, 2.1x merus, with midventral penicillate seta; ischium with ventral seta; merus 1.3 L:W, 0.8x carpus, with two ventrodistal setae; carpus 2.0 L:W, 1.1x propodus, with dorsodistal spine, short ventrodistal spine and short blade-like spine (0.2x propodus); propodus 3.6 L:W, 1.6x dactylus and unguis combined length, with ventrodistal spine (0.3x dactylus); dactylus 0.8x unguis.
Pereopod-4 (Figure 11E) overall 5.0 L:W; basis 2.3 L:W, 3.7x merus, naked; ischium with ventral seta (second seta not seen); merus 1.5 L:W, 0.6x carpus, with two ventrodistal spines and several comb-like scales; carpus 3.3 L:W, 1.2x propodus, with two long spines and short blade-like spine (0.1x propodus); propodus 4.2 L:W, 3.6x dactylus and unguis combined length, with penicillate dorsal setae, two serrate ventrodistal spines and one serrate dorsodistal seta (1.5x dactylus and unguis combined length); dactylus 1.8x unguis.
Pereopod-5 (Figure 11F) overall 4.6 L:W; basis 2.3 L:W, 3.9x merus, with two midventral penicillate setae; ischium with two ventral setae; merus 1.6 L:W, 0.6x carpus, with two spines; carpus 2.8 L:W, 1.1x propodus, with dorsodistal seta and short blade-like spine (0.1x propodus) (spines not seen); propodus 4.0 L:W, 3.3x dactylus and unguis combined length, with penicillate dorsal seta, two serrate ventrodistal spines, and one serrate dorsal seta (1.5x dactylus and unguis combined length); dactylus 3.0x unguis.
Pereopod-6 (Figure 11G) overall 5.4 L:W; basis 2.5 L:W, 3.3x merus, with minute ventroproximal seta; ischium with two ventral setae; merus 2.0 L:W, 0.8x carpus, with two spines; carpus 1.2 L:W, 0.9x propodus, with dorsodistal seta, two distal spines, and short blade-like spine (0.1x propodus); propodus 4.8 L:W, 6.0x dactylus and unguis combined length, with two serrate ventral setae and two serrate dorsal setae (longer setae 2.5x dactylus and unguis combined length); dactylus 2.0x unguis.
Pleopods (Figure 11H) rami long and slender, exopod with six, endopod with eight setae.
Uropod (Figure 11I) peduncle 1.3 L:W; exopod with two articles; 7.8 L:W; article-1 1.3 L:W, with distal seta; article-2 2.8 L:W, with two long setae; endopod 7.0 L:W; article-1 3.5 L:W, with one simple and two penicillate setae; article-2 4.2 L:W, with one subdistal and five distal setae. Exopod 0.7x endopod.
Distribution. The species is known from off Gippsland and Jervis Point SE Australia, at depths 400–1277 m.
Remarks. Pseudotanais shirazi sp. nov., with short conical blade like-spines on the carpus of pereopods 2–6, is the second species after P. intortus with this shape. Its maxilliped endites with a distinct medial cleft and each with one simple seta and two tubercles, distinguish it from P. intortus where the maxilliped endites are fused and each have only one tubercle. Additionally, the blade-like spine in pereopod-2 in P. shirazi is conical while, in P. intortus pereopod-2 is more flattened, with the cavity in the central part. A short propodal seta on pereopods 2–3 (0.2x dactylus and unguis combined length) in P. shirazi is different from P. intortus, where this spine is almost as long as dactylus and unguis combined length (0.8x). Finally, the pereopods 4–6 unguis is simple in contrast to P. intortus with a bifurcated unguis.
Discussion
The present study provides for the first time information about Pseudotanaidae species from the continental margin of SE Australia near Bass Strait. From five species, two occurred off eastern coast between Gippsland and Jervis Point (P. shirazi and P. chardonnayi), and three on the southern coast between Great Otway (Otway Point) and Kangaroo Island (P. caberneti, P. barossai and P. coonawarrai) (Figure 12). These five species bring the total number of described pseudotanaid species to 94. Until now the family was represented in Australian waters by only one species – the shallow-water Akanthinotanais scrappi (Bamber, 2005). Remarkably, the family is apparently absent in the well sampled Bass Strait (Błażewicz-Paszkowycz and Bamber, 2012; Bamber and Błażewicz-Paszkowycz, 2013), but they were recorded at the deeper shelf (around 100 m) and at the slope of West Australia (Bamber, 2005; McCallum et al., 2015; Poore et al., 2015); also, it was recorded in two locations of Great Barrier Reef e.g., Lizard and Heron Is; (Stȩpień et al., 2018). Unfortunately, these collections were not identified to species level.
FIGURE 12
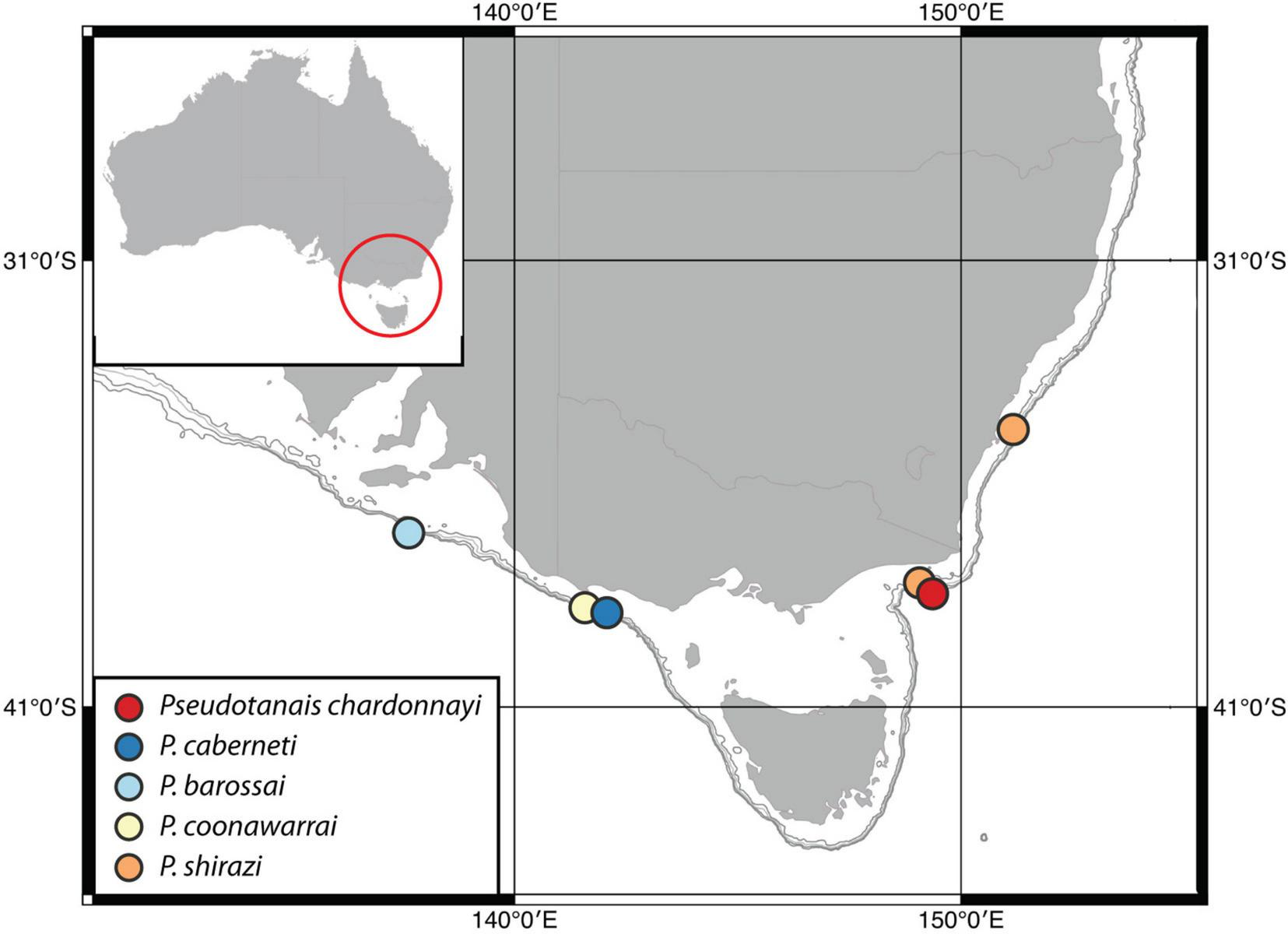
Distribution of Pseudotanaidae (described in this study) on the coast of SE Australia.
The Pseudotanaidae is cosmopolitan family that encompass all biogeographic zones (Watling et al., 2013). Collated literature date on the distribution of currently recognized pseudotanaid genera and the morpho-groups, allow to group pseudotanaids into few categories (Table 2 and Figure 13):
TABLE 2
| Group | Species | Ocean | Depth zone | Province |
| “affinis + longisetosus” | P. affinis | Atlantic | shelf | Arctic |
| “affinis + longisetosus” | P. macrocheles | Atlantic | shelf | Northern European Seas |
| “affinis + longisetosus” | P. rapunzelae | Southern | shelf | Scotia Sea |
| “affinis + longisetosus” | P. shackletoni | Southern | shelf | Scotia Sea |
| “affinis + longisetosus” | P. chardonnayi | Pacific | upper slope | NZ-Kermadec |
| “affinis + longisetosus” | P. caberneti | Pacific | upper slope | Subantarctic |
| “affinis + longisetosus” | P. longispinus | Atlantic | lower slope | North Atlantic |
| “affinis + longisetosus” | P. scalpellum | Atlantic | lower slope | North Atlantic |
| “affinis + longisetosus” | P. spatula | Atlantic | lower slope | North Atlantic |
| “affinis + longisetosus” | P. svavarssoni | Atlantic | lower slope | North Atlantic |
| “affinis + longisetosus” | P. nipponicus | Pacific | lower slope | Northern Pacific Boreal |
| “affinis + longisetosus” | P. longisetosus | Southern | lower slope | Antarctic |
| “affinis + longisetosus” | P. nordenskioldi | Southern | lower slope | Antarctic |
| “affinis + longisetosus” | P. gaiea | Pacific | abyssal | Equatorial Pacific |
| “affinis + longisetosus” | P. geralti | Pacific | abyssal | Equatorial Pacific |
| “affinis + longisetosus” | P. julietae | Pacific | abyssal | Equatorial Pacific |
| “affinis + longisetosus” | P. romeo | Pacific | abyssal | Equatorial Pacific |
| “affinis + longisetosus” | P. uranos | Pacific | abyssal | Equatorial Pacific |
| “affinis + longisetosus” | P. yenneferae | Pacific | abyssal | Equatorial Pacific |
| “affinis + longisetosus” | P. chanelae | Pacific | abyssal | North Pacific |
| “affinis + longisetosus” | P. curieae | Pacific | abyssal | North Pacific |
| “affinis + longisetosus” | P. monroeae | Pacific | abyssal | North Pacific |
| “affinis + longisetosus” | P. szymborskae | Pacific | abyssal | North Pacific |
| “affinis + longisetosus” | P. vitjazi | Pacific | abyssal | North Pacific |
| Akanthinotanais | A. breviaquas | Atlantic | shelf | Lusitanian |
| Akanthinotanais | A. siegi | Atlantic | shelf | Lusitanian |
| Akanthinotanais | A. similis | Atlantic | shelf | Northern European Seas |
| Akanthinotanais | A. mortenseni | Atlantic | shelf | Warm Temperate Northwest Atlantic |
| Akanthinotanais | A. scrappi | Indian | shelf | Southwest Australian Shelf |
| Akanthinotanais | A. gerlachi | Indian | shelf | Central Indian Ocean Islands |
| Akanthinotanais | A. malayensis | Pacific | shelf | Marshall, Gilbert, and Ellis Islands |
| Akanthinotanais | A. pedecerritulus | Indian | shelf | South Kuroshio |
| Akanthinotanais | A. gaussi | Southern | shelf | Scotia Sea |
| Akanthinotanais | A. guillei | Southern | shelf | Scotia Sea |
| Akanthinotanais | A. rossi | Southern | shelf | Scotia Sea |
| Akanthinotanais | A. kurchatovi | Atlantic | upper slope | N Atlantic |
| Akanthinotanais | A. makrothrix | Pacific | upper slope | Cocos Plate |
| Akanthinotanais | A. longipes | Atlantic | lower slope | Northern Atlantic Boreal |
| Beksitanais | B. vanhoeffeni | Southern | shelf | Amundsen/Bellingshausen Sea |
| Beksitanais | B. abyssi | Atlantic | lower slope | Northern Atlantic Boreal |
| Beksitanais | B. apocalyptica | Pacific | abyssal | Equatorial Pacific |
| “colonus” | P. borceai | Atlantic | shelf | Black Sea |
| “colonus” | P. lilljeborgi | Atlantic | shelf | Northern European Seas |
| “colonus” | P. falcifer | Atlantic | upper slope | N Atlantic |
| “colonus” | P. sigrunis | Atlantic | upper slope | N Atlantic |
| “colonus” | P. colonus | Atlantic | lower slope | North Atlantic |
| “colonus” | P. baresnauti | Atlantic | abyssal | North Atlantic |
| “denticulatus + abathagastor” | P. crassicornis | Atlantic | shelf | Arctic |
| “denticulatus + abathagastor” | P. amundseni | Southern | shelf | Amundsen/Bellingshausen Sea |
| “denticulatus + abathagastor” | P. barnesi | Southern | shelf | Amundsen/Bellingshausen Sea |
| “denticulatus + abathagastor” | P. biopearli | Southern | shelf | Amundsen/Bellingshausen Sea |
| “denticulatus + abathagastor” | P. kitsoni | Southern | shelf | Amundsen/Bellingshausen Sea |
| “denticulatus + abathagastor” | P. elephas | Southern | shelf | Scotia Sea |
| “denticulatus + abathagastor” | P. livingstoni | Southern | shelf | Scotia Sea |
| “denticulatus + abathagastor” | P. palmeri | Southern | shelf | Scotia Sea |
| “denticulatus + abathagastor” | P. abathagastor | Pacific | upper slope | N Pacific Boreal |
| “denticulatus + abathagastor” | P. denticulatus | Atlantic | lower slope | North Atlantic |
| “denticulatus + abathagastor” | P. corollatus | Atlantic | lower slope | Northern Atlantic Boreal |
| “denticulatus + abathagastor” | P. coonawarrai | Pacific | lower slope | Subantarctic |
| “denticulatus + abathagastor” | P. barrossai | Pacific | lower slope | Subantarctic |
| “denticulatus + abathagastor” | P. chaplini | Pacific | abyssal | Equatorial Pacific |
| “denticulatus + abathagastor” | P. chopini | Pacific | abyssal | Equatorial Pacific |
| “denticulatus + abathagastor” | P. georgesandae | Pacific | abyssal | Equatorial Pacific |
| “denticulatus + abathagastor” | P. mariae | Pacific | abyssal | Equatorial Pacific |
| “denticulatus + abathagastor” | P. oloughlini | Pacific | abyssal | Equatorial Pacific |
| “denticulatus + abathagastor” | P. locueolae | Pacific | abyssal | North Pacific |
| “forcipatus” | P. isabelae | Atlantic | shelf | Mediterranean Sea |
| “forcipatus” | P. mediterraneus | Atlantic | shelf | Mediterranean Sea |
| “forcipatus” | P. stiletto | Atlantic | shelf | Mediterranean Sea |
| “forcipatus” | P. unicus | Atlantic | shelf | Mediterranean Sea |
| “forcipatus” | P. forcipatus | Atlantic | shelf | Northern European Seas |
| “forcipatus” | P. jonesi | Atlantic | shelf | Northern European Seas |
| “forcipatus” | P. mexikolpos | Atlantic | shelf | Warm Temperate Northwest Atlantic |
| “forcipatus” | P. californensis | Pacific | shelf | Tropical East Pacific |
| “forcipatus” | P. enduranceae | Southern | shelf | Amundsen/Bellingshausen Sea |
| “forcipatus” | P. discoveryae | Southern | shelf | Scotia Sea |
| “forcipatus” | P. scotti | Southern | shelf | Scotia Sea |
| “forcipatus” | P. artoo | Atlantic | upper slope | S Atlantic |
| “forcipatus” | P. soja | Pacific | upper slope | N Pacific Boreal |
| “forcipatus” | P. falcicula | Atlantic | lower slope | North Atlantic |
| “forcipatus” | P. vulsella | Atlantic | lower slope | North Atlantic |
| “forcipatus” | P. misericorde | Atlantic | lower slope | Northern Atlantic Boreal |
| “forcipatus” | P. inflatus | Pacific | abyssal | North Pacific |
| not classified | P. intortus | Pacific | upper slope | N Pacific Boreal |
| Mystriocentrus | M. serratus | Atlantic | lower slope | North Atlantic |
| Mystriocentrus | M. biho | Atlantic | lower slope | Northern Atlantic Boreal |
| Mystriocentrus | M. hollandae | Pacific | abyssal | North Pacific |
| not classified | P. oculatus | Atlantic | shelf | Arctic |
| Parapseudotanais | P. abyssalis | Atlantic | abyssal | Arctic Basin |
| not classified | P. shirazi | Pacific | upper slope | NZ-Kermadec |
| spicatus | P. tympanobaculum | Pacific | upper slope | N Pacific Boreal |
| spicatus | P. spicatus | Atlantic | lower slope | North Atlantic |
| spicatus | P. kobro | Pacific | abyssal | Equatorial Pacific |
Classification of Pseudotanaidae to genera and morpho-groups according to Bird and Holdich (1989b) and McLelland (2007).
The zoogeographical and bathymetrical classification according to Spalding et al. (2007) and Watling et al. (2013).
FIGURE 13
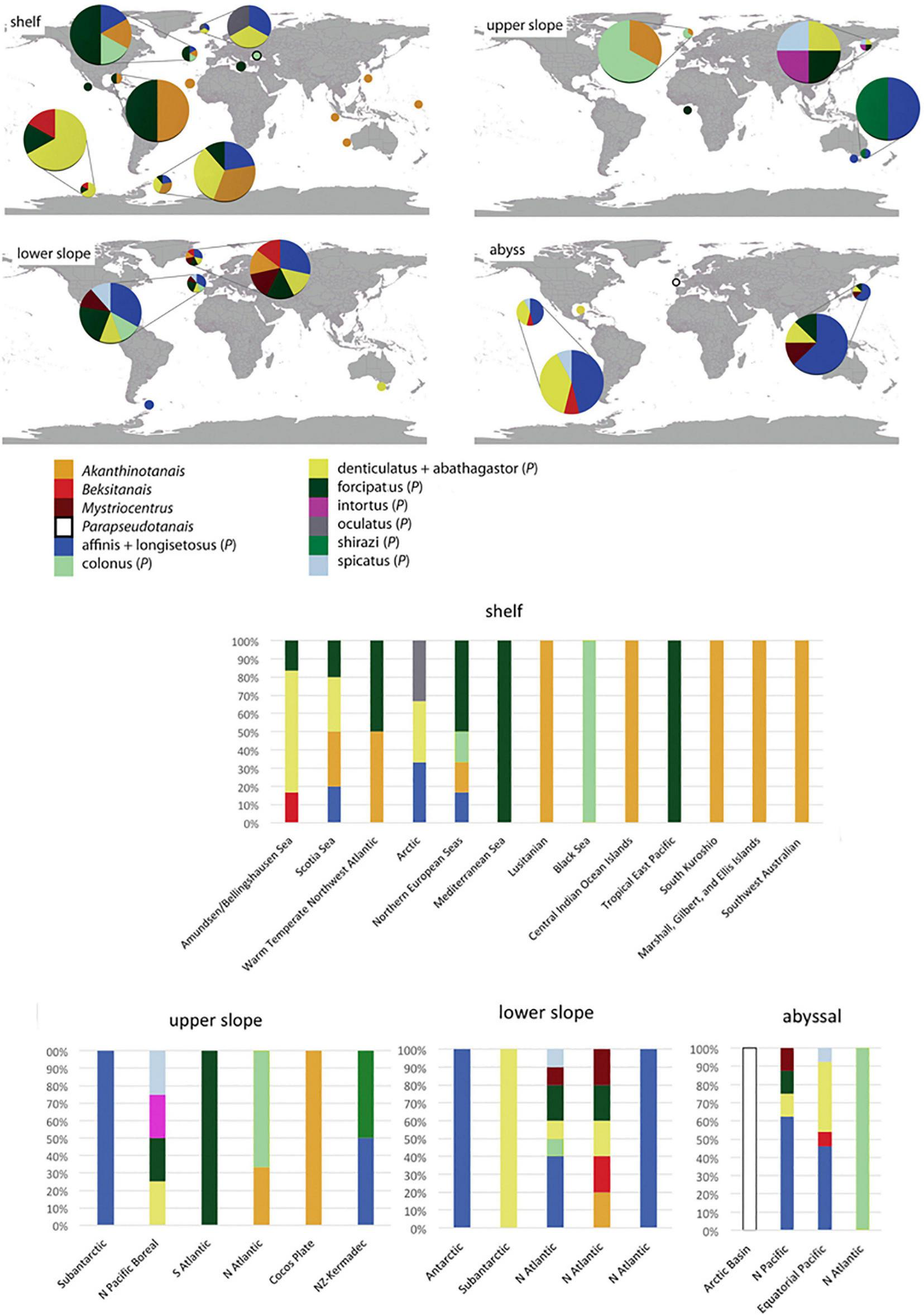
Distribution of pseudotanaid genera and morpho-groups according to Bird and Holdich (1989b) and McLelland (2007) (for details see Table 2). The zoogeographical and bathymetrical classification according to Spalding et al. (2007) and Watling et al. (2013). The size of the pies corresponds to the number of species recorded.
-
-
Akanthinotanais and “forcipatus” can be common on the shelf from the tropics to polar regions and have been only occasionally recorded below the continental margin (Sieg, 1977; Bird and Holdich, 1989a,b) or the abyssal (Jakiel et al., 2019). The former is still relatively understudied because of its relative scarcity, and exhibits a range of morphologies that may encompass several genera, even in a separate family;
-
-
“denticulatus + abathagastor” and “affinis + longisetosus” represent deep-sea fauna, but several species have been recorded on the shelf of polar regions. This distribution supports a polar emergence phenomenon observed for several taxa (Wilson, 1998; Berkman et al., 2004; Błażewicz-Paszkowycz, 2005; Raupach et al., 2012). With some probability, this group could also be represented by Beksitanais, although this assumption could be revised when more records become available;
-
-
“spicatus” is recorded on the upper, lower slope and in the abyss;
-
-
Parapseudotanais is recorded only from the abyss;
-
-
Mystriocentrus is known from lower slope and the abyss.
To confirm that Parapseudotanais and Mystriocentrus are deep-water genera requires more data. The species provisionally classified to the “colonus” group does not reveal a clear distribution pattern that suggest an artificial (non-monophyletic) character of the group.
Apart from the Pseudotanaidae, in general, the peracarid fauna of Australian coast is very diverse (Poore et al., 1994; Lowry and Stoddard, 2003; Poore and Bruce, 2012). With that background, tanaids are represented by 162 species in 66 genera (Edgar, 1997, 2008, 2012; Bamber, 2005, 2008; Błażewicz-Paszkowycz and Bamber, 2007, 2009, 2012; Jóźwiak and Błażewicz, 2021). This situation is apparently worse at the shelf break where only nine species from seven genera and four families (Apseudidae: one species; Agathotanaidae: two species, Anarthruridae six species, Paratanaidae: one species) are formally described (three species were described from SE Australia) (Larsen and Heard, 2001; Jóźwiak and Jakiel, 2012; Bamber and Błażewicz-Paszkowycz, 2013; Gellert and Błażewicz, 2018). For this reason tanaids are regarded as a comparatively non-diverse group, especially when compared to the other well studied taxa as Isopoda being represented in SE Australia by 51 families (Poore et al., 1994). However, exploration of the deeper shelf and slope of W Australia (McCallum et al., 2015; Poore et al., 2015) proves that tanaids below the continental break are diverse and the perceived lack of diversity mentioned above may be an illusion. The collection of Pseudotanaidae that we studied here is too limited to draw a conclusion about zoogeographical relationships and their link to the complex geological/tectonic history of SE Australia.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
This article is registered in ZooBank number: LSID urn:lsid:zoobank.org:pub:B15F3ACF-2FCC-4A3F-9EDC-0811EE99B9C6.
Author contributions
MB did the general concept and identification of the material. MB and AJ did the species, description, manuscript editing and figures editing. GB did the discussion and manuscript editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by NCN Sonatina (2020/36/C/NZ8/005 76) and NCN OPUS (2018/31/B/3115 NZ8/03198).
Acknowledgments
We would like to thank to the staff from the Department Marine Invertebrate of Melbourne Museum (VIC, Australia), especially to Gary Poore for collecting the material, and to Mel McKenzie and Jo Taylor for careful curating that lovely and unique tanaid collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Bamber R. N. (2005). The tanaidaceans (Arthropoda: Crustacea: Peracarida: Tanaidacea) of Esperance, Western Australia, Australia.Mar. Flora Fauna Esperance West. Aust. West. Aust. Museum Perth1963613–728.
2
Bamber R. N. (2008). Tanaidaceans (Crustacea: Peracarida: Tanaidacea) from Moreton Bay, Queensland.Mem. Queensl. Museum54143–218.
3
Bamber R. N. Błażewicz-Paszkowycz M. (2013). Another inordinate fondness: diversity of the tanaidacean fauna of Australia, with description of three new taxa.J. Nat. Hist.4725–28. 10.1080/00222933.2012.742164
4
Bamber R. N. Bird G. Błażewicz-Paszkowycz M. Galil B. (2009). Tanaidaceans (Crustacea: Malacostraca: Peracarida) from soft-sediment habitats off Israel, eastern Mediterranean.Zootaxa21091–44.
5
Berkman P. A. Cattaneo-Vietti R. Chiantore M. Howard-Williams C. (2004). Polar emergence and the influence of increased sea-ice extent on the Cenozoic biogeography of pectinid molluscs in Antarctic coastal areas.Deep. Res. Part II Top. Stud. Oceanogr.511839–1855. 10.1016/j.dsr2.2004.07.017
6
Bird G. J. (1999). A new species of Pseudotanais (Crustacea, Tanaidacea) from cold seeps in the deep Caribbean, collected by the French submersible Nautile.Zoosystema21445–451.
7
Bird G. J. Holdich D. M. (1984). New deep-sea leptognathiid tanaids (Crustacea, Tanaidacea) from the north-east Atlantic. Zool. Scr.13, 285–315. 10.1111/j.1463-6409.1984.tb00044.x
8
Bird G. J. Holdich D. M. (1989b). Tanaidacea (Crustacea) of the north−east Atlantic: the subfamily Pseudotanainae (Pseudotanaidae) and the family Nototanaidae.Zool. J. Linn. Soc.97233–298. 10.1111/j.1096-3642.1989.tb00548.x
9
Bird G. J. Holdich D. M. (1989a). Recolonisation of artificial sediments in the deep Bay of Biscay by tanaidaceans (Crustacea: Peracarida), with a description of a new species of Pseudotanais.J. Mar. Biol. Assoc. U K.69307–317. 10.1017/S0025315400029428
10
Błażewicz M. Jakiel A. Bamber R. N. Bird G. J. (2021). Pseudotanaidae Sieg, 1976 (Crustacea: Peracarida) from the Southern Ocean: diversity and bathymetric pattern.Eur. Zool. J.20211–76.
11
Błażewicz M. Stȩpień A. Jakiel A. Palero F. (2020). “Biogeographic atlas of the deep NW Pacific fauna,” in Biogeographic Atlas of the Deep NW Pacific Fauna, edsSaeediH.BrandtA. (Sofia: Pensoft), 462–500. 10.3897/ab.e51315
12
Błażewicz-Paszkowycz M. (2005). Revision of the genus Peraeospinosus Sieg, 1986 (Crustacea: Peracarida: Tanaidacea).J. Nat. Hist.393847–3901. 10.1080/00222930500450879
13
Błażewicz-Paszkowycz M. Bamber R. N. (2007). New apseudomorph tanaidaceans (Crustacea: Peracarida: Tanaidacea) from eastern Australia: Apseudidae, Whiteleggiidae, Metapseudidae and Pagurapseudidae.Mem. Museum Victoria64107–148.
14
Błażewicz-Paszkowycz M. Bamber R. N. (2009). A new genus of a new austral family of paratanaoid tanaidacean (Crustacea: Peracarida: Tanaidacea), with two new species.Mem. Museum Victoria665–15.
15
Błażewicz-Paszkowycz M. Bamber R. N. (2011). Tanaidomorph tanaidacea (crustacea: Peracarida) from mud-volcano and seep sites on the Norwegian Margin.Zootaxa30611–35.
16
Błażewicz-Paszkowycz M. Bamber R. N. (2012). The shallow-water Tanaidacea (Arthropoda: Malacostraca: Peracarida) of the Bass Strait, Victoria, Australia (other than the Tanaidae).Mem. Museum Victoria691–235. 10.24199/j.mmv.2012.69.01
17
Edgar G. J. (1997). A new genus and three new species of Apseudomorph Tanaidacean (Crustacea) From the Darwin region.Proc. Sixth Int. Mar. Biol. Work. Mar. Flora Fauna Darwin Harbour North Territ. Aust.1997279–299.
18
Edgar G. J. (2008). Shallow water Tanaidae (Crustacea: Tanaidacea) of Australia.Zootaxa18361–92.
19
Edgar G. J. (2012). New Leptocheliidae (Crustacea: Tanaidacea: Tanaidomorpha) from Australian seagrass and macro-algal habitats, and a redescription of the poorly-known Leptochelia ignota from Sydney Harbour.Zootaxa32761–37. 10.11646/zootaxa.3926.4.10
20
Francesca P. Mevenkamp L. Pape E. Błażewicz M. Bonifácio P. Riehl T. et al (2021). A local scale analysis of manganese nodules influence on the Clarion-Clipperton Fracture Zone macrobenthos.Deep Res. Part I Oceanogr. Res. Pap.168:103449. 10.1016/j.dsr.2020.103449
21
Gellert M. Błażewicz M. (2018). New species of Anarthruridae (Tanaidacea: Crustacea) of the western Australian slope.Mar. Biodivers.49583–601. 10.1007/s12526-017-0826-9
22
Jakiel A. Palero F. Błażewicz M. (2019). Deep ocean seascape and Pseudotanaidae (Crustacea: Tanaidacea) diversity at the Clarion-Clipperton Fracture Zone.Sci. Rep.9:17305.
23
Jakiel A. Palero F. Błażewicz M. (2020). Secrets from the deep: Pseudotanaidae (Crustacea: Tanaidacea) diversity from the Kuril–Kamchatka Trench.Prog. Oceanogr.183:102288. 10.1016/j.pocean.2020.102288
24
Jakiel A. Stȩpień A. Błażewicz M. (2018). A tip of the iceberg — Pseudotanaidae (Tanaidacea) diversity in the North Atlantic.Mar. Biodivers.48859–895. 10.1007/s12526-018-0881-x
25
Jakiel A. Stȩpień A. Jóźwiak P. Serigstad B. Błażewicz-Paszkowycz M. (2015). First record of Tanaidacea (Crustacea) from a deep-sea coral reef in the Gulf of Guinea.Zootaxa3991203–228. 10.11646/zootaxa.3995.1.18
26
Jennings R. M. Etter R. J. Ficarra L. (2013). Population differentiation and species formation in the deep sea: the potential role of environmental gradients and depth.PLoS One8:e77594. 10.1371/journal.pone.0077594
27
Jóźwiak P. Błażewicz M. (2021). Muvi schmallenbergi gen. nov., sp. nov. (Crustacea, Tanaidacea) from the southeast Australian coast, with comments on the distribution and habitat preferences of Chondropodinae.PeerJ9:e11607. 10.7717/peerj.11607
28
Jóźwiak P. Jakiel A. (2012). A new genus and new species of Agathotanaidae (Crustacea, Tanaidacea) from West Australia.Zookeys24315–26. 10.3897/zookeys.243.3408
29
Kaiser S. Lörz A.-N. Bird G. Malyutina M. Bowden D. (2018). Benthic boundary layer macrofauna from the upper slope of the Chatham Rise (SW Pacific).Mar. Ecol.2018:e12521. 10.1111/maec.12521
30
Kudinova-Pasternak R. (1993). Tanaidacea (Crustacea, Malacostraca) collected in the 43 cruize of the R/V “Dmitri Mendeleev” in the South-western Atlantic and the Weddell Sea.– Trudy Instituta okeanologii.Akad. Nauk SSSR127134–145.
31
Lang K. (1949). Contribution to the systematics and synonymics of the Tanaidacea.Ark. För Zool.421–14.
32
Larsen K. Heard R. W. (2001). A new tanaidacean subfamily, Bathytanaidinae (Crustacea: Paratanaididae), from the Australian continental shelf and slope.Zootaxa191–22. 10.11646/zootaxa.19.1.1
33
Lowry J. K. Stoddard H. E. (2003). Zoological catalogue of Australia, 19.2B. Crustacea: Malacostraca: Peracarida: Amphipoda, Cumacea, Mysidacea.Canberra: CISRO Publishing.
34
McCallum A. W. Woolley S. Błażewicz-Paszkowycz M. Browne J. Gerken S. Kloser R. et al (2015). Productivity enhances benthic species richness along an oligotrophic Indian Ocean continental margin.Glob. Ecol. Biogeogr.24462–471. 10.1111/geb.12255
35
McLelland J. A. (2007). Family Pseudotanaidae Sieg, 1976.Zootaxa159987–99. 10.11646/zootaxa.1599.1.5
36
McLelland J. A. (2008). A Systematic and Taxonomic Review of the Family Pseudotanaidae (Crustacea: Peracarida: Tanaidacea) Based Primarily on Morphometric Cladistic Analyses. Ph. Dissetation, 298.
37
Menot L. Sibuet M. Carney R. S. Levin L. A. Rowe G. T. David S. M. et al (2010). “New perceptions of continental margin biodiversity,” in Life in the World’s Oceans: Diversity, Distribution, and Abundance, ed.McIntyreA. D. (Hoboken, NJ: Blackwell Publishing Ltd), 79–101.
38
O’Hara T. D. Williams A. Althaus F. Ross A. S. Bax N. J. (2020). Regional-scale patterns of deep seafloor biodiversity for conservation assessment.Divers. Distrib.26479–494. 10.1111/ddi.13034
39
Pabis K. Błażewicz-Paszkowycz M. Jóźwiak P. Barnes D. K. A. (2014). Tanaidacea of the Amundsen and Scotia seas: An unexplored diversity.Antarct. Sci.2719–30. 10.1017/S0954102014000303
40
Pabis K. Jóźwiak P. Lörz A.-N. N. Schnabel K. Błażewicz-Paszkowycz M. (2015). First insights into the deep-sea tanaidacean fauna of the Ross Sea: species richness and composition across the shelf break, slope and abyss.Polar Biol.381429–1437. 10.1007/s00300-015-1706-z
41
Poore G. C. B. Bruce N. L. (2012). Global diversity of marine isopods (except Asellota and crustacean symbionts).PLoS One7:e43529. 10.1371/journal.pone.0043529
42
Poore G. C. B. Avery L. Błażewicz-Paszkowycz M. Browne J. Bruce N. L. Gerken S. et al (2015). Invertebrate diversity of the unexplored marine western margin of Australia: taxonomy and implications for global biodiversity.Mar. Biodivers.45271–286. 10.1007/s12526-014-0255-y
43
Poore G. C. B. Just J. Cohen B. F. (1994). Composition and diversity of Crustacea Isopoda of the southeastern Australian continental slope.Deep Sea Res. Part I Oceanogr. Res. Pap.41677–693. 10.1016/0967-0637(94)90049-3
44
Raupach M. J. Mayer C. Malyutina M. Wägele J. W. Wagele J.-W. (2012). Multiple origins of deep-sea Asellota (Crustacea: Isopoda) from shallow waters revealed by molecular data.Proc. R. Soc. B Biol. Sci.276799–808. 10.1098/rspb.2008.1063
45
Sieg J. (1977). Taxonomische Monographie der Familie Pseudotanaidae (Crustacea, Tanaidacea).Mitteilungen Museum Naturkd Berlin Zool. Museum Inst. Spez. Zool.533–109. 10.1002/mmnz.4830530102
46
Spalding M. D. Fox H. E. Allen G. R. Davidson N. Ferdaña Z. A. Finlayson M. et al (2007). Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas.Bioscience57573–583. 10.1641/B570707
47
Stȩpień A. Pabis K. Błażewicz M. (2018). Small-scale species richness of the Great Barrier Reef tanaidaceans — results of the CReefs compared with worldwide diversity of coral reef tanaidaceans.Mar. Biodivers.491169–1185. 10.1007/s12526-018-0894-5
48
Watling L. Guinotte J. Clark M. R. Smith C. R. (2013). A proposed biogeography of the deep ocean floor.Prog. Oceanogr.11191–112. 10.1016/j.pocean.2012.11.003
49
Wilson G. D. F. (1998). Historical Influences on Deep-sea Isopod Diversity in the Atlantic Ocean.Oxford: Pergamon Press.
50
Zardus J. D. Etter R. J. Chase M. R. Rex M. A. Boyle E. E. (2006). Bathymetric and geographic population structure in the pan-Atlantic deep-sea bivalve Deminucula atacellana (Schenck, 1939).Mol. Ecol.15639–651. 10.1111/j.1365-294X.2005.02832.x
Summary
Keywords
Peracarida, taxonomy, diversity, continental margin, Pseudotanaidae
Citation
Błażewicz M, Jakiel A and Bird GJ (2021) Pseudotanais Sars, 1882 (Crustacea: Tanaidacea) From the SE Australian Slope: A Gap in Our Knowledge. Front. Mar. Sci. 8:779001. doi: 10.3389/fmars.2021.779001
Received
17 September 2021
Accepted
11 October 2021
Published
29 November 2021
Volume
8 - 2021
Edited by
Clara F. Rodrigues, University of Aveiro, Portugal
Reviewed by
Juliana Lopes Segadilha, National Museum, Federal University of Rio de Janeiro, Brazil; Pedro L. Ardisson, Center for Research and Advanced Studies - Mérida Unit, Mexico
Updates
Copyright
© 2021 Błażewicz, Jakiel and Bird.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Magdalena Błażewicz, magdalena.blazewicz@biol.uni.lodz.pl
This article was submitted to Deep-Sea Environments and Ecology, a section of the journal Frontiers in Marine Science
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.