Abstract
The greater duckweed Spirodela polyrhiza (Lemnaceae) is a free-floating freshwater macrophyte. The effect of dietary inclusion of duckweed in the feed of common carp Cyprinus carpio fry was evaluated. The control feed (SP0) contained soybean meal as the primary protein source. In four experimental feeds, greater duckweed was incorporated at levels of 5% (SP5), 10% (SP10), 15% (SP15), and 20% (SP20) replacing soybean meal. Broken-line regression showed that incorporation of greater duckweed at 10 and 13.4% levels were the breakpoint for final weight and specific growth rate (SGR) of fish, respectively. The final weight and SGR of common carp fed diet SP20 were significantly higher compared with those of others. The feed conversion ratio was lowest in SP20 treatment. The inclusion of greater duckweed in the fish feeds showed linear relationships with amylase, trypsin, chymotrypsin, and lipase activities. The content of crude protein was significantly higher in SP10, SP15, and SP20 treatments compared with that of others. Significantly higher crude lipid and ash contents were found in SP20 diet-fed fish compared with other diet-fed fish. The essential amino acids composition was similar in five different diet-fed fish. The greater duckweed supplemented feeds influenced the fatty acid contents of fish. The monounsaturated fatty acids (MUFA) showed an inverse relationship with the inclusion level of greater duckweed in the feed. The highest MUFA content was found in fish fed SP0 diet. The highest level of linoleic acid was found in SP20 diet fed fish. The n-3 PUFA contents of fish showed an increasing trend with the increasing inclusion of greater duckweed, and a significantly higher level was found in SP20 compared with that of others. A significantly higher expression of fas was found in SP5 and fads2d6 in SP5 and SP10 compared with that of others. The expressions of elovl2 and elovl5 were significantly higher in SP5, SP10, and SP15 diet-fed fish compared with other diet-fed fish. The incorporation of greater duckweed in diets improved the growth performance and nutritional value of common carp.
Introduction
The application of freshwater macrophytes as fish feed ingredients is an emerging area of research. There is an increasing demand for quality ingredients that can replace fishmeal and fish oil without affecting the survival, growth performance, and quality of the farmed products. As an alternative to fish meal, plant protein is widely used in aquaculture as well as the poultry and swine feed industries (Hardy, 2010). The nutritional value (e.g., amino acid and fatty acid compositions, fiber content, and flavorings) of ingredients should be considered during fish feed formulation (Gatlin et al., 2007; Glencross et al., 2020). Meals and other products of soybean are the most commonly used plant-based ingredients in the aqua feed industry. However, soybean meal has great market demand as it is also used extensively by other animal feed industries. Therefore, there is a need to find other, non-conventional ingredients that have less or no use in other feed sectors, but that still have high-quality nutritional profiles with all the required amino acids and fatty acids. The greater duckweed Spirodela polyrhiza (family: Lemnaceae) is a free-floating freshwater macrophyte that has been considered as a suitable feed ingredient for both fish and livestock (FAO, 2001; Hasan and Chakrabarti, 2009; Cruz-Velásquez et al., 2014; Chakrabarti, 2017).
The study of the proximate composition showed that the crude protein content of soybean meal (460.7 g/kg) is higher compared with greater duckweed (366.5 g/kg), whereas crude lipid and ash levels are higher in greater duckweed (crude lipid: 76.2 g/kg, ash: 181.9 g/kg) compared with soybean meal (crude lipid: 11.0 g/kg, ash: 71.1 g/kg) (Lee et al., 2013). However, greater duckweed is a rich source of essential and non-essential amino acids (Sharma et al., 2019). The amino acid profile of greater duckweed fulfills all the recommended essential amino acid requirements of common carp Cyprinus carpio and Nile tilapia Oreochromis niloticus (NRC, 1998, 2011). Duckweeds are also known to be good sources of vitamins and fatty acids (Appenroth et al., 2017), with the fatty acid profile of greater duckweed being favorable in comparison with soybean meal. The n-3 PUFA content is 7.5-fold higher in greater duckweed than in soybean meal, with α-linolenic acid (ALA, 18:3n-3) being the predominant fatty acid in greater duckweed contributing 35.75% of total fatty acids (Sharma et al., 2019).
Supply of sufficient amounts of the essential n-3 long-chain PUFA (LC-PUFA), specifically eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3), to support optimal human nutrition is a global problem that transcends geographical and political boundaries. While the n-3 LC-PUFA is completely absent in conventional plant meals and vegetable oils, their precursor ALA can be abundant in terrestrial and freshwater plants. The conversion of ALA (and linoleic acid, LOA, 18:2n-6) to LC-PUFA requires a series of fatty acyl desaturase (fads) and elongation of very long-chain fatty acid (elovl) enzymes such as elovl5 and elovl2 (Kuhajda et al., 1994; Torstensen and Tocher, 2010; Castro et al., 2016; Monroig et al., 2016; Xie et al., 2021). The products of the Δ6fads and elovl5 genes are key enzymes in the biosynthesis of EPA and DHA (Fonseca-Madrigal et al., 2005; Torstensen and Tocher, 2010). Importantly, many freshwater fishes including common carp and Nile tilapia have the metabolic capacity to convert dietary ALA to the n-3 LC-PUFA, EPA, and DHA (Tocher et al., 2002; Glencross, 2009; Tocher, 2010; Taşbozan and Gökçe, 2017). Therefore, supplementation of the greater duckweed S. polyrhiza as a rich source of ALA in the feed of freshwater carp is a useful and cost-effective way to increase the n-3 LC-PUFA content of farmed fish for human consumption.
The omnivore common carp C. carpio (family: Cyprinidae) is the fourth most cultured freshwater fish and contributed 7% of total aquaculture (fish) production in 2018 (FAO, 2020) and is extensively used in composite fish culture in India (Rathore et al., 2005). The digestibility of ingredients plays a very significant role in the overall bioavailability of the nutrients present in feed (Chakrabarti and Rathore, 2009), and, recently, an in vitro digestibility study showed the potential suitability of greater duckweed as an ingredient in fish feed (Sharma et al., 2016). Rathore et al. (2005) have reported the variations in the activities of digestive enzyme in common carp during ontogenic development and observed significantly increased amylase activity in 30-day-old fish. This finding confirmed the capacity of common carp to digest plant-based feed. The presence of anti-nutritional factors is a major constraint to the application of plant-based ingredients in aquafeeds (Alarcón et al., 1998; Olsen et al., 2007; Hansen and Hemre, 2013).
Several studies have investigated the effects of freshwater macrophytes in feeds for different fish species. Dietary supplementation of Lemna minor (20%) and Azolla pinnata had no negative impacts on growth performance or feed utilization of common carp (Yılmaz et al., 2004; Gangadhar et al., 2017). Rohu Labeo rohita-fed diets containing 20 and 30% L. minor showed highest weight gain, SGR, and lowest FCR compared with the control diet without duckweed (Bairagi et al., 2002; Mer et al., 2016). The supplementation of duckweed in the diet of common carp increased the antioxidant capacity as evidenced by enhanced activities of superoxide dismutase (SOD) and glutathione peroxidase (GPx) and reduced levels of malondialdehyde, MDA (Yang et al., 2019). The aim of the present study was to investigate the effects of feeding diets containing greater duckweed S. polyrhiza on the survival, growth performance, digestive enzyme activities, and biochemical composition of common carp C. carpio. The greater duckweed was included in a graded manner at levels of 0%, 5%, 10%, 15%, and 20% of total diet in carp feeds. The expression of genes involved in the metabolic conversion of ALA was also studied to determine impacts on n-3 LC-PUFA biosynthesis.
Materials and Methods
Feed Formulation
The greater duckweed S. polyrhiza was cultured using organic manures viz., cattle manure mustard oil-cake and poultry dropping (Sharma et al., 2019). These manures are cheap and easily available. The production cost of greater duckweed in this study was around Rs.14.0/kg. The greater duckweed was collected, cleaned, dried, and ground. The meal was stored at 4°C prior to further use. The moisture, crude protein, crude lipid, carbohydrate, and ash contents of the greater duckweed meal were 75.2, 366.5, 76.2, 300.2, and 181.9 g/kg, respectively. Five isoproteic, isolipidic, and isoenergetic experimental feeds were prepared with graded inclusion of S. polyrhiza meal replacing soybean meal as the primary protein source (Table 1). Fish feeds were formulated using the Winfeed 2.8 software package (WinFeed UK Limited, Cambridge, United Kingdom). The control feed (SP0) contained soybean meal as the only primary source of protein, while in the four experimental feeds, greater duckweed was incorporated at the levels of 5%, 10%, 15%, and 20% of total feed at the expense of soybean meal, wheat flour, corn meal, and sunflower oil (to maintain constant crude protein, crude lipid, and gross energy levels) to produce feeds SP5, SP10, SP15, and SP20. The soybean meal was replaced in a graded manner, which resulted in changes in the proportions of amino acids in the feeds. Therefore, some specific amino acids such as histidine, methionine, lysine, and threonine were supplemented to the feeds based on the reported requirements of common carp (NRC, 2011). The inclusion levels of these four amino acids were determined using the Winfeed software to ensure the requirements of the fish were satisfied. All dry feed ingredients were blended for 10 min and mixed with the oil before warm water was added slowly and everything mixed thoroughly. The entire mixture was placed in the hopper of the twin-screw-extruder (Basic Technology Private Limited, Kolkata, India), and feed pellets were formed with extrusion conditions as follows: cutter 134 rpm; feeder 10 rpm; extrusion 190 rpm; extrusion torque 9.22; heater 1 temperature 65°C; heater 2 temperature 70°C; and final mass temperature 75°C. The diameter of the produced pellets was 1 mm. All feeds were stored at 4°C prior to use. A common difficulty in the use of feeds based on plant ingredients is their palatability to the fish (Rodriguez et al., 1996), but this can be mitigated by the extrusion process. Antinutritional factors such as trypsin inhibitor, phytic acid tannins, oxalates etc., are found in greater duckweed (Cruz et al., 2011). However, the preparation of the feeds by the extrusion technique helped to mitigate the impact of antinutritional factors as high temperature and pressure inactivate many of these factors and control enzymatic rancidity of nutrients (Rokey, 2004; Stadtlander et al., 2019). In this study, the preparation of feed using the extrusion technology improved the digestibility of proteins and starches and destroyed the antinutritional factors present in the feed. The amino acid and fatty acid compositions of the feeds were measured and are presented in Supplementary Tables 1, 2.
TABLE 1
| Ingredients (g/Kg) | SP0 | SP5 | SP10 | SP15 | SP20 |
| Soybean meal | 500 | 475 | 450 | 425 | 400 |
| Wheat flour | 245 | 224 | 204 | 183 | 162 |
| Corn flour | 147 | 144 | 141 | 138 | 135 |
| Sunflower oil | 62 | 60 | 57 | 55 | 52 |
| S. polyrhiza powder | 0 | 50 | 100 | 150 | 200 |
| Vitamin/minerals premix | 5 | 5 | 5 | 5 | 5 |
| Mono calcium phosphate | 20 | 20 | 20 | 20 | 20 |
| Choline chloride | 1 | 1 | 1 | 1 | 1 |
| Histidine | 1 | 1 | 2 | 2 | 2 |
| Methionine | 12 | 12 | 12 | 12 | 12 |
| Lysine | 5 | 6 | 6 | 7 | 7 |
| Threonine | 2 | 3 | 3 | 4 | 4 |
|
|
|||||
| Proximate composition (g/kg) | |||||
|
|
|||||
| Moisture | 63.3 ± 0.81 | 63.6 ± 0.20 | 63.2 ± 0.63 | 63.3 ± 0.84 | 62.5 ± 0.42 |
| Crude Protein | 317.6 ± 0.50 | 318.4 ± 2.20 | 318.2 ± 3.10 | 322.2 ± 3.61 | 322.0 ± 0.70 |
| Crude Lipid | 73.3 ± 0.81 | 73.4 ± 0.91 | 73.7 ± 0.25 | 73.2 ± 0.10 | 72.8 ± 0.17 |
| Carbohydrate | 474.6 ± 4.10 | 474.3 ± 1.72 | 472.0 ± 0.25 | 464.2 ± 2.21 | 460.6 ± 3.1 |
| Ash | 71.2 ± 0.28 | 70.3 ± 0.40 | 72.9 ± 0.60 | 77.1 ± 0.21 | 84.1 ± 1.1 |
Formulations and measured proximate compositions of experimental diets.
SP0, soybean meal only; SP5, soybean meal + 5% S. polyrhiza; SP10, soybean meal + 10% S. polyrhiza; SP15, soybean meal + 15% S. polyrhiza; SP20, soybean meal + 20% S. polyrhiza.
Culture of Fish and Sampling
Common carp were cultured and sampled following the guidelines of the University of Delhi Institutional Animal Ethics Committee (DU/ZOOL/IAEC-R/2015/07). The fish were collected from a local fish farm and acclimated in the aquarium at the University of Delhi for 1 week during which time the fry were fed the soybean-based control feed. The fry (0.473–0.479 g) were then distributed randomly into 15 glass aquaria (50 L each) with 30 fry per aquarium. The fry of common carp was selected to determine the influence of the plant-based diet on the digestive physiology of fish and to understand the suitability of these diets for early life stages. Each aquarium was connected to an external filtration unit (Sera fil bioactive 130, Germany). Water from the fish culture units was constantly filtered through the filtration unit to maintain ammonia levels of the units. The dissolved oxygen level of water was maintained with the help of an aerator. The carp were then fed one of the five different feeds SP0, SP5, SP10, SP15, and SP20 with three replicate aquaria per dietary treatment. The feeds were distributed ad libitum two times daily at 09:00 and 17:00 h and the weight of feed measured before distribution. Excess (uneaten) feed was collected from each aquarium 1 h after feeding, oven drying, and recording weight. Water quality parameters, namely, temperature, pH, dissolved oxygen, and conductivity were monitored in each aquarium using a probe connected to a portable meter (HQ40d Multiparameter, Hach, United States). The ammonia (NH3) level was estimated using a probe, connected to Orion Versastar (Thermo Scientific, United States). The nitrite (NO2–), nitrate (NO3–), and phosphate (PO43–) contents were analyzed regularly (APHA, 2017). There was no significant difference in water quality parameters among the dietary treatments throughout the culture period. Temperature, pH, and dissolved oxygen ranged from 25.8 to 28.1°C, 6.66 to 7.56, and 6.45 to 7.48 mg/L, respectively, during the experimental period. The range of ammonia, nitrite, nitrate, and phosphate levels were 0.003–0.0530, 0.291–0.906, 0.956–3.84, and 0.013–0.097 mg/L, respectively, in different treatments and conductivity ranged from 636–811 μS/cm.
After 60 days of culture, the feeding experiment was terminated, and fish was sampled. Fish were starved for 24 h before harvesting, and then, all fish were euthanized with tricaine methane sulfonate (MS222, Sigma, United States), and the weight of individual fish was measured. Four fish from each tank were pooled (four fish/replicate) and stored at -80°C for the assay of whole-body proximate, amino acid, and fatty acid compositions. Three tank replicates were used for all assays (three replicates per diet, n = 3). The digestive tracts of two individual fish per aquarium were collected (two fish/replicate, three replicates; 2 × 3 = 6 fish/diet) for the assay of digestive enzyme activities. The hepatopancreas from individual fish was collected (100 mg) and stored in 1 ml of TRIzol reagent (Ambion, Life Technologies, United States) for the gene expression analysis (four fish/treatment; from two aquaria 1 + 1 fish and two fish from the third aquarium). Specific growth rate (SGR) and feed conversion ratio (FCR) of fish were calculated as follows.
SGR (% body weight/day) = (In final body mass - In initial body mass) × 100/duration of experiment (days).
FCR = feed (dry weight) consumed by individual fish during feeding trial/weight gain (wet weight) of individual fish.
Digestive Enzymes Activities
Intestinal samples were freeze-dried and homogenized in ice-cold Milli-Q® water (1:10) to maintain neutral pH of the extracts. Homogenates were centrifuged for 30 min at 10,000 × g at 4°C, and supernatants were collected for the assay of digestive enzyme activities using fluorimetry (Multimode reader, BioTek Synergy H1 Hybrid, United States) using three replicates per dietary treatment. The amylase activity was determined using an assay kit (E33651; Invitrogen, United States) with fluorescence measured at 485 (i.e., excitation) and 520 nm (i.e., emission). The enzyme activity was expressed as mU/mg protein/min. A protease kit (E6638; Invitrogen) was used to measure the total protease activity with fluorescence measured at 485 (i.e., excitation) and 530 nm (i.e., emission). The protease activity was expressed as fluorescence change/unit. The substrate N-benzoyl-L-arginine-methyl-coumarinylamide (Sigma-Aldrich) was used for the estimation of serine proteases trypsin (Ueberschär, 1988). The fluorescence was measured at 380 (i.e., excitation) and 440 nm (i.e., emission). The chymotrypsin was measured using succinyl-Leu-Val-Tyr-4-methyl-coumaryl-7-amide (Sigma-Aldrich) as the substrate (Cao et al., 2000). The fluorescence was measured at 380 (i.e., excitation) and 450 nm (i.e., emission). The enzyme activities were expressed as μM 7-amino-4-methylcoumarin (AMC)/mg protein/min. The neutral lipase activity was determined following the method of Roberts (1985) using the substrate 4-methylumbelliferyl butyrate, 4-MU (Sigma-Aldrich). The fluorescence was measured at 365 nm (excitation) and 450 nm (emission). The enzyme activity was expressed as μM 4-MU/mg protein/min. Protein content was estimated using bovine serum albumin (BSA, Sigma-Aldrich) as standard (Bradford, 1976).
Proximate Composition
Samples of feed were ground, and the fish samples (four fish pooled per replicate) were blended to form homogeneous paste prior to biochemical composition analyses. Proximate compositions of feeds and cultured fish were determined (three replicates for each assay) following the standard protocols of the Association of Official Analytical Chemists International (AOAC, 2000). The moisture level was calculated after drying samples at 110°C for 24 h, and ash content was measured after incineration of samples at 600°C for 16 h. The nitrogen content was first assayed using micro Kjeldahl method, and then, crude protein was calculated (N × 6.25). The crude lipid content was measured gravimetrically following extraction of total lipid using chloroform/methanol (2:1, v/v) (Folch et al., 1957). The subtraction method was applied for the calculation of carbohydrate levels in the feeds (Aksnes and Opstvedt, 1998).
Amino Acid Analysis
Feed and whole fish samples were processed as described above and amino acid compositions assayed with an Automatic Amino Acid Analyzer L-8900 (Hitachi Co., Ltd., Tokyo, Japan) using three replicates for each sample. For all amino acids other than cysteine, methionine, and tryptophan, samples were hydrolyzed using 6 N HCl at 110°C for 24 h (Bassler and Buchholz, 1993; Chakrabarti et al., 2018). The sulfur-containing amino acids were analyzed after oxidizing the sample with performic acid prior to treating the sample with 6 N HCl. For the estimation of tryptophan, the sample was hydrolyzed using 4 M methanesulfonic acid with 0.2% 3-(2-aminoethyl). A nitrogen evaporator (PCi Analytic Private Limited, Maharashtra, India) was used to dry the digested samples, and 0.02 N HCl was added to obtain a protein concentration of 0.5 mg/ml in the sample, and 1.5 ml was placed in a glass vial of the auto sampler. A cation-exchange resin column (4.6 mm ID × 60 mm L) with 3 μm particle size was used for the separation of amino acids with the following analytical conditions: column temperature 30-70°C, reaction temperature 135°C, and a ninhydrin flow rate of 0.35 ml/min. All amino acids were monitored at 570 nm, except proline and hydroxyproline that were monitored at 440 nm. The concentration of individual amino acids was compared with a standard solution (Wako Pure Chemical Industries Limited, United States) and expressed as g/kg.
Fatty Acid Analysis
After processing feed and whole fish samples as described above, fatty acid compositions were analyzed by gas chromatography and flame ionization detection (GC-FID) using a Clarus 580 (PerkinElmer, Waltham, United States). In brief, crude lipid was extracted from the samples using chloroform/methanol (2:1, v/v) following the protocol of Folch et al. (1957) with three replicates per dietary treatment. Fatty acid methyl esters (FAME) were prepared from crude lipid extracts by acidic transesterification, treating the lipid with 1% sulfuric acid in methanol for 16 h at 50°C (Christie, 2003). After extraction and purification of FAME (Tocher and Harvie, 1988), a 1 ml aliquot was placed in a glass vial of the GC autosampler. Fatty acids were separated using a 60 m ZB-wax GC column, internal diameter of 0.32 mm, and film thickness of 0.25 μm (Phenomenex, Hyderabad, India). Data were collected using preinstalled programmed software (TotalChrom Workstation Ver6.3; PerkinElmer, United States). The FAME were identified and quantified by the comparison with standards (Supelco FAME 37 mix; Sigma-Aldrich, United States) and published data (Tocher and Harvie, 1988) and concentration expressed as mg/100 g.
Gene Expression
The levels of mRNA expression of delta-6 fatty acyl desaturase (fads2d6), elongation of very-long-chain fatty acids protein 2 (elovl2), elongation of very-long-chain fatty acids protein 5 (elovl5), and fatty acid synthase (fas) genes were determined in the hepatopancreas of common carp. Total RNA was extracted using the TRIzol reagent (Ambion, Life Technologies, United States) following the protocol of the manufacturer. The absorbance of extracted RNA was examined at 260 and 280 nm using a Nanodrop spectrophotometer (Thermo Scientific, United States) to determine the concentration and quality. The extracted RNA was treated with 1 U of DNase I (Sigma-Aldrich, United States) to avoid DNA contamination, and the quality of RNA treated with DNase was checked with 1% agarose gel electrophoresis. Subsequently, total RNA was reverse transcribed into cDNA by the reverse transcription reaction using high-capacity cDNA reverse transcription kit (Applied Biosystems, United States), using the protocol provided by the manufacturer.
Quantification of gene expression was carried out by using quantitative reverse transcription polymerase chain reaction (qRT-PCR) using a Quant Studio 6 Flex system (Applied Biosystems) and PowerUp SYBR™ Green Master Mix (Applied Biosystems). Primers were designed using the online primer design tool of NCBI with β−actin used as the reference (housekeeping) gene (Supplementary Table 3). The efficiency of primers was evaluated by using the melt curve and standard curve analysis using the QuantStudio 6 Flex Real-Time PCR system software v1 (Applied Biosystems). The 10 μl reaction mixture for qRT-PCR was composed of 0.25 μl PCR forward primer (2.5 μM), 0.25 μl PCR reverse primer (2.5 μM), 1 μl of cDNA (1:3), 5 μl of 2 × PowerUp™ SYBR™ Green PCR Master Mix (Applied Biosystems), and nuclease-free water (3.5 μl). Samples were run in duplicate for each target gene with non-template control (NTC). The thermal cycling conditions were as follows: predenaturation of nucleic acid at 95°C for 10 min followed by either 40 cycles of 15 s at 95°C and 1 min at 60°C (primer Tm 60°C) or 40 cycles of 15 s at 95°C, 15 s at 55°C, and 1 min at 72°C for (primer Tm < 60°C). The data of qRT−PCR were calculated using the 2–ΔΔCt (Livak and Schmittgen, 2001) method with β−actin as the internal control.
Statistical Analysis
Data are presented as means with standard error (SEM) with n values as stated. The IBM SPSS 25.0 software (SPSS Inc., Michigan Avenue, Chicago, IL, United States) was used for the statistical analysis. Data were analyzed using one-way analysis of variance, and the Tukey’s test was performed to compare the differences among experimental groups. The linear regression analysis was performed to check the effect of inclusion level of S. polyrhiza in the diets on growth performance, digestive enzyme activities, proximate composition, and amino acid and fatty acid profiles of fish. The broken-line regression analysis was performed for average weight and SGR to determine the breakpoint, BP (Muggeo, 2008) with final BP estimated based on the least sum of squares of deviation (LS method) using R package. The significance was accepted at p < 0.05 level.
Results
Performance of Fish
After 60 days of culture, the number of common carp in each aquarium was recorded. There was no mortality of fish; all fish survived (Table 2). The broken-line regression analysis showed the impact of greater duckweed on the growth performance and SGR of common carp. The estimated break point for final weight and SGR were 10 and 13.4% of greater duckweed, respectively. The final weight of SP20 diet-fed common carp was significantly higher compared with other diet-fed fish. Minimum weight was found in the SP0 treatment. Similar trend was also found with SGR. SGR was maximum in SP20 diet-fed carp. FCR was minimum and maximum in SP20 and SP0 treatment, respectively.
TABLE 2
| Parameters | SP0 | SP5 | SP10 | SP15 | SP20 | ANOVA | Linear regression R2 value | |
|
|
||||||||
| P value | F value | |||||||
| Initial weight (g) | 0.473 ± 0.001a | 0.479 ± 0.001a | 0.473 ± 0.003a | 0.473 ± 0.001a | 0.477 ± 0.002a | 0.093 | 2.692 | 0.518 |
| Survival (%) | 100 | 100 | 100 | 100 | 100 | - | - | - |
| Final weight (g) | 1.60 ± 0.004e | 1.88 ± 0.012d | 2.15 ± 0.007c | 2.49 ± 0.003b | 2.74 ± 0.013a | < 0.01 | 7,507.44 | 0.999 |
| Specific growth rate (SGR% BW/day) | 2.01 ± 0.002e | 2.30 ± 0.011d | 2.52 ± 0.007c | 2.75 ± 0.001b | 2.93 ± 0.001a | < 0.01 | 3,419.05 | 0.999 |
| Feed conversion ratio (FCR) | 1.25 ± 0.010a | 1.23 ± 0.012a | 1.11 ± 0.011b | 1.06 ± 0.013bc | 1.01 ± 0.010c | < 0.01 | 74.394 | 0.967 |
| Amylase (mU/mg protein/min) | 126.48 ± 3.80a | 98.91 ± 2.30b | 102.28 ± 2.13b | 125.41 ± 1.87a | 125.14 ± 1.82a | < 0.01 | 30.70 | 0.925 |
| Protease (Fluorescence change/unit) | 249.95 ± 1.40b | 247.31 ± 0.88b | 265.35 ± 5.30a | 264.78 ± 1.75a | 278.82 ± 6.28a | 0.091 | 2.715 | 0.521 |
| Trypsin (μM AMC/mg protein/min) | 799.93 ± 4.11c | 588.87 ± 6.18d | 768.03 ± 9.42c | 1,073.91 ± 6.72b | 1,335.45 ± 9.52a | < 0.01 | 1,532.15 | 0.998 |
| Chymotrypsin (μM AMC/mg protein/min) | 683.17 ± 17.86b | 813.10 ± 10.84a | 617.5 ± 4.30c | 477.24 ± 10.24d | 406.38 ± 15.20e | < 0.01 | 147.52 | 0.983 |
| Lipase (μM 4-MU/mg protein/min) | 1,000.97 ± 17.26c | 950.40 ± 10.80c | 992.27 ± 14.82c | 1,137.10 ± 16.30a | 1,074.27 ± 15.32b | < 0.01 | 27.50 | 0.917 |
Initial weight, survival rate, final weight, specific growth rate, feed conversion ratio, and digestive enzyme activities of Cyprinus carpio fed five different diets.
Values (n = 3) with different letters in the same row are significantly different (p < 0.05).
SP0, soybean meal only; SP5, soybean meal + 5% S. polyrhiza; SP10, soybean meal + 10% S. polyrhiza; SP15, soybean meal + 15% S. polyrhiza; SP20, soybean meal + 20% S. polyrhiza; BW, body weight.
Digestive Enzymes
The amylase activity ranged from 98.91 to 126.48 mU/mg protein/min in five different diet-fed common carp with a minimum amylase activity in SP5 diet-fed fish (Table 2). The amylase activity was significantly higher in SP0, SP15, and SP20 diet-fed fish compared with other diet-fed fish. The total protease activity was significantly higher in SP10, SP15, and SP20 diet-fed fish compared with other diet-fed fish. Trypsin activity was significantly higher in SP20 diet-fed fish compared with other diet-fed fish. Significantly higher chymotrypsin and lipase activities were found in SP5 and SP15 diet-fed common carp, respectively, compared with other treatments. The inclusion of greater duckweed in the carp diet showed linear relationships (R2 = 0.917–0.998) with amylase, trypsin, chymotrypsin, and lipase activities.
Biochemical Composition of Fish
Proximate Composition of the Whole Body
The moisture contents of fish varied from 747.6 to 756.4 g/kg in five different diet-fed common carp (Table 3). The crude protein content was significantly higher in SP10, SP15, and SP20 diet-fed common carp compared with other diet-fed fish. Significantly higher crude lipid and ash contents were found in SP20 diet-fed fish compared with other diet-fed fish.
TABLE 3
| Parameters | SP0 | SP5 | SP10 | SP15 | SP20 | ANOVA | Linear Regression R2 Value | |
|
|
||||||||
| P value | F value | |||||||
| Moisture | 756.4±3.6a | 752.8 ± 3.7a | 750.7 ± 1.02a | 750.5 ± 5.7a | 747.6 ± 1.2a | 0.524 | 0.852 | 0.254 |
| Crude Protein | 150.3 ± 0.66c | 152.34 ± 0.25b | 156.73 ± 0.25a | 156.79 ± 0.12a | 157.06 ± 0.1a | < 0.01 | 81.09 | 0.970 |
| Crude Lipid | 64.7 ± 0.1c | 64.4 ± 0.10c | 65.2 ± 0.1bc | 65.9 ± 0.2b | 68.1 ± 0.5a | < 0.01 | 33.949 | 0.931 |
| Ash | 19.6 ± 0.3c | 21.4 ± 0.1b | 21.8 ± 0.2b | 21.9 ± 0.3b | 22.5 ± 0.3a | < 0.01 | 28.401 | 0.919 |
Proximate compositions (g/kg, wet weight) of five different diets-fed Cyprinus carpio.
Values (n = 3) with different letters in the same row are significantly different (p < 0.05).
SP0, soybean meal only; SP, soybean meal + 5% S. polyrhiza; SP10, soybean meal + 10% S. polyrhiza; SP15, soybean meal + 15% S. polyrhiza; SP20, soybean meal + 20% S. polyrhiza.
Amino Acid Composition
The essential amino acids composition was similar in common carp cultured in five different feeding schemes (Table 4). The arginine level was 1.55- to 1.67-fold higher in 10–20% greater duckweed supplemented diet-fed common carp compared with SP0 and SP5 diet-fed fish. Histidine (3.64–3.72 g/kg), isoleucine (5.13–5.39 g/kg), leucine (9.59–10.12 g/kg), and lysine (11.54–12.75 mg/kg) contents were significantly higher in SP0 and SP5 treatments compared with other diet-fed fish. Significantly higher levels of methionine and valine were recorded in SP10; threonine and tryptophan levels were maximum in SP20 diet-fed common carp.
TABLE 4
| Amino acids | SP0 | SP5 | SP10 | SP15 | SP20 | ANOVA | Linear regression R2 value | |
|
|
||||||||
| Essential | P value | F value | ||||||
| Arginine (Arg) | 6.77 ± 0.2c | 6.40 ± 0.1d | 10.7 ± 0.1a | 10.21 ± 0.03b | 9.94 ± 0.04b | < 0.01 | 905.511 | 0.997 |
| Histidine (His) | 3.72 ± 0.1a | 3.64 ± 0.2a | 2.85 ± 0.05b | 2.87 ± 0.06b | 2.73 ± 0.03b | < 0.01 | 156.009 | 0.984 |
| Isoleucine (Ile) | 5.13 ± 0.12ab | 5.39 ± 0.07a | 4.98 ± 0.01b | 4.25 ± 0.1c | 4.44 ± 0.13c | < 0.01 | 67.119 | 0.964 |
| Leucine (Leu) | 9.59 ± 0.19a | 10.12 ± 0.33a | 8.59 ± 0.04b | 8.28 ± 0.11bc | 8.01 ± 0.25c | < 0.01 | 53.555 | 0.955 |
| Lysine (Lys) | 11.54 ± 0.04b | 12.75 ± 0.08a | 10.1 ± 0.1c | 9.15 ± 0.05d | 9.47 ± 0.24d | < 0.01 | 323.243 | 0.992 |
| Methionine (Met) | 2.94 ± 0.04c | 3.37 ± 0.04ab | 3.63 ± 0.21a | 2.77 ± 0.09c | 3.01 ± 0.30c | < 0.01 | 12.611 | 0.835 |
| Phenylalanine (Phe) | 5.52 ± 0.12ab | 5.83 ± 0.1a | 5.16 ± 0.01bc | 4.91 ± 0.20c | 4.99 ± 0.14a | < 0.01 | 14.657 | 0.854 |
| Threonine (Thr) | 4.73 ± 0.01d | 5.16 ± 0.03c | 6.20 ± 0.1b | 6.12 ± 0.1b | 6.67 ± 0.08a | < 0.01 | 293.479 | 0.992 |
| Tryptophan (Trp) | 2.27 ± 0.03c | 1.60 ± 0.05d | 1.56 ± 0.003d | 2.32 ± 0.01b | 2.48 ± 0.002a | < 0.01 | 1,705.8 | 0.998 |
| Valine (Val) | 5.98 ± 0.05d | 6.49 ± 0.05bc | 7.14 ± 0.06a | 6.25 ± 0.01c | 6.63 ± 0.17b | < 0.01 | 69.776 | 0.965 |
| Σ Essential | 58.2±1.1b | 60.8±0.1a | 61.1±0.4a | 57.1±0.7b | 58.4±0.4b | < 0.01 | 21.402 | 0.895 |
|
|
||||||||
| Non-essential | SP0 | SP5 | SP10 | SP15 | SP20 |
ANOVA
|
Linear regression R2 value | |
| P value | F value | |||||||
|
|
||||||||
| Alanine (Ala) | 8.03 ± 0.03b | 7.65 ± 0.03c | 8.03 ± 0.06b | 9.85 ± 0.07a | 9.94 ± 0.15a | < 0.01 | 363.836 | 0.993 |
| Aspartate (Asp) | 11.7 ± 0.03b | 11.4 ± 0.12b | 12.4 ± 0.36a | 11.3 ± 0.02b | 12.7 ± 0.12a | < 0.01 | 33.477 | 0.931 |
| Cysteine (Cys) | 1.14 ± 0.002c | 1.69 ± 0.07a | 1.12 ± 0.07c | 1.30 ± 0.17bc | 1.39 ± 0.02b | < 0.01 | 18.725 | 0.882 |
| Glutamic Acid (Glu) | 20.3 ± 0.08a | 18.8 ± 0.10c | 17.3 ± 0.01d | 17.3 ± 0.05d | 19.6 ± 0.17b | < 0.01 | 535.013 | 0.995 |
| Glycine (Gly) | 8.91 ± 0.09d | 10.0 ± 0.3c | 8.58 ± 0.01d | 10.80 ± 0.14b | 11.62 ± 0.03a | < 0.01 | 77.612 | 0.969 |
| Proline (Pro) | 27.8 ± 0.1c | 29.0 ± 0.1b | 31.37 ± 0.01a | 31.10 ± 0.20a | 25.1 ± 0.2d | < 0.01 | 387.422 | 0.991 |
| Serine (Ser) | 4.83 ± 0.02c | 4.81 ± 0.07c | 5.62 ± 0.01b | 5.48 ± 0.03b | 6.32 ± 0.08a | < 0.01 | 221.76 | 0.989 |
| Tyrosine (Tyr) | 4.10 ± 0.2a | 3.67 ± 0.34ab | 3.57 ± 0.02ab | 3.24 ± 0.16b | 3.34 ± 0.04b | < 0.01 | 7.311 | 0.745 |
| Phosphoserine (p-Ser) | 0.06 ± 0.00bc | 0.05 ± 0.00c | 0.10 ± 0.05bc | 0.28 ± 0.06a | 0.15 ± 0.01b | < 0.01 | 28.851 | 0.920 |
| Taurine (Tau) | 1.76 ± 0.03bc | 1.51 ± 0.15c | 1.74 ± 0.16bc | 1.96 ± 0.13b | 2.73 ± 0.04a | < 0.01 | 48.115 | 0.951 |
| α Amino -n- butyric acid (α-ABA) | − | − | 0.19 ± 0.02a | 0.20 ± 0.03a | 0.18 ± 0.00a | 0.221 | 1.961 | 0.395 |
| Citrulline (Cit) | 0.13 ± 0.01b | 0.15 ± 0.03b | 0.44 ± 0.00a | 0.54 ± 0.20a | 0.56 ± 0.00a | < 0.01 | 16.081 | 0.865 |
| Cystathionine (Cysthi) | 0.28 ± 0.05d | 0.11 ± 0.01d | 2.13 ± 0.14b | 2.60 ± 0.01a | 1.38 ± 0.04c | < 0.01 | 301.177 | 0.992 |
| β -Alanine (β -Ala) | − | 0.08 ± 0.02a | 0.06 ± 0.00ab | 0.11 ± 0.001a | 0.11 ± 0.05a | 0.185 | 2.051 | 0.435 |
| β Amino isobutyric acid (β-AiBA) | 0.04 ± 0.00b | − | − | − | 0.08 ± 0.00a | - | - | − |
| γ-Amino-n-butyric acid (γ-ABA) | 0.04 ± 0.00a | − | − | − | − | - | - | − |
| Hydroxylysine (Hylys) | 0.16 ± 0.05b | − | − | 0.24 ± 0.002a | − | < 0.01 | 518.664 | 0.992 |
| 3 Methyl histidine (3 Mehis) |
− | − | 0.07 ± 0.000a | − − | 0.06 ± 0.000a | 0.02 | 13.914 | 0.777 |
| Hydroxy proline (Hypro) | 1.58 ± 0.09c | 2.28 ± 0.08b | 2.91 ± 0.22a | 3.13 ± 0.002a | 3.07 ± 0.16a | < 0.01 | 301.177 | 0.992 |
| Σ Non- essential | 90.95±0.31c | 91.51±0.21c | 95.76±0.4b | 99.56±0.50a | 98.16±0.06a | < 0.01 | 136.54 | 0.982 |
Amino acid compositions (g/kg, wet weight) of five different diets-fed Cyprinus carpio.
Values (n = 3) with different letters in the same row are significantly different (p < 0.05).
SP0, soybean meal only; SP5, soybean meal + 5% S. polyrhiza; SP10, soybean meal + 10% S. polyrhiza; SP15, soybean meal + 15% S. polyrhiza; SP20, soybean meal + 20% S. polyrhiza. The bold values means summation/total value.
Among non-essential amino acids, alanine, aspartate, glycine, serine, taurine, and β-alanine contents were maximum in SP20 diet-fed common carp. Highest levels of glutamic acid and tyrosine were observed in control diet (SP0)-fed common carp. Aspartate contents were significantly higher in SP10 and SP20 diet-fed common carp. Highest levels of phosphoserine, cystathionine, and hydroxyproline were found in SP15 diet-fed fish. The α-amino-n-butyric acid content was highest in SP15, and the citrulline content was maximum in SP15 and SP20 treatments. The α-amino-n-butyric acid and 3-methyl histidine were absent in SP0 and SP5 diet-fed fish. The β-amino-isobutyric and γ-amino-butyric acids were absent in SP5, SP10, and SP15 diet-fed common carp. The inclusion of greater duckweed in the carp diet showed linear relationships (R2 = 0.745–0.998) with different amino acids, except α-amino-n-butyric acid and β-alanine.
Fatty Acid Composition
The feeding of common carp with greater duckweed supplemented diets influenced the fatty acid composition of fish (Table 5). Among saturated fatty acids (SFA), palmitic acid (16:0) was the dominant one regardless of treatments with myristic acid (14:0), the second most dominant SFA. Highest SFA was found in carp fed the SP5 diet. Monounsaturated fatty acids (MUFA) showed an inverse relationship with the inclusion level of greater duckweed in the diet. Significantly higher MUFA content was found in the control diet (SP0)-fed common carp compared with that of others. Among MUFA, oleic acid (18:1n-9) was the dominant one in all treatments. Nervonic acid (24:1) was absent in fish fed diets SP15 and SP20. The greater duckweed supplemented diets enhanced the n-6 PUFA and LOA (18:2 n-6) content in fish. The highest level of LOA was found in SP20 diet-fed fish. The n-3 PUFA contents of fish showed an increasing trend with the increasing inclusion of greater duckweeds in the diet. The ALA (18:3n-3), EPA (20:5n-3), docosapentanoic acid (22:5 n-3, DPA), and DHA (22: 6n-3) contents were significantly lower in SP0 diet fed common carp. The n-3 PUFA content was significantly higher in SP20 diet-fed common carp compared with other diet-fed fish. The inclusion of greater duckweed in the carp diet showed linear relationships (R2 = 0.745–0.998) with different fatty acids, except stearic acid (18:0).
TABLE 5
| Fatty acids | SP0 | SP5 | SP10 | SP15 | SP20 | ANOVA | Linear regression R2 value | |
|
|
||||||||
| P value | F value | |||||||
| 14:0 | 22.92 ± 1.23b | 30.64 ± 1.00a | 22.80 ± 0.34b | 22.42 ± 0.11b | 15.78 ± 0.07c | < 0.01 | 156.593 | 0.984 |
| 15:0 | 4.21 ± 0.23c | 6.52 ± 0.25b | 4.89 ± 0.61c | 6.21 ± 0.34b | 7.29 ± 0.29a | < 0.01 | 22.074 | 0.898 |
| 16:0 | 374.00 ± 9.01d | 548.07 ± 20.3a | 507.88 ± 6.26b | 435.9 ± 1.41c | 369.36 ± 2.75d | < 0.01 | 176.633 | 0.986 |
| 18:0 | 1.90 ± 0.01a | 2.81 ± 0.68a | 3.00 ± 0.20a | 2.05 ± 0.29a | 1.24 ± 0.21a | 0.081 | 2.856 | 0.533 |
| 24:0 | 10.53 ± 0.01b | 14.68 ± 2.17a | 12.49 ± 0.04a | 7.21 ± 0.77c | 9.25 ± 0.11b | < 0.01 | 79.245 | 0.969 |
| Σ SFA | 413.58±10.00d | 602.75±22.66a | 551.09.1±7.05b | 473.8±3.20c | 403.20±3.50d | < 0.01 | 165.49 | 0.985 |
|
|
||||||||
| 16:1 n-9 | 26.44 ± 0.02a | 14.10 ± 1.0b | 12.25 ± 2.35b | 24.44 ± 0.72a | 4.46 ± 0.73c | < 0.01 | 164.648 | 0.985 |
| 18:1 n-9 | 1,013.68 ± 0.02a | 963.71 ± 46.81a | 889.60 ± 5.90b | 905.3 ± 1.80b | 699.36 ± 10.84c | < 0.01 | 91.965 | 0.974 |
| 24:1 | 2.51 ± 0.04b | 3.86 ± 0.44a | 2.33 ± 0.29b | − | − | < 0.01 | 22.290 | 0.881 |
| Σ MUFA | 1,042.6±1.75a | 981.688±46.26b | 904.18±3.83c | 929.79±2.52bc | 703.82±11.57d | < 0.01 | 107.854 | 0.977 |
|
|
||||||||
| 18:2 n-6 | 911.99 ± 8.58b | 989.75 ± 9.10a | 1,030.2 ± 5.18a | 1,016.81 ± 3.76a | 1,053.6 ± 0.06a | < 0.01 | 11.145 | 0.817 |
| 20:2 n-6 | 32.67 ± 1.24b | 20.60 ± 0.03d | 32.55 ± 0.05b | 37.51 ± 1.82a | 24.48 ± 2.07c | < 0.01 | 72.707 | 0.967 |
| 20:3 n-6 | 13.44 ± 0.22b | 11.91 ± 0.78c | 10.77 ± 0.02d | 14.60 ± 0.30a | 10.85 ± 0.52d | < 0.01 | 55.671 | 0.957 |
| 20:4 n-6 | 26.89 ± 0.22b | 22.96 ± 0.32d | 28.17 ± 0.83a | 21.75 ± 0.15d | 25.12 ± 0.38c | < 0.01 | 102.122 | 0.976 |
| 22:5 n-6 | 71.09 ± 0.22ab | 66.27 ± 0.17c | 46.22 ± 0.002d | 69.43 ± 2.17b | 72.80 ± 0.144a | < 0.01 | 282.796 | 0.991 |
| Σ n-6 PUFA | 1,056.1±11.25b | 1,111.5±10ab | 1,147.96±62.51a | 1,160.11±8.01a | 1,186.95±2.2a | < 0.01 | 9.048 | 0.784 |
|
|
||||||||
| 18:3 n-3 | 15.10 ± 0.115d | 13.65 ± 0.18e | 18.09 ± 0.30c | 19.53 ± 0.40b | 32.63 ± 0.19a | < 0.01 | 2,563.494 | 0.999 |
| 20:4 n-3 | 126.51 ± 0.07c | 137.58 ± 0.33b | 144.32 ± 0.56a | 125.9 ± 3.42c | 145.15 ± 0.80a | < 0.01 | 111.460 | 0.978 |
| 20:5 n-3 | 3.94 ± 0.06c | 5.01 ± 0.33bc | 4.09 ± 1.04c | 8.99 ± 0.01a | 6.00 ± 0.40b | < 0.01 | 46.860 | 0.949 |
| 22:5 n-3 | 5.39 ± 0.01c | 9.29 ± 0.311b | 11.32 ± 1.36a | 13.00 ± 0.58a | 8.06 ± 0.16b | < 0.01 | 51.941 | 0.954 |
| 22:6 n-3 | 48.03 ± 0.04d | 63.07 ± 0.240c | 65.64 ± 0.30c | 74.05 ± 0.41b | 81.92 ± 1.00a | < 0.01 | 315.64 | 0.992 |
| Σ n-3 PUFA | 198.97±2.31d | 228.6±0.54c | 243.46±3.52b | 241.47±5.69b | 273.77±0.45a | < 0.01 | 473.046 | 0.995 |
| EPA + DHA | 51.97±2.19d | 68.08±0.75c | 69.73±1.28c | 83.04±1.29b | 87.93±0.60a | < 0.01 | 598.073 | 0.996 |
Fatty acid compositions (mg/100 g, wet weight) of five different diets-fed Cyprinus carpio.
Values (n = 3) with different letters in the same row are significantly different (p < 0.05).
SP0, soybean meal only; SP5, soybean meal + 5% S. polyrhiza; SP10, soybean meal + 10% S. polyrhiza; SP15, soybean meal + 15% S. polyrhiza; SP20, soybean meal + 20% S. polyrhiza. The bold values means summation/total value.
Gene Expression
Expressions of various genes involved in the metabolism of fatty acid were recorded in the hepatopancreas of common carp fed the five diets. The expression of fads2d6 was significantly higher in fish fed diet SP5 compared with others (Figure 1). The expression levels of elovl2 and elovl5 were significantly lower in SP20 diet-fed common carp compared with others (Figures 2, 3). The significantly higher expression of fas was observed in SP5 compared with others (Figure 4). The mRNA expressions showed polynomial 3 order relationships with different treatments.
FIGURE 1
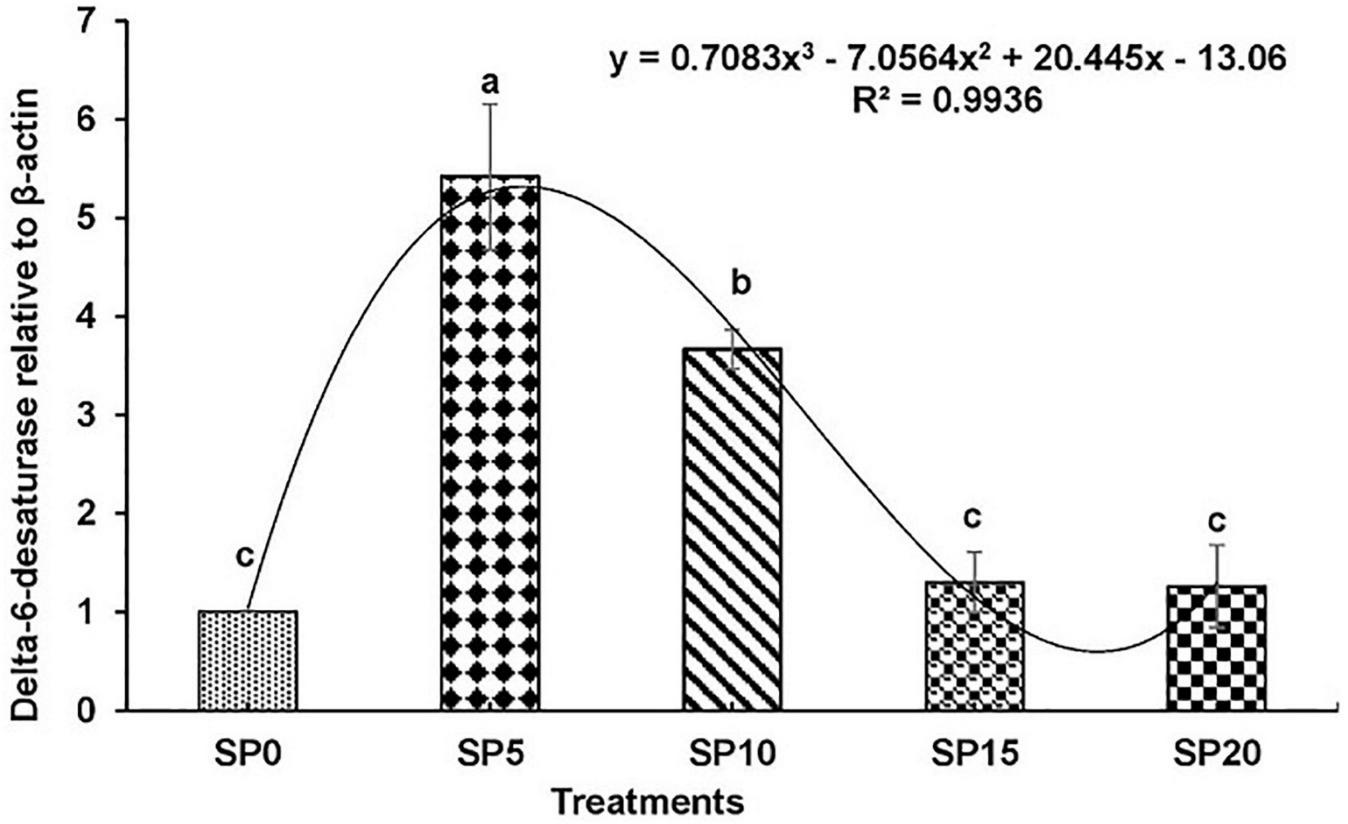
Expression of delta-6-desaturase (fads2d6) relative to β-actin in hepatopancreas of five different diet-fed Cyprinus carpio. Bars with different superscripts are significantly different (n = 3). A polynomial (order 3) relationship was found between the diet and expression of fads2d6 (R2 = 0.994).
FIGURE 2
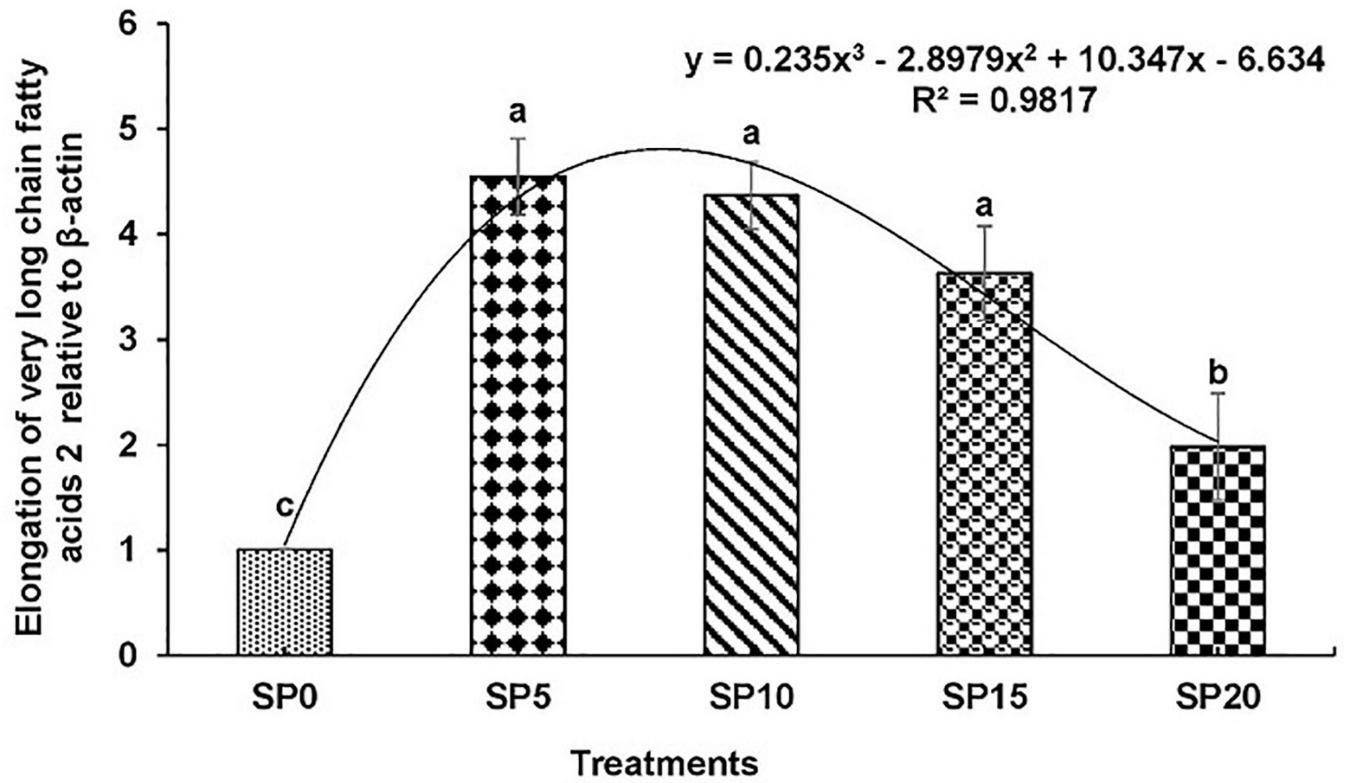
Expression of elongation of very long chain fatty acids protein 2 (elovl2) relative to β-actin in hepatopancreas of five different diet-fed Cyprinus carpio. Bars with different superscripts are significantly different (n = 3). A polynomial (order 3) relationship was found between the diet and expression of fads2d6 (R2 = 0.982).
FIGURE 3
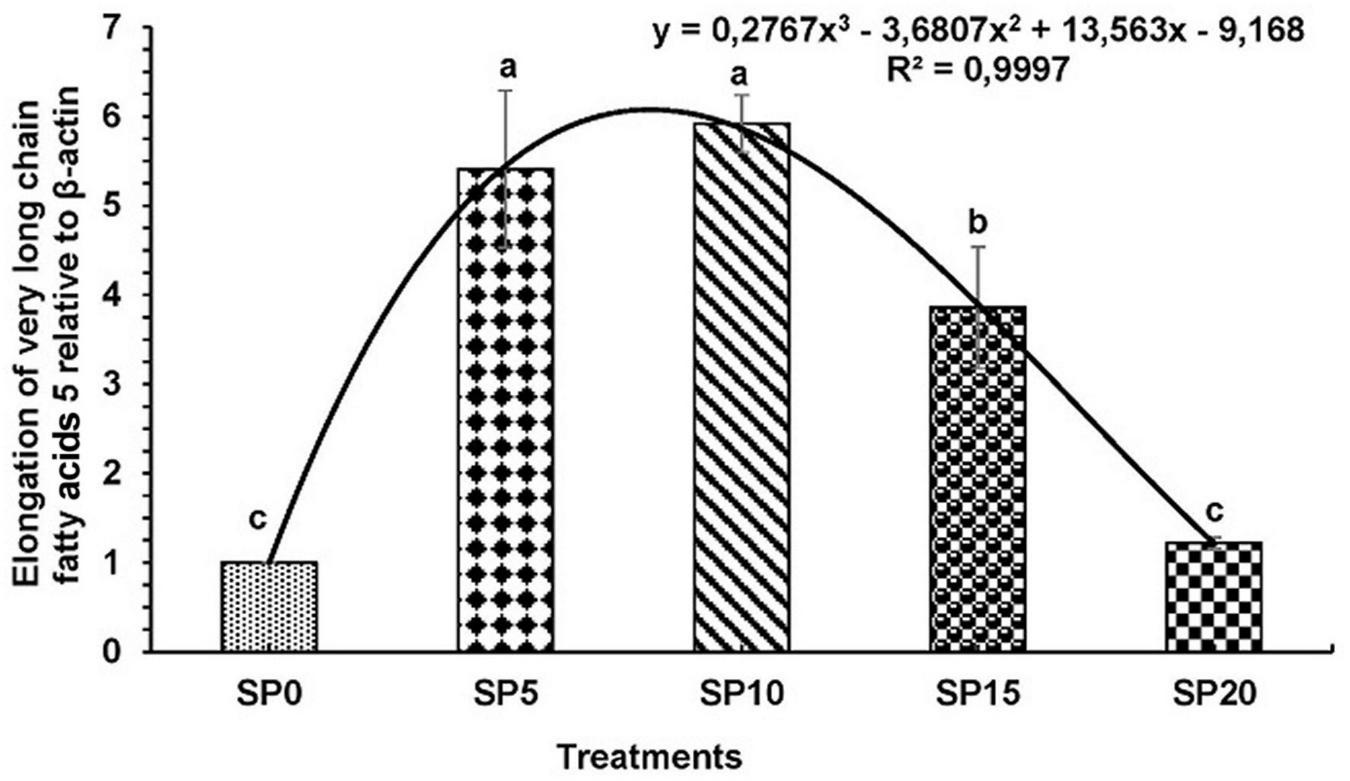
Expression of elongation of very long chain fatty acids protein 5 (elovl5) relative to β-actin in hepatopancreas of five different diet-fed Cyprinus carpio. Bars with different superscripts are significantly different (n = 3). A polynomial (order 3) relationship was found between the diet and expression of fads2d6 (R2 = 0.999).
FIGURE 4
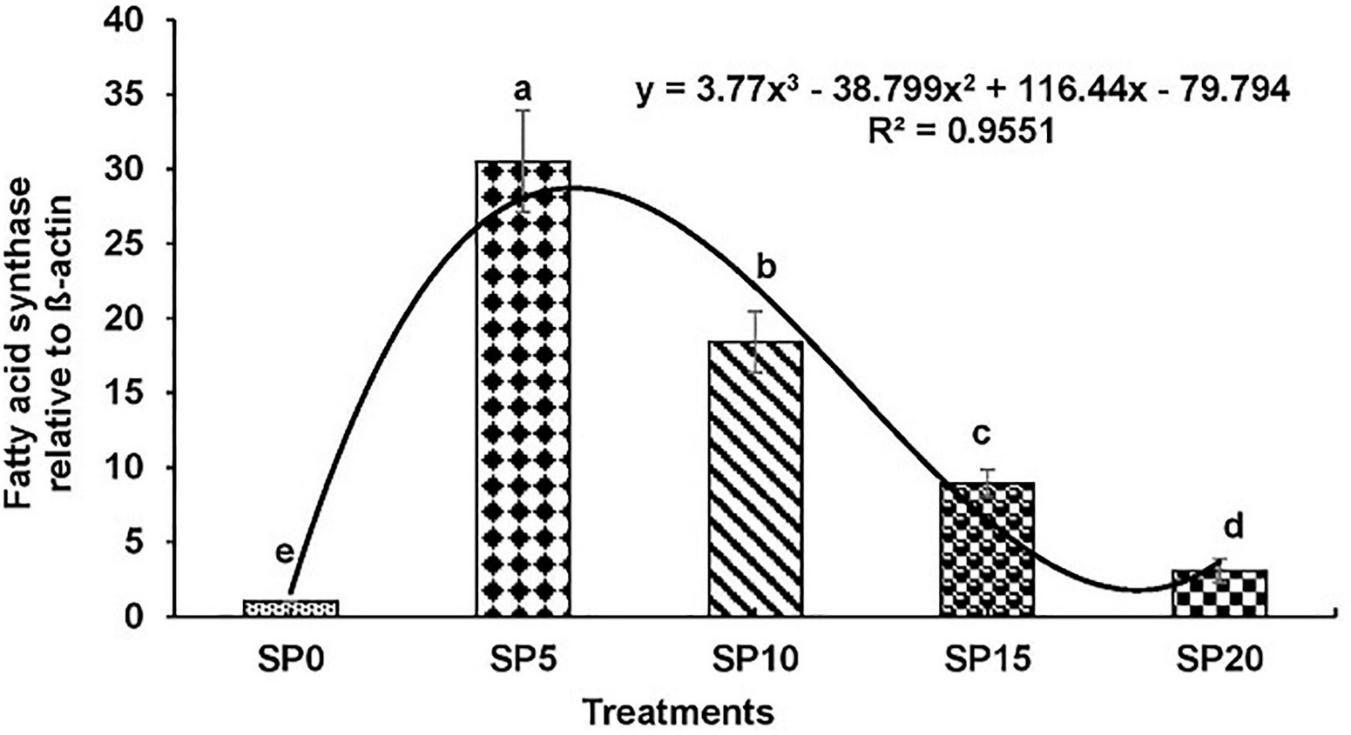
Expression of fatty acid synthase (fas) relative to β-actin in hepatopancreas of five different diet-fed Cyprinus carpio. Bars with different superscripts are significantly different (n = 3). A polynomial (order 3) relationship was found between the diet and expression of fads2d6 (R2 = 0.955).
Discussion
Performance of Common Carp
The effect of dietary inclusion of greater duckweed at four different levels (replacing soybean meal) on the performance of common carp was recorded in this experiment. The survival rate of fish was not affected with the inclusion of greater duckweed in the diets. The earlier study showed mixed results. The inclusion of greater duckweed more than 20% in diet resulted into mortality of tilapia (Fasakin et al., 1999, 2001), supplementation of L. minor (20%) in diets affected the survival rate of common carp (Yılmaz et al., 2004). El-Shafai et al. (2004) reported that the inclusion of duckweed in the feed of tilapia improved the survival rate of fish. The incorporation canola meal at 50% level in the diet was not affecting the survival rate of tilapia (Iqbal et al., 2021).
The broken-line regression showed that incorporation of greater duckweed at 10% level as a breakpoint for final weight of common carp, and it was 13.4% for SGR of fish. Highest growth performance was observed in SP20 diet-fed fish. Food was efficiently utilized in this treatment as minimum FCR was recorded. In L. minor supplemented diet-fed common carp and tilapia, similar trends of growth performance, SGR, and FCR were found (El-Shafai et al., 2004; Yılmaz et al., 2004). The inclusion of L. polyrhiza in the diets of mrigal Cirrhinus mrigala and rohu Lebeo rohita improved the weight gain, SGR, and FCR (Bairagi et al., 2002; Ghosh and Ray, 2014). Inclusion of fermented L. minor at 2.5% level and canola meal at 50% level increased the growth of tilapia (Herawati et al., 2020; Iqbal et al., 2021). In this study, the SGR of common carp (initial weight: 0.473–0.479 g) ranged from 2.01 to 2.93%. Similar results were reported in earlier study like, in L. minor supplemented diet-fed common carp (initial weight: 0.283–0.295 g), SGR ranged from 1.96 to 2.26% (Yılmaz et al., 2004), and in soy protein concentrate (SPC)-incorporated diet-fed common carp (initial weight: 2.43–2.47 g), SGR was 2.01–2.93% (Zhu et al., 2020). Xie et al. (2021) reported that feeding of common carp with diets containing fishmeal and ultra-micro-ground mixed plant proteins (uPP)-based diets resulted in 540–560% growth of fish after 112 days of culture. In this study with common carp, 238–474% weight gain of fish was recorded after 60 days of culture.
Digestive Enzyme Activities
The study of digestive enzyme activities in five different diet-fed common carp explained the reason of efficient utilization of consumed diet in SP20. Total protease and trypsin activities were maximum in SP20 diet-fed fish; considerable amylase and lipase activities were also found in SP20 diet-fed common carp. The digestive enzymes, namely, protease, lipase, and amylase played a significant role in digestion and absorption of nutrient (Zhou et al., 2010). Fish fed with different diets are able to adjust the activity of their digestive enzymes (Shiping and Zhao, 2005). The inclusion of duckweed in the diets of rohu and tilapia enhanced digestive enzyme activities like amylase, trypsin, and chymotrypsin (Goswami et al., 2020; Zhao et al., 2020).
Biochemical Composition of Fish
The proximate composition study showed that the inclusion (10–20%) of greater duckweed enhanced the crude protein content of common carp in this study. The crude lipid and ash contents of common carp increased in a graded manner with the enhanced inclusion of greater duckweed in the diet. An earlier study showed that supplementation of duckweed improved the crude protein and crude lipid contents in fish (El-Shafai et al., 2004; Yılmaz et al., 2004; Fasakin, 2008; Abou et al., 2011). Aslam et al. (2021) reported significantly higher crude protein contents in L. minor-incorporated diet-fed grass carp Ctenopharyngodon idella and silver carp Hypophthalmichthys molitrix compared with the soybean-supplemented diet-fed fishes. The inclusion of duckweed increased the ash content in fish (Fasakin et al., 1999; El-Shafai et al., 2004; Fasakin, 2008). This indicated that greater duckweed-supplemented diets fulfilled the nutritional requirements of common carp. The proximate composition study showed that the crude protein, crude lipid, and ash contents of greater duckweed were 36.65, 7.62, and 18.19 g/100 g (Sharma et al., 2019). The amino acid profile of greater duckweed is comparable with soybean meal. Feeding with greater duckweed-supplemented diets improved the non-essential amino acid contents in the common carp. The supplementation of fermented L. minor in the diet enhanced the lysine content in tilapia (Herawati et al., 2020).
In this study, fatty acid composition of fish was influenced by the supplementation of greater duckweed. Highest and lowest SFA contents were found in SP5 and SP20 diet-fed common carp, respectively. MUFA content showed an inverse relationship with the increased inclusion of greater duckweed in diet of common carp. A direct relationship was found between the amount of greater duckweed in the diet and n-6 PUFA and n-3 PUFA contents in common carp. Inclusion of greater duckweed in the diet enhanced the ALA, DHA, and EPA contents in common carp. The duckweeds are a rich source of fatty acids (Appenroth et al., 2017; Chakrabarti et al., 2018; Sharma et al., 2019). The feeding of Azolla filiculoides enhanced the total n-3 PUFA (especially EPA and DHA) content (Abou et al., 2011), and fermented L. minor enhanced LOA (Herawati et al., 2020) in Nile tilapia. Similarly, the contents of EPA and DHA in common carp increased linearly with increasing greater duckweed level in the diet.
Expression of Genes Involved in the Biosynthesis of Fatty Acids
In this study, the expression levels of key genes involved in the biosynthesis of fatty acids like fads2d6, elovl2, elovl5, and fas were evaluated in the common carp. Upregulation of all these genes was found in fish fed greater duckweed supplemented diets compared with control diet-fed fish. This might be due to the presence of LOA and ALA in the experimental diets. Earlier study showed that the higher contents of LOA and ALA upregulated the expression of desaturases/elongases (Tocher et al., 2004; Turchini et al., 2006; Francis et al., 2007; Li et al., 2008). However, an excess of ALA in diet can inhibit the transcription of fads2d6 gene (Bell et al., 1993). In this study, among the fish fed the experimental diets, the highest expression levels of genes were recorded in the SP5 treatment, and then the expression gradually decreased. The ALA content increased with increasing inclusion of greater duckweed in diet. EPA and DHA contents increased with decreasing expression of genes elovl2, elovl5, and fads2d6. Similar results were found in common carp and rainbow trout Oncorhynchus mykiss where the expressions of desaturases and elongases were higher in fish with lower contents of EPA and DHA (Ren et al., 2012; Lazzarotto et al., 2018).
Conclusion
Greater duckweed (S. polyrhiza) may replace soybean meal up to 20% in the diet of C. carpio without affecting the digestive physiology and growth performance of fish even at an early life stage. Inclusion of greater duckweed enhanced growth performance and improved the quality of fish in terms of amino acids and n-3 PUFA, especially EPA and DHA.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The animal study was reviewed and approved by Institutional Animal Ethics Committee, University of DelhiDU/ZOOL/IAEC-R/2015/07.
Author contributions
RC, DT, JS, and BG designed the study. AS, RC, JS, and GK cultured the fish and analyzed the samples. JS, RC, PM, DT, BG, and AS performed statistical analysis and wrote the manuscript. AS, RC, JS, and GK prepared graphs and tables. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors would like to thank the Department of Biotechnology (DBT), Government of India, New Delhi, India (Dy. No. 102/IFD/SAN/4678/2015-2016, dated 28.3.2016) and the Biotechnology and Biological Science Research Council (BBSRC) Newton Fund Global Research Partnership Project (BB/N005031/1) for providing financial support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.788455/full#supplementary-material
References
1
Abou Y. Fiogbé E. D. Beckers Y. Micha J. C. (2011). Approximate compositional values and tissue fatty acid profiles of Nile tilapia (Oreochromis niloticus L.) fed Azolla-diets in earthen ponds.Food Nutr. Sci.2964–973. 10.4236/fns.2011.29131
2
Aksnes A. Opstvedt J. (1998). Content of digestible energy in fish feed ingredients determined by the ingredient-substitution method.Aquaculture16145–53. 10.1016/S0044-8486(97)00255-X
3
Alarcón F. J. Díaz M. Moyano F. J. Abellán E. (1998). Characterization and funtional properties of digestive proteases in two sparids; gilthead sea bream (Sparus aurata) and common dentex (Dentex dentex).Fish Physiol. Biochem.19257–267.
4
AOAC (2000). Official Methods of Analysis.Washington, DC: Association of Official Analytical Chemists Inc.
5
APHA (2017). Standard Methods for the Examination of Water and Waste Water, 22nd Edn. Washington DC: American Public Health Association, American Water Works Association, Water Environment Federation.
6
Appenroth K. J. Sree K. S. Böhm V. Hammann S. Vetter W. Leiterer M. et al (2017). Nutritional value of duckweeds (Lemnaceae) as human food.Food Chem.217266–273. 10.1016/j.foodchem.2016.08.116
7
Aslam S. Zuberi A. Chan M. W. H. Mustaquim J. (2021). Effect of Lemna minor and Glycine max on haematological parameters, glucose level, total protein content and anti-oxidant enzyme activities in Ctenopharyngodon idella and Hypophthalmichthys molitrix.Aquac. Rep.19:100616. 10.1016/j.aqrep.2021.100616
8
Bairagi A. Ghosh K. S. Sen S. K. Ray A. K. (2002). Duckweed (Lemna polyrhiza) leaf meal as a source of feedstuff in formulated diets for rohu (Labeo rohita Ham.) fingerlings after fermentation with a fish intestinal bacterium.Bioresour. Technol.8517–24. 10.1016/s0960-8524(02)00067-6
9
Bassler R. Buchholz H. (1993). Amino Acid Analysis Methodenbuch, vol III, 4.1 1.1. Die chemische Untersuchung von Futtermitteln.Darmstadt: VDLUFA-Verlag, 1–5.
10
Bell J. G. Dick J. R. Vicar M. C. Sargent J. R. Thompson K. D. (1993). Dietary sunflower, linseed and fish oils affect phospholipid fatty acid composition, development of cardiac lesions, phospholipase activity and eicosanoid production in Atlantic salmon.Prostaglandins Leukot. Essent. Fatty Acids49665–673. 10.1016/0952-3278(93)90075-8
11
Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.J. Anal. Biochem.72248–254. 10.1016/0003-2697(76)90527-3
12
Cao M. J. Osatomi K. Suzuki M. Hara K. Tachibana K. Ishihara T. (2000). Purification and characterization of two anionic trypsins from the hepatopancreas of carp.Fish. Sci.661172–1179. 10.1046/j.1444-2906.2000.00185.x
13
Castro L. F. C. Tocher D. R. Monroig Ó (2016). Long-chain polyunsaturated fatty acid biosynthesis in chordates: insights into the evolution of fads and elovl gene repertoire.Prog. Lipid Res.6225–40. 10.1016/j.plipres.2016.01.001
14
Chakrabarti R. (2017). “Culture of zooplankton and aquatic macrophytes as non-conventional livelihood,” in Aquaculture for Nutritional and Livelihood Security, edsDhanzeR.NinaweA. S.DhanzeJ. R. (New Delhi: Narendra Publishing House), 189–203.
15
Chakrabarti R. Clark W. D. Sharma J. G. Goswami R. K. Shrivastav A. K. Tocher D. R. (2018). Mass production of Lemna minor and its amino acid and fatty acid profiles.Front. Chem.6:479. 10.3389/fchem.2018.00479
16
Chakrabarti R. Rathore R. M. (2009). Ontogenic changes in the digestive enzyme patterns and characterization of proteases in Indian major carp Cirrhinus mrigala.Aquac. Nutr.16569–581. 10.1111/j.1365-2095.2009.00694.x
17
Christie W. W. (2003). Lipid Analysis, 3rd Edn. Bridgewater, UK: Oily Press.
18
Cruz Y. Kijora C. Wedler E. Danier J. Schulz C. (2011). Fermentation properties and nutritional quality of selected aquatic macrophytes as alternative fish feed in rural areas of the neotropics.Livest. Res. Rural Dev.23239–246.
19
Cruz-Velásquez Y. Kijora C. Vergara-Hernández W. Schulz C. (2014). On-farm evaluation of Cachama blanca and Nile tilapia fed fermented aquatic plants in a polyculture.Orinoquia18269–277. 10.1016/j.aquaculture.2020.735176
20
El-Shafai S. A. El-Gohary F. A. Verreth J. A. J. Schrama J. W. Gijzen H. J. (2004). Apparent digestibility coefficient of duckweed (Lemna minor), fresh and dry for Nile tilapia (Oreochromis niloticus L.).Aquac. Res.35574–586. 10.1111/j.1365-2109.2004.01055.x
21
FAO (2001). Duckweed: a Tiny Aquatic Plant with Enormous Potential for Agriculture and Environment.Geneva: Food and Agricultural Organization of the United Nations.
22
FAO (2020). The State of World Fisheries and Aquaculture 2020. Sustainability in action.Rome: Food and Agricultural Organization of the United Nations, 10.4060/ca9229en
23
Fasakin E. A. (2008). Fish as food yesterday, today and forever. Inaugural Lecture Series 48.Akure: The Federal University of Technology, 52.
24
Fasakin E. A. Balogun A. M. Fagbenro O. A. (2001). Evaluation of sun-dried water fern, Azolla africana and duckweed, Spirodela polyrrhiza in practical diets for Nile tilapia, Oreochromis niloticus fingerlings.J. Appl. Aquac.1183–92. 10.1300/J028v11n04_09
25
Fasakin E. A. Balogun A. M. Fasuru B. E. (1999). Use of duckweed, Spirodela polyrrhiza L. Schleiden, as a protein feedstuff in practical diets for tilapia, Oreochromis niloticus L.Aquac. Res.30313–318. 10.1046/j.1365-2109.1999.00318.x
26
Folch J. Lees M. Sloane-Stanley G. H. (1957). A simple method for the isolation and purification of total lipids from animal tissues.J. Biol. Chem.226497–509. 10.1007/s11745-002-1004-1
27
Fonseca-Madrigal J. Karalazos V. Campbell P. J. Bell J. G. Tocher D. R. (2005). Influence of dietary palm oil on growth, tissue fatty acid compositions and fatty acid metabolism in liver and intestine in rainbow trout (Oncorhynchus mykiss).Aquac. Nutr.11241–250. 10.1111/j.1365-2095.2005.00346.x
28
Francis D. S. Turchini G. M. Jones P. L. De Silva S. S. (2007). Dietary lipid source modulates in vivo fatty acid metabolism in the freshwater fish, Murray cod (Maccullochella peelii peelii).J. Agric. Food Chem.551582–1591. 10.1021/jf062153x
29
Gangadhar B. Umalatha H. Hegde G. Sridhar N. (2017). Digestibility of dry matter and nutrients from three ingredients by the carps Labeo fimbriatus (Bloch, 1795) and Cyprinus carpio Linnaeus, 1758 with a note on digestive enzyme activity.Indian J. Fish.6475–84. 10.21077/ijf.2017.64.3.69091-11
30
Gatlin D. M. Barrows F. T. Brown P. Dabrowski K. Gaylord T. G. Hardy R. W. et al (2007). Expanding the utilization of sustainable plant products in aquafeeds: a review.Aquac. Res.38551–579. 10.1111/j.1365-2109.2007.01704.x
31
Ghosh P. Ray A. K. (2014). Effects of duckweed (Lemna polyrhiza) meal incorporated diet on enzyme producing autochthonous gut bacteria in fingerling mrigal, Cirrhinus mrigala (Hamilton).Int. J. Fish. Aquat. Stud.272–78.
32
Glencross B. D. (2009). Exploring the nutritional demand for essential fatty acids by aquaculture species.Rev. Aquac.171–124. 10.1111/j.1753-5131.2009.01006.x
33
Glencross B. D. Baily J. Berntssen M. H. G. Hardy R. W. Mackenzie S. Tocher D. R. (2020). Risk assessment of the use of alternative animal and plant raw material resources in aquaculture feeds.Rev. Aquac.12703–758. 10.1111/raq.12347
34
Goswami R. K. Shrivastav A. K. Sharma J. G. Tocher D. R. Chakrabarti R. (2020). Growth and digestive enzyme activities of rohu Labeo rohita fed diets containing macrophytes and almond oil-cake.Anim. Feed Sci. Technol.263:114456. 10.1016/j.anifeedsci.2020.114456
35
Hansen A. C. Hemre G. I. (2013). Effects of replacing fish meal and oil with plant resources in on-growing diets for Atlantic cod Gadus morhua L.Aquac. Nutr.19641–650. 10.1111/anu.12078
36
Hardy R. W. (2010). Utilization of plant proteins in fish diets: effects of global demand and supplies of fishmeal.Aquac. Res.41770–776. 10.1111/j.1365-2109.2009.02349.x
37
Hasan M. R. Chakrabarti R. (2009). Use of Algae and Aquatic Macrophytes as Feed in Small-Scale Aquaculture. A Review. Food and Agricultural Organization Fisheries Technical Paper, 531.Geneva: Food and Agricultural Organization.
38
Herawati V. E. Pinandoyo P. Darmanto Y. Rismaningsih N. Radjasa O. K. (2020). The effect of fermented duckweed (Lemna minor) in feed on growth and nutritional quality of tilapia (Oreochromis niloticus).Biodivers. J.213350–3358. 10.13057/biodiv/d210759
39
Iqbal M. Yaqub A. Ayub M. (2021). Partial and full substitution of fishmeal and soybean meal by canola meal in diets for genetically improved farmed tilapia (O. niloticus): growth performance, carcass composition, serum biochemistry, immune response, and intestine histology.J. Appl. Aquac.1–26. 10.1080/10454438.2021.1890661
40
Kuhajda F. P. Jenner K. Wood F. D. Hennigar R. A. Jacobs L. B. Dick J. D. et al (1994). Fatty acid synthesis: a potential selective target for antineoplastic therapy.Proc. Natl. Acad. Sci. U. S. A.916379–6383. 10.1073/pnas.91.14.6379
41
Lazzarotto V. Me‘dale F. Larroquet L. Corraze G. (2018). Long-term dietary replacement of fishmeal and fish oil in diets for rainbow trout (Oncorhynchus mykiss): effects on growth, whole body fatty acids and intestinal and hepatic gene expression.PLoS One13:190730. 10.1371/journal.pone.0190730
42
Lee J. W. Kil D. Y. Keever B. D. Killefer J. McKeith F. K. Sulabo R. C. et al (2013). Carcass fat quality of pigs is not improved by adding corn germ, beef tallow, palm kernel oil, or glycerol to finishing diets containing distillers dried grains with solubles.J. Anim. Sci.912426–2437. 10.2527/jas.2012-5328
43
Li Y. Hu C. Zheng Y. Xia X. Xu W. Wang S. et al (2008). The effects of dietary fatty acids on liver fatty acid composition and delta-6 desaturase expression differ with ambient salinities in Siganus canaliculatus.Comp. Biochem. Physiol. B Biochem. Mol. Biol.151183–190. 10.1016/j.cbpb.2008.06.013
44
Livak K. J. Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method.Methods25402–408. 10.1006/meth.2001.1262
45
Mer R. R. Vadher K. H. Patel M. R. (2016). Effect of partial supplementation of duckweed (Lemna minor) on growth and survival of Labeo rohita (Hamilton, 1822) fry.Bioscan Int. Q. J. Life Sci.11101–106.
46
Monroig Ó Lopes-Marques M. Navarro J. C. Hontoria F. Ruivo R. Santos M. M. et al (2016). Evolutionary wiring of the polyunsaturated fatty acid biosynthetic pathway.Sci. Rep.6:20510. 10.1038/srep20510
47
Muggeo V. M. (2008). Segmented: an R package to fit regression models with broken-line relationships.R News820–25.
48
NRC (1998). National Research Council. Nutrient Requirements of Swine.Washington DC, USA: The National Academic Press.
49
NRC (2011). Nutrient Requirements of Fish and Shrimp.Washington, DC, USA: The National Academic Press.
50
Olsen R. E. Hansen A. C. Rosenlund G. Hemre G. I. Mayhew T. M. Knudsen D. L. et al (2007). Total replacement of fish meal with plant proteins indiets for Atlantic cod (Gadus morhua L.) II-Health aspects.Aquaculture272612–624. 10.1016/j.aquaculture.2007.05.010
51
Rathore R. M. Kumar S. Chakrabarti R. (2005). Digestive enzyme profile of Cyprinus carpio during ontogenic development.J. World Aquac. Soc.3637–39. 10.1016/j.cbpc.2005.06.007
52
Ren H. T. Yu J. H. Xu P. Tang Y. K. (2012). Influence of dietary fatty acids on muscle fatty acid composition and expression levels of Δ6 desaturase-like and elovl5-like elongase in common carp (Cyprinus carpio var. Jian).Comp. Biochem. Physiol. B Biochem. Mol. Biol.163184–192. 10.1016/j.cbpb.2012.05.016
53
Roberts I. M. (1985). Hydrolysis of 4-methylumbelliferyl butyrate: a convenient and sensitive fluorescent assay for lipase activity.Lipids20243–247. 10.1007/bf02534195
54
Rokey G. (2004). “Raw material for extrusion processing,” in Feeds and Pet Food Extrusion Manual, edsRiazM. N.BarronM. (College Station, TX: Taxas A & M University), 5.
55
Rodriguez S. M. Olvera N. M. A. Carmona O. C. (1996). Nutritional value of animal by-product meal in practical diets for Nile tilapia, Oreochromis niloticus (L) fry.Aquac. Res.2767–73. 10.1111/j.1365-2109.1996.tb00967.x
56
Sharma J. Clark W. D. Shrivastav A. K. Goswami R. K. Tocher D. R. Chakrabarti R. (2019). Production potential of greater duckweed Spirodela polyrhiza (L. Schleiden) and its biochemical composition evaluation.Aquaculture513:734419. 10.1016/j.aquaculture.2019.734419
57
Sharma J. G. Kumar A. Saini D. Targay N. L. Khangembam B. K. Chakrabarti R. (2016). In vitro digestibility study of some plant protein sources as aquafeed for carps Labeo rohita and Cyprinus carpio using pH-stat method.Indian J. Exp. Biol.54606–611.
58
Shiping S. U. Zhao X. W. (2005). The influence of different diets on the digestive enzyme activities of Amur sturgeon Acipenseride schrencki Brandt larvae.J. Biol.2227–29.
59
Stadtlander T. Förster S. Rosskothen D. Leiber F. (2019). Slurry-grown duckweed (Spirodela polyrhiza) as a means to recycle nitrogen into feed for rainbow trout fry.J. Clean. Prod.22886–93. 10.1016/j.jclepro.2019.04.196
60
Taşbozan O. Gökçe M. A. (2017). “Fatty Acids in Fish,” in Fatty Acids, ed.CatalaA. (London: IntechOpen), 143–159. 10.5772/68048
61
Tocher D. R. (2010). Fatty acid requirements in ontogeny of marine and freshwater fish.Aquac. Res.41717–732. 10.1111/j.1365-2109.2008.02150.x
62
Tocher D. R. Agaba M. Hastings N. Bell J. G. Dick J. R. Teale A. J. (2002). Nutritional regulation of hepatocyte fatty acid desaturation and polyunsaturated fatty acid composition in zebrafish (Danio rerio) and tilapia (Oreochromis niloticus).Fish Physiol. Biochem.24309–320.
63
Tocher D. R. Fonseca-Madrigal J. Dick J. R. Ng W. Bell J. G. Campbell P. J. (2004). Effects of water temperature and diet containing palm oil on fatty acid desturation and oxidation in hepatocytes and intestinal enterocytes of rainbow trout (Onchorhynchus mykiss).Comp. Biochem. Physiol. B Biochem. Mol. Biol.13749–63. 10.1016/j.cbpc.2003.10.002
64
Tocher D. R. Harvie D. G. (1988). Fatty acid compositions of the major phosphoglycerides from fish neural tissues; (n-3) and (n-6) polyunsaturated fatty acids in rainbow trout (Salmo gairdneri) and cod (Gadus morhua) brains and retinas.Fish Physiol. Biochem.5229–239. 10.1007/BF01874800
65
Torstensen B. E. Tocher D. R. (2010). “The effects of fish oil replacement on lipid metabolism of fish,” in Fish Oil Replacement and Alternative Lipid Sources in Aquaculture Feeds, edsTurchiniG. M.NgW.TocherD. R. (Boca Raton, Florida: Taylor & Francis, CRC Press), 405–437
66
Turchini G. M. Francis D. S. De Silva S. S. (2006). Fatty acid metabolism in the freshwater fish Murray cod (Maccullochella peelii) deduced by the whole-body fatty acid balance method.Comp. Biochem. Physiol. B Biochem. Mol. Biol.144110–118. 10.1016/j.cbpb.2006.01.013
67
Ueberschär B. (1988). Determination of the nutritional condition of individual marine fish larvae by analyzing their proteolytic enzyme activities with a highly sensitive fluorescence technique.Meeresforschung32144–154.
68
Xie D. Chen C. Dong Y. You C. Wang S. Monroig Ó et al (2021). Regulation of long-chain polyunsaturated fatty acid biosynthesis in teleost fish.Prog. Lipid Res.82:101095. 10.1016/j.plipres.2021.101095
69
Yang S. Luo J. Long Y. Du J. Xu G. Zhao L. et al (2019). Mixed diets reduce the oxidative stress of common carp (Cyprinus carpio): based on microRNA sequencing.Front. Physiol.10:631. 10.3389/fphys.2019.00631
70
Yılmaz E. Akyurt I. Gunal G. (2004). Use of duckweed Lemna minor as a protein feedstuff in practical diets for common carp, Cyprinus carpio, fry.Turkish J. Fish. Aquat. Sci.4105–109.
71
Zhao L. Luo J. Liu Q. Du J. Yang H. Li B. et al (2020). Different diets can affect the digestion and immunity of common carp (Cyprinus carpio) according to enzyme activity assay and transcriptome sequencing.Aquaculture523:735176.
72
Zhou X. Q. Zhao C. R. Lin Y. (2010). Compare the effect of diet supplementation with uncoated or coated lysine on juvenile Jian carp (Cyprinus carpio Var. Jian).Aquac. Nutr.13457–461. 10.1111/j.1365-2095.2007.00498.x
73
Zhu R. Lia L. Lia M. Yua Z. Wanga H. Wu L. (2020). The effects of substituting fish meal with soy protein concentrate on growth performance, antioxidant capacity and intestinal histology in juvenile golden crucian carp, Cyprinus carpio × Carassius auratus.Aquac. Rep.18:100435. 10.1016/j.aqrep.2020.100435
Summary
Keywords
Cyprinus carpio , Spirodela polyrhiza , digestive enzymes, linoleic acid, eicosapentaenoic acid, docosahexaenoic acid, fads2d6 , elovl2
Citation
Shrivastav AK, Kumar G, Mittal P, Tocher DR, Glencross BD, Chakrabarti R and Sharma J (2022) Effect of Greater Duckweed Spirodela polyrhiza Supplemented Feed on Growth Performance, Digestive Enzymes, Amino and Fatty Acid Profiles, and Expression of Genes Involved in Fatty Acid Biosynthesis of Juvenile Common Carp Cyprinus carpio. Front. Mar. Sci. 9:788455. doi: 10.3389/fmars.2022.788455
Received
02 October 2021
Accepted
03 January 2022
Published
14 February 2022
Volume
9 - 2022
Edited by
Songlin Li, Shanghai Ocean University, China
Reviewed by
Omid Safari, Ferdowsi University of Mashhad, Iran; Sudhir Garg, Chaudhary Charan Singh Haryana Agricultural University, India; Jingjing Tian, Chinese Academy of Fishery Sciences, China
Updates
Copyright
© 2022 Shrivastav, Kumar, Mittal, Tocher, Glencross, Chakrabarti and Sharma.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: JaiGopal Sharma, sharmajaigopal@yahoo.com
This article was submitted to Marine Fisheries, Aquaculture and Living Resources, a section of the journal Frontiers in Marine Science
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.