Abstract
Each individual cetacean is an ecosystem itself, potentially harboring a great variety of animals that travel with it. Despite being often despised or overlooked, many of these epizoites have been proven to be suitable bio-indicators of their cetacean hosts, informing on health status, social interactions, migration patterns, population structure or phylogeography. Moreover, epizoites are advantageous over internal parasites in that many of them can be detected by direct observation (e.g., boat surveys), thus no capture or dissection of cetaceans are necessary. Previous reviews of epizoites of cetaceans have focused on specific geographical areas, cetacean species or epibiotic taxa, but fall short to include the increasing number of records and scientific findings about these animals. Here we present an updated review of all records of associations between cetaceans and their epibiotic fauna (i.e., commensals, ecto- or mesoparasites, and mutualists). We gathered nearly 500 publications and found a total of 58 facultative or obligate epibiotic taxa from 11 orders of arthropods, vertebrates, cnidarians, and a nematode that are associated to the external surface of 66 cetacean species around the globe. We also provide information on the use as an indicator species in the literature, if any, and about other relevant traits, such as geographic range, host specificity, genetic data, and life-cycle. We encourage researchers, not only to provide quantitative data (i.e., prevalence, abundance) on the epizoites they find on cetaceans, but also to inform on their absence. The inferences drawn from epizoites can greatly benefit conservation plans of both cetaceans and their epizoites.
Introduction
General Features of Epibiosis in Cetaceans
Cetaceans have developed a number of symbiotic associations (sensuLeung and Poulin, 2008) with other organisms, including endo-, meso- and ectoparasitism, commensalism, and mutualism (e.g., Arvy, 1982; Raga, 1994). Some of these organisms, the epibionts (also known as episymbionts or ectosymbionts), are associated to the external surface of cetaceans and can be classified into two basic types. On the one hand, ectoparasites live in/on the skin and cause a variable degree of harm by feeding on hosts’ integument (e.g., Smyth, 1962; Geraci and St. Aubin, 1987; Hopla et al., 1994). On the other hand, commensals or phoronts do not trophically depend on the tissues of cetaceans (also named basibionts in this case), thus they are generally harmless but benefit from epibiosis in multiple ways, e.g., via an improved feeding performance, reduction of predation, favored intraspecific contacts for reproduction, or offspring dispersion (Anderson, 1994; Seilacher, 2005; Carrillo et al., 2015). Not surprisingly, though, the limits of each type of interaction are not always clear-cut. For instance, whale-lice (fam. Cyamidae) are considered ectoparasites that primarily feed on hosts’ skin, but it has been speculated that they may opportunistically feed on plankton, even helping whales to detect plankton blooms, leading to a potentially mutualistic relationship (Rowntree, 1996). Or, high loads of commensal epibionts could increase the swimming drag or damage the skin on the site of settlement, thus producing indirect harm to cetaceans (Tomilin, 1957).
Given the high variety of life cycles of the epibionts of cetaceans, it is perhaps not surprising that their specific interactions are similarly diverse. Some epibionts depend strictly on cetaceans during their whole life (e.g., whale lice; Leung, 1976), whereas others use them only at some stages (e.g., barnacles; Nogata and Matsumura, 2006). Among commensals, many species are obligate epibionts, settling exclusively on cetaceans (e.g., coronulid barnacles; Hayashi et al., 2013), but others can colonize also inanimate substrata such as vessels or floating debris (e.g., Conchoderma spp. and Lepas spp.; Frick and Pfaller, 2013). The degree of host/basibiont specificity is also variable. For instance, many whale lice are known only from single, or a few, host species (Iwasa-Arai and Serejo, 2018), but other epibionts have a very broad host spectrum (e.g., Xenobalanus globicitipitisSteenstrup, 1852 or Pennella balaenopteraKoren & Danielssen, 1877; Kane et al., 2008; Fraija-Fernández et al., 2018). Finally, there are examples of hyperepibiosis in which some epibionts, e.g., barnacles, can act as basibionts for other epibionts, e.g., Conchoderma spp. or cyamids (Cornwall, 1927; Matthews, 1937; Leung, 1970a).
Susceptibility and Health Impact of Cetacean Epibiosis
As many other symbionts, epibionts must succeed twice to live their associative life. This two-step process is mediated by the so-called encounter and compatibility filters (Combes, 2001). First, spatial and temporal overlap must take place for initial settlement. Second, whether the host is a suitable substratum will determine survival and/or reproduction on it. Epidermis renewal and hydrolytic substances of cetacean skin may prevent fouling, at least to some extent (Hicks 1985; Baum et al., 2000; Baum et al., 2001), but skin regeneration and immune functions are seemingly lower in debilitated dolphins (J. R. Geraci and S. H. Ridgway pers comm. in Aznar et al., 1994). Poor health can also result in slower swimming (Aznar et al., 1994; Lehnert et al., 2021), fostering better conditions for epibiotic settlement (e.g., providing more time for contact with blooms of free-living infective stages, or mild water flow over the host’s body, thus reducing drag and facilitating initial colonization). For instance, striped dolphins, Stenella coeruleoalba (Meyen, 1833), infected by morbillivirus and in poor nutritional condition harbored high loads of parasitic and commensal epizoites (Aguilar and Raga, 1993; Aznar et al., 1994; Aznar et al., 2005). Also, higher prevalence of cyamids in porpoises could hint a higher incidence of disease-related skin injuries, where they attach (Lehnert et al., 2021). Another example is the massive infestation of cyamids on a stranded humpback whale, Megaptera novaeangliae (Borowski, 1781), that suffered from severe discospondylitis and, as a result, reduced mobility (Groch et al., 2018).
Once settled, the impact of epibionts on cetacean health varies among taxa (especially between ectoparasites and commensals; see above). For instance, the mesoparasite Pennella balaenoptera penetrates the skin and blubber of its hosts; this process has been related to both macro- and microscopic lesions such as abscesses, inflammation, and dermatitis (Cornaglia et al., 2000; Gomerčić et al., 2006; IJsseldijk et al., 2018). In contrast, no direct damage has been related to whale lice infections (e.g., Migaki, 1987; Lehnert et al., 2021), although it has been speculated that their occurrence may hinder skin healing processes (Lehnert et al., 2021). On the other hand, the possibility that some cetacean epibionts can act as viral or bacterial vectors is an open question, as it has been observed for ectoparasitic crustaceans parasitizing fish (Smit et al., 2019) or lice infecting seals (La Linn et al., 2001). Climate changes have shifted the geographical distribution of arthropod-borne viruses (Gould and Higgs, 2008) and whether these may emerge in cetaceans and even be transmitted by their epibonts (e.g., ectoparasitic lice, see Van Bressem et al., 2009) remains unknown.
Epibionts as Cetacean Indicators
Due to temporal or permanent association with their hosts/basibionts, both endoparasites and epibionts represent a cost-effective tool to study multiple facets of cetacean biology (e.g., Dailey and Vogelbein, 1991; Balbuena et al., 1995; Gomes et al., 2021). However, epibionts are advantageous over endoparasites in that many of them are detectable in the field (e.g., using boat-based photography; see Hermosilla et al., 2015; Siciliano et al., 2020; Flach et al., 2021), and can often be easily found and counted on stranded hosts, be alive or dead, with minimum dissection, if at all (Balbuena et al., 1995). Most studies using epibionts as markers only require basic data to be gathered, i.e. genus- or, preferably, species-level identification, and quantification of population size at host individual or population scales. More elaborated research may require additional information on (1) degree of host specificity, (2) size measurements as an estimate of time since attachment, (3) distribution patterns on hosts’ body, (4) geographic range, and/or (5) selected molecular markers (e.g., Bushuev, 1990; Kaliszewska et al., 2005; Ten et al., 2019; Moreno-Colom et al., 2020; Lehnert et al., 2021).
At present, cetacean epibionts have been used, inter alia, as ‘tags’ to trace past (e.g., Collareta et al., 2018a; Taylor et al., 2019) or present-day (e.g., Pearson et al., 2020; Visser et al., 2020) migratory routes and habitat use; shed light on phylogeography, population structure, and ecological stock delimitation (e.g., Bushuev, 1990; Kaliszewska et al., 2005; Iwasa-Arai et al., 2018); give insight into hydrodynamics (e.g., Kasuya and Rice, 1970; Briggs and Morejohn, 1972; Fish and Battle, 1995; Carrillo et al., 2015; Moreno-Colom et al., 2020), assist in individual recognition (e.g., Visser et al., 2020); and act as sentinels of health status (Mackintosh and Wheeler, 1929; Van Waerebeek et al., 1993; Aznar et al., 1994; Aznar et al., 2005; Lehnert et al., 2007; Vecchione and Aznar, 2014; Lehnert et al., 2021; for more references see Results). Nonetheless, there is plently of further opportunities to exploit the full potential of these organisms as biological indicators.
Aims
Studies including information on cetacean epibionts have usually focused on particular geographical areas (e.g., Kane et al., 2008; Lehnert et al., 2019), host species (e.g., Rice, 1978; Stimmelmayr and Gulland, 2020) or epibiotic taxa (e.g., Kane et al., 2008; Iwasa-Arai and Serejo, 2018). Furthermore, in the last decades a number of nomenclatural changes, new associations, and geographical records have been accumulating, thus we think that the available comprehensive reviews and checklists on this subject (Beneden, 1870; Dailey and Brownell, 1972; Arvy, 1977; Arvy, 1982; Raga, 1994) should be updated. On the other hand, few articles have reviewed the use of marine mammal parasites as biological tags (Balbuena et al., 1995; Mackenzie, 2002), and none gathered information about the whole epibiotic fauna of cetaceans.
The present systematic review aims to compile and update all records of cetacean epibiotic fauna (= epizoites) to date as a thorough, handy catalogue for researchers. Other organisms, i.e. diatoms and cookie-cutter shark, Isistius brasiliensis (Quoy & Gaimard, 1824) are also included in a specific section of this review to provide a complete picture of other externally-associated organisms that have been proven to be valuable biological indicators for cetaceans. Finally, we identify information gaps and future research directions and highlight the value of cetacean epibionts as indicator tools, encouraging their application in cetacean research.
Methods
Literature Search
A systematic literature review was performed following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Moher et al., 2015; Figure 1). We conducted a thorough bibliographic search in the following databases: Google Scholar (https://scholar.google.com), Scopus (https://www.scopus.com), ScienceDirect (https://www.sciencedirect.com), Web of Science (https://www.webofscience.com), and Sage (https://journals.sagepub.com). The following search string was used for Scopus, ScienceDirect, Web of Science, and Sage: (epibiont OR epibiotic OR epibiosis OR epizoite OR epizoic OR barnacle OR ectoparasite OR mesoparasite) AND (balaena OR eubalaena OR balaenoptera OR megaptera OR eschrichtius OR caperea OR cephalorhynchus OR delphinus OR feresa OR globicephala OR grampus OR lagenodelphis OR lagenorhynchus OR lissodelphis OR orcaella OR orcinus OR peponocephala OR pseudorca OR sotalia OR “Sousa chinensis” OR “Sousa plumbea” OR “Sousa sahulensis” OR “Sousa teuszii” OR stenella OR “Steno bredanensis” OR tursiops OR “Inia geoffrensis” OR kogia OR delphinapterus OR “Monodon monoceros” OR neophocaena OR phocoena OR phocoenoides OR physeter OR platanista OR pontoporia OR berardius OR hyperoodon OR mesoplodon OR tasmacetus OR ziphius OR indopacetus)
Figure 1
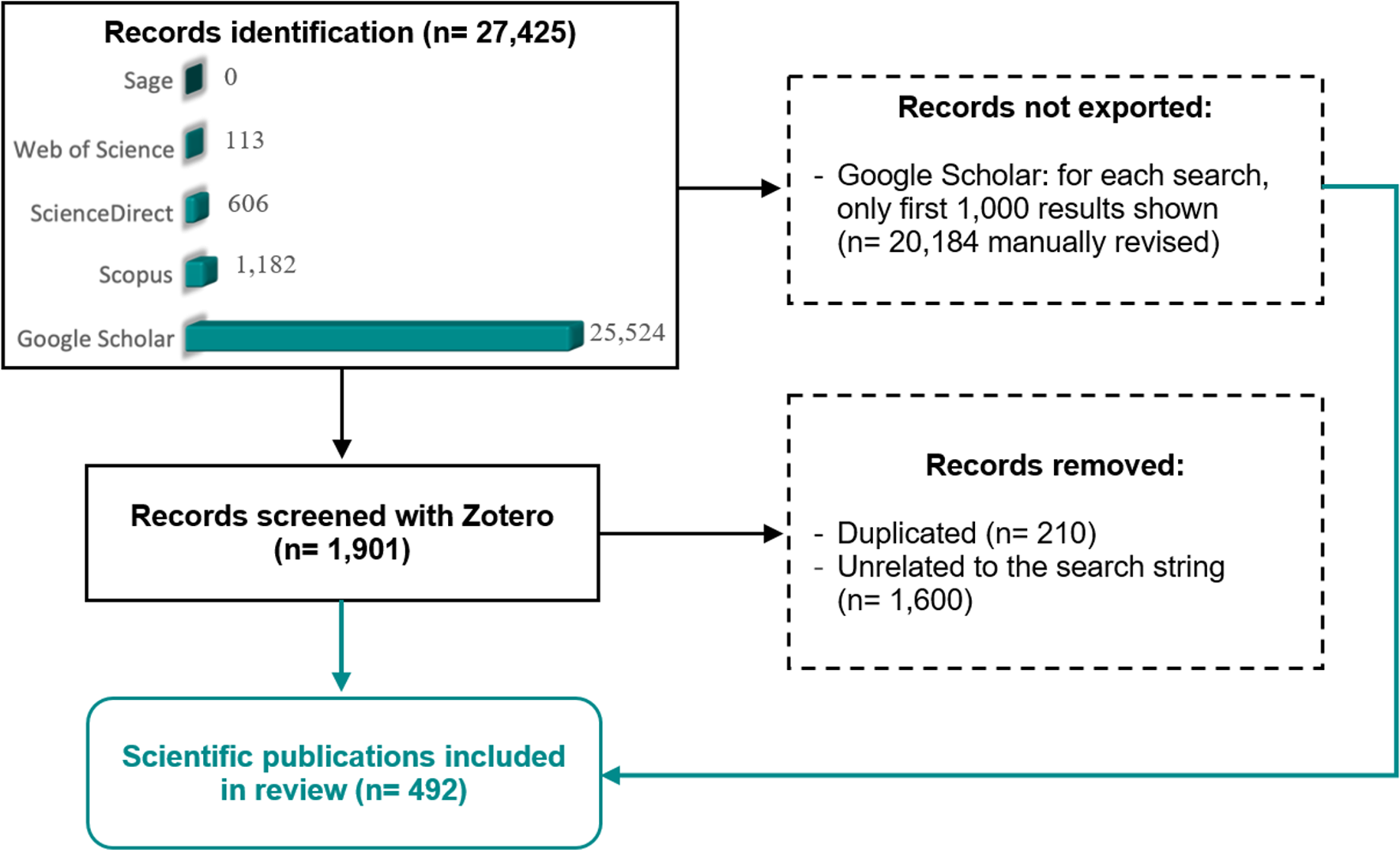
Flow diagram of the methodology used in the literature search performed in this systematic review. Adaptation from PRISMA (Preferred Reporting Items for Systematic Reviews) template (Page et al., 2021).
Note that the use of genus name in some cetacean genera, i.e., MonodonLinnaeus, 1758, Sousa Gray, 1866, and Steno Gray, 1846 yielded many records of unrelated taxa, thus full species name was included in these cases. The output was exported and checked for duplicates and non-relevant papers with the open-source reference management software Zotero.
In the case of Google Scholar, only the first 100 result pages are available, thus we used the search strings “(epibiont OR epibiotic OR epibiosis OR epizoite OR epizoic OR barnacle OR ectoparasite OR mesoparasite) AND i”, where i stands for a cetacean genus, to maximize the number of obtainable records. The output of each search was checked manually. In addition, we searched each epibiotic species in GBIF.org and included those associations and geographic locations that had not been reported in scientific publications. For all publications obtained, we looked up their references to search for potential missing records.
The final list includes the literature published until December 2021 that provides information on cetacean-epibiont(s) associations (Figure 1). These results are listed according to the epibiotic (see the Results) and the cetacean taxa (Supplementary Table 1). For each selected record, we extracted the following information, when available: cetacean species, epibiotic species, geographic area(s), prevalence (i.e., percent occurrence of the epibiont in each cetacean species of the sample), location on the cetacean, and any information related to indicator potential. Current species nomenclature and synonyms were checked in WoRMS (https://www.marinespecies.org/) and recent literature. Geographical locations were also classified at the scale of Large Marine Ecosystem (LME) (see e.g., Brotz et al., 2012).
For comparative purposes, we investigated research effort on each cetacean species using the number of results in Google Scholar as a proxy. For each species, we used the scientific name in quotation marks as search string. For the 6 species that previously constituted the Lagenorynchus genus (see Vollmer et al., 2019), we used the former nomenclature for the search to avoid understimation (i.e., "Lagenorynchus" followed by species name).
Results
General Patterns
A total of 492 published documents, including 7 unpublished manuscripts, and 9 GBIF records were found. Three additional reliable records were serendipitously found in internet photo-catalogues and were also included in the final list (Supplementary Table 1). A roughly exponential trend in the number of publications was found throughout the period covered (1655-2021), with a peak in the 2010s decade (Figure 2); 2020 was the most productive year with 21 publications.
Figure 2
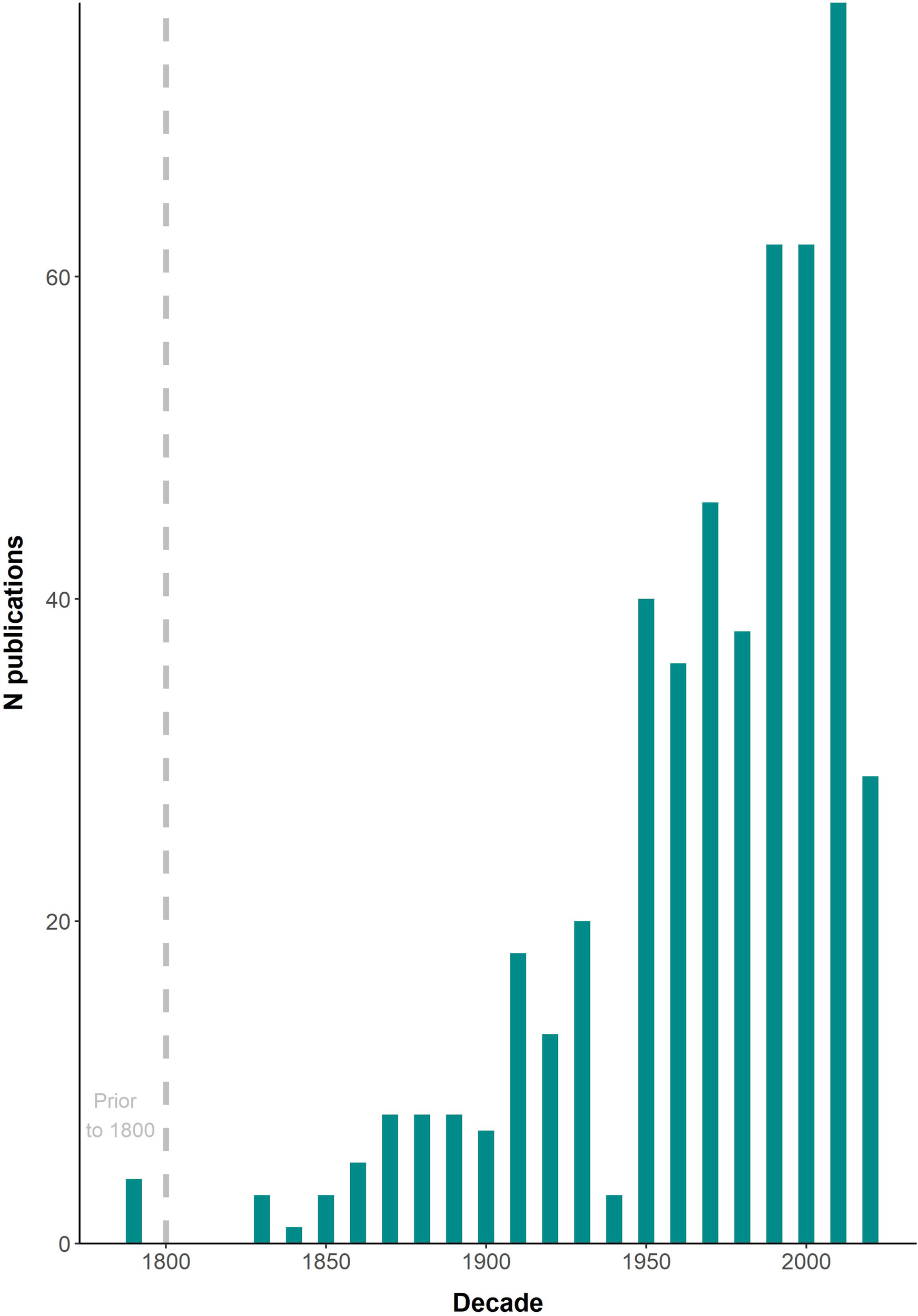
Number (N) of publications including data on cetacean epibiotic fauna at a decadal scale from 1655 to 2021.
Baleen whales, and particularly Megaptera novaeangliae (Borowski, 1781), show the highest diversity of epibionts, followed by Tursiops spp. (Figure 3). However, it is difficult to ascertain the extent to which this pattern is affected by sampling effort (Figure 3). Likewise, 26 cetacean species from four genera have no published records of epibiotic fauna to date (Supplementary Table 1), but these hosts have also been generally little studied (< 4,000 publications in Google Scholar, Figure 3). Research effort varies also among geographic regions (Figure 4). The Mediterranean Sea and Antarctica are, by far, the geographic areas with the highest number of publications of cetacean epizoites, and some areas still lack such studies.
Figure 3
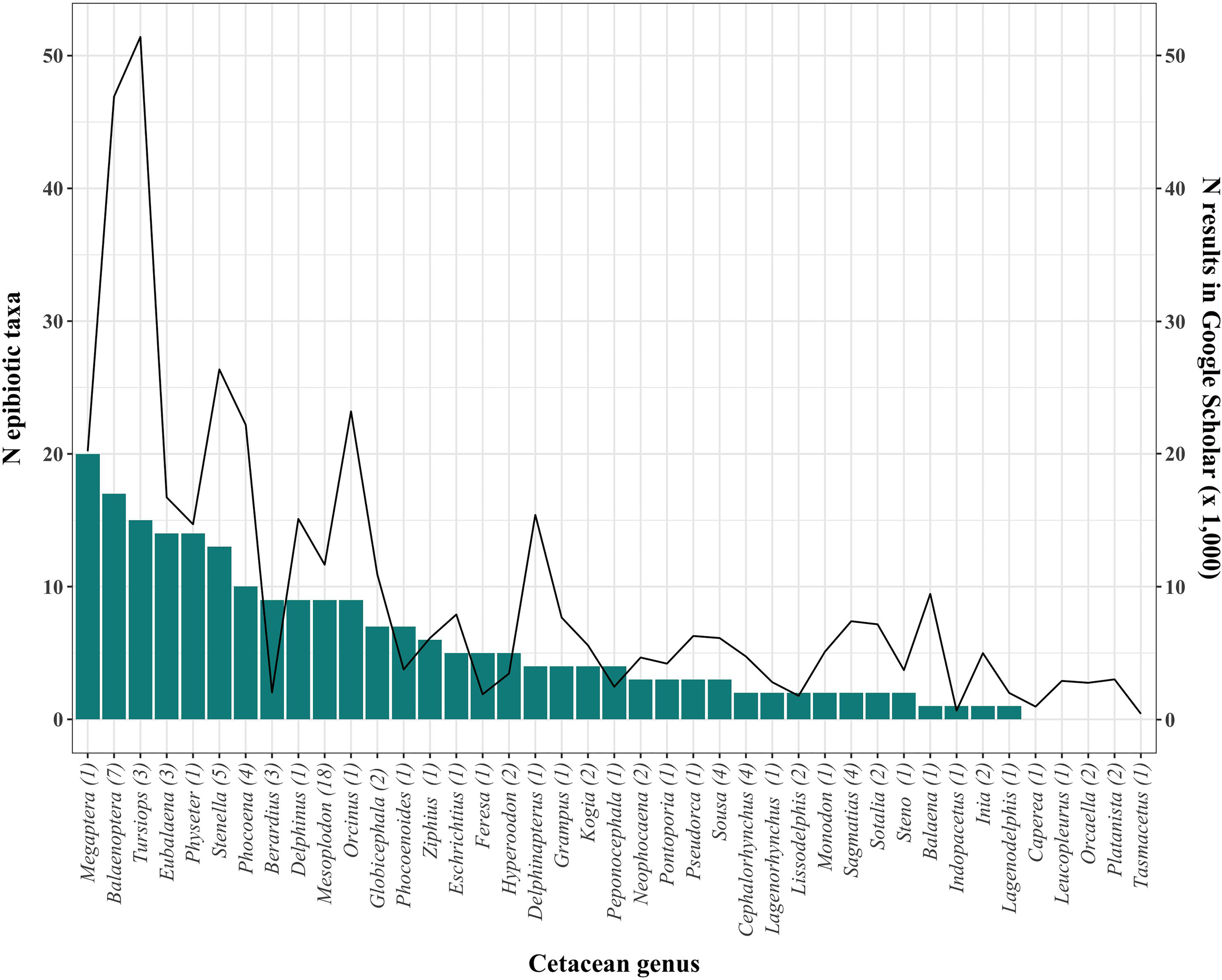
Number of epibiotic species (bars, left y-axis) and total number of general results in Google Scholar (line, right y-axis) of each cetacean genus. The number of cetacean species in each genus is shown in parentheses.
Figure 4
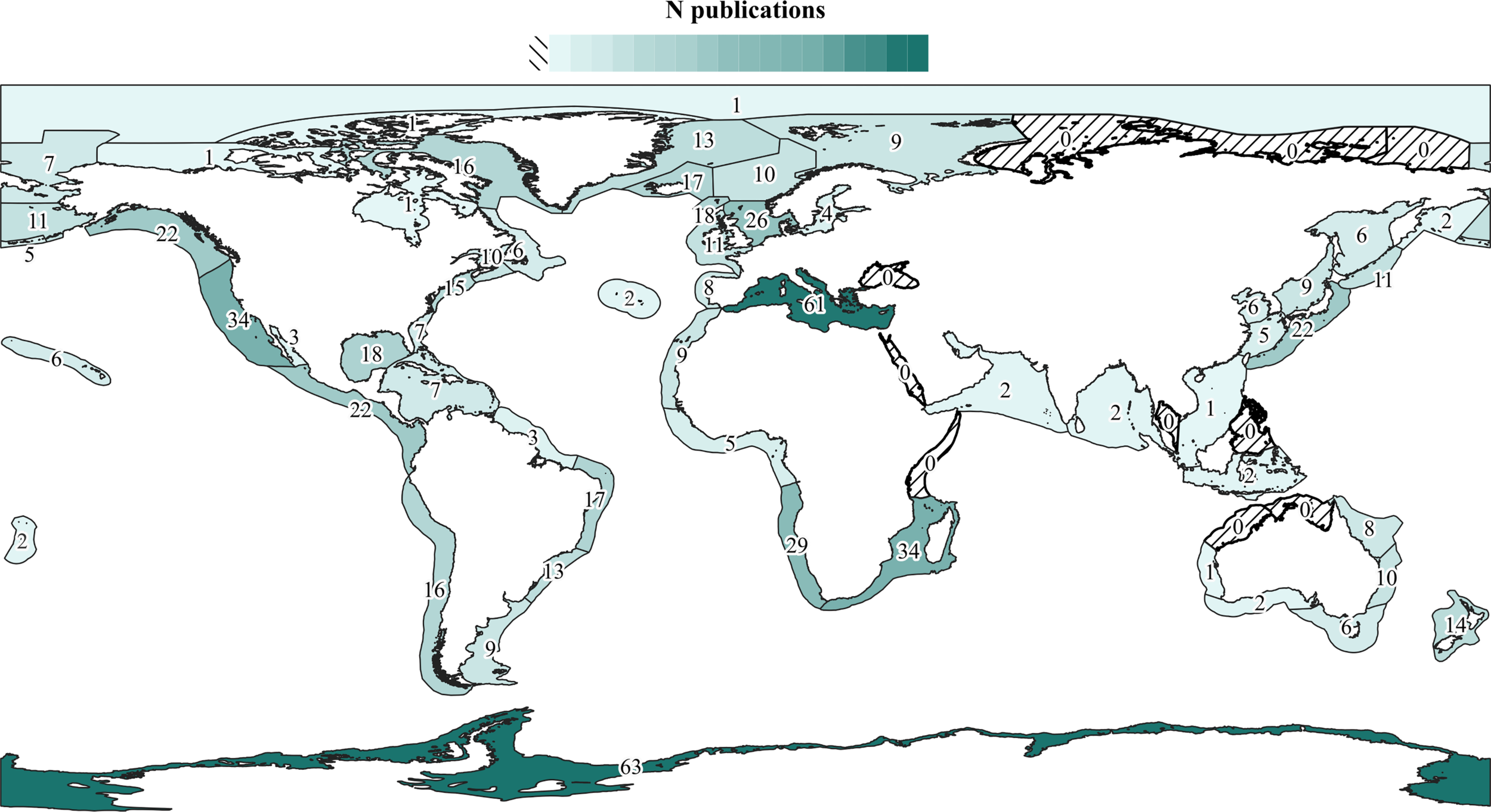
Number of publications (indicated by numbers and color gradient) on cetaceans that contain data on their epibiotic fauna at least to genus level grouped by Large Marine Ecosystems (LME). When the same publication includes data for several LMEs, it is counted separately for each one. Azores (NE Atlantic) and Tonga (SW Pacific) are not in the LME system but were included as additional areas.
Systematic List
A systematic list of the 58 epizoic taxa (53 at species level) found to date on cetaceans follows. For each one, we provide information on (i) taxonomic synonyms; (ii) a subset of selected references that provide a complete overview of the species morphology; (iii) molecular sequences available on GenBank (https://www.ncbi.nlm.nih.gov/genbank/), with references or with Accession Number whenever no published manuscript was available; (iv) primary type of association, including parasitic (34 spp.), obligate commensal (8-9 spp.), facultative commensal (8 spp.), mutualistic (possibly 1 sp.), or unknown (2 spp.); (v) a list of cetacean hosts/basibionts; (vi) geographic range; (vii) life-cycle; and (viii) microhabitat, i.e., the location(s) on the cetacean body, with references; and (ix) indicator use or potential, with references. Any other relevant data are reported in the ‘Remarks’ section, and all records of association between epizoites and cetaceans are cited in the ‘References’ section.
-
Phylum Arthropoda von Siebold, 1848
-
Class Malacostraca Latreille, 1802
-
Subclass Eumalacostraca, Grobben, 1892
-
Order Amphipoda Latreille, 1816
-
Family Cyamidae Rafinesque, 1815
The Cyamidae (‘whale lice’) comprises a group of amphipods that are found exclusively on marine cetaceans (see, e.g., Iwasa-Arai and Serejo, 2018). These 3-30 mm creatures use their pereopods to cling to areas of reduced water flow (e.g., ventral grooves, blowhole, genital slit), where they spend their whole life feeding primarily on cetacean skin (Rowntree, 1983; Rowntree, 1996; Schell et al., 2000); thus, they are all considered ectoparasites. However, evidence that they cause any harm is rather scarce, so some authors support the use of the term ‘ectocommensals’ for them (Leung, 1976; Kenney, 2009). Rowntree (1996) discussed the possibility that some cyamids from whales may also feed on plankton, having perhaps developed mutualistic associations with their hosts. In particular, the cyamid species covering the sensory hairs of whales could increase their activity during plankton blooms, amplifying the signal for prey detection by whales. In addition, it has also been suggested that cyamids could feed on cetaceans’ dead skin and epibiotic algae, thus cleaning up wounds and speeding up healing (Williams and Bunkley-Williams, 2019). Lehnert et al. (2021), on the contrary, hypothesized that cyamids’ feeding activity could actually hinder the healing of skin injuries, and some authors have suggested that heavy cyamid infections may contribute to the death of their hosts (Mignucci-Giannoni et al., 1998).
Since cyamids lack swimming stages, transmission must occur through bodily contacts (Fransen and Smeenk, 1991; Pfeiffer, 2009). Males are typically larger than females (but see Fraija-Fernández et al., 2017) and, at least in some species, have been observed to perfom pre-copulatory mate guarding (Rowntree, 1996; Oliver and Trilles, 2000). Females mate after molting (Conlan, 1991) and incubate eggs and protect the hatchling in a ventral brood pouch (Leung, 1976; Williams and Bunkley-Williams, 2019).
Balaenocyamus balaenopterae (Barnard K.H. 1931)
Synonyms
Cyamus balaenopterae Barnard K.H. 1931
Morphological Description
Barnard, 1932; Margolis, 1959; Leung, 1967; Iwasa-Arai and Serejo, 2018
Molecular Sequences
18S rRNA (Ito et al., 2011)
Association
Ectoparasite
Cetacean Hosts/Basibionts
Balaenoptera acutorostrata Lacépède, 1804, B. bonaerensis Burmeister, 1867, B. musculus (Linnaeus, 1758), B. physalus (Linnaeus, 1758)
Geographic Range
Atlantic, Pacific, Mediterranean, Indian Ocean, Antarctica
Life Cycle
In common minke whales, Balaenoptera acutorostrata, captured off Iceland, a one-year long life cycle is assumed; similar to other whale lice, hatching occurs in autumn, juveniles are released from the females’ pouch in mid-winter, and they reach sexual maturity in spring or summer (Ólafsdóttir and Shinn, 2013). This life cycle may be synchronized with whales’ seasonal migration (Raga and Sanpera, 1986).
Microhabitat
Natural orifices, i.e., ventral grooves, eyes, umbilicus, mammary slits, anus, and genital slit (Ohsumi et al., 1970; Ivashin, 1975; Raga and Sanpera, 1986)
Use as Indicator
Used to delineate ecological stocks and detect sex segregation in migrating cetaceans (Kawamura, 1969; Bushuev, 1990; Ólafsdóttir and Shinn, 2013).
Remarks
-
References
Mackintosh and Wheeler, 1929; Barnard, 1931; Barnard, 1932; Margolis, 1959; Leung, 1965; Kawamura, 1969; Ohsumi et al., 1970; Lincoln and Hurley, 1974a; Ivashin, 1975; Rice, 1978; Berzin and Vlasova, 1982; Best, 1982; Raga and Sanpera, 1986; Avdeev, 1989; Bushuev, 1990; Sedlak-Weinstein, 1990 (unpubl.); Dailey and Vogelbein, 1991; Kuramochi et al., 1996; Araki et al., 1997; Uchida, 1998; Kuramochi et al., 2000; Margolis et al., 2000; Uchida and Araki, 2000; Ólafsdóttir and Shinn, 2013; Iwasa-Arai and Serejo, 2018; Ten et al., unpubl.
Cyamus boopis (Lütken, 1870)
Synonyms
Cyamus elongatus Hiro, 1938, C. pacificus Lütken, 1873, C. suffuses Dall, 1872, Paracyamus boopis (Lütken, 1870)
Morphological Description
Sars, 1895; Barnard, 1932; Leung, 1967; Margolis et al., 2000; Iwasa-Arai et al., 2016
Molecular Sequences
COI (Iwasa-Arai et al., 2017a, Iwasa-Arai et al., 2018; GenBank FJ751158; FJ751159; MT551876; OK562816-OK562832), COII, COIII, ATP6, ATP8, ND3 (Kaliszewska et al., 2005) and the complete mitochondrial genome (GenBank MT458501)
Association
Ectoparasite
Cetacean Hosts/Basibionts
Typically on Megaptera novaeangliae, but once reported on Berardius bairdii Duvernoy, 1851, Eubalaena australis (Desmoulins, 1822), and Tursiops truncatus (Montagu, 1821)
Geographic Range
Arctic, Atlantic, Pacific, Mediterranean, Indian Ocean, Antarctica
Life Cycle
Transmission may regularly occur during contacts between migrating hosts or at the feeding areas (Iwasa-Arai et al., 2018).
Microhabitat
Ubiquitous, i.e., head tubercles, eye, jaw, ventral grooves, genital slit, fins (Matthews, 1937; Cockrill, 1960; Ivashin, 1965; Rowntree, 1996). Sometimes attached to the epibiotic cirripedes Coronula diadema (Linnaeus, 1767) and Conchoderma spp. (Dall, 1872; Matthews, 1937; Stephensen, 1942; Angot, 1951; Cockrill, 1960).
Use as Indicator
Haplotype and nucleotide diversities have been used to assess inter-mixing between different breeding populations of humpback whales (Iwasa-Arai et al., 2018). Also, its presence on a southern right whale suggests an interspecific interaction with humpback whales in Brazilian waters (Iwasa-Arai et al., 2017a). The presence of an alive unidentified cyamid (likely C. boopis) on a humpback whale was used to infer that the stranding occurred less than three days before (Bortolotto et al., 2016).
Remarks
Some records of C. boopis on sperm whales (e.g., Barnard, 1932) were re-classified as C. catodontis by Margolis (1955) and later authors (e.g., Stock, 1973a; Iwasa-Arai and Serejo, 2018).
References
Lütken, 1870; Dall, 1872; Scammon, 1874; Pouchet, 1888; Pouchet, 1892; Sars, 1895; Collet, 1912; Chevreux, 1913a; Liouville, 1913; Ishi, 1915; Cornwall, 1928; Barnard, 1932; Matthews, 1937; Hiro, 1938; Scheffer, 1939; Angot, 1951; Hurley, 1952; Rees, 1953; Margolis, 1954a; Cockrill, 1960; Rice, 1963; Ivashin, 1965; Leung, 1965; Leung, 1970b; Lincoln and Hurley, 1974a; Berzin and Vlasova, 1982; Sedlak-Weinstein, 1991; Rowntree, 1996; Abollo et al., 1998; Osmond and Kaufman, 1998; Margolis et al., 2000; Alonso de Pina and Giuffra, 2003; Carvalho et al., 2010; Iwasa-Arai et al., 2016; Iwasa-Arai et al., 2017b; Iwasa-Arai et al., 2018; Groch et al., 2018; Iwasa-Arai et al., 2021; Iwasa-Arai et al., 2018; Qiao et al., 2020
Cyamus catodontis (Margolis, 1954)
Synonyms
Cyamus bahamondei Buzeta, 1963
Morphological Description
Margolis, 1954a; Margolis, 1955; Buzeta, 1963; Leung, 1967; Stock, 1973a; Margolis et al., 2000
Molecular Sequences
-
Association
Ectoparasite
Cetacean Hosts/Basibionts
Typically on Physeter macrocephalusLinnaeus, 1758, but once reported on Balaenoptera acutorostrata, B. bonaerensis, B. musculus, B. physalus, and Berardius bairdii
Geographic Range
Eastern Atlantic, Pacific, Indian Ocean, Antarctica
Life Cycle
-
Microhabitat
One record on a sperm whale’s deformed jaw (Buzeta, 1963)
Use as Indicator
Used to detect social segregation in sperm whales; large males, but not females nor male bachelors, were infected with C. catodontis, suggesting that the former leave their natal pods at puberty (Best, 1969a; Best, 1979).
Remarks
-
References
Barnard, 1932; Margolis, 1954a; Clarke, 1956; Buzeta, 1963; Rice, 1963; Leung, 1965; Best, 1969a; Best, 1969b; Best, 1979; Stock, 1973b; Lincoln and Hurley, 1974a; Berzin and Vlasova, 1982; Fransen and Smeenk, 1991; Iwasa-Arai and Serejo, 2018
Cyamus ceti (Linnaeus, 1758)
Synonyms
Oniscus ceti Linnaeus, 1758
Morphological Description
Krøyer, 1843; Leung, 1967; Margolis et al., 2000; Iwasa-Arai and Serejo, 2018
Molecular Sequences
COI (GenBank FJ751160-FJ751180)
Association
Ectoparasite
Cetacean Hosts/Basibionts
Typically on Balaena mysticetusLinnaeus, 1758, but once reported on Eschrichtius robustus (Lilljeborg, 1861) and Eubalaena japonica (Lacépède, 1818)
Geographic Range
Artic, North Pacific
Life Cycle
Similar to C. scammoni (see below), but juveniles reach maturity before whales’ northern migration to summer grounds (Leung, 1976). Females carry 150-240 eggs in the brood pouch, of which about 75% are fertilized (Leung, 1976).
Microhabitat
Creases of the lips, flippers, flukes, and thin areas, e.g., armpit and genital slit (Stephensen, 1942; Leung, 1976)
Use as Indicator
-
Remarks
-
References
Linnaeus, 1758; Lütken, 1870; Dall, 1872; Scammon, 1874; Margolis, 1955; Omura, 1958; Rice, 1963; Lincoln and Hurley, 1974a; Leung, 1976; Berzin and Vlasova, 1982; Heckmann et al., 1987; Margolis et al., 2000; Kaliszewska et al., 2005; Von Duyke et al., 2016; Chernova et al., 2017; Iwasa-Arai and Serejo, 2018
Cyamus erraticus (Roussel de Vauzème, 1834)
Synonyms
Paracyamus erraticus Roussel de Vauzème, 1834
Morphological Description
Barnard, 1932; Iwasa, 1934; Margolis, 1955; Leung, 1967
Molecular Sequences
COI, COII, COIII, ATP6, ATP8, ND3 (Kaliszewska et al., 2005), EF1a (Seger et al., 2010)
Association
Ectoparasite
Cetacean Hosts/Basibionts
Typically on Eubalaena australis, E. glacialis (Müller, 1776), and E. japonica; also found on Megaptera novaeangliae
Geographic Range
Atlantic, Pacific, Indian Ocean, Antarctica
Life Cycle
-
Microhabitat
Genital, mammary, and anal slits, armpits, and opportunistically on wounds (Stephensen, 1942; Rowntree, 1996; see Remarks)
Use as Indicator
Sequence variation in mitochondrial DNA was used to investigate associations among right whale individuals and subpopulations, to estimate the time of past divergence of right whale populations, and to infer possible changes in their population sizes (Kaliszewska et al., 2005).
Remarks
Transmission probably occurs from mothers’s genital slit to calves’ head at birth. As callosity tissue develops, calves are colonized by the putative competitor Cyamus ovalisRoussel de Vauzème, 1834, likely by head-to-head contact with the mother; the distribution of C. erraticus is then restricted to skin folds and wounds (Rowntree, 1996).
References
Rossel de Vauzème, 1834; Lütken, 1873; Collet, 1912; Chevreux, 1913a; Liouville, 1913; Barnard, 1932; Iwasa, 1934; Margolis, 1955; Lincoln and Hurle7y, 1974a; Berzin and Vlasova, 1982; Rowntree, 1996; Margolis et al., 2000; Iwasa-Arai and Serejo, 2018
Cyamus eschrichtii (Margolis, McDonald & Bousfield, 2000)
Synonyms
-
Morphological Description
Molecular Sequences
-
Association
Ectoparasite
Cetacean Hosts/Basibionts
Eschrichtius robustus
Geographic Range
California (eastern North Pacific)
Life Cycle
-
Microhabitat
-
Use as Indicator
-
Remarks
-
References
Cyamus gracilis (Roussel de Vauzème, 1834)
Synonyms
Paracyamus gracilis (Roussel de Vauzème, 1834)
Morphological Description
Barnard, 1932; Leung, 1967; Iwasa-Arai and Serejo, 2018
Molecular Sequences
COI, COII, COIII, ATP6, ATP8, ND3 (Kaliszewska et al., 2005), EF1a (Seger et al., 2010)
Association
Ectoparasite
Cetacean Hosts/Basibionts
Eubalaena australis, E. glacialis, E. japonica
Geographic Range
Atlantic, Pacific, Antarctica
Life Cycle
-
Microhabitat
Head callosities (Barnard, 1932; Rowntree, 1996)
Use as Indicator
See C. erraticus.
Remarks
In a South African sample, C. gracilis co-occurred with C. ovalisRoussel de Vauzème, 1834 (Barnard, 1932).
References
Rossel de Vauzème, 1834; Lütken, 1873; Barnard, 1932; Margolis, 1955; Leung, 1965, Leung 1967; Lincoln and Hurley, 1974a; Berzin and Vlasova, 1982; Rowntree, 1996; Alonso de Pina and Giuffra, 2003; Iwasa-Arai and Serejo, 2018
Cyamus kessleri (A. Brandt, 1873)
Synonyms
-
Morphological Description
Brandt, 1872; Leung, 1967; Margolis et al., 2000; Iwasa-Arai and Serejo, 2018
Molecular Sequences
COI (GenBank FJ751215-FJ751224)
Association
Ectoparasite
Cetacean Hosts/Basibionts
Eschrichtius robustus
Geographic Range
From Chukchi Sea to California (eastern North Pacific)
Life Cycle
Similar to C. scammoni (see below), but juveniles reach maturity before whales’ northern migration to summer grounds (Leung, 1976). Females carry up to 300 eggs in the brood pouch, of which 75-80% are fertilized (Leung, 1976).
Microhabitat
Umbilicus, genital slit, and anal aperture (Leung, 1976)
Use as Indicator
-
Remarks
-
References
Hurley and Mohr, 1957; Leung, 1976; Berzin and Vlasova, 1982; Margolis et al., 2000; Kaliszewska et al., 2005; Iwasa-Arai and Serejo, 2018
Cyamus mesorubraedon (Margolis, McDonald & Bousfield, 2000)
Synonyms
-
Morphological Description
Molecular Sequences
-
Association
Ectoparasite
Cetacean Hosts/Basibionts
Physeter macrocephalus
Geographic Range
Vancouver Island (eastern North Pacific)
Life Cycle
-
Microhabitat
-
Use as Indicator
-
Remarks
-
References
Cyamus monodontis (Lütken, 1870)
Synonyms
-
Morphological Description
Leung, 1967; Margolis et al., 2000; Iwasa-Arai et al., 2017b; Iwasa-Arai and Serejo, 2018
Molecular Sequences
-
Association
Ectoparasite
Cetacean Hosts/Basibionts
Delphinapterus leucas (Pallas, 1776), Monodon monocerosLinnaeus, 1758, Ziphius cavirostris Cuvier, 1823
Geographic Range
Arctic, western North Atlantic, eastern North Pacific
Life Cycle
-
Microhabitat
Tusk base, caudal fin along with C. nodosus, skin injuries (Porsild, 1922; Stephensen, 1942)
Use as Indicator
-
Remarks
-
References
Lütken, 1870; Porsild, 1922; Lincoln and Hurley, 1974a; Heyning and Dahlheim, 1988; Mignucci-Giannoni et al., 1998; Margolis et al., 2000; Iwasa-Arai et al., 2017a
Cyamus nodosus (Lütken, 1861)
Synonyms
Paracyamus nodosus (Lütken, 1861)
Morphological Description
Leung, 1967; Iwasa-Arai et al., 2017b; Iwasa-Arai and Serejo, 2018
Molecular Sequences
-
Association
Ectoparasite
Cetacean Hosts/Basibionts
Delphinapterus leucas, Monodon monoceros
Geographic Range
Greenland (Arctic, western North Atlantic)
Life Cycle
-
Microhabitat
Tusk base, caudal fin along with C. monodontis, skin injuries (Porsild, 1922; Stephensen, 1942)
Use as Indicator
-
Remarks
-
References
Lütken, 1870; Porsild, 1922; Margolis, 1954b; Margolis, 1955; Lincoln and Hurley, 1974a; Iwasa-Arai et al., 2017a
Cyamus orubraedon (Waller, 1989)
Synonyms
-
Morphological Description
Molecular Sequences
-
Association
Ectoparasite
Cetacean Hosts/Basibionts
Berardius bairdii
Geographic Range
North Pacific
Life Cycle
-
Microhabitat
Lower jaw (Waller, 1989)
Use as Indicator
-
Remarks
-
References
Waller, 1989; Margolis et al., 2000; Iwasa-Arai and Serejo, 2018
Cyamus ovalis (Roussel de Vauzème, 1834)
Synonyms
-
Morphological Description
Roussel de Vauzème, 1834; Iwasa, 1934; Leung, 1967; Margolis et al., 2000; Iwasa-Arai and Serejo, 2018
Molecular Sequences
COI (Kaliszewska et al., 2005; Seger et al., 2010), COII, COIII, ATP6, ATP8, ND3 (Kaliszewska et al., 2005), EF1a (Seger et al., 2010)
Association
Ectoparasite
Cetacean Hosts/Basibionts
Eubalaena australis, E. glacialis, E. japonica, Physeter macrocephalus; once reported on Megaptera novaeangliae
Geographic Range
Atlantic, Pacific, Antarctica
Life Cycle
-
Microhabitat
Head callosities, sometimes with C. erraticus (Stephensen, 1942; Rowntree, 1996; see C. erraticus, above)
Use as Indicator
See C. erraticus.
Remarks
Once misidentified as Cyamus rhytinae (J. F. Brandt, 1846), ectoparasitic on the extinct Steller’s sea cow, Hydrodamalis gigas (Zimmermann, 1780) Palmer, 1895 (see Leung, 1967; O'Clair and O'Clair, 1998).
References
Roussel de Vauzème 1834; Lütken, 1873; Collet, 1912; Liouville, 1913; Barnard, 1932; Iwasa, 1934; Margolis, 1955; Leung, 1967; Lincoln and Hurley, 1974a; Berzin and Vlasova, 1982; Rowntree, 1996; Margolis et al., 2000; Pettis et al., 2004; Iwasa-Arai and Serejo, 2018
Cyamus scammoni (Dall, 1872)
Synonyms
-
Morphological Description
Lütken, 1887; Leung, 1967; Margolis et al., 2000; Iwasa-Arai and Serejo, 2018
Molecular Sequences
COI (GenBank FJ751214), hemocyanin mRNA (Terwilliger and Ryan, 2006)
Association
Ectoparasite
Cetacean Hosts/Basibionts
Eschrichtius robustus
Geographic Range
North Pacific
Life Cycle
Females can carry about 1,000 eggs in the brood pouch, although only about a 60% are fertilized (Leung, 1976). Eggs hatch in autumn, when gray whales arrive in California, and the young remain in the female’s pouch for 2-3 months and then find shelter in host’s crevices (Leung, 1976). Juveniles reach maturity during the winter northward migration of whales, and have full-grown brood upon arrival to summer grounds. The whole cycle takes 8-9 months to complete and there is probably some overlap in the life cycle of different individuals, given that juveniles are present throughout the year (Leung, 1976). The number of instars is presumed to be at least 7 or 8, but the number of ecdysis was untraceable (Leung, 1976).
Microhabitat
Ventral grooves, i.e., jaw and belly; flukes; on the cirriped Cryptolepas rachianectiDall, 1872 (Leung, 1976; Dailey et al., 2000)
Use as Indicator
-
Remarks
Chonotrichous ciliates can infest its ventral surface (Leung, 1976).
References
Dall, 1872; Scammon, 1874; Lütken, 1887; Margolis, 1954a; Rice, 1963; Leung, 1965; Lincoln and Hurley, 1974a; Leung, 1976; Sullivan and Houck, 1979; Berzin and Vlasova, 1982; Dailey et al., 2000; Margolis et al., 2000; Kaliszewska et al., 2005; Takeda and Ogino, 2005; Murase et al., 2014; Iwasa-Arai and Serejo, 2018
Isocyamus antarcticensis (Vlasova in Berzin & Vlasova, 1982)
Synonyms
Cyamus antarcticensis Vlasova in Berzin & Vlasova, 1982
Morphological Description
Molecular Sequences
-
Association
Ectoparasite
Cetacean Hosts/Basibionts
Orcinus orca (Linnaeus, 1758)
Geographic Range
Antarctica
Life Cycle
-
Microhabitat
Pectoral fins, umbilicus (Berzin and Vlasova, 1982)
Use as Indicator
-
Remarks
-
References
Isocyamus delphinii (Guérin-Méneville, 1836)
Synonyms
Cyamus delphinii Guérin-Méneville, 1836, C. globicipitis Lütken, 1870
Morphological Description
Barnard, 1932; Leung, 1967; Stock, 1973a; Stock, 1973b; Stock, 1977; Sedlak-Weinstein, 1991; Margolis et al., 2000; Lehnert et al., 2007; Lehnert et al., 2021
Molecular Sequences
COI (Lehnert et al., 2021)
Association
Ectoparasite
Cetacean Hosts/Basibionts
Typically found on Globicephala melas (Traill, 1809); some records on Delphinus delphisLinnaeus, 1758, Grampus griseus (G. Cuvier, 1812), Lagenorhynchus albirostris (Gray, 1846), Phocoena phocoena (Linnaeus, 1758), and Pseudorca crassidens (Owen, 1846); once reported on Globicephala macrorhynchus Gray, 1846, Megaptera novaeangliae, Mesoplodon europaeus (Gervais, 1855), Peponocephala electra (Gray, 1846), Phocoena dioptrica Lahille, 1912, Steno bredanensis (G. Cuvier in Lesson, 1828), and Tursiops truncatus
Geographic Range
Arctic, Atlantic, Pacific, Mediterranean, Indian Ocean
Life Cycle
-
Microhabitat
Ubiquitous; i.e., blowhole, eyes, jaw, insertion of pectoral fin, wounds (Stock, 1973a; Stock, 1977; Greenwood et al., 1979; Raga et al., 1988; Balbuena et al., 1989; Balbuena and Raga, 1991; Raga and Balbuena, 1993; Jauniaux et al., 2002; Lehnert et al., 2007; Batista et al., 2012; Lehnert et al., 2021)
Use as Indicator
The higher prevalence and intensity of I. delphinii on mature long-finned pilot whale males (vs. females and immature males) may identify the males that are dominant in sexual fights, given that the resulting wounds serve as shelter for this cyamid species (Balbuena and Raga, 1991; Raga and Balbuena, 1993).
Remarks
Lehnert et al. (2021) pose that some records around the 1970-90s misidentified this species and refer to Isocyamus deltobranchium Sedlak-Weinstein, 1992, which has triangular accessory gills (vs. cylindrical in I. delphinii).
References
Lütken, 1870; Lütken, 1893; Collet, 1912; Chevreux, 1913b; Hiro, 1938; Bowman, 1955; Sergeant, 1962; Leung, 1965; Stock, 1973a; Stock, 1973b; Lincoln and Hurley, 1974a; Stock, 1977; Van Bree and Smeenk, 1978; Greenwood et al., 1979; Berzin and Vlasova, 1982; Raga et al., 1983a; Rappé, 1985; Raga et al., 1988; Balbuena et al., 1989; Mead, 1989; Rappé, 1991; Balbuena and Raga, 1991; Fransen and Smeenk, 1991; Sedlak-Weinstein, 1991; Raga and Balbuena, 1993; Abollo et al., 1998; Gibson et al., 1998; Margolis et al., 2000; Wardle et al., 2000; Haelters, 2001; Jauniaux et al., 2002; Haney et al., 2004; Lehnert et al., 2007; Batista et al., 2012; Lehnert et al., 2021; Iwasa-Arai and Serejo, 2018
Isocyamus deltobranchium (Sedlak-Weinstein, 1992)
Synonyms
-
Morphological Description
Sedlak-Weinstein, 1992a; Martínez et al., 2008; Lehnert et al., 2021
Molecular Sequences
COI (Lehnert et al., 2021)
Association
Ectoparasite
Cetacean Hosts/Basibionts
Phocoena phocoena; once reported on Delphinus delphis, Globicephala macrorhynchus, G. melas, Mesoplodon mirus True, 1913, and Orcinus orca
Geographic Range
Eastern North Atlantic, western north Pacific, Indian Ocean
Life Cycle
-
Microhabitat
Skin wounds (Sedlak-Weinstein, 1992a; Martínez et al., 2008; Lehnert et al., 2021)
Use as Indicator
Higher prevalence in some harbor porpoise populations may reveal more interspecific contacts than in other areas (Lehnert et al., 2021). Also, temporal changes in prevalence could trace trends in the health status of cetacean hosts, given that it has been suggested that poor nutritional status may increase the susceptibility of porpoises to whale lice infections (Lehnert et al., 2021).
Remarks
Diatoms have been reported between I. deltobranchium forearms (Lehnert et al., 2021).
References
Sedlak-Weinstein, 1992a; Martínez et al., 2008; Iwasa-Arai and Serejo, 2018; Lehnert et al., 2021
Isocyamus indopacetus (Iwasa-Arai & Serejo, 2017)
Synonyms
-
Morphological Description
Iwasa-Arai et al., 2017b; Iwasa-Arai and Serejo, 2018; Kobayashi et al., 2021
Molecular Sequences
-
Association
Ectoparasite
Cetacean Hosts/Basibionts
Indopacetus pacificus (Longman, 1926)
Geographic Range
Japan, New Caledonia (western Pacific)
Life Cycle
-
Microhabitat
Mouth, mammary slits, and scars provoked by Isistius sp. (Kobayashi et al., 2021)
Use as Indicator
-
Remarks
-
References
Iwasa-Arai et al., 2017a; Kobayashi et al., 2021
Isocyamus kogiae (Sedlak-Weinstein, 1992)
Synonyms
-
Morphological Description
Molecular Sequences
-
Association
Ectoparasite
Cetacean Hosts/Basibionts
Kogia breviceps (de Blainville, 1838)
Geographic Range
Australia (western South Pacific)
Life Cycle
-
Microhabitat
Skin wounds (Sedlak-Weinstein, 1992b)
Use as Indicator
-
Remarks
-
References
Neocyamus physeteris (Pouchet, 1888)
Synonyms
Cyamus fascicularis Verrill, 1901, C. physeteris Pouchet, 1888, Paracyamus physeteris (Pouchet, 1888)
Morphological Description
Pouchet, 1892; Leung, 1967; Margolis et al., 2000; Iwasa-Arai and Serejo, 2018
Molecular Sequences
-
Association
Ectoparasite
Cetacean Hosts/Basibionts
Typically on Physeter macrocephalus; single record on Phocoenoides dalli (True, 1885)
Geographic Range
Eastern Pacific, Atlantic
Life Cycle
-
Microhabitat
-
Use as Indicator
Used to detect social segregation in sperm whales: females and male bachelors, but not large males, harbour N. physeteris, suggesting that the later leave their natal pods at puberty (Best, 1969a; Best, 1979).
Remarks
-
References
Pouchet, 1888; Pouchet, 1892; Verrill, 1902; Clarke, 1956; Margolis, 1959; Buzeta, 1963; Leung, 1965; Leung, 1967; Best, 1969a; Lincoln and Hurley, 1974a; Best, 1979; Berzin and Vlasova, 1982; Mignucci-Giannoni et al., 1998; Margolis et al., 2000; Iwasa-Arai and Serejo, 2018
Orcinocyamus orcini (Leung, 1970)
Synonyms
Cyamus orcini Leung, 1970b
Morphological Description
Leung, 1970b; Margolis et al., 2000
Molecular Sequences
-
Association
Ectoparasite
Cetacean Hosts/Basibionts
Orcinus orca
Geographic Range
Senegal (eastern South Atlantic)
Microhabitat
-
Use as Indicator
-
Remarks
-
References
Platycyamus flaviscutatus (Waller, 1989)
Synonyms
-
Morphological Description
Molecular Sequences
-
Association
Ectoparasite
Cetacean Hosts/Basibionts
Berardius bairdii
Geographic Range
North Pacific
Life Cycle
-
Microhabitat
Head, back, flanks, flukes (Waller, 1989)
Use as Indicator
-
Remarks
-
References
Waller, 1989; Margolis et al., 2000
Platycyamus thompsoni (Gosse, 1855)
Synonyms
Cyamus thompsoni Gosse, 1855
Morphological Description
Gosse, 1855; Lütken, 1873; Wolff, 1958; Leung, 1967; Sedlak-Weinstein, 1991; Iwasa-Arai and Serejo, 2018
Molecular Sequences
-
Association
Ectoparasite
Cetacean Hosts/Basibionts
Typically on Hyperoodon ampullatus (Forster, 1770); once reported on H. planifrons Flower, 1882 and Mesoplodon grayi von Haast, 1876
Geographic Range
North Atlantic, Pacific, Antarctica
Life Cycle
At least four instars have been distinguished in females (Wolff, 1958). Males are more difficult to classify by morphological features and could die and fall off the whale after copulation (Wolff, 1958).
Microhabitat
Ubiquitous on skin, i.e., eyes, beak, corners of the mouth (Tomilin, 1957; Wolff, 1958; Lincoln and Hurley, 1974a; Sedlak-Weinstein, 1991)
Use as Indicator
-
Remarks
-
References
Gosse, 1855; Lütken, 1870; Vosseler, 1889; Collet, 1912; Liouville, 1913; Tomilin, 1957; Wolff, 1958; Stock, 1973b; Lincoln and Hurley, 1974a; Berzin and Vlasova, 1982; Fransen and Smeenk, 1991; Sedlak-Weinstein, 1991; Iwasa-Arai and Serejo, 2018
Scutocyamus antipodensis (Lincoln & Hurley, 1980)
Synonyms
-
Morphological Description
Molecular Sequences
-
Association
Ectoparasite
Cetacean Hosts/Basibionts
Cephalorhynchus hectori (Lacépède, 1804), Phocoena dioptrica, Sagmatias obscurus (Gray, 1828)
Geographic Range
Off Namibia (eastern South Atlantic) and New Zealand (western South Pacific)
Life Cycle
-
Microhabitat
Ubiquitous on skin (Lincoln and Hurley, 1980; Best and Meÿer, 2010; Lehnert et al., 2017)
Use as Indicator
-
Remarks
-
References
Lincoln and Hurley, 1980; Best and Meÿer, 2010; Lehnert et al., 2017
Scutocyamus parvus (Lincoln & Hurley, 1974)
Synonyms
-
Morphological Description
Molecular Sequences
-
Association
Ectoparasite
Cetacean Hosts/Basibionts
Lagenorhynchus albirostris
Geographic Range
North Sea
Life Cycle
-
Microhabitat
-
Use as Indicator
-
Remarks
-
References
Lincoln and Hurley, 1974a, Lincoln and Hurley, 1974b; Stock, 1977; Fransen and Smeenk, 1991
Syncyamus aequus (Lincoln & Hurley, 1981)
Synonyms
See Remarks.
Morphological Description
Lincoln and Hurley, 1981; Raga, 1988; Sedlak-Weinstein, 1991
Molecular Sequences
-
Association
Ectoparasite
Cetacean Hosts/Basibionts
Delphinus delphis, Stenella coeruleoalba; once reported on Sousa chinensis (Osbeck, 1765), Stenella longirostris (Gray, 1828), Tursiops aduncus (Ehrenberg, 1832 [1833]), and T. truncatus
Geographic Range
Mediterranean, western South Pacific, Indian Ocean
Life Cycle
-
Microhabitat
Blowhole, eyes, corner of mouth, snout, jaw, axilla (Lincoln and Hurley, 1981; Raga and Raduan, 1982; Aznar et al., 1994; Cerioni and Mariniello, 1996; Haney, 1999; Haney et al., 2004; Fraija-Fernández et al., 2017)
Use as Indicator
-
Remarks
On the one hand, Mediterranean striped dolphins, Stenella coeruleoalba, harbored low prevalence and intensity of S. aequus (27% and 3 ind./host, respectively; Fraija-Fernández et al., 2017). Since striped dolphins are highly social animals (Carlucci et al., 2015), transmission success would be hardly hampered by the scarcity of contacts, but rather by the low sizes of source populations. These small populations may result from the extreme limitation of suitable microhabitats to shelter on these fast-swimming dolphins (Fraija-Fernández et al., 2017). This phenomenon seems also to impact the reproductive strategy of this species (Fraija-Fernández et al., 2017). On the other hand, the species Cyamus chelipes was first described by Costa (1866) and later re-classified in the genus Syncyamus by Bowman (1958). It is considered a nomen dubium (Haney, 1999), the type series is lost (Bowman, 1958), and it was not included in later reviews of the Cyamidae (Leung, 1965; Iwasa-Arai and Serejo, 2018|). Thus, it is possible that S. chelipes is a synonym of S. aequus, later described and common in the Mediterranean Sea (see above, Supplementary Table 1).
References
Lincoln and Hurley, 1981; Raga and Raduan, 1982; Raga et al., 1983; Raga and Carbonell, 1985; Raga, 1988; Sedlak-Weinstein, 1991; Aznar et al., 1994; Mariniello et al., 1994; Ross et al., 1994; Cerioni and Mariniello, 1996; Margolis et al., 2000; Fraija-Fernández et al., 2017
Syncyamus ilheusensis (Haney, de Almeida & Reid, 2004)
Synonyms
-
Morphological Description
Haney et al., 2004; Iwasa-Arai et al., 2017b; Iwasa-Arai and Serejo, 2018
Molecular Sequences
-
Association
Ectoparasite
Cetacean Hosts/Basibionts
Globicephala macrorhynchus, Peponocephala electra, Stenella clymene (Gray, 1850)
Geographic Range
Brazil (western South Atlantic)
Life Cycle
-
Microhabitat
Eyes, blowhole (Haney et al., 2004; Batista et al., 2012)
Use as Indicator
-
Remarks
-
References
Haney et al., 2004; Batista et al., 2012; Iwasa-Arai et al., 2017a; Iwasa-Arai et al., 2018
Syncyamus pseudorcae (Bowman, 1955)
Synonyms
-
Morphological Description
Molecular Sequences
-
Association
Ectoparasite
Cetacean Hosts/Basibionts
Delphinus delphis, Pseudorca crassidens, Stenella clymene
Geographic Range
North Atlantic, Pacific
Life Cycle
-
Microhabitat
Blowhole, mouth, snout, jaw (Carvalho et al., 2010)
Use as Indicator
-
Remarks
-
References
Bowman, 1955; Leung, 1970a; Sedlak-Weinstein, 1991; Jefferson et al., 1995; Carvalho et al., 2010
Order Isopoda Latreille, 1817
Family Cymothoidae Leach, 1818
Representatives from the family Cymothoidae are obligate parasites of mainly marine but also freshwater fish (Smit et al., 2014). Identification of cymothoid isopods is often difficult because species often show high morphological variation (Trilles et al., 2013). Many species of Nerocila Leach, 1818 require taxonomic revision (Aneesh et al., 2019).
Nerocila sp.
Synonyms
-
Morphological Description
A general account of the genus Nerocila and of some of its species can be found Hai-yan and Xin-zheng (2002) and Trilles et al. (2013).
Molecular Sequences
COI, LSU rRNA, 16S rRNA, and 18S rRNA of nine Nerocila spp. (see GenBank)
Association
Unknown
Cetacean Hosts/Basibionts
Pontoporia blainvillei (Gervais & d’Orbigny, 1844)
Geographic Range
-
Life Cycle
See Brusca (1978) and Smit et al. (2014) for a description of the cymothoid cycle.
Microhabitat
Neck region (Brownell, 1975)
Use as Indicator
-
Remarks
Brownell (1975) reported this ectoparasite on some La Plata dolphins that had been captured accidentally in gillnets, and interpreted that it could have been transmitted from sharks or other fish while all were trapped in the gillnet. Thus, the association with cetaceans should be viewed as accidental.
References
Class Thecostraca Gruvel, 1905
Subclass Copepoda Milne Edwards, 1840
Order Harpacticoida Sars G.O., 1903
Family Balaenophilidae Sars G.O., 1910
The genus Balaenophilus Aurivillius P.O.C., 1879 contains two species that live in close association with marine vertebrates. B. unisetus Aurivillius P.O.C., 1879 is considered an obligate commensal of baleen whales that feeds on algae and/or baleen tissue (Vervoort and Tranter, 1961; Fernandez-Leborans, 2001; Badillo et al., 2007), causing no harm to hosts (Ogawa et al., 1997; Badillo et al., 2007). In contrast, B. manatorum (Ortiz et al., 1992) infects manatees and sea turtles; in the latter they can feed on healthy skin (Badillo et al., 2007; Domènech et al., 2017), sometimes producing extensive lesions (Crespo-Picazo et al., 2017). Thus, this species is considered an ectoparasite.
Balaenophilus unisetus (Aurivillius P.O.C., 1879)
Synonyms
-
Morphological Description
Aurivillius, 1879; Vervoort and Tranter, 1961; Bannister and Grindley, 1966
Molecular Sequences
-
Association
Obligate commensal
Cetacean Hosts/Basibionts
Balaenoptera borealis Lesson, 1828, B. edeni Anderson, 1878, B. musculus, B. physalus
Geographic Range
Arctic, Atlantic, eastern Pacific, Indian Ocean, Antarctica
Life Cycle
Aurivillius (1879) describes a nauplius and five copepodite stages preceding the adult phase. In the allied species B. manatorum nauplii and early copepodite stages are unable to swim, and copepodite V and adults can perform only short swimming excursions (Domènech et al., 2017). Thus, host bodily contact or closeness is likely necessary for transmission in both species.
Microhabitat
Baleen plates (Aurivillius, 1879; Cocks, 1885; Lillie, 1910; Scharff, 1913; Matthews, 1938b; Vervoort and Tranter, 1961; Rice, 1963; Gambell, 1964; Bannister and Grindley, 1966; Ichihara, 1966; Ichihara, 1978; Collet, 1986; Raga and Sanpera, 1986; Dalla Rosa and Secchi, 1997; Esteves et al., 2020), corner of the mouth (Raga and Sanpera, 1986)
Use as Indicator
-
Remarks
The presence of this species is likely underestimated since it can be easily overlooked without exhaustive inspection of baleen plates (Aurivillius, 1879; Vervoort and Tranter, 1961). It can sometimes be colonized by chonotrichous ciliates, acting as basibiont (Fernandez-Leborans, 2001).
References
Cocks, 1885; Aurivillius, 1879; Lillie, 1910; Collet, 1912; Scharff, 1913; Allen, 1916; Cornwall, 1927; Cornwall, 1928; Matthews, 1938b; Vervoort and Tranter, 1961; Rice, 1963; Gambell, 1964; Bannister and Grindley, 1966; Ichihara, 1966; Kawamura, 1969; Rice, 1977; Ichihara, 1978; Collet, 1986; Raga and Sanpera, 1986; Dalla Rosa and Secchi, 1997; Esteves et al., 2020
Family Harpacticidae Dana, 1846
Members of this family are mostly marine or brackishwater macroalgal associates, with a few freshwater species (Joon and Young, 1993).
Harpacticus pulex (Humes, 1964)
Synonyms
-
Morphological Description
Molecular Sequences
-
Association
Unknown
Cetacean Hosts/Basibionts
Tursiops truncatus
Geographic Range
-
Life Cycle
Unknown for this species, but naupliar and copepodite stages have been described for other Harpacticus spp. (e.g., Itô, 1976; Walker, 1981; Choi and Kim, 1994). Harpacticoids generally lack planktonic larval stages, but adults are active swimmers (e.g., Hicks, 1985; Palmer, 1988). It is thus plausible that transmission to bottlenose dolphin occurred during the adult phase.
Microhabitat
On ulcerated and sloughed skin (Humes, 1964)
Use as Indicator
-
Remarks
This species was described by Humes (1964) on captive marine mammals and has never been reported again. Species of Harpacticus Milne Edwards H., 1840 typically colonize seagrass, algal clumps or sandy and muddy bottoms (Ólafsson, 2001 and references therein), thus the occurrence of H. pulex on cetaceans is intriguing and perhaps forced by confinement conditions (Humes, 1964). Future re-examination of the taxonomic status of H. pulex is advisable.
References
Order Siphonostomatoida Burmeister, 1835
Family Caligidae Burmeister, 1835
The family Caligidae (“sea lice”) contains 30 genera (Walter and Boxshall, 2020); species of Caligus Müller O. F., 1785 and Lepeophtheirus Nordmann, 1832 have great economic relevance due to their impact on salmonid fish mariculture (Costello, 2006; Hemmingsen et al., 2020). Caligids use their siphon and a pair of mandibles to feed on fish skin (Kabata, 1974), causing ulcerations and even death to their hosts (Tørud and Håstein, 2008), but their impact on cetaceans has not yet been reported.
Caligus elongatus (Nordmann, 1832)
Synonyms
Caligus arcticus Brandes, 1956, C. kroyeri Milne Edwards, 1840, C. latifrons Wilson C.B., 1905, C. leptochilus Leuckart in Frey & Leuckart, 1847, C. lumpi Krøyer, 1863, C. rabidus Leigh-Sharpe, 1936, C. rissoanus Milne Edwards, 1840, C. trachypteri Krøyer, 1863
Morphological Description
Hemmingsen et al., 2020 and references therein
Molecular Sequences
COI (Øines and Heuch, 2005; Raupach et al., 2015; GenBank AY386272; AY386273; EF452647), 16S rRNA (Øines and Schram, 2008; GenBank AY660020), 18S rRNA (Huys et al., 2006; Øines and Schram, 2008; Mohrbeck et al., 2015; Khodami et al., 2017; GenBank JX845119-JX845131), 28S rRNA (Khodami et al., 2017; GenBank DQ180336; DQ180337; EU118301; EU118302)
Association
Ectoparasite
Cetacean Hosts/Basibionts
Balaenoptera acutorostrata, Hyperodoon ampullatus
Geographic Range
North Atlantic (Hemmingsen et al., 2020)
Life Cycle
Two free-living planktonic nauplius stages, one free-swimming infective copepodid stage, and four chalimus stages and one adult stage attached to the host (Maran et al., 2013).
Microhabitat
Skin (O’Reilly, 1998; Ólafsdóttir and Shinn, 2013)
Use as Indicator
-
Remarks
This is a typical fish ectoparasite that has been reported on more than 80 species (Kabata, 1979; Agusti-Ridaura et al., 2019). Infections in cetaceans are exceptional and likely related to their occurrence close to cage farms (Ólafsdóttir and Shinn, 2013). The hyperparasitic monogenean Udonella caligorum Johnston, 1835, which typically attaches to fish copepods (Freeman and Ogawa, 2010), has been found on C. elongatus infecting common minke whales (Ólafsdóttir and Shinn, 2013).
References
Caligus rufimaculatus (Wilson C.B., 1905)
Synonyms
-
Morphological Description
Wilson, 1905; Takemoto and Luque, 2002; Kim et al., 2019
Molecular Sequences
-
Association
Ectoparasite
Cetacean Hosts/Basibionts
Tursiops truncatus
Geographic Range
Western Atlantic (Benz et al., 2011)
Life Cycle
See C. elongatus (above).
Microhabitat
Skin (Benz et al., 2011)
Use as Indicator
-
Remarks
This species typically infects fish, but there is an exceptional record of adult individuals, including ovigerous females, on a carcass of bottlenose dolphin (Benz et al., 2011).
References
Lepeophtheirus crassus (Wilson & Bere, 1936)
Synonyms
Gloiopotes crassus Wilson & Bere, 1936
Morphological Description
Molecular Sequences
-
Association
Ectoparasite
Cetacean Hosts/Basibionts
Delphinus delphis
Geographic Range
Western Atlantic, North Pacific, Indian Ocean (Lewis, 1967)
Life Cycle
Species of Lepeophtheirus have 2-4 chalimus stages and two preadult stages. The latter can be distinguished by their ability to detach and move over the surface of the host (Krøyer, 1834; see Hamre et al., 2013).
Microhabitat
Hyperparasitic on Remora australis (Bennett, 1840; Radford and Klawe, 1965)
Use as Indicator
-
Remarks
-
References
Family Pennellidae Burmeister, 1835
Unlike other families of the order Siphonostomatoida, members of the family Pennellidae do have intermediate hosts, usually a fish or invertebrate (Kabata, 1979; Nagasawa et al., 1985; Suyama et al., 2021a and references therein). Mating seemingly occurs in the intermediate host and fertilized females attach to the final host in which they produce and release the eggs (Arroyo et al., 2002).
Pennella balaenoptera (Koren & Danielssen, 1877)
Synonyms
Pennella antarctica Quidor, 1913, P. anthonyi Quidor, 1913, P. balaenopterae Koren & Danielssen, 1877, P. cettei Quidor, 1913, P. charcoti Quidor, 1913
Morphological Description
Koren and Danielssen, 1877; Turner, 1905; Hogans, 1987, Hogans, 2017; Abaunza et al., 2001; Vecchione and Aznar, 2014; Suyama et al., 2021b
Molecular Sequences
COI (Fraija-Fernández et al., 2018)
Association
Mesoparasite. The head penetrates the blubber and musculature to feed on blood and expands as 2-3 cephalic horns in host’s tissue to enable attachment, whereas the trunk, genital complex, and abdominal plumes protrude and hang on the host body (Hogans, 1987; Abaunza et al., 2001; Schmidt and Roberts, 2009; Hogans, 2017).
Cetacean Hosts/Basibionts
Balaenoptera acutorostrata, B. bonaerensis, B. borealis, B. edeni, B. musculus, B. physalus, Delphinus delphis, Eubalaena australis, Feresa attenuata Gray, 1874, Globicephala melas, Grampus griseus, Hyperoodon ampullatus, Kogia breviceps, Lissodelphis borealis (Peale, 1848), Megaptera novaeangliae, Mesoplodon bidens (Sowerby, 1804), M. carlhubbsi Moore, 1963, M. mirus, Orcinus orca, Phocoena phocoena, Physeter macrocephalus, Stenella coeruleoalba, Tursiops truncatus, Ziphius cavirostris
Geographic Range
Atlantic, Pacific, Mediterranean, Indian Ocean, Antarctica
Life Cycle
Based on information from other penellids, its life cycle is believed to include a pelagic naupliar stage and several copepodid and chalimus instars on the intermediate (squid) hosts; females are fertilized as late chalimi and undergo a pelagic phase to search out the definitive host, where they metamorphose into the adult stage (Schmidt and Roberts, 2009). In the case of P. balaenoptera, only adult females and the first naupliar stage are known (Arroyo et al., 2002). However, the copepodid and chalimus stages have been described for P. filosa (Linnaeus, 1758) collected from squids (Rose and Hamon, 1953; see also Arroyo et al., 2002), and P. filosa is now considered conspecific with P. balaenoptera (Fraija-Fernández et al., 2018; see also the Discussion). The life cycle of P. balaenoptera could be primarily oceanic because this species is more prevalent on pelagic versus coastal cetaceans (Fraija-Fernández et al., 2018).
Microhabitat
Commonly on the flanks (Raga and Sanpera, 1986; Aznar et al., 1994; Gomerčić et al., 2006; Souza et al., 2005; Ciçek et al., 2007; Foskolos et al., 2017), but occasionally reported on the head (Pouchet and Beauregard, 1889; Foskolos et al., 2017) and flukes (Foskolos et al., 2017). A single record on a whale sucker, Remora australis (Bennett, 1840) attached to a dolphin (Radford and Klawe, 1965).
Use as Indicator
It may be an indicator of compromised health in cetacean hosts (Mackintosh and Wheeler, 1929; Aznar et al., 2005; Vecchione and Aznar, 2014).
Remarks
Since P. balaenoptera is the only recognized species of Pennella Oken, 1815 parasitizing cetaceans, we consider that the published records of Pennella sp. in cetaceans could be assigned to this species, unless proven otherwise. Dailey et al. (2002) reported P. balaenoptera in one northern elephant seal, Mirounga angustirostris (Gill, 1866). Recently, molecular analyses revealed that specimens of P. balaenoptera collected from several cetaceans in western Mediterranean could be conspecific with P. filosa from swordfish, Xiphias gladiusLinnaeus, 1758, collected in the same area (Fraija-Fernández et al., 2018). This finding begs further attention (see the Discussion).
References
Steenstrup and Lütken, 1861; Sars, 1866; Pouchet and Beauregard, 1889; Anthony and Calvet, 1905; Turner, 1905; Bouvier, 1910; Japha, 1910; Mörch, 1911; Collet, 1912; Quidor, 1912; Liouville, 1913; Olsen, 1913; Scharff, 1913; Cornwall, 1927; Cornwall, 1928; Mackintosh and Wheeler, 1929; Van Oorde-de Lint and Schuurmans-Stekhoven, 1936; Matthews, 1938b; Allen, 1941; Stephensen, 1942; Mizue, 1950; Nishiwaki and Hayashi, 1950; Mizue and Murata, 1951; Nishiwaki and Oye, 1951; Ohno and Fujino, 1952; Kakuwa et al., 1953; Barnard, 1955; Chapman and Santler, 1955; Clarke, 1956; Zenkovich, 1956; Tomilin, 1957; Rice, 1963; Radford and Klawe, 1965; Kawamura, 1969; Berzin, 1972; Rice, 1977; Rice, 1978; Dailey and Stroud, 1978; Dailey and Walker, 1978; Ivashin and Golubovsky, 1978; Greenwood et al., 1979; Best, 1982; Raga and Carbonell, 1985; Raga and Sanpera, 1986; Smiddy, 1986; Mead, 1989; Bushuev, 1990; Dorsey et al., 1990; Sedlak-Weinstein, 1990 (unpubl.); Dailey and Vogelbein, 1991; Raga and Balbuena, 1993; Aznar et al., 1994; Aznar et al., 2005, unpubl.; Raga, 1994; Vecchione, 1994; Cerioni and Mariniello, 1996; Kuramochi et al., 1996; Araki et al., 1997; Kuramochi et al., 2000; McAlpine et al., 1997; Terasawa et al., 1997; Uchida, 1998; Walker and Hanson, 1999; Cornaglia et al., 2000; Uchida and Araki, 2000; Abaunza et al., 2001; Arroyo et al., 2002; Brzica, 2004; Gomerčić et al., 2006; Souza et al., 2005; Ciçek et al., 2007; Kautek et al., 2008; Martín et al., 2011; Rosso et al., 2011; Bertulli et al., 2012; Ólafsdóttir and Shinn, 2013; Tonay and Dede, 2013; Danyer et al., 2014; Öztürk et al., 2015; Delaney et al., 2016; Birincioğlu et al., 2017; Foskolos et al., 2017; Hogans, 2017; Fraija-Fernández et al., 2018; IJsseldijk et al., 2018; Marcer et al., 2019; Methion and Díaz López, 2019; Herr et al., 2020; Orrell, 2020; Ten et al., unpubl.
Subclass Cirripedia Burmeister, 1834
Order Balanomorpha Pilsbry, 1916
Family Balanidae Leach, 1817
Thoracic barnacles (Infraclass Thoracica) are sessile, hermaphroditic crustaceans that attach to diverse substrata and have specialized cirri to filter organic particles from water for feeding (Anderson, 1994). The life cycle typically includes a free-swimming nauplius larva that undergoes several (usually 6) moults, and a non-feeding cypris larva that searchs out, and attaches to, an appropriate substratum. Subsequent metamorphosis leads to a juvenile filter-feeding version of the adult (Darwin, 1854; Cornwall, 1955; Maruzzo et al., 2012). The cyprid stage is unique to barnacles and shows little morphological variability across species, even though they can attach to strikingly different substrata (Maruzzo et al., 2012; Dreyer et al., 2020).
This family originally encompassed all sessile barnacles (Leach, 1817), but whale barnacles and most sea turtles were later re-classified (Pitombo, 2004; see below). Most members of Balanidae are intertidal, although some species are facultative epibionts, e.g., those found on sea turtles, such as Balanus trigonus (Ten et al., 2019).
Balanus trigonus (Darwin, 1854)
Synonyms
-
Morphological Description
Molecular Sequences
COI (Chen et al., 2013; Ashton et al., 2016; GenBank JQ035523; JQ035524; MF974362; MK308152; MK308163; MK308322; MK496572; MT258956; MW277718; MW277822), EF1a (Chan et al., 2017), RPII (Chan et al., 2017), 12S rRNA (Endo et al., 2010; Kamiya et al., 2012; Pérez-Losada et al., 2014; Chan et al., 2017; GenBank GU983669; GU983670), 16S rRNA (Chan et al., 2017; GenBank JQ035491; JQ035492), 18S rRNA (Pérez-Losada et al., 2014; Chan et al., 2017), 28S rRNA (Pérez-Losada et al., 2014), and the complete mitochondrial genome (GenBank MW646099; MZ049958; NC_056392)
Association
Facultative commensal
Cetacean Hosts/Basibionts
Megaptera novaeangliae
Geographic Range
Cosmopolitan (Werner, 1967)
Life Cycle
Metamorphosis from nauplius to cyprid stage is speeded up at higher water temperature, i.e., 4-11 days (Thiyagarajan et al., 2003). Recruitment is seasonal and takes place at approximately 24°C (Lam, 2000).
Microhabitat
As a hyperepibiont on the barnacle Coronula diadema (Cornwall, 1928)
Use as Indicator
-
Remarks
-
References
Balanus spp.
Synonyms
-
Morphological Description
A general account of Balanus spp. can be found in Darwin (1854); Newman and Ross (1976), and Pitombo (2004).
Molecular Sequences
> 5,000 results in GenBank
Association
Presumably facultative commensal
Cetacean Hosts/Basibionts
Megaptera novaeangliae
Geographic Range
-
Life Cycle
Information for Balanus spp. is available from Brown and Roughgarden (1985) and Maruzzo et al. (2012).
Microhabitat
As a hyperepibiont on the barnacle Coronula spp. (Rice, 1963)
Use as Indicator
-
Remarks
Balanus spp., as in Rice (1963), may correspond to a single or several species.
References
Megabalanus tintinnabulum (Linnaeus, 1758)
Synonyms
Balanus tintinnabulum (Linnaeus, 1758), Lepas tintinnabulum Linnaeus, 1758
Morphological Description
Molecular Sequences
COI (Chen et al., 2013; Ashton et al., 2016; GenBank JQ035525-JQ035527), H3 (Pérez-Losada et al., 2004), 12S rRNA (Pérez-Losada et al., 2004), 16S rRNA (Pérez-Losada et al., 2004; GenBank JQ035505-JQ035508), 18S rRNA, 28S rRNA (Pérez-Losada et al., 2004), and the complete mitochondrial genome (Che et al., 2019; GenBank MW281857; NC_056162)
Association
Facultative commensal
Cetacean Hosts/Basibionts
Unidentified whale
Geographic Range
Tropical or sub-tropical to warm temperate waters (Otani et al., 2007)
Life Cycle
In the Arabian Sea, barnacles breed at lower temperatures, i.e., less than 24 °C in winter vs. > 28 °C in summer; and grow at a rate of 0.44-0.63 mm/year (Ali and Ayub, 2021).
Microhabitat
As a hyperepibiont on the barnacle Coronula diadema (Barnard, 1924)
Use as Indicator
-
Remarks
-
References
Family Coronulidae Leach, 1817
Coronulids are typically obligate epibionts of sea turtles, sirenians or cetaceans (Marlow, 1962; Hayashi et al., 2013). One species, Chelonibia testudinaria (Linnaeus, 1758), can also be found on crustaceans and sea snakes, and even on inanimate substrata (Frazier and Margaritoulis, 1990; Cheang et al., 2013).
Cetopirus complanatus (Mörch, 1852)
Synonyms
Coronula balaenaris (Gmelin, 1791), C. complanata (Mörch, 1852)
Morphological Description
Darwin, 1854; Pilsbry, 1916; Scarff, 1986; Pastorino and Griffin, 1996; Seilacher, 2005
Molecular Sequences
-
Association
Obligate commensal
Cetacean Hosts/Basibionts
Eubalaena australis, E. glacialis
Geographic Range
Arctic, Atlantic, eastern North Pacific, Antarctica
Life Cycle
-
Microhabitat
Lips, fins (Guiler, 1956; Best, 1991)
Use as Indicator
Shell plate remains of C. complanatus in Nerja Cave (Málaga, southern Spain) were used as indirect evidence of whale consumption by humans in the Upper Magdalenian (Álvarez-Fernández et al., 2013) and of the presence and migration of right whales (Balaenidae) in the Mediterranean during the Early Pleistocene (Collareta et al., 2016; Bosselaers et al., 2017).
Remarks
There is a single record on Megaptera novaeangliae (Guiler, 1956), but it was probably confused with Coronula reginae (Holthuis et al., 1998).
References
Chemnitz, 1785; Chemnitz and Martini, 1790; Darwin, 1854; Gruvel, 1903; Pilsbry, 1916; Nilsson-Cantell, 1931; Best, 1991
Coronula diadema (Linnaeus, 1767)
Synonyms
-
Morphological Description
Darwin, 1854; Dall, 1872; Cornwall, 1955; Scarff, 1986; Anderson, 1994
Molecular Sequences
H3, 12S rRNA, 16S rRNA, 18S rRNA, 28S rRNA (Hayashi et al., 2013)
Association
Obligate commensal
Cetacean Hosts/Basibionts
Typical from Megaptera novaeangliae but some records on Balaenoptera bonaerensis, B. borealis, B. musculus, B. physalus, Eubalaena glacialis, Hyperoodon ampullatus and Physeter macrocephalus
Geographic Range
Atlantic, Pacific, Indian Ocean, Antarctica
Life Cycle
A one-year life cycle has been proposed (Angot, 1951; Newman and Abbott, 1980). Larval release and settlement seem to occur in warm waters (20-25°C in September-October off Madagascar), whereas adult development may take place during whale migration to the poles (Angot, 1951). Details of development from the embryo to the juvenile stage have been studied in vitro (Nogata and Matsumura, 2006). Larval settlement is likely induced by chemical cues from whale skin, such as alpha-2-macroglobulin (Nogata and Matsumura, 2006).
Microhabitat
Rostrum, lips, lower jaw, fins (Dall, 1872; Pilsbry, 1916; Nilsson-Cantell, 1930a, Nilsson-Cantell, 1930c; Stephensen, 1938; Scheffer, 1939; Tomilin, 1957; Scarff, 1986)
Use as Indicator
Isotope analyses (δ18O) of shells of C. diadema and its direct ancestor C. bifida (Dominici et al., 2011) accurately trace current and Pleistocene-Miocene whale migration routes (Buckeridge et al., 2018; Collareta et al., 2018a; Collareta et al., 2018b; Buckeridge et al., 2019; Taylor et al., 2019). Fossil remains have also been used to infer humpback whale migration routes and breeding areas in the Late Pliocene-Pleistocene (Bianucci et al., 2006a; Bianucci et al., 2006b). Present-day observations of Coronula sp. (Olsen, 1913; Angot, 1951) helped to elucidate right whales’ migration from warmer waters (Best, 1991). The co-occurrence of C. bifida with Cetopirus complanatus may indicate that whales belonging to Balaenopteridae and Balaenidae shared breeding grounds during the Early Pleistocene (Collareta et al., 2016). Interestingly, the presence of C. diadema on cetaceans other than humpback whales could also indicate some geographical overlap between species (see the Discussion). Coronula spp. have been suggested as natural marks for individual photo-identification (Franklin et al., 2020). The pattern of attachment of barnacles (presumably C. diadema) indicates non-uniform water flow over humpback whale flippers and has shed light on the function of leading-edge tubercles (Fish and Battle, 1995). Rubbing against rocks and the sea bottom has been observed in humpback whales, which may be an attempt to remove these barnacles (Tomilin, 1957) and could limit its application as an indicator.
Remarks
This species serves as a basibiont of the facultative epibionts Balanus spp., Conchoderma auritum (Linnaeus, 1767), and Megabalanus tintinnabulum, and of the hydroid Obelia dichotoma (Linnaeus, 1758) (Liouville, 1913; Barnard, 1924; Cornwall, 1928; Stephensen, 1938; Rice, 1963; Kim et al., 2020).
References
Dall, 1872; Scammon, 1874; Fischer, 1884; Sars, 1890-1895; Borradaile, 1903; Liouville, 1913; Pilsbry, 1916; Cornwall, 1924; Cornwall, 1927; Cornwall, 1928; Nilsson-Cantell, 1930a; Nilsson-Cantell, 1930c, Hiro, 1935; Hiro, 1938; Stephensen, 1938; Nilsson-Cantell, 1939; Scheffer, 1939; Mizue and Murata, 1951; Rees, 1953; Tomilin, 1957; Nishiwaki, 1959; Cockrill, 1960; Wolff, 1960; Rice, 1963; Nilsson-Cantell, 1978; O’Riordan, 1979; Scarff, 1986; Paterson and Van Dyck, 1991; Young, 1991; Holthuis and Fransen, 2004; Félix et al., 2006; Nogata and Matsumura, 2006; Wirtz et al., 2006; Jones, 2010; Ávila et al., 2011; Jiménez et al., 2011; Hayashi, 2012; Angeletti et al., 2014; Kim et al., 2020; Minton et al., 2020 (in press.); Tasmanian Museum and Art Gallery, 2020; Ueda, 2020; Ten et al., unpubl.
Coronula reginae (Darwin, 1854)
Synonyms
-
Morphological Description
Molecular Sequences
-
Association
Obligate commensal
Cetacean Hosts/Basibionts
Balaenoptera bonaerensis, B. borealis, B. musculus, B. physalus, Eubalaena glacialis, Megaptera novaeangliae; single report on Delphinapterus leucas and Physeter macrocephalus
Geographic Range
Arctic, Atlantic, North Pacific, Indian Ocean, Antarctica
Life Cycle
-
Microhabitat
Lower jaw, flukes (Cockrill, 1960; Scarff, 1986)
Use as Indicator
See Coronula diadema (above).
Remarks
-
References
Collet, 1912; Pilsbry, 1916; Cornwall, 1927; Cornwall, 1928; Mackintosh and Wheeler, 1929; Nilsson-Cantell, 1930a; Nilsson-Cantell, 1930b; Hiro, 1938; Stephensen, 1938; Scheffer, 1939; Rees, 1953; Guiler, 1956; Tomilin, 1957; Cockrill, 1960; Rice, 1963; Klinkhart, 1966; Kawamura, 1969; Rice, 1977; Nilsson-Cantell, 1978; Silva-Brum, 1985; Scarff, 1986; Bushuev, 1990; Smiddy and Berrow, 1992; Holthuis and Fransen, 2004; Ten et al., unpubl.
Cryptolepas rhachianecti (Dall, 1872)
Synonyms
-
Morphological Description
Dall, 1872; Cornwall, 1955; Achituv, 1998; Seilacher, 2005
Molecular Sequences
H3, 12S rRNA, 16S rRNA, 18S rRNA, 28S rRNA (Hayashi et al., 2013)
Association
Obligate commensal, although Tomilin (1957) considered this species to be potentially harmful because it can impede whales’ movement and damage their skin.
Cetacean Hosts/Basibionts
Eschrichtius robustus; once reported on Delphinapterus leucas and Orcinus orca
Geographic Range
North Pacific; one record in the Gulf of Mexico (eastern North Atlantic)
Life Cycle
Gray whales wintering in waters off California and Mexico bear large and small specimens of C. rhachianecti when migrating northward, but only large barnacles when sighted during the southbound migration (Rice and Wolman, 1971). This would suggest that larval settlement occurs in wintering areas. This interpretation is supported by the observation that belugas held captive in San Diego Bay have C. rhachianecti in synchrony with gray whale northward migration (Rice and Wolman, 1971; Ridgway et al., 1997). Vertical shell growth is 0.12 mm/day (Killingley, 1980).
Microhabitat
Rostrum, lips, throat, peduncle, fins (Kasuya and Rice, 1970; Briggs and Morejohn, 1972)
Use as Indicator
Isotope analysis (δ18O) and geographical patterns of occurrence of fossilized remains have helped to reveal gray whale migration routes (Killingley, 1980; Bosselaers and Collareta, 2016; Taylor et al., 2019). Small size of barnacles and other features (appearance and associated scarring) have been used to identify calves of gray whale in photo-identification studies (Bradford et al., 2011). Barnacle orientation reflects waterflow patterns on gray whales (Kasuya and Rice, 1970; Briggs and Morejohn, 1972). Greater abundance of C. rachianecti on the left side of the head of gray whales may indicate that the right side is used predominantly for benthic feeding (Kasuya and Rice, 1970). In fact, right-sided feeding bias has been observed in some cetaceans (e.g., Clapham et al., 1995; Marino and Stowe, 1997; Karenina et al., 2016), including gray whales (e.g., Woodward and Winn, 2006).
Remarks
-
References
Dall, 1872; Pilsbry, 1916; Rice, 1963; Roest, 1970; Rice and Wolman, 1971; Briggs and Morejohn, 1972; Leung, 1976; Wellington and Anderson, 1978; Sullivan and Houck, 1979; Achituv, 1998; Weller et al., 1999; Takeda and Ogino, 2005; Sokolov and Arsen'ev, 2006; Murase et al., 2014; Scordino et al., 2017; Kasuya and Rice, 1970; Killingley, 1980; Swartz, 1981; Samaras, 1989; Ridgway et al., 1997; Findley and Vidal, 2002
Tubicinella major (Lamarck, 1802)
Synonyms
-
Morphological Description
Molecular Sequences
-
Association
Obligate commensal, although Tomilin (1957) considered this species to be potentially harmful because it can impede whales’ movement and damage their skin.
Cetacean Hosts/Basibionts
Eubalaena australis; once reported on Balaenoptera borealis and E. glacialis
Geographic Range
Atlantic, western South Pacific, Antarctica
Life Cycle
-
Microhabitat
Upper jaw, callosities, forehead, over the eye (Pilsbry, 1916; Scarff, 1986)
Use as Indicator
Shell plate remains of T. major found in Nerja Cave (Málaga, southern Spain) were used as indirect evidence of whale consumption by humans in the Upper Magdalenian (Álvarez-Fernández et al., 2013).
Remarks
Reported as a basibiont of facultative epibionts of the genus Conchoderma (Liouville, 1913).
References
Worm, 1655; Marloth, 1900; Gruvel, 1903; Liouville, 1913; Pilsbry, 1916; Reeb et al., 2007
Xenobalanus globicipitis (Steenstrup, 1852)
Synonyms
-
Morphological Description
Darwin, 1854; Cornwall, 1955; Rajaguru and Shantha, 1992; Anderson, 1994; Seilacher, 2005
Molecular Sequences
COI (Pérez-Losada et al., 2014), H3, 12S rRNA, 16S rRNA, 18S rRNA, 28S rRNA (Hayashi et al., 2013)
Association
Obligate commensal
Cetacean Hosts/Basibionts
Balaenoptera acutorostrata, B. bonaerensis, B. borealis, B. edeni, B. musculus, B. physalus, Delphinus delphis, Feresa attenuata, Globicephala macrorhynchus, G. melas, Grampus griseus, Kogia sp., Lagenodelphis hosei Fraser, 1956, Lissodelphis borealis, Megaptera novaeangliae, Mesoplodon bidens, M. mirus, Neophocaena asiaeorientalis Pilleri & Gihr, 1972, N. phocaenoides (Cuvier, 1829), Orcinus orca, Peponocephala electra, Phocoena phocoena, P. sinus Norris & McFarland, 1958, P. spinnipinnis (Burmeister, 1865), Physeter macrocephalus, Pontoporia blainvillei, Pseudorca crassidens, Sagmatias obliquidens (Gill, 1865), S. obscurus, Sotalia fluviatilis (Gervais & Deville in Gervais, 1853), S. guianensis (Van Beneden, 1864), Sousa plumbea (G. Cuvier, 1829), Stenella attenuata, S. clymene, S. coeruleoalba, S. frontalis (Cuvier, 1829), S. longirostris, Steno bredanensis, Tursiops aduncus, T. truncatus, Ziphius cavirostris
Geographic Range
Cosmopolitan (Arctic, Atlantic, Pacific, Mediterranean, South China Sea, Indian Ocean, Antarctica)
Life Cycle
Under experimental conditions at 28°C, the nauplii develop into cyprids in c. 8 days of hatching (Dreyer et al., 2020). Cyprids are similar to those of other barnacles but show variation in the structures that contact the substratum (Dreyer et al., 2020). In Guiana dolphins, Sotalia fluviatilis, off southern Brazil, field observations suggest that barnacle growth rate is initially fast and slows down after c. 30 days; sexual maturity seems to be reached in 40-45 days, and life span does not exceed one year (Flach et al., 2021).
Microhabitat
Trailing edge of dorsal fin, pectoral flippers, and mostly tail flukes (Calman, 1920; Barnard, 1924; Cornwall, 1927; Cornwall, 1928; Pope, 1958; Caldwell et al., 1971; Devaraj and Bennet, 1974; Bryden, 1976; Rice, 1978; Greenwood et al., 1979; Bane and Zullo, 1980; Spivey, 1980; Raga et al., 1983b; Ross, 1984; Raga and Sanpera, 1986; Brownell et al., 1987; Mead and Potter, 1990; Rajaguru and Shantha, 1992; Van Waerebeek et al., 1993; Watson et al., 1994; Jefferson et al., 1995; Reyes and Van Waerebeek, 1995; Araki et al., 1997; Orams and Schuetze, 1998; Rittmaster et al., 1999; Vidal et al., 1999; Barros and Stolen, 2001; Parsons et al., 2001; Resendes et al., 2002; Berland et al., 2003; Di Beneditto and Ramos, 2004; Palacios et al., 2004; Kane et al., 2008; Bearzi and Patonai, 2010; Best and Meÿer, 2010; Carvalho et al., 2010; Ribeiro et al., 2010; Foote et al., 2011; Karaa et al., 2011; Martín et al., 2011; Oliveira et al., 2011; Rosso et al., 2011; Díaz-Aguirre et al., 2012; González et al., 2012; Ólafsdóttir and Shinn, 2013; Towers et al., 2013; Whitehead et al., 2014; Díaz-Gamboa, 2015; Kim and Sohn, 2016; Methion and Díaz López, 2019; Pacheco et al., 2019; Herr et al., 2020; Matthews et al., 2020; Siciliano et al., 2020; Visser et al., 2020; Flach et al., 2021); also reported on the head (Samaras, 1989; Engel, 1994) and on a facial lesion (Alves-Motta et al., 2020).
Use as Indicator
The high detectability of X. globicipitis from visual surveys makes it applicable for individual marking of cetaceans (Visser et al., 2020) and as a multifaceted indicator. First, differences in its prevalence have been used to trace cetacean long-distance migrations (Best, 1982; Bushuev, 1990; Matthews et al., 2020; Ten et al., unpubl.) and to discriminate ecological stocks (Kawamura, 1969; Bushuev, 1990; Toth et al., 2012; Towers et al., 2013; Urian et al., 2019; Silva et al., 2020) and climate change-derived shifts in cetacean distribution (Visser et al., 2020). Second, its settlement patterns on hosts, which seem mainly driven by water flow, have been used to investigate cetacean swimming and hydrodynamics (Carrillo et al., 2015; Moreno-Colom et al., 2020). Lastly, the higher prevalence on immunosuppressed hosts highlights its potential as an indicator of health status in cetacean populations (Aznar et al., 1994; Aznar et al., 2005).
Remarks
-
References
Steenstrup, 1852; Darwin, 1854; Hoek, 1883; True, 1890; Richard and Neuville, 1897; Weltner, 1897; Gruvel, 1905; Gruvel, 1912; Collet, 1912; Liouville, 1913; Gruvel, 1920; Calman, 1920; Nilsson-Cantell, 1921; Barnard, 1924; Broch, 1924; Cornwall, 1927; Cornwall, 1928; Mackintosh and Wheeler, 1929; Nilsson-Cantell, 1930a; Richard, 1936; Matthews, 1938b; Heldt, 1950; Cornwall, 1955; Pope, 1958; Rice, 1963; Zullo, 1963; Stubbings, 1965; Pilleri, 1967; Dollfus, 1968; Kawamura, 1969; Pilleri and Gihr, 1969; Pilleri and Knuckey, 1969; Pilleri, 1970; Rice, 1977; Rice, 1978; Caldwell et al., 1971a; Devaraj and Bennet, 1974; Brownell, 1975; Mead, 1975; Bryden, 1976; Spivey, 1977; Dailey and Walker, 1978; Greenwood et al., 1979; Bane and Zullo, 1980; Spivey, 1980; Raga et al., 1982; Raga et al., 1983b; Ross, 1984; Raga and Carbonell, 1985; Gittings et al., 1986; Raga and Sanpera, 1986; Brownell et al., 1987; Rappé, 1988; Rappé and Van Waerebeek, 1988; Pinedo et al., 1989; Samaras, 1989; Bushuev, 1990; Mead and Potter, 1990; Van Waerebeek et al., 1990; Young, 1991; Duignan et al., 1992; Rajaguru and Shantha, 1992; Aguilar and Raga, 1993; Raga and Balbuena, 1993; Van Waerebeek et al., 1993; Aznar et al., 1994; Aznar et al., 2005; Aznar et al., 2016, unpubl.; Engel, 1994; Fertl, 1994; Watson et al., 1994; Jefferson et al., 1995; Reyes and Van Waerebeek, 1995; Azevedo et al., 1996; Fertl et al., 1996; Araki et al., 1997; Orams and Schuetze, 1998; Uchida, 1998; Rittmaster et al., 1999; Vidal et al., 1999; Di Beneditto and Ramos, 2001; Guerrero-Ruiz and Urbán, 2000; Kuramochi et al., 2000; Uchida and Araki, 2000; Addink and Smeenk, 2001; Barros and Stolen, 2001; Parsons et al., 2001; Danilewicz et al., 2002; Louella and Dolar, 2002; Resendes et al., 2002; Berland et al., 2003; Di Beneditto and Ramos, 2004; Karuppiah et al., 2004; Palacios et al., 2004; Watson and Gee, 2005; Bellido et al., 2006; Sakai et al., 2006; Best, 2007; Pitman et al., 2007; Toth-Brown and Hohn, 2007; Kane et al., 2008; Kautek et al., 2008; Rotstein et al., 2009; Sakai et al., 2009; Bearzi and Patonai, 2010; Best and Meÿer, 2010; Carvalho et al., 2010; Ribeiro et al., 2010; Weir, 2010; Foote et al., 2011; Karaa et al., 2011; Martín et al., 2011; Oliveira et al., 2011; Rosso et al., 2011; Bertulli et al., 2012; Díaz-Aguirre et al., 2012; González et al., 2012; Hayashi, 2012; Pugliese et al., 2012; Toth et al., 2012; Ólafsdóttir and Shinn, 2013; Towers et al., 2013; Lane et al., 2014; Whitehead et al., 2014; Díaz-Gamboa, 2015; Carrillo et al., 2015; Blum and Fong, 2016; Prestridge, 2016; Kim and Sohn, 2016; Denkinger and Alarcon, 2017; Donnelly et al., 2018; Ronje et al., 2018; Cortés-Peña, 2019; Methion and Díaz López, 2019; Pacheco et al., 2019; Urian et al., 2019; Alves-Motta et al., 2020; Gagnon and Torgersen, 2020; Gómez-Hernández et al., 2020; Herr et al., 2020; Matthews et al., 2020; Minton et al., 2020 (in press); Minussi, 2020; Moreno-Colom et al., 2020; Natural History Museum, 2020; Orrell, 2020; Siciliano et al., 2020; Silva et al., 2020; Ueda, 2020; Vargas-Bravo et al., 2020; Visser et al., 2020; CW Azores, 2021; Flach et al., 2021; iNaturalist, 2021; Ten et al., unpubl.
Order Scalpellomorpha Buckeridge & Newman, 2006
Family Lepadidae Darwin, 1852
Lepadids are oceanic fugitive species with relatively rapid growth and require a hard substratum to settle (e.g., Skerman, 1958; Patel, 1959; Southward, 1987; Harper, 1995; Hinojosa et al., 2006; Fraser et al., 2011; Wegner and Cartamil, 2012; Frick and Pfaller, 2013; Schiffer and Herbig, 2016). Overall, they are generalistic settlers on floating objects, be living or inanimate. This feature makes it often difficult to ascertain whether settlement on putative basibionts is pre- or postmortem (e.g., Magni et al., 2015; Ten et al., 2019). However, some degree of specialization for living cetaceans seems to be apparent especially for Conchoderma auritum (see below). Apart from cetaceans, other basibionts for species of Lepas and Conchoderma are, inter alia, bull kelps (Fraser et al., 2011; López et al., 2017), sea turtles (Ten et al., 2019), and even human corpses (Magni et al., 2015). Extensive description of the metamorphosis for species of this family is provided by Darwin (1854).
Conchoderma auritum (Linnaeus, 1767)
Synonyms
Conchoderma leporinum Olfers, 1814, Lepas aurita Linnaeus, 1767, Otion stimpsoni Dall, 1872
Morphological Description
Darwin, 1854; Dall, 1872; Monod, 1938; Cornwall, 1955
Molecular Sequences
COI (Ashton et al., 2016; GenBank MT563423; MT563438; MT563441), H3 (Pérez-Losada et al., 2008), 12S rRNA (Endo et al., 2010), 16S rRNA (Tomioka et al., 2020), 18S rRNA, 28S rRNA (Pérez-Losada et al., 2008)
Association
Facultative commensal. However, Newman and Abbott (1980) considered that this species might actually be an obligate commensal on cetaceans because most records of this species involve, as substrata, the shells of coronulid barnacles and/or on exposed hard surfaces of these mammals, e.g., baleens or tusks of ziphids. Rasmussen (1980) postulated that C. auritum prefers hard substrates in motion, although this species has also been reported on animate objects (see below).
Cetacean Hosts/Basibionts
Balaenoptera acutorostrata, B. bonaerensis, B. borealis, B. musculus, B. physalus, Berardius bairdii, Eschrichtius robustus, Eubalaena glacialis, Feresa attenuata, Globicephala macrorhynchus, G. melas, Hiperoodon ampullatus, H. planifrons, Megaptera novaeangliae, Mesoplodon bidens, M. densirostris (de Blainville, 1817), M. europaeus, M. hectori (Gray, 1871), M. layardii (Gray, 1865), M. mirus, M. stejnegeri True, 1885, Neophocaena phocaenoides, Peponocephala electra, Physeter macrocephalus, Pontoporia blainvillei, Stenella attenuata, S. frontalis, S. longirostris, Tursiops aduncus, T. truncatus, Ziphius cavirostris
Geographic Range
Cosmopolitan
Life Cycle
Growth rate of metamorphosed individuals are available only from inanimate substrata (0.1-1.0 mm/day; Il'in et al., 1978; Rasmussen, 1980; Dalley and Crisp, 1981). At a mean temperature of 23°C, the capitulum of newly recruited individuals can reach 1 mm long in just two days, and 6 mm in 9 days; in older individuals, growth rate estabilizes at 0.55 mm/day (Dalley and Crisp, 1981). Cyprids of C. auritum sampled along the Atlantic Ocean were found in low concentration between 25° N and 34 °S (Dalley and Crisp, 1981).
Microhabitat
On baleen plates (Nilsson-Cantell, 1930a; Nilsson-Cantell, 1939; Omura, 1950a; Christensen, 1985; Raga and Sanpera, 1986; Ólafsdóttir and Shinn, 2013); odontocete teeth (Beneden, 1870; Ohlin, 1893; Lillie, 1910; Hamilton, 1914; Broch, 1924; Nansen, 1925; Nilsson-Cantell, 1930a; Nilsson-Cantell, 1930c; Gauthier, 1938; Monod, 1938; Nilsson-Cantell, 1939; Scheffer, 1939; Fabian, 1950; Mizue, 1950; Omura, 1950a; Omura et al., 1955; Sergeant and Fisher, 1957; Tomilin, 1957; Wolff, 1960; Marlow, 1963; Rice, 1963; Morris and Mowbray, 1966; Pilleri, 1969a; Pilleri, 1969b; Caldwell et al., 1971b; Van Bree, 1971; Fordyce et al., 1979; Dixon, 1980; Baker, 1983; Pastene et al., 1990; Balbuena, 1991; Debrot, 1992; Rodríguez-López and Mignucci-Giannoni, 1999; Soto, 2001; O’Connor and Franco, 2003; Bermúdez-Villapol et al., 2006; Van Waerebeek et al., 2008; Holmes and Franco, 2010; Martín et al., 2011; Bachara and Gullan, 2016; Foskolos et al., 2017; Tomioka et al., 2020), and on the coronulid barnacles C. diadema (Beneden, 1870; Dall, 1872; Sars, 1880; Gruvel, 1911; Mörch, 1911; Liouville, 1913; Borradaile, 1916; Pilsbry, 1916; Broch, 1924; Cornwall, 1924; Cornwall, 1927; Cornwall, 1928; Nilsson-Cantell, 1930a; Nilsson-Cantell, 1930c; Hiro, 1935; Stephensen, 1938; Nilsson-Cantell, 1939; Scheffer, 1939; Tomilin, 1957; Rice, 1963; Clarke, 1966; Newman and Ross, 1971; Holthuis and Fransen, 2004; Kim et al., 2020), C. reginae (Nilsson-Cantell, 1930a; Nilsson-Cantell, 1939; Wolff, 1960; Rice, 1963; Clarke, 1966; Newman and Ross, 1971), and X. globicipitis (Ten et al., unpubl.). Also, on deformed or injured jaws that leave the teeth exposed (Davis, 1874; Mörch, 1911; Matthews, 1938c; Chapman and Santler, 1955; Clarke, 1956; Nasu, 1958; Cockrill, 1960; Wolff, 1960; Slijper, 1962; Spaul, 1964; Clarke, 1966; Pilleri, 1969b; Beach, 2015). Once recorded as an hyperepibiont on P. balaenoptera (Nilsson-Cantell, 1930a).
Use as Indicator
Holmes and Franco (2010) observed several individuals of C. auritum on the left tooth of Sowerby’s beaked whale, Mesoplodon bidens, but none on the right tooth. These authors speculated that the barnacles could indicate some type of chirality during feeding, which may hinder barnacle development on the right side (see Cryptolepas rachianecti above). On the other hand, the presence of C. auritum has been suggested as an indicator of previous interaction of cetaceans with fisheries since these barnacles can attach on scarred mouth injuries (Beach, 2015; Welch, 2017). Finally, knowledge of growth rates of C. auritum makes this species potentially suitable to make temporal calibrations of time since settlement. This could inform on basibiont movements or interaction with fisheries (see, e.g., Dalley and Crisp, 1981; Wegner and Cartamil, 2012; Zettler, 2021), although this application has not been used yet in cetaceans.
Remarks
Also recorded on inanimate substrata (e.g., ship hulls, moorings, ropes; Foster and Willan, 1979; Rasmussen, 1980; Farrapeira et al., 2007) and elephant seals, Mirounga spp. (Best, 1971; Joseph et al., 1986).
References
Bennet, 1837; Bennett, 1840; Hallas, 1868; Beneden, 1870; Dall, 1872; Davis, 1874; Sars, 1880; Ohlin, 1893; Lillie, 1910; Gruvel, 1911; Mörch, 1911; Collet, 1912; Liouville, 1913; Hamilton, 1914; Allen, 1916; Borradaile, 1916; Pilsbry, 1916; Broch, 1924; Cornwall, 1924; Hinton, 1925; Nansen, 1925; Cornwall, 1927; Cornwall, 1928; Mackintosh and Wheeler, 1929; Nilsson-Cantell, 1930a; Nilsson-Cantell, 1930c; Matthews, 1937; Matthews, 1938c; Gauthier, 1938; Hiro, 1938; Monod, 1938; Stephensen, 1938; Nilsson-Cantell, 1939; Scheffer, 1939; Fabian, 1950; Mizue, 1950; Omura, 1950a; Omura, et al., 1955; Angot, 1951; Ohno and Fujino, 1952; Kakuwa et al., 1953; Rees, 1953; Chapman and Santler, 1955; Clarke, 1956; Sergeant and Fisher, 1957; Tomilin, 1957; Nasu, 1958; Symons and Weston, 1958; Cockrill, 1960; Wolff, 1960; Sergeant, 1962; Slijper, 1962; Marlow, 1963; Rice, 1963; Spaul, 1964; Clarke, 1966; Morris and Mowbray, 1966; Perrin, 1969; Pilleri, 1969b; Newman and Ross, 1971; Van Bree, 1971; Monod and Serene, 1976; Fordyce et al., 1979; Dixon, 1980; Baker, 1983; Christensen, 1985; Raga and Sanpera, 1986; Mead, 1989; Bushuev, 1990; Pastene et al., 1990; Bordino and González, 1992; Debrot, 1992; García-Godos, 1992; Raga and Balbuena, 1993; Mignucci-Giannoni et al., 1998; Rodríguez-López and Mignucci-Giannoni, 1999; Huang et al., 2000; Soto, 2001; O’Connor and Franco, 2003; Holthuis and Fransen, 2004; Bermúdez-Villapol et al., 2006; Van Waerebeek et al., 2008; Holmes and Franco, 2010; Ávila et al., 2011; Martín et al., 2011; Ólafsdóttir and Shinn, 2013; Angeletti et al., 2014; Insacco et al., 2014; Beach, 2015; Elorriaga-Verplancken et al., 2015; Bachara and Gullan, 2016; Foskolos et al., 2017; Iwasa-Arai et al., 2017b; Wheeler and McIntosh, 2018; Kim et al., 2020; Natural History Museum, 2020; Tomioka et al., 2020; Ueda, 2020; Ten et al., unpubl.
Conchoderma virgatum (Spengler, 1789)
Synonyms
Conchoderma virgata (Spengler, 1790), Lepas virgata Spengler, 1790
Morphological Description
Darwin, 1854; Nilsson-Cantell, 1928
Molecular Sequences
COI (Chen et al., 2013), H3 (Pérez-Losada et al., 2008), 12S rRNA (Endo et al., 2010), 18S rRNA (Pérez-Losada et al., 2008; Yusa et al., 2012), 28S rRNA (Pérez-Losada et al., 2008)
Association
Facultative commensal
Cetacean Hosts/Basibionts
Balaenoptera acutorostrata, B. bonaerensis, B. borealis, B. musculus, B. physalus, Delphinus delphis, Feresa attenuata, Megaptera novaeangliae, Neophocaena phocaenoides, Physeter macrocephalus, Stenella coeruleoalba
Geographic Range
Cosmopolitan
Life Cycle
Most growth rate estimates of this species have been studied on inanimate substrata (0.1-1.5 mm/day; Darwin, 1851; Annandale, 1909; MacIntyre, 1966; Tsikhon-Lukanina et al., 1977; Il'in et al., 1978; Dalley and Crisp, 1981). For instance, at a mean temperature of 23°C and 14 days after metamorphosis, individuals grew 0.66 mm/day on an experimental torpedo (Dalley and Crisp, 1981). Eckert and Eckert (1987) provide a von Bertalanffy’s growth equation obtained from C. virgatum measurements on nesting sea turtles, which shows an asymptotic trend comparable to that of previous studies. Differences in growth rate estimates and maximum size between studies suggest an effect of the ecological conditions (Eckert and Eckert, 1987).
Microhabitat
Mostly as a hyperepibiont of Pennella balaenoptera (Sars, 1866; Koren and Danielssen, 1877; Turner, 1905; Nilsson-Cantell, 1930a; Clarke, 1956; Clarke, 1966; Raga and Sanpera, 1986; Araki et al., 1997; Terasawa et al., 1997; Uchida, 1998; Ólafsdóttir and Shinn, 2013), but it can also attach directly to odontocete teeth (Lillie, 1910; Aznar et al., 1994). Once reported on C. auritum (Clarke, 1966), Neocyamus physeteris (Oliver and Trilles, 2000), and on the shell of Xenobalanus globicipitis (Ten et al., unpubl.).
Use as Indicator
Knowledge of growth rates of C. virgatum makes this species potentially suitable to make temporal calibrations of time since settlement (see C. auritum). Indeed, unusual attachment of C. virgatum and Lepas spp. on dolphin teeth may have occurred after dolphin death, when teeth remain exposed (Aznar et al., 1994). This provides the opportunity to infer the approximate time of death, as it has been done in sea turtles (Ten et al., 2019). The finding of Conchoderma sp. (presumably C. virgatum) attached to a marlin spear that was inserted into the jaw of an Antarctic minke whale suggested that spearing occurred a few months before the finding (Ohsumi, 1973). Lastly, its presence and size has been used as an indicator of oceanic habitat use by sea turtles (Casale et al., 2004; Casale et al., 2012; Ten et al., 2019) and of interaction with pelagic fisheries (Wegner and Cartamil, 2012; Ten et al., 2019).
Remarks
It is typical settler of inanimate substrata, e.g., ship vessels, buoys (Foster and Willan, 1979; Farrapeira et al., 2007; González et al., 2012; Wegner and Cartamil, 2012), but also attaches to multiple marine animals, including fish (e.g., Crozier, 1916; Hastings, 1972; Ohsumi, 1973), sea turtles (e.g., Eckert and Eckert, 1987; Alonso et al., 2010), elephant seals (Joseph et al., 1986), sea snakes (Annandale, 1909; Yamato et al., 1996), and pelagic crabs (Jerde, 1967; Moazzam and Rizvi, 1979). It has also been reported as a hyperepibiont of fish copepods (e.g., Williams, 1978; Williams and Williams, 1986).
References
Sars, 1866; Koren and Danielssen, 1877; Turner, 1905; Lillie, 1910; Collet, 1912; Liouville, 1913; Mackintosh and Wheeler, 1929; Nilsson-Cantell, 1930a; Clarke, 1956; Clarke, 1966; Kawamura, 1969; Berzin, 1972; Rice, 1977; Greenwood et al., 1979; Raga and Carbonell, 1985; Raga and Sanpera, 1986; Bushuev, 1990; Aguilar and Raga, 1993; Aznar et al., 1994, unpubl.; Araki et al., 1997; Terasawa et al., 1997; Uchida, 1998; Huang et al., 2000; Kuramochi et al., 2000; Oliver and Trilles, 2000; Uchida and Araki, 2000; Ólafsdóttir and Shinn, 2013; Ten et al., unpubl.
Lepas (Anatifa) hillii (Leach, 1818)
Synonyms
Lepas hillii (Leach, 1818)
Morphological Description
Molecular Sequences
-
Association
Facultative commensal
Cetacean Hosts/Basibionts
Once reported on Stenella coeruleoalba
Geographic Range
Pantropical (González et al., 2012)
Life Cycle
At temperatures ca. 25 °C, individuals attached to a ship in central Atlantic Ocean reached maturity after 30-43 days for a capitulum 13-17 mm long (i.e., a growth rate of 0.5 mm/day; Evans, 1958). Similarly as in Conchoderma spp. (see above), growth was asymptotic and fell to 0.03 mm/day after maturity (Evans, 1958).
Microhabitat
Teeth (Aznar et al., 1994)
Use as Indicator
Deeper knowledge of growth rates of L. hillii would refine estimates of time since settlement (see C. virgatum). Some applications include the estimation of the time of death of basibionts (Aznar et al., 1994; Ten et al., 2019), interaction with fisheries (Wegner and Cartamil, 2012; Ten et al., 2019), and oceanic habitat use (Casale et al., 2004; Casale et al., 2012; Ten et al., 2019).
Remarks
On inanimate substrata, e.g., buoys, ship hulls, a rope (Il'in et al., 1978; Dalley and Crisp, 1981; Farrapeira et al., 2007; Wegner and Cartamil, 2012) and on marine vertebrates, including fish (Dulčić et al., 2015), sea turtles (Domènech et al., 2015; Ten et al., 2019), and elephant seals (Joseph et al., 1986).
References
Lepas (Anatifa) pectinata (Spengler, 1793)
Synonyms
Lepas pectinata Spengler, 1793
Morphological Description
Molecular Sequences
COI (Chen et al., 2013; Schiffer and Herbig, 2016; Aguilar et al., 2018; Rech et al., 2018; GenBank KY639421-KY639424; MF974366-MF974369), H3 (Pérez-Losada et al., 2008), 18S rRNA (Pérez-Losada et al., 2008; Schiffer and Herbig, 2016), 28S rRNA (Pérez-Losada et al., 2008)
Association
Facultative commensal
Cetacean Hosts/Basibionts
Stenella coeruleoalba
Geographic Range
Cosmopolitan (González et al., 2012)
Life Cycle
This is the most abundant lepadid in the Northeast Atlantic, where its development has been studied (Ellis et al., 1983; Conway et al., 1990). Interestingly, L. pectinata presumably performs ontogenetic depth migrations, i.e., nauplii feed in the upper 150 m and the non-feeding cyprids distribute at 300-400 m (Conway et al., 1990). Nauplii show similar feeding and swimming features as other barnacle larvae (Moyse, 1984).
Microhabitat
Teeth (Aznar et al., 1994)
Use as Indicator
See L. hillii (above).
Remarks
Closely associated to Sargassum spp. weed (Fine, 1970; Conway et al., 1990); also found on inanimate substrata (e.g., floating crude oil, plastic debris; Horn et al., 1970; Minchin, 1996; Bergami et al., 2021) and on sea turtles (Domènech et al., 2015; Ten et al., 2019).
References
Aguilar and Raga, 1993; Aznar et al., 1994
Phylum Chordata Haeckel, 1874
Class Actinopteri Cope, 1871
Subclass Teleostei Müller, 1846
Order Carangiformes Jordan, 1963
Family Echeneidae Rafinesque, 1810
Remoras or diskfishes include 8 species of specialized teleosts that use their dorsal fin as an adhesive disc to attach to a great variety of marine vertebrates from which they benefit through, e.g., ventilation, protection from predators, and increased contact with conspecifics (Fertl and Landry, 1999a; Fertl and Landry, 1999b). The fact that remoras live in association with elasmobranchs, teleosts, sea turtles, and cetaceans (Cressey and Lachner, 1970) has hampered research on basic biological features such as growth and reproduction for most species (Battaglia et al., 2016).
Echeneis naucrates (Linnaeus, 1758)
Synonyms
Echeneis chiromacer Duméril, 1858, E. fasciata Gronow, 1854, E. fusca Gronow, 1854, E. guaican Poey, 1860, E. lunata Bancroft, 1831, E. metallica Poey, 1860, E. naucratus Linnaeus, 1758, E. neucrates Linnaeus, 1758, E. scaphecrates Duméril, 1858, E. vittate Rüppell, 1838, Echensis naucrates Linnaeus, 1758, Echneis naucrates Linnaeus, 1758, Leptecheneis flaviventris Seale, 1906, L. naucrates (Linnaeus, 1758)
Morphological Description
Collette, 2003; Skaramuca et al., 2009
Molecular Sequences
> 40,000 results in GenBank
Association
Facultative commensal
Cetacean Hosts/Basibionts
Sotalia guianensis, Tursiops truncatus
Geographic Range
Cosmopolitan (Collette et al., 2015)
Life Cycle
In the eastern Gulf of Mexico females show slower growth but achieve larger size than males; spawning takes place in August (Bachman et al., 2018).
Microhabitat
Flanks and both dorsal and ventral sides
Use as Indicator
-
Remarks
It can free swim in the water column while feeding on small fishes and plankton (O’Toole, 2002), but also attach to a broad spectrum of basibionts, including reef teleosts, sharks, and sea turtles (O’Toole, 2002; Sazima and Grossman, 2006; Gray et al., 2009), nearshore dolphins (above), and even to conspecifics (Brunnschweiler and Sazima, 2006). It is considered a sister-species of E. neucratoides (O’Toole, 2002).
References
Fertl and Landry, 1999b; Fertl et al., 2002; Noke, 2004; Santos and Sazima, 2005
Echeneis neucratoides (Zuiew, 1789)
Synonyms
-
Morphological Description
-
Molecular Sequences
COI (GenBank KF461171), EGR1, EGR2B, EGR3 (Campbell et al., 2013), ITS1 (Gray et al., 2009), ND2 (Gray et al., 2009), RAG1, RH1 (Campbell et al., 2013), VCPIP, ZIC1 (Betancur et al., 2013), 5.8S rRNA, 12S rRNA, 16S rRNA, 18S rRNA (Gray et al., 2009)
Association
Presumably facultative commensal
Cetacean Hosts/Basibionts
Two unidentified cetaceans
Geographic Range
Western Atlantic Ocean (Fertl and Landry, 1999a; Fertl and Landry, 1999b)
Life Cycle
-
Microhabitat
-
Use as Indicator
-
Remarks
Typical commensal of sharks and once observed on a West Indian manatee captured in Puerto Rico (Mignucci-Giannoni et al., 1999).
References
Remora australis (Bennett, 1840)
Synonyms
Echeneis australis Bennett, 1840, E. scutata Günther, 1860, Remilegia australis (Bennett, 1840), Remora australia (Bennett, 1840), R. scutata (Günther, 1860)
Morphological Description
Molecular Sequences
COI (GenBank GU440495; OK030822), CYTB (Sanciangco et al., 2016), ITS1, ND2 (Gray et al., 2009), RAG1 (GenBank EU167871), 5.8S rRNA, 12S rRNA, 16S rRNA, 18S rRNA (Gray et al., 2009)
Association
Obligate commensal/mutualist. Although previously considered as an obligate commensal (Rice and Caldwell, 1961), later evidence has shown that this species can feed on host’s ectoparasites (O’Toole, 2002). However, remoras may potentially disrupt the flow over cetaceans’ body, increasing drag, and their sucking disk may produce irritation (Fish et al., 2006).
Cetacean Hosts/Basibionts
Balaenoptera borealis, B. edeni, B. musculus, Delphinus delphis, Orcinus orca, Physeter macrocephalus, Stenella attenuata, S. frontalis, S. longirostris, Tursiops truncatus
Geographic Range
eastern Pacific, Atlantic, Indian Ocean, Indonesian Sea
Life Cycle
Off Brazil, remoras of the smallest size class (i.e., < 10 cm) were the most abundant size class in May and their frequency fell until none were reported in October (Wingert et al., 2021).
Microhabitat
Ubiquitous on skin (Wingert et al., 2021)
Use as Indicator
Remoras on blue whales preferentially attach to regions with reduced drag. Therefore, they could evince patterns of water flow over swimming whales, which could optimize tag deployment for extended ecological monitoring (Flammang et al., 2020).
Remarks
The records from B. edeni, O. orca, S. attenuata, and T. truncatus above provide only identification to genus level, but are here assigned to R. australis since it is the only species of Remora associated to cetaceans (O’Toole, 2002). Individuals of R. australis appear to disengage from whales during whaling (Pike, 1951; Rice and Caldwell, 1961), which might result in gross underestimations of actual prevalence in nature. Prior to towing, the prevalence of R. australis on blue whales, Balaenoptera musculus, captured in California and Peru was close to 100 percent (Rice and Caldwell, 1961). Attachment marks of this species on the host’s epidermis are superficial, and scarring is not typically observed (Rice and Caldwell, 1961; Visser, pers. obs.). There is a single record of two copepod hyperparasites on R. australis, namely Pennella balaenoptera and Lepeophtheirus crassus (Radford and Klawe, 1965).
References
Carl and Wilby, 1945; Cadenat, 1953; Krefft, 1953; Follet and Dempster, 1960; Mahnken and Gilmore, 1960; Rice and Caldwell, 1961; Rice, 1963; Radford and Klawe, 1965; Rice, 1977; Rice, 1978; Notarbartolo di Sciara and Watkins, 1979; Fertl and Landry, 1999a; Fertl and Landry, 1999b; Wingert et al., 2021
Order Siluriformes -
Family Trichomycteridae Bleeker, 1858
Catfishes (Siluriformes) are widely distributed in freshwater, estuarine, and marine habitats of continental shelves (de Pinna, 1998). Members of the family Trichomycteridae, known as pencil or parasitic catfishes (de Pinna and Wosiacki, 2003), inhabit continental freshwaters from Costa Rica to Patagonia (de Pinna and Wosiacki, 2003; Eschmeyer et al., 2017).
Ochmacanthus sp.
Synonyms
-
Morphological Description
Molecular Sequences
COI, CYTB, H3, ND4, MYH6, RAG1, RAG2, 12S rRNA, and 16S rRNA of three Ochmacanthus spp. (see GenBank)
Association
Presumably obligate commensal
Cetacean Hosts/Basibionts
Inia geoffrensis (Blainville, 1817)
Geographic Range
South American rivers (Koch, 2002)
Life Cycle
-
Microhabitat
On lateral and ventral surfaces (Araújo-Wang et al., 2019)
Use as Indicator
-
Remarks
Candirus are generally commensal on various freshwater fishes (Adriaens et al., 2010), but Araújo-Wang et al. (2019) reported year-round observations on Inia geoffrensis.
References
Class Hyperoartia Müller, 1844
Order Petromyzontiformes Berg, 1940
Family Petromyzontidae Bonaparte, 1831
Anadromous lampreys (Petromyzontiformes) are jawless fishes distributed antitropically around the world. They develop in estuaries and oceans, where they parasitize large vertebrates consuming their blood, fluids, and flesh, and then migrate into freshwater streams to spawn and die (Renaud, 2011; Johnson et al., 2015; Clemens et al., 2019). Species of Petromyzontidae are exclusively found in the Northern Hemisphere (Renaud, 2019; Miller et al., 2021). The family Petromyzontidae is described in Renaud (2019).
Entosphenus tridentatus (Richardson, 1836)
Synonyms
Entosphenus epihexodon Gill, 1862, E. tridentatus tridentatus (Richardson, 1836), Lampetra tridentatus (Richardson, 1836), Petromyzon astori Girard, 1858, P. ciliatus Ayres, 1855, P. epihexodon (Gill, 1862), P. lividus Girard, 1858, P. tridentatus Richardson, 1836
Morphological Description
Molecular Sequences
COI (Yamazaki et al., 2006; April et al., 2011; Carim et al., 2017; GenBank GU440367; KF918874; KF918875; KF929845; KY570333), CR (GenBank AY205567), CYTB (Docker et al., 1999; Lorion et al., 2000; Yamazaki et al., 2006; Boguski et al., 2012; GenBank DW022992; GQ206157; KR422618; KR422619; KU672473-KU672485), ETR-1, ETR-2, ETR-3, ETR-4, ETR-5, ETR-6 (Spice et al., 2011), GnRH-III (Silver et al., 2004), ND1, ND2, ND4, ND5 (Docker et al., 2007), RT (GenBank AJ244558), 12S rRNA (GenBank LC091545; LC091546), 16S rRNA (GenBank KJ010762), and the whole genome (Hess et al., 2020)
Association
Ectoparasite
Cetacean Hosts/Basibionts
Balaenoptera borealis, B. musculus, B. physalus, Berardius bairdii, Megaptera novaeangliae, Physeter macrocephalus
Geographic Range
North Pacific, from Baja California north to the Bering and Chukchi seas and westward into Russia and Japan, showing the greatest latitudinal range of any lamprey (Renaud, 2011)
Life Cycle
Laboratory observations hypothesized that the time of residence in the ocean is ≤ 3.5 years (Beamish, 1980). Movements in the ocean are poorly understood, but they are typically caught between the surface and 500 m (see Clemens et al., 2019).
Microhabitat
-
Use as Indicator
Based on the degree of healing of the marks of Pacific lampreys on several species of whales, Pike (1951) inferred that lamprey attacks took place during the northward migration in the North Pacific. Therefore, marks could be used to trace whale’s migration.
Remarks
Typically parasitizes fish (Clemens et al., 2019).
References
Carl, 1950; Pike, 1951; Nemoto, 1955; Rice, 1963; Rice, 1977; Rice, 1978
Petromyzon marinus (Linnaeus, 1758)
Synonyms
Ammocoetes bicolor Lesueur, 1818, Batymyzon bairdii (Gill, 1883), Lampetra marina (Linnaeus, 1758), Oceanomyzon wilsoni Fowler, 1908, Petromyzon adriaticus Nardo, 1847, P. americanus Lesueur, 1818, P. bairdii Gill, 1883, P. concolor Wright, 1892, P. lampetra Pallas, 1814, P. maculosus Gronow, 1854, P. marinus dorsatus Wilder, 1883, P. marinus unicolor Gage, 1928, P. maximus Cuvier, 1816, P. nigricans Lesueur, 1818, P. ruber Lacepède, 1800
Morphological Description
Molecular Sequences
> 193,000 results in GenBank
Association
Ectoparasite, inferred from resulting wounds and scars (Silva et al., 2014)
Cetacean Hosts/Basibionts
Balaenoptera acutorostrata, B. borealis, B. physalus, Eubalaena glacialis, Grampus griseus, Megaptera novaeangliae, Mesoplodon bidens, Orcinus orca, Physeter macrocephalus, Tursiops truncatus, Ziphius cavirostris
Geographic Range
Atlantic coast of North America and Europe, including the central Mediterranean Sea (Holčík et al., 2004; Kottelat and Freyhof, 2007)
Life Cycle
This hematophagous species grows to adult size in 1 year; the complete metamorphosis and reproduction takes 1.5 years (Silva et al., 2013).
Microhabitat
Flanks of middle and posterior body areas (Bertulli et al., 2012; Ólafsdóttir and Shinn, 2013)
Use as Indicator
In some cases, the individuals are still attached to the host when found, being easier to detect (Nichols and Hamilton, 2004; Nichols and Tscherter, 2011; Samarra et al., 2012; Miočić-Stošić et al., 2020). In others, however, only the remaining marks are visible. The applicability of these marks is still to be determined. Samarra et al. (2012) stated that they apparently disappear within 1 year, whereas Miočić-Stošić et al. (2020) claim that they are seemingly short-lived, thus not being suitable markings in photo-identification. In the past years, it has been more commonly found in Icelandic waters, and this change in distribution seems to be due to a gradual increase in water temperatures around Iceland (Astthorsson and Palsson, 2006).
Remarks
This species is often found on freshwater and marine fishes (Collette and Klein-MacPhee, 2002).
References
Japha, 1910; Collet, 1912; Nichols and Hamilton, 2004; Nichols and Tscherter, 2011; Rosso et al., 2011; Bertulli et al., 2012; Samarra et al., 2012; Ólafsdóttir and Shinn, 2013; Silva et al., 2014; Bertulli et al., 2016; Miočić-Stošić et al., 2020
Phylum Cnidaria Hatschek, 1888
Class Hydrozoa Owen, 1843
Subclass Hydroidolina Collins, 2000
Order Leptothecata Cornelius, 1992
Family Campanulariidae Johnston, 1836
Members of this family of thecate hydroids are ubiquitous in marine benthic communities. Given that the morphology of colonies and polips are highly variable within species, it is difficult to find diagnostic morphological characters to separate congeneric species (Cunha et al., 2015), which may hinder correct identification to species level.
Obelia dichotoma (Linnaeus, 1758)
Synonyms
Multiple; see Schuchert (2021).
Morphological Description
Molecular Sequences
COI (Govindarajan et al., 2006; Cunha et al., 2015; Cunha et al., 2017; GenBank MG791815; MW277711; MW277730; MZ580517; MZ580890), calmodulin (Govindarajan et al., 2006), LSU rRNA (Pruski and Miglietta, 2019; Penney and Rawlings, 2021; GenBank MG786561; MG786562), SSU rRNA (MG792325), 5.8S rRNA (Cunha et al., 2015), 16S rRNA (Bridge et al., 1995; Govindarajan et al., 2006; Cunha et al., 2015; Cunha et al., 2017; Rech et al., 2018), 18S rRNA, 28S rRNA (Bridge et al., 1995; Govindarajan et al., 2006; Cunha et al., 2015; Maronna et al., 2016; Cunha et al., 2017)
Association
Unknown, although a commensalist or even mutualistic association cannot be ruled out since newly released medusae of this species are bacteriophagous (Boero et al., 2007).
Cetacean Hosts/Basibionts
Once reported on Megaptera novaeangliae
Geographic Range
Nearly cosmopolitan (Orejas et al., 2012)
Life Cycle
Kubota (1999) reported the complete life cycle of O. dichotoma in Northern Japan.
Microhabitat
As a hyperepibiont on the barnacle Coronula diadema (Cornwall, 1928)
Use as Indicator
-
Remarks
It can be found on hard substrata, such as floats, pilings, rocks, and shells (Orejas et al., 2012).
References
Obelia sp.
Synonyms
-
Morphological Description
Cornelius (1990) provides extensive descriptions of European Obelia spp.
Molecular Sequences
> 400 results in GenBank
Association
Unknown
Cetacean Hosts/Basibionts
Once reported on Megaptera novaeangliae
Geographic Range
-
Life Cycle
See Cornelius (1990).
Microhabitat
As a hyperepibiont on Coronula spp. (Rice, 1963)
Use as Indicator
-
Remarks
-
References
Phylum Nematoda Cobb, 1932
Class Chromadorea Inglis, 1983
Subclass Chromadoria Pearse, 1942
Order Monhysterida Filipjev, 1929
Family Monhysteridae de Man, 1876
This family is composed of terrestrial, freshwater, and marine forms. Some species are free-living in the sediment (e.g., Fonseca and Decraemer, 2008), bacterivorous on plants (Alkemade et al., 1992), associated to pack ice (Blome and Riemann, 1999) or living epibiotically on crustaceans in marine, limnetic, and terrestrial habitats (Lorenzen, 1986).
Odontobius ceti (Roussel de Vauzème, 1834)
Synonyms
-
Morphological Description
Roussel de Vauzème, 1834; Baylis, 1923; Lorenzen, 1986
Molecular Sequences
-
Association
Obligate commensal; it probably feeds primarily on organic particles from whales’ diet (Baylis, 1923).
Cetacean Hosts/Basibionts
Balaenoptera borealis, B. musculus, B. physalus, Eubalaena australis, Megaptera novaeangliae
Geographic Range
Atlantic, North Pacific, Antarctica
Life Cycle
Eggs are laid on the baleen plates but, since no larval stages have been found on cetaceans, further development may take place in the sea (Baylis, 1923).
Microhabitat
Baleen plates (Roussel de Vauzème, 1834; Baylis, 1923; Skrjabin, 1959; Rice, 1963; Lorenzen, 1986), in association with the ciliated protozoon Haematophagus megaptere Woodcock & Lodge, 1921 (Baylis, 1923).
Use as Indicator
-
Remarks
Considered a taxon inquirendum (WoRMS, 2021).
References
Roussel de Vauzème, 1834; Baylis, 1923; Skrjabin, 1959; Rice, 1963; Lorenzen, 1986
Other Taxa With Indicator Value
Some organisms have been reported on cetaceans but cannot be considered epibiotic animals (i.e., they belong to another kingdom or are not intimately associated to cetaceans). For instance, the cirolanid isopods Natatolana spp. or the hagfish Myxine glutinosaLinnaeus, 1758 are scavengers (Hale, 1926; Bowman, 1971; Pinedo et al., 1989; Martini, 1998; Keable, 2006; Zintzen et al., 2011) and records on living cetaceans are unusual (Pace et al., 2016). The following taxa, despite not being intimate associates or not belonging to the animal kingdom, can provide valuable information on cetacean biology.
At least 14 genera of diatoms (Chromista: Bacillariophyceae) have been recorded on over a dozen cetacean species (e.g., Hart, 1935; Matthews, 1938b; Hustedt, 1952; Nemoto, 1958; Nemoto et al., 1977; Heckman et al., 1987; Ferrario et al., 2018). Several species belonging to genera such as Bennettella Holmes, 1985, Epipellis Holmes, 1985, Epiphalaina Holmes, Nagasawa & Takano, 1993, Plumosigma Nemoto, 1956, and Tursiocola Holmes, Nagasawa & Takano, 1993 are believed to be exclusive to cetaceans. It has been proposed that these animal-specific diatoms settle on cetaceans in polar waters and take approximately one month to develop into a yellowish-brown film visible to the naked eye (Omura, 1950b). Therefore, it can be inferred that whales in polar areas that are covered by diatom films are at least one-month visitors, whereas those at lower latitudes and still showing skin colouration returned recently from polar regions (Hart, 1935; Matthews, 1938b; Omura, 1950b; Cockrill, 1960; Bannister, 1968; Sekiguchi et al., 1993). In South Africa, diatom films were detected more frequently as the Antarctic whaling season advanced (Cockrill, 1960) vs. at the beginning of the season (Best, 1969b). Diatom films have also been used to investigate population segregation, i.e., they were almost absent on sperm whale females and young males, which coincides with inferences of social segregation based in cyamid infections (see C. catodontis and N. physeteris; Best, 1969a; Best, 1969b).
The cookie-cutter shark Isistius brasiliensis (Quoy & Gaimard, 1824) preys on multiple marine organisms, including finfish (Papastamatiou et al., 2010), elasmobranchs (Yamaguchi and Nakaya, 1997), pinnipeds (Gallo-Reynoso and Figueroa-Carranza, 1992; Hiruki et al., 1993), sirenians (Reddacliff, 1988), and cetaceans (Dwyer and Visser, 2011). About 25% of stomach content consists of marine mammal remains, i.e., tissue plugs, skin, blubber (Carlisle et al., 2021), thus being considered a cetacean (micro)predator (Barros and Stolen, 2001). It has been hypothesized that cookie-cutter sharks use an ambush style of hunting; when potential preys are close enough, they latch and remove large plugs of tissue (Widder, 1998). This feeding mode has been catalogued as ectoparasitic (Carlisle et al., 2021). Despite its widespread distribution (Dwyer and Visser, 2011), its common range lies within equatorial and tropical waters (Nakano and Tabuchi, 1990; Yamaguchi and Nakaya, 1997). Accordingly, marks of I. brasiliensis on cetaceans at higher latitudes have been used as a migration tag (Tomilin, 1957 -who refers to them as ‘light spots’; Renner and Bell 2009; Foote et al., 2011; Bertulli et al., 2016). Interestingly, this species has not been reported on the southern right whale, Eubalaena australis (Matthews, 1938a), which is found only further south than 13°S (Peters and Barendse, 2016). Also, due to the long duration of the marks it leaves on cetaceans, it has been suggested as a tool for individual recognition and marking (Dorsey et al., 1990; Visser, 1999; Gill et al., 2000; McSweeney et al., 2007; Visser et al., 2010; Rosso et al., 2011; Bertulli et al., 2016; Visser et al., 2020; Franklin et al., 2020). Other applications of this biological tag include distinguishing cetacean age classes (McSweeney et al., 2007), populations (Sherchenko, 1970; Best, 1977; Moore et al., 2003), and orca ecotypes (Dwyer and Visser, 2011; Visser et al., 2020); characterizing whale wintering grounds (Bushuev, 1990); and as an indicator of swimming in deep waters (Baird et al., 2006) and of emaciation (Gasparini and Sazima, 1996). Its congeneric member, the largetooth cookiecutter shark, Isistius plutodus Garrick & Springer, 1964, once observed on a Cuvier’s beaked whale, Ziphius cavirostris (Pérez-Zayas et al., 2002), has a poorly known distribution (Moore et al., 2003). It leaves larger flesh “plugs” different from the wounds produced by I. brasiliensis (Compagno, 1984). Scars of Isistius spp. can harbor high loads of cyamids (Kobayashi et al., 2021).
As a final anecdotal remark, Ohsumi (1973) found the broken spear of a marlin, Makaira sp., stuck in the jaw of an Antarctic minke whale, Balaenoptera bonaerensis, which this author used to infer migration of this whale from tropical and sub-tropical waters, where marlins are distributed.
Discussion
Gaps and Biases
The present review includes records covering over three and a half centuries, a fact that attests to the curiosity that cetacean epibionts have sparked among naturalists, probably due to their often bizarre appearance and conspicuousness. As a result, a reasonable account of the associations between cetaceans and their metazoan epibionts has been achieved. However, important biases and gaps still remain. First of all, the vast majority of studies has not primarily focused on epibiosis and thus provides little quantitative information on these associations. For instance, less than a quarter (110 out of 493) of the publications in this review include data on prevalence. This ‘quantitative gap’ problem is worsened by the selective ‘picking’ of positive records, i.e., there is a tendency to report on the occurrence, but not on the absence, of epibionts in descriptive surveys on cetaceans. Consequently, it can be difficult to draw accurate pictures of the degree of specificity and, especially, geographic distribution of epizoic taxa. Another source of bias concerns epibiont size. Studies on large, visible barnacles such as Xenobalanus globicipitis are far more numerous than those focusing on minute creatures such as Balaenophilus unisetus (Badillo et al., 2007) or species of Cyamidae infecting dolphins (Fraija-Fernández et al., 2017). The genetic information available also varies among epibiotic taxa: 28 out of 54 species lack sequenced genetic material. Among these, some are poorly known species, but others have a long study history and numerous records (e.g., Odontobius ceti vs. Coronula reginae).
There is also an uneven coverage and research effort on cetaceans as basibionts, which can result in somewhat biased impressions on epibiont diversity among cetaceans. For instance, baleen whales as a group exhibit the greatest epibiont diversity most likely because they are large, slow-swimming hosts with a number of skin folds and callosities that provide suitable microhabitats for epibiont settlement (Berzin and Vlasova, 1982; see Fraija-Fernández et al., 2017). Moreover, the occurrence of certain epibionts on whales (e.g., coronulids) promotes the settlement and/or population growth of others (e.g., lepadids, cyamids), acting as pioneers (e.g., Matthews, 1937; Rice, 1963). However, mysticetes also are a well-studied cetacean group and, not surprinsingly, only the pygmy right whale, Caperea marginata (Gray, 1846), and Omura’s whale, Balaenoptera omuraiWada et al., 2003, described in 2003 (Wada et al., 2003), still lack records of epibiotic fauna. Conversely, odontocetes may exhibit relatively poor epizoic fauna because many of them (e.g., delphinids) are fast-swimming hosts with small, smooth surfaces. Moreover, there are riverine dolphins, i.e. species of Inia, Neophocaena, Orcaella, Platanista, and Sotalia that can seldom, if at all, be exposed to epibiotic taxa of marine origin. Research effort is also low for many odontocetes, and no studies are indeed available from species of Orcaella Gray, 1866, Platanista Wagler, 1830, and Tasmacetus Oliver, 1937. The overall point is, therefore, that epibiotic richness in the less studied cetaceans likely has an unassessed degree of underestimation.
The spatial distribution of data is also heterogeneous. First, records from oceanic waters are far less common than those from coastal areas. Second, most geographic records concentrate in the Southern Ocean, Mediterranean Sea, off South Africa, and California, followed by other Northern Pacific regions (Eastern waters and Japan) and the North Sea. However, other vast areas have few surveys, or even none, including the Arctic, Black Sea, Red Sea, Indian Ocean (except South African waters), and the Southwestern Pacific and adjacent seas (e.g., Sulu-Celebes Sea). In this context, it is worth noting that the higher number of records in particular regions does not necessarily result from higher epizoite diversity or abundance, but rather from higher sampling effort. Whaling was a fundamental source of data but focused mainly on areas and seasons where the target species occurred at higher densities, e.g., Antarctic whaling during the austral summer or Saldanha and Durban whaling stations in South Africa (see Findlay and Best, 2016; IWC, 2021). Also, the Mediterranean and U.S. stranding networks have been working for several decades (Becker et al., 1994), while other areas have recently started to gather data on cetaceans (e.g., the Western Indian Ocean region; Plön et al., 2020) or lack active stranding or research programs (i.e., eastern Russian Arctic).
Finally, we still know very little about biology of the epibiotic fauna of cetaceans; a problem which results, at least in part, from the difficulties of dealing with organisms that depend on marine hosts whose accessibility is often limited due to economical, logistic, and legal constraints for sampling. We call this the ‘association gap’; we often do not know basic aspects of many epibiont taxa, such as the complete life cycle or the actual nature of the interactions (commensal, parasitic or mutualistic).
The Nature of Epibiotic Associations and Their Indicator Potential
The origin of epibiotic associations of some animal groups with cetaceans is an exciting evolutionary issue since this epibiont fauna was acquired after the ancestors of these mammals colonized the sea (Aznar et al., 2001). Thus, there are instances of a simple use of cetaceans as additional substrata for facultative epibionts such as the Lepadidae (Newman and Abbott, 1980); host-switching events from prior obligate associations with other marine vertebrates, resulting in co-speciation, e.g., the Coronulidae (Frick et al., 2011; Hayashi et al., 2013; Buckeridge et al., 2019) and, perhaps, B. unisetus (Badillo et al., 2007); or putative colonization without speciation, e.g., in the case of Pennella balaenoptera (Fraija-Fernández et al., 2018). As far as we are aware, the Cyamidae could represent the only case of a potential primary adaptation to parasitism on cetaceans from a putative marine free-living ancestor (see Lowry and Myers, 2013). The nature of each type association brought about a variable degree of modifications in morphology, dependency of host/basibiont, and life history traits yet to be investigated in detail (see, e.g., Pugliese et al., 2012; Dreyer et al., 2020, for the case of X. globicipitis). These features define the potential of each epibiont as a tool to uncover aspects of cetaceans’ biology. In what follows, we condense the key biological data shown above for the main epibiotic groups, i.e., amphipods, cirripeds, and copepods; we also summarize their use as indicators. Other members of the epibiotic fauna of cetaceans are certainly interesting from ecological and evolutionary points of view, e.g., the roundworm Odontobius ceti or the whalesucker Remora australis. However, their usefulness as indicators are, in principle, more limited, and will not be further discussed here.
The level of host specificity varies greatly among whale lice species; some species have been reported only, or preferentially, on single cetacean species (e.g., Cyamus boopis, C. catodontis, C. ceti, C. eschrichtii, Neocyamus physeteris) or clades (e.g., Balaenocyamus balaenopterae, C. erraticus, C. gracilis), whereas others appear to be more generalist (e.g., Syncyamus aequus). The combination of bodily transmission and high specificity makes cyamids especially useful to shed light on phylogeography and social interactions of cetaceans (see references above). Moreover, cyamids can outlive their host for several days, thus providing a rough proxy of the time of death of cetaceans (Leung, 1976; Lehnert et al., 2007). However, when dealing with stranded cetaceans (a common scenario nowadays), these parasites can readily dislodge from hosts, which represents a potential drawback if quantitative infection data are to be used (Fraija-Fernández et al., 2017).
All epibiotic barnacles of cetaceans are filter-feeders whose life cycle includes a series of planktotrophic naupliar stages followed by a non-feeding cyprid, which permanently attaches to the basibiont (Darwin, 1851; Anderson, 1994; Høeg et al., 2003). Coronulids typical from whales tend to be selective and preferentially settle on single host species. For instance, Coronula diadema is associated to humpback whales (ca. 70% of records of C. diadema) and occurs on nearly all whales examined in surveys (Nishiwaki, 1959; Rice, 1963). In contrast, the basal representative of coronulids colonizing cetaceans, namely, Xenobalanus globicipitis, has been found on a total of 41 odontocete and mysticete species worldwide. The actual and potential indicator value of coronulids are thus defined by the commensal mode of feeding, the strict dependence on cetacean epidermis for attachment, and the variable degree of basibiont specificity. Species of this family have been used to unveil hydrodynamic features of cetaceans (Kasuya and Rice, 1970; Fish and Battle, 1995; Carrillo et al., 2015; Moreno-Colom et al., 2020) or systemic disease (Aznar et al., 1994; Aznar et al., 2005; Flach et al., 2021). However, their utility to inform on other aspects of cetacean biology, particularly movements and stock identification, are still far from full exploitation. For instance, Bushuev (1990) found significant differences of prevalence of X. globicipitis on Antartic minke whales from different Antarctic sectors, and interpreted them as evidence that whales used different wintering areas and did not mix in the Southern Ocean. However, this interpretation relies on the untested assumption that barnacle recruitment can only occur at low latitudes.
The second group of barnacles occurring on cetaceans, i.e., members of the Lepadidae, includes generalist dwellers on any type of hard substrata available in oceanic waters (e.g., Farrapeira et al., 2007; Wegner and Cartamil, 2012). Perhaps the most interesting species in this respect is Conchoderma auritum because, as noted above, it tends to be associated to cetaceans, either directly (on teeth) or indirectly (via the shell of coronulids, or the body of the mesoparasite P. balaenoptera). This raises the interesting question over the extent to which individuals of C. auritum recognize cetaceans as preferential substrata, and whether their populations depend on these basibionts for long-term stability. In any event, lepadids are fast-growing organisms that can be amenable for observational and experimental studies to determine their growth rate at different temperatures (Evans, 1958; Rasmussen, 1980; Dalley and Crisp, 1981; Eckert and Eckert, 1987; Inatsuchi et al., 2010). This makes them suitable as indicators of drifting time of their ‘living platforms’ (Fraser et al., 2011; Magni et al., 2015; López et al., 2017; Ten et al., 2019), and other aspects yet to be explored in cetaceans.
Two copepods have also developed intimate associations with cetaceans. Balaenophilus unisetus occurs on the baleen plates of four Balaenoptera spp. and is believed to feed on baleen’s keratin (or the associated microfilm) as an obligate commensal (Vervoort and Tranter, 1961; Ogawa et al., 1997; Fernandez-Leborans, 2001; Badillo et al., 2007). Interestingly, available evidence for the congeneric species B. manatorum suggests that direct contact is necessary for transmission of Balaenophilus spp. (Domènech et al., 2017). This feature has allowed to draw striking inferences on unexpected contacts between otherwise solitary juveniles of marine turtles (Domènech et al., 2017). However, the indicator value of B. unisetus seems much more limited because accessibility to whale samples is very restricted.
Females of the world-largest known copepod, Pennella balaenoptera, act as mesoparasite of at least 24 cetacean species, penetrating the blubber and musculature to feed on blood (Schmidt and Roberts, 2009; Hogans, 2017). Recent evidence has shown that there are not clear diagnostic morphological traits to differentiate this species from its congener P. filosa except for the use of different hosts (Abaunza et al., 2001; Hogans, 2017). Moreover, molecular data do not support segregation between specimens collected from cetaceans and the swordfish in the western Mediterranean (Fraija-Fernández et al., 2018). Pennella filosa parasitizes a broad spectrum of large marine fishes in the oceanic realm (Román-Reyes et al., 2019 and references herein). Apparently, then, the occurrence of P. balaenoptera (= filosa) in oceanic cetaceans could have resulted from and co-accommodation of the parasite on further hosts sharing the same habitat. However, this conclusion should be confirmed by analyzing more specimens of P. balaenoptera collected from other fish and cetaceans in other geographical regions. This is paramount because both P. balaenoptera and P. filosa exhibit low host specificity, contrary to other members of the family Pennellidae, which infect one or two hosts (Hogans, 2017). Thus, the possibility that cryptic speciation have occurred in P. balaenoptera (= filosa) cannot be ruled out. This taxonomic issue is also relevant to assess the usefulness of P. balaenoptera as an indicator species. So far, the species has been used as an indicator of host’s health status, i.e., heavy loads of this parasite could reflect poor health of the affected cetacean (Vecchione and Aznar, 2014). However, population inferences are more dependent on whether or not fishes should be included as part of the actual host community supporting the local population of P. balaenoptera (= filosa).
Concluding Remarks
Every epibiotic organism must first contact a potential basibiont, attach, and then successfully thrive on it (Crisp and Barnes, 1954; Crisp, 1955; Mullineaux and Butman, 1991). Accordingly, its presence on a vagile animal implies prior coincidence in time and space between both organisms and the suitability of the basibiont/host as a habitat. In addition, since epibionts essentially live in the ecotone between the basibiont/host surface and the marine environment, abiotic conditions (e.g., temperature, salinity) must also fit the auto-ecological requirements of the epibionts during all their life-span, regardless of the migratory activity of the basibiont/host (see Moreno-Colom et al., 2020, and references therein). All these features are the ones that potentially allow to draw inferences on hosts’ biology and ecology at individual, population, or community levels. However, the absence of epibionts is also informative, particularly at population level. For instance, investigating marine-mammal breeding and feeding grounds, and migratory routes, is especially important for conservation (Pompa et al., 2011), and can be elucidated, not only by the presence of selected epibionts, but also by their absence. We therefore encourage cetologists to report on both the presence or absence of epibionts whenever possible. Also, quantitative data (e.g., prevalence, mean number of individuals per host) would be most welcome.
Lastly, it is not an overstatement to claim that cetacean epibionts bear intrinsic value, thus should benefit from explicit consideration in conservation policies (see Whiteman and Parker, 2005; Aznar et al., 2011; Kwak et al., 2020). This becomes highly relevant for specific taxa associated to threatened cetaceans (e.g., whale lice), which are also on the verge of unnoticed extinction (see Buckeridge, 2012).
Funding
The work of ST was partially supported by UV-INV-PREDOC19F1-1007742. This study is supported by project AICO/2021/022 granted by Conselleria d’Innovacioó, Universitats, Ciència i Societat Digital, Generalitat Valenciana, and the Biodiversity Foundation of the Ministry for the Ecological Transition and Demographic Challenge (VARACOMVAL project) (with NextGeneration EU funds).
Acknowledgments
Special gratitude is to Julio Cruz Vila for his assistance during literature search and to the Libraries and Documentation Service of the University of Valencia (UV) for provision of some papers that were not easily accessible.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
ST carried out literature search, wrote the initial version of the manuscript, and prepared the figures. FJA participated in developing the ideas and organizing and writing the manuscript, and JAR revised the text. All authors read manuscript drafts and contributed content to the developing paper. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.846558/full#supplementary-material
References
1
Abaunza P. Arroyo N. L. Preciado I. (2001). A Contribution to the Knowledge on the Morphometry and the Anatomical Characters of Pennella balaenopterae (Copepoda, Ciphonostomatoida, Pennellidae), With Special Reference to the Buccal Complex. Crustaceana74 (2), 193–210. doi: 10.1163/156854001750096292
2
Abollo E. López A. Gestal C. Benavente P. Pascual S. (1998). Macroparasites in Cetaceans Stranded on the Northwestern Spanish Atlantic Coast. Dis. Aquat. Org.32 (3), 227–231. doi: 10.3354/dao032227
3
Achituv Y. (1998). Cirral Activity in the Whale Barnacle Cryptolepas Rhachianechi. J. Mar. Biolog. Assoc. U.K.78, 1203–1213. doi: 10.1017/S0025315400044428
4
Addink M. J. Smeenk C. (2001). Opportunistic Feeding Behaviour of Rough-Toothed Dolphins Steno bredanensis Off Mauritania. Zool. Verh.334, 37–48.
5
Adriaens D. Baskin J. N. Coppens H. (2010). “Evolutionary Morphology of Trichomycterid Catfishes: About Hanging on and Digging in” in Origin and Phylogenetic Interrelationships of Teleosts. Eds. NelsonJ. S.SchultzeH. P.WilsonM. V. (München: Verlag Dr Friedrich Pfeil), 337–362.
6
Aguilar R. Ogburn M. B. Hines A. H. (2018). Chesapeake Bay Barcode Initiative: Invertebrates Fy17 (EMBL/GenBank/DDBJ databases).
7
Aguilar A. Raga J. A. (1993). The Striped Dolphin Epizootic in the Mediterranean-Sea. AMBIO22, 524–528.
8
Agusti-Ridaura C. Hamre L. A. Espedal P. G. Øines Ø. Horsberg T. E. Kaur K. (2019). First Report on Sensitivity of Caligus elongatus Towards Anti-Louse Chemicals and Identification of Mitochondrial Cytochrome C Oxidase I Genotypes. Aquaculture507, 190–195. doi: 10.1016/j.aquaculture.2019.04.022
9
Ali S. N. Ayub Z. (2021). Growth and Reproduction of Megabalanus tintinnabulum (Crustacea: Cirripedia) in Coastal Waters of Pakistan, North Arabian Sea. Reg. Stud. Mar. Sci.43, 101662. doi: 10.1016/j.rsma.2021.101662
10
Alkemade R. Wielemaker A. Hemminga M. A. (1992). Stimulation of Decomposition of Spartina Anglica Leaves by the Bacterivorous Marine Nematode Diplolaimelloides bruciei (Monhysteridae). J. Exp. Mar. Bio. Ecol.159 (2), 267–278. doi: 10.1016/0022-0981(92)90041-8
11
Allen G. M. (1916). The Whalebone Whales of New England. Mem. Boston Soc Nat. Hist.8 (2), 107–322. doi: 10.5962/bhl.title.25585
12
Allen G. M. (1941). Pygmy Sperm Whale in the Atlantic. Field Mus. Nat. Hist. Zool. Ser.27, 17–36.
13
Alonso de Pina G. M. Giuffra R. (2003). Taxonomía, Distribución Y Notas Sobre Cuatro Especies De Ectoparásitos De Cetacea (Crustacea: Amphipoda: Cyamidae). Rev. del Museo Argentino Cienc. Naturales5 (1), 39–62. doi: 10.22179/REVMACN.5.29
14
Alonso L. Estrades A. Scarabino F. Calcagno J. (2010). Conchoderma Virgatum (Spengler 1970) (Cirripedia: Pedunculata) Associated With Sea Turtles in Uruguayan Shallow Coastal Waters. Pan-Am. J. Aquat. Sci.5 (1), 166–168.
15
Álvarez-Fernández E. Carriol R. P. Jordá J. F. Aura J. E. Avezuela B. Badal E. et al . (2013). Occurrence of Whale Barnacles in Nerja Cave (Málaga, Southern Spain): Indirect Evidence of Whale Consumption by Humans in the Upper Magdalenian. Quat. Int.337, 163–169. doi: 10.1016/j.quaint.2013.01.014
16
Alves-Motta M. R. Luz-Carvalho V. Nunes-Pinheiro D. C. S. Groch K. R. Gonçalves-Pereira L. Sánchez-Sarmiento A. M. et al . (2020). Facial Squamous Cell Carcinoma and Abdominal Peripheral Nerve Sheath Tumour With Rhabdomyoblastic Differentiation in a Rough-Toothed Dolphin (Steno bredanensis). J. Comp. Pathol.176, 122–127. doi: 10.1016/j.jcpa.2020.02.013
17
Anderson D. T. (1994). Barnacles: Structure, Function, Development and Evolution (London: Chapman & Hall).
18
Aneesh P. T. Valarmathi K. Mitra S. (2019). Redescription of Nerocila Recurvispina Schiödte and Meinert 1881:(Crustacea: Isopoda) From the Hooghly River, Kolkata, India. Mar. Biodivers.49 (1), 301–313. doi: 10.1007/s12526-017-0799-8
19
Angeletti S. Cervellini P. M. Massola V. (2014). Nuevo Registro De Ballena Jorobada (Megaptera novaeangliae) Para El Mar Argentino Y Notas Sobre Sus Epibiontes. Mastozoología Neotropical21 (2), 319–324.
20
Angot M. (1951). Rapport Scientifique Sur Les Expeditions Baleinieres Autour De Madagascar (Saisons 1949 Et 1950). Mém. Inst. Sci. Madagascar Sér. A6 (2), 439–486.
21
Annandale N. (1909). The Rate of Growth in Conchoderma and Lepas. Rec. India. Mus.3, 295.
22
Anthony R. Calvet J. (1905). Recherches Faites Sur Le Cétacé Capturé a Cètte, Le 6 Octobre 1904 – Balaenoptera Physalus (Linné). Bull. Soc Phil. 7(2), 75–85.
23
April J. Mayden R. L. Hanner R. H. Bernatchez L. (2011). Genetic Calibration of Species Diversity Among North America's Freshwater Fishes. Proc. Natl. Acad. Sci. U.S.A.108 (26), 10602–10607. doi: 10.1073/pnas.1016437108
24
Araki J. Kuramochi T. Machida M. Nagasawa K. Uchida A. (1997). A Note on the Parasite Fauna of the Western North Pacific Minke Whale (Balaenoptera Acutorostrata). Rep. Int. Whal. Commn.47, 565–567.
25
Araújo-Wang C. Schormans E. Wang J. (2019). Ecological Interaction of a “Parasitic” Candiru Catfish and Botos (Inia Geoffrensis). Mar. Mamm. Sci.35 (4), 1347–1354. doi: 10.1111/mms.12593
26
Arroyo N. L. Abaunza P. Preciado I. (2002). The First Naupliar Stage of Pennella balaenopterae Koren and Danielssen 1877 (Copepoda: Siphonostomatoida, Pennellidae). Sarsia: North Atlantic Marine Sci.87 (5), 333–337. doi: 10.1080/0036482021000155785
27
Arvy L. (1977). La Peau, Les Phaneres Et Leurs Parasites Chez Les Cetaces. Vie Milieu27, 137–190.
28
Arvy L. (1982). Phoresis and Parasitism in Cetaceans: A Review. Invest. Cetacea.14, 233–335.
29
Ashton G. V. Davidson I. C. Geller J. B. Ruiz G. M. (2016). Disentangling the Biogeography of Ship Biofouling: Barnacles in the Northeast Pacific. Glob. Ecol. Biogeogr.25 (6), 739–750. doi: 10.1111/geb.12450
30
Astthorsson O. S. Palsson J. (2006). New Fish Records and Records of Rare Southern Species in Icelandic Waters in the Warm Period 1996-2005. ICES Document CM 2006/C: 20, 22 pp.
31
Aurivillius P. O. C. (1879). On a New Genus and Species of Harpacticida. Bihang K. Svenska Vet. Akad. Handl.5 (18), 1–16.
32
Avdeev V. V. (1989). Parasitic Amphipods of the Family Cyamidae and the Problem of Cetacea Origin. Biologija Morja4, 27–33.
33
Ávila I. C. Cuellar L. M. Cantera J. R. (2011). Crustacean Ectoparasites and Epibionts of Humpback Whales, Megaptera novaeangliae (Cetacea: Balaenopteridae), in the Colombian Pacific. Res. J. Costa Rican Dist. Educ. Univ.2, 177–185.
34
Azevedo A. Soares M. P. de Castro M. C. T. Lailson-Brito J. Jr. Gurgel M. I. (1996). Ocorrência De Epizoítos Em Cetáceos Na Costa do Estado do Rio De Janeiro, Brasil (Porto Alegre, Brasil: Resumos do XXI Congresso Brasileiro de Zoologia), 254.
35
Aznar F. J. Balbuena J. A. Fernández M. Raga J. A. (2001). “Living Together: The Parasites of Marine Mammals,” in Marine Mammals. Eds. EvansP. G. H.RagaJ. A. (Boston, MA: Springer), 385–423. doi: 10.1007/978-1-4615-0529-7_11
36
Aznar F. J. Balbuena J. A. Raga J. A. (1994). Are Epizoites Biological Indicators of a Western Mediterranean Striped Dolphin Die-Off? Dis. Aquat. Org.18, 159–163. doi: 10.3354/dao018159
37
Aznar F. J. Fernández M. Balbuena J. A. (2011). “Why We Should Care About the Parasite Fauna of Cetaceans: A Plea for Integrative Studies,” in Whales and Dolphins: Behavior, Biology and Distribution. Ed. MurrayC. A. (New York:Nova Publishers).
38
Aznar F. J. Fernández M. Raga J. A. Balbuena J. A. (2016). Long-Term Population Trend of the Epibiont Barnacle Xenobalanus globicipitis in the Western Mediterranean: Is There a Role for Viral Outbreaks in its Cetacean Hosts? [Poster]. Front. Mar. Sci.3. doi: 10.3389/conf.fmars.2016.04.00086
39
Aznar F. J. Perdiguero D. Del Olmo A. P. Repullés A. Agustí C. Raga J. A. (2005). Changes in Epizoic Crustacean Infestations During Cetacean Die-Offs: The Mass Mortality of Mediterranean Striped Dolphins Stenella Coeruleoalba Revisited. Dis. Aquat. Org.67 (3), 239–247. doi: 10.3354/dao067239
40
Bachara W. Gullan A. (2016). First Stranding Record of a True’s Beaked Whale (Mesoplodon Mirus) in Mozambique, Report WB2016/1. [Unpubl. report to Cetal Fauna]
41
Bachman B. A. Kraus R. Peterson C. T. Grubbs R. D. Peters E. C. (2018). Growth and Reproduction of Echeneis Naucrates From the Eastern Gulf of Mexico. J. Fish Biol.93 (4), 755–758. doi: 10.1111/jfb.13790
42
Badillo F. J. Puig L. Montero F. E. Raga J. A. Aznar F. J. (2007). Diet of Balaenophilus spp. (Copepoda: Harpacticoida): Feeding on Keratin at Sea? Mar. Biol.151 (2), 751–758. doi: 10.1007/s00227-006-0521-z
43
Baird R. W. Webster D. L. McSweeney D. J. Ligon A. D. Schorr G. S. Barlow J. (2006). Diving Behaviour of Cuvier’s (Ziphius cavirostris) and Blainville’s (Mesoplodon densirostris) Beaked Whales in Hawaii. Can. J. Zool.84 (8), 1120–1128. doi: 10.1139/z06-095
44
Baker A. N. (1983). Whales and Dolphins of Australia and New Zealand (Wellington: Victoria University Press).
45
Balbuena J. A. (1991). Estudio Taxonómico Y Ecológico De La Parasitofauna Del Calderón Común, Globicephala melas (Traill 1809), En Las Aguas De Europa. [PhD Thesis] (Valencia, Spain: Universitat de València).
46
Balbuena J. A. Aznar F. J. Fernández M. Raga J. A. (1995). Parasites as Indicators of Social Structure and Stock Identity of Marine Mammals. Dev. Mar. Biol.4, 133–139). doi: 10.1016/S0163-6995(06)80017-X
47
Balbuena J. A. Raga J. A. (1991). Ecology and Host Relationships of the Whale-Louse Isocyamus delphini (Amphipoda: Cyamidae) Parasitizing Long-Finned Pilot Whales (Globicephala melas) Off the Faroe Islands (Northeast Atlantic). Can. J. Zool.69 (1), 141–145. doi: 10.1139/z91-021
48
Balbuena J. A. Raga J. A. Fernández M. Ortiz T. (1989). “Ectoparasites and Epizoits of the Long-Finned Pilot Whale Globicephala melas Off the Faroe Islands, With Special Reference to Isocyamus delphinii (Amphipoda: Cyamidae),” in European Research on Cetaceans. Eds. EvansR. G. H.SmeenkC. (La Rochelle, France: Proceedings of the third annual conference of the European Cetacean Society), 115–117.
49
Bane G. W. Zullo V. A. (1980). Observations on a Stranded Goosebeaked Whale (Ziphius cavirostris, Cuvier 1823) and its Ectocommensal Barnacles (Xenobalanus globicipitis). J. Elisha Mitchell Sci. Soc96 (1), 1–3.
50
Bannister J. L. (1968). An Aerial Survey for Sperm Whales Off the Coast of Western Australia 1963-1965. Aust. J. Mar. Freshw. Res.19, 31–51. doi: 10.1071/MF9680031
51
Bannister J. L. Grindley J. R. (1966). Notes on Balaenophilus Unisetus POC Aurivillius 1879, and its Occurrence in the Southern Hemisphere (Copepoda, Harpacticoida). Crustaceana10 (3), 296–302. doi: 10.1163/156854066X00199
52
Barnard K. H. (1924). Contribution to the Crustacean Fauna of South Africa. No. 7. Cirripedia. Ann. S. Afr. Mus.20, 1–103.
53
Barnard K. H. (1931). Diagnoses of New Genera and Species of Amphipod Crustacea Collected During the 'Discovery' Investigations 1925-1927. Ann. Mag. Nat. Hist. Ser.7, 425–430. doi: 10.1080/00222933108673327
54
Barnard K. H. (1932). Amphipoda: Cyamidae. Discovery Rep.5, 1–326. doi: 10.5962/bhl.part.27664
55
Barnard K. H. (1955). South African Parasitic Copepoda. Ann. S. Afr. Mus.41 (5), 223–312.
56
Barros N. B. Stolen M. K. (2001). Biology of Offshore Bottlenose Dolphins From East Florida (Sarasota, FL: Mote Marine Laboratory).
57
Batista R. L. G. Schiavetti A. Santos U. A. D. Reis M. D. S. S. D. (2012). Cetaceans Registered on the Coast of Ilhéus (Bahia), Northeastern Brazil. Biota Neotropica12 (1), 31–38. doi: 10.1590/S1676-06032012000100003
58
Battaglia P. Potoschi A. Valastro M. Andaloro F. Romeo T. (2016). Age, Growth, Biological and Ecological Aspects of Remora Osteochir (Echeneidae) in the Mediterranean Sea. J. Mar. Biolog. Assoc. U.K.96, 639–645. doi: 10.1017/S0025315415000867
59
Baum C. Stelzer R. Meyer W. Fleischer L. G. and Siebers D. . (2000). A Cryo-Scanning Electron Microscopic Study of the Skin Surface of the Pilot Whale Globicephala Melas. Aquat. Mamm.26(1), 7–16.
60
Baum C. Meyer W. Roessner D. Siebers D. Fleischer L. (2001). A Zymogel Enhances the Self-Cleaning Abilities of the Skin of the Pilot Whale (Globicephala melas). Comp. Biochem. Physiol. Mol. Integr. Physiol.130 (4), 835–847. doi: 10.1016/s1095-6433(01)00445-7
61
Baylis H. A. (1923). On Odontobius Ceti, Roussel De Vauzème, a Nematode Living on the Baleen of Whales. Ann. Mag. Nat. Hist.9 (12), 617–623. doi: 10.1080/00222932308632985
62
Beach K. A. (2015). Mouthline Injuries as an Indicator of Fisheries Interactions in Hawaiian Odontocetes. [Ph.D. Thesis] (Chicago (IL: Evergreen State College).
63
Beamish R. J. (1980). Adult Biology of the River Lamprey (Lampetra Ayresi) and the Pacific Lamprey (Lampetra Tridentata) From the Pacific Coast of Canada. Can. J. Fish Aquat. Sci.37, 1906–1923. doi: 10.1139/f80-232
64
Bearzi M. Patonai K. (2010). Occurrence of the Barnacle (Xenobalanus globicipitis) on Coastal and Offshore Common Bottlenose Dolphins (Tursiops Truncatus) in Santa Monica Bay and Adjacent Areas, California. Bull. S. Calif. Acad. Sci.109, 37–44. doi: 10.3160/0038-3872-109.2.37
65
Becker P. R. Wilkinson D. M. Lillestolen T. I. (1994). “Marine Mammal Health and Stranding Response Program: Program Development Plan,” in NOAA Technical Memorandum NMFS-OPR-94-2 (Silver Spring, MD: National Oceanic and Atmospheric Administration).
66
Bellido J. J. Castillo J. J. Farfán M. Á. Martín J. J. Mons J. L. Real R. (2006). Ejemplar enfermo de marsopa Phocoena phocoena. Galemys18, 1–2.
67
Beneden P. (1870). Les Cétacés, Leurs Commensaux Et Leurs Parasites. Bull. Acad. R. Belg. Cl. Sci.29 (2), 347–368.
68
Bennet F. D. (1837). On the Natural History of the Spermaceti Whale (Physeter Macrocephalus, Lac.). Proc. Zool. Soc Lond.1, 39–42.
69
Bennett F. D. (1840). Narrative of a Whaling Voyage Round the Globe, From the Year 1833 to 1836 Vol. 2 (London:Richard Bennet). doi: 10.5962/bhl.title.19771
70
Benz G. Greiner E. Bowen S. Goetz L. Evou N. (2011). Odd Association and Range Extension of Caligus Rufimaculatus Wilson 1905; Caligidae, Siphonostomatoida, Copepoda. Gulf Caribb. Res.23 (1), 49–53. doi: 10.18785/gcr.2301.05
71
Bergami E. Caroselli E. Vaccari L. Corsi I. Semenov A. Macali A. (2021). Pioneer Settlement of the Cold-Water Coral Desmophyllum Dianthus (Esper 1794) on Plastic. Coral Reefs40, 1355–1360. doi: 10.1007/s00338-021-02131-9
72
Berland B. Krakstad J.-O. Nöttestad L. Axelsen B. E. Vaz-Velho F. Bauleth- D’Almeida G. (2003). Xenobalanus globicipitis (Crustacea: Cirripedia) on Dusky Dolphins (Lagenorhynchus Obscurus) Off Namibia: Hitch-Hiker's Guide to the Seas. 15th Biennial Conference on the Biology of Marine Mammals (NC, U. S: Greensboro).
73
Bermúdez-Villapol L. A. Sayegh A. J. Estevez M. A. Rangel M. S. Rosso C. Vera N. I. (2006). Notes on the Pygmy Killer Whale Feresa Attenuata Gray 1874 (Cetacea: Delphinidae) in Venezuela, Southeastern Caribbean. Lat. Am. J. Aquat. Mamm.5(2), 135–139. doi: 10.5597/lajam00103
74
Bertulli C. G. Cecchetti A. Van Bressem M. F. Van Waerebeek K. (2012). Skin Disorders in Common Minke Whales and White-Beaked Dolphins Off Iceland, a Photographic Assessment. J. Mar. Anim. Ecol.5, 29–40.
75
Bertulli C. G. Rasmussen M. H. Rosso M. (2016). An Assessment of the Natural Marking Patterns Used for Photo-Identification of Common Minke Whales and White-Beaked Dolphins in Icelandic Waters. J. Mar. Biolog. Assoc. U.K.96 (04), 807–819. doi: 10.1017/S0025315415000284
76
Berzin A. A. (1972). The Sperm Whale (Jerusalem: Israel Program). Sci. Transl.
77
Berzin A. A. Vlasova L. P. (1982). Fauna of the Cetacea Cyamidae (Amphipoda) of the World Ocean. Invest. Cet.13, 149–164.
78
Best P. B. (1969a). The Sperm Whale (Physeter Catodon) Off the West Coast of South Africa. 3. Reproduction in the Male. Investl. Rep. Div. Sea Fish. S. Afr.78, 1–20.
79
Best P. B. (1969b). The Sperm Whale (Physeter Catodon) Off the West Coast of South Africa. 4. Distribution and Movements. Investl. Rep. Div. Sea Fish. S. Afr.78, 1–12.
80
Best P. B. (1971). Stalked Barnacles Conchoderma auritum on an Elephant Seal: Occurrence of Elephant Seals on South African Coast. Afr. Zool.6 (2), 181–185. doi: 10.1080/00445096.1971.11447410
81
Best P. B. (1977). Two Allopatric Forms of Bryde’s Whale Off South Africa. Rep. Int. Whal. Commn.1, 10–38.
82
Best P. B. (1979). “Social Organization in Sperm Whales, Physeter Macrocephalus,” in Behavior of Marine Animals. Eds. WinnH. E.OllaB. L. (Boston, MA: Springer), 227–289. doi: 10.1007/978-1-4684-2985-5_7
83
Best P. B. (1982). Seasonal Abundance, Feeding, Reproduction, Age and Growth in Minke Whales Off Durban (With Incidental Observations From the Antarctic). Rep. Int. Whal. Commn.32, 759–786.
84
Best P. B. (1991). The Presence of Coronuline Barnacles on a Southern Right Whale Eubalaena Australis. S. Afr. J. Mar. Sci.11 (1), 585–587. doi: 10.2989/025776191784287763
85
Best P. B. (2007). Whales and Dolphins of the Southern African Subregion (Cambridge: Cambridge University Press).
86
Best P. B. Meÿer M. A. (2010). “Neglected But Not Forgotten—Southern Africa’s Dusky Dolphins,” in The Dusky Dolphin. Eds. WürsigB.WürsigM. (San Diego: Academic Press), 291–311, ISBN: 9780123737236. doi: 10.1016/B978-0-12-373723-6.00014-X
87
Betancur R. R. Broughton R. E. Wiley E. O. Carpenter K. Lopez J. A. Li C. et al . (2013). The Tree of Life and a New Classification of Bony Fishes. PloS Curr.1, 1–45. doi: 10.1371/currents.tol.53ba26640df0ccaee75bb165c8c26288
88
Bianucci G. Di Celma C. Landini W. Buckeridge J. (2006a). Palaeoecology and Taphonomy of an Extraordinary Whale Barnacle Accumulation From the Plio-Pleistocene of Ecuador. Palaeogeogr. Palaeoclimatol. Palaeoecol.242 (3-4), 326–342. doi: 10.1016/j.palaeo.2006.07.004
89
Bianucci G. Landini W. Buckeridge J. (2006b). Whale Barnacles and Neogene Cetacean Migration Routes. N. Z. J. Geol. Geophys.49 (1), 115–120. doi: 10.1080/00288306.2006.9515152
90
Birincioğlu S. S. Aypak S. Avcı H. Birincioğlu B. İpek E. Akkoç A. N. (2017). Pathological and Parasitological Investigations in an Adult Bottlenose Dolphin (Tursiops Truncatus). Kafkas Univ Vet. Fak Derg23 (6), 1011–1014. doi: 10.9775/kvfd.2017.18016
91
Blome D. Riemann F. (1999). Antarctic Sea Ice Nematodes, With Description of Geomonhystera Glaciei Sp. Nov. (Monhysteridae). Mitt. Hamb. Zool. Mus. Inst.96, 15–20.
92
Blum S. Fong J. (2016). CAS Invertebrate Zoology (IZ). Version 14.2 (California Academy of Sciences). Available at: https://www.gbif.org/occurrence/609385701 (Accessed October 6, 2021).
93
Boero F. Bucci C. Colucci A. M. R. Gravili C. Stabili L. (2007). Obelia (Cnidaria, Hydrozoa, Campanulariidae): A Microphagous, Filter-Feeding Medusa. Mar. Ecol.28, 178–183. doi: 10.1111/j.1439-0485.2007.00164.x
94
Boguski D. A. Reid S. B. Goodman D. H. Docker M. F. (2012). Genetic Diversity, Endemism and Phylogeny of Lampreys Within the Genus Lampetra Sensu Stricto (Petromyzontiformes: Petromyzontidae) in Western North America. J. Fish Biol.81 (6), 1891–1914. doi: 10.1111/j.1095-8649.2012.03417.x
95
Bordino P. González R. (1992). Presencia Del Parásito Phyllobothrium Sp. (Cestoda) Y Del Foronte Conchoderma auritum (Crestacea, Cirripedia) Sobre Mesoplodon Layardii (Cetacea, Ziphiidae) (Buenos Aires, Argentina: V Reunión de Trabajo de Especialistas en Mamíferos Acuáticos de América del Sur).
96
Borradaile L. A. (1903). “Marine Crustaceans. VII. The Barnacles,” in The Fauna and Geography of the Maldive and Laccadive Archipelagoes, Being an Account of the Work Carried on and of Collections Made by an Expedition During Years 1899 and 1900. Ed. GardinerJ. S. (Cambridge: Cambridge University Press), 440–443.
97
Borradaile L. A. (1916). Crustacea. Part III. - Cirripedia. Br. Antarct. Terra Nova Exped. 1910. Zool.3, 127–136.
98
Bortolotto G. A. Kolesnikovas C. K. M. Freire A. S. Simões-Lopes P. C. (2016). Young Humpback Whale Megaptera Novaeangliae Feeding in Santa Catarina Coastal Waters, Southern Brazil, and a Ship Strike Report. Mar. Biodivers. Rec.9 (1), 1–6. doi: 10.1186/s41200-016-0043-4
99
Bosselaers M. Collareta A. (2016). The Whale Barnacle Cryptolepas Rhachianecti (Cirripedia: Coronulidae), a Phoront of the Grey Whale Eschrichtius Robustus (Cetacea: Eschrichtiidae), From a Sandy Beach in The Netherlands. Zootaxa4154 (3), 331–338. doi: 10.11646/zootaxa.4154.3.8
100
Bosselaers M. Van Nieulande F. Collareta A. (2017). A New Record of Cetopirus Complanatus (Cirripedia: Coronulidae), an Epibiont of Right Whales (Cetacea: Balaenidae: Eubalaena Spp.), From a Beach Deposit of Mediterranean Spain. Atti Soc Toscana Sci. Nat.124, 43–48. doi: 10.2424/ASTSN.M.2017.17
101
Bouvier E. L. (1910). Quelques Arthropodes Recueillis Aux Iles Kerguelen. Bull. Mus. Nat. d'Hist. Nat.26 (2), 190–193.
102
Bowman T. E. (1955). A New Genus and Species of Whale-Louse (Amphipoda: Cyamidae) From the False Killer Whale. Bull. Mar. Sci.5, 315–320.
103
Bowman T. E. (1958). First Pacific Record of the Whale-Louse Genus Syncyamus (Amphipoda: Cyamidae). Pac. Sci.12, 181–182.
104
Bowman T. E. (1971). Cirolana Narica N. Sp., a New Zealand Isopod (Crustacea) Found in the Nasal Tract of the Dolphin Cephalorhynchus Hectori. Beaufortia19 (252), 107–112.
105
Bradford A. L. Weller D. W. Lang A. R. Tsidulko G. A. Burdin A. M. Brownell R. L. Jr. (2011). Comparing Observations of Age at First Reproduction in Western Gray Whales to Estimates of Age at Sexual Maturity in Eastern Gray Whales Vol. 140 (Agadir: Publications, Agencies and Staff of the U.S. Department of Commerce).
106
Brandt A. (1872). Bericht Über Die Cyamiden Des Zoologischen Museum Der Kaiserlichen Academie Der Wissenscheften Zu St. Petersbourg. Bull. Acad. Imp. Sci. St. Petersbourg18, 113–134.
107
Bridge D. Cunningham C. W. DeSalle R. Buss L. W. (1995). Class-Level Relationships in the Phylum Cnidaria: Molecular and Morphological Evidence. Mol. Biol. Evol.12 (4), 679–689. doi: 10.1093/oxfordjournals.molbev.a040246
108
Briggs K. T. Morejohn G. V. (1972). Barnacle Orientation and Water Flow Characteristics in California Grey Whales. J. Zool.167, 287–292. doi: 10.1111/j.1469-7998.1972.tb03112.x
109
Broch H. (1924). Cirripedia Thoracica Von Norwegen Und Dem Norwegischen Nordmeere. Eine Systematische Und Biologisch-Tiergeographische Studie. Skr. Vidensk. Selsk.17, 121 pp.
110
Brotz L. Cheung W. W. Kleisner K. Pakhomov E. Pauly D. (2012). “Increasing Jellyfish Populations: Trends in Large Marine Ecosystems,” in Jellyfish Blooms. Eds. PittK. A.LucasC. H. (Dordrecht: Springer), 3–20. doi: 10.1007/978-94-007-5316-7_2
111
Brownell R. L. (1975). Progress Report on the Biology of the Franciscana Dolphin in Uruguayan Waters. J. Fish. Res. Board Can.32 (7), 1073–1078. doi: 10.1139/f75-127
112
Brownell R. L. Jr. Findley L. T. Vidal O. Robles A. Silvia Manzanilla N. (1987). External Morphology and Pigmentation of the Vaquita, Phocoena Sinus (Cetacea: Mammalia). Mar. Mamm. Sci.3 (1), 22–30. doi: 10.1111/j.1748-7692.1987.tb00149.x
113
Brown S. K. Roughgarden J. (1985). Growth, Morphology, and Laboratory Culture of Larvae of Balanus Glandula (Cirripedia: Thoracica). J. Crust. Biol.5 (4), 574–590. doi: 10.2307/1548236
114
Brunnschweiler J. Sazima I. (2006). A New and Unexpected Host for the Sharksucker (Echeneis Naucrates) With a Brief Review of the Echeneid-Host Interactions. JMBA2-Biodivers. Rec., 1–3. doi: 10.1017/S1755267206004349
115
Brusca R. C. (1978). Studies on the Cymothoid Fish Symbionts of the Eastern Pacific (Isopoda, Cymothoidae) I. Biology of Nerocila californica. Crustaceana34 (2), 141–154. doi: 10.1163/156854078X00718
116
Bryden M. M. (1976). Observations on a Pygmy Killer Wale, Feresa Attenuata, Stranded on the East Coast of Australia. Wildl. Res.3 (1), 21–28. doi: 10.1071/WR9760021
117
Brzica H. (2004). Morphological and Morphometric Characteristics of the Ectoparasite Pennella balaenopterae (Copepoda, Siphonostomatida, Pennellidae) of Whales (Cetacea) From the Adriatic Sea. [Student Research Paper] (Faculty of Veterinary Medicine of Zagreb). [In Croatian].
118
Buckeridge J. S. (2012). Opportunism and the Resilience of Barnacles (Cirripedia: Thoracica) to Environmental Change. Integr. Zool.7 (2), 137–146. doi: 10.1111/j.1749-4877.2012.00286.x
119
Buckeridge J. S. Chan B. K. K. Lee S. W. (2018). Accumulations of Fossils of the Whale Barnacle Coronula Bifida Bronn 1831 (Thoracica: Coronulidae) Provides Evidence of a Late Pliocene Cetacean Migration Route Through the Straits of Taiwan. Zool. Stud.57, e54. doi: 10.6620/ZS.2018.57-54
120
Buckeridge J. S. Chan B. K. K. Lin J. P. (2019). Paleontological Studies of Whale Barnacles in Taiwan Reveal New Cetacean Migration Routes in the Western Pacific Since the Miocene. Zool. Stud.58, e38. doi: 10.6620/ZS.2019.58-39
121
Bushuev S. G. (1990). A Study of the Population Structure of the Southern Minke Whale (Balaenoptera Acutorostrata Lacépède) Based on Morphological and Ecological Variability. Rep. Int. Whal. Commn.40, 317–324.
122
Buzeta R. (1963). Cyamidae (Crustacea: Amphipoda) En Physeter Catodon L. Capturados En Chile Con Descripción De Una Nueva Especie, Cyamus Bahamondei. Rev. Biol. Mar.3 (1-2), 126–137.
123
Cadenat J. (1953). Les Rémoras Des Côtes Du Sénégal. Bull. Inst. Fr. Afr. Noire15 (2), 672–683.
124
Caldwell D. K. Caldwell M. C. Rathjen W. F. Sullivan J. R. (1971a). Cetaceans From the Lesser Antillean Island of St Vincent.. Fish. Bull.69 (2), 303–312.
125
Caldwell D. K. Neuhauser H. Caldwell M. C. Coolidge H. W. (1971b). Recent Records of Marine Mammals from the Coasts of Georgia and South Carolina. Cetology5, 1–12.
126
Calman W. T. (1920). A Whale-Barnacle of the Genus Xenobalanus From Antarctic Seas. Ann. Mag. Nat. Hist.6, 165–166. doi: 10.1080/00222932008632427
127
Campbell M. A. Chen W. J. Lopez J. A. (2013). Are Flatfishes (Pleuronectiformes) Monophyletic? Mol. Phylogenet. Evol.69 (3), 664–673. doi: 10.1016/j.ympev.2013.07.011
128
Carim K. J. Dysthe J. C. Young M. K. McKelvey K. S. Schwartz M. K. (2017). A Noninvasive Tool to Assess the Distribution of Pacific Lamprey (Entosphenus Tridentatus) in the Columbia River Basin. PloS One12 (1), e0169334. doi: 10.1371/journal.pone.0169334
129
Carlisle A. B. Allan E. A. Kim S. L. Meyer L. Port J. Scherrer S. et al . (2021). Integrating Multiple Chemical Tracers to Elucidate the Diet and Habitat of Cookiecutter Sharks. Sci. Rep.11 (1), 1–16. doi: 10.1038/s41598-021-89903-z
130
Carlucci R. Ricci P. Miccoli Sartori S. Cipriano G. Cosentino A. Lionetti A. et al . (2015). Changes in Behaviour and Group Size of Stenella Coeruleoalba in the Gulf of Taranto (Northern Ionian Sea, Central Mediterranean Sea). Biol. Mar. Mediterr.22, 266–270.
131
Carl G. C. (1945). Personal Communication. British Columbia Provincial Museum
132
Carl G. C. Wilby G. V. (1945). Some Marine Fish Records for British Columbia. Can. Field Nat.39 (1), 28–30. doi: 10.2307/1438189
133
Carrillo J. M. Overstreet R. M. Raga J. A. Aznar F. J. (2015). Living on the Edge: Settlement Patterns by the Symbiotic Barnacle Xenobalanus globicipitis on Small Cetaceans. PloS One10 (6), e0127367. doi: 10.1371/journal.pone.0127367
134
Carvalho V. L. Bevilaqua C. M. L. Iñiguez A. M. Mathews-Cascond H. Bezerra Ribeiro F. Bezerra Pessoae L. M. et al . (2010). Metazoan Parasites of Cetaceans Off the Northeastern Coast of Brazil. Vet. Parasitol.173, 116–122. doi: 10.1016/j.vetpar.2010.06.023
135
Casale P. D’Addario M. Freggi D. Argano R. (2012). Barnacles (Cirripedia, Thoracica) and Associated Epibionts From Sea Turtles in the Central Mediterranean. Crustaceana85, 533–549. doi: 10.1163/156854012X634393
136
Casale P. Freggi D. Basso R. Argano R. (2004). Epibiotic Barnacles and Crabs as Indicators of Caretta Caretta Distribution and Movements in the Mediterranean Sea. J. Mar. Biolog. Assoc. U.K.84 (5), 1005–1006. doi: 10.1017/S0025315404010318h
137
Cerioni S. Mariniello L. (1996). Metazoi Parassiti Di Stenella Coeruleoalba (Cetacea: Delphinidae) Spiaggiata Lungo Le Coste Laziali Dal 1985 Al 1991. Parassitologia38, 505–510.
138
Chan B. Corbari L. Rodríguez Moreno P. Tsang L. (2017). Molecular Phylogeny of the Lower Acorn Barnacle Families (Bathylasmatidae, Chionelasmatidae, Pachylasmatidae and Waikalasmatidae) (Cirripedia: Balanomorpha) With Evidence for Revisions in Family Classification. Zool. J. Linn. Soc180 (3), 542–555. doi: 10.1093/zoolinnean/zlw005
139
Chapman G. Santler J. E. (1955). Aspects of the Fauna and Flora of the Azores. V. Crustacea. Ann. Mag. Nat. Hist.8, 371–376. doi: 10.1080/00222935508655652
140
Cheang C. C. Tsang L. M. Chu K. H. Cheng I. J. Chan B. K. K. (2013). Host-Specific Phenotypic Plasticity of the Turtle Barnacle Chelonibia testudinaria: A Widespread Generalist Rather than a Specialist. PLOS ONE8(3): e57592. doi: 10.1371/journal.pone.0057592
141
Chemnitz J. H. (1785). Neues Systematisches Conchylien-Cabinet (Nürnberg: Raspe). doi: 10.5962/bhl.title.120065
142
Chemnitz J. H. Martini F. H. W. (1790). Systematiches Conchilien-Cabinet Von Martini Und Chemnitz (Nurnberg: Bauer and Raspe).
143
Chen H. N. Hoeg J. T. Chan B. K. (2013). Morphometric and Molecular Identification of Individual Barnacle Cyprids From Wild Plankton: An Approach to Detecting Fouling and Invasive Barnacle Species. Biofouling29 (2), 133–145. doi: 10.1080/08927014.2012.753061
144
Chernova O. F. Shpak O. V. Kiladze A. B. Rozhnov V. V. (2017). Epidermal Molting in the Bowhead Whale Balaena Mysticetus. Biol. Bull. Russ. Acad. Sci.44, 591–602. doi: 10.1134/S1062359017050065
145
Chevreux E. (1913a). Amphipodes. Deuxième Expédition Antarctique Française, (1908–1910) Commandée Par Le Dr. Jean Charcot. Sci. naturelles: Documents scientifiques, 79–186. doi: 10.5962/bhl.title.6956
146
Chevreux E. (1913b). Sur Quelques Intéressantes Espèces D'amphipodes Provenant Des Parages De Monaco Et Des Pêches Pélagiques De La Princesse-Alice Et De I'Hirondelle II En Méditerranée. Bull. Inst. Océanogr. Monaco262, 1–26.
147
Choi K. H. Kim C. H. (1994). Naupliar Development of Harpacticus Nipponicus Ito (Copepoda: Harpacticoida: Harpacticidae) Reared in the Laboratory. Korean J. Syst. Zool.10 (2), 217–229.
148
Christensen I. (1985). First Record of Gooseneck Barnacles (Conchoderma auritum) on a Minke Whale (Balaenoptera Acutorostrata). ICES C.M. 1985/N:9ICES.
149
Ciçek E. Oktener A. Capar O. B. (2007). First Report of Pennella balaenopterae Koren and Danielssen 1877 (Copepoda: Pennelidae) From Turkey. Turkiye Parazitol. Derg.31 (3), 239–241.
150
Clapham P. J. Leimkuhler E. Gray B. A. Mattila D. K. (1995). Do Humpback Whales Exhibit Lateralized Behaviour? Anim. Behav.50 (1), 73–82. doi: 10.1006/anbe.1995.0222
151
Clarke R. (1956). Sperm Whales of the Azores. Discovery Rep.28, 237–298.
152
Clarke R. (1966). The Stalked Barnacle Conchoderma, Ectoparasitic on Whales. Norsk Hvalfangst-Tidende55, 153–168.
153
Clemens B. J. Weitkamp L. Siwicke K. Wade J. Harris J. Hess J. et al . (2019). Marine Biology of the Pacific Lamprey Entosphenus Tridentatus. Rev. Fish Biol. Fish.29 (4), 767–788. doi: 10.1007/s11160-019-09578-8
154
Clua E. E. Manire C. A. Garrigue C. (2014). Biological Data of Pygmy Killer Whale (Feresa Attenuata) From a Mass Stranding in New Caledonia (South Pacific) Associated With Hurricane Jim in 2006. Aquat. Mamm.40 (2), 162–172. doi: 10.1578/AM.40.2.2014.162
155
Cockrill W. R. (1960). Pathology of the Cetacea. A veterinary study on whales. Brit. Vet. J.116, 1–28. doi: 10.1016/S0007-1935(17)44304-1
156
Cocks A. H. (1885). Additional Notes on the Fin-Whale Fishery on the North European Coast. Zoologist3 (9), 134–142.
157
Collareta A. Bosselaers M. Bianucci G. (2016). Jumping From Turtles to Whales: A Pliocene Fossil Record Depicts an Ancient Dispersal of Chelonibia on Mysticetes. Riv. It. Paleont. Strat.122 (2), 35–44. doi: 10.13130/2039-4942/7229
158
Collareta A. Insacco G. Reitano A. Catanzariti R. Bosselaers M. Montes M. et al . (2018b). Fossil Whale Barnacles From the Lower Pleistocene of Sicily Shed Light on the Coeval Mediterranean Cetacean Fauna. Carnets Géologie18 (2), 9–22. doi: 10.4267/2042/65747
159
Collareta A. Regattieri E. Zanchetta G. Lambert O. Catanzariti R. Bosselaers M. et al . (2018a). New Insights on Ancient Cetacean Movement Patterns From Oxygenisotope Analyses of a Mediterranean Pleistocene Whale Barnacle. Neues Jahrb. Geol. Paläontol.288 (2), 143–159. doi: 10.1127/njgpa/2018/0729
160
Collet R. (1912). Norges Pattedyr (Kristiania:H. Aschehoug & Co. (W. Nygaard). doi: 10.5962/bhl.title.14929
161
Collet R. (1986). On the External Characters of Rudolphi's Rorqual (Balaenoptera Borealis). Proc. Zool. Soc Lond.17-18, 243–265.
162
Collette B. B. (2003). “Family Echeneidae,” in The Living Marine Resources of the Western Central Atlantic. Ed. CarpenterK. E. (Rome: FAO Species Identification Guide for Fishery Purposes and Amer. Soc. Ich. Herp. Spec. Publ), 1414–1419.
163
Collette B. B. Curtis M. Williams J. T. Smith-Vaniz W. F. Pina Amargos F. (2015). “Echeneis naucrates” in IUCN Red List of Threatened Species. e.T190393A76649216. doi: 10.2305/IUCN.UK.2015-4.RLTS.T190393A15603110.en
164
Collette B. B. Klein-MacPhee G. (2002). Bigelow and Schroeder’s Fishes of the Gulf of Maine (Washington, DC: Smithsonian Institution Press).
165
Combes C. (2001). Parasitism: The Ecology and Evolution of Intimate Interactions (Chicago: University of Chicago Press).
166
Compagno L. J. V. (1984). FAO Species Catalogue. Vol. 4. Sharks of the World. An Annotated and Illustrated Catalogue of Shark Species Known to Date. Part 1. Hexanchiformes to Lamniformes. FAO Fisheries Synopsis125, 93–96.
167
Conlan K. E. (1991). Precopulatory Mating Behaviour and Sexual Dimorphism in the Amphipod Crustacea. Hydrobiologia223, 255–282. doi: 10.1007/BF00047644
168
Conway D. V. P. Ellis C. J. Humpheryes I. G. (1990). Deep Distributions of Oceanic Cirripede Larvae in the Sargasso Sea and Surrounding North Atlantic Ocean. Mar. Biol.105 (3), 419–428. doi: 10.1007/BF01316313
169
Cornaglia E. Rebora L. Gili C. Di Guardo G. (2000). Histopathological and Immunohistochemical Studies on Cetaceans Found Stranded on the Coast of Italy Between 1990 and 1997. J. Vet. Med. Ser. A47 (3), 129–142. doi: 10.1046/j.1439-0442.2000.00268.x
170
Cornelius P. F. (1990). European Obelia (Cnidaria, Hydroida): Systematics and Identification. J. Nat. Hist.24 (3), 535–578. doi: 10.1080/00222939000770381
171
Cornwall I. E. (1924). Notes on West American Whale Barnacles. Proc. Calif. Acad. Sci.13, 421–431.
172
Cornwall I. E. (1927). Some North Pacific Whale Barnacles. Contrib. Can. Biol. Fish.3 (23), 503–517. doi: 10.1139/f26-023
173
Cornwall I. E. (1928). Collecting at Cachalot Whaling Station. Can. Field-Nat.42, 9–12.
174
Cornwall I. E. (1955). The Barnacles of British Columbia. Br. Col. Prov. Mus. Dept.7, 5–69.
175
Cortés-Peña D. (2019) Orca at Chañaral De Aceituno, Freirina, Atacama Region, Chile. Available at: https://www.facebook.com/groups/CetalFauna/permalink/206670.2686783266 (Accessed May 20, 2021).
176
Costa A. (1866). Descrizione Di Una Especie Di Cyamus Parassita De Delfini: C. Chelipes. Ann. Mus. Zool. Napoli3, 82–83.
177
Costello M. J. (2006). Ecology of Sea Lice Parasitic on Farmed and Wild Fish. Trends Parasitol.22 (10), 475–483. doi: 10.1016/j.pt.2006.08.006
178
Creaser C. W. Hubbs C. L. (1922). A Revision of the Holarctic Lampreys Vol. 120 (University of Michigan Mus. Zool).
179
Crespo-Picazo J. L. García-Parraga D. Domènech F. Tomás J. Aznar F. J. Ortega J. et al . (2017). Parasitic Outbreak of the Copepod Balaenophilus Manatorum in Neonate Loggerhead Sea Turtles (Caretta Caretta) From a Head-Starting Program. BMC V. Res.13 (1), 1–7. doi: 10.1186/s12917-017-1074-8
180
Cressey R. F. Lachner E. A. (1970). The Parasitic Copepod Diet and Life History of Diskfishes (Echeneidae). Copeia, 310–318. doi: 10.2307/1441652
181
Crisp D. J. (1955). The Behaviour of Barnacle Cyprids in Relation to Water Movement Over a Surface. J. Exp. Biol.32, 569–590. doi: 10.1242/jeb.32.3.569
182
Crisp D. J. Barnes H. (1954). The Orientation and Distribution of Barnacles at Settlement With Particular Reference to Surface Contour. J. Anim. Ecol.23 (1), 142. doi: 10.2307/1664
183
Crozier W. J. (1916). On a Barnacle, Conchoderma Virgatum, Attached to a Fish, Diodon Hystrix. Am. Nat.50 (598), 636–640. doi: 10.1086/279573
184
Cunha A. F. Collins A. G. Marques A. C. (2017). Phylogenetic Relationships of Proboscoida Broch 1910 (Cnidaria, Hydrozoa): Are Traditional Morphological Diagnostic Characters Relevant for the Delimitation of Lineages at the Species, Genus, and Family Levels? Mol. Phylogenet. Evol.106, 118–135. doi: 10.1016/j.ympev.2016.09.012
185
Cunha A. F. Genzano G. N. Marques A. C. (2015). Reassessment of Morphological Diagnostic Characters and Species Boundaries Requires Taxonomical Changes for the Genus Orthopyxis L. Agassiz 1862 (Campanulariidae, Hydrozoa) and Some Related Campanulariids. PloS One10 (2), e0117553. doi: 10.1371/journal.pone.0117553
186
Dailey M. D. Brownell J. R.L. (1972). “A Checklist of Marine Mammal Parasites,” in Mammals of the Sea: Biology and Medicine. Ed. RidgwayS. H. (Springfield, IL: Charles C. Thomas), 528–589.
187
Dailey M. D. Gulland F. M. Lowenstine L. J. Silvagni P. Howard D. (2000). Prey, Parasites and Pathology Associated With the Mortality of a Juvenile Gray Whale (Eschrichtius Robustus) Stranded Along the Northern California Coast. Dis. Aquat. Org.42 (2), 111–117. doi: 10.3354/dao042111
188
Dailey M. D. Haulena M. Lawrence J. (2002). First Report of a Parasitic Copepod (Pennella balaenopterae) Infestation in a Pinniped. J. Zoo Wildl. Med.33 (1), 62–65. doi: 10.1638/1042-7260(2002)033[0062:FROAPC]2.0.CO;2
189
Dailey M. Stroud R. (1978). Parasites and Associated Pathology Observed in Cetaceans Stranded Along the Oregon Coast. J. Wildl. Dis.14, 503–511. doi: 10.7589/0090-3558-14.4.503
190
Dailey M. D. Vogelbein W. (1991). Parasite Fauna of 3 Species of Antarctic Whales With Reference to Their Use as Potential Stock Indicators. Fish. Bull.89 (3), 355–365.
191
Dailey M. D. Walker W. A. (1978). Parasitism as a Factor (?) in Single Strandings of Southern California Cetaceans. J. Parasitol.64 (4), 593–596. doi: 10.2307/3279939
192
Dall W. H. (1872). On the Parasites of the Cetaceans of the N.W. Coast of America, With Descriptions of New Forms. Proc. Calif. Acad. Sci.4, 299–301. doi: 10.1080/00222937308696808
193
Dalla Rosa L. Secchi E. R. (1997). Stranding of a Blue Whale (Balaenoptera Musculus) in Southern Brazil: ‘True’or Pygmy. Rep. Int. Whal. Commn.47, 425–430.
194
Dalley R. Crisp D. J. (1981). Conchoderma: A Fouling Hazard to Ships Underway. Mar. Biol. Lett.2 (3), 141–152.
195
Danilewicz D. Rosas F. Bastida R. Marigo J. Muelbert M. Rodriguez D. et al . (2002). Report of the Working Group on Biology and Ecology. Lat. Am. J. Aquat. Mamm.1, 25–42. doi: 10.5597/lajam00005
196
Danyer E. Tonay A. M. Aytemiz I. Dede A. Yildirim F. Gurel A. (2014). First Report of Infestation by a Parasitic Copepod (Pennella balaenopterae) in a Harbour Porpoise (Phocoena Phocoena) From the Aegean Sea: A Case Report. Veterinarni Medicina59 (8), 403. doi: 10.17221/7661-VETMED
197
Darwin C. (1851). A Monograph on the Subclass Cirripedia. Vol. 1. The Lepadidae (London: The Ray Society).
198
Darwin C. (1854). A Monograph on the Sub-Class Cirripedia, Vol. 2. The Balaenidae. (London: The Ray Society).
199
Davis W. M. (1874). Nimrod of the Sea; or, the American Whaleman (New York: Harper & Brothers). doi: 10.5962/bhl.title.18173
200
Debrot A. O. (1992). Notes on a Gervais’beaked Whale, Mesoplodon Europaeus, and a Dwarf Sperm Whale, Kogia Simus, Stranded in Curaçao, Netherlands Antilles. Mar. Mamm. Sci.8 (2), 172–178. doi: 10.1111/j.1748-7692.1992.tb00379.x
201
Delaney M. A. Ford J. K. B. Tang K. Gaydos J. K. (2016). Mesoparasitic Popepod (Pennella balaenopterae) Infestation of a Stranded Offshore Orca (Orcinus Orca) in Southeast Alaska: Review of Significance as a Health Indicator in Cetaceans. Int. Assoc. Aquat. Anim. Med. 21-26 May. [Poster]
202
Denkinger J. Alarcon D. (2017). Orcas of the Galápagos Islands 21-26 May. [Poster] (CETACEA Project).
203
de Pinna M. C. C. (1998). “Phylogenetic Relationships of Neotropical Siluriformes (Teleostei: Ostariophysi): Historical Overview and Synthesis of Hypotheses,” in Phylogeny and Classification of Neotropical Fishes. Eds. MalabarbaL. R.ReisR. E.VariR. P.LucenaZ. M.LucenaC. A. S. (Porto Alegre: EDIPUCRS), 279–330.
204
de Pinna M. C. C. Wosiacki W. B. (2003). “Family Trichomycteridae (Pencil or Parasitic Catfishes),” in Check List of the Freshwater Fishes of South and Central America. Eds. ReisR. E.KullanderS. O.FerrarisC. J. (Porto Alegre: EDIPUCRS), EDIPUCRS), 270–290.
205
Devaraj M. Sam Bennett P. (1974). Occurrence of Xenobalanus globicipitis (Steenstrup) on the Finless Black Porpoise, Neomeris Phocoenoides in Indian Seas. India. J. Fish.21, 579–581.
206
Díaz-Aguirre F. Salinas C. Navarrete S. Castillo V. Castilla C. (2012). First Record of the Commensal Barnacle (Xenobalanus globicipitis) on Common Bottlenose Dolphins (Tursiops Truncatus) in Chile. Aquat. Mamm.38 (1), 76–80. doi: 10.1578/AM.38.1.2012.76
207
Díaz-Gamboa R. E. (2015). Varamiento De Orcas Pigmeas (Feresa Attenuata Gray 1874) En Yucatán: Reporte De Caso. Bioagrociencias8 (1), 36–43.
208
Di Beneditto A. P. M. Ramos R. M. A. (2000). Records of the Barnacla Xenobalanus globicipitis (Steenstrup 1851) on Small Cetaceans of Brazil. Biotemas13 (2), 159–165.
209
Di Beneditto A. P. M. Ramos R. M. A. (2001). Biology and Conservation of the Franciscana (Pontoporia Blainvillei) in the North of Rio De Janeiro State, Brazil. J. Cetacean Res. Manage.3 (2), 185–192. doi: 10.5007/%x
210
Di Beneditto A. P. M. Ramos R. M. A. (2004). Biology of the Marine Tucuxi Dolphin (Sotalia Fluviatilis) in South-Eastern Brazil. J. Mar. Biolog. Assoc. U.K.84 (6), 1245–1250. doi: 10.1017/S0025315404010744h
211
Dixon J. M. (1980). A Recent Stranding of the Strap-Toothed Whale, Mesoplodon Layardi (Gray) (Ziphiidae) From Victoria, and a Review of Australian Records of the Species. Vict. Nat.97, 34–41.
212
Docker M. F. Haas G. R. Goodman D. H. Reid S. B. Heath D. D. (2007). PCR-RFLP Markers Detect 29 Mitochondrial Haplotypes in Pacific Lamprey (Entosphenus Tridentatus). Mol. Ecol. Notes7, 350–353. doi: 10.1111/j.1471-8286.2006.01605.x
213
Docker M. F. Youson J. H. Beamish R. J. Devlin R. H. (1999). Phylogeny of the Lamprey Genus Lampetra Inferred From Mitochondrial Cytochrome B and ND3 Gene Sequences. Can. J. Fish. Aquat. Sci.56, 2340–2349. doi: 10.1139/f99-171
214
Dollfus R. (1968). Xenobalanus globicipitis Steenstrup (Cirripedia, Thoracica): Collected on Tursiops Truncatus (Montagu) Near the Northern Coast of Morocco. Bull. Inst. Pêches Marit. Maroc.16, 55–59. [In French].
215
Domènech F. Badillo F. J. Tomás J. Raga J. A. Aznar F. J. (2015). Epibiont Communities of Loggerhead Marine Turtles (Caretta Caretta) in the Western Mediterranean: Influence of Geographic and Ecological Factors. J. Mar. Biolog. Assoc. U.K.95 (4), 851–861. doi: 10.1017/S0025315414001520
216
Domènech F. Tomás J. Crespo-Picazo J. L. García-Párraga D. Raga J. A. Aznar F. J. (2017). To Swim or Not to Swim: Potential Transmission of Balaenophilus Manatorum (Copepoda: Harpacticoida) in Marine Turtles. PloS One12 (1), e0170789. doi: 10.1371/journal.pone.0170789
217
Dominici S. Bartalini M. Benvenuti M. Balestra B. (2011). Large Kings with Small Crowns: A Mediterranean Pleistocene Whale Barnacle. Bull. Soc Paleontol. Ital.50 (2), 95–101. doi: 10.4435/BSPI.2011.10
218
Donnelly D. McInnes J. D. Morrice M. Andrews C. (2018). Killer Whales of Eastern Australia. Victoria: Killer Whales Australia.
219
Dorsey E. M. Stern J. S. Hoelzel A. R. Jacobsen J. (1990). Minke Whales (Balaenoptera Acutorostrata) From the West Coast of North America: Individual Recognition and Small-Scale Site Fidelity. Rep. Int. Whal. Commn.12, 357–368.
220
Dreyer N. Zardus J. D. Høeg J. T. Olesen J. Yu M. C. Chan B. K. (2020). How Whale and Dolphin Barnacles Attach to Their Hosts and the Paradox of Remarkably Versatile Attachment Structures in Cypris Larvae. Org. Divers. Evol.20 (2), 233–249. doi: 10.1007/s13127-020-00434-3
221
Duignan P. J. Geraci J. R. Raga J. A. Calzada N. (1992). Pathology of Morbillivirus Infection in Striped Dolphins (Stenella Coeruleoalba) From Valencia and Murcia, Spain. Can. J. Vet. Res.56, 242–248.
222
Dulčić J. Dragičević B. Despalatović M. Cvitković I. Bojanić-Varezić D. Štifanić M. (2015). Lepadid Barnacles Found Attached to a Living Lobotes Surinamensis (Pisces). Crustaceana88 (6), 727–731. doi: 10.1163/15685403-00003435
223
Dwyer S. L. Visser I. N. (2011). Cookie Cutter Shark (Isistius Sp.) Bites on Cetaceans, With Particular Reference to Killer Whales (Orca) (Orcinus Orca). Aquat. Mamm.37 (2), 111–138. doi: 10.1578/AM.37.2.2011.111
224
Eckert K. L. Eckert S. A. (1987). Growth Rate and Reproductive Condition of the Barnacle Conchoderma Virgatum on Gravid Leatherback Sea Turtles in Caribbean Waters. J. Crust. Biol.7 (4), 682–690. doi: 10.2307/1548651
225
Ellis C. J. Billett D. S. M. Angel M. V. (1983). The Distribution of Oceanic Cirripedes in the North-East Atlantic in Summer 1983 and the Connotations of the Results to the Problems of Conchoderma Fouling. Inst. Oceanogr. Sci.193.
226
Elorriaga-Verplancken F. R. Tobar-Hurtado S. Medina-López M. A. de la Cruz D. B. Urbán J. R. (2015). Potential Morphological Contributions to a Live Stranding: Abnormal Snout and Conchoderma Auritum Infestation in a Bottlenose Dolphin (Tursiops Truncatus). Aquat. Mamm.41 (2), 198. doi: 10.1578/AM.41.2.2015.198
227
Endo N. Sato K. Matsumura K. Yoshimura E. Odaka Y. Nogata Y. (2010). Species-Specific Detection and Quantification of Common Barnacle Larvae From the Japanese Coast Using Quantitative Real-Time PCR. Biofouling26 (8), 901–911. doi: 10.1080/08927014.2010.531389
228
Engel M. (1994). Encalhe De Um Cachalote, Physeter Macrocephalus, Provocado Por Emalhamento Em Rede De Pesca No Litoral Da Bahia, Brasil. Florianópolis: Anais da VI Reunião de Trabalhos de Especialistas em Mamíferos Aquáticos da América do Sul. 24–28.
229
Eschmeyer W. N. Fricke R. N. van der Laan R. (2017). Catalog of fishes: genera, species, references. Available at: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp.
230
Esteves J. M. Acosta R. Bermúdez L. Lira C. Figueredo A. (2020). Epibiosis Por Balaenophilus Unisetus (Copepoda: Harpacticoida) En Rorcual Común, Balaenoptera Physalus (Mysticeti: Balaenopteridae), Varado En Isla De Margarita, Venezuela. MAFIS - Marine Fishery Sci.33 (2), 265–276. doi: 10.47193/mafis.3322020301108
231
Evans F. (1958). Growth and Maturity of the Barnacles Lepas Hillii and Lepas Anatifera. Nature182, 1245–1246. doi: 10.1038/1821245b0
232
Fabian H. (1950). “Bohr” Höhlen an Pottwalzähnen. Zool. Anz. Ergänzungsband zu Band145, 147–162.
233
Faria M. A. DeWeerdt J. Pace F. Mayer F. X. (2013). Observation of a Humpback Whale (Megaptera novaeangliae) Birth in the Coastal Waters of Sainte Marie Island, Madagascar. Aquat. Mamm.39 (3), 296. doi: 10.1578/AM.39.3.2013.296
234
Farrapeira C. M. R. Melo A. V. D. O. M. D. Barbosa D. F. Silva K. M. E. D. (2007). Ship Hull Fouling in the Port of Recife, Pernambuco. Braz. J. Oceanogr.55 (3), 207–221. doi: 10.1590/S1679-87592007000300005
235
Félix F. Bearson B. Falconí J. (2006). Epizoic Barnacles Removed From the Skin of a Humpback Whale After a Period of Intense Surface Activity. Mar. Mamm. Sci.22 (4), 979–984. doi: 10.1111/j.1748-7692.2006.00058.x
236
Fernandez-Leborans G. (2001). A Review of the Species of Protozoan Epibionts on Crustaceans. III. Chonotrich Ciliates. Crustaceana74 (6), 581–607. doi: 10.1163/156854001300228852
237
Ferrario M. E. Cefarelli A. O. Fazio A. Bordino P. Romero O. E. (2018). Bennettella Ceticola (Nelson Ex Bennett) Holmes on the Skin of Franciscana Dolphin (Pontoporia Blainvillei) of the Argentinean Sea: An Emendation of the Generic Description. Diatom Res.33 (4), 485–497. doi: 10.1080/0269249X.2019.1572651
238
Fertl D. (1994). Occurrence Patterns and Behavior of Bottlenose Dolphins (Tursiops Truncatus) in the Galveston Ship Channel, Texas. Texas J. Sci.46, 299–317.
239
Fertl D. Acevedo-Gutiérrez A. Darby F. L. (1996). A Report of Killer Whales (Orcinus Orca) Feeding on a a Carcharhinid Shark in Costa Rica. Mar. Mamm. Sci.12 (4), 606–611. doi: 10.1111/j.1748-7692.1996.tb00075.x
240
Fertl D. Landry A. M. Jr (1999a). First Report of a Sharksucker (Echeneis Naucrates) on a Bottlenose Dolphin (Tursiops Truncatus), and a Re-Evaluation of Remora-Cetacean Associations. Eur. Res. Cetaceans12, 88–90. doi: 10.1111/j.1748-7692.1999.tb00849.x
241
Fertl D. Landry A. M. Jr (1999b). Sharksucker (Echeneis Naucrates) on a Bottlenose Dolphin (Tursiops Truncatus) and a Review of Other Cetacean-Remora Associations. Mar. Mamm. Sci.15, 859–863. doi: 10.1111/j.1748-7692.1999.tb00849.x
242
Fertl D. Landry A. M. Jr. Barros N. B. (2002). Sharksucker (Echeneis Naucrates) on a Bottlenose Dolphin (Tursiops Truncatus) From Sarasota Bay, Florida, With Comments on Remora-Cetacean Associations in the Gulf of Mexico. Copeia20 (2), 151–152. doi: 10.18785/goms.2002.07
243
Findlay K. P. Best P. B. (2016). Distribution and Seasonal Abundance of Large Cetaceans in the Durban Whaling Grounds Off KwaZulu-Natal, South Africa 1972–1975. Afr. J. Mar. Sci.38 (2), 249–262. doi: 10.2989/1814232X.2016.1191042
244
Findley L. T. Vidal O. (2002). Gray Whale (Eschrichtius Robustus) at Calving Sites in the Gulf of California, México. J. Cetacean Res. Manage.4 (1), 27–40.
245
Fine M. L. (1970). Faunal Variation on Pelagic Sargassum. Mar. Biol.7 (2), 112–122. doi: 10.1007/BF00354914
246
Fischer P. (1884). Cirrhipèdes De L’archipel De La Nouvelle-Calédonie. Bull. Soc Zool. France9, 355–360.
247
Fish F. E. Battle J. M. (1995). Hydrodynamic Design of the Humpback Whale Flipper. J. Morph.225 (1), 51–60. doi: 10.1002/jmor.1052250105
248
Fish F. E. Nicastro A. J. Weihs D. (2006). Dynamics of the Aerial Maneuvers of Spinner Dolphins. J. Exp. Bio.209 (4), 590–598. doi: 10.1242/jeb.02034
249
Flach L. Van Bressem M. F. Pitombo F. Aznar F. J. (2021). Emergence of the Epibiotic Barnacle Xenobalanus globicipitis in Guiana Dolphins After a Morbillivirus Outbreak in Sepetiba Bay, Brazil. Estuar. Coast. Shelf Sci.263, 107632. doi: 10.1016/j.ecss.2021.107632
250
Flammang B. E. Marras S. Anderson E. J. Lehmkuhl O. Mukherjee A. Cade D. E. et al . (2020). Remoras Pick Where They Stick on Blue Whales. J. Exp. Bio.223 (20), jeb226654. doi: 10.1242/jeb.226654
251
Follet W. I. Dempster L. J. (1960). First Records of the Echeneidid Fish Remilegia Australis (Bennett) From California, With Meristic Data. Proc. Calif. Acad. Sci.31, 169–184. doi: 10.2307/1440203
252
Fonseca G. Decraemer W. (2008). State of the Art of the Free-Living Marine Monhysteridae (Nematoda). J. Mar. Biolog. Assoc. U.K.88 (7), 1371–1390. doi: 10.1017/S0025315408001719
253
Foote A. D. Vilstrup J. T. de Stephanis R. Verborgh P. Able Nielsen S. C. Deaville R. et al . (2011). Genetic Differentiation Among North Atlantic Killer Whale Populations. Mol. Ecol.20, 629–641. doi: 10.1111/j.1365-294X.2010.04957.x
254
Fordyce R. E. Mattlin R. H. Wilson G. J. (1979). Stranding of a Cuvier's Beaked Whale, Ziphius cavirostris Cuvier 1823, at New Brighton, New Zealand. Mäuri orä7, 73–82.
255
Foskolos I. Provata M. T. Frantzis A. (2017). First Record of Conchoderma auritum (Cirripedia: Lepadidae) on Ziphius cavirostris (Cetacea: Ziphiidae) in Greece. In Ann. Ser. Hist. Nat.27, 29–34. doi: 10.19233/ASHN.2017.04
256
Foster B. A. Willan R. C. (1979). Foreign Barnacles Transported to New Zealand on an Oil Platform. N. Z. J. Mar. Freshw. Res.13 (1), 143–149. doi: 10.1080/00288330.1979.9515788
257
Fraija-Fernández N. Fernández M. Gozalbes P. Revuelta O. Raga J. A. Aznar F. J. (2017). Living in a Harsh Habitat: Epidemiology of the Whale Louse, Syncyamus Aequus (Cyamidae), Infecting Striped Dolphins in the Western Mediterranean. J. Zool.303 (3), 199–206. doi: 10.1111/jzo.12482
258
Fraija-Fernández N. Hernández-Hortelano A. Ahuir-Baraja A. E. Raga J. A. Aznar F. J. (2018). Taxonomic Status and Epidemiology of the Mesoparasitic Copepod Pennella Balaenoptera in Cetaceans From the Western Mediterranean. Dis. Aquat. Org.128 (3), 249–258. doi: 10.3354/dao03226
259
Franklin T. Franklin W. Brooks L. Harrison P. Burns D. Holmberg J. et al . (2020). Photo-Identification of Individual Southern Hemisphere Humpback Whales (Megaptera novaeangliae) Using All Available Natural Marks: Managing the Potential for Misidentification. J. Cetacean Res. Manage.21 (1), 71–83. doi: 10.47536/jcrm.v21i1.186
260
Fransen C. H. J. M. Smeenk C. (1991). Whale-Lice (Amphipoda: Cyamidae) Recorded From The Netherlands. Zool. Med.65, 393–405.
261
Frantzis A. (2018). A Long and Deep Step in Range Expansion of an Alien Marine Mammal in the Mediterranean: First Record of the Indian Ocean Humpback Dolphin Sousa Plumbea (G. Cuvier 1829) in the Greek Seas. BioInvasions Rec.7 (1), 83–87. doi: 10.3391/bir.2018.7.1.13
262
Fraser C. I. Nikula R. Waters J. M. (2011). Oceanic Rafting by a Coastal Community. Proc. R. Soc B Biol. Sci.278, 649–655. doi: 10.1098/rspb.2010.1117
263
Frazier J. Margaritoulis D. (1990). The Occurrence of the Barnacle, Chelonibia Patula (Ranzani 1818), on an Inanimate Substratum (Cirripedia, Thoracica). Crustaceana59, 213–218. doi: 10.1163/156854090X00688
264
Freeman M. A. Ogawa H. (2010). Variation in the Small Subunit Ribosomal DNA Confirms That Udonella (Monogenea: Udonellidae) is a Species-Rich Group. Int. J. Parasitol.40, 244–264. doi: 10.1016/j.ijpara.2009.08.006
265
Frick M. G. Pfaller J. B. (2013). “Sea Turtle Epibiosis,” in The Biology of Sea Turtles. Eds. WynekenJ.LohmannK. J.MusickJ. A. (Boca Raton, FL: CRC Press), 399–426.
266
Frick M. G. Zardus J. D. Ross A. Senko J. Montano-Valdez D. Bucio-Pacheco M. et al . (2011). Novel Records and Observations of the Barnacle Stephanolepas Muricata (Cirripedia: Balanomorpha: Coronuloidea); Including a Case for Chemical Mediation in Turtle and Whale Barnacles. J. Nat. Hist.45 (11-12), 629–640. doi: 10.1080/00222933.2010.534563
267
Gagnon J. Torgersen J. (2020) Canadian Museum of Nature Crustacea Collection. Available at: https://www.gbif.org/occurrence/1804299388 (Accessed June 20, 2021).
268
Gallo-Reynoso J. P. Figueroa-Carranza A. L. (1992). A Cookiecutter Shark Wound on a Guadalupe Fur Seal Male. Mar. Mamm. Sci.8 (4), 428–430. doi: 10.1111/j.1748-7692.1992.tb00060.x
269
Gambell R. (1964). A Pygmy Blue Whale at Durban. Norsk Hvalfangst-Tid.53 (3), 66–68.
270
García-Godos I. (1992). Captura Estacional De Cetáceos Menores En La Caleta De Ancón (Lima: Memoria X Congreso Nacional de Biología).
271
Gasparini J. L. Sazima I. (1996). A Stranded Melonheaded Whale, Peponocephala Electra, in Southeastern Brazil, With Comments on Wounds From the Cookiecutter Shark, Isistius Brasiliensis. Mar. Mamm. Sci.12, 308–312. doi: 10.1111/j.1748-7692.1996.tb00582.x
272
Gauthier H. (1938). Observations Sur Un Cetacé Du Genre Ziphius Mort Au Large D'alger. Bull. Stn. Aquic. Pêche Castiglione1, 181–204.
273
Geraci J. R. St. Aubin D. J. (1987). Effects of Parasites on Marine Mammals. Int. J. Parasitol.17 (2), 407–414. doi: 10.1016/0020-7519(87)90116-0
274
Gibson D. I. Harris E. A. Bray R. A. Jepson P. D. Kuiken T. Baker J. R. et al . (1998). A Survey of the Helminth Parasites of Cetaceans Stranded on the Coast of England and Wales During the Period 1990-1994. J. Zool.244 (4), 563–574. doi: 10.1111/j.1469-7998.1998.tb00061.x
275
Gill A. Fairbairns B. Fairbairns R. (2000). Photo-Identification of the Minke Whale (Balaenoptera Acutorostrata) Around the Isle of Mull, Scotland. Report to the Hebridean Whale and Dolphin Trust88 pp.
276
Gittings S. R. Dennis G. D. Harry H. W. (1986). Annotated Guide to the Barnacles of the Northern Gulf of Mexico. Biol. Oceanogr.402, 1–36.
277
Gomerčić H. Gomerčić M. D. Gomerčić T. Lucić H. Dalebout M. Galov A. et al . (2006). Biological Aspects of Cuvier’s Beaked Whales (Ziphius cavirostris) Recorded in the Croatian Part of the Adriatic Sea. Eur. J. Wildl. Res.52, 182–187. doi: 10.1007/s10344-006-0032-8
278
Gomes T. Quiazon K. Kotake M. Fujise Y. Ohizumi H. Itoh N. et al . (2021). Anisakis Spp. In Toothed and Baleen Whales From Japanese Waters With Notes on Their Potential Role as Biological Tags. Parasitol. Int.80, 102228. doi: 10.1016/j.parint.2020.102228
279
Gómez-Hernández I. Serrano A. Becerril-Gómez C. Basañez-Muñoz A. Naval-Ávila C. (2020). Prevalencia Y Abundancia Relativa De Balanos Xenobalanus globicipitis Presentes En Poblaciones De Delfín Nariz De Botella Tursiops Truncatus En El Golfo De México Sur. Rev. Biol. Mar. Oceanogr.55 (2), 172–176. doi: 10.22370/rbmo.2020.55.2.2503
280
González J. A. Martín L. Herrera R. González-Lorenzo G. Espino F. Barquín-Diez J. et al . (2012). Cirripedia of the Canary Islands: Distribution and Ecological Notes. J. Mar. Biolog. Assoc. U.K.92 (1), 129–141. doi: 10.1017/S002531541100066X
281
Gosse P. H. (1855). Notes on Some New or Little-Known Marine Animals. Ann. Mag. Nat. Hist.16 (91), 27–36. doi: 10.1080/037454809495473
282
Gould E. A. Higgs S. (2008). Impact of Climate Change and Other Factors on Emerging Arbovirus Diseases. Trans. R. Soc Trop. Med. Hyg.103 (2), 109–121. doi: 10.1016/j.trstmh.2008.07.025
283
Govindarajan A. F. Boero F. Halanych K. M. (2006). Phylogenetic Analysis With Multiple Markers Indicates Repeated Loss of the Adult Medusa Stage in Campanulariidae (Hydrozoa, Cnidaria). Mol. Phylogenet. Evol.38 (3), 820–834. doi: 10.1016/j.ympev.2005.11.012
284
Gray K. N. McDowell J. R. Collette B. B. Graves J. E. (2009). A Molecular Phylogeny of the Remoras and Their Relatives. Bull. Mar. Sci.84 (2), 183–197.
285
Greenwood A. G. Taylor D. C. Gauckler A. (1979). Odontocete Parasites-Some New Host Records. Aquat. Mamm.7, 23–25.
286
Groch K. R. Diaz-Delgado J. Marcondes M. C. Colosio A. C. Santos-Neto E. B. Carvalho V. L. et al . (2018). Pathology and Causes of Death in Stranded Humpback Whales (Megaptera novaeangliae) From Brazil. PloS One13 (5), e0194872. doi: 10.1371/journal.pone.0194872
287
Groch K. Jerdy H. Marcondes M. Barbosa L. Ramos H. Pavanelli L. et al . (2020). Cetacean Morbillivirus Infection in a Killer Whale (Orcinus Orca) From Brazil. J. Comp. Pathol.181, 26–32. doi: 10.1016/j.jcpa.2020.09.012
288
Gruvel J. A. (1903). Revision Des Cirrhipedes Appartenant a La Collection Du Musee D'histoire Naturelle. Nouv. Arch. Des. Musees Ser.45, 95–170.
289
Gruvel J. A. (1905). Monographie Des Cirrhipèdes Ou Thécostracés (Paris: Masson et cie).
290
Gruvel J. A. (1911). Expédition Antarctique Française Du Pourquoi-Pas Dirigée Par M. Le Dr. J.-B. Charcot, (1908-1910). Liste de Cirrhipèdes. Bull. Mus. Nat. Hist. Nat.5, 292.
291
Gruvel J. A. (1912). Mission Gruvel Sur La Côte Occidentale D’afrique, (1909–1910) Et Collection Du Museum D’histoire Naturelle. Les Cirripedes Vol. 18 (Paris: Bulletin du Museum National d’Histoire Naturelle), 344–350.
292
Gruvel J. A. (1920). “Cirrhipedes Provenant Des Campagnes Scientifiques De S.A.S. Le Prince De Monaco, (1885– 1913),” in Résultas Des Campagnes Scientifiques Accomplies Sur Son Yacht Par Albert Ler (Monaco: Prince Souverain de Monaco), 1–88.
293
Guerrero-Ruiz M. Urbán J. R. (2000). First Report of Remoras on Two Killer Whales (Orcinus Orca) in the Gulf of California, Mexico. Aquat. Mamm.26 (2), 148–150.
294
Guiler E. R. (1956). Supplement to a List of the Crustacea of Tasmania. Records Queen Victoria Museum5, 1–8.
295
Høeg J. T. Lagersson N. C. Glenner H. (2003). “The Complete Cypris Larva and its Significance in Thecostracan Phylogeny,” in Evolutionary and Developmental Biology of Crustacea. Ed. ScholtzG. (Lisse, Abingdon, Exton (PA), Tokyo: A. A. Balkema Publishers), 197–215.
296
Haelters J. (2001). De Walvisluis Isocyamus delphinii (Guerin-Meneville 1837) Aangetroffen Op Een Witsnuitdolfijn Lagenorhynchus Albirostris (Gray 1846) En Massaal Parasiterend Op Een Bruinvis Phocoena Phocoena (L. 1758). Strandvlo21 (3), 107–112.
297
Hai-yan Y. Xin-zheng L. (2002). A New Species of the Genus Nerocila (Isopoda: Cymothoidae) From the East China Sea. Chin. J. Oceanol. Limnol.20 (3), 266–269. doi: 10.1007/BF02848857
298
Hale H. M. (1926). Review of Australian Isopods of Cymothoid Group, Part 1. Trans. R. Soc South Aust.49, 128–185.
299
Hallas S. (1868). Optegnelser Om Nogle Paa Et Hvalfangst-Tog I Havet Omkring Island Iagttagne Hvaler. Videnskabelige Meddelelser fra Dansk Naturhistorisk Forening1, 150–177.
300
Hamilton J. E. (1914). Report of the Committee Appointed to Investigate Biological Problems Incidental to Belmullet Whaling Station. Br. Assoc. Adv. Sci., 125–161.
301
Hamre L. A. Eichner C. Caipang C. M. A. Dalvin S. T. Bron J. E. Nilsen F. et al . (2013). The Salmon Louse Lepeophtheirus Salmonis (Copepoda: Caligidae) Life Cycle Has Only Two Chalimus Stages. PloS One8 (9), e73539. doi: 10.1371/journal.pone.0073539
302
Haney T. A. (1999). Phylogenetic Analysis of the Whale-Lice (Amphipoda: Cyamidae). [Master’s Thesis] (South Carolina: University of Charleston).
303
Haney T. A. De Almeida A. O. Reis M. S. S. (2004). A New Species of Cyamid (Crustacea: Amphipoda) From a Stranded Cetacean in Southern Bahia, Brazil. Bull. Mar. Sci.75 (3), 409–421.
304
Harper D. E. (1995). Fouling of towed seismic streamers off central Africa by the lepadomorph barnacle Conchoderma virgatum. Crustaceana68(6), 779–781. doi: 10.1163/156854095x00287
305
Harper D. E. Jr. . Fouling of Towed Seismic Streamers Off Central Africa by the Lepadomorph Barnacle Conchoderma Virgatum. Crustaceana (2001) 68, 779–781.
306
Hart T. J. (1935). On the Diatoms of the Skin Film of Whales, and Their Possible Bearing on Problems of Whale Movements (Cambridge: Cambridge University Press).
307
Hastings R. W. (1972). The Barnacle, Conchoderma Virgatum (Spengler), in Association With the Isopod, Nerocila Acuminata Schioedte & Meinert, and the Orange Filefish, Alutera Schoepfi (Walbaum). Crustaceana22 (3), 274–278. doi: 10.1163/156854072X00552
308
Hayashi R. (2012). Atlas of the Barnacles on Marine Vertebrates in Japanese Waters Including Taxonomic Review of Superfamily Coronuloidea (Cirripedia: Thoracica). J. Mar. Biolog. Assoc. U.K.92, 107–127. doi: 10.1017/S0025315411000737
309
Hayashi R. Chan B. Simon-Blecher N. Watanabe H. Guy-Haim T. Yonezawa T. et al . (2013). Phylogenetic Position and Evolutionary History of the Turtle and Whale Barnacles (Cirripedia: Balanomorpha: Coronuloidea). Mol. Phyl. Evol.67 (1), 9–14. doi: 10.1016/j.ympev.2012.12.018
310
Heckmann R. A. Jensen L. A. Warnock R. G. Coleman B. (1987). Parasites of the Bowhead Whale, Balaena Mysticetus. Great Basin Nat.47 (3), 355–372.
311
Heldt J. H. (1950). Note Au Sujet De Xenobalanus globicipitis Steenstrup Sur Balaenoptera Borealis Lesson En Méditerranée. Bull. la Société d’Histoire Naturelle Tunisie3, 25–28.
312
Hemmingsen W. MacKenzie K. Sagerup K. Remen M. Bloch-Hansen K. Dagbjartarson Imsland A. K. (2020). Caligus elongatus and Other Sea Lice of the Genus Caligus as Parasites of Farmed Salmonids: A Review. Aquaculture522, 735160. doi: 10.1016/j.aquaculture.2020.735160
313
Hermosilla C. Silva L. M. Prieto R. Kleinertz S. Taubert A. Silva M. A. (2015). Endo-And Ectoparasites of Large Whales (Cetartiodactyla: Balaenopteridae, Physeteridae): Overcoming Difficulties in Obtaining Appropriate Samples by non-and Minimally-Invasive Methods. Int. J. Parasitol.: Parasites Wildl.4 (3), 414–420. doi: 10.1016/j.ijppaw.2015.11.002
314
Herr H. Burkhardt-Holm P. Heyer K. Siebert U. Selling J. (2020). Injuries, Malformations, and Epidermal Conditions in Cetaceans of the Strait of Gibraltar. Aquat. Mamm.46 (2), 215–235. doi: 10.1578/AM.46.2.2020.215
315
Hess J. E. Smith J. J. Timoshevskaya N. Baker C. Caudill C. C. Graves D. et al . (2020). Genomic Islands of Divergence Infer a Phenotypic Landscape in Pacific Lamprey. Mol. Ecol.29 (20), 3841–3856. doi: 10.1111/mec.15605
316
Heyning J. E. Dahlheim M. E. (1988). Orcinus Orca. Mamma. Species304, 1–9. doi: 10.2307/3504225
317
Hicks G. F. R. (1985). “Meiofauna Associated With Rocky Shore Algae” in The Ecology of Rocky Coasts. Eds. MooreP. G.SeedR. (London: Hodder and Stoughton), 36–56.
318
Hinojosa I. Boltana S. Lancellotti D. Macaya E. Ugalde P. Valdivia N. et al . (2006). Geographic Distribution and Description of Four Pelagic Barnacles Along the South East Pacific Coast of Chile-A Zoogeographical Approximation. Rev. Chil. Hist. Natural79 (1), 13–27. doi: 10.4067/S0716-078X2006000100002
319
Hinton M. A. C. (1925). Reports on Papers Left by the Late Major G. E. H. Barrett-Hamilton Relating to the Whales of South Georgia (London: Crown Agents for the Colonies).
320
Hiro F. (1935). The Fauna of Akkeshi Bay. II. Cirripedia. J. Faculty Sci. Hokkaido University.4, 213–229.
321
Hiro F. (1938). Cyamus Elongatus N. Sp., a New Whale-Lice From Japan. Annot. Zool. Jap.17 (1), 71–77.
322
Hiruki L. M. Gilmartin W. G. Becker B. L. Stirling I. (1993). Wounding in Hawaiian Monk Seals (Monachus Schauinslandi). Can. J. Zool.71 (3), 458–468. doi: 10.1139/z93-066
323
Hoek P. P. C. (1883). Report on the Cirripedia Collected by H.M.S. Challenger During the Years 1873–1876. Report of the Scientific Results From the Exploratory Voyages of H.M.S. Challenger Zoology8, 1–169. doi: 10.5962/bhl.title.12873
324
Hogans W. E. (1987). Morphological Variation in Pennella Balaenoptera and P. Filosa (Copepoda: Pennellidae) With a Review of the Genus Pennella Oken 1816 Parasitic on Cetacea. Bull. Mar. Sci.40 (3), 442–453.
325
Hogans W. E. (2017). Review of Pennella Oken 1816 (Copepoda: Pennellidae) With a Description of Pennella Benzi Sp. Nov., a Parasite of Escolar, Lepidocybium Flavobrunneum (Pisces) in the Northwest Atlantic Ocean. Zootaxa4244 (1), 1–38. doi: 10.11646/zootaxa.4244.1.1
326
Holcík J. Delic A. Kucinic M. Bukvic V. Vater M. . Distribution and Morphology of the Sea Lamprey from the Balkan Coast of the Adriatic Sea. J. Fish Biol. (2004) 64(2), 514–527.
327
Holmes M. Franco J. M. F. (2010). Goose Barnacle Conchoderma auritum (L.) Attached to Tooth of Stranded Sowerby’s Beaked Whale Mesoplodon Bidens Sowerby. Ir. Nat.' J.31 (2), 136.
328
Holthuis L. B. Fransen C. H. (2004). Interesting Records of Whale Epizoic Crustaceans From the Dutch North Sea Coast (Cirripedia, Amphipoda). Nederlandse Faunistische Mededelingen21, 11–16.
329
Holthuis L. B. Smeenk C. Laarman F. J. (1998). The Find of a Whale Barnacle, Cetopirus Complanatus (Mörch 1853), in 10th Century Deposits in the Netherlands. Zool. Verh.323 (27), 349–363.
330
Hopla C. Durden L. Keirans J. (1994). Ectoparasites and Classification. Rev. Sci. Tech.13, 985–1034. doi: 10.20506/rst.13.4.815
331
Horn M. H. Teal J. M. Backus R. H. (1970). Petroleum Lumps on the Surface of the Sea. Science168, 245–246. doi: 10.1126/science.168.3928.245
332
Huang Z. Liu W. Zheng C. LI C. Wang J. Jefferson T. A. (2000). Finless Porpoises in Southern Coastal Waters of Fujian, China. Acta Oceanologica Sin.22, 114–119. [In Chinese, English summary].
333
Humes A. G. (1964). Harpacticus Pulex, a New Species of Copepod From the Skin of a Porpoise and a Manatee in Florida. Bull. Mar. Sci.14 (4), 517–528.
334
Hurley D. E. (1952). Studies on the New Zealand Amphipodan Fauna. I. The Family Cyamidae: The Whale-Louse Paracyamus Boopis. Trans. R. Soc N. Z. Zool.80, 63–68.
335
Hurley D. E. Mohr J. L. (1957). On Whale-Lice (Amphipoda: Cyamidae) From the California Gray Whale E. Glaucus. Parasitology43 (3), 352–357. doi: 10.2307/3274363
336
Hustedt F. (1952). Diatomeen Aus Der Lebensgemeinschaft Der Buckelwals (Megaptera Nodosa Bonn.). Archiv für Hydrobiologie46 (2), 286–298.
337
Huys R. Llewellyn-Hughes J. Olson P. D. Nagasawa K. (2006). Small Subunit rDNA and Bayesian Inference Reveal Pectenophilus Ornatus (Copepoda Incertae Sedis) as Highly Transformed Mytilicolidae, and Support Assignment of Chondracanthidae and Xarifiidae to Lichomolgoidea (Cyclopoida). Biol. J. Linn. Soc Lond.87 (3), 403–425. doi: 10.1111/j.1095-8312.2005.00579.x
338
Ichihara T. (1966). “The Pigmy Blue Whale, Balaenoptera Musculus Brevicauda, a New Subspecies From the Antarctic” in Whales, Dolphins and Porpoises. Ed. NorrisK. S. (Berkeley and LA: University of California Press), 79–113. doi: 10.1525/9780520321373-008
339
Ichihara T. (1981). “Review of Pygmy Blue Whale Stock in the Antarctic”. Mammals Seas (FAO)3, 211–218.
340
IJsseldijk L. L. Van Neer A. Deaville R. Begeman L. van de Bildt M. van den Brand J. M. et al . (2018). Beached Bachelors: An Extensive Study on the Largest Recorded Sperm Whale Physeter Macrocephalus Mortality Event in the North Sea. PloS One13 (8), e0201221. doi: 10.1371/journal.pone.0201221
341
Il'in I. I. Kuznetsova L. A. Starostin I. V. (1978). Oceanic Fouling in the Equatorial Atlantic. Oceanology18, 597–599.
342
Inatsuchi A. Yamato S. Yusa Y. (2010). Effects of Temperature and Food Availability on Growth and Reproduction in the Neustonic Pedunculate Barnacle. Lepas anserifera. Mar. Biol.157, 899–905. doi: 10.1007/s00227-009-1373-0
343
iNaturalist users, iNaturalist (2021) Inaturalist Research-Grade Observations (iNaturalist.org). Available at: https://www.gbif.org/occurrence/1880668572 (Accessed May 20, 2021).
344
Insacco G. Buscaino G. Buffa G. Cavallaro M. Crisafi E. Grasso R. et al . (2014). Il Patrimonio Delle Raccolte Cetologiche Museali Della Sicilia. Museologia Scientifica Memorie12, 391–405. [In Italian].
345
International Whaling Comission, IWC (2021) Total Catches (International Whaling Comission). Available at: https://iwc.int/total-catches (Accessed May 3, 2021).
346
Ishi S. (1915). A Cyamid Obtained in the Province Awa Vol. 27 (Tokio: Dobutsugaku Zasshi), 157–159. [In Japanese].
347
Itô T. (1976). Descriptions and Records of Marine Harpacticoid Copepods From Hokkaido. J. Fac. Sci., Hokkaido Univ.20 (3), 448–567.
348
Ito A. Aoki M. Yahata K. Wada H. (2011). Complicated Evolution of the Caprellid (Crustacea: Malacostraca: Peracarida: Amphipoda) Body Plan, Reacquisition or Multiple Losses of the Thoracic Limbs and Pleons. Dev. Genes Evol.221 (3), 133–140. doi: 10.1007/s00427-011-0365-5
349
Ivashin M. V. (1965). Obrastaniya I Ektoparasity U Gorbatykh KitovV kn.Morskie mlekopitayuschie.. 80–86.
350
Ivashin M. V. (1965). Vneshnie parazity malykh polosatikov Antarktiki [External parasites on lesser rorquals in the Antarctic]. Kiev: Naukova Dumka, 125–127. [In Russian].
351
Ivashin M. V. Golubovsky Y. P. (1978). On the Cause of Appereance of White Scars on the Body of Whales. Rep. Int. Whal. Commn.28, 199.
352
Iwasa M. (1934). Two Species of Whale-Lice (Amphipoda, Cyamidae) Parasitic on a Right Whale. Fac. Sci. Hokkaido Imperial Univ. Ser. 6 Zool.3, 33–40.
353
Iwasa-Arai T. Carvalho V. L. Serejo C. S. (2017b). Updates on Cyamidae (Crustacea: Amphipoda): Redescriptions of Cyamus Monodontis Lütken 1870 and Cyamus Nodosus Lütken 1861, a New Species of Isocyamus, and New Host Records for Syncyamus Ilheusensis Haney, De Almeida and Reis 2004. J. Nat. Hist.51 (37-38), 2225–2245. doi: 10.1080/00222933.2017.1365965
354
Iwasa-Arai T. da Silva Santana F. Barbosa C. B. Werneck M. R. (2021). One Crawled Over the Dolphin's Back: Unusual Record of the Whale Louse Cyamus Boopis (Crustacea: Amphipoda: Cyamidae) on the Bottlenose Dolphin (Tursiops Truncatus). Zoologischer Anzeiger295, 117–119. doi: 10.1016/j.jcz.2021.10.002
355
Iwasa-Arai T. Santarosa Freire A. Castaldo Colosio A. Serejo C. S. (2016). Ontogenetic Development and Redescription of the Whale Louse Cyamus Boopis Lütken 1870 (Crustacea: Amphipoda: Cyamidae), Ectoparasite of Humpback Whale Megaptera novaeangliae (Mammalia: Cetacea: Balaenopteridae). Mar. Biodiv.47 (3), 929–939. doi: 10.1007/s12526-016-0532-z
356
Iwasa-Arai T. Serejo C. S. (2018). Phylogenetic Analysis of the Family Cyamidae (Crustacea: Amphipoda): A Review Based on Morphological Characters. Zool. J. Linn. Soc184 (1), 66–94. doi: 10.1093/zoolinnean/zlx101
357
Iwasa-Arai T. Serejo C. S. Siciliano S. Ott P. H. Freire A. S. Elwen S. et al . (2018). The Host-Specific Whale Louse (Cyamus Boopis) as a Potential Tool for Interpreting Humpback Whale (Megaptera novaeangliae) Migratory Routes. J. Exp. Mar. Bio. Ecol.505, 45–51. doi: 10.1016/j.jembe.2018.05.001
358
Iwasa-Arai T. Siciliano S. Serejo C. Rodríguez-Rey G. (2017a). Life History Told by a Whale-Louse: A Possible Interaction of a Southern Right Whale Eubalaena Australis Calf With Humpback Whales Megaptera novaeangliae. Helgoland Marine Res.71 (1). doi: 10.1186/s10152-017-0486-y
359
Japha A. (1910). Weitere Beitrage Zur Kenntnis Der Walhaut. Zool. JahrbuchSuppl. 12, 711–718.
360
Jauniaux T. Petitjean D. Brenez C. Borrens M. Brosens L. Haelters J. et al . (2002). Post-Mortem Findings and Causes of Death of Harbour Porpoises (Phocoena Phocoena) Stranded From 1990 to 2000 Along the Coastlines of Belgium and Northern France. J. Comp. Path.126 (4), 243–253. doi: 10.1053/jcpa.2001.0547
361
Jefferson T. A. Odell D. K. Prunier K. T. (1995). Notes on the Biology of the Clymene Dolphin (Stenella Clymene) in the Northern Gulf of Mexico. Mar. Mamm. Sci.11 (4), 564–573. doi: 10.1111/j.1748-7692.1995.tb00679.x
362
Jerde C. W. (1967). On the Distribution of Portunus (Achelous) Affinis and Euphylax Dovii (Decapoda Brachyura, Portunidae) in the Eastern Tropical Pacific. Crustaceana13, 11–22. doi: 10.1163/156854067X00026
363
Jiménez I. C. A. Reina L. M. C. Kintz J. R. C. (2011). Crustáceos Ectoparásitos Y Epibiontes De Ballenas Jorobadas, Megaptera novaeangliae (Cetacea: Balaenopteridae) En El Pacífico Colombiano Vol. 3 (San José:Cuadernos de Investigación UNED), 177–185. doi: 10.22458/urj.v3i2.146
364
Johnson N. S. Buchinger T. J. Kintz J. R. C. (2015). “Reproductive ecology of lampreys” in Lampreys: Biology, Conservation, and Control, ed. DockerM. F. (New York: Springer), 265–303
365
Jones D. S. (2010). “The Littoral and Shallow-Water Barnacles (Crustacea: Cirripedia) of South-Eastern Queensland,” in Proceedings of the Thirteenth International Marine Biological Workshop, The Marine Fauna and Flora of Moreton Bay, Queensland, vol. 54 . Eds. DavieP. J. F.PhillipsJ. A. (Memoirs of the Queensland Museum. Nature), 199–233.
366
Joon S. S. Young C. C. (1993). Eight Harpactiocoid Species of Harpacticidae (Copepoda, Harpacticoida) From Korea. Anim. Syst. Evol. Divers.9 (2), 203–220.
367
Joseph B. E. Cornell L. H. Osborn K. G. (1986). Occurrence of Ectoparasitic Barnacles on Northern Elephant Seals (Mirounga Angustirostris). J. Mammal.67 (4), 772–772. doi: 10.2307/1381148
368
Kabata Z. (1974). Mouth and Mode of Feeding of Caligidae (Copepoda), Parasites of Fishes, as Determined by Light and Scanning Electron Microscopy. J. Fish. Res. Board Can.31, 1583–1588. doi: 10.1139/f74-199
369
Kabata Z. (1979). Parasitic Copepoda of British Fishes (London: The Ray Society).
370
Kakuwa Z. Kawakami T. Iguchi K. (1953). Biological Investigation on the Whales Caught by the Japanese Antarctic Whaling Fleets in the 1951-52 Season. Sci. Rep. Whales Res. Inst.8, 147–213.
371
Kaliszewska Z. A. Seger J. O. N. Rowntree V. J. Barco S. G. Benegas R. Best P. et al . (2005). Population Histories of Right Whales (Cetacea: Eubalaena) Inferred From Mitochondrial Sequence Diversities and Divergences of Their Whale Lice (Amphipoda: Cyamus). Mol. Ecol.14 (11), 3439–3456. doi: 10.1111/j.1365-294X.2005.02664.x
372
Kamiya K. Yamashita K. Yanagawa T. Kawabata T. Watanabe K. (2012). Cypris Larvae (Cirripedia: Balanomorpha) Display Auto-Fluorescence in Nearly Species-Specific Patterns. Zool. Sci.29 (4), 247–253. doi: 10.2108/zsj.29.247
373
Kane E. A. Olson P. A. Gerodette T. Fiedler P. C. (2008). Prevalence of the Commensal Barnacle Xenobalanus globicipitis on Cetacean Species in the Eastern Tropical Pacific Ocean, and Review of Global Occurrence. Fish. Bull.106, 395–404.
374
Karaa S. Insacco G. Bradai M. N. Scaravelli D. (2011). Records of Xenobalanus globicipitis on Balaenoptera Physalus and Stenella Coeruleoalba in Tunisian and Sicilian Waters. Natura Rerum.1, 55–59.
375
Karenina K. Giljov A. Ivkovich T. Malashichev Y. (2016). Evidence for the Perceptual Origin of Right-Sided Feeding Biases in Cetaceans. Anim. Cogn.19 (1), 239–243. doi: 10.1007/s10071-015-0899-4
376
Karuppiah S. Subramanian A. Obbard J. P. (2004). The Barnacle, Xenobalanus globicipitis (Cirripedia, Coronulidae), Attached to the Bottle-Nosed Dolphin, Tursiops Truncatus (Mammalia, Cetacea) on the Southeastern Coast of India. Crustaceana77, 879–882. doi: 10.1163/156854004774248753
377
Kasuya T. Rice D. W. (1970). Notes of Baleen Plates and on Arrangement of Parasitic Barnacles of Gray Whale. Sci. Rep. Whale Res. Inst.22, 39–43.
378
Kautek G. Van Bressem M. F. Ritter F. (2008). External Body Conditions in Cetaceans From La Gomera, Canary Islands, Spain. J. Marine Anim. Their Ecol.11 (2), 4–17.
379
Kawamura A. (1969). Some Consideration on the Stock Unit of Sei Whales by the Aspect of Ectoparasitic Organisms on the Body. Bull. Jap. Soc Fish. Oceanogr.14, 38–43. [In Japanese].
380
Keable S. J. (2006). Taxonomic Revision of Natatolana (Crustacea: Isopoda: Cirolanidae). Rec. Aust. Mus.58(2),133–244. doi: 10.3853/j.0067-1975.58.2006.1469
381
Kenney R. D. (2009). “Right Whales: Eubalaena Glacialis, E. Japonica, and E. Australis,” in Encyclopedia of Marine Mammals. Eds. PerrinW.WürsigB.ThewissenJ. (San Diego: Academic Press), 962–972. doi: 10.1016/B978-0-12-373553-9.00220-0
382
Khodami S. McArthur J. V. Blanco-Bercial L. Martínez Arbizu P. (2017). Molecular Phylogeny and Revision of Copepod Orders (Crustacea: Copepoda). Sci. Rep.7 (1), 9164. doi: 10.1038/s41598-017-06656-4
383
Killingley J. (1980). Migrations of California Gray Whales Tracked by Oxygen-18 Variations in Their Epizoic Barnacles. Scie207, 759–760. doi: 10.1126/science.207.4432.759
384
Kim H. K. Chan B. K. K. Kang C. Kim H. W. Kim W. (2020). How do Whale Barnacles Live on Their Hosts? Functional Morphology and Mating-Group Sizes of Coronula Diadema (Linnaeus 1767) and Conchoderma auritum (Linnaeus 1767) (Cirripedia: Thoracicalcarea). J. Crust. Biol.40 (6), 808–824. doi: 10.1093/jcbiol/ruaa075
385
Kim M. J. Sohn H. (2016). Rescue, Rehabilitation and Release of Finless Porpoise (Neophocaena Asiaeorientalis) in Korea. J. Fish. Mar. Sci. Educ.28 (3), 861–871. doi: 10.13000/JFMSE.2016.28.3.861
386
Kim I. H. Suárez-Morales E. Márquez-Rojas B. (2019). Caligid Copepods (Copepoda: Siphonostomatoida: Caligidae) as Zooplankters Off the Venezuelan Coast, Western Caribbean Sea. Thalassas35, 607–618. doi: 10.1007/s41208-019-00130-w
387
Klinkhart E. G. (1966). The bBeluga Whale in Alaska. Juneau: Alaska Dep. Fish. Game, Fed. Aid Wildl. Restor. Proj. Rep.7 W–6–R and W–14–R.
388
Kobayashi N. Tokutake K. Yoshida H. Okabe H. Miyamoto K. Ito H. et al . (2021). The First Stranding Record of Longman’s Beaked Whale (Indopacetus Pacificus) in Okinawa, Japan. Aquat. Mamm.47 (2), 153–174. doi: 10.1578/AM.47.2.2021.153
389
Koch W. R. (2002). Revisão taxonômica do gênero Homodiaetus (Teleostei, Siluriformes, Trichomycteridae). Iheringia, Série Zoologia, Porto Alegre92, 33–46.
390
Koren J. Danielssen D. C. (1877). En Ny Art Af Slaegten Pennella. (A New Species of the Genus Penella). Fauna Littoralis Norvegiae3, 157–163.
391
Kottelat M. Freyhof J. (2007). Handbook of European Freshwater Fishes (Cornol: Publications Kottelat).
392
Krøyer H. (1843). Om Cyamus Ceti (Med Et Par Bemaerkninger, Betraeffende Den Mulige Anvendelse Af De Paa Hvalerne Levende Smaa Dyr Ved Hvalarternes Adskillelse). Naturhistorisk Tidsskrift, 474–489.
393
Krefft G. (1953). Ichthyologische Mitteilungen Aus Dem Institut Für Seefischerei Der Bundesanstalt Für Fischerei. Zool. Anz.150, 275–282.
394
Kubota S. (1999). Fauna of Obelia (Cnidaria, Hydrozoa) in Japanese Waters, With Special Reference to Life Cycle of Obelia Dichotoma (L. 1758). Zoosystematica Rossica1, 67–76.
395
Kuramochi T. Araki J. Uchida Moriyama N. Takeda Y. Hayashi N. et al . (2000). “Summary of Parasite and Epizoit Investigations During JARPN Surveys 1994-1999, With Reference to Stock Structure Analysis for the Western North Pacific Minke Whales,” in IWC Scientific Committee Workshop to Review the Japanese Whaling Programme Under Special Permit for North Pacific Minke Whales (JARPN). SC/F2K/J19
396
Kuramochi T. Machida M. Araki J. Uchida A. Kishiro T. Nagasawa K. (1996). Minke Whales (Balaenoptera Acutorostrata) are One of the Major Final Hosts of Anisakis Simplex (Nematoda: Anisakidae) in the Northwestern North Pacific Ocean. Rep. Int. Whal. Commn.46, 415–420.
397
Kwak M. L. Heath A. C. Cardoso P. (2020). Methods for the Assessment and Conservation of Threatened Animal Parasites. Biol. Conserv.248, 108696. doi: 10.1016/j.biocon.2020.108696
398
La Linn M. Gardner J. Warrilow D. Darnell G. A. McMahon C. R. Field I. et al . (2001). Arbovirus of Marine Mammals: A New Alphavirus Isolated From the Elephant Seal Louse, Lepidophthirus Macrorhini. J. Virol.75, 4103–4109. doi: 10.1128/JVI.75.9.4103-4109.2001
399
Lam K. K. Y. (2000). Algal and Sessile Invertebrate Recruitment Onto an Experimental PFA-Concrete Artificial Reef in Hong Kong. Asian Mar. Biol.17, 55–76.
400
Lane E. P. De Wet M. Thompson P. Siebert U. Wohlsein P. Plön S. (2014). A Systematic Health Assessment of Indian Ocean Bottlenose (Tursiops Aduncus) and Indo-Pacific Humpback (Sousa Plumbea) Dolphins Incidentally Caught in Shark Nets Off the KwaZulu-Natal Coast, South Africa. PloS One9 (9), e107038. doi: 10.1371/journal.pone.0107038
401
Leach W. E. (1817). Distribution Systématique De La Classe Cirripède. J. Phys. Chim. Hist. Nat.85, 67–69.
402
Lehnert K. Fonfara S. Wohlsein P. Siebert U. (2007). Whale Lice (Isocyamus delphinii) on a Harbour Porpoise (Phocoena Phocoena) From German Waters. Vet. Rec.161, 526–528. doi: 10.1136/vr.161.15.526
403
Lehnert K. IJsseldijk L. Uy M. Boyi J. van Schalkwijk L. Tollenaar E. et al . (2021). Whale Lice (Isocyamus Deltobranchium & Isocyamus delphinii; Cyamidae) Prevalence in Odontocetes Off the German and Dutch Coasts – Morphological and Molecular Characterization and Health Implications. Int. J. Parasitol.: Parasites Wildl.15, 22–30. doi: 10.1016/j.ijppaw.2021.02.015
404
Lehnert K. Poulin R. Presswell B. (2019). Checklist of Marine Mammal Parasites in New Zealand and Australian Waters. J. Helminthol.93 (6), 649–676. doi: 10.1017/s0022149x19000361
405
Lehnert K. Randhawa H. Poulin R. (2017). Metazoan Parasites From Odontocetes Off New Zealand: New Records. Parasitol. Res.116, 2861–2868. doi: 10.1007/s00436-017-5573-0
406
Leung Y. M. (1965). A Collection of Whale-Lice (Cyamidae: Amphipoda). Bull. California Acad. Sci.64, 132–143.
407
Leung Y. M. (1967). An Illustrated Key to the Species of Whale-Lice (Amphipoda, Cyamidae), Ectoparasites of Cetacea, With a Guide to the Literature. Crustaceana12 (3), 279–291. doi: 10.1163/156854067X00251
408
Leung Y. M. (1970a). First Record of the Whale-Louse Genus Syncyamus (Cyamidae: Amphipoda) From the Western Mediterr. Invest. Cet.582, 243–247.
409
Leung Y. M. (1970b). Cyamus Orcini, a New Species of Whale-Louse (Cyamidae, Amphipoda) From a Killer Whale. Bull. Inst. Franç. Afrique Noire32, 669–675.
410
Leung Y. M. (1976). Life Cycle of Cyamus Scammoni (Amphipoda: Cyamidae), Ectoparasite of Gray Whale, With a Remark on the Associated Species. Sci. Rep. Whales Res. Inst.28, 153–160.
411
Leung T. L. F. Poulin R. (2008). Parasitism, Commensalism, and Mutualism: Exploring the Many Shades of Symbioses. Vie Milieu/Life Environ., 107–115.
412
Lewis A. G. (1967). Copepod Crustaceans Parasitic on Teleost Fishes of the Hawaiian Islands. Proc. U. S. Nat. Mus.121, 1–204. doi: 10.5479/si.00963801.121-3574.1
413
Lillie D. G. (1910). Observations on the Anatomy and General Biology of Some Members of the Larger Cetacea. Proc. Zool. Soc. Lond.3, 769–792. doi: 10.1111/j.1096-3642.1910.tb01916.x
414
Lincoln R. J. Hurley D. E. (1974a). Catalogue of the Whale-Lice (Crustacea, Amphipoda, Cyamidae) in the Collections of the British Museum (Natural History). Bull. Br. Mus. Nat. Hist. Zool.27, 65–72. doi: 10.5962/bhl.part.22972
415
Lincoln R. J. Hurley D. E. (1974b). Scutocyamus Parvus, a New Genus and Species of Whale-Louse (Amphipoda: Cyamidae) Ectoparasitic on the North Atlantic White-Beaked Dolphin. Bull. Brit. Mus. Nat. Hist. Zool.27 (2), 59–64. doi: 10.5962/bhl.part.22971
416
Lincoln R. J. Hurley D. E. (1980). Scutocyamus Antipodensis N. Sp. (Amphipoda: Cyamidae) on Hector’s Dolphin (Cephalorhynchus Hectori) From New Zealand. N. Z. J. Mar. Freshwater Res.14, 295–301. doi: 10.1080/00288330.1980.9515872
417
Lincoln R. J. Hurley D. E. (1981). A New Species of the Whale-Louse Syncyamus (Crustacea: Amphipoda: Cyamidae) Ectoparasitic on Dolphins From South Africa. Ann. Cape Prov. Mus. Nat. Hist.13 (13), 187–194.
418
Linehan E. J. (1979). The Trouble With Dolphins. Natl. Geographic Mag.155 (4), 540.
419
Linnaeus C. (1758). “Systema Naturae Per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, Cum Characteribus, Differentiis, Synonymis, Locis,” in Editio decima, reformata, 10th revised edition, vol. 1, 824 pp . doi: 10.5962/bhl.title.542
420
Liouville J. (1913). Cétacés De L'antarctique (Paris: Deuxième Expédition Antarctique Française), 1908–1910. *824 pp.
421
Lockyer C. (1979). Response of Orcas to Tagging. Carnivore11, 19–21.
422
López B. A. Macaya E. C. Tala F. Tellier F. Thiel M. (2017). The Variable Routes of Rafting: Stranding Dynamics of Floating Bull Kelp Durvillaea Antarctica (Fucales, Phaeophyceae) on Beaches in the SE Pacific. J. Phycol.53 (1), 70–84. doi: 10.1111/jpy.12479
423
Lorenzen S. (1986). Odontobius (Nematoda, Monhysteridae) From the Baleen Plates of Whales and its Relationship to Gammarinema Living on Crustaceans. Zool. Scr.15 (2), 101–106. doi: 10.1111/j.1463-6409.1986.tb00213.x
424
Lorion C. M. Markle D. F. Reid S. B. Docker M. F. (2000). Redescription of the Presumed-Extinct Miller Lake Lamprey, Lampetra Minima. Copeia100 (4), 1019–1028. doi: 10.1643/0045-8511(2000)000[1019:ROTPEM]2.0.CO;2
425
Louella M. Dolar L. (2002). “Fraser's Dolphin Lagenodelphis Hosei” in Encyclopedia of Marine Mammals. Eds. PerrinW. F.WursigB.ThewissenJ. G. M. (London: Academic Press), 485–487.
426
Lowry J. K. Myers A. A. (2013). A Phylogeny and Classification of the Senticaudata Subord. Nov. Crustacea: Amphipoda. Zootaxa3610 (1), 1–80. doi: 10.11646/zootaxa.3610.1.1
427
Lütken C. F. (1870). Conspectus Cyamidarum Borealium Hujusque Cognitarum. Vidensk. Selsk. Forhandl. Crustaceana13, 279–280. doi: 10.5962/bhl.part.9800
428
Lütken C. F. (1873). Bidrag Til Kundskab Om Arterne Af Slaegten Cyamus Latr. Eller Hvallusene. Vidensk. Selsk. Skr.10 (3), 230–284.
429
Lütken C. F. (1887). Tillaeg Til “Bidrag Til Kundskab Om Arterne Af Slaegten Cyamus Latr. Eller Hvallu Sene”. Vidensk. Selsk. Skr.4, 316–322.
430
Lütken C. F. (1893). Andet Tillaeg Til “Bidrag Til Kundskab Om Arterne Af Slaegten Cyamus Latr. Eller Hvallusene. Vidensk. Selsk. Skr.9, 421–434.
431
MacIntyre R. J. (1966). Rapid Growth in Stalked Barnacles. Nature212, 637–638. doi: 10.1038/212637a0
432
Mackenzie K. (2002). Parasites as Biological Tags in Population Studies of Marine Organisms: An Update. Parasitology124 (7), 153–163. doi: 10.1017/S0031182002001518
433
Mackintosh N. A. Wheeler J. F. G. (1929). Southern Blue and Fin Whales. Discovery Rep.1, 257–540.
434
Magni P. A. Venn C. Aquila I. Pepe F. Ricci P. Di Nunzio C. et al . (2015). Evaluation of the Floating Time of a Corpse Found in a Marine Environment Using the Barnacle Lepas Anatifera L. (Crustacea: Cirripedia: Pedunculata). Forensic Sci. Int.247, e6–e10. doi: 10.1016/j.forsciint.2014.11.016
435
Mahnken T. Gilmore R. M. (1960). Suckerfish on a Porpoise. J. Mammal.41 (1), 134. doi: 10.2307/1376540
436
Maran B. A. V. Moon S. Y. Ohtsuka S. Oh S. Y. Soh H. Y. Myoung J. G. et al . (2013). The Caligid Life Cycle: New Evidence From Lepeophtheirus Elegans Reconciles the Cycles of Caligus and Lepeophtheirus (Copepoda: Caligidae). Parasite20, 15. doi: 10.1051/parasite/2013015
437
Marcer F. Marchiori E. Centelleghe C. Ajzenberg D. Gustinelli A. Meroni V. et al . (2019). Parasitological and Pathological Findings in Fin Whales Balaenoptera Physalus Stranded Along Italian Coastlines. Dis. Aquat. Org.133 (1), 25–37. doi: 10.3354/dao03327
438
Margolis L. (1954a). Three Kinds of Whale-Lice (Cyamidae: Amphipoda) From the Pacific Coast of Canada, Including a New Species. J. Fish. Res. Board Can.11 (3), 319–325. doi: 10.1139/f54-020
439
Margolis L. (1954b). Delphinapterus Leucas, a New Host Record for the Whale-Louse, Paracyamus Nodosus (Lütken). J. Parasitol.40, 365. doi: 10.2307/3273757
440
Margolis L. (1955). Notes on the Morphology, Taxonomy and Synonymy of Several Species of Whale-Lice (Cyamidae: Amphipoda). J. Fish. Res. Board Can.12 (l), 121–133. doi: 10.1139/f55-009
441
Margolis L. (1959). Records of Cyamus Balaenopterae Barnard and Neocyamus Physeteris (Pouchet), Two Species of Whale-Lice (Amphipoda), From the Northeast Pacific. Can. J. Zool.37 (6), 895–897. doi: 10.1139/z59-086
442
Margolis L. McDonald T. E. Bousfield E. L. (2000). The Whale Lice (Amphipoda: Cyamidae) of the Northeastern Pacific Region. Amphipacifica II4, 63–117.
443
Mariniello L. Cerioni S. Di Cave D. (1994). Ridescrizione Di Syncyamus Aequus Lincoln Ed Hurley 1981 (Amphipoda: Cyamidae), Ectoparassita Di Stenella Coeruleoalba (Meyen 1833) E Prima Segnalazione Per Le Acque Italiane. Parassitologia36, 313–316.
444
Marino L. Stowe J. (1997). Lateralized Behavior in a Captive Beluga Whale (Delphinapterus Leucas). Aquat. Mamm.23, 101–104.
445
Marloth R. (1900). Notes on the Mode of Growth of Tubicinella Trachaelis, the Barnacle of the Southern Right Whale. Trans. S. Afr. Phil. Soc11, 1–6. doi: 10.1080/21560382.1900.9525952
446
Marlow B. (1962). A Recent Record of the Dugong, Dugong Dugon, From New South Wales. J. Mammal.43, 433–433. doi: 10.2307/1376973
447
Marlow B. G. (1963). Rare Whale Washed Up on Sydney Beach. Aust. Nat. Hist.15, 164.
448
Maronna M. M. Miranda T. P. Pena Cantero A. L. Barbeitos M. S. Marques A. C. (2016). Towards a Phylogenetic Classification of Leptothecata (Cnidaria, Hydrozoa). Sci. Rep.6, 18075. doi: 10.1038/srep18075
449
Martínez R. Segade P. Martínez-Cedeira J. A. Arias C. García-Estévez J. M. Iglesias R. (2008). Occurrence of the Ectoparasite Isocyamus Deltobranchium (Amphipoda: Cyamidae) on Cetaceans From Atlantic Waters. J. Parasitol.94 (6), 1239–1242. doi: 10.1645/GE-1518.1
450
Martini F. (1998). “The Ecology of Hagfishes,” in The Biology of Hagfishes. Eds. JorgensenJ. M.MalteH. (London: Chapman & Hall), 57–77. doi: 10.1007/978-94-011-5834-3_5
451
Martín V. Tejedor M. Pérez-Gil M. Dalebout M. L. Arbelo M. Fernández A. (2011). A Sowerby's Beaked Whale (Mesoplodon Bidens) Stranded in the Canary Islands: The Most Southern Record in the Eastern North Atlantic. Aquat. Mamm.37 (4), 512–519. doi: 10.1578/AM.37.4.2011.512
452
Maruzzo D. Aldred N. Clare A. S. Høeg J. T. (2012). Metamorphosis in the Cirripede Crustacean Balanus Amphitrite. PloS One7 (5), e37408. doi: 10.1371/journal.pone.0037408
453
Matthews L. H. (1937). The Humpback Whale, Megaptera novaeangliae. Discovery Rep.17, 7–92.
454
Matthews L. H. (1938a). Notes on the Southern Right Whale, Eubalaena Australis. Discovery Rep.17, 169–182.
455
Matthews L. H. (1938b). The Sei Whale Balaenoptera Borealis. Discovery Rep.17, 183–290.
456
Matthews L. H. (1938c). The Sperm Whale, Physeter Catodon. Discovery Rep.17, 93–168.
457
Matthews C. Ghazal M. Lefort K. Inuarak E. (2020). Epizoic Barnacles on Arctic Killer Whales Indicate Residency in Warm Waters. Mar. Mamm. Sci.36 (3), 1010–1014. doi: 10.1111/mms.12674
458
McAlpine D. (2003). “Pygmy and Dwarf Sperm Whales (Kogia Breviceps and K. Sima),” in Encyclopedia of Marine Mammals. Eds. PerrinW. F.WürsigB.ThewissenJ. G. M. (San Diego, CA: Academic Press), 1007–1009.
459
McAlpine D. F. Murison L. D. Hoberg E. P. (1997). New Records for the Pygmy Sperm Whale, Kogia Breviceps (Physeteridae) From Atlantic Canada With Notes on Diet and Parasites. Mar. Mamm. Sci.13 (4), 701–704. doi: 10.1111/j.1748-7692.1997.tb00093.x
460
McCann C. (1975). A Study of the Genus Berardius Duvernoy. Sci. Rep. Whales Res. Inst.27, 111–137.
461
McSweeney D. J. Baird R. W. Mahaffy S. D. (2007). Site Fidelity, Associations, and Movements of Cuvier’s (Ziphius cavirostris) and Blainville’s (Mesoplodon densirostris) Beaked Whales Off the Island of Hawaii. Mar. Mamm. Sci.23, 666–687. doi: 10.1111/j.1748-7692.2007.00135.x
462
Mead J. G. (1975). Preliminary Report on the Former Net Fisheries for Tursiops Truncatus in the Western North Atlantic. Fish. Res. Board Can.32 (7), 1155–1162. doi: 10.1139/f75-136
463
Mead J. G. (1989). “Beaked Whales of the Genus Mesoplodon,” in Handbook of Marine Mammals. Volume 4. River Dolphins and the Larger Toothed Whales. Eds. RidgwayS. H.HarrisonR.(London:Academic Press), 349–430.
464
Mead J. G. Potter C. W. (1990). “Natural History of Bottlenose Dolphins Along the Central Atlantic Coast of the United States,” in The Bottlenose Dolphin. Eds. LeatherwoodS.ReevesR. (San Diego, CA: Academic Press), 165–195. doi: 10.1016/B978-0-12-440280-5.50013-5
465
Methion S. Díaz López B. (2019). First Record of Atypical Pigmentation Pattern in Fin Whale Balaenoptera Physalus in the Atlantic Ocean. Dis. Aquat. Org.135 (2), 121–125. doi: 10.3354/dao03385
466
Migaki G. (1987). 21 Selected Dermatoses of Marine Mammals. Clin. Dermatol.5 (3), 155–164. doi: 10.1016/s0738-081x(87)80022-6
467
Mignucci-Giannoni A. A. Beck C. A. Montoya-Ospina R. A. Williams E. H. Jr. (1999). Parasites and Commensals of the West Indian Manatee From Puerto Rico. Skin66 (1), 87812.
468
Mignucci-Giannoni A. A. Hoberg E. P. Siegel-Causey D. Williams E. H. Jr. (1998). Metazoan Parasites and Other Symbionts of Cetaceans in the Caribbean. J. Parasitol.84(5), 939–946. doi: 10.2307/3284625
469
Miller A. K. Baker C. Kitson J. C. Yick J. L. Manquel P. E. I. Alexander A. et al . (2021). The Southern Hemisphere Lampreys (Geotriidae and Mordaciidae). Rev. Fish Biol. Fish.31, 201–232. doi: 10.1007/s11160-021-09639-x
470
Minchin D. (1996). Tar Pellets and Plastics as Attachment Surfaces for Lepadid Cirripedes in the North Atlantic Ocean. Mar. Pollut. Bull.32 (12), 855–859. doi: 10.1016/S0025-326X(96)00045-8
471
Minton G. Van Bressem M. F. Willson A. Collins T. Harthy S. Willson M. (2020). Visual Health Assessment and Evaluation of Anthropogenic Threats to Arabian Sea Humpback Whales in Oman. Annual IWC Scientific Committee 2020 (Cambridge, UK). [In press]
472
Minussi Rama A. C. (2020). Prevalência, Intensidade E Distribuição De Xenobalanus globicipitis (Cirripedia: Coronulidae) Em Cetáceos Na Bacia De Santos, Brasil, Florianópolis: Universidade Federal de Santa Catarina [Bachelor Thesis].
473
Miočić-Stošić J. Pleslić G. Holcer D. (2020). Sea Lamprey (Petromyzon Marinus) Attachment to the Common Bottlenose Dolphin (Tursiops Truncatus). Aquat. Mamm.46 (2), 152–166. doi: 10.1578/AM.46.2.2020.152
474
Mizue K. (1950). Factory Ship Whaling Around Bonin Islands in 1948. Sci. Rep. Whales Res. Inst. Tokyo3, 106–118.
475
Mizue K. Murata T. (1951). Biological Investigation on the Whales Caught by the Japanese Antarctic Whaling Fleets Season 1949–50. Sci. Rep. Whales Res. Inst. Tokyo6, 73–131.
476
Moazzam M. Rizvi S. H. N. (1979). Some Observations of the Post-Cyprid Stages and Population Structure of Conchoderma Virgatum Var. Hunteri From Offshore Waters of Pakistan. Pakistan J. Sci. Ind. Res.22, 305–307.
477
Moher D. Shamseer L. Clarke M. Ghersi D. Liberati A. Petticrew M. et al . (2015). Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev.4, 1–9. doi: 10.1186/2046-4053-4-1
478
Mohrbeck I. Raupach M. J. Martinez Arbizu P. Knebelsberger T. Laakmann S. (2015). High-Throughput Sequencing-The Key to Rapid Biodiversity Assessment of Marine Metazoa? PloS One10 (10), E0140342. doi: 10.1371/journal.pone.0140342
479
Monod T. (1938). Conchoderma auritum (L. 1767) Olfers 1814 Sur Un Ziphius Cf. Cavirostris (?) G. Cuvier 1823. Bull. Trav. Sta. Aquicult. Pêche Castiglione1, 205–210. [In French].
480
Monod T. H. Serene R. (1976). Parasitic, Commensal, and Inquiline Crustaceans Collected During the Rumphius Expedition II. Osean. Indones.6, 23–27.
481
Moore M. Steiner L. Jann B. (2003). Cetacean Surveys in the Cape Verde Islands and the Use of Cookiecutter Shark Bite Lesions as a Population Marker for Fin Whales. Aquat. Mamm.29 (3), 383–389. doi: 10.1578/01675420360736569
482
Mörch J. A. (1911). On the Natural History of Whalebone Whales. Proc. Zool. Soc Lond.47, 661–670. doi: 10.1111/j.1096-3642.1911.tb01952.x
483
Moreno-Colom P. Ten S. Raga J. A. Aznar F. J. (2020). Spatial Distribution and Aggregation of Xenobalanus globicipitis on the Flukes of Striped Dolphins, Stenella Coeruleoalba: An Indicator of Host Hydrodynamics? Mar. Mamm. Sci.36 (3), 897–914. doi: 10.1111/mms.12691
484
Morris R. A. Mowbray L. S. (1966). An Unusual Barnacle Attachment on the Teeth of the Hawaiian Spinning Dolphin. Nor. Hvalfangst-lid. (Norw. Whaling Gaz.)55, 15–16.
485
Moyse J. (1984). Some Observations on the Swimming and Feeding of the Nauplius Larvae of Lepas Pectinata (Cirripedia: Crustacea). Zool. J. Linn. Soc80 (2-3), 323–336. doi: 10.1111/j.1096-3642.1984.tb01981.x
486
Moyse J. (1987). Larvae of lepadomorph barnacles. Barnacle BiologySouthwardA. J.BalkemaA. A.(Rotterdam: Crustacean Issues), 329–362.
487
Mullineaux L. Butman C. (1991). Initial Contact, Exploration and Attachment of Barnacle (Balanus Amphitrite) Cyprids Settling in Flow. Mar. Biol.110 (1), 93–103. doi: 10.1007/BF01313096
488
Murase M. Tajima Y. Okamoto M. Matsuishi T. Yamada T. K. Asakawa M. (2014). An Ectoparasite and Epizoite From a Western Gray Whale (Eschrichtius Robustus) Stranded on Tomakomai, Hokkaido, Japan. J. Rakuno Gakuen Univ.38 (2), 149–152.
489
Nagasawa K. Imai Y. Ishida K. (1985). Distribution, Abundance, and Effects of Pennella sp. (Copepoda: Pennellidae), Parasitic on the Saury, Cololabis Saira (Brevoort), in the Western North Pacific Ocean and Adjacent Seas, 1984. Bull. Biogeogr. Soc. Japan40(5), 35–42.
490
Nakano H. Tabuchi M. (1990). Occurrence of the Cookiecutter Shark Isistius Brasiliensis in Surface Waters of the North Pacific Ocean. Japanese J. Ichthyol.37 (1), 60–63.
491
Nansen F. (1925). Hunting and Adventure in the Arctic (London: J. M. Dent & Sons).
492
Nasu K. (1958). Deformed Lower Jaw of Sperm Whale. Sci. Rep. Whales Res. Inst. Tokyo13, 211–212.
493
Natural History Museum (2020) Natural History Museum (London) Collection Specimens. Available at: https://www.gbif.org/occurrence/1056411821 (Accessed April 2, 2021).
494
Nemoto T. (1955). White Scars on Whales (I) Lamprey Marks. Sci. Rep. Whales Res. Inst.10, 69–77.
495
Nemoto T. (1958). Cocconeis Diatoms Infected on Whales in the Antarctic. Sci. Rep. Whales Res. Inst. Tokyo13, 185–192.
496
Nemoto T. Brownell F. L. Jr. Isfiimaru T. (1977). Cocconeis Diatoms on the Skin of Franciscana. Sci. Rep. Whales Res. Inst.29, 101–105.
497
Newman W. A. Abbott D. P. (1980). “Cirripedia: The Barnacles” in Intertidal Invertebrates of California. Eds. MorrisR. H.AbbottD. P.HaderlieE. C. (Palo Alto, CA: Stanford University Press), 504–535.
498
Nelson E. w. . On the Occurrence of Diatoms on the Skin of Whales by A. G. Bennett. Appendix by E. W. Nelson. (Cocconeis Ceticola). Proc. Royal Soc. B (1920) 91(641), 352–357.
499
Newman W. A. Ross A. (1971). Antarctic Cirripedia. Antarctic Res. Ser.14, 1–257. doi: 10.1029/AR014
500
Newman W. A. Ross A. (1976). Revision of the Balanomorph Barnacles Including a Catalogue of the Species. San Diego Soc Nat. Hist.9, 1–108.
501
Nichols O. C. Hamilton P. K. (2004). Occurrence of the Parasitic Sea Lamprey, Petromyzon Marinus, on Western North Atlantic Right Whales, Eubalaena Glacialis. Env. Biol. Fish.71, 413–417. doi: 10.1007/s10641-004-0776-5
502
Nichols O. C. Tscherter U. T. (2011). Feeding of Sea Lampreys Petromyzon Marinus on Minke Whales Balaenoptera Acutorostrata in the St Lawrence Estuary, Canada. J. Fish Biol.78 (1), 338–343. doi: 10.1111/j.1095-8649.2010.02842.x
503
Nicklin C. (1963). Whale Tale. Skin Diver Magazine.12 (11), 12–15. doi: 10.1080/05775132.1963.11469513
504
Nilsson-Cantell C. A. (1921). Cirripeden-Studien. Zur Kenntnis Der Biologie, Anatomie Und Systematik Dieser Gruppe. Zoologiska Bidrag Fra˚n Uppsala7, 75.395. doi: 10.5962/bhl.title.10682
505
Nilsson-Cantell C. A. (1928). Studies on Cirripeds in the British Museum (Natural History). Ann. Mag. Nat. Hist.10 (2), 1–39. doi: 10.1080/00222932808672845
506
Nilsson-Cantell C. A. (1930a). Thoracic Cirripedes Collected in 1925–1927. Discovery Rep.2, 223–260.
507
Nilsson-Cantell C. A. (1930b). Cirripédes. Résultats Scientifiques Du Voyage Aux Indes Orientales Néerlandaise De LL. AA. RR. Le Prince Et La Princesse Léopold De Belgique. Mémoires du Musée R. d’Histoire Naturelle Belgique3, 1–24.
508
Nilsson-Cantell C. A. (1930c). Cirripedien Von Der Stewart Insel Und Von Südgeorgien. Senckenbergiana12, 210–213.
509
Nilsson-Cantell C. A. (1931). Revision Der Sammlung Recenter Cirripedien Des Naturhistorischen Museums in Basel. Verhandl. Naturf Gesell Basel42, 103–137.
510
Nilsson-Cantell C. A. (1939). Thoracic Cirripedes Collected in 1925–1936. Discovery Rep.18, 1925–1936.
511
Nilsson-Cantell C. A. (1978). Cirripedia Thoracica and Acrothoracica Vol. 5 (Oslo: Marine Invertebrates of Scandinavia, Universitetsforlaget), 1–133.
512
Nishiwaki M. (1959). Humpback Whales in Ryukyuan Waters. Sci. Rep. Whales Res. Inst.14, 49–87.
513
Nishiwaki M. Hayashi K. (1950). Biological Survey of Fin and Blue Whales Taken in the Antarctic Season 1947–48 by the Japanese Fleet. Sci. Rep. Whales Res. Inst. Tokyo3, 132–190.
514
Nishiwaki M. Oye T. (1951). Biological Investigation on Blue Whales (Balaenoptera Musculus) and Fin Whales (Balaenoptera Physalus) Caught by the Japanese Antarctic Whaling Fleets. Sci. Rep. Whales Res. Inst.5, 91–167.
515
Nogata Y. Matsumura K. (2006). Larval Development and Settlement of a Whale Barnacle. Biol. Lett.2, 92–93. doi: 10.1098/rsbl.2005.0409
516
Noke W. (2004). The Association of Echeneids With Bottlenose Dolphins (Tursiops Truncatus) in the Indian River Lagoon, Florida, USA. Aquat. Mamm.30 (2), 296–298. doi: 10.1578/AM.30.2.2004.296
517
Notarbartolo di Sciara G. Watkins W. A. (1979). A Remora, Remilegia Australis, Attached to an Atlantic Spinner Dolphin, Stenella Longirostris. Bull. S. California Acad. Sci.79 (3), 119–121.
518
O'Clair R. M. O'Clair C. E. (1998). Southeast Alaska's Rocky Shores. Animals (Auke Bay, AK: Plant Press).
519
O’Connor B. Franco J. M. F. (2003). Conchoderma auritum (L.) (Cirripedia) Recorded From a Sperm Whale Physeter Catodon L. Washed Up at Claddaghduff, Co Galway. Ir. Nat. J.27 (6), 236–236.
520
Ogawa K. Matsuzaki K. Misaki H. (1997). A New Species of Balaenophilus (Copepoda: Harpacticoida), an Ectoparasite of a Sea Turtle in Japan. Zool. Sci.14 (4), 691–700. doi: 10.2108/zsj.14.691
521
Ohlin A. (1893). Some Remarks on the Bottlenose-Whale (Hyperoodon). Lunds. Univ. Arskr.29, 1–14.
522
Ohno M. Fujino K. (1952). Biological Investigation on the Whales Caught by the Japanese Antarctic Whaling Fleets, Season 1950/51. Sci. Rep. Whales Res. Inst.7, 125–188.
523
Ohsumi S. (1973). Find of Marlin Spear From the Antarctic Minke Whales. Sci. Rep. Whales Res. Inst. Tokyo25, 237–239.
524
Ohsumi S. Masaki Y. Kawamura A. (1970). Stock of the Antarctic Minke Whale. Sci. Rep. Whales Res. Inst.22, 75–125.
525
Øines Ø. Heuch P. A. (2005). Identification of Sea Louse Species of the Genus Caligus Using mtDNA. J. Mar. Biolog. Assoc. U.K.85, 73–79. doi: 10.1017/S0025315405010854h
526
Øines Ø. Schram T. (2008). Intra- or Inter-Specific Difference in Genotypes of Caligus elongatus Nordmann 1832? Acta Parasitol.53 (1), 93–105. doi: 10.2478/s11686-008-0002-2
527
Ólafsson E. Ingólfsson R. Steinarsdóttir M. B. . Harpacticoid Copepod Communities of Floating Seaweed: Controlling Factors and Implications for Dispersal. Hydrobiologia (2001) 453(1), 189–200.
528
Ólafsdóttir D. Shinn A. (2013). Epibiotic Macrofauna on Common Minke Whales, Balaenoptera Acutorostrata Lácepede 1804, in Icelandic Waters. Parasites Vectors6 (1), 105. doi: 10.1186/1756-3305-6-105
529
Ólafsson E. Ingólfsson A. Steinarsdóttir M.B. (2001). Harpacticoid copepod communities of floating seaweed: controlling factors and implications for dispersal. Hydrobiologia453, 189–200. doi: 10.1023/A:1013196724039
530
Oliveira J. B. Morales J. A. González-Barrientos R. C. Hernández-Gamboa J. Hernández-Mora G. (2011). Parasites of Cetaceans Stranded on the Pacific Coast of Costa Rica. Vet. Parasitol.182 (2-4), 319–328. doi: 10.1016/j.vetpar.2011.05.014
531
Oliver G. Trilles J. P. (2000). Crustacés Parasites Et Epizoïtes Du Cachalot, Physeter Catodon Linnaeus 1758 (Cetacea, Odontoceti), Dans Le Golfe Du Lion (Méditerranée Occidentale). Parasite7 (4), 311–321. doi: 10.1051/parasite/2000074311
532
Olsen O. (1913). On the External Characters and Biology of Bryde’s Whales (Balaenoptera Brydei), a New Rorqual From the Coast of South Africa. Proc. Zool. Soc Lond., 1073–1090. doi: 10.1111/j.1096-3642.1913.tb02005.x
533
Omura H. (1950a). Whales in the Adjacent Waters of Japan. Sci. Rep. Whales Res. Inst. Tokyo4, 27–113.
534
Omura H. (1950b). Diatom Infection on Blue and Fin Whales in the Antarctic Whaling Area V (the Ross Sea Area). Sci. Rep. Whales Res. Inst.4, 14–26.
535
Omura H. (1958). North Pacific Right Whale. Sci. Rep. Whales Res. Inst.13, 1–52.
536
Omura H. Fujino K. Kimura S. (1955). Beaked Whale Berardius Bairdii of Japan With Notes on Ziphius cavirostris. Sci. Rep. Whales Res. Inst. Tokyo10, 89–132.
537
Orams M. B. Schuetze C. (1998). Seasonal and Age/Size-Related Occurrence of a Barnacle (Xenobalanus globicipitis) on Bottlenose Dolphins (Tursiops Truncatus). Mar. Mamm. Sci.14, 186–189. doi: 10.1111/j.1748-7692.1998.tb00706.x
538
O’Reilly M. (1998). Whale-Lice (Amphipoda: Cyamidae) and Sea Lice (Copepoda: Caligidae) From Stranded Whales in the Firth of Forth. Glasg. Nat.23 (3), 24–26.
539
Orejas C. Rossi S. Peralba À. García E. Gili J. M. Lippert H. (2012). Feeding Ecology and Trophic Impact of the Hydroid Obelia Dichotoma in the Kongsfjorden (Spitsbergen, Arctic). Polar Biol.36 (1), 61–72. doi: 10.1007/s00300-012-1239-7
540
O’Riordan C. E. (1979). Marine Fauna Notes From the National Museum of Ireland 6. Ir. Nat.' J.19, 356–358.
541
Orrell T. (2020) NMNH Extant Specimen Records. Version 1.36 (National Museum of Natural History, Smithsonian Institution). Available at: https://www.gbif.org/occurrence/1320470940 (Accessed June 23, 2021).
542
Osmond M. G. Kaufman G. D. (1998). A Heavily Parasitized Humpback Whale (Megaptera novaeangliae). Mar. Mamm. Sci.14 (1), 146–149. doi: 10.1111/j.1748-7692.1998.tb00698.x
543
Otani M. Oumi T. Uwai S. Hanyuda T. Prabowo R. E. Yamaguchi T. et al . (2007). Occurrence and Diversity of Barnacles on International Ships Visiting Osaka Bay, Japan, and the Risk of Their Introduction. Biofouling23 (4), 277–286. doi: 10.1080/08927010701315089
544
O’Toole B. (2002). Phylogeny of the Species of the Superfamily Echeneoidea (Perciformes: Carangoidei: Echeneidae, Rachycentridae, and Coryphaenidae), With an Interpretation of Echeneid Hitchhiking Behaviour. Can. J. Zool.80 (4), 596–623. doi: 10.1139/z02-031
545
Öztürk A. A. Dede A. Tonay A. M. Danyer E. Aytemiz I. (2015). Stranding of a Minke Whale on the Eastern Mediterranean Coast of Turkey. J. Black Sea/Mediter. Envir.21 (2), 232–237.
546
Pace D. S. Mussi B. Miragliuolo A. Vivaldi C. Ardizzone G. (2016). First Record of a Hagfish Anchored to a Living Bottlenose Dolphin in the Mediterranean Sea. J. Mammal.97 (3), 960–965. doi: 10.1093/jmammal/gyw022
547
Pacheco A. S. Castro C. Carnero-Hauman R. Villagra D. Pinilla S. Denkinger J. et al . (2019). Sightings of an Adult Male Killer Whale Match Humpback Whale Breeding Seasons in Both Hemispheres in the Eastern Tropical Pacific. Aquat. Mamm.45, 320–326. doi: 10.1578/AM.45.3.2019.320
548
Page M. J. McKenzie J. E. Bossuyt P. M. Boutron I. Hoffmann T. C. Mulrow C. D. et al . (2021). The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ372, n71. doi: 10.1136/bmj.n71
549
Palacios D. M. Salazar S. K. Day D. (2004). Cetacean Remains and Strandings in the Galapagos Islands 1923-2003. Lat. Am. J. Aquat. Mamm.3(2), 127–150. doi: 10.5597/lajam00058
550
Palmer M. A. (1988). Dispersal of Marine Meiofauna: A Review and Conceptual Model Explaining Passive Transport and Active Emergence With Implications for Recruitment. Mar. Ecol. Prog. Series48 (1), 81–91. doi: 10.3354/meps048081
551
Papastamatiou Y. P. Wetherbee B. M. O’Sullivan J. Goodmanlowe G. D. Lowe C. G. (2010). Foraging Ecology of Cookiecutter Sharks (Isistius Brasiliensis) on Pelagic Fishes in Hawaii, Inferred From Prey Bite Wounds. Env. Biol. Fish.88 (4), 361–368. doi: 10.1007/s10641-010-9649-2
552
Parsons E. C. M. Overstreet R. M. Jefferson T. A. (2001). Parasites From Indo-Pacific Hump-Backed Dolphins (Sousa Chinensis) and Finless Porpoises (Neophocaena Phocaenoides) Stranded in Hong Kong. Vet. Rec.148, 776–780. doi: 10.1136/vr.148.25.776
553
Pastene L. A. Jofre K. Acevedo M. Joyce M. (1990). First Record of the Blainville's Beaked Whale, Mesoplodon densirostris Blainville 1817 (Cetacea: Ziphiidae) in the Eastern Pacific. Mar. Mamm. Sci.6, 82–84. doi: 10.1111/j.1748-7692.1990.tb00229.x
554
Pastorino G. Griffin M. (1996). An Extant Whale Barnacle (Cirripedia, Coronulidae) From Holocene Deposits of Buenos Aires (Argentina). Crustaceana69 (6), 769–772. doi: 10.1163/156854096X00781
555
Patel B. (1959). The Influence of Temperature on the Reproduction and Moulting of Lepas Anatifera L. Under Laboratory Condition. J. Mar. Biolog. Assoc. U.K.38, 589–597. doi: 10.1017/S0025315400007013
556
Paterson R. A. Van Dyck S. (1991). Studies of Two Humpback Whales, Megaptera novaeangliae, Stranded at Fraser Island, Queensland. Mem. Queensl. Mus.30, 343–350.
557
Pearson R. M. van de Merwe J. P. Connolly R. M. (2020). Global Oxygen Isoscapes for Barnacle Shells: Application for Tracing Movement in Oceans. Sci. Total. Environ.705, 135782. doi: 10.1016/j.scitotenv.2019.135782
558
Penney M. S. Rawlings T. A. (2021). An Examination of Shallow-Water Hydroids (Cnidaria, Hydrozoa, Hydroidolina) in Cape Breton, Nova Scotia, Using Morphology and DNA Barcoding. Northeastern Nat.28 (m18), 1–38. doi: 10.1656/045.028.m1801
559
Pérez-Losada M. Høeg J. T. Crandall K. A. (2004). Unraveling the Evolutionary Radiation of the Thoracican Barnacles Using Molecular and Morphological Evidence: A Comparison of Several Divergence Time Estimation Approaches. Syst. Biol.53 (2), 244–264. doi: 10.1080/10635150490423458
560
Pérez-Losada M. Høeg J. Simon-Blecher N. Achituv Y. Jones D. Crandall K. (2014). Molecular Phylogeny, Systematics and Morphological Evolution of the Acorn Barnacles (Thoracica: Sessilia: Balanomorpha). Mol. Phylogenet. Evol.81, 147–158. doi: 10.1016/j.ympev.2014.09.013
561
Pérez-Losada M. Harp M. Høeg J. T. Achituv Y. Jones D. Watanabe H. et al . (2008). The Tempo and Mode of Barnacle Evolution. Mol. Phylogenet. Evol.46 (1), 328–346. doi: 10.1016/j.ympev.2007.10.004
562
Pérez-Zayas J. Mignucci-Giannoni A. Toyos-González G. Rosario-Delestre R. Williams E. (2002). Incidental Predation by a Largetooth Cookiecutter Shark on a Cuvier’s Beaked Whale in Puerto Rico. Aquat. Mamm.28, 308–311.
563
Perrin W. (1969). The Barnacle, Conchoderma auritum, on a Porpoise (Stenella graffmani). J. Mammal.50(1), 149. doi: 10.2307/1378651
564
Peters I. T. Barendse J. (2016). “A Conservation Assessment of Eubalaena Australis,” in The Red List of Mammals of South Africa, Swaziland and Lesotho. Eds. ChildM. F.RoxburghL.Do LinhS. E.RaimondoD.Davies-MostertH. T. (South Africa: South African National Biodiversity Institute and Endangered Wildlife Trust).
565
Pettis H. M. Rolland R. M. Hamilton P. K. Brault S. Knowlton A. R. Kraus S. D. (2004). Visual Health Assessment of North Atlantic Right Whales (Eubalaena Glacialis) Using Photographs. Can. J. Zool.82 (1), 8–19. doi: 10.1139/z03-207
566
Pfeiffer C. J. (2009). ““Whale Lice”,” in Encyclopedia of Marine Mammals. Eds. PerrinW. F.WürsigB.ThewissenJ. G. M. (San Diego:Academic Press), 1220–1223. doi: 10.1016/B978-0-12-373553-9.00279-0
567
Pike G. C. (1951). Lamprey Marks on Whales. J. Fish. Res. Board Can.8, 275–280. doi: 10.1139/f50-017
568
Pilleri G. (1967). Behaviour of the Pseudorca Crassidens (Owen) Off the Spanish Mediterranean Coasts. Rev. Suisse Zoologie74, 679–683. doi: 10.5962/bhl.part.75874
569
Pilleri G. (1969a). The Barnacle, Conchoderma auritum, on a Porpoise (Stenella Graffmani). J. Mammal.50 (1), 149–151. doi: 10.2307/1378651
570
Pilleri G. (1969b). Zahnbefall Durch Conchoderma auritum (Cirripedia) Bei Einem Pottwal Aus Den Natalgewässern. Invest. Cet.1, 192.
571
Pilleri G. (1970). Xenobalanus globicipitis Steenstrup on Delphinus Delphus, Stenella Styx, Tursiops Truncatus in the Western Mediterranean. Invest. Cet.2, 248–249.
572
Pilleri G. Gihr M. (1969). Zur Anatomie Und Pathologie Von Inia Geoffrensis De Blainville 1817 (Cetacea, Sussuidae) Aus Dem Beni, Bolivien. Invest. Cet.1, 96–106.
573
Pilleri G. Knuckey J. (1969). Behaviour Patterns of Some Delphinidae Observed in the Western Mediterranean. Z. Tierpsychol.26, 48–72.
574
Pilsbry H. A. (1916). The Sessile Barnacles (Cirripedia) Contained in the Collections of the U.S. National Museum; Including a Monograph of the American Species. Bull. U.S. Natl. Mus.93, 1–366. doi: 10.5479/si.03629236.93.1
575
Pinedo M. C. Praderi R. Brownell R. L. (1989). “Review of the Biology and Status of the Franciscana, Pontoporia Blainvillei,” in Biology and Conservation of the River Dolphins, vol. No. 3 . Eds. PerrinW. F.BrownellR. L.ZhouK.LiuJ. (Wuhan: Occasional Papers of the International Union for Conservation of Nature Species Survival Commission), 46–51.
576
Pitman R. L. Fearnbach H. LeDuc R. Gilpatrick J. W. J. Ford J. K. B. Ballance L. T. (2007). Killer Whales Preying on a Blue Whale Calf on the Costa Rica Dome: Genetics, Morphometrics, Vocalisations and Composition of the Group. J. Cetacean Res. Manage.9, 151–157.
577
Pitombo F. B. (2004). Phylogenetic Analysis of the Balanidae (Cirripedia, Balanomorpha). Zool. Scr.33 (3), 261–276. doi: 10.1111/j.0300-3256.2004.00145.x
578
Plön S. Adam P. A. Andrianarivelo N. Braulik G. Bunbury N. Collins T. et al . (2020). “IndoCet Stranding Network-Report on Strandings for the Western Indian Ocean Region,” in International Whaling Commission. Report of the Scientific Committee Meeting.
579
Pompa S. Ehrlich P. R. Ceballos G. (2011). Global Distribution and Conservation of Marine Mammals. Proc. Nat. Acad. Sci.108 (33), 13600–13605. doi: 10.1073/pnas.1101525108
580
Pope E. C. (1958). The Barnacle, Xenobalanus globicipitis Steenstrup in Australian Seas. Proc. R. Zool. Soc1956–57, 159–161.
581
Porsild M. P. (1922). Scattered Observations on Narwhals. J. Mammal.41, 8–13. doi: 10.2307/1373444
582
Pouchet G. (1888). Sur Un Nouveau Cyamus (Physeteris) Parasite Du Cachalot. Compt. Rend. Acad. Sei. Paris107 (18), 698–699.
583
Pouchet G. (1892). Contribution Ir I'histoire Des Cyames. J. Anat. Physiol. Norm. Path.28, 99–108.
584
Pouchet G. Beauregard H. (1889). Recherches Sur Le Cachalot: Anatomie I-V. Nouv. Arch. Mus. Hist. Nat.3, 1–96.
585
Prestridge H. (2016) Biodiversity Research and Teaching Collections - TCWC Marine Invertebrates. Available at: https://www.gbif.org/occurrence/1234577280 (Accessed March 2, 2021).
586
Pruski S. Miglietta M. P. (2019). Fluctuation and Diversity of Hydromedusae (Hydrozoa, Cnidaria) in a Highly Productive Region of the Gulf of Mexico Inferred From High Frequency Plankton Sampling. Peer J.7, e7848. doi: 10.7717/peerj.7848
587
Pugliese M. C. Boettger S. A. Fish F. E. (2012). Barnacle Bonding: Morphology of Attachment of Xenobalanus globicipitis to its Host Tursiops Truncatus. J. Morphol.273 (4), 453–459. doi: 10.1002/jmor.20006
588
Qiao Y. Ma X. Chen B. Zhong S. Chen X. (2020). First Report of Cyamus Boopis From a Humpback Whale (Megaptera novaeangliae) in the Coastal East China Sea. Res. Sq. doi: 10.21203/rs.3.rs-44880/v1 [Preprint]
589
Quidor A. (1912). Copépodes Parasites. Deuxieme Expedition Antarctique Francaise, (1908-1910) (Paris: Scientifics Naturals, Documents Scientifiques), 197–214.
590
Radford K. W. Klawe W. L. (1965). Biological Observations on the Whale Sucker, Remilegia Australis-Echeneiformes: Echeneidae. Trans. San Diego Nat. Hist. Soc14 (6), 65–72.
591
Raga J. A. (1988). On Some Morphological Variations of Syncyamus Aequus Lincoln & Hurley 1981 (Amphipoda, Cyamidae) From the Mediterranean Sea. Crustaceana60(1), 149–152. doi: 10.1163/156854088X00050
592
Raga J. A. (1994). “Parasitismus Bei Den Cetacea,” in Handbuch Der Säugetiere Europas. Eds. RobineauD.DuguyR.KlimaM. (Wiesbaden: AULA-Verlag), 132–179.
593
Raga J. A. Balbuena J. A. (1993). Parasites of the Long-Finned Pilot Whale, Globicephala melas (Traill 1809), in European Waters. Rep. Int. Whal. Commn.14, 391–406.
594
Raga J. A. Balbuena J. A. Abril E. (1988). “Preliminary Report on Parasitological Research on Pilot Whales in the Faroe Islands,” in European Research on Cetaceans. Ed. EvansP. G. H. (Lisboa: European Cetacean Society), 65–69.
595
Raga J. A. Carbonell E. (1985). New Dates About Parasites on Stenella Coeruleoalba (Meyen 1833) (Cetacea: Delphinidae) in the Western Mediterranean Sea. Invest. Cet.17, 207–213.
596
Raga J. A. Carbonell E. Raduan M. A. (1982). Incidencias De Parásitos En Los Cetáceos Varados En Las Costas Españolas Del Mediterráneo. Memórias do Museu do Mar, Vol. 2. 1–11.
597
Raga J. A. Raduan M. A. (1982). First Record of Syncyamus Aequus Lincoln and Hurley 1981 (Amphipoda: Cyamidae) in the Mediterranean Sea. Invest. Cet.14, 22–23.
598
Raga J. A. Raduan A. Blanco C. (1983a). Sobre La Presencia De Isocyamus delphinii (Guerin-Meneville 1836) (Amphipoda: Cyamidae) En Aguas Del Mediterráneo Español. Actas del I Congreso Ibérico de Entomologia, Facultad de Biología. León1 (2), 627–630.
599
Raga J. A. Raduan M. A. Blanco C. Carbonell E. (1983b). Étude Parasitologique Du Dauphin Bleu Et Blanc Stenella Coeruleoalba Dans La Mediterranée Occidentale Vol. 28 (Monaco:Rapport P.-V Réunions—Commission Internationale pour l’Exploration Scientifique de la Mer Mediterranée), 211–212.
600
Raga J. A. Sanpera C. (1986). Ectoparásitos Y Epizoítos De Balaenoptera Physalus (L. 1758) En Aguas Atlánticas Ibéricas. Inv. Pesq.50, 489–498.
601
Rajaguru A. Shantha G. (1992). Association Between the Sessile Barnacle Xenobalanus globicipitis (Coronulidae) and the Bottlenose Dolphin Tursiops Truncatus (Delphinidae) From the Bay of Bengal, India, With a Summary of Previous Records From Cetaceans. Fish. Bull.90 (1), 197–202.
602
Rappé G. (1985). Isocyamus delphinii (Guérin 1836), Eerste Vondst Van Een Walvisluis (Amphipoda, Cyamidae) Aan Onze Kust. Strandvlo5 (3), 63–65.
603
Rappé G. (1988). Een Vondst Van Xenobalanus globicipitis in De Noordzee? Strandvlo8 (2), 100–101.
604
Rappé G. (1991). “Isocyamus delphinii (Crustacea, Amphipoda, Cyamidae), a Possible Biological Indicator in the North Sea,” in European Research on Cetaceans. Eds. EvansP. G. H.AguilarA.SmeenkC. (Palma de Mallorca: Proceedings of the fourth annual conference of the European Cetacean Society), 121–122.
605
Rappé G. Van Waerebeek K. (1988). “Xenobalanus globicipitis (Crustacea: Cirripedia) on Cetaceans in the Northeast Atlantic and the Mediterranean: A Review,” in European Research on Cetaceans. Ed. EvansP. G. H. (Lisboa: European Cetacean Society), 75–78.
606
Rasmussen T. (1980). Notes on the Biology of the Shipfouling Gooseneck Barnacle Conchoderma auritum Linnaeus 1776 (Cirripedia; Lepadomorpha). Biol. Mar.2, 37–44.
607
Raupach M. J. Barco A. Steinke D. Beermann J. Laakmann S. Mohrbeck I. et al . (2015). The Application of DNA Barcodes for the Identification of Marine Crustaceans From the North Sea and Adjacent Regions. PloS One10 (9), E0139421. doi: 10.1371/journal.pone.0139421
608
Rech S. Borrell Pichs Y. J. Garcia-Vazquez E. (2018). Anthropogenic Marine Litter Composition in Coastal Areas may be a Predictor of Potentially Invasive Rafting Fauna. PloS One13 (1), e0191859. doi: 10.1371/journal.pone.0191859
609
Reddacliff G. (1988). “Crater Wounds in Marine Mammals” in Marine Mammals of Australasia: Field Biology and Captive Management, ed. AugeeM. L.(Sydney: Royal Society of New South Wales), 133–134. doi: 10.7882/RZSNSW.1988.008
610
Reeb D. Best P. B. Kidson S. H. (2007). Structure of the Integument of Southern Right Whales, Eubalaena Australis. Anatom. Rec.290, 596–613. doi: 10.1002/ar.20535
611
Rees G. (1953). A Record of Some Parasitic Worms From Whales in the Ross Sea Area. Parasitology43, 27–34. doi: 10.1017/S003118200001831X
612
Renaud C. B. (2011). Lampreys of the World: An Annotated and Illustrated Catalogue of Lamprey Species Known to Date (Rome: Species Catalogue for Fishery Purposes, FAO).
613
Renaud C. B. (2019). “Family Petromyzontidae–Lampreys, Lamproies,” in Marine Fishes of Arctic CanadaCoadB. W.ReistJ. D. (Toronto/Buffalo/London: University of Toronto Press), 164–167. doi: 10.3138/9781442667297-023
614
Renner M. Bell K. (2009). A White Killer Whale in the Central Aleutians. ARCTIC61 (1), 102. doi: 10.14430/arctic10
615
Resendes A. R. Juan-Sallés C. Almeria S. Majó N. Domingo M. Dubey J. P. (2002). Hepatic Sarcocystosis in a Striped Dolphin (Stenella Coeruleoalba) From the Spanish Mediterranean Coast. J. Parasitol.88 (1), 206–209. doi: 10.1645/0022-3395(2002)088[0206:HSIASD]2.0.CO;2
616
Reyes J. C. Van Waerebeek K. (1995). Aspects of the Biology of Burmeister’s Porpoise From Peru. Rep. Int. Whal. Commn.16, 349–364.
617
Ribeiro F. B. Carvalho V. L. Bevilaqua C. M. L. Bezerra L. E. A. (2010). First Record of Xenobalanus globicipitis (Cirripedia: Coronulidae) on Stenella Coeruleoalba (Cetacea: Delphinidae) in the Oligotrophic Waters of North-Eastern Brazil. Mar. Biodivers. Rec.3, 1–5. doi: 10.1017/S1755267209991151
618
Rice D. W. (1963). Progress Report on Biological Studies of the Larger Cetacea in the Waters Off California. Norsk Hvalfangst-Tid.52 (7), 181–187.
619
Rice D. (1977). Synopsis of Biological Data on the Sei Whale and Bryde’s Whale in the Eastern North Pacific. Rep. Int. Whal. Commn.1, 333–336.
620
Rice D. W. (1978). “Blue Whale,” in Marine Mammals of Eastern North Pacific and Arctic Waters. Ed. HaleyD. (Washington: Pacific Search Press), 30–35.
621
Rice D. W. Caldwell D. K. (1961). Observations on the Habits of the Whale Sucker (Remilegia Australis). Norsk Hvalfangsttid.5, 181–189.
622
Rice D. W. Wolman A. A. (1971). The Life History and Ecology of the Gray Whale (Eschrichtius Robustus). Am. Soc. Mammal.3, 142 pp. doi: 10.5962/bhl.title.39537
623
Richard J. (1936). Résultats Des Campagnes Scientifiques Accomplies Sur Son Yacht Par Albert 1er Prince Souverain De Monaco. Fascicule XCIV94, 34–71.
624
Richard J. Neuville H. (1897). Sur Quelques Cétacés Observés Pendant Les Campagnes Du Yacht Princesse Alice Vol. 10 (Paris: Memoires de la Societeí Zoologique de France), 100–109.
625
Ridgway S. H. Linder E. Mahoney K. A. Newman W. A. (1997). Grey Whale Barnacles Cryptolepas Rhachianecti Infest White Whales, Delphinapterus Leucas, Housed in San Diego Bay. Bull. Mar. Sci.61, 377–385.
626
Risch D. Norris T. Curnock M. Friedlaender A. (2019). Common and Antarctic Minke Whales: Conservation Status and Future Research Directions. Front. Mar. Sci.6. doi: 10.3389/fmars.2019.00247
627
Rittmaster K. A. Bowles N. L. Mallon-Day S. G. R. B. Odell D. K. (1999). “Xenobalanus Barnacles on Tursiops Dorsal Fins in Beaufort, North Carolina,” in Wailea: 13th Biennial Conference on the Biology of Marine Mammals, 158 pp.
628
Rodríguez-López M. A. Mignucci-Giannoni A. A. (1999). A Stranded Pygmy Killer Whale (Feresa Attenuata) in Puerto Rico. Aquat. Mamm.25 (2), 119–121.
629
Roest A. I. (1970). Kogia Simus and Other Cetaceans From San Luis Obispo County, California. J. Mammal.51 (2), 410–417. doi: 10.2307/1378507
630
Román-Reyes J. C. Ortega-García S. Galván-Magaña F. Grano-Maldonado M. I. (2019). First Record of Pennella Filosa L. (Copepoda, Siphonostomatoida, Pennellidae) Parasitising the Yellowfin Tuna Thunnus Albacares (Bonnaterre 1788) From the Mexican Pacific Coast. Neotrop. Helminthol.13 (1), 109–114. doi: 10.24039/rnh2019131628
631
Ronje E. I. Whitehead H. R. Piwetz S. Mullin K. D. (2018). Field Summary for Common Bottlenose Dolphin Surveys on the Texas, Gulf of Mexico Ciast 2014-2016. NOAA Southeast Fisheries Science Center reference document PRBD‐2018‐02. Pascagoula: US Department of Commerce.
632
Rose M. Hamon M. (1953). A Propos De Pennella Varians Steenstrup Et Lütken 1861, Parasite Des Branchies De Céphalopodes. Bull. Soc Hist. Nat. Afrique N.44, 172–183.
633
Ross G. J. B. (1984). The Smaller Cetaceans of the South East Coast of Southern Africa. Ann. Cape Prov. Mus. Nat. Hist.15, 173–410.
634
Ross G. J. Heinsohn G. E. Cockcroft V. G. (1994). “Humpback Dolphins Sousa Chinensis (Osbeck 1765), Sousa Plumbea (G. Cuvier 1829) and Sousa Teuszii (Kukenthal 1892),” in Handbook of Marine Mammals, vol. 5 . Eds. RidgwayS. H.HarrisonR. J. (London: Academic Press), 23–42.
635
Rosso M. Ballardini M. Moulins A. Würtz M. (2011). Natural Markings of Cuvier's Beaked Whale Ziphius cavirostris in the Mediterranean Sea. Afr. J. Mar. Sci.33 (1), 45–57. doi: 10.2989/1814232X.2011.572336
636
Rotstein D. S. Burdett L. G. McLellan W. Schwacke L. Rowles T. Terio K. A. et al . (2009). Lobomycosis in Offshore Bottle Nose Dolphins (Tursiops Truncatus), North Carolina. Emerging Infect. Dis.15, 588–590. doi: 10.3201/eid1504.081358
637
Roussel de Vauzème D. M. (1834). Mémoire Sur Le Cyamus Ceti (Latr.) De La Classe Des Crustacés. Ann. Sci. Nat. Zoo.1, 239–265. doi: 10.5962/bhl.part.25117
638
Rowntree V. (1983). Cyamids: The Louse That Moored. Whalewatcher. J. Am. Cetacean Soc17, 14–17.
639
Rowntree V. J. (1996). Feeding, Distribution, and Reproductive Behavior of Cyamids (Crustacea: Amphipoda) Living on Humpback and Right Whales. Can. J. Zool.74, 103–109. doi: 10.1139/z96-014
640
Sakai Y. Hayashi R. Murata K. Yamada T. Asakawa M. (2009). Records of Barnacle, Xenobalanus globicipitis Steenstrup 1851 and Whale Lice, Cyamus Sp. From a Wild Killer Whale Captured in the Western North Pacific, Off Kii Peninsula, Japan. Japanese J. Zoo Wildl. Med.14, 81–84. doi: 10.5686/jjzwm.14.81
641
Sakai M. Hishii T. Takeda S. Kohshima S. (2006). Flipper Rubbing Behaviors in Wild Bottlenose Dolphins (Tursiops Aduncus). Mar. Mamm. Sci.22, 966–978. doi: 10.1111/j.1748-7692.2006.00082.x
642
Samaras W. F. (1989). New Host Record for the Barnacle Cryptolepas rhachianecti Dall, 1872 (Balanomorpha: Coronulidae). Mar. Mamm. Sci.5, 84–87. doi: 10.1111/j.1748-7692.1989.tb00216.x
643
Samarra I. P. F. Fennell A. Deecke F. B. Miller J. O. (2012). Persistence of Skin Marks on Killer Whales (Orcinus Orca) Caused by the Parasitic Sea Lamprey (Petromyzon Marinus). Iceland. Mar. Mamm. Sci.28, 395–409. doi: 10.1111/j.1748-7692.2011.00486.x
644
Sanciangco M. D. Carpenter K. E. Betancur R. ,. R. (2016). Phylogenetic Placement of Enigmatic Percomorph Families (Teleostei: Percomorphaceae). Mol. Phylogenet. Evol.94 (PT B), 565–576. doi: 10.1016/j.ympev.2015.10.006
645
Santos M. C. Sazima I. (2005). The Sharksucker (Echeneis Naucrates) Attached to a Tucuxi Dolphin (Sotalia Guianensis) in Estuarine Waters in South-Eastern Brazil. JMBA2-Biodivers. Rec., 1, 1–3. doi: 10.1017/S1755267205000746.
646
Sars G. O. (1866). “Beskrivelse Af En Ved Lofoten Indbjerget Erhval (Balaenoptera Musculus)” Forhandlinger I Videnskabs-Selskabet I Christiana. 280.
647
Sars G. O. (1880). Fortsatte Bidrag Til Kundskaben Om Vore Bardehvaler. Finnhvalen. Og ,Knölhvalen (Kristiania: Videnskabsselskabs Selskab ), 12.
648
Sars (1890-1895). An Account of the Crustacea of Norway, With Short Descriptions and Figures of All SpeciesChristiania: A. Cammermeyer., Vol. 1. 3–711. doi: 10.5962/bhl.title.1164
649
Sars G. O. (1895). An Account of the Crustacea of Norway: Amphipoda Vol. 1 and 2 (Copenhagen: A. Cammermeyer).
650
Savage K. N. Burek-Huntington K. Wright S. K. Bryan A. L. Sheffield G. Webber M. et al . (2021). Stejneger's Beaked Whale Strandings in Alaska 1995–2020. Mar. Mamm. Sci.37, 843–869. doi: 10.1111/mms.12780
651
Sazima I. Grossman A. (2006). Turtle Riders: Remoras on Marine Turtles in Southwest Atlantic. Neotrop. Ichthyol.4 (1), 123–126. doi: 10.1590/S1679-62252006000100014
652
Scammon C. M. (1874). The Marine Mammals of the Northwestem Coast of North America (San Francisco: John M. Carmany).
653
Scarff J. E. (1986). Occurrence of the Barnacles Coronula Diadema, C. Reginae and Cetopirus Complanatus (Cirripedia) on Right Whales. Sci. Rep.Whales Res. Inst.37, 129–153.
654
Scharff R. F. (1913). The Whale-Fishery in Ireland. Ir. Nat. J.22 (8), 145–147.
655
Scheffer V. B. (1939). Organisms Collected From Whales in the Aleutian Islands. Murrelet20 (3), 67–69. doi: 10.2307/3533790
656
Schell D. Rowntree V. Pfeiffer C. J. (2000). Stable-Isotope and Electron-Microscopic Evidence That Cyamids (Crustacea: Amphipoda) Feed on Whale Skin. Can. J. Zool.78 (5), 721–727. doi: 10.1139/z99-249
657
Schiffer P. H. Herbig H. G. (2016). Endorsing Darwin: Global Biogeography of the Epipelagic Goose Barnacles Lepas Spp. (Cirripedia, Lepadomorpha) Proves Cryptic Speciation. Zool. J. Linn. Soc177 (3), 507–525. doi: 10.1111/zoj.12373
658
Schmidt G. D. Roberts L. S. (2009). “Parasitic Crustaceans” in Foundations of Parasitology. Eds. SchmidtG. D.RobertsL. S. (New York: McGraw-Hill), 537–559.
659
Schuchert P. (2021)World Hydrozoa Database. In: Obelia Dichotoma (Linnaeus 1758). Available at: https://www.marinespecies.org/aphia.php?p=taxdetails&id=117386 (Accessed September 28, 2021).
660
Schuurmans-Stekhoven J. H. van Oorde-de Lint G. (1936). “Copepoda Parasitica,” in Grimpe Wagler, Tierwelt Der Nord- Und Ostsee. Liefg, 31.
661
Scordino J. J. Gosho M. Gearin P. J. Akmajian A. Calambokidis J. Wright N. (2017). Individual Gray Whale Use of Coastal Waters Off Northwest Washington During the Feeding Season 1984–2011: Implications for Management. J. Cetacean Res. Manage.16, 57–69.
662
Sedlak-Weinstein E. (1991). Three New Records of Cyamids (Amphipoda) From Australian Cetaceans. Crustaceana60 (1), 90–104. doi: 10.1163/156854091X00290
663
Sedlak-Weinstein E. (1992a). The Occurrence of a New Species of Isocyamus (Crustacea, Amphipoda) From Australian and Japanese Pilot Whales, With a Key to Species of Isocyamus. J. Nat. Hist.26, 937–946. doi: 10.1080/00222939200770561
664
Sedlak-Weinstein E. (1992b). A New Species of Isocyamus (Amphipoda: Cyamidae) From Kogia Breviceps (De Blainville 1838) in Australian Waters. Sist. Parasitol.23, 1–6. doi: 10.1007/BF00008001
665
Seger J. Smith W. A. Perry J. J. Hunn J. Kaliszewska Z. A. Sala L. L. et al . (2010). Gene Genealogies Strongly Distorted by Weakly Interfering Mutations in Constant Environments. Genetics184 (2), 529–545. doi: 10.1534/genetics.109.103556
666
Seilacher A. (2005). Whale Barnacles: Exaptational Access to a Forbidden Paradise. Paleobiology31, 27–35. doi: 10.1666/0094-8373(2005)031[0027:WBEATA]2.0.CO;2
667
Sekiguchi K. Klages N. T. W. Best P. B. (1996). The Diet of Strap-Toothed Whales (Mesoplodon Layardii). J. Zool.239 (3), 453–463. doi: 10.1111/j.1469-7998.1996.tb05935.x
668
Sekiguchi K. Klages N. Findlay K. (1993). Feeding Habits and Possible Movements of Southern Bottlenose Whales (Hyperodon Planifrons). Proc. NIPR Symp. Polar Biol.6, 84–97.
669
Sergeant D. E. (1962). The Biology of the Pilot or Pothead Whale. Globicephala Melaena (Traill) in Newfoundland Waters. Bull. Fish. Res. Board Can.132, 84.
670
Sergeant D. E. Fisher H. D. (1957). The Smaller Cetacea of Eastern Canadian Waters. J. Fish. Res. Board Can.14 (1), 83–115. doi: 10.1139/f57-003
671
Shane S. (1978). Suckerfish Attached to a Bottlenose Dolphin in Texas. J. Mammal.59 (2), 439–440. doi: 10.2307/1379936
672
Sherchenko V. (1970). Puzzling White Scars on Whale Body. Priroda6, 72–73. [In Russian].
673
Siciliano S. Cardoso J. Francisco A. De Souza S. P. Hauser-Davis R. A. Iwasa-Arai T. (2020). Epizoic Barnacle (Xenobalanus globicipitis) Infestations in Several Cetacean Species in South-Eastern Brazil. Mar. Biol. Res.16, 1–13. doi: 10.1080/17451000.2020.1783450
674
Silva S. Araújo M. J. Bao M. Mucientes G. Cobo F. (2014). The Haematophagous Feeding Stage of Anadromous Populations of Sea Lamprey Petromyzon Marinus: Low Host Selectivity and Wide Range of Habitats. Hydrobiologia734 (1), 187–199. doi: 10.1007/s10750-014-1879-4
675
Silva-Brum I. N. (1985). “Primeira Ocorrência No Brasil De Duas Espécies Da Família Coronulidae Leach 1825 (Cirripedia),” in Resumos do In Campinas, Brazil, 55.
676
Silva S. Servia M. J. Vieira-Lanero R. Barca S. Cobo F. (2013). Life Cycle of the Sea Lamprey Petromyzon Marinus: Duration of and Growth in the Marine Life Stage. Aquat. Biol.18 (1), 59–62. doi: 10.3354/ab00488
677
Silva D. Young R. F. Lavin A. O'Shea C. Murray E. (2020). Abundance and Seasonal Distribution of the Southern North Carolina Estuarine System Stock (USA) of Common Bottlenose Dolphins (Tursiops Truncatus). J. Cetacean Res. Manage.21 (1), 33–43. doi: 10.47536/jcrm.v21i1.175
678
Silver M. R. Kawauchi H. Nozaki M. Sower S. A. (2004). Cloning and Analysis of the Lamprey GnRH-III cDNA From Eight Species of Lamprey Representing the Three Families of Petromyzoniformes. Gen. Comp. Endocrinol.139 (1), 85–94. doi: 10.1016/j.ygcen.2004.07.011
679
Skaramuca D. Skaramuca B. Dulčić J. (2009). Record of a Live Sharksucker, Echeneis Naucrates (Osteichthyes: Echeneidae) From the South-Eastern Adriatic (Croatian Coast). Mar. Biodivers. Rec.2, e80. doi: 10.1017/s1755267209000694
680
Skerman T. M. (1958). Rates of Growth in Two Species of Lepas (Cirripedia). N. Z. J. Sci.1, 402–411.
681
Skrjabin A. S. (1959). “New Helminth Species From Marine Mammals in the Pacific Ocean and Far-East Sea,” in Izvestiya Krimskovo Pedagogicheskogo Instituta M. V. Frunze, vol. 34. , 99–118. [In Russian].
682
Slijper E. J. (1962). Whales (London: Hutchinson).
683
Smiddy P. (1986). Bottle-Nosed Whale Hyperdoon Ampullatus (Forster). Ir. Nat. J.22, 165.
684
Smiddy P. Berrow S. D. (1992). Humpback Whale Megaptera novaeangliae (Borowski). Ir. Nat. J.24 (4), 162.
685
Smit N. J. Bruce N. L. Hadfield K. A. (2014). Global Diversity of Fish Parasitic Isopod Crustaceans of the Family Cymothoidae. Int. J. Parasitol.: Parasites Wildl.3 (2), 188–197. doi: 10.1016/j.ijppaw.2014.03.004
686
Smit N. J. Bruce N. L. Hadfield K. A. (2019). Introduction to Parasitic Crustacea: State of Knowledge and Future Trends. Zool. Monogr.30, 1–6. doi: 10.1007/978-3-030-17385-2_1
687
Smyth J. D. (1962). Introduction to Animal Parasitology (London: The English Universities Press Ltd).
688
Sokolov V. E. Arsen'ev V. A. (2006). Baleen Whales of Russia and Adjacent Regions (Washington, DC: Science Publishers).
689
Soto J. M. R. (2001). Annotated Systematic Checklist and Bibliography of the Coastal and Oceanic Fauna of Brazil. I. Sharks. Mare Magnum1 (1), 51–120.
690
Souza S. P. Siciliano S. Cuenca S. Sanctis B. (2005). A True’s Beaked Whale (Mesoplodon Mirus) on the Coast of Brazil: Adding a New Beaked Whale Species to the Western Tropical Atlantic and South America. Lat. Am. J. Aquat. Res.4 (2), 129–136. doi: 10.5597/lajam00077
691
Spaul E. A. (1964). Deformity in the Lower Jaw of the Sperm Whale (Physeter Catodon). Proc. Zool. Soc Lond.142, 391–395. doi: 10.1111/j.1469-7998.1964.tb04505.x
692
Spice E. K. Whitesel T. A. McFarlane C. T. Docker M. F. (2011). Characterization of 12 Microsatellite Loci for the Pacific Lamprey, Entosphenus Tridentatus (Petromyzontidae), and Cross-Amplification in Five Other Lamprey Species. Genet. Mol. Res.10 (4), 3246–3250. doi: 10.4238/2011.December.22.2
693
Spivey H. R. (1977). Those Tenacious Travelers of Florida’s Atlantic Coast. Florida Nat.50, 6–10.
694
Spivey H. R. (1980). Occurrence of the Balanamorph Barnacle Xenobalanus globicipitis Steenstrup 1851 (Coronulidae) on the Atlantic Bottlenosed Dolphin, Tursiops Truncatus in the Gulf of Mexico. Florida Sci.43 (4), 292–293.
695
Steenstrup J. J. S. (1852). “Om Xenobalanus globicipitis, En Ny Cirriped-Slaegt Af Coronula Familien,” in Vidensk. Medd. Dan. Naturh. Foren., 62–64.
696
Steenstrup J. J. S. Lütken C. F. (1861). Bidrag Til Kundskab Om Det Aabne Havs Snyltekrebs Og Lernæer Samt Om Nogle Andre Nye Eller Hidtil Kun Ufuldstændigt Kjendte Parasitiske Copepoder. Kongelige Danske Videnskabernes Selskabs Skrifter, Naturhistorisk Og Mathematisk Afdeling. Kjöbenhavn5)5, 343–432. doi: 10.5962/bhl.title.59539
697
Stephensen K. (1938). Cirripedia (Including Rhizocephala). Zoology Iceland3, 1–11.
698
Stephensen K. (1940). Parasitic and Semiparasitic Copepoda. Zool Iceland34, 24.
699
Stephensen K. (1942). The Amphipoda of Norway and Spitzbergen With Adjacent Waters. Fasx. IY. Tromso Mus. Skrift.3 (4), 363–526.
700
Stimmelmayr R. Gulland F. (2020). Gray Whale (Eschrichtius Robustus) Health and Disease: Review and Future Directions. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.588820
701
Stock J. H. (1973a). Whale-Lice (Amphipoda: Cyamidae) in Dutch Waters. Bull. Zool. Mus. Univ. Amsterdam3 (12), 73–77.
702
Stock J. H. (1973b). Een Bruinvis Met Luizen. Levende Natuur76, 107–109.
703
Stock J. H. (1977). Whale-Lice (Amphipoda, Cyamidae) on Lagenorhynchus Albirostris in Dutch Waters. Crustaceana32 (2), 206. doi: 10.1163/156854077X00601
704
Stubbings H. G. (1965). West African Cirripedia in the Collection of the Institut Français D’afrique Noire, Dakar, Senegal. Bull. l’Institut Fran. d’Afrique Noire27, 876–907.
705
Sullivan R. Houck W. (1979). Sightings and Strandings of Cetaceans From Northern California. J. Mamm.60 (4), 828–833. doi: 10.2307/1380200
706
Suyama S. Miyamoto H. Fuji T. Tamura T. Shiozaki K. Ohshimo S. et al . (2021a). Infection by the parasitic copepod Pennella sp. induces mortality in the Pacific saury Cololabis saira. Fish. Sci.87 (3), 187–202. doi: 10.1007/s12562-020-01490-6
707
Suyama S. Yanagimoto T. Nakai K. Tamura T. Shiozaki K. Ohshimo S. et al . (2021b). A Taxonomic Revision of Pennella Oken, 1815 based on morphology and genetics (Copepoda: Siphonostomatoida: Pennellidae). J. Crust. Biol.41 (3), ruab040. doi: 10.1093/jcbiol/ruab040
708
Swartz S. L. (1981). Cleaning Symbiosis Between Topsmelt, Atherinops Affinis, and Gray Whale, Eschrichtius Robustus, in Laguna San Ignacio, Baja California Sur. Mexico. F. Bull.79, 360.
709
Symons H. W. Weston R. D. (1958). Studies on the Humpback Whale (Megaptera Nodosa) in the Bellinghausen Sea. Norsk Hvalfangsttid.47 (2), 53–81.
710
Tørud B. Håstein T. (2008). Skin Lesions in Fish: Causes and Solutions. Acta Vet. Scand.50, S7. doi: 10.1186/1751-0147-50-S1-S7
711
Tajima Y. Maeda K. Yamada T. K. (2015). Pathological Findings and Probable Causes of the Death of Stejneger’s Beaked Whales (Mesoplodon Stejnegeri) Stranded in Japan From 1999 and 2011. J. Vet. Med. Sci.13, 454. doi: 10.1292/jvms.13-0454
712
Takeda M. Ogino M. (2005). Record of a Whale Louse, Cyamus Scammoni Dall (Crustacea: Amphipoda: Cyamidae), From the Gray Whale Strayed Into Tokyo Bay, the Pacific Coast of Japan. Bull. Natn. Sci. Mus.31 (4), 151–156.
713
Takemoto R. M. Luque J. L. (2002). Parasitic Copepods on Oligoplites Spp. (Osteichthyes, Carangidae) From the Brazilian Coastal Zone, With the Redescription of Tuxophorus Caligodes Wilson 1908 (Siphonostomatoida, Tuxophoridae). Acta Scientiarum. Biol. Sci.24 (2), 481–487.
714
Tasmanian Museum and Art Gallery (2020) Tasmanian Museum and Art Gallery Provider for OZCAM. Available at: https://www.gbif.org/occurrence/1806052502 (Accessed May 14, 2021).
715
Taylor L. O’Dea A. Bralower T. Finnegan S. (2019). Isotopes From Fossil Coronulid Barnacle Shells Record Evidence of Migration in Multiple Pleistocene Whale Populations. Proc. Natl. Acad. Sci.116 (15), 7377–7381. doi: 10.1073/pnas.1808759116
716
Ten S. Pascual L. Pérez-Gabaldón M. I. Tomás J. Domènech F. Aznar F. J. (2019). Epibiotic Barnacles of Sea Turtles as Indicators of Habitat Use and Fishery Interactions: An Analysis of Juvenile Loggerhead Sea Turtles, Caretta Caretta, in the Western Mediterranean. Ecol. Indic.107, 105672. doi: 10.1016/j.ecolind.2019.105672
717
Terasawa F. Yamagami T. Kitamura M. Fujimoto A. (1997). A Pygmy Killer Whale (Feresa Attenuata) Stranded at Sagami Bay, Japan. Aquat. Mamm.23, 69–72.
718
Terwilliger N. B. Ryan M. C. (2006). Functional and Phylogenetic Analyses of Phenoloxidases From Brachyuran (Cancer Magister) and Branchiopod (Artemia Franciscana, Triops Longicaudatus) Crustaceans. Biol. Bull.210 (1), 38–50. doi: 10.2307/4134535
719
Thiyagarajan V. Harder T. Qian P. Y. (2003). Combined Effects of Temperature and Salinity on Larval Development and Attachment of the Subtidal Barnacle Balanus Trigonus Darwin. J. Exp. Mar. Bio. Ecol.287 (2), 223–236. doi: 10.1016/S0022-0981(02)00570-1
720
Tomilin A. G. (1957). Mammals of the SSSR and Adjacent Countries. Mammals of Eastern Europe and Adjacent Countries. Izdatel’stvo Akademi Nauk SSSR (Jerusalem: Israel Program for Scientific Translations). [In Russian].
721
Tomioka S. Kado R. Kakui K. Sato M. (2020). First Record of Conchoderma auritum (Linnaeus 1767) (Crustacea: Cirripedia: Lepadidae) From Rishiri Island. Rishiri Stud.39, 7–10.
722
Tonay A. M. Dede A. (2013). First Stranding Record of a Harbour Porpoise (Phocoena Phocoena) in the Southern Aegean Sea. Growth19 (1), 132–137.
723
Toth-Brown J. Hohn A. A. (2007). Occurrence of the Barnacle, Xenobalanus globicipitis, on Coastal Bottlenose Dolphins (Tursiops Truncatus) in New Jersey. Crustaceana80 (10), 1271–1279. doi: 10.1163/156854007782321137
724
Toth J. L. Hohn A. A. Able K. W. Gorgone A. M. (2012). Defining Bottlenose Dolphin (Tursiops Truncatus) Stocks Based on Environmental, Physical, and Behavioral Characteristics. Mar. Mamm. Sci.28 (3), 461–478. doi: 10.1111/j.1748-7692.2011.00497.x
725
Towers J. R. Mcmillan C. J. Malleson M. Hildering J. Ford J. K. B. Ellis G. M. (2013). Seasonal Movements and Ecological Markers as Evidence for Migration of Common Minke Whales Photo-Identified in the Eastern North Pacific. J. Cetacean Res. Manage.13, 221–229.
726
Trilles J. P. Rameshkumar G. Ravichandran S. (2013). Nerocila Species (Crustacea, Isopoda, Cymothoidae) From Indian Marine Fishes. Parasitol. Res.112 (3), 1273–1286. doi: 10.1007/s00436-012-3263-5
727
True F. W. (1890). Observations of the Life History of the Bottlenose Porpoise. Proc. U. S. Natl. Mus.13, 197–203. doi: 10.5479/si.00963801.13-812.197
728
Tsikhon-Lukanina V. A. Soldatova I. N. Kuznetsova I. A. Il'in I. I. (1977). Macrofouling Community in the Strait of Tunisia (Sicily). Oceanology16, 519–522.
729
Turner W. (1905). On Pennella Balænopteræ: A Crustacean, Parasitic on a Finner Whale, Balaenoptera Musculus. Earth. Environ. Sci. Trans. R. Soc Edinb.41 (2), 409–434. doi: 10.1017/S0080456800034487
730
Uchida A. (1998). Prevalence of Parasites and Histopathology of Parasitisation in Minke Whales (Balaenoptera Acutorostrata) From the Western North Pacific Ocean and the Southern Sea of Okhotsk. Rep. Int. Whal. Commn.48, 465–479. doi: 10.1016/S1383-5769(98)81064-7
731
Uchida A. Araki J. (2000). Ectoparasites and Endoparasites in the Minke Whale (Balaenoptera Acutorostrata) From the North-Western Pacific Ocean. J. Japan Vet. Med. Assoc.53 (2), 85–88. doi: 10.12935/jvma1951.53.85
732
Ueda K. (2020) Inaturalist Research-Grade Observations (iNaturalist.org). Available at: https://www.gbif.org/occurrence/2563485235 (Accessed June 4, 2021). doi: 10.3897/biss.4.59133
733
Urian K. W. Kaufmann R. Waples D. M. Read A. J. (2019). The Prevalence of Ectoparasitic Barnacles Discriminates Stocks of Atlantic Common Bottlenose Dolphins (Tursiops Truncatus) at Risk of Entanglement in Coastal Gill Net Fisheries. Mar. Mamm. Sci.35 (1), 290–299. doi: 10.1111/mms.12522
734
Van Bree P. J. H. (1971). The Rabbit-Eared Barnacle, Conchoderma auritum, on the Teeth of the Dolphin Stenella Frontalis. Z. f. Saugetierkunde36 (5), 316–317.
735
Van Bree P. J. H. Smeenk C. (1978). Strandingen Van Cetacea Op De Nederlandse Kust in 1976 En 1977. Lutra20 (1-3), 13–18.
736
Van Bressem M. Raga A. Di Guardo G. Jepson P. Duignan P. Siebert U. et al . (2009). Emerging Infectious Diseases in Cetaceans Worldwide and the Possible Role of Environmental Stressors. Dis. Aquat. Org.86, 143–157. doi: 10.3354/dao02101
737
Van Oorde-de Lint G. Schuurmans-Stekhoven J. H. . (2009). Copepoda parasitica. Tierwelt der Nord- und OstseeGrimpeG.WaglerE. (Leipzig: Geest & Portig)
738
Van Waerebeek K. Hazevoet C. J. López Suarez P. Rodrigues M. S. D. Gatt G. (2008). Preliminary Findings on the Mass Stranding of Melon-Headed Whale Peponocephala Electra on Boavista Island in November 2007, With Notes on Other Cetaceans From the Cape Verde Islands. Technical Report (Fondation Internationale du Banc d’Arguin).
739
Van Waerebeek K. Reyes J. C. Alfaro J. (1993). Helminth Parasites and Phoronts of Dusky Dolphins Lagenorhynchus Obscurus (Gray 1828) From Peru. Aquat. Mamm.19, 159–159.
740
Van Waerebeek K. Waerebeek K. V. Reyes J. C. Read A. J. McKinnon J. S. (1990). “Preliminary Observations of Bottlenose Dolphins From the Pacific Coast of South America,” in The Bottlenose Dolphin. Eds. LeatherwoodS.ReevesR. (San Diego: Academic Press). doi: 10.1016/B978-0-12-440280-5.50011-1
741
Vargas-Bravo M. H. Elorriaga-Verplancken F. R. Olivos-Ortiz A. Morales-Guerrero B. Lin-Cabello M. A. Ortega-Ortiz C. D. (2020). Ecological Aspects of Killer Whales From the Mexican Central Pacific Coast: Revealing a New Ecotype in the Eastern Tropical Pacific. Mar. Mamm. Sci.37, 1–16. doi: 10.1111/mms.12748
742
Vecchione A. (1994). Pennella Parasite in Stenella Coeruleoalba. Italian Marine Ecosystem of the Coast of Latium (Seminario Internazionale di Studi di Ecosistema Marino 96-98).
743
Vecchione A. Aznar F. J. (2014). The Mesoparasitic Copepod Pennella balaenopterae and its Significance as a Visible Indicator of Health Status in Dolphins (Delphinidae): A Review. J. Mar. Anim. Ecol.7 (4), 4–11.
744
Verrill A. E. (1902). The Bermuda Islands (New Haven, Ct: Published by the author).
745
Vervoort W. Tranter D. (1961). Balaenophilus Unisetus P.O.C. Aurivillius (Copepoda Harpacticoida) From the Southern Hemisphere. Crustaceana3 (1), 70–84. doi: 10.1163/156854061X00545
746
Vidal O. Brownell R. L. Jr. Findley L. T. (1999). “Vaquita,” in Handbook of Marine Mammals. Eds. RidgwayS. H.HarrisonR. J. (London: Academic Press).
747
Visser I. N. (1999). Propeller Scars on and Known Home Range of Two Orca (Orcinus Orca) in New Zealand Waters. N. Z. J. Mar. Freshw. Res.33 (4), 635–642. doi: 10.1080/00288330.1999.9516906
748
Visser I. N. Cooper T. Grimm H. (2020). Duration of Pseudo-Stalked Barnacles (Xenobalanus globicipitis) on a New Zealand Pelagic Ecotype Orca (Orcinus Orca), With Comments on Cookie Cutter Shark Bite Marks (Isistius Sp.); can They be Used as Biological Tags? Biod. J.11 (4), 1067–1086. doi: 10.31396/biodiv.jour.2020.11.4.1067.1086
749
Visser I. N. Zaeschmar J. Halliday J. Abraham A. Ball P. Bradley R. et al . (2010). First Record of Predation on False Killer Whales (Pseudorca Crassidens) by Killer Whales (Orcinus Orca). Aquat. Mamm.36 (2), 195–204. doi: 10.1578/AM.36.2.2010.195
750
Vollmer N. Ashe E. Brownell R. Cipriano F. Mead J. Reeves R. et al . (2010). Taxonomic revision of the dolphin genus Lagenorhynchus. Mar. Mamm. Sci.36(3), 957–1057. doi: 10.1111/mms.12573
751
Von Duyke A. Stimmelmayr R. Sheffield G. Sformo T. Givens G. George J. (2016). Prevalence and Abundance of Cyamid “Whale Lice” (Cyamus Ceti) on Subsistence Harvested Bowhead Whales (Balaena Mysticetus). ARCTIC69 (4), 331. doi: 10.14430/arctic4593
752
Vosseler J. (1889). Amphipoden Und Isopoden Von Spitzbergen. Arch. Naturgesch.55, 151–162.
753
Wada S. Oishi M. Yamada T. K. (2003). A Newly Discovered Species of Living Baleen Whale. Nature426, 278–281. doi: 10.1038/nature02103
754
Walker L. M. (1981). Reproductive Biology and Development of a Marine Harpacticoid Copepod Reared in the Laboratory. J. Crust. Biol.1 (3), 376–388. doi: 10.2307/1547969
755
Walker W. A. Hanson M. B. (1999). Biological Observations on Stejneger’s Beaked Whale, Mesoplodon Stejnegeri, From Strandings on Adak Alaska. Mar. Mamm. Sci.15 (4), 1314–1329. doi: 10.1111/j.1748-7692.1999.tb00893.x
756
Wallace B. (1977). Cover Photo. Sea Front.23, (3).
757
Waller G. N. H. (1989). Two New Species of Whale Lice (Cyamidae) From the Ziphioid Whale Berardius Bairdii. Invest. Cet.22, 292–297.
758
Walter T. C. Boxshall G. (2020) World of Copepods Database. Caligidae Burmeister. Available at: http://www.marinespecies.org/aphia.php?p-taxdetails&id=135566 (Accessed June 3, 2021).
759
Wardle W. J. Haney T. A. Worthy G. A. J. (2000). New Host Record for the Whale Louse Isocyamus delphinii (Amphipoda, Cyamidae). Crustaceana73, 639–641. doi: 10.1163/156854000504615
760
Watson A. Gee L. E. (2005). Laryngeal Displacement and Asphyxiation by a Beheaded Sheepshead (Archosargus Probatocephalus) in a Bottlenose Dolphin (Tursiops Truncatus). Aquat. Mamm.31, 447–452. doi: 10.1578/AM.31.4.2005.447
761
Watson A. G. Stein L. E. Marshall C. Henry G. A. (1994). Polydactyly in a Bottlenose Dolphin, Tursiops Truncatus. Mar. Mamm. Sci.10 (1), 93–100. doi: 10.1111/j.1748-7692.1994.tb00393.x
762
Wegner N. C. Cartamil D. P. (2012). Effects of Prolonged Entanglement in Discarded Fishing Gear With Substantive Biofouling on the Health and Behavior of an Adult Shortfin Mako Shark, Isurus Oxyrinchus. Mar. Poll. Bull.64 (2), 391–394. doi: 10.1016/j.marpolbul.2011.11.017
763
Weir C. R. (2010). Cetaceans Observed in the Coastal Waters of Namibe Province, Angola, During Summer and Winter 2008. Mar. Biodivers. Rec.3, e27. doi: 10.1017/S1755267210000230
764
Welch J. (2017). Mouthline Pigmentation Loss and Fisheries Associated Injuries of Rough-Toothed Dolphins (Steno bredanensis) in Hawaii. [Ph.D. Thesis] (Chicago (IL: Evergreen State College).
765
Weller D. Würsig B. Bradford A. L. Burdin A. M. Blokhin A. S. Minakuchi H. et al . (1999). Gray Whales (Eschrichtius Robustus) Off Sakhalin Island, Russia: Seasonal and Annual Patterns of Occurrence. Mar. Mamm. Sci.15, 1208–1227. doi: 10.1111/j.1748-7692.1999.tb00886.x
766
Wellington G. M. Anderson S. (1978). Surface Feeding by a Juvenile Gray Whale, Eschrichtius Robustus. Fish. Bull.76, 290–293.
767
Weltner W. (1897). Verzeichnis Der Bisher Beschriebene Recenten Cirripedienarten. Mit Angabe Der Im Berliner Museum Vorhandenen Species Und Ihrer Fundorte. Archiv fuür Naturgeschichte63, 227–280.
768
Werner J. W.E. (1967). The Distribution and Ecology of the Barnacle Balanus Trigonus. Bull. Mar. Sci.17 (1), 64–84.
769
Whales and Dolphins Package - CW Azores (2021). Available at: https://www.cwazores.com/whales-and-dolphins-package (Accessed August 16, 2021).
770
Wheeler E. McIntosh H. (2018) Royal BC Museum - Invertebrates Collection. Version 1.1. Royal British Columbia Museum. Available at: https://www.gbif.org/occurrence/1898261060 (Accessed May 4, 2021).
771
Whitehead T. O. Rollinson D. P. Reisinger R. R. (2014). Pseudostalked Barnacles Xenobalanus globicipitis Attached to Killer Whales Orcinus Orca in South African Waters. Mar. Biodivers. Rec.45, 873–876. doi: 10.1007/s12526-014-0296-2
772
Whiteman N. K. Parker P. G. (2005). Using Parasites to Infer Host Population History: A New Rationale for Parasite Conservation. Animal Conservation Forum. Anim. Conserv.8(2), 175–181. doi: 10.1017/S1367943005001915
773
Widder E. A. A. (1998). Predatory Use of Counter Illumination by the Squaloid Shark, Isistius Brasiliensis. Environ. Biol. Fishes53, 267–273. doi: 10.1023/A:1007498915860
774
Williams J. E.H. (1978). Conchoderma Virgatum (Spengler) (Cirripedia, Thoracica) in Association With Dinemoura Latifolia (Steenstrup and Lutken) (Copepoda, Caligidea), a Parasite of the Shortfin Mako, Isurus Oxyrhynchus Rafinesque (Pisces, Chondrichthyes). Crustaceana34, 109–111. doi: 10.1163/156854078X00655
775
Williams E. H. Bunkley-Williams L. (2019). “Life Cycle and Life History Strategies of Parasitic Crustacea,” in Parasitic Crustacea (Cham: Springer), 179–266. doi: 10.1007/978-3-030-17385-2_5
776
Williams E. H. Jr. Williams L. B. (1986). The First Association of Conchoderma Virgatum (Spengler) (Cirripedia: Thoracica) With a Euryphodid Copepod in the Mouth of a Fish. Galaxea5, 209–211.
777
Wilson C. B. (1905). North American Parasitic Copepods Belonging to the Family Caligidae, 1: The Caliginae. Proc. U. S. Nat. Mus.28, 479–672. doi: 10.5479/si.00963801.28-1404.479
778
Wingert N. Milmann L. Baumgarten M. Danilewicz D. Sazima I. Ott P. H. (2021). Relationships Between Common Bottlenose Dolphins (Tursiops Truncatus) and Whalesuckers (Remora Australis) at a Remote Archipelago in the Equatorial Atlantic Ocean. Aquat. Mamm.47 (6), 585–598. doi: 10.1578/AM.47.6.2021.585
779
Wirtz P. Araujo R. Southward A. J. (2006). Cirripedia of Madeira. Helgoland Mar. Res.60, 207–212. doi: 10.1007/s10152-006-0036-5
780
Wolff T. (1958). On the Rare Whale-Louse Platycyamus Thompsoni (Gosse) (Amphipoda, Cyamidae). Vidensk. Medd. Dansk naturh. Foren.120, 1–14.
781
Wolff T. (1960). Rankefodderne Conchoderma Og Coronula Pa Hvaler. Flora Fauna66 (1), 1–8.
782
Woodard J. C. Zam S. G. Caldwell D. K. Caldwell M. C. (1969). Some Parasitic Diseases of Dolphins. Pathol. Vet.6, 257–272. doi: 10.1177/030098586900600307
783
Woodward B. L. Winn J. P. (2006). Apparent Lateralized Behavior in Gray Whales Feeding Off the Central British Columbia Coast. Mar. Mamm. Sci.22 (1), 64–73. doi: 10.1111/j.1748-7692.2006.00006.x
784
Worm O. (1655). Museum Wormianum. Amstelodami, Apud Ludouicum & Danielem Elzeririos.
785
WoRMS - World Register of Marine Species (2021) Odontobius Ceti De Vauzème. Available at: https://www.marinespecies.org/aphia.php?p=taxdetails&id=229481 (Accessed September 16, 2021).
786
Yamaguchi R. Nakaya K. (1997). Fukuoka Megamouth, a Probable Victim of the Cookiecutter Shark. Biology of the Megamouth Shark (Tokyo: Tokai University Press), 171.
787
Yamato S. Yusa Y. Tanase H. (1996). Distribution of Two Species of Conchoderma (Cirripedia: Thoracica) Over the Body of a Sea Snake, Laticauda Semifasciata (Reinwardt), From the Kii Peninsula, Southwestern Japan. Publ. Seto Mar. Biol. Lab.37 (3-6), 337–343. doi: 10.5134/176259
788
Yamazaki Y. Yokoyama R. Nishida M. Goto A. (2006). Taxonomy and Molecular Phylogeny of Lethenteron Lampreys in Eastern Eurasia. J. Fish Biol.68 (SUPPL. B), 251–269. doi: 10.1111/j.0022-1112.2006.01070.x
789
Young P. S. (1991). The Superfamily Coronuloidea Leach (Cirripedia, Balanomorpha) From the Brazilian Coast, With Redescription of Stomatolepas Species. Crustaceana61, 190–212. doi: 10.1163/156854091X00678
790
Yusa Y. Yoshikawa M. Kitaura J. Kawane M. Ozaki Y. Yamato S. et al . (2012). Adaptive Evolution of Sexual Systems in Pedunculate Barnacles. Proc. Biol. Sci.279 (1730), 959–966. doi: 10.1098/rspb.2011.1554
791
Zenkovich (Senkowitsch) B. A. (1956). Jagd Aud Meeresriesen Rund Um Die Welt Auf Walfang (Leipzig: F. A. Brockhaus Verlag).
792
Zettler M. L. (2021). An Example for Transatlantic Hitchhiking by Macrozoobenthic Organisms With a Research Vessel. Helgoland Mar. Res.75 (1), 1–7. doi: 10.1186/s10152-021-00549-w
793
Zintzen V. Roberts C. D. Anderson M. J. Steward A. L. Struthers C. D. Harvey E. S. (2011). Hagfish Predatory Behavior and Slime Defence Mechanism. Sci. Rep.1, 131. doi: 10.1038/srep00131
794
Zullo V. A. (1963). A Preliminary Report on the Systematics and Distribution of Barnacles (Cirripedia) of the Cape Cod Region. Massachusetts and Ecology Program, Marine Biological Laboratory, Woods Hole, Massachusetts Contribution, Vol. 3. 1–33.
Summary
Keywords
epibiotic, fauna, cetacean, indicator, systematic review, checklist
Citation
Ten S, Raga JA and Aznar FJ (2022) Epibiotic Fauna on Cetaceans Worldwide: A Systematic Review of Records and Indicator Potential. Front. Mar. Sci. 9:846558. doi: 10.3389/fmars.2022.846558
Received
31 December 2021
Accepted
19 April 2022
Published
22 July 2022
Volume
9 - 2022
Edited by
Roksana Majewska, North-West University, South Africa
Reviewed by
Kristina Lehnert, University of Veterinary Medicine Hannover, Germany; Tammy Iwasa-Arai, State University of Campinas, Brazil
Updates
Copyright
© 2022 Ten, Raga and Aznar.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: S. Ten, sofia.ten@uv.es
This article was submitted to Marine Biology, a section of the journal Frontiers in Marine Science
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.