Abstract
A search of records from different kinds of sources namely, scientific and grey literature, social media, and zoological museum collections, has been carried out to review the incidence of Physalia physalis (Linnaeus, 1758), the Portuguese man-of-war, in the Mediterranean Sea. The temporal frame of the records, considered valid if documented with images or collected specimens, ranged from the second half of the eighteenth century to the year 2021. Thanks to colonies preserved in some Italian historical museum collections, originating from the western basin, it was possible to date the putative first documented record of P. physalis of the Mediterranean Sea in 1850. The dataset shows some massive strandings that occurred in localities of the Strait of Gibraltar and Alboran Sea, the area of the entrance, from the Atlantic toward the Mediterranean waters, and the starting point from where the species spread toward the western and central basin. Physalia physalis does not reach the eastern area of the Mediterranean Sea. As the records of this species from the Italian maritime regions were abundant in the summertime and considering the danger related to contact with humans, they were subdivided into three categories of risk according to the months of occurrence. These categories were created to assign a level of danger for swimmers. The increasing sightings of such a poisonous cnidarian in coastal waters can represent a risk to human health, and a threat to all those activities linked to the marine tourism sector. The overview given goes beyond scientific purposes and aims to reach society and public administrators. The involvement of citizens and touristic structures for the early detection of P. physalis can play a key role in preventing encounters with the species, allowing marine tourist facilities to operate within a range of reasonable security.
Introduction
The presence or even the blooms of gelatinous zooplankton (cnidarians, ctenophores, and pelagic tunicates) can have several negative impacts on ecosystems, on the economy (e.g., tourism, fisheries, aquaculture) and human health (Boero et al., 2008; De Donno et al., 2014; Bosch-Belmar et al., 2017; Marambio et al., 2021). About this latter aspect, it is well-known that zooplanktonic cnidarians, commonly named jellyfish, can generate serious wounds and poisoning to humans, and in some cases death (Burnett and Gable, 1989; Burke, 2002). However, since this gelatinous zooplankton is not usually targeted by fisheries and not easily surveyed or collected by research activities, data on dispersal and impact on human life are sometimes lacking (Condon et al., 2012; De Donno et al., 2014; Rossi et al., 2019; Gravili and Rossi, 2021). Hence, we cannot state with absolute certainty for several species that a general increase of their abundance is occurring or will occur in a specific region. In this regard, Citizen Science and local public networks can be of great help in collecting data on a large spatial-temporal scale in order to better understand the dynamics of jellyfish populations, as demonstrated by several projects (Boero et al., 2010; Baumann and Schernewski, 2012; Fleming et al., 2013; Bellido et al., 2020; Gutiérrez-Estrada et al., 2021; Marambio et al., 2021), though some species remains overlooked, such as Physalia physalis (Linnaeus, 1758).
One of the most dangerous pelagic species in the Mediterranean Sea is the Portuguese man-of-war, P. physalis, a pleustonic colonial organism rarely observed in the open Mediterranean basin, whose direct observations mainly concern stranded specimens (Berdar and Cavallaro, 1980; Deidun, 2010; Boero, 2013; Prieto et al., 2015; Castriota et al., 2017; Mghili et al., 2020; Deidun et al., 2020; Macías et al., 2021). This species, belonging to the Cnidarian group—order Siphonophores, is part of the floating community of ocean organisms that live at the interface between water and air (Boero, 2013; Munro et al., 2019). Though the whole body seems a single individual, it is a colony in which small individuals (zooids) perform different functions such as floating, feeding, defence and reproduction (Lane, 1960). The structure of the colony is complex; on the whole, it can be described as composed of two portions: a portion visible floating on the ocean surface—pneumatophore—and a not visible tentacular portion expanding below the water surface—the complex of zooids (Munro et al., 2019).
Physalia physalis is easily recognized by its bluish pneumatophore, an air bladder developed from one of the zooids that can reach 30 cm in length (Bardi and Marques, 2007; Munro et al., 2019), surmounted by a crest that acts like a sail that helps to navigate and float on the surface of the sea (Lane, 1960; Iosilevskii and Weihs, 2009). The tentacles of this species, maybe up to 30 m long and containing more than 750,000 stinging cells each (Munro et al., 2019), contain the “physaliatoxin” (Bardi and Marques, 2007) that is the cause of its painful sting, which causes a series of symptoms, ranging from local skin necrosis to neurological and cardiorespiratory problems that can cause death (Labadie et al., 2012). Furthermore, unlike other cnidarian species, the Portuguese man-of-war nematocysts seem to penetrate surgical gloves (Pierce, 2006). Physalia physalis behaves like a predator, plays an important ecological role and influences the abundance of populations of small animals, in particular juveniles of fishes and larvae that it captures with tentacles (Purcell, 1984). On the other hand, the species can be preyed upon by fishes, turtles, lepadomorph barnacles, and glaucid nudibranchs (Wangersky and Lane, 1960; Bieri, 1966; Thompson and Bennett, 1970; Arai, 2005), and other taxa not disclosed yet (Arai, 2005).
Physalia physalis is a cosmopolitan species, distributed from tropical to temperate waters roughly comprised from 51°N to 38°S (Shannon and Chapman, 1983; Kirkpatrick and Pugh, 1984; Haddad et al., 2002; Elston, 2007; Pontin et al., 2011; Oliveira et al., 2016). The species is surface-dwelling thanks to the chamber filled with air and carbon monoxide which represents a sail, thus the colony moves with the prevailing wind (Mapstone, 2014).
It is interesting to underline that the cohorts of P. physalis originate from unknown marine georeferenced points (Graham et al., 2001; Ferrer and Pastor, 2017; Ferrer and González, 2021). These colonies move toward coastal areas, where they find optimal trophic conditions, and float along the shoreline, as a consequence of the direction and energy of prevailing winds and surface currents (Ferrer and Pastor, 2017). Thus, the continuously updating of records is much relevant to foresee the dispersal routes.
Up to date, the first documented record of P. physalis for the Mediterranean Sea dates back to 1980 (Berdar and Cavallaro, 1980). The species has been subsequently reported sporadically, in the western and central basins, mostly around Sicily and Malta (Deidun, 2010; Deidun et al., 2020; Boero, 2013). The latest records in Sicily date back to 2019 and 2021, in the Aegadian Sicilian Islands (Deidun et al., 2020) and Donnalucata (Sicily), respectively.
Gathering the baseline information on this species records over time and space constitutes an important task for the elaboration of management programs aimed to alert the local human population and touristic structures (Macías et al., 2021). Physalia physalis represents an important species to be aware of, and for which dissemination of data about its presence should be improved.
Here we introduce a dataset of sightings, strandings, and museum records of the species in the Mediterranean Sea, and the first documented Mediterranean records, originating from the southern Tyrrhenian basin. Relevant biodiversity information can be obtained from materials and data collected in the past and preserved in the Natural History Museums, which can provide overlooked and, in many cases, still unpublished data (Tedesco et al., 2020; Lo Brutto et al., 2021). Additionally, the sightings and strandings herein reported highlight the potential role of the citizens’ participatory science. The data from social media and local Citizen Science projects can have a key role if well-managed; the information is often uploaded on the web but not archived in the scientific literature or summarized in any report, thus risking being lost. The case of P. physalis fills such a gap.
The paper gives an overview on (i) the first records of P. physalis in the Mediterranean Sea through analysis of zoological collections; (ii) the recent records detected in the literature and data from civil society; (iii) the recent records in Italian waters and an assessment of risk for humans.
All data here reported can represent an important reference point for the development of models and predictive tools based on the presence, abundance, and jellyfish trends over a large timeframe.
Materials and Methods
A survey on the presence of P. physalis in the Mediterranean Sea was carried out. Information was obtained from scientific and grey literature, from the web and social media (mainly Facebook, Instagram and Twitter) and analysed.
As the zoological records of museum collections are not reported in the literature, a further search of historical museum specimens in Italy and Malta was integrated to find out old data never published. In particular, information was acquired from the Natural History Museum of the University of Florence, Italy, the “Darwin Dohrn” Museum of the Anton Dohrn Zoological Station of Naples, Italy, the Museum of Zoology “P. Doderlein” of the University of Palermo, Italy, the Museum of the Department of Biology of the University of Malta, Malta, and the Museum of Zoology, University of Cagliari, Italy. A very recent record of the species is preserved at the Civic Museum of Natural History of Comiso, Italy.
For each source, only information documented with images or collected specimens were validated for the aims of the present study. The records were assigned to the main Mediterranean sectors based on the sampling locality; from west to east: Strait of Gibraltar, Alboran Sea, Algerian-Balearic Sea, Western Mediterranean, Liguro-Provençal basin, Central Tyrrhenian Sea, Southern Tyrrhenian Sea, Channel of Sicily, Strait of Messina.
In accordance with the results by Macías et al. (2021), apart from the Spanish coast, the colonies in the summer period are more frequent in the southern Italian zone. Consequently, the records from the Italian maritime regions were subdivided into three “categories of risk” according to the season of occurrence. The records of P. physalis detected in February-March were included in the low-risk category; the records occurred in April-May in the medium-risk one; the records happened in June-August in the high-risk category. These categories were created to designate a level of danger for swimmers in such a particular area, specifically it was assumed that the sightings of P. physalis in the summer period (June-August) could be considered the most dangerous (high-risk), according to the higher number of swimmers than the remaining months of the year; while medium and low-risk were associated to a lower frequency of human activities accordingly. The records from this zone belonging to the three categories of risk were counted and grouped following the biogeographical sectors proposed by Bianchi (2004). A final map showed such clustering.
Results
We can assert that at least 8,744 colonies of P. physalis entered the Mediterranean Sea, in a timescale comprised between 1850 and 2021 (August). Thirteen colonies were counted in the museum collections explored, 8,346 colonies were counted from scientific literature, information about 16 colonies was found on the web, and 369 records are herein published for the first time. Most of the colonies were reported stranded. The highest incidence was recorded in the Alboran Sea, in a limited area extending from the Strait of Gibraltar to the Algerian-Balearic Sea; while the central Mediterranean area was the one where P. physalis mainly spread (Table 1). None information was found from the Eastern Mediterranean, Adriatic Sea and Aegean and Levantine basin.
All data are shown in Table 1; the records are listed according to chronological order, and each locality is associated with a marine sector indicated by a point in the map of Figure 1 (see the ID numbers). The marine sectors where P. physalis was been recorded are the following: Strait of Gibraltar (corresponding to the ID number 1 on the map of Figure 1), Alboran Sea (ID 2), Algerian-Balearic Sea—Western Mediterranean (ID 3 and 4), the Liguro-Provençal basin (ID 4 and 5), coastline of Corsica—Liguro-Provençal basin (ID 6 and 8), the coastline of Sardinia—Western Mediterranean (ID 7), Tunisian waters—Channel of Sicily (ID 9 and 10), Pelagie Archipelago—Channel of Sicily (ID 11), Northern Tyrrhenian Sea (ID 12), Aegadian Archipelago – Southern Tyrrhenian Sea (ID 13), Southern Tyrrhenian Sea (ID 14), Central Tyrrhenian Sea (ID 15), Maltese Archipelago—Channel of Sicily (16), Strait of Messina (17) (Table 1 and Figure 1).
The First Mediterranean Records of Physalia physalis
Due to the scarcity of scientific literature documenting the presence of Physalis physalis in the past, some of the oldest zoological museum collections in Italy, which preserve specimens collected in the western area, were explored. The aim was to track down the first records of P. physalis in the Mediterranean Sea.
TABLE 1
| Year | Month | Locality | Mediterranean marine sector |
ID on map | Documented | Number of colonies | Coordinates/link to coordinates |
References | |
| Lat | Lon | ||||||||
| 1850 | Unknown | Nice (France) | Liguro-Provençal basin | 5 | Yes | 1 | 43.697551 | 7.265345 | Present paper MZUF |
| 1863 | Unknown | Livorno (Italy) | Liguro-Provençal basin | 5 | Yes | 1 | 43.540578 | 10.279674 | Present paper MZUF |
| 1914 | Unknown | Gulf of Naples (Italy) | Central Tyrrhenian Sea | 15 | Yes | 2 | 40.814625 | 14.271982 | Present paper DDM |
| Antecedent 1972 | Unknown | Gulf of Naples (Italy) | Central Tyrrhenian Sea | 15 | Yes | 5 | 40.814625 | 14.271982 | Present paper DDM |
| Antecedent 1980 | Unknown | Gulf of Palermo (Italy) | Southern Tyrrhenian Sea | 14 | Yes | 1 | 38.137995 | 13.391992 | Present paper MZPA |
| 1980 | February | Coast of Messina (Italy) | Strait of Messina | 17 | Yes | 1 | 38.247346 | 15.625401 | Berdar and Cavallaro, 1980 MDBM |
| 2001 | May | Golden Bay (Malta) | Maltese Archipelago Channel of Sicily | 16 | Yes | 1 | 35.934551 | 14.343233 | Deidun, 2010 |
| 2008 | August | Málaga, Granada (Spain) | Alboran Sea | 2 | Yes | 12 | https://static-content.springer.com/esm/art%3A10.1038%2Fsrep11545/Media Objects/41598_2015_BFsrep11545 _MOESM1_ESM.pdf | Prieto et al., 2015 | |
| 2009 | April-July | Murcia (Spain) | Algerian-Balearic Sea | 3 | Yes | 67 | https://static-content.springer.com/esm/art%3A10.1038%2Fsrep11545/Media Objects/41598_2015_ BFsrep11545_MOESM1_ESM.pdf | Prieto et al., 2015 | |
| 2009 | June | Ceuta (Spain) | Strait of Gibraltar | 1 | Yes | 3 | https://static-content.springer.com/esm/art%3A10.1038%2Fsrep11545/MediaObjects/41598_ 2015_BFsrep11545_MOESM1_ESM.pdf | Prieto et al., 2015 | |
| 2009 | February | Golden Bay (Malta) | Maltese Archipelago Channel of Sicily | 16 | Yes | 1 | 35.936178 | 14.343233 | Deidun, 2010 And P. J. Schembri pers. comm. |
| 2009 | April | Mgarr ix-Xini; Cirkewwa (Malta) | Maltese Archipelago Channel of Sicily | 16 | Yes | 2 | 36.020173 | 14.271735 | Deidun, 2010 |
| 35.988402 | 14.326594 | And P. J. Schembri pers. comm. | |||||||
| 2009 | July | Ghar Lapsi (Malta) | Maltese Archipelago Channel of Sicily | 16 | Yes | 1 | 35.827474 | 14.424703 | Deidun, 2010 And P. J. Schembri pers. comm. |
| 2009 | March | Capo Peloro (Italy) | Strait of Messina | 17 | Yes | 2 | 38.264997 | 15.651731 | Castriota et al., 2017 |
| 2009 | April | Lampedusa Island (Italy) | Pelagie Archipelago Channel of Sicily | 16 | Yes | 1 | 35.513284 | 12.557236 | Castriota et al., 2017 |
| 2009 | August | Crystal Lagoon, | Maltese Archipelago | 16 | Yes | 1 | 36.010346 | 14.327940 | Deidun, 2010 |
| Comino (Malta) | Channel of Sicily | ||||||||
| 2010 | February-July | Cadiz and Ceuta (Spain) | Strait of Gibraltar | 1 | Yes | 161 | https://static-content.springer.com/esm/art% 3A10.1038%2Fsrep11545/MediaObjects/41598 _2015_BFsrep11545_MOESM1_ESM.pdf | Prieto et al., 2015 | |
| 2010 | March | Málaga, Granada (Spain) | Alboran Sea | 2 | Yes | 316 | https://static-content.springer.com/esm/art% 3A10.1038%2Fsrep11545/MediaObjects/41598 _2015_BFsrep11545_MOESM1_ESM.pdf | Prieto et al., 2015 | |
| 2010 | March-July | Almeria, Murcia, Alicante (Spain) | Algerian-Balearic Sea | 3 | Yes | 363 | https://static-content.springer.com/esm/art% 3A10.1038%2Fsrep11545/MediaObjects/41598 _2015_BFsrep11545_MOESM1_ESM.pdf | Prieto et al., 2015 | |
| 2010 | March-June | Cirkewwa; St. ThomasBay; Xlendi (Malta) | Maltese Archipelago Channel of Sicily | 16 | Yes | 3 | 35.988402 | 14.326594 | Deidun, 2010 |
| 35.852088 | 14.566352 | ||||||||
| 36.028826 | 14.213850 | ||||||||
| 2010 | March | N-E coast of Sicily (Italy) | Strait of Messina | 17 | No | 1 | Unknown | Unknown | Boero, 2013 |
| 2010 | March-August | Torre dei corsari; Funtanamare; Island San Pietro; Villaputzu (Italy) | coastline of Sardinia Western Mediterranean | 7 | No | 4 | Unknown | Unknown | Boero, 2013 |
| 2010 | March-August | Gozo Island (Malta) | Maltese Archipelago | 16 | No | 2 | Unknown | Unknown | Boero, 2013 |
| Channel of Sicily | |||||||||
| 2010 | June-August | Corsica Island (France) | Coastline of Corsica | 06-Aug | No | 2 | Unknown | Unknown | Boero, 2013 |
| Liguro-Provençal basin | |||||||||
| 2010 | July | Sant’Antioco Island (Italy) | Coastline of Sardinia Western Mediterranean | 7 | Yes | 1 | 38.967609 | 8.439221 | Present paper MZCA |
| 2010 | July | Porto Ercole (Italy) | Northern Tyrrhenian Sea | 12 | No | 1 | Unknown | Unknown | Boero, 2013 |
| 2010 | July | Sicily (Italy) | Southern Tyrrhenian Sea | 14 | No | 1 | Unknown | Unknown | Boero, 2013 |
| 2011 | January | Cadiz (Spain) | Strait of Gibraltar | 1 | Yes | 1 | https://static-content.springer.com/esm/art% 3A10.1038%2Fsrep11545/MediaObjects/415 98_2015_BFsrep11545_MOESM1_ESM.pdf | Prieto et al., 2015 | |
| 2011 | January | Málaga, Granada (Spain) | Alboran Sea | 2 | Yes | 3 | https://static-content.springer.com/esm/art% 3A10.1038%2Fsrep11545/MediaObjects/415 98_2015_BFsrep11545_MOESM1_ESM.pdf | Prieto et al., 2015 | |
| 2011 | January | Alicante (Spain) | Algerian-Balearic Sea | 4 | Yes | 3 | https://static-content.springer.com/esm/art% 3A10.1038%2Fsrep11545/MediaObjects/415 98_2015_BFsrep11545_MOESM1_ESM.pdf | Prieto et al., 2015 | |
| 2011 | March | Sa Maison (Malta) | Maltese Archipelago | 16 | Yes | 1 | 35.894999 | 14.499521 | Deidun et al., 2020 |
| Channel of Sicily | |||||||||
| 2012 | March | Barcelona (Spain) | Algerian-Balearic Sea | 4 | Yes | 8 | https://static-content.springer.com/esm/art% 3A10.1038%2Fsrep11545/MediaObjects/41 598_2015_BFsrep11545_MOESM1_ESM.pdf | Prieto et al., 2015 | |
| 2012 | March | Ghajn Tuffieha (Malta) | Maltese Archipelago Channel of Sicily | 16 | Yes | 1 | 35.928234 35.928217 | 14.343357 14.343260 | Deidun et al., 2020 |
| 2013 | February-April | Cadiz (Spain) | Strait of Gibraltar | 1 | Yes | 121 | https://digital.csic.es/handle/10261/244755 | Macías et al., 2021 | |
| 2013 | April/July | Málaga, Granada (Spain) | Alboran Sea | 2 | Yes | 425 | https://digital.csic.es/handle/10261/244755 | Macías et al., 2021 | |
| 2013 | April/June | Almeria, Valencia (Spain) | Algerian-Balearic area | 4 | Yes | 40 | https://digital.csic.es/handle/10261/244755 | Macías et al., 2021 | |
| 2013 | July | Gnejna Bay (Malta) | Maltese Archipelago | 16 | Yes | 1 | 35.921039 | 14.342470 | Social network |
| Channel of Sicily | |||||||||
| 2014 | March | Capo Peloro (Italy) | Strait of Messina | 17 | Yes | 1 | 38.264997 | 15.651731 | Castriota et al., 2017 |
| 2014 | April | Xlendi (Malta) | Maltese Archipelago | 16 | Yes | 1 | 36.029434 | 14.215403 | Deidun et al., 2020 |
| Channel of Sicily | |||||||||
| 2014 | April | Levanzo Island (Italy) | Aegadian Archipelago | 13 | Yes | 1 | 38.017357 | 12.331665 | Deidun et al., 2020 |
| Southern Tyrrhenian Sea | |||||||||
| 2018 | February | Granada (Spain) | Alboran Sea | 2 | Yes | 13 | https://digital.csic.es/handle/10261/244755 | Macías et al., 2021 | |
| 2018 | March | Cadiz and Ceuta (Spain) | Strait of Gibraltar | 1 | Yes | 1125 | https://digital.csic.es/handle/10261/244755 | Macías et al., 2021 | |
| 2018 | March | Gibraltar (UK) | Strait of Gibraltar | 1 | Yes | 8 | https://digital.csic.es/handle/10261/244755 | Macías et al., 2021 | |
| 2018 | March | Strait of Gibraltar (Morocco) | Strait of Gibraltar | 1 | Yes | 8 | See Table 1 in Mghili et al. (2020) | Mghili et al., 2020 | |
| 2018 | March | Málaga, Granada (Spain) | Alboran Sea | 2 | Yes | 397 | https://digital.csic.es/handle/10261/244755 | Macías et al., 2021 | |
| 2018 | March | Almeria, Murcia (Spain) | Algerian-Balearic Sea | 3 | Yes | 76 | https://digital.csic.es/handle/10261/244755 | Macías et al., 2021 | |
| 2018 | April | Cadiz and Ceuta (Spain) | Strait of Gibraltar | 1 | Yes | 1425 | https://digital.csic.es/handle/10261/244755 | Macías et al., 2021 | |
| 2018 | April | M’diq, Fnideq, Martil, Azla (Morocco) | Strait of Gibraltar | 1 | Yes | 169 | See Table 1 in Mghili et al. (2020) | Mghili et al., 2020 | |
| 2018 | April | Gibraltar (UK) | Strait of Gibraltar | 1 | Yes | 184 | https://digital.csic.es/handle/10261/244755 | Macías et al., 2021 | |
| 2018 | April | Málaga, Granada (Spain) | Alboran Sea | 2 | Yes | 1474 | https://digital.csic.es/handle/10261/244755 | Macías et al., 2021 | |
| 2018 | April | Almeria, Murcia, Alicante, Balearic Islands (Spain) | Algerian-Balearic Sea | 3 | Yes | 1668 | https://digital.csic.es/handle/10261/244755 | Macías et al., 2021 | |
| 2018 | May | Martil, M’diq, Fnideq (Morocco) | Strait of Gibraltar | 1 | Yes | 46 | See Table 1 in Mghili et al. (2020) | Mghili et al., 2020 | |
| 2018 | May | Cadiz (Spain) | Strait of Gibraltar | 1 | Yes | 3 | https://digital.csic.es/handle/10261/244755 | Macías et al., 2021 | |
| 2018 | May | Málaga, Granada (Spain) | Alboran Sea | 2 | Yes | 53 | https://digital.csic.es/handle/10261/244755 | Macías et al., 2021 | |
| 2018 | April | Almeria, Murcia, Balearic Islands (Spain) | Algerian-Balearic Sea | 3 | Yes | 133 | https://digital.csic.es/handle/10261/244755 | Macías et al., 2021 | |
| 2018 | May | Ghasri valley, Gozo; Valletta Grand Harbour (Malta) | Maltese Archipelago Channel of Sicily | 16 | Yes | 2 | 36.078960 35.902643 | 14.228525 14.519831 | Deidun et al., 2020 |
| 2018 | May | Favignana Island (Italy) | Aegadian Archipelago | 13 | Yes | 1 | 37.938497 | 12.271064 | Deidun et al., 2020 |
| Southern Tyrrhenian Sea | |||||||||
| 2018 | June | Málaga, Granada (Spain) | Alboran Sea | 2 | Yes | 4 | https://digital.csic.es/handle/10261/244755 | Macías et al., 2021 | |
| 2018 | June | Almeria, Valencia (Spain) | Algerian-Balearic Sea | 3 | Yes | 2 | https://digital.csic.es/handle/10261/244755 | Macías et al., 2021 | |
| 2018 | July | Alicante, Valencia, Castellón (Spain) | Algerian-Balearic Sea | 3 | Yes | 5 | https://digital.csic.es/handle/10261/244755 | Macías et al., 2021 | |
| 2019 | January and April | Ghajn Tuffieha; North Comino Channel (Malta) | Maltese Archipelago | 16 | Yes | 2 | 35,930082 | 14,342845 | Deidun et al., 2020 |
| Channel of Sicily | 36,020037 | 14,335388 | |||||||
| 2019 | March | Cadiz (Spain) | Strait of Gibraltar | 1 | Yes | 3 | https://digital.csic.es/handle/10261/244755 | Present paper | |
| 2019 | March | Alicante, Valencia, Castellón (Spain) | Algerian-Balearic Sea | 3 | Yes | 9 | https://digital.csic.es/handle/10261/244755 | Present paper | |
| 2019 | April-May | Cadiz (Spain) | Strait of Gibraltar | 1 | Yes | 2 | https://digital.csic.es/handle/10261/244755 | Present paper | |
| 2019 | April | Granada (Spain) | Alboran Sea | 2 | Yes | 1 | https://digital.csic.es/handle/10261/244755 | Present paper | |
| 2019 | April-May | Balearic Islands (Spain) | Algerian-Balearic Sea | 4 | Yes | 10 | https://digital.csic.es/handle/10261/244755 | Present paper | |
| 2019 | June | Cadiz, Ceuta (Spain) | Strait of Gibraltar | 1 | Yes | 121 | https://digital.csic.es/handle/10261/244755 | Present paper | |
| 2019 | June-August | Almeria, Murcia, Alicante, Valencia, Balearic Islands (Spain) | Algerian-Balearic Sea | 3 | Yes | 14 | https://digital.csic.es/handle/10261/244755 | Present paper | |
| 2019 | September | Balearic Islands (Spain) | Algerian-Balearic Sea | 4 | Yes | 1 | https://digital.csic.es/handle/10261/244755 | Present paper | |
| 2020 | May | Balearic Islands (Spain) | Algerian-Balearic Sea | 4 | Yes | 1 | https://digital.csic.es/handle/10261/244755 | Present paper | |
| 2020 | May | S-W coastline of Sardinia Island (Italy) | coastline of Sardinia Western Mediterranean | 7 | Yes | 1 | 39.702275 | 8.454307 | Deidun et al., 2020 |
| 2020 | August | Ceuta (Spain) | Strait of Gibraltar | 1 | Yes | 4 | https://digital.csic.es/handle/10261/244755 | Present paper | |
| 2020 | August | Granada (Spain) | Alboran Sea | 2 | Yes | 1 | https://digital.csic.es/handle/10261/244755 | Present paper | |
| 2021 | February- March | Cadiz (Spain) | Strait of Gibraltar | 1 | Yes | 61 | https://digital.csic.es/handle/10261/244755 | Present paper | |
| 2021 | February | Balestrate, N-W Sicily Island (Italy) | Southern Tyrrhenian Sea | 14 | Yes | 1 | 38.048130 | 12.997849 | Present paper |
| 2021 | February-April | Maamoura (Tunisia) | Tunisian waters Channel of Sicily | 10 | Yes | 2 | 36.463409 | 10.815337 | Social network/web |
| 2021 | March | Balearic Islands (Spain) | Algerian-Balearic Sea | 4 | Yes | 2 | https://digital.csic.es/handle/10261/244755 | Present paper | |
| 2021 | March | Lampedusa Island (Italy) | Pelagie archipelago Channel of Sicily | 11 | Yes | 2 | 35.509704 | 12.563556 | Social network/web |
| 2021 | March | Donnalucata (Italy) | Channel of Sicily | 16 | Yes | 1 | 36.759035 | 14.629979 | Present paper |
| MSNC | |||||||||
| 2021 | March-April | Nabeul-Chebba (Tunisia) | Tunisian waters Channel of Sicily | 10 | Yes | 2 | 36.435326 | 10.712459 | Social network/web |
| 2021 | April | Málaga (Spain) | Alboran Sea | 2 | Yes | 12 | https://digital.csic.es/handle/10261/244755 | Present paper | |
| 2021 | April | Rades; Amilcar Beach; Carthage; Bizerte (Tunisia) | Tunisian waters Channel of Sicily | 9 | Yes | 6 | 36.774053 | 10.298923 | Social network/web |
| 36.878652 | 10.347118 | ||||||||
| 37.282300 | 9.886873 | ||||||||
| 2021 | April | San Pietro Island (Italy) | Coastline of Sardinia | 7 | Yes | 1 | 39.246798 | 8.248352 | Social network/web |
| Western Mediterranean | |||||||||
| 2021 | April | Capo Peloro (Italy) | Strait of Messina | 17 | Yes | 1 | 38.271362 | 15.656095 | Social network |
| 2021 | April | Malta | Maltese Archipelago | 16 | Yes | 1 | 35.975497 | 14.451138 | Social network |
| Channel of Sicily | |||||||||
| 2021 | April-May | Almeria, Balearic Islands (Spain) | Algerian-Balearic Sea | 4 | Yes | 63 | https://digital.csic.es/handle/10261/244755 | Present paper | |
| 2021 | June | Balearic Islands (Spain) | Algerian-Balearic Sea | 4 | Yes | 60 | https://digital.csic.es/handle/10261/244755 | Present paper | |
Records of Physalia physalis in the Mediterranean Sea, pointed out in the map of Figure 1.
The records are listed following a chronological order; year and month, when noticed, are specified. The locality and the marine sector are indicated, the last according to the biogeographical division of the Mediterranean Sea and Italian waters.
MZUF, Natural History Museum of the University of Florence, Italy; DDM, Darwin Dohrn Museumof the Anton Dohrn Zoological Station of Naples, Italy; MZPA, Museum of Zoology “P. Doderlein” of the University of Palermo, Italy; MDBM, Museum of the Department of Biology of the University of Malta; MZCA Museum of Zoology, University of Cagliari; MSNC, Comiso Natural History Museum.
FIGURE 1
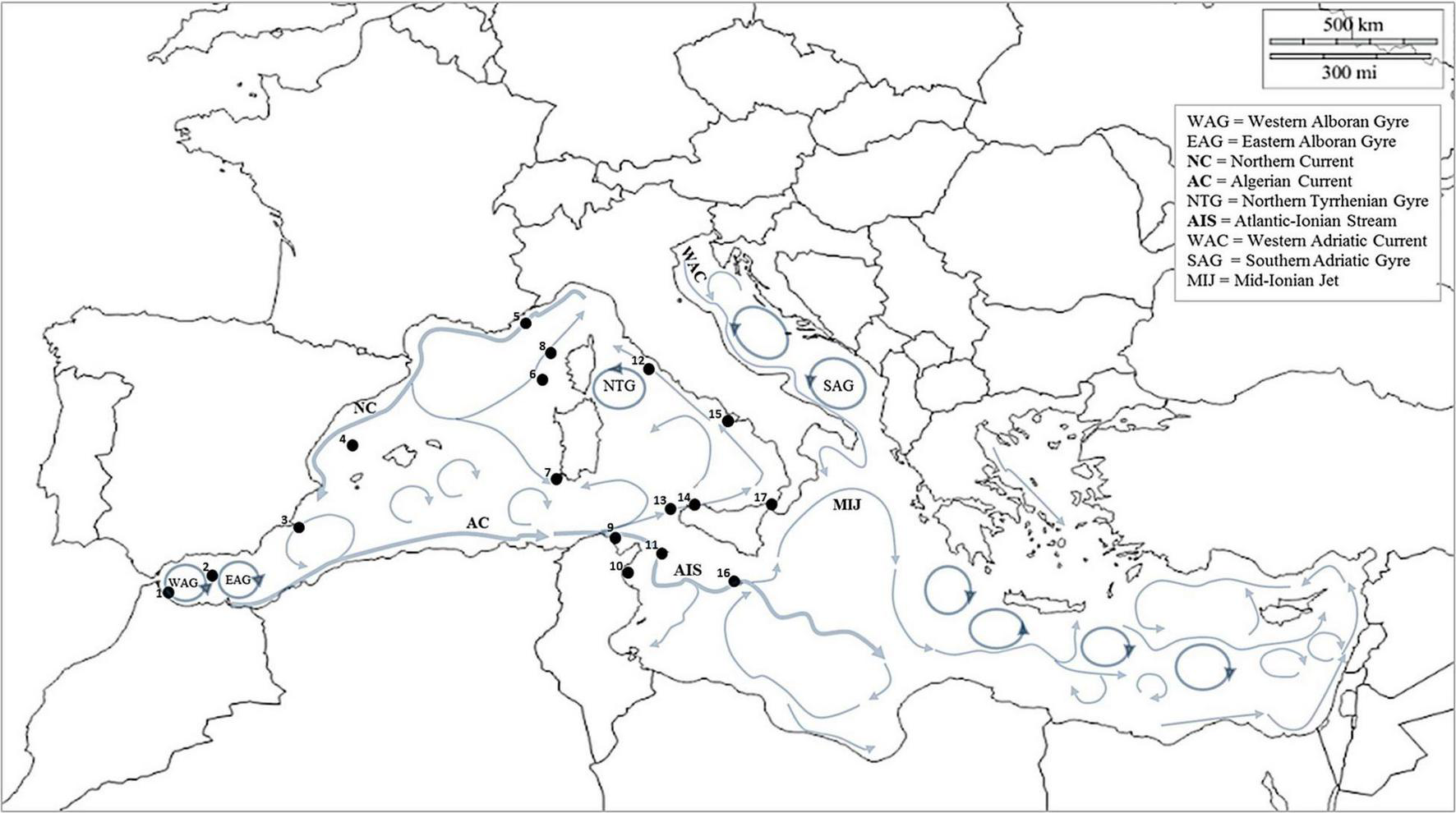
Map of thePhysalia physalis records in the Mediterranean Sea (1850 – 2021). The black points with the ID number indicate the area where P. physalis was found. The localities reported in Table 1 are associated with the ID number of such areas according to a neighbouring criterion. The arrows indicate the model of the main surface currents present in the Mediterranean Sea, according to Millot (1999) and Poulain et al. (2012).
The oldest documented colonies in the Mediterranean Sea were collected in the north-western area. The oldest uncovered records are preserved at the Natural History Museum of the University of Florence, a colony collected near Nice, France, dated back to 1850 (Figure 2; acquired by Gal Frères in June 1884—MZUF-uncatalogued) and a colony sampled in front of Livorno in 1863 (A. Targioni Tozzetti collection–voucher code MZUF-Cnidaria 1208). In 1914 a colony was collected in the Gulf of Naples, now preserved at the “Darwin Dohrn” Museum of the Anton Dohrn Zoological Station in Naples (voucher code HYD001; Figure 3). Further, by consulting the archive of the Museum of Zoology “Pietro Doderlein” of the University of Palermo, it was also possible to date a colony of P. physalis, collected along the north-western coast of Sicily Island (southern Italy), in a period antecedent to 1980, around 1960 (Silvano Riggio pers. comm.) (voucher code MZPA-IM-545). Presumably, further five colonies, preserved at the “Darwin Dohrn” Museum of the Anton Dohrn Zoological Station in Naples (voucher codes HYD002A, HYD002B, HYD174) and lacking date of capture, can be dated in a period antecedent to 1972 (Andrea Travaglini pers. comm.).
FIGURE 2
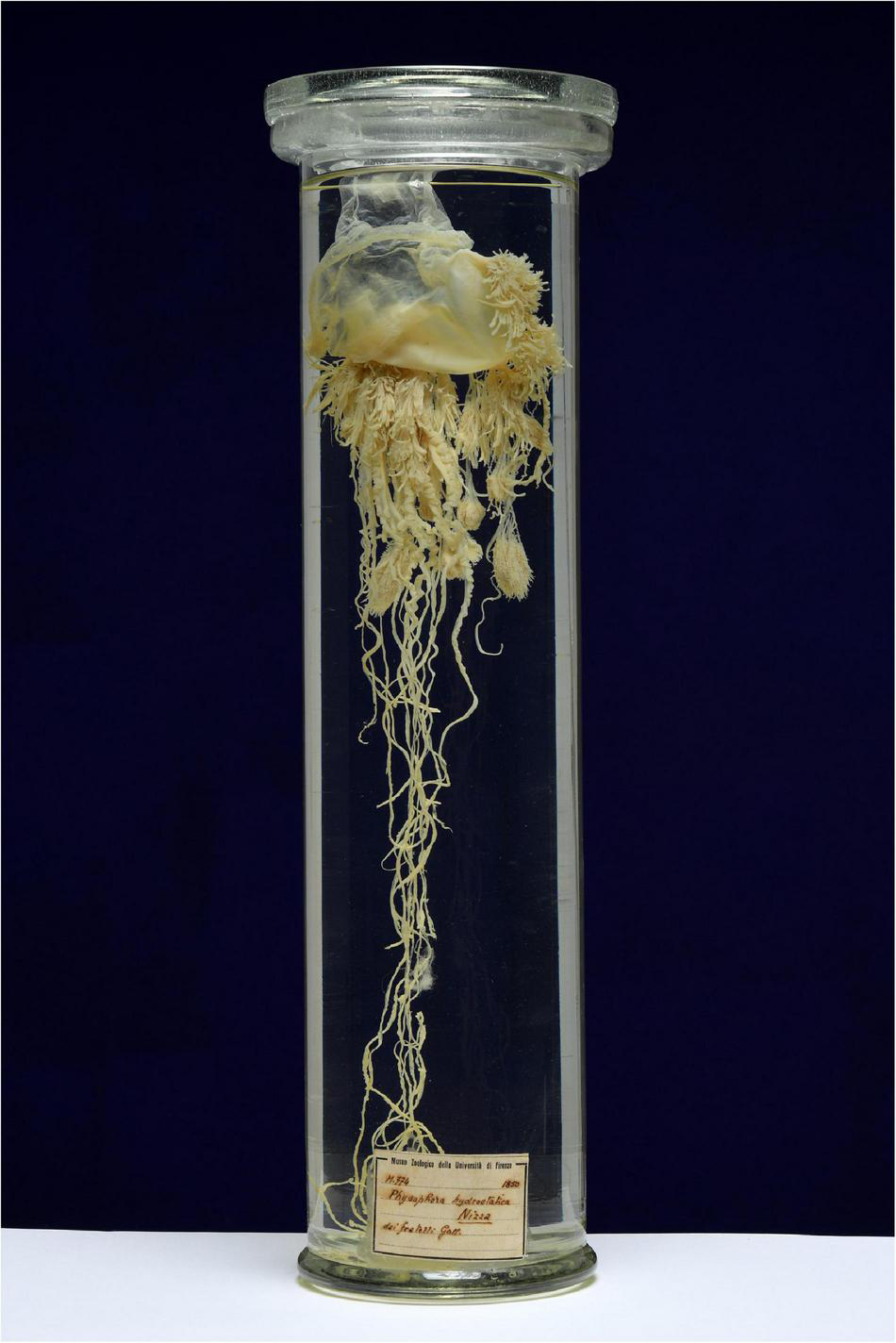
The colony of P. physalis collected in Mediterranean French waters (Nice) in 1850, voucher code MZUF-uncatalogued, preserved at the Natural History Museum of the University of Florence (photo ©Saulo Bambi, Museo di Storia NaturaleUniversitàdeglistudi di Firenze).
FIGURE 3

The two colonies of P. physalis collected in the Gulf of Naples (Italy) in 1914, preserved at the “Darwin Dohrn” Museum of Naples (photo by Akira Kihara, Science Center, Hosei University, Tokyo; ©2007 Zoological Collection Database Stazione Zoologica “Anton Dohrn”).
All the colonies were found stored in glass jars and were in a good state of conservation.
Recent Records
The recent records here assumed are the ones reported from 1980 to 2021. The year 1980 was the year of the presumptive first Mediterranean record, as highlighted by Berdar and Cavallaro (1980), however not confirmed by the museum collections above listed. The specimens preserved in the zoological collections did not further corroborate a record that was presumptively thought of as the first on the north-western coast of Sicily. Such colony stranded on the 17th February 2021 (Balestrate beach, Gulf of Castellammare) was photographically documented (Figure 4) and is here stated as new information. It was found still alive and showed the typical bluish colour of the species; it measured approximately 20 cm in total length. Considering that the sail of P. physalis can reach 30 cm in total length, the colony can be considered of medium-large size (Wilson, 1947; Prieto et al., 2015). In the same year (2021), from February to April, P. physalis strandings occurred along the coast of Tunisia and Lampedusa Island (Channel of Sicily sector), in the Strait of Messina and the waters of Sardinia Island (Western Mediterranean sector) (Table 1 and Figure 1). The sightings were photographed and published on the web and social networks. On the 19th of August 2021, a colony was found stranded in Donnalucata (Channel of Sicily sector—Sicily). The colony is currently preserved at the Civic Museum of Natural History of Comiso. The pneumatophore has a length of 111 mm (Gianni Insacco pers. comm.).
FIGURE 4

The colony of P. physalis recorded in the north-western coast of Sicily, southern Tyrrhenian basin in 2021 (photo by Rosario Badalamenti).
The records of P. physalis successive to the 1980 start in 2001. There is a gap of 20 years, from 1980 to 2000.
In 2001, the presence of this species was documented in Malta Island, although local fishermen affirmed that they had encountered the species previously (Calleja, 2009). In Maltese waters, this species has been frequently registered: in May 2001, from February to August 2009, from March to June 2010, and in the period 2011-2020 [Patrick J. Schembri pers. comm.; Deidun (2010) and Deidun et al. (2020)]. The sample registered in May 2001, stranded in Golden Bay, Malta, is preserved in the Museum of the Department of Biology of the University of Malta (Patrick J. Schembri pers. comm.). In this latter period (2011–2020), two records were also reported from the Aegadian archipelago—Southern Tyrrhenian sector, and one from the Island of Sardinia—Western Mediterranean sector (Deidun et al., 2020; Table 1 and Figure 1).
In February-July 2010, 840 colonies were registered along the Spanish coast (Prieto et al., 2015). The Western Mediterranean basin experienced several swarms, recording hundreds of colonies in different sites on the Spanish coast (Alboran Sea sector). A colony captured along Sardinia coast is preserved at the Museum of Zoology of the University of Cagliari (Table 1); it represents a record that supports the information extrapolated from Boero (2013) of which documentation was not retrieved.
In the following years, 2011, 2012, and 2013, the number of sightings decreased significantly, in fact, lesser colonies were recorded in the same Spanish monitored sites (Prieto et al., 2015).
In 2018, between March and April, an increasing number of colonies of P. physalis were registered for the first time on the Mediterranean coast of Morocco—Alboran Sea—Algerian-Balearic Sea sectors (Mghili et al., 2020; Macías et al., 2021) (see Table 1).
The flow of P. physalis from the Atlantic Ocean toward the western Mediterranean basin seemed to be attributed to an unusual combination of meteorological and oceanographic factors during the winter period (Poulain et al., 2012; Prieto et al., 2015). In the spring of 2010 (Prieto et al., 2015), 2013 (Macías et al., 2021), 2018 (Mghili et al., 2020; Macías et al., 2021; Prieto, 2021), 2019 and 2021 intense strandings were recorded in the Alboran Sea area, probably due to the action of the Western Alboran Gyre (WAG) and Eastern Alboran Gyre (EAG) (Poulain et al., 2012). Such surface currents of the Alboran Sea can behave also as physical barriers to the flow toward the western Mediterranean, as suggested by Prieto et al. (2015), limiting the easternmost circulation of a large number of P. physalis colonies. Only a few colonies that escape the front manage to circulate by the benefit of the surface currents present in the Mediterranean Sea (Poulain et al., 2012). By creating a map of the P. physalis records documented in the last century, and superimposing it with the trajectories of the surface currents that drive the colonies (Poulain et al., 2012), it has been shown a general pattern of occurrence of the Portuguese man-of-war within the Mediterranean Sea (Figure 1).
Records of Physalia physalis in the Italian Maritime Regions
A portion of the records of P. physalis was grouped in four Italian biogeographical sectors: Sardinian waters, Channel of Sicily, Tyrrhenian Sea, Strait of Messina, mainly around the Sicily Island, central Mediterranean. Such sectors are the ones supposed by Macías et al. (2021) as the zone where the species mainly occurs in the summer period; thus the clustering was arranged to identify those marine areas most affected by P. physalis sightings and strandings. The validated records of P. physalis were subdivided into three categories of risk (Figure 5) according to the season of occurrence and the level of danger for swimmers.
FIGURE 5
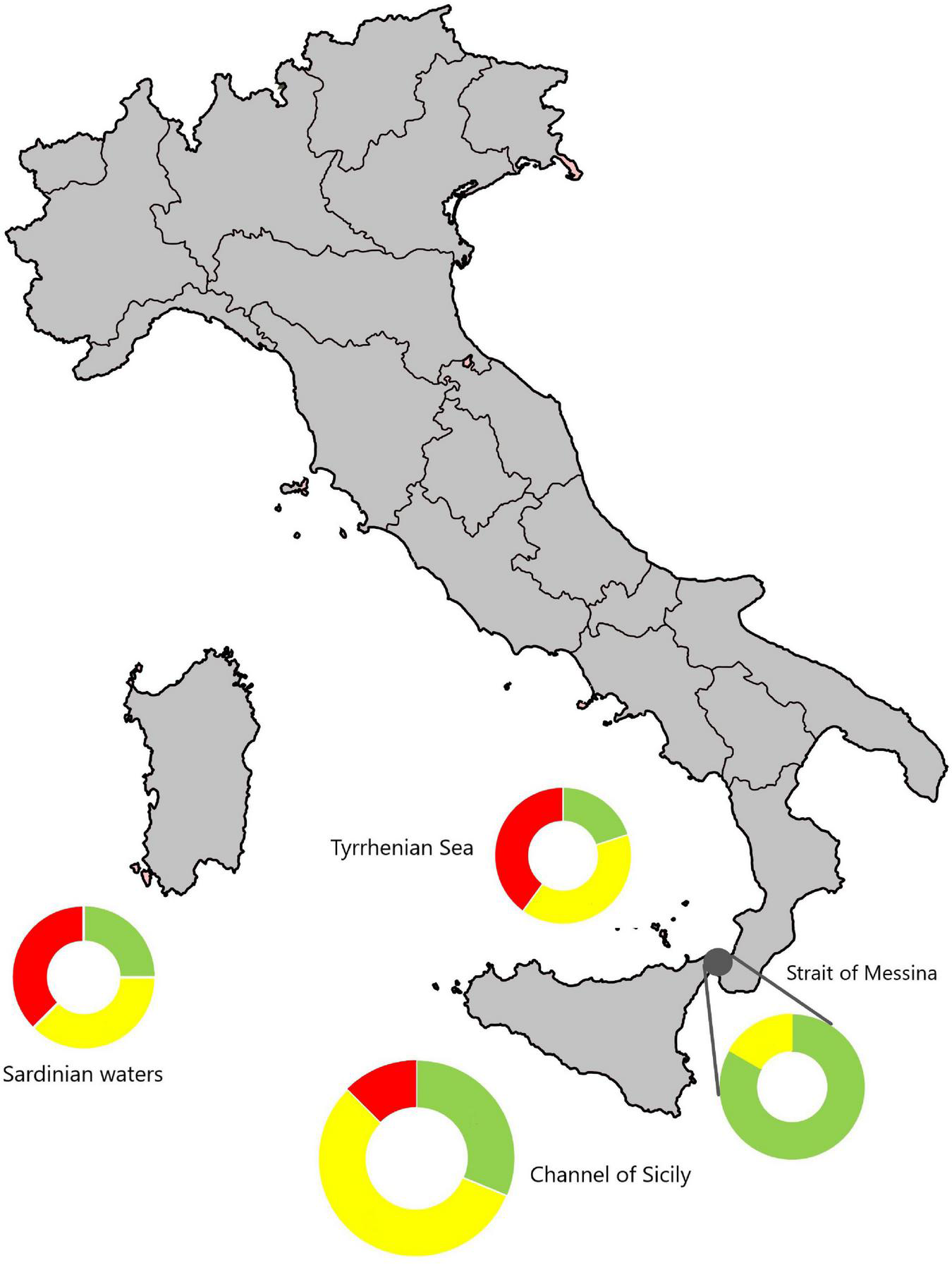
A schematic clustering of the records of P. physalis in the Italian maritime zone (see Table 1 for details); the records were aggregated in four different geographic sectors (from west to east, Sardinian waters; Channel of Sicily; Tyrrhenian Sea;Strait of Messina), and categorizedto a level of risk as tool for tourism sector. The green records indicate the number of colonies sighted in the period February–March (low-risk); yellow is the number of colonies sighted in the period April–May (medium-risk); red is the number of colonies sighted in the period June–August (high-risk). The map can be be used by local and regional managers to assess the risk of having free floating P. physalis colonies intheir area of interest.
A total of eight colonies were recorded in Sardinian waters, two belonging to the low-risk category, three to medium-risk and three to high-risk class (Figure 4). The Channel of Sicily showed the largest number of colonies sighted and stranded, thirty-two colonies of which ten were at low-risk, eighteen at medium-risk and four at high-risk. In the Tyrrhenian marine sector five colonies occurred, of which the only one at low-risk, two at medium-risk and two at high-risk. In this marine sector, the colonies of the Gulf of Naples and the Gulf of Palermo recorded in the museum collections were excluded because they were catalogued without a precise date. A total of six colonies were registered in the Strait of Messina, of which five were at low-risk and one at medium-risk.
Discussion
Physalia physalis is one of the most dangerous species of cnidarians that threaten public health. This is because the sting of this species is very painful and the venom present in nematocysts can cause health complications for people attacked. In some documented cases, these incidents have had fatal outcomes (Burke, 2002; Labadie et al., 2012). P. physalis can be harmful also in an indirect way, as prey of neustonic nudibranchs; in fact, some planktonic molluscs ingest the cnidarian and undischarged nematocysts as a defensive strategy (Thompson and Bennett, 1969). The nematocysts maintain toxicity in the predators (Thompson and Bennett, 1969; Ottuso, 2009). The same harmfulness has been detected in the stranded P. physalis individuals on a beach, even after several days of dehydration (Tibballs, 2006).
In the “Guidelines for safe recreational water environments” of the World Health Organization (WHO), P. physalis is included among the most dangerous aquatic organisms for human health, suggesting avoiding bathing in waters where Portuguese man-of-war is concentrated (World Health Organization [WHO], 2003). In order to develop policies for controlling and managing the well-being in recreational activities, a wide spatial and temporal scale of data is needed for an adequate assessment of the risk of contact with the Portuguese man-of-war within the Mediterranean Sea. Further, the launch of a series of activities based on scientific dissemination campaigns can allow the local authorities in charge to plan effective actions regarding the protection of public health and tourism (De Donno et al., 2014; Montgomery et al., 2016).
This is particularly relevant within the Mediterranean Sea where dispersal of some cnidarian species need major attention (Rossi et al., 2019) and where a great portion of the population still ignore the real hazard of most of the species, even though the high number of sightings and strandings recently detected through the media and social networks have helped to deepen the knowledge of the animal group.
Thus data were herein collected from different sources: social networks—scientific literature—museum zoological collections, as current and historical information on the distribution of the Portuguese man-of-war in the Mediterranean had not been organized yet and presented in a single article.
Under this operational framework, it was possible to track the putative first records of P. physalis in the Mediterranean Sea. The oldest documented capture of P. physalis occurred in Nice in 1850. Furthermore, it was possible to date further colonies of P. physalis, preserved in museums and captured in a wide area of the Western Mediterranean region in a period antecedent to 1980, even older than the record attributed as the first by Berdar and Cavallaro (1980) in the Strait of Messina.
The role of museums was fundamental for reconstructing the past distribution of P. physalis. A new scenario on its presence in the Mediterranean Sea has been depicted, up to now suspected by the scientific community (Boero, 2013). Indeed, based on these new data and those reported in the literature and here examined, it is very likely that P. physalis has always entered the Mediterranean Sea.
The occurrence of P. physalis in the Mediterranean had been considered rare till the last decades, due to the lack of documented records in the literature (Castriota et al., 2017). Only in recent years, an increase of P. physalis sightings in the central Mediterranean Sea was reported. The reasons are attributable to the advancement of technological innovations (smartphones), which make sightings validated with photographs or videos (not possible in the past) as the case of the Maltese fishermen who were not able to provide evidence of sightings (Calleja, 2009).
From 2001 to 2020, several P. physalis strandings have been documented in the Mediterranean Sea [Patrick J. Schembri pers. comm.; Deidun (2010), Focus (2010), Boero (2013), Mare Nostrum Italia (2013), Prieto et al. (2015); Castriota et al. (2017), Deidun et al. (2020); Mghili et al. (2020), Marambio et al. (2021), and Macías et al. (2021)] and lead us to assess the pathway of the colonies in the Mediterranean Sea. The route and the extent of flow depend on the particular climatic-oceanographic conditions which regulate the entry from the Atlantic Ocean toward the Mediterranean, as argued by Poulain et al. (2012).
The data showed massive strandings (thousands of colonies) recorded in the Alboran Sea (Prieto et al., 2015; Mghili et al., 2020; Macías et al., 2021), probably due to the action of the strong gyres in shallow water (Poulain et al., 2012), which behave like a physical barrier, as suggested by Prieto et al. (2015). The Atlantic water in the Alboran Sea describes two anticyclonic (clockwise rotating) gyres that dominate the surface flow pattern: a quasi-permanent anticyclonic gyre in the west (Western Alboran Gyre, WAG) and a more variable circuit in the east (Eastern Alboran Gyre, EAG) (Figure 1; Millot, 1999). In such a situation, the vein flowing from Spain to Algeria, named “the Almeria-Oran jet” (Millot, 1999), stops a large number of colonies, preventing their eastward dispersal, except some colonies that find a favourable oceanographic route and move toward the western and central Mediterranean.
As the circulation features of the Alboran sector is largely controlled by meteorological conditions (Macías et al., 2021), some concerns regard climate change which could alter the role of natural barrier in the future, allowing a greater entry of P. physalis from the Atlantic Ocean. Under such a hypothetical scenario of an increasing number of colonies of P. physalis, we should limit human activities eventually exposed to risks in the central Mediterranean.
The pattern described in the map of Figure 1 gives an idea of how the Portuguese man-of-war circulates in the Mediterranean. The records follow the surface currents and cover a limited geographical range maybe due to biological constraints, as it is probable that P. physalis dies during its “travel” in the Mediterranean Sea and new colonies enter through the Strait of Gibraltar the successive year. Based on this, at least for now, we cannot consider this species as an invasive or non-indigenous one.
The tracking of this species in the Mediterranean Sea should not be neglected, as the species threaten human health. For this reason, we selected some areas of risk by grouping the records of P. physalis in the most concerned four Italian maritime sectors: Sardinian waters; Channel of Sicily; Tyrrhenian Sea; Strait of Messina. The marine sectors most involved by P. physalis passage were identified and associated with levels of danger for swimmers according to the seasons of occurrence of the colonies (Figure 5). The highest number of colonies were recorded in the Channel of Sicily, probably also thanks to the active monitoring that has taken place in recent years. The area showed records belonging to the three categories of risk, low- medium- and high-risk category; it means that the species occurs from February to August. The Strait of Messina seems to be the area less involved by the passage of the Portuguese man-of-war. Concerning some records here reported, such as the specimen of the Gulf of Castellammare (Balestrate, southern Tyrrhenian Sea), the particular morphology of gulfs or bays with their currents regime could contribute to maintaining the colonies of P. physalis within the bay once has entered, with no or little chance to leaving the area. Hence the need for a better understanding and prediction through models of the currents and wind regime in these particular areas is auspicate.
The recent expansion of Citizen Science projects and the use of social media for the detection of “strange-looking species” or charismatic species may have certainly contributed to increasing the number of observations in nature, including Physalia physalis. In this regard, we observed, especially in the last decade, how Citizen Science is experiencing an upsurge of interest and was demonstrated to be particularly effective for data collection and maritime monitoring (Devictor et al., 2010; Dickinson et al., 2010). In the Mediterranean Sea, several Citizen Science projects are currently active and contribute to the early detection and monitoring of uncommon or non-indigenous species (Giovos et al., 2019; Tiralongo et al., 2019, 2020). These projects have often benefited from the involvement of the public through social media (Azzurro and Tiralongo, 2020; Al Mabruk et al., 2021). Citizen Science could be an efficient tool for information campaigns to introduce the Portuguese man-of-war to the public and better track the early detection of the species, as already done for other harmful organisms (Andaloro et al., 2016).
The results suggest that a national multidisciplinary summer surveillance program in the central Mediterranean Sea coast is required to provide alerts to the public, and hopefully to improve the quality of health care. Local authorities should set up a monitoring and alert system, with the participation of territorial organizations, and the contribution of the scientific community, as proposed in other seas (Labadie et al., 2012). In this regard, engaging tourists as citizen scientists can be a useful and low-cost method for the monitoring of this and other dangerous species in general and on a large spatial-temporal scale (Schaffer and Tham, 2019). Continuous monitoring could beneficial for marine tourist facilities.
In general, the data reported can be considered an important reference point for the development and calibration of predictive models on jellyfish abundance and distribution over a large timeframe. The importance of obtaining time-series data to monitor biodiversity changes in the Mediterranean Sea is crucial and acquire a relevant role in respect to the more frequent extreme events and temperature anomalies.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SL and FT conceived the project and contributed to final review and editing of the manuscript. LP provided some data. SL, LP, and RB analysed the data. SL, FT, and RB drafted the manuscript. SL, LP, FT, and VA reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Open Access Funding provided by Università degli Studi di Palermo. This work was carried out within the framework of the Ph.D. project of RB funded by Università degli Studi di Palermo. This research was also supported by the Project Agreement “Sistema de Observación y Predicción de Medusas en el Mar Balear” among Govern des Illes Balears, SOCIB and CSIC (Disposición 15052, BOE núm. 310, 2020).
Acknowledgments
We thank Cecilia Volpi (Natural History Museum of the University of Florence, Florence, Italy), Gianni Insacco [Museo Civico di Storia Naturale, Comiso (RG), Italy], Claudia Gili and Andrea Travaglini (Darwin Dohrn Museum of the Anton Dohrn Zoological Station, Naples, Italy), Enrico Bellia (Museum of Zoology P. Doderlein of the University of Palermo, Palermo, Italy), Susanna Salvadori (Museum of Zoology, University of Cagliari), Maria Linda Tumbiolo and Paola Pepe for helping in catalouging the marine invertebrates collection of the Museum of Zoology P. Doderlein of the University of Palermo, and Silvano Riggio and Patrick J. Schembri for being very helpful in getting information about museum specimens. We thank the three reviewers for their helpful suggestions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Al Mabruk S. A. A. Abdulghani A. Nour O. M. Adel M. Crocetta F. Doumpas N. et al (2021). The role of social media in compensating for the lack of field studies: five new fish species for Mediterranean Egypt.J. Fish Biol.99673–678. 10.1111/jfb.14721
2
Andaloro F. Castriota L. Falautano M. Azzurro E. Deidun A. Fenech-Farrugia A. (2016). Public feedback on early warning initiatives undertaken for hazardous non-indigenous species: the case of Lagocephalus sceleratus from Italian and Maltese waters.Manag. Biol. Invasions7313–331. 10.3391/mbi.2016.7.4.01
3
Arai M. N. (2005). Predation on pelagic coelenterates: a review.J. Mar. Biol. Assoc. U. K.85523–536. 10.1017/s0025315405011458
4
Azzurro E. Tiralongo F. (2020). First record of the mottled spinefoot Siganus fuscescens (Houttuyn, 1782) in Mediterranean waters: a Facebook based detection.Mediterr. Mar. Sci.21448–451.
5
Bardi J. Marques A. C. (2007). Taxonomic redescription of the Portuguese man-of-war, Physalia physalis (Cnidaria, Hydrozoa, Siphonophorae, Cystonectae) from Brazil.Iheringia Ser. Zool.97425–433. 10.1590/s0073-47212007000400011
6
Baumann S. Schernewski G. (2012). Occurrence and public perception of jellyfish along the German Baltic coastline.J. Coast. Conserv.16555–566. 10.1007/s11852-012-0199-y
7
Bellido J. Báez J. Souviron-Priego L. Ferri-Yañez F. Salas C. López J. A. et al (2020). Atmospheric indices allow anticipating the incidence of jellyfish coastal swarms.Mediterr. Mar. Sci.21289–297.
8
Berdar A. Cavallaro G. (1980). Celenterati, ctenofori e tunicati spiaggiati lungo la costa siciliana dello Stretto di Messina.Mem. Biol. Mar. Oceanogr.1019–26.
9
Bianchi C. N. (2004). Proposta di suddivisione dei mari Italiani in settori biogeografici.Not. SIBM4657–59. 10.1016/j.quip.2011.06.003
10
Bieri R. (1966). Feeding preferences and rates of the snail, Ianthina prolongata, the barnacle, Lepas anserifera, the nudibranchs, Glaucus atlanticus and Fiona pinnata, and the food web in the marine neuston.Publ. Seto Mar. Biol. Lab.14161–170. 10.5134/175429
11
Boero F. (2013). Review of jellyfish blooms in the mediterranean and black sea. General fisheries commission for the mediterranean.Stud. Rev.921–53.
12
Boero F. Bouillon J. Gravili C. Miglietta M. P. Parsons T. Piraino S. (2008). Gelatinous plankton: irregularities rule the world (sometimes).Mar. Ecol. Prog. Ser.356299–310. 10.3354/meps07368
13
Boero F. Gennari A. Tresca F. Miglietta A. M. (2010). Il plancton gelatinoso e la campagna“ Occhio alla medusa”.Caspur Ciber Publ.136–64.
14
Bosch-Belmar M. Azzurro E. Pulis K. Milisenda G. Fuentes V. Kéfi-Daly Yahia O. et al (2017). Jellyfish blooms perception in Mediterranean finfish aquaculture.Mar. Policy761–7. 10.1016/j.marpol.2016.11.005
15
Burke W. A. (2002). Cnidarians and human skin.Dermatol. Ther.1518–25. 10.1046/j.1529-8019.2002.01508.x
16
Burnett J. A. Gable W. D. (1989). Fatal jellyfish envenomation by the Portuguese man-of-war.Toxicon27823–824. 10.1016/0041-0101(89)90050-0
17
Calleja C. (2009). Jellyfish: The Man-of-War was here. Available Online at: https://timesofmalta.com/articles/view/jellyfish-the-man-of-war-was-here.260909[accessed March 27, 2020].
18
Castriota L. Falautano M. Battaglia P. Maraventano G. Prazzi E. Ammendolia G. et al (2017). First record of Physalia physalis in the Pelagie Islands (Strait of Sicily) and additional records in the Strait of Messina.Cah. Biol. Mar.581–4. 10.1163/9789004364189_002
19
Condon R. H. Graham W. M. Duarte C. M. Pitt K. A. Lucas C. H. Haddock S. H. D. et al (2012). Questioning the rise of gelatinous zooplankton in the world’s oceans.Bioscience62160–169. 10.1525/bio.2012.62.2.9
20
De Donno A. Idolo A. Bagordo F. Grassi T. Leomanni A. Serio F. et al (2014). Impact of stinging jellyfish proliferations along south Italian coasts: human health hazards, treatment and social costs.Int. J. Environ. Res. Public Health112488–2503. 10.3390/ijerph110302488
21
Deidun A. (2010). Notes on the recent occurrence of uncommon pelagic “jellyfish” species in Maltese coastal waters.Nat. Sicil.IV, XXXIV, 375–384.
22
Deidun A. Balistreri P. Zava B. (2020). Spreading further east: documenting the further penetration of the Portuguese man o’war Physalia physalis (Linnaeus, 1758) within the central Mediterranean. In: Bo et al. new records of rare species in the Mediterranean Sea.Mediterr. Mar. Sci.21608–630.
23
Devictor V. Whittaker R. J. Beltrame C. (2010). Beyond scarcity: citizen science programmes as useful tools for conservation biogeography.Divers. Distrib.16354–362. 10.1111/j.1472-4642.2009.00615.x
24
Dickinson J. Zuckerberg B. Bonter D. N. (2010). Citizen science as an ecological research tool: challenges and benefits.Annu. Rev. Ecol. Evol. Sci.41149–172. 10.1146/annurev-ecolsys-102209-144636
25
Elston D. M. (2007). Aquatic antagonists: Portuguese man-of-water (Physalia physalis).Cutis80186–188.
26
Ferrer L. González M. (2021). Relationship between dimorphism and drift in the Portuguese man-of-war.Cont. Shelf Res.212:104269. 10.1016/j.csr.2020.104269
27
Ferrer L. Pastor A. (2017). The Portuguese man-of-war: gone with the wind.Reg. Stud. Mar. Sci.1453–62. 10.1016/j.rsma.2017.05.004
28
Fleming N. E. C. Harrod C. Houghton J. D. R. (2013). Identifying potentially harmful jellyfish blooms using shoreline surveys.Aquac. Environ. Interact.4263–272. 10.3354/aei00086
29
Focus (2010). Prima Vittima di Medusa nel Mediterraneo. Available Online at: https://www.focus.it/temi/physalia-physalis[accessed July 10, 2021].
30
Giovos I. Kleitou P. Poursanidis D. Batjakas I. Bernardi G. Crocetta F. et al (2019). Citizen-science for monitoring marine invasions and stimulating public engagement: a case project from the eastern Mediterranean.Biol. Invasions213707–3721. 10.1007/s10530-019-02083-w
31
Graham W. M. Pagès F. Hamner W. M. (2001). A physical context for gelatinous zooplankton aggregations: a review.Hydrobiologia451199–212. 10.1007/978-94-010-0722-1_16
32
Gravili C. Rossi S. (2021). Who’s next. Non-indigenous cnidarian and ctenophoran species approaching to the Italian waters.Water13:1062. 10.3390/w13081062
33
Gutiérrez-Estrada J. C. Pulido-Calvo I. Peregrín A. García-Gálvez A. Báez J. C. Bellido J. J. et al (2021). Integrating local environmental data and information from non-driven citizen science to estimate jellyfish abundance in Coastal del Sol (southern Spain).Estuar. Coast. Shelf Sci.249:107112. 10.1016/j.ecss.2020.107112
34
Haddad V. da Silveira F. L. Cardoso J. L. C. Morandini A. C. (2002). A report of 49 cases of cnidarian envenoming from Southeastern Brazilian coastal waters.Toxicon401445–1450. 10.1016/s0041-0101(02)00162-9
35
Iosilevskii G. Weihs D. (2009). Hydrodynamics of sailing of the Portuguese man-of-war Physalia physalis.J. R. Soc. Interface6613–626. 10.1098/rsif.2008.0457
36
Kirkpatrick P. A. Pugh P. (1984). Siphonophores and velellids. Keys and notes for the identification of the species. Synopses of the British fauna.Lond. New Ser.29:154.
37
Labadie M. Aldabe B. Ong N. Joncquiert-Latarjet A. Groult V. Poulard A. et al (2012). Portuguese man-of-war (Physalia physalis) envenomation on the Aquitaine coast of France: an emerging health risk.Clin. Toxicol.50567–570. 10.3109/15563650.2012.707657
38
Lane C. E. (1960). The Portuguese man-of-war.Sci. Am.202158–171. 10.1038/scientificamerican0360-158
39
Lo Brutto S. Calascibetta A. Pavan G. Buffa G. (2021). Cetacean strandings and museum collections: a focus on Sicily Island crossroads for Mediterranean species.Diversity13:104. 10.3390/d13030104
40
Macías D. Prieto L. García-Gorriz E. (2021). A model-based management tool to predict the spread of Physalia physalis in the Mediterranean Sea. Minimizing risks for coastal activities.Ocean Coast. Manag.212:105810. 10.1016/j.ocecoaman.2021.105810
41
Mapstone G. M. (2014). Global diversity and review of Siphonophorae (Cnidaria: Hydrozoa).PLos One9:e87737. 10.1371/journal.pone.0087737
42
Marambio M. Canepa A. Lòpez L. Gauci A. A. Gueroun S. K. M. Zampardi S. et al (2021). Unfolding jellyfish bloom dynamics along the mediterranean basin by transnational citizen science initiatives.Diversity13:274. 10.3390/d13060274
43
Mare Nostrum Italia (2013). Physalia Physalis - Caravella Portoghese. Available Online at: http://marenostrumitalia.weebly.com/biologia-marina/physaliaphysalis-caravella-portoghese[accessed July 10, 2016].
44
Mghili B. Analla M. Aksissou M. (2020). First records of the jellyfish Physalia physalis (Linnaeus, 1758) on the Mediterranean coast of Morocco.Cah. Biol. Mar.61263–269.
45
Millot C. (1999). Circulation in the Western Mediterranean Sea.J. Mar. Syst.20423–442. 10.1016/s0924-7963(98)00078-5
46
Montgomery L. Seys J. Mees J. (2016). To pee, or not to pee: a review on envenomation and treatment in European Jellyfish species.Mar. Drugs14:127. 10.3390/md14070127
47
Munro C. Vue Z. Behringer R. R. Dunn C. W. (2019). Morphology and development of the Portuguese man of war, Physalia physalis.Sci. Rep.9:15522.
48
Oliveira O. M. P. Miranda T. P. Araujo E. M. Ayón P. Cedeño-Posso C. M. Cepeda-Mercado A. A. et al (2016). Census of Cnidaria (Medusozoa) and Ctenophora from South American marine waters.Zootaxa4194:zootaxa.4194.1.1. 10.11646/zootaxa.4194.1.1
49
Ottuso P. T. (2009). Aquatic antagonists: indirect nematocyst envenomation and acute allergic contact dermatitis due to nudibranchs.Cutis83237–239.
50
Pierce J. (2006). Aquarium design for the Portuguese man-of-war Physalia physalis.Int. Zoo. Yearb.40221–231. 10.1111/j.1748-1090.2006.00221.x
51
Pontin D. R. Schliebs S. Worner S. P. Watts M. J. (2011). Determining factors that influence the dispersal of a pelagic species: a comparison between artificial neural networks and evolutionary algorithms.Ecol. Modell.2221657–1665. 10.1016/j.ecolmodel.2011.03.002
52
Poulain P. M. Menna M. Mauri E. (2012). Surface geostrophic circulation of the Mediterranean Sea derived from drifter and satellite altimeter data.J. Phys. Oceanogr.42973–990. 10.1175/jpo-d-11-0159.1
53
Prieto L. (2021). Physalia physalis abundance on the shores in the Mediterranean Sea during 2010, 2013 and 2018 compiled by ICMAN (CSIC).Madrid: DIGITAL.CSIC. 10.20350/digitalCSIC/13920
54
Prieto L. Macias D. Peliz A. Ruiz J. (2015). Portuguese man-of-war (Physalia physalis) in the Mediterranean: a permanent invasion or a casual appearance?Sci. Rep.5:11545.
55
Purcell J. E. (1984). Predation on fish larvae by Physalia physalis, the Portuguese man of war.Mar. Ecol. Prog. Ser.19189–191. 10.3354/meps019189
56
Rossi S. Gravili C. Milisenda G. Bosch-Belmar M. De Vito D. Piraino S. (2019). Effects of global warming on reproduction and potential dispersal of Mediterranean Cnidarians.Eur. Zool. J.86255–271. 10.1080/24750263.2019.1631893
57
Schaffer V. Tham A. (2019). Engaging tourists as citizen scientists in marine tourism.Tourism Rev.75333–346. 10.1108/tr-10-2018-0151
58
Shannon L. V. Chapman P. (1983). Incidence of Physalia on beaches in the South Western Cape Province during January 1983.S. Afr. J. Sci.79454–458.
59
Tedesco M. R. Mandalà G. Consentino M. C. Ientile R. Lo Valvo F. Movalli P. et al (2020). The first inventory of birds of prey in Sicilian Museum collections (Italy).Museol. Sci.1467–80.
60
Thompson T. E. Bennett I. (1969). Physalia nematocysts: utilized by mollusks for defense.Science1661532–1533. 10.1126/science.166.3912.1532
61
Thompson T. E. Bennett I. (1970). Observations on Australian Glaucidae (Mollusca: Opisthobranchia).Zool. J. Linn. Soc.49187–197. 10.1111/j.1096-3642.1970.tb00735.x
62
Tibballs J. (2006). Australian venomous jellyfish, envenomation syndromes, toxins and therapy.Toxicon48830–859. 10.1016/j.toxicon.2006.07.020
63
Tiralongo F. Crocetta F. Riginella E. Lillo A. O. Tondo E. Macali A. et al (2020). Snapshot of rare, exotic and overlooked fish species in the Italian seas: a citizen science survey.J. Sea Res.164:101930. 10.1016/j.seares.2020.101930
64
Tiralongo F. Lillo A. O. Tibullo D. Tondo E. Lo Martire C. D’Agnese R. et al (2019). Monitoring uncommon and non-indigenous fishes in Italian waters: one year of results for the alien Fish project.Reg. Stud. Mar. Sci.28:100606. 10.1016/j.rsma.2019.100606
65
Wangersky E. D. Lane C. E. (1960). Interaction between the plasma of the loggerhead turtle and toxin of the Portuguese man-of-war.Nature185330–331. 10.1038/185330b0
66
World Health Organization [WHO] (2003). Guidelines for Safe Recreational Water Environment: Coastal and Freshwater.Geneva: World Health Organization.
67
Wilson D. P. (1947). The Portuguese man-of-war, Physalia physalis L., in British and adjacent seas.J. Mar. Biol. Assoc. U. K.27139–172. 10.1017/s0025315400014156
Summary
Keywords
Physalia physalis , zoological collections, dangerous species, citizen science, tourism sector, Mediterranean biodiversity
Citation
Tiralongo F, Badalamenti R, Arizza V, Prieto L and Lo Brutto S (2022) The Portuguese Man-of-War Has Always Entered the Mediterranean Sea—Strandings, Sightings, and Museum Collections. Front. Mar. Sci. 9:856979. doi: 10.3389/fmars.2022.856979
Received
18 January 2022
Accepted
28 February 2022
Published
29 March 2022
Volume
9 - 2022
Edited by
Pierluigi Carbonara, COISPA Tecnologia & Ricerca, Italy
Reviewed by
Dimitris Poursanidis, Terrasolutions Marine Environment Research, Greece; Juan Jesús Bellido López, Aula del Mar Málaga, Spain; Joe El Rahi, Ghent University, Belgium
Updates
Copyright
© 2022 Tiralongo, Badalamenti, Arizza, Prieto and Lo Brutto.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sabrina Lo Brutto, sabrina.lobrutto@unipa.it
This article was submitted to Marine Biology, a section of the journal Frontiers in Marine Science
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.