Abstract
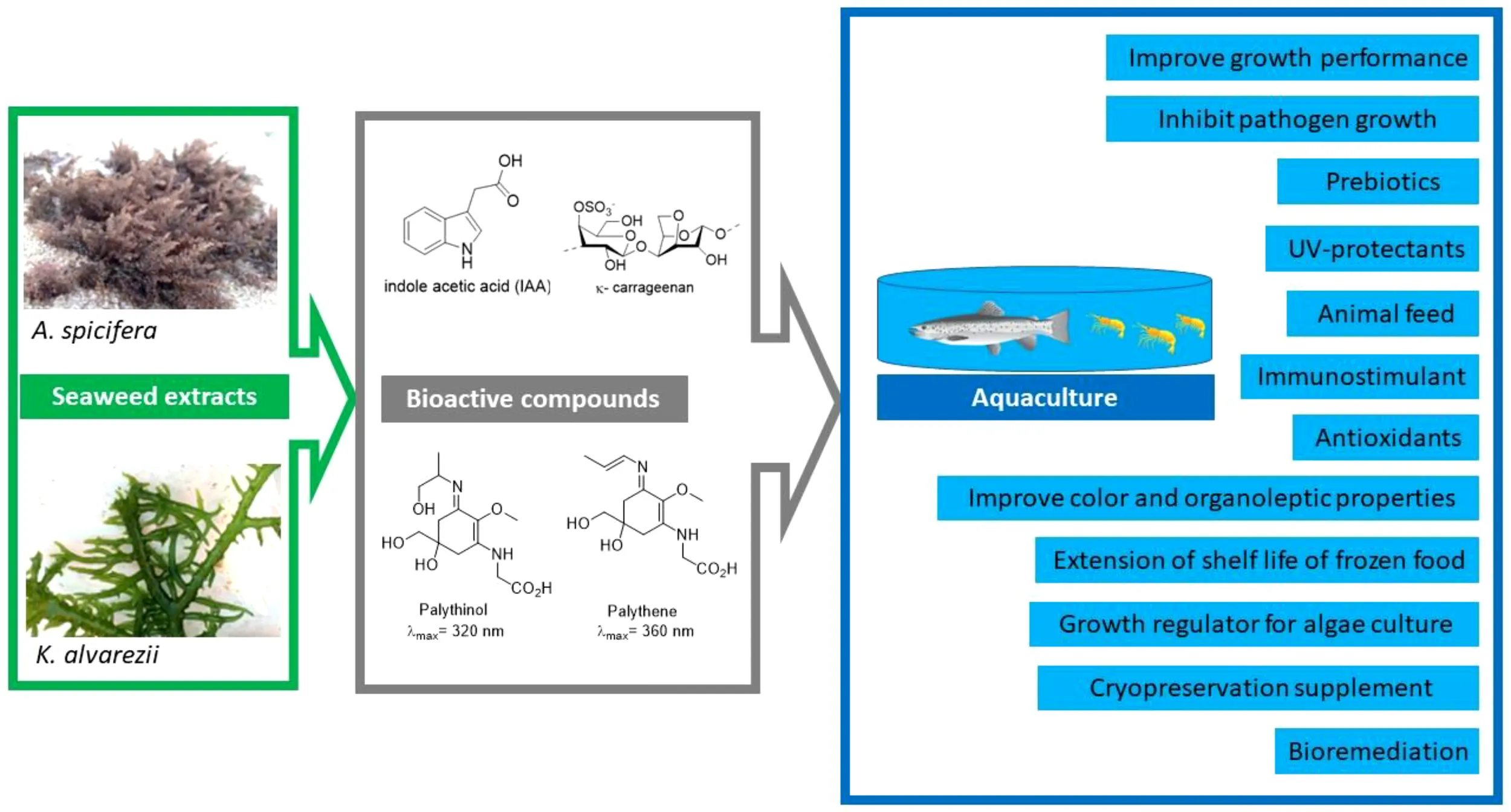
The role that seaweeds play as primary producers and ecosystems engineers in marine coastal ecosystems is widely acknowledged. Seaweeds, however, are also important drivers in the development of the blue bioeconomy due to their vast diversity of unique chemicals with a broad range of industrial and biotechnological applications. In tropical regions, seaweed production has been focused on a few species only, because of their hydrocolloids used in the food industry. There is a strong need to identify new applications of red seaweed species in other sectors such as aquaculture. Therefore, to diversify the culture of red seaweeds, more tropical species need to be investigated for their chemical composition and potential application in aquaculture, and then, to develop a method for a sustainable cultivation of new seaweed candidates and enhance their economic potential. Based on this context, we analyze the potential value of the red edible seaweed Acanthophora spp., an under-valued seaweed species which is naturally abundant in tropical countries, and Kappaphycus spp., a commercially valuable seaweed commonly used for polysaccharide extraction. The vast chemical diversity of seaweeds (polysaccharides, phytohormones, amino acids, and pigments) has led to research on a wide range of applications in aquaculture, including pathogen control, immunostimulant, antioxidant, bioremediation, feed, UV protectants, increase in seafood shelf life, animal colorant, and growth regulator for microalga culture. This review hopes to stimulate the interest among seaweed researchers to investigate other local seaweed species and seek greater added value of their biomass and chemical compounds and their applications in the aquaculture sector. Additionally, this information will help stakeholders to benefit from these two red seaweeds by contributing to the diversification of the blue bioeconomy in tropical countries.
1 Introduction
Seaweeds are photosynthetic and multicellular organisms commonly found in shallow coastal marine ecosystems. Seaweeds biosynthesize a wide range of natural products originating from different metabolic pathways (Cardozo et al., 2007) with a wide range of applications in food processing, pharmaceutical, nutraceutical and cosmetic industries as well in the agriculture and aquaculture sector. In recent years, the aquaculture sector has become more diverse and experienced an intensive growth around the world, especially in tropical regions (FAO, 2020). Seaweed cultivation has increased through the years around the world particularly in Asia (Naylor et al., 2021). Kappaphycus spp. and Eucheuma spp. have become the most cultivated species for carrageenan extraction, while other seaweeds, like Undaria pinnatifida, Porphyra/Pyropia spp., and Caulerpa spp., are being cultivated for human consumption. Recent studies have covered advances of seaweed nursery techniques and the importance to identify adequate environmental conditions for economically successful production in aquaculture systems (Hwang et al., 2022; Jiksing et al., 2022).
Seaweeds are also increasingly cultivated for bioremediation purposes in the aquaculture sector to help reducing the environmental impact, i.e. Integrated Multi-Trophic Aquaculture (IMTA) (Stévant et al., 2017). The implementation of land-based shrimp farms negatively impacts the environment causing eutrophication and polluting mangrove forest. The poor quality of water of shrimp farms can negatively affect growth of shrimp and increase the probabilities of shrimp disease outbreaks, which generate severe economic losses.
The incorporation of seaweeds into aquaculture systems has the potential to help the improvement of the marine ecosystem through their capacity to absorb nutrients from the water while synthesizing secondary metabolites with high-added value (Tanaka et al., 2020). IMTA cultivation of the kelp Saccharina latissima with the blue mussel Mytilus edulis improved the biomass of the kelp by 38%, reduced ephiphytes to 6% and increased the concentration of pigments such as chlorophyll a, fucoxanthin and phaeophytin, compared to monoculture (Hargrave et al., 2022). The seaweed Chaetomorpha linum cultivated in a IMTA system was found to be a prolific source of omega-3 and omega-6 fatty acids as a by-product of the bioremediation system (Stabili et al., 2019). Other seaweeds such as Codium and Ulva cultivated in land-based IMTA systems have shown to be alternative sources of commercially important polar lipids such as glycolipids, phospholipids, and betaine lipids (da Costa et al., 2015; Moreira et al., 2020), while IMTA cultures of Gracilaria vermiculophylla represent a promising source of photoprotective compounds known as mycosporine-like amino acids (MAAs) (Barceló-Villalobos et al., 2017). The chemical composition of seaweeds is highly variable when collected in the wild. Cultivation under control conditions through land-based IMTA systems presents an alternative to provide a continuous supply of compounds of commercial and nutritional interest.
One of the economically most significant and widespread cultivated seaweeds in tropical Asian countries is the red seaweed Kappaphycus alvarezii. Other countries, such as Mexico (Muñoz et al., 2004) and Brazil (Bulboa and De Paula, 2005), successfully cultivate introduced strains of K. alvarezii and K. striatus. This seaweed has also been introduced to other tropical regions around the world for mariculture including East Africa (Rönnbäck et al., 2002; Msuya, 2020) and the tropical Eastern Pacific (Hayashi et al., 2017).
Kappaphycus species are of interest for their high carrageenan content. Furthermore, among the three taxonomic groups of macroalgae; green (Chlorophyta), red (Rhodophyta) and brown (Ochrophyta and Phaeophyceae), red seaweeds are characterized by being the most important source of bioactive metabolites with higher protein content and for being the group with higher species diversity in the tropical eastern pacific (TEP) (Cortés et al., 2017). Additionally, the presence of abundant biomass of other red seaweed species in the coast of tropical countries should also be considered for their investigation and potential applications of their biomass and chemical constituents along with the ecology and social economic impact. For example, the red seaweed Acanthophora spp., is found abundantly in the wild, but its potential applications in different sectors is still to be evaluated.
Acanthophora is commonly found in tropical, marine ecosystems. Acanthophora spicifera has been considered as the most widespread and invasive species reported in the Eastern Pacific Ocean (Ávila et al., 2012). Its morphological plasticity, high capacity to adapt to different environmental conditions and its capacity to reproduce vía sexual or vegetative reproduction, makes it a threat to native marine biodiversity. A. spicifera can grow on different types of substrate such as rocky, sandy, shells, concrete and buoys exposed to diverse physical and environmental parameters such as water movement, light, salinity, and water temperature that have strong influence in its propagation (Russell, 1992). Harvesting the biomass of A. spicifera from the natural environment might be a way to reach two goals: (1) conservation of the natural marine environment with respect to control of invasive seaweed species and (2) developing a local bioeconomy by valorizing the harvested biomass. Although there is a scarce information about the ecological impact of Acanthophora spp. on native species in tropical region, a study carried out in Hawaii demonstrated that Acanthophora spicifera did not have a negative effect on epifaunal distribution (Fukunaga et al., 2014).
Even though, the ecological impact of invasive seaweed species should be carefully considered in all marine ecosystems, chemical characterization and valorizing its potential industrial applications could be worth it. For example, the presence of high feedstock of invasive seaweed species in the Iberian Peninsula (Pacheco et al., 2020) and the coast of Spain (Pereira et al., 2021) were reported and evaluated for their potential applications in different industries, suggesting their use as an alternative to decrease their population while achieving environmental and economic benefits. However, other seaweed such as Undaria pinnatifida, which is considered an invasive species in several countries such as France, Ireland and New Zealand (Kraan, 2017), but cultivation permits have been granted by local authorities to cultivate the species due to its economic potential in the food industry.
In this study, we analyze the potential use of the biomass and the chemical constituents, such as polysaccharides, pigments, amino acids, and peptides, and phytohormones of two tropical red seaweed species Kappaphycus and Acanthophora and their high value-added applications in the aquaculture sector.
2 General characteristics
2.1 Kappaphycus spp.
The commonly cultured Kappaphycus strains have been extensively introduced to various tropical countries as a source of carrageenan for the food industry (Ask and Azanza, 2002; Zuccarello et al., 2006). The genus Kappaphycus is comprised of five taxonomically accepted species: K. alvarezii, K. inermis, K. malesianus, K. procrusteanus and K. striatus (Dumilag et al., 2022). K. alvarezii includes three strains (red, green, and brown) and has been widely studied for their growth rate and carrageenan yield (Muñoz et al., 2004). There is a long list of cultivars of different Kappaphycus spp. For example, in the Phillipines over 60 different cultivars have been investigated (Dumilag et al., 2022). Recent studies have focused on the germination of tetraspores and carpospores from wild, harvested K. alvarezii (Hinaloc and Roleda, 2021).
Temperature is the most important factor that determines the growth rate in K. alvarezii and K. striatus (Muñoz et al., 2004; Bulboa and De Paula, 2005). Both species have shown higher growth rates during warm seasons but limited growth below 18°C for longer periods. Although K. striatus has shown a good growth rate and carrageenan yield, the presence of viable tetraspores during in vitro and in the sea experiments under different light and temperature conditions, suggest considering only K. alvarezii for commercial cultivation purposes to prevent propagation and possible environmental damage (Bulboa and De Paula, 2005). However, the negative impact caused by K. alvarezii on the coral Acropora sp., and bleaching of the fire coral Millepora alcicornis was reported in India (Chandrasekaran et al., 2008) and Venezuela (Barrios et al., 2007) respectively. Therefore, cultivation near biodiverse ecosystems should be highly reconsidered. Analysis of farming techniques based on biotechnological tools, ecological interactions and the challenges involved in K. alvarezii culture has been reviewed (Bindu and Levine, 2011). To increase the economic viability, sequential extractions of the same biomass of K. alvarezii to obtain carrageenan, chlorophyll, β-carotene, essential amino acids and phytohormones have been suggested for a green biorefinery development (Rudke et al., 2020). Several investigations have been focused on improving biomass cultivation methods of Kappaphycus as a monoculture such as micropropagation (Rama et al., 2018; Ali et al., 2020), culture on biofloc effluents (Pires et al., 2021), tissue culture (Budiyanto and Abadi, 2019), or as part of an IMTA using different systems like floating bamboo raft (Mantri et al., 2022), tubular net (Reis et al., 2015; Periyasamy and Subba Rao, 2017), floating cages and longlines (Mustafa, 2017), and stratified double net rounded cage (Putro et al., 2015).
The proximal composition of Kappaphycus species have been reported in different studies (Table 1).
Table 1
| Parameter(g/100 g dw)* | K. alvarezii | K. striatus | K. malesianus | K. inermis |
|---|---|---|---|---|
| Lipids | 0.03-1.50 a,b,c,d,e,f,g,h,i,j | 0.06-0.08 c,f | ** | ** |
| Protein content | 1.03-18.16 a,b,c,d,e,f,g,h,i,j | 0.73-1.25 c,f,g | ** | ** |
| Carbohydrate content | 8.67-71.83 b,c,d,g,h,i,j,l | 5.15-8.35 c,f,g | ** | ** |
| Sulfated polysaccharide content | 6-67.30 a,e,m,n,o,p,q | 30-56.40 n,p,r | 37 p | 29 n |
| Ash content | 5.01-45.37 a,b,c,d,e,f,g,h,k | 4.80-35.88 c,f,g | ** | ** |
| Fiber | 0.87-34.09 a,b,c,f,g,j,k,l | 1.18-15.81 c,g | ** | ** |
Proximal composition of Kappaphycus spp.
*Ranges of all values found in literature
a(Hurtado, 1995); b (Hong et al., 2007); c (Balasubramaniam et al., 2013); d (Suresh Kumar et al., 2015); e (Yong et al., 2014); f (Ariano et al., 2021); g (Adharini et al., 2019); h (Alcantara and Lazaro-Llanos, 2020); i (Abirami and Kowsalya, 2011); j (Kumar et al., 2014); k (Xiren and Aminah, 2017); l (Fayaz et al., 2005); m (Castelar et al., 2016); n (Santos, 1989); ° (Estevez et al., 2000); p (Bui et al., 2019); q (Ohno et al., 1994); r (Hung and Trinh, 2021).
** No scientific information available.
2.2 Acanthophora spp.
The genus Acanthophora is currently composed of 7 accepted species according to Algaebase (Guiry and Guiry, 2022): A. spicifera, A. muscoides, A. aokii, A. dendroides, A. nayadiformis, A. pacifica and A. ramulosa. Although, most species are restricted to tropical regions, some species such as A. spicifera and A. muscoides extend into temperate regions (Jong et al., 1999).
The presence of A. spicifera in mangrove ecosystems and results of experimental studies, indicate the species can grow under a large salinity range (25 to 40 g/100 g water) (Cordeiro-Marino et al., 1992; Pereira et al., 2017). Up till now, no studies on this seaweed biomass production in mangrove ecosystems have been recorded to evaluate its sustainable production.
Morphological changes, such as increased cell wall thickness and chloroplast disruption, reduced cell viability and growth rate, were also observed in A. spicifera after exposure to UV-B radiation which increases the formation of reactive oxygen species (ROS) within the cell (Pereira et al., 2017). Stress response was more obvious when plants were cultivated under higher salinity conditions. When A. spicifera was exposed to UV radiation in combination with higher nutrient availability, a reduction on these negative effects was observed. The fact that increased nutrient concentration can offset the negative effects of UV radiation could be explained by the capacity of the seaweed to incorporate nutrients that transform through different metabolic pathways into specific UV-absorbing compounds such as mycosporine-like amino acids (Häder et al., 2015).
The proximal composition of Acanthophora species have been reported (Table 2).
Table 2
| Parameter* (g/100 g dw) | A. spicifera | A. nayadiformis | A. muscoides |
|---|---|---|---|
| Lipids | 0.30-5.43 a,b,c,d,e,f,g,h,i | 0.29-2.19 j | ** |
| Protein content | 5.29-20.59 a,b,c,d,e,h,i, | 1.71-3.15 j | 21.83k |
| Carbohydrate content | 26.20-88.26 a,b | ** | ** |
| Sulfated polysaccharide content | 9.1-39.3 l,m,n | ** | ** |
| Ash content | 14.80-47.04 a,c,d,e,h | ** | ** |
Proximal composition of Acanthophora species.
* Ranges of all values found in literature
a (Herrera et al., 2019); b (Abomohra et al., 2018); c (Kailas and Nair, 2015); d (Dixit et al., 2018); e (Lawanyawut et al., 2002); f (Bhaskar et al., 2004); g (Marolia et al., 1982); h (Ganesan et al., 2020); i (Seenivasan et al., 2012); j (Polat and Ozogul, 2008); k (Rao, 1970); l (Schnoller et al., 2020); m (Anand et al., 2018); n (Ganesan et al., 2018);
** No scientific information available.
3 Seaweed biomass with applications in aquaculture
3.1 Kappaphycus spp.
Aquaculture applications of Kappaphycus spp. are numerous. They include biomass cultivation, bioremediation, and animal feed production.
The economic and environmental benefits of culturing Kappaphycus species with commercially important aquatic animals in diverse IMTA systems has been investigated in several studies (Qian et al., 1996; Lombardi et al., 2006; Rodriguez and Montaño, 2007; Hayashi et al., 2008; Putro et al., 2015; Kambey et al., 2020; da Silva et al., 2022). In those studies, the productivity of the cultivated species in open water and land-based systems was increased supporting IMTA technology as a viable method for seaweed production. These systems provide a sustainable method for the development of the aquaculture industry with ecological benefits, especially in developing countries. For example, an improved growth rate of K. alvarezii and the pearl oyster Pinctada martensi was observed in a co-culture system established in a subtidal zone in the coast of Hainan Island in China. Both organisms showed higher growth rates when seawater temperature was over 23°C, due to the efficient nutrient uptake by K. alvarezii removing nitrogen waste, particularly ammonium, and improved seawater quality (Qian et al., 1996). Although, K. alvarezii, K. striatum and Kappaphycus sp., all significantly reduced the ammonium concentration in fish farm effluent, K. striatum showed the best performance compared to the two other species (Rodriguez and Montaño, 2007). Also, the growth of K. striatum improved the growth rate of the sea cucumber Holothuria scabra in a co-culture system established in a tropical lagoon in Tanzania (Beltran-Gutierrez et al., 2016). The implementation of integrated aquaculture systems in lagoons, represents an alternative for the economic development in this area, particularly in countries where space for marine aquaculture is a limitation. Additionally, alternative methods to improve seaweed biomass production by increasing its resistance to diseases without affecting its polysaccharide quality have been reported. For example, a short immersion (12 hours) of K. alvarezii in a high nitrogen medium enhanced the growth, nitrogen uptake, and carrageenan quality and reduced the incidence of the “ice-ice” disease during its cultivation in the sea for 45 days (Luhan et al., 2015). Furthermore, the use of a bioflocs system with effluents released from farming of white Pacific shrimp (L. vannamei) improved the growth and biosynthesis of secondary metabolites with antioxidant activity, such as phenolics, carotenoids and flavonoids, during in vitro cultivation of K. alvarezii (Pedra et al., 2017).
From a bioremediation point of view, K. alvarezii was capable of removing 23–34% of ammonium and 5–30% of phosphate after 42 days of cultivation using seaweed biomass inside a fish cage system (Kambey et al., 2020). The dried biomass of K. alvarezii also showed high biosorption capacity for the removal of phosphate and heavy metals (Pb2+, Cu2+, Fe2+ and Zn2+) from contaminated waters (Rathod et al., 2014; Rahman and Sathasivam, 2015) and under laboratory conditions (Lee et al., 2011). The K. alvarezii brown strain showed higher biosorption of cadmium, cobalt, and chromium with values of 3.064, 3.365 and 2.799 mg/100 g fresh weight respectively, compared to the red and pale yellow strains during the experiments undertaken in the laboratory (Kumar et al., 2007).
Additionally, K. alvarezii biomass and its extracts have been included in animal feed and to improve the physiological and immunological response in economic important aquaculture species. The nutritional composition and low heavy metal content in K. alvarezii and K. striatus suggest their use for animal feed (Ariano et al., 2021). Supplementing with 15 g of seaweed/kg feed, resulted in a 10% increase in the survival rate of the white Pacific shrimp L. vannamei in a Vibrio harveyi challenge test (Suantika et al., 2018) (Table 3). K. alvarezii-enriched artemia used to feed the white shrimp L. vannamei increased the resistance against V. harveyi during the post-larvae phase and improved shrimp growth in a high-salinity environment (Suantika et al., 2017). The amino acid and fatty acid content increased through the fermentation of K. alvarezii with 10% of activated Saccharomyces cerevisiae provided a better alternative as a feed supplement for the white shrimp compared to the non-fermented one (Hardjani et al., 2017). Other studies demonstrated that higher lipid content improved the growth and survival rate and enhanced the larval immune system and stress tolerance in shrimps (Zhang et al., 2013; Xie et al., 2018). The use of raw and fermented K. alvarezii in the diet of up to 10 and 30%, respectively, for the feed of freshwater prawn Macrobrachium rosenbergi increased growth rate, protein and lipid digestibility and feed utilization efficiency (Felix and Brindo, 2014). The use of up to 5% of K. alvarezii as a binder in shrimp food to improve the survival rate of Penaeus monodon and to reduce organic waste generated by commonly used binder ingredients, such as wheat flour, has been suggested over 25 years ago (Peñaflorida and Golez, 1996). Also, higher growth rate and weight gain of the seabass Later calcarifer was observed when supplemented with 6% of cooked K. alvarezii in its diet (Shapawi et al., 2015).
Table 3
| Parameter | K. alvareziia | K. alvareziib | A. spiciferac |
|---|---|---|---|
| Preparation type |
Artemia nauplii enriched with seaweed paste (0.5 g/L artemia enrichment suspension) |
Mixture of seaweed powder and Artemia nauplii (15 g/kg feed) | Mixture of seaweed extract and lab-prepared feed |
| Animal used | L. vannamei (post-larvae) | L. vannamei (nursery phase) | C. punctatus |
| Growth rate after challenge: control (% body weight/day) | 9.65 ± 0.20 | 6.49 ± 1.84 | |
| Growth Rate* after challenge: seaweed (% bwt/day) | 10.51 ± 0.19 | 6.56 ± 2.28 | |
| Pathogen | Vibrio harveyi | Vibrio harveyi | Aeromonas hydrophila |
| Survival rate after challenge: control(%) | 77.7 ± 3.1 | 63.8 ± 1.6 | 0 |
| Survival rate after challenge: seaweed(%) | 90.2 ± 7.0 | 73.3 ± 1.6 | 60 |
Overview of aquaculture growth and pathogen challenge tests.
Interesting applications of Kappaphycus spp. extract (sap) in aquaculture have been reported with potential benefits for the industry. The use of the sap as a feed supplement for the commercial shrimp Penaeus monodon improved the survival from 73.7% (control) to 89.7% (treatment) (Anil et al., 2011). In addition, K. alvarezii ethyl acetate extract displayed inhibitory activity against Vibrio harveyi, one of the most important pathogens in the shrimp industry (Sivakumar et al., 2014). Although, some compounds, such as fatty acids and hydrocarbons, were identified through GC-MS analysis, further studies to isolate and characterize the bioactive compounds present in this extract and other types of extracts are required. Furthermore, the K. alvarezii methanolic extract showed an anti-genotoxic activity in the marine fish Therapon jarbua (Nagarani et al., 2012). The DNA damage in the fish was induced by exposure to mercury chloride, but after addition of the extract of this seaweed of up to 5 mg/L of tank water, DNA damage was notably reduced.
3.2 Acanthophora spp.
Aquaculture applications of Acanthophora spp. are numerous. They include biomass cultivation and animal feed production and the extension of shelf life of food.
Cultivation of Acanthophora was accomplished using 5 cm vegetative fragments, tied to polypropylene straws and fixed to nylon fishing lines at 1m depth in the nearshore area of Gulf of Mannar, India. An 2.6-fold increase of the initial weight (4.85 kg) was noted after 25 days from cultivation with a harvest weight of 12.85 kg (Kaliaperumal et al., 1987). However, no studies using Acanthophora in IMTA systems have been reported in the literature.
Acanthophora species are a rich source of carbohydrates, lipids, proteins, minerals, fatty acids, essential amino acids, and bioactive compounds (Table 2) that could be used for aquatic animals as a functional feed to enhance their growth, increase survival rate, protection against diverse pathogens or as a mechanism of chemical defense (Lawanyawut et al., 2002). Another reason is to reduce the use of animal-based protein in animal feed and the feed production costs (if collected seaweeds are being used). In natural ecosystems, this seaweed is an important natural food source for aquatic animals such as the green turtle Chelonia mydas L. (Russell and Balazs, 1994) and the blue striped angelfish Chaetodontoplus septentrionalis (Leu et al., 2010).
The shrimp industry is one of the most important economic sectors in tropical and subtropical countries. However, increasing shrimp farming has resulted in negative environmental impacts caused by pollution of mangrove forest and the nutrient enrichment of seawater. The use of Acanthophora species as bioindicators in shrimp farms and natural habitats in the vicinity of shrimp farms is an interesting alternative to reduce the ecological and environmental impact generated by the presence of the excessive nutrient concentration in the sea. This is because this excessive nutrient concentration in the sea leads to an increased presence of Acanthophora sp. In fact, the growth, N content in tissue and δ15N values in A. spicifera were used as a bioindicator in commercial shrimp farms (Lin and Fong, 2008). The growth and tissue N content was found to be higher in the sample collected closer to shrimp farming than those obtained at further distances. Additionally, analyzing isotopic ratios is a very sensitive technique that could be applied to detect effluent effects at very distant places and determine the main nutrient source in a specific location of a marine ecosystem. The increase in the growth and higher absorption of phosphorus by A. muscoides in a high salinity environment was evidenced by (Tanaka et al., 2020). Concentration of UV-absorbing metabolites with maximal UV absorption at 331 nm was highest when grown at normal salinity. The considerable capacity of the seaweed to absorb and store nutrients and use them for growth and to produce a wide diversity of chemicals could be used as additional material for the elaboration of high-added value bioproducts.
Some studies have reported on the bioactivity of Acanthophora crude extracts in aquaculture. For example, the aqueous extract of A. spicifera displayed immunostimulant activity against the fish pathogen Aeromona hydrophila providing a survival rate of 60% of the striped murrel Channa punctatus compared to 0% survival rate of the control group (Muthukrishnan and Raja, 2021).
Feeding studies of A. muscoides in the juvenile top shell Trochus niloticus showed a daily growth rate of 0.022 mm in diameter and 6 mg in wet weight, while the control group slightly decreased its weight daily (Lee and Amos, 1997).
Another reported application is the extension of the shelf life of frozen stored shrimp after being treated with a 5% ethanolic extract of A. muscoides (Arulkumar et al., 2020). A reduction of the formation of toxic biogenic amines in shrimp demonstrated the biopreservative potential of this red seaweed. The presence of diverse metabolites with antioxidant activity in Acanthophora (Zakaria et al., 2011; Dixit et al., 2018), contributes to preventing food lipid peroxidation and therefore its potential use as natural source of antioxidants.
4 Bioactive metabolites with applications in aquaculture
4.1 Polysaccharides
Hydrocolloids are high-molecular weight polysaccharides with unique physicochemical properties including gelling, thickening, and emulsifying at determined thermal conditions (Gupta and Raghava, 2008). Red seaweeds are the only source of carrageenans, and agars, two of the most economically important polysaccharides (phycolloids) characterized by their diverse rheological properties with different industrial applications. The structural diversity of carrageenan and agar have been reviewed (Usov, 2011; Ciancia et al., 2020).
A comprehensive review by (Mohan et al., 2019) describes the wide range of potential applications of marine-derived polysaccharides in aquaculture such as feeding, immunostimulant, antioxidant, antiviral, antifungal, antiparasitic and antibacterial activity. The different physico-chemical properties, biodegradability, non-toxicity, biocompatibility, and diverse biological activities make seaweed polysaccharides a promising source for the discovery of novel applications in the aquaculture sector. Therefore, it is important to characterize the chemical composition of other native and non-indigenous seaweeds, especially characterize their chemical composition and phycolloid content to enhance their potential use in aquaculture.
In addition, the chemical or enzymatic hydrolysis of seaweed polysaccharides will lead to the production of bioactive oligosaccharides with multiple pharmacological and cosmeceutical applications (Cheong et al., 2018). The biological activity displayed by oligosaccharides is dependent on the degree of depolymerization of the polysaccharide of origin. In aquaculture, oligosaccharides have also shown beneficial results for their capacity to improve the productivity, their antibacterial activity, and as efficient prebiotics (Sardari and Nordberg, 2018). The application of 0.5 and 1% of mannan oligosaccharides (commonly found in yeast) in the diet of the Thinlip grey mullet (Liza ramada) showed an enhanced specific growth rate of 2.57 and 2.54%/day, respectively, compared to the control (2.34%/day). Also, an increase of the digestive enzyme activity, such as lipase, amylase and protease, blood immunity and antioxidant activity, were observed (Magouz et al., 2021).
4.1.1 Kappaphycus spp.
The sulfated polysaccharide κ-carrageenan is the major cell wall component in K. alvarezii (Santos, 1989; Hurtado, 1995) and its presence has also been reported in K. inerme, K. striatus (Santos, 1989; Mendoza et al., 2002) and K. cottonii (Santos, 1989). However, very small amounts of ι-carrageenan and agaroids were also found in K. alvarezii when extracted at room temperature (Estevez et al., 2000). Furthermore, other polysaccharides, such as β-(1,4)-ɒ-glucomannan and β-(1,4/1,3)-ɒ-glucan 6-sulfate, have also been found in the latter (Lechat et al., 2000). K. alvarezii is one of the most widely cultivated seaweeds in the world for the extraction of κ-carrageenan which has an important economic value in the food industry, but it has also shown promising applications in the pharmaceutical industry for its wide range of biological activities or as a biomaterial for drug delivery systems (Cunha and Grenha, 2016; Hentati et al., 2020). Although very few studies have focused on the potential application of K. alvarezii polysaccharides in aquaculture, the use of different marine-derived polysaccharides as binders for feeding (Paolucci et al., 2015), as growth promoters, immunomodulators, to induce disease resistance and gut health maintenance or as immunostimulants have been reported (Mohan et al., 2019). In fact, κ-carrageenan induces immunostimulatory activity, enhances growth and resistance to diseases in the Asia seabass Lates calcarifer (Sakthivel et al., 2015) and postlarvae of tiger shrimp P. monodon (Jumah et al., 2020).
4.1.2 Acanthophora spp.
Studies have also been carried out to determine the hydrocolloid composition of Acanthophora species, with A. spicifera being the most studied species. Some of these studies demonstrated that the sulfated galactan λ-carrageenan is the major polysaccharide present in A. spicifera (Parekh et al., 1989; Gomaa and Elshoubaky, 2016; Anand et al., 2018). However, other authors reported the presence of agar as major component in the polysaccharide extracts of A. spicifera (Gonçalves et al., 2002; Duarte et al., 2004; Schnoller et al., 2020; Júnior et al., 2021) and A. muscoides (Rodrigues et al., 2016a).
Various factors, including geographical location, the different environmental conditions or potentially the presence of cryptic seaweeds, can potentially explain the variation in the major polysaccharide constituent of A. spicifera. Interestingly, the phycolloids reported from Brazil and Mexico corresponded to the agaran type, while the carrageenan types are reported from the Indian Ocean (India and Saudi Arabia). Further studies are required on the genetic diversity and structural analysis of phycolloids of Acanthophora specimens from various locations to investigate the underlying variation in cell wall characteristics.
An unidentified sulfated polysaccharide from A. muscoides was used, due to its antioxidant properties, to evaluate its performance in semen cryopreservation of the commercial fish Colossoma macropomum (tambaqui) (Pereira et al., 2020). Although, no significant difference in the improvement of semen quality was found when A. muscoides polysaccharide was added as a supplement in the cryopreservation medium, the author concluded that further studies were required to exploit its potential as a supplement for semen cryopreservation of the most commercially important fishes. Since the antioxidant activity displayed by seaweed polysaccharides varies depending on the season and geographical location of the collected sample, more studies should be carried out to determine the best spatial and temporal conditions when maximum antioxidant activity is displayed. Also, it is important for future studies on the antioxidant and total phenolic content assays from seaweeds to have a standardized methodology for these analysis. Currently, there are different methodologies used by several researchers that makes it very complicated to compare the data with the reported literature.
4.1.3 Potential based on results of other species
No studies have been reported on the application of oligosaccharides from Acanthophora and Kappaphycus in aquaculture. However, methodological studies to optimize isolation of oligosaccharides from K. alvarezii (Bouanati et al., 2020), K. striatus (Yu et al., 2017), A. spicifera (Duarte et al., 2004) and A. muscoides (Rodrigues et al., 2016b) are available. Therefore, it is important to investigate and evaluate the potential application of polysaccharides and oligosaccharides isolated from Acanthophora and Kappaphycus in aquaculture, based on the different uses of these chemical compounds obtained from other seaweeds.
The different physico-chemical properties displayed by polysaccharides shows potential use in the development of matrices for vaccine micro-encapsulation (Borgogna et al., 2011), functional bio-composites (Paolucci et al., 2015), prebiotics (Vidhya Hindu et al., 2019), and anti-biofilm agent (Zammuto et al., 2022) for aquaculture purposes. In fact, Artemia nauplii encapsulated with the unidentified crude polysaccharide of the red seaweed Amphiroa fragilissima for the feeding of the shrimp Litopenaeus vannamei enhanced its growth, biochemical composition, digestive enzyme activities and antioxidant properties which led to the improvement of larval quality (Muttharasi et al., 2021). Furthermore, the application of agar-based pellets in the feeding of the sea urchin Paracentrotus lividus showed higher water stability in water recirculating system and was easily consumed by the animal without affecting its gonad growth (Fabbrocini et al., 2012).
Also, the immunostimulant, antibacterial and antiviral activity reported for seaweed polysaccharides, enhances their use as a natural and environmentally friendly alternative to treat and control several diseases affecting fish, shrimp cultivation and other organisms produced through aquaculture (Marudhupandi and Inbakandan, 2015; Rizzo et al., 2017; Raguraman et al., 2020). An increased immunological response, upregulation of immune-related genes and resistance to pathogens was reported through in vitro and in vivo studies after the shrimp L. vannamei was exposed to λ-carrageenan via immersion and orally (Chen et al., 2014). It is suggested that carrageenan triggers shrimp innate immunity and improves immune parameters such as haemolymph sampling, haemocyte counts, phenoloxidase (PO), respiratory burst (RBs), superoxide dismutase (SOD) and lysozyme activity.
For years, the brine shrimp Artemia and the microalgae Chlorella have been traditionally used as a food source in the shrimp industry. Many investigations intended to improve the nutritional characteristics that could enhance shrimp’s survival at the different stages of their life cycle. For example, micro-encapsulated, micro-bound and micro-coated diets have been prepared with different nutrients depending on the nutritional requirements of the shrimp or larvae and evaluated for their capacity to increase the productivity and health (Kanazawa, 1989). In the formulation of this micro-bound diet for shrimps, κ-carrageenan has been used as a binder and showed an improvement on the survival rate and growth of shrimp’s larvae and post-larval stages when it was combined with Artemia salina (Bautista et al., 1989). Other studies showed a boost in the immunological response of shrimps and fishes when supplemented with κ-carrageenan in their diet. An improved immunological activity and pathogen resistance of L. vannamei against the infectious myonecrosis virus (IMNV) (Febriani and Nuryati, 2013) and of P. monodon against Vibrio harveyi (Traifalgar et al., 2013) were reported after feeding at 1.5% and 0.2% of κ-carrageenan, respectively. An improved growth, survival rate and immunological response of the Nile tilapia Oreochromis niloticus was observed by increasing the expression of immune-related genes including transferrin, IL-1β and appetite-related gene GH after administration of 0.5% of κ-carrageenan (Villamil et al., 2019). Similar results were reported with cobia Rachycentron canadum after feeding with 20 g/kg of κ-carrageenan (Harikrishnan et al., 2021). On the other hand, (Mariot et al., 2021) did not find significant difference in shrimp immunological response upon addition of up to 1.5% of κ-carrageenan to their diet. However, a small variation was noticed in the gut bacterial composition in a dose-dependent manner to carrageenan. Two unidentified bacteria of the families Rhodobacteraceae and Caldilineaceae with two bacteria of the family Rubritaleaceae were found in higher abundance when the concentration of κ-carrageenan was increased along with the shrimp resistance to White Spot Syndrome Virus (WSSV) disease. Recent studies have shown improvement in the metabolism of the white shrimp L. vannamei using the seaweeds Ulva lactuca and Eisenia sp., which increases the digestive enzymatic activity and variation in the gut microbiota while reducing the presence of pathogenic bacteria (Schleder et al., 2020; Omont et al., 2021). The prebiotic potential of marine polysaccharides and oligosaccharides has been recently reviewed (Gurpilhares et al., 2019; Zheng et al., 2020). The elicitor activity displayed by oligosaccharides provides a natural alternative to reduce disease outbreaks and improve the cultivation of seaweeds in aquaculture. The application of synthetic oligoagar at 100 µg/mL for 2 hours during the cultivation of the seaweed Pyropia haitanensis improved the seaweed growth, oxygen consumption and enhanced the upregulation of defense-related genes (Chen et al., 2016) and triggered oxidative burst releasing hydrogen peroxide in seaweeds such as Gracilaria spp. (Weinberger and Friedlander, 2000) and Laminaria digitata (Kupper et al., 2001), among others. Seaweed oligosaccharides have been studied for their use in the agriculture, medical and food industry (Zhu et al., 2021), however, their use in aquaculture as natural elicitors and as prebiotic remains to be exploited.
4.2 Phytohormones
Phytohormones, such as auxins, cytokinins, polyamines, abscisic acid, betaines, and gibberellins, are well known as plant growth regulators. These molecules are involved in important physiological functions such as mediating growth, signaling environmental alterations, initiating biotic or abiotic stress response and as indicator molecules during plant growth (Shanab and Shalaby, 2021). In seaweeds, phytohormones control different biochemical and physiological processes (Tarakhovskaya et al., 2007) and the mechanism of action of certain phytohormones such as indole acetic acid (IAA), N6-(Δ2-isopentenyl) adenine (iP), and abscisic acid in red seaweeds is suggested to be different from those reported from terrestrial plants (Mikami et al., 2016). Although, the presence of these molecules in red seaweeds (Yokoya et al., 2010; Wang et al., 2014), and the diverse extraction methods used (Górka and Wieczorek, 2017; Mori et al., 2017; Mohanty and Adhikary, 2018) have been reported in the literature, there are very limited studies on their identification and quantification in Kappaphycus and Acanthophora species and their application in aquaculture.
4.2.1 Kappaphycus spp.
The content of growth-hormone regulators in organic extracts and saps of K. alvarezii has been reported (Zodape et al., 2009; Prasad et al., 2010; Sedayu and Basmal, 2013; Layek et al., 2015; Fadilah et al., 2016; Cokrowati et al., 2021; Trivedi et al., 2022). In those studies, the presence of auxins such as indole-3-acetic acid (IAA), gibberellic acid (GA3, GA7), cytokinins (cis-zeatin, trans-zeatin, kinetin), choline, glycine betaine and betaine aldehyde have been found at different concentrations (Table 4). Two key factors in their quantification are: (1) the different extraction methods and solvents used for phytohormone extraction, and (2) the seaweed collection time and location. Nevertheless, the application of these hormones has been widely used to improve the biomass growth, photosynthesis and chemical composition of seaweeds including Kappaphycus species. Higher auxin content (16.28 mg/kg fresh weight) was found in the young thallus of K. alvarezii green strain from Indonesia compared to the old thallus of the green and brown strains (Cokrowati et al., 2021). The concentration of phytohormones present in K. alvarezii sap was determined by comparing three different methodologies, being spectrophotometric, HPLC, and ESI-MS (Prasad et al., 2010). The values obtained through ESI-MS analysis are more reliable than the other methodologies, since the interference caused by impurities is significantly reduced by their identification through their MS fingerprint.
Table 4
| Parameter * | A. spicifera | K. alvarezii | |
|---|---|---|---|
| (µg/g fresh weight) | (µg/kg fresh weight) | (µg/mL of extract) | |
| Auxin content | – | 5500 a,b,c | – |
| Indole acetic acid (IAA) | – | – | 3.87-160 b,c,d,e,g |
| Gibberellins content (GA3, GA7) |
157 a | – | – |
| GA3 | – | – | 23.65-128 c,d,e |
| GA7 | – | – | 110 d |
| Cytokinins content (trans-zeatin, kinetin) |
15.3 a | – | – |
| Cis-zeatin | – | – | 20.10-117 c,d,e,f,g |
| Trans-zeatin | – | – | 12.4 b |
| Kinetin | – | – | 7.94-73 c,d,f,g |
| Choline | – | – | 57.30 e |
| Glycine betaine | – | – | 79.33 e |
Phytohormone content in A. spicifera and K. alvarezii..
* Ranges of all values found in literature.
a (Hong et al., 2007); b (Das and Prasad, 2015); c (Prasad et al., 2010); d (Sedayu and Basmal, 2013); e (Layek et al., 2015); f (Zodape et al., 2009); g (Mondal et al., 2015).
For cultivation purposes, these plant growth regulators have been used alone or in combination to improve strains of K. alvarezii and increase the stock production through micropropagation. In addition, it has been proven that colchicine inhibits spindle fibers formation during cell division. The production of new strains using colchicine alone or in combination with phytohormones has potential to enhance micropropagation of seaweeds (Hayashi et al., 2007). Earlier studies demonstrated the efficacy of the use of phytohormones in enriched media to enhance branch culturing, reduce epiphyte contamination, improve daily growth rate and regenerate callus of K. alvarezii for micropropagation (Dawes and Koch, 1991; Dawes et al., 1993; Dawes et al., 1994; Hurtado and Biter, 2007). An improved callus induction of K. alvarezii with the plant growth regulators 1-naphthaleneacetic acid (NAA) and 6-benzylaminopurine (6-BA) and a reduction of the callus induction time with the addition of spermine has been reported (Muñoz et al., 2006). Additional studies demonstrated an increased growth rate, regeneration of explants, and shoot formation in micropropagules using spindle inhibitors such as colchicine and oryzalin alone or in combination with indole-3-acetic acid (IAA), 6-BA, spermine and kinetin (Hayashi et al., 2007; Neves et al., 2015) and the Acadian marine plant extract powder (Yunque et al., 2011; Tibubos et al., 2017).
The successful use of airlift bioreactors to produce seaweed biomass involving prior stimulation with phytohormones was reported by (Yong et al., 2014). In their in vitro study, an improved regeneration and growth of K. alvarezii propagules was observed using a dose-dependent application of 6-BA and IAA under control parameters such as pH, salinity, light, and culture intensity in Provasoli’s enriched seawater media.
Another alternative to promote the production of seed stock for commercial cultivation of Kappaphycus species is using seaweed liquid fertilizers. Application of the commercially available extract of Ascophyllum nodosum containing plant hormone regulators, macro- and micronutrients in a dose-dependent manner improved the daily growth rate of K. alvarezii strains with reaching almost the double growth rate of the control and reduced the presence of epiphytes and the “ice-ice” disease (Loureiro et al., 2010). It was noticed that brown and green strains of K. alvarezii had better growth rates than the red strain. The use of Acadian marine plant extract alone or in combination with phytohormones to promote micropropagation of K. alvarezii microplantlets has been recommended (Hurtado et al., 2008).
Commercially cultivated microalgae are other organisms that showed significant improvement in their growth and higher yield of their bioproducts through the application of phytohormones from seaweeds. Microalgae represent an important and sustainable source for animal feed in aquaculture based on their high nutritional content and as a bioremediation tool to prevent eutrophication in a high-ammonia-nitrogen environment (Zhao et al., 2019; Nagarajan et al., 2021). Different concentrations of K. alvarezii sap were used as biostimulant to enhance the growth and biochemical composition of Chlorella variabilis (Sati et al., 2021). The lipid and carbohydrate concentration of the latter microalga were increased up to 50 and 100% when sap concentration was used at 0.6 and 1%, respectively. Even though, very little investigation has been carried out with Kappaphycus sap containing phytohormones, several studies demonstrated the potential use of plant growth regulators to improve biomass productivity and chemical composition of microalgae (Hunt et al., 2010; Babu et al., 2017; Touliabah and Almutairi, 2021), especially under stressful conditions (Sun et al., 2018; Zhao et al., 2019).
4.2.2 Acanthophora spp.
The phytohormone composition and content of Acanthophora species have not been evaluated in detail. To the best of our knowledge, there is currently only one scientific report of the phytohormone content of A. spicifera, namely of one collected in Vietnam (Table 4), and no publications on the phytohormone content of extracts of this species have been reported.
4.3 Pigments
Red seaweeds are known to produce a diverse group of pigments, such as (1) the phycobiliproteins Rhodophyta phycoerythrin (R-PE) (red color), Rhodophyta phycocyanin (R-PC) (blue color) and allophycocyanin (APC), (2) carotenoids, (3) xanthophylls and (4) chlorophyll a (Chl a) (Freitas et al., 2021). These compounds have an important role in seaweed growth and development by harvesting solar energy and transforming it into chemical energy. As a high-added value, these compounds have displayed important bioactivities in the pharmaceutical industry as antioxidant (β-carotene) and anticancer agents used in photodynamic therapy (Chl a), in the textile industry as natural dyes (carotenoids, Chl a) (Ab Kadir et al., 2014) and as human-safe food colorant (Heriyanto et al., 2015). In the aquaculture sector, pigments like lutein, astaxanthin, chlorophylls, phycobiliproteins and β-carotene are used to improve the color, health, and organoleptic properties of commercially important marine organisms (Serive and Bach, 2018). However, the cost of production of these pigments is very high and has been limited by production technologies (Yusoff et al., 2020). It is necessary to do more research on the development of more effective, low cost and sustainable pigment production to be used in aquaculture. Although, most of the pigments used for aquaculture are obtained from microalgae (Yusoff et al., 2020), it is important to search for other natural sources such as seaweeds, that could provide good quality and quantity of pigments and find their way as functional food in the aquaculture industry.
4.3.1 Kappaphycus spp.
Studies in K. alvarezii reported the presence of R-PE (Naguit and Tisera, 2009; Heriyanto et al., 2015; Banu et al., 2017; Deepika, 2018; Ganesan and Shanmugam, 2020; Uju et al., 2020), R-PC, Chl a, chlorophyllide a, pheophytin, α-cryptoxanthin, violaxanthin, antheraxanthin, zeaxanthin, β-carotene, α-carotene, trans-fucoxanthin and other unidentified pigments (Naguit and Tisera, 2009; Heriyanto et al., 2015; Brotosudarmo et al., 2018) (Table 5). Variation of their composition between strains, depths of cultivation and subjection to stress conditions such as UV radiation have also been documented (Eswaran et al., 2001; Schmidt et al., 2010).
Table 5
| Parameter * | A. spicifera | A. nayadiformis | K. alvarezii |
|---|---|---|---|
| Chl a | 0.056 - 0.50 a,o ** 0.094 – 0.37 b,c *** |
0.20 – 1.60 f ** | 0.012 – 2.7 g,h,i,j,k,l,m,n *** |
| APC | 0.031 – 0.5 a,c,d ** 0.121 e *** |
nd | nd |
| R-PC | 0.057 – 0.9 a,d ** 0.24 – 0.34 c,e *** |
1.68 – 6.04 f ** | 0.203 – 0.491 l,m *** |
| R-PE | 0.143 – 2.6 a,d ** 0.42 – 0.49 c,e *** |
2.20 – 10.03 f ** | 1.2 – 1.7 l, m *** |
| Zeaxanthin | nd | 0.02 – 0.05 f ** | 0.0013 – 0.003 i *** |
| α-carotene | nd | nd | 0.002 – 0.054 i,n *** |
| β-carotene | nd | 0.02 – 0.08 f ** | 0.05 – 0.514 i,n *** |
| α-cryptoxanthin | nd | nd | 0.005 – 0.037 n *** |
| Antheraxanthin | nd | nd | 0.007 – 0.053 n *** |
| Lutein | nd | 0.02 – 0.09 f ** | nd |
| Total carotenoids | 0.245 – 0.379 a** 0.31 b *** |
nd | nd |
Pigment content in Acanthophora and Kappaphycus species.
* Ranges of all values found in literature; nd= not determined
** values expressed as mg/g dry weight (dw)
*** values expressed as mg/g fresh weight (fw)
a (Pereira et al., 2017); b (Seenivasan et al., 2012); c (Arunkumar et al., 2014); d (Martins et al., 2018); e (Senthilkumar et al., 2013); f (Petrocelli and Felicini, 1995); g (Hong et al., 2007); h (Naguit and Tisera, 2009); i (Brotosudarmo et al., 2018); j (Paransa et al., 2020); k (Rajaram et al., 2021); l (Eswaran et al., 2001); m (Periyasamy et al., 2019); n (Heriyanto et al., 2015), ° (Dawes et al., 1978).
Although, the application of pigments isolated from Kappaphycus spp., in aquaculture has not been reported, the application of K. alvarezii sap could be used during the cultivation of astaxanthin-rich organisms such as Haematococcus pluvialis or β-carotene-rich sources such as Dunaliella salina to enhance its yield (Xie et al., 2018). The use of carotenoids as food ingredients for aquaculture has been reviewed (Pereira da Costa and Campos, 2020). Studies on C-PC produced by cyanobacteria (Spirulina platensis) have shown to improve growth and color of the guppy fish Poecilia reticulata (Biabani Asrami et al., 2019), reduce cannibalism and enhance survival and disease resistance against Vibrio alginolyticus in the Asian seabass Lates calcarifer larvae (Gora et al., 2019) and induce a non-specific immune response in the fish carp Cyprinus carpio (Muchtar et al., 2019). R-PE isolated from the red alga Colaconema sp. induced an immunostimulatory effect on the shrimp Litopenaeus vannamei with increasing resistance against Vibrio parahaemolyticus and white spot syndrome virus (Lee et al., 2021). The strategies to improve yield and chemical stability of PE and PC have been recently reviewed (Hsieh-Lo et al., 2019).
Extraction optimization of pigments such as R-PE (Uju et al., 2020), β-carotene and chlorophyll (Baskararaj et al., 2019) and carrageenan (Mahyati and Azis, 2019) from K. alvarezii has been reported. (Freitas et al., 2021) provided an interesting review of the chemical diversity of the different pigments produced by red seaweeds and their biotechnological applications along with the number of patents registered.
4.3.2 Acanthophora spp.
Information on the pigment content in Acanthophora spp. is rather limited. A. spicifera contained 0.34 mg of R-PC, 0.121 mg of R-APC and 1.061 mg of R-PE per gram fresh weight (Senthilkumar et al., 2013; Pereira et al., 2019) while A. nayadiformis contained 0.20-0.28 mg/g dry weight of Chl a, 2.20-5.54 mg/g dry weight of R-PE and 1.68-3.91 mg/g dry weight of R-PC (Petrocelli and Felicini, 1995) (Table 5).
Concentrations of 0.79-2.39 mg/g dry weight of lutein, 5.79-11.28 mg/g dry weight of zeaxanthin, 1.59-16.16 mg/g dry weight of α-carotene were reported (Pereira et al., 2019), while antheraxanthin, β-cryptoxanthin and β-carotene were identified in A. spicifera based on their retention time and comparison with standards (Aihara and Yamamoto, 1968). Also, the concentration of 0.02 mg/g dry weight of β-carotene, 0.02 mg/g dry weight of zeaxanthin, and 0.02 mg/g dry weight of lutein were reported for A. nayadiformis (Petrocelli and Felicini, 1995).
4.4 Amino acids and peptides
Seaweed-derived peptides such as lectins and phycobiliproteins and the mycosporine-like amino acids (MAAs) have been studied for their wide diversity of applications in food, pharmaceutical and cosmeceutical sector (Lafarga et al., 2020; Vega et al., 2021; Echave et al., 2022). One of the most important seaweed peptides with aquaculture applications are lectins, of which the advances in the development of extraction methods and the wide range of biological activities was recently reviewed (Maliki et al., 2022). In this review, phycobiliproteins are being considered within the pigment section.
Lectins are glycoproteins of non-immune origin characterized for their high capacity and selectivity to reversibly bind to carbohydrate moieties present in pathogens through a carbohydrate recognition domain (Mishra et al., 2019). Seaweeds are known to be a good source of a vast diversity of novel bioactive lectins also called phycolectins. Red seaweed lectins are classified depending on their carbohydrate specificity in complex-type specific (complex N-glycan or complex O-glycan or both), high-mannose type specific or both complex and high-mannose glycan specific lectins (Singh and Walia, 2018). The gamete recognition during sexual reproduction is suggested to be one of the most important roles of lectins in marine algae.
The MAAs are low molecular weight and nitrogen-containing compounds with strong photoprotective activity against ultraviolet radiation (Oren and Gunde-Cimerman, 2007). These UV-absorbing compounds are found in many marine organisms like cyanobacteria, cnidarians, fungi, but are in greater content present in red seaweeds (Bedoux et al., 2020). The distribution, concentration, and types of MAAs in seaweeds and the development of an open database for these metabolites have been reported (Sun et al., 2020). These secondary metabolites play an important role in the photosynthesis as light-harvesting pigments, provide protection to the organism against desiccation or thermal stress, as antioxidants, with anti-lipid oxidation activity, and as intracellular nitrogen reservoir. These molecules are widely used in the cosmeceutical industry based on their antioxidant, anti-inflammatory, and collagen, elastin and DNA protective activities, and their distribution in seaweeds has been reviewed (Vega et al., 2021). Although, there is little application of MAAs in aquaculture, their presence in seaweeds and other aquatic animals provides them protection against solar radiation conditions and accumulation of these important metabolites in seaweeds would provide an economic alternative to be exploited for the cosmeceutical industry.
4.4.1 Kappaphycus spp.
Kappaphycus species are a rich source of essential amino acids required for human and animal consumption.
The presence of lectins has been reported in K. alvarezii (Hung et al., 2009b; Hung et al., 2009c; Sato and Hori, 2011; Yong et al., 2014) and K. striatus (Hung et al., 2009b; Hung et al., 2009c; Hung et al., 2019; Hung and Trinh, 2021). They have been shown to be highly specific for high-mannose N-glycans and have displayed potential biological activities as antiviral and anticancer agents and possess strong divalent cation-independent hemagglutination activity (Hung et al., 2009b; Sato and Hori, 2011; Hirayama et al., 2016; Hung and Trinh, 2021). Other studies reported that the three color morphotypes of K. alvarezii red, brown, and green strains, also contain similar lectin content, however, the lectin yield of the red strain was higher than the green and brown strains (Hung et al., 2009a). It has been suggested that lectin biosynthesis in macroalgae is enhanced by an increased uptake of inorganic nitrogen in ammonium (NH4+) form rather than nitrate (NO3-), especially during low seawater temperature, solar radiation, and low tide (Hung et al., 2009b). Whereas the application of seaweed-derived mannose-specific lectins has been focused mostly on the pharmacological potential for humans (Barre et al., 2019), very little research has been undertaken in the application of Kappaphycus-derived lectins in aquaculture. The only study reported by (Hung et al., 2015), revealed inhibition of the growth of the shrimp pathogens Enterobacter cloacae and Vibrio alginolyticus by the isolated lectin KSA-2 from K. striatus. Even though this lectin did not show growth inhibition against V. parahaemolyticus and V. harveyi in the same study, it has great potential to target aquaculture pathogens with glycoproteins with high-mannose N-glycans in their surface.
To the best of our knowledge, to date, there are no studies determining the MAAs composition and their concentration in Kappaphycus species.
4.4.2 Acanthophora spp.
Seaweeds are becoming an important source of high-nutritional and low-cost material for the development of animal feed. Analysis of the amino acid and peptides content of A. spicifera (Wahidulla et al., 1991; Lourenço et al., 2002; Vinoj Kumar and Kaladharan, 2007; Dixit et al., 2018), A. muscoides (Rao, 1970) and A. nayadiformis (Lewis and Gonzalves, 1962; Impellizzeri et al., 1975) have been reported. Acanthophora species are not a very good source of essential amino acids, as the estimated protein content is only in the range of 5-25% of its dry weight. Protein is one of the most important and expensive ingredients in feed for aquatic animals. The quality of a protein in feed depends mainly on its amino acid profile and digestibility. Therefore, the analysis of the amino acid profile is necessary for the elaboration of animal diets, in accordance with the amino acid’s requirements of the animal of interest.
The presence of lectins in A. spicifera was first deducted by the strong agglutinin activity of rabbit erythrocytes (Chiles and Bird, 1989) and the enzyme treated erythrocytes from chicken, rabbit, goat, pig, and human O type (Lima Ainouz et al., 1992; Mangaiyarkarasi et al., 2014) and from A. muscoides (Anam et al., 2019). However, there are no studies on the application of Acanthophora lectins in aquaculture.
The MAAs, such as asterina-330, gadusol, mycosporine-glycine, mycosporine-2-glycine, mycosporine-taurine, mycosporine-methylamine-serine, mycosporine-methylamine-threonine, palythinol, palythene, palythine, palythine-serine, palythine-serine-sulfate, palythine-threonine, porphyra-334, shinorine, and an unknown compound with maximum absorption at 299 nm have only been reported for A. spicifera (Karsten et al., 1998; Rosic et al., 2015; Muthiah et al., 2017). Although the UV-absorbing compounds asterina-330, mycosporine-glutamic acid and palythinol have been suggested to be present in A. muscoides (Tanaka et al., 2020), further studies are required to confirm their presence and concentration in this and other species of Acanthophora.
An increased metabolic rate and faster hatching egg hatchering were observed in the sea hare Aplysia dactylomela after feeding with A. spicifera as a rich source of MAAs (Carefoot et al., 1998). Further studies suggested the role of MAAs as UV protectant in A. dactylomela as these metabolites were distributed in the skin (Carefoot et al., 2000) and the fish Thalassoma duperrey after feeding with A. spicifera (Zamzow, 2004). The increase in MAAs content in the fish mucus was only observed when exposed to UV radiation, and accumulation of MAAs was higher in male fish than female. The UV photo-protective effect on other marine organisms such as the sea urchin Strongylocentrotus droebachiensis (Adams and Shick, 1996), and different species of holothuroids (Shick et al., 1992) has been reported. The presence of UV-absorbing compounds in the sea hare and fishes is resulting from their diet with seaweeds rich in MAAs. The chemical and ecological role of MAAs and their distribution among marine organisms have been reviewed (Carreto and Carignan, 2011).
Despite the few studies on the role of MAAs from Acanthophora species in aquaculture, these metabolites play a very important role in the protection of animals against radiation. The protective role of MAAs in aquatic animals has been reported in different animals. For example, studies on the sea hare Aplysia californica indicated the presence of MAAs and their role as a mechanism of chemical defense or as intraspecific alarm cues. Two undescribed MAAs such as aplysia palythine A and aplysia palythine B and the known asterina-330 were found in the sea hare after feeding with the red algae Gracilaria ferox and Agardhiella subulate (Kicklighter et al., 2011).
MAAs can be transferred from different trophic levels, and might play different roles in the marine environment, especially increasing the survival of species under high UV radiation and predation environments. Solar radiation causes damage to the marine environment and its fauna by reducing productivity, growth rate and development, and the mutation rate in eggs and larval stages of marine animals and algae is increased (Häder et al., 2007). Feeding commercially important species of marine animals with seaweeds rich in MAAs in tropical zones with higher exposure to solar radiation could enhance their survival, growth rate and reproduction with reduced economic impact. Additionally, when culturing seaweeds in high nitrogen environments, the concentration of MAAs in seaweeds would increase, as reported by a previous study on Gracilaria tenuistipitata (Barufi et al., 2012).
5 Discussion and research gaps
Further valorization of Kappaphycus spp. and Acanthophora spp. in the aquaculture sector will need to bridge the following research gaps:
-
(1) For at least one of the studied species the proof-of-principle of certain innovative applications of seaweed biomass, extracts and biochemicals in the aquaculture sector has been shown. These applications remain to be confirmed for the other species as well to open commercial opportunities for the seaweed biomass and their bioproducts.
-
(2) Similarly, other innovative aquaculture applications have been demonstrated for other seaweed species and their chemical arsenal. This suggests that some opportunities are available to explore these applications for Kappaphycus spp. and Acanthophora spp. by means of scientific research.
-
(3) Another remaining challenge is to standardize the methods to analyze the chemical profile and to quantify their concentration/content. Furthermore, concerted efforts are needed towards the standardization of the methodology to report data. This will allow a more accurate comparison of different studies, especially with respect to units (reporting of the content of chemical compound per fresh weight seaweed, dry weight seaweed, and volume of extract).
-
(4) To discover new biochemical compounds and identify new applications of these compounds, an in-depth chemical profiling of Kappaphycus spp. and Acanthophora spp. in different geographical locations and temporal variations needs to be carried out.
The available information highlights the great potential application of both genera of red seaweeds in aquaculture that will allow researchers to further develop the production of innovative seaweed-based bioproducts.
Local tropical seaweed species are yet to be fully utilized, especially as a primary product to be used in the development of natural bioproducts to achieve a more resilient aquaculture and agriculture. Therefore, more attention should be given to underutilized local tropical seaweed species.
6 Conclusions
The chemical diversity (polysaccharides, phytohormones, pigments, amino acids and peptides) of Kappaphycus and Acanthophora has shown promising applications for aquaculture, including pathogen control, immunostimulant, antioxidant, bioremediation, feed, UV protectants, increase in seafood shelf life, animal colorant, and growth regulator for microalgae culture. This review justifies further research of the latter species to guarantee seaweed biomass availability, to know the temporal and spatial variation of the chemical composition of seaweeds, to standardize research methodologies, and to confirm the bioactivity of phytochemicals, amongst others. This study contributes to boosting the applications of these and other red seaweeds in the aquaculture sector and, by this, helping stakeholders to benefit from these seaweeds by diversifying the blue bioeconomy in tropical countries. The authors would like to thank the reviewers for their valuable comments to improve the manuscript.
Funding
This study has been carried out within the framework of the projects (1) “Desarollo e implementación piloto de sistemas de maricultura que generen incentivos a la conservación del manglar (DIPSIMAR)” funded by the program REM Ecuador of the Ministry of Environment, Water and Ecological Transition of Ecuador (MAATE), administered by Fondo de Inversión Ambiental Sostenible (FIAS), co-financed by Norways International Climate and Forest Initiative (NICFI), Kreditanstalt für Wiederaufbau (KfW), and by ESPOL, and (2) “Seaweed-based innovations for a resilient aquaculture and agriculture (SIRENA)” funded by the program South Initiatives of Vlaamse Interuniversitaire Raad - Universitaire Ontwikkelingssamenwerking (VLIR-UOS), and co-funded by Ghent University and ESPOL.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Author contributions
PG, PM, SM, OC, PB and SH contributed to the initial idea and design of the manuscript. PG collected the literature and wrote the original draft of the manuscript. PM contributed to table data collection and reference listing. SH did literature survey, contributed to writing the abstract, research gaps, conclusions, and edited the total manuscript. SM revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Abirami R. Kowsalya S. (2011). Nutrient and nutraceutical potentials of seaweed biomass Ulva lactuca and Kappaphycus alvarezii. J. Agric. Sci. Technol.5, 109–115.
2
Ab Kadir M. Wan Ahmad W. Ahmad M. Abdul Jabbar H. Ngalib K. Ismail ,. A. (2014). "Dyeing properties and absorption study of natural dyes from seaweeds, Kappaphycus alvarezii". Proc. Int. Colloq. Text. Eng. Fash. Appar. Des., 99–105. doi: 10.1007/978-981-287-011-7
3
Abomohra A. El-Naggar A. Baeshen A. (2018). Potential of macroalgae for biodiesel production: screening and evaluation studies. J. Biosci. Bioeng.125, 231–237. doi: 10.1016/j.jbiosc.2017.08.020
4
Adams N. L. Shick J. M. (1996). Mycosporine-like amino acids provide protection against ultraviolet radiation in eggs of the green sea urchin Strongylocentrotus droebachiensis. Photochem. Photobiol.64, 149–158. doi: 10.1111/j.1751-1097.1996.tb02435.x
5
Adharini R. Suyono E. Jayanti A. Setyawan A. (2019). A comparison of nutritional values of Kappaphycus alvarezii, Kappaphycus striatum, and Kappaphycus spinosum from the farming sites in gorontalo province, sulawesi, Indonesia. J. Appl. Phycol.31, 725–730. doi: 10.1007/s10811-018-1540-0
6
Aihara M. Yamamoto H. (1968). Occurrence of antheraxanthin in two rhodophyceae Acanthophora spicifera and Gracilaria lichenoides. Phytochemistry7, 497–499. doi: 10.1016/S0031-9422(00)90896-3
7
Alcantara J. Lazaro-Llanos N. (2020). Mineral availability, dietary fiber contents, and short-chain fatty acid fermentation products of Caulerpa lentillifera and Kappaphycus alvarezii seaweeds. Kimika31, 1–10. doi: 10.26534/kimika.v31i1.1-10
8
Ali M. Critchley A. Hurtado A. (2020). Micropropagation and sea-based nursery growth of selected commercial Kappaphycus species in penang, Malaysia. J. Appl. Phycol. .32, 1301–1309. doi: 10.1007/s10811-019-02003-4
9
Anam C. Praseptiangga D. Fajarningsih N. D. Chasanah E. Yunus A. (2019). Preliminary characterization of crude lectins fractions of red macroalgae species collected from the southern coast of gunungkidul Indonesia. Int. J. Adv. Sci. Eng. Inf. Technol.9, 1309–1316. doi: 10.18517/ijaseit.9.4.5200
10
Anand J. Sathuvan M. Babu G. V. Sakthivel M. Palani P. Nagaraj S. (2018). Bioactive potential and composition analysis of sulfated polysaccharide from Acanthophora spicifera (Vahl) borgeson. Int. J. Biol. Macromol.111, 1238–1244. doi: 10.1016/j.ijbiomac.2018.01.057
11
Anil K. Balakrishanan G. Kanji J. Jitesh S. Kumaran R. (2011). Comparison of penaeus monodon (Crustacea, penaeidae) growth between commercial feed vs commercial shrimp feed supplemented with Kappaphycus alvarezii (Rhodophyta, solieriaceae) seaweed sap. Aquacult. Aquarium Conserv. Legis.4, 292–300.
12
Ariano A. Musco N. Severino L. De Maio A. Tramice A. Tommonaro G. et al . (2021). Chemistry of tropical eucheumatoids: Potential for food and feed applications. Biomolecules11, 804. doi: 10.3390/biom11060804
13
Arulkumar A. Satheeshkumar K. Paramasivam S. Rameshthangam P. Miranda J. M. (2020). Chemical biopreservative effects of red seaweed on the shelf life of black tiger shrimp (Penaeus monodon). Foods9, 634. doi: 10.3390/foods9050634
14
Arunkumar K. Palanivelu A. Darsis A. (2014). Proximate composition, nutraceutical constituents and fatty acid profile on GCMS of seaweeds collected from balk bay (Thondi), India. Int. J. Curr. Sci.12, 57–71.
15
Ask E. Azanza R. (2002). Advances in cultivation technology of commercial eucheumatoid species: a review with suggestions for future research. Aquaculture206, 257–277. doi: 10.1016/S0044-8486(01)00724-4
16
Ávila E. Méndez-Trejo M. Riosmena-Rodríguez R. López-Vivas J. Sentíes A. (2012). Epibiotic traits of the invasive red seaweed Acanthophora spicifera in la paz bay, south Baja California (Eastern pacific). Mar. Ecol.33, 470–480. doi: 10.1111/j.1439-0485.2012.00511.x
17
Babu A. Wu X. Kabra A. Kim D. (2017). Cultivation of an indigenous Chlorella sorokiniana with phytohormones for biomass and lipid production under n-limitation. Algal Res.23, 178–185. doi: 10.1016/j.algal.2017.02.004
18
Balasubramaniam V. Mustar S. Mustafa Khalid N. Abd Rashed A. Mohd Noh M. Wilcox M. et al . (2013). Inhibitory activities of three Malaysian edible seaweeds on lipase and α-amylase. J. Appl. Phycol.25, 1405–1412. doi: 10.1007/s10811-012-9964-4
19
Banu S. Santhosh S. Hemalatha V. Venkatakrishnan V. Dhandapani R. (2017). Optimization study on extraction & purification of phycoerythrin from red algae Kappaphycus alvarezii. Asian J. Pharm. Clin. Res.10, 1–6. doi: 10.22159/ajpcr.2017.v10i2.15598
20
Barceló-Villalobos M. Figueroa F. Korbee N. Álvarez-Gómez F. Abreu M. (2017). Production of mycosporine-like amino acids from Gracilaria vermiculophylla (Rhodophyta) cultured through one year in an integrated multi-trophic aquaculture (IMTA) system. Mar. Biotechnol.19, 246–254. doi: 10.1007/s10126-017-9746-8
21
Barre A. Simplicien M. Benoist H. Van Damme E. Rougé P. (2019). Mannose-specific lectins from marine algae: diverse structural scaffolds associated to common virucidal and anti-cancer properties. Mar. Drugs17, 440. doi: 10.3390/md17080440
22
Barrios J. Bolaños J. López R. (2007). Blanqueamiento de arrecifes coralinos por la invasión de Kappaphycus alvarezii (Rhodophyta) en isla cubagua, estado nueva esparta, Venezuela. Bol. Inst. Oceanogr. Venezuela.46, 147–152.
23
Barufi J. Mata M. Oliveira M. Figueroa F. (2012). Nitrate reduces the negative effect of UV radiation on photosynthesis and pigmentation in Gracilaria tenuistipitata (Rhodophyta): the photoprotection role of mycosporine-like amino acids. Phycologia51, 636–648. doi: 10.2216/10.77.1
24
Baskararaj S. Theivendren P. Palanisamy P. Kannan S. Pavadai P. Arunachalam S. et al . (2019). Optimization of bioactive compounds extraction assisted by microwave parameters from Kappaphycus alvarezii using RSM and ANFIS modeling. J. Food Meas. Charact.13, 2773–2789. doi: 10.1007/s11694-019-00198-1
25
Bautista M. Millamena O. Kanazawa A. (1989). Use of kappa-carrageenan microbound diet (C-MBD) as feed for Penaeus monodon larvae. Mar. Biol.103, 169–173. doi: 10.1007/BF00543344
26
Bedoux G. Pliego-Cortés H. Dufau C. Hardouin K. Boulho R. Freile-Pelegrin Y. et al . (2020). "Production and properties of mycosporine-like amino acids isolated from seaweeds,". Adv. Bot. Res.95, 213–245. doi: 10.1016/bs.abr.2019.11.004
27
Beltran-Gutierrez M. Ferse S. Kunzmann A. Stead S. Msuya F. Hoffmeister T. et al . (2016). Co-Culture of sea cucumber Holothuria scabra and red seaweed Kappaphycus striatum. Aquac. Res.47, 1549–1559. doi: 10.1111/are.12615
28
Bhaskar N. Kinami T. Miyashita K. Park S. Endo Y. Fujimoto K. (2004). Occurrence of conjugated polyenoic fatty acids in seaweeds from the Indian ocean. Z Naturforsch. C J. Biosci. .59, 310–314. doi: 10.1515/znc-2004-5-602
29
Biabani Asrami M. Sudagar M. Shahraki N. Vahdat S. (2019). Effect of extracted phycocyanin from Spirulina platensis on growth parameters, colorations, digestive enzymes and body chemical compositions of guppy fish (Poecilia reticulata). J. Surv. Fish. Sci.6, 1–8. doi: 10.18331/sfs2019.6.1.1
30
Bindu M. Levine I. (2011). The commercial red seaweed Kappaphycus alvarezii-an overview on farming and environment. J. Appl. Phycol.23, 789–796. doi: 10.1007/s10811-010-9570-2
31
Borgogna M. Bellich B. Cesàro A. (2011). Marine polysaccharides in microencapsulation and application to aquaculture:”from sea to sea”. Mar. Drugs9, 2572–2604. doi: 10.3390/md9122572
32
Bouanati T. Colson E. Moins S. Cabrera J. Eeckhaut I. Raquez J. et al . (2020). Microwave-assisted depolymerization of carrageenans from Kappaphycus alvarezii and Eucheuma spinosum: Controlled and green production of oligosaccharides from the algae biomass. Algal Res.51, 102054. doi: 10.1016/j.algal.2020.102054
33
Brotosudarmo T. Heriyanto H. Shioi Y. Indriatmoko I. Adhiwibawa M. Indrawati R. et al . (2018). Composition of the main dominant pigments from potential two edible seaweeds. Philipp. J. Sci.147, 47–55.
34
Budiyanto K. Abadi S. (2019). Growth and carrageenan content of local and tissue culture seed of Kappaphycus alvarezii cultivated in floating cage. AACL Bioflux.12, 167–178.
35
Bui V. Nguyen B. Renou F. Nicolai T. (2019). Structure and rheological properties of carrageenans extracted from different red algae species cultivated in cam ranh bay, Vietnam. J. Appl. Phycol.31, 1947–1953. doi: 10.1007/s10811-018-1665-1
36
Bulboa C. De Paula E. (2005). Introduction of non-native species of Kappaphycus (Rhodophyta, gigartinales) in subtropical waters: Comparative analysis of growth rates of Kappaphycus alvarezii and Kappaphycus striatum in vitro and in the sea in south-eastern Brazil. Phycol. Res.53, 183–188. doi: 10.1111/j.1440-183.2005.00385.x/full
37
Cardozo K. Guaratini T. Barros M. Falcão V. Tonon A. Lopes N. et al . (2007). Metabolites from algae with economical impact. Comp. Biochem. Physiol. C Toxicol. Pharmacol.146, 60–78. doi: 10.1016/j.cbpc.2006.05.007
38
Carefoot T. Harris M. Taylor B. Donovan D. Karentz D. (1998). Mycosporine-like amino acids: possible UV protection in eggs of the sea hare Aplysia dactylomela. Mar. Biol.130, 389–396. doi: 10.1007/s002270050259
39
Carefoot T. Karentz D. Pennings S. Young C. (2000). Distribution of mycosporine-like amino acids in the sea hare Aplysia dactylomela: effect of diet on amounts and types sequestered over time in tissues and spawn. Comp. Biochem. Physiol. - C Pharmacol. Toxicol. Endocrinol.126, 91–104. doi: 10.1016/S0742-8413(00)00098-0
40
Carreto J. Carignan M. (2011). Mycosporine-like amino acids: relevant secondary metabolites. chemical and ecological aspects. Mar. Drugs9, 387–446. doi: 10.3390/md9030387
41
Castelar B. Reis R. Azeredo F. Mattos P. Berardinelli G. (2016). Hypnea musciformis: alternative or complement to the production of Kappaphycus alvarezii introduced in tropical countries? Aquac. Res.47, 3538–3550. doi: 10.1111/are.12804
42
Chandrasekaran S. Nagendran N. Pandiaraja D. Krishnankutty N. Kamalakannan B. (2008). Bioinvasion of Kappaphycus alvarezii on corals in the gulf of mannar, India. Curr. Sci., 1167–1172.
43
Chen Y. Chen J. Lin Y. Putra D. Kitikiew S. Li C. et al . (2014). Shrimp that have received carrageenan via immersion and diet exhibit immunocompetence in phagocytosis despite a post-plateau in immune parameters. Fish Shellfish Immunol.36, 352–366. doi: 10.1016/j.fsi.2013.12.004
44
Chen H. Jian Q. Luo Q. Zhu Z. Yang R. Yan X. (2016). Application of oligoagars as elicitors for field aquaculture of Pyropia haitanensis. J. Appl. Phycol.28, 1783–1791. doi: 10.1007/s10811-015-0734-y
45
Cheong K. Qiu H. Du H. Liu Y. Khan B. (2018). Oligosaccharides derived from red seaweed: Production, properties, and potential health and cosmetic applications. Molecules23, 2451. doi: 10.3390/molecules23102451
46
Chiles T. Bird K. (1989). A comparative study of animal erythrocyte agglutinins from marine algae. Comp. Biochem. Physiol. – Part B Biochem.94, 107–111. doi: 10.1016/0305-0491(89)90018-7
47
Ciancia M. Matulewicz M. Tuvikene R. (2020). Structural diversity in galactans from red seaweeds and its influence on rheological properties. Front. Plant Sci.11. doi: 10.3389/fpls.2020.559986
48
Cokrowati N. Risjani Y. Andayani S. Firdaus M. Honiar R. (2021) Identification and determination of growth hormones of Kappaphycus alvarezii cultivated in Ekas Bay East Lombok. IOP Conf. Ser. Earth Environ. Sci., 890. doi: 10.1088/1755-1315/890/1/012040
49
Cordeiro-Marino M. Braga M. Eston V. Fujii M. Yokoya N. (1992). "Mangrove macroalgal communities of Latin America: the state of art and perspectives". Coast. Plant communities Latin America, 51–64. doi: 10.1016/B978-0-08-092567-7.50009-X
50
Cortés J. Enochs I. Sibaja-Cordero J. Hernández L. Alvarado J. Breedy O. et al . (2017). “"Marine biodiversity of Eastern tropical pacific coral reefs,",” in Coral reefs of the Eastern tropical pacific. Eds. GlynnP.ManzelloD.EnochsI. (Dordrecht: Springer), 203–250. doi: 10.1007/978-94-017-7499-4_7
51
Cunha L. Grenha A. (2016). Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs14, 42. doi: 10.3390/md14030042
52
da Costa E. Melo T. Moreira A. Alves E. Domingues P. Calado R. et al . (2015). Decoding bioactive polar lipid profile of the macroalgae Codium tomentosum from a sustainable IMTA system using a lipidomic approach. Algal Res.12, 388–397. doi: 10.1016/j.algal.2015.09.020
53
da Silva E. Castilho-Barros L. Henriques M. (2022). Economic feasibility of integrated multi-trophic aquaculture (mussel Perna perna, scallop Nodipecten nodosus and seaweed Kappaphycus alvarezii) in southeast Brazil: A small-scale aquaculture farm model. Aquaculture552, 738031. doi: 10.1016/j.aquaculture.2022.738031
54
Das K. Prasad K. (2015). Extraction of plant growth regulators present in Kappaphycus alvarezii sap using imidazolium based ionic liquids: detection and quantification by using HPLC-DAD technique. Anal. Methods7, 9064–9067. doi: 10.1039/C5AY02210J
55
Dawes C. Koch E. (1991). Branch, micropropagule and tissue culture of the red algae Eucheuma denticulatum and Kappaphycus alvarezii farmed in the Philippines. J. Appl. Phycol.3, 247–257. doi: 10.1007/BF00003583
56
Dawes C. Lluisma A. Trono G. (1994). Laboratory and field growth studies of commercial strains of Eucheuma denticulatum and Kappaphycus alvarezii in the Philippines. J. Appl. Phycol.6, 21–24. doi: 10.1007/BF02185899
57
Dawes C. Moon R. Davis M. (1978). The photosynthetic and respiratory rates and tolerances of benthic algae from a mangrove and salt marsh estuary: a comparative study. Estuar. Coast. Shelf Sci.6, 175–185. doi: 10.1016/0302-3524(78)90099-3
58
Dawes C. Trono G. Lluisma A. (1993). Clonal propagation of Eucheuma denticulatum and Kappaphycus alvarezii for Philippine seaweed farms. Hydrobiologia260, 379–383. doi: 10.1007/BF00049044
59
Deepika C. (2018). Extraction and spectral characterization of r-phycoerythrin from macroalgae – Kappaphycus alvarezii. Int. Res. J. Engin. Technol.5, 1181–1185.
60
Dixit D. Reddy C. Balar N. Suthar P. Gajaria T. Gadhavi D. (2018). Assessment of the nutritive, biochemical, antioxidant and antibacterial potential of eight tropical macro algae along kachchh coast, India as human food supplements. J. Aquat. Food Prod. Technol.27, 61–79. doi: 10.1080/10498850.2017.1396274
61
Duarte M. Cauduro J. Noseda D. Noseda M. Gonçalves A. Pujol C. et al . (2004). The structure of the agaran sulfate from Acanthophora spicifera (Rhodomelaceae, ceramiales) and its antiviral activity. relation between structure and antiviral activity in agarans. Carbohydr. Res.339, 335–347. doi: 10.1016/j.carres.2003.09.028
62
Dumilag R. Crisostomo B. Aguinaldo Z. Hinaloc L. Liao L. Roa-Quiaoit H. et al . (2022). The diversity of eucheumatoid seaweed cultivars in the Philippines. Rev. Fish. Sci. Aquac., 1–19. doi: 10.1080/23308249.2022.2060038
63
Echave J. Otero P. Garcia-Oliveira P. Munekata P. Pateiro M. Lorenzo J. et al . (2022). Seaweed-derived proteins and peptides: promising marine bioactives. Antioxidants11, 176. doi: 10.3390/antiox11010176
64
Estevez J. Ciancia M. Cerezo A. (2000). The system of low-molecular-weight carrageenans and agaroids from the room-temperature-extracted fraction of Kappaphycus alvarezii. Carbohydr. Res.325, 287–299. doi: 10.1016/S0008-6215(00)00006-9
65
Eswaran K. Subba Rao P. Mairh O. (2001). Impact of ultraviolet-b radiation on a marine red alga Kappaphycus alvarezii (Solieriaceae, rhodophyta). Indian J. Mar. Sci.30, 105–107.
66
Fabbrocini A. Volpe M. Di Stasio M. D'adamo R. Maurizio D. Coccia E. et al . (2012). Agar-based pellets as feed for sea urchins (Paracentrotus lividus): Rheological behaviour, digestive enzymes and gonad growth. Aquac. Res.43, 321–331. doi: 10.1111/j.1365-2109.2011.02831.x
67
Fadilah S. Pong-Masak P. Santoso J. Parenrengi A. (2016). Growth, morphology and growth related hormone level in Kappaphycus alvarezii produced by mass selection in gorontalo waters, Indonesia. HAYATI J. Biosci.23, 29–34. doi: 10.1016/j.hjb.2015.09.004
68
FAO (2020). El Estado mundial de la pesca y acuicultura 2020 (Roma: La sostenibilidad en acción). doi: 10.4060/ca9229es
69
Fayaz M. Namitha K. Murthy K. Swamy M. Sarada R. Khanam S. et al . (2005). Chemical composition, iron bioavailability, and antioxidant activity of Kappaphycus alvarezii (Doty). J. Agric. Food Chem.53, 792–797. doi: 10.1021/jf0493627
70
Febriani D. Nuryati S. (2013). Kappa-carrageenan as immunostimulant to control infectious myonecrosis (IMN) disease in white shrimp Litopenaeus vannamei. J. Akuakultur Indones.12, 70–78. doi: 10.19027/jai.12.70-78
71
Felix N. Brindo R. (2014). Substituting fish meal with fermented seaweed, Kappaphycus alvarezii in diets of juvenile freshwater prawn Macrobrachium rosenbergii. Int. J. Fish. Aquat. Stud.1, 261–265.
72
Freitas M. Pacheco D. Cotas J. Mouga T. Afonso C. Pereira L. (2021). Red seaweed pigments from a biotechnological perspective. Phycology2, 1–29. doi: 10.3390/phycology2010001
73
Fukunaga A. Peyton K. Thomas F. (2014). Epifaunal community structure and ammonium uptake compared for the invasive algae, Gracilaria salicornia and Acanthophora spicifera, and the native alga, Padina thivyi. J. Exp. Mar. Biol. Ecol. .456, 78–86. doi: 10.1016/j.jembe.2014.03.013
74
Ganesan A. Shanmugam M. (2020). Isolation of phycoerythrin from Kappaphycus alvarezii: a potential natural colourant in ice cream. J. Appl. Phycol.32, 4221–4233. doi: 10.1007/s10811-020-02214-0
75
Ganesan A. Shanmugam M. Palaniappan S. Rajauria G. (2018). Development of edible film from Acanthophora spicifera: Structural, rheological and functional properties. Food Biosci.23, 121–128. doi: 10.1016/j.fbio.2017.12.009
76
Ganesan A. Subramani K. Shanmugam M. Seedevi P. Park S. Alfarhan A. et al . (2020). A comparison of nutritional value of underexploited edible seaweeds with recommended dietary allowances. J. King Saud Univ. Sci.32, 1206–1211. doi: 10.1016/j.jksus.2019.11.009
77
Gomaa H. Elshoubaky G. (2016). Antiviral activity of sulfated polysaccharides carrageenan from some marine seaweeds. Int. J. Curr. Pharm. Rev. Res.7, 34–42.
78
Gonçalves A. Ducatti D. Duarte M. Noseda M. (2002). Sulfated and pyruvylated disaccharide alditols obtained from a red seaweed galactan: ESIMS and NMR approaches. Carbohydr. Res.337, 2443–2453. doi: 10.1016/s0008-6215(02)00318-x
79
Gora A. Ambasankar K. Sandeep K. Rehman S. Agarwal D. Ahamad I. et al . (2019). Effect of dietary supplementation of crude microalgal extracts on growth performance, survival and disease resistance of Lates calcarifer (Bloch 1790) larvae. Indian J. Fish.66, 64–72. doi: 10.21077/ijf.2019.66.1.79076-09
80
Górka B. Wieczorek P. (2017). Simultaneous determination of nine phytohormones in seaweed and algae extracts by HPLC-PDA. J. Chromatogr. B Anal. Technol. Biomed. Life Sci.1057, 32–39. doi: 10.1016/j.jchromb.2017.04.048
81
Guiry M. Guiry W. (2022). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. Available at: https://www.algaebase.org.
82
Gupta M. Raghava S. (2008). “Smart systems based on polysaccharides,” in Natural-based polymers for biomedical applications. Eds. NevesN.ManoJ.GomesM.MarquesA.AzevedoH. (London: Elsevier), 129–161.
83
Gurpilhares D. Cinelli L. Simas N. Pessoa A. Sette L. (2019). Marine prebiotics: Polysaccharides and oligosaccharides obtained by using microbial enzymes. Food Chem.280, 175–186. doi: 10.1016/j.foodchem.2018.12.023
84
Häder D. Kumar H. Smith R. Worrest R. (2007). Effects of solar UV radiation on aquatic ecosystems and interactions with climate change. Photochem. Photobiol. Sci.6, 267–285. doi: 10.1039/b700020k
85
Häder D. Williamson C. Wängberg S. Rautio M. Rose K. Gao K. et al . (2015). Effects of UV radiation on aquatic ecosystems and interactions with other environmental factors. Photochem. Photobiol. Sci.14, 108–126. doi: 10.1039/C4PP90035A
86
Hardjani D. Suantika G. Aditiawati P. (2017). Nutritional profile of red seaweed Kappaphycus alvarezii after fermentation using Saccharomyces cerevisiae as a feed supplement for white shrimp Litopenaeus vannamei nutritional profile of fermented red seaweed. J. Pure Appl. Microbiol.11, 1637–1645. doi: 10.22207/JPAM.11.4.01
87
Hargrave M. Nylund G. Enge S. Pavia H. (2022). Co-Cultivation with blue mussels increases yield and biomass quality of kelp. Aquaculture550, 737832. doi: 10.1016/j.aquaculture.2021.737832
88
Harikrishnan R. Devi G. Van Doan H. Balamurugan P. Arockiaraj J. Balasundaram C. (2021). Hepatic antioxidant activity, immunomodulation, and pro-anti-inflammatory cytokines manipulation of κ-carrageenan (κ-CGN) in cobia, Rachycentron canadum against Lactococcus garvieae. Fish Shellfish Immunol.119, 128–144. doi: 10.1016/j.fsi.2021.09.024
89
Hayashi L. Reis R. Santos A. Castelar B. Robledo D. Vega G. et al . (2017). “The cultivation of kappaphycus and eucheuma in tropical and sub-tropical waters,” in Tropical seaweed farming trends, problems and opportunities. Eds. HurtadoA.CritchleyA.NeishI. (Dordrecht: Springer), 55–90. doi: 10.1007/978-3-319-63498-2_4
90
Hayashi L. Yokoya N. Kikuchi D. Oliveira E. (2007). Callus induction and micropropagation improved by colchicine and phytoregulators in Kappaphycus alvarezii (Rhodophyta, solieriaceae). J. Appl. Phycol.20, 653–659. doi: 10.1007/s10811-007-9234-z
91
Hayashi L. Yokoya N. Ostini S. Pereira R. Braga E. Oliveira E. (2008). Nutrients removed by Kappaphycus alvarezii (Rhodophyta, solieriaceae) in integrated cultivation with fishes in re-circulating water. Aquaculture277, 185–191. doi: 10.1016/j.aquaculture.2008.02.024
92
Hentati F. Tounsi L. Djomdi D. Pierre G. Delattre C. Ursu A. et al . (2020). Bioactive polysaccharides from seaweeds. Molecules25, 1–29 . doi: 10.3390/molecules25143152
93
Heriyanto I. Limantara L. Brotosudarmo T. (2015). Composition of photosynthetic pigments in a red alga Kappaphycus alvarezi cultivated in different depths. Proc. Chem.14, 193–201. doi: 10.1016/j.proche.2015.03.028
94
Herrera D. Andrea D. Muñoz M. Hernández R. Hernández G. (2019). Biostimulant activity of individual and blended seaweed extracts on the germination and growth of the mung bean. J. Appl. Phycol.31, 2025–2037. doi: 10.1007/s10811-018-1680-2
95
Hinaloc L. Roleda M. (2021). Phenotypic diversity, growth and sexual differentiation in the progeny of wild Kappaphycus alvarezii (Gigartinales, florideophyceae). Phycologia60, 1–11. doi: 10.1080/00318884.2021.1946307
96
Hirayama M. Shibata H. Imamura K. Sakaguchi T. Hori K. (2016). High-mannose specific lectin and its recombinants from a carrageenophyta Kappaphycus alvarezii represent a potent anti-HIV activity through high-affinity binding to the viral envelope glycoprotein gp120. Mar. Biotechnol.18, 144–160. doi: 10.1007/s10126-015-9677-1
97
Hong D. Hien H. Son P. (2007). Seaweeds from Vietnam used for functional food, medicine and biofertilizer. J. Appl. Phycol.19, 817–826. doi: 10.1007/s10811-007-9228-x
98
Hsieh-Lo M. Castillo G. Ochoa-Becerra M. Mojica L. (2019). Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res.42, 101600. doi: 10.1016/j.algal.2019.101600
99
Hung L. Hirayama M. Ly B. Hori K. (2015). Biological activity, cDNA cloning and primary structure of lectin KSA-2 from the cultivated red alga Kappaphycus striatum (Schmitz) doty ex Silva. Phytochem. Lett.14, 99–105. doi: 10.1016/j.phytol.2015.09.012
100
Hung L. Hoa L. Hau L. Trung D. (2019). The lectin accumulation, growth rate, carrageenan yield, and quality from the red alga Kappaphycus striatus cultivated at camranh bay, Vietnam. J. Appl. Phycol.31, 1991–1998. doi: 10.1007/s10811-018-1692-y
101
Hung L. Hori K. Nang H. Kha T. Hoa L. (2009b). Seasonal changes in growth rate, carrageenan yield and lectin content in the red alga Kappaphycus alvarezii cultivated in camranh bay, Vietnam. J. Appl. Phycol.21, 265–272. doi: 10.1007/s10811-008-9360-2
102
Hung L. Kanj H. Nang Q. (2009c). Screening and preliminary characterization of hemagglutinins in Vietnamese marine algae. J. Appl. Phycol.21, 89. doi: 10.1007/s10811-008-9330-8
103
Hung D. Tomomi S. Hiromi S. Kanji H. (2009a). Biochemical comparison of lectins among three different color strains of the red alga Kappaphycus alvarezii. Fish. Sci.75, 723–730. doi: 10.1007/s12562-009-0088-y
104
Hung L. Trinh P. (2021). Structure and anticancer activity of a new lectin from the cultivated red alga, Kappaphycus striatus. J. Nat. Med.75, 223–231. doi: 10.1007/s11418-020-01455-0
105
Hunt R. Chinnasamy S. Bhatnagar A. Das K. (2010). Effect of biochemical stimulants on biomass productivity and metabolite content of the microalga, Chlorella sorokiniana. Appl. Biochem. Biotechnol.162, 2400–2414. doi: 10.1007/s12010-010-9012-2
106
Hurtado A. (1995). Carrageenan properties and proximate composition of three morphotypes of Kappaphycus alvarezii doty (Gigartinales, rhodophyta) grown at two depths. Bot. Mar.38, 215–220. doi: 10.1515/botm.1995.38.1-6.215
107
Hurtado A. Biter A. (2007). Plantlet regeneration of Kappaphycus alvarezii var. adik-adik by tissue culture. J. Appl. Phycol.19, 783–786. doi: 10.1007/s10811-007-9269-1
108
Hurtado A. Yunque D. Tibubos K. Critchley A. (2008). Use of acadian marine plant extract powder from Ascophyllum nodosum in tissue culture of Kappaphycus varieties. J. Appl. Phycol.21, 633. doi: 10.1007/s10811-008-9395-4
109
Hwang E. Boo G. Graf L. Yarish C. Yoon H. Kim J. (2022). Kelps in Korea: from population structure to aquaculture to potential carbon sequestration. Algae37, 85–103. doi: 10.4490/algae.2022.37.3.3
110
Impellizzeri G. Mangiafico S. Oriente G. Piattelli M. Sciuto S. Fattorusso E. et al . (1975). Amino acids and low-molecular-weight carbohydrates of some marine red algae. Phytochemistry14, 1549–1557. doi: 10.1016/0031-9422(75)85349-0
111
Jiksing C. Ongkudon M. Thien V. Rodrigues K. Yong W. (2022). Recent advances in seaweed seedling production: a review of eucheumatoids and other valuable seaweeds. Algae37, 105–121. doi: 10.4490/algae.2022.37.5.11
112
Jong Y. Hitipeuw C. van Reine W. (1999). A taxonomic, phylogenetic and biogeographic study of the genus Acanthophora (Rhodomelaceae, rhodophyta). Blumea: Biodiversity Evol. Biogeogr. Plants.44, 217–249.
113
Jumah Y. Tumbokon B. Serrano A. (2020). Effects of dietary κ-carrageenan on growth and resistance to acute salinity stress in the black tiger shrimp Penaeus monodon post larvae. Isr. J. Aquac. - Bamidgeh.72, 1–11. doi: 10.46989/IJA.72.2020.1227608
114
Júnior L. Nascimento F. Oliveira S. Lima G. Chagas F. Sombra V. et al . (2021). Protective effect against gastric mucosa injury of a sulfated agaran from Acanthophora spicifera. Carbohydr. Polym.261, 117829. doi: 10.1016/j.carbpol.2021.117829
115
Kailas A. Nair S. (2015). Comparison of nutrient compositions and calorific values of eight tropical seaweeds. Phykos45, 62–74.
116
Kaliaperumal N. Chennubhotla V. Kalimuthu S. Ramalingam J. Selvaraj M. Najmuddin M. (1987). "Chemical composition of seaweeds". Ed. BullC. (India: C.M.F.R. Institute).
117
Kambey C. Sondak C. Chung I. (2020). Potential growth and nutrient removal of Kappaphycus alvarezii in a fish floating-net cage system in sekotong bay, lombok, Indonesia. J. World Aquac. Soc51, 944–959. doi: 10.1111/jwas.12683
118
Kanazawa A . (1989). “Microparticulate feeds for penaeid larvae. Adv. Trop. Aquac.9, 395–404.
119
Karsten U. Sawall T. Wiencke C. (1998). A survey of the distribution of UV-absorbing substances in tropical macroalgae. Phycol. Res.46, 271–279. doi: 10.1046/j.1440-1835.1998.00144.x
120
Kicklighter C. Kamio M. Nguyen L. Germann M. Derby C. (2011). Mycosporine-like amino acids are multifunctional molecules in sea hares and their marine community. Proc. Natl. Acad. Sci. U. S. A.108, 11494–11499. doi: 10.1073/pnas.1103906108
121
Kraan S. (2017). Undaria marching on; late arrival in the republic of Ireland. J. Appl. Phycol.29, 1107–1114. doi: 10.1007/s10811-016-0985-2
122
Kumar K. Ganesan K. Rao P. (2007). Phycoremediation of heavy metals by the three-color forms of Kappaphycus alvarezii. J. Hazard. Mater.143, 590–592. doi: 10.1016/j.jhazmat.2006.09.061
123
Kumar K. Ganesan K. Selvaraj K. Rao P. (2014). Studies on the functional properties of protein concentrate of Kappaphycus alvarezii (Doty) doty–an edible seaweed. Food Chem.153, 353–360. doi: 10.1016/j.foodchem.2013.12.058
124
Kupper F. Kloareg B. Guern J. Potin P. (2001). Oligoguluronates elicit an oxidative burst in the brown algal kelp Laminaria digitata. Plant Physiol.125, 278–291. doi: 10.1104/pp.125.1.278
125
Lafarga T. Acién-Fernández F. Garcia-Vaquero M. (2020). Bioactive peptides and carbohydrates from seaweed for food applications: natural occurrence, isolation, purification, and identification. Algal Res.48, 101909. doi: 10.1016/j.algal.2020.101909
126
Lawanyawut K. Warotaipan S. Kaewkong A. (2002). Nutritional composition and Ca, p and fe quantities of seaweed in Thailand. Fish. Sci.68, 1321–1322. doi: 10.2331/fishsci.68.sup2_1321
127
Layek J. Das A. Ramkrushna G. Trivedi K. Yesuraj D. Chandramohan M. et al . (2015). Seaweed sap: a sustainable way to improve productivity of maize in north-East India. Int. J. Environ. Stud.72, 305–315. doi: 10.1080/00207233.2015.1010855
128
Lechat H. Amat M. Mazoyer J. Buléon A. Lahaye M. (2000). Structure and distribution of glucomannan and sulfated glucan in the cell walls of the red alga Kappaphycus alvarezii (Gigartinales, rhodophyta). J. Phycol.36, 891–902. doi: 10.1046/j.1529-8817.2000.00056.x
129
Lee C. Amos M . (1997) “Current status of topshell trochus niloticus hatcheries in Australia, Indonesia and the pacific–a review,” in Trochus: status, hatchery practice and nutrition (Darwin: Australian Centre for International Agricultural Research), 38–42.
130
Lee P. Huang J. Huang C. Liu Z. Yeh H. Huang H. et al . (2021). Phycoerythrin from colaconema sp. has immunostimulatory effects on the whiteleg shrimp Litopenaeus vannamei and increases resistance to Vibrio parahaemolyticus and white spot syndrome virus. Animals11, 2371. doi: 10.3390/ani11082371
131
Lee K. Ramli N. Said M. Ahmad M. Yasir S. Ariff A. (2011). Competitive metal sorption and desorption onto Kappaphycus alvarezii, seaweed waste biomass. Malays. J. Anal. Sci.15, 252–257.
132
Leu M. Meng P. Huang C. Tew K. Kuo J. Liou C. (2010). Spawning behaviour, early development and first feeding of the bluestriped angelfish [Chaetodontoplus septentrionalis (Temminck & schlegel 1844)] in captivity. Aquac. Res.41, 39–52. doi: 10.1111/j.1365-2109.2009.02454.x
133
Lewis E. Gonzalves E. (1962). The protein, peptide, and free amino-acid contents of some species of marine algae from Bombay. Ann. Bot.26, 301–316. doi: 10.1093/oxfordjournals.aob.a083796
134
Lima Ainouz I. Holanda Sampaio A. Barros Benevides N. Ponte Freitas A. Costa F. Carvalho M. et al . (1992). Agglutination of enzyme treated erythrocytes by Brazilian marine algal extracts. Bot. Mar.35, 475–479. doi: 10.1515/botm.1992.35.6.475
135
Lin D. T. Fong P. (2008). Macroalgal bioindicators (growth, tissue n, δ15N) detect nutrient enrichment from shrimp farm effluent entering opunohu bay, moorea, French Polynesia. Mar. Pollut. Bull.56, 245–249. doi: 10.1016/j.marpolbul.2007.09.031
136
Lombardi J. de Almeida Marques H. Pereira R. Barreto O. de Paula E. (2006). Cage polyculture of the pacific white shrimp Litopenaeus vannamei and the Philippines seaweed Kappaphycus alvarezii. Aquaculture258, 412–415. doi: 10.1016/j.aquaculture.2006.04.022
137
Loureiro R. Reis R. Critchley A. (2010). In vitro cultivation of three Kappaphycus alvarezii (Rhodophyta, areschougiaceae) variants (green, red and brown) exposed to a commercial extract of the brown alga Ascophyllum nodosum (Fucaceae, ochrophyta). J. Appl. Phycol.22, 101–104. doi: 10.1007/s10811-009-9412-2
138
Lourenço S. Barbarino E. De-Paula J. Pereira L. Marquez U. (2002). Amino acid composition, protein content and calculation of nitrogen-to-protein conversion factors for 19 tropical seaweeds. Phycol. Res.50, 233–241. doi: 10.1046/j.1440-1835.2002.00278.x
139
Luhan M. Avañcena S. Mateo J. (2015). Effect of short-term immersion of Kappaphycus alvarezii (Doty) doty in high nitrogen on the growth, nitrogen assimilation, carrageenan quality, and occurrence of “ice-ice” disease. J. Appl. Phycol.27, 917–922. doi: 10.1007/s10811-014-0365-8
140
Magouz F. Bassuini M. Khalafalla M. Abbas R. Sewilam H. Aboelenin S. et al . (2021). Mannan oligosaccharide enhanced the growth rate, digestive enzyme activity, carcass composition, and blood chemistry of thinlip grey mullet (Liza ramada). Animals11, 1–13. doi: 10.3390/ani11123559
141
Mahyati K. Azis A. (2019) Optimization of temperature and time in carrageenan extraction of seaweed (Kappaphycus alvarezii) using ultrasonic wave extraction methods. IOP Conf. Ser. Earth Environ. Sci.370, 1–7. doi: 10.1088/1755-1315/370/1/012076
142
Maliki I. Misson M. Teoh P. Rodrigues K. Yong W. (2022). Production of lectins from marine algae: current status, challenges, and opportunities for non-destructive extraction. Mar. Drugs20, 102. doi: 10.3390/md20020102
143
Mangaiyarkarasi R. Kannan L. Girija M. Gnanamurthy S. (2014). Screening of Indian marine macro algae (seaweeds) for haemagglutinin activity. Int. J. Adv. Res.2, 43–52.
144
Mantri V. Dineshkumar R. Yadav A. Eswaran K. Shanmugam M. Gajaria T. (2022). Projections for profitability assessment parameters under short-term, medium-term and long-term evaluation for three farming techniques of Kappaphycus alvarezii along eastern coast of India. Aquaculture.551, 737912. doi: 10.1016/j.aquaculture.2022.737912
145
Mariot L. Bolívar N. Coelho J. Goncalves P. Colombo S. do Nascimento F. et al . (2021). Diets supplemented with carrageenan increase the resistance of the pacific white shrimp to WSSV without changing its growth performance parameters. Aquaculture.545, 737172. doi: 10.1016/j.aquaculture.2021.737172
146
Marolia V. Joshi S. Mathur H. (1982). Fatty acid composition of neutral lipids from some red algae. Indian J. Mar. Sci.11, 102–103.
147
Martins A. Zambotti L. Yokoya N. Colepicolo P. (2018). Biotechnological potential of benthic marine algae collected along the Brazilian coast. Algal Res.33, 316–327. doi: 10.1016/j.algal.2018.05.008
148
Marudhupandi T. Inbakandan D. (2015). Polysaccharides in aquatic disease management. Fish. Aquac. J.6, 1–3. doi: 10.4172/2150-3508.1000135
149
Mendoza W. Montaño N. Ganzon-Fortes E. Villanueva R. (2002). Chemical and gelling profile of ice-ice infected carrageenan from Kappaphycus striatum (Schmitz) doty “sacol” strain (Solieriaceae, gigartinales, rhodophyta). J. Appl. Phycol.14, 409–418. doi: 10.1023/A:1022178119120
150
Mikami K. Mori I. Matsuura T. Ikeda Y. Kojima M. Sakakibara H. et al . (2016). Comprehensive quantification and genome survey reveal the presence of novel phytohormone action modes in red seaweeds. J. Appl. Phycol.28, 2539–2548. doi: 10.1007/s10811-015-0759-2
151
Mishra A. Behura A. Mawatwal S. Kumar A. Naik L. Mohanty S. et al . (2019). Structure-function and application of plant lectins in disease biology and immunity. Food Chem. Toxicol.134, 110827. doi: 10.1016/j.fct.2019.110827
152
Mohan K. Ravichandran S. Muralisankar T. Uthayakumar V. Chandirasekar R. Seedevi P. et al . (2019). Application of marine-derived polysaccharides as immunostimulants in aquaculture: A review of current knowledge and further perspectives. Fish Shellfish Immunol.86, 1177–1193. doi: 10.1016/j.fsi.2018.12.072
153
Mohanty D. Adhikary S. (2018). Standardization of protocol for preparation of liquid extracts from seaweeds, quantification of their phytohormones and application for crop improvement. Indian J. Geo-Marine Sci.47, 1364–1372.
154
Mondal D. Ghosh A. Prasad K. Singh S. Bhatt N. Zodape S. et al . (2015). Elimination of gibberellin from Kappaphycus alvarezii seaweed sap foliar spray enhances corn stover production without compromising the grain yield advantage. Plant Growth Regul.75, 657–666. doi: 10.1007/s10725-014-9967-z
155
Moreira A. da Costa E. Melo T. Sulpice R. Cardoso S. Pitarma B. et al . (2020). Seasonal plasticity of the polar lipidome of Ulva rigida cultivated in a sustainable integrated multi-trophic aquaculture. Algal Res.49, 101958. doi: 10.1016/j.algal.2020.101958
156
Mori I. Ikeda Y. Matsuura T. Hirayama T. Mikami K. (2017). Phytohormones in red seaweeds: a technical review of methods for analysis and a consideration of genomic data. Bot. Mar.60, 153–170. doi: 10.1515/bot-2016-0056
157
Msuya F. (2020). Seaweed resources of Tanzania: status, potential species, challenges and development potentials. Bot. Mar.63, 371–380. doi: 10.1515/bot-2019-0056
158
Muchtar M. Sukenda S. Nuryati S. Hidayatullah D. (2019). The use of immunostimulant from phycocyanin of Spirulina platensis to control motile aeromonad septicaemia (MAS) disease in common carp Cyprinus carpio. J. Akuakultur Indones.18, 101–109. doi: 10.19027/jai.18.1.101-109
159
Muñoz J. Cahue-López A. Patiño R. Robledo D. (2006). Use of plant growth regulators in micropropagation of Kappaphycus alvarezii (Doty) in airlift bioreactors. J. Appl. Phycol.18, 209–218. doi: 10.1007/s10811-006-9105-z
160
Muñoz J. Freile-Pelegrín Y. Robledo D. (2004). Mariculture of Kappaphycus alvarezii (Rhodophyta, solieriaceae) color strains in tropical waters of yucatán, méxico. Aquaculture.239, 161–177. doi: 10.1016/j.aquaculture.2004.05.043
161
Mustafa A. (2017). Comparison growth of Kappaphycus alvarezii (Rhodophyta, solieriaceae) cultivation in floating cage and longline in Indonesia. Aquac. Rep.6, 49–55. doi: 10.1016/j.aqrep.2017.03.004
162
Muthiah R. Kannu K. Muthuraj V. Sornalatha N. (2017). MAA-palythine compound of three red (macro) algae: Gelidiella acerosa, Acanthophora spicifera and Hypnea musciformis of gulf of mannar, southeast coast of India. Int. J. Curr. Microbiol. App. Sci.6, 607–615. doi: 10.20546/ijcmas.2017.607.074
163
Muthukrishnan S. Raja P. (2021). Immunostimulant effect of seaweeds in Channa punctatus challenged by Aeromonas hydrophila. J. Drug Deliv. Ther.11, 20–23. doi: 10.22270/jddt.v11i4.4948
164
Muttharasi C. Gayathri V. Muralisankar T. Mohan K. Uthayakumar V. Radhakrishnan S. et al . (2021). Growth performance, digestive enzymes and antioxidants activities in the shrimp Litopenaeus vannamei fed with Amphiroa fragilissima crude polysaccharides encapsulated Artemia nauplii. Aquaculture545, 737263. doi: 10.1016/j.aquaculture.2021.737263
165
Nagarajan D. Varjani S. Lee D. Chang J. (2021). Sustainable aquaculture and animal feed from microalgae–nutritive value and techno-functional components. Renew. Sustain. Energy Rev.150, 111549. doi: 10.1016/j.rser.2021.111549
166
Nagarani N. JanakiDevi V. YokeshBabu M. Kumaraguru A. (2012). Protective effect of Kappaphycus alvarezii (Rhodophyta) extract against DNA damage induced by mercury chloride in marine fish. Toxicol. Environ. Chem.94, 1401–1410. doi: 10.1080/02772248.2012.707792
167
Naguit M. Tisera W. (2009). Pigment analysis on Eucheuma denticulatum (Collins & hervey) and Kappaphycus alvarezii (Doty) cultivars cultured at different depths. Threshold4, 29–37.
168
Naylor R. Hardy R. Buschmann A. Bush S. Cao L. Klinger D. et al . (2021). A 20-year retrospective review of global aquaculture. Nature591, 551–563. doi: 10.1038/s41586-021-03308-6
169
Neves F. Simioni C. Bouzon Z. Hayashi L. (2015). Effects of spindle inhibitors and phytoregulators on the micropropagation of Kappaphycus alvarezii (Rhodophyta, gigartinales). J. Appl. Phycol.27, 437–445. doi: 10.1007/s10811-014-0309-3
170
Ohno M. Largo D. Ikumoto T. (1994). Growth rate, carrageenan yield and gel properties of culturedkappa-carrageenan producing red alga Kappaphycus alvarezii (Doty) doty in the subtropical waters of Shikoku, Japan. J. Appl. Phycol.6, 1–5. doi: 10.1007/BF02185896
171
Omont A. Elizondo-González R. Escobedo-Fregoso C. Tovar-Ramírez D. Hinojosa-Baltazar P. Peña-Rodríguez A. (2021). Bacterial communities and digestive enzymatic activities of Litopenaeus vannamei shrimp fed pre-digested seaweeds as a functional ingredient. J. Appl. Phycol.33, 1239–1251. doi: 10.1007/s10811-021-02381-8
172
Oren A. Gunde-Cimerman N. (2007). Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett.269, 1–10. doi: 10.1111/j.1574-6968.2007.00650.x
173
Pacheco D. Araújo G. Cotas J. Gaspar R. Neto J. Pereira L. (2020). Invasive seaweeds in the Iberian peninsula: A contribution for food supply. Mar. Drugs18, 560. doi: 10.3390/md18110560
174
Paolucci M. Fasulo G. Volpe M. (2015). Employment of marine polysaccharides to manufacture functional biocomposites for aquaculture feeding applications. Mar. Drugs13, 2680–2693. doi: 10.3390/md13052680
175
Paransa D. Mantiri D. Kepel R. Rumengan A. (2020). Pigment concentration of red algae, Kappaphycus alvarezii (Doty) doty ex Silva during the cultivation in the coastal waters of nain island, north sulawesi, Indonesia. AACL Bioflux.13, 2788–2797.
176
Parekh R. Doshi Y. Chauhan V. (1989) Polysaccharides from marine red algae Acanthophora spicifera, Grateloupia indica and Halymenia porphyroides. Indian J. Mar. Sci.18, 139–140.
177
Pedra A. Ramlov F. Maraschin M. Hayashi L. (2017). Cultivation of the red seaweed Kappaphycus alvarezii with effluents from shrimp cultivation and brown seaweed extract: Effects on growth and secondary metabolism. Aquaculture479, 297–303. doi: 10.1016/j.aquaculture.2017.06.005
178
Peñaflorida V. Golez N. (1996). Use of seaweed meals from Kappaphycus alvarezii and Gracilaria heteroclada as binders in diets for juvenile shrimp Penaeus monodon. Aquaculture143, 393–401. doi: 10.1016/0044-8486(96)01282-3
179
Pereira da Costa D. Campos K. (2020). The use of carotenoid pigments as food additives for aquatic organisms and their functional roles. Rev. Aquac.12, 1567–1578. doi: 10.1111/raq.12398
180
Pereira V. de Alencar D. de Araújo I. Rodrigues J. Lopes J. Nunes L. et al . (2020). Supplementation of cryodiluent media with seaweed or Nile tilapia skin sulfated polysaccharides for freezing of Colossoma macropomum (Characiformes: Serrasalmidae) semen. Aquaculture528, 735553. doi: 10.1016/j.aquaculture.2020.735553
181
Pereira A. Fraga-Corral M. Garcia-Oliveira P. Lourenço-Lopes C. Carpena M. Prieto M. et al . (2021). The use of invasive algae species as a source of secondary metabolites and biological activities: Spain as case-study. Mar. Drugs19, 178. doi: 10.3390/md19040178
182
Pereira D. Pereira B. Fonseca A. Ramlov F. Maraschin M. Álvarez-Gómez F. et al . (2019). Effects of ultraviolet radiation (UV-a+ UV-b) on the antioxidant metabolism of the red macroalga species Acanthophora spicifera (Rhodophyta, ceramiales) from different salinity and nutrient conditions. Photochem. Photobiol.95, 999–1009. doi: 10.1111/php.13094
183
Pereira D. Simioni C. Filipin E. Bouvie F. Ramlov F. Maraschin M. et al . (2017). Effects of salinity on the physiology of the red macroalga, Acanthophora spicifera (Rhodophyta, ceramiales). Acta Bot. Brasilica31, 555–565. doi: 10.1590/0102-33062017abb0059
184
Periyasamy C. Subba Rao P. (2017). Growth rate and carrageenan yield of cultivated Kappaphycus alvarezii (Doty) in the coastal waters of bay of Bengal at chepala timmapuram, andhra pradesh, east coast of India. J. Appl. Phycol.29, 1977–1987. doi: 10.1007/s10811-017-1099-1
185
Periyasamy C. Subba Rao P. Anantharaman P. (2019). Spatial and temporal variation of biochemical contents in relation to carrageenan yield of cultivated Kappaphycus alvarezii (Doty) doty at different locations of palk bay waters, Tamil nadu, southeast coast of India. J. Appl. Phycol.31, 1355–1368. doi: 10.1007/s10811-018-1645-5
186
Petrocelli A. Felicini G. (1995). Photosynthetic pigments of Acanthophora najadiformis (Ceramiales, rhodophyta). G. Bot. Ital.129, 1281–1284.
187
Pires C. Bazzo G. Barreto P. do Espírito Santo C. Ventura F. Pedra A. et al . (2021). Cultivation of the red seaweed Kappaphycus alvarezii using biofloc effluent. J. Appl. Phycol.33, 1047–1058. doi: 10.1007/s10811-020-02335-6
188
Polat S. Ozogul Y. (2008). Biochemical composition of some red and brown macro algae from the northeastern Mediterranean Sea. Int. J. Food Sci. Nutr.59, 566–572. doi: 10.1080/09637480701446524
189
Prasad K. Das A. Oza M. Brahmbhatt H. Siddhanta A. Meena R. et al . (2010). Detection and quantification of some plant growth regulators in a seaweed-based foliar spray employing a mass spectrometric technique sans chromatographic separation. J. Agric. Food Chem.58, 4594–4601. doi: 10.1021/jf904500e
190
Putro S. Widowati W. Suhartana S. Muhammad F. (2015). The application of integrated multi trophic aquaculture (IMTA) using stratified double net rounded cage (SDFNC) for aquaculture sustainability. Int. J. Sci. Eng.9, 85–89. doi: 10.12777/ijse.9.2.85-89
191
Qian P. Wu C. Wu M. Xie Y. (1996). Integrated cultivation of the red alga Kappaphycus alvarezii and the pearl oyster Pinctada martensi. Aquaculture147, 21–35. doi: 10.1016/S0044-8486(96)01393-2
192
Raguraman V. Ravindran N. Selvaraju K. Kasivelu G. (2020). Seaweed polysaccharides as potential therapeutic agents against white spot syndrome virus (WSSV): A mini review. Aquac. Int.28, 2333–2343. doi: 10.1007/s10499-020-00587-0
193
Rahman M. Sathasivam K. (2015). Heavy metal adsorption onto Kappaphycus sp. from aqueous solutions: the use of error functions for validation of isotherm and kinetics models. BioMed. Res. Int.2015, 1–13. doi: 10.1155/2015/126298
194
Rajaram R. Muralisankar T. Paray B. Al-Sadoon M. (2021). Phytochemical profiling and antioxidant capacity of Kappaphycus alvarezii (Doty) doty collected from seaweed farming sites of tropical coastal environment. Aquac. Res.52, 3438–3448. doi: 10.1111/are.15188
195
Rama R. Iba W. Nurdin A. Armin A. Yusnaeni Y. (2018). Seaweed cultivation of micropropagated seaweed (Kappaphycus alvarezii) in bungin permai coastal waters, tinanggea sub-district, south konawe regency, South East Sulawesi. IOP Conf. Ser. Earth Environ. Sci.1–9. doi: 10.1088/1755-1315/175/1/012219
196
Rao M. (1970). Economic seaweeds of India (Ramanathapuram (India): Central Marine Fisheries Research Institute).
197
Rathod M. Mody K. Basha S. (2014). Efficient removal of phosphate from aqueous solutions by red seaweed, Kappaphycus alverezii. J. Clean. Prod.84, 484–493. doi: 10.1016/j.jclepro.2014.03.064
198
Reis R. das Chagas Pereira R. de Góes H. (2015). The efficiency of tubular netting method of cultivation for Kappaphycus alvarezii (Rhodophyta, gigartinales) on the southeastern Brazilian coast. J. Appl. Phycol.27, 421–426. doi: 10.1007/s10811-014-0330-6
199
Rizzo C. Genovese G. Morabito M. Faggio C. Pagano M. Spanò A. et al . (2017). Potential antibacterial activity of marine macroalgae against pathogens relevant for aquaculture and human health. J. Pure Appl. Microbiol.11, 1695–1706. doi: 10.22207/JPAM.11.4.07
200
Rodrigues J. de Queiroz I. Quinderé A. Benevides N. Tovar A. de Souza Mourão P. (2016a). Extraction and structural properties of Acanthophora muscoides (Rhodophyceae) extracellular matrix sulfated polysaccharides and their effects on coagulation. Acta Sci. Technol.38, 273–282. doi: 10.4025/actascitechnol.v38i3.26146
201
Rodrigues J. de Queiroz I. Quinderé A. Benevides N. Tovar A. de Souza Mourão P. (2016b). Mild-acid hydrolysis of a native polysulfated fraction from Acanthophora muscoides generates sulfated oligosaccharides displaying in vitro thrombin generation inhibition. Acta Sci. Biol. Sci.38, 7–15. doi: 10.4025/actascibiolsci.v38i1.28257
202
Rodriguez M. Montaño M. (2007). Bioremediation potential of three carrageenophytes cultivated in tanks with seawater from fish farms. J. Appl. Phycol.19, 755–762. doi: 10.1007/s10811-007-9217-0
203
Rönnbäck P. Bryceson I. Kautsky N. (2002). Coastal aquaculture development in Eastern Africa and the Western Indian ocean: Prospects and problems for food security and local economies. Ambio31, 537–542. doi: 10.1579/0044-7447-31.7.537
204
Rosic N. Braun C. Kvaskoff D. (2015). “Extraction and analysis of mycosporine-like amino acids in marine algae,” in Natural products from marine algae. methods in molecular biology. Eds. StengelD.ConnanS. (New York: Springer), 119–129. doi: 10.1007/978-1-4939-2684-8_6
205
Rudke A. de Andrade C. Ferreira S. (2020). Kappaphycus alvarezii macroalgae: An unexplored and valuable biomass for green biorefinery conversion. Trends Food Sci. Technol.103, 214–224. doi: 10.1016/j.tifs.2020.07.018
206
Russell D. (1992). The ecological invasion of Hawaiian reefs by two marine red algae, Acanthophora spicifera (Vahl) Boerg. and Hypnea musciformis (Wulfen) J. Ag., and their association with two native species, Laurencia nidifica J. Ag. and Hypnea cervicornis. J. Ag. ICES J. Mar Sci. Symp.194, 110–125.
207
Russell D. Balazs G. (1994). Colonization by the alien marine alga Hypnea musciformis (Wulfen) j. Ag.(Rhodophyta: Gigartinales) in the Hawaiian islands and its utilization by the green turtle, Chelonia mydas l. Aquat. Bot.47, 53–60. doi: 10.1016/0304-3770(94)90048-5
208
Sakthivel M. Deivasigamani B. Rajasekar T. Kumaran S. Alagappan K. (2015). Immunostimulatory effects of polysaccharide compound from seaweed Kappaphycus alvarezii on Asian seabass (Lates calcarifer) and it’s resistance against Vibrio parahaemolyticus. J. Mar. Biol. Oceanogr.4, 2–10. doi: 10.4172/2324-8661.1000144
209
Santos G. (1989). Carrageenans of species of Eucheuma j. agardh and Kappaphycus doty (Solieriaceae, rhodophyta). Aquat. Bot.36, 55–67. doi: 10.1016/0304-3770(89)90091-0
210
Sardari R. Nordberg E. (2018). Marine poly-and oligosaccharides as prebiotics. J. Agric. Food Chem.66, 11544–11549. doi: 10.1021/acs.jafc.8b04418
211
Sati H. Chokshi K. Soundarya R. Ghosh A. Mishra S. (2021). Seaweed-based biostimulant improves photosynthesis and effectively enhances growth and biofuel potential of a green microalga Chlorella variabilis. Aquac. Int.29, 963–975. doi: 10.1007/s10499-021-00667-9
212
Sato Y. Hori K. (2011). High-mannose n-glycan-specific lectin from the red alga Kappaphycus striatum (Carrageenophyte). Phytochemistry72, 855–861. doi: 10.1016/j.phytochem.2011.03.009
213
Schleder D. Blank M. Peruch L. Poli M. Goncalves P. Rosa K. et al . (2020). Impact of combinations of brown seaweeds on shrimp gut microbiota and response to thermal shock and white spot disease. Aquaculture519, 734779. doi: 10.1016/j.aquaculture.2019.734779
214
Schmidt É. Maraschin M. Bouzon Z. (2010). Effects of UVB radiation on the carragenophyte Kappaphycus alvarezii (Rhodophyta, gigartinales): changes in ultrastructure, growth, and photosynthetic pigments. Hydrobiologia649, 171–182. doi: 10.1007/s10750-010-0243-6
215
Schnoller V. Hernández G. Hernández E. López J. Muñoz-Ochoa M. (2020). Chemical characterization of soluble polysaccharides in the red alga Acanthophora spicifera from la paz bay, Baja California sur, Mexico. Cienc. Mar.46, 165–176. doi: 10.7773/cm.v46i3.3090
216
Sedayu B. Basmal J. (2013). Identification of growth promoting hormones in the sap of Eucheuma cottonii. J. Pascapanen dan Bioteknol. Kelaut. dan Perikan.8, 1–8. doi: 10.15578/jpbkp.v8i1.48
217
Seenivasan R. Rekha M. Indu H. Geetha S. (2012). Antibacterial activity and phytochemical analysis of selected seaweeds from mandapam coast, India. J. Appl. Pharm. Sci.2, 159–169. doi: 10.7324/JAPS.2012.21030
218
Senthilkumar N. Suresh V. Thangam R. Kurinjimalar C. Kavitha G. Murugan P. et al . (2013). Isolation and characterization of macromolecular protein r-phycoerythrin from Portieria hornemannii. Int. J. Biol. Macromol.55, 150–160. doi: 10.1016/j.ijbiomac.2012.12.039
219
Serive B. Bach S. (2018). Marine pigment diversity: Applications and potential. Blue Biotechnol.2, 643–681. doi: 10.1002/9783527801718.ch20
220
Shanab S. Shalaby E. (2021). “Production of plant hormones from algae and its relation to plant growth,” in Plant growth-promoting microbes for sustainable biotic and abiotic stress management. Eds. MohamedH.El-BeltagiH.Abd-ElsalamK. (Switzerland: Springer), 395–423. doi: 10.1007/978-3-030-66587-6_14
221
Shapawi R. Safiin N. Senoo S. (2015). Improving dietary red seaweed Kappaphycus alvarezii (Doty) doty ex. p. Silva meal utilization in Asian seabass Lates calcarifer. J. Appl. Phycol.27, 1681–1688. doi: 10.1007/s10811-014-0454-8
222
Shick J. Dunlap W. Chalker B. Banaszak A. Rosenzweig T. (1992). Survey of ultraviolet radiation-absorbing mycosporine-like amino acids in organs of coral reef holothuroids. Mar. Ecol. Prog. Ser.90, 139–148. doi: 10.3354/meps090139
223
Singh R. Walia A. (2018). Lectins from red algae and their biomedical potential. J. Appl. Phycol.30, 1833–1858. doi: 10.1007/s10811-017-1338-5
224
Sivakumar K. Kannappan S. Dineshkumar M. Patil P. (2014). Antagonism of marine macro alga Kappaphycus alvarezii extract against luminescence disease causing Vibrio harveyi during Penaeus monodon larviculture. Afr. J. Microbiol. Res.8, 458–469. doi: 10.5897/ajmr2013.6232
225
Stabili L. Cecere E. Licciano M. Petrocelli A. Sicuro B. Giangrande A. (2019). Integrated multitrophic aquaculture by-products with added value: The polychaete Sabella spallanzanii and the seaweed Chaetomorpha linum as potential dietary ingredients. Mar. Drugs17, 677. doi: 10.3390/md17120677
226
Stévant P. Rebours C. Chapman A. (2017). Seaweed aquaculture in Norway: recent industrial developments and future perspectives. Aquac. Int.25, 1373–1390. doi: 10.1007/s10499-017-0120-7
227
Suantika G. Situmorang M. Aditiawati P. Khakim A. Suryanaray S. Nori S. et al . (2017). Effect of red seaweed Kappaphycus alvarezii on growth, salinity stress tolerance and vibriosis resistance in shrimp Litopenaeus vannamei hatchery. J. Fish. Aquat. Sci.12, 127–133. doi: 10.3923/jfas.2017.127.133
228
Suantika G. Situmorang M. Khakim A. Wibowo I. Aditiawati P. Suryanarayan S. et al . (2018). Effect of red seaweed Kappaphycus alvarezii on growth, survival, and disease resistance of pacific white shrimp Litopenaeus vannamei against Vibrio harveyi in the nursery phase. J. Aquac. Res. Dev.9, 1–7. doi: 10.4172/2155-9546.1000523
229
Sun X. Ren L. Zhao Q. Ji X. Huang H. (2018). Microalgae for the production of lipid and carotenoids: a review with focus on stress regulation and adaptation. Biotechnol. Biofuels.11, 1–16. doi: 10.1186/s13068-018-1275-9
230
Sun Y. Zhang N. Zhou J. Dong S. Zhang X. Guo L. et al . (2020). Distribution, contents, and types of mycosporine-like amino acids (MAAs) in marine macroalgae and a database for MAAs based on these characteristics. Mar. Drugs18, 43. doi: 10.3390/md18010043
231
Suresh Kumar K. Ganesan K. Subba Rao P. (2015). Seasonal variation in nutritional composition of Kappaphycus alvarezii (Doty) doty–an edible seaweed. J. Food Sci. Technol.52, 2751–2760. doi: 10.1007/s13197-014-1372-0
232
Tanaka Y. Ashaari A. Mohamad F. Lamit N. (2020). Bioremediation potential of tropical seaweeds in aquaculture: Low-salinity tolerance, phosphorus content, and production of UV-absorbing compounds. Aquaculture518, 734853. doi: 10.1016/j.aquaculture.2019.734853
233
Tarakhovskaya E. Maslov Y. Shishova M. (2007). Phytohormones in algae. Russ. J. Plant Physiol.54, 163–170. doi: 10.1134/S1021443707020021
234
Tibubos K. Hurtado A. Critchley A. (2017). Direct formation of axes in new plantlets of Kappaphycus alvarezii (Doty) doty, as influenced by the use of AMPEP k+, spindle inhibitors, and plant growth hormones. J. Appl. Phycol.29, 2345–2349. doi: 10.1007/s10811-016-0988-z
235
Touliabah H. E.-S. Almutairi A. W. (2021). Effect of phytohormones supplementation under nitrogen depletion on biomass and lipid production of Nannochloropsis oceanica for integrated application in nutrition and biodiesel. Sustain13, 592. doi: 10.3390/su13020592
236
Traifalgar R. Corre V. Serrano A. (2013). Efficacy of dietary immunostimulants to enhance the immunological responses and vibriosis resistance of juvenile Penaeus monodon. J. Fish. Aquat. Sci.8, 340–354. doi: 10.3923/jfas.2013.340.354
237
Trivedi K. Anand K. Kubavat D. Ghosh A. (2022). Role of Kappaphycus alvarezii seaweed extract and its active constituents, glycine betaine, choline chloride, and zeatin in the alleviation of drought stress at critical growth stages of maize crop. J. Appl. Phycol.34, 1791–1804. doi: 10.1007/s10811-022-02722-1
238
Uju S. Dewi N. Santoso J. Setyaningsih I. Hardingtyas S . (2020). Extraction of phycoerythrin from Kappaphycus alvarezii seaweed using ultrasonication". IOP Conf. Ser. Earth Environ. Sci. 414, 012028 (IOP Publishing).
239
Usov A. (Cambridge, Massachusetts: 2011). “Polysaccharides of the red algae,” in Advances in carbohydrate chemistry and biochemistry. Ed. HortonD. (Cambridge, Massachusetts: Elsevier), 115–217. doi: 10.1016/B978-0-12-385520-6.00004-2
240
Vega J. Schneider G. Moreira B. Herrera C. Bonomi J. Figueroa F. (2021). Mycosporine-like amino acids from red macroalgae: UV-photoprotectors with potential cosmeceutical applications. Appl. Sci.11, 1–23. doi: 10.3390/app11115112
241
Vidhya Hindu S. Chandrasekaran N. Mukherjee A. Thomas J. (2019). A review on the impact of seaweed polysaccharide on the growth of probiotic bacteria and its application in aquaculture. Aquac. Int.27, 227–238. doi: 10.1007/s10499-018-0318-3
242
Villamil L. Villamil S. Rozo G. Rojas J. (2019). Effect of dietary administration of kappa carrageenan extracted from hypnea musciformis on innate immune response, growth, and survival of Nile tilapia (Oreochromis niloticus). Aquac. Int.27, 53–62. doi: 10.1007/s10499-018-0306-7
243
Vinoj Kumar V. Kaladharan P. (2007). Amino acids in the seaweeds as an alternate source of protein for animal feed. J. Mar. Biol. Assoc. India.49, 35–40.
244
Wahidulla S. D'Souza L. Kamat S. (1991). Dipeptides from the red alga Acantophora spicifera. Phytochemistry30, 3323–3325. doi: 10.1016/0031-9422(91)83202-V
245
Wang X. Zhao P. Liu X. Chen J. Xu J. Chen H. et al . (2014). Quantitative profiling method for phytohormones and betaines in algae by liquid chromatography electrospray ionization tandem mass spectrometry. Biomed. Chromatogr.28, 275–280. doi: 10.1002/bmc.3018
246
Weinberger F. Friedlander M. (2000). Response of Gracilaria conferta (Rhodophyta) to oligoagars results in defense against agar-degrading epiphytes. J. Phycol.36, 1079–1086. doi: 10.1046/j.1529-8817.2000.00003.x
247
Xie S. Fang W. Wei D. Liu Y. Yin P. Niu J. et al . (2018). Dietary supplementation of Haematococcus pluvialis improved the immune capacity and low salinity tolerance ability of post-larval white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol.80, 452–457. doi: 10.1016/j.fsi.2018.06.039
248
Xiren G. Aminah A. (2017). Proximate composition and total amino acid composition of Kappaphycus alvarezii found in the waters of langkawi and sabah, Malaysia. Int. Food Res. J.241255–1260.
249
Yokoya N. Stirk W. Van Staden J. Novák O. Turečková V. Pěnčí k A. et al . (2010). Endogenous cytokinins, auxins, and abscisic acid in red algae from brazil. J. Phycol.46, 1198–1205. doi: 10.1111/j.1529-8817.2010.00898.x
250
Yong W. Ting S. Yong Y. Thien V. Wong S. Chin W. et al . (2014). Optimization of culture conditions for the direct regeneration of Kappaphycus alvarezii (Rhodophyta, solieriaceae). J. Appl. Phycol.26, 1597–1606. doi: 10.1007/s10811-013-0191-4
251
Yunque D. Tibubos K. Hurtado A. Critchley A. (2011). Optimization of culture conditions for tissue culture production of young plantlets of carrageenophyte Kappaphycus. J. Appl. Phycol.23, 433–438. doi: 10.1007/s10811-010-9594-7
252
Yusoff F. Banerjee S. Nagao N. Imaizumi Y. Shariff M. Toda T. (2020). “Use of microalgae pigments in aquaculture,” in Pigments from microalgae handbook. Eds. Jacob-LopesE.QueirozL.QueirozM. (Zug: Springer), 471–513. doi: 10.1007/978-3-030-50971-2_19
253
Yu Y. Yang M. Yang J. Su Q. Mou H. (2017). Composition and characteristics of continuous enzymatic hydrolysis products from Kappaphycus striatum. J. Appl. Phycol.29, 1647–1656. doi: 10.1007/s10811-017-1064-z
254
Zakaria N. Ibrahim D. Sulaiman S. Supardy A. (2011). Assessment of antioxidant activity, total phenolic content and in-vitro toxicity of Malaysian red seaweed, Acanthophora spicifera. J. Chem. Pharm. Res.3, 182–191.
255
Zammuto V. Rizzo M. Spanò A. Genovese G. Morabito M. Spagnuolo D. et al . (2022). In vitro evaluation of antibiofilm activity of crude extracts from macroalgae against pathogens relevant in aquaculture. Aquaculture549, 737729. doi: 10.1016/j.aquaculture.2021.737729
256
Zamzow J. (2004). Effects of diet, ultraviolet exposure, and gender on the ultraviolet absorbance of fish mucus and ocular structures. Mar. Biol.144, 1057–1064. doi: 10.1007/s00227-003-1286-2
257
Zhang S. Li J. Wu X. Zhong W. Xian J. Liao S. et al . (2013). Effects of different dietary lipid level on the growth, survival and immune-relating genes expression in pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol.34, 1131–1138. doi: 10.1016/j.fsi.2013.01.016
258
Zhao P. Wang Y. Lin Z. Zhou J. Chai H. He Q. et al . (2019). The alleviative effect of exogenous phytohormones on the growth, physiology and gene expression of Tetraselmis cordiformis under high ammonia-nitrogen stress. Bioresour. Technol.282, 339–347. doi: 10.1016/j.biortech.2019.03.031
259
Zheng L. Chen X. Cheong K. (2020). Current trends in marine algae polysaccharides: The digestive tract, microbial catabolism, and prebiotic potential. Int. J. Biol. Macromol.151, 344–354. doi: 10.1016/j.ijbiomac.2020.02.168
260
Zhu B. Ni F. Xiong Q. Yao Z. (2021). Marine oligosaccharides originated from seaweeds: Source, preparation, structure, physiological activity and applications. Crit. Rev. Food Sci. Nutr.61, 60–74. doi: 10.1080/10408398.2020.1716207
261
Zodape S. Mukherjee S. Reddy M. Chaudhary D. (2009). Effect of Kappaphycus alvarezii (Doty) doty ex silva. extract on grain quality, yield and some yield components of wheat (Triticum aestivum l.). Int. J. Plant Prod.3, 97–101. doi: 10.22069/IJPP.2012.646
262
Zuccarello G. Critchley A. Smith J. Sieber V. Lhonneur G. West J. (2006). Systematics and genetic variation in commercial shape Kappaphycus and shape Eucheuma (Solieriaceae, rhodophyta). J. Appl. Phycol.18, 643–651. doi: 10.1007/s10811-006-9066-2
Summary
Keywords
Acanthophora , Kappaphycus , aquaculture, tropical seaweed, bioproduct, metabolites, blue economy, chemistry
Citation
Guillén PO, Motti P, Mangelinckx S, De Clerck O, Bossier P and Van Den Hende S (2022) Valorization of the chemical diversity of the tropical red seaweeds Acanthophora and Kappaphycus and their applications in aquaculture: A review. Front. Mar. Sci. 9:957290. doi: 10.3389/fmars.2022.957290
Received
30 May 2022
Accepted
05 October 2022
Published
27 October 2022
Volume
9 - 2022
Edited by
Erik-Jan Malta, CTAQUA Aquaculture Tecnological Centre, Spain
Reviewed by
Tonmoy Ghosh, Indian Institute of Technology Indore, India; Moslem Sharifinia, Iranian Fisheries Science Research Institute, Iran
Updates
Copyright
© 2022 Guillén, Motti, Mangelinckx, De Clerck, Bossier and Van Den Hende.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sofie Van Den Hende, sofie_vdhende@yahoo.com; Paúl O. Guillén, pguille@espol.edu.ec
This article was submitted to Marine Biotechnology and Bioproducts, a section of the journal Frontiers in Marine Science
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.