Abstract
Introduction:
Intertidal habitat preferences and spatiotemporal variation in the abundance of juvenile Chinese mitten crab Eriocheir sinensis in Yangtze Estuary are reported.
Methods:
The size and abundance of this crab are reported for mud flat, gravel, root belt, and marsh habitats in this estuary’s lower, middle, and upper reaches from June 2021 (spring) to February 2022 (winter) using quadrat method.
Results:
Juvenile E. sinensis of carapace length (CL) 5.5 ± 2.1 mm (mean ± standard deviation) were collected; no juveniles were found in February 2022. Crab abundance in root belt and gravel habitats usually exceeded that of marsh habitat; no juveniles were found in mud flat habitat. The greatest abundances and smallest individuals were found when megalopa recruited in early spring (June); juvenile abundance decreased sharply afterwards, and crabs were absent from the intertidal during winter. Size and relative growth rate of juvenile crabs were greater in root belt and gravel habitat than in marsh habitat from June to August. Recruitment primarily drove changes in crab abundance and size during June and July, and temperature best correlated with changes in the winter. Many stage I juveniles (CL < 3.1 mm) occurred in the lower estuarine reaches, while stage III and IV juveniles (CL 3.9–6.5 mm) primarily occurred in the middle and upper estuarine reaches.
Discussion:
Although intertidal wetland habitat in Yangtze Estuary is severely degraded and reduced in area, it remains important for recruitment and maintenance of mitten crab populations. An understanding of the habitat requirements of this species will benefit management of this crab resource and the prioritized restoration of intertidal habitat.
1 Introduction
Estuaries represent transitional zones between rivers and oceans, and provide nursery habitat for myriad fish and benthic invertebrates (Lipcius et al., 2005; Johnson and Eggleston, 2010). Survival and growth can be promoted by the quality of shelter and foraging habitats that these environments provide (Beck et al., 2001; Vermeiren and Sheaves, 2015). The value of estuarine habitat to juveniles varies with factors such as ontogenetic stage, predation risk, prey availability, three-dimensionality of seabed structure, or condition of the habitat itself (Pfirrmann et al., 2023).
Many field and laboratory studies have demonstrated that juvenile crabs tend to associate with structurally complex habitats (Amaral et al., 2009), with seagrass beds, salt marshes, oyster reefs, and gravels having higher juvenile densities than less-structured habitats, and growth–survival ratios in vegetated habitats being higher than in non-vegetated ones (Beck et al., 2001; Polte et al., 2005; Fonseca et al., 2006; Lefcheck et al., 2019). However, highly structured habitats may not always be higher in quality (Taylor and Fehon, 2021), with growth and resource availability for the blue crab Callinectes sapidus (Seitz et al., 2005) and Dungeness crab Cancer magister (Holsman et al., 2006) greater in unstructured intertidal habitats such as mud flats and sand than in adjacent, more structured habitats.
The Chinese mitten crab Eriocheir sinensis, a native freshwater species that is widely distributed throughout China, is traditionally regarded as a culinary delicacy and a high‐valued aquatic product (Wang et al., 2016). Yield increased from 8.4 × 103 t in 1991 to 8.1 × 105 t in 2021, and its value has increased from an estimated 6.0 × 107 United States Dollars (USD) in 1991 to 1.0 × 1010 USD in 2021; wild capture of E. sinensis in China was 2.5 × 104 t in 2021 (FAO, 2024). While many studies have examined the ecology and resource dynamics of adults of this species, few have examined those of its juveniles.
Within the Yangtze River system, E. sinensis breeds exclusively within the estuary, wherein mating, spawning, hatching, and early development occur (Du, 2004; Chen and Du, 2017). During winter, females spawn where salt and freshwater mix, where hatched larvae ultimately settle (Chen and Du, 2017). Megalopa and juveniles are transported by the tides into the upper estuarine reaches (Du, 2004), where mortality and growth vary significantly with habitat type and environmental condition (Liu, 2015, 2017). Therefore, Yangtze River estuarine habitat may play an important role in the migration, early development, and supplementation of the wild-capture E. sinensis fisheries resource.
Few studies have investigated juvenile E. sinensis habitat use in Yangtze Estuary (Zhao et al., 2020). We report (1) crab abundance in different habitats, (2) spatiotemporal variation in juvenile abundance and size, and (3) correlations between abundance of juveniles and environmental parameters. An improved understanding of each of these is important for more sustainable management of this species and its habitat.
2 Materials and methods
2.1 Sampling area
The Yangtze Estuary has irregular semi-diurnal tides, with mean tidal range of 2.67 m and a maximum of 4.62 m. With a mouth approximately 90 km wide (Yan et al., 2013), the Yangtze River discharges approximately 470 × 106 t of sediment annually, which over time has formed Chongming Island and two tributaries, the northern and southern branch. The southern branch discharges > 99% of the freshwater to the East China Sea, whereas the northern branch runs almost perpendicular to the main channel and discharges only a small proportion of the total flow (Zhang et al., 2012). The complicated topography and influence of tides and saltwater intrusion result in variable current and salinity conditions (Qiu et al., 2012). Sampling was conducted in the southern branch off Chongming Island (Figure 1).
Figure 1
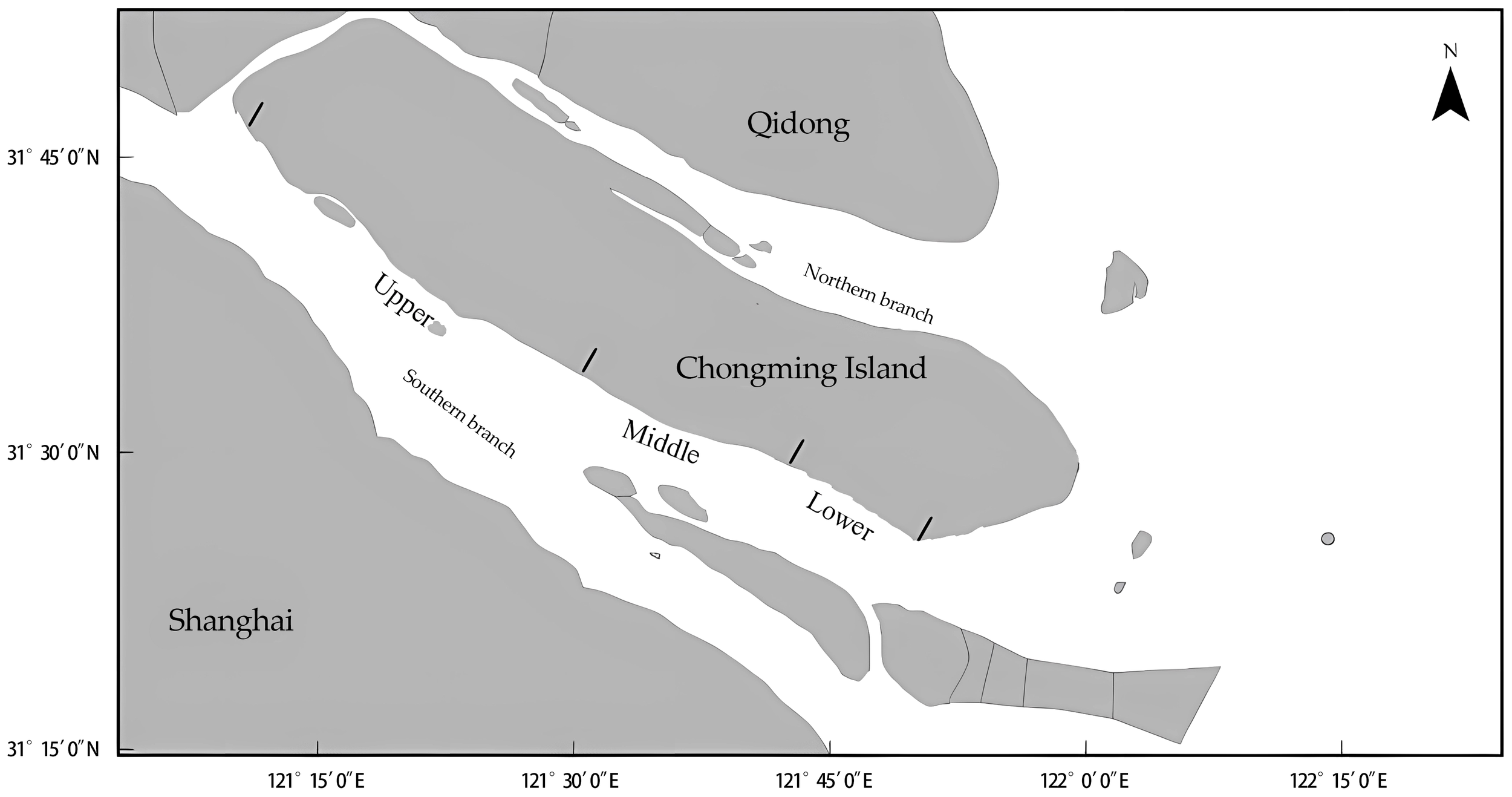
Study area, southern branch of Yangtze Estuary, China. Sampling of juvenile Eriocheir sinensis occurred in the upper, middle, and lower reaches of the estuary.
Local estuarine habitat includes marshes dominated by the reed Phragmites australis, sedge Scirpus triqueter, cordgrass Spartina alterniflora, gravels, and mud flats. Reeds are most common at high water, and sedges seaward of them. Mud flats mostly occur in the mid- and lower intertidal, within which patches of coarse gravel (mostly comprising material of diameter 3–10 cm) sporadically occur.
Juvenile E. sinensis density was measured in the upper, middle, and lower reaches of the estuary (Figure 1) twice monthly from June 2021 to February 2022. Density was estimated in three 0.5 m × 0.5 m quadrats in marsh (reed and sedge), root belt (the boundary between marsh and mud flat habitats, including some roots of marsh vegetation exposed because of tidal erosion), gravel, and mud flat habitats. Juvenile crabs were collected from the surface and the crab burrows within quadrats. We excavated crab burrows, collecting sediment from around them and sieving it through a 0.5 mm mesh. Carapace length (CL, Figure 2) of juvenile crabs was measured by vernier calipers in the laboratory. Sampling was performed during the diurnal ebb of the semi-diurnal tide. Salinity (‰), temperature (°C), and dissolved oxygen (mg/L) of nearshore water were measured using an in situ multi-parameter water quality analyzer (Pro Plus, YSI, OH, USA).
Figure 2

Schematic diagram of carapace length (CL).
2.2 Data analysis
Crabs were categorized by CL (Figure 2) into five instar stages (J1–J5) following Liu (2015) (Table 1). Juvenile crab (stage J1–J5) densities were measured for each habitat and reach. A preponderance of zero values during some sampling events meant those analyses requiring normally distributed data had to be abandoned in favor of non-parametric approaches (Anderson and Millar, 2004). By way of univariate PERMANOVA (p< 0.05, with 4999 permutations of raw data units) we tested our null hypothesis, that no significant differences in crab stage abundances or in total densities existed between habitat (fixed, 3 levels), month (fixed, 8 levels), and reach (random, 3 levels nested in habitat). Analyses were performed using a Euclidian distance matrix. Because some factors generated few possible permutations, a Monte-Carlo-based p-value was used (Anderson, 2001; McArdle and Anderson, 2001; Anderson and Robinson, 2003). This test is also recommended when data are too sparse or unbalanced for typical asymptotic methods (Senchaudhuri et al., 1995). Significant results were assessed post-hoc using PERMANOVA pairwise comparisons (T-test), which also used 4999 random permutations to obtain p-values.
Table 1
| Stage | J1 | J2 | J3 | J4 | J5 |
|---|---|---|---|---|---|
| CL (mm) | < 3.1 | 3.1–3.8 | 3.9–4.9 | 5–6.5 | > 6.6 |
| CL/CW | 1.05 | 0.98 | 0.94 | 0.91 | 0.90 |
Carapace length (CL) ranges used to categorize juvenile Eriocheir sinensis into stages; CL and carapace width (CW) ratios calculated from fitted curves (Liu, 2015).
A Chi-squared test was used to determine if the frequency of juvenile stages varied between months and between areas. The relative growth rate (RGR) was calculated using the following formula:
In the formula, CL1 represents the mean CL of juvenile crabs in the first month and CL2 represents the mean CL of juvenile crabs in subsequent months.
An unrepeated two-factor ANOVA was used to study variation in environmental factors among the three reaches. Pearson correlations were performed to evaluate the relationship between juvenile stage abundance and environmental variables.
3 Results
Juvenile E. sinensis were found between June 2021 and January 2022; no juvenile crabs were found in February 2022. During sampling, 4567 juveniles (mean CL ± standard deviation (SD), 5.5 ± 2.1 mm) of CL 2.3–15.5 mm were collected. These crabs were attributed to stages J1 (1406 ind, 31%), J2 (556, 12%), J3 (1096, 24%), J4 (1019, 22%), and J5 (490, 11%). Juveniles were collected from marsh, and root belt and gravel habitats, but not from mud flats. No megalopa was collected intertidally.
3.1 Habitat-specificity and temporal abundance
Juvenile density differed significantly between habitats and over time (Table 2). Total densities in root belt (mean ± SD, 107.95 ± 95.31 ind/m2) and gravel (79.14 ± 80.72 ind/m2) habitats were significantly higher than those in marshes (16.03 ± 17.60 ind/m2); no significant difference was apparent between total crab densities in root belt and gravel habitats (t = 3.1881, p = 0.0808).
Table 2
| Source | Component | df | J1 | J2 | J3 | J4 | J5 | Total density | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P(MC) | F | P(MC) | F | P(MC) | F | P(MC) | F | P(MC) | F | P(MC) | |||
| Habitat | Fixed | 2 | 30.81 | 0.0044 | 12.03 | 0.0204 | 71.31 | 0.0008 | 151 | 0.0008 | 189.53 | 0.0002 | 150.46 | 0.0006 |
| Month | Fixed | 7 | 230.04 | 0.0002 | 42.59 | 0.0002 | 15.66 | 0.0002 | 40.36 | 0.0002 | 10.55 | 0.0002 | 22.84 | 0.0002 |
| Reach (Habitat) | Random | 4 | 1.8 | 0.1314 | 2.63 | 0.0432 | 2.11 | 0.0798 | 1.72 | 0.1574 | 0.78 | 0.5412 | 2.82 | 0.0266 |
| Habitat × Month | Fixed | 14 | 7.57 | 0.0002 | 3.73 | 0.0002 | 2.16 | 0.0434 | 4.77 | 0.0014 | 2.44 | 0.0278 | 2.08 | 0.0568 |
| Reach (Habitat) × Month | Random | 24 | 1.48 | 0.07 | 1.93 | 0.009 | 3.51 | 0.0002 | 4.65 | 0.0002 | 2.81 | 0.0002 | 7.77 | 0.0002 |
Univariate PERMANOVA results for Eriocheir sinensis stages, and for total density in each habitat.
Bold numbers indicate significant values (p< 0.05).
Significant differences in abundances of stage J1–J5 crabs with time and habitat were apparent (Figure 3; Table 2). For several months, more J1–J5 stage crabs occurred in root belt and gravel habitats than in marsh habitats.
Figure 3
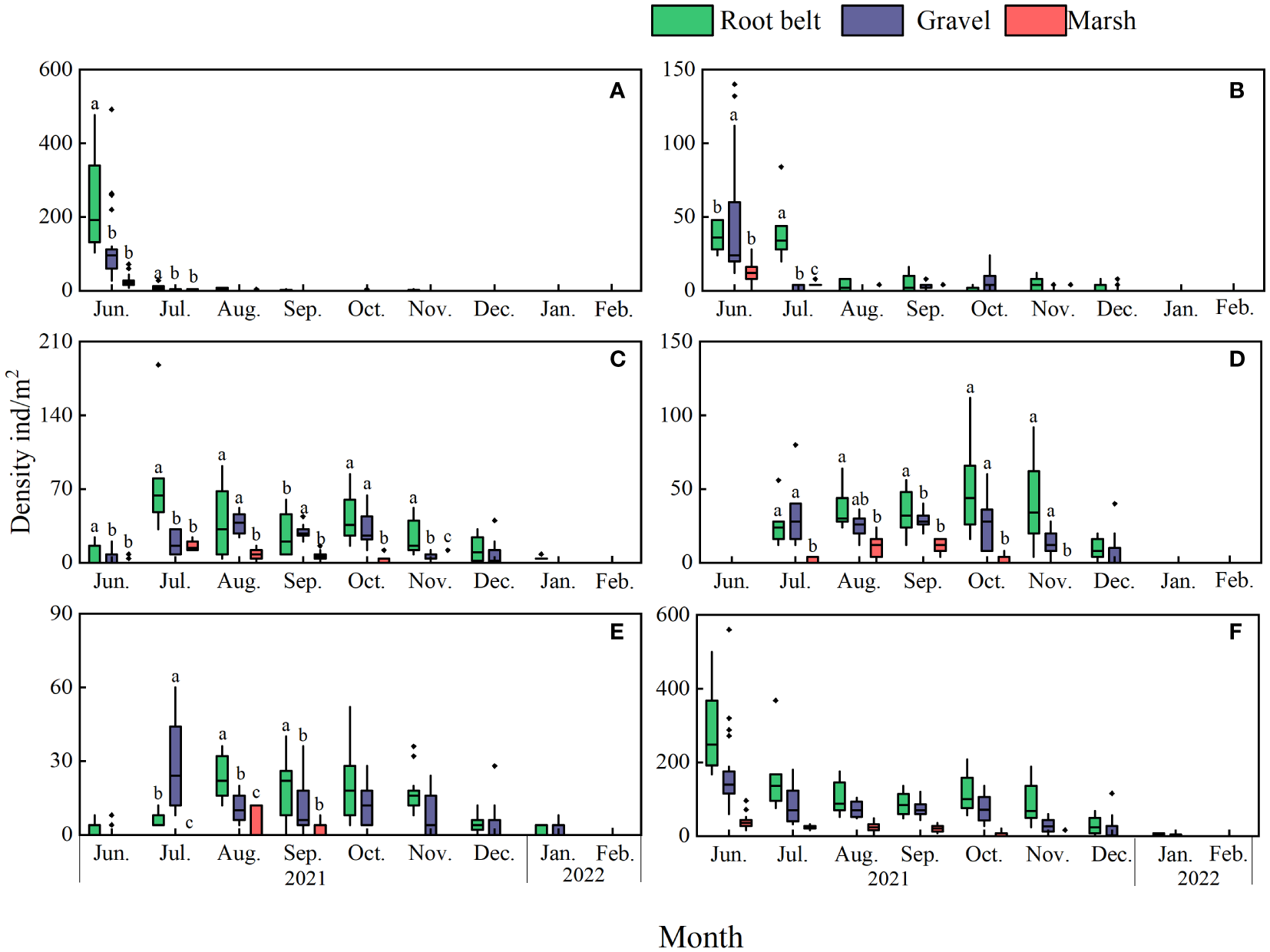
Spatial and temporal variation in Eriocheir sinensis densities. Stages: (A) J1, (B) J2, (C) J3, (D) J4, (E) J5, and (F) total juveniles. Lowercase letters denote significant differences. Because no “habitat” and “month” interaction was apparent for total juvenile density, bars in (F) are unlabeled.
3.2 Spatiotemporal variation in juvenile abundance
The distribution of juveniles was associated with sampling reach (p< 0.05). Proportionally more J1 stage crabs (33%) were collected in lower estuarine reaches than elsewhere. Proportions of J3 (31% in middle reaches, 34% in upper reaches) and J4 (37% and 34% in middle and upper reaches, respectively) stage crabs were higher than in lower reaches (Figure 4).
Figure 4
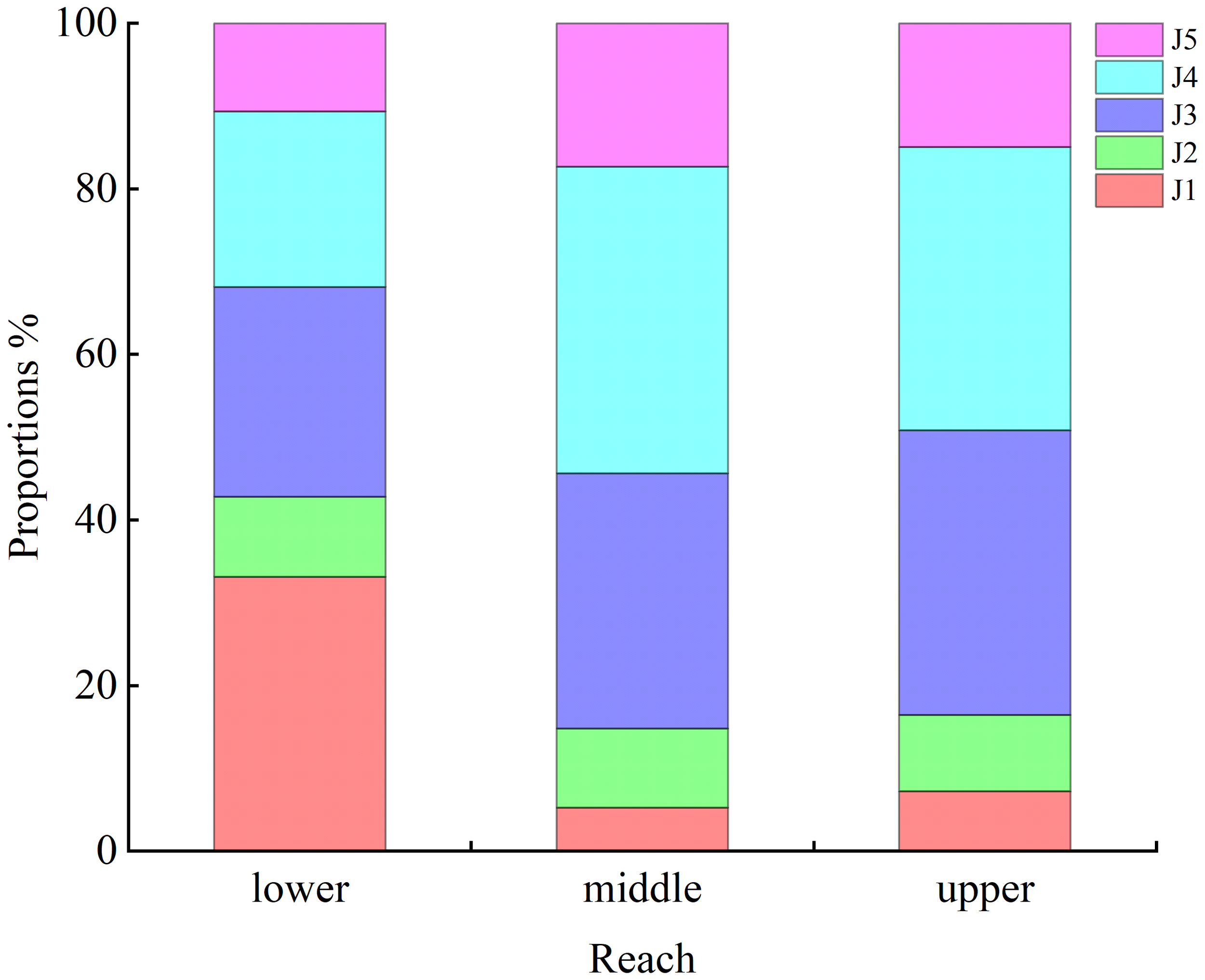
Spatial distribution of juvenile Eriocheir sinensis in the upper, middle, and lower reaches of the southern branch of the Yangtze Estuary.
Juvenile distributions were also associated with sampling month (p< 0.05). In June, stage J1 and J2 individuals dominated size-class frequencies (97%), while after June, the greatest proportion of juveniles were stage J3–J5 crabs (Figure 5).
Figure 5
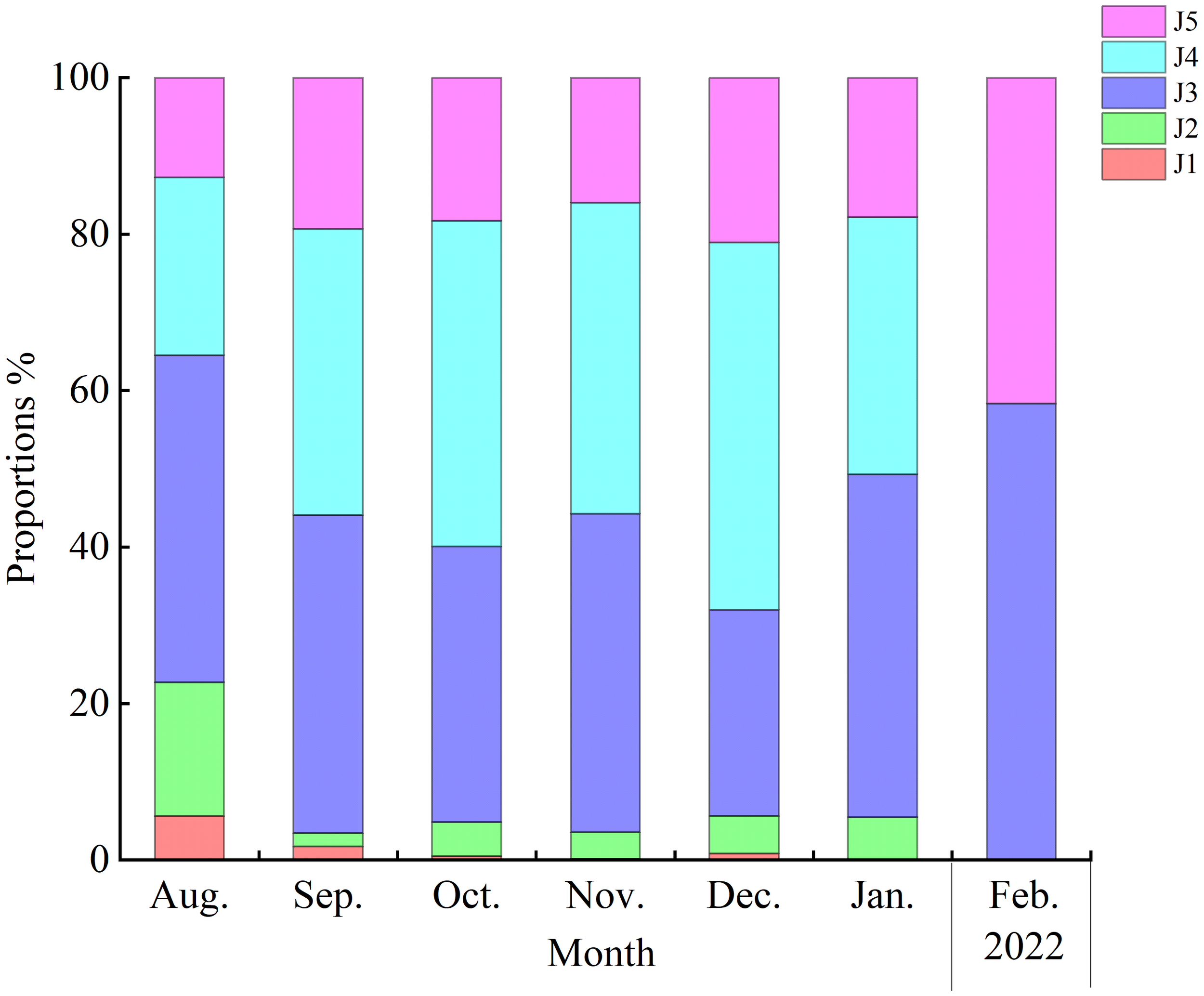
Distribution of juvenile Eriocheir sinensis stages over time along the southern branch of the Yangtze Estuary.
There were also differences in the size and RGR of juvenile E. sinensis in different habitats. From June to August, size and RGR of juvenile crabs were greater in root belt and gravel habitats than in marsh habitat (Table 3). After August, growth rates declined.
Table 3
| Year | Month | Root belt | Gravel | Marsh | |||
|---|---|---|---|---|---|---|---|
| Mean CL (mm) | RGR (%) | Mean CL (mm) | RGR (%) | Mean CL (mm) | RGR (%) | ||
| 2021 | Jun. | 3.13 ± 0.64 | – | 3.22 ± 0.74 | – | 3.23 ± 0.47 | – |
| Jul. | 4.78 ± 1.41 | 52.7 | 7.13 ± 2.68 | 121.40 | 4.48 ± 0.68 | 38.7 | |
| Aug. | 6.24 ± 2 | 99.4 | 6.32 ± 2.63 | 96.3 | 5.89 ± 1.11 | 82.4 | |
| Sep. | 6.36 ± 2.05 | 103.2 | 5.98 ± 2.69 | 85.7 | 5.91 ± 1.15 | 83.0 | |
| Oct. | 6.41 ± 2.25 | 104.8 | 6.26 ± 2.64 | 94.4 | 5.46 ± 0.83 | 69.0 | |
| Nov. | 6.56 ± 2.49 | 109.6 | 6.98 ± 2.34 | 116.8 | 4.38 ± 0.4 | 35.6 | |
| Dec. | 5.91 ± 1.89 | 88.8 | 6.75 ± 2.99 | 109.6 | – | – | |
| 2022 | Jan. | 5.94 ± 2.29 | 89.8% | – | – | – | – |
| Feb. | – | – | – | – | – | – | |
Changes in carapace length (CL) and relative growth rate (RGR) of juvenile Eriocheir sinensis in different habitats.
“-” indicates that the sample size was small and was not calculated.
3.3 Relationship between abundance and environmental variables
Significantly higher juvenile densities occurred in June 2021, when a recruitment peak occurred (Figure 3). There were no significant differences in salinity, dissolved oxygen, or water temperature among the upper, middle, and lower estuarine reaches (p > 0.05). Salinity ranged 0.09–1.09, dissolved oxygen ranged 6.78–11.62 mg/L, and water temperature ranged 9.4–27.8°C (Figure 6). Pearson analysis revealed total juvenile abundance to significantly, positively correlate with water temperature (Table 4) but not with dissolved oxygen concentrations or salinity. There was no significant correlation between the abundance of any stage J1–J5 crab and water temperature, dissolved oxygen, or salinity (Table 4).
Figure 6
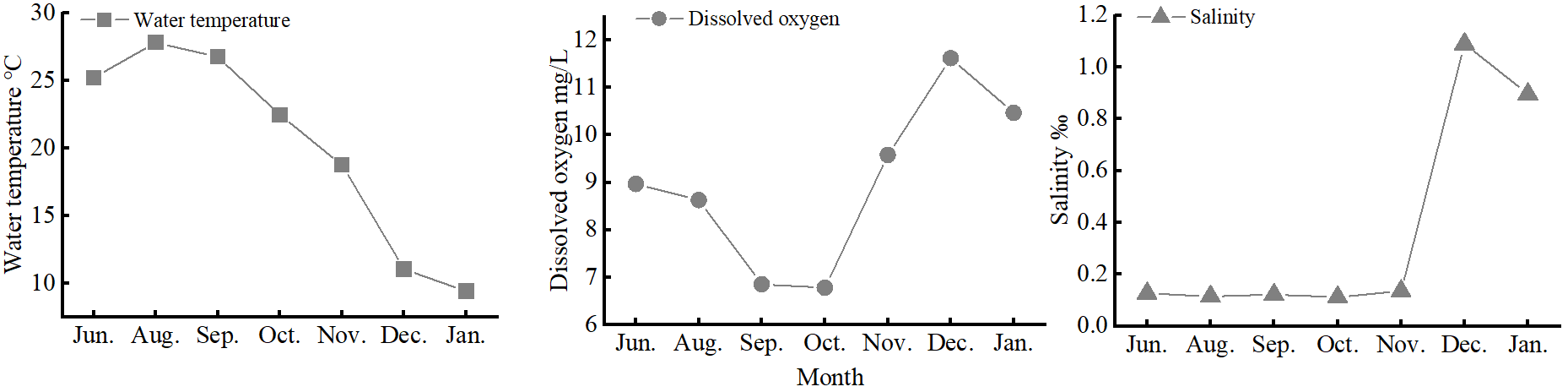
Mean dissolved oxygen, water temperature, and salinity values over time along the southern branch of the Yangtze Estuary.
Table 4
| Factor | J1 | J2 | J3 | J4 | J5 | Total abundance | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | |
| Salinity | 0.26 | 0.618 | 0.289 | 0.578 | −0.612 | 0.197 | −0.33 | 0.524 | −0.359 | 0.484 | −0.091 | 0.864 |
| Water temperature | 0.507 | 0.305 | 0.399 | 0.433 | 0.382 | 0.455 | 0.013 | 0.981 | 0.28 | 0.591 | 0.872 | 0.024 |
| Dissolved oxygen | −0.069 | 0.896 | −0.187 | 0.723 | −0.605 | 0.203 | −0.311 | 0.548 | −0.494 | 0.32 | −0.649 | 0.163 |
Pearson correlation test results between Eriocheir sinensis abundance and salinity, water temperature, and dissolved oxygen along the southern branch of the Yangtze Estuary.
Bold numbers indicate significant values (p< 0.05).
4 Discussion
We report the distribution of juvenile E. sinensis in intertidal habitat along the southern branch of the Yangtze Estuary from June 2021 to January 2022. Because the greatest juvenile crab densities occur in root belt and gravel habitats, followed by marshes, and no juveniles were found on mud flats, juvenile E. sinensis appears to prefer more complex habitat. This conclusion is consistent with accounts of E. sinensis mainly occurring in pebble habitat in the intertidal of the Thames Estuary (Gilbey et al., 2007), and in addition to pebbles, in Scirpus sp. and Typha latifolia habitats in the intertidal San Francisco Bay (Rudnick et al., 2003). Subtidally, juveniles mainly frequent densely vegetated shallow waters, with the largest populations found in the waterweed Egeria densa (Rudnick et al., 2003). Crustaceans typically prefer structurally complex habitats. For example, juvenile Callinectes sapidus are significantly more abundant in vegetated habitats than in unstructured mud flat and sand habitats (Hovel and Lipcius, 2001), larval Cancer magister densities are significantly higher in mussel than mud flat habitats (Fernandez et al., 1993), and larval numbers of green crab Carcinus maenas are significantly higher in filamentous algal, diatom, and mussel bed habitats than they are in open sandy areas (Moksnes, 2002).
The quality of refugia regulates populations of crab species (Shervette et al., 2004). Juvenile E. sinensis are abundant in root belt habitats, which may be related to habitat-specific food resource availability (Taylor and Fehon, 2021). Vegetation, rich in organic matter and detritus, supports high densities of benthic in- and epifauna, including important prey for juvenile E. sinensis. Root belt habitat might also offer size-suitable refugia for juveniles. Because juvenile decapod crustaceans benefit from shelter (Stevens and Swiney, 2005), and the structure of root belt and gravel habitats is more complex than that of marshes, they might provide more refugia for juvenile crabs (Shervette et al., 2011). A lack of suitably sized shelter might also explain why no large E. sinensis were collected intertidally (Bromilow and Lipcius, 2017).
Many indoor experiments have reported habitats such as aquatic plants and simulated shelters to significantly increase the developmental rate of juvenile E. sinensis (Liu, 2015). We report juvenile E. sinensis to have higher CL and RGR in root belt and gravel habitat than in marsh habitat from June to August. This complex habitat structure helps juveniles to avoid predators, and to store energy for development. Additionally, because crabs when molting are relatively weak and vulnerable to attack, this complex habitat provides suitable shelter to avoid attacks, thereby increasing molting success. Therefore, although the intertidal wetland in the Yangtze Estuary has been reduced by 36% over the past three decades (Chen et al., 2016) because of frequent large-scale intertidal reclamation, invasion by Spartina, and artificial wetland alteration (Zou et al., 2016), what remains is still important for recruitment and maintenance of E. sinensis populations.
Juvenile E. sinensis density varies over time. The greatest abundances occur in June, when stage J1 and J2 crabs are prevalent. Early juvenile abundance is closely related to spatiotemporal variation in megalopa settlement (Etherington and Eggleston, 2000). Eriocheir sinensis recruitment in Yangtze Estuary occurs in early June, over the space of one week (Li et al., 1997), and crab densities decrease rapidly after June. During recruitment from 2003 to 2013, megalopa catch varied from 3 to 32 t (Wang, 2019). Following recruitment, juveniles migrate into the upper reaches of the Yangtze Estuary. Additionally, the decrease in density of juvenile crabs may correlate with juvenile survivorship, with marine invertebrate mortality often being higher during earlier life stages (Wang and Haywood, 1999). Survival of juvenile crabs often increases with size (Johnson et al., 2008; Hultgren and Stachowicz, 2010). In aquaculture, the survival rate of early juvenile E. sinensis is low (Yang et al., 2018). Liu (2017) reported crabs to have a survival rate of 48% from megalopa to stage J5, and 79% from J5 to J10. Early juvenile crab mortality in the field usually occurs because of predation (Wilson et al., 1990; Thiel and Dernedde, 1994). Large-sized conspecific crabs, intertidal fishes, and migratory birds may also all be predators of juvenile crabs (Hedvall et al., 1998; Lipcius et al., 2005; Choi et al., 2017), with indoor experiments having reported cannibalism to occur in juvenile E. sinensis during periods of food shortage (Zeng et al., 2018). Migratory shorebirds stopping over at the Chongming Wetland also prey extensively on benthic macroinvertebrates (Zhu et al., 2007; Li et al., 2023). Although we might have expected the juvenile population to decline over time because of this migration, densities (mainly comprising stage J3–J5 juveniles) remained relatively stable from July to November of 2021. This may be because of continued juvenile recruitment, because a small number of J1 crabs were collected over the sampling period, or as a function of abnormal growth. In E. sinensis culture, frequent water level changes, high population densities, low dissolved oxygen levels, low vegetation cover, food shortage, and irregular feeding can contribute to abnormal growth (Sun and Wu, 1996), producing small-sized “lazy crabs” that remain in their burrows and neither feed nor move. Lazy crabs are extremely small, usually the size of an early juvenile crab (Sun and Wu, 1996). The semi-diurnal tides in Yangtze Estuary cause frequent changes in tidal height, which may result in lazy crabs. Reductions in intertidal wetland habitat may also lead to high densities of juvenile crabs in the Yangtze Estuary and food shortage, resulting in more lazy crabs.
Juvenile crabs in the upper estuarine reaches are generally larger than those in the lower reaches, consistent with the early migratory life history of the E. sinensis. The spatial and temporal distributions of decapod juveniles are closely related to biotic and abiotic factors (González-Ortegón et al., 2023). We report water temperature to positively, significantly correlate with juvenile E. sinensis density. After December 2021, numbers of juvenile E. sinensis decreased rapidly. It is possible that juvenile E. sinensis migrate to deeper riverbeds, where water temperatures are higher, and that temperature affects their spatial clustering. Consistent with our results, juvenile E. sinensis densities in intertidal zones during winter were also significantly lower than during summer (Gilbey et al., 2007). Low water temperature may hinder crustacean feeding and growth (Frederich et al., 2000) and contribute to juvenile E. sinensis migration during this period.
Understanding juvenile E. sinensis habitat requirements is important for their conservation and management. Understanding the role of estuaries in recruitment requires knowledge of appropriate physicochemical conditions, prey abundance, habitat availability, and interactions with other organisms. Our results from the southern branch of the Yangtze River along Chongming Island indicate that vegetated and gravel habitats should be prioritized for conservation as essential nursery grounds for this species.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
YW: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. SW: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. FZ: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Resources, Writing – review & editing. ZQ: Data curation, Investigation, Writing – review & editing. ZG: Data curation, Investigation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the National Natural Science Foundation of China (32072982; 32102800), the Program of Shanghai Academic Research Leader (21XD1405000), and the Natural Science Foundation of Shanghai (23ZR1479000).
Acknowledgments
We thank Wen Xie and Cong Fang for their help in field sampling, and Steve O’Shea, from Edanz (https://www.edanz.com/ac) for editing a draft of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Amaral V. Cabral H. N. Jenkins S. Hawkins S. Paula J. (2009). Comparing quality of estuarine and nearshore intertidal habitats for Carcinus maenas. Estuar. Coast. Shelf Sci.83, 219–226. doi: 10.1016/j.ecss.2009.03.029
2
Anderson M. J. (2001). A new method for non-parametric multivariate analysis of variance. Austral Ecol.26, 32–46. doi: 10.1111/j.1442–9993.2001.01070.x
3
Anderson M. J. Millar R. B. (2004). Spatial variation and effects of habitat on temperate reef fish assemblages in northeastern New Zealand. J. Exp. Mar. Biol. Ecol.305, 191–221. doi: 10.1016/j.jembe.2003.12.011
4
Anderson M. J. Robinson J. (2003). Generalized discriminant analysis based on distances. Aust. Nz J. Stat.45, 301–318. doi: 10.1111/1467-842X.00285
5
Beck M. W. Heck K. L. Able K. W. Childers D. L. Eggleston D. B. Gillanders B. M. et al . (2001). The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience51, 633–641. doi: 10.1641/0006-3568(2001)051[0633:TICAMO]2.0.CO;2
6
Bromilow A. M. Lipcius R. N. (2017). Mechanisms governing ontogenetic habitat shifts: Role of trade-offs, predation, and cannibalism for the blue crab. Mar. Ecol. Prog. Ser.584, 145–159. doi: 10.3354/meps12405
7
Chen Y. Dong J. W. Xiao X. M. Zhang M. Tian B. Zhou Y. X. et al . (2016). Land claim and loss of tidal flats in the Yangtze Estuary. Sci. Rep.6, 24018. doi: 10.1038/srep24018
8
Chen L. Du N. S. (2017). Biology of Eriocheir sinensis (Beijing: Science Press), 158–160.
9
Choi C. Y. Battley P. F. Potter M. A. Ma Z. J. Melville D. S. Sukkaewmanee P. (2017). How migratory shorebirds selectively exploit prey at a staging site dominated by a single prey species. Auk134, 76–91. doi: 10.1642/AUK-16-58.1
10
Du N.S. (2004). Migration of the Chinese mitten crab (Eriocheir sinensis). Fisheries Science & Technology Information02, 56–57. doi: 10.3969/j.issn.1001-1994.2004.02.001
11
Etherington L. L. Eggleston D. B. (2000). Large-scale blue crab recruitment: Linking postlarval transport, post-settlement planktonic dispersal, and multiple nursery habitats. Mar. Ecol. Prog. Ser.204, 179–198. doi: 10.3354/meps204179
12
FAO (2024) Fisheries & Aquaculture. Available online at: https://www.fao.org/fishery/statistics-query/en/home (Accessed 1 Jan 2024).
13
Fernandez M. Iribame O. Armstrong D. A. (1993). Habitat selection by young-of-the-year Dungeness crab Cancer magister and predation risk in intertidal habitats. Mar. Ecol. Prog. Ser.92, 171–177. doi: 10.3354/meps092171
14
Fonseca V. F. Vinagre C. Cabral H. N. (2006). Growth variability of juvenile soles Solea solea and Solea Senegalensis, and comparison with RNA: DNA ratios in the Tagus estuary, Portugal. J. Fish Biol.68, 1551–1562. doi: 10.1111/j.0022-1112.2006.001042.x
15
Frederich M. Sartoris F. J. Arntz W. E. Prtner H. O. (2000). Haemolymph Mg2+ regulation in decapod crustaceans: Physiological correlates and ecological consequences in polar areas. J. Exp. Biol.203, 1383–1393. doi: 10.1242/jeb.203.8.1383
16
Gilbey V. Attrill M. J. Coleman R. A. (2007). Juvenile Chinese mitten crabs (Eriocheir sinensis) in the Thames estuary: Distribution, movement and possible interactions with the native crab Carcinus maenas. Biol. Invasions10, 67–77. doi: 10.1007/s10530-007-9110-4
17
González-Ortegón E. de Carvalho-Souza G. F. Vilas C. Baldó F. Cuesta J. A. (2023). Trends in the decapod crustacean community at the southernmost estuary of the Atlantic coast of Europe. Sci. Rep.13, 22857. doi: 10.1038/s41598–023-50049–9
18
Hedvall O. Moksnes P. O. Pihl L. (1998). Active habitat selection by megalopae and juvenile shore crabs Carcinus maenas: a laboratory study in an annular flume. Hydrobiologia375, 89–100. doi: 10.1023/A:1017081611077
19
Holsman K. K. McDonald P. S. Armstrong D. A. (2006). Intertidal migration and habitat use by subadult Dungeness crab Cancer magister in a NE Pacific estuary. Mar. Ecol. Prog. Ser.308, 183–195. doi: 10.3354/meps308183
20
Hovel K. A. Lipcius R. N. (2001). Habitat fragmentation in a seagrass landscape: Patch size and complexity control blue crab survival. Ecology82, 1814–1829. doi: 10.1890/0012-9658(2001)082[1814:HFIASL]2.0.CO;2
21
Hultgren K. M. Stachowicz J. J. (2010). Size-related habitat shifts facilitated by positive preference induction in a marine kelp crab. Behav. Ecol.21, 329–336. doi: 10.1093/beheco/arp192
22
Johnson E. G. Eggleston D. B. (2010). Population density, survival and movement of blue crabs in estuarine salt marsh nurseries. Mar. Ecol. Prog. Ser.407, 135–147. doi: 10.3354/meps08574
23
Johnson E. G. Hines A. H. Kramer M. A. Young A. C. (2008). Importance of season and size of release to stocking success for the blue crab in Chesapeake Bay. Rev. Fish Sci. Aquac16, 243–253. doi: 10.1080/10641260701696837
24
Lefcheck J. S. Hughes B. B. Johnson A. J. Pfirrman B. W. Rasher D. B. Smyth A. R. et al . (2019). Are coastal habitats important nurseries? A meta-analysis. Conserv. Lett.12, e12645. doi: 10.1111/conl.12645
25
Li C. M. Chen J. S. Liao X. L. Ramus A. P. Angelini C. Liu L. L. et al . (2023). Shorebirds-driven trophic cascade helps restore coastal wetland multifunctionality. Nat. Commun.14, 8076. doi: 10.1038/s41467-023-43951-3
26
Li C. S. Yu L. F. Dai G. L. Yu L. C. Tang J. H. Shen D. H. (1997). Survey for megolopa of Ericheir sinensis and other species megolopa in the estuary of Changjiang river and adjacent waters. J. fish. ChinaS1, 111–114.
27
Lipcius R. N. Seitz R. D. Seebo M. S. Colón-Carrión D. (2005). Density, abundance and survival of the blue crab in seagrass and unstructured salt marsh nurseries of Chesapeake Bay. J. Exp. Mar. Biol. Ecol.319, 69–80. doi: 10.1016/j.jembe.2004.12.034
28
Liu Q. B. (2015). The preliminary study of growth of Chinese mitten crab (Eriocheir sinensis) in larval and juvenile stages. Shanghai Ocean University, Shanghai, China.
29
Liu Q. H. (2017). Effects of aquatic plants on growth, nutritional quality and gene expression of Chinese mitten crab Eriocheir sinensis. Shanghai Ocean University, Shanghai, China.
30
McArdle B. H. Anderson M. J. (2001). Fitting multivariate models to community data: A comment on distance based redundancy analysis. Ecology82, 290–297. doi: 10.1890/0012-9658(2001)082[0290:FMMTCD]2.0.CO;2
31
Moksnes P. O. (2002). The relative importance of habitat-specific settlement, predation and juvenile dispersal for distribution and abundance of young juvenile shore crabs Carcinus maenas L. J. Exp. Mar. Biol. Ecol.271, 41–73. doi: 10.1016/S0022-0981(02)00041-2
32
Pfirrmann B. W. Dunn R. P. Kimball M. E. Levesque E. M. (2023). Experimental laboratory tests of short-term habitat selection by hatchery-reared juvenile red drum (Sciaenops ocellatus). J. Exp. Mar. Biol. Ecol.559, 151852. doi: 10.1016/j.jembe.2022.151852
33
Polte P. Schanz A. Asmus H. (2005). The contribution of seagrass beds (Zostera noltii) to the function of tidal flats as a juvenile habitat for dominant, mobile epibenthos in the Wadden Sea. Mar. Biol.147, 813–822. doi: 10.1007/s00227-005-1583-z
34
Qiu C. Zhu J. R. Gu Y. L. (2012). Impact of seasonal tide variation on saltwater intrusion in the Changjiang River estuary. Chin. J. Oceanol. Limn.30, 342–351. doi: 10.1007/s00343-012-1115-x
35
Rudnick D. A. Hieb K. Grimmer K. F. Resh V. H. (2003). Patterns and processes of biological invasion: The Chinese mitten crab in San Francisco Bay. Basic Appl. Ecol.4, 249–262. doi: 10.1078/1439-1791-00152
36
Seitz R. D. Lipcius R. N. Seebo M. S. (2005). Food availability and growth of the blue crab in seagrass and unvegetated nurseries of Chesapeake Bay. J. Exp. Mar. Biol. Ecol.319, 57–68. doi: 10.1016/j.jembe.2004.10.013
37
Senchaudhuri P. Mehta C. R. Patel N. R. (1995). Estimating exact p values by the method of control variates or Monte Carlo rescue. J. Am. Stat. Assoc.90, 640–648. doi: 10.2307/2291077
38
Shervette V. R. Gelwick F. Hadley N. (2011). Decapod utilization of adjacent oyster, vegetated marsh, and non-vegetated bottom habitats in a Gulf of Mexico Estuary. J. Crustacean Biol.31, 660–667. doi: 10.1651/10-3360.1
39
Shervette V. R. Perry H. M. Rakocinski C. F. Biesiot P. M. (2004). Factors influencing refuge occupation by stone crab menippe adina juveniles in mississippi sound. J. Crustacean Biol.24, 652–665. doi: 10.1651/C-2453
40
Stevens B. G. Swiney K. M. (2005). Post-settlement effects of habitat type and predator size on cannibalism of glaucothoe and juveniles of red king crab. J. Exp. Mar. Biol. Ecol.321, 1–11. doi: 10.1016/j.jembe.2004.12.026
41
Sun J. F. Wu S. (1996). Causes and control measures of lazy crab. Fish. Sci.4, 27–28.
42
Taylor D. L. Fehon M. M. (2021). Blue crab (Callinectes sapidus) population structure in southern new england tidal rivers: Patterns of Shallow-Water, unvegetated habitat use and quality. Estuar. Coast.44, 1320–1343. doi: 10.1007/s12237-020-00867-1
43
Thiel M. Dernedde T. (1994). Recruitment of shore crabs Carcinus maenas on tidal flats: mussel clumps as an important refuge for juveniles. Helgoland Mar. Res.48, 321–332. doi: 10.1007/BF02367044
44
Vermeiren P. Sheaves M. (2015). Predictable habitat associations of four crab species across the low intertidal landscape of a tropical estuary over time. Estuaries Coasts38, 285–295. doi: 10.1007/s12237-014-9799-0
45
Wang H. H. (2019). Resource Changes and Conservation Strategies of Chinese Mitten Crabs in the Middle and Lower reaches of the Yangtze River. Shanghai Ocean University, Shanghai, China.
46
Wang Y. G. Haywood M. D. E. (1999). Size-dependent natural mortality of juvenile banana prawns Penaeus merguiensis in the Gulf of Carpentaria, Australia. Mar. Freshw. Res.50, 313–317. doi: 10.1071/MF97229
47
Wang Q. D. Liu J. S. Zhang S. Y. Lian Y. X. Ding H. Y. Du X. et al . (2016). Sustainable farming practices of the Chinese mitten crab (Eriocheir sinensis) around Hongze Lake, lower Yangtze River Basin, China. Ambio45, 361–373. doi: 10.1007/s13280-015-0722-0
48
Wilson K. A. Able K. W. Heck K. L. (1990). Predation rates on juvenile blue crabs in estuarine nursery habitats: evidence for the importance of macroalgae (Ulva lactuca). Mar. Ecol. Prog. Ser.58, 243–251. doi: 10.3354/meps058243
49
Yan C. X. Yang Y. Zhou J. L. Liu M. Nie M. H. Shi H. et al . (2013). Antibiotics in the surface water of the Yangtze Estuary: Occurrence, distribution and risk assessment. Environ. pollut.175, 22–29. doi: 10.1016/j.envpol.2012.12.008
50
Yang Z. G. Wei B. H. Liu Q. B. Cheng Y. X. Zhou J. Y. (2018). Individual growth pattern of juvenile stages of the Chinese mitten crab (Eriocheir sinensis) reared under laboratory conditions. Aquacult. Int.26, 645–657. doi: 10.1007/s10499-018-0239-1
51
Zeng Q. F. Jeppesen E. Gu X. H. Mao Z. G. Chen H. H. (2018). Cannibalism and habitat selection of cultured Chinese mitten crab: effects of submerged aquatic vegetation with different nutritional and refuge values. Water10, 1542. doi: 10.3390/w10111542
52
Zhang E. F. Savenije H. H. G. Chen S. L. Mao X. H. (2012). An analytical solution for tidal propagation in the Yangtze Estuary, China. Hydrol. Earth Syst. Sc16, 3327–3339. doi: 10.5194/hess-16-3327-2012
53
Zhao F. Huang X. R. Song C. Zhang T. Yang G. Zhuang P. (2020). Selection and use of artificial floating wetland habitats for larval Chinese mitten crab in the Yangtze Estuary. J. Fish. Sci. China27, 1003–1009. doi: 10.3724/SP.J.1118.2020.20035
54
Zhu J. Jing K. Gan X. J. Ma Z. J. (2007). Food supply in intertidal area for shorebirds during stopover at Chongming Dongtan, China. Acta Ecol. Sin.27, 2149–2159. doi: 10.1016/S1872-2032(07)60045-6
55
Zou Y. A. Tang C. D. Niu J. Y. Wang T. H. Xie Y. H. Guo H. (2016). Migratory waterbirds response to coastal habitat changes: conservation implications from long-term detection in the Chongming Dongtan Wetlands, China. Estuaries Coast.39, 273–286. doi: 10.1007/s12237-015-9991-x
Summary
Keywords
crab recruitment, Eriocheir sinensis , juvenile, habitat, Yangtze estuary
Citation
Wu Y, Wang S, Qin Z, Geng Z and Zhao F (2024) Spatiotemporal variation in size and abundance of juvenile Chinese mitten crab (Eriocheir sinensis) in Yangtze Estuary. Front. Mar. Sci. 11:1395991. doi: 10.3389/fmars.2024.1395991
Received
05 March 2024
Accepted
03 June 2024
Published
19 June 2024
Volume
11 - 2024
Edited by
Alberto Basset, University of Salento, Italy
Reviewed by
Vania Freitas, University of Porto, Portugal
Baoming Ge, Yancheng Teachers University, China
Updates
Copyright
© 2024 Wu, Wang, Qin, Geng and Zhao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Zhao, zhaof@ecsf.ac.cn; Sikai Wang, wangsk@ecsf.ac.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.