Abstract
To investigate the effect of the frozen Antarctic krill meal (AKM) as an nutritional supplement in Cherax quadracarinatus culturing, an experiment was designed with the following groups: a control group (C) fed with basic feed throughout the experiment; Experimental Group 1 (E1) fed basic feed for 2 d and then compound feed (50% AKM + 50% basic feed) for 1 d; Experimental Group 2 (E2) fed basic feed for 1 d and then compound feed (50% AKM + 50% basic feed) for 1 d; Experimental Group 3 (E3) fed compound feed (50% AKM + 50% basic feed) daily. After 10 weeks of feeding, growth results revealed that both E1 and E2 groups exhibited significantly higher weight gain rate (WGR) and specific growth rate (SGR) compared to groups C and E3 (p < 0.05), with E2 achieving the highest survival rate (SR). Regarding muscle nutrition, the contents of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in C. quadracarinatus muscle showed a gradual increase with higher AKM feeding levels (p < 0.05), while the highest astaxanthin content was observed in group E2. Analysis of digestive enzyme activity and fluorescence quantification demonstrated that feeding AKM every other day (E2) not only enhanced the activities of trypsin and lipase in the hepatopancreas but also upregulated the expression levels of immune-related and molting-related genes (p < 0.05), whereas daily AKM feeding showed no significant improvements. Additionally, AKM feeding influenced the intestinal microbiota structure and abundance, with a notable increase in Bacteroidota phylum abundance in group E2.In conclusion, this study demonstrates that alternate-day AKM feeding (E2) optimizes growth performance, enhances muscle nutritional quality, improves digestive and immune capacities, and modifies intestinal microbiota composition in red claw crayfish. In contrast, daily AKM feeding, while elevating certain nutritional indices (e.g., EPA+DHA), exhibits limited benefits for overall growth performance and physiological function enhancement. Therefore, alternate-day AKM feeding is recommended as a superior strategy, providing scientific evidence for its application in C. quadracarinatus aquaculture.
1 Introduction
In the past 10 years, the global aquaculture industry has experienced rapid development. The scale of shrimp and crab farming has been continuously expanding, and production has been rapidly increasing (FAO (Food and Agriculture Organization of the UN), 2024). While the aquaculture industry grows rapidly, it has raised higher demands for the diversity and functionality of aquatic feed nutrition. In practice, aquaculture enterprises typically select meat as nutritional supplements to fulfill the growth requirements of farmed species. Current aquatic nutrition research primarily focuses on replacing fishmeal in feeds or supplementing specific trace elements, while studies on aquaculture nutritional supplements remain limited (Liu et al., 2022). Moreover, the fresh frozen trash fish commonly used as nutritional supplements in production carries drawbacks such as aquaculture water pollution and disease outbreaks (Zeng et al., 2022). Therefore, identifying new and efficient nutrition sources as nutritional supplements in crustacean farming is the primary task to ensure healthy and sustainable development of the aquaculture industry.
In recent years, Antarctic krill (Euphausia superba), which also belongs to crustacean classes, has attracted the attention of many aquaculture nutrition researchers as a potential nutritional source for aquatic feed (Wang et al., 2021). Antarctic krill is a small marine crustacean, mainly distributed in Antarctic waters. It is currently the largest single-species biological resource on Earth, with an annual catch of ≤ 100 million tons (Zuo and Zuo, 2019). Antarctic krill has high nutritional value and is rich in 20:5n-3 (EPA) and 22:6n-3 (DHA) (Mai et al., 2016), which play important roles in disease resistance, immune function, material metabolism, and energy regulation in aquatic animals (Yang et al., 2006), and their endogenous synthesis is extremely low (Zhu and Zhu, 2022). Antarctic krill is also rich in astaxanthin, chitin, phospholipids, and other essential substances for aquatic animals. These substances are beneficial for enhancing the growth and development performance and disease resistance of crustaceans (Liu et al., 2018; Wang et al., 2021; Shi et al., 2023). Moreover, Antarctic krill meets the ideal protein pattern recommended by the Food and Agriculture Organization/World Health Organization, making it a natural high-quality feed (FAO/WHO, 1991).
Numerous studies have confirmed that addition of Antarctic krill to feed helps promote aquatic animal growth. Researchers such as Torrecillas et al. (2021) have found that replacing fishmeal in feed with Antarctic krill meal significantly improved the final body weight, protein and fat efficiency, specific growth rate (SGR), and feed conversion ratio (FCR) of European seabass (Dicentrarchus labrax), and effectively maintained liver health. Among crustaceans, Gao et al. (2020) found that adding Antarctic krill meal to feed significantly increased the weight gain rate, feed coefficient, and survival rate of Procambarus clarkii, and enhanced its immune function. In addition, addition of Antarctic krill to feed helps to enhance the digestive and antioxidant capacities of aquatic animals. For example, addition of Antarctic krill shell powder to feed significantly improved the growth performance of Macrobrachium nipponense and enhanced its digestive and antioxidant capacities (Yan et al., 2023). Similar results were reported in studies on Litopenaeus vannamei and Portunus trituberculatus (Guo et al., 2022; Li et al., 2023). Therefore, feeding with Antarctic krill has a promoting effect on the growth performance, antioxidant and digestive enzyme activity, and immune function of aquatic animals.
The red claw crawfish (Cherax quadracarinatus) belongs to the family Parastacidae and genus Cherax and is native to northern Australia. It is highly favored by consumers for its appealing taste, high meat yield, and rich muscle nutrition (Wang et al., 2019; Sun et al., 2023). Current research on C. quadracarinatus mainly focuses on farming methods (Huang et al., 2024a), environmental factors (Huang et al., 2024b), and immunogenomics (Liu et al., 2025); however, studies on its nutritional requirements remain limited. The majority of current nutritional research has focused on exploring effects of protein (Wu, 2019), fat levels (Chen et al., 2022), and trace additives (Kong et al., 2024) in feed on growth and reproduction. There is a lack of research on nutritional supplements, particularly on the effects of Antarctic krill nutrition on red claw crayfish growth, which, to the best of our knowledge, has not previously been reported. Therefore, this study aimed to explore the effects of frozen Antarctic krill meat as a nutritional supplement on the growth performance, muscle nutrition, immune function, and gut microbiota structure of red claw crayfish to provide a theoretical basis for nutritional research on C. quadracarinatus.
2 Materials and methods
2.1 Experimental diets and design
Frozen Antarctic krill meat was provided by Hengzhao Lanlong (Guangdong) Aquatic Co., Ltd. (Jiangmen, Guangdong, China), and its nutritional composition is shown in Table 1. Basal feed was purchased from Charoen Pokphand Aquaculture Co., Ltd. (Yangjiang, Guangdong, China), with the main raw materials being fishmeal, soybean meal, flour, and fish oil, etc. Crude protein, crude fat, crude ash, and moisture contents in this feed were 35.0%, 4.0%, 16.0%, and 12.0%, respectively. To simulate the actual practice of supplementary feeding in aquaculture production, this study adopted an alternating feeding strategy. To avoid uneven feeding among redclaw crayfish individuals due to low single-feeding amounts and physiological issues caused by excessive daily protein intake from high single-feeding amounts, the feeding amount of Antarctic krill per session was controlled at 50% of the daily total feeding amount. The experiment included a control group (C) fed with basal feed throughout the experiment; Experimental Group 1 (E1) fed with basal feed for 2 d followed by 1 d of feeding with mixed feed (50% Antarctic krill meal [AKM[+ 50% basal feed); Experimental Group 2 (E2): fed with basal feed for 1 d followed by 1 d of feeding with mixed feed (50% AKM + 50% basal feed), and; Experimental Group 3 (E3) fed with mixed feed (50% AKM + 50% basal feed) daily for a total of 10 weeks of rearing. The frozen krill meat is preserved using a -20°C refrigeration unit. Before feeding, the AKM should be thawed for half an hour and then mixed with commercial feed for feeding.
Table 1
| Formulation | Content |
|---|---|
| Amino acids(g/100g) | |
| Asp Thr Ser Glu Pro Gly Ala Val Met Ile Leu Tyr Phe Lys His Arg Total amino acids |
0.64 0.27 0.24 0.95 0.23 0.33 0.35 0.29 0.19 0.28 0.48 0.26 0.29 0.54 0.13 0.33 5.80 |
| Fatty acid(% of total fatty acids) | |
| C14:0 C15:0 C16:0 C16:1 C18:0 C18:1n9c C18:2n6c C18:3n3 C20:5n3 C20:4n6 C22:1n9 C22:6n3 |
6.70 1.46 25.91 2.21 2.24 12.33 4.41 3.36 17.25 2.68 1.58 26.38 |
| Proximate composition(g/100g) | |
| Protein Fat Ash Astaxanthin |
6.85 1.3 0.8 9.47 |
Nutrient composition of Antarctic krill.
2.2 Experimental animals and operation
Juvenile C. quadracarinatus were sourced from Hengzhao Lanlong (Guangdong) Aquatic Co., Ltd. (Jiangmen, Guangdong, China) and acclimated for 2 weeks prior to the experiment to adapt to the experimental environment. After acclimation, 180 red claw crayfish of uniform size (initial weight: 6.77 ± 0.78 g) were randomly allocated to 12 glass tanks (150 × 58 × 31 cm), with 15 crayfish per tank, divided into 4 groups, each with 3 replicates. During the experiment, feeding was conducted twice daily at 08:00 and 17:00, with a daily feeding rate of 4% of total body weight. Feces and leftover feed at the bottom of the tanks were removed using a siphoning method 1–2 h after feeding. Throughout the rearing period, water quality parameters were regularly monitored using an Octadem W-II water quality analyzer (Octadem Technology, Inc., Wuxi, China) to ensure stable water quality.
2.3 Sample collection and determination
Sample collection was conducted in compliance with the animal collection guidelines established by the Animal Experiment Ethics Committee of the Pearl River Fisheries Research Institute, with due regard for animal welfare. Upon completion of the 10-week rearing experiment, juvenile crayfish were subjected to a 24-h fasting period. Juvenile crayfish in each tank were then enumerated, and the survival rate was calculated. Body weight, hepatopancreas wet weight, and tail muscle weight of juvenile crayfish in each group were determined using a Mettler Toledo AL-204 analytical balance (Mettler Toledo, Inc., Shanghai, China). The hepatopancreas from 15 red claw crayfish in each group were harvested for assessments of enzyme activity and gene expression levels. Nine individual red claw crayfish from each group were sampled to determine the astaxanthin content and muscle amino acid and fatty acid composition. The remaining red claw crayfish from each group were collected for gut microbiota analysis. All tissues were rapidly frozen in liquid nitrogen and stored in a -80 °C freezer. Growth indices were calculated using the methods described by Liu et al. (2010) and Zhou et al. (2023) to determine the survival rate (SR), weight gain rate (WGR), SGR, Meat yield (MY), and hepatosomatic index (HSI). The formulas are as follows:
2.4 Biochemical analysis
Red claw crayfish liver–pancreas samples were collected for determination of antioxidant and digestive enzyme activities. Activities of superoxide dismutase (SOD), glutathione peroxidase (GSH-PX), trypsin (TPS), and lipase (LPS) in hepatopancreas tissue were measured using the WST-1, ammonium molybdate, colorimetric, microplate enzyme labeling, and microplate methods, respectively. These measurements were used to evaluate the effects of feeding Antarctic krill on antioxidant and digestive enzyme activities of red claw crayfish. All indicators were determined using kits produced by Nanjing Jiancheng Bioengineering Institute (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Sample pre-treatment, reagent preparation, and sample measurement were strictly carried out according to the manufacturer’s instructions.
2.5 Amino acid analysis in the muscle of C.quadricarinatus
The amino acid composition in the muscle was determined according to the method described in GB 5009.124-2016 (Ministry of Health and Family Planning of China, 2016b). First, the muscle of the C. quadricarinatus was ground, and 1.00 g of the homogenized muscle was taken. Then, 10 mL of 6 mol/L hydrochloric acid was added, and the mixture was frozen for 5 minutes. After vacuum treatment and nitrogen flushing, the mixture was hydrolyzed at 110°C for 22 hours. After cooling, the hydrolysate was filtered into a 50 mL volumetric flask and diluted to the mark with water. Then, 1.0 mL of the filtrate was taken and dried under reduced pressure at 50°C. The residue was dissolved in 2 mL of water, dried again under reduced pressure, and finally evaporated to dryness. The residue was dissolved in pH 2.2 sodium citrate buffer, filtered through a 0.22 μm membrane, and the resulting solution was used for analysis. The amino acid content was measured using an amino acid analyzer (Sykam S-433Dup, Germany).
The amino acid content in the sample solution was calculated using the following formula:
In which:
: Content of amino acid i in the sample solution, in nanomoles per milliliter (nmol/mL);
: Peak area of amino acid i in the sample solution;
: Peak area of amino acid s in the standard working solution;
: Content of amino acid s in the standard working solution, in nanomoles per milliliter (nmol/mL).
The content of each amino acid in the sample was calculated using the following formula:
In which:
: Content of amino acid i in the sample, in grams per 100 grams (g/100g)
: Content of amino acid i in the sample solution, in nanomoles per milliliter (nmol/mL)
F: Dilution factor
V: Volume of the hydrolyzed sample solution, in milliliters (mL)
M: Molar mass of amino acid i, in grams per mole (g/mol);
m: Sample weight, in grams (g); : Conversion factor from nanograms (ng) to grams (g);
100: Conversion factor.
2.6 Fatty acid analysis in the muscle of C. quadricarinatus
The fatty acid composition was determined according to the GB 5009.168–2016 method (Ministry of Health and Family Planning of China, 2016a). First, the muscle of the C. quadricarinatus was ground, and 3.00-5.00 g of homogenized sample was weighed and transferred into a 250 mL flat-bottom flask. Sequentially, 100 mg of pyrogallic acid and 2 mL of 95% ethanol were added and mixed thoroughly, followed by 8 mL of 2% NaOH-methanol solution. The flask was connected to a reflux condenser and refluxed in an 80°C ± 1°C water bath until oil droplets disappeared. Then, 7 mL of 15% boron trifluoride-methanol solution was added through the top of the reflux condenser, and refluxing was continued for 2 minutes. After cooling, 10 mL to 15 mL of n-heptane was added, shaken for 2 minutes, followed by the addition of saturated sodium chloride solution. The mixture was allowed to stand for phase separation. A 5 mL aliquot of the n-heptane layer was dehydrated with anhydrous Na2SO4 and analyzed by gas chromatography (HP 6890, USA). The percentage of each fatty acid relative to the total fatty acids was calculated using the area normalization method.
The percentage of a specific fatty acid () in the total fatty acids was calculated using the formula:
In which:
: Percentage of a specific fatty acid in the total fatty acids, %;
Peak area of the fatty acid methyl ester in the sample solution;
: Conversion factor from FAME ii to its corresponding fatty acid;
Sum of the peak areas of all FAMEs in the sample test solution.
2.7 Astaxanthin content determination
Muscle and shell samples (5 g) were collected from each tank and the astaxanthin content was determined according to the SN/T 2327-2009 7.2 method. (General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China, 2009). Samples were extracted using acetonitrile, defatted with n-hexane, and concentrated. Determination was carried out using a high-performance liquid chromatograph (Shanghai Zhuangrun International Trading Co., Ltd., Shanghai, China).
2.8 Immune- and molting-related gene expression
Total RNA was isolated from hepatopancreas samples using TRIzol reagent (Life technologies, USA) according to the manufacturer’s instructions. The RNA concentration, integrity, and quality were analyzed by spectrophotometry (OD260/OD280) using a Nanodrop8000 spectrophotometer (Thermo Scientific, USA) and 1.0% agarose gel electrophoresis. cDNA was synthesized using an M-MLV reverse transcription kit (Invitrogen, Waltham, MA, USA) following the manufacturer’s protocol. RNA and cDNA samples were stored at -80°C until use. Primer sequences for target and reference genes are shown in Table 2. Real-time quantitative PCR (qPCR) was performed using the Step One Plus Real-time PCR system (Applied Biosystems, Foster City, CA, USA) to evaluate immune- and molting-related gene expression levels. The reaction mixture consisted of 1 µL (50 ng/µL) cDNA, 10 µL 2×SYBR Green Premix (Universal), 1 µL (10 pmol/µL) of each primer, and 7 µL double-distilled water, with a final volume of 20 µL. The qPCR program included initial denaturation at 95°C for 30 min, followed by 35 cycles of denaturation at 95°C for 40 s, annealing at 60°C for 45 s, and extension at 72°C for 30 s, with a final extension at 72°C for 10 min. Relative gene expression levels were calculated using the 2^-ΔΔct method.
Table 2
| Primer Name | Primer Sequences (5′ –3′) | Efficiency Values | Sources |
|---|---|---|---|
|
ACTB-F ACTB-R Toll-F Toll-R HEM-F HEM-R EcR-F EcR-R RXR-F RXR-R |
CCCCATGCTATCTTGCGTCT CGTCAGGAAGCTCGTAGGAT ATGGCCTCACGGATGATACTGG TCAAGGGTTTTGTTTGGAATTTGAT ACATAGCAAGGACTGGCAAAC TGACCTCGTAAAGTGGTGGAA GGTTTCGGCACTCTTCAACG ACAGATTGCGACAAAAGCGG AGGAGATGCCGTAACCAACA ATGCTTCGGTGTGAGAAGGA |
98.5 97.6 97.4 98.8 97.9 |
MN396754.1 Li et al., 2017 Lu et al., 2021 OL963596.1 KM016907.1 |
The primer sequences used in the real-time quantitative PCR.
2.9 16S rRNA gene sequencing
Intestines of red claw crayfish from each group were collected for microbial diversity analysis. Following the method of Liu et al. (2019), genomic DNA was extracted from samples, and the V3 + V4 region of the 16S rDNA was amplified using specific primers with barcodes. The selected primer sequences were: 341F: CCTACGGGNGGCWGCAG; 806R: GGACTACHVGGGTATCTAAT. The purified amplification products (i.e., amplicons) were connected to sequencing adaptors to construct a sequencing library, which was then sequenced on an Illumina platform (Illumina, Inc., San Diego, USA). Based on the operational taxonomic unit (OTU) sequences and abundance data, species annotation, species composition analysis, indicator species analysis, α-diversity analysis, and functional prediction of the microbial community were conducted (Liu et al., 2024).
2.10 Statistical analysis
Data are presented as the mean ± standard error (SE) of three replicates. All statistical analyses were performed using GraphPad Prism 9.5 software. First, the normality of data distribution in each group was verified by the Shapiro-Wilk test, and homogeneity of variances among groups was confirmed by the Levene test. If the data met the assumptions of normality and homogeneity of variances, one-way ANOVA was applied, followed by post hoc multiple comparisons using the Tukey test. If the variances were unequal, Welch’s ANOVA test was used. If the data violated the assumptions of normality or homogeneity of variances, the nonparametric Kruskal-Wallis test was employed. A statistically significant difference was defined when p<0.05.
3 Results
3.1 Growth performance
Growth indices of C. quadracarinatus in each group after 10 weeks of rearing are shown in Table 3. The SR of red claw crayfish in groups C, E1, E2, and E3 were 66.67 ± 0.00%, 60.00 ± 6.67%, 93.33 ± 0.00%, and 70.00 ± 3.33%, respectively, and was significantly higher in E2 than in other groups (p < 0.05); whereas, no significant differences were observed among the other three groups (p > 0.05). WGR ranged from 134.91–218.61%, with that of E1 and E2 being 218.61 ± 10.36% and 203.60 ± 11.04%, respectively, which were significantly higher than those of C (154.40 ± 7.62%) and E3 (134.91 ± 8.83%) (p < 0.05). SGR ranged from 1.22–1.67% day-1, with those of E1 and E2 being significantly higher than those of other groups (p < 0.05). Meat yield ranged from 16.29–18.32%, with that of E1 being significantly higher than that of group C (p < 0.05); whereas, no significant differences were observed between the other three groups (p > 0.05). HSI ranged from 6.61–7.90%, with no significant differences among groups (p > 0.05). The growth indices indicated that E2 exhibited better overall growth performance.
Table 3
| Parameters | Groups | |||
|---|---|---|---|---|
| C | E1 | E2 | E3 | |
| SR (%) | 66.67 ± 0.00b | 60.00 ± 6.67b | 93.33 ± 0.00a | 70.00 ± 3.33b |
| WGR (%) | 154.40 ± 7.62b | 218.61 ± 10.36a | 203.60 ± 11.04a | 134.91 ± 8.83b |
| SGR (% day-1) | 1.342 ± 0.04b | 1.67 ± 0.05a | 1.56 ± 0.05a | 1.22 ± 0.06b |
| MY(%) | 16.29 ± 0.42b | 18.32 ± 0.51a | 17.56 ± 0.56ab | 17.07 ± 0.42ab |
| HSI (%) | 7.90 ± 0.20 | 7.67 ± 0.57 | 7.47 ± 0.53 | 6.61 ± 0.21 |
Effects of Antarctic krill supplementation on growth performance in C. quadricarinatus after the 10-week feeding trial.
Data are reported as the mean ± SE of three replicates. Data with different letters were significantly different (p < 0.05) among all the treatments. SR, survival rate; WGR, weight gain rate; SGR, specific growth rate; MY, Meat yield; HIS, hepatosomatic index.
C: Basal diet.
E1: 2-day basal diet +1-day compound (50% Antarctic krill meat +50% basal diet).
E2: 1-day basal diet +1-day compound (50% Antarctic krill meat +50% basal diet).
E3: Feeding compound (50% Antarctic krill meat +50% basal diet).
3.2 Muscle amino acid composition
The amino acid composition in muscle of C. quadracarinatus from each group is shown in Table 4. A total of 16 amino acids, including 9 essential amino acids (EAA) and 7 non-essential amino acids (NEAA), were detected in crayfish muscle from all groups. The total amino acid (TAA) content ranged from 18.95–19.33 g/kg, with no significant differences among groups (p > 0.05). The EAA content ranged from 9.50–9.92 g/kg, with no significant differences among groups (p > 0.05). The NEAA content ranged from 9.45–9.60 g/kg, with no significant differences among groups (p > 0.05). EAA/TAA and NEAA/TAA ratios were both 0.50, with no significant differences among groups (p > 0.05). The glycine (Gly) content in E3 was significantly higher than that in the other three groups (p < 0.05), whereas other amino acid contents showed no significant differences among groups (p > 0.05). Overall, feeding AKM had no significant effect on the amino acid composition of C. quadracarinatus.
Table 4
| Amino acids | Groups | |||
|---|---|---|---|---|
| (g kg-1) | C | E1 | E2 | E3 |
| Threonine | 0.79 ± 0.02 | 0.80 ± 0.01 | 0.78 ± 0.02 | 0.80 ± 0.02 |
| Valine | 0.87 ± 0.03 | 0.89 ± 0.03 | 0.85 ± 0.02 | 0.87 ± 0.01 |
| Methionine | 0.44 ± 0.02 | 0.44 ± 0.01 | 0.43 ± 0.01 | 0.45 ± 0.01 |
| Isoleucine | 0.79 ± 0.01 | 0.84 ± 0.2 | 0.82 ± 0.02 | 0.84 ± 0.01 |
| Leucine | 1.55 ± 0.02 | 1.58 ± 0.01 | 1.54 ± 0.06 | 1.57 ± 0.04 |
| Phenylalanine | 0.81 ± 0.01 | 0.81 ± 0.02 | 0.82 ± 0.01 | 0.84 ± 0.01 |
| Lysine | 1.69 ± 0.04 | 1.70 ± 0.03 | 1.68 ± 0.01 | 1.70 ± 0.01 |
| Arginine | 2.15 ± 0.03 | 2.21 ± 0.02 | 2.21 ± 0.05 | 2.29 ± 0.01 |
| Histidine | 0.44 ± 0.01 | 0.45 ± 0.01 | 0.45 ± 0.02 | 0.43 ± 0.01 |
| Serine | 0.80 ± 0.03 | 0.78 ± 0.02 | 0.77 ± 0.02 | 0.79 ± 0.01 |
| Glutamic acid | 3.41 ± 0.05 | 3.36 ± 0.03 | 3.38 ± 0.10 | 3.35 ± 0.11 |
| Proline | 0.66 ± 0.02 | 0.70 ± 0.02 | 0.67 ± 0.02 | 0.67 ± 0.01 |
| Glycine | 0.91 ± 0.00b | 0.90 ± 0.01b | 0.88 ± 0.02b | 0.97 ± 0.02a |
| Alanine | 1.04 ± 0.02 | 1.05 ± 0.01 | 1.00 ± 0.02 | 1.04 ± 0.02 |
| Aspartic acid | 2.09 ± 0.07 | 2.11 ± 0.05 | 2.04 ± 0.05 | 2.06 ± 0.02 |
| Tyrosine | 0.68 ± 0.03 | 0.71 ± 0.01 | 0.71 ± 0.02 | 0.71 ± 0.01 |
| TAA | 19.18 ± 0.39 | 19.33 ± 0.16 | 18.95 ± 0.41 | 19.33 ± 0.38 |
| EAA | 9.59 ± 0.20 | 9.73 ± 0.08 | 9.50 ± 0.22 | 9.92 ± 0.01 |
| NEAA | 9.59 ± 0.19 | 9.60 ± 0.08 | 9.45 ± 0.20 | 9.60 ± 0.20 |
| EAA/TAA | 0.50 ± 0.00 | 0.50 ± 0.00 | 0.50 ± 0.00 | 0.50 ± 0.00 |
| NEAA/TAA | 0.50 ± 0.00 | 0.50 ± 0.00 | 0.50 ± 0.00 | 0.50 ± 0.00 |
Amino acid composition in muscle of C. quadricarinatus after the 10-week feeding trial.
TAA, sum of total amino acids; EAA, sum of essential amino acids, included threonine, valine, leucine, isoleucine, histidine, arginine, lysine, phenylalanine, methionine; NEAA, sum of non-essential amino acids, included serine, glutamic acid, glycine, alanine, tyrosine, proline, aspartic acid. (Mean ± SE).
Different superscripts indicate significant differences (p<0.05) or highly significant differences (p<0.01).
3.3 Muscle fatty acid compositions
Regarding muscle fatty acid composition, dietary AKM significantly influenced the fatty acid profile in C. quadricarinatus muscle tissue. The fatty acid composition in muscle of C. quadracarinatus from each group is shown in Table 5. A total of 16 fatty acids, including 5 saturated fatty acids (SFA), 4 monounsaturated fatty acids (MUFA), and 7 polyunsaturated fatty acids (PUFA), were detected in crayfish muscle from all four groups. The SFA content ranged from 28.70–31.06%, with that of the non-fed group (C) being significantly higher than that of the fed groups (E1, E2, and E3) (p < 0.05). The MUFA content ranged from 24.62–26.34%, with no significant differences among groups (p > 0.05). The n-3 PUFA content ranged from 14.92–28.52%, which increased significantly with an increasing amount of Antarctic krill feed (p < 0.05). The n-6 PUFA content ranged from 18.63–24.94%, with that of E3 being significantly lower than those of C and E1 (p < 0.05). The PUFA content ranged from 42.52–47.60%, with no significant differences among groups (p > 0.05). The EPA+DHA content ranged from 14.93–25.43%, with that of E3 being significantly higher than those of E1 and E2 (p < 0.05) and extremely significantly higher than that of C (p < 0.01).
Table 5
| Fatty acid | Groups | |||
|---|---|---|---|---|
| (% fatty acid) | C | E1 | E2 | E3 |
| C16:0 | 16.94 ± 0.51 | 16.87 ± 0.16 | 16.74 ± 0.15 | 15.73 ± 0.03 |
| C16:1 | 1.87 ± 0.07ab | 1.90 ± 0.05ab | 2.01 ± 0.08a | 1.79 ± 0.01b |
| C17:0 | 0.37 ± 0.04b | 0.41 ± 0.02ab | 0.39 ± 0.03ab | 0.54 ± 0.04a |
| C18:0 | 9.27 ± 0.23 | 9.04 ± 0.17 | 9.01 ± 0.06 | 9.00 ± 0.06 |
| C18:1n9c | 22.40 ± 0.23a | 22.00 ± 0.15a | 21.45 ± 0.05ab | 20.63 ± 0.47b |
| C18:2n6c | 21.15 ± 1.14a | 19.49 ± 0.31a | 18.00 ± 0.029ab | 14.27 ± 1.38b |
| C18:3n3 | 0.32 ± 0.01b | 0.32 ± 0.01b | 0.32 ± 0.01b | 2.51 ± 0.05a |
| C20:0 | 1.97 ± 0.09 | 1.93 ± 0.06 | 1.92 ± 0.10 | 2.03 ± 0.03 |
| C20:1 | 0.87 ± 0.01a | 0.79 ± 0.02ab | 0.82 ± 0.01ab | 0.72 ± 0.03b |
| C20:2 | 1.87 ± 0.01a | 1.65 ± 0.02b | 1.73 ± 0.0ab | 1.71 ± 0.06b |
| C20:5n3 | 10.33 ± 0.73c | 13.16 ± 0.32b | 14.17 ± 0.17b | 18.98 ± 0.01a |
| C20:4n6 | 3.79 ± 0.22ab | 3.61 ± 0.06b | 3.90 ± 0.11ab | 4.34 ± 0.19a |
| C20:3n3 | 0.56 ± 0.01 | 0.51 ± 0.01 | 0.55 ± 0.01 | 0.56 ± 0.01 |
| C22:0 | 1.56 ± 0.12 | 1.36 ± 0.04 | 1.32 ± 0.08 | 1.45 ± 0.01 |
| C22:1n9 | 0.98 ± 0.00b | 0.90 ± 0.01d | 0.92 ± 0.01c | 1.11 ± 0.01a |
| C22:6n3 | 4.61 ± 0.16c | 5.36 ± 0.07b | 5.48 ± 0.10b | 6.45 ± 0.01a |
| SFA | 31.06 ± 0.01a | 29.50 ± 0.40b | 29.40 ± 0.36b | 28.70 ± 0.09b |
| MUFA | 26.34 ± 0.06 | 25.80 ± 0.16 | 25.57 ± 0.15 | 24.62 ± 0.84 |
| n-3 PUFA | 14.92 ± 0.02d | 19.62 ± 0.01c | 20.77 ± 0.01b | 28.52 ± 0.04a |
| n-6 PUFA | 24.94 ± 0.92a | 22.78 ± 0.38a | 21.90 ± 0.40ab | 18.63 ± 1.19b |
| PUFA | 42.52 ± 0.14 | 43.68 ± 0.77 | 44.04 ± 0.77 | 47.60 ± 0.01 |
| DHA+EPA | 14.93 ± 0.10c | 18.52 ± 0.25b | 19.65 ± 0.27b | 25.43 ± 0.01a |
| n-3/n-6 PUFA | 0.58 ± 0.00d | 0.85 ± 0.00c | 0.93 ± 0.00b | 1.64 ± 0.01a |
Fatty acid composition in muscle of C. quadricarinatus after the 10-week feeding trial.
SFA, Sum of saturated fatty acids; MUFA, Sum of monounsaturated fatty acids; n-3 PUFA, Sum of n-3 polyunsaturated fatty acids; n-6 PUFA, Sum of n-6 polyunsaturated fatty acids. (Mean ± SE).
Different superscripts indicate significant differences (p<0.05) or highly significant differences (p<0.01).
3.4 Astaxanthin content in muscle and shell
The astaxanthin content in C. quadracarinatus shell and muscle is shown in Table 6. The astaxanthin content in shell ranged from 28.23–39.27 mg/kg among the groups, with E2 having a significantly higher content than C (p < 0.05) and an extremely significantly higher content than E1 and E3 (p < 0.01). The astaxanthin content in muscle ranged from 0.35–1.15 mg/kg, with that of E2 being significantly higher than those of E1 and E3 (p < 0.05) and extremely significantly higher than that of C (p < 0.01).
Table 6
| Parameters | Groups | |||
|---|---|---|---|---|
| C | E1 | E2 | E3 | |
| Shell Muscle |
31.8 ± 0.23b 0.35 ± 0.00c |
28.23 ± 0.23c 0.55 ± 0.01b |
39.27 ± 0.17a 1.15 ± 0.01a |
28.23 ± 0.23c 0.55 ± 0.01b |
Effects of AKM supplementation on content of astaxanthin in muscle and shell of C. quadricarinatus after the 10-week feeding trial (mg/kg).
Data are reported as the mean ± SE of three replicates. Data with different letters were significantly different (p < 0.05) among all the treatments.
3.5 Antioxidant capacity and digestive enzyme activities
Antioxidant enzyme activity results are shown in Figures 1A, B. The activity levels of SOD and GSH-Px ranged from 57.90–67.78 U/mgprot and 362.81–453.80 U/mgprot, respectively. Compared with the C, AKM supplementation showed no significant effects on these enzyme activities (p > 0.05). However, it should be noted that the GSH-Px activity in E1 was significantly higher than that in E3 (p < 0.05). The digestive enzyme activity results are shown in Figures 1C, D. The TPS content ranged from 2.59–4.94 U/gprot, with that of E2 being significantly higher than that of the other three groups (p > 0.05) (Figure 1C). The LPS content ranged from 0.53–1.43 U/gprot, with that of E2 being significantly higher than those of E1 and E3 (p > 0.05) and extremely significantly higher than that of C (p < 0.01) (Figure 1D).
Figure 1
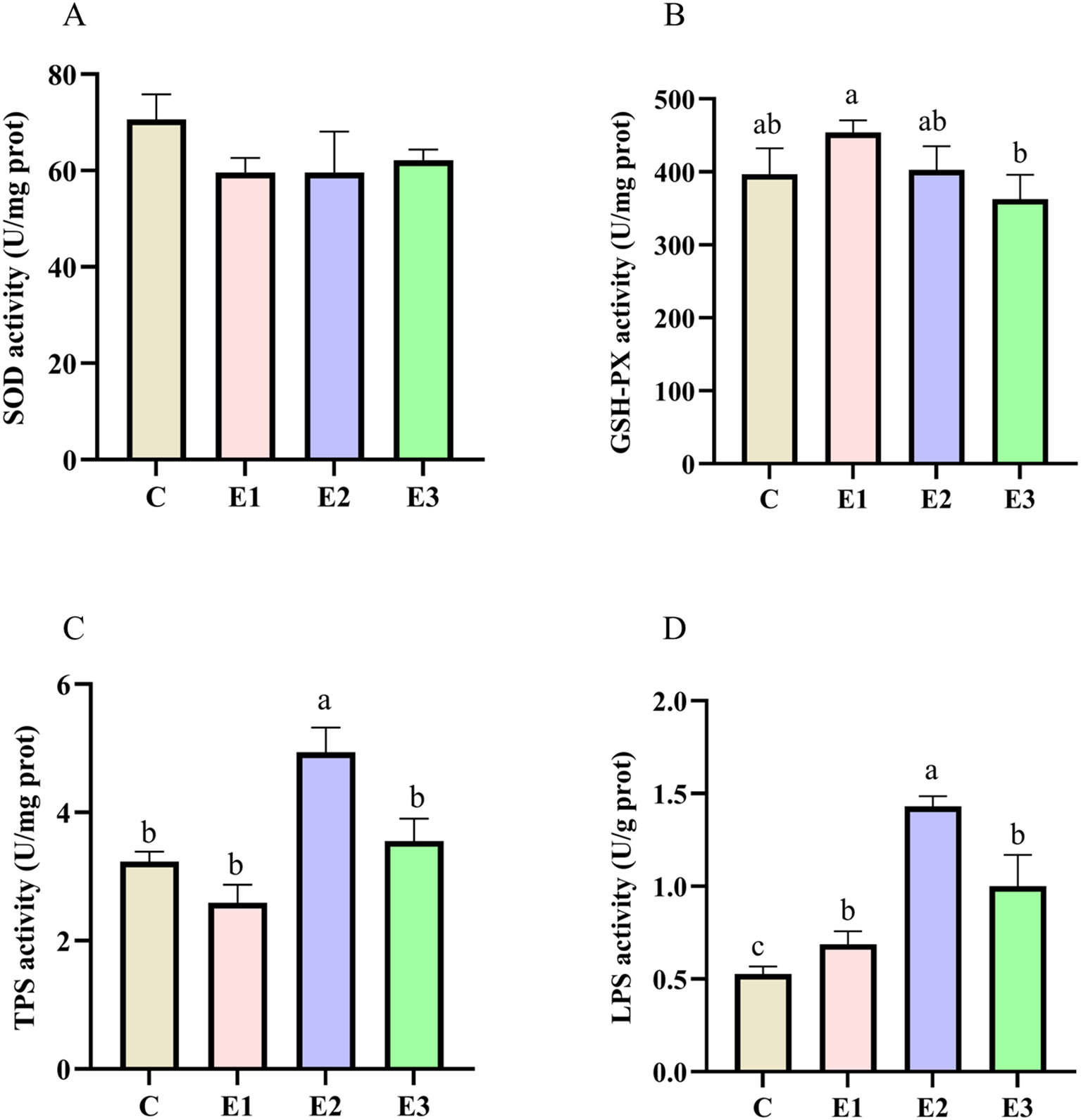
Effects of feeding different amounts of AKM on antioxidant and digestive enzyme activity in hepatopancreas of (C) quadricarinatus. (A) superoxide dismutase (SOD), (B) glutathione peroxidase (GSH-XP), (C) trypsin (TPS), (D) lipase (LPS). Different letters above the error line indicate significant differences (P < 0.05).
3.6 Immune- and molting-gene expression
Immune gene expression results in crayfish are shown in Figures 2A, B. The Expression levels of the Toll gene in E1 and E2 were significantly higher than those in C and E3 (p < 0.05) (Figure 2A). Expression level of the hemocyanin (HEM) gene in E2 was significantly higher than that in the other three groups, with no significant differences among the remaining three groups (p > 0.05) (Figure 2B). The results of molting-related genes are shown in Figures 2C, D. The EcR expression level in E2 was significantly higher than that in E1 (p < 0.05) and extremely significantly higher than that in C and E3 (p < 0.01) (Figure 2C); the retinoid X receptor gene (RXR) expression level in E2 was significantly higher than that in the other three groups, with no significant differences among the remaining three groups (p > 0.05) (Figure 2D).
Figure 2
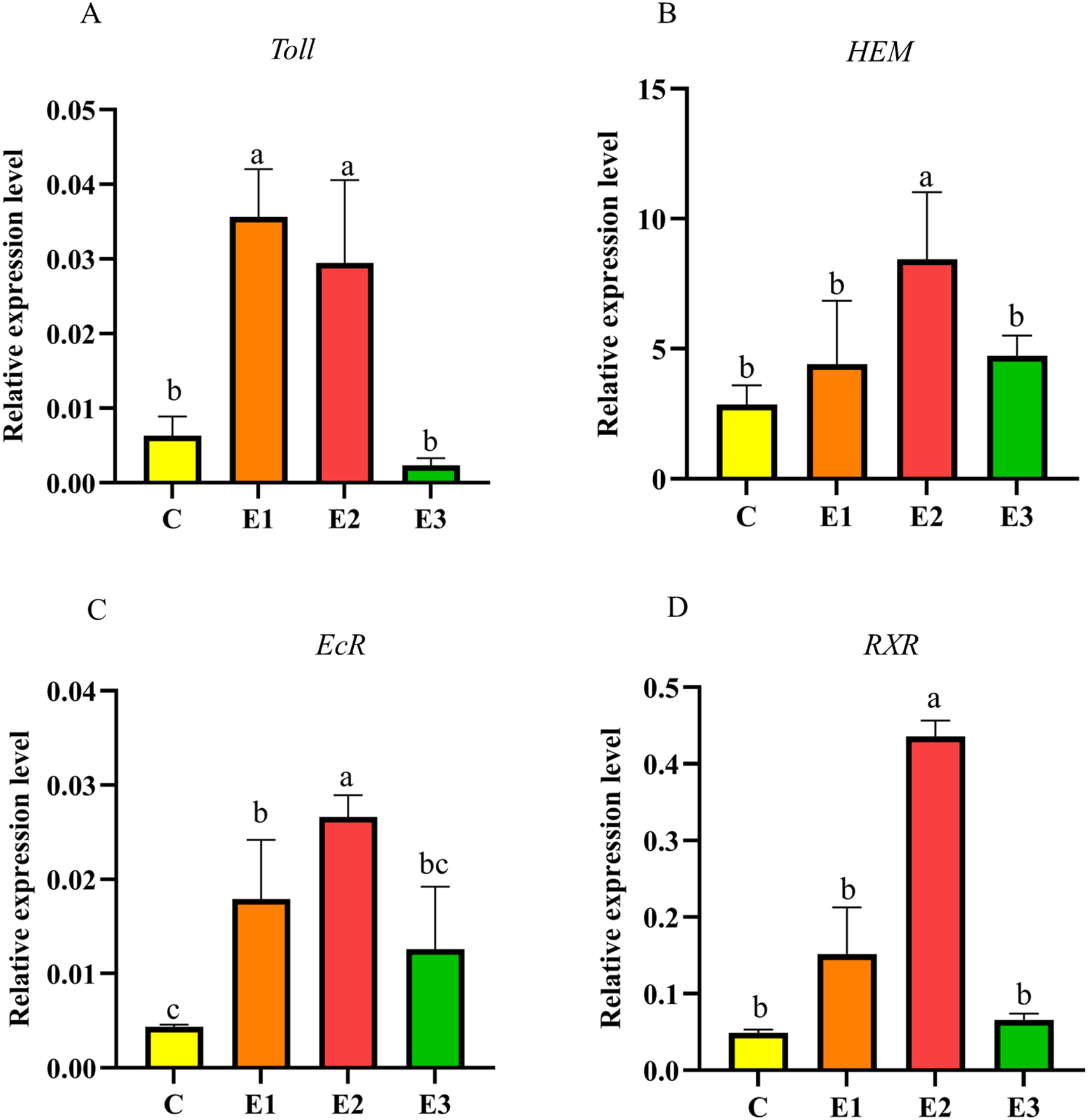
Effects of feeding different amounts of AKM on immunity, and molting gene expression levels in hepatopancreas of C. quadricarinatus. (A)Toll; (B)HEM, hemocyanin; (C)EcR, ecdysone receptor; (D)RXR, retinoic acid receptor. Different letters above the error line indicate significant differences (P < 0.05).
3.7 Intestinal microbiota structure
α-diversity results of intestinal microbiota in the four groups, analyzed using non-parametric tests, are shown in Table 7. The Chao1 index ranged from 460.80–700.90 among the groups, with that of C and E2 being significantly higher than that of E1 (p < 0.05) and showing no significant difference compared to that of E3 (p > 0.05). The Shannon index ranged from 3.39–5.87 and the Simpson’s index ranged from 0.73–0.94, with no significant differences among the four groups (p > 0.05). The coverage index for sequencing of gut samples from red claw crayfish in each group was 0.99, demonstrating the high reliability of the obtained diversity profiles. After high-throughput sequencing analysis, a total of 2,833 OTUs were identified in the gut of the four groups, among which 271 OTUs were common to all four groups. The number of unique OTUs in C, E1, E2, and E3 was 282, 119, 169, and 109, respectively (Figure 3A). The top 10 phyla in relative abundance in intestinal microbiota of the four groups are shown in Figure 3B. At the phylum level, the common dominant phyla in the gut of the four groups (relative abundance > 10%) were Firmicutes and Proteobacteria. The Firmicutes abundance in E1 was significantly higher than that in E3 (p < 0.05) and extremely significantly higher than that in C and E2 (p < 0.01); In contrast, the Proteobacteria abundance in E1 was significantly lower than that in the other three groups (p < 0.05). In addition, C had a unique dominant phylum, i.e., Actinobacteriota (10.47%), and E2 had a unique dominant phylum, i.e., Bacteroidota (16.03%). E1 and E3 did not have any unique dominant phyla. The top 10 genera in relative abundance in the gut microbiota of the four groups are shown in Figure 3C. Genera with a relative abundance > 10% were considered dominant. In C, dominant genera were Candidatus_Bacilloplasma (12.65%), Hypnocyclicus (11.32%), and Vibrio (11.09%). In E1, dominant genera were Candidatus_Hepatoplasma (27.06%) and Candidatus_Bacilloplasma (15.51%). In E2, dominant genera were Aeromonas (12.86%) and Flavobacterium (11.04%). In E3, dominant genera were Acinetobacter (20.16%) and Candidatus_Bacilloplasma (16.13%). There were no common dominant genera among the four groups.
Table 7
| Alpha diversity indices | Groups | |||
|---|---|---|---|---|
| C | E1 | E2 | E3 | |
| Chao1 | 700.90 ± 46.63a | 460.80 ± 41.99b | 671.70 ± 59.60a | 546.40 ± 25.24ab |
| Shannon | 5.252 ± 1.02 | 3.39 ± 0.74 | 5.87 ± 0.29 | 4.86 ± 0.13 |
| Simpson | 0.88 ± 0.04 | 0.73 ± 0.14 | 0.94 ± 0.01 | 0.89 ± 0.01 |
| Coverage | 0.99 ± 0.00 | 0.99 ± 0.00 | 0.99 ± 0.00 | 0.99 ± 0.00 |
Alpha diversity indices of intestinal microbiota of C. quadricarinatus after the 10-week feeding trial.
Data are reported as the mean ± SE of three replicates. Data with different letters were significantly different (p < 0.05) among all the treatments.
Figure 3
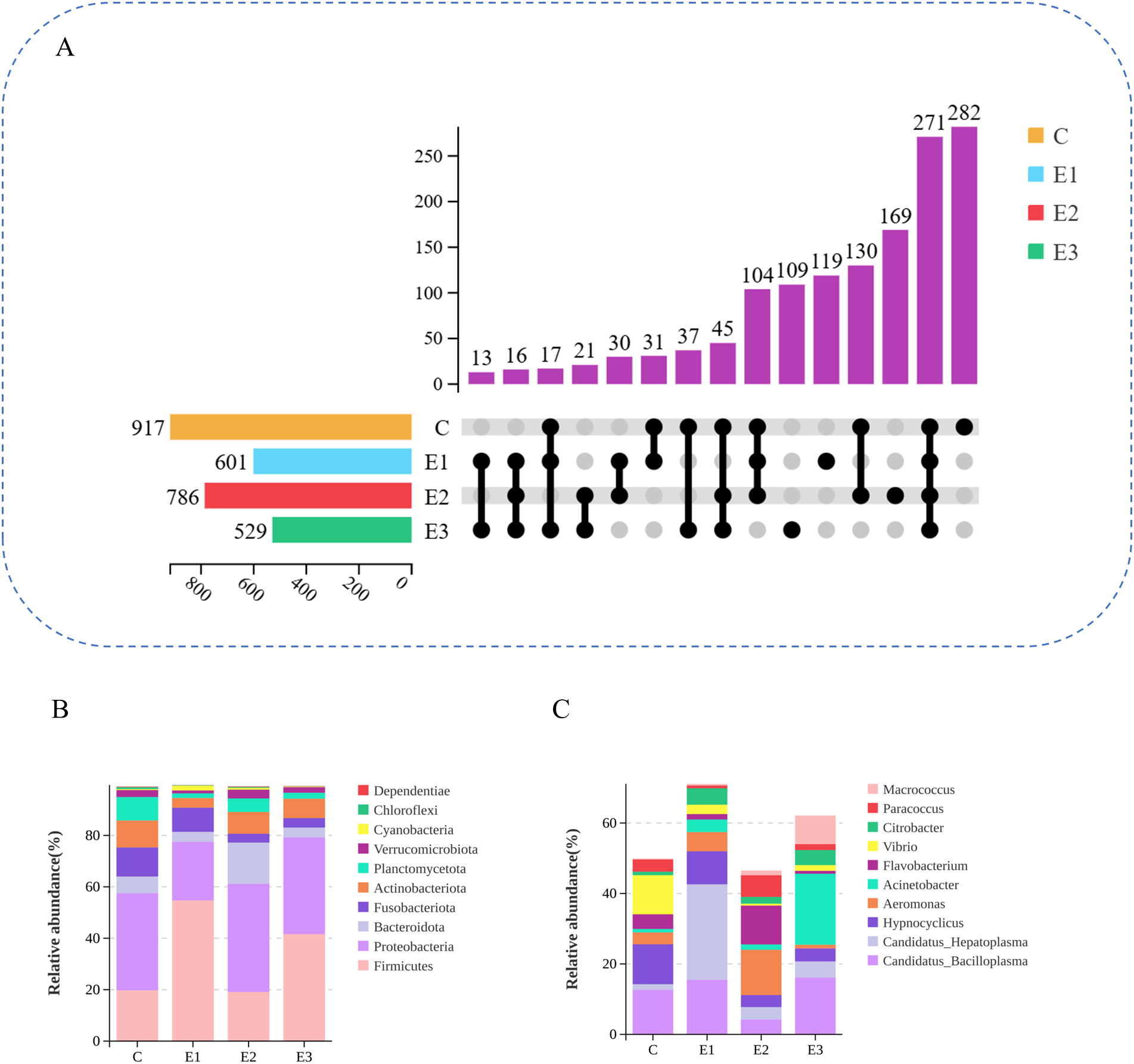
Effects of feeding different amounts of AKM on intestinal microbiota of C. quadricarinatus. (A) Upset map of shared and specific OTUs among four groups. (B) The relative abundance of top 10 intestinal bacterial at phylum level. (C) The relative abundance of top 10 intestinal bacterial at genus level.
4 Discussion
Antarctic krill has gained attention from aquaculture nutrition researchers in recent years due to its rich astaxanthin, EPA, and DHA contents, which are essential nutrients for aquatic animals. Currently, studies have reported that addition of an appropriate amount of Antarctic krill to feed can significantly improve the growth performance of aquatic animals such as Salmo salar L (Olsen et al., 2006), Oncorhynchus mykiss (Wei et al., 2019), gilthead seabream (Saleh et al., 2018), and D. labrax (Torrecillas et al., 2021). However, feeding excessive amounts of Antarctic krill is not necessarily beneficial for growth. For example, when feed of M. nipponense and Verasper variegatus is supplemented with excessive Antarctic krill, it can lead to a decrease in SGR (Yan et al., 2018, Yan et al., 2023). The results of the present study showed that feeding AKM once every 3 d or once every 2 d enhanced the growth performance of red claw crayfish, whereas feeding a high dose (i.e., daily feeding) of AKM did not promote its growth. Antarctic krill is rich in palatable components such as nucleotides, Gly, and glutamate (Olsen et al., 2006; Hatlen et al., 2017; Yan et al., 2018). We speculated that these palatable components can enhance the appetite of crayfish, thereby promoting their growth. In addition, Antarctic krill is also rich in fatty acids such as EPA and DHA, as well as astaxanthin (Araújo et al., 2019; Deng et al., 2024). Its unique nutritional substances are beneficial for EPA, DHA, and astaxanthin intake by crayfish, thus promoting their growth. According to the nutritional analysis by Sands et al. (1998), Antarctic krill is rich in chitin and fluoride, and it has been proven that high chitin and fluoride intake by aquatic animals can negatively affect their growth performance (Sands et al., 1998; Shiau and Yu, 1999; Julshamn et al., 2004; Yoshitomi et al., 2006; Yoshitomi and Nagano, 2012). Therefore, this study suggested that feeding AKM once every 3 d or once every 2 d is beneficial for C. quadracarinatus growth, whereas daily feeding of AKM does not produce beneficial effects on growth and other indicators.
The nutritional value of aquatic food has been a hot topic of concern for many years. Analyzing the fatty acid composition and unique nutritional substances in food is a means of evaluating its nutritional value. From the perspective of human fatty acid requirements, excessive SFA intake can increase the risk of coronary heart disease (Mensink, 2013); therefore, compared to SFA, PUFA is more in line with human nutritional needs. n-3 PUFA has been proven to be beneficial for human health and growth. In particular, EPA and DHA play positive roles in infant development, cancer prevention, cardiovascular disease control, and treatment of mental illnesses. Since they cannot be synthesized in the human body (Riediger et al., 2008), they must be obtained through food intake (Ruxton et al., 2004). Current research has shown that adding Antarctic krill to feed can increase the EPA and DHA levels in crustaceans such as L. vannamei and P. trituberculatus (Ambasankar et al., 2022; Guo et al., 2022). The present study found that with the increase of AKM feeding, the n-3PUFA, EPA, and DHA contents in muscle of C. quadracarinatus increased, and the SFA content decreased (Table 5). This indicated that feeding AKM effectively promoted intake of n-3PUFA, particularly EPA and DHA, in C. quadracarinatus, while reducing the SFA content, thereby enhancing the nutritional value of its muscle. Antarctic krill is also rich in natural astaxanthin, which is a nutrient with extensive health benefits for the human body, has been proven to prevent various human diseases, including diabetes and intestinal diseases, and has a positive promoting effect on human immune response, brain function, and skin health (Guerin et al., 2003; Hussein et al., 2006; Visioli and Artaria, 2017). Analysis of astaxanthin content showed that feeding AMK increased the astaxanthin content in C. quadracarinatus muscle (Table 6), indicating that feeding AKM was conducive to astaxanthin absorption by C. quadracarinatus. Therefore, this study suggested that feeding AKM can improve the fatty acid composition of C. quadracarinatus muscle and increase the astaxanthin content in its muscle, thereby enhancing the nutritional value of C. quadracarinatus muscle.
The hepatopancreas is an important part of the crustacean digestive system, and its digestive enzyme activity is an important indicator for evaluating the digestive function of the organism. It can reflect the ability of an organism to digest and absorb nutrients (Yan et al., 2023). TPS is among the important digestive enzymes in the hepatopancreas of crustaceans, and its activity level reflects the ability of an organism to break down and utilize feed protein (Dali et al., 2024). LPS is involved in regulating fat metabolism and can promote absorption of energy required for growth and development of the organism (Olivecrona, 2016). Previous studies have shown that adding an appropriate amount of Antarctic krill to feed can enhance TPS activity in M. nipponense and promote TPS secretion in Seriola quinqueradiata (Morimoto Kofuji et al., 2006; Yan et al., 2023). This demonstrates that adding Antarctic krill to feed is beneficial for improving the ability of aquatic animals to utilize proteins. The present study found that feeding AKM every 2 d (E2) enhanced TPS activity (Figure 1C), indicating that feeding AKM every 2 d is beneficial for C. quadracarinatus to utilize protein in feed. Analysis of LPS activity in C. quadracarinatus (Figure 1D) showed that with the increase of AKM feeding, LPS activity first increased and then decreased, and was significantly higher than that in the non-fed group, proving that feeding AKM was conducive to improving the ability of C. quadracarinatus to break down and utilize fat in feed. Moreover, feeding AKM every 2 d had a more obvious effect on its ability to utilize fat in feed. In addition, antioxidant enzyme activity results (Figures 1A, B) showed that feeding AKM resulted in no significant difference in the antioxidant enzyme activity of C. quadracarinatus (p > 0.05), indicating that feeding AKM did not significant affect its antioxidant capacity.
Since crustaceans lack specific immunity, when facing pathogen invasion, they completely rely on non-specific immune reactions to enhance their resistance (Liu et al., 2020). The non-specific immune system of crustaceans can resist pathogen invasion by regulating immune-related gene expression (Fredrick and Ravichandran, 2012). Therefore, immune-related gene expression levels are often used to evaluate the immune capacity of an organism. Toll plays an important role in the non-specific immunity of crustaceans as it can regulate antimicrobial peptide production to combat pathogenic bacteria (Akira, 2001; Li et al., 2017). For example, when the Toll gene of C. quadracarinatus is silenced, expression of the antimicrobial peptide ALF decreases, leading to enhanced transcription and replication of white spot syndrome virus (WSSV), proving that the Toll plays a significant role in the immune process of C. quadracarinatus (Li et al., 2017). The results of the present study showed that the Toll expression levels in E1 and E2 were significantly higher than those in the non-fed and daily-fed groups (p < 0.05) (Figure 2A), indicating that feeding AKM every 2 or 3 d is beneficial for enhancing the immune response capability of red claw crayfish. In addition, HEM has multiple immune functions such as antiviral, antibacterial, and hemolytic activities, and is also a factor used to assess the non-specific immune response of crustaceans (Destoumieux-Garzón et al., 2001; Zhuang et al., 2015). In L. vannamei, the HEM expression level increases and then decreases with an increase in Antarctic krill feeding, and it is significantly enhanced when 4% and 6% Antarctic krill powder are added to the feed, indicating that feeding an appropriate amount of Antarctic krill is beneficial for enhancing the immune response of the organism (Ambasankar et al., 2022). The present study found that the HEM expression pattern in the hepatopancreas of C. quadracarinatus after feeding AKM was consistent with previous research on L. vannamei, and the effect was most significant when feeding AKM every 2 d (Figure 2B), indicating that feeding AKM every 2 d is beneficial for enhancing the immune response of the organism. Molting is an important physiological process in the entire life cycle of crustaceans and is of great significance to their growth, development, and reproduction. Molting in crustaceans is regulated by multiple factors, among which the ecdysteroid receptor gene (EcR) and retinoid X receptor(RXR) are important regulatory factors (Priya et al., 2009; Techa and Chung, 2013). The present study found that feeding AKM every 2 d significantly increased the EcR and RXR gene expression levels in C. quadracarinatus (p < 0.05), indicating that feeding AKM every 2 d is beneficial for enhancing the molting ability of C. quadracarinatus. Therefore, this study suggested that feeding AKM every 2 d is beneficial for improving the immune response and enhancing the molting ability of C. quadracarinatus.
Aquatic animals have a rich microbial community in their intestines, the structural characteristics of which can affect physiological activities such as nutrient absorption, energy regulation, and metabolism of the host organism. A stable and healthy microbial community structure is beneficial for promoting the growth performance of aquatic animals (He et al., 2018; Sehnal et al., 2021). Analyzing the microbial community diversity and structure is a common method for assessing the structural characteristics of the intestinal microbiota. Currently, indices such as Chao1, Shannon, and Simpson’s are commonly used to evaluate intestinal microbiota diversity. Chao1 and Shannon are indicators of microbial diversity and richness, and Simpson’s is a parameter used to determine the evenness of microbial composition (Hildebrand et al., 2013). The diversity results of the intestinal microbiota of red claw crayfish in the present study (Table 7) showed that, except for the Chao1 index of the group fed AKM every 3 d being lower than that of the non-fed group and the group fed every 2 d, there were no significant differences in Shannon and Simpson’s indices among the four groups (p > 0.05). Although feeding AKM every 3 d had a slight effect on the intestinal microbiota diversity of C. quadracarinatus, based on the overall assessment of Shannon and Simpson’s indices, it was concluded that feeding AKM did not significantly affect the intestinal microbiota diversity. In addition, structural analysis of phyla and genera is commonly used to assess intestinal microbiota characteristics. Analysis of intestinal phyla in the present study found that the common dominant phyla among the four groups were Firmicutes and Proteobacteria. Firmicutes play an important role in promoting nutrient utilization in the intestines of aquatic animals and maintaining stable intestinal function, which has been verified by analysis of the intestinal microbiota structure of P. clarkii (Smriga et al., 2010; Zhang et al., 2018, Zhang et al., 2021; Liu et al., 2024). Phylum Proteobacteria includes many pathogens, such as Escherichia coli, Vibrio, and Helicobacter pylori. A high abundance of Proteobacteria can easily cause intestinal digestive and absorptive disorders (Xiao et al., 2023). According to previous research, Firmicutes and Proteobacteria are commonly found in the intestines of crustaceans such as P. clarkii and Macrobrachium rosenbergii, and play a role in nutrient utilization in crustaceans (Xie et al., 2021; Liu et al., 2024). In addition, at the phylum level, it was found that only the group fed AKM every other day had a unique dominant phylum (Bacteroidetes) (relative abundance > 10%) (Figure 3B). It has been reported that Bacteroidetes participate in carbohydrate and fiber decomposition in the digestive tract of aquatic animals, thereby promoting digestion and nutrient absorption in the host (Zhou et al., 2022). Therefore, we speculate that feeding AKM every other day is conducive to increasing the Bacteroidetes abundance in the intestine, thereby enhancing the intestinal digestive function of C. quadracarinatus. Although there were no common dominant genera among the four groups at the genus level, Vibrio abundance in intestines of crayfish in the unfed group was significantly higher than that in intestines of crayfish in all fed groups (p < 0.05) (Figure 3C). Since Vibrio is the most common pathogenic bacterium in aquatic animals (Xiong et al., 2017; Baron et al., 2020), we speculate that feeding AKM can reduce the risk of bacterial disease infection in C. quadracarinatus.
5 Conclusion
Antarctic krill, rich in nutrients, is commonly used as a high-quality nutritional supplement in aquatic animal feed. This study compared different AKM feeding strategies during juvenile C.quadricarinatus cultivation. The results revealed that AKM feeding not only enhanced the content of fatty acids such as EPA and DHA, as well as astaxanthin in the muscle, but also reduced the abundance of Vibrio genus in the intestine. Particularly, feeding AKM every two days significantly improved growth performance including SR, WGR, and SGR, while enhancing digestive capacity, immune function, and molting capability. In contrast, daily AKM feeding demonstrated weaker comprehensive effects on growth performance and physiological function promotion. In conclusion, the two-day interval AKM feeding strategy shows potential for improving growth performance and nutritional quality in C.quadricarinatus, though dosage control should be emphasized to avoid potential negative effects from excessive supplementation. This study provides scientific guidance for practical aquaculture practices.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Laboratory Animal Ethics Committee Pearl River Fisheries Research Institute, CAFS. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
ZM: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. JW: Conceptualization, Funding acquisition, Writing – review & editing. YW: Conceptualization, Project administration, Writing – review & editing. ZZ: Resources, Writing – review & editing. FL: Formal Analysis, Writing – review & editing. HL: Formal Analysis, Writing – review & editing. QS: Formal Analysis, Writing – review & editing. KH: Data curation, Writing – review & editing. QZ: Methodology, Writing – review & editing. TJ: Formal Analysis, Writing – review & editing. TY: Methodology, Writing – review & editing. LY: Conceptualization, Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Scientific Institution Basal Research Fund, PRFRI (2024SJRC1), China ASEAN Maritime Cooperation Fund (No. CAMC-2018F), the special fund for seed industry revitalization project of Guangdong Province (2022-SPY-00-008), the Central Public-Interest Scientific Institution Basal Research Fund, CAFS (2022SJ-XT1, 2020TD35, 2020ZJTD01), Guangdong Rural Revitalization Strategy Special Provincial Organization and Implementation Project Funds (2022-SBH-00-001), National Freshwater Genetic Resource Center (FGRC18537).
Acknowledgments
We thank to Hengzhao Lanlong Aquatic Products Co., Ltd. for providing the juvenile shrimp, experimental site, and facilities for this study, and thank Editage’s scholar for English language editing.
Conflict of interest
Author ZZ was employed by the company Hengzhao Lanlong Aquatic Products Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Akira S. (2001). Toll-like receptors and innate immunity. Adv. Immunol.78, 1–56. doi: 10.1016/S0065-2776(01)78001-7
2
Ambasankar K. Dayal J. S. Vasagam K. K. Sivaramakrishnan T. Sandeep K. P. Panigrahi A. et al . (2022). Growth, fatty acid composition, immune-related gene expression, histology and haematology indices of Penaeus vannamei fed graded levels of Antarctic krill meal at two different fishmeal concentrations. Aquaculture533, 738069. doi: 10.1016/j.aquaculture.2022.738069
3
Araújo B. C. Mata-Sotres J. A. Viana M. T. Tinajero A. Braga A. (2019). Fish oil-free diets for Pacific white shrimp Litopenaeus vannamei: The effects of DHA-EPA supplementation on juvenile growth performance and muscle fatty acid profile. Aquaculture511, 734276–734276. doi: 10.1016/j.aquaculture.2019.734276
4
Baron S. Ceccarelli D. Dalsgaard I. Granier S. A. Haenen O. Jansson E. et al . (2020). Influence of incubation time on antimicrobial susceptibility testing of pathogenic Vibrio Anguillarum and Vibrio vulnificus isolated from fish. Aquaculture524, 735258–735258. doi: 10.1016/j.aquaculture.2020.735258
5
Chen C. Xu C. Yang X. Jia Y. Gu Z. Li E. (2022). The optimum lipid level for the juvenile redclaw crayfish Cherax quadricarinatus: Practical diets with soybean oil as the lipid source. Aquaculture Nutr.2022, 2640479. doi: 10.1155/2022/2640479
6
Dali F. A. Nurjanah N. Lioe H. N. Suhartono M. T. (2024). Biochemical characterization of trypsin from Indonesian skipjack tuna (Katsuwonus pelamis) viscera. Open Agric.9 (1), 20220308. doi: 10.1515/opag-2022-0308
7
Deng Y. Xie S. Zhan W. Peng H. Cao H. Tang Z. et al . (2024). Dietary astaxanthin can promote the growth and motivate lipid metabolism by improving antioxidant properties for swimming crab, portunus trituberculatus. Antioxidants13, 522. doi: 10.3390/antiox13050522
8
Destoumieux-Garzón D. Saulnier D. Garnier J. Jouffrey C. Bulet P. Bachere E. (2001). Crustacean immunity. Antifungal peptides are generated from the C terminus of shrimp hemocyanin in response to microbial challenge. J. Biol. Chem.276, 47070–47077. doi: 10.1074/jbc.M103817200
9
FAO/WHO (1991). Protein quality evaluation report of joint FAO/WHO expert consultation (Rome: Food and Agriculture Organization of the United Nations).
10
FAO (Food and Agriculture Organization of the UN) (2024). The State of World Fisheries and Aquaculture 2024: Blue Transformation in Action (Rome, Italy: FAO).
11
Fredrick S. W. Ravichandran S. (2012). Hemolymph proteins in marine crustaceans. Asian Pacific J. Trop. Biomedicine2, 496–502. doi: 10.1016/s2221-1691(12)60084-7
12
Gao R. Chen L. Zhang W. Zhang S. Rao J. Hu J. (2020). Effect of dietary Antarctic krill Euphausia superba on the growth performance and nonspecific immunity of red swamp crayfish Procambarus clarkia. Fish Shellfish Immunol.96, 122–125. doi: 10.1016/j.fsi.2019.12.004
13
General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China , (2009). Determination of canthaxanthin and astaxanthin in foods of animal origin for import and export (SN/T 2327-2009) (Beijing: Standards Press of China).
14
Guerin M. Huntley M. E. Olaizola M. (2003). Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol.21, 210–216. doi: 10.1016/s0167-7799(03)00078-7
15
Guo C. Jin M. Jiao L. Xie S. Zhang X. Luo J. et al . (2022). Evaluation of krill meal in commercial diets for juvenile swimming crab (Portunus trituberculatus). Aquaculture Nutr.2022 (1), 3007674. doi: 10.1155/2022/3007674
16
Hatlen B. Berge K. Nordrum S. Johnsen K. Kolstad K. Mørkøre T. (2017). The effect of low inclusion levels of Antarctic krill (Euphausia superba) meal on growth performance, apparent digestibility and slaughter quality of Atlantic salmon (Salmo salar). Aquaculture Nutr.23, 721–729. doi: 10.1111/anu.12439
17
He J. Wang P. Feng J. Lou Y. (2018). Effects of fermented soybean meal on the growth and intestinal histology and microbiota of juvenile large yellow croaker Larimichthys crocea. Acta Hydrobiologica Sin.2(5), 919–928. doi: 10.7541/2018.113
18
Hildebrand F. Nguyen T. L. A. Brinkman B. Yunta R. G. Cauwe B. Vandenabeele P. et al . (2013). Inflammation-associated enterotypes, host genotype, cage and interindividual effects drive gut microbiota variation in common laboratory mice. Genome Biol.14, 1–15. doi: 10.1186/gb-2013-14-1-r4
19
Huang L. Lu T. Lu X. Shi J. Huang Y. Du X. et al . (2024a). Comparison of the intestinal microbiota composition and function of red claw crayfish (Cherax quadricarinatus) cultured in ponds and rice fields. Fishes9, 345–345. doi: 10.3390/FISHES9090345
20
Huang C. Nie X. Wei J. Wang Y. Hong K. Mu X. et al . (2024b). Effects of light spectrum on survival, growth, physiological, and biochemical indices of redclaw crayfish (Cherax quadricarinatus) juveniles. Aquaculture Res. 2024 (1), 8897473. doi: 10.1155/2024/8897473
21
Hussein G. Sankawa U. Goto H. Matsumoto K. Watanabe H. (2006). Astaxanthin, a carotenoid with potential in human health and nutrition. J. Natural Products69, 443–449. doi: 10.1021/np050354
22
Julshamn K. Malde M. K. Bjorvatn K. Krogedal P. (2004). Fluoride retention of Atlantic salmon (Salmo salar) fed krill meal. Aquaculture Nutr.10, 9–13. doi: 10.1046/j.1365-2095.2003.00273.x
23
Kong D. Ji Y. Li K. Wang Q. Liao Z. Zhang L. et al . (2024). Effects of Zn-MHA on growth performance, digestive enzyme activity and antioxidant capacity of Australian red claw crayfish (Cherax quadricarinatus). J. Economic Anim., 1–8.
24
Li Y. Chen X. Lin Y. Chen F. Ma Y. Liu P. (2017). CqToll participates in antiviral response against white spot syndrome virus via induction of anti-lipopolysaccharide factor in red claw crayfish Cherax quadricarinatus. Dev. Comp. Immunol.74, 217–226. doi: 10.1016/j.dci.2017.04.020
25
Li W. Wang H. Shan H. Gao C. Li J. (2023). Effects of dietary supplementation with powder of Antarctic krill (Euphausia superba) on growth performance, body composition and color of Litopenaeus vannamei. South China Fisheries Sci.19, 97–105. doi: 10.12131/20220074
26
Liu S. Cao J. Huang Y. Zhao H. Lan H. Yan J. et al . (2010). Effects of replacement of dietary fish oil by soybean oil on growth performance and hepatosomatic index in Litopenaeus vannamei. J. South China Agric. Univ.31, 95–99.
27
Liu F. Qu Y. Geng C. Wang A. Zhang J. Chen K. et al . (2020). Effects of hesperidin on the growth performance, antioxidant capacity, immune responses and disease resistance of red swamp crayfish (Procambarus clarkii). Fish Shellfish Immunol.99, 154–166. doi: 10.1016/j.fsi.2020.02.014
28
Liu X. Wang B. Li Y. Wang L. Liu J. (2018). Effects of dietary botanical and synthetic astaxanthin on E/Z and R/S isomer composition, growth performance, and antioxidant capacity of white shrimp, Litopenaeus vannamei, in the nursery phase. ISJ-Invert Surviv J.15, 131–140. doi: 10.25431/1824-307X/isj.v15i1.131-140
29
Liu M. Wei J. Hong K. Wang Y. Liu F. Zhu X. et al . (2024). Comparison of intestinal flora structure, nutritional components and muscle flavor of Macrobrachium rosenbergii under two culture modes. J. Shanghai Ocean Univ.33(5), 1132–1143. doi: 10.12024/jsou.20231204366
30
Liu L. Yang L. Jiang Q. Wu W. Liu H. (2025). Proteomic analysis of global protein acetylation in response to WSSV infection in a crustacean Cherax quadricarinatus. Aquaculture596, 741754–741754. doi: 10.1016/j.aquaculture.2024.741754
31
Liu B. Zhou Q. Miao L. Liang H. Sun C. Zheng X. et al . (2022). Research progress in nutrition and immunity of aquatic animals. J. Fisheries China46, 1761–1775. doi: 10.11964/jfc.20220613568
32
Lu Y. Zheng P. Zhang X. Wang L. Li J. Zhang Z. et al . (2021). Effects of dietary trehalose on growth, trehalose content, non-specific immunity, gene expression and desiccation resistance of juvenile red claw crayfish (Cherax quadricarinatus). Fish Shellfish Immunol.119, 524–532. doi: 10.1016/J.FSI.2021.10.043
33
Mai K. Wei Y. Wang J. Zhang W. (2016). Main nutrient compositions of Antarctic Krill and its application in aqua-feeds. Periodical Ocean Univ. China46(10), 1–15. doi: 10.16441/j.cnki.hdxsb.20160337
34
Mensink R. P. (2013). “Fatty acids: Health effects of saturated fatty acids,” in Encyclopedia of Human Nutrition, (America: Elsevier) 215–219. doi: 10.1016/b978-0-12-375083-9.00101-x
35
Ministry of Health and Family Planning of China (2016a). Food safety national standard: Determination of amino acids in food (GB 5009.168-2016) (Beijing: Standards Press of China).
36
Ministry of Health and Family Planning of China (2016b). Food safety national standard: Determination of amino acids in food (GB 5009.124-2016) (Beijing: Standards Press of China).
37
Morimoto Kofuji P. Y. Hosokawa H. Masumoto T. (2006). Effects of dietary supplementation with feeding stimulants on yellowtail Seriola quinqueradiata (Temminck Schlegel; Carangidae) protein digestion at low water temperatures. Aquaculture Res.37, 366–373. doi: 10.1111/j.1365-2109.2005.01435.x
38
Olivecrona G. (2016). Role of lipoprotein lipase in lipid metabolism. Curr. Opin. Lipidology27, 233–241. doi: 10.1097/MOL.0000000000000297
39
Olsen R. E. Suontama J. Langmyhr E. Mundheim H. Ringø E. Melle W. et al . (2006). The replacement of fish meal with Antarctic krill, Euphausia superba in diets for Atlantic salmon, Salmo salar. Aquaculture Nutr.12, 280–290. doi: 10.1111/j.1365-2095.2006.00400.x
40
Priya T. A. J. Li F. Zhang J. Wang B. Zhao C. Xiang J. (2009). Molecular characterization and effect of RNA interference of retinoid X receptor (RXR) on E75 and chitinase gene expression in Chinese shrimp Fenneropenaeus chinensis. Comp. Biochem. Physiology Part B153, 121–129. doi: 10.1016/j.cbpb.2009.02.009
41
Riediger N. D. Othman R. A. Suh M. Moghadasian M. H. (2008). A systemic review of the roles of n-3 fatty acids in health and disease. J. Am. Dietetic Assoc.109, 668–679. doi: 10.1016/j.jada.2008.12.022
42
Ruxton C. H. S. Reed S. C. Simpson M. J. A. Millington K. J. (2004). The health benefits of omega-3 polyunsaturated fatty acids: a review of the evidence. J. Hum. Nutr. Dietetics17, 449–459. doi: 10.21037/jphe.2017.03.04
43
Saleh R. Burri L. Benitez-Santana T. Turkmen S. Castro P. Izquierdo M. (2018). Dietary krill meal inclusion contributes to better growth performance of gilthead seabream juveniles. Aquaculture Res.49, 3289–3295. doi: 10.1111/are.13792
44
Sands M. Nicol S. McMinn A. (1998). Fluoride in Antarctic marine crustaceans. Marine Biol.132, 591–598. doi: 10.1007/s002270050424
45
Sehnal L. Brammer-Robbins E. Wormington A. M. Blaha L. Bisesi J. Larkin I. et al . (2021). Microbiome composition and function in aquatic vertebrates: small organisms making big impacts on aquatic animal health. Front. Microbiol.12. doi: 10.3389/fmicb.2021.567408
46
Shi W. Shao C. Liu Y. Li W. Qu Y. Zhu G. (2023). Comparison of nutrient qualities of Antarctic Krill harvested in different months. J. Guangdong Ocean Univ.43, 100–105. doi: 10.3969/j.issn.1673-9159.2023.05
47
Shiau S. Yu Y. (1999). Dietary supplementation of chitin and chitosan depresses growth in tilapia, Oreochromis niloticus× O. aureus. Aquaculture179, 439–446. doi: 10.1016/s0044-8486(99)00177-5
48
Smriga S. Sandin S. A. Azam F. (2010). Abundance, diversity, and activity of microbial assemblages associated with coral reef fish guts and feces. FEMS Microbiol. Ecol.73, 31–42. doi: 10.1111/j.1574-6941.2010.00879.x
49
Sun L. Li Q. Jiang J. Chen J. Gao L. Guo J. (2023). Analysis on phenotypic traits and muscle nutritional composition of Cherax quadricarinatus in different specifications. Oceanologia Limnologia Sin.54, 885–894. doi: 10.11693/hyhz20220800220
50
Techa S. Chung S. J. (2013). Ecdysone and retinoid-X receptors of the blue crab, Callinectes sapidus: Cloning and their expression patterns in eyestalks and Y-organs during the molt cycle. Gene527, 139–153. doi: 10.1016/j.gene.2013.05.035
51
Torrecillas S. Montero D. Carvalho M. Benitez-Santana T. Izquierdo M. (2021). Replacement of fish meal by Antarctic krill meal in diets for European sea bass Dicentrarchus labrax: Growth performance, feed utilization and liver lipid metabolism. Aquaculture545, 737166. doi: 10.1016/j.aquaculture.2021.737166
52
Visioli F. Artaria C. (2017). Astaxanthin in cardiovascular health and disease: mechanisms of action, therapeutic merits, and knowledge gaps. Food Funct.8, 39–63. doi: 10.1039/c6fo01721e
53
Wang G. Sun Y. Yu E. Zhang J. Li Z. Zhang K. et al . (2019). Analysis and quality evaluation of nutrient components in muscle of Cherax quadricarinatus and Procambarus clarkii. Chin. J. Anim. Nutr.31, 4339–4348. doi: 10.3969/j.issn.1006-267x.2019.09.049
54
Wang Y. Zhao L. Guo X. Zhang X. (2021). Nutritional characteristics of Antarctic Krill meal and its application in aquatic feed. Chin. J. Anim. Nutr.33, 6601–6611. doi: 10.3969/j.issn.1006-267x.2021.12.001
55
Wei Y. Chen H. Jia M. Zhou H. Zhang Y. Xu W. et al . (2019). Effects of dietary Antarctic krill Euphausia superba meal on growth performance and muscle quality of triploid rainbow trout Oncorhynchus mykiss farmed in sea water. Aquaculture509, 72–84. doi: 10.1016/j.aquaculture.2019.05.013
56
Wu G. (2019). Study on the optimum dietary protein level of Cherax Quadricarinatus at different growth stages. China Fisheries09, 78–80.
57
Xiao W. Hu B. Cui X. Cao M. Yao H. Li P. et al . (2023). Effects of egg product on growth performance, muscle nutrients, and intestinal microflora of Procambarus clarkii. South China Fisheries Sci.19(1), 116–127. doi: 10.12131/20220128
58
Xie M. Zhang S. Xu L. Jiang F. Yuan J. Wu Z. et al . (2021). The intestinal microbiota diversities of Procambarus clarkia at different sexes and growth stages. Acta Hydrobiologica Sin.45, 1243–1254. doi: 10.7541/2021.2020.130
59
Xiong J. Zhu J. Dai W. Dong C. Qiu Q. Li C. (2017). Integrating gut microbiota immaturity and disease-discriminatory taxa to diagnose the initiation and severity of shrimp disease. Environ. Microbiol.19, 1490–1501. doi: 10.1111/1462-2920.13701
60
Yan J. Chang Q. Chen S. Wang Z. Lu B. Liu C. et al . (2018). Effect of dietary antarctic krill meal on growth performance, muscle proximate composition, and antioxidative capacity of juvenile spotted halibut, verasper variegatus. J. World Aquaculture Soc.49, 761–769. doi: 10.1111/jwas.12455
61
Yan Y. Lin Y. Zhang L. Gao G. Chen S. Chi C. et al . (2023). Dietary supplementation with fermented antarctic krill shell improved the growth performance, digestive and antioxidant capability of Macrobrachium nipponense. Aquaculture Rep.30, 101587. doi: 10.1016/j.aqrep.2023.101587
62
Yang J. Wang A. Huo X. Yao L. (2006). Application of different fatty acids in aquatic animals. Marine Sci. Bull.02, 64–73.
63
Yoshitomi B. Aoki M. Oshima S. Hata K. (2006). Evaluation of krill (Euphausia superba) meal as a partial replacement for fish meal in rainbow trout (Oncorhynchus mykiss) diets. Aquaculture261, 440–446. doi: 10.1016/j.aquaculture.2006.06.036
64
Yoshitomi B. Nagano I. (2012). Effect of dietary fluoride derived from Antarctic krill (Euphausia superba) meal on growth of yellowtail (Seriola quinqueradiata). Chemosphere86, 891–897. doi: 10.1016/j.chemosphere.2011.10.042
65
Zeng Y. Wang L. Xu D. Tan P. Chen R. Zhu Q. (2022). Effect of compound feed and chilled trash fish on growth performance, feed utilization, growth-related gene expression and cultured water quality of Nibea albiflora. J. Zhejiang Ocean Univ. (Natural Science)04, 299–307.
66
Zhang X. Deeke S. Ning Z. Starr A. Butcher J. Li J. et al . (2018). Metaproteomics reveals associations between microbiome and intestinal extracellular vesicle proteins in pediatric inflammatory bowel disease. Nat. Commun.9, 2873. doi: 10.1038/s41467-018-05357-4
67
Zhang Y. Sun K. Li Z. Chai X. Fu X. Kholodkevich S. et al . (2021). Effects of acute diclofenac exposure on intestinal histology, antioxidant defense, and microbiota in freshwater crayfish (Procambarus clarkii). Chemosphere263, 128130. doi: 10.1016/j.chemosphere.2020.128130
68
Zhou H. Tang B. Gong H. Lei Y. Du S. Yu G. et al . (2023). Correlation analysis for feeding habits, morphological parameters, weight parameters and abdomen meat percentage of Procambarus clarkii. Chin. J. Anim. Nutr.35, 2455–2464. doi: 10.12418/CJAN2023.230
69
Zhou H. Xu Y. Jiang Y. Cui A. Wang B. Liu X. (2022). Microecological regulation of gastrointestinal microflora in the growth of yellowtail kingfish (Seriola lalandi) juveniles under indoor tank culture and cage culture modes. J. Fishery Sci. China29, 1437–1448. doi: 10.12264/JFSC2022-0185
70
Zhu J. Zhu G. (2022). Trophic linkage between mackerel icefish (Champsocephalus gunnari) and Antarctic krill (Euphausia superba) at South Georgia. Fisheries Res.253, 106366. doi: 10.1016/J.fishers.2022.106366
71
Zhuang J. Coates J. C. Zhu H. Zhu P. Wu Z. Xie L. (2015). Identification of candidate antimicrobial peptides derived from abalone hemocyanin. Dev. Comp. Immunol.49, 96–102. doi: 10.1016/j.dci.2014.11.008
72
Zuo Q. Zuo H. (2019). Outlook and present antarctic krill development. Cereal Food Industry26, 13–16.
Summary
Keywords
Antarctic krill meal, Cherax quadracarinatus , growth performance, fatty acid, immunity-related gene
Citation
Mai Z, Wei J, Wang Y, Zeng Z, Liu F, Li H, Su Q, Hong K, Zhou Q, Jiao T, Yang T and Yu L (2025) Effects of different feeding strategies for the frozen Antarctic krill meal on growth, muscle nutrition, digestive enzyme activity, immunity, molting, and gut microbiota structure of Cherax quadricarinatus. Front. Mar. Sci. 12:1573152. doi: 10.3389/fmars.2025.1573152
Received
08 February 2025
Accepted
10 April 2025
Published
06 May 2025
Volume
12 - 2025
Edited by
Christian Larbi Ayisi, University of Environment and Sustainable Development, Ghana
Reviewed by
Adnan H. Gora, Central Marine Fisheries Research Institute (ICAR), India
Amit Ranjan, Tamil Nadu Fisheries University, India
Updates
Copyright
© 2025 Mai, Wei, Wang, Zeng, Liu, Li, Su, Hong, Zhou, Jiao, Yang and Yu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingyun Yu, lysnp@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.