Abstract
The biomass of mesopelagic fishes is estimated to be on the order of or to exceed that of fishes in the epipelagic. Despite their abundance and importance as an ecological link between surface and deep ocean habitats, there is a dearth of basic life history data for mesopelagic fishes. Reproductive biology data are critical for understanding population dynamics and estimating production of a species, particularly when age and growth data are lacking. Between July 2018 and August 2022, collections were made in the western North Atlantic utilizing multiple net types to capture a broad size-range of mesopelagic fishes. Histological analysis of gonad tissue from four numerically dominant species—Argyropelecus aculeatus (Sternoptychidae), Benthosema glaciale (Myctophidae), Scopelogadus beanii (Melamphaidae), and Sigmops elongatus (Gonostomatidae)—were examined to describe aspects of reproduction. We determined that A. aculeatus and B. glaciale are gonochoristic batch spawners with indeterminate fecundity, and the standard length at which 50% of females were mature (L50) was 39.45 and 33.77 mm, respectively. S. beanii were found to be gonochoristic, iteroparous, and likely have multi-year oocyte development with an L50 of 90.38 mm. S. elongatus was confirmed as a protandrous hermaphrodite, iteroparous, and had an L50 of 200.45 mm. This study is the first to present regional maturity ogives for all four species and to describe detailed reproductive patterns in A. aculeatus, S. beanii, and S. elongatus. These results contribute to the data necessary for quantifying the role of mesopelagic fishes in global biogeochemical cycles and for ensuring responsible use of mesopelagic resources.
1 Introduction
The ocean’s mesopelagic zone (comprising pelagic waters of approximately 200–1000 m depth) is a vast habitat occupying roughly 20% of the global ocean volume (ICES, 2021). While the mesopelagic is unfamiliar to many, remote, and extremely difficult to access, it is not safe from anthropogenic impacts. Advancements in technology and depletion of nearshore resources is pushing commercial fisheries into deep sea habitats (Victorero et al., 2018; Watson and Morato, 2013). Increasing temperatures associated with climate change are expected to affect the mesopelagic through shifting oxyclines (Seibel and Wishner, 2019), shoaling of the deep scattering layer (Proud et al., 2017), deoxygenation (Gong et al., 2021), increased ocean acidification (Yang et al., 2022), and shifting vertical and latitudinal species distributions (Iglesias et al., 2024; Liu et al., 2024). Ultimately, the effects of climate change are complicated and not expected to be uniform across taxa, regions, or ocean basins (Koslow et al., 2019). These physical, chemical, biological, and ecological shifts are occurring simultaneously as the high biomass present in the mesopelagic zone is garnering attention in the development of commercial fisheries, either for direct or indirect (as aquaculture feed) consumption (Fjeld et al., 2023; Grimaldo et al., 2020), putting this ecologically important habitat at risk for major degradation.
Fishes of the mesopelagic are among the most numerous vertebrates on the planet and are estimated to comprise 5–15 billion metric tons (Irigoien et al., 2014; Kaartvedt et al., 2012; Proud et al., 2019), or approximately 50% to 94% of the total global fish biomass (Bianchi et al., 2021; Gjosater and Kawaguchi, 1980; Jennings et al., 2008). Due in large part to the prevalence of diel vertical migration in many species, these midwater fishes are an important ecological link between surface production and the deep sea (Isaacs et al., 1974; Sutton, 2013). Mesopelagic fish feeding activity in epipelagic waters and respiration and excretion at depth make mesopelagic fishes a significant component of the biological carbon pump (Boyd et al., 2019; Longhurst et al., 1990), though the precise magnitude of their influence in the global biogeochemical cycle remains unknown (McMonagle et al., 2024). In addition to their role in vertical ecological connectivity, these primarily zooplanktivorous and micronektivorous fishes connect lower and upper trophic levels, serving as prey for larger, deep-dwelling fishes as well as for apex predators such as billfishes, sharks, marine mammals, and seabirds (Choy et al., 2013; Iglesias et al., 2023; Potier et al., 2007; Young et al., 2015).
Despite their numerical abundance, biomass, and critical ecological role, little is known about mesopelagic fishes’ biology, ecology, and basic life history compared to their epipelagic and nearshore counterparts (Caiger et al., 2021). Age, growth, and reproductive analyses, which provide data for metrics such as population structure, growth rates, mortality rates, size and age at maturity, spawning seasonality, and productivity, are fundamental to understanding population dynamics of a species (Morgan, 2017). Of the studies to comment on reproductive parameters of mesopelagic fishes, few present detailed histological analysis (Caiger et al., 2021), which is generally considered the most accurate method of assessment (West, 1990). With a few exceptions (Garcia-Seoane et al, 2014; Knorrn et al., 2024; Marks et al., 2020), maturity ogives (estimates of the proportion of females mature at a given length or age) are lacking, despite that ogives are key metrics in population stock assessment models and are important for management. To fully appreciate how mesopelagic fishes will respond to ocean changes and anthropogenic influences, a more complete understanding of their population sizes and reproductive dynamics is needed.
This study describes aspects of the reproductive life history of four mesopelagic fish species collected in the Slope Sea of the western North Atlantic. The study species were selected based on their numerical dominance in net collections and ecological importance in the region. Argyropelecus aculeatus (Sternoptychidae) is broadly distributed in the Atlantic, Pacific, and Indian Oceans (Howell and Krueger, 1987; Schultz, 1964). No formal reproductive studies were found in the literature for any members of the genus, though aspects of reproduction based on visual observation of whole gonads from fish collected in the Bermuda Ocean Acre were described in Howell and Krueger (1987). Benthosema glaciale is a member of the species-rich Myctophidae family, a group that is estimated to represent the highest amount of vertebrate biomass on Earth (Catul et al., 2011). B. glaciale is found throughout the North Atlantic and in the Mediterranean Sea and is the numerically dominant myctophid north of 35°C (Sutton et al., 2008). Studies in other regions have described the reproductive strategies and estimated maturity parameters for B. glaciale (Garcia-Seoane et al, 2014; Halliday, 1970; Mazhirinia, 1988), but substantial variability has been observed, indicating that the full diversity of reproductive parameters has not yet been fully circumscribed. Scopelogadus beanii (Melamphaidae) has a broad distribution that overlaps with A. aculeatus (Froese and Pauly, 2024) and is associated with the Gulf Stream in the North Atlantic (Kotlyar, 2019). The reproductive biology of S. beanii has not been previously described. Sigmops elongatus, from the most numerically abundant vertebrate family (Gonostomatidae), is a predatory fish with a worldwide distribution. Limited reproductive data from the tropical Pacific and Gulf of Mexico have been collected for this hermaphroditic species. All these species of interest, except S. beanii, are known to participate in diel vertical migration, and due to their numerical abundance in our collections, are likely to play important roles in the regional biological carbon pump.
The primary objectives of this study were to describe the gender systems and patterns of reproduction, as well as to estimate regional female maturity parameters from histological examination of gonad tissue from A. aculeatus, B. glaciale, S. beanii, and S. elongatus. These results further the knowledge base of some of the mesopelagic region’s key players and can be used to further investigate their ecology, behavior, and role in biogeochemical cycles.
2 Materials and methods
2.1 Sample collection
All fish in this study were collected in the “Slope Sea” off the US Atlantic coast (Csanady and Hamilton, 1988; Richardson et al., 2016) approximately 165 to 275 km south-southwest of Martha’s Vineyard over water depths of 1300 m to 2800 m (Figure 1). Collections occurred primarily during four cruises specifically devoted to better understanding the ocean’s mesopelagic zone as part of the Woods Hole Oceanographic Institution’s Ocean Twilight Zone Project (OTZ) between July 2018 and August 2022 (Figure 1; Table 1). Collections were supplemented from five Northeast U.S. Shelf Long-Term Ecological Research (NES-LTER) cruises between February 2019 and July 2021 (Figure 1; Table 1). Sampling occurred both night and day from the surface to a maximum of 1000 m depth, utilizing multiple gear types, as detailed below, to catch a range of fish species and sizes (Figure 1; Table 1).
Figure 1
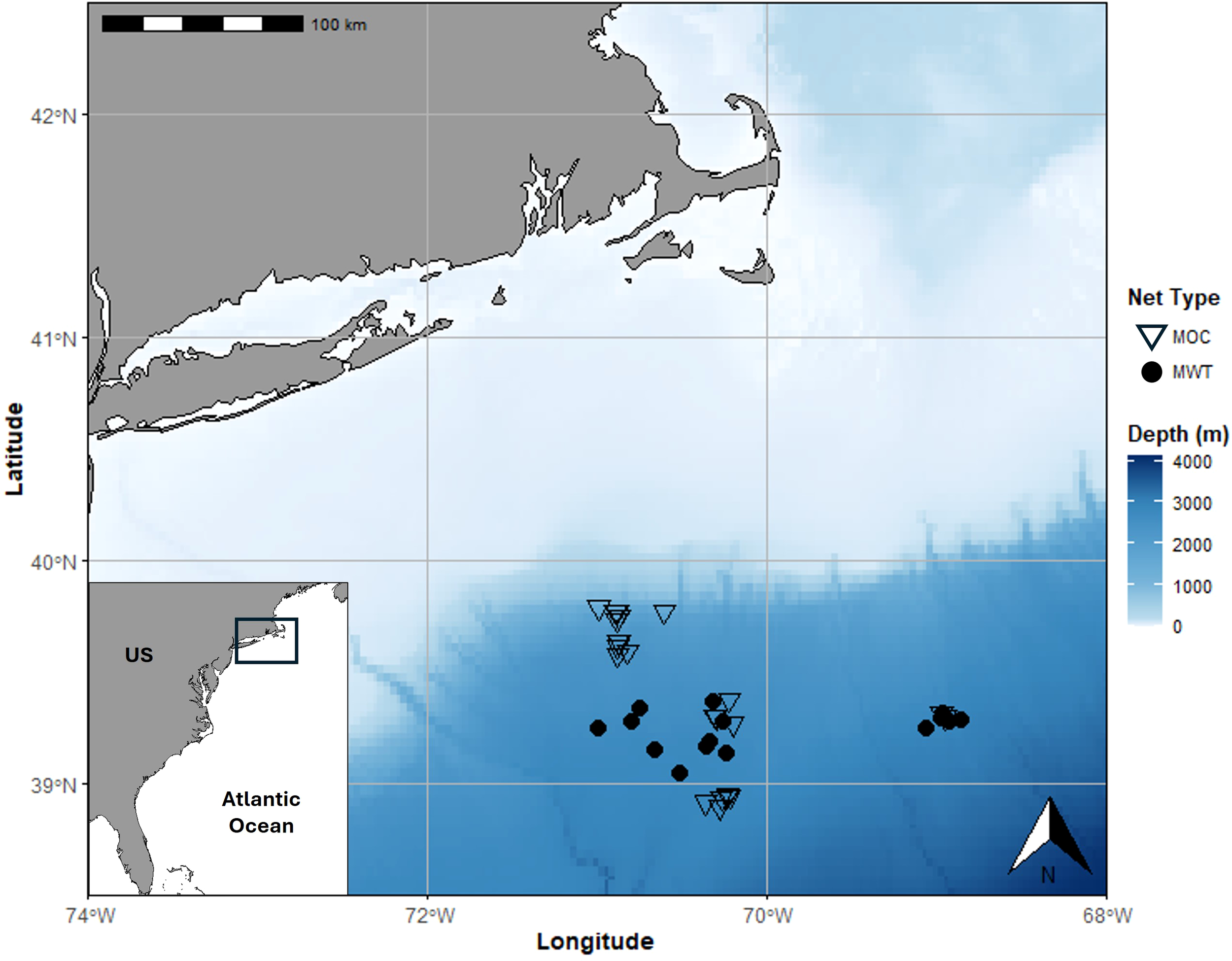
Map of tow locations by gear type for the collection of mesopelagic fish species in the Slope Sea of the Northwestern Atlantic Ocean. Net type used for each tow is denoted by the symbol shape. MOC, Multiple Opening/Closing Net and Environmental Sensing System (10 m2; open triangle); MWT, Marinovich midwater trawl (closed circle).
Table 1
| Cruise ID | Date | Project | Tow Type | Net used | Ntow | Nnet | Nsoi | Nhisto |
|---|---|---|---|---|---|---|---|---|
| HB1805 | 11–21 Aug 2018 | OTZ | MWT | MARMWT | 5 | 5 | 117 | 72 |
| EN627 | 4 Feb 2019 | NES-LTER | MWT | MOC10 | 1 | 1 | 9 | 3 |
| HB1907 | 24 Jul - 8 Aug 2019 | OTZ | MWT | MARMWT | 9 | 9 | 131 | 79 |
| EN649 | 3 Feb 2020 | NES-LTER | MWT | MOC10 | 1 | 1 | 10 | 5 |
| AR43 | 11–16 Mar 2020 | OTZ | MOC | MOC10 | 5 | 22 | 116 | 68 |
| EN657 | 15 Oct 2020 | NES-LTER | MWT | MOC10 | 1 | 1 | 33 | 30 |
| EN661 | 5 Feb 2021 | NES-LTER | MWT | MOC10 | 1 | 1 | 15 | 14 |
| EN668 | 18 Jul 2021 | NES-LTER | MWT | MOC10 | 1 | 1 | 10 | 9 |
| HB2205 | 4–18 Aug 2022 | OTZ | MOC | MOC10 | 6 | 23 | 181 | 51 |
| MWT | MOC10 | 1 | 1 | 5 | 3 |
Summary of cruises during which mesopelagic fishes were collected for reproductive and associated studies.
Species of interest (SOI) included Argyropelecus aculeatus, Benthosema glaciale, Scopelogadus beanii, and Sigmops elongatus. MARMWT, Marinovich Midwater Trawl; MOC10, 10m2 multiple opening-closing net and environmental sensing system (MOCNESS); MWT, midwater trawl type single net tow; MOC, MOCNESS style tow; NES-LTER Northeast Shelf Longterm Ecological Research; Nhisto, number of SOI processed for histology; Nnet, number of nets sampled; Nsoi=number of SOI collected; Ntow, number of tows conducted; OTZ, Ocean Twilight Zone.
A modified Marinovich midwater trawl (MARMWT) was used on OTZ cruises in July and August of 2018 and 2019 (Table 1). The MARMWT had an approximate mouth area of 49 m2, with graduated mesh sizes from 7 cm at the mouth to 3.8 cm at the cod-end. The MARMWT was towed obliquely, and maximum tow depths ranged from 50 m to 780 m over bottom depths of 2300 to 2800 m. The trawl catch was cursorily examined immediately upon retrieval: an unmeasured subsample of fish, visually selected to approximate the diversity of the catch, was removed, wrapped in foil, and quickly frozen in liquid nitrogen. After a minimum of 24 hours, flash frozen samples were transferred to -800C freezers where they were stored until processing. The remainder of the catch was sorted to the lowest taxonomic level practical with each taxon weighed en masse; lengths were recorded for up to 150 individuals per taxon and discarded.
A five-net multiple opening-closing net and environmental sensing system (MOCNESS; Wiebe et al., 1985) with a mouth area of 10 m2 was used on OTZ cruises in March 2020 and August 2022 (Table 1). Each tow produced up to four obliquely towed depth-binned samples from nets with 333 µm mesh and one obliquely towed sample from the surface to the tow’s maximum depth (400 m to 1000 m) with a 3 mm mesh net. After net retrieval, large organisms (>20 mm approximately in length) were removed, identified to the lowest taxonomic level practical, photographed, wrapped in foil, and frozen in liquid nitrogen. After a minimum of 24 hours, flash frozen samples were transferred to -800C freezers where they were stored until processing. On the March 2020 cruise, prior to flash freezing of large organisms 13 specimens of interest (n=8 S. beanii; n=5 S. elongatus) were placed on ice for at-sea dissection to obtain fresh gonads for comparison to flash frozen samples.
NES-LTER sampling occurred at the farthest offshore station (L11, ~39° 46.4´ N, 70° 53´ W) over bottom depths of approximately 1600 m of water (Figure 1; Table 1). Fish were collected via a midwater, Tucker-style trawl (MWT) that used a 10-m2 MOCNESS net with 3-mm mesh strung between two MOCNESS net bars (Wiebe et al., 1985); the upper bar was outfitted with a tow bridle and a 36 kg clump weight was attached to each end of the lower bar. The net was deployed to approximately 1000 m and either immediately retrieved (oblique tow; three cruises) or held at depth for two hours before retrieval (two cruises). Once aboard, large fish (approximately >20 mm in length) were removed, identified to the lowest taxonomic level practical, photographed, wrapped in foil, and frozen in liquid nitrogen. The remainder of the catch was discarded. After a minimum of 24 hours, flash frozen samples were transferred to -800C freezers, where they were stored until processing. On the July 2021 cruise, prior to flash freezing of large organisms, 11 specimens of interest (n=5 B. glaciale; n=3 S. beanii; n=2 S. elongatus) were placed on ice for at-sea dissection to obtain fresh gonads for comparison to flash frozen samples.
2.2 Sample processing
Fish that were dissected at-sea were identified to species, photographed, and measured for SL. Gonads were excised and fixed in 10% neutral buffered formalin.
In the laboratory, all flash frozen fish from MOCNESS and MARMWT samples were identified morphologically to the lowest taxonomic level possible. Standard length (SL) to the nearest mm and wet weight to the nearest 0.01 g were recorded. The four species of interest in this study were selected based on their numerical abundance in these collections. For flash frozen MWT collections, only individuals of the species of interest were identified and further processed. All following methods and analyses refer only to the four species of interest.
In addition to SL and weight, muscle tissue for genetic identification was removed, a subset of which was genotyped to confirm species identification. When possible, gonads were excised and weighed to the nearest 0.0001 g. The gonadosomatic index (GSI) was calculated as the quotient of gonad weight and somatic weight (total weight minus gonad weight) * 100. Depending on gonad size, a portion of or the entire gonad lobe was placed into a biopsy bag within a tissue cassette and fixed in 10% neutral buffered formalin. Gonads were not available from all collected fish due to the poor condition of thawed specimens, small size of the fish, loss of the tissue in histological processing, or early lab protocols that did not preserve tissue (see Table 1 for number of histology samples per cruise).
2.3 Histology
All available gonad tissue samples for specimen of interest were processed histologically (n=335) with standard techniques, using paraffin as a blocking medium (Humason, 1972). Thin sections (5 µm) were mounted on glass slides and stained with Schiff’s-Mallory trichrome. A subset thought to represent a variety of stages of ovarian development were stained and counter-stained with hematoxylin and Eosin Y for comparison (Humason, 1972).
Without reference to size or date of capture, histological sections were examined with a compound microscope (40-400x) to determine sex and comment on reproductive strategy (e.g. described as gonochoristic/hermaphroditic, semelparous/iteroparous, and total/batch spawner; see Murua and Saborido-Rey, 2003). An exact binomial test was performed to determine if the observed sex ratio in histology samples was significantly different from 1 male to 1 female. For hermaphrodites, the sex was recorded as male or female when a single gonad tissue type was evident or transitional when both male and female tissue were present. Males were not analyzed further as seasonal cycles are less distinct and due to artifacts of freezing that made distinguishing stages of spermatogenesis uncertain. For females, the diameter of the most advanced oocyte stage (where the nucleus was present) and thickness of the ovarian wall (when present in the section) was measured to the nearest µm using an ocular micrometer. For A. aculeatus, B. glaciale, and S. beanii specimens, each ovary was assigned a reproductive phase based on the most advanced oocyte stage present, incidence and stage of atresia, and the presence of postovulatory follicle complexes (POFs; Table 2). Reproductive phases were not assigned to S. elongatus due to low sample sizes and difficulty distinguishing between freezing artifacts and typical oogenesis. Reproductive phases were based on descriptions of oocyte development in Wallace and Selman (1981) and Grier et al. (2009) and utilized standard terminology suggested by Lowerre-Barbieri et al. (2023) with species-specific modifications (Table 2).
Table 2
| Reproductive State | Phase | General Description/Histological Indicators | Species-specific modifications | Freezing Artifacts Considerations |
|---|---|---|---|---|
| Immature | Immature | Oogonia, PG, or CA; tissue appears compact and well organized; OW thin | OW range (µm): <50 (AA); <20 (BG);<30 (SB) | Early stages of CA difficult to detect due to disruption of cytoplasm, but are distinguished from PG based on the marked increase in size; compactness of immature tissue unaffected by freezing |
| Mature or first time maturing | Early Developing | Early vitellogenesis; small vitellogenin granules evident in periphery of cell and size of these oocytes distinctly larger than PG/CA. Tissue compact and well organized in first maturing; tissue more dispersed with older atresia in repeat spawners | Vtg range (µm) = 150-260 (AA); 130-270 (BG); 200-350 (SB) | General melted appearance of cytoplasm but individual yolk granules can still be seen; OD may resemble coalescence but occurs in all oocyte stages |
| Late Developing | Vitellogenesis pronounced: vitellogenin evident throughout cytoplasm; increase in oil droplets throughout the cell. POFs may be present in repeat spawners | Vtg range (µm) = 220-450 (AA); 245-450 (BG); 340-500 (SB) | General melted appearance of cytoplasm but individual yolk granules can still be seen; OD may resemble coalescence but occurs in all oocyte stages | |
| Mature | Spawning Capable | Oocyte maturation evident as GVM, GVB, or hydration; coalescence of oil droplets and vitellogenin. | OM range (µm) = 440-620 (AA); 410-450 (BG); 450-600 (SB) | General melted appearance of cytoplasm but individual yolk granules can still be seen; OD coalescence distinguished from artifact because not evident in PG/CA oocytes |
| Between Spawning | Regressing/Regenerating | PG or CA most advanced-non atretic oocytes; atresia present (alpha, beta, and/or later stages). Tissue not densely packed; POFs may be present; OW thick | General melted appearance of cytoplasm; POFs evident due to SMT staining | |
| Sex change (protandrous, sequential hermaphrodite) | Transitional | Obvious male and female tissue present; male tissue degrading; only oogonia and PG present in female tissue. Sex recorded as dominant tissue | Only applies to S. elongatus | Transition may start earlier and evidence may be visible later but freezing artifact precludes |
Descriptions of reproductive states, phases, and histological criteria for reproductive studies of Argyropelecus aculeatus (AA), Benthosema glaciale (BG), Scopelogadus beanii (SB), and Sigmops elongatus collected in the Northwestern Atlantic between 2018 and 2022.
Reproductive phases were based on descriptions of oocyte development in Wallace and Selman (1981) and Grier et al. (2009) and utilized standard terminology suggested by Lowerre-Barbieri et al. (2023) with species specific modifications. The majority of fish were flash frozen in liquid nitrogen prior to the removal of the gonads: histological artifacts resulting from the freezing and thawing process were considered. CA, cortical alveoli/cortical alveolar stage oocytes; GVB, germinal vesicle breakdown; GVM, germinal vesicle migration; OD, oil droplet; OM, oocyte maturation; OW, ovarian wall; PG, primary growth stage oocyte; POF, post-ovulatory follicle complex; Vtg, vitellogenic.
Artifacts due to freezing precluded precise descriptions of discrete stages of cortical alveolar, vitellogenic, and maturation-stage oocytes. However, within histological sections, enough oocytes with internal structure were present to confidently identify the most advanced oocyte stage as having cortical alveoli or vitellogenin and to determine when later stages of oocyte maturation were occurring (germinal vesicle breakdown and lipid coalescence). Furthermore, histological sections from gonads fixed at sea were examined to inform interpretation of frozen tissue such as diameter and appearance of different stage oocytes.
Oocyte size frequency distribution diagrams were completed to inform the reproductive strategies. For each species, a single female with ovaries in the closest reproductive phase to spawning (actively spawning for A. aculeatus and B. glaciale; developing for S. beanii; phase not applicable for S. elongatus) and which had >100 oocytes with nuclei visible in histological slides was selected for analysis. For each selected individual, multiple non-overlapping photomicrographs were captured with a Leica ICC50 HD camera mounted to Leica DM 1000 LED compound microscope using the 4x objective. All oocytes with a visible nucleus and a diameter >65 µm for A. aculeatus, B. glaciale, and S. beanii and >30 µm for S. elongatus were measured using Fiji (vers. 2.14.0). Small primary growth oocytes and oogonia were not measured as these stages would not be available for use in the forthcoming reproductive events.
Based on histological markers and the assigned ovarian phase, females were classified as immature or mature. Immature females were those with primary growth oocytes or, to be conservative, cortical alveolar oocytes and no evidence of prior reproductive activity (e.g., no POFs, minimal atresia, thin ovarian walls). These individuals were presumed to have never participated in spawning. Mature females were those staged as early developing and later for A. aculeatus, B. glaciale, and S. beanii or with vitellogenic oocytes or POFs for S. elongatus (Table 2). The ovaries of mature females had cortical alveolar oocytes with evidence of reproductive activity (thick ovarian walls, POFs, or pronounced alpha or beta atresia) as the most advanced oocyte stage or further advanced oocytes with or without prior evidence of spawning. These individuals were physiologically capable of reproducing and/or had reproduced previously.
Maturity ogives were modeled for each species using generalized linear models with binomial error structure (0=immature; 1=mature) and logit link functions as:
where Px is the proportion of females mature at length x, α is the intercept of the linear predictor, and β is the slope. The length at which 50% and 95% of females were mature (L50 and L95, respectively), and model parameters were reported in addition to the minimum observed length at maturity (Lmin).
All analyses were completed using R version 4.1.2 (R Core Team, 2024) and results were considered significant at P<0.05. The “MASS” (Venables and Ripley, 2002) and “ggplot2” (Wickham, 2016) packages were used to plot and estimate parameters of the generalized linear models.
3 Results
In total, 2423 fish were collected, of which 627 were specimens of interest (lengths available for 602; Table 3), with 71% of samples collected in July and August. All species were represented in collections from both MARMWT and MOCNESS nets (Figure 2). The body lengths of fish collected were dependent on net type, with the MOCNESS collecting the broadest range of lengths. In general, more large individuals were collected in the MARMWT compared to MOCNESS nets (Figure 2). Within a species, male and female length ranges were similar, except for S. elongatus (Table 3).
Table 3
| Species | Sex | N | SL Range (mm) | Mean SL ± stdev | Weight Range (g) | Mean Weight ± stdev |
|---|---|---|---|---|---|---|
| Argyropelecus aculeatus | 156 | 10 - 73 | 50 ± 11 | 0.05 - 14.90 | 4.58 ± 2.37 | |
| Female | 64 | 25 - 73 | 42 ± 8 | 0.75 - 14.19 | 6.09 ± 3.48 | |
| Male | 61 | 20 - 63 | 50 ± 11 | 0.34 - 8.47 | 3.38 ± 1.57 | |
| Benthosema glaciale | 274 | 14 - 63 | 31 ± 13 | 0.02 - 3.40 | 0.62 ± 0.75 | |
| Female | 68 | 19 - 63 | 41 ± 9 | 0.08 - 3.40 | 1.17 ± 0.74 | |
| Male | 33 | 31 - 62 | 44 ± 9 | 0.34 - 2.83 | 1.30 ± 0.67 | |
| Scopelogadus beanii | 83 | 25 - 120 | 89 ± 24 | 0.37 - 42.22 | 20.48 ± 11.40 | |
| Female | 45 | 35 - 120 | 93 ± 23 | 0.93 - 42.22 | 22.53 ± 11.65 | |
| Male | 25 | 45 - 119 | 94 ± 18 | 2.36 - 34.15 | 21.99 ± 8.98 | |
| Sigmops elongatus | 89 | 29 - 270 | 132 ± 57 | 0.14 - 65.16 | 14.84 ± 16.70 | |
| Female | 22 | 145 - 270 | 199 ± 30 | 11.42 - 65.16 | 36.27 ± 16.33 | |
| Male | 11 | 71 - 151 | 112 ± 26 | 1.09 - 15.03 | 6.79 ± 4.84 | |
| Transitional | 5 | 103 - 161 | 132 ± 11 | 4.81 - 19.42 | 10.28 ± 5.16 |
Summary of collections of mesopelagic fish collected in the Slope Sea between 2018 and 2022 for reproductive studies.
The total number (N) of fish collected and the total that were successfully sexed through histological analysis are listed. g, grams; SL, standard length; stdev, standard deviation.
Figure 2
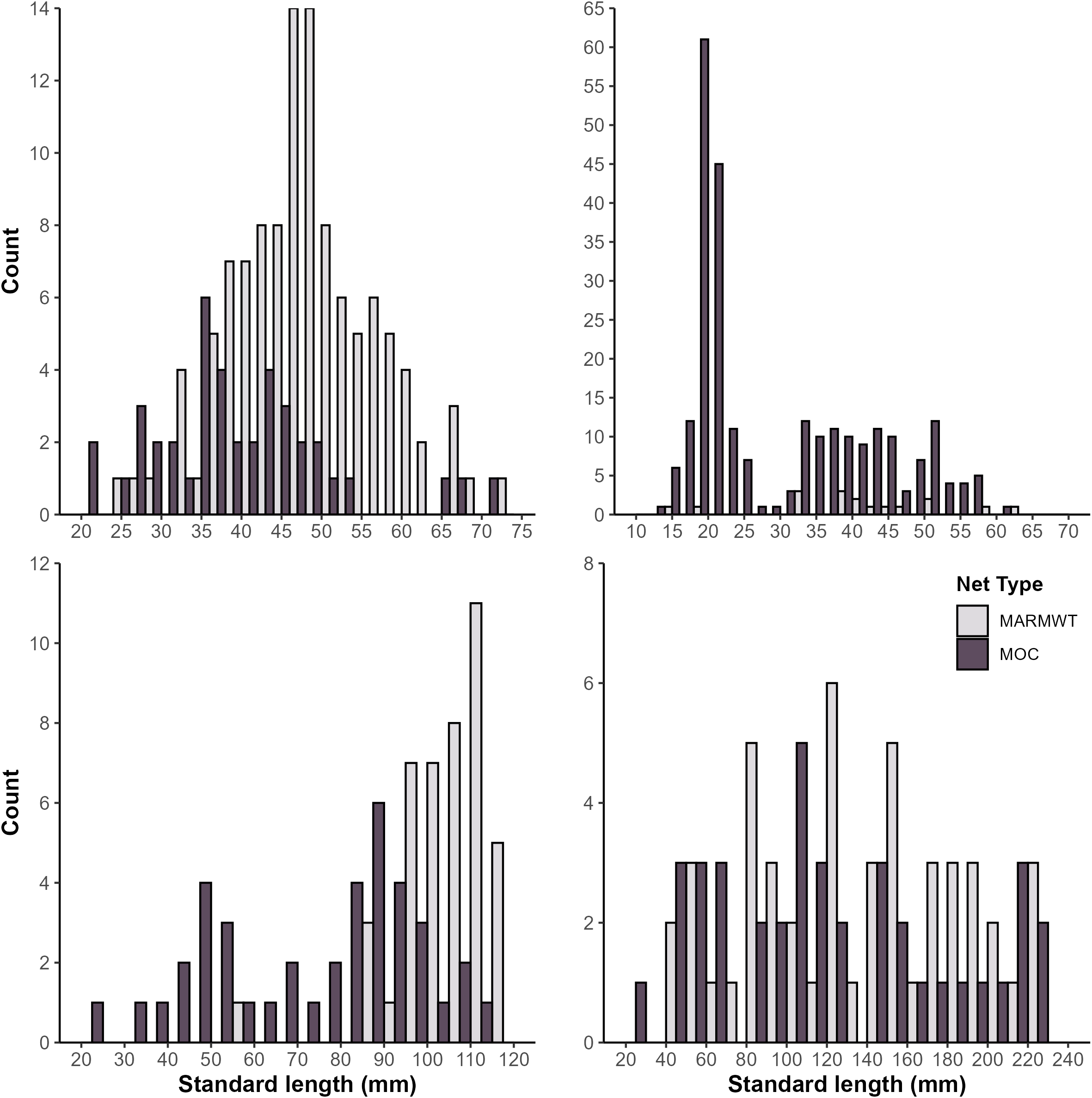
Length frequency distribution of male and female mesopelagic fish collected in the Slope Sea between July 2018 and August 2022 by net type and species. (A)Argyropelecus aculeatus (n=156) (B)Benthosema glaciale (n=274) (C)Scopelogadus beanii (n=83) (D)Sigmops elongatus (n=89). MOC, Multiple Opening/Closing Net and Environmental Sensing System (10 m2); MRMWT, Marinovich Midwater Trawl; n, number of fish.
Gonad samples were available for histology from 335 fish (Table 1). Freshly fixed gonads were available for B. glaciale (n=4); S. beanii (n=11), and S. elongatus (n=3). Artifacts in frozen tissue included: (1) a “melted” appearance of intracellular and interlamellar areas due to rupturing of cell membranes and cellular components; (2) the formation of vacuoles (reminiscent of oil droplet coalescence that occurs during oocyte maturation); and (3) the premature disintegration of the nuclear membrane (Figure 3). Artifacts present in frozen tissue precluded detailed descriptions of oogenesis such as descriptions of discrete stages of cortical alveolar, vitellogenic, and maturation stage oocytes. However, general patterns could be discerned due to the presence of structural characteristics of oocyte stages (e.g. cortical alveoli, early vitellogenin accumulation, germinal vesicle migration visible; POFs evident) and general appearance of tissue sections (e.g. compact, well-organized tissue still being distinguishable from the less organized tissue of repeat spawners). Schiff’s Mallory trichrome was the preferred stain due to the distinct staining of cortical alveoli and the follicular basement membrane in POFs. The subset of tissues stained with hematoxylin and eosin-Y were useful for confirming the presence of vitellogenin in frozen tissues.
Figure 3
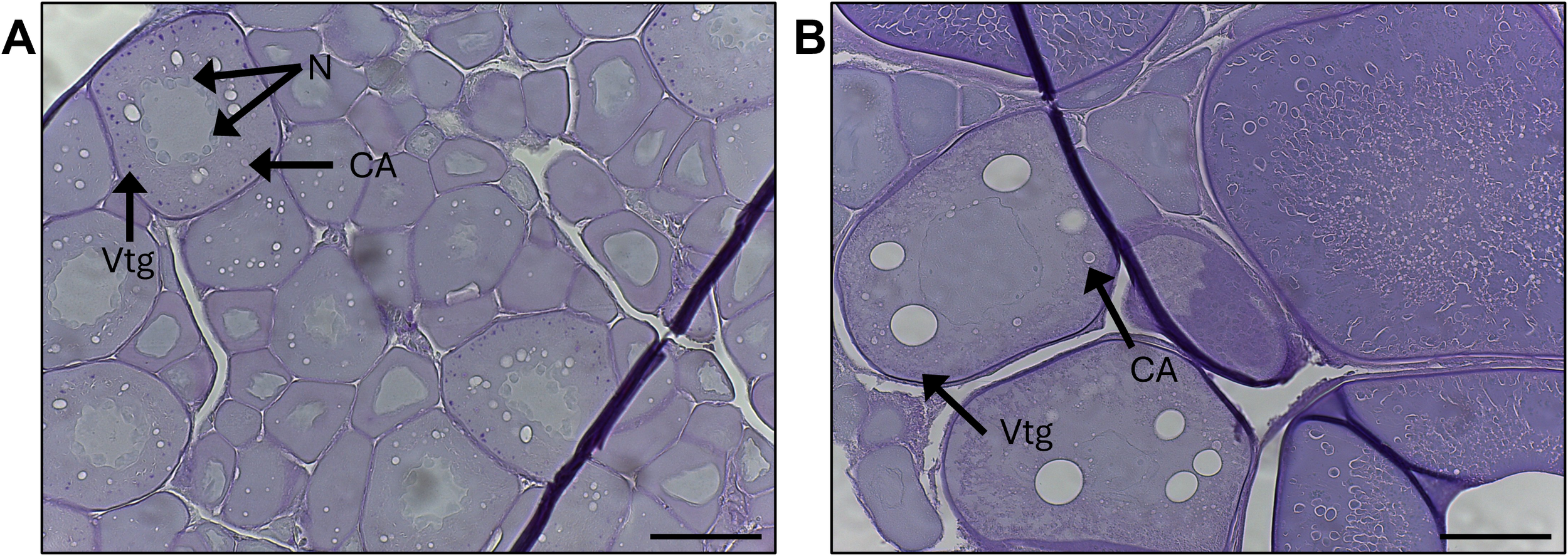
Histological micrographs of ovarian tissue from Benthosema glaciale stained with Schiff’s Mallory Trichrome demonstrating some artifacts of freezing on tissue quality. (A) Tissue collected from freshly caught specimen (58 mm standard length [SL]) with ovaries in an early developing stage. Vitellogenin droplets (Vtg), cortical alveoli (CA), and nucleoli (N) are distinct. (B) Tissue collected from a specimen (39 mm SL) frozen in liquid nitrogen and stored at -80°C. Vtg are diffuse and less distinct. Nucleoli may be ruptured. Pre-oocyte maturation stage vitellogenic oocytes resemble lipid coalescence due to rupturing of Vtg. Scale bars = 100 µm.
3.1 Gender systems & sex ratios
A. aculeatus, B. glaciale, and S. beanii were found to be gonochoristic: male and female lengths overlapped throughout their size ranges, and no simultaneous histological evidence of testicular and ovarian tissue in a sample was observed (Tables 3, 4). S. elongatus were found to be protandrous hermaphrodites. All fish ≤102 mm SL were male, and those ≥179 mm SL were female (Tables 3, 4). Five fish 103–147 mm SL were observed to have both testicular and ovarian tissue present; three had predominantly male tissue with primary growth oocytes present while two had predominately female tissue with degrading male tissue still evident.
Table 4
| Species | Gender System | Female Lifetime Reproductive Schedule | Spawning Strategy | Female Fecundity Type |
|---|---|---|---|---|
| Argyropelecus aculeatus | Gonochorisim | Iteroparous | Batch | Indeterminate |
| Benthosema glaciale | Gonochorisim | Iteroparous | Batch | Indeterminate |
| Scopelogadus beanii | Gonochorisim | Iteroparous | Unknown | Unknown |
| Sigmops elongatus | Protandrous hermaphroditism | Iteroparous | Unknown | Unknown |
Summary of reproductive traits of Arygyropelecus aculeatus, Benthosema glaciale, Scopelogadus beanii, and Sigmops elongatus, as determined through histological analysis of male and female gonad tissue.
The observed sex ratio for A. aculeatus (1:1.05) was not significantly different from 1:1 male:female (p=0.86). The observed sex ratios for B. glaciale and S. beanii were 1:2.1 (p<<0.05) and 1:1.8 (p=0.03), respectively. Considering individuals with transitional gonads the sex of the dominant tissue type, the observed sex ratio was 1:2.2 in S. elongatus (p<<0.05).
3.2 Female reproductive strategies & maturity
All phases of ovarian development were observed in A. aculeatus (Figure 4A). GSI values ranged from 0.25 to 11.75 (n=112) and were significantly different among ovaries staged as early developing, developing, and spawning (p<0.05; Figure 5A). We classified A. aculeatus as iteroparous (capable of participating in multiple spawning events; Murua et al., 2003) based on the observation of POFs in tissues with early secondary growth oocytes (cortical alveolar and early vitellogenic; Figure 6A). Oocyte development was group synchronous, with up to two distinct clutches of vitellogenic oocytes present (e.g. early and late vitellogenic clutches; Figure 7A). One female (67 mm SL) had primary growth, cortical alveolar, early vitellogenic oocytes, maturing oocytes, and POFs present, indicating the fish had spawned previously, was nearing a spawning event, and had additional oocytes developing and in reserve for future reproduction. Summer (July and August) was a period of reproductive activity for A. aculeatus based on the preponderance of females with developing and spawning capable ovaries and POFs. However, spawning frequency and seasonality could not be determined due to lack of sufficient seasonal coverage: all but two females (both immature) were collected in summer. Of females collected at night (n=52), 19% (n=10) had ovaries in the spawning capable phase indicating they were closer to spawning than females collected during day tows (1 out of 12 with spawning capable ovaries in day tows). One female (58 mm SL) had regressing ovaries with evidence of recent cessation of spawning (POFs and atretic cortical alveolar and vitellogenic oocytes) and a reserve stock of primary growth oocytes, suggesting this fish would be capable of participating in the next spawning season. The smallest mature female A. aculeatus was 34 mm SL. Estimates of L50 and L95 were 39.5 and 45.7 mm SL, respectively (Table 5; Figure 8A).
Figure 4
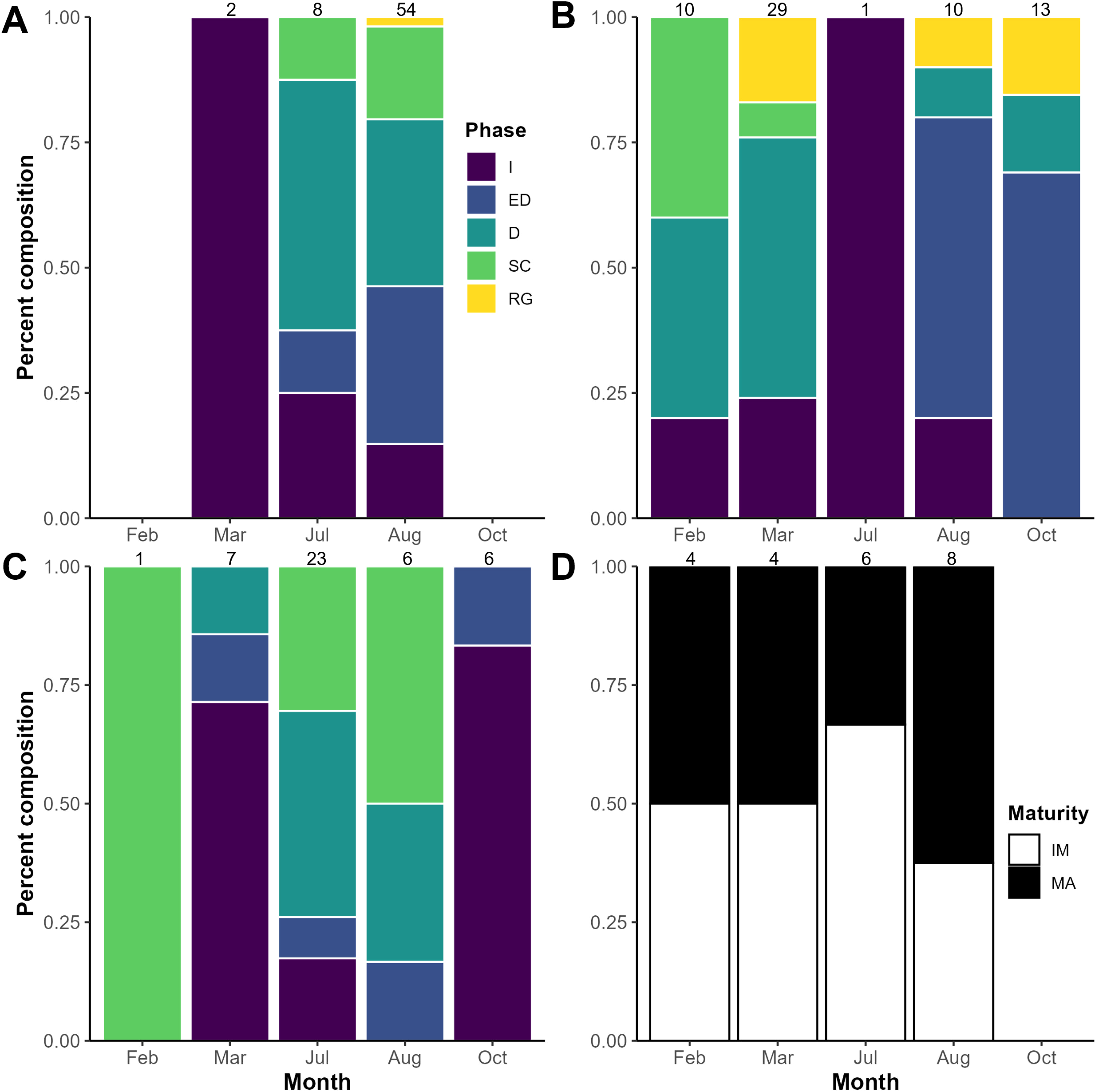
Reproductive phases or maturity by month for mesopelagic fishes collected in the Slope Sea between 2018 and 2022. Percent composition of female in each reproductive phase for (A)A. aculeatus, (B)B. glaciale, and (C)S. beanii, respectively. (D) Maturity status by month for female Sigmops elongatus. Numbers above the bars denote sample size by month. I, immature; ED, Early developing; D, Developing; SC, Spawning Capable; RG, Regressing/regenerating; IM, immature; MA, mature.
Figure 5
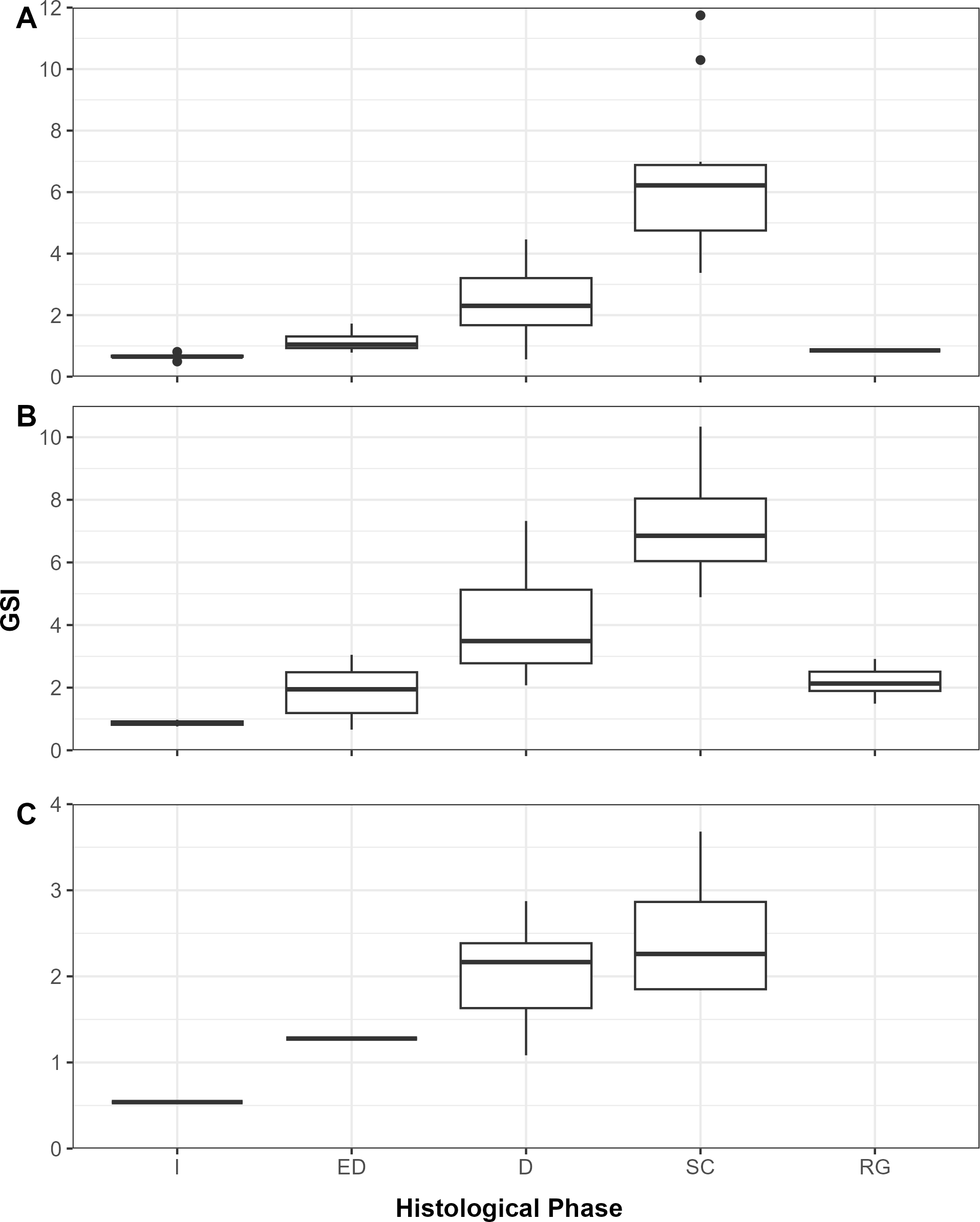
Female gonadosomatic index (GSI) by histologically determined reproductive phase in three mesopelagic fishes, (A)Argyropelecus aculeatus (n=53), (B)Benthosema glaciale (n=39), and (C)Scopelogadus beanii (n=18), collected in the Slope Sea between 2018 and 2022. GSI values were significantly different (p<0.05) for females with ED, D, and SC ovaries in all species. For B. glaciale, GSI values between ED and RG ovaries were not significantly different (p>0.05) I, immature; D, developing; ED, early developing; N, number of fish; RG, regressing/regenerating; SC, spawning capable.
Figure 6
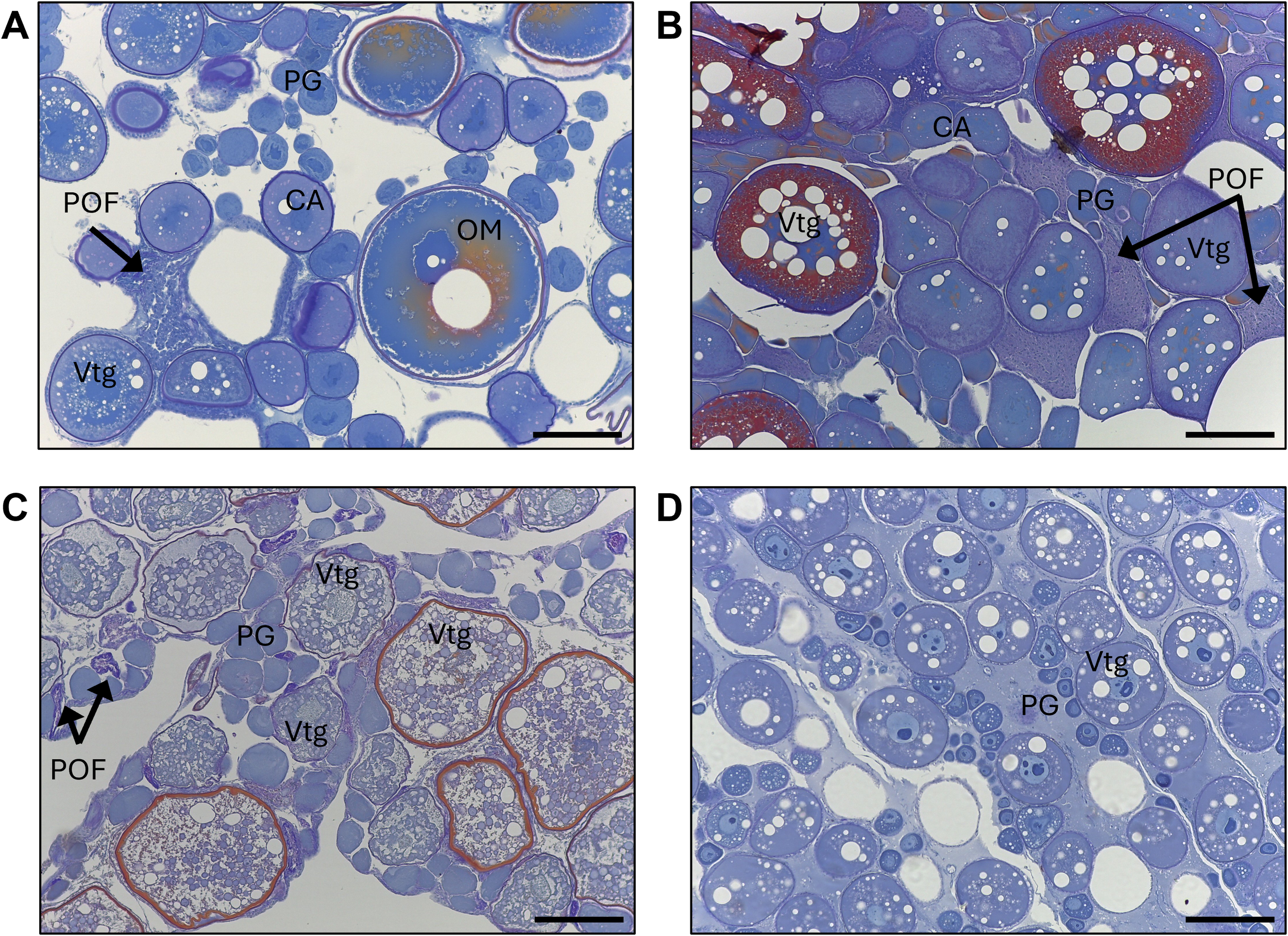
Histological micrographs from ovarian tissue stained with Schiff’s Mallory Trichrome from mature mesopelagic fishes collected in the Slope Sea between 2018 and 2022. (A)Argyropelecus aculeatus (standard length [SL]=54 mm) (B)Benthosema glaciale (SL=38 mm) (C)Scopelogadus beanii (SL=117 mm (D)Sigmops elongatus (SL=179mm). CA, cortical alveolar oocyte; OM, oocyte maturation stage oocyte; PG, primary growth oocyte; POF, postovulatory follicle complex; Vtg, vitellogenic oocyte. Scale bars = 200µm.
Figure 7
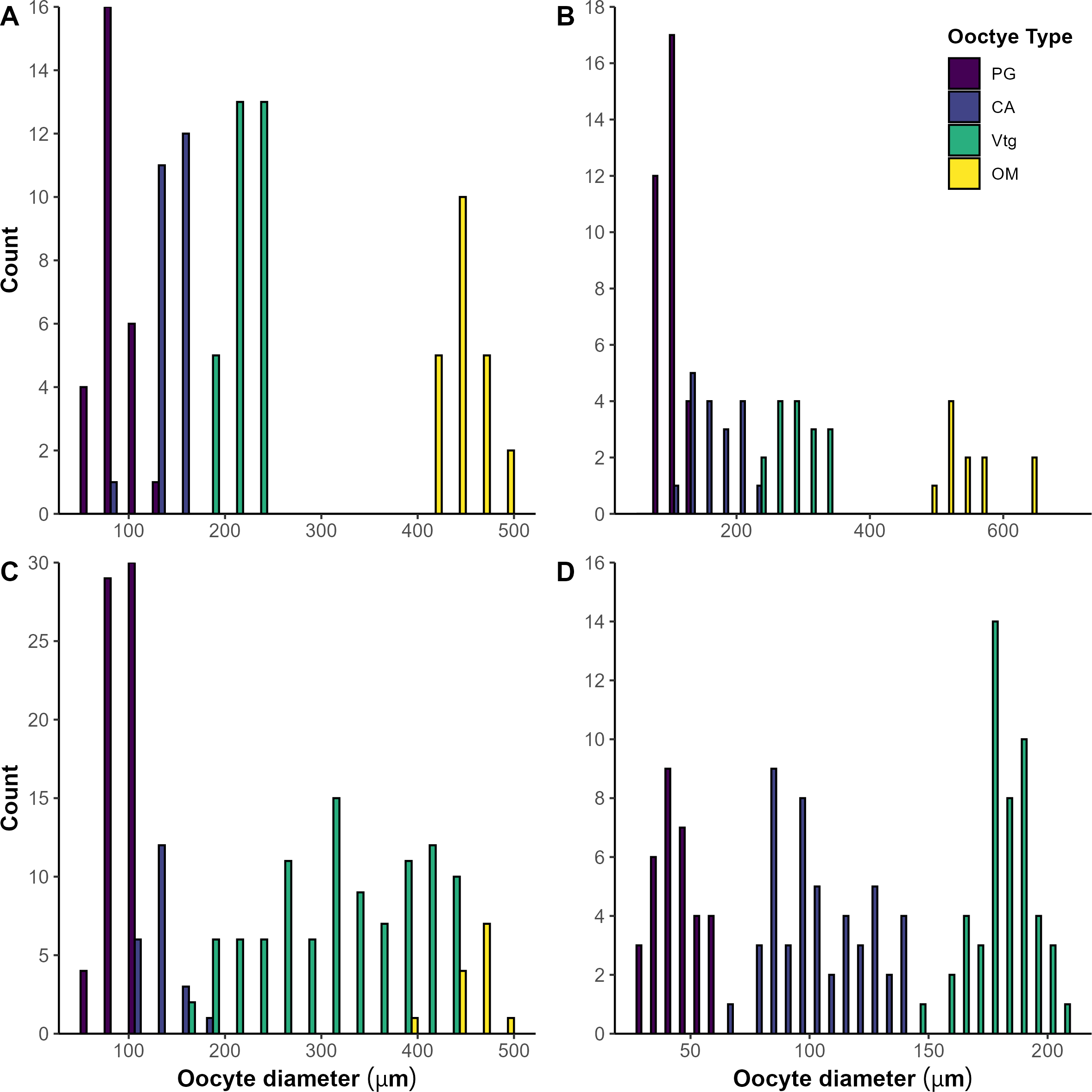
Oocyte size frequency distribution diagrams from representatives of each of four mesopelagic fish species collected in the Slope Sea between 2018 and 2022. The representatives for (A)Argyropelecus aculeatus and (B)Benthosema glaciale had ovaries in the spawning capable phase with high GSI values (5.68 and 4.90 respectively). The pattern observed in (A, B) depicts group synchronous development, with a hiatus forming between vitellogenic (Vtg) and maturation (OM) stage oocytes. The (C)Scopelogadus beanii individual had ovaries in the developing phase with low GSI values (1.67) and showed a pattern of asynchronous oocyte development. The (D)Sigmops elongatus individual had a pattern suggestive of group synchronous development; however, the size range of oocytes is small and the most advanced stage oocytes in this ovary were in very early stages of vitellogenesis. CA, cortical alveoli; PG, primary growth.
Table 5
| Species | N | α | β | Lmin (mm) | L50 (mm) | SEL50 | L95 (mm) | SEL95 |
|---|---|---|---|---|---|---|---|---|
| Argyropelecus aculeatus | 64 | -18.71 | 0.47 | 34 | 39.45 | 1.32 | 45.66 | 2.13 |
| Benthosema glaciale | 63 | -14.66 | 0.43 | 33 | 33.77 | 1.26 | 40.55 | 1.98 |
| Scopelogadus beanii | 43 | -34.53 | 0.38 | 85 | 90.38 | 2.01 | 98.09 | 3.46 |
| Sigmops elongatus | 22 | -15.20 | 0.08 | 179 | 200.45 | 7.46 | 239.27 | 17.63 |
Model parameters of binomial linear regression with logit error structures performed to determine the lengths of maturity for female mesopelagic fishes collected in the Slope Sea between 2018 and 2022.
α, intercept of the linear predictor; β, slope; Lmin, standard length at first maturity; L50, standard length at which 50% of females are mature; L95, standard length at which 95% of females are mature; SE, standard error.
Figure 8
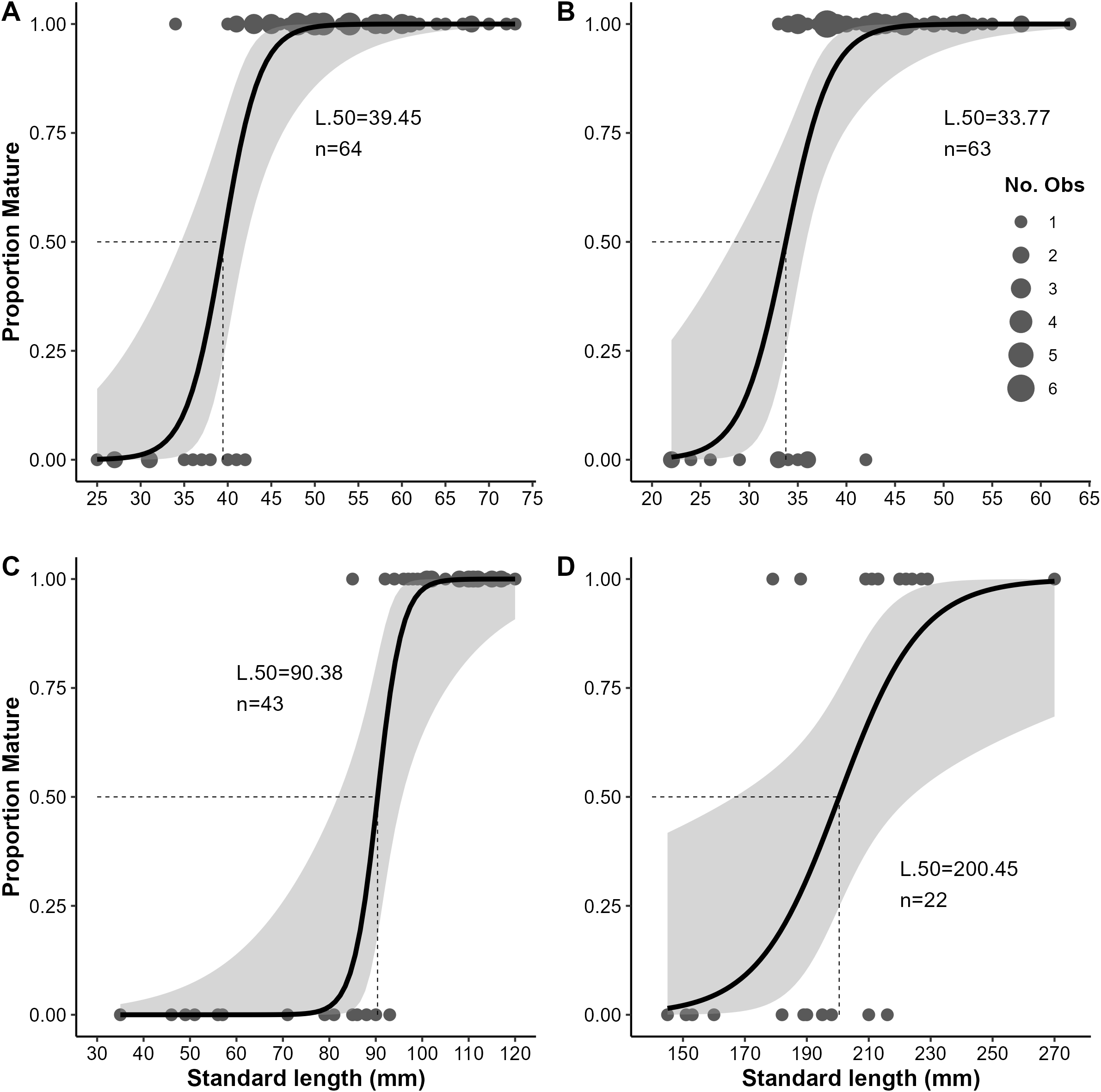
Logistic regression for the estimated proportion of females mature at a given standard length for female mesopelagic fishes collected in the Slope Sea between 2018 and 2022, (A)Argyropelecus aculeatus, (B)Benthosema glaciale, (C)Scopelogadus beanii, and (D)Sigmops elongatus. The length at which 50% of the females were mature (L50) is denoted by dashed lines. Shading indicates 95% confidence intervals. N, number of samples; No. Obs, number observed at given length for immature (0) and mature (1) females.
All phases of ovarian development were observed in B. glaciale (Figure 4B). GSI values ranged from 0.67 to 10.34 (n=39) and were significantly different among all phases except between early developing and regenerating (p=0.38; Figure 5B). We classified B. glaciale as iteroparous based on the observation of POFs and secondary growth oocytes within the same tissue (Figure 6B). Oocyte development was group synchronous with up to four distinct batches of secondary growth oocytes (Figure 7B): one female (38 mm SL) had primary growth, cortical alveolar, early vitellogenic, late vitellogenic, and hydrating oocytes present. Winter (February and March, n=39) was a period of reproductive activity based on the prevalence of females with ovaries staged as developing and the collection of fish with spawning capable ovaries exclusively in this season. Only three fish with developing ovaries were collected in the summer (Figure 8B). Diel periodicity of spawning was unclear as spawning capable fish were collected in both day (n=4) and night (n=2) tows. The only fish collected with hydrating oocytes, indicating imminent spawning, was collected around 1030 hrs local time. Estimates of L50 and L95 were 33.77 mm and 40.55 mm SL, respectively (Table 5; Figure 8B).
All phases of ovarian development except regenerating were observed in S. beanii (Figure 4C). GSI values ranged from 0.54 to 3.68 (n=18) and were not significantly different between developing and spawning capable phases (Figure 5C). We classified S. beanii as iteroparous due to the presence of POFs and developing oocytes in the same individual and the presence of a reserve stock of primary growth oocytes (Figure 6C). Oocyte development was asynchronous, with a continuum of secondary growth oocytes from the cortical alveolar through vitellogenic stages in developing ovaries and through maturation stage oocytes in spawning capable ovaries (Figure 7C). All fish with spawning capable ovaries (n=10) were collected in the summer at night; reproductive seasonality and diel periodicity was ambiguous, as 67% (n=30) of samples analyzed were collected in July and August. Most of those fish (90%; n=27) were collected at night. S. beanii observed in this study began to mature at 85 mm SL (Lmin) with L50 and L95 estimated at 90.4 mm and 98.1 mm, respectively (Table 5; Figure 8C).
Ovaries from S. elongatus were not assigned reproductive phases due to low sample sizes and difficulty in distinguishing freezing artifacts from typical oogenesis. With SMT staining, cortical alveoli appeared to be present in oocytes as small as 40 µm, and vitellogenesis appeared to be advanced in relatively small oocytes (200-280 µm; Figure 6D) based on intense eosinophilic staining in comparison stain slides. The average GSI of females with vitellogenic oocytes was 1.76 compared to 0.58 in those with less advanced oocytes. Assignment of female maturity was based on presence of vitellogenic oocytes or POFs. The smallest individual with vitellogenic oocytes (Lmin) was 179 mm SL. S. elongatus appeared to be iteroparous as females based on the observation of primary and secondary growth oocytes, beta atresia, and POFs in the same tissue of two females 213 and 270 mm SL (Figure 4D). POFs were estimated to be old based on their size and appearance and were present in 5 individuals (209–270 mm SL). Oogenesis appeared to be group synchronous with the observation of a single group of vitellogenic oocytes when present (Figure 7D). Spawning seasonality could not be assessed based on the available samples. Excluding transitional individuals, S. elongatus females had an estimated L50 of 200.45 and L95 of 239.27 mm SL (Table 5; Figure 8D), and the smallest mature female was 179 mm SL.
4 Discussion
To our knowledge, the work described here is the first to detail the reproductive patterns in A. aculeatus, S. beanii, and S. elongatus; presents the first histologically determined maturity ogives for any Argyropelecus species, S. beanii, and S. elongatus; and presents the first maturity ogive in this region of the North Atlantic for B. glaciale, to the best of our knowledge. The use of multiple types of sampling gear allowed for collection of a broad size range, up to the reported maximum size for each taxon of interest. These novel data were obtained despite the challenges of using fragile frozen tissues, which were mitigated by comparing fresh tissues and using alternate stains.
4.1 Gender systems & sex ratios
Gonochorism was the dominant gender system observed in the taxa in this study, consistent with that of teleosts more generally. While less than 1% of vertebrates demonstrate hermaphroditism, the majority of those that do are fishes (Kuwamura et al., 2020). Hermaphroditism in general, and protandry (male to female sex-change) specifically, is thought to be favored when mating is random and size-selective increases in reproductive success are more pronounced in females (Sunobe, 2023; Warner, 1988). The high relative abundances of Myctophids, Melamphaids, and Sternoptychids observed in many studies (Olivar et al., 2017; Sutton et al., 2008) suggest that conspecifics aggregate; presumably, aggregations formed due to increased food availability associated with oceanographic features that concentrated prey would also provide enhanced spawning opportunities. S. elongatus are primed for protandry if they are more solitary than the other species of interest in this study and given that in many fish taxa, female reproductive success increases disproportionately with size (Barneche et al., 2018; Fitzhugh et al., 2012; Hixon et al., 2014).
The sex ratios we observed in this study skewed female when different from parity (i.e. in all but A. aculeatus). Skewed sex ratios can occur when there are sex-based differential mortality, longevity, or growth rates (Charnov, 1982; Clarke, 1983; Linkowski et al., 1993). Sex ratios that skew female increase the egg producing biomass of a population and have been observed in other mesopelagic species (Andresen et al., 2025; Clarke, 1983). It should be noted, however, that the observed ratios in this or any study may be influenced by accessibility, discernability, and robustness of ovaries compared to testes, and may not be a precise representation of the catch or the overall population. In this study, testes were more difficult to distinguish in the body cavities of these relatively small species, especially in presumably immature fish. Flash freezing made the internal organs, and the testes in particular, difficult to extract as they were more delicate compared to those from freshly dissected individuals and from females. Furthermore, beyond the differences in accessibility of gonads, there may be diel, seasonal, size and/or depth patterns in distribution by sex seen in other mesopelagic taxa (Almeida and Rossi-Wongtschowski, 2007; Halliday, 1970; Howell and Krueger, 1987; Kawaguchi and Mauchline, 1982) that are not evident in the limited collections of any single study.
4.2 Female reproductive strategies
All species we examined appeared to be iteroparous, capable of participating in multiple reproductive events over the course of their lifetimes (Murua and Saborido-Rey, 2003). Evidence of iteroparity in female specimens included the presence of oogonia and primary growth oocytes in ovaries of all developmental stages, but particularly those staged as developing and spawning-capable: these oocyte stages are not found in reproductively active semelparous fishes (Heins and Brown-Peterson, 2022; Lowerre-Barbieri et al., 2011). Furthermore, POFs, the remnants of ovarian follicles that remain and slowly degrade after mature oocytes are ovulated, were observed in ovaries from all species. Additional evidence we observed in A. aculeatus and B. glaciale included individuals that appeared to be between spawning seasons (i.e. those with regressing/regenerating ovaries). Iteroparity, which is the dominant reproductive strategy in teleosts (Wootton and Smith, 2015), is typically viewed as a bet-hedging strategy, acting to increase lifetime reproductive potential by spreading risk over multiple years and environmental conditions. Iteroparity is thus thought to be favored in temporally heterogeneous systems with high variability in reproductive success and is associated with relatively delayed adult maturity (Bell, 1976; Wilbur and Rudolf, 2006). The temperate waters of the Slope Sea from which our fish were collected are characterized by large seasonal fluctuations in productivity. These fluctuations are associated with interannual variability in the timing of the spring bloom (Dutkiewicz et al., 2001), as well as highly stochastic effects of Labrador Sea and Gulf Stream waters and frequent passage of warm and cold core eddies with complex ephemeral impacts on regional chemical and biological conditions (Csanady and Hamilton, 1988). The mesopelagic depths of these regions, with their integral connection to variations in surface flow and productivity, are likely to exhibit both spatial and temporal patchiness in resource availability. This unpredictability would make semelparity highly risky for mesopelagic fishes in these regions and would favor repeat spawning as an adaptive strategy for maximizing lifetime reproductive output.
Among iteroparous fishes, many species exhibit batch spawning: repeated spawning throughout a reproductive season. The development of multiple stages of secondary growth oocytes that we observed in A. aculeatus and B. glaciale is consistent with a batch spawning strategy (Murua and Saborido-Rey, 2003). The discrete batches of vitellogenic and maturing oocytes along with primary growth and cortical alveolar oocytes that we found also suggest these species have indeterminate fecundity, with the reproductive potential of the species being a product of batch fecundity and the number of spawning events throughout the season (Hunter et al., 1992; Murua and Saborido-Rey, 2003). The spawning frequency (duration between spawning events) could not be determined in this study due to small sample sizes and lack of seasonal coverage; however, A. acuelatus and B. glaciale could produce a minimum of three and four batches, respectively. The indeterminate batch spawning strategy and minimum batch number we report is consistent with those for B. glaciale in other regions (Garcia-Seoane et al, 2014; Mazhirinia, 1988) and myctophids more generally (Dalpadado, 1988; Sarmiento-Lezcano et al., 2020; Sassa and Takahashi, 2022). We did not find earlier reports on reproductive strategy for Argyroplecus sp., though other Sternoptychid species have an indeterminate batch fecundity strategy (Almeida and Rossi-Wongtschowski, 2007; Goodson et al., 1995; Salvanes and Stockley, 1996; Young et al., 1987).
Diel periodicity of spawning in B. glaciale and A. aculeatus could not be determined in this study. Given that eggs are positively buoyant (Sundby and Kristiansen, 2015) and larvae are found in epipelagic waters (Daudén-Bengoa et al., 2020; Sassa et al., 2004; Wang et al., 2021), it is possible spawning occurs at night in shallow waters. This strategy is hypothesized to confer a survival advantage and optimize larval growth by releasing eggs in warmer and more productive waters compared to the deeper layers (Gartner, 1993). Conversely, as temperature is positively correlated with rates of oogenesis (Kjesbu et al., 2010; Kurita et al., 2011), it is also possible that exposure to shallower warmer water temperatures at night allows for more rapid development of the forthcoming oocyte batch which is then released at mesopelagic depths during the day. Myctophid species show both strategies (Gartner, 1993; Sassa, 2019), and our data do not clarify the strategy taken by the species of interest in our study. Whether spawning occurs in the epi- or mesopelagic, species that participate in diel vertical migration, such as A. aculeatus and B. glaciale, likely have more rapid oogenesis compared to deep-dwelling species due to increased metabolic rates associated with an increase in mean ambient temperature.
We observed multiple stages of secondary growth oocytes present in ovaries of S. beanii, but these fishes demonstrated asynchronous oocyte development and a lack of distinct batches. S. beanii occupy the deeper mesopelagic and shallow bathypelagic and do not participate in diel vertical migration (Cook et al., 2013; Govindarajan et al., 2023; Marohn et al., 2021). The continuum of secondary growth oocytes we observed may reflect prolonged oogenesis due to the low mesopelagic temperatures (2 to 5°C; (Helfman et al., 2009). S. beanii may experience multi-year oocyte development, as observed in a variety of cold-water species (Gunnarsson et al., 2006; Junquera et al., 2003; Lefebvre and Field, 2015; McBride et al., 2022). Whether spawning occurs annually or at another interval remains unclear.
4.3 Female reproductive seasonality & maturity
Seasonality of spawning in A. aculeatus could not be conclusively determined due to most fish being collected in July and August in the current study; the spawning activity we observed during this time was consistent with the summer through fall peak suggested for the species in the Sargasso Sea based on the abundance of postlarvae in plankton collections (Howell and Krueger, 1987). Our observation of a regressing/regenerating female of 58 mm SL in August, as well as the late summer collection by Howell and Krueger (1987) of seven fish 57–71 mm SL with resting ovaries, suggests that even if there are not distinct spawning seasons, mature individuals experience interspawning periods.
The L50 we estimated for A. aculeatus (39.5 mm SL) was similar to the upper end of “subadults” for the species suggested by Howell and Krueger (1987). In the earlier study, ovaries were examined macroscopically and a coarse estimate of Lmin of 52 mm SL was based on “large ova near the size at spawning” being visible over 90% of the ovary (Howell and Krueger, 1987). However, the authors noted a 35 mm female in “breeding condition”, suggesting some individuals mature at a smaller size. Our maturity criteria were conservative, in general, using vitellogenesis as the indicator of maturity: reproductive studies often use presence of first stage of secondary growth oocytes (cortical alveolar) to indicate maturity (Lowerre-Barbieri et al., 2011; Wallace and Selman, 1981). Because there are observed differences between physiological maturity (i.e. when an individual is physiologically capable of reproducing) and functional maturity (i.e. when an individual begins to contribute to production), as well as skipped spawning (i.e. missed reproduction events) in mature adults, documented across a wide variety of taxa (Kennedy et al., 2011; Lefebvre and Field, 2015; McBride et al., 2022; Rideout and Tomkiewicz, 2011), we conducted sensitivity analyses to determine how our L50 estimates would change using an even more conservative criteria of intermediate vitellogenesis (Supplementary Figure S1A). This sensitivity analysis resulted in an L50 of 48.69 mm SL for A. aculeatus and could be considered as the most conservative estimate for population assessment models of the species, given its unknown population dynamics. Additional collections of freshly fixed gonads throughout the year would assist in clarifying the most appropriate assignment of maturity.
We observed B. glaciale spawning activity in the Slope Sea during the winter (February and March), and fish were in the early stages of development for the subsequent spawning season in the summer and fall (July, August, and October). The winter and early-spring spawning in the western North Atlantic we documented was supported in other studies of the species based on the condition of ovaries of mature females in the summer and winter (Garcia-Seoane et al, 2014; Halliday, 1970) as well the presence of postlarvae in midwater collections from May and June (Halliday, 1970). Consistent with the current study, Garcia-Seoane et al (2014) presented evidence of a seasonal, batch spawning and indeterminate fecundity pattern for B. glaciale in the Flemish Cap off Nova Scotia. The early spring spawning we observed for B. glaciale in the Slope Sea would result in larvae residing in the epipelagic during the seasonal zooplankton production maxima (Friedland et al., 2015). The species is estimated to live up to seven years (Garcia-Seoane et al, 2014; Gjosater, 1973; Halliday, 1970; Kristoffersen and Salvanes, 2009), and preliminary aging efforts associated with the current study suggest females are mature by at least age three (unpublished data). The maximum age, age at maturity, and observation of regressing/regenerating females implies individuals participate in multiple spawning seasons.
The L50 for B. glaciale reported here (33.46 mm SL) is notably smaller than that reported for the species off Nova Scotia by Garcia-Seoane et al., 2014 (47.6 mm SL) and the Lmin reported in the same area by Mazhirinia, 1988 (46 mm SL). In both previous studies, regional differences in size at maturity were noted, with a decrease from west to east across the Atlantic (L50 = 24.5 mm in the Balearic Sea [Garcia-Seoane et al., 2014]; Lmin =33 mm in the Hatton Plateau [Mazhirinia, 1988). The criteria we used to define maturity are different from that of Garcia-Seoane et al (2014), who used the cortical alveolar stage to define maturity (the maturity criteria used by Mazhirinia, 1988 is not well defined). However, more conservative maturity criteria, as we used, would result in an increased L50 if all else were equal. Instead, the comparatively low size at maturity reported here is most attributable to regional differences: the collections off Nova Scotia occurred north of the 42nd latitude in the North Atlantic Drift biogeographic zone, while the current collections occurred at 39-40°N in what is considered the Central North Atlantic ecoregion (Sutton et al., 2017). There are generally positive relationships between asymptotic length and latitude (Gertseva et al., 2017) as well as asymptotic length and length at maturity (Froese and Binohlan, 2000) in marine fishes. Furthermore, intraspecific differences in length or age at maturity and other reproductive parameters (i.e. fecundity and spawning frequency) across biogeographical breaks are well documented in other taxa (Head et al., 2014; Lefebvre et al., 2018; McBride et al., 2013). We performed a sensitivity analysis by temporal restricting samples to those from the reproductive season (February and March) and using a more conservative criteria of mid-vitellogenesis, which resulted in a minimal increase in L50 to 34.32 mm SL (Supplementary Figure S1B).
Summer was a period of reproduction for S. beanii based on our observations, although it remains unknown if spawning occurs in other seasons due to the apparent size selectivity and seasonal usage of collection gears in the current study. The MARMWT collected primarily larger, mature individuals and was only deployed in July and August. To the best of our knowledge, this is the first report of the reproductive biology of S. beanii. Their congener Scopelogadus mizolepis were reproductively active in March and April in the eastern central Atlantic (Knorrn et al., 2024). No fish with regressing/regenerating ovaries were collected in this study, potentially due to the afore mentioned bias in sampling gear and season or because, upon reaching maturity, this species spawns continuously and dies. The latter “functionally semelparous” strategy has been suggested for other Melamphaids (Keene et al., 1987; Knorrn et al., 2024).
The maturity ogive presented is the first reported for S. beanii and the estimated L50 (90 mm SL) is notably larger than that reported for its congener S. mizolepis (46 mm SL, Knorrn et al., 2024). S. beanii reside in higher latitudes than S. mizolepis and attain higher maximum lengths (Ebeling and Weed, 1963; Keene et al., 1987; Kotlyar, 2019, Kotlyar, 2020). This stark difference in L50 is unsurprising considering the afore discussed trend in latitude and maturity and that metabolic growth rates are likely different between tropical and temperate species. A sensitivity analysis on maturity estimates was not possible for S. beanii due to a lack of model convergence based on our samples (i.e. all fish below 98 mm SL were immature; all above were mature).
The paucity of S. elongatus females with spawning capable ovaries in this study precluded interpretation of oogenesis, fecundity, or seasonal patterns. The maximum observed oocyte diameter (280 µm) was much smaller than that observed in other the species examined in this study (400-650 µm). While freezing artifacts made the determination of discrete vitellogenic stages impossible, the largest oocytes observed in S. elongatus appeared to be advanced due to the amount of vitellogenin present (as evidenced in comparison slides stained with hematoxylin and eosin-y). Therefore, it is possible that S. elongatus may have smaller eggs compared to the other taxa observed, a strategy that would maximize fecundity and spread risk among many gametes in a species with unpredictable spawning opportunities.
The estimated L50 for female S. elongatus in the current study (200 mm SL) is the first reported based on histological analysis for the species. The Lmin observed here (179 mm SL) was similar to that reported for females in the central Pacific (182 mm SL; Clarke, 1983). Fisher (1983) reported a Lmin of 125 mm SL in the Gulf of Mexico based primarily on microscopic viewing of whole mounts and limited histological sections. Similarly, the size range of transitional fish was lower in the Gulf of Mexico (86–117 mm; Fisher, 1983) compared to in the Slope Sea (103–147 mm). Off Hawaii, the largest male observed was 10 mm smaller than the smallest mature female (Clarke, 1983); while the author declared the species to be gonochoristic, transitional tissue is difficult to detect barring histological analysis (Sadovy and Shapiro, 1987).
4.4 Conclusions
As resources of the mesopelagic garner attention for potential extraction and as we aim to understand how climate change will impact the global oceans and biogeochemical cycling, life history data, and reproductive life history in particular, remain a critical data gap necessary to understand the impact mesopelagic fishes have on these systems. The female reproductive strategy of iteroparous batch spawning observed in A. aculeatus and B. glaciale in this study are consistent with the dominant strategies seen in epipelagic and shallow-water demersal fish species. Iteroparity is theoretically consistent with a life history that relies upon longevity to reach full reproductive potential, highlighting the risk of overharvesting smaller individuals. The probable multi-year oocyte development in S. beanii and the confirmed protandrous hermaphroditism in S. elongatus make these species vulnerable to mortality prior to contributing to reproduction. Furthermore, the taxa observed here are larger at maturity than congeners and conspecifics in other regions, underscoring the vulnerability of these populations to overexploitation due to directed or incidental fishing of immature individuals. Gaining species-level resolution and a fuller understanding of geographical and taxonomic diversity of reproductive traits within and among taxa is critical for management of some of the world’s most abundant vertebrates, many of which are highly vulnerable to the looming threat of deep-water commercial extraction. Careful consideration of these (and similar) data will be necessary for the development of a sustainable mid-water fishing management plan.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Woods Hole Oceanographic Institution Institutional Animal Care and Use Committee (WHOI IACUC), Project ID numbers 24708.01 and BI26870.01. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
LL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. HS: Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. JL: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Ocean Twilight Zone project (Woods Hole Oceanographic Institution and the Audacious Project housed at TED). NES-LTER cruises were funded by the National Science Foundation (#OCE-1655686, #OCE-2322676).
Acknowledgments
We would like to thank the crew of the R/V Armstrong, R/V Endeavour, and NOAA ship Henry Bigelow, and the scientists, technicians, students, and volunteers who participated in data collection. We would specifically like to thank Mike Jech of NOAA (Northeast Fisheries Science Center) and the members of the FOLFE lab, Paul Caiger, Julia Cox, Sarah Glancy, and Helena McMonagle for their at-sea and in-lab contributions. A special thanks to Martha Hauff (Stonehill College/WHOI) for coordination and review of the manuscript. Thank you to the Govindarajan lab at WHOI for genetic confirmation of species ID; Peter Wiebe for processing MOCNESS data; and the co-primary investigators and project managers of the Ocean Twilight Zone. NES-LTER collections were funded by the National Science Foundation (#OCE-1655686, #OCE-2322676). This project was funded by the Ocean Twilight Zone project (Woods Hole Oceanographic Institution and the Audacious Project housed at TED).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1582706/full#supplementary-material
References
1
Almeida E. M. Rossi-Wongtschowski C. L. D. B. (2007). Maurolicus stehmanni Parin & Kobyliansky 1993 (Sternoptychidae): length of first maturation, and spawning seasons in the south-southeast Brazilian region. Braz. J. Oceanography.55, 309–322. doi: 10.1590/S1679-87592007000400007
2
Andresen H. Eduardo L. N. Olivar M. P. Van Denderen P. D. Spitz J. Maureaud A. A. et al . (2025). Mesopelagic fish traits: functions and trade-offs. Fish Fisheries.26, 83–103. doi: 10.1111/faf.12867
3
Barneche D. R. Roberston D. R. White C. R. Marshall D. J. (2018). Fish reproductive-energy output increases disproportionately with body size. Science.360, 642–645. doi: 10.1126/science.aao6868
4
Bell G. (1976). On breeding more than once. Am. Naturalist.110, 57–77. doi: 10.1086/283048
5
Bianchi D. Carozza D. A. Galbraith E. D. Guiet J. Devries T. (2021). Estimating global biomass and biogeochemical cycling of marine fish with and without fishing. Sci. Advances.7, eabd7554. doi: 10.1126/sciadv.abd7554
6
Boyd P. W. Claustre H. Levy M. Siegel D. A. Weber T. (2019). Multi-faceted particle pumps drive carbon sequestration in the ocean. Nature.568, 327–335. doi: 10.1038/s41586-019-1098-2
7
Caiger P. E. Lefebvre L. S. Llopiz J. K. Proud R. (2021). Growth and reproduction in mesopelagic fishes: a literature synthesis. ICES J. Marine Science.78, 765–781. doi: 10.1093/icesjms/fsaa247
8
Catul V. Gauns M. Karuppasamy P. K. (2011). A review on mesopelagic fishes belonging to family Myctophidae. Rev. Fish Biol. Fisheries.21, 339–354. doi: 10.1007/s11160-010-9176-4
9
Charnov E. L. (1982). The theory of sex allocation. Monogr. Population Biol.18, 1–355.
10
Choy C. A. Portner E. Iwane M. Drazen J. C. (2013). Diets of five important predatory mesopelagic fishes of the central North Pacific. Marine Ecol. Prog. Series.492, 169–184. doi: 10.3354/meps10518
11
Clarke T. A. (1983). Sex ratios and sexual differences in size among mesopelagic fishes from the Central Pacific Ocean. Marine Biol.73, 203–209. doi: 10.1007/BF00406889
12
Cook A. B. Sutton T. T. Galbraith J. K. Vecchione M. (2013). Deep-pelagic (0–3000m) fish assemblage structure over the Mid-Atlantic Ridge in the area of the Charlie-Gibbs Fracture Zone. Deep Sea Res. Part II: Topical Stud. Oceanography.98, 279–291. doi: 10.1016/j.dsr2.2012.09.003
13
Csanady G. T. Hamilton P. (1988). Circulation of slopewater. Continental Shelf Res.8, 565–624. doi: 10.1016/0278-4343(88)90068-4
14
Dalpadado P. (1988). Reproductive biology of the lanternfish Benthosema pterotum from the Indian Ocean. Marine Biol.98, 307–316. doi: 10.1007/BF00391106
15
Daudén-Bengoa G. Jiménez-Rosenberg S. P. A. Compaire J. C. Del Pilar Echeverri-Garcia L. Pérez-Brunius P. Herzka S. Z. (2020). Larval fish assemblages of myctophids in the deep water region of the southern Gulf of Mexico linked to oceanographic conditions. Deep Sea Res. Part I: Oceanographic Res. Papers.155, 103181. doi: 10.1016/j.dsr.2019.103181
16
Dutkiewicz S. Follows M. Marshall J. Gregg W. W. (2001). Interannual variability of phytoplankton abundances in the North Atlantic. Deep Sea Res. Part II: Topical Stud. Oceanography.48, 2323–2344. doi: 10.1016/S0967-0645(00)00178-8
17
Ebeling A. W. Weed W. H. (1963). Melamphaidae III. Systematics and distribution of the species in the bathypelagic fish genus Scopelogadus Vaillant. Dana Rep. 60, 1–58
18
Fisher R. A. (1983). Protandric sex reversal in Gonostoma elongatum (Pisces: Gonostomatidae) from the Eastern Gulf of Mexico. Copeia.1983, 554–567. doi: 10.2307/1444411
19
Fitzhugh G. R. Shertzer K. W. Kellison G. T. Wyanski D. M. (2012). Review of size- and age-dependence in batch spawning: implications for stock assessment of fish species exhibiting indeterminate fecundity. Fishery Bulletin.110, 413–425.
20
Fjeld K. Tiller R. Grimaldo E. Grimsmo L. Standal I.-B. (2023). Mesopelagics–New gold rush or castle in the sky? Marine Policy.147, 105359. doi: 10.1016/j.marpol.2022.105359
21
Friedland K. D. Leaf R. T. Kane J. Tommasi D. Asch R. G. Rebuck N. et al . (2015). Spring bloom dynamics and zooplankton biomass response on the US Northeast Continental Shelf. Continental Shelf Res.102, 47–61. doi: 10.1016/j.csr.2015.04.005
22
Froese R. Binohlan C. (2000). Empirical relationships to estimate asymptotic length, length at first maturity and length at maximum yield per recruit in fishes, with a simple method to evaluate length frequency data. J. Fish Biol.56, 758–773. doi: 10.1111/j.1095-8649.2000.tb00870.x
23
Froese R. Pauly D. Editors. (2024). FishBase. World Wide Web electronic publication. Available online at: www.fishbase.org (Accessed June 2024).
24
Garcia-Seoane E. Bernal A. Saborido-Rey F. (2014). Reproductive ecology of the glacier lanternfish Benthosema glaciale. Hydrobiologia.727, 137–149. doi: 10.1007/s10750-013-1796-y
25
Gartner J. V. (1993). Patterns of reproduction in the dominant lanternfish species (Pisces: Myctophidae) of the Eastern Gulf of Mexico, with a review of reproduction among tropical-subtropical myctophidae. Bull. Marine Science.52, 721–750.
26
Gertseva V. Matson S. E. Cope J. (2017). Spatial growth variability in marine fish: example from Northeast Pacific groundfish. ICES J. Marine Science74 (6), 1602–1613. doi: 10.1093/icesjms/fsx016
27
Gjosater J. (1973). Age, growth, and mortality of the myctophid fish, Benthosema glaciale (Reinhardt), from western Norway. Sarsia.52, 1–14.
28
Gjosater J. Kawaguchi K. (1980). “A review of the world resources of mesopelagic fish,” in FAO fisheries technical paper. (Rome: Food and Agriculture Organization of the United Nations)
29
Gong H. Li C. Zhou Y. (2021). Emerging global ocean deoxygenation across the 21st century. Geophysical Res. Lett.48, e2021GL095370. doi: 10.1029/2021GL095370
30
Goodson M. S. Giske J. Rosland R. (1995). Growth and ovarian development of Maurolicus muelleri during spring. Marine Biol.124, 185–195. doi: 10.1007/BF00347122
31
Govindarajan A. F. Llopiz J. K. Caiger P. E. Jech J. M. Lavery A. C. Mcmonagle H. et al . (2023). Assessing mesopelagic fish diversity and diel vertical migration with environmental DNA. Front. Marine Science.10. doi: 10.3389/fmars.2023.1219993
32
Grier H. J. Uribe Aranzabal M. C. Patiño R. (2009). “The ovary, follicululogenesis, and oogenesis in teleosts,” in Reproductive biology and phylogeny of fishes (Agnathans and bony fishes). Ed. JamiesonB. G. M. (Science Publishers, Enfield, New Hampshire).
33
Grimaldo E. Grimsmo L. Alvarez P. Herrmann B. Moen Tveit G. Tiller R. et al . (2020). Investigating the potential for a commercial fishery in the Northeast Atlantic utilizing mesopelagic species. ICES J. Marine Science.77, 2541–2556. doi: 10.1093/icesjms/fsaa114
34
Gunnarsson A. Hjorleifsson E. Thorainsson K. Marteinsdottir G. (2006). Growth, maturity and fecundity of wolffish Anarhichas lupus L. in Icelandic waters. J. Fish Biol.68, 1158–1176. doi: 10.1111/j.0022-1112.2006.00990.x
35
Halliday R. G. (1970). Growth and vertical distribution of the glacier lanternfish, Benthosema glaciale, in the Northwestern Atlantic. J. Fisheries Res. Board Canada.27, 105–116. doi: 10.1139/f70-011
36
Head M. A. Keller A. A. Bradburn M. (2014). Maturity and growth of sablefish, Anoplopoma fimbria, along the US West Coast. Fisheries Res.159, 56–67. doi: 10.1016/j.fishres.2014.05.007
37
Heins D. C. Brown-Peterson N. J. (2022). The reproductive biology of small fishes and the clutch concept: Combining macroscopic and histological approaches. Aquaculture Fish Fisheries.2, 253–264. doi: 10.1002/aff2.49
38
Helfman G. S. Collette B. B. Facey D. E. Bowen B. B. (2009). The diversity of fishes: biology, evolution, and ecology (Chichester, UK: John-Wiley & Sons).
39
Hixon M. A. Johnson D. W. Sogard S. M. (2014). BOFFFFs: on the importance of conserving old-growth age structure in fishery populations. ICES J. Marine Science: J. du Conseil.71, 2171–2185. doi: 10.1093/icesjms/fst200
40
Howell H. Krueger W. H. (1987). “Family Sternoptychidae, marine hatchetfishes and related species,” in Biology of midwater fishes of the Bermuda ocean acre. Eds. GibbsR. H. J.KruegerW. H. (Smithsonian Institute Press, Washington D.C).
41
Humason G. L. (1972). Animal tissue techniques (San Francisco and London: W. H. Freeman and Co).
42
Hunter J. R. Macewicz B. J. Lo N. C. Kimbrell C. A. (1992). Fecundity, spawning, and maturity of female Dover sole, Microstomus pacificus, with an evaluation of assumptions and precision. Fishery Bulletin.90, 101–128.
43
ICES (2021). Working Group on Zooplankton Ecology (WGZE; outputs from 2020 meeting). ICES Sci. Rep. 3 (7), 52. doi: 10.17895/ices.pub.7689
44
Iglesias I. S. Fiechter J. Santora J. A. Field J. C. (2024). Vertical distribution of mesopelagic fishes deepens during marine heatwave in the California Current. ICES J. Marine Sci. 81 (9), 1837–1849. doi: 10.1093/icesjms/fsae129
45
Iglesias I. S. Santora J. A. Fiechter J. Field J. C. (2023). Mesopelagic fishes are important prey for a diversity of predators. Front. Mar. Sci. 10, 1220088. doi: 10.3389/fmars.2023.1220088
46
Irigoien X. Klevjer T. A. Rostad A. Martinez U. Boyra G. Acuña J. L. et al . (2014). Large mesopelagic fishes biomass and trophic efficiency in the open ocean. Nat. Communications.5, 3271. doi: 10.1038/ncomms4271
47
Isaacs J. D. Tont S. A. Wick G. L. (1974). Deep scattering layers: vertical migration as a tactic for finding food. Deep Sea Res. Oceanographic Abstracts.21, 651–656. doi: 10.1016/0011-7471(74)90049-7
48
Jennings S. Mélin F. Blanchard J. L. Forster R. M. Dulvy N. K. Wilson R. W. (2008). Global-scale predictions of community and ecosystem properties from simple ecological theory. Proc. Biol. Sci.275, 1375–1383. doi: 10.1098/rspb.2008.0192
49
Junquera S. Roman E. Morgan J. Sainza M. Ramilo G. (2003). Time scale of ovarian maturation in Greenland halibut (Reinhardtius hippoglossoides, Walbaum). Int. Council Explor. Sea.60, 767–773. doi: 10.1016/S1054-3139(03)00073-0
50
Kaartvedt S. Staby A. Aksnes D. L. (2012). Efficient trawl avoidance by mesopelagic fishes causes large underestimation of their biomass. Marine Ecol. Prog. Series.456, 1–6. doi: 10.3354/meps09785
51
Kawaguchi K. Mauchline J. (1982). Biology of myctophidae fishes (family Myctophidae) in the Rockall Trough, Northeastern Atlantic Ocean. Biol. Ocean.1, 337–373. doi: 10.1080/01965581.1982.10749447
52
Keene M. J. Gibbs R. H. J. Krueger W. H. (1987). “Family Melamphaidae, big scales,” in Biology of midwater fishes of the Bermuda ocean acre. Eds. GibbsR. H. J.KruegerW. H. (Smithsonian Institution Press, Washington D. C).
53
Kennedy J. Nash R. D. M. Slotte A. Kjesbu O. S. (2011). The role of fecundity regulation and abortive maturation in the reproductive strategy of Norwegian spring-spawning herring (Clupea harengus). Marine Biol.158, 1287–1299. doi: 10.1007/s00227-011-1648-0
54
Kjesbu O. S. Righton D. Krüger-Johnsen M. Thorsen A. Michalsen K. Fonn M. et al . (2010). Thermal dynamics of ovarian maturation in Atlantic cod (Gadus morhua). Can. J. Fisheries Aquat. Sci.67, 605–625. doi: 10.1139/F10-011
55
Knorrn A. H. Wieben K. L. Fock H. O. Andresen H. (2024). Reproductive biology of the electric lanternfish Electrona risso (Myctophidae) and the bigscale fishes Melamphaes polylepis and Scopelogadus mizolepis (Melamphaidae). J. Fish Biol.104, 252–264. doi: 10.1111/jfb.15575
56
Koslow J. A. Davison P. Ferrer E. Jiménez Rosenberg S. P. A. Aceves-Medina G. Watson W. (2019). The evolving response of mesopelagic fishes to declining midwater oxygen concentrations in the southern and central California Current. ICES J. Marine Science.76, 626–638. doi: 10.1093/icesjms/fsy154
57
Kotlyar A. N. (2019). Revision of the genus Scopelogadus (Melamphaidae): 1. S. beanii. J. Ichthyology.59, 641–655. doi: 10.1134/S0032945221010094
58
Kotlyar A. N. (2020). Revision of genus scopelogadus (Melamphaidae): 2. S. mizolepis. J. Ichthyology.60, 1–12. doi: 10.1134/s0032945220010087
59
Kristoffersen J. B. Salvanes A. G. V. (2009). Distribution, growth, and population genetics of the glacier lanternfish (Benthosema glaciale) in Norwegian waters: contrasting patterns in fjords and the ocean. Marine Biol. Res.5, 596–604. doi: 10.1080/17451000903042479
60
Kurita Y. Fujinami Y. Amano M. (2011). The effect of temperature on the duration of spawning markers–migratory-nucleus and hydrated oocytes and postovulatory follicles–in the multiple-batch spawner Japanese flounder (Paralichthys olivaceus). Fishery Bulletin.109, 79–89.
61
Kuwamura T. Sunobe T. Sakai Y. Kadota T. Sawada K. (2020). Hermaphroditism in fishes: an annotated list of species, phylogeny, and mating system. Ichthyological Res.67, 341–360. doi: 10.1007/s10228-020-00754-6
62
Lefebvre L. S. Beyer S. G. Stafford D. M. Kashef N. S. Dick E. J. Sogard S. M. et al . (2018). Double or nothing: Plasticity in reproductive output in the chilipepper rockfish (Sebastes goodei). Fisheries Res.204, 258–268. doi: 10.1016/j.fishres.2018.03.002
63
Lefebvre L. S. Field J. C. (2015). Reproductive complexity in a long-lived deepwater fish, Blackgill rockfish Sebastes melanostomus. Trans. Am. Fisheries Society.144, 383–399. doi: 10.1080/00028487.2014.1001039
64
Linkowski T. B. Radtke R. L. Lenz P. H. (1993). Otolith microstructure, age and growth of two species of Ceratoscopelus (Oosteichthyes: Myctophidae) from the eastern North Atlantic. J. Exp. Marine Biol. Ecology.167, 237–260. doi: 10.1016/0022-0981(93)90033-K
65
Liu S. Liu Y. Teschke K. Hindell M. A. Downey R. Woods B. et al . (2024). Incorporating mesopelagic fish into the evaluation of conservation areas for marine living resources under climate change scenarios. Mar Life Sci. Technol.6, 68–83. doi: 10.1007/s42995-023-00188-9
66
Longhurst A. R. Bedo A. W. Harrison W. G. Head E. J. H. Sameoto D. D. (1990). Vertical flux of respiratory carbon by oceanic diel migrant biota. Deep Sea Res. Part A. Oceanographic Res. Papers.37, 685–694. doi: 10.1016/0198-0149(90)90098-G
67
Lowerre-Barbieri S. K. Brown-Peterson N. J. Murua H. Tomkiewicz J. Wyanski D. M. Saborido-Rey F. (2011). Emerging issues and methodological advances in fisheries reproductive biology. Marine Coastal Fisheries: Dynamics Management Ecosystem Science.3, 32–51. doi: 10.1080/19425120.2011.555725
68
Lowerre-Barbieri S. K. Brown-Peterson N. J. Wyanski D. M. Moncrief-Cox H. E. Kolmos K. J. Menendez H. S. et al . (2023). A unified framework and terminology for reproductive traits integral to understanding fish population productivity. Marine Coastal Fisheries.15, e, 10276. doi: 10.1002/mcf2.10276
69
Marks A. D. Kerstetter D. W. Wyanski D. M. Sutton T. T. (2020). Reproductive ecology of dragonfishes (Stomiiformes: stomiidae) in the gulf of Mexico. Front. Marine Sci.7. doi: 10.3389/fmars.2020.00101
70
Marohn L. Schaber M. Freese M. Pohlmann J. D. Wysujack K. Czudaj S. et al . (2021). Distribution and diel vertical migration of mesopelagic fishes in the Southern Sargasso Sea — observations through hydroacoustics and stratified catches. Marine Biodiversity.51, 87. doi: 10.1007/s12526-021-01216-6
71
Mazhirinia G. P. (1988). Some information on the development of ovaries in Benthosema glaciale from different areas of the North Atlantic (Halifax, Nova Scotia: Northwest Atlantic Fisheries Organization).
72
McBride R. S. Fairchild E. A. Press Y. K. Elzey S. P. Adams C. F. Bentzen P. (2022). A life history study of atlantic wolffish resolves bias and imprecision in length- and age-at-maturity schedules by recognizing abortive maturation. Marine Coastal Fisheries.14, e10222. doi: 10.1002/mcf2.10222
73
McBride R. S. Wuenschel M. J. Nitscheke P. Thorton G. King J. R. (2013). Latitudinal and stock-specific variation in size- and age-at-maturity of female winter flounder, Pseudopleuronectes americanus, as determined with gonad histology. J. Sea Res.75, 41–51. doi: 10.1016/j.seares.2012.04.005
74
McMonagle H. Llopiz J. K. Maas A. E. Steinberg D. K. Govindarajan A. F. Essington T. E. (2024). Quantifying uncertainty in the contribution of mesopelagic fishes to the biological carbon pump in the Northeast Atlantic Ocean. ICES J. Marine Science.81, 2037–2051. doi: 10.1093/icesjms/fsae149
75
Morgan M. J. (2017). Understanding biology to improve advice for fisheries management. ICES J. Marine Sci. 75 (3), 923–931. doi: 10.1093/icesjms/fsx229
76
Murua H. Kraus G. Saborido-Rey F. Witthames P. R. Thorsen A. Junquera S. (2003). Procedures to estimate fecundity of marine fish species in relation to their reproductive strategy. J. Northwest Atlantic Fishery Science.33, 33–54. doi: 10.2960/J.v33.a3
77
Murua H. Saborido-Rey F. (2003). Female reproductive strategies of marine fish species of the North Atlantic. J. Northwest Atlantic Fishery Science.3, 23–31. doi: 10.2960/J.v33.a2
78
Olivar M. P. Hulley P. A. Castellón A. Emelianov M. López C. Tuset V. M. et al . (2017). Mesopelagic fishes across the tropical and equatorial Atlantic: Biogeographical and vertical patterns. Prog. Oceanography.151, 116–137. doi: 10.1016/j.pocean.2016.12.001
79
Potier M. Marsac F. Cherel Y. Lucas V. Sabatié R. Maury O. et al . (2007). Forage fauna in the diet of three large pelagic fishes (lancetfish, swordfish and yellowfin tuna) in the western equatorial Indian Ocean. Fisheries Res.83, 60–72. doi: 10.1016/j.fishres.2006.08.020
80
Proud R. Cox M. J. Brierley A. S. (2017). Biogeography of the global ocean’s mesopelagic zone. Curr. Biol.27, 113–119. doi: 10.1016/j.cub.2016.11.003
81
Proud R. Handegard N. O. Kloser R. J. Cox M. J. Brierley A. S. (2019). From siphonophores to deep scattering layers: uncertainty ranges for the estimation of global mesopelagic fish biomass. ICES J. Marine Science.76, 718–733. doi: 10.1093/icesjms/fsy037
82
Richardson D. E. Marancik K. E. Guyon J. R. Lutcavage M. E. Galuardi B. Lam C. H. et al . (2016). Discovery of a spawning ground reveals diverse migration strategies in Atlantic bluefin tuna (Thunnus thynnus). Proc. Natl. Acad. Sci.113, 3299–3304. doi: 10.1073/pnas.1525636113
83
Rideout R. M. Tomkiewicz J. (2011). Skipped spawning in fishes: more common than you might think. Marine Coastal Fisheries: Dynamics Management Ecosystem Science.3, 176–189. doi: 10.1080/19425120.2011.556943
84
Sadovy Y. Shapiro D. Y. (1987). Criteria for the diagnosis of hermaphroditism in fishes. Copeia.1987, 136–156. doi: 10.2307/1446046
85
Salvanes A. G. V. Stockley B. M. (1996). Spatial variation of growth and gonadal developments of Maurolicus muelleri in the Norwegian Sea and in a Norwegian fjord. Marine Biol.126, 321–332. doi: 10.1007/BF00347456
86
Sarmiento-Lezcano A. N. Triay-Portella R. Guerra-Marrero A. Jiménez-Alvarado D. Rubio-Rodríguez U. Núñez-González R. et al . (2020). Contribution to the reproductive ecology of Notoscopelus resplendens (Richardson 1845) (Myctophidae) in the Central-Eastern Atlantic. Sci. Reports.10, 15821. doi: 10.1038/s41598-020-72713-0
87
Sassa C. (2019). Estimation of the spawning biomass of myctophids based on larval production and reproductive parameters: the case study of Benthosema pterotum in the East China Sea. ICES J. Marine Science.76, 743–754. doi: 10.1093/icesjms/fsy051
88
Sassa C. Kawaguchi K. Hirota Y. Ishida M. (2004). Distribution patterns of larval myctophid fish assemblages in the subtropical–tropical waters of the western North Pacific. Fisheries Oceanography.13, 267–282. doi: 10.1111/j.1365-2419.2004.00289.x
89
Sassa C. Takahashi M. (2022). Diurnal maturation rhythm, spawning frequency and fecundity of Diaphus fulgens (Teleostei: Myctophidae) in the Kuroshio waters of the East China Sea. Deep Sea Res. Part I: Oceanographic Res. Papers.184, 103768. doi: 10.1016/j.dsr.2022.103768
90
Schultz L. P. (1964). “Family sternoptychidae,” in Fishes of the western north atlantic. Part four: order isospondyli, in part (Suborder argentinoidea, suborder stomiatoidea, suborder esocoidea, suborder bathylaconoidea) and order giganturoidei. Eds. BigelowH. B.MeadG. (Sears Foundation for Marine Research, New Haven).
91
Seibel B. A. Wishner K. F. (2019). “The significance of ocean deoxygenation for mesopelagic communities,” in Ocean deoxygenation: Everyone’s problem - Causes, impacts, consequences and solutions. Eds. LaffoleyD.BaxterJ. M. (IUCN, Gland, Switzerland).
92
Sundby S. Kristiansen T. (2015). The principles of buoyancy in marine fish eggs and their vertical distributions across the world oceans. PloS One10, e0138821. doi: 10.1371/journal.pone.0138821
93
Sunobe T. (2023). “Protandry in fishes,” in Hermaphroditism and mating systems in fish. Eds. KuwamuraT.SawadaK.SunobeT.SakaiY.KadotaT. (Springer Nature Singapore, Singapore).
94
Sutton T. T. (2013). Vertical ecology of the pelagic ocean: classical patterns and new perspectives. J. Fish Biol.83, 1508–1527. doi: 10.1111/jfb.12263
95
Sutton T. T. Clark M. R. Dunn D. C. Halpin P. N. Rogers A. D. Guinotte J. et al . (2017). A global biogeographic classification of the mesopelagic zone. Deep Sea Res. Part I: Oceanographic Res. Papers.126, 85–102. doi: 10.1016/j.dsr.2017.05.006
96
Sutton T. T. Porteiro F. M. Heino M. Byrkjedal I. Langhelle G. Anderson C. I. H. et al . (2008). Vertical structure, biomass and topographic association of deep-sea pelagic fishes in relation to a mid-ocean ridge system. Deep-Sea Res. II.55, 161–184. doi: 10.1016/j.dsr2.2007.09.013
97
Team R. C. (2024). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing).
98
Venables W. N. Ripley B. D. (2002). Modern applied statistics with S. 7.3.54 ed (New York: Springer).
99
Victorero L. Watling L. Palomares M. L. D. Nouvian C. (2018). Out of sight, but within reach: A global history of bottom trawled deep-sea fisheries from >400 m depth. Front. Marine Sci.5. doi: 10.3389/fmars.2018.00098
100
Wallace R. A. Selman K. (1981). Cellular and dynamic aspects of oocyte growth in teleosts. Am. Zoologist.21, 325–343. doi: 10.1093/icb/21.2.325
101
Wang V. H. Zapfe C. R. Hernandez F. J. (2021). Assemblage structure of larval fishes in epipelagic and mesopelagic waters of the northern gulf of Mexico. Front. Mar. Sci. 8, 766369. doi: 10.3389/fmars.2021.766369
102
Warner R. R. (1988). Sex change in fishes: hypotheses, evidence, and objections. Environ. Biol. Fishes.22, 81–90. doi: 10.1007/BF00001539
103
Watson R. A. Morato T. (2013). Fishing down the deep: Accounting for within-species changes in depth of fishing. Fisheries Res.140, 63–65. doi: 10.1016/j.fishres.2012.12.004
104
West G. (1990). Methods of assessing ovarian development in fishes: a review. Aust. J. Marine Freshwater Res.41, 199–222. doi: 10.1071/MF9900199
105
Wickham H. (2016). ggplot2: Elegant graphics for data analysis. 3.3.6 ed (Spring International Publishing).
106
Wiebe P. H. Morton A. W. Bradley A. M. Backus R. H. Craddock J. E. Barber V. et al . (1985). New development in the MOCNESS, an apparatus for sampling zooplankton and micronekton. Marine Biol.87, 313–323. doi: 10.1007/BF00397811
107
Wilbur H. M. Rudolf V. H. (2006). Life-history evolution in uncertain environments: bet hedging in time. Am. Nat.168, 398–411. doi: 10.1086/506258
108
Wootton R. J. Smith C. (2015). Reproductive biology of teleost fishes (Chichester, West Sussex: John Wiley & Sons, Ltd).
109
Yang W. Zhao X. Zheng M. (2022). Slow-sinking particulate organic carbon and its attenuation in the mesopelagic water of the South China Sea. Front. Marine Sci.9. doi: 10.3389/fmars.2022.1018825
110
Young J. W. Blaber S. J. M. Rose R. (1987). Reproductive biology of three species of midwater fishes associated with the continental slope of eastern Tasmania, Australia. Marine Biol.95, 323–332. doi: 10.1007/BF00409562
111
Young J. W. Hunt B. P. V. Cook T. R. Llopiz J. K. Hazen E. L. Pethybridge H. R. et al . (2015). The trophodynamics of marine top predators: Current knowledge, recent advances and challenges. Deep Sea Res. Part II: Topical Stud. Oceanography.113, 170–187. doi: 10.1016/j.dsr2.2014.05.015
Summary
Keywords
maturity ogive, reproduction, histology, Myctophidae, Melamphaidae, Sternoptychidae, Gonostomatidae, ocean twilight zone
Citation
Lefebvre LS, Sosik HM and Llopiz JK (2025) Spawning in the deep: reproductive life history of four mesopelagic fishes of the northwestern Atlantic Ocean. Front. Mar. Sci. 12:1582706. doi: 10.3389/fmars.2025.1582706
Received
24 February 2025
Accepted
05 May 2025
Published
27 May 2025
Volume
12 - 2025
Edited by
Maria Lourdes D. Palomares, University of British Columbia, Canada
Reviewed by
Ajit Kumar Mohanty, Indira Gandhi Centre for Atomic Research (IGCAR), India
Claudio D’Iglio, University of Messina, Italy
Updates
Copyright
© 2025 Lefebvre, Sosik and Llopiz.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lyndsey S. Lefebvre, llefebvre@whoi.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.