Abstract
The massive influx of pelagic Sargassum spp. species, also known as Sargassum inundation events (SIEs), first arrived at the Caribbean’s coastal waters in 2011. These events have been linked to hypoxia, among other ecological disturbances. Here, we report data from 2022 on (1) an assessment of the relative magnitude of particulate organic carbon (POC) load arising from SIEs into the La Parguera Marine Reserve (LPMR) basin off the southwest coast of Puerto Rico and (2) the biogeochemical impact of SIE in a nearshore mangrove key within the reserve, Monsio Jose Key Bay (MJKB). Our analysis yields that the carbon influx increased by 20% in the LPMR basin and by 103% in MJKB. Weekly observations of Sargassum input, along with the collection and analysis of water samples in MJKB, evidenced a cause-effect relation between Sargassum carbon loading and frequency of hypoxic (DO < 2 mg·L-1) and critically acidic conditions (Aragonite saturation, Ω < 2.0). During the 2022 Sargassum season, hypoxic conditions were detected in 43% of samples collected in MJKB. Considering the modulation of biogeochemical parameters by changes in tide height (Δh) and wind speed (m·s-1), stepwise multiple regression analyses (RDA-AIC model selection) showed that significant parameters influencing DO, pH, and Ω include the Sargassum carbon influx and Δh (p < 0.05). These findings strongly support the hypothesis that the additional input of POC influx enhances microbial mineralization rates responsible for depressed oxygen concentrations and acidic conditions, which could be detrimental to coastal ecosystems. This is particularly concerning in areas prone to SIEs where geomorphological features facilitate the entrainment of floating materials. Proper management requires the identification of vulnerable sites and Sargassum removal. Ongoing efforts towards that goal are underway for LPMR.
1 Introduction
Pelagic Sargassum blooms form aggregations or ‘rafts’ (Brooks et al., 2018), of which significant amounts are advected into Caribbean waters. Rafts support a drifting ecosystem hosting a wide variety of marine species (Weis, 1968; Casazza and Ross, 2008; Brown, 2020) and are recognized by the South-Atlantic Fisheries Council of the National Oceanic and Atmospheric Administration as an essential fish habitat (NOAA, 2003; Huffard et al., 2014; Cashman and Nagdee, 2017). Since 2011, the seasonal occurrence of large pelagic blooms of Sargassum, including two predominant species (S. fluitans and S. natans), has become the new normal in the Tropical and Subtropical North Atlantic from Brazil to Africa (de Széchy et al., 2012; Hu et al., 2016; Putman et al., 2018; Wang et al., 2019; Johns et al., 2020). Pelagic Sargassum blooms have attracted attention due to their substantial arrival in vast quantities, also known as Sargassum inundation events (SIEs), along the coasts of the Greater Caribbean and the Tropical Atlantic Regions (Moreira and Alfonso, 2013; Mendez-Tejeda and Rosado, 2019; Wang et al., 2019). Once brought ashore by currents and winds (Putman et al., 2018; Wang et al., 2019), the accumulation of Sargassum on the coast has been reported to lead to detrimental conditions for coastal ecosystems, fisheries, and tourism (Cashman and Nagdee, 2017; van Tussenbroek et al., 2017; Cabanillas-Teran et al., 2019; Mendez-Tejeda and Rosado, 2019; Brown, 2020; Bernard et al., 2022; Sánchez et al., 2023). Although SIEs have been considered a temporary phenomenon (Marsh et al., 2022), recent studies indicate that recurrent blooms could be associated with climate change, fluctuations in hydrodynamic patterns, and the introduction of anthropogenic nutrients (Djakouré et al., 2017; Sonter et al., 2017; Putman et al., 2018; Wang et al., 2019; Gouvêa et al., 2020). Sargassum accumulates seasonally under the Intertropical Convergence Zone (ITCZ) (Johns et al., 2020). In this zone the equatorial and Northwest Africa coastal upwelling regions, the Amazon and Orinoco River outflows, and the Saharan dust transported by the easterly trade winds supply a significant amount of nutrients (Wang et al., 2019; Oviatt et al., 2019), providing optimal conditions to sustain a Sargassum bloom in the North Equatorial Recirculation Region (NERR) (Gower et al., 2013; Wang and Hu, 2016; Djakouré et al., 2017). Following the bloom, the Sargassum is transported westward and eastward, creating the great Atlantic Sargassum belt (Wang et al., 2019; Johns et al., 2020; Skliris et al., 2022).
Given its effectiveness as a primary producer and storehouse of organic carbon (Krause-Jensen and Duarte, 2016; Gouvêa et al., 2020), SIEs can represent a significant exogenous source of particulate organic carbon (POC) (Valiela et al., 1997). POC influx can be expected to result in hypoxia and ocean acidification due to increased metabolic demands (Burkholder et al., 2007; Lee et al., 2007; Martínez-Lüscher and Holmer, 2010; van Tussenbroek et al., 2017). Hypoxic conditions associated with Sargassum have been linked to neritic fish and crustacean mortality (Rodríguez-Martínez et al., 2019). However, although Sargassum’s role in carbon dynamics in the Tropical and Subtropical Atlantic oceanic domains has been well-documented (Krause-Jensen and Duarte, 2016; Wang et al., 2018; Gouvêa et al., 2020; Hu et al., 2021), the impact of pelagic Sargassum carbon that inundates Caribbean coastal ecosystems remains to be adequately assessed. Further studies identifying the magnitude and frequency of SIEs driven hypoxia and acidification events in representative critical ecosystems should provide a baseline for the development of models predicting biomass influx and retention as well as the resulting hypoxia and acidification. Said forecasting tools would support resource managers responsible for deploying impact mitigation measures.
Here, we present data from a year-long (2022) time-series of observations focused on assessing the temporal variability of Sargassum biomass influx rates into the La Parguera Marine Reserve (LPMR) basin and at Monsio Jose Key Bay (MJKB) within the basin. The relative increase in POC loading resulting from Sargassum influx, both at the basin-wide scale and at MJKB, is estimated using available data on mangrove litterfall (Vega-Rodríguez et al., 2008; Pérez-Pérez et al., 2022) and seagrass production for LPMR basin (Liboy, 1976; Hertler, 2002). Below we report serial observations of dissolved oxygen (DO) concentration, total alkalinity (TA) and pH at MJKB, collected in parallel to biomass influx measurements provided for assessing the magnitude, frequency and duration of hypoxia and acidification events in a mangrove key, a typical ecosystem in LPMR basin, arising from SIEs.
2 Materials and methods
2.1 Area of study
This study was conducted in the coastal waters of LPMR off the southwestern coast of Puerto Rico (Figure 1), an area designated as a Nature Reserve in September 1979. The reserve consists of a series of reef cays with a dispersed distribution along the interior insular shelf hosting ecosystems, including coral reefs, seagrass meadows, and mangroves (Valdés-Pizzini and Schärer-Umpierre, 2014). Due to the prevalence of south-southeasterly winds, the area is particularly susceptible to SIEs (Hernández et al., 2022). Meteorologically, the LPMR basin is characterized by a wet season extending from August to November, and semiarid conditions prevail during the rest of the year (García-Troche et al., 2021; Ayala-Torres and Otero, 2023). Mangrove litterfall and seagrasses are the major organic carbon sources in LPMR. Net carbon production by planktonic autotrophs was not included as a source of POC in LPMR basin, as the only published information available (Odum et al., 1959) reports net autotrophy presumably supported by dissolved organic carbon (DOC) exported by mangrove forests. Moreover, Meléndez et al. (2022), using data from a decade of observations collected by La Parguera MapCO2 buoy, located off a mid-shelf reef key in the LPMR basin, reported net heterotrophic conditions during the year as slightly autotrophic conditions only prevailing during winter months.
Figure 1
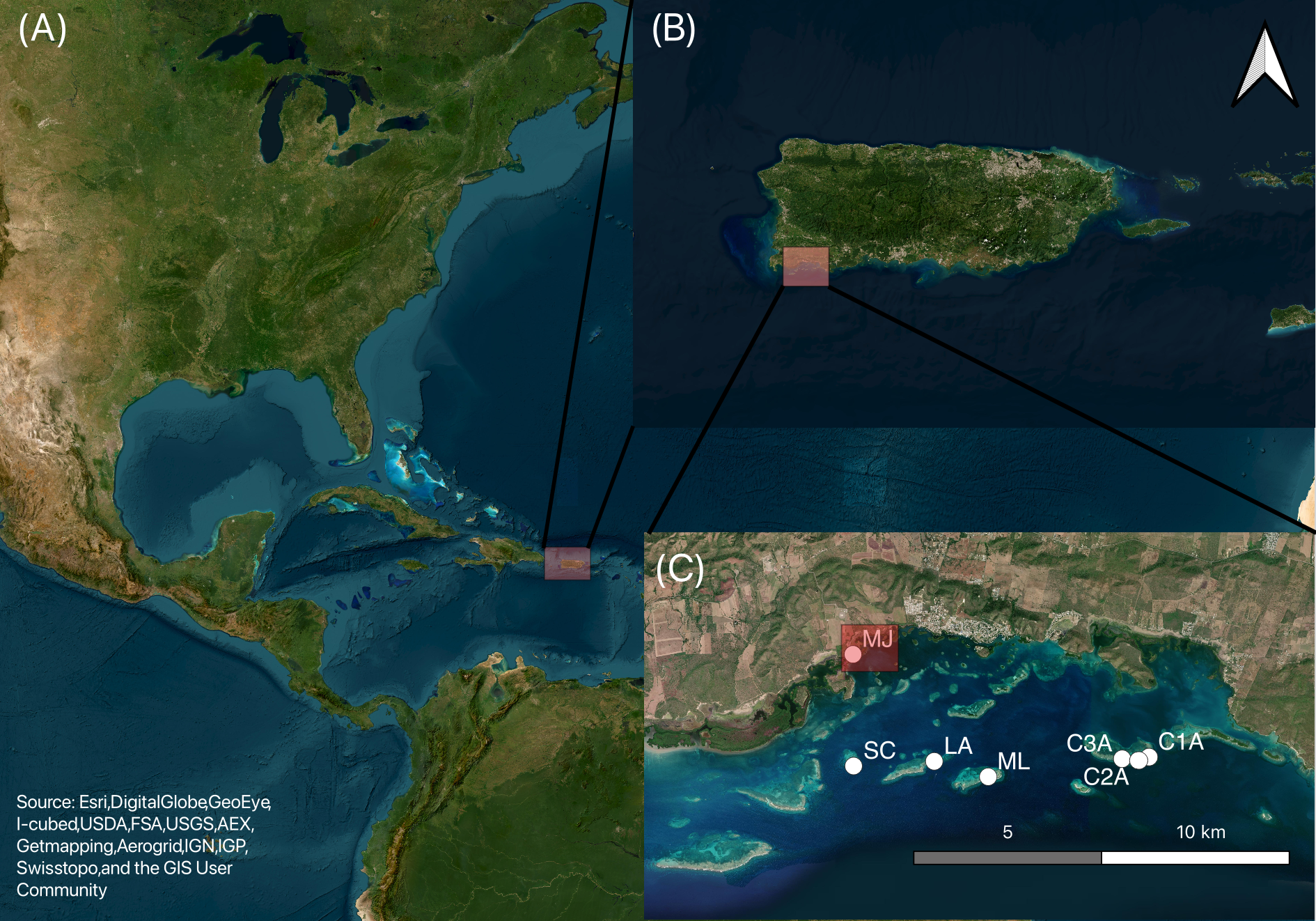
Satellite images showing (A) Puerto Rico in the Caribbean Sea, (B) the study area off the southwestern coast of Puerto Rico, and (C) the geographical distribution of Sargassum trap location around La Parguera Marine Reserve, Lajas, Puerto Rico. The white circles indicate study sites: SC (San Cristobal key; 17.942074°N, 67.076714°W), LA (Laurel key; 17.943191°N, 67.056441°W), ML (Media Luna key; 17.9395°N, 67.042871°W), C3A (17.9438°N, 67.009188°W), C2A (17.9434°N, 67.005127°W), and C1A (17.9442°N, 67.002603°W) (Corral Key) and MJKB (Monsio José Key Bay; 17.9688°N, 67.076871°W). The red squared in MJKB marks the location where a Sargassum trap was located, and biogeochemical samples were collected.
2.2 Estimation of Sargassum carbon influx
Six (6) Sargassum traps (Supplementary Figure A.1 in Supplementary Materials), constructed using PVC pipe and plastic mesh and measuring 0.63 x 0.5 x 0.63 m (depth x width x height), were deployed facing the prevailing southeasterly winds on the seaward edge of four reef islands on the outer southern boundary of LPMR basin. An additional trap was deployed in Monsio Jose Key Bay (MJKB) (17.9688°N, 67.076871°W), a nearshore mangrove-lined embayment (Figure 1). Traps were placed in 10 cm deep water to ensure water inflow even at low tide. Weekly sampling facilitated trap maintenance, allowing for continuous assessment of their condition. Traps were replaced as needed to ensure optimal functionality and uninterrupted sampling.
Quantification of the weekly Sargassum biomass influx (kg·m-1·Wk-1) into the LPMR basin and MJKB was estimated by collecting the Sargassum accumulated in the traps, transferring it to a mesh bag, and weighing it on-site using an electronic fish scale. The Sargassum wet weight was converted to POC using an averaged carbon-to-wet weight ratio of 0.05 ± 0.01 published by Laffoley et al. (2014); Wang et al. (2018, 2019); and Gouvêa et al. (2020) (Supplementary Table A.1 in Supplementary Materials). The total weekly Sargassum carbon influx to the LPMR basin was estimated using the weekly mean capture of all traps located in the outer reefs, normalized by the trap width (meters) and multiplied by the width of the basin’s windward boundary (10.4 km). For MJKB, weekly mean values were multiplied by the width of the channel (69 m) facing the prevailing wind. Sargassum carbon influx rates to MJKB nearshore station were contrasted with estimates of carbon influx from mangrove litterfall. To achieve a more comprehensive assessment of the carbon contribution, we also estimated DOC from Sargassum using values reported by Powers et al. (2019).
2.3 Estimating mangrove POC influx
The mangrove litterfall rate for the LPMR basin and MJKB were estimated using the mean of reported litterfall observations in LPMR, 476 dry weights·m-2·yr-1 (Vega-Rodríguez et al., 2008; Pérez-Pérez et al., 2022) and area estimates were derived from satellite imagery. Mangrove litter mass was converted to carbon using the 0.5 carbon/litter weight ratio reported by Golley et al. (1962) (Supplementary Table A.2 in Supplementary Materials). The estimated POC was converted to DOC using the 0.13 reported by Adame and Lovelock (2011).
2.4 Estimating seagrass POC input
Estimates of net carbon input from seagrasses are based on seagrass growth studies carried out in LPMR basin by Liboy (1976) and Hertler (2002). The mean seagrass productivity rate (4.56 ± 2.01 g·m-2·day-1) calculated from data from both studies was used to obtain the seagrass POC production rate for the basin. Said rate is consistent with reports from other areas in the Caribbean (Linton and Fisher, 2004; Juman, 2005). Seagrass biomass was converted to carbon using the carbon-to-biomass ratio (0.32) reported by Bay et al. (1996) (Supplementary Table A.3 in Supplementary Materials). We estimated the exudation carbon by using the POC-to-DOC ratio (0.126) reported by Robertson et al. (1982).
2.5 Biogeochemical observations at MJKB
Although Sargassum traps were deployed throughout LPMR, the analysis of weekly seawater samples for assessing the biogeochemical impact of SIEs was exclusively conducted for MJKB in this study. Samples were collected within 3.5 meters of the MJKB Sargassum trap (17.968766°N, 67.076871°W) from January 2022 to December 2022 between 7:00 and 10:00 a.m. (local time) at 1-meter depth using a Van Dorn 3.5 L sampler, following the best practices guidelines (Dickson et al., 2007). One seawater sample was collected for each parameter, which allowed for duplicate analyses in the lab. Conductivity and temperature data were collected with an SBE25 CTD. Seawater samples for pH and TA were fixed immediately with a saturated solution of mercury chloride (HgCl2) to prevent biological alteration. Analysis for pH on the Total Scale was performed using a spectrophotometer with m-cresol purple indicator dye (pHT ± 0.003) (Dickson and Goyet, 1996; Grasshoff et al., 2007). Total alkalinity determinations (TA ± 2 μmol·kg-1) (Dickson et al., 2007) were carried out following the protocol described by García-Troche et al. (2021). DO sample analysis was performed following the Winkler method (DO ± 0.50 mg·L-1) (Grasshoff et al., 2007; Astor et al., 2013). Aragonite saturation state (Ω) values were estimated from pH and TA measurements using the CO2SYS program (Lewis and Wallace, 1998).
2.6 Statistical analysis
Pearson’s correlation analysis was used to identify significant time-lagged correlations between the explanatory variable (i.e., weekly Sargassum carbon influx) and the dependent variables (i.e., DO). A MATLAB function was created to identify different weekly lags between the variables and show the significant Pearson’s correlation coefficient. The lagged Sargassum carbon influx (kgC·m-1) and physical parameters, such as wind speed (m·s-1) and changes in tide height, calculated as Δh = (tide height at sampling time)/(mean low tide), were included in data analyses to determine their significance in modulating the measured and calculated biogeochemical parameters (i.e., DO, pH, Ω). Wind speed data were sourced from the National Buoy Center, and tidal data were obtained from NOAA Tides & Currents for Station 9759110, Magueyes Islands, PR.
The MATLAB Fathom toolbox (Jones, 2017) was used to perform a stepwise forward selection of explanatory variables in Redundancy Analysis (RDA) using Akaike Information Criteria (AIC). This analysis identified optimal variables that substantially explained the variation of biogeochemical parameters (i.e., response variables; DO, pH, Ω). Explanatory variables included in the RDA-AIC analysis were Sargassum carbon influx, wind speed, and Δh. Subsequently, a permutation-based RDA with 1000 iterations was conducted using the optimal explanatory variables identified through the RDA-AIC analyses to derive the model statistics. Lastly, to gain a clearer understanding of the individual effects of the optimal explanatory variables on the response variables, a permutation-based Multiple Linear Regression via Least Squares Estimation with 1000 iterations were performed for each response variable independently. This enabled a more precise interpretation of the impact of each explanatory variable on the response variables, offering insights into their distinct roles within the broader model.
3 Results
3.1 Sargassum biomass influx
During 2022, SIEs at LPMR basin started in April and extended until November. The mean weekly Sargassum biomass influx for the six (6) traps, located in the outer keys of the LPMR basin (Figure 2), ranged from non-detectable to a maximum of 24.80 kg·m-1·Wk-1 occurring on the second week of May. An estimate of the mean weekly Sargassum biomass influx into the basin yields 7.85 ± 6.60 kg·m-1·Wk-1 or 81,725 kg·Wk-1 for the whole basin. For the same period, the weekly Sargassum biomass influx rate into the MJKB averaged 2.13 ± 5.08 kg·m-1·Wk-1 with a maximum Sargassum input rate of 23.22 kg·m-1·Wk-1 (Figure 3). Extrapolation using the width of MJKB channel (69 m) aligned with the prevailing wind direction, yields a weekly mean Sargassum biomass loading rate for the embayment of 155 ± 327 kg·Wk-1.
Figure 2
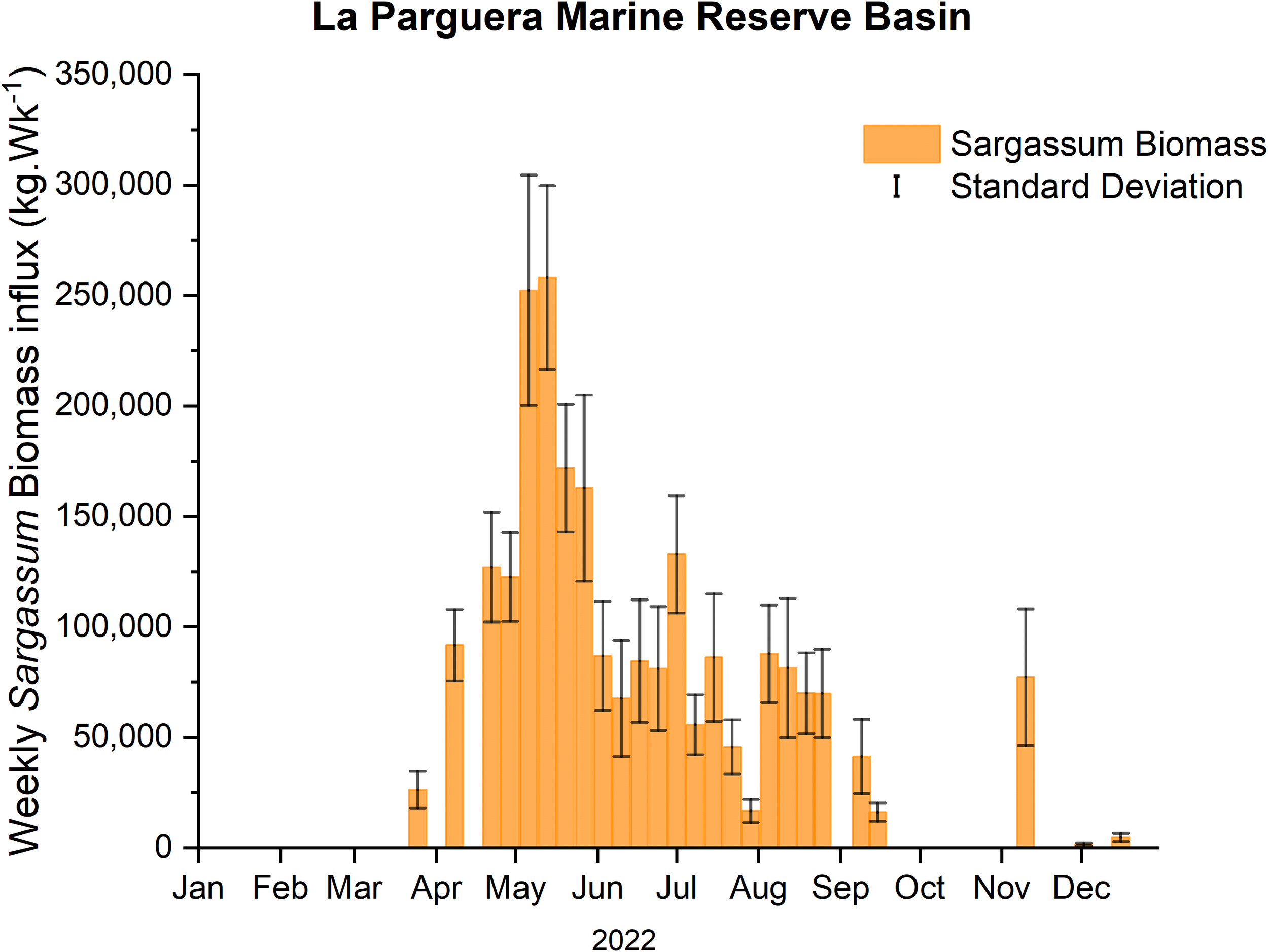
Estimated average over-trap (n = 6) of Sargassum biomass influx on the outer keys extrapolated by the width of the LPMR basin during the 2022 season.
Figure 3
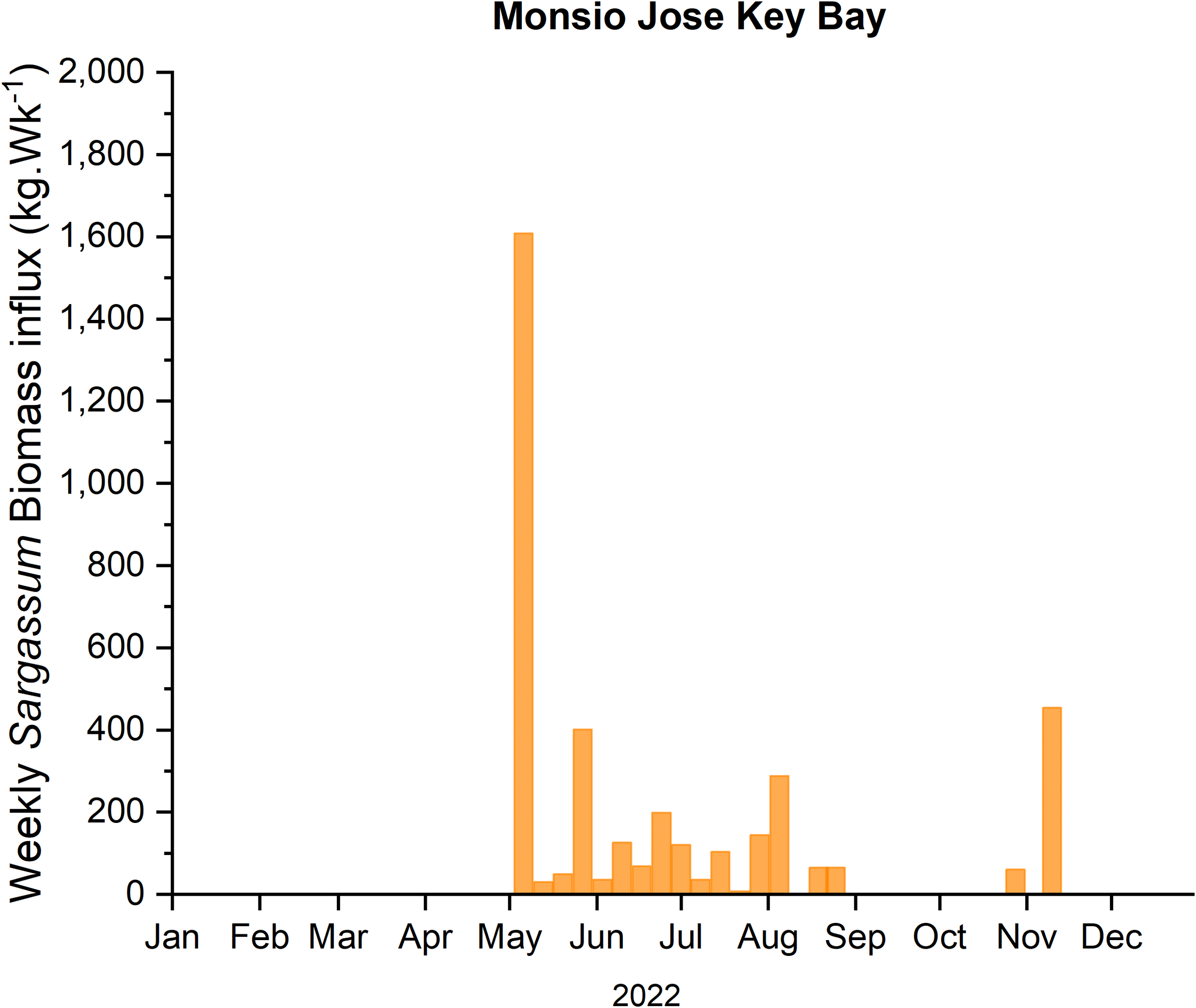
Estimated Sargassum biomass influx to MJKB, extrapolated by the width of the channel, during the 2022 season.
3.2 Carbon inputs to LPMR basin
Figure 4 presents the estimates of POC production by mangroves (as litterfall) and seagrasses (leaf growth) as well as estimates of Sargassum POC influx into the LPMR basin. The weekly POC production of seagrasses and mangroves totaled 18,209 kgC·Wk-1, while the POC loading arising from the Sargassum influx during high season in 2022 averaged 3,617 ± 3,452 kgC·Wk-1 with a standard error of 241 kgC·Wk-1, thus representing about 20 ± 19% increase in POC input to LPMR basin (Figure 4).
Figure 4
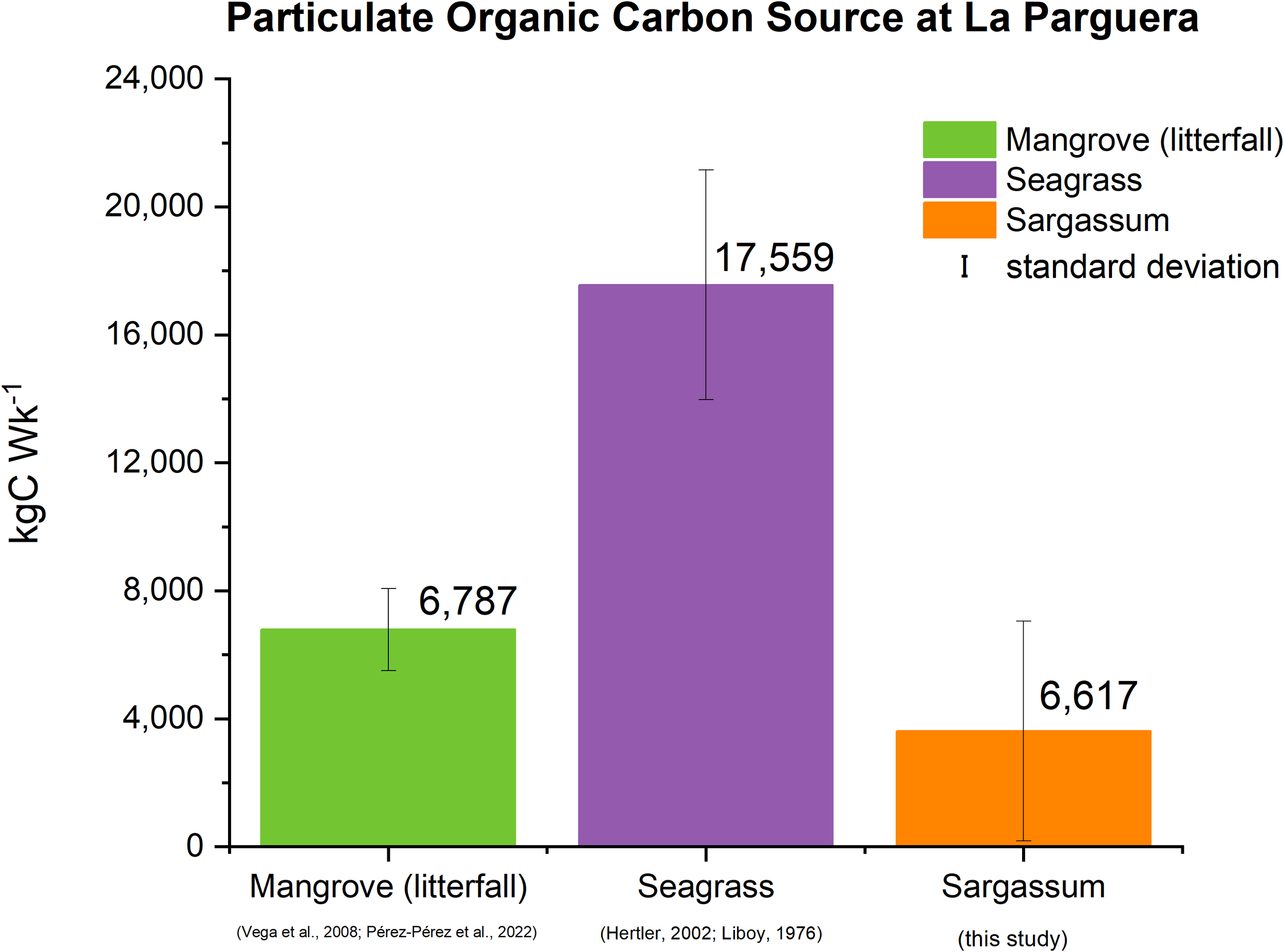
Estimated weekly POC input rates from Sargassum and other primary sources in LPMR basin.
The weekly estimated exudates of DOC from the production of seagrasses and mangroves in the LPMR basin are 3,041 kgC·Wk-1 (Supplementary Table A.4 in Supplementary Materials). At the same time, the DOC loading arising from the Sargassum influx during the high season in 2022 averaged 6 kgC·Wk-1, thus representing a 0.2% minor fraction increase in DOC input to the LPMR basin. The comparison of the calculated POC and DOC fractions from Sargassum suggests that POC is the predominant contributor to the organic carbon pool in the LPMR basin.
3.3 Carbon input to MJKB
Weekly estimates of carbon loading from mangroves (as litterfall) and Sargassum to MJKB are presented in Figure 5. Nonetheless, for MJKB, the seagrass carbon was not considered because the study site does not harbor seagrasses. While carbon production by mangroves for the area in the MJKB, is estimated at 7.5 ± 1.4 kgC·Wk-1, Sargassum, POC influx averaged 7.7 ± 16.3 kgC·Wk-1, thus representing a 103% net increase in POC.
Figure 5
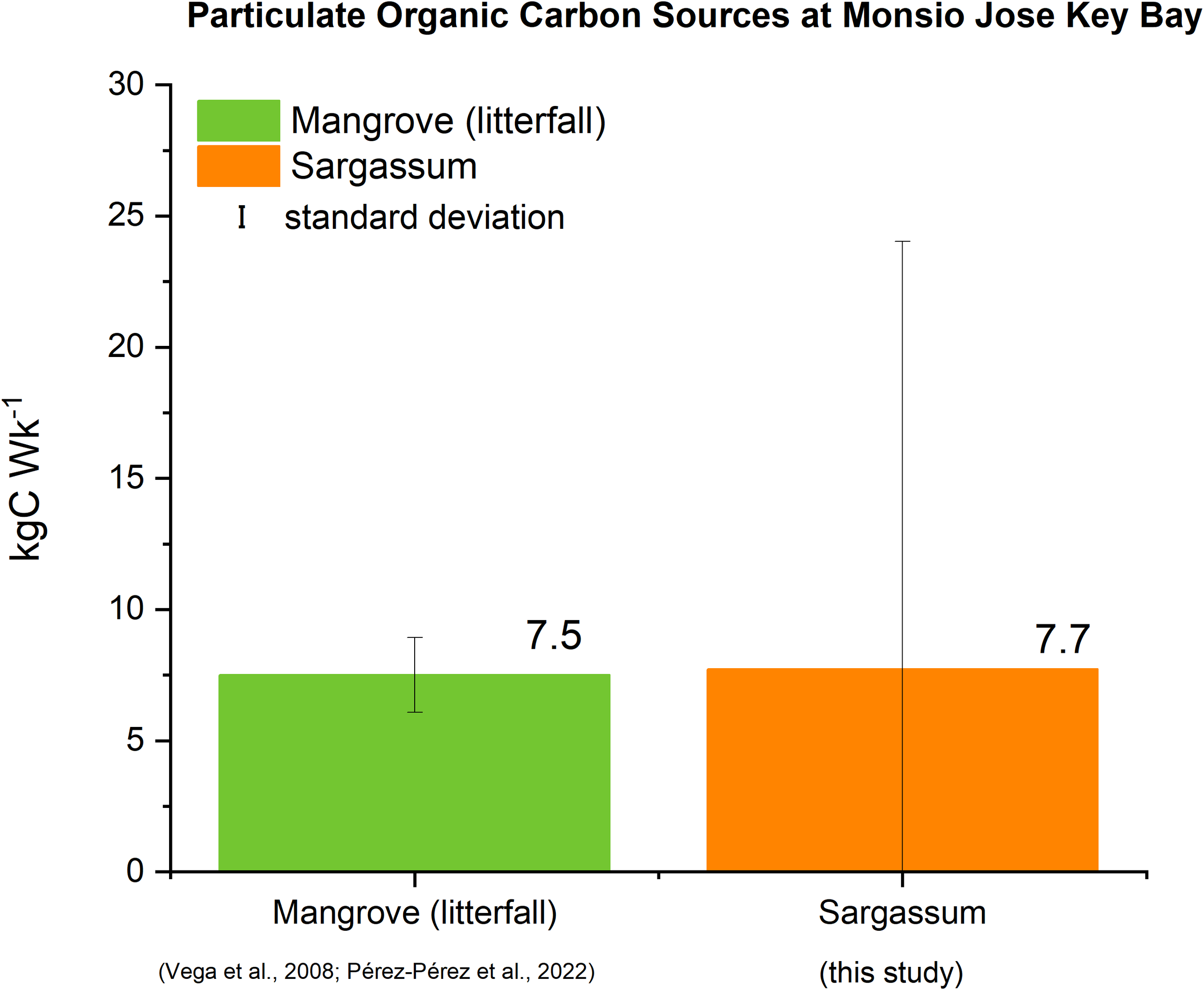
Estimates of POC sources at MJKB during the 2022 Sargassum season.
The carbon exudation estimates by mangroves, primarily through litterfall, for MJKB are approximately 1.0 kgC·Wk-1 (Supplementary Table A.5 in Supplementary Materials). In contrast, the DOC influx from Sargassum into the MJKB averaged 0.013 kgC, contributing to a minor increase of 1.3% in DOC.
3.4 Biogeochemistry at MJKB
Observations of biogeochemical data indicate that during the months before the arrival of Sargassum (winter season), pH ranged from 7.7 to 7.9, while Ω aragonite ranged from 2.2 to 3.0. DO values ranged from 3.28 to 5.24 mg·L-1, while temperature ranged from 26.11 to 28.21°C (Supplementary Table A.6 in Supplementary Materials). After the onset of the Sargassum season in early May, we observed a sharp decrease in pH, Ωaragonite, and DO (Figure 6). Simultaneously, we observed a warmer seawater temperature. For this period, pH values ranged between 7.0 – 7.8, with increased seawater acidity observed during the summer months when Ω aragonite ranged between 0.5 – 2.8, values under critical levels are Ω < 2.0. During the same period, DO values ranged from non-detectable to 4.67 mg·L-1, frequently reaching hypoxic or anoxic conditions. Temperature values ranged from 27.90 to 30.77°C, with higher temperature levels occurring between late summer and fall. The ecosystem’s gradual and modest recovery is evident towards the end of the season, albeit with sustained low DO and pH levels. These conditions persisted from mid-June to September.
Figure 6
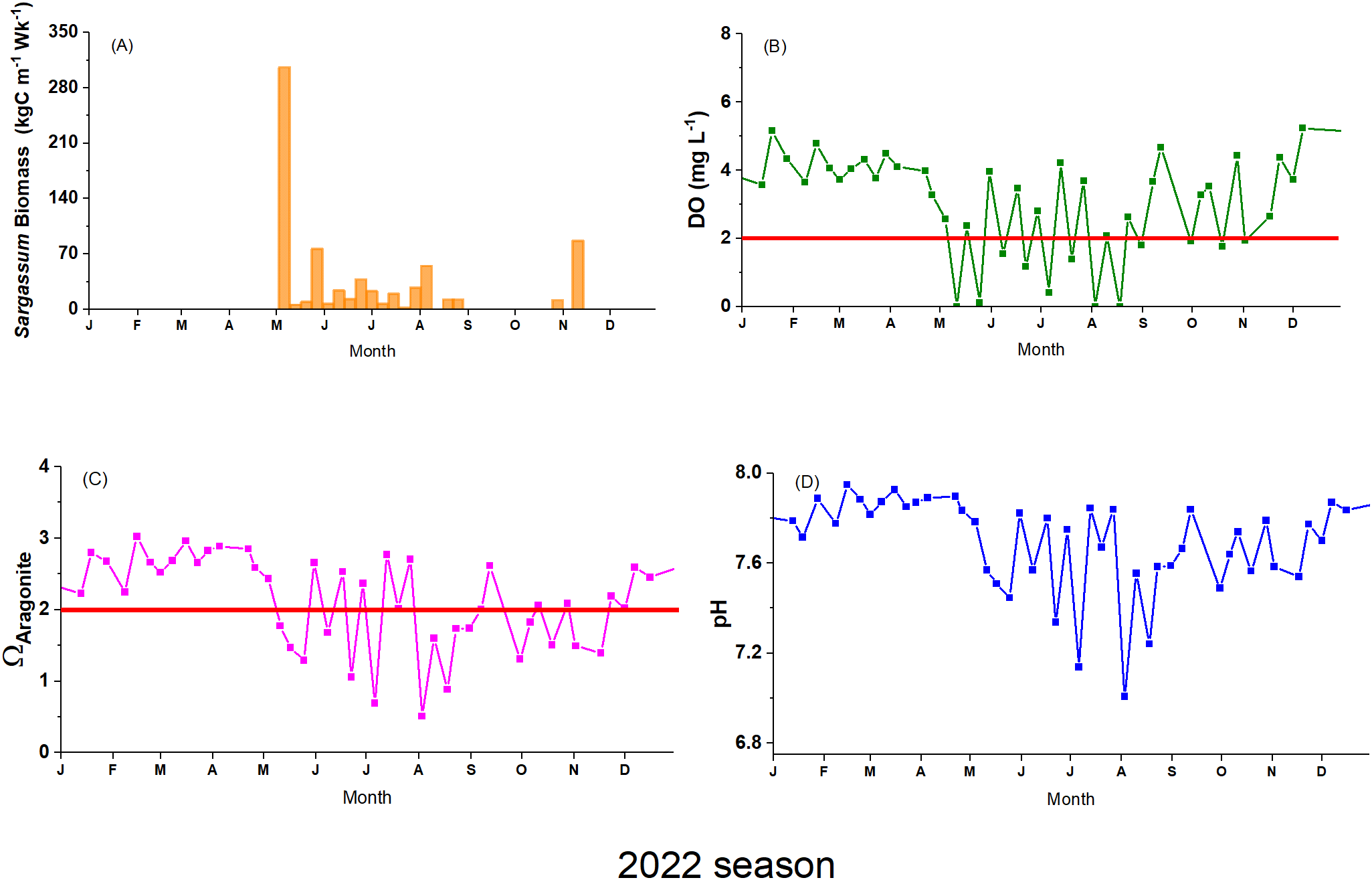
Time series of (A)Sargassum carbon influx, (B) Dissolved oxygen, (C) Ω aragonite, and (D) pH, for the 2022 season at Monsio Jose Key Bay (MJKB). The red line represents the critical level for DO (<2 mg·L-1) and Ω (< 2).
Data analyses were performed using a one-week lag on the Sargassum carbon influx based on the significant time lagged correlations identified by the Pearson’s correlation analysis using DO, pH and Ω data (Supplementary Table A.7 in Supplementary Materials). These results suggest that biogeochemical parameters exhibit measurable changes one week after a SIE, indicating Sargassum impact on the system’s chemical dynamics. The stepwise RDA-AIC analyses showed that Δh and Sargassum carbon influx were the optimal explanatory variables for the variance of DO, pH and Ω (Table 1). A significant portion of the response variables is explained by Δh independently, but adding Sargassum carbon influx further improves the model. Wind speed was not identified as an optimal explanatory variable by the AIC analyses. The RDA permutation test demonstrated that the model incorporating the optimal explanatory variables identified through RDA-AIC accounted for a significant proportion of the variance in the response variables (p < 0.05, r² = 0.34; Supplementary Table A.8 in Supplementary Materials).
Table 1
| Variable | r2 | r2Adjusted | AIC |
|---|---|---|---|
| Δh | 0.21 | 0.19 | 34.69 |
| Sargassum carbon influx | 0.32 | 0.28 | 31.27 |
Akaike Information Criterion (AIC) model with explanatory variables (Δh, Sargassum carbon influx) that explained DO, pH, Ω at Monsio Jose.
Multiple linear regressions to assess the individual effects of Δh and Sargassum carbon influx on response variables (i.e., DO, pH, Ω) showed that DO is significantly influenced by Δh and Sargassum carbon influx (p < 0.05, r2 = 0.35; Table 2), with Sargassum carbon influx having a negative relationship and Δh having a positive relationship with DO. However, the response of pH and Ω is less well explained by Δh and Sargassum carbon influx (r2 = 0.14). The variation in Δh has a marginal influence on pH, whereas the influx of carbon from Sargassum has a minimal impact on Ω (Table 2).
Table 2
| Response variable | r2 | Adjusted r2 | p | Intercept (beta, p) | Sargassum carbon influx (beta, p) | Δh (beta, p) |
|---|---|---|---|---|---|---|
| DO | 0.35 | 0.32 | 1·10−3 | 1.75, 2.00·10−3 | -0.03, 2.00·10−3 | 1.33, 2.00·10−3 |
| pH | 0.14 | 0.10 | 1·10−3 | 7.56, 2.00·10−3 | -3.12·10−3, 0.06 | 0.13, 2.00·10−3 |
| Ω | 0.14 | 0.09 | 1·10−3 | 1.86, 2.00·10−3 | -0.01, 2.00·10−3 | 0.30, 0.08 |
Results of multiple regressions for DO, pH and Ω against Δh and Sargassum carbon influx.
Significant p values are in bold.
4 Discussion
Sargassum inundation events in LPMR basin and MJKB exhibit a marked seasonal variability, with peak influx rates occurring in spring and summer and quickly subsiding between August and December. In May 2022, the LPMR basin and MJKB experienced a major influx of Sargassum biomass, which has been corroborated by satellite image analyses reported by the University of South Florida Optical Oceanography Lab, which sets a new historical record for the month of May for all Caribbean regions, exceeding all major Sargassum blooms in previous years (Hu, 2022). The mean estimate of Sargassum biomass loading in LPMR basin indicates a substantial influx. The assumption is that the Sargassum collected in the traps represents the total Sargassum accumulation in the LPMR basin, which can lead to an overestimation of biomass influx. We emphasize that the purpose of this research is to estimate the amount of Sargassum POC entering the basin compared to well-known local POC sources. However, we compared our 2022 data on Sargassum biomass (wet weight) with data from Mexico in 2015. This comparison provides an insight into the influx estimate. Our data indicate that the LPMR basin received a monthly influx of 49,360 kg·km-1 for July-August, an amount notably lower compared to the monthly ~817,000 kg·km-1 accumulated on Mexico’s coastline in 2015 (van Tussenbroek et al., 2017).
The estimates reported in Section 4 for POC inputs to the LPMR basin indicate that, at the basin scale, Sargassum input represents a significant increase in carbon load (20%) over POC inputs from seagrass and mangrove litter. However, in MJKB, where the shoreline favors entrainment of buoyant material, the Sargassum inundation represented a 103% increase in carbon loading comparable with local carbon input from mangrove litterfall. This means that environmental conditions (e.g., prevailing winds, hydrodynamics) at specific geographical areas with shoreline characteristics that are conducive to the retention of Sargassum, are particularly vulnerable to SIE (León-Pérez et al., 2023). In MJKB, this additional input of POC into the ecosystem most probably leads to hypoxia and acidification enhancement due to increased metabolic demands in the benthos and water column (Burkholder et al., 2007; Lee et al., 2007; Martínez-Lüscher and Holmer, 2010; van Tussenbroek et al., 2017; Valiela et al., 1997; Rabalais et al., 2002; Wallace et al., 2014).
The tidal height differential (Δh) plays a significant role in regulating DO, pH, and Ω through physical and biogeochemical processes. During periods of larger Δh, the influx of offshore water into MJKB facilitates water mass flushing, promoting oxygenation and mitigating declines in pH and Ω. Conversely, when Δh is minimal and tidal exchange is limited, the “residence time” of water masses in MJKB may become stagnant, allowing biological processes such as respiration and the decomposition of Sargassum and other organic matter to drive reductions in DO, pH, and Ω. These findings indicate that tides actively shape the ecological and biogeochemical conditions at MJKB, even during SIEs.
Monsio Jose Key Bay is characterized by fringe mangroves, which are host to a varied community of autotrophs and heterotrophs and function as essential nursery grounds for juvenile fish (Nagelkerken et al., 2008), SIEs may disrupt these ecosystems, leading to direct mortality, forced migration, heightened vulnerability to predation, shifts in food availability, and changes to life cycles (Rabalais et al., 2002; Vaquer-Sunyer and Duarte, 2008; Dubuc et al., 2019; Pérez-Posada et al., 2023). Therefore, the constant arrival of Sargassum poses a threat to both flora and fauna. Hernández et al. (2022) suggest that the persistent influx of Sargassum may have negatively impacted vegetation cover, including mangroves and seagrasses, resulting in a decline in La Parguera. MJKB experienced 10 weeks of hypoxic conditions due to the accumulation of carbon-rich Sargassum biomass, with DO levels decreasing below the critical lethal concentration 50% (LC50) threshold of 2 mg O2/L (Vaquer-Sunyer and Duarte, 2008). According to Vaquer-Sunyer and Duarte (2008), fish and crustaceans would perish from hypoxia in these circumstances before they could reach the critical threshold.
The evolution of hypoxia was paralleled by a decline in aragonite saturation, which dropped below the critical threshold of Ω < 2.0 following SIEs (Sánchez-Beristain et al., 2016), as illustrated in Figure 6. The decrease in Ω could be disadvantageous for many marine organisms, such as corals, clams, echinoderms, mussels, oysters, etc (Morse et al., 2006; Bates et al., 2009; Millero, 2013; Mollica et al., 2018). Also, the low pH and Ω levels could affect commercially important species’ breeding areas and the food web dynamics at lower trophic levels (Branch et al., 2013; Sutton et al., 2016; Clements and Chopin, 2017).
The SIEs have a significant impact on the ecosystem and socio-economic consequences, disrupting tourism, limiting local recreational activities, and constraining fisheries due to reduced fish availability (Rodríguez-Martínez et al., 2019; Hamel et al., 2024). Additionally, there are challenges in developing cost-effective management strategies to remove Sargassum from shorelines (Hamel et al., 2024; León-Pérez et al., 2024). Our observations highlight the need for further assessment of impacts arising from Sargassum and the development of tools capable of forecasting SIEs and their biogeochemical impacts. The time series presented in this study was used to develop the CARICOOS’ coastal Sargassum inundation forecasting products (caricoos.org/sargassum) and is an ongoing effort to validate the models. In this way we are enhancing predictive models and providing tools for coastal management strategies.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: http://dm1.caricoos.org/thredds/catalog/content/Parguera_Sargassum/catalog.html.
Author contributions
PM-C: Writing – review & editing, Project administration, Writing – original draft, Formal analysis, Methodology, Data curation, Conceptualization, Investigation. JMM: Funding acquisition, Resources, Project administration, Formal analysis, Supervision, Conceptualization, Writing – review & editing, Methodology. LM-B: Formal analysis, Methodology, Writing – review & editing. LR-M: Methodology, Writing – review & editing, Investigation. JEM: Methodology, Investigation, Writing – review & editing. MV-R: Formal analysis, Writing – review & editing, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was funded by the NOAA Ocean Service, National Centers for Coastal Ocean Science, Competitive Research Program under NOAA award #NA23NOS4780291. This is contribution number 267 from the NOAA Monitoring and Event Response for Harmful Algal Blooms (MERHAB) Research Program.
Acknowledgments
We acknowledge the financial support provided by NOAA’s NCCOS and IOOS programs and the Department of Marine Science at the University of Puerto Rico Mayagüez for their assistance with field logistics.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1612438/full#supplementary-material
References
1
Adame M. F. Lovelock C. E. (2011). Carbon and nutrient exchange of mangrove forests with the coastal ocean. Hydrobiologia663, 23–50. doi: 10.1007/s10750-010-0554-7
2
Astor Y. Fanning K. Guzman L. Li X. Lorenzoni L. Masserini R. et al . (2013). Cariaco Time Series Study Handbook of Methods for the Analysis of Oceanographic Parameters at the CARIACO Time-series Station Serie Ciencia y Tecnología N ° 12 Fundación La Salle de Ciencias Naturales Caracas 2013. Fundación La Salle de Ciencias Naturales Caracas: CARIACO Time‑Series Study Handbook of Methods Methods.
3
Ayala-Torres R. Otero E. (2023). Seasonal dissolved oxygen depletion in bottom waters may be linked to bioluminescence in a shallow Caribbean bay. Regional. Stud. Mar. Sci.66, 103139. doi: 10.1016/j.rsma.2023.103139
4
Bates N. R. Mathis J. T. Cooper L. W. (2009). Ocean acidification and biologically induced seasonality of carbonate mineral saturation states in the western Arctic Ocean. J. Geophys. Res. Oceans.114, 1–21. doi: 10.1029/2008JC004862
5
Bay C. Lee K.-S. Dunton K. H. (1996). Production and carbon reserve dynamics of the seagrass Thalassia testudinum in Corpus Christi Bay, Texas, USA.
6
Bernard D. Biabiany E. Cécé R. Chery R. Sekkat N. (2022). Clustering analysis of the Sargassum transport process: application to beaching prediction in the Lesser Antilles. Ocean. Sci.18, 915–935. doi: 10.5194/os-18-915-2022
7
Branch T. A. DeJoseph B. M. Ray L. J. Wagner C. A. (2013). Impacts of ocean acidification on marine seafood. Trends Ecol. Evol.28, 178–186. doi: 10.1016/j.tree.2012.10.001
8
Brooks M. T. Coles V. J. Hood R. R. Gower J. F. R. (2018). Factors controlling the seasonal distribution of pelagic Sargassum. Mar. Ecol. Prog. Ser.599, 1–18. doi: 10.3354/meps12646
9
Brown P. (2020). Rethinking Sargassum Seaweed: Could It Be the New Normal in Jamaica? Available online at: https://nacla.org/news/2020/03/12/rethinking-sargassum-seaweed-Jamaica (Accessed April 26, 2020).
10
Burkholder J. A. M. Tomasko D. A. Touchette B. W. (2007). Seagrasses and eutrophication. J. Exp. Mar. Biol. Ecol.350, 46–72. doi: 10.1016/j.jembe.2007.06.024
11
Cabanillas-Teran N. Hernandez-Arana H. A. Ruiz-Zarate M. A. Vega-Zepeda A. Sanchez-Gonzalez A. (2019). Sargassum blooms in the Caribbean alter the trophic structure of the sea urchin Diadema antillarum. PeerJ2019, 1–32. doi: 10.7717/peerj.7589
12
Casazza T. L. Ross S. W. (2008). Fishes associated with pelagic Sargassum and open water lacking Sargassum in the Gulf Stream off North Carolina. Available online at: http://hdl.handle.net/1834/25466 (Accessed October 11, 2023).
13
Cashman A. Nagdee M. R. (2017). Impacts of climate change on settlements and infrastructure in the coastal and marine environments of caribbean small island developing states (SIDS). Sci. Rev.2017, 155–173.
14
Clements J. C. Chopin T. (2017). Ocean acidification and marine aquaculture in North America: potential impacts and mitigation strategies. Rev. Aquac.9, 326–341. doi: 10.1111/raq.12140
15
de Széchy M. T. M. Guedes P. M. Baeta-Neves M. H. Oliveira E. N. (2012). Verification of Sargassum natans (Linnaeus) Gaillon (Heterokontophyta: Phaeophyceae) from the Sargasso Sea off the coast of Brazil, western Atlantic Ocean. Check. List.8, 638–641. doi: 10.15560/8.4.638
16
Dickson A. G. Andrew G. Sabine C. L. Christian J. R. North Pacific Marine Science Organization (2007). Guide to best practices for ocean CO2 measurements (Sidney, British Columbia: North Pacific Marine Science Organization).
17
Dickson A. G. Goyet C. (1994). “Determination of the pH of sea water using the indicator dye m-cresol purple,” in Handbook of Methods for the Analysis of the various parameters of the carbon dioxide system in Sea Water (Oak Ridge, TN: Oak Ridge National Lab. (ORNL)), 3–10. doi: 10.2172/10107773
18
Djakouré S. Araujo M. Hounsou-Gbo A. Noriega C. Bourlès B. (2017). On the potential causes of the recent Pelagic Sargassum blooms events in the tropical North Atlantic Ocean. Biogeosci. Discuss.2017, 1–20. doi: 10.5194/bg-2017-346
19
Dubuc A. Baker R. Marchand C. Waltham N. J. Sheaves M. (2019). Hypoxia in mangroves: Occurrence and impact on valuable tropical fish habitat. Biogeosciences16, 3959–3976. doi: 10.5194/bg-16-3959-2019
20
García-Troche E. M. Morell J. M. Meléndez M. Salisbury J. E. (2021). Carbonate chemistry seasonality in a tropical mangrove lagoon in La Parguera, Puerto Rico. PloS One16, e0250069. doi: 10.1371/journal.pone.0250069
21
Golley F. Odum H. T. Wilson R. F. (1962). The structure and metabolism of a puerto rican red mangrove forest in may. Ecology43, 9–19. doi: 10.2307/1932034
22
Gouvêa L. P. Assis J. Gurgel C. F. D. Serrão E. A. Silveira T. C. L. Santos R. et al . (2020). Golden carbon of Sargassum forests revealed as an opportunity for climate change mitigation. Sci. Total. Environ.729, 138745. doi: 10.1016/j.scitotenv.2020.138745
23
Gower J. Young E. King S. (2013). Satellite images suggest a new Sargassum source region in 2011. Remote Sens. Lett.4, 764–773. doi: 10.1080/2150704X.2013.796433
24
Grasshoff K. Kremling K. Ehrhardt M. (2007). Methods of Seawater Analysis: Third, Completely Revised and Extended Edition (Weinheim, Germany: Wiley-VCH). doi: 10.1002/9783527613984
25
Hamel K. Garcia-Quijano C. Jin D. Dalton T. (2024). Perceived Sargassum event incidence, impacts, and management response in the Caribbean Basin. Mar. Policy165, 106214. doi: 10.1016/j.marpol.2024.106214
26
Hernández W. J. Morell J. M. Armstrong R. A. (2022). Using high-resolution satellite imagery to assess the impact of Sargassum inundation on coastal areas. Remote Sens. Lett.13, 24–34. doi: 10.1080/2150704X.2021.1981558
27
Hertler H. (2002). Implications of resource management in La Parguera, Puerto Rico. Available online at: https://www.researchgate.net/publication/28673803 (Accessed October 20, 2023).
28
Hu C. (2022). Outlook of 2022 Sargassum blooms in the Caribbean Sea and Gulf of Mexico. Available online at: https://optics.marine.usf.edu/projects/SaWS/pdf/Sargassum_outlook_2022_bulletin05_USF.pdf (Accessed May 22, 2024).
29
Hu C. Hardy R. Ruder E. Geggel A. Feng L. Powers S. et al . (2016). Sargassum coverage in the northeastern Gulf of Mexico during 2010 from Landsat and airborne observations: Implications for the Deepwater Horizon oil spill impact assessment. Mar. pollut. Bull.107, 15–21. doi: 10.1016/j.marpolbul.2016.04.045
30
Hu C. Wang M. Lapointe B. E. Brewton R. A. Hernandez F. J. (2021). On the Atlantic pelagic Sargassum’s role in carbon fixation and sequestration. Sci. Total. Environ.781, 146801. doi: 10.1016/j.scitotenv.2021.146801
31
Huffard C. L. von Thun S. Sherman A. D. Sealey K. Smith K. L. (2014). Pelagic Sargassum community change over a 40-year period: temporal and spatial variability. Mar. Biol.161, 2735–2751. doi: 10.1007/s00227-014-2539-y
32
Johns E. M. Lumpkin R. Putman N. F. Smith R. H. Muller-Karger F. E. T. Rueda-Roa D. et al . (2020). The establishment of a pelagic Sargassum population in the tropical Atlantic: Biological consequences of a basin-scale long distance dispersal event. Prog. Oceanogr.182, 102269. doi: 10.1016/j.pocean.2020.102269
33
Jones D. L. (2017). Fathom Toolbox for MATLAB: software for multivariate ecological and oceanographic data analysis (St. Petersburg, FL, USA: College of Marine Science, University of South Florida). Available online at: https://www.usf.edu/marine-science/research/matlab-resources/index.aspx/ (Accessed March 3, 2025).
34
Juman R. A. (2005). The structure and productivity of the Thalassia testudinum community in Bon Accord Lagoon,Tobago. Rev. Biol. Trop.53, 219–227. Available at: http://www.scielo.sa.cr/scielo.php?script=sci_arttext&pid=S0034-77442005000300027&lng=en&nrm=iso&tlng=en.
35
Krause-Jensen D. Duarte C. M. (2016). Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci.9, 737–742. doi: 10.1038/ngeo2790
36
Laffoley D. Baxter J. M. Thevenon F. Oliver J. D. Baxter J. M. Thevenon F. et al . (2014). The significance and management of natural carbon stores in the open ocean (Gland, Switzerland: IUCN (International Union for Conservation of Nature)).
37
Lee K. S. Park S. R. Kim Y. K. (2007). Effects of irradiance, temperature, and nutrients on growth dynamics of seagrasses: A review. J. Exp. Mar. Biol. Ecol.350, 144–175. doi: 10.1016/j.jembe.2007.06.016
38
León-Pérez M. C. McLaughlin R. J. Gibeaut J. C. Carrubba L. Colón-Rivera R. J. Esteves R. (2024). First steps towards untangling the sargassum legal regime in Puerto Rico. Mar. Policy165, 106202. doi: 10.1016/j.marpol.2024.106202
39
León-Pérez M. C. Reisinger A. S. Gibeaut J. C. (2023). Spatial-temporal dynamics of decaying stages of pelagic Sargassum spp. along shorelines in Puerto Rico using Google Earth Engine. Mar. pollut. Bull.188, 114715. doi: 10.1016/j.marpolbul.2023.114715
40
Lewis E. Wallace D. (1998). Program developed for CO2 system calculations (Oak Ridge, Tennessee, USA: Carbon Dioxide Information Analysis Center (CDIAC), Oak Ridge National Laboratory).
41
Liboy J. G. (1976). An Examination of the Present Condition of Seagrass Meadows in La Parguera, Puerto Rico (Puerto Rico: Department of Natural Resources, Puerto Rico).
42
Linton D. Fisher T. (2004). CARICOMP: Caribbean Coastal Marine Productivity Program, 1993-2003. (Kingston, Jamaica: CARICOMP).
43
Marsh R. Oxenford H. A. Cox S. A. L. Johnson D. R. Bellamy J. (2022). Forecasting seasonal sargassum events across the tropical Atlantic: Overview and challenges. Front. Mar. Sci.9. doi: 10.3389/fmars.2022.914501
44
Martínez-Lüscher J. Holmer M. (2010). Potential effects of the invasive species Gracilaria vermiculophylla on Zostera marina metabolism and survival. Mar. Environ. Res.69, 345–349. doi: 10.1016/j.marenvres.2009.12.009
45
Meléndez M. Salisbury J. Gledhill D. Langdon C. Morell J. M. Manzello D. et al . (2022). Net ecosystem dissolution and respiration dominate metabolic rates at two western Atlantic reef sites. Limnol. Oceanogr.67, 527–539. doi: 10.1002/lno.12009
46
Mendez-Tejeda R. Rosado J. G. A. (2019). Influence of climatic factors on Sargassum arrivals to the coasts of the Dominican Republic. J. Oceanogr. Mar. Sci.10, 22–32. doi: 10.5897/joms2019.0156
47
Millero F. (2013). Chemical Oceanography, 4th ed. (Boca Raton, Florida, USA: CRC Press). doi: 10.1201/b14753
48
Mollica N. R. Guo W. Cohen A. L. Huang K. F. Foster G. L. Donald H. K. et al . (2018). Ocean acidification affects coral growth by reducing skeletal density. Proc. Natl. Acad. Sci. U.S.A.115, 1754–1759. doi: 10.1073/pnas.1712806115
49
Moreira Á. Alfonso G. (2013). Inusual arribazón de Sargassum fluitans (Børgesen) Børgesen en la costa centro-sur de Cuba. Rev. Investig. Mar.33, 17–20.
50
Morse J. W. Andersson A. J. Mackenzie F. T. (2006). Initial responses of carbonate-rich shelf sediments to rising atmospheric pCO2 and “ocean acidification”: Role of high Mg-calcites. Geochim. Cosmochim. Acta70, 5814–5830. doi: 10.1016/j.gca.2006.08.017
51
Nagelkerken I. Blaber S. J. M. Bouillon S. Green P. Haywood M. Kirton L. G. et al . (2008). The habitat function of mangroves for terrestrial and marine fauna: A review. Aquat. Bot.89, 155–185. doi: 10.1016/j.aquabot.2007.12.007
52
NOAA (2003). Rule to implement fishery management plan for pelagic sargassum habitat of the south atlantic region. Fed. Regist.68.
53
Odum H. Burkholder P. Rivero J. (1959). Measurement of productivity of turtle grass flats, reefs, and the bahia fosforescente of southern Puerto Rico. Inst. Mar. Sci.VI, 159–170.
54
Oviatt C. A. Huizenga K. Rogers C. S. Miller W. J. (2019). What nutrient sources support anomalous growth and the recent Sargassum mass stranding on Caribbean beaches? A review. Mar. pollut. Bull.145, 517–525. doi: 10.1016/j.marpolbul.2019.06.049
55
Pérez-Pérez J. Cruz Motta J. J. Hernández López W. J. Morales Payán J. P. (2022). Impacts of floating Sargassum accumulation on the fringing mangrove Rhizophora mangle in Southwestern Puerto Rico: A Case Study (Mayagüez, Puerto Rico: University of Puerto Rico at Mayagüez).
56
Pérez-Posada I. Cabanillas-Terán N. Rosas-Luis R. Hernández-Arana H. A. Sánchez-Gonzalez A. (2023). Isotopic niche shift in the sea urchins Echinometra lucunter and E. viridis after massive arrivals of Sargassum in the Mexican Caribbean. Regional. Stud. Mar. Sci.65, 103064. doi: 10.1016/j.rsma.2023.103064
57
Powers L. C. Hertkorn N. McDonald N. Schmitt-Kopplin P. Del Vecchio R. Blough N. V. et al . (2019). Sargassum sp. Act as a large regional source of marine dissolved organic carbon and polyphenols. Global Biogeochem. Cycles.33, 1423–1439. doi: 10.1029/2019GB006225
58
Putman N. F. Goni G. J. Gramer L. J. Hu C. Johns E. M. Trinanes J. et al . (2018). Simulating transport pathways of pelagic Sargassum from the Equatorial Atlantic into the Caribbean Sea. Prog. Oceanogr.165, 205–214. doi: 10.1016/j.pocean.2018.06.009
59
Rabalais N. N. Turner R. E. Wiseman W. J. (2002). Gulf of Mexico hypoxia, a.k.a. “The dead zone. Annu. Rev. Ecol. Syst.33, 235–263. doi: 10.1146/annurev.ecolsys.33.010802.150513
60
Robertson M. L. Mills A. L. Zieman J. C. (1982). Microbial synthesis of detritus-like particulates from dissolved organic carbon released by tropical seagrasses. Mar. Ecol. Prog. Ser.7, 279–285. doi: 10.3354/meps007279
61
Rodríguez-Martínez R. E. Medina-Valmaseda A. E. Blanchon P. Monroy-Velázquez L. V. Almazán-Becerril A. Delgado-Pech B. et al . (2019). Faunal mortality associated with massive beaching and decomposition of pelagic Sargassum. Mar. pollut. Bull.146, 201–205. doi: 10.1016/j.marpolbul.2019.06.015
62
Sánchez A. Gonzalez-Jones P. Camacho-Cruz K. A. Anguas-Cabrera D. Ortiz-Hernández M. C. Rey-Villiers N. (2023). Influence of pelagic sargassum influxes on the δ15N in Thalassia testudinum of the Mexican Caribbean coastal ecosystem. Mar. pollut. Bull.192, 115091. doi: 10.1016/j.marpolbul.2023.115091
63
Sánchez-Beristain F. García-Barrera P. Calvillo-Canadell L. (2016). Mares calcíticos y aragoníticos: efectos en organismos formadores de arrecifes a través del tiempo. TIP19, 45–53. doi: 10.1016/j.recqb.2016.02.005
64
Skliris N. Marsh R. Appeaning Addo K. Oxenford H. (2022). Physical drivers of pelagic sargassum bloom interannual variability in the Central West Atlantic over 2010–2020. Ocean. Dynamics.72, 383–404. doi: 10.1007/s10236-022-01511-1
65
Sonter L. J. Herrera D. Barrett D. J. Galford G. L. Moran C. J. Soares-Filho B. S. (2017). Mining drives extensive deforestation in the Brazilian Amazon. Nat. Commun.8, 1013. doi: 10.1038/s41467-017-00557-w
66
Sutton A. J. Sabine C. L. Feely R. A. Cai W. J. Cronin M. F. McPhaden M. J. et al . (2016). Using present-day observations to detect when anthropogenic change forces surface ocean carbonate chemistry outside preindustrial bounds. Biogeosciences13, 5065–5083. doi: 10.5194/bg-13-5065-2016
67
Valdés-Pizzini M. Schärer-Umpierre M. (2014). People, habitats, Species, and Governance: An Assessment of the Social-Ecological System of La Parguera, Puerto Rico. Available online at: http://www.seagrantpr.org/catalog/files/books/La_Parguera.pdf (Accessed February 18, 2025).
68
Valiela I. McClelland J. Hauxwell J. Behr P. J. Hersh D. Foreman K. (1997). Macroalgal blooms in shallow estuaries: Controls and ecophysiological and ecosystem consequences. Limnol. Oceanogr.42, 1105–1118. doi: 10.4319/lo.1997.42.5_part_2.1105
69
van Tussenbroek B. I. Hernández Arana H. A. Rodríguez-Martínez R. E. Espinoza-Avalos J. Canizales-Flores H. M. González-Godoy C. E. et al . (2017). Severe impacts of brown tides caused by Sargassum spp. on near-shore Caribbean seagrass communities. Mar. pollut. Bull.122, 272–281. doi: 10.1016/j.marpolbul.2017.06.057
70
Vaquer-Sunyer R. Duarte C. M. (2008). Thresholds of hypoxia for marine biodiversity. Proc. Natl. Acad. Sci.105, 15452–7. doi: 10.1073/pnas.0803833105
71
Vega-Rodríguez M. Armstrong R. A. Gilbes F. López J. Fernandez del Viso D. (2008). Estimating primary productivity of red mangroves in southwestern Puerto Rico from remote sensing and field measurements (Master’s thesis). (Mayagüez, Puerto Rico: University of Puerto Rico at Mayagüez).
72
Wallace R. B. Baumann H. Grear J. S. Aller R. C. Gobler C. J. (2014). Coastal ocean acidification: The other eutrophication problem. Estuar. Coast. Shelf. Sci.148, 1–13. doi: 10.1016/j.ecss.2014.05.027
73
Wang M. Hu C. (2016). Mapping and quantifying Sargassum distribution and coverage in the Central West Atlantic using MODIS observations. Remote Sens. Environ.183, 356–367. doi: 10.1016/j.rse.2016.04.019
74
Wang M. Hu C. Barnes B. B. Mitchum G. Lapointe B. Montoya J. P. (2019). The great Atlantic Sargassum belt. Sci. (1979).365, 83–87. doi: 10.1126/science.aaw7912
75
Wang M. Hu C. Cannizzaro J. English D. Han X. Naar D. et al . (2018). Remote sensing of sargassum biomass, nutrients, and pigments. Geophys. Res. Lett.45, 12,359–12,367. doi: 10.1029/2018GL078858
76
Weis J. S. (1968). Fauna associated with pelagic sargassum in the gulf stream. Source.: Am. Midland. Nat.80, 554–558. doi: 10.2307/2423550
Summary
Keywords
carbon input, tropical coastal ecosystem, pelagic Sargassum, hypoxia, biogeochemistry, ocean acidification
Citation
Molina-Cora PN, Morell JM, Martell-Bonet L, Rodriguez-Matos LR, Morell JE and Vélez-Rivera M (2025) Observations of Sargassum carbon influx and biogeochemical impact in La Parguera Marine Reserve. Front. Mar. Sci. 12:1612438. doi: 10.3389/fmars.2025.1612438
Received
15 April 2025
Accepted
18 June 2025
Published
04 July 2025
Volume
12 - 2025
Edited by
Betina J. Lomovasky, Institute of Marine and Coastal Research (IIMyC), Argentina
Reviewed by
Emma Rocke, University of Cape Town, South Africa
Alberto Sánchez-González, National Polytechnic Institute (IPN), Mexico
Updates
Copyright
© 2025 Molina-Cora, Morell, Martell-Bonet, Rodriguez-Matos, Morell and Vélez-Rivera.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Priscilla N. Molina-Cora, priscilla.molina@upr.edu; Julio M. Morell, julio.morell@upr.edu; Loraine Martell-Bonet, loraine.martelll@upr.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.