- 1Department of Biological, Geological and Environmental Sciences (BIGEA), University of Bologna (UNIBO), Bologna, Italy
- 2National Research Council (CNR), Institute for Biological Resources and Marine Biotechnologies (IRBIM), Ancona, Italy
- 3NBFC, National Biodiversity Future Center, Palermo, Italy
- 4Department of Marine Sciences, University of the Aegean, Mytilene, Greece
The introduction of invasive alien species (IAS) and the occurrence of jellyfish blooms and harmful algal blooms (HABs) can significantly alter native biodiversity and disrupt ecosystem functioning. This study expands the Cumulative IMPacts of invasive ALien species (CIMPAL) index to assess the cumulative impacts of IAS, HABs, and jellyfish blooms, also accounting for interspecific interactions. The approach is implemented in the Aegean Sea, analyzing data on 26 alien species (including one jellyfish), seven phytoplankton species responsible for HABs, and four native jellyfish species known to cause blooms. The application of CIMPAL revealed the spatial patterns and the relative importance of impacts across the Aegean Sea, identifying the most affected areas and ranking species based on four impact indicators. The results indicated that IAS contributed the most to cumulative impacts, with the highest scores observed in confined southern coastal areas of the Aegean Sea. Consequently, highly impacted coastal regions due to IAS were more prevalent in the southern Aegean compared to the north. In contrast, cumulative impacts in open waters decreased from the northern to the southern Aegean. HABs and jellyfish blooms also caused considerable impacts, particularly in certain gulfs. This study provides essential spatially explicit information to support effective management and mitigation of these environmental challenges in the Aegean Sea.
1 Introduction
Alien species are organisms that have been intentionally or unintentionally introduced beyond their native geographic range due to human activities. Their introduction into new ecosystems can significantly alter the structure of native communities and disrupt ecosystem functioning (Howard et al., 2019; Katsanevakis et al., 2014a), often leading to dramatic declines in native biodiversity (Doherty et al., 2016; García-Gómez et al., 2020; Tsirintanis et al., 2022) and major losses in ecosystem services (Castro-Díez et al., 2019; Katsanevakis et al., 2014b). Human activities have led to the introduction of over 37,000 alien species across various taxonomic groups and geographic regions worldwide. The rate of new introductions is currently around 200 species per year, with an unprecedented increase in the spread of alien species (Roy et al., 2023). When alien species negatively impact their new ecosystems, they are classified as invasive alien species (IAS) (CBD, 2008). IAS cause significant and often irreversible changes to biodiversity and ecosystems, contributing to 60% of recorded global extinctions. They promote biotic homogenization, alter ecosystems properties, and their impacts are predicted to escalate in the future (Roy et al., 2023).
Jellyfish blooms are defined as sudden increases in gelatinous zooplankton populations from various taxonomic groups, including Cnidaria (Hydrozoa, Scyphozoa, and Cubozoa), Chordata (Tunicata), and Ctenophora (Boero, 2013; Sagarminaga et al., 2024). Jellyfish populations naturally fluctuate, experiencing periodic surges, and thus blooms are generally considered normal ecological phenomena (Boero, 2013). However, numerous studies have identified various anthropogenic drivers behind the increasing frequency and intensity of jellyfish outbreaks (Sagarminaga et al., 2024), such as eutrophication (Richardson et al., 2009), overfishing (Boero, 2013), climate change (Boero et al., 2016), and the introduction of alien species (Qu et al., 2014). Additionally, jellyfish densities are influenced by environmental factors. Although responses vary among species, temperature is a primary factor (Qu et al., 2014), followed by light, salinity, nutrient availability (Graham et al., 2001; Purcell, 2005), as well as physical factors such as currents, pressure, and turbulence (Graham et al., 2001). Jellyfish blooms can have significant consequences for marine ecosystems and coastal human activities, with impacts ranging from localized disturbances to broad regional effects (Sagarminaga et al., 2024). The adverse effects on biodiversity (e.g., Báez et al., 2022; Dinasquet et al., 2012; Helmholz et al., 2010; Sagarminaga et al., 2024), fisheries and aquaculture (e.g., Doyle et al., 2008; Quiñones et al., 2013; Sagarminaga et al., 2024), tourism (Canepa et al., 2014; Ghermandi et al., 2015), and human health (Cegolon et al., 2013) are well-documented. The increasing frequency of jellyfish blooms in recent decades across various regions (Boero et al., 2008) highlights the urgent need for effective management strategies to mitigate their ecological and socio-economic impacts (Sagarminaga et al., 2024).
Harmful Algal Blooms (HABs) are events of proliferation of specific microalgal and macroalgal species that result in ecological, social, economic, and health-related impacts (Sagarminaga et al., 2023). These blooms are triggered by particular physical, biological, and chemical conditions, and can be exacerbated by human activities (West et al., 2021). The impacts of HABs are diverse. Even non-toxic algal blooms can disrupt ecosystems by reducing light penetration - contributing, for example, to seagrass decline (Burkholder et al., 2007) - and by depleting oxygen during decay, often leading to mass mortalities (Anderson, 2009). Additional harms include mechanical damage, acting as potential vectors for diseases, and degradation of water quality (Landsberg, 2002). When organisms ingest toxic phytoplankton, toxins can accumulate in their tissues to levels that pose substantial health risks to humans and other consumers, resulting in various poisoning syndromes. Toxins may also be released directly into the water, further threatening aquatic life and public health (Anderson, 2009). HABs can also lead to significant economic losses, including monitoring costs, fishery closures, fish and shellfish mortalities, reduced seafood sales, tourism disruption, and medical expenses for affected populations (Anderson, 2009). Although HABs are widely believed to be increasing in frequency and severity, further analysis is required to confirm a global trend (Hallegraeff et al., 2021). However, the expansion of aquaculture is expected to exacerbate HAB events in certain areas, particularly in developing regions already facing serious challenges from such outbreaks (Anderson, 2014). Given their extensive environmental and socio-economic consequences, effective monitoring and management strategies are fundamental (Sagarminaga et al., 2023; Azzurro et al., 2024).
Conducting impact assessments is essential for identifying and highlighting threats to biodiversity and ecosystem functioning. This understanding is crucial for developing effective adaptive management strategies in marine ecosystems, as it accounts for both the nature and spatial distribution of current impacts (Levin et al., 2009). The CIMPAL (Cumulative IMPacts of invasive ALien species) index, developed by Katsanevakis et al. (2016), provides a structured framework for estimating and mapping the impacts of IAS. It has been extensively applied across terrestrial (e.g., pan-European IAS pressure mapping; Polce et al., 2023), freshwater (e.g., European river basins; Magliozzi et al., 2020), and marine (e.g., Mediterranean Sea; Katsanevakis et al., 2016) ecosystems. Among its key advantages are the ability to identify impact hotspots, prioritize management actions, and target specific sites, habitats, and species for intervention (Katsanevakis et al., 2016; Tsirintanis et al., 2023). CIMPAL supports informed decision-making and enhances the effectiveness of conservation efforts by providing a standardized, quantitative method for assessing cumulative impacts (Katsanevakis et al., 2016). To date, CIMPAL has been limited to assessing and mapping the impacts of IAS.
Recognizing that both native and alien species can exhibit invasive characteristics, this study adopts a broader ecological perspective on invasiveness, treating it not merely as a biogeographical phenomenon associated to alien species (Valéry et al., 2009). Accordingly, IAS are treated as a subset of the broader category of invasive species (Katsanevakis et al., 2023), which include also native invaders (Simberloff, 2011). Specifically, for the purposes of this study, we grouped IAS, HABs, and Jellyfish blooms into a single operational category, hereafter referred to as Invasive Native or Alien Species (INAS). Thus, this research expands the CIMPAL framework by integrating the impacts of all these species while also accounting for interspecific interactions. The extended framework is applied to the Aegean Sea as a case study.
2 Materials and methods
2.1 Expanding CIMPAL
CIMPAL is expanded to incorporate (1) the impacts of INAS, and (2) interspecific interactions. The new index is called CIMPAL-JH (Cumulative IMPacts of ALien species, Jellyfish blooms and HABs) and is calculated using the following Equation 1:
Ic is the cumulative impact score for a given study area cell. Ai denotes the population state of species i in the specific cell, standardized between 0 and 1. Hj represents the extent of habitat j within the cell. n is the total number of INAS; and m is the total number of marine habitats included in the analysis. wi,j is the impact weight of species i on habitat j, estimated as a combination of species i impact magnitude (following Blackburn et al., 2014; Volery et al., 2020) and the strength of available evidence (following Katsanevakis et al., 2014b)The estimations of the ‘impact magnitude’ and the ‘strength of available evidence’ are provided in the Supplementary Figures S1 and S2”. Ak represents the population state of species k≠i, another INAS interacting with species i, exhibiting either positive or negative effects, with Ak values standardized between 0 and 1. fi,k refers to the interaction effect of species k on species i, ranging from -1 (maximum negative interaction) up to a maximum value constrained by the condition (corresponding to the threshold for ‘massive’ impacts; i.e., the overall impact of a species, including synergistic interactions through the second term of Equation 1, cannot exceed this value); for i = k, fi,k = 0.
For INAS with continuous impacts (such as for most IAS) Ai should ideally be a standardized measure of species abundance. In case of limited data, proxies such as the probability of presence derived from species distribution models or even binary presence-absence may be used. However, species causing blooms (jellyfish or phytoplankton) differ fundamentally, as they typically occur in high concentrations seasonally or episodically, and for limited durations. Therefore, the overall severity of their impacts depends not only on the abundance (or concentration) of the blooming species but also on the average annual duration of blooms. To account for this, we propose estimating Ai for bloom-forming species using species-specific thresholds, derived from historical data on bloom concentration and duration. Ai can thus be defined as a composite measure combining both metrics (see Supplementary Figure S3 for details).
2.2 Aegean Sea case study
The Aegean Sea (north-eastern Mediterranean) is renowned for its rich marine biodiversity and complex ecosystems. Despite its ecological importance, the Aegean Sea faces substantial challenges for effective management and spatial planning (Sini et al., 2017). These challenges are intensified by human activities, including overfishing, pollution, and the introduction of alien species (Anagnostou et al., 2024). Over the past two decades, the number of established alien species in the region has substantially increased (Ragkousis et al., 2023), leading to important negative impacts on native biodiversity (Tsirintanis et al., 2023). Additionally, the frequency of jellyfish blooms has risen, disrupting tourism and posing risks to human health (Isinibilir et al., 2021; Özgür and Öztürk, 2008). HABs have also been recorded in the Aegean’s coastal waters, with documented adverse effects on the environment and ecosystem services (Tsikoti and Genitsaris, 2021). These events have resulted in water discoloration, mucilage formation, habitat degradation, marine species mortality, localized anoxia, and economic losses in tourism and aquaculture, along with public health concerns (Economou et al., 2007; Tsikoti and Genitsaris, 2021).
To apply CIMPAL-JH, the Aegean Sea was partitioned into 321,346 grid cells, each measuring 0.01° in latitude and longitude, as described by Tsirintanis et al. (2023). For each grid cell, the percent cover of ten broad habitat types was retrieved from Tsirintanis et al. (2023), derived from the habitat maps developed by Sini et al. (2017). These habitat types included seagrass meadows, shallow soft substrates (0–60 m depth), deep soft substrates (60–200 m depth), soft substrates of the dysphotic zone (deeper than 200 m), shallow hard substrates (0–60 m depth), deep hard substrates (60–200 m), hard substrates of the dysphotic zone (deeper than 200 m), submarine caves, coralligenous formations, and the pelagic habitat. Depth information was integrated by overlaying bathymetric contours with the distribution of soft and hard substrates to estimate the coverage of each habitat type within each grid cell.
IAS occurrence data were sourced from Tsirintanis et al. (2023). Overall, 26 IAS were found to impact at least one of the studied habitats. These species are Amathia verticillata, Amphistegina lobifera, Asparagopsis spp., Brachidontes pharaonis, Callinectes sapidus, Caulerpa cylindracea, Codium fragile, Conomurex persicus, Fistularia commersonii, Halophila stipulacea,Hydroides elegans, Lagocephalus sceleratus, Lophocladia lallemandii,Mnemiopsis leidyi (a jellyfish), Oculina patagonica,Parupeneus forsskali, Pempheris rhomboidea, Pinctada radiata,Pterois miles, Sargocentron rubrum, Siganus luridus, Siganus rivulatus, Styela plicata, Stypopodium schimperi,Upeneus pori and Womersleyella setacea.
Jellyfish bloom data were compiled from published studies, the CIESM Jelly Watch Program, and iNaturalist, while HAB occurrence data were sourced through a systematic literature review. The ctenophore Mnemiopsis leidyi, although a bloom-forming species, was included in the IAS dataset and consequently excluded from the jellyfish bloom records. Among all the studied species, only two interspecific interactions were identified: the consumption of the chlorophyte Caulerpa cylindracea by the two herbivorous fish Siganus luridus and S. rivulatus (Azzurro et al., 2007; Tsirintanis et al., 2023). In both cases, fi,k was taken equal to -1, as these two herbivore fish, when abundant, completely eradicate C. cylindracea. This means that the impact of C. cylindracea in such cases becomes (from Equation 1):
The CIMPAL-JH index was calculated for each grid cell, integrating the cumulative impacts of IAS, HABs, and jellyfish blooms. The population state of IAS, Ai, was derived from species distribution models (SDMs) as reported by Tsirintanis et al. (2023). For jellyfish and HAB species, only binary presence (1) or absence (0) data were used, due to the unavailability of consistent and reliable information on bloom duration and concentration. Impact magnitude and strength of evidence for each IAS were retrieved from Tsirintanis et al. (2023), whereas data for jellyfish and phytoplankton species were obtained through literature reviews (see Supplementary Table 1). Species lacking documented impacts were excluded from the cumulative impact score. This applied to the jellyfish Chrysaora hysoscella and Pennaria disticha and the phytoplankton species Alexandrium insuetum, Alexandrium minutum, Chaetoceros spp., Chattonella globosa, Chroococcus gelatinosus, Cylindrotheca closterium, Gonyaulax fragilis, Gonyaulax sp., Gonyaulax spinifera, Gymnodinium catenatum, Leptocylindrus danicus, Leptocylindrus minimus, Lyngbya agardhii, Microcystis aeruginosa, Noctiluca scintillans, Phaeocystis pouchetii, Prorocentrum micans, Prorocentrum minimum, Prorocentrum redfeldii, Pseudo-nitzschia, Skeletonema costatum, Spatulodinium pseudonoctiluca, Synechocystis sallensis, Synechocystis spp. and Trichodesmium erythraeum.
Species were ranked by their negative impacts using four indicators as proposed by Tsirintanis et al. (2023). The first (D1) measured the total area of occurrence, defined as the number of grid cells where the species was present. The second (D2) counted the number of cells where the species had an impact score greater than zero. The third (D3) calculated the cumulative impact score of each species by summing its impact values across the study area. The fourth (D4) determined the mean impact score across the species’ range, excluding grid cells with values below 0.1.
3 Results
Cumulative impact scores for IAS, jellyfish blooms, and HABs per grid cell ranged from 0 to 45.6 (Figures 1, 2), with an average score of 1.6 per cell. Shallow coastal habitats (<60 m depth) exhibited significantly higher cumulative impact scores than offshore waters, with impacts being spatially localized rather than widespread (Figure 2). In offshore waters, cumulative impacts gradually declined from the northern to the southern Aegean (Figure 1). In contrast, high-impact coastal areas (i.e., the highest CIMPAL-JH scores) were more abundant in the southern Aegean compared to the north (Figures 1, 2). Certain regions, particularly the Saronikos and Thermaikos Gulfs, emerged as hotspots of cumulative impact, affected by all three categories of INAS (Figures 1–3).
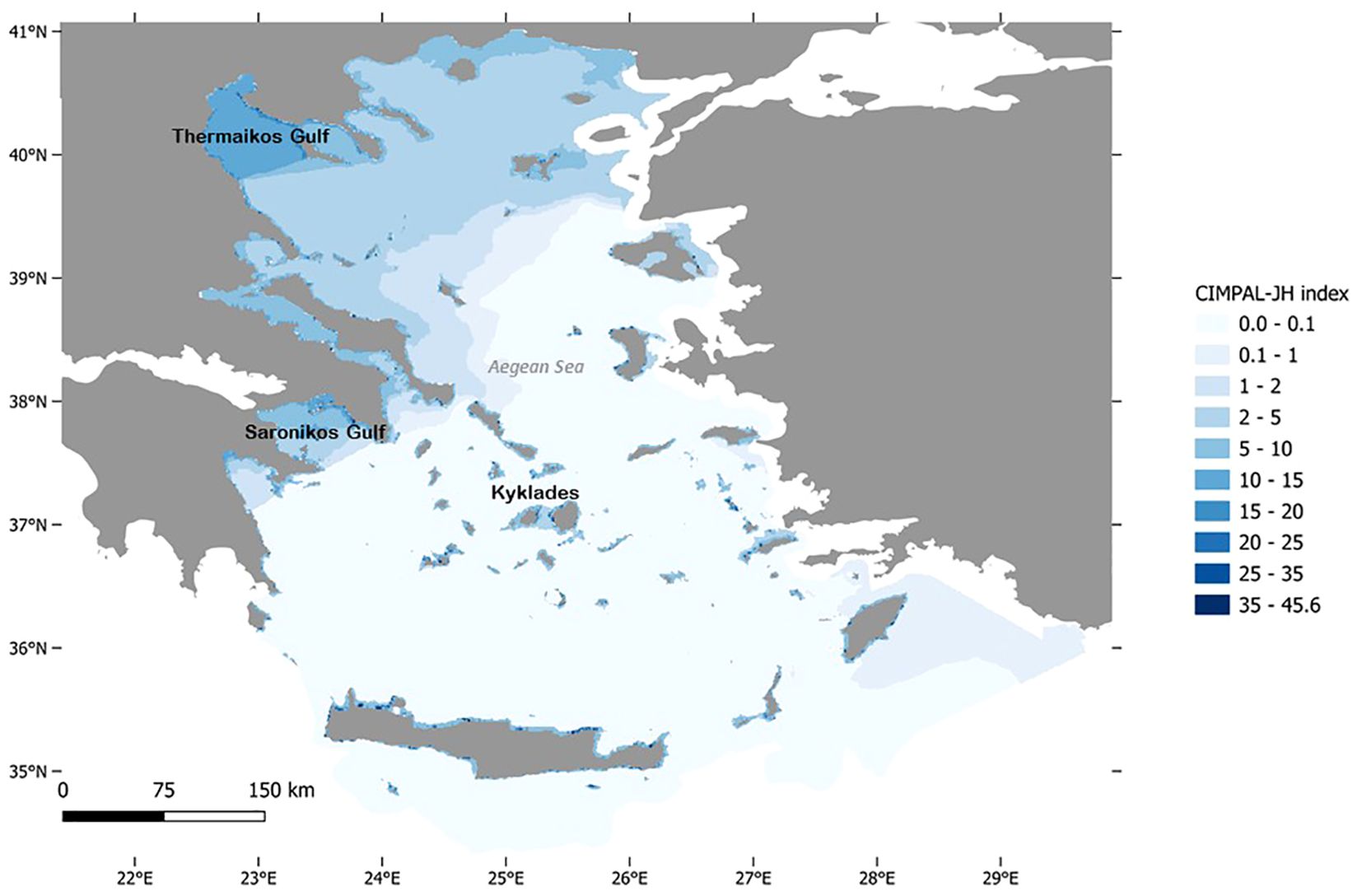
Figure 1. Map of cumulative impacts of invasive alien species (IAS), jellyfish blooms, and harmful algal blooms (HABs) in the Aegean Sea using CIMPAL-JH index scores.
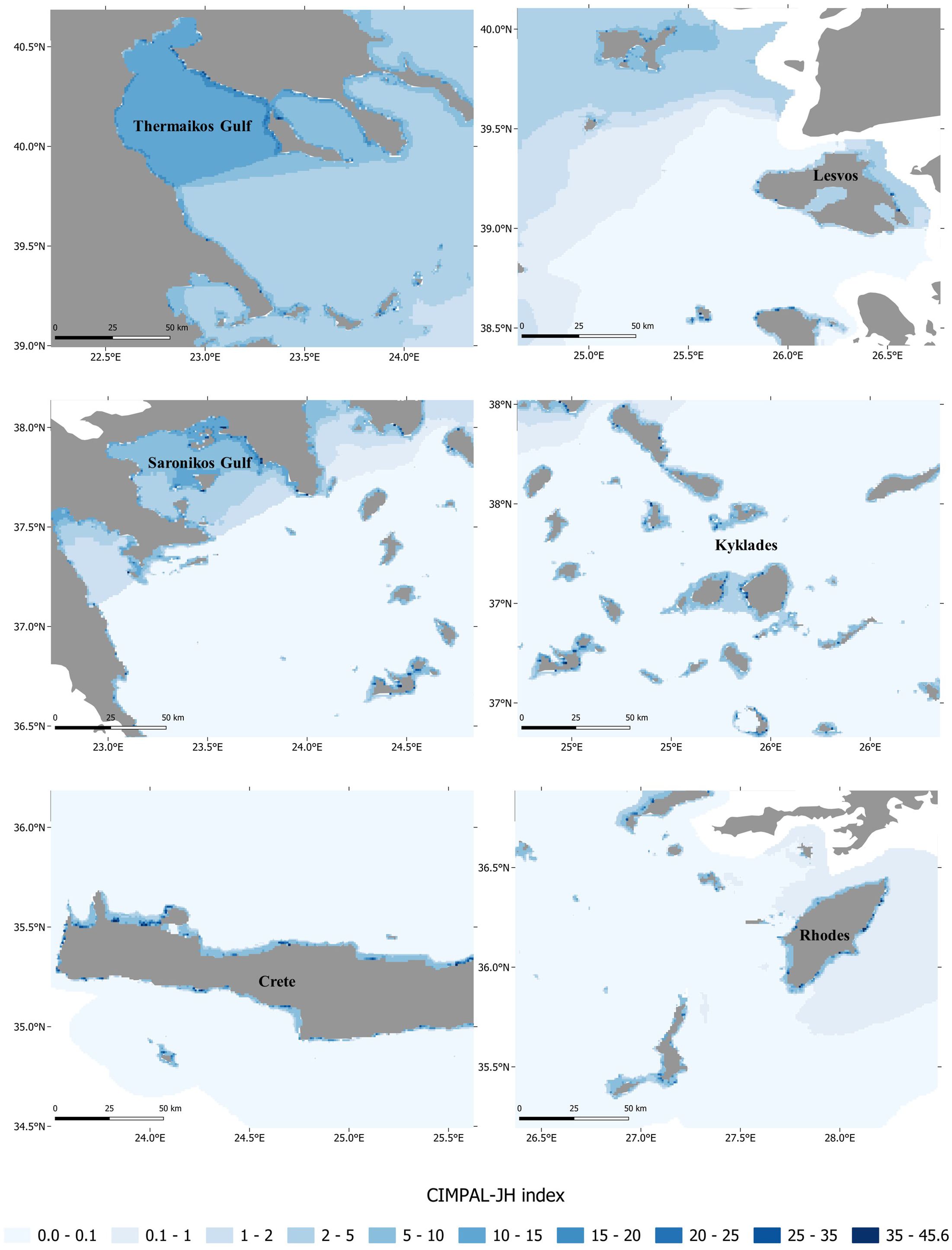
Figure 2. Zoomed-in views of cumulative impacts of invasive alien species (IAS), jellyfish blooms, and harmful algal blooms (HABs), in selected coastal areas of the Aegean Sea using CIMPAL-JH index scores. These close-up maps highlight spatial variations and fine-scale details of cumulative impact distribution in coastal regions.
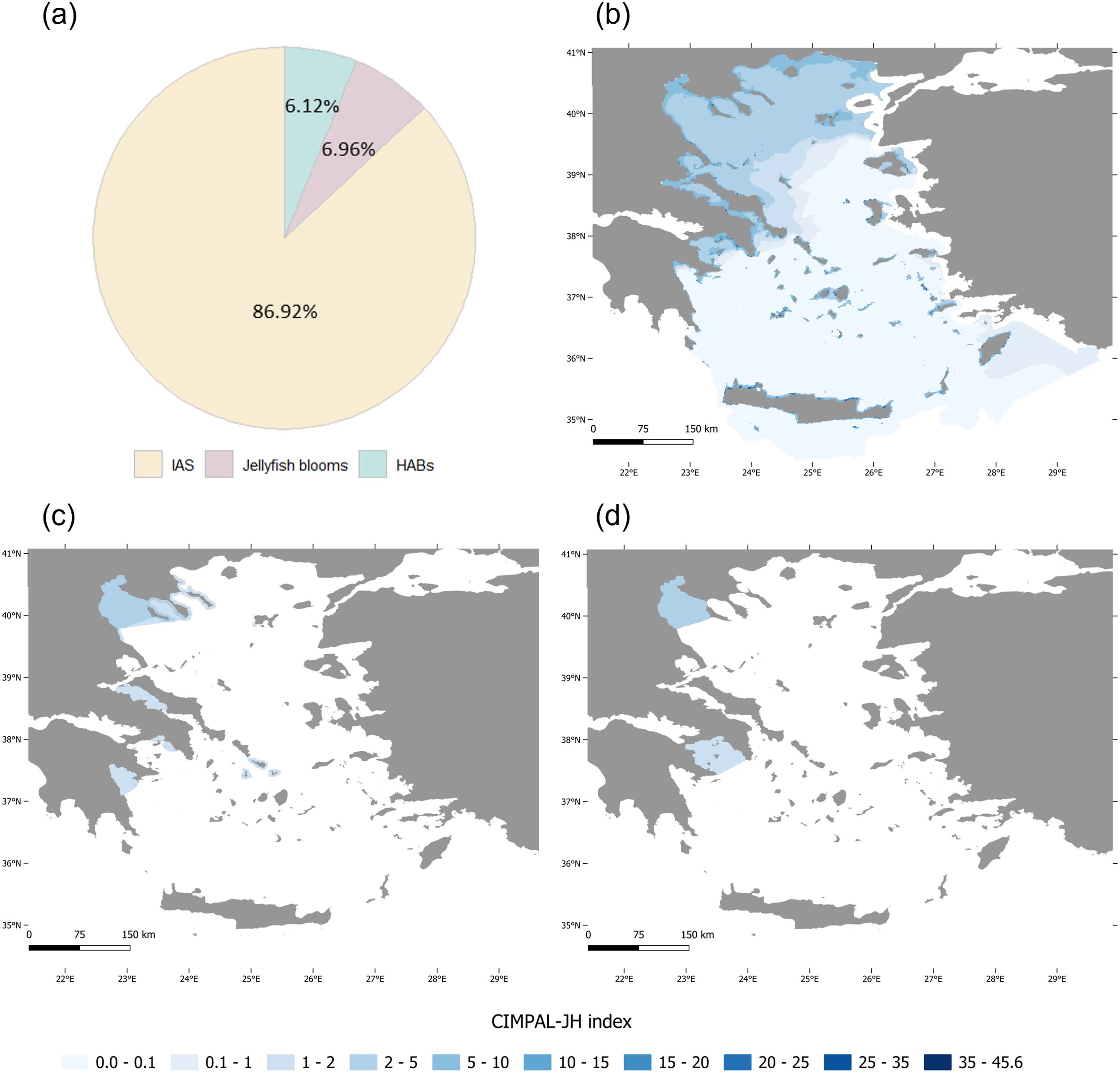
Figure 3. (a) Disaggregation of the total CIMPAL-JH score by its three components (IAS, jellyfish blooms, and HABs), and maps of the cumulative impacts in the Aegean Sea of (b) IAS, (c) jellyfish blooms, and (d) HABs.
IAS are responsible for the most extensive impacts recorded among the CIMPAL-JH components in the Aegean Sea (Figure 3b). Among the most widespread IAS, Mnemiopsis leidyi, Hydroides elegans, Lagocephalus sceleratus, Fistularia commersonii, and Pinctada radiata exhibited the broadest distribution (D1). When considering number of impacted cells (D2), M. leidyi, L. sceleratus, F. commersonii, Parupeneus forsskali, and Caulerpa cylindracea ranked highest. The cumulative impact scores (D3) identified M. leidyi, L. sceleratus, Amphistegina lobifera, F. commersonii, and Lophocladia lallemandii as the most impactful species. In terms of average impact within each species’ range (D4), M. leidyi, L. lallemandii, Siganus luridus, Siganus rivulatus, and Womersleyella setacea were the most impactful species in the Aegean Sea (Figure 4).
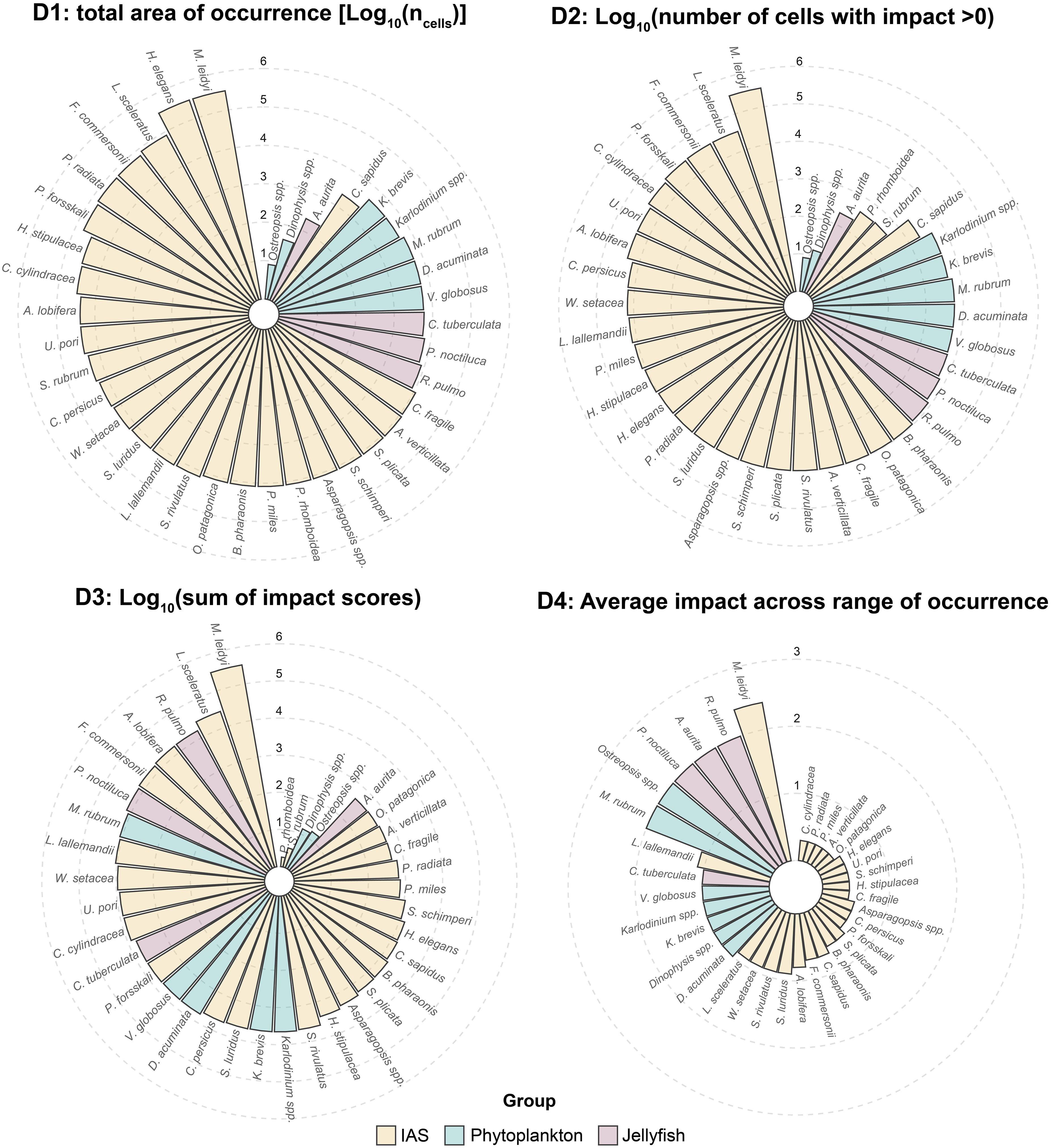
Figure 4. Ranking of the species impacts in the Aegean Sea according to the indicators D1 (Top Left), D2 (Top Right), D3 (Bottom Left), D4 (Bottom Right). The data related to D1, D2 and D3 are log-transformed.
Four jellyfish species were identified as impactful: Aurelia aurita, Cotylorhiza tuberculata, Pelagia noctiluca, and Rhizostoma pulmo. Cumulative impact scores of jellyfish blooms per cell ranged from 0 to 5 (Figure 3d) with an average of 0.11 per cell, contributing 7.0% of the total CIMPAL-JH impact (Figure 3a). Data from different sources showed a consistent pattern, indicating that the western Aegean is the most affected area by jellyfish blooms, as well as some islands in the Kyklades. Based on D1, D2, and D3, the species ranking was: R. pulmo, P. noctiluca, C. tuberculata and A. aurita. For D4 (average impact per cell within their distribution range), P. noctiluca, A. aurita, and R. pulmo were tied, followed by C. tuberculata (Figure 4).
Seven phytoplankton taxa were identified as impactful in the Aegean Sea: Dinophysis sp., Dinophysis acuminata, Karenia brevis, Karlodinium spp., Mesodinium rubrum, Ostreopsis spp., and Vicicitus globosus. HABs exhibited cumulative impact scores per cell ranging from 0 to 6 (Figure 3c), with an average of 0.10, contributing 6.1% to the total CIMPAL-JH score (Figure 3a). These impacts were mainly concentrated in the Thermaikos and Saronikos gulfs. Based on both D1 and D2, D. acuminata and V. globosus were the top-ranked species. According to D3, M. rubrum had the highest impact, followed by D. acuminata and V. globosus. In terms of D4, M. rubrum and Ostreopsis spp. had the highest average impacts within their distribution ranges (Figure 4).
4 Discussion
Assessing cumulative impacts is a fundamental principle of ecosystem-based management (Papadopoulou et al., 2025). In the context of biological invasions, such assessments allow for a comprehensive evaluation of the combined effects of multiple invasive species on marine ecosystems, ensuring that management strategies effectively account for complex ecological interactions and mitigate associated risks (Carneiro et al., 2025; Mačic et al., 2018; McGeoch et al., 2016). While earlier assessments have primarily focused on IAS, other native invaders, such as those causing HABs and jellyfish blooms, can also cause significant disruptions to marine biodiversity (Sagarminaga et al., 2024, 2023). By expanding the CIMPAL framework to include other environmental drivers and their interspecific interactions, CIMPAL-JH provides a more comprehensive tool for assessing and mapping invasion-related impacts across marine habitats (Borja et al., 2024). It offers an integrated approach to identify high-risk areas, rank species based on their ecological impact, and support evidence-based management strategies.
The Aegean Sea faces a range of environmental challenges driven by population growth, wastewater discharge, agricultural runoff, aquaculture, industrialization, tourism, maritime transport, and climate change (Anagnostou et al., 2024). These stressors have contributed to increased occurrences of IAS, HABs, and jellyfish blooms, leading to the cumulative impacts documented in this study.
According to the CIMPAL-JH index, coastal habitats consistently exhibited higher cumulative impact scores than offshore waters, echoing the findings of (Tsirintanis et al., 2023). This trend reflects the thermophilic affinity of most Mediterranean IAS, thriving in warmer, shallower waters (Katsanevakis et al., 2014a), though it may also be influenced by research effort bias, as most ecological surveys are conducted in nearshore zones (Tsirintanis et al., 2023).
The Thermaikos Gulf stood out as the most affected pelagic area, with significant impacts from jellyfish blooms and HABs. This can be linked to multiple anthropogenic stressors, including sewage discharge, industrial waste, agricultural runoff, shipping activities, and harbor operations. Furthermore, five major rivers transport nutrients, heavy metals, and organic compounds into the gulf, exacerbating these issues (Androulidakis et al., 2024). Both Thermaikos and Saronikos Gulfs are hotspots for alien invertebrates belonging to Mollusca, Arthropoda, and Annelida, though they do not exhibit the same degree of invasion by Chordata, particularly fish (Katsanevakis et al., 2013). Notably, both Thermaikos and Saronikos are among the Greek coastal regions most severely affected by human-induced eutrophication (Pagou, 2005), which is consistent with our findings of elevated HAB impacts.
Differences between this study and Tsirintanis et al. (2023) are largely due to the inclusion of interspecific interactions, which reduced the cumulative impact of Caulerpa cylindracea. In Tsirintanis et al. (2023), C. cylindracea ranked first in cumulative impact sum across all cells (D3) but, in this study, it has dropped to eighth place. Similarly, in terms of the average impact within a species’ range (D4), C. cylindracea fell from first in Tsirintanis et al. (2023) to 20th in the current assessment. This lower ranking more accurately reflects its current status, as recent observations indicate a substantial decline in C. cylindracea populations in the eastern Mediterranean, with the species even disappearing from several areas due to predation by Siganus spp (Dimitriadis et al., 2021).
Among jellyfish, Rhizostoma pulmo emerged as the most impactful species across D1, D2, and D3, partly due to its role as a vector for bacterial pathogens, which may spread diseases to marine organisms and humans (Stabili et al., 2020). Additionally, R. pulmo blooms can threaten planktonic populations and disrupt fish larvae recruitment by reducing their zooplanktonic prey (Gueroun et al., 2022). Pelagia noctiluca, ranked second, exerts strong predatory pressure on zooplankton, including eggs and larvae of both benthic and pelagic species (Mariottini et al., 2008). Cotylorhiza tuberculata, ranked third in D1–D3 but lower in D4, indicating widespread but relatively lower localized impacts. However, its high abundance in enclosed coastal zones can disrupt resource availability and alter competition dynamics (Astorga et al., 2012). Aurelia aurita, while ranked lowest in D1, D2, and D3, had a high average impact within its range (D4), indicating it can still exert significant localized ecological pressures, particularly through zooplankton predation and microbial community shifts (Zoccarato et al., 2016).
Although HABs had the lowest cumulative impact overall, they remain an ecological concern, particularly in the western Aegean. Like other Dinophysis species, D. acuminata produces okadaic acid (OA) and dinophysistoxins (DTXs), which are causative agents of diarrhetic shellfish poisoning (DSP) in humans. However, the impacts of HABs extend beyond human health, as marine organisms are also vulnerable to their toxins. Research on DSP toxin exposure, accumulation, and effects on marine life remains limited, though available studies indicate potential behavioral and morphological changes, as well as mortality in fish (Corriere et al., 2021). Vicicitus globosus is known for its cytotoxic effects, which can be harmful to a wide range of marine organisms (Chang, 2015). Mesodinium rubrum, which had the highest cumulative impact score (D3) and (together with Ostreopsis spp.) the greatest average impact within its range (D4), is particularly notable for its ability to turn water into a distinct red color. Its blooms can disrupt local marine ecosystems by outcompeting other phytoplankton species for resources (Zhang et al., 2018). Karenia cf. brevis is well known for causing red tides and producing brevetoxins, which are highly toxic to marine life and can cause Neurotoxic Shellfish Poisoning (NSP) in humans (Stumpf et al., 2022). Ostreopsis spp., although more localized, are also of concern, as they produce palytoxins and ovatoxins, which are harmful to marine organisms and can become aerosolized, leading to respiratory issues, skin irritation, and other health effects in humans (Pavaux et al., 2020).
The impacts of HABs are strongly influenced by bloom density, as even the most toxic species must reach a threshold cell concentration to cause significant ecological harm. Although many harmful species are widespread, their actual impact depends on both bloom intensity and duration (Zingone and Oksfeldt Enevoldsen, 2000). Notably, some taxa, such as Dinophysis spp., can still induce toxic contamination at very low concentrations (Zingone and Oksfeldt Enevoldsen, 2000). To improve future assessments, it is essential to determine species-specific cell concentration thresholds and consistently report bloom intensity and duration. A similar approach applies to jellyfish blooms, whose severity also depends on concentration and persistence - factors not yet reflected in the Ai term in the Aegean case study due to data limitations. Incorporating these parameters would improve impact estimations by more accurately quantifying the severity of these phenomena and moving beyond the constraints of simple presence-absence data, thereby avoiding under- or overestimation of impacts. This would also allow for the detection of subtle trends or changes in bloom dynamics and their associated impacts over time.
Furthermore, the current implementation of CIMPAL-JH was static, assuming that the impacts of a species remain constant in space and time. However, impacts can be dynamic, influenced by environmental changes, fluctuations in resource availability, or adaptation of the native biodiversity, with ‘boom-bust’ dynamics reported for many species, challenging the assumption of persistent impacts (Santamaría et al., 2022).
Future refinements should therefore aim to integrate species-specific concentration thresholds and spatiotemporal dynamics into the Ai and wi,j terms to enhance the assessment of cumulative impacts. Moreover, while the impacts of individual harmful species are relatively well-documented, the interactions among multiple species and their combined effects remain complex and poorly understood. HAB events commonly involve multiple co-occurring species. Further research is needed to fully assess the cumulative impacts of co-occurring species and incorporate this understanding into the CIMPAL-JH index.
This study expanded the CIMPAL framework, originally designed to assess IAS impacts, by incorporating jellyfish blooms, HABs, and interspecific interactions. Despite limitations in data availability, the application of CIMPAL-JH in the Aegean Sea has offered valuable insights into the spatial distribution of cumulative impacts of INAS, highlighting key hotspots of environmental impact. Beyond the Aegean, CIMPAL-JH is a versatile and scalable tool for assessing the impacts of both native and invasive alien species across diverse regions. By integrating multiple environmental drivers and accounting for species interactions, the index can be adapted to different aquatic ecosystems, helping to identify high-risk areas, prioritize management efforts, and support conservation strategies.
CIMPAL-JH may support existing monitoring and management efforts. For example, the European Union’s Marine Strategy Framework Directive (MSFD) requires Member States to monitor and manage biological disturbances as part of efforts to achieve Good Environmental Status (GES). Although to implement MSFD, Member States need to monitor and assess the occurrence and impacts of IAS, HABs, and jellyfish blooms, MSFD does not propose specific methodologies for evaluating their cumulative impacts on marine biodiversity. CIMPAL-JH addresses this gap by combining INAS and habitats distributions with habitat vulnerability to produce spatially explicit cumulative impact assessments. In this way, the framework contributes to the implementation of the MSFD by offering a structured approach to evaluating IAS and bloom-related pressures and supporting progress toward GES. Furthermore, the spatial outputs of the CIMPAL-JH framework can directly inform the actions of regional environmental managers by identifying high-risk zones where IAS and bloom events are likely to have the greatest impact. This information can guide monitoring priorities, optimize resource allocation, and inform the design of localized response strategies.
Future applications could further refine the methodology by incorporating additional factors such as bloom intensity and species-specific thresholds or consider further environmental challenges. This would enhance the utility of the index as a decision-support tool for mitigating cumulative impacts on biodiversity and ecosystem services in the face of growing ecological challenges.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Author contributions
MC: Conceptualization, Investigation, Methodology, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. YS: Formal Analysis, Investigation, Writing – review & editing. CK: Formal Analysis, Investigation, Writing – review & editing. KT: Formal Analysis, Investigation, Writing – review & editing. GT: Formal Analysis, Investigation, Writing – review & editing. EA: Writing – review & editing. SK: Conceptualization, Formal Analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This manuscript is a result of GES4SEAS (Achieving Good Environmental Status for maintaining ecosystem services, by assessing integrated impacts of cumulative pressures) project, funded by the European Union under the Horizon Europe program (grant agreement No. 101059877) (www.ges4seas.eu). MC was also supported by the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4 - Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union – NextGenerationEU; Award Number: Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP D33C22000960007, Project title “National Biodiversity Future Center - NBFC”. SK was also supported by the European Union’s Horizon Europe HORIZON-CL6-2024-BIODIV-01 project ‘GuardIAS - Guarding European Waters from IAS’, under grant agreement no. 101181413 (Katsanevakis et al., 2024).
Acknowledgments
This study represents partial fulfillment of the requirements for the doctoral thesis of M. Chiappi, within the international Program “Innovative Technologies and Sustainable Use of Mediterranean Sea Fishery and Biological Resources” (FishMed-PhD; www.FishMed-PhD.org) at the University of Bologna, Italy.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1631423/full#supplementary-material
References
Anagnostou C. L., Kostianoy A. G., Mariolakos I. D., Panayotidis P., Soilemezidou M., and Tsaltas G. (2024). “The aegean sea: environment, the biodiversity of the natural system,” in The handbook of environmental chemistry, vol. 129. (Springer). doi: 10.1007/978-3-031-59415-1
Anderson D. M. (2009). Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean Coast. Manage. 52, 342–347. doi: 10.1016/j.ocecoaman.2009.04.006
Anderson D. M. (2014). “HABs in a changing world: a perspective on harmful algal blooms, their impacts, and research and management in a dynamic era of climactic and environmental change,” in Harmful algae 2012: proceedings of the 15th international conference on harmful algae: october 29-november 2, 2012(CECO, Changwon, Gyeongnam, Korea: Society for the Study of Harmful Algae (ISSHA)).
Androulidakis Y., Makris C., Kombiadou K., Krestenitis Y., Stefanidou N., Antoniadou C., et al. (2024). Oceanographic research in the thermaikos gulf: A review over five decades. J. Mar. Sci. Engeneering 12, 795. doi: 10.3390/jmse12050795
Astorga D., Ruiz J., and Prieto L. (2012). Ecological aspects of early life stages of Cotylorhiza tuberculata (Scyphozoa: Rhizostomae) affecting its pelagic population success. Hydrobiologia 690, 141–155. doi: 10.1007/s10750-012-1036-x
Azzurro E., Bahri T., Valbo-Jørgensen J., Ma X., Strafella P., and Vasconcellos M. (Eds.) (2024). “Fisheries responses to invasive species in a changing climate – Lessons learned from case studies,” in FAO fisheriesTechnical paper, no. 704 (FAO, Rome). doi: 10.4060/cd1400en
Azzurro E., Fanelli E., Mostarda E., Catra M., and Andaloro F. (2007). Resource partitioning among early colonizing Siganus luridus and native herbivorous fish in the Mediterranean: An integrated study based on gut-content analysis and stable isotope signatures. J. Mar. Biol. Assoc. United Kingdom 87, 991–998. doi: 10.1017/S0025315407056342
Báez J. C., Pennino M. G., Albo-Puigserver M., Coll M., Giraldez A., and Bellido J. M. (2022). Effects of environmental conditions and jellyfish blooms on small pelagic fish and fisheries from the Western Mediterranean Sea. Estuarine Coast. Shelf Sci. 264. doi: 10.1016/j.ecss.2021.107699
Blackburn T. M., Essl F., Evans T., Hulme P. E., Jeschke J. M., Kühn I., et al. (2014). A unified classification of alien species based on the magnitude of their environmental impacts. PloS Biol. 12 (5), 1–11. doi: 10.1371/journal.pbio.1001850
Boero F. (2013). “Review of jellyfish blooms in the Mediterranean and Black Sea,” in Studies and reviews. General fisheries commission for the mediterranean. No. 92, vol. 53. (FAO, Rome).
Boero F., Bouillon J., Gravili C., Miglietta M. P., Parsons T., and Piraino S. (2008). Gelatinous plankton: Irregularities rule the world (sometimes). Mar. Ecol. Prog. Ser. 356, 299–310. doi: 10.3354/meps07368
Boero F., Brotz L., Gibbons M. J., Piraino S., and Zampardi S. (2016). Ocean Warming 3.10 Impacts and effects of ocean warming on jellyfish. Explaining ocean warming: Causes scale effects consequences, 213–237.
Borja A., Elliott M., Teixeira H., Stelzenmüller V., Katsanevakis S., Coll M., et al. (2024). Addressing the cumulative impacts of multiple human pressures in marine systems, for the sustainable use of the seas. Front. Ocean Sustainability 1. doi: 10.3389/focsu.2023.1308125
Burkholder J. M., Tomasko D. A., and Touchette B. W. (2007). Seagrasses and eutrophication. J. Exp. Mar. Biol. Ecol. 350, 46–72. doi: 10.1016/j.jembe.2007.06.024
Canepa A., Fuentes V., Sabatés A., Piraino S., Boero F., and Gili J. M. (2014). Pelagia noctiluca in the mediterranean sea. Jellyfish Blooms, 237–266. doi: 10.1007/978-94-007-7015-7_11
Carneiro L., Miiller N., Prestes J. G., Vitule J., and Cuthbert R. N. (2025). Impacts and mechanisms of biological invasions in global protected areas. Biol. Invasions 27. doi: 10.1007/s10530-024-03498-w
Castro-Díez P., Vaz A. S., Silva J. S., van Loo M., Alonso Á., Aponte C., et al. (2019). Global effects of non-native tree species on multiple ecosystem services. Biol. Rev. 94, 1477–1501. doi: 10.1111/brv.12511
CBD (2008). “COP 9 decisions. VI/23,” in Alien species that threaten ecosystems, habitats or species, Meeting of the Conference of the Parties to the Convention on Biological Diversity (CBD)(Germany: Secretariat of the Convention on Biological Diversity, United Nations Environment Programme (UNEP)).
Cegolon L., Heymann W. C., Lange J. H., and Mastrangelo G. (2013). Jellyfish stings and their management: A review. Mar. Drugs 11, 523–550. doi: 10.3390/md11020523
Chang F. H. (2015). Cytotoxic effects of Vicicitus globosus (Class Dictyochophyceae) and Chattonella marina (class Raphidophyceae) on rotifers and other microalgae. J. Mar. Sci. Eng. 3, 401–411. doi: 10.3390/jmse3020401
CIESM Jelly Watch program. Available online at: https://www.ciesm.org/gis/JW/build/JellyBlooms.php (Accessed July 23, 2024).
Corriere M., Soliño L., and Costa P. R. (2021). Effects of the marine biotoxins okadaic acid and dinophysistoxins on fish. J. Mar. Sci. Eng. 9. doi: 10.3390/jmse9030293
Dimitriadis C., Fournari–konstantinidou I., Sourbès L., Koutsoubas D., and Katsanevakis S. (2021). Long term interactions of native and invasive species in a marine protected area suggest complex cascading effects challenging conservation outcomes. Diversity (Basel) 13. doi: 10.3390/d13020071
Dinasquet J., Titelman J., Møller L. F., Setälä O., Granhag L., Andersen T., et al. (2012). Cascading effects of the ctenophore Mnemiopsis leidyi on the planktonic food web in a nutrient-limited estuarine system. Mar. Ecol. Prog. Ser. 460, 49–61. doi: 10.3354/meps09770
Doherty T. S., Glen A. S., Nimmo D. G., Ritchie E. G., and Dickman C. R. (2016). Invasive predators and global biodiversity loss. Proc. Natl. Acad. Sci. 113, 11261–11265. doi: 10.1073/pnas.1602480113
Doyle T. K., De Haas H., Cotton D., Dorschel B., Cummins V., Houghton J. D. R., et al. (2008). Widespread occurrence of the jellyfish Pelagia noctiluca in Irish coastal and shelf waters. J. Plankton Res. 30, 963–968. doi: 10.1093/plankt/fbn052
Economou V., Papadopoulou C., Brett M., Kansouzidou A., Charalabopoulos K., Filioussis G., et al. (2007). Diarrheic shellfish poisoning due to toxic mussel consumption: The first recorded outbreak in Greece. Food additives contaminants 24, 297–305. doi: 10.1080/02652030601053139
García-Gómez J. C., Sempere-Valverde J., González A. R., Martínez-Chacón M., Olaya-Ponzone L., Sánchez-Moyano E., et al. (2020). From exotic to invasive in record time: The extreme impact of Rugulopteryx okamurae (Dictyotales, Ochrophyta) in the strait of Gibraltar. Sci. Total Environ. 704. doi: 10.1016/j.scitotenv.2019.135408
Ghermandi A., Galil B., Gowdy J., and Nunes P. A. L. D. (2015). Jellyfish outbreak impacts on recreation in the Mediterranean Sea: Welfare estimates from a socioeconomic pilot survey in Israel. Ecosystem Serv. 11, 140–147. doi: 10.1016/j.ecoser.2014.12.004
Graham W. M., Pagès F., and Hamner W. M. (2001). A physical context for gelatinous zooplankton aggregations: A review. Hydrobiologia. 451, 199–212. doi: 10.1023/A:1011876004427
Gueroun S. K. M., Piraino S., Kéfi-Daly Yahia O., Daly Yahia M. N., and Koski M. (2022). Jellyfish diversity, trends and patterns in Southwestern Mediterranean Sea: a citizen science and field monitoring alliance. J. Plankton Res. 44, 819–837. doi: 10.1093/plankt/fbac057
Hallegraeff G., Enevoldsen H., and Zingone A. (2021). Global harmful algal bloom status reporting. Harmful Algae 102. doi: 10.1016/j.hal.2021.101992
Helmholz H., Johnston B. D., Ruhnau C., and Prange A. (2010). Gill cell toxicity of northern boreal scyphomedusae Cyanea capillata and Aurelia aurita measured by an in vitro cell assay. Hydrobiologia 645, 223–234. doi: 10.1007/s10750-010-0216-9
Howard B. R., Francis F. T., Côté I. M., and Therriault T. W. (2019). Habitat alteration by invasive European green crab (Carcinus maenas) causes eelgrass loss in British Columbia, Canada. Biol. Invasions 21, 3607–3618. doi: 10.1007/s10530-019-02072-z
Isinibilir M., Yüksel E., and Güreşen O. (2021). The first record of geryonia proboscidalis (Forskål 1775), (Cnidaria: hydrozoa) on the coasts of gökçeada, the aegean sea, Turkey. Aquat. Sci. Eng. 36, 216–218. doi: 10.26650/ASE2021962514
Katsanevakis S., Coll M., Piroddi C., Steenbeek J., Lasram F. B. R., Zenetos A., et al. (2014a). Invading the Mediterranean Sea: Biodiversity patterns shaped by human activities. Front. Mar. Sci. 1. doi: 10.3389/fmars.2014.00032
Katsanevakis S., Olenin S., Puntila-Dodd R., Rilov G., Stæhr P. A. U., Teixeira H., et al. (2023). Marine invasive alien species in Europe: 9 years after the IAS Regulation. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1271755
Katsanevakis S., Tempera F., and Teixeira H. (2016). Mapping the impact of alien species on marine ecosystems: The Mediterranean Sea case study. Diversity Distributions 22, 694–707. doi: 10.1111/ddi.12429
Katsanevakis S., Wallentinus I., Zenetos A., Leppäkoski E., Çinar M. E., Oztürk B., et al. (2014b). Impacts of invasive alien marine species on ecosystem services and biodiversity: A pan-European review. Aquat. Invasions 9, 391–423. doi: 10.3391/ai.2014.9.4.01
Katsanevakis S., Zaiko A., Olenin S., Costello M. J., Gallardo B., Tricarico E., et al. (2024). GuardIAS – guarding european waters from invasive alien species. Manage. Biol. Invasions 15, 701–730. doi: 10.3391/mbi.2024.15.4.14
Katsanevakis S., Zenetos A., Poursanidis D., Nunes A. L., Deriu I., Bogucarskis K., et al. (2013). ELNAIS meets EASIN: Distribution of marine alien species in Greece using EASIN mapping services and ELNAIS spatial data. Mediterr. Mar. Sci. 14, 95–98. doi: 10.12681/mms.329
Landsberg J. H. (2002). The effects of harmful algal blooms on aquatic organisms. Rev. Fisheries Sci. 10, 113–390. doi: 10.1080/20026491051695
Levin P. S., Fogarty M. J., Murawski S. A., and Fluharty D. (2009). Integrated ecosystem assessments: Developing the scientific basis for ecosystem-based management of the ocean. PloS Biol. 7 (1), 23–28. doi: 10.1371/journal.pbio.1000014
Mačic V., Albano P. G., Almpanidou V., Claudet J., Corrales X., Essl F., et al. (2018). Biological invasions in conservation planning: A global systematic review. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00178
Magliozzi C., Tsiamis K., Vigiak O., Deriu I., Gervasini E., and Cardoso A. C. (2020). Assessing invasive alien species in European catchments: Distribution and impacts. Sci. Total Environ. 732. doi: 10.1016/j.scitotenv.2020.138677
Mariottini G. L., Giacco E., and Pane L. (2008). The mauve stinger Pelagia noctiluca (Forsskål 1775). Distribution ecology toxicity Epidemiol. stings. A review. Mar. Drugs 6, 496–513. doi: 10.3390/md20080025
McGeoch M. A., Genovesi P., Bellingham P. J., Costello M. J., McGrannachan C., and Sheppard A. (2016). Prioritizing species, pathways, and sites to achieve conservation targets for biological invasion. Biol. Invasions 18, 299–314. doi: 10.1007/s10530-015-1013-1
Özgür E. and Öztürk B. (2008). A population of the alien jellyfish, Cassiopea andromeda (Forsskål 1775) [Cnidaria: scyphozoa: rhizostomea] in the ölüdeniz lagoon, Turkey. Aquat. Invasions 3, 423–428. doi: 10.3391/ai.2008.3.4.8
Papadopoulou N., Smith C. J., Franco A., Elliott M., Borja A., Andersen J. H., et al. (2025). ‘Horses for courses’ – an interrogation of tools for marine ecosystem-based management. Front. Mar. Sci. 12. doi: 10.3389/fmars.2025.1426971
Pavaux A. S., Berdalet E., and Lemée R. (2020). Chemical ecology of the benthic dinoflagellate genus ostreopsis: review of progress and future directions. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00498
Polce C., Cardoso A. C., Deriu I., Gervasini E., Tsiamis K., Vigiak O., et al. (2023). Invasive alien species of policy concerns show widespread patterns of invasion and potential pressure across European ecosystems. Sci. Rep. 13. doi: 10.1038/s41598-023-32993-8
Purcell J. E. (2005). Climate effects on formation of jellyfish and ctenophore blooms: A review. J. Mar. Biol. Assoc. United Kingdom 85, 461–476. doi: 10.1017/S0025315405011409
Qu C. F., Song J. M., and Li N. (2014). Causes of jellyfish blooms and their influence on marine environment. Chin. J. Appl. Ecol. 25 (12), 3701–3709.
Quiñones J., Monroy A., Acha E. M., and Mianzan H. (2013). Jellyfish bycatch diminishes profit in an anchovy fishery off Peru. Fisheries Res. 139, 47–50. doi: 10.1016/j.fishres.2012.04.014
Ragkousis M., Sini M., Koukourouvli N., Zenetos A., and Katsanevakis S. (2023). Invading the greek seas: spatiotemporal patterns of marine impactful alien and cryptogenic species. Diversity (Basel) 15. doi: 10.3390/d15030353
Richardson A. J., Bakun A., Hays G. C., and Gibbons M. J. (2009). The jellyfish joyride: causes, consequences and management responses to a more gelatinous future. Trends Ecol. Evol. 24, 312–322. doi: 10.1016/j.tree.2009.01.010
Roy H. E., Pauchard A., Stoett P., Renard Truong T., Bacher S., Galil B. S., et al. (2023). Summary for policymakers of the thematic assessment report on invasive alien species and their control of the intergovernmental science-policy platform on biodiversity and ecosystem services, environmental indicators. IPBES Invasive alien species Assess., 1–56.
Sagarminaga Y., Garcés E., Francé J., Stern R., Revilla M., Magaletti E., et al. (2023). New tools and recommendations for a better management of harmful algal blooms under the European Marine Strategy Framework Directive. Front. Ocean Sustainability 1. doi: 10.3389/focsu.2023.1298800
Sagarminaga Y., Piraino S., Lynam C. P., Leoni V., Nikolaou A., Jaspers C., et al. (2024). Management of jellyfish outbreaks to achieve good environmental status. Front. Ocean Sustainability 2. doi: 10.3389/focsu.2024.1449190
Santamaria J., Golo R., Verdura J., Tomas F., Ballesteros E., Alcoverro T., et al. (2022). Learning takes time: Biotic resistance by native herbivores increases through the invasion process. Ecol. Lett. 25, 2525–2539. doi: 10.1111/ele.v25.11
Simberloff D. (2011). Native invaders, in: Encyclopedia of Biological Invasions (Berkeley and Los Angeles, CA: University of California Press), 472–475. doi: 10.1525/9780520948433-106
Sini M., Katsanevakis S., Koukourouvli N., Gerovasileiou V., Dailianis T., Buhl-Mortensen L., et al. (2017). Assembling ecological pieces to reconstruct the conservation puzzle of the Aegean Sea. Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00347
Stabili L., Rizzo L., Basso L., Marzano M., Fosso B., Pesole G., et al. (2020). The microbial community associated with Rhizostoma pulmo: Ecological significance and potential consequences for marine organisms and human health. Mar. Drugs 18 (9), 1–24. doi: 10.3390/MD18090437
Stumpf R. P., Li Y., Kirkpatrick B., Wayne Litaker R., Hubbard K. A., Currier R. D., et al. (2022). Quantifying Karenia brevis bloom severity and respiratory irritation impact along the shoreline of Southwest Florida. PloS One 17. doi: 10.1371/journal.pone.0260755
Tsikoti C. and Genitsaris S. (2021). Review of harmful algal blooms in the coastal Mediterranean Sea, with a focus on Greek waters. Diversity (Basel) 13. doi: 10.3390/d13080396
Tsirintanis K., Azzurro E., Crocetta F., Dimiza M., Froglia C., Gerovasileiou V., et al. (2022). Bioinvasion impacts on biodiversity, ecosystem services, and human health in the Mediterranean Sea. Aquat. Invasions 17 (3), 308–352. doi: 10.3391/ai.2022.17.3.01
Tsirintanis K., Sini M., Ragkousis M., Zenetos A., and Katsanevakis S. (2023). Cumulative negative impacts of invasive alien species on marine ecosystems of the aegean sea. Biol. (Basel) 12. doi: 10.3390/biology12070933
Valéry L., Fritz H., Lefeuvre J. C., and Simberloff D. (2009). Invasive species can also be native. Trends Ecol. Evol. 24. doi: 10.1016/j.tree.2009.07.003
Volery L., Blackburn T. M., Bertolino S., Evans T., Genovesi P., Kumschick S., et al. (2020). Improving the Environmental Impact Classification for Alien Taxa (EICAT): a summary of revisions to the framework and guidelines. NeoBiota 62, 547–567. doi: 10.3897/neobiota.62.52723
West J. J., Järnberg L., Berdalet E., and Cusack C. (2021). Understanding and managing harmful algal bloom risks in a changing climate: lessons from the european coCliME project. Front. Climate 3. doi: 10.3389/fclim.2021.636723
Zhang Y., Song X., Harrison P. J., Liu S., Yu Z., Kan J., et al. (2018). Regeneration and utilization of nutrients during collapse of a Mesodinium rubrum red tide and its influence on phytoplankton species composition. Sci. China Earth Sci. 61, 1384–1396. doi: 10.1007/s11430-017-9233-x
Zingone A. and Oksfeldt Enevoldsen H. (2000). The diversity of harmful algal blooms: A challenge for science and management. Ocean Coast. Manage. 43, 725–748. doi: 10.1016/S0964-5691(00)00056-9
Keywords: alien species, HABs, jellyfish blooms, impacts, CIMPAL, impact mapping, management, Mediterranean Sea
Citation: Chiappi M, Stranga Y, Kalloniati C, Tsirintanis K, Tsirtsis G, Azzurro E and Katsanevakis S (2025) CIMPAL expanded: unraveling the cumulative impacts of invasive alien species, jellyfish blooms, and harmful algal blooms. Front. Mar. Sci. 12:1631423. doi: 10.3389/fmars.2025.1631423
Received: 19 May 2025; Accepted: 23 June 2025;
Published: 11 July 2025.
Edited by:
Francesco Tiralongo, University of Catania, ItalyReviewed by:
Vasco Manuel Nobre de Carvalho da Silva Vieira, University of Lisbon, PortugalChiara Magliozzi, Joint Research Centre (Italy), Italy
Copyright © 2025 Chiappi, Stranga, Kalloniati, Tsirintanis, Tsirtsis, Azzurro and Katsanevakis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marina Chiappi, bWFyaW5hY2hpYXBwaUBnbWFpbC5jb20=
 Marina Chiappi
Marina Chiappi Yolanda Stranga
Yolanda Stranga Chrysanthi Kalloniati4
Chrysanthi Kalloniati4 Konstantinos Tsirintanis
Konstantinos Tsirintanis George Tsirtsis
George Tsirtsis Stelios Katsanevakis
Stelios Katsanevakis