Abstract
The release of cesium (Cs) isotopes from treated nuclear-contaminated water at Japan’s Fukushima Daiichi Nuclear Power Plant (FDNPP) has raised global concern due to their potential long-term environmental impacts. Some of these isotopes have a physical half-life of 30.17 years, posing a potential threat to marine environments and marine life. This study used the stable isotope 133Cs to simulate exposure and assess the ecological risks associated with Cs isotopes in marine environments. The black porgy (Acanthopagrus schlegelii) was selected as the model organism and was exposed to various concentrations of 133Cs (0.02, 0.2, 2, and 20 mg/L). Although 133Cs exhibited low bioaccumulation in black porgy, it still showed potential for biomagnification. The fish demonstrated a strong stress response and some antioxidant adaptation at 3 days, but significant cellular and tissue damage occurred after 14 days of exposure. Analysis of the Integrated Biomarker Response version 2 (IBRv2) further revealed that the black porgy was more sensitive to Cs at 3 days, with toxic effects intensifying over time. This study provides a scientific basis and experimental reference for assessing the ecological risks of Cs isotopes in marine ecosystems.
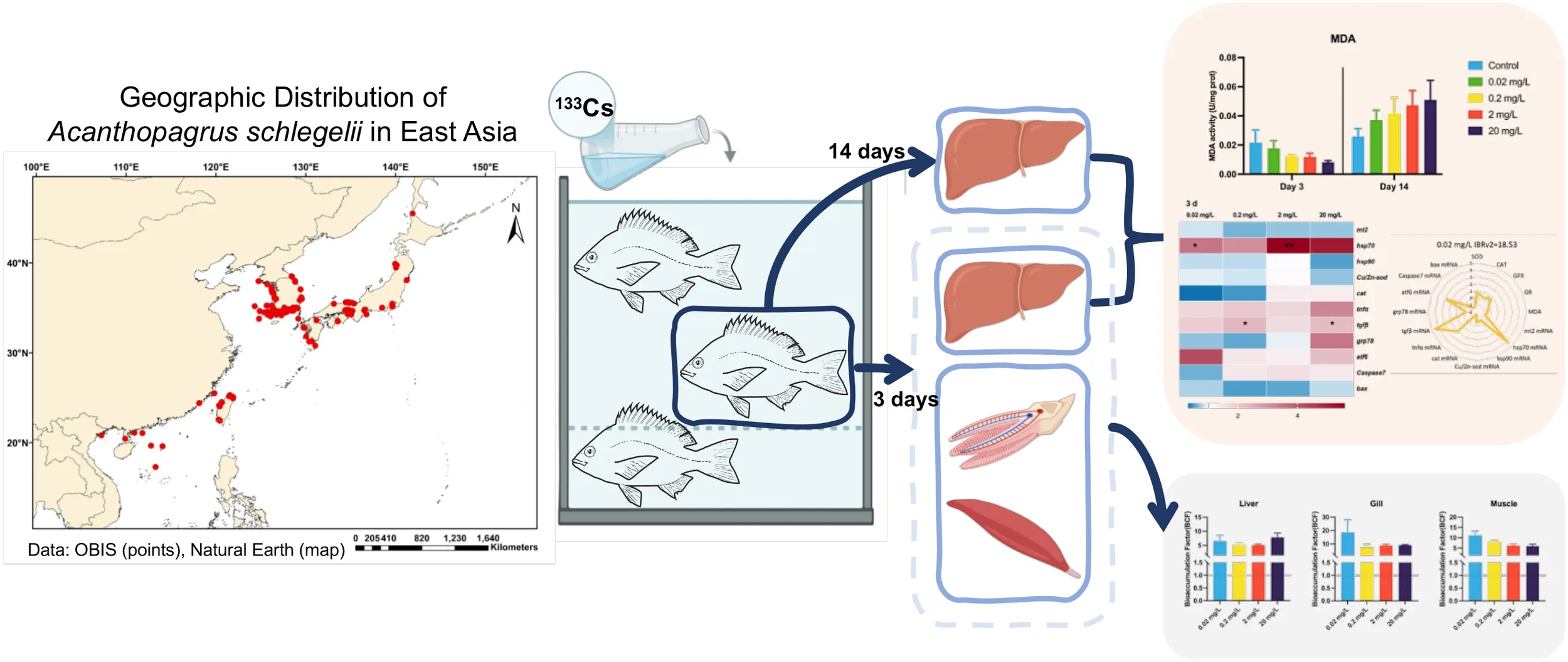
1 Introduction
On 24 August 2023, Japan began discharging treated nuclear-contaminated water from the Fukushima Daiichi Nuclear Power Plant (FDNPP) into the Pacific Ocean, drawing global concern. This release reintroduces radionuclides into the marine environment, reminiscent of the 2011 FDNPP incident, which contaminated shallow-water organisms along the Pacific coast of eastern Japan (Tateda et al., 2024b). Indeed, radioactive cesium (Cs) levels in pelagic fish have already exceeded the Japanese seafood safety limit of 100 Bq kg-ww-1 (Fukushima Prefecture (FP), 2023). Furthermore, between 2019 and 2022, the concentration of Cs isotopes in suspended solids from inflow water in an urban pond in Koriyama City, Fukushima Prefecture, Japan, was 0.041 m2/kg, higher than in other urban catchments (Kurosawa et al., 2025). Such findings underscore the potential long-term threat of Cs isotopes to marine ecosystems has been noted globally (Liu et al., 2025).
Cs is an alkali metal with one stable isotope (133Cs) and 32 radioactive isotopes. Among these, 137Cs and 134Cs have half-lives of radioactive decay, lasting 30.17 years and 2.06 years, respectively (Mohammad et al., 2015). Environmental monitoring has revealed the widespread distribution of Cs isotopes across aquatic systems. For example, in the Kaniv Reservoir, 133Cs in suspended particles was measured at 4.4 ± 0.2 mg/kg (Sansone et al., 2002). In coastal São Paulo, 137Cs levels in surface seawater ranged from 1.7 to 1.9 Bq m−³ (Cunha et al., 1993), while in the Arabian Gulf, 137Cs concentrations ranged between 1.04 and 1.18 Bq m−³ (Uddin et al., 2017). From 2011 to 2013, 137Cs concentration along the eastern coast of Indonesia varied between 0.12 and 0.32 Bq m−³ (Suseno and Prihatiningsih, 2014). Offshore measurements in the central Pacific Ocean in January 2012 recorded 137Cs at 1.5 ± 0.1 Bq L−³ (Kameník et al., 2013). These findings highlight the widespread presence of Cs isotopes in aquatic systems. Although 133Cs is less frequently detected, its stability and chemical similarity to radioactive isotopes such as 137Cs (Rühm et al., 1999; Tsukada and Hasegawa, 2003), make it crucial for understanding the biological effects of Cs in ecosystems. Given its stability and chemical properties, studying the biological impact of 133Cs, particularly in fish, is essential.
Apart from Cs isotopes being present in the environment, their accumulation has also been observed in aquatic organisms. Dissolved Cs is a major route for the bioconcentration of its isotopes in marine invertebrates (Thomas and Fisher, 2019), and tends to concentrate in scallop soft tissues (Metian et al., 2011). As a potassium analogue, Cs accumulates in organisms during biological growth (Lacoue-Labarthe et al., 2010), entering the food chain through potassium exchange. In marine fish, Cs isotopes are mainly acquired through feeding and drinking seawater (Fowler and Fisher, 2005). Between 2018 and 2021, increased Cs isotopes levels in zooplankton led to higher radioactivity in surface water fish (Tateda et al., 2024a). Notably, radioactive Cs, like mercury (Hg), biomagnifies at the top of the marine food chain (Xiguang et al., 2001). Additionally, 133Cs has been shown to induce immunotoxicity and impair immune function in Mytilus edulis (Xu et al., 2023). Chronic effects of 137Cs on fish include delayed reproduction, reduced fertility, increased embryo mortality, and adult sterility (Xu et al., 2024). Cs accumulation in organisms can lead to localized high doses, causing tissue-specific damage and even organ failure (Xu et al., 2023). Despite these known risks, the impact of Cs on marine ecosystems remains poorly understood.
In light of these concerns, this study aims to clarify the ecological risks of stable Cs (133Cs) in marine fish by examining its bioaccumulation and effects in black porgy (Acanthopagrus schlegelii). Black porgy, a fast-growing species resilient to environmental stressors and widely distributed along the coasts of China, Japan, and Korea (Mao et al., 2024; Sun et al., 2024), serves as our model organism. We first quantified bioconcentration factor (BCF) of 133Cs in various tissues and then measured hepatosomatic index (HSI) and condition factor (K) after 3- and 14-day exposures to assess impacts on growth and health. Next, biochemical responses were examined by assessing antioxidant enzyme activities and corresponding gene transcription levels. Finally, these physiological and molecular endpoints were integrated using the integrated biomarker response version 2 (IBRv2) to comprehensively gauge 133Cs-induced disruption. Our findings provide a scientific basis and experimental reference for assessing the ecological risks of Cs isotopes in coastal ecosystems.
2 Materials and methods
2.1 Fish maintenance
Three hundred healthy juvenile black porgy (body weight: 14.12 ± 7.67 g, body length: 9.5 ± 1.5 cm) were purchased from the Zhangzhou Aquaculture Company, Fujian, China. Before the experiment, fish were acclimated for one week in a flow-through system with constant aeration in a 1000 L Polyvinyl chloride (PVC) tank containing sand-filtered seawater. Water conditions were maintained at 28 ± 0.5°C, salinity 15 ± 1 Practical Salinity Unit (psu), and pH 8.0 ± 0.1. The fish were fed once daily, equivalent to 1% of their body weight. The fish feed was supplied by Zhangzhou Aquaculture Company. All animal procedures were approved by the Ethics Committee of the Third Institute of Oceanography, Ministry of Natural Resources, China (No. TIO-IACUC-01-2024-03-08).
2.2 Experimental design
2.2.1 The simulative system of 133Cs exposure
The experiment was designed to assess the dose-dependent toxicity of 133Cs in juvenile fish through a controlled 14-day exposure period. The setup included a control group and four treatment groups, each in triplicate, receiving 133Cs at concentrations of 0.02, 0.20, 2.00, and 20 mg/L. These specific concentrations were chosen based on preliminary trials to represent a gradient of exposure levels, allowing assessment of potential toxicity patterns and dose-responsive behavior. Analytical-grade cesium chloride (Shanghai Macklin Biochemical Technology Company, Shanghai, China) was used to prepare the solutions. Each tank held 30 uniformly sized juvenile fish in 75 liters of artificial seawater to minimize background ion interference and ensure reproducibility. The health of all fish was confirmed before starting the experiment. To maintain consistent exposure conditions, two-thirds of the solution in each petri tank was renewed daily with freshly prepared Cs solution at the same concentration. Throughout the 14-day period, environmental parameters such as temperature, humidity, and lighting were kept stable to ensure reliable experimental outcomes.
2.2.2 Tissue sampling
The experimental sample collection was carried out on days 3 and 14 of the exposure period. During tissue sampling, fish were anesthetized using 200 mg/L MS-222 (Sigma Aldrich, St. Louis, MO, USA), and their body length and weight were recorded. the liver was then selected for analysis due to its crucial role in processing toxicants entering the organism. As the primary site of detoxification and elimination, the liver is particularly susceptible to damage from environmental chemicals. Consequently, it is one of the most valuable tissues for toxicological research, directly reflects an organism’s exposure to harmful compounds (Bo et al., 2023; Ishaq et al., 2023). After sampling, livers were immediately flash-frozen in liquid nitrogen and stored at −80°C for subsequent analysis. The HSI and CF were calculated using the following standard equations.
2.3 Concentration determination of 133Cs in water and fish tissues
At the beginning of the experiment, 50 mL of water was collected from each concentration group, with three replicates per group. Samples were transferred to polyethylene (PE) tubes at time points 0 hours, 24 hours (prior to the initial water renewal), and 48 hours (after subsequent water renewal). This approach verified concentration stability over time, ensuring that observed biological responses could be reliably correlated with actual exposure levels.
The concentration of 133Cs+ in each water sample was determined by inductively coupled plasma mass spectrometry (ICP-MS, Model 7700x, Agilent Technologies, Santa Clara, CA, USA). Cs standard solutions were prepared at 0, 0.2, 1, 5, 20, 50, 100, and 500 μg/L and measured by ICP-MS to create a calibration curve via linear regression. All calibration curves had correlation coefficients within acceptable analytical criteria. Following this, Control and experimental water samples were analyzed using the same procedure. To ensure accuracy, an internal standard solution was introduced continuously throughout analysis, and sample concentrations were corrected according to internal standard recovery rates (92.5%–108.2%). The method detection limit (MDL) for Cs was determined to be 0.04 μg/L.
Liver, muscle, and gill samples were collected after a 3-day exposure period to assess 133Cs enrichment. Three replicate samples were taken from each concentration group, with each replicate consisting of tissue from ten fish. Tissue samples were removed from cold storage, thawed at room temperature, and weighed to obtain fresh weight. They were then dried at 80 °C until constant weight and reweighed for dry weight. Next, approximately 1:10 (w/v) concentrated nitric acid was added, and samples were left to digest overnight at room temperature. This was followed by sequential heating at 60 °C for 1 h, 80 °C for 1 h, and 120 °C for 6 h, until the solution became clear. Blank digestions using nitric acid alone were prepared in parallel. After digestion, samples were diluted to 15 mL with ultrapure water, mixed thoroughly, filtered through a syringe filter, and analyzed for Cs concentration by ICP-MS. For water samples, 5% nitric acid was added immediately after collection for fixation. Prior to analysis, these water samples were diluted 100-fold with 2% nitric acid, filtered through a syringe filter, and measured for Cs concentrations using the same ICP-MS protocol.
Samples were removed from cold storage, thawed at room temperature, and weighed to obtain fresh weight. They were then dried at 80°C until they reached a constant weight, after which they were reweighed for dry weight determination. Next, approximately 1:10 (w/v) concentrated nitric acid was added, and samples were left to digest overnight at room temperature. Sequential heating was then performed at 60°C for 1 h, 80°C for 1 h, and 120°C for 6 h, until the solution became clear. Blank digestions using nitric acid alone were run in parallel. After digestion, each sample was diluted to 15 mL with ultrapure water, mixed thoroughly, filtered through a syringe filter, and analyzed for Cs concentration by ICP-MS. For water samples, 5% nitric acid was added immediately after collection to fix the samples. Before ICP-MS analysis, these water samples were diluted 100-fold with 2% nitric acid, filtered through a syringe filter, and then measured for Cs concentration using the same protocol.
To evaluate the efficiency of metal accumulation in the liver, gills, and muscle, the BCF were calculated using the standard equation.
2.4 Antioxidant enzymatic activity measurement
After sampling, antioxidant enzyme activity was measured using five replicate liver samples from each concentration group. Liver tissues were first homogenized in a pre-cooled sodium chloride solution (1:9 w/v) to prepare a 10% (w/v) tissue homogenate. Homogenization was performed in a high-throughput vertical cryogenic grinding instrument (MB-LD 48; Zhejiang Meibi Instruments Company Limited, Zhejiang, China) at 10,000 rpm for 10 minutes at 4°C. After centrifugation, the supernatant was collected carefully and used to assess antioxidant enzymatic activity. The activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), glutathione reductase (GR), total protein (TP), and malondialdehyde (MDA) were measured using commercial kits sourced from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
2.5 Real-time quantitative polymerase chain reaction analysis
Total RNA was isolated from five replicate liver samples per concentration group using TRIZOL® reagent (Invitrogen, Carlsbad, California, USA). The concentration and purity of the extracted RNA were assessed with a microspectrophotometer (K5600, Kaiao Technology, Beijing, China). For cDNA synthesis, 1 μg of total RNA was reverse-transcribed using the PrimeScripte™ RT-PCR Kit (Takara, Tokyo, Japan). Real-time quantitative polymerase chain reaction (RT-qPCR) was subsequently conducted using the SYBR Green qPCR Master Mix Kit (Promega, Madison, Wisconsin, USA), with amplification and data-analysis protocols adapted from (Bo et al., 2012).
The transcriptional levels of various genes related to metallothionein regulation, oxidative stress, immune response, inflammation, endoplasmic reticulum (ER) stress pathways, and pro-apoptotic mechanisms were analyzed. The genes examined included mt2 (metallothionein II), hsp70 (heat shock protein 70), hsp90 (heat shock protein 90), Cu/Zn-sod (copper-zinc superoxide dismutase), cat (catalase), tnfα (tumor necrosis factor-alpha), tgfβ (transforming growth factor-β), grp78 (glucose-regulated protein 78kD), atf6 (activating transcription factor 6), caspase7 (cysteinyl aspartate specific proteinase), and bax (bcl-2 associated X protein). β-actin was used as the reference gene. The RT-qPCR primer sequences were adopted from (Zhao et al., 2024), with gene-specific primer details provided in Supplementary Table S1 in Supplementary Information (SI).
2.6 IBRv2 analysis
To comprehensively assess biological responses across different biomarkers (antioxidant enzyme activities and genes expression), the IBRv2 index was employed. This method provides a visual and quantitative means of evaluating organismal stress by integrating various biomarker responses into a single comparative value. Calculations were performed following established methodologies from previous studies (Gao et al., 2024; Leite et al., 2024; Zheng et al., 2024). Displayed as a star plot, IBRv2 is independent of biomarker arrangement and is based on reference deviations between undisturbed and disturbed physiological states (Pollicelli et al., 2023). IBRv2 was calculated using the following equations.
Xi is the treatment mean and X0 is the control mean. The treatment group and the control group are represented by Zi and Z0, respectively. μ is the mean and σ is the standard deviation of Yi. A represents the biomarker deviation index. The IBRv2 value was calculated by summing the Ai value (absolute value).
2.7 Statistical analysis
The results are presented as the mean ± standard error (S.E.). Initially, the data were tested for normality and homogeneity. A one-way analysis of variance (ANOVA) was then conducted, followed by Tukey’s Honest Significant Difference (HSD) test to identify significant differences between the treatment and control groups. In cases where the data did not follow a normal distribution, a Kruskal-Wallis ANOVA was used to detect significant differences. Statistical significance was considered at *p< 0.05 and **p< 0.01. All statistical analyses were performed using IBM SPSS Statistics 29.0.1.0 (Armonk, New York, USA), while graphical representations were created using GraphPad Prism 10 (GraphPad Software, San Diego, California, USA).
3 Results
3.1 133Cs+ exposure levels in water, biometric changes, and tissue bioaccumulation in black porgy
To evaluate the internal and external exposure of black porgy to 133CsCl, both water concentrations and biological responses were monitored across a range of treatment levels. At 0 hours, the actual 133Cs+ concentrations in water were approximately 75% of the nominal values for the 0.02, 0.2, and 2 mg/L groups, and around 55% for the highest concentration group (20 mg/L) (Table 1). These levels remained relatively stable at 24 hours and showed a slight increase by 48 hours. In terms of biometric responses, a significant decrease in HSI was observed after 14 days, with mean values of 1.06 in the 0.02 mg/L group and 0.85 in the 20 mg/L group (Figure 1). In contrast, body length, weight, and CF showed no significant alterations (Figure 1).
Table 1
| Nominal concentration (mg/L) | Actual concentration (mg/L) | ||
|---|---|---|---|
| Control | 0 h | 24 h | 48 h |
| < MDL | < MDL | < MDL | |
| 0.02 | 0.033 ± 0.004 | 0.020 ± 0.002 | 0.054 ± 0.030 |
| 0.2 | 0.167 ± 0.005 | 0.155 ± 0.004 | 0.199 ± 0.034 |
| 2 | 1.478 ± 0.052 | 1.500 ± 0.014 | 1.495 ± 0.034 |
| 20 | 11.415 ± 0.279 | 11.507 ± 0.109 | 11.597 ± 0.092 |
Actual Cs+ concentration in the waters.
MDL (method detection limit), values reported as “< MDL” indicate that the concentration was below the method detection limit of 0.04 μg/L.
Figure 1
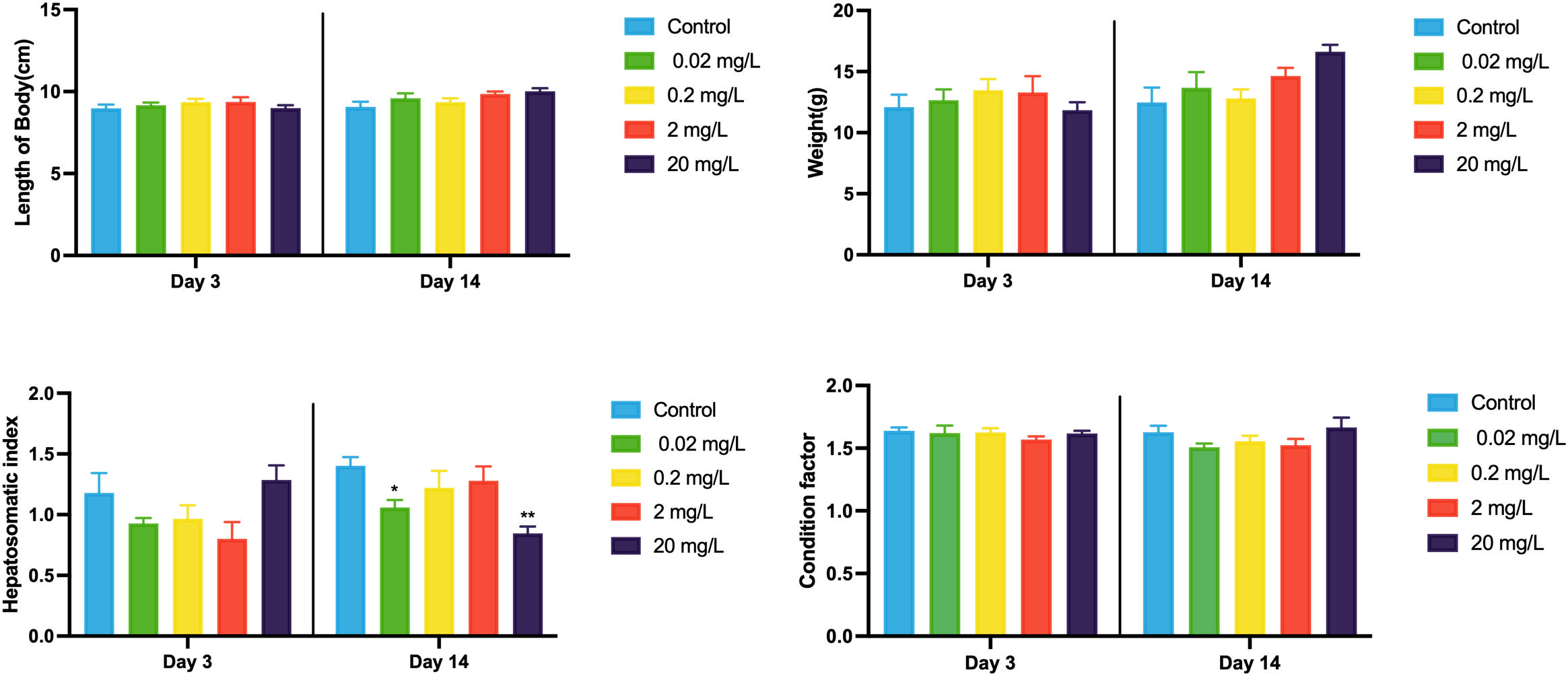
Biometric responses of black porgy following 3- and 14-day exposures to 0.02, 0.2, 2, and 20 mg/L of 133CsCl. Data are expressed as mean ± standard error (SE). Statistically significant differences between the exposure and control groups are indicated at *p< 0.05.
Regarding bioaccumulation, 133Cs+ concentrations in tissues increased in a dose-dependent manner (Table 2). At lower exposure levels (0.02, 0.2, and 2 mg/L), 133Cs+ accumulated in the order: gill > muscle > liver. However, at the highest concentration (20 mg/L), the distribution shifted, with the gill still showing the highest accumulation, followed by the liver and then muscle. BCF values for all three tissues remained above 1 across all exposure levels (Figure 2). Notably, the liver BCF decreased with rising concentrations but rose again at 20 mg/L, suggesting a non-linear uptake pattern. The gill BCF peaked at the lowest concentration and stabilized at higher doses, whereas the muscle BCF declined progressively with increasing exposure.
Table 2
| Concentration (mg/L) | Cs bioaccumulation in different tissues (mg/kg) | ||
|---|---|---|---|
| Control | Liver | Gill | Muscle |
| < MDL | < MDL | < MDL | |
| 0.02 | 0.348 ± 0.198 | 0.994 ± 0.894 | 0.599 ± 0.191 |
| 0.2 | 1.016 ± 0.258 | 1.431 ± 0.857 | 1.566 ± 0.276 |
| 2 | 7.655 ± 0.668 | 13.258 ± 2.054 | 9.328 ± 1.679 |
| 20 | 90.929 ± 30.224 | 105.250 ± 8.605 | 69.103 ± 18.776 |
Levels of Cs+ bioaccumulation in different tissues of the black porgy.
MDL (method detection limit), values reported as “< MDL” indicate that the concentration was below the method detection limit of 0.04 μg/L.
Figure 2
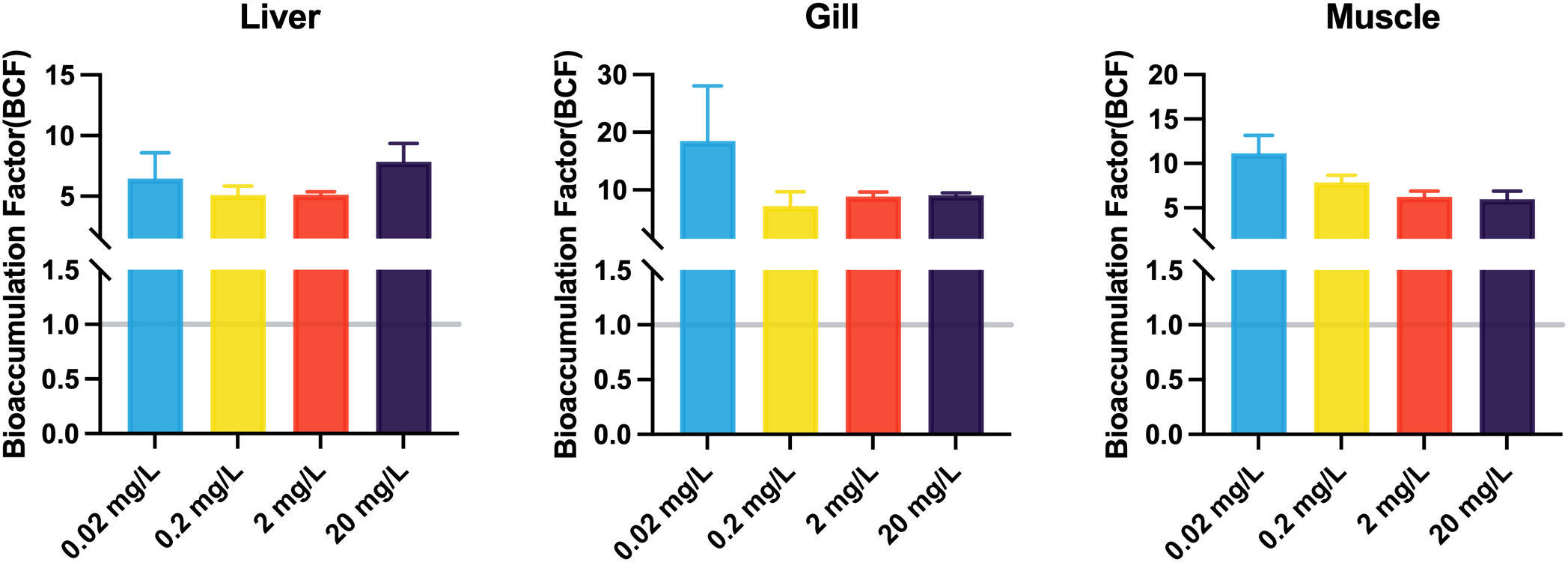
The BCF in liver, gill, and muscle of the black porgy following 3-day exposures to 0.02, 0.2, 2, and 20 mg/L of 133CsCl. Data are expressed as mean ± standard error (SE).
3.2 Antioxidant responses after exposure to 133CsCl
MDA levels showed a decreasing trend with rising concentrations of 133CsCl during the 3-day acute exposure. However, during the 14-day chronic exposure, MDA levels increased with higher doses compared to the control, though the differences were not statistically significant (Figure 3). This shift in MDA patterns over time may reflect a delayed oxidative response, prompting further examination of key antioxidant enzymes. Throughout both acute and chronic exposures, SOD activity remained stable. GR activity decreased at day 14 compared to day 3, whereas GPX activity showed a dose-dependent increase followed by a decline, peaking at 2 mg/L after 14 days. In contrast, CAT activity declined gradually with increasing 133CsCl concentrations at day 3. By 14 days, a significant reduction was observed in the treated groups compared to the control, with mean activity of 292.89 U/mg prot (Figure 4).
Figure 3
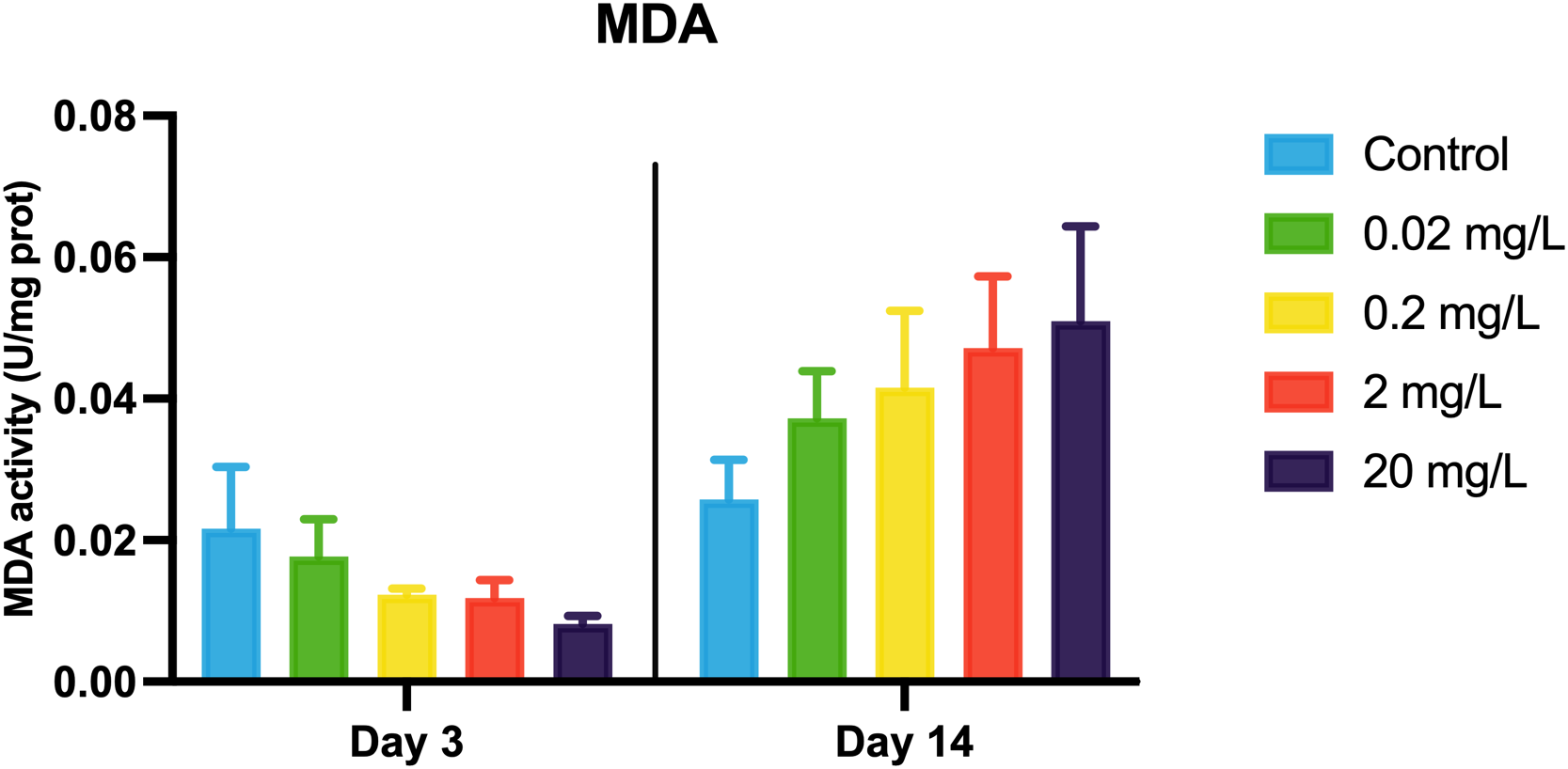
MDA activity in the black porgy following 3- and 14-day exposures to 0.02, 0.2, 2, and 20 mg/L of 133CsCl. Data are expressed as mean ± standard error (SE).
Figure 4
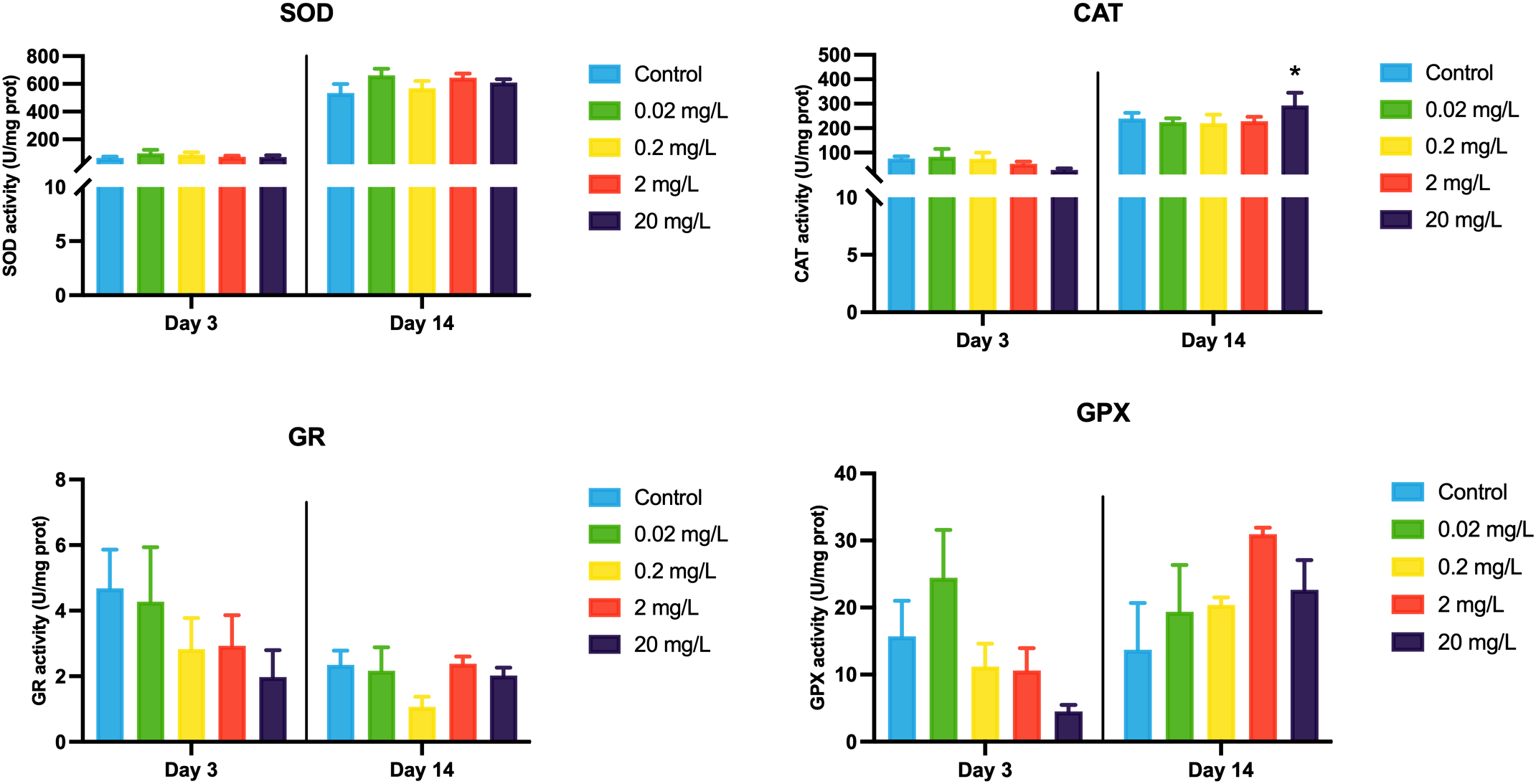
Antioxidant enzyme activities of the black porgy following 3- and 14-day exposures to 0.02, 0.2, 2, and 20 mg/L of 133CsCl. Data are expressed as mean ± standard error (SE). Statistically significant differences between the exposure and control groups are indicated at *p< 0.05.
3.3 Transcriptional expression profiles of target genes after exposure to 133CsCl
Significant differences in mRNA expression were observed for hsp70 and tgfβ during acute exposure (3 days). Specifically, the expression of hsp70 was 3.76 and 6.20 times higher in the 0.02 mg/L and 2 mg/L groups, respectively, compared to the control. Similarly, tgfβ expression was 1.99-fold higher in the 0.2 mg/L group and 1.96-fold higher in the 20 mg/L group compared to the control. However, no significant differences were observed in the expression of mt2, hsp90, Cu/Zn-sod, cat, tnfα, grp78, atf6, caspase7, and bax.
During the chronic exposure (14 days), significant changes were found for Cu/Zn-sod and grp78. Cu/Zn-sod expression was significantly induced by 2.43 and 1.91-fold at 0.2 mg/L and 20 mg/L groups, respectively. Conversely, grp78 expression was markedly suppressed to 0.17-fold in both 0.02 and 2 mg/L groups. No significant alterations were observed in the expression of mt2, hsp70, hsp90, cat, tnfα, tgfβ, atf6, caspase7, and bax after 14 days of exposure (Figure 5).
Figure 5
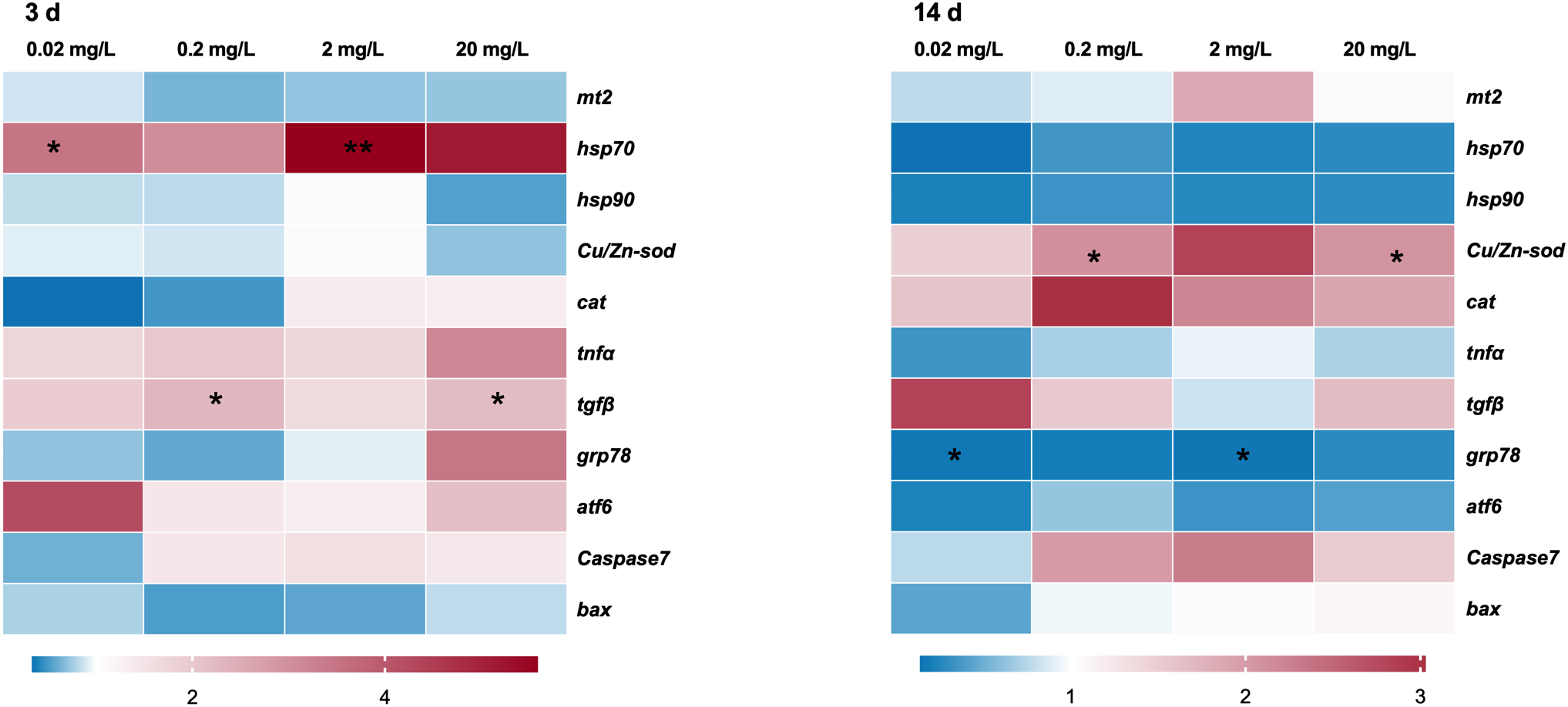
Transcription profiles of the selected genes in the black porgy following 3- and 14-day exposures to 0.02, 0.2, 2, and 20 mg/L of 133CsCl. Data are expressed as mean ± standard error (SE). Statistically significant differences between the exposure and control groups are indicated at *p< 0.05 and **p< 0.01.
3.4 IBRv2 values
In terms of IBRv2 values, the different concentration groups exhibited varying degrees of deviation following 133CsCl exposure. At 3 days, the IBRv2 value increased gradually from 18.53 in the 0.02 mg/L group to 31.88 in the 20 mg/L group. However, by 14 days, the trend reversed, with the IBRv2 values decreasing from 49.48 in the 0.02 mg/L group to 37.48 in the 20 mg/L group (Figure 6).
Figure 6
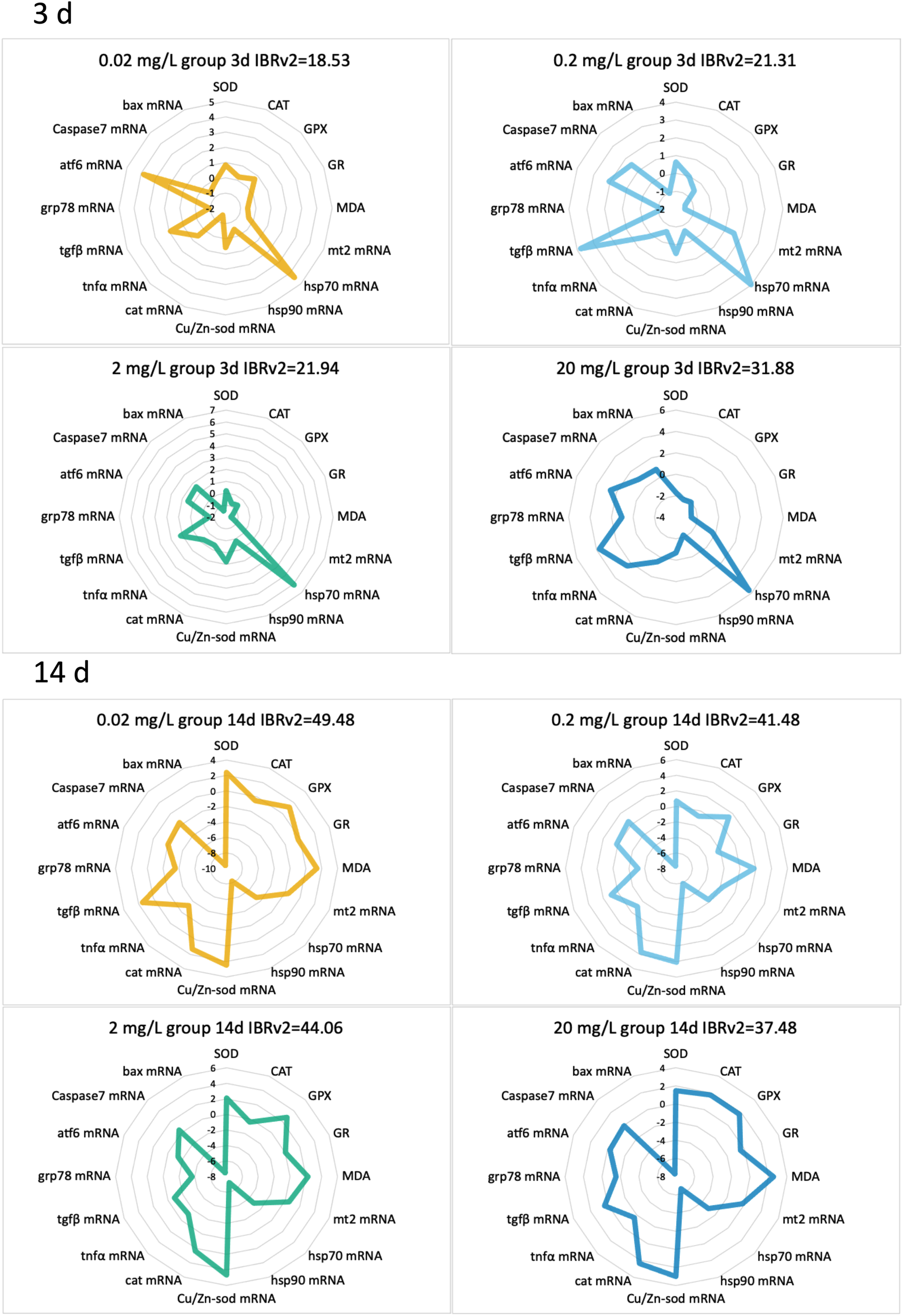
IBRv2 radargrams of various biomarkers in the black porgy following 3- and 14-day exposures to 0.02, 0.2, 2, and 20 mg/L of 133CsCl.
4 Discussion
Using controlled laboratory conditions, this study systematically investigated the integrated biological effects of black porgy following exposure to a gradient of 133CsCl concentrations under controlled laboratory conditions. Our results demonstrate that 133CsCl exposure perturbs multiple biological functions, with evidence indicating potential hepatic impairment. Despite tissue bioaccumulation levels remained relatively low, BCFs suggested a risk of biomagnification through aquatic food webs. At the molecular level, key metabolic pathways involved in heat shock response, oxidative stress, immune regulation, and ER stress were markedly affected.
To quantify bioaccumulation, BCFs were calculated for liver, gill, and muscle tissues. The BCF is a critical metric for evaluating a contaminant’s accumulation potential in aquatic organisms (Kowalska et al., 2024). In this study, ¹³³Cs+ BCF values ranged from 5 to 30, aligning with bioaccumulation profiles observed for metals such as cadmium (Jing et al., 2019). According to Organization for Economic Co-operation and Development (OECD) guidelines (OECD, 2012), BCF values below 100 denote low bioaccumulation potential. However, any value exceeding 1 confirms the bioaccumulative nature of ¹³³Cs+ and implies a potential for biomagnification within aquatic ecosystems (Parkerton et al., 2008).
Beyond BCF measures, oxidative stress biomarkers were evaluated at multiple time points to capture their temporal dynamics under 133CsCl exposure. During the acute phase (3 days), activities of CAT, GPX, and GR declined as 133CsCl concentration increased in this study, mirroring previous reports of antioxidant enzyme inhibition following short-term metal exposure (Fatima and Ahmad, 2005; Zheng et al., 2016). This decline may reflect toxic inhibition, substrate competition (Freitas et al., 2020) or decreased glutathione availability (Özkan-Yılmaz et al., 2014). Notably, SOD activity remained stable, consistent with its role as a first-line defense enzyme that is less sensitive to moderate oxidative stress (Eyckmans et al., 2011). Unexpectedly, MDA levels decreased during acute exposure despite elevated 133Cs+ doses. This observation differs from the typical increase in lipid peroxidation under oxidative stress (Chakraborty et al., 2024; Wang et al., 2024), but parallels findings in mercury-exposed Dicentrarchus labrax juveniles (Barboza et al., 2018), suggesting either effective initial antioxidant scavenging or insufficient exposure duration to induce lipid peroxidation (Zheng et al., 2016). Given that MDA typically rises within 48 hours post-stress (Chakraborty et al., 2024; Liu et al., 2024), measurements at 96 hours underscore a robust early antioxidant response, consistent with enhanced aldehyde detoxification (Li et al., 2024). Conversely, chronic exposure (14 days) induced increased activities of SOD, CAT, and GPX alongside dose-dependent MDA elevation, indicating that prolonged 133CsCl exposure overwhelms antioxidant defenses, culminating in oxidative damage. This pattern aligns with chronic metal toxicity observed in other fish species, where sustained exposure leads to lipid peroxidation and compensatory enzyme induction (Vinodhini and Narayanan, 2009; Tabrez and Ahmad, 2011; Javed et al., 2017). Despite increased enzyme activities, the response appears insufficient to prevent oxidative injury, potentially impairing mitochondrial function and immune defense (Halliwell and Gutteridge, 2015; Sun et al., 2022).
To investigate whether these enzymatic changes correspond to gene-level regulation, we examined transcriptional profiles of key antioxidant genes over time. At the transcriptional level, antioxidant genes exhibited complex regulation: Cu/Zn-sod and cat mRNA levels increased at 14 days, mirroring enzymatic antioxidant activation in the chronic phase. However, discrepancies between gene expression and enzyme activity were observed, reflecting common post-transcriptional regulatory mechanisms and temporal delays in protein synthesis (Craig et al., 2007; Li et al., 2013; Regoli and Giuliani, 2014) This underscores the necessity of interpreting molecular and enzymatic data conjointly. Gene expression patterns also revealed a temporal shift between early and late exposure. During early exposure, antioxidant genes such as Cu/Zn-sod and mt2 were downregulated, while stress-related genes (hsp70, tnfα, tnfβ, grp78, and atf6) were progressively upregulated with increasing contaminant levels. Such patterns suggest a cellular strategy prioritizing protein repair and immune responses over immediate antioxidant defense, balancing reactive oxygen species detoxification with proteostasis maintenance mediated by heat shock proteins (Pockley and Henderson, 2018; Junprung et al., 2021). Additionally, hsp70, a molecular chaperone vital for protein stabilization and refolding under stress, was notably upregulated, reflecting its conserved protective function in metal-exposed fish (Safari et al., 2014; Jeyachandran et al., 2023). This adaptive response may be mediated via the Nrf2-ARE pathway, linking antioxidant gene activation with heat shock responses to mount a comprehensive defense against oxidative insults (Dayalan Naidu et al., 2015). Contrastingly, mt2 expression remained low during acute exposure and showed no clear correlation with 133Cs concentration, aligning with previous observations of weak or inverse relationships between MT expression and metal burden in short-term exposures (De Boeck et al., 2003; Huang et al., 2014; Lee and Nam, 2016). Possible explanations include limited Cs accumulation or species-specific metal handling mechanisms.
In addition to antioxidant responses, inflammatory and apoptotic pathways were activated under 133Cs exposure, as oxidative stress can trigger these processes. Pro-inflammatory cytokine tnfα was dose-dependently upregulated in a dose-dependent manner, peaking at 20 mg/L, which is indicative of an immune response to Cs-induced inflammation (Abdel-Latif et al., 2022). Tnfα activation can initiate macrophage and monocyte signaling cascades culminating in apoptosis (Harikrishnan et al., 2021; Gao et al., 2023). Supporting this, apoptotic gene induction coincided with ER stress markers upregulation grp78, atf6, and caspase7 pointing to unfolded protein response (UPR) activation (Qian et al., 2001; Biagioli et al., 2008). Chronic ER stress may lead to apoptosis, as demonstrated in other metal toxicity models (Szegezdi et al., 2006; Kitamura and Hiramatsu, 2010). Collectively, these intertwined pathways of oxidative stress, inflammation, ER stress, and apoptosis delineate a coordinated cellular response to Cs exposure. Although antioxidant defenses and heat shock proteins offer some protection, inflammation and apoptosis contribute substantially to cellular damage during acute exposure. After 14 days of exposure, expression of heat shock proteins, inflammatory mediators, and ER stress markers decreased, while mt2, antioxidant, and apoptotic genes increased, especially at lower doses (2 mg/L). This pattern suggests reactivation of antioxidant and metal regulation mechanisms, although progressive Cs accumulation may impair liver function and reduce mt2 expression at higher doses (George, 1989; De Boeck et al., 2003; Huang et al., 2014). Notably, mt2 expression peaked at 2 mg/L but declined at higher concentrations, possibly indicating cellular saturation or toxicity thresholds, raising concerns about its reliability as a biomarker for chronic metal exposure (Rainbow, 2002; Lin et al., 2004; Long and Wang, 2005; Zhang and Wang, 2005; Cho et al., 2008; Le Croizier et al., 2018). Despite increased Cu/Zn-sod and cat expression, their activation was insufficient to counter prolonged oxidative stress, as evidenced by dose-dependent MDA increases. Simultaneously, hsp70 expression was suppressed, potentially weakening cellular resilience and exacerbating damage (Lin et al., 2023). Chronic exposure also suppressed inflammatory pathways but enhanced apoptotic signaling through caspase7 and bax, indicating severe oxidative damage, tissue injury, and programmed cell death in black porgy.
Finally, the IBRv2 index proved valuable for quantifying cumulative biological stress. Our study demonstrated that acute exposure elicited slightly greater variability in IBRv2 values (IBRv2: 18.53–31.88) than chronic exposure (IBRv2: 37.48–49.48), consistent with previous reports (Cao et al., 2022; Ramesh et al., 2024). Interestingly, during acute exposure, the highest deviation occurred at the highest dose (20 mg/L), while at 14 days, the greatest deviation appeared at the lowest dose (0.02 mg/L). Although this inverse dose response pattern is uncommon in IBRv2 studies, it highlights the complex dynamics of toxicant effects over time.
5 Conclusion
This study examined the physiological and biochemical responses of black porgy to stable isotopic 133Cs at different doses over both 3-day and 14-day exposures. The accumulation of Cs+ in black porgy suggests a potential risk of biomagnification within the food chain. While the fish demonstrated a robust stress response, its antioxidant system (SOD, CAT, GR and GPX) was capable of rapidly adapting to acute exposure. However, chronic exposure to 133Cs led to cellular and organ damage, as indicated by changes in altered gene expression profiles and increased antioxidant enzyme activity. Additionally, the IBRv2 index showed a dose-dependent response, further revealing the compound toxic effects of 133Cs. These findings highlight that ¹³³Cs poses toxic risks to economically important marine fish. Further research is needed to assess the impact of other isotopes on aquatic organisms and to better understand its toxicological mechanisms. This will help improve ecological risk assessments, particularly in regions affected by nuclear wastewater discharge and where seafood is a major food source.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The animal procedures were approved by the ethics committee of Third Institute of Oceanography, Ministry of Natural Resources, China (No. TIO-IACUC-01-2024-03-08). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
CZ: Investigation, Writing – original draft, Data curation, Formal Analysis, Methodology. GL: Investigation, Writing – review & editing, Formal Analysis. LS: Writing – review & editing, Formal Analysis, Investigation. RBZ: Writing – review & editing. LW: Formal Analysis, Investigation, Writing – review & editing. HZ: Formal Analysis, Writing – review & editing, Investigation. RHZ: Formal Analysis, Investigation, Writing – review & editing. CF: Writing – review & editing. YH: Supervision, Validation, Writing – review & editing. TY: Supervision, Writing – review & editing, Validation. JB: Conceptualization, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was sponsored by the National Key Research and Development Program of China (No. 2023YFC3108303) and Sichuan Science and Technology Program (No. 2025YFHZ0168).
Acknowledgments
The authors acknowledge BioRender.com for providing the graphical resources employed in this study. Illustrations of liver, muscle, gill, fish tank, and conical flask used in the graphical abstract were created with BioRender.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1635128/full#supplementary-material
References
1
Abdel-Latif H. M. R. Shukry M. Abd-elaziz R. A. (2022). Clinico-pathological findings and expression of inflammatory cytokines, apoptosis, and oxidative stress-related genes draw mechanistic insights in Nile tilapia reared under ammonia-N exposure and Aeromonas hydrophila challenge. Fish Shellfish Immunol.127, 1–12. doi: 10.1016/j.fsi.2022.06.001
2
Barboza L. G. A. Vieira L. R. Branco V. Carvalho C. Guilhermino L. (2018). Microplastics increase mercury bioconcentration in gills and bioaccumulation in the liver, and cause oxidative stress and damage in Dicentrarchus labrax juveniles. Sci. Rep.8, 15655. doi: 10.1038/s41598-018-34125-z
3
Biagioli M. Pifferi S. Ragghianti M. Bucci S. Rizzuto R. Pinton P. (2008). Endoplasmic reticulum stress and alteration in calcium homeostasis are involved in cadmium-induced apoptosis. Cell Calcium43, 184–195. doi: 10.1016/j.ceca.2007.05.003
4
Bo J. Giesy J. P. Ye R. Wang K.-J. Lee J.-S. Au D. W. T. (2012). Identification of differentially expressed genes and quantitative expression of complement genes in the liver of marine medaka Oryzias melastigma challenged with Vibrio parahaemolyticus. Comp. Biochem. Physiol. D Genomics Proteomics7, 191–200. doi: 10.1016/j.cbd.2012.02.005
5
Bo J. Zheng R. Jiang Y. Chen J. Fang C. Bailey C. et al . (2023). Physiological-dependent alterations on transcriptomic and proteomic patterns of the single and combined temperature and salinity-exposed hybrid grouper, Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂. Aquaculture575, 739746. doi: 10.1016/j.aquaculture.2023.739746
6
Cao B. Lv H. Nie T. Ma Y. Jiang Z. Hu Y. et al . (2022). Combined toxicity of acetochlor and metribuzin on earthworm Eisenia fetida: Survival, oxidative stress responses and joint effect. Appl. Soil Ecol.178, 104583. doi: 10.1016/j.apsoil.2022.104583
7
Chakraborty D. Mandal A. H. Ghosh S. Sadhu A. Das D. Saha N. C. et al . (2024). The physiological effects of acute and sub-lethal exposure to phenol on antioxidant enzyme activity in the freshwater sludge worm Tubifex tubifex. Toxicol. Rep.13, 101717. doi: 10.1016/j.toxrep.2024.101717
8
Cho Y. S. Lee S. Y. Kim K.-Y. Bang I. C. Kim D. S. Nam Y. K. (2008). Gene structure and expression of metallothionein during metal exposures in Hemibarbus mylodon. Ecotoxicol. Environ. Saf.71, 125–137. doi: 10.1016/j.ecoenv.2007.08.005
9
Craig P. M. Wood C. M. McClelland G. B. (2007). Oxidative stress response and gene expression with acute copper exposure in zebrafish (Danio rerio). Am. J. Physiol. Regul. Integr. Comp. Physiol.293, R1882–R1892. doi: 10.1152/ajpregu.00383.2007
10
Cunha I. I. L. Munita C. S. Paiva R. P. Teixeira A. (1993). Levels of cesium-137 in seawater and fish from the Brazilian coast. Sci. Total Environ.139-140, 431–435. doi: 10.1016/0048-9697(93)90040-D
11
Dayalan Naidu S. Kostov R. V. Dinkova-Kostova A. T. (2015). Transcription factors Hsf1 and Nrf2 engage in crosstalk for cytoprotection. Trends Pharmacol. Sci.36, 6–14. doi: 10.1016/j.tips.2014.10.011
12
De Boeck G. Ngo T. T. H. Van Campenhout K. Blust R. (2003). Differential metallothionein induction patterns in three freshwater fish during sublethal copper exposure. Aquat. Toxicol.65, 413–424. doi: 10.1016/S0166-445X(03)00178-4
13
Eyckmans M. Celis N. Horemans N. Blust R. De Boeck G. (2011). Exposure to waterborne copper reveals differences in oxidative stress response in three freshwater fish species. Aquat. Toxicol.103, 112–120. doi: 10.1016/j.aquatox.2011.02.010
14
Fatima R. A. Ahmad M. (2005). Certain antioxidant enzymes of Allium cepa as biomarkers for the detection of toxic heavy metals in wastewater. Sci. Total Environ.346, 256–273. doi: 10.1016/j.scitotenv.2004.12.004
15
Fowler S. W. Fisher N. S. (2005). “Chapter 6 Radionuclides in the biosphere,” in Radioactivity in the Environment. Ed. LivingstonH. D. (Amsterdam: Elsevier), 167–203. doi: 10.1016/s1569-4860(05)80007-5
16
Freitas R. Silvestro S. Coppola F. Costa S. Meucci V. Battaglia F. et al . (2020). Toxic impacts induced by Sodium lauryl sulfate in Mytilus galloprovincialis. Comp. Biochem. Physiol. A Mol. Integr. Physiol.242, 110656. doi: 10.1016/j.cbpa.2020.110656
17
Fukushima Prefecture (FP) (2023). Fukushima Prefecture Agriculture Forestry and Fisheries Products Process Food Monitoring Information (Fukushima Prefecture).
18
Gao F. Zheng R. Zhang K. Ma L. Liu K. Huang D. et al . (2024). Effects of thermal stress from nuclear power plants on the survival rate, behavioral changes, and biochemical and molecular responses of abalone. Aquac. Rep.37, 102239. doi: 10.1016/j.aqrep.2024.102239
19
Gao J. Guo H.-Y. Liu M.-J. Zhu K.-C. Liu B. Liu B.-S. et al . (2023). Transcriptome Analysis of the Immune Process of Golden Pompano (Trachinotus ovatus) Infected with Streptococcus agalactiae. Fishes8, 52. doi: 10.3390/fishes8010052
20
George S. G. (1989). Cadmium effects on plaice liver xenobiotic and metal detoxication systems: dose-response. Aquat. Toxicol.15, 303–309. doi: 10.1016/0166-445X(89)90043-X
21
Halliwell B. Gutteridge J. M. C. (2015). Free Radicals in Biology and Medicine. 2th Edn (Oxford: Oxford Univ. Press).
22
Harikrishnan R. Devi G. Van Doan H. Arockiaraj J. Jawahar S. Balasundaram C. et al . (2021). Influence of bamboo vinegar powder (BVP) enriched diet on antioxidant status, immunity level, and pro-anti-inflammatory cytokines modulation in Asian sea bass, Lates calcarifer (Bloch 1790) against Vibrio Anguillarum. Fish Shellfish Immunol.119, 462–477. doi: 10.1016/j.fsi.2021.10.026
23
Huang G.-Y. Ying G.-G. Liang Y.-Q. Liu S.-S. Liu Y.-S. (2014). Expression patterns of metallothionein, cytochrome P450 1A and vitellogenin genes in western mosquitofish (Gambusia affinis) in response to heavy metals. Ecotoxicol. Environ. Saf.105, 97–102. doi: 10.1016/j.ecoenv.2014.04.012
24
Ishaq S. Jabeen G. Arshad M. Kanwal Z. Un Nisa F. Zahra R. et al . (2023). Heavy metal toxicity arising from the industrial effluents repercussions on oxidative stress, liver enzymes and antioxidant activity in brain homogenates of Oreochromis niloticus. Sci. Rep.13, 19936. doi: 10.1038/s41598-023-47366-4
25
Javed M. Ahmad M. I. Usmani N. Ahmad M. (2017). Multiple biomarker responses (serum biochemistry, oxidative stress, genotoxicity and histopathology) in Channa punctatus exposed to heavy metal loaded waste water. Sci. Rep.7, 1675. doi: 10.1038/s41598-017-01749-6
26
Jeyachandran S. Chellapandian H. Park K. Kwak I.-S. (2023). A review on the involvement of heat shock proteins (Extrinsic chaperones) in response to stress conditions in aquatic organisms. Antioxidants12 (7), 1444. doi: 10.3390/antiox12071444
27
Jing W. Lang L. Lin Z. Liu N. Wang L. (2019). Cadmium bioaccumulation and elimination in tissues of the freshwater mussel Anodonta woodiana. Chemosphere219, 321–327. doi: 10.1016/j.chemosphere.2018.12.033
28
Junprung W. Supungul P. Tassanakajon A. (2021). Structure, gene expression, and putative functions of crustacean heat shock proteins in innate immunity. Dev. Comp. Immunol.115, 103875. doi: 10.1016/j.dci.2020.103875
29
Kameník J. Dulaiova H. Šebesta F. Šťastná K. (2013). Fast concentration of dissolved forms of cesium radioisotopes from large seawater samples. J. Radioanal. Nucl. Chem.296, 841–846. doi: 10.1007/s10967-012-2007-4
30
Kitamura M. Hiramatsu N. (2010). The oxidative stress: endoplasmic reticulum stress axis in cadmium toxicity. BioMetals23, 941–950. doi: 10.1007/s10534-010-9296-2
31
Kowalska D. Sosnowska A. Zdybel S. Stepnik M. Puzyn T. (2024). Predicting bioconcentration factors (BCFs) for per- and polyfluoroalkyl substances (PFAS). Chemosphere364, 143146. doi: 10.1016/j.chemosphere.2024.143146
32
Kurosawa H. Wakiyama Y. Wada T. Nanba K. (2025). Long-term 137Cs dynamics after decontamination of an urban pond in Fukushima Prefecture, Japan. J. Environ. Radioact.281, 107573. doi: 10.1016/j.jenvrad.2024.107573
33
Lacoue-Labarthe T. Warnau M. Oberhänsli F. Teyssié J.-L. Bustamante P. (2010). Contrasting accumulation biokinetics and distribution of 241Am, Co, Cs, Mn and Zn during the whole development time of the eggs of the common cuttlefish, Sepia officinalis. J. Exp. Mar. Biol. Ecol.382, 131–138. doi: 10.1016/j.jembe.2009.10.008
34
Le Croizier G. Lacroix C. Artigaud S. Le Floch S. Raffray J. Penicaud V. et al . (2018). Significance of metallothioneins in differential cadmium accumulation kinetics between two marine fish species. Environ. pollut.236, 462–476. doi: 10.1016/j.envpol.2018.01.002
35
Lee S. Y. Nam Y. K. (2016). Transcriptional responses of metallothionein gene to different stress factors in Pacific abalone (Haliotis discus hannai). Fish Shellfish Immunol.58, 530–541. doi: 10.1016/j.fsi.2016.09.030
36
Leite C. Russo T. Cuccaro A. Pinto J. Polese G. Soares A. M. V. M. et al . (2024). Rare earth elements and warming: Implications for adult mussel health and sperm quality. Mar. Environ. Res.201, 106666. doi: 10.1016/j.marenvres.2024.106666
37
Li S. Liu H. Huang W. Yang S. Xie M. Zhou M. et al . (2024). Effects of three polychaete species on growth and reproductive performance, biochemical indices, and histological condition of differing tissues in male broodstock of Pacific white shrimp, Litopenaeus vannamei. Aquaculture598, 742055. doi: 10.1016/j.aquaculture.2024.742055
38
Li M. Zheng Y. Liang H. Zou L. Sun J. Zhang Y. et al . (2013). Molecular cloning and characterization of cat, gpx1 and Cu/Zn-sod genes in pengze crucian carp (Carassius auratus var. Pengze) and antioxidant enzyme modulation induced by hexavalent chromium in juveniles. Comp. Biochem. Physiol. C157, 310–321. doi: 10.1016/j.cbpc.2013.02.003
39
Lin C.-H. John J. A. C. Ou L. W. Chen J.-C. Lin C.-H. Chang C.-Y. (2004). Cloning and characterization of metallothionein gene in ayu Plecoglossus altivelis. Aquat. Toxicol.66, 111–124. doi: 10.1016/j.aquatox.2003.06.003
40
Lin W. Wu J. Luo H. Liu X. Cao B. Hu F. et al . (2023). Sub-chronic ammonia exposure induces hepatopancreatic damage, oxidative stress, and immune dysfunction in red swamp crayfish (Procambarus clarkii). Ecotoxicol. Environ. Saf.254, 114724. doi: 10.1016/j.ecoenv.2023.114724
41
Liu X. Li Z. Li Q. Bao X. Jiang L. Yang J. (2024). Acute exposure to polystyrene nanoplastics induced oxidative stress in Sepia esculenta Larvae. Aquac. Rep.35, 102004. doi: 10.1016/j.aqrep.2024.102004
42
Liu Y. Li Y.-L. Min Y.-T. Chen S. Yang W. Gu J.-T. et al . (2025). Fukushima contaminated water risk factor: global implications. Environ. Sci. Technol.59, 3703–3712. doi: 10.1021/acs.est.4c08145
43
Long A. Wang W. (2005). Assimilation and bioconcentration of Ag and Cd by the marine black bream after waterborne and dietary metal exposure. Environ. Toxicol. Chem.24, 709–716. doi: 10.1897/03-664.1
44
Mao X. Zhao Y. Li Z. (2024). Microsatellite analysis of genetic diversity and population differentiation of Acanthopagrus schlegelii in China. Reg. Stud. Mar. Sci.77, 103662. doi: 10.1016/j.rsma.2024.103662
45
Metian M. Warnau M. Teyssié J.-L. Bustamante P. (2011). Characterization of 241Am and 134Cs bioaccumulation in the king scallop Pecten maximus: investigation via three exposure pathways. J. Environ. Radioact.102, 543–550. doi: 10.1016/j.jenvrad.2011.02.008
46
Mohammad A. Yang Y. Khan M. A. Faustino P. J. (2015). A long-term stability study of PRussian blue: A quality assessment of water content and cesium binding. J. Pharm. Biomed. Anal.103, 85–90. doi: 10.1016/j.jpba.2014.10.030
47
OECD (2012). “Test No. 305: bioaccumulation in fish: aqueous and dietary exposure, OECD guidelines for the testing of chemicals,” in Section 3 (OECD Publishing, Paris).
48
Özkan-Yılmaz F. Özlüer-Hunt A. Gündüz S. G. Berköz M. Yalın S. (2014). Effects of dietary selenium of organic form against lead toxicity on the antioxidant system in Cyprinus carpio. Fish Physiol. Biochem.40, 355–363. doi: 10.1007/s10695-013-9848-9
49
Parkerton T. F. Arnot J. A. Weisbrod A. V. Russom C. Hoke R. A. Woodburn K. et al . (2008). Guidance for evaluating in vivo fish bioaccumulation data. I. Environ. Assess. Manage.4, 139–155. doi: 10.1897/IEAM_2007-057.1
50
Pockley A. G. Henderson B. (2018). Extracellular cell stress (heat shock) proteins—immune responses and disease: an overview. Phil. Trans. R. Soc B373, 20160522. doi: 10.1098/rstb.2016.0522
51
Pollicelli M. Márquez F. Pollicelli M. D. Idaszkin Y. L. (2023). Screening of tolerance of Atriplex vulgatissima under zinc or lead experimental conditions. An integrative perspective by using the integrated biological response index (IBRv2). Chemosphere341, 140110. doi: 10.1016/j.chemosphere.2023.140110
52
Qian Y. Falahatpisheh M. H. Zheng Y. Ramos K. S. Tiffany-Castiglioni E. (2001). Induction of 78 kD glucose-regulated protein (GRP78) expression and redox-regulated transcription factor activity by lead and mercury in C6 rat glioma cells. Neurotoxicol. Res.3, 581–589. doi: 10.1007/BF03033212
53
Rainbow P. S. (2002). Trace metal concentrations in aquatic invertebrates: why and so what? Environ. pollut.120, 497–507. doi: 10.1016/S0269-7491(02)00238-5
54
Ramesh M. Selvaraju S.-G. Poopal R.-K. Ren Z. Li B. (2024). Impact of continuous Triazophos exposure on Labeo rohita: Physiological, biochemical, and histological alterations and IBRv2 index assessment. Pestic. Biochem. Physiol.204, 106043. doi: 10.1016/j.pestbp.2024.106043
55
Regoli F. Giuliani M. E. (2014). Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res.93, 106–117. doi: 10.1016/j.marenvres.2013.07.006
56
Rühm W. Yoshida S. Muramatsu Y. Steiner M. Wirth E. (1999). Distribution patterns for stable 133Cs and their implications with respect to the long-term fate of radioactive 134Cs and 137Cs in a semi-natural ecosystem. J. Environ. Radioact.45, 253–270. doi: 10.1016/S0265-931X(98)00104-0
57
Safari R. Shabani A. Ramezanpour S. Imanpour M. R. Rezvani S. (2014). Alternations of heat shock proteins (hsp70) gene expression in liver and gill of Persian sturgeon (Acipenser persicus Borodin 1987) exposed to cadmium chloride. Iran. J. Fish. Sci.13, 979–997. doi: 10.22092/ijfs.2018.114411
58
Sansone U. Belli M. Jeran Z. Kanivets V. V. Radojko J. Riccardi M. et al . (2002). Suspended particle adhesion on aquatic plant surfaces: implications for 137Cs and 133Cs uptake rates and water-to-plant concentration ratios. J. Environ. Radioact.59, 257–271. doi: 10.1016/S0265-931X(01)00078-9
59
Sun Q. Li Y. Shi L. Hussain R. Mehmood K. Tang Z. et al . (2022). Heavy metals induced mitochondrial dysfunction in animals: Molecular mechanism of toxicity. Toxicology469, 153136. doi: 10.1016/j.tox.2022.153136
60
Sun P. Yuan X. Gao X. Ling J. Jiang Y. (2024). Reduced rearing density improved the growth and welfare of Acanthopagrus schlegelii: An integrated analysis using transcriptomics and metabolomics. Aquac. Rep.38, 102344. doi: 10.1016/j.aqrep.2024.102344
61
Suseno H. Prihatiningsih W. R. (2014). Monitoring 137Cs and 134Cs at marine coasts in Indonesia between 2011 and 2013. Mar. pollut. Bull.88, 319–324. doi: 10.1016/j.marpolbul.2014.08.024
62
Szegezdi E. Duffy A. O’Mahoney M. E. Logue S. E. Mylotte L. A. O’Brien T. et al . (2006). ER stress contributes to ischemia-induced cardiomyocyte apoptosis. Biochem. Biophys. Res. Commun.349, 1406–1411. doi: 10.1016/j.bbrc.2006.09.009
63
Tabrez S. Ahmad M. (2011). Oxidative stress-mediated genotoxicity of wastewaters collected from two different stations in northern India. Mutat. Res. Genet. Toxicol. Environ. Mutagen.726, 15–20. doi: 10.1016/j.mrgentox.2011.07.012
64
Tateda Y. Aoyama M. Hamajima Y. Tsumune D. Ishimaru T. Ito Y. et al . (2024a). Radioecological behaviour of 137Cs in rockfish of the southern coastal waters off Fukushima during 2017–2021. J. Environ. Radioact.273, 107386. doi: 10.1016/j.jenvrad.2024.107386
65
Tateda Y. Nishikawa J. Aoyama M. Takata H. Hamajima Y. Aono T. (2024b). Status of the transfer state of 137Cs in zooplankton and surface water fish off Fukushima during 2018–2021. J. Environ. Radioact.278, 107496. doi: 10.1016/j.jenvrad.2024.107496
66
Thomas D. M. Fisher N. S. (2019). Evaluation of body size and temperature on 137Cs uptake in marine animals. J. Environ. Radioact.202, 25–31. doi: 10.1016/j.jenvrad.2019.02.005
67
Tsukada H. Hasegawa H. (2003). Uptake and distribution of 137Cs, stable Cs and K in rice plants (Japan: IAEA International Nuclear Information System).
68
Uddin S. Aba A. Behbehani M. Al-Ghadban A. N. Al-Zekri W. Al-Shammari H. (2017). Plutonium and cesium baseline concentrations in seawater from northern Arabian Gulf. Mar. pollut. Bull.120, 396–400. doi: 10.1016/j.marpolbul.2017.05.006
69
Vinodhini R. Narayanan M. (2009). Biochemical changes of antioxidant enzymes in common carp (Cyprinus carpio L.) after heavy metal exposure. Turk. J. Vet. Anim. Sci.33, Article 32. doi: 10.3906/vet-0711-18
70
Wang Y. Hou Y. He C. Zhao Y. Duan C. Nie X. et al . (2024). Toxic effects of acute and chronic atorvastatin exposure on antioxidant systems, autophagy processes, energy metabolism and life history in Daphnia magna. Chemosphere369, 143792. doi: 10.1016/j.chemosphere.2024.143792
71
Xiguang Z. Wen-Xiong W. Yu K. N. Paul K. S. L. (2001). Biomagnification of radiocesium in a marine piscivorous fish. Mar. Ecol. Prog. Ser.222, 227–237. doi: 10.3354/meps222227
72
Xu Y. Men W. Yu Y. Wang F. (2024). Assessment of radiation in fishes derived from radiocesium in the port of Fukushima Daiichi nuclear power plant. Mar. pollut. Bull.202, 116301. doi: 10.1016/j.marpolbul.2024.116301
73
Xu M. Zhang Y. Cao S. Li Y. Wang J. Dong H. et al . (2023). A simulated toxic assessment of cesium on the blue mussel Mytilus edulis provides evidence for the potential impacts of nuclear wastewater discharge on marine ecosystems. Environ. pollut.316, 120458. doi: 10.1016/j.envpol.2022.120458
74
Zhang L. Wang W.-X. (2005). Effects of Zn pre-exposure on Cd and Zn bioaccumulation and metallothionein levels in two species of marine fish. Aquat. Toxicol.73, 353–369. doi: 10.1016/j.aquatox.2005.04.001
75
Zhao X. Zhao W. Xu F. Shen Y. Bao Y. Yang B. et al . (2024). Toxicity and detoxication assessment of juvenile black seabream (Acanthopagrus schlegelii) in response to dietary cadmium exposure: Based on growth performance and stress indicators. Aquac. Rep.34, 101897. doi: 10.1016/j.aqrep.2023.101897
76
Zheng R. Lai X. Fang C. Lin H. Huang Y. Zheng J. et al . (2024). Quantitatively characterize the response of the hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) under elevated temperature stress. Mar. Environ. Res.202, 106758. doi: 10.1016/j.marenvres.2024.106758
77
Zheng J.-L. Yuan S.-S. Wu C.-W. Lv Z.-M. (2016). Acute exposure to waterborne cadmium induced oxidative stress and immunotoxicity in the brain, ovary and liver of zebrafish (Danio rerio). Aquat. Toxicol.180, 36–44. doi: 10.1016/j.aquatox.2016.09.012
Summary
Keywords
bioaccumulation, gene expression, antioxidant response, malondialdehyde level, integrated biological effects
Citation
Zheng C, Liu G, Shao L, Zeb R, Wang L, Zhang H, Zheng R, Fang C, Huang Y, Yu T and Bo J (2025) Enrichment characteristics and biological effects of 133CsCl in black porgy Acanthopagrus schlegelii. Front. Mar. Sci. 12:1635128. doi: 10.3389/fmars.2025.1635128
Received
26 May 2025
Accepted
16 June 2025
Published
30 June 2025
Volume
12 - 2025
Edited by
Gopalakrishnan Singaram, krishkash Envirotech Private Limited, India
Reviewed by
Thiagarajan Raman, Ramakrishna Mission Vivekananda College, India
Rajamanickam Krishnamurthy, Arignar Anna Government Arts and Science College Chennai, India
Chezhian Eswer, Dharmapuram Gnanambigai Government Arts College for Women, India
Updates
Copyright
© 2025 Zheng, Liu, Shao, Zeb, Wang, Zhang, Zheng, Fang, Huang, Yu and Bo.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Youyou Huang, yyhuang_cwnu@163.com; Tao Yu, yutao@tio.org.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.