- 1First Institute of Oceanography, Ministry of Natural Resources, Qingdao, China
- 2Fuzhou Haixia Electricity Generation Co., Ltd, Fuzhou, China
- 3Zhejiang Institute of Hydrogeology and Engineering Geology, Ningbo, China
- 4Qingdao Institute of Marine Geology, China Geological Survey, Qingdao, China
Northeastern Fuqing Bay is crucial for the marine ecosystem in Fujian Province and plays an important role in regional economic development and ecological balance. However, rapid economic and population growth has led to severe heavy metal (HM) pollution from anthropogenic sources and atmospheric deposition. This study comprehensively assessed HMs in surface sediments, surface seawater, and marine organisms in northeastern Fuqing Bay, Fujian Province. A total of 37 surface seawater samples, 22 surface sediment samples, and 4 marine organism samples were collected. The results indicated that certain HMs, such as Hg and Cd, exhibited high coefficients of variation in surface sediments. The concentrations of HMs in both surface seawater and sediments met Class I standards; however, some sediment samples were contaminated with Cd, As, and Hg, and the Cr levels in marine organisms exceeded the permissible limits at certain sampling sites. Analysis of various indices revealed that the mean potential ecological risk index (RI) value was 193.12, indicating moderate ecological risk, primarily influenced by Cd and Hg, whereas seawater was classified as having a low ecological risk, with mean RI value of 13.52. Marine organisms demonstrated a strong bioaccumulation capacity for certain HMs in seawater. Principal component analysis indicated that HM sources in sediments were mainly wastewater discharge from chemical enterprises, port operations, rock weathering, metal smelting, and agricultural activities. In contrast, HM sources in seawater were partly natural and partly related to anthropogenic activities, such as urban and rural sewage discharge. This study provides an important reference for the ecological restoration of this region.
1 Introduction
As a crucial part of the marine ecosystem in Fujian Province, northeastern Fuqing Bay plays a significant role in the regional economic development and maintenance of ecological balance (Liu et al., 2025). This area is rich in fishery resources and serves as a habitat and breeding ground for numerous marine organisms (Fan et al., 2022). It is also an important area for economic activities such as maritime transportation and coastal tourism (Lv et al., 2019). However, with rapid economic development and continuous population growth in coastal areas, large amounts of heavy metal (HM) pollutants enter the area through various channels, including industrial wastewater discharge, agricultural non-point source pollution, urban domestic sewage discharge, and atmospheric deposition. Consequently, it is facing an increasingly severe HM pollution problem (Lv et al., 2019).
HMs, such as Hg, Cd, Pb, Cr, and As, are characterized by high toxicity, difficult degradation, and easy accumulation (Avvari et al., 2022). Once they enter the marine environment, they not only migrate and transfer to seawater, but also continuously accumulate in sediments. Biological magnification of HMs in the food chain poses a serious threat to marine life and human health (Feng et al., 2023; Qiu et al., 2019). Research shows that HM pollution can interfere with the physiological functions of marine organisms, affecting their growth, development, reproduction, and immune processes, thereby leading to a decline in biodiversity and damage to the structure and function of ecosystems (Nour et al., 2022). In seawater, the existing forms and concentrations of HMs are affected by various factors such as pH, redox potential, salinity, and particulate matter content. Changes in these factors can alter the migration and transformation of HMs, as well as their bioavailability (Madadi et al., 2023). As an important reservoir of HMs, the composition, structure, and physicochemical properties of sediments play key roles in HM adsorption, desorption, precipitation, and dissolution. In addition, microbial activity in sediments affects the morphological transformation and bioavailability of HMs (Leung et al., 2021). HM absorption, accumulation, and metabolism in organisms are not only related to the physiological characteristics of the organisms themselves but are also closely related to the concentration and form of HMs in the environment and food chain relationships (Zhang et al., 2023).
Although the ecological status of Fuqing Bay and its adjacent areas has garnered increasing research attention in recent years (Li et al., 2008; Ruan et al., 2000), most previous studies have focused primarily on HM distribution and contamination in surface sediments (Luo et al., 2004; Li et al., 2010; Lin, 2012). Few have provided an integrated assessment across multiple environmental compartments, particularly simultaneous evaluations in seawater columns and marine biota. Moreover, there remains a limited understanding of the transport mechanisms and biogeochemical cycling of HMs within this ecosystem. These knowledge gaps highlight the need for a more holistic approach to HM pollution assessment in the region.
In response, this study presents a comprehensive evaluation of HM pollution in northeastern Fuqing Bay by examining three interlinked compartments: surface sediments, seawater, and marine organisms. The main objectives are: (1) to analyze the spatial distribution and variability of HMs across these media; (2) to identify and apportion potential contamination sources using multivariate statistical methods; (3) to assess ecological risks through established sediment and water quality indices; and (4) to provide a scientific foundation for designing targeted ecological restoration and pollution control strategies. By integrating multiple environmental matrices and applying advanced analytical techniques, this research aims to offer novel insights into the fate, transport, and impacts of HMs in Fuqing Bay, thereby addressing critical gaps in existing literature and supporting informed environmental management.
2 Materials and methods
2.1 Sample collection and analytical methods
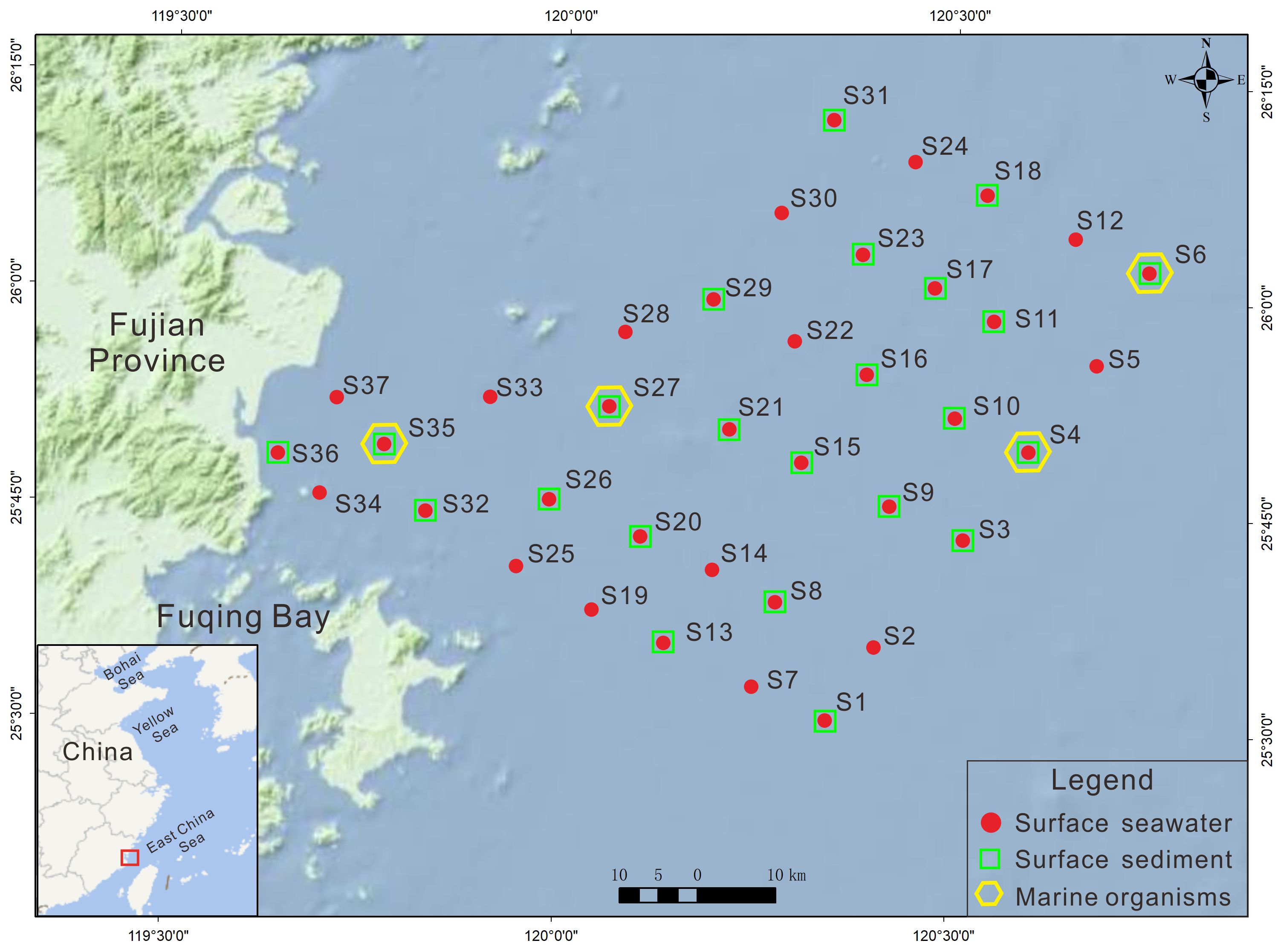
In April 2022, 37 surface seawater samples, 22 surface sediment samples, and 4 marine organism samples were collected from northeastern Fuqing Bay (Figure 1). The procedures for collecting, preserving, and transporting these samples adhered to the guidelines outlined in the “Specifications for Marine Surveys” (GB/T12763-2007).
2.1.1 Seawater sampling and pretreatment
Surface seawater samples (~0.5 m depth) were collected using a plexiglass water sampler. Prior to storage, sample bottles were rinsed twice with the sampled seawater to minimize contamination. Polyethylene bottles were used to preserve Zn, Cu, Cr, Cd, Pb, and As samples, whereas Hg samples were stored in pre-cleaned glass bottles. All samples were immediately stored under dark, chilled conditions during transportation.
For stabilization, 2 mL of HNO3 (ultrapure grade) was added to samples designated for Zn, Cu, Cr, Cd, and Pb analysis, whereas 2 mL of H2SO4 was used for As and Hg preservation. Filtration was performed using 0.45 μm glass fiber membranes to remove particulate matter. Digestion involved adding 10 mL HNO3 to 500 mL seawater, followed by heating on an electric hotplate until concentrated to ~10 mL. The digestate was diluted to 25 mL with 1% HNO3 and homogenized prior to instrumental analysis. Procedural blanks and certified reference materials were processed simultaneously to ensure quality. The accuracy and repeatability of the analytical procedures were verified through elemental recovery rates, which fell within the acceptable range of 90% to 110% for all measured elements. To evaluate reproducibility, 10% of the samples were analyzed in three replicates, yielding relative standard deviations (RSD) between 0.05% and 2.5% for the determined heavy metal concentrations.
2.1.2 Sediment sampling and pretreatment
Surface sediments were obtained using a grab sampler, and approximately 200 g of surface mud was collected using a polyethylene spoon. Samples were sealed in polyethylene bags, stored in the dark, and refrigerated until laboratory processing. Freeze-drying was conducted for 40 h to eliminate moisture, followed by manual homogenization using an agate mortar. The dried sediment was sieved through a 160-mesh nylon screen to ensure a uniform particle size.
For acid digestion, 1.0 g of sediment was treated with a 9:3 (v/v) mixture of HNO3 and HCl (aqua regia) in Teflon vessels.
2.1.3 Biological sample collection and processing
Marine organisms were collected using a single-vessel trawl net (40 × 94 m/49.3 m) with wing and cod-end configuration. Sampling was conducted during daylight, accounting for variables such as vessel speed (3–4 knots), current direction, and wind conditions. Trawling was performed 2–4 nautical miles from the designated stations to ensure representative sampling.
Organisms were taxonomically identified, and biometric data (length and mass) were recorded. The samples were categorized into crustaceans (Parapenaeopsis hardwickii and Metapenaeus joyneri) and fish (Cynoglossus abbreviates and Collichthys lucidus). Muscle tissues from the pectoral fins (fish) and abdominal segments (crustaceans) were excised, rinsed with ultrapure water, and stored in pre-weighed 10 mL centrifuge tubes at –20°C.
Digestion followed the HY/T132–2010 protocols, where 0.1 g of tissue was treated with 9 mL HNO33 and 3 mL H2O2 in a microwave-assisted digestion system.
2.1.4 Instrumental analysis and quality control
Cu, Pb, Zn, and Cd in seawater were quantified using graphite furnace atomic absorption spectrometry. The sediment and biological samples were analyzed similarly, except for Zn, which was determined using flame atomic absorption spectrometry. A Varian 240 FS AAS (USA) was used for the measurements. As and Hg were detected using an XGY-1011A atomic fluorescence spectrometer.
Method validation included reagent blanks, duplicate samples, and certified reference materials, with recovery rates of 90–110%. Precision was assessed via triplicate analysis (10% of samples), yielding relative standard deviations of 0.05–2.5%, confirming method reproducibility.
2.2 Analytical assessments
Several indices were employed to evaluate HM pollution risks and identify the sources, including the geoaccumulation index (Igeo), pollution load index (PLI), potential ecological risk index (RI), single factor pollution index (Cf), water quality index (WQI), single pollution index (Pi), bioaccumulation factor (BAF), and principal component analysis (PCA).
The Igeo was first proposed by Muller in 1969 and was determined using the following formula (Equation 1):
where Ci represents the HM concentration in the sediments and Bi denotes the background values from Fujian Province, China (Chen et al., 1992). The Igeo scale ranges from uncontaminated (Igeo ≤ 0) to severely contaminated (Igeo > 5), as defined by Muller (1981).
The PLI, which was initially proposed by Tomlinson et al. (1980), is a comprehensive indicator of the integrated contamination status of multiple HMs. The calculation followed the following expression (Equations 2 and 3):
where CFi is the contamination coefficient of the ith metallic element, Ci is the measured concentration, and C0 is the natural geochemical baseline concentration. According to the classification established by Chakravarty and Patgiri (2009), PLI values below 1 indicate uncontaminated conditions, whereas values exceeding 1 indicate varying degrees of pollution.
The RI, originally developed by Hakanson (1980), provides a quantitative framework for evaluating potential ecological hazards by incorporating metal-specific toxicity coefficients and regional background concentration. It is calculated as (Equation 4):
where is the contamination factor for element i, calculated as the ratio of the measured concentration () to the background level (). The parameter represents the toxic response factor specific to each metal, with established values for common HMs, such as Zn (1), Cr (2), Cu, Pb (5), As (10), Cd (30), and Hg (40) (Hakanson, 1980). This index provides a cumulative assessment of the ecological risks from multiple metallic contaminants.
Individual metal risks () were classified into five progressive categories: minimal risk (<40), moderate (40-79), substantial (80-159), severe (160-319), and extreme (≥320). Similarly, the composite RI was categorized into four tiers: low (RI< 105), intermediate (105 ≤ RI< 210), significant (210 ≤ RI< 420), and very high (RI ≥ 420), as per the classification system updated by Xu et al. (2021).
For marine water quality assessment, Cf and WQI have been widely adopted (Küikrer and Mutlu, 2019) and were calculated as follows (Equations 5 and 6):
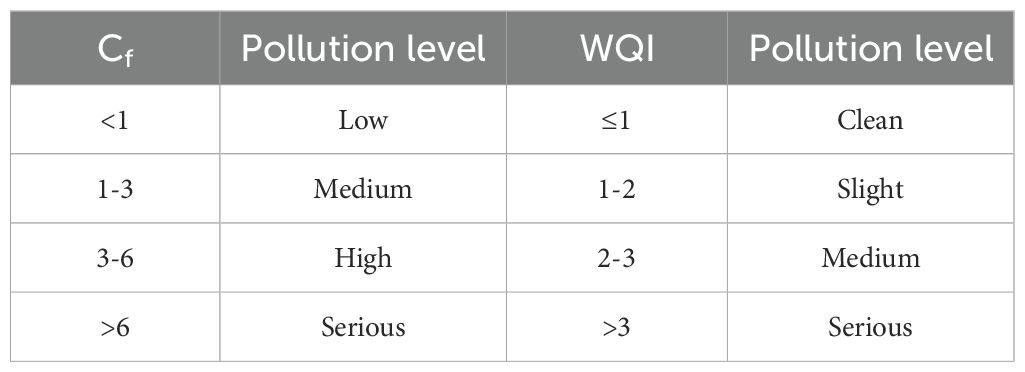
where Cf quantifies the pollution magnitude of individual metals, Ci is the analytical measurement, and Cs represents the regulatory limit of China’s Seawater Quality Standard (GB 3097-1997). The variable n indicates the number of monitored elements. The interpretive framework correlating Cf and WQI values and contamination levels has been comprehensively documented in previous studies (Baltas et al., 2017; Macdonald et al., 1996), as summarized in Table 1.
Pi was implemented to evaluate HM bioaccumulation in marine organisms and was calculated using the following expression (Equation 7):
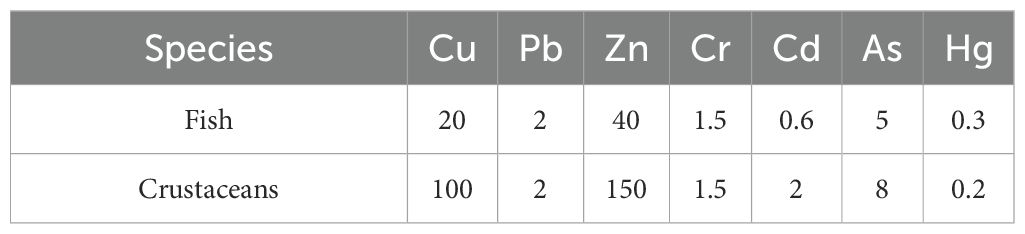
This index compares the measured tissue concentrations (Ci) against biological reference standards (Si) derived from China’s “Coastal and Marine Resource Comprehensive Survey Guidelines (Table 2).” Current evaluation protocols, as described by Zhou et al. (2022), consider Pi values below 1.0 as compliant with biological quality standards, whereas values above this threshold indicate excessive contamination.
BAF was employed to quantify HM transfer from environmental matrices to biota. Specifically, this study calculated two variants, BWAF and BSAF, which were determined as follows (Equations 8 and 9):
These metrics relate organismal metal concentrations (Ci) to their corresponding levels in seawater (Cw) and sedimentary compartments (Cs).
Spatial distribution patterns of the data were visualized through planar maps created with Surfer 23.0. Statistical evaluations, such as principal component analysis and Pearson correlation, were conducted using SPSS 27.0 (IBM Corp.). Preliminary data organization and descriptive statistics were carried out in Microsoft Excel 2016 (Microsoft Corp.).
The use of varimax rotation and the Kaiser criterion (eigenvalues >1) is a standard and widely accepted practice in environmental source apportionment studies using PCA. Varimax rotation was chosen because it maximizes the variances of the squared loadings within each factor, thereby enhancing the interpretability of the factors by making high loadings higher and low loadings lower for each component, which helps in clearer source identification.
3 Results and discussion
3.1 Concentration of HMs in sediments, surface seawater, and organisms
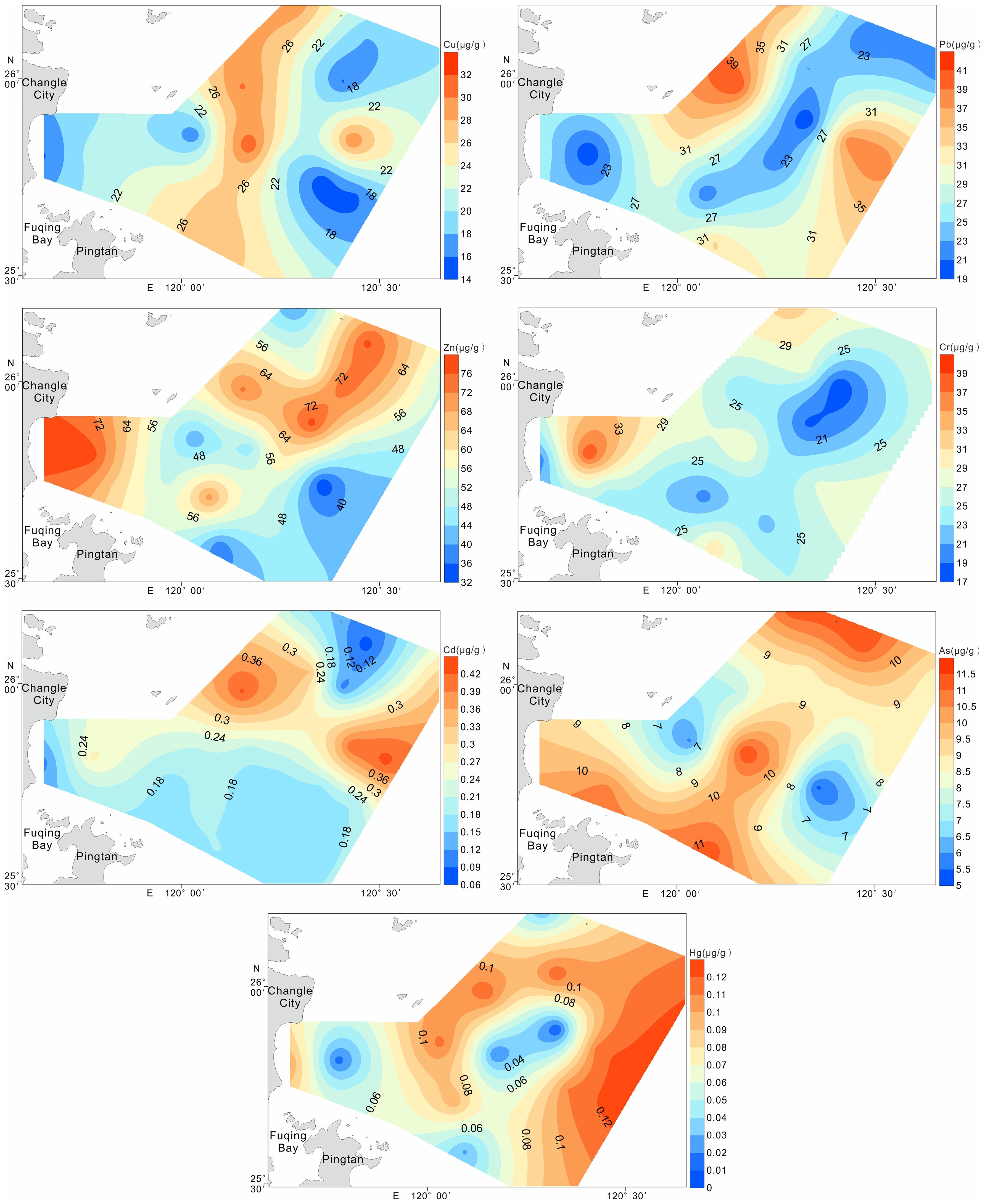
Surface sediment analysis in northeastern Fuqing Bay revealed the following HM concentration ranges (μg/g dry weight): Cu (14.6-31.8), Pb (19.1-40.9), Zn (32.6-77.9), Cr (17.4-39.2), Cd (0.08-0.43), As (5.4-11.5), and Hg (0.009-0.122), with corresponding mean values of 22.36, 28.33, 56.40, 25.56, 0.23, 9.04, and 0.08 μg/g (Table 3), respectively. All the measured concentrations complied with the Class I standards specified in China’s Marine Sediment Quality (GB 18668-2002) (Administration of quality supervision, inspection and quarantine of the People’s Republic of China (AQSIQ), 2002).
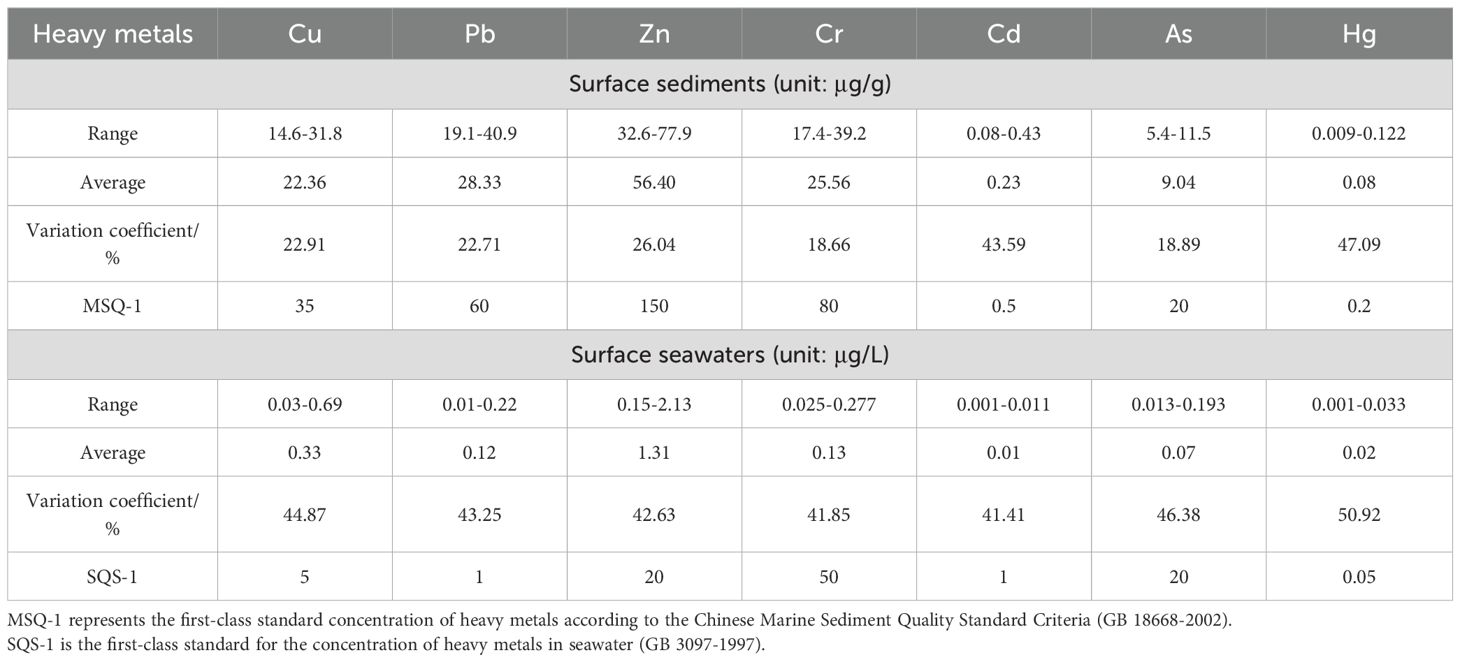
Table 3. Heavy metal concentrations in the surface sediments and surface seawater in northeast Fuqing Bay.
Variation coefficients were calculated to assess the anthropogenic influence on metal distribution. The metals exhibited the following order of variability: Hg (47.09%) > Cd (43.59%) > Zn > Cu > Pb > As > Cr (18.66-26.04%). Notably, Hg and Cd demonstrated substantial variation (exceeding 35%), suggesting significant external influences and a heterogeneous spatial distribution. The other elements showed moderate variability, indicating relatively uniform distribution patterns across the sampling sites.
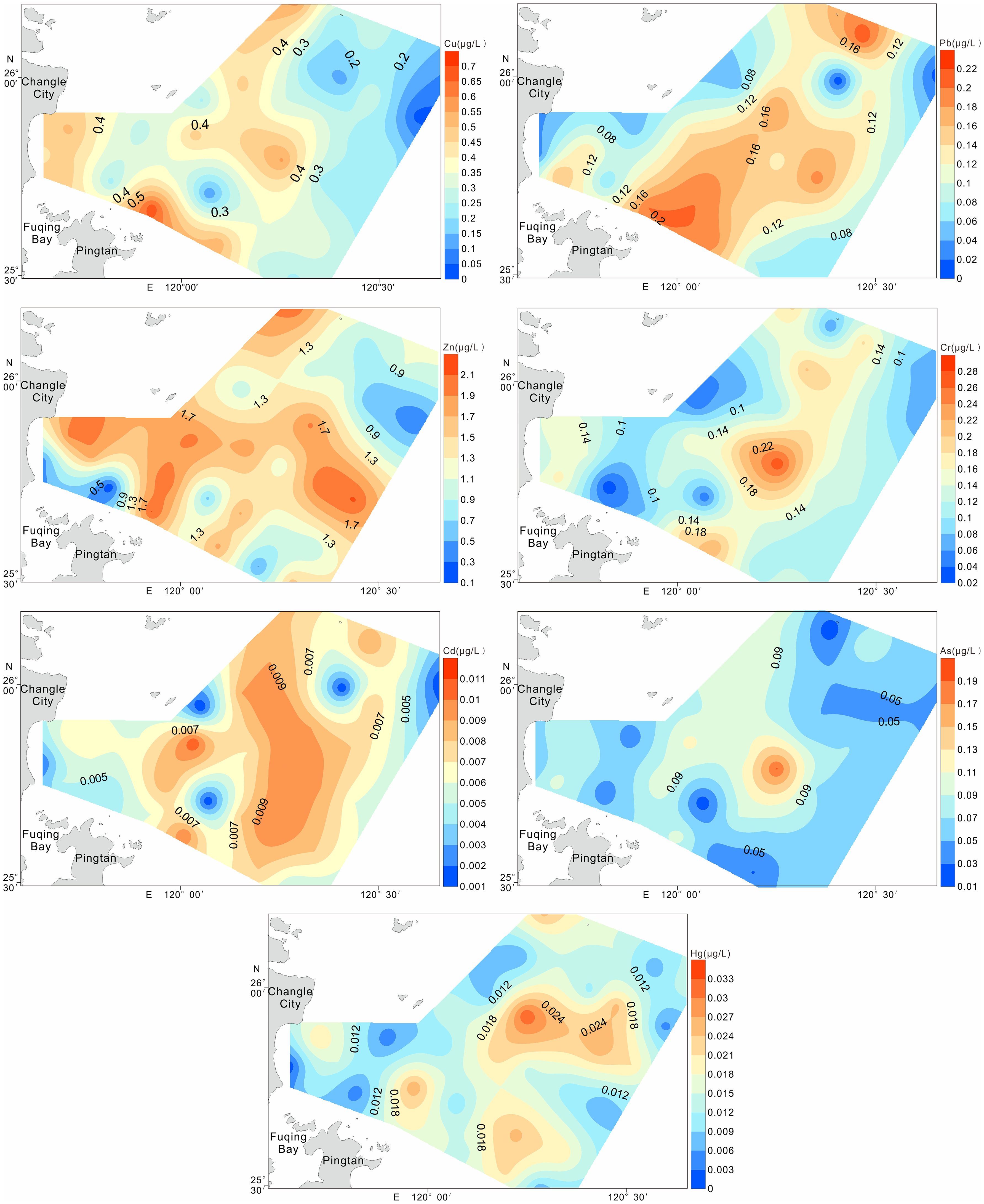
In the water column, dissolved metal concentrations (μg/L) ranged as follows: Cu (0.03–0.69), Pb (0.01–0.22), Zn (0.15–2.13), Cr (0.025–0.277), Cd (0.001–0.011), As (0.013–0.193), and Hg (0.001–0.033), with mean values of 0.33, 0.12, 1.31, 0.13, 0.01, 0.07, and 0.02 μg/L respectively. All measurements satisfied Class I seawater quality criteria (GB 3097–1997).
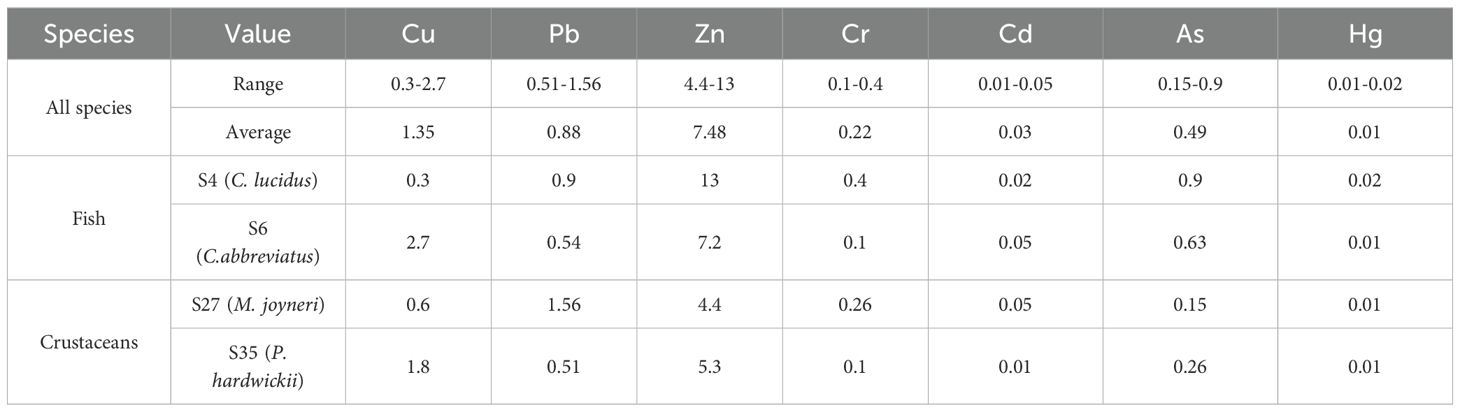
Biological samples exhibited species-specific metal accumulation patterns (μg/g wet weight): Cu (0.3–2.7), Pb (0.51–1.56), Zn (4.4–13), Cr (0.1–0.4), Cd (0.01–0.05), As (0.15–0.9), and Hg (0.01–0.02), with mean concentrations of 1.35, 0.88, 7.48, 0.22, 0.03, 0.49, and 0.01 respectively (Table 4). Maximum concentrations of Zn, Cr, As, and Hg were found in C. lucidus, whereas C. abbreviatus accumulated the highest Cu and Cd levels. M. joyneri showed the highest Pb and Cd concentrations in crustaceans, whereas P. hardwickii showed minimal accumulation of Pb, Cr, and Cd.
The spatial patterns of HMs in the surface sediments from northeastern Fuqing Bay are shown in Figure 2. High concentrations of Cu, Zn, Cd, and As were observed in the central region, in contrast to the lower levels of Pb, Cr, and Hg in the same area. Notably, higher accumulations of Zn, Cr, and As were detected near Changle City, likely attributable to anthropogenic influences.
Figure 3 illustrates the spatial variability of dissolved HMs in surface waters across the study area. The distributions of Pb, Zn, and Cd exhibited similar trends, with peak concentrations clustered in the central zone. Cu, Cr, As, and Hg also displayed elevated levels at mid-region stations, although their distributions were more spatially dispersed.
3.2 Assessment of sediment pollution using ecological risk indices
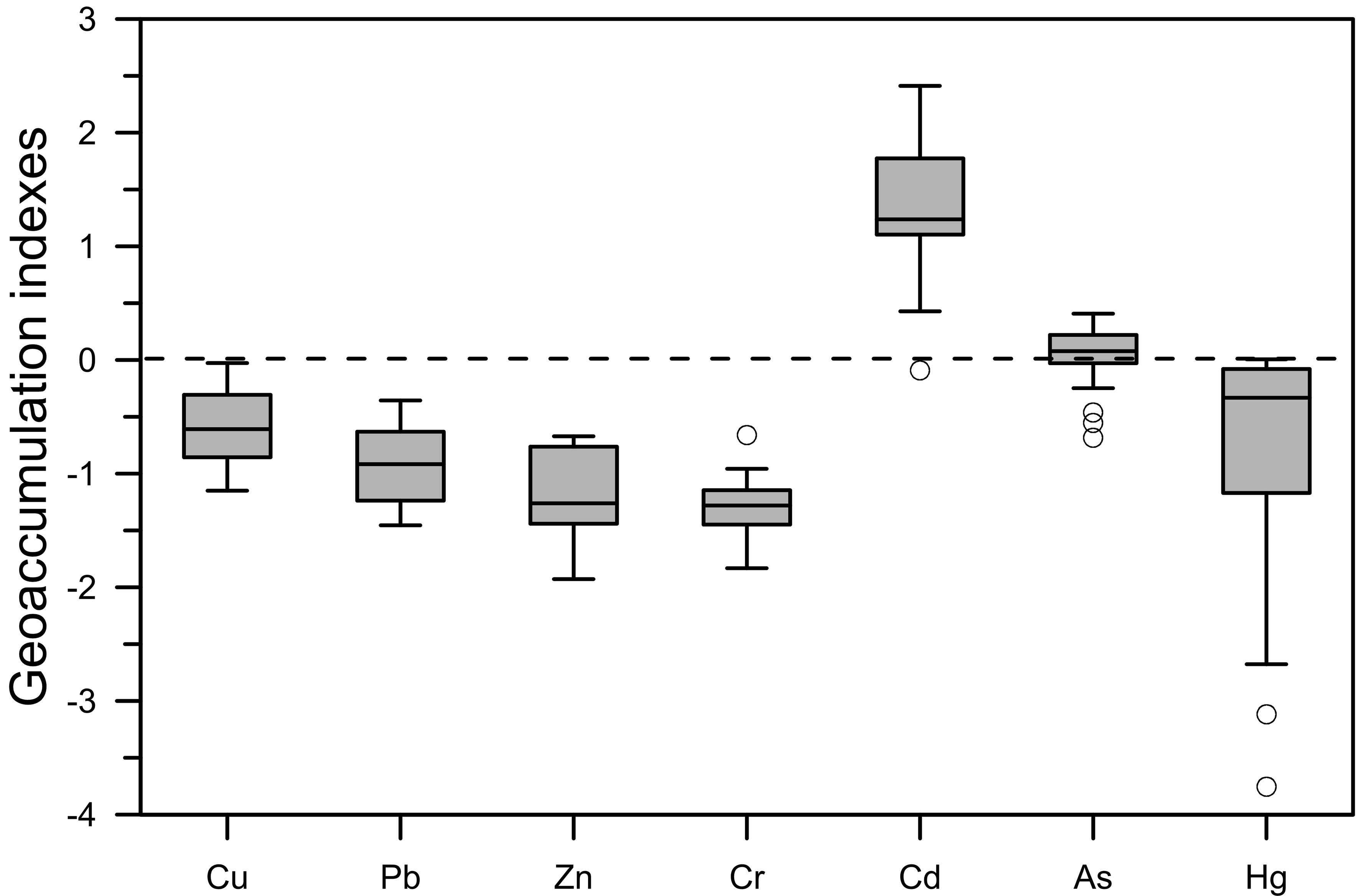
The mean and range of the Igeo values for the seven HMs in the sediments in northeast Fuqing Bay are presented in Table 5. The mean degree of contamination was the highest for Cd, followed by As, Cu, Hg, Pb, Zn, and Cr. The Igeo values of Cd, As, and Hg were greater than zero in 95.5%, 63.6%, and 4.5% of the samples, respectively (Figure 4). More than half of the sediments in the middle of the study area were slightly contaminated (0–1) by As. Evidently, the majority of the area was slightly to strongly contaminated (0–3) by Cd (Muller, 1981).
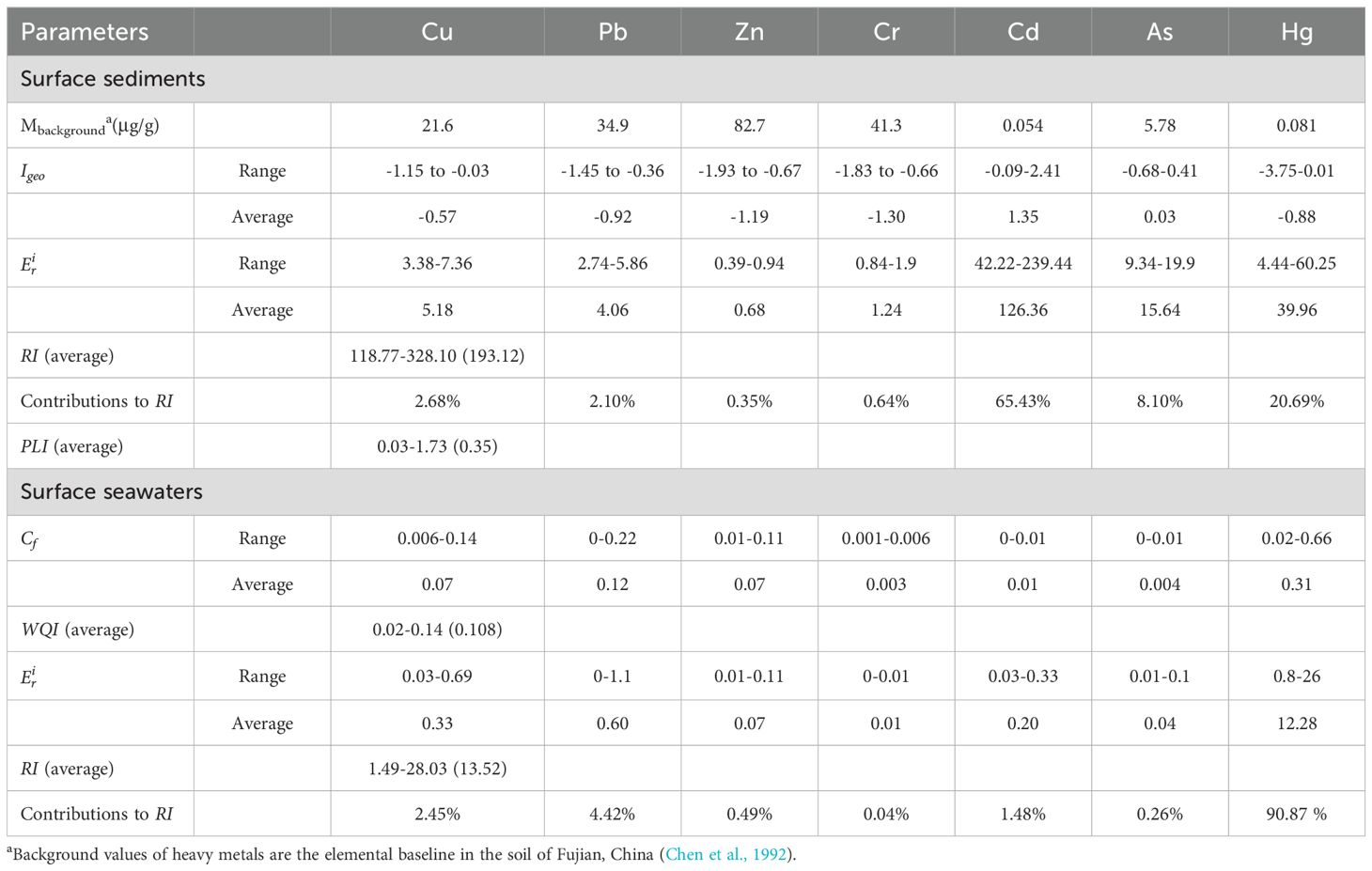
Table 5. Background value, Igeo, , RI, PLI values for surface sediments and Cf, WQI, RI values for surface seawaters of heavy metals in northeast Fuqing Bay.
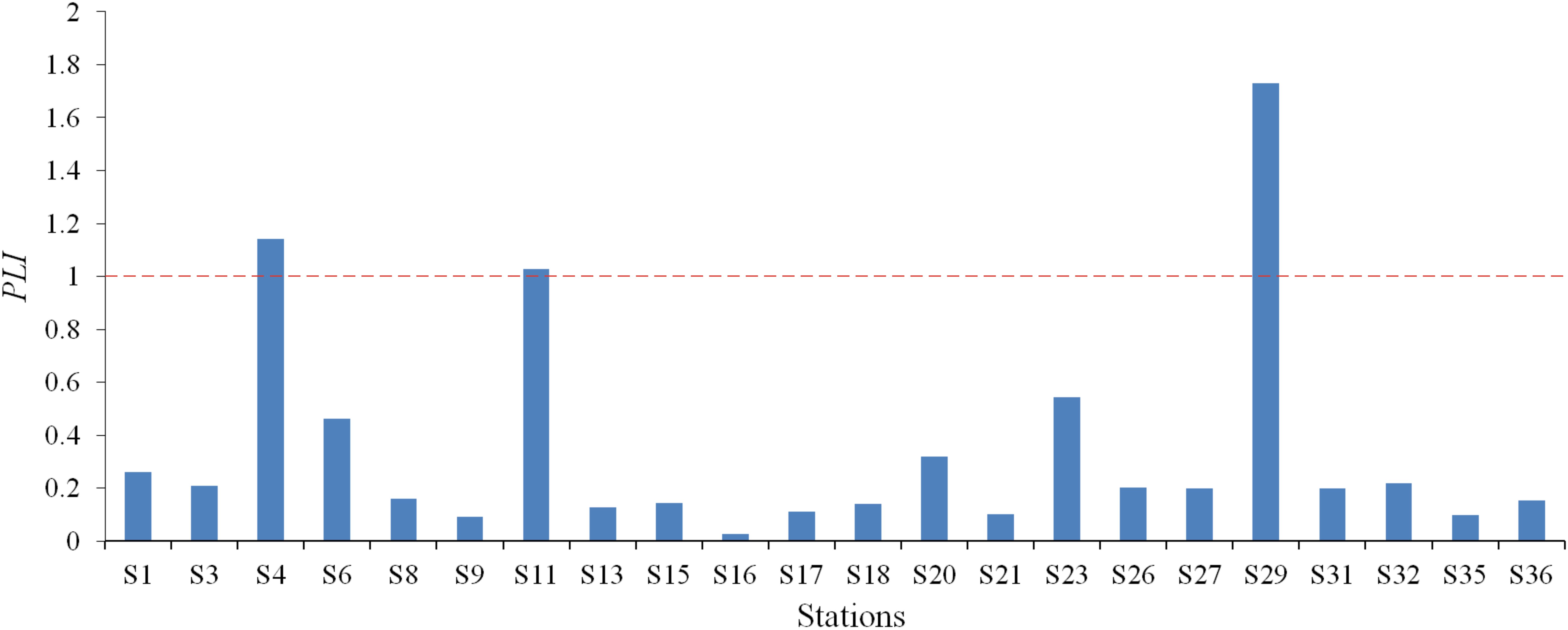
The PLI analysis revealed considerable spatial variation across the study area, with values ranging from 0.03 to 1.73 (mean = 0.35) at the 22 sampling stations (Table 5). Spatial mapping (Figure 5) identified three northern stations (S4, S11, and S29) with PLI values > 1, suggesting a significant anthropogenic influence on HM accumulation at these locations.
The ecological risk assessment demonstrated distinct patterns among the measured metals. The risk coefficients followed the order: Cd (highest) > Hg > As > Cu > Pb > Cr > Zn (lowest) (Table 5). While most metals exhibited low ecological risk (average < 40), Cd showed moderate–high risk potential ( range: 42.22-239.44). Hg is of particular concern, with 59.1% of the stations showing moderate ecological risk despite relatively low concentrations, reflecting its exceptionally high toxicity (Tam and Wong, 2000).
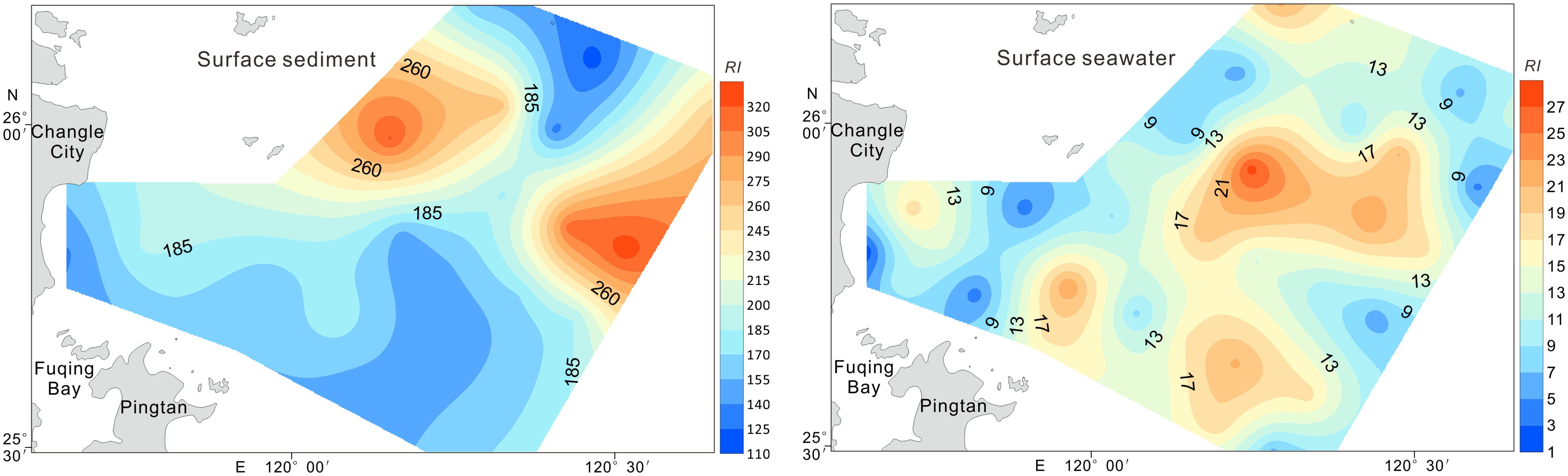
The RI values ranged from118.77 to 328.1 (mean = 193.12), indicating a moderate ecological risk across the study area (Table 5). Five stations in the eastern and western sectors exceeded RI value of 210, reaching the significant risk category (Figure 6). Source apportionment analysis identified Cd (65.43%) and Hg (20.69%) as the primary risk contributors (Table 5), highlighting their disproportionate ecological impact relative to their concentration levels (Di Bella et al., 2024). This phenomenon reflects the enhanced biological toxicity of these elements compared with that of the other measured metals. The RI values in Luoyuan Bay in northern eastern coast of Fujian Province ranged from 43.96 to 182.73, with mean level of 68 (Fan et al., 2022), which were lower than those in study area.
3.3 Assessment of HM contamination in surface waters
The Cf analysis revealed the following HM concentration ranges in the surface waters of northeastern Fuqing Bay: Cu (0.006–0.14), Pb (0–0.22), Zn (0.01–0.11), Cr (0.001–0.006), Cd (0–0.01), As (0–0.01), and Hg (0.02–0.66). The corresponding mean values were 0.07, 0.12, 0.07, 0.003, 0.01, 0.004, and 0.31, respectively (Table 5). WQI values across sampling stations varied between 0.02 and 0.14, consistently below the threshold value of 1. This demonstrated compliance with Class I seawater quality standards for all monitored HMs throughout the study period, reflecting good water quality conditions (Ramadan et al., 2021).
The ecological risk assessment results followed the order of single-element potential ecological risk coefficients as follows: Hg > Pd > Cu > Cd > Zn > As > Cr (Table 5). All individual risk coefficients remained below 40 at every sampling location, suggesting minimal ecological concern. The comprehensive potential ecological risk index (RI) values ranged from 1.49 to 28.03 (mean = 13.52, Table 5 and Figure 6), confirming an overall low ecological risk status in the study area (Küikrer and Mutlu, 2019).
3.4 Accumulation of HMs in organisms
Statistical analysis revealed distinct distribution patterns of HM concentrations in the two marine organism groups in northeastern Fuqing Bay. The descending order of metal concentrations in fish was Cr > Hg > Pb > Cu > Cd > Zn > As, with crustaceans also showing a similar pattern. Notably, elevated levels of Cr and Hg in both groups suggest potential environmental contamination. When evaluated against the biological quality criteria established in China’s Coastal Zone and Marine Resource Comprehensive Survey Guidelines, the standardized indices presented in Table 6 demonstrate that Cr concentrations exceeded the permissible limits at four sampling locations. Among the measured metals, Cr exhibited the highest standardized indices in both fish (6.74) and crustaceans (3.23). Other HMs remained within the acceptable thresholds according to the national standards. Cr, particularly in its hexavalent form (Cr(VI)), is highly toxic to aquatic organisms. Studies have indicated that chromium exposure can induce various adverse physiological effects in aquatic invertebrates and fish, including but not limited to: triggering oxidative stress, causing damage to tissues such as gills and hepatopancreas, inhibiting growth and development, and impairing immune and reproductive functions.
The bioaccumulation potential was assessed using two key indicators: BWAF and BSAF. Values exceeding unity for BSAF signify significant metal accumulation capacity, whereas BWAF > 1 indicates pronounced bioaccumulation in aqueous environments. As shown in Table 7, all BSAF measurements remained below 1, suggesting limited HM uptake from sedimentary sources (Dean et al., 2007). This finding contrasts with the aqueous bioaccumulation pattern, where despite generally low dissolved metal concentrations in seawater, most BWAF values exceeded 1 (excluding Cr at S35 and Hg at S4/S6). This demonstrates the efficient biological concentration of metals from the aqueous phase through trophic transfer, which is consistent with the findings of previous studies (Harada, 2016; Hoai et al., 2020; Islam and Tanaka, 2004). The observed differential accumulation patterns between the sedimentary and aqueous sources highlight the complex dynamics of metal transfer in marine ecosystems.
3.5 Source identification of HMs
To identify the potential HM sources in the environment, PCA was performed using SPSS software. For surface sediments, three principal components (PCs) with eigenvalues >1 were extracted, which collectively explained 77.94% of the total variance (Table 8), thereby capturing most of the variability in the dataset (Waykar and Petare, 2016).
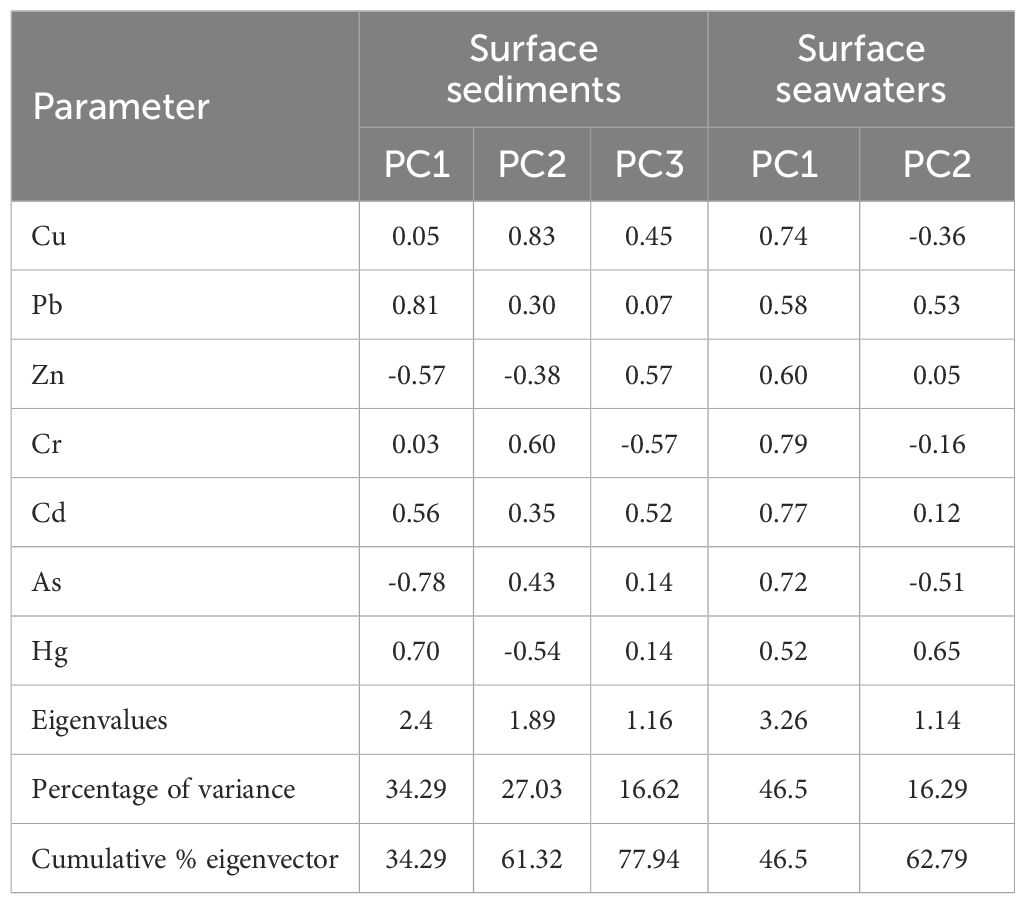
Table 8. Extracted three principal components in surface sediments and two principal components for surface seawater.
PC1 accounted for 34.29% of the variance and exhibited strong loadings for Pb (0.81), Cd (0.56), and Hg (0.70), thereby implying a shared origin for these elements. The spatial distribution of Cd indicates slight to moderate contamination (0–3) across most of the study area (Table 5 and Figure 6). Potential anthropogenic sources include industrial effluents from chemical plants that often discharge wastewater containing Cd and Hg into rivers and coastal waters. Additionally, port operations, machinery maintenance, and related industrial activities generate metal-laden dust and wastewater, and Pb is a notable contaminant in these maintenance processes.
PC2 explained 27.03% of the variance, and was dominated by Cu (0.83), Cr (0.60), and As (0.43). These metals may originate from the natural weathering of local geological formations, such as granite and basalt, which release Cu, Cr, and Zn into aquatic systems via surface runoff and atmospheric deposition. Furthermore, metal smelting activities in Fuqing contribute to Cu and Zn emissions from industrial wastewater and airborne particulates.
PC3 contributed 16.62% of the variance with notable loadings for Zn (0.57) and Cd (0.52). Agricultural runoff is another plausible source, as pesticides (containing As) and fertilizers (containing Zn) can be introduced into marine sediments via rainwater erosion and riverine transport.
For surface seawater in northeastern Fuqing Bay, two principal components were extracted, which explained 62.79% of the total variance (Table 8).
PC1 (46.5% variance) showed high positive loadings (>0.7) for Cu, Cr, Cd, and As, suggesting a natural origin because their concentrations remained below background levels and showed minimal anthropogenic influence. However, Pb, Zn, and Hg also exhibited moderate loadings on PC1, indicating partial contributions from these components.
PC2 (21.44% variance) was strongly associated with Pb (0.53) and Hg (0.65), likely reflecting anthropogenic input. Domestic wastewater, particularly that from urban and rural sewage systems, often contains Hg (from cosmetics, batteries, and electronic waste) and Pb (from industrial and household sources). Inadequate wastewater treatment may lead to the discharge of residual HMs into coastal waters, ultimately accumulating in sediments.
While PCA provides valuable insights into potential pollution sources, several limitations should be considered. Firstly, the results are dependent on the initial selection of variables and data normalization techniques. Secondly, the extracted factors require subjective interpretation based on loading patterns and ancillary knowledge of the study area, which introduces an element of expert judgment. Furthermore, PCA assumes linear relationships among variables and may not fully capture complex interactions or minor sources. Lastly, unlike absolute receptor models, PCA does not directly quantify the mass contribution from each identified source. Therefore, the findings presented here should be viewed as a preliminary identification of major source categories rather than an exact quantification of their contributions.
4 Conclusion
This study focused on the evaluation of surface sediments, seawater, and HMs in organisms in northeastern Fuqing Bay, Fujian Province. Results revealed that the concentrations of HMs in surface sediments and seawater in the area were consistent with the relevant standard classes. However, spatial variations in the distributions of certain HMs were also observed. In the sediments, Cd, As, and Hg were present in some samples, posing potential moderate ecological risk primarily due to Cd and Hg. The ecological risk posed by seawater was determined to be low, with fairly uncontaminated water quality. Regarding marine organisms, Cr levels exceeded the standard at some stations, and organisms exhibited a significant enrichment capacity for most HMs in seawater. PCA indicated wastewater discharge from chemical enterprises, port operations, rock weathering, metal smelting, and agricultural activities as the principal sources of HMs in the sediments. Notably, a proportion of the HMs detected in seawater has a natural origin, whereas others are attributable to anthropogenic activities, including urban and rural domestic sewage discharge. This study provides a fundamental foundation for research on regional ecological environments. To effectively mitigate the identified pollution, future efforts should translate these findings into concrete actions. For key anthropogenic sources identified by PCA, such as chemical industries and ports, we recommend implementing technology-based effluent standards that mandate the installation of advanced wastewater treatment systems (e.g., electrocoagulation, membrane filtration) and establishing a real-time online monitoring network for their discharges to ensure continuous compliance.
Regarding agricultural non-point source pollution, the promotion of Best Management Practices (BMPs) is essential, including precision farming to reduce pesticide and fertilizer use, the construction of riparian buffer zones to intercept runoff, and incentives for adopting organic alternatives.
Furthermore, policy makers should consider enacting stricter discharge limits for heavy metals, imposing stronger penalties for violations, and incorporating heavy metal pollution control metrics into the environmental performance assessments of local authorities. Continued monitoring, coupled with these targeted regulatory and management measures, will provide essential scientific support for the effective restoration and protection of the ecological environment in Fuqing Bay.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
EW: Conceptualization, Formal Analysis, Funding acquisition, Writing – original draft. YY: Investigation, Project administration, Writing – original draft. YS: Software, Supervision, Writing – original draft. JW: Resources, Visualization, Writing – original draft. XC: Methodology, Project administration, Writing – original draft. JY: Data curation, Formal Analysis, Writing – original draft. JQW: Investigation, Validation, Writing – original draft. CD: Data curation, Resources, Writing – original draft. JQ: Conceptualization, Formal Analysis, Methodology, Writing – original draft. ZW: Methodology, Resources, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
Author YY was employed by the company Fuzhou Haixia Electricity Generation Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Administration of quality supervision, inspection and quarantine of the People’s Republic of China (AQSIQ). (2002). Marine Sediment Quality of China (GB 18668-2002) (Beijing: Standards Press of China).
Avvari L., Basuri C., Chari H., Tirukkovalluri S., and Gollapalli N. (2022). Assessment of heavy metal distribution in seawaters of Kakinada Bay, a tropical mangrove-rich coastal environment. Mar. pollut. Bull. 181, 113877. doi: 10.1016/j.marpolbul.2022.113877
Baltas H., Sirin M., Dalgic G., Bayrak E. Y., and Akdeniz A. (2017). Assessment of metal concentrations (Cu, Zn, and Pb)in seawater, sediment and biota samples in the coastal area of Eastern black Sea, Turkey. Mar. pollut. Bull. 122, 475–482. doi: 10.1016/j.marpolbul.2017.06.059
Chakravarty M. and Patgiri A. D. (2009). Metal pollution assessment in sediments of the Dikrong River, NE India. J. Hum. Ecol. 27, 63–67. doi: 10.1080/09709274.2009.11906193
Chen Z., Chen C., Liu Y., Wu Y., Yang S., and Lu C. (1992). Study on soil environmental background value in Fujian Province. Environ. Sci. 13, 70–76. doi: 10.13227/j.hjkx.1992.04.020
Dean R. J., Shimmield T. M., and Black K. D. (2007). Copper, zinc and cadmium in marine cage fish farm sediments: an extensive survey. Environ. pollut. 145, 84–95. doi: 10.1016/j.envpol.2006.03.050
Di Bella G., El-Sorogy A. S., Giacobbe S., Nava V., Al-Kahtany K., and Nour H. E. (2024). Risk assessment of potentially toxic elements in intermittent rivers, “fiumara”, flowing in the gulf of milazzo (sicily, Italy). Environ. Earth Sci. 83, 321. doi: 10.1007/s12665-024-11631-0
Fan Y., Chen X., Chen Z., Zhou X., and Liu J. (2022). Pollution characteristics and source analysis of heavy metals in surface sediments of Luoyuan Bay, Fujian. Environ. Res. 203, 111911. doi: 10.1016/j.envres.2021.111911
Feng S., Li Y., Lin Z., Xie Y., Hong C., Lu X., et al. (2023). Spatial distribution and source analysis of heavy metals in the Haizhou Bay marine ranch. J. Hydroecol. 44, 114–122. doi: 10.15928/j.1674-3075.202108170282
Hakanson L. (1980). An ecological risk index for aquatic pollution control.a sedimentological approach. Water Res. 14, 975–1001. doi: 10.1016/0043-1354(80)90143-8
Harada Y. (2016). Determination of heavy metal concentrations in squid organs of the East China Sea and evaluation as environmental indicators. Tokyo. Ocean. Univ. 4, 25–43.
Hoai N., Thanh ‘T., Manh H., Thung D., and Johnstone R. (2020). An assessment of heavy metal contamination in the surface sediments of Ha Long Bay, Vietnam. Environ. Earth Sci. 79, 436. doi: 10.1007/s12665-020-09192-z
Islam M. S. and Tanaka M. (2004). Impacts of pollution on coastal and marine ecosystems including coastal and marine fisheries and approach for management: a review and synthesis. Mar. pollut. Bull. 48, 624–649. doi: 10.1016/j.marpolbul.2003.12.004
Küikrer S. and Mutlu E. (2019). Assessment of surface water quality using water quality index and multivariate statistical analyses in saraydüzŭ dam lake, Turkey. Environ. Monit. Assess. 191, 1–16.
Leung H., Cheung K., Chi K., Yung K., and Li W. (2021). An assessment of heavy metal contamination in the marine soil/sediment of Coles Bay area, svalbard, and greater bay area, China: A baseline survey from a rapidly developing bay. Environ. Sci. pollut. Res. 28, 22170–22178. doi: 10.1007/s11356-021-13489-2
Li Y., Chen J., Huang C., Wang A., and Li D. (2010). Distribution patterns of heavy metals in surface sediments of the Quanzhou Bay and environmental quality assessment. Environ. Sci. 31, 931–938. doi: 10.4028/www.scientific.net/AMM.482.287
Li J., Zhang Y., Gong X., Yao H., Wang X., Li J., et al. (2008). Pollution and assessment of heavy metals in surface sediments of Xinghua Bay, Fujian Province. Environ. Sci. Tech. 31, 125–128. doi: 10.3969/j.issn.1003-6504.2008.01.034
Lin X. (2012). Distribution and ecological risk assessment of heavy metals in surface sediments of Fuqing Bay. J. Fujian. Fish. 43, 214–219.
Liu C., Cao S., Li Y., Zhang Y., Li J. F., Li J., et al. (2025). Distribution and sources of heavy metals in bottom sediments of the Xiamen Bay, Fujian Province and its effects on ecological environment. Geol. China 52, 232–245. doi: 10.12029/gc20230214002
Luo D., Ruan J., Xu C., and Liao D. (2004). Concentration of the heavy metals and organic matter in surface sednents from the main shellfish culture areas of Fujian and their correlativity. Mar. Environ. Sci. 23, 33–36.
Lv L., Li Z., Dong S., Liu Y., and Lin T. (2019). Distribution and pollution assessment of heavy metals in surface sediments in the north of Meizhou Bay in 2010. Trans. Oceanol. Limnol. 3, 60–68. doi: 10.13984/j.cnki.cn37-1141
Macdonald D. D., Carr R. S., Calder F. D., Long E. R., and Ingersoll C. G. (1996). Development and evaluation of sediment quality guidelines for Florida coastal waters. Ecotoxicology 5, 253–278. doi: 10.1007/BF00118995
Madadi R., Mejjad N., and De-la-Torre G. E. (2023). Geochemical speciation, ecological risk, and source identification of heavy metal(loid)s in sediments and waters from Musa Estuary, Persian Gulf. Mar. pollut. Bull. 190, 114836. doi: 10.1016/j.marpolbul.2023.114836
Muller G. (1981). Die Schwermetallbelastung der sediment des Neckars und seiner Nebenflusse: eine Bestandsaufnahme. Chemiker. Zeitung. 105, 157–164.
Nour H. E., Helal S. A., and Wahab M. A. (2022). Contamination and health risk assessment of heavy metals in beach sediments of red sea and Gulf of Aqaba, Egypt. Mar. pollut. Bull. 177, 113517. doi: 10.1016/j.marpolbul.2022.113517
Qiu J., Yin P., Liu J., Cao K., and Wang S. (2019). Historical records of trace metals in core sediments from Jiangsu coastal are, China. Mar. pollut. Bull. 149, 110625. doi: 10.1016/j.marpolbul.2019.110625
Ramadan F., Nour H., Aita S., and Zahran H. (2021). Evaluation of heavy metals accumulation risks in water of the Qalubiya drain in East Delta, Egypt. Arab. J. Geosci. 14, 1750. doi: 10.1007/s12517-021-08198-6
Ruan J., Xu C., and Luo D. (2000). Assessment of heavy metals in sea water, surface sediments and fishery organisms at Xinghua Bya, Fujian Province. Trop. Oceanol. 19, 52–57.
Tam N. F. Y. and Wong Y. S. (2000). Spatial variation of heavy metals in surface sediments of Hong Kong mangrove swamps. Environ. pollut. 110, 195–205. doi: 10.1016/S0269-7491(99)00310-3
Tomlinson D. L., Wilson J. G., Harris C. R., and Jeffrey D. W. (1980). Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgol. Ermeeresun. Tersuchungen. 33, 566–575. doi: 10.1007/BF02414780
Waykar B. and Petare R. (2016). Studies on monitoring the heavy metal concentrations in the water, sediment and snail species in Latipada reservoir. J. Environ. Biol. 37, 585–589.
Xu Y., Gao H., Wei X., and Zhu J. (2021). Heavy metals and their ecological risk in the surface sediments of Laizhou Bay. Period. Ocean. Uni. China 51, 74–85. doi: 10.16441/j.cnki.hdxb.20200326
Zhang S., Fu K., Gao S., Liang B., and Fu G. (2023). Bioaccumulation of heavy metals in the water, sediment, and organisms from the sea ranching areas of Haizhou Bay in China. Water 15, 2218. doi: 10.3390/w15122218
Keywords: heavy metal pollution, Fuqing Bay, sediments, seawater, marine organisms, ecological risk assessment
Citation: Wang E, Yang Y, Sun Y, Wang J, Chen X, Yang J, Wang J, Dong C, Qiu J and Wang Z (2025) Comprehensive assessment of heavy metal pollution in northeast Fuqing Bay: integrating sediments, seawater, and marine organisms analysis with multivariate techniques. Front. Mar. Sci. 12:1684021. doi: 10.3389/fmars.2025.1684021
Received: 12 August 2025; Accepted: 18 September 2025;
Published: 01 October 2025.
Edited by:
Qi Wang, Shandong Jianzhu University, ChinaReviewed by:
Junliang Gao, Jiangsu University of Science and Technology, ChinaChao Chen, Suzhou University of Science and Technology, China
Copyright © 2025 Wang, Yang, Sun, Wang, Chen, Yang, Wang, Dong, Qiu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiandong Qiu, amlhbmRvbmdxaXVAMTYzLmNvbQ==; Zhao Wang, MTM1MzUwOTk1OEBxcS5jb20=
 Enkang Wang
Enkang Wang Yongchao Yang2
Yongchao Yang2 Yonggen Sun
Yonggen Sun Chao Dong
Chao Dong Jiandong Qiu
Jiandong Qiu Zhao Wang
Zhao Wang