- 1The Second Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu, China
- 2Shenzhen Eye Hospital, Shenzhen Eye Medical Center, Southern Medical University, Shenzhen, Guangdong, China
Myopia is a significant global health challenge, with the incidence of pathologic myopia (PM) on the rise. PM-related fundus diseases have become a leading cause of irreversible blindness. Early detection and treatment are crucial for the prevention and control of myopia. Recent advancements in artificial intelligence (AI), particularly in machine learning and deep learning algorithms, have shown promising results in the field of PM in ophthalmology. This review explores the latest developments in AI technology for managing PM, emphasizing its role in screening and diagnosis, grading and classification, and predictive assessment. AI has shown significant potential for clinical application in PM management, enhancing its intelligent, precise, and efficient practices.
1 Introduction
Myopia, a prevalent global condition, is characterized by focused light in front of the retina due to the eye's extended axial length (AL) or changes in the refractive medium (e.g., cornea and lens) or abnormal accommodation of the eye, leading to blurred vision (1). Myopia can progress to high myopia (HM) and pathologic myopia (PM). HM is defined as a significant lengthening of the AL of the eye with a refractive error usually at or above −6.00 D. Typically, the AL of the eye is more than 26.5 mm. In comparison, PM is defined as structural changes in the eye due to excessive elongation of AL, resulting in severe visual impairment and complications such as posterior scleral staphyloma, lacquer cracks, choroidal neovascularization, macular hemorrhage, and myopic maculopathy (MM). PM emerges with characteristic fundus lesions, indicating a more advanced stage compared to HM, which highlights a significantly higher disease-related risk (2). With advances in ophthalmic technology, significant breakthroughs have been achieved in the diagnosis and treatment of PM. Advanced imaging tools such as color fundus photography (CFP) and optical coherence tomography (OCT) have revealed new pathologic features such as leopard-shaped fundus and myopia-associated MM. At the same time, new treatments for these lesions and their complications have shown remarkable results. For example, anti-VEGF therapy has been shown to be effective in treating macular neovascularization triggered by PM, while vitreoretinal surgery has emerged as an effective means of managing traction macular detachment. Posterior scleral reinforcement has been proposed as a treatment option to address the problem of increasing axial growth, and its effectiveness and limitations have been clinically.
The prevalence of myopia, particularly in East and Southeast Asia, presents a major health challenge. Predictions suggest a significant rise in HM by 2050, with the prevalence of myopia in the world population from 22.9% to 49.8% (3–5). The widespread occurrence of myopia (30%−50%), especially PM (1%−3%), underlines the urgent need for effective management strategies to reduce its heavy societal impact (6). Early detection and regular monitoring are essential for managing PM, emphasizing the gap between growing diagnostic needs and limited medical resources, posing a significant public health challenge.
In the realm of managing such challenges, AI emerges as a pivotal tool, encompassing machine learning (ML) and deep learning (DL) which employ deep neural networks (DNNs) to solve complex problems. Convolutional neural networks (CNNs), a specific type of DNN, are adept at processing image data, making them particularly useful in ophthalmology. The capability of AI to analyze vast amounts of data offers unprecedented support in diagnosing, grading, and predicting ocular diseases (7). Its application in PM, through accurate analysis and interpretation of fundus images, marks a significant advancement in ophthalmology, potentially revolutionizing PM management (8–13). The China Alliance of Research in High Myopia (CHARM), a large-scale research project planned to cover more than 100,000 Chinese patients, aims to investigate the diagnosis, progression, and genetic factors of HM (14). AI applications for myopia have now been identified and synthesized by many researchers, but there is not a complete narrative of AI applications for PM. Complications of PM bring serious health hazards and economic burdens to patients and society, so it is urgent to fully recognize the application of AI in PM.
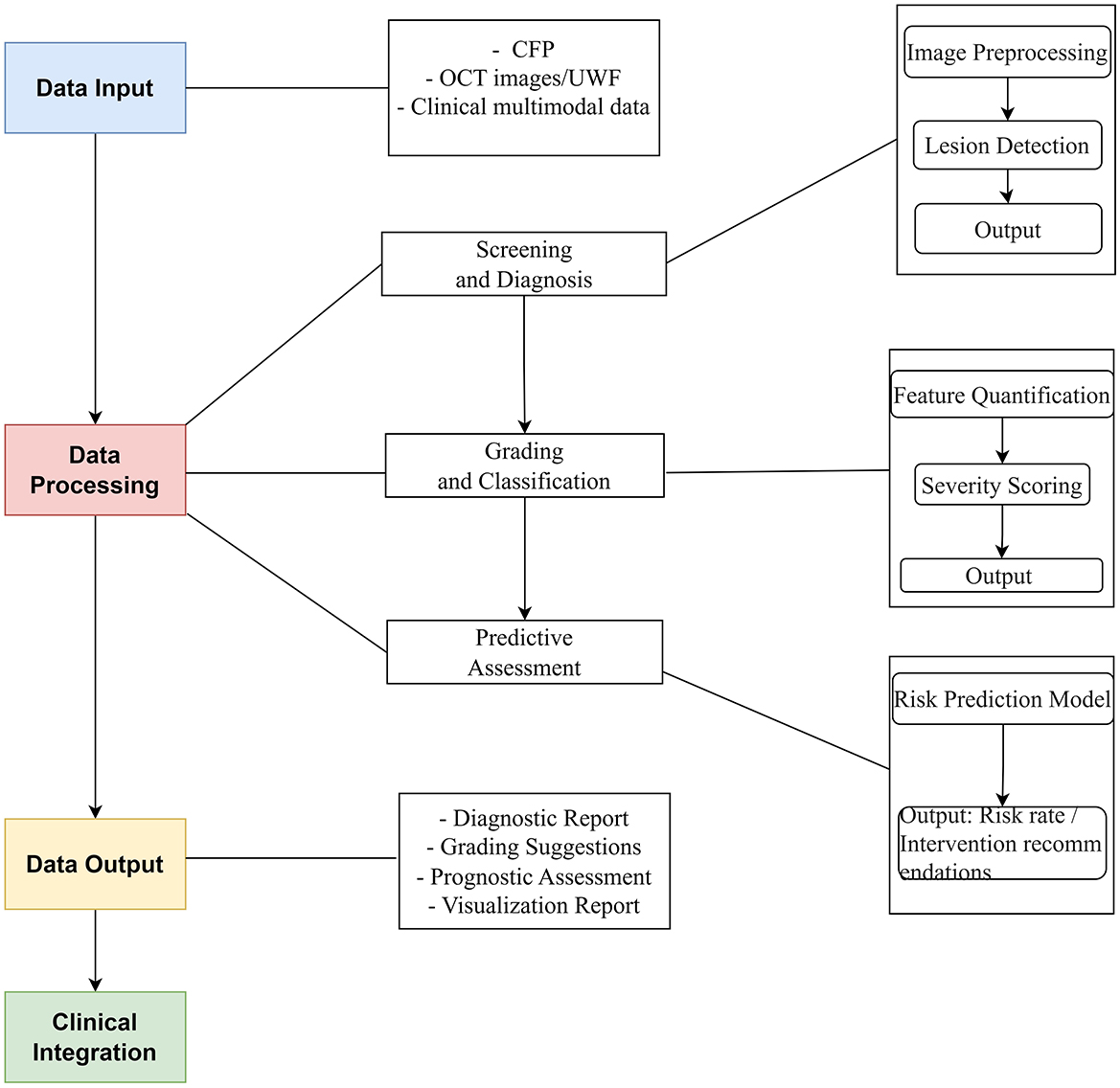
This review delves into the application of AI technology in PM, highlighting significant research breakthroughs and their value in clinical practice. AI is transforming PM management through a stepwise approach:
1. Screening and Diagnosis: AI-powered image analysis automates early detection of PM lesions, significantly improving diagnostic sensitivity compared to manual review.
2. Grading and Classification: Building upon diagnostic findings, AI further quantifies lesion features to objectively classify disease stages.
3. Predictive Assessment: By integrating longitudinal data and lesion characteristics, AI models generate personalized progression risks, enabling proactive interventions.
This pipeline (Figure 1)—from detection to stratification to prognosis—demonstrates AI's capability to address the full clinical spectrum of PM.
2 Retrieval methods
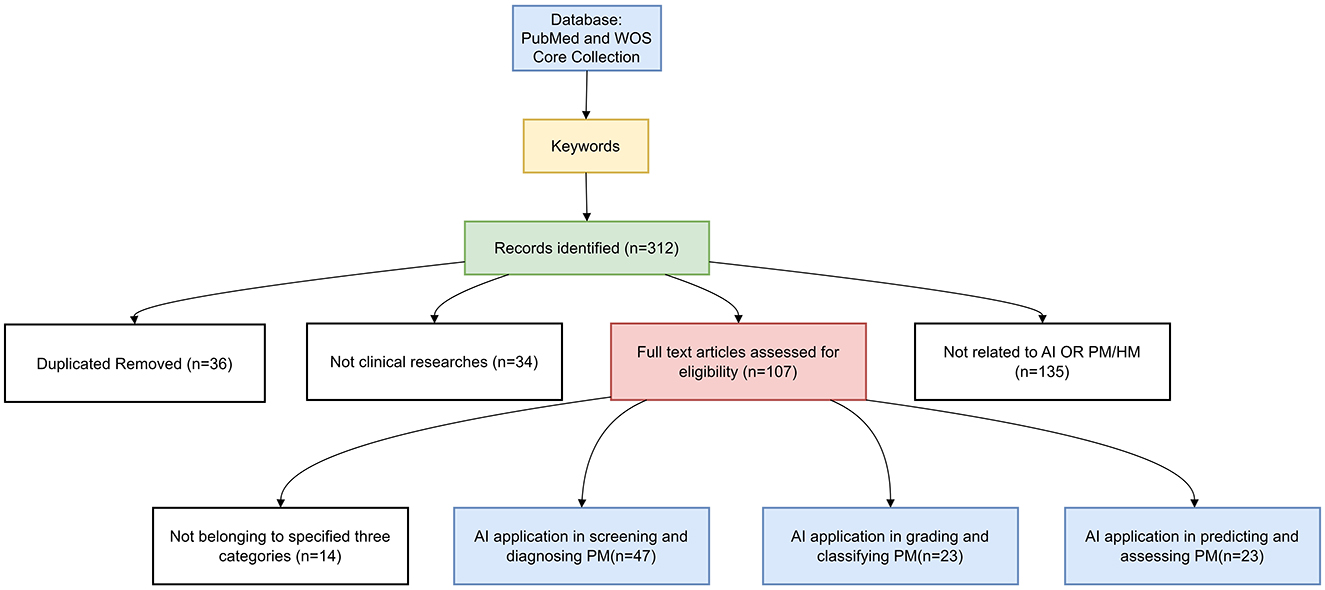
On 31 January 2025, we conducted a thorough search of the PubMed database and WOS Core Collection. We reviewed the latest research on AI in the field of PM. Our focus was on screening and diagnostic methods, classification and grading systems, and prediction models for disease progression. We used search terms including “artificial intelligence”, “machine learning”, “deep learning”, “ensemble learning”, “reinforcement learning”, “transfer network”, “neural network”, “supervised learning”, “computer vision system”, “computational intelligence”, “evolutionary computation”, “large language model”, “pathologic myopia”, “high myopia”, “myopic maculopathy”, and “progressive myopia”.
Inclusion criteria: The inclusion criteria were as follows:
1. AI technology application: Research studies that utilize AI technology for the diagnosis, grading, and prognostic evaluation of PM.
2. Detailed model development: Research studies that provide a comprehensive description of the AI model construction, including the training and validation processes.
Exclusion criteria: The exclusion criteria were as follows:
1. Non-human research studies: Research studies that involve animal models or in vitro experiments rather than human subjects.
2. Review, conference papers, and non-peer-reviewed articles: Research studies that are review articles, conference papers, or articles that have not undergone peer review.
3. Clinical research articles unrelated to AI or PM.
After collecting the relevant literature, we manually screened the titles and abstracts to match the research, and the screening process details are provided in Figure 2.
Analyzing the latest AI research findings in the field of PM and categorizing them based on their content, the application of AI in managing PM is prominently showcased in three primary aspects: screening and diagnosis, grading and classification, and predictive modeling for assessing PM progression.
3 Applications of AI in screening and diagnosis of PM
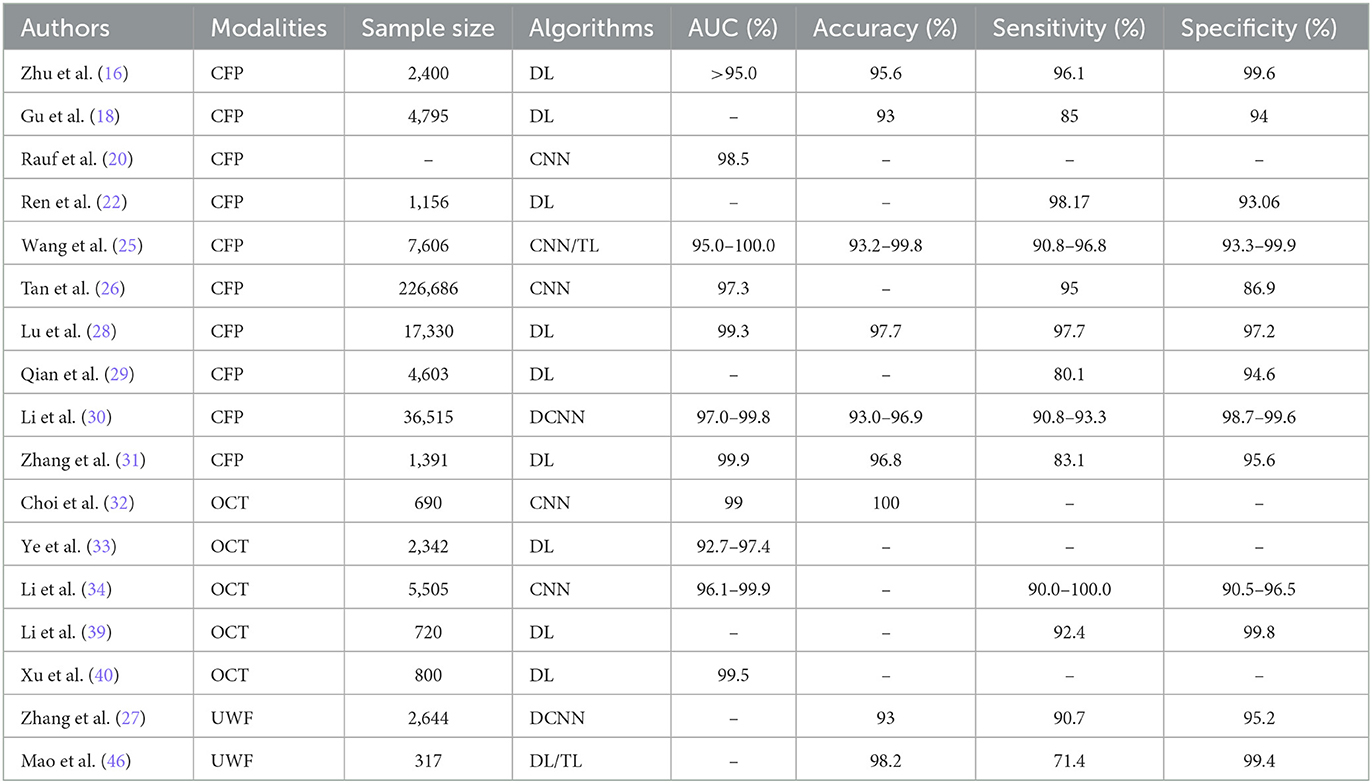
Early identification and accurate diagnosis of PM are crucial for the effective prevention and management of its complications (15). Table 1 lists AI applications in the diagnosis of PM (Literature not listed is not clinical research studies in multiple case centers and could not be included in the table).
3.1 AI screening and diagnosis of fundus images for PM
AI plays a pivotal role in enhancing the capabilities of primary care providers in detecting and managing PM by automating the screening process and facilitating timely referrals. This advancement is further supported by the development of imaging technologies ranging from CFP and OCT to ultra-widefield (UWF) imaging (16), which has dramatically improved the understanding of myopic fundus lesions (17). The target population for this task is at-risk individuals in the general population, especially adolescents and children, as they are at a critical period in the development of myopia.
3.1.1 Color fundus photography
Research initiatives have successfully used various AI models (e.g., EfficientNet) based on CFP to diagnose common retinal diseases such as PM, glaucoma, and diabetic retinopathy with high accuracy (18–21). The multi-disease diagnostic tool PADAr has been developed for primary eye screening, demonstrating the potential of AI to increase the rate of early diagnosis of PM in the primary care setting (22).
Developing an automatic, accurate, and non-invasive screening system for PM patients through AI can significantly conserve time and resources for both clinicians and patients. CNNs, specialized in processing lattice-structured data, have demonstrated superior performance in the automatic screening and diagnosis of PM. The pre-processed fundus photographs were feature extracted by the CNN model and categorized into normal fundus images and pathologic myopic fundus, achieving the best AUC of 0.9845 (23). However, the problem of overfitting cannot be avoided, and data augmentation mode was matched to the image dataset, achieving an accuracy increase of 2.85% (24). DL models, such as the PM-AI system (25) and MyopiaDETR (26), not only effectively detect the location of lesions but can also suggest the presence of myopic choroidal neovascularization (mCNV) (27–30). MM is a series of macular lesions associated with PM, characterized by macular atrophy, lacquer cracks, choroidal neovascularization, and posterior staphyloma. DL models show great potential in screening the diagnosis of MM (31–33). Furthermore, self-supervised learning is integrated with DL to make a more precise diagnosis (34).
3.1.2 Optical coherence tomography and ultra-widefield images
Fundus imaging presents significant challenges for PM. The evolution of modalities for ophthalmic imaging provides a solid foundation for entering the era of healthcare intelligence. Choi et al. (35) comparatively assessed the ability of three DL models to screen for HM using OCT imaging, with ResNet 50 performing the best. Innovations such as the application of models like Inception ResnetV2 and ResNeSt101 (36) for detecting fundus complications (retinoschisis, macular hole, retinal detachment, and mCNV) affirm the value of CNNs in achieving high diagnostic accuracy (37). Anatomically, Ota-Itadani et al. (38) used DL on 3D OCT imaging to detect and classify lamina cribrosa defects, assisting in early screening for PM.
The advent of UWF technology has also solved many of the challenges of PM imaging (30). UWF OCT improves the imaging of mCNV, the vitreoretinal interface, and the progression of myopic traction maculopathy (17).
These advancements underscore the transformative potential of AI in the management of PM, highlighting AI's role in refining diagnostic methodologies through enhanced image analysis and the integration of novel technologies.
3.2 AI applications of imaging markers and multimodal data analysis for PM
The applications of AI in employing imaging markers and bioinformation for the diagnosis of PM have shown significant advancements. Choroidal thickness and the degree of peripapillary atrophy (PPA) at the optic disc are recognized as effective indicators for assessing PM-related lesions (39–41).
Utilizing AI for precise segmentation and data collection on choroidal characteristics greatly aids in disease diagnosis. Recent research using DL algorithms has demonstrated a high degree of accuracy in distinguishing between the choroidal thickness measured automatically and manually, highlighting AI's capability in achieving detailed analysis with high agreement levels (42–44). Research studies utilizing mask region CNN models have automated the process of segmenting the choroidal region in OCT images of myopic patients, enabling the assessment of myopia severity based on choroidal thickness (45). Innovations such as the Boundary Enhancement Module and the utilization of parallel branches in AI architecture have aimed to improve segmentation accuracy by enhancing boundary features from varied perspectives (46). DL has also facilitated the automatic quantification of the vascular structure of the choroid, allowing the extraction of valuable metrics such as choroidal thickness, area, volume index, and vessel density through automated segmentation (47, 48), while thinner choroidal thicknesses tend to be associated with elongation of AL over 2 years (49). Furthermore, DL models have been employed successfully for noise reduction in OCT-scanned images, achieving the automated three-dimensional reconstruction and segmentation of intrachoroidal cavitation (50). AI applications extend to enhance the accuracy of segmenting high myopic choroid, with U-Net-based sequence models showing promising results (51). Although UWF imaging is primarily used to capture images of the peripheral retina, its 200° panoramic field of view and high resolution also make it possible to display details of the posterior pole clearly. The analysis of UWF imaging has revealed an association between the severity of mCNV and increased retinal blood vessel density and branching, highlighting AI's potential to provide foundational data for PM screening and diagnosis (52). Moreover, AI-measured lacquer crack extension is proved to be related to the progression of mCNV that a larger initial LC area or a faster rate of LC expansion may predict earlier CNV onset (53).
Advancements in AI have enhanced the segmentation accuracy of the optic disc and PPA (54). Measurement of vascular parameters and PPA area with a DL model confirms pathologic mechanisms in the development of PM (55). Through the integration of vision transformer and CNNs in model development, modern algorithms, based on UWF imaging, significantly outperform classical models in segmenting the optic disc and PPA (56). Its ability to handle complex backgrounds and fuzzy boundaries, multi-scale feature extraction, feature fusion, and boundary information processing enable transformers to more accurately segment PPA, fundamentally aiding ophthalmologists in diagnosing and managing conditions related to PM. However, incorporating transformers typically requires larger training datasets, so a lightweight image segmentation network named simple CNN-UNet is proposed for precise segmentation of the optic disc (57). In response to the limitations of retinal atrophy such as unclear boundaries and irregular shapes, ARA-Net-segmented retina has been proposed to detect retinal atrophy in various area sizes (58).
AI significantly enhances the diagnosis of PM by integrating more biological data alongside the direct screening and identification of distinctive fundus images. Zhang et al. (59) developed the PM-BMII, a computer-aided diagnostic framework that smartly merges diverse biomedical data from various sources, such as genetic, demographic, clinical information, and imaging data, improving the diagnostic accuracy of ~4.2–46.3% of PM. Furthermore, AI's capability to discern correlations between multiple potential factors and HM enriches our understanding, as evidenced by Zhang et al.'s (60) discovery of a significant link between increased serum vitamin A levels and HM prevalence. The role of AI extends beyond diagnostic advancement to enhancing healthcare service quality and the efficacy of medical interventions. A comparative research by Fang et al. (61) highlighted the superior educational effectiveness of an AI-driven PM auto-recognition system over traditional teaching methods in improving students' diagnostic accuracy and learning efficiency, showcasing the impact of AI in medical education.
The deployment of AI in the diagnosis of PM presents multiple advantages, such as convenience, speed, and medical resource conservation, alongside ensuring accuracy and enhancing management efficiency. Its automated screening and diagnostic capabilities play a pivotal role in early detection, raising awareness, and mitigating complications. This not only improves the ophthalmologist's ability to deliver timely and personalized interventions but also improves the overall patient experience through more accurate diagnostic results. Consequently, AI's contributions are vital in preserving visual health and alleviating societal burdens.
4 Applications of AI in grading and classification of PM
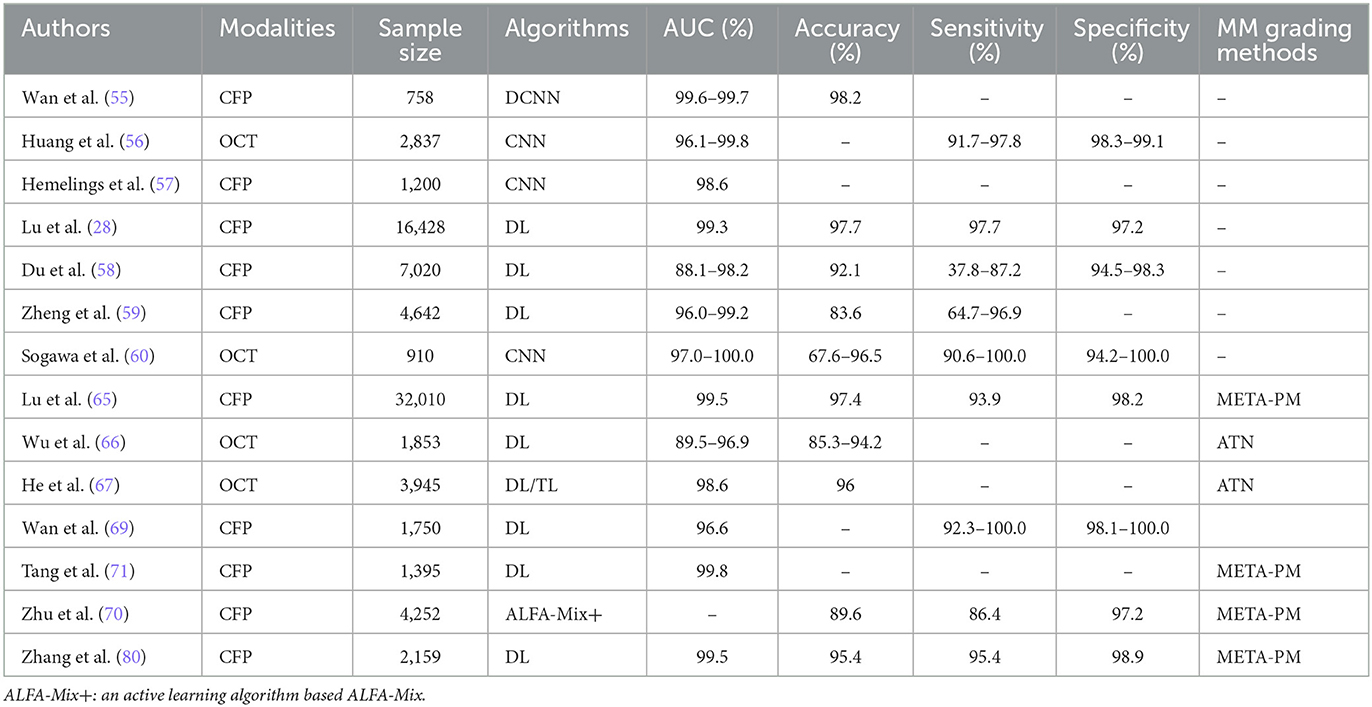
The application of AI in grading and classifying PM is indispensable for determining the severity of damage, the risk of complications, and its impact on visual acuity. This categorization is essential to develop specific treatments, ranging from monitoring the progression in mild cases to preventing complications in severe conditions. The target population for this task is patients who have been diagnosed with PM, which facilitates clinicians to regularly track the progress of PM, assess the effectiveness of treatment, and make timely adjustments to the treatment plan. Table 2 lists AI applications in the grading and classification of PM.
4.1 General advances in AI for grading and classification of PM
In general, many advances have been made by many researchers in AI for PM grading and classification. Wan et al. (62) have successfully utilized a DL model for intelligent risk grading of HM in fundus images. The model's ability to automatically classify fundus images into normal fundus, low-risk HM, and high-risk HM categories demonstrates its superiority over traditional assessments by ophthalmologists, with sensitivity and specificity close to 100%. In another research, Huang et al. (63) employed the ResNet-34 architecture to enhance the grading of traction retinopathy in highly myopic eyes across five categories, achieving significant accuracy in identifying varying severities of traction retinopathy. To optimize current automated diagnostic tools, Wang et al. (18) applied lightweight classification models such as MobileNetV3 and ShufflenetV2, which offer the benefits of shorter diagnosis times along with reduced storage and parameter requirements. Hemelings et al. (64) combined PM classification with lesion segmentation for PM complications using 2D fundus images and the transformer architecture, leading to a reduced false-positive rate for PM cases. Furthermore, the recent contributions of Lu (31) and Du et al. (65) in employing the International Classification System (META-PM) for PM identification and classification have been notable. While Du R's research is primarily aimed at predicting the risk of developing HM in children, Lu L's research focused more on identifying and classifying PM, with a particular emphasis on pathologic retinal changes.
4.2 Advances in AI for grading and classifying MM
Advances in AI have significantly improved the grading and classifying of MM, a major complication of PM that substantially affects vision quality. MM refers to characteristic degenerative changes in the macula, including chorioretinal atrophy, Fuchs spot, lacquer cracks, posterior staphyloma, and optic disc changes. A CNN model was designed for automatic extraction and quantification of features of the posterior scleral wall from OCT images, which were finally presented as 3D-MRI images (66). The classification of MM is crucial, given its implications on treatment and prognosis. Zheng et al. (67) utilized the EfficientNet model applied to fundus photographs of MM, achieving notable sensitivity and specificity. By exploiting SS-OCT imaging, Sogawa et al. (68) found that DL models could precisely diagnose MM, distinguishing it from non-degenerate conditions. In addition, Du et al. (69) investigated novel imaging biomarkers within the optic disc region of fundus images through ML and radiomics, uncovering features that effectively differentiate between varying degrees of MM severity. Tessellated fundus (TF), well-defined choroidal vessels at the posterior fundus pole, is associated with the development and progression of MM. The choroidal area per unit area exposed on fundus photographs can be used as an assessment metric for deep learning models, reflecting the severity of MM (70–73). Quantified FT was found to migrate toward the macula as myopia progressed by DL techniques, which has potential applications for predicting HM and its complications.
Currently, the META-PM and ATN (A—atrophy; T—traction; N—neovascularization) grading systems are the primary methods for MM assessment. Lu et al. (74) utilized these systems to train DL algorithms for grading retinal fundus images, achieving an accuracy comparable to that of expert evaluations. Based on the META-PM system, DL models were automatically extracted from fundus images of diffuse chorioretinal atrophy (DCA) regions from fundus images, which were quantitatively graded into four classes based on size, density, and ocular biology (75). It has been shown that the presence of DCA and its association with visual function impairment is linked to a reduction in choroidal perfusion and stromal composition (76). However, challenges remain, such as the algorithm's inability to detect posterior staphyloma or accurately diagnose “PLUS” lesions. Wu et al. (77) investigated myopic macular lesions using the ATN system, employing CNNs (ResNet) and MB-ASPP techniques for finer subgroup classifications. This DL approach demonstrated high accuracy, as reflected in the AUC values for A, T, and N classifications. He et al. (78) employed macular OCT images from the ATN system, developing two algorithms, where the latter, utilizing transfer learning, showed improved performance. A gated attention mechanism network (GAMNet) achieves 93.3% accuracy in classifying myopic tractional macular degeneration (79). Ye et al. (36), adopting the ResNet 501 architecture across five distinct models, achieved differentiation of various MM features such as macular choroidal atrophy and myopic tractional maculopathy among others. The ensemble model made by combining different deep learning models performs better on MM classification (80). Comparative research studies of AI models by Wan et al. (81), Zhu et al. (82), and Tang et al. (83) showcased advancements in MM detection and classification, employing techniques such as VOLO-D2 models and the DeepLabv3+ network for lesion segmentation and classification. On the basis of the above META-PM grading, Meng et al. (84) proposed to grade HM by combining the visual index of contrast sensitivity function (CSF) and fundus features, emphasizing the non-negligible role of functional indexes, which help to identify the hidden visual damage in early stage (84). These research studies have enhanced our understanding of myopic retinopathy onset signs and provided innovative approaches for monitoring myopia progression.
5 Applications of AI in predicting and assessing PM
The application of AI in predicting and assessing PM is essential for identifying individuals at the highest risk and accurately forecasting future trends early in the disease (Table 3). AI's capability in DL-based classification has demonstrated considerable potential in ophthalmic research for the early prediction and assessment of PM.
5.1 AI prediction for risk assessment of PM
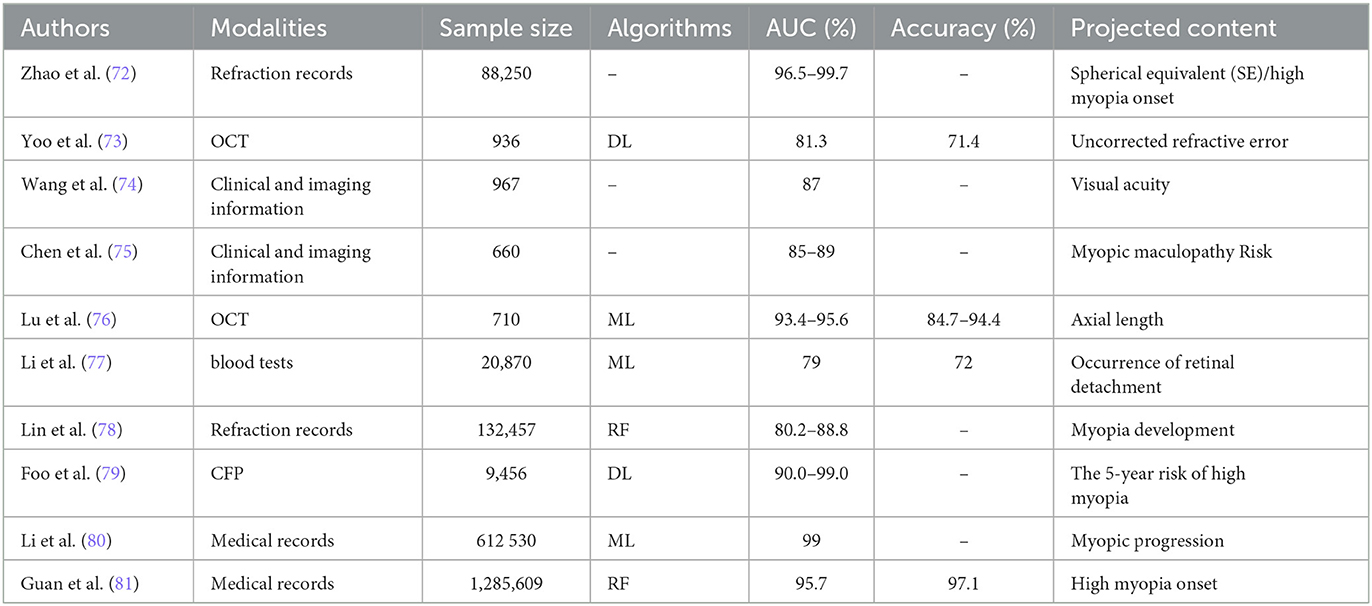
In the realm of risk assessment for PM, AI methods have been deployed to gauge prospective risk and predict the tendency toward progressive myopia in patients with HM. Zhao et al. (85) employed random forest and gradient boosting decision tree (GBDT) models to predict refraction and the likelihood of developing HM using a refractive data span of 15 years. This approach aids in the early identification and prevention of myopia. Yoo et al. (86) extracted structural features from the posterior segment of OCT images and utilized ML to predict uncorrected refraction, which reduces the risk of overlooking problems associated with PM or HM. Similarly, machine learning models for predicting long-term visual acuity in patients with high myopia have also achieved good results using fundus imaging information (87). In addition, machine learning based on SS-OCT can make predictions about the progression of MM after 10 years based on relevant predictors such as longer AL or thinner choroidal thickness (88). Although AL and choroidal thickness are important predictors of PM, they have no discriminative ability sufficient for prediction. Kim et al. (89) utilized a support vector machine (SVM) to accurately measure the relative tomographic elevation of posterior sclera on OCT images, increasing accuracy by 10% compared to that which only measured AL and choroidal thickness. Lu et al. (90) developed classifiers to predict ocular AL using OCT scanning, while Li et al. (91) continuously monitor and predict retinal detachment in patients with HM using the conditional probability algorithm based on blood parameters designed, with an AUC of ~0.96.AI prediction for myopia risk in visually developing children.
Regarding the assessment of myopia risk in visually developing children, it is crucial to predict refractive changes during this critical period to manage the prevalence of HM and PM adequately. Multiple machine learning algorithms were constructed to confirm the correlation between BMI and HM with an AUC of 85%, with implications for the etiologic progression of HM (92). Lin et al. (93) used random forest on large datasets, alongside factors such as age and spherical lenses, to forecast HM incidence over 8 years. Foo et al. (94) combined DenseNet-121, a deep neural network model, with random forest to evaluate fundus images and clinical data, assessing the likelihood of children developing HM within 5 years. Li et al. (95) aggregated data from various cohorts to predict myopia progression and the risk of HM using ML, achieving an AUC of 0.99. Guan et al. (96) explored risk factors for HM through ML, with random forest showing notable accuracy. Clark et al. (97) introduced “polygenic scoring” (PGS) to predict myopia in children by assessing genetic susceptibility through DL and examining refractive error across different European populations. Ota-Itadani et al. and Liu et al. have further illustrated the potential of AI in PM research, focusing on structural abnormalities in PM patients. Liu et al. (98) and Kang et al. (99) observed optic disc and peripapillary atrophy (PPA) changes related to refractive errors in school-aged children, suggesting the PPA to optic disc area ratio as an early indicator of myopia progression. Tiny retinal vascular changes in children with HM can also be recognized by AI, such as a decrease in arterial branching angle and vascular arteriovenous ratio, which can help identify trends in HM earlier (100, 101).
5.2 AI prediction for treatment outcomes in PM
AI has demonstrated its potential to effectively predict treatment outcomes and efficacy in patients with PM. For instance, Zhou et al. (102) applied AI algorithms to calculate lens power during intraocular lens (IOL) implantation in highly myopic eyes, achieving high prediction accuracy. Notably, AI models such as XGBoost, Hill-RBF, and Kane excelled in cases with an AL exceeding 26 mm. In another research, Guo et al. (103) utilized extreme gradient boosting and support vector regression algorithms to formulate the Zhu-Lu formula, a novel ML approach for predicting post-surgical lens dioptric power in highly myopic patients. Wei et al. (104) developed and compared five ML algorithms based on OCT scans, all of which showed high accuracy and stability in predicting the optimal corrected visual acuity after cataract surgery in highly myopic patients. Yang et al. (105) and Kang et al. utilized a deep neural network to predict visual acuity in patients with PM and mCNV treated with anti-VEGF, achieving an accuracy rate of over 80%. Sawai et al. (106) investigated the impact of the denoising process on the prediction of PM using single-frame optical coherence tomography angiography (OCTA) imaging, highlighting its role in providing fast and high-quality image analysis. Despite these advancements, the AI-based prediction of PM progression remains in its early stages. To enhance the management of PM and improve patient outcomes, it is crucial to increase scientific research investment, foster interdisciplinary collaboration, and conduct more rigorous clinical trials.
6 Limitations of AI in PM
While AI technology has made significant strides in various fields, its application in the clinical research and treatment of PM faces notable challenges. One such challenge is the poor quality of retinal images, such as artifacts caused by eye movements during imaging, which impede the accurate identification and diagnosis of ocular microscopic lesions. To address this, Cheng et al. (107) proposed the structure-preserving guided retinal image filtering (SGRIF) method, which enhances image quality by simulating the natural attenuation and scattering of the human eye lens, thereby improving contrast and aiding in the automated diagnosis of ophthalmic diseases such as optic disc analysis. Improving image quality in clinical work is the basis for AI in ophthalmology clinical work.
In addition, several overarching issues persist in AI research and application for PM. First, the issue of data heterogeneity deficits and model generalization bottlenecks represents the foundational barrier to advancing AI applications in PM research studies. Research studies have predominantly utilized single datasets, which may lack the size and diversity to represent the global spectrum of PM, thus limiting the models' generalizability. In classification tasks, the limited availability of imbalanced lesion distribution (e.g., “plus” lesions) in the META-PM classification of MM can result in overfitting. Second, lack of algorithm interpretability, particularly in tasks such as lesion localization (e.g., posterior staphyloma) and progression prediction, directly undermines clinicians' trust in AI outputs and their integration into practice. Third, deficiencies in clinical validation frameworks affect the spread and the use of AI for PM in clinical management. Of the included research studies above, overreliance on retrospective single-center data with limited real-world prospective validation limited prospective real-world validation research studies. The impact of variations in imaging equipment and quality remains understudied, despite its critical importance—especially given the heterogeneous image quality in primary care settings (e.g., county/township hospitals), which may limit the generalizability of AI models. Moreover, there is a need for a more in-depth exploration of model parameter tuning to enhance the accuracy of screening, grading, and prediction. While most algorithms struggle with detecting or localizing complications such as posterior staphyloma, some have shown promise in segmenting retinal lesion regions to improve detection rates. Last but not the least, the application of AI in PM and HM is still in the experimental stage and has not been widely put into clinical use, but some of the research studies mentioned above compared the accuracy of AI with the work of ophthalmic professionals, showing AI was better than or equal to the level of experts. Therefore, the development of AI and the advancement of its use in PM management is a major trend.
7 Conclusion
This review provides a comprehensive review of the recent advancements in using AI technologies to manage PM. It highlights the significant contributions of AI in improving screening and diagnostic processes, as well as in grading, classification, and predictive assessment. Integrating AI has notably enhanced the accuracy and efficiency of PM diagnosis and its complications, surpassing traditional methods. AI enables the segmentation and extraction of retinal lesions, which helps in understanding disease severity and predicting disease progression, crucial for its management. In summary, AI is revolutionizing PM clinical management by enhancing its intelligence, precision, and efficiency.
In summary, AI, through the application of DL and ML, has been effectively utilized in the domain of PM. Despite its demonstrated potential, several challenges remain. These include the need to address the limitations in processing low-quality images, refining disease progression models, and fostering interdisciplinary collaboration to advance research and application.
Looking ahead, the value of AI in the management of PM can be further enhanced by focusing on the following areas: (1) The development of more extensive and diverse multicenter PM datasets that include demographic parameters such as age, gender, and occupation, and increase the representation of rare findings such as “plus lesions”. (2) Researchers should concentrate on enhancing the image processing capabilities of AI models, particularly in improving clarity and noise reduction, while also considering the constraints of equipment in primary healthcare settings during clinical applications. (3) Although AI is not yet fully mature for predicting PM, future research can integrate predictive models with intervention strategies. For example, researchers can provide clinical interventions to patients at different risks and compare them with preclinical patients without interventions to see whether there is any difference in the patients' prognosis. By training AI models with substantial datasets and diverse cases, researchers can develop personalized prevention and treatment recommendations for PM patients based on their risk profiles.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
XH: Data curation, Writing – original draft. YW: Writing – review & editing, Data curation. XZ: Writing – review & editing, Conceptualization. WC: Writing – review & editing, Conceptualization, Funding acquisition, Methodology. WY: Writing – review & editing, Conceptualization, Funding acquisition, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Guangdong Basic and Applied Basic Research Foundation (2025A1515011627).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AUC, area under the curve; PM, pathologic myopia; HM, high myopia; MM, myopic maculopathy; PPA, peripapillary atrophy; AL, axial length; DCA, diffuse chorioretinal atrophy; mCNV, myopic choroidal neovascularization; CPF, color fundus photograph(y); OCT, optical coherence tomography; UWF, ultra-widefield; AI, artificial intelligence; DL, deep learning; ML, machine learning; CNN, convolutional neural network.
References
1. Ohno-Matsui K, Wu PC, Yamashiro K, Vutipongsatorn K, Fang Y, Cheung CMG, et al. IMI Pathologic Myopia. Invest Ophthalmol Vis Sci. (2021) 62:5. doi: 10.1167/iovs.62.5.5
2. Morgan IG, He M, Rose KA. Epidemic of pathologic myopia. What can laboratory studies and epidemiology tell us? Retina. (2017) 37:989–97. doi: 10.1097/IAE.0000000000001272
3. Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. (2016) 123:1036–42. doi: 10.1016/j.ophtha.2016.01.006
4. Morgan IG, French AN, Ashby RS, Guo X, Ding X, He M, et al. The epidemics of myopia: aetiology and prevention. Prog Retin Eye Res. (2018) 62:134–49. doi: 10.1016/j.preteyeres.2017.09.004
5. Wong TY, Ferreira A, Hughes R, Carter G, Mitchell P. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am J Ophthalmol. (2014) 157:9–25.e12. doi: 10.1016/j.ajo.2013.08.010
6. Liu H, Li R, Zhang Y, Zhang K, Yusufu M, Liu Y, et al. Economic evaluation of combined population-based screening for multiple blindness-causing eye diseases in China: a cost-effectiveness analysis. Lancet Global Health. (2023) 11:e456–e65. doi: 10.1016/S2214-109X(22)00554-X
7. Yang WH, Shao Y, Xu YW. Guidelines on clinical research evaluation of artificial intelligence in ophthalmology (2023). Int J Ophthalmol. (2023) 16:1361–72. doi: 10.18240/ijo.2023.09.02
8. Ting DSW, Pasquale LR, Peng L, Campbell JP, Lee AY, Raman R, et al. Artificial intelligence and deep learning in ophthalmology. Br J Ophthalmol. (2019) 103:167–75. doi: 10.1136/bjophthalmol-2018-313173
9. Li Y, Yip MYT, Ting DSW, Ang M. Artificial intelligence and digital solutions for myopia. Taiwan J Ophthalmol. (2023) 13:142–50. doi: 10.4103/tjo.TJO-D-23-00032
10. Hood DC, La Bruna S, Tsamis E, Thakoor KA, Rai A, Leshno A, et al. Detecting glaucoma with only OCT: Implications for the clinic, research, screening, and AI development. Prog Retin Eye Res. (2022) 90:101052. doi: 10.1016/j.preteyeres.2022.101052
11. Ting DSJ, Foo VH, Yang LWY, Sia JT, Ang M, Lin H, et al. Artificial intelligence for anterior segment diseases: emerging applications in ophthalmology. Br J Ophthalmol. (2021) 105:158–68. doi: 10.1136/bjophthalmol-2019-315651
12. Tan TE, Wong TY. Diabetic retinopathy: looking forward to 2030. Front Endocrinol. (2022) 13:1077669. doi: 10.3389/fendo.2022.1077669
13. Peng Z, Ma R, Zhang Y, Yan M, Lu J, Cheng Q, et al. Development and evaluation of multimodal AI for diagnosis and triage of ophthalmic diseases using ChatGPT and anterior segment images: protocol for a two-stage cross-sectional study. Front Artif Intell. (2023) 6:1323924. doi: 10.3389/frai.2023.1323924
14. He HL, Liu YX, Song H, Xu TZ, Wong TY, Jin ZB. Initiation of China Alliance of Research in High Myopia (CHARM): protocol for an AI-based multimodal high myopia research biobank. BMJ Open. (2023) 13:e076418. doi: 10.1136/bmjopen-2023-076418
15. Cho BJ, Shin JY Yu HG. Complications of pathologic myopia. Eye Contact Lens. (2016) 42:9–15. doi: 10.1097/ICL.0000000000000223
16. Sun G, Wang X, Xu L, Li C, Wang W, Yi Z, et al. Deep learning for the detection of multiple fundus diseases using ultra-widefield images. Ophthalmol Ther. (2023) 12:895–907. doi: 10.1007/s40123-022-00627-3
17. Ludwig CA, Moon J, Garg I, Miller JB. Ultra-Widefield Imaging for Evaluation of the Myopic Eye. Semin Ophthalmol. (2021) 36:185–90. doi: 10.1080/08820538.2021.1887904
18. Wang JD, Liu MR, Liu ML, Zhang R, Chen CX, Cao K. An auxiliary diagnostic tool for common fundus diseases based on fundus color photography and light-weight classification models. Graefe's Arch Clin Exp Ophthalmol. (2024) 262:223–9. doi: 10.1007/s00417-023-06182-2
19. Zhu S, Lu B, Wang C, Wu M, Zheng B, Jiang Q, et al. Screening of common retinal diseases using six-category models based on EfficientNet. Front Med. (2022) 9:808402. doi: 10.3389/fmed.2022.808402
20. Cao S, Zhang R, Jiang A, Kuerban M, Wumaier A, Wu J, et al. Application effect of an artificial intelligence-based fundus screening system: evaluation in a clinical setting and population screening. Biomed Eng Online. (2023) 22:38. doi: 10.1186/s12938-023-01097-9
21. Gu C, Wang Y, Jiang Y, Xu F, Wang S, Liu R, et al. Application of artificial intelligence system for screening multiple fundus diseases in Chinese primary healthcare settings: a real-world, multicentre and cross-sectional study of 4795 cases. Br J Ophthalmol. (2024) 108:424–31. doi: 10.1136/bjo-2022-322940
22. Lin PK, Chiu YH, Huang CJ, Wang CY, Pan ML, Wang DW, et al. PADAr: physician-oriented artificial intelligence-facilitating diagnosis aid for retinal diseases. J Med Imag. (2022) 9:044501. doi: 10.1117/1.JMI.9.4.044501
23. Rauf N, Gilani SO, Waris A. Automatic detection of pathological myopia using machine learning. Sci Rep. (2021) 11:16570. doi: 10.1038/s41598-021-95205-1
24. Cui J, Zhang X, Xiong F, Chen CL. Pathological myopia image recognition strategy based on data augmentation and model fusion. J Healthc Eng. (2021) 2021:5549779. doi: 10.1155/2021/5549779
25. Ren PF, Tang XY Yu CY, Zhu LL, Yang WH, Shen Y. Evaluation of a novel deep learning based screening system for pathologic myopia. Int J Ophthalmol. (2023) 16:1417–23. doi: 10.18240/ijo.2023.09.07
26. Li M, Liu S, Wang Z, Li X, Yan Z, Zhu R, et al. MyopiaDETR: End-to-end pathological myopia detection based on transformer using 2D fundus images. Front Neurosci. (2023) 17:1130609. doi: 10.3389/fnins.2023.1130609
27. Fang H, Li F, Wu J, Fu H, Sun X, Orlando JI, et al. Open fundus photograph dataset with pathologic myopia recognition and anatomical structure annotation. Scientific data. (2024) 11:99. doi: 10.1038/s41597-024-02911-2
28. Wang R, He J, Chen Q, Ye L, Sun D, Yin L, et al. Efficacy of a deep learning system for screening myopic maculopathy based on color fundus photographs. Ophthalmol Ther. (2023) 12:469–84. doi: 10.1007/s40123-022-00621-9
29. Tan TE, Anees A, Chen C, Li S, Xu X, Li Z, et al. Retinal photograph-based deep learning algorithms for myopia and a blockchain platform to facilitate artificial intelligence medical research: a retrospective multicohort study. Lancet Digital Health. (2021) 3:e317–e29. doi: 10.1016/S2589-7500(21)00055-8
30. Zhang W, Zhao X, Chen Y, Zhong J, Yi Z. DeepUWF: an automated ultra-wide-field fundus screening system via deep learning. IEEE J Biomed Health Inform. (2021) 25:2988–96. doi: 10.1109/JBHI.2020.3046771
31. Lu L, Zhou E, Yu W, Chen B, Ren P, Lu Q, et al. Development of deep learning-based detecting systems for pathologic myopia using retinal fundus images. Commun Biol. (2021) 4:1225. doi: 10.1038/s42003-021-02758-y
32. Qian B, Sheng B, Chen H, Wang X, Li T, Jin Y, et al. A competition for the diagnosis of myopic maculopathy by artificial intelligence algorithms. JAMA Ophthalmol. (2024). doi: 10.1001/jamaophthalmol.2024.3707
33. Li J, Wang L, Gao Y, Liang Q, Chen L, Sun X, et al. Automated detection of myopic maculopathy from color fundus photographs using deep convolutional neural networks. Eye Vision. (2022) 9:13. doi: 10.1186/s40662-022-00285-3
34. Zhang J, Xiao F, Zou H, Feng R, He J. Self-supervised learning-enhanced deep learning method for identifying myopic maculopathy in high myopia patients. iScience. (2024) 27:110566. doi: 10.1016/j.isci.2024.110566
35. Choi KJ, Choi JE, Roh HC, Eun JS, Kim JM, Shin YK, et al. Deep learning models for screening of high myopia using optical coherence tomography. Sci Rep. (2021) 11:21663. doi: 10.1038/s41598-021-00622-x
36. Ye X, Wang J, Chen Y, Lv Z, He S, Mao J, et al. Automatic screening and identifying myopic maculopathy on optical coherence tomography images using deep learning. Transl Vis Sci Technol. (2021) 10:10. doi: 10.1167/tvst.10.13.10
37. Li Y, Feng W, Zhao X, Liu B, Zhang Y, Chi W, et al. Development and validation of a deep learning system to screen vision-threatening conditions in high myopia using optical coherence tomography images. Br J Ophthalmol. (2022) 106:633–9. doi: 10.1136/bjophthalmol-2020-317825
38. Ota-Itadani M, Takahashi H, Mao Z, Igarashi-Yokoi T, Yoshida T, Ohno-Matsui K. Deep learning-based 3D OCT imaging for detection of lamina cribrosa defects in eyes with high myopia. Sci Rep. (2022) 12:22195. doi: 10.1038/s41598-022-26520-4
39. Liu R, Xuan M, Wang DC, Xiao O, Guo XX, Zhang J, et al. Using choroidal thickness to detect myopic macular degeneration. Int J Ophthalmol. (2024) 17:317–23.
40. Zhao XJ, Jiang HY Li YH, Liu BQ, Xu HX, Zhou J, et al. Correlations between the optic nerve head morphology and ocular biometrics in highly myopic eyes. Int J Ophthalmol. (2018) 11:997–1001.
41. Shi F, Cheng XN, Feng SL, Yang CQ, Diao SY, Zhu WF, et al. Group-wise context selection network for choroid segmentation in optical coherence tomography. Phys Med Biol. (2021) 66:245010. doi: 10.1088/1361-6560/ac3a23
42. Li M, Zhou J, Chen Q, Zou H, He J, Zhu J, et al. Choroid automatic segmentation and thickness quantification on swept-source optical coherence tomography images of highly myopic patients. Ann Transl Med. (2022) 10:620. doi: 10.21037/atm-21-6736
43. Xu X, Wang X, Lin J, Xiong H, Wang M, Tan H, et al. Automatic segmentation and measurement of choroid layer in high myopia for OCT imaging using deep learning. J Digit Imaging. (2022) 35:1153–63. doi: 10.1007/s10278-021-00571-x
44. Lin CY, Chen HJ, Chan YK, Hsia WP, Huang YL, Chang CJ. Automatic fovea detection and choroid segmentation for choroidal thickness assessment in optical coherence tomography. Int J Ophthalmol. (2024) 17:1763–71. doi: 10.18240/ijo.2024.10.01
45. Chen HJ, Huang YL, Tse SL, Hsia WP, Hsiao CH, Wang Y, et al. Application of artificial intelligence and deep learning for choroid segmentation in myopia. Transl Vis Sci Technol. (2022) 11:38. doi: 10.1167/tvst.11.2.38
46. Wu W, Gong Y, Hao H, Zhang J, Su P, Yan Q, et al. Choroidal layer segmentation in OCT images by a boundary enhancement network. Front Cell Dev Biol. (2022) 10:1060241. doi: 10.3389/fcell.2022.1060241
47. Wang Y, Chen S, Lin J, Chen W, Huang H, Fan X, et al. Vascular changes of the choroid and their correlations with visual acuity in pathological myopia. Invest Ophthalmol Vis Sci. (2022) 63:20. doi: 10.1167/iovs.63.12.20
48. Luo H, Sun J, Chen L, Ke D, Zhong Z, Cheng X, et al. Compartmental analysis of three-dimensional choroidal vascularity and thickness of myopic eyes in young adults using SS-OCTA. Front Physiol. (2022) 13:916323. doi: 10.3389/fphys.2022.916323
49. Xuan M, Wang D, Xiao O, Guo X, Zhang J, Yin Q, et al. Choroidal vascularity and axial length elongation in highly myopic children: a 2-year longitudinal investigation. Invest Ophthalmol Vis Sci. (2024) 65:7. doi: 10.1167/iovs.65.10.7
50. Fujimoto S, Miki A, Maruyama K, Mei S, Mao Z, Wang Z, et al. Three-dimensional volume calculation of intrachoroidal cavitation using deep-learning-based noise reduction of optical coherence tomography. Transl Vis Sci Technol. (2022) 11:1. doi: 10.1167/tvst.11.7.1
51. Cahyo DAY, Wong DWK, Yow AP, Saw SM, Schmetterer L. Volumetric choroidal segmentation using sequential deep learning approach in high myopia subjects. Annu Int Conf IEEE Eng Med Biol Soc. (2020) 2020:1286–9. doi: 10.1109/EMBC44109.2020.9176184
52. Mao J, Deng X, Ye Y, Liu H, Fang Y, Zhang Z, et al. Morphological characteristics of retinal vessels in eyes with high myopia: Ultra-wide field images analyzed by artificial intelligence using a transfer learning system. Front Med. (2022) 9:956179. doi: 10.3389/fmed.2022.956179
53. Crincoli E, Ferrara S, Miere A, Sacconi R, Battista M, Catania F, et al. Correlation between AI-measured lacquer cracks extension and development of myopic choroidal neovascularization. Eye. (2023) 37:2963–8. doi: 10.1038/s41433-023-02451-w
54. Wan C, Wu J, Li H, Yan Z, Wang C, Jiang Q, et al. Optimized-Unet: novel algorithm for parapapillary atrophy segmentation. Front Neurosci. (2021) 15:758887. doi: 10.3389/fnins.2021.758887
55. He HL, Liu YX, Liu H, Zhang X, Song H, Xu TZ, et al. Deep learning-enabled vasculometry depicts phased lesion patterns in high myopia progression. Asia-Pacific J Ophthal. (2024) 13:100086. doi: 10.1016/j.apjo.2024.100086
56. Wan C, Fang J, Li K, Zhang Q, Zhang S, Yang W, et al. new segmentation algorithm for peripapillary atrophy and optic disk from ultra-widefield photographs(1). Comput Biol Med. (2024) 172:108281. doi: 10.1016/j.compbiomed.2024.108281
57. Xiao Y, Zhao J, Yu Y, Ding X, Liu S, Bao W, et al. SimpleCNN-UNet: an optic disc image segmentation network based on efficient small-kernel convolutions. Exp Syst Appl. (2024) 256:124935. doi: 10.1016/j.eswa.2024.124935
58. Chen L, Zhou Y, Gao S, Li M, Tan H, Wan Z. ARA-net: an attention-aware retinal atrophy segmentation network coping with fundus images. Front Neurosci. (2023) 17:1174937. doi: 10.3389/fnins.2023.1174937
59. Zhang Z, Xu Y, Liu J, Wong DW, Kwoh CK, Saw SM, et al. Automatic diagnosis of pathological myopia from heterogeneous biomedical data. PLoS ONE. (2013) 8:e65736. doi: 10.1371/journal.pone.0065736
60. Zhang R, Dong L, Yang Q, Zhou W, Wu H, Li Y, et al. Screening for novel risk factors related to high myopia using machine learning. BMC Ophthalmol. (2022) 22:405. doi: 10.1186/s12886-022-02627-0
61. Fang Z, Xu Z, He X, Han W. Artificial intelligence-based pathologic myopia identification system in the ophthalmology residency training program. Front Cell Dev Biol. (2022) 10:1053079. doi: 10.3389/fcell.2022.1053079
62. Wan C, Li H, Cao GF, Jiang Q, Yang WH. An artificial intelligent risk classification method of high myopia based on fundus images. J Clin Med. (2021) 10:4488. doi: 10.3390/jcm10194488
63. Huang X, He S, Wang J, Yang S, Wang Y, Ye X. Lesion detection with fine-grained image categorization for myopic traction maculopathy (MTM) using optical coherence tomography. Med Phys. (2023) 50:5398–409. doi: 10.1002/mp.16623
64. Hemelings R, Elen B, Blaschko MB, Jacob J, Stalmans I, De Boever P. Pathological myopia classification with simultaneous lesion segmentation using deep learning. Comput Methods Programs Biomed. (2021) 199:105920. doi: 10.1016/j.cmpb.2020.105920
65. Du R, Xie S, Fang Y, Igarashi-Yokoi T, Moriyama M, Ogata S, et al. Deep learning approach for automated detection of myopic maculopathy and pathologic myopia in fundus images. Ophthalmology Retina. (2021) 5:1235–44. doi: 10.1016/j.oret.2021.02.006
66. Han YX, Guo XX, Wang YX, Jonas JB, Chen X, Wang XF. Automated posterior scleral topography assessment for enhanced staphyloma visualization and quantification with improved maculopathy correlation. Transl Vis Sci Technol. (2024) 13:41. doi: 10.1167/tvst.13.10.41
67. Zheng B, Zhang M, Zhu S, Wu M, Chen L, Zhang S, et al. Research on an artificial intelligence-based myopic maculopathy grading method using EfficientNet. Indian J Ophthalmol. (2024) 72:S53–s9. doi: 10.4103/IJO.IJO_48_23
68. Sogawa T, Tabuchi H, Nagasato D, Masumoto H, Ikuno Y, Ohsugi H, et al. Accuracy of a deep convolutional neural network in the detection of myopic macular diseases using swept-source optical coherence tomography. PLoS ONE. (2020) 15:e0227240. doi: 10.1371/journal.pone.0227240
69. Du Y, Chen Q, Fan Y, Zhu J, He J, Zou H, et al. Automatic identification of myopic maculopathy related imaging features in optic disc region via machine learning methods. J Transl Med. (2021) 19:167. doi: 10.1186/s12967-021-02818-1
70. He HL, Liu YX, Chen XY, Ling SG Qi Y, Xiong Y, et al. Fundus tessellated density of pathologic myopia. Asia-Pacific J Ophthalmol. (2023) 12:604–13. doi: 10.1097/APO.0000000000000642
71. Gong W, Wang J, Deng J, Chen J, Zhu Z, Seth I, et al. Quantification of fundus tessellation reflects early myopic maculopathy in a large-scale population of children and adolescents. Transl Vis Sci Technol. (2024) 13:22. doi: 10.1167/tvst.13.6.22
72. Sun Y, Li Y, Zhang F, Zhao H, Liu H, Wang N, et al. A deep network using coarse clinical prior for myopic maculopathy grading. Comput Biol Med. (2023) 154:106556. doi: 10.1016/j.compbiomed.2023.106556
73. Shao L, Zhang QL, Long TF, Dong L, Zhang C, Da Zhou W, et al. Quantitative assessment of fundus tessellated density and associated factors in fundus images using artificial intelligence. Transl Vis Sci Technol. (2021) 10:23. doi: 10.1167/tvst.10.9.23
74. Lu L, Ren P, Tang X, Yang M, Yuan M, Yu W, et al. AI-Model for Identifying Pathologic Myopia Based on Deep Learning Algorithms of Myopic Maculopathy Classification and “Plus” Lesion Detection in Fundus Images. Front Cell Dev Biol. (2021) 9:719262. doi: 10.3389/fcell.2021.719262
75. Niu YN, He HL, Chen XY, Ling SG, Dong Z, Xiong Y, et al. A novel grading system for diffuse chorioretinal atrophy in pathologic myopia. Ophthalmol Ther. (2024) 13:1171–84. doi: 10.1007/s40123-024-00908-z
76. Li Y, Li H, Rui X, Wang Y, Zhu S, Huang M, et al. Choroidal vascular changes in early-stage myopic maculopathy from deep learning choroidal analysis: a hospital-based SS-OCT study. Eye Vision. (2024) 11:32. doi: 10.1186/s40662-024-00398-x
77. Wu Z, Cai W, Xie H, Chen S, Wang Y, Lei B, et al. Predicting optical coherence tomography-derived high myopia grades from fundus photographs using deep learning. Front Med. (2022) 9:842680. doi: 10.3389/fmed.2022.842680
78. He X, Ren P, Lu L, Tang X, Wang J, Yang Z, et al. Development of a deep learning algorithm for myopic maculopathy classification based on OCT images using transfer learning. Front Publ Health. (2022) 10:1005700. doi: 10.3389/fpubh.2022.1005700
79. Zhou Y, Chen X, Li TY, Lin SQ, Sheng B, Liu RH, et al. GAMNet: a gated attention mechanism network for grading myopic traction maculopathy in OCT images. Visual Computer. (2025) 41:1097–108. doi: 10.1007/s00371-024-03386-3
80. Zhang Z, Gao Q, Fang D, Mijit A, Chen L, Li W, et al. Effective automatic classification methods via deep learning for myopic maculopathy. Front Med. (2024) 11:1492808. doi: 10.3389/fmed.2024.1492808
81. Wan C, Fang J, Hua X, Chen L, Zhang S, Yang W. Automated detection of myopic maculopathy using five-category models based on vision outlooker for visual recognition. Front Comput Neurosci. (2023) 17:1169464. doi: 10.3389/fncom.2023.1169464
82. Zhu SJ, Zhan HD, Wu MN, Zheng B, Liu BQ, Zhang SC, et al. Research on classification method of high myopic maculopathy based on retinal fundus images and optimized ALFA-Mix active learning algorithm. Int J Ophthalmol. (2023) 16:995–1004. doi: 10.18240/ijo.2023.07.01
83. Tang J, Yuan M, Tian K, Wang Y, Wang D, Yang J, et al. An artificial-intelligence-based automated grading and lesions segmentation system for myopic maculopathy based on color fundus photographs. Transl Vis Sci Technol. (2022) 11:16. doi: 10.1167/tvst.11.6.16
84. Meng J, Song Y, He W, Lu ZL, Chen Y, Wei L, et al. A novel artificial intelligence-based classification of highly myopic eyes based on visual function and fundus features. Transl Vis Sci Technol. (2024) 13:12. doi: 10.1167/tvst.13.9.12
85. Zhao J, Yu Y, Li Y, Li F, Zhang Z, Jian W, et al. Development and validation of predictive models for myopia onset and progression using extensive 15-year refractive data in children and adolescents. J Transl Med. (2024) 22:289. doi: 10.1186/s12967-024-05075-0
86. Yoo TK Ryu IH, Kim JK, Lee IS. Deep learning for predicting uncorrected refractive error using posterior segment optical coherence tomography images. Eye. (2022) 36:1959–65. doi: 10.1038/s41433-021-01795-5
87. Wang Y, Du R, Xie S, Chen C, Lu H, Xiong J, et al. Machine learning models for predicting long-term visual acuity in highly myopic eyes. JAMA Ophthalmol. (2023) 141:1117–24. doi: 10.1001/jamaophthalmol.2023.4786
88. Chen Y, Yang S, Liu R, Xiong R, Wang Y, Li C, et al. Forecasting myopic maculopathy risk over a decade: development and validation of an interpretable machine learning algorithm. Invest Ophthalmol Vis Sci. (2024) 65:40. doi: 10.1167/iovs.65.6.40
89. Kim YC, Chang DJ, Park SJ, Choi IY, Gong YS, Kim HA, et al. Machine learning prediction of pathologic myopia using tomographic elevation of the posterior sclera. Sci Rep. (2021) 11:6950. doi: 10.1038/s41598-021-85699-0
90. Lu HC, Chen HY, Huang CJ, Chu PH, Wu LS, Tsai CY. Predicting axial length from choroidal thickness on optical coherence tomography images with machine learning based algorithms. Front Med. (2022) 9:850284. doi: 10.3389/fmed.2022.850284
91. Li S, Li M, Wu J, Li Y, Han J, Cao W, et al. Development and validation of a routine blood parameters-based model for screening the occurrence of retinal detachment in high myopia in the context of PPPM. EPMA J. (2023) 14:219–33. doi: 10.1007/s13167-023-00319-3
92. Shi XH, Dong L, Zhang RH, Wei WB. Association between weight-adjusted waist index and myopia in adolescents and young adults: results from NHANES 1999-2008. BMC Ophthalmol. (2024) 24:14. doi: 10.1186/s12886-024-03282-3
93. Lin H, Long E, Ding X, Diao H, Chen Z, Liu R, et al. Prediction of myopia development among Chinese school-aged children using refraction data from electronic medical records: A retrospective, multicentre machine learning study. PLoS Med. (2018) 15:e1002674. doi: 10.1371/journal.pmed.1002674
94. Foo LL, Lim GYS, Lanca C, Wong CW, Hoang QV, Zhang XJ, et al. Deep learning system to predict the 5-year risk of high myopia using fundus imaging in children. NPJ Digit Med. (2023) 6:10. doi: 10.1038/s41746-023-00752-8
95. Li J, Zeng S, Li Z, Xu J, Sun Z, Zhao J, et al. Accurate prediction of myopic progression and high myopia by machine learning. Precis Clin Med. (2024) 7:pbae005. doi: 10.1093/pcmedi/pbae005
96. Guan J, Zhu Y, Hu Q, Ma S, Mu J, Li Z, et al. Prevalence patterns and onset prediction of high myopia for children and adolescents in southern china via real-world screening data: retrospective school-based study. J Med Internet Res. (2023) 25:e39507. doi: 10.2196/39507
97. Clark R, Lee SS, Du R, Wang Y, Kneepkens SCM, Charng J, et al. A new polygenic score for refractive error improves detection of children at risk of high myopia but not the prediction of those at risk of myopic macular degeneration. EBioMedicine. (2023) 91:104551. doi: 10.1016/j.ebiom.2023.104551
98. Liu F, Yu XH, Wang YC, Cao M, Xie LF, Liu J, et al. Quantitative analysis of optic disc changes in school-age children with ametropia based on artificial intelligence. Int J Ophthalmol. (2023) 16:1727–33. doi: 10.18240/ijo.2023.11.01
99. Kang EY, Yeung L, Lee YL, Wu CH, Peng SY, Chen YP, et al. A multimodal imaging-based deep learning model for detecting treatment-requiring retinal vascular diseases: model development and validation study. JMIR Med Inform. (2021) 9:e28868. doi: 10.2196/28868
100. Zhao Y, Zhao Z, Yang J, Li L, Nasseri MA, Zapp D. AI-based fully automatic analysis of retinal vascular morphology in pediatric high myopia. BMC Ophthalmol. (2024) 24:415. doi: 10.1186/s12886-024-03682-5
101. Liu L, Zhong L, Zeng L, Liu F, Yu X, Xie L, et al. Quantitative analysis of retinal vascular parameters changes in school-age children with refractive error using artificial intelligence. Front Med. (2024) 11:1528772. doi: 10.3389/fmed.2024.1528772
102. Zhou Y, Dai M, Sun L, Tang X, Zhou L, Tang Z, et al. The accuracy of intraocular lens power calculation formulas based on artificial intelligence in highly myopic eyes: a systematic review and network meta-analysis. Front Publ Health. (2023) 11:1279718. doi: 10.3389/fpubh.2023.1279718
103. Guo D, He W, Wei L, Song Y, Qi J, Yao Y, et al. The Zhu-Lu formula: a machine learning-based intraocular lens power calculation formula for highly myopic eyes. Eye Vision. (2023) 10:26. doi: 10.1186/s40662-023-00342-5
104. Wei L, He W, Wang J, Zhang K, Du Y, Qi J, et al. An optical coherence tomography-based deep learning algorithm for visual acuity prediction of highly myopic eyes after cataract surgery. Front Cell Dev Biol. (2021) 9:652848. doi: 10.3389/fcell.2021.652848
105. Yang M, Han J, Park JI, Hwang JS, Han JM, Yoon J, et al. Prediction of visual acuity in pathologic myopia with myopic choroidal neovascularization treated with anti-vascular endothelial growth factor using a deep neural network based on optical coherence tomography images. Biomedicines. (2023) 11:2238. doi: 10.3390/biomedicines11082238
106. Sawai Y, Miyata M, Uji A, Ooto S, Tamura H, Ueda-Arakawa N, et al. Usefulness of denoising process to depict myopic choroidal neovascularisation using a single optical coherence tomography angiography image. Sci Rep. (2020) 10:6172. doi: 10.1038/s41598-020-62607-6
Keywords: artificial intelligence, pathologic myopia, diagnosis, classification, prediction
Citation: He X, Wang Y, Zhang X, Chi W and Yang W (2025) Artificial intelligence in pathologic myopia: a review of clinical research studies. Front. Med. 12:1572750. doi: 10.3389/fmed.2025.1572750
Received: 12 February 2025; Accepted: 03 April 2025;
Published: 23 April 2025.
Edited by:
Yitian Zhao, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Peng Gao, Tongji University, ChinaYan-yan Zhang, The Affiliated Ningbo Eye Hospital of Wenzhou Medical University, China
Meng Wang, Agency for Science, Technology and Research (A*STAR), Singapore
Copyright © 2025 He, Wang, Zhang, Chi and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaojun Zhang, emhhbmd4aWFvanVuQG5qbXUuZWR1LmNu; Wei Chi, Y2hpd2VpQG1haWwuc3lzdS5lZHUuY24=; Weihua Yang, YmVuYmVuMDYwNkAxMzkuY29t
†These authors have contributed equally to this work and share first authorship
 Xinying He
Xinying He Yun Wang
Yun Wang Xiaojun Zhang1*
Xiaojun Zhang1* Wei Chi
Wei Chi Weihua Yang
Weihua Yang