Abstract
Lower extremity arterial disease (LEAD) is a major vascular complication of diabetes. Vascular endothelial cells dysfunction can exacerbate local ischemia, leading to a significant increase in amputation, disability, and even mortality in patients with diabetes combined with LEAD. Therefore, it is of great clinical importance to explore proper and effective treatments. Conventional treatments of diabetic LEAD include lifestyle management, medication, open surgery, endovascular treatment, and amputation. As interdisciplinary research emerges, regenerative medicine strategies have provided new insights to treat chronic limb threatening ischemia (CLTI). Therapeutic angiogenesis strategies, such as delivering growth factors, stem cells, drugs to ischemic tissues, have also been proposed to treat LEAD by fundamentally stimulating multidimensional vascular regeneration. Recent years have seen the rapid growth of tissue engineering technology; tissue-engineered biomaterials have been used to study the treatment of LEAD, such as encapsulation of growth factors and drugs in hydrogel to facilitate the restoration of blood perfusion in ischemic tissues of animals. The primary purpose of this review is to introduce treatments and novel biomaterials development in LEAD. Firstly, the pathogenesis of LEAD is briefly described. Secondly, conventional therapies and therapeutic angiogenesis strategies of LEAD are discussed. Finally, recent research advances and future perspectives on biomaterials in LEAD are proposed.

1 Introduction
Lower extremity arterial disease (LEAD) is a critical component of peripheral arterial disease (PAD), which defined as a vascular disease caused by occlusion of lower extremity arteries. It is widely recognized that LEAD is associated with type 2 diabetes mellitus (T2DM) (Fowkes et al., 2017). LEAD is an essential factor contributing to the increased mortality in T2DM patients (Nativel et al., 2018). Over the past century, preclinical and clinical research of LEAD have been intensively studied. Here, Figure 1 presented the time line of importance matter of LEAD. Since 1925, the risk factors associated with T2DM and LEAD have been gradually revealed, such as hyperlipidemia (Barker, 1939), hypertension (Adler et al., 2000) and oxidative stress (Belch et al., 1995). Meanwhile, different diagnosis methods (Naide, 1939; Campbell and Smith, 1950) and conventional treatments (Sethi et al., 1980; Jonason and Bergstrom, 1987) of LEAD have also emerged. For instance, there are several treatments commonly used in clinical practice, such as lifestyle management, medication, open surgery, and endovascular revascularization (Teraa et al., 2016). These treatments can increase blood perfusion and restore microcirculation in the ischemic limbs. Unfortunately, these may be ineffective in some patients.
FIGURE 1

Timeline of preclinical and clinical research on LEAD. The dates listed indicate essential milestones in the history of LEAD.
In 1994, the concept of therapeutic angiogenesis was put forward (Takeshita et al., 1994), and since then, researchers have started to use growth factors (Rajagopalan et al., 2003) or stem cells (Amann et al., 2009) to treat LEAD. With the continuous progress of medicine, therapeutic angiogenesis arises at the historical moment with aims to promote revascularization by changing the pathological conditions around ischemic tissues (Xing et al., 2021a). At present, therapeutic angiogenesis mainly includes growth factor therapy, stem cell therapy and gene therapy (Veith et al., 2019). However, since most studies remain in the laboratory stage, further clinical tests are needed to verify the efficiency of therapeutic angiogenesis in people who suffer from LEAD.
Biomaterial-based gene therapy for vascular diseases was introduced in 1997 (Schwartz and Moawad, 1997); from then on, researchers have turned their attention to the field of biomaterials (Delcroix et al., 2010; Moon et al., 2011; Agudelo et al., 2012; Kim et al., 2014; Hong et al., 2020). Biomaterials become suitable carriers for encapsulating and delivering biologically active substances or drugs, which provide new therapeutic ideas for promoting angiogenesis in diabetes patients with LEAD. Among different kinds of biomaterials, hydrogel has been extensively studied. Researchers have used natural or synthetic materials to create various hydrogels with diverse biological properties, of which injectable hydrogel has proved to improve blood perfusion in animals with lower limb ischemia. Given many advantages, such as biocompatibility, biodegradability and non-toxicity, hydrogel and other biomaterials, for example, scaffold, nanoparticle and spheroid, are hopefully being used in clinical research to increase the therapeutic efficiency of diabetic LEAD.
2 Overview of diabetic LEAD
The 10th edition of the IDF Diabetes Atlas indicates that over 500 million people worldwide are affected by T2DM (Sun et al., 2022), and it is reported that the number of people who suffer from LEAD are over 200 million globally (Fowkes et al., 2013). Compared to non-diabetic people, patients with both T2DM and LEAD show a worse prognosis, for example, the increased risks of CLTI, major adverse limb events (MALEs) and major adverse cardiac events (MACEs).
Although the pathogenesis of diabetic LEAD is still not fully clarified, it is believed that the key factor contributing to LEAD is atherosclerosis. The hallmark of atherosclerosis is endothelial dysfunction, which is caused by multiple interrelated risk factors, such as the overproduction of advanced glycation end products (AGEs), excessive oxidative stress and inflammation (Soyoye et al., 2021). Besides, the migration of low-density lipoprotein cholesterol (LDL-C) to the intima is a precursor of atherosclerosis (Suzuki et al., 2001; Taniwaki et al., 2001; Yang et al., 2017). Endothelial dysfunction and hyperglycemia are associated with abnormal clotting. Thus, abnormal platelet activation and aggregation are important factors in the development of atherosclerosis (American Diabetes, 2003).
Based on the pathological factors identified so far, researchers have further explored advanced treatments for LEAD by establishing animal models of limb ischemia. Ligation or resection of superficial femoral artery and vein are one of the most commonly used surgical methods (Masaki et al., 2002; Tan et al., 2007; Wang et al., 2020a). However, as the ligation and resection result in a state of acute ischemia, this differs from the vascular pathology of the lower extremity in clinical diabetic patients, whose vascular disease developed after a long period of ischemia. Luckily, this animal model is suitable for studying new strategies to treat LEAD due to the severe degree of ischemia and long postoperative blood flow recovery time, which simulates the vascular state of CLTI patients.
3 Conventional treatments of diabetic LEAD
Conventional treatments of diabetic LEAD include: 1) medication; 2) open surgery or endovascular treatment; 3) amputation; and 4) lifestyle management. Firstly, a good lifestyle is a needed for all other treatments. Secondly, patients are advised to take their medication as prescribed by their doctor. In addition, open surgery and endovascular treatment are indicated in patients for whom medication has failed or been ineffective. Finally, people with CLTI may experience an amputation. Here, we will describe each of these four treatments.
3.1 Medication
The drug treatments are also commonly used in clinical practice to slow down the progression of LEAD, or prevent acute cardiovascular or limb events.
Firstly, glycemic control is a primary treatment for patients with diabetic LEAD. The research revealed that combining glucagon-like peptide-1receptor agonists (GLP-1RAs) (e.g., liraglutide) with standard treatment can decrease amputation in T2DM people who suffer from diabetic foot ulcers (DFU) (Dhatariya et al., 2018). However, the relationship between sodium-glucose co-transporter 2 inhibitors (SGLT2is) and lower limb amputation remains unclear; the Canagliflozin Cardiovascular Assessment Study (CANVAS) reported that canagliflozin increased amputation rates (Minami et al., 2021). A meta-analysis showed no increased risk for SGLT2is compared to placebo (Heyward et al., 2020). A synthesis of the current studies leads to the conclusion that SGLT2is does not increase the risk of amputation and that GLP-1RA reduces this risk.
Secondly, the incidence of LEAD in DM patients is greatly associated with blood pressure (Mohammedi et al., 2016). The ACC and AHA guidelines suggest using angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor antagonists (ARBs) for patients with PAD (Gerhard-Herman et al., 2017).
Thirdly, high LDL-C is related to atherosclerosis, which leads to LEAD (Gebauer and Reinecke, 2018). There are large amounts of evidence prove the benefits of statins for LEAD (Gerhard-Herman et al., 2017). In addition, apolipoprotein B (apoB) and non-high-density lipoprotein cholesterol (non-HDL-C) have been shown to predict CVD, even at low levels of LDL-C (McQueen et al., 2008). A recent study suggests that increased non-HDL and apoB are better predictors of arterial stiffness than LDL-C (Jia et al., 2022).
Finally, patients with ischemia symptoms should be treated with anti-platelet or anticoagulation therapy. The AHA/ACC guidelines suggest using aspirin or clopidogrel alone to reduce vascular risk and mortality (Gerhard-Herman et al., 2017). As an anticoagulant, rivaroxaban has proven benefit in PAD. In the VOYAGER PAD trial, rivaroxaban 2.5 mg twice daily plus low-dose aspirin significantly reduced acute limb, brain and cardiac ischemic events compared with aspirin alone (Bonaca et al., 2020).
3.2 Open surgery and endovascular treatment
Lower extremity open surgery and endovascular treatment (EVT) have been used to restore blood perfusion in patients with CLTI (Farber and Eberhardt, 2016). EVT has the advantage of a simpler and less time-consuming procedure, as well as a faster recovery and lower recurrence rate (Papavassiliou et al., 2003; Adam et al., 2005; Slovut and Lipsitz, 2012). However, neither open surgery nor EVT can prevent restenosis (Biscetti et al., 2021). LEAD patients often accompany with occlusion of the distal arteries in the calf and foot, there will be a lack of suitable arterial inflow as well as an adequate vascular bed, leading to the failure of surgical treatment (Pan et al., 2019).
3.3 Amputation
Amputation is the ultimate clinical treatment for patients with CLTI (Norgren et al., 2007). It includes minor and major amputation. Minor amputation barely affects the patient’s motor function, whereas major amputation is usually performed above the ankle joint, reducing the independent motor ability after surgery (Nazri et al., 2019). It will seriously affect the quality of life of patients, so clinicians should intervene in the early stages of LEAD to reduce the occurrence of amputation.
3.4 Lifestyle management
As we known, lifestyle changes are an efficient way in treating abnormal glucose tolerance. However, most patients are not very compliant and have difficulty adhering to good lifestyle habits. We recommend all pre-diabetics and diabetics to initiate lifestyle management which will reduce the incidence of diabetic complications. Firstly, smoking ranks first in all risk factors for PAD. Stopping smoking can slow the procession of limb ischemia and reduce amputation, even mortality in patients (Armstrong et al., 2014). Secondly, the LEAD patient need physical exercise along with glycemic control, this benefit weight loss, lowering blood pressure and improving insulin sensitivity (Abrignani, 2018; Lee et al., 2018). It also helps patients alleviate ischemic symptoms and decrease the incidence of cardiovascular disease (CVD) (Lane et al., 2017).
Diet is an important way to both prevention and treatment of diseases, including DM (Martin-Pelaez et al., 2020). Studies have demonstrated that the Geriatric Nutrition Risk Index (GNRI) and Controlled Nutritional Status (CONUT) scores are predictors of prognosis for patients with LEAD. Therefore, improving malnutrition is a new therapeutic target in elder population (Yokoyama et al., 2018; Jhang et al., 2020).
4 Therapeutic angiogenesis
Although conventional treatments of diabetic LEAD are commonly used in the clinical stage, the methods mentioned above still have certain limitations, such as the inability to allow revascularization of the ischemic limb itself (Xing et al., 2021a). Moreover, the overall prognosis is less than ideal, and the mortality after amputation remains high. To fill these gaps, researchers have explored “therapeutic angiogenesis” since the 1990s. It mainly includes protein therapies, gene and nucleic acid-based therapies, drug and drug-like compounds therapies and stem cell-based therapies (Veith et al., 2019). Therapeutic angiogenesis strategies are extensively studied in animal experiments, and the safety of growth factor and stem cell-based biologic therapies has also been confirmed in randomized clinical trials.
4.1 The conception of therapeutic angiogenesis
Therapeutic angiogenesis was firstly proposed in an experiment in which vascular endothelial growth factor (VEGF) was injected into the ischemic hind limb of rabbits to demonstrate revascularization (Takeshita et al., 1994). Therapeutic angiogenesis can be divided into three types (Figure 2) (Xing et al., 2021a): 1) Angiogenesis: a new capillary network is sprouted based on the inherent blood vessels. This process includes the activation of VEGF, hypoxia-inducible factor-1α (HIF-1α) and erythropoietin (EPO). These cytokines act on endothelial cells and promote angiogenesis; 2) Arteriogenesis: including collateral vessel formation and artery maturation (Chalothorn et al., 2007). When the microenvironment changes, the expression of tumor necrosis factor (TNF) and fibroblast growth factor (FGF) increases, which further induces arteriogenesis (de Muinck and Simons, 2004); 3) Vasculogenesis: new blood vessels form in situ. Firstly, endothelial progenitor cells (EPCs) gather with each other, then fuse with the surrounding cells, gradually form the vascular lumen structure, and finally become the mature blood vessel (Kamei et al., 2006; Xing et al., 2020). With more in-depth studies of therapeutic angiogenesis, researchers found that this novel treatment strategy can help the body form endogenous revascularization and restore blood perfusion in ischemic tissue. Therefore, therapeutic angiogenesis is expected to become a new scheme to treat limb ischemia in patients with diabetic LEAD.
FIGURE 2

Schematic diagram of different types of therapeutic angiogenesis. (A) Angiogenesis: revascularization formed through capillary sprouting based on the intrinsic vessels. (B) Arteriogenesis: collateral vessel formation and artery maturation through capillary sprouting. (C) Vasculogenesis: blood vessels formed through the accumulation of EPCs. Figure adapted with permission from ref. (Xing et al., 2021a).
4.2 Growth factor therapy
At present, many studies have been conducted on growth factors such as FGF, VEGF, HGF, PDGF, and EGF to induce angiogenesis. Interestingly, in the experimental animal stage, exogenous growth factors showed an excellent function of stimulating ischemic collateral circulation; however, the effect was slightly inadequate in clinical trials. Makarevich et al. (2012) demonstrated that intramuscular injection of a mixture of plasmids that contain VEGF 165 and HGF could restore the blood flow in ischemic skeletal muscle. Upon this, they developed a novel bicistronic plasmid expressing both VEGF165 and HGF. The results showed that HGF/VEGF165 plasmid could induce angiogenesis in human umbilical vein endothelial cells (HUVECs) and improve blood perfusion in a hind limb ischemia mouse model (Figures 3A,B) (Slobodkina et al., 2020). Regarding the effectiveness of growth factor therapy in clinical trials, its therapeutic effect on patients with LEAD is less significant. Gu et al. studied the efficiency of HGF plasmid NL003 for the treatment of limb ischemia. They found those 6 months after intramuscular injection of NL003, patients experienced relief of ischemic symptoms and had a higher rate of ulcer healing. However, no statistical differences were found in transcutaneous oxygen pressure (TcPO2), ankle-brachial index (ABI), or toe-brachial index (TBI) in both control and experimental groups (Figures 3C,D) (Gu et al., 2019). It shows that the clinical value of growth factors in LEAD patients still needs a large number of in-depth studies to verify their effectiveness.
FIGURE 3

Schematic diagrams of growth factor therapy to improve angiogenesis. (A) Tube formation assay of HUVECs after HGF/VEGF165 plasmid treatment. Scale bar = 300 μm. (B) Laser doppler perfusion imaging (LDPI) images of ischemic hind limbs and analytical diagrams of the perfusion rate. (C) The relief of rest pain after HGF plasmid treatment. (D) Rate of completely pain relief at the end of HGF plasmid treatment. (A,B) are reprinted with permission from ref. (Slobodkina et al., 2020) and (C,D) are reprinted with permission from ref. (Gu et al., 2019).
4.3 Stem cell therapy
Stem cell therapy can be used for patients who cannot undergo open surgery or EVT. Many types of stem cells are already being explored, like EPCs, bone marrow mononuclear cells (BMMNCs), peripheral blood mononuclear cells (PBMNCs) and mesenchymal stem cells (MSCs), etc. At present, numerous researches have proved the effectiveness, such as improving blood perfusion, relieving rest pain and increasing ABI and TcPO2 (Jonsson et al., 2012; Moazzami et al., 2014; Rigato et al., 2017). In addition to conducting studies about stem cell transplants, researchers have also combined growth factors with stem cells to explore their effects on angiogenesis. Park et al. (2019) used VEGF and enhanced green fluorescent protein (EGFP) to transfect MSCs, and then transplanted the MSCs into the ischemic hind limbs to observe their effect of revascularization. They found that this MSCs-based transplantation therapy could not only enhance the expression of VEGF, but also improve blood perfusion and induce angiogenesis (Figure 4).
FIGURE 4

Schematic diagrams of MSCs transplantation to treat hind limbs ischemia in a mouse model. (A) LDPI images of ischemic hind limbs and analytical graphs of the perfusion recovery rate. (B) Representative pictures of Masson’s trichrome staining showing fibrosis and H&E staining showing necrosis. Figure adapted with permission from ref. (Park et al., 2019).
Similar to growth factor therapy, the results of stem cell therapy in clinical trials are not as favorable as in animal experiments, and the results vary from one researcher to another. Ai et al. (2016) and Rigato et al. (2017) found that cell-based transplantation may reduce amputation and increase the survival rate of non-amputation. Xie et al. (2018) and Gao et al. (2019) found that although the total amputation rate decreased after stem cell therapy, it did not improve the preservation of major limbs. In addition, a large-scale randomized controlled trial showed that the total amputation rate, mortality and non-amputation survival rate in the treatment group might not be significantly improved after stem cell transplantation (Peeters Weem et al., 2015; Liew et al., 2016; Pan et al., 2018). It remains unclear whether stem cell therapy can actually benefit patients with diabetic LEAD, we sincerely hope that its efficacy can be verified and therefore we summarize some of the current studies on stem cell therapy in Table 1.
TABLE 1
| Author | Year | Cell type | Study population | Delivery | Outcome | Mean follow-up | Refs. |
|---|---|---|---|---|---|---|---|
| Lara et al. | 2010 | EPCs | 28 | Intramuscular injection | Increased ABI, decreased pain, improved tissue perfusion and obtained high amputation-free rates | 14 months | Lara-Hernandez et al. (2010) |
| Benoit et al. | 2011 | BMSCs | 48 | Intramuscular injection | Decreased amputation and improved ulcer healing | 6 months | Benoit et al. (2011) |
| Ozturk et al. | 2012 | PBMNCs | 40 | Intramuscular injection | Increased ABI, TcPO2, and 6-min walking distance, decreased pain score and limb ulcers | 12 months | Ozturk et al. (2012) |
| Lasala et al. | 2012 | MSCs and EPCs | 26 | Intramuscular injection | Improved walking time and ABI, increased perfusion | 4 months | Lasala et al. (2012) |
| Szabo et al. | 2013 | PBSCs | 20 | Intramuscular injection | Increased ulcer healing and TcPO2 | 24 months | Szabo et al. (2013) |
| Gupta et al. | 2013 | BMMSCs | 20 | Intramuscular injection | Increased ABI, decreased amputation, and improved ulcer healing | 6 months | Gupta et al. (2013) |
| Skóra et al. | 2015 | BMMNCs | 32 | Intramuscular injection | Increased ABI, developed collateral vessels, and decreased amputation | 3 months | Skora et al. (2015) |
Clinical trials based on cell therapy for LEAD patients.
Based on current research reports, stem cell-based therapies may have better efficacy than other therapeutic angiogenesis strategies (Ho et al., 2022), as cell therapy has been shown to induce therapeutic angiogenesis in both animal models and human experiments (Van Raemdonck et al., 2015; Novakova et al., 2016). Compared to other stem cells, induced pluripotent stem cells (iPSC) and their derivative cells may have enhanced therapeutic potential. It reported that human iPSC-derived smooth muscle cells (hiPSC-SMC) can mediate angiogenesis, thereby improving hind limb ischemia. Stem cell therapy using iPSC-derived cells may represent a novel therapeutic angiogenesis strategy for the treatment of LEAD (Gao et al., 2021; Park et al., 2021).
The therapeutic angiogenesis strategy was carried out as early as 1990s and it is currently under great investigation. Although promising preclinical studies in animal models, cell-based therapies in LEAD patients still show limitations. It has been demonstrated that ion channels differ between humans and animal models and that the disease state in humans cannot be fully replicated in animal models (Tanner and Beeton, 2018) . Most previous investigations used autologous cells, which damaged by chronic hyperglycemia, and have a low survival rate in the ischemic environment (Qadura et al., 2018). However, we can initially screen some effective drugs through animal studies, and then verify their safety and effectiveness through clinical trials. In the future, multicenter, larger-scale clinical trials are needed to verify the effectiveness of therapeutic angiogenesis in clinical LEAD patients.
5 Application of biomaterials in the treatment of diabetic LEAD
Researchers have verified the effectiveness of therapeutic angiogenesis in the experimental animal stage, but when it turns to clinical trials, this therapeutic strategy remains to be further explored. At present, most studies transport growth factors, stem cells or other bioactive molecules to ischemic tissue in order to restore blood perfusion. However, how to maintain the activity of these substances and how to improve the accuracy of delivery still requires more in-depth research. Due to the excellent biocompatibility, biodegradability and non-toxicity, biomaterials can be used as a carrier of various bioactive molecules to treat LEAD. As reported, the vascular scaffold has been used in patients with PAD, and no amputations or deaths have been observed over a 2-years clinical trial (Lammer et al., 2016). Therefore, biomaterials are of great clinical importance in the treatment of LEAD.
5.1 Biomaterials overview
Biomaterials are one of the significant components of tissue engineering, acting as an extracellular matrix (ECM) and promoting cell migration, tube formation, tissue maturation and wound healing (Zhang et al., 2013; Lee et al., 2014). There are numerous ways to classify biomaterials; for example, according to the origins, biomaterials can be classified as natural or synthetic materials (Troy et al., 2021). Researchers use these biomaterials to prepare tissue engineering materials with different structural forms and diverse functions, such as hydrogels, microneedles, nanoparticles and scaffolds, etc. (Figure 5). Taking hydrogel as an example, on the one hand, it has good biological properties and mechanical properties; on the other hand, the preparation is more straightforward compared with other biomaterials. Therefore, in biomaterial-based therapeutic angiogenesis strategies, hydrogels have become promising candidates (Buwalda et al., 2017). Here, we will mainly focus on some of the explorations that have been completed in the field of hydrogels and list some studies in the treatment of LEAD based on other biomaterials.
FIGURE 5
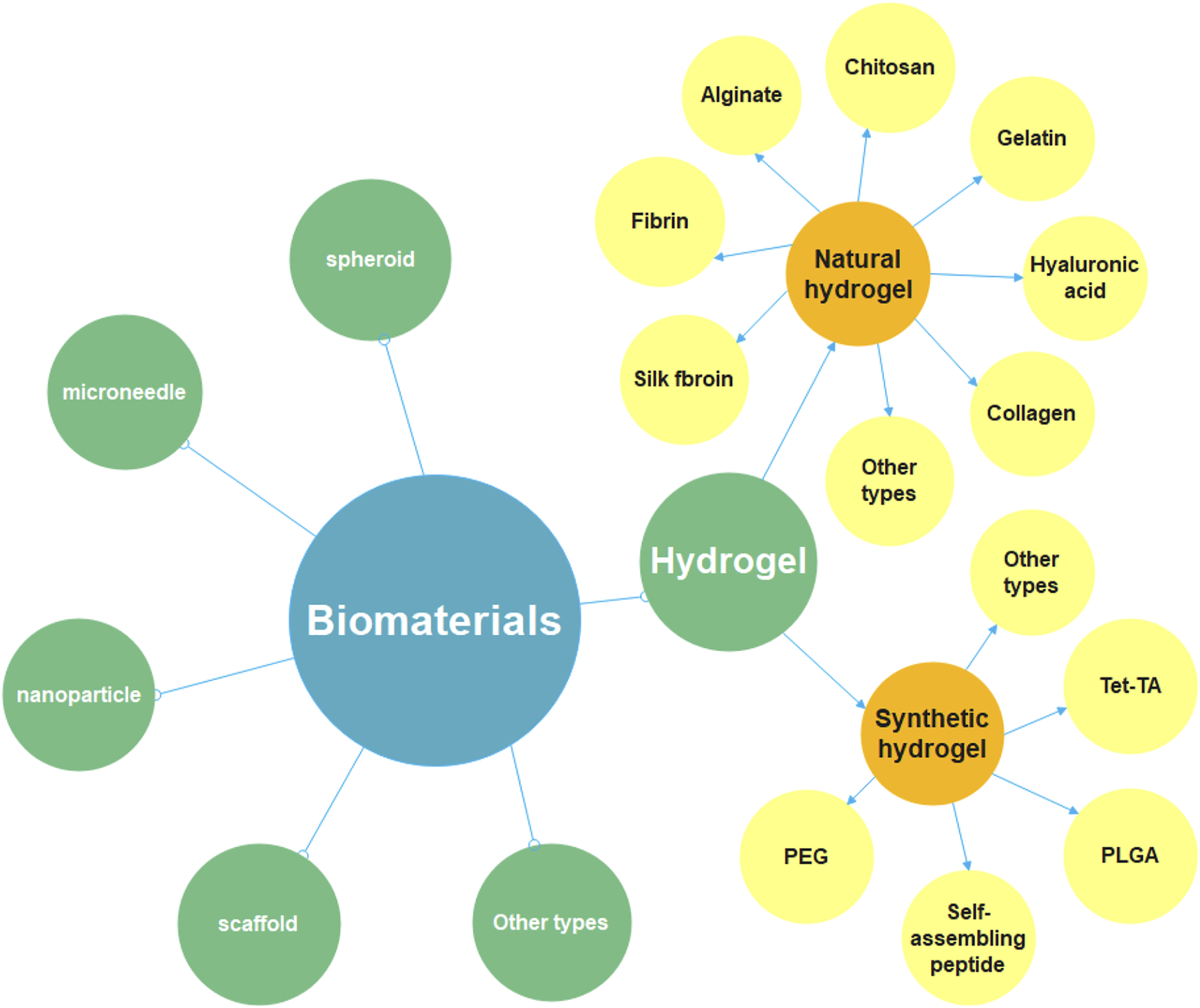
Diagram of the classification of biomaterials. Biomaterials include hydrogel, spheroid, microneedle, nanoparticle, scaffold and so on, this review focuses on hydrogel-related research. It can be divided into natural hydrogel and synthetic hydrogel. The natural materials for preparing hydrogels include chitosan, gelatin, fibrin, alginate, collagen, hyaluronic acid, silk fibroin, etc. The synthetic materials for preparing hydrogels include polyethylene glycol (PEG), poly (lactic-co-glycolic Acid) (PLGA), self-assembling peptide (SAP), tetronic-tyramine (Tet-TA), etc.
5.2 Hydrogel overview
Hydrogel is a kind of hydrophilic polymer material, which is extensively used in the medical stage because of its excellent water absorption, biocompatibility, and ion transferability (Xing et al., 2021a). Due to the conventional treatments of LEAD and therapeutic angiogenesis strategies having certain limitations and side effects, researchers have made an in-depth exploration of therapeutic angiogenesis based on hydrogels. They have designed various studies to treat LEAD by encapsulating various angiogenic factors or stem cells in hydrogels and then transporting them to ischemic tissues effectively. According to the different sources of materials for preparing hydrogel, it can be divided into natural hydrogel and synthetic hydrogel.
5.2.1 Natural hydrogel
The natural materials for preparing hydrogels include chitosan (CS), gelatin, fibrin, alginate, collagen, hyaluronic acid (HA), silk fibroin (SF), etc. (Figure 6). Chitosan has been broadly used in biomedical fields for its biocompatibility, degradability and antibacterial (Jayakumar et al., 2010; Kalantari et al., 2019). Recently, Wang et al. (2020b) synthesized a temperature-responsive chitosan hydrogel encapsulated with ferulic acid (FA). The hydrogel is capable of the stable release of FA, which has the effects of both antioxidant and pro-angiogenic. They validated the efficacy of the FA-chitosan hydrogel in a hind limb ischemia mouse model and demonstrated its remarkable ability to promote blood perfusion and angiogenesis (Figure 7A). Gelatin has good temperature responsiveness; when the temperature is below 40°C, it forms into a transparent gel solution (Chu and Wang, 2012). Lee et al. (2020) prepared a microchannel network hydrogel with gelatin solution and poly (N-isopropyl acrylamide) (PNIPAM) fibers. The in vivo results proved that this hydrogel could increase blood perfusion (Figure 7B). Fibrin has a large number of binding sites to growth factors, which allows angiogenesis (Loureiro et al., 2019). Recently, Pei et al. (2020) prepared a fibrin hydrogel encapsulating cardiac stem cells (CSCs) and transfected HIF-1α into CSCs. The in vivo results of animal experiments showed that the hydrogel has a solid ability to promote angiogenesis and restore the blood perfusion of ischemic limbs (Figure 7C). Alginate is a kind of natural anionic polymer, which is widely used in the preparation of hydrogel due to its easy gelation. Ruvinov et al. (2010) prepared an alginate hydrogel encapsulated with HGF, which released HGF in a rapid and controlled manner. Then, they established a mouse model to verify the effect of the HGF-hydrogel. The in vivo results proved that the HGF-hydrogel could improve blood perfusion by improving angiogenesis through its pro-angiogenesis effect (Figure 7D). Since many research used natural hydrogels for LEAD, we summarized some of studies in Table 2.
FIGURE 6
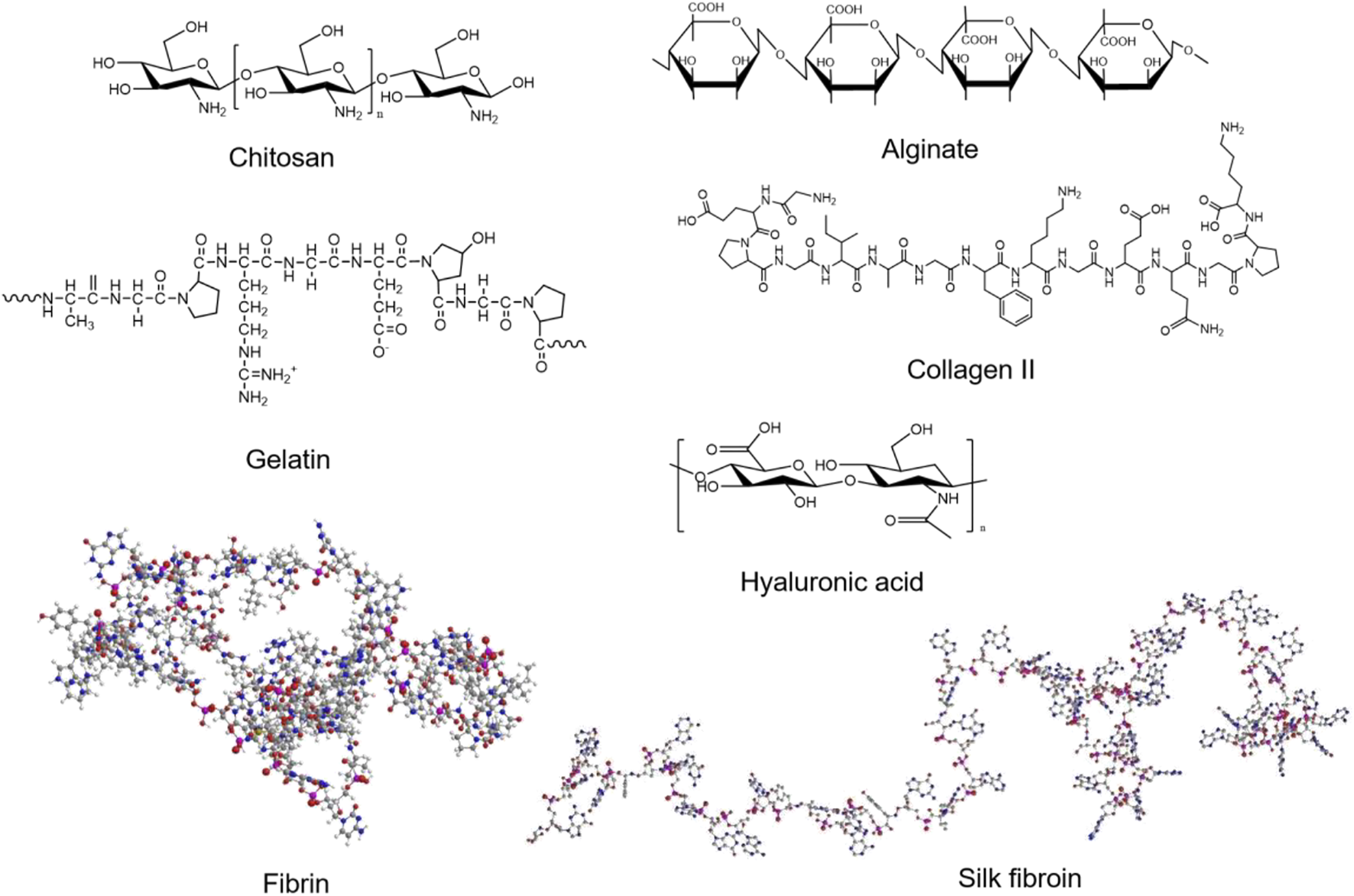
The structure of natural materials for preparing hydrogels.
FIGURE 7

Schematic diagrams of several natural hydrogels to improve angiogenesis. (A) The SEM image of the FA-chitosan hydrogel and the result of animal study to promote angiogenesis. Scale bar = 10 μm. (B) LDPI images in a hind limb ischemia mouse model. (C) Confocal microscopy images of a micro-architecture of injectable fibrin hydrogel with different concentrations of fibrinogen solution and the representative LDPI images. Scale bar = 50 μm. (D) LDPI images proved the angiogenesis effect of the HGF-alginate hydrogel. (A) is reprinted with permission from ref. (Wang et al., 2020b). (B) is reprinted with permission from ref. (Lee et al., 2020). (C) is reprinted with permission from ref. (Pei et al., 2020). (D) is reprinted with permission from ref. (Ruvinov et al., 2010).
TABLE 2
| Materials | Cargo | Animals | Injury model | Blood perfusion | Revascularization | Others | Refs. |
|---|---|---|---|---|---|---|---|
| Chitosan | VEGF, ECs, CD31+ cells | Mice | Femoral artery ligation | Increased | Increased capillary density | Increased mRNA level of angiogenic factors | Lee et al. (2015) |
| Chitosan‐HP | FGF-2 | Rats | Femoral artery ligation | Increased | Increased capillary density | Enhanced early vascular maturation | Nillesen et al. (2007) |
| Alginate | HGF | Mice | Femoral artery excised | Increased | Increased arteriole density | Not mentioned | Ruvinov et al. (2010) |
| Alginate | IGF + VEGF | Rabbits | Lateral circumflex artery and femoral artery ligation | Increased | Increased capillary density | Increased muscle fibers size | Anderson et al. (2017) |
| Alginate | CD4+ T cells | CD4 KO mice | Femoral artery and vein ligation | Increased | Increased blood vessel density | Enhanced myogenesis | Kwee et al. (2018) |
| Gelatin | G-CSF, FGF-2 | Mice | Femoral artery excised | Increased | Increased capillary density | Promoted mature vessel formation | Layman et al. (2009) |
| Gelatin | FGFs, plasma derived GFs mixture | Mice | Iliac and femoral artery and vein excised | Increased | Increased blood vessel density | Not mentioned | Matsui and Tabata, (2012) |
| Fibrin | HIF-1α overexpressed CSCs | Mice | Femoral artery excised | Increased | Not mentioned | Attenuated tissue degeneration and fibrosis | Pei et al. (2020) |
| Fibrin | Recombinant VEGF164 | Mice | Femoral artery excised | Increased | Increased the mature capillaries | Improved wound-healing | Sacchi et al. (2014) |
| Fibrin | FGF2, HO-1 gene | Nude mice | Femoral artery excised | Not mentioned | Not mentioned | Reduced tissue apoptosis and necrosis, enhanced angiogenic paracrine factor expression | Bhang et al. (2009) |
| Collagen‐HP | FGF-2 | Rabbits | Femoral artery excised | Not mentioned | Increased capillary density | Increased oxygen perfusion | Zhou et al. (2012) |
| Catechol modified HA | ADSCs | Nude mice | Femoral artery excised | Increased | Increased capillary and arteriole density | Attenuated tissue degeneration and fibrosis | Park et al. (2016) |
Preclinical studies of natural hydrogels for LEAD.
5.2.2 Synthetic hydrogel
The synthetic materials for preparing hydrogels include polyethylene glycol (PEG), poly (Lactic-co-glycolic Acid) (PLGA), self-assembling peptide (SAP), tetronic-tyramine (Tet-TA), etc. (Figure 8). Polyethylene glycol (PEG) hydrogel has good biocompatibility, which is widely used in biomedical fields (Lin and Anseth, 2009). Mulyasasmita et al. (2014) prepared a protein-PEG hybrid hydrogel, which could co-deliver human induced pluripotent stem cell-derived endothelial cells (hiPSC-ECs) and VEGF. The in vivo results of a PAD mouse model revealed that the protein-PEG hydrogel could lower inflammation and decrease ischemia-reperfusion injury (Figure 9A). Poly (Latic-co-glycolic Acid) (PLGA) is also widely used in the preparation of biomaterials, and some researchers have made it into micron or nanoparticles and 3D scaffolds (Bharadwaz and Jayasuriya, 2020). In a recent study, Hong et al. (2020) prepared a PLGA-Cys-mPEG porous hydrogel and realized the “gel-solution” transition with glutathione (GSH). The hydrogel can not only support the formation of adipose-derived stem cells (ASCs) spheroids, but also treat hind limb ischemia by minimally invasive injection. The in vivo results indicated that the hydrogel had an apparent therapeutic effect in limb ischemia, which could enhance paracrine secretion of ASCs, promote angiogenesis of ischemic limbs (Figure 9B).
FIGURE 8

The structures of synthetic materials for preparing hydrogels.
FIGURE 9
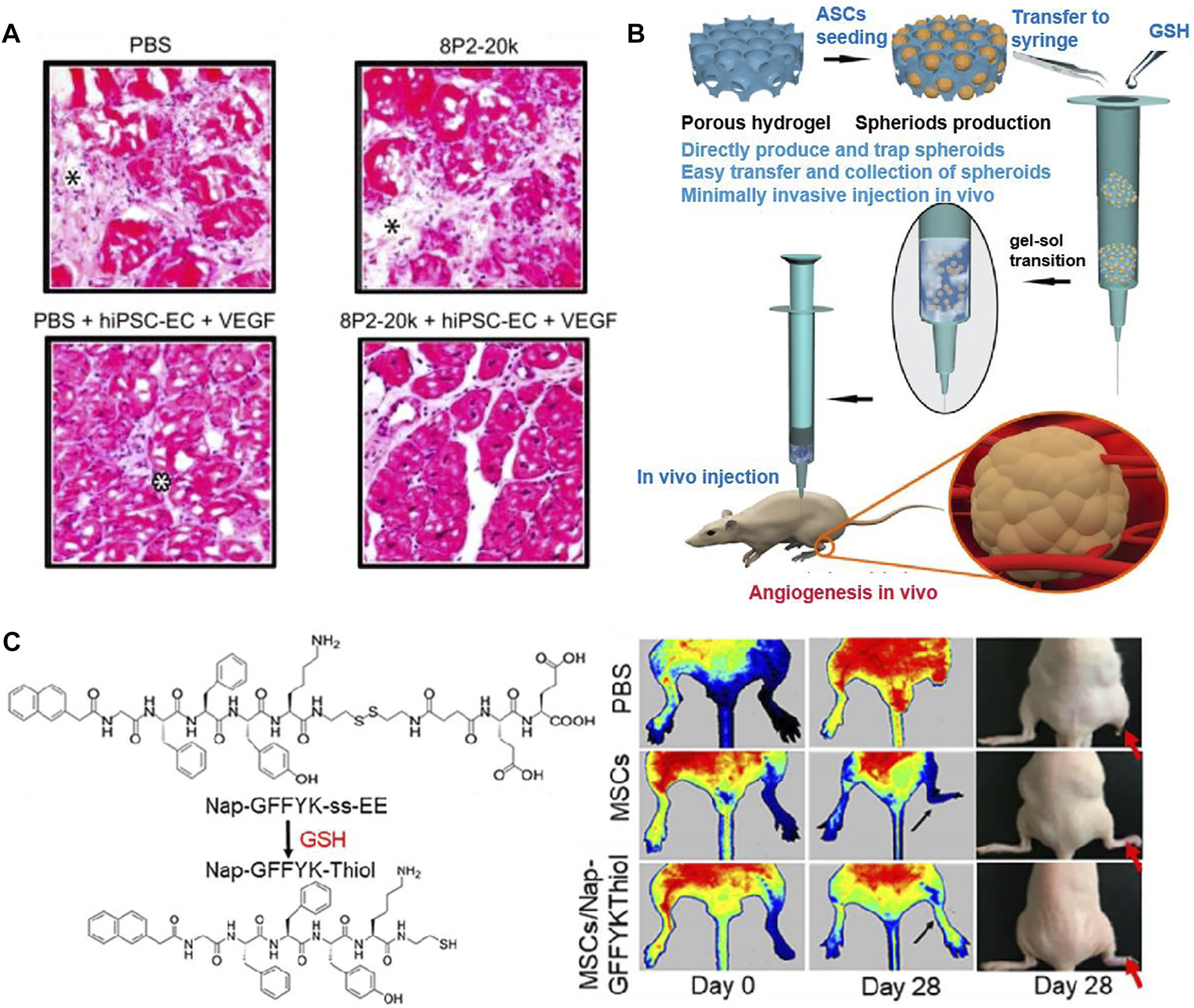
Schematic diagrams of several synthetic hydrogels to improve angiogenesis. (A) Representative images of H&E staining showed the effect of PEG hydrogel, which co-deliver hiPSC-ECs and VEGF. Only the protein-PEG co-deliver system group showed no significant inflammation. Black asterisk indicates the expansion of connective tissue and white asterisk indicates inflammatory cells. (B) General diagram of the preparation of PLGA-Cys-mPEG porous hydrogel and the in vivo result of angiogenesis. (C) Chemical structures of Nap-GFFYK-Thiol hydrogel and LDPI images of blood perfusion. (A) is reprinted with permission from ref. (Mulyasasmita et al., 2014). (B) is reprinted with permission from ref. (Hong et al., 2020). (C) is reprinted with permission from ref. (Huang et al., 2019a).
The development of self-assembling peptide (SAP) is inspired by the phenomenon of molecular self-assembly in vivo, such as protein winding, folding and the construction of DNA double helix (Palmer and Stupp, 2008). Huang et al. (2019a) synthesized a novel small molecular hydrogel by disulfide bond reduction, named Nap-GFFYK-Thiol hydrogel. The results showed that the hydrogel could not only improve the activity of human placental mesenchymal stem cells (hP-MSCs), but also promote blood flower recovery (Figure 9C). Since many research used synthetic hydrogels for LEAD, we summarized some of the studies in Table 3.
TABLE 3
| Materials | Cargo | Animal | Injury model | Blood perfusion | Revascularization | Others | Refs. |
|---|---|---|---|---|---|---|---|
| PLGA-mPEG | ADSCs | Nude mice | Femoral artery and its branches ligation | Increased | Increased capillary and arteriole formation | Reduced tissue damage, increased the level of proangiogenic factors | Park et al. (2016) |
| Protein modified PEG | iPSC-ECs, VEGF | Nude mice | Femoral artery ligation | Not mentioned | Not mentioned | Induced tissue regeneration and decrease necrosis | Mulyasasmita et al. (2014) |
| PLGA-PEG-RADA peptide | VEGF, EPCs | Mice | Femoral artery ligation | Increased | Increased blood vessel density | Diminished inflammation, tissue fibrosis and degeneration | Li et al. (2017) |
| FN/Tet-TA copolymer | MSCs, ECs | Nude mice | Femoral artery and its branches excised | Not mentioned | Increased arteriole density | Reduced inflammation and tissue fibrosis | Jun et al. (2017) |
| RADA peptide | MSCs | Rats | Femoral artery excised | Increased | Not mentioned | Increased level of angiogenic markers, inhibited tissue apoptosis and fibrosis | Park et al. (2018) |
Preclinical studies of synthetic hydrogels for LEAD.
5.2.3 Hydrogel loaded with growth factors or stem cells
With the boosting studies of biomaterials, more and more research is focusing on the combination of biomaterials with growth factors and stem cells. Among all these materials, hydrogel is frequently used due to its 3D porous structure and efficient water content. Thus, hydrogels are chosen as carriers for growth factors and stem cells to treat LEAD. In a recent study, Xing et al. (2021b) transfected VEGF and TFEB plasmids into extracellular vesicles (EVs) derived from endothelial cells. Then prepared a hydrogel loaded with VEGF/TFEB-engineered EVs. Their work demonstrated that the EV-loaded hydrogel could improve angiogenesis (Figure 10A). Huang et al. (2019b) designed a functionalized SAP hydrogel with RADA16-GGQQLK (QLK) and RADA16-GGLRKKLGKA (LRK) sequences. The LRK sequence is a cofactor that could help growth factors to encapsulate in this SAP hydrogel. The results revealed that the VEGF/HGF-loaded SAP hydrogel could promote tube formation in vitro and angiogenesis in vivo (Figures 10B,C). Stem cells therapy has been proved to treat limb ischemia, but it is still limited by problems such as inaccurate cell release and poor cell survival (Tang et al., 2017). Due to a lack of blood perfusion, the temperature of ischemic limb is often lower than normal tissues (Fernandez et al., 2018). Inspired by this, Wang et al. (2019) created a temperature-responsive hydrogel, which based on Methylcellulose (MC), encapsulated with human placental mesenchymal stem cells (PMSCs). The results showed that MC-based hydrogel can effectively release PMSCs into ischemic area, and then improve blood perfusion, enhance revascularization. The above experiments confirm that hydrogels as carriers have a good synergistic effect with growth factors or stem cells in promoting angiogenesis. Therefore, it is reasonable to believe that this type of research deserves to be further explored to facilitate the treatment of LEAD in the clinical field.
FIGURE 10

Schematic diagrams of hydrogels loaded with growth factors or stem cells to treat LEAD. (A) LDPI images of ischemic hind limbs in a mouse model showed that engineered-EV/hydrogel significantly improved blood perfusion and preserved the ischemic limb. (B) Representative images of H&E staining showed that the GF-loaded hydrogel could promote tube formation. Scale bar = 60 µm. (C) Representative images of immunohistochemistry staining showed that the GF-loaded hydrogel could improve angiogenesis. Scale bar = 20 µm. (A) is reprinted with permission from ref. (Xing et al., 2021b). (B,C) are reprinted with permission from ref. (Huang et al., 2019b).
5.3 Other biomaterials
In addition to hydrogels, there are still many other types of biomaterials that have been studied to treat LEAD, such as scaffold (Li et al., 2018; Carrabba et al., 2020; Leclerc et al., 2021; Mu et al., 2021), nanoparticle (NP) (Matsumoto et al., 2020; Park et al., 2020; Liu et al., 2017; Albrecht-Schgoer et al., 2017) and spheroid (Park et al., 2014; Choi et al., 2021; Lee et al., 2021).
5.3.1 Scaffold
Many researchers have begun to focus on scaffold-based tissue engineering strategies to restore blood perfusion in ischemic limbs. Recently, Mu et al. (2021) fabricated an electrospun glucomannan decanoate (GMDE) scaffold to simulate microbial invasion and isolate endogenous galectin-1. The in vivo results found that GMDE scaffold can effectively promote blood perfusion (Figures 11A,B). Li et al. (2018) designed a nanoparticle-anchoring hydrogel scaffold and evaluated the therapeutic effect on ischemic hind limbs of mice. The in vivo results showed the scaffold could significantly promote the restoration of blood perfusion (Figures 11C,D).
FIGURE 11

Schematic diagrams of scaffolds to improve blood perfusion. (A) LDPI images of ischemic hind limbs showed that GMDE scaffold group had the best angiogenic effect. (B) Immunofluorescent staining for CD31 and α-SMA of thigh muscle showed that GMDE scaffold could promote angiogenesis. Scale bar = 50 µm. (C) LDPI images of ischemic hind limbs showed that T-RNPs-anchoring hydrogel scaffold loaded with EPCs had the best angiogenic effect. (D) Immunofluorescent images of CD31, H&E, and Masson’s trichrome staining of ischemic muscles. The E + R + T-RNPs group had highest blood vessel density and lowest levels of inflammation and fibrosis. Scale bar = 200 μm. (A,B) are reprinted with permission from ref. (Mu et al., 2021). (C,D) are reprinted with permission from ref. (Li et al., 2018).
5.3.2 Nanoparticle
Nanoparticles (NPs) have emerged as a new model of drug delivery system with advantages such as targeting and slow release. Matsumoto et al. (2020) designed a NP-mediated, targeted drug delivery system using PLGA and statin as raw materials. The results revealed that Pitavastatin-NPs could significantly improve the therapeutic effect on limb ischemia (Figures 12A,B). According to many studies, cerium oxide nanoparticles (CNP) have shown therapeutic effects both in vivo (Naganuma and Traversa, 2014) and in vitro (Kwon et al., 2016). Park et al. (2020) confirmed the angiogenesis-promoting properties of CNP in a hind limb ischemia mouse model and further investigated molecular mechanisms by which it exerts its promoting effects. The results showed increased expression of pro-angiogenic markers, vascular maturation and muscle remodeling after intramuscular injection of CNP. Moreover, such an efficient pro-angiogenic effect may be due to the upregulation of the Ref-1/APE1 pathway (Figure 12C).
FIGURE 12

Schematic diagrams of nanoparticles to improve blood perfusion. (A) Representative angio-CT images demonstrated the development of collateral arteries. (B) Representative images of angiography of ischemic lower extremities. The pitavastain-NPs group showed the revascularization. (C) Representative images of limb loss in different treatment groups and quantification of limb loss, foot necrosis, and limb salvage. The CNP 0.6 mg group showed the best effect of limb salvage. (A,B) are reprinted with permission from ref. (Matsumoto et al., 2020). (C) is reprinted with permission from ref. (Park et al., 2020).
5.3.3 Spheroid
As mentioned above, stem cell therapy in therapeutic angiogenesis is a reliable treatment for LEAD. The therapeutic benefit of single-cell transplantation is not ideal. Still, the efficiency will significantly enhance when cells become spheroids, because the 3D spheroid system could create a more biomimetic microenvironment (Lee et al., 2021). Therefore, cell spheroids that act synergistically with biomaterials have been extensively studied. Choi et al. (2021) cultured human adipose-derived stem cells (hASCs) in an FGF-2 matrix, which in turn developed into an enhanced cells spheroid (FECs-Ad) and were able to secrete a variety of angiogenic growth factors. Their work proved that the injection of FECs-Ad promotes angiogenesis in ischemic hind limbs, which also increases the expression of VEGF (Figures 13A,B).
FIGURE 13

Schematic diagrams of spheroids to improve blood perfusion. (A) Representative images of LDPI and hind limb morphology. FECS-Ad treatment resulted in the best recovery of blood flow and it reduced limb loss. (B) Laminin staining of the thigh and TA muscles showed that FECS-Ad promotes muscle fiber regeneration and restores muscle structure. Scale bar = 20 μm. Figure adapted with permission from ref. (Choi et al., 2021).
Since there have been many studies using other biomaterials to investigate the treatment of LEAD, so we summarize some of the studies in Table 4.
TABLE 4
| Material forms | Materials | Cargo | Animal | Injury model | Blood perfusion | Revascularization | Others | Refs. |
|---|---|---|---|---|---|---|---|---|
| Nanoparticles | Dextran‐gelatin | VEGF | Mice | Femoral artery and its branches excised | Increased | Increased capillary density | Not mentioned | Xie et al. (2013) |
| Nanoparticles | Fragmine/protamine | bFGF | Rabbits | Femoral artery excised | Not mentioned | Increased collateral arteries | Not mentioned | Fujita et al. (2013) |
| Nanoparticles | Fragmine/protamine | bFGF | Mice | Femoral artery excised | Increased | Increased arterioles | Limb salvage and improved oxygen reperfusion | Nakamura et al. (2012) |
| Nanoparticles | PLGA | bFGF | Mice | Occlusion of the saphenous artery | Not mentioned | Increased number and diameter of arterioles | Not mentioned | Chappell et al. (2008) |
| Microspheres | Dextran‐PLGA | VEGF | Rats | Femoral artery excised | Not mentioned | Increased capillary and arteriole density | Enhanced the proliferation of endothelial cells | Zhang et al. (2019) |
| Microspheres | Gelatin | bFGF | Dogs | Iliac artery ligation and femoral artery excised | Increased | Increased capillary and arteriole density | Not mentioned | Zhao et al. (2011) |
| Porous scaffold | Collagen | BMMSCs | Rabbits | Femoral artery excised | Increased | Increased capillary and arteriole density | Increased oxygen saturation ratio | Wang et al. (2012) |
| Electrospun scaffold | Polycaprolactone/gelatin scaffolds | iPSCs‐ECs | Mice | Subcutaneous | Increased | Increased capillary and arteriole density | Increased VEGF expression and reduction of the inflammatory | Tan et al. (2018) |
Preclinical studies of other biomaterials for LEAD.
6 Conclusion and future perspectives
As diabetes continues to increase around the world, complication also arises. In all vascular complications of diabetes, LEAD is an essential cause of death for patients. The end-stage of LEAD is called CLTI, and the study found that the 5-years survival rate of diabetic patients with CLTI is lower than those without limb ischemia. Therefore, they need more effective treatments to repair ischemic limbs, minimize amputation and mortality. Conventional treatments include medication, open surgery, endovascular treatment, amputation and lifestyle management are not always enough. Crucial aspect is the lack of awareness of the disease among both physicians and patients. This means that patients often present to the physician’s attention already with an advanced disease: CLTI. In these cases, currently available treatment options often perform poorly. The therapeutic angiogenesis strategy proposed in recent years aims to promote the spontaneous formation of revascularization and restore the blood perfusion of ischemic limbs through growth factors and stem cell transplantation. However, all of the above treatments have their limitations, urging us to explore novel methods to treat diabetic LEAD. With the continuous development of biomedicine and tissue engineering, many natural or synthetic biomaterials have been used to research of limb ischemia mouse models due to their promising biological properties. At present, many researchers have confirmed the effectiveness of biomaterials to rescue limb ischemia. Unfortunately, most of the studies are still in the experimental animal stage, and the animal models established are not as complicated as many other complications of clinical diabetic patients. Although some clinical trials have already verified the safety of the biomaterials they use, however, the translation of biomaterials into clinical products still needs to face many questions. For example, whether biomaterial-based therapeutic angiogenesis strategies can produce good angiogenesis-promoting effects in real-world patients is still debatable; and how to translate various biomaterials from small-scale application in the laboratory stage to high-volume use in treating patients in the clinic. Therefore, randomized clinical trials are needed to clarify the role of these new technologies in treating patients with diabetic LEAD. In conclusion, we hope that biomaterials will be further developed and gradually applied to clinical treatment from the current animal research stage, thus providing more ways to treat diabetic LEAD.
Statements
Author contributions
HH and HZ conceived the presented idea of this review. YP, JH, and LD collected the data and wrote the original draft manuscript in consultation with HH and HZ. HH and HZ review and editing the paper. All authors discussed the results and commented on the manuscript.
Funding
HZ acknowledge the financial support from Zhejiang Provincial Medical Science and Technology Plan (2022491161). JH acknowledge the financial support from the Wenzhou Municipal Science and Technology Bureau (Y20180609).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- ABI
ankle-brachial index
- ACEIs
angiotensin-converting enzyme inhibitors
- ADSCs
adipose-derived stem cells
- AGEs
advanced glycation end products
- apoB
apolipoprotein B
- ARBs
angiotensin receptor antagonists
- bFGF/FGF-2
basic fibroblast growth factor
- BMMNCs
bone marrow mononuclear cells
- BMMSCs
bone marrow mesenchymal stem cells
- BMSCs
bone marrow stem cells
- CLTI
chronic limb threatening ischemia
- CNP
cerium oxide nanoparticle
- CSCs
cardiac stem cells
- CV
cardiovascular
- CVD
cardiovascular disease
- DFU
diabetic foot ulcer
- DPP-4is
dipeptidyl peptidase-4 inhibitors
- E + R + T-RNPs
EPCs + RADA16 + tacrolimus-loaded RADA16 peptide-modified nanoparticles
- ECM
extracellular matrix
- ECs
endothelial cells
- EGF
epidermal growth factor
- EGFP
enhanced green fluorescent protein
- EGM-2
endothelial growth medium-2
- EMCV
encephalomyocarditis virus
- EPCs
endothelial progenitor cells
- EPO
erythropoietin
- EVs
extracellular vesicles
- EVT
endovascular treatment
- FA
ferulic acid
- FECS-Ad
functionally enhanced cells spheroid
- FGF
fibroblast growth factor
- FN
fibronectin
- G-CSF
granulocyte-colony stimulating factor
- GFs
growth factors
- GLP-1RAs
glucagon-like peptide-1 receptor agonists
- GMDE
glucomannan decanoate
- GSH
glutathione
- hASCs
human adipose-derived stem cells
- HbA1c
haemoglobin A1c
- HGF
hepatocyte growth factor
- HIF-1α
hypoxia-inducible factor-1α
- HLI
hindlimb ischemia
- HUVECs
human umbilical vein endothelial cells
- IGF
insulin-like growth factor
- IL-1a
interleukin-1a
- iPSCs-ECs
induced pluripotent stem cells-endothelial cells
- LDL-C
low-density lipoprotein cholesterol
- LDPI
laser doppler perfusion imaging
- LEAD
lower extremity arterial disease
- MACE
major adverse cardiovascular event
- MALE
major adverse limb event
- mPEG
modified polyethylene glycol
- MSCs
mesenchymal stem cells 5
- NO
nitric oxide
- non-HDL-C
non-high-density lipoprotein cholesterol
- NPs
nanoparticles
- PAD
peripheral arterial disease
- PBMNCs
peripheral blood mononuclear cells
- PBSCs
peripheral blood stem cells
- PCL
polycaprolactone
- PCSK9
proprotein convertase subtilisin/kexin type 9
- PDGF
platelet derived growth factor
- PGE1
prostaglandin E1
- PLGA
poly (lactic-co-glycolic Acid)
- SEM
scanning electron microscope
- SGLT2is
sodium glucose co-transporter 2 inhibitors
- T2DM
type 2 diabetes mellitus
- TA
tibialis anterior
- TBI
toe-brachial index
- TcPO2
transcutaneous oxygen pressure
- Tet-TA
tetronic-tyramine
- TNF-α
tumor necrosis factor-α
- VEGF
vascular endothelial growth factor
- vWF
von Willebrand factor
- α-SMA
α-Smooth muscle actin
References
1
Abrignani M. G. (2018). Physical exercise and risk of arterial hypertension and diabetes mellitus. Let's move, it is never too late. Eur. J. Prev. Cardiol.25 (10), 1063–1064. 10.1177/2047487318781116
2
Adam D. J. Beard J. D. Cleveland T. Bell J. Bradbury A. W. Forbes J. F. et al (2005). Bypass versus angioplasty in severe ischaemia of the leg (BASIL): Multicentre, randomised controlled trial. Lancet366 (9501), 1925–1934. 10.1016/S0140-6736(05)67704-5
3
Adler A. I. Stratton I. M. Neil H. A. Yudkin J. S. Matthews D. R. Cull C. A. et al (2000). Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): Prospective observational study. Br. Med. J.321 (7258), 412–419. 10.1136/bmj.321.7258.412
4
Agudelo C. A. Tachibana Y. Hurtado A. F. Ose T. Iida H. Yamaoka T. (2012). The use of magnetic resonance cell tracking to monitor endothelial progenitor cells in a rat hindlimb ischemic model. Biomaterials33 (8), 2439–2448. 10.1016/j.biomaterials.2011.11.075
5
Ai M. Yan C. F. Xia F. C. Zhou S. L. He J. Li C. P. (2016). Safety and efficacy of cell-based therapy on critical limb ischemia: A meta-analysis. Cytotherapy18 (6), 712–724. 10.1016/j.jcyt.2016.02.009
6
Albrecht-Schgoer K. Barthelmes J. Schgoer W. Theurl M. Nardin I. Lener D. et al (2017). Nanoparticular delivery system for a secretoneurin derivative induces angiogenesis in a hind limb ischemia model. J. Control. Release250, 1–8. 10.1016/j.jconrel.2017.02.004
7
Amann B. Lüdemann C. Ratei R. Schmidt-Lucke J. A. (2009). Autologous bone-marrow stem-cell transplantation for induction of arteriogenesis for limb salvage in critical limb ischaemia. Zentralbl. Chir.134 (4), 298–304. 10.1055/s-0029-1224532
8
American Diabetes A. (2003). Peripheral arterial disease in people with diabetes. Diabetes Care26 (12), 3333–3341. 10.2337/diacare.26.12.3333
9
Anderson E. M. Silva E. A. Hao Y. Martinick K. D. Vermillion S. A. Stafford A. G. et al (2017). VEGF and IGF delivered from alginate hydrogels promote stable perfusion recovery in ischemic hind limbs of aged mice and young rabbits. J. Vasc. Res.54 (5), 288–298. 10.1159/000479869
10
Armstrong E. J. Wu J. Singh G. D. Dawson D. L. Pevec W. C. Amsterdam E. A. et al (2014). Smoking cessation is associated with decreased mortality and improved amputation-free survival among patients with symptomatic peripheral artery disease. J. Vasc. Surg.60 (6), 1565–1571. 10.1016/j.jvs.2014.08.064
11
Barker N. W. (1939). Occlusive arterial disease of the lower extremities associated with lipemia and xanthoma tuberosum. Ann. Intern. Med.12 (11), 1891–1895.
12
Belch J. J. Mackay I. R. Hill A. Jennings P. McCollum P. (1995). Oxidative stress is present in atherosclerotic peripheral arterial disease and further increased by diabetes mellitus. Int. Angiol.14 (4), 385–388.
13
Benoit E. O'Donnell T. F. Jr. Iafrati M. D. Asher E. Bandyk D. F. Hallett J. W. et al (2011). The role of amputation as an outcome measure in cellular therapy for critical limb ischemia: Implications for clinical trial design. J. Transl. Med.9, 165. 10.1186/1479-5876-9-165
14
Bhang S. H. Kim J. H. Yang H. S. La W. G. Lee T. J. Sun A. Y. et al (2009). Combined delivery of heme oxygenase-1 gene and fibroblast growth factor-2 protein for therapeutic angiogenesis. Biomaterials30 (31), 6247–6256. 10.1016/j.biomaterials.2009.07.058
15
Bharadwaz A. Jayasuriya A. C. (2020). Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mat. Sci. Eng. C Mat. Biol. Appl.110, 110698. 10.1016/j.msec.2020.110698
16
Biscetti F. Nardella E. Rando M. M. Cecchini A. L. Gasbarrini A. Massetti M. et al (2021). Outcomes of lower extremity endovascular revascularization: Potential predictors and prevention strategies. Int. J. Mol. Sci.22 (4), 2002. 10.3390/ijms22042002
17
Bonaca M. P. Bauersachs R. M. Anand S. S. Debus E. S. Nehler M. R. Patel M. R. et al (2020). Rivaroxaban in peripheral artery disease after revascularization. N. Engl. J. Med.382 (21), 1994–2004. 10.1056/NEJMoa2000052
18
Buwalda S. J. Vermonden T. Hennink W. E. (2017). Hydrogels for therapeutic delivery: Current developments and future directions. Biomacromolecules18 (2), 316–330. 10.1021/acs.biomac.6b01604
19
Campbell D. A. Smith R. G. (1950). The arteriographic examination of the lower extremity. Angiology1 (1), 100–105. 10.1177/000331975000100108
20
Carrabba M. Jover E. Fagnano M. Thomas A. C. Avolio E. Richardson T. et al (2020). Fabrication of new hybrid scaffolds for in vivo perivascular application to treat limb ischemia. Front. Cardiovasc. Med.7, 598890. 10.3389/fcvm.2020.598890
21
Chalothorn D. Clayton J. A. Zhang H. Pomp D. Faber J. E. (2007). Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains. Physiol. Genomics30 (2), 179–191. 10.1152/physiolgenomics.00047.2007
22
Chappell J. C. Song J. Burke C. W. Klibanov A. L. Price R. J. (2008). Targeted delivery of nanoparticles bearing fibroblast growth factor-2 by ultrasonic microbubble destruction for therapeutic arteriogenesis. Small4 (10), 1769–1777. 10.1002/smll.200800806
23
Choi J. Choi W. Joo Y. Chung H. Kim D. Oh S. J. et al (2021). FGF2-primed 3D spheroids producing IL-8 promote therapeutic angiogenesis in murine hindlimb ischemia. NPJ Regen. Med.6 (1), 48. 10.1038/s41536-021-00159-7
24
Chu H. Wang Y. (2012). Therapeutic angiogenesis: Controlled delivery of angiogenic factors. Ther. Deliv.3 (6), 693–714. 10.4155/tde.12.50
25
de Muinck E. D. Simons M. (2004). Re-evaluating therapeutic neovascularization. J. Mol. Cell. Cardiol.36 (1), 25–32. 10.1016/j.yjmcc.2003.10.002
26
Delcroix G. J. Schiller P. C. Benoit J. P. Montero-Menei C. N. (2010). Adult cell therapy for brain neuronal damages and the role of tissue engineering. Biomaterials31 (8), 2105–2120. 10.1016/j.biomaterials.2009.11.084
27
Dhatariya K. Bain S. C. Buse J. B. Simpson R. Tarnow L. Kaltoft M. S. et al (2018). The impact of liraglutide on diabetes-related foot ulceration and associated complications in patients with type 2 diabetes at high risk for cardiovascular events: Results from the LEADER trial. Diabetes Care41 (10), 2229–2235. 10.2337/dc18-1094
28
Farber A. Eberhardt R. T. (2016). The current state of critical limb ischemia: A systematic review. JAMA Surg.151 (11), 1070–1077. 10.1001/jamasurg.2016.2018
29
Fernandez S. Parodi J. C. Moscovich F. Pulmari C. (2018). Reversal of lower-extremity intermittent claudication and rest pain by hydration. Ann. Vasc. Surg.49, 1–7. 10.1016/j.avsg.2018.01.074
30
Fowkes F. G. Aboyans V. Fowkes F. J. McDermott M. M. Sampson U. K. Criqui M. H. (2017). Peripheral artery disease: Epidemiology and global perspectives. Nat. Rev. Cardiol.14 (3), 156–170. 10.1038/nrcardio.2016.179
31
Fowkes F. G. Rudan D. Rudan I. Aboyans V. Denenberg J. O. McDermott M. M. et al (2013). Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet382 (9901), 1329–1340. 10.1016/S0140-6736(13)61249-0
32
Fujita M. Horio T. Kishimoto S. Nakamura S. Takikawa M. Nakayama T. et al (2013). Effects of platelet-rich plasma-containing fragmin/protamine microparticles in enhancing endothelial and smooth muscle cell growth and inducing collateral vessels in a rabbit model of hindlimb ischemia. J. Biomed. Mat. Res. B Appl. Biomater.101 (1), 36–42. 10.1002/jbm.b.32808
33
Gao W. Chen D. Liu G. Ran X. (2019). Autologous stem cell therapy for peripheral arterial disease: A systematic review and meta-analysis of randomized controlled trials. Stem Cell Res. Ther.10 (1), 140. 10.1186/s13287-019-1254-5
34
Gao X. Gao M. Gorecka J. Langford J. Liu J. Luo J. et al (2021). Human-induced pluripotent stem-cell-derived smooth muscle cells increase angiogenesis to treat hindlimb ischemia. Cells10 (4), 792. 10.3390/cells10040792
35
Gebauer K. Reinecke H. (2018). PCSK9 inhibition for LDL lowering and beyond - implications for patients with peripheral artery disease. Vasa.47 (3), 165–176. 10.1024/0301-1526/a000689
36
Gerhard-Herman M. D. Gornik H. L. Barrett C. Barshes N. R. Corriere M. A. Drachman D. E. et al (2017). 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: Executive summary: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation135 (12), e686–e725. 10.1161/CIR.0000000000000470
37
Gu Y. Cui S. Wang Q. Liu C. Jin B. Guo W. et al (2019). A randomized, double-blind, placebo-controlled phase II study of hepatocyte growth factor in the treatment of critical limb ischemia. Mol. Ther.27 (12), 2158–2165. 10.1016/j.ymthe.2019.10.017
38
Gupta P. K. Chullikana A. Parakh R. Desai S. Das A. Gottipamula S. et al (2013). A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. J. Transl. Med.11, 143. 10.1186/1479-5876-11-143
39
Heyward J. Mansour O. Olson L. Singh S. Alexander G. C. (2020). Association between sodium-glucose cotransporter 2 (SGLT2) inhibitors and lower extremity amputation: A systematic review and meta-analysis. PLoS One15 (6), e0234065. 10.1371/journal.pone.0234065
40
Ho J. Yue D. Cheema U. Hsia H. C. Dardik A. (2022). Innovations in stem cell therapy for diabetic wound healing. Adv. Wound Care (New Rochelle)2022. 10.1089/wound.2021.0104
41
Hong Y. Chen J. Fang H. Li G. Yan S. Zhang K. et al (2020). All-in-One hydrogel realizing adipose-derived stem cell spheroid production and in vivo injection via "Gel-Sol" transition for angiogenesis in hind limb ischemia. ACS Appl. Mat. Interfaces12 (10), 11375–11387. 10.1021/acsami.9b23534
42
Huang A. Liu D. Qi X. Yue Z. Cao H. Zhang K. et al (2019). Self-assembled GFFYK peptide hydrogel enhances the therapeutic efficacy of mesenchymal stem cells in a mouse hindlimb ischemia model. Acta Biomater.85, 94–105. 10.1016/j.actbio.2018.12.015
43
Huang L. C. Wang H. C. Chen L. H. Ho C. Y. Hsieh P. H. Huang M. Y. et al (2019). Bioinspired self-assembling peptide hydrogel with proteoglycan-assisted growth factor delivery for therapeutic angiogenesis. Theranostics9 (23), 7072–7087. 10.7150/thno.35803
44
Jayakumar R. Prabaharan M. Nair S. V. Tamura H. (2010). Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol. Adv.28 (1), 142–150. 10.1016/j.biotechadv.2009.11.001
45
Jhang J. Y. Tzeng I. S. Chou H. H. Jang S. J. Hsieh C. A. Ko Y. L. et al (2020). Association rule mining and prognostic stratification of 2-year longevity in octogenarians undergoing endovascular therapy for lower extremity arterial disease: Observational cohort study. J. Med. Internet Res.22 (12), e17487. 10.2196/17487
46
Jia X. Qi Y. Zheng R. Lin L. Hu C. Zhu Y. et al (2022). Discordance of apolipoprotein B, non-HDL-cholesterol, and LDL-cholesterol predicts risk of increased arterial stiffness and elevated carotid intima-media thickness in middle-aged and elderly Chinese adults. Front. Cardiovasc. Med.9, 906396. 10.3389/fcvm.2022.906396
47
Jonason T. Bergstrom R. (1987). Cessation of smoking in patients with intermittent claudication. Effects on the risk of peripheral vascular complications, myocardial infarction and mortality. Acta Med. Scand.221 (3), 253–260. 10.1111/j.0954-6820.1987.tb00891.x
48
Jonsson T. B. Larzon T. Arfvidsson B. Tidefelt U. Axelsson C. G. Jurstrand M. et al (2012). Adverse events during treatment of critical limb ischemia with autologous peripheral blood mononuclear cell implant. Int. Angiol.31 (1), 77–84.
49
Jun I. Ahmad T. Bak S. Lee J. Y. Kim E. M. Lee J. et al (2017). Spatially assembled bilayer cell sheets of stem cells and endothelial cells using thermosensitive hydrogels for therapeutic angiogenesis. Adv. Healthc. Mat.6 (9), 1601340. 10.1002/adhm.201601340
50
Kalantari K. Afifi A. M. Jahangirian H. Webster T. J. (2019). Biomedical applications of chitosan electrospun nanofibers as a green polymer - Review. Carbohydr. Polym.207, 588–600. 10.1016/j.carbpol.2018.12.011
51
Kamei M. Saunders W. B. Bayless K. J. Dye L. Davis G. E. Weinstein B. M. (2006). Endothelial tubes assemble from intracellular vacuoles in vivo. Nature442 (7101), 453–456. 10.1038/nature04923
52
Kim P. H. Yim H. G. Choi Y. J. Kang B. J. Kim J. Kwon S. M. et al (2014). Injectable multifunctional microgel encapsulating outgrowth endothelial cells and growth factors for enhanced neovascularization. J. Control. Release187, 1–13. 10.1016/j.jconrel.2014.05.010
53
Kwee B. J. Budina E. Najibi A. J. Mooney D. J. (2018). CD4 T-cells regulate angiogenesis and myogenesis. Biomaterials178, 109–121. 10.1016/j.biomaterials.2018.06.003
54
Kwon H. J. Cha M. Y. Kim D. Kim D. K. Soh M. Shin K. et al (2016). Mitochondria-targeting ceria nanoparticles as antioxidants for alzheimer's disease. ACS Nano10 (2), 2860–2870. 10.1021/acsnano.5b08045
55
Lammer J. Bosiers M. Deloose K. Schmidt A. Zeller T. Wolf F. et al (2016). Bioresorbable everolimus-eluting vascular scaffold for patients with peripheral artery disease (ESPRIT I): 2-Year clinical and imaging results. JACC. Cardiovasc. Interv.9 (11), 1178–1187. 10.1016/j.jcin.2016.02.051
56
Lane R. Harwood A. Watson L. Leng G. C. (2017). Exercise for intermittent claudication. Cochrane Database Syst. Rev.12, CD000990. 10.1002/14651858.CD000990.pub4
57
Lara-Hernandez R. Lozano-Vilardell P. Blanes P. Torreguitart-Mirada N. Galmes A. Besalduch J. (2010). Safety and efficacy of therapeutic angiogenesis as a novel treatment in patients with critical limb ischemia. Ann. Vasc. Surg.24 (2), 287–294. 10.1016/j.avsg.2009.10.012
58
Lasala G. P. Silva J. A. Minguell J. J. (2012). Therapeutic angiogenesis in patients with severe limb ischemia by transplantation of a combination stem cell product. J. Thorac. Cardiovasc. Surg.144 (2), 377–382. 10.1016/j.jtcvs.2011.08.053
59
Layman H. Sacasa M. Murphy A. E. Murphy A. M. Pham S. M. Andreopoulos F. M. (2009). Co-delivery of FGF-2 and G-CSF from gelatin-based hydrogels as angiogenic therapy in a murine critical limb ischemic model. Acta Biomater.5 (1), 230–239. 10.1016/j.actbio.2008.07.024
60
Leclerc C. J. Cooper T. T. Bell G. I. Lajoie G. A. Flynn L. E. Hess D. A. (2021). Decellularized adipose tissue scaffolds guide hematopoietic differentiation and stimulate vascular regeneration in a hindlimb ischemia model. Biomaterials274, 120867. 10.1016/j.biomaterials.2021.120867
61
Lee E. J. Kasper F. K. Mikos A. G. (2014). Biomaterials for tissue engineering. Ann. Biomed. Eng.42 (2), 323–337. 10.1007/s10439-013-0859-6
62
Lee J. B. Kim D. H. Yoon J. K. Park D. B. Kim H. S. Shin Y. M. et al (2020). Microchannel network hydrogel induced ischemic blood perfusion connection. Nat. Commun.11 (1), 615. 10.1038/s41467-020-14480-0
63
Lee J. Y. Ryu S. Sung K. C. (2018). Association of baseline level of physical activity and its temporal changes with incident hypertension and diabetes mellitus. Eur. J. Prev. Cardiol.25 (10), 1065–1073. 10.1177/2047487318774419
64
Lee N. H. Bayaraa O. Zechu Z. Kim H. S. (2021). Biomaterials-assisted spheroid engineering for regenerative therapy. BMB Rep.54 (7), 356–367. 10.5483/bmbrep.2021.54.7.059
65
Lee S. Valmikinathan C. M. Byun J. Kim S. Lee G. Mokarram N. et al (2015). Enhanced therapeutic neovascularization by CD31-expressing cells and embryonic stem cell-derived endothelial cells engineered with chitosan hydrogel containing VEGF-releasing microtubes. Biomaterials63, 158–167. 10.1016/j.biomaterials.2015.06.009
66
Li R. Liang J. He Y. Qin J. He H. Lee S. et al (2018). Sustained release of immunosuppressant by nanoparticle-anchoring hydrogel scaffold improved the survival of transplanted stem cells and tissue regeneration. Theranostics8 (4), 878–893. 10.7150/thno.22072
67
Li R. Pang Z. He H. Lee S. Qin J. Wu J. et al (2017). Drug depot-anchoring hydrogel: A self-assembling scaffold for localized drug release and enhanced stem cell differentiation. J. Control. Release261, 234–245. 10.1016/j.jconrel.2017.07.008
68
Liew A. Bhattacharya V. Shaw J. Stansby G. (2016). Cell therapy for critical limb ischemia: A meta-analysis of randomized controlled trials. Angiology67 (5), 444–455. 10.1177/0003319715595172
69
Lin C. C. Anseth K. S. (2009). PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm. Res.26 (3), 631–643. 10.1007/s11095-008-9801-2
70
Liu G. Fang Z. Yuan M. Li W. Yang Y. Jiang M. et al (2017). Biodegradable carriers for delivery of VEGF plasmid DNA for the treatment of critical limb ischemia. Front. Pharmacol.8, 528. 10.3389/fphar.2017.00528
71
Loureiro J. Torres A. L. Neto T. Aguiar P. Barrias C. C. Pinto M. T. et al (2019). Conjugation of the T1 sequence from CCN1 to fibrin hydrogels for therapeutic vascularization. Mat. Sci. Eng. C Mat. Biol. Appl.104, 109847. 10.1016/j.msec.2019.109847
72
Makarevich P. Tsokolaeva Z. Shevelev A. Rybalkin I. Shevchenko E. Beloglazova I. et al (2012). Combined transfer of human VEGF165 and HGF genes renders potent angiogenic effect in ischemic skeletal muscle. PLoS One7 (6), e38776. 10.1371/journal.pone.0038776
73
Martin-Pelaez S. Fito M. Castaner O. (2020). Mediterranean diet effects on type 2 diabetes prevention, disease progression, and related mechanisms. A review. Nutrients12 (8), 2236. 10.3390/nu12082236
74
Masaki I. Yonemitsu Y. Yamashita A. Sata S. Tanii M. Komori K. et al (2002). Angiogenic gene therapy for experimental critical limb ischemia: Acceleration of limb loss by overexpression of vascular endothelial growth factor 165 but not of fibroblast growth factor-2. Circ. Res.90 (9), 966–973. 10.1161/01.res.0000019540.41697.60
75
Matsui M. Tabata Y. (2012). Enhanced angiogenesis by multiple release of platelet-rich plasma contents and basic fibroblast growth factor from gelatin hydrogels. Acta Biomater.8 (5), 1792–1801. 10.1016/j.actbio.2012.01.016
76
Matsumoto T. Yamashita S. Yoshino S. Kurose S. Morisaki K. Nakano K. et al (2020). Therapeutic arteriogenesis/angiogenesis for peripheral arterial disease by nanoparticle-mediated delivery of Pitavastatin into vascular endothelial cells. Ann. Vasc. Dis.13 (1), 4–12. 10.3400/avd.ra.19-00130
77
McQueen M. J. Hawken S. Wang X. Ounpuu S. Sniderman A. Probstfield J. et al (2008). Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): A case-control study. Lancet372 (9634), 224–233. 10.1016/S0140-6736(08)61076-4
78
Minami T. Kameda A. Terauchi Y. (2021). An evaluation of canagliflozin for the treatment of type 2 diabetes: An update. Expert Opin. Pharmacother.22 (16), 2087–2094. 10.1080/14656566.2021.1939675
79
Moazzami K. Moazzami B. Roohi A. Nedjat S. Dolmatova E. (2014). Local intramuscular transplantation of autologous mononuclear cells for critical lower limb ischaemia. Cochrane Database Syst. Rev.12, CD008347. 10.1002/14651858.CD008347.pub2
80
Mohammedi K. Woodward M. Hirakawa Y. Zoungas S. Williams B. Lisheng L. et al (2016). Microvascular and macrovascular disease and risk for major peripheral arterial disease in patients with type 2 diabetes. Diabetes Care39 (10), 1796–1803. 10.2337/dc16-0588
81
Moon S. H. Kim J. S. Park S. J. Lee H. J. Do J. T. Chung H. M. (2011). A system for treating ischemic disease using human embryonic stem cell-derived endothelial cells without direct incorporation. Biomaterials32 (27), 6445–6455. 10.1016/j.biomaterials.2011.05.026
82
Mu R. Zhang Y. Yan L. Liao Z. Yang Y. Su H. et al (2021). A "Bridge-Building" glycan scaffold mimicking microbial invasion for in situ endothelialization. Adv. Mater.33, e2103490. 10.1002/adma.202103490
83
Mulyasasmita W. Cai L. Dewi R. E. Jha A. Ullmann S. D. Luong R. H. et al (2014). Avidity-controlled hydrogels for injectable co-delivery of induced pluripotent stem cell-derived endothelial cells and growth factors. J. Control. Release191, 71–81. 10.1016/j.jconrel.2014.05.015
84
Naganuma T. Traversa E. (2014). The effect of cerium valence states at cerium oxide nanoparticle surfaces on cell proliferation. Biomaterials35 (15), 4441–4453. 10.1016/j.biomaterials.2014.01.074
85
Naide Oscillometry (1939). Diagnosis of arteriosclerosis of the lower extremities. Archives Intern. Med.63 (6), 1158–1162.
86
Nakamura S. Ishihara M. Takikawa M. Kishimoto S. Isoda S. Fujita M. et al (2012). Attenuation of limb loss in an experimentally induced hindlimb ischemic model by fibroblast growth factor-2/fragmin/protamine microparticles as a delivery system. Tissue Eng. Part A18 (21-22), 2239–2247. 10.1089/ten.TEA.2011.0741
87
Nativel M. Potier L. Alexandre L. Baillet-Blanco L. Ducasse E. Velho G. et al (2018). Lower extremity arterial disease in patients with diabetes: A contemporary narrative review. Cardiovasc. Diabetol.17 (1), 138–151. 10.1186/s12933-018-0781-1
88
Nazri M. Y. Aminudin C. A. Ahmad F. S. Mohd Jazlan M. A. Jamalludin Ab R. Ramli M. (2019). Quality of life of diabetes amputees following major and minor lower limb amputations. Med. J. Malays.74 (1), 25–29.
89
Nillesen S. T. Geutjes P. J. Wismans R. Schalkwijk J. Daamen W. F. van Kuppevelt T. H. (2007). Increased angiogenesis and blood vessel maturation in acellular collagen-heparin scaffolds containing both FGF2 and VEGF. Biomaterials28 (6), 1123–1131. 10.1016/j.biomaterials.2006.10.029
90
Norgren L. Hiatt W. R. Dormandy J. A. Nehler M. R. Harris K. A. Fowkes F. G. et al (2007). Inter-society consensus for the management of peripheral arterial disease (TASC II). J. Vasc. Surg.45, S5–S67. 10.1016/j.jvs.2006.12.037
91
Novakova V. Sandhu G. S. Dragomir-Daescu D. Klabusay M. (2016). Apelinergic system in endothelial cells and its role in angiogenesis in myocardial ischemia. Vasc. Pharmacol.76, 1–10. 10.1016/j.vph.2015.08.005
92
Ozturk A. Kucukardali Y. Tangi F. Erikci A. Uzun G. Bashekim C. et al (2012). Therapeutical potential of autologous peripheral blood mononuclear cell transplantation in patients with type 2 diabetic critical limb ischemia. J. Diabetes Complicat.26 (1), 29–33. 10.1016/j.jdiacomp.2011.11.007
93
Palmer L. C. Stupp S. I. (2008). Molecular self-assembly into one-dimensional nanostructures. Acc. Chem. Res.41 (12), 1674–1684. 10.1021/ar8000926
94
Pan T. Liu H. Fang Y. Wei Z. Gu S. Fang G. et al (2019). Predictors of responders to mononuclear stem cell-based therapeutic angiogenesis for no-option critical limb ischemia. Stem Cell Res. Ther.10 (1), 15–27. 10.1186/s13287-018-1117-5
95
Pan T. Wei Z. Fang Y. Dong Z. Fu W. (2018). Therapeutic efficacy of CD34(+) cell-involved mononuclear cell therapy for no-option critical limb ischemia: A meta-analysis of randomized controlled clinical trials. Vasc. Med.23 (3), 219–231. 10.1177/1358863X17752556
96
Papavassiliou V. G. Walker S. R. Bolia A. Fishwick G. London N. (2003). Techniques for the endovascular management of complications following lower limb percutaneous transluminal angioplasty. Eur. J. Vasc. Endovasc. Surg.25 (2), 125–130. 10.1053/ejvs.2002.1822
97
Park H. J. Jin Y. Shin J. Yang K. Lee C. Yang H. S. et al (2016). Catechol-functionalized hyaluronic acid hydrogels enhance angiogenesis and osteogenesis of human adipose-derived stem cells in critical tissue defects. Biomacromolecules17 (6), 1939–1948. 10.1021/acs.biomac.5b01670
98
Park H. S. Choi G. H. Kim D. Jung T. W. Jung I. M. Chung J. K. et al (2018). Use of self-assembling peptides to enhance stem cell function for therapeutic angiogenesis. Stem Cells Int.2018, 4162075. 10.1155/2018/4162075
99
Park I. S. Chung P. S. Ahn J. C. (2014). Enhanced angiogenic effect of adipose-derived stromal cell spheroid with low-level light therapy in hind limb ischemia mice. Biomaterials35 (34), 9280–9289. 10.1016/j.biomaterials.2014.07.061
100
Park I. S. Mahapatra C. Park J. S. Dashnyam K. Kim J. W. Ahn J. C. et al (2020). Revascularization and limb salvage following critical limb ischemia by nanoceria-induced Ref-1/APE1-dependent angiogenesis. Biomaterials242, 11991. 10.1016/j.biomaterials.2020.119919
101
Park J. J. Kwon Y. W. Kim J. W. Park G. T. Yoon J. W. Kim Y. S. et al (2021). Coadministration of endothelial and smooth muscle cells derived from human induced pluripotent stem cells as a therapy for critical limb ischemia. Stem Cells Transl. Med.10 (3), 414–426. 10.1002/sctm.20-0132
102
Park J. S. Bae S. H. Jung S. Lee M. Choi D. (2019). Enrichment of vascular endothelial growth factor secreting mesenchymal stromal cells enhances therapeutic angiogenesis in a mouse model of hind limb ischemia. Cytotherapy21 (4), 433–443. 10.1016/j.jcyt.2018.12.007
103
Peeters Weem S. M. Teraa M. de Borst G. J. Verhaar M. C. Moll F. L. (2015). Bone marrow derived cell therapy in critical limb ischemia: A meta-analysis of randomized placebo controlled trials. Eur. J. Vasc. Endovasc. Surg.50 (6), 775–783. 10.1016/j.ejvs.2015.08.018
104
Pei X. Kim H. Lee M. Wang N. Shin J. Lee S. et al (2020). Local delivery of cardiac stem cells overexpressing HIF-1α promotes angiogenesis and muscular tissue repair in a hind limb ischemia model. J. Control. Release322, 610–621. 10.1016/j.jconrel.2020.03.017
105
Qadura M. Terenzi D. C. Verma S. Al-Omran M. Hess D. A. (2018). Concise review: Cell therapy for critical limb ischemia: An integrated review of preclinical and clinical studies. Stem Cells36 (2), 161–171. 10.1002/stem.2751
106
Rajagopalan S. Mohler E. 3rd Lederman R. J. Saucedo J. Mendelsohn F. O. Olin J. et al (2003). Regional angiogenesis with vascular endothelial growth factor (VEGF) in peripheral arterial disease: Design of the RAVE trial. Am. Heart J.145 (6), 1114–1118. 10.1016/S0002-8703(03)00102-9
107
Rigato M. Monami M. Fadini G. P. (2017). Autologous cell therapy for peripheral arterial disease: Systematic review and meta-analysis of randomized, nonrandomized, and noncontrolled studies. Circ. Res.120 (8), 1326–1340. 10.1161/CIRCRESAHA.116.309045
108
Ruvinov E. Leor J. Cohen S. (2010). The effects of controlled HGF delivery from an affinity-binding alginate biomaterial on angiogenesis and blood perfusion in a hindlimb ischemia model. Biomaterials31 (16), 4573–4582. 10.1016/j.biomaterials.2010.02.026
109
Sacchi V. Mittermayr R. Hartinger J. Martino M. M. Lorentz K. M. Wolbank S. et al (2014). Long-lasting fibrin matrices ensure stable and functional angiogenesis by highly tunable, sustained delivery of recombinant VEGF164. Proc. Natl. Acad. Sci. U. S. A.111 (19), 6952–6957. 10.1073/pnas.1404605111
110
Schwartz L. B. Moawad J. (1997). Gene therapy for vascular disease. Ann. Vasc. Surg.11 (2), 189–199. 10.1007/s100169900034
111
Sethi G. K. Scott S. M. Takaro T. (1980). Effect of intraarterial infusion of PGE1 in patients with severe ischemia of lower extremity. J. Cardiovasc. Surg.21 (2), 185–192.
112
Skora J. Pupka A. Janczak D. Barc P. Dawiskiba T. Korta K. et al (2015). Combined autologous bone marrow mononuclear cell and gene therapy as the last resort for patients with critical limb ischemia. Arch. Med. Sci.11 (2), 325–331. 10.5114/aoms.2013.39935
113
Slobodkina E. Boldyreva M. Karagyaur M. Eremichev R. Alexandrushkina N. Balabanyan V. et al (2020). Therapeutic angiogenesis by a "dynamic duo": Simultaneous expression of HGF and VEGF165 by novel bicistronic plasmid restores blood flow in ischemic skeletal muscle. Pharmaceutics12 (12), 1231. 10.3390/pharmaceutics12121231
114
Slovut D. P. Lipsitz E. C. (2012). Surgical technique and peripheral artery disease. Circulation126 (9), 1127–1138. 10.1161/CIRCULATIONAHA.111.059048
115
Soyoye D. O. Abiodun O. O. Ikem R. T. Kolawole B. A. Akintomide A. O. (2021). Diabetes and peripheral artery disease: A review. World J. Diabetes12 (6), 827–838. 10.4239/wjd.v12.i6.827
116
Sun H. Saeedi P. Karuranga S. Pinkepank M. Ogurtsova K. Duncan B. B. et al (2022). IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract.183, 109119. 10.1016/j.diabres.2021.109119
117
Suzuki E. Kashiwagi A. Nishio Y. Egawa K. Shimizu S. Maegawa H. et al (2001). Increased arterial wall stiffness limits flow volume in the lower extremities in type 2 diabetic patients. Diabetes Care24 (12), 2107–2114. 10.2337/diacare.24.12.2107
118
Szabo G. V. Kovesd Z. Cserepes J. Daroczy J. Belkin M. Acsady G. (2013). Peripheral blood-derived autologous stem cell therapy for the treatment of patients with late-stage peripheral artery disease-results of the short- and long-term follow-up. Cytotherapy15 (10), 1245–1252. 10.1016/j.jcyt.2013.05.017
119
Takeshita S. Zheng L. P. Brogi E. Kearney M. Pu L. Q. Bunting S. et al (1994). Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J. Clin. Invest.93 (2), 662–670. 10.1172/JCI117018
120
Tan R. P. Chan A. H. P. Lennartsson K. Miravet M. M. Lee B. S. L. Rnjak-Kovacina J. et al (2018). Integration of induced pluripotent stem cell-derived endothelial cells with polycaprolactone/gelatin-based electrospun scaffolds for enhanced therapeutic angiogenesis. Stem Cell Res. Ther.9 (1), 70. 10.1186/s13287-018-0824-2
121
Tan Y. Shao H. Eton D. Yang Z. Alonso-Diaz L. Zhang H. et al (2007). Stromal cell-derived factor-1 enhances pro-angiogenic effect of granulocyte-colony stimulating factor. Cardiovasc. Res.73 (4), 823–832. 10.1016/j.cardiores.2006.12.015
122
Tang J. Cui X. Caranasos T. G. Hensley M. T. Vandergriff A. C. Hartanto Y. et al (2017). Heart repair using nanogel-encapsulated human cardiac stem cells in mice and pigs with myocardial infarction. ACS Nano11 (10), 9738–9749. 10.1021/acsnano.7b01008
123
Taniwaki H. Shoji T. Emoto M. Kawagishi T. Ishimura E. Inaba M. et al (2001). Femoral artery wall thickness and stiffness in evaluation of peripheral vascular disease in type 2 diabetes mellitus. Atherosclerosis158 (1), 207–214. 10.1016/s0021-9150(01)00414-2
124
Tanner M. R. Beeton C. (2018). Differences in ion channel phenotype and function between humans and animal models. Front. Biosci.23 (1), 43–64. 10.2741/4581
125
Teraa M. Conte M. S. Moll F. L. Verhaar M. C. (2016). Critical limb ischemia: Current trends and future directions. J. Am. Heart Assoc.5 (2), e002938. 10.1161/JAHA.115.002938
126
Troy E. Tilbury M. A. Power A. M. Wall J. G. (2021). Nature-based biomaterials and their application in biomedicine. Polym. (Basel)13 (19), 3321. 10.3390/polym13193321
127
Van Raemdonck K. Van den Steen P. E. Liekens S. Van Damme J. Struyf S. (2015). CXCR3 ligands in disease and therapy. Cytokine Growth Factor Rev.26 (3), 311–327. 10.1016/j.cytogfr.2014.11.009
128
Veith A. P. Henderson K. Spencer A. Sligar A. D. Baker A. B. (2019). Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev.146, 97–125. 10.1016/j.addr.2018.09.010
129
Wang C. Y. Hsiao C. Y. Tsai K. L. Cheng Y. H. (2020). Injectable thermosensitive chitosan-based hydrogel containing ferulic acid for treating peripheral arterial disease. J. Tissue Eng. Regen. Med.14 (10), 1438–1448. 10.1002/term.3109
130
Wang J. Cui W. Ye J. Ji S. Zhao X. Zhan L. et al (2012). A cellular delivery system fabricated with autologous BMSCs and collagen scaffold enhances angiogenesis and perfusion in ischemic hind limb. J. Biomed. Mat. Res. A100 (6), 1438–1447. 10.1002/jbm.a.34081
131
Wang K. Dai X. He J. Yan X. Yang C. Fan X. et al (2020). Endothelial overexpression of metallothionein prevents diabetes-induced impairment in ischemia angiogenesis through preservation of HIF-1α/SDF-1/VEGF signaling in endothelial progenitor cells. Diabetes69 (8), 1779–1792. 10.2337/db19-0829
132
Wang R. Yao X. Li T. Li X. Jin M. Ni Y. et al (2019). Reversible thermoresponsive hydrogel fabricated from natural biopolymer for the improvement of critical limb ischemia by controlling release of stem cells. Adv. Healthc. Mat.8 (20), e1900967. 10.1002/adhm.201900967
133
Xie B. Luo H. Zhang Y. Wang Q. Zhou C. Xu D. (2018). Autologous stem cell therapy in critical limb ischemia: A meta-analysis of randomized controlled trials. Stem Cells Int.2018, 7528464. 10.1155/2018/7528464
134
Xie J. Wang H. Wang Y. Ren F. Yi W. Zhao K. et al (2013). Induction of angiogenesis by controlled delivery of vascular endothelial growth factor using nanoparticles. Cardiovasc. Ther.31 (3), e12–8. 10.1111/j.1755-5922.2012.00317.x
135
Xing Z. Zhao C. Liu H. Fan Y. (2020). Endothelial progenitor cell-derived extracellular vesicles: A novel candidate for regenerative medicine and disease treatment. Adv. Healthc. Mat.9 (12), e2000255. 10.1002/adhm.202000255
136
Xing Z. Zhao C. Wu S. Yang D. Zhang C. Wei X. et al (2021). Hydrogel loaded with VEGF/TFEB-Engineered extracellular vesicles for rescuing critical limb ischemia by a dual-pathway activation strategy. Adv. Healthc. Mat.11, e2100334. 10.1002/adhm.202100334
137
Xing Z. Zhao C. Wu S. Zhang C. Liu H. Fan Y. (2021). Hydrogel-based therapeutic angiogenesis: An alternative treatment strategy for critical limb ischemia. Biomaterials274, 120872. 10.1016/j.biomaterials.2021.120872
138
Yang S. L. Zhu L. Y. Han R. Sun L. L. Li J. X. Dou J. T. (2017). Pathophysiology of peripheral arterial disease in diabetes mellitus. J. Diabetes9 (2), 133–140. 10.1111/1753-0407.12474
139
Yokoyama M. Watanabe T. Otaki Y. Watanabe K. Toshima T. Sugai T. et al (2018). Impact of objective malnutrition Status on the clinical outcomes in patients with peripheral artery disease following endovascular therapy. Circ. J.82 (3), 847–856. 10.1253/circj.CJ-17-0731
140
Zhang Z. D. Xu Y. Q. Chen F. Luo J. F. Liu C. D. (2019). Sustained delivery of vascular endothelial growth factor using a dextran/poly(lactic-co-glycolic acid)-combined microsphere system for therapeutic neovascularization. Heart Vessels34 (1), 167–176. 10.1007/s00380-018-1230-5
141
Zhang Z. Gupte M. J. Ma P. X. (2013). Biomaterials and stem cells for tissue engineering. Expert Opin. Biol. Ther.13 (4), 527–540. 10.1517/14712598.2013.756468
142
Zhao Y. Liu Z. Pan C. Li Z. Zhou J. Wang J. et al (2011). Preparation of gelatin microspheres encapsulated with bFGF for therapeutic angiogenesis in a canine ischemic hind limb. J. Biomater. Sci. Polym. Ed.22 (4-6), 665–682. 10.1163/092050610X489880
143
Zhou J. Zhao Y. Wang J. Zhang S. Liu Z. Zhen M. et al (2012). Therapeutic angiogenesis using basic fibroblast growth factor in combination with a collagen matrix in chronic hindlimb ischemia. ScientificWorldJournal.2012, 652794. 10.1100/2012/652794
Summary
Keywords
biomaterials, lower extremity arterial disease, therapeutic angiogenesis, critical limb ischemia, peripheral arterial disease
Citation
Pan Y, Luo Y, Hong J, He H, Dai L, Zhu H and Wu J (2022) Advances for the treatment of lower extremity arterial disease associated with diabetes mellitus. Front. Mol. Biosci. 9:929718. doi: 10.3389/fmolb.2022.929718
Received
27 April 2022
Accepted
19 July 2022
Published
17 August 2022
Volume
9 - 2022
Edited by
Yifeng Cao, Zhejiang University of Technology, China
Reviewed by
Ting-Heng Chou, Nationwide Children’s Hospital, United States
Giacomo Buso, University of Lausanne, Switzerland
Kentaro Jujo, Tokyo Women’s Medical University, Japan
Updates
Copyright
© 2022 Pan, Luo, Hong, He, Dai, Zhu and Wu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huacheng He, hehc@wzu.edu.cn; Hong Zhu, zhuhong@wmu.edu.cn
This article was submitted to Molecular Diagnostics and Therapeutics, a section of the journal Frontiers in Molecular Biosciences
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.