Abstract
Macrophages are involved in the whole process of atherosclerosis, which is characterized by accumulation of lipid and inflammation. Presently, clinically used lipid-lowering drugs cannot completely retard the progress of atherosclerosis. Liver X receptor (LXR) plays a key role in regulation of lipid metabolism and inflammation. Accumulating evidence have demonstrated that synthetic LXR agonists can significantly retard the development of atherosclerosis. However, these agonists induce sever hypertriglyceridemia and liver steatosis. These side effects have greatly limited their potential application for therapy of atherosclerosis. The rapid development of drug delivery system makes it possible to delivery interested drugs to special organs or cells using nanocarriers. Macrophages express various receptors which can recognize and ingest specially modified nanocarriers loaded with LXR agonists. In the past decades, a great progress has been made in this field. These macrophage-targeted nanocarriers loaded with LXR agonists are found to decrease atherosclerosis by reducing cholesterol accumulation and inflammatory reactions. Of important, these nanocarriers can alleviate side effects of LXR agonists. In this article, we briefly review the roles of macrophages in atherosclerosis, mechanisms of action of LXR agonists, and focus on the advances of macrophage-targeted nanocarriers loaded with LXR agonists. This work may promote the potential clinical application of these nanocarriers.
Introduction
It is known that the onset and progression of atherosclerosis are associated with elevated low-density lipoprotein (LDL) particles as well as raised TG-rich lipoproteins (Vekic et al., 2022; Zhang B. H et al., 2022). Although the first line lipid-lowering drugs, statins, are capable of lowering LDL cholesterol (LDL-C) and suppress inflammation, these drugs can only reduce ∼30% cardiovascular disease (CVD) events. Furthermore, clinical studies have found various side effects of statin therapy (Qiao et al., 2022; Zhang W et al., 2022). A promising alternative to statin therapy is the inhibition of the proprotein convertase subtilisin/kexin-9 type, thereby increasing the uptake of circulating LDL particles by the liver (Qiao et al., 2022). However, they are not suitable for primary prevention because they are not cost-effectiveness. Additionally, the clinically used TG-lowering drugs, fibrates, are still inconclusive for therapy of atherosclerotic CVD (Zhang B. H et al., 2022; Jin et al., 2023). Currently, some novel therapeutic strategies have been proposed, such as gene-guided therapy, anti-inflammatory therapy, regulation of immunity, endothelial function, and gut microbiota (Björkegren and Lusis, 2022; Chan and Ramji, 2022). Presently, there is no effective strategies to successfully reduce or eliminate atherosclerotic plaques that have formed.
Atherosclerosis is characterized by the formation of atherosclerotic plaques, which are composed of lipids, necrotic nuclei, calcified areas, inflammatory cells, endothelial cells, immune cells, and foam cells derived from macrophages (Liu et al., 2021). Macrophages play an important role in innate and adaptive immunity as well in tissue repair and internal homeostasis. On the one hand, macrophages differentiate into different subtypes and exert distinct functions in the microenvironment of atherosclerotic plaques (Lin et al., 2021). On the other hand, macrophages play a pivotal role in regulation of lipid homeostasis within atherosclerotic plaques (Lin et al., 2021; Björkegren and Lusis, 2022). However, the normal physiological function of macrophages is disturbed in atherosclerosis. For instance, macrophages are the main inflammatory cells in atherosclerotic lesions, playing a leading role in the formation, development, and regression of atherosclerotic plaques (Björkegren and Lusis, 2022). Targeting the recruitment and activation of normal macrophages or regulation of dysfunctional macrophages are promising therapeutic strategies.
Liver X receptor (LXR) belongs to the nuclear receptor family and has two isoforms, LXRα and LXRβ. LXR controls lipid metabolism by regulating the expression of related genes through dimerization with retinoid X receptors (RXRs) (Savla et al., 2022). The expression of LXR varies in different tissues. LXRα is highly expressed in tissues with high lipid metabolism, such as liver, lung, and adipose tissue. LXRβ is widely expressed in distinct tissues. Synthetic LXR agonists have been at the center of active research by pharmaceutical companies, not only because they can combat atherosclerosis, but also due to their potential applications for treatment of other metabolic or neurological disorders as well as cancer (Viennois et al., 2011). Accumulating evidence have shown that the activation of LXR can effectively prevent the development of atherosclerosis by modulating lipid metabolism and inflammation in atherosclerotic plaques (Steffensen et al., 2013; Savla et al., 2022). However, the synthetic ligands that have been developed so far lead to elevated blood triglyceride (TG) levels, fatty liver, and neurological disorders (Schultz et al., 2000; Cave et al., 2016). Of note, targeted drug delivery system may successfully reduce these side effects.
Well-designed nanocarriers can target special tissue and/or cells through specific interaction with biomacromolecules and receptors (Liu et al., 2022; Bhaladhare and Bhattacharjee, 2023). Since the approval of adriamycin liposomes in 1995, nearly 250 kinds of nanotherapeutic drugs have been studied in preclinical or clinical research, especially in cancer therapy (Zhang et al., 2015; Xu et al., 2023). It is worth noting that there are increasing nanotherapeutic studies focusing on atherosclerosis therapy (Soumya and Raghu, 2023). With the development of nanotechnology, nanocarriers can effectively target macrophages in atherosclerosis, enhancing therapeutic effects and reducing the side effects of loaded drugs. Over the last decades, researchers tried to deliver LXR agonists to macrophages within atherosclerotic plaques by distinct nanocarriers, which promote intracellular cholesterol efflux without inducing fatty liver, providing new opportunities for treatment of atherosclerosis by LXR agonists. In this review, we will summarize the advances in the field of macrophage-targeted nanotherapeutic strategies that have been used for therapy of atherosclerosis by focusing on LXR agonists. To make the whole article readable, we will briefly review the roles of macrophages in atherosclerosis and mechanisms of action of LXR agonists. The literatures are searching results of PubMed and Web of Science mainly using “macrophage, atherosclerosis, LXR agonist” as keywords.
Macrophages in atherosclerosis
The pathogenic mechanism of atherosclerosis involved the persistent injuries induced by accumulation of LDL and inflammatory factors resulting in irreparable damage to the structure and function of vascular endothelium. And then, the damaged endothelial cells release monocyte chemokines to recruit large numbers of inflammatory cells which will release inflammatory factors to further accelerate disease progression (Björkegren and Lusis, 2022). Subsequently, LDL in the blood vessels is modified to oxidized LDL (ox-LDL) and can be specifically recognized and taken up by macrophages of monocyte origin to form foam cells ultimately (Lin et al., 2021). In the following sections, we will briefly review the actions of macrophages in lipid metabolism (Figure 1) and inflammation (Figure 2) in atherosclerosis.
FIGURE 1

Cholesterol metabolism and macrophages in atherosclerosis. Nascent high-density lipoprotein (HDL) particles derived from apolipoprotein A-I (apo A-I) that is secreted either by liver or small intestine, uptake cholesterol from different cell types, including macrophages via ATP binding cassette transporter (ABC) A1. ABCG1 mediates cholesterol transport from peripheral cells to mature HDL. HDL-cholesterol can be esterified by lecithin-cholesterol acyltransferase (LCAT) to promote the formation of mature HDL. HDL is absorbed by hepatocytes mainly by scavenger receptor, class B type 1 and is metabolized in the liver. Hepatic synthesized very low-density lipoprotein (VLDL) particles can be transformed to low-density lipoprotein (LDL). These lipoproteins can be uptake by macrophages through receptors such as VLDL receptor (VLDLR), cluster of differentiation 36 (CD36), scavenger receptor A (SR-A). The excess accumulation of lipids in macrophages under the arterial endothelium will promote the occurrence and development of atherosclerosis. These abbreviations are suitable for the following figures.
FIGURE 2

Macrophages and inflammation in atherogenesis. Chronic inflammation promotes the accumulation of monocytes on the surface of endothelial cells of the aorta and induces monocytes transition and transformation. Granulocyte-macrophage colony-stimulating factor and macrophage colony-stimulating factor mediate the polarization of M1 and M2 macrophages, respectively. The polarization of M1 macrophages is promoted by pro-inflammatory factors, such as tumor necrosis factor (TNF), interleukin (IL)-6, monocyte chemoattractant protein 1, and interferon-γ (IFN-γ), while the polarization of M2 macrophages is mediated by anti-inflammatory factors such as IL-4, IL-10, and transforming growth factor β. Different signaling pathways are involved in these phenotype shifts. Furthermore, monocyte/macrophage can be differentiated into Mox, M4 and Mhem subtypes upon distinct stimuli. AKT: serine/threonine-protein kinases; CXCL: chemokine (C-X-C motif) ligand; GM-CSF: granulocyte-macrophage colony stimulating factor; JAK: janus kinase; LPS, lipopolysaccharide; MAPK: mitogen activated protein kinases; M-CSF: macrophage colony stimulating factor; mTOR: mechanistic target of rapamycin; NF-κB: nuclear factor kappa-B; PI3K: phosphoinositide 3-kinase; STAT: signal transducers and activators of transcription; TLR: toll-like receptors. These abbreviations are suitable for the following figures.
Macrophages and lipid metabolism in atherosclerosis
Foam cells in atherosclerotic plaques are derived from macrophages cells and vascular smooth muscle cells. Macrophages play a central role in the occurrence and development of atherosclerotic CVD (Barrett, 2021; Lin et al., 2021). Cholesterol in normal macrophages come from the internalization of plasma lipoproteins, endocytosis of apoptotic cells, and intracellular cholesterol synthesis. Macrophages absorb cholesterol in lipoproteins, including high-density lipoprotein (HDL) and LDL, through related receptors such as cluster of differentiation 36 (CD36), scavenger receptor, class B type 1 (SR-B1), and LDL receptor (LDLR). As an important scavenger receptor in macrophages, CD36 absorbs ox-LDL and promotes the formation of foam cells (Yang et al., 2018; Vazquez et al., 2020). SR-B1 is a multiligand membrane receptor protein that function as physiologically related HDL receptor (Meslier et al., 2020). The first step in SR-B1-mediated selective uptake of cholesterol ester (CE) is the binding of CE-rich lipoproteins to the extracellular domain of SR-B1. After binding, CE moves down the concentration gradient in HDL particles to cell plasma membranes. When CE is separated from HDL, HDL is released into circulation again to continue peripheral cholesterol transport. Within the cell, CE hydrolase hydrolyzes CE to free cholesterol. Other lipids in HDL particles, such as free cholesterol and TG, are also selectively transferred to macrophages (Shen et al., 2018; Straub, 2019).
Macrophages excrete excess cholesterol to extracellular receptors or convert cholesterol into CE and store it as lipid droplets, thus avoiding the cytotoxic effect of cholesterol accumulation. Several pathways are involved in cholesterol efflux from macrophages: 1) adenosine triphosphate binding cassette A1 (ABCA1) mediated cholesterol efflux to lipid-free apolipoprotein (apo), especially apoA-I; 2) unidirectional cholesterol efflux to mature HDL particles mediated by ABCG1; and 3) passive diffusion of cholesterol promoted by SR-B1 to mature HDL particles (Shen et al., 2018; Matsuo., 2021). ABCA1 and ABCG1 play a vital role in regulating cholesterol homeostasis in macrophages (Skarda et al., 2021). Of note, functions of ABCA1 and ABCG1 need ATP to transport free cholesterol from endoplasmic reticulum to cell membrane. ABCA1-mediates efflux of cholesterol and phosphatidylcholine to lipid-free ApoA-I, promoting the generation of newborn nascent HDL, and ABCG1 mediates the outflow of cholesterol, phosphatidylcholine, and sphingomyelin to nascent HDL and mature HDL. Dysfunction of ABCA1 and ABCG1 significantly reduces serum HDL levels, thereby greatly impairing cholesterol and lipid transport (Matsuo., 2021; Wu et al., 2022). Of note, SR-B1 not only mediates the selective entry of HDL into macrophages but also promotes the outflow of intracellular free cholesterol, preventing free cholesterol from accumulation in the arterial wall (Figure 1). Therefore, macrophages play a pivotal role in reverse cholesterol transport (RCT). Abnormal RCT accelerates the occurrence and development of atherosclerosis (Rohatgi, 2018; Ma et al., 2021).
Macrophages and inflammation in atherosclerosis
Macrophages contribute to the sustained local inflammatory response by secreting chemokines/cytokines as well as factors leading to oxidative stress (Khoury et al., 2021). A growing body of evidence suggests that resident macrophages can proliferate under microenvironment stimuli, such as oxidized lipids accumulation, inflammation response, and cytokine secretion which will in turn influence plaque microenvironment (Lin et al., 2021; Wang et al., 2023). For instance, in the atherosclerotic plaque environment, macrophages can also be initiated by oxidized lipids, induce a “foamy” state of activation, and exhibit an anti-inflammatory phenotype (Kuznetsova et al., 2020). Interestingly, macrophages can differentiate into distinct subtypes with different functions under different stimuli (Khoury et al., 2021). According to the dichotomy, macrophages are divided into two classes: classically activated pro-inflammatory M1 macrophages and alternatively activated anti-inflammatory M2 macrophages (Naumann et al., 2023). As shown in Figure 2, pro-inflammatory M1 macrophages are typically triggered by T helper (Th) 1 cytokines and lead to an inflammatory response, whereas M2 macrophages are mainly induced by Th2 cytokines and alleviate inflammation via producing anti-inflammatory cytokines (Liu et al., 2021).
Of note, high levels of the glycoprotein Ly6C monocytes in mice, known as CD14 (CD14++/CD16− subtype) in humans, differentiate into M1 macrophages, while low levels of the glycoprotein Ly6C monocytes differentiate into M2 macrophages (Liu et al., 2021; Yang et al., 2022). In the arterial intima, monocytes differentiate into macrophages by multiple pro-differentiation factors (Lin et al., 2021). Granulocyte-macrophage colony-stimulating factor and macrophage colony-stimulating factor mediate the polarization of M1 and M2 macrophages, respectively (Jiemy et al., 2020; Lin et al., 2021). Macrophages engulf modified LDL in the intima to form lipid-laden foam cells, giving rise to early atherosclerotic lesions. Generally, macrophages exert their atheroprotective effects through endocytic clearance of lipoprotein deposits and efferocytosis (phagocytosis clearance of apoptotic cells), thereby counteracting inflammatory processes involved in plaque formation (Yin et al., 2020; Kong et al., 2022). If the pro-inflammatory state persists, the atherosclerotic lesions progress to an advanced stage characterized by increased macrophage apoptosis and defective clearance of apoptotic cells (Lin et al., 2021; Chen et al., 2022).
Present data suggest that the ratio of pro-inflammatory M1 to anti-inflammatory M2 macrophages, within the atherosclerosis plaques may determine the progression and regression of atherosclerosis (Lin et al., 2021). M1 macrophages are the major inflammatory macrophage cell population in lipid cores of atherosclerotic plaques (Zang et al., 2021). M2 macrophages can be divided into three subtypes, namely, M2a, M2b and M2c. The polarization of M1 macrophages is promoted by pro-inflammatory factors, such as tumor necrosis factor (TNF), interleukin (IL)-6, monocyte chemoattractant protein 1, and interferon-γ, while the polarization of M2 macrophages is mediated by anti-inflammatory factors such as IL-4, IL-10, and transforming growth factor β (Lin et al., 2021; Yang et al., 2022). Interestingly, TNF-α decreases the expression of ABCA1, exaggerating foam cell formation (Hu et al., 2021). IL-10 secreted by M2 type macrophages not only stimulates the function of ABCA1 and ABCG1 but also inhibits CD36-mediated ox-LDL uptake by macrophages (Hu et al., 2021; Li H et al., 2022). Two main signaling pathways, the Akt/mTORC/LXR pathway and the JAK/STAT6 pathway, are two major signaling pathway for M2 polarization. (Bi et al., 2019; Fang et al., 2022). Besides, Th2 cell-secreted molecules, such as IL-4, can also induce the differentiation of macrophages toward M2 phenotype, thereby alleviating inflammation by inhibiting the mitogen-activated protein kinase signaling pathway (Zhao et al., 2016). The shift of macrophages from M1 to M2 phenotype makes it possible to maintain a basal anti-inflammatory environment in atherosclerosis plaques.
Furthermore, additional plaque-specific macrophage phenotypes have been recently identified (Figure 2), including oxidized phospholipid-induced macrophages (Mox), chemokine (C-X-C motif) ligand 4 (CXC-4) or platelet factor 4-induced macrophages (M4), erythrocyte and hemoglobin-induced macrophages (HA-mac, M(Hb), and Mhem) (Maretti-Mira et al., 2021; Nakai, 2021). Mox macrophages, a pro-atherogenic subset induced by oxidized phospholipids, express redox-regulatory genes as well as pro-inflammatory genes via Toll-like receptor 2 and display a proatherogenic ability (Li Z et al., 2022). In atherosclerotic lesions of LDLR-deficient (LDLR−/−) mice, Mox macrophages are extensively distributed in plaque and account for 30% of the total macrophages (Li H et al., 2022). Notably, the phagocytosis and migration capacities of Mox macrophages are inferior to M1 and M2 subsets. M4 macrophages are polarized by CXC-4 (Domschke and Gleissner, 2019) and they are associated with reduced phagocytosis and elevated production of inflammatory cytokines and molecules, such as IL-6, TNF-α, and MMP-7 (Skuratovskaia et al., 2020). The M4 subset is dominatingly presented in the adventitia and intima of human arteries to trigger inflammation and promote plaque instability (Lin et al., 2021). Hemorrhage-residing Mhem macrophages participate in hemo-globin clearance via phagocytosis of erythrocyte and improve cholesterol efflux by enhancing the expression of cholesterol transporters, ABCA1 and ABCG1, through LXRα and LXRβ (Li Z et al., 2022; Lin et al., 2021; Kong et al., 2022).
LXR agonists for therapy of atherosclerosis
Studies have shown that when mice are fed a high-cholesterol diet, the deficiency of LXR leads to pathological accumulation of cholesterol in the liver. When mice are fed a normal chow diet, the loss of liver LXR mainly leads to metabolic disorder of fatty acids and phospholipids (Bideyan et al., 2022). LXR are involved in reduction of intestinal cholesterol absorption and increase of intestinal excretion via down-regulating Niemann-Pick C1-like 1 and elevating ABCG5/8 (Muscoli et al., 2022). Furthermore, LXR participate in the repair and anti-inflammation of vascular cells through multiple pathways (Xu et al., 2020). However, LXR agonists lead to elevated blood TG levels, resulting in significant fat accumulation in the liver, leading to hepatic steatosis and even liver lesions, which limit their application (Schultz et al., 2000).
Mechanisms of action of LXR signaling pathway
LXR can be activated with oxysterols, which are derivatives of cholesterol. Upon activation, LXRα and LXRβ interact with RXRs to form specific heterodimers, which bind to promoters of multiple downstream genes involved in lipid metabolism (Abe et al., 2022). For instance, LXR activation promote the expression of ABC transporters, which play important roles in macrophage cholesterol efflux, hepatic and intestinal lipid homeostasis (Tanigawa et al., 2009; Van der Velde, 2010; Peng et al., 2019). As discussed above, activation of ABCA1 and ABCG1 through LXR enhances macrophage cholesterol efflux and promotes the generation of mature HDL and upregulation of RCT (Kasbi Chadli et al., 2013; Guo Y et al., 2018). The expression of ABCA1 and ABCG1 is mainly regulated by LXRα. Several lines of evidence have shown that ABCG1 upregulation promotes cholesterol transport from peripheral cells to HDL and inhibits transformation of macrophages to foam cells (Matsuo., 2021; Zhang X et al., 2021). Moreover, LXR upregulate the specific expression of ABCG5 and ABCG8 on the surface of hepatocytes and intestinal cells. ABCG5 and ABCG8 form a heterodimer that affects the hepatic cholesterol excretion and intestinal cholesterol absorption. LXR also upregulate the expression of ApoE, which is involved in transformation and metabolism of lipoproteins and is an important regulator of cholesterol homeostasis (Li et al., 2019). In addition, the expression of NPC1L1 can be downregulated by LXR activation, thus reducing absorption of intestinal cholesterol (Li et al., 2017).
It is worth noting that LXR not only play a key role in cholesterol metabolism but also elevate hepatic lipogenesis (Figure 3). Upon activation, LXR induce the expression of adipogenesis-related genes, such as sterol regulatory element-binding protein-1c (SREBP-1c), fatty acid synthase, stearoyl-coenzyme A dehydrogenase-1 and carbohydrate response element-binding protein, resulting in elevated plasma and hepatic TG levels (Schultz et al., 2000). SREBP-1c, the main transcription regulator of fatty acid and TG synthesis, is mainly regulated by LXRα is in liver. As previously reviewed, LXRβ knockout has no effect on the expression of SREBP-1c (Golub et al., 2023). As a cholesterol sensor, LXRα activates SREBP1c to enhance synthesis of fatty acids which are substrates for CE synthesis, thereby alleviating intracellular cholesterol accumulation (WHO, 2023). However, this process induces the main side effects of LXR activation.
FIGURE 3

Effects of liver X receptor (LXR) activation. (1) activation of LXR can promote reverse cholesterol transport by upregulating the expression of ABCA1 and ABCG1 genes in peripheral cells, thereby reducing intracellular cholesterol content; LXR activation also promote cholesterol secretion from the liver and small intestine by upregulating the expression of ABCG5 and ABCG8. (2) LXR activation inhibits NF-κB signaling pathway to suppress inflammatory factors, including the expression of TNF-α, IL-5, IL-6, and IL-1β. (3) LXR activation induces hypertriglyceridemia and liver steatosis by enhancing the expression of sterol regulatory element-binding protein-1c (SREBP-1c), fatty acid synthase (FAS), stearoyl-coenzyme A dehydrogenase-1 (SCD-1), and carbohydrate response element-binding protein (ChREBP). RXR, retinoid X receptors; TG, triglyceride.
In addition, LXR activation influences the biological properties of related inflammatory cells and inhibits gene expression of inflammatory mediators (Figure 3). According to the pathological formation process of atherosclerosis, the inflammatory cells mainly include vessel endothelial cells, macrophages, T lymphocytes, and smooth muscle cells (Ross, 1999). LXR are expressed in these cells and their activation can modulate the inflammatory states. For instance, the endogenous LXR activator, desmosterol, suppresses inflammatory genes in foam cells of atherosclerotic plaques (Spann et al., 2012; Zhang S et al., 2021). It has been demonstrated that LXR activation inhibits the nuclear factor kappa B signaling pathway (Marathe et al., 2006). Furthermore, LXR activation inhibits the production of a series of pro-inflammatory factors which are stimulated by bacteria, bacterial endotoxin lipopolysaccharide (LPS), TNF-α, and IL-1β. LXR agonists play an important regulatory role in inhibiting the expression of proinflammatory genes in macrophages and other types of cells (Findeisen et al., 2022). LXRα can selectively regulate the expression of genes related to apoptosis and leukocyte migration. Furthermore, LXRα plays an important role in the development of autoimmune hepatitis (Khoury et al., 2020). The function of LXRβ is closely related to lymphocyte differentiation and selection.
The coupling process between the agonist-bound LXR and the small ubiquitin-related modifier (SUMO) is called SUMOylation. In macrophages, SUMOylated LXR interacts with actin-binding protein CORONIN 2A to prevent actin from recruiting inflammatory gene promoters (Huang et al., 2011). Upon interferon-γ stimuli, both SUMOylated LXRα and LXRβ can inhibit the transcriptional response of mouse macrophages (Glaría et al., 2020). Of note, LXR activation inhibits the production of pro-inflammatory cytokine IL-18 directly and indirectly. LXR indirectly induces the expression of IL-18 binding protein, which is an effective endogenous inhibitor of IL-18 in mice and human systems, by up-regulating interferon regulatory factor 8 (Pourcet et al., 2016). Moreover, LXR agonists promote the expansion of regulatory T cells and inhibit the differentiation of Th17 cells that can secrete pro-inflammatory factor IL-17 in mice and humans (Yasuda et al., 2019).
Anti-atherosclerotic effects of LXR agonists
Given the pivotal roles of LXR in inhibiting inflammation and promoting RCT, LXR agonists can effectively prevent the development of atherosclerosis. As shown in Figure 4, some synthetic LXR agonists have been reported. The synthetic LXR agonist GW3965 upregulates the expression of ABCA1 and ABCG1, thereby promoting cholesterol efflux from murine RAW264.7 macrophages and reducing aortic plaque formation in LDLR−/− mice (Venkateswaran et al., 2000; Joseph et al., 2002). The anti-atherosclerosis effects of the synthetic LXR agonists GW3965 and T0901317 have been further demonstrated in other atherosclerosis models, such as apoE-deficient (apoE−/−) and LDLR−/− mice (Joseph et al., 2002; Terasaka et al., 2003). In 2006, another study demonstrates that GW3965 promotes cholesterol efflux from mouse macrophages and enhances cholesterol secretion from liver, inhibiting atherosclerosis by improving RCT (Wang et al., 2007). As shown in Table 1, LXR agonists have demonstrated powerful anti-atherosclerosis effects in distinct animal models (Rasheed and Cummins, 2018).
FIGURE 4

Structures of several mostly studied synthetic liver X receptor agonists.
TABLE 1
| LXR agonists | Model and dosage | Beneficial effects | Disadvantages | References |
|---|---|---|---|---|
| GW3965 | LDLR−/− mice, 1 mg/kg and 10 mg/kg ApoE−/− mice, 10 mg/kg; Feed for 12 weeks | Reduction in aortic root injury; In ApoE−/− mice, TC levels and HDL cholesterol levels were unchanged | Elevated plasma TG significantly; Tolerance or compensation may occur with chronic GW3965 administration | Joseph et al. (2002) |
| LDLR−/− mice treated with GW3965(1μ mol/L), once every 2 weeks for 8 weeks | Decrease the endothelial defects associated | Upregulation of hypertriglyceridemia | Rasheed et al. (2021) | |
| T0901317 | LDLR−/− mic, 0.2, 0.5, 1, 2 mg/kg T0901317; Gavage; Once a day for 2 weeks | Produced a 54%, 75% and 94% decline in atherosclerosis plaque area; Inhibit expression of pro-inflammatory molecules perhaps by limiting the activation of NF-κB | Elevated VLDL-CH and total TG significantly | Schultz et al., (2000), Peng et al., (2009) |
| ApoE−/− mice, T090317(0.5 mg/kg) in combination with notch receptor inhibitors (1.0 mg/kg), feed for 4 weeks | Reduced liver cholesterol and TG, upregulation of ABCA and RCT. | Elevated serum alanine amino-transferase aspartate aminotransferase and hepatic injury slightly | Hao et al. (2020) | |
| LXR-623 | LDLR−/− mice, 15 mg/kg/day or 50 mg/kg/day; Golden Gopher, 20, 60, 120 mg/kg/day; Crab-eating macaques, 1.15, 2.50 and 50 mg/kg/day | Reduced area of aortic arch injury, plasma total cholesterol and LDLC; not elevated plasma and liver cholesterol and TG; less potent for inducing SREBP1c and associated TG accumulation in liver cells | It was discontinued due to significant loss of certain neurons in the brain in the central nervous system | Katz et al., (2009), Quinet et al., (2009), Wang et al., (2017) |
| C57BL/6 mice, tunicamycin 1 mg/kg and LXR-623 15 mg/kg, injected intraperitoneally, observed after 48 h | Elevated ABCA1 and ABCG1 in hepatocytes and macrophages. | Elevated liver total cholesterol and TG slightly | Wang T et al. (2020) | |
| GW6340 | Wild-type mice, 30 mg/kg; primary macrophages deficient in LXRα/β | Upregulation of ABCA1, ABCG5 and ABCG8. It does not modulate TG content | Elevated total TG. | Yasuda et al. (2010) |
| XL-652 | Male cynomolgus | Upregulation of ABCA1 and ABCG1 | Elevated plasma TG and LDL cholesterol cannot be completely avoided | Kirchgessner et al. (2015) |
| Monkeys. 1 mg/kg; or 0.3, 1, and 10 mg/kg/day | ||||
| XL-041 | C57BL/6J mice, 0.03, 0.1, 1, or 3 mg/kg/day; LDLR−/−mice, 0.03–3 mg/day; Male cynomolgus Monkeys, 0.1, 0.3, 1, or 3 mg/kg/day | Upregulation of RCT by 70% | Elevated plasma TG. Terminated due to undisclosed reasons in 2013 | Kirchgessner et al. (2016) |
| CS8080 | Healthy Volunteers, 1, 3, 10, 20, 50 or 100 mg/day | No study results posted on llinicalTrials.gov for this study | By late 2008, the project was terminated due to undisclosed safety concerns | NCT00796575. 2008. Available from: clinicaltrials.gov |
| AZ876 | Female ApoE*3 leiden transgenic mice. 5 mmol/kg/day and 20 mmol/kg/day | AZ876 20 mmol/kg increased total lipids by 75%, reduced lesion area by 91%, and had anti-inflammatory effects; 5 mmol/kg/day decreased lesion area by 47% | Elevated liver TG level | van der Hoorn et al. (2011) |
| IMB-808 | RAW264.7 macrophages, HepG2, HEK293T, Caco-2 cells and THP-1 cells. From 0.001 μM to 30 μM | Upregulation of ABCA1, ABCG1, apoE, ABCG8 and ABCG5. Downregulation of NPC1L1. Promotes cholesterol efflux, reduces cellular lipid accumulation and almost does not induce lipogenic gene expression | Not reported | Li et al. (2017) |
Summary of synthetic LXR agonists for therapy of atherosclerosis.
Abbreviations: ABC, ATP-binding cassette transporter; ApoE, apolipoprotein E; ApoE−/−, Apolipoprotein E-deficient; HDL, High-density lipoprotein; LDL, Low-density lipoprotein; LDLR−/−, Low-density lipoprotein receptor-deficient; LXR, Liver X receptor; NF-κB, Nuclear factor kappa-B; NPC1L1, Niemann-Pick C1 like 1; RCT, Reverse cholesterol transport; SREBP-1c, Sterol regulatory element-binding protein-1c; TG, Triglyceride; VLDL-C, Very low-density lipoprotein cholesterol.
However, LXR pan-agonists are found to activate fatty genes in liver, such as fatty acid synthase and acetyl-Co A carboxylase 1, which induce hypertriglyceridemia and liver steatosis (Schultz et al., 2000). Furthermore, LXR agonists downregulate the expression of apoA5, resulting in hypertriglyceridemia by modulating ANGPTL3/8-mediated LPL activity (Chen et al., 2021). It seems that selective activation of LXRβ or tissue-selective activation are more feasible for treatment of atherosclerosis. For instance, the intestinal LXR agonist GW6340 activates the expression of intestinal ABCA1, ABCG5, and ABCG8 with little effect on hepatic LXR target genes (Yasuda et al., 2010). The selective LXRβ agonist LXR-623 (WAY 252623) improves RCT without boosting fatty acid synthesis in the liver. However, the phase I clinical trial of LXR-623 has been terminated due to its adverse reactions in the central nervous system (Katz et al., 2009). Moreover, several other compounds, including CS8080, BMS-779788 (XL-652), and BMS-852927 (XL-041), were terminated due to their unexpected side effects after entering phase I clinical trial (Loren et al., 2013). The side effects of these synthetic LXR agonists are also listed in Table 1. Interestingly, LXR agonists in combination with other drugs may potentially alleviate the side effects of these agonists. For instance, LXR-623 combined with simvastatin induces atherosclerotic plaque regression in New Zealand white rabbits at a lower dose and effectively reduces side effects (Giannarelli et al., 2012). The combination of U0126, a MEK1/2 inhibitor, and LXRα T0901317 synergistically reduces the formation of atherosclerotic plaques and avoids lipogenesis induced by T0901317 (Hayashi et al., 2014). Metformin, a first-line agent for the treatment of type II diabetes, can also synergistically inhibit atherosclerosis and reduce lipogenesis induced by T0901317 (Kim et al., 2015). Although combination therapy may partially reduce the side effects of LXR agonists, the long-term beneficial effects need to be investigated in future and in other animal models.
In addition to lipid-lowering, LXR activation attenuates the expression of inflammatory genes in macrophages (Pourcet et al., 2016). For instance, LXR activation suppresses the expression of inflammatory genes by multiple mechanisms including cis-repression (Thomas et al., 2018), trans-repression (Ghisletti et al., 2007), and cholesterol efflux-mediated regulation (Yvan-Charvet et al., 2008; Ito et al., 2015). Similarly, LXR activation represses inflammatory genes and upregulates cholesterol efflux genes in myelin damage-associated microglia (Berghoff et al., 2021). In addition, macrophages have a favorable homeostatic role in atherosclerosis, helping to clear lipid debris derived from circulating lipoproteins and necrotic cells while tamping down inflammation (Endo-Umeda et al., 2022).
Targeting macrophages in atherosclerosis using nanocarriers
Macrophage receptors
Macrophages play an important role in many biological processes, such as phagocytosis and killing of microorganisms (Fogelman et al., 1988). These biological activities are determined by their surface membrane receptors, including mannose receptor (MR) (Martinez-Pomares, 2012), folate receptor (FR) (Shen et al., 2014), T-cell immunoglobulin and mucin domain containing protein 4 (TIM-4) (Miyanishi et al., 2007), brain-specific angiogenesis inhibitor 1 (BAI-1) (Park et al., 2007), and pattern recognition receptors (PRRs) (Terpstra et al., 1997). These surface receptors of macrophage mediate internalization of exogenous nanoparticles and can be used for designing specific macrophage-targeted drug delivery systems (Figure 5).
FIGURE 5
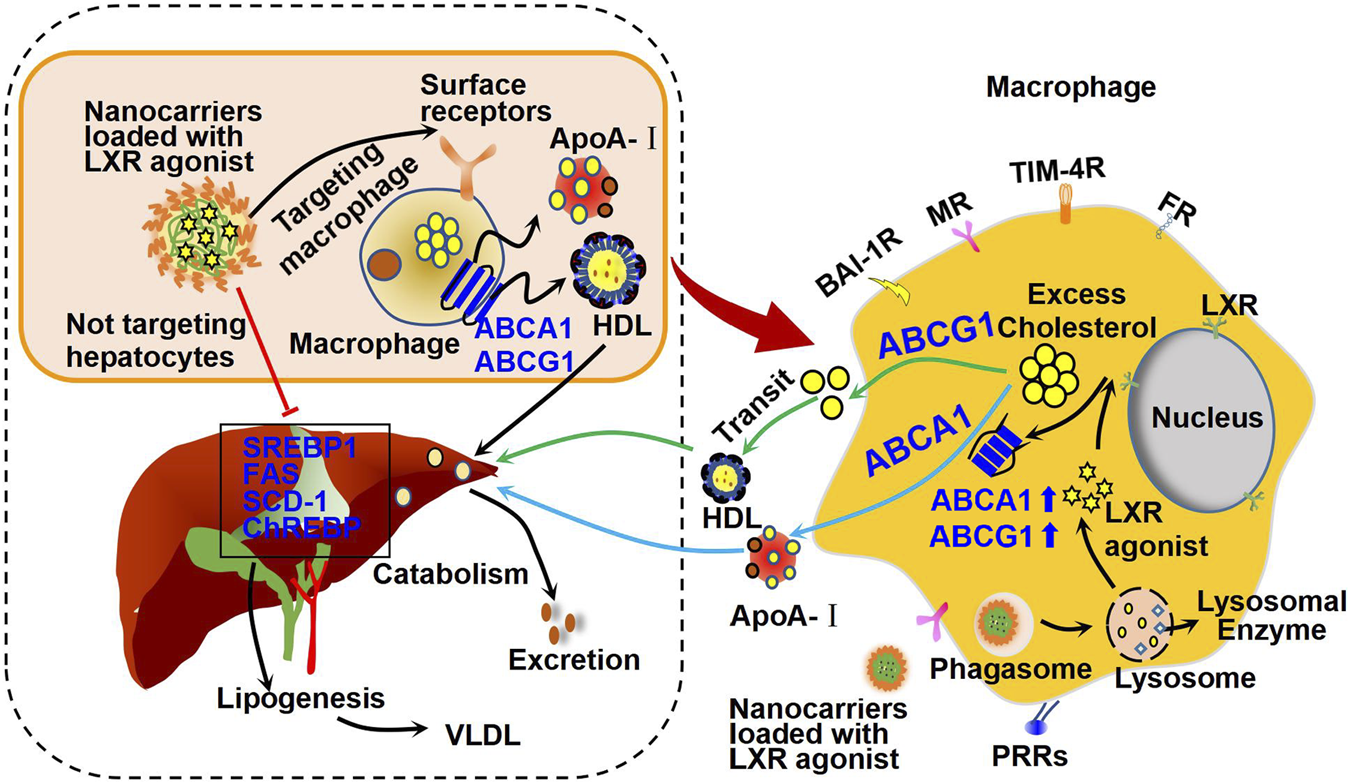
Mechanism of action of macrophage-targeted nanocarriers loaded with LXR agonists. Specially designed nanocarriers loaded with LXR agonists can be recognized and ingested by macrophages through the corresponding surface receptors, such as brain-specific angiogenesis inhibitor 1 receptor (BAI-1R), mannose receptor (MR), T-cell immunoglobulin and mucin domain containing protein 4 receptor (TIM-4R), folate receptor (FR), and pattern recognition receptors (PRRs). Therefore, these nanocarriers may activate cholesterol efflux and reduce inflammation as shown in Figure 3. Importantly, these nanocarriers are supposed to be seldomly absorbed by hepatocytes, and thus they may have minor effect on hepatic lipogenesis genes, including SREBP1, FAS, and SCD-1 as shown in Figure 3. Therefore, these nanocarriers show minor effect on fat accumulation in liver and may reduce side effects of LXR agonists in other organs.
MR, an endocytic protein, is a carbohydrate binding receptor that is highly expressed on macrophages (Ezekowitzet et al., 1991). The uptake of mannose glycopolymer was enhanced in M2-polarized macrophages. This carbohydrate-specific receptor has been used in gene vaccine by targeting human dendritic cells and macrophages through the phagocytic pathway (Wattendorf et al., 2008). FR beta (FR-β) is specifically expressed by activated macrophages and has a high affinity for folic acid (Puig-Kroger et al., 2009; Van Der Heijidan et al., 2009). Therefore, a folate-based nanocarrier may deliver therapeutic agents to activated macrophages without affecting normal cells and tissues. BAI-1, a phagocytic receptor, is widely distributed on the surface of macrophages and has an important role in macrophage phagocytosis. Studies have shown that BAI-1 mediates macrophage phagocytosis and can recognize LPS on the surface of microorganisms (Park et al., 2007; Das, et al., 2011). TIM-4, one of the cellular immunoglobulin mucin molecules, is selectively expressed on the surface of macrophages (Kobayashi et al., 2007; Rodriguez-Manzanet et al., 2008; Wong et al., 2010). TIM-4 is also a phosphatidylserine receptor and acts as a receptor involved in macrophage phagocytosis of apoptotic cells (Nurtanio and Yang, 2011). In addition, the PRRs of macrophages recognize modified LDL as damage associated molecular patterns or an ‘eat-me’ signal (Terpstra et al., 1997; Terpstra et al., 1998). Dectin-1, a member of the PRRs family, is expressed in macrophages and is essential for macrophage phagocytosis of yeast by recognizing the cellular component β-glucan of yeast (Herre et al., 2004). siRNAs with β-glucose shells targeting the dectin-1 receptor of macrophages have been successfully designed for oral treatment of systemic inflammation (Puig-Kröger et al., 2009). In a recent study, liposomes were functionalized with CD47-derived auto peptides and galactose in combination with carriers of “eat me/not eat me” signals to precisely deliver nanomedicines to M2 macrophages (Tang et al., 2021).
Last but not the least, most reported scavenger receptors are highly expressed by phagocytes including macrophages. These receptors can bind ligands of both pathogen- and self-origin and recognize modified LDL particles (such as oxidation or acetylation) (Graversen et al., 2012; Wilkinson and EI Khoury, 2012). In this aspect, Wang et al. reported that multi-walled carbon nanotubes (MWCNTs) cause apoptosis and inflammatory response in a dose-and time-dependent manner (Elgrabli et al., 2008). Apoptosis percentage among MWCNTs-treated RAW264.7 cells are significantly reduced when non-specific inhibitor of scavenger receptor (poly I) and the scavenger receptor-A specific inhibitor (2F8) is employed (Wang et al., 2012). These findings suggest that scavenger receptors can also be explored as a target of macrophages.
Macrophage-targeted nanocarriers for therapy of atherosclerosis
The rapid development of drug delivery system has provided new hope for alleviating drug side effects and improving the beneficial effects of LXR agonists (Figure 5). After entering the circulation, specially designed nanocarriers loaded with LXR agonists can be recognized and taken up by receptors on the surface of macrophages as mentioned earlier (Chen et al., 2022), but not by hepatocytes, thereby effectively blocking the effect of LXR agonists on hepatic adipogenesis. Upon uptake by macrophages, some macrophages infiltrate into plaques, where they are degraded by lysosomes, releasing LXR agonists that inhibit macrophage-derived foam cell formation and plaque inflammation (Ma et al., 2021). In the following, we will focus on the advances in macrophage-targeted nanocarriers that are loaded with LXR agonists for therapy of atherosclerosis (Table 2).
TABLE 2
| Nanocarriers | Target | Model and dosage | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| PLA-DSPE-mPEG1000/DLPC/Col IV-DSPE-PEG2000-GW3965-NPs | Collagen type IV of atherosclerotic plaque | LDLR−/− mice, 8 mg/kg GW3965, intravenous, twice weekly for 5 weeks | Macrophage content reduced by ∼30%; curative effect was enhanced by ∼18% without inducing serum TG compared to GW3965 alone; Excellent physical stability and biocompatibility | Minor hepatotoxicity; Cumbersome preparation steps | Yu et al. (2017) |
| PLGA-b-PEG-GW3965-NPs | Enhanced permeability and retention produced by the “leaky” endothelium | LDLR−/− mice, 10 mg/kg GW3965, retro-orbital injection, three times weekly for 2 weeks | The atherosclerotic area reduced by 50%; reduced hepatic TC and TG levels; Reduced inflammation; High biodegradability; Excellent biocompatibility | Entrapment and loading efficacy are not very high of nanocarriers | Zhang et al. (2015) |
| PS-PLGA-b-PEG-GW3965-NPs | TIM-4 receptor of macrophage | LDLR−/− mice, 10 mg/kg of GW3965, retro-orbital injection, three times weekly for 2 weeks | The atherosclerotic area reduced by 40%; reduced hepatic TG levels; Reduced inflammation; High biodegradability; Excellent biocompatibility | Raised hepatic TC levels; Entrapment and loading efficacy are not very high | Zhang et al. (2015) |
| PLGA-HDL-NPs | Natural targeting by mimicking the HDL nanoparticles | ApoE−/− mice, 0.2 mL of PLGA-HDL (10 mg/mL) | Improved cellular cholesterol efflux to PLGA–HDL; Controlled release properties | Did not show specific targeting for macrophages; Complex design and cumbersome steps | Sanchez-Gaytan et al. (2015) |
| mDNP-T0901317-NPs | Mannose receptor of macrophage | LDLR−/− mice, 200 μg per mouse. Tail vein injection, weekly for 4 weeks | Reduced atherosclerotic plaque progression, plaque necrosis, and plaque inflammation without affecting hepatic lipogenic genes or plasma lipids; High buffering capacity, extend the circulation time in the blood | Reducing atherosclerotic plaque that has already formed does not prevent plaque formation at all; Cumbersome preparation steps | He et al. (2018) |
| Lyp-1-GW3965-liposomes | P32 receptor of foam cells | LDLR−/− mice, 6.5 mg/mL GW3965, intravenous injections, twice a week for 5 weeks | The macrophage content reduced two-fold; stabilized atherosclerotic plaques without increasing plasma or hepatic lipid levels; Significant ability to penetrate atherosclerotic plaques | Uptake of liposomes by Kupffer cells in the liver; P32 target has been found to be expressed not only in macrophages | Benne et al. (2020) |
| D-napg-gffy-T0901317- nanohydrogel | Scavenger receptor class A of macrophage | C57BL/6J mice and ApoE−/− mice, 5 mg/kg and 15 mg/kg, subcutaneous injection, once every 3 days for 16 days | Reversed advanced lesions without inducing hypertriglyceridaemia, fatty liver and other liver injuries; inhibited formation of foam cells and production of pro-inflammatory cytokines; Nanocarriers have high stability in vivo | The curative effect on atherosclerosis was similar as oral T0901317; Small amounts of nanocarriers can be degraded by extracellular proteinase K | Ma et al. (2021) |
| SHDL-T0901317-NPs | Cholesterol receptor in atherosclerotic plaques | ApoE−/− mice, 1.5 mg/kg, intraperitoneal injection, three times weekly | Significantly reduced atherogenesis and hypertriglyceridemia compared to T0901317 alone; Small size, high purity of nanoparticles | Minor hepatotoxicity | Yuan et al. (2021) |
| ApoA-I(22A)-sHDL-T0901317-NPs | Cholesterol receptors in atherosclerotic plaques | C57BL/6J mice and ApoE−/− mice, 1.5 mg/kg T0901317, intraperitoneal injection, three times weekly for 6 weeks | Upregulation of ABCA1 and ABCG1. Had no influence on hepatic or serum TG levels; Good tolerability; long mean half-life | Difficult and expensive for preparation | Guo Y et al. (2018) |
| ApoA-Ⅰ protein-Phospholipids-GW3965-NPs | Cholesterol receptors of macrophages in atherosclerosis | ApoE−/− mice. Loaded with GW3965 (10 mg/kg). Injection every 2 days for 8 days | Small size (30 nm), accumulated in aortic plaques and exhibited local anti-inflammatory effects. Abolished liver toxicity; Long blood half-life | Did not show advantage compared to free drug in treatment of atherosclerosis; Cumbersome preparation steps | Tang et al. (2016) |
Macrophage-targeted nanocarriers loaded with synthetic LXR agonists for therapy of atherosclerosis.
Abbreviations: ABC, ATP-binding cassette transporter; ApoA-Ⅰ, apolipoprotein A-Ⅰ; ApoA-I(22A)-sHDL, a synthetic apoA-I mimetic peptide (22-amino acid peptide, 22A) is utilized to formulate sHDL; ApoE−/−, Apolipoprotein E-deficient; D-napg-gffy, naphthylacetic acid modified D-enantiomeric-glycine-phenylalanine-phenylalanine-tyrosine; LDLR−/−, Low-density lipoprotein receptor-deficient; Lyp-1, the cyclic peptide Lyp-1 (CGNKRTRGC); mDNP, mannose functionalized dendrimer nanoparticles; NPs, nanoparticles; PLA-DSPE-mPEG1000/DLPC/Col IV-DSPE-PEG2000, poly(d,l-lactide), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine, methoxy (polyethylene glycol), 1,2-dilauroyl-sn-glycero-3-phosphocholine, collagen IV, polyethylene glycol-coating molecules; PLGA-b-PEG, biodegrad-able diblock poly (lactide-co-glycolide)-b-poly(ethylene glycol); PLGA-HDL:polymer poly (lactic-co-glycolic acid)-synthetic high-density lipoprotein; PS-PLGA-b-PEG, phosphatidylserine and PLGA-b-PEG self-assembling; sHDL, synthetic high-density lipoprotein; TC, total cholesterol; TG, Triglyceride.
Nanoparticles loaded with LXR agonist GW3965
At the early stage of atherosclerosis, a large amount of collagen type IV (Col IV) are expressed in the plaques and are valuable targets for delivery of nanoparticles (Kamaly et al., 2016). With poly (D, L-lactide) (PLA)-based as the core, DSPE-mPEG1000/DLPC/Col IV-DSPE-PEG2000 as the lipid layer, these nanoparticles (Col IV-GW-NPs) show good binding to Col IV-targeted ligand linker. In vitro, Col IV-GW-NPs loaded with GW3965 upregulate LXR target genes and downregulate proinflammatory mediators in macrophages. In vivo, these nanoparticles can successfully reach the atherosclerosis lesion site. Compared with untargeted nanoparticles loaded with GW3965, the curative effect of Col IV-GW-NPs enhance approximately 18% in the LDLR−/− mice. Moreover, these Col IV-GW-NPs do not induce fatty liver or hyperlipidemia during treatment (Yu et al., 2017).
Poly(lactide-co-glycolide)-b-poly (ethylene glycol) copolymers has high biocompatible and good biodegradable and can be prepared by self-assembly. These copolymers have been widely used as effective nanocarriers for small and macromolecular drugs (Farokhzad, 2008). These nanocarriers loaded with GW3965 (10 mg/kg) reduce atherosclerotic plaque formation by 50% in LDLR−/− mice after retro-orbital injection for 2 weeks (three times weekly) (Zhang et al., 2015). Furthermore, the surface of these nanocarriers can be further modified by phosphatidylserine, which is a phagocytic target for phagocytes, such as macrophages (Fadok et al., 1992). In LDLR−/− mice, these modified nanocarriers induce a 40% macrophage reduction in atherosclerotic plaques. Importantly, these nanocarriers loaded with GW3965 also reduce inflammation and have no negative impact on either plasma or liver compared with free GW3965 (Zhang et al., 2015). Sanchez-Gaytan et al. developed a hybrid polymer/HDL nanocarriers with lipid/apolipoprotein as the shell and poly (lactic-co-glycolic acid) as the core. Compared to normal poly (lactic-co-glycolic acid) nanocarriers, these new type nanocarriers not only have a slower release characteristic but also have the characteristics of natural HDL, such as preferential uptake by macrophages and good ability to transport cholesterol out of atherosclerotic plaque. In apoE−/− mice, these hybrid polymer/HDL nanocarriers can successfully target atherosclerotic plaques and preferentially accumulate in plaque macrophages (Sanchez-Gaytan et al., 2015). These biomimetic nanocarriers provide a novel method for delivery of LXR agonists to macrophages in atherosclerosis.
Nanoparticles loaded with LXR agonist T0901317
Among the drug loading nanocarriers, polyamidoamine dendrimer provides unique advantages for development of multifunctional dendritic nanoparticles (DNPs). Polyamidoamine dendrimers have more than 120 terminal amino groups in generation 5.0, which can be surface modified with high buffer capacity, and can achieve a unique “proton sponge” effect for endosome escape. In addition, PEGylation of DNPs can reduce positive charge on the surface, prolonging the blood circulation time of theses DNPs (Zahr et al., 2006; Lipka et al., 2010; Pelaz et al., 2015). Interestingly, the mannose functionalized dendrimer nanoparticles loaded with T0901317 can specifically target the MR expressed on macrophages. In LDLR−/− mice, these intravenously injected mannose functionalized nanoparticles loaded with T0901319 (MDNP-T0901317) can successfully target macrophages in atherosclerotic plaques. Importantly, MDNP-T0901317 can be efficiently taken up by macrophages but not hepatocytes (He et al., 2018). This advantage is sure to reduce the side effects of T09011317 and other LXR agonists. A recent study reported a new nanoparticle, which uses mesoporous silica nanoparticles as carriers to generate nanoparticles that are simultaneously loaded with peroxisome proliferators-activated receptor alpha and LXRα agonists. These novel nanoparticles successfully reduce atherosclerotic plaque area in rats (Zhang W et al., 2022). It has been demonstrated that modification of nanoparticles with platelet membranes can significantly enhance the targeting property of nanocarriers (Wang H et al., 2020). Compared with uncoated nanoparticles, platelet membrane-coated nanoparticles can avoid complement activation, providing these nanoparticles with immune escape ability and making it possible for nanoparticles position in deep locations (Choi et al., 2020). This provides new ideas for the treatment of atherosclerosis using nanocarriers.
Liposomes loaded with LXR agonists
Modification of liposomes with cyclic peptide Lyp-1 can make these liposomes be recognized by p32 receptors that are expressed on foam cells. Compared with untargeted liposomes, the accumulation of GW3965-Lyp-1 liposomes in foam cells of atherosclerotic plaque increases by 1.7 folds. In male LDLR−/− mice, these modified liposomes reduce the number of macrophages in atherosclerotic plaques by 2 folds and increase the percentage of collagen by 3 folds. It is noteworthy that targeted liposomes loaded with GW3965-Lyp-1 can not only reduce atherosclerotic plaque formation but also stabilize atherosclerosis and reduce the side effects of LXR agonist. Furthermore, Lyp-1 is also used to functionalize other nanocarriers for targeting macrophages, such as heat shock protein cage designed by Uchida (Uchida et al., 2011). In another study using apoE−/− mice, liposomes loaded with GW3965-Lyp-1 has a high retention rate in aortic lesions (3 h) and shows a significant therapeutic effect at a relatively low dose of GW3965 (≈6.5 mg kg-1) without inducing high serum TG levels (Seo et al., 2014).
Nanohydrogels loaded with LXR agonists
Supramolecular hydrogels of short peptides have been widely studied due to their beneficial characteristics (Liu et al., 2015). D-glycine–phenylalanine–phenylalanine–tyrosine tetrapeptide (D-Nap-GFFY) modified with naphthyl acetic acid is not easy to be degraded by endogenous proteases due to the presence of amino acids in D-configuration. More importantly, these D-Nap-GFFY can successfully target macrophages. D-Nap-GFFY nanofiber hydrogels loaded with T0901317 are reported to inhibit atherosclerosis as that of oral T0901317 and do not induce hepatic lipogenesis. More importantly, D-Nap-GFFY-T0901317 hydrogels are found to reverse advanced atherosclerotic lesions (Ma et al., 2021).
Synthetic HDL particles loaded with LXR agonists
HDL, an endogenous lipidic nanoparticles with a diameter ranging from 7 nm to 13 nm in size, is mainly composed of phospholipids and apoA-I. HDL can transfer cholesterol from lipid-laden plaque macrophages to the liver through the process of RCT. As preparation of ApoA-I from human plasma is time-consuming, various genetic variants of ApoA-I (Terasaka et al., 2003; Nicholls et al., 2018a) or recombinant ApoA-I (Tardif et al., 2014; Nicholls et al., 2018b) have been used to replace human plasma ApoA-I to obtain reconstituted HDL or synthetic HDL (sHDL) particles with similar arteriosclerosis protective effects as endogenous HDL. sHDL can effectively target atherosclerotic plaques and has been clinically proved to be a cholesterol receptor with good safety. (Jayaraman et al., 2012). sHDL particles have therefore been widely studied as nanocarriers to deliver therapeutics or imaging agents for atherosclerosis management (Lameijer et al., 2018; Binderup et al., 2019). These sHDL nanoparticles (10.5 ± 0.1 nm) loaded with LXR agonist T0901317 shows a good therapeutic effect with fewer side effects due to the low dosage of T0901317. In apoE−/− mice fed a high fructose, high-fat diet for 6 weeks, these nanoparticles reduce aortic plaque areas by 15.0% (Yuan et al., 2021). In another study, phospholipid recombinant ApoA-I peptide (22A)-derived sHDL-nanoparticles loaded with T0901317 reduce the aortic root plaque area by 40.8% without induce side effects as that of free T0901317 (Guo T et al., 2018). HDL-like nanoparticles can also be prepared using full-length apoA-I protein, phospholipids, and polymers. The diameter of these HDL-like particles is about 30 nm, which is slightly greater than that of endogenous HDL (about 10–14 nm). In apoE−/− mice, GW3965-laden HDL particles accumulate in aortic plaques and exhibit local anti-inflammatory effects (Tang et al., 2016). However, apoA-I is a large protein (human apoA-I has 243 amino acid residues), which makes it difficult and expensive to synthesize high-quality HDL particles for intravenous administration.
The advantages and disadvantages of these nanocarriers are listed in Table 2. For example, type IV collagen exposed in atherosclerotic plaques is a target, and PLA-DSPE NPs modified with type IV collagen have excellent physical stability and biocompatibility, but the preparation steps are cumbersome (Yu et al., 2017). Although PLGA-b-PEG NPs has good biodegradability and biocompatibility, the entrapment and loading efficiency are not high (Zhang et al., 2015). Macrophage TIM-4 receptors can recognize phosphatidylserine, and phosphatidylserine-modified PLGA-b-PEG NPs promote macrophages shift to an anti-inflammatory mode (Zhang et al., 2015). Mannose receptors are abundantly expressed on the surface of macrophages, and DNP NPs modified by mannose improve buffering capacity and prolong blood circulation time, but the preparation steps need to be simplified (He et al., 2018). Foam cell P32 receptors recognize Lyp-1, and liposomes modified by Lyp-1 can significantly penetrate atherosclerotic plaques, but P32 receptors are not only expressed on macrophages (Benne et al., 2020). Macrophage class A scavenger receptors recognize D-Nap-GFFY, and D-Nap-GFFY-modified nanohydrogels are highly stable in vivo. Although a small number of theses nanocarriers can be degraded by extracellular proteinase K, this type of nanocarriers has an attractive application in future (Ma et al., 2021). Furthermore, ApoA-I(22A)-sHDL NPs are well tolerated and have a long mean half-life and PLGA-HDL NPs have controlled release properties. However, they are expensive for preparation at present (Tang et al., 2016; Guo T et al., 2018; Yuan et al., 2021).
Comparisons with other studies and what does the current work add to the existing knowledge
A recent article reviewed the application of nanoparticles for diagnostic imaging of CVD. They also discussed the applications of nanocarriers for therapy of hypertension, myocardial infarction, stroke, cardiomyopathy as well as atherosclerosis (Soumya and Raghu, 2023). Another review in 2022 described macrophage-targeted nanomedicines for diagnosis and treatment of atherosclerosis. The authors carefully reviewed nanoparticles for diagnostic imaging and discussed how rationally designed nanomaterials can efficiently carry and delivery therapeutics to macrophages (Chen et al., 2022). However, the above two review articles only mentioned a small portion of LXR agonists, which have a great potential application for therapy of atherosclerosis after successfully eliminating their side effects in the liver.
As described above, LXR expressed by macrophages are an excellent target for treatment of atherosclerosis. Activation of LXR not only inhibits macrophage-mediated inflammation but also promotes RCT, showing a good anti-atherosclerotic potential. However, LXR agonist-induced side effects, especially in the liver, have greatly limited the potential usage of these drugs. Targeted drug delivery system is designed to release drugs at the desired site, which can improve efficacy and reduce side effects. In this article, we provide useful information about the role of macrophages in atherosclerosis, the actions of LXR agonists (why they can induce side effects), how nanocarriers are designed and recognized by the receptors that are located on the surface of macrophages, and the advantages and disadvantages of these reported nanocarriers. This review will greatly enhance the research interest in macrophage-targeted therapy of atherosclerosis by LXR agonists as well as other drugs. Of importance, this review may promote the development of novel nanocarriers with greater targeting property and therapeutic effects, thereby promoting the potential clinical application of LXR agonists.
Conclusion and future directions
Atherosclerosis therapy is still a great challenge for our humans. Macrophages play pivotal roles in the onset and development of atherosclerotic CVD. LXR play a unique role in lipid homeostasis and inflammation, which has attracted much attention in the field of developing therapeutic agents for atherosclerosis. However, until now no LXR agonists have not been approved as treatment for atherosclerosis which are partially due to their adverse effects including hepatic lipogenesis, steatosis and hyperlipidemia. Interestingly, macrophages express various receptors which can be used for targeted drug delivery. Present data clearly demonstrate that macrophage-targeted nanocarriers loaded with LXR agonists can successfully retard the progress of atherosclerosis and significantly reduce the side effects of these agonists. The success of nanocarriers in preclinical studies of arteriosclerosis and their application in human cancer treatment indicate that macrophage-targeted drug delivery system can be used in the diagnosis and treatment of patients with CVD in future.
However, there are some questions need to be addressed before these macrophage-targeted nanocarriers loaded with LXR agonists: 1) several kinds of nanocarriers have been studies by different groups, which kind of nanocarrier is the best for targeting macrophage and has the best therapeutic effect need to be further investigated by comparation studies; 2) distinct nanocarriers may have different drug release rate, pharmacokinetic studies are needed to figure out which kind of nanodrug delivery system is suitable for macrophage-targeted therapy of atherosclerosis; 3) there are several subtypes of macrophages in atherosclerotic plaques, whether nanocarriers can target special subtypes of macrophages and promote their shift (such as from M1 to M2) are an interesting problem needs to be figured out in future; 4) as patients with atherosclerotic CVD need a long-term intervention, the safety of the selected nanocarriers loaded with LXR agonists need to be investigated in animal experiments that are designed for more than 6 months’ treatment; 5) presently, the preparation of these nanocarriers, especially those biomimetic nanoparticles (such as synthetic HDL), is still a great challenge for their large scale production; 6) most researches rely on the mouse models, whose atherosclerotic plaques are different from our humans; 7) the last but not the least, the development of selective ligands for each LXR isoform may reduce the side effects of LXR agonist.
Statements
Author contributions
T-MY, MM, W-QY, XW, and F-JX collected the references and prepared the manuscript; MM, W-QY, Y-JL, and T-MY drew the figures; Y-JL and S-DG re-edited the manuscript and provided funding support. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (82070469, 81770463) and the fund of Weifang Medical University’s official domestic visiting program (No. 20227-12).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abe R. J. Abe J. I. Nguyen M. T. H. Olmsted-Davis E. A. Mamun A. Banerjee P. et al (2022). Free cholesterol bioavailability and atherosclerosis. Curr. Atheroscler. Rep.24, 323–336. 10.1007/s11883-022-01011-z
2
Barrett T. J. (2021). Macrophages in atherosclerosis regression. Arteriosclerosis, Thrombosis, Vasc. Biol.40, 20–33. 10.1161/ATVBAHA.119.312802
3
Benne N. Martins Cardoso R. Boyle A. L. Kros A. Jiskoot W. Kuiper J. et al (2020). Complement receptor targeted liposomes encapsulating the liver x receptor agonist GW3965 accumulate in and stabilize atherosclerotic plaques. Adv. Healthc. Mat.9, e2000043. 10.1002/adhm.202000043
4
Berghoff S. A. Spieth L. Sun T. Hosang L. Schlaphoff L. Depp C. et al (2021). Microglia facilitate repair of demyelinated lesions via post-squalene sterol synthesis. Nat. Neurosci.24, 47–60. 10.1038/s41593-020-00757-6
5
Bhaladhare S. Bhattacharjee S. (2023). Chemical, physical, and biological stimuli-responsive nanogels for biomedical applications (mechanisms, concepts, and advancements): A review. Int. J. Biol. Macromol.226, 535–553. 10.1016/j.ijbiomac.2022.12.076
6
Bi Y. Chen J. Hu F. Liu J. Li M. Zhao L. (2019). M2 Macrophages as a potential target for antiatherosclerosis treatment. Neural Plast.2019, 6724903. 10.1155/2019/6724903
7
Bideyan L. Fan W. Kaczor-Urbanowicz K. E. Priest C. Casero D. Tontonoz P. (2022). Integrative analysis reveals multiple modes of LXR transcriptional regulation in liver. Proc. Natl. Acad. Sci. U. S. A.119, e2122683119. 10.1073/pnas.2122683119
8
Binderup T. Duivenvoorden R. Fay F. van Leent M. M. T. Malkus J. Baxter S. et al (2019). Imaging-assisted nanoimmunotherapy for atherosclerosis in multiple species. Sci. Transl. Med. 11, eaaw773611, eaaw7736. 10.1126/scitranslmed.aaw7736
9
Björkegren J. L. M. Lusis A. J. (2022). Atherosclerosis: Recent developments. Cell.185, 1630–1645. 10.1016/j.cell.2022.04.004
10
Cave M. C. Clair H. B. Hardesty J. E. Falkner K. C. Feng W. Clark B. J. et al (2016). Nuclear receptors and nonalcoholic fatty liver disease. Biochim. Biophys. Acta1859, 1083–1099. 10.1016/j.bbagrm.2016.03.002
11
Chan Y. H. Ramji D. P. (2022). Atherosclerosis: Pathogenesis and key cellular processes, current and emerging therapies, key challenges, and future research directions. Methods Mol. Biol.2419, 3–19. 10.1007/978-1-0716-1924-7_1
12
Chen W. Schilperoort M. Cao Y. Shi J. Tabas I. Tao W. (2022). Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat. Rev. Cardiol.19, 228–249. 10.1038/s41569-021-00629-x
13
Chen Y. Q. Pottanat T. G. Zhen E. Y. Siegel R. W. Ehsani M. Qian Y. W. et al (2021). ApoA5 lowers triglyceride levels via suppression of ANGPTL3/8-mediated LPL inhibition. J. Lipid Res.62, 100068. 10.1016/j.jlr.2021.100068
14
Choi B. Park W. Park S. B. Rhim W. K. Han D. K. (2020). Recent trends in cell membrane-cloaked nanoparticles for therapeutic applications. Methods177, 2–14. 10.1016/j.ymeth.2019.12.004
15
Das S. Owen K. A. Ly K. T. Park D. Black S. G. Wilson J. M. et al (2011). Brain angiogenesis inhibitor 1 (Bai1) is a pattern recognition receptor that mediates macrophage binding and engulfment of Gram-negative bacteria. Proc. Natl. Acad. Sci. U. S. A.108, 2136–2141. 10.1073/pnas.1014775108
16
Domschke G. Gleissner C. A. (2019). CXCL4-induced macrophages in human atherosclerosis. Cytokine122, 154141. 10.1016/j.cyto.2017.08.021
17
Elgrabli D. Abella-Gallart S. Robidel F. Rogerieux F. Boczkowski J. Lacroix G. (2008). Induction of apoptosis and absence of inflammation in rat lung after intratracheal instillation of multiwalled carbon nanotubes. Toxicology253, 131–136. 10.1016/j.tox.2008.09.004
18
Endo-Umeda K. Kim E. Thomas D. G. Liu W. Dou H. Yalcinkaya M. et al (2022). Myeloid LXR (Liver X Receptor) deficiency induces inflammatory gene expression in foamy macrophages and accelerates atherosclerosis. Arterioscler. Thromb. Vasc. Biol.42, 719–731. 10.1161/ATVBAHA.122.317583
19
Ezekowitz R. A. Williams D. J. Koziel H. Armstrong M. Y. Warner A. Richards F. F. et al (1991). Uptake of pneumocystis carinii mediated by the macrophage mannose receptor. Nature.351, 155–158. 10.1038/351155a0
20
Fadok V. A. Voelker D. R. Campbell P. A. Cohen J. J. Bratton D. L. Henson P. M. (1992). Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol.148, 2207–2216. 10.4049/jimmunol.148.7.2207
21
Fang J. Ou Q. Wu B. Li S. Wu M. Qiu J. et al (2022). TcpC inhibits M1 but promotes M2 macrophage polarization via regulation of the MAPK/NF-κB and Akt/STAT6 pathways in urinary tract infection. Cells.11, 2674. 10.3390/cells11172674
22
Farokhzad O. C. (2008). Nanotechnology for drug delivery: The perfect partnership. Expert Opin. Drug Deliv.5, 927–929. 10.1517/17425247.5.9.927
23
Findeisen H. M. Voges V. C. Braun L. C. Sonnenberg J. Schwarz D. Körner H. et al (2022). LXRα regulates oxLDL-induced trained immunity in macrophages. Int. J. Mol. Sci.23, 6166. 10.3390/ijms23116166
24
Fogelman A. M. Van Lenten B. J. Warden C. Haberland M. E. Edwards P. A. (1988). Macrophage lipoprotein receptors. J. Cell. Sci. Suppl.9, 135–149. 10.1242/jcs.1988.supplement_9.7
25
Ghisletti S. Huang W. Ogawa S. Pascual G. Lin M. E. Willson T. M. et al (2007). Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPAR gamma. Mol. Cell.25, 57–70. 10.1016/j.molcel.2006.11.022
26
Giannarelli C. Cimmino G. Connolly T. M. Ibanez B. Ruiz J. M. Alique M. et al (2012). Synergistic effect of liver X receptor activation and simvastatin on plaque regression and stabilization: An magnetic resonance imaging study in a model of advanced atherosclerosis. Eur. Heart J.33, 264–273. 10.1093/eurheartj/ehr136
27
Glaría E. Letelier N. A. Valledor A. F. (2020). Integrating the roles of liver X receptors in inflammation and infection: Mechanisms and outcomes. Curr. Opin. Pharmacol.53, 55–65. 10.1016/j.coph.2020.05.001
28
Golub I. S. Termeie O. G. Kristo S. Schroeder L. P. Lakshmanan S. Shafter A. M. et al (2023). Major global coronary artery calcium guidelines. JACC Cardiovasc. Imaging.16, 98–117. 10.1016/j.jcmg.2022.06.018
29
Graversen J. H. Svendsen P. Dagnæs-Hansen F. Dal J. Anton G. Etzerodt A. et al (2012). Targeting the hemoglobin scavenger receptor CD163 in macrophages highly increases the anti-inflammatory potency of dexamethasone. Mol. Ther.20, 1550–1558. 10.1038/mt.2012.103
30
Guo, T T. Liu Q. Hou P. Li F. Guo S. Song W. et al (2018). Stilbenoids and cannabinoids from the leaves of cannabis sativa f. sativa with potential reverse cholesterol transport activity. Food Funct.9, 6608–6617. 10.1039/c8fo01896k
31
Guo, Y Y. Yuan W. Yu B. Kuai R. Hu W. Morin E. E. et al (2018). Synthetic high-density lipoprotein-mediated targeted delivery of liver X receptors agonist promotes atherosclerosis regression. EBioMedicine.28, 225–233. 10.1016/j.ebiom.2017.12.021
32
Hao Y. Wang X. Zhang F. Wang M. Wang Y. Wang H. et al (2020). Inhibition of notch enhances the anti-atherosclerotic effects of LXR agonists while reducing fatty liver development in ApoE-deficient mice. Toxicol. Appl. Pharmacol.406, 115211. 10.1016/j.taap.2020.115211
33
Hayashi T. Kotani H. Yamaguchi T. Taguchi K. Iida M. Ina K. et al (2014). Endothelial cellular senescence is inhibited by liver X receptor activation with an additional mechanism for its atheroprotection in diabetes. Proc. Natl. Acad. Sci. U. S. A.111, 1168–1173. 10.1073/pnas.1322153111
34
He H. Yuan Q. Bie J. Wallace R. L. Yannie P. J. Wang J. et al (2018). Development of mannose functionalized dendrimeric nanoparticles for targeted delivery to macrophages: Use of this platform to modulate atherosclerosis. Transl. Res.193, 13–30. 10.1016/j.trsl.2017.10.008
35
Herre J. Gordon S. Brown G. D. (2004). Dectin-1 and its role in the recognition of beta-glucans by macrophages. Mol. Immunol.40, 869–876. 10.1016/j.molimm.2003.10.007
36
Hu D. Wang Z. Wang Y. Liang C. (2021). Targeting macrophages in atherosclerosis. Target. macrophages Atheroscler. Curr. Pharm. Biotechnol.22, 2008–2018. 10.2174/1389201022666210122142233
37
Huang W. Ghisletti S. Saijo K. Gandhi M. Aouadi M. Tesz G. J. et al (2011). Coronin 2A mediates actin-dependent de-repression of inflammatory response genes. Nature.470, 414–418. 10.1038/nature09703
38
Ito A. Hong C. Rong X. Zhu X. Tarling E. J. Hedde P. N. et al (2015). LXRs link metabolism to inflammation through Abca1-depen-dent regulation of membrane composition and TLR signaling. Elife. 44, e08009. 10.7554/eLife.08009e08009
39
Jayaraman S. Cavigiolio G. Gursky O. (2012). Folded functional lipid-poor apolipoprotein A-I obtained by heating of high-density lipoproteins: Relevance to high-density lipoprotein biogenesis. Biochem.442, 703–712. 10.1042/BJ20111831
40
Jiemy W. F. van Sleen Y. van der Geest K. S. Berge T. H. A. Abdulahad W. H. Sandovici M. et al (2020). Distinct macrophage phenotypes skewed by local granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) are associated with tissue destruction and intimal hyperplasia in giant cell arteritis. Clin. Transl. Immunol.9, e1164. 10.1002/cti2.1164
41
Jin L. Hua H. Ji Y. Jia Z. Peng M. Huang S. (2023). Anti-inflammatory role of fenofibrate in treating diseases. Biomol. Biomed.2023, 8534. 10.17305/bb.2022.8534
42
Joseph S. B. McKilligin E. Pei L. Watson M. A. Collins A. R. Laffitte B. A. et al (2002). Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc. Natl. Acad. Sci. U. S. A.99, 7604–7609. 10.1073/pnas.112059299
43
Kamaly N. Fredman G. Fojas J. J. Subramanian M. Choi W. I. Zepeda K. et al (2016). Targeted interleukin-10 nanotherapeutics developed with a microfluidic chip enhance resolution of inflammation in advanced atherosclerosis. Acs. Nano.10, 5280–5292. 10.1021/acsnano.6b01114
44
Kasbi Chadli F. Nazih H. Krempf M. Nguyen P. Ouguerram K. (2013). Omega 3 fatty acids promote macrophage reverse cholesterol transport in hamster fed high fat diet. PLoS One8, e61109. 10.1371/journal.pone.0061109
45
Katz A. Udata C. Ott E. Hickey L. Burczynski M. E. Burghart P. et al (2009). Safety, pharmacokinetics, and pharmacodynamics of single doses of LXR-623, a novel liver X receptor agonist, in healthy participants. J. Clin. Pharmacol.49, 643–649. 10.1177/0091270009335768
46
Khoury M. K. Yang H. Liu B. (2021). Macrophage biology in cardiovascular diseases. Macrophage Biol. Cardiovasc. Dis. Arterioscler. Thromb. Vasc. Biol.41, e77–e81. 10.1161/ATVBAHA.120.313584
47
Khoury S. J. C. M. De Haas R. Smeitink J. A. M. Rongen G. A. Russel F. G. M. Schirris T. J. J. (2020). Brothers in arms: ABCA1-and ABCG1-mediated cholesterol efflux as promising targets in cardiovascular disease treatment. Annu. Rev. Pharmacol. Toxicol.72, 152–190. 10.1124/pr.119.017897
48
Kim G. H. Oh G. S. Yoon J. Lee G. G. Lee K. U. Kim S. W. et al (2015). Hepatic TRAP80 selectively regulates lipogenic activity of liver X receptor. J. Clin. Invest.125, 183–193. 10.1172/JCI73615
49
Kirchgessner T. G. Martin R. Sleph P. Grimm D. Liu X. Lupisella J. et al (2015). Pharmacological characterization of a novel liver X receptor agonist with partial LXRα activity and a favorable window in nonhuman primates. J. Pharmacol. Exp. Ther.2015352 (2), 305–314. 10.1124/jpet.114.219923
50
Kirchgessner T. G. Sleph P. Ostrowski J. Lupisella J. Ryan C. S. Liu X. et al (2016). Beneficial and adverse effects of an LXR agonist on human lipid and lipoprotein metabolism and circulating neutrophils. Cell. Metab.24, 223–233. 10.1016/j.cmet.2016.07.016
51
Kobayashi N. Karisola P. Peña-Cruz V. Dorfman D. M. Jinushi M. Umetsu S. E. et al (2007). TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Cell. Metab.27, 927–940. 10.1016/j.immuni.2007.11.011
52
Kong P. Cui Z. Y. Huang X. F. Zhang D. D. Guo R. J. Han M. (2022). Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Signal Transduct. Target. Ther.7, 131. 10.1038/s41392-022-00955-7
53
Kuznetsova T. Prange K. H. M. Glass C. K. de Winther M. P. J. (2020). Transcriptional and epigenetic regulation of macrophages in atherosclerosis. Transcr. epigenetic Regul. macrophages Atheroscler. Nat. Rev. Cardiol.17, 216–228. 10.1038/s41569-019-0265-3
54
Lameijer M. Binderup T. van Leent M. M. T. Senders M. L. Fay F. Malkus J. et al (2018). Efficacy and safety assessment of a TRAF6-targeted nanoimmunotherapy in atherosclerotic mice and non-human primates. Nat. Biomed. Eng.2, 279–292. 10.1038/s41551-018-0221-2
55
Li H. Cao Z. Wang L. Liu C. Lin H. Tang Y. et al (2022). Macrophage subsets and death are responsible for atherosclerotic plaque formation. Front. Immunol.13, 843712. 10.3389/fimmu.2022.843712
56
Li N. Wang X. Xu Y. Lin Y. Zhu N. Liu P. et al (2017). Identification of a novel liver X receptor agonist that regulates the expression of key cholesterol homeostasis genes with distinct pharmacological characteristics. Mol. Pharmacol.91 (4), 264–276. 10.1124/mol.116.105213
57
Li R. Liu Y. Shi J. Yu Y. Lu H. Yu L. et al (2019). Diosgenin regulates cholesterol metabolism in hypercholesterolemic rats by inhibiting NPC1L1 and enhancing ABCG5 and ABCG8. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids1864, 1124–1133. 10.1016/j.bbalip.2019.04.010
58
Li Z. Ma D. Wang Y. Wu S. Wang L. Jiang Y. et al (2022). Astragali radix-coptis rhizoma herb pair attenuates atherosclerosis in ApoE-/- mice by regulating the M1/M2 and Th1/Th2 immune balance and activating the STAT6 signaling pathway. Evid. Based. Complement. Altern. Med.2022, 7421265. 10.1155/2022/7421265
59
Lin P. Ji H. H. Li Y. J. Guo S. D. (2021). Macrophage plasticity and atherosclerosis therapy. Macrophage plasticity Atheroscler. Ther. Front. Mol. Biosci.8, 679797. 10.3389/fmolb.2021.679797
60
Lipka J. Semmler-Behnke M. Sperling R. A. Wenk A. Takenaka S. Schleh C. et al (2010). Biodistribution of peg-modified gold nanoparticles following intratracheal instillation and intravenous injection. Biomaterials31, 6574–6581. 10.1016/j.biomaterials.2010.05.009
61
Liu N. Zhang B. H. Sun Y. L. Song W. Guo S. D. (2021). Atherosclerosis. Arterioscler. Thromb. Vasc. Biol.35, 485–491. 10.1161/ATVBAHA.115.305380
62
Liu S. Tang A. Xie M. Zhao Y. Jiang J. Liang G. (2015). Oligomeric hydrogels self-assembled from reduction-controlled condensation. Angew. Chem. Int. Ed. Engl.54, 3639–3642. 10.1002/anie.201409952
63
Liu W. Ma Z. Wang Y. Yang J. (2022). Multiple nano-drug delivery systems for intervertebral disc degeneration: Current status and future perspectives. Bioact. Mat.23, 274–299. 10.1016/j.bioactmat.2022.11.006
64
Loren J. Huang Z. Laffitte B. A. Molteni V. (2013). Liver X receptor modulators: A review of recently patented compounds (2009-2012). Expert Opin. Ther. Pat.23, 1317–1335. 10.1517/13543776.2013.814640
65
Ma C. Feng K. Yang X. Yang Z. Wang Z. Shang Y. et al (2021). Targeting macrophage liver X receptors by hydrogel-encapsulated T0901317 reduces atherosclerosis without effect on hepatic lipogenesis. Br. J. Pharmacol.178, 1620–1638. 10.1111/bph.15387
66
Marathe C. Bradley M. N. Hong C. Lopez F. Galarreta C. M. R. Tontonoz P. et al (2006). The arginase ii gene is an anti-inflammatory target of liver x receptor in macrophages. J. Biol. Chem.281, 32197–32206. 10.1074/jbc.M605237200
67
Maretti-Mira A. C. Golden-Mason L. Salomon M. P. Kaplan M. J. Rosen H. R. (2021). Cholesterol-Induced M4-Like macrophages recruit neutrophils and induce netosis. Front. Immunol.12, 671073. 10.3389/fimmu.2021.671073
68
Martinez-Pomares L. (2012). The mannose receptor. J. Leukoc. Biol.92, 1177–1186. 10.1189/jlb.0512231
69
Matsuo M. (2021). ABCA1 and ABCG1 as potential therapeutic targets for the prevention of atherosclerosis. J. Pharmacol. Sci.148, 197–203. 10.1016/j.jphs.2021.11.005
70
Meslier V. Laiola M. Roager H. M. De Filippis F. Roume H. Quinquis B. et al (2020). Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut.69, 1258–1268. 10.1136/gutjnl-2019-320438
71
Miyanishi M. Tada K. Koike M. Uchiyama Y. Kitamura T. Nagata S. (2007). Identification of tim4 as a phosphatidylserine receptor. Nature.450, 435–439. 10.1038/nature06307
72
Muscoli S. Ifrim M. Russo M. Candido F. Sanseviero A. Milite M. et al (2022). Current options and future perspectives in the treatment of dyslipidemia. J. Clin. Med.11, 4716. 10.3390/jcm11164716
73
Nakai K. (2021). Multiple roles of macrophage in skin. J. Dermatol. Sci.104, 2–10. 10.1016/j.jdermsci.2021.08.008
74
Naumann M. Arend N. Guliev R. R. Kretzer C. Rubio I. Werz O. et al (2023). Label-free characterization of macrophage polarization using Raman spectroscopy. Int. J. Mol. Sci.24, 824. 10.3390/ijms24010824
75
Nicholls S. J. Andrews J. Kastelein J. J. P. Merkely B. Nissen S. E. Ray K. K. et al (2018b). Effect of serial infusions of CER-001, a pre-β high-density lipoprotein mimetic, on coronary atherosclerosis in patients following acute coronary syndromes in the CER-001 atherosclerosis regression acute coronary syndrome trial: A randomized clinical trial. JAMA Cardiol.3, 815–822. 10.1001/jamacardio.2018.2121
76
Nicholls S. J. Puri R. Ballantyne C. M. Jukema J. W. Kastelein J. J. P. Koenig W. et al (2018a). Effect of infusion of high-density lipoprotein mimetic containing recombinant apolipoprotein A-I milano on coronary disease in patients with an acute coronary syndrome in the MILANO-PILOT trial: A randomized clinical trial. JAMA Cardiol.3, 806–814. 10.1001/jamacardio.2018.2112
77
Nurtanio N. Yang P. C. (2011). Role of TIM-4 in innate or adaptive immune response. N. Am. J. Med. Sci.3, 217–221. 10.4297/najms.2011.3217
78
Park D. Tosello-Trampont A. C. Elliott M. R. Lu M. Haney L. B. Ma Z. et al (2007). Bai1 is an engulfment receptor for apoptotic cells upstream of the elmo/dock180/rac module. Nature.450, 430–434. 10.1038/nature06329
79
Pelaz B. del Pino P. Maffre P. Hartmann R. Gallego M. Rivera-Fernández S. et al (2015). Surface functionalization of nanoparticles with polyethylene glycol: Effects on protein adsorption and cellular uptake. Acs. Nano9, 6996–7008. 10.1021/acsnano.5b01326
80
Peng D. Hiipakka R. A. Reardon C. A. Getz G. S. Liao S. (2009). Differential anti-atherosclerotic effects in the innominate artery and aortic sinus by the liver X receptor agonist T0901317. Atherosclerosis.203, 59–66. 10.1016/j.atherosclerosis.2008.05.058
81
Peng Y. Xu J. Zeng Y. Chen L. Xu X. L. (2019). Polydatin attenuates atherosclerosis in apolipoprotein E-deficient mice: Role of reverse cholesterol transport. Phytomedicine.62, 152935. 10.1016/j.phymed.2019.152935
82
Pourcet B. Gage M. C. León T. E. Waddington K. E. Pello O. M. Steffensen K. R. et al (2016). The nuclear receptor LXR modulates interleukin-18 levels in macrophages through multiple mechanisms. Sci. Rep.6, 25481. 10.1038/srep25481
83
Puig-Kröger A. Sierra-Filardi E. Domínguez-Soto A. Samaniego R. Corcuera M. T. Gómez-Aguado F. et al (2009). Folate receptor beta is expressed by tumor-associated macrophages and constitutes a marker for M2 anti-inflammatory/regulatory macrophages. Cancer Res.69, 9395–9403. 10.1158/0008-5472.CAN-09-2050
84
Qiao Y. N. Zou Y. L. Guo S. D. (2022). Low-density lipoprotein particles in atherosclerosis. Front. Physiol.13, 931931. 10.3389/fphys.2022.931931
85
Quinet E. M. Basso M. D. Halpern A. R. Yates D. W. Steffan R. J. Clerin V. et al (2009). LXR ligand lowers LDL cholesterol in primates, is lipid neutral in hamster, and reduces atherosclerosis in mouse. J. Lipid Res.50, 2358–2370. 10.1194/jlr.M900037-JLR200
86
Rasheed A. Cummins C. L. (2018). Beyond the foam cell: The role of LXRs in preventing atherogenesis. Int. J. Mol. Sci.19, 2307. 10.3390/ijms19082307
87
Rasheed A. Shawky S. A. Tsai R. Jung R. G. Simard T. Saikali M. F. et al (2021). The secretome of liver X receptor agonist-treated early outgrowth cells decreases atherosclerosis in Ldlr-/- mice. Stem Cells Transl. Med.10, 479–491. 10.1002/sctm.19-0390
88
Rodriguez-Manzanet R. Meyers J. H. Balasubramanian S. Slavik J. Kassam N. Dardalhon V. et al (2008). TIM-4 expressed on APCs induces T cell expansion and survival. J. Immunol.180, 4706–4713. 10.4049/jimmunol.180.7.4706
89
Rohatgi A. (2019). Reverse cholesterol transport and atherosclerosis. Arteriosclerosis, Thrombosis, Vasc. Biol.39, 2–4. 10.1161/ATVBAHA.118.311978
90
Ross R. (1999). Atherosclerosis–an inflammatory disease. N. Engl. J. Med.340, 115–126. 10.1056/NEJM199901143400207
91
Sanchez-Gaytan B. L. Fay F. Lobatto M. E. Tang J. Ouimet M. Kim Y. et al (2015). HDL-mimetic PLGA nanoparticle to target atherosclerosis plaque macrophages. Bioconjug. Chem.26, 443–451. 10.1021/bc500517k
92
Savla S. R. Prabhavalkar K. S. Bhatt L. K. (2022). Liver X receptor: A potential target in the treatment of atherosclerosis. Expert Opin. Ther. Targets26, 645–658. 10.1080/14728222.2022.2117610
93
Schultz J. R. Tu H. Luk A. Repa J. J. Medina J. C. Li L. et al (2000). Role of LXRs in control of lipogenesis. Genes. Dev.14, 2831–2838. 10.1101/gad.850400
94
Seo J. W. Baek H. Mahakian L. M. Kusunose J. Hamzah J. Ruoslahti E. et al (2014). (64) Cu-labeled LyP-1-dendrimer for PET-CT imaging of atherosclerotic plaque. Bioconjug. Chem.25, 231–239. 10.1021/bc400347s
95
Shen J. Hilgenbrink A. R. Xia W. Feng Y. Dimitrov D. S. Lockwood M. B. et al (2014). Folate receptor-β constitutes a marker for human proinflammatory monocytes. J. Leukoc. Biol.96, 563–570. 10.1189/jlb.2AB0713-372R
96
Shen W. J. Azhar S. Kraemer F. B. (2018). SR-B1: A unique multifunctional receptor for cholesterol influx and efflux. Annu. Rev. Physiol.8080, 95–116. 10.1146/annurev-physiol-021317-121550
97
Skarda L. Kowal J. Locher K. P. (2021). Structure of the human cholesterol transporter ABCG1. J. Pharm. Pharmacol.433, 167218. 10.1016/j.jmb.2021.167218
98
Skuratovskaia D. Vulf M. Khaziakhmatova O. Malashchenko V. Komar A. Shunkin E. et al (2020). Tissue-specific role of macrophages in noninfectious inflammatory disorders. Biomedicines.8, 400. 10.3390/biomedicines8100400,
99
Soumya R. S. Raghu K. G. (2023). Recent advances on nanoparticle-based therapies for cardiovascular diseases. J. Cardiol.81, 10–18. 10.1016/j.jjcc.2022.02.009
100
Spann N. J. Garmire L. X. McDonald J. G. Myers D. S. Milne S. B. Shibata N. et al (2012). Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell.151, 138–152. 10.1016/j.cell.2012.06.054
101
Steffensen K. R. Jakobsson T. GustafssonÅ J. (2013). Targeting liver X receptors in inflammation. Expert Opin. Ther. Targets.17, 977–990. 10.1517/14728222.2013.806490
102
Straub R. H. (2020). The memory of the fatty acid system. Prog. lipid Res.79, 101049. 10.1016/j.plipres.2020.101049
103
Tang J. Baxter S. Menon A. Alaarg A. Sanchez-Gaytan B. L. Fay F. et al (2016). Immune cell screening of a nanoparticle library improves atherosclerosis therapy. Proc. Natl. Acad. Sci. U. S. A.113, E6731–E6740. 10.1073/pnas.1609629113
104
Tang Y. Tang Z. Li P. Tang K. Ma Z. Wang Y. et al (2021). Precise delivery of nanomedicines to M2 macrophages by combining "eat me/don't eat me" signals and its anticancer application. ACS Nano.15, 18100–18112. 10.1021/acsnano.1c06707
105
Tanigawa H. Billheimer J. T. Tohyama J. Fuki I. V. Ng D. S. Rothblat G. H. et al (2009). Lecithin: Cholesterol acyltransferase expression has minimal effects on macrophage reverse cholesterol transport in vivo. Circulation.120, 160–169. 10.1161/CIRCULATIONAHA.108.825109
106
Tardif J. C. Ballantyne C. M. Barter P. Dasseux J. L. Fayad Z. A. Guertin M. C. et al (2014). Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: A randomized trial. Eur. Heart.35, 3277–3286. 10.1093/eurheartj/ehu171
107
Terasaka N. Hiroshima A. Koieyama T. Ubukata N. Morikawa Y. Nakai D. et al (2003). T-0901317, a synthetic liver X receptor ligand, inhibits development of atherosclerosis in LDL receptor-deficient mice. Febs. Lett.536, 6–11. 10.1016/s0014-5793(02)03578-0
108
Terpstra V. Bird D. A. Steinberg D. (1998). Evidence that the lipid moiety of oxidized low density lipoprotein plays a role in its interaction with macrophage receptors. Proc. Natl. Acad. Sci. U. S. A.95, 1806–1811. 10.1073/pnas.95.4.1806
109
Terpstra V. Kondratenko N. Steinberg D. (1997). Macrophages lacking scavenger receptor a show a decrease in binding and uptake of acetylated low-density lipoprotein and of apoptotic thymocytes, but not of oxidatively damaged red blood cells. Proc. Natl. Acad. Sci. U.S.A.94, 8127–8131. 10.1073/pnas.94.15.8127
110
Thomas D. G. Doran A. C. Fotakis P. Westerterp M. Antonson P. Jiang H. et al (2018). LXR suppresses inflammatory gene expression and neutrophil migration through cis-repression and cholesterol efflux. Cell. Rep.25, 3774–3785. 10.1016/j.celrep.2018.11.100
111
Uchida M. Kosuge H. Terashima M. Willits D. A. Liepold L. O. Young M. J. et al (2011). Protein cage nanoparticles bearing the LyP-1 peptide for enhanced imaging of macrophage-rich vascular lesions. Acs. Nano.5, 2493–2502. 10.1021/nn102863y
112
Van der Heijden J. W. Oerlemans R. Dijkmans B. A. Qi H. van der Laken C. J. Lems W. F. et al (2009). Folate receptor beta as a potential delivery route for novel folate antagonists to macrophages in the synovial tissue of rheumatoid arthritis patients. Arthritis Rheum.60, 12–21. 10.1002/art.24219
113
Van der Hoorn J. Lindén D. Lindahl U. Bekkers M. Voskuilen M. Nilsson R. et al (2011). Low dose of the liver X receptor agonist, AZ876, reduces atherosclerosis in APOE*3Leiden mice without affecting liver or plasma triglyceride levels. Br. J. Pharmacol.162, 1553–1563. 10.1111/j.1476-5381.2010.01168.x
114
Van der Velde A. E. (2010). Reverse cholesterol transport: From classical view to new insights. World J. Gastroenterol.16, 5908–5915. 10.3748/wjg.v16.i47.5908
115
Vazquez M. M. Gutierrez M. V. Salvatore S. R. Puiatti M. Dato V. A. Chiabrando G. A. et al (2020). Nitro-oleic acid, a ligand of CD36, reduces cholesterol accumulation by modulating oxidized-LDL uptake and cholesterol efflux in RAW264.7 macrophages. Redox Biol.36, 101591. 10.1016/j.redox.2020.101591
116
Vekic J. Zeljkovic A. Cicero A. F. G. Janez A. Stoian A. P. Sonmez A. et al (2022). Atherosclerosis development and progression: The role of atherogenic small, dense LDL. Med. Kaunas.58, 299. 10.3390/medicina58020299
117
Venkateswaran A. Laffitte B. A. Joseph S. B. Mak P. A. Wilpitz D. C. Edwards P. A. et al (2000). Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc. Natl. Acad. Sci. U. S. A.97, 12097–12102. 10.1073/pnas.200367697
118
Viennois E. Pommier A. J. Mouzat K. OumeddourEl A. Dufour J. Hajjaji F. Z. et al (2011). Targeting liver X receptors in human health: Deadlock or promising trail?Expert Opin. Ther. Targets.15, 219–232. 10.1517/14728222.2011.547853
119
Wang H. Liu Y. He R. Xu D. Zang J. Weeranoppanant N. et al (2020). Cell membrane biomimetic nanoparticles for inflammation and cancer targeting in drug delivery. Biomater. Sci.8, 552–568. 10.1039/c9bm01392j
120
Wang J. Z. Fang Y. Ji W. D. Xu H. (2017). LXR agonists promote the proliferation of neural progenitor cells through MEK-ERK pathway. Biochem. Biophys. Res. Commun.483, 216–222. 10.1016/j.bbrc.2016.12.163
121
Wang T. Zhao Y. You Z. Li X. Xiong M. Li H. et al (2020). Endoplasmic reticulum stress affects cholesterol homeostasis by inhibiting LXRα expression in hepatocytes and macrophages. Nutrients.12, 3088. 10.3390/nu12103088
122
Wang W. Liang M. Wang L. Bei W. Rong X. Xu J. et al (2023). Role of prostaglandin E2 in macrophage polarization: Insights into atherosclerosis. Biochem., Pharmacol.207, 115357. 10.1016/j.bcp.2022.115357
123
Wang X. Collins H. L. Ranalletta M. Fuki I. V. Billheimer J. T. Rothblat G. H. et al (2007). Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo but not SR-BI promote macrophage reverse cholesterol transport in vivo. J. Clin. Invest.117, 2216–2224. 10.1172/JCI32057
124
Wang X. Guo J. Chen T. Nie H. Wang H. Zang J. et al (2012). Multi-walled carbon nanotubes induce apoptosis via mitochondrial pathway and scavenger receptor. Toxicol. Vitro.26, 799–806. 10.1016/j.tiv.2012.05.010
125
Wattendorf U. Coullerez G. Vörös J. Textor M. Merkle H. P. (2008). Mannose-based molecular patterns on stealth microspheres for receptor-specific targeting of human antigen-presenting cells. Langmuir.24, 11790–11802. 10.1021/la801085d
126
WHO (2023). Cardiovascular diseases. Available at: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1.
127
Wilkinson K. El Khoury J. (2012). Microglial scavenger receptors and their roles in the pathogenesis of alzheimer's disease. Int. J. Alzheimers. Dis.2012, 489456. 10.1155/2012/489456
128
Wong K. Valdez P. A. Tan C. Yeh S. Hongo J. A. Ouyang W. (2010). Phosphatidylserine receptor Tim-4 is essential for the maintenance of the homeostatic state of resident peritoneal macrophages. Proc. Natl. Acad. Sci. U.S.A. 13. Proc. Natl. Acad. Sci. U. S. A.107 (19), 8712–8717. 10.1073/pnas.0910929107
129
Wu Y. Chen L. Xie Z. Wang C. Zhang J. Yan X. (2022). Effects of ABCG1 knockout on proteomic composition of HDL in mice on a chow diet and a high-fat diet. Proteomics22, e2100028. 10.1002/pmic.202100028
130
Xu H. Nie W. Dai L. Luo R. Lin D. Zhang M. et al (2023). Recent advances in natural polysaccharides-based controlled release nanosystems for anti-cancer phototherapy. Carbohydr. Polym.301, 120311. 10.1016/j.carbpol.2022.120311
131
Xu Y. Xu Y. Zhu Y. Sun H. Juguilon C. Li F. et al (2020). Macrophage miR-34a is a key regulator of cholesterol efflux and atherosclerosis. Mol. Ther.28, 202–216. 10.1016/j.ymthe.2019.09.008
132
Yang K. Xiao Q. Niu M. Pan X. Zhu X. (2022). Exosomes in atherosclerosis: convergence on macrophages. Int. J. Biol. Sci.18, 3266–3281. 10.7150/ijbs.71862
133
Yang M. Kholmukhamedov A. Schulte M. L. Cooley B. C. Scoggins N. O. Wood J. P. et al (2018). Platelet CD36 signaling through ERK5 promotes caspase-dependent procoagulant activity and fibrin deposition in vivo. Blood Adv.2, 2848–2861. 10.1182/bloodadvances.2018025411
134
Yasuda K. Takeuchi Y. Hirota K. (2019). The pathogenicity of Th17 cells in autoimmune diseases. Semin. Immunopathol.41, 283–297. 10.1007/s00281-019-00733-8
135
Yasuda T. Grillot D. Billheimer J. T. Briand F. Delerive P. Huet S. et al (2010). Tissue-specific liver X receptor activation pro-motes macrophage reverse cholesterol transport in vivo. Arterioscler. Thromb. Vasc. Biol.30, 781–786. 10.1161/ATVBAHA.109.195693
136
Yin C. Vrieze A. M. Rosoga M. Akingbasote J. Pawlak E. N. Jacob R. A. et al (2020). Efferocytic defects in early atherosclerosis are driven by GATA2 overexpression in macrophages. Front. Immunol.211, 594136. 10.3389/fimmu.2020.594136
137
Yu M. Amengual J. Menon A. Kamaly N. Zhou F. Xu X. et al (2017). Targeted nanotherapeutics encapsulating liver X receptor agonist GW3965 enhance antiatherogenic effects without adverse effects on hepatic lipid metabolism in Ldlr-/- Mice. Adv. Healthc. Mat.6, 10. 10.1002/adhm.201700313
138
Yuan W. Yu B. Yu M. Kuai R. Morin E. E. Wang H. et al (2021). Synthetic high-density lipoproteins delivering liver X receptor agonist prevent atherogenesis by enhancing reverse cholesterol transport. J. Control. Release.329, 361–371. 10.1016/j.jconrel.2020.11.016
139
Yvan-Charvet L. Welch C. Pagler T. A. Ranalletta M. Lamkanfi M. Han S. et al (2008). Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation.118, 1837–1847. 10.1161/CIRCULATIONAHA.108.793869
140
Zahr A. S. Davis C. A. Pishko M. V. (2006). Macrophage uptake of core-shell nanoparticles surface modified with poly (ethylene glycol). Langmuir22, 8178–8185. 10.1021/la060951b
141
Zang X. Cheng M. Zhang X. Chen X. (2021). Targeting macrophages using nanoparticles: ;a potential therapeutic strategy for atherosclerosis. J. Mat. Chem. B9, 3284–3294. 10.1039/d0tb02956d
142
Zhang B. H. Yin F. Qiao Y. N. Guo S. D. (2022). Triglyceride and triglyceride-rich lipoproteins in atherosclerosis. Front. Mol. Biosci.9, 909151. 10.3389/fmolb.2022.909151
143
Zhang S. Li L. Wang J. Zhang T. Ye T. Wang S. et al (2021). Recent advances in the regulation of ABCA1 and ABCG1 by lncRNAs. Clin. Chim. Acta516, 100–110. 10.1016/j.cca.2021.01.019
144
Zhang W. Lv Z. Zhang Y. Gopinath S. C. B. Yuan Y. Huang D. et al (2022). Targeted diagnosis, therapeutic monitoring, and assessment of atherosclerosis based on mesoporous silica nanoparticles coated with cRGD-Platelets. Oxid. Med. Cell. Longev.2022, 6006601. 10.1155/2022/6006601
145
Zhang X. McDonald J. G. Aryal B. Canfrán-Duque A. Goldberg E. L. Araldi E. et al (2021). Desmosterol suppresses macrophage inflammasome activation and protects against vascular inflammation and atherosclerosis. Proc. Natl. Acad. Sci. USA.118, e2107682118. 10.1073/pnas.2107682118
146
Zhang X. Q. Even-Or O. Xu X. van-Rosmalen M. Lim L. Gadde S. et al (2015). Nanoparticles containing a liver X receptor agonist inhibit inflammation and atherosclerosis. Adv. Healthc. Mat.4, 228–236. 10.1002/adhm.201400337
147
Zhao X. N. Li Y. N. Wang Y. T. (2016). Interleukin-4 regulates macrophage polarization via the MAPK signaling pathway to protect against atherosclerosis. Genet. Mol. Res. 15. 10.4238/gmr.15017348
Summary
Keywords
Liver X receptor agonists, macrophages, atherosclerosis, nanodrug delivery system, cholesterol homeostasis
Citation
Yang T-M, Miao M, Yu W-Q, Wang X, Xia F-J, Li Y-J and Guo S-D (2023) Targeting macrophages in atherosclerosis using nanocarriers loaded with liver X receptor agonists: A narrow review. Front. Mol. Biosci. 10:1147699. doi: 10.3389/fmolb.2023.1147699
Received
19 January 2023
Accepted
20 February 2023
Published
02 March 2023
Volume
10 - 2023
Edited by
Leming Sun, Northwestern Polytechnical University, China
Reviewed by
Xiaoxiao Yang, Hefei University of Technology, China
Hongliang He, Southeast University, China
Updates
Copyright
© 2023 Yang, Miao, Yu, Wang, Xia, Li and Guo.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan-Jie Li, liyj@wfmc.edu.cn; Shou-Dong Guo, sd-guo@hotmail.com
†These authors have contributed equally to this work
This article was submitted to Molecular Diagnostics and Therapeutics, a section of the journal Frontiers in Molecular Biosciences
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.