Abstract
Heavy metals are the metal compounds found in earth’s crust and have densities higher than that of water. Common heavy metals include the lead, arsenic, mercury, cadmium, copper, manganese, chromium, nickel, and aluminum. Their environmental levels are consistently rising above the permissible limits and they are highly toxic as enter living systems via inhalation, ingestion, or inoculation. Prolonged exposures cause the disruption of metabolism, altered gene and/or protein expression, and dysregulated metabolite profiles. Metabolomics is a state of the art analytical tool widely used for pathomolecular inv22estigations, biomarkers, drug discovery and validation of biotransformation pathways in the fields of biomedicine, nutrition, agriculture, and industry. Here, we overview studies using metabolomics as a dynamic tool to decipher the mechanisms of metabolic impairment related to heavy metal toxicities caused by the environmental or experimental exposures in different living systems. These investigations highlight the key role of metabolomics in identifying perturbations in pathways of lipid and amino acid metabolism, with a critical role of oxidative stress in metabolic impairment. We present the conclusions with future perspectives on metabolomics applications in meeting emerging needs.
1 Introduction
The profiling of metabolome is known as metabonomics or metabolomics, which is a branch of “omics” science including genomics, proteomics, transcriptomics, inomics, phenomics, and metabolomics (Deshmukh et al., 2014). Metabonomics has been frequently associated with nuclear magnetic resonance (NMR) spectroscopy while metabolomics is often associated with mass spectrometry (MS) based approaches and refers to the study of metabolites, metabolite composition, metabolome, or metabolism in the organism, organ, tissue, or cell. It includes the quantitative and qualitative characterization of small molecule (<1.5 kDa) metabolites, their intermediates, hormones, and other signaling molecules as well as secondary metabolites that show changes in response to genetic modifications or stimuli from the external and/or internal sources. The metabolome represents the global collection or complete set of all low-molecular weight metabolites that are expressed during the metabolic activity and provide a direct functional readout of the cellular activity and the physiological status (Patti et al., 2012; Dinis-Oliveira, 2014; Wishart, 2016).
Metabolomics is located at the bottom of the “omics” cascade and relates to both the downstream output of the genome and the upstream input of the environment and may, therefore, answers queries that might not be answered using other branches of “omics” science (Kettunen et al., 2012; Wishart, 2016; Zaitsu et al., 2016). Whereas, metabolome refers to the metabolites and biomolecules derived endogenously including steroids, fatty acids, lipids, vitamins, carbohydrates, and amino acids, the exo-metabolome refers to the extracellular metabolites and the xeno-metabolome relates to the metabolites derived exogenously from xenobiotics or their metabolites from phase I or phase II metabolism (David et al., 2014). Metabolomics helps detect endogenous substances in biospecimens such as tissues, hair, nails, blood, urine, cerebrospinal fluid, saliva, etc. in relation to changes in metabolite profiles under different conditions. Metabolomics is now being widely used in studies of biomarkers (Klein and Shearer, 2016; Zhang et al., 2016; Ambati et al., 2017), drug discovery (Lu and Chen, 2017; Mercier et al., 2018), biotransformation pathways validation (Ren et al., 2016; Zhang et al., 2016), disease pathogenesis (Klein and Shearer, 2016; Ren et al., 2016; Würtz et al., 2016), and the investigations related to toxicology, nutrition, pharmacology, and clinical trials (Brignardello et al., 2017; Korsholm et al., 2017; Cornelis et al., 2018; Wu et al., 2018).
The metals found in earth’s crust with density higher than that of water are called heavy metals (Tchounwou et al., 2012). Humans and animals are constantly being exposed to heavy metals and, unfortunately, their levels in the environment are exceeding the permissible limits. Contamination of heavy metals in water resources, air, and food is a metter of growing concern for both human and animal health and wellbeing, and hundreds of amillions of people are being affected worldwide (Balali-Mood et al., 2021). The levels of toxic heavy metals in the environment have reached the alarming thresholds, posing a serious global issue due to their potential adverse effects on living systems. These inorganic pollutants, commonly known as heavy metals, are continuously increasing in our surroundings as they are released into the atmosphere, soil, and water bodies. The primary sources of these heavy metals include the rapid expansion of metal industries, agricultural practices, the use of fertilizers, improper waste disposal, and the widespread application of pesticides (Rajkumar et al., 2023). The incidence and magnitude of heavy metal toxicities vary depending on the geographical location, soil content, human activities, social customs, types and location of industries, regulatory measures for containment of pollution, healthcare facilities for detection and toxicity intervention as well as nutritional status and genetics of local population (Tchounwou et al., 2012). The environmental pollution is particularly high in point source areas including mining, foundries, smelters, and other metal-related industrial operations (Bradl, 2005; He et al., 2005). Environmental contamination may also occur through other causes or activities such as metal corrosion, soil erosion of metal ions and leaching of heavy metals, atmospheric deposition, sediment resuspension and metal evaporation from water resources to soil and ground water (Nriagu, 1989). The World Health Organization (WHO) has listed 10 major pollutants, 4 of which include heavy metals (Rehman et al., 2018). Epidemiological studies have demonstrated the association between heavy metal exposure and chronic disorders such as diabetes, respiratory diseases, renal disease, neurodegenerative disorders, cutaneous ailments, cardiovascular disease, and cancer (Kim et al., 2015; Yang et al., 2015).
Such heavy metals include the lead (Liu et al.), arsenic (As), mercury (Hg), cadmium (Cd), copper (Cu), manganese (Rafati Rahimzadeh, Rafati Rahimzadeh, Kazemi, and Moghadamnia), chromium (Cr), nickel (Ni), and aluminum (Al); all of which are highly toxic when inhaled, ingested, or experimentally inoculated in living systems. Following entry into the body, these heavy metals damage the cellular organelles and inhibit biochemical and metabolic pathways to impair physiological functions of the organs. The heavy metals have specific mechanisms of toxicity in the living systems and most cause oxidative stress which leads to an excessive accumulation of highly toxic reactive oxygen species (ROS) and free radical-associated damage to the critical organs, tissues, cells, and macromolecules (Kettunen et al.). ROS can irreversibly damage the major organs like liver, kidney, brain, heart, lung, as well as the reproductive and immune systems. Heavy metals can also cause gene mutations, carcinogenesis, skin irritation, cell damage, and cell death. The metabolism of carbohydrates, lipids, and amino acids is also affected by heavy metals. Prolonged exposures to heavy metals cause altered gene and protein expression as well as disruption of metabolism and metabolites. The damages caused by heavy metals can become life threatening and irreversible (Jaishankar et al., 2014; Briffa et al., 2020; Balali-Mood et al., 2021; Mitra et al., 2022). The major sources of heavy metals’ exposure and associated health hazards are listed in Table 1.
TABLE 1
| Heavy metals | Permissible level (mg/L) | Sources of exposure | Health hazards | Ref |
|---|---|---|---|---|
| Pb | 0.1 | Pipes, Paints, Batteries, Sinkers in fishing, Ceramics, Paper dying, Automobile emission, Coal burning, Mining, Petrochemicals, Smoking, Manufacturing of lead-acid batteries | Effects on blood-brain barrier, Reproductive and neurological defects, Bladder and lung cancer, CVD, Hemolytic anemia | Wani et al. (2015), Rehman et al. (2018) |
| As | 0.02 | Wood preservatives, Herbicides, Agricultural pesticides, Tobacco smoke, Fungicides, Metal smelters, Contaminated water, Industrial waste | Gastrointestinal disturbances, Diarrhea, Cardiovascular disease risk, Severe vomiting, Hypertension, Diabetes mellitus, Carotid atherosclerosis, and Malignancies such as lung cancer | Chen et al. (2011), Hughes et al. (2011), Hubaux et al. (2013), Rehman et al. (2018) |
| Cd | 0.06 | Used as a stabilizer in several alloys, color pigments, and PVC products, Phosphate fertilizer, Cigarette smoking, Crops and vegetables grown in cadmium-contaminated soil due to sludge and phosphate fertilizer, Drinking water, Photovoltaic device in TV screens, Nuclear and coal power plants, Toys, Welding, Electroplating, and nuclear fission plants | Oxidative stress, Damage to the respiratory system, Neurotoxicity, Nephrotoxicity, Carcinogenic effects, Bone demineralization, and Diabetes mellitus | Rafati Rahimzadeh et al. (2017), Rehman et al. (2018) |
| Hg | 0.01 | Fluorescent light bulbs, Electrical switches, Incineration of municipal waste, Mercury bulbs, Coal power plants, Pesticides, Cosmetics, Batteries, Paper industry | Damage to the kidney, brain, and vision, Skin burns, and Nervous disorders | Rehman et al. (2018), Zulaikhah et al. (2020) |
| Cu | 0.1 | Pesticide production, Chemical industry, Mining, and Metal piping | Liver and kidney damage, Anemia, and GI tract irritation | Rehman et al. (2018), Rehman et al. (2019) |
Heavy metals, permissible limits, sources of exposure, and related health hazards.
Abbreviations: Pb: Lead; As: Arsenic; Cd: Cadmium; Hg: Mercury; Cu: Copper; CVD: Cardio-vascular disease; PVC: polyvinyl chloride; GI: gastrointestinal.
2 Metabolomics workflow
Typically, a biospecimen such as an organ, tissue biopsy, or cultured cells from an organism is metabolically quenched using nitrogen gas. To prepare a biospecimen containing thousands of metabolites, the sample is homogenized by an electric homogenizer to obtain the liquid mixture of the sample. Since the tissue extraction process is somewhat complex, it is rather more convenient to collect the biofluids such as cell culture supernatants, sweat, breast milk, bile, feces, cerebrospinal fluid, urine, saliva, and blood. Certain metabolites can be found in both blood and urine samples, while others may be more specific to each sample type. In contrast, saliva samples generally contain a limited number of metabolites compared to blood and urine. The selection of the appropriate sample for metabolomics studies is of utmost importance, as it significantly influences the outcomes. Therefore, it is essential to handle, store, and process the samples under specific conditions to ensure the accuracy and reliability of the results. Any deviation from these specified conditions can impact the identification of metabolites and their associated pathways. Once the sample is prepared, it can be injected into one or more analytical instruments such as gas chromatography/mass spectrometry (GC-MS), NMR, liquid chromatography/mass spectrometry (LC-MS), capillary electrophoresis/mass spectrometry (CE-MS), or ion mobility spectrometer/mass spectrometer (IMS-MS) systems.
Overall, to develop better workflows in heavy metal toxicity assessments using metabolomics, it is imperative to increase the reproducibility and translatability of toxicometabolomics data through the standardized approach or procedures based on appropriate selection of experimental design, optimal sample collection, storage, extraction and preparation, correct choice of analytical instruments/platform and proper data analysis, all of which can considerably affect the obtained results. Since the optimal and simultaneous extraction, detection, and quantification of all metabolites is unlikely while using a single method, use of multiple analytical platforms enhances the metabolite coverage. To date, NMR and MS are the commonest approaches used to generate metabolomics data (Araújo et al., 2021). It should also be noted that the use of in vitro models (primary cells, cell lines, co-cultures, 3D cultures, organ-on-chip culture, etc.) as well as using small cohorts might contribute as potential sources of bias and data variability. Furthermore, the preliminary metabolomics data obtained must be further validated, for which, putative biomarkers that have been identified using metabolomics must be accurately and precisely assessed in larger study cohorts. Special consideration should also be given to the data stored in databases and biobanks which, in case of heavy metal toxicometabolomics, still need to be further expanded and improved. A complete workflow of metabolomics is illustrated in Figure 1.
FIGURE 1

The workflow diagram of metabolomics.
3 Role of metabolomics in deciphering impairment caused by heavy metal toxicities
Heavy metals have a specific density of ˃5 g/cm3 (Järup, 2003) and a potential to adversely affect the environment as significant pollutants and prove noxious to living organisms when the levels exceed certain permissible thresholds. At very low, normal or at biologically relevant concentrations, these metals are quintessential for the homeostatic maintenance of diverse biochemical and physiological functions in living organisms. The most common heavy metal toxicants found in the environment include the As, Pb, Cd, Cr, Hg, Cu, and Al, all of which pose dire risks for human and animal health as well as for the environment (Tchounwou et al., 2012). The common sources of heavy metal toxicants include sewage discharge, soil erosion, urban runoff, industrial and mining operations and effluents, natural weathering of earth’s crust, pesticides and other disease control agents applied to crops, etc. (Oosthuizen, 2012). Mechanistically, most heavy metals have the ability to bind with proteins at critical sites by displacing original metals from their natural binding sites, thus leading to cellular dysfunction and toxicity or by binding to DNA and nuclear proteins which results in oxidative deterioration of the biological macromolecules (Flora et al., 2008).
“As” causes serious toxicities in humans and animals and exposures commonly occur via air, food, and water, leading to organ toxicity, hypertension, cardiometabolic disease, type-2 diabetes (T2D), neurological issues, and cancers of the lung, liver, bladder and skin (Smith et al., 2000). Regarding the effect of inorganic As in diabetic and non-diabetic individuals, Martin et al. found that metabolite alterations were associated with amino acid metabolism and TCA cycle and were related to oxidative stress and endocrine disturbances as potential indicators (Martin et al., 2015). A study in male Sprague-Dawley (SD) rats also reported changes in amino acid and lipid metabolism, leading to organ toxicity, metabolic disruption and altered gene expression (Wang et al., 2015). Similarly, Zhang and Shen et al. identified that the top 5 urinary biomarker metabolites were strongly associated with endocrine disturbances and oxidative stress, concluding that metabolomics was highly useful for identifying urinary biomarkers of As-associated toxicity (Zhang et al., 2014).
Pb is used in several industries, agriculture, and home items. Common toxicities occur via inhalation and ingestion. It induces expression of free radicals and increased ROS lead to changes in lipid metabolism, DNA damage, and dysregulated gene expression, also interrupting the heme pathway to cause anemia (Rehman et al., 2018). A Pb toxicity study of inhabitants living near the used lead acid battery (ULAB) recycling plants reported urinary metabolites that were associated with impairment in hemoglobin biosynthesis pathway, small-molecule transport, nephrotoxicity, and neurotoxicity (Eguchi et al., 2018). Kelly et al. investigated Pb levels in toenails and blood, with signature metabolites in the plasma and concluded that metabolomics was a promising tool for analyzing pathogenic mechanisms of Pb toxicity including oxidative stress and immune system dysfunction (Kelly et al., 2020). In a recent study, a group of 25 albino Wistar rats was divided into different groups and treated with lead acetate (PbAc). The aim was to investigate the toxic effects of lead exposure on metabolic pathways and evaluate the potential ameliorative effect of quercetin (Que), a known antioxidant. The metabolic study conducted in this research revealed interesting findings. Specifically, the analysis identified four lipid metabolites and six amino acids that demonstrated significant changes in response to lead exposure. These alterations in the lipid and amino acid metabolism provided evidence of impairment in these specific metabolic pathways due to the toxic effects of lead. To further explore the potential protective effect of quercetin, rats were treated with Que after PbAc exposure. The results indicated that the antioxidant properties of Que exerted an ameliorative effect on lead toxicity. This suggests that Que was able to mitigate or reduce the negative impacts of lead-induced oxidative stress on the metabolic pathways. Overall, this study confirms the toxicity of heavy metals, specifically lead, in impairing metabolic pathways through the induction of oxidative stress. The identification of altered lipid metabolites and amino acids provides the insight into the specific metabolic disturbances that are caused by lead exposure. Additionally, the study highlights the potential of quercetin as a protective agent against heavy metal toxicity, potentially through its antioxidant properties (Yaqoob et al., 2022).
Cd and chlorpyrifos (CPF) are the environmental contaminants and exposures occur via inhalation (tobacco smoking) and ingestion (dietary sources), resulting in acute and chronic intoxications. These can bind with cysteine, glutamate, histidine, and aspartate, causing iron deficiency and neurotoxicity. By replacing Zn2+ in metallothionein, they can interfere with free radical scavenging and lead to oxidative damage (Irfan et al., 2013). Regarding toxic effects of Cd on metabolism and renal function, increased levels were associated with changes in amino acid metabolism and urine creatine pathway, and the most specific biomarkers included creatine, creatinine, l-tryptophan, adenine, and uric acid (Zeng et al., 2021). Cd, CPF, or their mixture caused neuronal damage to the rat brain, and disrupted amino acid and energy metabolism (Xu et al., 2015). Metabolic profiling of volunteers identified the potential biomarkers of Cd exposure and smoking to guide future interventions for disease prevention (Ellis et al., 2012).
Cr is found in the industrial environment such as metallurgy, electroplating, chemicals, paints, pigments, tanning, wood, pulp, and paper production. Cr toxicity causes dysfunction of antioxidant enzymes such as catalase, peroxidase, and cytochrome oxidase, leading to ROS overexpression and oxidative stress which damages the DNA/RNA, lipids, and proteins (Stohs and Bagchi, 1995).
Hg is a potentially hazardous toxicant associated with acute heavy metal poisoning in humans. Methyl mercury (MeHg) is highly neurotoxic and causes free radical formation, lipid peroxidation, damage to the ribosomes, endoplasmic reticulum (ER) and mitochondria, disruption of mitochondrial membrane potential (ΔΨm) and Ca2+ homeostasis, and the accumulation of neurotoxic molecules including aspartate, glutamate, and serotonin which may lead to malfunctioning of nerves, brain, kidneys, and muscles (Patrick, 2002). A toxicity study of MeHg and perfluorooctanesulfonate (PFOS) in pregnant SD rats showed weight loss, and delayed behavioral and innate immune responses in newborns, anxiety in adolescents, and increased thigmotaxic or hyperactivity in young pups. The altered cortical metabolites were associated with excitatory and inhibitory neurotransmission and defective fetal development (Reardon et al., 2019).
Cu is found in high concentrations in the liver, brain, and kidneys. It binds to ceruloplasmin in the liver and is transported to the peripheral tissues. It acts as a catalytic cofactor in redox reactions of many proteins and a balance between uptake and efflux of Cu2+ ions is critical to cellular Cu homeostasis. Increased Cu levels lead to oxidative stress through Fenton reactions, DNA damage, reduced cell proliferation, and metabolic disruption (Oe et al., 2016). Xiao et al. found that Cu toxicity in the intestinal HT-29 human colon carcinoma cell line caused oxidative stress, apoptosis, and altered energy and lipid metabolism and mitochondrial β-oxidation (Xiao et al., 2016). A study of untargeted metabolomics in human lung epithelial A549 cells exposed to copper oxide nanoparticles (CuO NPs) identified biomarker metabolites that were associated with hypertonic and oxidative stresses and apoptosis (Boyles et al., 2016).
Al toxicities occur by ingestion of contaminated food and beverages. Using human colon carcinoma HT-29 cell line model, Yu et al. reported that Al exposure arrested the cellular growth and proliferation, with altered metabolites/pathways of pyruvate metabolism, glutathione metabolism and TCA cycle, and mechanisms associated with lipids/amino acid metabolism, apoptosis, oxidative stress, and bioenergetics (Yu et al., 2019).
In regard with the relationship between heavy metal toxicity and breast cancer progression, the top 4 heavy metals that were significantly increased in plasma of breast cancer patients included the Cd, Pb, As, and Cr, suggesting that altered lipids and metabolites influenced the breast cancer progression (Li et al., 2020). Likewise, metabolomics of urine samples of breast cancer patients were found to have elevated levels of Cd, As, and Cr, suggesting that environmental exposure to these heavy metals affected urine metabolites and correlated with breast cancer progression (Men et al., 2020). Regarding the relationship between heavy metal toxicity and T2D, a longitudinal study reported the link between exposure to heavy metals (Pb, Hg, and Cd), renal dysfunction biomarkers (RBP, NAG, and KIM-1), and plasma indicators for T2D (AAAs, BCAAs, leptin, and adiponectin) (Valcke et al., 2019). A cross-sectional metabolomics study was conducted in 2022, among the middle to older-aged Chinese adults to investigate the potential risk of developing T2D and acute coronary syndrome (ACS) associated with heavy metal toxicity. The study aimed to explore the association between plasma heavy metals and metabolites and their relationship with the identified diseases. The analysis involved the measurement of 17 plasma heavy metals and 189 metabolites. The results revealed significant associations between heavy metals and metabolites. Among the top five identified metabolites were uridine, aspartyl phenylalanine, lysophosphatidylcholines 18:2, carnitine C14:2, and free fatty acid 14:1. Further analysis using Bayesian kernel machine regression (BKMR) confirmed the positive correlations of plasma Mn, Ba, Pb, and Co with carnitine, as well as plasma Ba, Al, Zn, and As with aspartyl phenylalanine. These findings suggested that a relationship existed between heavy metal toxicity and the biosynthesis of aminoacyl-tRNA and linoleic acid metabolism pathways. Additionally, the study revealed two unique pathways, alpha-linolenic acid metabolism, and biosynthesis of unsaturated fatty acids, that showed associations with heavy metals. The study concluded that the identified metabolites provided evidence of the risk of developing T2D and ACS due to heavy metal toxicity (Lin et al., 2022a). Furthermore, Supplementary Table S1 provides a comprehensive overview of the most common heavy metal toxicities in humans, animals, and cell lines, including details such as study type, exposure source, study design, subjects, detected metabolites, sample types, analytical instruments, software, database, and statistical models used.
Collectively, these studies demonstrate the advantages of metabolomics in assessing heavy metal toxicity. Although heavy metal toxicity may not exhibit immediate phenotypic changes, it can contribute to enhance the future risk of various chronic diseases and metabolic impairments. Metabolomics studies provide valuable information on the qualitative and quantitative changes in metabolites resulting from heavy metal exposure. Since these metabolites are involved in various metabolic and biosynthetic pathways, any impairment in these pathways can contribute to the development of progressive diseases.
4 Key role of oxidative stress in heavy metal toxicity pathogenesis
Humans are exposed to heavy metals via the occupational or environmental exposures. Accumulation of heavy metals in the body causes suppression or imbalance of the enzymes that are involved in antioxidant defense such as catalase, superoxide dismutase, glutathione reductase (GR), and glutathione peroxidase (GPx), resulting in generation of highly reactive free radicals or ROS (superoxide O2•−, hydroxyl OH•, peroxyl RO2• and alkoxyl RO•) as well as certain non-radical species (peroxynitrite ONOO− and hydrogen peroxide H2O2) and mitochondrial dysfunction (Ranjbar et al., 2014; Jia et al., 2015; Sun et al., 2022). The imbalance in the generation and scavenging of free radicals leads to oxidative stress, DNA/RNA oxidation, lipid peroxidation, protein carbonylation, metabolic impairment, and hepatotoxicity in the body (Niture et al., 2021; Renu et al., 2021). These complications further lead to dysregulation of the ER (Yokouchi et al., 2007; Rana, 2020) and mitochondrial homeostasis (Branca et al., 2020), dysregulation of autophagy (Chatterjee et al., 2014; Wang et al., 2020), cell cycle arrest, DNA fragmentation and apoptosis (Hamada et al., 1997; Yedjou et al., 2015). Heavy metals cause alteration in the expression of DNA damage repair associated p53 tumor suppressor protein (Rana, 2008), downregulated expression of p21 cyclin-dependent kinase inhibitor (CKI) (Vogt and Rossman, 2001), DNA methylation (Vaiserman and Lushchak, 2021), other epigenetic alterations (Bitto et al., 2014; Ryu et al., 2015), and histone modifications; all these factors may lead to carcinogenicity (Chen et al., 2006; Ke et al., 2008; Chen et al., 2010).
Heavy metals also have deleterious effects on kidneys and are responsible for tubular proteinuria, renal glycosuria, aminoaciduria, glucosuria, phosphaturia, and Fanconi-like syndrome (Diamond and Zalups, 1998; Lentini et al., 2017; Orr and Bridges, 2017). Nephrotoxicity is mediated by elevated expression of transcription factors MAPK/NF-κB which triggers the inflammatory processes in renal tissue microenvironment (Li et al., 2021; Hassanein et al., 2022). Accumulation of heavy metals may lead to several neurological disorders including Parkinson’s disease (Montgomery, 1995; Vellingiri et al., 2022), Alzheimer’s disease (Kitazawa et al., 2009; Kabir et al., 2021), and related dementias (Bakulski et al., 2020), resulting in the loss of homeostatic regulation, neuroinflammation and neuronal apoptosis (Huat et al., 2019; Alquezar et al., 2020). The immune function is also compromised by exposure to heavy metals, leading to changes in major immune effector cells and expression of inflammatory cytokines/chemokines (Dong et al., 1998; Bonaventura et al., 2018; Hossein-Khannazer et al., 2020; Wang et al., 2021), autoimmunity (Rowley and Monestier, 2005; Anka et al., 2022), increased allergies (Büdinger and Hertl, 2000; Vas and Monestier, 2008; Riedel et al., 2021), hematological cancers (Kim et al., 2015; Chen et al., 2016; Gianì et al., 2021; Kiani et al., 2021; Vincent Salvatore and Juley, 2021), immunosuppression (Jadhav et al., 2007; Jin et al., 2020), and diseases involving opportunistic pathogens (Lokuge et al., 2004; Krueger and Wade, 2016; Eggers et al., 2018; Vats et al., 2022) and parasites (El-Hak et al., 2022).
Chronic heavy metal exposures can lead to hyperpigmentation (Granstein and Sober, 1981; Jagadeesan et al., 2021), hyperkeratosis (Huang et al., 2019), cutaneous cancers (Järup, 2003; Uddin et al., 2007; Surdu et al., 2013) as well as cardiovascular disease (CVD) associated metabolic pathologies including hypertension (Rahman et al., 1999), atherosclerosis (Hsieh et al., 2008), reduced NO availability (Simeonova and Luster, 2004), altered renin-angiotensin system (Akther et al., 2019), disrupted vascular smooth muscle Ca2+ signaling (Marchetti, 2013), thrombosis (Arbi et al., 2017; Ferrante et al., 2017), suppression of vasodilator (Tan et al., 2020) and overexpression of vasoconstrictor prostaglandins (Alissa and Ferns, 2011), and increased inflammatory responses (Zhang et al., 2020; Nguyen, Oh, Hoang and Kim, 2021).
Overall, oxidative stress from exposure to heavy metals leads to ROS-associated metabolic reprogramming, cellular and/or organelle dysfunction, as well as perturbations of critical pathways related to carbohydrate, protein, and lipid metabolism (Wu et al., 2016). Heavy metals toxicity mechanisms are depicted in Figure 2.
FIGURE 2
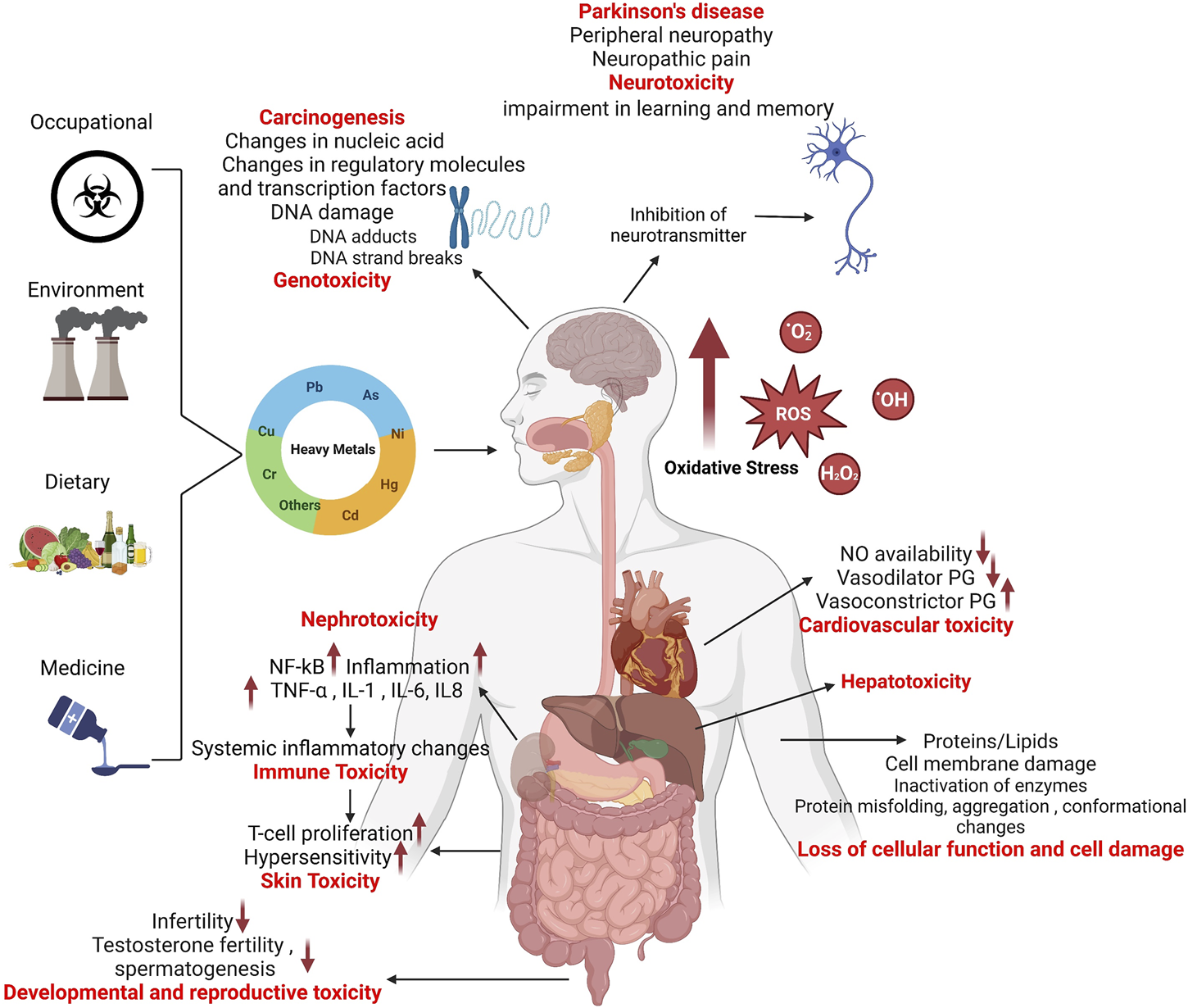
Mechanisms of heavy metal toxicity.
5 Conclusion and perspectives
Compared with other relatively more established “omics”, such as transcriptomics, we have just begun to realize the robustness and enormous potentials of metabolomics as a state of the art technique to investigate heavy metal toxicities at the cellular, tissue, and organismal levels. Metabolomics provides unique perspectives on cytotoxicity-induced changes in metabolic profiles and functions. Herein, we have reviewed the studies that investigated toxicity effects of heavy metals or derivatives such as Pb, As, Cd, Cr, Hg/MeHg, Ni, PFOS, Cu/CuO NPs, and Al, using metabolomics of blood, plasma, serum, urine, toenails, brain samples of humans and animals as well as cell lines. Heavy metals cause impairment of antioxidant defense, resulting in excessive accumulation of highly reactive free radicals or ROS which emerge as key players in pathophysiology of heavy metal toxicity. Apart from causing damage to the ER and mitochondria, ROS irreversibly damage the cellular biological macromolecules including nucleic acids, proteins, and lipids. ROS-induced genotoxic changes such as abstraction or addition of bases, strand breaks in DNA sugar-phosphate backbone, and other DNA lesions may lead to cancer development. While, protein and lipid per/oxidation metabolites give rise to multiple toxicities including molecular, cell and organ damage, altered transcriptional and translational regulation, reproductive and developmental disorders, and neurological, dermatological, hepatic, renal, pulmonary, cardiovascular, hematologic, and immunological morbidities. Metabolomics yields the unique information about key metabolic changes affecting morbid processes in a wide variety of living systems by identifying, quantifying, and characterizing small metabolites with high precision. Metabolic fingerprinting and footprinting help decipher metabolites that derange homeostasis, metabolism, physiology, and immune functioning in the body.
Nonetheless, there are some challenges involved that need to be pointed out. First, while using untargeted or knowledge-discovery approach, it might be difficult to pinpoint which metabolic pathways play a key role as this type of investigations aim to measure as many metabolites as possible, given that this information provides basis of hypothesis building for future work. It is encouraging to see that better tools are becoming available for enrichment analyses and network constructions which enhances capabilities for data interpretation. Second, metabolomics might be laborious and expensive, given the chemical diversity of metabolome and rapid turnover of metabolites, both of which affect reproducibility of data generated. Third, in order for metabolomics data to be more meaningful, data integration with other systems biology approaches like transcriptomics and proteomics might be required for temporal comparison. From broader perspectives, metabolomics is a dynamic tool for cutting edge research in healthcare, pharmaceutics, food sciences, agriculture, and chemical industries. Metabolomics is guiding decision making for drug safety and biomarker discovery. In the near future, individualized metabolomics will most likely be mainstay for human health and environment monitoring and emerge as a lead analytical tool for molecular diagnostics, drug phenotyping, customized therapeutics, and much more.
Statements
Author contributions
Conceptualization, MSHA, FAM, RA, and SS; data curation, MSHA and SS; writing–original draft preparation, MSHA, AY, KR, MI, MAA, FAR, FAM, RA, and SS; writing–review and editing, MSHA, FAR, FAM, RA, and SS; supervision, MSHA, FAM, and SS. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the Kuwait Foundation for the Advancement of Sciences (KFAS) (Grants #: RA 2015-027; RA HM-2019-022). The authors express their appreciation to the Deanship of Scientific Research at King Khalid University, Saudi Arabia for supporting this work through research group program under grant number RGP-2/370/44.
Acknowledgments
The authors thank BioRender.com for allowing creation of the illustrations used in this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2023.1218497/full#supplementary-material
References
1
Akther J. Nabi A. H. M. N. Ebihara A. (2019). Heavy metals as environmental risk factors for cardiovascular diseases: From the perspective of the renin angiotensin aldosterone system and oxidative stress. Rev. Agric. Sci.7, 68–83. 10.7831/ras.7.0_68
2
Alissa E. M. Ferns G. A. (2011). Heavy metal poisoning and cardiovascular disease. J. Toxicol.2011, 870125. 10.1155/2011/870125
3
Alquezar C. Felix J. B. McCandlish E. Buckley B. T. Caparros-Lefebvre D. Karch C. M. et al (2020). Heavy metals contaminating the environment of a progressive supranuclear palsy cluster induce tau accumulation and cell death in cultured neurons. Sci. Rep.10 (1), 569. 10.1038/s41598-019-56930-w
4
Ambati C. S. Yuan F. Abu-Elheiga L. A. Zhang Y. Shetty V. (2017). Identification and quantitation of malonic acid biomarkers of in-born error metabolism by targeted metabolomics. J. Am. Soc. Mass. Spectrom.28 (5), 929–938. 10.1007/s13361-017-1631-1
5
Anka A. U. Usman A. B. Kaoje A. N. Kabir R. M. Bala A. Kazem Arki M. et al (2022). Potential mechanisms of some selected heavy metals in the induction of inflammation and autoimmunity. Eur. J. Inflamm.20, 1721727X2211227. 10.1177/1721727X221122719
6
Araújo A. M. Carvalho F. Guedes de Pinho P. Carvalho M. (2021). Toxicometabolomics: Small molecules to answer big toxicological questions. Metabolites11 (10), 692. 10.3390/metabo11100692
7
Arbi S. Oberholzer H. M. Van Rooy M. J. Venter C. Bester M. J. (2017). Effects of chronic exposure to mercury and cadmium alone and in combination on the coagulation system of Sprague-Dawley rats. Ultrastruct. Pathol.41 (4), 275–283. 10.1080/01913123.2017.1327909
8
Bakulski K. M. Seo Y. A. Hickman R. C. Brandt D. Vadari H. S. Hu H. et al (2020). Heavy metals exposure and Alzheimer's disease and related dementias. J. Alzheimers Dis.76 (4), 1215–1242. 10.3233/JAD-200282
9
Balali-Mood M. Naseri K. Tahergorabi Z. Khazdair M. Sadeghi M. (2021). Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol.12, 643972. 10.3389/fphar.2021.643972
10
Bitto A. Pizzino G. Irrera N. Galfo F. Squadrito F. (2014). Epigenetic modifications due to heavy metals exposure in children living in polluted areas. Curr. Genomics15 (6), 464–468. 10.2174/138920291506150106153336
11
Bonaventura P. Lamboux A. Albarède F. Miossec P. (2018). Differential effects of TNF-α and IL-1β on the control of metal metabolism and cadmium-induced cell death in chronic inflammation. PLoS One13 (5), 0196285. 10.1371/journal.pone.0196285
12
Boyles M. S. Ranninger C. Reischl R. Rurik M. Tessadri R. Kohlbacher O. et al (2016). Copper oxide nanoparticle toxicity profiling using untargeted metabolomics. Part Fibre Toxicol.13 (1), 49. 10.1186/s12989-016-0160-6
13
Bradl H. (2005). Heavy metals in the environment: Origin, interaction and remediation. Elsevier. Academic Press.
14
Branca J. J. V. Pacini A. Gulisano M. Taddei N. Fiorillo C. Becatti M. (2020). Cadmium-induced cytotoxicity: Effects on mitochondrial electron transport chain. Front. Cell. Dev. Biol.8, 604377. 10.3389/fcell.2020.604377
15
Briffa J. Sinagra E. Blundell R. (2020). Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon6 (9), 04691. 10.1016/j.heliyon.2020.e04691
16
Brignardello J. Holmes E. Garcia-Perez I. (2017). Metabolic phenotyping of diet and dietary intake. Adv. Food. Nutr. Res.81, 231–270. 10.1016/bs.afnr.2016.12.002
17
Büdinger L. Hertl M. (2000). Immunologic mechanisms in hypersensitivity reactions to metal ions: An overview. Allergy55 (2), 108–115. 10.1034/j.1398-9995.2000.00107.x
18
Chatterjee S. Sarkar S. Bhattacharya S. (2014). Toxic metals and autophagy. Chem. Res. Toxicol.27 (11), 1887–1900. 10.1021/tx500264s
19
Chen C. Y. Peng H. C. Chen Y. Y. Chan C. C. Yu C. J. (2016). Association of environmental heavy metals exposure and lung cancer incidence and prognosis. Eur. Respir. J.48 (60), 2805. 10.1183/13993003.congress-2016.PA2805
20
Chen H. Ke Q. Kluz T. Yan Y. Costa M. (2006). Nickel ions increase histone H3 lysine 9 dimethylation and induce transgene silencing. Mol. Cell. Biol.26 (10), 3728–3737. 10.1128/MCB.26.10.3728-3737.2006
21
Chen H. Kluz T. Zhang R. Costa M. (2010). Hypoxia and nickel inhibit histone demethylase JMJD1A and repress Spry2 expression in human bronchial epithelial BEAS-2B cells. Carcinogenesis31 (12), 2136–2144. 10.1093/carcin/bgq197
22
Chen Y. Graziano J. H. Parvez F. Liu M. Slavkovich V. Kalra T. et al (2011). Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: Prospective cohort study. BMJ342, d2431. 10.1136/bmj.d2431
23
Cornelis M. C. Erlund I. Michelotti G. A. Herder C. Westerhuis J. A. Tuomilehto J. (2018). Metabolomic response to coffee consumption: Application to a three-stage clinical trial. J. Intern. Med.283 (6), 544–557. 10.1111/joim.12737
24
David A. Abdul-Sada A. Lange A. Tyler C. R. Hill E. M. (2014). A new approach for plasma (xeno)metabolomics based on solid-phase extraction and nanoflow liquid chromatography-nanoelectrospray ionisation mass spectrometry. J. Chromatogr. A1365, 72–85. 10.1016/j.chroma.2014.09.001
25
Deshmukh R. Sonah H. Patil G. Chen W. Prince S. Mutava R. et al (2014). Integrating omic approaches for abiotic stress tolerance in soybean. Front. Plant Sci.5, 244. 10.3389/fpls.2014.00244
26
Diamond G. L. Zalups R. K. (1998). Understanding renal toxicity of heavy metals. Toxicol. Pathol.26 (1), 92–103. 10.1177/019262339802600111
27
Dinis-Oliveira R. J. (2014). Metabolomics of drugs of abuse: A more realistic view of the toxicological complexity. Bioanalysis6 (23), 3155–3159. 10.4155/bio.14.260
28
Dong W. Simeonova P. P. Gallucci R. Matheson J. Flood L. Wang S. et al (1998). Toxic metals stimulate inflammatory cytokines in hepatocytes through oxidative stress mechanisms. Toxicol. Appl. Pharmacol.151 (2), 359–366. 10.1006/taap.1998.8481
29
Eggers S. Safdar N. Malecki K. M. (2018). Heavy metal exposure and nasal Staphylococcus aureus colonization: Analysis of the national health and nutrition examination survey (NHANES). Environ. Health17 (1), 2. 10.1186/s12940-017-0349-7
30
Eguchi A. Nomiyama K. Sakurai K. Kim Trang P. T. Viet P. H. Takahashi S. et al (2018). Alterations in urinary metabolomic profiles due to lead exposure from a lead–acid battery recycling site. Environ. Pollut.242, 98–105. 10.1016/j.envpol.2018.06.071
31
El-Hak H. N. G. Ghobashy M. A. Mansour F. A. El-Shenawy N. S. El-Din M. I. S. (2022). Heavy metals and parasitological infection associated with oxidative stress and histopathological alteration in the Clarias gariepinus. Ecotoxicology31 (7), 1096–1110. 10.1007/s10646-022-02569-9
32
Ellis J. K. Athersuch T. J. Thomas L. D. K. Teichert F. Pérez-Trujillo M. Svendsen C. et al (2012). Metabolic profiling detects early effects of environmental and lifestyle exposure to cadmium in a human population. BMC Med.10 (1), 61. 10.1186/1741-7015-10-61
33
Ferrante M. Fiore M. Conti G. O. Fiore V. Grasso A. Copat C. et al (2017). Transition and heavy metals compared to oxidative parameter balance in patients with deep vein thrombosis: A case-control study. Mol. Med. Rep.15 (5), 3438–3444. 10.3892/mmr.2017.6394
34
Flora S. J. Mittal M. Mehta A. (2008). Heavy metal induced oxidative stress and its possible reversal by chelation therapy. Indian J. Med. Res.128 (4), 501–523.
35
Gianì F. Masto R. Trovato M. A. Malandrino P. Russo M. Pellegriti G. et al (2021). Heavy metals in the environment and thyroid cancer. Cancers13 (16), 4052. 10.3390/cancers13164052
36
Granstein R. D. Sober A. J. (1981). Drug- and heavy metal--induced hyperpigmentation. J. Am. Acad. Dermatol5 (1), 1–18. 10.1016/s0190-9622(81)70072-0
37
Hamada T. Tanimoto A. Sasaguri Y. (1997). Apoptosis induced by cadmium. Apoptosis2 (4), 359–367. 10.1023/A:1026401506914
38
Hassanein E. H. M. Mohamed W. R. Ahmed O. S. Abdel-Daim M. M. Sayed A. M. (2022). The role of inflammation in cadmium nephrotoxicity: NF-κB comes into view. Life Sci.308, 120971. 10.1016/j.lfs.2022.120971
39
He Z. L. Yang X. E. Stoffella P. J. (2005). Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol.19 (2-3), 125–140. 10.1016/j.jtemb.2005.02.010
40
Hossein-Khannazer N. Azizi G. Eslami S. Alhassan Mohammed H. Fayyaz F. Hosseinzadeh R. et al (2020). The effects of cadmium exposure in the induction of inflammation. Immunopharmacol. Immunotoxicol.42 (1), 1–8. 10.1080/08923973.2019.1697284
41
Hsieh Y. C. Hsieh F. I. Lien L. M. Chou Y. L. Chiou H. Y. Chen C. J. (2008). Risk of carotid atherosclerosis associated with genetic polymorphisms of apolipoprotein E and inflammatory genes among arsenic exposed residents in Taiwan. Toxicol. Appl. Pharmacol.227 (1), 1–7. 10.1016/j.taap.2007.10.013
42
Huang H. W. Lee C. H. Yu H. S. (2019). Arsenic-induced carcinogenesis and immune dysregulation. Int. J. Environ. Res. Public Health16 (15), 2746. 10.3390/ijerph16152746
43
Huat T. J. Camats-Perna J. Newcombe E. A. Valmas N. Kitazawa M. Medeiros R. (2019). Metal toxicity links to Alzheimer's disease and neuroinflammation. J. Mol. Biol.431 (9), 1843–1868. 10.1016/j.jmb.2019.01.018
44
Hubaux R. Becker-Santos D. D. Enfield K. S. Rowbotham D. Lam S. Lam W. L. et al (2013). Molecular features in arsenic-induced lung tumors. Mol. Cancer.12, 20. 10.1186/1476-4598-12-20
45
Hughes M. F. Beck B. D. Chen Y. Lewis A. S. Thomas D. J. (2011). Arsenic exposure and toxicology: A historical perspective. Toxicol. Sci.123 (2), 305–332. 10.1093/toxsci/kfr184
46
Irfan M. Hayat S. Ahmad A. Alyemeni M. N. (2013). Soil cadmium enrichment: Allocation and plant physiological manifestations. Saudi J. Biol. Sci.20 (1), 1–10. 10.1016/j.sjbs.2012.11.004
47
Jadhav S. H. Sarkar S. N. Ram G. C. Tripathi H. C. (2007). Immunosuppressive effect of subchronic exposure to a mixture of eight heavy metals, found as groundwater contaminants in different areas of India, through drinking water in male rats. Arch. Environ. Contam. Toxicol.53 (3), 450–458. 10.1007/s00244-006-0177-1
48
Jagadeesan S. Duraisamy P. Panicker V. V. Anjaneyan G. Sajini L. Velayudhan S. et al (2021). Cutaneous mercury granulomas, hyperpigmentation and systemic involvement: A case of mercury toxicity following herbal medication for psoriasis. Indian J. Dermatol Venereol. Leprol.87 (6), 892. 10.25259/IJDVL_888_20
49
Jaishankar M. Tseten T. Anbalagan N. Mathew B. B. Beeregowda K. N. (2014). Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol.7 (2), 60–72. 10.2478/intox-2014-0009
50
Järup L. (2003). Hazards of heavy metal contamination. Br. Med. Bull.68, 167–182. 10.1093/bmb/ldg032
51
Jia G. Aroor A. R. Martinez-Lemus L. A. Sowers J. R. (2015). Mitochondrial functional impairment in response to environmental toxins in the cardiorenal metabolic syndrome. Arch. Toxicol.89 (2), 147–153. 10.1007/s00204-014-1431-3
52
Jin S. Muhammad N. Sun Y. Tan Y. Yuan H. Song D. et al (2020). Multispecific platinum(IV) complex deters breast cancer via interposing inflammation and immunosuppression as an inhibitor of COX-2 and PD-L1. Angew. Chem. Int. Ed.59 (51), 23313–23321. 10.1002/anie.202011273
53
Kabir M. T. Uddin M. S. Zaman S. Begum Y. Ashraf G. M. Bin-Jumah M. N. et al (2021). Molecular mechanisms of metal toxicity in the pathogenesis of Alzheimer's disease. Mol. Neurobiol.58 (1), 1–20. 10.1007/s12035-020-02096-w
54
Ke Q. Li Q. Ellen T. P. Sun H. Costa M. (2008). Nickel compounds induce phosphorylation of histone H3 at serine 10 by activating JNK-MAPK pathway. Carcinogenesis29 (6), 1276–1281. 10.1093/carcin/bgn084
55
Kelly R. S. Bayne H. Spiro A. Vokonas P. Sparrow D. Weiss S. T. et al (2020). Metabolomic signatures of lead exposure in the VA normative aging study. Environ. Res.190, 110022. 10.1016/j.envres.2020.110022
56
Kettunen J. Tukiainen T. Sarin A. P. Ortega-Alonso A. Tikkanen E. Lyytikäinen L. P. et al (2012). Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nat. Genet.44 (3), 269–276. 10.1038/ng.1073
57
Kiani B. Hashemi Amin F. Bagheri N. Bergquist R. Mohammadi A. A. Yousefi M. et al (2021). Association between heavy metals and colon cancer: An ecological study based on geographical information systems in north-eastern Iran. BMC Cancer21 (1), 414. 10.1186/s12885-021-08148-1
58
Kim H. S. Kim Y. J. Seo Y. R. (2015a). An overview of carcinogenic heavy metal: Molecular toxicity mechanism and prevention. J. Cancer Prev.20 (4), 232–240. 10.15430/jcp.2015.20.4.232
59
Kim N. H. Hyun Y. Y. Lee K. B. Chang Y. Ryu S. Oh K. H. et al (2015b). Environmental heavy metal exposure and chronic kidney disease in the general population. J. Korean Med. Sci.30 (3), 272–277. 10.3346/jkms.2015.30.3.272
60
Kitazawa M. Cheng D. Laferla F. M. (2009). Chronic copper exposure exacerbates both amyloid and tau pathology and selectively dysregulates cdk5 in a mouse model of AD. J. Neurochem.108 (6), 1550–1560. 10.1111/j.1471-4159.2009.05901.x
61
Klein M. S. Shearer J. (2016). Metabolomics and type 2 diabetes: Translating basic research into clinical application. J. Diabetes Res.2016, 3898502. 10.1155/2016/3898502
62
Korsholm A. S. Kjær T. N. Ornstrup M. J. Pedersen S. B. (2017). Comprehensive metabolomic analysis in blood, urine, fat, and muscle in men with metabolic syndrome: A randomized, placebo-controlled clinical trial on the effects of resveratrol after four months' treatment. Int. J. Mol. Sci.18 (3), 554. 10.3390/ijms18030554
63
Krueger W. S. Wade T. J. (2016). Elevated blood lead and cadmium levels associated with chronic infections among non-smokers in a cross-sectional analysis of NHANES data. Environ. Health15 (1), 16. 10.1186/s12940-016-0113-4
64
Lentini P. Zanoli L. Granata A. Signorelli S. S. Castellino P. Dell'Aquila R. (2017). Kidney and heavy metals - the role of environmental exposure (Review). Mol. Med. Rep.15 (5), 3413–3419. 10.3892/mmr.2017.6389
65
Li L. Zhang M. Men Y. Wang W. Zhang W. (2020). Heavy metals interfere with plasma metabolites, including lipids and amino acids, in patients with breast cancer. Oncol. Lett.19, 2925–2933. 10.3892/ol.2020.11402
66
Li Z. Chi H. Zhu W. Yang G. Song J. Mo L. et al (2021). Cadmium induces renal inflammation by activating the NLRP3 inflammasome through ROS/MAPK/NF-κB pathway in vitro and in vivo. Arch. Toxicol.95 (11), 3497–3513. 10.1007/s00204-021-03157-2
67
Lin Y. Yuan Y. Ouyang Y. Wang H. Xiao Y. Zhao X. et al (2022a). Metabolome-wide association study of multiple plasma metals with serum metabolomic profile among middle-to-older-aged Chinese adults. Environ. Sci. Technol.56 (22), 16001–16011. 10.1021/acs.est.2c05547
68
Liu J. de Vries P. S. Del Greco M, F. Johansson Å. Schraut K. E. Hayward C. et al (2022b). A multi-omics study of circulating phospholipid markers of blood pressure. Sci. Rep.12 (1), 574. 10.1038/s41598-021-04446-7
69
Lokuge K. M. Smith W. Caldwell B. Dear K. Milton A. H. (2004). The effect of arsenic mitigation interventions on disease burden in Bangladesh. Environ. Health Perspect.112 (11), 1172–1177. 10.1289/ehp.6866
70
Lu Y. Chen C. (2017). Metabolomics: Bridging chemistry and biology in drug discovery and development. Curr. Pharmacol. Rep.3, 16–25. 10.1007/s40495-017-0083-4
71
Marchetti C. (2013). Role of calcium channels in heavy metal toxicity. ISRN Toxicol.2013, 184360. 10.1155/2013/184360
72
Martin E. González-Horta C. Rager J. Bailey K. A. Sánchez-Ramírez B. Ballinas-Casarrubias L. et al (2015). Metabolomic characteristics of arsenic-associated diabetes in a prospective cohort in Chihuahua, Mexico. Toxicol. Sci.144 (2), 338–346. 10.1093/toxsci/kfu318
73
Men Y. Li L. Zhang F. Kong X. Zhang W. Hao C. et al (2020). Evaluation of heavy metals and metabolites in the urine of patients with breast cancer. Oncol. Lett.19 (2), 1331–1337. 10.3892/ol.2019.11206
74
Mercier K. A. Al-Jazrawe M. Poon R. Acuff Z. Alman B. (2018). A metabolomics pilot study on desmoid tumors and novel drug candidates. Sci. Rep.8 (1), 584. 10.1038/s41598-017-18921-7
75
Mitra S. Chakraborty A. Tareq A. Emran T. Nainu F. Khusro A. et al (2022). Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud. Univ. Sci.34, 101865. 10.1016/j.jksus.2022.101865
76
Montgomery E. B. (1995). Heavy metals and the etiology of Parkinson's disease and other movement disorders. Toxicology97 (1-3), 3–9. 10.1016/0300-483x(94)02962-t
77
Nguyen H. D. Oh H. Hoang N. H. M. Kim M. S. (2021). Association between heavy metals, high-sensitivity C-reaction protein and 10-year risk of cardiovascular diseases among adult Korean population. Sci. Rep.11 (1), 14664. 10.1038/s41598-021-94158-9
78
Niture S. Lin M. Qi Q. Moore J. T. Levine K. E. Fernando R. A. et al (2021). Role of autophagy in cadmium-induced hepatotoxicity and liver diseases. J. Toxicol.2021, 9564297. 10.1155/2021/9564297
79
Nriagu J. O. (1989). A global assessment of natural sources of atmospheric trace metals. Nature338, 47–49. 10.1038/338047a0
80
Oe S. Miyagawa K. Honma Y. Harada M. (2016). Copper induces hepatocyte injury due to the endoplasmic reticulum stress in cultured cells and patients with Wilson disease. Exp. Cell. Res.347 (1), 192–200. 10.1016/j.yexcr.2016.08.003
81
Oosthuizen J. (2012). Environmental health: Emerging issues and practice. BoD–Books on Demand. Neubrucke, Germany: University of Applied Sciences, Academic PresseBook ISBN: 9780080455006.
82
Orr S. E. Bridges C. C. (2017). Chronic kidney disease and exposure to nephrotoxic metals. Int. J. Mol. Sci.18 (5), 1039. 10.3390/ijms18051039
83
Patrick L. (2002). Mercury toxicity and antioxidants: Part 1: Role of glutathione and alpha-lipoic acid in the treatment of mercury toxicity. Altern. Med. Rev.7 (6), 456–471.
84
Patti G. J. Yanes O. Siuzdak G. (2012). Innovation: Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell. Biol.13 (4), 263–269. 10.1038/nrm3314
85
Rafati Rahimzadeh M. Rafati Rahimzadeh M. Kazemi S. Moghadamnia A. A. (2017). Cadmium toxicity and treatment: An update. Casp. J. Intern. Med.8 (3), 135–145. 10.22088/cjim.8.3.135
86
Rahman M. Tondel M. Ahmad S. A. Chowdhury I. A. Faruquee M. H. Axelson O. (1999). Hypertension and arsenic exposure in Bangladesh. Hypertension33 (1), 74–78. 10.1161/01.hyp.33.1.74
87
Rajkumar V. Lee V. R. Gupta V. (2023). “Heavy metal toxicity,” in StatPearls (Treasure Island (FL): StatPearls Publishing).
88
Rana S. V. (2008). Metals and apoptosis: Recent developments. J. Trace Elem. Med. Biol.22 (4), 262–284. 10.1016/j.jtemb.2008.08.002
89
Rana S. V. S. (2020). Endoplasmic reticulum stress induced by toxic elements-a review of recent developments. Biol. Trace Elem. Res.196 (1), 10–19. 10.1007/s12011-019-01903-3
90
Ranjbar A. Ghasemi H. Rostampour F. (2014). The role of oxidative stress in metals toxicity; mitochondrial dysfunction as a key player. Galen Med. J.3, 2–13. 10.31661/gmj.v3i1.100
91
Reardon A. J. F. Karathra J. Ribbenstedt A. Benskin J. P. MacDonald A. M. Kinniburgh D. W. et al (2019). Neurodevelopmental and metabolomic responses from prenatal coexposure to Perfluorooctanesulfonate (PFOS) and Methylmercury (MeHg) in Sprague-Dawley rats. Chem. Res. Toxicol.32 (8), 1656–1669. 10.1021/acs.chemrestox.9b00192
92
Rehman K. Fatima F. Waheed I. Akash M. S. H. (2018). Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem.119 (1), 157–184. 10.1002/jcb.26234
93
Rehman M. Liu L. Wang Q. Saleem M. H. Bashir S. Ullah S. et al (2019). Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. Res.26 (18), 18003–18016. 10.1007/s11356-019-05073-6
94
Ren S. Shao Y. Zhao X. Hong C. S. Wang F. Lu X. et al (2016). Integration of metabolomics and transcriptomics reveals major metabolic pathways and potential biomarker involved in prostate cancer. Mol. Cell. Proteomics.15 (1), 154–163. 10.1074/mcp.M115.052381
95
Renu K. Chakraborty R. Myakala H. Koti R. Famurewa A. C. Madhyastha H. et al (2021). Molecular mechanism of heavy metals (lead, chromium, arsenic, mercury, nickel and cadmium) - induced hepatotoxicity - a review. Chemosphere271, 129735. 10.1016/j.chemosphere.2021.129735
96
Riedel F. Aparicio-Soto M. Curato C. Thierse H. J. Siewert K. Luch A. (2021). Immunological mechanisms of metal allergies and the nickel-specific TCR-pMHC interface. Int. J. Environ. Res. Public Health18 (20), 10867. 10.3390/ijerph182010867
97
Rowley B. Monestier M. (2005). Mechanisms of heavy metal-induced autoimmunity. Mol. Immunol.42 (7), 833–838. 10.1016/j.molimm.2004.07.050
98
Ryu H. W. Lee D. H. Won H. R. Kim K. H. Seong Y. J. Kwon S. H. (2015). Influence of toxicologically relevant metals on human epigenetic regulation. Toxicol. Res.31 (1), 1–9. 10.5487/tr.2015.31.1.001
99
Simeonova P. P. Luster M. I. (2004). Arsenic and atherosclerosis. Toxicol. Appl. Pharmacol.198 (3), 444–449. 10.1016/j.taap.2003.10.018
100
Smith A. H. Lingas E. O. Rahman M. (2000). Contamination of drinking-water by arsenic in Bangladesh: A public health emergency. Bull. World Health Organ78 (9), 1093–1103.
101
Stohs S. J. Bagchi D. (1995). Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med.18 (2), 321–336. 10.1016/0891-5849(94)00159-h
102
Sun Q. Li Y. Shi L. Hussain R. Mehmood K. Tang Z. et al (2022). Heavy metals induced mitochondrial dysfunction in animals: Molecular mechanism of toxicity. Toxicology469, 153136. 10.1016/j.tox.2022.153136
103
Surdu S. Fitzgerald E. F. Bloom M. S. Boscoe F. P. Carpenter D. O. Haase R. F. et al (2013). Occupational exposure to arsenic and risk of nonmelanoma skin cancer in a multinational European study. Int. J. Cancer133 (9), 2182–2191. 10.1002/ijc.28216
104
Tan Q. Ma J. Zhou M. Wang D. Wang B. Nie X. et al (2020). Heavy metals exposure, lipid peroxidation and heart rate variability alteration: Association and mediation analyses in urban adults. Ecotoxicol. Environ. Saf.205, 111149. 10.1016/j.ecoenv.2020.111149
105
Tchounwou P. B. Yedjou C. G. Patlolla A. K. Sutton D. J. (2012). Heavy metal toxicity and the environment. Exp. Suppl.101, 133–164. 10.1007/978-3-7643-8340-4_6
106
Uddin A. N. Burns F. J. Rossman T. G. Chen H. Kluz T. Costa M. (2007). Dietary chromium and nickel enhance UV-carcinogenesis in skin of hairless mice. Toxicol. Appl. Pharmacol.221 (3), 329–338. 10.1016/j.taap.2007.03.030
107
Vaiserman A. Lushchak O. (2021). DNA methylation changes induced by prenatal toxic metal exposure: An overview of epidemiological evidence. Environ. Epigenetics7 (1), 007. 10.1093/eep/dvab007
108
Valcke M. Ouellet N. Dubé M. Laouan Sidi E. A. LeBlanc A. Normandin L. et al (2019). Biomarkers of cadmium, lead and mercury exposure in relation with early biomarkers of renal dysfunction and diabetes: Results from a pilot study among aging Canadians. Toxicol. Lett.312, 148–156. 10.1016/j.toxlet.2019.05.014
109
Vas J. Monestier M. (2008). Immunology of mercury. Ann. N. Y. Acad. Sci.1143, 240–267. 10.1196/annals.1443.022
110
Vats P. Kaur U. J. Rishi P. (2022). Heavy metal-induced selection and proliferation of antibiotic resistance: A review. J. Appl. Microbiol.132 (6), 4058–4076. 10.1111/jam.15492
111
Vellingiri B. Suriyanarayanan A. Abraham K. S. Venkatesan D. Iyer M. Raj N. et al (2022). Influence of heavy metals in Parkinson's disease: An overview. J. Neurol.269 (11), 5798–5811. 10.1007/s00415-022-11282-w
112
Vincent Salvatore G. Juley H. (2021). “Role of heavy metals in the incidence of human cancers,” in Heavy metals. Editors Mazen KhaledN.HongboZ. (Rijeka: IntechOpen).
113
Vogt B. L. Rossman T. G. (2001). Effects of arsenite on p53, p21 and cyclin D expression in normal human fibroblasts -- a possible mechanism for arsenite's comutagenicity. Mutat. Res.478 (1-2), 159–168. 10.1016/s0027-5107(01)00137-3
114
Wang C. Nie G. Zhuang Y. Hu R. Wu H. Xing C. et al (2020). Inhibition of autophagy enhances cadmium-induced apoptosis in duck renal tubular epithelial cells. Ecotoxicol. Environ. Saf.205, 111188. 10.1016/j.ecoenv.2020.111188
115
Wang X. Mu X. Zhang J. Huang Q. Alamdar A. Tian M. et al (2015). Serum metabolomics reveals that arsenic exposure disrupted lipid and amino acid metabolism in rats: A step forward in understanding chronic arsenic toxicity. Metallomics7 (3), 544–552. 10.1039/c5mt00002e
116
Wang Z. Sun Y. Yao W. Ba Q. Wang H. (2021). Effects of cadmium exposure on the immune system and immunoregulation. Front. Immunol.12, 695484. 10.3389/fimmu.2021.695484
117
Wani A. L. Ara A. Usmani J. A. (2015). Lead toxicity: A review. Interdiscip. Toxicol.8 (2), 55–64. 10.1515/intox-2015-0009
118
Wishart D. S. (2016). Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug Discov.15 (7), 473–484. 10.1038/nrd.2016.32
119
Wu S. Y. Phan N. N. Ho S. H. Lai Y. H. Tsai C. H. Yang C. H. et al (2018). Metabolomic assessment of arsenite toxicity and novel biomarker discovery in early development of zebrafish embryos. Toxicol. Lett.290, 116–122. 10.1016/j.toxlet.2018.03.014
120
Wu X. Cobbina S. J. Mao G. Xu H. Zhang Z. Yang L. (2016). A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. Int.23 (9), 8244–8259. 10.1007/s11356-016-6333-x
121
Würtz P. Cook S. Wang Q. Tiainen M. Tynkkynen T. Kangas A. J. et al (2016). Metabolic profiling of alcohol consumption in 9778 young adults. Int. J. Epidemiol.45 (5), 1493–1506. 10.1093/ije/dyw175
122
Xiao Y. Zhai Q. Wang G. Liu X. Zhao J. Tian F. et al (2016). Metabolomics analysis reveals heavy metal copper-induced cytotoxicity in HT-29 human colon cancer cells. RSC Adv.6 (82), 78445–78456. 10.1039/C6RA09320E
123
Xu M. Y. Sun Y. J. Wang P. Xu H. Y. Chen L. P. Zhu L. et al (2015). Metabolomics analysis and biomarker identification for brains of rats exposed subchronically to the mixtures of low-dose cadmium and chlorpyrifos. Chem. Res. Toxicol.28 (6), 1216–1223. 10.1021/acs.chemrestox.5b00054
124
Yang A. M. Cheng N. Pu H. Q. Liu S. M. Li J. S. Bassig B. A. et al (2015). Metal exposure and risk of diabetes and prediabetes among Chinese occupational workers. Biomed. Environ. Sci.28 (12), 875–883. 10.3967/bes2015.121
125
Yaqoob A. Rehman K. Akash M. S. H. Alvi M. Shoaib S. (2022). Biochemical profiling of metabolomics in heavy metal-intoxicated impaired metabolism and its amelioration using plant-based bioactive compound. Front. Mol. Biosci.9, 1029729. 10.3389/fmolb.2022.1029729
126
Yedjou C. G. Tchounwou H. M. Tchounwou P. B. (2015). DNA damage, cell cycle arrest, and apoptosis induction caused by lead in human leukemia cells. Int. J. Environ. Res. Public Health13 (1), 13010056. 10.3390/ijerph13010056
127
Yokouchi M. Hiramatsu N. Hayakawa K. Kasai A. Takano Y. Yao J. et al (2007). Atypical, bidirectional regulation of cadmium-induced apoptosis via distinct signaling of unfolded protein response. Cell. Death Differ.14 (8), 1467–1474. 10.1038/sj.cdd.4402154
128
Yu L. Wu J. Zhai Q. Tian F. Zhao J. Zhang H. et al (2019). Metabolomic analysis reveals the mechanism of aluminum cytotoxicity in HT-29 cells. PeerJ7, 7524. 10.7717/peerj.7524
129
Zaitsu K. Hayashi Y. Kusano M. Tsuchihashi H. Ishii A. (2016). Application of metabolomics to toxicology of drugs of abuse: A mini review of metabolomics approach to acute and chronic toxicity studies. Drug Metab. Pharmacokinet.31 (1), 21–26. 10.1016/j.dmpk.2015.10.002
130
Zeng T. Liang Y. Chen J. Cao G. Yang Z. Zhao X. et al (2021). Urinary metabolic characterization with nephrotoxicity for residents under cadmium exposure. Environ. Int.154, 106646. 10.1016/j.envint.2021.106646
131
Zhang A. Sun H. Yan G. Wang P. Wang X. (2016). Mass spectrometry-based metabolomics: Applications to biomarker and metabolic pathway research. Biomed. Chromatogr.30 (1), 7–12. 10.1002/bmc.3453
132
Zhang J. Shen H. Xu W. Xia Y. Barr D. B. Mu X. et al (2014). Urinary metabolomics revealed arsenic internal dose-related metabolic alterations: A proof-of-concept study in a Chinese male cohort. Environ. Sci. Technol.48 (20), 12265–12274. 10.1021/es503659w
133
Zhang Y. Huo X. Lu X. Zeng Z. Faas M. M. Xu X. (2020). Exposure to multiple heavy metals associate with aberrant immune homeostasis and inflammatory activation in preschool children. Chemosphere257, 127257. 10.1016/j.chemosphere.2020.127257
134
Zulaikhah S. Wahyuwibowo J. Pratama A. (2020). Mercury and its effect on human health: A review of the literature. Int. J. Publ. health Sci.9, 103–114. 10.11591/ijphs.v9i2.20416
Summary
Keywords
heavy metals, toxicity, metabolomics, metabolic impairment, oxidative stress
Citation
Akash MSH, Yaqoob A, Rehman K, Imran M, Assiri MA, Al-Rashed F, Al-Mulla F, Ahmad R and Sindhu S (2023) Metabolomics: a promising tool for deciphering metabolic impairment in heavy metal toxicities. Front. Mol. Biosci. 10:1218497. doi: 10.3389/fmolb.2023.1218497
Received
07 May 2023
Accepted
21 June 2023
Published
06 July 2023
Volume
10 - 2023
Edited by
Wentao Zhu, China Agricultural University, China
Reviewed by
Jasim Khan, University of Alabama at Birmingham, United States
Tianlin Gao, Qingdao University, China
Updates
Copyright
© 2023 Akash, Yaqoob, Rehman, Imran, Assiri, Al-Rashed, Al-Mulla, Ahmad and Sindhu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kanwal Rehman, kanwalrehman@wum.edu.pk; Sardar Sindhu, sardar.sindhu@dasmaninstitute.org
†These authors share last authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.