Abstract
Alzheimer’s disease (AD) is a neurodegenerative disease that primarily affects elderly individuals. Recent studies have found that sigma-1 receptor (S1R) agonists can maintain endoplasmic reticulum stress homeostasis, reduce neuronal apoptosis, and enhance mitochondrial function and autophagy, making S1R a target for AD therapy. Traditional experimental methods are costly and inefficient, and rapid and accurate prediction methods need to be developed, while drug repurposing provides new ways and options for AD treatment. In this paper, we propose HNNDTA, a hybrid neural network for drug–target affinity (DTA) prediction, to facilitate drug repurposing for AD treatment. The study combines protein–protein interaction (PPI) network analysis, the HNNDTA model, and molecular docking to identify potential leads for AD. The HNNDTA model was constructed using 13 drug encoding networks and 9 target encoding networks with 2506 FDA-approved drugs as the candidate drug library for S1R and related proteins. Seven potential drugs were identified using network pharmacology and DTA prediction results of the HNNDTA model. Molecular docking simulations were further performed using the AutoDock Vina tool to screen haloperidol and bromperidol as lead compounds for AD treatment. Absorption, distribution, metabolism, excretion, and toxicity (ADMET) evaluation results indicated that both compounds had good pharmacokinetic properties and were virtually non-toxic. The study proposes a new approach to computer-aided drug design that is faster and more economical, and can improve hit rates for new drug compounds. The results of this study provide new lead compounds for AD treatment, which may be effective due to their multi-target action. HNNDTA is freely available at https://github.com/lizhj39/HNNDTA.
1 Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease that mainly affects elderly people and whose etiology remains unclear. The symptoms of patients include a decline in cognitive abilities and a weakening of memory and thinking abilities (Hung and Fu, 2017; Srivastava et al., 2021; Briggs et al., 2016). Although there are some drugs currently used to treat AD, their effectiveness is limited. However, drug repurposing (DR) has provided a new approach and selection for the treatment of AD (Padhi and Govindaraju, 2022; Ihara and Saito, 2020). This method involves reanalyzing the biological effects of known drugs and applying them to new areas of disease treatment. DR can accelerate the development of new drugs, provide more treatment options, and reduce the risk of drug development.
Previous studies have suggested that sigma-1 receptor (S1R) has neuroprotective effects and that its physiological function has a direct impact on endogenous neuroprotective mechanisms (Voronin et al., 2023). As a protein chaperone, S1R locates on specialized lipid rafts of mitochondria-associated endoplasmic reticulum membranes (MAMs), which are known to form mitochondrial endoplasmic reticulum contacts (MERCs) with the outer mitochondrial membrane and play a role in various biochemical processes, such as autophagosome formation, cellular energy production, and maintenance of IR3R3-dependent calcium homeostasis. Thus, disruption of this structure is now considered an early stage in the pathogenesis of neurodegenerative diseases, including AD. Activation of S1R using agonists has been shown to maintain the structural and functional stability of MAMs and MERCs, thereby enhancing autophagic activity, restoring mitochondrial function, and regulating intracellular calcium balance (Barazzuol et al., 2021; Leal and Martins, 2021; Wilson and Metzakopian, 2021; Weng et al., 2017). In AD models, such as PS1-KI and APP-KI, dendritic spines of hippocampal neurons are lost both in vitro and in vivo, indicating that the loss of mushroom-shaped “memory spines” reflects cognitive decline, learning, and memory deficits in AD (Ryskamp et al., 2019; Fisher et al., 2015), suggesting the involvement of reduced S1R in AD pathology. The mixed muscarinic/S1R agonist AF710B stabilizes mature mushroom spines in hippocampal cultures derived from AD mice in vitro, while pridopidine, an S1R agonist, stabilizes mushroom spines in an Alzheimer’s mouse model through its action on S1R. S1R agonists have demonstrated preclinical efficacy in AD animal models (Ryskamp et al., 2019; Fisher et al., 2015). Donepezil, a potent acetylcholinesterase inhibitor used for AD treatment, is also a high-affinity S1R ligand. Precise pharmacological studies on the interaction between donepezil and S1R suggest that the drug exerts anti-amnesic effects primarily through S1R activation against scopolamine, β-amyloid, or carbon monoxide-induced memory impairments (Hassan et al., 2017). Overall, S1R agonists exhibit neuroprotective effects and modulate synaptic plasticity, making S1R a potential target for AD treatment.
In the past decade, the “one disease–one target–one drug” paradigm has dominated the approach to drug discovery. However, this paradigm has certain limitations, and recent advances in systems biology have shifted the focus from “single-target drugs to “multi-target drugs” (Noor et al., 2023). When treating a particular disease, it is not feasible to rely solely on a single target to identify drugs. Instead, a range of targets within an imbalanced pathway in the complex biological network must be considered as inhibiting a single enzyme alone may lead to cancer cells compensating by activating other enzymes (Ryskamp et al., 2019; Fisher et al., 2015; Hassan et al., 2017). Zhi et al. utilized network pharmacology and molecular docking to reveal dihydroorotate dehydrogenase (DHODH) as a therapeutic target for small-cell lung cancer. Subsequently, they constructed a prediction model using graph neural networks (GNNs) and traditional machine learning methods to screen for potential DHODH inhibitors (Noor et al., 2023; Zhi et al., 2021). Cantini et al. introduced a multi-network strategy by integrating multiple genomic information layers, particularly gene co-expression and protein–protein interactions, to identify cancer-related targets. They employed consensus clustering algorithms in a predictive network, revealing CD46, BTG2, ATF3, HDGF, and F11R as driver genes in cancer (Noor et al., 2023; Cantini et al., 2015).
In drug repurposing, artificial intelligence (AI) plays an important role. By analyzing data on existing drugs and diseases using machine learning and deep learning methods, potential drugs can be quickly and efficiently screened (Cheng and Cummings, 2022; Yin and Wong, 2021; Vatansever et al., 2021). In addition, simulating the interactions between drugs and proteins can predict drug activity and affinity, guiding drug repositioning research. In recent years, researchers have successfully screened many promising drugs using AI methods (Selvaraj et al., 2021; Malandraki-Miller and Riley, 2021; Patel et al., 2020). These studies indicate that drug repositioning has important clinical application prospects, and AI methods can provide more powerful support for drug repositioning.
The affinity between drugs and targets is the basis for drug action, and predicting the affinity between drugs and targets is an important part of drug repurposing (Pushpakom et al., 2019; Parisi et al., 2020). Traditional experimental methods have disadvantages such as high cost and low efficiency, making it necessary to develop a fast and accurate prediction method. In recent years, with the development of deep learning technology, using neural networks to predict the affinity between drugs and targets has gradually become a research hotspot (Thomas et al., 2022; Choudhury et al., 2022; Jiang et al., 2022; Wang and Dokholyan, 2022). Neural networks are powerful computational tools with the ability to deal with non-linear problems and have achieved some success in predicting the affinity between drugs and targets.
In recent years, more and more researchers have begun to explore the use of neural networks to construct computational models for drug repositioning prediction to screen drugs for treating AD (Chyr et al., 2022; Wu et al., 2022; Siavelis et al., 2016). Some related studies have made some progress. For example, Zhou et al. Fang et al. (2022) proposed an integrated network-based AI method that can quickly translate genome-wide association study findings and multi-omics data into genotype-based therapeutic discoveries in AD, and identified pioglitazone as a potential new method for treating AD using AI methods. Tsuji et al. (2021) developed a deep learning-based computational framework that can extract low-dimensional representations of high-dimensional protein–protein interaction network data and infer potential drug target genes using latent features and state-of-the-art machine learning techniques. The study inferred that tamoxifen, bosutinib, and dasatinib could serve as repositionable candidate compounds against the disease. Rodriguez et al. (2021) proposed a machine learning framework, DRIAD (drug repositioning in AD), which quantifies potential associations between the pathological severity of AD and molecular mechanisms encoded in a list of gene names, and identified a ranked list of repositioning candidates for treating AD from 80 FDA-approved and clinically tested drugs.
Using AI methods for drug repurposing has become an important approach in AD drug research, providing important ideas and directions for new drug discovery. Although many studies have used neural networks to predict drug–target affinity, their application in the field of AD treatment is still relatively limited. This study aims to use neural networks to predict drug–target affinity and screen potential drugs for the treatment of AD, providing new ideas and choices for AD treatment. At the same time, we will compare and analyze different neural network models to find the best prediction model.
In this paper, we propose HNNDTA, a hybrid neural network for drug–target affinity prediction, thereby enabling drug repurposing for the treatment of AD. As shown in Figure 1, starting from the pathogenic target of AD, S1R, we conducted protein–protein interaction (PPI) analysis, screened out proteins related to S1R, and constructed a dataset based on inhibitors of S1R and related proteins. Subsequently, we used the HNNDTA model to train the dataset, combined with network pharmacology analysis to screen FDA-approved drugs, and obtained a batch of candidate drugs. Then, we use the molecular docking of candidate drugs with S1R and its related proteins to find potential effective lead compounds, and predict their pharmacokinetics and toxicity to ensure the pharmacokinetics of these candidate drugs. The academic characteristics meet the requirements. Through this series of studies, we have obtained some lead compounds with potential therapeutic effects, which provide new ideas and options for the treatment of AD.
FIGURE 1

Flowchart of the overall process. In this paper, we started with the AD pathogenic target S1R and conducted PPI analysis to obtain S1R-related proteins. Based on the inhibitors of S1R and related proteins, we constructed a dataset. Subsequently, we trained the dataset using the HNNDTA model. Combining the prediction results of the HNNDTA model and network pharmacology analysis, we screened FDA-approved drugs and obtained candidate drugs. Finally, we performed molecular docking on these candidate drugs, identified lead compounds with potential efficacy, and predicted their ADMET properties.
2 Materials and methods
2.1 Dataset
2.1.1 Target
STRING (Szklarczyk et al., 2023) is a database of known and predicted PPIs. We used STRING to get the PPI network of S1R, as shown in Figure 2A; we marked the correlation scores of proteins related to S1R in the network, among which the scores of dopamine D2 receptor (DRD2) and binding-immunoglobulin protein (BIP) are highest, 0.983 and 0.990, respectively, so we picked them as primary targets for network pharmacology analysis. We obtained the sequences of S1R (Q99720), DRD2 (P14416), and BIP (P11021) from the UniProt repository (Consortium, 2019). In addition, we obtained S1R (PDB ID: 5HK1) (Schmidt et al., 2016), DRD2 (6 PDB ID: LUQ) (Fan et al., 2020), and BIP (PDB ID: 3LDN) (Macias et al., 2011) from the RCSB Protein Data Bank (PDB) (Berman et al., 2000), which are 2.51 Å, 3.10 Å, and 2.20 Å, respectively, and their structures are shown in Figure 2A.
FIGURE 2

PPI network of S1R and related proteins, DRD2 and BIP, and the distribution of their inhibitor datasets. (A) The PPI network of S1R was obtained using STRING, and the 3D structures of the most related proteins BIP and DRD2. (B) Distribution of SMILES string lengths and DTA values of the inhibitor datasets for S1R, DRD2, and BIP.
2.1.2 Inhibitors
The half-inhibitory concentration (IC50) refers to the concentration of the drug or inhibitor required to inhibit half of the specified biological process, and the inhibition constant Ki reflects the inhibitory strength of the inhibitor on the target. The smaller the value, the stronger the inhibitory ability. pIC50 is the negative logarithm of the IC50 value, which is usually used to characterize the activity of molecules in drug screening. The formula for converting IC50 values to pIC50 values is
We obtained data on inhibitors of S1R, DRD2, and BIP and their binding abilities to their targets from the ChEMBL database (Gaulton et al., 2012). Although both IC50 and Ki can reflect the activity of the inhibitor, for data consistency, we screened the inhibitor data with IC50 as the subsequent drug–target affinity (DTA) training data on the HNN. Similarly, under the premise of ensuring the number of datasets, we screened the data whose source description was scientific literature and excluded other data. Figure 2B shows the simplified molecular input line entry system (SMILES) length distribution and binding force distribution of the three protein inhibitors. The inhibitor distribution of S1R and DRD2 showed a Gaussian distribution trend, while the inhibitor distribution of BIP was relatively sparse.
2.1.3 Molecules for drug repurposing
The drug screening library used in this study comes from FDA-approved drugs in the DrugBank database (Wishart et al., 2008). DrugBank is a comprehensive pharmaceutical knowledge bank that provides pharmacists, pharmacologists, health professionals, and drug researchers with free academic resources to help advance drug development and clinical practice. We chose DrugBank as the screening bank because it contains extensive drug information and a list of FDA-approved drugs, which can be used to screen potential drugs for the treatment of AD. These drugs have been proven to be safe and effective treatments in human clinical trials, so they are expected to be used in the treatment of AD. We selected FDA-approved drugs in the DrugBank database as screening libraries, and a total of 2509 drug molecules were available for drug repurposing studies.
2.2 HNNDTA
2.2.1 Overview of the framework
The overview of the HNNDTA framework proposed in this study is shown in Figure 3. First, we used a network pharmacology approach to find other targets in the same pathway as the AD target S1R, namely, DRD2 and BIP. We searched the ChEMBL website for inhibitor data for these three targets. The target protein is encoded as a one-dimensional target embedding, and the drug molecule is encoded as a one-dimensional drug embedding. The two encoding vectors are spliced in zero dimension, and after the calculation of the deep neural network (DNN), the final DTA is obtained, which can be expressed as follows:where the function represents the splicing operation of the 1D a and b vectors, and vp and vd represent the encoding vectors of the target protein and the drug molecule, respectively. In this paper, there are 13 kinds of target encoders and 9 kinds of drug encoders, all of which are built by DeepPurpose (Huang et al., 2020). A suitable combined model will produce better prediction accuracy. During the training phase, the dataset was randomly divided into independent training, validation, and test sets in a ratio of 7:1:2. The training set was used to train the model, while the validation and test sets were used to evaluate its performance. Due to the nature of our HNNDTA framework, which was trained on datasets specific to individual targets, it exhibits higher predictive accuracy for single targets. We have observed that models trained on single targets exhibit higher accuracy than those trained on mixed-target datasets.
FIGURE 3

HNNDTA network framework. The framework consists of drug encoding, target encoding, general encoding, and decoding networks. The encoding networks are used to encode the SMILES of drugs and the sequences of proteins to obtain corresponding embeddings. Then, the embeddings are decoded through DNNs to obtain the prediction results of DTA.
2.2.2 Drug encoding network
The drug encoder receives SMILES sequences as input. The Morgan encoder first uses the ECFP (Rogers and Hahn, 2010) algorithm to generate the feature representation sequence of the circular substructure of the drug, with a length of 1,024 bits. A multi-layer perceptron (MLP) then processes the sequence of feature representations to obtain a vector representation that can be fed into a neural network. The Morgan encoder is expressed as follows:
Similar to the Morgan encoder, the daylight encoder also uses the ECFP algorithm to generate a feature sequence based on the channel substructure of the drug, which is used as the input of the multi-layer perceptron to generate a feature sequence with a length of 2048 bits. The daylight encoder is represented as follows:
The PubChem encoder (Kim et al., 2019) generates feature sequences using handcrafted important substructures and then generates a feature sequence with a length of 881 bits through a multi-layer perceptron. The PubChem encoder is represented as follows:
The rdkit_2d_normalize encoder (Reczko and Bohr, 1994) generates a feature sequence with a length of 200 bits according to the global pharmacophore of the drug and then normalizes the feature sequence by fitting the cumulative density function of a given molecule sample. The rdkit_2d_normalize encoder is represented as follows:
The extended reeb graph (ErG) method (Stiefl et al., 2006) mixes the simplified graph and the binding attribute pair to generate a feature sequence and uses the node description of the drug carrier type to encode the relevant molecular properties; the encoded features are obtained after the MLP calculation vector. The ErG coder is expressed as follows:
MLP obtains the output value through feedforward propagation and updates the model parameters through reverse transmission so that the model output value gradually approaches the real value. The output of the MLP forward propagation is expressed as follows:where AC is the activation function and the typical activation function is the modified linear unit ReLU; Ml is the number of neurons in the lth layer network, ωi,l is the weight of the ith neuron in the lth layer network; and the termination condition of in the aforementioned formula is the first layer of the neural network, that is, the input layer. The reverse transfer uses the Adam optimizer to update the model weights. The underlying algorithm is the gradient descent method. The update on the weight of ωi,l can be expressed as follows:where E is the difference between the predicted value and the real value, is the partial derivative of E to ωi,l, and η is the learning rate.
2.2.3 Target encoding network
The input to a target encoder is the amino acid sequence of the target. The signature sequence generated by the amino acid composition (AAC) coder is 8420 positions in length, where each position is consistent with the maximum length of overlapping subsequences (k-mers) of one amino acid. The amino acid composition coder is expressed as follows:where fi represents the number of occurrences of amino acid i in the protein and L represents the length of the amino acid sequence. The AAC encoder concatenates 20 AAC values for each position in the amino acid sequence to obtain a signature sequence of 8420 elements in length.
The pseudo amino acid composition (PseAAC) encoder adds the hydrophobic and hydrophilic pattern information on the protein based on AAC to generate a 30-bit feature vector representation. The pseudo-amino acid composition encoder is expressed as follows:where fk,i represents the frequency of amino acid i in the kth position in the protein sequence and wk,j represents the weight of the pattern of the kth amino acid and the relative position j. The PseAAC encoder concatenates 30 PseAAC values for each position in the amino acid sequence, resulting in a feature vector of 30 elements in length.
The conjoint triad (ConTriad) encoder (Shen et al., 2007) forms a 7-letter alphabet based on amino acid triplet features, generating a feature vector with a length of 343 elements. The ConTriad encoder is expressed as follows:where fj,i indicates that the three adjacent amino acids in the protein sequence are converted into a number according to the 7-letter alphabet, the ith element indicates the frequency of the jth triplet appearing in the protein sequence, and wj is the weight of the jth triplet. The ConTriad encoder concatenates 343 ConTriad values for each position in the amino acid sequence, resulting in a feature vector of 343 elements in length.
The quasi-sequential encoder consists of a 100-element feature vector of quasi-sequential descriptors (Chou, 2000). The feature vectors generated by the aforementioned manual feature encoder will be further processed as input to MLP to obtain the feature vector of the target. The quasi-sequential encoder is expressed as follows:where ρj represents the weight of the jth quasi-sequential descriptor and dij is the distance between the ith amino acid and the jth sequence descriptor. The quasi-sequential encoder concatenates 100 QuasiSeq values for each position in the amino acid sequence, resulting in a feature vector of 100 elements in length.
2.2.4 General encoding network
The aforementioned drug and target feature extraction methods are based on prior chemical knowledge and manual transformation, so these encoders cannot be mixed. The encoders introduced in this section are general-purpose encoders based on DNNs, including convolutional neural networks (CNNs) (Krizhevsky et al., 2017), gated recurrent units (GRUs) (Chung et al., 2014), long short-term memory (LSTM) (Hochreiter and Schmidhuber, 1997), and transformers (Vaswani et al., 2017). These neural networks treat amino acid sequences as one-dimensional data.
The CNN encoder is a multilayer 1D CNN (Krizhevsky et al., 2017). After encoding the amino acid sequence character by character, the obtained deep feature vector will pass through multiple 1D convolutional layers and finally pass through the one-dimensional maximum pooling layer to obtain the output of the target feature vector. The output of the 1D convolutional layer is the result of convolving the input with the convolution kernel, which can be expressed as follows:where ⊗ represents a convolution operation. Assuming that the convolution kernel size is 2k + 1, k ∈ N+, the ith convolution output can be expressed as follows:
GRU and LSTM encoders are types of recurrent neural networks. In both networks, each node will get an output based on the state at the last moment and the current input and update the state of the node. This can solve the problem of traditional convolutional networks without long-term memory to a certain extent. Specifically, the SMILES sequence or amino acid sequence will first pass through the CNN for feature extraction and then use the output of the CNN as the input of the recurrent network.
The transformer encoder applies a self-attention mechanism (Vaswani et al., 2017). Due to the computational time and memory cost of the transformer, amino acid sequences are decomposed into moderately sized protein substructures, such as motifs, and each segmentation is then treated as a token and fed into a self-attention-based encoder. If a SMILES sequence or amino acid sequence is treated as a sentence, cut into several meaningful phrases, and encoded into several vectors with the same number of phrases, denoted as x, then the output of the transformer can be expressed aswhere attn is a self-attention function, mask is a Boolean value about whether the input x is eliminated, feedforward is a feedforward neural network, and norm is a layer normalization operation.
2.2.5 Evaluation metrics
In mathematical statistics, mean-squared error (MSE) is a method used to measure the difference between the predicted and real values. It calculates the mean of the squared difference between predicted and true values, which is the expected value of the squared difference between predicted and true values. The smaller the value of the MSE, the higher the prediction accuracy of the prediction model. Assuming there are n samples, MSE can be expressed by the following formula:where yi and are the true and estimated values of the ith sample, respectively. In this paper, the MSE is used to evaluate the accuracy of the model to predict the binding affinity of the drug to the target.
Harrell’s C-index (also known as the concordance index, CI) is a widely used metric for evaluating the performance of risk models. It is commonly employed in survival analysis, especially when dealing with censored data (Harrell et al., 1982). The C-index measures the degree of concordance between predicted and observed rankings of survival times. It serves as an indicator of the model’s accuracy with values closer to 1, indicating a higher level of consistency between the predicted outcomes and the actual observed outcomes.
Suppose the data are represented by vectors , where is a possibly right-censored continuous survival time and is a vector of predictor variables. It is assumed that is the minimum of the true survival time Ti and an independent continuous censoring time Ci. The variable Δi≔I(Ti < = Ci) indicates whether Ti has been fully observed (Δi = 1) or not (Δi = 0). A one-dimensional score is estimated for each observation i = 1, …, n, by averaging the cumulative hazard estimates over all trees and all time points. The concordance index is given bywhere the indices i and j refer to pairs of observations in the sample (Schmid et al., 2016).
2.3 Network pharmacology
Network pharmacology (NP) (Hopkins, 2008) is a new drug development method based on systems biology. It reveals the multi-target action mechanism of drugs by integrating protein interaction and drug compound networks. To construct a network pharmacology-based analysis, we mapped protein–protein and protein–drug interaction networks (Hasan et al., 2020). We fetch the PPI network from the STRING database and select the protein most related to the target we need to study. We then used the HNNDTA model to predict the binding forces between these proteins and compounds. To identify the best compounds, we picked the top 20 most binding proteins for each protein and mapped them into a protein–compound network. We use Sankey diagrams (Lee et al., 2019) to visualize drug–protein interaction networks to better understand and analyze the mechanism of action of drugs in biological systems. In this network, we can identify which compounds may be the most promising drug candidates by analyzing the interactions between proteins and compounds. In particular, for those compounds that bind strongly to multiple proteins, we can select them as our drug candidates.
2.4 Molecular docking
In molecular docking tasks, AutoDock Vina (Eberhardt et al., 2021) is one of the widely used docking engines in AutoDock Suite, and its open-source code and fast docking speed are favored. We use AutoDock Vina 1.1.2 for molecular docking experiments. First, we obtained the 3D molecular structure files of all receptor molecules and processed them to remove crystal water and hydrogenate them to generate preprocessed receptors. Next, we first removed the crystal water and the original ligand, then added hydrogen and charge distribution, and manually set the active site area of the receptor as the grid box according to the feature information on the protein in UniProt. For the ligand file, we obtained its structure from PubChem (Kim et al., 2019) and then performed hydrogenation and charge addition to obtain the preprocessed ligand file. Then, we used AutoDock Vina for docking; exhaustiveness is set to 32; a total of 10 docking poses are generated; the top 5 best poses are kept, and finally, the binding energy value (in k/mol) of the best pose is used as the docking score. The results of molecular docking were output in pdbqt format and visualized and analyzed using PyMOL molecular visualization software. The docking results are evaluated by factors such as hydrogen bonds, van der Waals forces, and electron static energy.
3 Results
3.1 Performance evaluation
The HNNDTA framework was constructed using 13 drug encoders and 9 target encoders. We fixed the target encoder as AAC and constructed 13 different drug encoder models. The MSE and CI of the test models are shown in Figures 4A, B. The orange column is the test result of the ligand dataset of the S1R protein, and the sky blue column is the test result of the ligand dataset of the DRD2 protein. The smaller the MSE and the larger the CI, the smaller the difference between the predicted results of the model and the real results, and the higher the accuracy of the model. In the figure, the MSE value of the Morgan encoder is the smallest and the CI value is the largest, indicating that the Morgan encoder will make the model perform better, and the Morgan encoder should be considered in the subsequent grid search for the best drug encoder–target encoder combination. We fixed the drug encoder as Morgan, constructed nine models with different target encoders, and compared the MSE and CI of the test models, as shown in Figures 4C, D. The MSE values of each encoder are basically at the same level because there is only a very small amount of target data in the dataset, and the difference in information provided by the target is less.
FIGURE 4

Performance comparison of drugs and encoders. (A, B) Comparison of MSE and CI of drug encoders. (C, D) Comparison of MSE and CI of target encoders.
We have a total of 117 models of 13 drug encoders and 9 target encoders, and conduct a grid search on the ligand datasets of the three targets of S1R, DRD2, and BIP to find the best models. After testing, there are eight models with both MSE and CI in the top 10, as shown in Figure 5; Table 1. Among them, the Morgan encoder has the best encoding effect on drugs, and the transformer and PseudoAAC encoders have better encoding effects on protein targets. Overall, the performance of these eight models is comparable and complements each other. In the next step of screening candidate drugs, the average of the votes predicted by the eight models is taken as the drug–target interaction score.
FIGURE 5
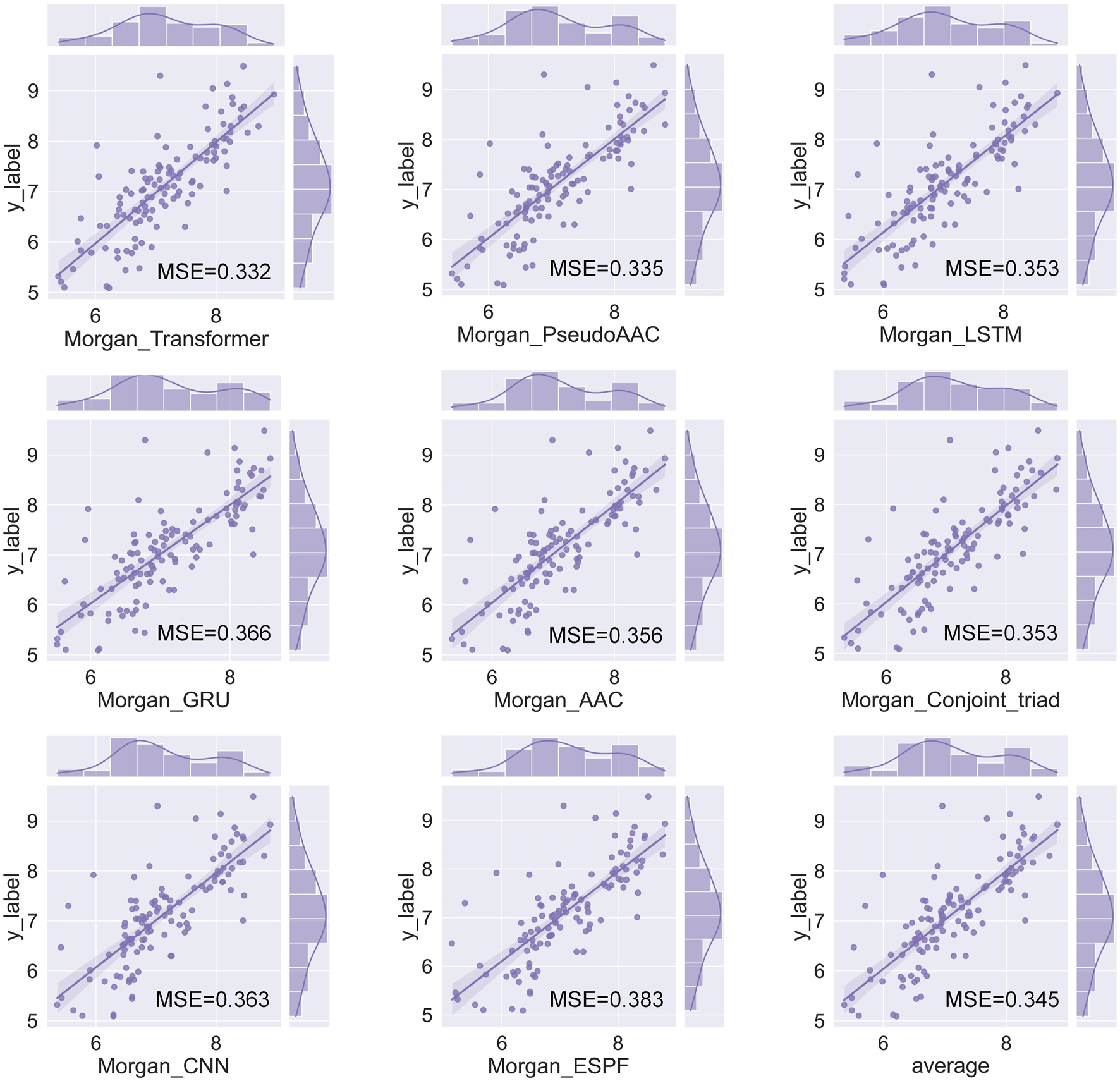
Fit plot of the best-performing model.
TABLE 1
| Drug encoder | Target encoder | MSE | CI |
|---|---|---|---|
| Morgan | Transformer | 0.332 | 0.816 |
| Morgan | PseudoAAC | 0.335 | 0.822 |
| Morgan | LSTM | 0.353 | 0.818 |
| Morgan | Conjoint_triad | 0.353 | 0.813 |
| Morgan | AAC | 0.356 | 0.817 |
| Morgan | CNN | 0.363 | 0.811 |
| Morgan | GRU | 0.366 | 0.814 |
| Morgan | ESPF | 0.383 | 0.815 |
Best-performing model.
3.2 Virtual screening of HNNDTA and network pharmacology
In this study, 2506 FDA-approved drugs were used as drug candidates for the AD target protein S1R and other targets of the same pathway, DRD2 and BIP. For S1R and DRD2, the respective models were trained using ligand datasets obtained from the ChEMBL website. For BIP, due to the lack of ligand data on BIP on the ChEMBL website, it is not enough to train a good model. We can pre-train the model with a large amount of ligand data for the same pathway target of S1R and then fine-tune the model with the ligand data on BIP itself.
The HNNDTA model was used to predict the activities of FDA-approved drugs and targets S1R, DRD2, and BIP, and the 20 drugs with the highest binding activities to these three targets are shown in Figure 6A. On the left side of the Sankey diagram are the three target proteins, and on the right side are the 20 drugs with the highest binding activity to these three targets. At the intersection, there are a total of 40 drugs. The prediction results show that most drugs can only have high activity with one or two targets, while the seven drugs DB13928, DB06287, DB00626, DB09265, DB00502, DB12401, and DB01369 have high binding activity with three targets, indicating that they can simultaneously inhibit these three AD-related targets. Therefore, these seven drugs can be used as alternative drugs for the treatment of AD. The DTA values of the aforementioned seven candidate drugs and S1R, DRD2, and BIP are shown in Figure 6B. The DTA values of these seven drugs and three targets stand out among more than 2,000 FDA drugs. The two drugs, DB00502 and DB12401, have the highest combined affinity for the three targets and are expected to become candidate drugs for the treatment of AD.
FIGURE 6

Sankey diagram of the DTI network for the top 20 drugs with the highest affinity to S1R, DRD2, BIP, and DTA heatmaps of the candidates. (A) Sankey diagram of the drug–target interaction network is shown, with the sky-blue nodes indicating the selected candidates. (B) Binding affinity heatmap of the candidates with S1R, DRD2, and BIP. Red represents the highest DTA value, while gray represents the lowest DTA value. Both DB12041 and DB00502 exhibit high affinity to S1R and DRD2.
3.3 Benchmark testing
To assess the accuracy of the model predictions and validate the efficacy of the drugs identified through network pharmacology (i.e., haloperidol and bromperidol), benchmark testing was conducted. Known high-affinity ligands for S1R, DRD2, and BIP were collected from the ChEMBL and BindingDB databases for validating the docked scores of the screened drugs as being higher than or comparable to the known high-affinity ligands. Conversely, known low-affinity ligands were gathered to demonstrate that the docked scores of the screened drugs are superior to them. The information on known ligands and their affinities is presented in Table 2.
TABLE 2
| Target | Inhibitor | Affinity | Type |
|---|---|---|---|
| S1R | Haloperidol | 8.54 | Ki |
| S1R | Donepezil | 7.84 | Ki |
| S1R | Fluvoxamine | 7.44 | Ki |
| S1R | Corticosterone | 4.45 | Ki |
| S1R | Cocaine | 5.05 | Ki |
| DRD2 | Haloperidol | 8.76 | Ki |
| DRD2 | Pimozide | 7.93 | Ki |
| DRD2 | Amisulpride | 7.90 | Ki |
| DRD2 | Procaterol | 4.07 | Ki |
| DRD2 | Isoproterenol | 4.32 | Ki |
| BIP | CHEMBL462871 | 7.22 | Kd |
| BIP | CHEMBL516197 | 4.85 | Kd |
Collected known drug–target pairs with high and low binding affinities.
First, the HNNDTA model was utilized to predict the binding affinities (pIC50) of the collected ligands to the three targets. The prediction results are shown in Table 3, where the green boxes and red boxes represent known high- and low-affinity drug–target pairs, respectively. Overall, the predicted affinities in the green boxes are higher than those in the red boxes, indicating that our model can accurately differentiate between high and low affinities among drug–target pairs. Subsequently, blind docking of ligand–protein was performed using QuickVina-W software (Hassan et al., 2017), and the docking scores are presented in Table 4. Lower docking scores indicate smaller binding energies and higher binding affinity. The docking scores in the green boxes are generally lower than those in the red boxes, suggesting the effectiveness of the docking procedure.
TABLE 3
| Ligand targets | DB00502 | DB12401 | DB00843 | DB00176 | DB04652 | DB00907 | DB01100 | DB06288 | DB01366 | DB01064 | CHEMBL462871 | CHEMBL516197 | DB15477 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1R | 7.5 | 7.3 | 6.8 | 6.3 | 6.7 | 6.5 | 6.9 | 6.0 | 6.3 | 6.3 | 6.3 | 5.6 | 6.8 |
| DRD2 | 7.8 | 7.9 | 5.9 | 5.7 | 5.7 | 5.4 | 6.4 | 8.5 | 5.4 | 5.2 | 5.9 | 5.2 | 5.9 |
| BIP | 6.8 | 6.7 | 5.3 | 5.0 | 5.4 | 5.0 | 5.4 | 7.3 | 4.4 | 4.5 | 6.3 | 4.4 | 5.4 |
DTA predicting results obtained from HNNDTA are presented. The green boxes and red boxes represent known high-affinity and low-affinity drug–target pairs, respectively.
TABLE 4
| Ligand targets | DB00502 | DB12401 | DB00843 | DB00176 | DB04652 | DB00907 | DB01100 | DB06288 | DB01366 | DB01064 | CHEMBL462871 | CHEMBL516197 | DB15477 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1R | −10.3 | −10.3 | −10.5 | −8.3 | −6.5 | −8.7 | −11.3 | −5.4 | −5.7 | −6.8 | −8.1 | −7.5 | −6.7 |
| DRD2 | −11.3 | −10.7 | −9.5 | −7.5 | −8.3 | −7.3 | −10.9 | −7.9 | −7.0 | −7.2 | −10.7 | −6.9 | −8.5 |
| BIP | −8.4 | −8.6 | −8.4 | −7.3 | −7.4 | −7.7 | −10.1 | −7.6 | −6.6 | −6.9 | −9.2 | −7.6 | −8.3 |
The molecular docking results obtained from QuickVina-W are presented, where a lower docking score indicates weaker binding energy and stronger binding affinity. The green boxes and red boxes represent known high-affinity and low-affinity drug–target pairs, respectively.
Our screened drugs, haloperidol and bromperidol, exhibit lower overall docking scores with the three targets compared to most other drugs. Furthermore, the docking scores of the screened drugs are comparable to those of known high-affinity ligands and significantly lower than those of known low-affinity ligands. This indicates that the HNNDTA model successfully identified high-affinity drugs suitable for multiple targets. It is worth noting that Table 4 shows that the drug pimozide has the best multi-target docking score. However, molecular docking requires manual preprocessing of 3D structures and is computationally time-consuming, making it difficult to apply to high-throughput drug target screening in network pharmacology. The HNNDTA model can expedite this process and successfully screen multi-target high-affinity drugs, even if it may represent a suboptimal solution.
3.4 Virtual screening of molecular docking
Small molecules have smaller molecular weights, which favor better pharmacokinetics and less toxicity. The molecular weight of antibiotics is large, and the metabolic process affects the drug’s efficacy. Small molecules have good medicinal properties, such as high bioavailability, good tissue specificity, and low toxicity and side effects, and are suitable for drug research and development. Therefore, we only choose small molecules with a weight of less than 500 as lead compounds. In AutoDock Vina docking, we use the binding energy value of the best pose as the docking score and tabulate the results in Table 5. The molecules of DB09265 and DB13928 are very large, beyond the active site region of the receptor, causing errors in Vina, which indicates that the binding between the two ligands and the receptor is difficult. Since Vina uses binding energy as a docking score, a smaller score indicates tighter binding between the two molecules, which generally indicates better docking. However, when the score is positive, it means that docking is difficult to produce. Both of these conditions can indicate a docking failure. Table 5 shows that although the ligands DB01369 and DB06287 have good docking effects on DRD2 and BIP receptors, they are difficult to bind to S1R receptors. Ligands DB00502 (bromperidol) and DB12401 (haloperidol) have good binding abilities to the three receptors, and the molecular weight is less than 500, meeting the screening requirements, so they may become potential drugs for AD.
TABLE 5
| DrugBank ID | Generic name | Summary | Docking score | ||
|---|---|---|---|---|---|
| S1R | DRD2 | BIP | |||
| DB00502 | Haloperidol | Antipsychotic | −8.856 | −7.265 | −8.585 |
| DB00626 | Bacitracin | Antibiotic | −7.979 | −6.412 | −6.35 |
| DB01369 | Quinupristin | Antibiotic | - | −9.455 | −9.689 |
| DB06287 | Temsirolimus | Antineoplastic | - | −9.674 | −9.858 |
| DB09265 | Lixisenatide | GLP-1 receptor agonist | - | - | - |
| DB12401 | Bromperidol | Antipsychotic | −8.516 | −7.031 | −8.245 |
| DB13928 | Semaglutide | Peptide 1 receptor agonist | - | - | - |
Overview of candidate compounds and their docking scores with S1R, DRD2, and BIP proteins. The docking scores were calculated using the molecular docking software application AutoDock Vina, with higher scores indicating stronger interactions.
The bold values indicate the docking scores of the top two drugs with the highest docking scores for a specific target.
3.5 Explanatory analysis of DTA
Figure 7 shows the 2D chemical structures of haloperidol and bromperidol, and the 2D poses resulting from docking with the target S1R. As shown in Figure 7A, their chemical structures are very similar, differing only by one halogen atom: haloperidol with a Cl atom and bromperidol with a Br atom. They are both high-affinity ligands for S1R, with only slight differences. This may be caused by the different interaction distances between the halogen atoms in the bromperidol molecule and the six amino acids of S1R. As shown in Figure 7B, both drugs produced hydrogen bonds with SER34, SER99, and LEU100 amino acids of S1R, and produced π − π interactions with TRP29, HIS72, LEU214, and TYR217 amino acids of S1R. The two molecules stabilize the association between them through their interaction with S1R.
FIGURE 7

2D chemical structures of haloperidol and bromperidol and their 2D poses generated by docking with the target S1R. In the 2D chemical structures (A) and 2D docking poses (B), the chemical structures of haloperidol and bromperidol are shown on the upper part and their 2D poses generated by docking with S1R are shown on the lower part. Although their chemical structures are very similar, their affinities to S1R differ when binding to it. This may be due to the different interaction distances between the halogen atoms in the bromperidol molecule and the six amino acids of S1R.
In order to further observe the docking poses of haloperidol and bromperidol with S1R, we also plotted the 3D docking simulation results, as shown in Figure 8. Both haloperidol and bromperidol dock at the S1R surface and interact with surrounding S1R amino acids. As shown in Figures 8A, B, the docking poses of haloperidol and bromperidol are very close to S1R, which is related to their similar chemical structures. They jointly participate in the stable combination with S1R and produce more interactions.
FIGURE 8

Simulation results of 3D docking of haloperidol and bromperidol with the target S1R. Through docking simulation, we demonstrated the surface and 3D docking poses of haloperidol (A) and bromperidol (B) with S1R. In the 3D docking poses, green represents hydrogen bonding and pink represents π − π interactions. The two molecules stabilize their binding through interactions with S1R.
To evaluate the ADMET, of haloperidol and bromperidol, we evaluated them using the ADMETlab 2.0 tool (Xiong et al., 2021), as shown in Figure 9. The evaluation results of haloperidol and bromperidol are roughly similar, except for logD and logP, and their compound properties are distributed between the upper and lower limits. This shows that haloperidol and bromperidol have better pharmacokinetic conditions and almost no toxicity. Haloperidol is an antipsychotic drug used to treat schizophrenia and other psychotic disorders, as well as symptoms of agitation, irritability, and delirium. Bromperidol is used to treat schizophrenia and other psychotic symptoms and has been used in trials investigating the treatment of dementia, depression, schizophrenia, anxiety disorders, and psychosomatic disorders, among others. It further illustrates the accuracy of our HNNDTA screening by finding a trial that is already in the treatment of AD and, at the same time, screening a new potential drug for the treatment of AD.
FIGURE 9

Results of the ADMET evaluation of haloperidol and bromperidol by the ADMETlab 2.0 tool. The evaluation results of haloperidol (A) and bromperidol (B) are generally similar, with compound properties distributed between the upper and lower limits, except for logD and logP.
4 Discussion
Alzheimer’s disease is a significant age-related illness that has garnered widespread attention in society. In this article, we propose a drug-screening framework that combines network pharmacology and hybrid neural networks to discover potential drugs for treating Alzheimer’s disease. Existing evidence supports S1R as a potential therapeutic target for Alzheimer’s disease. Initially, we conducted protein–protein interaction analysis using the STRING database to identify the most relevant targets associated with S1R, including DRD2 and BIP. These targets were then utilized in network pharmacology for drug screening. We developed a hybrid neural network framework to predict the binding affinity between targets and ligands, enabling the prediction of multi-target interactions for drug candidates. Benchmark testing was performed using a collection of known ligands with high and low affinity, demonstrating our model’s ability to differentiate between high- and low-affinity ligands. Furthermore, our model identified two drugs, haloperidol and bromperidol, with overall higher docking scores than other drugs, thereby validating the effectiveness of our proposed framework.
In PPI analysis, our results indicated that BIP and DRD2 have a higher combined score than other proteins related to S1R. A substantial body of evidence suggests that S1R, in combination with BIP, a regulator of endoplasmic reticulum stress (ERS), plays a pivotal role in the ERS pathway, which is a component of cellular stress and a core mechanism underlying synaptic loss and neurodegeneration in AD pathology (Ortega-Roldan et al., 2013; Venkataraman et al., 2022). S1R-dependent neuroprotection is likely to be mediated by the regulation of the unfolded protein response (UPR) in ERS (Voronin et al., 2023). Under ERS conditions, S1R agonists promote the dissociation of S1R-BIP calcium ion-sensitive chaperone complexes, resulting in enhanced chaperone activity of BIP toward misfolded proteins and S1R binding to client protein IRE1α. The regulatory effect of S1R agonists can increase the expression of BIP and brain-derived neurotrophic factor (BDNF) and decrease the expression of pro-inflammatory interleukin-6 (IL-6) (Hayashi and Su, 2007; Rosen et al., 2019; Zhemkov et al., 2021). Thus, S1R agonist regulation presents a viable strategy for the neuroprotective treatment of AD, aimed at reducing ERS and neuroinflammation while enhancing neural plasticity (Voronin et al., 2023).
It should be noted that the HNNDTA model does not differentiate between ligands as agonists or antagonists of the targets. Unfortunately, the existing literature reports that haloperidol is an antagonist of S1R (Maurice and Su, 2009), while S1R agonists are potential drugs for treating AD. Therefore, haloperidol is not suitable for the treatment of AD. On the other hand, bromperidol, which was selected by the HNNDTA model, may be the optimal candidate drug for AD treatment. The existing literature has discussed the potential of antipsychotic drugs, including bromperidol, on multiple targets related to AD (Kumar et al., 2017).
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
CY-C designed the research. XW, GC, ZL, and YY worked together to complete the experiment. XW and ZL contributed to analytic tools. GC and YY analyzed the data. XW, GC, ZL, YY, and CY-C wrote the manuscript together. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant No. 62176272), the Research and Development Program of Guangzhou Science and Technology Bureau (No. 2023B01J1016), the Key-Area Research and Development Program of Guangdong Province (No. 2020B1111100001), and China Medical University Hospital (DMR-112-085).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Barazzuol L. Giamogante F. Calì T. (2021). Mitochondria associated membranes (MAMs): Architecture and physiopathological role. Cell Calcium94, 102343. 10.1016/j.ceca.2020.102343
2
Berman H. M. Westbrook J. Feng Z. Gilliland G. Bhat T. N. Weissig H. et al (2000). The protein data bank. Nucleic acids Res.28, 235–242. 10.1093/nar/28.1.235
3
Briggs R. Kennelly S. P. O’Neill D. (2016). Drug treatments in alzheimer’s disease. Clin. Med.16, 247–253. 10.7861/clinmedicine.16-3-247
4
Cantini L. Medico E. Fortunato S. Caselle M. (2015). Detection of gene communities in multi-networks reveals cancer drivers. Sci. Rep.5, 17386. 10.1038/srep17386
5
Cheng F. Cummings J. (2022). “Artificial intelligence in Alzheimer's drug Discovery,” in Alzheimer's disease drug development: research and development ecosystem. Editors FillitH.KinneyJ.CummingsJ. (Cambridge: Cambridge University Press), 62–72. 10.1017/9781108975759.007
6
Chou K.-C. (2000). Prediction of protein subcellular locations by incorporating quasi-sequence-order effect. Biochem. biophysical Res. Commun.278, 477–483. 10.1006/bbrc.2000.3815
7
Choudhury C. Murugan N. A. Priyakumar U. D. (2022). Structure-based drug repurposing: Traditional and advanced ai/ml-aided methods. Drug Discov. Today27, 1847–1861. 10.1016/j.drudis.2022.03.006
8
Chung J. Gulcehre C. Cho K. Bengio Y. (2014). Empirical evaluation of gated recurrent neural networks on sequence modeling. arXiv preprint arXiv:1412.3555
9
Chyr J. Gong H. Zhou X. (2022). Dota: Deep learning optimal transport approach to advance drug repositioning for alzheimer’s disease. Biomolecules12, 196. 10.3390/biom12020196
10
Consortium U. (2019). Uniprot: A worldwide hub of protein knowledge. Nucleic acids Res.47, D506–D515. 10.1093/nar/gky1049
11
Eberhardt J. Santos-Martins D. Tillack A. F. Forli S. (2021). Autodock vina 1.2. 0: New docking methods, expanded force field, and python bindings. J. Chem. Inf. Model.61, 3891–3898. 10.1021/acs.jcim.1c00203
12
Fan L. Tan L. Chen Z. Qi J. Nie F. Luo Z. et al (2020). Haloperidol bound d2 dopamine receptor structure inspired the discovery of subtype selective ligands. Nat. Commun.11, 1074. 10.1038/s41467-020-14884-y
13
Fang J. Zhang P. Wang Q. Chiang C.-W. Zhou Y. Hou Y. et al (2022). Artificial intelligence framework identifies candidate targets for drug repurposing in alzheimer’s disease. Alzheimer’s Res. Ther.14, 7–23. 10.1186/s13195-021-00951-z
14
Fisher A. Bezprozvanny I. Wu L. Ryskamp D. A. Bar-Ner N. Natan N. et al (2015). AF710B, a novel M1/σ1 agonist with therapeutic efficacy in animal models of alzheimer’s disease. Neurodegener. Dis.16, 95–110. 10.1159/000440864
15
Gaulton A. Bellis L. J. Bento A. P. Chambers J. Davies M. Hersey A. et al (2012). Chembl: A large-scale bioactivity database for drug discovery. Nucleic acids Res.40, D1100–D1107. 10.1093/nar/gkr777
16
Harrell F. E. Jr Califf R. M. Pryor D. B. Lee K. L. Rosati R. A. (1982). Evaluating the yield of medical tests. JAMA247, 2543–2546. 10.1001/jama.1982.03320430047030
17
Hasan M. R. Paul B. K. Ahmed K. Bhuyian T. (2020). Design protein-protein interaction network and protein-drug interaction network for common cancer diseases: A bioinformatics approach. Inf. Med. Unlocked18, 100311. 10.1016/j.imu.2020.100311
18
Hassan N. M. Alhossary A. A. Mu Y. Kwoh C.-K. (2017). Protein-ligand blind docking using QuickVina-W with inter-process spatio-temporal integration. Sci. Rep.7, 15451. 10.1038/s41598-017-15571-7
19
Hayashi T. Su T.-P. (2007). Sigma-1 receptor chaperones at the er-mitochondrion interface regulate ca2+ signaling and cell survival. Cell131, 596–610. 10.1016/j.cell.2007.08.036
20
Hochreiter S. Schmidhuber J. (1997). Long short-term memory. Neural Comput.9, 1735–1780. 10.1162/neco.1997.9.8.1735
21
Hopkins A. L. (2008). Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol.4, 682–690. 10.1038/nchembio.118
22
Huang K. Fu T. Glass L. M. Zitnik M. Xiao C. Sun J. (2020). Deeppurpose: A deep learning library for drug–target interaction prediction. Bioinformatics36, 5545–5547. 10.1093/bioinformatics/btaa1005
23
Hung S.-Y. Fu W.-M. (2017). Drug candidates in clinical trials for alzheimer’s disease. J. Biomed. Sci.24, 47–12. 10.1186/s12929-017-0355-7
24
Ihara M. Saito S. (2020). Drug repositioning for alzheimer’s disease: Finding hidden clues in old drugs. J. Alzheimer’s Dis.74, 1013–1028. 10.3233/JAD-200049
25
Jiang H. Wang J. Cong W. Huang Y. Ramezani M. Sarma A. et al (2022). Predicting protein–ligand docking structure with graph neural network. J. Chem. Inf. Model.62, 2923–2932. 10.1021/acs.jcim.2c00127
26
Kim S. Chen J. Cheng T. Gindulyte A. He J. He S. et al (2019). Pubchem 2019 update: Improved access to chemical data. Nucleic acids Res.47, D1102-–D1109. 10.1093/nar/gky1033
27
Krizhevsky A. Sutskever I. Hinton G. E. (2017). Imagenet classification with deep convolutional neural networks. Commun. ACM60, 84–90. 10.1145/3065386
28
Kumar S. Chowdhury S. Kumar S. (2017). In silico repurposing of antipsychotic drugs for Alzheimer’s disease. BMC Neurosci.18, 76. 10.1186/s12868-017-0394-8
29
Leal N. S. Martins L. M. (2021). Mind the gap: Mitochondria and the endoplasmic reticulum in neurodegenerative diseases. Biomedicines9, 227. 10.3390/biomedicines9020227
30
Lee W.-Y. Lee C.-Y. Kim Y.-S. Kim C.-E. (2019). The methodological trends of traditional herbal medicine employing network pharmacology. Biomolecules9, 362. 10.3390/biom9080362
31
Macias A. T. Williamson D. S. Allen N. Borgognoni J. Clay A. Daniels Z. et al (2011). Adenosine-derived inhibitors of 78 kda glucose regulated protein (grp78) atpase: Insights into isoform selectivity. J. Med. Chem.54, 4034–4041. 10.1021/jm101625x
32
Malandraki-Miller S. Riley P. R. (2021). Use of artificial intelligence to enhance phenotypic drug discovery. Drug Discov. Today26, 887–901. 10.1016/j.drudis.2021.01.013
33
Maurice T. Su T.-P. (2009). The pharmacology of sigma-1 receptors. Pharmacol. Ther.124, 195–206. 10.1016/j.pharmthera.2009.07.001
34
Noor F. Asif M. Ashfaq U. A. Qasim M. Tahir ul Qamar M. (2023). Machine learning for synergistic network pharmacology: A comprehensive overview. Briefings Bioinforma.24, bbad120. 10.1093/bib/bbad120
35
Ortega-Roldan J. L. Ossa F. Schnell J. R. (2013). Characterization of the human sigma-1 receptor chaperone domain structure and binding immunoglobulin protein (bip) interactions. J. Biol. Chem.288, 21448–21457. 10.1074/jbc.M113.450379
36
Padhi D. Govindaraju T. (2022). Mechanistic insights for drug repurposing and the design of hybrid drugs for alzheimer’s disease. J. Med. Chem.65, 7088–7105. 10.1021/acs.jmedchem.2c00335
37
Parisi D. Adasme M. F. Sveshnikova A. Bolz S. N. Moreau Y. Schroeder M. (2020). Drug repositioning or target repositioning: A structural perspective of drug-target-indication relationship for available repurposed drugs. Comput. Struct. Biotechnol. J.18, 1043–1055. 10.1016/j.csbj.2020.04.004
38
Patel L. Shukla T. Huang X. Ussery D. W. Wang S. (2020). Machine learning methods in drug discovery. Molecules25, 5277. 10.3390/molecules25225277
39
Pushpakom S. Iorio F. Eyers P. A. Escott K. J. Hopper S. Wells A. et al (2019). Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov.18, 41–58. 10.1038/nrd.2018.168
40
Reczko M. Bohr H. (1994). The def data base of sequence based protein fold class predictions. Nucleic acids Res.22, 3616–3619.
41
Rodriguez S. Hug C. Todorov P. Moret N. Boswell S. A. Evans K. et al (2021). Machine learning identifies candidates for drug repurposing in alzheimer’s disease. Nat. Commun.12, 1033. 10.1038/s41467-021-21330-0
42
Rogers D. Hahn M. (2010). Extended-connectivity fingerprints. J. Chem. Inf. Model.50, 742–754. 10.1021/ci100050t
43
Rosen D. A. Seki S. M. Fernández-Castañeda A. Beiter R. M. Eccles J. D. Woodfolk J. A. et al (2019). Modulation of the sigma-1 receptor–ire1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci. Transl. Med.11, eaau5266. 10.1126/scitranslmed.aau5266
44
Ryskamp D. Wu L. Wu J. Kim D. Rammes G. Geva M. et al (2019). Pridopidine stabilizes mushroom spines in mouse models of Alzheimer’s disease by acting on the sigma-1 receptor. Neurobiol. Dis.124, 489–504. 10.1016/j.nbd.2018.12.022
45
Schmid M. Wright M. Ziegler A. (2016). On the use of Harrell’s C for clinical risk prediction via random survival forests. Nature Publishing Group. ArXiv:1507.03092 [stat].
46
Schmidt H. R. Zheng S. Gurpinar E. Koehl A. Manglik A. Kruse A. C. (2016). Crystal structure of the human σ1 receptor. Nature532, 527–530. 10.1038/nature17391
47
Selvaraj C. Chandra I. Singh S. K. Abhirami R. (2021). Artificial intelligence and machine learning approaches for drug design: Challenges and opportunities for the pharmaceutical industries. Mol. Divers.126, 1–38. 10.1016/bs.apcsb.2021.02.001
48
Shen J. Zhang J. Luo X. Zhu W. Yu K. Chen K. et al (2007). Predicting protein–protein interactions based only on sequences information. Proc. Natl. Acad. Sci.104, 4337–4341. 10.1073/pnas.0607879104
49
Siavelis J. C. Bourdakou M. M. Athanasiadis E. I. Spyrou G. M. Nikita K. S. (2016). Bioinformatics methods in drug repurposing for alzheimer’s disease. Briefings Bioinforma.17, 322–335. 10.1093/bib/bbv048
50
Srivastava S. Ahmad R. Khare S. K. (2021). Alzheimer’s disease and its treatment by different approaches: A review. Eur. J. Med. Chem.216, 113320. 10.1016/j.ejmech.2021.113320
51
Stiefl N. Watson I. A. Baumann K. Zaliani A. (2006). Erg: 2d pharmacophore descriptions for scaffold hopping. J. Chem. Inf. Model.46, 208–220. 10.1021/ci050457y
52
Szklarczyk D. Kirsch R. Koutrouli M. Nastou K. Mehryary F. Hachilif R. et al (2023). The string database in 2023: Protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res.51, D638–D646. 10.1093/nar/gkac1000
53
Thomas S. Abraham A. Baldwin J. Piplani S. Petrovsky N. (2022). Artificial intelligence in vaccine and drug design. Vaccine Des. Methods Protoc.1, 131–146. 10.1007/978-1-0716-1884-4_6
54
Tsuji S. Hase T. Yachie-Kinoshita A. Nishino T. Ghosh S. Kikuchi M. et al (2021). Artificial intelligence-based computational framework for drug-target prioritization and inference of novel repositionable drugs for alzheimer’s disease. Alzheimer’s Res. Ther.13, 92–15. 10.1186/s13195-021-00826-3
55
Vaswani A. Shazeer N. Parmar N. Uszkoreit J. Jones L. Gomez A. N. et al (2017). Attention is all you need. arXiv. 10.48550/arXiv.1706.03762
56
Vatansever S. Schlessinger A. Wacker D. Kaniskan H. Ü. Jin J. Zhou M.-M. et al (2021). Artificial intelligence and machine learning-aided drug discovery in central nervous system diseases: State-of-the-arts and future directions. Med. Res. Rev.41, 1427–1473. 10.1002/med.21764
57
Venkataraman A. V. Mansur A. Rizzo G. Bishop C. Lewis Y. Kocagoncu E. et al (2022). Widespread cell stress and mitochondrial dysfunction occur in patients with early alzheimer’s disease. Sci. Transl. Med.14, eabk1051. 10.1126/scitranslmed.abk1051
58
Voronin M. V. Abramova E. V. Verbovaya E. R. Vakhitova Y. V. Seredenin S. B. (2023). Chaperone-dependent mechanisms as a pharmacological target for neuroprotection. Int. J. Mol. Sci.24, 823. 10.3390/ijms24010823
59
Wang J. Dokholyan N. V. (2022). Yuel: Improving the generalizability of structure-free compound–protein interaction prediction. J. Chem. Inf. Model.62, 463–471. 10.1021/acs.jcim.1c01531
60
Weng T.-Y. Tsai S.-Y. A. Su T.-P. (2017). Roles of sigma-1 receptors on mitochondrial functions relevant to neurodegenerative diseases. J. Biomed. Sci.24, 74–14. 10.1186/s12929-017-0380-6
61
Wilson E. L. Metzakopian E. (2021). Er-Mitochondria contact sites in neurodegeneration: Genetic screening approaches to investigate novel disease mechanisms. Cell Death Differ.28, 1804–1821. 10.1038/s41418-020-00705-8
62
Wishart D. S. Knox C. Guo A. C. Cheng D. Shrivastava S. Tzur D. et al (2008). Drugbank: A knowledgebase for drugs, drug actions and drug targets. Nucleic acids Res.36, D901–D906. 10.1093/nar/gkm958
63
Wu Y. Liu H. Yan J. Hu X. (2022). Drug repositioning for alzheimer’s disease with transfer learning. arXiv. 10.48550/arXiv.2210.15271.
64
Xiong G. Wu Z. Yi J. Fu L. Yang Z. Hsieh C. et al (2021). Admetlab 2.0: An integrated online platform for accurate and comprehensive predictions of admet properties. Nucleic Acids Res.49, W5–W14. 10.1093/nar/gkab255
65
Yin Z. Wong S. T. (2021). Artificial intelligence unifies knowledge and actions in drug repositioning. Emerg. Top. life Sci.5, 803–813. 10.1042/ETLS20210223
66
Zhemkov V. Geva M. Hayden M. R. Bezprozvanny I. (2021). Sigma-1 receptor (s1r) interaction with cholesterol: Mechanisms of s1r activation and its role in neurodegenerative diseases. Int. J. Mol. Sci.22, 4082. 10.3390/ijms22084082
67
Zhi H.-Y. Zhao L. Lee C.-C. Chen C. Y.-C. (2021). A novel graph neural network methodology to investigate dihydroorotate dehydrogenase inhibitors in small cell lung cancer. Biomolecules11, 477. 10.3390/biom11030477
Summary
Keywords
Alzheimer’s disease, drug repurposing, hybrid neural network, molecular docking, sigma-1 receptor
Citation
Wu X, Li Z, Chen G, Yin Y and Chen CY-C (2023) Hybrid neural network approaches to predict drug–target binding affinity for drug repurposing: screening for potential leads for Alzheimer’s disease. Front. Mol. Biosci. 10:1227371. doi: 10.3389/fmolb.2023.1227371
Received
29 May 2023
Accepted
13 June 2023
Published
27 June 2023
Volume
10 - 2023
Edited by
Fabio Polticelli, Roma Tre University, Italy
Reviewed by
Haiping Zhang, Chinese Academy of Sciences (CAS), China
Jian Wang, The Pennsylvania State University, United States
Updates
Copyright
© 2023 Wu, Li, Chen, Yin and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Calvin Yu-Chian Chen, chenyuchian@mail.sysu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.