- 1Park Avenue Neurology, New York, NY, United States
- 2Departments of Neurology, Zucker School of Medicine, Northwell Health, New York, NY, United States
- 3Departments of Psychiatry, Zucker School of Medicine, Northwell Health, New York, NY, United States
Background: Women face a significantly higher lifetime risk of developing Alzheimer’s disease (AD) than men. This disparity is often attributed to longer female longevity, but growing evidence suggests a multifactorial origin, including hormonal, vascular, and immunologic contributions. Estrogen plays a critical neuroprotective role across multiple systems implicated in AD pathogenesis, including synaptic plasticity, mitochondrial function, and cerebrovascular integrity. However, clinical trials investigating hormone therapy (HT) for AD prevention have yielded mixed results, in part due to variability in study populations, timing of intervention, and formulation of hormones.
Aims/methods: This review examines the biological rationale for estrogen’s role in cognitive aging, synthesizes clinical and translational data on hormone therapy and AD risk, and highlights the importance of vascular comorbidity, including cerebral small vessel disease, in mediating AD pathology.
Conclusion: We propose that estrogen’s neuroprotective potential may be best realized in personalized treatment frameworks that account for age, timing, APOE genotype, and vascular burden. Interpretation of estrogen’s role in AD is further complicated by variability in diagnostic criteria, which may contribute to conflicting findings across studies. Recognition of menopause-related cognitive impairment as an early, hormonally modulated risk state may offer additional opportunity for timely intervention. Addressing this complexity is essential to refining AD prevention strategies in midlife women.
1 Introduction
Alzheimer’s disease (AD) disproportionately affects women, with nearly two-thirds of all diagnosed cases occurring in females. Biological, hormonal, and sociocultural factors are likely the primary drivers of the sex disparity in risk and clinical presentation, rather than increased longevity as previously believed (Beam et al., 2018; Jett et al., 2022; Vila-Castelar et al., 2023). Women not only exhibit a higher incidence and prevalence of AD but also tend to show faster cognitive decline and greater pathological burden once diagnosed (Lin et al., 2015).
Emerging research suggests that female-specific risk factors—especially those tied to hormonal transitions such as menopause—may critically influence brain aging and AD vulnerability (Toran-Allerand et al., 1999; Sherwin, 2009; Henderson and Sherwin, 2007). Estrogen, in particular, has garnered attention for its pleiotropic effects on neuronal resilience, cerebral perfusion, and immune modulation (Toran-Allerand et al., 1999). The perimenopausal and postmenopausal brain may thus experience a compound loss of estrogen’s protective influence at the very time when age-related neurodegeneration begins to accelerate (Henderson and Sherwin, 2007; Henderson and Rocca, 2012; Henderson et al., 2016). Interpretation of these findings is further complicated by inconsistencies in how AD is defined across the three major diagnostic frameworks, a factor that may obscure true associations between estrogen exposure and disease risk (Bieger et al., 2024).
Compounding this complex picture is the frequent co-occurrence of other primary brain co-pathology in aging women (Devi, 2023). AD almost never occurs in isolation. In fact, neuropathologic studies show that from 66% to 100% of AD cases exhibit coexisting primary brain pathologies—including vascular brain injury and Lewy body disease (Spina et al., 2021; Karanth et al., 2020). Mixed pathology is the norm, rather than the exception.
In addition to these structural and molecular risks, the menopausal transition itself is associated with measurable cognitive changes in a substantial proportion of women (Weber et al., 2014; Davis et al., 2015). Multiple longitudinal cohort studies demonstrate that up to 60% of midlife women report difficulties with memory, attention, and verbal fluency during perimenopause (Henderson and Sherwin, 2007; Weber et al., 2014; Davis et al., 2015). Objective testing confirms declines in verbal memory, working memory, and executive function, often correlating with fluctuations in estradiol and follicle-stimulating hormone (Davis et al., 2015). These changes, although subtle, can significantly impact quality of life and may be mistaken for early signs of dementia. Recognizing the menopausal origin of these symptoms is crucial for timely and appropriate management.
Menopause-related cognitive impairment (MeRCI), a recently defined clinical entity, describes the emergence of cognitive symptoms, including objective evidence of language, executive function and/or memory impairment, during the menopausal transition in the absence of other medical or psychiatric conditions (Devi, 2018; Maki and Henderson, 2012). MeRCI reflects the estrogen-sensitive cognitive phenotype often underrecognized in midlife women and may represent a modifiable early risk state within the Alzheimer’s disease continuum.
This review synthesizes current evidence on estrogen’s neuroprotective mechanisms, evaluates clinical trial data on hormone therapy and AD risk, and emphasizes the need to consider vascular comorbidity in both mechanistic understanding and therapeutic planning. By reframing AD as a spectrum disorder influenced by sex-specific biology and comorbid pathologies, we advocate for a precision medicine approach to prevention in midlife women.
2 Discussion
We begin by examining the multiple mechanisms through which estrogen may support neuronal health, including its influence on neuroplasticity, neurotransmitter regulation, oxidative stress, amyloid and tau pathology, neuroimmune function, and the integrity of the blood-brain barrier. Many of these areas have been extensively reviewed elsewhere and therefore will be summarized in this review.
We then discuss how the diagnostic criteria used in making a diagnosis of AD may significantly alter results of data. We review current evidence on estrogen’s role in cognitive impairment, drawing from both observational studies and randomized controlled trials. This includes an analysis of the specific estrogen formulations used, the cognitive effects of progestins, and the importance of the critical window for initiating hormone therapy.
Next, we explore the significant overlap between vascular and neurodegenerative pathology in women with Alzheimer’s disease and the potential modulatory role of estrogen in this context. We highlight the emerging clinical entity of menopause-related cognitive impairment, which may reflect a unique intersection of hormonal and neurodegenerative changes.
Finally, we discuss the clinical implications of these findings and consider how hormone therapy might be integrated into personalized treatment paradigms for women at risk of or experiencing cognitive decline.
2.1 Estrogen and the female brain: mechanisms of neuroprotection
Estrogen exerts extensive influence on the central nervous system (CNS), affecting not only reproductive regulation but also cognition, affect, and neuroprotection. Its effects are mediated through estrogen receptors—ERα and ERβ—widely distributed throughout the brain, including the hippocampus, prefrontal cortex, amygdala, and basal forebrain (McEwen et al., 2001; McEwen et al., 2012; Mitterling et al., 2010). These receptors facilitate both genomic transcriptional regulation and rapid non-genomic signaling, engaging intracellular cascades that impact brain aging and resilience to neurodegenerative stressors (McEwen, 2012; Ma et al., 2016).
2.1.1 Synaptic plasticity, neurogenesis, and cognitive performance
Estrogen enhances synaptic connectivity through promotion of long-term potentiation (LTP), increased dendritic spine density, and heightened expression of synaptic proteins important for synaptic plasticity (Toran-Allerand et al., 1999; Henderson et al., 2016; Smejkalova and Woolley, 2010). These effects are especially pronounced in the hippocampus and prefrontal cortex—regions integral to working memory, spatial navigation, and executive function (Shanmugan and Epperson, 2014). Estradiol also stimulates adult neurogenesis in the dentate gyrus, a capacity that declines with age and estrogen deprivation (Barha and Galea, 2010; Grodstein et al., 2003). These synaptic and neurogenic effects underlie the cognitive advantages observed in premenopausal women and in animal models treated with estradiol (Mishra et al., 2023).
In mouse models, early ovarian failure during perimenopause enhances astrocyte activation and regional amyloid accumulation in the hippocampus during early-stage cerebral amyloid angiopathy, suggesting emerging neurovascular dysfunction, which is often correlated with the development of dementia (Platholi et al., 2023). Another mouse model study found that chemically induced perimenopause in mice with early-stage Alzheimer’s pathology increased amyloid-beta accumulation and heightened astrocyte and microglial activation in specific hippocampal subregions, although cognitive deficits were not yet apparent. These results support the idea that perimenopause represents a critical window of emerging vulnerability to Alzheimer’s-related brain changes where intervention may be beneficial (Marongiu et al., 2025).
2.1.2 Neurotransmitter systems: acetylcholine, serotonin, dopamine, and norepinephrine
Estrogen exerts widespread influence on neurotransmitter systems critical to cognition, mood regulation, and behavioral flexibility—many of which are vulnerable to age-related decline and prominently implicated in AD. Estrogen receptors are expressed not only in cortical and hippocampal neurons but also in neurons producing acetylcholine and monoamines, including serotonin, dopamine, and norepinephrine (Ma et al., 2016; Ross and Van Bockstaele, 2021).
• Acetylcholine: Estrogen upregulates choline acetyltransferase, the enzyme responsible for acetylcholine synthesis, and enhances muscarinic receptor density in the basal forebrain and hippocampus. It also supports the integrity of cholinergic projections—among the earliest to deteriorate in AD—thereby preserving attention and memory processes. This cholinergic support underpins estrogen’s role in attention and memory (Russell et al., 2019; Newhouse and Dumas, 2015). The loss of this estrogenic support during perimenopause may compromise cholinergic tone and contribute to the early cognitive symptoms observed in prodromal AD (Kwakowsky et al., 2016).
• Serotonin: Estrogen modulates serotonergic function by increasing tryptophan hydroxylase expression—the rate-limiting enzyme in serotonin synthesis—and by regulating serotonin receptor subtypes, particularly 5-HT1A and 5-HT2A (MCEWEN Harold and Milliken, 2001; Amin et al., 2005; Bendis et al., 2024). These effects are associated with improvements in mood and affect and may explain the increased risk of depression during the menopausal transition (MCEWEN Harold and Milliken, 2001; Amin et al., 2005). Estrogen’s serotonergic actions are central to its role in maintaining emotional resilience and are increasingly recognized as part of its broader neuroprotective profile.
• Dopamine: Estrogen influences dopaminergic signaling by enhancing dopamine synthesis and turnover, and regulates D1 and D2 receptor expression, particularly in the prefrontal cortex, striatum, and nucleus accumbens. These regions govern executive function, working memory, motivation, and reward processing—domains commonly impaired in early AD (Almey et al., 2015). Membrane-associated estrogen receptors localized on dopaminergic neurons further modulate dopamine receptor expression and sensitivity (Bendis et al., 2024; Almey et al., 2015; Almey et al., 2012). These mechanisms underpin sex differences in dopamine-mediated cognition and highlight estrogen’s critical role in modulating frontostriatal function.
• Norepinephrine: Estrogen also modulates the locus coeruleus (LC) –norepinephrine system, which is involved in arousal, attention, vigilance, and stress regulation. Estrogen receptors are expressed in LC neurons, where estrogen preserves structural integrity and promotes norepinephrine synthesis and release. Notably, LC degeneration is one of the earliest pathologic changes in AD and contributes to neuroinflammatory priming and influences amyloid processing. Estrogen-mediated preservation of locus coeruleus function may be critical given its early degeneration in AD (Russell et al., 2019; Bangasser et al., 2016). Estrogen modulation of LC activity suggests another potential mechanism by which hormonal decline during perimenopause could amplify AD risk through disrupted arousal and neuroimmune regulation (Ross and Van Bockstaele, 2021).
Through this multifaceted support of key neurotransmitter systems, estrogen maintains the neurochemical infrastructure essential for cognitive and emotional regulation. The loss of estrogenic signaling during perimenopause may destabilize these systems, creating a period of heightened vulnerability to the neuropathological processes underlying Alzheimer’s disease.
2.1.3 Mitochondrial function and oxidative stress
Mitochondria are central to neuronal metabolism, and their dysfunction is a key feature of aging and Alzheimer’s disease (McGill Percy et al., 2025). Estrogen enhances mitochondrial biogenesis, increases ATP production, and reduces oxidative stress through upregulation of antioxidant enzymes such as superoxide dismutase and glutathione peroxidase (Lejri et al., 2018; Velarde, 2013). This contributes to reduced accumulation of reactive oxygen species, which are implicated in both amyloid aggregation and tau hyperphosphorylation.
2.1.4 Amyloid and tau pathophysiology
Estrogen influences amyloid precursor protein processing by promoting α-secretase activity and suppressing β-secretase, thereby favoring the non-amyloidogenic pathway (Cole and Vassar, 2007). In vivo models show that estrogen administration reduces Aβ levels and plaque formation and may reduce tau formation in post-menopausal women (Wang et al., 2024a; Henderson, 2014). Additionally, estrogen modulates kinases involved in tau phosphorylation, thereby mitigating neurofibrillary tangle development (Kantarci et al., 2016; Wang et al., 2024b; Depypere et al., 2023).
2.1.5 Cerebrovascular and blood-brain barrier (BBB) integrity
Estrogen has vasoprotective effects, including the promotion of nitric oxide synthesis, improved endothelial function, and preservation of cerebral autoregulation (McNeill et al., 2002). It also maintains blood-brain barrier integrity, reducing permeability and neuroinflammatory infiltration (Maggioli et al., 2016). With estrogen loss, particularly during the menopausal transition, cerebral perfusion diminishes and the BBB becomes more vulnerable—conditions that may synergize with amyloid and vascular pathology to accelerate cognitive decline (Russell et al., 2019; Maggioli et al., 2016).
2.1.6 Neuroimmune modulation
Microglia and astrocytes express estrogen receptors and are directly modulated by hormonal signaling (Mor et al., 1999). Estrogen promotes an anti-inflammatory glial phenotype, downregulates pro-inflammatory cytokines (e.g., IL-1β, IL-6, and TNF-α), and restrains chronic neuroinflammation—a hallmark of aging and AD (Mor et al., 1999; Villa et al., 2016). Estrogen deprivation in menopause may shift this balance toward a pro-inflammatory state, thereby exacerbating neuronal injury and synaptic loss (Villa et al., 2016).
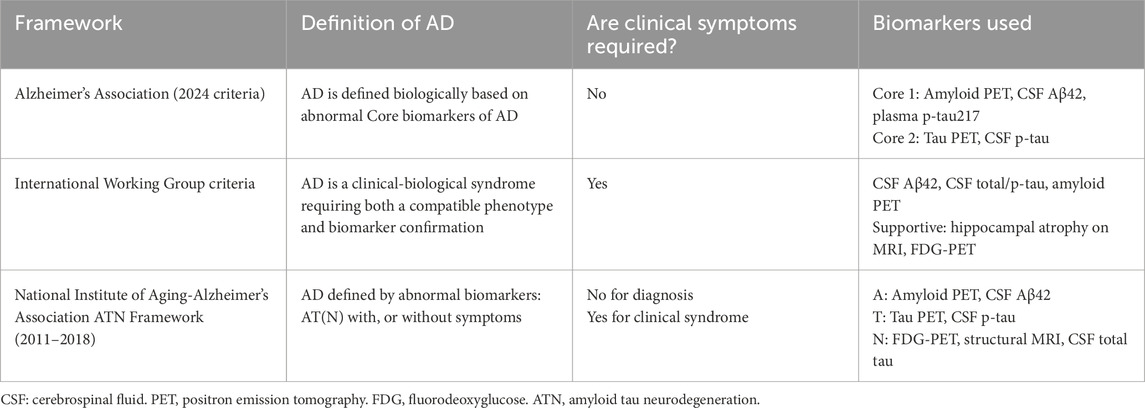
2.2 Diagnostic criteria discordance and implications for research in HT and AD
Differences in Alzheimer’s disease diagnostic frameworks—particularly between the two biomarker-only National Institute on Aging–Alzheimer’s Association (NIA-AA) and the Alzheimer’s Association (AA) criterion, and the clinico-biological International Working Group (IWG) criteria—have important implications for estrogen research (Table 1) (Villain and Paretsky, 2024).
The IWG requires both clinical symptoms and biomarker evidence for diagnosis (Dubois et al., 2021). In contrast, the NIA-AA and the AA frameworks permit a biological diagnosis based solely on amyloid, tau, and neurodegeneration biomarkers (AT [N]), even in asymptomatic individuals (Brum et al., 2022; Jack et al., 2018). A third approach, endorsed by an Alzheimer’s Association-led working group, allows for diagnosis based on a single abnormal biomarker (Ja et al., 2024). When applied to the same cohort, these frameworks yielded a 42% discrepancy in diagnoses, especially in individuals with only one abnormal biomarker (Bieger et al., 2024). Notably, among individuals with isolated amyloid-beta abnormalities, 65% remained cognitively intact (Bieger et al., 2024; Villain and Paretsky, 2024).
For estrogen studies that rely on AD diagnosis as an outcome or grouping variable, such variability can distort associations, especially among cognitively normal, biomarker-positive midlife women. Researchers should therefore clearly specify which diagnostic criteria are used and consider those that integrate both clinical and biological features to improve relevance for sex-specific risk and treatment response.
2.3 The timing hypothesis and clinical trials of hormone therapy in Alzheimer’s disease
The “timing hypothesis” posits that the neuroprotective effects of estrogen are contingent upon when HT is initiated in relation to the onset of menopause. Specifically, estrogen may confer cognitive and structural brain benefits when administered during the critical window of perimenopause or early post-menopause, whereas delayed initiation—often a decade or more after menopause—may yield no benefit or even harm (Sherwin, 2009; Henderson and Rocca, 2012; Erickson et al., 2010; Lord et al., 2008).
2.3.1 Observational evidence and the critical window
Early observational studies suggested a strong protective association between estrogen use and reduced AD risk. For example, data from the Cache County Study, which included persons aged 65 years or older, and the Baltimore Longitudinal Study on Aging, which included postmenopausal women ages 65 and older, indicated that women who used HT near the time of menopause had a lower incidence of AD (Shao et al., 2012; Nerattini et al., 2023). However, these studies were limited by healthy-user bias and lack of randomization.
The critical window hypothesis emerged from such findings, suggesting that the brain may retain estrogen sensitivity during a finite period following ovarian hormone withdrawal (Sherwin, 2009). Beyond this window, estrogen receptors may downregulate, and the brain may enter a pro-inflammatory, vulnerable state that does not respond favorably to exogenous hormones.
2.3.2 Randomized controlled trials: mixed results
Randomized controlled trials have yielded mixed results and are briefly summarized in Table 2. The Women’s Health Initiative Memory Study (WHIMS) remains the most widely cited trial in the field Espeland et al. (2024). In this large, randomized controlled trial (RCT) of women aged 65 and older, conjugated equine estrogen (CEE) with or without medroxyprogesterone acetate (MPA) increased the risk of dementia and cognitive decline. However, this trial enrolled women well beyond the menopausal transition (mean age 69), raising questions about generalizability to midlife populations. Additionally, while there was an increased risk of all-cause dementia (including normal pressure hydrocephalus, for example,), there was NOT an increased risk for AD (Nerattini et al., 2023).
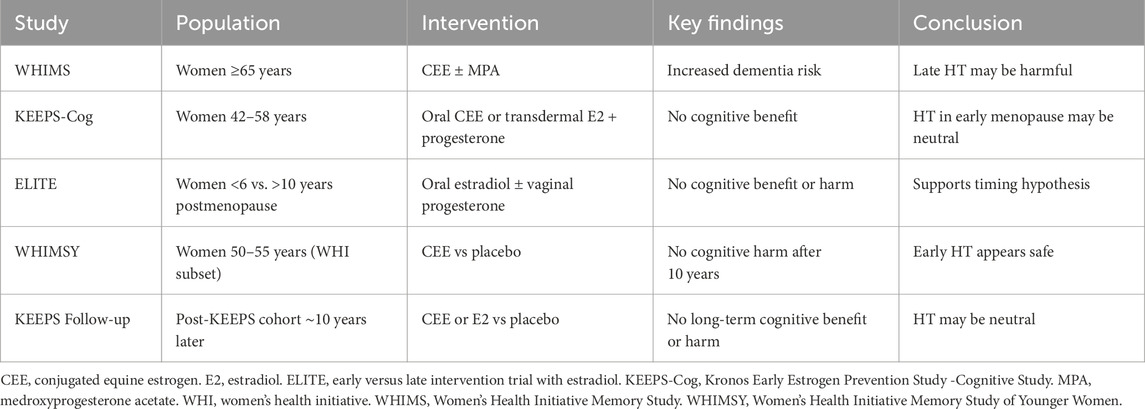
Table 2. Major clinical trials evaluating hormone therapy and cognitive outcomes in postmenopausal women.
Subsequent RCTs have painted a more nuanced picture:
• The KEEPS-Cog trial studied younger postmenopausal women (ages 42–58) and found no adverse cognitive effects of transdermal estradiol over 4 years, although it was not powered to detect AD incidence (Gleason et al., 2015).
• The ELITE study stratified women by time since menopause and found that estradiol improved carotid artery intima-media thickness in recently menopausal women (<6 years) but not in those further out (>10 years), indirectly supporting the timing hypothesis for vascular aging (Hodis et al., 2016).
• The WHIMSY trial, a follow-up to WHI, evaluated cognition in women who had received CEE between ages 50 and 55. It found no increased risk of cognitive impairment over 10 years of follow-up, suggesting that early use of estrogen may be neutral or beneficial Espeland et al. (2024); Maki and Henderson, 2012; Shumaker et al., 2004).
2.3.3 Formulation, route, and progestin effects
Not all estrogens or regimens are equivalent. The WHIMS trial employed oral CEE, a formulation derived from pregnant mare’s urine, along with MPA, a synthetic progestin Espeland et al. (2024); Shumaker et al., 2004). In contrast, bioidentical 17β-estradiol—particularly via transdermal routes—may have more favorable effects on cognition and cardiovascular health. Oral estrogens undergo first-pass hepatic metabolism, increasing coagulation factors and inflammatory markers, whereas transdermal estradiol bypasses the liver and maintains a more physiologic estrogen profile (Kantarci et al., 2016; Wharton et al., 2011).
Progestin co-administration further complicates interpretation. While necessary for endometrial protection in women with a uterus, synthetic progestins like MPA may attenuate estrogen’s beneficial effects (Stanczyk et al., 2013). Natural micronized progesterone appears to have a more favorable safety and neurocognitive profile but has not been extensively studied in long-term AD prevention trials.
These differences were directly explored in the Kronos Early Estrogen Prevention Study (KEEPS), which randomized healthy recently postmenopausal women (median age 42–58 years old; within 3 years of menopause) to either oral CEE, transdermal 17β-estradiol, or placebo, all with cyclic micronized progesterone (Gleason et al., 2015; Gleason et al., 2024). The KEEPS-Cog sub-study evaluated cognitive outcomes over 4 years and found no cognitive benefit from either formulation compared to placebo. Although neither route caused harm, the anticipated cognitive enhancement—particularly from transdermal estradiol—was not observed, challenging assumptions that bioidentical transdermal therapy would outperform CEE in preserving cognitive function (Gleason et al., 2024).
A long-term follow-up study of the KEEPS cohort approximately 10 years after cessation of therapy similarly found no lasting benefit or harm on cognitive outcomes from either hormone regimen. These findings suggest that in healthy, low-risk women, even early HT initiation may be cognitively neutral, and that timing, formulation, and population risk profile all influence therapeutic outcomes (Gleason et al., 2024).
This nuanced evidence underscores the importance of tailoring hormone therapy—not only in terms of age and timing, but also formulation, route, and individual risk factors, including cardiovascular and genetic vulnerability.
2.3.4 Limitations and remaining questions
Despite promising mechanistic data, current clinical trials remain underpowered, short in duration, and heterogeneous in design. Most have not targeted women at the highest risk—those with metabolic syndrome, cerebrovascular pathology, or APOE-ε4 genotype (Saleh et al., 2023). Furthermore, outcomes have focused on global cognition rather than sensitive biomarkers such as amyloid PET imaging, tau load, white matter hyperintensity volume, or regional perfusion.
Another prospective cohort of women tracked from midlife to late life, early initiation of hormone therapy—particularly within 5 years of menopause—was associated with reduced risk of cognitive impairment and dementia. Notably, the protective association was strongest in APOE-ε4 non-carriers, whereas APOE-ε4 carriers did not appear to benefit and, in some cases, showed potential harm with later or prolonged hormone use (Taxier et al., 2022a).
This study reinforces both the critical window hypothesis and the need for genotype-informed HT decision-making, aligning with other studies showing that APOE-ε4 carriers may have heightened sensitivity to hormonal and metabolic stressors. These findings argue for tailoring both timing and duration of hormone therapy to genetic and vascular risk (Saleh et al., 2023; Taxier et al., 2022a).
Such observational data, while subject to confounding factors, offer valuable direction for future trials and underscore the need for stratified prevention strategies.
2.4 The overlap of vascular and neurodegenerative pathology in women with Alzheimer’s disease
Alzheimer’s disease is almost never a pure neuropathological entity (Devi, 2023). Recent studies, including large autopsy series and biomarker-based analyses, confirm that nearly all cases of AD have coexisting primary brain pathology. Common vascular comorbities include cerebral small vessel disease (SVD), microinfarcts, white matter hyperintensities (WMHs), and arteriolosclerosis (Hodis et al., 2016; Simpkins et al., 2009). Vascular comorbidities are particularly prevalent in women, amplifying cognitive impairment and complicating diagnostic and therapeutic strategies.
2.4.1 Vascular aging and estrogen loss
Estrogen plays a central role in maintaining cerebrovascular health through its effects on endothelial function, nitric oxide production, arterial compliance, and blood-brain barrier (BBB) integrity (Maggioli et al., 2016). With the menopausal transition, the decline in estrogen leads to increased arterial stiffness, endothelial dysfunction, and enhanced vulnerability to ischemic injury. These vascular changes may precede or exacerbate neurodegenerative processes, particularly in women with underlying metabolic or hypertensive risk (Hodis et al., 2016).
Women also appear to have greater WMH burden than men for a given vascular risk profile, and WMHs are a well-established predictor of cognitive decline, especially executive dysfunction and processing speed—domains often affected early in women with AD (Hodis et al., 2016).
2.4.2 Interaction between vascular pathology and amyloid-tau cascade
Vascular dysfunction may synergize with amyloid and tau pathology to accelerate neurodegeneration. Chronic hypoperfusion impairs glymphatic clearance of Aβ, while blood-brain barrier breakdown facilitates neuroinflammation and toxic protein accumulation. Additionally, microvascular disease may sensitize neurons to injury from tau hyperphosphorylation.
In women, this interplay may be intensified by estrogen loss. For example, animal models of ovariectomy show increased cerebrovascular inflammation, reduced perfusion, and enhanced Aβ deposition—effects partially reversed by estrogen replacement (Hodis et al., 2016; Simpkins et al., 2009; Attems et al., 2014). These models suggest that estrogen’s protective role includes dampening the vascular contributions to AD pathology.
2.4.3 Cerebrovascular burden as a modifier of hormone therapy response
Vascular burden may also modulate the cognitive response to hormone therapy. Subgroup analyses from the WHIMS-MRI study revealed that older women on hormone therapy had increased WMH volume, particularly those with preexisting vascular risk factors (Coker et al., 2009). This has raised concern that in the presence of significant SVD, exogenous estrogen may exacerbate white matter injury, particularly when initiated late.
Conversely, studies in younger women with lower vascular burden suggest more neutral or even beneficial effects on brain perfusion and connectivity. These findings support a precision-medicine approach in which vascular status—assessed via imaging or biomarkers—guides HT candidacy and regimen.
2.4.4 Racial and metabolic disparities
It is important to recognize that vascular risk and estrogen deficiency intersect with social determinants of health, leading to disproportionate burden in Black and Hispanic women, who have higher rates of hypertension, diabetes, and stroke (Chinn et al., 2020). These populations are underrepresented in clinical trials, yet their risk profiles may make them more susceptible to both the benefits and harms of HT.
Incorporating vascular screening and risk stratification—particularly in midlife—may identify women most likely to benefit from early estrogen intervention and those in whom non-hormonal vascular risk management should take precedence.
2.5 Menopause-related cognitive impairment (MeRCI): a distinct clinical entity
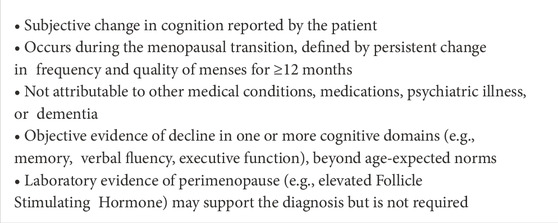
Menopause-related cognitive impairment (MeRCI) is a recognized syndrome describing cognitive deficits that emerge during the perimenopausal and early postmenopausal transition, often in otherwise healthy women. Symptoms commonly include reduced verbal fluency, memory lapses, and executive dysfunction, often occurring in the absence of mood disorders or clear structural brain abnormalities (Devi, 2018).
Studies show that 34%–62% of midlife women report memory changes during menopause (Devi et al., 2005; Drogos et al., 2013). These subjective complaints correlate with objective reductions in verbal memory and fluency on neuropsychological testing (Sherwin, 2009; Henderson and Sherwin, 2007). Critically, these deficits can mimic early signs of neurodegenerative disease—leading to misdiagnoses such as Alzheimer’s disease or frontotemporal dementia (Henderson and Sherwin, 2007; Weber et al., 2014; Devi, 2018; Leblanc et al., 2002).
Diagnostic criteria for MeRCI include subjective and objective cognitive changes, as noted in Table 3, temporal association with menstrual irregularity, and exclusion of other medical or psychiatric causes (Devi, 2018). Brain imaging is often unremarkable, and symptoms may stabilize or improve with short-term hormone therapy or cognitive remediation strategies (Devi, 2018).
Because MeRCI is underrecognized in routine neurological and gynecological evaluations, many affected women receive incorrect diagnoses or unnecessary treatment. Routine screening for cognitive changes during menopause should be incorporated into clinical practice, particularly in women reporting difficulty with language, memory, or multitasking. Early recognition can prevent stigmatizing misdiagnoses and facilitate appropriate interventions.
2.6 Integrating hormone therapy into Alzheimer’s prevention: future directions and clinical considerations
While the neuroprotective potential of estrogen is well-supported by preclinical data, translation into effective clinical prevention of Alzheimer’s disease has proven complex. Disparate trial results reflect the challenges of a “one-size-fits-all” approach to hormone therapy and underscore the need for precision targeting based on age, timing, genotype, vascular health, and formulation.
2.6.1 The need for risk-stratified prevention frameworks
Future prevention strategies should be individualized, not only by menopausal timing but also by risk phenotype. For example, a recently postmenopausal woman with a strong family history of AD, APOE-ε4 positivity, and minimal vascular burden may derive benefit from early transdermal estradiol, particularly if initiated within 5 years of menopause (Kantarci et al., 2016). In contrast, an older woman with metabolic syndrome, hypertension, and white matter hyperintensities may be better served by aggressive vascular risk reduction rather than hormone therapy.
This approach requires integrating biomarkers into decision-making, such as:
• Neuroimaging markers: hippocampal atrophy, cerebral perfusion, PET tau and amyloid (Coughlan et al., 2022; Coughlan et al., 2023; Coughlan et al., 2025).
• Plasma or CSF biomarkers: Aβ42/40, phosphorylated tau, neurofilament light (Coughlan et al., 2025).
• Genetic markers: Apolipoprotein E genotype and other AD risk polymorphisms (Saleh et al., 2023; Wang and Brinton, 2016; Taxier et al., 2022b).
2.6.2 Opportunities for combination and sequential therapies
Estrogen’s failure to show consistent benefit in isolation may reflect a need for combination strategies. Potential synergistic approaches include:
• HT + lifestyle intervention: Exercise, Mediterranean diet, and cognitive training may amplify HT effects on brain plasticity.
• HT + anti-hypertensive or anti-diabetic agents: Addressing vascular risk may unmask the cognitive benefits of HT.
• Sequential or cycling therapy: Intermittent HT may retain efficacy while minimizing long-term exposure risks.
Such strategies should be tested in next-generation trials that use brain biomarkers as primary outcomes and recruit enriched populations at midlife.
2.6.3 Clinical counseling: communicating nuance
For clinicians, translating this nuanced landscape into practice requires transparent, individualized risk-benefit counseling. Core principles include:
• Timing matters: Benefits are more likely when HT is initiated close to menopause onset (Hodis et al., 2016).
• Formulation matters: Transdermal estradiol and micronized progesterone are preferable to oral CEE and synthetic progestins (MŠ et al., 2023).
• Personal risk profile matters: APOE-ε4 status, vascular comorbidities, and cognitive symptoms must inform decisions (Riedel et al., 2016).
Hormone therapy should not be universally recommended for AD prevention, but neither should it be categorically dismissed—particularly in healthy women at midlife (Wharton et al., 2011). Reframing the conversation from binary risk to contextual, individualized potential empowers women to make informed choices.
2.6.4 Research priorities
Key directions for future investigation include:
• Large, long-duration trials of transdermal estradiol in at-risk midlife women, stratified by APOE status and vascular health
• Integration of neuroimaging and fluid biomarkers into HT studies
• Inclusion of diverse populations, especially underrepresented racial and ethnic groups
• Evaluation of brain outcomes beyond global cognition, such as hippocampal volume, WMHs, network connectivity, and centiloid clearance
Only through such refined approaches can we resolve the longstanding ambiguity around estrogen and AD and deliver truly personalized prevention to the women most at risk.
3 Conclusion
Alzheimer’s disease in women is not simply a consequence of living longer—it is a reflection of complex, sex-specific biological, vascular, and hormonal interactions that begin decades before clinical onset. The abrupt decline in estrogen during menopause appears to be a pivotal inflection point in this trajectory, contributing to measurable cognitive changes, modulation of neurotransmitter systems, vascular vulnerability, and the acceleration of AD-related pathology.
Yet, estrogen’s therapeutic potential has been obscured by inconsistencies in study design, population selection, and timing of intervention. Evidence suggests that hormone therapy may offer cognitive benefits—particularly when initiated early in the menopausal transition, using bioidentical formulations and appropriate delivery routes. Conversely, delayed or inappropriate hormone use may fail to protect or may even harm, especially in the context of existing vascular disease or APOE-ε4 carrier status.
Future studies should clearly define the diagnostic criteria used for AD, as differences in classification frameworks may significantly affect both participant selection and interpretation of estrogen-related outcomes.
Recognition of menopause-related cognitive impairment as a distinct clinical entity offers a valuable framework for identifying estrogen-sensitive cognitive decline and avoiding misdiagnosis. Integrating vascular risk profiling, neuroimaging, genotype information, and menopausal timing into cognitive assessments will allow clinicians to tailor better interventions.
Hormone therapy should not be universally recommended for Alzheimer’s prevention, but neither should it be uniformly dismissed. A nuanced, precision medicine approach—targeting the right women, at the right time, with the right formulation—holds promise for altering the course of cognitive aging in women. The next-generation of trials must rise to this complexity, and clinical practice must evolve to reflect it.
Author contributions
NM: Writing – original draft, Writing – review and editing. GD: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Almey, A., Filardo, E. J., Milner, T. A., and Brake, W. G. (2012). Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization. Endocrinology 153 (11), 5373–5383. doi:10.1210/en.2012-1458
Almey, A., Milner, T. A., and Brake, W. G. (2015). “Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Hormones Behav. 74. 125–138. doi:10.1016/j.yhbeh.2015.06.010
Amin, Z., Canli, T., and Epperson, C. N. (2005). Effect of estrogen-serotonin interactions on mood and cognition. Behav. Cognitive Neurosci. Rev. 4, 43–58. doi:10.1177/1534582305277152
Attems, J., and Jellinger, K. A. (2014). The overlap between vascular disease and Alzheimer’s disease - lessons from pathology. BMC Med. 12 (1), 206. doi:10.1186/s12916-014-0206-2
Bangasser, D. A., Wiersielis, K. R., and Khantsis, S. (2016). “Sex differences in the locus coeruleus-norepinephrine system and its regulation by stress, Brain Res. 1641. 177–188. doi:10.1016/j.brainres.2015.11.021
Barha, C. K., and Galea, L. A. M. (2010). Influence of different estrogens on neuroplasticity and cognition in the hippocampus. Biochimica Biophysica Acta - General Subj. 1800, 1056–1067. doi:10.1016/j.bbagen.2010.01.006
Beam, C. R., Kaneshiro, C., Jang, J. Y., Reynolds, C. A., Pedersen, N. L., and Gatz, M. (2018). Differences between women and men in incidence rates of dementia and alzheimer’s disease. J. Alzheimer’s Dis. 64 (4), 1077–1083. doi:10.3233/JAD-180141
Bendis, P. C., Zimmerman, S., Onisiforou, A., Zanos, P., and Georgiou, P. (2024). The impact of estradiol on serotonin, glutamate, and dopamine systems. Front. Neurosci., 18, 1348551, doi:10.3389/fnins.2024.1348551
Bieger, A., Brum, W. S., Borelli, W. V., Therriault, J., De Bastiani, M. A., Moreira, A. G., et al. (2024). Influence of different diagnostic criteria on alzheimer disease clinical research. Neurology. 103 (5), e209836. doi:10.1212/WNL.0000000000209753
Brum, W. S., de Bastiani, M. A., Bieger, A., Therriault, J., Ferrari-Souza, J. P., Benedet, A. L., et al. (2022). A three-range approach enhances the prognostic utility of CSF biomarkers in Alzheimer’s disease. Alzheimer’s Dementia Transl. Res. Clin. Interventions 8 (1), e12270. doi:10.1002/trc2.12270
Chinn, J. J., Martin, I. K., and Redmond, N. (2020). Health equity among black women in the United States. J. Womens Health 30 (2), 212–219. doi:10.1089/jwh.2020.8868
Coker, L. H., Hogan, P. E., Bryan, N. R., Kuller, L. H., Margolis, K. L., Bettermann, K., et al. (2009). Postmenopausal hormone therapy and subclinical cerebrovascular disease the WHIMS-MRI study. Neurology 72, 125–134. doi:10.1212/01.wnl.0000339036.88842.9e
Cole, S. L., and Vassar, R. (2007). The Alzheimer’s disease β-secretase enzyme, BACE1. Mol. Neurodegener. 2 (1), 22. doi:10.1186/1750-1326-2-22
Coughlan, G. T., Betthauser, T. J., Boyle, R., Koscik, R. L., Klinger, H. M., Chibnik, L. B., et al. (2023). Association of age at menopause and hormone therapy use with tau and β-Amyloid positron emission tomography. JAMA Neurol. 80, 462–473. doi:10.1001/jamaneurol.2023.0455
Coughlan, G. T., Koscik, R. L., Betthauser, T. J., Boyle, R. T., Jonaitis, E. M., Wenzel, A., et al. (2022). Menopause age and hormone therapy use moderate PET tau and amyloid association: findings from the Wisconsin registry for Alzheimer prevention. Alzheimer’s & Dementia 18 (S6). doi:10.1002/alz.062007
Coughlan, G. T., Rubinstein, Z., Klinger, H., Lopez, K. A., Hsieh, S., Boyle, R., et al. (2025). Associations between hormone therapy use and tau accumulation in brain regions vulnerable to Alzheimer’s disease. Sci. Adv. 11, eadt1288. doi:10.1126/sciadv.adt1288
Davis, S. R., Lambrinoudaki, I., Lumsden, M., Mishra, G. D., Pal, L., Rees, M., et al. (2015). Menopause. Nat. Rev. Dis. Prim. 1, 15004. doi:10.1038/nrdp.2015.4
Depypere, H., Vergallo, A., Lemercier, P., Lista, S., Benedet, A., Ashton, N., et al. (2023). Menopause hormone therapy significantly alters pathophysiological biomarkers of Alzheimer’s disease. Alzheimer’s Dementia 19 (4), 1320–1330. doi:10.1002/alz.12759
Devi, G. (2018). Menopause-related cognitive impairment. Obstetrics Gynecol. 132 (6), 1325–1327. doi:10.1097/AOG.0000000000002963
Devi, G. A how-to guide for a precision medicine approach to the diagnosis and treatment of Alzheimer’s disease (2023). Front. Aging Neurosci. 15, 1213968, doi:10.3389/fnagi.2023.1213968
Devi, G., Hahn, K., Massimi, S., and Zhivotovskaya, E. (2005). Prevalence of memory loss complaints and other symptoms associated with the menopause transition: a community survey. Gend. Med. 2 (4), 255–264. doi:10.1016/s1550-8579(05)80055-5
Drogos, L. L., Rubin, L. H., Geller, S. E., Banuvar, S., Shulman, L. P., and Maki, P. M. (2013). Objective cognitive performance is related to subjective memory complaints in midlife women with moderate to severe vasomotor symptoms. Menopause 20 (12), 1236–1242. doi:10.1097/GME.0b013e318291f5a6
Dubois, B., Villain, N., Frisoni, G. B., Rabinovici, G. D., Sabbagh, M., Cappa, S., et al. (2021). “Clinical diagnosis of Alzheimer’s disease: recommendations of the international working group,” Lancet Neurology 20, 484–496. doi:10.1016/S1474-4422(21)00066-1
Erickson, K. I., Voss, M. W., Prakash, R. S., Chaddock, L., and Kramer, A. F. (2010). A cross-sectional study of hormone treatment and hippocampal volume in postmenopausal women: evidence for a limited window of opportunity. Neuropsychology 24 (1), 68–76. doi:10.1037/a0017292
Espeland, M. A., Rapp, S. R., Shumaker, S. A., Brunner, R., Manson, J. E., Sherwin, B. B., et al. (2024). Conjugated equine estrogens and global cognitive function in postmenopausal women women’s health initiative memory study. doi:10.1001/jama.291.24.2959
Gleason, C. E., Dowling, N. M., Kara, F., James, T. T., Salazar, H., Ferrer, S. C. A., et al. (2024). Long-term cognitive effects of menopausal hormone therapy: findings from the KEEPS continuation study. PLoS Med. doi:10.1371/journal.pmed.1004435
Gleason, C. E., Dowling, N. M., Wharton, W., Manson, J. A. E., Miller, V. M., Atwood, C. S., et al. (2015). Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS–cognitive and affective study. PLoS Med. 12 (6), e1001833. doi:10.1371/journal.pmed.1001833
Henderson, V. W. (2014). “Alzheimer’s disease: review of hormone therapy trials and implications for treatment and prevention after menopause,”, J. Steroid Biochem. Mol. Biol. 142. 99–106. doi:10.1016/j.jsbmb.2013.05.010
Henderson, V. W., and Rocca, W. A. (2012). “Estrogens Alzheimer Dis. risk Is there a window opportunity?. Neurology. 79. 1840–1841. doi:10.1212/WNL.0b013e318271f88f
Henderson, V. W., and Sherwin, B. B. (2007). Surgical versus natural menopause: cognitive issues. Menopause 14, 572–579. doi:10.1097/gme.0b013e31803df49c
Henderson, V. W., St John, J. A., Hodis, H. N., McCleary, C. A., Stanczyk, F. Z., Shoupe, D., et al. (2016). Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology 87 (7), 699–708. doi:10.1212/WNL.0000000000002980
Hodis, H. N., Mack, W. J., Henderson, V. W., Shoupe, D., Budoff, M. J., Hwang-Levine, J., et al. (2016). Vascular effects of early versus late postmenopausal treatment with estradiol. N. Engl. J. Med. 374 (13), 1221–1231. doi:10.1056/NEJMoa1505241
Jack, C. R., Andrews, J. S., Beach, T. G., Buracchio, T., Dunn, B., Graf, A., et al. (2024). Revised criteria for diagnosis and staging of Alzheimer’s disease: alzheimer’s association workgroup. Alzheimer’s Dementia 20 (8), 5143–5169. doi:10.1002/alz.13859
Jack, C. R., Bennett, D. A., Blennow, K., Carrillo, M. C., Dunn, B., Haeberlein, S. B., et al. (2018). NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14 (4), 535–562. doi:10.1016/j.jalz.2018.02.018
Jett, S., Malviya, N., Schelbaum, E., Jang, G., Jahan, E., Clancy, K., et al. (2022). Endogenous and exogenous estrogen exposures: how women’s reproductive health can drive brain aging and inform alzheimer’s prevention. Front. Aging Neurosci. 14, 831807. doi:10.3389/fnagi.2022.831807
Kantarci, K., Lowe, V. J., Lesnick, T. G., Tosakulwong, N., Bailey, K. R., Fields, J. A., et al. (2016). Early postmenopausal transdermal 17β-Estradiol therapy and Amyloid-β deposition. J. Alzheimer’s Dis. 53 (2), 547–556. doi:10.3233/JAD-160258
Karanth, S., Nelson, P. T., Katsumata, Y., Kryscio, R. J., Schmitt, F. A., Fardo, D. W., et al. (2020). Prevalence and clinical phenotype of quadruple misfolded proteins in older adults. JAMA Neurol. 77 (10), 1299–1307. doi:10.1001/jamaneurol.2020.1741
Kwakowsky, A., Milne, M. R., Waldvogel, H. J., and Faull, R. L. (2016). “Effect of estradiol on neurotrophin receptors in basal forebrain cholinergic neurons: relevance for Alzheimer’s disease,” Int. J. Mol. Sci. 17. doi:10.3390/ijms17122122
Leblanc, E., Chan, MPHB, Heidi, D., and Nelson, M. P. H. (2002). Hormone replacement therapy and cognition: systematic evidence review. doi:10.1001/jama.285.11.1489
Lejri, I., Grimm, A., and Eckert, A. (2018). Mitochondria, estrogen and female brain aging. Front. Aging Neurosci. 10, 124. doi:10.3389/fnagi.2018.00124
Lin, K. A., Choudhury, K. R., Rathakrishnan, B. G., Marks, D. M., Petrella, J. R., Doraiswamy, P. M., et al. (2015). Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimer’s Dementia Transl. Res. Clin. Interventions 1 (2), 103–110. doi:10.1016/j.trci.2015.07.001
Lord, C., Buss, C., Lupien, S. J., and Pruessner, J. C. (2008). Hippocampal volumes are larger in postmenopausal women using estrogen therapy compared to past users, never users and men: a possible window of opportunity effect. Neurobiol. Aging 29 (1), 95–101. doi:10.1016/j.neurobiolaging.2006.09.001
Maggioli, E., McArthur, S., Mauro, C., Kieswich, J., Kusters, D. H. M., Reutelingsperger, C. P. M., et al. (2016). Estrogen protects the blood–brain barrier from inflammation-induced disruption and increased lymphocyte trafficking. Brain Behav. Immun. 51, 212–222. doi:10.1016/j.bbi.2015.08.020
Maki, P. M., and Henderson, V. W. (2012). Hormone therapy, dementia, and cognition: the Women’s health initiative 10 years on. Climacteric 15, 256–262. doi:10.3109/13697137.2012.660613
Marongiu, R., Platholi, J., Park, L., Yu, F., Sommer, G., Woods, C., et al. (2025). Promotion of neuroinflammation in select hippocampal regions in a mouse model of perimenopausal Alzheimer’s disease. Front. Mol. Biosci. 12, 1597130. doi:10.3389/fmolb.2025.1597130
Marrocco, J., and McEwen, B. S. (2016). Sex in the brain: hormones and sex differences. Dialogues Clin. Neurosci. 18 (4), 373–383. doi:10.31887/DCNS.2016.18.4/jmarrocco
McEwen, B., Akama, K., Alves, S., Brake, W. G., Bulloch, K., Lee, S., et al. (2001). Tracking the estrogen receptor in neurons: implications for estrogen-induced synapse formation. Proc. Natl. Acad. Sci. U. S. A. 98 (13), 7093–7100. doi:10.1073/pnas.121146898
McEwen, B. S. (2012). The ever-changing brain: cellular and molecular mechanisms for the effects of stressful experiences. Dev. Neurobiol. 72 (6), 878–890. doi:10.1002/dneu.20968
McEwen, B. S., Akama, K. T., Spencer-Segal, J. L., Milner, T. A., and Waters, E. M. (2012). Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav. Neurosci. 126 (1), 4–16. doi:10.1037/a0026708
Mcewen Harold, B. S., and Milliken, M. (2001). Invited review: estrogens effects on the brain: multiple sites and molecular mechanisms. J. Appl. Physiol. 91, 2785–2801. doi:10.1152/jappl.2001.91.6.2785
McGill Percy, K. C., Liu, Z., and Qi, X. (2025). “Mitochondrial dysfunction in Alzheimer’s disease: guiding the path to targeted therapies,”, Neurotherapeutics 22. doi:10.1016/j.neurot.2025.e00525
McNeill, A. M., Zhang, C., Stanczyk, F. Z., Duckles, S. P., and Krause, D. N. (2002). Estrogen increases endothelial nitric oxide synthase via estrogen receptors in rat cerebral blood vessels: effect preserved after concurrent treatment with medroxyprogesterone acetate or progesterone. Stroke 33 (6), 1685–1691. doi:10.1161/01.str.0000016325.54374.93
Mishra, P., Davies, D. A., and Albensi, B. C. (2023). The interaction between NF-κB and estrogen in alzheimer’s disease. Mol. Neurobiol. 60, 1515–1526. doi:10.1007/s12035-022-03152-3
Mitterling, K. L., Spencer, J. L., Dziedzic, N., Shenoy, S., McCarthy, K., Waters, E. M., et al. (2010). Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J. Comp. Neurology 518 (14), 2729–2743. doi:10.1002/cne.22361
Mor, G., Nilsen, J., Horvath, T., Bechmann, I., Brown, S., Garcia-Segura, L. M., et al. (1999). Estrogen and microglia: a regulatory system that affects the brain. J. Neurobiol. 40, 484–496. doi:10.1002/(sici)1097-4695(19990915)40:4<484::aid-neu6>3.0.co;2-c
Mš, G., Mikuš, M., Ferrari, F. A., Bosco, M., Uccella, S., Noventa, M., et al. (2023). Effects of transdermal versus oral hormone replacement therapy in postmenopause: a systematic review. Arch. Gynecol. Obstet. 307 (6), 1727–1745. doi:10.1007/s00404-022-06647-5
Nerattini, M., Jett, S., Andy, C., Carlton, C., Zarate, C., Boneu, C., et al. (2023). Systematic review and meta-analysis of the effects of menopause hormone therapy on risk of Alzheimer’s disease and dementia. Front. Aging Neurosci. 15, 1260427, doi:10.3389/fnagi.2023.1260427
Newhouse, P., and Dumas, J. (2015). Estrogen-cholinergic interactions: implications for cognitive aging. Horm. Behav. 74, 173–185. doi:10.1016/j.yhbeh.2015.06.022
Platholi, J., Marongiu, R., Park, L., Yu, F., Sommer, G., Weinberger, R., et al. (2023). Hippocampal glial inflammatory markers are differentially altered in a novel mouse model of perimenopausal cerebral amyloid angiopathy. Front. Aging Neurosci. 15, 1280218. doi:10.3389/fnagi.2023.1280218
Riedel, B. C., Thompson, P. M., and Brinton, R. D. (2016). “Age, APOE and sex: triad of risk of Alzheimer’s disease,”, J. Steroid Biochem. Mol. Biol. 160. 134–147. doi:10.1016/j.jsbmb.2016.03.012
Ross, J. A., and Van Bockstaele, E. J. (2021). The locus Coeruleus- norepinephrine system in stress and arousal: unraveling historical. Curr. Future Perspect. Front. Psychiatry. doi:10.3389/fpsyt.2020.601519
Russell, J. K., Jones, C. K., and Newhouse, P. A. (2019). The role of estrogen in brain and cognitive aging. Neurotherapeutics 16, 649–665. doi:10.1007/s13311-019-00766-9
Saleh, R. N. M., Hornberger, M., Ritchie, C. W., and Minihane, A. M. (2023). Hormone replacement therapy is associated with improved cognition and larger brain volumes in at-risk APOE4 women: results from the european prevention of Alzheimer’s Disease (EPAD) cohort. Alzheimers Res. Ther. 15 (1), 10. doi:10.1186/s13195-022-01121-5
Shanmugan, S., and Epperson, C. N. (2014). Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum. Brain Mapp. 35, 847–865. doi:10.1002/hbm.22218
Shao, H., Breitner, J. C. S., Whitmer, R. A., Wang, J., Hayden, K., Wengreen, H., et al. (2012). Hormone therapy and Alzheimer disease dementia new findings from the cache county study. Neurology 79, 1846–1852. doi:10.1212/WNL.0b013e318271f823
Sherwin, B. B. (2009). Estrogen therapy: is time of initiation critical for neuroprotection? Vol. 5. Nat. Rev. Endocrinol. 5, 620–627. doi:10.1038/nrendo.2009.193
Shumaker, S. A., Legault, C., Kuller, L., Rapp, S. R., Thal, L., Lane, D. S., et al. (2004). Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women women’s health initiative memory study. doi:10.1001/jama.291.24.2947
Simpkins, J. W., Perez, E., Wang, X., Yang, S., Wen, Y., and Singh, M. (2009). The potential for estrogens in preventing alzheimer's disease and vascular dementia. Ther. Adv. Neurological Disord. 2, 31–49. doi:10.1177/1756285608100427
Smejkalova, T., and Woolley, C. S. (2010). Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J. Neurosci. 30 (48), 16137–16148. doi:10.1523/JNEUROSCI.4161-10.2010
Spina, S., La Joie, R., Petersen, C., Nolan, A. L., Cuevas, D., Cosme, C., et al. (2021). Comorbid neuropathological diagnoses in early versus late-onset alzheimer’s disease. Brain 144 (7), 2186–2198. doi:10.1093/brain/awab099
Stanczyk, F. Z., Hapgood, J. P., Winer, S., and Mishell, Jr (2013). Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr. Rev. 34 (2), 171–208. doi:10.1210/er.2012-1008
Taxier, L. R., Philippi, S. M., Fleischer, A. W., York, J. M., LaDu, M. J., and Frick, K. M. (2022a). APOE4 homozygote females are resistant to the beneficial effects of 17β-estradiol on memory and CA1 dendritic spine density in the EFAD mouse model of Alzheimer’s disease. Neurobiol. Aging 118, 13–24. doi:10.1016/j.neurobiolaging.2022.06.005
Taxier, L. R., Philippi, S. M., York, J. M., LaDu, M. J., and Frick, K. M. (2022b). The detrimental effects of APOE4 on risk for Alzheimer’s disease May result from altered dendritic spine density, synaptic proteins, and estrogen receptor alpha. Neurobiol. Aging 112, 74–86. doi:10.1016/j.neurobiolaging.2021.12.006
Toran-Allerand, D., Singh, M., and Sétá, G. (1999). Novel mechanisms of estrogen action in the brain: new players in an old story. Front. Neuroendocrinol. 20, 97–121. doi:10.1006/frne.1999.0177
Velarde, M. C. (2013). Pleiotropic actions of estrogen: a mitochondrial matter. Physiol. Genomics 45, 106–109. doi:10.1152/physiolgenomics.00155.2012
Vila-Castelar, C., Udeh-Momoh, C., Aggarwal, N. T., and Mielke, M. M. (2023). “Sex and gender considerations in dementia: a call for global research,”, Nat. Aging 3, 463–465. doi:10.1038/s43587-023-00374-5
Villa, A., Vegeto, E., Poletti, A., and Maggi, A. (2016). Estrogens, neuroinflammation, and neurodegeneration. Endocr. Rev. 37, 372–402. doi:10.1210/er.2016-1007
Villain, N., and Paretsky, M. S. (2024). The great debate in diagnosing alzheimer disease: more than just a β test. Neurology 103, e209836. doi:10.1212/WNL.0000000000209836
Wang, Y., and Brinton, R. D. (2016). Triad of risk for late onset alzheimer’s: mitochondrial haplotype, apoe genotype and chromosomal sex. Front. Aging Neurosci. 8, 232. doi:10.3389/fnagi.2016.00232
Wang, Y. T., Therriault, J., Servaes, S., Tissot, C., Rahmouni, N., Macedo, A. C., et al. (2024b). Sex-specific modulation of amyloid-β on tau phosphorylation underlies faster tangle accumulation in females. Brain 147 (4), 1497–1510. doi:10.1093/brain/awad397
Wang, Y. T., Therriault, J., Tissot, C., Servaes, S., Rahmouni, N., Macedo, A. C., et al. (2024a). Hormone therapy is associated with lower Alzheimer’s disease tau biomarkers in post-menopausal females -evidence from two independent cohorts. Alzheimers Res. Ther. 16 (1), 162. doi:10.1186/s13195-024-01509-5
Weber, M. T., Maki, P. M., and McDermott, M. P. (2014). Cognition and mood in perimenopause: a systematic review and meta-analysis. J. Steroid Biochem. Mol. Biol. 142, 90–98. doi:10.1016/j.jsbmb.2013.06.001
Wharton, W., Baker, L. D., Gleason, C. E., Dowling, M., Barnet, J. H., Johnson, S., et al. (2011). Short-term hormone therapy with transdermal estradiol improves cognition for postmenopausal women with Alzheimer’s disease: results of a randomized controlled trial. J. Alzheimer’s Dis. 26 (3), 495–505. doi:10.3233/JAD-2011-110341
Keywords: APOE genotype and dementia risk in women, precision medicine in women’s cognitive aging, hormone therapy and brain health, menopause related cognitive impairment, estrogen and Alzheimer’s disease
Citation: Mervosh N and Devi G (2025) Estrogen, menopause, and Alzheimer’s disease: understanding the link to cognitive decline in women. Front. Mol. Biosci. 12:1634302. doi: 10.3389/fmolb.2025.1634302
Received: 24 May 2025; Accepted: 16 June 2025;
Published: 30 June 2025.
Edited by:
Roberta Marongiu, Cornell University, United StatesReviewed by:
Teresa A. Milner, Weill Cornell Medicine, United StatesCopyright © 2025 Mervosh and Devi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gayatri Devi, Z2RAbnlicmFpbi5vcmc=
 Nicholas Mervosh1
Nicholas Mervosh1 Gayatri Devi
Gayatri Devi