- 1Department of Medical Laboratory Sciences, College of Applied Medical Sciences, University of Hail, Hail, Saudi Arabia
- 2Department of Pharmacology, College of Pharmacy, University of Hail, Hail, Saudi Arabia
- 3Department of Biochemistry, SKS Hospital Medical College and Research Centre, Mathura, India
- 4Department of Diagnostic Radiology, College of Applied Medical Science, University of Hail, Hail, Saudi Arabia
- 5Department of Epidemic Disease Research, Institute for Research and Medical Consultations (IRMC), Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
- 6Applied Sciences and Humanities Section, Department of Chemistry, The University Polytechnic, Aligarh Muslim University, Aligarh, India
- 7Department of Internal Medicine, College of Medicine, University of Hail, Hail, Saudi Arabia
- 8Department of Biophysics, Institute for Research and Medical Consultations, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
- 9University Centre for Research and Development, Chandigarh University, Mohali, Punjab, India
- 10Department of Biotechnology and Life Sciences, Institute of Biomedical Education and Research, Mangalayatan University, Beswan, India
The nonenzymatic glycation process initiates a harmful cycle that generates various intermediate compounds, leading to carbonyl and oxidative stress, and ultimately resulting in the formation of advanced glycation end products (AGEs). AGEs have been implicated as key factors in the development of several diseases, including neurodegenerative conditions like Parkinson’s and Alzheimer’s diseases, as well as complications associated with diabetes. Given the potential impact of AGEs on these diseases, this study explores the role of copper ferrite nanoparticles (CuFe2O4NPs) in inhibiting the formation of these harmful intermediates and AGEs. Magnetic CuFe2O4 nanoparticles were synthesized using Aloe vera leaf extracts and their effects on AGE formation were assessed. Using both biophysical and biochemical approaches, the study demonstrates that CuFe2O4 NPs have significant anti-glycation properties, which help reduce or prevent AGE formation while maintaining protein structure. These findings suggest that CuFe2O4 NPs may offer therapeutic potential in addressing AGE-related diseases, particularly those linked to diabetes and its complications.
Introduction
Over the last three to four decades, studies have revealed the involvement of AGEs (advanced glycation end products) in human pathophysiology. AGEs are the products of a non-enzymatic glycation reaction, also known as the Maillard reaction (Monnier and Cerami, 1981). Normally, AGEs form under physiological conditions and during natural aging. However, their formation is accelerated in disease conditions, and high levels of AGEs have been reported in various diseases such as diabetes (Van Eupen et al., 2013), atherosclerosis (Hanssen et al., 2014), and obesity (Gaens et al., 2014). AGEs have been recognized as potential contributors to several diseases, including age-related neurodegenerative diseases like Parkinson’s and Alzheimer’s disease (Ahmed et al., 2005; Dalfo et al., 2005), as well as diabetic complications (Garay-Sevilla et al., 2005). The glycation reaction is also associated with oxidative stress, which generates species involved in the onset of several diseases, including neurodegenerative disorders (Dalfo et al., 2005; Garay-Sevilla et al., 2005; Leszek et al., 2016; Khan et al., 2016).
Glycation of macromolecules is not restricted to reducing sugars; non-sugar entities such as dicarbonyls have also been reported as potential glycating agents (Ashraf et al., 2015). Dicarbonyl compounds, such as methylglyoxal (MG), glyoxal, and 3-deoxyglucosone (3-DG), are naturally generated through various pathways, including the Maillard reaction, sugar auto-oxidation, amino acid metabolism, and lipid peroxidation (Chetyrkin et al., 2008). These highly reactive compounds have the ability to glycate molecules even at minimal concentrations (Thornalley, 1998). The intracellular accumulation of MG is particularly toxic due to its strong glycation properties (Allaman et al., 2015).
In recent years, systematic studies have been conducted to identify pharmacological agents that may reduce or inhibit AGE formation. Several potential inhibitors or anti-glycating agents, including aspirin, steroidal compounds, and aminoguanidine, have shown promise in reducing AGEs formation in neurological diseases (Reddy and Beyaz, 2006; Webster et al., 2005; Jaturapatporn et al., 2012). Additionally, many phytochemical compounds, such as phenols and flavonoids (Albarracin et al., 2012; Sutachan et al., 2012), as well as herbal drugs, have been identified as AGE inhibitors (Dey et al., 2017; Natarajan et al., 2013). However, despite their anti-glycating properties, some compounds have been discontinued due to side effects. For example, although aminoguanidine demonstrated anti-AGE activity, its use was prohibited after phase III clinical trials revealed adverse effects (Ahmed et al., 1986). As a result, finding safe and effective anti-glycating agents to counter AGE formation has become a significant challenge for scientists.
Nanotechnology offers a potential solution to various technological and medical challenges. Nanoparticles are small nano-sized inorganic particles that exhibit unique physical and chemical properties. They exhibit excellent biocompatibility, flexible conjugation with biomolecules (Nikalje, 2015), and the capability to interact both at the cellular surface and within cells, owing to their small size, which is approximately 100 to 10,000 times smaller than human cells. Nanoparticles can easily enter living cells via endocytosis because of their resemblance to cellular components (Leszek et al., 2017), making them useful in a wide range of physical, biological, biomedical, and pharmaceutical fields (Loureiro et al., 2016; Treuel et al., 2013).
Spinel ferrite nanoparticles (SFNPs) have recently garnered attention due to their superparamagnetic properties at the nanoscale, chemical stability, simple composition, and broad applications. Extensive research has been conducted on SFNPs with the general formula MFe2O4, which typically consists of iron oxide and one or more transition metals (Masunga et al., 2019). SFNPs are being used in biomedicine for cancer cell detection, magnetic hyperthermia for tumor diagnosis and treatment, magnetic drug delivery, cellular signaling, magnetic resonance imaging, magnetic recording, water and wastewater treatment, magneto-optical devices, ferromagnetic fluids, energy storage, magnetic separation, and biosensors, among other applications (Ansari et al., 2018; Kuznetsov et al., 2013; Srinivasan et al., 2018). Among various SFNPs, copper ferrite nanoparticles (CuFe2O4 NPs) have attracted significant interest due to their wide range of applications. These include their use as antibacterial agents (Ansari et al., 2018; Liu et al., 2019), catalysts in nanomedicine for treating breast cancer, magnetic resonance imaging materials, photocatalysts, photoanodes for solar water oxidation, catalysts, enzyme immobilization supports, and in water treatment and purification (Masunga et al., 2019). Certain nanoparticles, such as silver, gold, selenium, and ZnO NPs, have shown both anti-glycative and antioxidative activities (Ansari et al., 2016; Ashraf et al., 2018; Kim et al., 2012; Ashraf et al., 2014; Kim et al., 2012).
Given the hazardous role of AGEs in disease development, we are the first to explore the anti-glycative activity of magnetic CuFe2O4 NPs. This study presents the anti-glycation potential of biologically synthesized copper ferrite nanoparticles (CuFe2O4 NPs). The goal is to establish a safer and more effective therapeutic approach for AGE-related diseases.
This study presents an innovative approach by exploring the anti-glycation potential of biologically synthesized magnetic copper ferrite nanoparticles (CuFe2O4 NPs) for the first time. While AGEs (advanced glycation end-products) have been identified as contributors to various pathologies, including neurodegenerative diseases and diabetes-related complications, current pharmacological interventions face limitations due to side effects and inefficacy. Unlike traditional anti-glycation compounds, the unique physical and chemical properties of CuFe2O4 NPs—such as their superparamagnetic behaviour and compatibility with biological systems—could provide enhanced therapeutic effects. Furthermore, by leveraging nanotechnology, this study aims to overcome the challenges of safe and efficient AGE inhibition, positioning CuFe2O4 NPs as promising candidates for antiglycation therapy.
Material and methods
Materials
Preparation of the aqueous leaf extract of aloe vera
In this study, copper ferrite nanoparticles (CuFe2O4 NPs) were biosynthesized using Aloe vera leaf extract (Ashraf et al., 2018). Fresh, healthy Aloe vera leaves were collected, thoroughly washed with tap water, and then with double-distilled water to remove any impurities. About 25 g of the sterilized, chopped leaves were heated at 100°C in 100 mL of sterilized water for 10–15 min. The extract was centrifuged twice, and the supernatant was filtered through a 0.45 µm filter and stored at 4°C for further use in the synthesis of CuFe2O4 NPs. Millipore water was used as a blank to adjust the baseline, and the Aloe vera leaf extract was prepared as described in the materials and methods section (Ashraf et al., 2018).
Preparation of CuFe2O4 NPs:
For the biosynthesis of CuFe2O4 NPs, Cu(NO3)2·3H2O and Fe(NO3)3·9H2O were used as precursors. A 0.1 M solution of Cu(NO3)2·3H2O and a 0.2 M solution of Fe(NO3)3·9H2O were prepared by dissolving them in sterilized water to form a clear solution. Subsequently, 25 mL of Aloe vera extract was added to 75 mL of the Cu(NO3)2·3H2O and Fe(NO3)3·9H2O solution. The reaction mixture was stirred at 100°C for 10 h, resulting in a brown semi-solid gel. This gel was washed three times with hot water and twice with ethyl alcohol. The pellet was dried in an oven at 200°C for 6 h to obtain the CuFe2O4 nanoparticles.
Characterization of biosynthesized CuFe2O4 NPs
The biosynthesized CuFe2O4 NPs were characterized using UV-Vis spectroscopy (Shimadzu UV-1800) for preliminary analysis. The sample was placed in a quartz cuvette, and absorption spectra were recorded in the 200–800 nm range (Ansari et al., 2023; Anandan et al., 2019). FTIR (Shimadzu IRSpirit, Shimadzu, Kyoto, Japan) was used to assess the functional groups of the nanoparticles in transmittance mode with KBr pellets in the 4,000–400 cm-1 range (Ansari et al., 2023; Anandan et al., 2019). The morphology and size of the NPs were analyzed using SEM (TESCAN, VEG3, Berno-Czech Republic) at an acceleration voltage of 20 kV and TEM (FEI Morgagni 268, Czech Republic) at 200 kV (Ansari et al., 2023; Ansari and Alomary, 2024). SEM and TEM images were captured after applying a small amount of the NP solution to aluminum stubs and carbon-coated copper grids, respectively. Additionally, XRD analysis was performed using an X-ray powder diffractometer (Shimadzu XRD-7000) with CuKα radiation (λ = 1.54056) in the 2θ range of 20°–80° at 40 keV, following the protocol described in previous studies (Ashraf et al., 2018; Ansari and Alomary, 2024).
BSA in vitro glycation assay
Bovine serum albumin (BSA), methylglyoxal, and CuFe2O4 nanoparticles were used in this anti-glycation assay with slight modifications from the original method (Ashraf et al., 2018). The reaction mixture consisted of BSA (333 μg/mL), methylglyoxal (10 mM), aminoguanidine (2 mM) and 10 mM PBS (pH 7.4) with 1 mM sodium azide, along with varying concentrations of CuFe2O4 NPs (50 μg/mL, 100 μg/mL). Native BSA (without methylglyoxal or CuFe2O4 NPs) was used as the control. The mixtures were incubated at 37°C for 6 days. After incubation, the samples were dialyzed against sodium phosphate buffer at 4°C for 48 h to remove unbound methylglyoxal and CuFe2O4 NPs. The dialyzed samples were diluted in the same buffer for further analysis unless otherwise specified.
Absorbance spectroscopy:
The UV-visible absorption spectra of native, glycated, and NP-treated BSA samples were recorded using a UV-visible spectrophotometer (Labman) between 200 and 600 nm in a 1 cm quartz cuvette. Absorbance was measured at 280 nm (Ashraf et al., 2018).
Determination of amadori products
Amadori products/ketoamine moieties formed in native, glycated, and NP-treated BSA samples were determined using the NBT reduction assay, with slight modifications as described previously (Johnson et al., 1982). A 20 µL protein sample was mixed with 180 µL of 100 mM sodium carbonate-bicarbonate buffer (pH 10.8) containing 0.2 mM nitro blue tetrazolium (NBT) and incubated at 37°C for 25 min. Absorbance was measured at 525 nm using the same buffer as the blank. The ketoamine moiety concentration (nm/mL) was calculated by multiplying the absorbance by the molar extinction coefficient of 12,640 M-1 cm-1 for monoformazon.
Spectrofluorimetric analysis
AGE-specific fluorescence of samples was measured using an Agilent Cary Eclipse fluorescence spectrophotometer (G9550-64000). The samples were excited at 370 nm, and emission spectra were recorded at 450 nm with both slits set to 5 nm and a 1 cm path length. Fluorescence emission was calculated using the formula:
where Fg, Fn, and Fh represent the fluorescence intensities of glycated BSA, NPs-treated samples, and native BSA, respectively.
Intrinsic fluorescence measurements
Structural characteristics of the samples were studied using intrinsic fluorescence measurement. Native, glycated, and NP-treated samples were excited at 295 nm (specific for tryptophan residues), and the emission spectra were recorded from 300–600 nm (Ashraf et al., 2018).
HMF, CC, Free Lysine & Arginine, ANS, and ThT Assays:
The hydroxymethylfurfural (HMF) content, an early-stage glycation product, was quantified using the thiobarbituric acid assay (Ahmad et al., 2012). Carbonyl content (CC) in native, glycated, and NP-treated samples was measured using 2,4-dinitrophenylhydrazine (DNPH), as described with slight modifications (Levine et al., 1994). Free lysine modifications were assessed using the 2,4,6-trinitrobenzenesulfonic acid (TNBS) method (Sashidhar et al., 1994). Free arginine content was measured using the Phenanthroquinone method (Smith and MacQuarrie, 1978), and hydrophobicity changes were analyzed through ANS binding, excited at 380 nm (Hu et al., 2008). For aggregation studies, Thioflavin T (ThT) was used as described in previous research (Khurana et al., 2005).
Statistical analysis
All data were presented as mean ± standard deviation (SD) in triplicates (n = 3) using the XL. STAT 7.0. One-Way ANOVA using Dunnet’s’ post hoc test. Values were considered statistically significant when p < 0.01.
Results
Characterization of CuFe2O4 NPs
UV-Vis spectrum of the biosynthesized CuFe2O4 NPs
The aqueous extract of aloe vera leaves was utilized in this investigation for the purpose of synthesizing CuFe2O4 nanoparticles. The UV-Vis spectrum of the biosynthesized CuFe2O4 NPs reveals a prominent peak at 238 nm, indicating the successful formation of ferrite nanoparticles (Figure 1A).
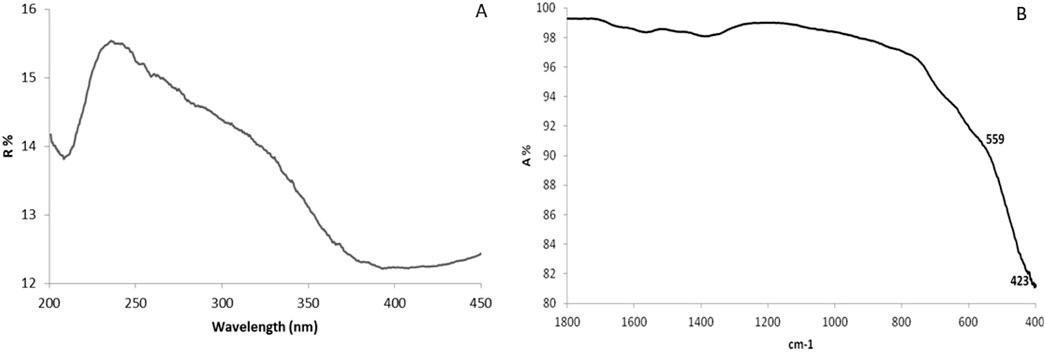
Figure 1. UV-Vis spectroscopy (A) and (B) FTIR spectra of CuFe2O4 NPs synthesized by Aloe vera leaf extract.
FT-IR spectra of the synthesized CuFe2O4 NPs
The FT-IR spectra of the synthesized CuFe2O4 NPs, shown in Figure 1B, were measured in the range of 400–4,000 cm-1, display two distinct absorption ranges between 400 and 600 cm-1, with peaks at ν1 (566 cm-1) and ν2 (431 cm-1), corresponding to the spinel structure of the ferrite nanoparticles. The ν1 peak reflects the Fe–O stretching vibration at the tetrahedral site, while ν2 is attributed to Cu–O stretching at the octahedral site.
Investigation of morphology of synthesized ferrite NPs by SEM and TEM
The size and shape of the synthesized nanoparticles were further confirmed through SEM and TEM analysis (Figures 2A–C). The SEM image shows that the NPs have an irregular, spinel spherical shape (Figure 2A). The TEM image reveals spherical particles, with the size distribution analyzed using ImageJ software showing an average particle size of 3.75 ± 2.21 nm (Figure 2B), which aligns with the XRD results (Figure 3).
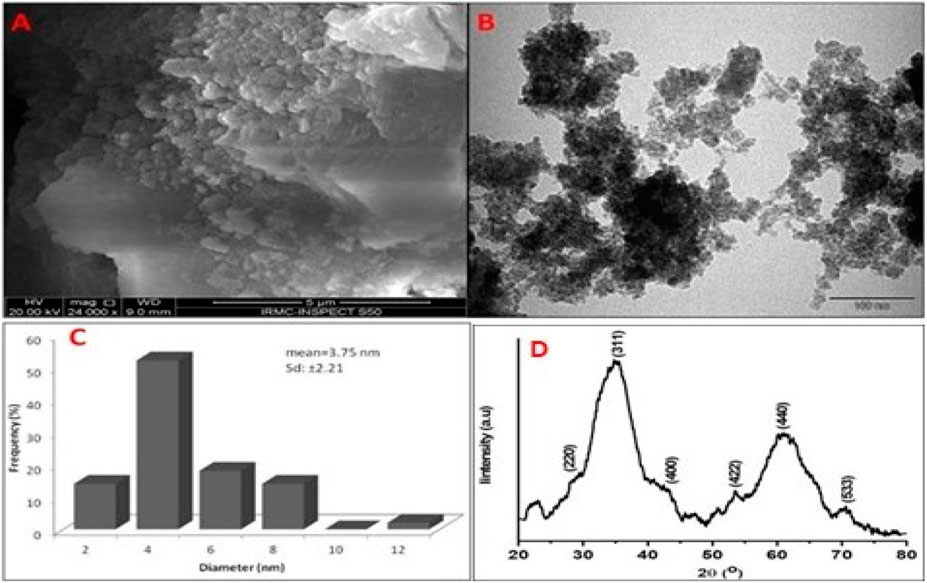
Figure 2. (A) SEM, (B) TEM, (C) histogram and (D) XRD pattern of CuFe2O4 NPs synthesized by Aloe vera leaf extract.
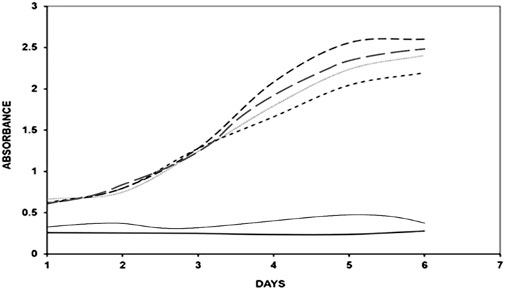
Figure 3. UV absorption spectra of native BSA (






XRD analysis of synthesized ferrite NPs
Further, the crystalline size and nature of synthesized CuFe2O4 NPs was analyzed by XRD method. Figure 2D illustrates the X-ray diffraction pattern of the CuFe2O4 nanoparticles. The XRD analysis indicates the presence of distinct peaks at 220, 311, 222, 400, 422, 511, and 440, corresponding to the cubic phase of CuFe2O4 NPs (JCPDS Card no. 75–1517).
UV profile of MG-Modified BSA with CuFe2O4 NPs
Pilot experiments were conducted to determine the optimal incubation time and concentration of BSA, methylglyoxal, aminoguanidine and Aloe vera leaf extract, with or without varying concentrations of CuFe2O4 NPs, over different time intervals (0–6 days) at 37°C. The UV-Vis absorbance of native BSA was measured at 280 nm. The absorbance (hyperchromicity) increased significantly at 280 nm after BSA glycation with methylglyoxal, compared to native BSA, while the NPs-treated BSA-methylglyoxal mixtures showed a reduction in hyperchromicity compared to the native protein (Figure 3). The hyperchromicity of glycated protein was 89% when compared to native protein, and the hyperchromicity of glycated samples was reduced to 8% and 17% respectively, after treatment of NPs (50 μg/mL and 100 μg/mL). Therefore, 100 µg/mL of NPs was selected for further studies. Aminoguanidine was taken as positive control. It showed 10% higher hyperchromicity as compared to that of CuFe2O4 NPs treated sample.
Determination of arginine and lysine by 2,4,6-trinitrobenzenesulfonic acid (TNBS) and phenanthroquinone
To assess the free amino groups of lysine and arginine residues in the samples, 2,4,6-trinitrobenzenesulfonic acid (TNBS) and phenanthroquinone were employed, respectively. After 6 days of BSA glycation, a 66% decrease in the availability of free lysine amino groups was observed compared to native BSA (Figure 4A). However, the availability of NH2 groups increased by 11% after treating the glycated BSA with 100 μg/mL of NPs. Similarly, glycation led to a 26% reduction in the availability of free arginine amino groups compared to native BSA (Figure 4B). The availability of NH2 groups increased by 6% in the NPs (100 μg/mL) treated sample.
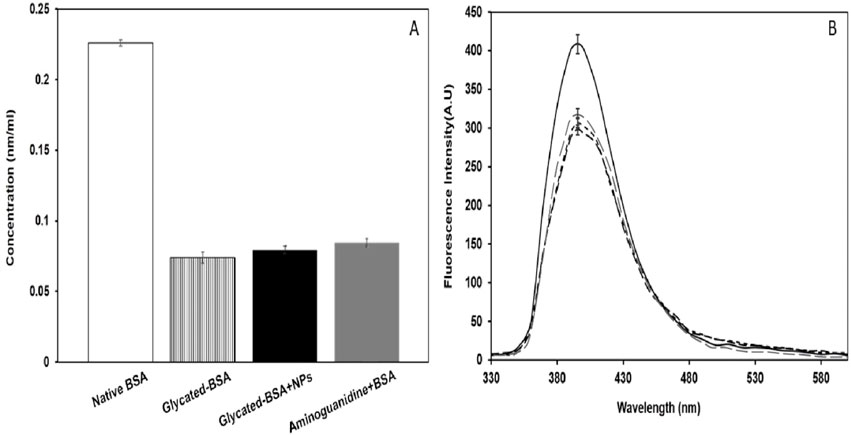
Figure 4. (A) Free lysine estimation for native BSA (








Nitroblue tetrazolium test (NBT) for ketoamine/early glycation products detection
NBT reduction assay is particularly for ketoamine/early glycation products detection, not for AGEs (Alouffi et al., 2022). Native BSA alone showed negligible amounts of ketoamine contents (14.5 nmol/mL) as compared to glycated BSA (35.8 nmol/mL) on the 3rd day of incubation (Figure 5A). Whereas, glycated sample treated with NPs (100 µg/mL) showed marked decrease in ketoamine contents (19.1 nmol/mL) as compared to that of other samples.
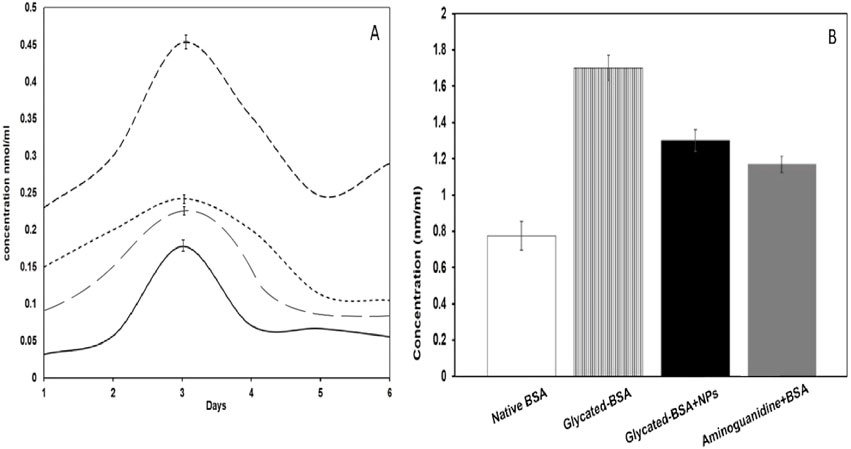
Figure 5. (A) NBT reduction assay of native BSA (








Analysis of hydroxymethylfurfural (HMF) by thiobarbituric acid assay
Hydroxymethylfurfural (HMF) is formed during early glycation reaction. Thiobarbituric acid assay was applied to investigate the HMF level in samples. The HMF contents in glycated BSA was high (1.7 ± 0.7 nmol/mL) as compared to that of control (0.77 ± 0.8 nmol/mL) (Figure 5B). The decline in HMF contents (1.3 ± 0.6 nmol/mL) was recorded in NPs (100 µg/mL) treated sample. CuFe2O4 NPs treated sample showed up to 23% decrease in HMF contents when compared to glycated BSA (Figure 7B).
Measurement of carbonyl content
The statistical significance of the results is evident from the observed differences in carbonyl content between the various groups. Methylglyoxal-glycated BSA showed a significant increase in carbonyl content (90.36 ± 1.5 nmol/mg) compared to native BSA (66.33 ± 0.8 nmol/mg), indicating a marked increase in protein oxidation due to glycation (Figure 6A). In contrast, treatment with CuFe2O4 NPs significantly reduced the carbonyl content to 55.82 ± 1.6 nmol/mg.
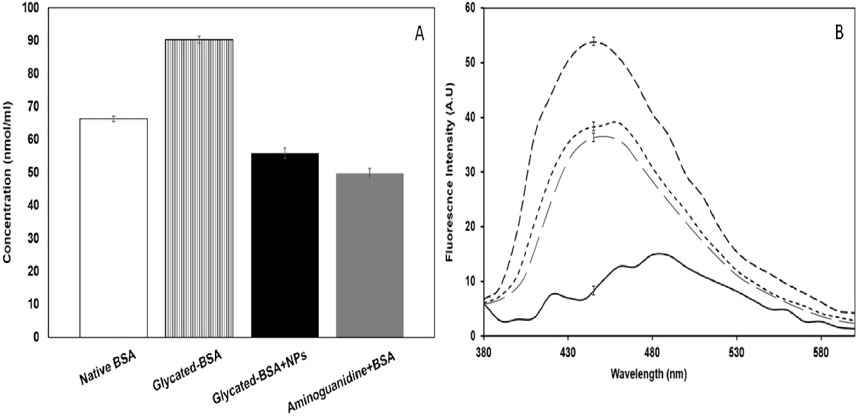
Figure 6. (A) Showing carbonyl content (cc) in native BSA (







Fluorescence studies of MG-Modified BSA with CuFe2O4 NPs
In the present study, the glycated sample displayed a significant increase in fluorescence intensity at around 450 nm, indicative of the formation of fluorophoric AGEs, which agrees with earlier reports (Alouffi et al., 2022; Ashraf et al., 2015). The fluorescence results from this study are in line with previous findings, confirming that AGEs exhibit fluorescence at specific excitation/emission wavelengths. The 28% reduction in fluorescence intensity upon treatment with CuFe2O4 NPs (100 μg/mL) suggests a strong inhibitory effect on AGE formation (Figure 6B).
Thioflavin T (ThT) fluorescence studies
Thioflavin T (ThT) fluorescence is used to detect protein aggregation. Confirmed that glycation induced significant aggregation in BSA, as evidenced by a 92.72% increase in fluorescence intensity (Figure 7A). However, the treated sample exhibited a 40.89% reduction in fluorescence intensity.
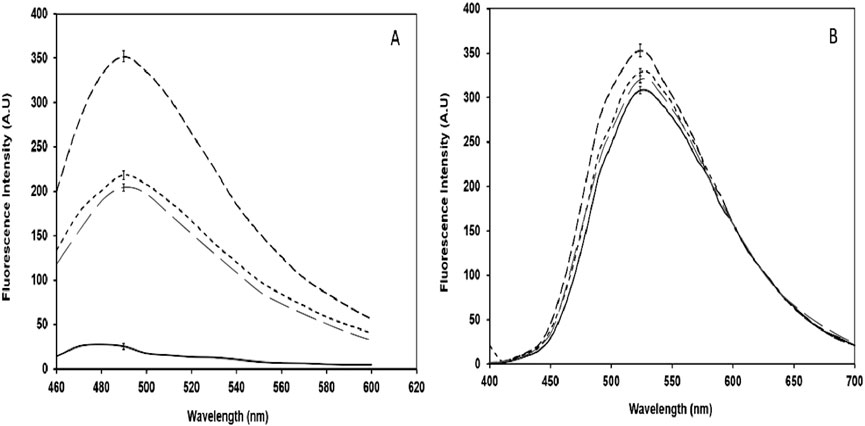
Figure 7. (A) ThT emission spectra of native BSA (








Analysis of structural change in samples by ANS dye
The ANS dye is used for the study of structural alteration in the proteins caused by damaging agents. The increase in ANS fluorescence in the glycated sample was observed while decrease in fluorescence intensity was recorded in the sample treated with CuFe2O4 NPs (Figure 7B).
Structural analysis of protein by fluorescence spectroscopy
Fluorescence spectroscopy is used to measure the intrinsic fluorescence of tryptophan in proteins. A radical decrease in fluorescence intensity in the glycated sample was observed (Figure 8). In contrast to that the NPs-treated sample showed that an 8% increase in fluorescence intensity.
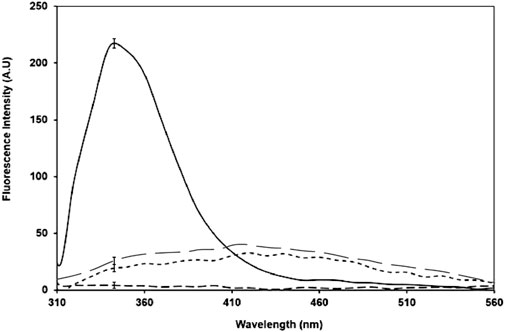
Figure 8. Intrinsic fluorescence for native BSA (




Discussion
The first confirmation of the successful synthesis of CuFe2O4 NPs using green approach came via UV-Vis spectroscopy. The production of the ferrite NPs was identified by the distinctive peak in the range of 230–500 nm (Mushtaq et al., 2017). In their UV-vis absorbance spectra, metal nanoparticles typically exhibit a distinctive plasmon resonance band because of the combined oscillation of their conducting electrons (Mushtaq et al., 2017).
FTIR analysis of ferrite nanoparticles exhibit two absorption peaks at v1 (566 cm-1) and v2 (431 cm-1), indicating their spinel structure. The v1 peak points to Fe–O stretching at the tetrahedral site, while ν2 represents Cu–O stretching at the site of the octahedral (Salavati-Niasari et al., 2012; Zekri and Fareghi-Alamdari, 2016). Our findings are consistent with those of Ramazani et al. (2017), who reported Fe–O and Cu–O bond stretching at 572 and 421 cm-1, respectively, in CuFe2O4 nanocrystals synthesized through a green method using tragacanth gum as a biotemplate. In contrast, another study using a thermal treatment method reported Fe–O and Cu–O bond stretching at 533 and 315 cm-1, respectively (Zakiyah et al., 2015). A broad absorption at 3,358 cm-1 reflects the stretching of OH groups, indicative of phytocompounds such as alcohols, phenols, acids, and their derivatives (Abbasi et al., 2020). The 2,951 cm-1 peak corresponds to symmetrical and asymmetrical C–H stretching in aliphatic groups, while the peaks at 1,638 cm-1 and 1,500 cm-1 represent the carbonyl C=O (primary amide) and carboxylate (-COO-) groups, respectively, in Aloe vera extract. The IR peaks at 1,414 cm-1 and 1,233 cm-1 correspond to the CH3 group of primary aromatic amines and C–O–C stretching of–COCH3 groups, respectively (Lim and Cheong, 2015). The 1,083 cm-1 peak is associated with C=C bonds in unsaturated five- or six-membered ring compounds (Abbasi et al., 2020), while the peaks at 827, 750, 659, and 566 cm-1 correspond to aromatic CH out-of-plane deformation (Benalia et al., 2022). These FTIR results suggest that phenols, proteins, and sterol phytocompounds in Aloe vera leaf extract may serve as reducing agents responsible for the formation of nanoparticles.
The shape and size of the biosynthesized nanoparticles were studied using SEM and TEM. Through TEM investigation, it was determined that the particles were spherical in shape, with an average particle size of 3.75 ± 2.21 nm. Zakiyah et al. (2015) (Ramazani et al., 2017) reported a particle size of 24–34 nm for NPs synthesized via the hydrothermal co-precipitation method. In another study, CuFe2O4 nanoparticles produced using Nasturtium officinale extract exhibited a particle size of 15–50 nm (Khan et al., 2024).
According to the XRD study, there are distinctive peaks at 220, 311, 222, 400, 422, 511, and 440 that represent the cubic phase of CuFe2O4 NPs. These reflection peaks align well with those of CuFe2O4 NPs synthesized by the green method using tragacanth gum (Zekri and Fareghi-Alamdari, 2016) and the hydrothermal co-precipitation method (Ramazani et al., 2017). The average particle size, determined using Scherrer’s formula from the FWHM of the (311) peak, was found to be 1.16 nm. Ramazani et al. (2017) (Zekri and Fareghi-Alamdari, 2016) reported an average particle size of 14 nm for CuFe2O4 NPs synthesized using tragacanth gum.
It is well known that protein glycation leads to the accumulation of advanced glycation end-products (AGEs) and oxidative stress, which disrupt the structure and function of proteins in various diseases (Jasim et al., 2022; Ahmad et al., 2024). Given the involvement of AGEs and oxidative stress in disease pathology, it is crucial to explore novel agents capable of inhibiting the glycation process and slowing AGE formation.
The UV analysis of the sample treated with NPs demonstrated the reduced hyperchromicity suggests inhibitory action of NPs on glycation reaction that corroborates with previous studies showing the inhibitory strength of various nanoparticles (Ansari et al., 2016; Ashraf et al., 2018; Kim et al., 2012; Ashraf et al., 2014; Kim et al., 2012). The increase in the absorbance or hyperchromicity may be due to exposure of aromatic amino acids resulting from unfolding of protein upon glycation (Alouffi et al., 2022).
It is well-established that the free amino groups of lysine and arginine are the primary sites of glycation in proteins (Allaman et al., 2015; Kim et al., 2012; Ashraf et al., 2015). The availability of lysine and arginine residues in presence of CuFe2O4 NPs, compared to without of CuFe2O4 NPs indicates anti-glycation potential of CuFe2O4 NPs. This may be caused by reduction of the interaction between residues lysine and arginine with methylglyoxal, thereby inhibiting the glycation reaction at an early stage, as demonstrated in other studies (Ansari et al., 2016; Ashraf et al., 2018; Ashraf et al., 2014).
The declined nitroblue tetrazolium (NBT) reduction is due to formation of lesser amount of ketoamines in presence of CuFe2O4 NPs as compared to glycated sample, indicating disturbance of reaction between BSA and methylglyoxal/early glycation reaction is due to interference of CuFe2O4 NPs. Since numerous studies have suggested that the reduction of NB could result from the formation of superoxide radicals and the degradation byproducts of ketoamines. The presence of ketoamines serves as an indicator of the early stages of the glycation process and is considered a crucial precursor to the formation of advanced glycation end products (AGEs) (Alyahyawi et al., 2023; Azevedo et al., 1988).
The high contents of HMF at sixth day of incubation in glycated sample corresponds to the NBT assay result (Ahmad et al., 2012). The decreased ketoamine and HMF contents clearly demonstrate CuFe2O4 NPs are capable to slow down the early glycation reaction, consequentially reduced AGEs formation.
It is well-recognized fact that oxidative stress is generated during glycation reaction in disease condition such as diabetes and in neurodegenerative diseases, causing severe damage to biomolecules (Leszek et al., 2016; Khan et al., 2016). Protein carbonyl content is indicative of one of the most important biomarkers of protein oxidation and AGEs formation (Albarracin et al., 2012; Levine et al., 1994). Ketoamines, which are early glycation products, are transformed into protein carbonyl compounds through a protein enediol intermediate, producing superoxide radicals that contribute to oxidative stress both in vivo and in vitro. This oxidative stress causes irreversible damage to proteins during the glycation process (Azevedo et al., 1988; Maarfi et al., 2023).
The statistically significant reduction in carbonyl content in the CuFe2O4 NPs-treated group (p < 0.01) supports the conclusion that CuFe2O4 NPs exhibit antiglycation properties, likely by blocking reactive groups involved in glycation. This inhibition of glycation is further supported by the observed decrease in lysine, arginine, ketoamines, HMF, and carbonyl content, implying that CuFe2O4 NPs may protect against glycation-induced protein damage (Ansari et al., 2016; Ashraf et al., 2014).
The decrease of fluorescence intensity in presence of CuFe2O4 NPs points towards its anti-glycative activity. This fluorescence study is consistent with studies demonstrating similar anti-glycating properties of other nanoparticles like silver and ZnO (Ansari et al., 2016; Kim et al., 2012; Kim et al., 2012). So, this reaffirms the hypothesis that CuFe2O4 NPs could serve as effective anti-glycating agents.
The reduction in fluorescence intensity indicates CuFe2O4 NPs effectively inhibited protein aggregation, potentially by disrupting the glycation process. This result complements previous work, which highlights the role of glycation in promoting protein aggregation and cross-linking, contributing to various metabolic disorders (Lo et al., 1994; bayashi et al., 1996).
Similarly, the analysis using ANS dye further validated the ability of CuFe2O4 NPs to mitigate the structural alterations in BSA caused by glycation (Figure 7B). The increase in ANS fluorescence in the glycated sample indicates the formation of hydrophobic patches, while a decrease in the treated sample suggests that the NPs prevented the exposure of these hydrophobic regions. These findings are congruent with earlier studies that used similar dyes to monitor protein conformational changes upon glycation and confirmed the protective role of nanoparticles in maintaining protein integrity (Arafat et al., 2014).
Finally, the measurement of intrinsic tryptophan fluorescence provided key insights into the preservation of protein structure. A severe decrease in fluorescence intensity in the glycated sample indicates significant tryptophan residue destruction due to glycation (Figure 8) (Ashraf et al., 2018; Shaklai et al., 1984). In contrast, the NPs-treated sample showed increase (8%) in fluorescence, confirming that CuFe2O4 NPs preserved the protein’s structural integrity, echoing findings from studies with other nanoparticles like ZnO (Bhogale et al., 2013).
One interesting and somewhat unexpected observation was the differential behavior of nanoparticles in protecting proteins. While CuFe2O4 NPs demonstrated strong protective effects in this study, the literature suggests that not all nanoparticles interact with proteins in the same way. For instance, gold NPs have been shown to cause conformational changes in BSA (Bhogale et al., 2013), while ZnO and carbon C60 nanoparticles reportedly do not induce significant structural modifications (Bhogale et al., 2013; Liu et al., 2012). This highlights the complexity of nanoparticle-protein interactions, which seem to depend on several factors, including the specific properties of the nanoparticles and the proteins involved.
Since, nanoparticles have received much attention due to their toxicity. Nanoparticles can easily cross the cell membranes and interact with intracellular metabolism (Hanley et al., 2009). In vivo studies have reported the astrocyte swelling, blood–brain barrier destruction, oxidative stress induced by free radicals, alteration of gene expression and neuronal degeneration on exposure of nanoparticles (Wang M. F. RahmanJ. et al., 2009; Sharma et al., 2010; Tang et al., 2009). Reactive oxygen species (ROS) generation is one of the mechanisms for nanoparticle toxicity (Wang et al., 2013; Elsaesser and Howard, 2012). Interaction of nanoparticle with cells instigating ROS formation, mitochondrial respiration, and NADPH-dependent enzyme systems (Regoli and Giuliani, 2014; Jomova et al., 2012; Chen et al., 2011). While, phagocytosis leads the generation of ROS upon internalization of nanoparticles (Regoli and Giuliani, 2014; Jomova et al., 2012; Soenen et al., 2011). Disproportionate production of free radical is considered the basis of apoptosis and DNA damage (Ryter et al., 2007; Li and Osborne, 2008).
Undeniably, as existing in vitro and in vivo toxicity testing methods are chiefly employed to evaluate the acute and subacute toxicity of NPs, while nanotoxicity testing methods for chronic long-term NPs exposure, which are critical for predicting chronic toxicity in humans, are still deficient. Therefore, there is an imperative necessity to develop new powerful tools to evaluate and understand the mechanisms of NP toxicity as well as addressing the knowledge gap.
While CuFe2O4 NPs demonstrated anti-glycating effects in vitro, their behavior in vivo may differ due to interactions with various biomolecules, such as proteins, nucleic acids, and lipids. Factors such as solvation forces, hydrogen bonding, and Van der Waals interactions at the nano-bio interface may affect their reactivity in biological environments. Moreover, it is important to account for external factors such as pH, temperature, and the type of reducing sugars or inhibitors present in glycation reactions, all of which could influence the efficacy of nanoparticles (Wangoo et al., 2008; Turci et al., 2010). For instance, the glycation reaction has been shown to be highly pH-dependent, with an increase in glycation under alkaline conditions. Understanding how CuFe2O4 NPs behave under varying conditions will be essential for optimizing their use as anti-glycating agents.
Further research should focus on elucidating the exact mechanisms of action of CuFe2O4 NPs, particularly their interaction with protein structures and free amino groups. Additionally, in vivo studies are needed to assess their safety, bioavailability, and efficacy in more complex biological systems. Nanomedicine continues to offer immense potential for treating diseases associated with AGEs, but a thorough evaluation of the physicochemical properties of nanoparticles and their impact on human health is critical for advancing this field (Wong et al., 2008; El-Ghorab et al., 2010).
Another limitation involves the potential toxicity of nanoparticles. Although CuFe2O4 NPs showed beneficial effects in this study, the literature on nanoparticle toxicity is inconsistent. For example, ZnO nanoparticles have been reported to exhibit both acute and subacute toxicity depending on the mode of administration and dosage (Tian et al., 2015; Sruthi et al., 2017; Wang et al., 2006; Hackenberg et al., 2011). Furthermore, some studies have highlighted the ability of nanoparticles to generate reactive oxygen species (ROS), which could lead to oxidative stress and associated pathological conditions like inflammation and fibrosis (Long et al., 2006). These findings emphasize the need for a more detailed investigation into the toxicity, bioavailability, and tissue distribution of CuFe2O4 NPs before they can be considered for clinical applications.
Additionally, the inhibition of AGE formation by CuFe2O4 NPs raises questions about the precise mechanism by which these nanoparticles exert their effects.
Since mechanism of AGEs formation involved various steps, hence, different compounds have been identified which can prevent this process at different steps (Khalifah et al., 1999; Rahbar and Figarola, 2002; Wang J. et al., 2009). Several classes of glycation inhibitors have been developed, including those that compete with carbohydrates for amino groups, react with aldoses and ketoses, interfere with side reactions, trap RCS, or act as AGE breakers. Examples of such inhibitors include aminoguanidine, pyridoxamine, carnosine, benfotiamine, aspirin and ALT-711, each demonstrating different mechanisms of action. Similarly, several studies have shown various nanoparticles may also act as antiglycating agents like that of naturals agents (Yu et al., 2015), acting at different stages of AGEs formation and on different reacting groups. As previous studies have suggested that several nanoparticles like SeNPs, GNPs, AuNPs, ZnONPs, etc. may inhibit the glycation process by competing with -NH2 groups of lysine and Arginine as well as arresting the reactive group of glycating agents (Kim et al., 2012; Rafia et al., 2023; Du et al., 2020; Hu et al., 2005). Therefore, mechanisms of inhibition of AGEs of CuFe2O4 NPs may be involved in the trapping of reactive amino groups, making them unable to react with MG and sequestration of reacting groups. Though, the exact mechanism of action of CuFe2O4 NPs is still ambiguous. Therefore, further investigation is needed to clarify these inhibitory pathways and their molecular details.
CuFe2O4 NPs offer several advantages over other oxide NPs due to their superior redox properties, protein binding, biocompatibility, and anti-glycation efficacy. This makes them particularly promising for therapeutic applications targeting AGE-related diseases, where stability, reduced toxicity, and potent anti-glycative actions are essential. Their performance surpasses that of commonly studied NPs like ZnO, TiO2 (Alenazi et al., 2022), Ag, Fe3O4, and others, positioning CuFe2O4 as a potent agent with multi-functional capabilities in glycation inhibition and disease prevention.
Conclusion
In conclusion, while CuFe2O4 NPs show great promise as anti-glycating agents with potential applications in treating AGE-related diseases, careful consideration of their biological interactions and toxicological profiles will be necessary for their successful integration into clinical practice.
The findings from this study highlight the potential of magnetically biosynthesized copper ferrite nanoparticles (CuFe2O4 NPs) as promising agents in the fight against advanced glycation end products (AGEs). AGEs are key contributors to the onset and progression of serious health conditions such as diabetic complications and neurodegenerative diseases, including Alzheimer’s and Parkinson’s. The demonstrated ability of CuFe2O4 NPs to inhibit AGE formation and protect protein integrity presents a new avenue in the development of therapies aimed at mitigating the harmful effects of AGEs, offering hope for improving patient outcomes.
This study offers the potential to develop nanoparticle-based therapeutics for managing diabetes and associated complications, which continue to rise globally. Additionally, the implications extend to neurodegenerative diseases, where AGE accumulation has been associated with neuronal damage and cognitive decline. Effective management of AGEs could contribute to delaying disease progression, improving quality of life, and reducing the burden on healthcare systems.
AGEs play a critical role in the pathophysiology of diabetic complications and neurodegenerative conditions. In diabetes, AGEs contribute to complications such as retinopathy, nephropathy, neuropathy, and cardiovascular disease by damaging proteins, lipids, and nucleic acids. Similarly, in neurodegenerative diseases, AGEs are implicated in promoting oxidative damage and neuronal cell death, thereby accelerating cognitive decline.
Mitigating the formation of AGEs is vital because it interrupts this destructive cycle, offering protection against oxidative stress and preserving the structural integrity of essential biomolecules. Effective antiglycation strategies could slow the progression of these diseases, prevent complications, and improve patient survival and quality of life.
While this study demonstrates the potential of CuFe2O4 NPs as antiglycation agents, future research is needed to fully explore their clinical applicability. The detailed mechanistic studies should be conducted to understand the precise molecular mechanisms by which CuFe2O4 NPs inhibit AGE formation and protect against protein damage. Before clinical application, the biocompatibility and potential toxicity of CuFe2O4 NPs must be rigorously evaluated to ensure safety for human use.
This research adds to the growing body of knowledge on the use of nanoparticles as therapeutic agents for AGE inhibition. While several studies have explored the potential of antioxidants and natural extracts in combating AGEs, the use of copper ferrite nanoparticles synthesized through a green, biosynthetic approach (using Aloe vera leaf extract) represents a novel and environmentally friendly method of producing potent antiglycation agents.
In comparison with other studies that focus on AGE inhibitors such as aminoguanidine and natural polyphenols, CuFe2O4 NPs offer distinct advantages, including their magnetic properties, which could be harnessed for targeted drug delivery. Moreover, the antioxidative capabilities of these nanoparticles offer an additional layer of protection against oxidative stress, a significant factor in both diabetic and neurodegenerative diseases.
Overall, this research lays the foundation for the development of nanoparticle-based therapeutics for AGE-related pathologies, expanding the horizon of potential treatments for complex chronic diseases.
Future direction
Despite promising findings regarding the use of nanoparticles in various in vitro and in vivo studies, several limitations are needed to be addressed for future studies. One key limitation is the incomplete understanding of nanoparticles’ biocompatibility and bioactivity in complex biological systems leading to its toxicity.
Finally, a comprehensive understanding of NP toxicity from in vivo studies is essential before reaching a definitive consensus on the overall toxicity of nanoparticles (NPs), about safety and potential risks. In a while, we believe that the convergence of related disciplines like medicine, material science, chemistry, and artificial intelligence holds huge potential to revolutionize nanotoxicity research and warrant the safer application of nanoparticles (NPs) in humans.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SAh: Supervision, Writing – original draft. US: Conceptualization, Writing – review and editing. JA: Formal Analysis, Writing – review and editing. AmA: Funding acquisition, Writing – review and editing. MA: Methodology, Writing – review and editing. SF: Methodology, Writing – review and editing. AhA: Methodology, Writing – review and editing. RA: Methodology, Writing – review and editing. SAs: Methodology, Writing – review and editing. PP: Funding acquisition, Methodology, Writing – review and editing. AK: Investigation, Methodology, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Deanship of scientific research, University of Ha’il. This research has been funded by Scientific Research Deanship at University of Hail - Saudi Arabia through project number RG-23 146.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbasi, M. S., Tahir, M. A., and Meer, S. (2020). FTIR Spectroscopic study of aloe vera barbadensis Mill Buds. Asian J. Chem. Sci. 7 (4), 1–6. doi:10.9734/ajocs/2020/v7i419026
Ahmad, S., Ahmad, M. F., Khan, S., Alouffi, S., Khan, M., Prakash, C., et al. (2024). Exploring aldose reductase inhibitors as promising therapeutic targets for diabetes-linked disabilities. Int. J. Biol. Macromol. 280, 135761. doi:10.1016/j.ijbiomac.2024.135761
Ahmad, S., Moinuddin, , Khan, R. H., and Ali, A. (2012). Physicochemical studies on glycation-induced structural changes in human IgG. IUBMB Life 64 (2), 151–156. doi:10.1002/iub.582
Ahmed, M. U., Thorpe, S. R., and Baynes, J. W. (1986). Identification of N epsilon-carboxymethyllysine as a degradation product of fructoselysine in glycated protein. J. Biol. Chem. 261 (11), 4889–4894. doi:10.1016/s0021-9258(19)89188-3
Ahmed, N., Ahmed, U., Thornalley, P. J., Hager, K., Fleischer, G., and Munch, G. (2005). Protein glycation, oxidation and nitration adduct residues and free adducts of cerebrospinal fluid in Alzheimer’s disease and link to cognitive impairment. J. Neurochem. 92, 255–263. doi:10.1111/j.1471-4159.2004.02864.x
Albarracin, S. L., Stab, B., Casas, Z., Sutachan, J. J., Samudio, I., Gonzalez, J., et al. (2012). Effects of natural antioxidants in neurodegenerative disease. Neurosci 15 (1), 1–9. doi:10.1179/1476830511y.0000000028
Alenazi, F., Saleem, M., Syed Khaja, A. S., Zafar, M., Alharbi, M. S., Al Hagbani, T., et al. (2022). Antiglycation potential of plant based TiO2 nanoparticle in D-ribose glycated BSA in vitro. Cell Biochem. Funct. 40 (7), 784–796. doi:10.1002/cbf.3744
Allaman, I., Belanger, M., and Magistretti, P. J. (2015). Methylglyoxal, the dark side of glycolysis. Front. Neurosci. 9, 23. doi:10.3389/fnins.2015.00023
Alouffi, S., Shahab, U., Khan, S., Khan, M., Khanam, A., Akasha, R., et al. (2022). Glyoxal induced glycative insult suffered by immunoglobulin G and fibrinogen proteins: a comparative physicochemical characterization to reveal structural perturbations. Int. J. Biol. Macromol. 205, 283–296. doi:10.1016/j.ijbiomac.2022.02.093
Alyahyawi, A. R., Khan, M. Y., Alouffi, S., Maarfi, F., Akasha, R., Khan, S., et al. (2023). Identification of glycoxidative lesion in isolated low-density lipoproteins from diabetes mellitus subjects. Life 13 (10), 1986. doi:10.3390/life13101986
Anandan, S., Mahadevamurthy, M., Ansari, M. A., Alzohairy, M. A., Alomary, M. N., Siraj, S. F., et al. (2019). Biosynthesized ZnO-NPs from morus indica attenuates methylglyoxal-induced protein glycation and RBC damage: in-vitro, in-vivo and molecular docking study. Biomolecules 9 (12), 882. doi:10.3390/biom9120882
Ansari, J. M. A. M. A., Khan, H. M., Alzohairy, M. A., and Choi, I. (2016). Green synthesis of silver nanoparticles and characterization of their inhibitory effects on AGEs formation using biophysical techniques. Sci. Rep. 6 (1), 20414. doi:10.1038/srep20414
Ansari, M. A., and Alomary, M. N. (2024). Bioinspired ferromagnetic NiFe2O4 nanoparticles: eradication of fungal and drug-resistant bacterial pathogens and their established biofilm. Microb. Pathog. 193, 106729. doi:10.1016/j.micpath.2024.106729
Ansari, M. A., Baykal, A., Asiri, S., and Rehman, S. (2018). Synthesis and characterization of antibacterial activity of spinel chromium-substituted copper ferrite nanoparticles for biomedical application. J. Inorg. Organomet. Polym. Mater. 28 (6), 2316–2327. doi:10.1007/s10904-018-0889-5
Ansari, M. A., Govindasamy, R., Begum, M. Y., Ghazwani, M., Alqahtani, A., Alomary, M. N., et al. (2023). Bioinspired ferromagnetic CoFe2O4 nanoparticles: potential pharmaceutical and medical applications. Nanotechnol. Rev. 12 (1), 20230575. doi:10.1515/ntrev-2023-0575
Arafat, M. Y., Ashraf, J. M., Arif, Z., Moinuddin, , and Alam, K. (2014). Fine characterization of glucosylated human IgG by biochemical and biophysical methods. Int. J. Biol. Macromol. 69, 408–415. doi:10.1016/j.ijbiomac.2014.05.069
Ashraf, J. M., Ansari, M. A., Choi, I., Khan, H. M., and Alzohairy, M. A. (2014). Antiglycating potential of gum Arabic capped-silver nanoparticles. Appl. Biochem. Biotechnol. 174, 398–410. doi:10.1007/s12010-014-1065-1
Ashraf, J. M., Ansari, M. A., Fatma, S., Abdullah, S. M. S., Iqbal, J., Madkhali, A., et al. (2018). Inhibiting effect of zinc oxide nanoparticles on advanced glycation products and oxidative modifications: a potential tool to counteract oxidative stress in neurodegenerative diseases. Mol. Neurobio. 55, 7438–7452. doi:10.1007/s12035-018-0935-x
Ashraf, J. M., Rabbani, G., Ahmad, S., Hasan, Q., Khan, R. H., Alam, K., et al. (2015). Glycation of H1 histone by 3-deoxyglucosone: effects on protein structure and generation of different advanced glycation end products. PLoS One 10 (6), e0130630. doi:10.1371/journal.pone.0130630
Azevedo, M., Falcao, J., and Manso, J. R. C. (1988). Superoxide radical generation by Amadori compounds. Free Radic. Res. Commun. 4, 331–335. doi:10.3109/10715768809066899
bayashi, O. H., Nakano, K., Shigeta, H., Yamaguchi, M., Yoshimori, K., Fukui, M., et al. (1996). Formation of crossline as a fluorescent advanced glycation end product in vitro and in vivo. Biochem. Biophys. Res. Commun. 226 (1), 37–41. doi:10.1006/bbrc.1996.1308
Benalia, A., Derbal, K., Khalfaoui, A., Pizzi, A., and Medjahdi, G. (2022). The use of as natural coagulant in algerian drinking water treatment plant. J. Renew. Mater. 10 (3), 625–637. doi:10.32604/jrm.2022.017848
Bhogale, A., Patel, N., Sarpotdar, P., Mariam, J., Dongre, P. M., Miotello, A., et al. (2013). Systematic investigation on the interaction of bovine serum albumin with ZnO nanoparticles using fluorescence spectroscopy. Colloids Surf. B Biointerfaces 102, 257–264. doi:10.1016/j.colsurfb.2012.08.023
Chen, H., Yoshioka, H., Kim, G. S., Jung, J., Okami, N., Sakata, H., et al. (2011). Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neu-roprotection. Antioxid. Redox. Signal. 14 (8), 1505–1517. doi:10.1089/ars.2010.3576
Chetyrkin, S. V., Mathis, M. E., Ham, A. J., Hachey, D. L., Hudson, B. G., and Voziyan, P. A. (2008). Propagation of protein glycation damage involves modification of tryptophan residues via reactive oxygen species: inhibition by pyridoxamine. Free Radic. Biol. Med. 44, 1276–1285. doi:10.1016/j.freeradbiomed.2007.09.016
Dalfo, E., Portero-Otin, M., Ayala, V., Martinez, A., Pamplona, R., and Ferrer, I. (2005). Evidence of oxidative stress in the neocortex in incidental lewy body disease. J. Neuropathol. Exp. Neurol. 64, 816–830. doi:10.1097/01.jnen.0000179050.54522.5a
Dey, A., Bhattacharya, R., Mukherjee, A., and Pandey, D. K. (2017). Natural products against Alzheimer’s disease: pharmaco-therapeutics and biotechnological interventions. Biotechnol. Adv. 35 (2), 178–216. doi:10.1016/j.biotechadv.2016.12.005
Du, P., Tu, Z., Wang, H., and Hu, Y. (2020). Mechanism of selenium nanoparticles inhibiting advanced glycation end products. J. Agric. Food Chem. 68, 10586–10595. doi:10.1021/acs.jafc.0c03229
El-Ghorab, A. H., Ashraf, I. F., Mohamed, A. F., Shaaban, H. A., El-massry, K. F., and Farouk, A. (2010). The effect of pH on flavor formation and antioxidant activity of amino acids and sugars interaction products. J. Arab. Soc. Med. Res. 5, 131–139.
Elsaesser, A., and Howard, C. V. (2012). Toxicology of nanoparticles. Adv. Drug Deliv. Rev. 64 (2), 129–137. doi:10.1016/j.addr.2011.09.001
Gaens, K. H. J., Goossens, G. H., Niessen, P. M., van Greevenbroek, M. M., van der Kallen, C. J. H., Niessen, H. W., et al. (2014). N’-(carboxymethyl)lysine-receptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arter. Throm. Vas. 34, 1199–1208. doi:10.1161/atvbaha.113.302281
Garay-Sevilla, M. E., Regalado, J. C., Malacara, J. M., Nava, L. E., Wróbel-Zasada, K., Castro-Rivas, A., et al. (2005). Advanced glycosylation end products in skin, serum, saliva and urine and its association with complications of patients with type 2 diabetes mellitus. J. Endocrinol. Invest. 28, 223–230. doi:10.1007/bf03345377
Hackenberg, S., Scherzed, A., Technau, A., Kessler, M., Froelich, K., Ginzkey, C., et al. (2011). Cytotoxic, genotoxic and pro-inflammatory effects of zinc oxide nanoparticles in human nasal mucosa cells in vitro. Toxicol. Vitro. 25 (3), 657–663. doi:10.1016/j.tiv.2011.01.003
Hanley, C., Thurber, A., Hanna, C., Punnoose, A., Zhang, J., and Wingett, D. G. (2009). The influences of cell type and ZnO nanoparticle size on immune cell cytotoxicity and cytokine induction. Nanoscale Res. Lett. 4 (12), 1409–1420. doi:10.1007/s11671-009-9413-8
Hanssen, N. M., Wouters, K., Huijberts, M. S., Gijbels, M. J., Sluimer, J. C., Scheijen, J. L., et al. (2014). Higher levels of advanced glycation endproducts in human carotid atherosclerotic plaques are associated with a rupture-prone phenotype. Eur. Heart J. 35, 1137–1146. doi:10.1093/eurheartj/eht402
Hu, D., Qin, Z., Xue, B., Fink, A. L., and Uversky, V. N. (2008). Effect of methionine oxidation on the structural properties, conformational stability, and aggregation of immunoglobulin light chain LEN. Biochemistry 47, 8665–8677. doi:10.1021/bi800806d
Hu, X., Cheng, W., Wang, T., Wang, E., and Dong, S. (2005). Well-ordered end-to-end linkage of gold nanorods. Nanotechnology 16, 2164–2169. doi:10.1088/0957-4484/16/10/032
Jasim, S. A., Patra, I., Opulencia, M. J., Hachem, K., Parra, R. M., Ansari, M. J., et al. (2022). Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity. Review 11 (1), 2483–2492. doi:10.1515/ntrev-2022-0143
Jaturapatporn, D., Isaac, M. G., McCleery, J., and Tabet, N. (2012). Aspirin, steroidal and non-steroidal anti-inflammatory drugs for the treatment of Alzheimer’s disease. Cochrane Database Syst. Rev. 2, CD006378. doi:10.1002/14651858.cd006378.pub2
Johnson, R. N., Metcalf, P. A., and Baker, J. R. (1982). Fructosamine: a new approach to the estimation of serum glycosylprotein. An index of diabetic control. Clin. Chim. Acta 127, 87–95. doi:10.1016/0009-8981(83)90078-5
Jomova, K., Baros, S., and Valko, M. (2012). Redox active metal-induced oxidative stress in biological systems. Met. Chem. 37 (2), 127–134. doi:10.1007/s11243-012-9583-6
Khalifah, R. G., Baynes, J. W., and Hudson, B. G. (1999). Amadorins: novel post Amadori inhibitors of advanced glycation reactions. Biochem. Biophys. Res. Commun. 257, 251–258. doi:10.1006/bbrc.1999.0371
Khan, H., Khanam, A., Khan, A. A., Ahmad, R., Husain, A., Habib, S., et al. (2024). The complex landscape of intracellular signalling in protein modification under hyperglycaemic stress leading to metabolic disorders. Protein J. 43 (3), 425–436. doi:10.1007/s10930-024-10191-3
Khan, T. A., Hassan, I., Ahmad, A., Perveen, A., Aman, S., Quddusi, S., et al. (2016). Recent updates on the dynamic association between oxidative stress and neurodegenerative disorders. CNS Neurol. Disord. Drug Targets 15 (3), 310–320. doi:10.2174/1871527315666160202124518
Khurana, R., Coleman, C., Ionescu-Zanetti, C., Carter, S. A., Krishna, V., Grover, R. K., et al. (2005). Mechanism of Thioflavin T binding to amyloid fibrils. J. Struct. Biol. 151 (3), 229–238. doi:10.1016/j.jsb.2005.06.006
Kim, J., Hong, C., Koo, Y., Choi, H., and Lee, K. (2012). Anti-glycation effect of gold nanoparticles on collagen. Biol. Pharm. Bull. 35, 260–264. doi:10.1248/bpb.35.260
Kuznetsov, M. V., Morozov, Y. G., and Belousova, O. V. (2013). Synthesis of copper ferrite nanoparticles. Inorg. Mater. 49 (6), 606–615. doi:10.1134/s0020168513050063
Leszek, J., Barreto, G. E., Gasiorowski, K., Koutsouraki, E., Avila- Rodrigues, M., and Aliev, G. (2016). Inflammatory mechanisms and oxidative stress as key factors responsible for progression of neurodegeneration: role of brain innate immune system. CNS Neurol. Disord. Drug Targets 15 (3), 329–336. doi:10.2174/1871527315666160202125914
Leszek, J., shraf, A. M. G., Tse, W. H., Zhang, J., Gasiorowski, K., Avila-Rodriguez, M. F., et al. (2017). Nanotechnology for alzheimer disease. Curr. Alzheimer Res. 14 (11), 1182–1189. doi:10.2174/1567205014666170203125008
Levine, R. L., Williams, J., Stadtman, E. R., and Shacter, E. (1994). Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 233, 346–357. doi:10.1016/s0076-6879(94)33040-9
Li, G.-Y., and Osborne, N. N. (2008). Oxidative-induced apoptosis to an immortalized ganglion cell line is caspase independent but involves the activation of poly (ADP-ribose) polymerase and apoptosis-inducing factor. Brain Res. 1188, 35–43. doi:10.1016/j.brainres.2007.10.073
Lim, Z. X., and Cheong, K. Y. (2015). Effects of drying temperature and ethanol concentration on bipolar switching characteristics of natural Aloe vera-based memory devices. Phys. Chem. Chem. Phys. 17 (40), 26833–26853. doi:10.1039/c5cp04622j
Liu, S., Sui, Y., Guo, K., Yin, Z., and Gao, X. (2012). Spectroscopic study on the interaction of pristine C60 and serum albumins in solution. Nanoscale Res. Lett. 7 (1), 433. doi:10.1186/1556-276x-7-433
Liu, Y., Guo, Z., Li, F., Xiao, Y., Zhang, Y., Bu, T., et al. (2019). Multifunctional magnetic copper ferrite nanoparticles as fenton-like reaction and near-infrared photothermal agents for synergetic antibacterial therapy. ACS Appl. Mater. and interfaces 11 (35), 31649–31660. doi:10.1021/acsami.9b10096
Lo, T. W. C., Westwood, M. E., McLellan, A. C., Selwood, T., and Thornalley, P. J. (1994). Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. J. Biol. Chem. 269, 32299–32305. doi:10.1016/s0021-9258(18)31635-1
Long, T. C., Saleh, N., Tilton, R. D., Lowry, G. V., and Veronesi, B. (2006). Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): implications for nanoparticle neurotoxicity. Environ. Sci. Technol. 40 (14), 4346–4352. doi:10.1021/es060589n
Loureiro, A., Azoia, N. G., Gomes, A. C., and Cavaco-Paulo, A. (2016). Albumin-based nanodevices as drug carriers. Curr. Pharm. Des. 22, 1371–1390. doi:10.2174/1381612822666160125114900
Maarfi, F., Ahmad, S., Alouffi, S., Akasha, R., Khan, M. S., Rafi, Z., et al. (2023). Differential impact of glycation on apolipoprotein AI of high-density lipoprotein: a review. Glycobiology. 33 (6), 442–453. doi:10.1093/glycob/cwad010
Masunga, N., Mmelesi, O. K., Kefeni, K. K., and Mamba, B. B. (2019). Recent advances in copper ferrite nanoparticles and nanocomposites synthesis, magnetic properties and application in water treatment: review. J. Environ. Chem. Eng. 7 (3), 103179. doi:10.1016/j.jece.2019.103179
Monnier, V. M., and Cerami, A. (1981). Nonenzymatic browning in vivo: possible process for aging of long-lived proteins. Science 211, 491–493. doi:10.1126/science.6779377
Mushtaq, M. W., Kanwal, F., Islam, A., Ahmed, K., Haq, Z. U., Jamil, T., et al. (2017). Synthesis and characterisation of doxorubicin-loaded functionalised cobalt ferrite nanoparticles and their in vitro anti-tumour activity under an AC-magnetic field. T. J. pharma. Res. 16 (7), 1663–1674. doi:10.4314/tjpr.v16i7.27
Natarajan, S., Shunmugiah, K. P., and Kasi, P. D. (2013). Plants traditionally used in age-related brain disorders (dementia): an ethanopharmacological survey. Pharm. Biol. 51 (4), 492–523. doi:10.3109/13880209.2012.738423
Nikalje, A. P. (2015). Nanotechnology and its applications in medicine. Med. Chem. 5, 81–89. doi:10.4172/2161-0444.1000247
Rafia, Z., Baig, M. H., Husain, F. M., Alomard, S. Y., Dongb, J., and Khan, M. S. (2023). Biological reaction mediated engineered AuNPs facilitated delivery encore the anticancer, antiglycation, and antidiabetic potential of garcinol. J. K. S. Uni. Sci. 35, 102524.
Rahbar, S., and Figarola, J. L. (2002). Inhibitors and breakers of advanced gly cation endproducts (AGEs): a review. Curr. Med. Chem. Endocr. Metab. Agents 2, 135–161.
Ramazani, A., Taghavi Fardood, S., Hosseinzadeh, Z., Sadri, F., and Joo, S. W. (2017). Green synthesis of magnetic copper ferrite nanoparticles using tragacanth gum as a biotemplate and their catalytic activity for the oxidation of alcohols. Iran. J. Catal. 7 (3), 181–185.
Reddy, V. P., and Beyaz, A. (2006). Inhibitors of the Maillard reaction and AGE breakers as therapeutics for multiple diseases. Drug Discov. Today 11 (13–14), 646–654. doi:10.1016/j.drudis.2006.05.016
Regoli, F., and Giuliani, M. E. (2014). Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 93, 106–117. doi:10.1016/j.marenvres.2013.07.006
Ryter, S. W., Kim, H. P., Hoetzel, A., Park, J. W., Nakahira, K., Wang, X., et al. (2007). Mechanisms of cell death in oxidative stress. Antioxid. Redox Signal. 9 (1), 49–89. doi:10.1089/ars.2007.9.49
Salavati-Niasari, M., Mahmoudi, T., Sabet, M., Hosseinpour-Mashkani, S. M., Soofivand, F., and Tavakoli, F. (2012). Synthesis and characterization of copper ferrite nanocrystals via coprecipitation. J. Clust. Sci. 23 (4), 1003–1010. doi:10.1007/s10876-012-0486-7
Sashidhar, R. B., Capoor, A. K., and Ramana, D. (1994). Quantitation of ϵ-amino group using amino acids as reference standards by trinitrobenzene sulfonic acid. J. Immunol. Methods 167 (1-2), 121–127. doi:10.1016/0022-1759(94)90081-7
Shaklai, N., Garlick, R. L., and Bunn, H. F. (1984). Nonenzymatic glycosylation of human serum albumin alters its conformation and function. J. Biol. Chem. 259 (6), 3812–3817. doi:10.1016/s0021-9258(17)43168-1
Sharma, H. S., Hussain, S., Schlager, J., Ali, S. F., and Sharma, A. (2010). “Influence of nanoparticles on blood–brain barrier permeability and brain edema formation in rats,” in Brain edema XIV (Berlin: Springer), 359–364.
Smith, R. E., and MacQuarrie, R. (1978). A sensitive fluorometric method for the determination of arginine using 9,10- phenanthrenequinone. Anal. Biochem. 90 (1), 246–255. doi:10.1016/0003-2697(78)90029-5
Soenen, S. J., Rivera-Gil, P., José-María, M., Parak, W. J., De Smedt, S. C., and Braeckmans, K. (2011). Cellular toxicity of inorganic nanoparticles: common aspects and guidelines for improved nanotoxicity evaluation. Nano Today 6 (5), 446–465. doi:10.1016/j.nantod.2011.08.001
Srinivasan, S. Y., Paknikar, K. M., Bodas, D., and Gajbhiye, V. (2018). Applications of cobalt ferrite nanoparticles in biomedical nanotechnology. Nanomedicine 13 (10), 1221–1238. doi:10.2217/nnm-2017-0379
Sruthi, S., Millot, N., and Mohanan, P. V. (2017). Zinc oxide nanoparticles mediated cytotoxicity, mitochondrial membrane potential and level of antioxidants in presence of melatonin. Int. J. Biol. Macromol. 103, 808–818. doi:10.1016/j.ijbiomac.2017.05.088
Sutachan, J. J., Casas, Z., Albarracin, S. L., Stab, B. R., Samudio, I., Gonzalez, J., et al. (2012). Cellular and molecular mechanisms of antioxidants in Parkinson’s disease. Nutr. Neurosci. 15 (3), 120–126. doi:10.1179/1476830511y.0000000033
Tang, J., Xiong, L., Wang, S., Wang, J., Liu, L., Li, J., et al. (2009). Distribution, translocation and accumulation of silver nanoparticles in rats. J. Nanosci. Nanotechnol. 9 (8), 4924–4932. doi:10.1166/jnn.2009.1269
Thornalley, P. J. (1998). Glutathione-dependent detoxification of alpha-oxoaldehydes by the glyoxalase system: involvement in disease mechanisms and antiproliferative activity of glyoxalase I inhibitors. Chem. Biol. Interact. 111-112, 137–151. doi:10.1016/s0009-2797(97)00157-9
Tian, L., Lin, B., Wu, L., Li, K., Liu, H., Yan, J., et al. (2015). Neurotoxicity induced by zinc oxide nanoparticles: age-related differences and interaction. Sci. Rep. 5 (1), 16117. doi:10.1038/srep16117
Treuel, L., Jiang, X., and Nienhaus, G. U. (2013). New views on cellular uptake and trafficking of manufactured nanoparticles. J. R. Soc. Interface 10, 20120939. doi:10.1098/rsif.2012.0939
Turci, F., Ghibaudi, E., Colonna, M., Boscolo, B., Fenoglio, I., and Fubini, B. (2010). An integrated approach to the study of the interaction between proteins and nanoparticles. Langmuir ACS J. Surf. Colloids 26 (11), 8336–8346. doi:10.1021/la904758j
Van Eupen, M. G., Schram, M. T., Colhoun, H. M., Hanssen, N. M., Niessen, H. W., Tarnow, L., et al. (2013). The methylglyoxal-derived AGE tetrahydropyrimidine is increased in plasma of individuals with type 1 diabetes mellitus and in atherosclerotic lesions and is associated with sVCAM-1. Diabetologia 56, 1845–1855. doi:10.1007/s00125-013-2919-8
Wang, B., Feng, W. Y., Wang, T. C., Jia, G., Wang, M., Shi, J. W., et al. (2006). Acute toxicity of nano- and micro-scale zinc powder in healthy adult mice. Toxicol. Lett. 161 (2), 115–123. doi:10.1016/j.toxlet.2005.08.007
Wang, F., Yu, L., Monopoli, M. P., Sandin, P., Mahon, E., Salvati, A., et al. (2013). The biomolecular corona is retained during nanoparticle uptake and protects the cells from the damage induced by cationic nanoparticles until degraded in the lysosomes. Nanomed. Nanotechnol. Biol. Med. 9 (8), 1159–1168. doi:10.1016/j.nano.2013.04.010
Wang, J., Sun, B., Cao, Y. P., and Tian, Y. (2009b). Protein glycation inhibitory activity of wheat bran feruloyl oligosaccharides. Food Chem. 112, 350–353. doi:10.1016/j.foodchem.2008.05.072
Wang, M. F. R. J., Patterson, T. A., Saini, U. T., Robinson, B. L., Newport, G. D., Murdock, R. C., et al. (2009a). Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol. Lett. 187 (1), 15–21. doi:10.1016/j.toxlet.2009.01.020
Wangoo, N., Suri, C. R., and Shekhawat, G. (2008). Interaction of gold nanoparticles with protein: a spectroscopic study to monitor protein conformational changes. Appl. Phys. Lett. 92 (13), 133104. doi:10.1063/1.2902302
Webster, J., Urban, C., Berbaum, K., Loske, C., Alpar, A., Gartner, U., et al. (2005). The carbonyl scavengers aminoguanidine and tenilsetam protect against the neurotoxic effects of methylglyoxal. Neurotox. Res. 7 (1–2), 95–101. doi:10.1007/bf03033780
Wong, K. H., Aziz, S. A., and Mohamed, S. (2008). Sensory aroma from Maillard reaction of individual and combinations of amino acids with glucose in acidic conditions. Int. J. Food Sci. Technol. 43 (9), 1512–1519.
Yu, S., Zhang, W., Liu, W., Zhu, W., Guo, R., Wang, Y., et al. (2015). The inhibitory effect of selenium nanoparticles on protein glycation in vitro. Nanotechnology 26, 145703. doi:10.1088/0957-4484/26/14/145703
Zakiyah, L. B., Saion, E., Al-Hada, N. M., Gharibshahi, E., Salem, A., Soltani, N., et al. (2015). Up-scalable synthesis of size-controlled copper ferrite nanocrystals by thermal treatment method. Mater. Sci. Semicond. Process 40, 564–569. doi:10.1016/j.mssp.2015.07.027
Keywords: methylglyoxal, ages, CuFe2O4 NPs, antiglycation, nanoparticles
Citation: Ahmad S, Shahab U, Ashraf JM, Alyahyawi AR, Ansari MA, Fatma S, Alshammari A, Akasha R, Asiri S, Puri P and Khanam A (2025) Antiglycating activity of biomimetically synthesized magnetic copper ferrite nanoparticles: inhibition of methylglyoxal-derived AGEs. Front. Nanotechnol. 7:1564954. doi: 10.3389/fnano.2025.1564954
Received: 22 January 2025; Accepted: 22 May 2025;
Published: 10 June 2025.
Edited by:
Kevin M. Koo, The University of Queensland, AustraliaReviewed by:
Deniz Özkan Vardar, Lokman Hekim University, TürkiyeAnushruti Ashok, Cleveland Clinic, United States
Copyright © 2025 Ahmad, Shahab, Ashraf, Alyahyawi, Ansari, Fatma, Alshammari, Akasha, Asiri, Puri and Khanam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saheem Ahmad, YWhtYWRzYWhlZW1AZ21haWwuY29t, cy5hbnNhcmlAdW9oLmVkdS5zYQ==; Jalaluddin M. Ashraf, am1hc2hyYWZAZ21haWwuY29t
 Saheem Ahmad
Saheem Ahmad Uzma Shahab2
Uzma Shahab2 Jalaluddin M. Ashraf
Jalaluddin M. Ashraf Mohammad Azam Ansari
Mohammad Azam Ansari Rihab Akasha
Rihab Akasha Sarah Asiri
Sarah Asiri