- 1Department of Biotechnology, Guru Jambheshwar University of Science and Technology, Hisar, Haryana, India
- 2Department of Physics and Astrophysics, School of Basic Sciences, Central University of Haryana, Mahendragarh, Haryana, India
- 3Department of Chemistry, College of Basic Sciences and Humanities, Chaudhary Charan Singh Haryana Agriculture University, Hisar, Haryana, India
- 4Department of Biochemistry, School of Interdisciplinary and Applied Sciences, Central University of Haryana, Mahendergarh, Haryana, India
- 5Department of Applied Sciences and Humanities (Physics, SOET), Central University of Haryana, Mahendergarh, Haryana, India
- 6Department of Physics, Punjab Engineering College (Deemed to be University), Chandigarh, India
Efficient electrode materials for energy storage device fabrication are in demand to address global energy needs. In this study, novel binder-free electrodes were based on NiCo2S4 (NCS) and an Ni-Co metal–organic framework (MOF). Electrodes were engineered using a convenient dual-step solvothermal method employing nickel (Ni) foam as a current collector. The electrode material was characterized for crystallinity and crystal phase purity (pXRD), morphology (FESEM), and surface elemental profiling (XPS). Cyclic voltammetry (CV) for redox behavior analysis, galvanostatic charge–discharge (GCD) for capacitance evaluation, and electrochemical impedance spectroscopy (EIS) for charge transfer resistance were employed to investigate electrochemical performance. The composite active electrode materials NCS/NCM@NF exhibited high specific capacitance (2,150.3 F g−1) at a scan rate of 2 mV s-1, with KOH (6M) as an electrolyte. The fabricated electrode was highly reusable—approximately 89% of capacitance was retained, even after 10,000 cycles of usage (charge–discharge). The composite material has high energy density, Ed (199.6 W h kg−1), and power density, Pd (1,500.2 W kg−1). The charge transfer resistance (Rct, 790 mΩ) and solution resistance (Rs, 1.52 Ω), computed through EIS, being low, show a quick charge transfer at the interface, making the composite material suitable for supercapacitor application.
1 Introduction
The use of fossil fuels negatively impacts the environment and, in turn, impacts human health. Consequently, progress in sustainable energy storage devices that offer low costs together with superior energy and power density has become an emerging area of research. Batteries and supercapacitors are primarily employed for energy storage. The sluggish movement of electrons and ions at fast rates makes batteries capable of producing lower power densities. Furthermore, batteries require long charging times in contrast to supercapacitors (Gao et al., 2023). Supercapacitors exhibit improved power density, suitable charge–discharge functioning, long life span, enhanced effectiveness under adverse circumstances, and eco-benign behavior (Pershaanaa et al., 2022; Khan et al., 2024). They have lower energy density than batteries, making them preferable over the latter for short-term energy storage (Yaseen et al., 2021). Supercapacitors, having a wide operating temperature range, are ideal for transport systems, hybrid electric vehicles, braking systems (regenerative), actuator systems, renewable energy systems, backup power supplies, sensing devices, grid stabilization, flexible screens, portable electronic devices, power quality enhancers, high-efficiency converters, and surge absorbers (Abdel Maksoud et al., 2021; Andrew et al., 2023; Ariyarathna et al., 2023; Bharathi Sankar Ammaiyappan et al., 2023; Bhat et al., 2023; Biswas et al., 2023). Supercapacitors are classified into pseudocapacitors and electrochemical double-layer capacitors (EDLCs) based on their energy storage mechanisms. Pseudocapacitors provide more improved energy density and longer life cycle than batteries and work via reversible reduction and reactions (case I: charge store via faradaic processes at or close to the electrode material’s surface with adsorption or intercalation of ions is the repercussion of electrons being transferred across the electrode and electrolyte in the set up) (Mahala et al., 2023; Seenivasan et al., 2023). EDLCs function only through electrostatic processes (case II: there are no chemical processes supported in the charging process; instead, ions associated with the electrolyte are electrostatically adsorbed onto the large surface area of an active electrode) (Pal et al., 2022; Kim et al., 2019).
The electrode material directly affects the working efficiency of a supercapacitor and hence plays a decisive role in energy storage device fabrication. There are three major categories of electrode materials: (a) carbonaceous materials (graphene, rGO, GO etc.) (Pathak et al., 2022), (b) metal oxides (ZnO, Fe2O3, MnO2 etc.) (Shah et al., 2024), and (c) conducting polymers (PANI, PPy, PTh etc.) (Shah et al., 2024; Xu et al., 2024). Each of these materials has inherent issues such as low capacitance (carbonaceous materials), lack of conductivity (metal oxides), and mechanical disintegration (conductive polymers) (Zou et al., 2013; Wu et al., 2015; Pan et al., 2019). The theoretical specific capacitance and electrochemical performance of metal oxides (transition metal oxides particularly) is relatively higher. Metal oxides, including single and transition double metal oxides, have multiple oxidation states, high conductivity, and low toxicity, and are thus cost-effective (Kumar et al., 2020; Kumar et al., 2024; Ni et al., 2022; Priya M et al., 2023; Shaheen et al., 2023; Mathivanan et al., 2024; Raskar et al., 2024). Spinel-type metal oxides/sulfides (AB2X4; A-divalent metal-and B-trivalent metal, O-oxygen, sulfur), especially Ni- and Co-based binary transition metal oxides (MCo2O4; M = Ni, Cu, Zn, Mg, Fe, Mn etc.), are potential active electrode materials owing to their variable oxidation states, high redox activity, environmentally benign nature, and improved capacitance (Jose et al., 2022; Zulqarnain et al., 2024). Nickel cobalt oxide nanostructures exhibit specific capacitance 2000 F g−1 with energy density (Ed) of approximately 280 Wh kg−1 and power density (Pd) of 55 kW kg−1 (Abdolahi, 2021). Nickel-oxide-anchored CoOx nanoparticles on modified commercial nickel foam (NF) exhibits specific capacitance of 475 Fg−1 at 1 mAcm−2 with an energy density of 2.43 Whkg−1 and power density of 0.18 kWkg−1 (Chen et al., 2020). NiCo binary oxide has a specific capacitance of 1607 F/g, having Ed (28 Wh kg-1) and Pd (3064 W kg−1) (Long et al., 2015). Metal–organic frameworks (MOFs) in combination with metal sulfides enhance stability, performance, and electron-transport capability due to tunable porosity, large specific surface area, and diverse surface chemistry. MOFs, known for their robust framework (Kumar et al., 2020), high porosity (Qian et al., 2020), large surface area (Xiao and Jiang, 2019), and adjustable pore size, are porous materials suitable for electrode preparation (Yang et al., 2020; Li et al., 2023). They have been used in several applications, including gas separation (Lawson et al., 2021), photocatalysis (Kreno et al., 2012), drug delivery (Xie et al., 2020), sensing (Jayaramulu et al., 2022), and energy storage. Nevertheless, the practical application of MOFs in electrochemical energy storage is hindered by their inherently low electrical conductivity and inadequate ion transport to the current collector (Chen et al., 2019; Le et al., 2022). Furthermore, derivatives of MOFs with oxides, sulfides, hydroxides, and selenides offer operative electrochemical sites, extensive electrode–electrolyte interfacial areas, and higher conductivity, making them suitable materials for energy storage. However, the electrochemical properties of MOF derivatives of single metals do not meet the expectation of an active electrode material. The electrochemical properties of MOF derivatives can be enhanced by incorporating metal ions into their frameworks, leading to multi-metal MOFs. This approach exploits the synergistic effects of metal ions to improve the overall electrochemical performance of MOFs. Moreover, the introduction of different metal ions can tailor the electronic structure and optimize the redox sites of materials to improve capacity, stability, and rate capability, fine-tune pore size and distribution, and enhance ion transport and diffusion within the electrode material. Multi-metal MOFs are, therefore, a popular material for fabricating high performance and durable energy storage devices (batteries and supercapacitors).
A number of combinations of metal ions, including bimetallic transition sulfides (BMTS) (Radhika et al., 2020), have been used to identify the most effective combination for intended energy storage requirement. NiCo2S4 hollow spheres grown over nickel foam (NF) were used to fabricate supercapacitor electrodes (specific capacitance: 1036 F g−1; at 1.0 A g−1, energy density: 42.3 W h kg−1, power density: 476 W kg−1 (Gao et al., 2023). The specific capacitance (738 F g−1, at current density of 4 A g−1) was reported to be retained up to 93.4% (after 4000 cycles of usage). However, composites of NiCo2S4 and MOFs deposited over porous texture like Ni foam have rarely been explored for electrochemical energy storage application.
In this research, NiCo2S4 and Ni-Co bimetallic MOF were prepared and deposited over highly porous Ni foam via a facile in situ solvothermal method. The resulting 3D flower-like structure with multiple redox active sites and high surface area improved the charged storage capacity of the as-prepared composite. The novel composite showed high specific capacitance with cyclic stability. Overall, the composite material could be a promising candidate in the scenario of the escalating demand for clean energy alternatives.
2 Materials and methods
Analytical-grade reagents were used directly without any additional purification. Nickel foam featuring a porosity of 96.5%, pore size ranging from 0.2 to 0.8 mm, and a thickness of 1.6 mm was obtained from Hefei Kejing Materials Technology, Hefei, China. Cobalt nitrate hexahydrate (Co(NO3)2·6H2O) (CDH, 99.9%), nickel nitrate hexahydrate (Ni(NO3)2·6H2O) (CDH, 99.9%), trimesic acid (98.0%), potassium hydroxide (KOH) (98.0%), and ethanol (98.0%) were sourced from Central Drug House (P) Ltd. (CDH, New Delhi, India). Thiourea (NH2CSNH2) (98.0%), dimethyl formamide (DMF) (99.8%), and hydrochloric acid (HCl) (0.01M) were purchased from Sisco Research Laboratories Pvt. Ltd. (Mumbai, India). Data processing and presentation were carried out using OriginLab 9.0 (2018) software.
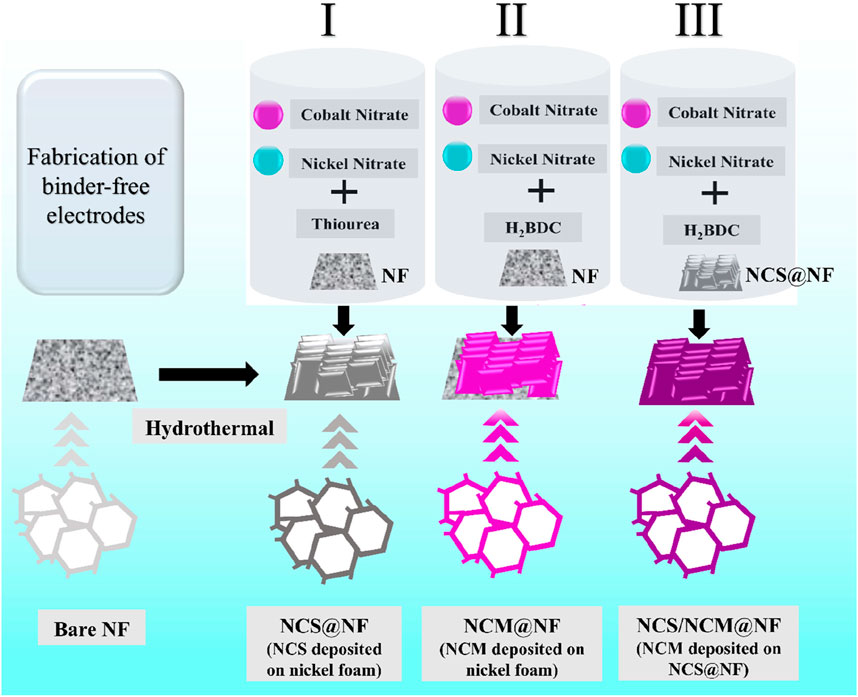
2.1 Fabrication of NiCo2S4-loaded Ni-foam (NF)
A piece of Ni-foam (1 × 1.5 cm2) was cleaned using HCl (37 wt% in H2O) and then ultra-sonicated (25 min) to eliminate the surface oxide layer. The resulting NF surface was dipped into double-distilled water (DDW) and ethanol (four times) to remove acid traces, followed by drying (60°C, 10 h) in a hot-air oven. The NiCo2S4 (NCS) was synthesized hydrothermally. Briefly, nickel nitrate hexahydrate (Ni (NO3)2 .6H2O, 1 mmol) including cobalt nitrate hexahydrate Co (NO3)2.6H2O (2 mmol) was mixed in DDW (70 mL) by 30 min stirring, followed by the addition of thiourea (20 mmol) and magnetic stirring (120 min) until a clear transparent pink solution was obtained. An NF was transferred into 100 mL of hydrothermal autoclave (Teflon-lined stainless steel, Technistro (Autoclave-PTFE-0100)), and the reaction mixture was allowed to proceed (5 h, 150°C) in an oven (Equitron, # 7051-250), followed by cooling to room temperature [40]. NCS-loaded NF (NCS@NF) was separated from the solution and cleaned (with ethanol and DDW) and dried (70°C, 10 h). The loaded NF (NCS@NF) was annealed in a tube furnace (3 h, 350°C, 2°C min−1).
2.2 Fabrication of NiCo-MOF@NF and NiCo2S4@NiCo-MOF @NF electrode
Nickel cobalt MOF-loaded NF (NCM@NF) was also synthesized hydrothermally. Briefly, nickel (II) nitrate hexahydrate (Ni (NO3)2·6H2O, 1.5 mmol) and cobalt (II) nitrate hexahydrate (Co (NO3)2·6H2O, 1.5 mmol) were dissolved in DMF (40 mL) at 26°C (room temperature), followed by the addition of trimesic acid (organic linker) to maintain a pre-decided molar ratio (1:6) relative to the combined moles of Ni2+ and Co2+ ions (Radhika et al., 2020). The vivid purple solution was further stirred (90 min) and poured into a Teflon-lined stainless steel autoclave (100 mL). Nickel foam (NF) was then introduced, and the suspension was subjected to hydrothermal treatment (160°C, 18 h), followed by cooling to an ambient temperature. The loaded NF (NCM@NF) was recovered and rinsed (with ethanol, DMF, and deionized water) to remove traces of solvents. Finally, the material was dried (80°C, 24 h) in a hot-air oven. NiCo2S4@NiCo MOF/NF (NCS/NCM @NF) electrodes were fabricated by following the process used for NCS @NF electrodes (refer to Section 2.2 for fabrication process) instead of bare NF in the above synthesis method (Section 2.3), as shown in Figure 1.
2.3 Characterizations
The electrodes fabricated in Sections 2.2 and 2.3 were further investigated for material characterizations such as XRD, FESEM (EDS), and XPS. The crystalline and amorphous characteristics of the materials were analyzed using a powder X-ray diffractometer (XRD, Rigaku Smart Lab 3 kW) equipped with a Cu–Kα radiation source (λ = 1.540 Å). The electrodes were directly mounted onto the sample holder of the instrument as fabricated. The instrument operated at a constant filament intensity of 25 mA and a power of 45 kV. Data collection was performed over a 2θ range of 5° to 80°, employing a slow scan rate of 2°/min with a step size of 0.02°. The surface morphology and topography of the prepared electrodes were examined using FESEM (FE-SEM, Zeiss Gemini SEM 500 Thermal field emission type). Prior to analysis, the electrode samples were attached over the carbon tape and gently air-blown to remove dust particles. Micrographs were captured at various magnifications with an accelerating voltage of 10 kV. The quantitative elemental composition of the materials was then determined through energy-dispersive X-ray spectroscopy (SEM-EDS). The elemental distribution and oxidation states were further investigated using X-ray photoelectron spectroscopy (XPS, SPECS System). The samples were vacuum-dried, mounted onto conductive sample holders using double-sided carbon tape, and then subjected to data acquistion.
2.4 Electrochemical studies
The electrochemical studies were performed on prepared electrodes (NCS/NF, NCM/NF, NCS/NCM@NF) in Sections 2.2 and 2.3 as working electrodes. The electrodes of the control material had a mass loading of 1.070 mg (≈1.0 mg). The clean NF was first weighed to obtain the initial mass of the active mass of NF. After the material’s deposition and drying, the coated NF was weighed again to record the final mass. The difference between initial and material-loaded NF was considered as mass loading and normalized to the coated surface area. The electrodes were electrochemically analyzed at room temperature (26°C) in a three-electrode assembly in a basic medium (2M, 4M, and 6M KOH) with platinum foil (as a counter-electrode) and Ag/AgCl electrode (as a reference electrode). CV, GCD, and EIS studies were performed using an electrochemical workstation (Auto lab PGSTAT 302N).
3 Results and discussion
3.1 Powder X-ray diffraction (PXRD) studies
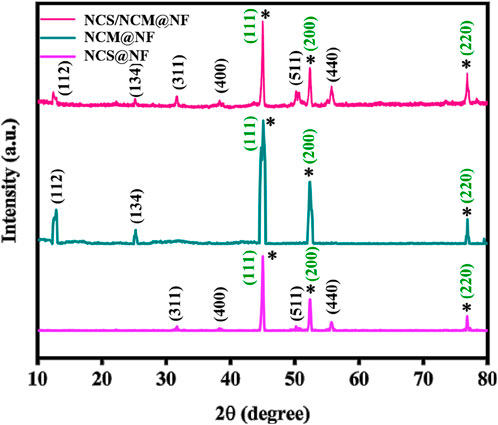
The structural information of all the synthesized samples was investigated using pXRD analysis. The three diffractogram intense peaks at 44.5°, 51.9°, and 76.4°, respectively corresponding to the planes (111), (200), and (220), were of Ni foam (shown by * in Figure 2) having space group (Fm3 m) (JCPDS card no. 00-004-0850) (Yolanda et al., 2020). The X-ray diffraction pattern of the NiCo2S4 material was consistent with JCPDS card No. 20-0782. The characteristic at 31.6°, 38.3°, 50.5°, and 55.3° corresponded to the (311), (400), (511), and (440) planes, respectively, with the Fd-3mspace group and a primitive unit cell a = 9.4177 Å (MadhusudanaRao et al., 2021). The XRD pattern of NiCo-MOF exhibits diffraction peaks which probably have a layered topology crystal structure as a result of connection between central metal ions and BDC ligands to form a 2D layered structure (Radhika et al., 2020). The two sharp peaks (2θ) at 12.9° and 25.8°, corresponding to planes (112) and (134), are due to the presence of Ni/Co-MOF and ZIF-67 (CCDC 671073). In the case of NCM@Ni, small peak splitting was observed in the XRD pattern at 44.5° due to the presence of multiple phases or the influence of strain or preferred orientation within the Ni foam structure.
Peaks at 31.6° (311), 38.3° (400), 50.5° (511), 55.3° (440), 12.9° (112), and 25.8° (134) are attributed to the composite sample (NCS/NCM). The above results confirm the preservation of the layered structures of both MOF and bimetal sulfide in the composite material.
3.2 Surface morphology
The surface morphology of Ni foam is porous, with an average pore size of 60 ±5 µm (Figure 3a), taking into account the standard deviation value calculated by ImageJ software. NCS has a 3D flower-like morphology with petals (sheet like structure) radiating outward from a central core grown on Ni foam (Jiang et al., 2022) (Figure 3b). The overall morphology of NCS is a cluster of 2D layers (size: 200–400 nm) (Figure 3b). Ni-Co MOF has a nano-layered sheet-like morphology (Figure 3c). The composite material has a mixed morphology (Figure 3d) possibly contributed by NiCo2S4 and NiCo-MOF, where MOF particles cover NCS layers to obtain support for their framework.
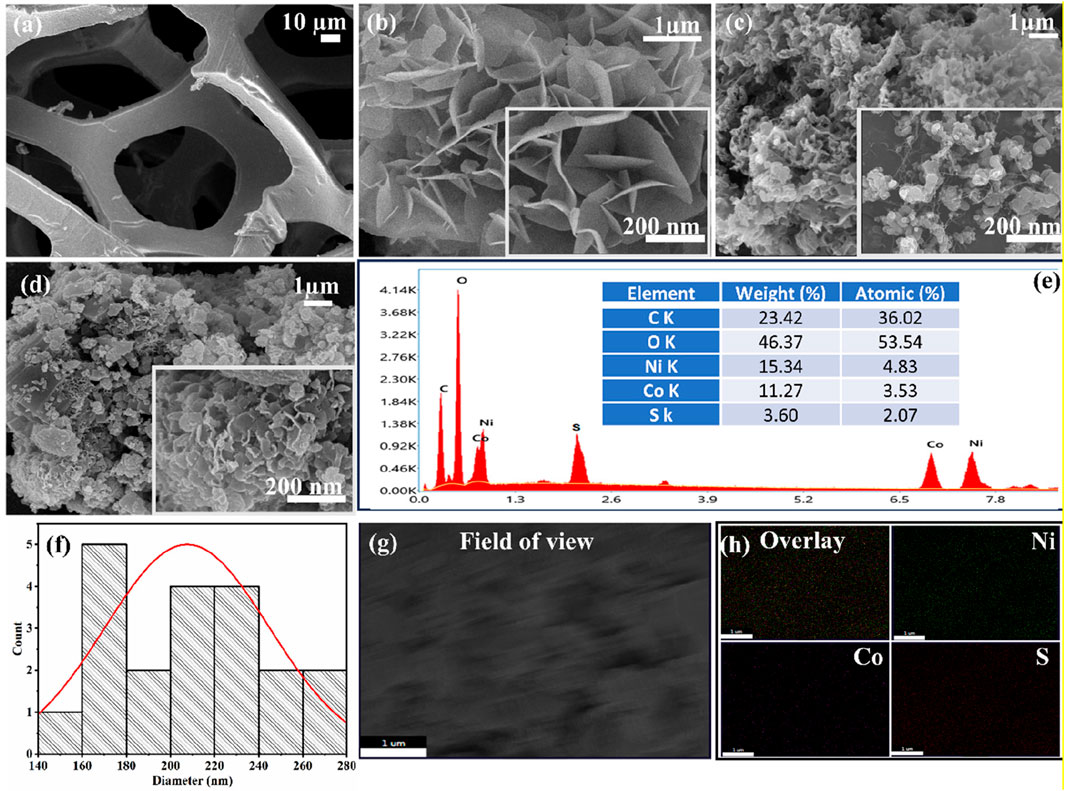
Figure 3. Morphology of (a) Ni Foam, (b) NCS@NF, (c) NCM@NF, (d) NCS/NCM@NF, (e) EDS of NCS/NCM@NF, (f) particle distribution curve of NCS/NCM@NF, (g) field of view for elemental mapping of NCS/NCM@NF, and (h) elemental mapping of NCS/NCM@NF.
The flower-like nanoarrays of NiCo2S4 remained structurally intact beneath the NiCo-MOF layer, suggesting that the distinctive surface morphologies of both materials were well preserved. This indicates successful interfacial integration and structural robustness of the resulting hybrid architecture. Energy-dispersive X-ray spectroscopy (EDX) analysis confirmed the presence of elements in the composite, with the following weight percentages: C (23.42%), O (46.37%), Ni (15.34%), Co (11.27%), and S (3.60%); the corresponding atomic percentages were C (36.02%), O (53.54%), Ni (4.83%), Co (3.53%), and S (2.07%) (Figure 3e). Figure 3f represents the particle size distribution curve for NCS/NCM@ NF and the average size of the particles lie between (140-280 nm) Figures 3g,h represents the field of view and elemental mapping of NCS/NCM@NF for composite material.
3.3 X-ray photoelectron spectroscopy (XPS)
The elemental profiling and oxidation states of NCS/NCM@NF were studied by X-ray photoelectron spectroscopy (XPS). The identical peaks corresponded to Ni2p, Co2p, and S2p, respectively, in the XPS spectrum (Figure 4) with additional contributions from O (from water) and C (CO2 or organic substances). The distinct peaks at 856.1 eV and 873.3 eV correspond to Ni2+ (Ni 2p3/2 and Ni 2p1/2) in the Ni 2p spectrum (Figure 4b). These peaks, complemented by shakeup satellites, confirm the presence of Ni2+. Shake-up satellite peaks are due to the excitation of electrons (valence band to a higher energy state) resulting from interaction between an outgoing electron and a valence electron. The presence of Co2+ and Co3+(in Co 2p spectrum (Figure 4c) may be confirmed by peaks at 778.5 eV, 793.8 eV, 780.9 eV, and 796.5 eV, respectively (Gong et al., 2021). The relatively sharp peaks at 161.2 eV and 162.2 eV in the S2p spectrum (Figure 4d) reveal the presence of S2p3/2 and S2p1/2, respectively (Samba, 2013). Overall, the XPS study provides a detailed picture of the chemical environment of NCS/NCM@NF and confirmed the presence of Ni2+, Co2+, Co3+, and partially oxidized sulfur.
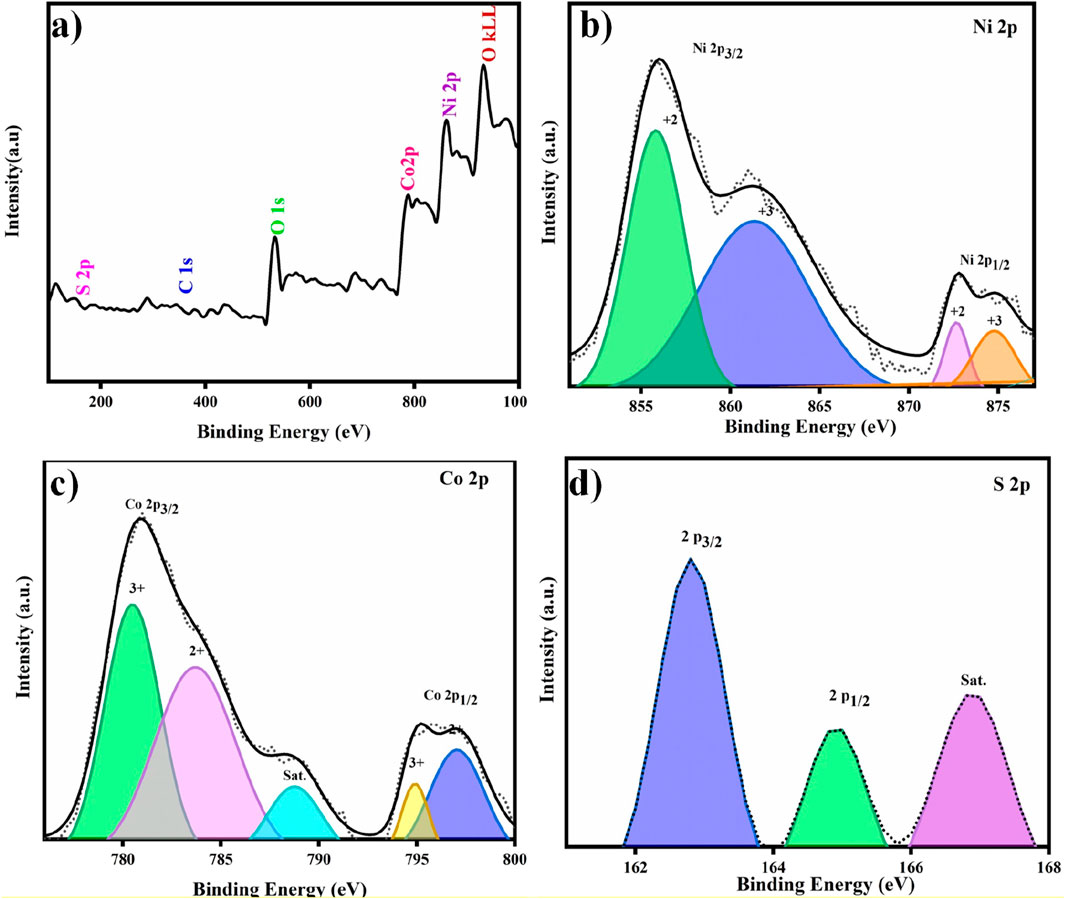
Figure 4. (a) Survey spectra of X-ray photoelectron spectroscopy (XPS) showing all main elements of NCS/NCM@NF, (b) Ni 2p, (c) Co 2p, and (d) S 2p spectra.
4 Electrochemical studies
The electrochemical performance of NCS@NF, NCM@NF, and NCS/NCM@NF electrodes was studied with the changing molarity of KOH (2M, 4M, and 6M) as the electrolyte. The basic (KOH) electrolyte was used as it enhances the conductivity and ensures ion transfer for specific electrochemical reactions.
Peaks in the CV research of NCS material in composite may be attributed to redox reactions. NCS/NCM@NF exhibits larger peak value current and higher enclosed surface area compared to its counterparts, possibly due to high electrochemical activity and specific capacity. The electrochemical property of active materials was evaluated on a three-electrode assembly system at different scan rates (2, 5, 10, 20, 50, and 100 mVs−1). On the other hand, redox peaks in CV research are attributed to faradic redox reactions (Wang et al., 2019). The anodic and cathodic peaks shift toward higher and lower voltages, respectively, with an increase in the scan rate (Keithley et al., 2011) due to the insufficient insertion of hydroxyl ions (OH−) in the basic electrolyte into the center of the NCS material. A higher specific capacitance Cs was achieved with 6M KOH because the concentration number of ions increased due to the high ionic mobility of K+ and OH− ion in aqueous media (Zhang et al., 2012). The molarity beyond 6M was avoided to avert the negative impact of corrosiveness, electrode degradation, and the hindrance of ion transfer due to higher viscosity. High molarity leads to the crystallization of base (KOH), which blocks ion pathways and impacts the electrode structure. The high molarity (6M) of KOH maintains a balance between efficient ion transfer and compatibility with materials to meet the requirements of electrochemical studies (Zhang et al., 2012). The capacitance of NCS/NCM@NF was relatively higher due to the synergistic effect of NiCO2S4 and Ni Co MOF in which NiCO2S4 nano sheets facilitate efficient electron transport and Ni Co MOF offers pathways for rapid ion diffusion, reducing resistance and enhancing overall conductivity.
4.1 Cyclic voltammetry studies (CV)
The specific capacitance (CS) of materials was determined using the formula of Wang et al. (2013) as given in Equation 1:
We used the data from the third cycle (n = 3) to calculate the specific capacitance because the first cycle often shows some initial changes such as surface activation or electrolyte wetting. The second cycle starts to stabilize and, by the third cycle, the current response becomes steady and more reliable. This approach is widely used to avoid early-stage variations (Han et al., 2017). The electrochemical stability of an electrolyte solution is intrinsically related to its potential window. In supercapacitors, the high potential window defines the maximum safe operating voltage range within which the device can undergo charge and discharge cycles without substantial degradation or failure (Shrestha, 2022). This upper voltage limit is critical as it determines the capacity of the device to maintain structural and functional integrity over prolonged use. The potential windows of NCM@NF, NCS@NF, and NCS/NCM@NF are 0.45 V, 0.95 V, and 1.0 V, respectively. Higher potential windows of the composite indicate that the material effectively facilitates redox reactions and ionic transport within the electrolyte, leading to overall advancement in energy storage applications.
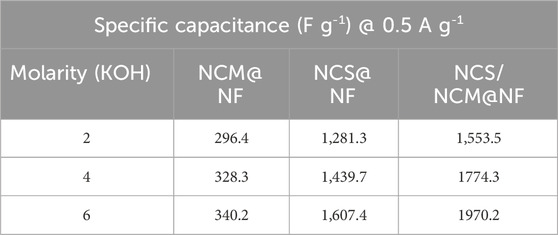
The specific capacitance of composite at 6M KOH (at 2 mV s−1) was relatively higher (2,150.3 F/g) than NCM@NF (393.1 F g−1) and NCS@NF (1,542.2 F g−1) (Figures 5a,c,e (Table 1). The increase in the closed area of the CV curve with rising scan rates, while maintaining its shape, indicates that the as-synthesized electrode material undergoes highly reversible redox reactions (Figure 5b,d,f; Table 2). Moreover, at high scan rates, the limited contact time between the electrode and electrolyte hinders ion diffusion into the deeper active sites of the electrode. This restricted interaction reduces the effective utilization of these sites, ultimately leading to a decrease in the charge storage capacity.
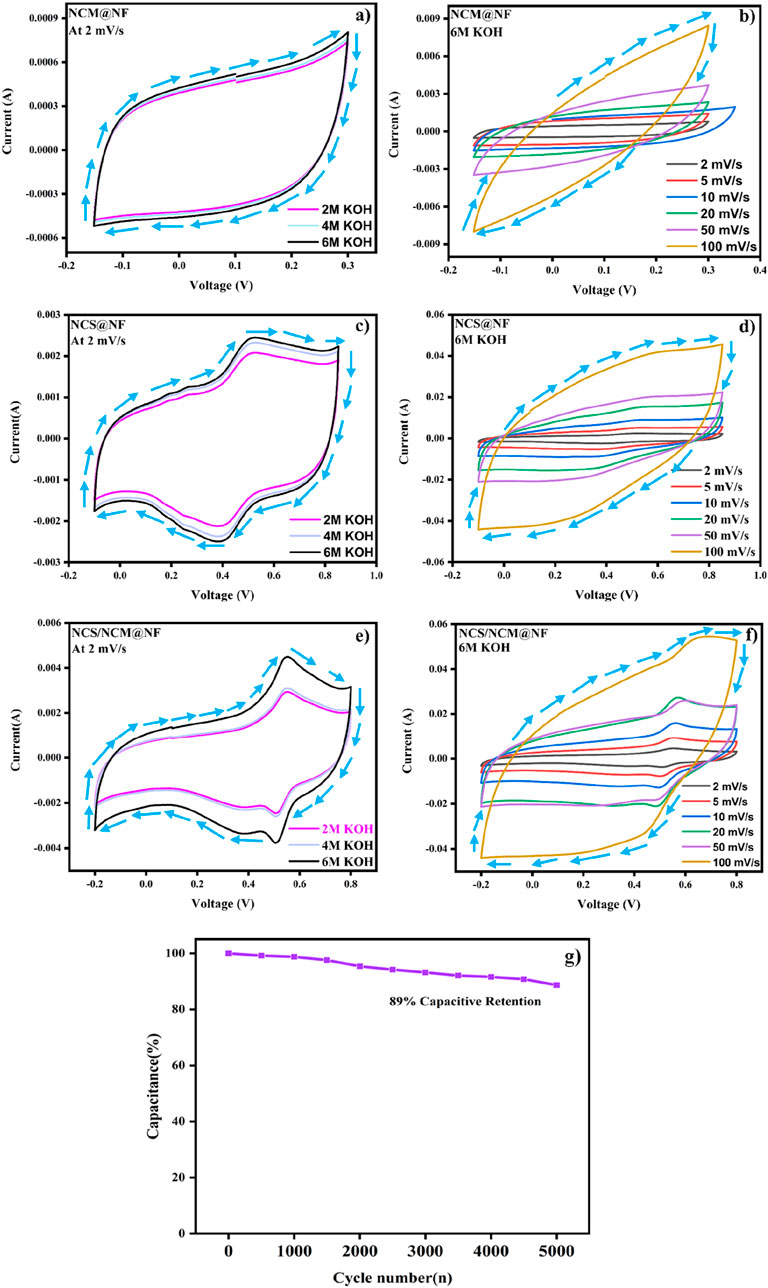
Figure 5. (a) Cyclic voltammetry curves of NCM@NF, (c) NCS@NF, and (e) NCM@NF at varying base (KOH) concentrations (2M, 4M, and 6M) at 2 mV/S; (b) cyclic voltammetry curves of NCM@NF, (d) NCS@NF, and (f) NCS/NCM@NF at different scan rates at 6M KOH; (g) cyclic stability of composite NCS/NCM@NF over 10,000 cycles.
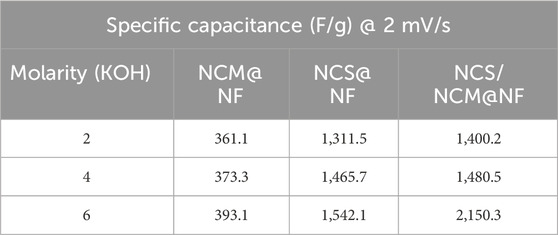
Table 1. Specific capacitance of NCM@NF, NCS@NF, and NCS/NCM@NF at different molarities of KOH (2M, 4M, and 6M) as electrolyte at 2 mV/s scan rate.
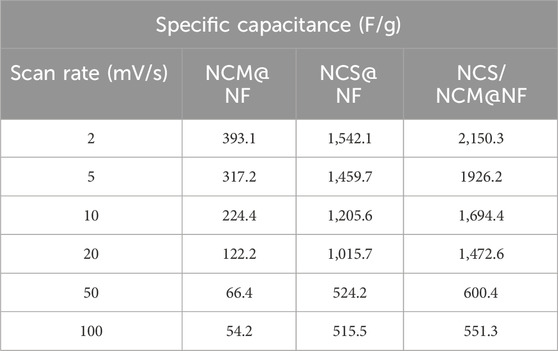
Table 2. Specific capacitance of NCM@NF, NCS@NF, and NCS/NCM@NF at different scan rates with a fixed molarity of electrolyte KOH (6M).
The durability of the electrode material plays a crucial role in ensuring its suitability for practical applications. The capacitance of the as-synthesized composite electrode material (NCS/NCM@NF) retained 89%, even after 10,000 cycles of usage; the same demonstrates the electrode material’s excellent stability and suitability for extended use (Figure 5g). These results highlight the effectiveness of the core-shell structure, which provides a robust and stable framework for energy storage. The enhanced capacitance of NiCo2S4 and NiCo MOF composite may be attributed to the unique structural properties of NiCo2S4, which facilitate rapid ion diffusion pathways and electron transport, thus leading to increased charge storage capacity. Furthermore, the presence of Ni-Co MOF contributes additional active sites for electrochemical reactions and enhanced capacitance. There is also the satellite reduction peak in the CV curve of the NCS/NCM@Ni composite, which is absent in the bare NCM and NCS electrodes. This is due to the synergistic effect between NiCo2S4 and MOF, not only improving energy density but also enhancing the cyclic performance, rendering the composite ideal for energy storage applications.
4.2 Galvanostatic charge/discharge analysis (GCD)
Galvanostatic charge–discharge (GCD) is a widely used approach in electrochemistry for charge and discharge characteristic assessment of energy storage devices (such as batteries and supercapacitors). This approach involves the application of a constant current to charge and discharge a material followed by the measurement of parameters like Cs, Ed, and Pd.
The GCD curves of NCM@NF, NCS@NF, and NCS/NCM@NF electrodes, with reference electrode (Ag/AgCl) at constant current densities (0.5 A/g, 1 A/g, 2 A/g, and 3 A/g), are depicted in Figures 6a–f. The performance of the composite material electrode was better than that of the precursor material electrode, possibly due to ionic transport capabilities in the former. The results underscore the composite material’s potential as a favorable candidate for advanced energy storage applications.
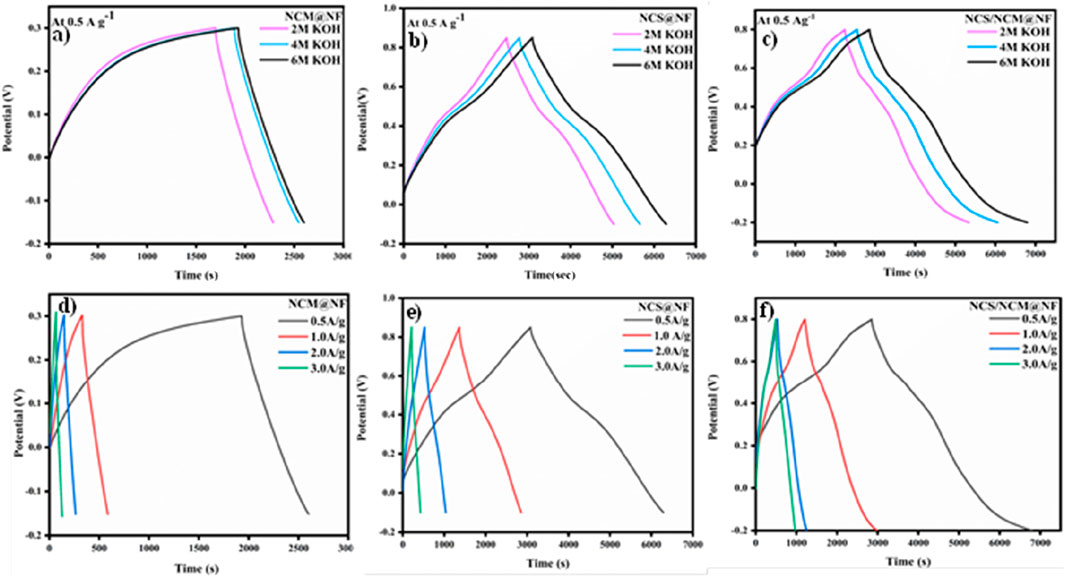
Figure 6. GCD curves of NCM@NF (a), NCS@NF (b), and NCS/NCM@NF (c) with varying KOH molarities (2M, 4M, and 6M) at 0.5 A/g and GCD curves of NCM@NF (d), NCS@NF (e), and NCS/NCM@NF (f) with changing current densities and 6M electrolyte.
The CS, Ed, and Pd of the electrode materials were calculated (Pal et al., 2021) using Equations 2–4 to provide a comprehensive understanding of the performance of electrode materials.
where I is current density (A g−1), ∆t is discharging time (s) as calculated from the GCD curve, m is mass (mg) deposited on the electrode, and ∆V is potential window (V).
The noticeable nonlinear behavior seen in the GCD patterns can be linked to quasi-reversible Faradaic reactions occurring within the system. These reactions cause fluctuations in the charge–voltage ratio, leading to dynamic variations throughout the discharge process. As a result, calculating the specific capacitance under such conditions requires an integration method that considers the changing charge–voltage relationship influenced by these Faradaic processes. The specific capacitance at different molarities (2M,4M and 6M KOH) at 0.5 A g-1 is shown in Table 3. and higher capacitance is acheived in case of 6M KOH. The specific capacitance (F g-1), at a current density of 0.5 A g−1, were 340.2 F g−1 (NCM@NF), 1,607.4 F g−1 (NCS@NF), and 1970.2 F g−1 (NCS/NCM @NF) at 6M KOH shown in Table 4. The significant rise in the capacitance of the composite (particularly at elevated current densities) offers the composite as a potential material for energy storage applications under diverse operational conditions. The increased current density restricts ion diffusion and reduces exposure to the electrode surface, thereby restricting efficient charge storage. Higher current densities can lead to polarization effects, non-uniform ion distribution, and increased internal resistance that impedes the efficient movement of ions and decrease overall capacitance. Furthermore, elevated current may instigate undesirable Faradaic reactions, altering the structural and electrochemical properties of electrode material and diminishing their capacitance. This phenomenon accounts for the reduction in CS with increasing current densities. The hiked Ed (199.6 W h kg−1) and Pd (1,500.2 W kg−1) at 6M KOH shows the composite to be viable for high-performance energy storage applications, particularly in meeting rapid charge and discharge requirements. The NCS/NCM@NF composite exhibits exceptional electrochemical performance, attributable to several key factors.
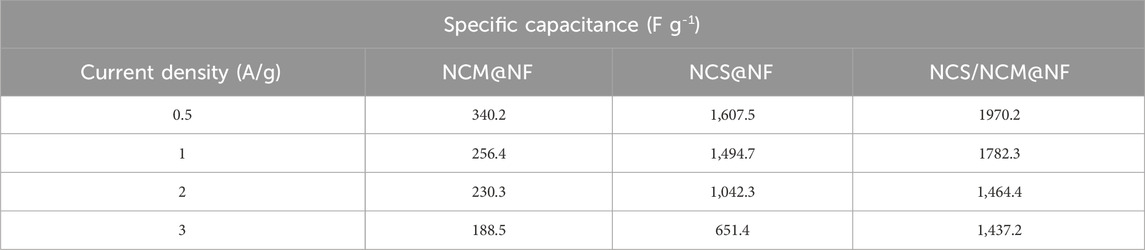
Table 4. Specific capacitance of NCM@NF, NCS@NF, and NCS/NCM@NF with varying molarity of electrolytes (2, 4, and 6M).
The active material was directly grown on nickel foam to eliminate the need for binders and conductive additives, which shortens the diffusion path of ions and relatively decreased internal and diffusion resistance. Moreover, the core-shell structure enhances the electrode–electrolyte interface, improves contact, increases active sites, and initiates accelerated electron transfer. The Ni-Co MOF serves as a core layer, providing abundant Faradaic redox reaction sites, while NiCo2S4 as a shell material enhances electron conductivity and contributes additional pseudo capacitance. The synergistic effect of these three materials further improved the capacitance of the electrode. This combination provides structural advantages and improved material properties as composite (NCS/NCM@NF), making it a suitable candidate for advanced energy storage applications. The comparison of different electrode materials with NCS/NCM @NF over Specific capacitance and capacitive retention is shown in Table 5.
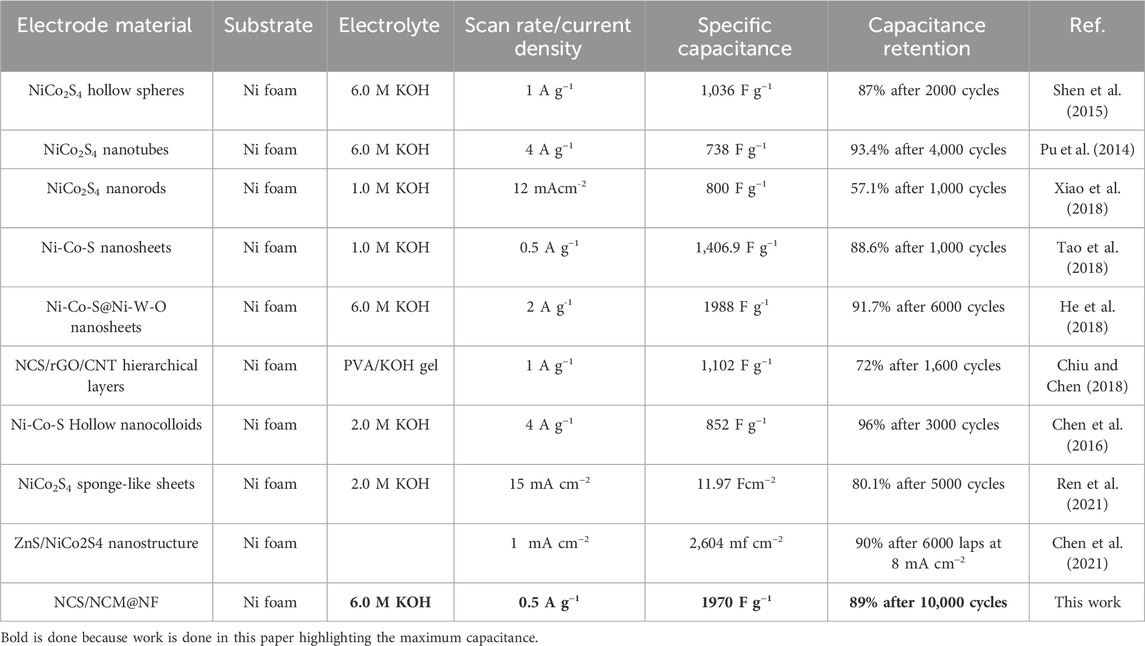
Table 5. Comparison of capacitance of materials reported herein with materials reported in the literature.
4.3 Electrochemical impedance spectroscopy (EIS)
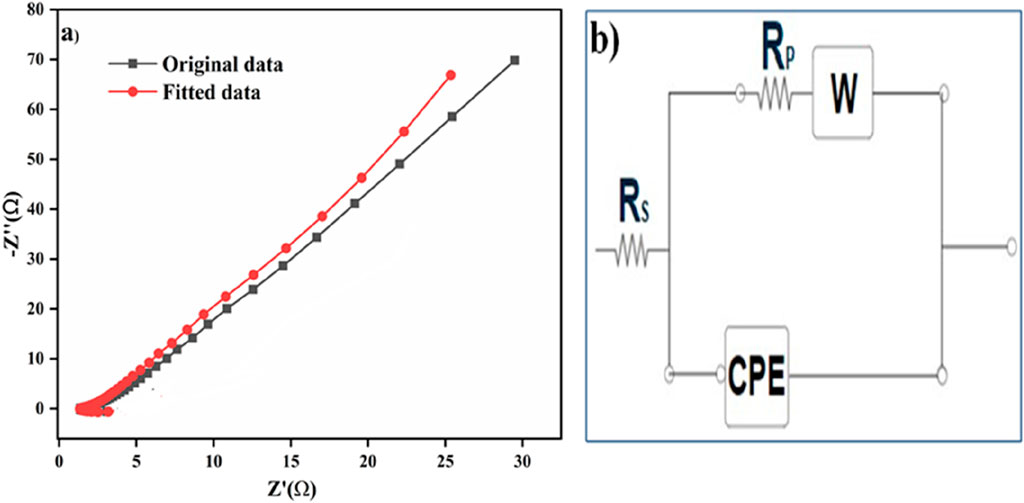
Electrochemical impedance spectroscopy (EIS) is a powerful technique that evaluates a system’s electrical response by applying an alternating current (AC) signal across a wide frequency range, typically from 0.01 Hz to 106 Hz. Through detailed analysis of the resulting impedance spectrum, crucial electrochemical parameters such as the solution resistance (Rs), charge transfer resistance (Rct), and interfacial properties can be extracted. This method provides valuable insights into ion transport kinetics, electrode–electrolyte interfaces, and overall electrochemical performance (Wu et al., 2010). The data obtained were fitted using an equivalent circuit model in NOVA 2.1 software. The Nyquist plot, which represents the imaginary (Zʺ) versus the real impedance (Z′), illustrates the system’s overall electrochemical behavior. Typically, Nyquist plots initiate at high frequencies due to restricted sample diffusion, influencing the impedance characteristics. The fitted circuit included several components such as Rs, Rp, a constant phase element (CPE), and a Warburg element (W) shown in (Figure 7b). The composite material NCS/NCM@NF exhibited a solution resistance of 1.52 Ω, indicating minimal resistance contribution from the electrolyte to the current flow. The Rp reflects the resistance at the electrode–electrolyte interface, which was found to be 790 mΩ, suggesting efficient charge transfer kinetics.
The presence of a constant phase element (CPE) highlights the system’s non-ideal capacitive behavior. CPE is frequency-dependent and provides insights into factors such as surface roughness, porosity, and material heterogeneity on the electrode surface. The Warburg element (W) is a crucial component in EIS analysis that characterizes ion diffusion behavior within the electrochemical system, exhibiting a frequency-dependent response proportional to √ω, where ω denotes the angular frequency of the applied AC voltage. In the Nyquist plot (Figure 7a), which represents the real (Z′) versus the imaginary (Zʺ) impedance, this diffusion behavior is typically observed as a linear region at low frequencies. The Nyquist plot is widely employed to extract key electrochemical parameters such as solution resistance (Rs), charge transfer resistance (Rct), and diffusion-related characteristics, providing comprehensive insights into the system’s ionic transport and interfacial properties. The accuracy of the fit has been evaluated using the chi-squared (χ2) value, which was found to be 0.848, as provided by software. This value reflects a reasonable agreement between the experimental and the fitted data.
5 Conclusion
Double-metal NiCo-MOF, NiCo2S4 spinel material, and their composite (namely: NCS/NF, NCM/NF, and NCS/NCM@NF) were successfully synthesized using a simple method and immobilized onto nickel foam to eliminate binder-related issues. The NCS/NF composite serves as an effective and uniform base platform, facilitating the predominant surface formation of the NCS/NCM@NF composite material. The overall electrochemical performance of the active materials was tuned by varying the KOH molarity (2M, 4M, and 6M). The binder-free NCS/NCM@NF composite electrode, which incorporates both Ni and Co, exhibited enhanced electrochemical performance in a 6M KOH electrolyte. The composite material achieved a remarkable CS of 2,150.3 F g−1 at a scan rate of 2 mV s−1, demonstrating its exceptional energy storage capability. The electrode’s durability was confirmed through cyclic stability tests, retaining approximately 89% of its capacitance even after 10,000 cycles. Furthermore, the composite delivered a high Ed of 199.6 Wh kg−1 and a Pd of 1,500.2 W kg−1, demonstrating its capability for practical energy storage device applications. EIS studies revealed efficient ionic and electronic transport within the electrodes, attributed to minimal charge transfer and solution resistance. Consequently, the Ni-Co MOF- and NiCo2S4-based electrodes hold significant promise for advanced energy storage systems.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
PW: Data curation, Formal Analysis, Writing – original draft, methodology. PS: Writing – review and editing, Formal analysis, Methodology. RS: Methodology, Data curation, Writing – original draft. KJ: Formal Analysis, Data curation, Writing – original draft. ND: Resources, Investigation, Methodology, Writing – review and editing, Supervision. RD: Investigation, Methodology, Writing – review editing, Supervision. NS: Writing – review and editing, Methodology, Formal analysis. MS: Formal analysis, Investigation, Methodology, Writing – review and editing. SK: Resources, Visualization, Formal analysis, Conceptualization, Methodology, Writing – review and editing, Supervision, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Sandeep Kumar thanks the Department of Science and Technology, Govt. of India for a DST-PURSE grant (No. SR/PURSE/2024/350 dated 14-10-2024).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel Maksoud, M. I. A., Fahim, R. A., Shalan, A. E., Abd Elkodous, M., Olojede, S. O., Osman, A. I., et al. (2021). Advanced materials and technologies for supercapacitors used in energy conversion and storage: a review. Springer International Publishing. doi:10.1007/s10311-020-01075-w
Abdolahi, B., Gholivand, M. B., Shamsipur, M., and Amiri, M. (2021). Engineering of nickel-cobalt oxide nanostructures based on biomass material for high performance supercapacitor and catalytic water splitting. Int. J. Energy Res. 45, 12879–12897. doi:10.1002/er.6618
Andrew, L. J., Gillman, E. R., Walters, C. M., Lizundia, E., and MacLachlan, M. J. (2023). Multi-responsive supercapacitors from chiral nematic cellulose nanocrystal-based activated carbon aerogels. Small 19, e2301947. doi:10.1002/smll.202301947
Ariyarathna, T., Kularatna, N., and Gunawardane, K. (2023). “Supercapacitor assisted extra low frequency power converters and surge protectors: applying Supercapacitor Assisted Loss Management Concept in practical applications,” in Conf. Proc. - IEEE appl. Power electron. Conf. Expo. - APEC 2023-march, 1840–1845. doi:10.1109/APEC43580.2023.10131267
Bharathi Sankar Ammaiyappan, A., Seyezhai, R., Someswaran, M., and Abinanthan Ram, M. (2023). Piezoelectric driven self-powered supercapacitor for wearable device applications. Mater. Today Proc. doi:10.1016/j.matpr.2023.09.102
Bhat, M. Y., Hashmi, S. A., Khan, M., Choi, D., and Qurashi, A. (2023). Frontiers and recent developments on supercapacitor’s materials, design, and applications: transport and power system applications. J. Energy Storage 58, 106104. doi:10.1016/j.est.2022.106104
Biswas, S., Shauloff, N., Bisht, R., and Jelinek, R. (2023). Anthraquinone-functionalized polydiacetylene supercapacitors. Adv. Sustain. Syst. 7, 1–9. doi:10.1002/adsu.202300035
Chen, B., Zhong, Y., Shen, G., Wang, F., Liu, Z., Chen, M., et al. (2020). Binder-free nickel oxide lamellar layer anchored CoO x nanoparticles on nickel foam for supercapacitor electrodes. Nanomater. (Basel). 10, 194. doi:10.3390/nano10020194
Chen, H. Q., Xiang, J., Zhao, R.Da, Guo, Y., Loy, S., Wu, F. F., et al. (2021). ZnS/NiCo2S4 arrays on nickel foam as an energy storage for supercapacitor. Ionics (Kiel) 27, 867–874. doi:10.1007/s11581-020-03868-z
Chen, Y., Zhai, B., Liang, Y., Li, Y., and Li, J. (2019). Preparation of CdS/g-C3N4/MOF composite with enhanced visible-light photocatalytic activity for dye degradation. J. Solid State Chem. 274, 32–39. doi:10.1016/j.jssc.2019.01.038
Chen, Z., Wan, Z., Yang, T., Zhao, M., Lv, X., Wang, H., et al. (2016). Preparation of nickel cobalt sulfide hollow nanocolloids with enhanced electrochemical property for supercapacitors application. Sci. Rep. 6, 25151–25158. doi:10.1038/srep25151
Chiu, C.-T., and Chen, D.-H. (2018). One-step hydrothermal synthesis of three-dimensional porous Ni–Co sulfide/reduced graphene oxide composite with optimal incorporation of carbon nanotubes for high performance supercapacitors. Nanotechnology 29, 175602. doi:10.1088/1361-6528/aaaff5
Gao, M., Wang, Z., Lek, D. G., and Wang, Q. (2023). Towards high power density aqueous redox flow batteries. Nano Res. Energy 2, e9120045–e9120048. doi:10.26599/NRE.2023.9120045
Gong, J., Yang, J., Wang, J., Lv, L., Wang, W., Pu, L., et al. (2021). A dual NiCo metal-organic frameworks derived NiCo2S4 core-shell nanorod arrays as high-performance electrodes for asymmetric supercapacitors. Electrochim. Acta 374, 137794. doi:10.1016/j.electacta.2021.137794
Han, J., Xi, H. Z., and Chen, C. C. (2017). Cycling stability of iron-based layered double hydroxide thin-films for battery-type electrode materials. J. Mater. Sci. Mater. Electron. 28, 2754–2762. doi:10.1007/s10854-016-5855-9
He, W., Liang, Z., Ji, K., Sun, Q., Zhai, T., and Xu, X. (2018). Hierarchical Ni-Co-S@Ni-W-O core–shell nanosheet arrays on nickel foam for high-performance asymmetric supercapacitors. Nano Res. 11, 1415–1425. doi:10.1007/s12274-017-1757-2
Jayaramulu, K., Mukherjee, S., Morales, D. M., Dubal, D. P., Nanjundan, A. K., Schneemann, A., et al. (2022). Graphene-based metal-organic framework hybrids for applications in catalysis, environmental, and energy technologies. Chem. Rev. 122, 17241–17338. doi:10.1021/acs.chemrev.2c00270
Jiang, J., Li, F., Su, H., Gao, Y., Li, N., and Ge, L. (2022). Flower-like NiCo2S4/NiFeP/NF composite material as an effective electrocatalyst with high overall water splitting performance. Chin. Chem. Lett. 33, 4367–4374. doi:10.1016/j.cclet.2021.12.028
Jose, V., Jose, V., Freeda Christy, C. E., and Nesaraj, A. S. (2022). Spinel-based electrode materials for application in electrochemical supercapacitors – present status and future prospects. Inorg. Nano-Metal Chem. 52, 1449–1462. doi:10.1080/24701556.2021.1956968
Keithley, R. B., Takmakov, P., Bucher, E. S., Belle, A. M., Owesson-White, C. A., Park, J., et al. (2011). Higher sensitivity dopamine measurements with faster-scan cyclic voltammetry. Anal. Chem. 83, 3563–3571. doi:10.1021/ac200143v
Khan, H. A., Tawalbeh, M., Aljawrneh, B., Abuwatfa, W., Al-Othman, A., Sadeghifar, H., et al. (2024). A comprehensive review on supercapacitors: their promise to flexibility, high temperature, materials, design, and challenges. Energy 295, 131043. doi:10.1016/j.energy.2024.131043
Kim, J., Park, S.-J., Chung, S., and Kim, S. (2019). Preparation and capacitance of Ni metal organic framework/reduced graphene oxide composites for supercapacitors as nanoarchitectonics. J. Nanosci. Nanotechnol. 20, 2750–2754. doi:10.1166/jnn.2020.17469
Kreno, L. E., Leong, K., Farha, O. K., Allendorf, M., Van Duyne, R. P., and Hupp, J. T. (2012). Metal-organic framework materials as chemical sensors. Chem. Rev. 112, 1105–1125. doi:10.1021/cr200324t
Kumar, S., Tahira, A., Bhatti, A. L., Bhatti, M. A., Ujjan, Z. A., Aftab, U., et al. (2024). Oxygen vacancies and tailored redox activity encountered with NiCo2O4 nanostructures for promising applications in supercapacitor and water oxidation. J. Energy Storage 77, 109994. doi:10.1016/j.est.2023.109994
Kumar, Y., Chopra, S., Gupta, A., Kumar, Y., Uke, S. J., and Mardikar, S. P. (2020). Low temperature synthesis of MnO2 nanostructures for supercapacitor application. Mater. Sci. Energy Technol. 3, 566–574. doi:10.1016/j.mset.2020.06.002
Lawson, H. D., Walton, S. P., and Chan, C. (2021). Metal-organic frameworks for drug delivery: a design perspective. ACS Appl. Mater. Interfaces 13, 7004–7020. doi:10.1021/acsami.1c01089
Le, Q. B., Nguyen, T.-H., Fei, H., Bubulinca, C., Munster, L., Bugarova, N., et al. (2022). Electrochemical performance of composite electrodes based on rGO, Mn/Cu metal–organic frameworks, and PANI. Sci. Rep. 12, 664. doi:10.1038/s41598-021-04409-y
Li, Z., Pei, D., Tian, R., and Lu, C. (2023). Screening the specific surface area for metal-organic frameworks by cataluminescence. Chemosensors 11, 292. doi:10.3390/chemosensors11050292
Long, C., Zheng, M., Xiao, Y., Lei, B., Dong, H., Zhang, H., et al. (2015). Amorphous Ni-Co binary oxide with hierarchical porous structure for electrochemical capacitors. Appl. Mater. $ Interfacesaterials $ Interfaces 7, 24419–24429. doi:10.1021/acsami.5b03036
MadhusudanaRao, K., Raghavendra, K. V. G., and Han, S. S. (2021). Facile synthesis of layered NiCo2S4/NiO/nickel foam nanoarchitecture as a sophisticated efficient electrode for next-generation supercapacitors. J. Energy Storage 41, 102839. doi:10.1016/j.est.2021.102839
Mahala, S., Khosravinia, K., and Kiani, A. (2023). Unwanted degradation in pseudocapacitors: challenges and opportunities. J. Energy Storage 67, 107558. doi:10.1016/j.est.2023.107558
Mathivanan, A., Jothibas, M., and Nesakumar, N. (2024). Synthesis and characterization of ZnCo2O4 nanocomposites with enhanced electrochemical features for supercapacitor applications. Surfaces Interfaces 49, 104443. doi:10.1016/j.surfin.2024.104443
Ni, H., Ke, Z., Hu, T., Liu, W., Tian, Y., Zhu, X., et al. (2022). Facile synthesis of dual-morphological MgCo2O4 with remarkable performance for pseudosupercapacitors. Mater. Adv. 4, 134–149. doi:10.1039/d2ma00889k
Pal, A., Ghosh, S., Singha, D., and Nandi, M. (2021). Morphology controlled synthesis of heteroatom-doped spherical porous carbon particles retaining high specific capacitance at high current density. ACS Appl. Energy Mater. 4, 10810–10825. doi:10.1021/acsaem.1c01786
Pal, B., Sarkar, K. J., Wu, B., Děkanovský, L., Mazánek, V., Jose, R., et al. (2022). Exploration of charge storage behavior of binder-free EDL capacitors in aqueous electrolytes. ACS Omega 8, 2629–2638. doi:10.1021/acsomega.2c07143
Pan, Y., Xu, K., and Wu, C. (2019). Recent progress in supercapacitors based on the advanced carbon electrodes. Nanotechnol. Rev. 8, 299–314. doi:10.1515/ntrev-2019-0029
Pathak, M., Tatrari, G., Karakoti, M., Pandey, S., Sahu, P. S., Saha, B., et al. (2022). Few layer graphene nanosheets from kinnow peel waste for high-performance supercapacitors: a comparative study with three different electrolytes. J. Energy Storage 55, 105729. doi:10.1016/j.est.2022.105729
Pershaanaa, M., Bashir, S., Ramesh, S., and Ramesh, K. (2022). Every bite of Supercap: a brief review on construction and enhancement of supercapacitor. J. Energy Storage 50, 104599. doi:10.1016/j.est.2022.104599
Priya, M., Udhayan, S., Vasantharani, P., and Sivakumar, G. (2023). Energy storage performance and photocatalytic activity of Diamond-like ZnCo2O4 nanostructure. Inorg. Chem. Commun. 153, 110762. doi:10.1016/j.inoche.2023.110762
Pu, J., Wang, T., Wang, H., Tong, Y., Lu, C., Kong, W., et al. (2014). Direct growth of NiCo2S4 nanotube arrays on nickel foam as high-performance binder-free electrodes for supercapacitors. Chempluschem 79, 577–583. doi:10.1002/cplu.201300431
Qian, Q., Asinger, P. A., Lee, M. J., Han, G., Mizrahi Rodriguez, K., Lin, S., et al. (2020). MOF-based membranes for gas separations. Chem. Rev. 120, 8161–8266. doi:10.1021/acs.chemrev.0c00119
Radhika, M. G., Gopalakrishna, B., Chaitra, K., Bhatta, L. K. G., Venkatesh, K., Sudha Kamath, M. K., et al. (2020). Electrochemical studies on Ni, Co and Ni/Co-MOFs for high-performance hybrid supercapacitors. Mater. Res. Express 7, 054003. doi:10.1088/2053-1591/ab8d5d
Raskar, N. D., Dake, D. V., Mane, V. A., Sonpir, R. B., Vasundhara, M., Asokan, K., et al. (2024). Designing reduced graphene oxide decorated Ni doped δ-MnO2 nanocomposites for supercapacitor applications. Mater. Sci. Semicond. process. 178, 108451. doi:10.1016/j.mssp.2024.108451
Ren, F., Ji, Y., Tan, S., and Chen, F. (2021). Sponge-like NiCo2S4nanosheets supported on nickel foam as high-performance electrode materials for asymmetric supercapacitors. Inorg. Chem. Front. 8, 72–78. doi:10.1039/d0qi01085e
Samba, R. (2013). Is the enhanced adhesion of PEDOT thin films on electrodes due to sulfur - gold interaction? - an XPS study. Open Surf. Sci. J. 5, 17–20. doi:10.2174/1876531901305010017
Seenivasan, S., Shim, K. I., Lim, C., Kavinkumar, T., Sivagurunathan, A. T., Han, J. W., et al. (2023). Boosting pseudocapacitive behavior of supercapattery electrodes by incorporating a Schottky junction for ultrahigh energy density. Nano-Micro Lett. 15, 62–21. doi:10.1007/s40820-023-01016-6
Shah, S. S., Niaz, F., Ehsan, M. A., Das, H. T., Younas, M., Khan, A. S., et al. (2024). Advanced strategies in electrode engineering and nanomaterial modifications for supercapacitor performance enhancement: a comprehensive review. J. Energy Storage 79, 110152. doi:10.1016/j.est.2023.110152
Shaheen, I., Hussain, I., Zahra, T., Javed, M. S., Shah, S. S. A., Khan, K., et al. (2023). Recent advancements in metal oxides for energy storage materials: design, classification, and electrodes configuration of supercapacitor. J. Energy Storage 72, 108719. doi:10.1016/j.est.2023.108719
Shen, L., Yu, L., Wu, H. B., Yu, X. Y., Zhang, X., and Lou, X. W. (2015). Formation of nickel cobalt sulfide ball-in-ball hollow spheres with enhanced electrochemical pseudocapacitive properties. Nat. Commun. 6, 6694–6698. doi:10.1038/ncomms7694
Shrestha, D. (2022). Evaluation of physical and electrochemical performances of hardwood and softwood derived activated carbons for supercapacitor application. Mater. Sci. Energy Technol. 5, 353–365. doi:10.1016/j.mset.2022.09.002
Tao, K., Han, X., Ma, Q., and Han, L. (2018). A metal-organic framework derived hierarchical nickel-cobalt sulfide nanosheet array on Ni foam with enhanced electrochemical performance for supercapacitors. Dalt. Trans. 47, 3496–3502. doi:10.1039/c7dt04942k
Wang, L., Wang, X., Xiao, X., Xu, F., Sun, Y., and Li, Z. (2013). Reduced graphene oxide/nickel cobaltite nanoflake composites for high specific capacitance supercapacitors. Electrochim. Acta 111, 937–945. doi:10.1016/j.electacta.2013.08.094
Wang, Q., Luo, Y., Hou, R., Zaman, S., Qi, K., Liu, H., et al. (2019). Redox tuning in crystalline and electronic structure of bimetal–organic frameworks derived cobalt/nickel boride/sulfide for boosted faradaic capacitance. Adv. Mater. 31, e1905744. doi:10.1002/adma.201905744
Wu, C., Cai, J., Zhang, Q., Zhou, X., Zhu, Y., Li, L., et al. (2015). Direct growth of urchin-like ZnCo2O4 microspheres assembled from nanowires on nickel foam as high-performance electrodes for supercapacitors. Electrochim. Acta 169, 202–209. doi:10.1016/j.electacta.2015.04.079
Wu, Q., Xu, Y., Yao, Z., Liu, A., and Shi, G. (2010). Supercapacitors based on flexible graphene/polyaniline nanofiber composite films. ACS Nano 4, 1963–1970. doi:10.1021/nn1000035
Xiao, J.-D., and Jiang, H.-L. (2019). Metal–organic frameworks for photocatalysis and photothermal catalysis. Acc. Chem. Res. 52, 356–366. doi:10.1021/acs.accounts.8b00521
Xiao, T., Li, J., Zhuang, X., Zhang, W., Wang, S., Chen, X., et al. (2018). Wide potential window and high specific capacitance triggered via rough NiCo2S4 nanorod arrays with open top for symmetric supercapacitors. Electrochim. Acta 269, 397–404. doi:10.1016/j.electacta.2018.03.026
Xie, L. S., Skorupskii, G., and Dincǎ, M. (2020). Electrically conductive metal-organic frameworks. Chem. Rev. 120, 8536–8580. doi:10.1021/acs.chemrev.9b00766
Xu, Y., Yu, S., Johnson, H. M., Wu, Y., Liu, X., Fang, B., et al. (2024). Recent progress in electrode materials for micro-supercapacitors. iScience 27, 108786. doi:10.1016/j.isci.2024.108786
Yang, S., Peng, L., Syzgantseva, O. A., Trukhina, O., Kochetygov, I., Justin, A., et al. (2020). Preparation of highly porous metal-organic framework beads for metal extraction from liquid streams. J. Am. Chem. Soc. 142, 13415–13425. doi:10.1021/jacs.0c02371
Yaseen, M., Khattak, M. A. K., Humayun, M., Usman, M., Shah, S. S., Bibi, S., et al. (2021). A review of supercapacitors: materials design, modification, and applications. Energies 14, 7779–7840. doi:10.3390/en14227779
Yolanda, Y., Ridwan, M., Wook Hong, J., and Anggarainum Ivandini, T. (2020). Preparation of nickel manganese oxide modified ni foam for anode catalyst direct urea fuel cell. E3S Web Conf. 211, 03004. doi:10.1051/e3sconf/202021103004
Zhang, X., Wang, X., Jiang, L., Wu, H., Wu, C., and Su, J. (2012). Effect of aqueous electrolytes on the electrochemical behaviors of supercapacitors based on hierarchically porous carbons. J. Power Sources 216, 290–296. doi:10.1016/j.jpowsour.2012.05.090
Zou, R., Xu, K., Wang, T., He, G., Liu, Q., Liu, X., et al. (2013). Chain-like NiCo2O4 nanowires with different exposed reactive planes for high-performance supercapacitors. J. Mater. Chem. A 1, 8560–8566. doi:10.1039/c3ta11361b
Keywords: metal–organic frameworks, specific capacitance, supercapacitor, binder free electrode, energy storage
Citation: Wadhwa P, Singh P, Saini R, Jain K, Dilbaghi N, Dhaka RK, Sangwan NS, Singh MK and Kumar S (2025) Binder-free Ni-foam bolstered NiCo2S4/NiCo-MOF composite for enhanced supercapacitor applications. Front. Nanotechnol. 7:1595022. doi: 10.3389/fnano.2025.1595022
Received: 17 March 2025; Accepted: 01 May 2025;
Published: 30 June 2025.
Edited by:
Karthik Ramasamy, Los Alamos National Laboratory (DOE), United StatesReviewed by:
Soubantika Palchoudhury, University of Dayton, United StatesAvishek Banik, Colorado State University, United States
Copyright © 2025 Wadhwa, Singh, Saini, Jain, Dilbaghi, Dhaka, Sangwan, Singh and Kumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sandeep Kumar, a3NhbmRlZXAzNkB5YWhvby5jb20=
 Priyanka Wadhwa
Priyanka Wadhwa Pratap Singh
Pratap Singh Robin Saini
Robin Saini Karuna Jain3
Karuna Jain3 Rahul Kumar Dhaka
Rahul Kumar Dhaka Neelam S. Sangwan
Neelam S. Sangwan Sandeep Kumar
Sandeep Kumar