Abstract
Magnetic hollow hydroxyapatite (HAp) microspheres have demonstrated great potential for biomedical applications and pollution treatment. However, it remains unclear how the rate of magnetic nanoparticle addition during synthesis affects the material’s structure and properties. In this study, samples with varying magnetic nanoparticle addition rates were prepared and analyzed. The results reveal that the rate of magnetic nanoparticle addition is associated with the composition, structure, and properties of magnetic hollow hydroxyapatite microspheres. The sample with a medium magnetic nanoparticle addition rate exhibits optimal composition and structure. The saturation magnetization of the optimal sample is 18.99 emu/g, and the specific surface area and pore volume are 61.66 m2/g and 0.17 cm3/g, respectively. This research provides valuable guidance for synthesizing magnetic hollow hydroxyapatite microspheres and offers insights into the development of other magnetic composite materials.
1 Introduction
Hydroxyapatite (HAp), as a naturally occurring substance in human bone, is widely utilized as a biomaterial due to its excellent biocompatibility and bioactivity. Numerous researchers have investigated its applications, such as bone repair and drug delivery (DileepKumar et al., 2022; Cheng et al., 2025). HAp is capable of forming various structures depending on the preparation process, including rod-shaped, plate-shaped, and spherical forms. Among these, hollow HAp microspheres have attracted researchers' attention due to their unique structure and larger specific surface area, which confer inherent advantages in applications such as adsorption and drug delivery (Lai et al., 2016; Li et al., 2023; Liu et al., 2023; Zeng et al., 2023; Li et al., 2025). However, as medicine continues to advance, the demand for biomedical materials is also gradually increasing. The functionality of HAp is too limited to satisfy modern medical needs.
Magnetic nanoparticles are a widely used biomaterial with applications across multiple fields, such as magnetic resonance imaging contrast agents and drug delivery systems (Wang et al., 2020; Zhang et al., 2022; Wang et al., 2023; Ahmad et al., 2025; Karimova et al., 2025; Ma et al., 2025). Incorporating magnetic nanoparticles, such as Fe3O4, into HAp can impart magnetic responsiveness to materials, enabling external-field manipulation, targeted delivery, magnetic hyperthermia, and magnetic resonance imaging. Furthermore, when magnetic HAp possesses mesoporous or specialized structures, its range of applications can be further expanded (Oni et al., 2023). Such multifunctional composites show promise as next-generation biomedical materials.
Extensive studies have examined the synthesis of magnetic HAp, focusing on particle type, doping, and surface modification. These works highlight how nanoparticle properties affect HAp nucleation and growth. Nevertheless, the addition rate of magnetic nanoparticles—a parameter that strongly influences dispersion, nucleation dynamics, and spatial distribution within microspheres—has received little attention. Variation in addition speed may alter the formation of hollow structures, ultimately modulating magnetic performance, drug-loading capacity, and biological response.
This study examines the impact of magnetic nanoparticle addition rate on the formation and properties of magnetic hollow HAp microspheres. By controlling the addition speed and combining structural characterization with functional evaluation, we demonstrate how the addition rate of magnetic nanoparticles governs nanoparticle distribution, hollow morphology, and composite performance. These findings provide new insight into the synthesis–structure–property relationship of magnetic hollow HAp microspheres and offer guidance for the controlled design of multifunctional biomaterials.
2 Methods and experiments
2.1 Synthetic methods
The magnetic hollow HAp microspheres were prepared using the previously reported method (Rong et al., 2023). Briefly, the MnFe2O4 magnetic nanoparticles were first prepared by the hydrothermal method (Ma et al., 2021). Then, the magnetic nanoparticles were added to the CaCl2, Na2CO3, and poly (styrene sulfonic acid) sodium salt (PSS) mixture at different rates during the synthesis of the CaCO3 microsphere template. The template was then used to prepare magnetic hollow HAp microspheres via a hydrothermal process in the presence of Na2HPO4. The addition rate of the MnFe2O4 magnetic nanoparticles was based on the rate of Na2CO3 addition. Samples prepared by adding MnFe2O4 at rates of 0.5, 1, and 2 mL/min were designated S0.5, S1, and S2, respectively. Detailed information on the materials used in the synthetic process is provided in Supplementary Table S1.
2.2 Characterization
To determine the composition and crystal structure of the samples, X-ray diffraction (XRD) patterns were obtained for all samples at 10°–90° using a X-ray diffractometer (SmartLab SE, Rigaku, Japan) with Cu Ka radiation.
To study the structure of the products, a scanning electron microscope (SEM, SU8600, Hitachi, Japan) and a transmission electron microscope (TEM, JEM-2100F, JEOL, Japan) were employed to obtain micrographs of the products. The energy-dispersive X-ray spectroscopy (EDS, Xplore, Oxford Instruments, UK) was also used to determine the distribution of elements on the surface of the products.
The magnetic properties, including the hysteresis loop and saturation magnetization, of the material were determined using a vibrating sample magnetometer (VSM, Quantum Design, USA), which was installed in a physical property measurement system (PPMS, Quantum Design, USA). After weighing, the samples were placed into VSM powder capsules (Quantum Design, USA) and then transferred to the VSM for testing. The test magnetic field range is −15000 to 15,000 Oe, with a magnetic field change rate of 200 Oe/min.
An accelerated surface area and porosimetry system (ASAP 2420, Micromeritics, USA) was used to measure surface area, pore size, and pore volume of the products. The adsorption–desorption isotherms were obtained under nitrogen, and the Brunauer–Emmett–Teller (BET) method was selected to calculate the specific surface area of all three samples.
3 Results
The XRD patterns of all samples are shown in Figure 1. The three samples exhibit similar XRD patterns. All three samples can be well matched with hydroxyapatite (PDF number 04-015–6216) (Jevtic et al., 2008) and MnFe2O4 (PDF number 04-016–8331) (Kim et al., 2009). The peaks located at 25.89°, 31.74°, and 32.86° can be determined as the (002), (211), and (300) phases of hydroxyapatite. The peak located at 35.67° can be associated with the (311) phase of MnFe2O4. Besides, the peaks of phase (321) in Mn0.9Fe4.1(PO4)3(OH)4(H2O) (PDF number 04-026–3572) (Grey et al., 2019) located at 27.78° can be indexed in the patterns of S0.5 and S2, which are not present in S1.
FIGURE 1

The XRD pattern of the prepared samples.
The XRD results of the three samples indicated that all these products were composites containing hydroxyapatite and MnFe2O4. Although the main components of the products are the same, the unique constituent ferrorockbridgeite in S0.5 and S2 requires more attention. Ferrorockbridgeite is a member of the rockbridgeite group, containing Mn, Fe, and P elements. Considering the chemical composition of the reagents employed in the synthesis process of magnetic hollow HAp microspheres, the presence of ferrorockbridgeite is likely due to the reaction between the MnFe2O4 nanoparticles and Na2HPO4 solution during the hydrothermal process. Additionally, compared to the standard PDF cards, the peaks of ferrorockbridgeite in the XRD patterns of S0.5 and S2 exhibit a slight leftward shift (approximately 1°), indicating larger lattice parameters and interplanar spacings in the products, which may be caused by the doping of Mn and Fe.
The SEM images of all the products are shown in Figure 2. Clearly, all samples are microspheres with diameters of approximately 3 μm. Although SEM images reveal that all three microspheres are made of primary particles, they show significant differences in detail. In S0.5 and S1, the microspheres are coated with rod-like particles. In S0.5, the coating appears to be partial, whereas in S1, the microspheres are thoroughly coated. Compared to the microspheres in S0.5 and S2, the coating layer on the microspheres in S2 seems to have disappeared or entirely fused with the microsphere wall. Our previous work reported the SEM image analysis and the corresponding EDS mapping results for the sample, which were prepared under the same conditions as S1 (Rong et al., 2023). Thus, this study will focus on the EDS mapping results for the S0.5 and S2 samples, which are shown in Figures 3, 4.
FIGURE 2

The SEM images of sample (A) S0.5, (B) S1, and (C) S2.
FIGURE 3

The SEM images and corresponding EDS mapping results of S0.5.
FIGURE 4

The SEM images and corresponding EDS mapping results of (A) a complete, and (B) a broken microsphere in S2.
The EDS mapping results can clearly reveal the elemental distribution on the surface of products. The results of S0.5 (Figure 3) clearly show that the main component of the coating layer in S0.5 was MnFe2O4 nanoparticles, and the content of MnFe2O4 in the microsphere was relatively low. Besides, the MnFe2O4 coating layer on the surface of the microsphere was nonuniform. A pronounced clustering phenomenon of MnFe2O4 nanoparticles is observable.
In S2 (Figure 4A), the EDS results indicated that the microsphere contains negligible amounts of Mn and Fe elements. To locate the MnFe2O4 nanoparticles added during the preparation of the magnetic hollow hydroxyapatite microspheres, a broken microsphere in S2 was identified and analyzed using EDS, as shown in Figure 4B. Mapping results suggest that MnFe2O4 nanoparticles are present in the inner surface of the microsphere in the S2 sample.
To better understand the distribution of MnFe2O4 nanoparticles in the S2, the TEM image and related EDS mapping results are obtained and presented in Figure 5. Given the thinner thickness of the sample observed in TEM, the EDS mapping results from TEM can provide a more accurate depiction of the element distribution on the microsphere’s wall. The mapping results confirmed the presence of Ca, P, Mn, and Fe elements, with Mn and Fe distributed evenly across the microsphere wall.
FIGURE 5
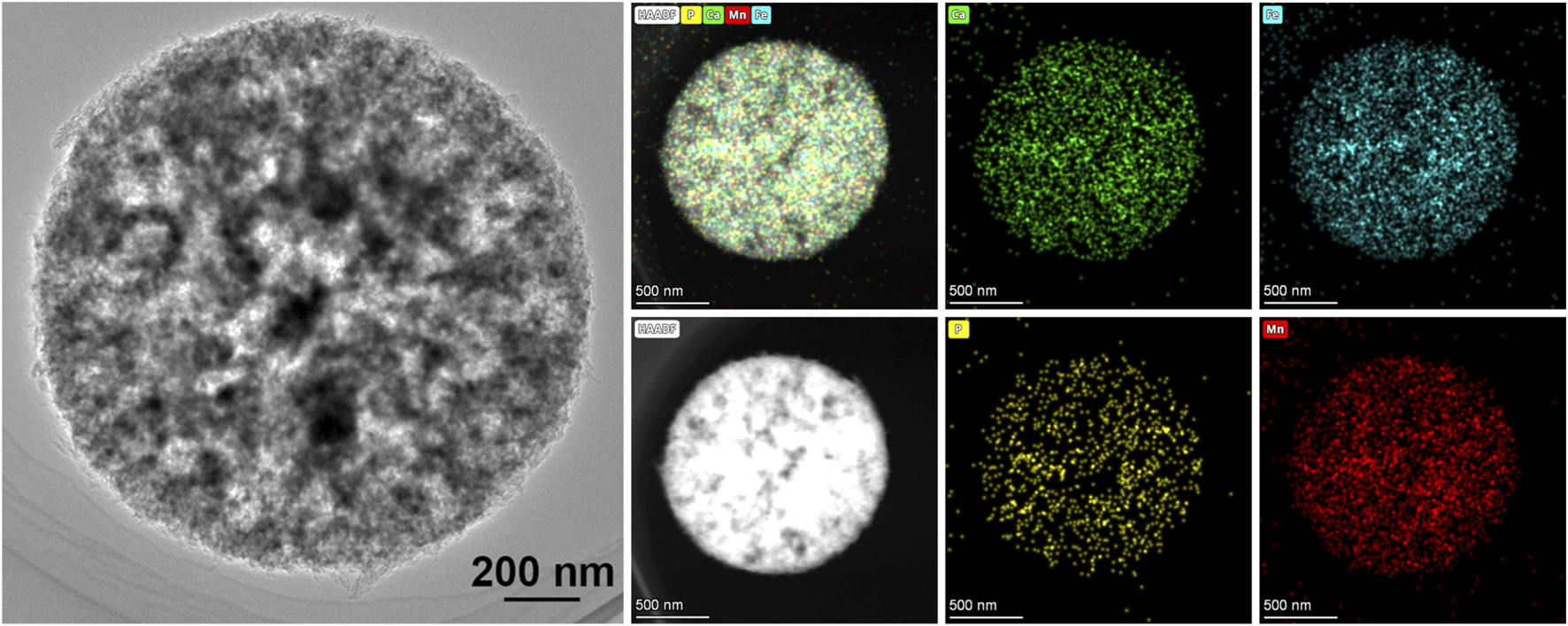
The TEM images and corresponding EDS results of sample S2.
Combining the SEM and TEM results of these samples, it is evident that the rate of adding MnFe2O4 nanoparticles affects the morphology of the magnetic hollow HAp microspheres and the distribution of the MnFe2O4 nanoparticles. At a lower rate, MnFe2O4 nanoparticles did not appear within the sphere’s shell, and the coating layer is nonuniform. As the rate increases, nanoparticles gradually penetrate deeper into the wall layer and ultimately seem to become the inner surface of the microsphere.
The results of the composition and morphology of the samples already indicated that the rate of adding MnFe2O4 nanoparticles will influence the final products, which may further affect the properties of magnetic hollow HAp microspheres. Our previous work and other research have already demonstrated that the saturation magnetization (Ms) and specific surface area are key properties of magnetic hollow hydroxyapatite microspheres when used as biomaterials, respectively associated with magnetic resonance imaging (MRI) contrast enhancement and drug delivery performance (Rong et al., 2024).
The magnetic property experiment results for all samples are shown in Figure 6. Hysteresis loops demonstrated that all three samples are superparamagnetic. The saturation magnetizations of S0.5, S1, and S2 are 23.00, 18.99, and 18.61 emu/g, respectively. The higher saturation magnetization in S0.5 may be related to its morphology.
FIGURE 6

The hysteresis loops of S0.5, S1, and S2.
Usually, during the synthesis of magnetic hollow hydroxyapatite microsphere templates, not all MnFe2O4 nanoparticles can be incorporated into the templates. Since the remaining particles are on a nanoscale, those not embedded in the templates can be easily removed during the washing steps. However, in S0.5, some magnetic nanoparticles bind to primary HAp particles, forming non-spherical components. At this stage, the agglomerate size increased to the micrometer scale, making it difficult to remove through washing. As a result, the magnetic content retained in the product increased, thereby enhancing the sample’s saturation magnetization. In S1 and S2, most magnetic nanoparticles were embedded into the walls of the magnetic hollow hydroxyapatite microspheres, thereby preventing this phenomenon. As a result, the saturation magnetization of both samples was similar.
The adsorption properties of the products are also affected by the addition rate of MnFe2O4. Figure 7 reveals the nitrogen adsorption–desorption experiment results of S0.5, S1, and S2. Based on the results, the isotherms of all samples are of type IV, with a type H3 loop, according to the classification of the International Union of Pure and Applied Chemistry. According to the isotherms, at the same relative pressure, S1 exhibits a higher adsorption capacity, indicating a greater specific surface area or pore volume.
FIGURE 7

The nitrogen adsorption–desorption isotherms of S0.5, S1, and S2.
The specific surface areas measured using the BET method of S0.5, S1, and S2 are 72.36, 61.66, and 50.66 m2/g, respectively. And the average pore diameter of S0.5, S1, and S2 are 8.05, 11.55, and 11.54 nm, respectively. The Barrett–Joyner–Halenda (BJH) adsorption cumulative volume of pores between 1.70 nm and 300.00 nm diameter is 0.14, 0.17, and 0.14 cm3/g for S0.5, S1, and S2, respectively.
Similar to magnetic properties, the addition rate of magnetic nanoparticles also influences the specific surface area, pore volume, and pore size of the samples. From S0.5 to S1, the specific surface area decreases gradually, while the pore size increases. The S2 and S1 have similar pore sizes. Meanwhile, the pore volume of S0.5 and S2 is comparable, and S1 has the highest pore volume.
To understand the nitrogen adsorption–desorption results, not only should the relationship between specific area, pore volume, and pore size be considered, but the hollow structure of the three samples also needs to be considered. The specific area is mainly controlled by micropores and mesopores formed by primary particles. Smaller particles will form more micropores, resulting in a larger specific surface area. The hollow structure and macropores have a minimal impact on the specific surface area but significantly influence the pore volume. The S1 and S2 have a larger pore size than S0.5, resulting in a smaller specific surface area. Considering that all three samples have a hollow structure, the larger pore volume of S1 is primarily due to the presence of macropores.
4 Discussion
In the previous section, the composition, microstructure, magnetic properties, and adsorption-related characteristics of products formed with different magnetic particle addition rates were discussed. All experimental results indicate that the rate of magnetic particle addition influences the product’s composition and microstructure, thereby impacting its properties. Researchers have reported the formation of hollow calcium carbonate particles in the presence of PSS, further indicating that CaCO3 crystal formation occurs within approximately 200 s (Beuvier et al., 2022; Gibaud et al., 2023). Considering that the magnetic hollow HAp microspheres were prepared by introducing magnetic nanoparticles into the hollow CaCO3 template, and the formation of the template is time sensitive. Therefore, the addition rate of magnetic nanoparticles, in other words, their concentration within the reaction system at various times, will inevitably influence the structure of the magnetic hollow HAp microspheres.
When the addition rate of magnetic nanoparticles is too slow, CaCO3 crystals will form first. Meanwhile, the concentration of magnetic nanoparticles remains relatively low. The already-formed CaCO3 crystals contain little to no magnetic nanoparticles and also hinder nanoparticles from binding to the spheres. Because the PSS sphere in the reaction system is limited, nanoparticles associate with other components, leading to non-spherical magnetic templates or magnetic particle agglomerates. These then further react with disodium hydrogen phosphate under the hydrothermal process. Under these conditions, magnetic particle agglomerates react with disodium hydrogen phosphate at high pressure, which can explain the presence of the ferrorockbridgeite. Additionally, the non-spherical magnetic HAp was also formed by primary particles, and its non-hollow structure provides numerous micropores, resulting in a higher specific surface area but smaller pore size and volume, like sample S0.5, which has the largest specific surface area 72.36 m2/g, but the smallest pore size 8.05 nm and pore volume 0.14 cm3/g.
Conversely, when the addition rate of magnetic nanoparticles is too high, the concentration of magnetic nanoparticles before CaCO3 crystal formation will be extremely high. In this case, magnetic nanoparticles will first occupy the spherical surface. Similarly, due to the limited number of PSS spheres, the CaCO3 crystals will form only on the remaining surfaces or on top of the magnetic nanoparticles. Subsequently, these templates were used to synthesize magnetic hollow HAp microspheres via hydrothermal synthesis. Under these conditions, since the magnetic nanoparticles are directly bound to the PSS surface without being encapsulated by CaCO3 crystals or the hydroxyapatite primary particles formed on these crystals, the magnetic particles may also react directly with disodium hydrogen phosphate under hydrothermal conditions to form ferrorockbridgeite. However, due to the CaCO3 crystals on top of the magnetic nanoparticles, the amount formed of ferrorockbridgeite is significantly less compared to conditions with a lower addition rate. Considering that under this situation, no or less non-spherical hydroxyapatite was formed, the specific surface area and pore size will be more similar to those of the magnetic hollow HAp microspheres synthesized at a suitable magnetic nanoparticles addition rate. As shown in the preceding section, the sample S2 has a similar specific surface area (50.66 m2/g vs. 61.66 m2/g) and pore size (11.54 nm vs. 11.55 nm) to sample S1.
Although experimental results show that products synthesized with lower magnetic nanoparticle addition rates have higher saturation magnetization (S0.5 has the highest, 23.00 emu/g) and specific surface area, based on the preceding analysis, these products are not suitable for biomedical applications. This is mainly because these two improved properties are not achieved by increasing the magnetic hollow HAp microspheres needed for biomedical use, but rather by the contribution of unnecessary, non-spherical components, such as ferrorockbridgeite. Moreover, a larger specific surface area is highly desirable for adsorption applications, whereas in drug delivery systems, the material’s pore volume is considered more important. Therefore, selecting the appropriate magnetic particle addition rate and achieving a balance between different properties is crucial. Based on all experimental results presented in this work, Sample S1 achieved a favorable balance among other properties. The magnetic particle addition rate used during its preparation may be an optimal condition for synthesizing magnetic hollow HAp microspheres.
5 Conclusion
In this study, magnetic hollow HAp microspheres were synthesized using three different addition rates of magnetic nanoparticles: fast (S2), medium (S1), and slow (S0.5). The composition, microstructure, and properties of all samples were thoroughly characterized. XRD and SEM results indicated that all samples are hollow microspheres composed of hydroxyapatite and MnFe2O4. However, an impurity, ferrorockbridgeite, was detected in both S2 and S0.5 samples. EDS results for all three samples indicate that the addition rate of magnetic nanoparticles influences their distribution within the magnetic hollow HAp microsphere. This suggests that during the formation of the CaCO3 template used to make magnetic hollow HAp microspheres, the magnetic nanoparticles may compete with CaCO3 crystals for surface space on PSS spheres, thereby affecting the microstructure and properties of the final product. Considering the biomedical application requirements, it is crucial to maintain a moderate nanoparticle addition rate during synthesis to achieve optimal performance. Among the samples, S1 exhibits the best balance of properties, including saturation magnetization (18.99 emu/g), specific surface area (61.66 m2/g), and pore volume (0.17 cm3/g). These findings not only provide practical guidance for tuning composition–structure–property relationships in magnetic HAp systems but also offer a valuable framework for designing materials suited for biomedical applications such as targeted drug delivery, magnetic-guided transport, and controlled release. The optimized synthesis conditions identified in this work are expected to facilitate the development of next-generation multifunctional magnetic hollow HAp microspheres and may inspire future studies exploring their in vivo behavior, loading efficiency for therapeutic agents, integration with external magnetic control strategies, and application as MRI contrast agents.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
MR: Conceptualization, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review and editing. SD: Conceptualization, Formal Analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review and editing. DL: Formal Analysis, Investigation, Methodology, Validation, Writing – original draft. XZ: Funding acquisition, Resources, Supervision, Writing – review and editing. XX: Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review and editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. XX is thankful for the financial support from the National Natural Science Foundation of China (Grant No. U23A20548). XZ is grateful for the financial support from the National Natural Science Foundation of China (Grant No. 82472459, 82172447), Beijing Natural Science Foundation (24L20439), and Beijing Municipal Science and Technology Commission, Adminitrative Commission of Zhongguancun Science Park (Z22111000740000).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author XZ declared that they were an editorial board member of Frontiers at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnano.2025.1716157/full#supplementary-material
References
1
Ahmad S. Shahab U. Ashraf J. M. Alyahyawi A. R. Ansari M. A. Fatma S. et al (2025). Antiglycating activity of biomimetically synthesized magnetic copper ferrite nanoparticles: inhibition of methylglyoxal-derived AGEs. Front. Nanotechnol.7, 1564954. 10.3389/fnano.2025.1564954
2
Beuvier T. Chushkin Y. Zontone F. Gibaud A. Cherkas O. Da Silva J. et al (2022). Self-transformation of solid CaCO(3) microspheres into core-shell and hollow hierarchical structures revealed by coherent X-ray diffraction imaging. IUCrJ9 (Pt 5), 580–593. 10.1107/S2052252522006108
3
Cheng S. Meng X.-H. Li Z. Han H.-H. Zhang Y.-F. (2025). Nanomaterial-mediated antibiotic delivery: a novel strategy for osteomyelitis therapy. Front. Bioeng. Biotechnol.13, 1671151. 10.3389/fbioe.2025.1671151
4
DileepKumar V. G. Sridhar M. S. Aramwit P. Krut'ko V. K. Musskaya O. N. Glazov I. E. et al (2022). A review on the synthesis and properties of hydroxyapatite for biomedical applications. J. Biomaterials Sci.33 (2), 229–261. 10.1080/09205063.2021.1980985
5
Gibaud A. Younas D. Matthews L. Narayanan T. Longkaew K. Hageberg I. U. et al (2023). Insights into the precipitation kinetics of CaCO(3) particles in the presence of polystyrene sulfonate using in situ small-angle X-ray scattering. J. Appl. Crystallogr.56 (Pt 4), 1114–1124. 10.1107/S1600576723005356
6
Grey I. E. Kampf A. R. Keck E. Cashion J. D. MacRae C. M. Gozukara Y. et al (2019). The rockbridgeite group approved and a new member, ferrorockbridgeite, (Fe2+, Mn2+) 2 (Fe3+) 3 (PO4) 3 (OH) 4 (H2O), described from the hagendorf süd pegmatite, oberpfalz, Bavaria. Eur. J. Mineralogy31 (2), 389–397. 10.1127/ejm/2019/0031-2823
7
Jevtic M. Mitric M. Skapin S. Jancar B. Ignjatovic N. Uskokovic D. (2008). Crystal structure of hydroxyapatite nanorods synthesized by sonochemical homogeneous precipitation. Cryst. Growth Des.8 (7), 2217–2222. 10.1021/cg7007304
8
Karimova A. Hajizada S. Shirinova H. Nuriyeva S. Gahramanli L. Mehdiyeva A. et al (2025). Dextran-coated Fe3O4 nanoparticles with ratio-dependent drug loading: structural characterization and cytotoxicity in colorectal cancer cells. Front. Nanotechnol.7, 1634225. 10.3389/fnano.2025.1634225
9
Kim J. Seo J. Cheon J. Kim Y. (2009). Rietveld analysis of nano-crystalline MnFe2O4 with electron powder diffraction. Bull. Korean Chem. Soc.30 (1), 183.
10
Lai W. Chen C. Ren X. Lee I. S. Jiang G. Kong X. (2016). Hydrothermal fabrication of porous hollow hydroxyapatite microspheres for a drug delivery system. Mater. Sci. Eng. C62, 166–172. 10.1016/j.msec.2016.01.055
11
Li S. Guo R. Chen S. Zhang J. Ikoma T. Li X. et al (2023). Facile synthesis, in vitro biocompatibility, and antibacterial activity of porous hollow hydroxyapatite microspheres. Int. J. Appl. Ceram. Technol.20 (1), 341–349. 10.1111/ijac.14195
12
Li L. Liu L. Lv Y. Yin P. Lei T. (2025). Porous and hollow hydroxyapatite microspheres: synthesis and application in pH-responsive drug release. Inorg. Chem. Commun.177, 114410. 10.1016/j.inoche.2025.114410
13
Liu X. Xie Y. Gao W. Zhan L. Hu L. Zuo L. et al (2023). Experimental study of dexamethasone-loaded hollow hydroxyapatite microspheres applied to direct pulp capping of rat molars. Front. Endocrinol.14, 1192420. 10.3389/fendo.2023.1192420
14
Ma Y. Xu X. Lu L. Meng K. Wu Y. Chen J. et al (2021). Facile synthesis of ultrasmall MnFe2O4 nanoparticles with high saturation magnetization for magnetic resonance imaging. Ceram. Int.47 (24), 34005–34011. 10.1016/j.ceramint.2021.08.308
15
Ma Y. Ma X. Rong M. Lu L. Yuan W. Xu X. et al (2025). Circulating catalysis nanocomposites for photodynamic and starvation treatment via long-persistent regulation of tumor hypoxia. Colloids Surfaces B Biointerfaces258, 115227. 10.1016/j.colsurfb.2025.115227
16
Oni O. P. Hu Y. Tang S. Yan H. Zeng H. Wang H. et al (2023). Syntheses and applications of mesoporous hydroxyapatite: a review. Mater. Chem. Front.7 (1), 9–43. 10.1039/d2qm00686c
17
Rong M. Xu X. Wang K. Lu L. Bai Y. Tian Y. et al (2023). A novel hydroxyapatite-based hollow microsphere nanocomposite for copper ion adsorption. Chem. Phys. Lett.824, 140548. 10.1016/j.cplett.2023.140548
18
Rong M. Liu D. Xu X. Li A. Bai Y. Yang G. et al (2024). A superparamagnetic composite Hydrogel scaffold as in vivo dynamic monitorable Theranostic platform for osteoarthritis regeneration. Adv. Mater.36 (35), e2405641. 10.1002/adma.202405641
19
Wang K. Xu X. Ma Y. Sheng C. Li L. Lu L. et al (2020). Fe3O4@Angelica sinensis polysaccharide nanoparticles as an ultralow-toxicity contrast agent for magnetic resonance imaging. Rare Met.40 (9), 2486–2493. 10.1007/s12598-020-01620-0
20
Wang K. Wang J. Xu X. Rong M. Lu L. Zhao X. et al (2023). Fe3O4-rhodamine 6G nanoparticles: an iron enhanced pH sensitive multimodal probe for fluorescence and magnetic resonance imaging of tumor cell. J. Mater. Sci. and Technol.160, 128–138. 10.1016/j.jmst.2023.03.022
21
Zeng J. Xiong S. Zhou J. Wei P. Guo K. Wang F. et al (2023). Hollow hydroxyapatite microspheres loaded with rhCXCL13 to recruit BMSC for osteogenesis and synergetic angiogenesis to promote bone regeneration in bone defects. Int. J. Nanomedicine18, 3509–3534. 10.2147/IJN.S408905
22
Zhang W. Zhang Z. Lou S. Chang Z. Wen B. Zhang T. (2022). Hyaluronic acid-stabilized Fe3O4 nanoparticles for promoting in vivo magnetic resonance imaging of tumors. Front. Pharmacol.13, 918819. 10.3389/fphar.2022.918819
Summary
Keywords
magnetic materials, hydroxyapatite, nanocomposites, biomaterials, superparamagnetic particles
Citation
Rong M, Deng S, Liu D, Zhang X and Xu X (2026) Effect of MnFe2O4 magnetic nanoparticle addition rate on the structure and properties of magnetic hollow hydroxyapatite microspheres. Front. Nanotechnol. 7:1716157. doi: 10.3389/fnano.2025.1716157
Received
30 September 2025
Revised
24 November 2025
Accepted
15 December 2025
Published
05 January 2026
Volume
7 - 2025
Edited by
Cristina Satriano, University of Catania, Italy
Reviewed by
Jagriti Behal, Sri Sai University, India
Jiabing Ran, China Three Gorges University, China
Anceu Murniati, Universitas Jenderal Achmad Yani, Indonesia
Updates
Copyright
© 2026 Rong, Deng, Liu, Zhang and Xu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoguang Xu, xgxu@ustb.edu.cn
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.