- 1Movement Disorder Unit, Department of Psychiatry and Neurology, University Hospital Grenoble, Grenoble, France
- 2Institut national de la santé et de la recherche médicale, Unité 836, Equipe 11, Grenoble Institut des Neurosciences, Grenoble, France
- 3Joseph Fourier University, Grenoble, France
Several prospective epidemiological studies on large cohorts have consistently reported an association between milk intake and a higher incidence of Parkinson’s disease (PD). Pesticide contamination of milk and milk’s urate-lowering effects have been put forward as risk factors to explain epidemiological data. This has led to considerable uncertainty among physicians and avoidance of dairy products by PD patients. However, neither factor stands up to the rational and detailed examination of the literature carried out in this mini-review. We suggest that changes in eating behavior related to pre-motor PD are an alternative potential explanation of correlations observed between milk intake and PD occurrence. Despite clear-cut associations between milk intake and PD incidence, there is no rational explanation for milk being a risk factor for PD. Based on current knowledge, limiting the consumption of dairy products does not seem to be a reasonable strategy in the prevention of the development and progression of PD.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disease, characterized by a progressive loss of dopaminergic neurons originating in the Substantia nigra pars compacta. The etiology is unknown, and environmental factors are thought to play a role in neurotoxicity, especially factors contributing to oxidative stress, such as pesticides or heavy metals (1). Dietary factors may be relevant as they can alter the redox balance in the brain or serve as a vehicle for environmental neurotoxins. Data from prospective epidemiological research has made the study of any link between PD and dietary factors possible. One such finding, recently recapitulated in a meta-analysis (2), was a positive association between milk intake and PD risk. Due to speculation and warnings in the lay press, on the internet and from physicians, many PD patients tend to avoid dairy products. This precaution may worsen the quality of their diet, especially that of elderly patients, who are at risk of under-nutrition, loss of weight and muscle mass (3), osteoporosis (4), and hip fracture (5).
Dairy Products in Nutrition
Most milk products consumed in human nutrition come from cow’s milk. The significance of milk in human nutrition is related to its content in protein (3%), fat (3–4%), carbohydrate (lactose) (4–5%), calcium (1200 mg/L), and other minerals, microelements, and vitamins (6). The protein fraction is of high quality in terms of digestibility and amino acid composition (6) and encourages skeletal muscle growth (7), which contributes to the prevention of sarcopenia, especially in the elderly.
Bioactive peptides resulting from enzymatic hydrolysis of milk proteins exert multiple biological – such as immunomodulatory and antihypertensive – actions, and are thought to play a protective role in human health (6). Milk and dairy products are the most important sources of calcium in human nutrition and their consumption is related to higher bone density, which may contribute to osteoporosis prevention (6, 8). Consumption of dairy products is also associated with improved metabolic health, and epidemiological evidence points to the contribution of milk in the prevention of metabolic disorders such as excess weight, type 2 diabetes, and cardiovascular disease (7).
Global recommended adult dietary calcium allowances range from 700 to 1300 mg/day (7) and milk products are a good source of calcium with high bioavailability. Because of their nutritional values, dairy products are highly recommended, especially for older people (6–8) and special recommendations also exist for ethnic groups with a high prevalence of lactose intolerance (9).
Dairy Products and PD Risk: Epidemiological Data
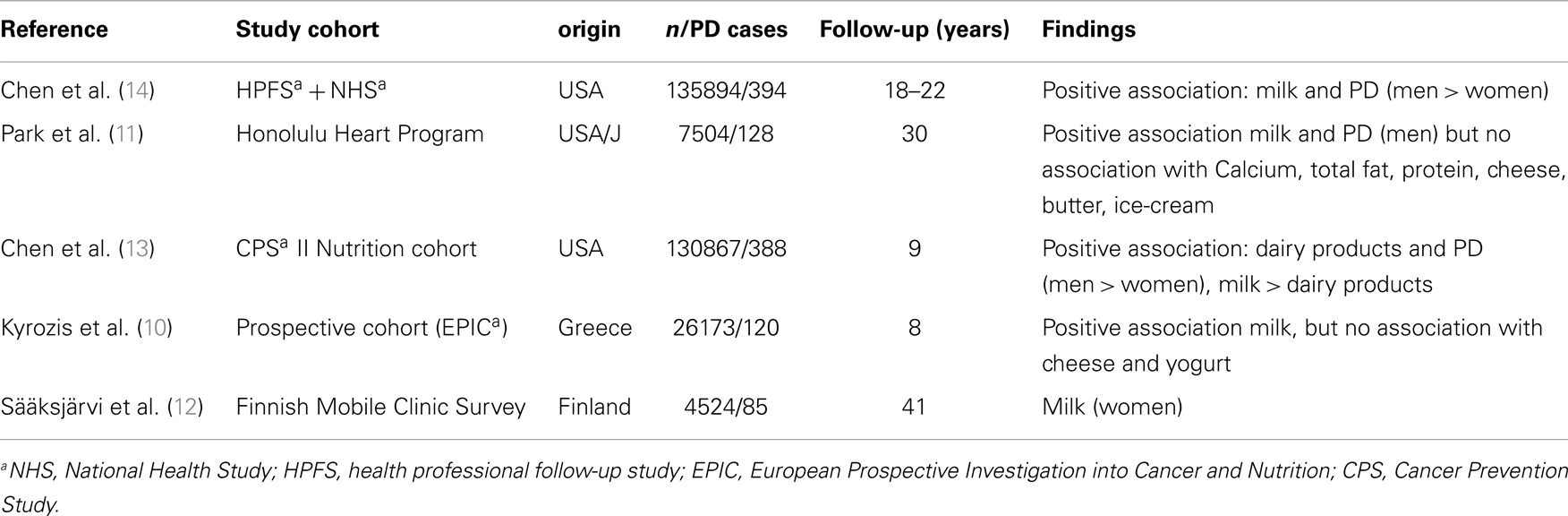
A positive association between milk consumption and PD risk has been found in large prospective cohort studies in the United States, Finland and Greece (10–14) (Table 1). A recent meta-analysis reported a combined risk of 1.4 for highest vs. lowest levels of dairy food intake, with a stronger association for men than for women (2).
Overall, the association between PD and milk was stronger than the association with other dairy products such as cheese, yogurt, or butter (2). We do not know which of the components of milk mediates this effect. It is unlikely to be due to milk compounds such as calcium, vitamin D, total fat, or total protein as these compounds are not associated with PD when derived from other sources (11, 14). It was hypothesized that low levels of pesticides or other neurotoxic chemicals may be present in milk and could accumulate in the brain and increase PD risk (15). However, this hypothesis is rather unlikely as skimmed products containing less fat, and therefore a decreased capacity for harboring pesticides, have the same effect (12). Meta-analysis of existing studies (2) has shown that the link between butter and the risk of PD is even lower. Park et al. speculated that genetic characteristics, such as lactose intolerance could be protective. However, the association between milk intake and PD persisted after men who consumed no milk whatsoever (presumably those with lactose intolerance) were excluded (11).
Another theory points to a milk-induced shift in the metabolic redox balance: it was shown that ingestion of milk can decrease serum urate levels in healthy volunteers (16). Urate is a potent endogenous antioxidant, which can inactivate potentially harmful neurotoxic substances, such as free radicals or iron (17). A recent meta-analysis confirmed low urate levels in PD, a finding, which could point to a neuro-protective role of urate (18).
However, we lack evidence of either (a) a role of low urate levels in neuro-degeneration or (b) an association between milk and metabolic antioxidative defense.
Consumption of Dairy Products in Pre-Motor PD: A Result of Altered Eating Behavior?
We hypothesize that psychological and emotional alteration in pre-motor PD may induce modifications in eating behavior. Non-motor symptoms, such as depression, anxiety, or apathy may be present in PD and precede motor symptoms (19). Associations between foods and moods, especially depressive symptoms, have been reported (20, 21). But why would these patients prefer milk to all other foods? One possible hypothesis is that milk consumption could be associated with mood alterations, as has been shown in an Australian epidemiologic study, where consumption of dairy products was related to the presence of depressive symptoms (22). This could be linked to alpha-lactalbumin, one of the main protein components of milk, which is rich in tryptophan, the precursor of serotonin. Serotonergic hypofunction has been described in early PD and may be involved in depression and anxiety (23). A significant rise in cortex tryptophan closely followed by an increase of serotonin synthesis occurred in rats following acute alpha-lactalbumin consumption (24) and this effect persisted chronically (25). In human volunteers an alpha-lactalbumin-enriched diet increases the ratio of tryptophan to the other large neutral amino acids, which is considered to be an indirect indication of increased brain serotonin function (26). In stress-vulnerable individuals, alpha-lactalbumin improved mood and attenuated the cortisol response following experimental stress (26). In individuals with high-trait anxiety, the administration of alpha-lactalbumin had an effect on food hedonics (27). Milk consumption in pre-motor PD may therefore be interpreted as spontaneous self-medication aimed at improving mood. Since the effect would be very discreet, it would only be observed in large-scale epidemiological studies. This hypothesis might also explain why milk is more strongly linked to PD than cheese: In the cheese-making process, the liquid whey containing alpha-lactalbumin is separated from the curd, and cheese does not contain alpha-lactalbumin.
Furthermore, in many Western countries, milk is traditionally considered as a sleep-inducing beverage and could be consumed – because of its content in melatonin or just on belief – by people suffering from sleep disorders (28), which are frequent in pre-motor PD (29).
However, as we have no strong evidence of the mood- or sleep-modifying effects of milk to date, other mechanisms should be considered to explain the epidemiological findings. Other non-motor symptoms that are frequent in pre-motor PD, such as hyposmia or constipation (30), for instance, may alter dietary habits. Dairy foods, especially fermented products, may relieve constipation, or constipated people may think they do. Another hypothesis is increased consumption of dairy products secondary to decreased consumption of other drinks. In fact, coffee consumption was reported to be low in pre-motor PD (31) and may have been replaced by dairy products. Finally, dopaminergic and homeostatic control of eating behavior may be disturbed in pre-motor PD (3), leading to alterations in dietary habits which may explain the findings.
Conclusion
To date, the explanation for the epidemiological link between dairy products and PD remains unknown. Evidence that milk constitutes a risk factor in PD is lacking. Limitation of milk consumption cannot therefore be recommended, just as smoking or coffee drinking cannot be recommended in order to prevent PD. Foods that supply calcium and high-quality protein should not be limited in view of the high prevalence of osteoporosis and hip fracture in PD (4, 5). Because of their high nutritional qualities, consumption of milk and dairy products should be encouraged in PD to the same degree as in the general population. Interference with dopaminergic treatment may be canceled out by respecting appropriate time lags between medication intake and meals. In the case of lactose intolerance, fermented dairy products, such as yogurt or cheese, may replace milk.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Jenner P. Oxidative stress as a cause of Parkinson’s disease. Acta Neurol Scand Suppl (1991) 136:6–15. doi: 10.1111/j.1600-0404.1991.tb05013.x
2. Jiang W, Ju C, Jiang H, Zhang D. Dairy foods intake and risk of Parkinson’s disease: a dose-response meta-analysis of prospective cohort studies. Eur J Epidemiol (2014). doi:10.1007/s10654-014-9921-4
3. Kistner A, Lhommee E, Krack P. Mechanisms of body weight fluctuations in Parkinson’s disease. Front Neurol (2014) 5:84. doi:10.3389/fneur.2014.00084
4. van den Bos F, Speelman AD, Samson M, Munneke M, Bloem BR, Verhaar HJ. Parkinson’s disease and osteoporosis. Age Ageing (2014) 42(2):156–62. doi:10.1093/ageing/afs161
5. Invernizzi M, Carda S, Viscontini GS, Cisari C. Osteoporosis in Parkinson’s disease. Parkinsonism Relat Disord (2009) 15(5):339–46. doi:10.1016/j.parkreldis.2009.02.009
6. Pereira PC. Milk nutritional composition and its role in human health. Nutrition (2014) 30(6):619–27. doi:10.1016/j.nut.2013.10.011
7. McGregor RA, Poppitt SD. Milk protein for improved metabolic health: a review of the evidence. Nutr Metab (Lond) (2013) 10(1):46. doi:10.1186/1743-7075-10-46
8. Caroli A, Poli A, Ricotta D, Banfi G, Cocchi D. Invited review: dairy intake and bone health: a viewpoint from the state of the art. J Dairy Sci (2011) 94(11):5249–62. doi:10.3168/jds.2011-4578
9. Bailey RK, Fileti CP, Keith J, Tropez-Sims S, Price W, Allison-Ottey SD. Lactose intolerance and health disparities among African Americans and Hispanic Americans: an updated consensus statement. J Natl Med Assoc (2013) 105(2):112–27.
10. Kyrozis A, Ghika A, Stathopoulos P, Vassilopoulos D, Trichopoulos D, Trichopoulou A. Dietary and lifestyle variables in relation to incidence of Parkinson’s disease in Greece. Eur J Epidemiol (2013) 28(1):67–77. doi:10.1007/s10654-012-9760-0
11. Park M, Ross GW, Petrovitch H, White LR, Masaki KH, Nelson JS, et al. Consumption of milk and calcium in midlife and the future risk of Parkinson disease. Neurology (2005) 64(6):1047–51. doi:10.1212/01.WNL.0000154532.98495.BF
12. Sääksjärvi K, Knekt P, Lundqvist A, Männistö S, Heliövaara M, Rissanen H, et al. A cohort study on diet and the risk of Parkinson’s disease: the role of food groups and diet quality. Br J Nutr (2013) 109(2):329–37. doi:10.1017/S0007114512000955
13. Chen H, O’Reilly E, McCullough ML, Rodriguez C, Schwarzschild MA, Calle EE, et al. Consumption of dairy products and risk of Parkinson’s disease. Am J Epidemiol (2007) 165(9):998–1006. doi:10.1093/aje/kwk089
14. Chen H, Zhang SM, Hernan MA, Willett WC, Ascherio A. Diet and Parkinson’s disease: a potential role of dairy products in men. Ann Neurol (2002) 52(6):793–801. doi:10.1002/ana.10381
15. Makino Y, Ohta S, Tachikawa O, Hirobe M. Presence of tetrahydroisoquinoline and 1-methyl-tetrahydro-isoquinoline in foods: compounds related to Parkinson’s disease. Life Sci (1988) 43(4):373–8. doi:10.1016/0024-3205(88)90115-4
16. Dalbeth N, Wong S, Gamble GD, Horne A, Mason B, Pool B, et al. Acute effect of milk on serum urate concentrations: a randomised controlled crossover trial. Ann Rheum Dis (2010) 69(9):1677–82. doi:10.1136/ard.2009.124230
17. Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA (1981) 78(11):6858–62. doi:10.1073/pnas.78.11.6858
18. Shen L, Ji HF. Low uric acid levels in patients with Parkinson’s disease: evidence from meta-analysis. BMJ Open (2013) 3(11):e003620. doi:10.1136/bmjopen-2013-003620
19. Agid Y, Arnulf I, Bejjani P, Bloch F, Bonnet AM, Damier P, et al. Parkinson’s disease is a neuropsychiatric disorder. Adv Neurol (2003) 91:365–70.
20. Konttinen H, Mannisto S, Sarlio-Lahteenkorva S, Silventoinen K, Haukkala A. Emotional eating, depressive symptoms and self-reported food consumption. A population-based study. Appetite (2010) 54(3):473–9. doi:10.1016/j.appet.2010.01.014
21. Ioakimidis I, Zandian M, Ulbl F, Bergh C, Leon M, Sodersten P. How eating affects mood. Physiol Behav (2011) 103(3–4):290–4. doi:10.1016/j.physbeh.2011.01.025
22. Meyer BJ, Kolanu N, Griffiths DA, Grounds B, Howe PR, Kreis IA. Food groups and fatty acids associated with self-reported depression: an analysis from the Australian National Nutrition and Health Surveys. Nutrition (2013) 29(7–8):1042–7. doi:10.1016/j.nut.2013.02.006
23. Fox SH, Chuang R, Brotchie JM. Serotonin and Parkinson’s disease: on movement, mood, and madness. Mov Disord (2009) 24(9):1255–66. doi:10.1002/mds.22473
24. Choi S, Disilvio B, Fernstrom MH, Fernstrom JD. Meal ingestion, amino acids and brain neurotransmitters: effects of dietary protein source on serotonin and catecholamine synthesis rates. Physiol Behav (2009) 98(1–2):156–62. doi:10.1016/j.physbeh.2009.05.004
25. Choi S, DiSilvio B, Fernstrom MH, Fernstrom JD. The chronic ingestion of diets containing different proteins produces marked variations in brain tryptophan levels and serotonin synthesis in the rat. Neurochem Res (2011) 36(3):559–65. doi:10.1007/s11064-010-0382-1
26. Markus CR, Olivier B, Panhuysen GE, Van DerGugten J, Alles MS, Tuiten A, et al. The bovine protein alpha-lactalbumin increases the plasma ratio of tryptophan to the other large neutral amino acids, and in vulnerable subjects raises brain serotonin activity, reduces cortisol concentration, and improves mood under stress. Am J Clin Nutr (2000) 71(6):1536–44.
27. Verschoor E, Finlayson G, Blundell J, Markus CR, King NA. Effects of an acute alpha-lactalbumin manipulation on mood and food hedonics in high- and low-trait anxiety individuals. Br J Nutr (2010) 104(4):595–602. doi:10.1017/S0007114510000838
28. Peuhkuri K, Sihvola N, Korpela R. Diet promotes sleep duration and quality. Nutr Res (2012) 32(5):309–19. doi:10.1016/j.nutres.2012.03.009
29. Iranzo A. Sleep-wake changes in the premotor stage of Parkinson disease. J Neurol Sci (2011) 310(1–2):283–5. doi:10.1016/j.jns.2011.07.049
30. Barichella M, Cereda E, Pezzoli G. Major nutritional issues in the management of Parkinson’s disease. Mov Disord (2009) 24(13):1881–92. doi:10.1002/mds.22705
Keywords: Parkinson’s disease, diet, milk, dairy products, risk factors, eating behavior
Citation: Kistner A and Krack P (2014) Parkinson’s disease: no milk today? Front. Neurol. 5:172. doi: 10.3389/fneur.2014.00172
Received: 09 July 2014; Accepted: 24 August 2014;
Published online: 05 September 2014.
Edited by:
Jaime Kulisevsky, Sant Pau Institute of Biomedical Research, SpainReviewed by:
Manuel Menéndez-González, Hospital Álvarez-Buylla, SpainPedro J. Garcia-Ruiz, Fundación Jiménez Díaz, Spain
Copyright: © 2014 Kistner and Krack. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Kistner, Department of Neurology, University Hospital Grenoble, BP 217, Grenoble Cedex 09 38043, France e-mail:YWtpc3RuZXJAY2h1LWdyZW5vYmxlLmZy
 Andrea Kistner
Andrea Kistner Paul Krack
Paul Krack