- 1Anatomy and Pathology, Adelaide Medical School, University of Adelaide, Adelaide, SA, Australia
- 2Andalusian Center for Molecular Biology and Regenerative Medicine-CABIMER, Unversity of Seville-CSIC-University Pablo de Olavide, Seville, Spain
A Commentary on
In case we needed reminding, age-related neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and motor neurone disease (MND) have two factors in common: (i) advancing age as the single biggest risk factor and (ii) the fact that they are characterized by neuronal degeneration. A recent article (1) encourages us to focus on some of the similarities of these diseases by demonstrating that features characteristically associated with AD are also commonly found in MND.
In their study (1), the group measured key biomarkers of the amyloid cascade [amyloid precursor protein (APP), transactive response DNA-binding protein 43 (TDP-43), phosphorylated TDP-43 (pho-TDP43), amyloid-beta peptide (Aβ), and amyloid precursor protein-binding protein family B (Fe 65)] immunohistochemically in postmortem samples of the hippocampus of amyotrophic lateral sclerosis (ALS) and ALS–frontotemporal dementia patients. Compared to controls, they report increased levels of APP and Aβ peptide in MND patients; the latter change also correlating with cytoplasmic pho-TDP-43 expression. In addition, they found decreased Fe65 expression and increased expression of pho-tau. Interestingly, these molecular alterations were similar for both ALS and ALS–FTD, albeit more pronounced in the latter group. This indicates that the “amyloid cascade,” resulting in the accumulation of amyloid β, is activated in the hippocampus of patients with ALS and ALS–FTD, and that such activation correlates with alterations in TDP-43.
This is an important finding, because it adds to the growing body of evidence that age-related neurodegenerative diseases, rather than being discrete entities, may in fact be different points on a continuum, and the corollary of this is that they may all have similar underlying mechanisms. Indeed, a brief survey of three age-related neurodegenerative diseases (AD, PD, and MND) reveals that there is much overlap in the features associated with these conditions (Table 1), and that they have more in common than anything else (Figure 1).
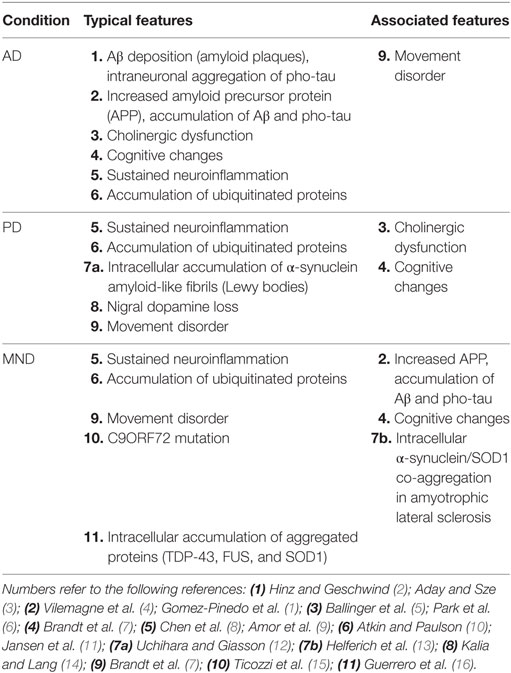
Table 1. Typical and associated features of Alzheimer’s disease (AD), Parkinson’s disease (PD), and motor neurone disease (MND).
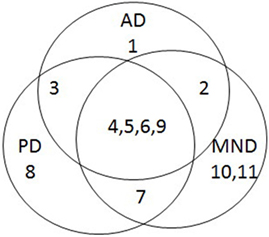
Figure 1. Venn diagram summarizing overlap of features associated with Alzheimer’s disease (AD), Parkinson’s disease (PD), and motor neurone disease (MND).
The paper by Gomez-Pinedo et al. (1) is a timely reminder that researchers should perhaps not get tied up with the details of these individual diseases that are so important for differential diagnoses. Instead, those seeking to illuminate basic underlying mechanisms might do well to pool data for neurodegenerative diseases in the hope that it will point them in the right direction. After all, these conditions have so far defied effective treatment or cure.
Author Contributions
IJ wrote the initial draft. CR revised the initial draft and contributed with further writing. Both collected data from literature and revised the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
CR acknowledges the funding by the Spanish Ministry of Economy (RTC-2015-3309-1) and Instituto de Salud Carlos III, Spain (CPII16/00058), with cofounding by FEDER.
References
1. Gomez-Pinedo U, Villar-Quiles R, Galan L, Matias-Guiu J, Benito-Martin M, Guerrero-Sola A, et al. Immunochemical markers of the amyloid cascade in the hippocampus in motor neuron diseases. Front Psychiatry (2016) 7:195. doi:10.3389/fneur.2016.00195
2. Hinz F, Geschwind D. Molecular genetics of neurodegenerative dementias. Cold Spring Harb Perspect Biol (2016):a023705. doi:10.1101/cshperspect.a023705
3. Aday S, Sze S. Insight of brain degenerative protein modifications in the pathology of neurodegeneration and dementia by proteomic profiling. Mol Brain (2016) 9:92. doi:10.1186/s13041-016-0272-9
4. Vilemagne V, Dore V, Bourget P, Burnham S, Laws S, Salvado O, et al. Aβ-amyloid and Tau imaging in dementia. Semin Nucl Med (2017) 47:75–88. doi:10.1053/j.semnuclmed.2016.09.00
5. Ballinger E, Anath M, Talmage D, Role L. Basal forebrain cholinergic circuits and signalling in cognition and cognitive decline. Neuron (2016) 91:1199–218. doi:10.1016/j.neuron.2016.09.006
6. Park H, Park I, Oh Y, Yang D, Lee K, Choi H, et al. Subcortical white matter hyperintensities within the cholinergic pathways of patients with dementia and Parkinsonism. J Neurol Sci (2015) 2015(353):44–8. doi:10.1016/j.jns.2015.03.046
7. Brandt T, Caplan L, Dichgans J, Diener H, Kennard C. Neurological Disorders. Course and Treatment. San Diego, CA: Academic Press (1997).
8. Chen WW, Zhang X, Huang WJ. Role of neuroinflammation in neurodegenerative diseases (review). Mol Med Rep (2016) 13:3391–6. doi:10.3892/mmr.2016.4948
9. Amor S, Peferoen L, Vogel D, Breur M, van der Valk P, Baker D, et al. Inflammation in neurodegenerative diseases – an update. Immunology (2013) 142:151–66. doi:10.1111/imm.12233
10. Atkin G, Paulson H. Ubiquitin pathways in neurodegenerative disease. Front Mol Neurosci (2014) 7:63. doi:10.3389/fnmol.2014.00063
11. Jansen AH, Reits EA, Hol EM. The ubiquitin proteasome system in glia and its role in neurodegenerative diseases. Front Mol Neurosci (2014) 7:73. doi:10.3389/fnmol.2014.00073
12. Uchihara T, Giasson BI. Propagation of alpha-synuclein pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies (review). Acta Neuropathol (2016) 131(1):49–73. doi:10.1007/s00401-015-1485-1
13. Helferich AM, Ruf WP, Grozdanov V, Freischmidt A, Feiler MS, Zondler L, et al. α-Synuclein interacts with SOD1 and promotes its oligomerization. Mol Neurodegener (2015) 10:66. doi:10.1186/s13024-015-0062-3
14. Kalia LV, Lang AE. Parkinson’s disease. Lancet (2015) 386(9996):896–912. doi:10.1016/S0140-6736(14)61393-3
15. Ticozzi N, Tiloca C, Calini D, Gagliardi S, Altieri A, Colombrita C, et al. C9orf72 repeat expansions are restricted to the ALS-FTD spectrum. Neurobiol Aging (2014) 35(4):e13–7. doi:10.1016/j.neurobiolaging.2013.09.037
Keywords: neurodegenerative diseases, aging, common features, amyloid cascade, translational medical research
Citation: Johnson IP and Roodveldt C (2017) Commentary: Immunochemical Markers of the Amyloid Cascade in the Hippocampus in Motor Neuron Diseases. Front. Neurol. 8:105. doi: 10.3389/fneur.2017.00105
Received: 23 January 2017; Accepted: 02 March 2017;
Published: 20 March 2017
Edited by:
Tibor Hortobágyi, University of Debrecen, HungaryReviewed by:
Patrick Vourc’H, Institut national de la santé et de la recherche médicale (INSERM), FranceCopyright: © 2017 Johnson and Roodveldt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ian Paul Johnson, aWFuLmpvaG5zb25AYWRlbGFpZGUuZWR1LmF1
 Ian Paul Johnson
Ian Paul Johnson Cintia Roodveldt
Cintia Roodveldt