- 1Department of Neurology, Mailman School of Public Health, College of Physicians and Surgeons, Columbia University, New York, NY, United States
- 2Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, NY, United States
- 3Department of Epidemiology, School of Public Health, Birmingham, AL, United States
- 4Department of Emergency Medicine, Thomas Jefferson University, Philadelphia, PA, United States
- 5Department of Neurology, University of Pennsylvania, Philadelphia, PA, United States
- 6Geriatric Research Education and Clinical Center (GRECC), Birmingham VA Medical Center, Birmingham, AL, United States
- 7Department of Neurology, School of Medicine, University of Alabama at Birmingham, Birmingham, AL, United States
- 8Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania, Philadelphia, PA, United States
- 9Leonard Davis Health Institute, University of Pennsylvania, Philadelphia, PA, United States
Background and purpose: Sex and race disparities in recombinant tissue plasminogen activator (rt-PA) use have been reported. We sought to explore sex and race differences in the utilization of rt-PA at primary stroke centers (PSCs) compared to non-PSCs across the US.
Methods: Data from the National (Nationwide) Inpatient Sample (NIS) 2004–2010 was utilized to assess sex differences in treatment for ischemic stroke in PSCs compared to non-PSCs.
Results: There were 304,152 hospitalizations with a primary diagnosis of ischemic stroke between 2004 and 2010 in the analysis: 75,160 (24.7%) patients were evaluated at a PSC. A little over half of the patients evaluated at PSCs were female (53.8%). A lower proportion of women than men received rt-PA at both PSCs (6.8 vs. 7.5%, p < 0.001) and non-PSCs (2.3 vs. 2.8%, p < 0.001). After adjustment for potential confounders the odds of being treated with rt-PA remained lower for women regardless of presentation to a PSC (OR 0.87, 95% CI 0.81–0.94) or non-PSC (OR 0.88, 95% CI 0.82–0.94). After stratifying by sex and race, the lowest absolute treatment rates were observed in black women (4.4% at PSC, 1.9% at non-PSC). The odds of treatment, relative to white men, was however lowest for white women (PSC OR = 0.85, 95% CI 0.78–0.93; non-PSC OR = 0.80, 95% CI 0.75–0.85). In the multivariable model, sex did not modify the effect of PSC certification on rt-PA utilization (p-value for interaction = 0.58).
Conclusion: Women are less likely to receive rt-PA than men at both PSCs and non-PSCs. Absolute treatment rates are lowest in black women, although the relative difference in men and women was greatest for white women.
Introduction
Intravenous recombinant tissue plasminogen activator (IV rt-PA) is a thrombolytic agent that improves outcomes in stroke patients when administered within 4.5 h of symptom onset (1–3). The utilization of this therapy in the United States is low despite consensus guidelines recommending its use (4, 5). Although patient-level factors, primarily a delay from symptom onset to emergency department arrival, are a major contributor to the low utilization of IV rt-PA, provider- and hospital-level factors may also contribute to underutilization. The Joint Commission (TJC) primary stroke center (PSC) certification, which requires an acute stroke team, a stroke unit, and written care protocols, has been associated with higher rt-PA utilization and lower mortality among patients when compared to patients treated at non-PSCs (6–10).
Sex disparities in IV rt-PA use have been reported; however, whether PSCs reduce these disparities is unknown (11–13). Given this, an analysis of IV rt-PA treatment comparing non-PSCs and PSCs across the US would fill a gap in our understanding. When compared with men, women have worse functional outcomes and lower quality of life after stroke (14, 15). While this may be attributable to patient-level factors, such as older age at time of stroke or differences in stroke subtype, there is also evidence that women receive lower quality of care than men, with less efficient stroke evaluation, delay in treatment with thrombolytics, and lower adherence to stroke care quality metrics (16–18).
Further contributing to the sex disparity in IV rt-PA utilization is evidence that sex differences may vary by race/ethnicity, with black women being treated less frequently than white women (19). Building on prior work evaluating differences in rt-PA by race/ethnicity (20), we used the National (Nationwide) Inpatient Sample (NIS), to explore differences in the utilization of rt-PA at PSCs compared to non-PSCs, stratified by both sex and race/ethnicity.
Materials and Methods
Study Design
A retrospective, cross-sectional analysis of data from the NIS from 2004 to 2010 was conducted. As part of the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project, the NIS is the largest publicly available all-payer inpatient care database (21). This analysis limits the data to 26 states that publicly identified both treating hospital and patient race/ethnicity (Table S1 in Supplementary Material). Data on PSC certification and date of initial certification for PSCs was obtained via personal communication from TJC on May 17th, 2011 (Jean Range, executive director of disease specific care, TJC, unpublished data, 2011). Methods for this work have been previously reported (7, 20).
We identified patients aged ≥18 years who had a primary diagnosis of ischemic stroke defined by International Classification of Diseases, 9th Revision (ICD-9) codes 433.x1, 434.x1, and 436. The positive predictive value of these codes for identifying acute ischemic stroke is greater than 85% (22). We excluded patients transferred from other hospitals. Patients who were missing information on death, sex, length of stay, or primary payer were excluded. Treatment with IV rt-PA, defined by ICD-9 procedure code 99.10, was the primary outcome. When compared to pharmacy billing data, this code has been shown to identify 77% of IV rt-PA cases (23). This study involved the analysis of de-identified data and was exempt from IRB approval.
Demographic Variables
The NIS classifies sex as male or female. Race/ethnicity is reported as non-Hispanic white, non-Hispanic black, Hispanic, Asian/Pacific Islander, Native American, or other. Race and ethnicity are not reported separately. Other patient-level variables included age, year of discharge, expected primary payer (Medicaid, Medicare, private, other), median household income in the patient’s ZIP code, comorbid conditions (Table S2 in Supplementary Material) (24), and an all-patient refined diagnosis related group (APR-DRG) measure of the expected risk of inpatient mortality. The APR-DRG uses diagnosis and procedure codes to estimate the likelihood of dying during the hospitalization as minor, moderate, major, or extreme (25). The APR-DRG marker is not specific to stroke and does not include a measure of stroke severity. Hospital-level variables included geographic region (Northeast, Midwest, South, West), rural or urban location, status as a teaching hospital (yes/no), and annual ischemic stroke case volume (<100, 100–299, ≥300).
Statistical Analysis
The statistical approach for this study parallels prior work from our group using the NIS to evaluate rt-PA use. Baseline characteristics were described for patients treated at PSCs and non-PSCs using measures of central tendency (means, medians) for continuous variables and proportions for categorical variables. Differences between the groups were evaluated using Student’s t, Wilcoxon rank-sum, and χ2 tests, as appropriate. Patients were stratified by sex, and then further stratified by race/ethnicity within sex. A multivariate model was constructed to determine independent associations including year of discharge, age, sex, primary expected payer, median income by ZIP code, hospital region, teaching status, urban/rural location, and ischemic stroke admission volume, the 29 Elixhauser comorbid conditions, and the APR-DRG measure of disease severity (20). Our analytic models used NIS survey statistics and Taylor series estimation to account for the survey design and clustering within hospitals. The analysis was conducted using SAS-callable-SUDAAN version 11.0.1. As this was an exploratory analysis, no adjustments were made for multiple comparisons (26). An alpha of 0.05 was set as the level of significance.
Results
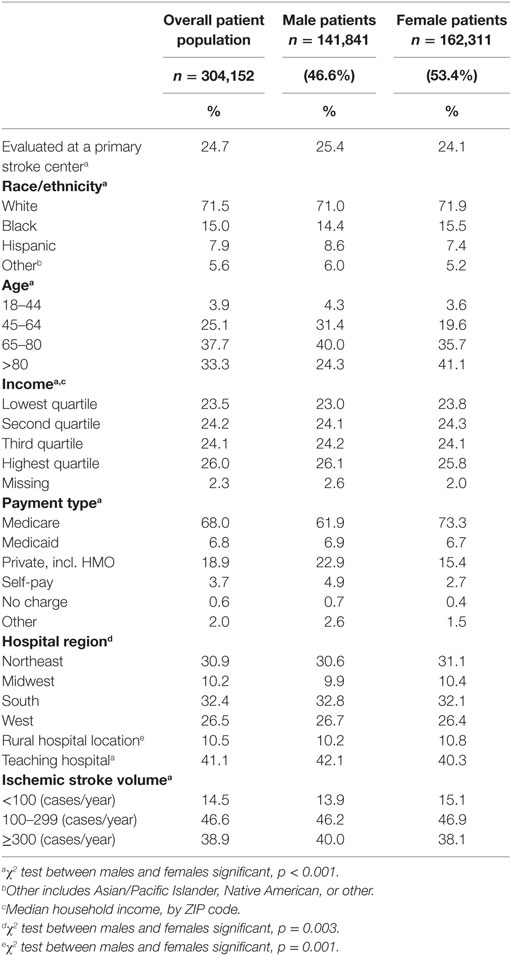
Between 2004 and 2010, acute ischemic stroke was the primary diagnosis for 598,606 hospitalizations in the NIS. The inclusion/exclusion criteria for the study population and the baseline patient- and hospital-level characteristics stratified by PSC status have been previously published (20). Table 1 describes the patient- and hospital-level characteristics for the sample, stratified by sex. Overall, 53.4% of the patients were women. Among women, 71.9% were white, 15.5% black, 7.4% Hispanic, and 5.2% were in other categories (e.g., Asian/Pacific Islander, Native American, other). Among men, 71.0% were white, 14.4% were black, 8.6% Hispanic, and 6.0% other. There were 75,160 (24.7%) patients evaluated at a PSC, and 228,992 (75.3%) evaluated at a non-PSC.
The proportion of patients evaluated at a PSC increased over time with similar proportions for men and women. The proportion of racial/ethnicity groups presenting to a PSC stratified by sex increased over time (Figure 1). A little over half of the patients evaluated at PSCs were women (53.8%), with 71.5% of white, 14.5% of black, 8.8% of Hispanic, and 5.3% of other patients evaluated at a PSC.
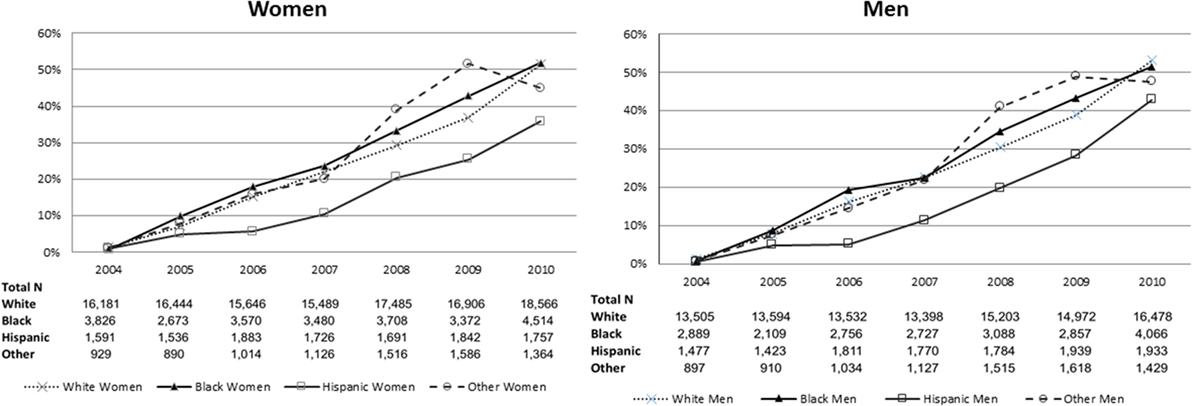
Figure 1. Proportion of patients presenting to a primary stroke center over time, stratified by sex and then by race/ethnicity.
A lower proportion of women than men received rt-PA at both PSCs (6.8 vs. 7.5%, p < 0.001) and non-PSCs (2.3 vs. 2.8%, p < 0.001). Figure 2 shows treatment rates stratified by sex and race. A higher proportion of patients received rt-PA at PSCs in all sex and race/ethnicity groups. The proportion of rt-PA treated patients at PSCs ranged from 4.4% in black women to 8.1% in white men. The proportion of rt-PA treated patients at non-PSCs ranged from 1.9% for black women to 3.0% for white men.
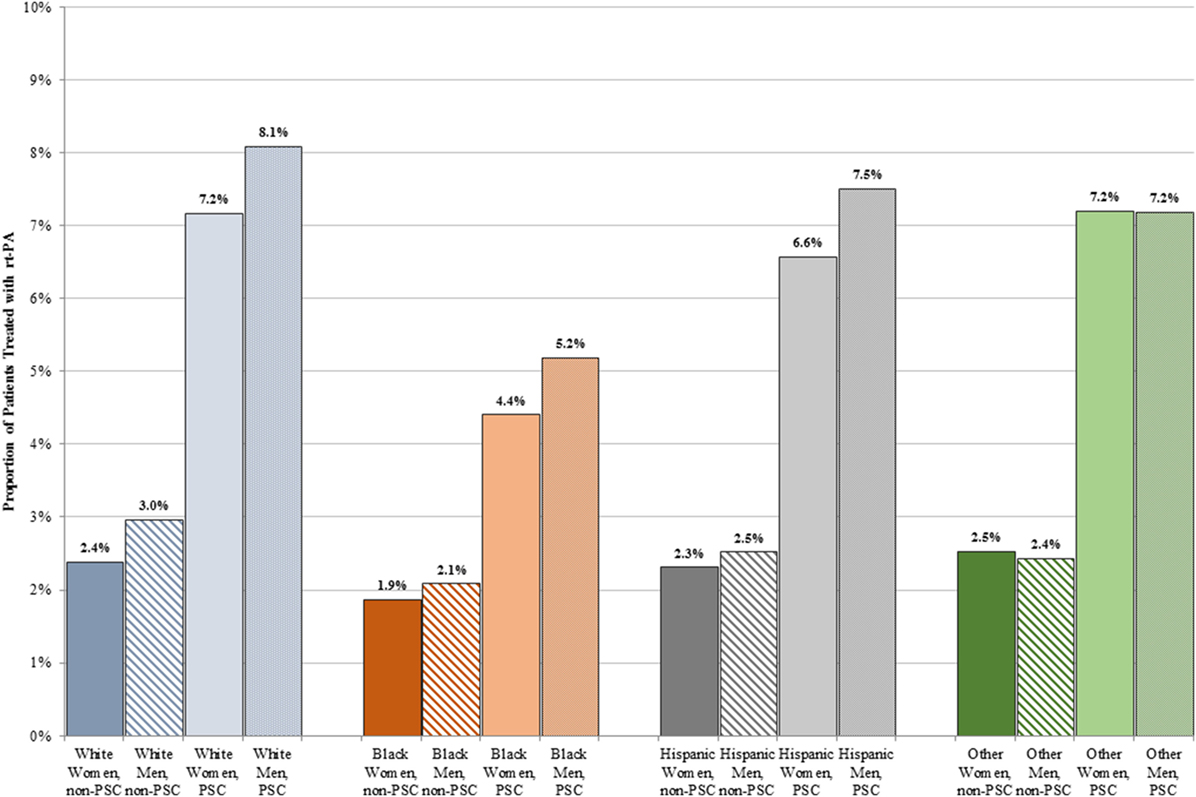
Figure 2. Proportion of men and women treated with rt-PA stratified by race/ethnicity and primary stroke center (PSC) versus non-PSC.
In an unadjusted model, women were at lower odds of being treated with rt-tPA compared to men at both PSCs and non-PSCs (Table 2). After adjusting for year, age, primary expected payer, median income quartiles by ZIP code, region, teaching hospital, urban hospital location, annual ischemic stroke case volume, 29 AHRQ individual comorbidities, and APR-DRG risk of mortality, the odds of being treated with rt-PA remained significantly lower for women regardless of presentation to a PSC (OR 0.87, 95% CI 0.81–0.94) or non-PSC (OR 0.88, 95% CI 0.0.82–0.94). After stratifying by race, white women had significantly lower odds of rt-tPA treatment in the adjusted model (Table 2). In the multivariable model, sex did not modify the effect of PSC status on rt-PA utilization (p-value for interaction = 0.58).
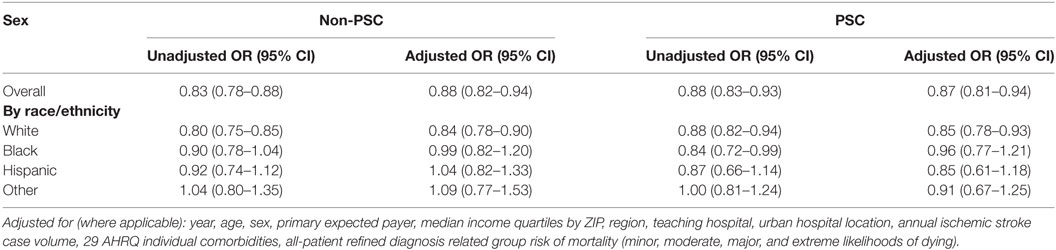
Table 2. Odds of being treated with rt-PA for women, relative to men, at non-PSCs and primary stroke centers (PSCs), stratified by race/ethnicity.
Discussion
Our results illustrate that the relationship between PSC certification and rt-PA utilization is similar in men and women. PSCs utilize rt-PA more than non-PSCs in both men and women, regardless of race/ethnicity. However, our results also illustrate that women are less likely to receive rt-PA than men at both PSCs and non-PSCs. In absolute treatment proportions, black patients are treated in lower proportions than other racial/ethnicity groups, and black women are treated less than any other group; however, the relative odds of treatment were lowest in white women relative to white men.
Sex disparities in the treatment of stroke remain a concern, particularly with the rise in incident stroke among women. IV rt-PA is effective for both men and women, but women may be even more likely to benefit from treatment than men (27, 28). Among patients not treated with rt-PA, women may have worse outcomes than men (29). It is therefore critical to ensure that women with stroke who are eligible for rt-PA receive treatment. PSCs administer more rt-PA than non-PSCs. This difference is equally true in men and in women. As more PSCs become certified throughout the US and as a greater proportion of stroke patients are evaluated at PSCs, IV rt-PA treatment in women should continue to increase. However, a better understanding of the drivers of sex differences in rt-PA treatment is needed to maximize the population benefit of developing systems of care and eliminate existing disparities. Identification of these drivers is of particular importance considering the evidence that rt-PA is associated with a greater improvement in stroke symptoms for women than for men, with no differences in adverse events after rt-PA administration between men and women (30, 31).
Lower rt-PA treatment rates in women may be related to patient factors. First, women tend to be older than men at the time of stroke. Second, women tend to present with more severe strokes (32). Third, knowledge of the signs and symptoms of stroke may be lower in women (14). This could potentially contribute to a delay in emergency department presentation, thereby preventing women from being treated if presentation is outside of the treatment time window (33). Unfortunately, the National Inpatient Sample does not contain the granular clinical data necessary to investigate these possibilities. More research is needed to determine why women are treated at a lower proportion than men so that sex disparities in acute stroke therapies can be eliminated.
Black women have the lowest proportion of rt-PA treatment, regardless of PSC status of the treating hospital. This finding supports prior data from two academic medical centers, which also found that black women were treated less frequently with rt-PA. In prior studies, black women were more likely to have delayed presentation, and once time from onset was accounted for, the sex and race disparity in rt-PA treatment no longer remained (19). It is possible that differences in time to presentation are responsible for the findings in the present study, but as noted above, symptom duration is not available in the NIS.
This study has limitations similar to prior work evaluating rt-PA use in the NIS (7, 20). As this study relies on administrative claims data defined by ICD-9 codes, we were unable to determine if there were differences in rt-PA eligibility, stroke severity, or time to presentation. We do not have information on functional outcomes after rt-PA in this dataset and cannot assess sex differences in outcomes. States that do not provide data on hospital identity or race were excluded, including a few with large minority populations, such as Georgia and Texas. In spite of this, the 26 states that provided data cover approximately 50% of the Hispanic and black population in the United States (34, 35). The definition of PSCs by TJC certification does not recognize other national or state-based certifications or identify hospitals that participate in national stroke care improvement programs, such as Get with the Guidelines. Misclassification of these hospitals as non-stroke centers would likely bias the results toward the null and would not change the observed disparities. The use of the ICD-9 procedure code 99.10 to define rt-PA may underestimate rt-PA use. This has the potential to introduce bias if coding varies between PSCs and non-PSCs or, less likely, between women and men (36). Finally, we are unable to investigate “drip and ship” cases. This could lead to underestimation of rt-PA utilization at non-PSCs.
Conclusion
The sex disparities seen in rt-PA utilization remain after accounting for race/ethnicity and other known factors that contribute to rt-PA underutilization. The increased utilization of rt-PA at PSCs is consistent between men and women and stable across racial and ethnic groups. Considering women may benefit from rt-PA utilization more than men, the disparity in utilization is concerning. Further research is needed to investigate what factors are contributing to this sex disparity.
Ethics Statement
This study was the analysis of de-identified data from the National Inpatient Sample and was exempt from IRB approval.
Author Contributions
AB is the primary author of this manuscript. BC and SK made critical revisions to the manuscript. KA contributed to the study idea, data interpretation, and made critical revisions. MK conducted the data analyses and made critical revisions. ME and CB made critical revisions. MM developed the study idea, obtained the data, interpreted the data, and made critical revisions.
Conflict of Interest Statement
BC spends a portion of his time in the Office of the Assistant Secretary for Preparedness and Response. The findings/conclusions of this report are those of the author and do not necessarily represent the views of the Department of Health and Human Services or its components. The other authors have no conflicts to report.
Funding
AB NINDS NIH T32 NS007153-31; MM NHLBI K12 HL083772 R01 HS018362; BC AHRQ K08HS17960 and R01 HS018362; and KA AHRQ T32 HS013852-10, American Heart Association 14CRP20380256, and the Geriatric Research Education and Clinical Center (GRECC) at the Birmingham VA Medical Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS, NIH, or the AHRQ.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fneur.2017.00500/full#supplementary-material.
References
1. Marler JR, Tilley BC, Lu M, Brott TG, Lyden PC, Grotta JC, et al. Early stroke treatment associated with better outcome: the NINDS rt-PA stroke study. Neurology (2000) 55:1649–55. doi:10.1212/WNL.55.11.1649
2. Del Zoppo GJ, Saver JL, Jauch EC, Adams HP; Council AHAS. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke (2009) 40:2945–8. doi:10.1161/STROKEAHA.109.192535
3. American College of Emergency Physicians, American Academy of Neurology. Clinical policy: use of intravenous tPA for the management of acute ischemic stroke in the emergency department. Ann Emerg Med (2013) 61:225–43. doi:10.1016/j.annemergmed.2012.11.005
4. Schwamm LH, Ali SF, Reeves MJ, Smith EE, Saver JL, Messe S, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at get with the guidelines-stroke hospitals. Circ Cardiovasc Qual Outcomes (2013) 6:543–9. doi:10.1161/CIRCOUTCOMES.111.000303
5. California Acute Stroke Pilot Registry (CASPR) Investigators. Prioritizing interventions to improve rates of thrombolysis for ischemic stroke. Neurology (2005) 64:654–9. doi:10.1212/01.WNL.0000151850.39648.51
6. Prabhakaran S, McNulty M, O’Neill K, Ouyang B. Intravenous thrombolysis for stroke increases over time at primary stroke centers. Stroke (2012) 43:875–7. doi:10.1161/STROKEAHA.111.640060
7. Mullen MT, Kasner SE, Kallan MJ, Kleindorfer DO, Albright KC, Carr BG. Joint commission primary stroke centers utilize more rt-PA in the nationwide inpatient sample. J Am Heart Assoc (2013) 2:e000071. doi:10.1161/JAHA.112.000071
8. Xian Y, Holloway RG, Chan PS, Noyes K, Shah MN, Ting HH, et al. Association between stroke center hospitalization for acute ischemic stroke and mortality. JAMA (2011) 305:373–80. doi:10.1001/jama.2011.22
9. Lichtman JH, Jones SB, Wang Y, Watanabe E, Leifheit-Limson E, Goldstein LB. Outcomes after ischemic stroke for hospitals with and without joint commission-certified primary stroke centers. Neurology (2011) 76:1976–82. doi:10.1212/WNL.0b013e31821e54f3
10. Kleindorfer D, de los Rios La Rosa F, Khatri P, Kissela B, Mackey J, Adeoye O. Temporal trends in acute stroke management. Stroke (2013) 44:S129–31. doi:10.1161/STROKEAHA.113.001457
11. Lisabeth LD, Brown DL, Morgenstern LB. Barriers to intravenous tissue plasminogen activator for acute stroke therapy in women. Gend Med (2006) 3:270–8. doi:10.1016/S1550-8579(06)80215-9
12. Gattringer T, Ferrari J, Knoflach M, Seyfang L, Horner S, Niederkorn K, et al. Sex-related differences of acute stroke unit care: results from the Austrian stroke unit registry. Stroke (2014) 45(6):1632–8. doi:10.1161/STROKEAHA.114.004897
13. Allen NB, Myers D, Watanabe E, Dostal J, Sama D, Goldstein LB, et al. Utilization of intravenous tissue plasminogen activator for ischemic stroke: are there sex differences? Cerebrovasc Dis (2009) 27:254–8. doi:10.1159/000196824
14. Bushnell CD, Reeves MJ, Zhao X, Pan W, Prvu-Bettger J, Zimmer L, et al. Sex differences in quality of life after ischemic stroke. Neurology (2014) 82:922–31. doi:10.1212/WNL.0000000000000208
15. Gall SL, Tran PL, Martin K, Blizzard L, Srikanth V. Sex differences in long-term outcomes after stroke: functional outcomes, handicap, and quality of life. Stroke (2012) 43:1982–7. doi:10.1161/STROKEAHA.111.632547
16. Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol (2008) 7:915–26. doi:10.1016/S1474-4422(08)70193-5
17. Smith MA, Lisabeth LD, Brown DL, Morgenstern LB. Gender comparisons of diagnostic evaluation for ischemic stroke patients. Neurology (2005) 65:855–8. doi:10.1212/01.wnl.0000176054.72325.0f
18. Reeves MJ, Fonarow GC, Zhao X, Smith EE, Schwamm LH; Get With The Guidelines-Stroke Steering Committee & Investigators. Quality of care in women with ischemic stroke in the GWTG program. Stroke (2009) 40:1127–33. doi:10.1161/STROKEAHA.108.543157
19. Boehme AK, Kumar AD, Lyerly MJ, Gillette MA, Siegler JE, Albright KC, et al. Persistent leukocytosis-is this a persistent problem for patients with acute ischemic stroke? J Stroke Cerebrovasc Dis (2014) 23:1939–43. doi:10.1016/j.jstrokecerebrovasdis.2014.02.004
20. Aparicio HJ, Carr BG, Kasner SE, Kallan MJ, Albright KC, Kleindorfer DO, et al. Racial disparities in intravenous recombinant tissue plasminogen activator use persist at primary stroke centers. J Am Heart Assoc (2015) 4:e001877. doi:10.1161/JAHA.115.001877
21. Overview of the Nationwide Inpatient Sample (NIS). (2013). Available from: https://www.hcup-us.ahrq.gov/nisoverview.jsp
22. Andrade SH, Tjia J, Cutrona S, Saczynski J, Goldberg R, Gurwitz J. Mini-Sentinel Systematic Evaluation of Health Outcome of Interest Definitions for Studies Using Administrative Data: Cerebrovascular/Transient Ischemic Attack Report. (2011).
23. Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke (2011) 42:1952–5. doi:10.1161/STROKEAHA.110.612358
24. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care (1998) 36:8–27. doi:10.1097/00005650-199801000-00004
25. Averill RF, Goldfield NI, Muldoon J, Steinbeck BA, Grant TM. A closer look at all-patient refined DRGS. J AHIMA (2002) 73:46–50.
26. Bender R, Lange S. Adjusting for multiple testing – when and how? J Clin Epidemiol (2001) 54:343–9. doi:10.1016/S0895-4356(00)00314-0
27. Kent DM, Price LL, Ringleb P, Hill MD, Selker HP. Sex-based differences in response to recombinant tissue plasminogen activator in acute ischemic stroke: a pooled analysis of randomized clinical trials. Stroke (2005) 36:62–5. doi:10.1161/01.STR.0000150515.15576.29
28. Yeo LL, Paliwal P, Teoh HL, Seet RC, Chan BP, Wakerley B, et al. Early and continuous neurologic improvements after intravenous thrombolysis are strong predictors of favorable long-term outcomes in acute ischemic stroke. J Stroke Cerebrovasc Dis (2013) 22:e590–6. doi:10.1016/j.jstrokecerebrovasdis.2013.07.024
29. Shobha N, Sylaja PN, Kapral MK, Fang J, Hill MD; Investigators of the Registry of the Canadian Stroke Network. Differences in stroke outcome based on sex. Neurology (2010) 74:767–71. doi:10.1212/WNL.0b013e3181d5275c
30. Lasek-Bal A, Puz P, Kazibutowska Z. Efficacy and safety assessment of alteplase in the treatment of stroke – gender differences. Neurol Res (2014) 36:851–6. doi:10.1179/1743132814Y.0000000331
31. Buijs JE, Uyttenboogaart M, Brouns R, de Keyser J, Kamphuisen PW, Luijckx GJ. The effect of age and sex on clinical outcome after intravenous recombinant tissue plasminogen activator treatment in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis (2016) 25:312–6. doi:10.1016/j.jstrokecerebrovasdis.2015.09.035
32. Practice advisory: thrombolytic therapy for acute ischemic stroke – summary statement. Report of the quality standards subcommittee of the American Academy of Neurology. Neurology (1996) 47:835–9. doi:10.1212/WNL.47.3.835
33. Ferris A, Robertson RM, Fabunmi R, Mosca L; American Heart Association, American Stroke Association. American Heart Association and American Stroke Association national survey of stroke risk awareness among women. Circulation (2005) 111:1321–6. doi:10.1161/01.CIR.0000157745.46344.A1
34. Ennis SR, Ríos-Vargas M, Albert NG. The Hispanic Population: 2010. (2011). Available from: https://www.census.gov/prod/cen2010/briefs/c2010br-04.pdf
35. Rastogi S, Johnson TD, Hoeffel EM, Drewery J, Malcolm P. The Black Population: 2010. (2011). Available from: https://www.census.gov/prod/cen2010/briefs/c2010br-06.pdf
Keywords: acute stroke care, thrombolysis, health policy, emergency care, healthcare delivery systems
Citation: Boehme AK, Carr BG, Kasner SE, Albright KC, Kallan MJ, Elkind MSV, Branas CC and Mullen MT (2017) Sex Differences in rt-PA Utilization at Hospitals Treating Stroke: The National Inpatient Sample. Front. Neurol. 8:500. doi: 10.3389/fneur.2017.00500
Received: 06 April 2017; Accepted: 07 September 2017;
Published: 27 September 2017
Edited by:
Ayrton R. Massaro, Hospital Sirio-Libanes, BrazilReviewed by:
Henry Ma, Monash University, AustraliaMuhib Khan, Michigan State University, United States
Sombat Muengtaweepongsa, Thammasat University, Thailand
Alexander Tsiskaridze, Tbilisi State University, Georgia
Copyright: © 2017 Boehme, Carr, Kasner, Albright, Kallan, Elkind, Branas and Mullen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael T. Mullen, bWljaGFlbC5tdWxsZW5AdXBocy51cGVubi5lZHU=
 Amelia K. Boehme
Amelia K. Boehme Brendan G. Carr4
Brendan G. Carr4 Scott Eric Kasner
Scott Eric Kasner Michael J. Kallan
Michael J. Kallan Michael T. Mullen
Michael T. Mullen