- 1Department of Medical, Surgical, Neurological, Metabolic, and Aging Sciences, Headache Center, University of Campania “Luigi Vanvitelli, ” Naples, Italy
- 2MRI Research Center SUN-FISM, University of Campania “Luigi Vanvitelli, ” Naples, Italy
- 3Research Unit of Neurophysiology of Vision and Neuro-Ophthalmology, G. B. Bietti Foundation-IRCCS, Rome, Italy
- 4Department of Medico-Surgical Sciences and Biotechnologies, Sapienza University of Rome Polo Pontino, Latina, Italy
- 5IRCCS Neuromed, Pozzilli, Italy
- 6Institute for Diagnosis and Care “Hermitage Capodimonte, ” Naples, Italy
Background: It is well-known that both inter- and intra-individual differences exist in the perception of pain; this is especially true in migraine, an elusive pain disorder of the head. Although electrophysiology and neuroimaging techniques have greatly contributed to a better understanding of the mechanisms involved in migraine during recent decades, the exact characteristics of pain threshold and pain intensity perception remain to be determined, and continue to be a matter of debate.
Objective: The aim of this review is to provide a comprehensive overview of clinical, electrophysiological, and functional neuroimaging studies investigating changes during various phases of the so-called “migraine cycle” and in different migraine phenotypes, using pain threshold and pain intensity perception assessments.
Methods: A systematic search for qualitative studies was conducted using search terms “migraine,” “pain,” “headache,” “temporal summation,” “quantitative sensory testing,” and “threshold,” alone and in combination (subject headings and keywords). The literature search was updated using the additional keywords “pain intensity,” and “neuroimaging” to identify full-text papers written in English and published in peer-reviewed journals, using PubMed and Google Scholar databases. In addition, we manually searched the reference lists of all research articles and review articles.
Conclusion: Consistent data indicate that pain threshold is lower during the ictal phase than during the interictal phase of migraine or healthy controls in response to pressure, cold and heat stimuli. There is evidence for preictal sub-allodynia, whereas interictal results are conflicting due to either reduced or no observed difference in pain threshold. On the other hand, despite methodological limitations, converging observations support the concept that migraine attacks may be characterized by an increased pain intensity perception, which normalizes between episodes. Nevertheless, future studies are required to longitudinally evaluate a large group of patients before and after pharmacological and non-pharmacological interventions to investigate phases of the migraine cycle, clinical parameters of disease severity and chronic medication usage.
Introduction
Migraine is one of the most prevalent neurological disorder and consistently ranks fifth to eighth among the top causes of disability, worldwide (1). Ictal migraine manifestations are characterized by episodes of unilateral moderate or severe throbbing and pulsating headache, frequently associated with nausea, vomiting, photophobia, phonophobia, and cutaneous allodynia (CA) (2, 3), defined as “pain due to a stimulus that does not normally provoke pain” (4). Current consensus is that migraine is an episodic, recurrent, genetically determined dysfunction of brain excitability that leads to the activation and sensitization of the trigeminovascular pain pathway. However, the neural components and mechanisms involved in the generation and recurrence of migraine headaches are still far from being understood.
Nevertheless, during recent decades, electrophysiology and neuroimaging techniques have greatly contributed to the understanding of the mechanisms involved in this elusive painful disorder. Several neurochemical, functional and microstructural alterations across various subcortical-cortical cerebral areas have been described thus far (5–7). However, despite enormous progress in the field, one of the biggest challenges of researchers worldwide remains the identification of the brain circuitry sub-serving and encoding the clinical perception of pain in migraine.
It is well-known that the perception of clinical pain per se appears to be vary greatly from person to person in the general population. However, there also exists intra-individual differences. Indeed, it is well-known that several factors, such as spontaneous neuronal fluctuations, attention, expectation of pain, cognitive and emotional states, sleep habits and stress, may influence pain perception (8).
Studies of pain perception in humans require an external stimulus that must be applied to recreate the experience of pain. Once produced, this artificial experience of pain can be evaluated by verbal, behavioral, and physiological measures (9). The single-point measure of “pain threshold” (PT)—defined as the minimum intensity of a stimulus that is perceived as painful (4)—is often used to easily assess subjective pain sensitivity. In an experimental setting, the subject indicates the moment in which a stimulus is perceived as painful, corresponding to a detailed definition of the stimulus itself (e.g., degrees centigrade for heat and cold stimuli, milliamperes for electrical stimuli, grams for pressure with Von Frey filament, etc.).
In contrast to the well-established response related to PT evaluation, the assessment of perceived pain intensity (PPI) consists of delivering a series of discrete supra-threshold stimuli of varying intensity in random order (9). This method assumes that subjects can judge the magnitude of a pain-inducing stimulus, primarily using a rating scale (e.g., Numerical Rating Scale from 0 to 10 or 100).
To better understand migraine-related changes in pain processing, several authors have evaluated pain perception during different phases of the migraine cycle using pressure, electrical, and thermal (cold or heat) stimuli, single or repetitive pulses of varying frequencies delivered over cephalic, cervical, or extra-cephalic areas.
The purpose of this review is to provide a comprehensive overview of the findings of clinical, electrophysiological and functional neuroimaging studies aimed to investigate changes in pain perception of migraine patients during the various phases of the so-called migraine cycle, using PT and PPI assessments.
Review Criteria
We initially searched the PubMed database to identify articles published up to December 2016. The search terms used were “migraine,” “pain,” “headache,” “temporal summation,” “quantitative sensory testing,” and “threshold,” alone and in combination. The literature search was updated using the additional keywords “pain intensity,” and “neuroimaging” to identify full-text papers written in English and published in peer-reviewed journals, using the PubMed and Google Scholar databases. In addition, we manually searched the reference lists of all research articles and review articles.
Data were presented with the criteria of following the various phases of the migraine cycle, and the progression of migraine (see Tables 1–3 for further information).
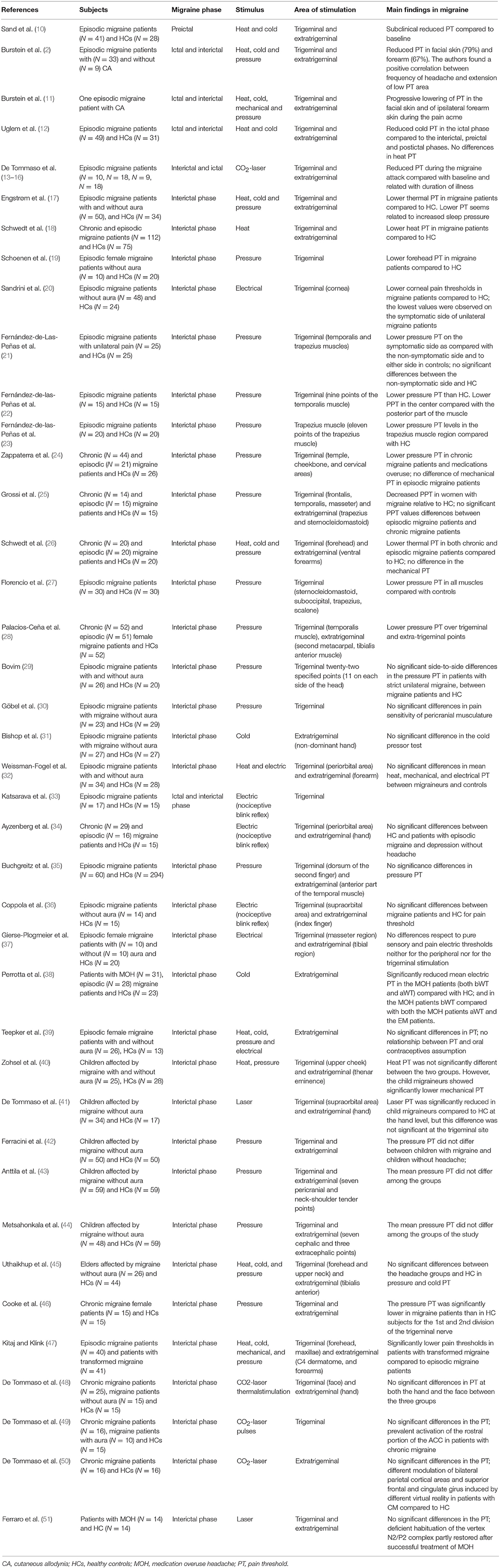
Table 1. Pain threshold findings from experimental studies during noxious stimulation in migraine patients.
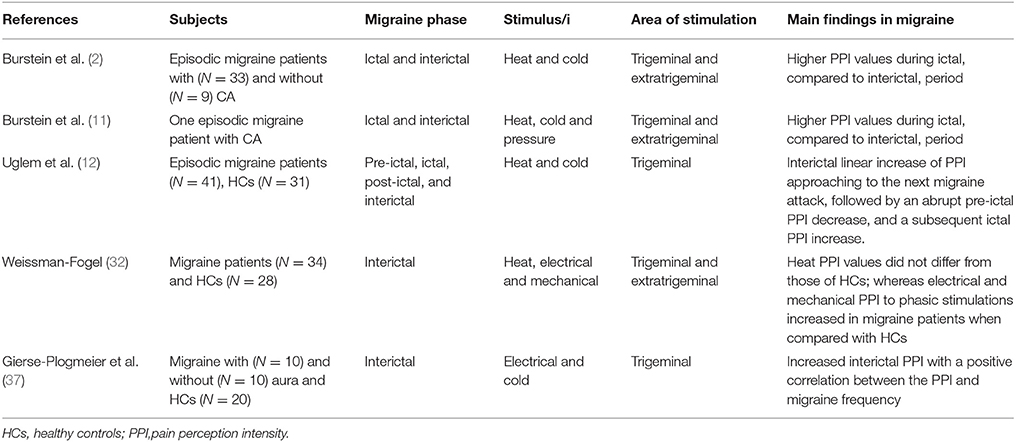
Table 2. Perceived pain intensity findings from experimental studies during heat, cold, pressure, or electrical noxious stimulation in migraine patients.
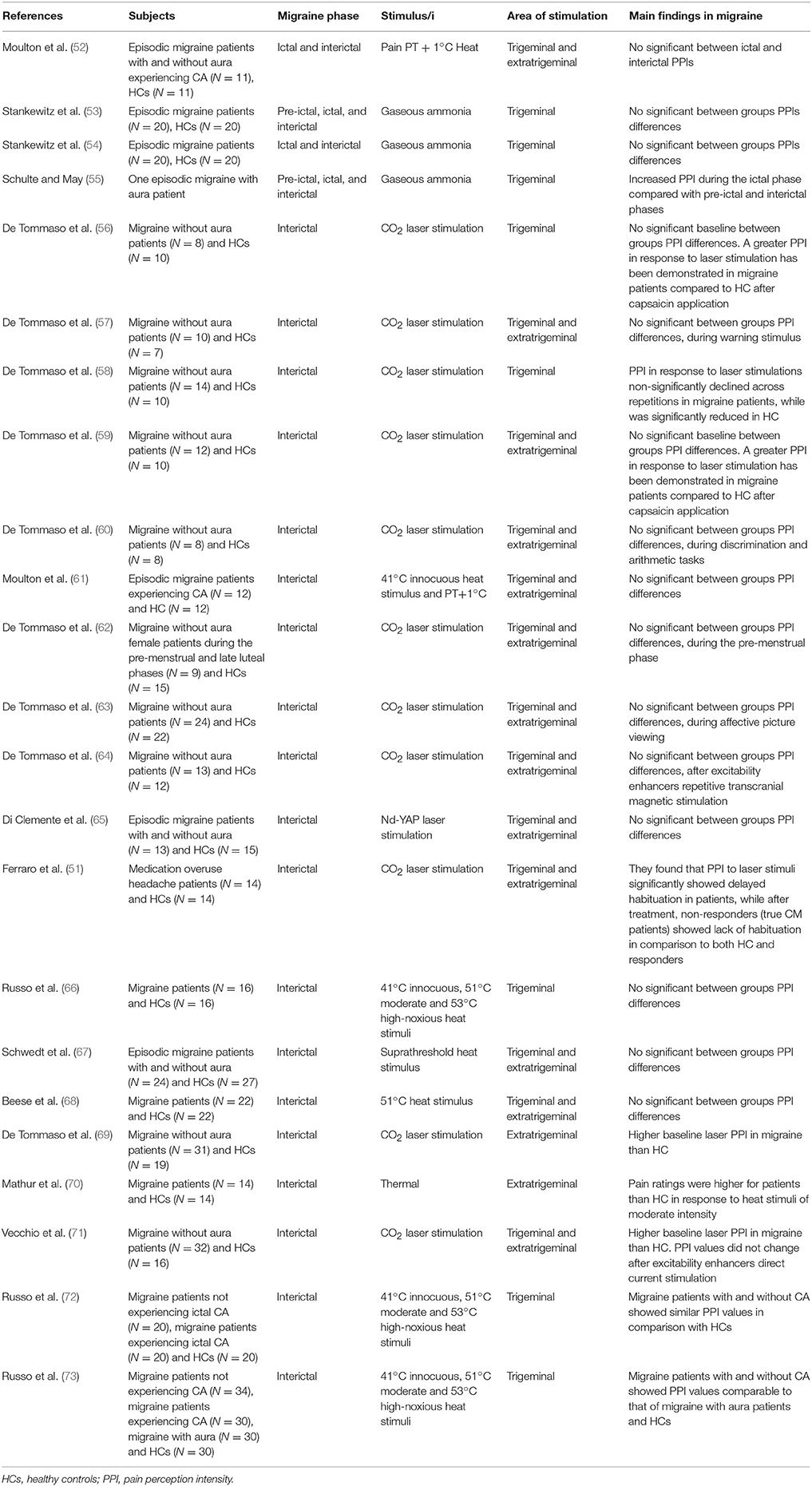
Table 3. Perceived pain intensity findings from experimental studies using pain-related evoked potentials and neuroimaging during noxious stimulation in migraine patients.
Data Overview
Ictal Phase of Episodic Migraine
Few studies were dedicated to the assessment of PT in migraine patients during a migraine headache (ictal phase).
Pain Threshold
Burstein and colleagues repeatedly measured PT both in the absence and during the course of moderate-to-severe migraine attacks in the same patients using bilateral heat, cold and pressure stimuli applied to the cephalic (forehead) and extra-cephalic (forearm) regions (2). In this study, 79% of patients showed reduced PT by at least one standard deviation from the baseline interictal control threshold on the facial skin ipsilateral to migraine pain, as assessed by one or more modalities. This was considered a clinical manifestation of CA. Frequently, CA was not restricted to the referred migrainous pain area, but spread contralaterally, and even to the ipsi- and contra-lateral forearms. Notably, patients' PT were compared among themselves (interictal vs. ictal) rather than to healthy controls (HC). Interestingly, non-allodynic patients were younger, with shorter disease duration, and less frequently reported aura symptoms compared to allodynic patients.
In a 42 year old male affected by migraine with aura (MwA), Burstein and colleagues (11) further investigated the temporal and spatial development of CA. In the first phase of a migraine attack, a reduced PT for mechanical and cold stimuli was found within the referred pain area. Two hours from the onset of migraine attack, further lowering of mechanical PT on the ipsilateral forehead and lowering of pressure and cold PT on the contralateral forehead and ipsilateral forearm were observed. Finally, during the acme of the migraine attack (4 h from the onset), heat PT was even further decreased in both the ipsilateral and contralateral forehead and in the ipsilateral forearm (11). Other observations based on the longitudinal study of a group of patients (10, 12) supported that PT is consistently lower during the ictal phase compared to the interictal phase. Focusing on a more recent study performed in a large cohort of participants, authors observed decreased cold PT of the forehead during the ictal phase compared to the interictal phase. However, no differences in the heat PT of the hand or forehead was observed between ictal and interictal phases (12). These contrasting data (2, 11) could be related to the time-point within the development of the migraine attack when testing took place. However, PT was not altered by controlling for aura or headache side.
Pain Perception Intensity as Judged by Pain-Related Evoked Potentials
In migraine, the PPI assessment during continuous suprathreshold heat pain stimulation on the temporal areas showed significantly higher values during the ictal period than during interictal period (2, 11). This was not altered by controlling for aura or the headache side. Notably, an alternative experimental approach to study pain processing is by delivering repetitive laser stimulations, and rating PPI while recording cortical laser evoked potentials (LEPs). In electrophysiological studies using CO2 laser stimulations, lowered basic PT and resulting increased cortical amplitudes during spontaneous or experimentally induced migraine attacks have been confirmed in the bilateral cephalic (supraorbital) and extra-cephalic areas (hand) ipsilateral to headache side compared to the interictal phase (13–16). Interestingly, administration of either almotriptan or lysine-acetylsalicylate could not increase PT to laser stimuli in migraine patients for up to 2 h after intake, though treatment provided relief from headache (16).
Pain Perception Intensity as Judged by Functional Neuroimaging
Recently, increased trigeminal PPI using trigeminal nociceptive stimuli (gaseous ammonia) has been demonstrated during the ictal phase compared with preictal and interictal phases in one migraine patient scanned daily for 30 days (55). In this patient, the hypothalamus was significantly more active immediately before the headache phase, when it also showed the greatest functional coupling with the spinal trigeminal nuclei. On the other hand, during the ictal state the hypothalamus was functionally coupled with the dorsal rostral pons. However, in a similar (though retrospective) study from the same research group, no differences in PPI were observed using olfactory stimulations in episodic migraine patients during the interictal, preictal, or ictal phases, or when compared with HC (53).
Overall, consistent data indicate that PT is lower during the ictal phase than both the interictal phase of migraine and in HC in response to pressure, cold, and heat stimuli applied over the cephalic (forehead) region on the usual headache side. The involvement of extracephalic territories (forearm or hand) ipsilateral and/or contralateral to the headache side depends mainly on the time that has passed since the beginning of the attack. PPI during continuous suprathreshold heat pain stimulation at the cephalic and extracephalic territories was significantly higher in patients in the ictal phase, than in those in the interictal phase or in HC. These changes in PT and PPI during the ictal phase are accompanied by a heightened cortical response and reorganization of brain areas devoted to nociception/antinociception.
Peri-Ictal Phases of Episodic Migraine
Although the abnormalities in the pain perception are more pronounced during migraine attacks, subtle changes may be present before and after the headache phase (i.e., during the so-called preictal and postictal phases). The preictal phase occurs a couple of hours to days before the onset of a migraine attack, and is manifested by various symptoms such as fatigue, difficulty in concentrating, neck stiffness, increased sensitivity to light and sound, nausea, blurred vision, yawning, and pallor. The postictal phase usually lasts 24 h, and is frequently associated with mood changes, muscle weakness, physical exhaustion, and reduced appetite (74).
Pain Threshold
Until now, few studies have directly or indirectly focused on PT in the preictal and postictal phases of the migraine cycle. Sand and colleagues (10) performed a blinded paired longitudinal investigation of 11 patients (about 24 h before the headache), and performed within-subject comparisons between the preictal and interictal phase. The authors found that preictally, heat PTs were slightly lower in the forehead, neck and hand, while cold PTs were lower in the neck and hand. Moreover, in preictal phase, significant lower cold (but not heat) PTs were detected on the symptomatic forehead side when compared with the asymptomatic side. Although the researchers did not check for possible differences in PT between preictal migraine patients and HC, only marginal differences between preictal data and HC can be observed in the raw data presented (10). The mild decrease in PT (remaining within the normal range) was considered suggestive of preictal heat sub-allodynia (12, 29). However, despite these encouraging results, another small study performed by the same research group did not find significant differences in pressure and thermal PT between interictal, preictal, and postictal migraine patients (17). These negative results were recently confirmed in a group of migraine patients comparing both interictal-ictal and interictal-postictal paired measurements (12). Nonetheless, they emphasized that both heat and cold PT values were lower in the ictal compared to the interictal phase, arguing that an interictal-preictal-ictal gradient could exist. Indeed, when interictal and preictal patients were pooled and analyzed over a 15-day range, it is possible that preictal thermal sub-allodynia manifests closer to the attack in many episodic migraine patients (12). The latter data seem to be in line with the previous observations of a positive correlation between heat PT (at the forehead and at the forearm) and the number of hours until the next migraine attack (18). This unusual pattern suggests that neural events affect pain processing the day before the migraine attack (12). However, study limitations included PT evaluation regardless of the side on which the patient most commonly experienced headaches, and the inclusion of patients taking acute medication, which may have biased analyses.
Moreover, in a study evaluating the relationship between PT and sleep quality in migraine, slow-wave sleep was negatively correlated with pressure PT, and slow bursts were negatively correlated with thermal PT, suggesting that sleep abnormalities might precipitate attacks and induce hyperalgesia (17).
Pain Perception Intensity as Judged by Quantitative Sensory Testing
The fluctuations in pain processing of migraine patients depending on the time from attack onset has been further confirmed by Uglem et al. (12). They assessed subjective PPI and showed a linear increase of PPI during the interictal period approaching the next migraine attack, followed by an abrupt preictal PPI decrease, and a subsequent ictal PPI increase.
Overall, during the preictal phase, at a time when premonitory symptoms may occur, patients tend to show only mildly significant lowered PT to heat and cold stimuli in the forehead, neck, and hand, mainly on the symptomatic side, compared to their own interictal phase or to HC. This condition was considered to be suggestive of preictal sub-allodynia and could be considered an early manifestation of (or a predisposing factor for) the development of the next attack. Some studies suggest that migraine patients experience fluctuations in pain processing depending on the distance from the attack, since PT decreases preictally approaching the next attack, while PT greatly decreased and PPI increased ictally.
Interictal Phase of Episodic Migraine
Pain Threshold
Several studies using different paradigms have investigated thermal, mechanical, or electrical PT in migraine patients in the interictal phase and compared them with HC. However, these studies have generated conflicting results, showing significant differences in PT between migraine patients and HC (17–28) or no differences (10, 29–39, 68, 69, 75). Among the work in which a significantly lower PT has been demonstrated in migraine patients, PT reduction was observed in cephalic (17–22, 24–26), cervical (21, 23, 25, 27), and extracephalic (18, 21, 25, 26, 28, 69) regions, in particular using mechanical painful stimuli (19, 21–25, 27, 28), to a lesser extent in thermal (18, 26, 69, 76), and in one case electric (20) stimuli.
Fernández-de-las-Peñas et al. showed that pressure PTs in patients with strictly unilateral migraine was significantly lower in the cervical (upper trapezius muscle), but not cephalic areas of the symptomatic side compared to the non-symptomatic side or HC (21, 23). Further, this effect was more evident in females than in males (21). However, another group of researchers failed to find a correlation between headache laterality and pressure PT values in a group of women with migraine tested interictally (25). In a large cohort of mixed episodic and chronic migraine (CM) patients, Schwedt et al. (18) failed to confirm their previous findings of a negative correlation between heat (but not cold or pressure) PT and CA symptom scores, as assessed at the time of testing or recalled during headache (26). This was despite the observation of increased (but within normal range) self-reported CA scores.
Taken together, these studies demonstrate great variability in results, likely due to a highly heterogeneous subject samples. For instance, studies included patients on prophylactic medication, or in some sub-analyses, intermixed (e.g., episodic and chronic) migraine patients (21, 27, 77). Nonetheless, a major source of inconclusiveness in studies is in the lack of precise information regarding the time-point during the migraine cycle in which patients were tested, as authors did not control for proximity to the next attack (18, 21–24, 26, 27, 77).
Pain Perception Intensity as Judged by Pain-Related Evoked Potentials
Using heat LEPs, the majority of studies did not report differences in laser PPI between migraine patients during the pain-free phase and HC (57, 59, 65), after visual warning stimulus (57), during discrimination and arithmetic tasks (60), during the pre-menstrual phase (62), during affective picture viewing (63), or after administration of excitability enhancers such as repetitive transcranial magnetic (64) or direct current (71) stimulations. Nonetheless, some exceptions exist which found higher baseline laser PPI in migraine patients compared to HC (69, 71).
The absence of PPI differences between migraine patients during the pain-free phase and HC has been supported by robust behavioral data collected during event-related neuroimaging studies using noxious heat stimuli, although based on small heterogeneous migraine patient populations (52–54, 61, 66, 67, 72). These findings have been confirmed in a subgroups of episodic migraine patients with or without CA compared to HC (72), when stimuli were applied both over cephalic (52–54, 61, 66, 67, 72) and extra-cephalic regions (52, 67). Moreover, PPI ratings did not differ between pain-free patients and HC using both variable heat PT+1°C stimulus intensity (52, 61, 67) or fixed moderate-to-high painful heat stimuli (66, 72), with one notable exception where pain ratings were higher for migraine patients in response to moderate heat stimuli (70).
A greater PPI in response to laser stimulation has been demonstrated in migraine patients compared to HC after capsaicin application, with less inhibition of laser evoked potential (LEP) amplitudes (56, 59). PPI and LEP amplitudes in response to laser stimulations non-significantly declined across repetitions in migraine patients between attacks, while both were significantly reduced in HC- a behavioral phenomenon ascribed to a sensitization mechanism rather than habituation (58). Similarly, some authors found increased PPI in migraine patients between attacks compared to HC by delivering repetitive trains of mechanical (32) and electrical (32, 37, 38) pain stimuli, an experimental model of sensitization also knows as temporal summation (or “wind-up”). Patients with increased temporal summation had increased frequency of attacks per month and tended to be closer to their last attack than patients without increased temporal summation (32, 37). The authors ascribed this phenomenon to sub-clinical activation-dependent plasticity of pain pathways, reflecting subtle increased membrane excitability that may be described clinically as a sub-allodynia state.
Pain Perception Intensity as Judged by Functional Neuroimaging
Neuroimaging studies in patients scanned during the migraine attacks either documented significant greater PPI than during the interictal phase (55), or no differences (53–54).
Compared to HC, migraine patients are characterized by an increased blood oxygenation level dependent (BOLD)–response in brain areas involved in nociception/antinociception and neurocognitive aspects of pain processing (i.e., insula, middle cingulate and anterior cingulate cortices, secondary somatosensory cortex, amygdala, cerebellum, caudate nuclei, and motor and pre-motor areas, temporal pole, lentiform nuclei, posterior thalamus, fusiform gyrus, subthalamic nucleus, pre- and post-central gyrus, hippocampus and parahippocampal gyrus, and dorsolateral prefrontal cortex) (52–54, 67, 70). Further, they exhibit reduced BOLD activation within the brainstem (trigeminal nucleus caudalis, nucleus cuneiformis) (53, 61), despite experiencing normal heat PPI in cephalic and extra-cephalic areas.
Interestingly, spinal trigeminal nucleus activation appears to fluctuate during the migraine cycle, being slightly greater in the preictal state compared to HC and significantly enhanced compared to interictal scans. and decreased during an acute attack compared to both HC and preictal migraine patients (53).
Russo and colleagues used fixed innocuous (41°C), moderate noxious (51°C), and severe noxious (53°C) heat stimuli over the innervation territory of the maxillary division of the trigeminal nerve delivered on the more affected head side in episodic migraine patients between attacks during MRI scans (66, 72). The authors did not observe differences in cerebral BOLD signals during the innocuous (41°C) stimulus sessions compared to HC, or in subgroups with or without CA. However, while PPI was not significantly different between patients and HC, an overall greater activation to a moderate painful stimulus was observed in the perigenual area of the anterior cingulate cortex (66), especially in patients experiencing CA (72). This was associated with further involvement of the left anterior pons within the brainstem (66) and the left middle frontal gyrus (72). During the severe painful stimulus, an overall increased activation of the secondary somatosensory cortex was observed bilaterally in migraine patients (66) and in the subgroups with and (even more so) without CA (72).
There were no statistically significant correlations between PPI at any level of experimental stimuli nor BOLD-fMRI signal changes in migraine patients with or without experience of CA (72). Furthermore, there was no significant correlation between pain-induced BOLD signal changes and depression or state-trait anxiety scores (67). Nonetheless, when clinical migraine features were regressed with pain-related BOLD signals, the time to next attack was positively related to activation strength within the trigeminal nuclei (53), intensity of headache was negatively related to middle prefrontal cortex and posterior cingulate cortex and positively to bilateral insula activation (70), attack frequency was related to activation strength within several brain areas (i.e., middle cingulate, insula, fusiform gyrus, hippocampus, dorsolateral prefrontal cortex, precentral gyrus) (52, 67, 70), and years with migraine was positively related to activation strength within the fusiform gyrus (67) and negatively related to that in the superior temporal gyrus (70).
More recently, Russo and colleagues (73) used trigeminal heat stimulation (THS) at three different predefined intensities (41, 51, and 53°C), and explored PPI in three drug-naïve patient groups characterized by homogeneous migraine phenotypes such as migraine without aura (MwoA) without CA (MwoA CA−), MwoA with ictal CA (MwoA CA+), and MwA without CA (MwA CA−). When compared to HC, the authors found no significant differences in PPI in any experimentally induced stimuli. Moreover, no significant correlations were found between clinical variables and PPI of the THS at any level of experimental stimulus.
Overall, the results obtained during the interictal period are conflicting, because either reduced or no differences in PT were observed. However, studies were mostly conducted using mechanical sensory testing, making it impossible to determine the contribution of the peripheral muscle (e.g., muscle tenderness) and central sensitization processes. Indeed, pressure PT was unrelated to the presence of overt interictal/ictal CA. Quantitative sensory testing and neuroimaging data support the concept that migraine patients may be characterized by a normal PPI, despite methodological limitations of the studies. Nonetheless, some authors detected increased PPI during concomitant sensitization-inducing stimulations (such as stimulus repetition and capsaicin application) in migraine patients between attacks compared to HC, especially when patients were tested closer to an attack or experienced higher attack frequency. Finally, neuroimaging studies reveal that, despite essentially normal PPI ratings, the migraine brain encodes and behaves differently to subjective PPI compared to HC, especially depending on patients' clinical features.
Pain Perception in Children and Elders With Migraine
Although migraine in children and the elderly may represent good models to test disease onset and progression, few studies have been dedicated to the assessment of the pain processing in childhood and elderly migraine patients. Nonetheless, research that has been done has also produced contradictory results.
Indeed, both significant differences (40–42), or no differences (43, 44) in PT have been documented in children with migraine during interictal period and HC. Among the research reporting significantly reduced PT in migraine patients, it has been observed in cephalic (40), cervical (42), and extracephalic (40, 41) regions, using mechanical (40, 42) and CO2 laser (41) painful stimuli.
In a small cohort of children with a short migraine history, unaltered heat PT and lower mechanical PT were observed following stimulation within the innervation territory of the second trigeminal branch territory (maxilla) as well as the thenar eminence of the non-dominant hand (40).
In a CO2 laser evoked potential study, laser PT was significantly lower in children with migraine compared to HC at the hand level; however, no significant differences were observed in the trigeminal field (41). Moreover, in children with headache increased amplitude and lack of habituation of the N2-P2 vertex complex was observed both in the trigeminal and extracephalic areas, which was correlated with acute CA and inter-critical pericranial tenderness (41).
Similar to studies performed in adult migraine patients, some biases affect these observations. For example, the inclusion of patients on prophylactic medication (42) or lack of precise information about the test subject's phase of migraine cycle (40–42) may affect results. Only one study has investigated the effects of pressure, heat, and cold PTs in a group of elderly patients suffering from episodic and CM, mostly experiencing headache on the testing day and taking prophylactic medication (45). The authors did not find significant differences for pressure and cold PT at any somatic location (cephalic, cervical, and extracephalic regions) between the migraine patients and HC. However, the migraine group showed significantly lower heat PT in the upper neck area compared to HC, which was significantly related to the presence of pain in the area, making the PT difference difficult to ascribe to the presence of headache. There were no significant correlations between any of the patients' clinical features and PTs (45).
To the best of our knowledge, there are no studies specifically exploring PPI in children or elders with migraine. Overall, some reports in children with migraine tend to indicate that abnormal noxious information processing could appear early in life, perhaps with a concomitant presence of an altered PT detection system.
Pain Perception in Chronic Migraine
A proportion of episodic migraine patients experience an attack frequency equal to or greater than 15 days per month for at least 3 months leading to CM. In clinical practice, the most frequent exogenous factor that leads to headache chronification is medication overuse, diagnosed in up to 80% of patients. The diagnostic criteria of CM are still hotly debated and thus in continuous evolution. Most published papers assessing PT in CM (25, 46, 47, 78) used the criteria of Silberstein-Lipton for transformed migraine (TM) (79) which shows 93% agreement with the International Classification of Headache Disorders 2004 edition (80).
In the assessment of PTs, researchers compared CM patients with HC (24–26, 28, 34, 38, 46, 48–51) and/or with episodic migraine patients (24–26, 28, 34, 38, 47–49, 78). Again, conflicting results were obtained by these studies, since either significantly reduced (24–26, 28, 38, 46), or normal (34, 48–51) PTs were detected in CM patients. Studies that compared CM with episodic migraine patients either documented equal to (25, 26, 28, 48–51) or greater (24, 47, 78) reduction in the former compared to HC. Among the research in which PTs appear to be significantly lower in CM patients, the reduction was observed in cephalic (24–26, 28, 46, 47, 78), cervical (24, 25, 46, 47, 78), and extracephalic (28, 38, 46, 47, 78) regions. This PT reduction was evident using mechanical (24, 25, 28, 46, 47, 78), thermal (26, 47), and electrical (38) painful stimuli.
In a group of CM due to medication overuse (MOH), Perrotta and colleagues (38) found a markedly reduced temporal summation threshold of the spinal noxious flexion reflex, with an increase in reflex area and PPI in MOH patients compared to both HC and episodic migraine patients. Further, they reported a marked improvement in MOH patients after drug withdrawal treatment. Interestingly, activation of the diffuse supraspinal inhibitory controls by means of cold pressor test did not produce any significant effect on either the neurophysiological or psychophysical parameters before detoxification, but not after (38). In a group of mixed episodic and CM patients, Schwedt and colleagues (26) found that patients showed a significant negative correlation between heat PT and number of symptoms of CA. The authors used a questionnaire assessment administered at the time of testing and recalled during headache, and concluded that lower PT during migraine is due to central sensitization phenomena. However, it must be mentioned that in a larger cohort of patients, the same authors failed to confirm their previous findings (18).
Ferraro et al. studied PPI and LEP amplitudes in response to repetitive laser stimulations in CM and MOH (before and after successful treatment) patients as well as HC. They found that PPI and LEP amplitudes to laser stimuli demonstrated significantly delayed habituation in both patient groups, while after treatment, non-responders (true CM patients) showed lack of habituation compared to both HC and MOH patients. While in those MOH patients who showed a treatment response (true MOH patients), authors found normal habituation (51).
In an intermixed group of chronic tension type headache and MOH patients, the duration of the chronic phase was associated with cutaneous PT reduction, while in the MOH only group, mean cutaneous PT values decreased with increasing daily drug intake (24). Finally, some researchers found that in women with CM, the presence of a widespread reduction in PT is negatively associated with the severity of migraine pain but unrelated to the presence of anxiety or depressive symptoms (28). Limitations of such studies can be the inclusion of CM patients on prophylactic medications (25, 46, 47, 78) intermixed with those overusing acute medications (46, 47), and patients with ongoing concomitant chronic pain conditions (47).
Overall, as for the interictal state, PT data obtained from migraine patients ranging from episodic to CM are inconclusive. However, some have documented lower PT in CM patients compared to HC and episodic migraine patients, almost exclusively using mechanical stimuli. Increased PPI ratings with reduced efficiency of supraspinal descending inhibition controls were documented in CM resulting from medication overuse, before and after drug detoxification. Nonetheless, the level of PT seems to be related to the severity of migraine and the daily drug intake level.
Discussion
Migraine is presently considered a disorder of the brain. Many independent research groups have observed that the brain of migraine patients abnormally processes all sensory information, with the exception of olfaction (81). These functional abnormalities are not constant, but rather exist under continuous fluctuations following various phases of the so-called migraine cycle (6). Recent evidence provided by modern MRI techniques tends to show that reversible plastic changes in brain micro-and macro- structure accompany functional abnormalities (53, 82–84). However, whatever the origin of these cerebral morpho-functional abnormalities, migraine manifestation requires ignition of the central and peripheral trigeminal system (2). Overt or silent cortical spreading depression (85), malfunctioning descending pain control systems in the frontal cortex (86) and brainstem (53, 87), and abnormal thalamic control (83, 88, 89)- alone or in combination- seem to be major permissive interictal factors for the preictal cascade of events that leads to sequential sensitization of first- and/or second-order trigeminovascular nociceptors resulting in transient (episodic migraine) or persistent (in CM) central sensitization (2, 90).
Here, we have summarized clinical, neurophysiological, and neuroimaging studies that have assessed the perception of pain in the various phases of the migraine cycle. The studies performed during the spontaneous occurrence of a migraine attack have detected lower PTs and increased PPI during the ictal phase compared to both the interictal phase and HC in response to various type of sensory stimuli applied over the cephalic region on the usual headache side. The involvement of ipsilateral and/or contralateral extracephalic territories depends mainly on the time passed from the beginning of an attack. These time and spatial changes in pain processing and sensitivity can be interpreted as reflecting subtle manifestations of the sequential activation of the peripheral and central sensitization processes, sometimes clinically manifesting as CA.
Contrary to what was observed during the ictal period, the results obtained during the interictal period are conflicting, as either reduced PT or no differences were observed. Moreover, PTs were unrelated to the presence of overt interictal/ictal CA, as well as the presence of CA was in any way related to PPI in response to different levels of stimulus intensity and induced only marginal changes in BOLD-fMRI signals. Despite these negative results, some studies show that migraine patients experience fluctuations in pain processing depending on the distance from the attack, as PT decreases preictally approaching the next attack, while PT greatly decreased and PPI increased ictally. Interestingly, a similar correlation with the number of days that had elapsed since the last attack was previously found in migraine with other sensory modalities, such as electrophysiology (91–94), psychophysical tests (95), and neuroimaging (53, 83, 96). Overall, these data indicate that a common neurobiological dysfunction might be responsible for abnormal information processing of both noxious and innocuous sensory stimuli. This interpretation is supported by the previous observation that the abnormal interictal processing of nociceptive blink reflex and innocuous visual evoked potentials are strictly interrelated when tested in the same patient between attacks (97).
As for the interictal state, PT data compared from patients evolving from episodic to CM to both episodic migraine patients and HCs are conflicting and inconclusive. Instead, higher PPI ratings were documented solely in CM patients overusing acute medications before drug withdrawal compared to HCs. The latter results may be explained as abnormal modulation of pain processing at the spinal level due to a decrease in antinociceptive activity of the supraspinal structures in MOH patients. This hypothesis is supported by recent neuroimaging studies showing altered structural integrity and functional connectivity of descending pain modulatory areas, such as periaqueductal gray (82, 98–100), and thalamic nuclei (101) in MOH patients.
Quantitative sensory testing (chiefly using LEPs) and functional neuroimaging studies in response to noxious stimuli show evidence of plastic reorganization in brain areas anchored to the so-called salience network (102), in response to the recurrence of migraine attacks and chronification, unrelated to the subjective PPI as assessed over the cephalic and extra-cephalic areas.
Future studies in this subject area in coming years should focus on the longitudinal investigation of a large group of patients before and after pharmacological and non-pharmacological interventions (103). This could help elucidate whether lower PT and increased pain sensitivity are primary risk factors or secondary consequences of migraine recurrence. To reduce discrepancies between studies, more attention should be given to the influence of chronic medication use on brain physiology, and the accurate collection of clinical data before, during and after the day of testing, to prospectively monitor the patients' clinical fluctuations, and be aware of changes resulting from increased consumption of acute medications and concomitant increase in attacks frequency.
Author Contributions
AR and GC: review the literature, manuscript drafting, and revision; GT, AT, and FP: manuscript revision; MS and VP: clinical and neurophysiological studies revision to perform Tables 1, 2. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The Italian Ministry of Health and Fondazione Roma financially supported GC and VP.
References
1. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet (2016) 388:1545–602. doi: 10.1016/S0140-6736(16)31678-6
2. Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol. (2000) 47:614–24. doi: 10.1002/1531-8249(200005)47:5<614::AID-ANA9>3.0.CO;2-N
3. Aguggia M, Saracco MG, Cavallini M, Bussone G, Cortelli P. Sensitization and pain. Neurol Sci. (2013) 34(Suppl. 1):S37–40. doi: 10.1007/s10072-013-1382-0
4. IASP. Task Force on Taxonomy IASP Taxonomy [online]. Available online at: http://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698 (Accessed Feb 8, 2013).
5. Sprenger T, Borsook D. Migraine changes the brain: neuroimaging makes its mark. Curr Opin Neurol. (2012) 25:252–62. doi: 10.1097/WCO.0b013e3283532ca3
6. Coppola G, Di Lorenzo C, Schoenen J, Pierelli F. Habituation and sensitization in primary headaches. J Headache Pain (2013) 14:65. doi: 10.1186/1129-2377-14-65
7. Lai TH, Protsenko E, Cheng YC, Loggia ML, Coppola G, Chen WT. Neural plasticity in common forms of chronic headaches. Neural Plasticity (2015) 2015:205985. doi: 10.1155/2015/205985
8. Kröger IL, Menz MM, May A. Dissociating the neural mechanisms of pain consistency and pain intensity in the trigemino-nociceptive system. Cephalalgia (2016) 36:790–9. doi: 10.1177/0333102415612765
9. Gracely RH. Wall and Melzack's Textbook of pain. In: Wall & Melzack's Textbook of Pain. McMahon SB, Koltzenburg M, Tracey I, Turk DC editors. Philadelphia, PA: Elsevier Saunders (2013), pp. 283–300.
10. Sand T, Zhitniy N, Nilsen KB, Helde G, Hagen K, Stovner LJ. Thermal pain thresholds are decreased in the migraine preattack phase. Eur J Neurol. (2008) 15:1199–205. doi: 10.1111/j.1468-1331.2008.02276.x
11. Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain (2000) 123(Pt 8):1703–9. doi: 10.1093/brain/123.8.1703
12. Uglem M, Omland PM, Nilsen KB, Tronvik E, Stovner LJ, Hagen K, et al. Does pain sensitivity change by migraine phase? A blinded longitudinal study. Cephalalgia (2017) 37:1337–49. doi: 10.1177/0333102416679955
13. De Tommaso M, Guido M, Libro G, Losito L, Sciruicchio V, Monetti C, et al. Abnormal brain processing of cutaneous pain in migraine patients during the attack. Neurosci Lett. (2002) 333:29–32. doi: 10.1016/S0304-3940(02)00967-9
14. de Tommaso M, Guido M, Libro G, Losito L, Difruscolo O, Puca F, et al. Topographic and dipolar analysis of laser-evoked potentials during migraine attack. Headache (2004) 44:947–60. doi: 10.1111/j.1526-4610.2004.04188.x
15. De Tommaso M, Libro G, Guido M, Difruscolo O, Losito L, Sardaro M, et al. Nitroglycerin induces migraine headache and central sensitization phenomena in patients with migraine without aura: a study of laser evoked potentials. Neurosci Lett. (2004) 363:272–5. doi: 10.1016/j.neulet.2004.04.029
16. De Tommaso M, Losito L, Libro G, Guido M, Di Fruscolo O, Sardaro M, et al. Effects of symptomatic treatments on cutaneous hyperalgesia and laser evoked potentials during migraine attack. Cephalalgia (2005) 25:359–68. doi: 10.1111/j.1468-2982.2004.00866.x
17. Engstrøm M, Hagen K, Bjørk MH, Stovner LJ, Gravdahl GB, Stjern M, et al. Sleep quality, arousal and pain thresholds in migraineurs: a blinded controlled polysomnographic study. J Headache Pain (2013) 14:12. doi: 10.1186/1129-2377-14-12
18. Schwedt TJ, Zuniga L, Chong CD. Low heat pain thresholds in migraineurs between attacks. Cephalalgia (2015) 35:593–599. doi: 10.1177/0333102414550417
19. Schoenen J, Bottin D, Hardy F, Gerard P. Cephalic and extracephalic pressure pain thresholds in chronic tension-type headache. Pain (1991) 47:145–9. doi: 10.1016/0304-3959(91)90198-7
20. Sandrini G, Proietti Cecchini A, Milanov I, Tassorelli C, Buzzi MG, Nappi G. Electrophysiological evidence for trigeminal neuron sensitization in patients with migraine. Neurosci Lett (2002) 317:135–8. doi: 10.1016/S0304-3940(01)02447-8
21. Fernández-de-Las-Peñas C, Cuadrado ML, Arendt-Nielsen L, Pareja JA. Side-to-side differences in pressure pain thresholds and pericranial muscle tenderness in strictly unilateral migraine. Eur J Neurol. (2008) 15:162–8. doi: 10.1111/j.1468-1331.2007.02020.x
22. Fernández-de-las-Peñas C, Madeleine P, Cuadrado ML, Ge HY, Arendt-Nielsen L, Pareja JA. Pressure pain sensitivity mapping of the temporalis muscle revealed bilateral pressure hyperalgesia in patients with strictly unilateral migraine. Cephalalgia (2009) 29:670–6. doi: 10.1111/j.1468-2982.2008.01831.x
23. Fernández-de-las-Peñas C, Madeleine P, Caminero AB, Cuadrado ML, Arendt-Nielsen L, Pareja JA. Generalized neck-shoulder hyperalgesia in chronic tension-type headache and unilateral migraine assessed by pressure pain sensitivity topographical maps of the trapezius muscle. Cephalalgia (2010) 30:77–86. doi: 10.1111/j.1468-2982.2009.01901.x
24. Zappaterra M, Guerzoni S, Cainazzo MM, Ferrari A, Pini LA. Basal cutaneous pain threshold in headache patients. J Headache Pain (2011) 12:303–10. doi: 10.1007/s10194-011-0313-9
25. Grossi DB, Chaves TC, Gonçalves MC, Moreira VC, Canonica AC, Florencio LL, et al. Pressure pain threshold in the craniocervical muscles of women with episodic and chronic migraine: a controlled study. Arq Neuropsiquiatr (2011) 69:607–12. doi: 10.1590/S0004-282X2011000500007
26. Schwedt TJ, Krauss MJ, Frey K, Gereau RW. IV Episodic and chronic migraineurs are hypersensitive to thermal stimuli between migraine attacks. Cephalalgia (2011) 31:6–12. doi: 10.1177/0333102410365108
27. Florencio LL, Giantomassi MC, Carvalho GF, Gonçalves MC, Dach F, Fernández-de-Las-Peñas C, et al. Generalized pressure pain hypersensitivity in the cervical muscles in women with migraine. Pain Med. (2015) 16:1629–34. doi: 10.1111/pme.12767
28. Palacios-Ceña M, Lima Florencio L, Natália, Ferracini G, Barón J, Guerrero ÁL, Ordás-Bandera C, et al. Women with chronic and episodic migraine exhibit similar widespread pressure pain sensitivity. Pain Med. (2016) 17:2127–33. doi: 10.1093/pm/pnw056
29. Bovim G. Cervicogenic headache, migraine, and tension-type headache. Pressure-pain threshold measurements. Pain (1992) 51:169–73.
30. Göbel H, Weigle L, Kropp P, Soyka D. Pain sensitivity and pain reactivity of pericranial muscles in migraine and tension-type headache. Cephalalgia (1992) 12:142–51.
31. Bishop KL, Holm JE, Borowiak DM, Wilson BA. Perceptions of pain in women with headache: a laboratory investigation of the influence of pain-related anxiety and fear. Headache (2001) 41:494–9. doi: 10.1046/j.1526-4610.2001.01087.x
32. Weissman-Fogel I. Repeated noxious stimulation of the skin enhances cutaneous pain perception of migraine patients in-between attacks: clinical evidence for continuous sub-threshold increase in membrane excitability of central trigeminovascular neurons. Pain (2003) 104:693–700. doi: 10.1016/S0304-3959(03)00159-3
33. Katsarava Z, Giffin N, Diener HC, Kaube H. Abnormal habituation of “nociceptive” blink reflex in migraine–evidence for increased excitability of trigeminal nociception. Cephalalgia (2003) 23:814–9. doi: 10.1046/j.1468-2982.2003.00591.x
34. Ayzenberg I, Obermann M, Nyhuis P, Gastpar M, Limmroth V, Diener HC, et al. Central sensitization of the trigeminal and somatic nociceptive systems in medication overuse headache mainly involves cerebral supraspinal structures. Cephalalgia (2006) 26:1106–14. doi: 10.1111/j.1468-2982.2006.01183.x
35. Buchgreitz L, Lyngberg AC, Bendtsen L, Jensen R. Frequency of headache is related to sensitization: a population study. Pain (2006) 123:19–27. doi: 10.1016/j.pain.2006.01.040
36. Coppola G, Di Clemente L, Fumal A, Magis D, De Pasqua V, Pierelli F, et al. Inhibition of the nociceptive R2 blink reflex after supraorbital or index finger stimulation is normal in migraine without aura between attacks. Cephalalgia (2007) 2:803–8. doi: 10.1111/j.1468-2982.2007.01323.x
37. Gierse-Plogmeier B, Colak-Ekici R, Wolowski A, Gralow I, Marziniak M, Evers S. Differences in trigeminal and peripheral electrical pain perception in women with and without migraine. J Headache Pain (2009) 10:249–54. doi: 10.1007/s10194-009-0118-2
38. Perrotta A, Serrao M, Sandrini G, Burstein R, Sances G, Rossi P, et al. Sensitisation of spinal cord pain processing in medication overuse headache involves supraspinal pain control. Cephalalgia (2010) 30:272–84. doi: 10.1111/j.1468-2982.2009.01914.x
39. Teepker M, Peters M, Kundermann B, Vedder H, Schepelmann K, Lautenbacher S. The effects of oral contraceptives on detection and pain thresholds as well as headache intensity during menstrual cycle in migraine. Headache (2011) 51:92–104. doi: 10.1111/j.1526-4610.2010.01775.x
40. Zohsel K, Hohmeister J, Oelkers-Ax R, Flor H, Hermann C. Quantitative sensory testing in children with migraine: preliminary evidence for enhanced sensitivity to painful stimuli especially in girls. Pain (2006) 123:10–8. doi: 10.1016/j.pain.2005.12.015
41. De Tommaso M, Sciruicchio V, Ricci K, Montemurno A, Gentile F, Vecchio E, et al. Laser-evoked potential habituation and central sensitization symptoms in childhood migraine. Cephalalgia (2016) 36:463–73. doi: 10.1177/0333102415597527
42. Ferracini GN, Stuginsk-Barbosa J, Dach F, Speciali JG. A comparison pressure pain threshold in pericranial and extracephalic regions in children with migraine. Pain Med. (2014) 15:702–9. doi: 10.1111/pme.12353
43. Anttila P, Metsähonkala L, Mikkelsson M, Aromaa M, Kautiainen H, Salminen J, et al. Muscle tenderness in pericranial and neck-shoulder region in children with headache. A controlled study. Cephalalgia (2002) 22:340–4. doi: 10.1046/j.1468-2982.2002.00352.x
44. Metsahonkala L, Anttila P, Laimi K, Aromaa M, Helenius H, Mikkelsson M, et al. Extracephalic tenderness and pressure pain threshold in children with headache. Eur J Pain (2006) 10:581–5. doi: 10.1016/j.ejpain.2005.08.005
45. Uthaikhup S, Sterling M, Jull G. Widespread sensory hypersensitivity is not a feature of chronic headache in elders. Clin J Pain (2009) 25:699–704. doi: 10.1097/AJP.0b013e3181a38f88
46. Cooke L, Eliasziw M, Becker WJ. Cutaneous allodynia in transformed migraine patients. Headache (2007) 47:531–9. doi: 10.1111/j.1526-4610.2006.00717.x
47. Kitaj MB, Klink M. Pain thresholds in daily transformed migraine versus episodic migraine headache patients. Headache (2005) 45:992–8. doi: 10.1111/j.1526-4610.2005.05179.x
48. De Tommaso M, Valeriani M, Guido M, Libro G, Specchio LM, Tonali P, et al. Abnormal brain processing of cutaneous pain in patients with chronic migraine. Pain (2003) 101:25–32. doi: 10.1016/S0304-3959(02)00299-3
49. De Tommaso M, Losito L, Difruscolo O, Libro G, Guido M, Livrea P. Changes in cortical processing of pain in chronic migraine. Headache J Head Face Pain (2005) 45:1208–18. doi: 10.1111/j.1526-4610.2005.00244.x
50. De Tommaso M, Ricci K, Laneve L, Savino N, Antonaci V, Livrea P. Virtual visual effect of hospital waiting room on pain modulation in healthy subjects and patients with chronic migraine. Pain Res Treat (2013) 2013:515730. doi: 10.1155/2013/515730
51. Ferraro D, Vollono C, Miliucci R, Virdis D, De Armas L, Pazzaglia C, et al. Habituation to pain in “medication overuse headache”: a CO2 laser-evoked potential study. Headache (2012) 52:792–807. doi: 10.1111/j.1526-4610.2012.02151.x
52. Moulton EA, Becerra L, Maleki N, Pendse G, Tully S, Hargreaves R, et al. Painful heat reveals hyperexcitability of the temporal pole in interictal and ictal migraine States. Cereb Cortex (2011) 21:435–48. doi: 10.1093/cercor/bhq109
53. Stankewitz A, Aderjan D, Eippert F, May A. Trigeminal nociceptive transmission in migraineurs predicts migraine attacks. J Neurosci. (2011) 31:1937–43. doi: 10.1523/JNEUROSCI.4496-10.2011
54. Stankewitz A, Schulz E, May A. Neuronal correlates of impaired habituation in response to repeated trigemino-nociceptive but not to olfactory input in migraineurs: an fMRI study. Cephalalgia (2013) 33:256–65. doi: 10.1177/0333102412470215
55. Schulte LH, May A. The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain (2016) 139:1987–93. doi: 10.1093/brain/aww097
56. De Tommaso M, Losito L, Difruscolo O, Sardaro M, Libro G, Guido M, et al. Capsaicin failed in suppressing cortical processing of CO2 laser pain in migraine patients. Neurosci Lett. (2005) 384:150–5. doi: 10.1016/j.neulet.2005.04.086
57. De Tommaso M, Marinazzo D, Stramaglia S. The measure of randomness by leave-one-out prediction error in the analysis of EEG after laser painful stimulation in healthy subjects and migraine patients. Clin Neurophysiol. (2005) 116:2775–82. doi: 10.1016/j.clinph.2005.08.019
58. De Tommaso M, Libro G, Guido M, Losito L, Lamberti P, Livrea P. Habituation of single CO2 laser-evoked responses during interictal phase of migraine. J Headache Pain (2005) 6:195–8. doi: 10.1007/s10194-005-0183-0
59. De Tommaso M, Difruscolo O, Sardaro M, Libro G, Pecoraro C, Serpino C, et al. Effects of remote cutaneous pain on trigeminal laser-evoked potentials in migraine patients. J Headache Pain (2007) 8:167–74. doi: 10.1007/s10194-007-0385-8
60. De Tommaso M, Baumgartner U, Sardaro M, Difruscolo O, Serpino C, Treede RD. Effects of distraction versus spatial discrimination on laser-evoked potentials in migraine. Headache (2008) 48:408–16. doi: 10.1111/j.1526-4610.2007.00857.x
61. Moulton EA, Burstein R, Tully S, Hargreaves R, Becerra L, Borsook D. Interictal dysfunction of a brainstem descending modulatory center in migraine patients. PLoS ONE (2008) 3:e3799. doi: 10.1371/journal.pone.0003799
62. De Tommaso M, Valeriani M, Sardaro M, Serpino C, Fruscolo OD, Vecchio E, et al. Pain perception and laser evoked potentials during menstrual cycle in migraine. J Headache Pain (2009) 10:423–9. doi: 10.1007/s10194-009-0150-2
63. De Tommaso M, Calabrese R, Vecchio E, De Vito Francesco V, Lancioni G, Livrea P. Effects of affective pictures on pain sensitivity and cortical responses induced by laser stimuli in healthy subjects and migraine patients. Int J Psychophysiol. (2009) 74:139–48. doi: 10.1016/j.ijpsycho.2009.08.004
64. De Tommaso M, Brighina F, Fierro B, Francesco VD, Santostasi R, Sciruicchio V, et al. Effects of high-frequency repetitive transcranial magnetic stimulation of primary motor cortex on laser-evoked potentials in migraine. J Headache Pain (2010) 11:505–12. doi: 10.1007/s10194-010-0247-7
65. Di Clemente L, Puledda F, Biasiotta A, Viganò A, Vicenzini E, Truini A, et al. Topiramate modulates habituation in migraine: evidences from nociceptive responses elicited by laser evoked potentials. J Headache Pain (2013) 14:25. doi: 10.1186/1129-2377-14-25
66. Russo A, Tessitore A, Esposito F, Marcuccio L, Giordano A, Conforti R, et al. Pain processing in patients with migraine: an event-related fMRI study during trigeminal nociceptive stimulation. J Neurol. (2012) 259:1903–12. doi: 10.1007/s00415-012-6438-1
67. Schwedt TJ, Chong CD, Chiang CC, Baxter L, Schlaggar BL, Dodick DW. Enhanced pain-induced activity of pain-processing regions in a case-control study of episodic migraine. Cephalalgia (2014) 34:947–58. doi: 10.1177/0333102414526069
68. Beese LC, Putzer D, Osada N, Evers S, Marziniak M. Contact heat evoked potentials and habituation measured interictally in migraineurs. J Headache Pain (2015) 16:1. doi: 10.1186/1129-2377-16-1
69. De Tommaso M, Trotta G, Vecchio E, Ricci K, Van de Steen F, Montemurno A, et al. Functional connectivity of EEG signals under laser stimulation in migraine. Front Hum Neurosci. (2015) 9:640. doi: 10.3389/fnhum.2015.00640
70. Mathur VA, Moayedi M, Keaser ML, Khan SA, Hubbard CS, Goyal M, et al. High frequency migraine is associated with lower acute pain sensitivity and abnormal insula activity related to migraine pain intensity, attack frequency, and pain catastrophizing. Front Hum Neurosci. (2016) 10:489. doi: 10.3389/fnhum.2016.00489
71. Vecchio E, Ricci K, Montemurno A, Delussi M, Invitto S, de Tommaso M. Effects of left primary motor and dorsolateral prefrontal cortex transcranial direct current stimulation on laser-evoked potentials in migraine patients and normal subjects. Neurosci Lett. (2016) 626:149–57. doi: 10.1016/j.neulet.2016.05.034
72. Russo A, Esposito F, Conte F, Fratello M, Caiazzo G, Marcuccio L, et al. Functional interictal changes of pain processing in migraine with ictal cutaneous allodynia. Cephalalgia (2017) 37:305–14. doi: 10.1177/0333102416644969
73. Russo A, Tessitore A, Bruno A, Siciliano M, Marcuccio L, Silvestro M, et al. Migraine does not affect pain intensity perception: a cross-sectional study. Pain Med. doi: 10.1093/pm/pnx174. [Epub ahead of print].
74. Zagami AS, Bahra A. Symptomatology of Migraines without Aura. In: Olesen J, Goadsby PJ, Ramadan NM, editors. The Headaches. Philadelphia, PA: Lippincott Williams and Wilkins (2006). pp. 399–405.
75. Buchgreitz L, Lyngberg AC, Bendtsen L, Jensen R. Increased pain sensitivity is not a risk factor but a consequence of frequent headache: a population-based follow-up study. Pain (2008) 137:623–30. doi: 10.1016/j.pain.2007.10.023
76. Engstrøm M, Hagen K, Bjørk M, Gravdahl GB, Sand T. Sleep-related and non-sleep-related migraine: interictal sleep quality, arousals and pain thresholds. J Headache Pain (2013) 14:68. doi: 10.1186/1129-2377-14-68
77. Mathur VA, Khan SA, Keaser ML, Hubbard CS, Goyal M, Seminowicz DA. Altered cognition-related brain activity and interactions with acute pain in migraine. NeuroImage Clin (2015) 7:347–58. doi: 10.1016/j.nicl.2015.01.003
78. LoPinto C, Young WB, Ashkenazi A. Comparison of dynamic (brush) and static (pressure) mechanical allodynia in migraine. Cephalalgia (2006) 26:852–6. doi: 10.1111/j.1468-2982.2006.01121.x
79. Silberstein SD, Lipton RB, Sliwinski M. Classification of daily and near-daily headaches: field trial of revised IHS criteria. Neurology (1996) 47:871–5. doi: 10.1212/WNL.47.4.871
80. Bigal ME, Tepper SJ, Sheftell FD, Rapoport AM, Lipton RB. Chronic daily headache: correlation between the 2004 and the 1988 International Headache Society Diagnostic Criteria. Headache J Head Face Pain (2004) 44:684–91. doi: 10.1111/j.1526-4610.2004.04128.x
81. De Tommaso M, Ambrosini A, Brighina F, Coppola G, Perrotta A, Pierelli F, et al. Altered processing of sensory stimuli in patients with migraine. Nat Rev Neurol. (2014) 10:144–55. doi: 10.1038/nrneurol.2014.14
82. Riederer F, Gantenbein AR, Marti M, Luechinger R, Kollias S, Sándor PS. Decrease of gray matter volume in the midbrain is associated with treatment response in medication-overuse headache: possible influence of orbitofrontal cortex. J Neurosci. (2013) 33:15343–9. doi: 10.1523/JNEUROSCI.3804-12.2013
83. Coppola G, Tinelli E, Lepre C, Iacovelli E, Di Lorenzo C, Di Lorenzo G, et al. Dynamic changes in thalamic microstructure of migraine without aura patients: a diffusion tensor magnetic resonance imaging study. Eur J Neurol. (2014) 21:287-e13. doi: 10.1111/ene.12296
84. Coppola G, Di Renzo A, Tinelli E, Iacovelli E, Lepre C, Di, Lorenzo C, et al. Evidence for brain morphometric changes during the migraine cycle: a magnetic resonance-based morphometry study. Cephalalgia (2015) 35:783–91. doi: 10.1177/0333102414559732
85. Lambert GA, Truong L, Zagami AS. Effect of cortical spreading depression on basal and evoked traffic in the trigeminovascular sensory system. Cephalalgia (2011) 31:1439–1451. doi: 10.1177/0333102411422383
86. Lev R, Granovsky Y, Yarnitsky D. Orbitofrontal disinhibition of pain in migraine with aura: an interictal EEG-mapping study. Cephalalgia (2010) 30:910–8. doi: 10.1177/0333102409357249
87. Welch KM, Nagesh V, Aurora SK, Gelman N. Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache (2001) 41:629–37. doi: 10.1046/j.1526-4610.2001.041007629.x
88. Magon S, May A, Stankewitz A, Goadsby PJ, Tso AR, Ashina M, et al. Morphological abnormalities of thalamic subnuclei in migraine: a multicenter MRI Study at 3 Tesla. J Neurosci. (2015) 35:13800–6. doi: 10.1523/JNEUROSCI.2154-15.2015
89. Porcaro C, Di Lorenzo G, Seri S, Pierelli F, Tecchio F, Coppola G. Impaired brainstem and thalamic high-frequency oscillatory EEG activity in migraine between attacks. Cephalalgia (2016) 37:915–26. doi: 10.1177/0333102416657146
90. Burstein R, Jakubowski M, Garcia-Nicas E, Kainz V, Bajwa Z, Hargreaves R, et al. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol. (2010) 68:81–91. doi: 10.1002/ana.21994
91. Coppola G, Parisi V, Di Lorenzo C, Serrao M, Magis D, Schoenen J, et al. Lateral inhibition in visual cortex of migraine patients between attacks. J Headache Pain (2013) 14:20. doi: 10.1186/1129-2377-14-20
92. Coppola G, Bracaglia M, Di Lenola D, Di Lorenzo C, Serrao M, Parisi V, et al. Visual evoked potentials in subgroups of migraine with aura patients. J Headache Pain (2015) 16:92. doi: 10.1186/s10194-015-0577-6
93. Coppola G, Bracaglia M, Di Lenola D, Iacovelli E, Di Lorenzo C, Serrao M, et al. Lateral inhibition in the somatosensory cortex during and between migraine without aura attacks: correlations with thalamocortical activity and clinical features. Cephalalgia (2016) 36:568–78. doi: 10.1177/0333102415610873
94. Cortese F, Coppola G, Di Lenola D, Serrao M, Di Lorenzo C, Parisi V, et al. Excitability of the motor cortex in patients with migraine changes with the time elapsed from the last attack. J Headache Pain (2017) 18:2. doi: 10.1186/s10194-016-0712-z
95. Shepherd AJ, Wyatt G, Tibber MS. Visual metacontrast masking in migraine. Cephalalgia (2011) 31:346–56. doi: 10.1177/0333102410380755
96. Deen M, Hansen HD, Hougaard A, da Cunha-Bang S, Nørgaard M, Svarer C, et al. Low 5-HT1B receptor binding in the migraine brain: a PET study. Cephalalgia (2018) 38:519–27. doi: 10.1177/0333102417698708
97. Di Clemente L, Coppola G, Magis D, Fumal A, De Pasqua V, Schoenen J. Nociceptive blink reflex and visual evoked potential habituations are correlated in migraine. Headache (2005) 45:1388–93. doi: 10.1111/j.1526-4610.2005.00271.x
98. Chen Z, Chen X, Liu M, Liu S, Ma L, Yu S. Disrupted functional connectivity of periaqueductal gray subregions in episodic migraine. J Headache Pain (2017) 18:36. doi: 10.1186/s10194-017-0747-9
99. Chen Z, Chen X, Liu M, Liu S, Ma L, Yu S. Texture features of periaqueductal gray in the patients with medication-overuse headache. J Headache Pain (2017) 18:14. doi: 10.1186/s10194-017-0727-0
100. Michels L, Christidi F, Steiger VR, Sándor PS, Gantenbein AR, Landmann G, et al. Pain modulation is affected differently in medication-overuse headache and chronic myofascial pain–A multimodal MRI study. Cephalalgia (2017) 37:764–79. doi: 10.1177/0333102416652625
101. Chen Z, Jia Z, Chen X, Liu M, Liu S, Ma L, et al. Volumetric abnormalities of thalamic subnuclei in medication-overuse headache. J Headache Pain (2017) 18:82. doi: 10.1186/s10194-017-0791-5
102. Uddin LQ. Anatomy of the salience network. In: Salience Network of the Human Brain. Academic Press. (2017) p. 46.
Keywords: migraine, headache, pain processing, pain measurement, pain threshold, pain intensity perception, temporal summation
Citation: Russo A, Coppola G, Pierelli F, Parisi V, Silvestro M, Tessitore A and Tedeschi G (2018) Pain Perception and Migraine. Front. Neurol. 9:576. doi: 10.3389/fneur.2018.00576
Received: 20 December 2017; Accepted: 26 June 2018;
Published: 02 August 2018.
Edited by:
Raquel Gil-Gouveia, Hospital da Luz, PortugalReviewed by:
Ruth Ruscheweyh, Klinikum der Universität München, GermanyJean Schoenen, University of Liège, Belgium
Copyright © 2018 Russo, Coppola, Pierelli, Parisi, Silvestro, Tessitore and Tedeschi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio Russo, ZG90dG9yLnJ1c3NvQGdtYWlsLmNvbQ==
† These authors have contributed equally to this work.
 Antonio Russo
Antonio Russo Gianluca Coppola
Gianluca Coppola Francesco Pierelli4,5
Francesco Pierelli4,5 Vincenzo Parisi
Vincenzo Parisi Alessandro Tessitore
Alessandro Tessitore