- Computational Neuroimaging Group, Academic Unit of Neurology, Biomedical Sciences Institute, Trinity College Dublin, Dublin, Ireland
Post-polio syndrome (PPS) is a neurological condition that affects polio survivors decades after their initial infection. Despite its high prevalence, the etiology of PPS remains elusive, mechanisms of progression are poorly understood, and the condition is notoriously under-researched. While motor dysfunction is a hallmark feature of the condition, generalized fatigue, sleep disturbance, decreased endurance, neuropsychological deficits, sensory symptoms, and chronic pain are also often reported and have considerable quality of life implications in PPS. The non-motor aspects of PPS are particularly challenging to evaluate, quantify, and treat. Generalized fatigue is one of the most distressing symptoms of PPS and is likely to be multifactorial due to weight-gain, respiratory compromise, poor sleep, and polypharmacy. No validated diagnostic, monitoring, or prognostic markers have been developed in PPS to date and the mainstay of therapy centers on symptomatic relief and individualized rehabilitation strategies such as energy conservation and muscle strengthening exercise regimes. Despite a number of large clinical trials in PPS, no effective disease-modifying pharmacological treatments are currently available.
Introduction
Poliomyelitis was one of the most acutely debilitating infections of the twentieth century that affected millions in the 1940 and 1950s and more recently in India during an outbreak in 1988 (1). Following the introduction of the polio vaccine in the mid-1950s and early 1960s, there has been a dramatic decline in the number of new polio cases and it is estimated to be 99% eradicated today. Despite the enormous progress in the eradication of the polio virus, 15–20 million people across the world still suffer from the sequelae of the infection (2). A large proportion of polio survivors has been presenting with a constellation of new neurological symptoms that has been described as Post-Polio Syndrome (PPS). The description of PPS is attributed to Jean-Martin Charcot in 1875 but was only widely recognized by the medical community in the early 1980s (3). PPS is characterized by new neurological deficits after a long period of neurological stability, typically at least 15 years after the initial polio infection. PPS may manifest as new, persistent, and progressive muscle weakness, atrophy, limb fatigability, myalgia, arthralgia, and dysphagia, but also as generalized fatigue, which typically has a considerable impact on the patients' quality of life. The estimates of the percentage of polio patients affected by PPS are inconsistent, varying between 20 and 85% (4, 5) depending on the diagnostic criteria applied (2). As a result, despite the rarity of acute polio infection in the modern world, PPS is likely to persist for the next few decades. Despite its prevalence, post-polio syndrome remains surprisingly under-researched and poorly characterized. The purpose of this review is to provide a comprehensive overview of the aetiological, genetic, diagnostic, prognostic factors, and treatment modalities in PPS while highlighting key gaps that require further research.
Methods
A literature search was performed on PubMed using the search term “post-polio syndrome,” “postpolio syndrome” or “post-polio syndrome” alone and in combination with “epidemiology,” “pathophysiology,” “clinical features,” “fatigue,” “neurophysiology,” “brain imaging,” “electromyography,” “inflammation,” “diagnosis,” “management,” “clinical trial,” “longitudinal,” “cross-sectional,” “case report,” “autopsy,” and “post mortem.” Only articles written in English and published between January 1980 and May 2019 were selected for literature review. Identified publications were categorized into “academic” papers discussing pathophysiology, genetic susceptibility, biology, and “clinical” papers focusing on diagnostic criteria, management, rehabilitation, and clinical trials.
Results
Pathophysiology
During the acute poliomyelitis infection, 95% of those infected remain asymptomatic or only suffer flu-like symptoms while the remaining 5% succumb to the paralytic form of the disease. Acute poliomyelitis is typically spinal, affecting the limbs and respiratory musculature, but bulbar manifestations affecting speech and swallow are also well-documented. Polioenterovirus type 1 is the main cause of meningeal, spinal cord and brain inflammation as it can cross the blood-brain barrier independently from poliovirus receptors (6, 7). Ensuing anterior horn degeneration, and apoptosis post infection has been widely recognized as the hallmark feature of paralytic poliomyelitis. Following the acute phase, axonal sprouting takes place reinnervating the muscle of the affected regions (8, 9). Motor units gradually become abnormally enlarged, up to 7-fold their original size (10) rendering them metabolically unsustainable (11). This process can take up to three decades from the acute infection to the development of PPS symptoms (12). The concomitant denervation-reinnervation process is evidenced by electromyography (EMG) findings (13–17) and muscle histology showing small angulated fibers (18, 19) and muscle fiber type-grouping (15). Metabolic stress (11, 20), overuse (21, 22), physiological aging (20, 23), and persistent inflammation (24) are also thought to contribute to gradual motor unit failure. Motor units loss has been consistently correlated to functional decline in longitudinal studies (13, 14, 25, 26). Overuse of functioning muscle units is thought to induce detrimental structural alterations (27, 28). Cellular adaptation in the muscles, such as fiber alteration from type II (fast) to type I (slow) (28), changes in contractile properties (29–31), and muscle hypertrophy (9) are likely to contribute to muscular fatigue and myalgia in PPS. The persistence or reactivation of polio virus in polio survivors has also been suggested with conflicting reports. Two research studies (7, 32) have identified polio-virus (PV) genomic sequences in the CSF and peripheral leucocytes as well as high serum IgM anti-PV antibody titres, which were absent in stable polio survivors and in other neurodegenerative groups (33). Other studies however could not confirm these findings (34). An inflammatory or autoimmune basis to post-polio syndrome has also been proposed. This hypothesis originates from post mortem observations of inflammatory changes in the spinal cord of PPS patients (35, 36). The role of inflammation is also supported by in vivo evidence. Increased serum and CSF levels of pro-inflammatory cytokines and peptides such as TNF-α, IFN-γ were repeatedly observed in PPS (37–39). Furthermore, TNF-α and IFN-γ levels respond to IVIg therapy in PPS, and remain unchanged in controls (37, 38, 40). However, no correlations have been detected between symptom severity (38), rate of decline (37), and pro-inflammatory peptide levels. Skeletal muscle biopsies also exhibit inflammatory changes and increased expression of prostaglandin E2 synthetic pathway enzymes (41). Relatively limited evidence exists to support the autoimmune basis of PPS. One study identified high titres of PV antibodies concurrently with high levels of regulatory T cells (42), while another study (43) found normal levels of immune complexes in PPS patients. No specific anti-muscle or anti-neuronal autoantibodies have been associated with PPS (44). A genetic predisposition for PPS has also been investigated, but no conclusive risk profile has been identified to date. SMN gene deletion (45, 46) associated with spinal muscular atrophy (SMA) was not reported in PPS, but Fc-gamma receptor IIIA polymorphisms may play a role in the predisposition to PPS (47).
Neuropathology and Neuroimaging
Post-mortem studies are conflicting with regards to cerebral involvement in post-polio syndrome. Post-mortem studies (48) from 50 to 70 years ago suggest that polio virus preferentially affects the reticular formation, posterior hypothalamus, thalamus, putamen, caudate, locus co-eruleus, and substantia nigra which may account for the late-onset fatigue and attention deficit (49–52). Interestingly, cortical involvement is relatively selective and preferentially involves the precentral gyrus and pre-motor areas. A more recent case report (53) and a retrospective analysis of formalin-fixed central nervous system (CNS) tissue of a small cohort of patients (33) arrived at a different conclusion. They identified no cerebral involvement at all, but selective spinal cord pathology affecting the anterior roots with dorsal root sparing. These studies detected enterovirus RNA in spinal cord only. There have also been rare reports of polio patients developing ALS with characteristic histopathological findings (54, 55). Compared to other motor neuron diseases (56), there is a striking paucity of brain (57) and spinal cord imaging studies in PPS (58). Magnetic resonance imaging (MRI) has been used to evaluate volumetric changes (59) and to correlate anatomical changes to post mortem findings (48). The main focus of existing brain imaging studies in PPS was to explore the substrate of fatigue. Multiple hyperintensities were identified in the reticular formation, putamen and medial lemniscus in the majority of PPS patients (48) which is consistent with previous post mortem studies (49–52). A large study of 118 participants compared the brain volume profile of 42 PPS patients, 49 multiple sclerosis patients and 27 controls, and no statistically significant volume reductions were identified in PPS (59). No association was identified between fatigue and brain volumes. The majority of existing studies are cross-sectional which provide limited insights into progressive longitudinal alterations (60). There is an ongoing longitudinal, case-control study to characterize spinal cord alterations in PPS (61).
Diagnosis
Post-polio syndrome is a clinical diagnosis, supported by electrophysiological findings and possible mimics need to be reassuringly ruled out. An extensive work-up including laboratory tests, imaging studies, cerebrospinal fluid sampling, detailed electrophysiological evaluation, and muscle biopsies may be required to exclude alternative diagnoses. The diagnostic criteria for PPS was first proposed by Halstead in 1991 (62) and evolved over time to the current March of Dimes diagnostic criteria (63, 64) which include:
1. Prior paralytic poliomyelitis with evidence of motor neuron loss, as confirmed by history of the acute paralytic illness, signs of residual weakness and muscle atrophy on examination, or signs of denervation on EMG.
2. A period of partial or complete functional recovery after acute paralytic poliomyelitis, followed by an interval (usually 15 years or more) of stable neuromuscular function.
3. Gradual onset (rarely abrupt) progressive and persistent new muscle weakness or abnormal muscle fatigability (decreased endurance), with or without generalized fatigue, muscle atrophy, or muscle and joint pain. Onset may at times follow trauma, surgery, or a period of inactivity. Less commonly, bulbar dysfunction or respiratory weakness occurs.
4. Symptoms that persist for at least a year.
5. Exclusion of alternative neuromuscular, medical, and orthopedic problems as causes of symptoms.
PCR amplification of poliovirus RNA in the CSF is indicative of prior history of poliomyelitis (6, 7, 32) and the presence of pro-inflammatory cytokines may also be detected (39, 65). Proteomic CSF markers such as gelsolin, hemopexin, peptidylglycine alpha-amidating monooxygenase, glutathione synthetase, and kallikrein 6 have been proposed as diagnostic markers but supporting evidence from larger studies is lacking (4). On muscle biopsy, hypertrophic muscle fibers type I (66, 67), indicative of compensatory reinnervation and small angulated fibers, indicative of active denervation (19) may be observed. CSF sampling and muscle biopsy also allows the exclusion of other neuromuscular mimics. People with PPS typically undergo detailed spinal imaging to rule out alternative structural, neoplastic, compressive, or inflammatory spinal etiologies which could manifest in lower motor neuron dysfunction (58, 68–70). Electromyography (EMG) is an invaluable tool to assess suspected post-polio cases, as it allows the confirmation of a prior history of poliomyelitis while excluding differential diagnoses (71). A variety of EMG techniques have been used in post-polio research studies including single fiber EMG (SFEMG), high density surface EMG (HDsEMG) (72), and macro-EMG. Ongoing denervation can be detected on conventional EMG by the presence of fibrillation and fasciculation potentials and increased jitter on SFEMG in newly weakened muscles (73). Needle EMG can also readily detect sub-clinically affected muscles in PPS (74). EMG measures correlate well with muscle strength and endurance (75, 76). While EMG provides important insights, EMG measures don't differ significantly between those with PPS and stable polio (77) and thus EMG is not regarded as an electrodiagnostic tool to confirm PPS (73). PPS is therefore a clinical diagnosis supported by laboratory tests.
The Spectrum of Clinical Manifestations
Post-polio patients characteristically experience new onset muscle weakness, decreased endurance, muscle atrophy, myalgia, and fasciculations (78). Additional symptoms often include generalized fatigue, cold intolerance, dysarthria, dysphagia, and respiratory compromise (79, 80). New symptoms typically occur in previously affected areas but sub-clinically affected body regions can also get affected (74). Ambulatory difficulties often necessitate assistive devices, and may lead to increased fall risk (81). PPS is also associated with a wide range of non-motor symptoms. Frank sensory deficits may be detected and paraesthesias are often reported by PPS patients. Changes in sensory evoked potentials have been linked to cord atrophy on MRI (82). There have been consistent reports of cognitive deficits (83) in PPS including word finding difficulties (84), poor concentration, limited attention, memory impairment (85), and mood disturbances (86). The non-motor aspects of PPS are often under evaluated despite their considerable quality of life implications (87). Due to the combination of motor disability (88) and non-motor symptoms, many patients engage less in social activities (89) which may lead to social isolation. Generalized fatigue is one of the most distressing sequelae of PPS which is likely to be multifactorial due to muscle unit pathology, weight-gain, respiratory compromise, polypharmacy, and poor sleep (Figure 1). The identification of the key “fatigue-factors” in individual patients is indispensable for the effective pharmacological and non-pharmacological management of fatigue. Fatigue is thought to exhibit circadian variations throughout the day (90). Sleep disorders such as restless leg syndrome (RLS) (87, 91–94), sleep related breathing disturbances (95), obstructive sleep apnoea (OSA) (96), excessive daytime somnolence (EDS), and periodic limb movement in sleep (PLMS) (97) are not only often reported in PPS but they are likely to play an important role in the pathogenesis of fatigue in PPS (98, 99). Fatigue is thought to be more severe in PPS with RLS, and correlate to the severity of RLS (87). The simultaneous onset of RLS and PPS symptoms (91) and the positive response to pramipexole in an uncontrolled trial by Kumru et al. (93) have been interpreted as a pathophysiological link between RLS and PPS (98). The putative link between RLS and neuroimmunological alterations (100, 101) may also suggest shared pathophysiological processes between PPS and RLS (99). Furthermore, a higher incidence of cauda equina syndrome (102) and renal impairment (103) has also been reported in PPS but the association between these syndromes remains to be elucidated.
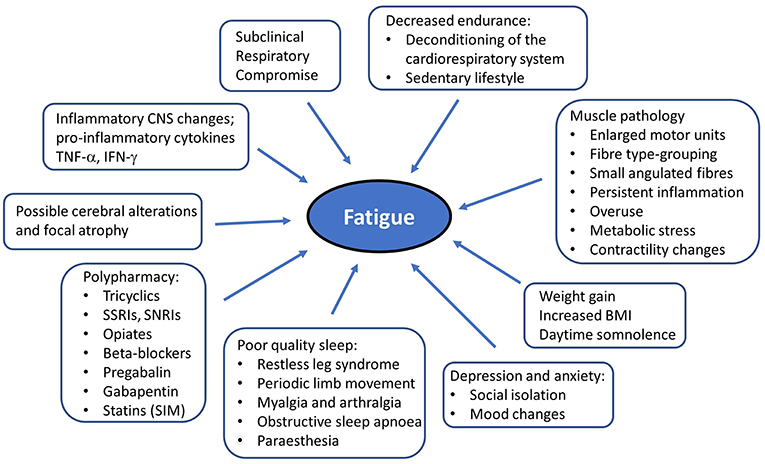
Figure 1. Putative factors in the etiology of generalized fatigue in post-polio syndrome. RLS, Restless leg syndrome; PLMS, periodic limb movement in sleep; CNS, Central nervous system.
Progression, Assessment, and Monitoring
The majority of longitudinal studies (14, 25, 104–107) detect progressive muscle weakness, which contributes to deteriorating gait performance (107) and declining mobility (105). Quantifying the rate of decline in PPS is challenging and no reliable functional predictors have been validated. Male gender is thought to be a negative prognostic indicator (108), but PPS is more common in females (12). Most PPS patients who participated in research studies have lived with PPS for over 13 years suggesting that PPS is a relatively slowly progressive condition. There have also been however sporadic reports of rapidly progressive and life-threatening forms of PPS (109), which raises the question of occasional misdiagnoses or a link between PPS and amyotrophic lateral sclerosis (ALS) (54). The severity of PPS-associate disability is typically evaluated clinically but a number of rating scales and questionnaires have been developed and validated for both clinical and research use. In addition to mobility and dexterity, these instruments evaluate the non-motor aspects of the condition such as fatigue, pain, sleeping disturbances, and mood (110). Clinical tests used to assess motor disability include the 6-min walking test (6MWT) (111) at self-preferred speed, the 2-min walking test (2MWT) at maximal speed (112), Timed-Up-and-Go test (TUG) (113), 10 meters walking test (10MWT), Sit-Stand-Sit test (SSS) (114). Muscle strength is typically appraised by manual muscle testing using the MRC scale, or more objectively using a dynamometer during maximal isokinetic and isometric voluntary contraction. Endurance is measured using isometric contraction peak torque, isometric endurance, tension time index (TTI) or recovery of torque after endurance test (76). Quantitative muscle mass assessment can be performed using ultrasound parameters such as muscle echo intensity and muscle thickness which are non-invasive tools for disease monitoring (115). The most commonly used instruments to assess non-motor domains include the Fatigue Severity Scale (FSS) (116), Fatigue Impact Scale (FIS), Piper Fatigue Scale (PFS), Short Fatigue Questionnaire (SFQ), Nottingham Health Profile (NHP), Physical activity scale for the elderly (PASE) (117), Polio Problem List (PPL), Visual analog scale (VAS) (118), Multidimensional Fatigue Inventory (MFI-20) (119), World Health Organization quality of life abbreviated scale (WHOQOL-BREF) (120), University of Washington Self-Efficacy Scale (UW-SES) (121), Sickness Impact Profile (SIP), 36-item Short Form Health Survey (SF-36) (112). Sleep disturbances (97) and respiratory function can be formally assessed through polysomnography and pulmonary function tests (PFT) (122, 123). RLS is typically diagnosed clinically (124) and most commonly evaluated using the validated international RLS rating scale (IRLS) (87, 93, 125). Maximal inspiratory and expiratory pressures (MIP and MEP), sniff nasal inspiratory pressure (SNIP) (126), and arterial blood gases are validate markers of respiratory function in PPS.
Non-pharmacological Interventions
The effective management of the heterogeneous symptoms of PPS requires individualized care in a multidisciplinary setting (127). Expert input from physiotherapists, occupational therapists, speech and language therapists, respiratory physicians, podiatrists, psychologists, dieticians, pain specialists, social workers, nurse specialists, and orthotists are needed to meet the multifaceted care and support needs of PPS patients (128). Individualized lifestyle modifications and energy conservation strategies are indispensable in the effective management of PPS (129). PPS-specific training regimens alternating active intervals and rest have been developed to improve cardiorespiratory fitness, conserve energy during routine activities, and maintain independence (130). Isokinetic, isometric, resistance, and endurance training are thought to improve muscle strength and endurance without further muscle unit degeneration (131–140). Combining aerobic and flexibility training is also thought to improve QoL. Supervised training is advised in those with significant disability (141). Training in a warm environment may have longer lasting effects than training in colder temperatures (142). Patients with arthralgia may benefit from dynamic water exercises (143) as well as exercising in a group setting (144). Deconditioning of the cardiorespiratory system (145) may limit the effectiveness of aerobic training in PPS (146), therefore aerobic regimens must be carefully tailored to individual fitness levels (147). While some studies show improved endurance following mid- to high-intensity aerobic exercises (139, 140), a recent study (148) highlights that high-intensity aerobic exercise may not be beneficial in PPS patients with fatigue. Due to the heterogeneity of disability profiles in PPS, individualized training regimes and exercises that don't rely on anti-gravity strength are particularly important (148–150). Home-based arm ergometry for example is a well-tolerated and safe form of aerobic exercise (149, 150). Whole body vibration (WBV) has been proposed as an alternative to exercise in PPS (151) and improved mobility was reported in a small study (152), but no improvement was noted in muscle strength or gait performance (153). Orthoses are commonly prescribed for PPS patients to improve mobility and reduce pain. New powered-type Knee Ankle Foot Orthosis (KAFOs) offer limited benefits on gait symmetry or walking speeds but were shown to improve base support, swing time, stance-phase, and knee flexion during swing phase (154). The emergence of novel, light-weight materials such as carbon fiber (155) and the biomechanical analysis of individual walking patterns have helped to optimize orthosis-design for patients. The use of MIG3 Bioceramics fabrics for example had beneficial effects on pain and periodic limb movement (156). Other lifestyle modification such as weight loss, smoking cessation, increased physical activity, and modification to daily activities have all been beneficial to patients with PPS (22). There are sporadic reports that anodal transcranial direct current stimulation (tDCS) of premotor regions (157), repetitive transcranial magnetic stimulation (rTMS) of the left prefrontal cortex (158) and static magnetic fields (159) may ameliorate fatigue, improve sleep, reduce pain, and even improve motor functions in PPS, but these studies have not been replicated. PPS patients with bulbar involvement require expert phonatory and swallowing assessments by a speech-and-language therapist (160) and careful follow-up. Instrumental modalities such as ultrasonography and videofluoroscopy (161) and clinical instruments (162) can be used to detect progressive bulbar dysfunction and appraise the risk aspiration. Compensatory swallowing techniques, dietician input for food consistency alterations, individualized speech therapy, and laryngeal muscle training may be helpful in PPS patients with bulbar involvement (163). PPS patients who suffer from respiratory compromise and sleep related breathing disorders benefit from lung volume recruitment (LVR) (164) and non-invasive ventilation (NIV) such as Bi-PAP (165) or nasal intermittent positive-pressure ventilators (NIPPV) (166). Invasive ventilatory support with a tracheostomy is seldom required in PPS (167).
Addressing the non-physical aspects of PPS; mitigating psychological responses, emotional reactions, frustration, and fear of falling are equally important aspects of multidisciplinary care (168). Despite its positive effects on self-esteem (169), cognitive behavioral therapy (CBT) is not superior to standard multidisciplinary care in the treatment of fatigue (170–172). Psychotherapy is primarily aimed at reducing anxiety, improving depressive symptoms (173), alleviating pain (174, 175), and enhancing subjective well-being (176). Hope-oriented psychotherapy and encouraging participation in work (177) promote resilience in polio survivors and is associated with improved social functioning (178), satisfaction with social roles, improved quality of life, and superior mental health (179). Peer-support groups are also instrumental in buffering the impact of a functional impairment on psychosocial well-being (180). Furthermore, a reduction of physical demands at work and ergonomic adaptations at the workplace not only help PPS patients to maintain their occupational activities but enjoy their work (181). Rehabilitation nurses also play an important role in the setting of realistic health goals, encouraging resiliency, and providing emotional support (182).
Pharmacological Trials
Several randomized controlled clinical trials (RCT) were conducted in PPS (Table 1). High-dose prednisone (183), amantadine (184), and modafinil (187, 188) showed no superiority to placebo in the management of fatigue. Prednisone therapy, showed a short-lived improvement in muscular strength but no meaningful functional improvement (183). The evidence for the benefit of pyridostigmine therapy remains conflicting. Some studies (185) identified no benefit on muscle function while others reported a slight improvement in walking performance (186). Co-enzyme Q10 supplements are thought to have no effect on muscle strength, endurance or fatigue in PPS (189, 190). A small RCT of lamotrigine, demonstrated improvements in VAS, NHP, and FSS suggesting that it may be beneficial to treat pain and fatigue and improve quality of life (191). Given the inflammatory and autoimmune hypothesis of PPS pathogenesis, intravenous immunoglobulin has been extensively investigated for its potential therapeutic effects. Its benefit with regards to pain, muscle strength, physical functioning, and quality of life is inconsistent. Improved pain control and overall vitality (192, 196) seem to be the main benefit of intravenous immunoglobulin (IVIg) treatment. Two small uncontrolled trials (38, 194) and two larger RCTs (40, 65) arrived to similar conclusions with regards to pain control and improvement in serum and CSF inflammatory markers. The main indicators for response to IVIg include severe pain, fatigue, <65 years of age, and paresis mainly affecting the lower extremities (194, 195, 198). Studies are somewhat conflicting on its effect on muscle strength (65, 193). These findings however encourage further large RCTs to establish the target PPS cohort for IVIg treatment, treatment intervals, and dose optimisation. A single-center, double-blind RCT trial of L-citrulline (197) is currently underway to investigate its effect on muscle metabolism and function. It is at clinical phase IIa and has proven to be of beneficial in muscular dystrophies in improving endurance in both aerobic and anaerobic exercise. The symptomatic management of non-motor symptoms in PPS also has considerable quality of life benefits. Restless leg syndrome in PPS often responds to dopamine agonists such as pramipexole (93, 199). The use of analgesics and antidepressants such as amitryptiline, duloxetine, and codeine may decrease physical discomfort and improve mood but need careful monitoring as they may worsen fatigue and lead to poor concentration. Adverse reactions to certain anesthetic agents are well-documented in PPS. Post-anesthesia fatigue, somnolence, and weakness are well-recognized, and fatal outcomes due to respiratory arrest have also been reported (200, 201). The diagnosis of PPS needs to be carefully discussed with the anaesthesiologists, so the appropriate muscle relaxants and anesthetics can be used, and patients should be advised of the possibility of a prolonged post-operative phase (202).
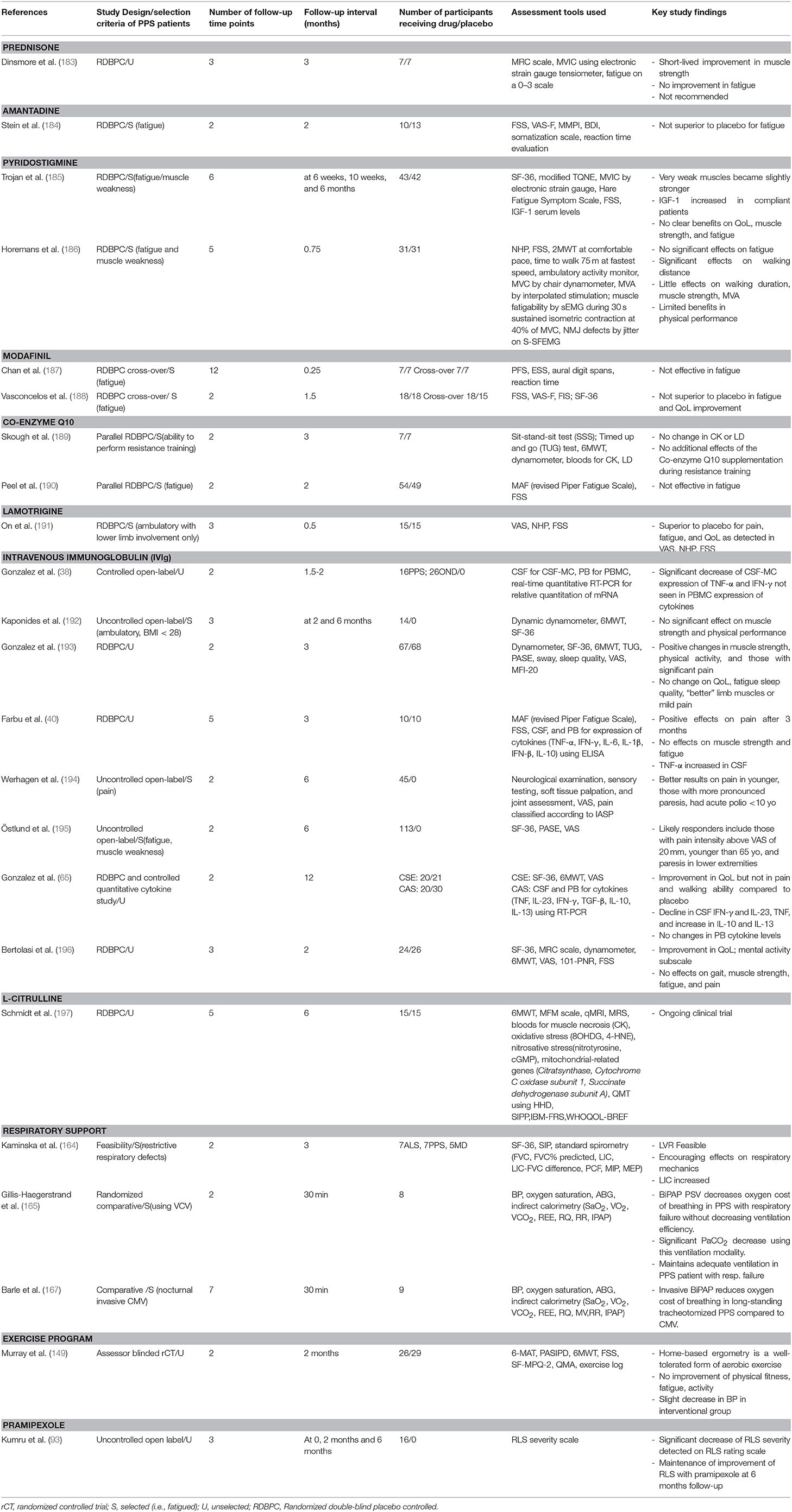
Table 1. Pharmaceutical and non-pharmaceutical clinical trials in post-polio syndrome; study characteristics and key outcomes.
Conclusions
Despite being one of the most devastating neurodegenerative conditions in the world, surprisingly limited research is undertaken in post-polio syndrome. Its pathogenesis remains elusive, no sensitive diagnostic tools have been developed, and validated prognostic and monitoring markers are lacking. Non-motor symptoms of PPS have considerable quality of life implications and are notoriously challenging to manage. The etiology of fatigue in PPS is yet to be elucidated and successful individualized management strategies are needed to maintain mobility, independence, and patient autonomy. There is striking a paucity of neuroimaging studies in PPS that could provide anatomical insights into the substrate of extra-motor symptoms. Ultimately, the characterization of PPS-associated pathology may help research efforts in other motor neuron diseases.
Author Contributions
The manuscript was drafted by SL and PB. The manuscript was edited, adjusted, and reviewed for intellectual content by RC, EF, DM, and OH.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Peter Bede and the Computational Neuroimaging Group are supported by the Health Research Board (HRB–Ireland; HRB EIA-2017-019), the Andrew Lydon Scholarship, the Irish Institute of Clinical Neuroscience (IICN)–Novartis Ireland Research Grant, the Iris O'Brien Foundation, and the Research Motor Neuron (RMN–Ireland) Foundation.
Abbreviations
101-PNR, 101- point numeric rating; 10MWT, 10-meter walk test; 2MWT, 2-minute walk test; 6MWT, 6-minute walk test; ALS, Amyotrophic lateral sclerosis; BDI, Beck depression inventory; BiPAP, Bilevel positive airway pressure; CAS, cytokine analysis study; CBT, Cognitive behavioral therapy; CK, Creatine kinase; CMAP, Compound muscle action potential; CMV, Controlled mechanical ventilation; CSE, Clinical study extension; CSF, Cerebrospinal fluid; CSF-MC, cerebrospinal fluid mononuclear cells; ELISA, Enzyme-linked immunosorbent assay; EMG, Electromyography; ESS, Epworth sleepiness scale; FIS, Fatigue impact scale; FSS, Fatigue severity scale; FVC, forced vital capacity; HDsEMG, High density surface electromyography; HHD, hand-held dynamometry; IASP, International Association for the Study of Pain; IBM-FRS, Inclusion body myositis functional rating scale; IPAP, inspiratory positive airway pressure; KAFO, Knee ankle foot orthosis; LIC, lung insufflation capacity; LVR, Lung volume recruitment; MAF, Multidimensional assessment of fatigue; MD, Myotonic dystrophy; MEP, Maximal expiratory pressure; MFI-20, Multidimensional functional inventory; MFM scale, Motor function measurement scale; MIP, Maximal inspiratory pressure; MMPI, Minnesota multiphasic personality inventory; MRC, Medical Research Council Scale for muscle strength; MRI, Magnetic resonance imaging; MRS, Magnetic resonance spectroscopy; MUAP, Motor unit action potential; MV, Minute ventilation; MVA, Maximal voluntary activation; MVC, Maximal voluntary contraction; MVIC, Maximal isometric voluntary contraction; NHP, Nottingham health profile; NIPPV, Nasal intermittent positive pressure ventilation; NIV, Non-invasive ventilation; OSA, Obstructive sleep apnea; PASE, Physical activity of the elderly; PBMC, peripheral blood mononuclear cells; PCF, unassisted peak cough flow; PFS, Piper fatigue scale; PFT, Pulmonary function test; PLMS, Periodic limb movements of sleep; PPL, Polio problem list; PPS, Post-polio syndrome; PV, Polio virus; qMRI, quantitative magnetic resonance imaging; QMT, Quantitative motor test; rCT, randomized controlled trial; RDBPC, Randomized double-blind placebo controlled; REE, resting energy expenditure; RLS, Restless leg syndrome; RNA, Ribonucleic acid; RQ, respiratory quotient; RR, respiratory rate; RT-PCR, Reverse transcription polymerase chain reaction; rTMS, Repetitive transcranial magnetic stimulation; S-SFEMG, Single fiber electromyography stimulation; SF-36, 36-item short form survey; SFEMG, Single fiber electromyography; SFQ, Short fatigue questionnaire; SIP, Sickness impact profile; SIPP, Self-reported impairments in persons with late effects of polio; SMN gene, Survival motor neuron gene; SNIP, Sniff nasal inspiratory pressure; SSS test, Sit-stand-sit test; tDCS, Transcranial direct current stimulation; TQNE, Turf's quantitative neuromuscular examination; TUG test, Timed-Up-and-Go test; UW-SES, University of Washington self-efficacy scale; VAS, Visual analog scale; VAS-F, Visual analog scale for fatigue; VCO2, carbon dioxide production; VO2, oxygen consumption; WBV, Whole body vibration; WHOQOL-BREF, World Health Organization quality of life abbreviated scale.
References
1. Cousins S. Accounting for polio survivors in the post-polio world. Lancet. (2017) 389:1503–4. doi: 10.1016/S0140-6736(17)30999-6
2. Gonzalez H, Olsson T, Borg K. Management of postpolio syndrome. Lancet Neurol. (2010) 9:634–42. doi: 10.1016/S1474-4422(10)70095-8
3. Gawne AC, Halstead LS. Post-polio syndrome: historical perspective, epidemiology and clinical presentation. NeuroRehabilitation. (1997) 8:73–81. doi: 10.1016/S1053-8135(96)00212-0
4. Trojan DA, Cashman NR. Post-poliomyelitis syndrome. Muscle Nerve. (2005) 31:6–19. doi: 10.1002/mus.20259
5. Farbu E, Gilhus NE, Barnes MP, Borg K, de Visser M, Driessen A, et al. EFNS guideline on diagnosis and management of post-polio syndrome. Report of an EFNS task force. Eur J Neurol. (2006) 13:795–801. doi: 10.1111/j.1468-1331.2006.01385.x
6. Baj A, Colombo M, Headley JL, McFarlane JR, Liethof MA, Toniolo A. Post-poliomyelitis syndrome as a possible viral disease. Int J Infect Dis. (2015) 35:107–16. doi: 10.1016/j.ijid.2015.04.018
7. Leon-Monzon ME, Dalakas MC. Detection of poliovirus antibodies and poliovirus genome in patients with the post-polio syndrome. Ann N Y Acad Sci. (1995) 753:208–18. doi: 10.1111/j.1749-6632.1995.tb27547.x
8. Luciano CA, Sivakumar K, Spector SA, Dalakas MC. Electrophysiologic and histologic studies in clinically unaffected muscles of patients with prior paralytic poliomyelitis. Muscle Nerve. (1996) 19:1413–20. doi: 10.1002/(SICI)1097-4598(199611)19:11<1413::AID-MUS5>3.0.CO;2-F
9. Luciano CA, Sivakumar K, Spector SA, Dalakas MC. Reinnervation in clinically unaffected muscles of patients with prior paralytic poliomyelitis. Correlation between macroelectromyography and histology. Ann N Y Acad Sci. (1995) 753:394–6. doi: 10.1111/j.1749-6632.1995.tb27570.x
10. Cashman NR, Trojan DA. Correlation of electrophysiology with pathology, pathogenesis, and anticholinesterase therapy in post-polio syndrome. Ann N Y Acad Sci. (1995) 753:138–50. doi: 10.1111/j.1749-6632.1995.tb27540.x
11. Wiechers DO, Hubbell SL. Late changes in the motor unit after acute poliomyelitis. Muscle Nerve. (1981) 4:524–8. doi: 10.1002/mus.880040610
12. Bertolasi L, Danese A, Monaco S, Turri M, Borg K, Werhagen L. Polio patients in Northern Italy, a 50 year follow-up. Open Neurol J. (2016) 10:77–82. doi: 10.2174/1874205X01610010077
13. Ivanyi B, Ongerboer de Visser BW, Nelemans PJ, de Visser M. Macro EMG follow-up study in post-poliomyelitis patients. J Neurol. (1994) 242:37–40. doi: 10.1007/BF00920572
14. Bickerstaffe A, van Dijk JP, Beelen A, Zwarts MJ, Nollet F. Loss of motor unit size and quadriceps strength over 10 years in post-polio syndrome. Clin Neurophysiol. (2014) 125:1255–60. doi: 10.1016/j.clinph.2013.11.003
15. Maselli RA, Cashman NR, Wollman RL, Salazar-Grueso EF, Roos R. Neuromuscular transmission as a function of motor unit size in patients with prior poliomyelitis. Muscle Nerve. (1992) 15:648–55. doi: 10.1002/mus.880150603
16. Sandberg A, Stalberg E. Changes in macro electromyography over time in patients with a history of polio: a comparison of 2 muscles. Arch Phys Med Rehabil. (2004) 85:1174–82. doi: 10.1016/j.apmr.2003.08.101
17. Sandberg A, Nandedkar SD, Stalberg E. Macro electromyography and motor unit number index in the tibialis anterior muscle: differences and similarities in characterizing motor unit properties in prior polio. Muscle Nerve. (2011) 43:335–41. doi: 10.1002/mus.21878
18. Emeryk B, Rowinska-Marcinska K, Ryniewicz B, Hausmanowa-Petrusewicz I. Disintegration of the motor unit in post-polio syndrome. Part II. Electrophysiological findings in patients with post-polio syndrome. Electromyogr Clin Neurophysiol. (1990) 30:451–8.
19. Jubelt B, Cashman NR. Neurological manifestations of the post-polio syndrome. Critic Rev Neurobiol. (1987) 3:199–220.
20. Gordon T, Hegedus J, Tam SL. Adaptive and maladaptive motor axonal sprouting in aging and motoneuron disease. Neurol Res. (2004) 26:174–85. doi: 10.1179/016164104225013806
21. Perry J, Barnes G, Gronley JK. The postpolio syndrome. An overuse phenomenon. Clin Orthop Relat Res. (1988) 1988:145–62. doi: 10.1097/00003086-198808000-00018
22. Perry J, Fontaine JD, Mulroy S. Findings in post-poliomyelitis syndrome. Weakness of muscles of the calf as a source of late pain and fatigue of muscles of the thigh after poliomyelitis. J Bone Joint Surg Am Vol. (1995) 77:1148–53. doi: 10.2106/00004623-199508000-00002
23. Trojan DA, Cashman NR, Shapiro S, Tansey CM, Esdaile JM. Predictive factors for post-poliomyelitis syndrome. Arch Phys Med Rehabil. (1994) 75:770–7.
24. Dalakas MC. Pathogenetic mechanisms of post-polio syndrome: morphological, electrophysiological, virological, and immunological correlations. Ann N Y Acad Sci. (1995) 753:167–85. doi: 10.1111/j.1749-6632.1995.tb27543.x
25. Daube JR, Sorenson EJ, Windebank AJ. Prospective 15-year study of neuromuscular function in a cohort of patients with prior poliomyelitis. Suppl Clin Neurophysiol. (2009) 60:197–201. doi: 10.1016/S1567-424X(08)00020-2
26. Sorenson EJ, Daube JR, Windebank AJ. A 15-year follow-up of neuromuscular function in patients with prior poliomyelitis. Neurology. (2005) 64:1070–2. doi: 10.1212/01.WNL.0000154604.97992.4A
27. Grimby G, Hedberg M, Henning GB. Changes in muscle morphology, strength and enzymes in a 4-5-year follow-up of subjects with poliomyelitis sequelae. Scand J Rehabil Med. (1994) 26:121–30.
28. Borg K, Borg J, Edstrom L, Grimby L. Effects of excessive use of remaining muscle fibers in prior polio and LV lesion. Muscle Nerve. (1988) 11:1219–30. doi: 10.1002/mus.880111206
29. Larsson L, Li X, Tollback A, Grimby L. Contractile properties in single muscle fibres from chronically overused motor units in relation to motoneuron firing properties in prior polio patients. J Neurol Sci. (1995) 132:182–92. doi: 10.1016/0022-510X(95)00138-R
30. Borg J, Borg K, Edstrom L, Grimby L, Henriksson J, Larsson L, et al. Motoneuron and muscle fiber properties of remaining motor units in weak tibialis anterior muscles in prior polio. Ann N Y Acad Sci. (1995) 753:335–42. doi: 10.1111/j.1749-6632.1995.tb27559.x
31. Borg K, Henriksson J. Prior poliomyelitis-reduced capillary supply and metabolic enzyme content in hypertrophic slow-twitch (type I) muscle fibres. J Neurol Neurosurg Psychiatry. (1991) 54:236–40. doi: 10.1136/jnnp.54.3.236
32. Julien J, Leparc-Goffart I, Lina B, Fuchs F, Foray S, Janatova I, et al. Postpolio syndrome: poliovirus persistence is involved in the pathogenesis. J Neurol. (1999) 246:472–6. doi: 10.1007/s004150050386
33. Muir P, Nicholson F, Spencer GT, Ajetunmobi JF, Starkey WG, Khan M, et al. Enterovirus infection of the central nervous system of humans: lack of association with chronic neurological disease. J Gen Virol. (1996) 77 (Pt 7):1469–76. doi: 10.1099/0022-1317-77-7-1469
34. Melchers W, de Visser M, Jongen P, van Loon A, Nibbeling R, Oostvogel P, et al. The postpolio syndrome: no evidence for poliovirus persistence. Ann Neurol. (1992) 32:728–32. doi: 10.1002/ana.410320605
35. Kaminski HJ, Tresser N, Hogan RE, Martin E. Pathological analysis of spinal cords from survivors of poliomyelitis. Ann N Y Acad Sci. (1995) 753:390–3. doi: 10.1111/j.1749-6632.1995.tb27569.x
36. Sharief MK, Hentges R, Ciardi M. Intrathecal immune response in patients with the post-polio syndrome. N Engl J Med. (1991) 325:749–55. doi: 10.1056/NEJM199109123251101
37. Bickerstaffe A, Beelen A, Lutter R, Nollet F. Elevated plasma inflammatory mediators in post-polio syndrome: no association with long-term functional decline. J Neuroimmunol. (2015) 289:162–7. doi: 10.1016/j.jneuroim.2015.10.019
38. Gonzalez H, Khademi M, Andersson M, Piehl F, Wallstrom E, Borg K, et al. Prior poliomyelitis-IVIg treatment reduces proinflammatory cytokine production. J Neuroimmunol. (2004) 150:139–44. doi: 10.1016/j.jneuroim.2004.01.010
39. Gonzalez H, Khademi M, Andersson M, Wallstrom E, Borg K, Olsson T. Prior poliomyelitis-evidence of cytokine production in the central nervous system. J Neurol Sci. (2002) 205:9–13. doi: 10.1016/S0022-510X(02)00316-7
40. Farbu E, Rekand T, Vik-Mo E, Lygren H, Gilhus NE, Aarli JA. Post-polio syndrome patients treated with intravenous immunoglobulin: a double-blinded randomized controlled pilot study. Eur J Neurol. (2007) 14:60–5. doi: 10.1111/j.1468-1331.2006.01552.x
41. Melin E, Lindroos E, Lundberg IE, Borg K, Korotkova M. Elevated expression of prostaglandin E2 synthetic pathway in skeletal muscle of prior polio patients. J Rehabil Med. (2014) 46:67–72. doi: 10.2340/16501977-1230
42. Wahid R. Regulatory T Cells as a Biomarker of Post-polio Syndrome. (2008). Available online at: http://www.post-polio.org/edu/pphnews/pph24-2p1-5.pdf
43. Melin E, Sohrabian A, Ronnelid J, Borg K. Normal serum levels of immune complexes in postpolio patients. Results Immunol. (2014) 4:54–7. doi: 10.1016/j.rinim.2014.06.001
44. Farbu E, Rekand T, Tysnes OB, Aarli JA, Gilhus NE, Vedeler CA. GM1 antibodies in post-polio syndrome and previous paralytic polio. J Neuroimmunol. (2003) 139:141–4. doi: 10.1016/S0165-5728(03)00123-1
45. Bartholdi D, Gonzalez H, Borg K, Melki J. Absence of SMN gene deletion in post-polio syndrome. Neuromuscul Disord. (2000) 10:99. doi: 10.1016/S0960-8966(99)00076-0
46. Querin G, El Mendili MM, Lenglet T, Behin A, Stojkovic T, Salachas F, et al. The spinal and cerebral profile of adult spinal-muscular atrophy: a multimodal imaging study. NeuroImage Clin. (2019) 21:101618. doi: 10.1016/j.nicl.2018.101618
47. Rekand T, Langeland N, Aarli JA, Vedeler CA. Fcgamma receptor IIIA polymorphism as a risk factor for acute poliomyelitis. J Infect Dis. (2002) 186:1840–3. doi: 10.1086/345769
48. Bruno RL, Cohen JM, Galski T, Frick NM. The neuroanatomy of post-polio fatigue. Arch Phys Med Rehabil. (1994) 75:498–504.
49. Bodian D. Histopathologic basis of clinical findings in poliomyelitis. Am J Med. (1949) 6:563–78. doi: 10.1016/0002-9343(49)90130-8
50. Barnhart M, Rhines R. Distribution of lesions of the brain stem in poliomyelitis. Arch Neurol Psychiatry. (1948) 59:368–77. doi: 10.1001/archneurpsyc.1948.02300380097008
51. Matzke HA, Baker AB. Poliomyelitis. IV. A study of the midbrain. AMA Arch Neurol Psychiatry. (1951) 65:1–15. doi: 10.1001/archneurpsyc.1951.02320010007001
52. Luhan JA. Epidemic poliomyelitis; some pathologic observations on human material. Arch Pathol (Chic). (1946) 42:245–60.
53. Miller DC. Post-polio syndrome spinal cord pathology. Case report with immunopathology. Ann N Y Acad Sci. (1995) 753:186–93. doi: 10.1111/j.1749-6632.1995.tb27544.x
54. Casula M, Steentjes K, Aronica E, van Geel BM, Troost D. Concomitant CNS pathology in a patient with amyotropic lateral sclerosis following poliomyelitis in childhood. Clin Neuropathol. (2011) 30:111–7. doi: 10.5414/NPP30111
55. Shimada A, Lange DJ, Hays AP. Amyotrophic lateral sclerosis in an adult following acute paralytic poliomyelitis in early childhood. Acta Neuropathol. (1999) 97:317–21. doi: 10.1007/s004010050991
56. Bede P, Hardiman O. Lessons of ALS imaging: pitfalls and future directions — a critical review. NeuroImage Clin. (2014) 4:436–43. doi: 10.1016/j.nicl.2014.02.011
57. Bede P, Querin G, Pradat PF. The changing landscape of motor neuron disease imaging: the transition from descriptive studies to precision clinical tools. Curr Opin Neurol. (2018) 31:431–8. doi: 10.1097/WCO.0000000000000569
58. El Mendili MM, Querin G, Bede P, Pradat PF. Spinal cord imaging in amyotrophic lateral sclerosis: historical concepts-novel techniques. Front Neurol. (2019) 10:350. doi: 10.3389/fneur.2019.00350
59. Trojan DA, Narayanan S, Francis SJ, Caramanos Z, Robinson A, Cardoso M, et al. Brain volume and fatigue in patients with postpoliomyelitis syndrome. PMR. (2014) 6:215–20. doi: 10.1016/j.pmrj.2013.09.009
60. Schuster C, Elamin M, Hardiman O, Bede P. Presymptomatic and longitudinal neuroimaging in neurodegeneration–from snapshots to motion picture: a systematic review. J Neurol Neurosurg Psychiatry. (2015) 86:1089–96. doi: 10.1136/jnnp-2014-309888
61. Spinal Cord Gray Matter Imaging in Post Polio Syndrome. (2018). Available online at: https://ClinicalTrials.gov/show/NCT03561623
62. Halstead LS. Assessment and differential diagnosis for post-polio syndrome. Orthopedics. (1991) 14:1209–17.
63. March of Dimes. Post-polio Syndrome: Identifying Best Practices in Diagnosis and Care. (2001). Available online at: http://www.post-polio.org/edu/pps.html
64. Farbu E. Update on current and emerging treatment options for post-polio syndrome. Ther Clin Risk Manag. (2010) 6:307–13. doi: 10.2147/TCRM.S4440
65. Gonzalez H, Khademi M, Borg K, Olsson T. Intravenous immunoglobulin treatment of the post-polio syndrome: sustained effects on quality of life variables and cytokine expression after one year follow up. J Neuroinflamm. (2012) 9:167. doi: 10.1186/1742-2094-9-167
66. Dalakas MC. Morphologic changes in the muscles of patients with postpoliomyelitis neuromuscular symptoms. Neurology. (1988) 38:99–104. doi: 10.1212/WNL.38.1.99
67. Grimby G, Einarsson G, Hedberg M, Aniansson A. Muscle adaptive changes in post-polio subjects. Scand J Rehabil Med. (1989) 21:19–26.
68. Bede P, Walsh R, Fagan AJ, Hardiman O. “Sand-watch” spinal cord: a case of inferior cervical spinal cord atrophy. J Neurol. (2013) 261:235–7. doi: 10.1007/s00415-013-7193-7
69. Lebouteux MV, Franques J, Guillevin R, Delmont E, Lenglet T, Bede P, et al. Revisiting the spectrum of lower motor neuron diseases with snake eyes appearance on magnetic resonance imaging. Eur J Neurol. (2014) 21:1233–41. doi: 10.1111/ene.12465
70. Bede P, Bokde AL, Byrne SC, Elamin M, Walsh RJ, Hardiman O. Waterskier's Hirayama syndrome. J Neurol. (2011) 258:2078–9. doi: 10.1007/s00415-011-6046-5
71. Sandberg A, Stalberg E. How to interpret normal electromyographic findings in patients with an alleged history of polio. J Rehabil Med. (2004) 36:169–76. doi: 10.1080/16501970410025135
72. Roeleveld K, Sandberg A, Stalberg EV, Stegeman DF. Motor unit size estimation of enlarged motor units with surface electromyography. Muscle Nerve. (1998) 21:878–86. doi: 10.1002/(SICI)1097-4598(199807)21:7<878::AID-MUS5>3.0.CO;2-3
73. Trojan DA, Gendron D, Cashman NR. Electrophysiology and electrodiagnosis of the post-polio motor unit. Orthopedics. (1991) 14:1353–61.
74. On AY, Sungur U. Patients with post-polio syndrome are more likely to have subclinical involvement as compared to polio survivors without new symptoms. Ann Indian Acad Neurol. (2016) 19:44–7. doi: 10.4103/0972-2327.167705
75. Rodriquez AA, Agre JC. Electrophysiologic study of the quadriceps muscles during fatiguing exercise and recovery: a comparison of symptomatic and asymptomatic postpolio patients and controls. Arch Phys Med Rehabil. (1991) 72:993–7.
76. Rodriquez AA, Agre JC, Franke TM. Electromyographic and neuromuscular variables in unstable postpolio subjects, stable postpolio subjects, and control subjects. Arch Phys Med Rehabil. (1997) 78:986–91. doi: 10.1016/S0003-9993(97)90062-9
77. Cashman NR, Maselli R, Wollmann RL, Roos R, Simon R, Antel JP. Late denervation in patients with antecedent paralytic poliomyelitis. N Engl J Med. (1987) 317:7–12. doi: 10.1056/NEJM198707023170102
78. Grabljevec K, Burger H, Kersevan K, Valencic V, Marincek C. Strength and endurance of knee extensors in subjects after paralytic poliomyelitis. Disabil Rehabil. (2005) 27:791–9. doi: 10.1080/09638280400020623
79. Soderholm S, Lehtinen A, Valtonen K, Ylinen A. Dysphagia and dysphonia among persons with post-polio syndrome - a challenge in neurorehabilitation. Acta Neurol Scand. (2010) 122:343–9. doi: 10.1111/j.1600-0404.2009.01315.x
80. Driscoll BP, Gracco C, Coelho C, Goldstein J, Oshima K, Tierney E, et al. Laryngeal function in postpolio patients. Laryngoscope. (1995) 105:35–41. doi: 10.1288/00005537-199501000-00010
81. Bertolasi L, Acler M, dall'Ora E, Gajofatto A, Frasson E, Tocco P, et al. Risk factors for post-polio syndrome among an Italian population: a case-control study. Neurol Sci. (2012) 33:1271–5. doi: 10.1007/s10072-012-0931-2
82. Prokhorenko OA, Vasconcelos OM, Lupu VD, Campbell WW, Jabbari B. Sensory physiology assessed by evoked potentials in survivors of poliomyelitis. Muscle Nerve. (2008) 38:1266–71. doi: 10.1002/mus.21093
83. Bruno RL, Galski T, DeLuca J. The neuropsychology of post-polio fatigue. Arch Phys Med Rehabil. (1993) 74:1061–5. doi: 10.1016/0003-9993(93)90062-F
84. Bruno RL, Zimmerman JR. Word finding difficulty as a post-polio sequelae. Am J Phys Med Rehabil. (2000) 79:343–8. doi: 10.1097/00002060-200007000-00005
85. Hazendonk KM, Crowe SF. A neuropsychological study of the postpolio syndrome: support for depression without neuropsychological impairment. Neuropsychiatry Neuropsychol Behav Neurol. (2000) 13:112–8.
86. Bruno RL, Sapolsky R, Zimmerman JR, Frick NM. Pathophysiology of a central cause of post-polio fatigue. Ann N Y Acad Sci. (1995) 753:257–75. doi: 10.1111/j.1749-6632.1995.tb27552.x
87. Romigi A, Pierantozzi M, Placidi F, Evangelista E, Albanese M, Liguori C, et al. Restless legs syndrome and post polio syndrome: a case-control study. Eur J Neurol. (2015) 22:472–8. doi: 10.1111/ene.12593
88. Murray D, Hardiman O, Meldrum D. Assessment of subjective and motor fatigue in Polio survivors, attending a Postpolio clinic, comparison with healthy controls and an exploration of clinical correlates. Physiother Theory Pract. (2014) 30:229–35. doi: 10.3109/09593985.2013.862890
89. Duncan A, Batliwalla Z. Growing older with post-polio syndrome: social and quality-of-life implications. SAGE Open Med. (2018) 6:2050312118793563. doi: 10.1177/2050312118793563
90. Viana CF, Pradella-Hallinan M, Quadros AA, Marin LF, Oliveira AS. Circadian variation of fatigue in both patients with paralytic poliomyelitis and post-polio syndrome. Arq Neuropsiquiatr. (2013) 71:442–5. doi: 10.1590/0004-282X20130059
91. Marin LF, Carvalho LBC, Prado LBF, Oliveira ASB, Prado GF. Restless legs syndrome is highly prevalent in patients with post-polio syndrome. Sleep Med. (2017) 37:147–50. doi: 10.1016/j.sleep.2017.06.025
92. Marin LF, Carvalho LB, Prado LB, Quadros AA, Oliveira AS, Prado GF. Restless legs syndrome in post-polio syndrome: a series of 10 patients with demographic, clinical and laboratorial findings. Parkinsonism Relat Disord. (2011) 17:563–4. doi: 10.1016/j.parkreldis.2011.02.011
93. Kumru H, Portell E, Barrio M, Santamaria J. Restless legs syndrome in patients with sequelae of poliomyelitis. Parkinsonism Relat Disord. (2014) 20:1056–8. doi: 10.1016/j.parkreldis.2014.06.014
94. De Grandis E, Mir P, Edwards MJ, Bhatia KP. Restless legs may be associated with the post-polio syndrome. Parkinsonism Relat Disord. (2009) 15:74–5. doi: 10.1016/j.parkreldis.2008.02.005
95. Hsu AA, Staats BA. “Postpolio” sequelae and sleep-related disordered breathing. Mayo Clin Proc. (1998) 73:216–24. doi: 10.4065/73.3.216
96. Dolmage TE, Avendano MA, Goldstein RS. Respiratory function during wakefulness and sleep among survivors of respiratory and non-respiratory poliomyelitis. Eur Respir J. (1992) 5:864–70.
97. Araujo MA, Silva TM, Moreira GA, Pradella-Hallinan M, Tufik S, Oliveira AS. Sleep disorders frequency in post-polio syndrome patients caused by periodic limb movements. Arq Neuropsiquiatr. (2010) 68:35–8. doi: 10.1590/S0004-282X2010000100008
98. Romigi A, Maestri M. Circadian fatigue or unrecognized restless legs syndrome? The post-polio syndrome model. Front Neurol. (2014) 5:115. doi: 10.3389/fneur.2014.00115
99. Romigi A, Placidi F, Evangelista E, Desiato MT. Circadian variation of fatigue in paralytic poliomyelitis and postpolio syndrome: just fatigue or masked restless legs syndrome? Arq Neuropsiquiatr. (2014) 72:475–6. doi: 10.1590/0004-282X20140046
100. Romigi A, Franco V, Placidi F, Liguori C, Rastelli E, Vitrani G, et al. Comparative sleep disturbances in myotonic dystrophy types 1 and 2. Curr Neurol Neurosci Rep. (2018) 18:102. doi: 10.1007/s11910-018-0903-x
101. Weinstock LB, Walters AS, Paueksakon P. Restless legs syndrome–theoretical roles of inflammatory and immune mechanisms. Sleep Med Rev. (2012) 16:341–54. doi: 10.1016/j.smrv.2011.09.003
102. Tseng WC, Wu ZF, Liaw WJ, Hwa SY, Hung NK. A patient with postpolio syndrome developed cauda equina syndrome after neuraxial anesthesia: a case report. J Clin Anesthesia. (2017) 37:49–51. doi: 10.1016/j.jclinane.2016.09.032
103. Leming MK, Breyer MJ. Renal failure in a patient with postpolio syndrome and a normal creatinine level. Am J Emergency Med. (2012) 30:247.e1-3. doi: 10.1016/j.ajem.2010.07.026
104. Willen C, Thoren-Jonsson AL, Grimby G, Sunnerhagen KS. Disability in a 4-year follow-up study of people with post-polio syndrome. J Rehabil Med. (2007) 39:175–80. doi: 10.2340/16501977-0034
105. Bickerstaffe A, Beelen A, Nollet F. Change in physical mobility over 10 years in post-polio syndrome. Neuromuscul Disord. (2015) 25:225–30. doi: 10.1016/j.nmd.2014.11.015
106. Klein MG, Whyte J, Keenan MA, Esquenazi A, Polansky M. Changes in strength over time among polio survivors. Arch Phys Med Rehabil. (2000) 81:1059–64. doi: 10.1053/apmr.2000.3890
107. Flansbjer UB, Lexell J, Brogardh C. Predictors of changes in gait performance over four years in persons with late effects of polio. NeuroRehabilitation. (2017) 41:403–11. doi: 10.3233/NRE-162057
108. Flansbjer UB, Brogardh C, Horstmann V, Lexell J. Men with late effects of polio decline more than women in lower limb muscle strength: a 4-year longitudinal study. PMR. (2015) 7:1127–36. doi: 10.1016/j.pmrj.2015.05.005
109. Kosaka T, Kuroha Y, Tada M, Hasegawa A, Tani T, Matsubara N, et al. A fatal neuromuscular disease in an adult patient after poliomyelitis in early childhood: consideration of the pathology of post-polio syndrome. Neuropathology. (2013) 33:93–101. doi: 10.1111/j.1440-1789.2012.01327.x
110. Nollet F, Trojan DA. Finding causes of and managing fatigue in PPS. (2009). Available online at: https://www.cvppsg.org/wp-content/uploads/2012/02/findingcausesmanagingfatiguepps.pdf
111. Skough K, Broman L, Borg K. Test-retest reliability of the 6-min walk test in patients with postpolio syndrome. Int J Rehabil Res. (2013) 36:140–5. doi: 10.1097/MRR.0b013e32835b669b
112. Stolwijk-Swuste JM, Beelen A, Lankhorst GJ, Nollet F, group Cs. SF36 physical functioning scale and 2-minute walk test advocated as core qualifiers to evaluate physical functioning in patients with late-onset sequelae of poliomyelitis. J Rehabil Med. (2008) 40:387–94. doi: 10.2340/16501977-0188
113. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. (1991) 39:142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x
114. Whitney SL, Wrisley DM, Marchetti GF, Gee MA, Redfern MS, Furman JM. Clinical measurement of sit-to-stand performance in people with balance disorders: validity of data for the Five-Times-Sit-to-Stand Test. Phys Ther. (2005) 85:1034–45.
115. Bickerstaffe A, Beelen A, Zwarts MJ, Nollet F, van Dijk JP. Quantitative muscle ultrasound and quadriceps strength in patients with post-polio syndrome. Muscle Nerve. (2015) 51:24–9. doi: 10.1002/mus.24272
116. Koopman FS, Brehm MA, Heerkens YF, Nollet F, Beelen A. Measuring fatigue in polio survivors: content comparison and reliability of the Fatigue Severity Scale and the Checklist Individual Strength. J Rehabil Med. (2014) 46:761–7. doi: 10.2340/16501977-1838
117. Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. (1999) 52:643–51. doi: 10.1016/S0895-4356(99)00049-9
118. Ostlund G, Wahlin A, Sunnerhagen KS, Borg K. Post polio syndrome: fatigued patients a specific subgroup? J Rehabil Med. (2011) 43:39–45. doi: 10.2340/16501977-0634
119. Dencker A, Sunnerhagen KS, Taft C, Lundgren-Nilsson A. Multidimensional fatigue inventory and post-polio syndrome - a Rasch analysis. Health Qual Life Outcomes. (2015) 13:20. doi: 10.1186/s12955-015-0213-9
120. Pomeroy IM, Tennant A, Young CA. Rasch analysis of the WHOQOL-BREF in post polio syndrome. J Rehabil Med. (2013) 45:873–80. doi: 10.2340/16501977-1186
121. Chung H, Kim J, Park R, Bamer AM, Bocell FD, Amtmann D. Testing the measurement invariance of the University of Washington Self-Efficacy Scale short form across four diagnostic subgroups. Qual Life Res. (2016) 25:2559–64. doi: 10.1007/s11136-016-1300-z
122. Silva TM, Moreira GA, Quadros AA, Pradella-Hallinan M, Tufik S, Oliveira AS. Analysis of sleep characteristics in post-polio syndrome patients. Arq Neuropsiquiatr. (2010) 68:535–40. doi: 10.1590/S0004-282X2010000400011
123. Orsini M, Lopes AJ, Guimaraes FS, Freitas MR, Nascimento OJ, Anna Mde SJ, et al. Currents issues in cardiorespiratory care of patients with post-polio syndrome. Arq Neuropsiquiatr. (2016) 74:574–9. doi: 10.1590/0004-282X20160072
124. Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria–history, rationale, description, and significance. Sleep Med. (2014) 15:860–73. doi: 10.1016/j.sleep.2014.03.025
125. Walters AS, LeBrocq C, Dhar A, Hening W, Rosen R, Allen RP, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. (2003) 4:121–32. doi: 10.1016/S1389-9457(02)00258-7
126. Soliman MG, Higgins SE, El-Kabir DR, Davidson AC, Williams AJ, Howard RS. Non-invasive assessment of respiratory muscle strength in patients with previous poliomyelitis. Respir Med. (2005) 99:1217–22. doi: 10.1016/j.rmed.2005.02.035
127. Natterlund B, Ahlstrom G. Problem-focused coping and satisfaction with activities of daily living in individuals with muscular dystrophy and postpolio syndrome. Scand J Caring Sci. (1999) 13:26–32. doi: 10.1111/j.1471-6712.1999.tb00511.x
128. Saeki S, Takemura J, Matsushima Y, Chisaka H, Hachisuka K. Workplace disability management in postpolio syndrome. J Occup Rehabil. (2001) 11:299–307. doi: 10.1023/A:1013352710035
129. Davidson AC, Auyeung V, Luff R, Holland M, Hodgkiss A, Weinman J. Prolonged benefit in post-polio syndrome from comprehensive rehabilitation: a pilot study. Disabil Rehabil. (2009) 31:309–17. doi: 10.1080/09638280801973206
130. Agre JC, Rodriquez AA. Intermittent isometric activity: its effect on muscle fatigue in postpolio subjects. Arch Phys Med Rehabil. (1991) 72:971–5.
131. Agre JC, Rodriquez AA, Franke TM, Swiggum ER, Harmon RL, Curt JT. Low-intensity, alternate-day exercise improves muscle performance without apparent adverse effect in postpolio patients. Am J Phys Med Rehabil. (1996) 75:50–8. doi: 10.1097/00002060-199601000-00014
132. Dean E, Ross J. Effect of modified aerobic training on movement energetics in polio survivors. Orthopedics. (1991) 14:1243–6.
133. Ernstoff B, Wetterqvist H, Kvist H, Grimby G. Endurance training effect on individuals with postpoliomyelitis. Arch Phys Med Rehabil. (1996) 77:843–8. doi: 10.1016/S0003-9993(96)90268-3
134. Chan KM, Amirjani N, Sumrain M, Clarke A, Strohschein FJ. Randomized controlled trial of strength training in post-polio patients. Muscle Nerve. (2003) 27:332–8. doi: 10.1002/mus.10327
135. Fillyaw MJ, Badger GJ, Goodwin GD, Bradley WG, Fries TJ, Shukla A. The effects of long-term non-fatiguing resistance exercise in subjects with post-polio syndrome. Orthopedics. (1991) 14:1253–6.
136. Feldman RM, Soskolne CL. The use of nonfatiguing strengthening exercises in post-polio syndrome. Birth Defects Original Article Ser. (1987) 23:335–41.
137. Spector SA, Gordon PL, Feuerstein IM, Sivakumar K, Hurley BF, Dalakas MC. Strength gains without muscle injury after strength training in patients with postpolio muscular atrophy. Muscle Nerve. (1996) 19:1282–90. doi: 10.1002/(SICI)1097-4598(199610)19:10<1282::AID-MUS5>3.0.CO;2-A
138. Agre JC, Rodriquez AA, Franke TM. Strength, endurance, and work capacity after muscle strengthening exercise in postpolio subjects. Arch Phys Med Rehabil. (1997) 78:681–6. doi: 10.1016/S0003-9993(97)90073-3
139. Kriz JL, Jones DR, Speier JL, Canine JK, Owen RR, Serfass RC. Cardiorespiratory responses to upper extremity aerobic training by postpolio subjects. Arch Phys Med Rehabil. (1992) 73:49–54.
140. Jones DR, Speier J, Canine K, Owen R, Stull GA. Cardiorespiratory responses to aerobic training by patients with postpoliomyelitis sequelae. JAMA. (1989) 261:3255–8. doi: 10.1001/jama.261.22.3255
141. Oncu J, Durmaz B, Karapolat H. Short-term effects of aerobic exercise on functional capacity, fatigue, and quality of life in patients with post-polio syndrome. Clin Rehabil. (2009) 23:155–63. doi: 10.1177/0269215508098893
142. Strumse YA, Stanghelle JK, Utne L, Ahlvin P, Svendsby EK. Treatment of patients with postpolio syndrome in a warm climate. Disabil Rehabil. (2003) 25:77–84. doi: 10.1080/dre.25.2.77.84
143. Willen C, Sunnerhagen KS, Grimby G. Dynamic water exercise in individuals with late poliomyelitis. Arch Phys Med Rehabil. (2001) 82:66–72. doi: 10.1053/apmr.2001.9626
144. Willen C, Scherman MH. Group training in a pool causes ripples on the water: experiences by persons with late effects of polio. J Rehabil Med. (2002) 34:191–7. doi: 10.1080/16501970213232
145. Stanghelle JK, Festvag L, Aksnes AK. Pulmonary function and symptom-limited exercise stress testing in subjects with late sequelae of poliomyelitis. Scand J Rehabil Med. (1993) 25:125–9.
146. Kilmer DD. Response to aerobic exercise training in humans with neuromuscular disease. Am J Phys Med Rehabil. (2002) 81(11 Suppl):S148–50. doi: 10.1097/00002060-200211001-00015
147. Voorn EL, Gerrits KH, Koopman FS, Nollet F, Beelen A. Determining the anaerobic threshold in postpolio syndrome: comparison with current guidelines for training intensity prescription. Arch Phys Med Rehabil. (2014) 95:935–40. doi: 10.1016/j.apmr.2014.01.015
148. Voorn EL, Koopman FS, Brehm MA, Beelen A, de Haan A, Gerrits KH, et al. Aerobic exercise training in post-polio syndrome: process evaluation of a randomized controlled trial. PLoS ONE. (2016) 11:e0159280. doi: 10.1371/journal.pone.0159280
149. Murray D, Hardiman O, Campion A, Vance R, Horgan F, Meldrum D. The effects of a home-based arm ergometry exercise programme on physical fitness, fatigue and activity in Polio survivors: a randomised controlled trial. Clin Rehabil. (2017) 31:913–25. doi: 10.1177/0269215516661225
150. Murray D, Meldrum D, Moloney R, Campion A, Horgan F, Hardiman O. The effects of a home-based arm ergometry exercise programme on physical fitness, fatigue and activity in polio survivors: protocol for a randomised controlled trial. BMC Neurol. (2012) 12:157. doi: 10.1186/1471-2377-12-157
151. Da Silva CP, Szot CL, deSa N. Whole body vibration on people with sequelae of polio. Physiother Theory Pract. (2018) 2018:1–11. doi: 10.1080/09593985.2018.1454559
152. Da Silva CP. Whole body vibration methods with survivors of polio. J Vis Exp. (2018) 2018:140. doi: 10.3791/58449
153. Brogardh C, Flansbjer UB, Lexell J. No effects of whole-body vibration training on muscle strength and gait performance in persons with late effects of polio: a pilot study. Arch Phys Med Rehabil. (2010) 91:1474–7. doi: 10.1016/j.apmr.2010.06.024
154. Arazpour M, Ahmadi F, Bahramizadeh M, Samadian M, Mousavi ME, Bani MA, et al. Evaluation of gait symmetry in poliomyelitis subjects: comparison of a conventional knee-ankle-foot orthosis and a new powered knee-ankle-foot orthosis. Prosthet Orthot Int. (2016) 40:689–95. doi: 10.1177/0309364615596063
155. Heim M, Yaacobi E, Azaria M. A pilot study to determine the efficiency of lightweight carbon fibre orthoses in the management of patients suffering from post-poliomyelitis syndrome. Clin Rehabil. (1997) 11:302–5. doi: 10.1177/026921559701100406
156. Silva TM, Moreira GA, Quadros AA, Pradella-Hallinan M, Tufik S, Oliveira AS. Effects of the use of MIG3 bioceramics fabrics use–long infrared emitter–in pain, intolerance to cold and periodic limb movements in post-polio syndrome. Arq Neuropsiquiatr. (2009) 67:1049–53. doi: 10.1590/S0004-282X2009000600016
157. Acler M, Bocci T, Valenti D, Turri M, Priori A, Bertolasi L. Transcranial direct current stimulation (tDCS) for sleep disturbances and fatigue in patients with post-polio syndrome. Restor Neurol Neurosci. (2013) 31:661–8. doi: 10.3233/RNN-130321
158. Pastuszak Z, Piusinska-Macoch R, Stepien A, Czernicki Z. Repetitive transcranial magnetic stimulation in treatment of post polio syndrome. Neurol Neurochir Pol. (2018) 52:281–4. doi: 10.1016/j.pjnns.2017.10.013
159. Vallbona C, Hazlewood CF, Jurida G. Response of pain to static magnetic fields in postpolio patients: a double-blind pilot study. Arch Phys Med Rehabil. (1997) 78:1200–3. doi: 10.1016/S0003-9993(97)90332-4
160. Sonies BC. Dysphagia and post-polio syndrome: past, present, and future. Semin Neurol. (1996) 16:365–70. doi: 10.1055/s-2008-1040995
161. Sonies BC, Dalakas MC. Dysphagia in patients with the post-polio syndrome. N Engl J Med. (1991) 324:1162–7. doi: 10.1056/NEJM199104253241703
162. Yunusova Y, Plowman EK, Green JR, Barnett C, Bede P. Clinical measures of bulbar dysfunction in ALS. Front Neurol. (2019) 10:106. doi: 10.3389/fneur.2019.00106
163. Silbergleit AK, Waring WP, Sullivan MJ, Maynard FM. Evaluation, treatment, and follow-up results of post polio patients with dysphagia. Otolaryngol Head Neck Surg. (1991) 104:333–8. doi: 10.1177/019459989110400308
164. Kaminska M, Browman F, Trojan DA, Genge A, Benedetti A, Petrof BJ. Feasibility of lung volume recruitment in early neuromuscular weakness: a comparison between amyotrophic lateral sclerosis, myotonic dystrophy, and postpolio syndrome. PMR. (2015) 7:677–84. doi: 10.1016/j.pmrj.2015.04.001
165. Gillis-Haegerstrand C, Markstrom A, Barle H. Bi-level positive airway pressure ventilation maintains adequate ventilation in post-polio patients with respiratory failure. Acta Anaesthesiol Scand. (2006) 50:580–5. doi: 10.1111/j.1399-6576.2006.001015.x
166. Bach JR, Alba AS, Shin D. Management alternatives for post-polio respiratory insufficiency. Assisted ventilation by nasal or oral-nasal interface. Am J Phys Med Rehabil. (1989) 68:264–71. doi: 10.1097/00002060-198912000-00002
167. Barle H, Soderberg P, Haegerstrand C, Markstrom A. Bi-level positive airway pressure ventilation reduces the oxygen cost of breathing in long-standing post-polio patients on invasive home mechanical ventilation. Acta Anaesthesiol Scand. (2005) 49:197–202. doi: 10.1111/j.1399-6576.2004.00566.x
168. Brogardh C, Lexell J, Hammarlund CS. Experiences of falls and strategies to manage the consequences of falls in persons with late effects of polio: a qualitative study. J Rehabil Med. (2017) 49:652–8. doi: 10.2340/16501977-2262
169. Bakker M, Schipper K, Koopman FS, Nollet F, Abma TA. Experiences and perspectives of patients with post-polio syndrome and therapists with exercise and cognitive behavioural therapy. BMC Neurol. (2016) 16:23. doi: 10.1186/s12883-016-0544-0
170. Koopman FS, Beelen A, Gerrits KH, Bleijenberg G, Abma TA, de Visser M, et al. Exercise therapy and cognitive behavioural therapy to improve fatigue, daily activity performance and quality of life in postpoliomyelitis syndrome: the protocol of the FACTS-2-PPS trial. BMC Neurol. (2010) 10:8. doi: 10.1186/1471-2377-10-8
171. Koopman FS, Brehm MA, Beelen A, Voet N, Bleijenberg G, Geurts A, et al. Cognitive behavioural therapy for reducing fatigue in post-polio syndrome and in facioscapulohumeral dystrophy: a comparison. J Rehabil Med. (2017) 49:585–90. doi: 10.2340/16501977-2247
172. Koopman FS, Voorn EL, Beelen A, Bleijenberg G, de Visser M, Brehm MA, et al. No reduction of severe fatigue in patients with postpolio syndrome by exercise therapy or cognitive behavioral therapy: results of an RCT. Neurorehabil Neural Repair. (2016) 30:402–10. doi: 10.1177/1545968315600271
173. Battalio SL, Glette M, Alschuler KN, Jensen MP. Anxiety, depression, and function in individuals with chronic physical conditions: a longitudinal analysis. Rehabil Psychol. (2018) 63:532–41. doi: 10.1037/rep0000231
174. Muller R, Gertz KJ, Molton IR, Terrill AL, Bombardier CH, Ehde DM, et al. Effects of a tailored positive psychology intervention on well-being and pain in individuals with chronic pain and a physical disability: a feasibility trial. Clin J Pain. (2016) 32:32–44. doi: 10.1097/AJP.0000000000000225
175. Hirsh AT, Kupper AE, Carter GT, Jensen MP. Psychosocial factors and adjustment to pain in individuals with postpolio syndrome. Am J Phys Med Rehabil. (2010) 89:213–24. doi: 10.1097/PHM.0b013e3181c9f9a1
176. Furrer A, Michel G, Terrill AL, Jensen MP, Muller R. Modeling subjective well-being in individuals with chronic pain and a physical disability: the role of pain control and pain catastrophizing. Disabil Rehabil. (2017) 2017:1–10. doi: 10.1080/09638288.2017.1390614
177. Shiri S, Gartsman I, Meiner Z, Schwartz I. Long-standing poliomyelitis and psychological health. Disabil Rehabil. (2015) 37:2233–7. doi: 10.3109/09638288.2015.1019007
178. Silverman AM, Molton IR, Alschuler KN, Ehde DM, Jensen MP. Resilience predicts functional outcomes in people aging with disability: a longitudinal investigation. Arch Phys Med Rehabil. (2015) 96:1262–8. doi: 10.1016/j.apmr.2015.02.023
179. Battalio SL, Silverman AM, Ehde DM, Amtmann D, Edwards KA, Jensen MP. Resilience and function in adults with physical disabilities: an observational study. Arch Phys Med Rehabil. (2017) 98:1158–64. doi: 10.1016/j.apmr.2016.11.012
180. Silverman AM, Molton IR, Smith AE, Jensen MP, Cohen GL. Solace in solidarity: disability friendship networks buffer well-being. Rehabil Psychol. (2017) 62:525–33. doi: 10.1037/rep0000128
181. Ten Katen K, Beelen A, Nollet F, Frings-Dresen MH, Sluiter JK. Overcoming barriers to work participation for patients with postpoliomyelitis syndrome. Disabil Rehabil. (2011) 33:522–9. doi: 10.3109/09638288.2010.503257
182. Pierini D, Stuifbergen AK. Psychological resilience and depressive symptoms in older adults diagnosed with post-polio syndrome. Rehabil Nurs. (2010) 35:167–75. doi: 10.1002/j.2048-7940.2010.tb00043.x
183. Dinsmore S, Dambrosia J, Dalakas MC. A double-blind, placebo-controlled trial of high-dose prednisone for the treatment of post-poliomyelitis syndrome. Ann N Y Acad Sci. (1995) 753:303–13. doi: 10.1111/j.1749-6632.1995.tb27556.x
184. Stein DP, Dambrosia JM, Dalakas MC. A double-blind, placebo-controlled trial of amantadine for the treatment of fatigue in patients with the post-polio syndrome. Ann N Y Acad Sci. (1995) 753:296–302. doi: 10.1111/j.1749-6632.1995.tb27555.x
185. Trojan DA, Collet JP, Shapiro S, Jubelt B, Miller RG, Agre JC, et al. A multicenter, randomized, double-blinded trial of pyridostigmine in postpolio syndrome. Neurology. (1999) 53:1225–33. doi: 10.1212/WNL.53.6.1225
186. Horemans HL, Nollet F, Beelen A, Drost G, Stegeman DF, Zwarts MJ, et al. Pyridostigmine in postpolio syndrome: no decline in fatigue and limited functional improvement. J Neurol Neurosurg Psychiatry. (2003) 74:1655–61. doi: 10.1136/jnnp.74.12.1655
187. Chan KM, Strohschein FJ, Rydz D, Allidina A, Shuaib A, Westbury CF. Randomized controlled trial of modafinil for the treatment of fatigue in postpolio patients. Muscle Nerve. (2006) 33:138–41. doi: 10.1002/mus.20437
188. Vasconcelos OM, Prokhorenko OA, Salajegheh MK, Kelley KF, Livornese K, Olsen CH, et al. Modafinil for treatment of fatigue in post-polio syndrome: a randomized controlled trial. Neurology. (2007) 68:1680–6. doi: 10.1212/01.wnl.0000261912.53959.b4
189. Skough K, Krossen C, Heiwe S, Theorell H, Borg K. Effects of resistance training in combination with coenzyme Q10 supplementation in patients with post-polio: a pilot study. J Rehabil Med. (2008) 40:773–5. doi: 10.2340/16501977-0245
190. Peel MM, Cooke M, Lewis-Peel HJ, Lea RA, Moyle W. A randomized controlled trial of coenzyme Q10 for fatigue in the late-onset sequelae of poliomyelitis. Complement Ther Med. (2015) 23:789–93. doi: 10.1016/j.ctim.2015.09.002
191. On AY, Oncu J, Uludag B, Ertekin C. Effects of lamotrigine on the symptoms and life qualities of patients with post polio syndrome: a randomized, controlled study. NeuroRehabilitation. (2005) 20:245–51.
192. Kaponides G, Gonzalez H, Olsson T, Borg K. Effect of intravenous immunoglobulin in patients with post-polio syndrome – an uncontrolled pilot study. J Rehabil Med. (2006) 38:138–40. doi: 10.1080/16501970500441625
193. Gonzalez H, Sunnerhagen KS, Sjoberg I, Kaponides G, Olsson T, Borg K. Intravenous immunoglobulin for post-polio syndrome: a randomised controlled trial. Lancet Neurol. (2006) 5:493–500. doi: 10.1016/S1474-4422(06)70447-1
194. Werhagen L, Borg K. Effect of intravenous immunoglobulin on pain in patients with post-polio syndrome. J Rehabil Med. (2011) 43:1038–40. doi: 10.2340/16501977-0884
195. Ostlund G, Broman L, Werhagen L, Borg K. IVIG treatment in post-polio patients: evaluation of responders. J Neurol. (2012) 259:2571–8. doi: 10.1007/s00415-012-6538-y
196. Bertolasi L, Frasson E, Turri M, Gajofatto A, Bordignon M, Zanolin E, et al. A randomized controlled trial of IV immunoglobulin in patients with postpolio syndrome. J Neurol Sci. (2013) 330:94–9. doi: 10.1016/j.jns.2013.04.016
197. Schmidt S, Gocheva V, Zumbrunn T, Rubino-Nacht D, Bonati U, Fischer D, et al. Treatment with L-citrulline in patients with post-polio syndrome: study protocol for a single-center, randomised, placebo-controlled, double-blind trial. Trials. (2017) 18:116. doi: 10.1186/s13063-017-1829-3
198. Ostlund G, Broman L, Werhagen L, Borg K. Immunoglobulin treatment in post-polio syndrome: identification of responders and non-responders. J Rehabil Med. (2015) 47:727–33. doi: 10.2340/16501977-1985
199. Scholz H, Trenkwalder C, Kohnen R, Riemann D, Kriston L, Hornyak M. Dopamine agonists for restless legs syndrome. Cochrane Database Syst Rev. (2011) 2011:CD006009. doi: 10.1002/14651858.CD006009.pub2
200. Schwartz A, Bosch LM. Anesthetic implications of postpolio syndrome: new concerns for an old disease. AANA J. (2012) 80:356–61.
201. Spencer GT, Reynolds F. Postoperative respiratory arrest in a post poliomyelitis patient. Anaesthesia. (2003) 58:609–10; author reply 10. doi: 10.1046/j.1365-2044.2003.03207_14.x
Keywords: postpolio syndrome, PPS, polio, poliomyelitis, neuroimaging, biomarker, clinical trials, motor neuron disease
Citation: Li Hi Shing S, Chipika RH, Finegan E, Murray D, Hardiman O and Bede P (2019) Post-polio Syndrome: More Than Just a Lower Motor Neuron Disease. Front. Neurol. 10:773. doi: 10.3389/fneur.2019.00773
Received: 31 March 2019; Accepted: 02 July 2019;
Published: 16 July 2019.
Edited by:
Francesca Trojsi, University of Campania, Luigi Vanvitelli Caserta, ItalyReviewed by:
Andrea Romigi, Mediterranean Neurological Institute (IRCCS), ItalyLouisa Ng, The University of Melbourne, Australia
Copyright © 2019 Li Hi Shing, Chipika, Finegan, Murray, Hardiman and Bede. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter Bede, YmVkZXBAdGNkLmll
 Stacey Li Hi Shing
Stacey Li Hi Shing Rangariroyashe H. Chipika
Rangariroyashe H. Chipika Eoin Finegan
Eoin Finegan Deirdre Murray
Deirdre Murray Orla Hardiman
Orla Hardiman Peter Bede
Peter Bede