- 1Laboratoire EA6310 HAVAE “Handicap, Activité, Vieillissement, Autonomie, Environnement,” Université de Limoges, Limoges, France
- 2Laboratoire EA 7369 URePSSS “Unité de Recherche Pluridisciplinaire Sport, Santé, Société”, Université du Littoral Côte d'Opale, Dunkerque, France
- 3Médecine Physique et de Réadaptation, CHU de Limoges, Limoges, France
- 4Laboratoire EA6314 MOVE “Mobilité Vieillissement et Exercice,” Université de Poitiers, Poitiers, France
Increasing cerebral oxygenation, more precisely the overactivation of the prefrontal cortex (PFC), reflects cortical control of gait in stroke disease. Studies about the relationship between brain activation and the functional status in stroke patients remain scarce. The aim of this study was to compare brain activation, gait parameters, and cognitive performances in single and dual tasks according to the functional status in subacute stroke patients. Twenty-one subacute stroke patients were divided in two groups according to Barthel Index (“low Barthel” and “high Barthel”) and randomly performed ordered walking, cognitive task (n-back task), and dual tasks (walking + n-back task). We assessed gait performances (speed, variability) using an electronic walkway system and cerebral oxygenation (ΔO2Hb) by functional near-infrared spectroscopy. Patients with better functional status (high Barthel) showed a lower PFC activation (ΔO2Hb) and better gait parameters in single and dual tasks compared to low-Barthel patients, who exhibited decreased gait performances despite a higher PFC activation, especially in the unaffected side (P < 0.001). PFC overactivation in less functional subacute stroke patients may be due to the loss of stepping automaticity. Our results underline the interest of proposing rehabilitation programs focused on walking, especially for patients with low functional capacity.
Introduction
Stroke is associated with gait disorders, mainly characterized by a decreased gait speed (1) and a greater variability (2). Walking is further affected by challenging conditions such as simultaneous cognitive and motor tasks [e.g., dual task (DT)] (3). In stroke patients, this increased cognitive demand of walking during DT was underlined by the key role of the prefrontal cortex (PFC) (4), whose activation can be assessed by functional near-infrared spectroscopy (fNIRS) (5). Studies using fNIRS reported a greater brain activity in the PFC during DT than in single task (ST) in chronic stroke patients, implying that executive functions were primary involved in this overactivation (6, 7). Recently, we observed no difference of oxygenated hemoglobin levels (ΔO2Hb) between motor ST and DT in subacute stroke patients (8), highlighting a ceiling effect on brain activity observed in DT and thus the loss of stepping automaticity in these patients.
Other recent findings suggest that people with poorer mobility such as elders or neurological patients exhibits a higher PFC activation than control groups during walking, reflecting a higher cognitive demand (7, 9). In stroke, lower mobility was associated with a higher (and saturated) recruitment of the PFC in walking tasks (7, 10). Hence, the challenge for upcoming studies investigating brain activation during walking in stroke patients relies on a better understanding of the relationship between the cortical control of gait and functional independence.
The aim of this study is to compare brain activation, gait parameters, and cognitive performances in ST and DT according to the functional status in subacute stroke patients. We hypothesize that the PFC activation and the decrease in cognitive/gait performances during ST and DT are greater in stroke patients with a lower functional status.
Participants and Methods
This was a retrospective study based on the analysis of complementary data of Hermand et al. (8).
Participants
Twenty-one subacute stroke patients (Table 1) participated in this study, at the Limoges University Hospital. Inclusion criteria included acute or subacute stroke (<3 months), first stroke (left or right middle cerebral artery), and being able to walk 10 m. Exclusion criteria included previous neurological disease (e.g., Parkinson disease, dementia).
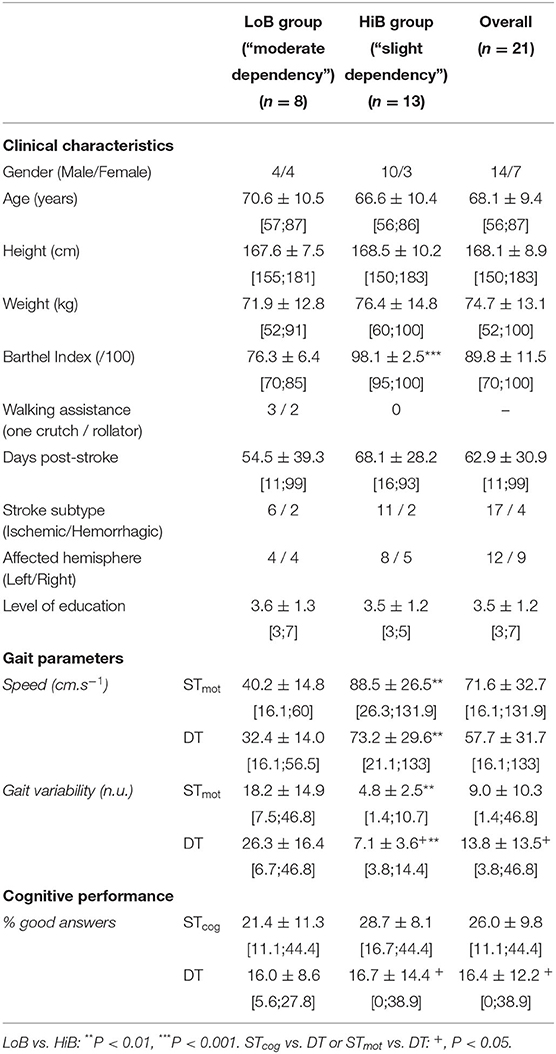
Table 1. Clinical characteristics of patients and mean values (± SD) of gait parameters and cognitive performance in ST and DT (mean ± SD).
Functional Status
The Barthel Index (BI) (11) for each patient was evaluated on test day by a trained hospital practitioner, on a 0- to 100-point scale. Patients were assigned in two groups: “slight dependency,” for a higher BI between 91 and 100 (HiB), and “moderate dependency,” for a lower BI between 61 and 90 (LoB) (12).
Design Protocol
The patients successively performed three randomly ordered tests: a cognitive ST (STcog), a walking ST (STmot), and a DT. Cognitive tasks for STcog and DT followed a 2-back task (8): the experimenter, facing the patient at a distance of 1 m during STcog or walking 1 m behind him/her during DT, read aloud, and clearly a series of 20 fixed random numbers, between 0 and 10, evenly spaced in a 30-s interval. Responses were recorded with a voice recorder. The percentage of correct answers was computed for each cognitive condition, as missing or incorrect answers were accounted for as errors (8). In walking STmot and DT, patients walked through an open space at a comfortable pace for 30 s, through an 8-m GAITRite walkway (Sparta, USA), which provided speed and stride variability. One practice trial for each ST and DT task was conducted prior to experimental testing to ensure proper hearing/vision and a good understanding of each task.
fNIRS Acquisition
Cerebral oxygenation was measured using an fNIRS system (Portalite, Artinis Medical, the Netherlands). Two optodes were placed on symmetrical prefrontal sites Fp1 and Fp2 according to the EEG 10/20 system. Acquisition was made through the Oxysoft software (version 3.0.97.1). Differential pathlength factor was set on 5 as its calculation formula does not apply to patients' age 50 years or older (13). In each condition, after a 30-s rest for baseline, patients performed the 30-s test, before a final 30-s rest phase. A 0.1-Hz low-pass filter was applied to the fNIRS signal to remove physiological and instrumental noise, and motion artifacts were corrected using MATLAB-based scripts when needed (8, 14). The relative concentrations in O2Hb (ΔO2Hb, ΔO2Hb-affected, and ΔO2Hb-unaffected in the PFC, μmol L−1) in the test interval (i.e., the last 20 s) were then normalized by subtracting to them the mean value of the last 10 s of baseline, immediately before the beginning of the task, that is, seated for STcog and standing for STmot and DT.
Statistical Analysis
A Shapiro-Wilk test confirmed the non-normal distribution of the ΔO2Hb/gait/cognitive data. Friedman and Wilcoxon tests were then conducted to compare and assess the respective effects of functional status (i.e., LoB and HiB) and conditions (STcog, STmot, and DT) on cerebral activity (ΔO2Hb, ΔO2Hb-affected, and ΔO2Hb-unaffected) and gait parameters (speed, gait variability). For all analyses, the statistical significance level was set at α <0.05. Statistical analyses were performed using IBM SPSS® Statistics version 23 (IBM Corp, Armonk, NY, USA).
Results
The LoB and HiB groups included stroke patients whose BIs range from 70 to 85 (n = 8) and from 95 to 100 (n = 13), respectively (Table 1).
Brain Activation
There was an overall BI effect on ΔO2Hb (P = 0.0022), ΔO2Hb-unaffected (P = 0.0009), and ΔO2Hb-affected (P = 0.040). More precisely, LoB patients exhibited a higher activation than HiB in STmot for ΔO2Hb (3.13 ± 1.67 vs. 1.48 ± 1.67 μmol L−1, P = 0.025, Figure 1A) and for ΔO2Hb-unaffected (1.70 ± 0.85 vs. 0.63 ± 0.92 μmol L−1, P = 0.011, Figure 1B) and in DT for ΔO2Hb-unaffected (2.18 ± 0.93 vs. 1.06 ± 1.87 μmol L−1, P = 0.036, Figure 1B). No difference was observed for ΔO2Hb-affected in both LoB and HiB patients (Figure 1C).
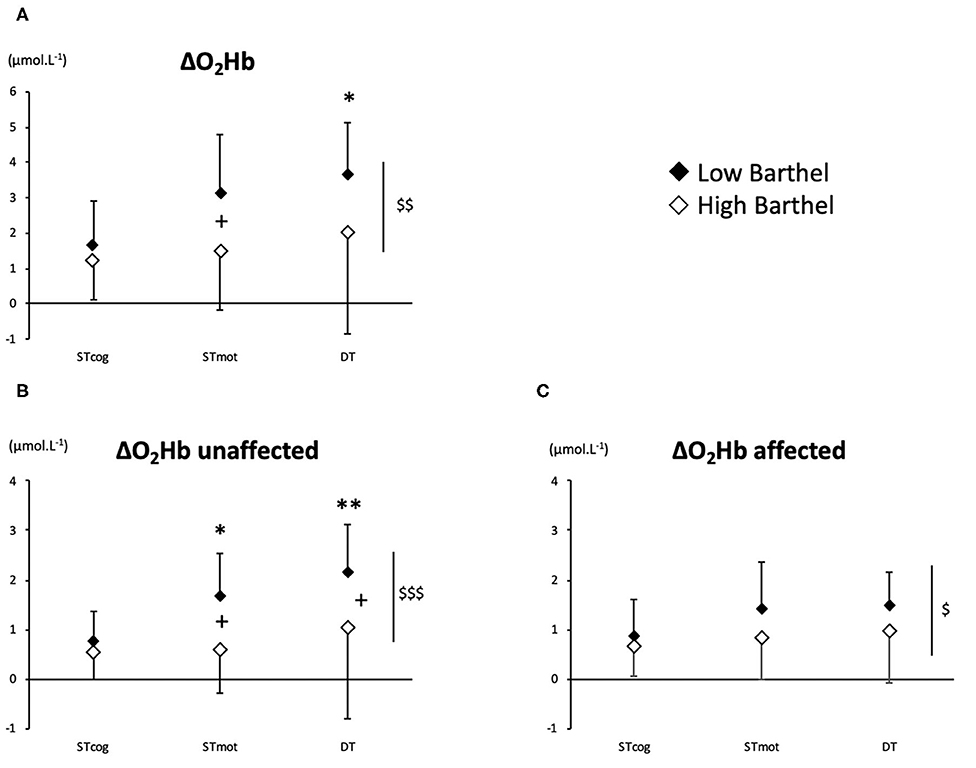
Figure 1. PFC oxygenation values (A: ΔO2Hb; B: ΔO2Hb-unaffected; C: ΔO2Hb-affected) for LoB (black dots) and HiB (white dots) patients in three tests: STcog, STmot, and DT (mean ± SD). LoB patients: condition vs. STcog (*P < 0.05, **P < 0.01); LoB vs. HiB (overall), +P < 0.05; LoB vs. HiB, $P < 0.05, $$P < 0.01, $$$P < 0.001.
Gait and Cognitive Performances
An overall BI effect was observed on both speed and gait variability. More precisely, speed was higher, and gait variability was lower in HiB patients than in LoB in STmot (P = 0.0017 and P = 0.0016, respectively) and in DT (P = 0.0018 and P = 0.0013, respectively) (Table 1).
There was no BI effect on cognitive performance across all conditions.
Effects of DT
No difference between ST and DT on PFC oxygenation was observed for the whole population (HiB and LoB patients pooled together) and for the HiB group (separately). In LoB patients, ΔO2Hb and ΔO2Hb-unaffected were lower in STcog than in DT (P < 0.05 and P < 0.01, respectively). In LoB patients, ΔO2Hb-unaffected was lower in STcog than in STmot (P = 0.028, Figure 1B).
No difference was observed between STmot and DT on gait parameters (except a trend for gait variability, P = 0.085) for all population. However, gait variability was higher in DT than in STmot for HiB patients only (P = 0.039).
There was an overall effect of DT on cognitive performances (P < 0.05), but this negative impact was observed only for HiB patients (P < 0.05) and not for LoB patients (P > 0.05).
Discussion
First, this study shows that patients with a better functional status (HiB) showed a lower PFC activation and better gait parameters in ST and DT compared to LoB patients, who exhibited decreased gait performances despite a higher PFC activation, especially in the unaffected side (Figure 2).
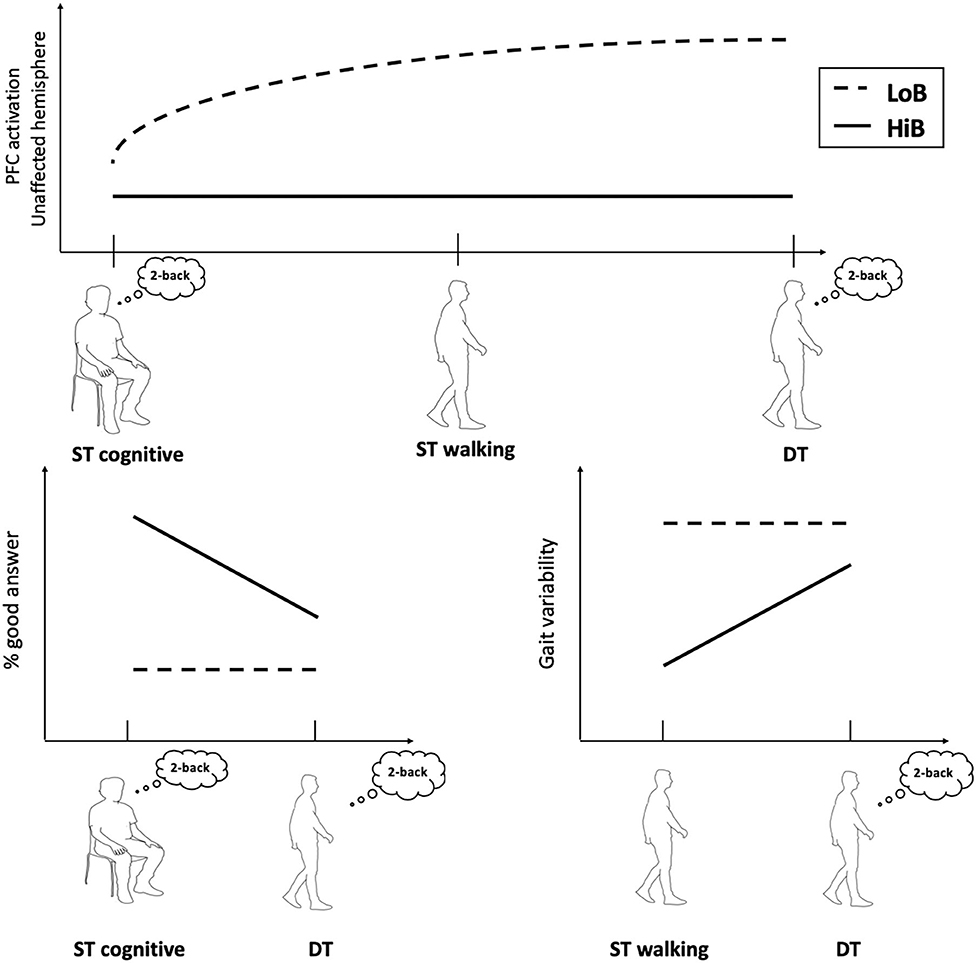
Figure 2. Conceptual framework illustrating cognitive–motor performances and cerebral oxygenation in walking tasks according to functional status.
LoB patients required additional attentional resources for walking, in accordance with our previous work in which STmot and cognitive–motor DT induced a PFC overactivation (vs. STcog) in subacute stroke patients (8). We had highlighted the existence of a “ceiling” phenomenon in brain oxygenation induced by walking: a brain overactivation in stroke patients could be triggered by STmot and could not be further augmented by an additional cognitive load in DT (8). The present study evidences a similar phenomenon only for LoB patients, which illustrates a greater reliance on cortical control of gait in patients with poor mobility. In literature, recently published reviews on fNIRS studies during DTs in aging have interpreted the recruitment of neuronal networks in PFC as compensatory mechanisms for declines in functional efficiency (9, 15). Also, the increased PFC activity in LoB patients only seems to be associated with changes of neuronal networks caused by stroke and could be explained by models of neural inefficiency and limited capacity (16, 17).
Moreover, it is interesting to note that the functional status is associated with a sided overactivation for LoB patients during STmot and DT (vs. STcog): the unaffected side was more activated (Figure 1B), as a compensatory mechanism for the affected PFC (Figure 1C), as previously observed during balance task for unaffected PFC compensating for various ipsilesional damages areas (18). Despite a higher PFC activation, LoB gait performances remained lower than HiB and confirm that a higher BI is associated with a lower gait variability (2). According to our data, we could assume that an increase of a central O2 availability in the unaffected PFC would not be enough to compensate for the affected PFC in LoB patients, potentially because the maximal cognitive capabilities might already be reached; as a consequence, performance in gait/cognitive tasks remain low, in ST and DT. The overactivation of the unaffected PFC could illustrate this disequilibrium between the affected and the unaffected sides, and the subsequent reassignment of cerebral tasks to the unaffected side, in whole or in part (19). We can also assume that LoB patients may exhibit a primary recruitment of unaffected PFC to compensate for the deficient side, less available to voluntary gait control. HiB patients who have better performance in gait and/or cognitive parameters would have interhemispheric activation balance in PFC, as observed in normal older subjects (7, 20). This also could involve another potential mechanism during recovery, relying on the interaction between PFC and other brain areas involved into stepping automaticity, such as premotor and primary motor cortices, which could enhance compensatory mechanisms in HiB patients, as observed in older normal subjects (21). However, in our study, we were not able to measure the activation of other brain areas, and fNIRS technology offers only a limited depth penetration that does not allow us to assess the activation of deeper cerebral structures.
Second, there was no difference of PFC oxygenation between gait conditions (STmot and DT), but we observed better gait performances (i.e., gait variability) in STmot compared to DT only for HiB. This highlights the key role of functional status on the cognitive–motor interference: unlike LoB patients, HiB patients with better recovered gait and/or cognition exhibit a decrease in their performance in DT. Compared to LoB patients in which DT does not impact the already low gait/cognitive performances, this decrease in HiB patients could be then associated to a “normal” behavior (3, 4) and hence may reflect a better recovery of walking capabilities. This discrepancy between LoB and HiB patients in subacute phase could lead to a further reflection on personalized rehabilitation modalities for stroke patients according to their functional status: LoB patients, more prone to fall risks (22), could benefit from rehabilitation strategies designed to improve stepping automaticity, whereas HiB patients may focus more on increasing the complexity of cognitive tasks.
In conclusion, our study highlights a PFC overactivation in the unaffected side for less functional stroke patients, triggered in walking conditions (STmot), potentially limited by an upper limit that may not be exceeded in DT (8). This would likely be due to the loss of stepping automaticity in ST (i.e., higher-level control of gait) and then is not observed in more autonomous stroke patients. This overactivation in PFC in patients with poor mobility confirms that basic motor tasks require most of their attention resources and could be interpreted as a neural inefficiency. Also, the functional status (i.e., the Barthel Index) may represent a valuable indicator to assess both motor and cerebral recovery in stroke patients. Future studies might need to include more subacute stroke patients with various functional status, evaluated with the Fugl–Meyer Assessment (23) or by a 10-m gait test (24), and controlled sociodemographic factors. Finally, the evolution of brain activation during a follow-up of a stroke patients' cohort during rehabilitation would be interesting to investigate from acute to chronic phase of stroke.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This was a retrospective study based on the analysis of complementary data of a previous study. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AP, J-CD, and EH: study concept and design. EH: acquisition of data. EH, OD, and AP: analysis and interpretation of data. EH, OD, AP, and J-CD: drafting of the manuscript. MC and J-YS: critical revision of the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Wonsetler EC, Bowden MG. A systematic review of mechanisms of gait speed change post-stroke. Part 1: spatiotemporal parameters and asymmetry ratios. Topics Stroke Rehabil. (2017) 24:435–46. doi: 10.1080/10749357.2017.1285746
2. Balasubramanian CK, Neptune RR, Kautz SA. Variability in spatiotemporal step characteristics and its relationship to walking performance post-stroke. Gait Posture. (2009) 29:408–14. doi: 10.1016/j.gaitpost.2008.10.061
3. Plummer P, Eskes G, Wallace S, Giuffrida C, Fraas M, Campbell G, et al. Cognitive-Motor Interference During Functional Mobility After Stroke: State of the Science and Implications for Future Research. Archives of Physical Medicine and Rehabilitation. (2013) 94:2565–2574.e6. doi: 10.1016/j.apmr.2013.08.002
4. Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. (2008) 23:329–42. doi: 10.1002/mds.21720
5. Gramigna V, Pellegrino G, Cerasa A, Cutini S, Vasta R, Olivadese G, et al. Near-infrared spectroscopy in gait disorders: is it time to begin? Neurorehabil Neural Repair. (2017) 31:402–12. doi: 10.1177/1545968317693304
6. Al-Yahya E, Johansen-Berg H, Kischka U, Zarei M, Cockburn J, Dawes H. Prefrontal cortex activation while walking under dual-task conditions in stroke: a multimodal imaging study. Neurorehabil Neural Repair. (2016) 30:591–9. doi: 10.1177/1545968315613864
7. Hawkins KA, Fox EJ, Daly JJ, Rose DK, Christou EA, McGuirk TE, et al. Prefrontal over-activation during walking in people with mobility deficits: interpretation and functional implications. Human Movement Sci. (2018) 59:46–55. doi: 10.1016/j.humov.2018.03.010
8. Hermand E, Tapie B, Dupuy O, Fraser S, Compagnat M, Salle JY, et al. Prefrontal cortex activation during dual task with increasing cognitive load in subacute stroke patients: a pilot study. Front Aging Neurosci. (2019) 11:160. doi: 10.3389/fnagi.2019.00160
9. Kahya M, Moon S, Ranchet M, Vukas RR, Lyons KE, Pahwa R, et al. Brain activity during dual task gait and balance in aging and age-related neurodegenerative conditions: a systematic review. Exp Gerontol. (2019) 128:110756. doi: 10.1016/j.exger.2019.110756
10. Chatterjee SA, Fox EJ, Daly JJ, Rose DK, Wu SS, Christou EA, et al. Interpreting prefrontal recruitment during walking after stroke: influence of individual differences in mobility and cognitive function. Front Hum Neurosci. (2019) 13:194. doi: 10.3389/fnhum.2019.00194
11. Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J. (1965) 14:61–5. doi: 10.1037/t02366-000
12. Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. (1989) 42:703–9. doi: 10.1016/0895-4356(89)90065-6
13. Duncan A, Meek JH, Clemence M, Elwell CE, Fallon P, Tyszczuk L, et al. Measurement of cranial optical path length as a function of age using phase resolved near infrared spectroscopy. Pediatr Res. (1996) 39:889–94. doi: 10.1203/00006450-199605000-00025
14. Fishburn FA, Ludlum RS, Vaidya CJ, Medvedev AV. Temporal Derivative Distribution Repair (TDDR): a motion correction method for fNIRS. NeuroImage. (2019) 184:171–9. doi: 10.1016/j.neuroimage.2018.09.025
15. Udina C, Avtzi S, Durduran T, Holtzer R, Rosso AL, Castellano-Tejedor C, et al. Functional near-infrared spectroscopy to study cerebral hemodynamics in older adults during cognitive and motor tasks: a review. Front Aging Neurosci. (2020) 11:367. doi: 10.3389/fnagi.2019.00367
16. Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci. (2008) 17:177–82. doi: 10.1111/j.1467-8721.2008.00570.x
17. Holtzer R, Rakitin BC, Steffener J, Flynn J, Kumar A, Stern Y. Age effects on load-dependent brain activations in working memory for novel material. Brain Res. (2009) 1249:148–61. doi: 10.1016/j.brainres.2008.10.009
18. Mihara M, Miyai I, Hattori N, Hatakenaka M, Yagura H, Kawano T, et al. Cortical control of postural balance in patients with hemiplegic stroke. NeuroReport. (2012) 23:314–9. doi: 10.1097/WNR.0b013e328351757b
19. Leone C, Feys P, Moumdjian L, D'Amico E, Zappia M, Patti F. Cognitive-motor dual-task interference: a systematic review of neural correlates. Neurosci Biobehav Rev. (2017) 75:348–60. doi: 10.1016/j.neubiorev.2017.01.010
20. Mori T, Takeuchi N, Izumi S-I. Prefrontal cortex activation during a dual task in patients with stroke. Gait Posture. (2018) 59:193–98. doi: 10.1016/j.gaitpost.2017.09.03
21. Beurskens R, Helmich I, Rein R, Bock O. Age-related changes in prefrontal activity during walking in dual-task situations: a fNIRS study. Int J Psychophysiol. (2014) 92:122–8. doi: 10.1016/j.ijpsycho.2014.03.005
22. Sheikh M, Hosseini HA. Investigating the relationship between spatiotemporal gait variability and falls self-efficacy in individuals with chronic stroke. Physiother Theory Practice. (2020). doi: 10.1080/09593985.2020.1771799. [Epub ahead of print].
23. de Oliveira R, Cacho EWA, Borges G. Post-stroke motor and functional evaluations: a clinical correlation using Fugl-Meyer assessment scale, Berg balance scale and Barthel index. Arq Neuropsiquiatr. (2006) 64:731–5. doi: 10.1590/S0004-282X2006000500006
Keywords: functional near-infrared spectroscopy, dual task, gait, cognition, prefrontal cortex
Citation: Hermand E, Compagnat M, Dupuy O, Salle J-Y, Daviet J-C and Perrochon A (2020) Functional Status Is Associated With Prefrontal Cortex Activation in Gait in Subacute Stroke Patients: A Functional Near-Infrared Spectroscopy Study. Front. Neurol. 11:559227. doi: 10.3389/fneur.2020.559227
Received: 06 May 2020; Accepted: 22 September 2020;
Published: 05 November 2020.
Edited by:
Margit Alt Murphy, University of Gothenburg, SwedenReviewed by:
Sudeshna Aloke Chatterjee, University of Florida, United StatesInbal Maidan, Tel Aviv Sourasky Medical Center, Israel
Copyright © 2020 Hermand, Compagnat, Dupuy, Salle, Daviet and Perrochon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anaick Perrochon, YW5haWNrLnBlcnJvY2hvbkB1bmlsaW0uZnI=
 Eric Hermand
Eric Hermand Maxence Compagnat
Maxence Compagnat Olivier Dupuy
Olivier Dupuy Jean-Yves Salle3
Jean-Yves Salle3 Anaick Perrochon
Anaick Perrochon