- The Elton Laboratory for Molecular Neuroendocrinology, Department of Human Molecular Genetics and Biochemistry, Sackler Faculty of Medicine, Sagol School of Neuroscience and Adams Super Center for Brain Studies, Tel Aviv University, Tel Aviv, Israel
Activity-dependent neuroprotective protein (ADNP) syndrome, also known as Helsmoortel-Van Der Aa syndrome, is a rare condition, which is diagnosed in children exhibiting signs of autism. Specifically, the disease is suspected when a child is suffering from developmental delay and/or intellectual disability. The syndrome occurs when one of the two copies of the ADNP gene carries a pathogenic sequence variant, mostly a de novo mutation resulting in loss of normal functions. Original data showed that Adnp+/− mice suffer from learning and memory deficiencies, muscle weakness, and communication problems. Further studies showed that the ADNP microtubule-interacting fragment NAP (called here CP201) resolves, in part, Adnp deficiencies and protects against ADNP pathogenic sequence variant abnormalities. With a clean toxicology and positive human adult experience, CP201 is planned for future clinical trials in the ADNP syndrome.
Background
The ADNP syndrome (https://www.orpha.net/consor/cgi-bin/OC_Exp.php?Lng=EN&Expert=404448; https://rarediseases.info.nih.gov/diseases/12931/adnp-syndrome) traits include limitations of social interactions and communication along with stereotypic, repetitive behavior, and restricted interest (1). ADNP de novo mutations (pathogenic sequence variants) causing syndromic autism were first described by O'Roak et al. and later extended by Helsmoortel et al. as reviewed in the laboratory of Illana Gozes, the discoverer of the ADNP gene (2–8).
The human ADNP gene is ~40 kilobases long and contains five exons and four introns (5). The gene is located on the q13.13 band of chromosome 20 (8, 9). The protein comprises 1,102 amino acids including asparagine–alanine–proline–valine–serine–isoleucine–proline–glutamine (NAPVSIPQ), which is an 8-amino-acid neuroprotective peptide called NAP (also discovered by the Gozes Laboratory). NAP is referred here to as CP201 (1, 4, 7, 8).
The ADNP gene is one of the most prevalent single mutated genes within the autism spectrum disorders (ASDs) (5, 6, 10, 11). According to the original description, the ADNP syndrome is estimated to account for 0.17% of all cases of ASD (4, 12).
More than 400 genes are regulated by ADNP, which are critical for brain formation, organ development, cognition, and motor function (6, 13–16). In the nucleus, ADNP is a member of a chromatin remodeling complex that is responsible for RNA transcription and splicing (13, 17–19). In the cytoplasm, ADNP has been shown to correlate with the microtubule (MT)–associated protein Tau, leading to dynamic Tau expression and protection against Tau pathology (hyperphosphorylation) (20). Tau hyperphosphorylation has been associated with neurodegeneration along with cognitive decline (6, 20, 21). Importantly, ADNP interacts directly with the MT end-binding proteins (EB1 and EB3). When there is a mutation and one of the ADNP alleles is lost (or dysfunctional), there is a disruption in the MT–EB protein interaction (6). This causes a negative impact on brain formation leading to decreased learning skills and memory (5).
The syndrome occurs when one of the two copies of the ADNP gene is mutated and loses its normal function (4). The mutation is most often a de novo (4). In this respect, Adnp+/− mice suffer from learning and memory deficiencies, muscle weakness, and communication problems. Data have shown the resolution of these symptoms with the administration of CP201, which also reduces neurodegeneration (20, 22). Mice with both Adnp genes deleted (Adnp−/−) do not survive, as Adnp is critical for neural tube closure and further brain formation (4, 23). Most recent data showed direct protection of CP201 against deleterious effects of ADNP pathogenic sequence variants spanning the ADNP protein (24, 25) as detailed below.
Symptoms
Children are delivered on time (normal length and weight) (1, 26). Dysmorphic facial features are common, including a prominent forehead, high hairline, widely spaced and down-sloping eyes, posteriorly rotated ears, large head, long flat philtrum, thin upper lip, and a flat/broad nasal bridge ((4); https://www.adnpfoundation.org/). Other symptoms include seizures, hypotonia, feeding difficulties, gastroesophageal reflux disease, constipation, vomiting, heart defects (atrial septal defects and mitral valve prolapse), brain abnormalities (anxiety, aggressiveness, obsessive compulsive disorder), delayed milestones, severe cognitive delays, language disorder, motor skill disorder, undescended testicles, bilateral cryptorchidism, congenital hernia, and visual disturbances (hypermetropia, strabismus, and ptosis) (1, 5, 26). The main, similar features include gross and fine motor delay, along with intellectual disability (ID) and speech delay (10, 26–28).
Musculoskeletal defects have also been noted. These include joint hyperlaxity and multiple hand abnormalities, including, but not limited, to clinodactyly and abnormal phalanges (1). These children are also plagued with recurrent infections of the upper respiratory and urinary tracts (1). Abnormalities seen on brain magnetic resonance imaging (MRI) include wide ventricles, white matter lesions, and choroid cysts (1).
Diagnosis
Diagnosis is usually made by identifying a heterozygous ADNP mutation through molecular genetic testing using whole-exome sequencing (4, 5, 10). Other molecular testing approaches are acceptable including single-gene testing and multigene panels (4). Commonly, the mutation is a de novo mutation, meaning it is a spontaneous pathogenic sequence variant within the DNA (5, 10).
Early tooth eruption is a common trait found in these children (6). Usually, by the end of their first birthday, the children have a full mouth of teeth including their molars. This premature teething can be an early diagnostic marker for ADNP mutations, which can pave the way to early intervention and personalized treatment (6).
Standard of Care
Currently, there is no cure for this disease, and the prognosis for this syndrome is unknown (26). Although there is no standard of care for these children, they are symptomatically treated with walkers and surgically treated for atrial septal defects, ventricular septal defects, cardiovascular valve prolapse, imperforate anus, and astigmatism, along with other anatomical defects (10). Occasional treatments with risperidone have also been reported. Specifically, one case study on a 2½-year-old patient described that application of antipsychotic medication resulted in a significant resolution of behavioral outbursts, leading to progress in language acquisition (29).
Additional current treatments include physical therapy, occupational therapy, behavioral therapy, sensory processing therapy, and music and water therapy. Improvement with therapeutic intervention would prove beneficial to these patients and to caregivers (5, 10).
Rationale for Drug Development
ADNP syndrome is a chronically debilitating disease to which there is no approved treatment. Although current pharmacological treatments can be effective at treating children symptomatically, a treatment to help with ID could potentially be life changing. Usually, treatment of these children involves multiple specialists that include neurologists, cardiologists, and surgeons (https://www.orpha.net/consor/cgi-bin/OC_Exp.php?Lng=EN&Expert=404448; https://rarediseases.info.nih.gov/diseases/12931/adnp-syndrome) (26).
This disease affects not only the child, but also the parents and the health care system. In the first few years of life, a child may go through multiple surgeries, including open heart surgery with ADNP involved in heart development (13) and affecting congenital heart diseases (5, 30). The cognitive impairments (ID) may be very severe; a 7-year-old may behave like a 16-month-old, and the language of a 3-year-old may be equivalent to a 12-month-old, as words are unrecognizable (5, 10, 31).
Looking at more than anecdotal case highlights, an extensive worldwide cohort of 78 individuals with ADNP syndrome was collected (2014–2016). The comprehensive results are published including clinician and parental interviews (26). In summary, clinical features include ID, autistic traits, severe motor and language development delays, and common facial appearances (outlined above). Behavioral problems, sleep irregularities, epilepsy, visual problems, hypotonia, congenital heart defects, gastrointestinal irregularities, short stature, endocrine (hormonal) deficiencies, and brain abnormalities (MRI) were described as common comorbidities. All these emphasize a need for further drug development.
Although rare, there have been at least two cases of childhood deaths (personal Facebook, https://www.facebook.com/TeamKnoxJoseph/; https://www.adnpkids.com/adnp-angels.html) with one recently published (25).
Postmortem analysis was conduct on a 7-year-old boy, heterozygous for ADNP de novo pathogenic sequence variant c.2244Adup/p.His559Glnfs*3. The child had autism, motor delays, severe ID, and seizures. He died following liver transplantation and multiple organ failure. A comparison to young adult with no tauopathy emphasizes the disease severity (25). Thus, a widespread child brain tauopathy paralleled by extensive transcriptomic alternations was discovered. Tauopathy was explained by direct ADNP mutation inhibition of Tau–MT binding (25). As tauopathy is a progressive condition, treatments halting tauopathy progression are required (24).
Therefore, the ADNP syndrome, in some cases, may be a devastating disease that does not allow children suffering from this disease to integrate into society due to multiple serious medical problems such as feeding difficulties, developmental delays (memory loss, limited speech), anatomical defects, and limited mobility.
To this end, CP201 is being developed for the treatment of the ADNP syndrome. This is based on reports of CP201 administration in heterozygous (haploinsufficient) mouse models of ADNP that has shown amelioration of some cognitive abnormalities along with restoration of learning and memory, skeletal strength, and vocalization with a reduction in neurodegeneration (22, 32–36). This is further based on CP201 mechanism of action as illustrated below.
Drug Candidate: CP201 (NAP) Mechanism of Action
ADNP is critical for the brain, influencing brain development, brain injury protection, and aging. ADNP has been associated with EB1/EB3 (end-binding proteins) through the CP201 active motif mediating MT neuroplasticity (37). ADNP deficiency in mice impedes axonal transport (6, 38). Neuronal communication depends on MT integrity, and disruption results in delayed cognition. CP201 is brain bioavailable, benefiting synaptic development by promoting neuronal cell survival, synaptic maturation, neuroplasticity, and axonal transport. Alternative names include AL-108, NAP, NAPVSIPQ (molecular weight, 824.9 Da), and davunetide. CP201 is an intranasal (IN) investigational drug product constituting a multidispensing, metered nasal spray pump device including an aqueous solution of davunetide. It is packaged in a mechanical multi-dose device designed for the IN application of solutions.
Specifically, CP201 exhibits brain bioavailability (39, 40) and cellular bioavailability (41). The mechanism of action of CP201 is through its interaction with the MT EB-interacting motif (SxIP) in ADNP (binding to the neuroactive proteins EB1 and EB3) (37). CP201 is shown to enhance ADNP-EB1/EB3/Tau interaction (6, 42, 43) even in the face of ADNP mutations (24, 25).
By binding to EB1/EB3 and promoting other SxIP-containing proteins including ADNP to associate with EBs, CP201 also enhances MT impact on neuroplasticity and neuroprotection (37). Furthermore, by binding CP201 and EB1/EB3, the Tau–MT interaction is dramatically increased leading to neuron/brain protection (6). As such, CP201 promotes formation of mature dendritic spines (post synapse) (17, 22), enhances MT invasion to the tip of the growth cone (pre-synapse) (33, 44) and protects MT-dependent axonal transport (6, 38). This explains the breadth and efficiency of CP201's neuroprotective capability along with its neurotrophic capacities (6, 37). Heterozygous mutations of Adnp (Adnp+/−) result in Tau (MT associated protein) hyperphosphorylation paralleled by cognitive deficits. CP201 enhances Tau–MT binding and inhibits Tau hyperphosphorylation and aggregation, therefore, reversing ADNP deficiency (21).
Specifically, cellular expression of heterozygous ADNP truncating mutations (representing the majority of the ADNP syndrome cases, e.g., ADNP p.Ser404* or p.Tyr719*, or p.Arg730*) reduced Tau–MT interactions (25) and impaired MT dynamics, in cell culture models (24). We have previously shown that CP201 enhanced the interaction of the intact ADNP with MT-Tau (6, 37). Thus, treating the ADNP-mutated cells with CP201 protected against ADNP mutation-induced MT dysfunction (24, 25). These results suggest that CP201-induces increased interaction of the intact ADNP with MT-Tau (6) and provides cellular protection (37), in the face of ADNP pathogenic sequence variants (25).
Furthermore, autophagy, a major cellular regulatory mechanism, is dependent on MTs, and ADNP binding to the MT associated protein 1 light chain 3 (LC3) is enhanced by CP201, protecting autophagy (45).
We propose CP201 as a first-in-class drug candidate, leading to the discovery of new routes to combat devastating brain diseases associated with the loss of essential cellular functions that culminate in loss of crucial daily functions (37).
Preclinical Studies
Toxicology and pharmacology studies in animals were conducted with davunetide, the Drug Substance (DS). The DS used was of similar purity and quality as the batch used to produce the clinical supplies described here. For intravenous (IV) administration in the non-clinical acute dog toxicity study and the safety pharmacology studies, davunetide was dissolved in sodium chloride for injection. For IN administration in the non-clinical toxicity studies, davunetide was dissolved either in sodium chloride for injection or in a solution containing 7.5 mg/mL sodium chloride, 1.7 mg/mL citric acid monohydrate, 3 mg/mL disodium phosphate dihydrate, and 0.01% benzalkonium chloride. The IN toxicology studies used the same formulation composition as for the davunetide clinical supplies, except that the concentration of benzalkonium chloride used in the toxicology studies was twice the concentration in the clinical formulation (0.01% for animal studies vs. 0.005% for clinical supplies). Benzalkonium chloride is used as a preservative or bacterial-static agent commonly found in IN drugs. Proof-of-concept studies are described below.
The safety of davunetide was studied in various modes of administration (IN or IV) in a broad spectrum of doses (up to 300 mg/kg per day) and in several animal model (rats, dogs, and mice), as well as studies in juvenile animal performed in 6-week-old rats and 4–5-month old beagle dogs. The studies included safety pharmacology, acute dose toxicity, repeat dose toxicity in various lengths and designs, genotoxicity, pharmacokinetic analysis, and drug–drug interaction where the inhibition of CYPs was studied. The product was well tolerated in the non-clinical studies and did not demonstrate any test article related adverse events (39, 40). Davunetide demonstrated a maximal NOAEL of 20 mg/kg per day in IN administration in dogs, which is equivalent to 11.11 mg/kg per day in humans.
Proof of Concept Studies
In vitro
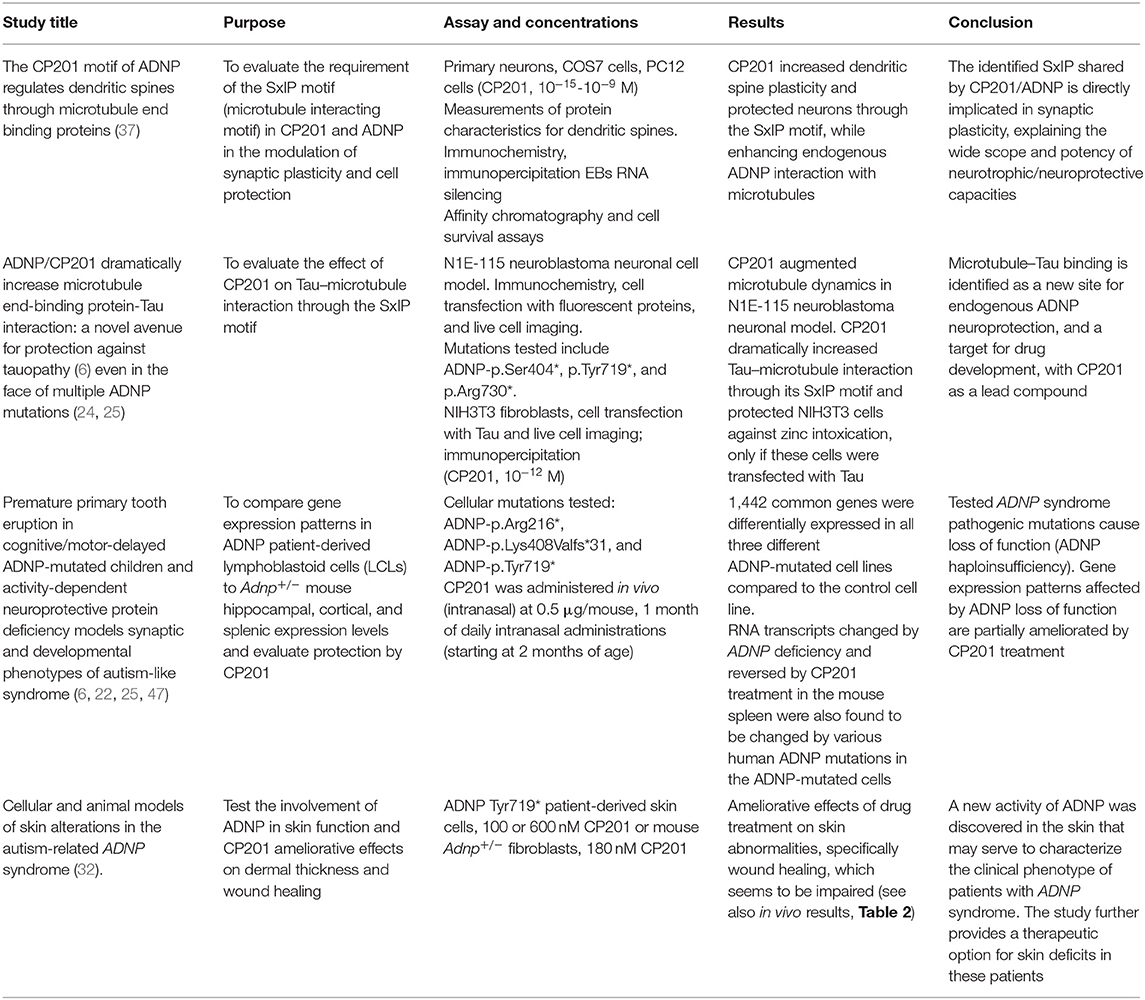
CP201 (NAP) was extensively studied in multiple in vitro studies. A previous review summarized the pharmacology up to 2017 (46). Here, in vitro studies related to the mechanism of ADNP are summarized in Table 1. These studies demonstrate that CP201 directly affects ADNP mechanisms. In neuronal cell cultures, CP201 increased dendritic spine plasticity and protected neurons through the SxIP motif, while enhancing endogenous ADNP interaction with MTs and Tau.
In vivo
The Adnp+/− Mouse Model
The Adnp+/− mouse model predicted the ADNP syndrome (20). This mouse line exhibits developmental delays and synaptic dysfunctions mimicking children with ADNP syndrome. A survey of 78 children carrying pathogenic ADNP sequence variants spanning the entire protein suggested partial loss of similar functions, with potentially some increased severity in ADNP p.Tyr719* children (the most prevalent group) (26). Importantly, the mutated (mostly truncated) and the intact ADNP alleles are both expressed in ADNP syndrome human cells (4), supporting the Adnp+/− mouse as a model predictive for ADNP heterozygous mutation deficiency in humans (6, 26). Furthermore, some children with ADNP syndrome show almost complete deletions of one allele (9), presenting a haploinsufficient loss-of-function phenotype (1, 9). Finally, there is a very high conservation of the ADNP gene between human and mouse (about 90% identity at the mRNA level) (8), and ADNP is critical for brain development in the mouse, like in human (23).
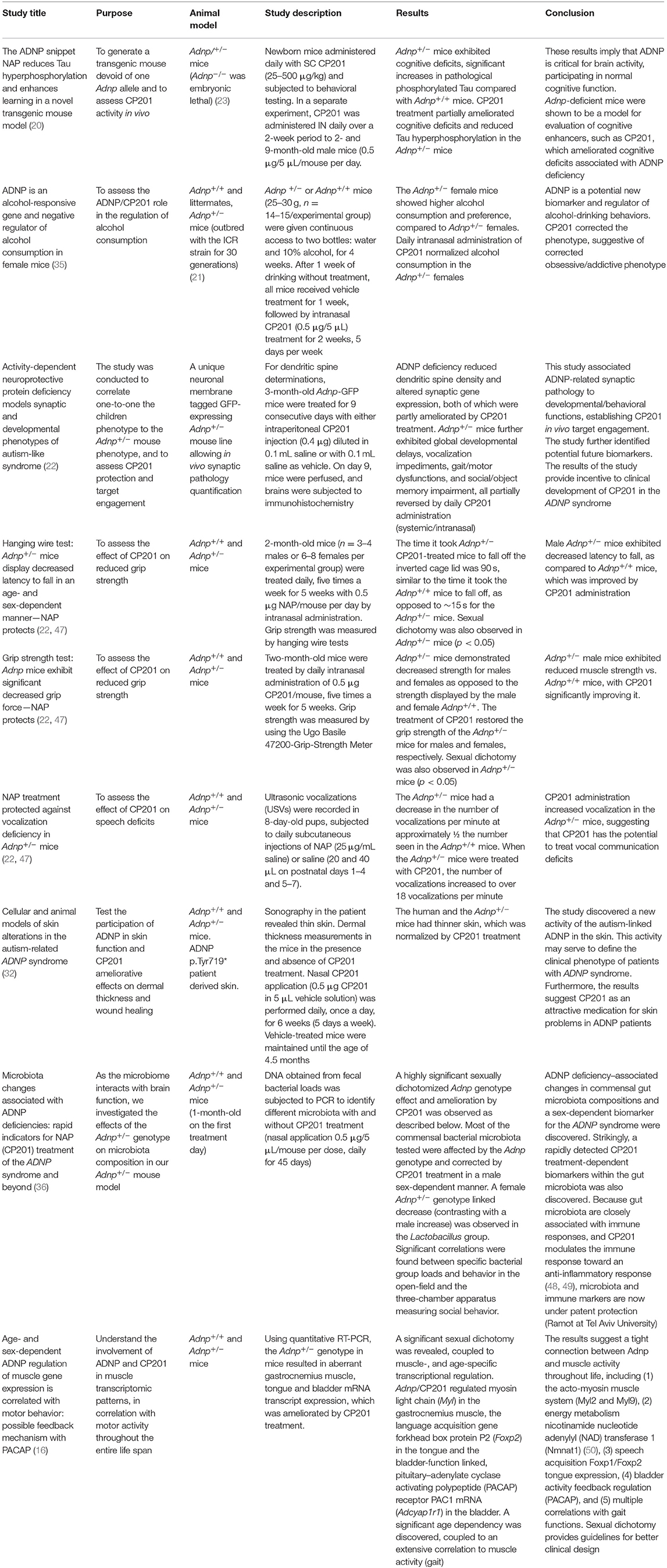
The Adnp+/− mouse model is representative of traits presented in children with ADNP syndrome as described before (22). The protective effect of CP201 was demonstrated by affecting animal traits that are equivalent to clinical symptoms in human patients with ADNP syndrome (20, 22). A summary of in vivo proof-of-concept studies in the Adnp+/− mouse model is provided in Table 2 and expended below.
ADNP Deficiency in Mice Models the ADNP Syndrome
Results comparing synaptogenesis, dendritic spine formation, and immunohistochemistry of excitatory synapses in the Adnp+/− mice to human ADNP syndrome MRI data have been collected (22, 33, 34). These results demonstrate parallels between the Adnp+/− mouse and patients with ADNP syndrome, at multiple levels (developmental, behavioral, and motor). Furthermore, the mouse model allowed quantitation of excitatory synapse density in the hippocampus and motor cortex and evaluation of transcriptomic data, correlating molecular, anatomical, and functional consequences as described (22). These results establish CP201 in vivo target engagement and identify potential biomarkers, paving the way toward clinically advancing CP201 for the ADNP syndrome.
The data in Adnp+/− mice further demonstrate that hyperphosphorylation of Tau is decreased following CP201 treatment (20). This is in line with the findings of tauopathy in the human postmortem ADNP case and with mutated human ADNP reducing Tau–MT interaction, which is corrected/normalized by CP201 treatment (25).
Collectively, the data from ADNP syndrome mouse model demonstrate CP201 to be a promising therapeutic candidate for the treatment of children who suffer from this debilitating disease (Table 2).
It should be added that although the current review may seem limited in cellular and animal models, a previous book chapter summarized CP201 (NAP) in vitro and in vivo pharmacology up to 2017. This previous report includes dozens of our own investigations, as well as independent research in versatile disease models corroborating the proposed efficacious mechanism of action (46).
Clinical Studies
CP201 has not been previously approved for the treatment of the ADNP syndrome; however, clinical trials for other indications have been conducted [progressive supranuclear palsy (PSP), mild cognitive impairment (MCI), and schizophrenia] (42).
CP201 was previously referred to as AL-108, developed by Allon Therapeutics and subsequently licensed by Coronis Neurosciences from Ramot at Tel Aviv University.
The legal owner of all Allon Therapeutics materials is Ramot. Previous clinical trials for IN administered davunetide by Allon include the following:
1. ClinicalTrials.gov identifier: NCT00422981—MCI
2. ClinicalTrials.gov identifier: NCT00505765—Schizophrenia
3. ClinicalTrials.gov identifier: NCT01056965—Tauopathies
4. ClinicalTrials.gov identifier: NCT01110720—PSP
Allon also conducted an IV administration trial:
ClinicalTrials.gov identifier: NCT00404014—MCI Following Coronary Artery Bypass Graft Surgery.
No significant side effects were reported. Minor side effects in a small minority of patients may have included some nasal discomfort (51), which could perhaps be associated with the application volume requiring repeated daily nasal administrations (52). In general, all studies have proven safety and tolerance of CP201 in hundreds of adult compromised patients. Efficacy was seen in enhancement of cognitive function and functional activities of daily living as reviewed (46).
Additional clinical studies have shown that ADNP levels correlate with disease status (cognitive impairments, and schizophrenia) and tauopathy as illustrated above, e.g., ClinicalTrials.gov identifier: NCT01403519—Innovative Biomarkers in Alzheimer's Disease and Frontotemporal Dementia: Preventative and Personalized (24, 53).
Current ADNP syndrome clinical trials feature natural history (e.g., ClinicalTrials.gov identifier: NCT01238250 and NCT03718936). Furthermore, ketamine is being tested in the ADNP syndrome patients ClinicalTrials.gov identifier: NCT04388774, and as noted above, risperidone treatment has shown some efficacy in a case study (29).
Coronis was granted an Orphan Drug Designation #DRU-2017-6243 by the US Food and Drug Administration (FDA) for the treatment of the ADNP syndrome with CP201. Coronis has further officially met with the FDA for a Pre-Investigational New Drug Application, paving the path to a CP201 clinical trial (54).
Author Contributions
IG designed, led and orchestrated the writing, provided funding, designed experiments, analyzed the data of many of the cited articles, and wrote the final mini review.
Funding
This work was partially supported by the following grants (IG): European Research Area Network (ERA-NET) Neuron ADNPinMED, the US–Israel Binational Science Foundation—US National Science Foundation (BSF-NSF 2016746), the Alberto Moscona Nisim (AMN) Foundation for the Advancement of Science, Art and Culture in Israel, as well as by Drs. Ronith and Armand Stemmer and Arthur Gerbi (French Friends of Tel Aviv University) and Anne and Alex Cohen (Canadian Friends of Tel Aviv University). Professor Illana Gozes, currently the Director of the Dr. Diana and Ziga Elton Laboratory for Molecular Neuroendocrinology, was the first incumbent of the Lily and Avraham Gildor Chair for the Investigation of Growth Factors.
Conflict of Interest
IG is the Chief Scientific Officer of Coronis Neurosciences. NAP (CP201) use is under patent protection (US patent nos. US7960334, US8618043, and USWO2017130190A1).
Acknowledgments
I thank the diligent employees of ADRES-Advanced Regulatory Services LTD for helping with regulatory aspects of the project and all my students and collaborators cited above.
References
1. Vandeweyer G, Helsmoortel C, Van Dijck A, Vulto-van Silfhout AT, Coe BP, Bernier R, et al. The transcriptional regulator ADNP links the BAF (SWI/SNF) complexes with autism. Am J Med Genet. (2014) 166c:315–26. doi: 10.1002/ajmg.c.31413
2. O'Roak BJ, Vives L, Fu W, Egertson JD, Stanaway IB, Phelps IG, et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. (2012) 338:1619–22. doi: 10.1126/science.1227764
3. O'Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. (2012) 485:246–50. doi: 10.1038/nature10989
4. Helsmoortel C, Vulto-van Silfhout AT, Coe BP, Vandeweyer G, Rooms L, van den Ende J, et al. A SWI/SNF-related autism syndrome caused by de novo mutations in ADNP. Nat Genet. (2014) 46:380–4. doi: 10.1038/ng.2899
5. Gozes I, Helsmoortel C, Vandeweyer G, Van der Aa N, Kooy F, Sermone SB. The compassionate side of neuroscience: tony sermone's undiagnosed genetic journey–ADNP mutation. J Mol Neurosci. (2015) 56:751–7. doi: 10.1007/s12031-015-0586-6
6. Ivashko-Pachima Y, Sayas CL, Malishkevich A, Gozes I. ADNP/NAP dramatically increase microtubule end-binding protein-Tau interaction: a novel avenue for protection against tauopathy. Mol Psychiatr. (2017) 22:1335–44. doi: 10.1038/mp.2016.255
7. Bassan M, Zamostiano R, Davidson A, Pinhasov A, Giladi E, Perl O, et al. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J Neurochem. (1999) 72:1283–93. doi: 10.1046/j.1471-4159.1999.0721283.x
8. Zamostiano R, Pinhasov A, Gelber E, Steingart RA, Seroussi E, Giladi E, et al. Cloning and characterization of the human activity-dependent neuroprotective protein. J Biol Chem. (2001) 276:708–14. doi: 10.1074/jbc.M007416200
9. Huynh MT, Boudry-Labis E, Massard A, Thuillier C, Delobel B, Duban-Bedu B, et al. A heterozygous microdeletion of 20q13.13 encompassing ADNP gene in a child with Helsmoortel-van der Aa syndrome. Eur J Human Genet. (2018) 26:1497–501. doi: 10.1038/s41431-018-0165-8
10. Gozes I. The eight and a half year journey of undiagnosed AD: gene sequencing and funding of advanced genetic testing has led to hope and new beginnings. Front Endocrinol. (2017) 8:107. doi: 10.3389/fendo.2017.00107
11. Satterstrom FK, Kosmicki JA, Wang J, Breen MS, De Rubeis S, An JY, et al. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of Autism. Cell. (2020) 180:568–84.e23. doi: 10.1016/j.cell.2019.12.036
13. Mandel S, Gozes I. Activity-dependent neuroprotective protein constitutes a novel element in the SWI/SNF chromatin remodeling complex. J Biol Chem. (2007) 282:34448–56. doi: 10.1074/jbc.M704756200
14. Gozes I. ADNP regulates cognition: a multitasking protein. Front Neurosci. (2018) 12:873. doi: 10.3389/fnins.2018.00873
15. Amram N, Hacohen-Kleiman G, Sragovich S, Malishkevich A, Katz J, Touloumi O, et al. Sexual divergence in microtubule function: the novel intranasal microtubule targeting SKIP normalizes axonal transport and enhances memory. Mol Psychiatr. (2016) 21:1467–76. doi: 10.1038/mp.2015.208
16. Kapitansky O, Sragovich S, Jaljuli I, Hadar A, Giladi E, Gozes I. Age and sex-dependent ADNP regulation of muscle gene expression is correlated with motor behavior: possible feedback mechanism with PACAP. Int J Mol Sci. (2020) 21:6715. doi: 10.3390/ijms21186715
17. Schirer Y, Malishkevich A, Ophir Y, Lewis J, Giladi E, Gozes I. Novel marker for the onset of frontotemporal dementia: early increase in activity-dependent neuroprotective protein (ADNP) in the face of Tau mutation. PLoS ONE. (2014) 9:e87383. doi: 10.1371/journal.pone.0087383
18. Ferrari R, de Llobet Cucalon LI, Di Vona C, Le Dilly F, Vidal E, Lioutas A, et al. TFIIIC binding to alu elements controls gene expression via chromatin looping and histone acetylation. Mol Cell. (2020) 77:475–87.e11. doi: 10.1016/j.molcel.2019.10.020
19. Kaaij LJT, Mohn F, van der Weide RH, de Wit E, Buhler M. The ChAHP complex counteracts chromatin looping at CTCF sites that emerged from SINE expansions in mouse. Cell. (2019) 178:1437–51.e14. doi: 10.1016/j.cell.2019.08.007
20. Vulih-Shultzman I, Pinhasov A, Mandel S, Grigoriadis N, Touloumi O, Pittel Z, et al. Activity-dependent neuroprotective protein snippet NAP reduces tau hyperphosphorylation and enhances learning in a novel transgenic mouse model. J Pharmacol Exp Therapeut. (2007) 323:438–49. doi: 10.1124/jpet.107.129551
21. Malishkevich A, Amram N, Hacohen-Kleiman G, Magen I, Giladi E, Gozes I. Activity-dependent neuroprotective protein (ADNP) exhibits striking sexual dichotomy impacting on autistic and Alzheimer's pathologies. Transl Psychiatr. (2015) 5:e501. doi: 10.1038/tp.2014.138
22. Hacohen-Kleiman G, Sragovich S, Karmon G, Gao AYL, Grigg I, Pasmanik-Chor M, et al. Activity-dependent neuroprotective protein deficiency models synaptic and developmental phenotypes of autism-like syndrome. J Clin Invest. (2018) 128:4956–69. doi: 10.1172/JCI98199
23. Pinhasov A, Mandel S, Torchinsky A, Giladi E, Pittel Z, Goldsweig AM, et al. Activity-dependent neuroprotective protein: a novel gene essential for brain formation. Brain Res Dev Brain Res. (2003) 144:83–90. doi: 10.1016/S0165-3806(03)00162-7
24. Ivashko-Pachima Y, Hadar A, Grigg I, Korenkova V, Kapitansky O, Karmon G, et al. Discovery of autism/intellectual disability somatic mutations in Alzheimer's brains: mutated ADNP cytoskeletal impairments and repair as a case study. Mol Psychiatr. (2019) 10. doi: 10.1038/s41380-019-0563-5
25. Grigg I, Ivashko-Pachima Y, Hait TA, Korenková V, Touloumi O, Lagoudaki R, et al. Tauopathy in the young autistic brain: novel biomarker and therapeutic target. Transl Psychiatr. (2020) 10:228. doi: 10.1038/s41398-020-00904-4
26. Van Dijck A, Vulto-van Silfhout AT, Cappuyns E, van der Werf IM, Mancini GM, Tzschach A, et al. Clinical presentation of a complex neurodevelopmental disorder caused by mutations in ADNP. Biol Psychiatr. (2019) 85:287–97. doi: 10.1016/j.biopsych.2018.02.1173
27. Arnett AB, Beighley JS, Kurtz-Nelson EC, Hoekzema K, Wang T, Bernier RA, et al. Developmental predictors of cognitive and adaptive outcomes in genetic subtypes of autism spectrum disorder. Autism Res. (2020) 13:1659–69. doi: 10.1002/aur.2385
28. Arnett AB, Rhoads CL, Hoekzema K, Turner TN, Gerdts J, Wallace AS, Bedrosian-Sermone S, Eichler EE, Bernier RA. The autism spectrum phenotype in ADNP syndrome. Autism Res. (2018) 11:1300–10. doi: 10.1002/aur.1980
29. Shillington A, Pedapati E, Hopkin R, Suhrie K. Early behavioral and developmental interventions in ADNP-syndrome: a case report of SWI/SNF-related neurodevelopmental syndrome. Mol Genet Genomic Med. (2020) 8:e1230. doi: 10.1002/mgg3.1230
30. Ji W, Ferdman D, Copel J, Scheinost D, Shabanova V, Brueckner M, et al. De novo damaging variants associated with congenital heart diseases contribute to the connectome. Sci Rep. (2020) 10:7046. doi: 10.1038/s41598-020-63928-2
31. Levine J, Cohen D, Herman C, Verloes A, Guinchat V, Diaz L, et al. Developmental phenotype of the rare case of DJ caused by a unique ADNP gene de novo mutation. J Mol Neurosci. (2019) 68:321–30. doi: 10.1007/s12031-019-01333-9
32. Mollinedo P, Kapitansky O, Gonzalez-Lamuno D, Zaslavsky A, Real P, Gozes I, et al. Cellular and animal models of skin alterations in the autism-related ADNP syndrome. Sci Rep. (2019) 9:736. doi: 10.1038/s41598-018-36859-2
33. Sragovich S, Malishkevich A, Piontkewitz Y, Giladi E, Touloumi O, Lagoudaki R, et al. The autism/neuroprotection-linked ADNP/NAP regulate the excitatory glutamatergic synapse. Transl Psychiatr. (2019) 9:2. doi: 10.1038/s41398-018-0357-6
34. Sragovich S, Ziv Y, Vaisvaser S, Shomron N, Hendler T, Gozes I. The autism-mutated ADNP plays a key role in stress response. Transl Psychiatr. (2019) 9:235. doi: 10.1038/s41398-019-0569-4
35. Ziv Y, Rahamim N, Lezmy N, Even-Chen O, Shaham O, Malishkevich A, et al. Activity-dependent neuroprotective protein (ADNP) is an alcohol-responsive gene and negative regulator of alcohol consumption in female mice. Neuropsychopharmacology. (2019) 44:415–24. doi: 10.1038/s41386-018-0132-7
36. Kapitansky O, Giladi E, Jaljuli I, Bereswill S, Heimesaat MM, Gozes I. Microbiota changes associated with ADNP deficiencies: rapid indicators for NAP (CP201) treatment of the ADNP syndrome and beyond. J Neural Transmiss. (2020) 127:251–63. doi: 10.1007/s00702-020-02155-5
37. Oz S, Kapitansky O, Ivashco-Pachima Y, Malishkevich A, Giladi E, Skalka N, et al. The NAP motif of activity-dependent neuroprotective protein (ADNP) regulates dendritic spines through microtubule end binding proteins. Mol Psychiatr. (2014) 19:1115–24. doi: 10.1038/mp.2014.97
38. Jouroukhin Y, Ostritsky R, Assaf Y, Pelled G, Giladi E, Gozes I. NAP (davunetide) modifies disease progression in a mouse model of severe neurodegeneration: protection against impairments in axonal transport. Neurobiol Dis. (2013) 56:79–94. doi: 10.1016/j.nbd.2013.04.012
39. Gozes I, Morimoto BH, Tiong J, Fox A, Sutherland K, Dangoor D, et al. NAP: research and development of a peptide derived from activity-dependent neuroprotective protein (ADNP). CNS Drug Rev. (2005) 11:353–68. doi: 10.1111/j.1527-3458.2005.tb00053.x
40. Morimoto BH, Fox AW, Stewart AJ, Gold M. Davunetide: a review of safety and efficacy data with a focus on neurodegenerative diseases. Exp Rev Clin Pharmacol. (2013) 6:483–502. doi: 10.1586/17512433.2013.827403
41. Ivashko-Pachima Y, Gozes I. Deciphering the Enigma: NAP (CP201) the active ADNP drug candidate enters cells by dynamin-associated endocytosis. J Mol Neurosci. (2020) 70:993–8. doi: 10.1007/s12031-020-01632-6
42. Ivashko-Pachima Y, Maor-Nof M, Gozes I. NAP (davunetide) preferential interaction with dynamic 3-repeat Tau explains differential protection in selected tauopathies. PLoS ONE. (2019) 14:e0213666. doi: 10.1371/journal.pone.0213666
43. Gozes I, Ivashko-Pachima Y, Sayas CL. ADNP, a microtubule interacting protein, provides neuroprotection through end binding proteins and tau: an amplifier effect. Front Mol Neurosci. (2018) 11:151. doi: 10.3389/fnmol.2018.00151
44. Oz S, Ivashko-Pachima Y, Gozes I. The ADNP derived peptide, NAP modulates the tubulin pool: implication for neurotrophic and neuroprotective activities. PLoS ONE. (2012) 7:e51458. doi: 10.1371/journal.pone.0051458
45. Sragovich S, Merenlender-Wagner A, Gozes I. ADNP plays a key role in autophagy: from autism to schizophrenia and alzheimer's disease. BioEssays. (2017) 39:1700054. doi: 10.1002/bies.201700054
46. Gozes I. Neuroprotective drug development: the story of ADNP, NAP (Davunetide), and SKIP. In: Gozes I, editor. Neuroprotection in Alzheimer's Disease. Academic Press/Elsevier (2017). p. 253–70. doi: 10.1016/B978-0-12-803690-7.00013-2
48. Heimesaat MM, Mousavi S, Klove S, Genger C, Weschka D, Giladi E, et al. Immune-modulatory properties of the octapeptide NAP in Campylobacter jejuni infected mice suffering from acute enterocolitis. Microorganisms. (2020) 8:802. doi: 10.3390/microorganisms8060802
49. Idan-Feldman A, Schirer Y, Polyzoidou E, Touloumi O, Lagoudaki R, Grigoriadis NC, Gozes I. Davunetide (NAP) as a preventative treatment for central nervous system complications in a diabetes rat model. Neurobiol Dis. (2011) 44:327–39. doi: 10.1016/j.nbd.2011.06.020
50. Kapitansky O, Gozes I. ADNP differentially interact with genes/proteins in correlation with aging: a novel marker for muscle aging. GeroScience. (2019) 41:321–40. doi: 10.1007/s11357-019-00079-x
51. Boxer AL, Lang AE, Grossman M, Knopman DS, Miller BL, Schneider LS, et al. Davunetide in patients with progressive supranuclear palsy: a randomised, double-blind, placebo-controlled phase 2/3 trial. Lancet. (2014) 13:676–85. doi: 10.1016/S1474-4422(14)70088-2
52. Javitt DC, Buchanan RW, Keefe RS, Kern R, McMahon RP, Green MF, et al. Effect of the neuroprotective peptide davunetide (AL-108) on cognition and functional capacity in schizophrenia. Schizophrenia Res. (2012) 136:25–31. doi: 10.1016/j.schres.2011.11.001
53. Malishkevich A, Marshall GA, Schultz AP, Sperling RA, Aharon-Peretz J, Gozes I. Blood-borne activity-dependent neuroprotective protein (ADNP) is correlated with premorbid intelligence, clinical stage, and alzheimer's disease biomarkers. J Alzheimer's Dis. (2016) 50:249–60. doi: 10.3233/JAD-150799
Keywords: ADNP, ADNP syndrome, CP201 (NAP, davunetide), microtubules (MT), Adnp+/− mice, tau
Citation: Gozes I (2020) The ADNP Syndrome and CP201 (NAP) Potential and Hope. Front. Neurol. 11:608444. doi: 10.3389/fneur.2020.608444
Received: 20 September 2020; Accepted: 20 October 2020;
Published: 24 November 2020.
Edited by:
Brahim Tabarki Melaiki, University of Sousse, TunisiaReviewed by:
Amelle Shillington, Cincinnati Children's Hospital Medical Center, United StatesCorrado Romano, Oasi Research Institute (IRCCS), Italy
Minh-Tuan Huynh, Centre Hospitalier Universitaire (CHU) de Nantes, France
Copyright © 2020 Gozes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Illana Gozes, aWdvemVzQHRhdWV4LnRhdS5hYy5pbA==
 Illana Gozes
Illana Gozes