- 1Department of Neurosurgery, Shinshu University School of Medicine, Matsumoto, Japan
- 2Department of Neurosurgery, Iida Municipal Hospital, Nagano, Japan
- 3Department of Neurosurgery, Juntendo University, Tokyo, Japan
Background: Recently, electrocorticographic (ECoG) studies have emphasized the importance of gamma band-based functional mapping in the presurgical localization of the eloquent cortex. Passive functional mapping using ECoG signals provides a reliable method for identifying receptive language areas without many of the risks and limitations associated with electrical cortical stimulation. We report a surgical case of left temporal malignant glioma with intraoperative passive language mapping.
Case Description: A 78-year-old woman was diagnosed with left temporal glioma with inspection of her language difficulty. MRI showed a left temporal tumor measuring 74.6 × 50.0 × 51.5 mm in size. Real-time CortiQ-based mapping using high-gamma activity by word-listening and story-listening tasks was performed. Significant listening task-evoked high gamma activities were detected in 5 channels in the superior temporal gyrus and one channel in the middle temporal gyrus. The tumor was grossly removed except for the region corresponding to listening task-evoked high gamma activities. Postoperatively, the patient's symptoms of language comprehension difficulty improved, and no new neurological deficits were observed.
Conclusion: Intraoperative passive language mapping was successfully performed, and the patient's language function was well-preserved. Intraoperative passive language mapping, which is applicable in a short time and under general anesthesia, can be an important tool for detecting language areas.
Introduction
Electrical cortical stimulation (ECS) mapping remains the gold standard for presurgical localization of brain function (1). Awake craniotomy is the only established method to intraoperatively verify the preservation of language function (2); however, precise functional mapping is still difficult in patients with language deficits (3). ECS has shortcomings, as it is time-consuming and may evoke after-discharges or seizures (4).
Recently, electrocorticographic (ECoG) studies have emphasized the importance of gamma band-based functional mapping in the presurgical localization of the eloquent cortex (5). Passive ECoG-based mapping is set to become one of the most important novel tools in presurgical functional mapping (4).
We report an elderly patient with left temporal glioma evaluated with intraoperative passive language mapping. Tumor removal was successfully performed, preserving her language function.
Case Description
Initial Presentation
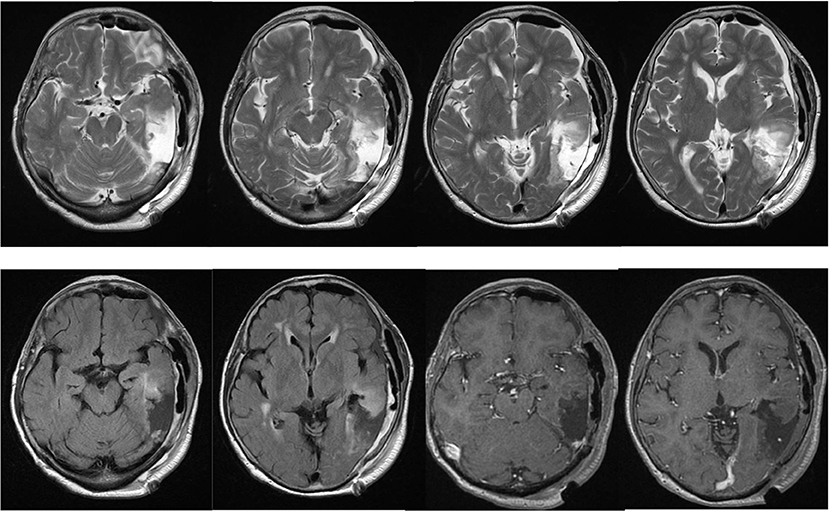
A previously healthy 78-year-old woman noticed a worsening of language difficulty and visited our hospital. CT showed a calcified lesion on the left temporal lobe (Figure 1A), and MRI revealed an intra-axial tumor showing high T2 signal and heterogeneous enhancement and a cystic component located medially (Figures 1B,C). The tumor was located in the inferior temporal and middle temporal regions and the fusiform gyrus and 74.6 × 50.0 × 51.5 mm (AP × LR × HT) in size. Preoperative fMRI could not show the Wernicke area clearly because of the large tumor and perifocal edema. Preoperatively, she had fluent aphasia, naming disorder, and difficulty in language comprehension.
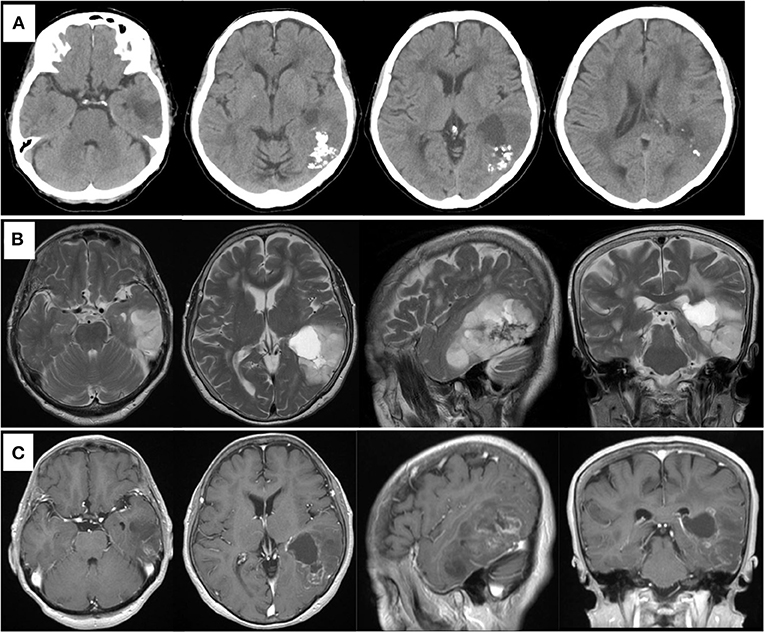
Figure 1. Preoperative computed tomography showing calcified and cystic lesions in the left temporal lobe (A). MR images showing high intensity in the left temporal lobe on T2-weighted image (B) and heterogeneous enhanced lesion on gadolinium-enhanced T1- weighted image (C).
Awake craniotomy was abandoned as a possibility considering her aphasia and age. Tumor removal with passive language mapping under general anesthesia was planned to remove as much of the tumor as possible to preserve her language function.
Intraoperative Passive Language Mapping and Surgery
We used a 20-channel ECoG grid with a 3-mm electrode diameter and a 10-mm interelectrode distance (Unique Medical), together with 4-channel strip electrodes. The exposed brain surface and grid were captured by a digital camera before resection (Figures 2A,B). Real-time CortiQ-based mapping (g.tec medication engineering GmbH, Austria) using high-gamma activity by word-listening (e.g., moon, book, flog, cake, etc.,) and Japanese story-listening tasks was performed. The listening tasks were delivered without sound for 20 s (rest phase) and then delivered sounds for 20 s (active phase). The active phase was composed of meaning and meaningless sounds. The rest and active phases were repeated alternately six times. The sampling rate was 1,200 Hz with a g.HIamp (g.tec medical engineering GmbH, Austria). The analysis was performed by MATLAB 2015a at 60–90 and 110–140 Hz, and over 25% task/rest band power was defined as significant. Anesthesia was maintained with propofol, muscle relaxant, and fentanyl. A bispectral index (BIS) monitor was used to assess the depth of anesthesia and it was maintained with a high BIS level (70–90) during passive language mapping. Significant listening task-evoked high gamma activities were detected (Figure 2C). We analyzed 5 channels in the superior temporal gyrus and one channel in the middle temporal gyrus that were most meaningful for the auditory and Wernicke areas (Figure 3A). The tumor was totally removed except for the region corresponding to the listening task-evoked high gamma activities in the middle temporal gyrus and was suspected to be a lower-grade glioma (Figure 3B).
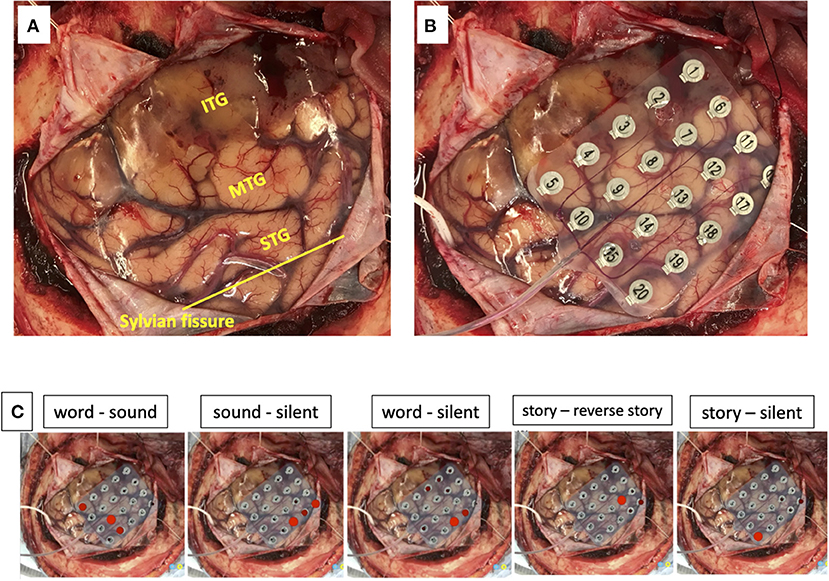
Figure 2. The exposed brain surface (A) and a 20-channel ECoG grid placement (B) before resection. Red bubble meaning significant listening task-evoked high gamma activities in each task during passive language mapping (C). STG, superior temporal gyrus; MTG, middle temporal gyrus; ITG, inferior temporal gyrus.
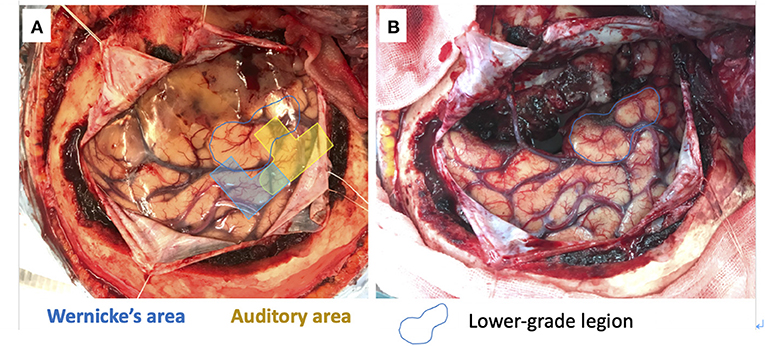
Figure 3. 5 channels in the superior temporal gyrus and one channel in the middle temporal gyrus meaning the auditory (yellow) and Wernicke (blue) areas (A). The gross tumor removal except for the lower-grade lesion in the middle temporal gyrus (blue circle) (B).
Postoperative Course
The patient's postoperative course was uneventful, and her symptoms of language comprehension difficulty improved, although, her naming disorder was unchanged. Postoperative MRI showed gross total resection of the enhancing component (Figure 4). A diagnosis of anaplastic oligodendroglioma (WHO grade 3) was made, and postoperative irradiation and chemotherapy (temozolomide) were performed. She was followed for 1 year without any new neurological deficits or radiological signs of recurrence.
Discussion
ECS has been the gold standard for functional mapping for decades (4). It is widely accepted that the application of conventional ECS methods produces specific and reliable outcomes at defined sites and that neurosurgical resective strategies guided by this method can eliminate or minimize sensorimotor and linguistic postoperative deficits (6, 7). Awake craniotomy is usually performed in patients aged 15–65 years, although, awake craniotomy is not only specified by age (8). Recently, there have been some reports that awake craniotomy can be successfully performed for elderly and younger patients (9–11); however, the procedure can be difficult to apply for patients with severe aphasia, intellectual disability, or psychiatric disorders, and elderly and younger patients who cannot tolerate awake craniotomy (12, 13). Furthermore, ECS has noteworthy limitations that include substantial time requirements (14, 15) and an increased risk for induced pathological brain activity, such as after-discharges or seizures (16, 17).
Gamma oscillation may be induced by synchronized activity of γ-aminobutyric acidergic (GABAergic) interneurons in the population network playing an important role in controlling the timing of action potential generation in pyramidal cells (18–21). Several researches have revealed that gamma-band power is positively correlated with neuronal firing rates (22–24).
Tamura et al. reported that the dynamics of oscillatory neuronal activity on ECoG have been proposed as potential neurophysiological indicators. Among these oscillatory changes, augmentation of high gamma activity from ~60 to 140 Hz was assumed to reflect localized cortical processing. Therefore, high gamma activity may be a strong candidate for an index of local cortical activation, and passive language mapping has possibilities of detecting physiological mechanisms related to language in the human brain (3).
Swift et al. reported that the qualitative comparison between cortical sites identified by ECoG and ECS shows a high concordance. However, the authors emphasized that it is important to evaluate language function by not only ECoG mapping but also other modalities, such as ECS, although, passive functional mapping using ECoG signals provides a fast, robust, and reliable method for identifying receptive language areas without many of the risks and limitations associated with ECS (4).
Wang et al. reported that electrode sites showing task-related high-gamma augmentation were spatially concordant with, but more extensive than, the language areas suggested by gold standard electrical stimulation mapping (25). Furthermore, Asano and Gotman reported that task-related high-gamma augmentation may be more extensive than electrical stimulation in localizing the cortex participating in language. As a result of this increased sensitivity, task-related high-gamma augmentation could involve a more extensive area than the region that, if removed, would result in clinically noteworthy language deficits (26). Therefore, we speculated that the possibility of postoperative language functional preservation can be higher when ECoG-positive regions are left intact. The reason for the improvement of the language comprehension could be the preservation and the decompression of the auditory and Wernicke areas. However, ECoG can be identified only in the region of the functional cortex, not white matter. Therefore, awake craniotomy and/or cortico-cortical evoked potential (CCEP) must be considered to reveal and preserve the language network (27).
It is noteworthy that passive language mapping was successfully performed under general anesthesia, and language function preservation was achieved postoperatively in the present case. Pre and postoperative language functions were evaluated using the Standard Language Test of Aphasia (SLTA) which is widely administered in Japan (28). The SLTA is a comprehensive Japanese language test battery including 26 subtests for language comprehension, speaking, reading comprehension, writing, and calculation abilities.
The influence of general anesthesia should be considered when passive functional mapping under general anesthesia is done. Suzuki et al. (29) reported that the amplitudes of corticocortical evoked potential were increased in accordance with BIS level, and there were statistically significant differences between the awake (>80 BIS level) and anesthesia (<65 BIS level) states. However, to our best knowledge, there were no articles about a correlation between passive functional mapping and the depth of general anesthesia. We speculated that functional language mapping can be affected by the depth of anesthesia, therefore, anesthesia was maintained with a high BIS level in the present case. Further, studies about the influences of anesthesia on passive functional mapping are necessary to evaluate the intraoperative passive functional mapping accurately.
A limitation of this case report is that we do not know how the sensitivity and specificity of ECoG compared with ECS were in this case because ECS was not performed. We presume that passive language mapping will be widely applicable for the detection of brain function with the least stress for patients. We believe that real-time passive language mapping can be one of the most powerful tools to detect the region of the receptive-language function in brain surgery, especially for those who are not able to tolerate awake craniotomy.
Conclusion
We performed real-time CortiQ-based passive language mapping under general anesthesia for an elderly patient with left temporal glioma. The patient's language function was well-preserved postoperatively. Further, studies are necessary to investigate the efficacy of intraoperative passive language mapping.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Author Contributions
KK, TM, TK, and SK contributed conception and design of the study. KK wrote the first draft of the manuscript. TM, TK, and SK critically revised the final manuscript draft. All authors contributed to manuscript revision, read and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.652401/full#supplementary-material
References
1. Arya R, Roth C, Leach JL, Middeler D, Wilson JA, Vannest J, et al. Neuropsychological outcomes after resection of cortical sites with visual naming associated electrocorticographic high-gamma modulation. Epilepsy Res. (2019) 151:17–23. doi: 10.1016/j.eplepsyres.2019.01.011
2. Kanno A, Mikuni N. Evaluation of language function under awake craniotomy. Neurol Med Chir. (2015) 55:367–73. doi: 10.2176/nmc.ra.2014-0395
3. Tamura Y, Ogawa H, Kapeller C, Prueckl R, Takeuchi F, Anei R, et al. Passive language mapping combining real-time oscillation analysis with cortico-cortical evoked potentials for awake craniotomy. J Neurosurg. (2016) 125:1580–8. doi: 10.3171/2015.4.JNS15193
4. Swift JR, Coon WG, Guger C, Brunner P, Bunch M, Lynch T, et al. Passive functional mapping of receptive language areas using electrocorticographic signals. Clin Neurophysiol. (2018) 129:2517–24. doi: 10.1016/j.clinph.2018.09.007
5. Kapeller C, Korostenskaja M, Prueckl R, Chen PC, Lee KH, Westerveld M, et al. CortiQ-based real-time functional mapping for epilepsy surgery. J Clin Neurophysiol. (2015) 32:e12–22. doi: 10.1097/WNP.0000000000000131
6. Haglund MM, Berger MS, Shamseldin M, Lettich E, Ojemann GA. Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery. (1994) 34:567–76. doi: 10.1227/00006123-199404000-00001
7. Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. (2008) 358:18–27. doi: 10.1056/NEJMoa067819
8. Kayama T. Guidelines committee of the Japan awake surgery conference. the guidelines for awake craniotomy guidelines committee of the Japan awake surgery conference. Neurol Med Chir. (2012) 52:119–41. doi: 10.2176/nmc.52.119
9. Grossman R, Nossek E, Sitt R, Hayat D, Shahar T, Barzilai O, et al. Outcome of elderly patients undergoing awake-craniotomy for tumor resection. Ann Surg Oncol. (2013) 20:1722–8. doi: 10.1245/s10434-012-2748-x
10. Lohkamp LN, Mottolese C, Szathmari A, Huguet L, Beuriat PA, Christofori I, et al. Awake brain surgery in children-review of the literature and state-of-the-art. Childs Nerv Syst. (2019) 35:2071–7. doi: 10.1007/s00381-019-04279-w
11. Lohkamp LN, Beuriat PA, Desmurget M, Cristofori I, Szathmari A, Huguet L, et al. Awake brain surgery in children-a single-center experience. Childs Nerv Syst. (2020) 36:967–74. doi: 10.1007/s00381-020-04522-9
12. Chitoku S, Otsubo H, Harada Y, Jay V, Rutka JT, Weiss SK, et al. Extraoperative cortical stimulation of motor function in children. Pediatr Neurol. (2001) 24:344–50. doi: 10.1016/S0887-8994(01)00264-8
13. Korostenskaja M, Chen PC, Salinas CM, Westerveld M, Brunner P, Schalk G, et al. Real-time functional mapping: potential tool for improving language outcome in pediatric epilepsy surgery. J Neurosurg Pediatr. (2014) 14:287–95. doi: 10.3171/2014.6.PEDS13477
14. Hamberger MJ. Cortical language mapping in epilepsy: a critical review. Neuropsychol Rev. (2007) 17:477–89. doi: 10.1007/s11065-007-9046-6
15. Ritaccio AL, Brunner P, Schalk G. Electrical stimulation mapping of the brain: basic principles and emerging alternatives. J Clin Neurophysiol. (2018) 35:86–97. doi: 10.1097/WNP.0000000000000440
16. Qian T, Zhou W, Ling Z, Gao S, Liu H, Hong B. Fast presurgical functional mapping using task-related intracranial high gamma activity. J Neurosurg. (2013) 119:26–36. doi: 10.3171/2013.2.JNS12843
17. Corley JA, Nazari P, Rossi VJ, Kim NC, Fogg LF, Hoeppner TJ, et al. Cortical stimulation parameters for functional mapping. Seizure. (2017) 45:36–41. doi: 10.1016/j.seizure.2016.11.015
18. Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. (1995) 378:75–8. doi: 10.1038/378075a0
19. Fries P, Nikolić D, Singer W. The gamma cycle. Trends Neurosci. (2007) 30:309–16. doi: 10.1016/j.tins.2007.05.005
20. Hasenstaub A, Shu Y, Haider B, Kraushaar U, Duque A, McCormick DA. Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron. (2005) 47:423–35. doi: 10.1016/j.neuron.2005.06.016
21. Traub RD, Cunningham MO, Gloveli T, LeBeau FE, Bibbig A, Buhl EH, et al. GABA-enhanced collective behavior in neuronal axons underlies persistent gamma-frequency oscillations. Proc Natl Acad Sci U S A. (2003) 100:11047–52. doi: 10.1073/pnas.1934854100
22. Manning JR, Jacobs J, Fried I, Kahana MJ. Broadband shifts in local field potential power spectra are correlated with singleneuron spiking in humans. J Neurosci. (2009) 29:13613–20. doi: 10.1523/JNEUROSCI.2041-09.2009
23. Nir Y, Fisch L, Mukamel R, Gelbard-Sagiv H, Arieli A, Fried I, et al. Coupling between neuronal firing rate, gamma LFP, and BOLD fMRI is related to interneuronal correlations. Curr Biol. (2007) 17:1275–85. doi: 10.1016/j.cub.2007.06.066
24. Ray S, Crone NE, Niebur E, Franaszczuk PJ, Hsiao SS. Neural correlates of high-gamma oscillations (60–200 Hz) in macaque local field potentials and their potential implications in electrocorticography. J Neurosci. (2008) 28:11526–36. doi: 10.1523/JNEUROSCI.2848-08.2008
25. Wang Y, Fifer MS, Flinker A, Korzeniewska A, Cervenka MC, Anderson WS, et al. Spatial-temporal functional mapping of language at the bedside with electrocorticography. Neurology. (2016) 86:1181–9. doi: 10.1212/WNL.0000000000002525
26. Asano E, Gotman J. Is electrocorticography-based language mapping ready to replace stimulation? Neurology. (2016) 86:1174–6. doi: 10.1212/WNL.0000000000002533
27. Saito T, Tamura M, Muragaki Y, Maruyama T, Kubota Y, Fukuchi S, et al. Intraoperative cortico-cortical evoked potentials for the evaluation of language function during brain tumor resection: initial experience with 13 cases. J Neurosurg. (2014) 121:827–38. doi: 10.3171/2014.4.JNS131195
28. Brain Function Test Committee. Japan Society for Higher Brain Dysfunction. The Standard Language Test of Aphasia. Tokyo: Sinkoh Igaku Shuppansha (2003).
Keywords: cortiq, awake craniotomy, intraoperative passive language mapping, real-time, high-gamma activity, glioma
Citation: Kanaya K, Mitsuhashi T, Kiuchi T and Kobayashi S (2021) The Efficacy of Intraoperative Passive Language Mapping for Glioma Surgery: A Case Report. Front. Neurol. 12:652401. doi: 10.3389/fneur.2021.652401
Received: 12 January 2021; Accepted: 09 July 2021;
Published: 02 August 2021.
Edited by:
Carlo Alberto Artusi, University of Turin, ItalyReviewed by:
Michael D. Staudt, Oakland University William Beaumont School of Medicine, United StatesJens Gempt, Technische Universität München, Germany
Alberto Romagnolo, University of Turin, Italy
Copyright © 2021 Kanaya, Mitsuhashi, Kiuchi and Kobayashi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kohei Kanaya, a2FuYXlhQHNoaW5zaHUtdS5hYy5qcA==
 Kohei Kanaya
Kohei Kanaya Takumi Mitsuhashi
Takumi Mitsuhashi Takafumi Kiuchi2
Takafumi Kiuchi2 Sumio Kobayashi
Sumio Kobayashi