- 1Vrije Universiteit Brussel (VUB), Universitair Ziekenhuis Brussel (UZ Brussel), Department of Neurology/Center for Neurosciences, Brussels, Belgium
- 2Centre Hospitalier Universitaire Brugmann (CHU Brugmann), Department of Neurology, Brussels, Belgium
- 3Department of Neurology, Sint-Maria Halle, Halle, Belgium
- 4Faculty of Medicine and Pharmacy, Vrije Universiteit Brussel, Brussels, Belgium
Background: A high Neutrophil-to-Lymphocyte ratio (NLR) in patients with acute ischemic stroke (AIS) has been associated with post-stroke infections, but it's role as an early predictive biomarker for post-stroke pneumonia (PSP) and urinary tract infection (UTI) is not clear.
Aim: To investigate the usefulness of NLR obtained within 24 h after AIS for predicting PSP and UTI in the first week.
Methods: Clinical and laboratory data were retrieved from the University Hospital Brussels stroke database/electronic record system. Patients were divided into those who developed PSP or UTI within the first week after stroke onset and those who didn't. Receiver operating characteristics (ROC) curves and logistic regression analysis were used to identify independent predictors.
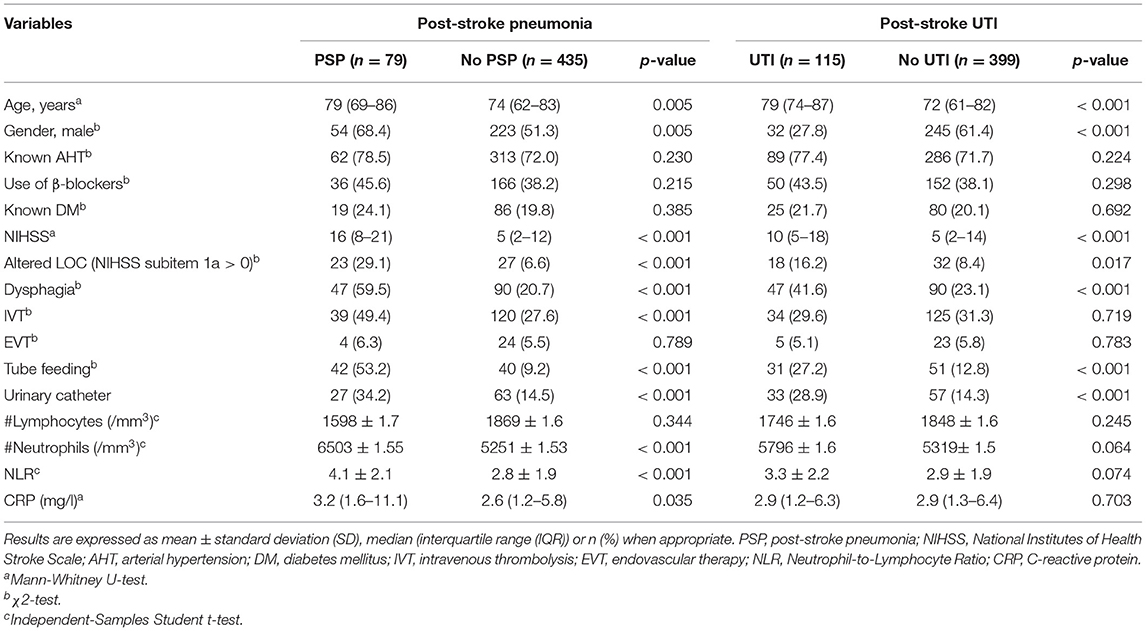
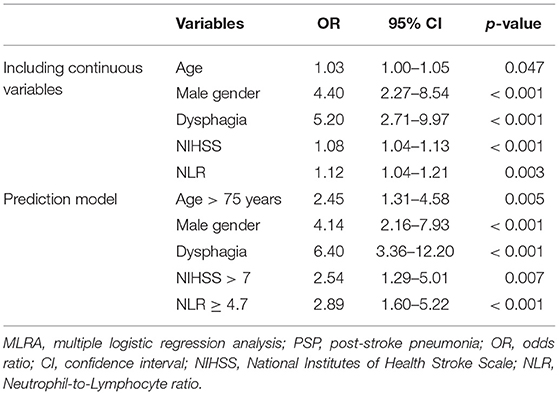
Results: Five hundred and fourteen patients were included, of which 15.4% (n = 79) developed PSP and 22% (n = 115) UTI. In univariate analysis, NLR was significantly higher in patients who developed PSP (4.1 vs. 2.8, p < 0.001) but not in those who developed UTI (3.3 vs. 2.9, p = 0.074). Multiple logistic regression analysis for PSP showed that NLR, male gender, dysphagia, and stroke severity measured by the National Institutes of Health Stroke Scale (NIHSS), were independent predictors of PSP. For NLR alone, the area under the curve (AUC) in the ROC curve was 0.66 (95% CI = 0.59–0.73). When combining NLR ≥ 4.7 with age >75 years, male gender, NIHSS > 7, and dysphagia, the AUC increased to 0.84 (95% CI = 0.79–0.89).
Conclusion: The NLR within 24 h after AIS appears to have no predictive value for post-stroke UTI, and is only a weak predictor for identifying patients at high risk for PSP. Its predictive value for PSP appears to be much stronger when incorporated in a prediction model including age, gender, NIHSS score, and dysphagia.
Introduction
Pneumonia and urinary tract infections (UTI) are the most common infectious complications after acute ischemic stroke (AIS), with an incidence of 12 and 16%, respectively (1). Post-stroke infections have been associated with poor outcome and mortality (2, 3). Therefore, there is an interest in finding early predictors of these post-stroke infections, which may help to select high-risk patients to start interventions in time. Most prediction scoring models for post-stroke pneumonia (PSP) are based on clinical features including age, gender, stroke severity measured by the National Institutes of Health Stroke Scale (NIHSS) (4) and the presence of dysphagia (5–9). A recent meta-analysis showed that age, female gender and post-void residual volume >100 ml were predictors of post-stroke UTI (10).
Next to clinical factors, a number of inflammatory parameters including C-reactive protein (CRP), white blood cell count, procalcitonin and copeptin (11), interleukin-13 and interferon-γ (12), elevated monocyte count and interleukin-10 (13), and high circulating natural killer cell count within the first hours after stroke followed by a drop in all lymphocyte subsets (14) have been associated with post-stroke infections. However, it is unclear how these parameters should be applied in clinical practice.
A biomarker, which has gained interest over the last years, is the Neutrophil-to-Lymphocyte Ratio (NLR). It is a marker of inflammation that is simply calculated from blood cell counts obtained on admission in every AIS patient. Nam et al. (15) found that a higher NLR in patients with AIS who were admitted within 7 days of symptoms onset independently predicted PSP during that 7-day period. Wang et al. (16) found that the NRL at multiple time points with a peak at 36 h after stroke onset was independently associated with PSP but not with UTI. The NLR on admission was not used separately in their study. Three other studies in patients with AIS in whom blood was collected within 24 h of symptom onset did not discriminate between PSP, UTI, and other infections. Two of them found that a higher NLR was independently associated with post stroke infections (17, 18), whereas the third study could not confirm this association (19).
Since most of these infections already manifest within the first days after AIS, we wanted to investigate the predictive value of NLR obtained on admission within 24 h after stroke onset for PSP and UTI separately.
Materials and Methods
Patients and Assessment Procedures
We extracted the data of 1,457 patients admitted to the Stroke Unit of the University Hospital Brussels (Belgium), which were prospectively collected in a database over a 6-year period. We included all patients with AIS, who had routine blood sampling within 24 h after stroke onset. AIS was defined as “a sudden onset of loss of global or focal cerebral function” (20) caused by brain ischemia of any origin, confirmed on cerebral computed tomography, or magnetic resonance imaging. Exclusion criteria were previous hematologic, inflammatory or autoimmune disorders, current cancer, infections preceding stroke, use of antibiotics <24 h before admission, use of immunosuppressants on admission, recent surgery, and stroke related death and/or palliative care started <48 h after stroke onset. A study population flowchart is shown in Figure 1. Demographic data (age, gender), medical history, use of beta-blockers prior to admission, pre-stroke modified Rankin Scale (mRS), NIHSS on admission, level of consciousness (LOC, determined by NIHSS subitem 1a) and information concerning intravenous thrombolysis (IVT) and endovascular therapy (EVT) were retrieved from the database. Dysphagia objectified by a professional speech therapist, nasogastric tube feeding, urinary catheter placement, and results of baseline blood measures (absolute neutrophil count, absolute lymphocyte count and CRP) were retrieved from the electronic record system.
Standard Protocol Approval
The study protocol was approved by the Ethics Committee of the University Hospital of Brussels (reference number B.U.N. 143201733949).
Neutrophil-to-Lymphocyte Ratio
The NLR was defined as the ratio of the absolute neutrophil count to the absolute lymphocyte count, which were counted in the peripheral blood sample on admission by use of fluorescent flowcytometric measurements (CELL-DYN Sapphire, Abbott Diagnostics, Abbott Park, IL) (14, 21).
Post-stroke Pneumonia
PSP during the first week after stroke onset was retrospectively diagnosed using Modified criteria of the US Center for Disease Control and Prevention: “at least one of the former and one of the latter criteria fulfilled: (A) abnormal respiratory examination, pulmonary infiltrates on chest x-rays; (B) productive cough with purulent sputum, microbiological cultures from lower respiratory tract or blood cultures, leukocytosis, elevated CRP” (14, 22).
Post-stroke UTI
UTI during the first week after stroke onset was retrospectively diagnosed and defined as having at least 2 of the 4 following criteria: urine sample positive for nitrite, urine culture with >100.0000 colonies/ml, urine culture with >25 white blood cells/μl or body temperature >38°C (22).
Statistics
Statistical analyses were performed using SPSS version 27.0 software package. Patients were divided into those who developed PSP/UTI and those who didn't. Normality was checked by using the Kolmogorov-Smirnov test and visual interpretation of histograms and Q-Q plots. Skewed variables were log-transformed to reach normality. Differences were detected using the Independent-Samples Student T-test (with back-transformation of the results, if applicable) and the Mann-Whitney U-test for continuous variables. The χ2- or Fisher Exact-test were used for categorical variables. Age and NIHSS on admission were dichotomized by using the values of the 50% percentile as cut-off. For NLR, the 75% percentile was used. Variables of clinical interest were enrolled in multiple logistic regression analysis (MLRA). The stepwise Backward Wald method and ROC curves were used to identify independent predictors. Variables most accessible on admission were combined to create a prediction model.
Results
Baseline Characteristics
Five hundred and fourteen patients met the selection criteria, of whom 15% (n = 79) developed PSP and 22% (n = 115) developed UTI (Figure 1). Table 1 presents the baseline characteristics of patients with PSP vs. without PSP, and of patients with post-stroke UTI vs. without post-stroke UTI.
Post-stroke Pneumonia
In univariate analysis, age, male gender, NIHSS, altered LOC, treatment with IVT, dysphagia, tube feeding and urinary catheter placement were associated with PSP (p < 0.05). Patients who developed PSP had significantly lower lymphocyte counts on admission. CRP, neutrophil count, and NLR within 24 h after stroke onset were significantly higher in patients with vs. without PSP. The NLR was not significantly different between patients who developed PSP during the first 3 days (71% of PSP cases) of admission and those who developed PSP between day 4 and 7 (29% of cases) of admission (4.29 ± 2.07 vs. 3.59 ± 1.99 respectively, p = 0.320). Of all patients, 145 patients were discharged before day 7. The mean length of their hospital stay was 4.75 ± 1.2 days, which exceeded the mean time to onset of PSP of 2.9 ± 1.7 days for the entire study population. The mean time to event did not significantly differ between patients who had a hospital stay of 7 days or more vs. those who were discharged before day 7 (3.0 ± 1.8 vs. 2.4 ± 1.2 days, p = 0.301).
Post-stroke UTI
In univariate analysis, age, female gender, pre-stroke mRS, NIHSS, dysphagia, tube feeding, altered LOC, and urinary catheter placement were associated with post-stroke UTI (p < 0.05). NLR within 24 h after stroke onset was not significantly higher in patients with post-stroke UTI compared to patients without post-stroke UTI. The NLR was not predictive in both patients discharged before day 7 and those who stayed for 7 days or more.
Multiple Logistic Regression
Since NLR was not significant in univariate analysis for UTI, we opted to perform multivariate analysis for PSP only. The following variables were enrolled in MLRA: age, gender, smoking, chronic obstructive pulmonary disease (COPD), diabetes mellitus (DM), NIHSS, LOC, dysphagia, and NLR. The results indicated that NLR, next to age, male gender, NIHSS on admission, and dysphagia, was an independent predictor of PSP (Table 2). To create a more easy-to-use prediction model, we dichotomized “NIHSS on admission” and “age” by using the 50% percentile values as cut-offs, which were >7 and >75 years, respectively. The cut-off for NLR was determined by the 75% percentile value, which was ≥4.7. Based on the results of the first multivariate analysis and the clinical usefulness of the variables, we repeated MLRA using the following variables: age > 75 years, male gender, dysphagia, NIHSS > 7 and NLR ≥ 4.7, which shows a significant predictive value for each of these variables when using this model (Table 2).
ROC Curve Analyses
For NLR, age, NIHSS, and male gender, AUC was to 0.66 (95% CI = 0.59–0.73), 0.60 (95% CI = 0.53–0.66), 0.75 (95% CI = 0.68–0.81) and 0.59 (95% CI = 0.52–0.66), respectively (see Figure 2). For the dichotomized variables, NLR ≥ 4.7, age > 75 years, and NIHSS > 7, AUC was 0.64 (95% CI = 0.56–0.71), 0.58 (95% CI = 0.50–0.65), and 0.68 (95% CI = 0.62–0.75), respectively (Figure 2). For a 5-item prediction model, which combines age > 75, male gender, dysphagia, NIHSS > 7, and NLR ≥ 4.7, AUC was 0.84 (95% CI = 0.79–0.89) (Figure 2).
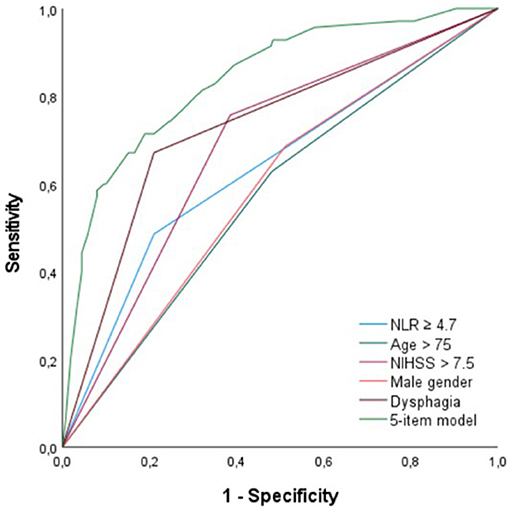
Figure 2. ROC curve analysis for NLR ≥ 4.7, age > 75 years, NIHSS > 7, male gender, dysphagia, and a 5-item prediction model (NLR ≥ 4.7, age > 75 years, dysphagia, NIHSS > 7, male gender). ROC, receiver operating characteristics; NLR, neutrophil-to-lymphocyte ratio; NIHSS, National Institutes of Health Stroke Scale.
Discussion
Previous studies have shown that NLR is a predictor of poor functional outcome and mortality after AIS, but the underlying mechanisms remain unclear (20, 23–26). Two studies found a link between the NLR and post-stroke infections but they lack information about the location of the infection (17, 18). A study by Nam et al. (15) found that a NLR cut-off value >2.43, which was based on the median of their cohort, was an independent predictor of PSP. However, NLR was determined within 7 days of stroke onset instead of 24 h. In another study, a higher NLR at different time points post-stroke, with a peak value at 36 h, has also been associated with post-stroke infection, and more specific PSP (16). A study of van Gemmeren did not show an independent predictive value of the 24 h NLR for PSP, but because numbers were small the study was likely underpowered to detect such an effect (19).
Our results provide additional evidence for NLR as a significant and independent predictor for PSP, although, its predictive value appears to be quite weak. ROC curve analysis of NLR alone showed an AUC of 0.66 (95% CI = 0.59–0.73). This could be explained by the fact that immunological changes are only one of the mechanisms leading to PSP. Our results further showed that age, male gender, dysphagia, and stroke severity (NIHSS) were, albeit also weak, independent predictors of PSP, which is in line with previous studies (5–9, 27–29). Because of its rather low predictive value for PSP, we reperformed MLRA with only dichotomized variables, to make it more clinically useful. Based on the results of our first multivariate analysis and the immediate availability upon admission of the enrolled clinical variables, we created a 5-item prediction model using NLR ≥ 4.7, age > 75 years, male gender, dysphagia, and NIHSS > 7. In this model, the AUC increased to 0.84 (95% CI = 0.79–0.89), indicating that NLR is especially useful in predicting PSP when incorporated into a model with these four clinical predictive factors.
The NLR was not significantly different in patients who developed PSP within 3 days of admission and those who developed PSP during day 4–7 after admission, suggesting that a high admission NLR is not solely due to an inflammatory response caused by aspiration, or a pneumonia, that was already started on admission.
Our study found that NLR within 24 h after stroke onset was not a significant predictor of UTI. This confirms the findings of Wang et al. (16) who also did not find a significantly higher NLR in patients with post-stroke UTI, although, they did not use the NLR on admission. A plausible explanation why NLR is predictive for PSP but not for UTI, is that the underlying mechanisms of these infections are at least partially different. After AIS, neutrophil counts increase and lymphocyte counts decrease (28, 30, 31) as part of the post-stroke immunodepression phenomenon, activated by the sympathetic nervous system and hypothalamic-pituitary-adrenal axis (30, 32). This may be a mechanism to prevent further damage by reducing local brain inflammation. The role of neutrophils and lymphocytes seems to be dual, with both beneficial and harmful effects (3, 14, 31, 33). The NLR could be used to estimate the degree to which this post-stroke immunodepression occurs, with a higher NLR suggestive of a more pronounced immunodepression. Since both NLR and pneumonia have been associated with poor prognosis after ischemic stroke (20, 24, 34–36), we hypothesize that a higher degree of immunodepression makes patients more susceptible to systemic infections, such as pneumonia, leading to a worse outcome. Preclinical evidence shows that mice subjected to ischemic stroke were more susceptible to spontaneous bacteriemia and pneumonia compared to mice who underwent sham procedure (37). An explanation might be that the post-stroke immunodepression phenomenon favors bacterial translocation and dissemination of commensal bacteria from the host gut microbiota, leading to systemic infections (38). Whereas, these mechanisms might contribute to PSP, the occurrence of post-stroke UTI seems to rather depend on other factors. Urinary tract infections, which can be seen as rather local than systemic infections, seem to be mainly explained by mechanical factors such as bladder dysfunction causing urinary retention (39), use of urinary catheter (29, 40) and the presence of a short urethra (female predominance). In addition, they are less clearly associated with worse prognosis after ischemic stroke, since although preventive antibiotics reduced UTI frequency in the PASS-study, no effect was seen on outcome (36).
It has been hypothesized that sympathetic nervous system activation might be one of the underlying mechanisms of post-stroke immunodepression, and that therefore beta-blockers might theoretically prevent post-stroke infections (32). In mice, blockade of the sympathetic pathways by beta-blockers reduced post-stroke infections and improved stroke outcome (41). However, in human studies, results have been conflicting. Sykora et al. (42) reported that pre-stroke and on-stroke beta-blocker treatment reduced PSP frequency. On the other hand, Maier and coworkers reported that beta-blocker exposure had no effect on PSP frequency, but that it reduced UTI rates (43, 44). Dromerick et al. (45) found the use of beta-blockers to be a predictor of post-stroke UTI. In our study, we did not find an association between beta-blocker use prior to AIS and PSP or UTI.
There are some limitations to this study. First, although data were gathered prospectively, the diagnosis of PSP and UTI was checked retrospectively, which could have caused some diagnostic errors. By using the modified CDC criteria for retrospective diagnosis of pneumonia, a positive chest x-ray was not necessary to reach diagnostic criteria. Therefore, diagnosis could also be made based on clinical features only, which might have decreased diagnostic accuracy. Second, the NLR was only investigated for its predictive role regarding PSP/UTI. It is possible that patients developed other infectious or inflammatory complications that might have influenced NLR. Third, we did not intend to exclude patients discharged before day 7, as we wanted to explore the role of NLR and the subsequent combined model in a situation consistent with real-life in which we do not know in advance how long patients will stay. We may have missed a number of cases with PSP and UTI in patients who were discharged before day 7. However, because the majority of patients (72%) was hospitalized for 7 days or more, it is unlikely that this will affect our main conclusions. In addition, for UTI, the NLR was not predictive in both patients discharged before day 7 and those who stayed for 7 days or more. For PSP, we found that the mean length of hospital stay for those discharged before day 7 exceeded the mean time to onset of PSP, which is usually within the first 2 to 3 days after stroke onset.
Prospective studies are required to investigate whether our proposed prediction model, which incorporates NLR, too can, with a high degree of certainty, identify patients prone to develop PSP, who may therefore be candidates for prophylactic measures. Prophylactic antibiotic treatment significantly decreases overall post-stroke infection rate, but its effect on reducing the incidence of PSP has not been established (46). Identifying patients at risk will lead to a better selection of patients who could benefit from this kind of treatment. In addition, new therapeutic approaches other than prophylactic antibiotic administration, such as treatment of the underlying mechanisms of post-stroke immunodepression, should be considered (32).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Commissie Medische Ethiek UZ Brussel (reference number B.U.N. 143201733949). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
RG: design and conceptualized study, major role in the acquisition of data, analyzed the data, and drafted the manuscript for intellectual content. AO: design and conceptualized study, major role in the acquisition of data, analyzed the data, and drafted the manuscript for intellectual content. AD: major role in the acquisition of data and revised the manuscript for intellectual content. JD: revised the manuscript for intellectual content. SD: design and conceptualized study, major role in the acquisition of data, and drafted the manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We gratefully acknowledge the advice on the statistical analysis of the data by Kurt Barbé (VUB).
References
1. Davenport RJ, Dennis MS, Wellwood I, Warlow CP. Complications after acute stroke. Stroke. (1996) 27:415–20. doi: 10.1161/01.STR.27.3.415
2. Aslanyan S, Weir CJ, Diener HC, Kaste M, Lees KR, GAIN International Steering Committee and Investigators. Pneumonia and urinary tract infection after acute ischaemic stroke: a tertiary analysis of the GAIN International trial. Eur J Neurol. (2004) 11:49–53. doi: 10.1046/j.1468-1331.2003.00749.x
3. Emsley HC, Hopkins SJ. Acute ischaemic stroke and infection: recent and emerging concepts. Lancet Neurol. (2008) 7:341–53. doi: 10.1016/S1474-4422(08)70061-9
4. Kwah LK, Diong J. National Institutes of Health Stroke Scale (NIHSS). J Physiother. (2014) 60:61. doi: 10.1016/j.jphys.2013.12.012
5. Smith CJ, Bray BD, Hoffman A, Meisel A, Heuschmann PU, Wolfe CD, et al. Can a novel clinical risk score improve pneumonia prediction in acute stroke care? A UK multicenter cohort study. J Am Heart Assoc. (2015) 4:e001307. doi: 10.1161/JAHA.114.001307
6. Hoffmann S, Malzahn U, Harms H, Koennecke HC, Berger K, Kalic M, et al. Development of a clinical score (A2DS2) to predict pneumonia in acute ischemic stroke. Stroke. (2012) 43:2617–23. doi: 10.1161/STROKEAHA.112.653055
7. Ji R, Shen H, Pan Y, Wang P, Liu G, Wang Y, et al. Novel risk score to predict pneumonia after acute ischemic stroke. Stroke. (2013) 44:1303–9. doi: 10.1161/STROKEAHA.111.000598
8. Kwon HM, Jeong SW, Lee SH, Yoon BW. The pneumonia score: a simple grading scale for prediction of pneumonia after acute stroke. Am J Infect Control. (2006) 34:64–8. doi: 10.1016/j.ajic.2005.06.011
9. Chumbler NR, Williams LS, Wells CK, Lo AC, Nadeau S, Peixoto AJ, et al. Derivation and validation of a clinical system for predicting pneumonia in acute stroke. Neuroepidemiology. (2010) 34:193–9. doi: 10.1159/000289350
10. Yan T, Liu C, Li Y, Xiao W, Wang S. Prevalence and predictive factors of urinary tract infection among patients with stroke: a meta-analysis. Am J Infect Control. (2018) 46:402–9. doi: 10.1016/j.ajic.2017.10.001
11. Fluri F, Morgenthaler NG, Mueller B, Christ-Crain M, Katan M. Copeptin, procalcitonin, and routine inflammatory markers-predictors of infection after stroke. PLoS ONE. (2012) 7:e48309. doi: 10.1371/journal.pone.0048309
12. Salat D, Penalba A, García-Berrocoso T, Campos-Martorell M, Flores A, Pagola J, et al. Immunological biomarkers improve the accuracy of clinical risk models of infection in the acute phase of ischemic stroke. Cerebrovasc Dis. (2013) 35:220–7. doi: 10.1159/000346591
13. Chamorro A, Amaro S, Vargas M, Obach V, Cervera A, Torres F, et al. Interleukin 10, monocytes and increased risk of early infection in ischaemic stroke. J Neurol Neurosurg Psychiatry. (2006) 77:1279–81. doi: 10.1136/jnnp.2006.100800
14. De Raedt S, De Vos A, Van Binst AM, De Waele M, Coomans D, Buyl R, et al. High natural killer cell number might identify stroke patients at risk of developing infections. Neurol Neuroimmunol Neuroinflamm. (2015) 2:e71. doi: 10.1212/NXI.0000000000000071
15. Nam KW, Kim TJ, Lee JS, Kwon HM, Lee YS, Ko SB, et al. High neutrophil-to-lymphocyte ratio predicts stroke-associated pneumonia. Stroke. (2018) 49:1886–92. doi: 10.1161/STROKEAHA.118.021228
16. Wang L, Guo W, Wang C, Yang X, Hao Z, Wu S, et al. Dynamic change of neutrophil to lymphocyte ratios and infection in patients with acute ischemic stroke. Curr Neurovasc Res. (2020) 17:294–303. doi: 10.2174/1567202617666200408091131
17. He L, Wang J, Wang F, Zhang L, Zhao W. Increased neutrophil-to-lymphocyte ratio predicts the development of post-stroke infections in patients with acute ischemic stroke. BMC Neurol. (2020) 20:328. doi: 10.1186/s12883-020-01914-x
18. Lan Y, Sun W, Chen Y, Miao J, Li G, Qiu X, et al. Nomogram including neutrophil-to-lymphocyte ratio for the prediction of stroke-associated infections. Front Neurol. (2020) 11:574280. doi: 10.3389/fneur.2020.574280
19. van Gemmeren T, Schuppner R, Grosse GM, Fering J, Gabriel MM, Huber R, et al. Early post-stroke infections are associated with an impaired function of neutrophil granulocytes. J Clin Med. (2020) 9:872. doi: 10.3390/jcm9030872
20. Fang YN, Tong MS, Sung PH, Chen YL, Chen CH, Tsai NW, et al. Higher neutrophil counts and neutrophil-to-lymphocyte ratio predict prognostic outcomes in patients after non-atrial fibrillation-caused ischemic stroke. Biomed J. (2017) 40:154–62. doi: 10.1016/j.bj.2017.03.002
21. Nous A, Peeters I, Nieboer K, Vanbinst AM, De Keyser J, De Raedt S. Post-stroke infections associated with spleen volume reduction: a pilot study. PLoS ONE. (2020) 15:e0232497. doi: 10.1371/journal.pone.0232497
22. Harms H, Prass K, Meisel C, Klehmet J, Rogge W, Drenckhahn C, et al. Preventive antibacterial therapy in acute ischemic stroke: a randomized controlled trial. PLoS ONE. (2008) 3:e2158. doi: 10.1371/journal.pone.0002158
23. Celikbilek A, Ismailogullari S, Zararsiz G. Neutrophil to lymphocyte ratio predicts poor prognosis in ischemic cerebrovascular disease. J Clin Lab Anal. (2014) 28:27–31. doi: 10.1002/jcla.21639
24. Tokgoz S, Keskin S, Kayrak M, Seyithanoglu A, Ogmegul A. Is neutrophil/lymphocyte ratio predict to short-term mortality in acute cerebral infarct independently from infarct volume? J Stroke Cerebrovasc Dis. (2014) 23:2163–8. doi: 10.1016/j.jstrokecerebrovasdis.2014.04.007
25. Xue J, Huang W, Chen X, Li Q, Cai Z, Yu T, et al. Neutrophil-to-Lymphocyte ratio is a prognostic marker in acute ischemic stroke. J Stroke Cerebrovasc Dis. (2017) 26:650–7. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.010
26. Tokgoz S, Kayrak M, Akpinar Z, Seyithanoglu A, Güney F, Yürüten B. Neutrophil lymphocyte ratio as a predictor of stroke. J Stroke Cerebrovasc Dis. (2013) 22:1169–74. doi: 10.1016/j.jstrokecerebrovasdis.2013.01.011
27. Walter U, Knoblich R, Steinhagen V, Donat M, Benecke R, Kloth A. Predictors of pneumonia in acute stroke patients admitted to a neurological intensive care unit. J Neurol. (2007) 254:1323–9. doi: 10.1007/s00415-007-0520-0
28. Haeusler KG, Schmidt WU, Föhring F, Meisel C, Helms T, Jungehulsing GJ, et al. Cellular immunodepression preceding infectious complications after acute ischemic stroke in humans. Cerebrovasc Dis. (2008) 25:50–8. doi: 10.1159/000111499
29. Wästfelt M, Cao Y, Ström JO. Predictors of post-stroke fever and infections: a systematic review and meta-analysis. BMC Neurol. (2018) 18:49. doi: 10.1186/s12883-018-1046-z
30. Shim R, Wong CH. Ischemia, immunosuppression and infection–tackling the predicaments of post-stroke complications. Int J Mol Sci. (2016) 17:64. doi: 10.3390/ijms17010064
31. Ruhnau J, Schulze J, Dressel A, Vogelgesang A. Thrombosis, neuroinflammation, and poststroke infection: the multifaceted role of neutrophils in stroke. J Immunol Res. (2017) 2017:5140679. doi: 10.1155/2017/5140679
32. De Raedt S, De Vos A, De Keyser J. Autonomic dysfunction in acute ischemic stroke: an underexplored therapeutic area? J Neurol Sci. (2015) 348:24–34. doi: 10.1016/j.jns.2014.12.007
33. Ruhnau J, Schulze K, Gaida B, Langner S, Kessler C, Bröker B, et al. Stroke alters respiratory burst in neutrophils and monocytes. Stroke. (2014) 45:794–800. doi: 10.1161/STROKEAHA.113.003342
34. Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. (2011) 11:110. doi: 10.1186/1471-2377-11-110
35. Qun S, Tang Y, Sun J, Liu Z, Wu J, Zhang J, et al. Neutrophil-to-lymphocyte ratio predicts 3-month outcome of acute ischemic stroke. Neurotox Res. (2017) 31:444–52. doi: 10.1007/s12640-017-9707-z
36. Westendorp WF, Vermeij JD, Zock E, Hooijenga IJ, Kruyt ND, Bosboom HJ, et al. The Preventive Antibiotics in Stroke Study (PASS): a pragmatic randomised open-label masked endpoint clinical trial. Lancet. (2015) 385:1519–26. doi: 10.1016/S0140-6736(14)62456-9
37. Prass K, Meisel C, Höflich C, Braun J, Halle E, Wolf T, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. (2003) 198:725–36. doi: 10.1084/jem.20021098
38. Stanley D, Mason LJ, Mackin KE, Srikhanta YN, Lyras D, Prakash MD, et al. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat Med. (2016) 22:1277–84. doi: 10.1038/nm.4194
39. Kong KH, Young S. Incidence and outcome of poststroke urinary retention: a prospective study. Arch Phys Med Rehabil. (2000) 81:1464–7. doi: 10.1053/apmr.2000.9630
40. Poisson SN, Johnston SC, Josephson SA. Urinary tract infections complicating stroke: mechanisms, consequences, and possible solutions. Stroke. (2010) 41:e180–4. doi: 10.1161/STROKEAHA.109.576413
41. Shi K, Wood K, Shi FD, Wang X, Liu Q. Stroke-induced immunosuppression and poststroke infection. Stroke Vasc Neurol. (2018) 3:34–41. doi: 10.1136/svn-2017-000123
42. Sykora M, Siarnik P, Diedler J, VISTA Acute Collaborators. β-Blockers, pneumonia, and outcome after ischemic stroke: evidence from virtual international stroke trials archive. Stroke. (2015) 46:1269–74. doi: 10.1161/STROKEAHA.114.008260
43. Maier IL, Karch A, Mikolajczyk R, Bähr M, Liman J. Effect of beta-blocker therapy on the risk of infections and death after acute stroke–a historical cohort study. PLoS ONE. (2015) 10:e0116836. doi: 10.1371/journal.pone.0116836
44. Dziedzic T, Slowik A, Pera J, Szczudlik A. Beta-blockers reduce the risk of early death in ischemic stroke. J Neurol Sci. (2007) 252:53–6. doi: 10.1016/j.jns.2006.10.007
45. Dromerick AW, Edwards DF. Relation of postvoid residual to urinary tract infection during stroke rehabilitation. Arch Phys Med Rehabil. (2003) 84:1369–72. doi: 10.1016/S0003-9993(03)00201-6
Keywords: acute ischemic stroke, post-stroke pneumonia, post-stroke urinary tract infection, post-stroke infections, neutrophil-to-lymphocyte ratio
Citation: Gens R, Ourtani A, De Vos A, De Keyser J and De Raedt S (2021) Usefulness of the Neutrophil-to-Lymphocyte Ratio as a Predictor of Pneumonia and Urinary Tract Infection Within the First Week After Acute Ischemic Stroke. Front. Neurol. 12:671739. doi: 10.3389/fneur.2021.671739
Received: 24 February 2021; Accepted: 19 April 2021;
Published: 13 May 2021.
Edited by:
Timo Uphaus, Johannes Gutenberg University Mainz, GermanyReviewed by:
David J. Seiffge, University Hospital Bern, SwitzerlandDaniel Richter, Ruhr University Bochum, Germany
Copyright © 2021 Gens, Ourtani, De Vos, De Keyser and De Raedt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robin Gens, cm9iaW4uZ2Vuc0B2dWIuYmU=
†These authors have contributed equally to this work and share first authorship
 Robin Gens
Robin Gens Anissa Ourtani
Anissa Ourtani Aurelie De Vos
Aurelie De Vos Jacques De Keyser
Jacques De Keyser Sylvie De Raedt
Sylvie De Raedt