- 1Department of Radiology and Nuclear Medicine, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Netherlands
- 2Department of Radiology and Nuclear Medicine, Erasmus University Medical Center, Rotterdam, Netherlands
- 3Department of Radiology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University Medical Center+, Maastricht, Netherlands
- 4Department of Radiology, University Medical Center Utrecht, Utrecht, Netherlands
- 5Department of Neurology, Erasmus University Medical Center, Rotterdam, Netherlands
- 6Department of Neurology, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Netherlands
- 7Department of Biomedical Engineering and Physics, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Netherlands
Background: A considerable proportion of acute ischemic stroke patients treated with endovascular thrombectomy (EVT) are dead or severely disabled at 3 months despite successful reperfusion. Ischemic core imaging biomarkers may help to identify patients who are more likely to have a poor outcome after endovascular thrombectomy (EVT) despite successful reperfusion. We studied the association of CT perfusion-(CTP), CT angiography-(CTA), and non-contrast CT-(NCCT) based imaging markers with poor outcome in patients who underwent EVT in daily clinical practice.
Methods: We included EVT-treated patients (July 2016–November 2017) with an anterior circulation occlusion from the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN) Registry with available baseline CTP, CTA, and NCCT. We used multivariable binary and ordinal logistic regression to analyze the association of CTP ischemic core volume, CTA-Collateral Score (CTA-CS), and Alberta Stroke Program Early CT Score (ASPECTS) with poor outcome (modified Rankin Scale score (mRS) 5-6) and likelihood of having a lower score on the mRS at 90 days.
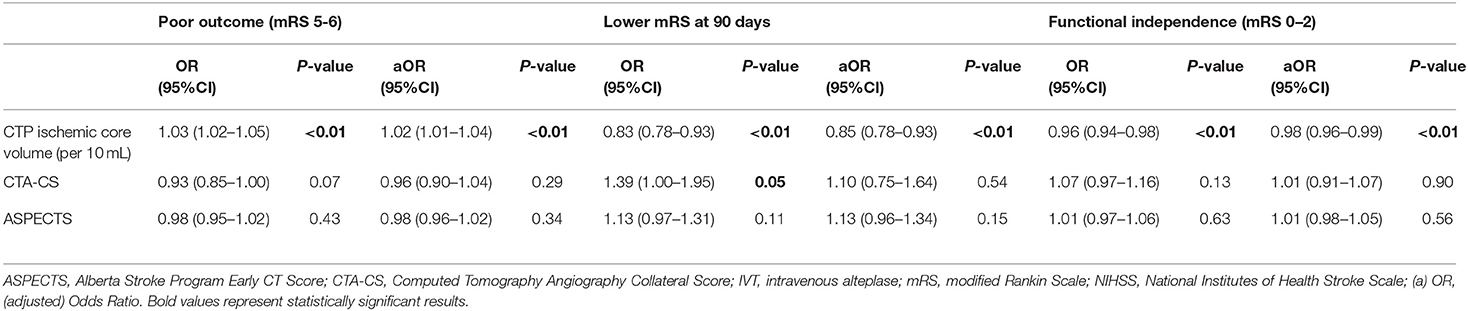
Results: In 201 patients, median core volume was 13 (IQR 5-41) mL. Median ASPECTS was 9 (IQR 8-10). Most patients had grade 2 (83/201; 42%) or grade 3 (28/201; 14%) collaterals. CTP ischemic core volume was associated with poor outcome [aOR per 10 mL 1.02 (95%CI 1.01–1.04)] and lower likelihood of having a lower score on the mRS at 90 days [aOR per 10 mL 0.85 (95% CI 0.78–0.93)]. In multivariable analysis, neither CTA-CS nor ASPECTS were significantly associated with poor outcome or the likelihood of having a lower mRS.
Conclusion: In our population of patients treated with EVT in daily clinical practice, CTP ischemic core volume is associated with poor outcome and lower likelihood of shift toward better outcome in contrast to either CTA-CS or ASPECTS.
Introduction
The efficacy of endovascular thrombectomy (EVT) is well-established for eligible patients with acute ischemic stroke due to anterior circulation large vessel occlusion (LVO) in both the early (0–6 h) and late (6–24 h) time window (1–7). Most of the early time window trials used non-contrast computed tomography (NCCT) and computed tomography angiography (CTA) to select patients, whereas the late time window trials used computed tomography perfusion (CTP) or magnetic resonance imaging (MRI) to select patients based on ischemic core volumes. In daily clinical practice, the most commonly used imaging parameters to evaluate patients for EVT are the Alberta Stroke Program Early CT Score (ASPECTS) on NCCT, occlusion location and collateral score on CTA (CTA-CS), and the estimated ischemic core volume on CTP. A pooled meta-analysis of the Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke (HERMES) collaboration data showed an independent association of CTP ischemic core volume and patients' functional outcome, although modification of benefit from EVT over standard care was not shown (8). The AHA/ASA and ESO/ESMINT guidelines currently do not recommend CTP for patient selection for EVT in the early time window (9, 10).
In the late time window, the DAWN and DEFUSE-3 trials showed benefit from EVT in patients who were selected using CTP or MRI (6, 7). Therefore, the AHA/ASA and ESO/ESMINT guidelines do recommend the use of advanced imaging for selection of EVT in the late time window (9, 10). The above-mentioned guidelines are based on data from trials with predetermined NCCT, CTA, or CTP in- and exclusion criteria.
The treatment effect of EVT is robust and persists in patients with premorbid disability (11). However, less than half of the acute ischemic stroke patients fail to recover to living independently at 3 months after EVT in both the early and late time window (12). If imaging biomarkers could identify patients with a high mortality risk or identify patients who are likely to be severely disabled despite successful EVT, this could potentially support physicians in their decision to treat or not to treat a patient. Consequently, this would enable an individualized, patient- and physiology-based stroke treatment selection rather than selection based on time only (13, 14).
Hence, in this post-hoc analysis of the Multicenter Randomized Controlled Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN) Registry, we aim to assess the association of CTP-, CTA-, and NCCT-based imaging biomarkers with poor functional outcome in EVT-treated patients with acute ischemic stroke.
Materials and Methods
Patient Selection
We used individual clinical and imaging data from patients in the MR CLEAN Registry (July 2016–November 2017) with available baseline CTP data in the 0–24-h time window. The MR CLEAN Registry was an observational, prospective registry of all patients undergoing EVT for acute ischemic stroke in the Netherlands (12). We included patients with a proximal occlusion of the anterior circulation aged >18 years old and treated in a MR CLEAN trial center. Patients were excluded if CTP quality was poor due to movement artifacts, insufficient intra-arterial contrast, or technical failure during post-processing. A schematic representation of our patient selection is given in Figure 1.
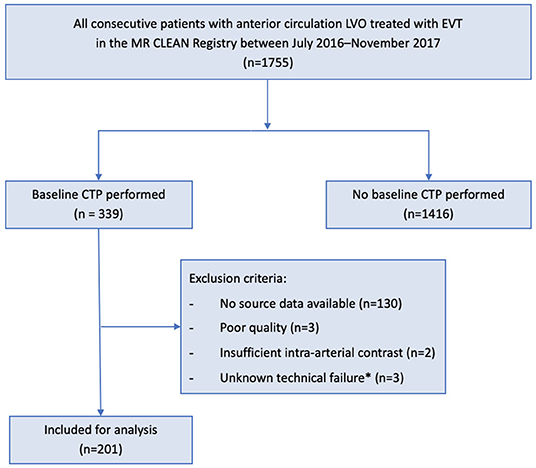
Figure 1. Flowchart for patient selection. EVT, endovascular treatment; CTP, computed tomography-perfusion; MR CLEAN, Multicenter Randomized Controlled Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands. *Unknown scan data issue causing failure in post-processing software functioning.
Baseline Imaging Assessment
NCCT and CTA data were assessed by an independent core laboratory of (neuro) radiologists (12). Observers were blinded to all clinical information except occlusion side. Observers evaluated occlusion location and CTA-CS on CTA, ASPECTS on NCCT, and expanded treatment in cerebral ischemia (eTICI) score on post-treatment digital subtraction angiography (DSA). CTA-CS ranges from 0 (absent collaterals) to 3 (excellent collaterals). Poor CTA-CS was defined as CTA-CS 0-1. ASPECTS is a 10-point quantitative NCCT score to quantify early ischemic changes on NCCT. Poor ASPECTS was defined as ASPECTS 0-5. The eTICI score ranges from 0 (no antegrade reperfusion) to 3 (complete antegrade reperfusion).
CTP Acquisition, Post-processing, and Visual Quality Assessment
CTP images were acquired according to local protocols per site. CTP data were centrally post-processed using syngo.via CT Neuro Perfusion (version VB30A, Siemens Healthineers, Forchheim, Germany). The ischemic core was estimated as CBV <1.2 mL/100 mL and critically hypoperfused tissue volume (including penumbra) as CBF <27 mL/100 mL/min. A default smoothing filter was applied (15). Visual quality assessment of the post-processed data was performed by an experienced neuroradiologist (CBLMM; >15 years of experience). Craniocaudal cropping of the ischemic core (available by default in syngo.via) was performed by two readers (MSK and JWH) to remove obvious artifacts at the skull base level. Penumbra was defined as critically hypoperfused volume minus ischemic core volume. The mismatch ratio was calculated as the critically hypoperfused volume divided by the ischemic core volume. Presence of a target mismatch profile (TMM) was defined using the EXTEND-IA criteria: mismatch ratio >1.2, penumbra volume >10 mL, and ischemic core volume <70 mL (2).
Outcomes
The primary outcome was poor outcome, defined as a 90-day modified Rankin Scale (mRS) score 5–6. Secondary outcomes were likelihood of having a lower mRS at 90 days, functional independence (mRS 0-2), and reperfusion after EVT (eTICI). Safety outcomes were mortality and symptomatic intracranial hemorrhage (sICH) – defined as an increase of ≥4 points on the National Institutes of Health Stroke Scale (NIHSS) and confirmation of intracranial hemorrhage on neuroimaging.
Missing Data
We used multiple imputation with five imputations to handle missing data. We adjusted our analyses for the following variables: age, baseline NIHSS score, pre-stroke mRS, baseline ASPECTS, occlusion segment, collateral status, time from symptom onset to groin, eTICI, and 90-day mRS score. The presented baseline, imaging, and outcome characteristics are crude and not imputed. We reported the number of known values for each baseline and outcome variable.
Statistical Analysis
Baseline, imaging, and outcome characteristics were reported using summary statistics and non-parametric tests appropriate to the type of data and were compared to the overall MR CLEAN Registry population. We used univariable and multivariable logistic regression to analyze the associations with poor outcome and functional independence of the CTP ischemic core volume, penumbra volume, CTA-CS, and ASPECTS for each biomarker separately. We used ordinal logistic regression for the likelihood of having a lower mRS at 90 days. We adjusted for the following pre-defined variables: age, sex, baseline National Institutes of Health Stroke Scale (NIHSS) score, prestroke mRS, administration of intravenous alteplase, time from stroke onset to groin, and site of vessel occlusion.
We investigated if the association of successful reperfusion (defined as eTICI 2b-3) with a shift toward better outcome was modified by CTP ischemic core volume, CTA-CS, and ASPECTS by adding interaction terms (CTP ischemic core volume*successful reperfusion; CTA-CS*successful reperfusion; ASPECTS*successful reperfusion) to the multivariable ordinal regression models. We adjusted for the above-mentioned variables.
We performed an exploratory subgroup analysis to determine the association of dichotomized CTP ischemic core volume (≥70 and ≥50 vs. <70 and <50 mL) with the primary and secondary outcomes. Finally, we assessed whether the absence of a TMM was associated with poor outcome.
We reported (adjusted) odds ratios [(a)ORs] with 95% confidence intervals (95% CI) and considered a two-sided p < 0.05 statistically significant. Statistical analyses were performed using R (R Statistical Software, V3.5.0, R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria).
Protocol Approval and Patient Consent
The central medical ethics committee of the Erasmus MC, Rotterdam, the Netherlands, evaluated the study protocol of the MR CLEAN Registry and granted permission to carry out the study as a registry (MEC-2014-235).
Results
We included 201 patients for analysis. A detailed overview of the baseline characteristics and outcomes is given in Table 1. Median age was 71 years. Most patients were female (58%). Median onset-to-imaging time was 79 min. Of the patients, seven patients (3%) received baseline imaging beyond 6 h after onset (median 440 min). Twelve patients (6%) had an onset-to-groin time >6 h (median 485 min). At 90 days, 51 (25%) patients had a poor functional outcome (mRS 5–6). The distribution of occlusion location, CTA-CS, and ASPECTS was comparable to the overall MR CLEAN Registry cohort (Table 1). The distributions of CTP ischemic core volumes per ASPECTS and CTA-CS category are given in the Supplementary Figures I, II.
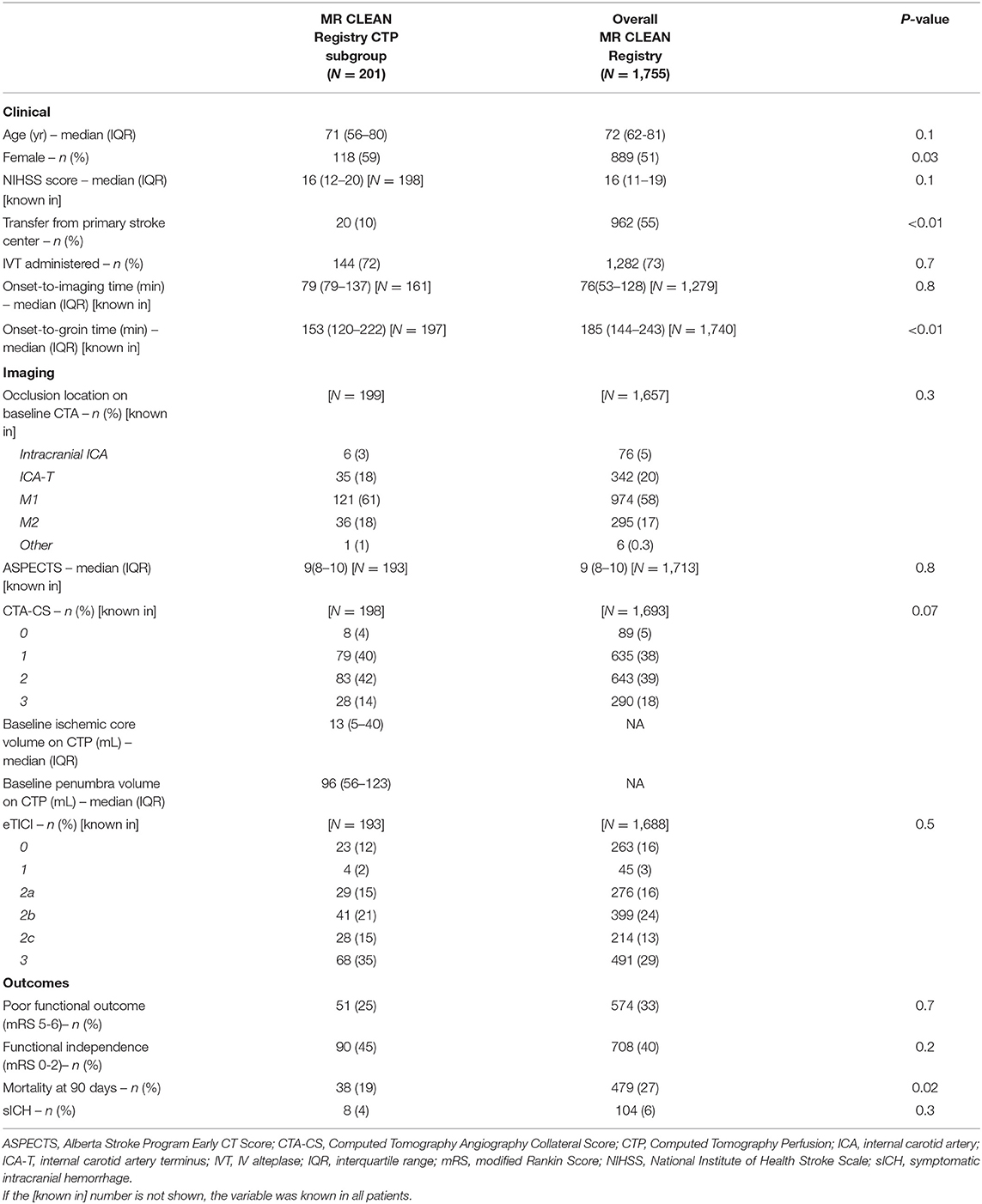
Table 1. Baseline characteristics and outcome of the MR CLEAN Registry CTP subgroup compared to the overall MR CLEAN Registry cohort.
Association of CTP Ischemic Core Volume With Outcome
Median CTP ischemic core volume was 13 mL. CTP ischemic core volume was significantly associated with poor outcome in univariable (OR per 10 mL 1.03 [95% CI 1.02–1.05]), and multivariable logistic regression analysis (aOR per 10 mL 1.02 [95% CI 1.01–1.04]) after adjusting for the predefined covariates (Table 2). Larger CTP ischemic core volume was associated with lower likelihood of having a lower mRS at 90 days [aOR per 10 mL 0.85 (95% CI 0.78–0.93)] and a lower chance of achieving functional independence [aOR per 10 mL 0.98 (95%CI 0.96–0.99)]. The association between ischemic core volume as a continuous variable and functional outcome is shown in Figure 2. The effect of successful reperfusion (eTICI2b-3) on ordinal mRS was not modified by CTP ischemic core volume (P for interaction 0.84). A detailed presentation of the associations of clinical and imaging and parameters with poor functional outcome is given in Table 2 and Supplementary Table I.
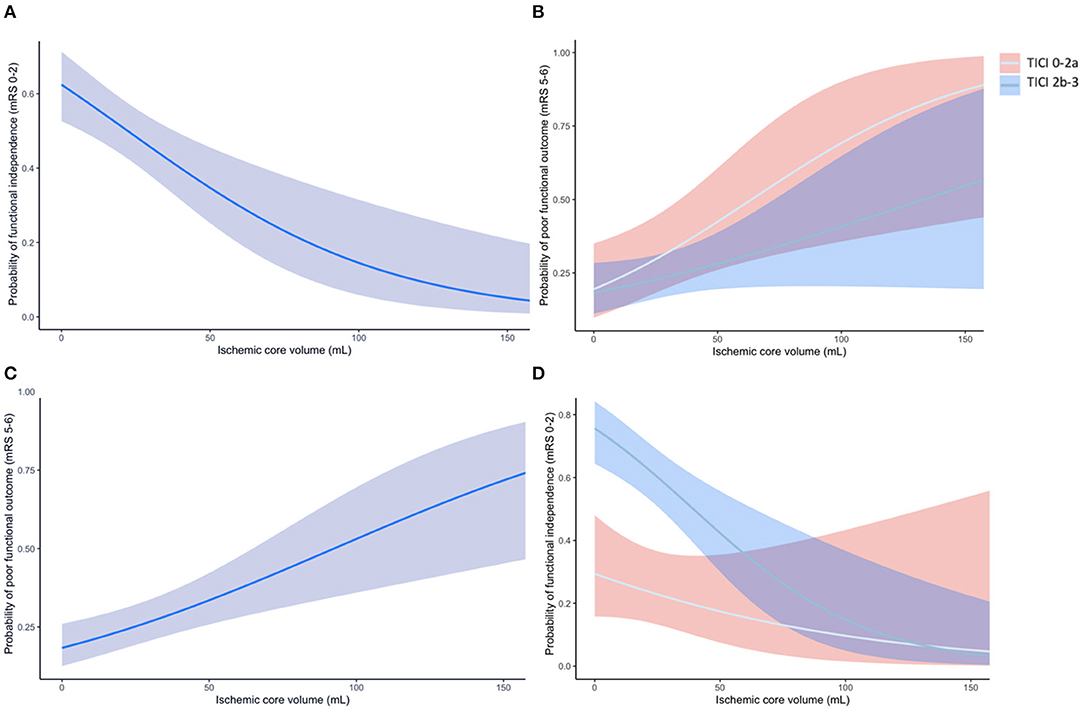
Figure 2. (A) The association of CTP ischemic core volume with the probability of poor outcome (mRS 5-6) at 90 days. (B) The association of CTP ischemic core volume with the probability of poor outcome for patients with successful reperfusion (eTICI 2b-3; blue line with blue area representing the 95%CI) and unsuccessful reperfusion (eTICI 0-2a; gray line with red area representing the 95%CI). (C) The association of CTP ischemic core volume with functional independence (mRS 0-2) at 90 days. (D) The association of CTP ischemic core volume with the probability of functional independence for patients with successful and unsuccessful reperfusion. The effect of successful reperfusion (eTICI 2b-3) on achieving functional independence was modified by CTP ischemic core (p for interaction <0.01).
Association of CTA-CS With Outcome
CTA-CS was not significantly associated with poor outcome [aOR 0.96 (95% CI 0.90–1.04)] in multivariable analysis (Table 2 and Supplementary Tables I, II). In univariable analysis, CTA-CS was associated with a lower mRS at 90 days [OR 1.39 (95%CI 1.00–1.95)]. This association was not significant after adjusting for the predefined covariates [aOR 1.10 (95%CI 0.75–1.64)]. CTA-CS was not associated with functional independence in univariable or multivariable analyses (Table 2). Poor collaterals were not significantly associated with poor outcome [aOR 1.05 (95%CI 0.91–1.16)]. The effect of successful reperfusion on ordinal mRS at 90 days was not modified by CTA-CS (P for interaction 0.22).
Association of ASPECTS With Outcome
We did not find a statistically significant association between ASPECTS and poor outcome, lower mRS at 90 days, or functional independence in either univariable nor multivariable analysis (Table 2 and Supplementary Tables I, II). Poor ASPECTS (N = 13) was not significantly associated with poor outcome [aOR 1.18 (95%CI 0.93–1.46), p = 0.15]. The effect of successful reperfusion on ordinal outcome was not modified by ASPECTS (P for interaction = 0.36).
Association of Dichotomized CTP Ischemic Core Volume With Outcome
Exploratory subgroup analysis showed that CTP ischemic core volume ≥70 mL was significantly associated with poor outcome [aOR 1.32 (95%CI 1.14–1.65)]. Twenty-two (11%) of 201 patients had a CTP ischemic core volume ≥70 mL, of which 13 (65%) had poor outcome at 90 days post-EVT, compared to 38 (23%) patients with CTP ischemic core volume <70 mL (P < 0.01). Mortality was more common in patients with CTP ischemic core volume ≥70 mL compared to patients with CTP ischemic core volume <70 mL (55 vs. 16%; P < 0.01). Two (10%) patients with CTP ischemic core volume ≥70 mL achieved functional independence compared to 88 (49%) patients with CTP ischemic core volume <70 mL (p < 0.01; Supplementary Figure III). Symptomatic intracranial hemorrhage occurred more frequently in patients with CTP ischemic core volume ≥70 mL compared to patients with CTP ischemic core volume <70 mL (13 vs. 3%; p = 0.01). Results of the subgroup analysis with a threshold of 50 mL are reported in the data supplement (Supplementary Table III, Supplementary Figure IV).
Target Mismatch Profile
The median penumbra volume was 96 mL and the median mismatch ratio was 6.6. In univariable analyses, penumbra volume was not associated with poor functional outcome [OR 1.00 (95% CI 0.99–1.01)]. On baseline imaging, 175 (87%) patients did have a TMM profile as applied in the EXTEND-IA trial (2). Thirteen (54%) patients without a TMM profile had a poor outcome after 90 days vs. 38 (23%) patients with a TMM profile [OR 1.36 (95% CI 1.13–1.64)]. In the group without a TMM profile, 25% of the patients achieved functional independence compared to 52% in the group with a TMM profile [OR 0.76 (95% CI 0.61–0.94)]. The rate of successful reperfusion was similar in both groups: 71% in the TMM profile group vs. 68% in the group without a TMM profile (p = 0.73).
Association of Imaging Biomarkers With Mortality and SICH
Both CTP ischemic core volume and CTA-CS were significantly associated with mortality and sICH in univariable analyses (Supplementary Table III). In contrast, ASPECTS was not significantly associated with either mortality or sICH.
Discussion
Our results showed that larger CTP ischemic core volume was associated with poor outcome, a lower likelihood of lower mRS at 90 days, and a lower chance of functional independence in a cohort of patients treated with EVT in daily clinical practice. The relative benefit of successful recanalization – as proxy for EVT – to prevent poor outcome was not modified by CTP ischemic core volume. Our results showed that a better CTA-CS was associated with a higher likelihood of having a lower mRS at 90 days in univariable analysis only. In contrast to CTP ischemic core volume, neither CTA-CS nor ASPECTS were associated with poor outcome, lower mRS at 90 days, or functional independence in multivariable analyses.
Although penumbra volume alone was not associated with outcome independent of ischemic core volume, we found that absence of a favorable perfusion imaging, or target mismatch (TMM) profile, was associated with poor outcome. The subgroup analysis of patients with CTP ischemic core ≥70 or <70 mL showed higher rates of mortality and intracranial hemorrhage in patients with CTP ischemic core volume ≥70 mL. In addition, patients with a CTP ischemic core volume ≥70 mL less often achieved functional independence. This is in line with previous results from the SELECT and TREVO studies (16). Moreover, other studies on the association of CTP ischemic core volume with functional outcome found similar results (17, 18).
Several studies have described the association between CTA-CS and ASPECTS and functional outcome in larger study populations. In a previous study in a larger subset of the MR CLEAN Registry population, good collateral status was found to be strongly associated with better functional outcome of patients with acute ischemic stroke due to intracranial LVO of the anterior circulation (19). Interestingly, this association was not found in our subgroup of patients included in the MR CLEAN Registry who received baseline CTP. The absence of a significant association between CTA-CS and functional outcome in our cohort might be caused by a significantly smaller sample size (201 vs. 1412 patients). Nonetheless, in line with our results, collateral status alone was unable to identify patients who are likely or less likely to benefit from EVT in the study by Jansen et al. (19).
The association between ASPECTS and functional outcome has also been studied in various studies. In a recent post-hoc analysis from the MR CLEAN Registry by Ospel et al., a significant association between ASPECTS and functional outcome was found (20). Most probably, the different results between this analysis and our study can be explained by the (much) larger sample size (201 vs. 3279 patients) in their analysis. The benefit of EVT in patients with low ASPECTS has been extensively explored in previous studies (21, 22). In a subgroup analysis of the MR CLEAN trial, ASPECTS 5-7 was associated with functional outcome (relative to ASPECTS 8-10 and ASPECTS 0-4), however no significant modification of treatment effect by ASPECTS was found (21). This is in line with our results, which showed no modification of successful reperfusion. Yet, we should note that only very few (N = 13) patients with low ASPECTS scores were included in our analysis.
Although there is no clear consensus yet on the additional value of selecting patients for EVT based on acute stroke imaging biomarkers, such as CTP ischemic core volume, CTA-CS, or ASPECTS, all three imaging biomarkers are commonly used to select patients for EVT and are often incorporated in the exclusion criteria of EVT trials (23, 24). All acute stroke imaging biomarkers have their specific limitations. For example, CTP is sensitive to patient motion, requires appropriate contrast administration, and results differ between processing software packages (15, 25, 26). On the other hand, for both CTA-CS and ASPECTS, the interobserver agreement is a major limiting factor (27, 28).
In our study population, poor outcome in EVT-treated patients was associated with age, NIHSS, and ischemic core volume. Previous studies have extensively developed prognostic scores or models to predict poor outcome prior to reperfusion therapy (13, 29). In the current transition toward more personalized acute stroke care, a clinically validated prediction model, such as MR PREDICTS, could support physicians in clinical decision-making based on patient-based physiological characteristics (29). Most current models, however, do not take CTP characteristics into account. Our results suggest that CTP ischemic core volume might be a valuable addition to such prognostic models to further improve their predictive power.
Several limitations to our study should be noted. First, only data of patients who underwent EVT were recorded in the MR CLEAN Registry. Therefore, we were not able to compare our results with a formal control group that did not undergo EVT. Instead, we used unsuccessful reperfusion as a proxy for the control group in our analysis. Second, the MR CLEAN Registry had no prespecified diagnostic imaging criteria for patient selection, therefore it is possible that patients presenting with poor clinical and imaging profiles were not considered as EVT candidates. Also, selection bias might have been introduced since no standardized CTP acquisition was required for inclusion in the MR CLEAN Registry (19% had baseline CTP) and was therefore not always performed according to the same protocol in all participating centers – if performed routinely at all. However, a comparison of the study population with the overall MR CLEAN Registry cohort showed no differences in the baseline distribution of ASPECTS or CTA-CS between the two groups. Third, CTA-CS was scored on single-phase CTA images. Therefore, our results might not be applicable to a setting in which multiphase CTA images are acquired. Finally, in this study, we assessed the association of commonly used acute stroke imaging biomarkers separately. Therefore, our results cannot be compared with performances of prognostic models using a combination of acute stroke imaging biomarkers. Future studies could potentially explore the performance of a prognostic model using a combination of acute stroke imaging biomarkers to further improve the outcome prediction in acute ischemic stroke patients.
It has been suggested that patients with fast core growth—and thus a higher chance of a larger core volume—are more likely to present early (30). Yet, this was not reflected by our study population as our patient sample—along with the overall MR CLEAN Registry cohort—included only a few patients with ischemic core volumes ≥70 mL. A possible explanation could be that patients with an estimated ischemic core volume ≥70 mL were excluded from treatment, although we could not confirm this. To date, the efficacy of EVT in patients with larger ischemic core volumes remains a topic of debate. Although a potential benefit from EVT is suggested in patients with larger core volumes, evidence is mainly based on non-randomized data (31). Most patients were enrolled before publication of the DAWN and DEFUSE-3 results. Therefore, our cohort consisted mainly of patients who were treated in the 0–6 h time window, which might underestimate the discriminating power of CTP in patient selection, especially in the 6–24 h window. Finally, our results might not be applicable to imaging data that are post-processed using different software. Previous studies have shown that different CTP post-processing software packages give different results, although these differences were less severe in patients with CTP ischemic core volumes <70 mL (15, 26).
Conclusion
In anterior circulation acute ischemic stroke patients treated with EVT in daily clinical practice, larger CTP ischemic core volume is associated with poor outcome, a lower likelihood of having a lower mRS at 90 days, and a lower probability of functional independence. ASPECTS and CTA-CS are not significantly associated with poor outcome or the chance of having a lower mRS at 90 days. None of the imaging biomarkers provided a strong argument to withhold EVT.
Data Availability Statement
The datasets presented in this article are not readily available since individual patient data cannot be made available under Dutch law if no consent was obtained.
Ethics Statement
The studies involving human participants were reviewed and approved by Central Medical Ethics Committee of the Erasmus MC, Rotterdam, Netherlands. The Ethics Committee waived the requirement of written informed consent for participation.
Mr Clean Registry Investigators
Diederik W. J. Dippel, Aad van der Lugt, Charles B. L. M. Majoie, Yvo B. W. E. M. Roos, Robert J. van Oostenbrugge, Wim H. van Zwam, Jelis Boiten, Jan Albert Vos, Ivo G. H. Jansen, Maxim J. H. L. Mulder, Robert-Jan B. Goldhoorn, Kars C. J. Compagne, Manon Kappelhof, Josje Brouwer, Sanne J. den Hartog, Wouter H. Hinsenveld, Bob Roozenbeek, Aad van der Lugt, Adriaan C. G. M. van Es, Bart J. Emmer, Jonathan M. Coutinho, Wouter J. Schonewille, Marieke J. H. Wermer, Marianne A. A. van Walderveen, Julie Staals, Jeannette Hofmeijer, Jasper M. Martens, Geert J. Lycklama à Nijeholt, Sebastiaan F. de Bruijn, Lukas C. van Dijk, H. Bart van der Worp, Rob H. Lo, Ewoud J. van Dijk, Hieronymus D. Boogaarts, J. de Vries, Paul L. M. de Kort, Julia van Tuijl, Jo P. Peluso, Puck Fransen, Jan S. P. van den Berg, Boudewijn A. A. M. van Hasselt, Leo A. M. Aerden, René J. Dallinga, Maarten Uyttenboogaart, Omid Eschgi, Reinoud P.H. Bokkers, Tobien H. C. M. L. Schreuder, Roel J. J. Heijboer, Koos Keizer, Lonneke S. F. Yo, Heleen M. den Hertog, Emiel J. C. Sturm, Paul J. A. M. Brouwers, Marieke E. S. Sprengers, Sjoerd F. M. Jenniskens, René van den Berg, Albert J. Yoo, Ludo F. M. Beenen, Alida A. Postma, Stefan D. Roosendaal, Bas F. W. van der Kallen, Ido R. van den Wijngaard, Lonneke S. F. Yo, Joost Bot, Pieter-Jan van Doormaal, Anton Meijer, Elyas Ghariq, Reinoud P. H. Bokkers, Marc P. van Proosdij, G. Menno Krietemeijer, Jo P. Peluso, Rob Lo, Dick Gerrits, Wouter Dinkelaar, Auke P. A. Appelman, Bas Hammer, Sjoert Pegge, Anouk van der Hoorn, Saman Vinke, Robert J. van Oostenbrugge, H. Zwenneke Flach, Hester F. Lingsma, Naziha el Ghannouti, Martin Sterrenberg, Wilma Pellikaan, Rita Sprengers, Marjan Elfrink, Michelle Simons, Marjolein Vossers, Joke de Meris, Tamara Vermeulen, Annet Geerlings, Gina van Vemde, Tiny Simons, Gert Messchendorp, Nynke Nicolaij, Hester Bongenaar, Karin Bodde, Sandra Kleijn, Jasmijn Lodico, Hanneke Droste, Maureen Wollaert, Sabrina Verheesen, D. Jeurrissen, Erna Bos, Yvonne Drabbe, Michelle Sandiman, Nicoline Aaldering, Berber Zweedijk, Jocova Vervoort, Eva Ponjee, Sharon Romviel, Karin Kanselaar, Denn Barning, Esmee Venema, Vicky Chalos, Ralph R. Geuskens, Tim van Straaten, Saliha Ergezen, Roger R. M. Harmsma Daan Muijres, Anouk de Jong, Anna M. M. Boers, J. Huguet, P. F. C. Groot, Marieke A. Mens, Katinka R. van Kranendonk, Kilian M. Treurniet, Manon L. Tolhuisen, Heitor Alves, Annick J. Weterings, Eleonora L. F. Kirkels, Lieve M. Schupp, Eva J. H. F. Voogd, Sabine Collette, Adrien E. D. Groot, Natalie E. LeCouffe, Praneeta R. Konduri, Haryadi Prasetya, Nerea Arrarte-Terreros and Lucas A. Ramos.
Author Contributions
MSK and JH collected and prepared data for this study, performed the statistical analysis, interpreted the results, and drafted the paper. HM, BE, OB, and CM assisted with the statistical analysis and interpretation of the results. MK, OB, LB, WZ, HJ, JD, DD, JC, HM, BE, and CM critically revised the paper. All authors contributed to the article and approved the submitted version.
Funding
The MR CLEAN Registry was funded and carried out by the Erasmus University Medical Center, the Academic Medical Center Amsterdam, and the Maastricht University Medical Center. The MR CLEAN Registry was additionally funded by the Applied Scientific Institute for Neuromodulation (TWIN).
Conflict of Interest
BE reports grants from LtC (ZonMW and TKI-PPP of Health Holland outside the submitted work. WZ reports personal fees from Cerenovus and from Stryker outside the submitted work. DD reports grants from the Dutch Heart Foundation, AngioCare, Medtronic/Covidien/EV3, MEDAC/LAMEPRO, Penumbra, Top Medical/Concentric, and Stryker during conduct of the study; consultation fees from Stryker, Bracco Imaging, and Servier, received by the Erasmus University Medical Centre, outside the submitted work. CM reports grants from TWIN, during the conduct of the study and grants from CVON/Dutch Heart Foundation, European Commission, Dutch Health Evaluation Program, and from Stryker outside the submitted work (paid to institution) and is shareholder of NICO.LAB. HM is co-founder and shareholder of NICO.LAB.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all MR CLEAN Registry trial centers and investigators, interventionists, core lab members, research nurses, Executive Committee, and PhD student coordinators for their endless efforts on data collection, completion, and methodological and logistical support (Appendix B in the Data Supplement).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.771367/full#supplementary-material
Abbreviations
ASPECTS, Alberta Stroke Program Early CT Score; CTA, Computed Tomography Angiography; CTA-CS, Computed Tomography Angiography Collateral Score; CTP, Computed Tomography Perfusion; IQR, Interquartile Range; EVT, Endovascular Thrombectomy; LVO, Large Vessel Occlusion; MR CLEAN, Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands; mRS, modified Rankin Scale; OR, Odds Ratio; TICI, Thrombolysis in Cerebral Infarction.
References
1. Berkhemer OA, Fransen PSS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl J Med. (2015) 372:11–20. doi: 10.1056/NEJMoa1411587
2. Campbell BCV, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. (2015) 372:1009–18. doi: 10.1056/NEJMoa1414792
3. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. (2015) 372:1019–30. doi: 10.1056/NEJMoa1414905
4. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. (2015) 372:2285–95. doi: 10.1056/NEJMoa1415061
5. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. (2015) 372:2296–306. doi: 10.1056/NEJMoa1503780
6. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. (2018) 378:708–18. doi: 10.1056/NEJMoa1713973
7. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. (2018) 378:11–21. doi: 10.1056/NEJMoa1706442
8. Campbell BCV, Majoie CBLM, Albers GW, Menon BK, Yassi N, Sharma G, et al. Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol. (2018) 18:P46–55. doi: 10.1016/S1474-4422(18)30314-4
9. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
10. Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, et al. European stroke organisation (ESO) - european society for minimally invasive neurological therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke endorsed by stroke alliance for Europe (SAFE). Eur Stroke J. (2019) 4:6–12. doi: 10.1177/2396987319832140
11. Regenhardt RW, Young MJ, Etherton MR, Das AS, Stapleton CJ, Patel AB, et al. Toward a more inclusive paradigm: thrombectomy for stroke patients with pre-existing disabilities. J Neurointerv Surg. (2021) 13:865–8. doi: 10.1136/neurintsurg-2020-016783
12. Jansen IGH, Mulder MJHLM, Goldhoorn RJB, investigators MCR. Endovascular treatment for acute ischaemic stroke in routine clinical practice: prospective, observational cohort study (MR CLEAN Registry). BMJ. (2018) 360:k949. doi: 10.1136/bmj.k949
13. Ramos LA, Kappelhof M, van Os HJA, Chalos V, Van Kranendonk K, Kruyt ND, et al. Predicting poor outcome before endovascular treatment in patients with acute ischemic stroke. Front Neurol. (2020) 11:1215. doi: 10.3389/fneur.2020.580957
14. Rabinstein AA, Albers GW, Brinjikji W, Koch S. Factors that may contribute to poor outcome despite good reperfusion after acute endovascular stroke therapy. Int J Stroke. (2019) 14:23–31. doi: 10.1177/1747493018799979
15. Koopman MS, Berkhemer OA, Geuskens RREG, Emmer BJ, Walderveen MAA, Jenniskens SFM, et al. Comparison of three commonly used CT perfusion software packages in patients with acute ischemic stroke. J Neurointerv Surg. (2019) 11:1249–56. doi: 10.1136/neurintsurg-2019-014822
16. Sarraj A, Hassan A, Nogueira R, Liebeskind D, Vora N, Abraham M. Endovascular Thrombectomy Outcomes in Large Core on CT are Strongly Associated With Perfusion Core Volume Time: Implications From Two Large Cohorts for Future Trials. Honolulu, HI: The American Heart Association (2019). Available online at: https://professional.heart.org/-/media/phd-files-2/science-news/e/endovascular_thrombectory_outcomes_ucm_503515.pdf?la=en
17. Borst J, Berkhemer OA, Roos YBWEM, van Bavel E, van Zwam WH, van Oostenbrugge RJ, et al. Value of computed tomographic perfusion-based patient selection for intra-arterial acute ischemic stroke treatment. Stroke. (2015) 46:3375–82. doi: 10.1161/STROKEAHA.115.010564
18. Haussen DC, Dehkharghani S, Rangaraju S, Rebello LC, Bouslama M, Grossberg JA, et al. Automated CT perfusion ischemic core volume and noncontrast CT ASPECTS (Alberta Stroke Program Early CT Score): correlation and clinical outcome prediction in large vessel stroke. Stroke. (2016) 47:2318–22. doi: 10.1161/STROKEAHA.116.014117
19. Jansen IGH, Mulder MJHL, Goldhoorn RJB, Boers AMM, van Es ACGM, Yo LSF, et al. Impact of single phase CT angiography collateral status on functional outcome over time: results from the MR CLEAN Registry. J Neurointerv Surg. (2019) 11:866–73. doi: 10.1136/neurintsurg-2018-014619
20. Ospel J, Kappelhof M, Groot AE, LeCouffe NE, Coutinho JM, Yoo AJ, et al. Combined effect of age and baseline alberta stroke program early computed tomography score on post-thrombectomy clinical outcomes in the MR CLEAN Registry. Stroke. (2020) 51:3742–5. doi: 10.1161/STROKEAHA.120.031773
21. Yoo AJ, Berkhemer OA, Fransen PSS, van den Berg LA, Beumer D, Lingsma HF, et al. Effect of baseline alberta stroke program early CT Score on safety and efficacy of intra-arterial treatment: a subgroup analysis of a randomised phase 3 trial (MR CLEAN). Lancet Neurol. (2016) 15:685–94. doi: 10.1016/S1474-4422(16)00124-1
22. Diestro JDB, Dmytriw AA, Broocks G, Chen K, Hirsch JA, Kemmling A, et al. Endovascular thrombectomy for low ASPECTS large vessel occlusion ischemic stroke: a systematic review and meta-analysis. Can J Neurol Sci. (2020) 47:612–9. doi: 10.1017/cjn.2020.71
23. MR CLEAN-LATE trial. Available online at: https://mrclean-late.nl/
24. Broocks G, Kniep H, Schramm P, Hanning U, Flottmann F, Faizy T, et al. Patients with low Alberta Stroke Program Early CT Score (ASPECTS) but good collaterals benefit from endovascular recanalization. J Neurointerv Surg. (2020) 12:747–52. doi: 10.1136/neurintsurg-2019-015308
25. Vagal A, Wintermark M, Nael K, Bivard A, Parsons M, Grossman AW, et al. Automated CT perfusion imaging for acute ischemic stroke: pearls and pitfalls for real-world use. Neurology. (2019) 93:888–98. doi: 10.1212/WNL.0000000000008481
26. Austein F, Riedel C, Kerby T, Meyne J, Binder A, Lindner T, et al. Comparison of Perfusion CT Software to predict the final infarct volume after thrombectomy. Stroke. (2016) 47:2311–7. doi: 10.1161/STROKEAHA.116.013147
27. Gupta AC, Schaefer PW, Chaudhry ZA, Leslie-Mazwi TM, Chandra RV, González RG, et al. Interobserver reliability of baseline noncontrast CT alberta stroke program early CT score for intra-arterial stroke treatment selection. Am J Neuroradiol. (2012) 33:1046–9. doi: 10.3174/ajnr.A2942
28. Berkhemer OA, Jansen IGHH, Beumer D, Fransen PSSS, Van Den Berg LA, Yoo AJ, et al. Collateral status on baseline computed tomographic angiography and intra-arterial treatment effect in patients with proximal anterior circulation stroke. Stroke. (2016) 47:768–76. doi: 10.1161/STROKEAHA.115.011788
29. Venema E, Mulder MJHL, Roozenbeek B, Broderick JP, Yeatts SD, Khatri P, et al. Selection of patients for intra-arterial treatment for acute ischaemic stroke: development and validation of a clinical decision tool in two randomised trials. BMJ. (2017) 357:j1710. doi: 10.1136/bmj.j1710
30. Lin L, Zhang H, Chen C, Bivard A, Butcher K, Garcia-Esperon C, et al. Stroke patients with faster core growth have greater benefit from endovascular therapy. Stroke. (2021) 52:3998–4006. doi: 10.1161/STROKEAHA.121.034205
Keywords: CT perfusion (CTP), ischemic core, thrombectomy, stroke, alberta stroke program early CT score (ASPECTS), collaterals
Citation: Koopman MS, Hoving JW, Kappelhof M, Berkhemer OA, Beenen LFM, van Zwam WH, de Jong HWAM, Dankbaar JW, Dippel DWJ, Coutinho JM, Marquering HA, Emmer BJ and Majoie CBLM (2022) Association of Ischemic Core Imaging Biomarkers With Post-Thrombectomy Clinical Outcomes in the MR CLEAN Registry. Front. Neurol. 12:771367. doi: 10.3389/fneur.2021.771367
Received: 06 September 2021; Accepted: 30 November 2021;
Published: 10 January 2022.
Edited by:
Amrou Sarraj, University of Texas Health Science Center at Houston, United StatesReviewed by:
Jean-Marc Olivot, Centre Hospitalier Universitaire de Toulouse, FranceMark William Parsons, The University of Newcastle, Australia
Copyright © 2022 Koopman, Hoving, Kappelhof, Berkhemer, Beenen, van Zwam, de Jong, Dankbaar, Dippel, Coutinho, Marquering, Emmer and Majoie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miou S. Koopman, bS5zLmtvb3BtYW5AYW1zdGVyZGFtdW1jLm5s
†These authors have contributed equally to this work
 Miou S. Koopman
Miou S. Koopman Jan W. Hoving
Jan W. Hoving Manon Kappelhof
Manon Kappelhof Olvert A. Berkhemer1,2
Olvert A. Berkhemer1,2 Ludo F. M. Beenen
Ludo F. M. Beenen Wim H. van Zwam
Wim H. van Zwam Jan Willem Dankbaar
Jan Willem Dankbaar Diederik W. J. Dippel
Diederik W. J. Dippel Jonathan M. Coutinho
Jonathan M. Coutinho Henk A. Marquering
Henk A. Marquering Charles B. L. M. Majoie
Charles B. L. M. Majoie