- 1Department of Neurosurgery, West China Longquan Hospital Sichuan University, Chengdu, China
- 2Department of Neurosurgery, The First People's Hospital of Longquanyi District, Chengdu, China
- 3Department of Neurosurgery, Affiliated Hospital of Chengdu University, Chengdu, China
- 4Department of Ophthalmology, West China Longquan Hospital Sichuan University, Chengdu, China
- 5Department of Ophthalmology, The First People's Hospital of Longquanyi District, Chengdu, China
- 6Department of Medical Oncology, Thomas Jefferson University, Philadelphia, PA, United States
- 7Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, PA, United States
- 8Key Laboratory of Molecular Biology for Infectious Diseases (Ministry of Education), Institute for Viral Hepatitis, Department of Infectious Diseases, The Second Affiliated Hospital, Chongqing Medical University, Chongqing, China
- 9Department of Neurosurgery, West China Hospital, Sichuan University, Chengdu, China
Objectives: To report the prevalence, clinical associations, and prognostic consequences of liver fibrosis in patients with aneurysmal subarachnoid hemorrhage (aSAH).
Methods: In a retrospective study of patients with aSAH, we evaluated three validated liver fibrosis indices and modeled them as continuous-exposure variables, including the aspartate aminotransferase/platelet ratio index (APRI), the fibrosis-4 (FIB-4) index, and the Forns index. The primary outcome was mortality at 90 days. We compared the addition of fibrosis indices to the predictors of the full Subarachnoid Hemorrhage International Trialists model.
Results: A total of 3,722 patients with aSAH were included in the study. According to the APRI, FIB-4 index, and Forns index, 21.7, 17.7, and 11.4% of patients had liver fibrosis. After adjusting for potential confounding factors, liver fibrosis indices were associated with increased 90-day mortality, with odds ratios of 1.35 (95% CI 1.02–1.77) for the FIB-4 index, 1.39 (95% CI.08–1.78) for APRI, and 1.53 (95% CI 1.11–2.12) for the Forns index. Similarly, high liver fibrosis indices were associated with an increased risk of rebleeding. However, the Forns index was not significantly associated with mortality and rebleeding. The addition of FIB-4 indices and APRI into the standard model improved the mortality prediction.
Conclusions: Liver fibrosis is common in patients with aSAH, and high liver fibrosis indices are associated with mortality and rebleeding. The addition of liver fibrosis indices to a standard clinical model significantly improves risk stratification.
Introduction
In the last decade, aneurysmal subarachnoid hemorrhage (aSAH) had an incidence of 6 to 9 per 100,000 persons per year, with substantial morbidity and a mortality rate of 35% (1). Many studies concluded that hypertension, smoking, and excessive alcohol consumption were risk factors for aSAH (2). Other researchers proved that cirrhosis was associated with an increased risk of hemorrhagic stroke (3). Recently, we found evidence that chronic liver disease was associated with mortality and rebleeding after aSAH (4). These studies highlight the association between advanced liver disease and poor outcomes after aSAH, but it is still uncertain whether there is a similar association between subclinical liver disease and clinical outcomes in patients with aSAH.
Liver fibrosis is a subclinical manifestation of chronic liver diseases, characterized by excessive and abnormal deposition of extracellular matrix components in the liver (5). Previous studies have shown a high prevalence of liver fibrosis in up to 15% of the general population without known liver disease and can be detected using no-ninvasive tests (6, 7).
Liver biopsy is still considered the gold standard for the evaluation of liver fibrosis (8). However, it is expensive, painful, and may be dangerous for patients. Recently, several laboratory indices of liver fibrosis have been reported to be useful predictors of liver fibrosis (9, 10). An elevated fibrosis-4 (FIB-4) index was associated with higher risks of worse outcomes for liver diseases (11). Similar predictive values for adverse liver events were seen in the aspartate aminotransferase/platelet ratio index (APRI) and the Forns index (12, 13).
Liver fibrosis has been correlated with admission hematoma volume, hematoma growth, and mortality in patients with intracerebral hemorrhage (14, 15). For aSAH, the impact of liver fibrosis on rebleeding risks is a significant clinical concern (16). However, its prognostic impact in patients with aSAH is not well known.
We aimed to investigate the prevalence, clinical associations, and prognostic consequences of subclinical liver disease, as defined via three liver fibrosis indices, in patients with aSAH.
Methods
Study Design and Data Source
The data that support the findings of this study are available from the corresponding author upon reasonable request. This is a single-center, retrospective cohort study of patients with aSAH. We retrospectively analyzed the data from the electronic medical records of patients with aSAH admitted to West China Hospital, Sichuan University, from January 2009 to June 2019. The ethics committees and the institutional review boards of West China Hospital approved the study and waived the requirement for informed consent.
Patient Selection
This analysis consisted of patients with aSAH. Aneurysmal subarachnoid hemorrhage was assessed according to neuroimaging (including CT, MRI, or angiography), cerebrospinal fluid analysis, or intraoperatively by a neurosurgeon. Patients were excluded for the following reasons: (1) having overt liver disease and (2) lack of liver fibrosis indices at admission within 24 h. We also excluded the patients whose household registrations were not found in the Household Registration Administration System of Sichuan province. We used personal identification numbers to identify death records from the Household Registration Administration System of Sichuan province. If the identification numbers of patients in the electronic medical record system were wrong or non-existent, or if their household registration was not in Sichuan province, we were unable to find their household registration in the system.
Clinical Variables
The primary variables were liver fibrosis indices measured at admission. All participants underwent the liver fibrosis assessment using three non-invasive liver fibrosis indices: APRI (17), FIB-4 index (18, 19), and the Forns index (9). Liver fibrosis indices were calculated from the demographic variables and the laboratory data, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), platelet count (PLT), and total cholesterol, which were collected from patients within the first 24 h after admission to the hospital. The formula of the liver fibrosis indices is shown in Figure 1A. We used 3.25 for the FIB-4 index (18, 19), 7 for APRI (17), and 6.9 for the Forns index (9) as cutoffs, according to published literature.
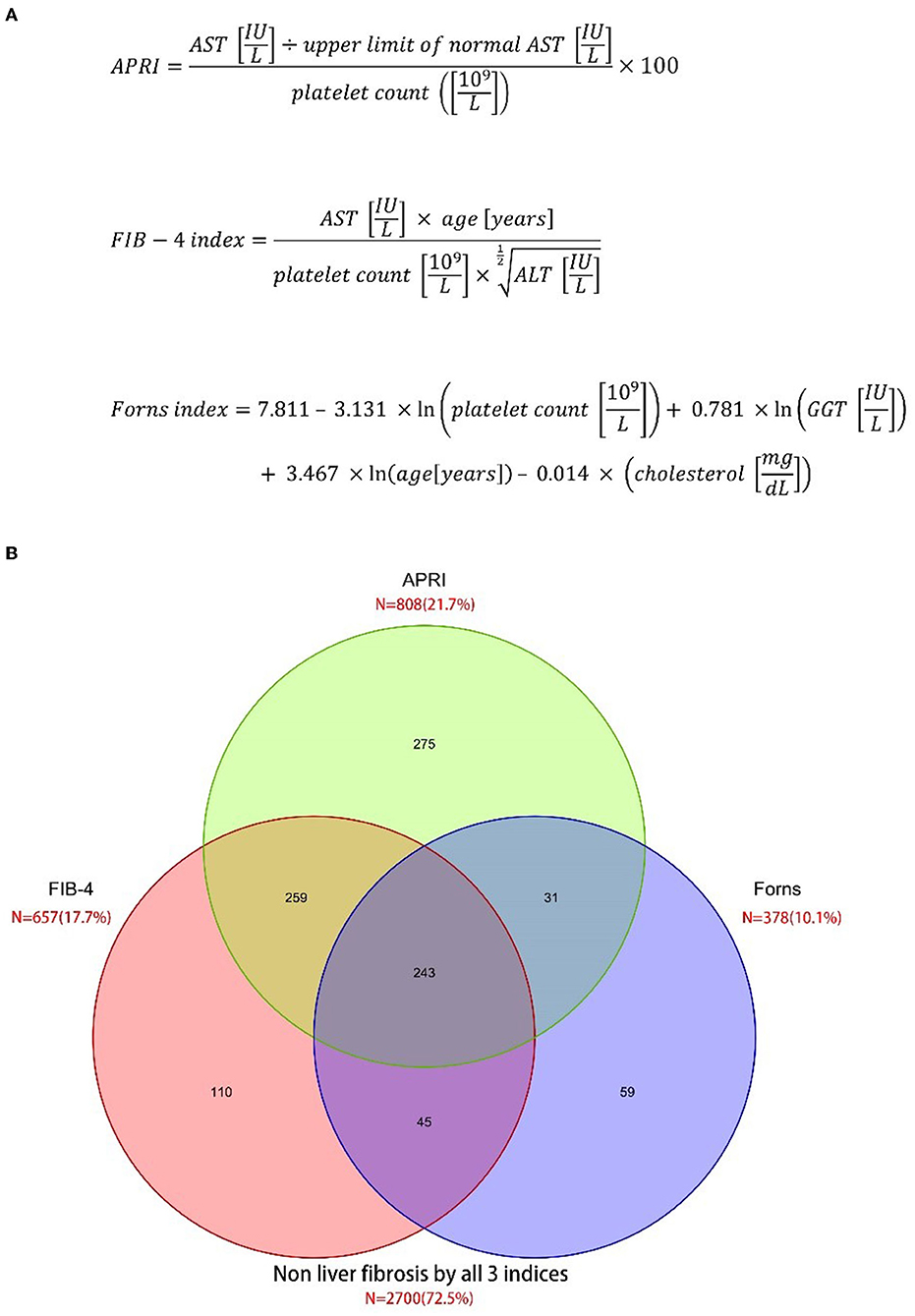
Figure 1. Prevalence of liver fibrosis according to three different scoring systems. (A) Three liver fibrosis indices (B) venn diagram.
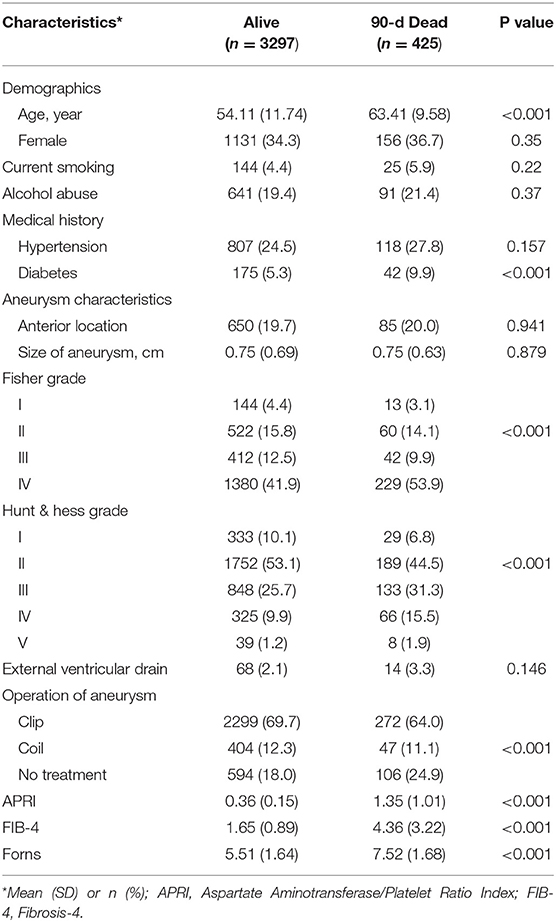
The data of the study population were as follows: demographic variables included age and sex, comorbid conditions (hypertension, diabetes, chronic obstructive pulmonary disease, coronary heart disease, and chronic renal failure), alcohol abuse, smoking, aneurysm characteristics (location and size), external ventricular drain, and aneurysm treatment (clip, coil, and no treatment), and we also collected the Hunt & Hess grade and the Fisher grade at baseline.
Clinical Outcomes and Definitions
The primary clinical outcome was mortality at 90 days. Other mortality included mortality at 180 days, mortality at 1 year, and mortality at 3 years. We obtained the death records from the Household Registration Administration System database up to 15 August 2021. In China, by law, upon death, the death certificate should be submitted to the household registration office of the Public Security Bureau within 30 days. Because the submission of the death certificate is enforced and the database is always updated, the loss of follow-up in our cohort is almost negligible.
Secondary clinical outcomes were in-hospital complications, including acute kidney injury, pneumonia, hydrocephalus, rebleeding, and delayed cerebral ischemia. Rebleeding was defined as acute worsening in the neurological status along with an increase in hemorrhage volume, which was confirmed in a repeat neuroimaging (CT or MRI scan).
Statistical Analysis
For all calculations in the statistical analysis, we used software R (version 4.0.3). The data were shown as means ± SD for continuous variables, as medians with the interquartile range, and as numbers (percentage) for categorical variables. All the significance tests were two sided, and p-values of <0.05 were considered statistically significant. Multiple imputations have become an appropriate and flexible way to solve missing data, so we repeated all outcomes using multiple regression imputations (20, 21).
According to univariate analyses, the included data were assessed for potential confounding factors. All baseline variables with a p value <0.10 in univariate analyses were added into a multivariate regression model. Measured confounders included age and sex, hypertension, diabetes, alcohol abuse, smoking, aneurysm characteristics (location and size), Hunt & Hess grade, Fisher grade, external ventricular drain, and aneurysm treatment (clip, coil, and no treatment).
We assessed improvement in model performance for the addition of liver fibrosis indices to a standard score model by calculating the change in the area under the curve (AUC) and net reclassification improvement (NRI), as recommended by the TRIPOD statement (22, 23). The Subarachnoid Hemorrhage International Trialists (SAHIT) model is a well-established prediction model (24). The SAHIT model was developed from over 10,000 patients and validated in external validation. The full model of SAHIT includes age, neurological grade on admission, history of hypertension, Fisher grade, aneurysm size, aneurysm location, and treatment.
Discrimination was evaluated with AUC curves. A comparison of AUC was performed using the DeLong test. The calibration was evaluated graphically with calibration plots and statistically by computing a goodness-of-fit test of the model using the Hosmer-Lemeshow test.
Results
A total of 3,722 eligible patients with aSAH were included in the cohort study (Supplementary Figure 1). Among them, 425 (11.2%) were dead at 90 days. According to the APRI, FIB-4 index, and Forns index, 21.7, 17.7, and 10.1% of patients had liver fibrosis (Figure 1B). All liver fibrosis indices were correlated with each other (APRI vs. FIB-4 index: r = 0.71; APRI vs. Forns index: r = 0.62; FIB-4 index vs. Forns index: r = 0.47; all P < 0.001). The baseline characteristics of the included patients are described in Table 1. The mean APRI in patients who died was 0.36 and in patients who survived was 1.35 (p < 0.001), the mean FIB-4 index in patients who died was 1.65 and in patients who survived was 4.36 (p < 0.001), and the mean Forns index in patients who died was 5.51 and in patients who survived was 7.52 (p < 0.001), which suggested that all three indices may be prognostic factors for predicting mortality. Other factors that were more common in patients who died included older age, medical history of diabetes, the larger size of the aneurysm, and higher Hunt & Hess grades and Fisher grades.
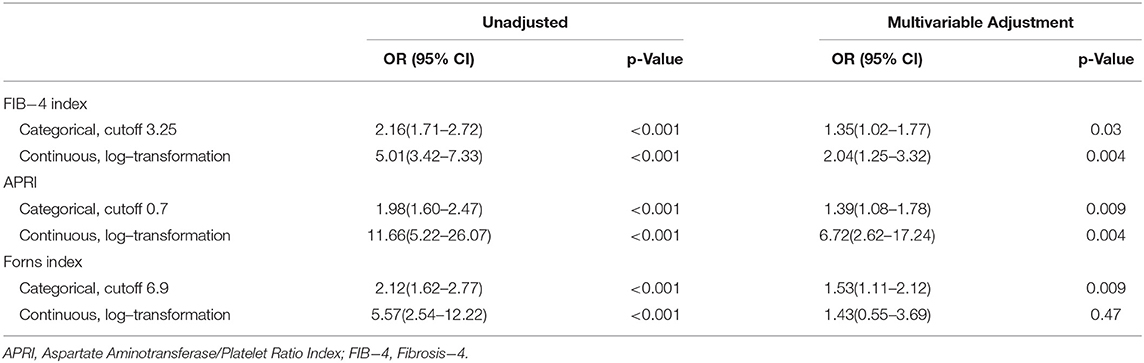
The logistic regression for the association between liver fibrosis indices and 90-day mortality is summarized in Table 2. In the univariate logistic analysis, all of the three liver fibrosis indices had an association with mortality. After adjusting for all covariates in the model, the multivariate logistic regression analysis still suggested the association between APRI/ FIB-4 index and 90-day mortality, including mortality. The Forns index as binary variables was associated with 90-day mortality in the multivariate logistic analysis, but the association was not significant when the Forns index was input as the continuous variable after logarithmic transformation.
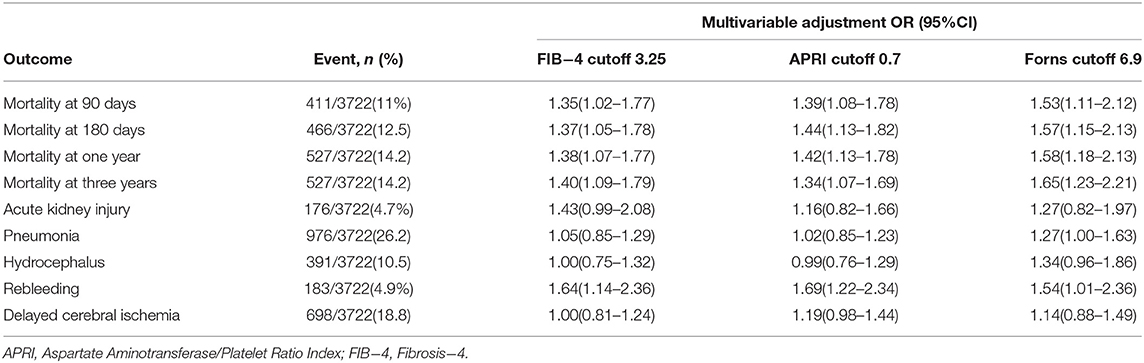
The association between liver fibrosis indices as binary variables and other outcomes is summarized in Table 3. The multivariate logistic regression models showed the associations with rebleeding for APRI (odds ratio (OR) 1.69, 95% CI 1.22–2.34), FIB-4 index (OR 1.64, 95% CI 1.14–2.36), and Forns index (OR 1.54, 95% CI 1.01–2.36). None of the liver fibrosis indices were associated with pneumonia, hydrocephalus, and delayed cerebral ischemia.
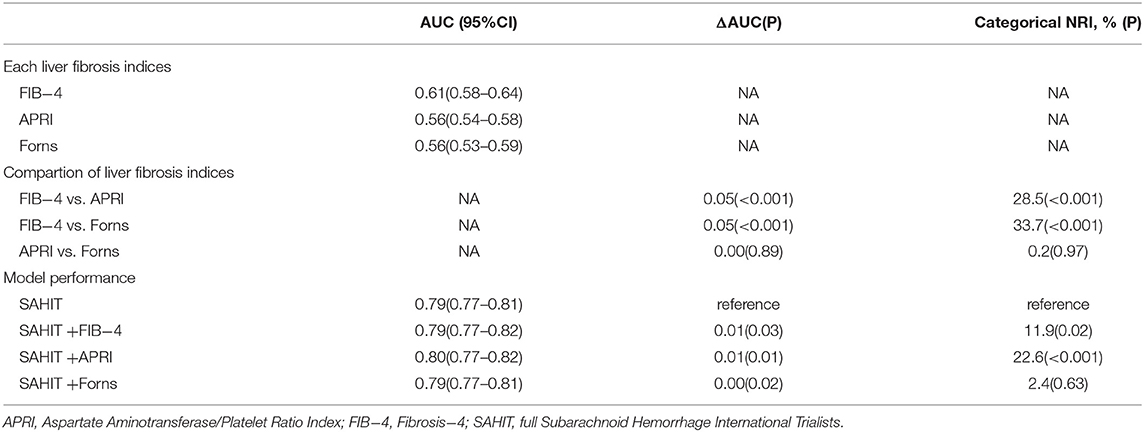
Compared to ARPI and the Forns index, the FIB-4 index outperformed the AUC and NRI at predicting mortality, as was seen by the discrimination index values (Table 4). Using the same predictors as the SAHIT model, our data produced a pooled AUC of 0.79. We added FIB-4, APRI, and Forns indices as predictors into the SAHIT model separately, and including them in the SAHIT model suggested a statistically significant, yet modest, improvement in mortality prediction (P = 0.03, 0.01, and 0.02). The Hosmer-Lemeshow test confirmed goodness-of-fit for the SAHIT model (P = 0.50, Supplementary Figure 2), SAHIT plus FIB-4 model (P = 0.65), SAHIT plus APRI model (P = 0.93), and SAHIT plus Forns model (P = 0.59).
Discussion
Liver fibrosis, as defined by existing scores, is common in patients with aSAH. Liver fibrosis indices are associated with mortality and rebleeding. Moreover, the addition of the liver fibrosis indices in a prediction model could accurately predict mortality.
Previous studies confirmed that liver disease was a risk factor for aSAH (4). Liver fibrosis is one of the main manifestations of chronic liver diseases. In recent studies, Parikh et al. found that cirrhosis may be an independent risk factor for aSAH among older individuals. However, they did not assess the predictive value of cirrhosis for significant clinical concerns of aSAH, including rebleeding. Moreover, many other important confounding factors, such as smoking (2), were not adjusted.
To our knowledge, this is the first study to explore the association between liver fibrosis indices and clinical outcomes in patients with aSAH. However, we found several small studies evaluating the association between liver fibrosis indices and poor stroke outcomes. In the study of 432 patients with intracerebral hemorrhage, Parikh et al. (14) explored the association between liver fibrosis indices and mortality. The study revealed that two liver fibrosis indices (APRI and FIB-4) were associated with mortality at 90 days, consistent with our findings; however, they did not explore whether this association remained in long-term outcomes. Baik et al. (25) investigated the association between liver fibrosis and long-term mortality in 395 patients with ischemic stroke. They assessed the degree of liver fibrosis using transient elastography but not liver fibrosis indices and found that liver fibrosis was associated with long-term mortality (median 2.7 years).
The underlying mechanisms of the association of liver fibrosis with mortality in patients with aSAH remain unclear and require further exploration. Hepatic stellate cells play an essential role in liver fibrogenesis and inflammation and are known to be associated with increased circulating levels of several forms of inflammation, endothelial dysfunction, oxidative stress markers, and procoagulant factors (16, 26–28).
This study is the first to investigate the association between liver fibrosis indices and mortality and complications based on patients with aSAH. The key strengths of the study included the large population, long-term follow-up, and comprehensive demographic characteristics. The outcome of patient mortality was based on a provincial population registry, the China Registered Residence system, with accurate records on mortality. We excluded patients with advanced liver disease to better analyze the impact of liver fibrosis indices on clinical outcomes.
Despite these study strengths, the study still had some limitations. First, although we made every effort to acquire data, bias and lack of clinical data are inevitable due to the retrospective cohort study. Second, as we measured liver fibrosis indices only once during admission, it was not possible to assess longitudinal changes in liver fibrosis indices on the risk of death. Third, we could not obtain the cause of death as that was not specified in the registry database.
Conclusions
Liver fibrosis is common in patients with aSAH. Liver fibrosis indices were associated with all-cause mortality and rebleeding. The inclusion of the liver fibrosis indices led to outcome predictions with superior predictive power compared to the SAHIT score model only. An adequate assessment of liver fibrosis can help improve the prognosis of patients with aSAH.x
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of West China Hospital (No. 20191133). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
YZ: study concept. PW, XG, WC, JK, FF, and YZ: acquisition, analysis, or interpretation of data. TL and PW: statistical analysis and drafting of the manuscript. All authors were involved in the design and critical revision of the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Key R&D Program of China (2018YFA0108604), the 1·3·5 project for disciplines of excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (21HXFH046), the project of Sichuan Science and Technology Bureau (22ZDYF0798), and Clinical Incubation Program of West China Hospital, SCU (2018HXFU008).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.850405/full#supplementary-material
References
1. Neifert SN, Chapman EK, Martini ML, Shuman WH, Schupper AJ, Oermann EK, et al. Aneurysmal subarachnoid hemorrhage: the last decade. Transl Stroke Res. (2021) 12:428–46. doi: 10.1007/s12975-020-00867-0
2. Andreasen TH, Bartek J Jr., Andresen M, Springborg JB, Romner B. Modifiable risk factors for aneurysmal subarachnoid hemorrhage. Stroke. (2013) 44:3607–12. doi: 10.1161/STROKEAHA.113.001575
3. Parikh NS, Navi BB, Schneider Y, Jesudian A, Kamel H. Association between cirrhosis and stroke in a nationally representative cohort. JAMA Neurol. (2017) 74:927–32. doi: 10.1001/jamaneurol.2017.0923
4. Zhang Y, Li L, Jia L, Chong W, Hai Y, Lunsford LD, et al. Association of chronic liver disease and mortality in patients with aneurysmal subarachnoid hemorrhage. Stroke. (1999) 8:3–15. doi: 10.1161/STROKEAHA.121.034136
5. Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. (2012) 18:1028–40. doi: 10.1038/nm.2807
6. L. Caballería, Pera G, Arteaga I, Rodríguez L, Alumà A, Morillas RM, et al. High prevalence of liver fibrosis among European adults with unknown liver disease: a population-based study. Clin Gastroenterol Hepatol. (2018) 16 1138–45.e5. doi: 10.1016/j.cgh.2017.12.048
7. You SC, Kim KJ, Kim SU, Kim BK, Park JY, Kim DY, et al. Factors associated with significant liver fibrosis assessed using transient elastography in general population. World J Gastroenterol. (2015) 21:1158–66. doi: 10.3748/wjg.v21.i4.1158
8. Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. (2001) 344:495–500. doi: 10.1056/NEJM200102153440706
9. Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. (2002) 36:986–92. doi: 10.1053/jhep.2002.36128
10. Rasmussen DN, Thiele M, Johansen S, Kjærgaard M, Lindvig KP, Israelsen M, et al. Prognostic performance of 7 biomarkers compared to liver biopsy in early alcohol-related liver disease. J Hepatol. (2021) 75:1017–25. doi: 10.1016/j.jhep.2021.05.037
11. Kim SW, Jeon JH, Moon JS, Kim MK. High fibrosis-4 index is related with worse clinical outcome in patients with Coronavirus disease 2019 and diabetes mellitus: a multicenter observational study. Endocrinol Metab (Seoul). (2021) 36:800–9. doi: 10.3803/EnM.2021.1040
12. Cheng CH, Chu CY, Chen HL, Lin IT, Wu CH, Lee YK, et al. Subgroup analysis of the predictive ability of aspartate aminotransferase to platelet ratio index (APRI) and fibrosis-4 (FIB-4) for assessing hepatic fibrosis among patients with chronic hepatitis C. J Microbiol Immunol Infect. (2020) 53:542–9. doi: 10.1016/j.jmii.2019.09.002
13. Güzelbulut F, Çetinkaya ZA, Sezikli M, Yaşar B, Ozkara S, Övünç AO. AST-Platelet ratio index, forns index and FIB-4 in the prediction of significant fibrosis and cirrhosis in patients with chronic hepatitis C. Turk J Gastroenterol. (2011) 22:279–85. doi: 10.4318/tjg.2011.0213
14. Parikh NS, Kamel H, Navi BB, Iadecola C, Merkler AE, Jesudian A, et al. Liver fibrosis indices and outcomes after primary intracerebral hemorrhage. Stroke. (2020) 51:830–7. doi: 10.1161/STROKEAHA.119.028161
15. Parikh NS, Jesudian A, Kamel H, Hanley DF, Ziai WC, Murthy SB. Liver Fibrosis and perihematomal edema growth in primary intracerebral hemorrhage. Neurocrit Care. (2021) 34:983–9. doi: 10.1007/s12028-020-01081-4
16. Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. (2011) 365:147–56. doi: 10.1056/NEJMra1011170
17. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. (2003) 38:518–26. doi: 10.1053/jhep.2003.50346
18. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. (2006) 43:1317–25. doi: 10.1002/hep.21178
19. Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. (2007) 46:32–6. doi: 10.1002/hep.21669
20. Hayati Rezvan P, Lee KJ, Simpson JA. The rise of multiple imputation: a review of the reporting and implementation of the method in medical research. BMC Med Res Methodol. (2015) 15:30. doi: 10.1186/s12874-015-0022-1
21. J. L. Schafer. Multiple imputation: a primer. Stat Methods Med Res. (1999) 8:3–15. doi: 10.1191/096228099671525676
22. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. (2015) 350:g7594. doi: 10.1136/bmj.g7594
23. Alba AC, Agoritsas T, Walsh M, Hanna S, Iorio A, Devereaux PJ, et al. Discrimination and calibration of clinical prediction models: users' guides to the medical literature. JAMA. (2017) 318:1377–84. doi: 10.1001/jama.2017.12126
24. Jaja BNR, Saposnik G, Lingsma HF, Macdonald E, Thorpe KE, Mamdani M, et al. Development and validation of outcome prediction models for aneurysmal subarachnoid haemorrhage: the SAHIT multinational cohort study. BMJ. (2018) 360:j5745. doi: 10.1136/bmj.j5745
25. Baik M, Kim SU, Kang S, Park HJ, Nam HS, Heo JH, et al. Liver fibrosis, not steatosis, associates with long-term outcomes in ischaemic stroke patients. Cerebrovasc Dis (Basel, Switzerland). (2019) 47:32–9. doi: 10.1159/000497069
27. You SC, Kim KJ, Kim SU, Kim BK, Park JY, Kim DY, et al. Hepatic fibrosis assessed using transient elastography independently associated with coronary artery calcification. J Gastroenterol Hepatol. (2015) 30:1536–42. doi: 10.1111/jgh.12992
Keywords: intracranial aneurysm, subarachnoid hemorrhage, liver fibrosis, mortality, prognosis
Citation: Li T, Wang P, Gong X, Chong W, Hai Y, You C, Kang J, Fang F and Zhang Y (2022) Prevalence and Prognostic Significance of Liver Fibrosis in Patients With Aneurysmal Subarachnoid Hemorrhage. Front. Neurol. 13:850405. doi: 10.3389/fneur.2022.850405
Received: 13 January 2022; Accepted: 28 April 2022;
Published: 02 June 2022.
Edited by:
Bedjan Behmanesh, Goethe University Frankfurt, GermanyReviewed by:
Juergen Konczalla, University Hospital Frankfurt, GermanyLuis Rafael Moscote-Salazar, Latinamerican Council of Neurocritical Care (CLaNi), Colombia
Copyright © 2022 Li, Wang, Gong, Chong, Hai, You, Kang, Fang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Zhang, dG50MTA1N0BvdXRsb29rLmNvbQ==; Juan Kang, a2owMzI5QGFsaXl1bi5jb20=
†These authors have contributed equally to this work
 Tiangui Li1,2†
Tiangui Li1,2† Peng Wang
Peng Wang Fang Fang
Fang Fang Yu Zhang
Yu Zhang