- 1The Fourth School of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou, China
- 2Department of Neurosurgery, Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 3Department of Intensive Care Unit, Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 4Department of Neurosurgery, Xixi Hospital Affiliated to Zhejiang Chinese Medical University, Hangzhou, China
- 5Central Laboratory, Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine, Hangzhou, China
Background: Cellular prion protein (PRPC) exerts brain-protective effects. We determined the relationship between plasma PRPC levels and disease severity plus clinical outcome after acute intracerebral hemorrhage (ICH).
Methods: A total of 138 ICH patients and 138 healthy controls were included in this prospective, observational study. Hematoma volume and Glasgow coma scale (GCS) score were used to assess disease severity. Glasgow outcome scale (GOS) scores of 1–3 and 4–5 at 90 days after stroke were defined as a poor outcome and good outcome, respectively. Using multivariate analysis, we discerned the relation of plasma PRPC levels to disease severity and poor outcome. The receiver operating characteristic (ROC) curve was built to evaluate the prognostic predictive capability.
Results: Plasma PRPC levels in ICH patients were significantly higher than those in healthy controls (median, 4.20 vs. 2.02 ng/ml; P < 0.001), and were independently correlated with GCS score (r = −0.645, P < 0.001) and hematoma volume (r = 0.627, P < 0.001). Plasma PRPC levels were highly correlated with GOS score (r = −0.762, P < 0.001), and were substantially higher in patients with poor outcomes than in those with the good outcomes. Using maximum Youden index, plasma PRPC levels >3.893 ng/ml distinguished the risk of poor outcome at 90 days, with a sensitivity of 86.4% and a specificity of 65.8% (area under the curve, 0.809; 95% confidence interval (CI), 0.737–0.881, P < 0.001). Plasma PRPC levels >3.893 ng/ml were independently associated with a poor 90-day outcome with an odds ratio of 12.278 (95% CI, 5.101–29.554).
Conclusion: Elevated plasma PRPC levels are significantly associated with disease severity and poor 90-day outcome in ICH patients, indicating that plasma PRPC may be used as a potential prognostic biomarker after ICH.
Introduction
Spontaneous intracerebral hemorrhage (ICH) is one of the most common severe diseases, characterized by acute onset, high disability and mortality, and poor outcome (1, 2). Secondary brain injury caused by ICH is considered to be an important factor affecting the outcome of patients. The pathophysiological mechanisms involved in secondary brain injury are very complex, including inflammatory response, destruction of the blood–brain barrier, brain edema, cytotoxic reaction, and oxidative stress (3–6). During recent decades, some biomarkers related to the occurrence and development of secondary brain injury have drawn interest for aiding in the assessment of clinical severity and prognostic prediction.
The cellular prion protein (PRPC), is a small, cell-surface glycoprotein notable primarily for its critical role in the pathogenesis of the neurodegenerative disorders known as prion diseases (7). PRPC can greatly be expressed in the central nervous system and could exert beneficial effects on the recovery of brain function (8, 9). PRPCs were substantially elevated in brain tissues of rats with acute ischemic stroke (10). In experimental traumatic brain injury, plasma PRPC levels of rats were significantly increased (11). Interestingly, patients with aneurysmal subarachnoid hemorrhage had a significant elevation of plasma PRPC levels, which were closely associated with severity and prognosis (12). Thus, PRPC is hypothesized to be released from injured brain tissues through the damaged blood–brain barrier after acute brain injury, and circulating PRPC may be a potential biomarker of acute brain injury. In the present study, we aimed to explore whether plasma PRPC levels are associated with the severity and clinical outcome of ICH.
Materials and Methods
Study Population
In this perspective, observational study, we recruited first-ever ICH patients admitted to the Department of Neurosurgery at our hospital from January 2019 to October 2021. We required that all patients should be hospitalized within 24 h after the onset of ICH symptoms. In this study, we required that ICH should result from amyloidosis or hypertensive arteriosclerosis. We further excluded those patients with (1) age of <18 years, (2) intracerebral bleeding as a result of head trauma, venous sinus thrombosis, intracranial arteriovenous malformation, moyamoya disease, hemorrhagic transformation of ischemic stroke, intracranial tumor, or intracranial aneurysm, (3) previous history of hemorrhagic or ischemic stroke or (4) comorbidities such as severe infection within a month, autoimmune diseases and known malignancies. Meanwhile, a group of healthy volunteers, who were recruited at our hospital from January 2019 to October 2021, were selected as controls. Controls had normal routine laboratory tests, had no history of surgery, and were free of other diseases, such as hypertension, diabetes mellitus, hyperlipidemia, malignancies, infection, and stroke.
Assessments
We collected the subjects' information, such as gender, age, previous specific diseases (such as hypertension, diabetes, and hyperlipidemia), cigarette smoking, alcohol drinking, vital signs, and medication. The clinical severity was assessed at admission using the Glasgow Coma Scale (GCS). The ICH volume was determined by the ABC/2 method (13). A noninvasive technique was performed at admission to measure systolic and diastolic blood pressures. We used the Glasgow outcome scale (GOS) at 90 days after stroke as the evaluation criterion of neurologic function and defined a GOS score of 1–3 as a poor outcome.
Determinations
Venous blood samples of ICH patients were immediately taken upon completion of the head CT scan, and those of healthy controls were collected at entry into the study. The blood samples were then centrifuged and frozen in a −80°C freezer until final measurement. Plasma PRPC levels were measured using a commercial enzyme-linked immunosorbent assay kit according to the designated instructions, and the same experimenter completed all testing procedures, and it was ensured that the experimenter did not know the information about the participants.
Statistical Analysis
SPSS 23.0 was used for statistical analysis. All quantitative variables were non-normally distributed and thereby were expressed as median (upper and lower quartiles). Mann–Whitney U rank-sum test was used for their intergroup comparisons. The categorical variables were expressed as the number of cases (percentage) and the comparison between two groups was performed by the χ2 test or Fisher's exact method. Spearman correlation coefficient analysis was utilized for the correlation test. Logistic regression analysis was done to assess the relationship between plasma PRPC levels and poor outcomes at 90 days in ICH patients, and the odds ratio (OR) value and 95% confidence interval (CI) were calculated. The receiver operating characteristic (ROC) curve was constructed to investigate the predictive value of plasma PRPC levels for poor outcomes in ICH patients, and the cut-off value was determined using the Youden method. Meanwhile, the corresponding sensitivity and specificity were generated. Areas under the ROC curves (AUCs) were compared using the Z test. The difference of P < 0.05 was defined as statistical significance.
Results
Patient Selection and Characteristics
In this study, a total of 172 patients with first-ever ICH, who were admitted to our hospital within 24 h of onset, were initially included. Afterward, we excluded seven patients with secondary ICH, 11 patients with the previous history of stroke, 13 patients with severe diseases, and 3 patients with loss to follow-up. Finally, a total of 138 patients were enrolled in the study (Figure 1). In addition, 138 healthy controls were recruited. In Table 1, while there were no significant differences between the patients and the healthy controls in age (P = 0.145), sex percentage (P = 0.807), smoking (P = 0.533), and alcohol consumption (P = 0.130); percentages of hypertension (P < 0.001), diabetes mellitus (P < 0.001), and hyperlipidemia (P = 0.044) were substantially higher in the patients than in the controls.
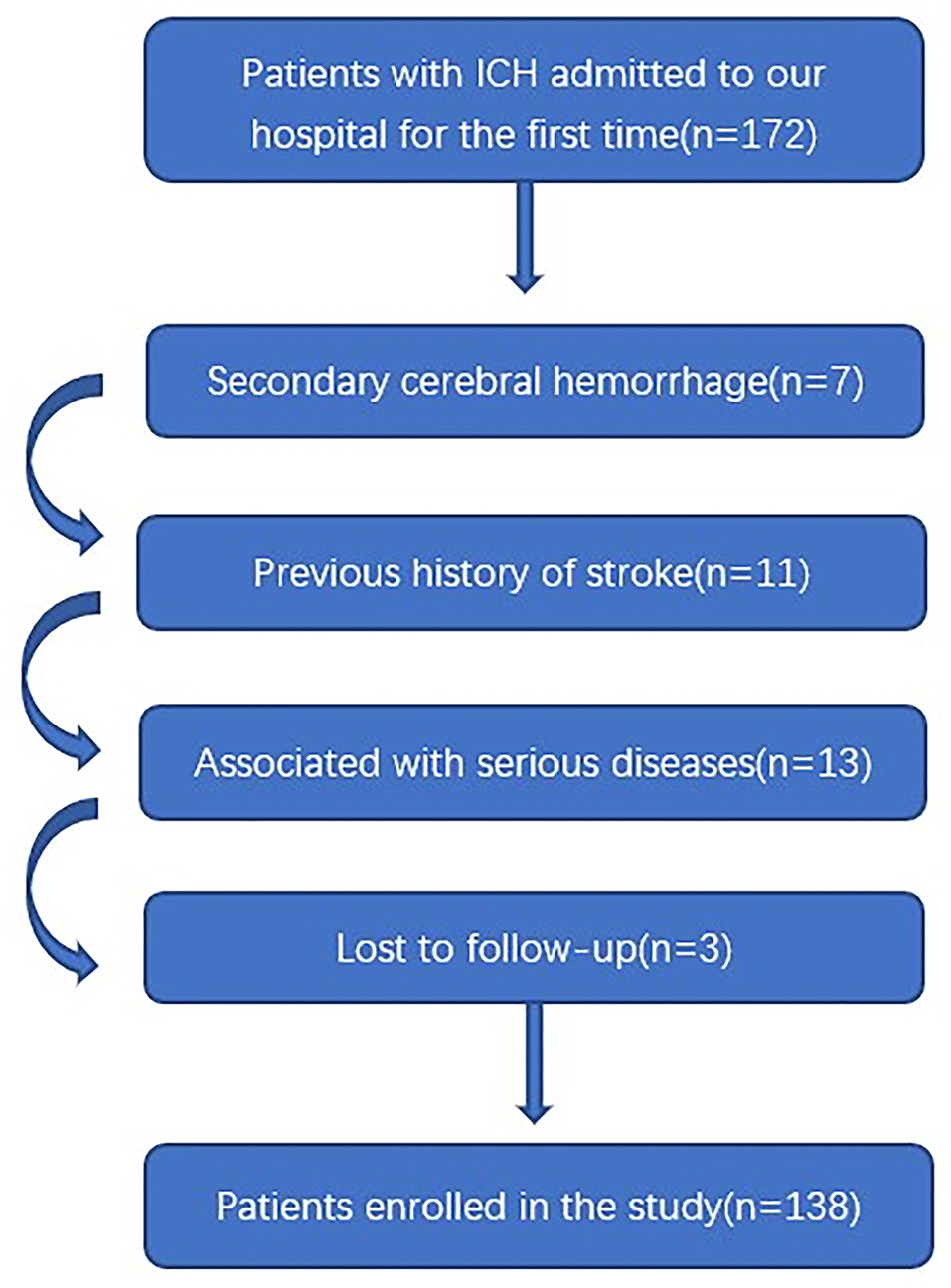
Figure 1. Flowing chart for screening eligible patients with intracerebral hemorrhage. ICH indicates intracerebral hemorrhage.
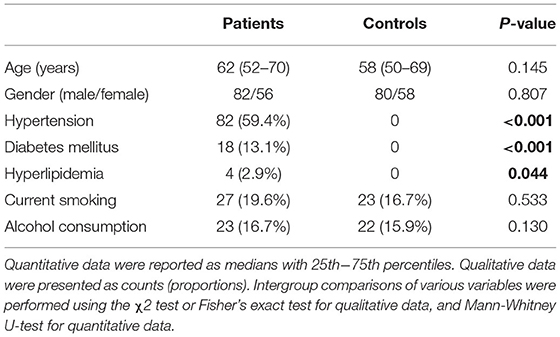
Table 1. Comparisons of demographic data and vascular risk factors between controls and patients with acute intracerebral hemorrhage.
There were 82 (59.4%) males and 56 (40.6%) females among ICH patients. Patients were aged from 23 to 89 years (median, 62 years; 25th−75th percentiles, 53–70 years). In total, 27 patients had a history of cigarette smoking and 32 patients had a history of alcohol drinking. We recorded previous medical histories, including hypertension (82 cases), diabetes (18 cases), and hyperlipidemia (4 cases). The median value of time between onset and admission was 6.7 h (range, 0.5–24 h; 25th−75th percentiles, 4–11.6 h), with their blood samples collected from 1 to 25 h (median, 7 h; 25th−75th percentiles, 4.8–12 h) after ICH. The median GCS score for severity assessment at admission was 13 (range, 4–15; 25th−75th percentiles, 9–15). According to imaging data, there were 24 patients (17.4%) with infratentorial hemorrhage and 21 patients (15.2%) with intraventricular hemorrhage. Median hematoma volume was 14.7 ml (25th−75th percentiles, 7.9–27.2 ml; range, 1.7–84.3 ml); median arterial systolic and diastolic blood pressures were 156 mmHg (range, 100–195 mmHg; 25th−75th percentiles, 140–167 mmHg) and 91 mmHg (range, 55–123 mmHg; 25th−75th percentiles, 78–100 mmHg). Laboratory examination showed a median blood leucocyte count of 8.7 × 109/L (range, 3.4–20.9 × 109/L; 25th−75th percentiles, 6.7–10.8 × 109/L), a median blood glucose level of 6.6 mmol/L (range, 2.5–21.2 mmol/L; 25th−75th percentiles, 5.3–8.2 mmol/L) and a median blood potassium level of 3.64 mmol/L (range, 2.76–5.20 mmol/L; 25th−75th percentiles, 3.43–3.91 mmol/L). At 90 days after ICH, a total of 59 patients had a poor outcome (GOS score 1–3).
Change of Plasma PRPC Levels
Plasma PRPC levels of ICH patients were 1.82–8.90 ng/ml, with a median of 4.20 ng/ml (25th−75th percentiles, 3.08–5.50 ng/ml). The median plasma PRPC levels in healthy controls were 2.02 ng/ml (25th−75th percentiles, 1.66–2.36 ng/ml). Using Mann–Whitney U-test, plasma PRPC levels were markedly higher in ICH patients than in healthy controls (P < 0.001, Figure 2).
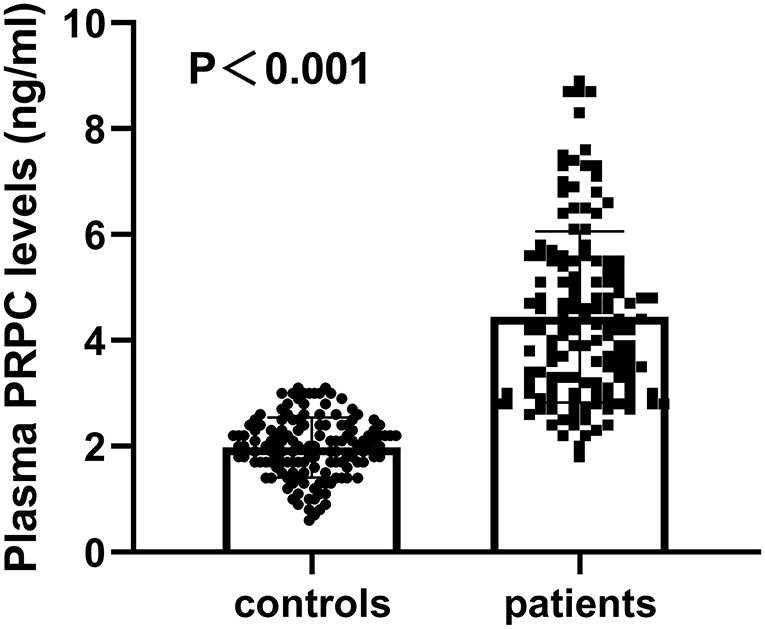
Figure 2. Differences in plasma cellular prion protein levels between healthy controls and patients with cerebral hemorrhage. Using the Mann–Whitney U-test, plasma cellular prion protein levels significantly higher in patients with ICH (n = 138) than in healthy controls (n = 138) (P < 0.001). Abbreviation: PRPC, cellular prion protein.
Correlation of Plasma PRPC Levels With Hemorrhagic Severity
In order to verify the correlation of plasma PRPC levels with hemorrhagic severity indicated by GCS score and hematoma volume, GCS score and hematoma volume were defined as quantitative data. ICH patients were divided into three groups by GCS score, with scores of 3–8, 9–12, and 13–15. At the same time, 30 ml hematoma volume was taken as the critical value to classify the ICH patients, with hematoma volume >30 ml as one group, and <30ml as the other group. Subsequently, plasma PRPC levels were significantly associated with GCS score (Figures 3A,B) and hematoma volume (Figures 3C,D) of ICH patients.
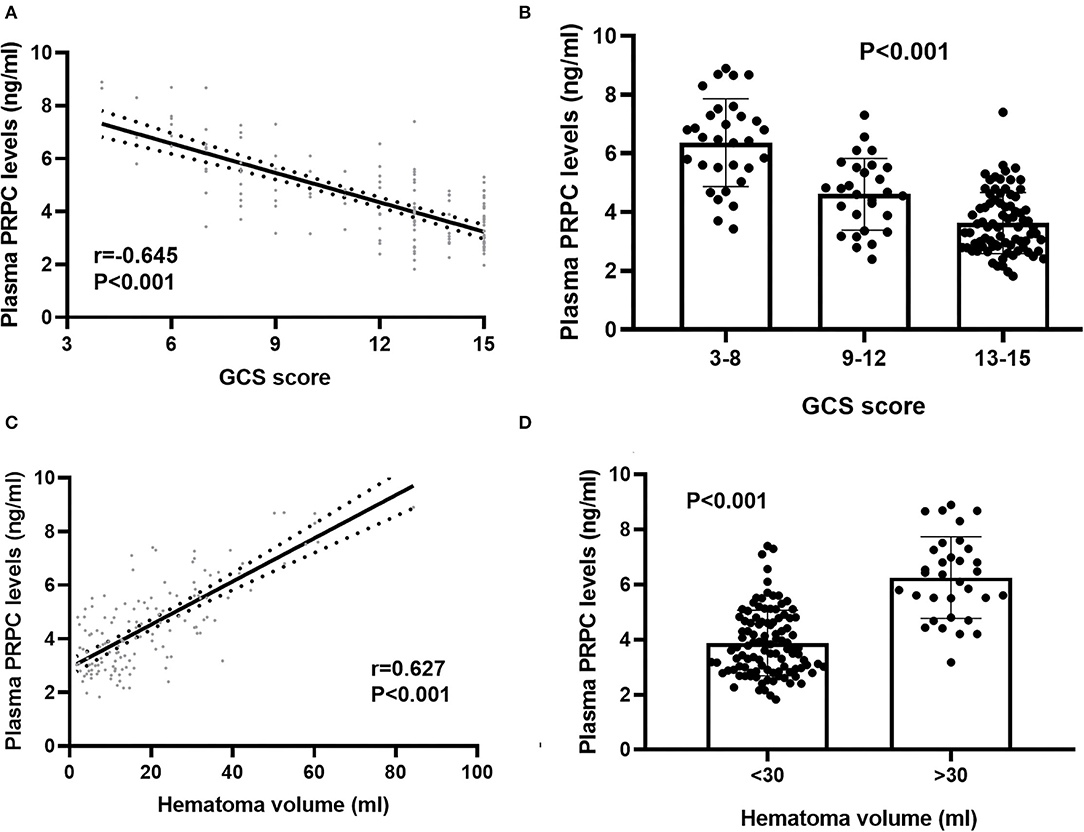
Figure 3. Relationship between plasma cellular prion protein levels and illness severity among intracerebral hemorrhage patients. (A) Correlation of plasma cellular prion protein levels with Glasgow Coma Scale score. Plasma cellular prion protein levels were significantly raised with decreasing Glasgow Coma Scale score using Spearman correlation coefficients (r = −0.645, P < 0.001). In a correlation graph, the solid line means the line of best fit, and the dashed line represents 95% confidence interval of a population mean. (B) Differences in plasma cellular prion protein levels among patients with different Glasgow Coma Scale scores. Glasgow Coma Scale score was identified as a categorical variable and subsequently, patients were divided into three groups in accordance with the Glasgow Coma Scale score, namely, 3–8 (n = 31), 9–12 (n = 28), and 13–15 (n = 79) and subsequently, patients with Glasgow Coma Scale score 3–8 had substantially highest plasma cellular prion protein levels, followed by Glasgow Coma Scale score 9–12 and then 13–15 using Kruskal–Wallis H-test (P < 0.001). (C) Correlation of plasma cellular prion protein levels with hematoma volume. Plasma cellular prion protein levels were substantially elevated with rising hematoma volume using Spearman correlation coefficients (r = 0.627, P < 0.001). In a correlation graph, the solid line means the line of best fit, and the dashed line represents 95% confidence interval of a population mean. (D) Differences in plasma cellular prion protein levels among patients with different hematoma volumes. Hematoma volume was identified as a categorical variable, patients with hematoma volume above 30 ml (n = 33) had substantially higher plasma cellular prion protein levels than those with hematoma volume below 30 ml (n = 105) using Mann–Whitney U-test (P < 0.001). Abbreviations: GCS, Glasgow Coma Scale; PRPC, cellular prion protein.
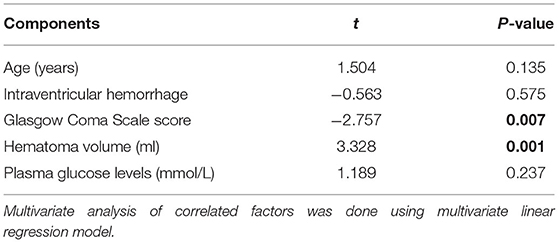
Bivariate correlation analysis showed that age (r = 0.189, P = 0.026), intraventricular hemorrhage (r = 0.346, P < 0.001), hematoma volume (r = 0.627, P < 0.001), GCS score (r = −0.645, P < 0.001), and blood glucose levels (r = 0.250, P = 0.003) were highly correlated with plasma PRPC levels (Tables 2, 3). Subsequent multivariate linear regression analysis of variables with significant differences in univariate analysis demonstrated that hematoma volume and GCS score remained independently correlated with plasma PRPC levels (Table 4).
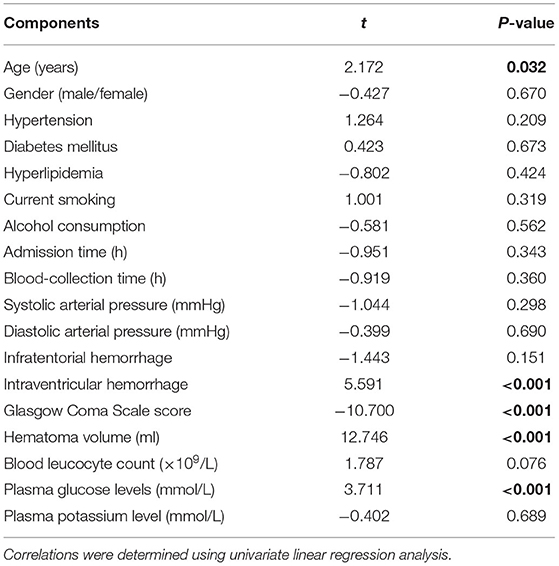
Table 2. Correlations between plasma cellular prion protein levels and other variables using univariate linear regression analysis in intracerebral hemorrhage.
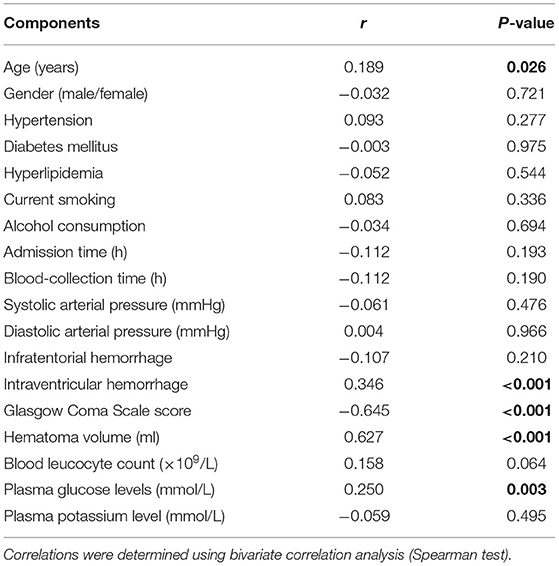
Table 3. Correlations between plasma cellular prion protein levels and other variables using bivariate correlation analysis in intracerebral hemorrhage.
Relationship Between Plasma PRPC Levels and Outcome
As shown in Figures 4A,B, plasma PRPC levels showed a significant downward trend when patients' GOS scores increased. In Figure 4C, plasma PRPC levels were significantly lower in patients with good outcomes than in those with poor outcomes. In addition, the ROC curve showed that plasma PRPC levels significantly predicted poor outcomes at 90 days after ICH (AUC, 0.809; 95% CI, 0.737–0.881), and plasma PRPC levels of 3.896 ng/ml was the cutoff value, yielding the corresponding sensitivity and specificity values of 86.4 and 65.8% (Maximum Youden index J, 0.522), respectively (Figure 5A). Intriguingly, Figure 5B showed that its discriminatory ability for poor outcome was equivalent to those of GCS score (AUC, 0.793; 95% CI, 0.716–0.857; P = 0.6462) and hematoma volume (AUC, 0.863; 95% CI, 0.795–0.916; P = 0.1062).
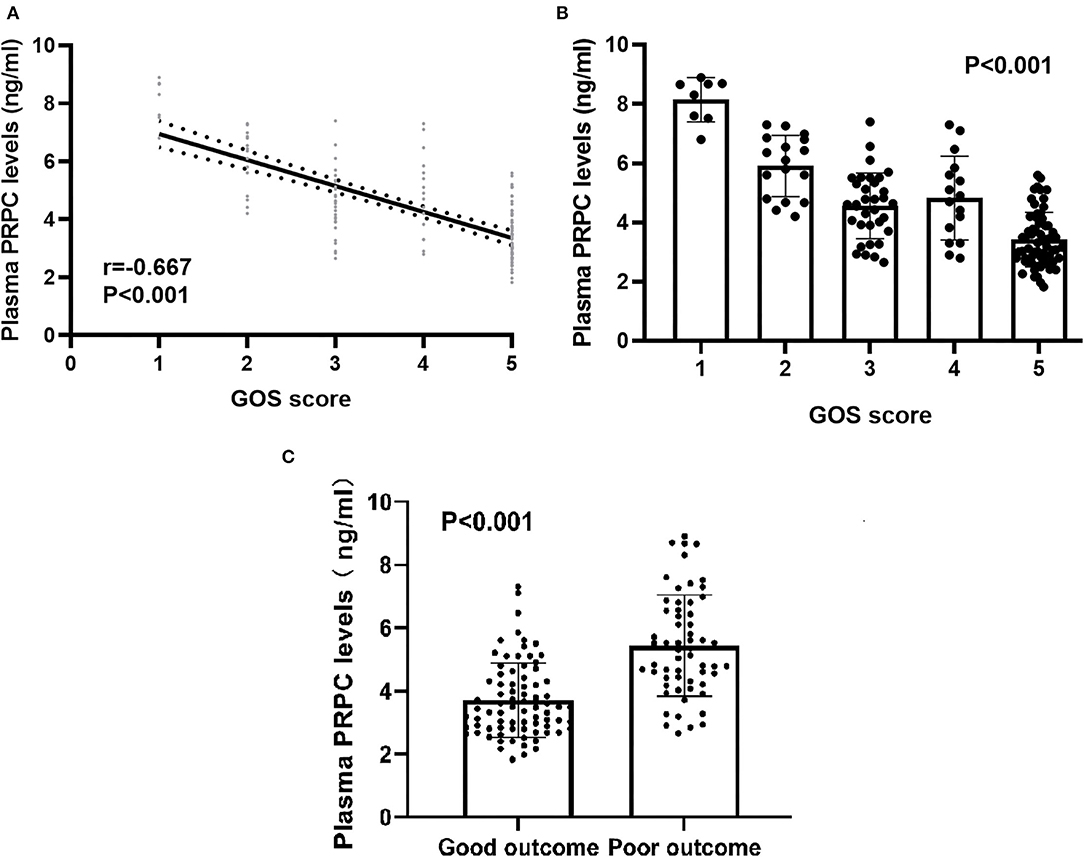
Figure 4. Relationship between plasma cellular prion protein levels and clinical outcome at 90 days after intracerebral hemorrhage. (A) Relationship between plasma cellular prion protein levels and Glasgow Outcome Scale score at 90 days after intracerebral hemorrhage. When the Glasgow Outcome Scale score was a continuous variable, plasma cellular prion protein levels were significantly declined with raised Glasgow Outcome Scale score using Spearman correlation coefficients (r = −0.667, P < 0.001). In a correlation graph, the solid line means the line of best fit, and the dashed line represents 95% confidence interval of a population mean. (B) Differences in plasma cellular prion protein levels among patients with Glasgow Outcome Scale score at 90 days after intracerebral hemorrhage. Plasma cellular prion protein levels significantly differed among patients with different Glasgow Outcome Scale scores using Kruskal–Wallis H-test (P < 0.001). (C) Comparison of plasma cellular prion protein levels between patients with good outcomes and those with poor outcomes at 90 days after intracerebral hemorrhage. Patients with a poor outcome (n = 59) had substantially higher plasma cellular prion protein levels than those with a good outcome (n = 79) using Mann–Whitney U-test (P < 0.001). Abbreviations: GOS, Glasgow Outcome Scale; PRPC, cellular prion protein.
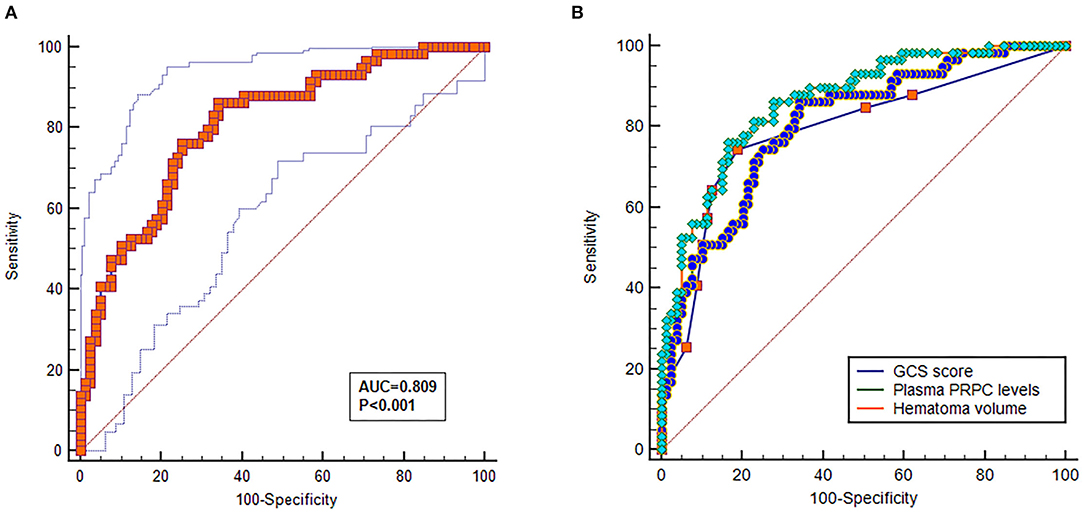
Figure 5. Predictive value of plasma cellular prion protein levels for poor outcome at 90 days after intracerebral hemorrhage. (A) Discriminatory ability of plasma cellular prion protein levels for poor outcome at 90 days after intracerebral hemorrhage. Under the receiver-operating characteristic curve, plasma cellular prion protein levels remarkably predicted the poor outcome at 90 days after intracerebral hemorrhage (area under the curve, 0.809; 95% confidence interval, 0.737–0.881); and plasma cellular prion protein levels more than 3.893 ng/ml distinguished patients with development of 90-day poor outcome with 86.4% specificity and 65.8% sensitivity, (Youden index J, 0.552). (B) Comparison of discriminatory capability with respect to plasma cellular prion protein levels, Glasgow Coma Scale score, and hematoma volume for 90-day poor outcome following acute intracerebral hemorrhage. Under receiver operating characteristic curve, prognostic predictive ability of plasma cellular prion protein levels (area under curve, 0.809; 95% confidence interval, 0.737–0.881) was similar to those of Glasgow Coma Scale score (area under curve, 0.793; 95% confidence interval, 0.716–0.857; P = 0.6462) and hematoma volume (area under curve, 0.863; 95% confidence interval, 0.795–0.916; P = 0.1062). Abbreviations: GOS, Glasgow Outcome Scale; PRPC, cellular prion protein.
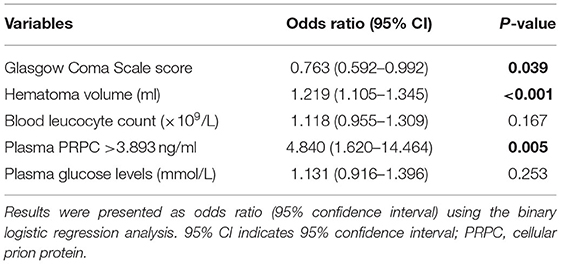
There was a significantly higher percentage of cases with plasma PRPC levels above 3.893 ng/ml in patients with poor outcomes (GOS score 1–3) than in those with good outcomes (GOS score 4–5). In addition, as compared to patients with good outcomes, those with poor outcomes had significantly higher hematoma volume, blood leukocyte count, and glucose levels, as well as displayed substantially lower GCS scores (Tables 5, 6). Those above-mentioned variables, which were significant in univariate analysis, were included in the multivariable model, and subsequently, it was revealed that the independent predictors for poor outcome at 90 days after ICH were hematoma volume, GCS score, and plasma PRPC levels higher than 3.893 ng/ml (Table 7).
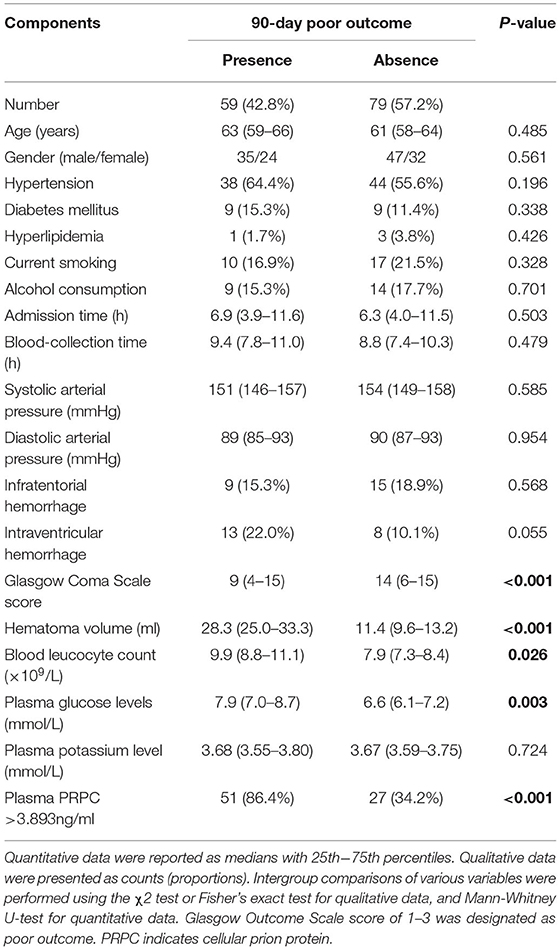
Table 5. Demographic, clinical, radiological and biochemical factors for 90-day poor outcome after acute intracerebral hemorrhage.

Table 6. Predictive factors of 90-day poor outcome among patients with intracerebral hemorrhage using univariable logistic regression analysis.
Discussion
To the best of our knowledge, this study is the first series to investigate the relationship between plasma PRPC levels and hemorrhagic severity in addition to clinical outcomes in ICH patients. Our study confirmed that (1) as compared with controls, PRPC levels in the plasma of patients with ICH were increased significantly; (2) plasma PRPC levels, as a continuous variable, were independently related to hematoma volume and GCS score; (3) plasma PRPC levels, as a categorical variable, were independently associated with 90-day poor outcome after ICH; (4) plasma PRPC levels exhibited similar prognostic predictive ability, as compared to GCS score and hematoma volume. Hence, it is assumed that plasma PRPC may be a potential biomarker for assessing disease severity and predicting poor outcomes in patients with hemorrhagic stroke.
Cellular prion protein (PRPC) is a ubiquitously expressed plasma membrane glycoprotein (14). Accumulating experimental data have shown that PRPC may exert neuroprotective effects via various forms, including anti-inflammatory and anti-oxidative stress, promoting neurogenesis and angiogenesis, mediating cell protection signaling pathways, and maintaining the integrity of the blood–brain barrier after acute brain injury (15–23). PRPC expressions were significantly elevated in the ischemic hemisphere of mice with permanent focal ischemia (24). Specifically, the expression of PRPC was mainly enhanced in the cell bodies of neurons, microvascular endothelial cells, and inflammatory cells of brain tissue around the ischemic infarction (8). In patients with acute stroke, PRPC expressions were also up-regulated substantially in peri-infarcted brain tissues (25). Presumably, due to its neuroprotective characteristics, up-regulation of PRPC expressions in injured brain tissues may be a supplement against brain damage.
Although a pilot study of 37 controls and 20 head trauma patients showed an insignificant elevation of plasma PRPC levels (26), other studies on acute ischemic stroke and aneurysmal subarachnoid hemorrhage have found similar results that plasma PRPC levels were substantially raised after acute brain injury (12, 27). In line with the two preceding studies (12, 27), the current study was supportive of the notion that circulating PRPC levels should be enhanced markedly after acute brain injury. It has been verified that PRPC was greatly expressed in surrounding brain tissues after acute ischemic stroke in humans or animals (27, 28). Hence, it is assumed that PRPC in the peripheral blood may be at least partly derived from damaged brain tissues.
In patients with ischemic infarction, plasma PRPC levels are highly correlated with NIHSS scores (27). Meanwhile, in the aneurysmal subarachnoid hemorrhage patients, plasma PRPC levels were intimately related to the World Federation of Neurological Surgeons scale score, GCS score, Hunt-Hess score, and modified Fisher score (12). The above-mentioned results indicate that plasma PRPC may be a potential biomarker for reflecting the degree of brain injury. However, only univariate correlation analysis was done in the preceding epidemiological investigations. Intriguingly, using the multivariate linear regression model, we found that plasma PRPC levels were independently correlated with GCS score and hematoma volume after ICH. So, our study further supplies solid evidence supporting the notion that plasma PRPC should have the potential to serve as a promising biomarker for severity assessment after ICH. PRPC is identified as a ubiquitously expressed plasma membrane glycoprotein (14), and therefore may be released when neurons are damaged. Overall, it is assumed that PRPC may reflect the degree of neuronal injury, and plasma PRPC levels may be related to the extent of cellular membrane disruption by the form of cellular insult.
In patients with aneurysmal subarachnoid hemorrhage, plasma PRPC emerged as an independent predictor for 90-day poor outcome, but not for delayed cerebral infarction (12). Our study defined GOS scores 1–3 at 90 days after ICH as a poor outcome. Besides GCS score and hematoma volume, plasma PRPC levels were revealed to be independently associated with 90-day poor outcomes in the current study. Of note, plasma PRPC levels displayed similar prognostic predictive ability, as compared to GCS score and hematoma volume. Overall, plasma PRPC may be a useful prognostic biomarker for ICH.
Conclusion
This is the first study to investigate the role of plasma PRPC levels in the prognosis of ICH patients and further demonstrate that elevated plasma PRPC levels, in close correlation with illness severity reflected by GCS score and hematoma volume, are independently associated with 90-day poor outcome after ICH. The preceding data substantialize plasma PRPC as a promising prognostic biomarker of ICH.
Limitation
The current study has several limitations. First, this is a single-center study, which is characterized by small sample size, and therefore, a further cohort study with a larger sample size is required to prove the current conclusions. Second, plasma PRPC levels were only tested at the admission time. Hence, investigation of its dynamic change may be of clinical significance. Finally, in this study, there were significant differences in terms of comorbid status (hypertension, diabetes mellitus, and hyperlipidemia) between controls and patients with ICH. So, this sort of heterogeneity may lead to false-positive errors in changes in plasma PRPC levels after ICH. In the future, a control group with well-balanced comorbid status should be selected to validate the results.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
XW and ML contributed to the conception and design of the study, analysis and interpretation of the data, as well as drafting, and revision of the manuscript. XD and KW contributed to the analysis of the data, revision of the manuscript, and final approval of the manuscript. TY and ZW participated in the study design and performed the follow-up. WY, QD, WH, ZZ, and YZ helped to sort data. All authors contributed to the article and approved the submitted version.
Funding
This work is financially supported by Key Research and Development Projects of Zhejiang Province (Grant. 2020C03071) and Construction Fund of Medical Key Disciplines of Hangzhou (Nos. OO20200485 and OO20200055).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Witsch J, Siegerink B, Nolte CH, Sprügel M, Steiner T, Endres M, et al. Prognostication after intracerebral hemorrhage: a review. Neurol Res Pract. (2021) 3:22. doi: 10.1186/s42466-021-00120-5
2. An SJ, Kim TJ, Yoon BW. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke. (2017) 19:3–10. doi: 10.5853/jos.2016.00864
3. Lan X, Han X, Li Q, Yang QW, Wang J. Modulators of microglial activation and polarization after intracerebral hemorrhage. Nat Rev Neurol. (2017) 13:420–33. doi: 10.1038/nrneurol.2017.69
4. Lan X, Han X, Liu X, Wang J. Inflammatory responses after intracerebral hemorrhage: from cellular function to therapeutic targets. J Cereb Blood Flow Metab. (2019) 39:184–6. doi: 10.1177/0271678X18805675
5. Keep RF, Hua Y, Xi G. Intracerebral hemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. (2012) 11:720–31. doi: 10.1016/S1474-4422(12)70104-7
6. Wang G, Wang L, Sun XG, Tang J. Hematoma scavenging in intracerebral hemorrhage: from mechanisms to the clinic. J Cell Mol Med. (2018) 22:768–77. doi: 10.1111/jcmm.13441
7. Crestini A, Santilli F, Martellucci S, Carbone E, Sorice M, Piscopo P, et al. Prions and neurodegenerative diseases: a focus on Alzheimer's disease. J Alzheimers Dis. (2022) 85:503–18. doi: 10.3233/JAD-215171
8. Puig B, Yang D, Brenna S, Altmeppen HC, Magnus T. Show me your friends and I tell you who you are: the many facets of prion protein in stroke. Cells. (2020) 9:1609. doi: 10.3390/cells9071609
9. Watts JC, Bourkas MEC, Arshad H. The function of the cellular prion protein in health and disease. Acta Neuropathol. (2018) 135:159–78. doi: 10.1007/s00401-017-1790-y
10. Shyu WC, Lin SZ, Chiang MF, Ding DC, Li KW, Chen SF, et al. Overexpression of PrPC by adenovirus-mediated gene targeting reduces ischemic injury in a stroke rat model. J Neurosci. (2005) 25:8967–77. doi: 10.1523/JNEUROSCI.1115-05.2005
11. Pham N, Sawyer TW, Wang Y, Jazii FR, Vair C, Taghibiglou C. Primary blast-induced traumatic brain injury in rats leads to increased prion protein in plasma: a potential biomarker for blast-induced traumatic brain injury. J Neurotrauma. (2015) 32:58–65. doi: 10.1089/neu.2014.3471
12. Yao H, Lv C, Luo F, He C. Plasma cellular prion protein concentrations correlate with severity and prognosis of aneurysmal subarachnoid hemorrhage. Clin Chim Acta. (2021) 523:114–9. doi: 10.1016/j.cca.2021.09.010
13. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. (1996) 27:1304–5. doi: 10.1161/01.STR.27.8.1304
14. Thellung S, Corsaro A, Bosio AG, Zambito M, Barbieri F, Mazzanti M, et al. Emerging role of cellular prion protein in the maintenance and expansion of glioma stem cells. Cells. (2019) 8:1458. doi: 10.3390/cells8111458
15. Sekar S, Zhang Y, Miranzadeh Mahabadi H, Parvizi A, Taghibiglou C. Low-field magnetic stimulation restores cognitive and motor functions in the mouse model of repeated traumatic brain injury: role of cellular prion protein. J Neurotrauma. (2019) 36:3103–14. doi: 10.1089/neu.2018.5918
16. Turu M, Slevin M, Ethirajan P, Luque A, Elasbali A, Font A, et al. The normal cellular prion protein and its possible role in angiogenesis. Front Biosci. (2008) 13:6491–500. doi: 10.2741/3169
17. Santos TG, Silva IR, Costa-Silva B, Lepique AP, Martins VR, Lopes MH. Enhanced neural progenitor/stem cells self-renewal via the interaction of stress-inducible protein 1 with the prion protein. Stem Cells. (2011) 29:1126–36. doi: 10.1002/stem.664
18. Kim BH, Lee HG, Choi JK, Kim JI, Choi EK, Carp RI, et al. The cellular prion protein (PrPC) prevents apoptotic neuronal cell death and mitochondrial dysfunction induced by serum deprivation. Brain Res Mol Brain Res. (2004) 124:40–50. doi: 10.1016/j.molbrainres.2004.02.005
19. Watt NT, Taylor DR, Gillott A, Thomas DA, Perera WS, Hooper NM. Reactive oxygen species-mediated beta-cleavage of the prion protein in the cellular response to oxidative stress. J Biol Chem. (2005) 280:35914–21. doi: 10.1074/jbc.M507327200
20. Bakkebø MK, Mouillet-Richard S, Espenes A, Goldmann W, Tatzelt J, Tranulis MA. The cellular prion protein: a player in immunological quiescence. Front Immunol. (2015) 6:450. doi: 10.3389/fimmu.2015.00450
21. Milhavet O, McMahon HE, Rachidi W, Nishida N, Katamine S, Mangé A, et al. Prion infection impairs the cellular response to oxidative stress. Proc Natl Acad Sci USA. (2000) 97:13937–42. doi: 10.1073/pnas.250289197
22. Hoshino S, Inoue K, Yokoyama T, Kobayashi S, Asakura T, Teramoto A, et al. Prions prevent brain damage after experimental brain injury: a preliminary report. Acta Neurochir Suppl. (2003) 86:297–9. doi: 10.1007/978-3-7091-0651-8_64
23. Martellucci S, Santacroce C, Santilli F, Piccoli L, Delle Monache S, Angelucci A, et al. Cellular and molecular mechanisms mediated by recPrPC involved in the neuronal differentiation process of mesenchymal stem cells. Int J Mol Sci. (2019) 20:345. doi: 10.3390/ijms20020345
24. Weise J, Sandau R, Schwarting S, Crome O, Wrede A, Schulz-Schaeffer W, et al. Deletion of cellular prion protein results in reduced Akt activation, enhanced post-ischemic caspase-3 activation, and exacerbation of ischemic brain injury. Stroke. (2006) 37:1296–300. doi: 10.1161/01.STR.0000217262.03192.d4
25. McLennan NF, Brennan PM, McNeill A, Davies I, Fotheringham A, Rennison KA, et al. Prion protein accumulation and neuroprotection in hypoxic brain damage. Am J Pathol. (2004) 165:227–35. doi: 10.1016/S0002-9440(10)63291-9
26. Persad A, Pham N, Moien-Afshari F, Gormley W, Yan S, Mannix R, et al. Plasma PrPC and ADAM-10 as novel biomarkers for traumatic brain injury and concussion: a pilot study. Brain Inj. (2021) 35:734–41. doi: 10.1080/02699052.2021.1900602
27. Mitsios N, Saka M, Krupinski J, Pennucci R, Sanfeliu C, Miguel Turu M, et al. Cellular prion protein is increased in the plasma and peri-infarcted brain tissue after acute stroke. J Neurosci Res. (2007) 85:602–11. doi: 10.1002/jnr.21142
Keywords: severity, prognosis, intracerebral hemorrhage, cellular prion protein, biomarker
Citation: Wu X, Liu M, Yan T, Wang Z, Yu W, Du Q, Hu W, Zheng Y, Zhang Z, Wang K and Dong X (2022) Plasma PRPC Levels Correlate With Severity and Prognosis of Intracerebral Hemorrhage. Front. Neurol. 13:913926. doi: 10.3389/fneur.2022.913926
Received: 07 April 2022; Accepted: 14 June 2022;
Published: 11 July 2022.
Edited by:
Changiz Taghibiglou, University of Saskatchewan, CanadaReviewed by:
Sathiya Sekar, University of British Columbia, CanadaPaulina Carmona-Mora, University of California, Davis, United States
Amit Persad, University of Saskatchewan, Canada
Copyright © 2022 Wu, Liu, Yan, Wang, Yu, Du, Hu, Zheng, Zhang, Wang and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keyi Wang, d2t5X2phY2t5QDE2My5jb20=; Xiaoqiao Dong, ZHhxaHl5QDE2My5jb20=
†These authors have contributed equally to this work
 Xiaoyu Wu1†
Xiaoyu Wu1† Xiaoqiao Dong
Xiaoqiao Dong