Abstract
Background:
Post-exertional malaise (PEM) is considered a hallmark characteristic of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). This may also apply to subgroups of patients with long COVID-induced ME/CFS. However, it is uncertain to what extent PEM is acknowledged in routine specialist healthcare for ME/CFS patients, and how this affects patient outcomes.
Objective:
This study aims to evaluate to what extent ME/CFS patients experienced focus on PEM in specialist healthcare practice and its significance for outcome and care quality.
Methods:
Data from two online cross-sectional surveys covering specialist healthcare services for ME/CFS patients at rehabilitation institutes in Norway and two regional hospitals, respectively, were analyzed. Evaluations of 788 rehabilitation stays, 86 hospital consultations, and 89 hospital interventions were included. Logistic regression models and Mann–Whitney U-tests were used to quantify the impact of addressing PEM on health and functioning, care satisfaction, or benefit. Spearman’s rank correlation and Cronbach’s alpha of focus on PEM with the respondents’ perception of healthcare providers’ knowledge, symptom acknowledgment, and suitability of intervention were assessed as measures for care quality and their internal consistency, respectively.
Results:
PEM was addressed in 48% of the rehabilitation stays, 43% of the consultations, and 65% of the hospital interventions. Failure to address PEM roughly doubled the risk of health deterioration, following rehabilitation (OR = 0.39, 95% CI 0.29–0.52; 40.1% vs. 63.2% P = <0.001) and hospital intervention (OR = 0.34, 95% CI 0.13–0.89; 22.4% vs. 45.2%, p = 0.026). The focus on PEM (PEM-focus) during the clinical contact was associated with significantly higher scores on patients’ rated care satisfaction and benefit of both consultation and intervention. Furthermore, addressing PEM was (inter)related to positive views about healthcare providers’ level of knowledge of ME/CFS, their acknowledgment of symptoms, obtained knowledge, and the perceived suitability of intervention (Cronbach’s alpha ≥0.80).
Discussion:
PEM is still frequently not acknowledged in specialist healthcare practice for ME/CFS patients in Norway. Not addressing PEM substantially increased the probability of a decline in health and functioning following the intervention and was strongly associated with reduced perceived care quality, satisfaction, and benefit. These findings may be related to the applied explanatory models for ME/CFS and are most likely of relevance to long COVID.
1 Introduction
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a long-term, severe multisystem disease with a distinctive clinical picture, often, but not necessarily, preceded by an infection. Its pathophysiology is still uncertain; therefore, some clinical and research settings apply a biopsychosocial explanatory model for ME/CFS. In these settings, ME/CFS is perceived as a fatigue illness, explained with a psychosomatic understanding as a maladaptive response to an infection or overload, perpetuated by dysfunctional personality factors or beliefs, health anxiety, and deconditioning (1–3). This approach has been criticized for overlooking the evidence of detectable pathophysiological disturbances explaining the symptoms of ME/CFS patients (4–7). Others, however, apply a biomedical approach and consider ME/CFS as a maladaptive pathophysiological response, following an infection or other trigger that remains inadequately studied.
This biomedical explanatory model is acknowledged in the diagnostic criteria sets for ME/CFS that have been defined during the last two decades (6, 8–11). These criteria are more specific than earlier criteria. Core symptoms are fatigue, exertion-induced worsening of disease and symptoms, cognitive dysfunction, and sleep dysfunction (11). Furthermore, immune dysfunction, orthostatic intolerance, neuroendocrine, circulatory, and gastrointestinal dysfunction are common symptoms, while mental illness as the cause of the symptoms is explicitly excluded.
Exertion-induced aggravation of symptoms in ME/CFS is generally called post-exertional malaise (PEM) or post-exertional symptom exacerbation (PESE). It involves a relatively long-lasting and severe worsening of symptoms and/or the appearance of new symptoms, with a further substantial reduction in functioning (9, 12, 13). It may be an immediate or a delayed, disproportionate response to physical, orthostatic, or cognitive effort, or sensory stimuli, which previously were tolerated. It can take days, weeks, or longer to return to baseline (12, 14, 15). Sometimes, a new, more severe baseline is established. The delayed onset and the broad constellation of symptom deteriorations distinguish ME/CFS from other diseases with severe fatigue or deconditioning (6, 16–21). PEM is widely recognized as the most debilitating and persistent feature of ME/CFS (22). The PEM phenomenon has been demonstrated in multiple studies, both with patient-reported outcome measures and with objective measures. Objective findings include new or increased structural and functional abnormalities, following controlled exertion situations (23–26). These findings indicate disturbances in energy metabolism and a dysfunctional autonomic nervous system, impairing the body’s ability to recover from exertion (27, 28). In current practice in Norway, PEM is at best evaluated by anecdotal described experiences; standardized questionnaires (18) or clinical objective PEM, e.g., repeated hand grip strength (29) or cardiopulmonary exercise tests (25), is not routine practice.
If a psychosomatic understanding is applied to approach ME/CFS, PEM is usually disregarded and rather considered as a dysfunctional cognition and extreme behavioral response (30). Interventions typically aim at interrupting the self-perpetuating vicious circle that is thought to maintain symptoms. Assumed mistaken illness beliefs, dysfunctional cognitions, and fear of activity are aimed to be corrected by increasing physical activity to overcome avoidance behavior and regain physical fitness (31, 32). In this view, commonly applied approaches are cognitive behavioral therapy (CBT) and graded exercise therapy (GET), respectively, or varieties that share central conceptual elements.
Current research and clinical recommendations that acknowledge a biomedical base and the PEM phenomenon, however, recognize that there is currently no scientific evidence for effective treatment of ME/CFS and explicitly discourage curative CBT and GET forms (10, 33–37). Instead, pacing strategies are considered to be the most effective approach to reduce the risk of PEM relapse and retain or improve physical functioning and quality of life (10, 36–40).
In Norway, the main responsibility of the diagnosing process of adults with ME/CFS symptoms is held by the general practitioner (GP), preferably a specialist in general medicine (41). In case of unclear differential diagnostic issues, the GP should refer to relevant clinical specialists for further evaluation. The European Network on ME/CFS (EUROMENE) expert consensus (36) recommends referral to specialist service for confirmation of diagnosis, drug treatment, and a range of service offerings, such as multidisciplinary rehabilitation, supportive counseling, education on self-management, and symptom-contingent pacing.
In several studies (42–45), various aspects of perceived care quality in specialist healthcare for ME/CFS patients have been evaluated, but they did not focus on the attention to PEM. In general, only a minority was satisfied with the obtained care and specialists’ knowledge about ME/CFS. In another, recent Norwegian study, however, perceived care quality was evaluated related to the specificity of the diagnosis and PEM severity (42). Patients meeting more specific criteria and patients with higher PEM scores reported more negative experiences with specialist care.
It appears essential to acknowledge PEM in the diagnostic process and therapeutic approach of ME/CFS. To our knowledge, it is inadequately documented to what degree PEM generally is addressed in ordinary healthcare practice or more specifically in specialist healthcare practice in Norway. Likewise, it is insufficiently documented what the consequences are of not addressing PEM for the patient-related outcome and the perceived quality of these services regarding clinical effectiveness, patient safety, and patient experiences.
Awareness and knowledge about PEM seem also of specific relevance for a new growing subgroup of patients facing similar symptoms and biological abnormalities, and PEM (46–52). In the patients with persistent, debilitating symptoms following acute COVID-19 (long COVID), approximately up to half of the patients will meet the diagnostic criteria for ME/CFS (46, 51, 53). Consequently, ME/CFS prevalence is increasing dramatically. Addressing PEM in the approach of long COVID patients is of specific importance as well (54–56).
The aim of this present study was to assess the significance of acknowledging the PEM phenomenon in the clinical approach of ME/CFS patients in specialist healthcare practice.
The first objective was to evaluate to what extent ME/CFS patients experienced focus on PEM during clinical consultations, hospital intervention, or rehabilitation.
The second objective was to estimate to what degree focus on PEM in the received care is related to patient-reported outcomes. The primary outcome is the impact of addressing PEM during an intervention on subsequent changes in health status. The secondary outcome measures are the reported care satisfaction or the perceived general benefit of the obtained care.
The third objective was to assess whether the acknowledgment of PEM in a clinical situation is associated with patient-reported experiences of perceived healthcare quality.
2 Materials and methods
This study is a non-prespecified secondary analysis, applying data from two patient surveys executed by the Norwegian ME Association (NMEF). The two patient surveys focused on different healthcare settings, but objectives, methods, and questionnaires are partly similar and described below.
Both surveys were retrospective, anonymous, Internet-based on the platform SurveyMonkey and limited to one response per IP address. There were no time restrictions on response during the study period as the questionnaire remained open until submitted.
The objective of the hospital survey (57) was to evaluate the experiences of ME/CFS patients with specialist healthcare services at two regional hospitals in Southeast Norway. These healthcare services covered two different types of clinical settings: consultations and interventions. Experiences with these settings were evaluated separately in the analyzes. Data collection was performed in the period 5–31 March 2022 and aimed at covering the period since 2017. If they in that period had received care at different departments, the respondents reported that separately, but for each department, only once per consultation and once per intervention.
The rehabilitation survey (58) aimed at retrospectively mapping the experiences of ME/CFS patients with Norwegian rehabilitation services. Data collection was carried out from 4 September 2017 until 15 October 2017 with no restrictions on region and date of stay at one of the rehabilitation facilities.
For the current study, analyzes were restricted to adult respondents included in the surveys. Respondents should have obtained hospital care at one of the two concerning hospitals or rehabilitation at an institute in Norway. Furthermore, they should have an ME/CFS diagnosis or long COVID with PEM and have answered the question concerning PEM-focus in the obtained healthcare setting. Figure 1 presents the flow chart of the study.
Figure 1

Study flow chart of survey 1 and survey 2, showing the inclusion of respondents and identification of corresponding evaluated care events for analyzes.
2.1 Subjects
For both surveys, invitations were shared on various relevant open and closed Norwegian Facebook groups for ME/CFS patients, their relatives, and other interested parties, both within and outside the ME Association’s auspices. Relatives could answer on behalf of patients who were too ill to answer themselves.
In the hospital survey, members of Vestfold and Telemark Regional ME Association were also directly approached by email. The survey was open for respondents who had been referred to the relevant hospitals during the last 5 years and had an ME/CFS or long COVID diagnosis, were in a diagnosing process for this, or considered themselves as having ME/CFS, post-viral syndrome, or long COVID with PEM. Before evaluating the occurrence of PEM, as well as other typical ME/CFS symptoms, PEM was explained in the survey. Then, the respondents reported which diagnosis they regarded as the most appropriate for them. Only the respondents that answered, “ME/CFS or ME,” “sequela after COVID-19 infection with PEM” or “Post-viral syndrome” and had PEM could progress further in the survey. Respondents not being adults (here below 20) were excluded from the analyzes in the analysis.
In the rehabilitation survey, respondents residing in Norway who previously had obtained an ME/CFS diagnosis G93.3 (59) from the specialist health service or A04 (60) from a GP specialist in general medicine were invited to participate.
2.2 Measures
All measures are presented in Tables 1, 2, including applied methods for dichotomization of variables, if relevant. The complete questionnaires (in Norwegian) can be found in the underlying reports (57, 58).
Table 1
| Domain | Survey 1 Hospital consultation |
Survey 1 Hospital intervention |
Survey 2 Rehabilitation |
|---|---|---|---|
| Respondents’ characteristics | |||
| Gender | Female/male | 1. Female, 2. male | - |
| Age | < 10, 10–19, 20–29, 30–39, 40–49, 50–59, 60–69, ≥ 70 | < 10, 10–19, 20–29, 30–39, 40–49, 50–59, 60–69, ≥ 70 | … over 18 |
| Participation work/education |
1. 0%, 2. 25%, 3. 50%, 4. 75%, 5. 100%
Sickness benefits (≥75%)/ No education lessons |
1. 0%, 2. 25%, 3. 50%, 4. 75%, 5. 100%
Sickness benefits (≥75%)/ No education lessons |
|
| Diagnostician | Who made the diagnosis? | Who made the diagnosis? | Who made the diagnosis? |
| Disease duration | For how long have you had CFS/ME fatigue symptoms? < 6 months, 6–12 months, 1–2 years, 2–5 years, 5–10 years, > 10 years |
For how long have you had CFS/ME fatigue symptoms? < 6 months, 6–12 months, 1–2 years, 2–5 years, 5–10 years, > 10 years |
When was the ME/CFS diagnosis set? (year)—was recalculated to the categories < 1 year, 1–2 years, 2–5 years, 5–10 years, > 10 years |
| Disease severity | What severity degree of ME/CFS do you have? 1. Below mild, 2. Mild, 3, mild–moderate, 4. Moderate, 5. Moderate–severe, 6. Severe, 7. Sever-very severe, 8. Very severe |
What severity degree of ME/CFS do you have? What severity degree of ME/CFS did you have at start of the intervention? 1. Below mild, 2. Mild, 3, mild–moderate, 4. Moderate, 5. Moderate–severe, 6. Severe, 7. Sever-very severe, 8. Very severe |
What was the severity degree at start of the rehabilitation stay? 2. Mild, 4. Moderate, 6. Severe, 8. Very severe |
| Situational context | |||
| Medical specialty | Which department or clinic? | Which department or clinic? What sort of intervention? | Which rehabilitation facility? |
| Intervention duration | How many days? (open answer) | How many weeks? 1 /2/3/4/other | |
| Type of treatment | Counseling, group course, training to increase flexibility, training to increase activity and fitness, relaxation, CBT aimed at coping of severe illness, CBT aimed at symptom reduction and activity increase, medications or supplements, or other | ||
| Group course-target patient group | ME/CFS, fatigue, or various health complaints | ||
Relevant questions describing the respondents’ characteristics and the situational context.
Answer alternatives are italicized.
Table 2
| Domain | Survey 1 Hospital consultation | Survey 1 Hospital intervention | Survey 2 Rehabilitation |
|---|---|---|---|
| Post-exertional malaise (PEM) | |||
| PEM-focus | Were you asked, directly or indirectly, if you had PEM? 1. No, 3. Unsure, 5. Yes | Did you gain any new knowledge or understanding about PEM? | Was PEM explained during the stay? 1. No, 5. Yes |
| 1. PEM was not seen as typical or relevant, 2. No information, 3. Nothing new, 4. Some, 5. A lot | |||
| Patient-reported outcome | |||
| Care satisfaction/Benefit | Overall, were you satisfied with the consultation? ** | What benefit have you had, overall, from the intervention? 1. No benefit, 2. Little benefit, 3. Some benefit, 4. Large benefit, 5. Very large benefit |
I am satisfied with my stay at the rehabilitation facility * |
| Impact on health | What severity degree of ME/CFS did you have the first following 2 weeks/the following 3 to 6 months? 1. below mild, 2. Mild, 3. Mild–moderate, 4. Moderate, 5. Moderate–severe, 6. Severe, 7. Sever-very severe, 8. Very severe | I felt better just after the stay than before I felt better 1 month after the stay than before 1. Strongly disagree, 2. Disagree, 3. Neither agree nor disagree, 4. Agree, 5. Strongly agree |
|
| Impact on various domains | How did you benefit from the intervention, when it comes to: physical health, cognitive effort, mental health, and ability to master daily tasks, ability to regulate activity level, quality of life? 1. Much worse, 2. Somewhat worse, 3. No, change, 4. Somewhat improved, 5. Strongly improved |
||
| Patient-reported experiences of perceived healthcare quality | |||
| Suitability of the intervention | Did you feel that the intervention was suitable for your situation? * | The activity level was adapted to my illness* | |
| Healthcare provider knowledge? | Do you think that the doctor or possibly other healthcare provider had a good knowledge of M//CFS? 1. Very little, 2. Not much, 3. Both, 4. Good, 5. Very good |
Do you think that this therapist/supervisor/institution had good knowledge of ME/CFS? 1. Very little, 2. Not much, 3. Both, 4. Good, 5. Very good |
The healthcare providers had good knowledge on ME/CFS* |
| Symptom acknowledgment? | Did you feel that your symptoms were taken seriously? * | Did you feel that your symptoms were taken seriously? ** | The staff at the rehabilitation facility were understanding when I told them about my symptoms * |
| Gained beneficial knowledge or skills | Did the intervention help you to be able to prevent and manage PEM? 1. PEM was not seen as typical or relevant, 2. No information, 3. Nothing, 4. Somewhat, 5. A lot |
I learned a lot that I have benefited from later * | |
| Incorrect treatment | Do you think you obtained incorrect treatment in some way?** | – | |
| Intervention completed | Did you complete the intervention? 1. No I quit because I got worse/ no I quit because I did not think it was helpful, 2. Yes |
Were you at the rehabilitation facility for the entire period? 1. No, I got worse and chose to go home/ no, I got worse and was sent home by the staff, 2. Yes |
|
Relevant questions that were applied in the analyzes: PEM-focus in the clinical contacts, variables assessing patient-reported outcome, and patient-reported experiences of perceived healthcare quality.
Answer alternatives are italicized, and in the case of dichotomized response options, the desirable responses are underlined. *1. Strongly disagree, 2. Disagree, 3. Neither agree nor disagree, 4. Agree, 5. Strongly agree; **1. Not at all, 2. To a small degree, 3. To some degree, 4. To a great degree, 5. To a very great degree.
2.2.1 Respondent characteristics
The operationalization of respondents’ characteristics is presented in Table 1. Sociodemographic characteristics covering gender, age, and participation degree in work and school were only evaluated in the hospital survey. Both surveys evaluated some disease characteristics such as diagnosis, disease duration, and severity grading.
2.2.2 Post-exertional malaise-focus as an explanatory variable
The primary variable of interest was the focus on PEM (PEM-focus) in specialist healthcare settings and its impact. PEM-focus in the three types of healthcare settings was operationalized with closed questions, but with different wording and different scales for each type of healthcare setting (see Table 2). In the analyzes, PEM-focus was dichotomized as PEM+ (PEM was addressed) or no-PEM (PEM was not addressed); this is described in Table 2 as well.
2.2.3 Patient-reported outcome
The assessments of the outcome measures are presented in Table 2. The impact of hospital intervention on health status was operationalized by computing changes in the evaluated disease severity before and after the intervention–in the first 2 weeks (post-intervention) and 3 to 6 months (short-term follow-up). Disease severity classification was based on the Norwegian National Guidelines for CFS/ME (41) and ICC (9). More severe disease severity after the intervention was classified as ‘deteriorated’. ‘Not deteriorated’ includes both unchanged and improved health status. In addition, the benefit of the hospital interventions was more specifically evaluated related to various domains: physical health, cognitive effort, mental health, ability to master daily tasks, ability to regulate activity level, and quality of life. The answer options “much worse” and “somewhat worse” were rated as “deteriorated.”
Post-intervention changes in health status following rehabilitation were operationalized as ‘deteriorated’ if the respondents strongly disagreed with the statement “I felt healthier just after the stay than before.” Short-term changes reported as “I felt better one month after the stay than before” were considered as “deteriorated” if the respondent strongly disagreed. Other replies were valued as ‘not deteriorated’.
In addition, satisfaction with the consultation or the rehabilitation program and perceived general benefit of the hospital intervention were assessed with 5-point Likert scales and applied as outcome measures.
2.2.4 Patient-reported experiences of perceived healthcare quality
Relevant items are presented in Table 2. In addition to treatment completion, all care quality variables were assessed with 5-point Likert scales. Operationalization varies by care setting. Appraisal of the quality of the clinical consultations (the hospital survey) was assessed by evaluating the patients’ view on the ME/CFS-specific knowledge and experienced symptom with respect to the healthcare professionals. Rated suitability of the intervention to the respondents’ condition and the proportion who completed treatment were considered as additional indicators for care quality of intervention and rehabilitation. An item of the rehabilitation survey that evaluated whether the respondents felt they had obtained useful knowledge was included. For hospital intervention, respondents’ opinion of the extent to which they had acquired PEM coping skills and whether they had obtained incorrect treatment was included as well.
2.2.5 Situational context
In both surveys, the intervention duration and the involved hospital, department, or rehabilitation institution were assessed with closed questions and an ‘other’ option (see Table 1).
In the hospital survey, the type of intervention options was assessed systematically as well: individual treatment, group course, or both. The types of treatment options were exercises to increase mobility, aerobic condition, or relaxation, cognitive behavioral therapy (CBT) aimed at reducing symptom focus and increasing activity, CBT focused on support and illness coping, or medication.
Apart from which particular rehabilitation institution was evaluated, no context variables were assessed systematically in the rehabilitation survey.
2.3 Analysis
Analyzes are based on available data from two surveys: a hospital survey (57) and a rehabilitation survey (58).
Perceived PEM-focus (PEM+ or no-PEM) in provided specialist healthcare is the main object of interest. For the different healthcare settings, PEM-focus, as evaluated by the respondents, is mainly analyzed as dichotomized variables.
Situational context variables, such as which hospital, department, or rehabilitation institution, as well as type of intervention, are not presented in detail. General context differences in PEM-focus were evaluated with chi-square tests.
To determine the impact of PEM-focus on the outcome, binary univariate and multivariate logistic regression were used with PEM-focus as the explanatory variable and disease duration and severity (14, 61, 62) included as covariates if available. The response variables were dichotomized outcome measures of satisfaction or rated general benefit, impact on health status following the intervention, and additionally for the hospital interventions, the impact on various ME/CFS-related domains. Because of the limited expected improvement in health status and the real possibility of deterioration following the intervention, health impact was evaluated as ‘no-deterioration’ versus ‘deterioration’.
Satisfaction with clinical consults was only evaluated with univariate analyzes; crude odds ratios (ORs) with corresponding 95% confidence intervals (95% CI) are presented. For both intervention settings, both crude and adjusted OR were calculated. Hence, OR > 1.0 indicates that the variable is associated with a higher probability of the response variable (satisfaction, benefit, health, or function deterioration), whereas OR < 1.0 indicates an association with a lower probability. The results were also presented as bar diagrams with full-scale outcome variables. Mann–Whitney U-test was applied to assess group differences. The impact of PEM-focus on changes in health status following hospital intervention was evaluated with paired-sample Wilcoxon signed rank-sum tests for PEM+ and no-PEM.
The care quality variables are presented with Spearman’s rank correlation coefficient (Spearman’s rho: ρ) as a measure of association with PEM-focus. In addition, Cronbach’s alpha was calculated as a measure of internal consistency of the care quality variables and the full scale of PEM-focus answers.
No power calculation was performed as the primary surveys were considered explorative. Differences between respondents that were included and excluded in the analyzes of this study are compared in the available disease characteristics within both surveys with chi-square tests. In the hospital survey, screening for ME/CFS diagnosis and possible exclusion if ME/CFS was not considered as their main diagnosis was done in the first part of the survey. Sociodemographics were questioned at the end of the survey and thus were not answered by most of the excluded respondents. In the rehabilitation survey, no sociodemographic characteristics were collected. This made it impossible to compare the sociodemographics of respondents who completed vs. not completed the surveys.
Data analyzes were performed using IBM SPSS Statistics for Windows, version 28 (IBM Corp., Armonk, N.Y., United States). A p-value of less than 0.05 was considered statistically significant.
3 Results
3.1 Subjects
Figure 1 shows the study flow chart including both surveys. In the hospital survey, 82 respondents were included in the analyzes. In total, 86 consultations and 89 interventions were evaluated. The majority had evaluated only one consultation (71%) or intervention (69%), 21 and 23%, respectively, had evaluated two, and 8% had evaluated consultations and interventions with three different departments. In the rehabilitation survey, 788 respondents who had participated in a rehabilitation program at a rehabilitation facility in Norway were included.
The non-completers of the hospital survey did not differ in illness duration, age of symptom debut, diagnosis and disease severity, the degree they experienced PEM, and fulfillment of the Canadian Consensus Criteria (8) as evaluated in this survey. The non-completers had less often obtained an ME/CFS or long COVID with PEM diagnosis (80.5% vs. 97.5%, p = 0.002). In the rehabilitation survey, there was no difference in how long ago the diagnosis was set and by whom, between the respondents that were included or excluded for analyzes (see Figure 1).
3.1.1 Sociodemographic and disease characteristics
In the hospital survey, 84.5% was female and the age distribution at the time of the survey was 26.4% 20–29 yr., 18.1% 30–39 yr., 27.8% 40–49 yr., 26.4% 50–59 yr., and one respondent older than 60. The majority (88.7%) was not working or studying at all, 5.6% worked or studied 1–5 h weekly, 4.2% 6–20 h, and only one respondent worked or studied more than 20 h weekly. There were no sociodemographic data available from the respondents of the rehabilitation survey.
ME/CFS was self-reported as the main diagnosis by 80 of the 82 respondents of the hospital survey; for 79, a physician had set this diagnosis as well. One respondent had obtained a ‘burnout or chronic fatigue’ diagnosis. Two respondents had long COVID; this was confirmed for one respondent. In total, 24% of the respondents were diagnosed by a GP only, 39% by a specialist of one of the hospitals only, or by other specialists in private practice only (7%). The remaining respondents were diagnosed by both a GP and a specialist (24%) or by both a hospital and a private specialist (5%). No respondents had reported that they ‘had a fatigue illness (including ME/CFS) before but not now’.
All patients in the rehabilitation survey self-reported that they had been diagnosed with ME/CFS. Twenty patients (0.9%) of the subjects that had started the survey reported they were neither a ME/CFS patient nor a relative and had been excluded. 20% of the respondents had received the diagnosis from their GP, 23% had consulted a specialist in private practice, and 49% had received the diagnosis from the local or regional hospital. 8% had received the diagnosis from the national CFS/ME center, a third-line service for advanced interdisciplinary assessment and guidance for adult patients.
Disease duration and severity are reported in Table 3.
Table 3
| Hospital survey | Rehabilitation survey | |||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| ME disease duration* | 82 | 770 | ||
| < 1 yr. | 0 | 0.0% | 21 | 2.7% |
| 1–2 yr. | 5 | 6.1% | 182 | 23.7% |
| 2–5 yr. | 19 | 23.2% | 247 | 32.2% |
| 5–10 yr. | 33 | 40.2% | 217 | 28.3% |
| > 10 yr. | 25 | 30.5% | 100 | 13.0% |
| Disease severity at intervention start | 52 | 788 | ||
| Mild | 2 | 3.7% | 162 | 20.6% |
| Mild to moderate | 20 | 37.0% | ||
| Moderate | 17 | 31.5% | 519 | 65.9% |
| Moderate to severe | 12 | 22.2% | ||
| Severe | 3 | 5.6% | 106 | 13.5% |
| Severe to very severe | 0 | 0.0% | ||
| Very Severe | 0 | 0.0% | 1 | 0.1% |
Disease duration and severity.
*Hospital survey: duration of ME/CFS symptoms at the date of survey response. Rehabilitation survey: time from year of diagnosis until intervention start.
3.2 Situational context and PEM-focus
The clinical consultations with a health provider at the two relevant hospitals (n = 86) were received at mainly six different types of departments. The majority had been at an ME/CFS Medicine Clinic (30.2%), a department of Physical Medicine (23.3%), or Neurology (20.9%). The others had been at a department of Infectious Diseases (8.1%), Mental Health (8.1%), or an ME/CFS Outpatient Clinic (5.8%), Gastroenterology (2.3%), or Pulmonary (1.2%). The hospital interventions (n = 89) were mainly received at a department of Physical Medicine (38.2%) or a department for Therapeutic Patient Education (38.2%). The remaining interventions were received at an ME/CFS Medicine Clinic (6.7%), departments of Mental Health (6.7%), Health and Work (5.6%), or Neurology (4.5%).
The type and duration of the hospital interventions varied. Intervention could include educational group courses (70.8%), individual consultation/one-to-one counseling (57.3%), or both. In addition to education in the group courses, the interventions comprised CBT aimed at reducing symptom focus and increasing activity (14.6%), CBT focused on support and illness coping (11.2%), exercises to increase mobility (4.5%), aerobic condition (3.4%), or relaxation (4.5%), as well as medication or dietary supplements (2.2%). Most hospital interventions were delivered on an outpatient basis, generally once or a few times. The educational courses were either intensive (3 days within 1 week) or spread over a longer period (6–8 times, once every 1 or 2 weeks). Only 67.2% of the respondents attended educational courses aimed specifically at ME/CFS, and the rest of the courses were aimed at patients with either general fatigue (21.8%) or other health complaints (10.9%).
Experiences of obtained rehabilitation services (n = 788) were evaluated for over 20 rehabilitation facilities in Norway. Two rehabilitation facilities were each evaluated by over 100 respondents (32.1% of respondents), four by over 50 respondents (35.3%), and four by at least 20 respondents. In the rehabilitation survey, applied intervention methods were not evaluated systematically. However, according to the open-ended comments in the survey, the rehabilitation institutions had different approaches to the rehabilitation of ME/CFS patients. Some encouraged CBT aimed at reducing symptom focus combined with a graded activity increase. Other rehabilitation facilities provided explanations about exertion-induced symptom exacerbation (PEM) and focused on the importance of managing and adjusting activity levels according to the patient’s capacity (“energy envelope theory”) to prevent PEM (58).
Overall, respondents reported that PEM was addressed in 43.0% of the consultations, 65.2% of the hospital interventions, and 47.5% of the rehabilitation stays. A more detailed distribution is presented in Figure 2. Whether PEM was addressed (PEM+) or not (no-PEM) varied significantly across the different settings from zero to 81% for clinical consultations (p < 0.001) and from 29 to 100% for hospital intervention (p < 0.001). Among the respondents who had participated in an educational group course or had received counseling, 71.4 and 45.1%, respectively, reported that PEM had been addressed. In the group courses specifically aimed at ME/CFS patients, 97.7% perceived PEM+, while PEM+ was 14.3% in the groups for fatigue and other health complaints. Among the rehabilitation facilities, reported PEM+ varied significantly as well, from 2.2 to 68.8% (p < 0.001).
Figure 2
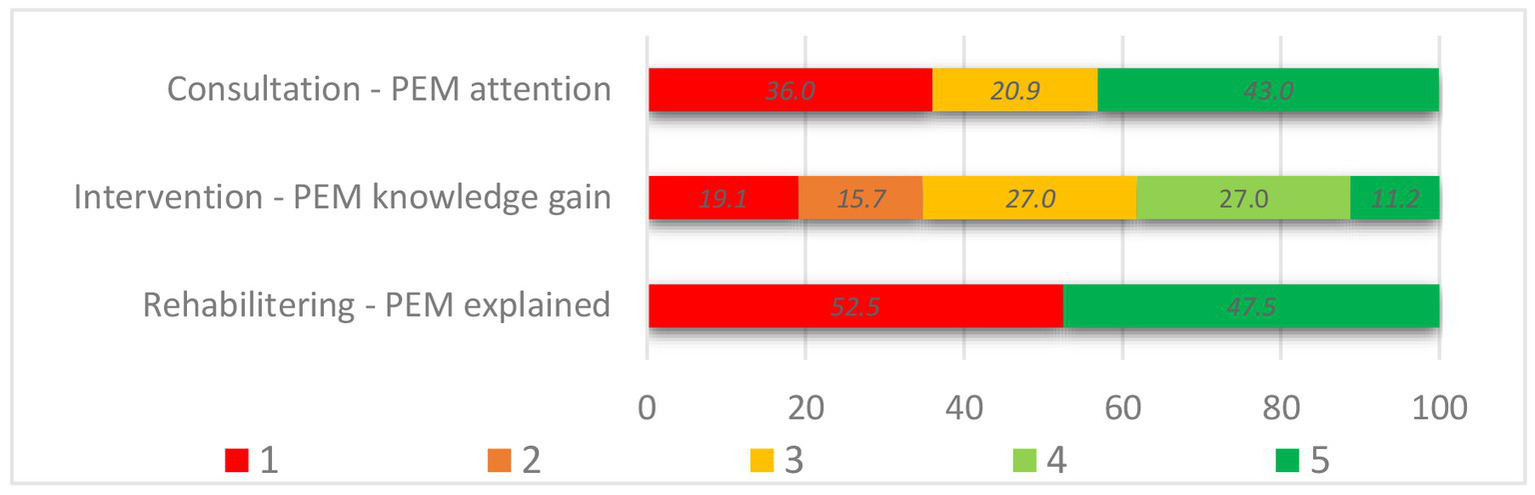
PEM-focus in the healthcare settings. Answer options of Assessment of PEM, in consultation (n = 86): 1. No (no-PEM), 3. Unsure (no-PEM), 5. Yes (PEM+). Knowledge gain following hospital intervention (n = 89): 1. PEM was not seen as typical or relevant (no-PEM), 2. No information (no-PEM) n, 3. Yes, but nothing new (PEM+), 4. Some (PEM+), 5. A lot (PEM+). PEM explained in rehabilitation setting (n = 788): 1. No (no-PEM), 5. Yes (PEM+).
3.3 Post-exertional malaise-focus as an explanatory variable for the outcome
Differences in several outcome measures stratified by PEM-focus (no-PEM or PEM+) are presented in Figures 3–5. In addition, Table 4 summarizes the results of logistic regression analyzes for the association between PEM-focus and binary outcome measures. Multivariate logistic regression analyzes produced nearly identical results as univariate logistic regression for the impact of PEM. The results of the univariate regression analyzes are, therefore, not presented here.
Figure 3
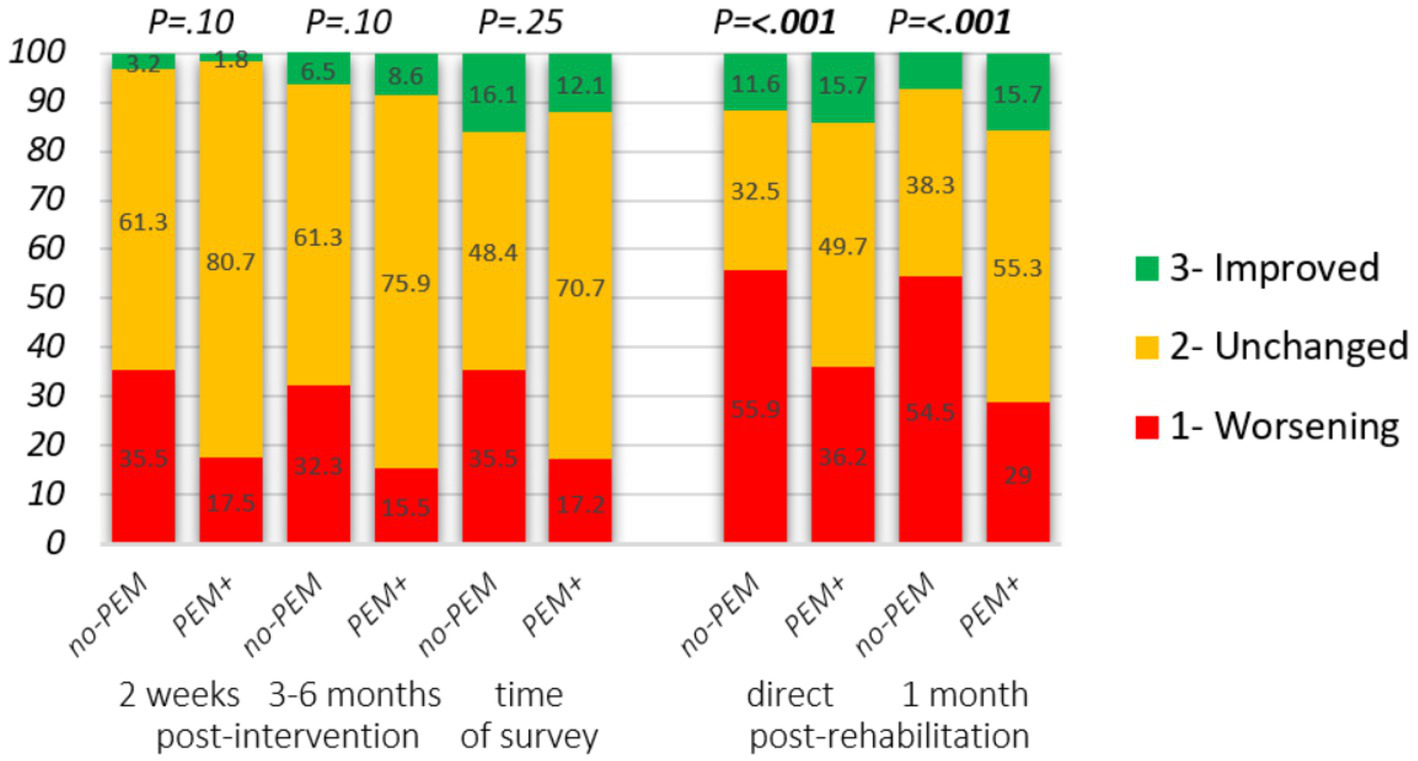
Impact of hospital intervention and rehabilitation on the state of health stratified by PEM-focus in the therapeutic approach. Changes from intervention start. Hospital intervention: self-reported severity degree at baseline, 2 weeks (n = 88), and 3 to 6 months (n = 89) following the intervention. Change in clinical severity degree: 1. Higher disease degree, 2. Unchanged, 3. Lower disease degree. Rehabilitation: reply to the statements “I felt better just after the stay than before” and “I felt better 1 month after the stay than before,” answer options: 1. Strongly disagree, 2. Disagree/neither agree nor disagree, 3. Strongly agree. Mann–Whitney U-test was applied to assess group differences.
Figure 4
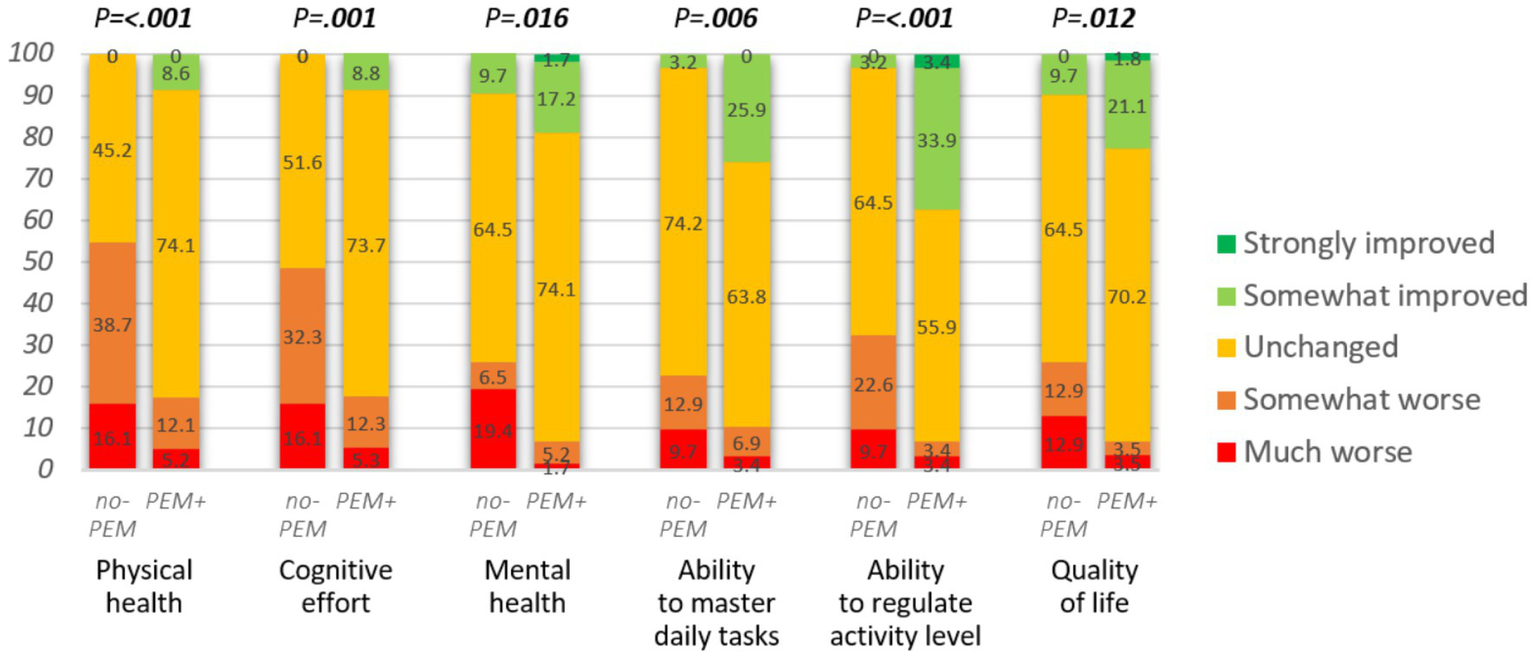
Impact of hospital intervention on various domains (n = 88 or 89). Mann–Whitney U-test was applied to assess the group differences.
Figure 5
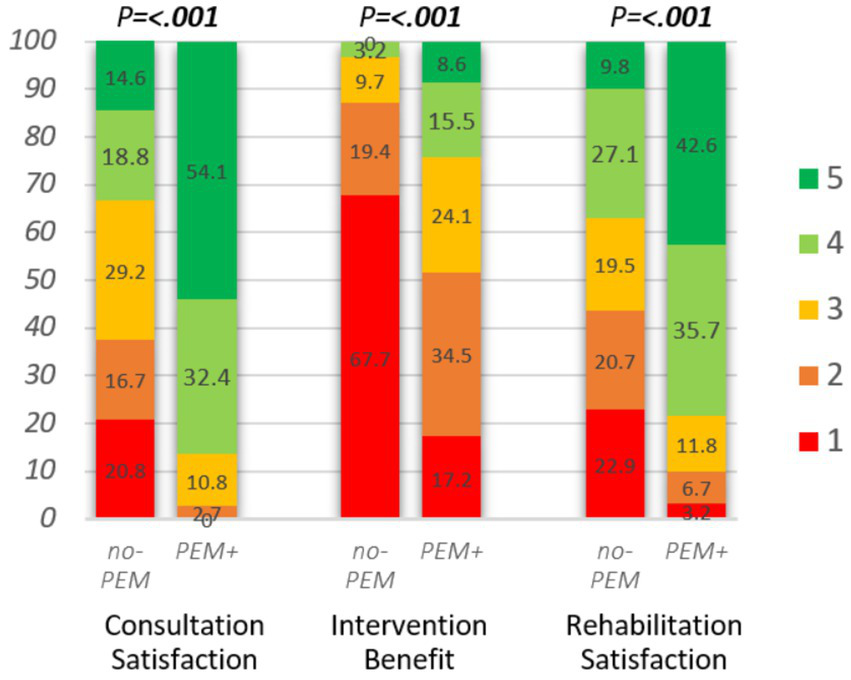
Impact of hospital consultation (n = 85), intervention (n = 89), and rehabilitation (n = 783) on rated satisfaction or benefit, stratified by PEM-focus during clinical contact. Consultation satisfaction: “All in all, were you satisfied with the consultation?,” answer options: 1. not at all, 2. to a small degree, 3. to some degree, 4. to a great degree, 5. to a very great degree. Intervention benefit, hospital intervention: “What benefit have you had, all in all, from the intervention?” answer options: 1. No, 2. Little, 3. Some, 4. Large, 5. Very large. Rehabilitation satisfaction: “I am satisfied with my stay at the rehabilitation facility,” answer options: 1. Strongly disagree, 2. Disagree, 3. Neither agree nor disagree, 4. Agree, 5. Strongly agree. Mann–Whitney U-test was applied to assess the differences between no-PEM and PEM+.
Table 4
| Setting | n | Response variables (Outcome) | Explanatory variables | OR | [95% CI] | p |
|---|---|---|---|---|---|---|
| Hospital consultation | 79 | Satisfaction | PEM-focus | 11.57 | [3.72–35.96] | <0.001 |
| Hospital intervention | 89 | Benefit | PEM-focus | 9.74 | [1,21–78.57] | 0.033 |
| Disease severitya | 0.93 | [0.83–1.05] | 0.26 | |||
| 89 | Function deteriorationb | PEM-focus | 0.13 | [0.05–0.37] | <0.001 | |
| Disease severitya | 1.12 | [1.01–1.24] | 0.034 | |||
| 88 | Worsening disease severity- post, 1–2 wkc | PEM-focus | 0.37 | [0.14–1.04] | 0.058 | |
| Disease severitya | 0.93 | [0.84–1.03] | 0.166 | |||
| 89 | Worsening disease severity- 3-6 mosc | PEM-focus | 0.38 | [0.14–1.08] | 0.07 | |
| Disease severitya | 0.97 | [0.88–1.08] | 0.57 | |||
| Rehabilitation | 742 | Satisfaction | PEM-focus | 5.75 | [4.14–7.98] | <0.001 |
| Disease severitya | 0.63 | [0.48–0.84] | 0.002 | |||
| Disease duration | 0.99 | [0.94–1,05] | 0.77 | |||
| 768 | Worsening health–post | PEM-focus | 0.46 | [0.34–0.63] | <0.001 | |
| Disease severitya | 1.29 | [1.00–1.67] | 0.052 | |||
| Disease duration | 0.98 | [0.93–1.03] | 0.44 | |||
| 769 | Worsening health–1 mo. | PEM-focus | 0.35 | [0.26–0.48] | <0.001 | |
| Disease severitya | 1.48 | [1.13–1,94] | 0.005 | |||
| Disease duration | 0.99 | [0.94–1.05] | 0.83 |
Results of logistic regression analysis for the association between PEM-focus (no-PEM or PEM+) and outcome.
Relevant disease variables were included as covariates, if available. ‘No-PEM’ is the reference category for PEM focus. aDisease severity at intervention start; bWorsening physical, cognitive, or mental functioning; cchanges in disease severity compared to baseline.
Figure 3 and Table 4 present the impact of PEM-focus on the health state after finishing the intervention. For the majority, their health state did not change. On average, for respondents in both groups, disease severity was worsened in the first 2 weeks following hospital intervention (p = 0.005 in no-PEM and p = 0.008 in PEM+). From baseline until 3 to 6 months following baseline, differences were only significant in no-PEM (p = 0.042 and p = 0.13 in PEM+).
However, there was a tendency that at both time points, around twice as many respondents from the no-PEM group experienced a deterioration of health status, following the intervention compared to the PEM+ group. Overall, if PEM had not been addressed in the intervention, logistic regression showed that the odds of experiencing health deterioration on at least one of the two time points increased significantly following both hospital intervention (proportion 22.4% in PEM+ vs. 45.2% in no-PEM, p = 0.026) and rehabilitation (40.1% vs. 63.2% P = <0.001) [adjusted OR: 0.34 (95% CI 0.13–0.89; p = 0.027) and 0.39 (95% CI 0.29–0.52; P = <0.001), respectively]. At the time of data collection (up to 5 years after hospital intervention), 35.5% of no-PEM respondents and 17.2% of PEM+ respondents had a more severe disease degree (OR 0.38, 95% CI 0.14–1.03, p = 0.058) compared to the start of the intervention. In the rehabilitation survey, changes in disease severity were not assessed.
The lack of focus on PEM in the hospital intervention had a significant impact on physical and mental health, cognitive effort, ability to master daily tasks, ability to regulate activity level, and quality of life (see Figure 4; Table 4). The respondents from the no-PEM group experienced over three times more often any physical, cognitive, or mental function worsening following hospital intervention [61.3% vs. 19.0%, p < 0.001, adjusted OR = 0.13 (95% CI 0.05–0.37), p < 0.001].
Figure 5 and Table 4 show the treatment outcome assessed as patient satisfaction or benefit following hospital consultations, interventions, or rehabilitation, stratified on PEM-focus. Evaluated satisfaction or benefit was generally significantly higher (all p < 0.001) in all three clinical settings when PEM was addressed. Satisfaction with consultation and rehabilitation was twice as high and over 4-fold as many respondents reported to have perceived at least some benefit of the hospital intervention.
In the educational group courses at the hospitals, outcome measures were strongly related to the specificity of the intervention. Deterioration of health and functioning and perceived benefit was significantly less frequently reported after the ME/CFS-specific courses, compared to the courses for general fatigue or health complaints. Worsening of health was experienced by 25.6% vs. 52.4% (p = 0.034), and deterioration of physical, cognitive, or mental function was reported by 18.6% vs. 71.4% (p < 0.001). Perceived benefit was low in both groups: 23.3% of the respondents that had participated in ME/CFS-specific courses and 9.5% of the participants of less specific courses (p = 0.19) had reported large or very large benefits of the education.
3.4 Care quality related to PEM-focus
Table 5 presents the correlation between focus on PEM in different clinical situations and care quality as perceived by the respondents. In all three types of healthcare settings, respondents’ perceptions of the healthcare provider’s level of ME/CFS knowledge and symptom acknowledgment were strongly associated with whether or not there had been attention to PEM. PEM-focus in hospital intervention and rehabilitation was also strongly correlated with respondents’ opinion on whether the intervention was suitable and sufficiently adjusted to their situation. Cronbach’s alpha of respective 0.89, 0.89, and 0.80 of the care quality variables and PEM-focus indicates high internal consistency.
Table 5
| Survey 1—hospital PEM-focus in consultation |
Survey 1–hospital PEM-focus in intervention |
Survey 2–rehabilitation PEM-focus in rehabilitation |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | no-PEM 49 (56%) |
PEM+ 38 (44%) |
P-value | n | no-PEM 31 (35%) |
PEM+ 58 (65%) |
P-value | n | no-PEM 414 (53%) |
PEM+ 374 (48%) |
P-value | |
| Test statistics | Test statistics | Test statistics | ||||||||||
| Healthcare provider ME/CFS knowledge | 86 | ρ = 0.62 | <0.001 | 89 | ρ = 0.74 | <0.001 | 786 | ρ = 0.56 | <0.001 | |||
| Very little | 11 | 22.4% | 0.0% | 18 | 51.6% | 3.4% | 125 | 28.4% | 2.1% | |||
| Not much | 10 | 18.4% | 2.7% | 9 | 25.8% | 1.7% | 115 | 23.3% | 5.1% | |||
| Both | 17 | 28.6% | 8.1% | 17 | 19.4% | 19.0% | 125 | 19.2% | 12.3% | |||
| Good | 16 | 16.3% | 21.6% | 23 | 3.2% | 37.9% | 228 | 21.1% | 37.6% | |||
| Very good | 32 | 14.3% | 67.6% | 22 | 0.0% | 37.9% | 193 | 8.0% | 42.8% | |||
| Symptom acknowledgment | 86 | ρ = 0.60 | <0.001 | 88 | ρ = 0.55 | <0.001 | 784 | ρ = 0.48 | <0.001 | |||
| Not at all | 10 | 20.4% | 0.0% | 12 | 30.0% | 5.2% | 73 | 16.8% | 1.1% | |||
| To a small degree | 9 | 18.4% | 0.0% | 9 | 23.3% | 3.4% | 72 | 14.1% | 3.8% | |||
| To some degree | 13 | 22.4% | 5.4% | 17 | 23.3% | 17.2% | 84 | 16.1% | 4.8% | |||
| To a great degree | 17 | 18.4% | 21.6% | 26 | 20.0% | 34.5% | 275 | 36.0% | 34.0% | |||
| To a very great degree | 37 | 20.4% | 73.0% | 24 | 3.3% | 39.7% | 280 | 17.0% | 56.3% | |||
| Suitability of intervention | 89 | ρ = 0.61 | <0.001 | 785 | ρ = 0.46 | <0.001 | ||||||
| Not at all | 17 | 45.2% | 5.2% | 161 | 34.2% | 5.4% | ||||||
| To a small degree | 10 | 22.6% | 5.2% | 127 | 18.9% | 13.1% | ||||||
| To some degree | 22 | 22.6% | 25.9% | 113 | 17.7% | 10.7% | ||||||
| To a great degree | 24 | 6.5% | 37.9% | 243 | 22.3% | 40.5% | ||||||
| To a very great degree | 16 | 3.2% | 25.9% | 141 | 6.8% | 30.3% | ||||||
| Completed intervention | 78 | ρ = 0.26 | 0.021 | 784 | ρ = 0.06 | 0.12 | ||||||
| No | 12 | 27.6% | 8.2% | 104 | 15.0% | 11.3% | ||||||
| Yes | 66 | 72.4% | 91.8% | 680 | 85.0% | 88.7% | ||||||
| Cronbach’s alpha | 0.89 | 0.89 | 0.80 | |||||||||
Distribution of degree of perceived care quality on several factors as reported by the respondents in the three types of care settings, stratified and tested by PEM-focus. Measures for internal consistencies calculated of all variables, including PEM-focus, are presented as well.
Spearman’s rank correlation coefficient (Spearman’s rho: ρ) as a measure of association with PEM-focus. Cronbach’s alpha as a measure of internal consistency (with the full scale of PEM-focus).
During rehabilitation, 28.4% of the no-PEM respondents vs. 73.5% of the PEM+ respondents (p < 0.001) had learned a lot which they had benefited from afterward. In hospital intervention, none of the no-PEM respondents versus 58.6% (p < 0.001) of PEM+ had obtained new knowledge or understanding about PEM, and 3.3% of no-PEM vs. 46.5% of PEM+ (p < 0.001) had obtained new PEM coping skills. Almost half (48.4%) of the no-PEM group vs. 5.2% (p < 0.001) of the PEM+ group felt that they had been treated incorrectly in the intervention obtained at the hospital.
For the educational group courses, most care quality measures were also strongly correlated with whether the target group was specific for ME/CFS patients or not [healthcare providers’ ME/CFS knowledge, ρ = 0.64 (p < 0.001); symptom acknowledgment, p = 0.59 (p < 0.001); and suitability of intervention, ρ = 0.63 (p < 0.001)]. There were, however, no significant differences in dropout ratios: 5.3% in the ME/CFS-specific education and 10.0% in the other courses (p = 0.50).
4 Discussion
The PEM phenomenon is a hallmark feature of ME/CFS and essential to acknowledge in both clinical consultation and intervention. This study was conducted in Norway, generally featuring high-quality care. Nevertheless, according to a significant proportion of the ME/CFS patients, PEM had frequently not been addressed during their contact with specialist healthcare services. This concerned both consultation services at the hospitals as well as the interventions delivered at the hospitals and rehabilitation institutions. This lack of focus on PEM increased the probability of experiencing deterioration, following hospital intervention and rehabilitation care. On the other hand, addressing PEM was related to increased rated care satisfaction, healthcare quality, and benefit.
4.1 Addressing PEM in the intervention
Over one-third of the respondents of the hospital interventions and half of the individuals who had stayed at a rehabilitation institute reported that PEM had not been addressed. This doubled the number of respondents that acquired a more severe disease degree for a long time; for the hospital respondents, the data demonstrated that these differences were still present at the time of data collection (i.e., up to 5 years after the intervention).
From a psychosomatic point of view (3, 63, 64), PEM is ignored as a direct physiological response to physical or mental exertion. From this perspective, GET and CBT are considered as effective therapies. Although the Norwegian Guidelines regard PEM as a cardinal symptom, GET and CBT are still suggested as effective treatment approaches in these guidelines (41). This is despite there currently being no research evidence of convincing effects of these approaches for ME/CFS patients with PEM (10, 33, 34, 65, 66) and despite the fact that several surveys actually reported that over half of the ME/CFS patients experience substantial deterioration after GET and usually do no benefit from CBT (67–69).
GET and curative CBT were seldom explicitly mentioned as applied method in both our hospital and rehabilitation surveys. Yet, many patients reported that they encountered elements of CBT and GET, such as being encouraged to believe their disease is not serious or physical, encouragement to increase activity levels, and disregarding symptoms. This usually happened in settings where PEM was not addressed. Some of the citations that the respondents had added in comments text fields in both surveys testify to this (see Table 6).
Table 6
| Psychosomatic approach |
|
“The doctor said it should not be called ME but rather ‘BE’ because it is Between the Ears.”
“We were met by a psychologist who claimed that if you felt exhaustion coming over you, you should think of something pleasant and that ‘feeling’ would go away!.” “There was a great deal of focus on stress management and stuck-thought- patterns.” “I felt that ME was not taken very seriously; all forms of exhaustion seemed to be taken under the same umbrella.” “PEM and exhaustion were seen as complaints and depression, and as an excuse not to exercise.” |
| Sustained arousal hypothesis |
|
“The doctor believed that I could recover completely with their approach; a sustained stress response, which is cured with the right mind-set and individually adapted training.”
“They were only concerned with the body’s stress response.” |
| Consequences of opposing explanatory models of ME/CFS |
| “Because of their perception that ME comes from a biopsychosocial model of explanation, I was never able to become fully comfortable with them. I have a completely different experience of the disease and they focused far too much on the psychological side.” |
| Poor disease understanding among healthcare providers |
| “The healthcare providers barely knew anything about ME/CFS, but they tried their best. The stay was too much. Just being there. It took many years for me to get back to the same level I was before I left.” |
| Ignoring symptoms |
|
“I told them about all the symptoms, but was then told that we had too much focus on symptoms.”
“It was just about not thinking about the symptoms, that you get well as long as you increase your activity and think positively.” “The (rehabilitation) stay is based on CBT and GET, the patient himself must be well aware of his own limits, otherwise it can become too much.” |
| Addressing PEM perceived as more positively |
|
“It was a very nice stay and it was nice to meet more people like me. I did not get any better, but I brought home some tips on everyday life that make it a little easier.”
“The first time I met healthcare providers who believed in me and took my illness into account” “If you could not handle an activity, they said, ‘It’s great that you are taking care of yourself!” |
| Failure to acknowledge PEM may cause potential iatrogenic harm |
|
“Became bedridden for 1 year after rehabilitation because I had to exercise four times a day on weekdays. It was not adapted to ME at all. The basic philosophy at the center was that one could become healthy through exercise.”
“Now, 4 months later, I am still worse than when I went to the rehabilitation institution. But the place is very good; one just has to be healthier than I was to benefit from the stay.” |
| New knowledge and strategies may take time before potential benefit is recognized |
| “It took a long time, approximately 6 months, before there was an effect of the changes I made.” |
When evidence for curative treatments for ME/CFS is lacking, intervention should at least aim at educating the patient to optimize their ability to maintain function in everyday activities and reduce PEM. This may help to alleviate symptoms and increase quality of life (35, 36). Therefore, in updated clinical recommendations for ME/CFS, educational approaches are included. They typically aim at empowering the patient for self-management with a focus on pacing strategies to conserve energy and focus on coping with a disease with substantial function loss and symptom burden.
In the rehabilitation survey, applied intervention methods were not evaluated systematically, but the programs are usually multidisciplinary and patient education is often part of a rehabilitation program. In the case PEM was addressed in the rehabilitation, nearly three-quarters of respondents reported that they had learned a lot which they had benefited from afterward. This applied to less than a third of the patients if PEM had not been discussed.
In the hospital survey, a considerable portion of the respondents had received educational group courses as well. Some patients received educational courses that were aimed exclusively at ME/CFS patients, while others were included in courses aimed at patients with more general fatigue or health problems. Nearly all participants of ME/CFS-specific courses reported to have obtained information about PEM, but only one of seven participants of the less specific courses reported the same. Apparently, the focus on education, and counseling had been delivered from clinical settings with different explanatory approaches to ME/CFS. Not informing ME/CFS patients about their main disabling symptoms is both worrying and unacceptable and may lead to severe consequences for the patients. In our study, functional deterioration was reported by over seven out of 10 participants of the non-specific courses, but only by less than two out of 10 of the participants of the ME/CFS-specific education. Understandably, the perceived impact on health and functioning and rated care quality was associated with this. Half of the patients who had not received information about PEM during their hospital intervention, versus only one in each 20 patients who had received this, felt that they had been treated incorrectly.
Generally, in intervention effect studies, clinical effectiveness is evaluated. Unfortunately, as reported in our study, even when PEM was addressed in the therapeutic approach, clinical improvements were generally absent. Due to the nature of the disease, some deterioration can be expected after out-of-home interventions, particularly among patients with higher disease degrees. The combined burden of travel, social interaction, coping with time schedules, etc. will often be far beyond the patients’ day-to-day activity level.
Compared to our study, higher improvement rates were reported following specialist ME/CFS services in England (70). At 1-year follow-up, 28% reported overall improvement, and only 8% worsened health. One reason might be that the specialist services are indeed better tailored to this specific patient group. Other reasons might be that the evaluated patients had a shorter duration of ME/CFS and were only mildly affected (70). Our data did not cover treatment at a specialist ME/CFS service.
4.2 Is addressing PEM related to the explanatory view of me/CFS?
The PEM phenomenon challenges existing medical assumptions of the health benefits of exercise and other physical and mental activity and sensory stimuli (71). As knowledge and understanding of PEM are crucial for diagnosis and maintaining optimal functioning in ME/CFS, early screening and explaining explicitly about PEM are essential in clinical consultations where ME/CFS is suspected (14, 35). Failure to recognize ME/CFS and PEM may result in poor management in daily life and in the clinical approach, which may hamper recovery potential and aggravate the disease (62, 72).
Only two out of five respondents had noticed that PEM had been addressed in the clinical consultations. The main reason for not discussing PEM in a clinical consultation is probably that the clinician does not acknowledge PEM as an essential feature in ME/CFS. The applied explanatory model in the various clinical settings was not explicitly evaluated in the surveys. However, not acknowledging PEM as a key phenomenon, which in this study was associated with little focus on the patients’ symptoms and poor specific suitability of intervention, is in our opinion an obvious indication of a psychosomatic view. Some of the citations confirm an apparent psychosomatic approach at some of the evaluated healthcare services (see Table 6).
One of the assumptions derived from a biopsychosocial perspective is the sustained arousal hypothesis (73), based on ‘the cognitive activation theory of stress’ (CATS) (74). According to CATS, the sustained stress responses may originate from different precipitating factors (interacting with predisposing factors (genetic traits, personality) and learned expectations (classical and operant conditioning)). Although this theory has not been confirmed, the sustained arousal hypothesis has strong support in Norway, including in some of the evaluated departments, as mirrored in some of the comments (see Table 6).
Because of the presence of a strong psychosomatic network in Norway (3), and the equivocal explanatory view and recommendations for approaching ME/CFS of both the National Advisory Unit on CFS/ME and the Norwegian CFS/ME guidelines (41), it was not surprising to meet a psychosomatic view in several of the specialist healthcare services evaluated in our study.
The respondents’ own underlying assumptions explaining their symptoms had not been assessed. However, for majority of the ME/CFS patients, a predominantly biomedical explanation of their disease usually fits their experiences better than a psychosomatic approach (32, 75, 76). Generally, many ME/CFS patients feel that the doctors psychologize too much, trivialize the symptoms, or tell them that their symptoms are psychosomatic (43, 77–79). If patients meet an opposing explanatory model in healthcare practice, negative patient experiences and dissatisfaction with received care may arise (75, 79). In our study, failure to address PEM led to ineffective, harmful healthcare and respondents reported poor disease understanding of ME/CFS among healthcare providers and a lack of validation of their illness experiences (see also Table 6). This has also been reported in previous studies (42, 43, 45, 79, 80). The high internal consistency of not addressing PEM and a reported approach that was poorly customized to ME/CFS suggests that these elements may measure a similar notion of viewing ME/CFS (58).
Illnesses that lack clear pathophysiology, that has inconsistent diagnostic criteria, inadequate research focus, and lack of proper training, seem frequently to be related to negative consequences or iatrogenesis for the patient (80–82). As in our study, Geraghty and Blease (32) recognized several modalities of iatrogenesis in ME/CFS such as high levels of patient dissatisfaction, challenges to the patients’ narratives and experiences, and negative responses to therapy. In addition, other modalities were identified, such as difficulties in reaching an acceptable diagnosis of ME/CFS and access to medical care and social support.
4.3 Methodological issues, strengths, and limitations
To our knowledge, this is the first study to evaluate the significance of addressing PEM in the clinical approach to ME/CFS patients in naturalistic settings of specialist healthcare practice. The evaluation of PEM-focus was in fact not the primary outcome of the initial surveys. This may have reduced respondent bias because they were unaware of the aim of the present analyzes of assessing the significance of acknowledging the PEM phenomenon with regard to their health and perceived care quality.
The inclusion of two comparable surveys, together covering specialist healthcare for ME/CFS patients in Norway, and the large sample size from a large geographical area in the rehabilitation survey were also strengths of this study. Another key feature of this study is the focus on intervention-induced ‘deterioration’ versus ‘no-deterioration’ instead of evaluating clinical effectiveness. This seems especially relevant in the evaluation of ‘real-life’ interventions for ME/CFS because of general limited improvement in health status. Instead, exacerbations are frequently described in patient surveys but usually ignored or camouflaged in the presentation of average scores.
In the analyzes of our study, the occurrences of provided healthcare are in fact the main study focus and not the individual respondents. Therefore, in the hospital survey, some respondents assessed their experiences from more than one department. These have been analyzed as independent occurrences. We considered this as acceptable since ME/CFS is a chronic disease with very limited recovery potential (35, 72, 83), the provided healthcare could cover a time frame of 5 years and the order in which the setting was evaluated was random. Notably, each respondent could evaluate each department only once. The patients’ view concerning PEM-focus and outcome seemed independent of order and number of assessed settings.
This current study has some limitations, mainly concerning methodological issues. The low sample size of the hospital survey may have reduced the statistical power and the chance of detecting true consequences. This might especially concern the analyzes concerning the impact of the interventions on health. In addition, it limited the opportunity to conduct analysis more specific per clinical specialty. Furthermore, the limited diversity of potential covariates in the available data reduced the number of possible factors of interest to adjust for in the regression analyzes.
As a consequence of performing non-prespecified analyzes based on an exploration of two retrospective surveys, some applied measures and scales were not optimal and inconsistent. This applied also to the assessment of PEM-focus that was operationalized with different wording and different scales for the three types of healthcare settings. However, we do not expect this to be a major drawback. Additionally, we were not able to assess the actual focus on PEM in the clinical settings. We were dependent on patients’ perception of its acknowledgment and recall bias may have occurred. This may also have affected the outcome measures that assessed satisfaction and impact on functioning and health status. The retrospective design, however, might have been a methodological plus as the participants gained the opportunity to put their experiences into a longer-term perspective. It may take time to implement new knowledge and learned strategies in daily life before the potential benefit is recognized (see Table 6). Psychosomatic approaches may aim at influencing how patients interpret and report their health state and thus may easily bias subjective outcome measures immediately after the intervention.
A strength of recruiting respondents outside the healthcare settings and collecting anonymous feedback is a better chance of obtaining objective opinions. Patients may hesitate to share negative experiences with healthcare providers because they fear they will appear unmotivated and non-cooperative. This could negatively affect the approval of health benefit allowances.
The recruitment method with open online surveys may, however, have affected the representativeness of the study population. Because of the anonymity, diagnoses could not be verified. ME/CFS status was self-reported by the respondents, therefore is misclassification possible (53). We have limited descriptive data on the respondents, and we have no insight into the population of eligible patients who have visited the hospitals or rehabilitation institutions in the studied period. Invitation of participation to the surveys was shared online among groups that are interested in ME/CFS. However, subjects who are active on social media or are members of the Norwegian ME Association may be overrepresented. Former ME/CFS patients had the possibility to participate in the hospital survey as well. However, none had selected the diagnostic alternative ‘had a fatigue illness before but not now’. In the rehabilitation survey, 20 respondents (0.9%) were excluded because they were neither a patient nor a relative. Some might have been former patients.
Notably, patients with a severe or very severe degree of the disease are poorly represented. An obvious reason is that this group of patients might be less active on social media and has limited energy to answer a questionnaire. They are also less likely to have obtained secondary healthcare because their severe disease status might hamper access to specialist healthcare. In the region of the hospital survey, ambulant healthcare services are not available for this patient group. Challenges in obtaining adequate healthcare have been confirmed in a recent Norwegian study where this was the case for around seven out of ten ME/CFS patients with a severe or very severe sickness degree (84). Some respondents reported that they no longer dared to have contact with healthcare providers due to frequent negative experiences with various healthcare providers.
The hospital survey had aimed at including long COVID patients as well but did not succeed in this. Only two long COVID patients with PEM are part of the study population. Although a relatively high proportion of long COVID patients are expected to develop ME/CFS (47, 85–87), this was not common knowledge at the beginning of 2022, and many long COVID patients with ME/CFS symptoms may not have identified themselves as an ME/CFS patient.
4.4 Implications for research and clinical practice
Quality of healthcare is typically described in terms of clinical effectiveness, patient safety, and patient experience. This study evaluated ‘real-life’ experiences of ME/CFS with routine specialist healthcare service in a country with generally high-quality healthcare. The quality of care services delivered to ME/CFS patients seemed strongly related to the acknowledgment of the disease and its cardinal symptom PEM in particular. Ignoring PEM in the approach of ME/CFS appears as a reckless maltreatment of patients.
The findings seem relevant for long COVID as well. Alertness to the possibility of the development of COVID-induced PEM and ME/CFS is, therefore, essential in patients with post-COVID symptoms. In patients with (suspected) ME/CFS or long COVID, early identification and management of PEM may be a cost-effective and the most important method for stabilizing symptoms and improving prognosis and patients’ quality of life (10, 35, 87–89).
In general, ME/CFS-specific knowledge seems limited in many healthcare providers (80, 81, 90–93) and usually ignored in their education (93). The reported iatrogenesis may be traced back to this but also to the fact that at present, ME/CFS is not covered by a defined clinical specialty. As seen from our study, patients had been referred to several medical specialties, both for clinical consultations and intervention. Although ME/CFS is regarded as a multisystem disease, with a neuroimmunological base, often proceeded by an infection, neither the disciplines of infectious diseases, immunology, nor neurology has claimed ‘ownership’ over the diagnosis. This ‘orphaned’ position may have significant implications for whether medical specialists feel an interest or obligation to keep up to date in the field. This might be a reason that still, among many healthcare providers, skepticism is established about whether the disease is primarily ‘physical’ (80, 81, 90, 91). This affects care quality. It has been demonstrated that health providers’ view of ME/CFS being a psychosomatic disorder is associated with worse outcomes than views of ME/CFS as a physical illness (38). Immediate large-scale investment in updated education of (future) healthcare providers about the management of ME/CFS, long COVID, and PEM is essential. In our study, the inter-variability between the departments of how patients rated PEM-focus and related care quality was substantial. This provides opportunities to learn from each other’s clinical practice if interested and open-minded about alternative approaches to ME/CFS.
In healthcare, there is a growing need and recognition of patient experiences as an important aspect of evidence-based practice. Patient experiences as described in our study may contribute to the improvement of the quality of specialist healthcare practice for ME/CFS. The significance of acknowledging the PEM phenomenon for outcome and healthcare quality in ME/CFS or long COVID has not been studied systematically before. It seems unethical to study this in an experimental design, therefore evaluating this in pragmatic settings seems most appropriate. The analyzes and findings presented here can be considered exploratory. Further well-designed research is needed to validate these findings and investigate the value of acknowledging PEM in the approach of ME/CFS and long COVID.
5 Conclusion
Despite the inclusion of PEM as a core symptom of ME/CFS in updated diagnostic criteria sets, and the biomedical evidence of the existence of the phenomenon, PEM is still not always accepted and taken into consideration in specialist healthcare practice in Norway.
PEM was not addressed in more than half of the evaluated consultations and rehabilitation stays, and one-third of the hospital interventions. Not addressing PEM doubled the probability of a decline in health and functioning following the intervention and was strongly associated with reduced perceived care quality, satisfaction, and benefit. Acknowledgment of PEM by the healthcare provider was correlated with a more positive rating by the patients of the healthcare providers’ recognition of patient’s symptoms, level of ME/CFS knowledge, and suitability of the intervention to their condition.
This study confirmed the significance of acknowledging the PEM phenomenon in the clinical approach of ME/CFS patients in specialist healthcare practice. When disregarding the PEM phenomenon, healthcare for ME/CFS patients can be described as ineffective, harmful, and of poor quality. In this respect, it seems essential to raise awareness among healthcare providers in specialist healthcare about ME/CFS and PEM.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the studies involving humans because the study was based on the anonymous replies on two online surveys. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements because the study was based on anonymous replies.
Author contributions
MW conceptualized and designed the study, conducted the hospital survey and the analyzes related to the hospital survey, and wrote the first draft of the manuscript. SR conducted the analyzes related to the rehabilitation survey and contributed to the final draft of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors are grateful to all respondents who volunteered their time and energy to answer the surveys so that we might learn more about their experiences which we hope can contribute to better care for ME/CFS patients. The authors want to thank Trude Schei and Arild Angelsen (Norwegian ME Association) for providing them data from the rehabilitation survey (58). The authors also thank Jens Egeland (Oslo University and Vestfold Hospital Trust) and Trude Schei for their comments on the manuscript.
Conflict of interest
MW was employed by one of the evaluated hospitals.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views and opinions expressed in this paper are ours and do not necessarily reflect those of our institutions.
References
1.
Marks DF . The rise and fall of the psychosomatic approach to medically unexplained symptoms, Myalgic encephalomyelitis and chronic fatigue syndrome. Arch Epidemiol Pub Health Res. (2022) 1:97–143. doi: 10.33140/AEPHR.01.02.06
2.
Wright A Fisher PL Baker N O’Rourke L Cherry MG . Perfectionism, depression and anxiety in chronic fatigue syndrome: a systematic review. J Psychosom Res. (2021) 140:110322. doi: 10.1016/j.jpsychores.2020.110322
3.
Alme TN Andreasson A Asprusten TT Bakken AK Beadsworth MB Boye B et al . Chronic fatigue syndromes: real illnesses that people can recover from. Scand J Prim Health Care. (2023) 2023:1–5. doi: 10.1080/02813432.2023.2235609
4.
Sweetman E Noble A Edgar C Mackay A Helliwell A Vallings R et al . Current research provides insight into the biological basis and diagnostic potential for Myalgic encephalomyelitis/chronic fatigue syndrome (me/Cfs). Diagnostics. (2019) 9:73. doi: 10.3390/diagnostics9030073
5.
Holden S Maksoud R Eaton-Fitch N Cabanas H Staines D Marshall-Gradisnik S . A systematic review of mitochondrial abnormalities in Myalgic encephalomyelitis/chronic fatigue syndrome/systemic exertion intolerance disease. J Transl Med. (2020) 18:290. doi: 10.1186/s12967-020-02452-3
6.
IOM . Beyond Myalgic encephalomyelitis/chronic fatigue syndrome: Redefining an illness. Washington DC: US Institute of Medicine (2015).
7.
Maksoud R Magawa C Eaton-Fitch N Thapaliya K Marshall-Gradisnik S . Biomarkers for Myalgic encephalomyelitis/chronic fatigue syndrome (me/Cfs): a systematic review. BMC Med. (2023) 21:1–18. doi: 10.1186/s12916-023-02893-9
8.
Carruthers BM Jain AK de Meirleir KL Peterson DL Klimas NG Lerner AM et al . Myalgic encephalomyelitis/chronic fatigue syndrome: clinical working case definition, diagnostic and treatment protocols. J Chronic Fatigue Syndr. (2003) 11:2. doi: 10.1300/J092v11n01_02
9.
Carruthers BM van de Sande MI De Meirleir KL Klimas NG Broderick G Mitchell T et al . Myalgic encephalomyelitis: international consensus criteria. J Intern Med. (2011) 270:327–38. doi: 10.1111/j.1365-2796.2011.02428.x
10.
NICE . Myalgic encephalomyelitis (or encephalopathy)/chronic fatigue syndrome: Diagnosis and management. Nice Guideline [Ng206]. National Institute for Health and Care Excellence. (2021). Available at: https://www.nice.org.uk/guidance/ng206.
11.
Conroy KE Islam MF Jason LA . Evaluating case diagnostic criteria for Myalgic encephalomyelitis/chronic fatigue syndrome (me/Cfs): toward an empirical case definition. Disabil Rehabil. (2022) 45:840–7. doi: 10.1080/09638288.2022.2043462
12.
Chu L Valencia IJ Garvert DW Montoya JG . Deconstructing post-exertional malaise in Myalgic encephalomyelitis/ chronic fatigue syndrome: a patient-centered, cross-sectional survey. PLoS One. (2018) 13:e0197811. doi: 10.1371/journal.pone.0197811
13.
May M Milrad SF Perdomo DM Czaja SJ Fletcher MA Jutagir DR et al . Post-exertional malaise is associated with greater symptom burden and psychological distress in patients diagnosed with chronic fatigue syndrome. J Psychosom Res. (2020) 129:109893. doi: 10.1016/j.jpsychores.2019.109893
14.
Hartle M Bateman L Vernon SD . Dissecting the nature of post-exertional malaise. Fatigue. (2021) 9:33–44. doi: 10.1080/21641846.2021.1905415
15.
Stussman B Williams A Snow J Gavin A Scott R Nath A et al . Characterization of post–exertional malaise in patients with Myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurol. (2020) 11:1025. doi: 10.3389/fneur.2020.01025
16.
Jason LA Damrongvachiraphan D Hunnell J Bartgis L Brown A Evans M et al . Myalgic encephalomyelitis: case definitions. Auton Control Physiol State Funct. (2012) 1:1–14. doi: 10.4303/acpsf/K110601
17.
Mateo LJ Chu L Stevens S Stevens J Snell CR Davenport T et al . Post-exertional symptoms distinguish Myalgic encephalomyelitis/chronic fatigue syndrome subjects from healthy controls. Work. (2020) 66:265–75. doi: 10.3233/WOR-203168
18.
Cotler J Holtzman C Dudun C Jason LA . A brief questionnaire to assess post-exertional malaise. Diagnostics (Basel). (2018) 8:66. doi: 10.3390/diagnostics8030066
19.
Hodges LD Nielsen T Baken D . Physiological measures in participants with chronic fatigue syndrome, multiple sclerosis and healthy controls following repeated exercise: a pilot study. Clin Physiol Funct Imaging. (2018) 38:639–44. doi: 10.1111/cpf.12460
20.
Klebek L Sunnquist M Jason LA . Differentiating post-polio syndrome from Myalgic encephalomyelitis and chronic fatigue syndrome. Fatigue. (2019) 7:196–206. doi: 10.1080/21641846.2019.1687117
21.
van Campen C Visser FC . Comparing idiopathic chronic fatigue and Myalgic encephalomyelitis/chronic fatigue syndrome (me/Cfs) in males: response to two-day cardiopulmonary exercise testing protocol. Healthcare (Basel). (2021) 9:683. doi: 10.3390/healthcare9060683
22.
Brown A Jason LA . Meta-analysis investigating post-exertional malaise between patients and controls. J Health Psychol. (2018) 25:2053–71. doi: 10.1177/1359105318784161
23.
Lim EJ Kang EB Jang ES Son CG . The prospects of the two-day cardiopulmonary exercise test (Cpet) in me/Cfs patients: a Meta-analysis. J Clin Med. (2020) 9:40. doi: 10.3390/jcm9124040
24.
Nijs J Nees A Paul L De Kooning M Ickmans K Meeus M et al . Altered immune response to exercise in patients with chronic fatigue syndrome/Myalgic encephalomyelitis: a systematic literature review. Exerc Immunol Rev. (2014) 20:94–116. PMID:
25.
Stevens S Snell C Stevens J Keller B VanNess JM . Cardiopulmonary exercise test methodology for assessing exertion intolerance in Myalgic encephalomyelitis/chronic fatigue syndrome. Front Pediatr. (2018) 6:242. doi: 10.3389/fped.2018.00242
26.
Cook DB Light AR Light KC Broderick G Shields MR Dougherty RJ et al . Neural consequences of post-exertion malaise in Myalgic encephalomyelitis/chronic fatigue syndrome. Brain Behav Immun. (2017) 62:87–99. doi: 10.1016/j.bbi.2017.02.009
27.
Joseph P Arevalo C Oliveira RKF Faria-Urbina M Felsenstein D Oaklander AL et al . Insights from invasive cardiopulmonary exercise testing of patients with Myalgic encephalomyelitis/chronic fatigue syndrome. Chest. (2021) 160:642–51. doi: 10.1016/j.chest.2021.01.082
28.
Fluge O Tronstad KJ Mella O . Pathomechanisms and possible interventions in Myalgic encephalomyelitis/chronic fatigue syndrome (me/Cfs). J Clin Invest. (2021) 131:377. doi: 10.1172/JCI150377
29.
Jäkel B Kedor C Grabowski P Wittke K Thiel S Scherbakov N et al . Hand grip strength and fatigability: correlation with clinical parameters and diagnostic suitability in me/Cfs. J Transl Med. (2021) 19:1–12. doi: 10.1186/s12967-021-02774-w
30.
Grande T Grande B Gerner P Hammer S Stingl M Vink M et al . The role of psychotherapy in the Care of Patients with Myalgic encephalomyelitis/chronic fatigue syndrome. Medicina. (2023) 59:719. doi: 10.3390/medicina59040719
31.
Surawy C Hackmann A Hawton K Sharpe M . Chronic fatigue syndrome: a cognitive approach. Behav Res Ther. (1995) 33:535–44. doi: 10.1016/0005-7967(94)00077-w
32.
Geraghty KJ Blease C . Myalgic encephalomyelitis/chronic fatigue syndrome and the biopsychosocial model: a review of patient harm and distress in the medical encounter. Disabil Rehabil. (2019) 41:3092–102. doi: 10.1080/09638288.2018.1481149
33.
Wormgoor MEA Rodenburg SC . The evidence base for physiotherapy in Myalgic encephalomyelitis/chronic fatigue syndrome when considering post-exertional malaise: a systematic review and narrative synthesis. J Transl Med. (2021) 19:1. doi: 10.1186/s12967-020-02683-4
34.
Ahmed SA Mewes JC Vrijhoef H . Assessment of the scientific rigour of randomized controlled trials on the effectiveness of cognitive Behavioural therapy and graded exercise therapy for patients with Myalgic encephalomyelitis/chronic fatigue syndrome: a systematic review. J Health Psychol. (2020) 25:240–55. doi: 10.1177/1359105319847261
35.
Bateman L Bested AC Bonilla HF Chheda BV Chu L Curtin JM et al . Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin Proc. (2021) 96:2861–78. doi: 10.1016/j.mayocp.2021.07.004
36.
Nacul L Authier FJ Scheibenbogen C Lorusso L Helland IB Martin JA et al . European network on Myalgic encephalomyelitis/chronic fatigue syndrome (Euromene): Expert consensus on the diagnosis, service provision, and Care of People with me/Cfs in Europe. Medicina (Kaunas). (2021) 57:510. doi: 10.3390/medicina57050510
37.
Grach SL Seltzer J Chon TY Ganesh R . Diagnosis and Management of Myalgic Encephalomyelitis/chronic fatigue syndrome. Mayo Clin Proc. (2023) 96:2861–78. doi: 10.1016/j.mayocp.2023.07.032
38.
Geraghty K Hann M Kurtev S . Myalgic encephalomyelitis/chronic fatigue syndrome Patients' reports of symptom changes following cognitive Behavioural therapy, graded exercise therapy and pacing treatments: analysis of a primary survey compared with secondary surveys. J Health Psychol. (2019) 24:1318–33. doi: 10.1177/1359105317726152
39.
Brown M Khorana N Jason LA . The role of changes in activity as a function of perceived available and expended energy in nonpharmacological treatment outcomes for me/Cfs. J Clin Psychol. (2011) 67:253–60. doi: 10.1002/jclp.20744
40.
Sanal-Hayes NE Mclaughlin M Hayes LD Mair JL Ormerod J Carless D et al . A scoping review of ‘Pacing’for Management of Myalgic Encephalomyelitis/chronic fatigue syndrome (me/Cfs): lessons learned for the long Covid pandemic. J Transl Med. (2023) 21:1–22. doi: 10.1186/s12967-023-04587-5
41.
Helsedirektoratet. Nasjonal Veileder . Pasienter Med Cfs/Me: Utredning, Diagnostikk, Behandling, Rehabilitering, Pleie Og Omsorg. Oslo: Helsedirektoratet (2015).
42.
Kielland A Liu J Jason LA . Do diagnostic criteria for me matter to patient experience with services and interventions? Key results from an online Rds survey targeting fatigue patients in Norway. J Health Psychol. (2023) 28:1189–203. doi: 10.1177/13591053231169191
43.
Tschopp R König RS Rejmer P Paris DH . Health system support among patients with me/Cfs in Switzerland. J Taibah Univ Med Sci. (2023) 18:876–85. doi: 10.1016/j.jtumed.2022.12.019
44.
Sunnquist M Nicholson L Jason LA Friedman KJ . Access to medical Care for Individuals with Myalgic encephalomyelitis and chronic fatigue syndrome: a call for centers of excellence. Mod Clin Med Res. (2017) 1:28–35. doi: 10.22606/mcmr.2017.11005
45.
Froehlich L Hattesohl DB Jason LA Scheibenbogen C Behrends U Thoma M . Medical care situation of people with Myalgic encephalomyelitis/chronic fatigue syndrome in Germany. Medicina. (2021) 57:646. doi: 10.3390/medicina57070646
46.
Davis HE McCorkell L Vogel JM Topol EJ . Long Covid: major findings, mechanisms and recommendations. Nat Rev Microbiol. (2023) 21:133–46. doi: 10.1038/s41579-022-00846-2
47.
Wong TL Weitzer DJ . Long Covid and Myalgic encephalomyelitis/chronic fatigue syndrome (me/Cfs)-a systemic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas). (2021) 57:418. doi: 10.3390/medicina57050418
48.
Marks DF . Converging evidence of similar symptomatology of me/Cfs and Pasc indicating multisystemic Dyshomeostasis. Biomedicine. (2023) 11:180. doi: 10.3390/biomedicines11010180
49.
Joseph P Singh I Oliveira R Capone CA Mullen MP Cook DB et al . Exercise pathophysiology in Myalgic encephalomyelitis/chronic fatigue syndrome and post-acute sequelae of Sars-Cov-2: more in common than not?Chest. (2023) 164:717–26. doi: 10.1016/j.chest.2023.03.049
50.
Vernon SD Hartle M Sullivan K Bell J Abbaszadeh S Unutmaz D et al . Post-exertional malaise among people with long Covid compared to Myalgic encephalomyelitis/chronic fatigue syndrome (me/Cfs). Work. (2023) 74:1179–86. doi: 10.3233/wor-220581
51.
Komaroff AL Lipkin WI . Me/Cfs and long Covid share similar symptoms and biological abnormalities. Front Med. (2023) 10:1059. doi: 10.3389/fmed.2023.1187163
52.
Singh I Joseph P Heerdt PM Cullinan M Lutchmansingh DD Gulati M et al . Persistent exertional intolerance after Covid-19: insights from invasive cardiopulmonary exercise testing. Chest. (2022) 161:54–63. doi: 10.1016/j.chest.2021.08.010
53.
Jason LA Dorri JA . Me/Cfs and post-exertional malaise among patients with long Covid. Neurol Int. (2022) 15:1–11. doi: 10.3390/neurolint15010001
54.
World Health Organisation . Clinical Management of Covid-19: Living Guideline. Geneva: World Health Organization (2022).
55.
Ghali A Lacombe V Ravaiau C Delattre E Ghali M Urbanski G et al . The relevance of pacing strategies in managing symptoms of post-Covid-19 syndrome. J Transl Med. (2023) 21:375. doi: 10.1186/s12967-023-04229-w
56.
Scheibenbogen C Bellmann-Strobl JT Heindrich C Wittke K Stein E Franke C et al . Fighting post-Covid and me/Cfs–development of curative therapies. Front Med. (2023) 10:1194754. doi: 10.3389/fmed.2023.1194754
57.
Wormgoor MEA . Pasienterfaringer med Utredning Og Behandling Av me Ved Sykehuset I Vestfold Og Telemark Sykehus [patient experiences with clinical evaluations and treatment of me at Vestfold hospital and Telemark hospital]. (2023).
58.
Schei T Angelsen A . Me-Pasienters Erfaring med Rehabiliteringsopphold [me Patients' experience with rehabilitation stays]. Norges Myalgisk Encefalopati Forening, (2018).
59.
WHO . Icidh-2: International classification of function and disability, Prefinal draft. Geneva, Switzerland: World Health Organization (2000).
60.
WHO . International Classification of Primary Care, -Icpc-2. Geneva: WHO (2009).
61.
Vink M Vink-Niese F . Work rehabilitation and medical retirement for Myalgic encephalomyelitis/chronic fatigue syndrome patients. A review and appraisal of diagnostic strategies. Diagnostics (Basel). (2019) 9:124. doi: 10.3390/diagnostics9040124
62.
Ghali A Lacout C Fortrat J-O Depres K Ghali M Lavigne C . Factors influencing the prognosis of patients with Myalgic encephalomyelitis/chronic fatigue syndrome. Diagnostics. (2022) 12:2540. doi: 10.3390/diagnostics12102540
63.
Wessely S David A Butler S Chalder T . Management of Chronic (post-viral) fatigue syndrome. J R Coll Gen Pract. (1989) 39:26–9. PMID:
64.
Knoop H Prins JB Moss-Morris R Bleijenberg G . The central role of cognitive processes in the perpetuation of chronic fatigue syndrome. J Psychosom Res. (2010) 68:489–94. doi: 10.1016/j.jpsychores.2010.01.022
65.
Vink M Vink-Niese A . Cognitive Behavioural therapy for Myalgic encephalomyelitis/chronic fatigue syndrome is not effective. Re-Analysis of a Cochrane Review. Health Psychol Open. (2019) 6:84061. doi: 10.1177/2055102919840614
66.
McPhee G Baldwin A Kindlon T Hughes BM . Monitoring treatment harm in Myalgic encephalomyelitis/chronic fatigue syndrome: a freedom-of-information study of National Health Service Specialist Centres in England. J Health Psychol. (2021) 26:975–84. doi: 10.1177/1359105319854532
67.
Shepherd C . Pacing and exercise in chronic fatigue syndrome. Physiotherapy. (2001) 87:395–6. doi: 10.1016/S0031-9406(05)65457-0
68.
Gladwell PW Pheby D Rodriguez T Poland F . Use of an online survey to explore positive and negative outcomes of rehabilitation for people with Cfs/me. Disabil Rehabil. (2014) 36:387–94. doi: 10.3109/09638288.2013.797508
69.
Dawes H . Results of the forward me survey for Cbt and get. Oxford: Oxford Brookes University (2019).
70.
Collin SM Crawley E . Specialist treatment of chronic fatigue syndrome/me: a cohort study among adult patients in England. BMC Health Serv Res. (2017) 17:488. doi: 10.1186/s12913-017-2437-3
71.
Reid H Ridout AJ Tomaz SA Kelly P Jones N . Benefits outweigh the risks: a consensus statement on the risks of physical activity for people living with long-term conditions. Br J Sports Med. (2022) 56:427–38. doi: 10.1136/bjsports-2021-104281
72.
Nacul L O'Boyle S Palla L Nacul FE Mudie K Kingdon CC et al . How Myalgic encephalomyelitis/chronic fatigue syndrome (me/Cfs) progresses: the natural history of me/Cfs. Front Neurol. (2020) 11:826. doi: 10.3389/fneur.2020.00826
73.
Wyller VB Eriksen HR Malterud K . Can sustained arousal explain the chronic fatigue syndrome?Behav Brain Funct. (2009) 5:10. doi: 10.1186/1744-9081-5-10
74.
Ursin H Eriksen HR . The cognitive activation theory of stress. Psychoneuroendocrinology. (2004) 29:567–92. doi: 10.1016/S0306-4530(03)00091-X
75.
Larun L Malterud K . Identity and coping experiences in chronic fatigue syndrome: a synthesis of qualitative studies. Patient Educ Couns. (2007) 69:20–8. doi: 10.1016/j.pec.2007.06.008
76.
De Boer ML . Epistemic in/Justice in Patient Participation. A discourse analysis of the Dutch me/Cfs health council advisory process. Sociol Health Illn. (2021) 43:1335–54. doi: 10.1111/1467-9566.13301
77.
Åsbring P Närvänen A-L . Women’s experiences of stigma in relation to chronic fatigue syndrome and fibromyalgia. Qual Health Res. (2002) 12:148–60. doi: 10.1177/104973230201200202
78.
Mengshoel AM Helland IB Meeus M Castro-Marrero J Pheby D Bolle SE . Patients' experiences and effects of non-pharmacological treatment for Myalgic encephalomyelitis/chronic fatigue syndrome - a scoping mixed methods review. Int J Qual Stud Health Wellbeing. (2020) 15:1764830. doi: 10.1080/17482631.2020.1764830
79.
Gilje AM Söderlund A Malterud K . Obstructions for quality care experienced by patients with chronic fatigue syndrome (Cfs)—a case study. Patient Educ Couns. (2008) 73:36–41. doi: 10.1016/j.pec.2008.04.001
80.
Hng KN Geraghty K Pheby DFH . An audit of Uk hospital Doctors' knowledge and experience of Myalgic encephalomyelitis. Medicina (Kaunas). (2021) 57:885. doi: 10.3390/medicina57090885
81.
Komaroff AL . Myalgic encephalomyelitis/chronic fatigue syndrome: a real illness. Ann Intern Med. (2015) 162:871–2. doi: 10.7326/M15-0647
82.
Bellman V Zolnikov TR . Understanding patient-provider interaction, treatment acceptance, and outcomes in medically unexplained symptoms. Cureus. (2022) 14:e32915. doi: 10.7759/cureus.32915
83.
Cairns R Hotopf M . A systematic review describing the prognosis of chronic fatigue syndrome. Occup Med (Lond). (2005) 55:20–31. doi: 10.1093/occmed/kqi013
84.
Sommerfelt K Schei T Angelsen A . Severe and very severe Myalgic encephalopathy/chronic fatigue syndrome me/Cfs in Norway: symptom burden and access to care. J Clin Med. (2023) 12:1487. doi: 10.3390/jcm12041487
85.
Komaroff AL Bateman L . Will Covid-19 Lead to Myalgic encephalomyelitis/chronic fatigue syndrome?Front Med (Lausanne). (2020) 7:606824. doi: 10.3389/fmed.2020.606824
86.
Twomey R DeMars J Franklin K Culos-Reed SN Weatherald J Wrightson JG . Chronic fatigue and Postexertional malaise in people living with long Covid: an observational study. Phys Ther. (2022) 102:5. doi: 10.1093/ptj/pzac005
87.
Decary S Gaboury I Poirier S Garcia C Simpson S Bull M et al . Humility and acceptance: working within our limits with long Covid and Myalgic encephalomyelitis/chronic fatigue syndrome. J Orthop Sports Phys Ther. (2021) 51:197–200. doi: 10.2519/jospt.2021.0106
88.
Pheby DFH Araja D Berkis U Brenna E Cullinan J de Korwin JD et al . The role of prevention in reducing the economic impact of me/Cfs in Europe: A report from the socioeconomics working Group of the European Network on me/Cfs (Euromene). Medicina (Kaunas). (2021) 57:388. doi: 10.3390/medicina57040388
89.
Oliveira CR Jason LA Unutmaz D Bateman L Vernon SD . Improvement of long Covid symptoms over one year. Front Med. (2023) 9:1065620. doi: 10.3389/fmed.2022.1065620
90.
Holgate ST Komaroff AL Mangan D Wessely S . Chronic fatigue syndrome: understanding a complex illness. Nat Rev Neurosci. (2011) 12:539–44. doi: 10.1038/nrn3087
91.
Wojcik W Armstrong D Kanaan R . Is chronic fatigue syndrome a neurological condition? A survey of Uk neurologists. J Psychosom Res. (2011) 70:573–4. doi: 10.1016/j.jpsychores.2011.02.007
92.
Pheby DF Araja D Berkis U Brenna E Cullinan J de Korwin J-D et al . A literature review of Gp knowledge and understanding of me/Cfs: a report from the socioeconomic working Group of the European Network on me/Cfs (Euromene). Medicina. (2020) 57:7. doi: 10.3390/medicina57010007
93.
Muirhead N Muirhead J Lavery G Marsh B . Medical school education on Myalgic encephalomyelitis. Medicina. (2021) 57:542. doi: 10.3390/medicina57060542
Summary
Keywords
ME/CFS, myalgic encephalomyelitis, chronic fatigue syndrome, post-exertional malaise, post-exertional symptom exacerbation, patient experience, specialist healthcare, healthcare quality
Citation
Wormgoor MEA and Rodenburg SC (2023) Focus on post-exertional malaise when approaching ME/CFS in specialist healthcare improves satisfaction and reduces deterioration. Front. Neurol. 14:1247698. doi: 10.3389/fneur.2023.1247698
Received
03 July 2023
Accepted
31 October 2023
Published
01 December 2023
Volume
14 - 2023
Edited by
Nuno Sepulveda, Warsaw University of Technology, Poland
Reviewed by
Geoffrey Moore, Cornell University, United States; Nina Muirhead, Buckinghamshire Healthcare NHS Trust, United Kingdom
Updates
Copyright
© 2023 Wormgoor and Rodenburg.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marjon E. A. Wormgoor, rodeworm@online.no; marwor@siv.no
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.