- 1Affiliated Hospital of Hebei University, Baoding, China
- 2Clinical Medical College, Hebei University, Baoding, China
- 3Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 4Puyang Oilfield General Hospital, Puyang, China
- 5China National Clinical Research Center for Neurological Diseases, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 6Beijing Advanced Innovation Center for Biomedical Engineering, School of Biological Science and Medical Engineering, Beihang University, Beijing, China
Background: Data on the association between serum alkaline phosphatase (ALP) levels and clinical outcomes in patients with ischemic stroke (IS) are inconsistent and limited. Therefore, this study aimed to investigate the correlation between ALP and prognosis in patients with IS.
Methods: Patients with acute ischemic stroke (AIS) or transient ischemic attack (TIA) from the Third China National Stroke Registry were divided into four groups according to the quartiles of serum ALP levels on admission. Cox proportional hazards and logistic regression models were used to evaluate the correlation between ALP and the risk of all-cause mortality, disability (modified Rankin Scale (mRS) score 3–5), and poor functional outcomes (mRS score 3–6).
Results: A total of 11,405 patients were included in the study. Higher levels of ALP were associated with all-cause mortality at 3 months (adjusted hazard ratio [HR] per standard deviation [SD]: 1.16; 95% confidence interval (CI): 1.07–1.27; p = 0.001) and 1 year (adjusted HR: 1.11; 95% CI: 1.03–1.20; p = 0.010). At the 3-month follow-up, each SD increase of ALP was associated with a 12 and 14% higher risk of disability (adjusted odds ratio (OR): 1.12; 95% CI: 1.06–1.18; p < 0.001) and poor functional outcomes (adjusted OR: 1.14; 95% CI: 1.08–1.20; p < 0.001). Similar results were observed at the 1-year follow-up. Higher ALP levels were associated with an increased risk of all-cause mortality, disability, and poor functional outcomes in patients with “others” subtypes (including other determined etiology and undetermined etiology) (p < 0.05).
Conclusion: Elevated ALP levels were associated with an increased risk of all-cause mortality, disability, and poor function outcomes in patients with IS. Heterogeneity was observed among the subtypes of different etiologies.
1 Introduction
Stroke is the second leading cause of death and the third leading cause of disability worldwide (1, 2). Globally, China faces the most significant stroke burden, with ischemic stroke (IS) accounting for over 82% of all stroke cases (3). Therefore, identifying reliable blood markers for stroke prognosis is crucial for optimizing healthcare resource allocation (4).
Alkaline phosphatase (ALP), a widely expressed enzyme in human tissues, has been implicated in vascular calcification and the development of atherosclerosis (5–7). Inhibition of ALP has been shown to prevent the formation of vascular atherosclerosis (8). Traditionally recognized as a marker for skeletal or hepatobiliary dysfunction (9, 10), ALP is now considered indicative of atherosclerosis and inflammatory responses (6, 11). Studies have indicated that elevated serum ALP levels are linked to increased atherosclerosis in coronary and peripheral arteries and that higher ALP levels are independently associated with the risk of cardiovascular disease (CVD) and mortality events (12–14). However, conflicting findings exist among epidemiological investigations regarding the association between higher serum ALP levels and adverse clinical outcomes in stroke patients. Multiple studies have suggested that high serum ALP levels are associated with an increased incidence of stroke, higher post-stroke mortality rates, and poor functional outcomes (15–19). However, other studies have concluded that there was no significant association between increased ALP levels and poor functional outcomes (18, 20). Therefore, at present, there is no research consensus on the association between ALP levels and clinical outcomes in patients with stroke, and studies on this topic have been limited. Furthermore, the different etiologies of stroke have not yet been examined.
Therefore, this study aims to utilize a large sample from the China National Stroke Registry III (CNSR-III) to investigate the correlation between serum ALP levels and clinical outcomes (mortality, disability, and poor functional outcomes) in patients with acute ischemic stroke (AIS) and transient ischemic attack (TIA), analyze the association between serum ALP levels and stroke subtypes, and further explore the underlying mechanism of ALP.
2 Methods
2.1 Study population
CNSR-III is a nationwide prospective registry of consecutive patients with AIS or TIA. Patients were enrolled from 201 hospitals between August 2015 and March 2018. The detailed design and description of the CNSR-III have been published previously (21). The inclusion criteria were as follows: (1) age 18 years or older and (2) diagnosis of ischemic stroke or TIA within 7 days from the onset of symptoms to enrollment. The exclusion criteria were as follows: (1) silent cerebral infarction with no manifestation of symptoms and signs, and (2) refusal to participate in the registry.
The CNSR-III study was approved by the ethics committee of Beijing Tiantan Hospital (NO. KY2015-001-01), and written informed consent was obtained from patients or their legally authorized representatives. The study complied with the principles of the Declaration of Helsinki.
2.2 Data collection and management
After admission, the participants were collected by a trained neurologist in the hospital and the following baseline data were recorded: age, sex, body mass index (BMI, calculated as kg/m2), smoking and alcohol consumption status, medical history (previous diabetes, hypertension, dyslipidemia, coronary heart disease, and stroke), treatment during hospitalization (intravenous thrombolysis, mechanical thrombectomy, antiplatelet aggregation therapy, anticoagulation therapy, and lipid-lowering therapy), National Institutes of Health Stroke Scale (NIHSS) score on admission, and pre-stroke modified Rankin Scale (mRS) score. In addition, serum ALP, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were obtained through venous puncture within 24 h. Total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglyceride (TG), estimated glomerular filtration rate (eGFR), and high-sensitivity C-reactive protein (hs-CRP) samples were transported to the central laboratory of Beijing Tiantan Hospital for centralized testing through the cold chain.
All imaging data were collected on disks in the DICOM format, analyzed by two professional neurologists, and classified by etiological TOAST (Org10172 trial in the treatment of acute stroke). As there were a few patients with a stroke of other determined etiology, these patients were combined as a stroke of undetermined etiology and defined together as the “others.” Hence, patients in this study were classified into four subtypes: large-artery atherosclerosis (LAA), cardioembolism, small-vessel occlusion, and others (including other determined etiology and undetermined etiology).
2.3 Patient follow-up and clinical outcome assessment
The clinical outcomes were obtained by trained research coordinators who were unaware of the participants’ baseline characteristics, through a face-to-face interview at 3 months and via the telephone at 1 year after the onset of symptoms. Clinical outcomes included all-cause mortality, disability, and poor functional outcomes at the 3-month and 1-year follow-up. All-cause mortality was either confirmed by a death certification from the attended hospital or the local citizen registry, and the mRS score ranged from 0 (no symptoms) to 6 (death); poor functional outcome was determined by an mRS score of 3–6, while major disability was determined by an mRS score of 3–5.
2.4 Statistical analysis
This study’s population characteristics were presented as medians (interquartile ranges, IQRs) or numbers (proportions) by quartiles of serum ALP levels. The associations of ALP with all-cause mortality, disability, and poor functional outcomes at 3 months and 1 year were assessed. For all-cause mortality, we used the Kaplan–Meier method to estimate the cumulative incidence in the ALP quartile groups, and the difference across groups was compared using the log-rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using the Cox proportional hazards models. The proportional hazards assumption was checked using Schoenfeld residuals over time, and no deviations from the assumption were found. For disability and poor functional outcomes, odds ratios (ORs) with 95% CIs were estimated using logistic regression models. Serum ALP was included in the models, both as a categorized variable (in quartiles) and as a continuous variable.
Based on the clinical experience and relevant literature (16–18), we selected covariates and fitted three adjusted models. Model 1 was adjusted for age and gender. Model 2 was further adjusted for BMI, current smoking, heavy drinking, pre-stroke mRS score, TOAST classification, hypertension, diabetes, dyslipidemia, coronary heart disease, and previous stroke. Model 3 was further adjusted for antiplatelet agents, anticoagulant agents, estimated glomerular filtration rate, and high-sensitivity C-reactive protein. To visualize the potential non-linear associations of serum ALP with death, disability, and poor functional outcomes, we constructed restricted cubic splines with three knots at the 10th, 50th, and 90th percentiles. Stratified analyzes were performed in the subgroups of TOAST types. All statistical analyzes were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, United States) and R software version 4.1.3 (R Foundation for Statistical Computing). The statistical significance was determined as two-sided p-values of <0.05.
3 Results
3.1 Baseline characteristics
We excluded 3,761 patients from the initial 15,166 patients due to underlying conditions, such as liver disease (n = 100), kidney disease (n = 131), arthritis (n = 329), cancer (n = 134), infection within 2 weeks before admission (n = 450), or missing ALP values (n = 2,337), as well as mRS scores at the 1-year (n = 349) or 3-month (n = 170) follow-up (Supplementary Figure S1). The final analysis encompassed 11,405 patients. The baseline characteristics of both included and excluded patients are presented in Supplementary Table S1, demonstrating a balanced distribution between the two groups. Table 1 summarizes the baseline characteristics of the included patients, with a median age of 63 (54.0–70.0) years. Among the included patients, 10,525 (92.3%) were diagnosed with AIS, 7,784 (68.3%) were male patients, 3,605 (31.6%) were current smokers, 1,628 (14.3%) were heavy drinkers, 949 (8.3%) received intravenous thrombolysis, and 32 (0.3%) of them underwent mechanical thrombectomy. The median NIHSS score was 3 (1.0, 6.0). In the higher quartile ALP groups, patients were more likely to have a history of hypertension, coronary heart disease, and stroke, while they were less likely to have diabetes, be smokers, and consume alcohol. In TOAST classification, all ALP quartile groups had a relatively higher number of LAA and undetermined etiology stroke patients. The levels of hs-CRP increased with the increase in serum ALP levels (Table 1).
3.2 All-cause mortality
A total of 160 (1.4%) and 355 (3.1%) patients died during the 3-month and 1-year follow-up, respectively. The Kaplan–Meier curves showed that the cumulative incidence of all-cause mortality increased in patients with higher serum ALP levels within 3-month (log-rank p = 0.015) and 1-year (log-rank p = 0.058) follow-up (Figure 1). Higher levels of ALP were associated with all-cause mortality at 3 months (adjusted HR per standard deviation [SD]: 1.16; 95% CI: 1.07–1.27; p = 0.001) and 1 year (adjusted HR: 1.11; 95% CI: 1.03–1.20; p = 0.010) (Table 2). In addition, there was a linear correlation between the increase in ALP and all-cause mortality (p < 0.001; Figures 2A,D).
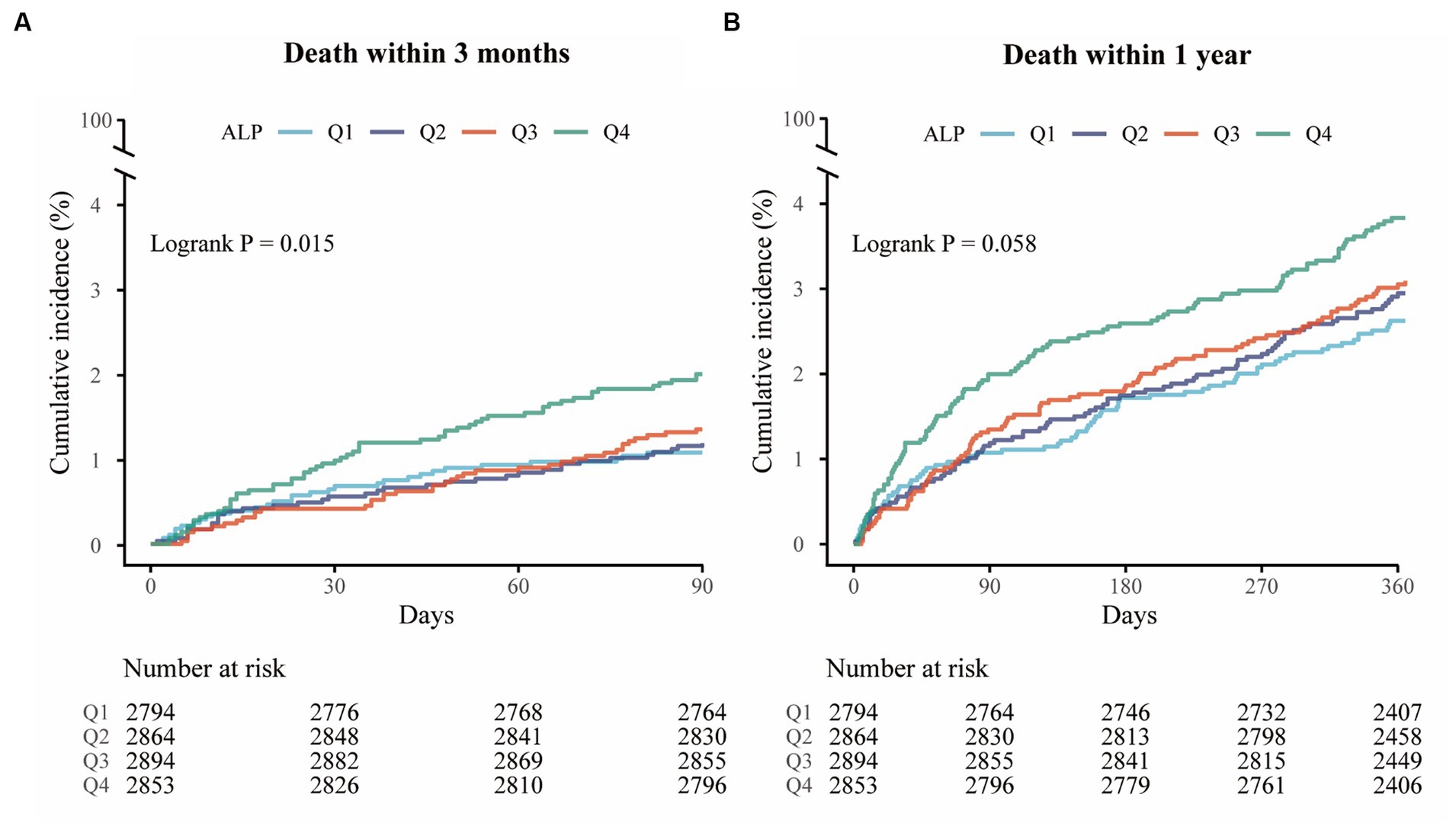
Figure 1. Kaplan–Meier curves for all-cause mortality. (A) Kaplan–Meier curves for all-cause mortality within 3 months (log-rank test; p = 0.015). (B) Kaplan–Meier curves for all-cause mortality within 1 year (log-rank test; p = 0.058).
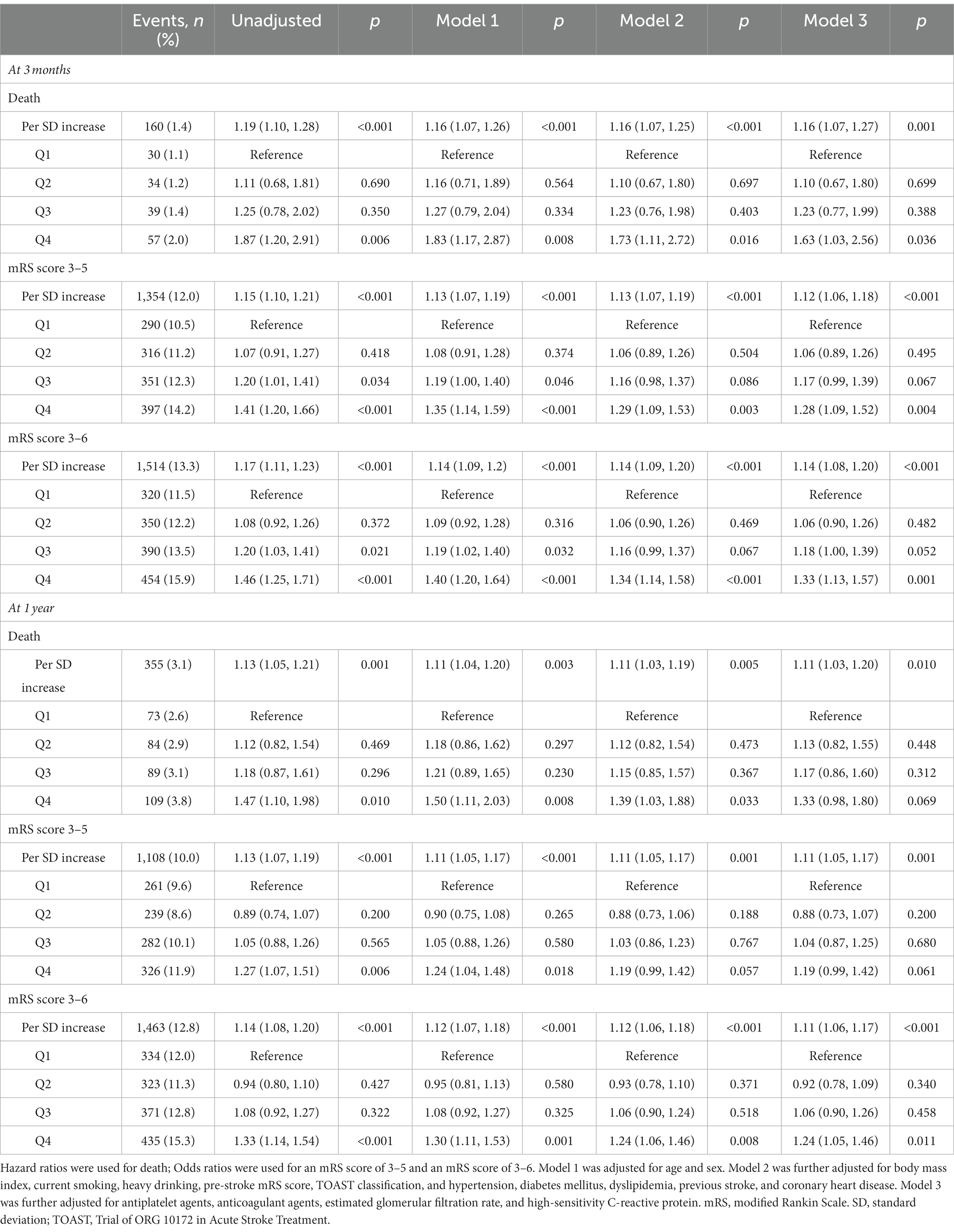
Table 2. Associations of alkaline phosphatase with all-cause mortality, disability, and poor functional outcomes.
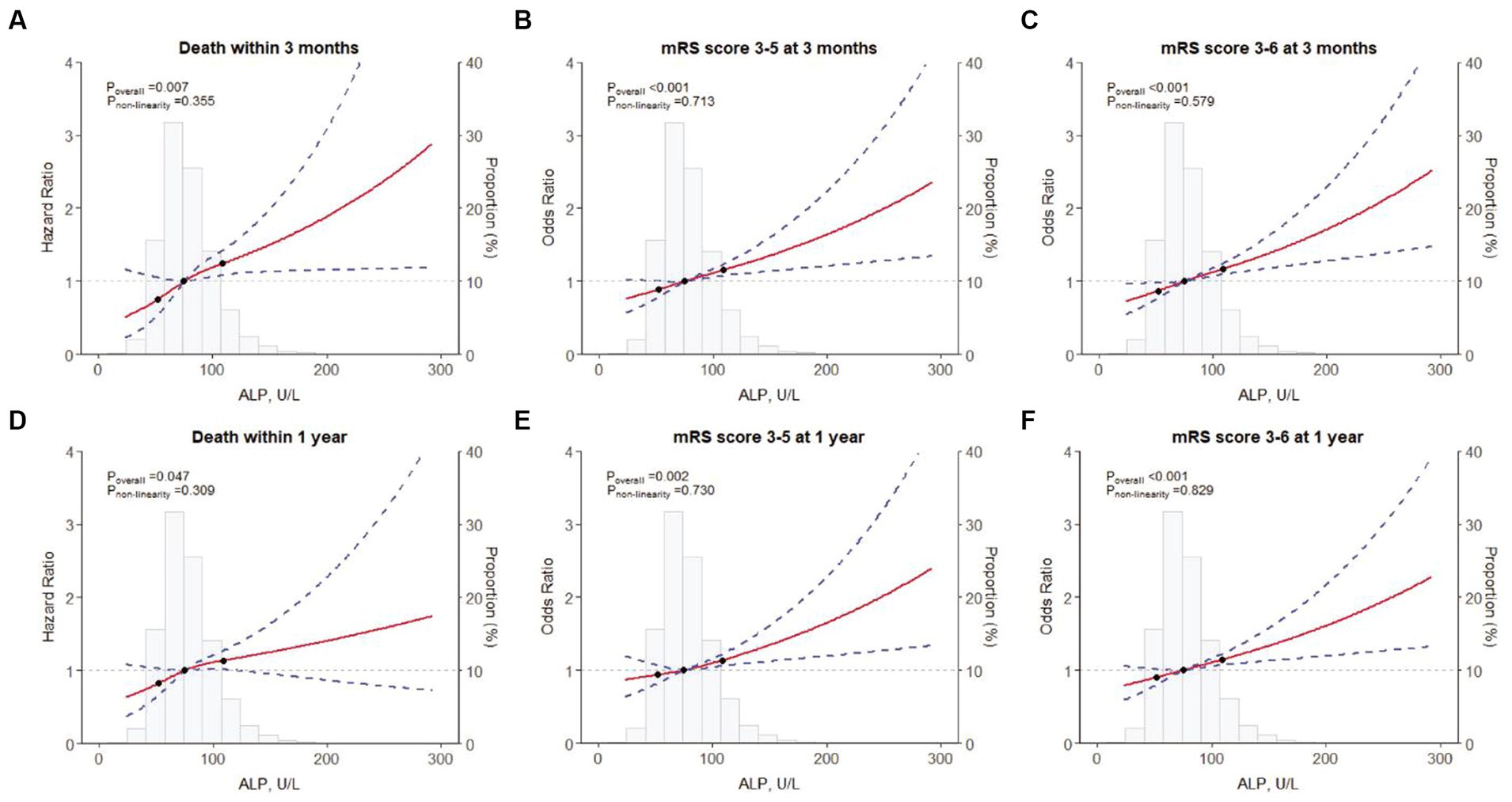
Figure 2. Restricted cubic spline for associations between ALP and clinical outcomes. (A) Death within 3 months; (B) an mRS score of 3–5 at 3 months; (C) an mRS score of 3–6 at 3 months; (D) Death within 1 year; (E) an mRS score of 3–5 at 1 year; (F) an mRS score of 3–6 at 1 year. ALP, alkaline phosphatase; mRS, modified Rankin Scale.
3.3 Functional outcome
In total, 1,354 patients (12.0%) had an mRS score of 3–5, and 1,514 patients (13.3%) had an mRS score of 3–6 at 3 months. At the 1-year assessment, 1,108 patients (10.0%) had an mRS score of 3–5, and 1,463 patients (12.8%) had an mRS score of 3–6. The higher the level of ALP grouping, the higher the proportion of patients with high mRS scores (Supplementary Figure S2).
At the 3-month follow-up, compared with the lowest quartile, patients in the highest quartile had a 28 and 33% greater risk of disability and poor functional outcomes, respectively (adjusted OR: 1.28; 95% CI: 1.09–1.52; p = 0.004 and 1.33; 95% CI: 1.13–1.57; p = 0.001). Similarly, at the 1-year follow-up, the risk of poor functional outcomes was found to increase in the highest quartile compared with the lowest quartile, with adjusted OR 1.24 (95% CI: 1.05–1.46; p = 0.011) (Table 2).
At the 3-month follow-up, each SD increase of ALP levels was associated with 12 and 14% higher risk of disability (adjusted OR: 1.12; 95% CI: 1.06–1.18; p < 0.001) and poor functional outcomes (adjusted OR: 1.14; 95% CI: 1.08–1.20; p < 0.001) in the fully adjusted model, respectively. Similar results were found at the 1-year follow-up (Table 2). In addition, the restricted cubic spline regression analysis showed a linear and positive correlation between serum ALP and functional poor outcomes at 3 months and 1 year (Figures 2B,C,E,F).
3.4 TOAST classification
As for TOAST etiologies, for each 1 SD increase of ALP in the “others” subtype, the risk of death at 3 months increased by 19% (adjusted HR: 1.19; 95% CI: 1.04–1.36; p = 0.014). Similarly, the risk of death at 1 year increased by 16% (adjusted HR: 1.16; 95% CI: 1.04–1.30; p = 0.008) (Supplementary Table S2). However, no correlation with mortality was found among LAA, SVO, and CE subtypes.
After adjusting for potential confounding factors (Model 3), in the “others” subtype, higher levels of ALP were associated with poor functional outcomes at 3 months (OR per SD: 1.15 [95% CI: 1.06–1.24; p = 0.001]) and 1 year (OR per SD: 1.16; 95% CI: 1.07–1.26, p < 0.001). Similar results were found for disability. In LAA and SVO subtypes, elevated ALP levels were associated with an increased risk of poor functional outcomes at 3 months, with ORs were 1.11 (95% CI: 1.01–1.22, p = 0.023) and 1.18 (95% CI: 1.04–1.34, p = 0.010), respectively. Similar trends were observed for disability. Furthermore, compared to the lowest quartile, higher levels of ALP were associated with an increased risk of disability and poor functional outcomes in patients with LAA at 3 months (p < 0.05), and there was also an increased risk of poor functional outcomes in patients with the “others” subtype at 3 months and 1 year (p < 0.05) (Figure 3; Supplementary Table S2). However, no correlation between disability and poor functional prognosis was found in the SVO and CE subtypes.
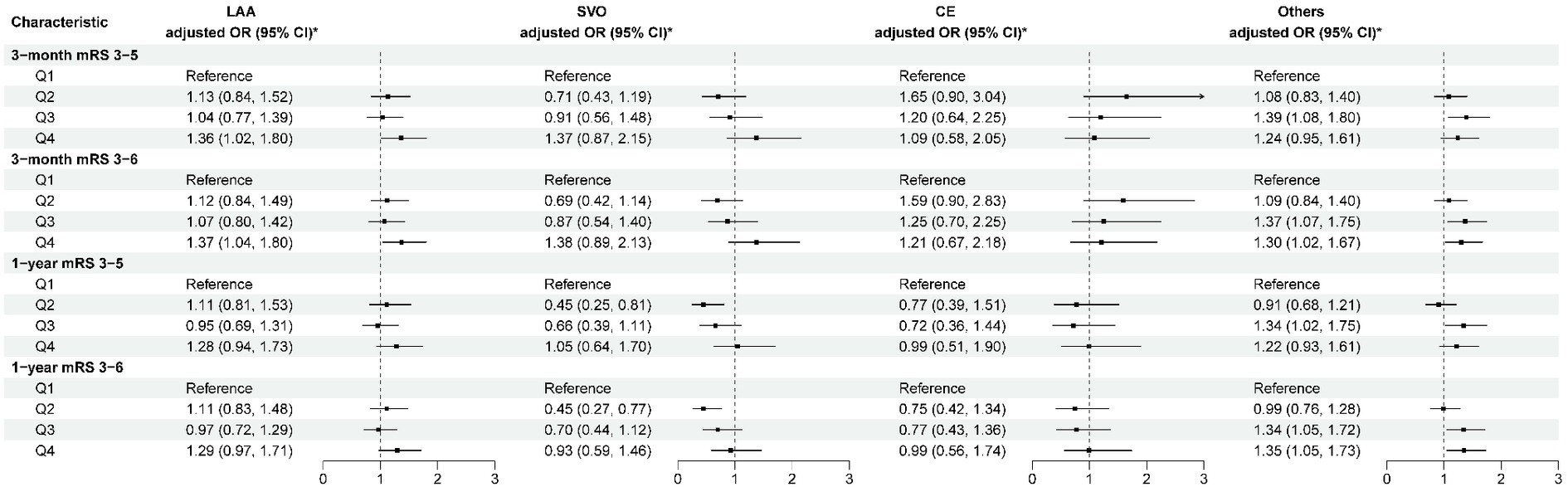
Figure 3. Multivariable analysis of disability and poor functional outcomes according to TOAST classification. *Model 3 is adjusted for age, sex, body mass index, current smoking, heavy drinking, pre-stroke mRS score, TOAST classification, hypertension, diabetes, dyslipidemia, coronary heart disease, previous stroke, antiplatelet agents, anticoagulant agents, estimated glomerular filtration rate, and high-sensitivity C-reactive protein. CE, cardioembolism; LAA, large-artery atherosclerosis; mRS, modified Rankin Scale; others, other determined etiology and undetermined etiology; SVO, small-vessel occlusion; TOAST, Trial of ORG 10172 in Acute Stroke Treatment.
4 Discussion
This study revealed a positive association between elevated ALP levels and an increased risk of mortality, disability, and poor functional outcomes in patients with AIS. Specifically, elevated ALP levels were linked to adverse clinical outcomes in the others subtype and were correlated with an increased risk of disability and poor functional outcomes in the LAA and SVO subtypes at 3 months. These findings offer new insights into the role of ALP levels in the AIS prediction. The Chinese Stroke Registry II and the Xi’an multicenter study reported that higher ALP levels were associated with increased patient mortality, without identifying a linear correlation (17, 18). Conversely, a Korean single-center study indicated a positive linear correlation between elevated ALP levels and mortality (16). In line with these findings, our study indicated that elevated ALP levels were associated with an increased risk of all-cause mortality, with a linear correlation observed. The differences in the studies primarily resulted from the included populations. The linear correlation study focused on IS patients, while the non-linear correlation studies included mixed stroke (including ischemic stroke and hemorrhagic stroke) patients. Ryu et al. also revealed that hemorrhagic stroke patients in the elevated ALP group had a higher risk of death than ischemic patients, which may affect the linear and non-linear relationships (16). Furthermore, Zhong et al. (22) observed similar results in their study of 2,944 enrolled AIS patients. We also performed an analysis of poor functional outcomes, and similar to Zhu et al. and Kim et al., we found that higher ALP levels were associated with an increased risk of poor functional outcomes (11, 19). However, Guo et al. and Liu et al. found that elevated ALP levels were not associated with poor functional outcomes in stroke patients (18, 20). The difference in conclusions may be related to the participant characteristics and sample size. Furthermore, we analyzed the disability and discovered that elevated levels of ALP could serve as a predictor for disability in patients with AIS and TIA.
In CVD studies, the elevated mortality associated with increased ALP levels was related to atherosclerosis (23). Unlike CVD, IS is a heterogeneous disease with a distinct pathogenesis. The mechanism between elevated serum ALP and prognosis in patients with IS remains unclear; no studies have investigated the role of ALP in different subtypes of IS. However, in this study, an increase in ALP was not found to be associated with all-cause mortality in patients with the LAA subtype, and only LAA and SVO subtypes were associated with poor functional outcomes and disability during the short-term follow-up. Kim et al. also found that higher ALP levels were not associated with cerebral atherosclerosis (19). Therefore, the association between higher ALP levels and an increased risk of adverse outcomes in stroke patients might be unrelated to the mechanism of atherosclerosis. This study discovered a significant association between elevated levels of ALP and the prognosis of patients with an undetermined etiology stroke subtype. Furthermore, it revealed that patients with a history of coronary heart disease exhibited elevated serum ALP levels. The occurrence of adverse clinical outcomes may be related to unstable and easy detachment of calcified plaques or cardioembolism (24, 25), which requires further research.
Systemic inflammation has been recognized as a significant factor influencing the short-term and long-term outcomes of patients with stroke (26, 27). The pathophysiological mechanisms underlying the adverse clinical outcomes in patients with elevated ALP levels may be associated with the interplay between elevated ALP, neuroinflammation, blood–brain barrier (BBB) permeability, and vascular homeostasis (28). The immune rescue mechanism of neuroinflammation was reported to be activated after cerebral ischemia (29), resulting in an increase in ALP (7, 30, 31), which was consistent with the current proposal that peripheral immunity is involved in complex brain immune networks (32). Furthermore, tissue-non-specific alkaline phosphatase (TNAP), the isoenzyme of ALP, is abundant in brain endothelial cells and neurons (33) and regulates neuroinflammatory responses (34, 35). After the breakdown of the BBB, TNAP is lost to the periphery, and the decrease in TNAP levels further exacerbates brain damage (36, 37). Thus, we hypothesized that elevated serum ALP levels in the acute phase could indicate a significant depletion of ALP in the brain, reflecting the extent of neurological impairment and ultimately leading to a poor prognosis. The potential of oral ALP or TNAP administration for the treatment of nerve damage following IS presents a compelling area for future investigation.
This study is a large-scale, multicenter prospective study with a substantial sample size, including patients from 201 hospitals, which enhances the generalizability of the research findings. This study explores, for the first time, the impact of ALP on various TOAST subtypes. However, the study also has some limitations. First, this was an observational study, controlling for some important potential confounding factors in the multivariable adjustment model; however, it is still difficult to entirely eliminate the possibility of residual confounding. Second, we did not collect information on vitamin D deficiency in our study, despite the known impact of vitamin D on serum ALP levels. To minimize the potential confounding effects, we collected blood samples at a predetermined time (the next morning after the admission with overnight fasting). Third, our study only examined ALP levels in the acute phase and did not assess their continuity over time. Therefore, it remains unclear whether changes in ALP levels may in turn impact the outcomes of IS. Fourth, the types of ALP isoenzymes have not been evaluated, and it was not possible to assess which types of ALP are associated with adverse stroke outcomes. Further studies are needed to confirm the role of isoenzymes in AIS and TIA, which might provide more valuable information for understanding the mechanism of ALP on clinical outcomes. Additionally, since all participants in the study were Chinese, the generalizability to other races and ethnicities may be limited.
5 Conclusion
In summary, this study showed that elevated ALP levels were associated with an increased risk of all-cause mortality, disability, and poor function outcomes in patients with IS. Furthermore, heterogeneity was observed among the subtypes of different stroke etiologies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Beijing Tiantan Hospital (No. KY2015-001-01). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZW: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. JLi: Methodology, Writing – original draft, Writing – review & editing. JJ: Data curation, Project administration, Supervision, Writing – review & editing. ZZ: Data curation, Project administration, Supervision, Writing – review & editing. QX: Data curation, Formal analysis, Writing – review & editing. TL: Data curation, Project administration, Supervision, Writing – review & editing. JLin: Data curation, Formal analysis, Investigation, Writing – review & editing. YJ: Data curation, Methodology, Writing – review & editing. YW: Conceptualization, Supervision, Writing – review & editing. AW: Conceptualization, Methodology, Supervision, Writing – review & editing. XM: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Key Research and Development Program of China (2022YFC3600600, 2022YFC3600603, 2022YFC3501100), the National Natural Science Foundation of China (81870905, U20A20358), the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2019-I2M-5-029), the Training Fund for Open Projects at Clinical Institutes and Departments of Capital Medical University (CCMU2022KYXZ009), the Capital’s Funds for Health Improvement and Research (2020-1-2041), the Beijing Municipal Science & Technology Commission (D171100003017002), and the Beijing Municipal Administration of Hospitals Incubating Program (PX2020021).
Acknowledgments
The authors appreciate all study participants, their relatives, and the members of the survey teams of the CNSR-III study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1336069/full#supplementary-material
References
1. Feigin, VL , Stark, BA , Johnson, CO , Roth, GA , Bisignano, C , Abady, GG, et al. Global, regional, and National Burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
2. Feigin, VL , Brainin, M , Norrving, B , Martins, S , Sacco, RL , Hacke, W, et al. World stroke organization (Wso): global stroke fact sheet 2022. Int J Stroke. (2022) 17:18–29. doi: 10.1177/17474930211065917
3. Wang, YJ , Li, ZX , Gu, HQ , Zhai, Y , Zhou, Q , Jiang, Y, et al. China stroke statistics: an update on the 2019 report from the National Center for healthcare quality Management in Neurological Diseases, China National Clinical Research Center for neurological diseases, the Chinese Stroke Association, National Center for chronic and non-communicable disease control and prevention, Chinese Center for Disease Control and Prevention and institute for global neuroscience and stroke collaborations. Stroke Vasc Neurol. (2022) 7:415–50. doi: 10.1136/svn-2021-001374
4. Makris, K , Haliassos, A , Chondrogianni, M , and Tsivgoulis, G . Blood biomarkers in ischemic stroke: potential role and challenges in clinical practice and research. Crit Rev Clin Lab Sci. (2018) 55:294–328. doi: 10.1080/10408363.2018.1461190
5. Harmey, D , Hessle, L , Narisawa, S , Johnson, KA , Terkeltaub, R , and Millán, JL . Concerted regulation of inorganic pyrophosphate and Osteopontin by Akp2, Enpp1, and Ank: an integrated model of the pathogenesis of mineralization disorders. Am J Pathol. (2004) 164:1199–209. doi: 10.1016/S0002-9440(10)63208-7
6. Naito, H , Nezu, T , Hosomi, N , Kuzume, D , Aoki, S , Morimoto, Y, et al. Increased serum alkaline phosphatase and functional outcome in patients with acute ischemic stroke presenting a low ankle- brachial index. J Atheroscler Thromb. (2022) 29:719–30. doi: 10.5551/jat.62795
7. Haarhaus, M , Brandenburg, V , Kalantar-Zadeh, K , Stenvinkel, P , and Magnusson, P . Alkaline phosphatase: a novel treatment target for cardiovascular disease in Ckd. Nat Rev Nephrol. (2017) 13:429–42. doi: 10.1038/nrneph.2017.60
8. Bessueille, L , Kawtharany, L , Quillard, T , Goettsch, C , Briolay, A , Taraconat, N, et al. Inhibition of alkaline phosphatase impairs dyslipidemia and protects mice from atherosclerosis. Transl Res. (2023) 251:2–13. doi: 10.1016/j.trsl.2022.06.010
9. Siller, AF , and Whyte, MP . Alkaline phosphatase: discovery and naming of our favorite enzyme. J Bone Miner Res. (2018) 33:362–4. doi: 10.1002/jbmr.3225
10. Poupon, R . Liver alkaline phosphatase: a missing link between Choleresis and biliary inflammation. Hepatology (Baltimore, Md). (2015) 61:2080–90. doi: 10.1002/hep.27715
11. Zhu, HJ , Sun, X , Guo, ZN , Qu, Y , Sun, YY , Jin, H, et al. Prognostic values of serum alkaline phosphatase and globulin levels in patients undergoing intravenous thrombolysis. Front Mol Neurosci. (2022) 15:932075. doi: 10.3389/fnmol.2022.932075
12. Liu, K , Yu, Y , Yuan, Y , Xu, X , Lei, W , Niu, R, et al. Elevated levels of serum alkaline phosphatase are associated with increased risk of cardiovascular disease: a prospective cohort study. J Atheroscler Thromb. (2023) 30:795–819. doi: 10.5551/jat.63646
13. Kabootari, M , Raee, MR , Akbarpour, S , Asgari, S , Azizi, F , and Hadaegh, F . Serum alkaline phosphatase and the risk of coronary heart disease, stroke and all-cause mortality: Tehran lipid and glucose study. BMJ Open. (2018) 8:e023735. doi: 10.1136/bmjopen-2018-023735
14. Panh, L , Ruidavets, JB , Rousseau, H , Petermann, A , Bongard, V , Bérard, E, et al. Association between serum alkaline phosphatase and coronary artery calcification in a sample of primary cardiovascular prevention patients. Atherosclerosis. (2017) 260:81–6. doi: 10.1016/j.atherosclerosis.2017.03.030
15. Kitamura, H , Yamada, S , Hiyamuta, H , Yotsueda, R , Taniguchi, M , Tokumoto, M, et al. Serum alkaline phosphatase levels and increased risk of brain hemorrhage in hemodialysis patients: the Q-cohort study. J Atheroscler Thromb. (2022) 29:923–36. doi: 10.5551/jat.62885
16. Ryu, WS , Lee, SH , Kim, CK , Kim, BJ , and Yoon, BW . Increased serum alkaline phosphatase as a predictor of long-term mortality after stroke. Neurology. (2010) 75:1995–2002. doi: 10.1212/WNL.0b013e3181ff966a
17. Zong, LX , Wang, XW , Li, ZX , Zhao, XQ , Liu, LP , Li, H, et al. Alkaline phosphatase and outcomes in patients with preserved renal function Results from China National Stroke Registry. Stroke. (2018) 49:1176–82. doi: 10.1161/STROKEAHA.118.020237
18. Guo, WY , Liu, ZZ , Lu, QL , Liu, P , Lin, XM , Wang, J, et al. Non-linear association between serum alkaline phosphatase and 3-month outcomes in patients with acute stroke: results from the Xi'an stroke registry study of China. Front Neurol. (2022) 13:859258. doi: 10.3389/fneur.2022.859258
19. Kim, J , Song, TJ , Song, D , Lee, HS , Nam, CM , Nam, HS, et al. Serum alkaline phosphatase and phosphate in cerebral atherosclerosis and functional outcomes after cerebral infarction. Stroke. (2013) 44:3547–9. doi: 10.1161/STROKEAHA.113.002959
20. Liu, Y , Liang, X , Xu, XM , Dong, MX , Jia, SY , Lu, CQ, et al. Increased serum alkaline phosphatase in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. (2019) 28:21–5. doi: 10.1016/j.jstrokecerebrovasdis.2018.09.011
21. Wang, YJ , Jing, J , Meng, X , Pan, YS , Wang, YL , Zhao, XQ, et al. The third China National Stroke Registry (Cnsr-iii) for patients with acute Ischaemic stroke or transient Ischaemic attack: design, rationale and baseline patient characteristics. Stroke Vasc Neurol. (2019) 4:158–64. doi: 10.1136/svn-2019-000242
22. Zhong, CK , You, SJ , Chen, JP , Zhai, GJ , Du, HP , Luo, Y, et al. Serum alkaline phosphatase, phosphate, and in-hospital mortality in acute ischemic stroke patients. J Stroke Cerebrovasc Dis. (2018) 27:257–66. doi: 10.1016/j.jstrokecerebrovasdis.2017.08.041
23. Tonelli, M , Curhan, G , Pfeffer, M , Sacks, F , Thadhani, R , Melamed, ML, et al. Relation between alkaline phosphatase, serum phosphate, and all-cause or cardiovascular mortality. Circulation. (2009) 120:1784–92. doi: 10.1161/CIRCULATIONAHA.109.851873
24. Yang, J , Pan, XJ , Zhang, B , Yan, YH , Huang, YB , Woolf, AK, et al. Superficial and multiple calcifications and ulceration associate with Intraplaque hemorrhage in the carotid atherosclerotic plaque. Eur Radiol. (2018) 28:4968–77. doi: 10.1007/s00330-018-5535-7
25. Ntaios, G . Embolic stroke of undetermined source: Jacc review topic of the week. J Am Coll Cardiol. (2020) 75:333–40. doi: 10.1016/j.jacc.2019.11.024
26. Simats, A , and Liesz, A . Systemic inflammation after stroke: implications for post-stroke comorbidities. EMBO Mol Med. (2022) 14:e16269. doi: 10.15252/emmm.202216269
27. Endres, M , Moro, MA , Nolte, CH , Dames, C , Buckwalter, MS , and Meisel, A . Immune pathways in etiology, acute phase, and chronic sequelae of ischemic stroke. Circ Res. (2022) 130:1167–86. doi: 10.1161/CIRCRESAHA.121.319994
28. Nezu, T , Hosomi, N , Yoshimura, K , Kuzume, D , Naito, H , Aoki, S, et al. Predictors of stroke outcome extracted from multivariate linear discriminant analysis or neural network analysis. J Atheroscler Thromb. (2022) 29:99–110. doi: 10.5551/jat.59642
29. Pike, AF , Kramer, NI , Blaauboer, BJ , Seinen, W , and Brands, R . A novel hypothesis for an alkaline Phosphatase 'Rescue' mechanism in the hepatic acute phase immune response. BBA-Mol Basis Dis. (2013) 1832:2044–56. doi: 10.1016/j.bbadis.2013.07.016
30. Kim, JH , Lee, HS , Park, HM , and Lee, YJ . Serum alkaline phosphatase level is positively associated with metabolic syndrome: a Nationwide population-based study. Clin Chim Acta. (2020) 500:189–94. doi: 10.1016/j.cca.2019.10.015
31. Webber, M , Krishnan, A , Thomas, NG , and Cheung, BMY . Association between serum alkaline phosphatase and C-reactive protein in the United States National Health and nutrition examination survey 2005-2006. Clin Chem Lab Med. (2010) 48:167–73. doi: 10.1515/CCLM.2010.052
32. Castellani, G , Croese, T , Ramos, JMP , and Schwartz, M . Transforming the understanding of brain immunity. Science (New York, NY). (2023) 380:eabo7649. doi: 10.1126/science.abo7649
33. Brichacek, AL , Benkovic, SA , Chakraborty, S , Nwafor, DC , Wang, W , Jun, SJ, et al. Systemic inhibition of tissue-nonspecific alkaline phosphatase alters the brain-immune Axis in experimental Sepsis. Sci Rep. (2019) 9:18788. doi: 10.1038/s41598-019-55154-2
34. Rader, BA . Alkaline phosphatase, an unconventional immune protein. Front Immunol. (2017) 8:897. doi: 10.3389/fimmu.2017.00897
35. Goettsch, C , Strzelecka-Kiliszek, A , Bessueille, L , Quillard, T , Mechtouff, L , Pikula, S, et al. Tnap as a therapeutic target for cardiovascular calcification: a discussion of its pleiotropic functions in the body. Cardiovasc Res. (2022) 118:84–96. doi: 10.1093/cvr/cvaa299
36. Pike, AF , Kramer, NI , Blaauboer, BJ , Seinen, W , and Brands, R . An alkaline phosphatase transport mechanism in the pathogenesis of Alzheimer's disease and neurodegeneration. Chem Biol Interact. (2015) 226:30–9. doi: 10.1016/j.cbi.2014.12.006
Keywords: alkaline phosphatase, mortality, disability, poor functional outcomes, stroke
Citation: Wang Z, Li J, Jing J, Zhang Z, Xu Q, Liu T, Lin J, Jiang Y, Wang Y, Wang A and Meng X (2024) Impact of alkaline phosphatase on clinical outcomes in patients with ischemic stroke: a nationwide registry analysis. Front. Neurol. 15:1336069. doi: 10.3389/fneur.2024.1336069
Edited by:
Mohamed F. Doheim, University of Pittsburgh Medical Center, United StatessReviewed by:
Mostafa Meshref, Al-Azhar University, EgyptRobrecht Knapen, Maastricht University Medical Centre, Netherlands
Wei Li, First Affiliated Hospital of Hainan Medical University, China
Mohamed Elfil, University of Nebraska Medical Center, United States
Copyright © 2024 Wang, Li, Jing, Zhang, Xu, Liu, Lin, Jiang, Wang, Wang and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anxin Wang, d2FuZ2FueGluQGJqdHRoLm9yZw==; Xia Meng, bWVuZ3hpYUBuY3JjbmQub3JnLmNu
†These authors have contributed equally to this work
 Zhaobin Wang
Zhaobin Wang Jing Li3,5†
Jing Li3,5† Jing Jing
Jing Jing Zhe Zhang
Zhe Zhang Tao Liu
Tao Liu Yong Jiang
Yong Jiang Yongjun Wang
Yongjun Wang Anxin Wang
Anxin Wang Xia Meng
Xia Meng