- 1Department of Anesthesiology, The Second Clinical Medical College, North Sichuan Medical College, Nanchong Central Hospital, Nanchong, China
- 2School of Humanities and Management, Key Laboratory for Quality of Life and Psychological Assessment and Intervention, Guangdong Medical University, Dongguan, China
- 3Nanchong Center for Disease Control and Prevention, Nanchong, China
Postoperative cognitive dysfunction (POCD) is a common neurological complication in elderly patients after surgery and general anesthesia. The occurrence of POCD seriously affects the postoperative recovery of patients, and leads to prolonged hospital stay, reduced quality of life, increased medical costs, and even higher mortality. There is no definite and effective drug treatment for POCD. More evidence shows that perioperative non-pharmacological intervention can improve postoperative cognitive function and reduce the incidence of POCD. Therefore, our studies summarize the current non-pharmacological interventions of POCD from the aspects of cognitive training, physical activity, transcutaneous electrical acupoint stimulation, noninvasive brain stimulation, non-pharmacological sleep improvement, music therapy, environment, and multimodal combination Interventions, to provide more data for clinical application and research.
1 Introduction
With an aging population and advances in global healthcare, more elderly patients are undergoing surgical procedures, and neurocognitive impairment related to surgery and anesthesia has further attracted widespread attention (1). Postoperative Cognitive Dysfunction (POCD) is a common neurological complication after surgery and general anesthesia, which refers to the impairment and regression of cognitive function after surgery in patients who had no preoperative cognitive dysfunction. POCD often occurs in elderly patients and is characterized by impaired thinking, memory, orientation, and executive ability (2, 3). The incidence of POCD has been reported to be 7–35% after noncardiac surgery (4) and up to 30–80% 1 month after cardiac surgery (5, 6). The occurrence of POCD seriously affects the postoperative recovery of patients, and leads to prolonged hospital stay, reduced quality of life, increased medical costs, and even higher mortality (7).
However, the exact mechanism of POCD remains unclear, and current research has found that it may be associated with many risk factors, including age, cognitive status, concomitant chronic diseases, nutritional status, anesthesia, surgery, and pain (8–10). Current evidence suggests that drug interventions have an uncertain effect on cognitive impairment (11, 12), so non-pharmacological interventions for POCD may be an effective means of prevention and a focus for future research. Many randomized controlled trials (RCT) and meta-analyses have shown that non-pharmacological interventions have a positive effect on the improvement and prevention of POCD (13, 14). Therefore, our studies summarize the current non-pharmacological interventions of POCD from the aspects of cognitive training, physical activity, transcutaneous electrical acupoint stimulation, noninvasive brain stimulation, non-pharmacological sleep improvement, music therapy, environment, and multimodal combination Interventions, to provide more data for clinical application and research.
2 Methods
2.1 Search strategy
PubMed and Cochrane databases were searched for studies published through October 2023. The search terms used were as follows: (postoperative cognitive dysfunction OR POCD OR postoperative delirium OR POD OR Perioperative neurocognitive disorders OR cognitive function) AND (non-pharmacological OR cognitive training OR cognitive intervention OR physical activity OR exercise OR acupoint stimulation OR noninvasive brain stimulation OR repetitive transcranial magnetic stimulation OR transcranial direct current stimulation OR music OR sleep OR communication). Only studies written in English are considered. The results of the Pubmed search are provided in Supplementary file S1. We examined references to some of the studies to find other relevant papers. We did not present this study as a systematic literature review, but rather as a narrative review, given the great heterogeneity of different interventions and the variety of outcome measures reported in different studies.
2.2 Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) The intervention group received one or more perioperative non-pharmacological interventions. (2) Outcome measures included incidence of POCD, cognitive function scores, or other evaluations of cognitive function. (3) This study does not restrict the study population. (4) There are no restrictions on the type of study, which can be randomized controlled trials, reviews, meta-analyses, etc.
We excluded studies from which data could not be extracted or used for analysis.
3 Results
3.1 Cognitive function training
Cognitive function training has been defined as a training program that provides structured practice on specific cognitive tasks to improve cognitive functioning by training patients in cognitive aspects such as memory, executive processing, language skills, attention, and perceptual awareness (15). Current studies believe that the mechanism of cognitive function training to prevent POCD may mainly be as follows: First, cognitive training may increase the density of cortical dopamine D1 receptor, and human cognitive function depends on adequate dopamine neurotransmission (16, 17). Secondly, some studies have found that cognitive training can improve cognitive reserve, and the incidence of POCD after surgery has been proven to be lower in patients with higher cognitive reserve (represented by a higher educational background). Therefore, cognitive training may improve POCD by improving cognitive reserve (18, 19). Third, previous studies have found that cognitive function, perception, and memory function will gradually decline during the aging process. Still, the brain retains lifelong plasticity and adaptive reorganization ability, so the use of appropriately designed training programs can improve some cognitive functions of the brain (20, 21). At present, there are various ways of cognitive function training. According to cognitive training tools, it can be divided into traditional cognitive training based on paper, pen, reading, reciting, etc., and cognitive training based on computer programs.
3.1.1 Cognitive training based on traditional training methods
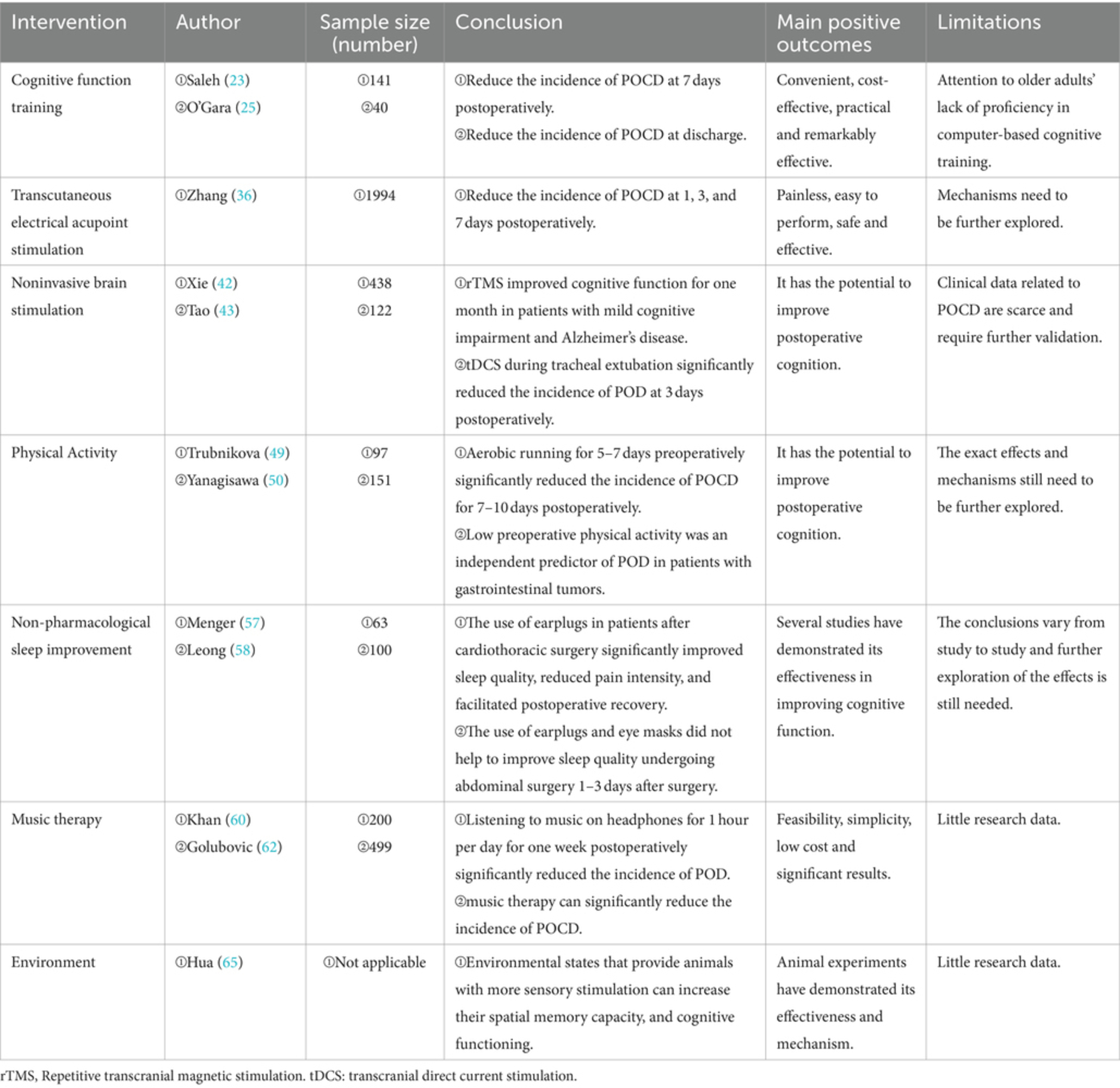
An RCT of 94 patients undergoing cardiac surgery by Butz et al. found that 36 min of paper and pen-based cognitive training per day for 3 weeks post-surgery significantly reduced the incidence of POCD and remained statistically different 15 months after the intervention (22). In an RCT of 141 elderly patients undergoing surgery for gastrointestinal tumors, Saleh et al. found that the incidence of POCD in elderly patients at 7 days postoperatively could be significantly reduced by preoperative spatial memory training (23). In addition, in an RCT of 72 elderly patients undergoing coronary artery bypass grafting, Li et al. found that preoperative training of patients in memory, orientation, language, and attention significantly reduced the incidence of POCD at two weeks postoperatively (24).
A large number of studies have confirmed that the traditional cognitive training approach can significantly improve postoperative cognitive function and reduce the incidence of POCD, especially in elderly patients. In addition, it has advantages in terms of convenience, economy, and practicality.
3.1.2 Cognitive training based on computer programs
With the proliferation of computers, cognitive function training through the use of designed apps is gaining popularity. O’Gara et al.’s RCT of 40 patients undergoing cardiac surgery found that 30 min of daily training with a mobile phone app in the preoperative period and for 4 weeks postoperatively significantly reduced the incidence of POCD (25). Song et al.’s RCT of 42 lung transplant patients found that after eight consecutive weeks of 40-min-a-day computer-based cognitive training in three cognitive domains, including memory, attention, and processing speed, beginning at postoperative week 5. The cognitive training group scored significantly higher than the control group on both the numerical and verbal fluency tests when administered at 12 weeks post-intervention (26). However, an RCT of 52 elderly patients by Vlisides et al. found that after 20 min of home computer-based cognitive training per day for 1 week preoperatively, there were no statistically significant differences between the cognitive training group and controls when memory, attention, and processing speed were assessed at 3 days postoperatively (27). In addition, an RCT of 36 elderly patients undergoing coronary artery bypass grafting by Greaves et al. found that after 13 weeks of home computer-based cognitive training targeting 4 domains of attention, memory, reaction, and executive function, there were no statistical differences between the cognitive training group and the control group in any of the 4 cognitive domains at the time of discharge, at 4 months after discharge, and at the time of assessment 6 months later (28).
There is currently a large discrepancy in the outcome performance of POCD using cognitive training with computer programs, leading to the following possibilities for inconsistency in POCD outcomes. First, most of the study participants were elderly, who are usually not fluent with computers. Second, the cognitive training programs of Song et al. (26), Vlisides et al. (27), and Greaves et al. (28) were completed by the patients themselves at home rather than in the hospital using computers, which exacerbated the low compliance of the elderly patients. Therefore the effectiveness of the cognitive training was greatly diminished. The study by Vlisides et al. (27) mentioned that only 5 (out of 29) completed all the cognitive training, and the study by Humeidan et al. (29) mentioned that only 11 (out of 125) completed all the cognitive training, which confirmed the low adherence to computerized cognitive training in elderly patients. Thirdly, the sample sizes of the studies by Vlisides et al. (27) and Greaves et al. (28) were small, which reduced the strength of the evidence for the outcome. The use of computer programs has the advantage of portability compared to traditional cognitive training methods, but their utility and cognitive training effectiveness for older adults are controversial, so this needs to be validated in more RCTs.
3.2 Transcutaneous electrical acupoint stimulation
Transcutaneous electrical acupoint stimulation (TEAS) is an important part of traditional Chinese medicine in China (30). TEAS is an emerging acupuncture therapy guided by the meridian theory of Chinese medicine and combined with transcutaneous electrical nerve stimulation technology. TEAS uses noninvasive electrical stimulation technology to add perceptible pulsed stimulation to electrodes placed on the surface of acupoints. Unlike conventional acupuncture, TEAS does not puncture the skin and avoids the risk of infection to a large extent (31). TEAS can play a protective role in the brain by hindering microglia activation, reducing oxidative stress, inhibiting central and peripheral inflammatory responses, and lowering inflammatory factor levels (32).
Hua et al.’s RCT of 97 elderly patients undergoing thoracoscopic surgery found that after TEAS from 30 min before until the end of the procedure, the TEAS group had significantly higher scores on the mini-mental state examination (MMSE) scale than the control group on days 1, 3, 5, and 7 postoperatively (33). An RCT of 84 elderly patients undergoing total intravenous anesthesia by Wu et al. found that after TEAS during surgery, MMSE and Montreal cognitive assessment (MoCA) scores were significantly higher in the TEAS group than in the control group at 1, 3, and 7 days postoperatively (34). In addition, a meta-analysis including 13 RCTs with a total of 999 elderly patients found that the incidence of POCD in the TEAS group was significantly lower than that in the control group at 3 months postoperatively, and subgroup analyses showed that patients in the TEAS group had a lower incidence of POCD than those in the control group in both orthopedic and abdominal surgery (35). Zhang et al.’s meta-analysis including 29 RCTs with a total of 1994 patients similarly found that the incidence of POCD was significantly lower in the TEAS group than in the control group at 1, 3, and 7 days postoperatively (36).
It has been widely verified that TEAS can reduce the incidence of POCD, and it has the advantages of painlessness, parameter unification, simple operation, and safety, so it is worthy of extensive clinical promotion (37).
3.3 Noninvasive brain stimulation
Noninvasive brain stimulation may improve cognitive function by modulating the excitatory of cortical neurons, enhancing the sensory-motor network, the left fronto-parietal network, and the functions related to the level of consciousness (38, 39). There is evidence that noninvasive brain stimulation enhances cognitive abilities, including memory, attention, and perception (40, 41).
Repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS), as the two main noninvasive and safe brain stimulation techniques, are now widely used in the diagnosis and treatment of various neuropsychiatric disorders. A meta-analysis of 438 participants found that rTMS improved cognitive function for one month in patients with mild cognitive impairment and Alzheimer’s disease (42). Tao et al.’s RCT of 122 elderly patients undergoing orthopedic surgery found that 20 min of tDCS during tracheal extubation significantly reduced the incidence of postoperative delirium (POD) at 3 days postoperatively (43). Wu et al. selected POCD-susceptible mice for rTMS 10 days before surgery, and neurocognitive assessment on days 3 to 10 after surgery found that rTMS significantly improved postoperative cognitive decline and spatial memory impairment in mice (44).
There have been many studies demonstrating that noninvasive brain stimulation can improve cognitive function in patients with mild cognitive impairment and Alzheimer’s disease (45–47), but there is not enough data to support the prevention and treatment of POCD for the time being, which needs to be explored in more RCTs.
3.4 Physical activity
Physical activity is widely believed to have positive health benefits and is associated with improved cognitive function and brain health (48). Trubnikova et al.’s RCT of 97 patients undergoing coronary artery bypass grafting found that aerobic running for 5–7 days preoperatively significantly reduced the brain’s susceptibility to ischemia and lowered the incidence of POCD for 7–10 days postoperatively (49). In a retrospective study of 151 patients, Yanagisawa et al. found that low preoperative physical activity was an independent predictor of POD in patients with gastrointestinal tumors (50). Chapman et al. found that shorter periods of aerobic exercise can also aid in neurological recovery, improve cognitive function, and benefit brain health (51).
In general, although many hypotheses have been proposed regarding the improvement of cognitive function by physical activity (52), they have not been confirmed, and RCTs directly evaluating the effect of physical activity on POCD are relatively few. In addition, physical activity intensity and physical activity type have different effects on POCD improvement, and further research is needed to determine which exercise mode is more effective for POCD high-risk groups.
3.5 Non-pharmacological sleep improvement
Postoperative patients often suffer from sleep disturbances. Poor sleep quality is closely associated with the development of POCD, and improving sleep is an important part of POCD prevention strategies (53). Maintaining a quiet and dimly lit environment on the ward, reducing distractions from nighttime nursing activities, and using eye masks and earplugs are some common measures (54). A meta that included 1,455 patients found that the use of earplugs at night in ICU patients significantly reduced the incidence of delirium (55). Kamdar et al. found that using a combination of sleep-promoting measures for ICU patients significantly improved sleep disturbances and reduced the incidence of delirium. These measures included turning off the television, dimming the lights, reducing nursing activities, using earplugs, and eye masks, and playing soothing music (56). Menger et al. found that the use of earplugs in patients on the first night after cardiothoracic surgery significantly improved sleep quality as well as patient satisfaction, reduced pain intensity, facilitated postoperative recovery, and reduced hospitalization costs (57). However, some studies have found the opposite conclusion. Leong et al. found that the use of earplugs and eye masks did not help to improve sleep quality in 100 patients undergoing abdominal surgery 1–3 days after surgery (58).
For post-surgical patients, the effect of using non-pharmacological sleep interventions on improving POCD remains inconclusive. Studies directly examining the effects of nonpharmacologic sleep interventions on POCD are still scarce, so the effects of non-pharmacological sleep aids on POCD need to be further explored.
3.6 Music therapy
Music therapy is the use of music and rhythm to treat physical and psychological disorders (59). Music therapy has the function of regulating mood and stress and reducing the release of inflammatory factors (60). Khan et al.’s RCT of 200 coronary artery bypass grafts found that listening to music on headphones for 1 h per day for one week postoperatively significantly reduced the incidence of POD (60). An RCT of 115 surgical patients with type A aortic coarctation by Wu et al. found that Beck’s cognitive training combined with music therapy for two months postoperatively significantly reduced the incidence of POCD (61). A meta-analysis by Golubovic et al. found that music therapy can significantly reduce the incidence of POCD (62).
Music therapy has the feasibility of clinical application, with a simple and cost-effective process and objective and stable results, providing new evidence for perioperative non-pharmacological interventions to improve cognitive function. However, there are relatively few studies evaluating music therapy for POCD. In addition, the effect of the timing, duration, and choice of music intervention on POCD improvement remains unclear.
3.7 Environment
Enrich environment has profound effects on the central nervous system of adults. Several studies have found that an enrich environment alters animal behavior and improves cognitive function, especially learning and memory (63). In some animal model studies, it has been found that environmental states that provide animals with more sensory stimulation can increase their spatial memory capacity, and cognitive functioning (64, 65). However, studies investigating the Enrich environment and POCD in humans are scarce. For perioperative patients, an appropriate environment may improve postoperative cognitive function.
3.8 Multimodal combination of non-pharmacological interventions
To further improve the effect on postoperative cognitive function, many studies have used multiple intervention modalities. Some studies have explored the effects of cognitive training combined with physical activity on POCD. Duan et al.’s RCT of 86 elderly patients undergoing orthopedic surgery found that 30 min of reading-based cognitive training per day perioperative period combined with 40 min of physical activity per day postoperatively significantly reduced the incidence of POCD within 1 week postoperatively (66). Linying found that intellectual training combined with physical activity was effective in reducing the incidence of postoperative inflammatory factors and POCD in elderly patients undergoing fracture surgery (67).
Elderly people have special characteristics such as the coexistence of multiple diseases, and deterioration of body functions and cognitive functions, so the risk of POCD and its severity increase accordingly. The Hospital Elder Life Program (HELP) is a targeted, multidisciplinary, collaborative strategy that has been shown to be effective and cost-efficient in preventing delirium, cognitive decline, and other common complications in hospitalized older adults (68–70). HELP is a combination of multiple non-pharmacological interventions, including orientation training, cognitive training, early activity, sleep assistance, visual stimulation, auditory stimulation, dietary guidance, and early multidisciplinary consultation. A meta-analysis of 3,605 patients found that HELP-based interventions reduced the odds of delirium by 45% and significantly reduced the length of hospitalization (71). Wang et al. found that HELP was effective in reducing POD, improving postoperative cognitive function, and shortening the length of hospitalization in elderly patients (72). Liang et al. found in 140 elderly fracture patients that modified HELP significantly improved cognitive function at 1 and 12 months after surgery (73).
4 Limitations
This study had some limitations. First, the mechanisms by which many non-pharmacological interventions improve cognitive function remain unclear. Secondly, some studies have shown contrary results, and the effectiveness of interventions still needs to be validated in more large-sample randomized controlled trials. In addition, some interventions have less research data, which still needs to be validated in more studies. Another limitation is that only two databases were used for the literature search.
5 Conclusion
With the increasing demand for perioperative comfort treatment, more studies have begun to focus on postoperative complications (74). The pathogenesis of POCD, one of the most common postoperative complications, is not fully understood, and there are no specific drugs for POCD. Therefore, this study summarized the perioperative non-pharmacological interventions regarding POCD by reviewing the literature, including cognitive training, physical activity, transcutaneous electrical acupoint stimulation, noninvasive brain stimulation, non-pharmacological sleep improvement, music therapy, environment, and multimodal combination Interventions. All of these interventions have been shown to be effective in slowing disease progression and improving postoperative cognitive function (Table 1). In addition, non-pharmacological interventions have become a hot research topic in recent years because they are usually low-cost, easy to implement, and have few adverse effects. However, there is still controversy about the therapeutic effects of different studies on the same method. Influencing factors that lead to differences in efficacy may be different ages, literacy levels, intensity of the intervention, and outcome assessment indicators. In future studies, it is necessary to set more uniform study inclusion criteria to achieve higher comparability of efficacy. In addition, many studies have only evaluated the short-term cognitive function after surgery, and the long-term effects on cognitive function improvement have yet to be verified. Therefore, future studies can continue to explore the potential mechanisms of nonpharmacologic interventions for the prevention of POCD and investigate more forms of non-pharmacologic interventions, which will have a great deal of room for development and prospects.
Author contributions
LiZ: Writing – review & editing, Writing – original draft, Investigation, Conceptualization. YG: Writing – review & editing, Writing – original draft, Investigation, Conceptualization. XZ: Writing – original draft, Software, Methodology, Formal analysis, Data curation. WM: Writing – original draft, Software, Methodology, Formal analysis, Data curation. HZ: Writing – review & editing, Project administration, Formal analysis. LC: Writing – review & editing, Project administration, Formal analysis. XL: Writing – original draft, Methodology. LoZ: Writing – original draft, Methodology. YX: Writing – review & editing, Visualization, Validation, Supervision, Resources, Funding acquisition. LL: Writing – review & editing, Visualization, Validation, Supervision, Resources, Funding acquisition.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by research funds from Nanchong Science and Technology Bureau (22SXQT0293).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1369821/full#supplementary-material
References
1. Safavynia, SA, and Goldstein, PA. The role of neuroinflammation in postoperative cognitive dysfunction: moving from hypothesis to treatment. Front Psych. (2018) 9:752. doi: 10.3389/fpsyt.2018.00752
2. Evered, L, Silbert, B, Knopman, DS, Scott, DA, DeKosky, ST, Rasmussen, LS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth. (2018) 121:1005–12. doi: 10.1016/j.bja.2017.11.087
3. Terrando, N, Brzezinski, M, Degos, V, Eriksson, LI, Kramer, JH, Leung, JM, et al. Perioperative cognitive decline in the aging population. Mayo Clin Proc. (2011) 86:885–93. doi: 10.4065/mcp.2011.0332
4. Monk, TG, Weldon, BC, Garvan, CW, Dede, DE, van der Aa, MT, Heilman, KM, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. (2008) 108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e
5. Kotekar, N, Shenkar, A, and Nagaraj, R. Postoperative cognitive dysfunction – current preventive strategies. Clin Interv Aging. (2018) 13:2267–73. doi: 10.2147/CIA.S133896
6. Newman, MF, Mathew, JP, Grocott, HP, Mackensen, GB, Monk, T, Welsh-Bohmer, KA, et al. Central nervous system injury associated with cardiac surgery. Lancet (London, England). (2006) 368:694–703. doi: 10.1016/S0140-6736(06)69254-4
7. Steinmetz, J, Christensen, KB, Lund, T, Lohse, N, and Rasmussen, LS. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. (2009) 110:548–55. doi: 10.1097/ALN.0b013e318195b569
8. Kong, H, Xu, L, and Wang, D. Perioperative neurocognitive disorders: a narrative review focusing on diagnosis, prevention, and treatment. CNS Neurosci Ther. (2022) 28:1147–67. doi: 10.1111/cns.13873
9. Evered, L, Atkins, K, Silbert, B, and Scott, DA. Acute peri-operative neurocognitive disorders: a narrative review. Anaesthesia. (2022) 77:34–42. doi: 10.1111/anae.15613
10. Xu, X, Hu, Y, Yan, E, Zhan, G, Liu, C, and Yang, C. Perioperative neurocognitive dysfunction: thinking from the gut? Aging. (2020) 12:15797–817. doi: 10.18632/aging.103738
11. Li, L, Wang, C, Fang, M, Xu, H, Lu, H, and Zhang, H. Effects of dexamethasone on post-operative cognitive dysfunction and delirium in adults following general anaesthesia: a meta-analysis of randomised controlled trials. BMC Anesthesiol. (2019) 19:113. doi: 10.1186/s12871-019-0783-x
12. Siddiqi, N, Stockdale, R, Britton, AM, and Holmes, J. Interventions for preventing delirium in hospitalised patients. Cochrane Database Syst Rev. (2007) 18:CD 5563. doi: 10.1002/14651858.CD005563.pub2
13. Li, Z, Yiping, G, Xuelei, Z, Wei, M, and Linji, L. Preoperative cognitive training improves postoperative cognitive function: A meta-analysis and systematic review of randomized controlled trials. Front Neurol. (2024) 14:1293153. doi: 10.3389/fneur.2023.1293153
14. Zhao, L, Zhu, H, Mao, W, Zhou, X, Xie, Y, and Li, L. Effects of perioperative cognitive function training on postoperative cognitive dysfunction and postoperative delirium: a systematic review and meta-analysis. Front Neurol. (2023) 14:1146164. doi: 10.3389/fneur.2023.1146164
15. Guglietti, B, Hobbs, D, and Collins-Praino, LE. Optimizing cognitive training for the treatment of cognitive dysfunction in Parkinson's disease: current limitations and future directions. Front Aging Neurosci. (2021) 13:709484. doi: 10.3389/fnagi.2021.709484
16. Bäckman, L, Lindenberger, U, Li, S, and Nyberg, L. Linking cognitive aging to alterations in dopamine neurotransmitter functioning: recent data and future avenues. Neurosci Biobehav Rev. (2010) 34:670–7. doi: 10.1016/j.neubiorev.2009.12.008
17. McNab, F, Varrone, A, Farde, L, Jucaite, A, Bystritsky, P, Forssberg, H, et al. Changes in cortical dopamine d1 receptor binding associated with cognitive training. Science (New York, N.Y.). (2009) 323:800–2. doi: 10.1126/science.1166102
18. Feinkohl, I, Winterer, G, Spies, CD, and Pischon, T. Cognitive reserve and the risk of postoperative cognitive dysfunction. Dtsch Arztebl Int. (2017) 114:110–7. doi: 10.3238/arztebl.2017.0110
19. Petrelli, A, Kaesberg, S, Barbe, MT, Timmermann, L, Rosen, JB, Fink, GR, et al. Cognitive training in Parkinson's disease reduces cognitive decline in the long term. Eur J Neurol. (2015) 22:640–7. doi: 10.1111/ene.12621
20. Smith, GE, Housen, P, Yaffe, K, Ruff, R, Kennison, RF, Mahncke, HW, et al. A cognitive training program based on principles of brain plasticity: results from the improvement in memory with plasticity-based adaptive cognitive training (impact) study. J Am Geriatr Soc. (2009) 57:594–603. doi: 10.1111/j.1532-5415.2008.02167.x
21. Mahncke, HW, Connor, BB, Appelman, J, Ahsanuddin, ON, Hardy, JL, Wood, RA, et al. Memory enhancement in healthy older adults using a brain plasticity-based training program: a randomized, controlled study. Proc Natl Acad Sci USA. (2006) 103:12523–8. doi: 10.1073/pnas.0605194103
22. Butz, M, Gerriets, T, Sammer, G, El-Shazly, J, Tschernatsch, M, Huttner, HB, et al. Effects of postoperative cognitive training on neurocognitive decline after heart surgery: a randomized clinical trial. Eur J Cardiothorac Surg. (2022) 62:ezac251. doi: 10.1093/ejcts/ezac251
23. Saleh, AJ, Tang, G, Hadi, SM, Yan, L, Chen, M, Duan, K, et al. Preoperative cognitive intervention reduces cognitive dysfunction in elderly patients after gastrointestinal surgery: a randomized controlled trial. Med Sci Monit. (2015) 21:798–805. doi: 10.12659/MSM.893359
24. Chao-liang, LOFZ. Effect of early cognitive behavior intervention on cognitive function in elderly patients after coronary artery bypass grafting. Chinese Journal of the Frontiers of Medical Science (Electronic Version). (2018) 10:96–9. doi: 10.12037/YXQY.2018.07-18
25. O'Gara, BP, Mueller, A, Gasangwa, DVI, Patxot, M, Shaefi, S, Khabbaz, K, et al. Prevention of early postoperative decline: a randomized, controlled feasibility trial of perioperative cognitive training. Anesth Analg. (2020) 130:586–95. doi: 10.1213/ANE.0000000000004469
26. Song, Y, Cui, X, Zhang, Y, Gao, H, Cai, Q, and Mu, Z. Home-based computerized cognitive training for postoperative cognitive dysfunction after lung transplantation in elderly population: a randomized controlled trial. J Nerv Ment Dis. (2019) 207:693–9. doi: 10.1097/NMD.0000000000001032
27. Vlisides, PE, Das, AR, Thompson, AM, Kunkler, B, Zierau, M, Cantley, MJ, et al. Home-based cognitive prehabilitation in older surgical patients: a feasibility study. J Neurosurg Anesthesiol. (2019) 31:212–7. doi: 10.1097/ANA.0000000000000569
28. Greaves, D, Astley, J, Psaltis, PJ, Lampit, A, Davis, DH, Ghezzi, ES, et al. The effects of computerised cognitive training on post-cabg delirium and cognitive change: a prospective randomised controlled trial. Delirium (Bielefeld, Germany). (2023) 1:67976. doi: 10.56392/001c.67976
29. Humeidan, ML, Reyes, JC, Mavarez-Martinez, A, Roeth, C, Nguyen, CM, Sheridan, E, et al. Effect of cognitive prehabilitation on the incidence of postoperative delirium among older adults undergoing major noncardiac surgery: the neurobics randomized clinical trial. JAMA Surg. (2021) 156:148–56. doi: 10.1001/jamasurg.2020.4371
30. Wang, L, Liang, W, Wang, B, Guo, M, Zhou, J, Chen, L, et al. Transcutaneous electrical acupoint stimulation for reducing cognitive dysfunction in lumbar spine surgery: a randomized, controlled trail. Front Aging Neurosci. (2022) 14:1034998. doi: 10.3389/fnagi.2022.1034998
31. Chen, J, Tu, Q, Miao, S, Zhou, Z, and Hu, S. Transcutaneous electrical acupoint stimulation for preventing postoperative nausea and vomiting after general anesthesia: a meta-analysis of randomized controlled trials. Int J Surg (London, England). (2020) 73:57–64. doi: 10.1016/j.ijsu.2019.10.036
32. Yuan, S, Zhang, X, Bo, Y, Li, W, Zhang, H, and Jiang, Q. The effects of electroacupuncture treatment on the postoperative cognitive function in aged rats with acute myocardial ischemia-reperfusion. Brain Res. (2014) 1593:19–29. doi: 10.1016/j.brainres.2014.10.005
33. Wei, H, Huang, J, Zhao, F, Xie, Z, Xia, Z, and Gan, J. Transcutaneous electrical acupoint stimulation improves postoperative cognitive function in senior patients undergoing video-assisted thoracoscopic surgery: a randomized controlled trial. Chin J Integr Med. (2022) 28:730–5. doi: 10.1007/s11655-022-3516-1
34. Wu, Y, and Luo, H. Effect of electrical acupoint stimulation on postoperative cognitive dysfunction after total intravenous anesthesia. Shanghai J Acupunct Moxib. (2020) 39:1161–5. doi: 10.13460/j.issn.1005-0957.2020.09.1161
35. Li, S, Jiang, H, Liu, W, Yin, Y, Yin, C, Chen, H, et al. Transcutaneous electrical acupoint stimulation for the prevention of perioperative neurocognitive disorders in geriatric patients: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). (2022) 101:e32329. doi: 10.1097/MD.0000000000032329
36. Zhang, T, Ou, L, Chen, Z, Li, J, Shang, Y, and Hu, G. Transcutaneous electrical acupoint stimulation for the prevention of postoperative cognitive dysfunction: a systematic review and meta-analysis. Front Med (Lausanne). (2021) 8:756366. doi: 10.3389/fmed.2021.756366
37. Zhang Penghui, WX. Progress in the prevention of postoperative cognitive dysfunction in elderly patients by transcutaneous electrical stimulation of acupoints. Journal of Clinical Anesthesiology (China), (2018):720–2. doi: 10.12089/jca.2018.07.024,
38. Chan, MMY, Yau, SSY, and Han, YMY. The neurobiology of prefrontal transcranial direct current stimulation (tdcs) in promoting brain plasticity: a systematic review and meta-analyses of human and rodent studies. Neurosci Biobehav Rev. (2021) 125:392–416. doi: 10.1016/j.neubiorev.2021.02.035
39. Lefaucheur, J, Antal, A, Ayache, SS, Benninger, DH, Brunelin, J, Cogiamanian, F, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tdcs). Clin Neurophysiol. (2017) 128:56–92. doi: 10.1016/j.clinph.2016.10.087
40. Majdi, A, van Boekholdt, L, Sadigh-Eteghad, S, and Mc, LM. A systematic review and meta-analysis of transcranial direct-current stimulation effects on cognitive function in patients with Alzheimer's disease. Mol Psychiatry. (2022) 27:2000–9. doi: 10.1038/s41380-022-01444-7
41. Siegert, A, Diedrich, L, and Antal, A. New methods, old brains-a systematic review on the effects of tdcs on the cognition of elderly people. Front Hum Neurosci. (2021) 15:730134. doi: 10.3389/fnhum.2021.730134
42. Xie, Y, Li, Y, Nie, L, Zhang, W, Ke, Z, and Ku, Y. Cognitive enhancement of repetitive transcranial magnetic stimulation in patients with mild cognitive impairment and early Alzheimer's disease: a systematic review and meta-analysis. Front Cell Dev Biol. (2021) 9:734046. doi: 10.3389/fcell.2021.734046
43. Tao, M, Zhang, S, Han, Y, Li, C, Wei, Q, Chen, D, et al. Efficacy of transcranial direct current stimulation on postoperative delirium in elderly patients undergoing lower limb major arthroplasty: a randomized controlled trial. Brain Stimul. (2023) 16:88–96. doi: 10.1016/j.brs.2023.01.839
44. Wu, T, Li, M, Tian, L, Cong, P, Huang, X, Wu, H, et al. A modified mouse model of perioperative neurocognitive disorders exacerbated by sleep fragmentation. Exp Anim. (2023) 72:55–67. doi: 10.1538/expanim.22-0053
45. Zhang, F, Qin, Y, Xie, L, Zheng, C, Huang, X, and Zhang, M. High-frequency repetitive transcranial magnetic stimulation combined with cognitive training improves cognitive function and cortical metabolic ratios in Alzheimer's disease. J Neural Transm (Vienna). (2019) 126:1081–94. doi: 10.1007/s00702-019-02022-y
46. Zhao, J, Li, Z, Cong, Y, Zhang, J, Tan, M, Zhang, H, et al. Repetitive transcranial magnetic stimulation improves cognitive function of Alzheimer's disease patients. Oncotarget. (2017) 8:33864–71. doi: 10.18632/oncotarget.13060
47. Rabey, JM, Dobronevsky, E, Aichenbaum, S, Gonen, O, Marton, RG, and Khaigrekht, M. Repetitive transcranial magnetic stimulation combined with cognitive training is a safe and effective modality for the treatment of Alzheimer's disease: a randomized, double-blind study. J Neural Transm (Vienna). (2013) 120:813–9. doi: 10.1007/s00702-012-0902-z
48. Lee, SS, Lo, Y, and Verghese, J. Physical activity and risk of postoperative delirium. J Am Geriatr Soc. (2019) 67:2260–6. doi: 10.1111/jgs.16083
49. Trubnikova, OA, Tarasova, IV, Moskin, EG, Kupriyanova, DS, Argunova, YA, Pomeshkina, SA, et al. Beneficial effects of a short course of physical prehabilitation on neurophysiological functioning and neurovascular biomarkers in patients undergoing coronary artery bypass grafting. Front Aging Neurosci. (2021) 13:699259. doi: 10.3389/fnagi.2021.699259
50. Yanagisawa, T, Tatematsu, N, Horiuchi, M, Migitaka, S, Yasuda, S, Itatsu, K, et al. Preoperative low physical activity is a predictor of postoperative delirium in patients with gastrointestinal cancer: a retrospective study. Asian Pac J Cancer Prev. (2022) 23:1753–9. doi: 10.31557/APJCP.2022.23.5.1753
51. Chapman, SB, Aslan, S, Spence, JS, Defina, LF, Keebler, MW, Didehbani, N, et al. Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Front Aging Neurosci. (2013) 5:75. doi: 10.3389/fnagi.2013.00075
52. Cassilhas, RC, Tufik, S, and de Mello, MT. Physical exercise, neuroplasticity, spatial learning and memory. Cell Mol Life Sci. (2016) 73:975–83. doi: 10.1007/s00018-015-2102-0
53. Cho, M, Song, S, and Ryu, C. Sleep disturbance strongly related to the development of postoperative delirium in proximal femoral fracture patients aged 60 or older. Hip & Pelvis. (2020) 32:93–8. doi: 10.5371/hp.2020.32.2.93
54. Hu, R, Jiang, X, Chen, J, Zeng, Z, Chen, XY, Li, Y, et al. Non-pharmacological interventions for sleep promotion in the intensive care unit. Cochrane Database Syst Rev. (2015) 2015:CD008808. doi: 10.1002/14651858.CD008808.pub2
55. Litton, E, Carnegie, V, Elliott, R, and Webb, SAR. The efficacy of earplugs as a sleep hygiene strategy for reducing delirium in the icu: a systematic review and meta-analysis. Crit Care Med. (2016) 44:992–9. doi: 10.1097/CCM.0000000000001557
56. Kamdar, BB, King, LM, Collop, NA, Sakamuri, S, Colantuoni, E, Neufeld, KJ, et al. The effect of a quality improvement intervention on perceived sleep quality and cognition in a medical icu. Crit Care Med. (2013) 41:800–9. doi: 10.1097/CCM.0b013e3182746442
57. Menger, J, Urbanek, B, Skhirtladze-Dworschak, K, Wolf, V, Fischer, A, Rinösl, H, et al. Earplugs during the first night after cardiothoracic surgery may improve a fast-track protocol. Minerva Anestesiol. (2018) 84:49–57. doi: 10.23736/S0375-9393.17.11758-X
58. Leong, RW, Davies, LJ, Fook-Chong, S, Ng, SY, and Lee, YL. Effect of the use of earplugs and eye masks on the quality of sleep after major abdominal surgery: a randomised controlled trial. Anaesthesia. (2021) 76:1482–91. doi: 10.1111/anae.15468
59. Walsh, S, Causer, R, and Brayne, C. Does playing a musical instrument reduce the incidence of cognitive impairment and dementia? A systematic review and meta-analysis. Aging Ment Health. (2021) 25:593–601. doi: 10.1080/13607863.2019.1699019
60. Khan, SH, Xu, C, Purpura, R, Durrani, S, Lindroth, H, Wang, S, et al. Decreasing delirium through music: a randomized pilot trial. Am J Crit Care. (2020) 29:e31–8. doi: 10.4037/ajcc2020175
61. Xin, WNQY. Effect of 49elaxation training combined with baker's cognitive therapy on cardiac muscle contraction reserve and cognitive status in patients with pocd after aortic dissection. J Int Psychiatry. (2022) 49:175–8. doi: 10.13479/j.cnki.jip.2022.01.024
62. Golubovic, J, Neerland, BE, Aune, D, and Baker, FA. Music interventions and delirium in adults: a systematic literature review and meta-analysis. Brain Sci. (2022) 12:12. doi: 10.3390/brainsci12050568
63. Goodman, CS, and Shatz, CJ. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. (1993) 72:77–98. doi: 10.1016/S0092-8674(05)80030-3
64. Baroncelli, L, Braschi, C, Spolidoro, M, Begenisic, T, Sale, A, and Maffei, L. Nurturing brain plasticity: impact of environmental enrichment. Cell Death Differ. (2010) 17:1092–103. doi: 10.1038/cdd.2009.193
65. Hua, M, and Min, J. Postoperative cognitive dysfunction and the protective effects of enriched environment: a systematic review. Neurodegener Dis. (2020) 20:113–22. doi: 10.1159/000513196
66. Duan, S, Liao, Y, Tang, Y, Zhang, B, Peng, M, Tong, J, et al. Short-term perioperative cognitive therapy combined with rehabilitation exercise reduces the incidence of neurocognitive disorder in elderly patients: a randomized controlled trial. Minerva Anestesiol. (2022) 88:145–55. doi: 10.23736/S0375-9393.21.15877-8
67. Linying, P. Short-term exercise reduced the incidence of postoperative cognitive dysfuction (pocd) and inflammatory cytokines in the elderly patients. Central South University (China) (2014).
68. Huson, K, Stolee, P, Pearce, N, Bradfield, C, and Heckman, GA. Examining the hospital elder life program in a rehabilitation setting: a pilot feasibility study. BMC Geriatr. (2016) 16:140. doi: 10.1186/s12877-016-0313-3
69. Yue, J, Tabloski, P, Dowal, SL, Puelle, MR, Nandan, R, and Inouye, SK. Nice to help: operationalizing national institute for health and clinical excellence guidelines to improve clinical practice. J Am Geriatr Soc. (2014) 62:754–61. doi: 10.1111/jgs.12768
70. Inouye, SK, Bogardus, STJ, Charpentier, PA, Leo-Summers, L, Acampora, D, Holford, TR, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. (1999) 340:669–76. doi: 10.1056/NEJM199903043400901
71. Hshieh, TT, Yang, T, Gartaganis, SL, Yue, J, and Inouye, SK. Hospital elder life program: systematic review and meta-analysis of effectiveness. Am J Geriatr Psychiatry. (2018) 26:1015–33. doi: 10.1016/j.jagp.2018.06.007
72. Wang, Y, Yue, J, Xie, D, Carter, P, Li, Q, Gartaganis, SL, et al. Effect of the tailored, family-involved hospital elder life program on postoperative delirium and function in older adults: a randomized clinical trial. JAMA Intern Med. (2020) 180:17–25. doi: 10.1001/jamainternmed.2019.4446
73. Liang, C, Chu, C, Hsu, Y, Chou, M, Wang, Y, Lin, Y, et al. Effects of modified version of the hospital elder life program on post-discharge cognitive function and activities of daily living among older adults undergoing total knee arthroplasty. Arch Gerontol Geriatr. (2021) 93:104284. doi: 10.1016/j.archger.2020.104284
Keywords: cognitive function training, non-pharmacological interventions, perioperative neurocognitive dysfunction, postoperative cognitive dysfunction, review
Citation: Zhao L, Guo Y, Zhou X, Mao W, Zhu H, Chen L, Liu X, Zhang L, Xie Y and Li L (2024) The research progress of perioperative non-pharmacological interventions on postoperative cognitive dysfunction: a narrative review. Front. Neurol. 15:1369821. doi: 10.3389/fneur.2024.1369821
Edited by:
Ismael Luis Calandri, Fundación Para la Lucha Contra las Enfermedades Neurológicas de la Infancia (FLENI), ArgentinaReviewed by:
Guido Santiago Dorman, Instituto de Neurología Cognitiva, ArgentinaLucia Crivelli, Fundación Para la Lucha Contra las Enfermedades Neurológicas de la Infancia (FLENI), Argentina
Copyright © 2024 Zhao, Guo, Zhou, Mao, Zhu, Chen, Liu, Zhang, Xie and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linji Li, bGxqLXN0ZXBoZW5AMTYzLmNvbQ==
†These authors have contributed equally to this work
 Li Zhao
Li Zhao Yiping Guo
Yiping Guo Xuelei Zhou
Xuelei Zhou Wei Mao
Wei Mao Hongyu Zhu1
Hongyu Zhu1 Xianchun Liu
Xianchun Liu Linji Li
Linji Li