Abstract
Background and objectives:
Several recent clinical studies have indicated that vamorolone is comparable in effectiveness to glucocorticosteroids for treating Duchenne muscular dystrophy (DMD). However, there is a lack of extensive data regarding the efficacy and safety of various doses of vamorolone. We conducted a study to evaluate the efficacy of different doses of vamorolone in boys with DMD, and compare the safety of vamorolone vs. glucocorticosteroids, prednisone or deflazacort in boys with DMD.
Methods:
We performed systematic searches of the PubMed, Embase, and Cochrane Library databases for vamorolone, glucocorticosteroids, prednisone or deflazacort in boys with DMD. We assessed statistical heterogeneity across trials based on the Newcastle Ottawa scale (NOS) tool test and I2 values, and mean differences were pooled using the random-effects model. We used traditional meta-analysis to evaluate efficacy and safety of vamorolone 6.0 mg/kg/d vs. vamorolone 2.0 mg/kg/d and vamorolone vs. prednisone. A network meta-analysis was applied to estimated the safety of vamorolone in comparison to glucocorticosteroids, prednisone and deflazacort. Our meta-analysis were performed using Revman 5.4 software, and our network meta-analysis were performed using Stata/MP 18.0.
Results:
In the meta-analysis, a total of 193 patients were analyzed across four clinical trials (97 patients receiving vamorolone 2 mg/kg per day; 96 patients receiving vamorolone 2 mg/kg per day). We observed that there were statistically significant differences in boys with DMD between vamorolone 6.0 mg/kg/d and vamorolone 2.0 mg/kg/d in TTSTANDV (MD = 0.03, 95%CI = 0.00–0.06, p = 0.04), TTRWV (MD = 0.13, 95%CI = 0.08–0.19, p < 0.01), 6MWT (MD = 24.54, 95%CI = 4.46–44.82, p = 0.02), TTCLIMBV (MD = 0.04, 95%CI = 0.01–0.06, p = 0.009), no significant difference in BMI z score (MD = 0.09, 95%CI = −0.03–0.20, p = 0.13). Indirect comparisons derived from network meta-analysis did not show significant differences among vamorolone, glucocorticosteroids, prednisone and deflazacort in BMI z score.
Conclusion:
Our findings implied that boys with DMD who took vamorolone 6 mg/kg daily instead of 2 mg/kg daily may be safer and have superior motor function. However, more large sample randomized controlled trials are needed to confirm our results.
Systematic Review Registration:
This systematic review and meta-analysis has been registered in the International Prospective Register of Ongoing Systematic Reviews PROSPERO (registration number: CRD42024562916).
Introduction
Duchenne muscular dystrophy (DMD) is an inherited X-linked recessive neuromuscular disease, which is caused by a mutations in the DMD gene resulting in deficiency of structural protein myotonic dystrophy proteins (1). The main clinical manifestations are progressive quadriceps lumbar muscle weakness, and loss of the ability to walk by the age of 10-14 years (1). DMD is the most common type of muscular dystrophy, with an incidence rate of about 30 per 100,000 male infants and seems to be the same between regions (1, 2). The condition is marked by a gradual worsening of muscle weakness and atrophy in the trunk and limbs, along with the development of muscle pseudohypertrophy. Additionally, many individuals experience myocardial damage and typically lose the ability to walk by the age of 12 years (3). Due to the absence of a targeted treatment for DMD, the majority of patients typically succumb to respiratory infections and cardiac failure around the age of 20–30 years (4). There have been significant advancements in therapeutic agents for DMD in recent years. These include treatments that address genetic defects (5–7), such as exon skipping, gene replacement therapy, gene editing, and stop codon read-through therapy. Additionally, there are treatments that target the secondary pathological changes of DMD (8, 9), such as glucocorticosteroids, novel anti-inflammatory compounds (vamorolone), inhibitors of muscle growth inhibitors, inhibitors of nuclear factors, cardio-protective compounds, and stem cell therapy. Thus, the natural history of individuals with DMD has undergone significant changes in the last three decades as a result of the adoption of care standards (10).
Over the last decade, clinical studies have found that vamorolone has the same efficacy as glucocorticosteroids in DMD, but lacks the adverse effects associated with glucocorticosteroids (growth retardation, bone disease, muscle atrophy and so on) (11, 12). Glucocorticosteroids and vamorolone are both drugs with pleiotropic effects and multiple mechanisms of action. However, they have distinct differences: vamorolone reduces inflammation without causing the immunosuppressive effects on lymphocyte viability and function that prednisone does (11, 13, 14). A number of animal studies have shown that vamorolone lowered inflammation and enhanced function in mouse models of inflammatory disorders including inflammatory bowel disease, multiple sclerosis, allergic asthma, and myotonic dystrophy (11, 15). First-in-class anti-inflammatory research drug vamorolone has demonstrated safety and efficacy in DMD at 2.0 or 6.0 mg/kg/day (8, 16, 17). Following six months of therapy, the high-dose group (≥2.0 mg/kg/d) in an initial open-label, multiple ascending dose exploratory study in boys with DMD showed notable improvements in motor function (16). Based on the results of multiple studies (8, 16–18), it has been observed that vamorolone at a dosage of 6 mg/kg/day is more effective in maintaining motor outcomes compared to vamorolone at a dosage of 2 mg/kg/day.
Studies have shown that glucocorticosteroids have a notable positive impact on exercise and cardiac function in individuals with DMD. As a result, they are currently recommended for early use in clinical practice. Furthermore, it is suggested that glucocorticosteroids be used to maintain late-stage functional abilities, as well as respiratory and cardiac function after the loss of ambulation (9). Although deflazacort was linked to less weight gain than prednisone, daily use of both drugs is effective in preserving muscle strength for up to 12 weeks (19, 20). Deflazacort offers patients with nonsense mutation DMD clinically significant delays in the loss of physical milestones over 48 weeks when compared to prednisone (21). Although only deflazacort is FDA approved for DMD treatment, both prednisone and deflazacort are used as the standard of care (22–25). But glucocorticosteroids have certain side effects, thus their clinical use is more limited.
There has been controversy regarding the efficacy and safety of different doses of vamorolone in boys with DMD. Currently, there is a lack of data to compare it with deflazacort. In view of this, we conducted a meta-analysis to assess the efficacy and safety of vamorolone at doses of 2.0 or 6.0 mg/kg/day. In addition, we aimed to compare the safety of vamololone with glucocorticosteroids.
Methods
This study utilized a systematic review and network meta-analysis approach, adhering to the PRISMA guidelines (26). This systematic review and meta-analysis has been registered in the International Prospective Register of Ongoing Systematic Reviews PROSPERO (registration number: CRD42024562916).
Search strategy
The databases of PubMed, EMBASE and the Cochrane Library were searched from inception to April 2024. We used the following terms: “Vamorolone” OR “Prednisone” OR “Deflazacort” OR “Glucocorticosteroid” OR “Corticosteroid” AND “Duchenne muscular dystrophy.”At least one of the following predetermined outcomes of interest had to be reported by studies: time to stand from supine velocity (TTSTANDV), 6-min walk test distance (6MWD), time to run/walk 10 m velocity (TTRWV), time to climb 4 stairs velocity (TTCLIMBV), and North Star Ambulatory Assessment (NSAA). For the three timed function tests, velocities were computed by dividing 10 by time in seconds (TTRWV) or by taking the reciprocal of the test duration (TTSTANDV, TTCLIMBV). Growth stunting and weight gaining are recognised adverse outcomes associated with glucocorticosteroids, which may be troublesome for boys with DMD during adolescence and is the greatest limitation of current glucocorticosteroids therapy for boys with DMD. Therefore, safety was evaluated by height percentile and BMI z score, which are good indicators of changes in weight and height.
Article selection and data extraction
The article search was concluded on April, 2024. We selected studies that met the following inclusion criteria: (i) clinical trials; (ii) inclusion of at least one outcome variable; (iii) studies comparing vamorolone, deflazacort, prednisone, orglucocorticosteroids (alone or in combination) for DMD; (iv) the data could be directly obtained or indirectly estimated from figures for statistical analysis.
The exclusion criteria consisted of: (i) duplicate studies; (ii) case reports, reviews, and animal studies; (iii) studies without relevant outcomes; (iv) studies unrelated to the topic; (v) studies published in languages other than English.
Two independent authors (Qin Wang and Yaqing Zeng) reviewed the titles and/or abstracts of the papers that were searched in order to identify appropriate studies. Subsequently, the two authors separately reviewed and modified the complete texts of the obtained reports. The third author (Zhibing Song) resolved any issues that arose among the authors.
We collected the following details: (i) study ID, year of publication, phase of the study, therapy used; (ii) number of patients per arm, age (mean and range) and baseline characteristics. and (iii) outcome data: TTSTANDV or TTSTAND, 6MWD, TTRWV or TTRW, TTCLIMBV or TTCLIMB, BMI z score and Height percentile.
Quality assessment
The Newcastle Ottawa scale method was employed to evaluate the quality of the primary research (27). Two writers conducted an independent assessment of the quality of the trial’s primary outcome using the Newcastle Ottawa scale (NOS) methodology. This scale was assessed on the basis of individual selection, comparability between groups, and outcome, with a total score of 9 points. Achieving higher scores indicates superior quality in investigations, with a score of 7–9 being seen as indicative of high-quality research. Disputes were settled by an intermediary author.
Data synthesis
Traditional meta-analysis
An analysis was conducted using RevMan software, version 5.4, to assess the effectiveness and safety of vamorolone at different dosages and compare it to Prednisone. The mean difference (MD) of continuous data was reported along with a 95% confidence interval (CI). The heterogeneity test primarily relied on the I2 statistic, and the random-effects model was utilized. Results were deemed statistically significant if the p < 0.05.
Network meta-analysis
To assess the safety of vamorolone in comparison to glucocorticosteroids such as prednisone or deflazacort, a network meta-analysis was conducted on boys with DMD using Stata/MP 18.0. The matrix is represented by the abbreviation HR, and 95% confidence interval (CI) were utilized to assess the significance of differences in effectiveness between each pair of regimens.
Results
Study selection
After removing duplicates, we obtained a total of 154 studies. Eventually, seven studies were selected for the analysis (Figure 1). 193 patients of four clinical trials were analyzed in the meta-analysis, including 97 patients receiving vamorolone 2 mg/kg per day and 96 patients receiving vamorolone 2 mg/kg per day. Our network meta-analysis included 363 patients in 4 studies, 53 patients in the deflazacort group, 106 patients in the prednisone group, 129 patients in the vamorolone group, 75 patients in the glucocorticosteroids group. The characteristics of the studies included are reported in Table 1. Table 2 displays the quality of each study that was taken into consideration for analysis. The quality of all studies was good, with a score of 7–8.
Figure 1

Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow diagram describing the systematic literature search and study selection.
Table 1
| Study | Year of publication | Study type | Study duration | Drug | Sample size | Age (year) | BMI (kg/m2) | Height percentile | Height (cm) | Weight (kg) | TTSTAND(s)/TTSTANDV (event/s) | TTRW(s)/TTRWV (meters/s) | 6MWT (m) | NSAA score | TTCLIMB(s)/TTCLIMBV (event/s) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shieh et al. (23) | 2018 | A post hoc analysis | 48 weeks | Deflazacort | 53 | 9.2 ± 1.7 | 18.6 ± 4.7 | N/A | 127 ± 10.6 | 30.9 ± 11.9 | N/A | N/A | N/A | N/A | N/A |
| Prednisone | 61 | 8.8 ± 1.6 | 19.0 ± 3.5 | N/A | 125.7 ± 10.4 | 30.5 ± 9.2 | N/A | N/A | N/A | N/A | N/A | ||||
| Hoffman et al. (16) | 2019 | An open-label clinical trial | 24 weeks | Vamorolone0.25 mg/kg/d | 12 | 5.2 ± 1.0 | 17.4 ± 1.1 | N/A | N/A | N/A | 6.1 ± 2.1 | 6.5 ± 1.4 | 316 ± 59 | 19.0 ± 5.1 | 5.4 ± 1.5 |
| Vamorolone0.75 mg/kg/d | 12 | 4.8 ± 0.8 | 16.5 ± 1.5 | N/A | N/A | N/A | 5.1 ± 2.3 | 5.9 ± 1.2 | 331 ± 53 | 20.5 ± 5.6 | 4.6 ± 2.8 | ||||
| Vamorolone2.0 mg/kg/d | 12 | 4.7 ± 0.9 | 17.2 ± 0.8 | N/A | N/A | N/A | 5.3 ± 2.0 | 5.6 ± 1.1 | 354 ± 65 | 20.0 ± 4.9 | 4.6 ± 2.8 | ||||
| Vamorolone6.0 mg/kg/d | 12 | 4.8 ± 0.8 | 16.5 ± 1.0 | N/A | N/A | N/A | 5.9 ± 2.8 | 6.3 ± 1.2 | 337 ± 63 | 19.7 ± 4.9 | 5.0 ± 2.4 | ||||
| All vamorolone groups | 48 | 4.9 ± 0.9 | 16.9 ± 1.2 | N/A | N/A | N/A | 5.6 ± 2.3 | 6.1 ± 1.2 | 334 ± 59 | 19.8 ± 5.0 | 4.9 ± 2.4 | ||||
| Prednisone | 14 | 4.9 ± 0.8 | 15.2 ± 1.6 | N/A | N/A | N/A | 5.9 ± 3.2 | 6.6 ± 2.0 | N/A | N/A | 6.3 ± 4.1 | ||||
| Steroid naive | 31 | 5.7 ± 0.7 | 16.3 ± 1.7 | N/A | N/A | N/A | 10.03 ± 12.1 | 6.2 ± 2.4 | N/A | N/A | 6.1 ± 5.7 | ||||
| Smith et al. (14) | 2020 | A non-randomized openlabel extension study | 18 months | Vamorolone2.0 + 6.0 mg/kg/d | 23 | 5.2 ± 0.9 | 17.0 ± 0.9 | N/A | 107 ± 6.8 | 19.5 ± 2.5 | 0.206 ± 0.07 | 1.735 ± 0.331 | 343.2 ± 64.3 | 19.9 ± 4.9 | 0.266 ± 0.134 |
| Prednisone | 12 | 5.7 ± 0.66 | 16.5 ± 1.9 | N/A | 110.3 ± 6.8 | 20.1 ± 3.5 | N/A | N/A | N/A | N/A | N/A | ||||
| Corticosteroid-treated | 68 | 5.9 ± 0.64 | 17.2 ± 1.9 | N/A | 109.2 ± 5.7 | 20.6 ± 3.4 | N/A | N/A | N/A | N/A | N/A | ||||
| Steroid naive | 19 | 5.03 ± 0.55 | 16.4 ± 0.9 | N/A | 105.4 ± 5.1 | 18.3 ± 2.0 | 0.202 ± 0.055 | 1.619 ± 0.483 | N/A | NA | 0.218 ± 0.098 | ||||
| Mah et al. (17) | 2022 | A nonrandomized controlled trial | 30 months | Vamorolone2.0 + 6.0 mg/kg/d | 23 | 5.83 ± 0.88 | 17.68 ± 1.23 | N/A | 111.8 ± 6.94 | 21.98 ± 3.78 | 0.25 ± 0.10 | 1.90 ± 0.34 | 377.9 ± 64.77 | 23.3 ± 4.72 | 0.31 ± 0.13 |
| Corticosteroid-treated | 75 | 6.08 ± 0.81 | 16.68 ± 1.55 | N/A | 109.86 ± 6.86 | 20.35 ± 3.55 | 0.25 ± 0.10 | 1.91 ± 0.52 | N/A | N/A | 0.32 ± 0.14 | ||||
| Guglieri et al. (28) | 2022 | A randomized, double-blind, placebo clinical trial | 24 weeks | Vamorolone2.0 mg/kg/d | 30 | 5.3 ± 0.9 | 16.2 ± 1.2 | 30 ± 29 | 108 ± 9 | 19 ± 4 | 0.18 ± 0.05 | 1.6 ± 0.3 | 316 ± 58 | 17.2 ± 4.7 | 0.21 ± 0.09 |
| Vamorolone 6.0 mg/kg/d | 28 | 5.4 ± 0.9 | 16.6 ± 1.4 | 23 ± 25 | 107 ± 7 | 19 ± 3 | 0.19 ± 0.06 | 1.6 ± 0.4 | 313 ± 56 | 18.9 ± 4.1 | 0.2 ± 0.05 | ||||
| Prednisone 0.75 mg | 31 | 5.5 ± 0.9 | 16.8 ± 1.3 | 37 ± 29 | 111 ± 6 | 21 ± 3 | 0.22 ± 0.06 | 1.9 ± 0.4 | 343 ± 56 | 21.2 ± 5.5 | 0.29 ± 0.11 | ||||
| Steroid naive | 28 | 5.4 ± 0.8 | 16.3 ± 1.1 | 33 ± 29 | 109 ± 9 | 20 ± 3 | 0.20 ± 0.06 | 1.7 ± 0.3 | 355 ± 78 | 18.9 ± 5.3 | 0.25 ± 0.09 | ||||
| Dang et al. (8) | 2024 | A Randomized controlled Trial | 48 weeks | Vamorolone2.0 mg/kg/d | 27 | 5.3 ± 0.9 | 16.2 ± 1.2 | 30 ± 29 | 108 ± 9 | 19 ± 4 | 0.18 ± 0.05 | 1.6 ± 0.3 | 316 ± 58 | 17.2 ± 4.7 | 0.21 ± 0.09 |
| Vamorolone6.0 mg/kg/d | 28 | 5.4 ± 0.9 | 16.6 ± 1.4 | 23 ± 25 | 107 ± 7 | 19 ± 3 | 0.19 ± 0.06 | 1.6 ± 0.4 | 313 ± 56 | 18.9 ± 4.1 | 0.2 ± 0.05 | ||||
| Leinonen et al. (18) | 2023 | A randomized and double-blind study | 48 weeks | Vamorolone2.0 mg/kg/d | 28 | 5.3 ± 0.9 | N/A | 43.1 ± 29 | N/A | N/A | 6.0 ± 2.4 | N/A | 317 ± 60 | 17.5 ± 4.6 | N/A |
| Vamorolone6.0 mg/kg/d | 28 | 5.4 ± 0.9 | N/A | 43.7 ± 26.7 | N/A | N/A | 6.0 ± 2.0 | N/A | 313 ± 56 | 18.9 ± 4.1 | N/A |
Baseline characteristics.
Table 2
| ID | Selection | Comparability | Outcome | Total point | AHRQ Standards | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) Representativeness of the exposed cohort | (2) Selection of the non-exposed cohort | (3) Ascertainment of exposure | (4) Demonstration that outcome of interest was not oresent at start of study | (1) Comparability of cohorts on the basis of design or ananlysis | (2) Comparabiliy of cohorts on the basis of measurement | (1) Assessment of outcome | (2) Was follow-up long enough for outcomes to occur | (3) Adequacy of followup of cohorts | |||
| Shieh et al. (23) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 7 | Good |
| Hoffman et al. (16) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 8 | Good |
| Smith et al. (14) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 8 | Good |
| Mah et al. (17) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 7 | Good |
| Guglieri et al. (28) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 8 | Good |
| Dang et al. (8) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 8 | Good |
| Leinonen et al. (18) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 8 | Good |
Quality assessment for each study considered for analysis.
Meta-analysis
Vamorolone 6.0 mg/kg/d vs. vamorolone 2.0 mg/kg/d
TTSTAND velocity
Figure 2 presented the forest plot illustrating the velocity change in TTSTAND between vamorolone 6.0 mg/kg/d and vamorolone 2.0 mg/kg/d in each study. Our observation indicates a significant difference in TTSTANDV (MD = 0.03, 95%CI = 0.00–0.06, p = 0.04), along with notable heterogeneity among studies (p = 0.01, I2 = 74%) (Figure 2) (8, 16, 18, 28).
Figure 2

Forest plot of TTSTAND velocity outcome data for vamorolone 6.0 mg/kg/d vs. vamorolone 2.0 mg/kg/d.
TTRW velocity
A notable disparity was observed in TTRWV between vamorolone 6.0 mg/kg/d and vamorolone 2.0 mg/kg/d (MD = 0.13, 95%CI = 0.08–0.19, p < 0.00001). There was no significant heterogeneity among the studies (p = 0.86, I2 = 0%) (Figure 3) (8, 16, 18, 28).
Figure 3

Forest plot of TTRW velocity outcome data for vamorolone 6.0 mg/kg/d vs. vamorolone 2.0 mg/kg/d.
6MWT
The pooled analysis showed a significant difference between vamorolone 6.0 mg/kg/d and vamorolone 2.0 mg/kg/d in 6MWT (MD = 24.54, 95%CI = 4.46–44.82, p = 0.02), and a significant heterogeneity among studies (p = 0.07, I2 = 57%) (Figure 4) (8, 16, 18, 28).
Figure 4

Forest plot of 6MWT velocity outcome data for vamorolone 6.0 mg/kg/d vs. vamorolone 2.0 mg/kg/d.
TTCLIMB velocity
The combined analysis revealed no significant heterogeneity across studies (p = 0.49, I2 = 0%) and a significant difference in TTCLIMBV between vamorolone 6.0 mg/kg/d and vamorolone 2.0 mg/kg/d (MD = 0.04, 95%CI = 0.01–0.06, p = 0.009) (Figure 5) (8, 16, 18, 28).
Figure 5
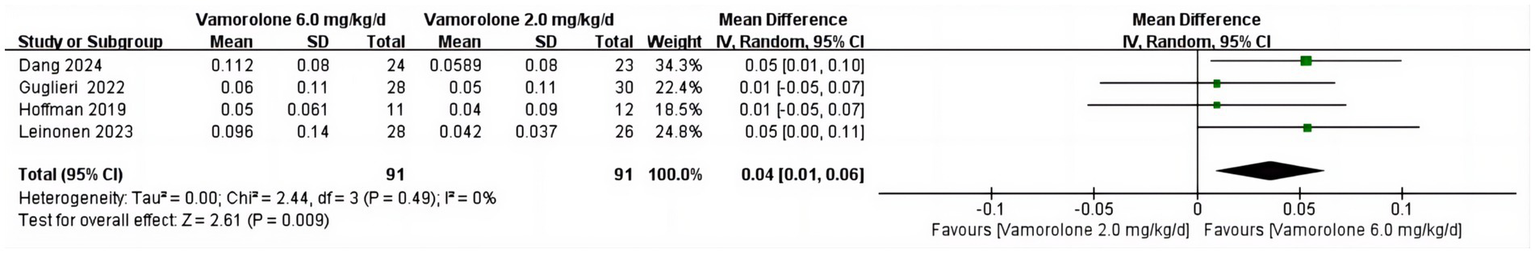
Forest plot of TTCLIMB velocity outcome data for vamorolone 6.0 mg/kg/d vs. vamorolone 2.0 mg/kg/d.
BMI z score
We found no statistically significant difference in BMI z score between vamorolone 2.0 mg/kg/d and vamorolone 6.0 mg/kg/d (MD = 0.09, 95%CI = −0.03–0.20, p = 0.13) or significant heterogeneity amongst studies (p = 0.25, I2 = 27%) (Figure 6) (8, 16, 18, 28).
Figure 6
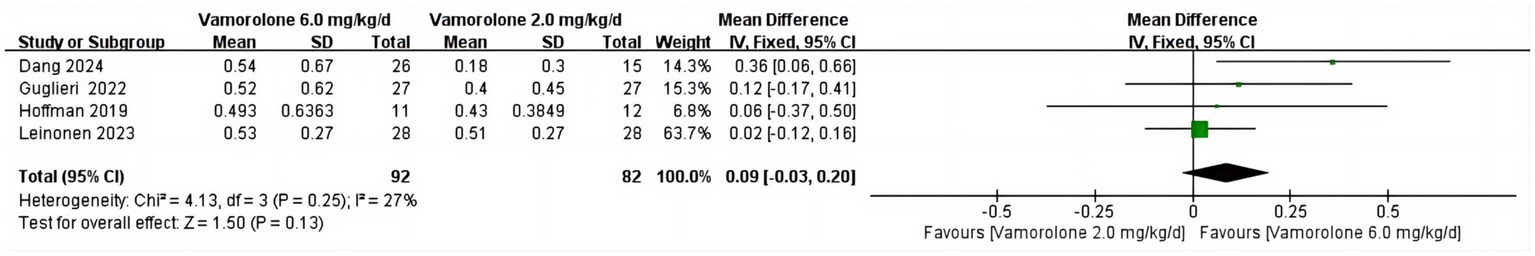
Forest plot of BMI z score outcome data for vamorolone 6.0 mg/kg/d vs. vamorolone 2.0 mg/kg/d.
Vamorolone vs. prednisone
BMI z score
The combined analysis revealed no statistically significant difference in the BMI z score between vamorolone and prednisone (MD = 0.03, 95%CI = −0.13–0.19, p = 0.71) or in the heterogeneity among the studies (p = 0.72, I2 = 0%) (Figure 7) (14, 16, 28).
Figure 7

Forest plot of BMI z score outcome data for vamorolone vs. prednisone.
Mean height percentile for age
The pooled analysis revealed a statistically significant disparity in the Mean height percentile for age between vamorolone and prednisone (MD = 4.38, 95%CI = −1.40–7.36, p = 0.004). There was no significant variation among the studies (p = 0.45, I2 = 0%) (Figure 8) (14, 28).
Figure 8

Forest plot of mean height percentile for age outcome data for vamorolone vs. prednisone.
Network meta-analysis
BMI z score
The network meta-analysis did not find any significant differences in BMI z score among vamorolone, glucocorticosteroid, prednisone, and deflazacort, based on indirect comparisons (Figure 9) (16, 17, 23, 28).
Figure 9
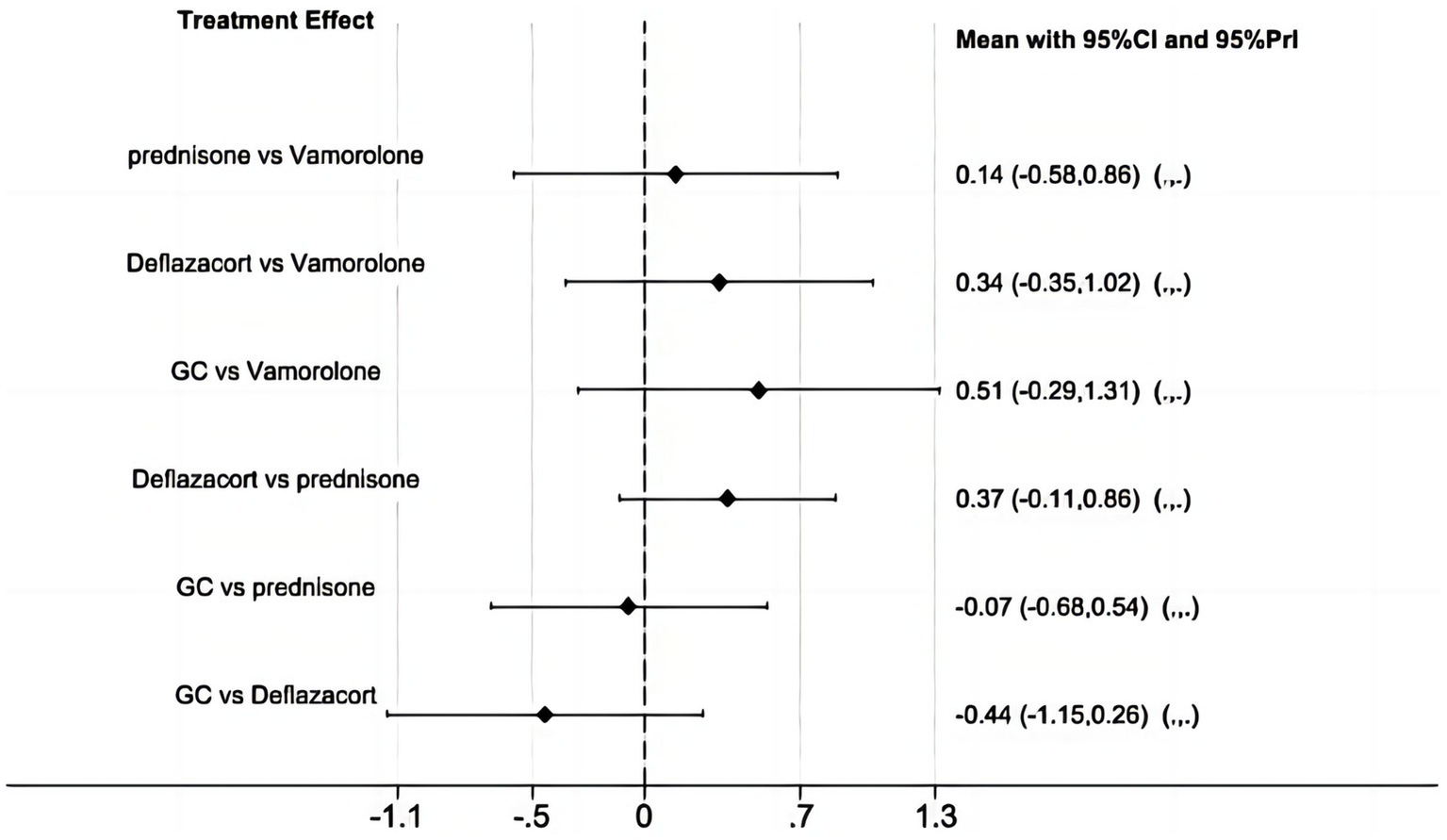
Network meta-analysis for vamorolone vs. glucocorticosteroid, prednisone and deflazacort of BMI z score.
Discussion
To our knowledge, this is the first meta-analysis to compared vamorolone 6 mg/kg per day with vamorolone 2 mg/kg per day in boys with DMD. From the results of our study, we conclude DMD patients may have greater response to vamorolone 6 mg/kg per day without causing weight gain.
A new drug called vamorolone seems to maximize conventional steroidal anti-inflammatory effects by retaining transrepression (NF-κB inhibition anti-inflammatory activity) and losing transactivation (gene transcription via GRE-mediated binding of ligand/receptor dimers) (11, 13). Comparing vamorolone to prednisolone, studies in animal models of chronic inflammatory states, including DMD mouse models, have demonstrated retention of anti-inflammatory efficacy and reduction of side effects (11, 12, 29). Similar to glucocorticosteroids in pharmacokinetics and metabolism, vamorolone is given orally once a day (30, 31). In contrast to glucocorticosteroids, vamorolone does not interact with 11 β-hydroxysteroid dehydrogenase regulatory enzymes and effectively blocks the mineralocorticoid receptor (15). Vamorolone has demonstrated efficacy in preserving muscle function and extending walking time in patients with DMD, as evidenced by multiple clinical trials (13, 14). Consequently, it represents a promising and groundbreaking therapeutic option for the treatment of DMD (32). Vamorolone received approval in October 2023 for treating DMD in patients aged 2 and older in the USA. It also received a positive opinion in the EU in October 2023 for treating DMD in patients aged 4 and older (33). However, the specific dosage remains uncertain, as it is only indicated that a dose of 2-6 mg/kg might be suitable.
Boys with DMD who received vamorolone 6 mg/kg/day compared to vamorolone 2 mg/kg/day improved in several functional end points, including TTSTANDV, TTRWV, 6MWT and TTCLIMBV, according to this meta-analysis. But BMI z score is not statistically significant. Our results support the conclusions of Dang et al. (8), who found that for most motor outcomes, vamorolone at a dose of 6 mg/kg/d demonstrated better maintenance of effect than vamorolone at a dose of 2 mg/kg/d. Like us, Leinonen et al. (18) found that in TTSTAND velocity, the effect observed at week 24 for vamorolone 6 mg/kg/day persisted until week 48, but not for vamorolone 2 mg/kg/day at week 48. That study also revealed that, compared to TTRW or NSAA, DMD patients taking 6 mg/kg of vamorolone daily improved more in 6MWT and TTCLIMB. Hoffman et al. (16) discovered that vamorolone 6.0 mg/kg/d improved 6MWT and TTRW more than 2.0 mg/kg/d, but this dose also raised more safety concerns, with a mean rise in BMI z score comparable to that with prednisone. Although Guglieri et al. (28) found that the variations in TTSTANDV and, 6MWT were clinically significant, this study did not compare the functional end points of vamorolone 6 mg/kg daily with vamorolone 2 mg/kg daily.
During the initial investigation into the exposure-response relationships of vamorolone, it was discovered that significant enhancements in clinical efficacy were observed at a daily dose of 2 mg/kg to 6 mg/kg after 24 weeks. A study found that higher-dose groups (≧2.0 mg/kg/d) experienced significant motor function improvement after 6 months of treatment (14). Additionally, the long-term results of this study revealed that vamorolone was associated with the maintenance of muscle strength and function for up to 30 months. This effect was similar to standard-of-care glucocorticoid therapy. Furthermore, vamorolone showed improved height velocity compared to the growth deceleration observed with glucocorticoid therapy. These findings suggest that vamorolone may be a promising option for the treatment of DMD (17). Significant increases in TTSTAND, TTRW, and TTCLIMB were observed in a meta-analysis of vamolodone versus placebo and steroids, which suggests that vamolodone has benefits in the treatment of DMD patients (34). Thus, our combined analysis and the studies mentioned above suggest that boys with DMD who took vamorolone 6 mg/kg daily may have better motor function than those who took vamorolone 2 mg/kg daily and that there is no appreciable difference in the impact on body weight. Unfortunately, the studies we included were unable to access the mean height percentile for age in boys with DMD, who recieved vamorolone 6 mg/kg/day or vamorolone 2 mg/kg/day.
Administering glucocorticosteroids to children with DMD can enhance muscle mobility and postpone damage to the heart muscle, resulting in a longer period of walking and ultimately improving the survival rate of patients (22, 24). Glucocorticosteroids, which are commonly used to treat children with DMD, have been found to cause several negative effects. These include stunted growth, hormonal imbalances, weakened bones, metabolic disorders, increased body weight, and delays in puberty development (9, 19, 25). More previous studies have identified two measures of safety concerns related to glucocorticoids: growth deceleration (growth retardation measured as change in mean height-for-age percentile) and body mass index (BMI). In children, chronic glucocorticosteroids treatment often results in growth deceleration (8, 9, 14, 17). Vamorolone and prednisone differed significantly in the mean height percentile for age in the pooled analysis. Like us, Hoffman et al. (16) found that boys with DMD who took vamorolone 6 mg/kg daily had a mean rise in BMI z score comparable to that of prednisone. However, our findings indicated that vamorolone has little effect on bone formation. Mah et al. (17) reported similar findings to ours, showing that vamorolone increased height velocity in contrast to growth deceleration associated with glucocorticoid treatment. Dang et al. (8) further found that switching from prednisone to vamolone therapy reversed the bone morbidity of prednisone (growth retardation and decreased serum bone biomarkers). The Guglieri study also revealed the analogous results that height percentile decreased in participants receiving prednisone (but not vamorolone) (28). It can be concluded from these studies and our meta-analysis that vamorolone has a far smaller impact on height development than glucocorticoid.
Glucocorticosteroids are utilized as the standard treatment for DMD. However, it is important to note that only deflazacort has received FDA approval specifically for DMD (35). We conducted a safety comparison indirectly between vamorolone, glucocorticosteroid, prednisone, and deflazacort. However, our findings indicate that there are no significant differences in BMI z score among these medications. More trials are needed to explore the safety comparisons among vamorolone, glucocorticosteroid, prednisone, and deflazacort.
Future implications
The results of our meta-analysis suggest that DMD boys taking 6 mg/kg per day instead of 2 mg/kg per day vamorolone may be more advantaged in timed functional tests like TTSTANDV, TTRWV, TTCLIMBV and 6MWT, without affecting BMI. Our study suggests that DMD boys taking 6 mg/kg of vamorolone per day may have greater improvement in motor function. However, safety studies on 2 mg/kg per day vamorolone and 6 mg/kg per day vamorolone are still relatively scarce and need to be further explored. Due to the fact that there are no studies with larger doses, while whether there is a better effect at doses higher than 6 mg/kg per day vamorolone still needs to be discovered in more studies.
Strengths and limitations
This meta-analysis compared the efficacy and safety of different doses of vamorolone, and safety of vamorolone vs. glucocorticosteroid, prednisone or deflazacort in boys with DMD. But this study has several limitations. First, BMI z score comparisons between vamorolone, glucocorticosteroid, predinisone or deflazacort in this meta-analysis were indirect. And we failed to analyse comparative data on motor function between vamorolone and glucocorticoids, due to differences in study evaluations. Second, since there was no information in the current studies about comparison of height percentile between the 2 mg/kg and 6 mg/kg dose, this meta-analysis did not compare vamorolone 6 mg/kg per day compared with vamorolone 2 mg/kg per day in mean height percentile for age. Third, some of our studies were clinical trials but not randomized. In addition, the study periods and age ranges of the study population are not the same, because of limitations on the number of studies.
Conclusion
Our study indicates that TTSTANDV, TTRWV, 6MWT, and TTCLIMBV of boys with DMD who received vamorolone 6 mg/kg daily improved better compared to vamorolone 2 mg/kg daily. But the BMI z score is not statistically significant. Boys with DMD who take vamorolone 6 mg/kg daily may have better function and be safer. But more large sample size randomized clinical trials are required to investigate the long-term safety and effectiveness of various vamorolone dosages.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
QW: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Supervision. YZ: Data curation, Supervision, Writing – review & editing. LJ: Data curation, Investigation, Writing – review & editing. JH: Data curation, Supervision, Writing – review & editing. BL: Data curation, Investigation, Methodology, Writing – review & editing. YG: Data curation, Supervision, Writing – review & editing. ZS: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
- 6MWD
6-minute walk distance
- BMI
Body mass index
- DMD
Duchenne muscular dystrophy
- TTCLIMB
Time to climb 4 steps
- NSAA
North Star Ambulatory Assessment
- TTRW
Time to run/walk 10 m
- TTSTAND
Time to stand from supine
- TTCLIMBV
Time to climb 4 steps velocity
- TTRWV
Time to run/walk 10 m velocity
- TTSTANDV
Time to stand from supine velocity
Glossary
References
1.
Duan D Goemans N Takeda S Mercuri E Aartsma-Rus A . Duchenne muscular dystrophy. Nat Rev Dis Primers. (2021) 7:13. doi: 10.1038/s41572-021-00248-3
2.
Happi Mbakam C Lamothe G Tremblay JP . Therapeutic strategies for dystrophin replacement in Duchenne muscular dystrophy. Front Med (Lausanne). (2022) 9:859930. doi: 10.3389/fmed.2022.859930
3.
Deng J Zhang J Shi K Liu Z . Drug development progress in duchenne muscular dystrophy. Front Pharmacol. (2022) 13:950651. doi: 10.3389/fphar.2022.950651
4.
Verhaart IEC Aartsma-Rus A . Therapeutic developments for Duchenne muscular dystrophy. Nat Rev Neurol. (2019) 15:373–86. doi: 10.1038/s41582-019-0203-3
5.
Berling E Nicolle R Laforêt P Ronzitti G . Gene therapy review: Duchenne muscular dystrophy case study. Rev Neurol (Paris). (2023) 179:90–105. doi: 10.1016/j.neurol.2022.11.005
6.
Happi Mbakam C Tremblay JP . Gene therapy for Duchenne muscular dystrophy: an update on the latest clinical developments. Expert Rev Neurother. (2023) 23:905–20. doi: 10.1080/14737175.2023.2249607
7.
Patterson G Conner H Groneman M Blavo C Parmar MS . Duchenne muscular dystrophy: current treatment and emerging exon skipping and gene therapy approach. Eur J Pharmacol. (2023) 947:175675. doi: 10.1016/j.ejphar.2023.175675
8.
Dang UJ Damsker JM Guglieri M Clemens PR Perlman SJ Smith EC et al . Efficacy and safety of Vamorolone over 48 weeks in boys with Duchenne muscular dystrophy: a randomized controlled trial. Neurology. (2024) 102:e208112. doi: 10.1212/wnl.0000000000208112
9.
Schiava M Lofra RM Bourke JP Díaz-Manera J James MK Elseed MA et al . Functional abilities, respiratory and cardiac function in a large cohort of adults with Duchenne muscular dystrophy treated with glucocorticoids. Eur J Neurol. (2024) 31:e16267. doi: 10.1111/ene.16267
10.
Broomfield J Hill M Guglieri M Crowther M Abrams K . Life expectancy in Duchenne muscular dystrophy: reproduced individual patient data Meta-analysis. Neurology. (2021) 97:e2304–e 2314. doi: 10.1212/wnl.0000000000012910
11.
Heier CR Damsker JM Yu Q Dillingham BC Huynh T van der Meulen JH et al . VBP15, a novel anti-inflammatory and membrane-stabilizer, improves muscular dystrophy without side effects. EMBO Mol Med. (2013) 5:1569–85. doi: 10.1002/emmm.201302621
12.
Akkad H Cacciani N Llano-Diez M Corpeno Kalamgi R Tchkonia T Kirkland JL et al . Vamorolone treatment improves skeletal muscle outcome in a critical illness myopathy rat model. Acta Physiol (Oxf). (2019) 225:e13172. doi: 10.1111/apha.13172
13.
Li X Conklin LS van den Anker J Hoffman EP Clemens PR Jusko WJ . Exposure-response analysis of Vamorolone (VBP15) in boys with Duchenne muscular dystrophy. J Clin Pharmacol. (2020) 60:1385–96. doi: 10.1002/jcph.1632
14.
Smith EC Conklin LS Hoffman EP Clemens PR Mah JK Finkel RS et al . Efficacy and safety of vamorolone in Duchenne muscular dystrophy: an 18-month interim analysis of a non-randomized open-label extension study. PLoS Med. (2020) 17:e1003222. doi: 10.1371/journal.pmed.1003222
15.
Heier CR Yu Q Fiorillo AA Tully CB Tucker A Mazala DA et al . Vamorolone targets dual nuclear receptors to treat inflammation and dystrophic cardiomyopathy. Life Sci Alliance. (2019) 2:e201800186. doi: 10.26508/lsa.201800186
16.
Hoffman EP Schwartz BD Mengle-Gaw LJ Smith EC Castro D Mah JK et al . Vamorolone trial in Duchenne muscular dystrophy shows dose-related improvement of muscle function. Neurology. (2019) 93:e1312–23. doi: 10.1212/wnl.0000000000008168
17.
Mah JK Clemens PR Guglieri M Smith EC Finkel RS Tulinius M et al . Efficacy and safety of Vamorolone in Duchenne muscular dystrophy: a 30-month nonrandomized controlled open-label extension trial. JAMA Netw Open. (2022) 5:e2144178. doi: 10.1001/jamanetworkopen.2021.44178
18.
Leinonen M Hasham S Guglieri M Clemens PR Perlman S Smith EC et al . Efficacy and safety of vamorolone during 48-week treatment in patients with Duchenne muscular dystrophy (DMD) in VBP15-004 study. Conf Abstr Acta Myol. (2023) 42:96.
19.
Griggs RC Miller JP Greenberg CR Fehlings DL Pestronk A Mendell JR et al . Efficacy and safety of deflazacort vs prednisone and placebo for Duchenne muscular dystrophy. Neurology. (2016) 87:2123–31. doi: 10.1212/wnl.0000000000003217
20.
Reddy C Patil AN Suthar R Sankhyan N Sirari T Kumar A et al . Deflazacort dose optimization and safety evaluation in Duchenne muscular dystrophy (DOSE): a randomized, double-blind non-inferiority trial. Eur J Paediatr Neurol. (2022) 38:77–84. doi: 10.1016/j.ejpn.2022.04.004
21.
Shieh PB Elfring G Trifillis P Santos C Peltz SW Parsons JA et al . Meta-analyses of deflazacort versus prednisone/prednisolone in patients with nonsense mutation Duchenne muscular dystrophy. J Comp Eff Res. (2021) 10:1337–47. doi: 10.2217/cer-2021-0018
22.
Bonifati MD Ruzza G Bonometto P Berardinelli A Gorni K Orcesi S et al . A multicenter, double-blind, randomized trial of deflazacort versus prednisone in Duchenne muscular dystrophy. Muscle Nerve. (2000) 23:1344–7. doi: 10.1002/1097-4598(200009)23:9<1344::aid-mus4>3.0.co;2-f
23.
Shieh PB McIntosh J Jin F Souza M Elfring G Narayanan S et al . Deflazacort versus prednisone/prednisolone for maintaining motor function and delaying loss of ambulation: a post HOC analysis from the ACT DMD trial. Muscle Nerve. (2018) 58:639–45. doi: 10.1002/mus.26191
24.
Guglieri M Bushby K McDermott MP Hart KA Tawil R Martens WB et al . Developing standardized corticosteroid treatment for Duchenne muscular dystrophy. Contemp Clin Trials. (2017) 58:34–9. doi: 10.1016/j.cct.2017.04.008
25.
Guglieri M Bushby K McDermott MP Hart KA Tawil R Martens WB et al . Effect of different corticosteroid dosing regimens on clinical outcomes in boys with Duchenne muscular dystrophy: a randomized clinical trial. JAMA. (2022) 327:1456–68. doi: 10.1001/jama.2022.4315
26.
Page MJ McKenzie JE Bossuyt PM et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
27.
Stang A . Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
28.
Guglieri M Clemens PR Perlman SJ Smith EC Horrocks I Finkel RS et al . Efficacy and safety of Vamorolone vs placebo and prednisone among boys with Duchenne muscular dystrophy: a randomized clinical trial. JAMA Neurol. (2022) 79:1005–14. doi: 10.1001/jamaneurol.2022.2480
29.
Dillingham BC Knoblach SM Many GM Harmon BT Mullen AM Heier CR et al . VBP15, a novel anti-inflammatory, is effective at reducing the severity of murine experimental autoimmune encephalomyelitis. Cell Mol Neurobiol. (2015) 35:377–87. doi: 10.1007/s10571-014-0133-y
30.
Conklin LS Damsker JM Hoffman EP Jusko WJ Mavroudis PD Schwartz BD et al . Phase IIa trial in Duchenne muscular dystrophy shows vamorolone is a first-in-class dissociative steroidal anti-inflammatory drug. Pharmacol Res. (2018) 136:140–50. doi: 10.1016/j.phrs.2018.09.007
31.
Li X Sabbatini D Pegoraro E Bello L Clemens P Guglieri M et al . Assessing Pharmacogenomic loci associated with the pharmacokinetics of Vamorolone in boys with Duchenne muscular dystrophy. J Clin Pharmacol. (2024). doi: 10.1002/jcph.2446
32.
Grounds MD Lloyd EM . Considering the promise of Vamorolone for treating Duchenne muscular dystrophy. J Neuromuscul Dis. (2023) 10:1013–30. doi: 10.3233/jnd-230161
33.
Keam SJ . Vamorolone: first approval. Drugs. (2024) 84:111–7. doi: 10.1007/s40265-023-01986-2
34.
Elhalag RH Motawea KR Talat NE Rouzan SS Shah J . Efficacy of vamorolone in treatment of Duchene muscle dystrophy. A meta-analysis. Front Neurol. (2023) 14:1107474. doi: 10.3389/fneur.2023.1107474
35.
Liu G Lipari P Mollin A Jung S Teplova I Li W et al . Comparison of pharmaceutical properties and biological activities of prednisolone, deflazacort, and vamorolone in DMD disease models. Hum Mol Genet. (2024) 33:211–23. doi: 10.1093/hmg/ddad173
Summary
Keywords
Duchenne muscular dystroph, vamorolone, glucocorticosteroids, prednisone, deflazacort
Citation
Wang Q, Zeng Y, Jiao L, He J, Li B, Guo Y and Song Z (2024) Efficacy and safety of different doses of vamorolone in boys with Duchenne muscular dystrophy: a systematic review and network meta-analysis. Front. Neurol. 15:1456559. doi: 10.3389/fneur.2024.1456559
Received
01 July 2024
Accepted
09 August 2024
Published
21 August 2024
Volume
15 - 2024
Edited by
Jens Schmidt, Immanuel Klinik Rüdersdorf, Germany
Reviewed by
Ori Bar-Nur, ETH Zürich, Switzerland
Jonathan Broomfield, University of Leicester, United Kingdom
Updates
Copyright
© 2024 Wang, Zeng, Jiao, He, Li, Guo and Song.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhibin Song, frandk@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.