- 1Department of Neurosurgery, University Hospital Basel, Basel, Switzerland
- 2Faculty of Medicine, University of Basel, Basel, Switzerland
- 3Department of Neurology, University Hospital Basel, Basel, Switzerland
- 4Department of Biomedicine, University of Basel, Basel, Switzerland
- 5Rehabilitation Center Rheinfelden, Rheinfelden, Switzerland
Background: Whether minimally invasive endoscopic surgery (ES) improves survival and functional outcome in people with spontaneous supratentorial intracerebral hemorrhage (SSICH) is unknown.
Methods: This is a single-center pilot study performed between July 2021 to January 2023. Any supratentorial hematoma with a volume between 20 mL and 100 mL was endoscopically evacuated within 24 h after bleeding onset. Participants were followed-up for 6 months, assessing clinical and radiological outcomes. The primary feasibility outcome was satisfactory hematoma removal (<15 mL residual volume on the first postinterventional CT study) and the primary efficacy outcome was reaching a modified Rankin Scale 0–3 (mRS) at 6 months. Secondary outcomes were mortality and morbidity rates.
Results: Ten participants (median age 72.5 years [IQR 67–81], 70% male, median baseline hematoma volume 34.1 [IQR 25.5–58.0]) were included. Satisfactory hematoma evacuation was achieved in 70% (7/10) with a median evacuation percentage of 69.5% [IQR 45.3–93.9%]. The median duration of surgery was 91 min [IQR 73–111]. Favorable outcome at 6 months was observed in 60% of the participants and improved from within 24 h before the intervention to the last follow-up (6 months). Five participants (50%) experienced a total of six complications, two recurrent bleedings, three pneumonias and one epilepsy. Mortality rate was 30%, while one participant died from pneumonia, one from a recurrent bleeding, and one participant due to a glioblastoma.
Conclusion: ES appears to be feasible, with satisfactory hematoma removal being achieved in the majority of participants. Based on the descriptive results of this pilot trial, a national multicenter RCT comparing ES to best medical treatment is currently ongoing
Clinical trial registration: https://clinicaltrials.gov/, identifier NCT05681988.
1 Introduction
Intracerebral hemorrhage (ICH) is one of the most devastating forms of stroke, affecting around 3.41 million people annually (1, 2). Mortality rates reach up to 40% while the burden of disease for survivors remains catastrophic (3, 4). In SSICH, the hematoma volume directly contributes to poorer outcomes, due to its mass effect and toxic break down products causing further secondary damage, previous treatment trials aimed to prevent hematoma growth or reduce hematoma volume (5–13). As opposed to infratentorial ICH, where surgical treatment seems superior, in SSICH previous surgical trials failed to reduce mortality or improve functional outcome compared to best medical treatment (BMT) alone (5, 9, 10, 12–14). Therefore, BMT remains the mainstay of SSICH treatment, which includes a care bundle approach of a combination of active blood pressure management, intensive care unit surveillance, prevention of further complications and early rehabilitation (13).
Early minimal invasive endoscopic hematoma evacuation (ES) has been introduced years ago and showed some promising results (14, 15). A recent systematic review and meta-analysis showed a reduction in mortality and improved functional outcome after ES evacuation of SSICH compared to BMT. However, the included studies were of low power and quality (16). Recently the ENRICH trial has been completed showing for the first time beneficial outcome using minimally invasive surgery in lobar hematomas with current trials being ongoing to confirm these results (17). Furthermore, uncertainty remains about the ideal time point of the evacuation of SSICH. MISTIE III suggested an improved functional outcome in people treated early (<48 h) compared to a later treatment start (>48 h), however confirmatory studies are lacking (12, 18, 19). Given the current lack of evidence, further investigation regarding early, minimally invasive endoscopic evacuation of SSICH is needed.
We herein report our phase II pilot trial, assessing feasibility and safety of early minimally invasive endoscopic surgery for SSICH.
2 Methods
In this single-center, phase II, prospective pilot trial we included people between 18 and 85 years of age, without disabilities prior to SSICH (mRS 0–1), with any supratentorial lobar or deep hematomas of a volume between 20 and 100 mL and a neurological deficit, assessed with the NIH Stroke Scale Score (NIHSS) of ≥8 points or a Glasgow Coma Scale (GCS) between 5 and 13 points (20). People with vascular malformations, tumor, or trauma as well as people with active bleeding or coagulation disorder (i.e., spot sign and an INR of >1.5) were excluded (full eligibility criteria can be found in Supplementary Table S1). A spot sign was defined as an extravasation of contrast agent as demonstrate by computed tomography angiography. Oral anticoagulation was not an exclusion criterion if the effects could be reversed before surgery (i.e., within 24 h after SSICH onset). Oral anticoagulants were reversed according to current guidelines for SSICH and our in-house recommendations using the recommended antidots if available or prothrombin complex concentrates (21). We planned to include at least 10 participants in this exploratory and feasibility study. No formal sample size calculation was done.
Consecutive enrolment took place at the emergency department of the University Hospital Basel from July 2021 until January 2023 with some delay in recruitment due to the COVID-19 pandemic. All participants included underwent ES for the removal of the SSICH within 24 h after symptom onset. Follow-up visits took place at 24 h and 3 days after surgery, as well as at discharge, 1 month, and 6 months (Supplementary Table S2). The surgical procedure has been previously reported (22). In brief, a trajectory with the shortest distance to the long axis of the hematoma is calculated in our navigational software (Brainlab Inc., Munich, Germany). After completing a burr-hole trepanation the endoscope is inserted through a transparent trocar and guided by navigation to the distal end of the hematoma cavity. Using constant irrigation and suction, the hematoma is evacuated from distal to proximal. After completing the hematoma evacuation a postoperative CT scan for quality control is completed (outside of the OR in a standard CT scanner) (22). In addition to surgery, BMT is applied in all participants according to institutional guidelines (13).
The primary feasibility outcome was a satisfactory reduction in hematoma volume to <15 mL residual volume directly after but not later than 24 h after surgery, measured by the volumetric function of our navigational software. For calculation of volumetric differences before and after surgery, high resolution CT scans were compared. This threshold was chosen in accordance with findings from previous studies, leading to increased odds of favorable outcome in affected patients (12, 23). The primary efficacy outcome was a modified Rankin Scale 0–3 (mRS) at 6 months. Secondary outcomes were mortality at 6 months, relative reduction of the hematoma volume (admission compared to the first postoperative CT scan) and neurological deficits measured by the NIHSS at 6 months. Additionally, consecutive blood sampling was performed to assess fluid biomarkers for brain damage which will be presented in a future report.
All data were collected prospectively. CT imaging studies were conducted upon admission, 24 h postoperative and 3 days after surgery (MRI). For screening and including participants into the study the AxBxC/2 method was used to measure hematoma volume, while all volumes were secondarily validated using the volumetric function of the Brainlab (Brainlab Inc., Munich, Germany) navigation station using the admission CT and the first CT after surgery (20).
Data were reported descriptively. No formal comparison of safety outcomes and functional outcomes was possible due to the lack of a comparator. Safety and functional outcome results were reported in context with contemporary literature of comparable trials (24–26). Only patients with completed follow-ups were included in the final analysis. Within group comparison (mRS admission vs. mRS 6-month follow-up; NIHSS admission vs. NIHSS 6-month follow-up and GCS admission vs. GCS 6-month follow-up) was done using Wilcoxon signed rank sum- or McNemars test. An alpha level of 5% was deemed significant. Statistical analysis was performed using SPSS (V 28, IBM, Armonk, NY, United States). This trial was approved by the local ethics committee (EKNZ 2021–00161) and conducted according to the Declaration of Helsinki (27). The trial was registered with the identifier NCT04805177 and reported in agreement with the Consort Statement and the extension for pilot and feasibility trials (28, 29). All participants were informed about the study and consented for participation. An anonymized dataset is available with the corresponding author upon reasonable request.
3 Results
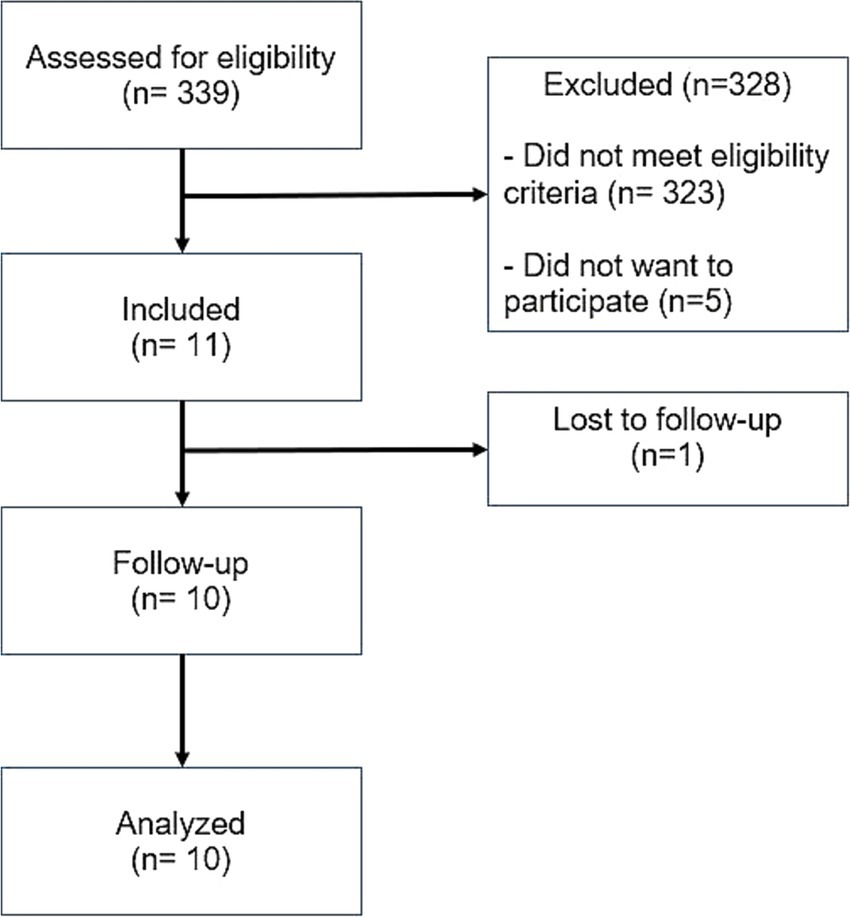
Between July 2021 and January 2023, 339 people with ICH of various etiologies, were screened. Sixteen participants were found eligible, and 11 were included. The most common reason for non-eligibility were the non-spontaneous etiology of the ICH (i.e., traumatic ICH) and inadequate hematoma volume (too large/too small). One participant was lost to follow-up (at visit 5/1 month after intervention) and 10 participants were included for the final analysis (Figure 1).
3.1 Baseline demographic data
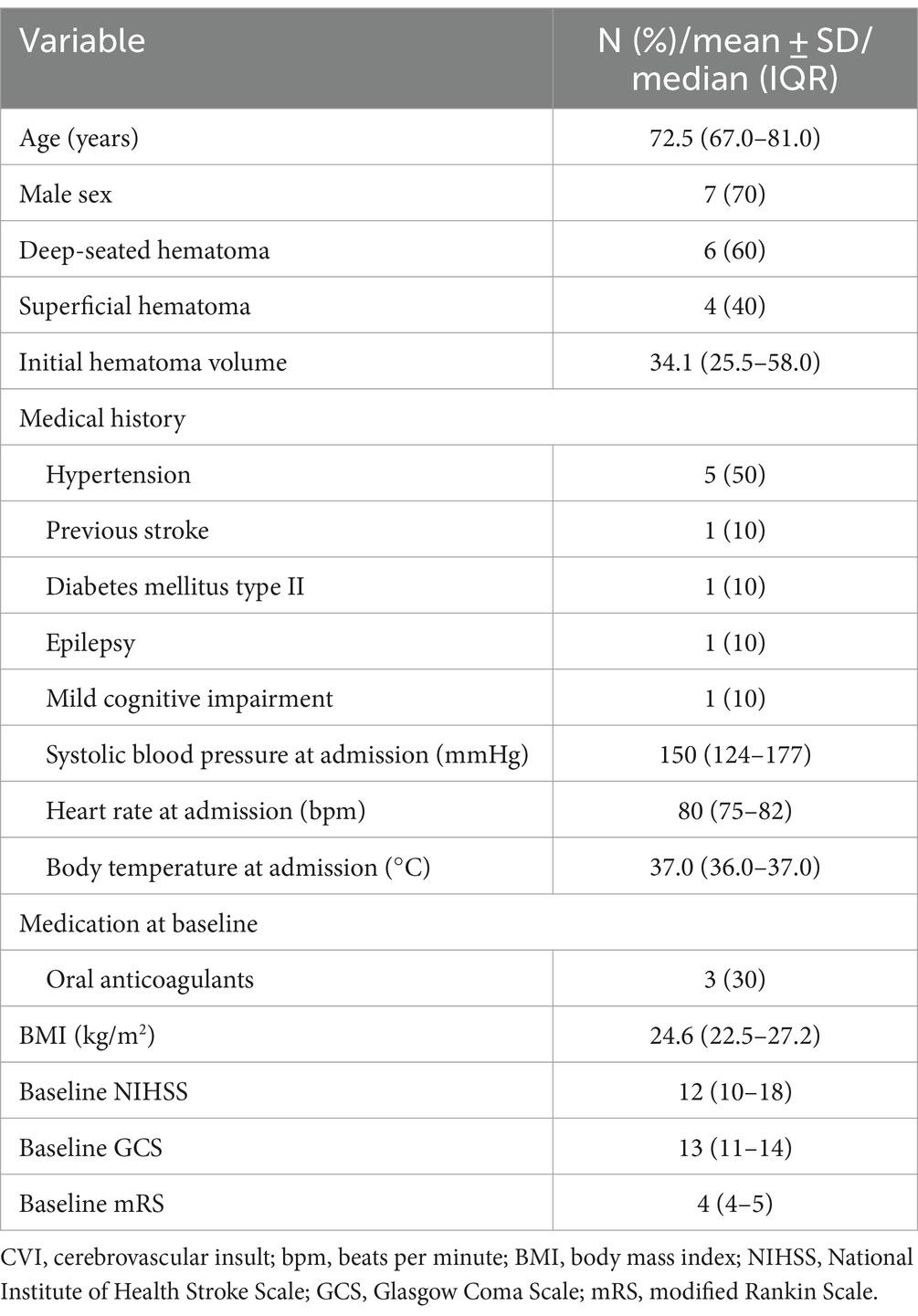
The median age was 72.5 years (IQR 67–81) with a male predominance (n = 7, 70.0%). Deep-seated hematomas were more commonly observed (n = 6, 60%) with a median pre-interventional hematoma volume of 34.1 mL (IQR 25.5–58.0). Three participants (30%) suffered their ICH under oral anticoagulation. Five participants (50%) had a history of hypertension, while previous cerebrovascular ischemia, diabetes mellitus type II, epilepsy, and mild cognitive impairment were reported in one case each. Median baseline systolic blood pressure at admission was 150 (IQR 124–177) mmHg. Median baseline NIHSS score was 12 (IQR 10–18), median baseline GCS was 13 (IQR 11–14) and median mRS was 4 (IQR 4–5) points (Table 1).
3.2 Primary and secondary outcomes
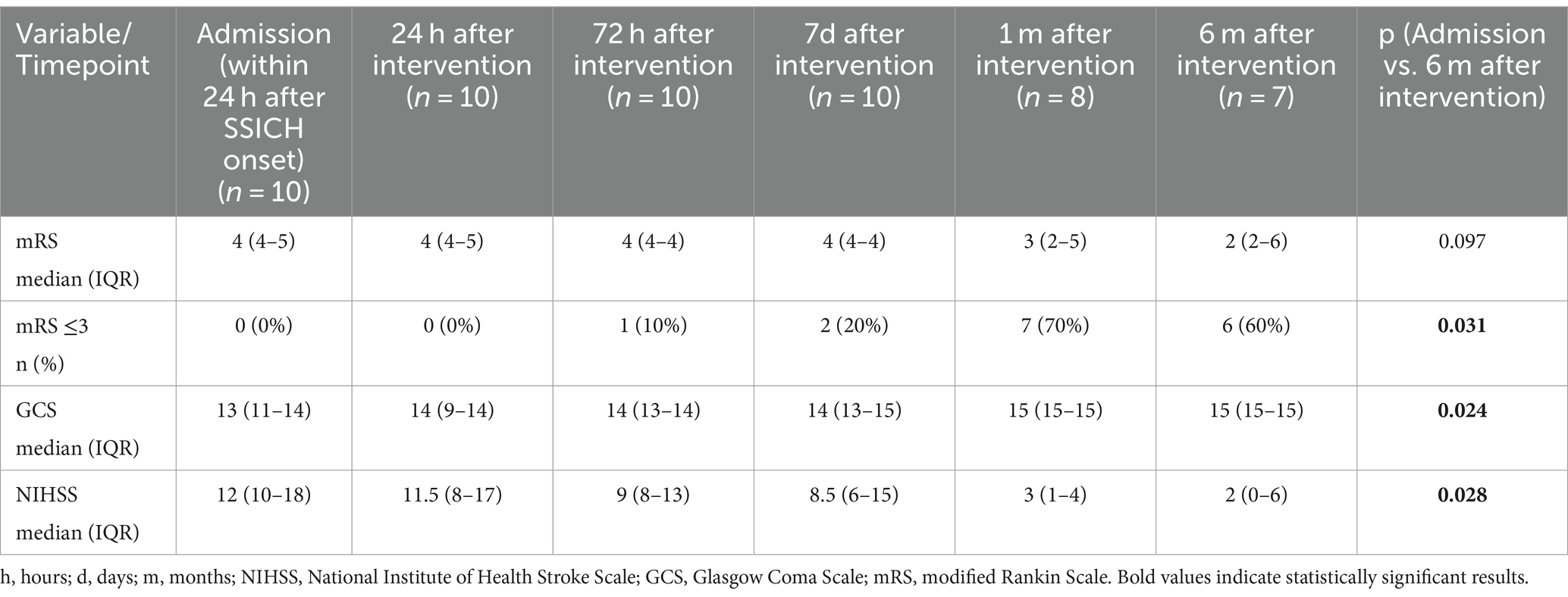
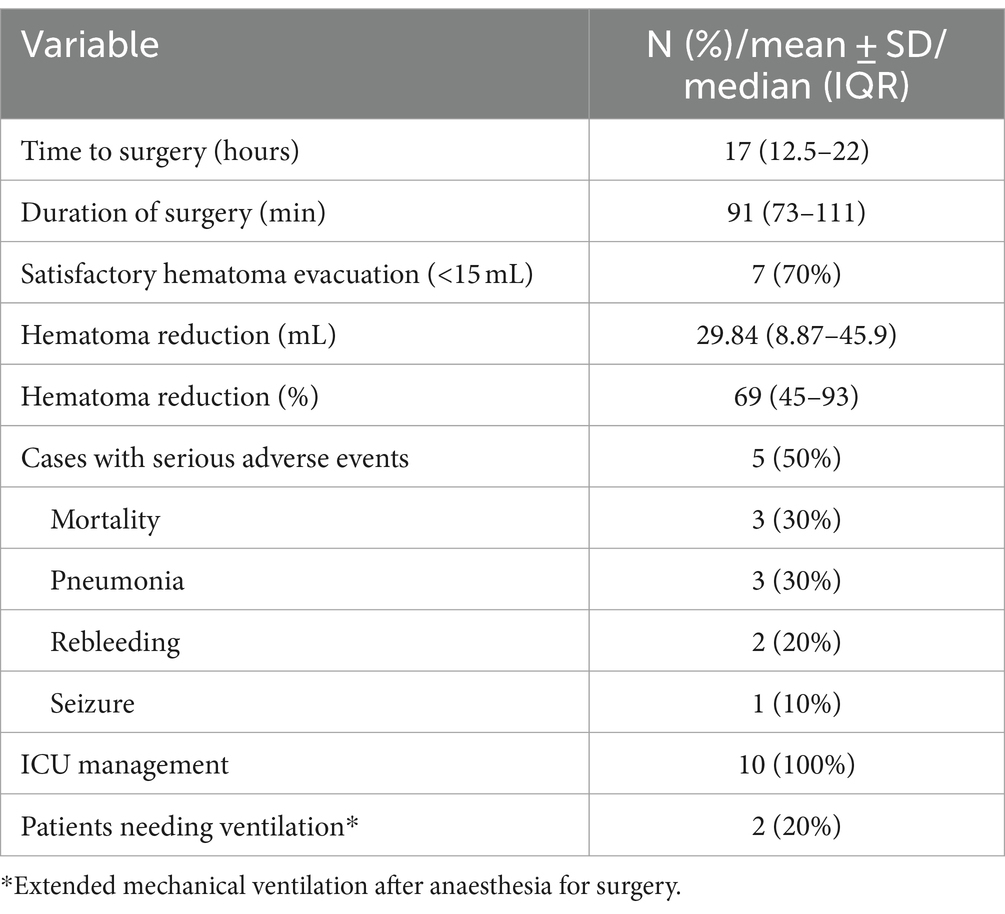
Satisfactory hematoma evacuation was observed in seven participants (70%). We observed a trend toward improvement in the median evacuation rate from the first to the last participant (median evacuation rate of the first five participants: 62.5% (IQR 40–78%) and last five participants: 92.8% (IQR 47–96%), p = 0.251). We recorded six (60%) patients with favorable outcome (Tables 2, 3) with the primary efficacy outcome of a mRS 0–3 at 6 months. The location of the hematoma (deep vs. lobar) appeared to have no influence on satisfactory hematoma evacuation rate or functional outcome at 6 months. Satisfactory hematoma evacuation rate did not influence the rate of favorable outcome at 6 months.
Three participants (30%) died. In two the cause of death may be related to the intervention or sequelae of the underlying disease, respectively, (pneumonia and recurrent hematoma with palliative treatment decision, both between hospital discharge and 1-month follow-up) and one participant died due to a GBM WHO grade IV not related to the intervention or the initial bleeding 6 months after intervention. Median change in hematoma volume was 29.8 (IQR 8.9–45.9) ml, corresponding to a median percentage of 69.51% (IQR 45.3–93.9%) hematoma reduction after surgery. Both, NIHSS and the GCS improved significantly at 6 months compared to baseline (p = 0.024 and p = 0.028, respectively).
Median duration of surgery was 91 (IQR 73–111) minutes (Table 3). Median time to surgery was 17 (IQR 12.5–22) hours after symptom onset while participants were treated in a postoperative ICU setting for the median time of 20 (IQR 15.5–87.0) hours.
3.3 Safety outcomes
A total of six adverse events were seen in five cases (50%); rebleeding (one patient within 72 h after intervention and one around 5 months after intervention. The latter patient presented with an underlying glioblastoma which led to rebleeding in the hematoma cavity) in two participants, pneumonia in three, and epilepsy in one participant (Table 3).
4 Discussion
The EMINENT-ICH pilot trial showed technical feasibility of early minimally invasive endoscopic hematoma evacuation as well as a good safety profile over time without being able to prove efficacy of the intervention by lack of a control group in this pilot study.
4.1 Feasibility and technique of endoscopic hematoma evacuation
This study primarily assessed the feasibility of ES, which is not routinely offered for SSICH removal. Therefore, and according to the IDEAL guidelines, a phase II pilot study is mandatory before conducting a large multicenter randomized trial (30). Due to our small sample size, we refrained from performing an assessment of a potential learning curve. Nevertheless, both junior and senior attendings showed a swift understanding of the technique and key learning points (workflow with the transparent trocar, planning the access and trajectory) could be rapidly implemented. Consequently, the median evacuation rate could be improved from the first to the last participant (median evacuation rate of the first five participants: 62.5% (IQR 40–78%) and last five participants: 92.8% (IQR 47–96%), p = 0.251), similar to reports from previous studies (26, 31). With a median evacuation rate of 69.5% and a proportion of satisfactory hematoma evacuations of 70%, we are well within the technical efficacy reported in previous studies ranging from 54 to 97% (12, 25, 31–34). Our technique is based on the SCUBA technique described by Kellner et al. (22, 35). However, our technique differs from other trials using either the Apollo/Artemis device (DIST, MIND) or the NICO Myriad system (ENRICH) (22, 26, 35–37). We use a transparent sheath (Viewsite Brain Access Device, Vycor medical) as an access device to the hematoma, while hematoma is evacuated using a standard suction cannula and forceps if needed (22). The use of a transparent sheath carries the benefit of a 360° vision during hematoma evacuation which helps detect and evacuate residual hematoma around the sheath (31, 32). As observed in this pilot trial, future phase III trials should promote and adapt pragmatic, real-world eligibility criteria to facilitate enrolment and provide a real-world setting to enhance generalizability. Further patient and public involvement should be implemented to ensure adequate patient retention during the trial.
4.2 Safety and adverse events
We report three deaths (30%) and six adverse events (60%). The mortality rate is comparable to the current literature described for endoscopic hematoma evacuation ranging from 10 to 34.3% (16, 26, 31).
None of the adverse events were directly related to the surgery itself, but rather to the underlying disease. The most frequent adverse event was rebleeding, which occurred in two participants. Factors described to be potentially associated with rebleeding are timing of hematoma evacuation and the presence of a spot sign on the initial CT scan (38, 39). Controversies exist in the literature on what should be the ideal time of hematoma removal. Clearly, early evacuation should be the goal, since in hemorrhagic stroke reports show, that hematoma evacuation within 24 h leads to better functional outcome (18, 19). On the other hand, ultra early evacuation (<4 h) might lead to an unacceptable high percentage of rebleeding (40%) (40). Although some reports have shown that hematoma removal within 8 h might be safe, the rebleeding rate might still be higher than when surgery is delayed to 12 or even 24 h (40, 41). Furthermore, the current literature includes mostly people undergoing craniotomy and not endoscopic hematoma removal, making an inference to endoscopic surgery difficult (41). Based on the preliminary results of some endoscopic trials it seems that the rebleeding rate is overall lower than after surgical evacuation by craniotomy (26). However, earlier treatment could potentially reduce the secondary brain damage produced by toxic breakdown products and as such, the ideal time for ES, causing the least risk for rebleeding but the best chance for improving functional outcome, lies most likely somewhere within 24 h after bleeding onset, but overall still remains a matter of debate (7). We report a median time to surgery of 17 h, which is well within the 24-h time limit, however given that we did not include an active comparator or compared different time windows, no inference on whether 17 h are early enough or not can be concluded. Based on the time points of rebleeding and the eligibility criteria in this trial, it seems unlikely that the rebleeding was related to either early surgery or the presence of an arterial spot-sign. The ongoing studies on endoscopic evacuation of SSICH all have different time windows set for their cohorts, which will potentially provide us with more robust data on the ideal time point of surgical evacuation EVACUATE (NCT04434807), MIND (NCT03342664), and EMINENT-ICH (NCT05681988) (37, 42).
In the earliest endoscopic surgery reports, the presence of a spot sign was independently associated with intraoperative bleeding, requiring skill to manage the bleedings (38). Furthermore, in people with spot sing outcome at 90-days was poorer than in non-spot sign people, as it directly leads to rebleeding/hematoma enlargement (43, 44). However, recent publications have shown, that endoscopic surgery may mitigate the risk of intraoperative bleeding by direct cauterization and therefore surgery in people with a spot sign might not present a higher risk for rebleeding (26, 45).
4.3 Functional outcome
Although this pilot trial was not powered to show efficacy in improving functional outcome, we report comparable favorable outcome rates of ES (60%) with the current largest cohort of endoscopically operated participants in a single arm cohort (33). These observations are in line with previously reported data from several meta-analyses highlighting potential improvement of functional outcome rates in ES (16, 18, 46–49). Compared to other surgical procedures used in STICH I (mostly craniotomy) and MISTIE III (stereotactic catheter placement), ES in our cohort lead to higher rates of improved functional outcome (60% in ES vs. 44% in MISTIE III and 33% in STICH I) although based on our sample size this could be random at best (5, 12). The EMINENT-ICH trial is therefore designed to assess whether ES plus BMT compared to BMT alone improves functional outcome in people with SSICH.
4.4 Limitations
This study comes with several limitations inherent to the study design of any phase II feasibility study. First, we report no active control group, thus limiting the conclusion drawn from this study, besides conclusion on the surgical feasibility and safety. Furthermore, we used rather strict eligibility criteria, which restricted and slowed down our enrolment reduced the generalizability of our results. Due to the rather small sample size, drawing meaningful conclusions on the potential benefits of endoscopic hematoma evacuation on mortality and functional outcome is difficult.
Our pilot trial provides crucial data for the large multicenter randomized controlled trial EMINENT-ICH. The strengths of this phase II pilot trial are a meticulous screening log, screening every person that was admitted to our hospital with any kind of ICH. We used a standardized, published surgical protocol which made a uniform treatment approach possible. Lastly, we designed a pragmatic trial, adding only very little expenditure to the hospital staff, allowing adherence to the study procedures.
5 Conclusion
Early minimally invasive endoscopic surgery in intracerebral hemorrhage appears to be safe, feasible, and leads to satisfactory hematoma evacuation. Based on these results, EMINENT-ICH, a national, multicenter RCT has been launched, with the aim to assess the benefit of ES hematoma removal plus BMT on functional outcome and mortality compared to BMT alone.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors upon reasonable request.
Ethics statement
The studies involving humans were approved by the Ethikkommission Nordwest und Zentralschweiz (EKNZ). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
TH: Data curation, Formal analysis, Investigation, Project administration, Writing – original draft, Writing – review & editing. UF: Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. NG: Data curation, Resources, Writing – original draft, Writing – review & editing. JK: Resources, Writing – original draft, Writing – review & editing. RG: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. LB: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. JS: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by a study grant from the Swiss Heart Foundation (Received by L. Bonati, Co-Applicants Guzman and Soleman) and Swiss National Science Foundation (Grant No. 213616).
Acknowledgments
We would like to thank Birsel Klein-Reesink for her help with all administrative aspects of the trial. We also want to thank our participants and their families for volunteering to participate in this trial.
Conflict of interest
Tim Hallenberger receives a personal scholarship jointly by the Swiss National Science Foundation and the Swiss Academy of Medical Sciences. Soleman and Guzman receive a grant from the Swiss National Science Foundation for the EMINENT-ICH RCT (Grant. No. 213616).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1484255/full#supplementary-material
Abbreviations
BMT, Best medical treatment; ES, Endoscopic surgery; GCS, Glasgow Coma Scale; ICH, Intracerebral hemorrhage; mRS, Modified Rankin Scale; SSICH, Spontaneous supratentorial intracerebral hemorrhage.
References
1. Krishnamurthi, RV, Ikeda, T, and Feigin, VL. Global, regional and country-specific burden of ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage: a systematic analysis of the global burden of disease study 2017. Neuroepidemiology. (2020) 54:171–9. doi: 10.1159/000506396
2. Global, regional, and national burden of stroke and its risk factors. A systematic analysis for the global burden of disease study 2019 (2021). Lancet Neurol. (1990-2019) 20:795–820. doi: 10.1016/s1474-4422(21)00252-0
3. van Asch, CJ, Luitse, MJ, Rinkel, GJ, van der Tweel, I, Algra, A, and Klijn, CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. (2010) 9:167–76. doi: 10.1016/s1474-4422(09)70340-0
4. Poon, MT, Fonville, AF, and Al-Shahi Salman, R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. (2014) 85:660–7. doi: 10.1136/jnnp-2013-306476
5. Mendelow, AD, Gregson, BA, Fernandes, HM, Murray, GD, Teasdale, GM, Hope, DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the international surgical trial in intracerebral Haemorrhage (STICH): a randomised trial. Lancet. (2005) 365:387–97. doi: 10.1016/s0140-6736(05)17826-x
6. Davis, SM, Broderick, J, Hennerici, M, Brun, NC, Diringer, MN, Mayer, SA, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. (2006) 66:1175–81. doi: 10.1212/01.wnl.0000208408.98482.99
7. Xi, G, Keep, RF, and Hoff, JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. (2006) 5:53–63. doi: 10.1016/s1474-4422(05)70283-0
8. Anderson, CS, Heeley, E, Huang, Y, Wang, J, Stapf, C, Delcourt, C, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. (2013) 368:2355–65. doi: 10.1056/NEJMoa1214609
9. Mendelow, AD, Gregson, BA, Rowan, EN, Murray, GD, Gholkar, A, and Mitchell, PM. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. (2013) 382:397–408. doi: 10.1016/s0140-6736(13)60986-1
10. Hanley, DF, Thompson, RE, Muschelli, J, Rosenblum, M, McBee, N, Lane, K, et al. Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (MISTIE): a randomised, controlled, open-label, phase 2 trial. Lancet Neurol. (2016) 15:1228–37. doi: 10.1016/s1474-4422(16)30234-4
11. Qureshi, AI, Palesch, YY, Barsan, WG, Hanley, DF, Hsu, CY, Martin, RL, et al. Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med. (2016) 375:1033–43. doi: 10.1056/NEJMoa1603460
12. Hanley, DF, Thompson, RE, Rosenblum, M, Yenokyan, G, Lane, K, McBee, N, et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. (2019) 393:1021–32. doi: 10.1016/s0140-6736(19)30195-3
13. Greenberg, SM, Ziai, WC, Cordonnier, C, Dowlatshahi, D, Francis, B, Goldstein, JN, et al. 2022 guideline for the Management of Patients with Spontaneous Intracerebral Hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. (2022) 53:e282–361. doi: 10.1161/STR.0000000000000407
14. Cordonnier, C, Demchuk, A, Ziai, W, and Anderson, CS. Intracerebral haemorrhage: current approaches to acute management. Lancet. (2018) 392:1257–68. doi: 10.1016/S0140-6736(18)31878-6
15. de Oliveira Manoel, AL . Surgery for spontaneous intracerebral hemorrhage. Crit Care. (2020) 24:45. doi: 10.1186/s13054-020-2749-2
16. Hallenberger, TJ, Guzman, R, Bonati, LH, Greuter, L, and Soleman, J. Endoscopic surgery for spontaneous supratentorial intracerebral haemorrhage: a systematic review and meta-analysis. Front Neurol. (2022) 13:1054106. doi: 10.3389/fneur.2022.1054106
17. Pradilla, G, Ratcliff, JJ, Hall, AJ, Saville, BR, Allen, JW, Paulon, G, et al. Trial of early minimally invasive removal of intracerebral hemorrhage. N Engl J Med. (2024) 390:1277–89. doi: 10.1056/NEJMoa2308440
18. Scaggiante, J, Zhang, X, Mocco, J, and Kellner, CP. Minimally invasive surgery for intracerebral hemorrhage. Stroke. (2018) 49:2612–20. doi: 10.1161/strokeaha.118.020688
19. Kellner, CP, Song, R, Ali, M, Nistal, DA, Samarage, M, Dangayach, NS, et al. Time to evacuation and functional outcome after minimally invasive endoscopic intracerebral hemorrhage evacuation. Stroke. (2021) 52:e536–9. doi: 10.1161/strokeaha.121.034392
20. Kothari, RU, Brott, T, Broderick, JP, Barsan, WG, Sauerbeck, LR, Zuccarello, M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. (1996) 27:1304–5. doi: 10.1161/01.str.27.8.1304
21. Hemphill, JC, Greenberg, SM, Anderson, CS, Becker, K, Bendok, BR, Cushman, M, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2015) 46:2032–60. doi: 10.1161/str.0000000000000069
22. Hallenberger, TJ, Guzman, R, and Soleman, J. Minimally invasive image-guided endoscopic evacuation of intracerebral haemorrhage: how I do it. Acta Neurochir. (2022) 165:1597–602. doi: 10.1007/s00701-022-05326-3
23. Dammers, R, Beck, J, Volovici, V, Anderson, CS, and Klijn, CJM. Advancing the surgical treatment of intracerebral hemorrhage: study design and research directions. World Neurosurg. (2022) 161:367–75. doi: 10.1016/j.wneu.2022.01.084
24. Miller, CM, Vespa, P, Saver, JL, Kidwell, CS, Carmichael, ST, Alger, J, et al. Image-guided endoscopic evacuation of spontaneous intracerebral hemorrhage. Surg Neurol. (2008) 69:441–6. doi: 10.1016/j.surneu.2007.12.016
25. Vespa, P, Hanley, D, Betz, J, Hoffer, A, Engh, J, Carter, R, et al. ICES (intraoperative stereotactic computed tomography-guided endoscopic surgery) for brain hemorrhage: a multicenter randomized controlled trial. Stroke. (2016) 47:2749–55. doi: 10.1161/strokeaha.116.013837
26. Sondag, L, Schreuder, F, Pegge, SAH, Coutinho, JM, Dippel, DWJ, Janssen, PM, et al. Safety and technical efficacy of early minimally invasive endoscopy-guided surgery for intracerebral haemorrhage: the Dutch intracerebral haemorrhage surgery trial pilot study. Acta Neurochir. (2023) 165:1585–96. doi: 10.1007/s00701-023-05599-2
27. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 310:2191–4. doi: 10.1001/jama.2013.281053
28. Schulz, KF, Altman, DG, and Moher, D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. (2010) 8:18. doi: 10.1186/1741-7015-8-18
29. Eldridge, SM, Chan, CL, Campbell, MJ, Bond, CM, Hopewell, S, Thabane, L, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. (2016) 355:i5239. doi: 10.1136/bmj.i5239
30. MacDougall, M, Cameron, HS, and Maxwell, SRJ. Medical graduate views on statistical learning needs for clinical practice: a comprehensive survey. BMC Med Educ. (2019) 20:1. doi: 10.1186/s12909-019-1842-1
31. Ma, L, Hou, Y, Zhu, R, and Chen, X. Endoscopic evacuation of basal ganglia hematoma: surgical technique, outcome, and learning curve. World Neurosurg. (2017) 101:57–68. doi: 10.1016/j.wneu.2017.01.072
32. Nishihara, T, Teraoka, A, Morita, A, Ueki, K, Takai, K, and Kirino, T. A transparent sheath for endoscopic surgery and its application in surgical evacuation of spontaneous intracerebral hematomas: technical note. J Neurosurg. (2000) 92:1053–5. doi: 10.3171/jns.2000.92.6.1053
33. Kellner, CP, Song, R, Pan, J, Nistal, DA, Scaggiante, J, Chartrain, AG, et al. Long-term functional outcome following minimally invasive endoscopic intracerebral hemorrhage evacuation. J Neurointerventi Surg. (2020) 12:489–94. doi: 10.1136/neurintsurg-2019-015528
34. Sun, G-C, Chen, X-L, Hou, Y-Z, Yu, X-G, Ma, X-D, Liu, G, et al. Image-guided endoscopic surgery for spontaneous supratentorial intracerebral hematoma. J Neurosurg. (2017) 127:537–42. doi: 10.3171/2016.7.JNS16932
35. Kellner, CP, Chartrain, AG, Nistal, DA, Scaggiante, J, Hom, D, Ghatan, S, et al. The stereotactic intracerebral hemorrhage underwater blood aspiration (SCUBA) technique for minimally invasive endoscopic intracerebral hemorrhage evacuation. J Neurointervent Surg. (2018) 10:771–6. doi: 10.1136/neurintsurg-2017-013719
36. Song, R, Ali, M, Smith, C, Jankowitz, B, Hom, D, Mocco, J, et al. Initial experience with the NICO myriad device for minimally invasive endoscopic evacuation of intracerebral hemorrhage. Operat Neurosurg. (2022) 23:194–9. doi: 10.1227/ons.0000000000000304
37. Ratcliff, JJ, Hall, AJ, Porto, E, Saville, BR, Lewis, RJ, Allen, JW, et al. Early minimally invasive removal of intracerebral hemorrhage (ENRICH): study protocol for a multi-centered two-arm randomized adaptive trial. Front Neurol. (2023) 14:1126958. doi: 10.3389/fneur.2023.1126958
38. Miki, K, Yagi, K, Nonaka, M, Iwaasa, M, Abe, H, Morishita, T, et al. Intraoperative active bleeding in endoscopic surgery for spontaneous intracerebral hemorrhage is predicted by the spot sign. World Neurosurg. (2018) 116:e513–8. doi: 10.1016/j.wneu.2018.05.022
39. Demchuk, AM, Dowlatshahi, D, Rodriguez-Luna, D, Molina, CA, Blas, YS, Dzialowski, I, et al. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. Lancet Neurol. (2012) 11:307–14. doi: 10.1016/S1474-4422(12)70038-8
40. Morgenstern, LB, Demchuk, AM, Kim, DH, Frankowski, RF, and Grotta, JC. Rebleeding leads to poor outcome in ultra-early craniotomy for intracerebral hemorrhage. Neurology. (2001) 56:1294–9. doi: 10.1212/wnl.56.10.1294
41. Gregson, BA, Broderick, JP, Auer, LM, Batjer, H, Chen, X-C, Juvela, S, et al. Individual patient data subgroup Meta-analysis of surgery for spontaneous Supratentorial intracerebral hemorrhage. Stroke. (2012) 43:1496–504. doi: 10.1161/STROKEAHA.111.640284
42. Klijn, CJ, and Dammers, R (2022) Dutch ICH surgery trial; minimally invasive endoscopy-guided surgery for spontaneous supratentorial intracerebral hemorrhage.
43. Miki, K, Yagi, K, Nonaka, M, Iwaasa, M, Abe, H, Morishita, T, et al. Spot sign as a predictor of rebleeding after endoscopic surgery for intracerebral hemorrhage. J Neurosurg. (2019) 130:1485–90. doi: 10.3171/2017.12.JNS172335
44. Miki, K, Abe, H, Nonaka, M, Morishita, T, Iwaasa, M, Arima, H, et al. Impact of spot sign etiology in Supratentorial intracerebral hemorrhage on outcomes of endoscopic surgery. World Neurosurg. (2020) 133:e281–7. doi: 10.1016/j.wneu.2019.08.244
45. Nagasaka, T, Inao, S, and Wakabayashi, T. What does the CT angiography "spot sign" of intracerebral hemorrhage mean in modern neurosurgical settings with minimally invasive endoscopic techniques? Neurosurg Rev. (2013) 36:341–8. doi: 10.1007/s10143-012-0437-7
46. Yao, Z, Hu, X, You, C, and He, M. Effect and feasibility of endoscopic surgery in spontaneous intracerebral hemorrhage: a systematic review and meta-analysis. World Neurosurg. (2018) 113:348–356.e2. doi: 10.1016/j.wneu.2018.02.022
47. Guo, G, Pan, C, Guo, W, Bai, S, Nie, H, Feng, Y, et al. Efficacy and safety of four interventions for spontaneous supratentorial intracerebral hemorrhage: a network meta-analysis. J Neurointerv Surg. (2020) 12:598–604. doi: 10.1136/neurintsurg-2019-015362
48. Li, M, Mu, F, Su, D, Han, Q, Guo, Z, and Chen, T. Different surgical interventions for patients with spontaneous supratentorial intracranial hemorrhage: a network meta-analysis. Clin Neurol Neurosurg. (2020) 188:105617. doi: 10.1016/j.clineuro.2019.105617
Keywords: endoscopic surgery, intracerebral hemorrhage, functional outcome, pilot trial, neurosurgery
Citation: Hallenberger TJ, Fischer U, Ghosh N, Kuhle J, Guzman R, Bonati LH and Soleman J (2024) Early minimally invasive image-guided eNdoscopic evacuation of iNTracerebral hemorrhage: a phase II pilot trial. Front. Neurol. 15:1484255. doi: 10.3389/fneur.2024.1484255
Edited by:
Maurizio Acampa, Siena University Hospital, ItalyReviewed by:
Simon Fandler-Höfler, Medical University of Graz, AustriaTom Moullaali, University of Edinburgh, United Kingdom
Chaohua Cui, Affiliated Liutie Central Hospital of Guangxi Medical University, China
Radek Frič, Oslo University Hospital, Norway
Copyright © 2024 Hallenberger, Fischer, Ghosh, Kuhle, Guzman, Bonati and Soleman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tim Jonas Hallenberger, dGltLmhhbGxlbmJlcmdlckB1c2IuY2g=
†These authors share last authorship
 Tim Jonas Hallenberger
Tim Jonas Hallenberger Urs Fischer
Urs Fischer Nilabh Ghosh4
Nilabh Ghosh4 Jens Kuhle
Jens Kuhle Raphael Guzman
Raphael Guzman Jehuda Soleman
Jehuda Soleman